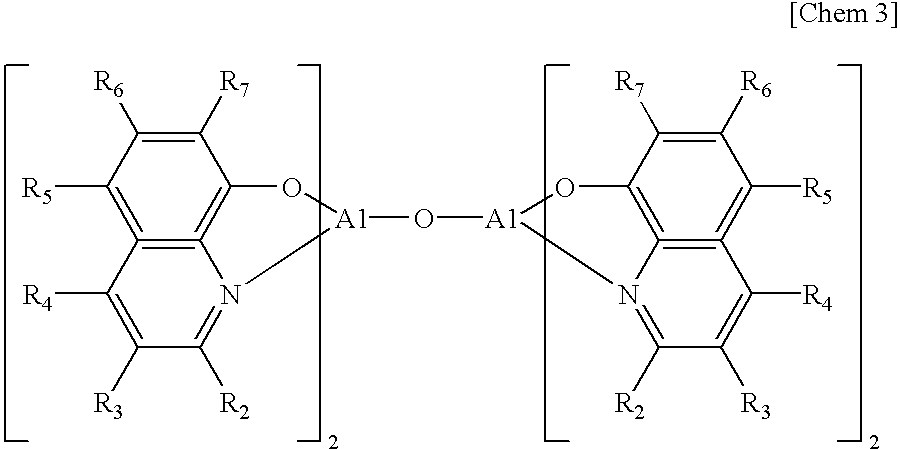
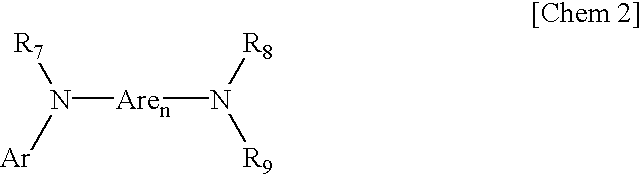Patents
Literature
69132 results about "Moisture" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Moisture is the presence of a liquid, especially water, often in trace amounts. Small amounts of water may be found, for example, in the air (humidity), in foods, and in various commercial products. Moisture also refers to the amount of water vapour present in the air.
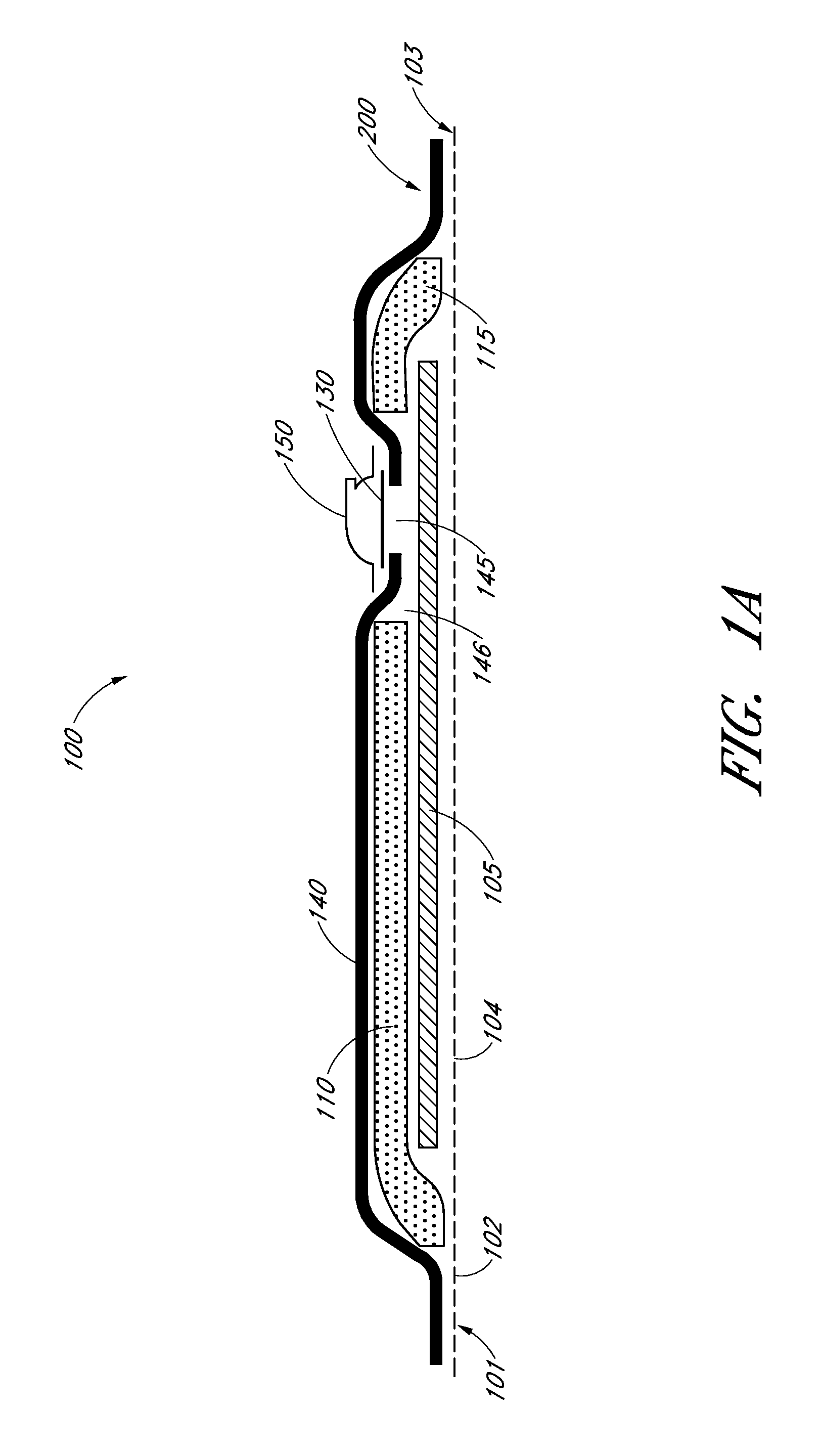
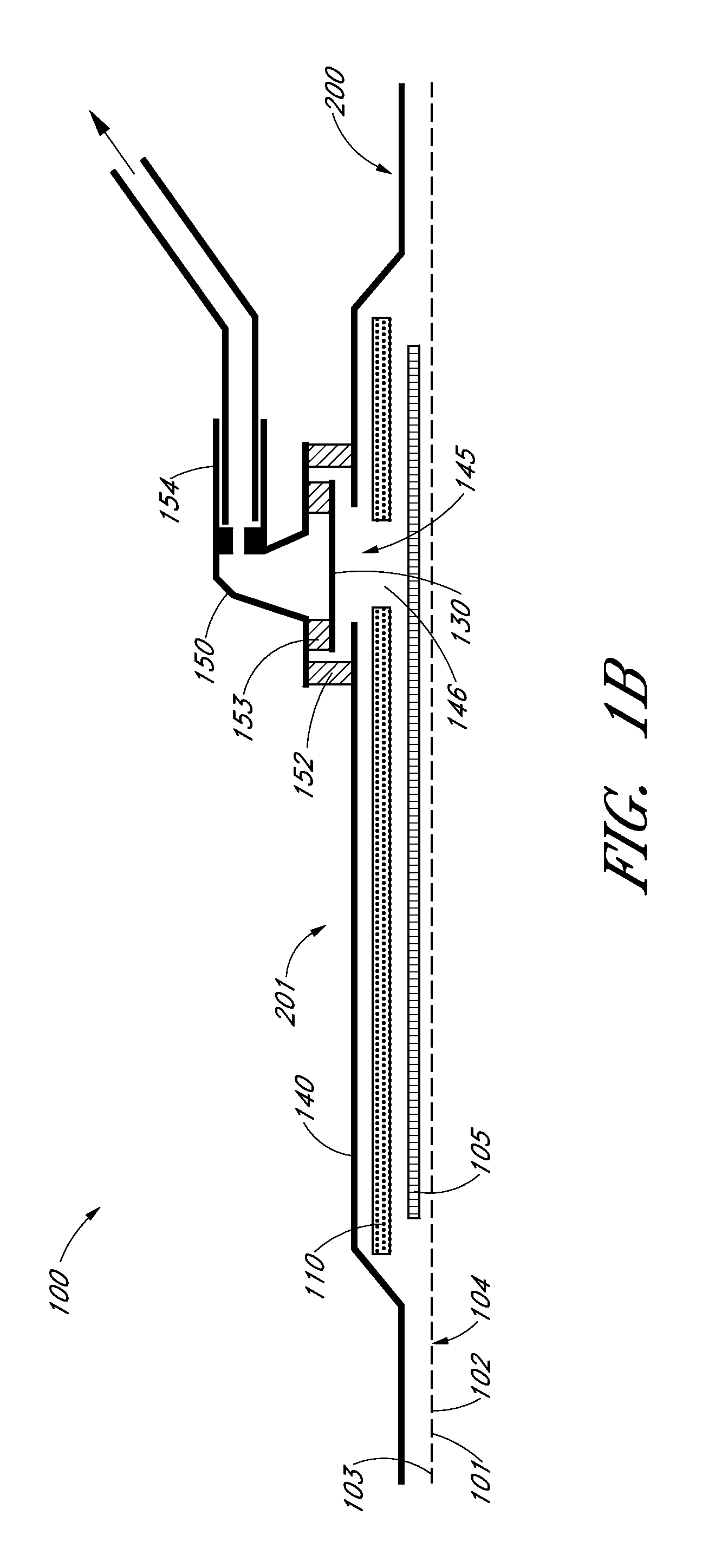
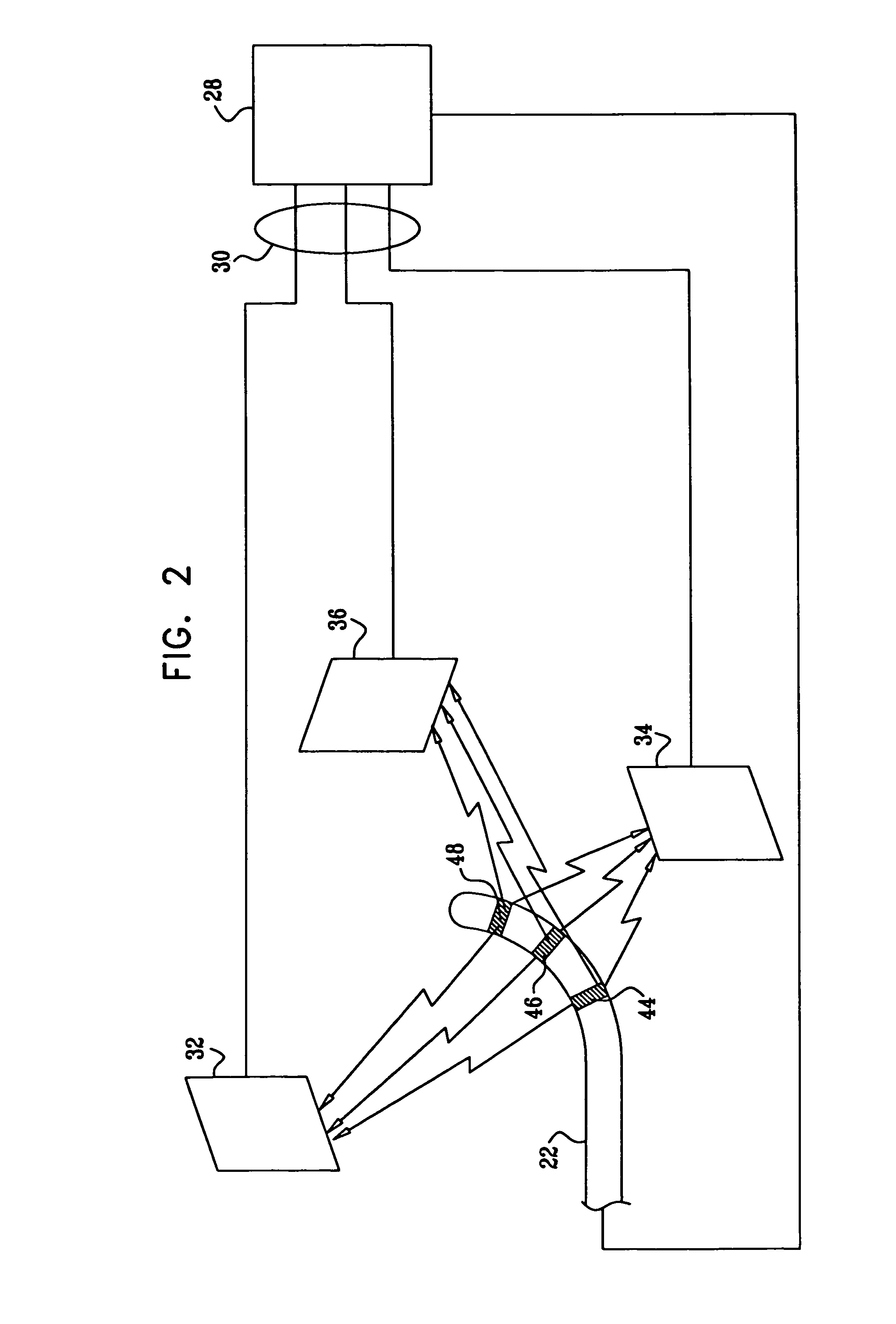
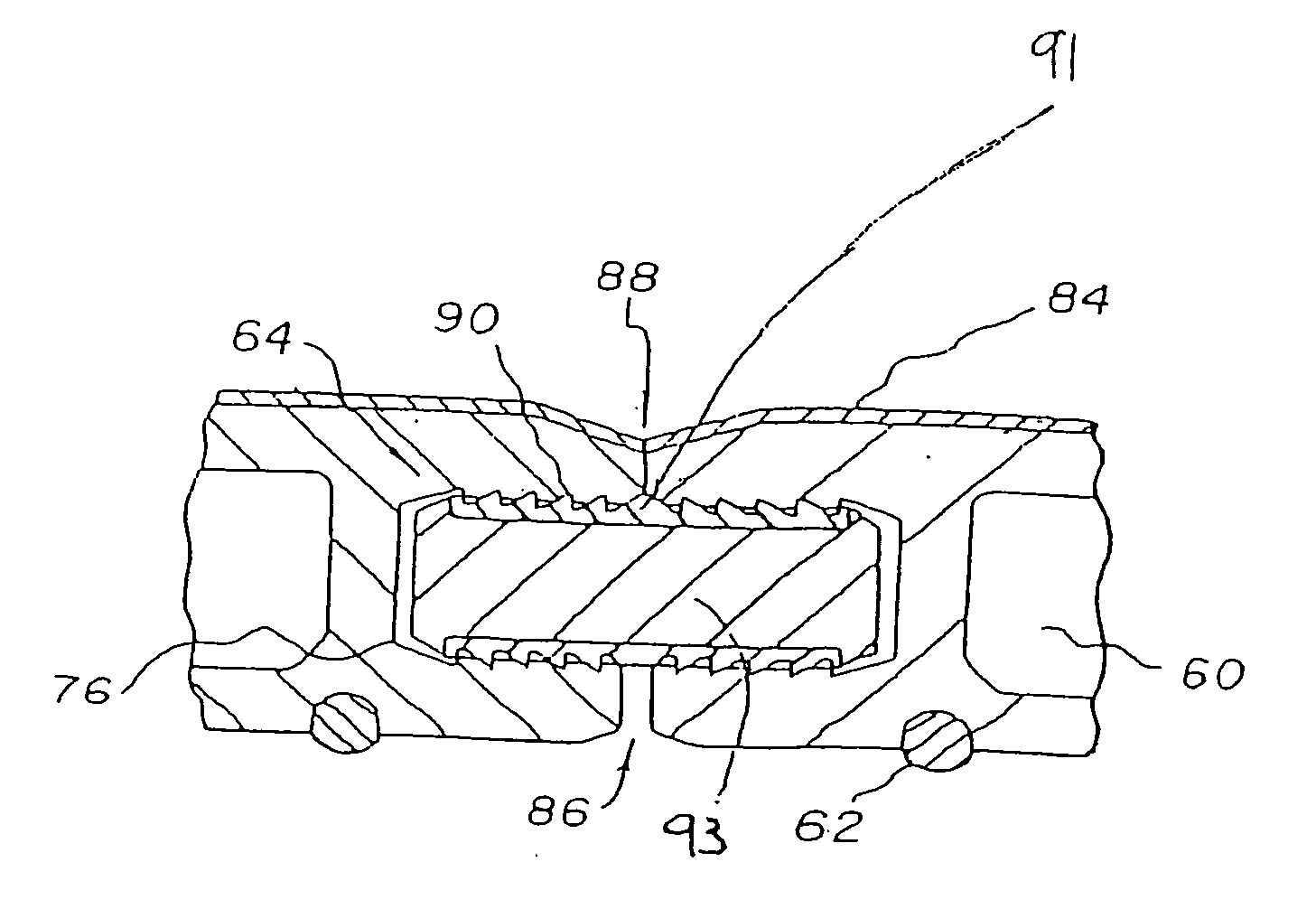
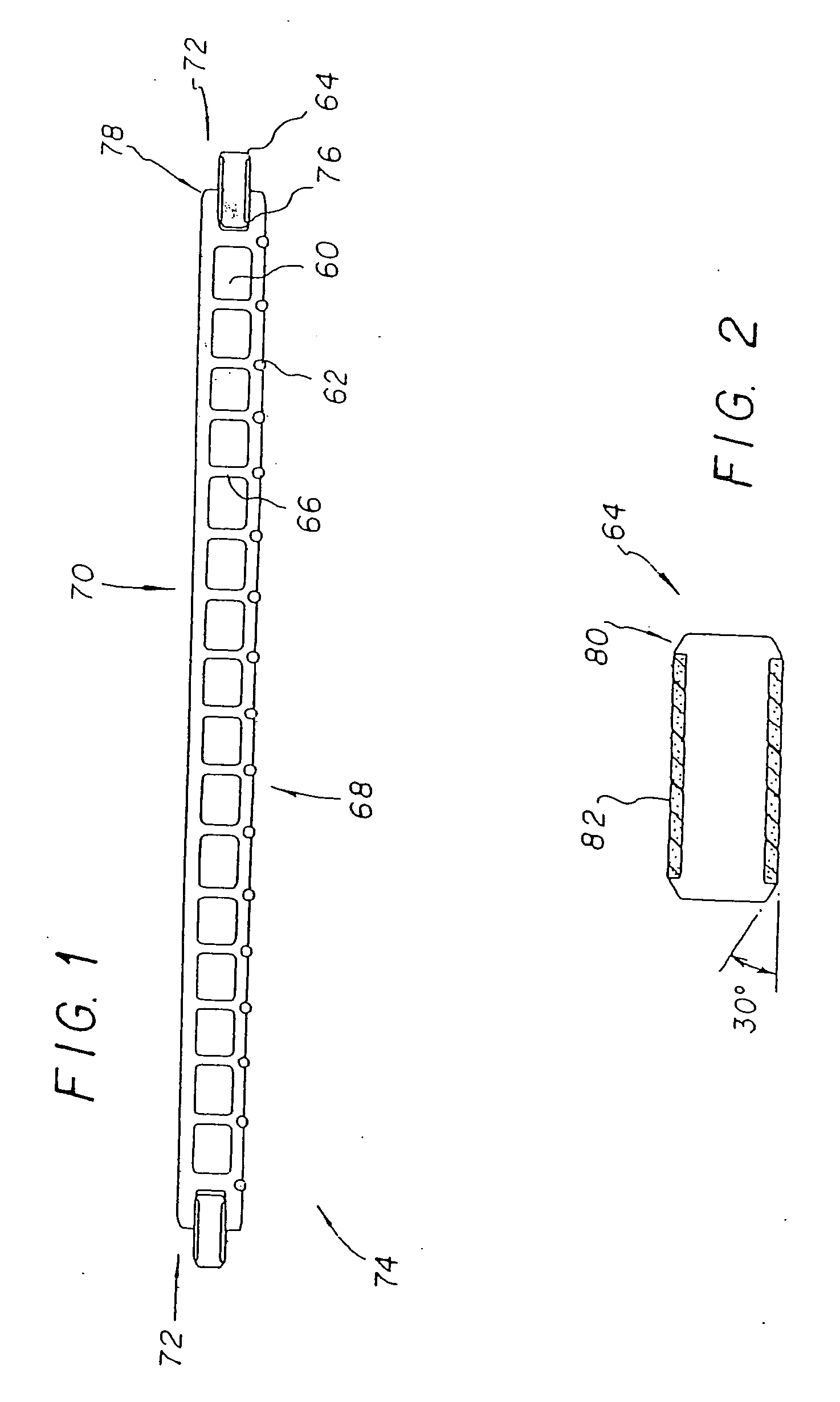
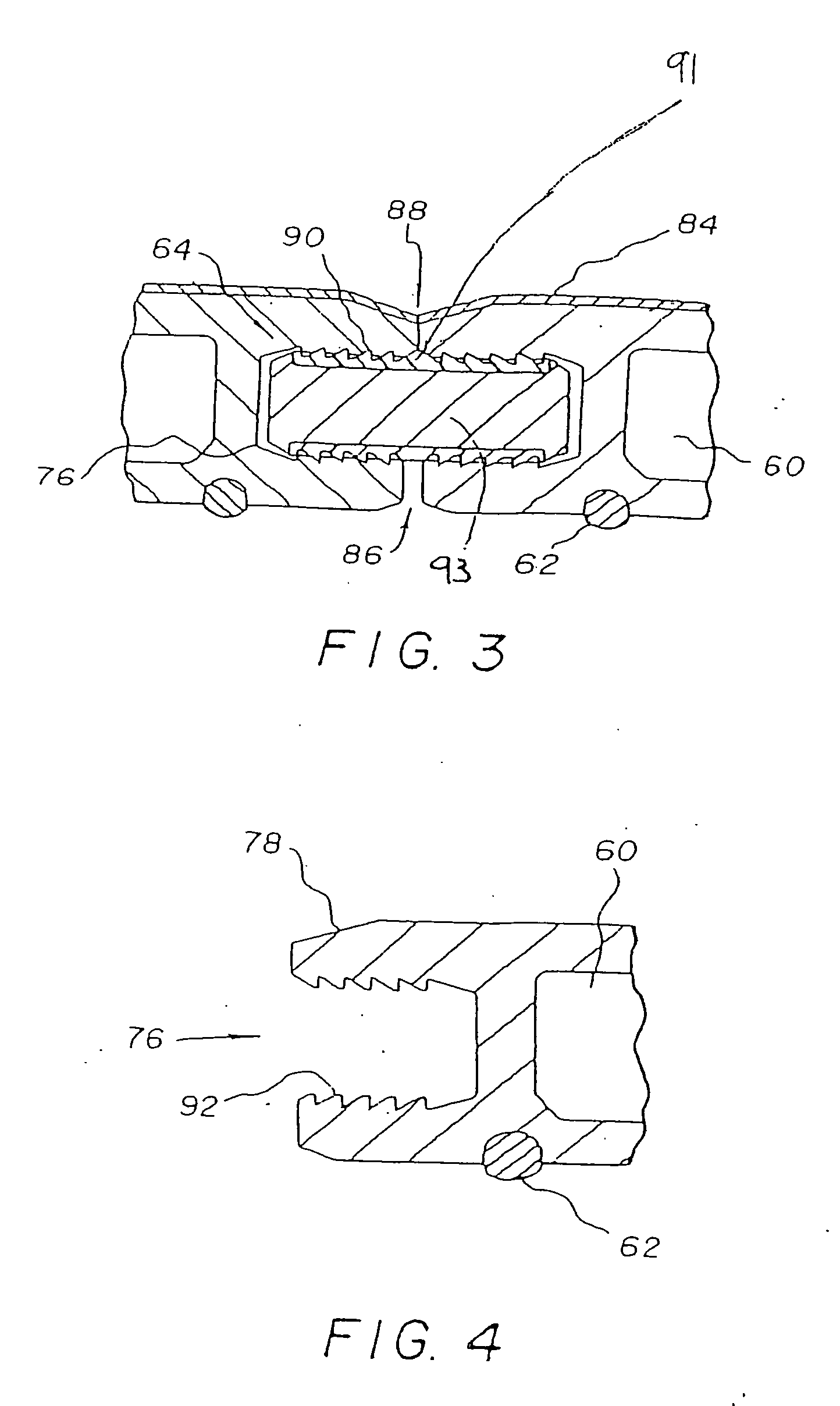
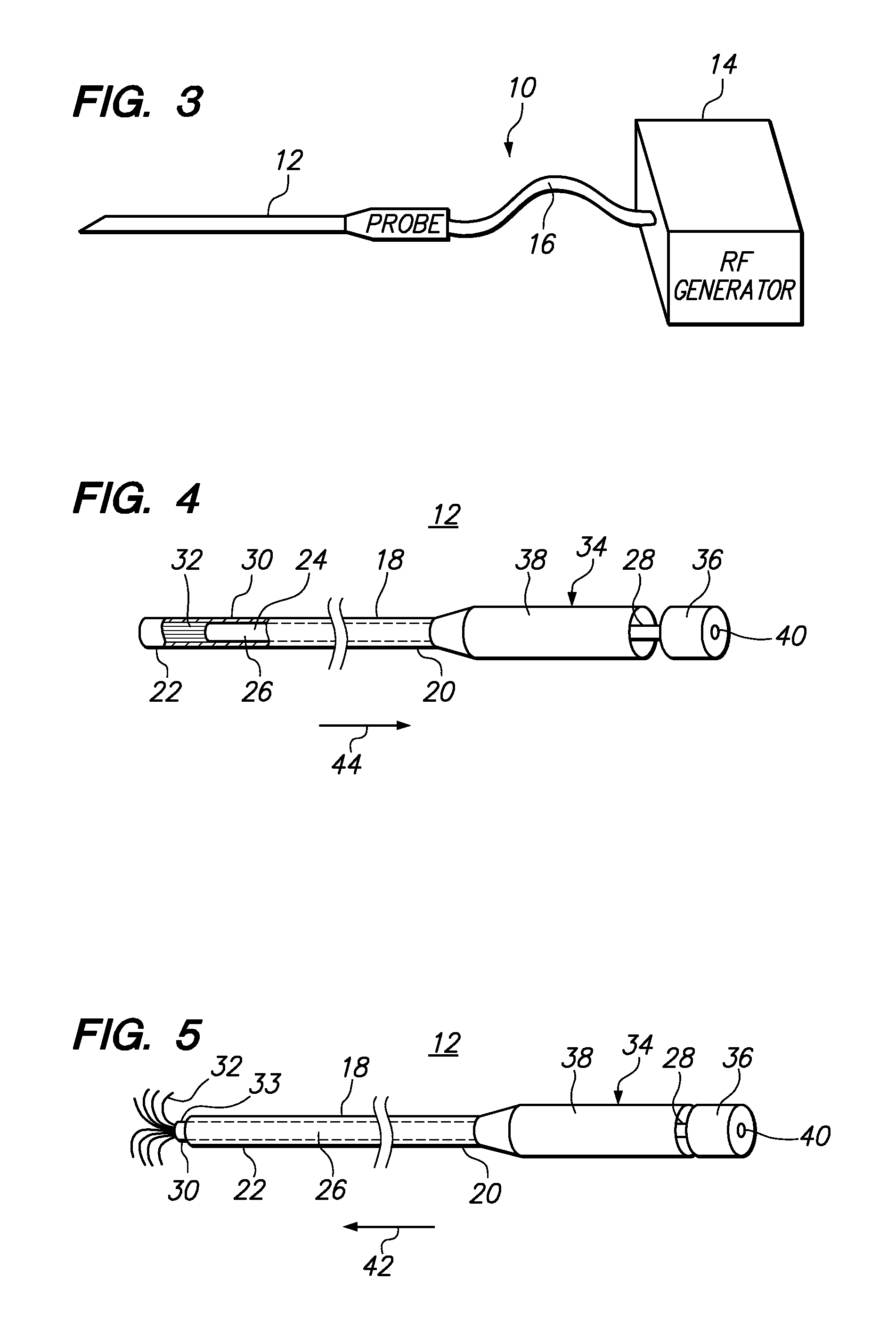
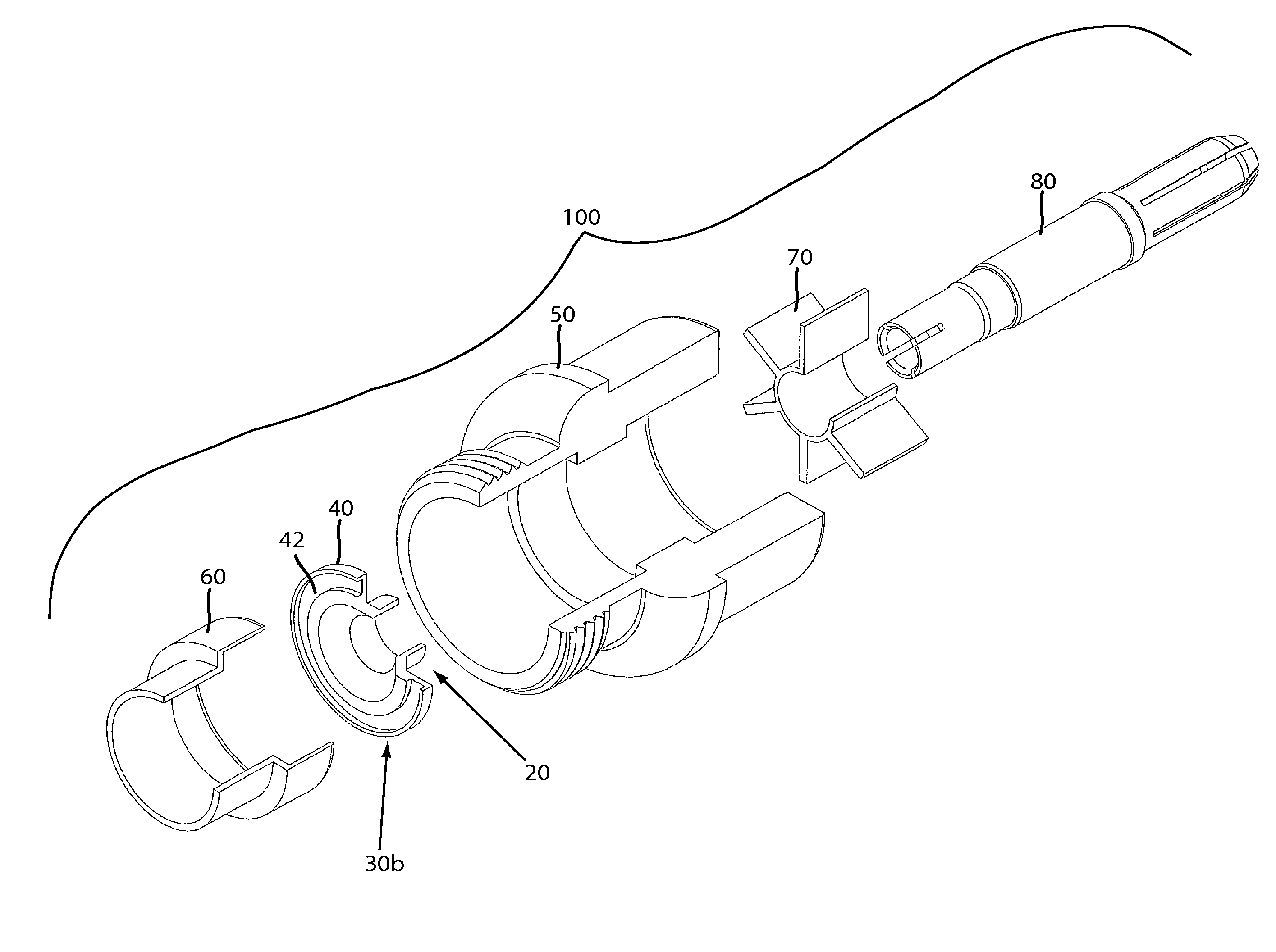
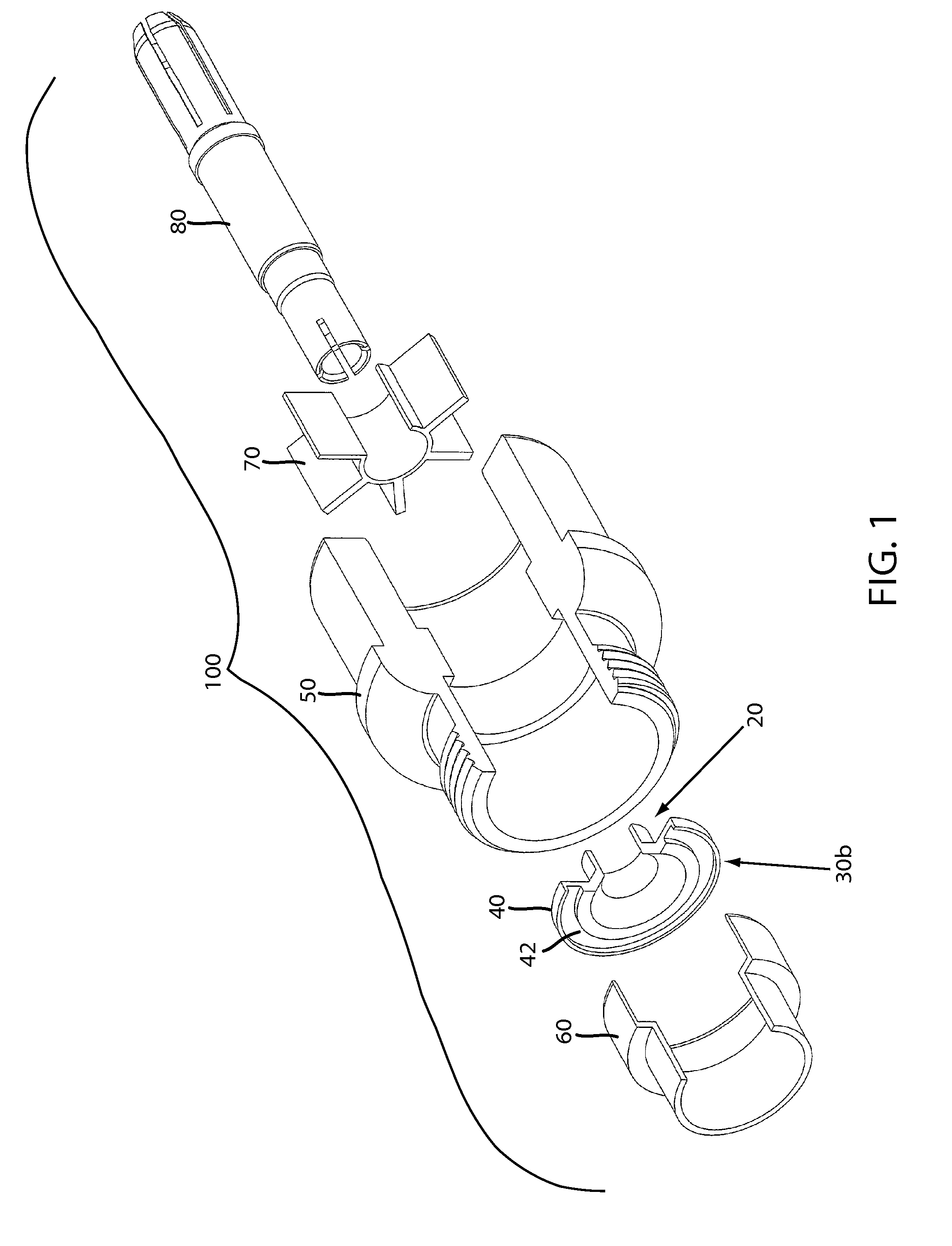
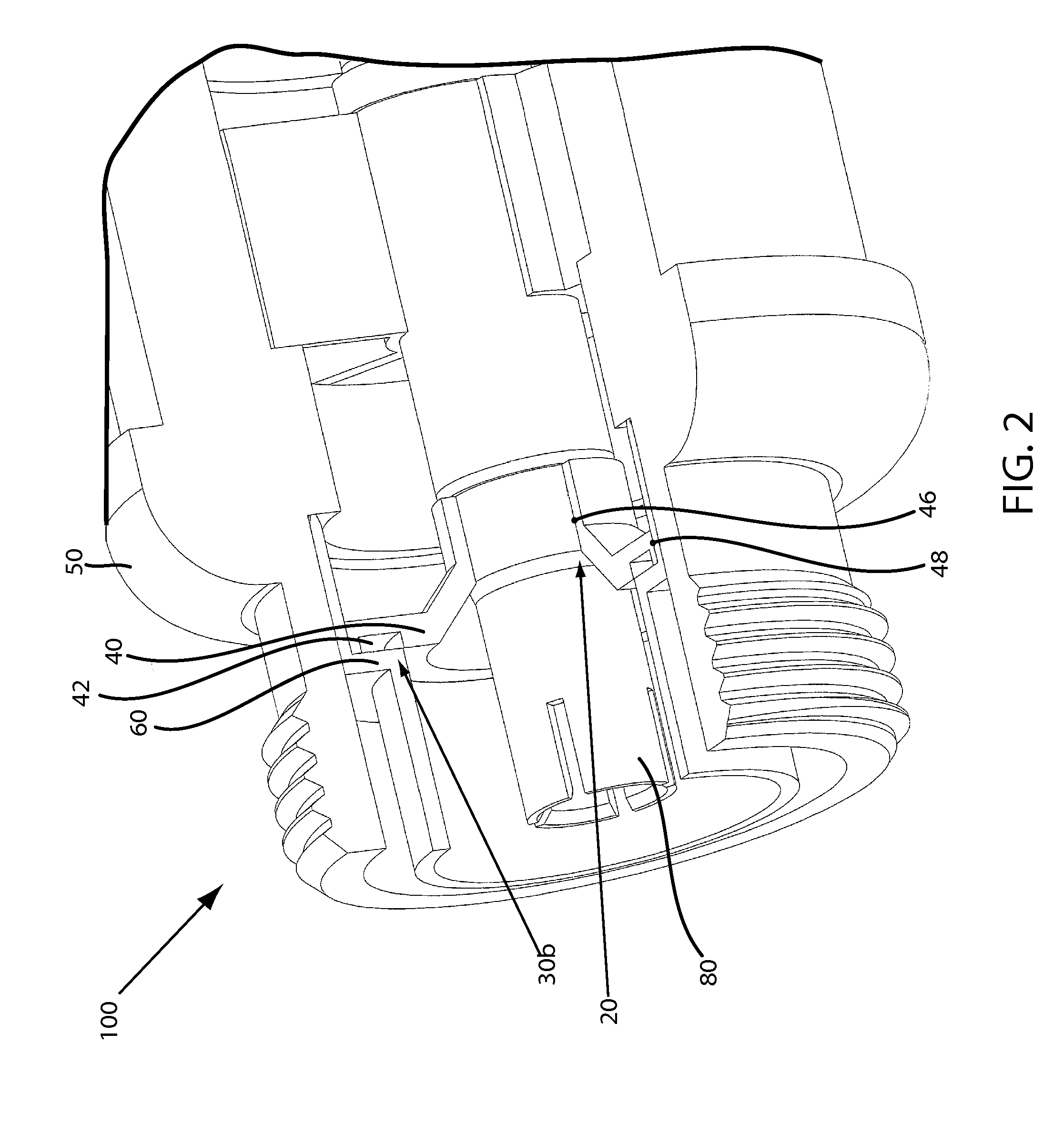
Moisture-detecting shaft for use with an electro-mechanical surgical device
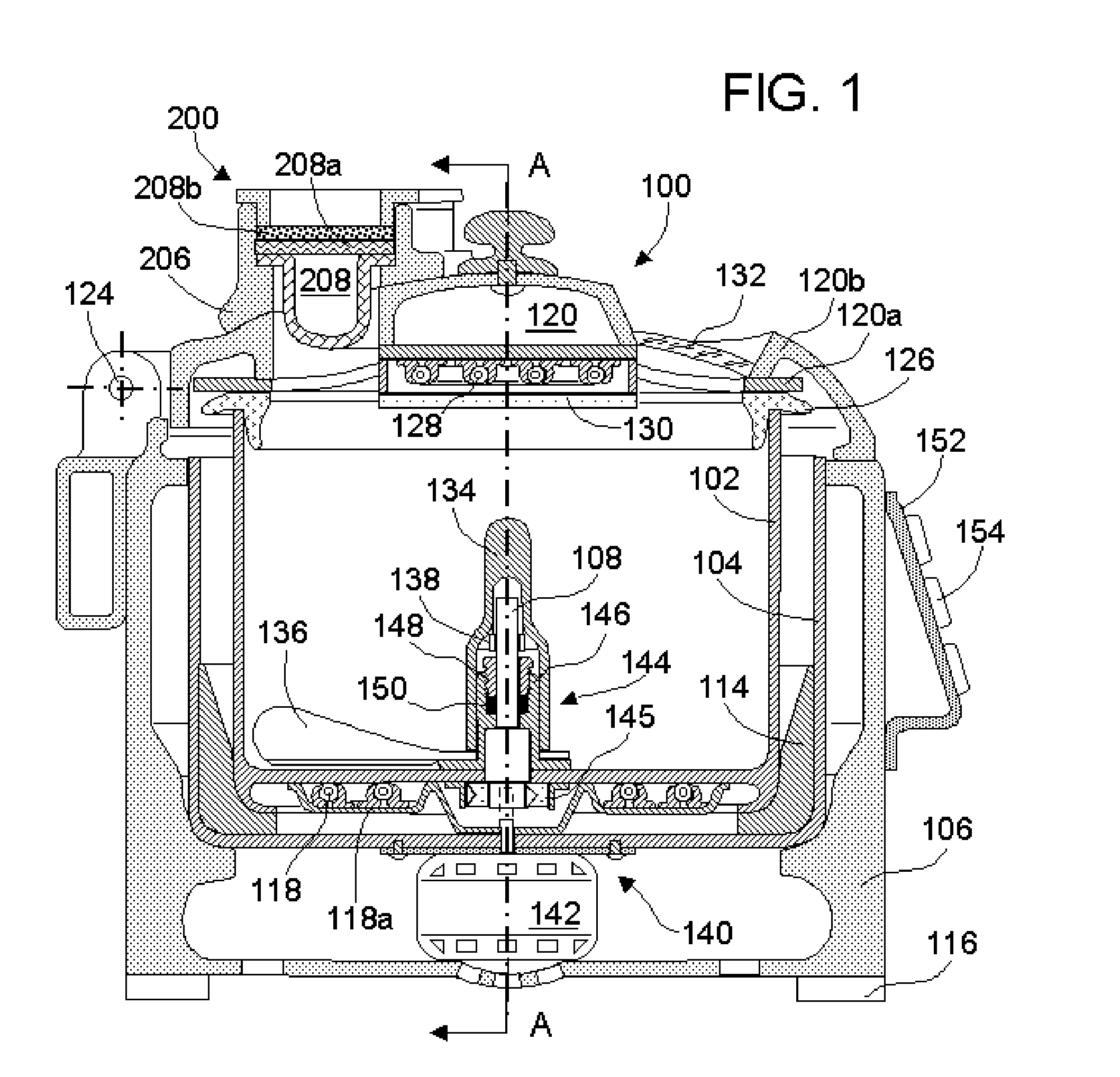
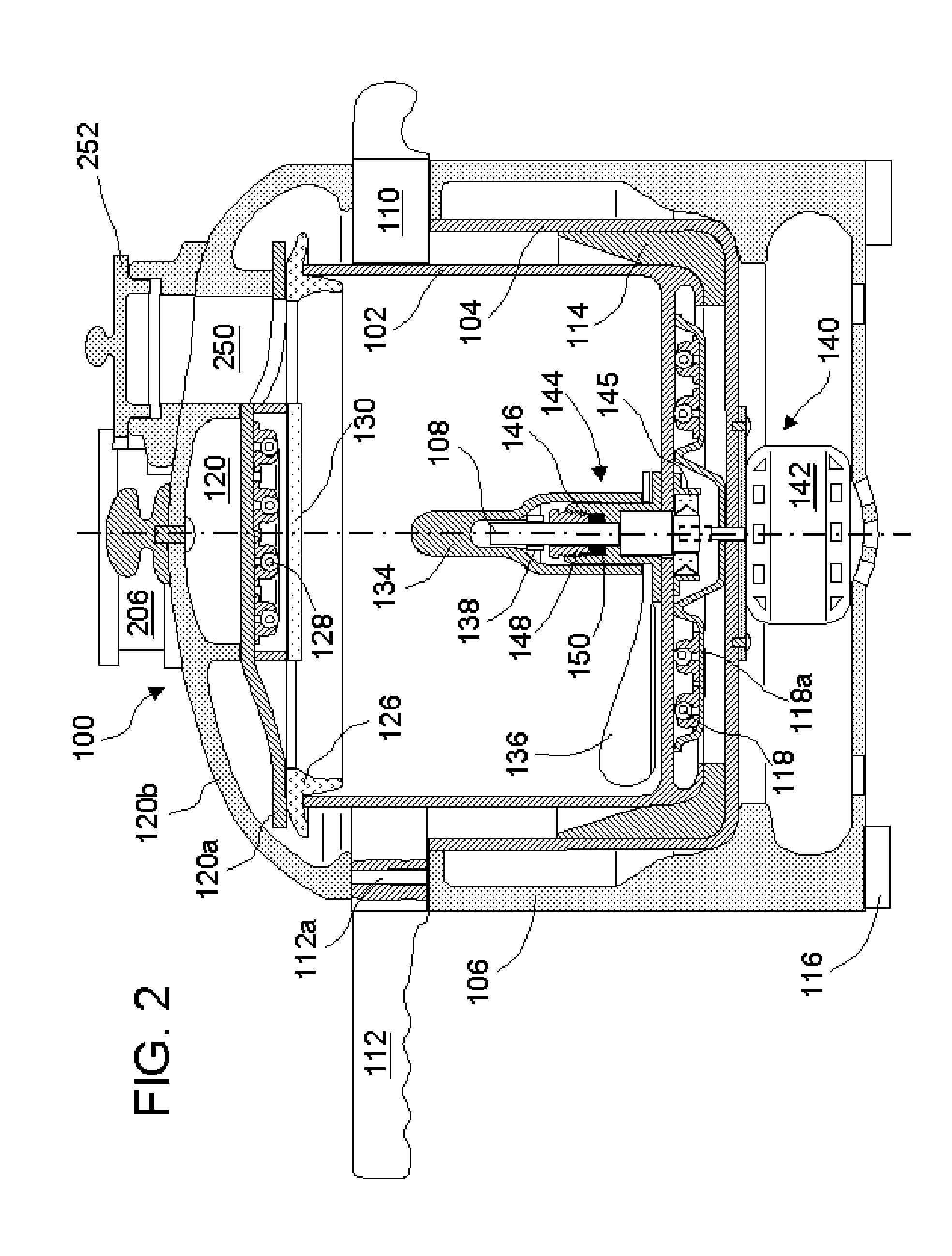
A flexible shaft includes a flexible, elongated outer sheath, at least one drive shaft disposed within the outer sheath and a moisture sensor disposed within the outer sheath configured to detect moisture within the outer sheath. Another flexible shaft includes a flexible, elongated outer sheath, at least one flexible drive shaft disposed within the outer sheath and a coupling connected to a distal end of the outer sheath configured to couple to a surgical attachment.A sleeve includes an elongated shaft configured to receive a flexible shaft therein and a securing arrangement configured to selectively and variably retain the elongated shaft in any one of a number of longitudinal positions along the flexible shaft.A surgical system includes an electro-mechanical driver, an elongated, flexible sheath, at least one drive shaft disposed within the flexible sheath, a surgical attachment coupled to the at least one drive shaft, the electro-mechanical driver configured to drive the surgical attachment, a shape-retaining sleeve, at least a portion of the flexible sleeve being disposed in the shape-retaining sleeve, the shape-retaining sleeve configured to maintain the at least portion of the flexible sheath in a predetermined shape, and an arrangement variably securing the shape-retaining member to the flexible sheath in any one of a number of longitudinal positions along the flexible sheath.
Owner:TYCO HEALTHCARE GRP LP
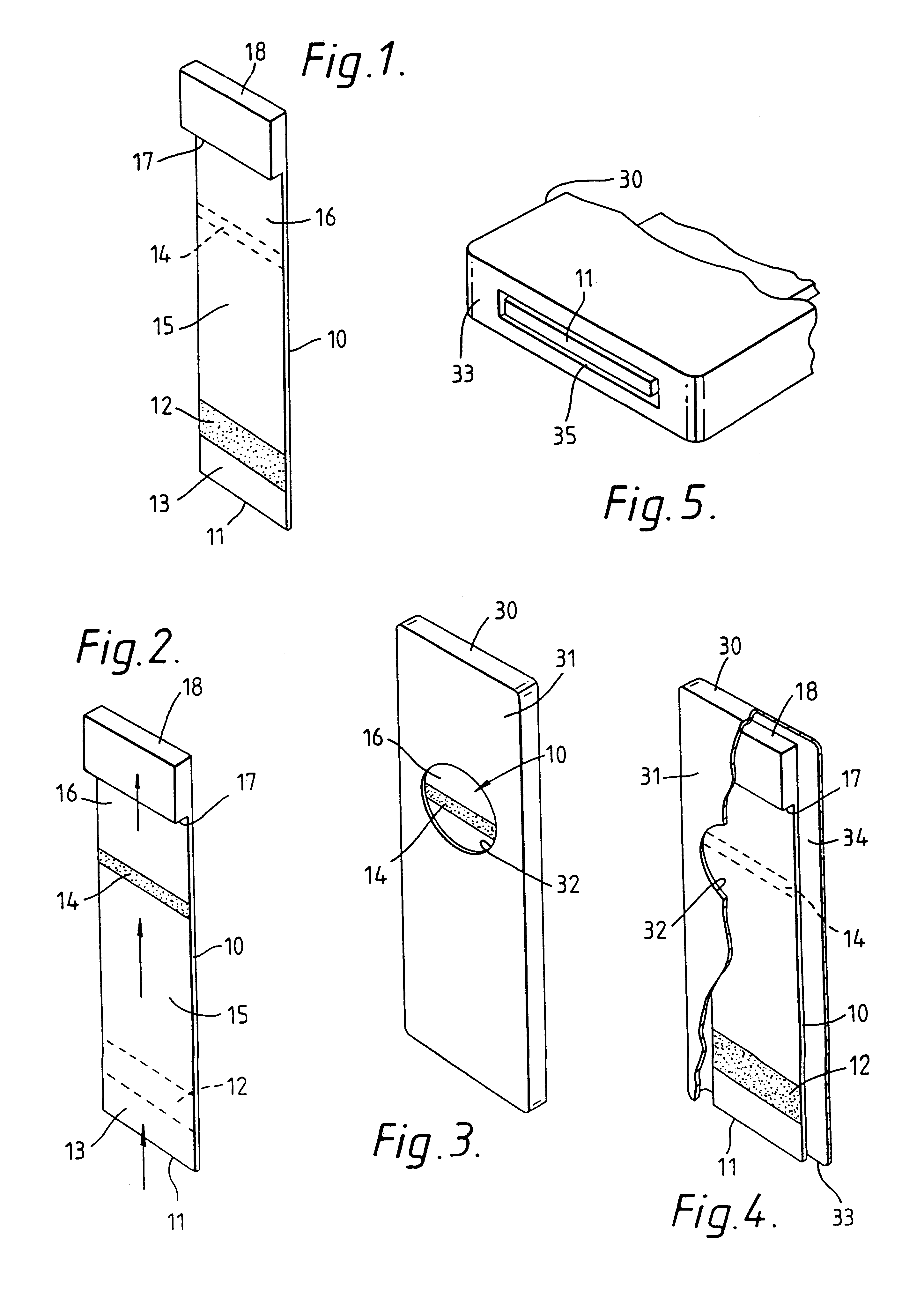
Wound dressing and method of use
ActiveUS20110282309A1Reduce decreaseExtended service lifeNon-adhesive dressingsPlastersWound.exudateMoisture
A system, method, and apparatus are disclosed for dressing a wound. The apparatus comprises a liquid and gas permeable transmission layer, an absorbent layer for absorbing wound exudate, the absorbent layer overlying the transmission layer, a gas impermeable cover layer overlying the absorbent layer and comprising a first orifice, wherein the cover layer is moisture vapor permeable.
Owner:SMITH & NEPHEW INC
Position sensing and detection of skin impedance
ActiveUS7756576B2Improve accuracyUltrasonic/sonic/infrasonic diagnosticsDiagnostic recording/measuringLiving bodyMoisture
Apparatus and methods are provided for determining in near realtime the position of a probe placed within a living body. Electric currents are driven between one or more electrodes on the probe and electrodes placed on the body surface. The impedance between the probe and each of the body surface electrodes is measured, and three-dimensional position coordinates of the probe are determined based on the impedance measurements. Dynamic compensation is provided for changing impedance of the body surface and its interface with the electrodes, resulting from such causes as electrode peel-off and changes in moisture and temperature. The compensation improves the accuracy of, inter alia, medical procedures, such as mapping the heart or performing ablation to treat cardiac arrhythmias.
Owner:BIOSENSE WEBSTER INC
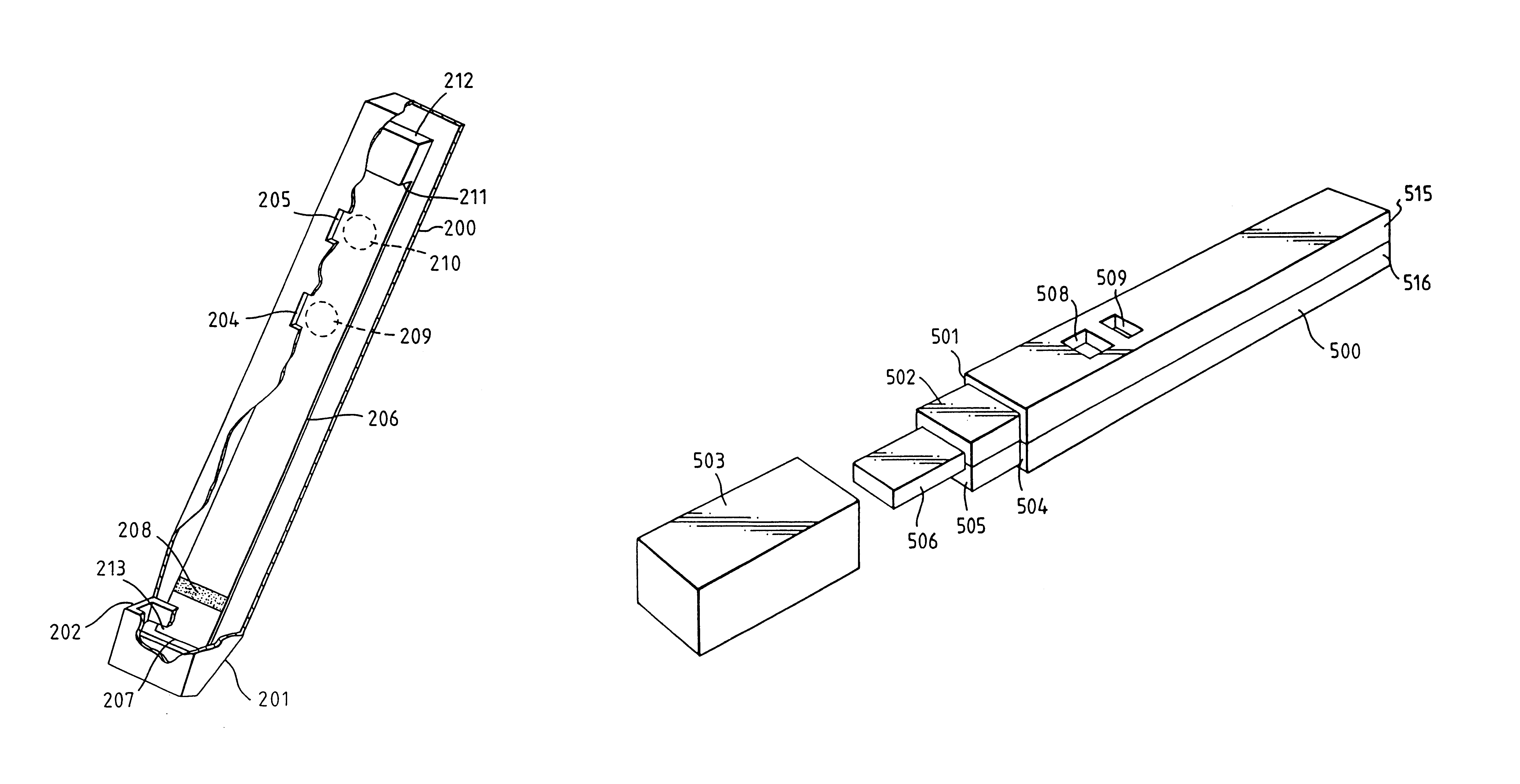
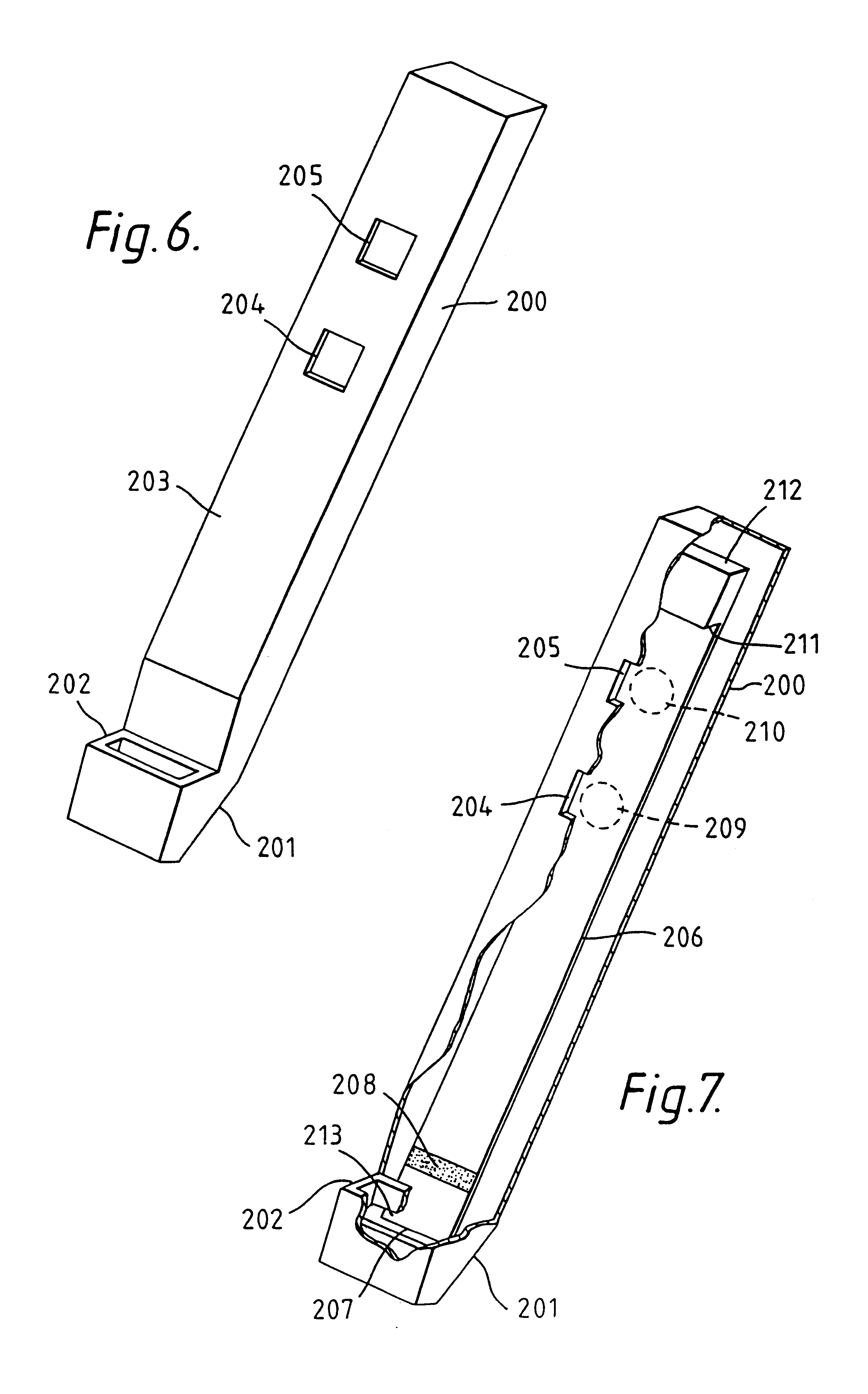
Capillary immunoassay and device therefor comprising mobilizable particulate labelled reagents
InactiveUS6228660B1Improve completenessAnalysis using chemical indicatorsComponent separationParticulatesAnalyte
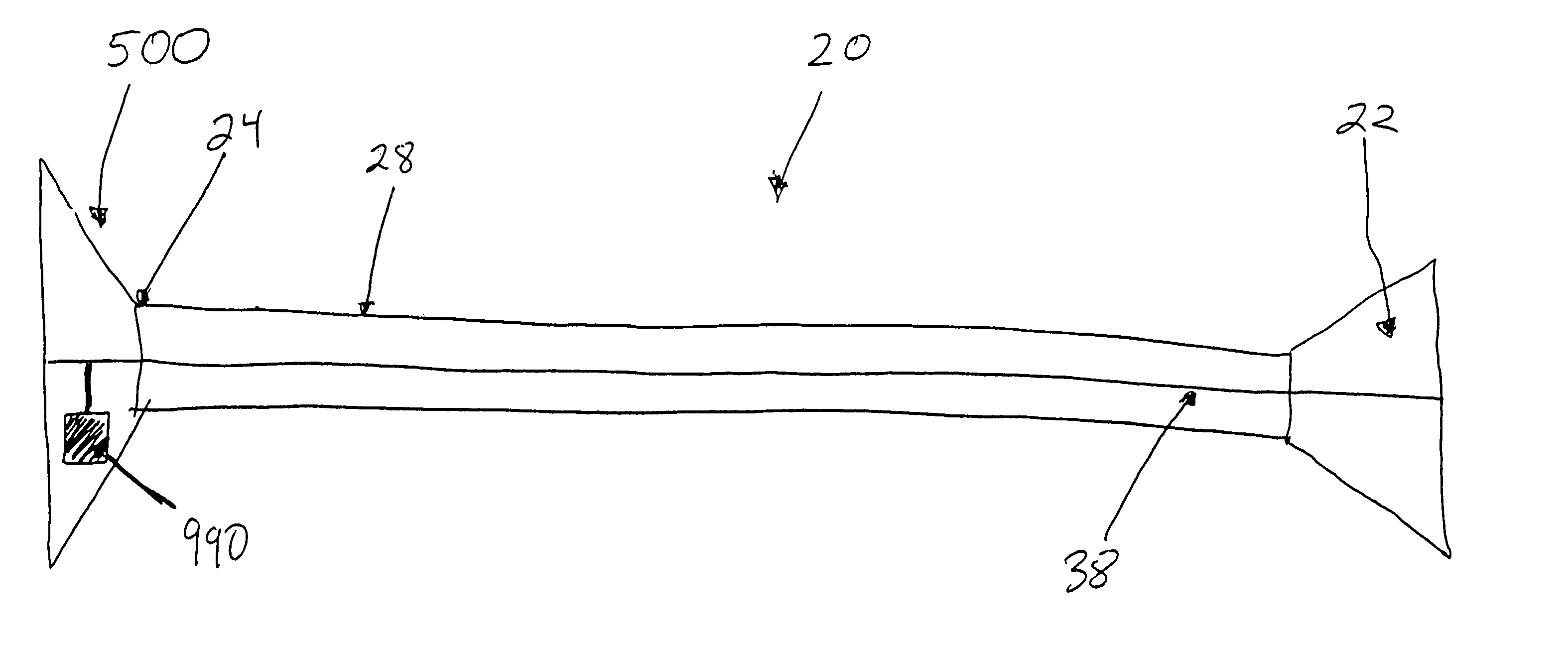
An analytical test device useful for example in pregnancy testing, comprises a hollow casing (500) constructed of moisture-impervious solid material, such as plastics materials, containing a dry porous carrier (510) which communicates indirectly with the exterior of the casing via a bibulous sample receiving member (506) which protrudes from the casing such that a liquid test sample can be applied to the receiving member and permeate therefrom to the porous carrier, the carrier containing in a first zone a labelled specific binding reagent is freely mobile within the porous carrier when in the moist state, and in a second zone spatially distinct from the first zone unlabelled specific binding reagent for the same analyte which unlabelled reagent is permanently immobilized on the carrier material and is therefore not mobile in the moist state, the two zones being arranged such that liquid sample applied to the porous carrier can permeate via the first zone into the second zone, and the device incorporating means, such as an aperture (508) in the casing, enabling the extent (if any) to which the labelled reagent becomes bound in the second zone to be observed. Preferably the device includes a removable cap for the protruding bibulous member.
Owner:CONOPCO INC D B A UNILEVER
Flexible high-temperature ultrabarrier
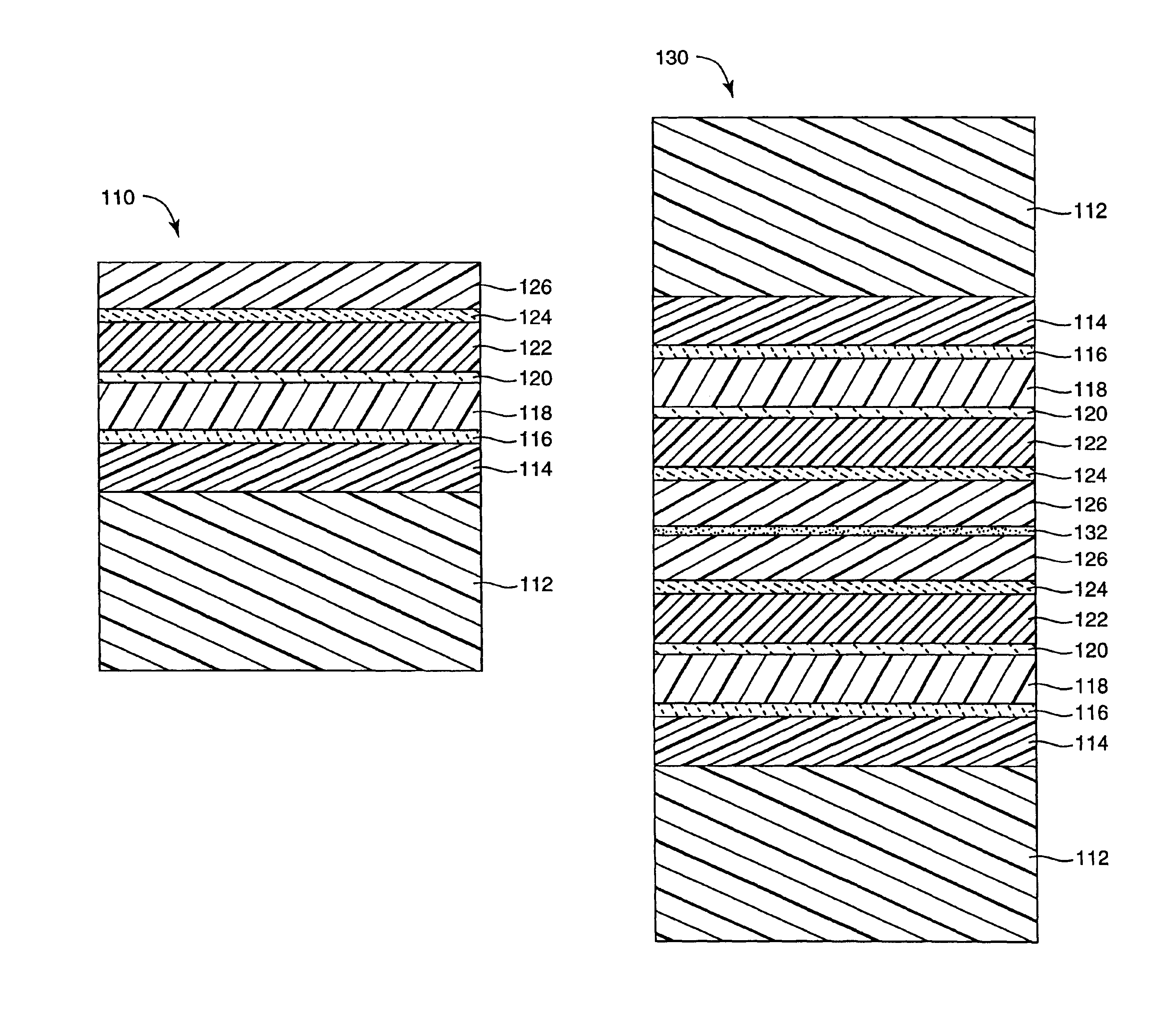
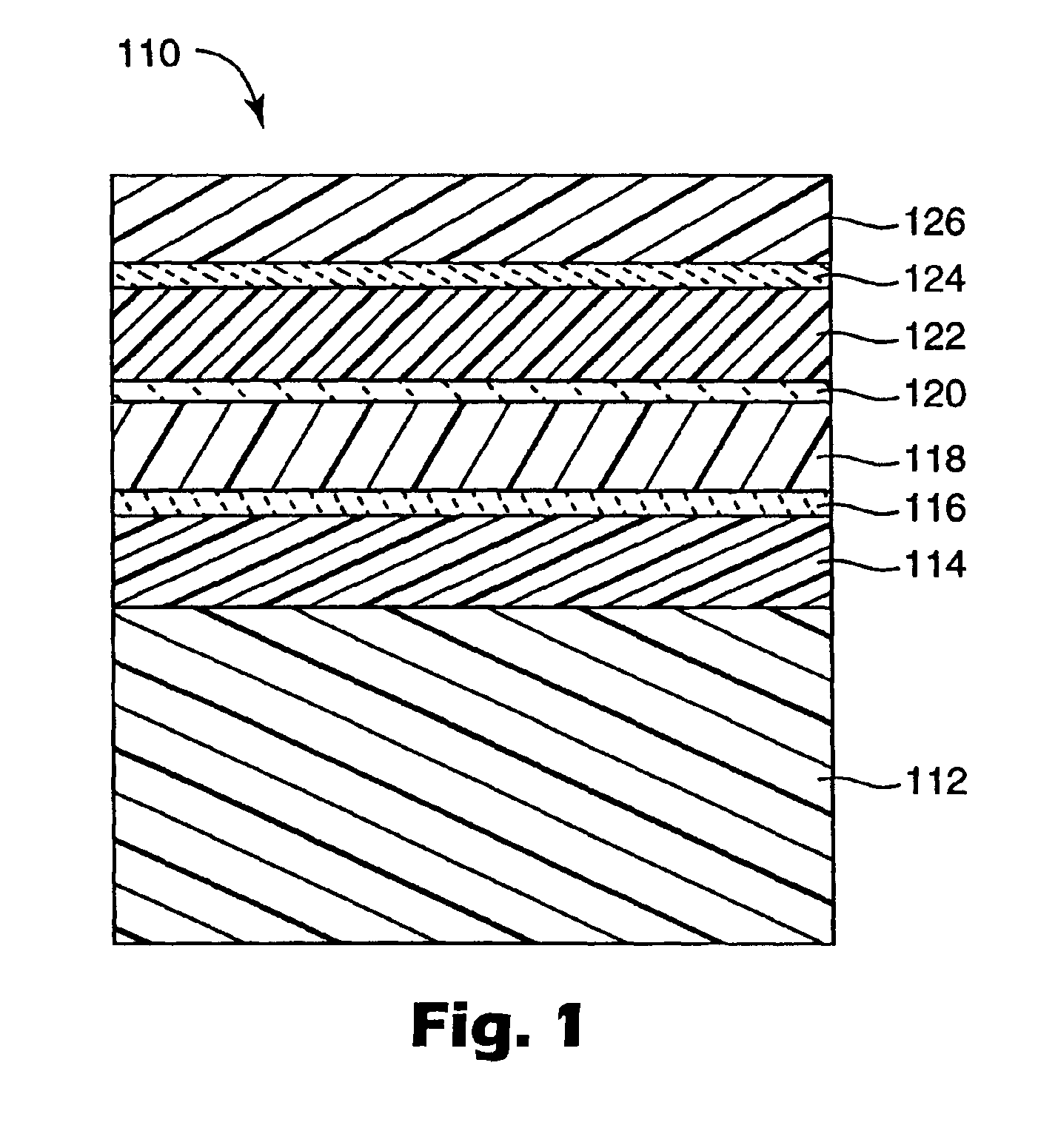
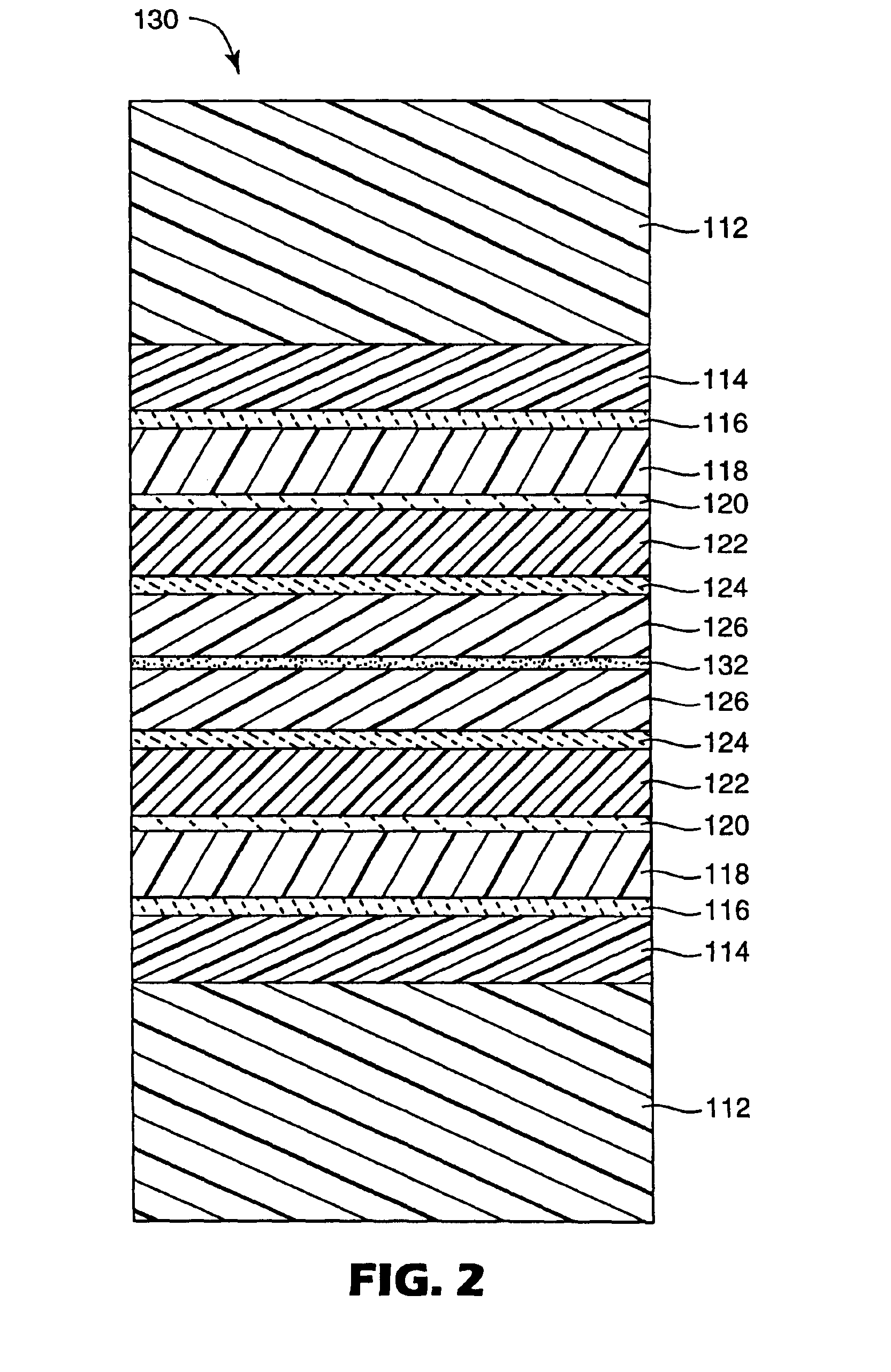
InactiveUS7018713B2Final product manufactureSynthetic resin layered productsPolyethylene terephthalateOrganic light emitting device
A flexible barrier assembly having a flexible visible light-transmissive substrate having a Tg greater than or equal to that of heat-stabilized polyethylene terephthalate (“HSPET”) overcoated with a first polymer layer having a Tg greater than or equal to that of HSPET and further overcoated with at least two visible light-transmissive inorganic barrier layers separated by at least one second polymer layer having a Tg greater than or equal to that of HSPET can be used to mount, cover, encapsulate or form moisture- and oxygen-sensitive articles such as organic light emitting devices and light valves.
Owner:3M INNOVATIVE PROPERTIES CO
Electro-optical device and electronic device
InactiveUS6689492B1Reduce heatReduce releaseDischarge tube luminescnet screensSemiconductor/solid-state device detailsDisplay deviceEngineering
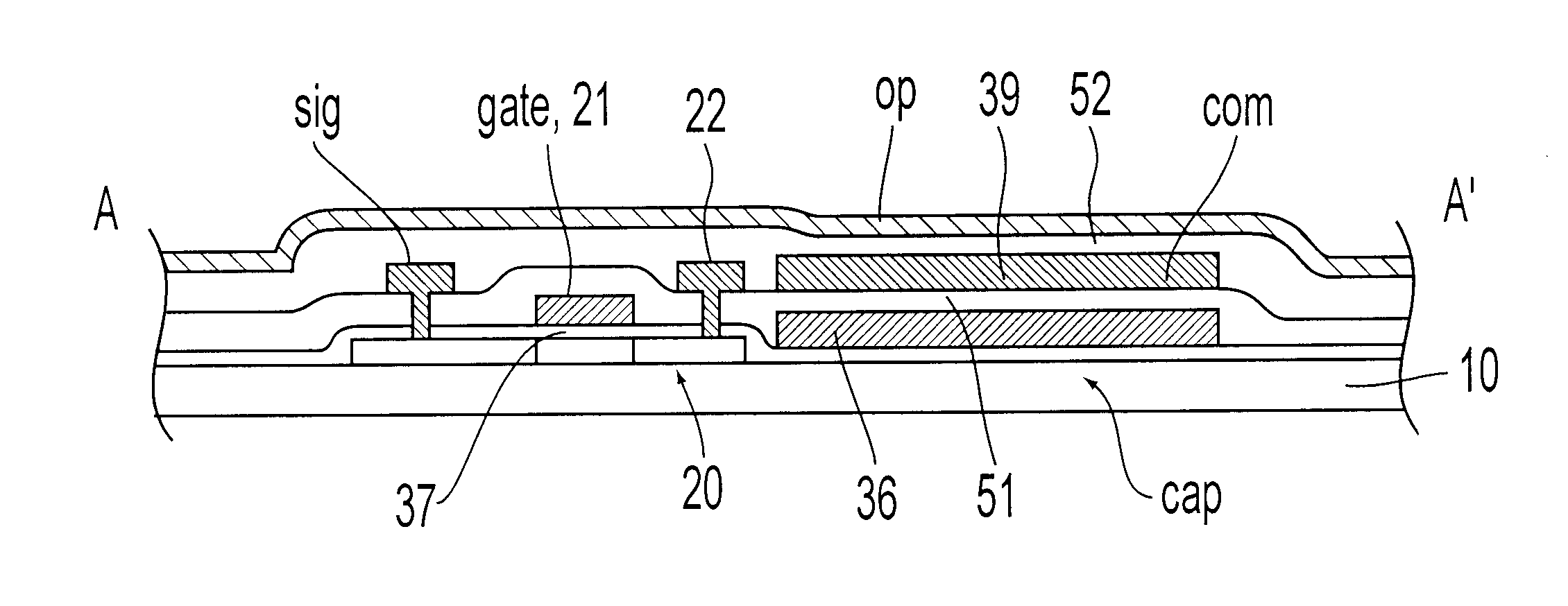
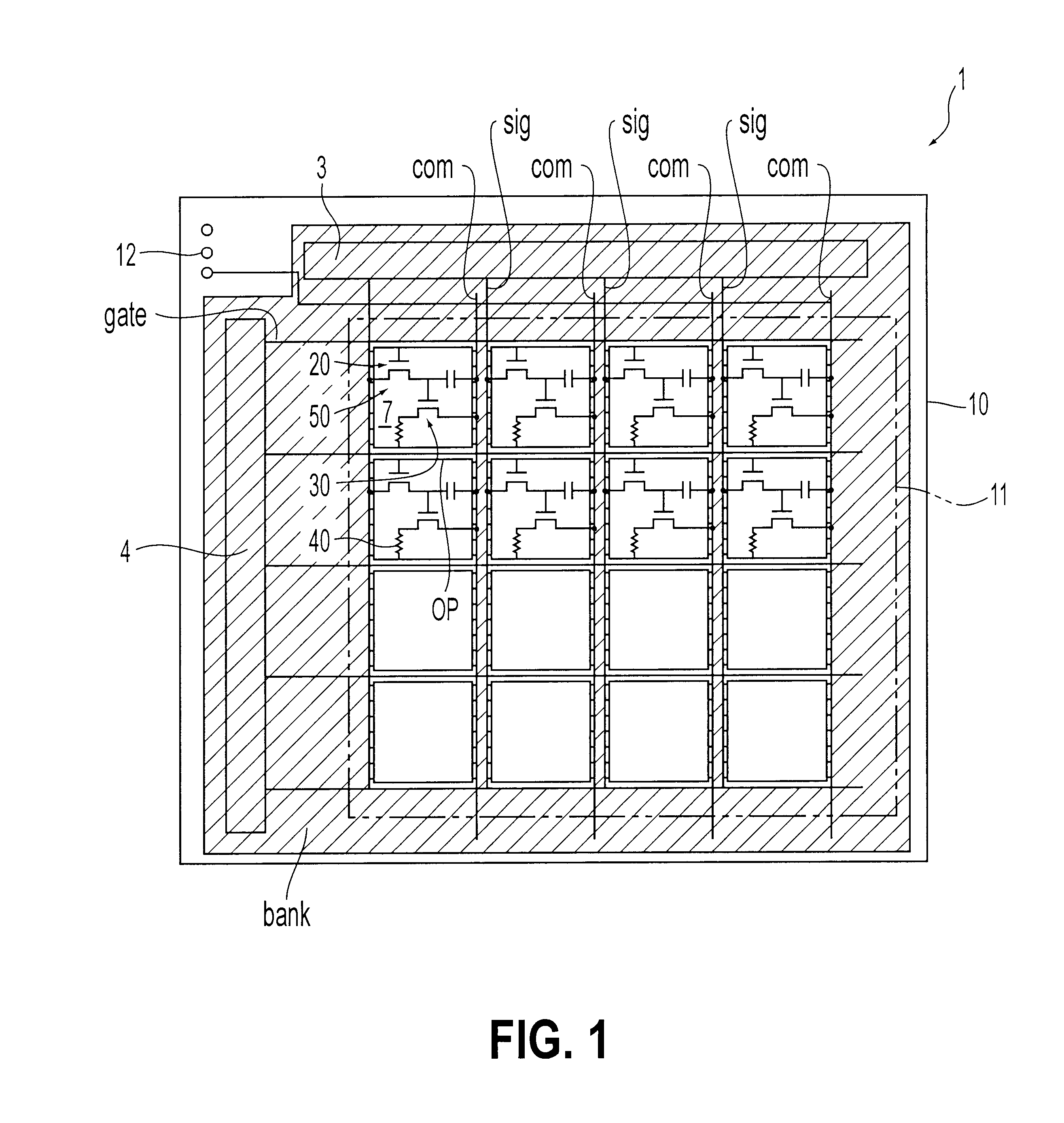
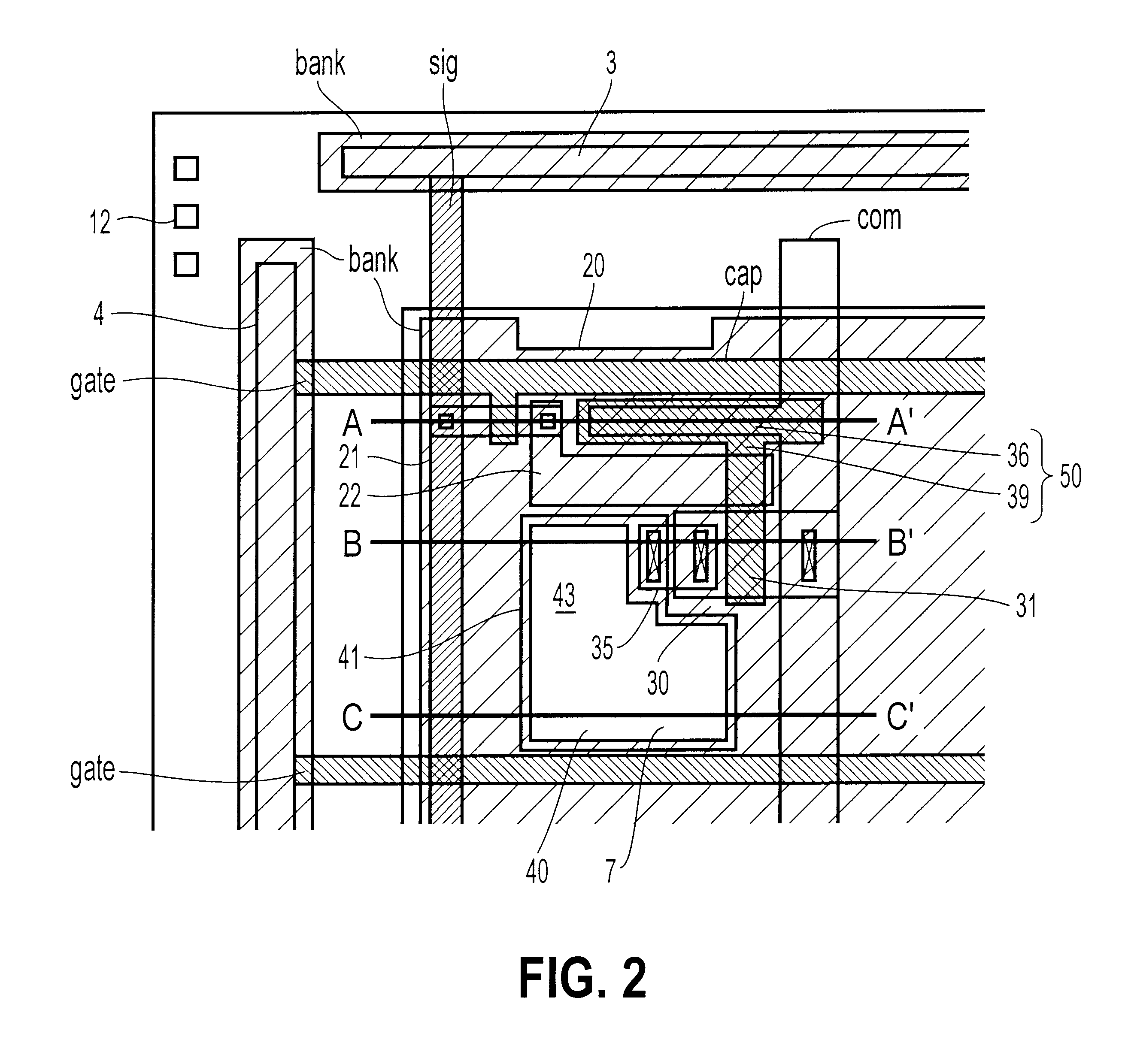
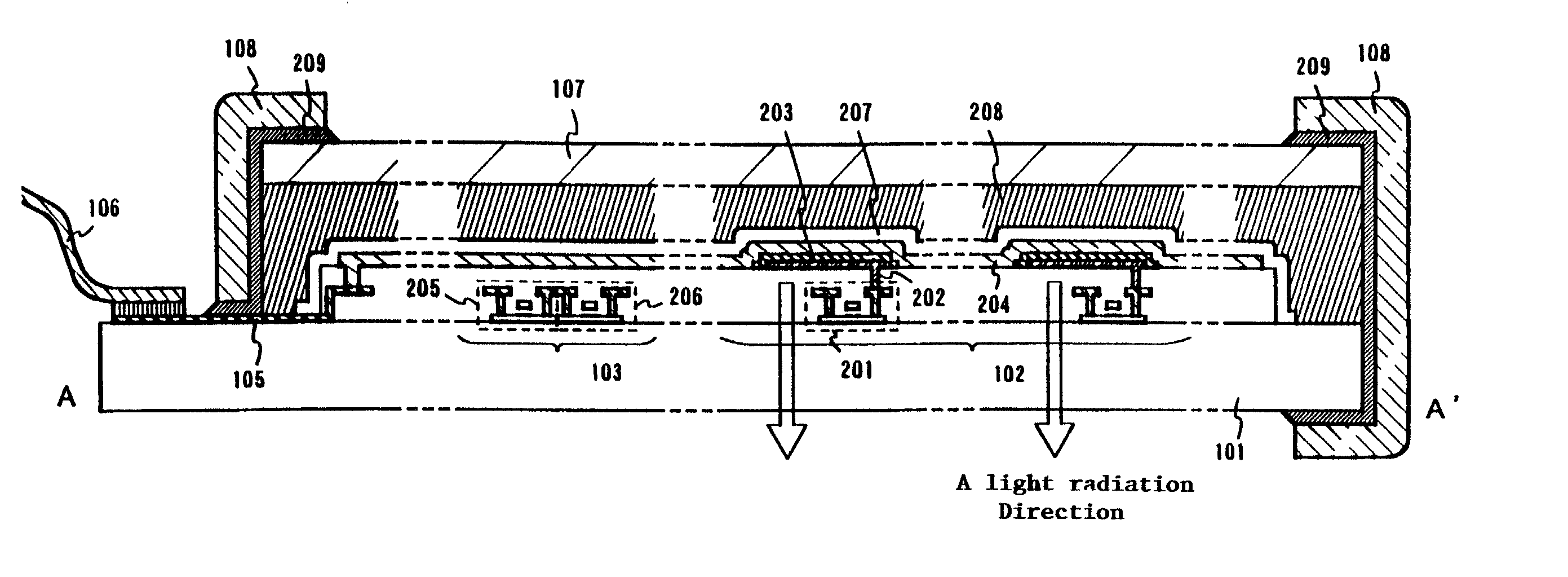
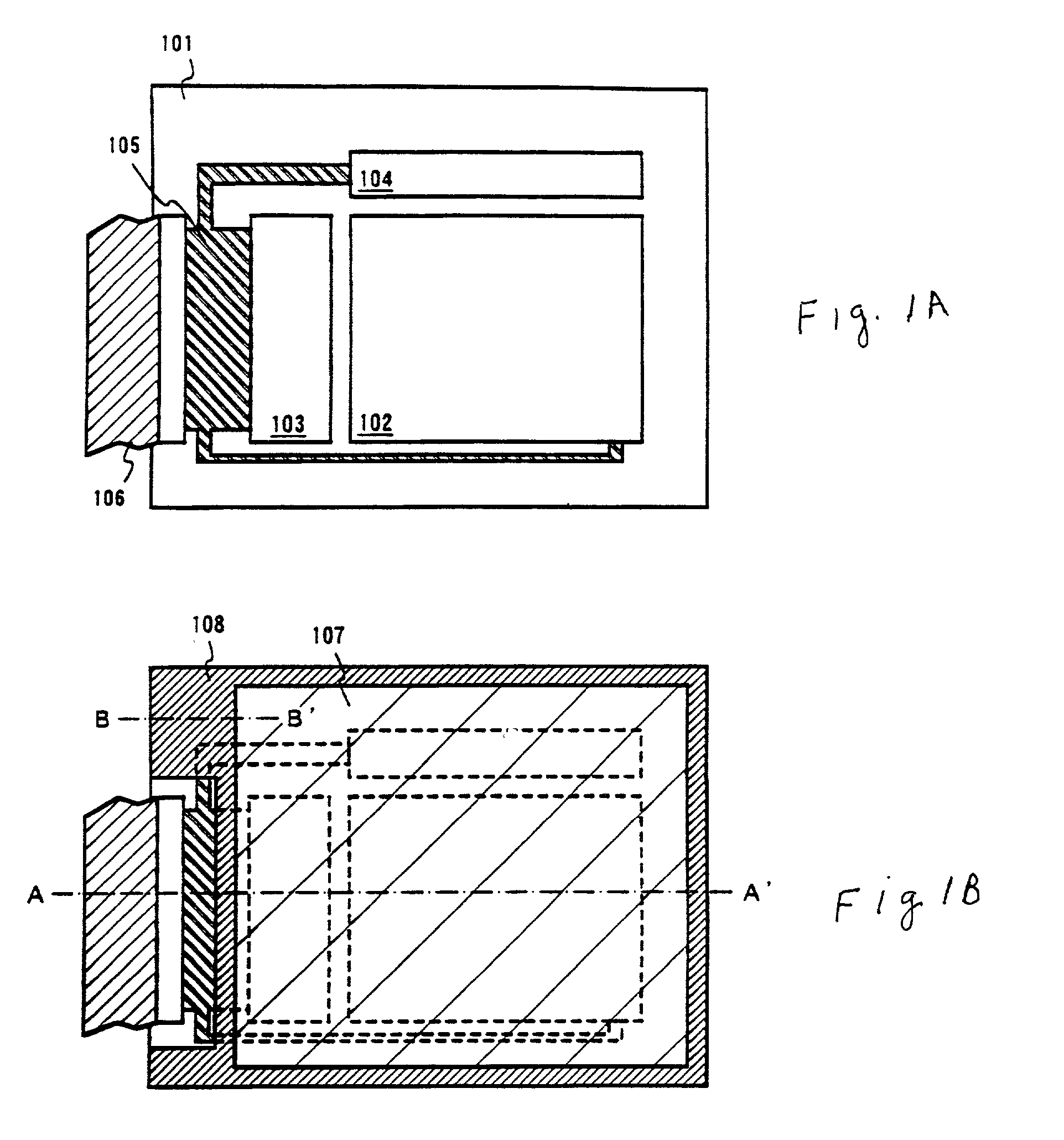
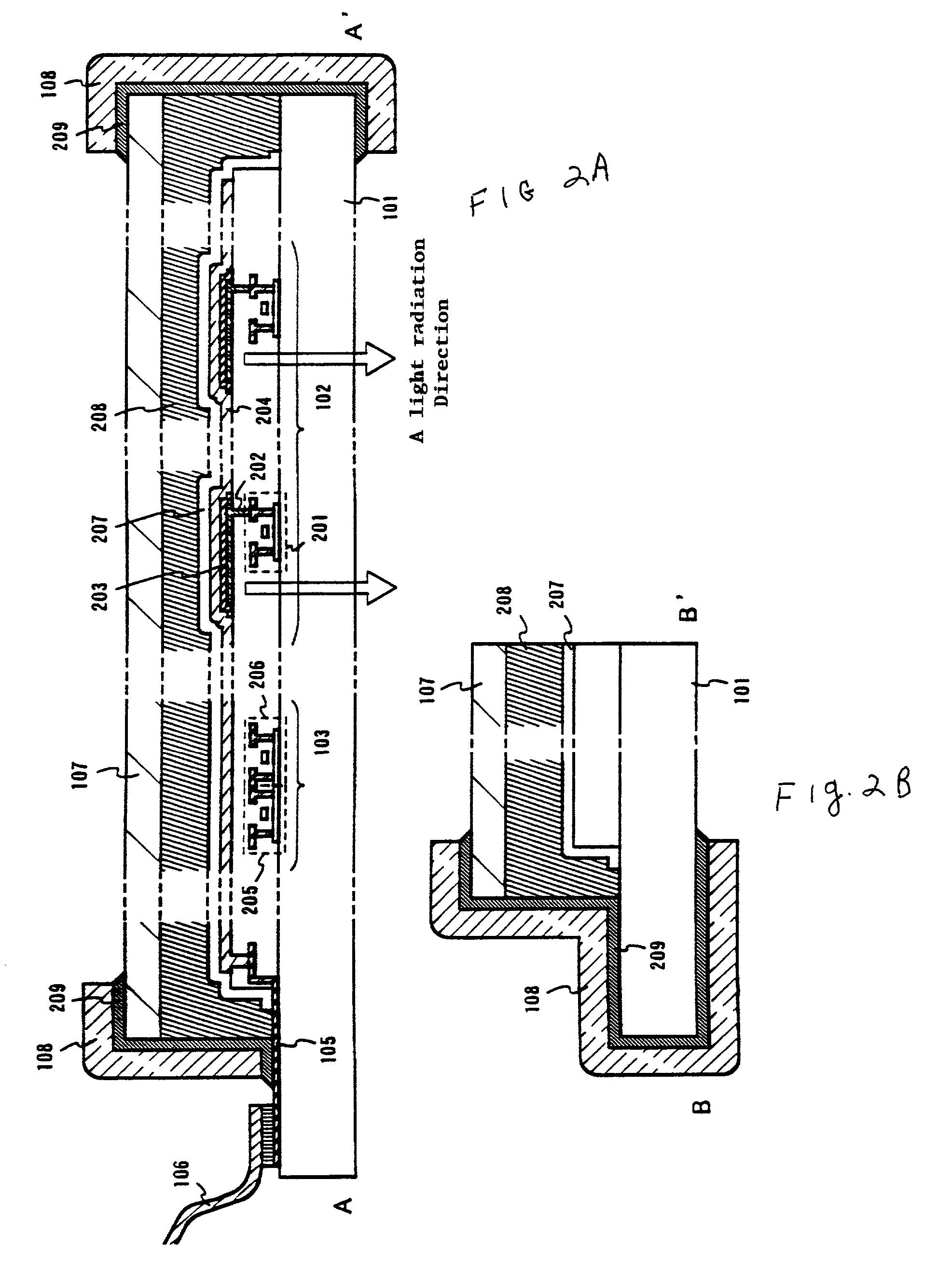
An object of the present invention is to provide an EL display device, which has a high operating performance and reliability. A third passivation film 45 is disposed under an EL element 203 which comprises a pixel electrode (anode) 46, and EL layer 47 and a cathode 48, to make a structure in which heat generated by the EL element 203 is radiated. Further, the third passivation film 45 prevents alkali metals within the EL element 203 from diffusing into the TFTs side, and prevents moisture and oxygen of the TFTs side from penetrating into the EL element 203. More preferably, heat radiating effect is given to a fourth passivation film 50 to make the EL element 203 to be enclosed by heat radiating layers.
Owner:SEMICON ENERGY LAB CO LTD
Three-Dimensional Adhesive Device Having a Microelectronic System Embedded Therein
InactiveUS20080275327A1Simple and inexpensive wayModerate viscosityWave amplification devicesLayered productsCapacitanceElectronic systems
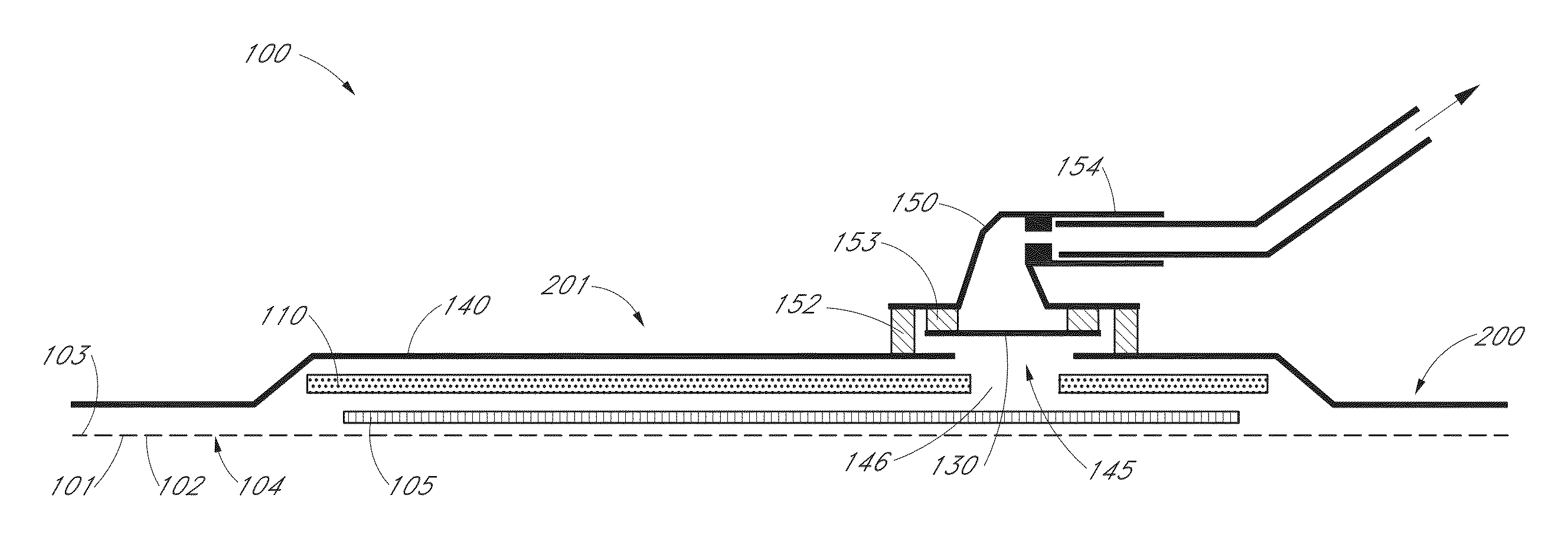
Accordingly, the present invention relates to a three-dimensional adhesive device to be attached to the body surface of a mammal comprising a microelectronic sensing system characterized by(a) a three-dimensional adhesive body made of a pressure sensitive adhesive having an upper surface and a bottom surface;(b) a microelectronic system embedded in the body of the pressure sensitive adhesive;(c) one or more cover layer(s) attached to the upper surface; and(d) optionally a release liner releasable attached to the bottom surface of the adhesive device.Suitably the microelectronic system is a microelectronic sensing system capable of sensing physical input such as pressure, vibration, sound, electrical activity (e.g. from muscle activity), tension, blood-flow, moisture, temperature, enzyme activity, bacteria, pH, blood sugar, conductivity, resistance, capacitance, inductance or other chemical, biochemical, biological, mechanical or electrical properties.
Owner:BRAEMAR MFG +2
Electroluminescence display device having a desiccant
InactiveUS6833668B1Increase the aperture ratioReliable sealing structureDischarge tube luminescnet screensElectroluminescent light sourcesDesiccantDisplay device
Powder desiccant is mixed into a seal which adheres a first substrate to a second substrate, thereby sealing a display region. Almost no moisture permeates through the outer surfaces of the substrates sandwiching the display region, while moisture permeating through the seal is adsorbed by the desiccant. An emissive layer is thereby prevented from deterioration due to moisture. By covering the display region with a resin sealing layer composed of resin having desiccant mixed therein, the display region can reliably be protected from moisture. By forming a groove in the substrate and placing a desiccant therein, moisture can be further reliably adsorbed.
Owner:SANYO ELECTRIC CO LTD
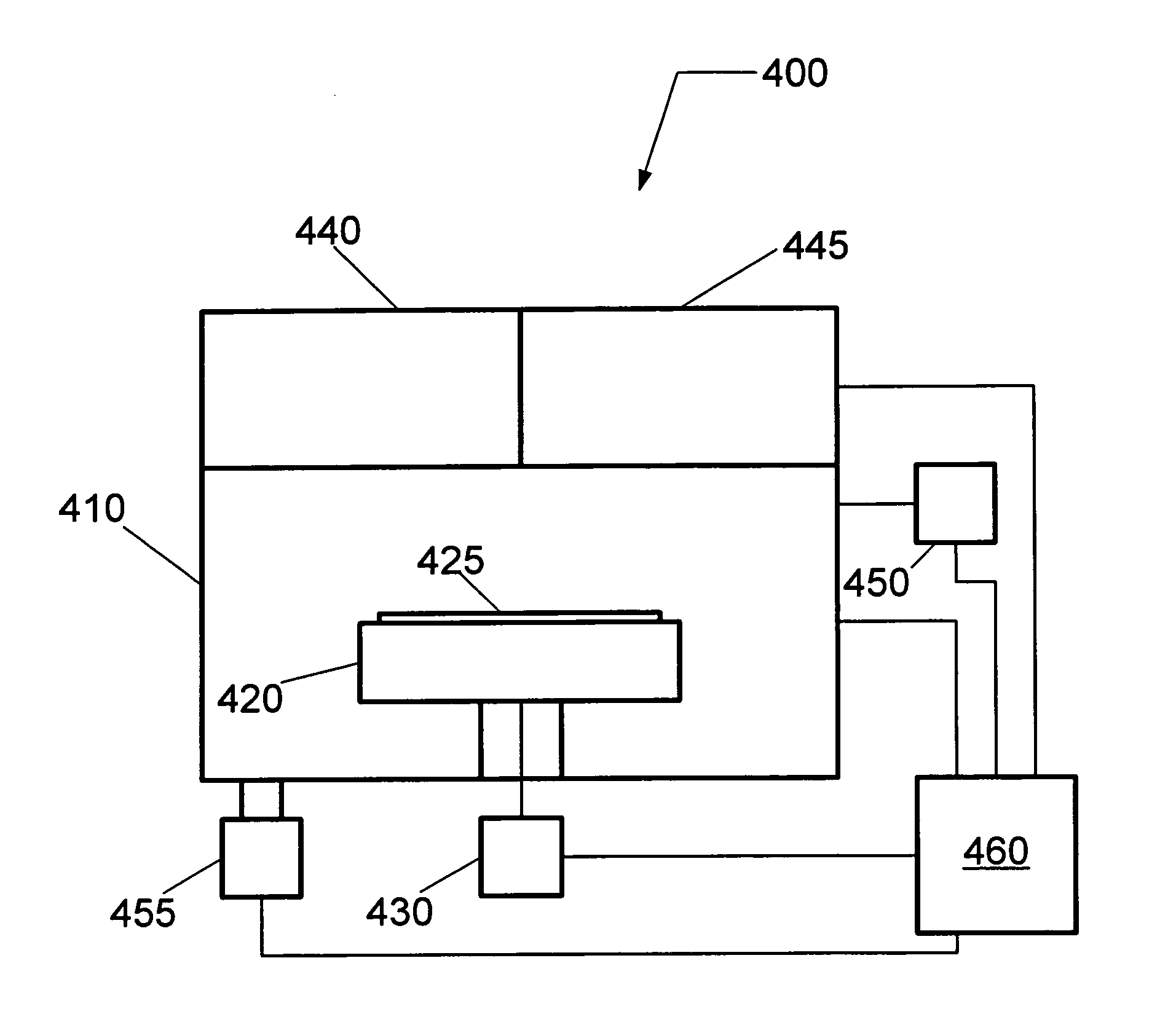
In vacuum optical wafer heater for cryogenic processing
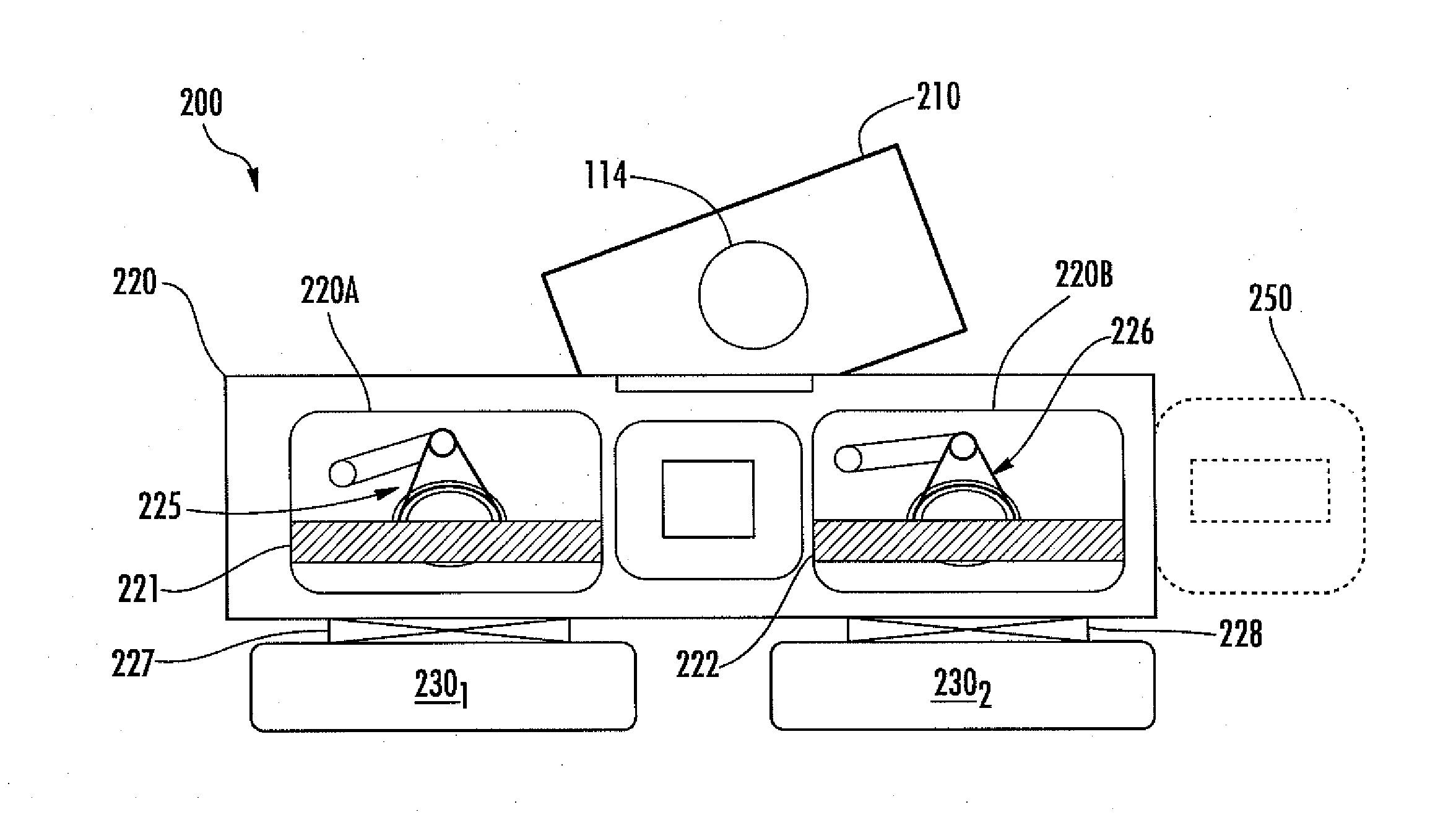
ActiveUS20110143461A1Prevent unwanted condensationConveyorsSemiconductor/solid-state device testing/measurementEngineeringMoisture
A vacuum assembly used for warming processed substrates above the dew point to prevent unwanted moisture on the processed substrate surfaces as well as reducing negative impact on manufacturing throughput. The vacuum assembly includes a processing chamber, a substrate handling robot, and a heater which may be an optical heater. The processing chamber is configured to cryogenically process one or more substrates. The transfer chamber is connected to the processing chamber and houses the substrate handling robot. The substrate handling robot is configured to displace one or more substrates from the processing chamber to the transfer chamber. The heater is connected to the transfer chamber above the substrate handling robot such that the heater emits energy incident on the substrate when the substrate handling robot displaces the substrate in the transfer chamber.
Owner:VARIAN SEMICON EQUIP ASSOC INC
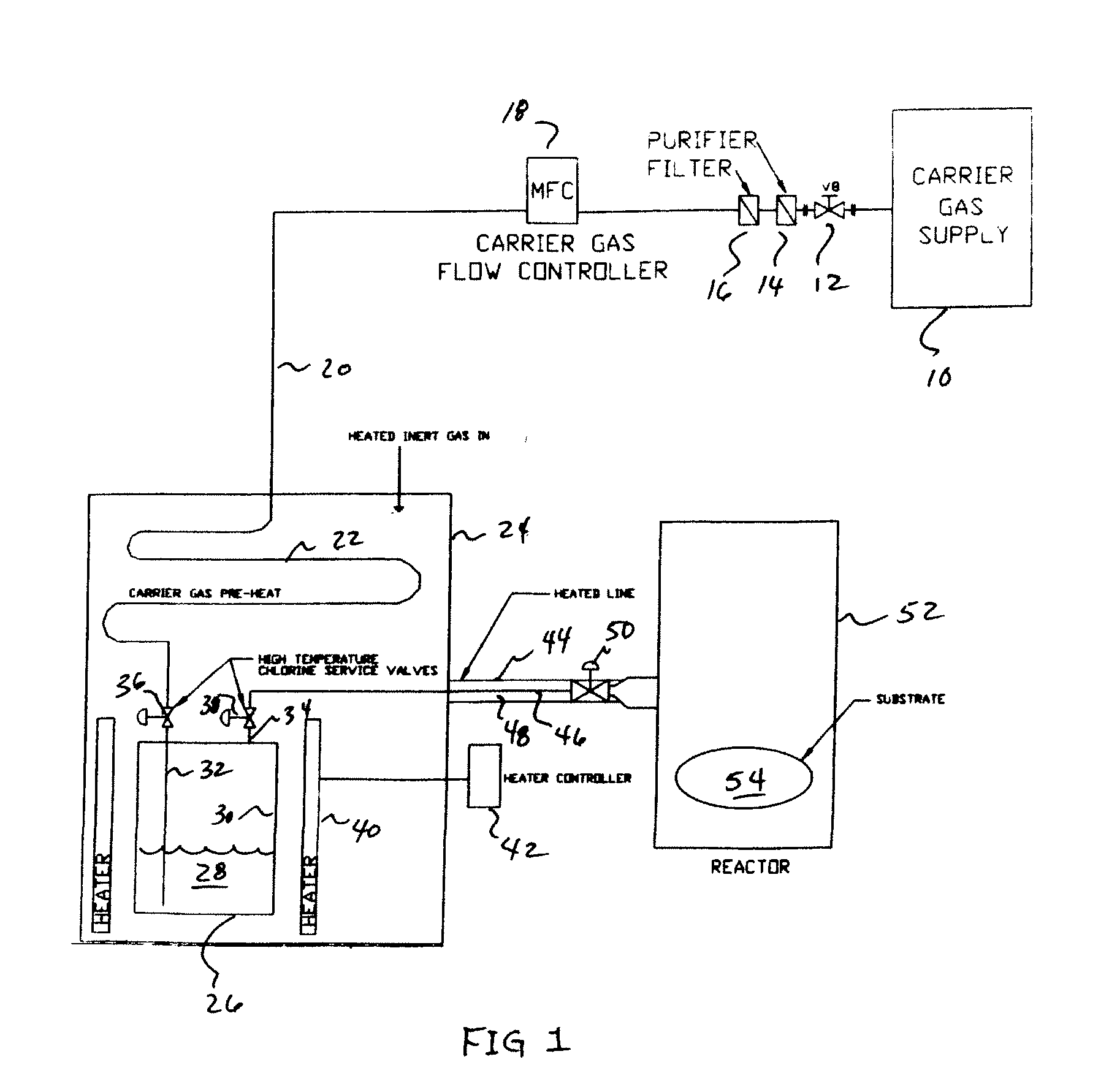
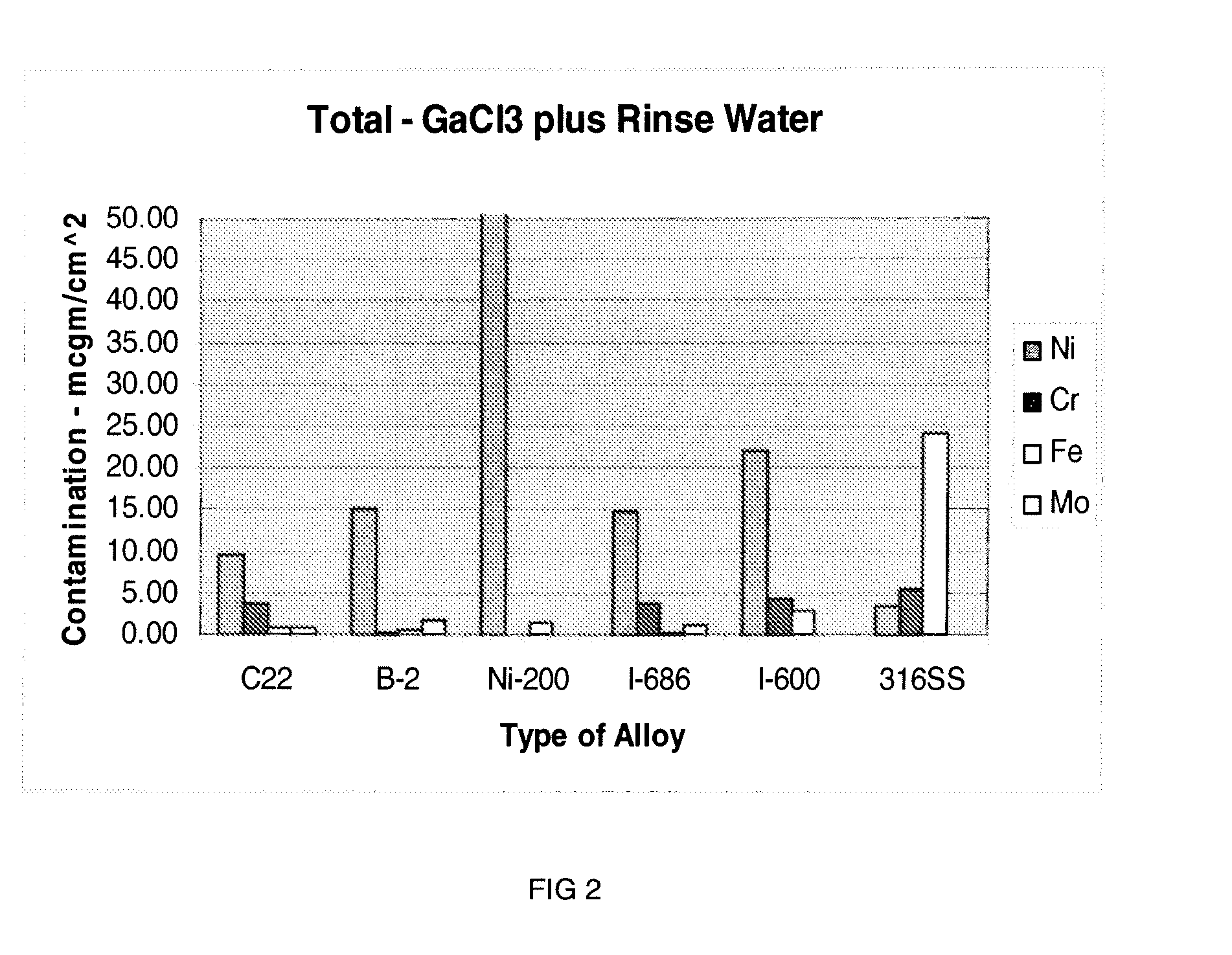
High Flow GaCl3 Delivery
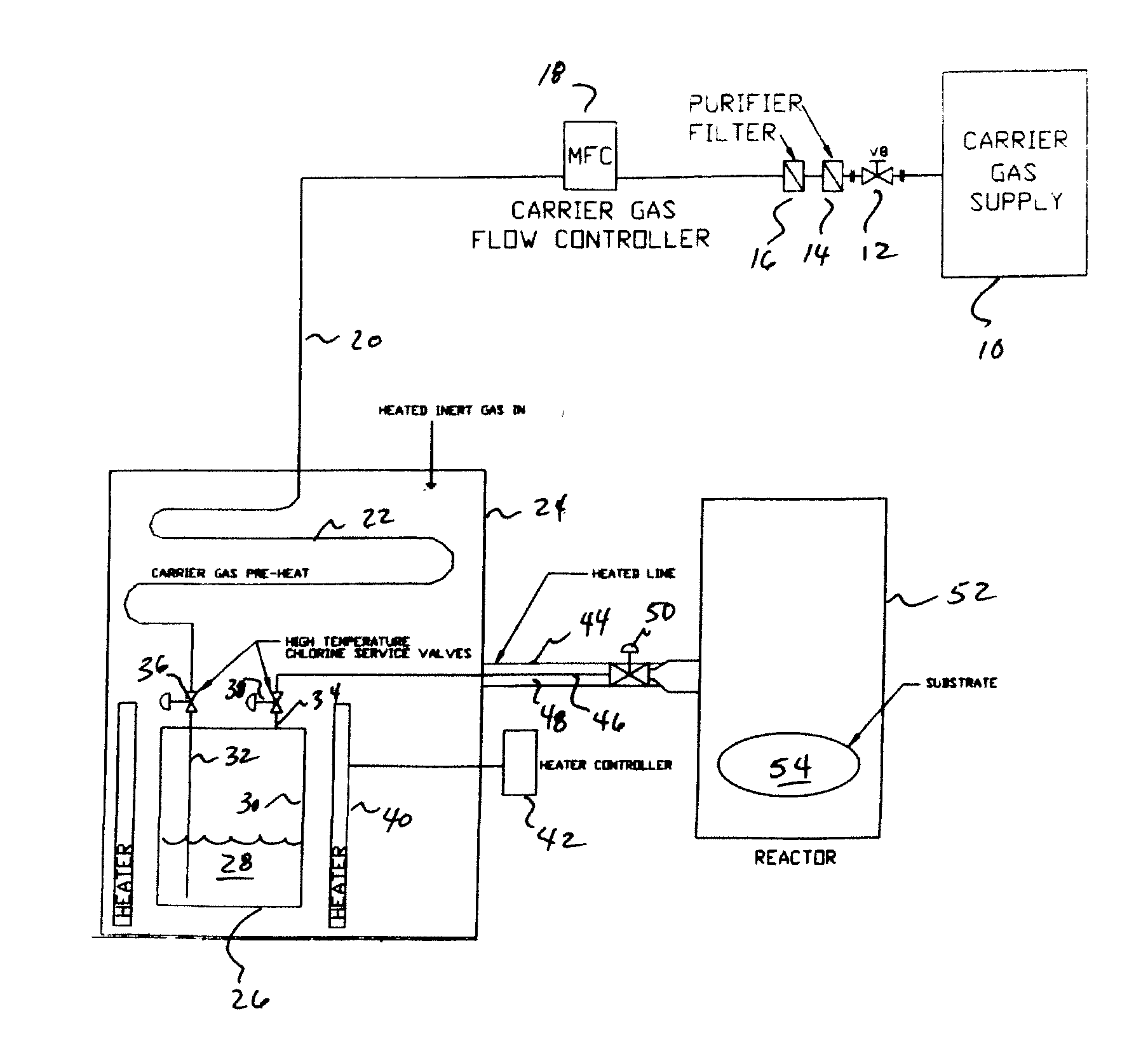
InactiveUS20080018004A1Avoid insufficient heatingCarburetting airPolycrystalline material growthGas phaseReaction zone
The present invention is an apparatus for deliverying high purity gallium trichloride in the vapor phase to a gallium nitride reactor, comprising; a source of carrier gas at an elevated pressure; a purifier to remove moisture from the carrier gas; a heater capable of heating the carrier gas to at least 80° C.; a container having a supply of gallium trichloride, a valve controlled inlet for the carrier gas having a dip tube with an outlet below the level of the gallium trichloride, a valve controlled outlet for removing the carrier gas and entrained gallium trichloride; a heater capable of heating sufficient to melt the gallium trichloride; a delivery line connected to the valve controlled outlet for carrying the entrained gallium trichloride to a reaction zone for gallium nitride. A process is also described for the apparatus.
Owner:VERSUM MATERIALS US LLC
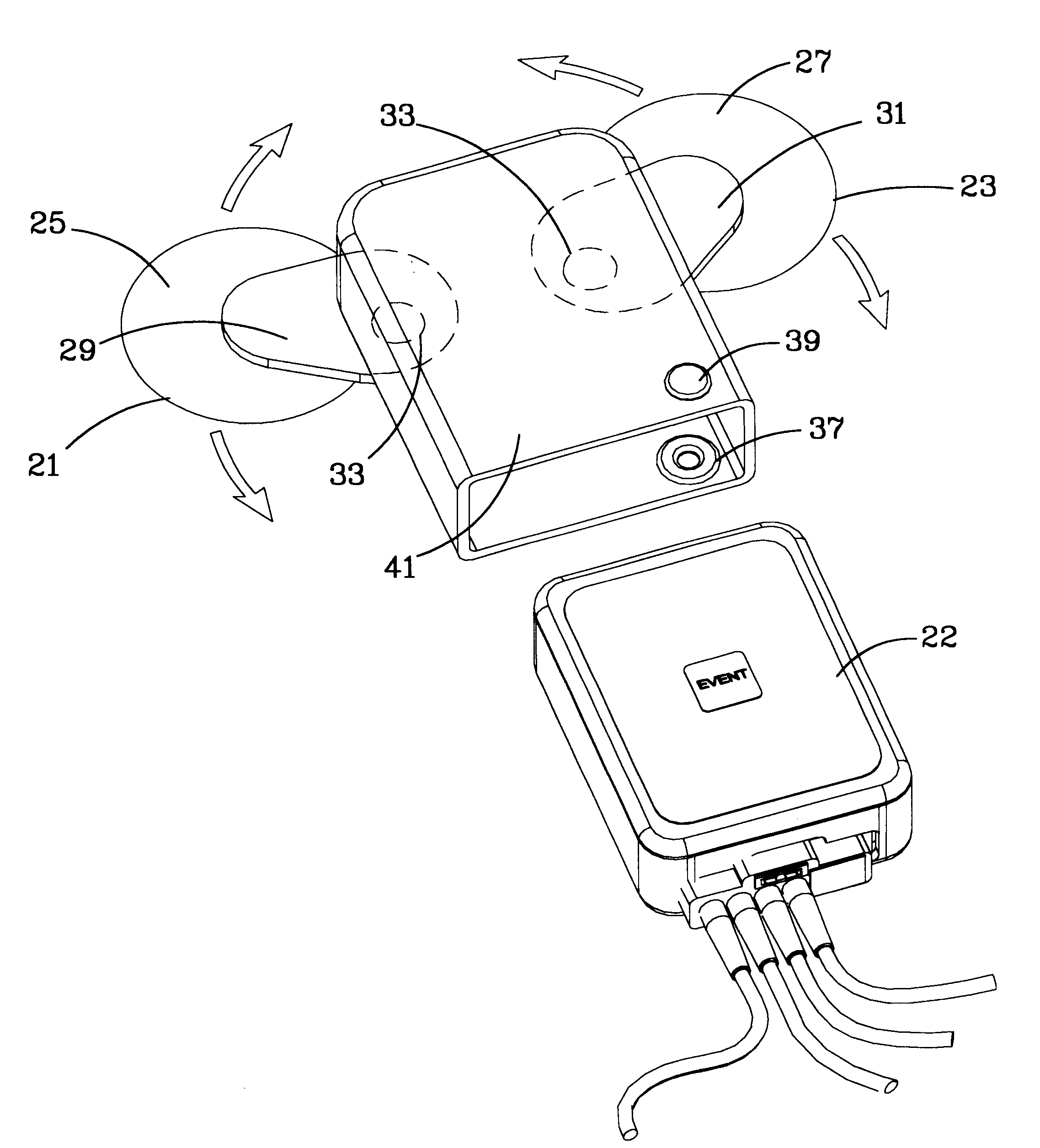
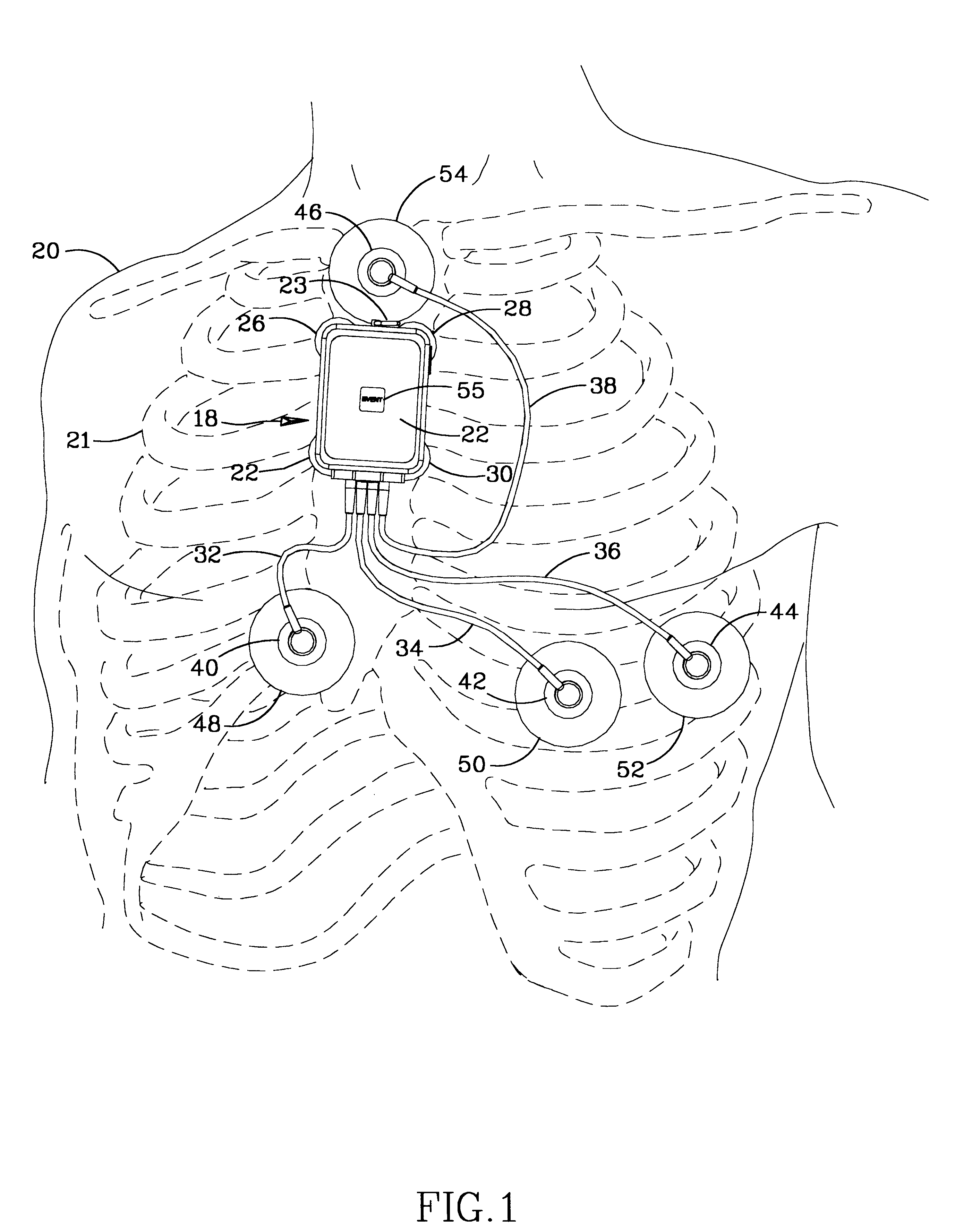
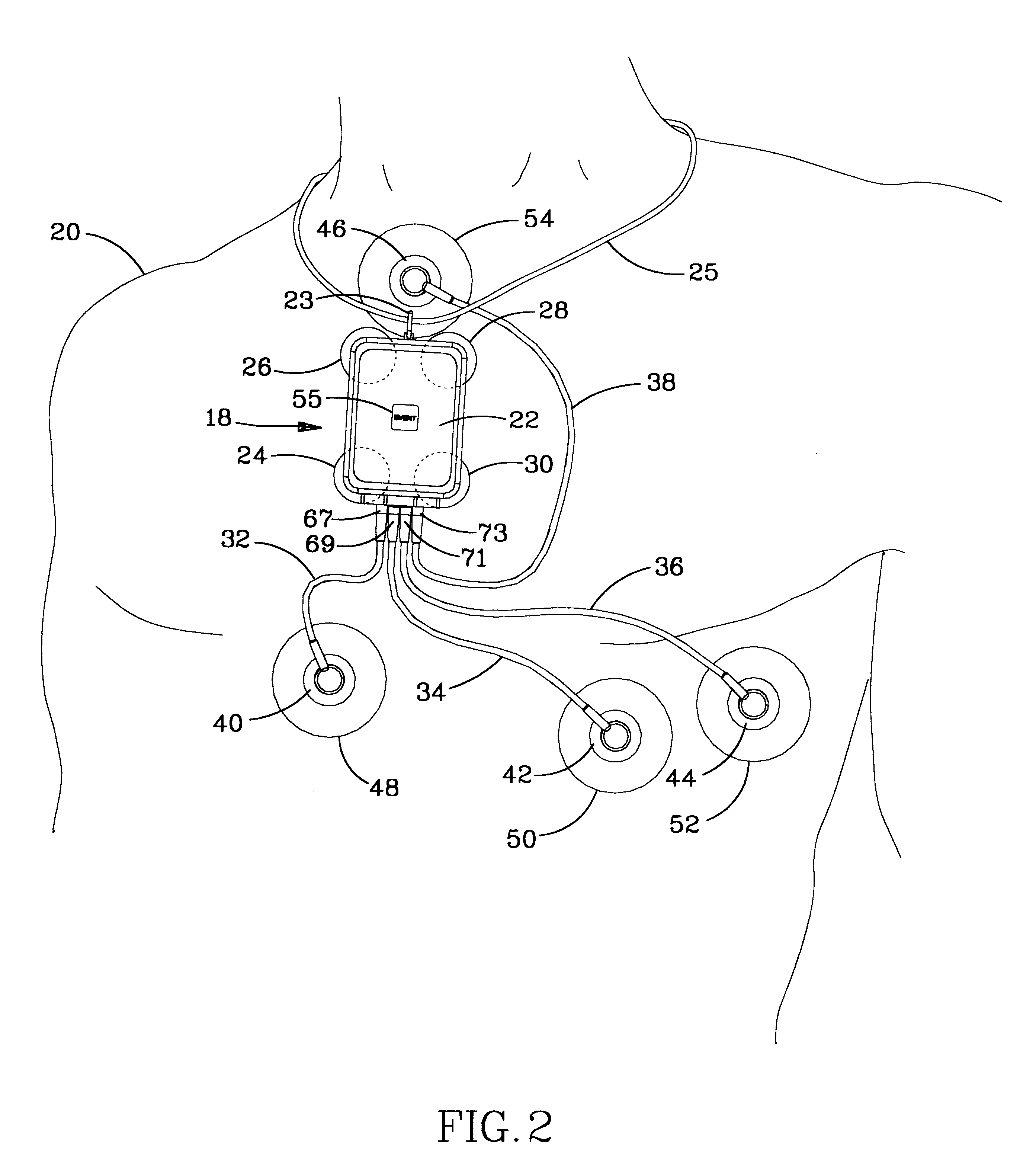
Ambulatory physio-kinetic monitor with envelope enclosure
InactiveUS6605046B1Complete and reliableShorten the lengthElectrocardiographySurgeryAmbulatoryAccelerometer
A water and moisture sealed, self contained, compact, long term, ambulatory physio-kinetic monitor is designed for mounting directly to the skin of an athlete or fitness performer, preferably immediately adjacent to the organ or system that is to be monitored, and is adhesively held there in place, covertly and comfortably, under clothing by disposable electrode, adhesive skin pads. At least three positive electrodes and a common negative electrode extend from the monitor and attach by similar disposable adhesive electrode pads to detect physiological, e.g. ECG data. Accelerometer means disposed within the monitor detects body movement and likewise stores that data on a third ECG data channel.
Owner:SPACELABS HEALTHCARE LLC
Multi-step system and method for curing a dielectric film
ActiveUS7622378B2Pretreated surfacesSemiconductor/solid-state device manufacturingUltravioletMoisture
A multi-step system and method for curing a dielectric film in which the system includes a drying system configured to reduce the amount of contaminants, such as moisture, in the dielectric film. The system further includes a curing system coupled to the drying system, and configured to treat the dielectric film with ultraviolet (UV) radiation and infrared (IR) radiation in order to cure the dielectric film.
Owner:TOKYO ELECTRON LTD
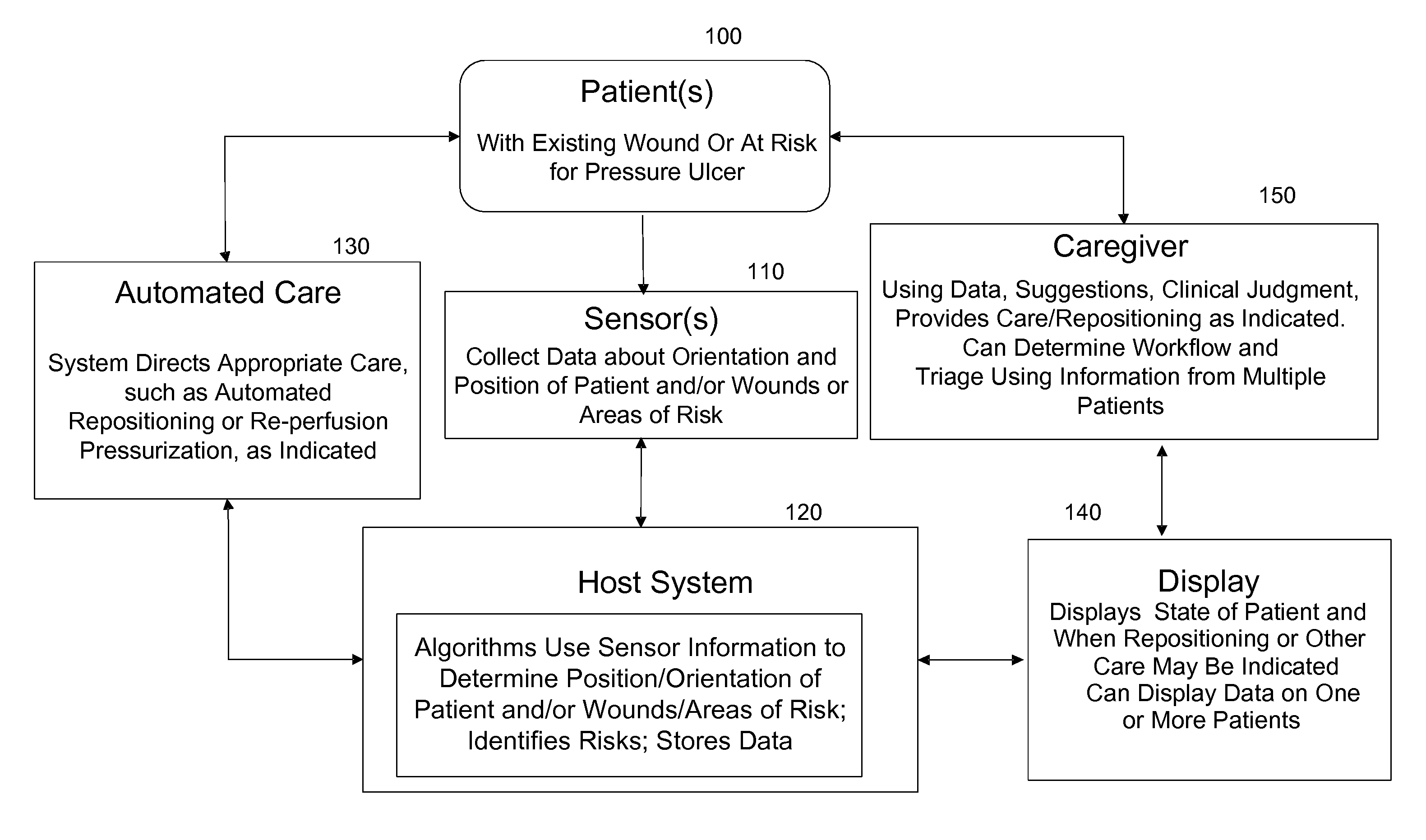
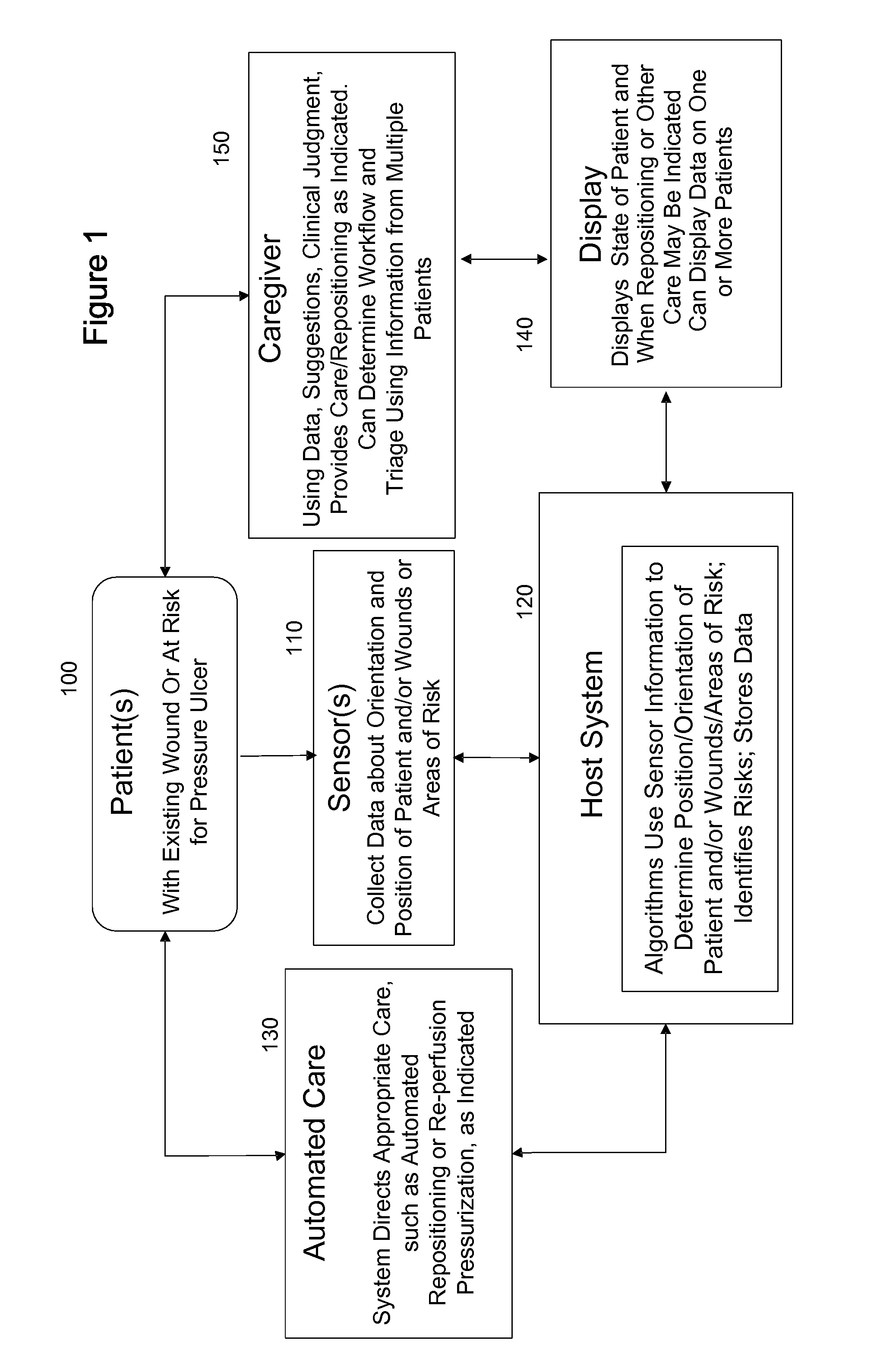
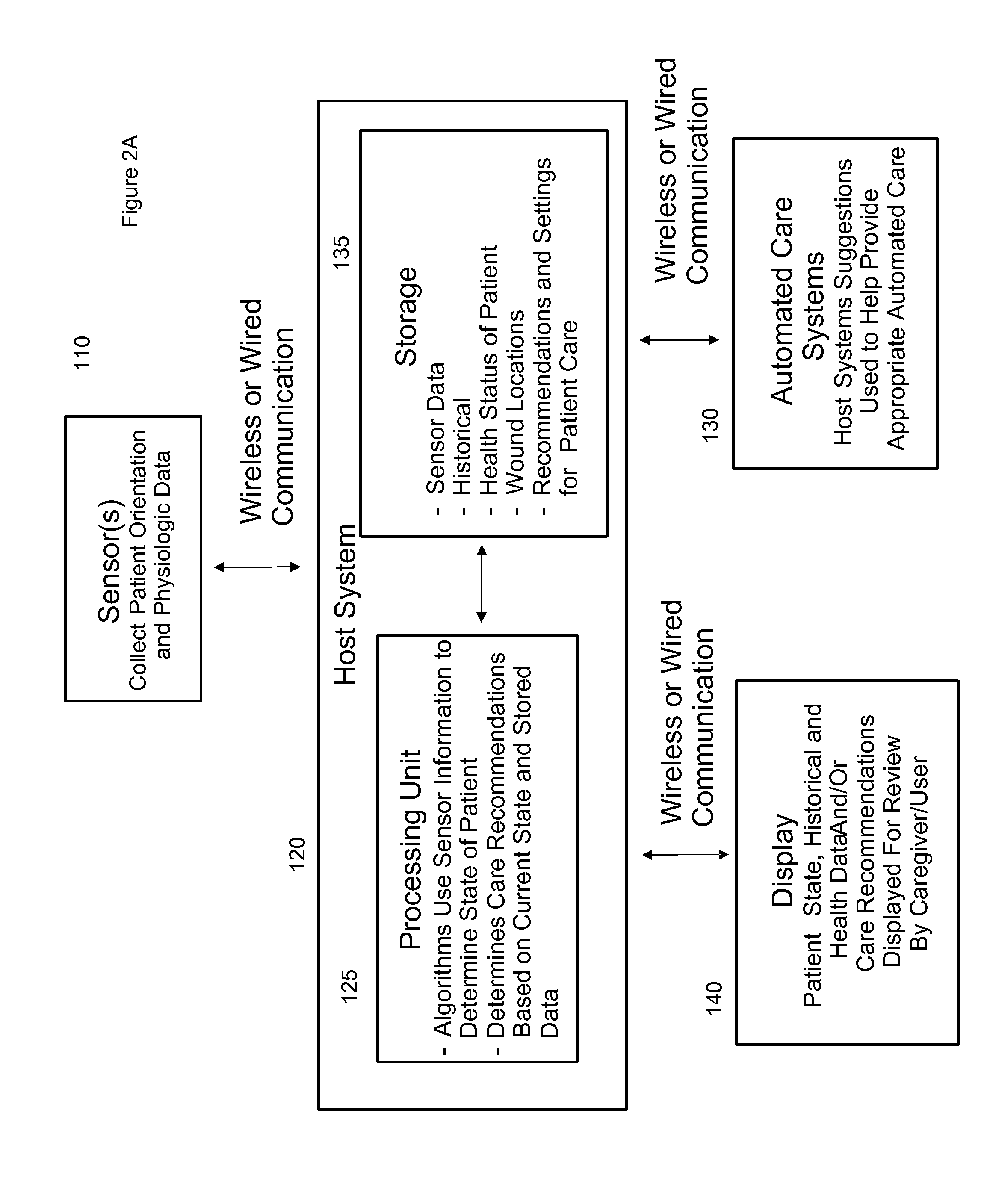
Systems, devices and methods for preventing, detecting and treating pressure-induced ischemia, pressure ulcers, and other conditions
ActiveUS20110263950A1Minimize and eliminate physical contactPromote blood circulationMechanical/radiation/invasive therapiesOperating chairsAccelerometerPatient characteristics
A system for monitoring medical conditions including pressure ulcers, pressure-induced ischemia and related medical conditions comprises at least one sensor adapted to detect one or more patient characteristic including at least position, orientation, temperature, acceleration, moisture, resistance, stress, heart rate, respiration rate, and blood oxygenation, a host for processing the data received from the sensors together with historical patient data to develop an assessment of patient condition and suggested course of treatment. In some embodiments, the system can further include a support surface having one or more sensors incorporated therein either in addition to sensors affixed to the patient or as an alternative thereof. The support surface is, in some embodiments, capable of responding to commands from the host for assisting in implementing a course of action for patient treatment. The sensor can include bi-axial or tri-axial accelerometers, as well as resistive, inductive, capactive, magnetic and other sensing devices, depending on whether the sensor is located on the patient or the support surface, and for what purpose.
Owner:LEAF HEALTHCARE
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Active matrix display
InactiveUS6359606B1Improve display qualityDischarge tube luminescnet screensElectroluminescent light sourcesTectorial membraneActive matrix
In an active matrix display device, each pixel is provided with a pixel electrode, an organic semiconductor film deposited on the upper layer side of the pixel electrode, and a thin film luminescent element provided with an opposing electrode formed on the upper layer side of the organic semiconductor film. A protective film covering almost the entire surface of a substrate is formed on the upper layer of the opposing electrode. The protective film prevents the entry of moisture or oxygen to inhibit the deterioration of the thin film luminescent element.
Owner:INTELLECTUAL KEYSTONE TECH LLC
Flooring products and methods of making the same
InactiveUS20060032175A1Improve moisture resistanceStrutsSynthetic resin layered productsThick plateNatural fiber
A plank is described wherein the plank comprises a core, and optionally, a print layer, and optionally an overlay. The core includes from about 30 wt % to about 95 wt % at least one polymeric material, by weight of the core, and from about 5 wt % to about 70 wt % of least one natural fiber or flour, by weight of the core, wherein the core includes a top surface and a bottom surface, and opposing sides, wherein said plank is substantially moisture resistant, having a swelling property of from about 0.5% to about 5% by NALFA Thickness Test Section 3.2 LF 01-2003 standard, and wherein said plank includes a bow of from about 0.5% to about 4%. In addition, a method of making the plank is further described.
Owner:MANNINGTON MILLS
Stir-frying Apparatus with Overhead Heating Device
InactiveUS20050223906A1Free handsEnvironmentally friendlyRoasters/grillsBaking plantsFresh airKnife blades
A stir-frying apparatus (100) with an overhead-heating device (128). Apparatus (100) comprises a container (102) for holding foods, a lid (120) covering on top of container (102), a bottom heating device (118) installed underneath container (102) and an overhead heating device installed on lid (120), a stirring blade (136) rotatably and removably installed inside container (102) for stirring foods, a power-drive assembly (140) operationally coupled with blade (136) for driving blade (136) through repeating stirring cycles, a coupling device (134) for coupling assembly (140) and blade (136), a venting device (200) for filtering and deodorizing cooking fumes, a blowing device (202) for forcing fresh air into apparatus (100) for the removal of moisture from therein-side, and an ingredient adding conduit (250) for use of adding ingredients during a frying process.
Owner:XU ZHAOXIA +1
Tissue ablation using pulse modulated radio frequency energy
ActiveUS8152801B2Quantity maximizationIncrease and decrease in moisture concentrationSurgical instruments for heatingRadio frequencyTissue ablation
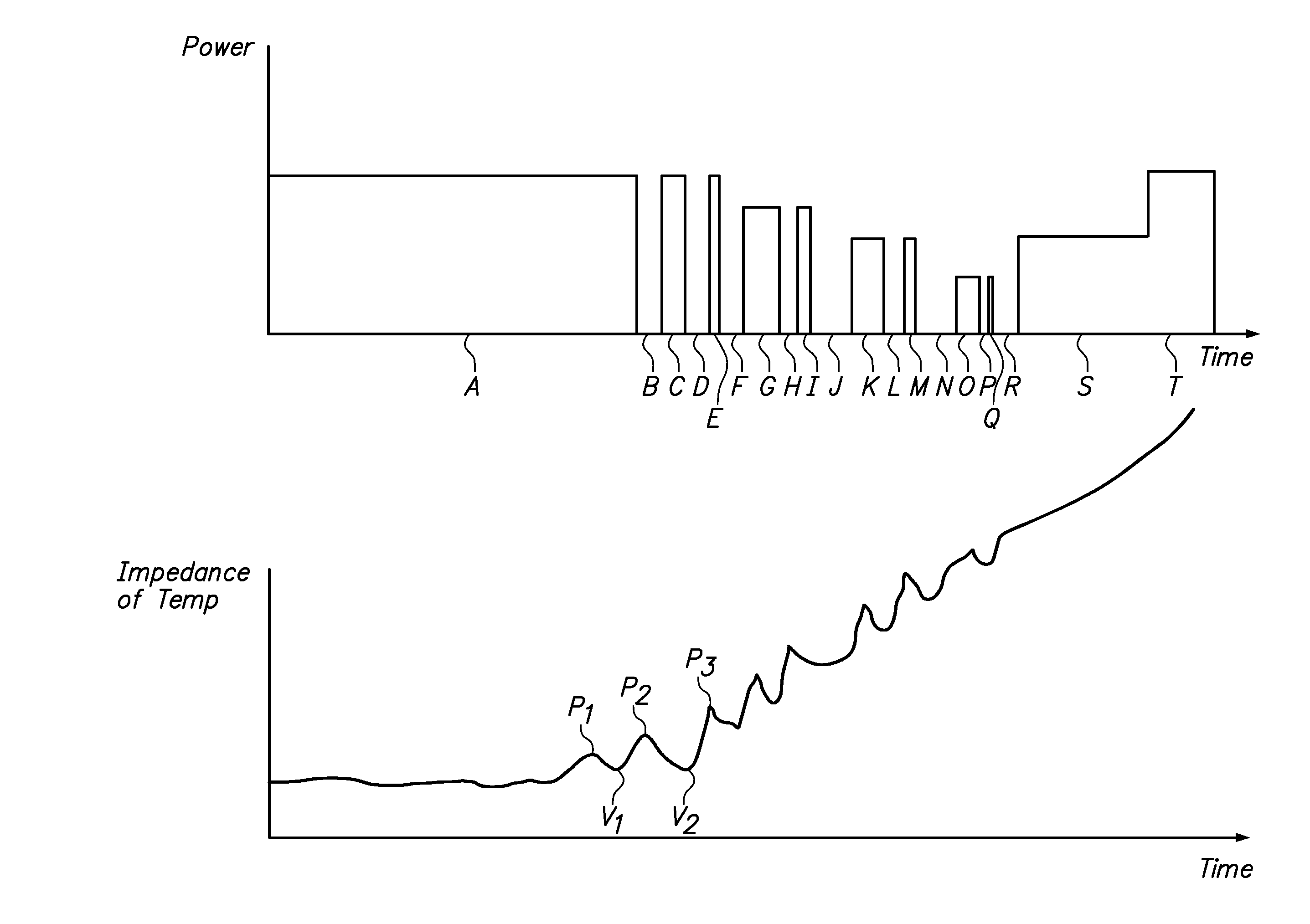
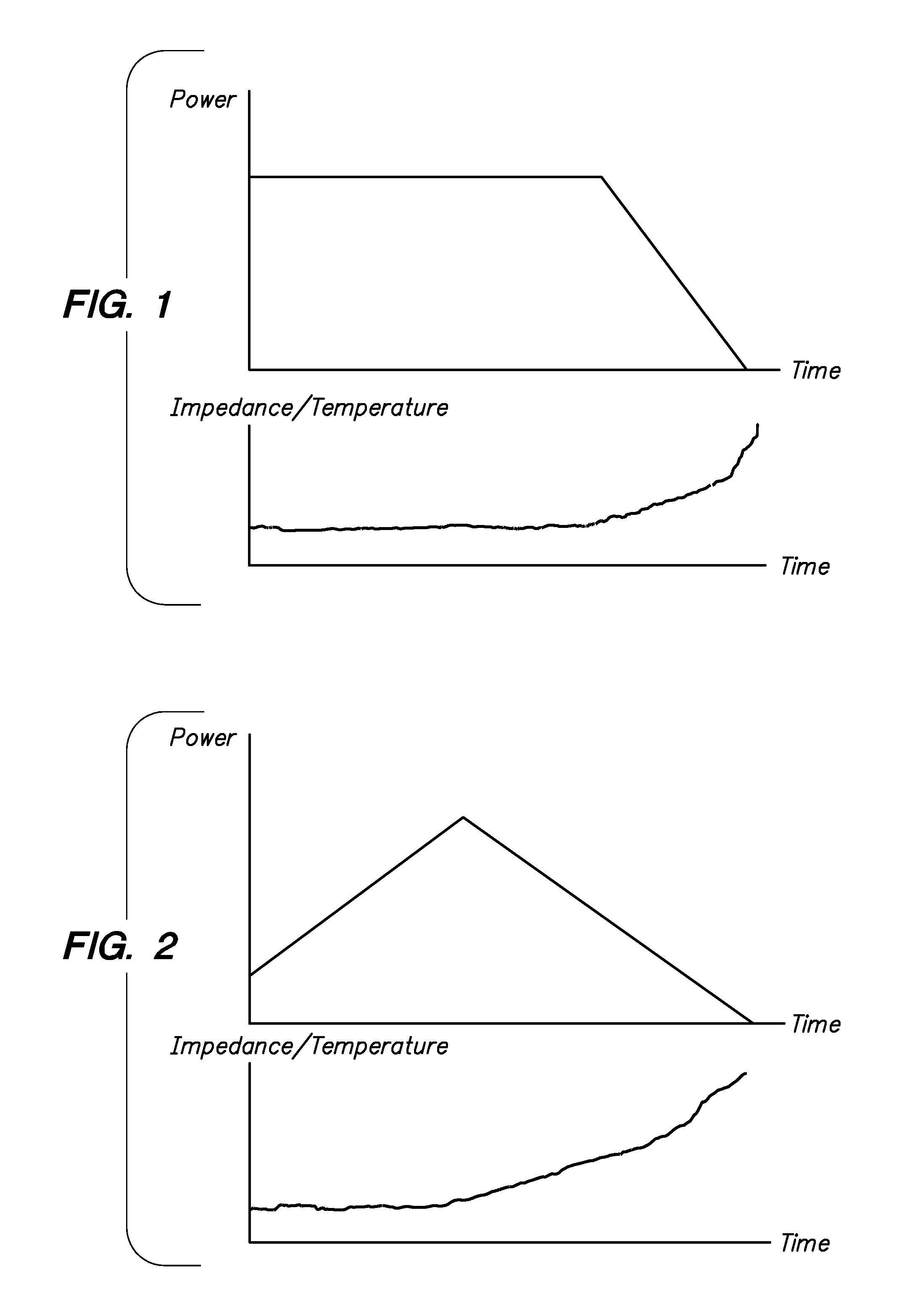
Tissue ablation systems and methods are provided. Ablation energy (e.g., radio frequency energy) is delivered to the tissue and a physiological parameter (e.g., impedance and / or temperature) indicative of a change in moisture concentration of the tissue is sensed. The ablation energy is alternately pulsed on and off to generate an energy pulse train, with the ablation energy being pulsed on if the sensed physiological parameter crosses a threshold value indicative of an increase in the moisture concentration, and being pulsed off if the sensed physiological parameter crosses a threshold value indicative of a decrease in the moisture concentration.
Owner:BOSTON SCI SCIMED INC
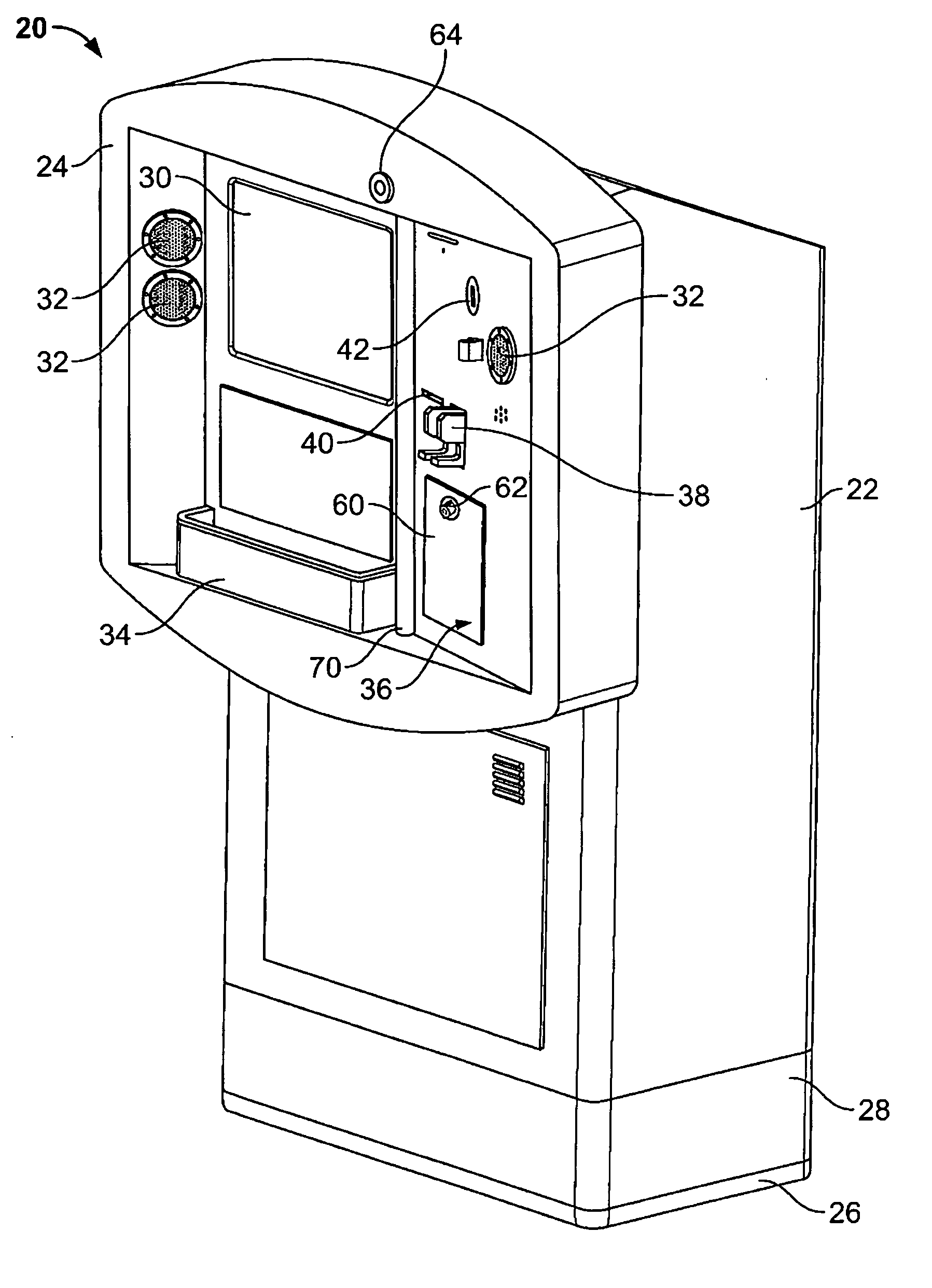
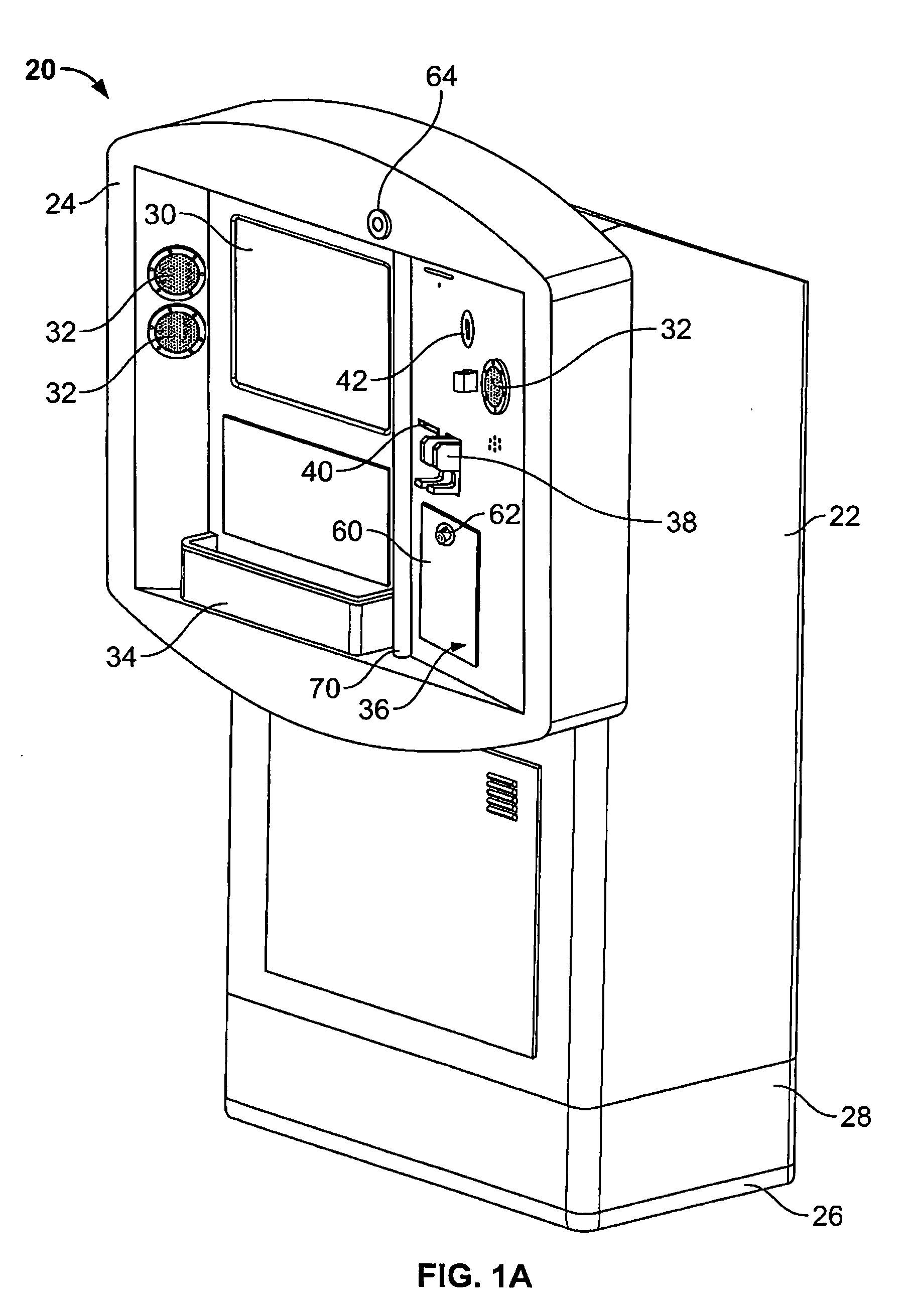
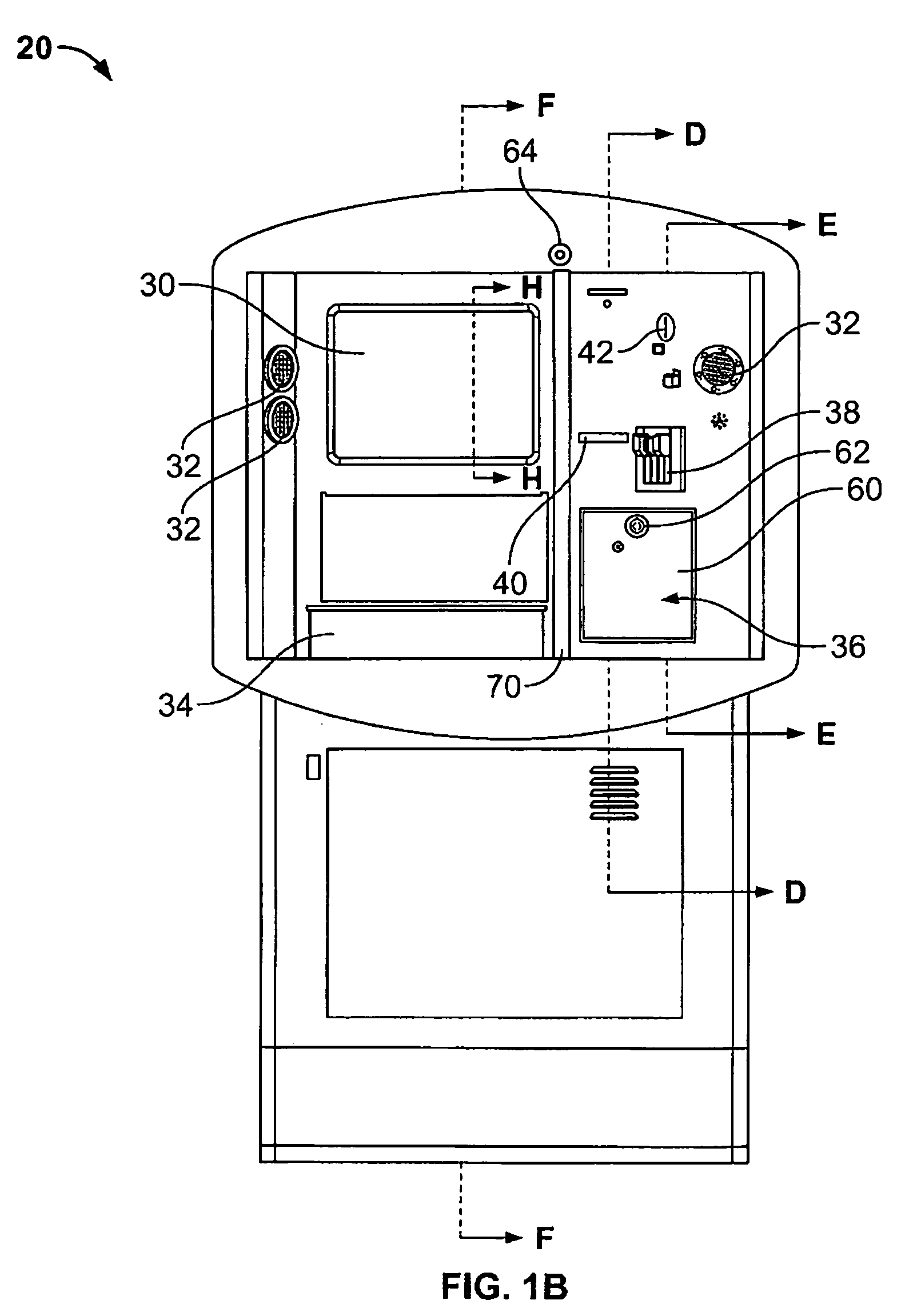
Kiosk systems and methods
A kiosk includes a front panel defining an opening in a body and an interactive screen positioned within the opening. A moisture resistant support structure is disposed between the front panel and the interactive screen. A central processing unit is disposed within the body and the central processing unit is operatively connected to the interactive screen.
Owner:RYKO MFG
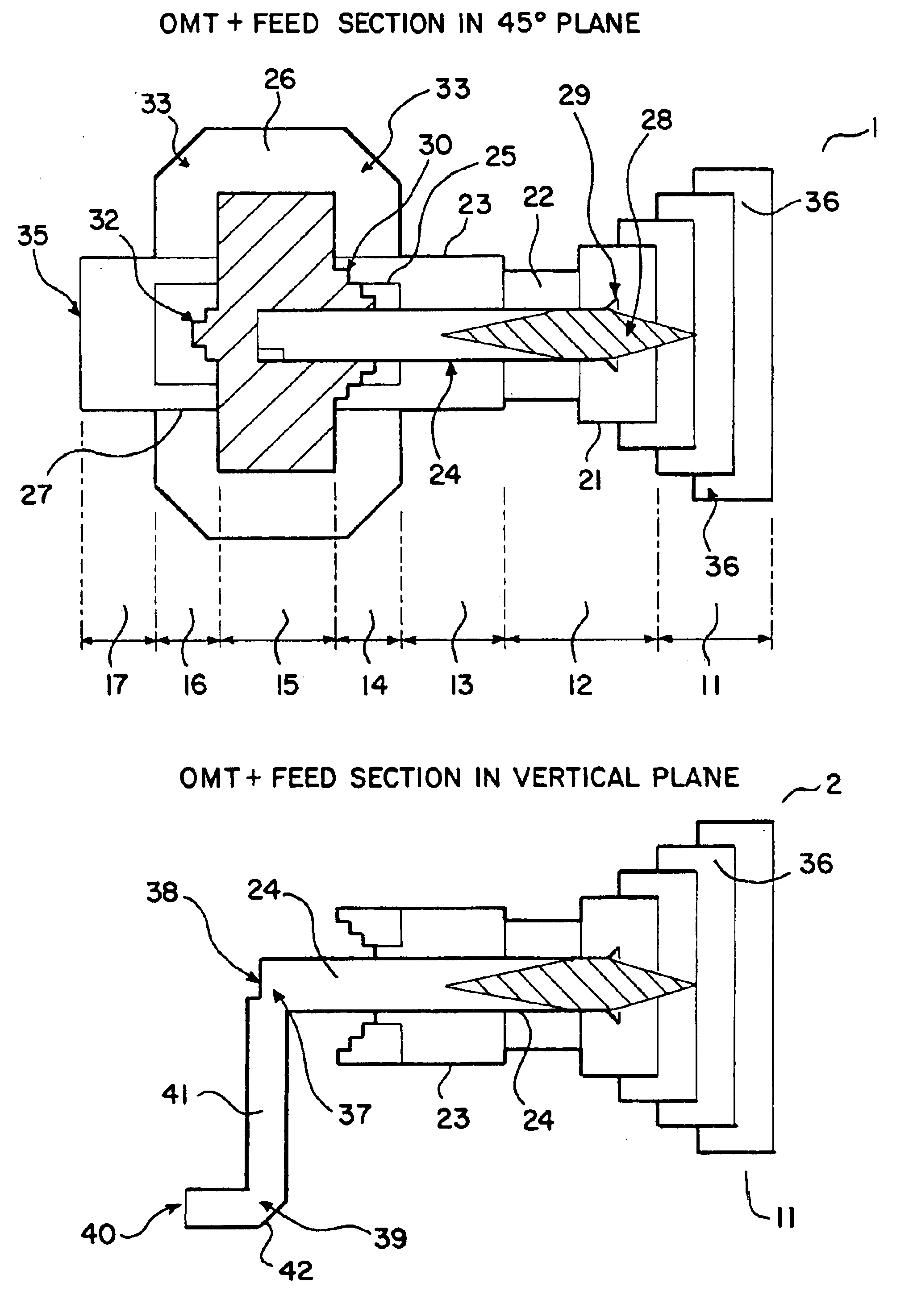
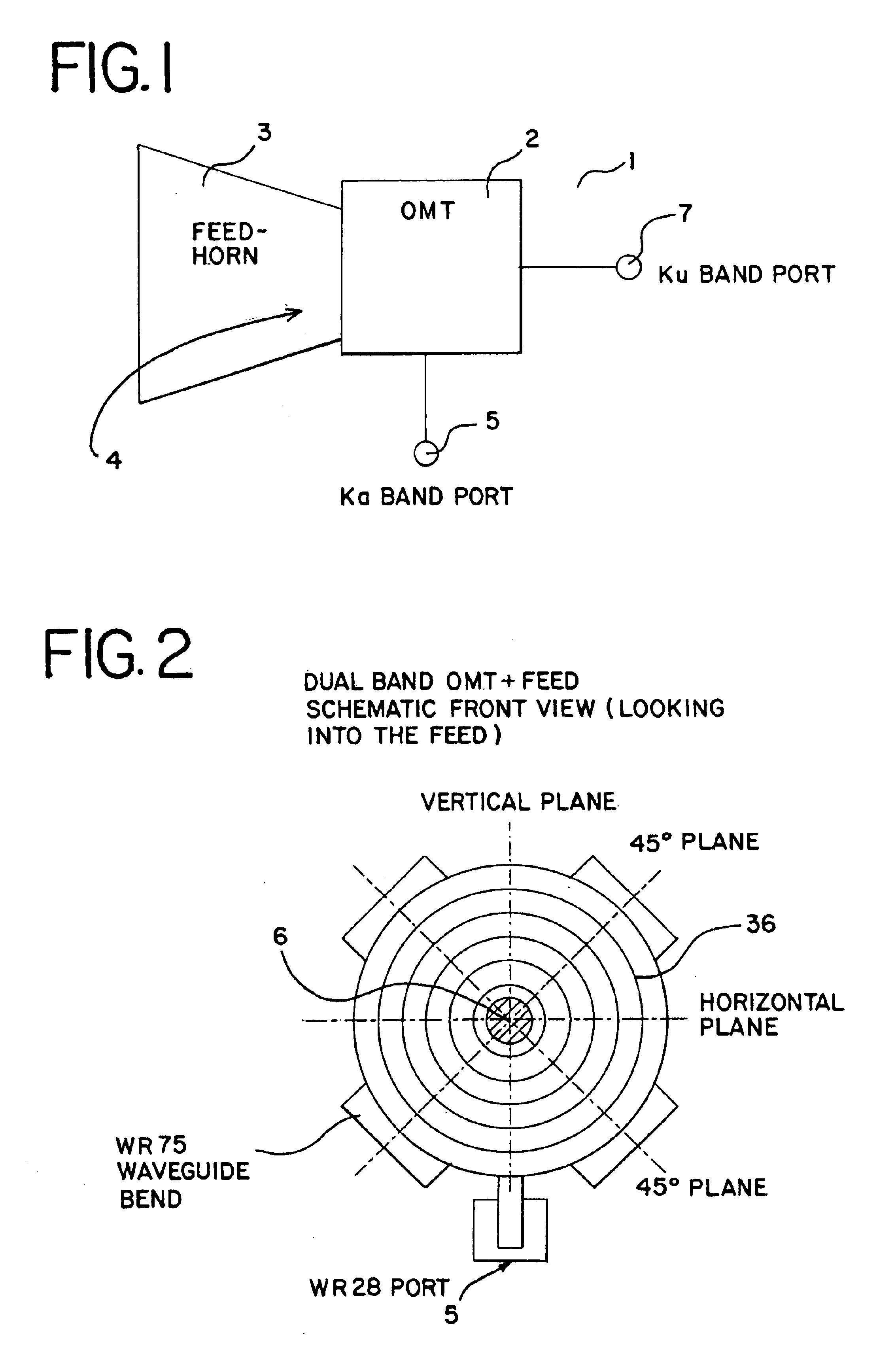
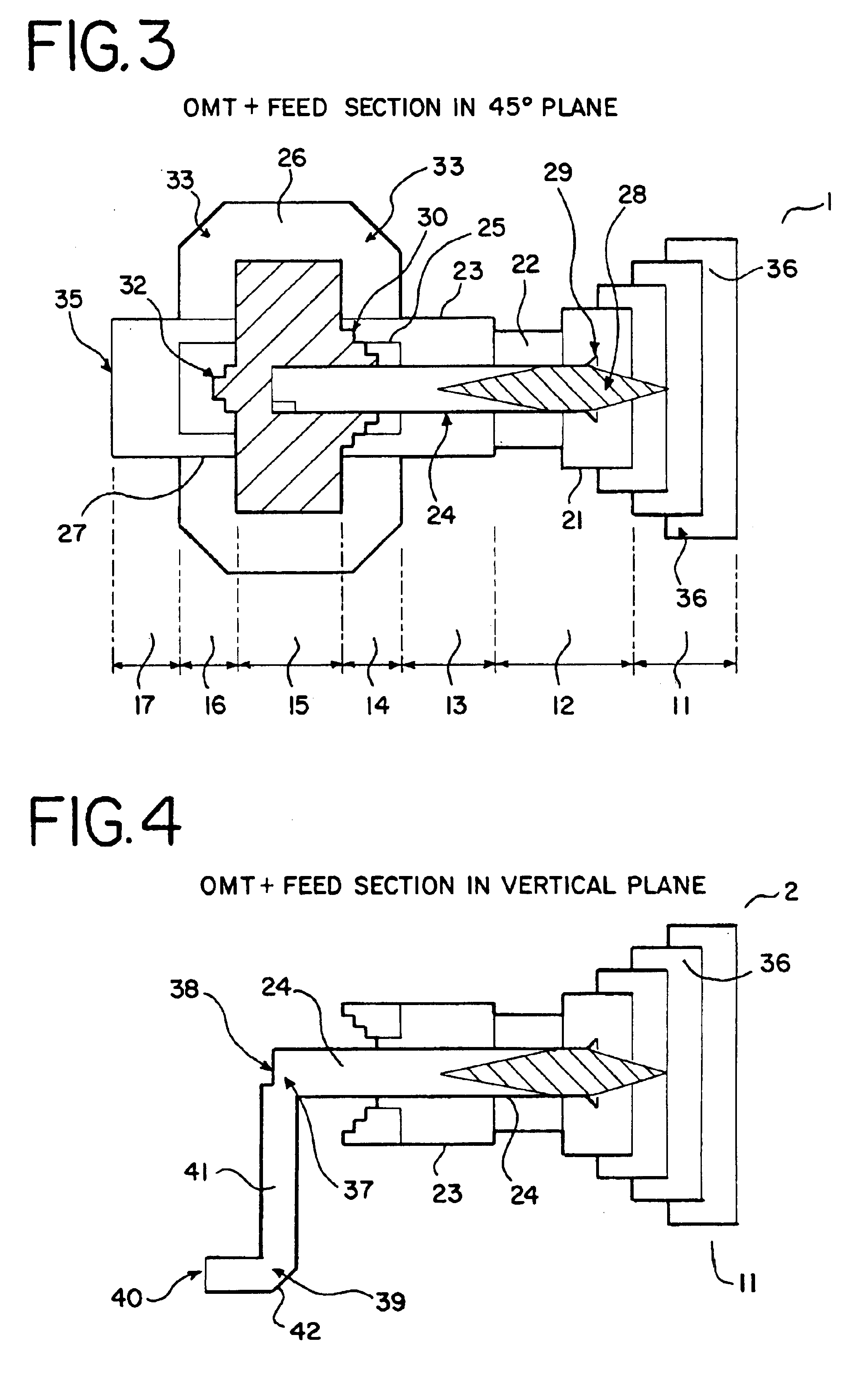
Ka/Ku dual band feedhorn and orthomode transduce (OMT)
InactiveUS6714165B2Simultaneous aerial operationsAntennas earthing switches associationRidge waveguidesTransducer
A dual band, higher and lower frequency range transducer with a circular coaxial waveguide feed is described having a first junction for connection of a lower frequency range outer waveguide of the coaxial waveguide feed to at least two rectangular or ridge waveguides offset from the longitudinal axis of the transducer and a second junction for connection of the at least two rectangular or ridge waveguides to a further waveguide. A third junction is provided for connecting an inner waveguide of the coaxial waveguide feed to a higher frequency range waveguide. The transducer comprises at least first and second parts joined across a first plane substantially perpendicular to the longitudinal axis and including at least a portion of the higher frequency range waveguide extending within the first plane of the join. A seal such as an "O" ring seal may be placed easily in the plane of the join thus preventing moisture ingress. Similarly, a feed horn and input and output ports may be sealingly attached to the first and second parts of the transducer. The first and second junctions are preferably impedance matched turnstile junctions.
Owner:NEWTEC CY
Filament loading system in an extrusion apparatus
InactiveUS6923634B2Protect environmentEasy loadingConfectionerySweetmeatsDrive wheelDimensional modeling
An apparatus which extrudes flowable material from a liquifier includes a system for loading filament supplied in a cassette. The cassette is loaded into a loading bay of the apparatus. A strand of filament from the cassette is engaged and advanced along a path to the liquifier using a drive wheel or roller pair. A conduit having an entrance in the loading bay guides the filament as it is advanced. The filament loading system of the present invention provides a convenient manner of loading and unloading filament in a three-dimensional modeling machine, and can be implemented in a manner that protects the filament from environmental moisture.
Owner:STRATSYS INC
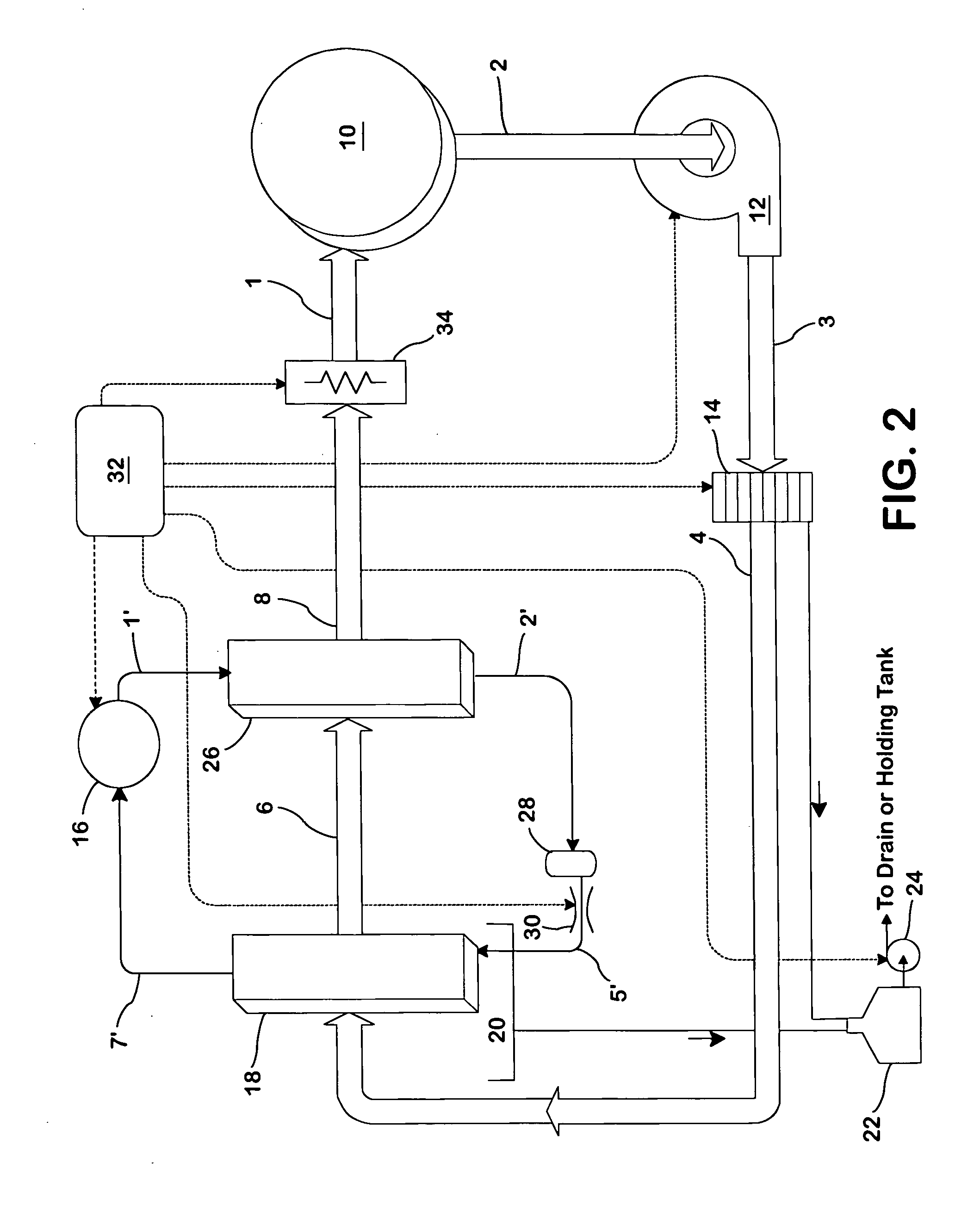
Heat pump clothes dryer
InactiveUS20050066538A1Improve performanceImprove efficiencyDrying gas arrangementsDrying chambers/containersEngineeringRefrigerant
A drying apparatus for drying articles such as clothing is provided. The drying apparatus includes a chamber for containing articles to be dried and a system for supplying heated dry air at a first temperature to the chamber. The air supplying system comprises an air flow pathway having an evaporator for removing moisture from air exiting the chamber and for decreasing the temperature of the air to below dew point temperature. The air supply system further has a condenser for increasing the temperature of the air exiting the evaporator to the first temperature. The drying apparatus further has a heat pump system having a refrigerant loop which includes a compressor, the condenser, a TEV valve, and the evaporator.
Owner:FLI HLDG COMPANY
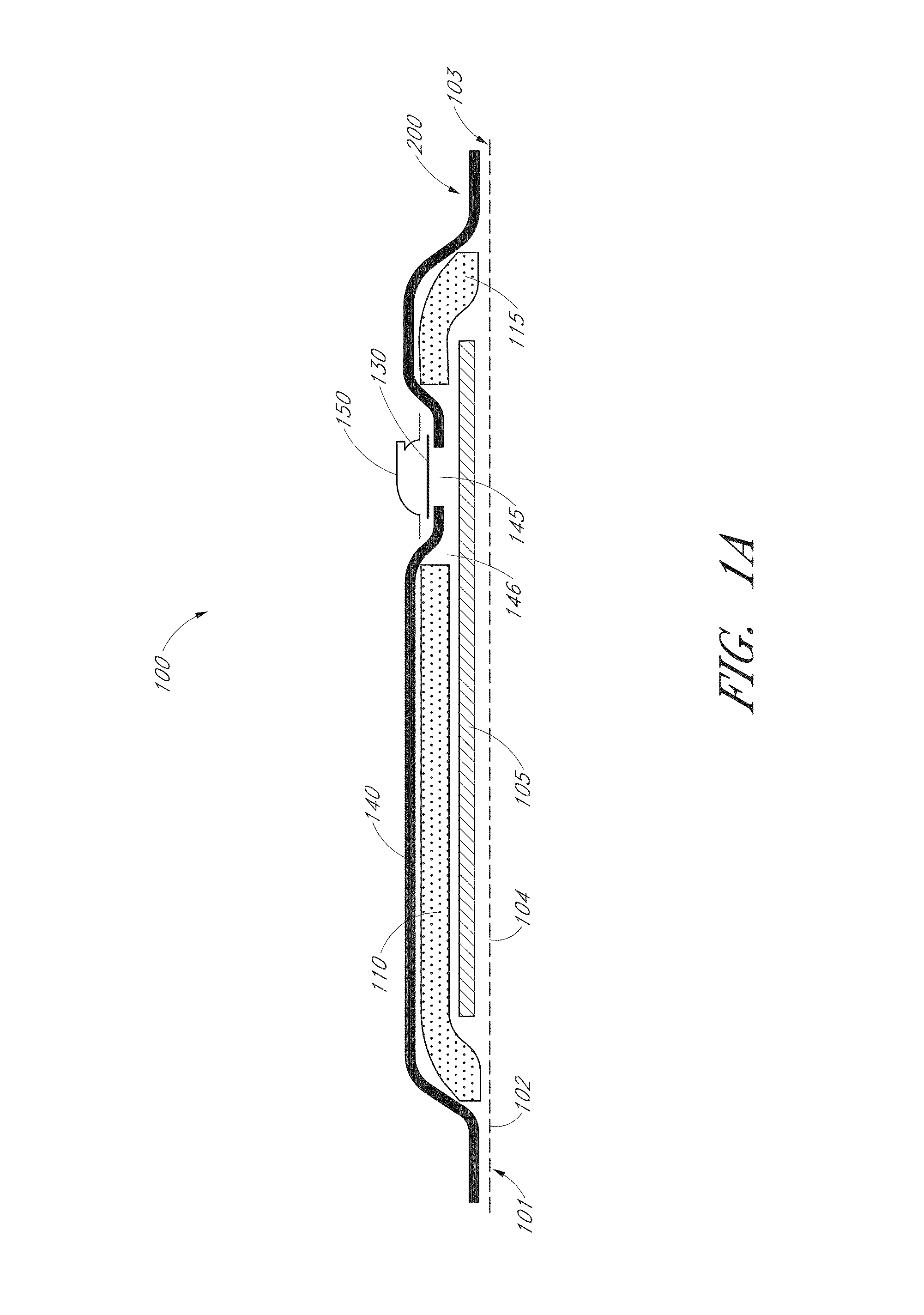
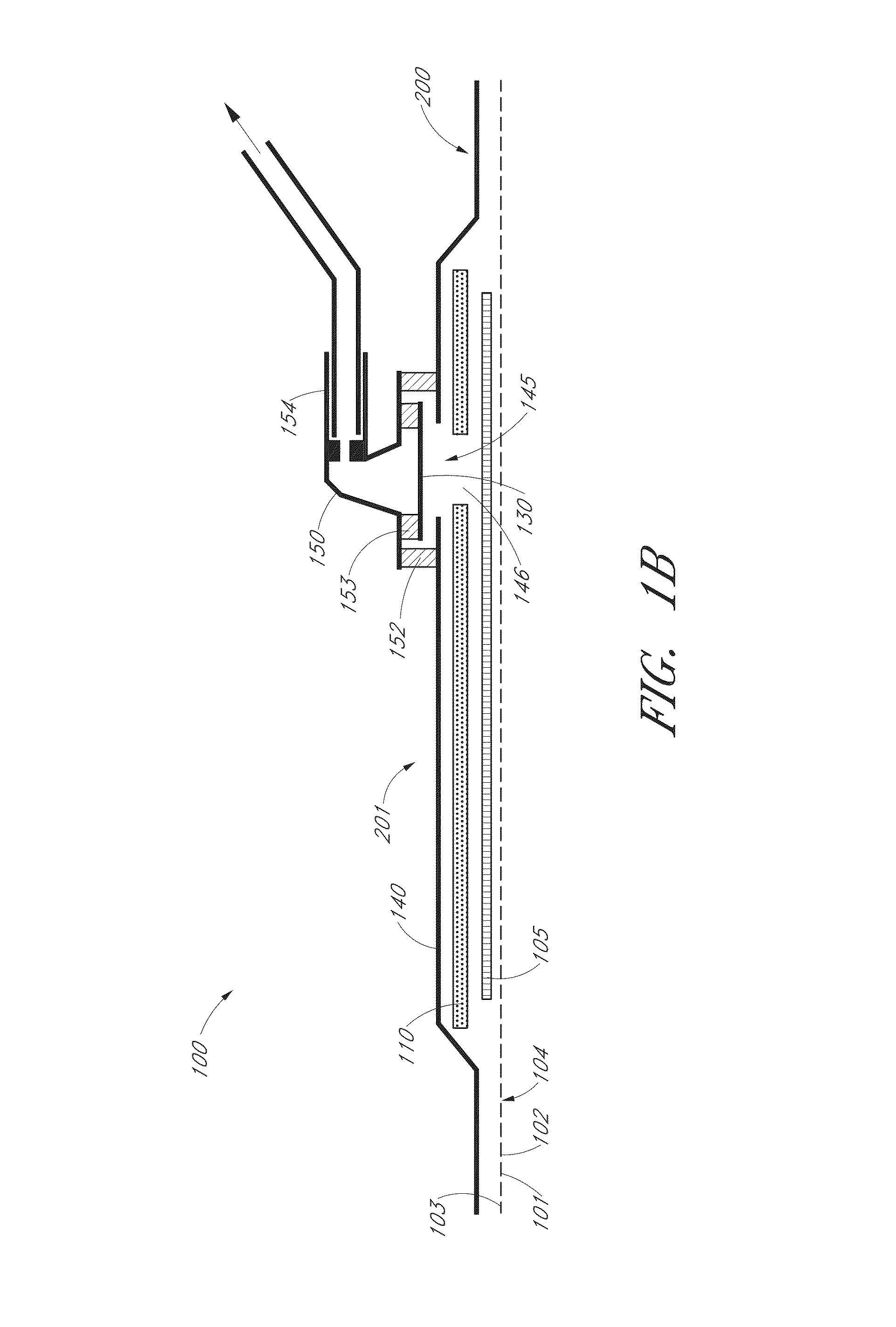
Reusable powered surgical devices having improved durability
InactiveUS20180242970A1Durable for re-useSurgical furnitureDiagnosticsEngineeringElectronic component
A surgical device includes at least one electronic component having a moisture collection agent sealed therein that is configured to protect electronic elements of the electronic component and / or a moisture mitigation system including a moisture migration component disposed within the surgical device and a moisture collection agent disposed adjacent to the moisture migration component to remove moisture from within the surgical device. The at least one electronic component may include a substrate, electronic elements disposed on a surface of the substrate, a coating covering the surface of the substrate and the electronic elements disposed thereon, and a moisture collection agent disposed between the substrate and the coating to control moisture at the surface of the substrate. The at least one electronic component may include a substrate having a base and a cover defining a sealed cavity therein, and electronic elements and a moisture collection agent disposed within the sealed cavity.
Owner:TYCO HEALTHCARE GRP LP
EL display device and electronic device
InactiveUS20020125817A1Improve reliabilityDischarge tube luminescnet screensElectroluminescent light sourcesAdhesiveDisplay device
A cover member 107 is adhered onto a substrate 101 on which an EL element is formed with a filler 208 as an adhesive. Further, a sealing member 209 is provided so as to cover a side surface (an exposing surface) of the filler 208, and a frame member 108 is adhered using the sealing member 209. With this structure, the EL element is completely sealed by the filler 208, and shut from moisture and the like from the outside. Thus, deterioration of an EL layer due to oxidization can be prevented.
Owner:SEMICON ENERGY LAB CO LTD
Wound dressing and method of use
A system, method, and apparatus are disclosed for dressing a wound. The apparatus comprises a liquid and gas permeable transmission layer, an absorbent layer for absorbing wound exudate, the absorbent layer overlying the transmission layer, a gas impermeable cover layer overlying the absorbent layer and comprising a first orifice, wherein the cover layer is moisture vapor permeable.
Owner:SMITH & NEPHEW PLC
Test device with means for storing and dispensing diagnostic strips
The invention provides a substantially moisture-proof, air-tight apparatus for both dispensing a plurality of diagnostic test strips and testing a biological fluid dispensed onto the strip. One strip may be advanced for use in testing using a single, translational movement.
Owner:LIFESCAN IP HLDG LLC
Methods of spray-drying a drug and a hydrophobic amino acid
InactiveUS6372258B1High level of stabilityEasy to manufacturePowder deliveryOrganic active ingredientsMoistureParticle-size distribution
According to the subject invention, dispersible dry powder pharmaceutical-based compositions are provided, including methods for their manufacture and dry powder dispersion devices. A dispersible dry powder pharmaceutical-based composition is one having a moisture content of less than about 10% by weight (% w) water, usually below about 5% w and preferably less than about 3% w; a particle size of about 1.0-5.0 mum mass median diameter (MMD), usually 1.0-4.0 mum MMD, and preferably 1.0-3.0 mum MMD; a delivered dose of about >30%, usually >40%, preferably >50%, and most preferred >60%; and an aerosol particle size distribution of about 1.0-5.0 mum mass median aerodynamic diameter (MMAD), usually 1.5-4.5 mum MMAD, and preferably 1.5-4.0 MMAD. Such composition are of pharmaceutical grade purity.
Owner:NOVARTIS FARMA
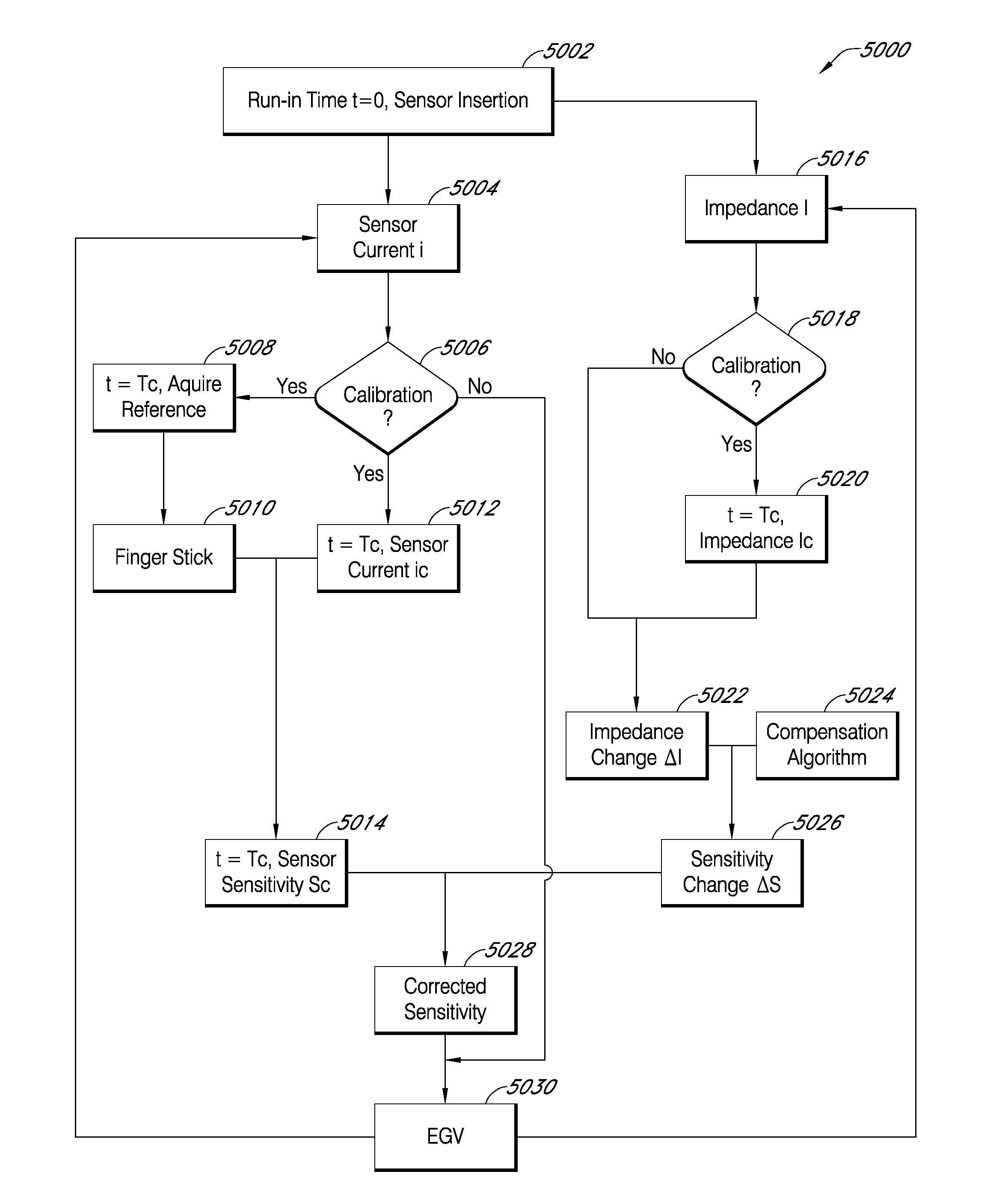
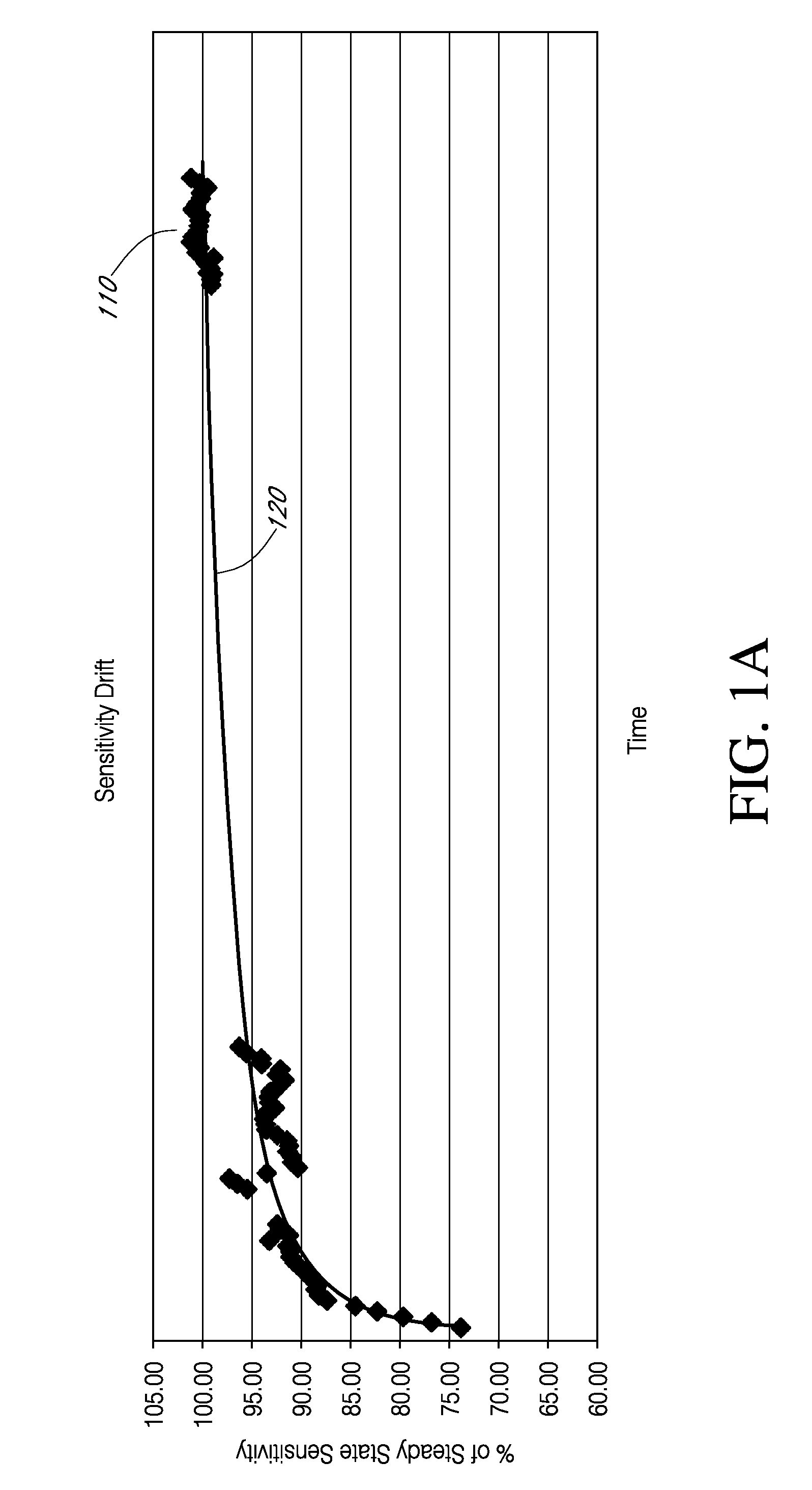
Advanced analyte sensor calibration and error detection
Systems and methods for processing sensor data and self-calibration are provided. In some embodiments, systems and methods are provided which are capable of calibrating a continuous analyte sensor based on an initial sensitivity, and then continuously performing self-calibration without using, or with reduced use of, reference measurements. In certain embodiments, a sensitivity of the analyte sensor is determined by applying an estimative algorithm that is a function of certain parameters. Also described herein are systems and methods for determining a property of an analyte sensor using a stimulus signal. The sensor property can be used to compensate sensor data for sensitivity drift, or determine another property associated with the sensor, such as temperature, sensor membrane damage, moisture ingress in sensor electronics, and scaling factors.
Owner:DEXCOM
Internal coaxial cable connector integrated circuit and method of use thereof
InactiveUS8414326B2Improve reliabilityElectrically conductive connectionsTwo pole connectionsCoaxial cableElectrical devices
A structure is provided. The structure includes a signal retrieval circuit formed within a disk located within a coaxial cable connector. The signal retrieval circuit is located in a position that is external to a signal path of an electrical signal flowing through the coaxial cable connector. The signal retrieval circuit is configured to extract an energy signal from the electrical signal flowing through the coaxial cable connector. The energy signal is configured to apply power to an electrical device located within the coaxial cable connector. The sensing circuit is configured to sense physical parameter such as condition of the RF electrical signal flowing through the connector or presence of moisture in the connector. The structure may include an integrated circuit configured to convert the parameter signal into a data acquisition signal readable by the integrated circuit.
Owner:ROCHESTER INSTITUTE OF TECHNOLOGY
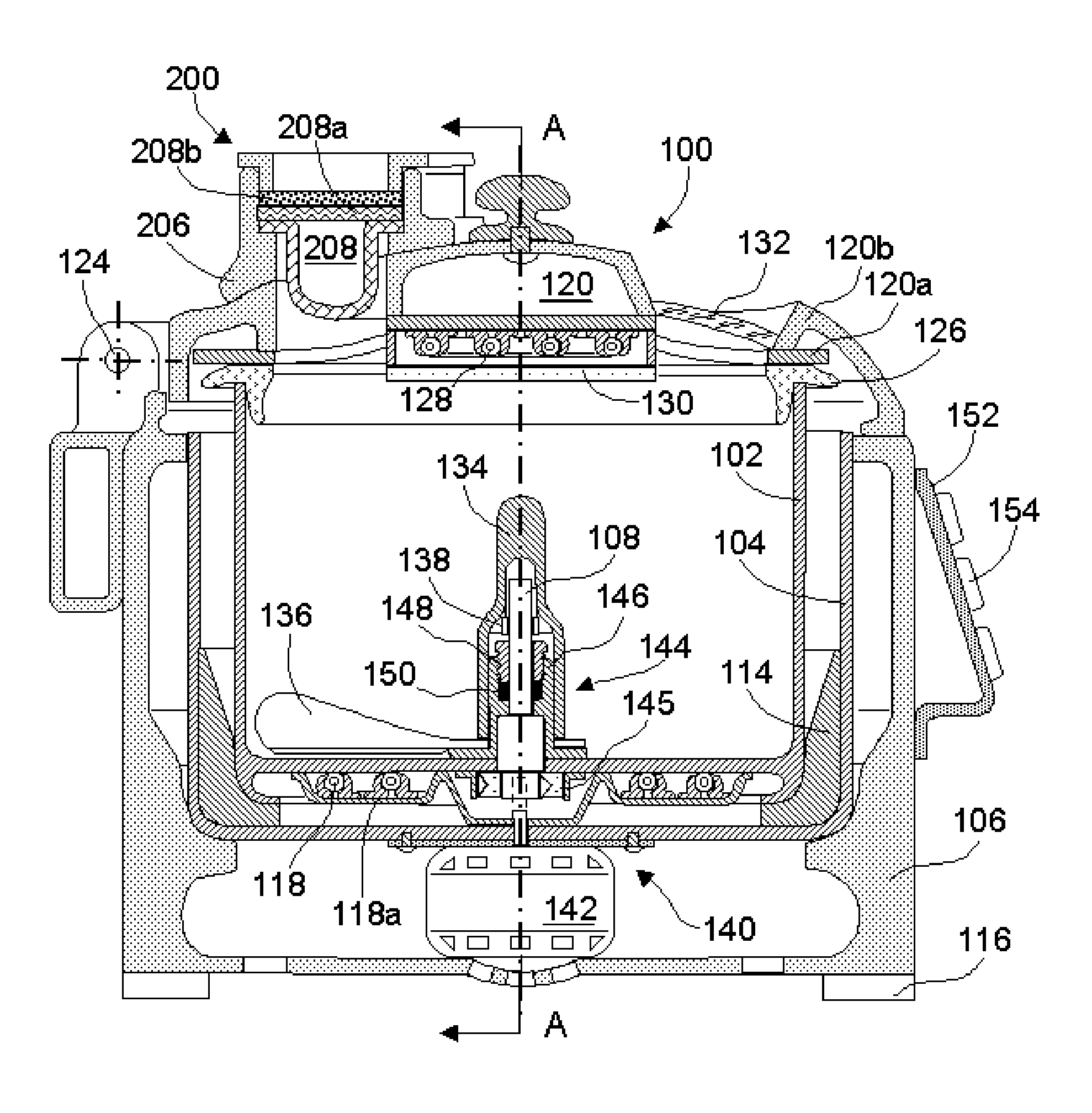
Wafer handling chamber with moisture reduction
ActiveUS20190371640A1Reduce amountReduce cleaning volumeSemiconductor/solid-state device manufacturingEngineeringMoisture
An apparatus and method for reducing moisture within a wafer handling chamber is disclosed. The moisture reduction results in reduced oxidation of a wafer. The moisture reduction is made possible through use of valves and purging gas. Operation of the valves may result in improved localized purging.
Owner:ASM IP HLDG BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com