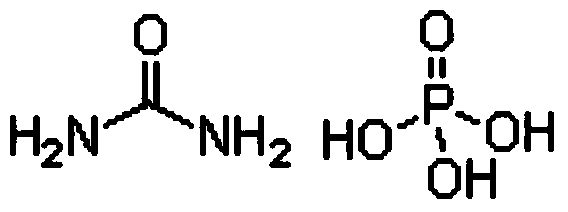Production method for monoammonium phosphate
A monoammonium phosphate and production method technology, which is applied in the direction of phosphate, phosphorus oxyacid, etc., can solve the problems of high product cost, unstable product quality, long process flow, etc., to simplify the production process and benefit the value of environmental protection , the effect of reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
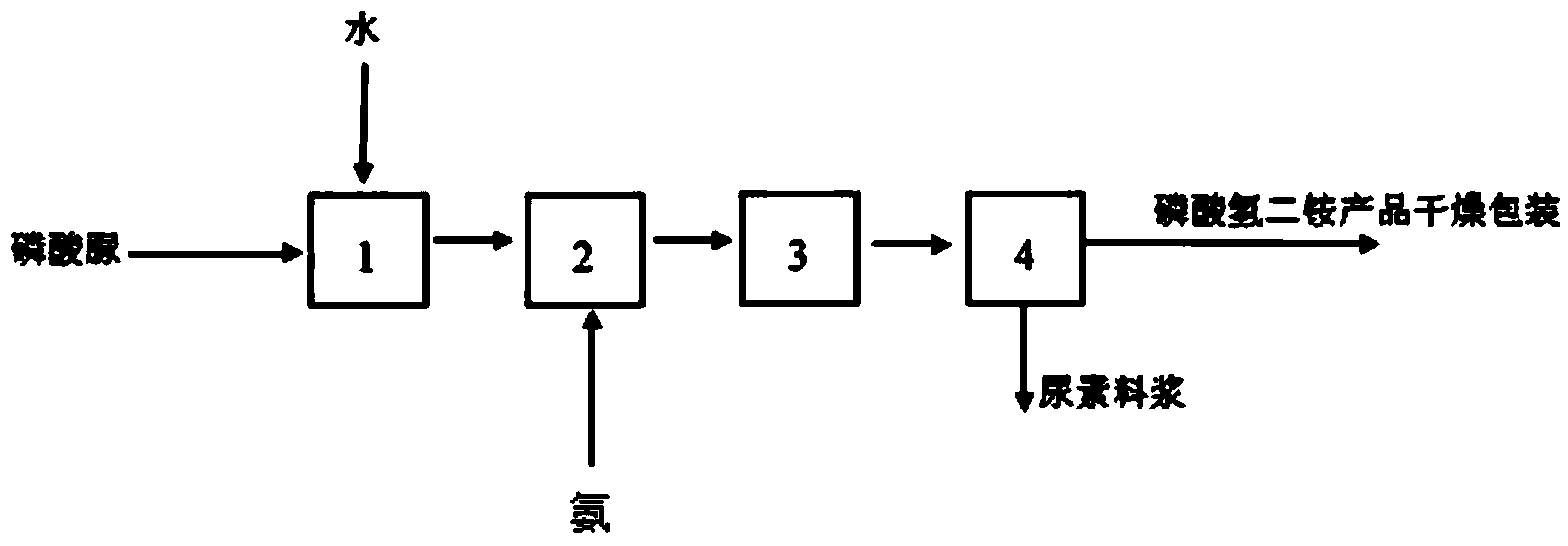
[0040] A kind of production method of monoammonium phosphate, adopts high-purity urea phosphate and ammonia to directly carry out metathesis reaction to prepare monoammonium phosphate, and its reaction equation is:
[0041] CO(NH 2 ) 2 ·H 3 PO 4 +NH 3 =NH 4 h 2 PO 4 +CO(NH 2 ) 2
[0042] Specifically, the following steps are included:
[0043] (1) Preparation of urea phosphate solution: add water to the urea phosphate solid with a purity of 98% in a dissolution tank and measure it, heat it to a temperature of 40°C, stir to dissolve and mix evenly, and control the stirring speed to 30r / min to obtain phosphoric acid Urea solution;
[0044] (2) Synthesis of monoammonium phosphate: input the urea phosphate solution into the synthesis tank with a transfer pump, control the temperature at 40°C, and add ammonia to react according to the molar ratio of urea phosphate and ammonia at a ratio of 0.99:1; Ammonia was added, the stirring speed was 60-70r / min, and when the reaction...
Embodiment 2
[0048] A kind of production method of monoammonium phosphate, adopts high-purity urea phosphate and ammonia to directly carry out metathesis reaction to prepare monoammonium phosphate, and its reaction equation is:
[0049] CO(NH 2 ) 2 ·H 3 PO 4 +NH 3 =NH 4 h 2 PO 4 +CO(NH 2 ) 2
[0050] Specifically, the following steps are included:
[0051] (1) Preparation of urea phosphate solution: Add water to the urea phosphate solid with a purity of 98% in the dissolution tank and measure it. After heating up to 80°C, stir to dissolve and mix evenly, and control the stirring speed to 50r / min to obtain phosphoric acid Urea solution;
[0052] (2) Synthesis of monoammonium phosphate: input the urea phosphate solution into the synthesis tank with a transfer pump, control the temperature at 80°C, and add ammonia to react according to the molar ratio of urea phosphate and ammonia at a ratio of 1.05:1; Ammonia was added while stirring, the stirring speed was 70r / min, when the stir...
Embodiment 3
[0056] A kind of production method of monoammonium phosphate, adopts high-purity urea phosphate and ammonia to directly carry out metathesis reaction to prepare monoammonium phosphate, and its reaction equation is:
[0057] CO(NH 2 ) 2 ·H 3 PO 4 +NH 3 =NH 4 h 2 PO 4 +CO(NH 2 ) 2
[0058] Specifically, the following steps are included:
[0059] (1) Preparation of urea phosphate solution: Add water to the urea phosphate solid with a purity of 98% and measure it in a dissolution tank. After heating up to 60°C, stir to dissolve and mix evenly, and control the stirring speed to 40r / min to obtain phosphoric acid Urea solution;
[0060] (2) Synthesis of monoammonium phosphate: Input the urea phosphate solution into the synthesis tank with a delivery pump, control the temperature at 60°C, and add ammonia to react according to the molar ratio of urea phosphate and ammonia at a ratio of 1:1; Ammonia was added, the stirring speed was 65r / min, and when the stirring reaction wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com