Method for recovery of CO2 from gas streams
a gas stream and co2 technology, applied in the field of gas stream recovery, can solve the problems of limited process choice to a few, harmful emissions of co2 in the atmosphere, and wide adoption of technology, and achieve the effects of increasing the ionic strength of the co2 absorbent, high level of amine or inorganic salts, and high ionic strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0141]Amine solutions were tested for ability to dissolve CO2 by sparging 2 molar aqueous solutions of the amine with pure CO2 through a fritted glass disperser. The amine sample was held at constant temperature (either 25° C. or 50° C.) until the weight of the sample remained constant. The CO2 concentration was calculated as moles of CO2 per mole of amine. The data are presented in Table 2.
example 2
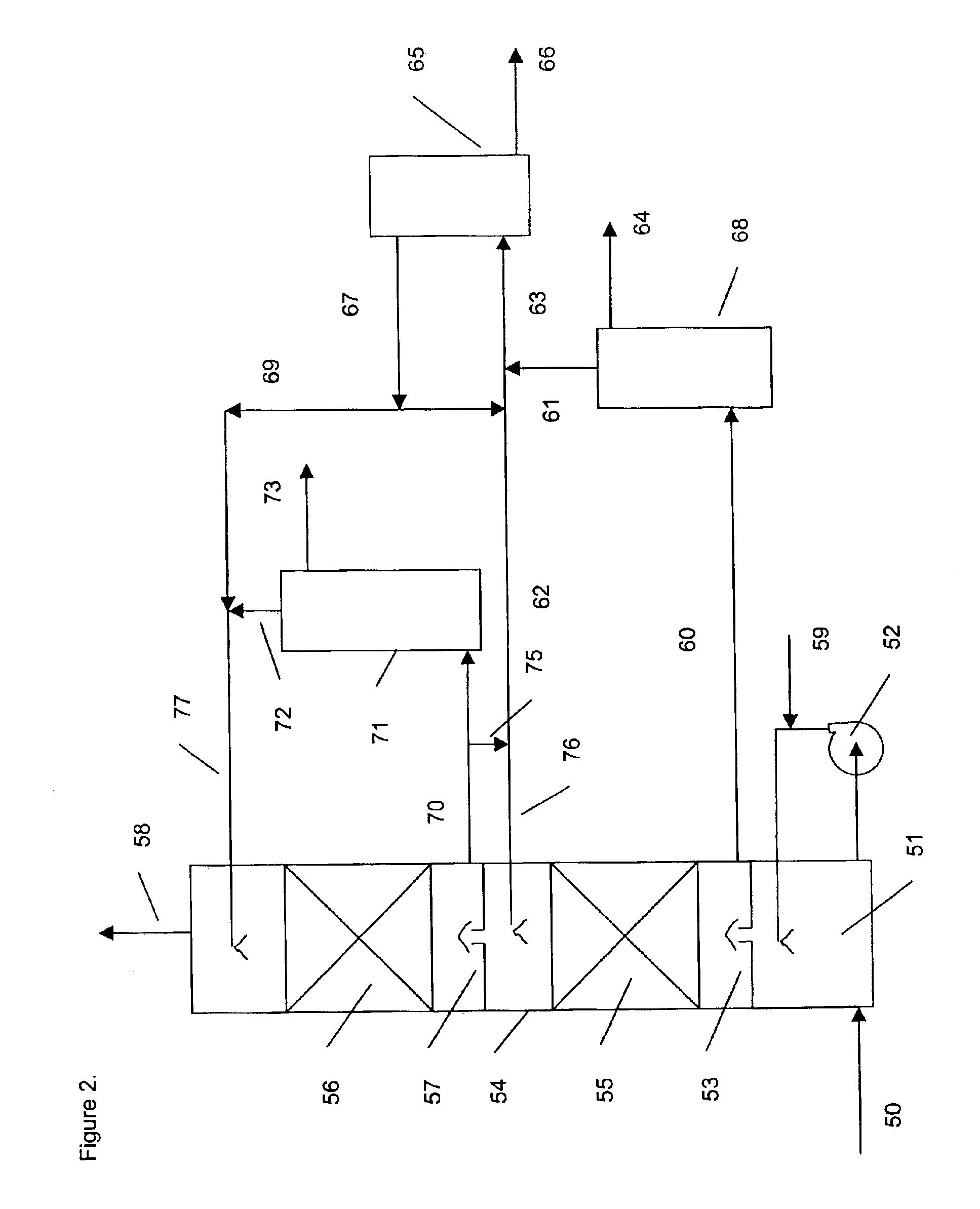
[0142]Absorbents were tested in a laboratory scale pilot apparatus, using a synthetic mixture of gases obtained by mixing mass flow controlled streams of the individual pure gases from gas cylinders. The test apparatus consisted of an 1 inch outer diameter glass absorbing tower containing a 12 inch bed of wire mesh saddles. The test absorbents were pumped to the absorbing tower by a variable speed metering pump. The absorbent at the bottom of the tower collected in a flask immersed in a thermostated bath set at 60° C. The bottom sump liquid level was controlled by means of another variable speed metering pump, which pumped the rich solvent to the top of a 5 sieve tray regeneration tower. The bottom sump of the regeneration tower was immersed in another thermostated bath, set at about 130° C., which then provided the stripping steam. An overhead condenser condensed most of the water vapor in the overhead stream of CO2 and steam and returned the water to the regeneration tower, in ord...
example 3
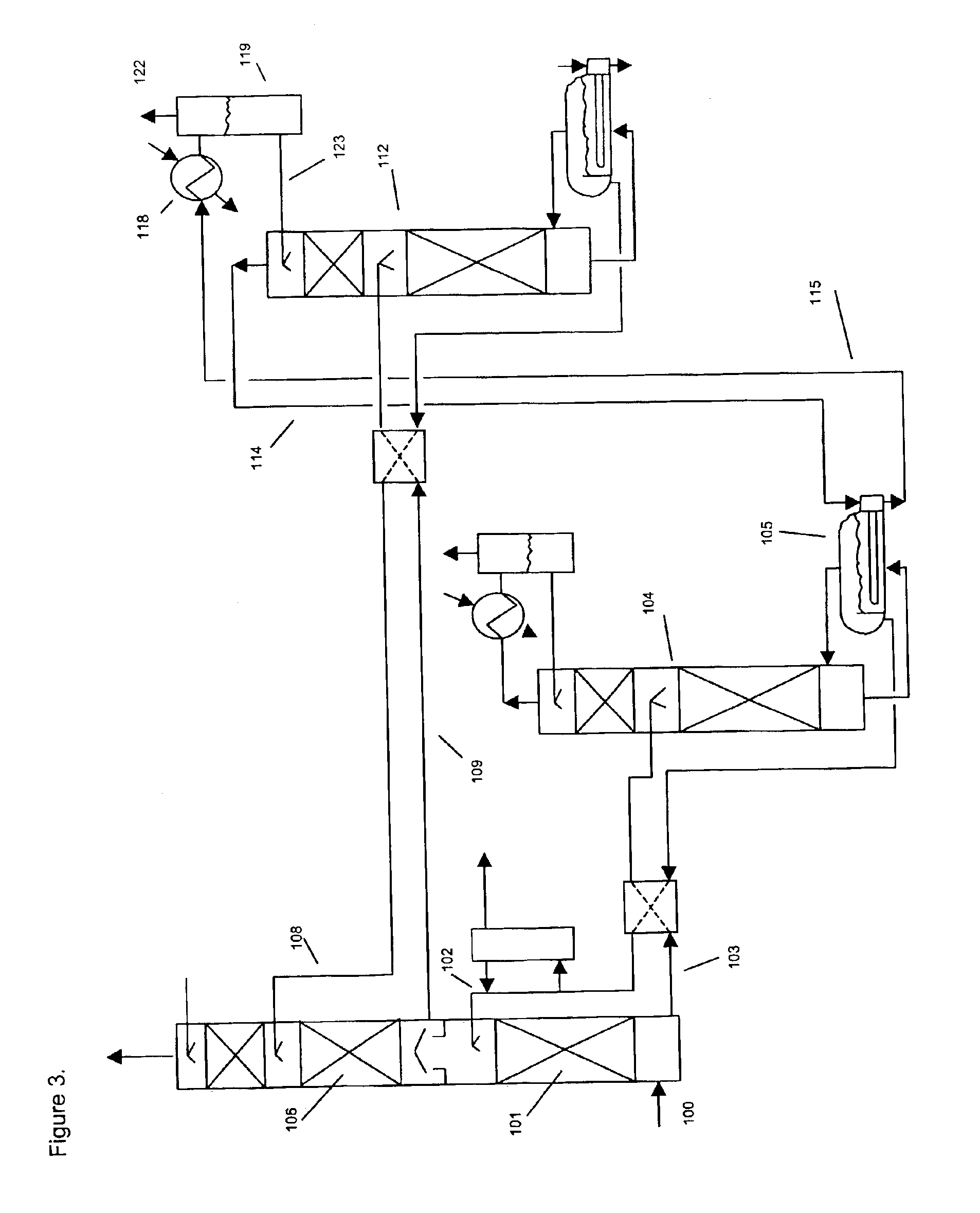
[0143]The apparatus of Example 2 was used to test the simultaneous removal of CO2 and nitric oxide, NO. The absorbent liquid was 3 molar in triethanolamine, 0.05 molar in FeEDTA and containing 2% sodium sulfite, with the balance being water. The feed gas flow of 1.9 liters per minute contained 9% vol. CO2 and 360 ppmv of NO, with the balance nitrogen. Analysis for NO was performed with a non-dispersive infrared gas analyzer and CO2 was determined with Gastec detector tubes. An absorbent flow rate of 15 ml / minute was used. The absorber tower bottom was thermostated to 60° C. and the regenerator bottom sump was held at about 100° C. The absorber pressure was equal to ambient. The test was run for 5 hours. The NO and CO2 removal remained essentially constant during the At the end, the outlet NO concentration was 17 ppmv for a removal of 95%. The outlet CO2 was 5%, for a removal of 44%.
[0144]
TABLE 1Tertiary Amines for CO2 CaptureMolecularCompoundWeightpKaN-methyldiethanolamine (MDEA)119...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com