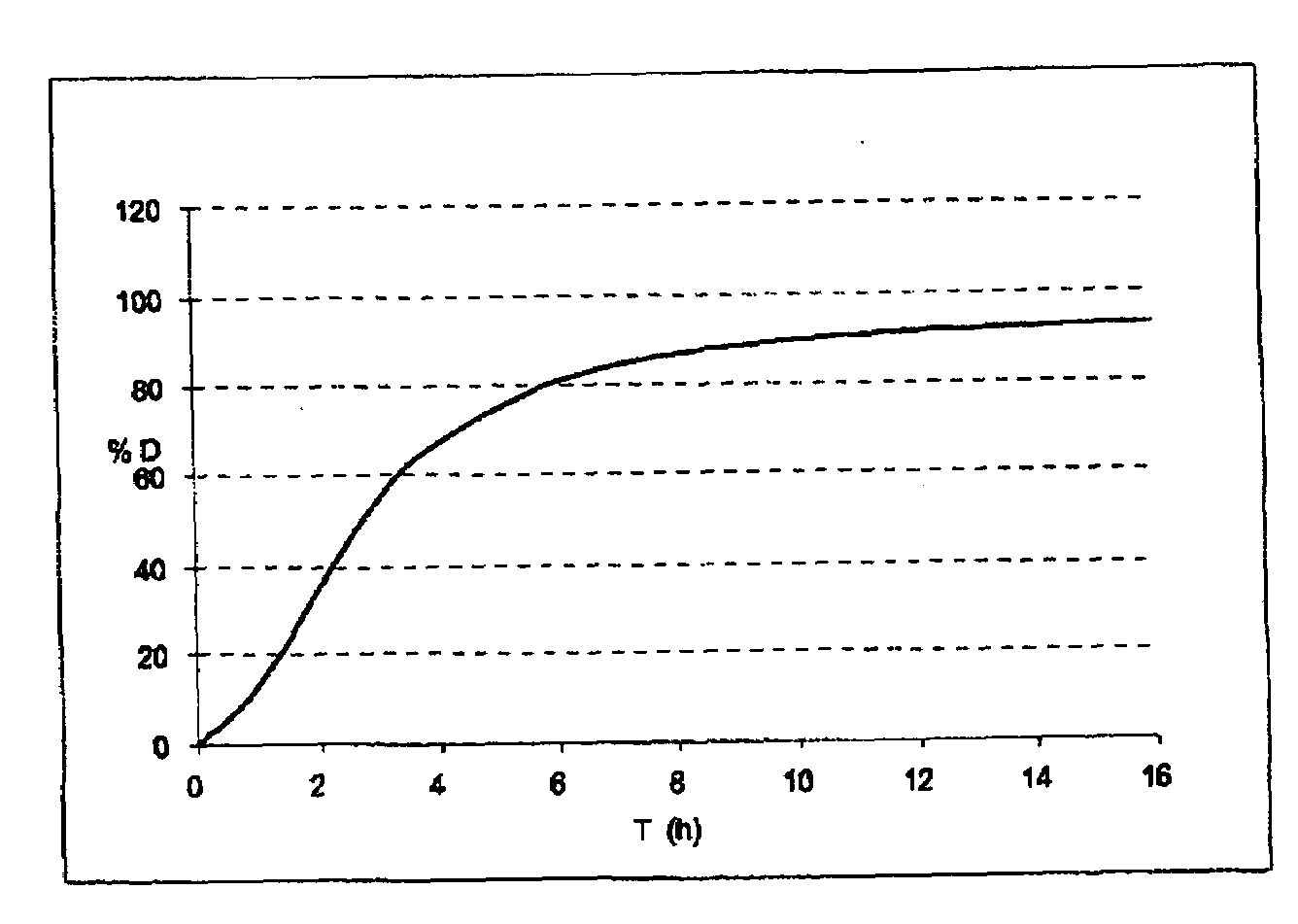
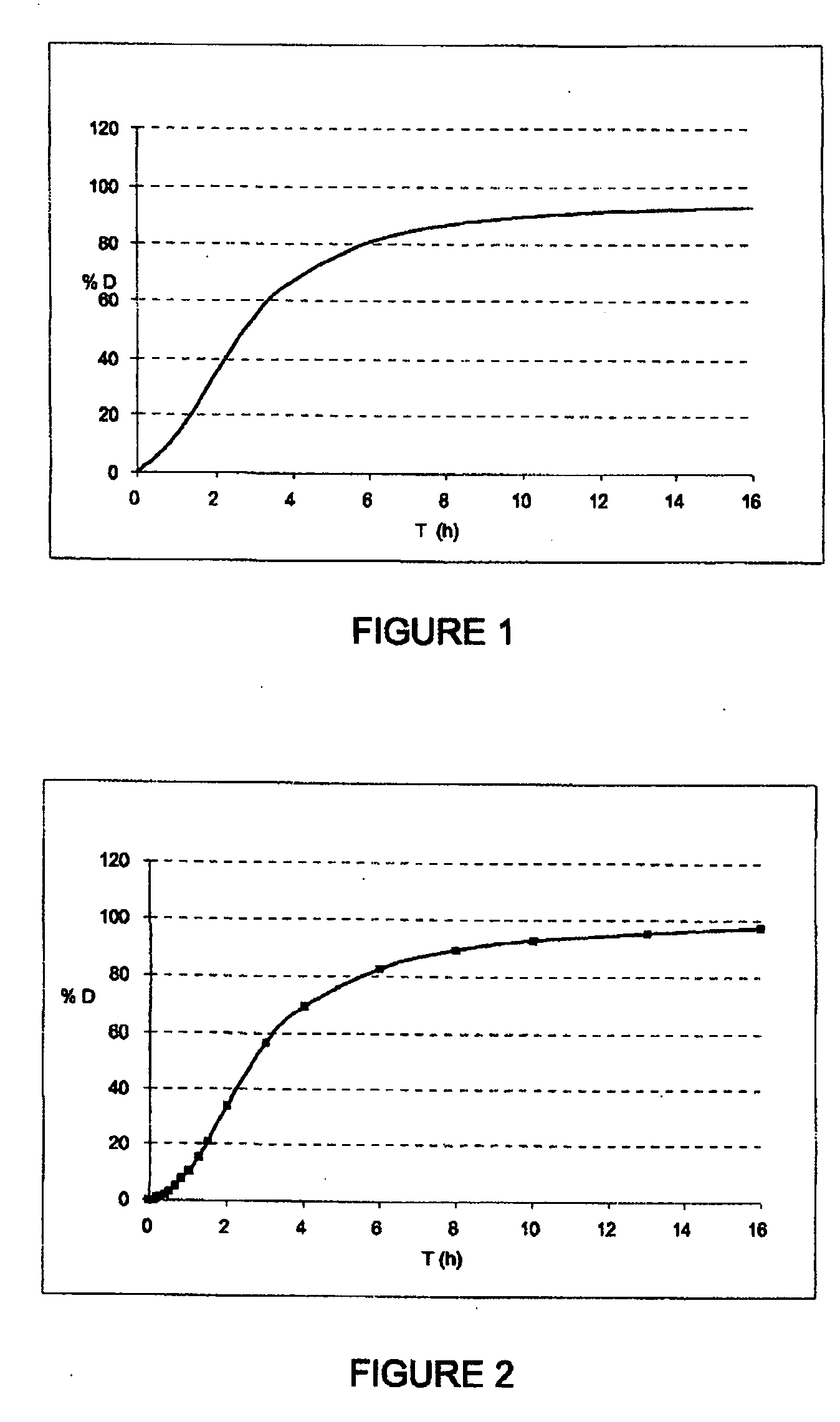
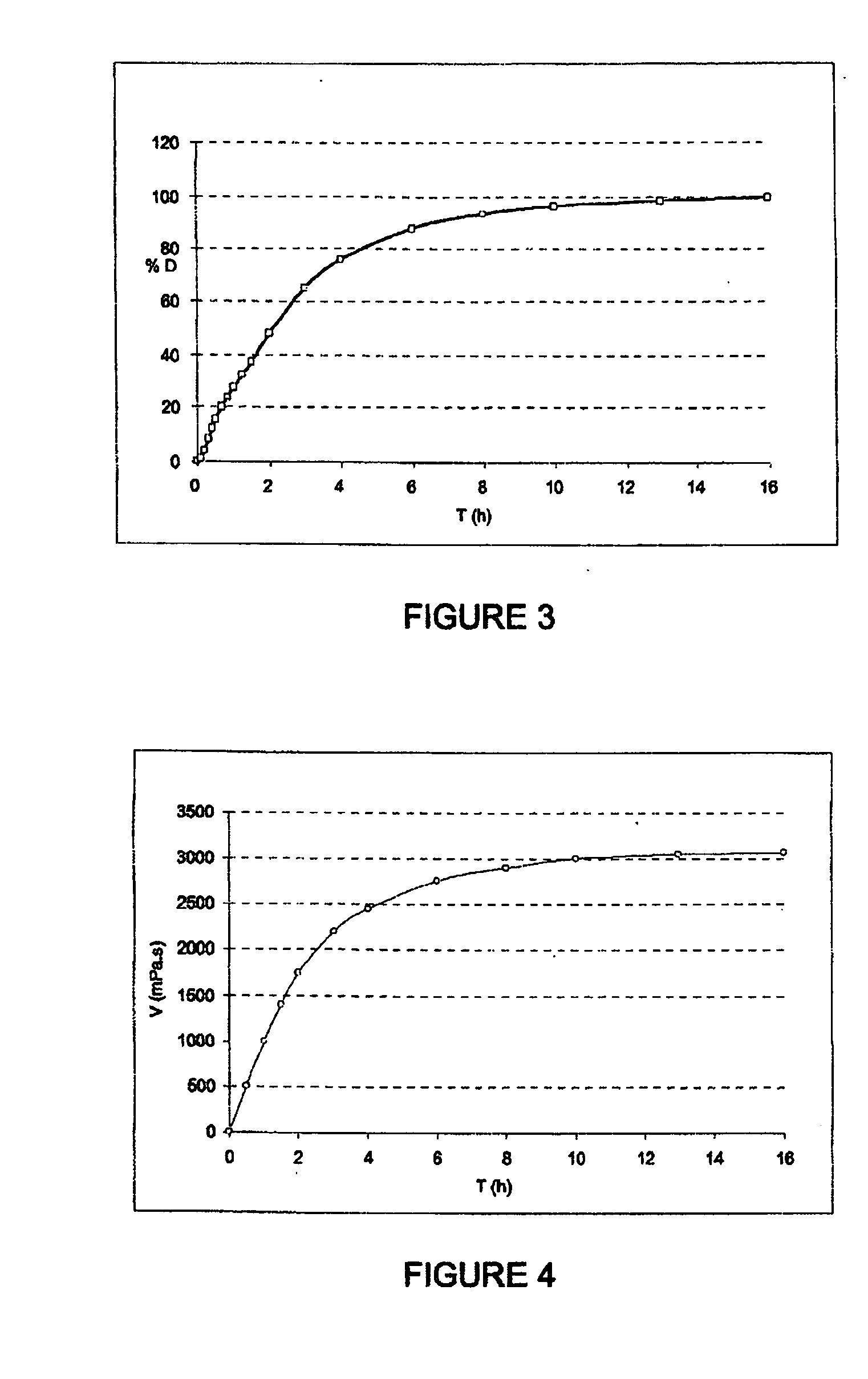
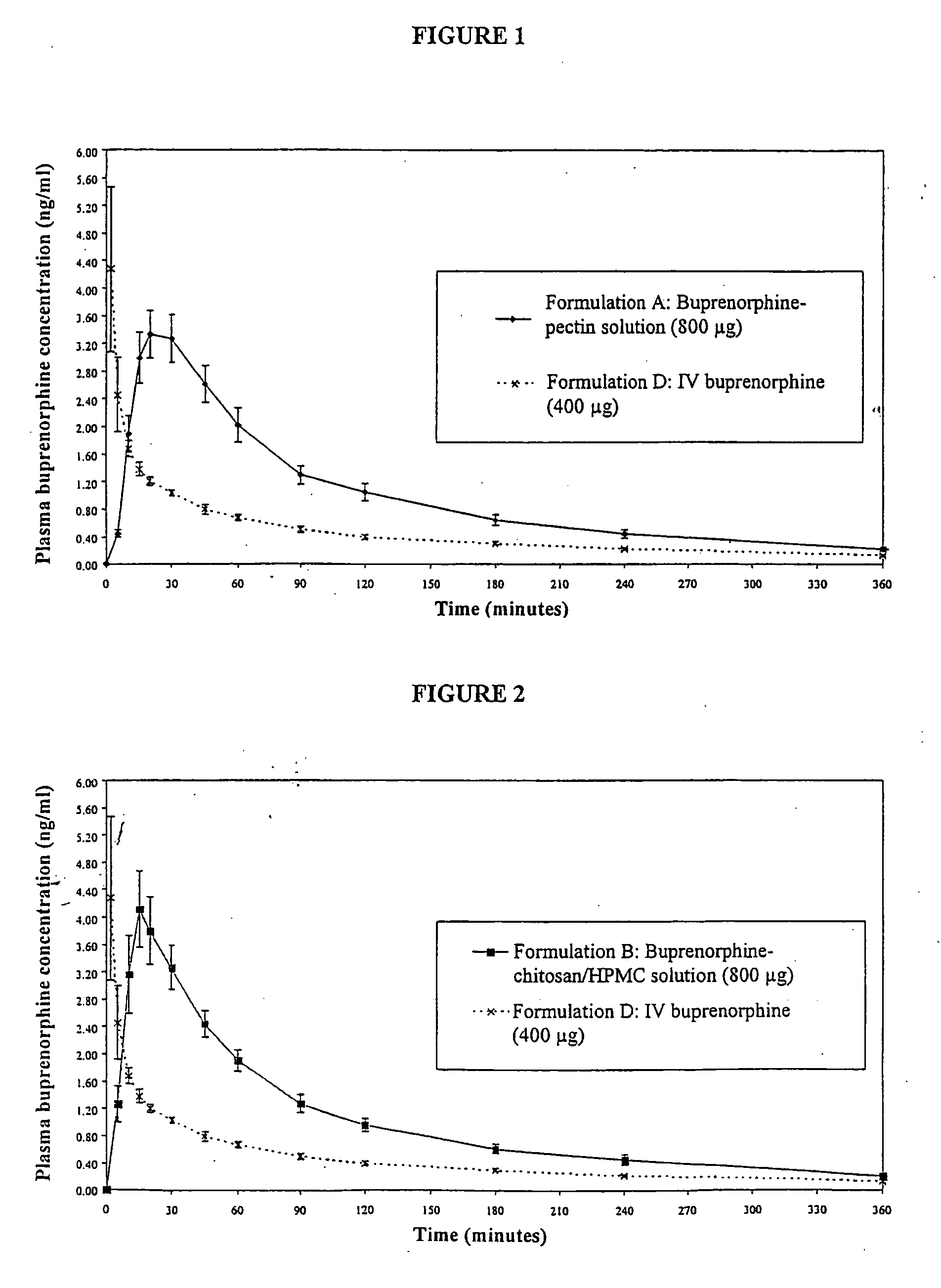
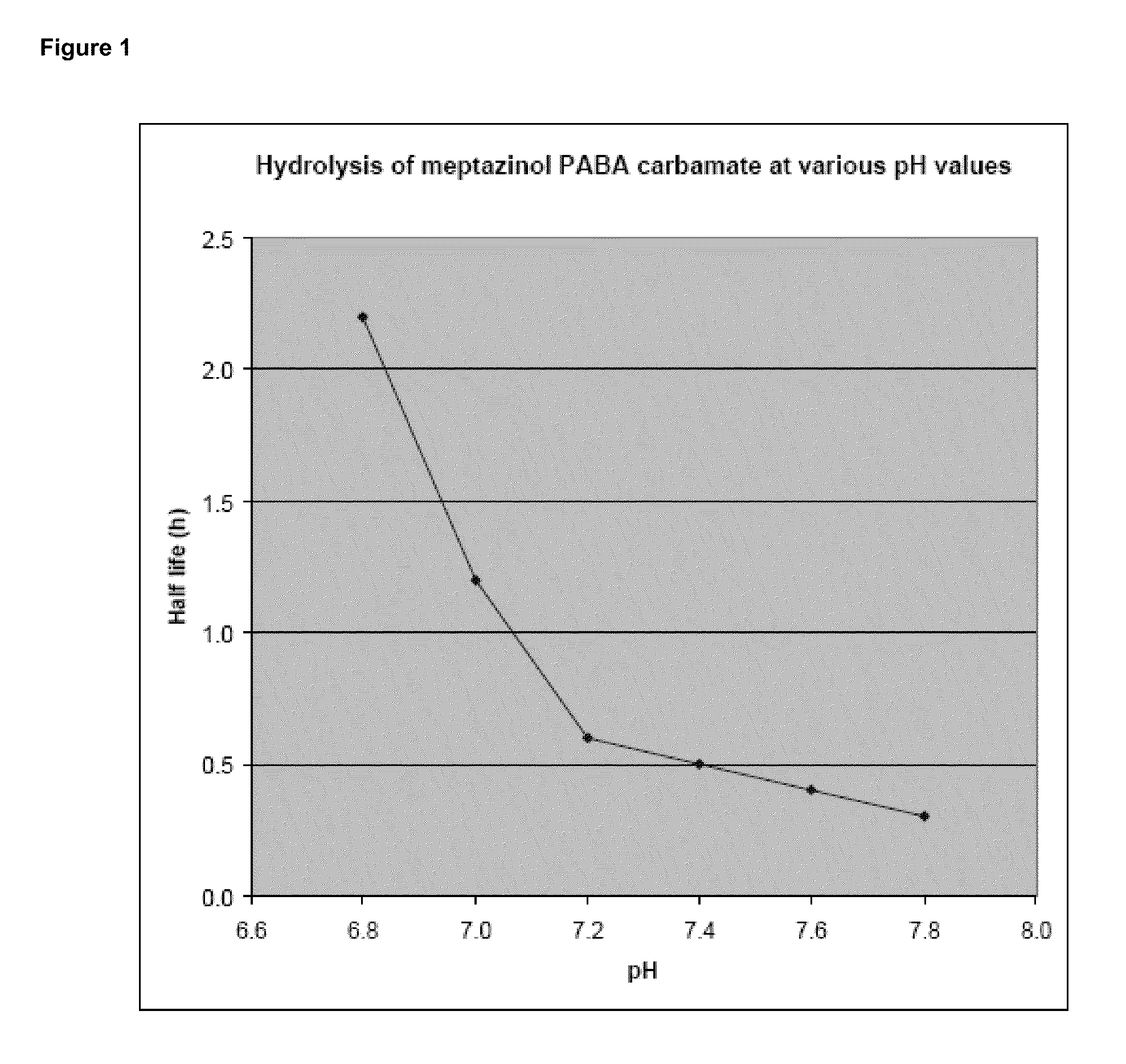
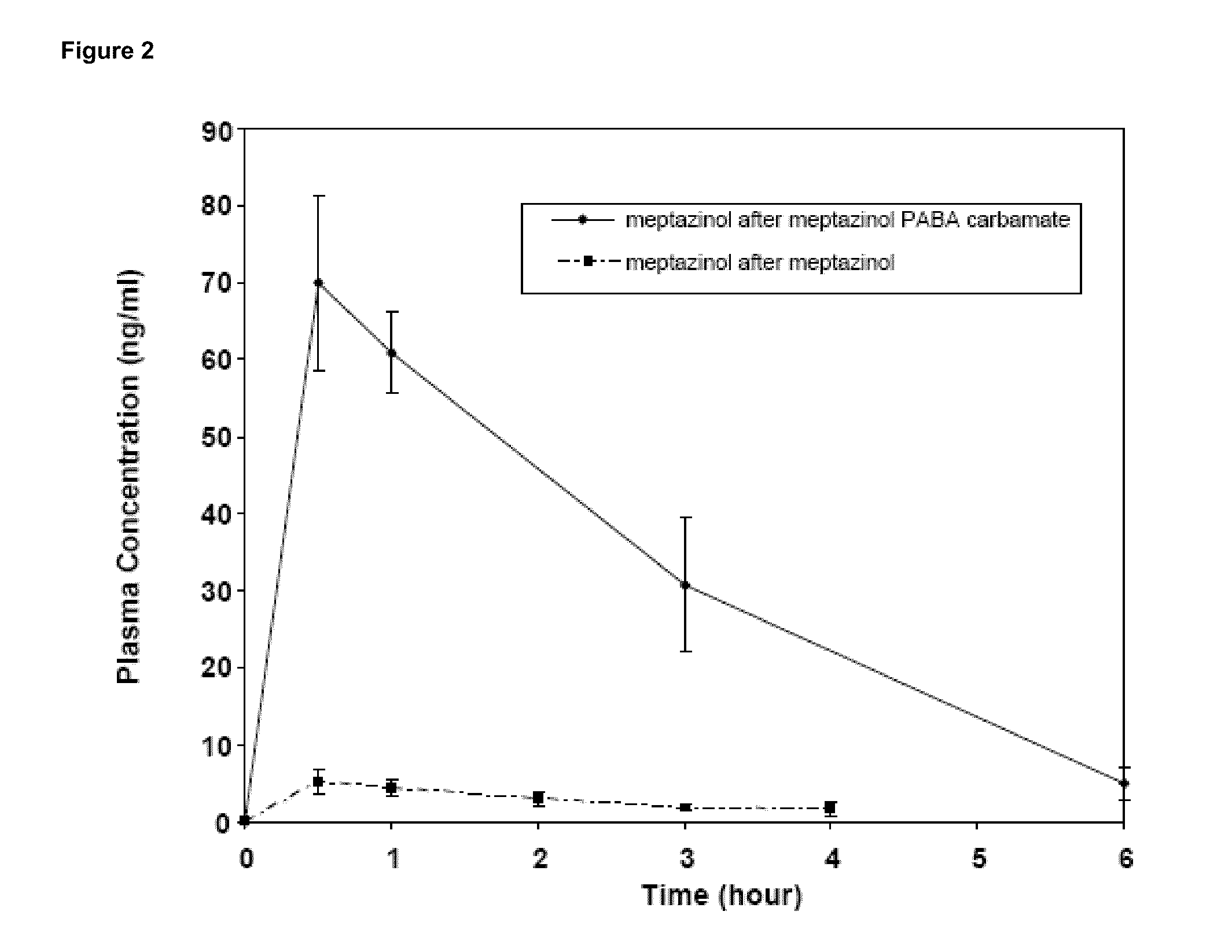
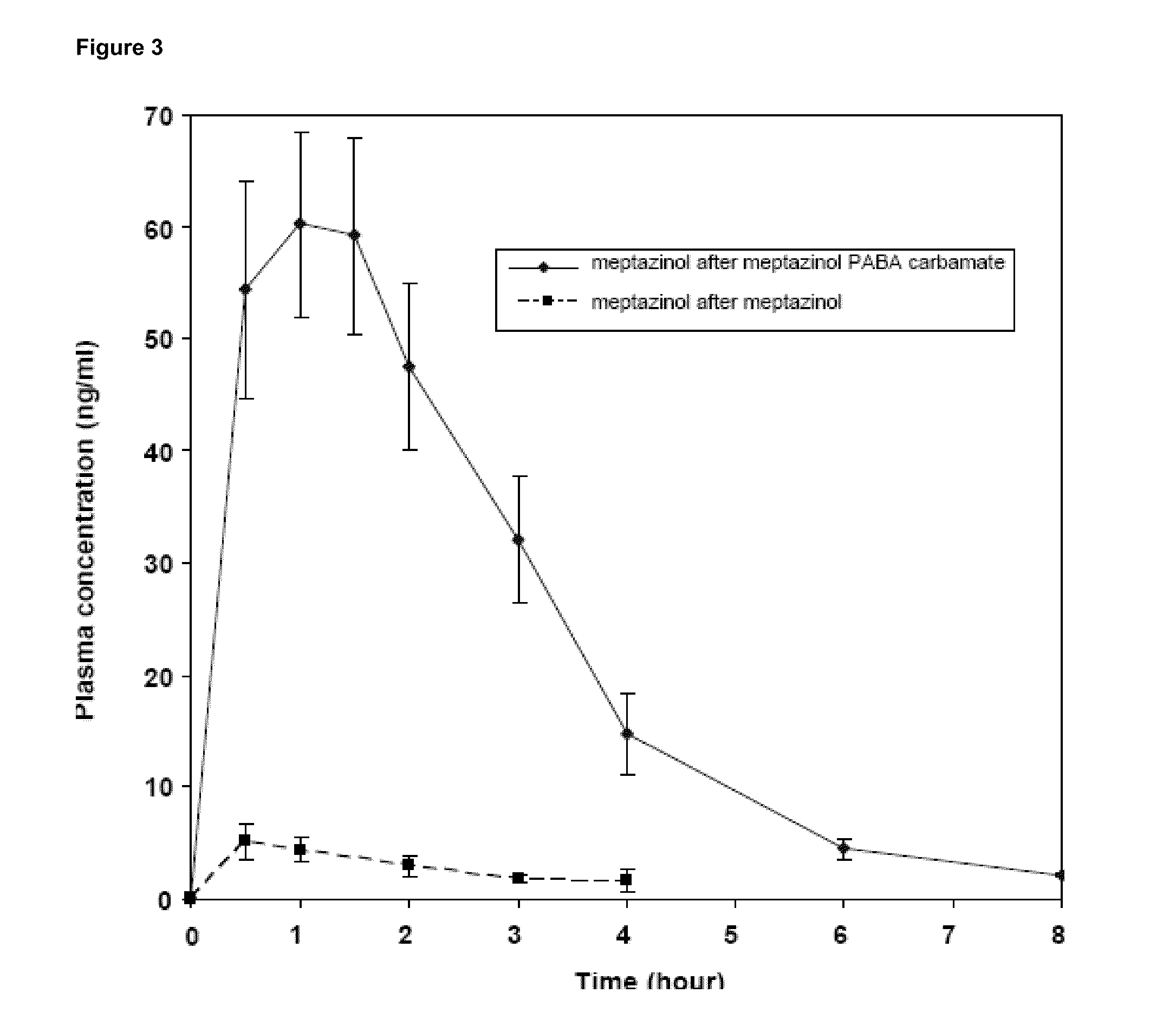
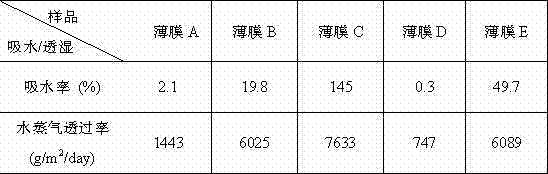
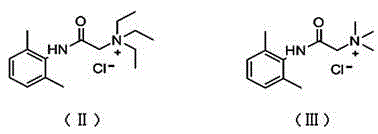Patents
Literature
312 results about "Analgesics drugs" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
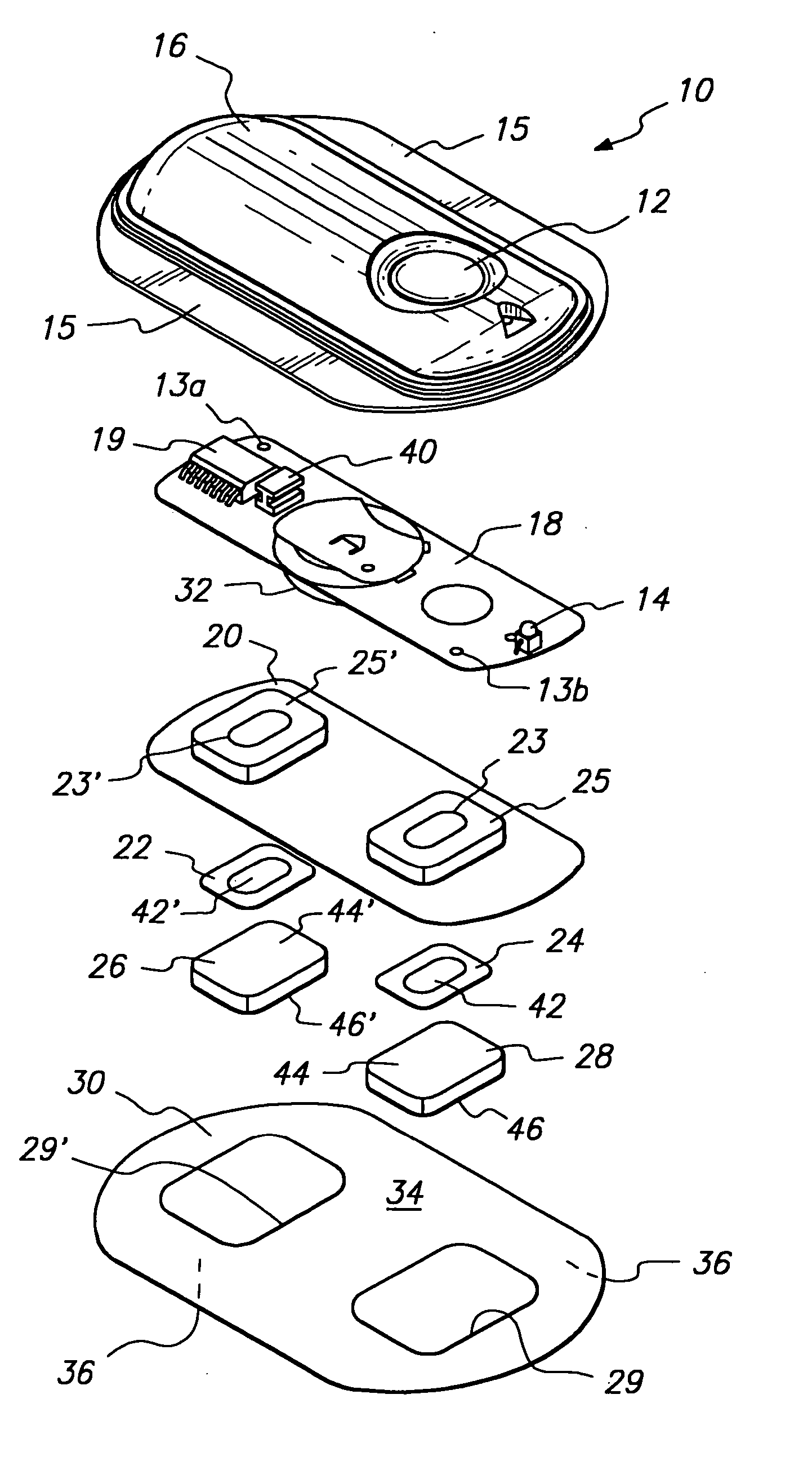
Analgesics are drugs designed specifically to relieve pain. There are several types of analgesics: acetaminophen (Tylenol), which is available without a prescription, and a variety of opioid analgesics, which are available only with a prescription.
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
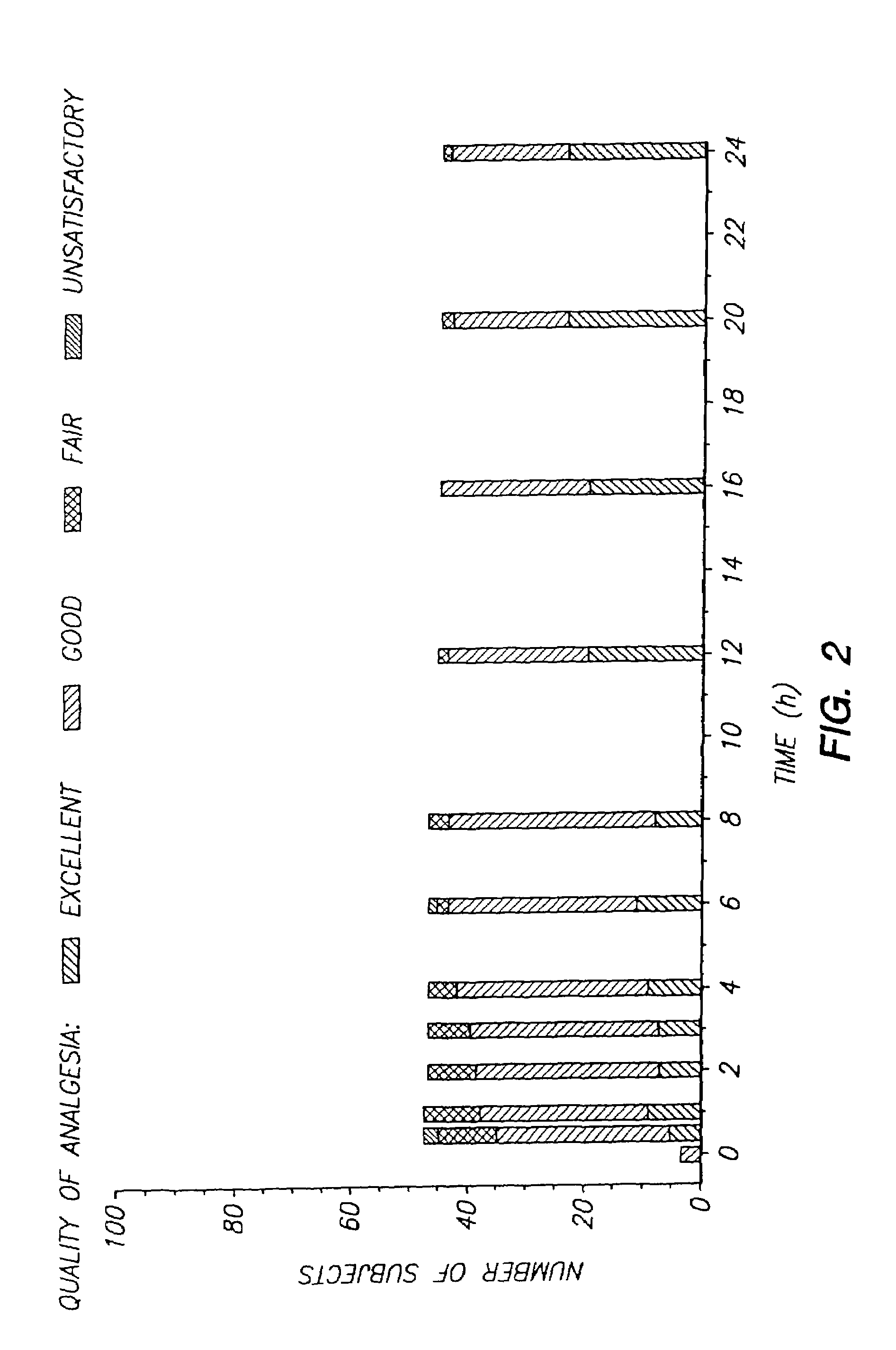
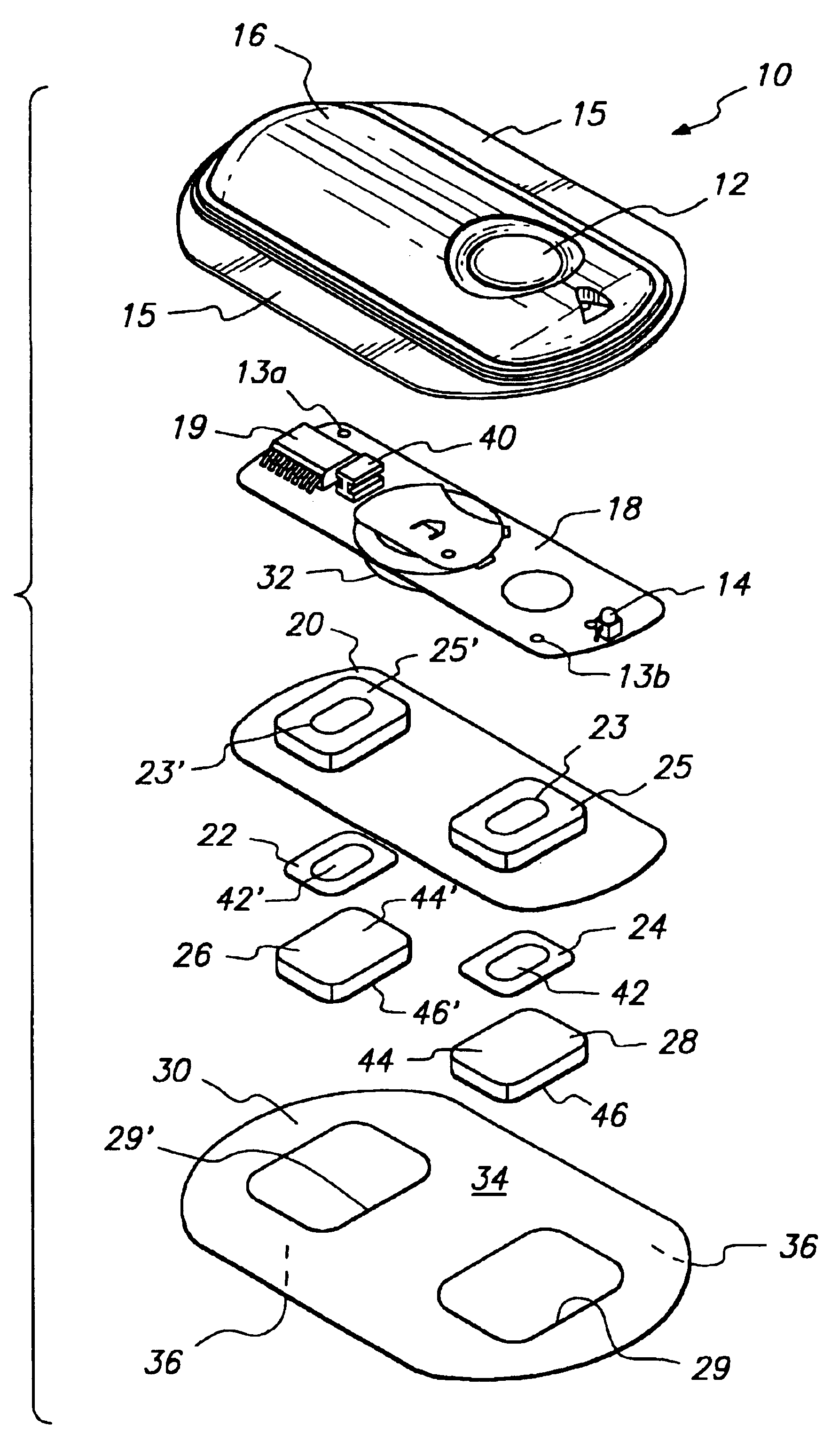
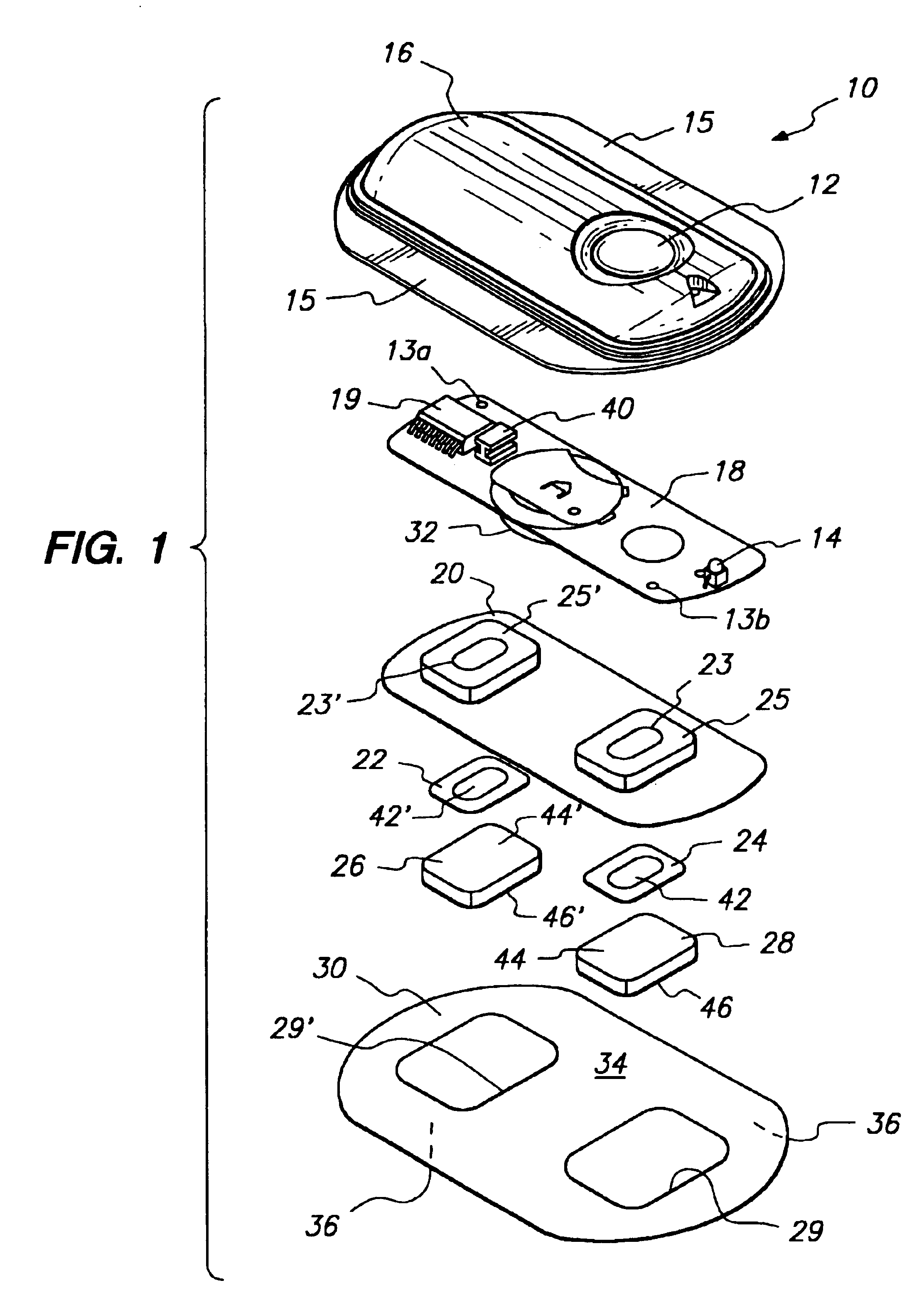
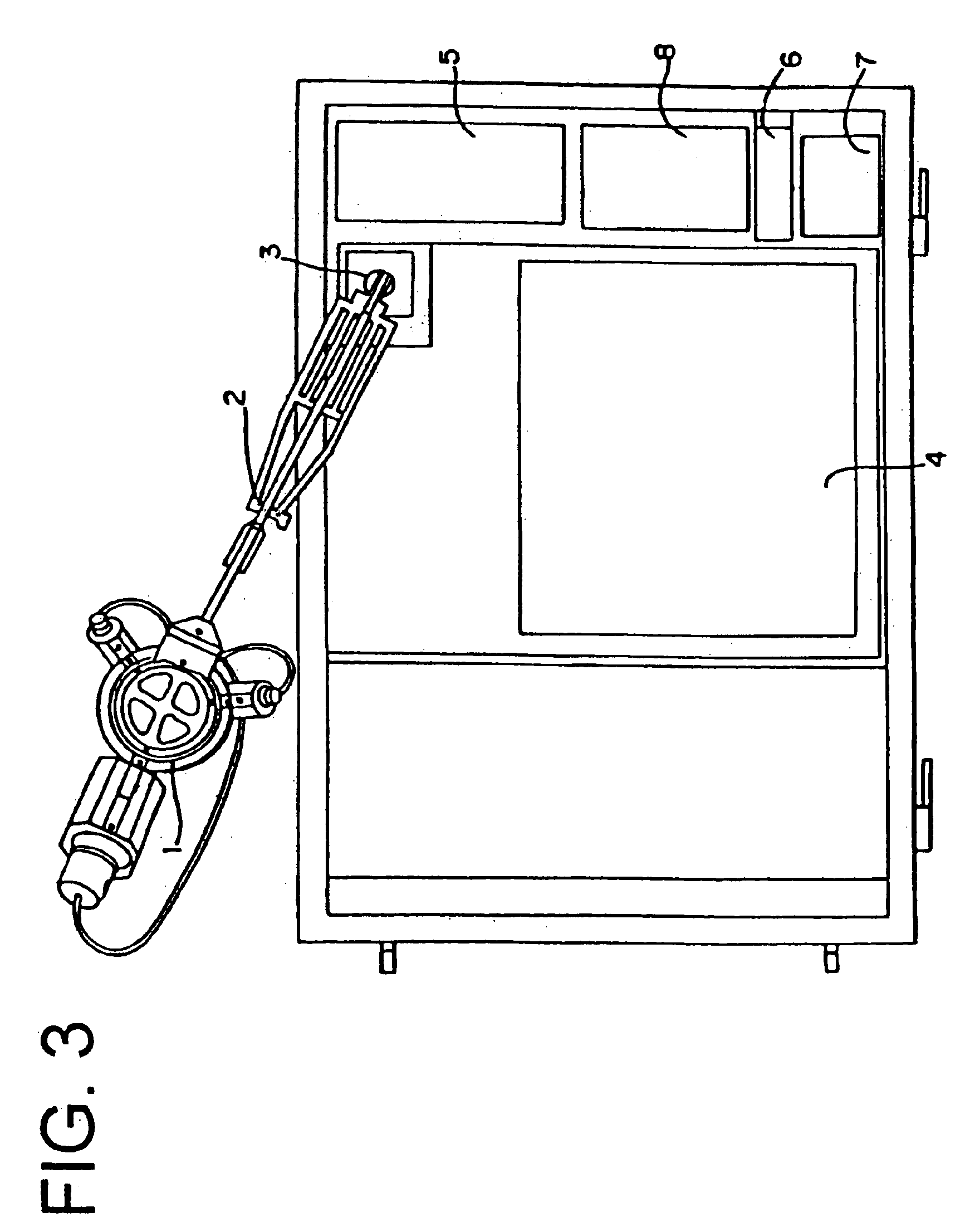
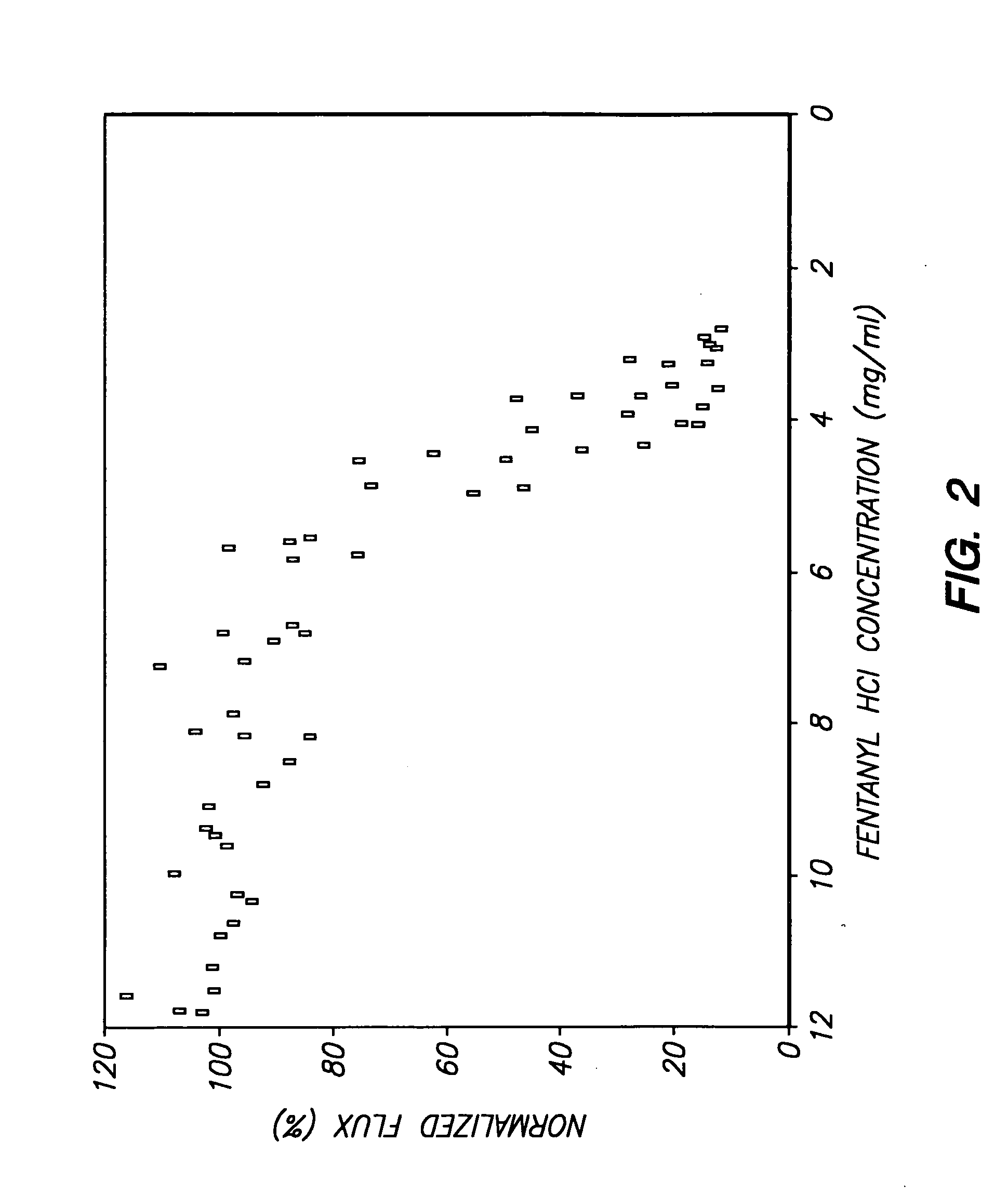
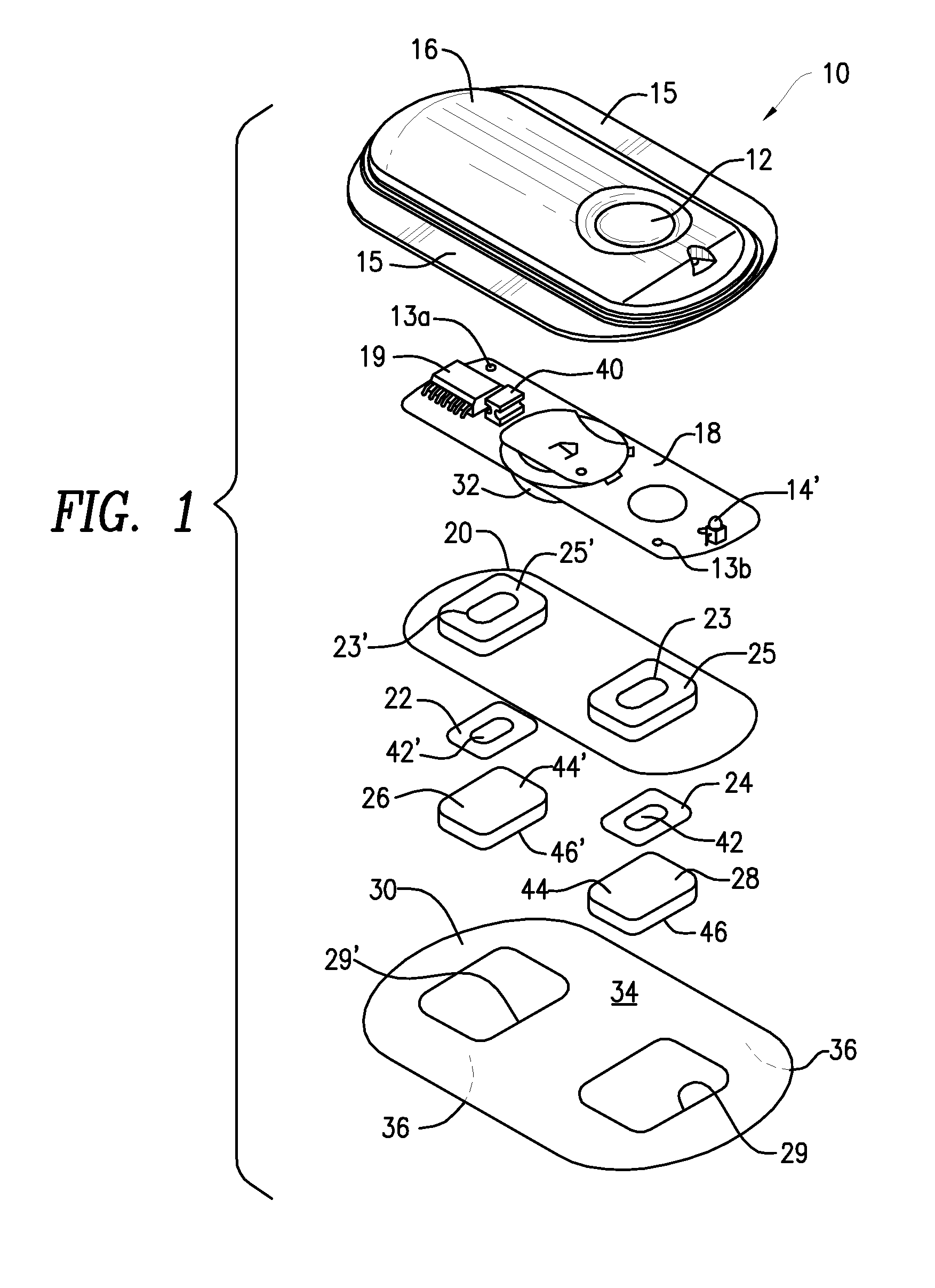
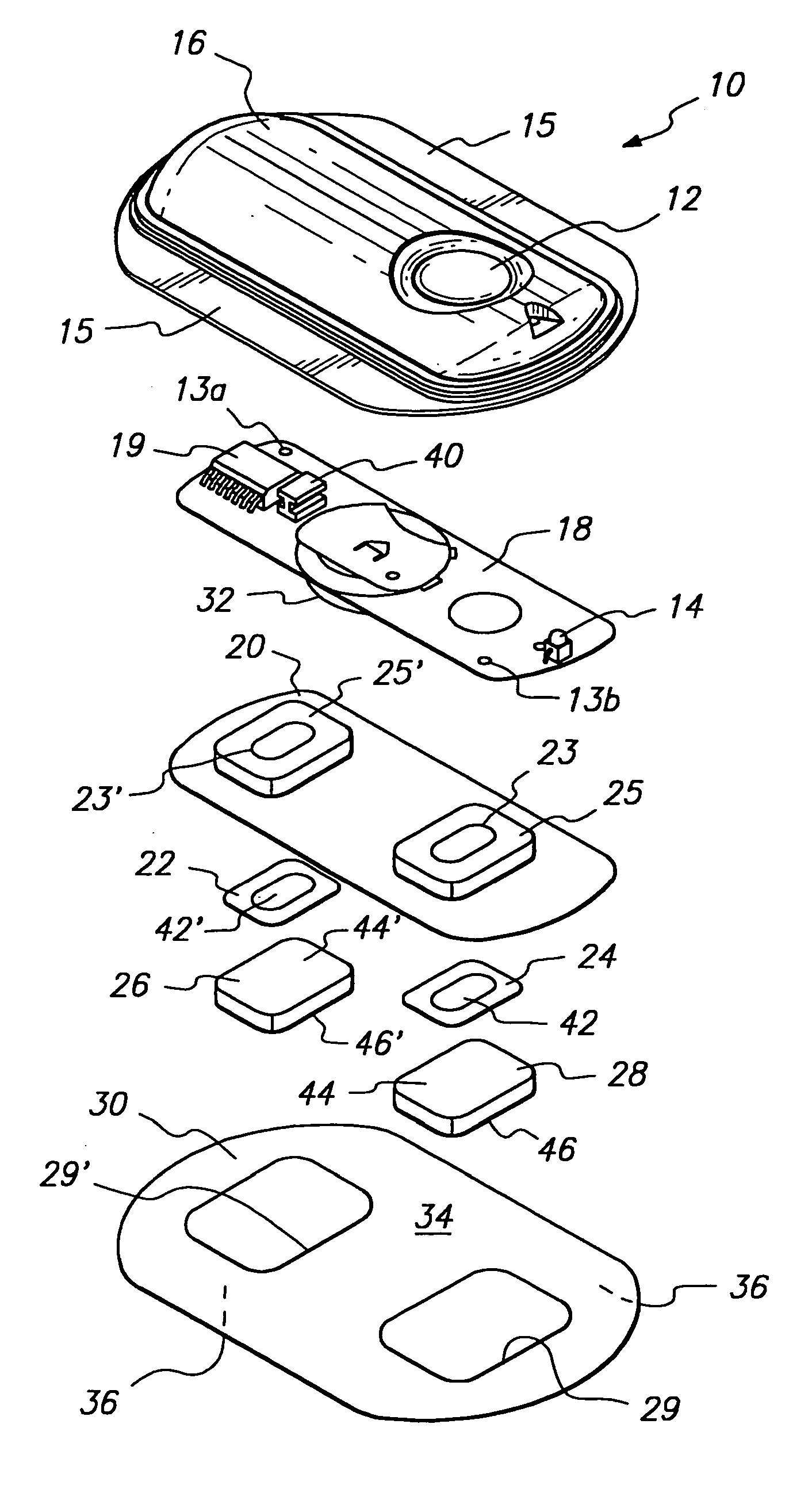
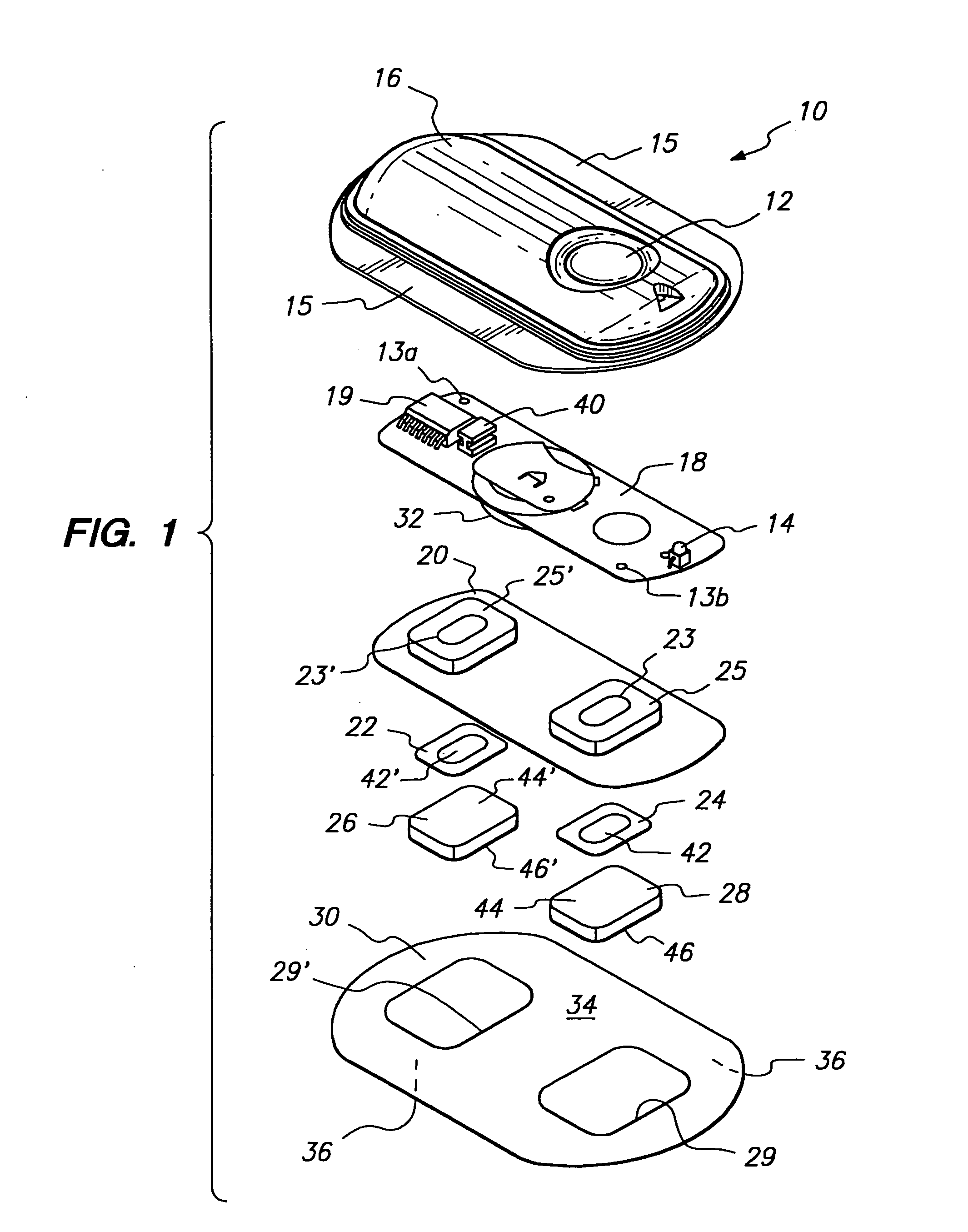
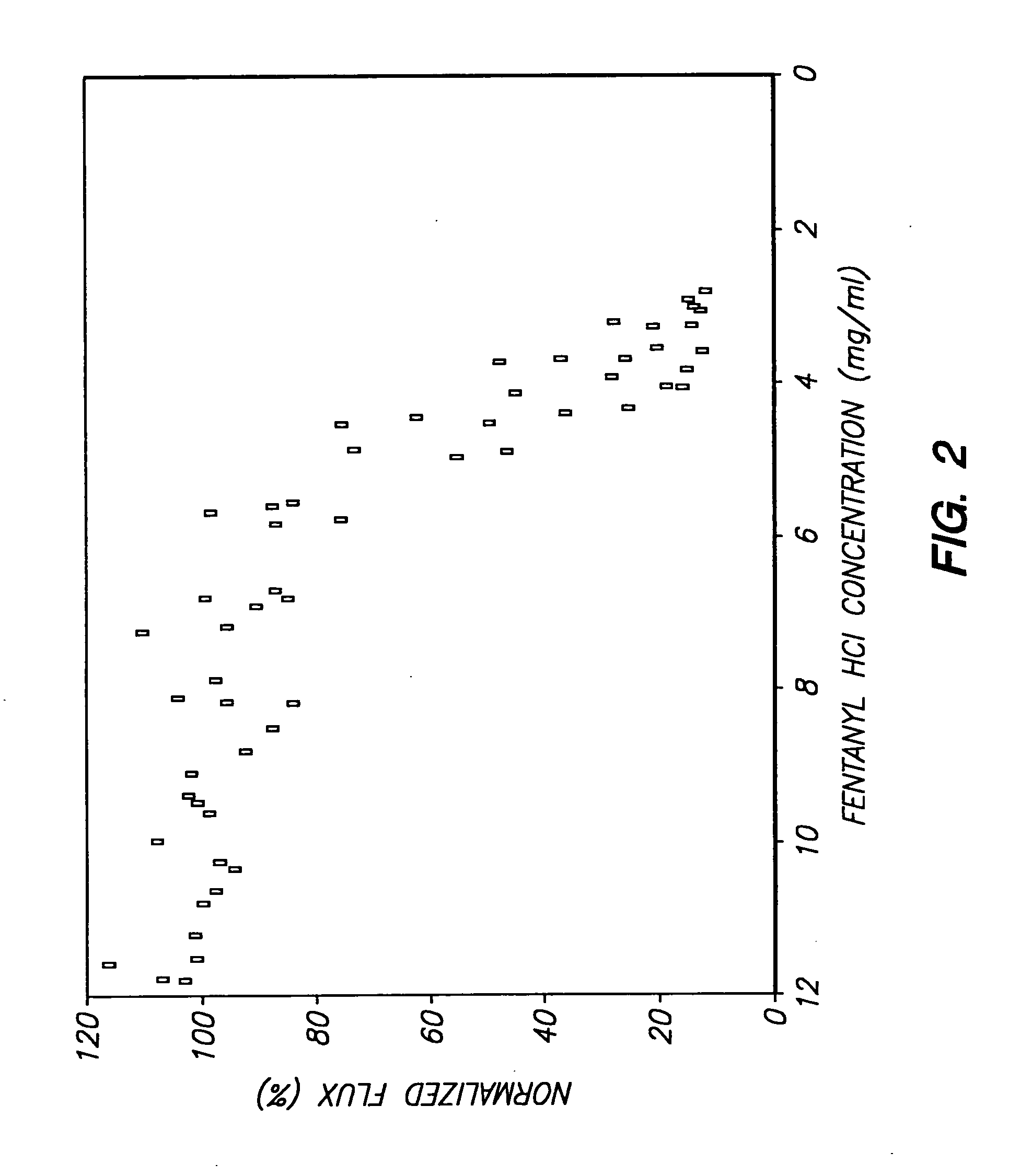
Device for transdermal electrotransport delivery of fentanyl and sufentanil
InactiveUS7018370B2Improved transdermal electrotransport deliveryImprove efficiencyOrganic active ingredientsElectrotherapyAnalgesics drugsHuman patient
The invention provides an improved electrotransport drug delivery system for analgesic drugs, namely fentanyl and sufentanil. The fentanyl / sufentanil is provided as a water soluble salt (e.g., fentanyl hydrochloride), preferably in a hydrogel formulation, for use in an electrotransport device (10). In accordance with the present invention, a transdermal electrotransport delivered dose of fentanyl / sufentanil is provided which is sufficient to induce analgesia in (e.g., adult) human patients suffering from moderate-to-severe pain associated with major surgical procedures.
Owner:ALZA CORP
Method and device for transdermal electrotransport delivery of fentanyl and sufentanil
InactiveUS6881208B1Improved transdermal electrotransport deliveryImprove efficiencyOrganic active ingredientsNervous disorderAnalgesics drugsMedicine
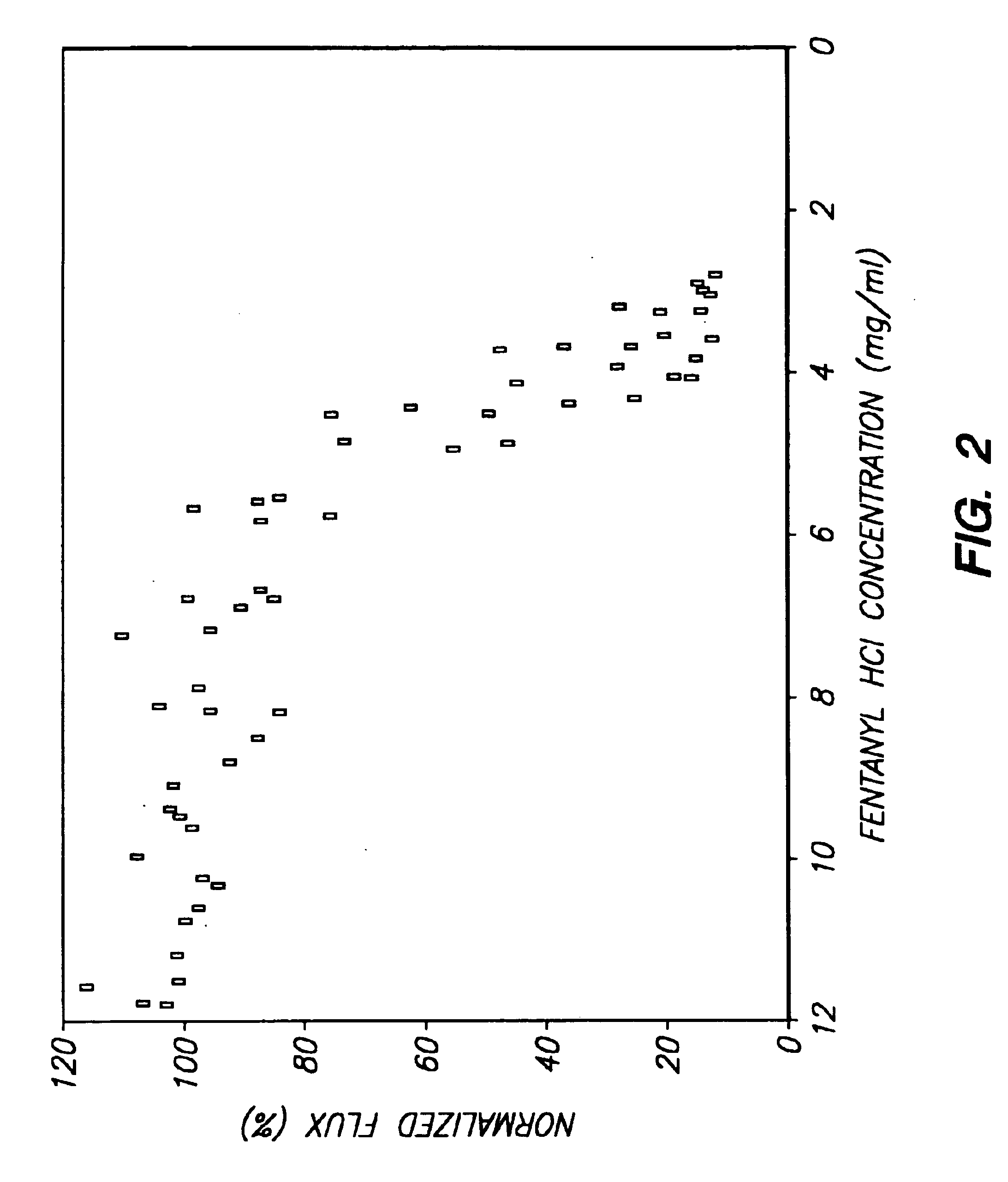
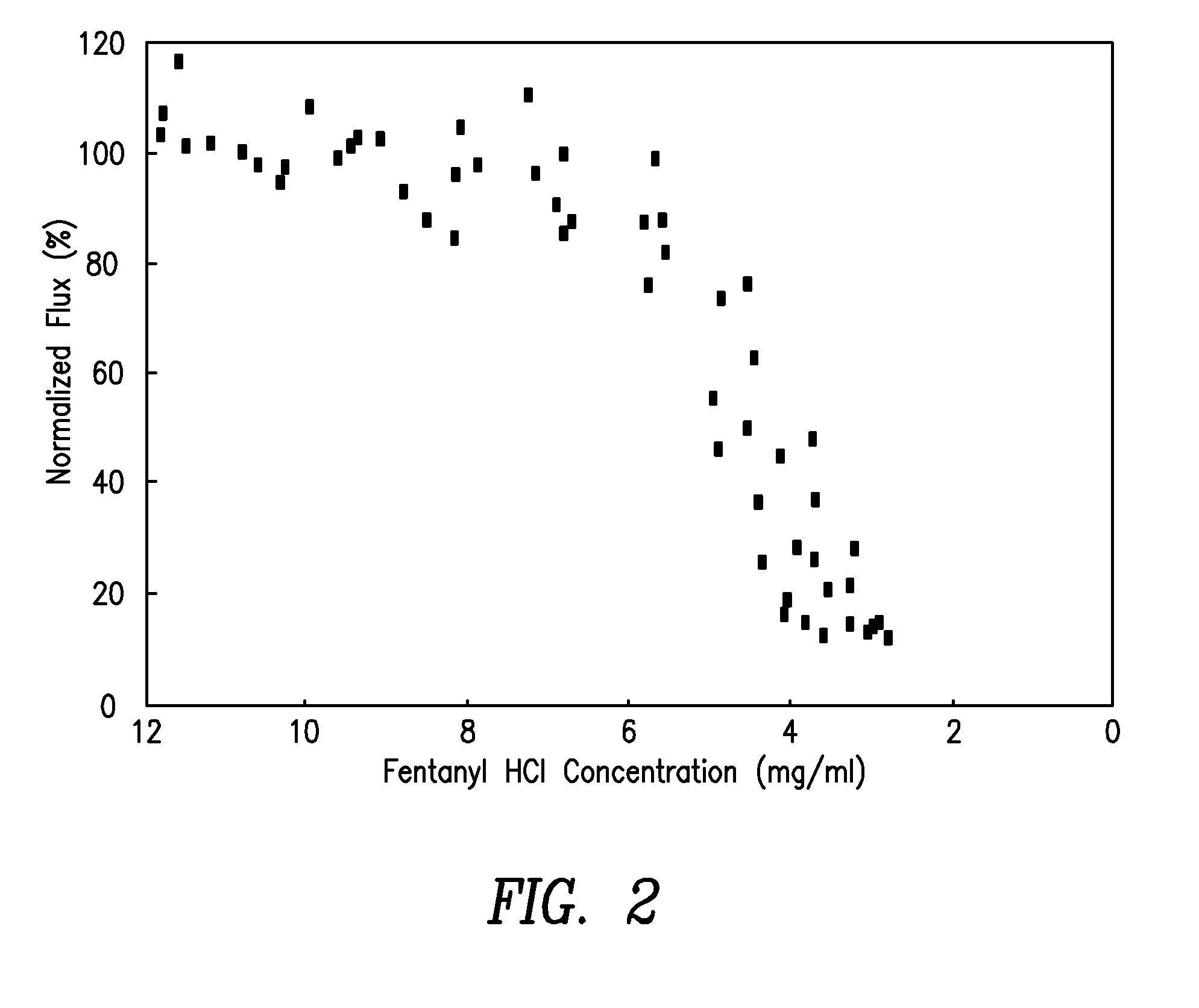
The invention provides an improved electrotransport drug delivery system for analgesic drugs, namely fentanyl and sufentanil. The fentanyl / sufentanil is provided as a water soluble salt (eg, fentanyl hydrochloride) dispersed in a hydrogel formulation for use in an electrotransport device (10). In accordance with one aspect of the invention, the concentration of fentanyl / sufentanil in the donor reservoir (26) solution is above a predetermined minimum concentration, whereby the transdermal electrotransport flux of fentanyl / sufentanil is maintained independent of the concentration of fentanyl / sufentanil in solution. In accordance with a second aspect of the present invention, the donor reservoir (26) of the electrotransport delivery device (10) is comprised of silver and the donor reservoir (26) contains a predetermined “excess” loading of fentanyl / sufentanil halide to prevent silver ion migration with attendant skin discoloration. In accordance with a third aspect of the present invention, a transdermal electrotransport delivered dose of fentanyl / sufentanil is provided which is sufficient to induce analgesia in (eg, adult) human patients suffering from moderate-to-severe pain associated with major surgical procedures.
Owner:ALZA CORP
Anti-Misuse Microparticulate Oral Drug Form
InactiveUS20090041838A1Avoid misuseAvoid fraudulent abuseOrganic active ingredientsPowder deliveryAdditive ingredientMicroparticle
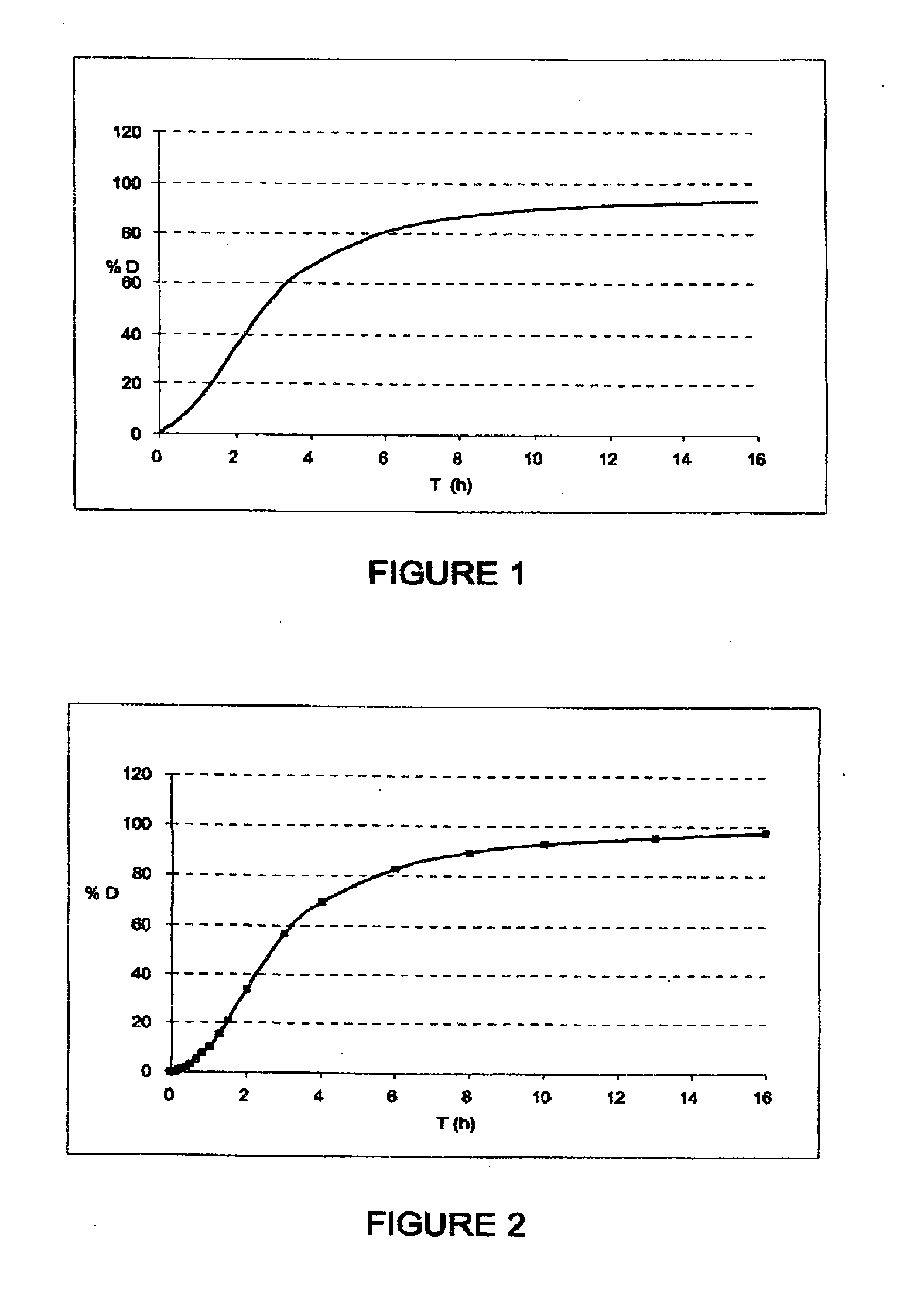
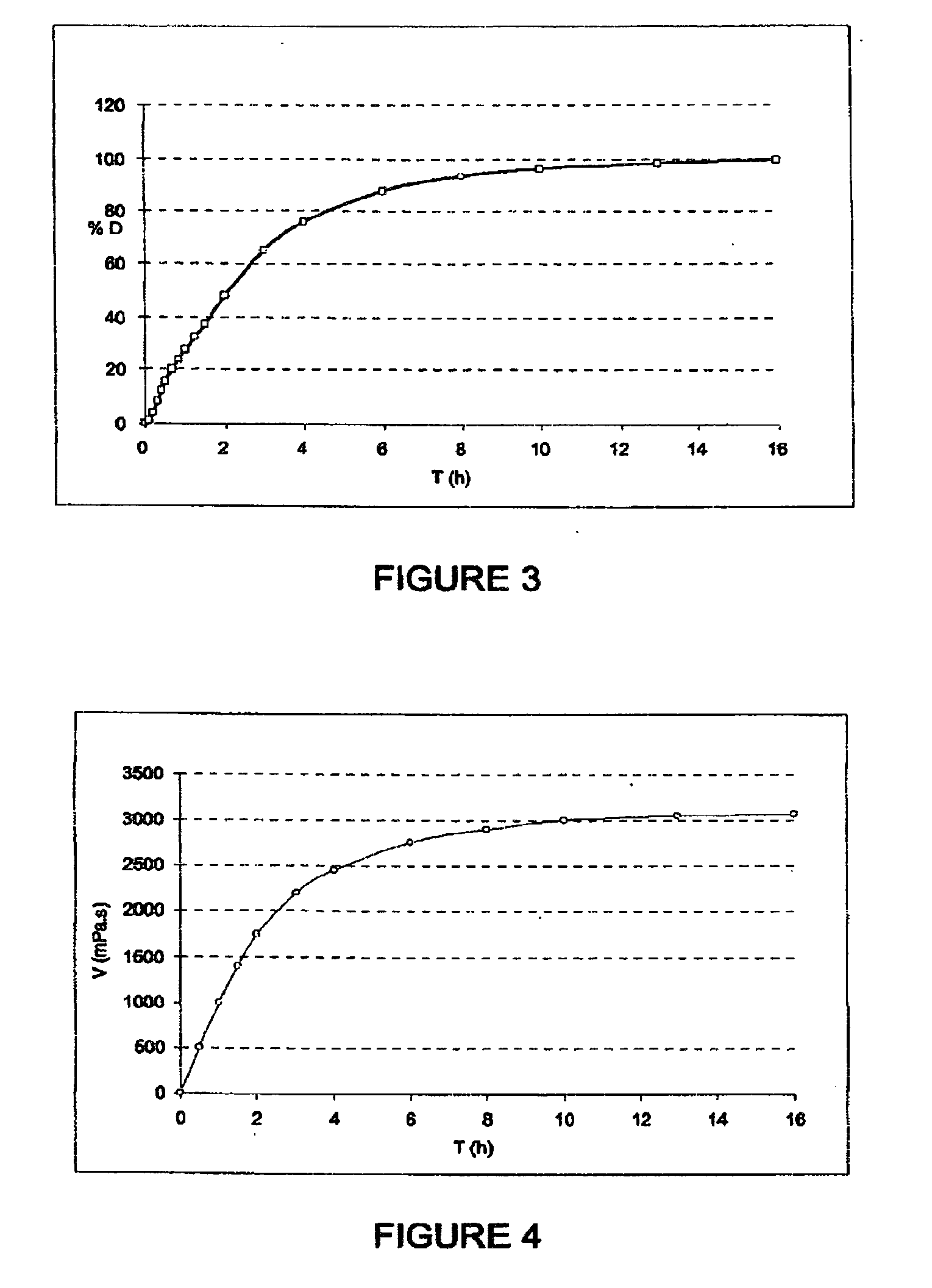
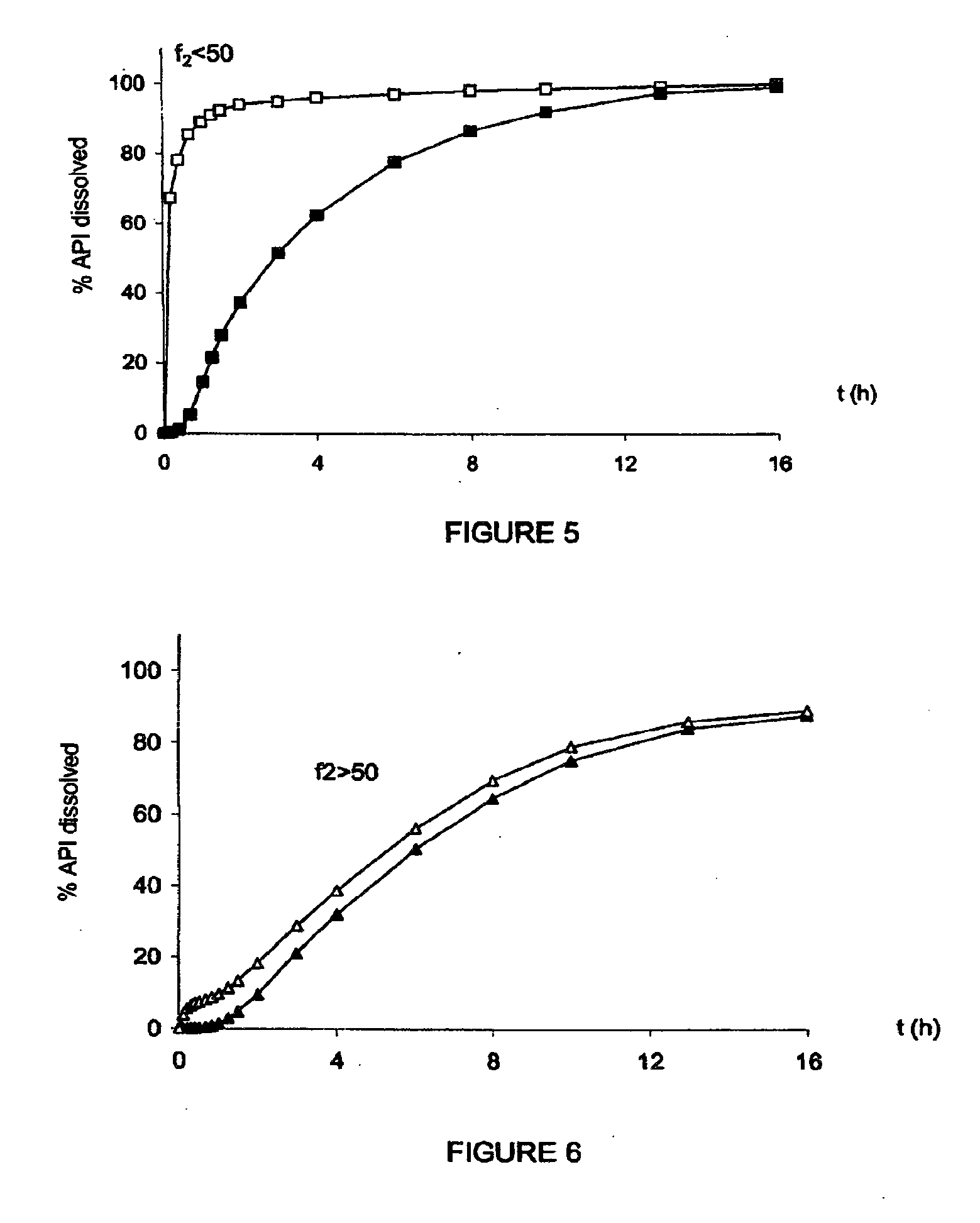
The invention relates to solid microparticulate oral dosage forms having a composition that prevents the misuse of the active pharmaceutical ingredient (API) contained therein. The aim of the invention is to prevent the improper use of solid oral drugs for any use other than the therapeutic use(s) officially approved by the appropriate public health authorities. Another aim of the invention is to provide novel analgesic drugs which can be used to: prevent the misuse of, and addiction to certain analgesics and / or to control plasma concentration variability and / or to facilitate oral; administration; and / or to combine analgesics with one another and / or with one or more active ingredients in the same oral form. More specifically, the invention relates to a solid oral drug form comprising anti-misuse means and at least one active ingredient, which is characterized in that: at least part of the active ingredient is contained in microparticles; and the anti-misuse means comprise anti-crushing means (a) which enable the microparticles of the active ingredient to resist crushing, such as to prevent the misuse thereof. According to the invention, the drug form can also comprise means (b) for preventing the misuse of the active ingredient following a possible liquid extraction process.
Owner:FLAMEL TECHNOLOGIES
Analgesics for nasal administration
InactiveUS20050142072A1Rapid uptakeRapid onsetPowder deliveryBiocideNasal Cavity EpitheliumBlood plasma
An analgesic and a delivery agent are combined in a pharmaceutical composition such that, on introduction into the nasal cavity of a patient to be treated, the analgesic may be delivered to the bloodstream to produce within 30 minutes a therapeutic plasma concentration, Cther, of 0.2 ng / ml or greater which is maintained for a duration Tmaint of at least 2 hours. The analgesic may be an opioid analgesic or a non-steroidal anti-inflammatory drug.
Owner:IONIX PHARMA +1
Combined immediate release and extended release analgesic composition
InactiveUS20060240128A1Lower potentialGood effectPowder deliveryBiocideAnalgesics drugsImmediate release
The present invention pertains to an analgesic composition comprising an analgesic drug in an extended release form in combination with an analgesia-enhancing amount of a nontoxic N-methyl-D-aspartate receptor antagonist in an immediate release form.
Owner:ENDO PHARMA INC
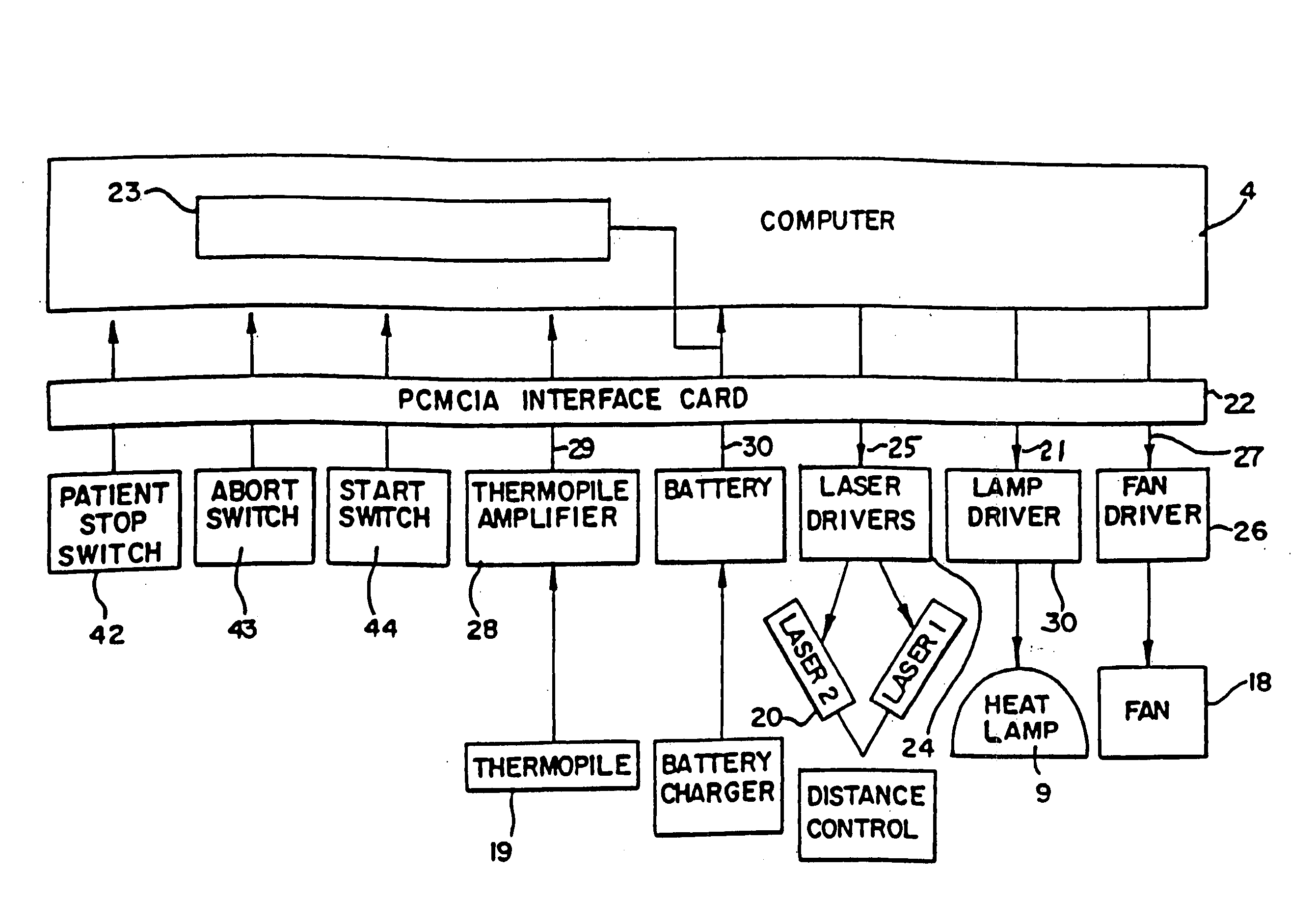
Comprehensive pain assessment systems and methods
Systems, devices, and methods are provided for simultaneous assessment of a subject's subjective and objective pain states. These include a dolorimeter arrangement for determining a subject's cutaneous pain tolerance level at any site on the body. In certain preferred embodiments, the dolorimeter is hand-held and utilizes a sonar distance-measuring device. In another aspect, the systems, devices, and methods of the current invention include platforms effective for implementing pain monitoring methods that include delivering pain questionnaires to patients over a period of time points. The platforms may harvest analgesic drug data from nurses attending the patients, and may provide simple statistical analysis of collected data useful both at the bedside and at central base-stations. The platforms may provide additional functions based on analysis of patient pain data. Preferred embodiments of the current invention combine the dolorimeter arrangement with the platforms for implementing pain monitoring methods.
Owner:NEUROSCI TOOLWORKS
Anti-misuse microparticulate oral drug form
InactiveUS20100266701A1Avoid fraudulent abuseReduce riskBiocidePowder deliveryAnalgesics drugsPublic health
The invention relates to solid microparticulate oral dosage forms having a composition that prevents the misuse of the active pharmaceutical ingredient (API) contained therein. The aim of the invention is to prevent the improper use of solid oral drugs for any use other than the therapeutic use(s) officially approved by the appropriate public health authorities. Another aim of the invention is to provide novel analgesic drugs which can be used to: prevent the misuse of, and addiction to certain analgesics and / or to control plasma concentration variability and / or to facilitate oral; administration; and / or to combine analgesics with one another and / or with one or more active ingredients in the same oral form. More specifically, the invention relates to a solid oral drug form comprising anti-misuse means and at least one active ingredient, which is characterized in that: at least part of the active ingredient is contained in microparticles; and the anti-misuse means comprise anti-crushing means (a) which enable the microparticles of the active ingredient to resist crushing, such as to prevent the misuse thereof. According to the invention, the drug form can also comprise means (b) for preventing the misuse of the active ingredient following a possible liquid extraction process.
Owner:FLAMEL TECHNOLOGIES
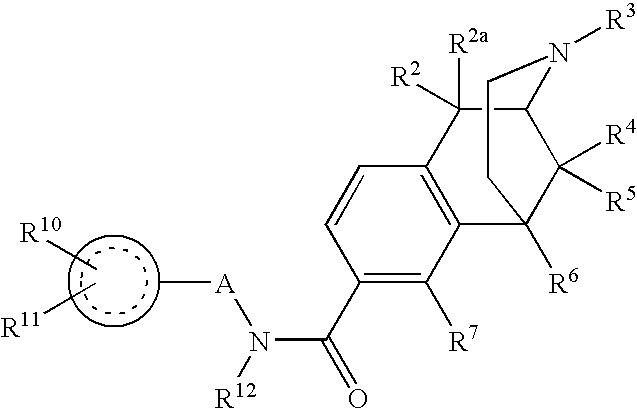
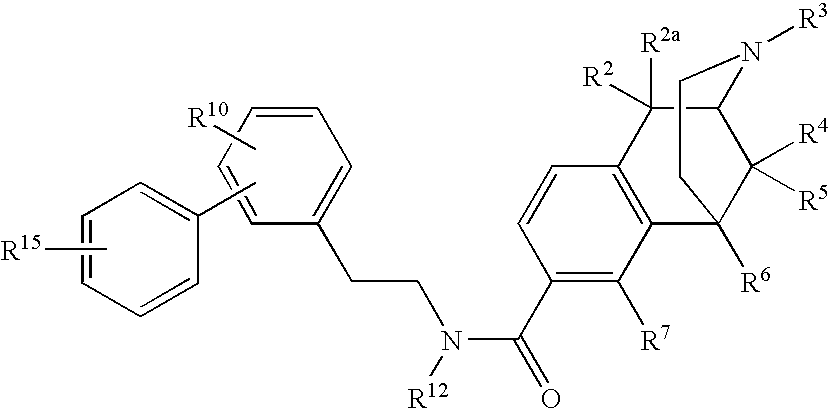
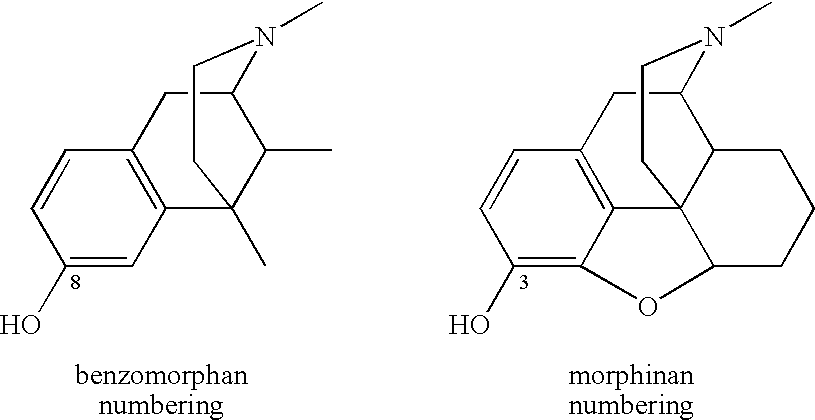
Large Substituent, Non-Phenolic Opioids
ActiveUS20070021457A1Excellent opioid bindingGood metabolic stabilityBiocideNervous disorderBenzazocineAnalgesic agents
8-Substituted-2,6-methano-3-benzazocines of general structure are useful as analgesics, anti-diarrheal agents, anticonvulsants, antitussives and anti-addiction medications. One embodiment is the subgenus of biphenylethyl compounds:
Owner:RENESSELAER POLYTECHNIC INST
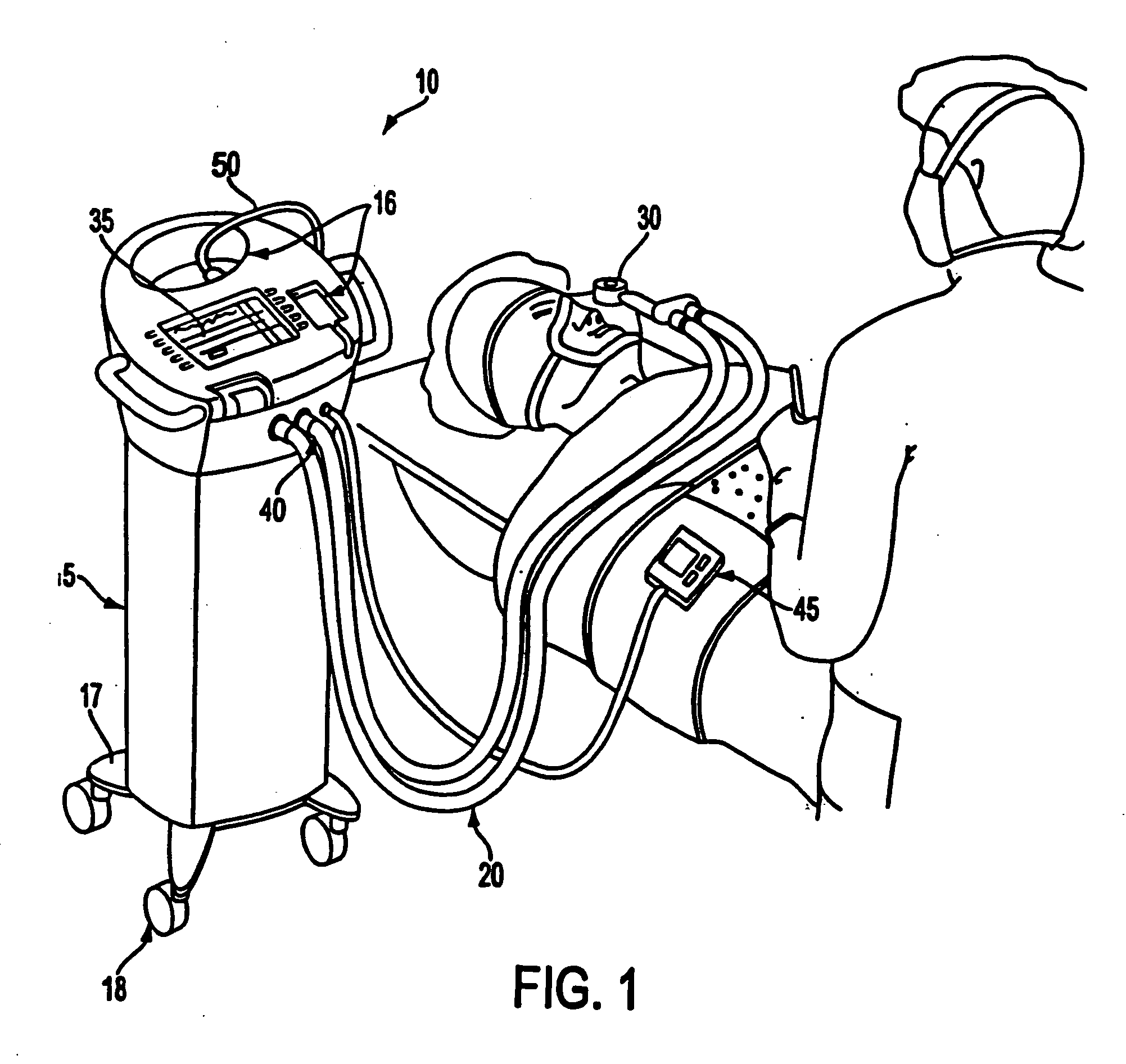
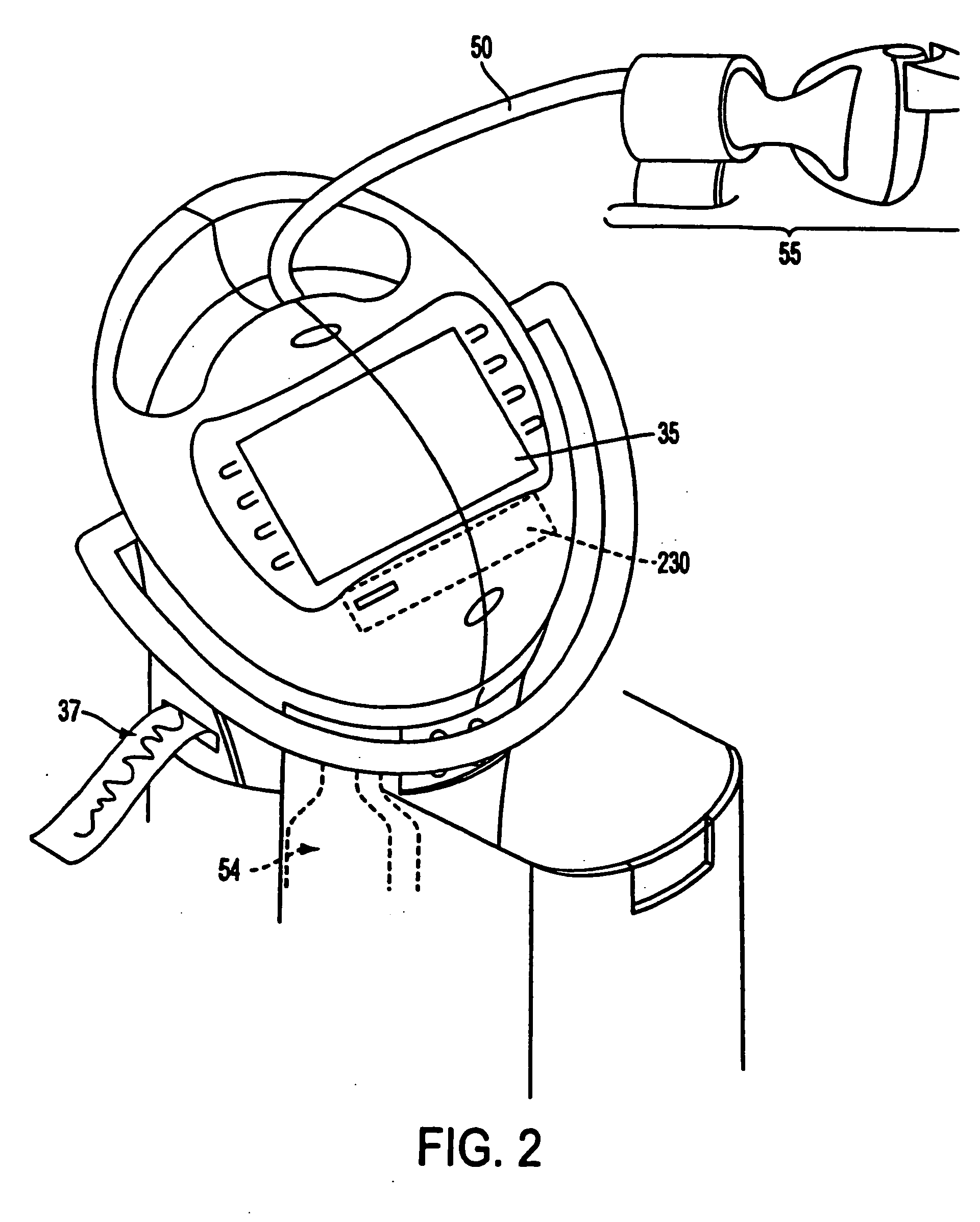
Apparatus, method and drug products for providing a conscious patient relief from pain and anxiety associated with medical or surgical procedures
InactiveUS20060093785A1Safe and effectiveRelieve painRespiratorsNervous disorderSurgical operationAnalgesics drugs
Disclosed is drug delivery for facilitating medical and / or procedures that are performed without “general anesthesia,” which is also described in the specification as the state of patient “unconsciousness” resulting from a drug administered by an anesthetist or anesthesiologist. Mixtures of sedative and analgesic drugs are adapted for safe and effective administration by devices to provide and maintain drug infusions that do not push the patient into unconsciousness and / or general anesthesia. Drug delivery devices are also disclosed that include the use of stored parameters and / or values that correlate to drug mixture delivery during a procedure, and a patient health monitor to measure and send signals regarding a patient health condition to a processor.
Owner:SCOTT LAB
Method and device for transdermal delivery of fentanyl and sufentanil
InactiveUS20050131337A1Improved transdermal electrotransport deliveryImprove efficiencyBiocideNervous disorderAnalgesics drugsMedicine
Owner:ALZA CORP
Medical operation suture thread
InactiveCN101380484AReduce dosageAchieve long-lasting pain reliefSuture equipmentsAnalgesics drugsEnteral administration
The invention discloses a medical suture that contains an analgesic drug which can slowly release in a body. The suture is combined with the analgesic drug. The medicine administration is simultaneously accomplished during suture, and the medicine is slowly released from the suture, thereby achieving the long term acesodyne effect on the sutured part. Due to the partial medicine administration, the medicine dosage is greatly reduced compared with the whole body medicine administration, and the side effect on the whole body is remarkably reduced while the partial effective pain stop is achieved.
Owner:李捷
Systems and methods for providing gastrointestinal pain management
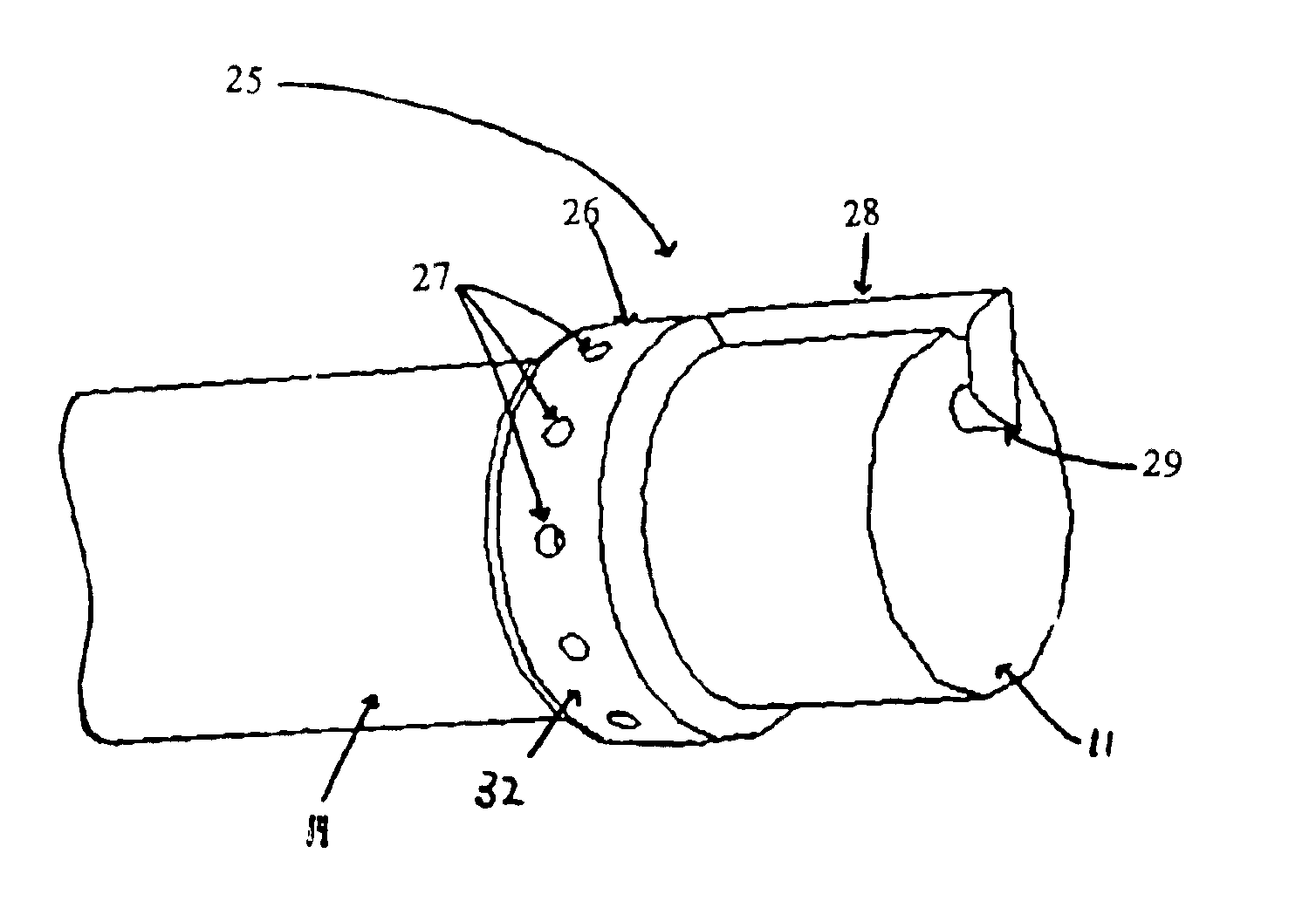
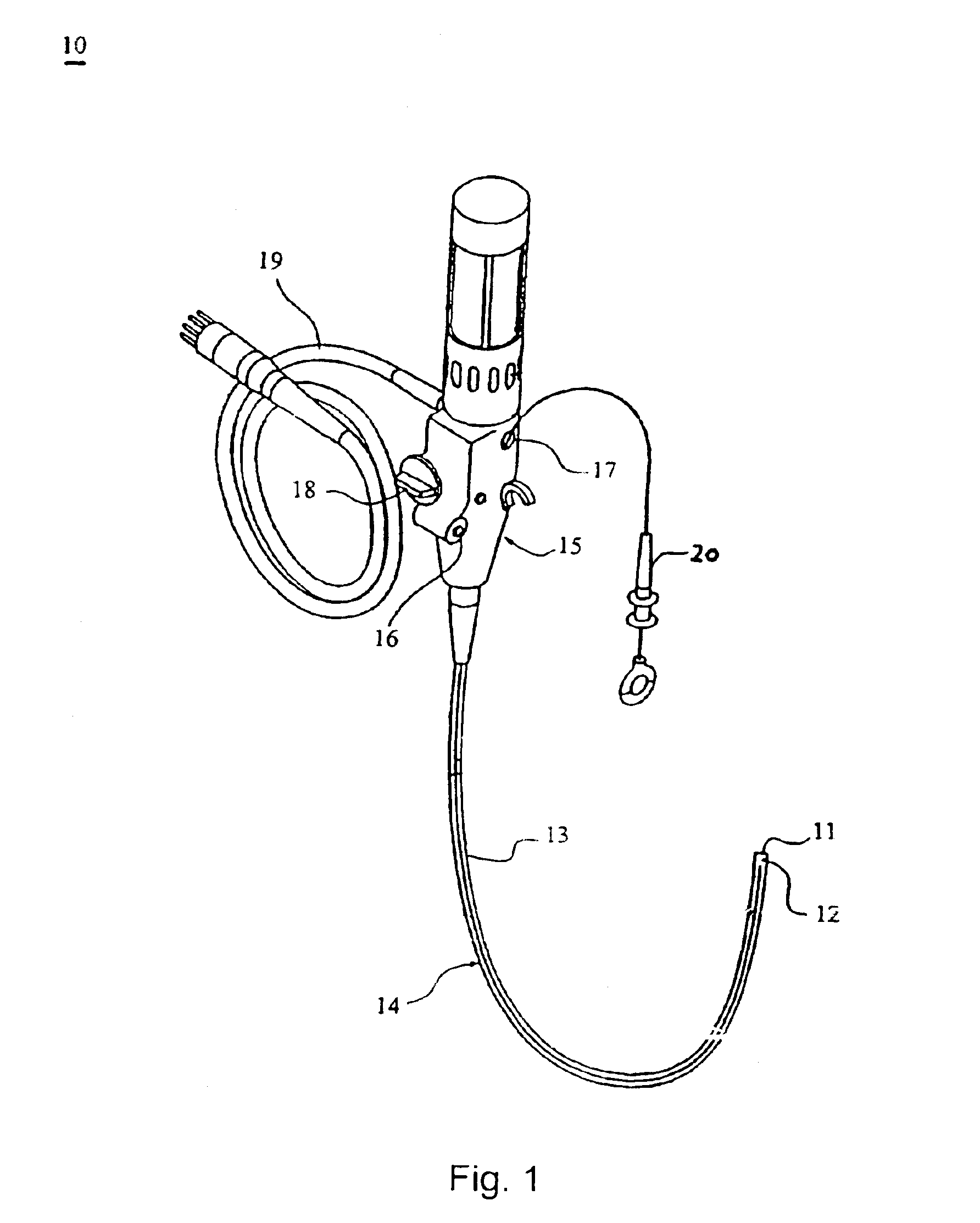
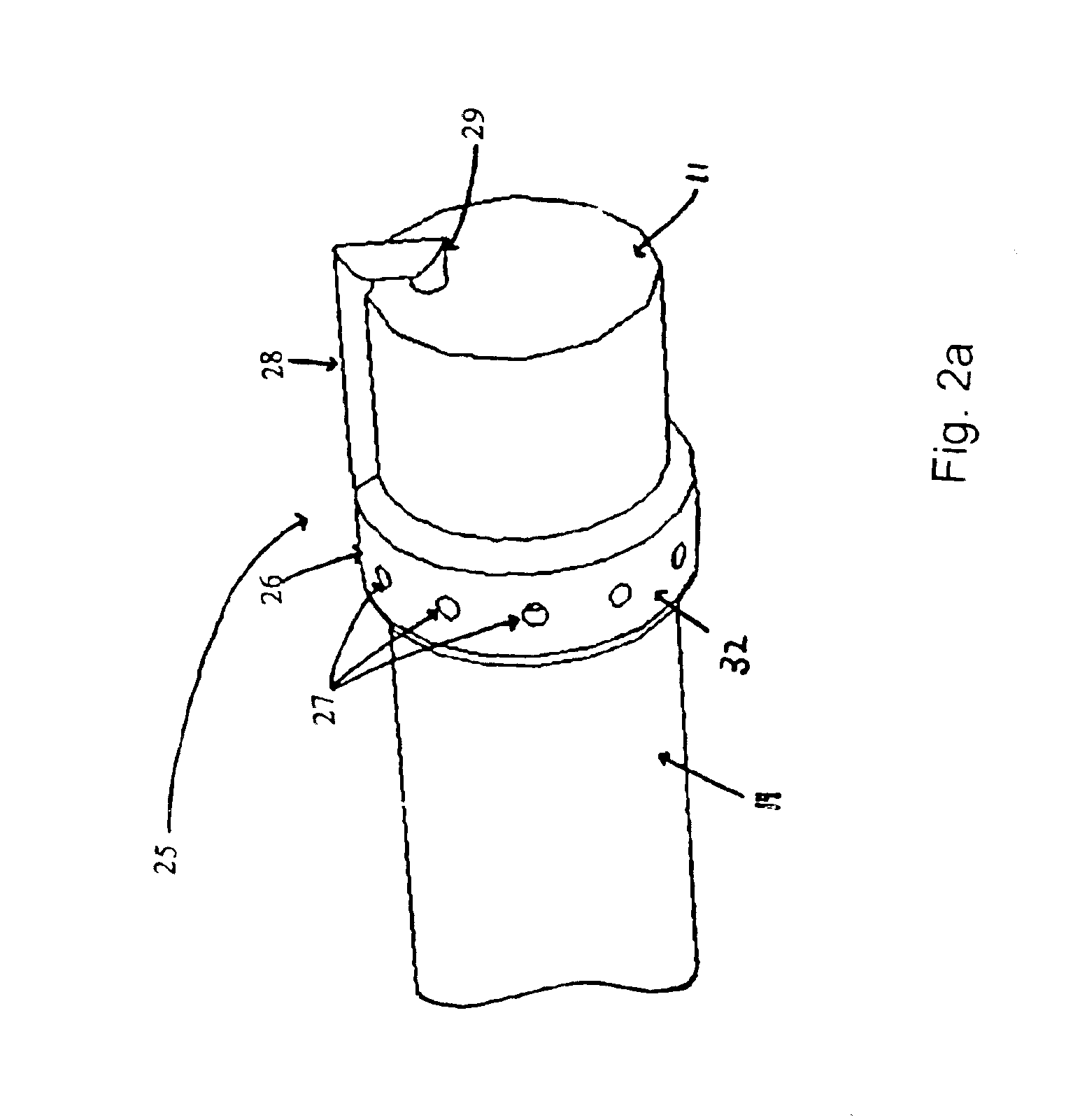
The present invention includes systems and methods for decreasing the pain and discomfort commonly associated with endoscopic procedures, where such procedures may be performed with lower dosage levels of sedative and analgesic drugs. The invention includes use of an anesthetic collar coupled to an endoscope with a flexible shaft. The anesthetic collar allows lubricants, local anesthetics, dyes, and / or other desirable fluids to be passed through the existing lumen of the flexible shaft into an annulus, where the fluid may be distributed through expulsion pores into the gastrointestinal tract. Utilizing the existing lumens found in endoscopes, the present invention allows those fluids that may reduce the pain and discomfort associated with endoscopies such as, for example, local anesthetics and lubricants, to be distributed in an even fashion throughout the gastrointestinal tract or throughout the length and circumference of the endoscope, where such fluids may reduce the drug level requirements for sedative and analgesic agents. Alternatively, the endoscope may be redesigned for streamlined integration with the anesthetic collar or to accomplish the same function of distributing local anesthetics and lubricants, in an even fashion throughout the gastrointestinal tract or throughout the length and circumference of the endoscope, The invention can also be used with endoscopes without existing lumens.
Owner:SCOTT LAB
Method and Device for Transdermal Electrotransport Delivery of Fentanyl and Sufentanil
InactiveUS20090264855A1Improved transdermal electrotransport deliveryImprove efficiencyOrganic active ingredientsNervous disorderAnalgesics drugsMedicine
The invention provides an improved electrotransport drug delivery system for analgesic drugs, namely fentanyl and sufentanil. The fentanyl / sufentanil is provided as a water soluble salt (eg, fentanyl hydrochloride) dispersed in a hydrogel formulation for use in an electrotransport device (10). In accordance with one aspect of the invention, the concentration of fentanyl / sufentanil in the donor reservoir (26) solution is above a predetermined minimum concentration, whereby the transdermal electrotransport flux of fentanyl / sufentanil is maintained independent of the concentration of fentanyl / sufentanil in solution. In accordance with a second aspect of the present invention, the donor reservoir (26) of the electrotransport delivery device (10) is comprised of silver and the donor reservoir (26) contains a predetermined “excess” loading of fentanyl / sufentanil halide to prevent silver ion migration with attendant skin discoloration. In accordance with a third aspect of the present invention, a transdermal electrotransport delivered dose of fentanyl / sufentanil is provided which is sufficient to induce analgesia in (eg, adult) human patients suffering from moderate-to-severe pain associated with major surgical procedures.
Owner:ALZA CORP
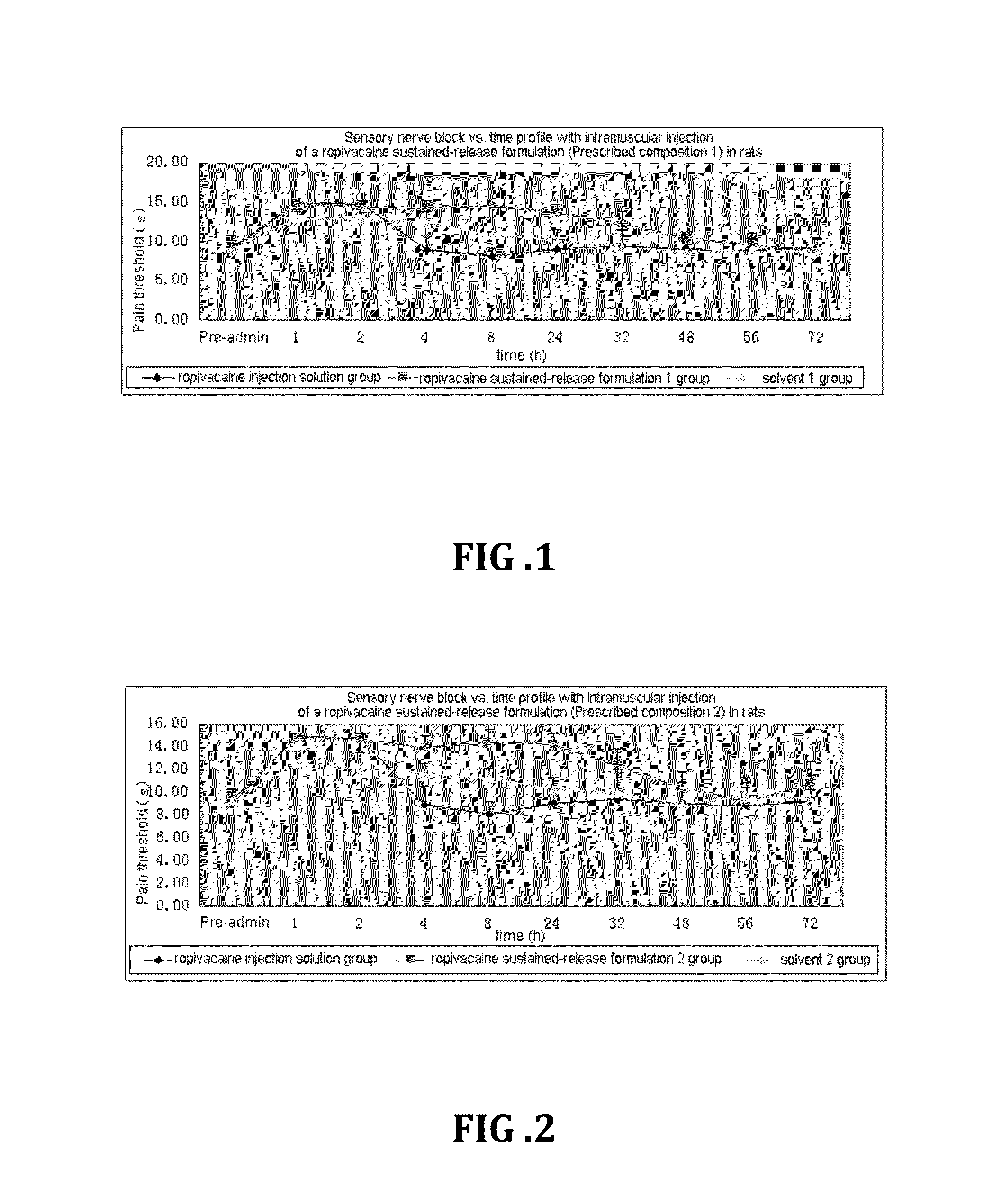
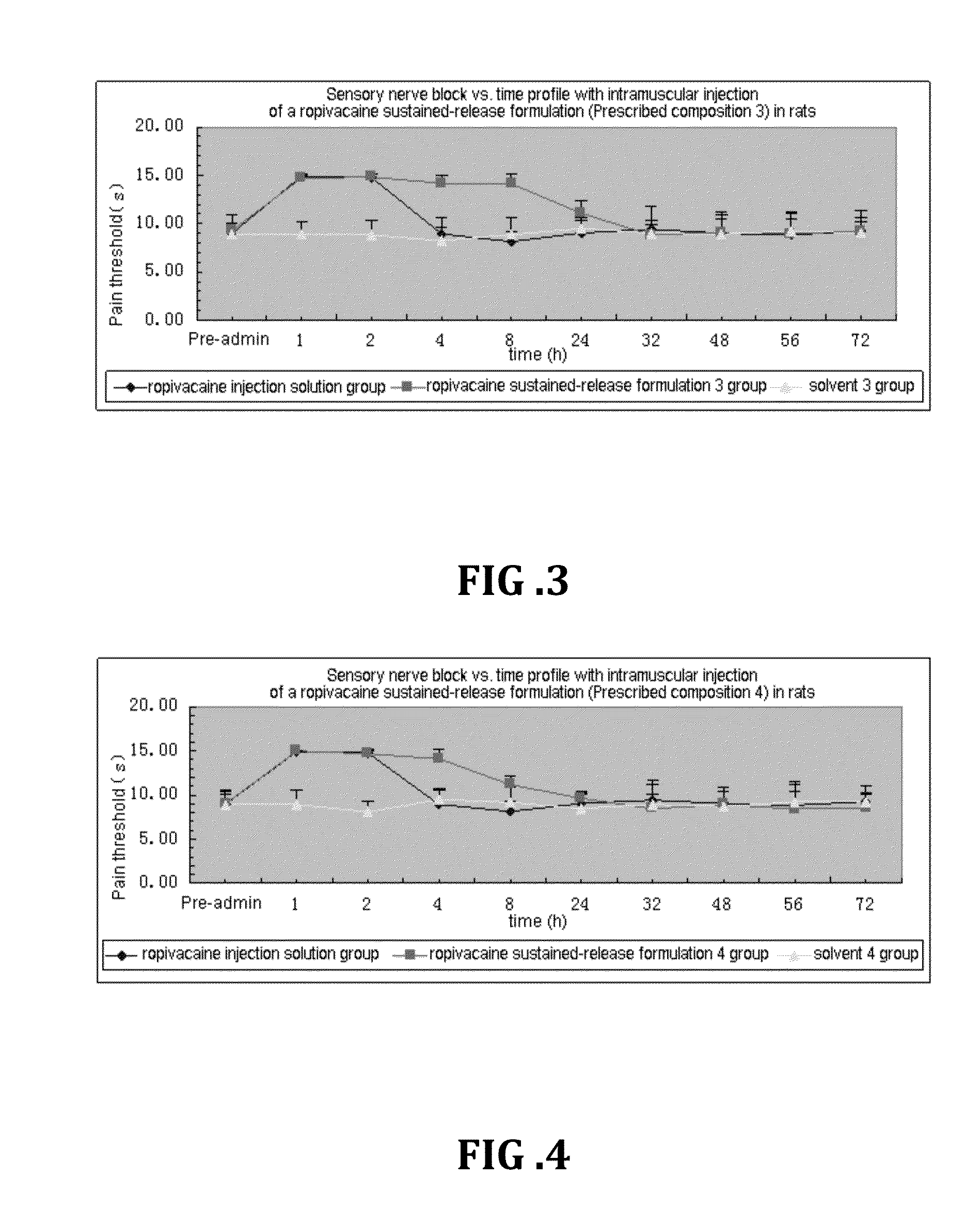
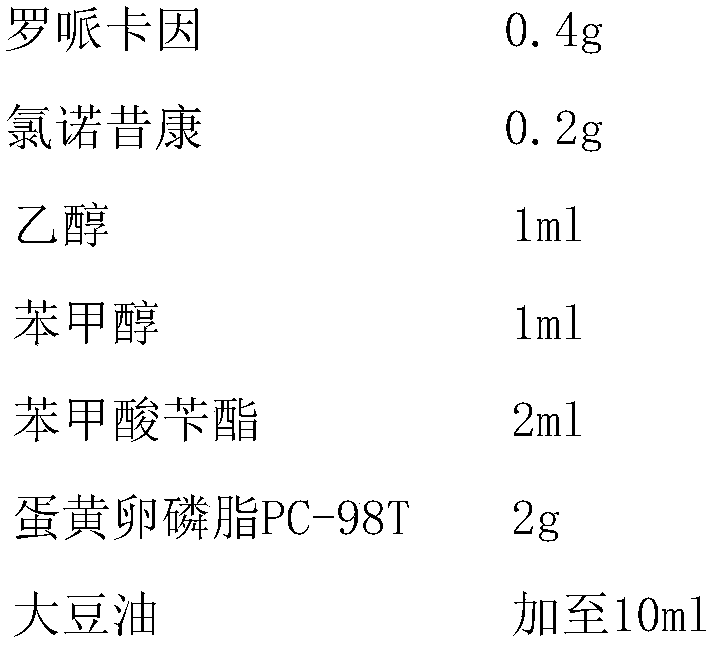
Non-addictive analgesic sustained-release drug delivery system and preparation method thereof
ActiveUS20150359891A1Improve drug deliveryExcellent mutual solubilizationBiocideNervous disorderEthyl lactateBENZYL ALCOHOL/WATER
A non-addictive analgesic sustained-release drug delivery system, comprising: (1) a narcotic analgesic drug having a concentration of 1 mg / ml-160 mg / ml, the drug being selected from a group consisting of: a local analgesic drug, and the combination of the local analgesic drug and a nonsteroidal analgesic drug and / or an opioid analgesic drug; (2) a drug menstruum in a proportion of 1%-75% (v / v), the menstruum being selected from a group consisting of benzyl alcohol, ethanol, benzyl benzoate, ethyl lactate, and tetrahydrofurfuryl polyethylene glycol ether; and (3) a drug sustained-release formulation having a proportion of 25%-99% (v / v), the sustained-release formulation being selected from a group consisting of natural vegetable oil, synthetic lipid, artificially improved half-natural lipid and derivative thereof. Also disclosed are a preparation process and use of the sustained-release drug delivery system.
Owner:LIPONT PHARMA
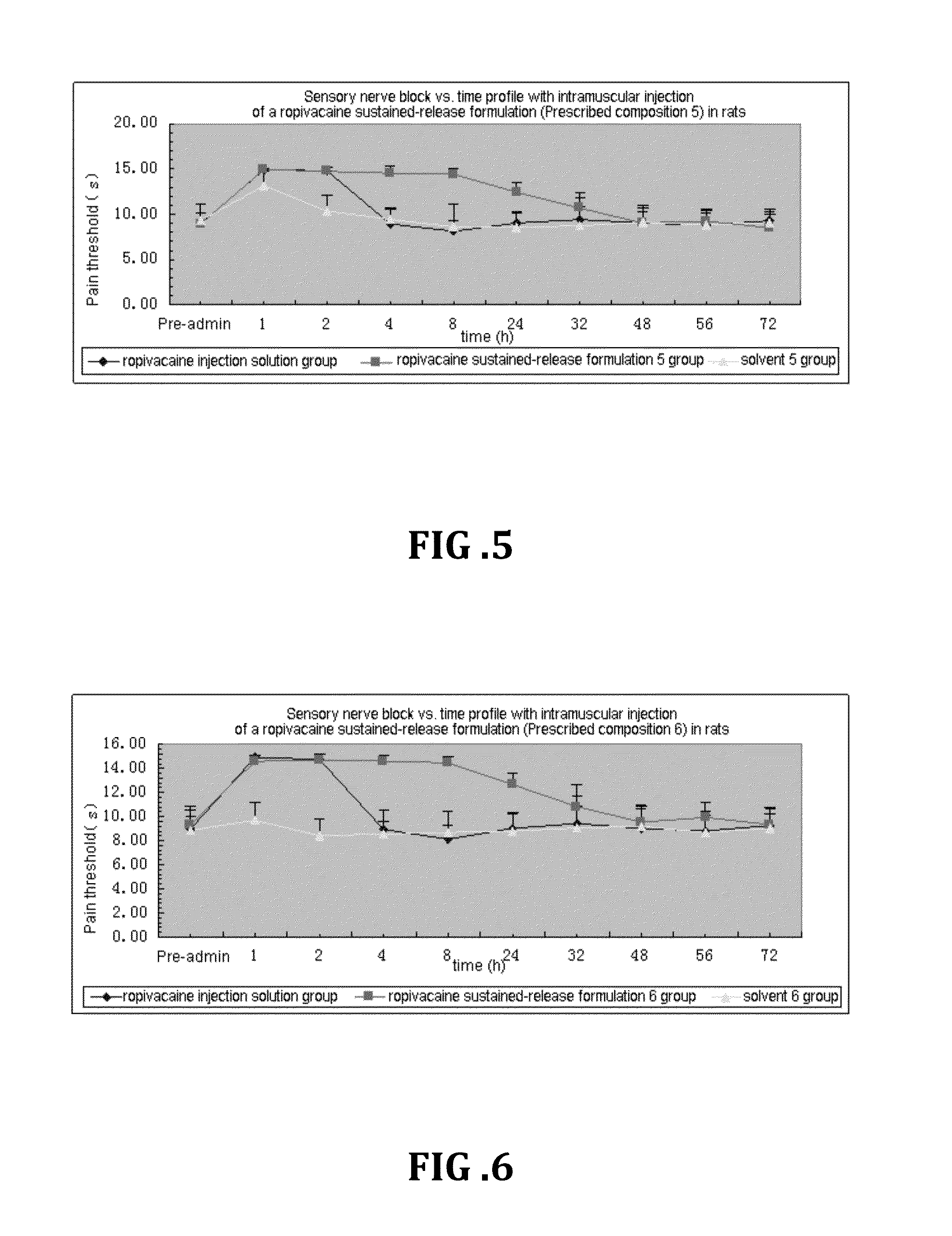
Use of the non-opiate analgesic drug flupirtine for the treatment of overactive bladder and associated diseases including urge incontinence, urinary flow problems as a result of prostate hyperplasia and irritable bowel syndrome
InactiveUS7309713B2Reduce concentrationReduce maintenanceBiocideDigestive systemAnalgesics drugsFecal incontinence
The present invention is directed to the prevention, reversal and medical treatment of lower urinary tract dysfunction including bladder instability and other related diseases as described below including urinary flow problems, urgency and incontinence as a result of prostate hyperplasia (BPH) and to the prevention, reversal and medical treatment of irritable bowl syndrome (IBS) with special focus on the diarrhea-predominant and mixed diarrhea-constipation type IBS, both in human beings and animals.
Owner:RUNDFELDT DR CHRISTIAN
Method and device for transdermal electrotransport delivery of fentanyl and sufentanil
InactiveUS20050171464A1Improved transdermal electrotransport deliveryImprove efficiencyOrganic active ingredientsNervous disorderAnalgesics drugsMedicine
The invention provides an improved electrotransport drug delivery system for analgesic drugs, namely fentanyl and sufentanil. The fentanyl / sufentanil is provided as a water soluble salt (eg, fentanyl hydrochloride) dispersed in a hydrogel formulation for use in an electrotransport device (10). In accordance with one aspect of the invention, the concentration of fentanyl / sufentanil in the donor reservoir (26) solution is above a predetermined minimum concentration, whereby the transdermal electrotransport flux of fentanyl / sufentanil is maintained independent of the concentration of fentanyl / sufentanil in solution. In accordance with a second aspect of the present invention, the donor reservoir (26) of the electrotransport delivery device (10) is comprised of silver and the donor reservoir (26) contains a predetermined “excess” loading of fentanyl / sufentanil halide to prevent silver ion migration with attendant skin discoloration. In accordance with a third aspect of the present invention, a transdermal electrotransport delivered dose of fentanyl / sufentanil is provided which is sufficient to induce analgesia in (eg, adult) human patients suffering from moderate-to-severe pain associated with major surgical procedures.
Owner:ALZA CORP
Composition of compound sustained-release drug delivery system capable of alleviating pain for long term and promoting wound healing and application
PendingCN109316602AControlled burstExtended release timeAntipyreticAnalgesicsAnalgesics drugsPharmacy
The invention belongs to the field of pharmacy, and mainly relates to a composition of a compound sustained-release drug delivery system capable of alleviating pain for a long term and promoting woundhealing and application. The main drug of the system is a composition of a local analgesic drug and a non-steroidal anti-inflammatory drug, and further comprises a solvent and a corresponding sustained-release material. The main drug adopts a local analgesic drug and non-steroidal anti-inflammatory drug compound, the local analgesic drug can remedy the poor pain alleviating effect of the non-steroidal anti-inflammatory drug used alone, and can enhance the pain alleviating effect by reducing the pain sensitivity of an organism, inhibit inflammatory response and promote wound healing; and in addition, the combined use of the local analgesic drug and the non-steroidal anti-inflammatory drug can reduce the administration dosages of the local analgesic drug and the non-steroidal anti-inflammatory drug to reduce respective adverse reactions.
Owner:XIAN LIBANG PHARMA TECH
Antiinflammatory And Analgesic Preparation For External Use
ActiveUS20080031835A1Prevent photosensitivityInhibition effectCosmetic preparationsBiocideAnalgesics drugsSide effect
It is intended to provide an antiinflammatory and analgesic preparation for external use whereby side effects of a nonsteroidal antiinflammatory and analgesic drug on the skin can be regulated, the nonsteroidal antiinflammatory and analgesic drug can be prevented from degeneration with time due to a dibenzoylmethane derivative contained as an UV absorbent therein, the dibenzoylmethane derivative can be prevented from crystallization and sedimentation due to an oily phase component contained therein, and the inherent effects of the nonsteroidal anti-inflammatory and analgesic drug can be fully exerted without showing skin irritation caused by the oily phase component, an emulsifier and a thickener. The above problem can be solved by providing an antiinflammatory and analgesic preparation for external use which comprises a base for external use containing an oily phase component, a nonsteroidal antiinflammatory and analgesic drug and a dibenzoylmethane derivative and in which the total amount of fatty acid esters in the oily phase component is at least thrice as much as the content of the dibenzoylmethane derivative.
Owner:HISAMITSU PHARM CO INC
Medical composite chitosan gel containing analgesic drug
InactiveCN105903068AImprove performanceKeep a humid environmentBandagesAnalgesics drugsMolecular materials
The invention discloses medical composite chitosan gel containing analgesic drug. The medical composite chitosan gel is prepared from, by weight, 0.5-10 parts of chitosan high molecular materials, 0.5-7 parts of analgesic drug, 0-10 parts of thickening agent, 1-25 parts of moistening agent and 48-98 parts of purified water. The medical composite chitosan gel containing the analgesic drug can effectively keep the wet environment for a wound and relieve wound pain; according to the dressing, the chitosan high molecular materials are used as a matrix, the analgesic drug is adopted in an assisted mode, the advantages of moisture absorption, moisturizing, bacteriostasis, hemostasis and the like and the effect of analgesia of the analgesic drug are combined, and the gel with excellent performance is prepared.
Owner:SICHUAN KUIXING MEDICAL POLYMER PROD CO LTD
Endomorphin-1 analogs, synthesis thereof and application of endomorphin-1 analogs in preparation of analgesic medicines
ActiveCN102241737AHigh affinity activityGood analgesic effectAntipyreticAnalgesicsAnalgesics drugsAssay
The invention synthesizes four novel endomorphin-1 analogs, and belongs to the technical field of biochemistry. The endomorphin-1 analogs are prepared by performing N-end guanidyl formation at the first-position Tyr of endomorphin-1, replacing the second-position Pro by D-Ala, replacing the third-position Trp by Gly, and replacing the fourth-position Phe by Trp or performing para-position chlorination and fluorination modification or no modification on a benzene ring of Phe. The synthesized endomorphin-1 analogs are subjected to a series of physiological and pharmacological activity identification such as radio-ligand receptor binding assays, isolated organ bioactivity identification experiments, isolated enzymolysis stability experiments, analgesic tests with water bath and medicine tolerance experiments, show high affinity activity, high enzymolysis stability and high analgesic activity, and hardly cause tolerance. Therefore, the four novel endomorphin-1 analogs can be used for preparing clinical analgesic medicines.
Owner:LANZHOU UNIVERSITY
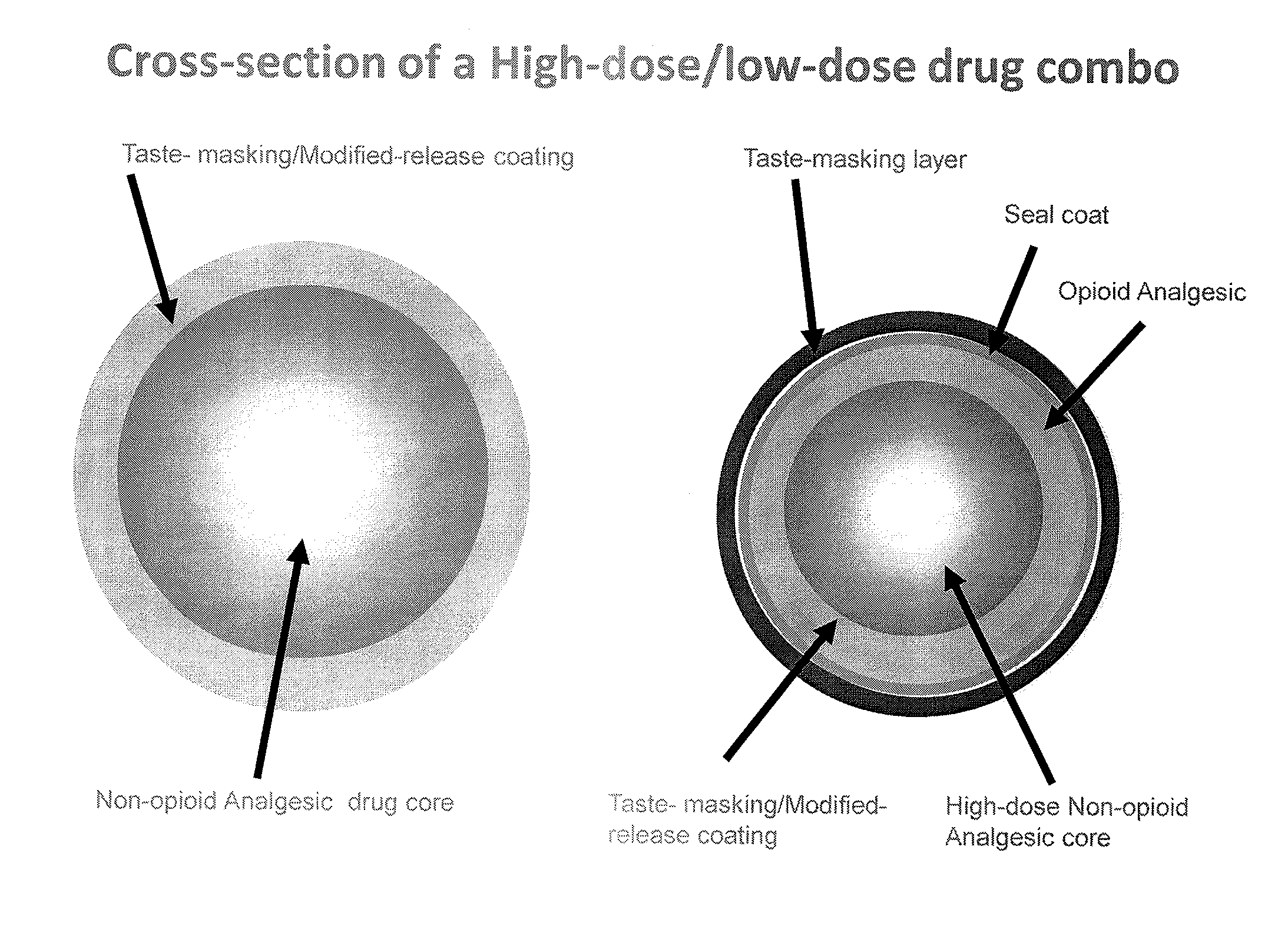
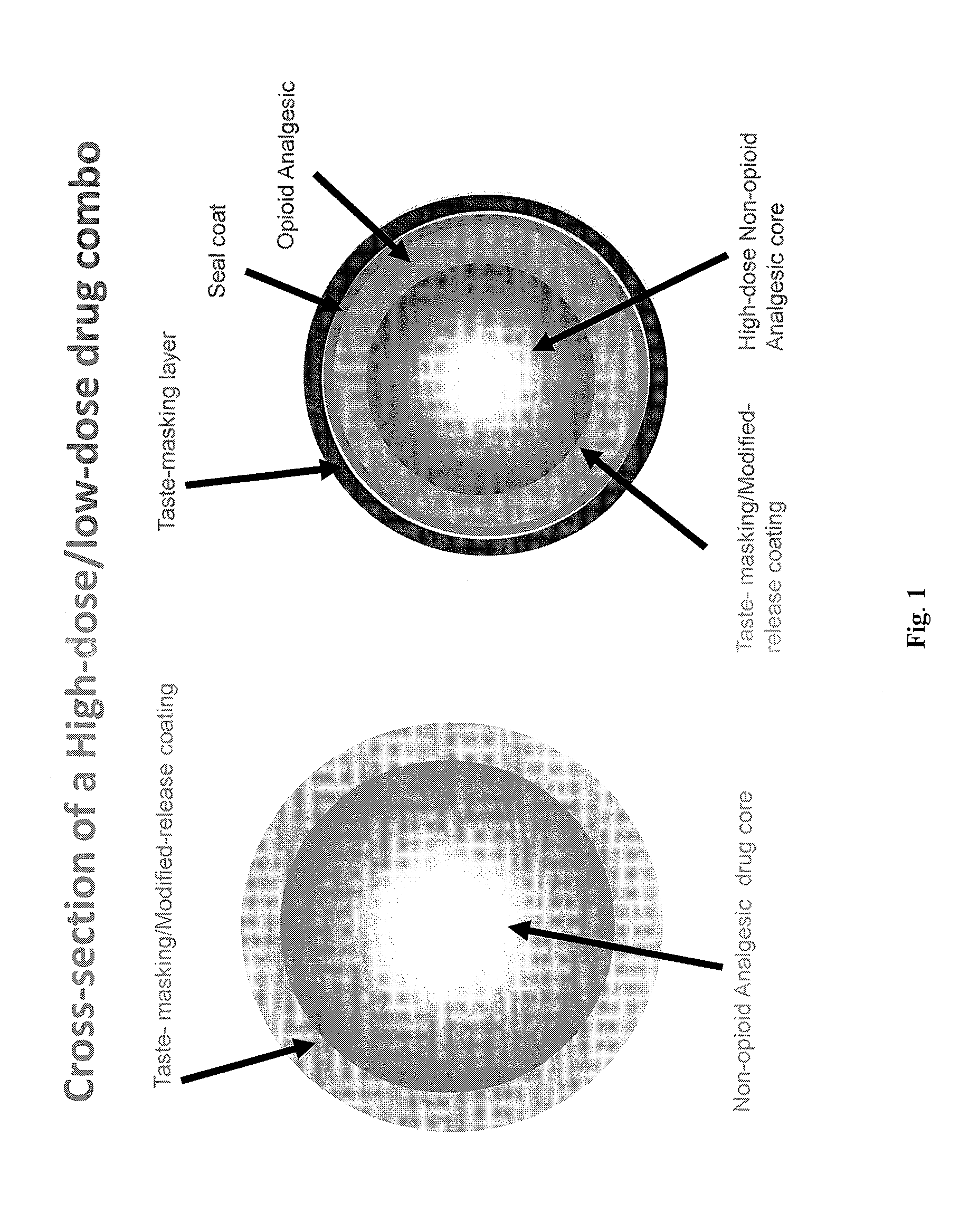
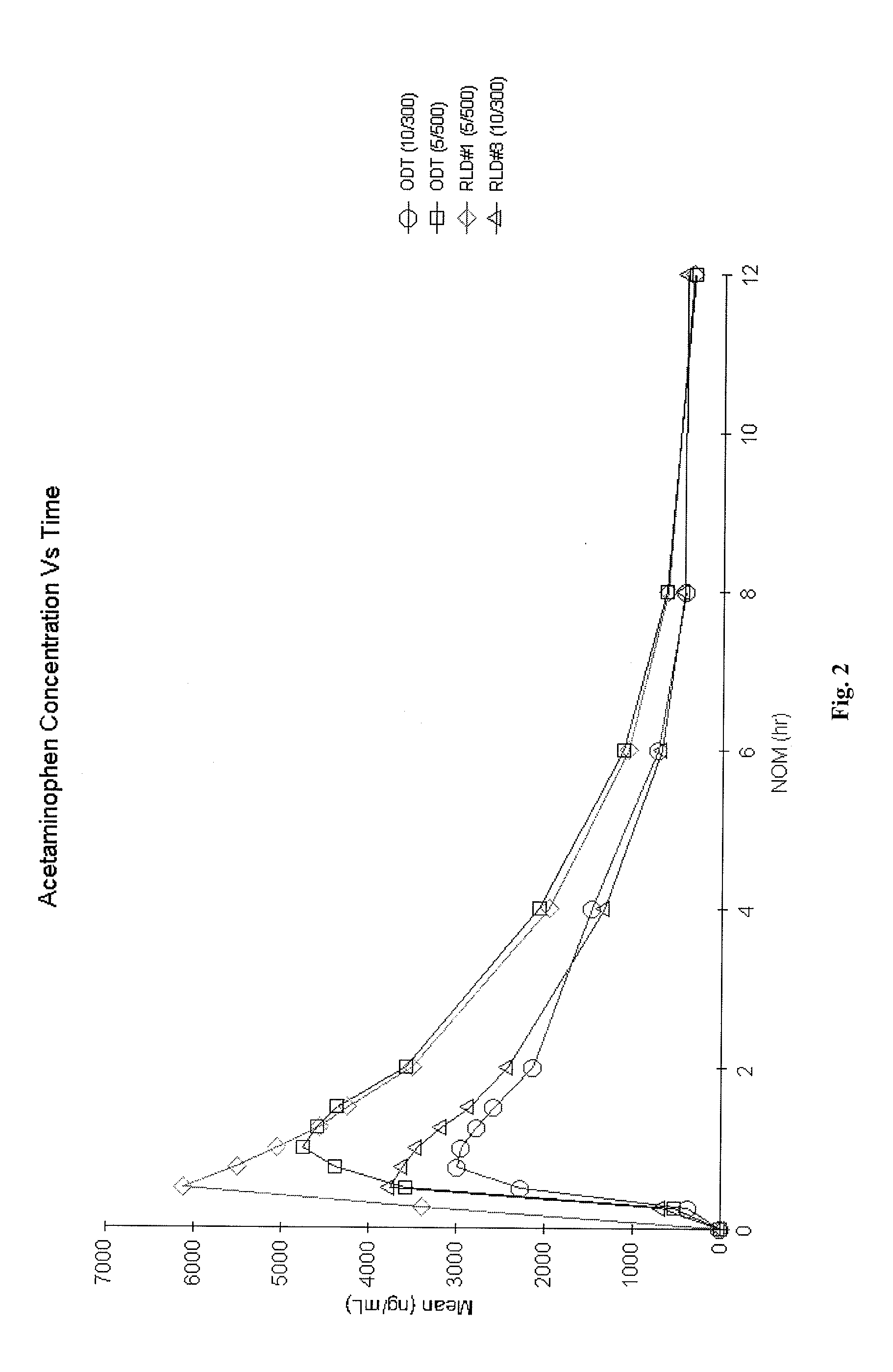
Orally Disintegrating Tablet Compositions Comprising Combinations of Non-Opioid and Opioid Analgesics
The present invention is directed to pharmaceutical compositions comprising a plurality of taste-masked non-opioid analgesic / opioid analgesic drug-containing microparticles, dosage forms comprising such pharmaceutical compositions (such as an orally disintegrating tablet), and methods of making the pharmaceutical compositions and dosage forms of the present invention. Dosage forms comprising the pharmaceutical compositions of the present invention are improved homogeneous blends of non-opioid and opioid analgesics which provide for more convenient and palatable administration of drug combinations, for example for treating pain.
Owner:ADARE PHARM INC
Multi-target peptide molecules of opium and neuropeptide FF receptors, and preparation and application thereof
ActiveCN106084001AStrong central analgesic activityNo significant regulationNervous disorderPeptide/protein ingredientsAnalgesics drugsSide effect
The invention discloses multi-target polypeptides of opium and neuropeptide FF receptors; amino acid substitution is performed based on an opium peptide Biphalin and an NPFF chimeric peptide BN-9, and a series of multi-target polypeptides which can activate various receptors of an opium and NPFF system at the same time are obtained. By in-vitro cAMP function identification and identification of body analgesic activity and central side effects and other pharmacological activities, the multi-target polypeptides are indicated to activate the opium and NPFF receptors at the same time, have high-efficiency analgesic activity and cannot generate the analgesic tolerance phenomenon, and moreover, have the advantages of showing low side effects on body temperature, gastrointestinal motility, cardiovascular activity and the like. Therefore, the multi-target polypeptides have high application value in preparation of clinical analgesic drugs.
Owner:SHANGHAI TIANCI LIFE SCI DEV CO LTD
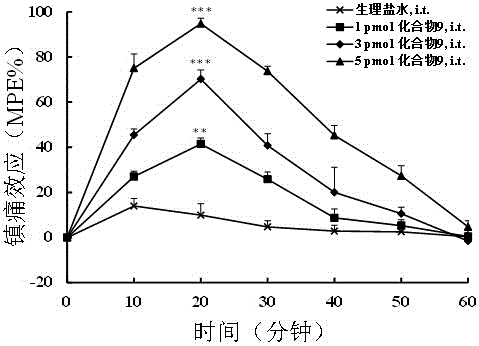
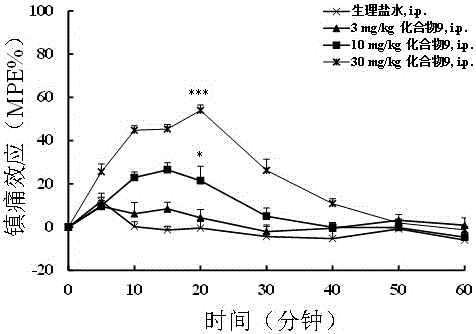
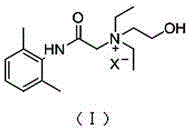
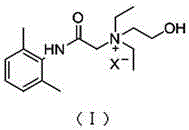
Application of N-acetanilide cationic compound in preparation of local nerve blocking drug
The invention discloses an application of an N-acetanilide cationic compound in preparation of a local nerve blocking drug. The cationic compound is a quaternary ammonium salt compound and has a structure as shown in a formula (I), wherein X is a halogen atom. When used alone, the compound has the characteristics of good safety performance, strong nerve blocking effect and the like, can take a reversible and durable local anaesthesia effect in vivo and can be used as a local anaesthesia drug or an analgesic drug which is long-acting and / or capable of realizing selective blocking. Particularly, a composition composed of the compound and other local anaesthesia drugs has the remarkable characteristics of high effect taking speed, strong effect, long acting time, little nerve injury and the like when used for nerve blocking.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV +1
Comprehensive pain assessment systems and methods
InactiveUS20050267338A1Cost-effectivePrecise heatingCatheterDiagnostic recording/measuringAnalgesics drugsPain assessment
Owner:NEUROSCI TOOLWORKS
Prodrugs of opioids and uses thereof
InactiveUS20110190267A1Improve oral bioavailabilityReduced bioavailabilityBiocideNervous disorderSide effectBioavailability
The present invention concerns prodrugs of opioid analgesics and pharmaceutical compositions containing such prodrugs. Methods for providing more consistent pain relief by increasing the bioavailability of the opioid analgesic with the aforementioned prodrugs are provided. The invention also provides for decreasing the adverse GI side effects of opioid analgesics.
Owner:SHIRE PHARMA INC
Preparation method of polyurethane and sericin medical composite film with water absorption and moisture permeability
InactiveCN102294048AImprove hydrophilicityGood water absorption and moisture permeabilityAbsorbent padsBandagesAnalgesics drugsComposite film
The invention discloses a preparation method of a polyurethane and sericin medical composite film with water absorption and moisture permeability. The steps are as follows: (1) preparation of polyurethane solution; (2) preparation of sericin suspension; (3) configuration of aliphatic polyurethane and sericin blend solution; (4) pouring membrane-forming liquid into glassine On the paper, the smear stick is evenly applied, and two methods of dry film formation and wet film formation are used to make a uniform film. Using sericin as the filling material, introducing sericin particles into the matrix not only forms micropores around the sericin particles, but also sericin contains a variety of amino acids with polar groups, and its good hydrophilicity provides a bridge for water transfer , so that the prepared film has good water absorption and moisture permeability. Bioactive ingredients can be loaded during the preparation process, and the bioactive ingredients can include various antibacterial, hemostatic, anti-inflammatory, analgesic drugs and / or growth factors and other active ingredients required for treatment to promote wound healing.
Owner:ZHEJIANG SCI-TECH UNIV
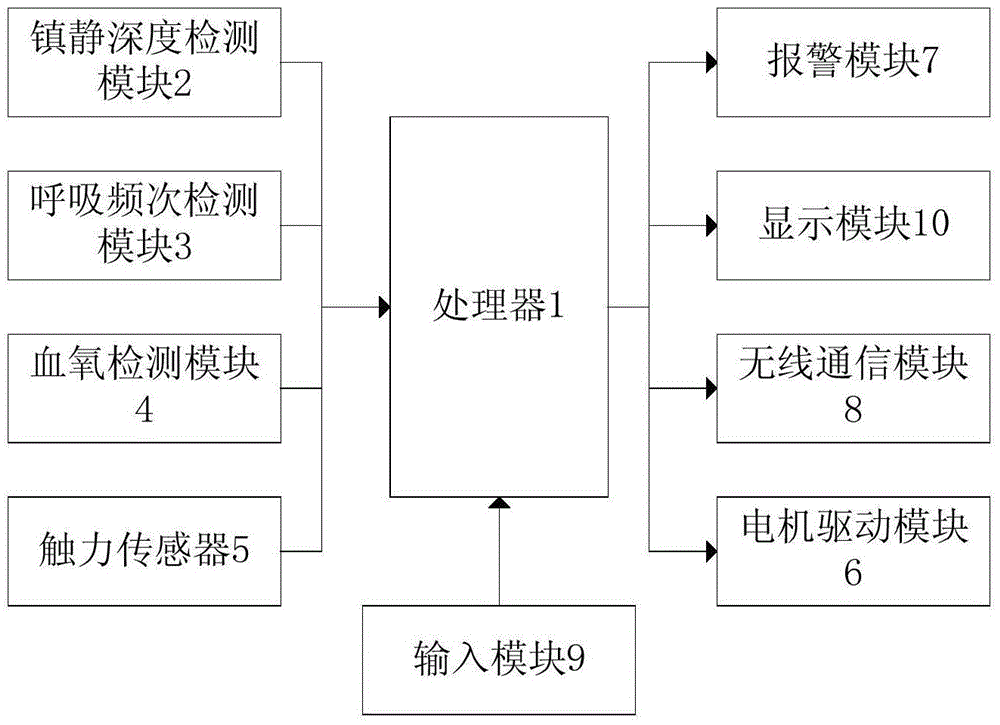
Analgesia pump control method, device and equipment capable of achieving intelligent infusion
ActiveCN104784784APrevent over-sedationAvoid accidentsMedical devicesFlow monitorsStopped workAnalgesics drugs
The invention discloses analgesia pump control method, device and equipment capable of achieving intelligent infusion. The equipment comprises a processor, a motor drive module and an analgesia depth detection module and / or breathing frequency detection module and / or blood oxygen monitoring module. The analgesia depth detection module and / or breathing frequency detection module and / or blood oxygen monitoring module and the motor drive module are electrically connected with the processor. The processor is used for obtaining detection signals sent by the analgesia depth detection module and / or breathing frequency detection module and / or blood oxygen monitoring module, judging the obtained detection signals respectively and sending a control signal to the motor drive module if any one of the detection signals is abnormal so as to drive a motor to make a analgesia pump stop working or lower the infusion speed. The analgesia pump control method has the advantages that multiple kinds of detection are combined, side effects caused by unreasonable analgesic drug infusion are detected, an alarm is given out, and the analgesia infusion risk is avoided.
Owner:JIANGSU REHN MEDICAL INSTR TECH
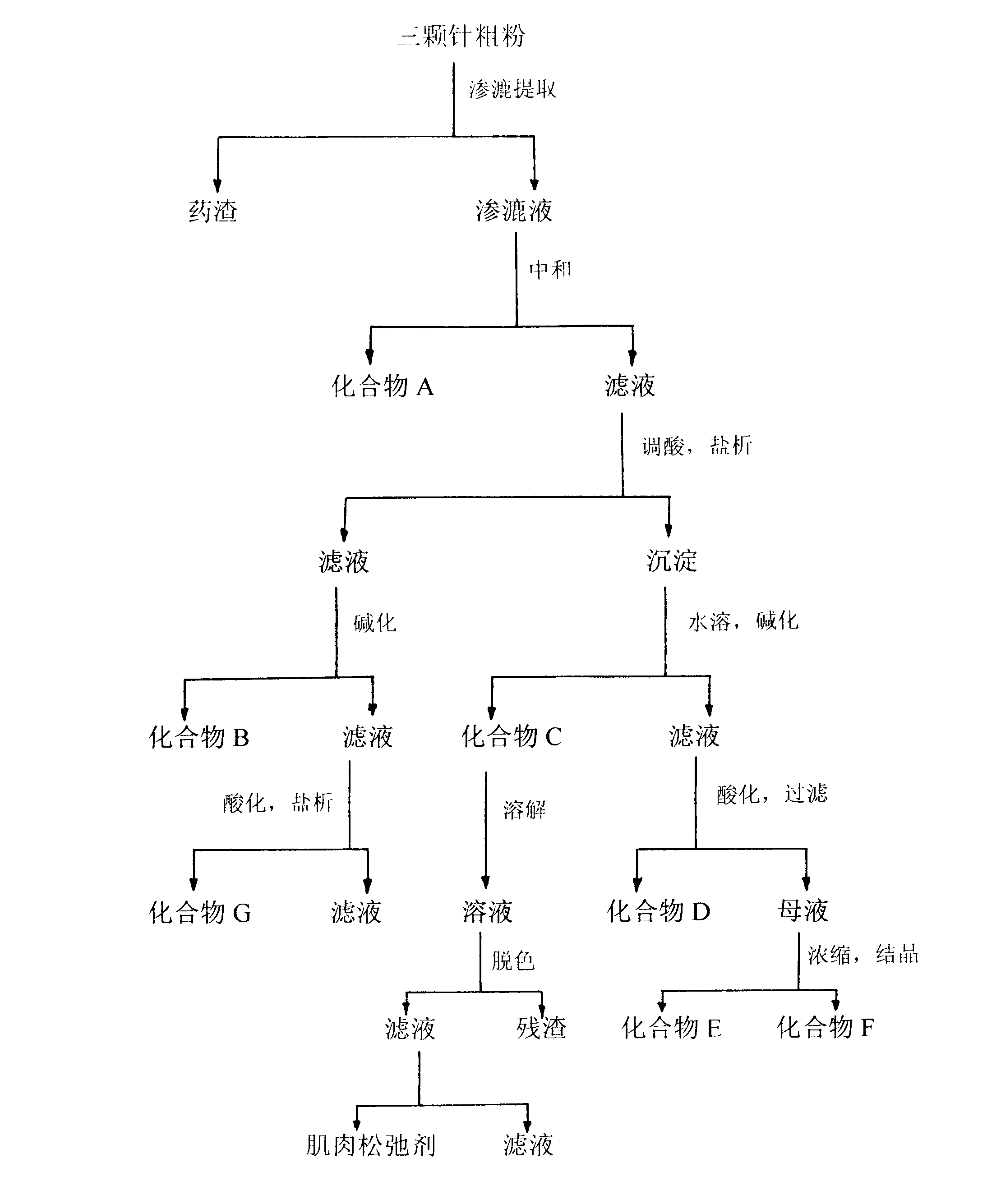
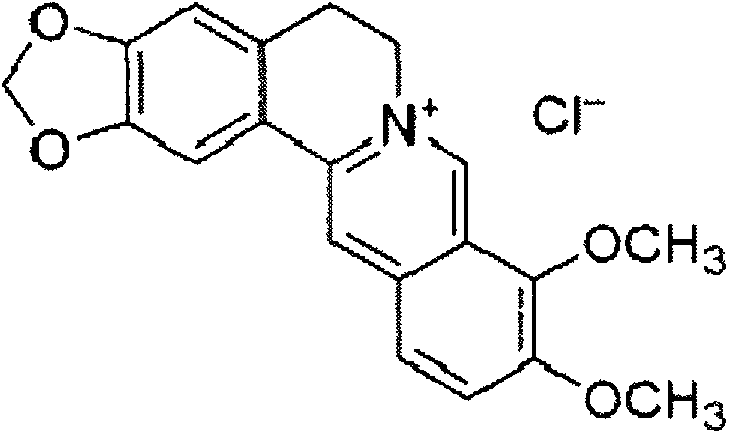
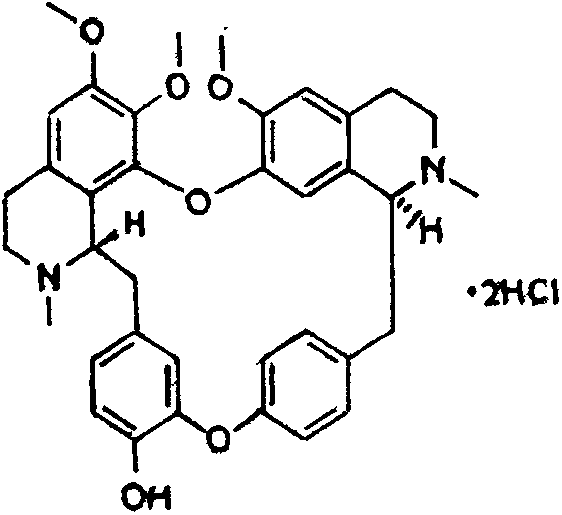
Design of comprehensive extraction process for barberry plant resources
The invention discloses a design of a comprehensive extraction process for barberry plant resources. Berbamine hydrochloride, jatrorrhizine hydrochloride, palmatine hydrochloride and a muscle relaxant are synchronously, efficiently and comprehensively extracted while berberine hydrochloride is extracted, and berbamine is used as a raw material for synthesizing an analgesic drug. Therefore, the barberry plant resources are fully utilized, the added medicinal value is increased, and the comprehensive utilization rate of medicinal barberry is increased. For a traditional Chinese medicine, one medicinal part is often utilized only and other parts are discarded. Various components in the barberry are synchronously extracted and comprehensively utilized, so that the economic value of the barberry is greatly increased.
Owner:李玉山
Intelligent pharmaceutical delivery system with automatic shutoff and method of using
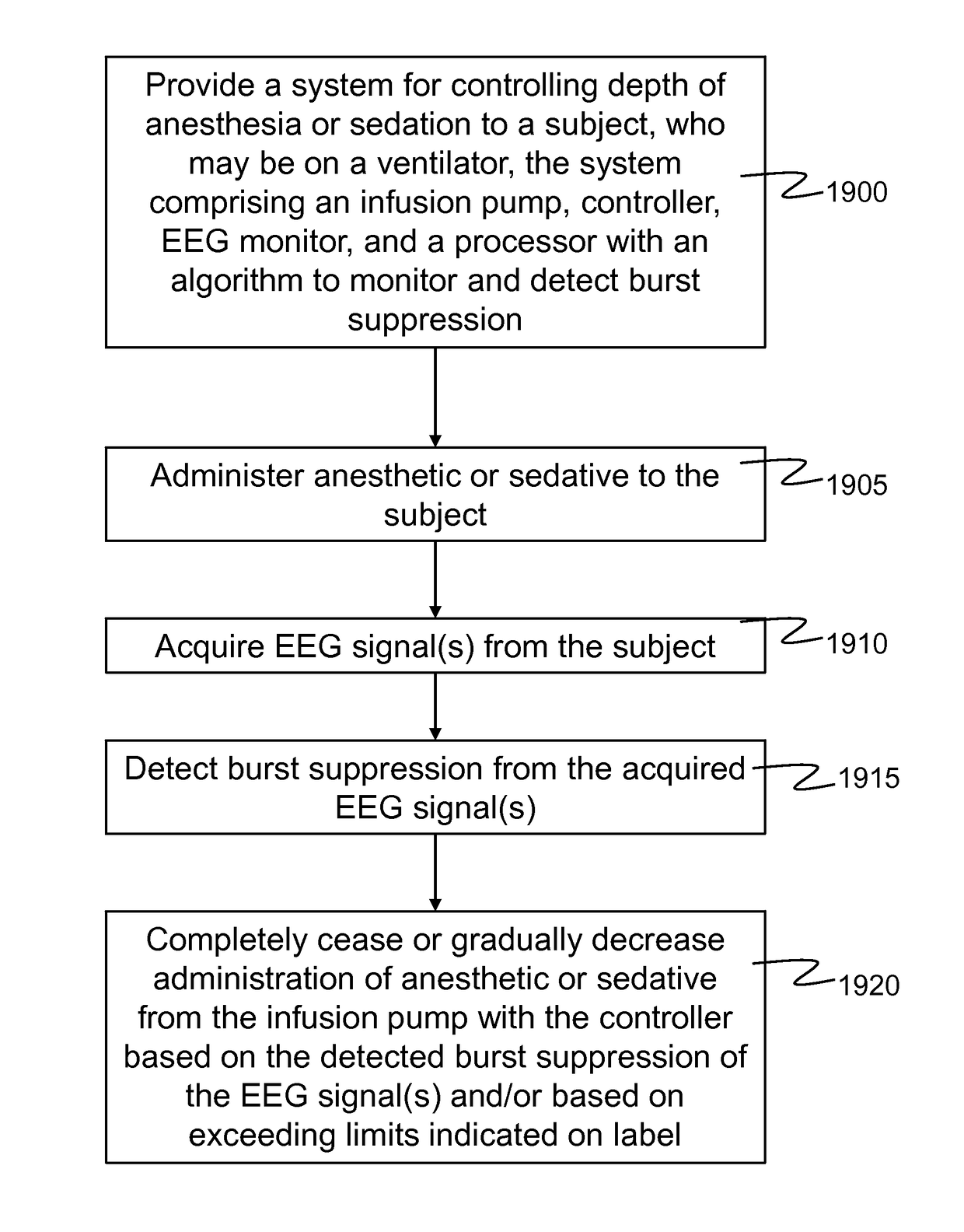
ActiveUS10130766B1Reduce riskEasy and fast data transferElectroencephalographyRespiratorsAnalgesics drugsBurst suppression
A system and method provides closed-loop sedation, anesthesia, or analgesia by monitoring EEG and automatically adjusting the delivery of sedative, anesthetic, and / or analgesic drugs to maintain that desired or predetermined level of cortical at all echelons of care. The system and method further monitor the subject's cortical activity to detect occurrence of burst suppression which can be indicative of unsafe depth of anesthesia or sedation. Further, the system and methods provide for alteration or cessation of the administration of anesthesia or sedation based on the occurrence of burst suppression to mitigate harm to the subject.
Owner:NEUROWAVE SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com