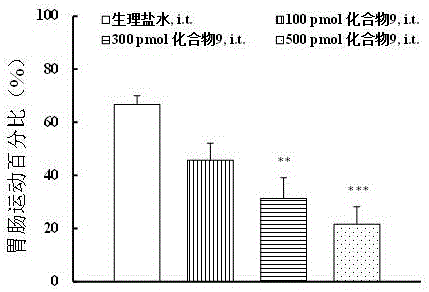
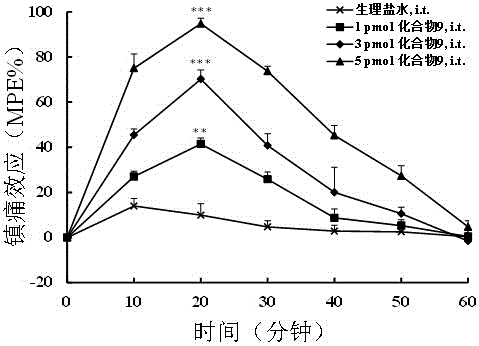
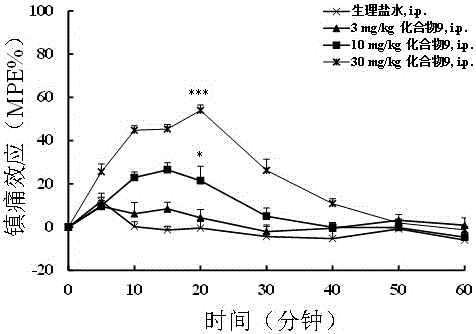
Multi-target peptide molecules of opium and neuropeptide FF receptors, and preparation and application thereof
A neuropeptide, multi-target technology, applied in the field of biochemical peptide drugs, can solve the problems of unsatisfactory analgesic effect intensity and drug effect time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0152] Embodiment 1, the synthesis of compound 1
[0153] (1) Resin pretreatment: Weigh 600 mg of Rink-Amide-MBHA resin with a substitution value of 0.43 mmol / g and add it to the synthesizer, then add DCM and stir for 30 min to fully swell the resin and dry the solvent under reduced pressure.
[0154] (2) Removal of Fmoc group protection: Add a mixed solution of hexahydropyridine, DBU, and DMF (the volume ratio of the three is 1:1:98) to the resin that has been swollen and drained of solvent, stir for 5 minutes and then pump Dry, repeat 3 times and then drain. The DMF that was added at the end was stirred for 3 min and then sucked dry. This was repeated 4 times to obtain a resin sample from which the protection of the Fmoc group was removed.
[0155] (3) Indene test: add to the test tube in the following order: resin sample, 0.1 ml reagent ①, 0.2 ml reagent ②, 0.1 ml reagent ③, observe after boiling water bath for 3-10 min. Both the solution and the resin are blue, indicatin...
Embodiment 2
[0160] Embodiment two, the synthesis of compound 2
[0161] (1) Resin pretreatment: Weigh 600 mg of Rink-Amide-MBHA resin with a substitution value of 0.43 mmol / g and add it to the synthesizer, then add DCM and stir for 30 min to fully swell the resin and dry the solvent under reduced pressure.
[0162] (2) Removal of Fmoc group protection: same as Example 1.
[0163] (3) Indene detection: Same as Example 1.
[0164] (4) Condensation of amino acids: N-α-Fmoc protected Fmoc-Phe-OH, N-hydroxybenzotriazole, O-benzotriazole-N,N,N',N'-tetramethyl Urea-hexafluorophosphate was completely dissolved in DMF at a molar ratio of 1:1:1, then added diisopropylethylamine (DIEA) twice the amount of Fmoc-Phe-OH and mixed thoroughly to obtain a mixed solution ; the mixed solution and step (2) obtained by removing the Fmoc group protected resin (the molar ratio of Fmoc-Phe-OH to the resin removed from the Fmoc group protected resin is 1:2.5) was added to the synthesizer, under argon The react...
Embodiment 3
[0168] Embodiment three, the synthesis of compound 3
[0169] (1) Resin pretreatment: Weigh 600 mg of Rink-Amide-MBHA resin with a substitution value of 0.5 mmol / g and add it to the synthesizer, then add DCM and stir for 30 min to fully swell the resin and dry the solvent under reduced pressure.
[0170] (2) Removal of Fmoc group protection: same as Example 1.
[0171] (3) Indene detection: Same as Example 1.
[0172] (4) Condensation of amino acids: N-α-Fmoc protected Fmoc-Phe-OH, N-hydroxybenzotriazole, O-benzotriazole-N,N,N',N'-tetramethyl Urea-hexafluorophosphate is completely dissolved in DMF at a molar ratio of 1:1:1, and then diisopropylethylamine (DIEA) is added to twice the molar amount of Fmoc-Phe-OH and mixed thoroughly to obtain a mixture solution; the mixed solution and the resin obtained in step (2) to remove the Fmoc group protection (the molar ratio of Fmoc-Phe-OH to the resin removed from the Fmoc group protection is 1:2.5) was added to the synthesizer, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com