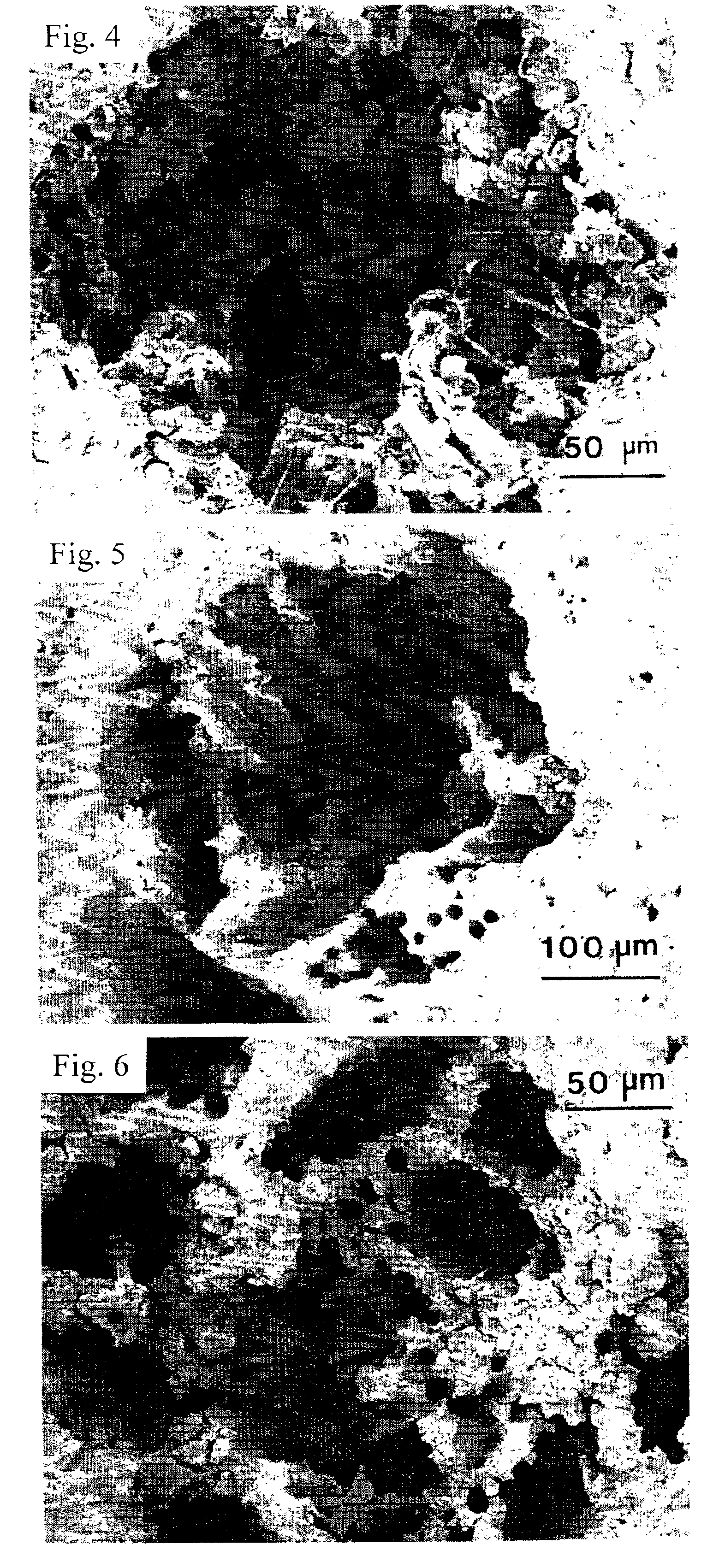
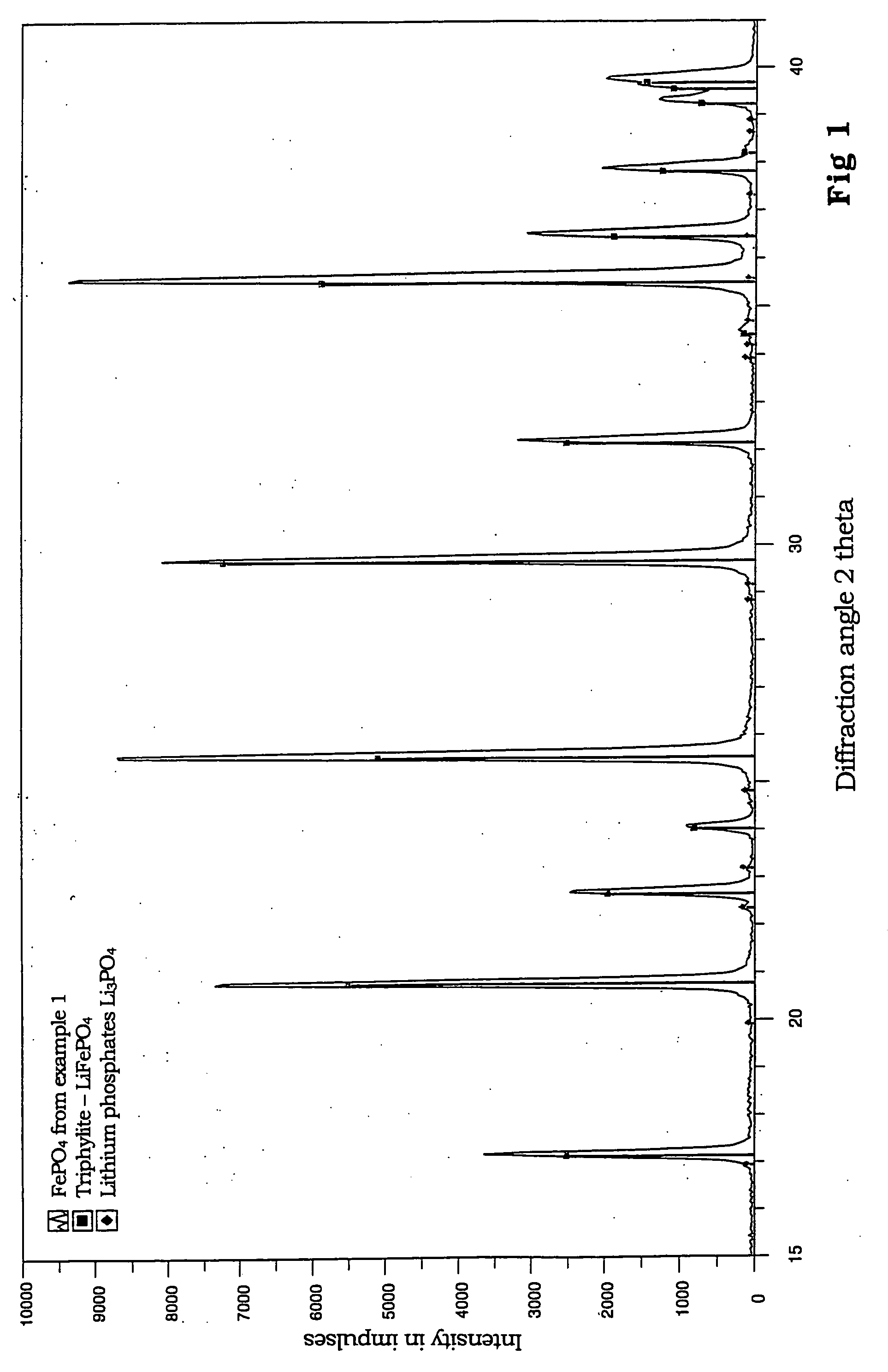
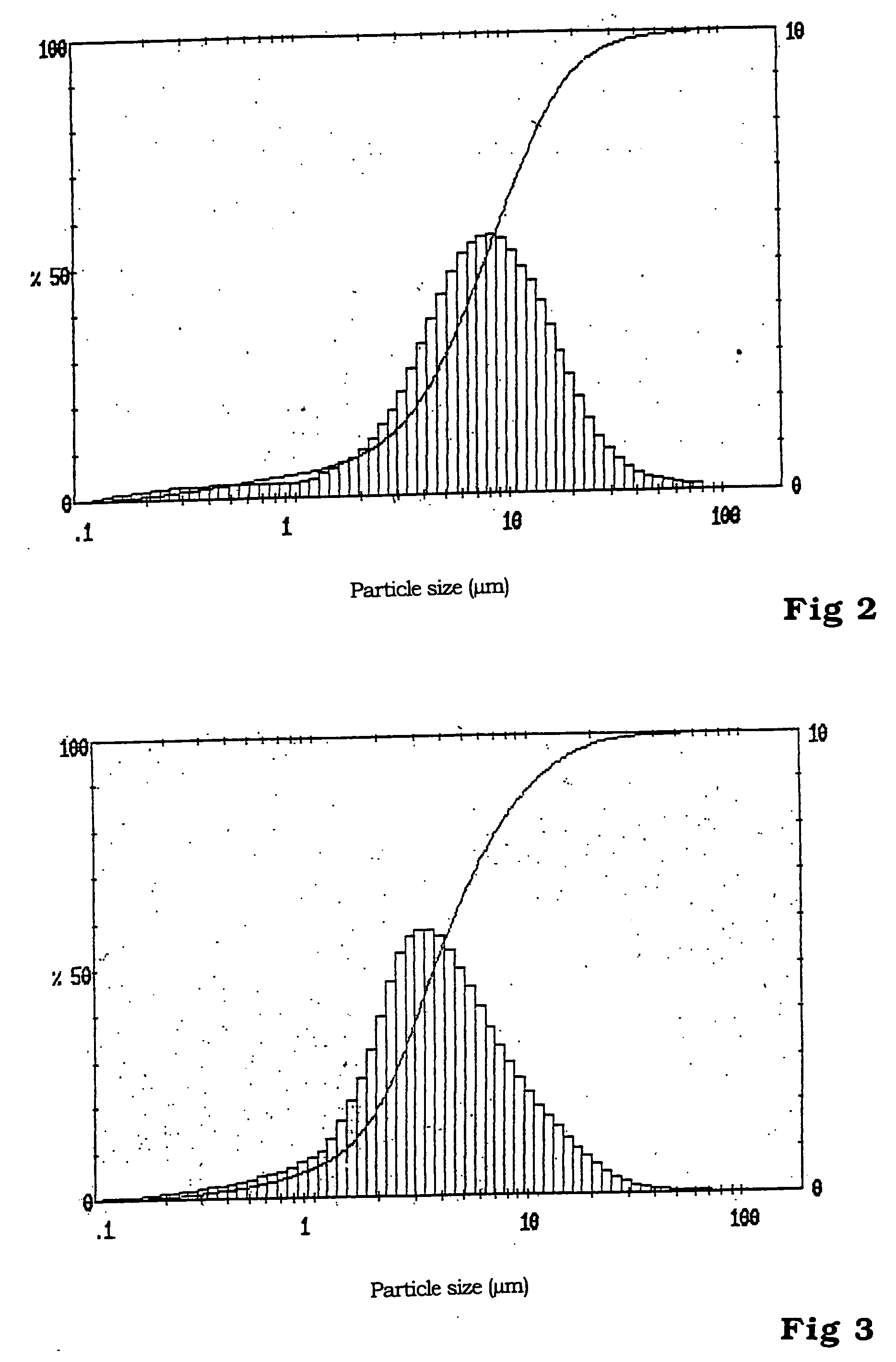
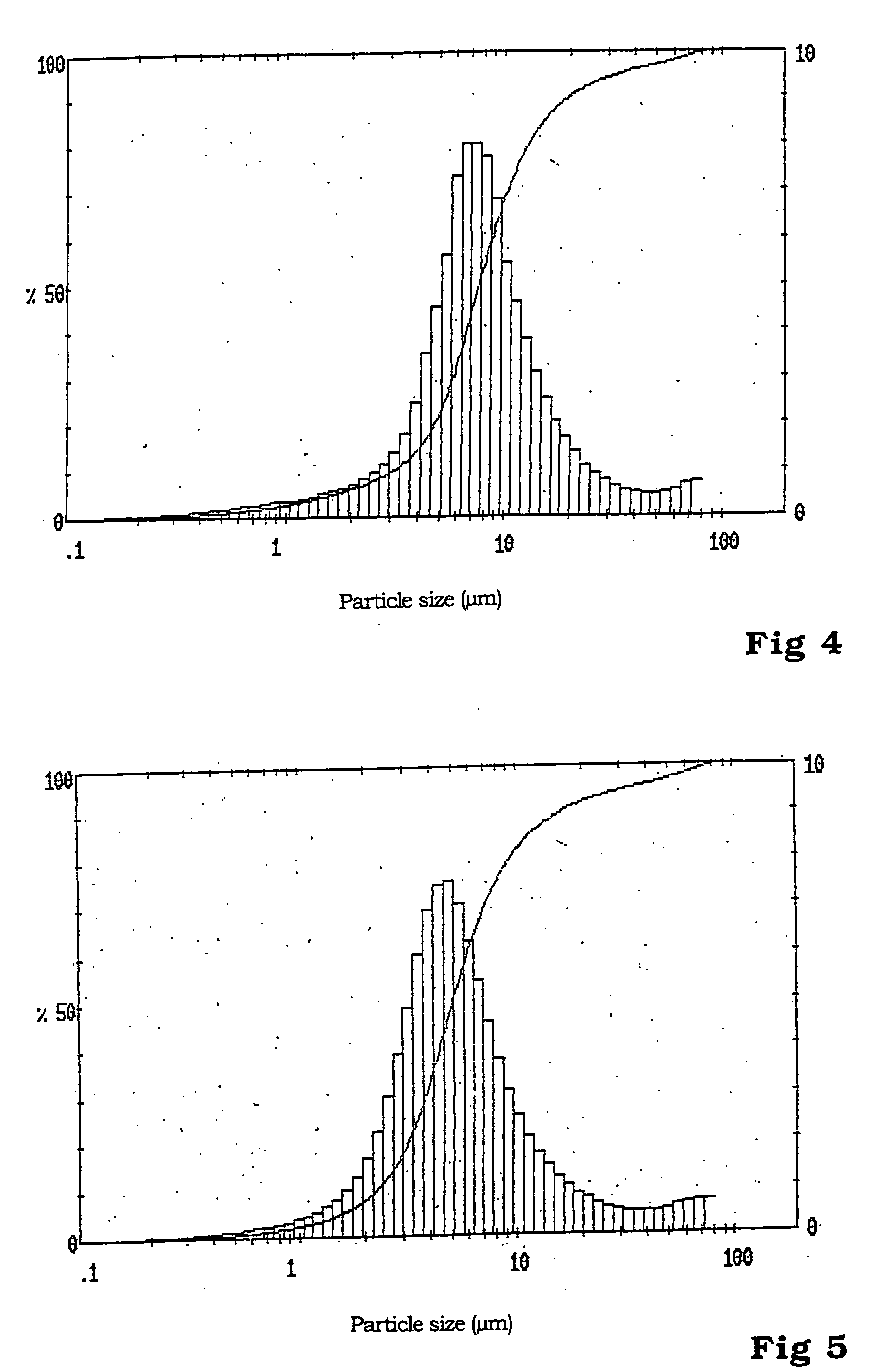
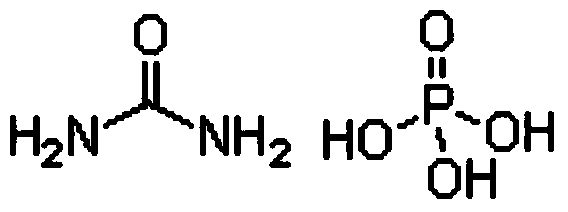Patents
Literature
1748results about "Phosphates" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
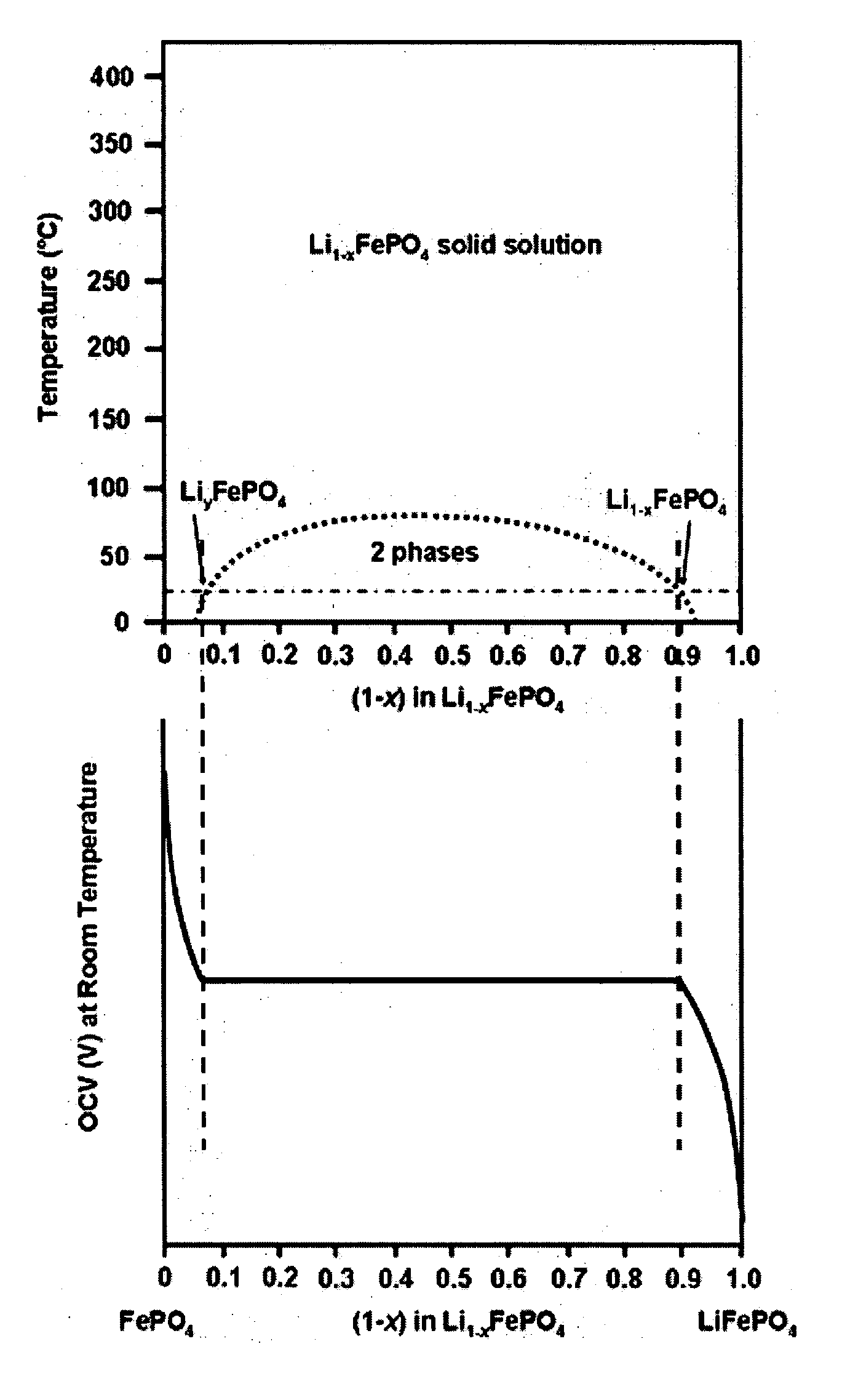
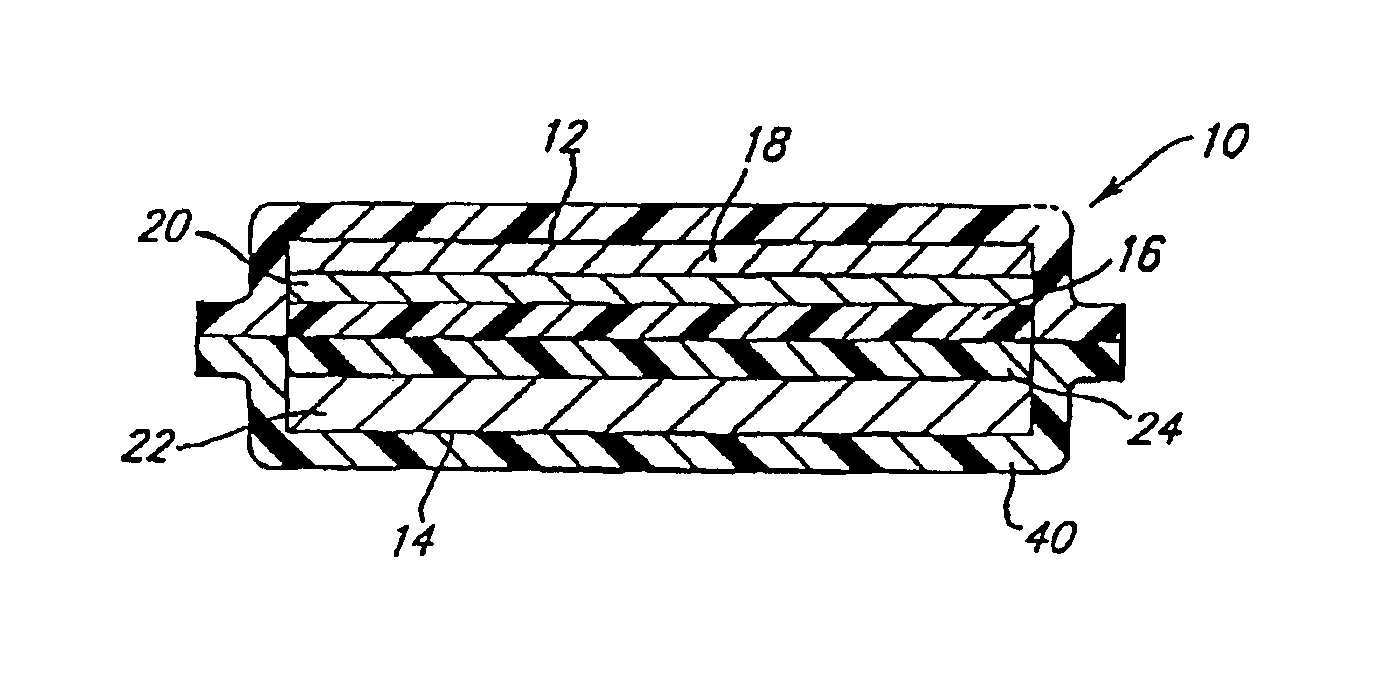
Conductive lithium storage electrode
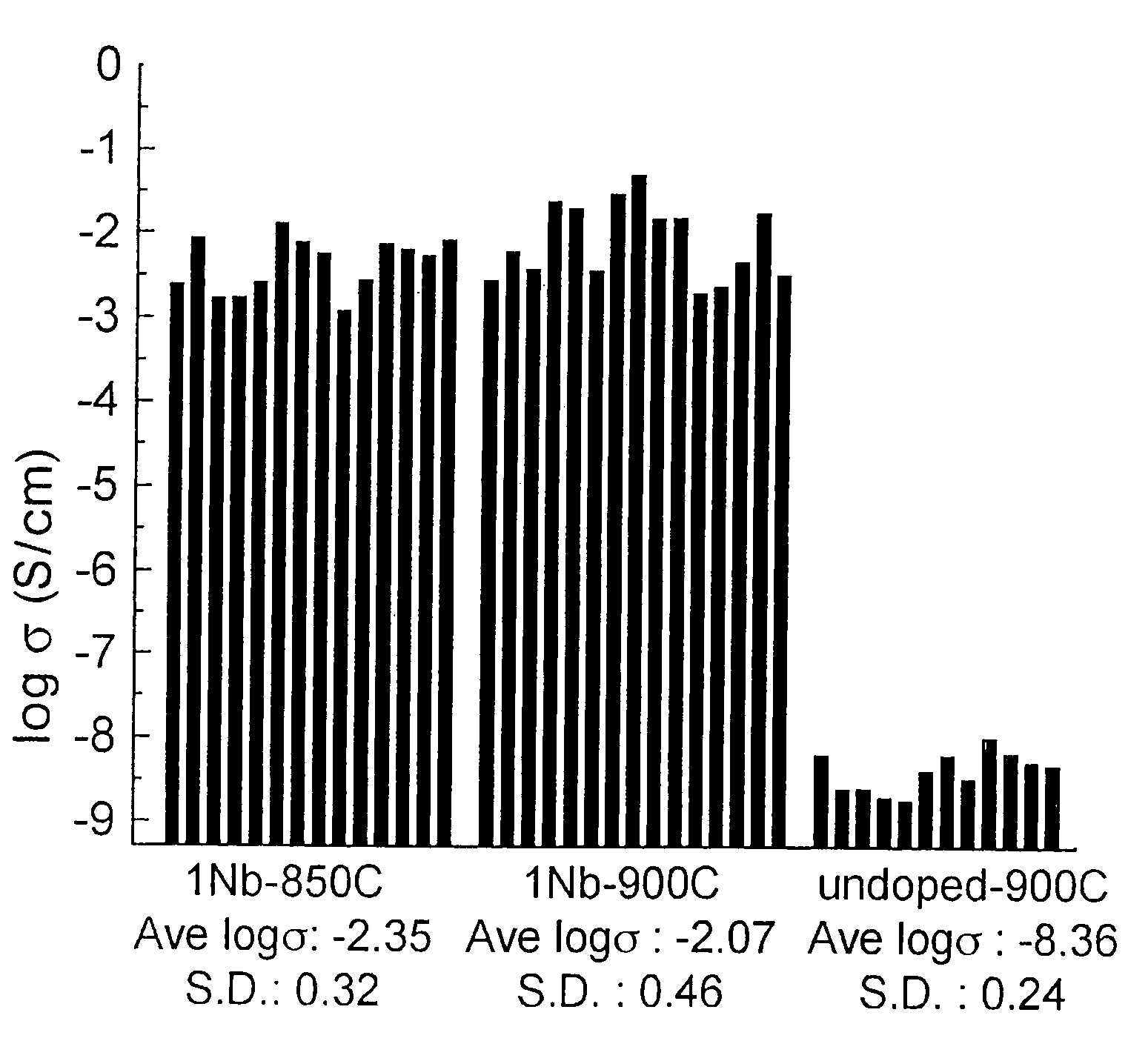
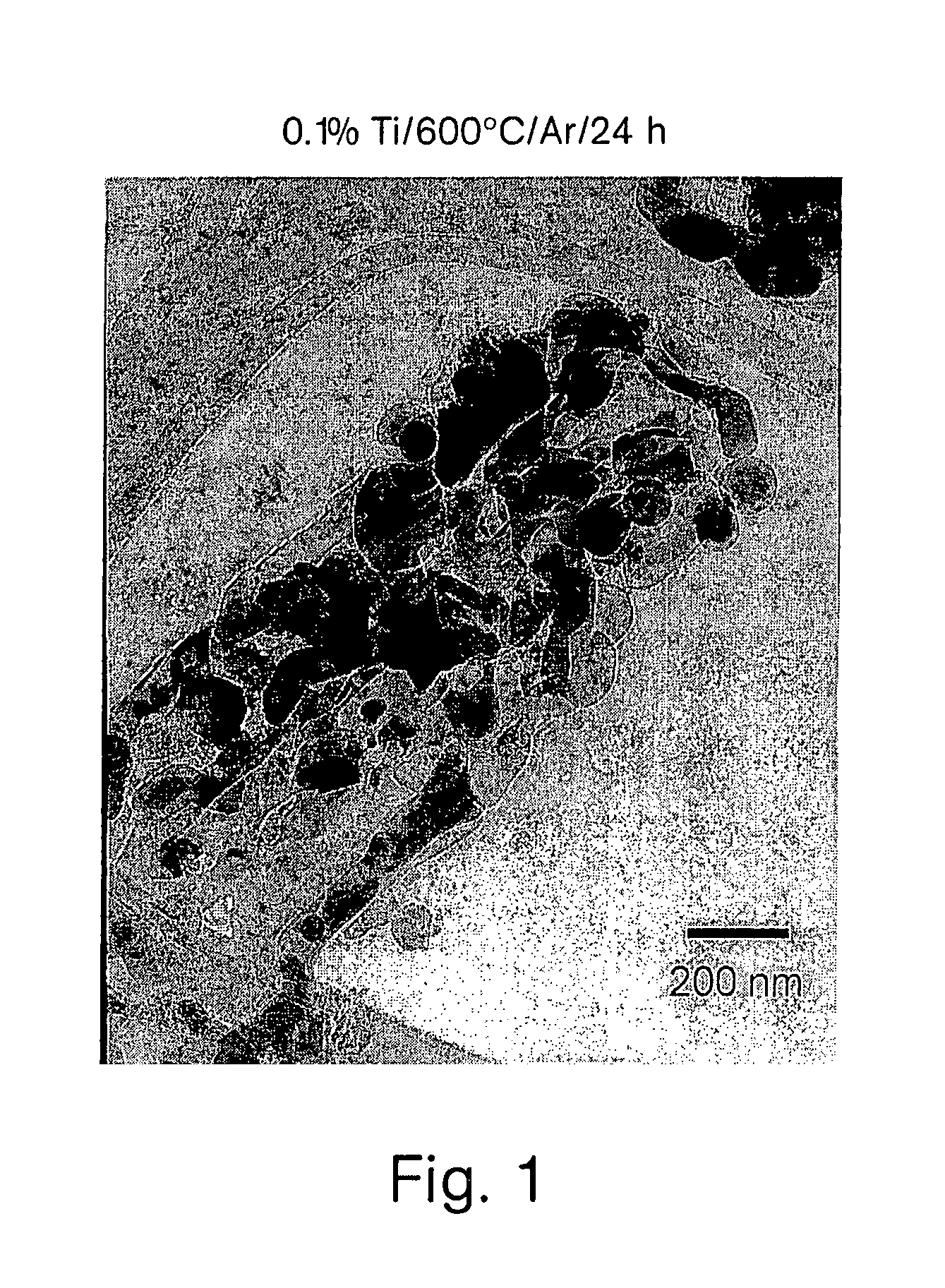
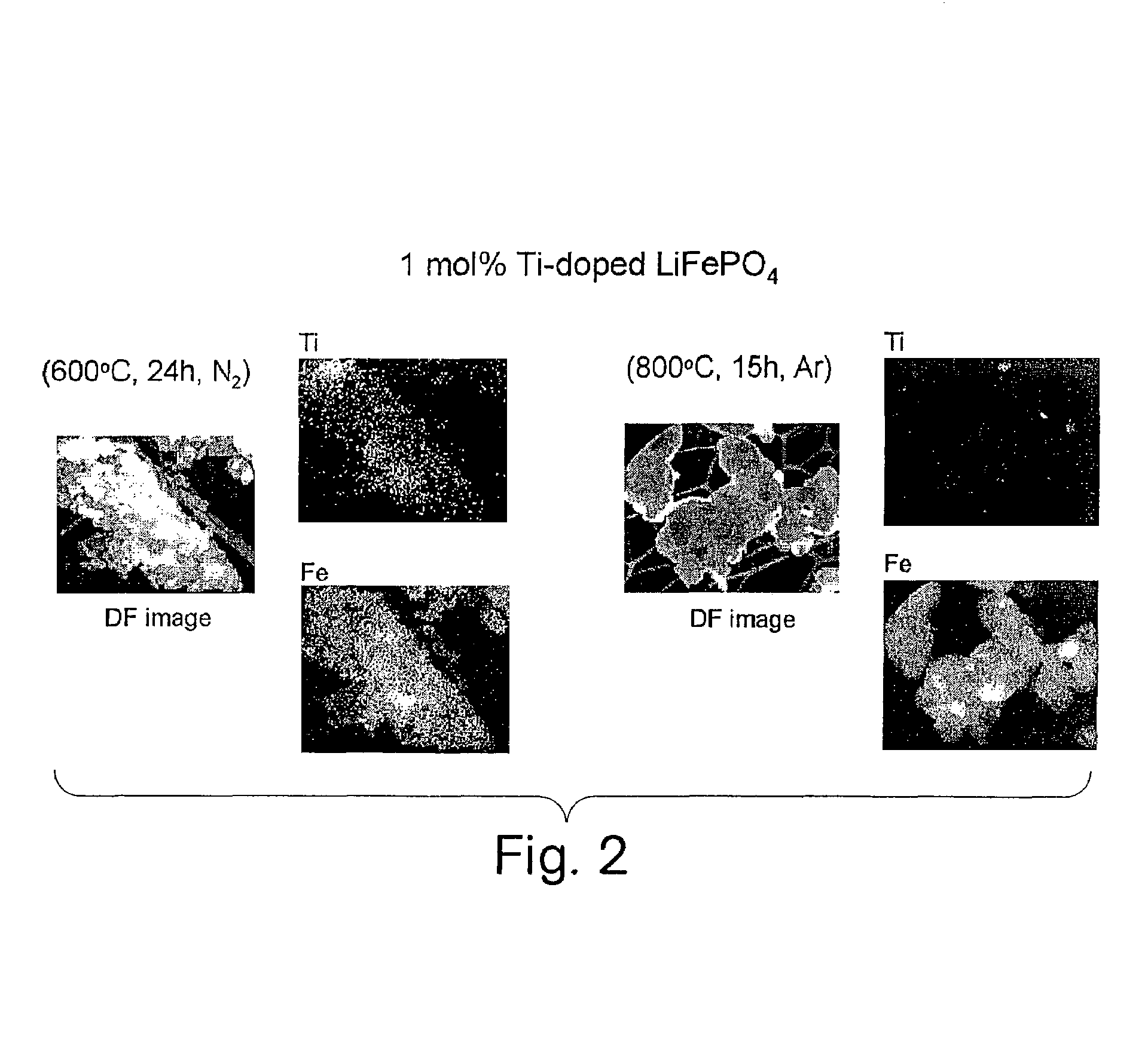
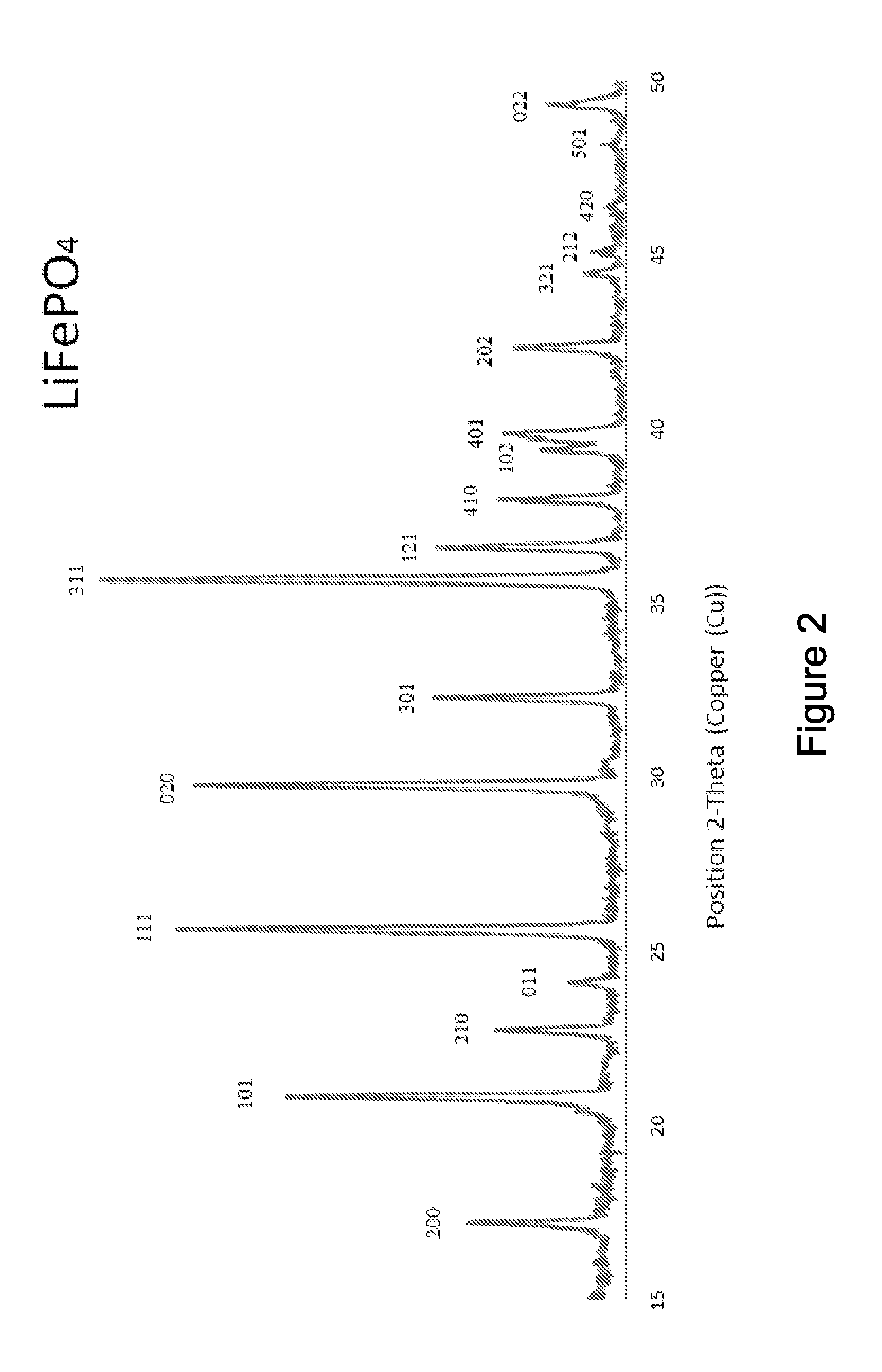
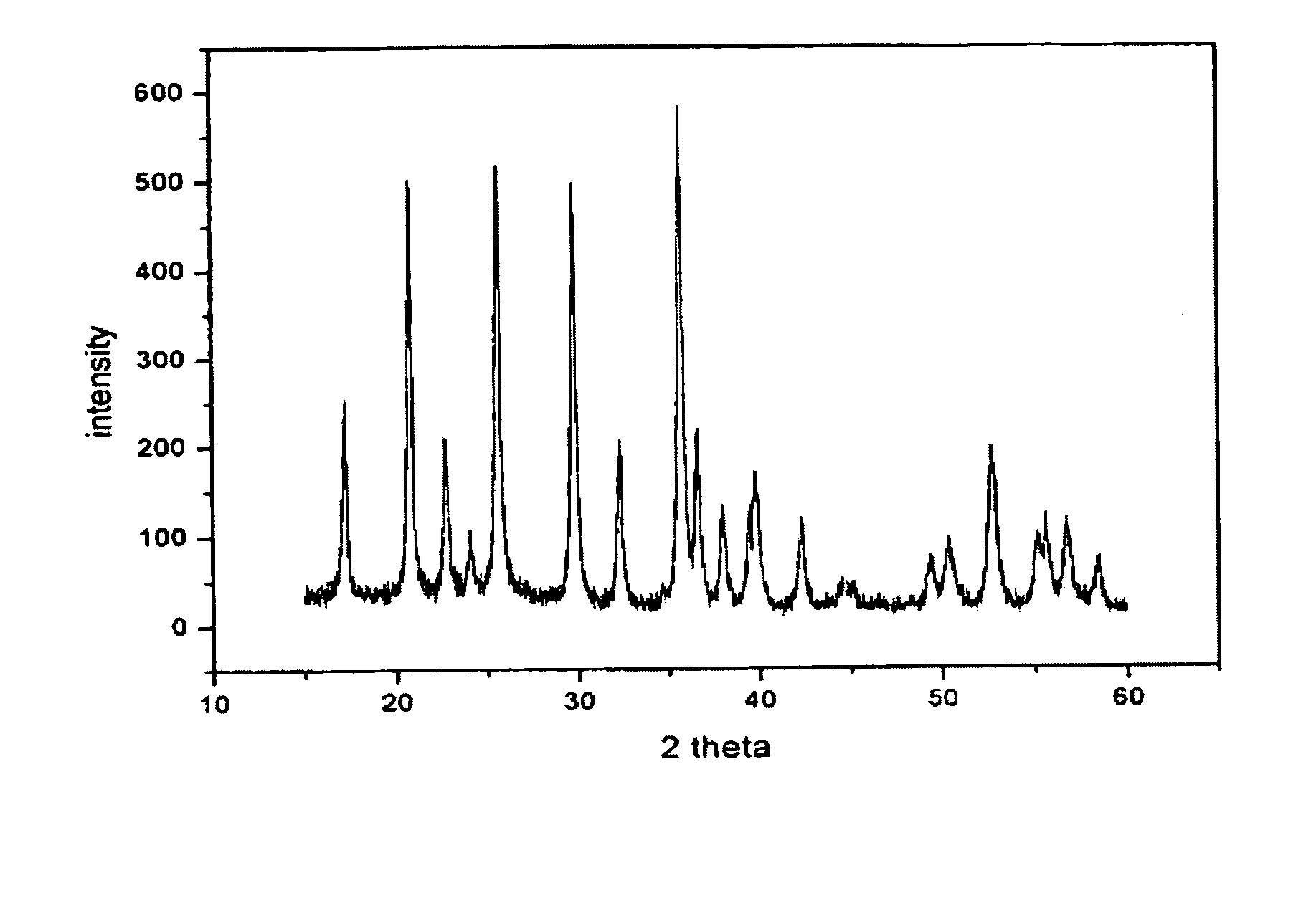
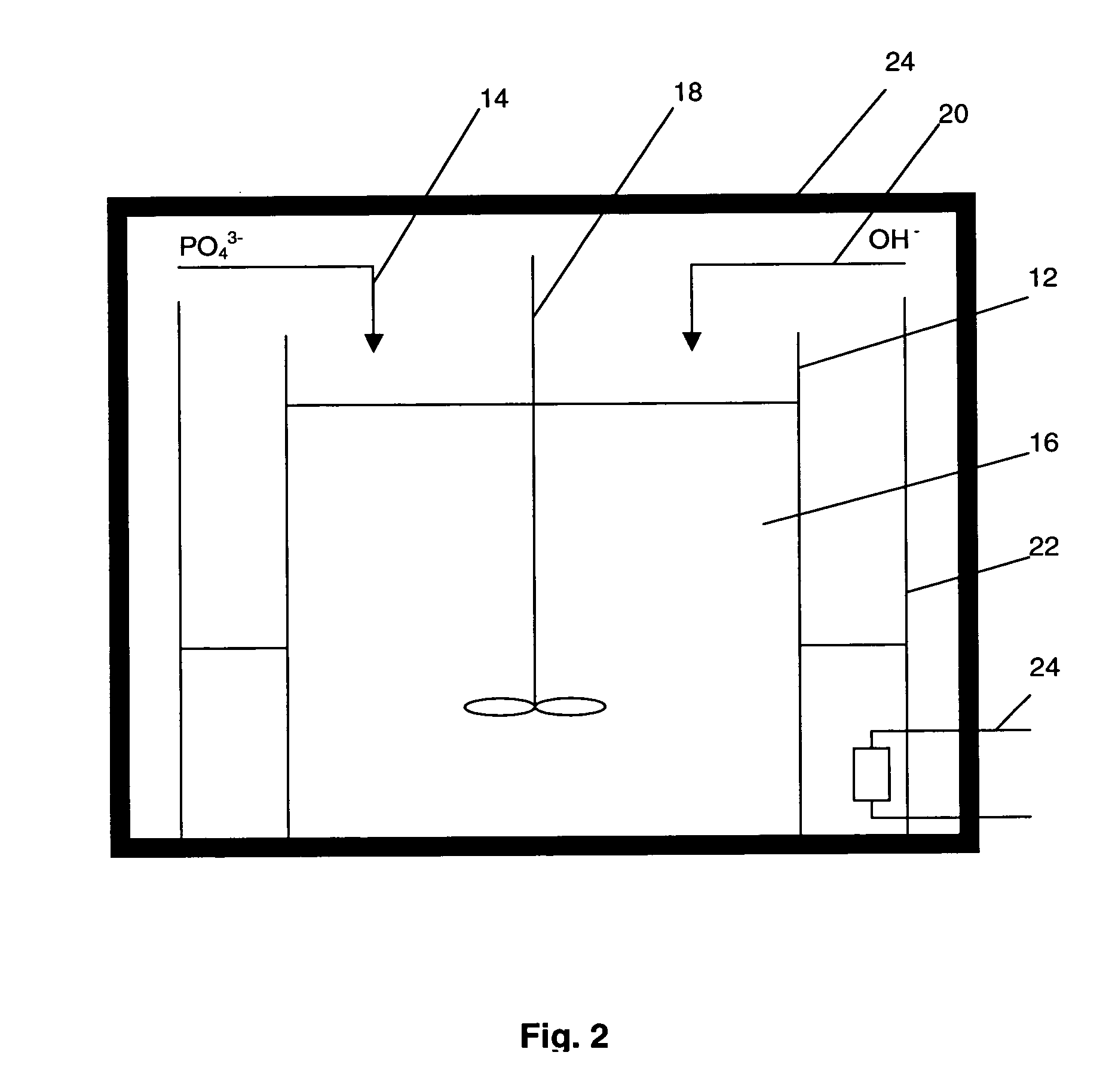
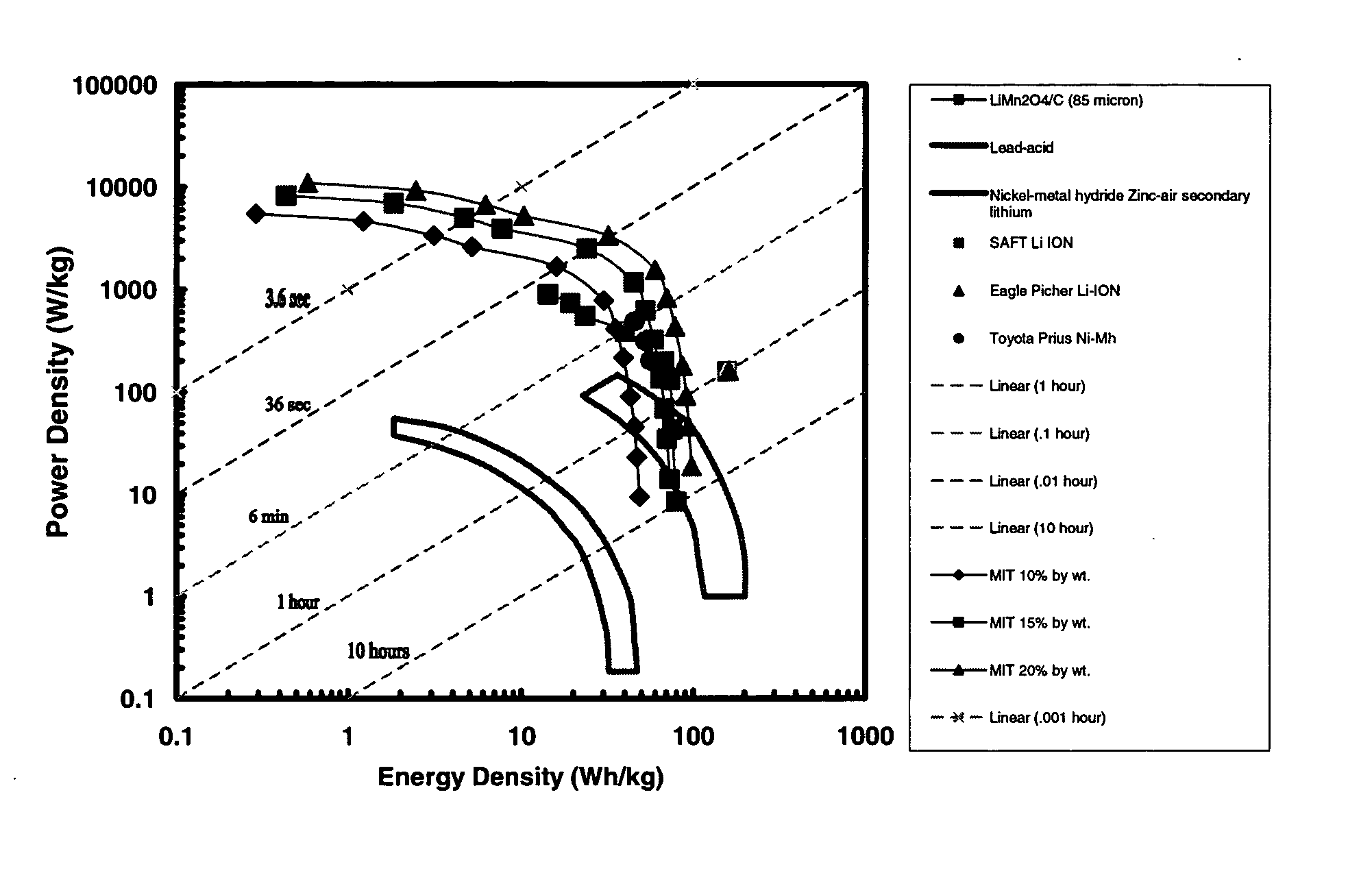
A compound comprising a composition Ax(M′1-aM″a)y(XD4)z, Ax(M′1-aM″a)y(DXD4)z, or Ax(M′1-aM″a)y(X2D7)z, and have values such that x, plus y(1-a) times a formal valence or valences of M′, plus ya times a formal valence or valence of M″, is equal to z times a formal valence of the XD4, X2D7, or DXD4 group; or a compound comprising a composition (A1-aM″a)xM′y(XD4)z, (A1-aM″a)xM′y(DXD4)z(A1-aM″a)xM′y(X2D7)z and have values such that (1-a)x plus the quantity ax times the formal valence or valences of M″ plus y times the formal valence or valences of M′ is equal to z times the formal valence of the XD4, X2D7 or DXD4 group. In the compound, A is at least one of an alkali metal and hydrogen, M′ is a first-row transition metal, X is at least one of phosphorus, sulfur, arsenic, molybdenum, and tungsten, M″ any of a Group IIA, IIIA, IVA, VA, VIA, VIIA, VIIIA, IB, IIB, IIIB, IVB, VB, and VIB metal, D is at least one of oxygen, nitrogen, carbon, or a halogen, 0.0001<a≦0.1, and x, y, and z are greater than zero. The compound can have a conductivity at 27° C. of at least about 10−8 S / cm. The compound can be a doped lithium phosphate that can intercalate lithium or hydrogen. The compound can be used in an electrochemical device including electrodes and storage batteries and can have a gravimetric capacity of at least about 80 mAh / g while being charged / discharged at greater than about C rate of the compound.
Owner:MASSACHUSETTS INST OF TECH
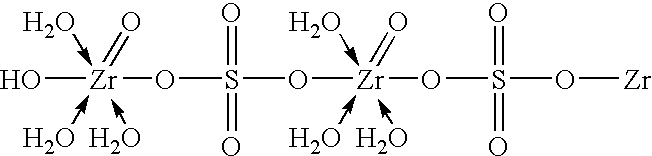
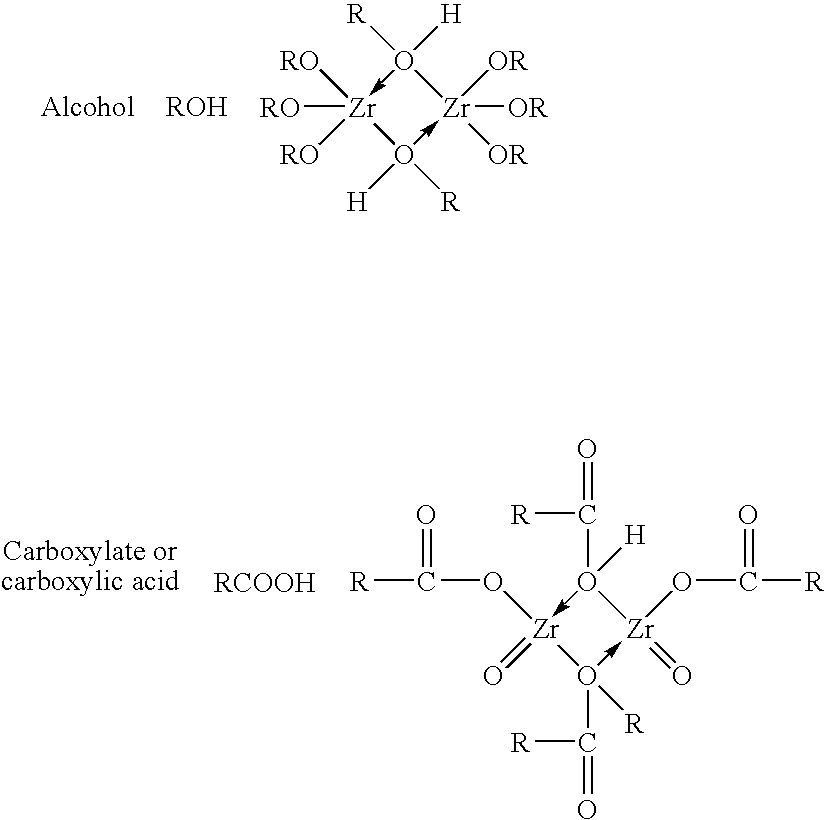
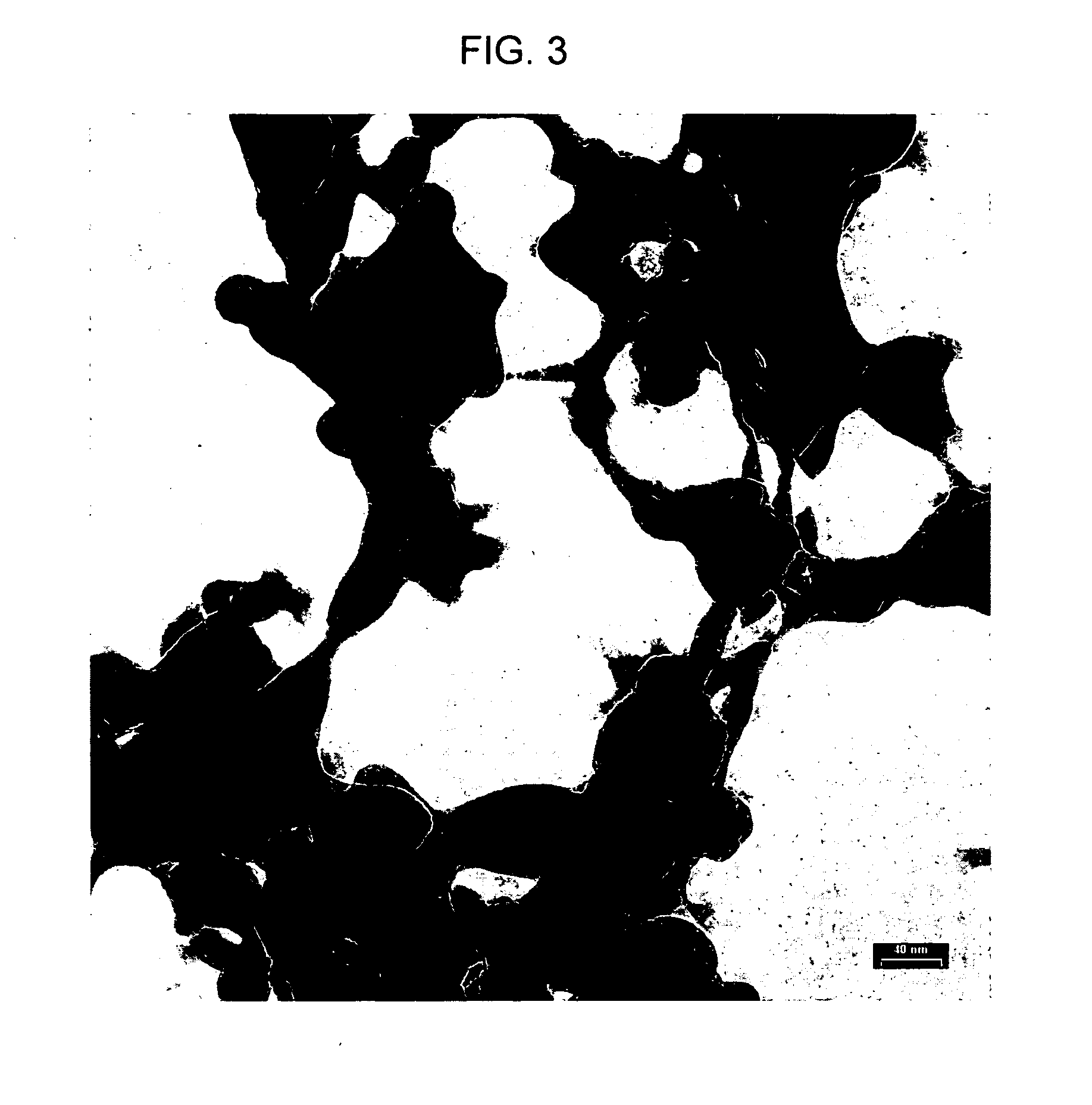
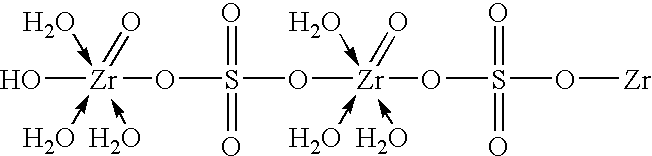
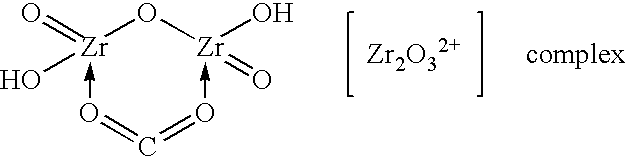
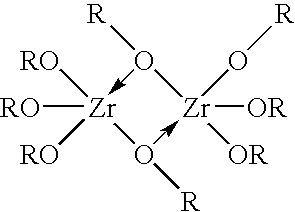
Method of synthesizing zirconium phosphate particles
InactiveUS7566432B2Inhibition of agglomerationReduce moistureSemi-permeable membranesPhosphatesO-Phosphoric AcidZirconium oxychloride
Zirconium phosphate particles are synthesized by providing a solution of zirconium oxychloride in an aqueous solvent, adding at least one oxygen-containing additive to the solution, the oxygen-containing additive being selected to form a complex with zirconium ions in the solution of zirconium oxychloride and thereby reduce hydration of the zirconium ions, and combining this solution with phosphoric acid or a phosphoric acid salt to obtain zirconium phosphate particles by sol gel precipitation.
Owner:RENAL SOLUTIONS
Thin film battery and electrolyte therefor
A solid amorphous electrolyte composition for a thin-film battery. The electrolyte composition includes a lithium phosphorus oxynitride material containing a sulfide ion dopant wherein the atomic ratio of sulfide ion to phosphorus ion (S / P) in the electrolyte ranges greater than 0 up to about 0.2. The composition is represented by the formula: where 2x+3y+2z=5+w, x ranges from about 3.2 to about 3.8, y ranges from about 0.13 to about 0.46, z ranges from greater than zero up to about 0.2, and w ranges from about 2.9 to about 3.3. Thin-film batteries containing the sulfide doped lithium oxynitride electrolyte are capable of delivering more power and energy than thin-film batteries containing electrolytes without sulfide doping.
Owner:OAK RIDGE MICRO ENERGY
Metal-containing compounds
The invention relates to a novel solid state process for the preparation of metal-containing compounds comprising the steps i) forming a reaction mixture comprising one or more metal-containing precursor compounds and optionally one or more non-metal-containing reactants, and ii) using one or more hypophosphite-containing materials as a reducing agent; wherein one or more of the hypophosphite-containing materials is used as an agent to reduce one or more of the metal-containing precursor compounds; and further wherein the process is performed in the absence of an oxidizing atmosphere. Materials made by such a process are useful, for example, as electrode materials in alkali metal-ion battery applications.
Owner:LITHIUM WERKS TECH BV
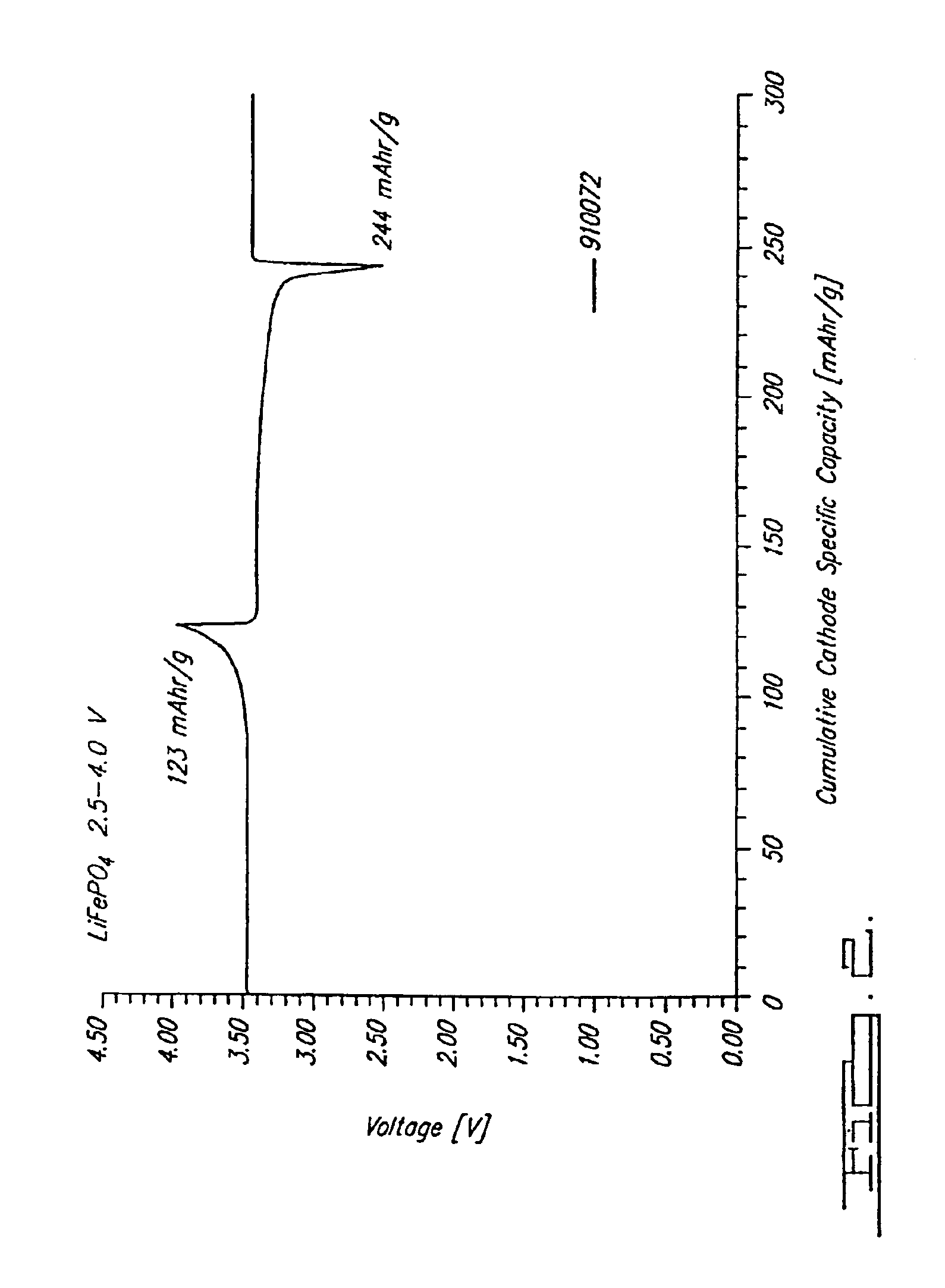
Lithium iron phosphate cathode materials with enhanced energy density and power performance
InactiveUS20090155689A1Powerful performanceHigh discharge ratePhosphatesPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesFiberPhosphate
The invention is related to a cathode material comprising particles having a lithium metal phosphate core and a pyrolytic carbon deposit, said particles having a synthetic multimodal particle size distribution comprising at least one fraction of micron size particles and one fraction of submicron size particles, said lithium metal phosphate having formula LiMPO4 wherein M is at least Fe or Mn.Said material is prepared by method comprising the steps of providing starting micron sized particles and starting submicron sized particles of at least one lithium metal phosphate or of precursors of a lithium metal phosphate; mixing by mechanical means said starting particles; making a pyrolytic carbon deposit on the lithium metal phosphate starting particles before or after the mixing step, and on their metal precursor before or after mixing the particles; optionally adding carbon black, graphite powder or fibers to the said lithium metal phosphate particles before the mechanical mixing.
Owner:PHOSTECH LITHIUM
Zirconium Phosphate Particles Having Improved Adsorption Capacity and Method Of Synthesizing The Same
InactiveUS20100084330A1Avoid disadvantagesHigh porosityPhosphatesDialysis systemsPhosphoric acidOxygen
Zirconium phosphate particles are synthesized by providing a solution of zirconium oxychloride in an aqueous solvent, adding at least one low molecular weight, oxygen containing, monofunctional, organic additive to the solution, and combining this solution with heated phosphoric acid or a phosphoric acid salt to obtain zirconium phosphate particles by sol gel precipitation.
Owner:FRESENIUS MEDICAL CARE HLDG INC
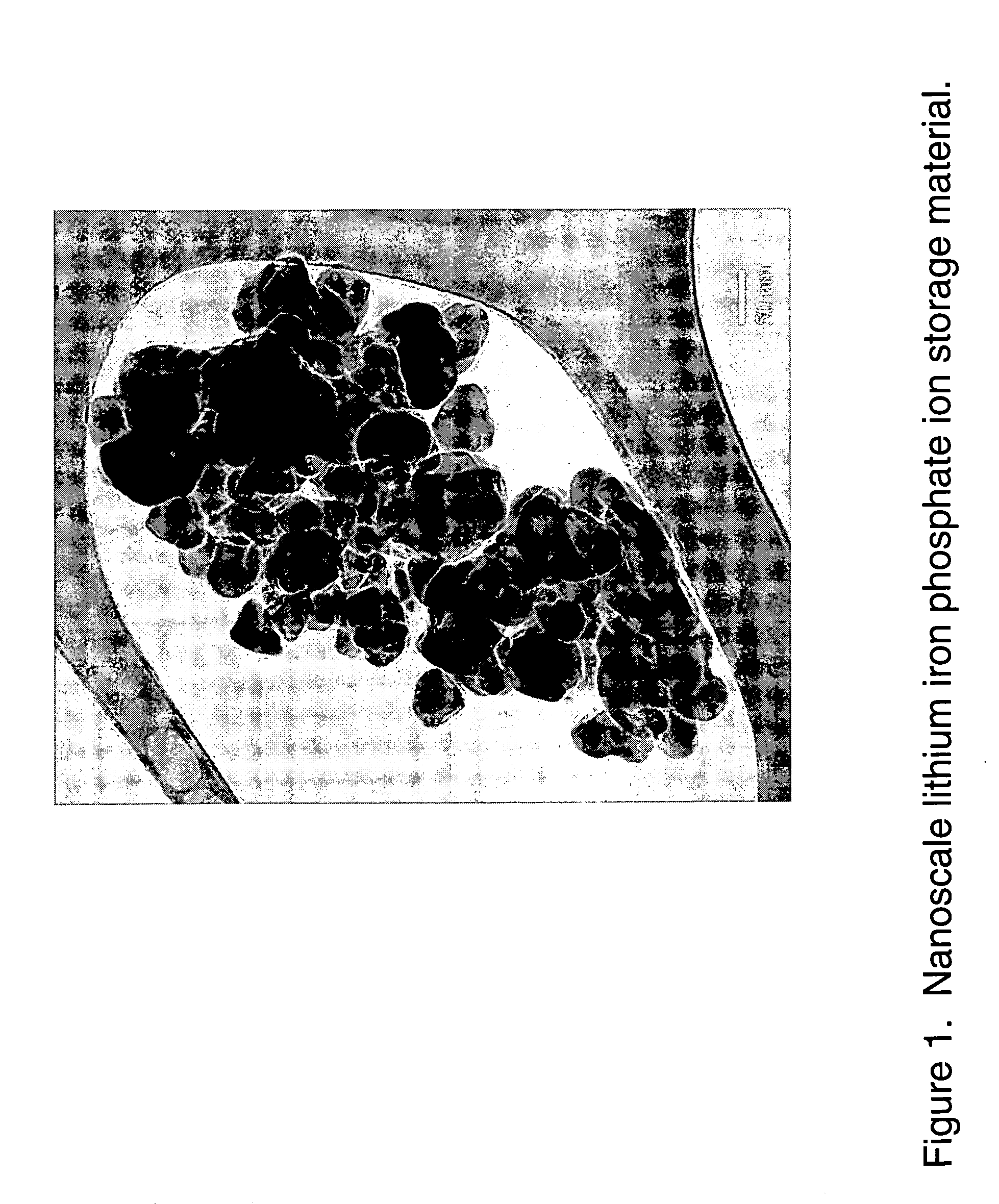
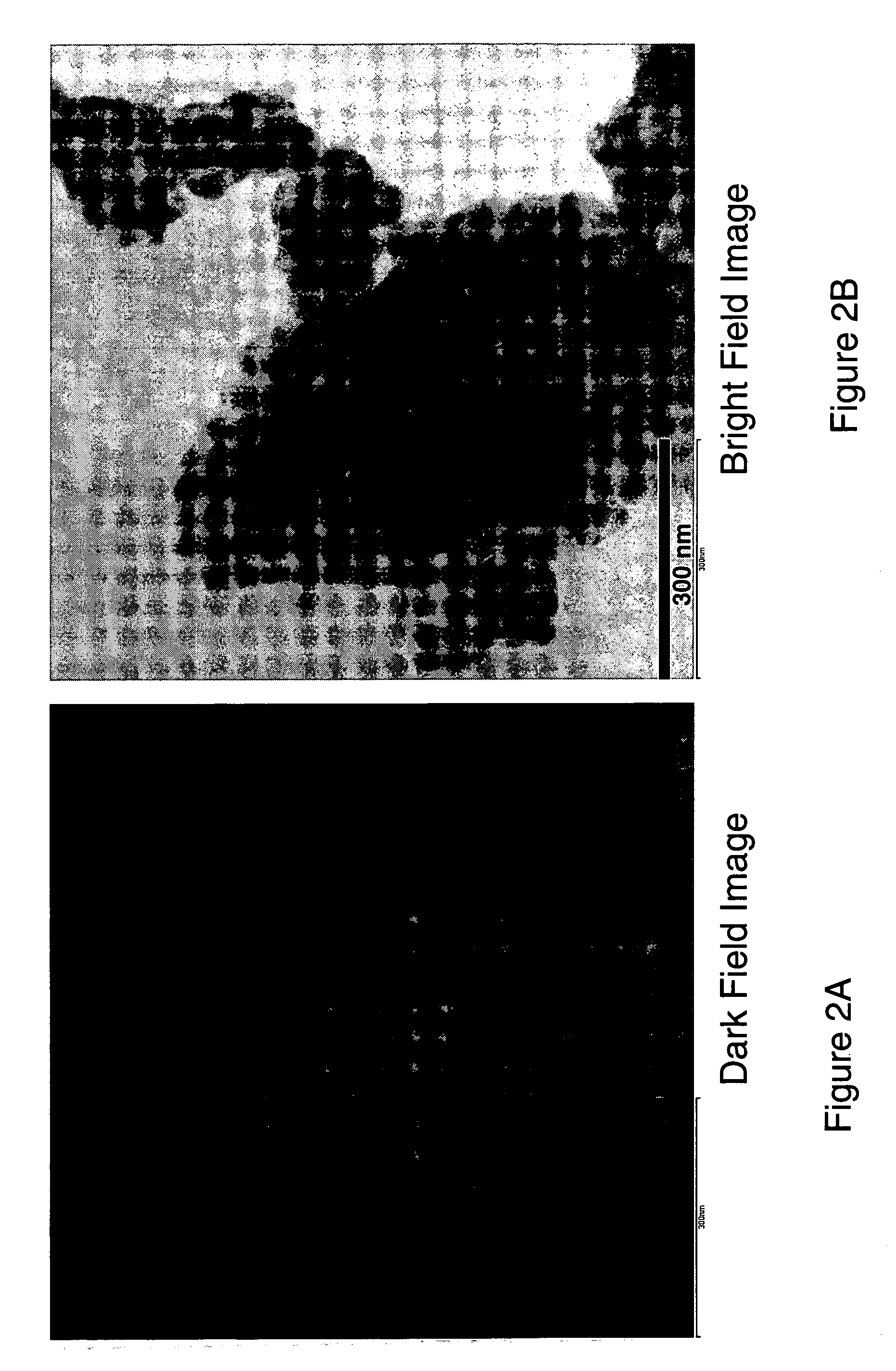
Nanoscale ion storage materials
ActiveUS20070031732A1Improve electronic conductivityImproved electromechanical stabilityMaterial nanotechnologyPhosphatesHigh ratePhosphate
Nanoscale ion storage materials are provided that exhibit unique properties measurably distinct from their larger scale counterparts. For example, the nanoscale materials can exhibit increased electronic conductivity, improved electromechanical stability, increased rate of intercalation, and / or an extended range of solid solution. Useful nanoscale materials include alkaline transition metal phosphates, such as LiMPO4, where M is one or more transition metals. The nanoscale ion storage materials are useful for producing devices such as high energy and high power storage batteries, battery-capacitor hybrid devices, and high rate electrochromic devices.
Owner:RIL USA INC +1
Lithium ion conductive solid electrolyte and method for manufacturing the same
ActiveUS20070087269A1Increase battery capacityImprove discharge characteristicsSolid electrolytesPhosphatesLithiumPorosity
Owner:OHARA
Method for making a lithium mixed metal compound having an olivine structure
ActiveUS20070207080A1Mitigate such drawbackNon-metal conductorsLiquid surface applicatorsLithiumCarbon layer
Owner:AQUIRE ENERGY CO LTD
Self-hardening calcium phosphate materials with high resistance to fracture, controlled strength histories and tailored macropore formation rates
InactiveUS6955716B2Strong and tough self-hardeningHigh strengthPhosphatesOther chemical processesFiberHigh resistance
A bone replacement material and therapy comprises the combination of calcium phosphate compounds and two or more soluble fillers in the form of fibers, mesh or other materials which have the dual functions of reinforcing an in vivo implant while dissolving at a programmed rate to form macropores capable of receiving natural bone ingrowth.
Owner:ADA FOUND
Binary, ternary and quaternary lithium phosphates, method for the production thereof and use of the same
ActiveUS20040151649A1High specific capacitySimple and inexpensivePhosphatesPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesRoom temperatureReducing atmosphere
The invention relates to binary, ternary and quaternary lithium phosphates of general formula Li(FexM<1>yM<2>z)PO4 wherein M<1 >represents at least one element of the group comprising Sc, Ti, V, Cr, Mn, Co, Ni, Cu, Zn, Be, Mg, Ca, Sr, Ba, Al, Zr, and La; M<2 >represents at least one element of the group comprising Sc, Ti, V, Cr, Mn, Co, Ni, Cu, Zn, Be, Mg, Ca, Sr, Ba, Al, Zr, and La; x=between 0.5 and 1, y=between 0 and 0.5, z=between 0 and 0.5, provided that x+y+z=1, or x=0, y =1 and z=0. The said lithium phosphates can be obtained according to a method whereby precursor compounds of elements Li, Fe, M<1 >and / or M<2 >are precipitated from aqueous solutions and the precipitation product is dried in an inert gas atmosphere or a reducing atmosphere at a temperature which is between room temperature and approximately 200° C. and tempered at a temperature of between 300° C. and 1000° C. The inventive lithium phosphates have a very high capacity when used as cathode material in lithium accumulators.
Owner:ZENT FUR SONNENENERGIE & WASSERSTOFF FORSCHUNG BADEN WURTTEMBERG GEMEINNUTZIGE STIFTUNG
Cathode material for manufacturing a rechargeable battery
A cathode material has one of olivine and NASICON structures and includes micrometer-sized secondary particles having a particle size ranging from 1 to 50 μm. Each of the micrometer-sized secondary particles is composed of crystalline nanometer-sized primary particles of a metal compound having a particle size ranging from 10 to 500 nm.
Owner:ADVANCED LITHIUM ELECTROCHEMISTRY CO LTD
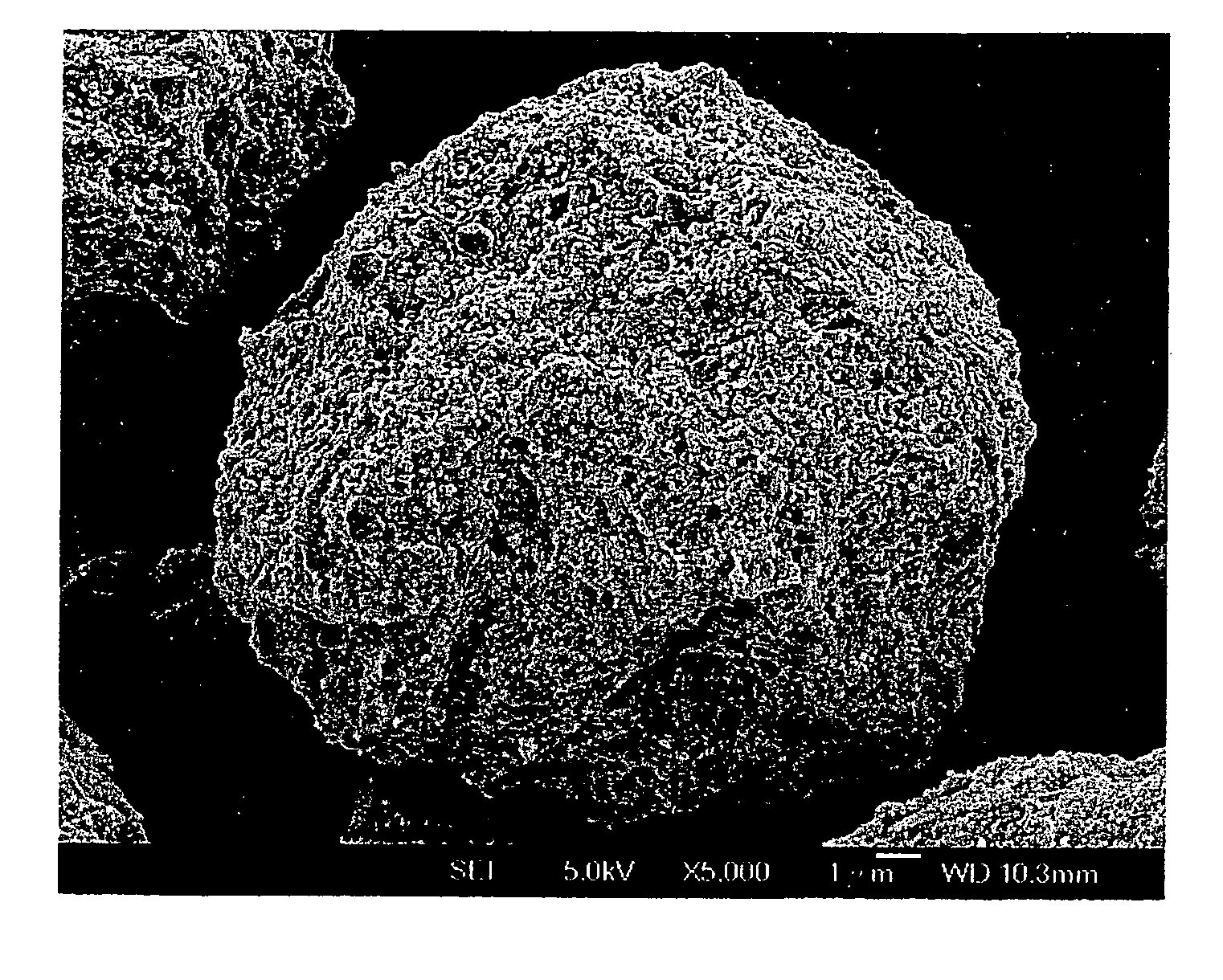
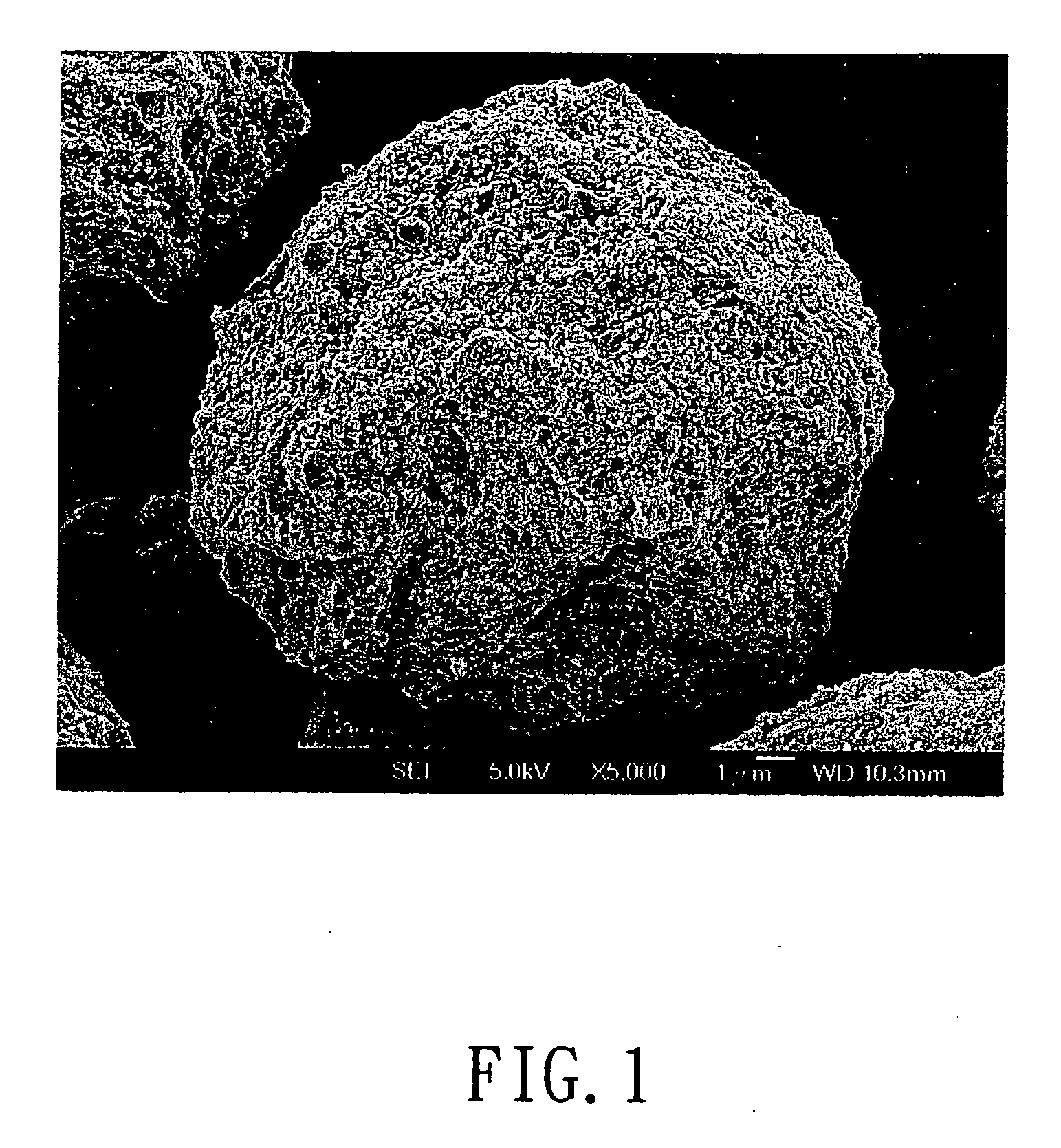
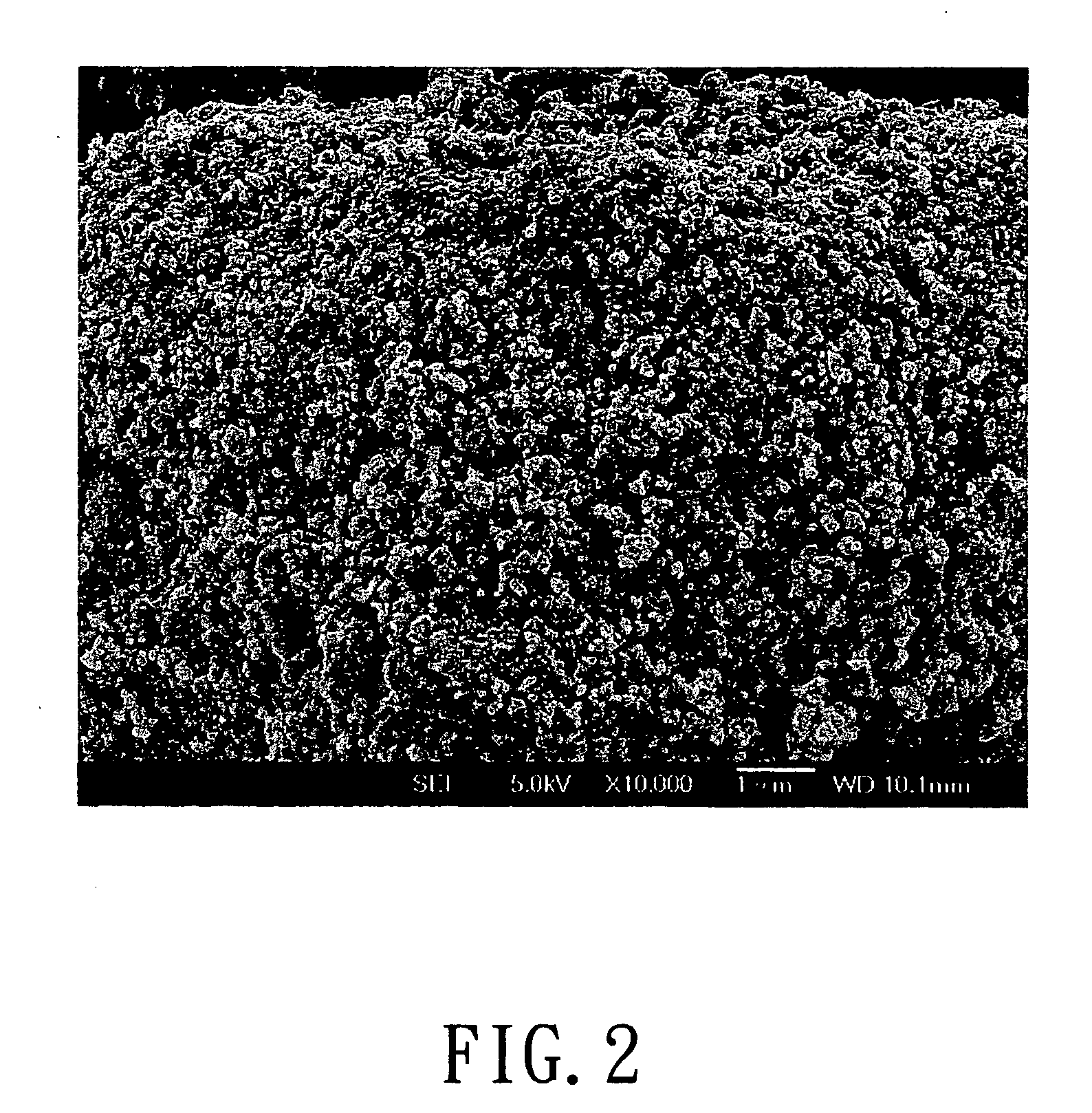
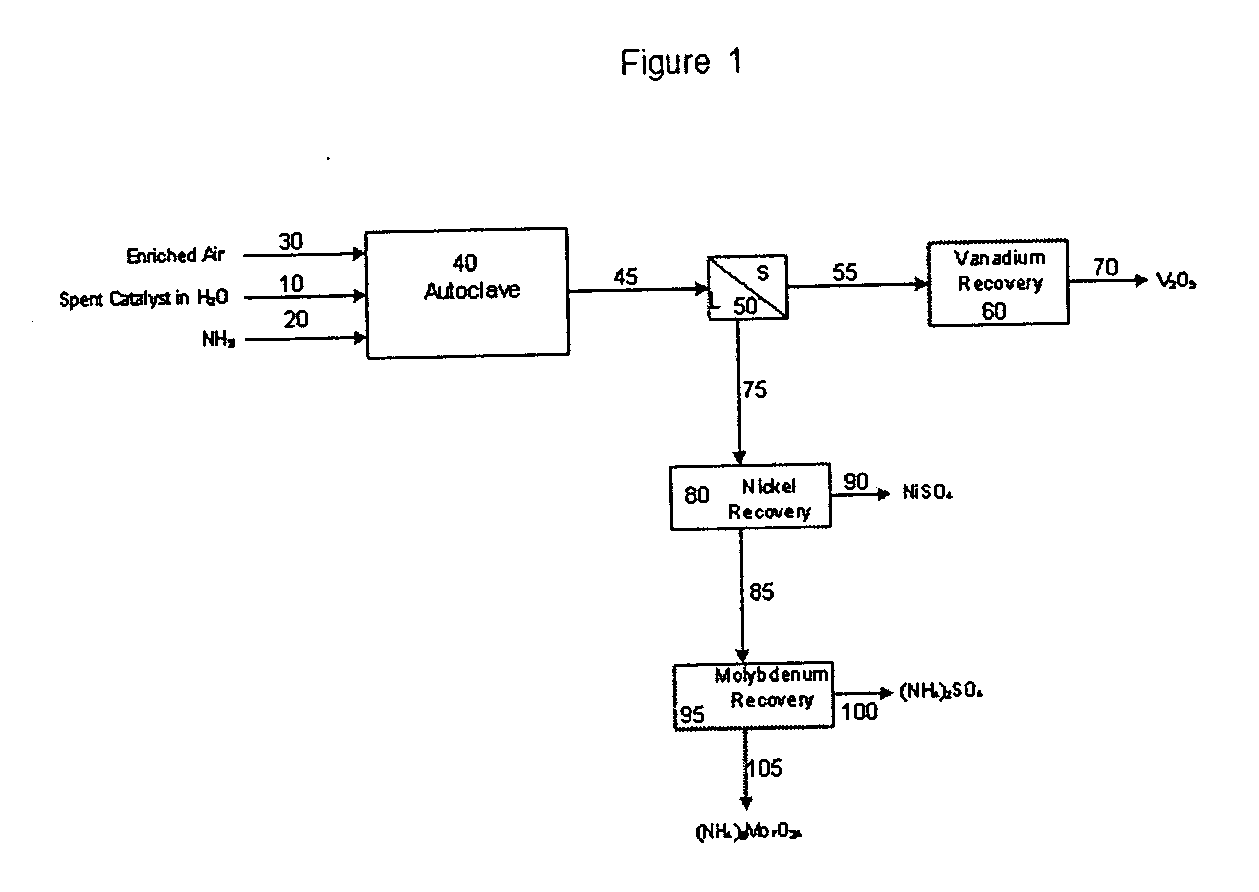
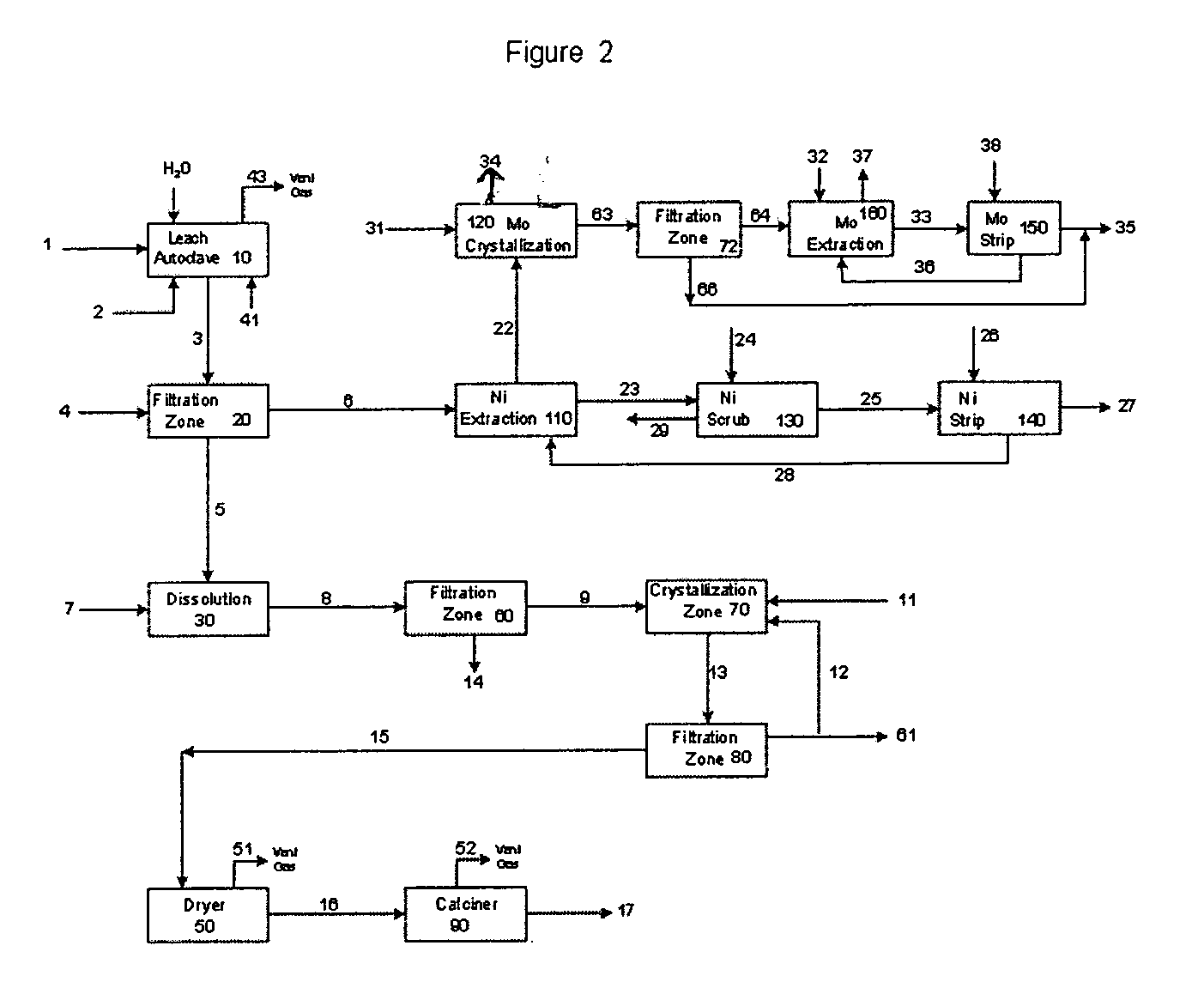
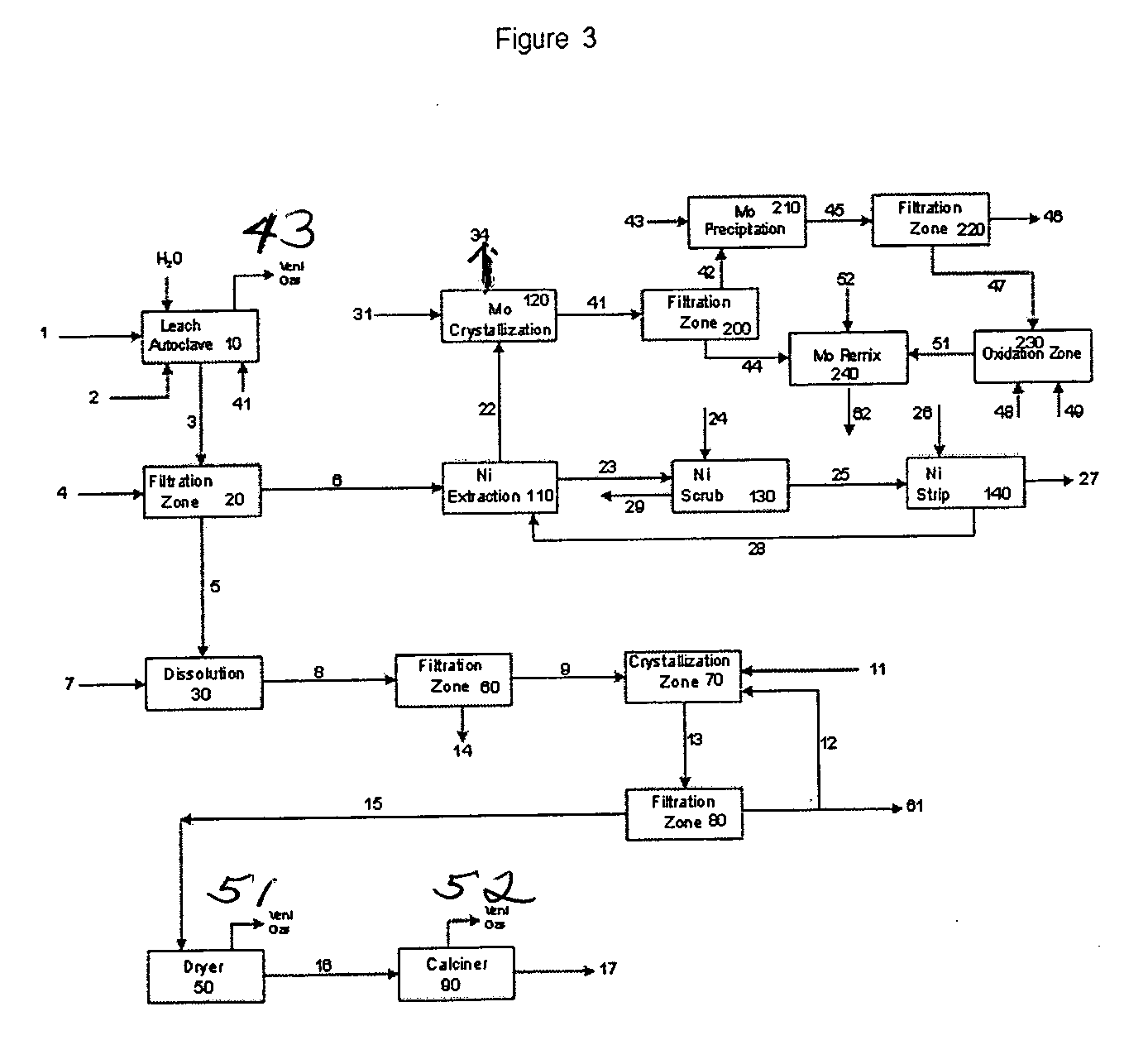
Process for metals recovery from spent catalyst
The process of this invention is directed to the removal of metals from an unsupported spent catalyst. The catalyst is subjected to leaching reactions. Vanadium is removed as a precipitate, while a solution comprising molybdenum and nickel is subjected to further extraction steps for the removal of these metals. Molybdenum may alternately be removed through precipitation.
Owner:CHEVROU USA INC
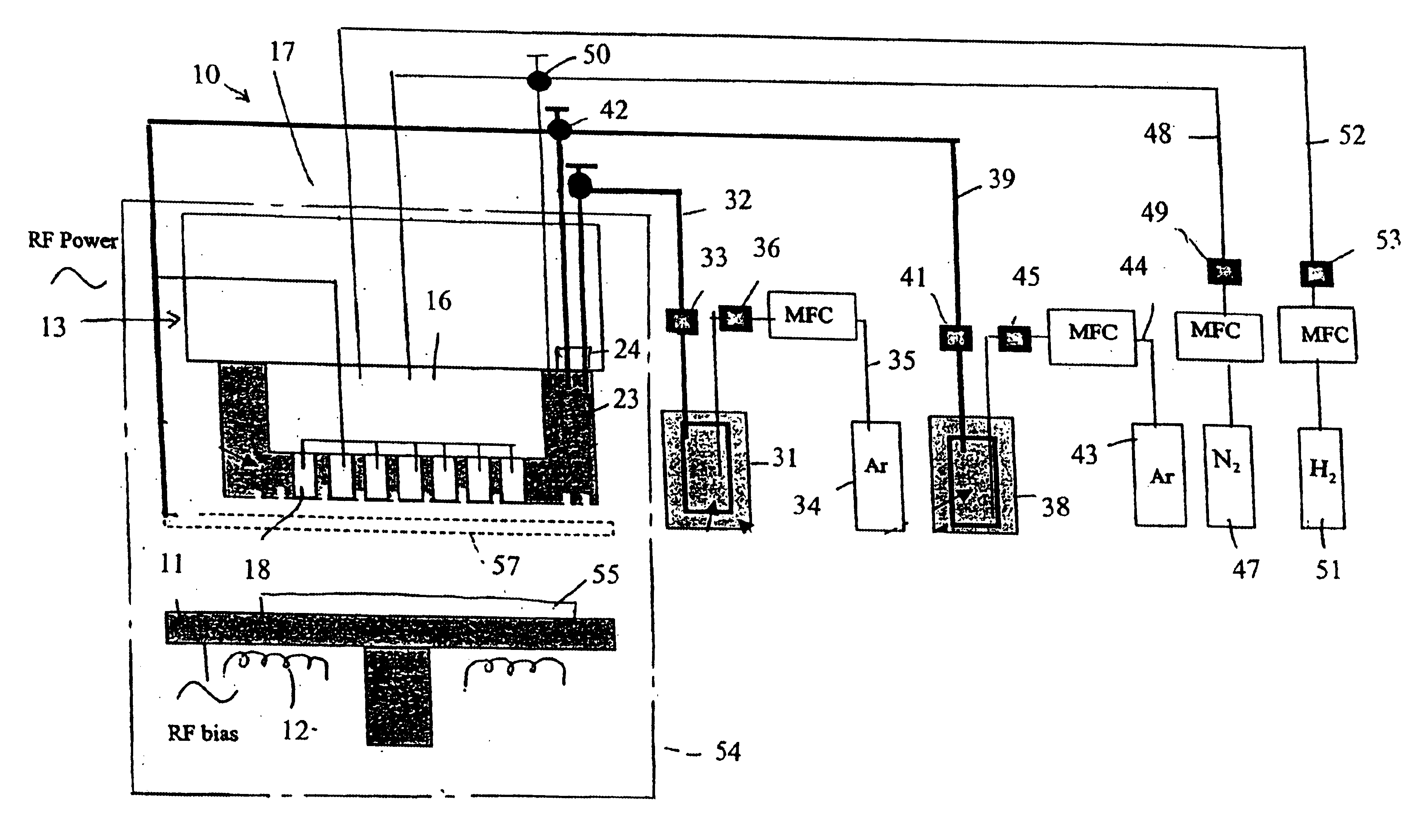
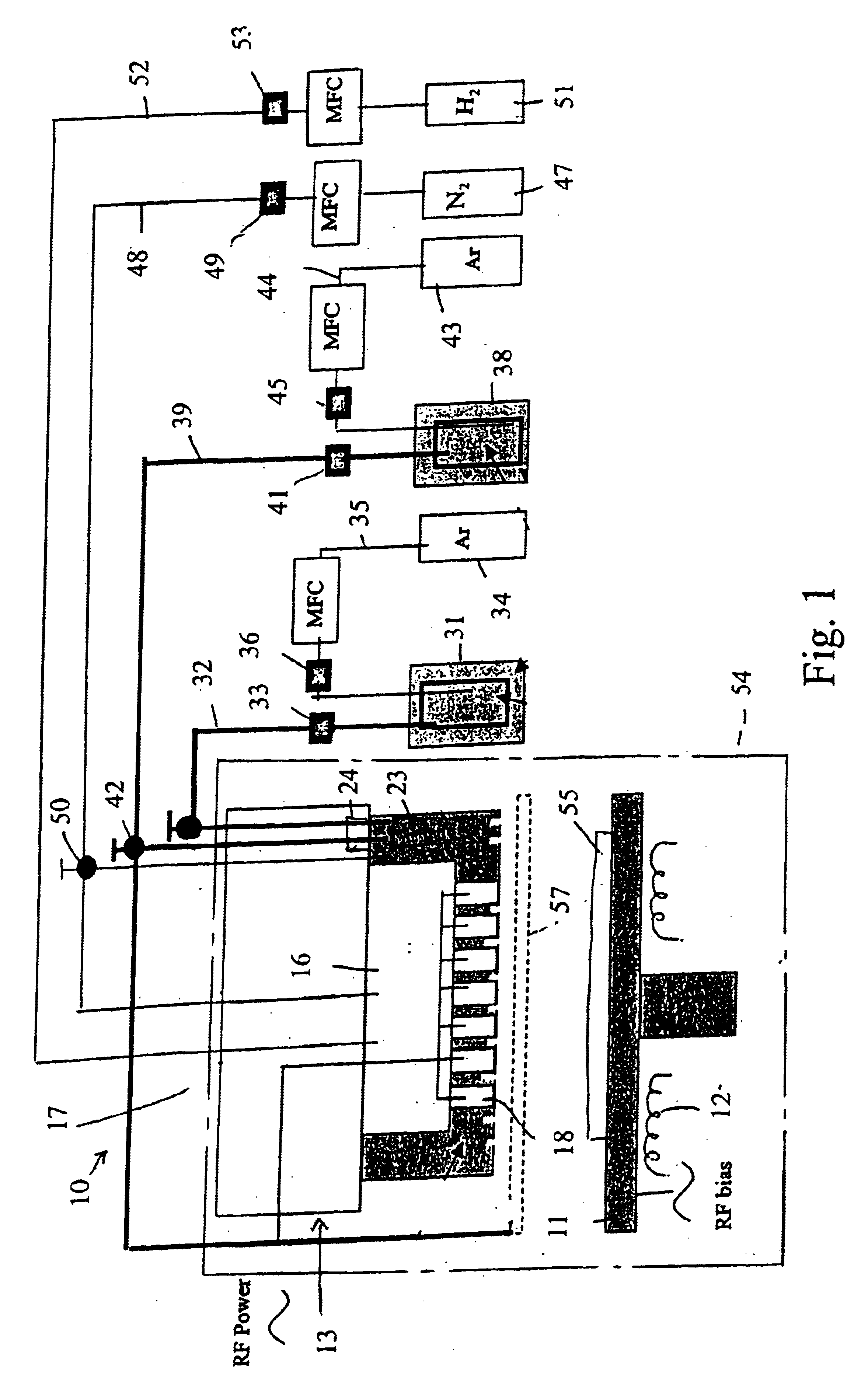
System and method of producing thin-film electrolyte
InactiveUS6852139B2PhosphatesPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesLithiumHydrogen
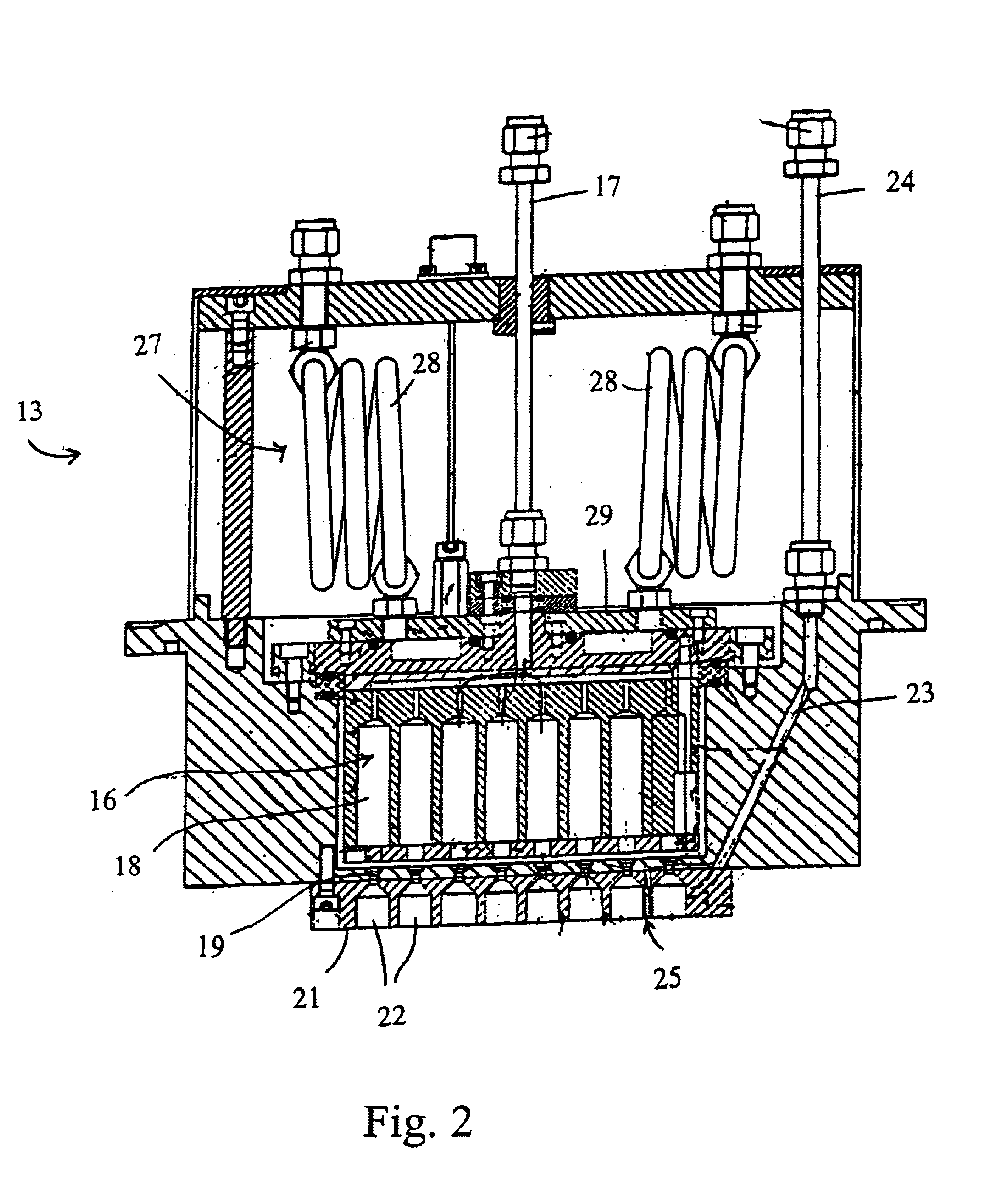
A process of producing a thin film electrolyte is provided wherein a volatile lithium-containing precursor and a volatile phosphate-containing precursor are mixed into a plasma generated from a plasma source. The mixture is then deposited upon a substrate. The process is conducted with the use of a system (11) having a plasma source (13) having a primary plenum (16) and a secondary plenum (23). The primary plenum is in fluid communication with a source of nitrogen gas (47) and a source of hydrogen gas (51). The secondary plenum is in fluid communication with a first bubbler (31) and a second bubbler (38).
Owner:JOHNSON IP HLDG LLC
Method of hydrothermal liquid phase sintering of ceramic materials and products derived therefrom
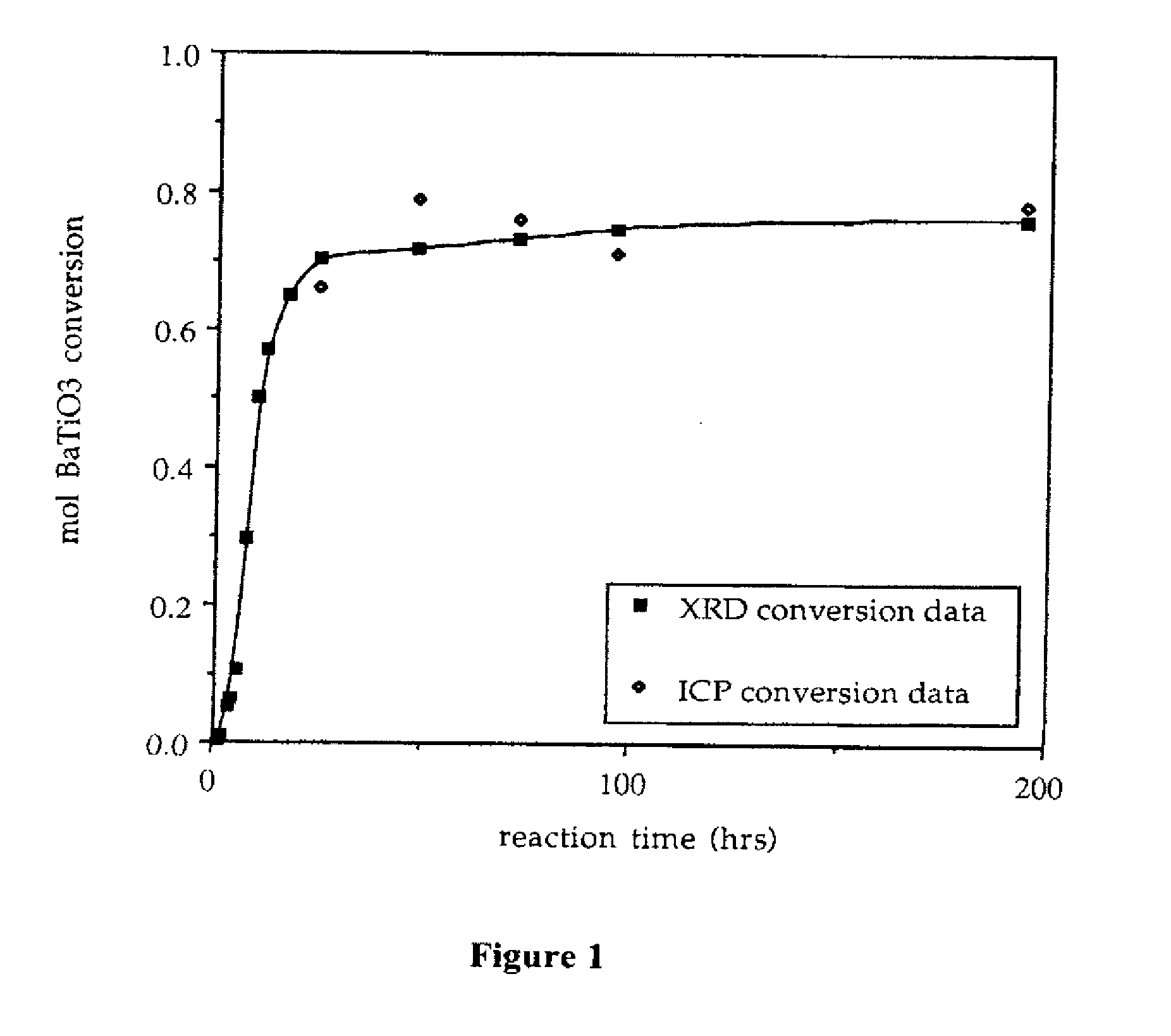
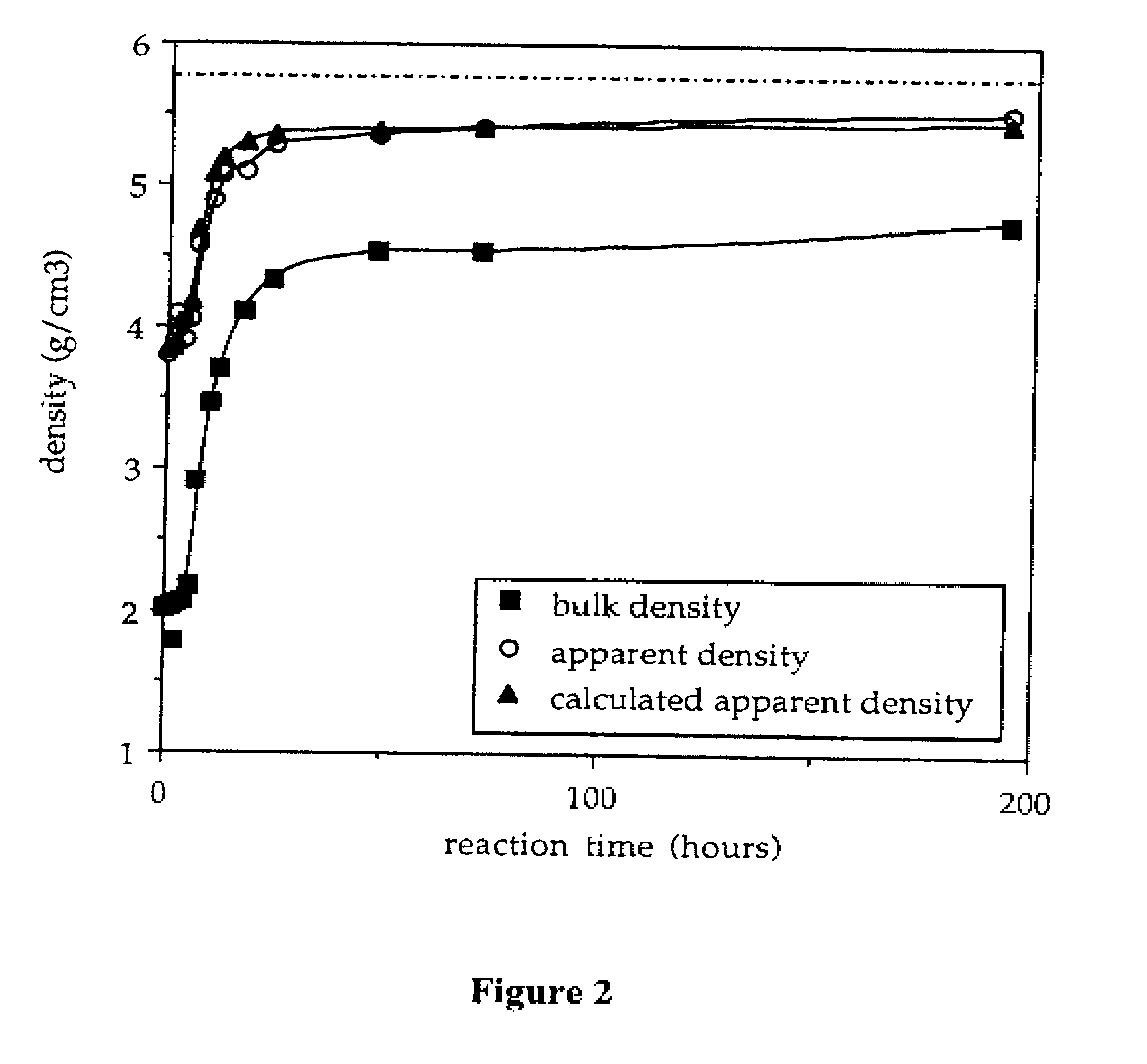
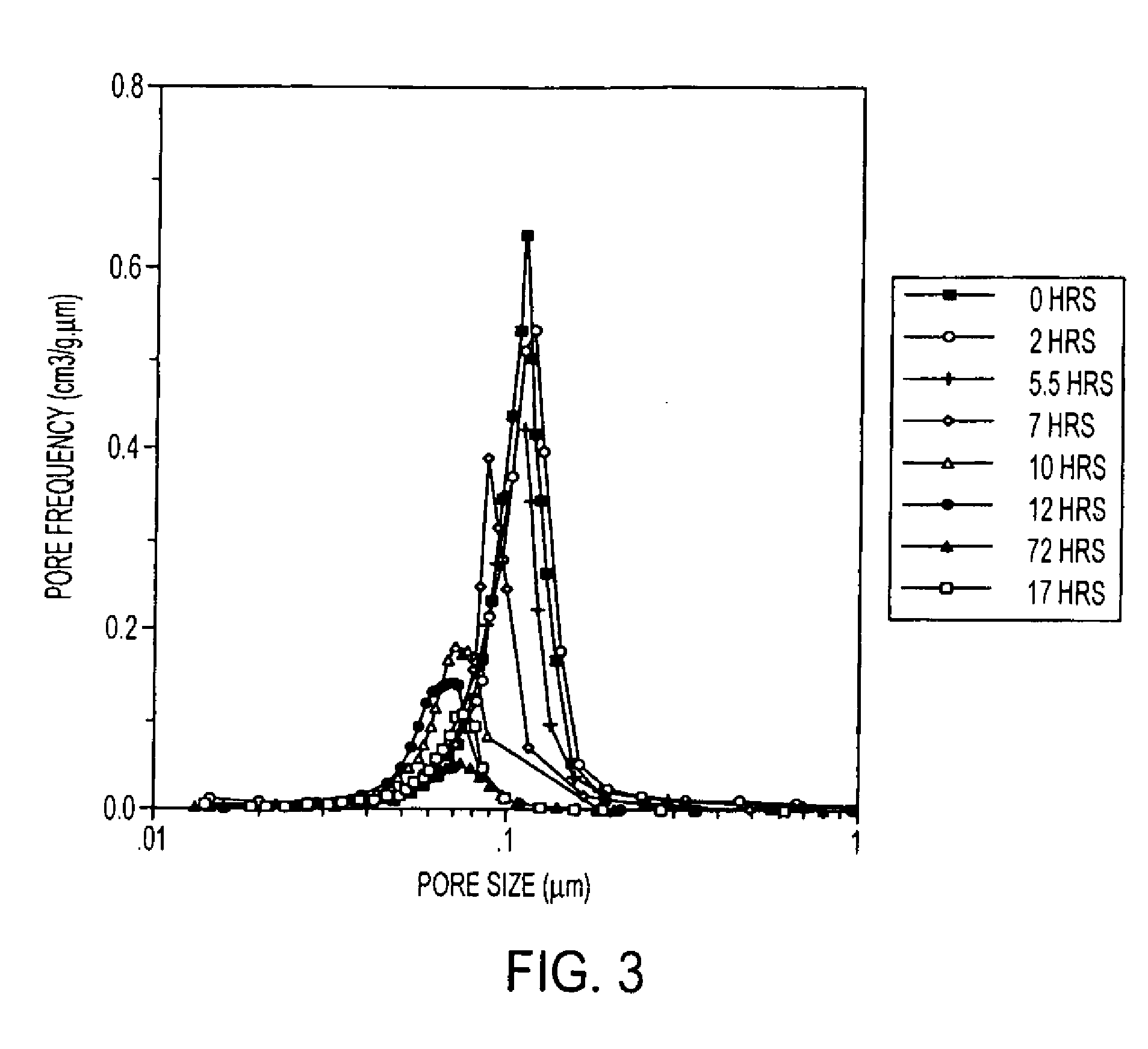
Provided here is a method of producing a monolithic body from a porous matrix, comprising: (i) providing a porous matrix having interstitial spaces and comprising at least a first reactant; (ii) contacting the porous matrix with an infiltrating medium that carries at least a second reactant; (iii) allowing the infiltrating medium to infiltrate at least a portion of the interstitial spaces of the porous matrix under conditions that promote a reaction between the at least first reactant and the at least second reactant to provide at least a first product; and (iv) allowing the at least first product to form and fill at least a portion of the interstitial spaces of the porous matrix, thereby producing a monolithic body, wherein the monolithic body does not comprise barium titanate.
Owner:RUTGERS THE STATE UNIV
Lithium-based active materials and preparation thereof
InactiveUS6884544B2Easy to adaptHigh capacity retentionElectrode manufacturing processesPhosphatesElectrochemical cellChemistry
The invention provides novel active material represented by the general formula LiaMI1-yMIIyPO4, wherein MI is selected from the group consisting of Fe, Ni, Mn, V, Sn, Ti, Cr, and mixtures thereof; MII is selected from the group consisting of Zn, Cd, and a mixture thereof; a is about 1; and 0<y<1, which, upon electrochemical interaction, releases lithium ions, and is capable of reversibly cycling lithium ions. The invention provides a rechargeable lithium battery which comprises an electrode formed from the novel active material, wherein MI is selected from the group consisting of Fe, Co, Ni, Mn, Cu, V, Sn, Ti, Cr, and mixtures thereof. Methods for making the novel active material and methods for using such a material in electrochemical cells are also provided.
Owner:LITHIUM WERKS TECH BV
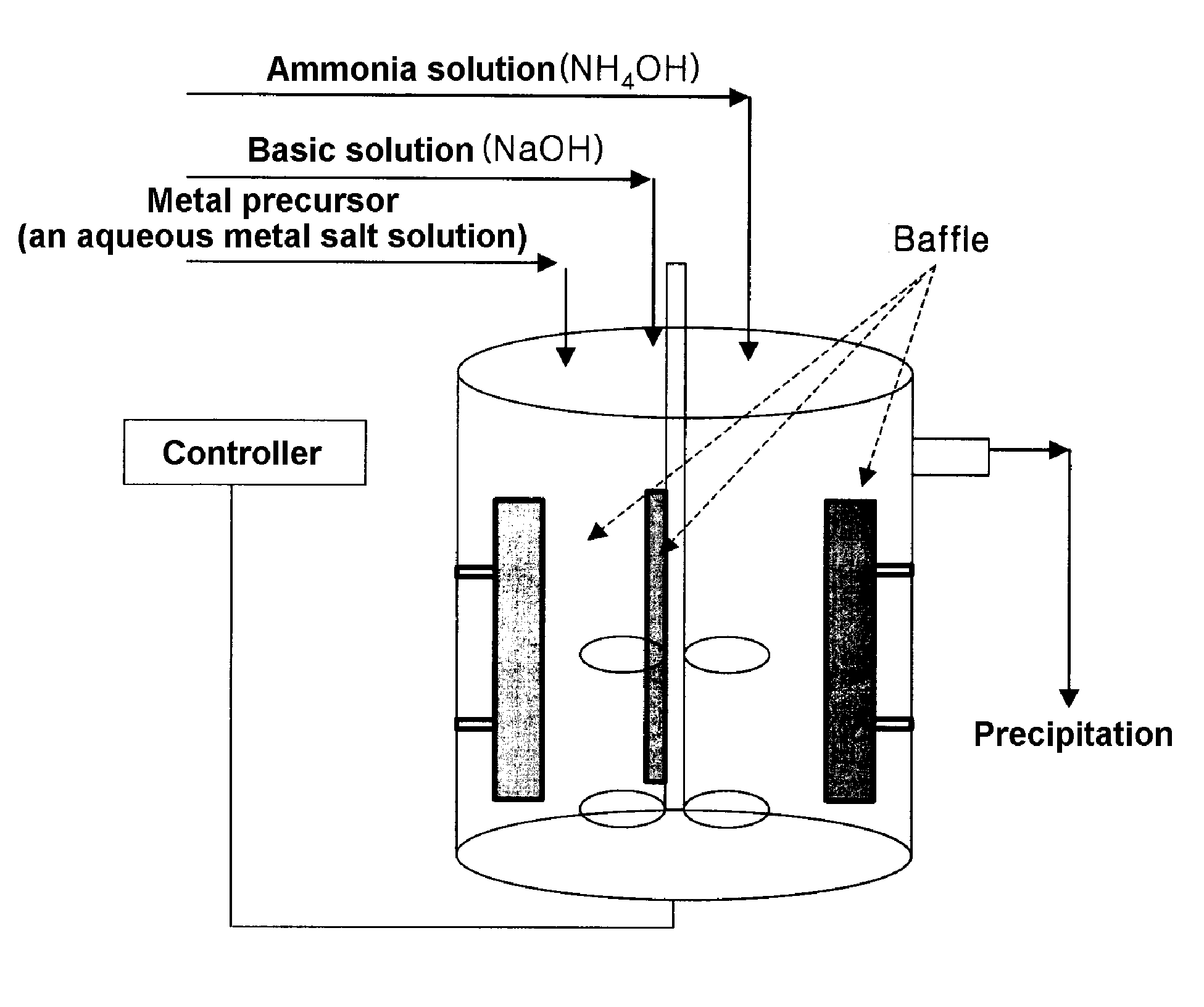
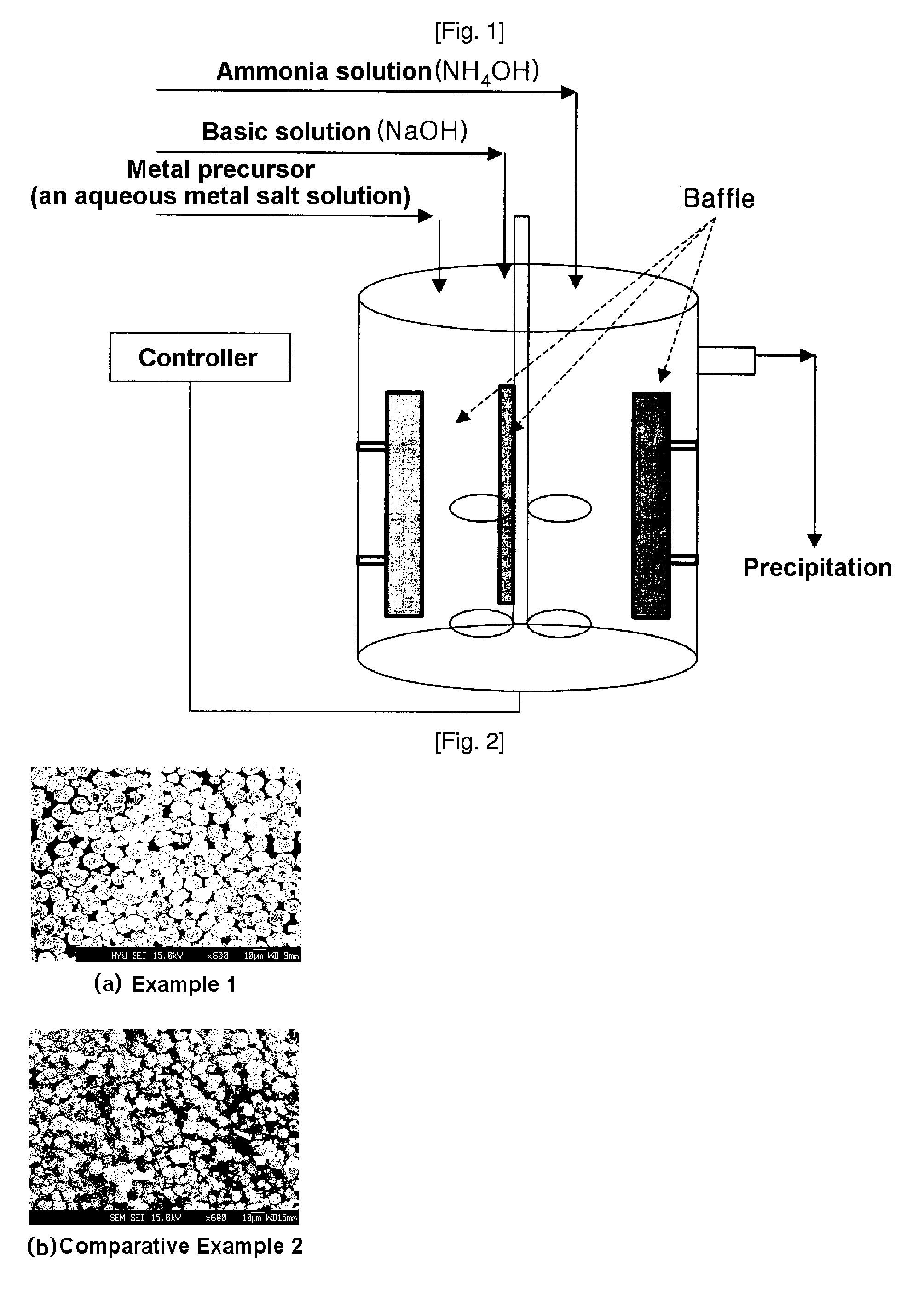
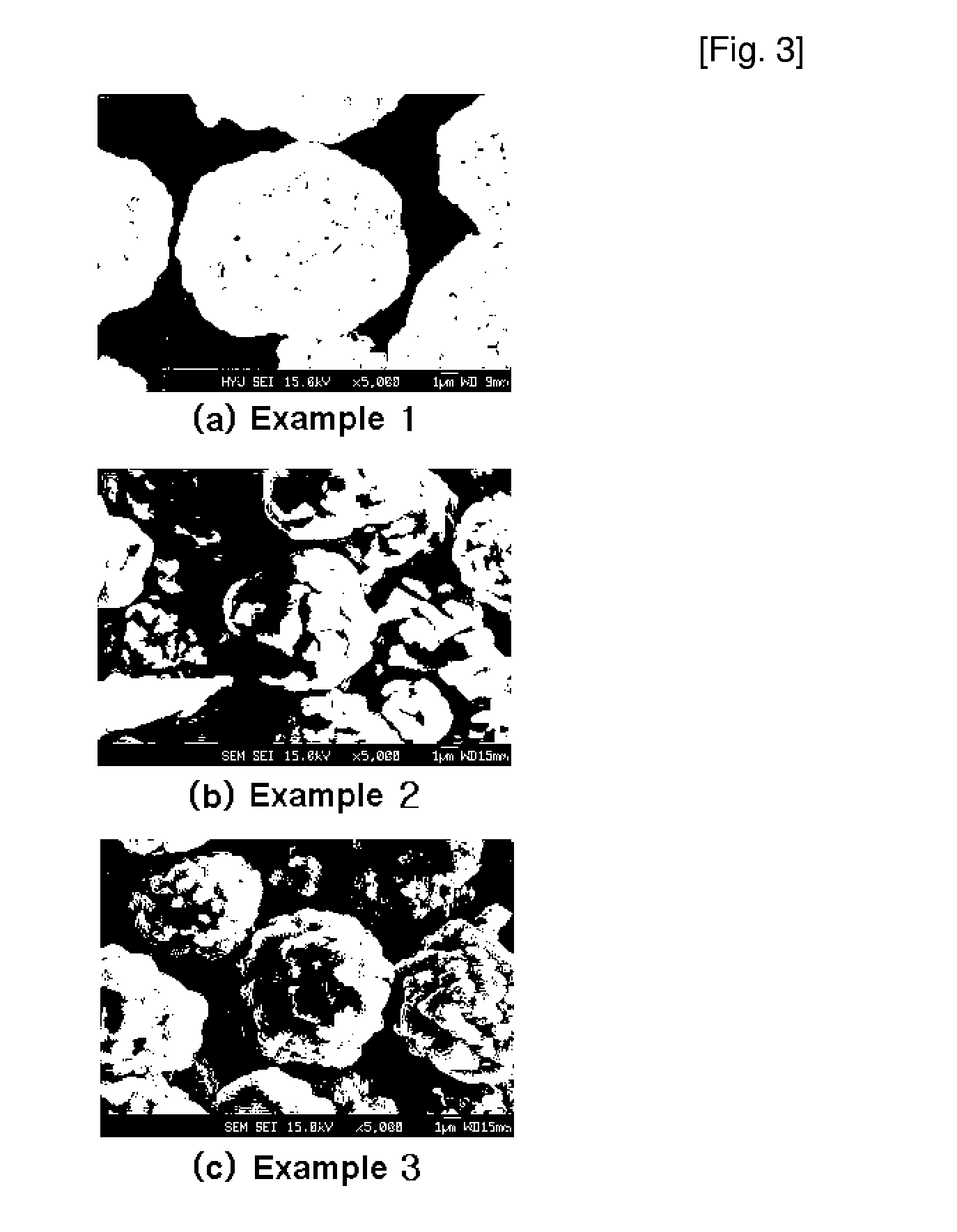
Cathode active material for lithium secondary battery, process for preparing the same and reactor for use in the same process
ActiveUS20070111098A1Large capacityHigh tap densityElectrode manufacturing processesPhosphatesLithiumHigh rate
The present invention relates to a cathode active material for a lithium secondary battery and a process for preparing the same. In accordance with the present invention, the cathode active material having a high packing density was designed and synthesized and thus provided is a cathode active material for a lithium secondary battery exhibiting structural stability such as improved characteristics for charge / discharge, service life and high-rate and thermal stability, by modifying surface of the electrode active material with amphoteric or basic compounds capable of neutralizing acid produced around the cathode active material.
Owner:LG CHEM LTD
Method of synthesizing electrochemically active materials from a slurry of precursors
InactiveUS6913855B2Quality improvementHigh-quality materialPhosphatesElectrode thermal treatmentCompound (substance)Slurry
A method for making an active material comprises the steps of forming a slurry, spray drying the slurry to form a powdered precursor composition, and heating the powdered precursor composition at a temperature and for a time sufficient to form a reaction product. The slurry has a liquid phase and a solid phase, and contains at least an alkali metal compound and a transition metal compound. Preferably the liquid phase contains dissolved alkali metal compound, and the solid phase contains an insoluble transition metal compound, an insoluble carbonaceous material compound, or both. Electrodes and batteries are provided that contain the active materials.
Owner:RIL USA INC +1
Aluminum phosphate or polyphosphate particles for use as pigments in paints and method of making same
InactiveUS20060045831A1PhosphatesPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesAluminium sulfateOrganic acid
An aluminum phosphate or polyphosphate-based pigment product is made by a process comprising contacting phosphoric acid with aluminum sulfate and an alkaline solution to produce an aluminum phosphate based product; and optionally calcining the aluminum phosphate based product at an elevated temperature, wherein the process is substantially free of an organic acid. The aluminum phosphate or polyphosphate-based pigment is amorphous. The amorphous aluminum phosphate or polyphosphate characterized by a bulk density of less than 2.30 grams per cubic centimeter and a phosphorus to aluminum mole ratio of greater than 0.8. The composition is useful in paints and as a substitute for titanium dioxide.
Owner:BUNGE AMORPHIC SOLUTIONS +1
Use of alkaline earth metal containing small pore non-zeolitic molecular sieve catalysts in oxygenate conversion
A method for converting starting material to olefins comprising contacting the starting material with a small pore non-zeolitic molecular sieve catalyst under effective conditions to produce olefins, wherein the non-zeolitic molecular sieve has been prepared in-situ or modified after synthesis by incorporation using an alkaline earth metal compound, wherein the alkaline earth metal ion is selected from the group consisting of strontium, calcium, barium, and mixtures thereof.
Owner:EXXON CHEM PAT INC
Method of synthesizing zirconium phosphate particles
InactiveUS20060140840A1Avoid disadvantagesInhibition of agglomerationSemi-permeable membranesPhosphatesPhosphoric acidSolvent
Zirconium phosphate particles are synthesized by providing a solution of zirconium oxychloride in an aqueous solvent, adding at least one oxygen-containing additive to the solution, the oxygen-containing additive being selected to form a complex with zirconium ions in the solution of zirconium oxychloride and thereby reduce hydration of the zirconium ions, and combining this solution with phosphoric acid or a phosphoric acid salt to obtain zirconium phosphate particles by sol gel precipitation.
Owner:RENAL SOLUTIONS
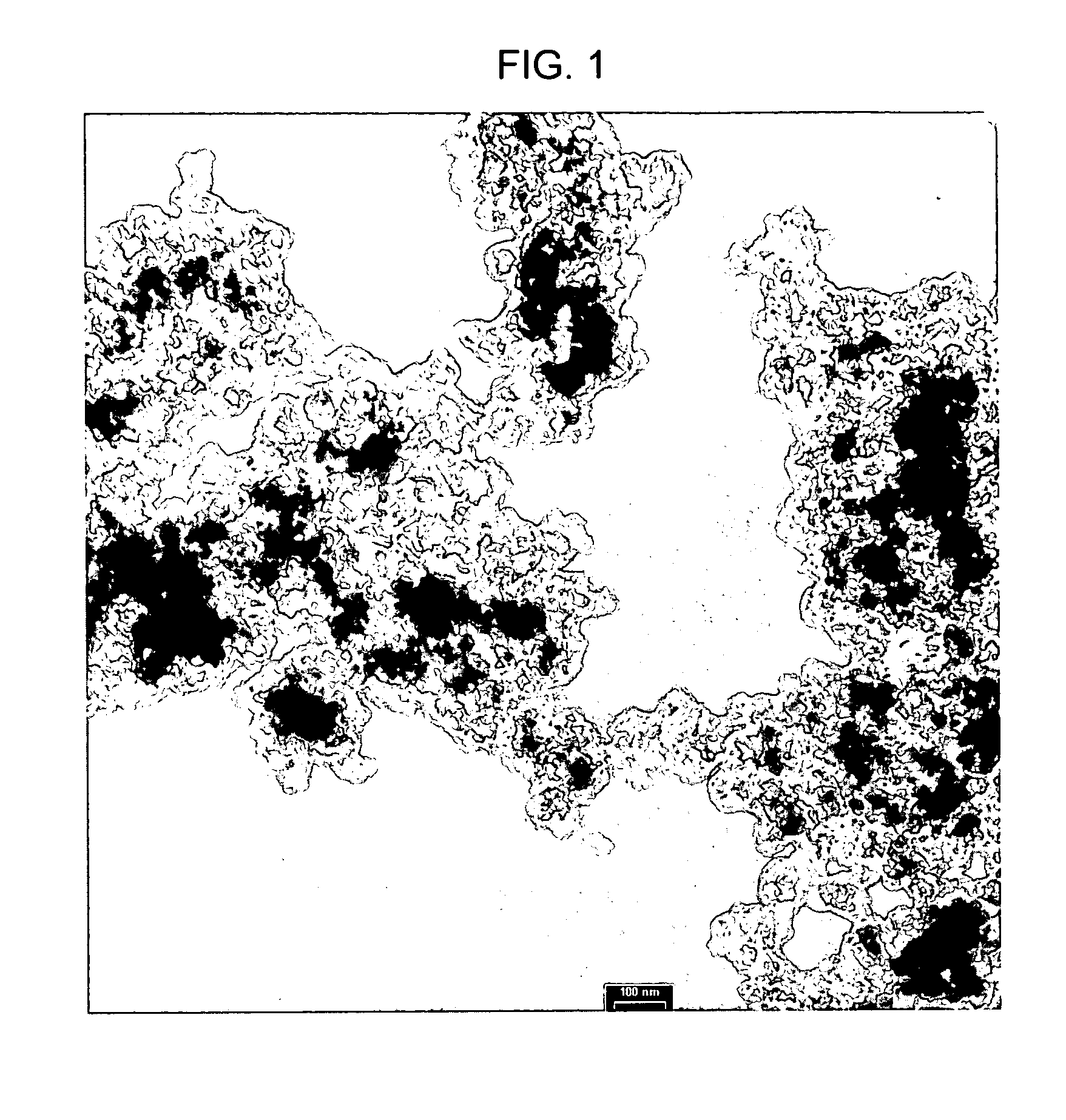
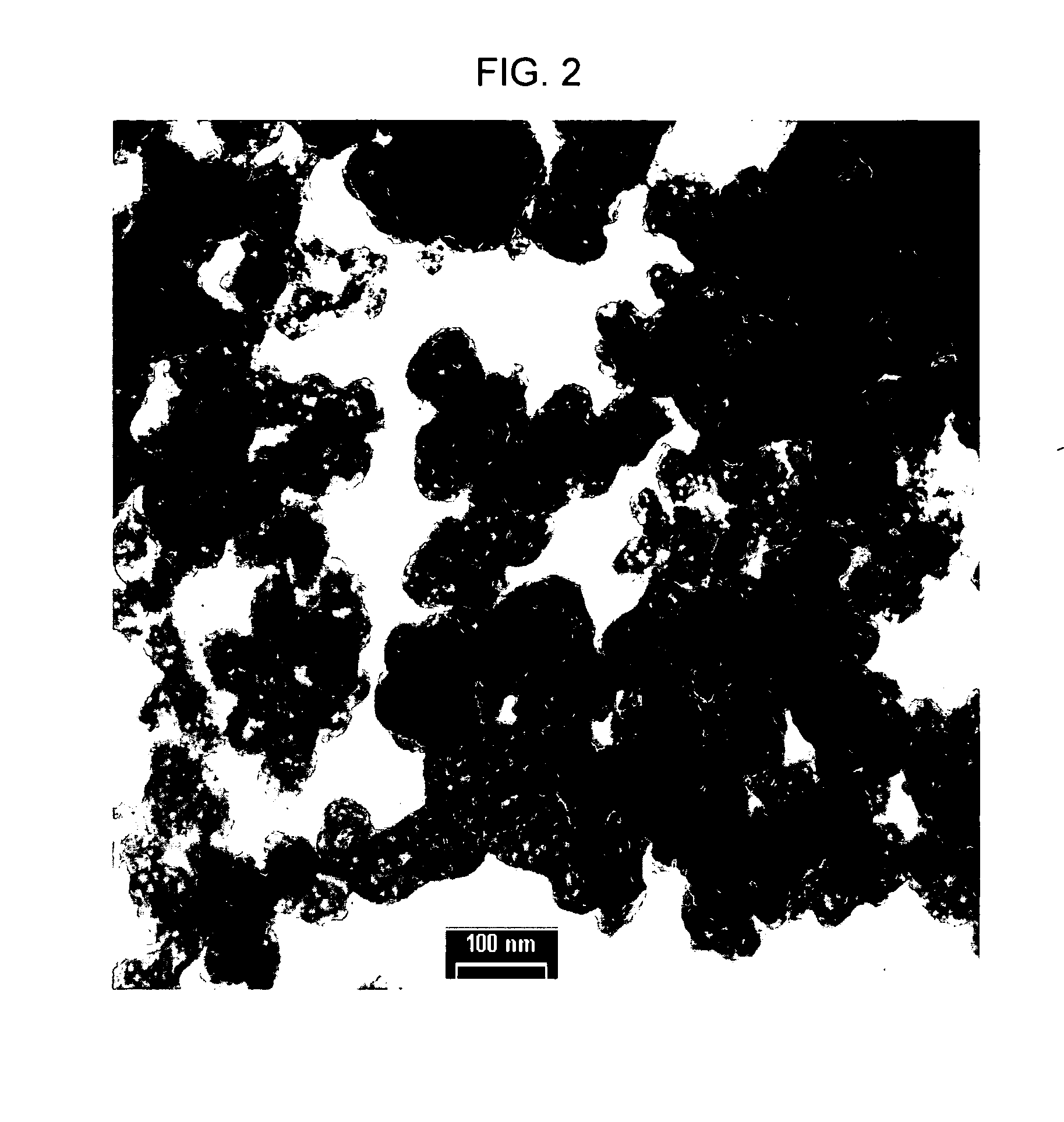
Electrode active composite materials and methods of making thereof
ActiveUS20100279117A1PhosphatesPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesNanoparticleLithium metal
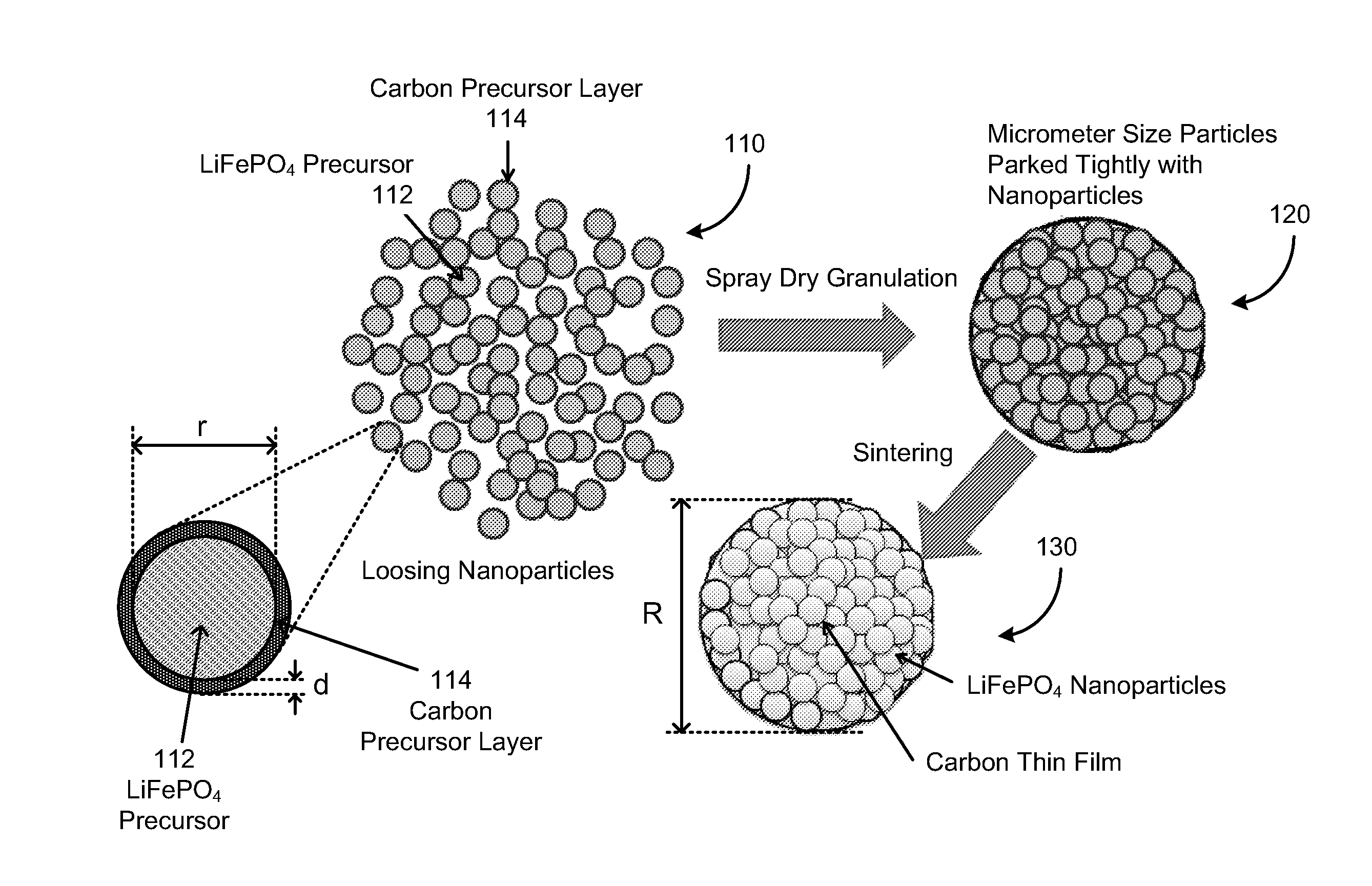
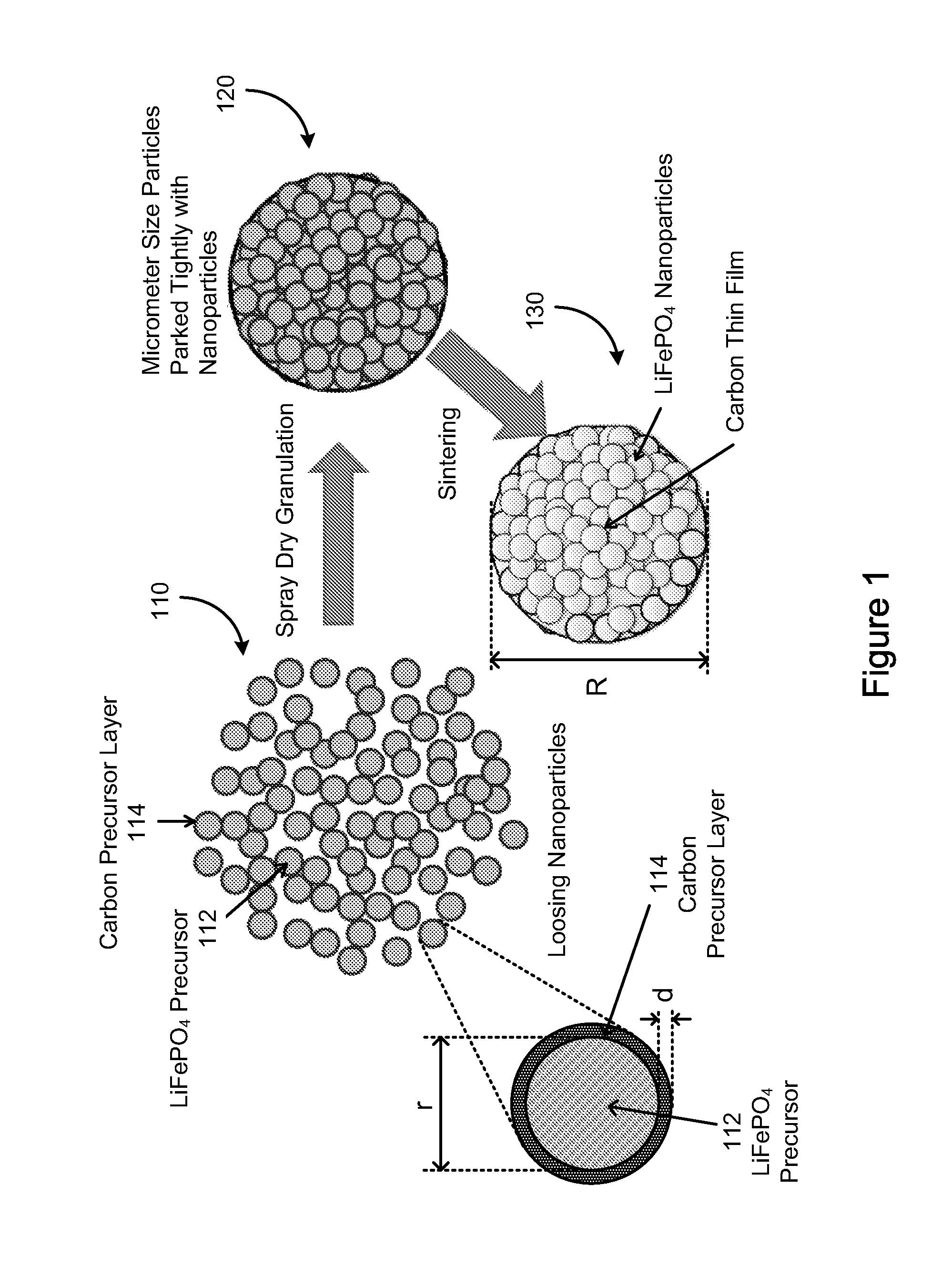
In one aspect of the invention, a method of synthesizing a lithium metal phosphate composite usable for a lithium secondary battery includes the steps of forming a nanometer-size precursor comprising lithium source and metal phosphate nanoparticles having each nanoparticle at least partially coated a layer of carbon precursor, spray drying the nanometer-size precursor at a first desired temperature to form micron-size particles packed with the lithium metal phosphate precursor nanoparticles, and sintering the micron-size particles at a second desired temperature under an inert and / or reduction atmosphere to form a micron-size lithium metal phosphate composite.
Owner:MEECOTECH
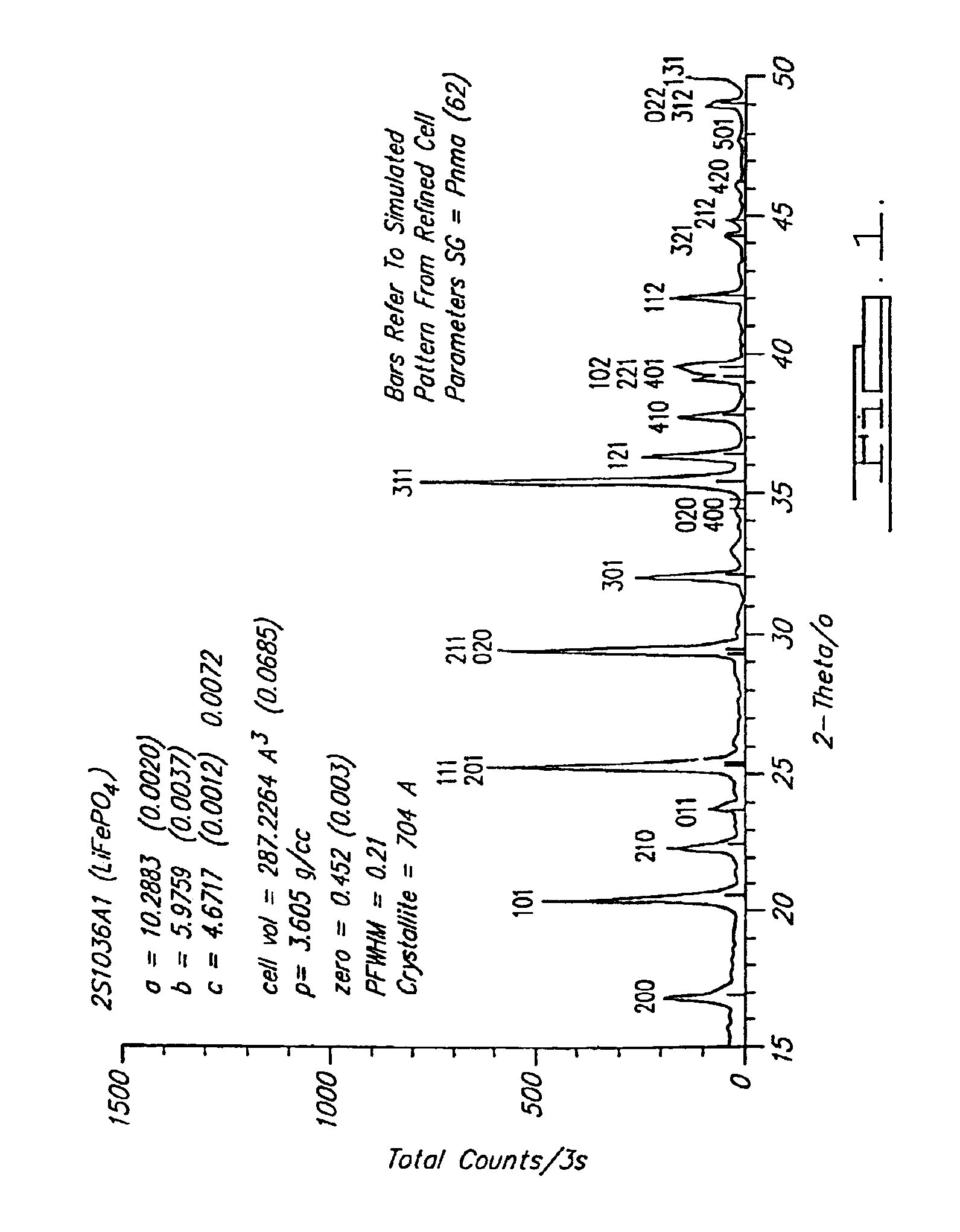
Preparation of olivine Li Fe PO4 cathode materials for lithium batteries via a solution method
InactiveUS20060263286A1Low costImprove conductivityPhosphatesPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesIron powderPhosphate
A preparation method of olivine Li1+xFe1+yPO4 is disclosed, wherein −0.2≦x≦0.2 and −0.2≦y≦0.2, which includes the following steps: (A) adding iron powder, lithium salt, and phosphate into an acid solution to form a mixture, wherein the molar ratio of Li+:Fe2+:PO43− is 1+x:1+y:y; (B) stirring the mixture; (C) drying the mixture to obtain solid precursor powder; and (D) heating the precursor solid powder at a temperature over 500° C. to form olivine structured powders.
Owner:TATUNG COMPANY
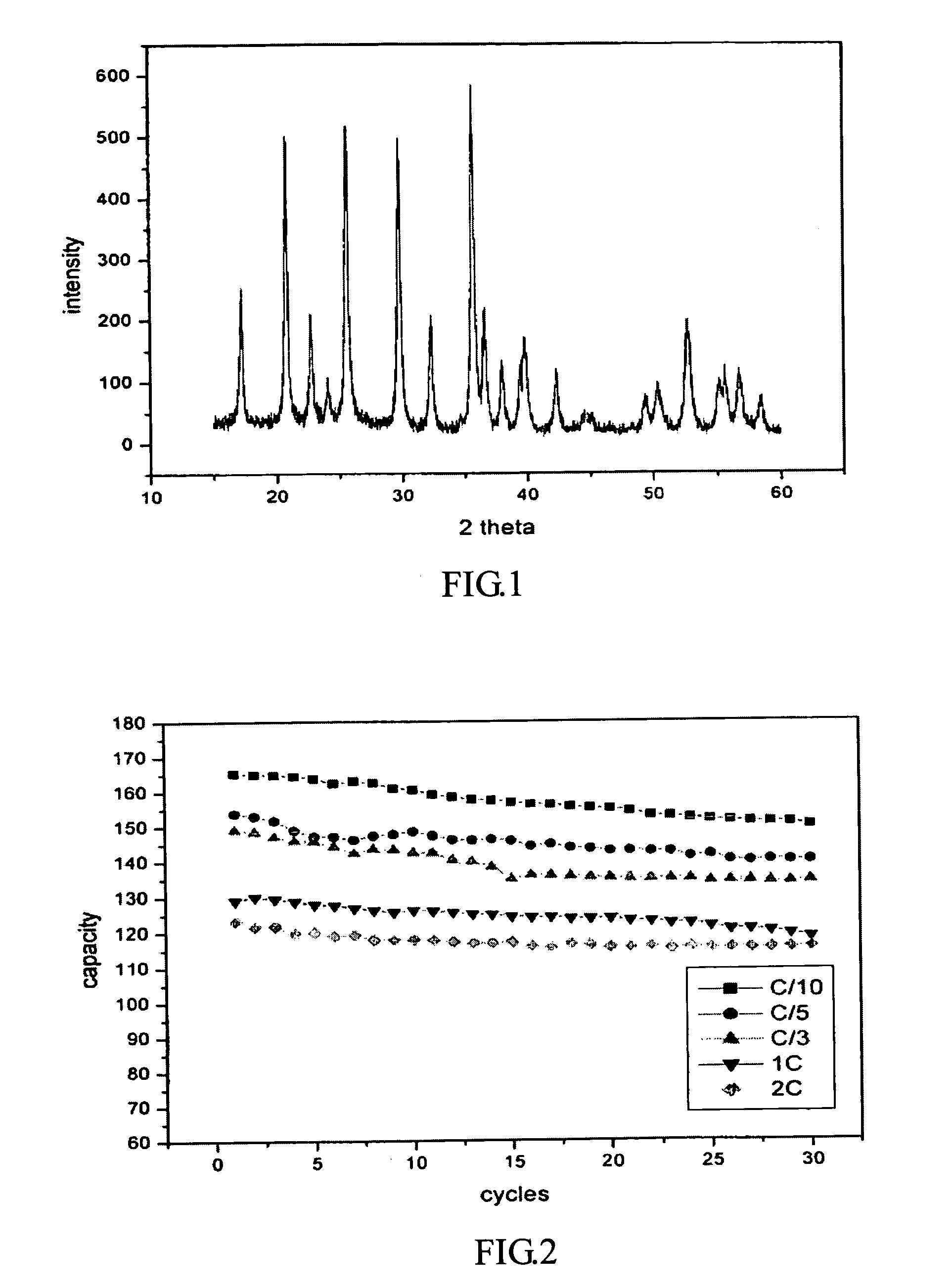
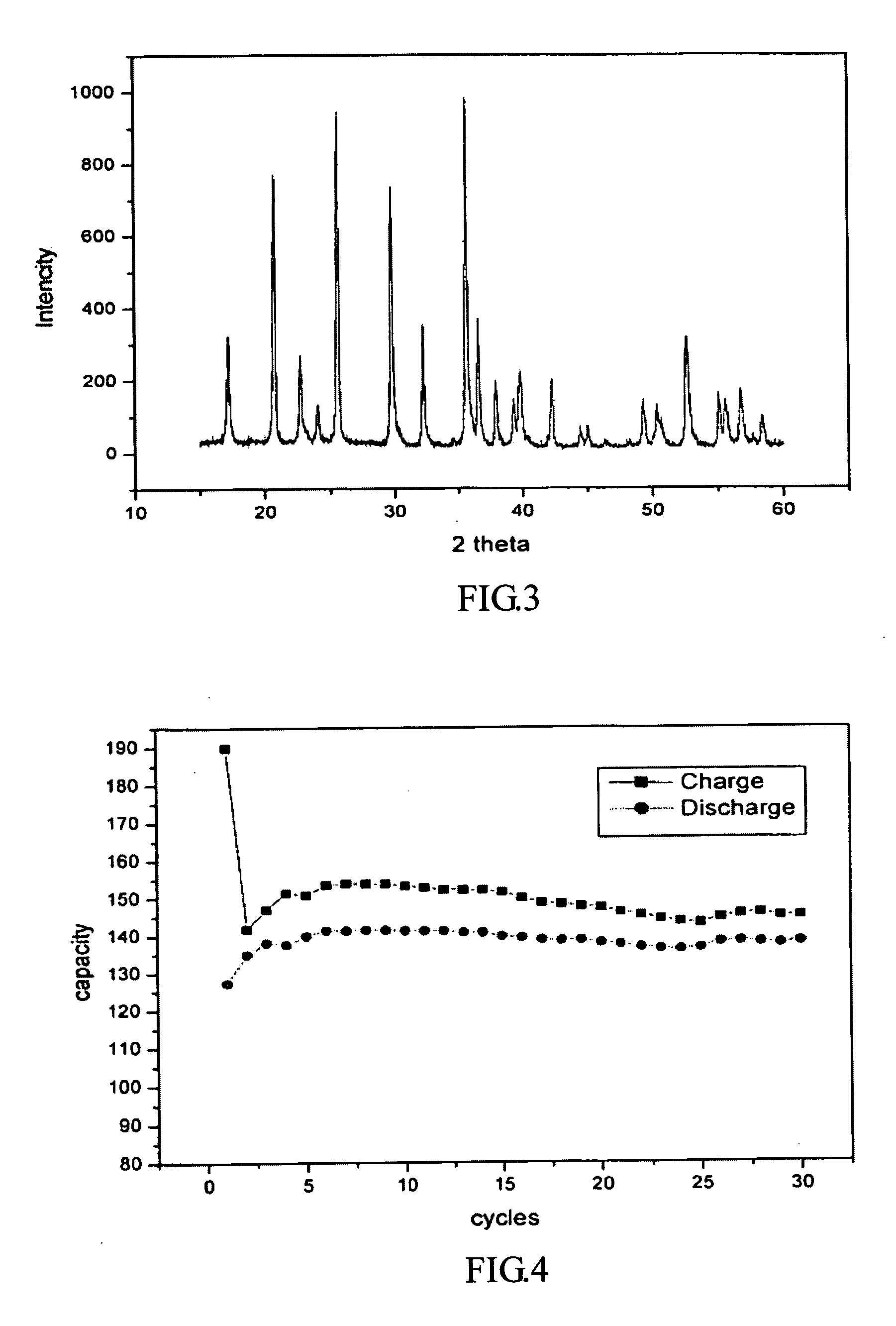
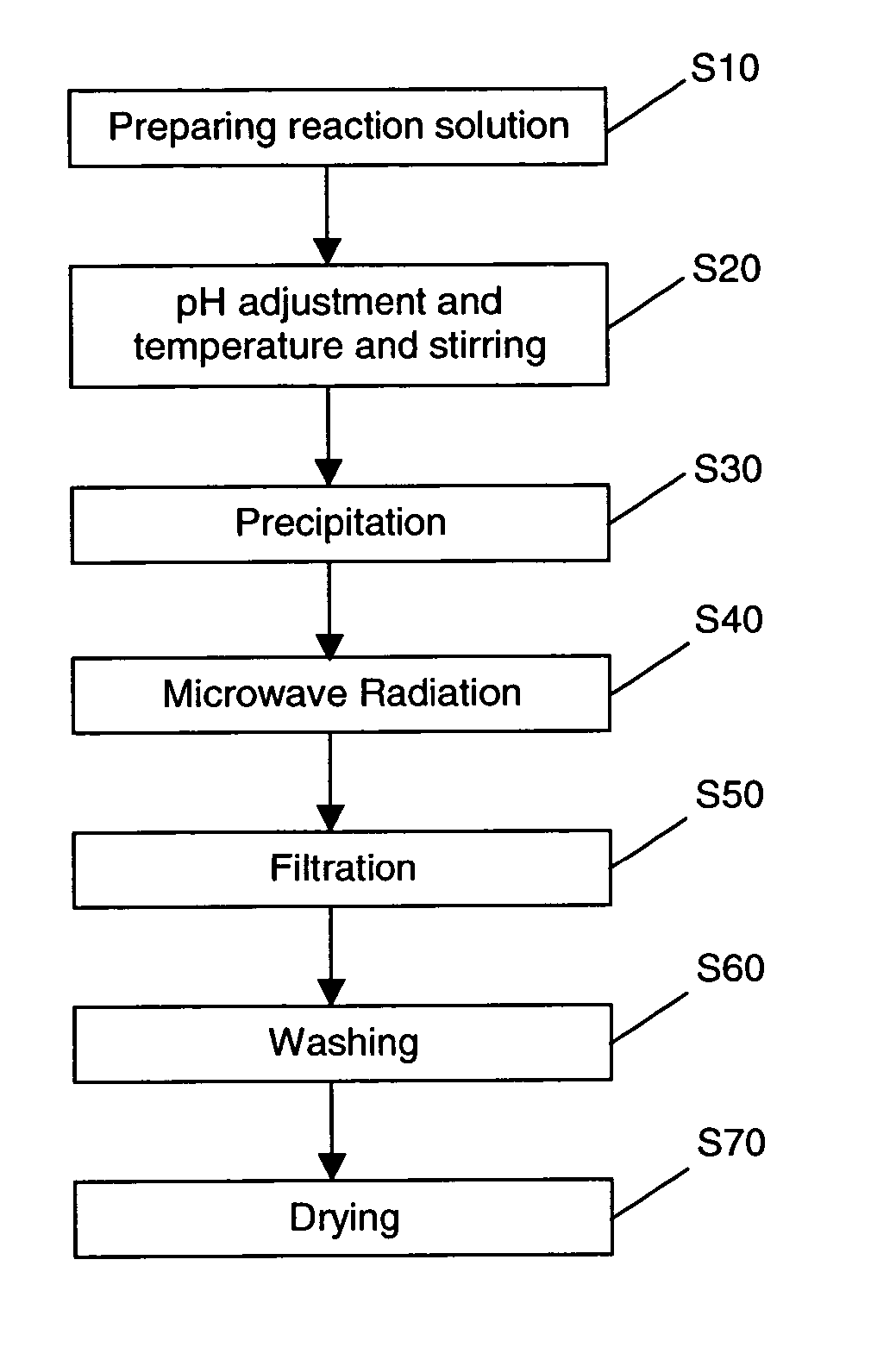
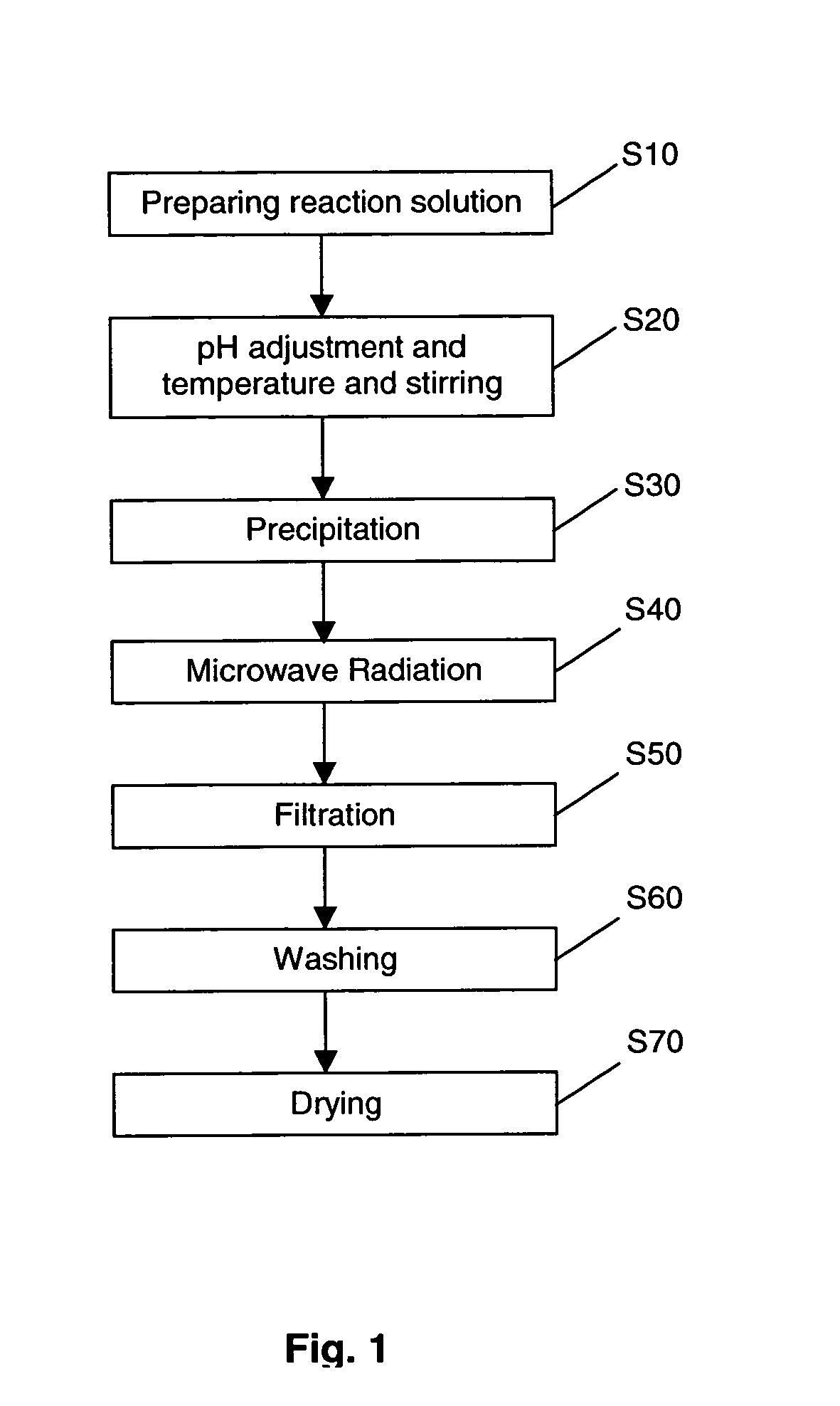
Production of nano-sized hydroxyapatite particles
Nano-sized hydroxyapatite particles are formed in a method comprising the steps of preparing a reaction solution containing a mixture of calcium ions and phosphate ions, stirring the reaction solution at a defined stirring speed, at a defined pH range and at a defined temperature range to form a suspension of hydroxyapatite seed particles, and subjecting the suspension of hydroxyapatite particles to microwave radiation for a defined time period, while maintaining the stirring speed, to form a suspension of nano-sized hydroxyapatite particles. The suspension of hydroxyapatite particles may preferably be aged for a period of time prior to the microwave radiation.
Owner:NAT UNIV OF SINGAPORE
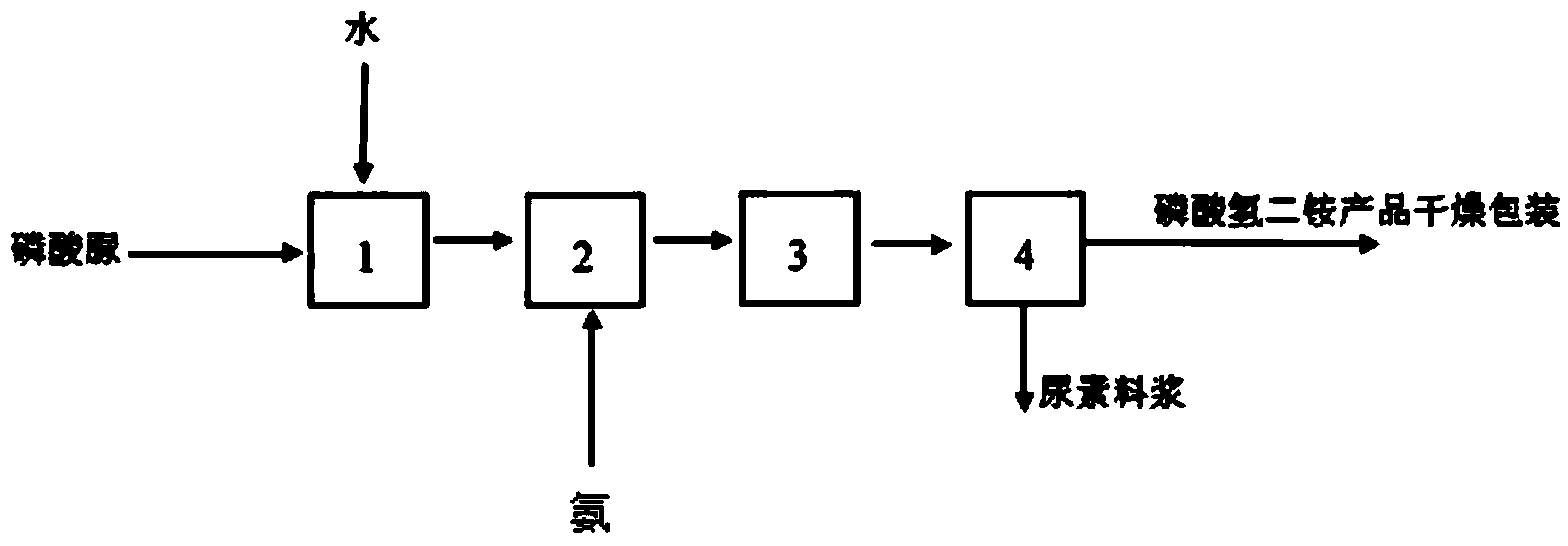
Production method for monoammonium phosphate
InactiveCN104016323AReduce energy consumptionReduce manufacturing costPhosphatesWater insolubleDecomposition
The invention relates to the technical field of chemical engineering, and particularly relates to a production method for monoammonium phosphate. A monoammonium phosphate product is prepared by directly subjecting high pure urea phosphate and ammonia to a double decomposition reaction and reacting for 20-60 min with stirring by controlling a reaction temperature at 40-80 DEG C and a pH value at a reaction endpoint being 4.2-4.6, and through the steps of cooling for crystallization; separating by centrifugation and drying. Measurements of physical and chemical indexes of the produced product show that the physical and chemical indexes of the produced monoammonium phosphate product meet an industrial first level standard. Specifically, the total nutrient content of monoammonium phosphate is higher than 73%; the content of total nitrogen is 12.01-12.23%; the content of phosphorus pentoxide is 61.05-61.25%; the content of moisture is 0.35-0.52%; a pH value is 4.2-4.6; the content of water insoluble materials is 0.090-0.096%; and the content of fluoride is 0.15-0.19%.
Owner:GUIYANG KAILIN FERTILIZER CO LTD
Methods of making lithium metal cathode active materials
The invention provides a novel method for making lithium mixed metal materials in electrochemical cells. The lithium mixed metal materials comprise lithium and at least one other metal besides lithium. The invention involves the reaction of a metal compound, a phosphate compound, with a reducing agent to reduce the metal and form a metal phosphate. The invention also includes methods of making lithium metal oxides involving reaction of a lithium compound, a metal oxide with a reducing agent.
Owner:LITHIUM WERKS TECH BV
Lithium ion conductive sulfide-based solid electrolyte and all-solid lithium battery using same
InactiveUS20090159839A1Improve lithium ion conductivityImprove conductivityPhosphatesFinal product manufactureElectrolyteAnalytical chemistry
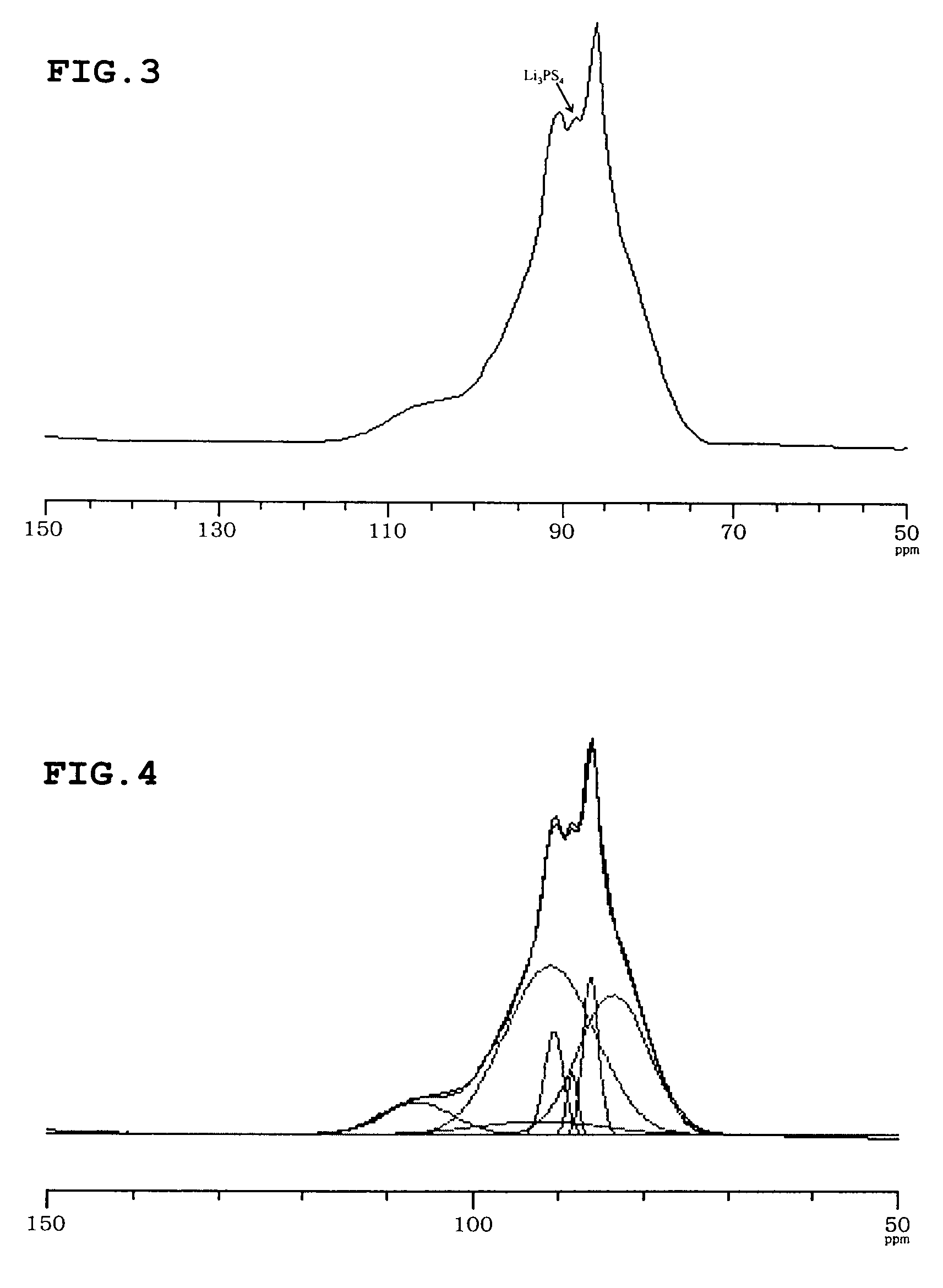
A solid electrolyte including a lithium (Li) element, a phosphorus (P) element and a sulfur (S) element, the 31P MAS NMR spectrum thereof having a peak ascribed to a crystal at 90.9±0.4 ppm and 86.5±0.4 ppm; and the ratio (xc) of the crystal in the solid electrolyte being from 60 mol % to 100 mol %.
Owner:IDEMITSU KOSAN CO LTD
Synthesis of molecular sieves having the chabazite framework type and their use in the conversion of oxygenates to olefins
The synthesis of a crystalline aluminophosphate or silicoaluminophosphate molecular sieve having a chabazite-type framework type is conducted in the presence of an organic directing agent having the formula (I) [R1R2R3N—R4]+X− (I) wherein R1, R2 and R3 are independently selected from the group consisting of alkyl groups having from 1 to 3 carbon atoms and hydroxyalkyl groups having from 1 to 3 carbon atoms; R4 is selected from the group consisting of 4- to 8-membered cycloalkyl groups, optionally substituted by 1 to 3 alkyl groups having from 1 to 3 carbon atoms; 4- to 8-membered heterocyclic groups having from 1 to 3 heteroatoms, said heterocyclic groups being optionally substituted by 1 to 3 alkyl groups having from 1 to 3 carbon atoms and the heteroatoms in said heterocyclic groups being selected from the group consisting of O, N, and S; and aromatic groups optionally substituted by 1 to 3 alkyl groups, said alkyl groups having from 1 to 3 carbon atoms; and X− is an anion.
Owner:EXXONMOBIL CHEM PAT INC
High energy and power density electrochemical cells
ActiveUS20060292444A1Improve electrochemical performanceExtreme safetyPhosphatesPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesHigh energyMetalloid
The energy density of the entire cell may be improved while retaining high power density by use of an alkali metal transition metal polyanion compound as the cathode and a thin film metal or metalloid anode. The thin film anode may be initially unalloyed or partially unalloyed. During use, the thin film anode may be only partially unalloyed relative to the theoretical maximum. The high volumetric capacity of the metal anode makes it possible to use a dense or porous thin film anode in conjunction with a relatively thin particle-based cathode to thereby improve the energy density of the cell.
Owner:A123 SYSTEMS LLC
Method for making a lithium mixed metal compound
A method for making a lithium mixed metal compound includes: preparing a reactant mixture that contains a metal compound, a lithium compound, and optionally, a phosphate-containing compound; and exposing the reactant mixture to an atmosphere in the presence of suspended carbon particles, and conducting a reduction to reduce oxidation state of at least one metal ion of the reactant mixture at a temperature sufficient to form a reaction product containing lithium and the reduced metal ion.
Owner:AQUIRE ENERGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com