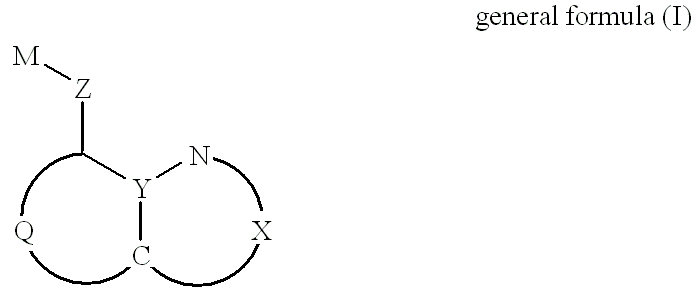
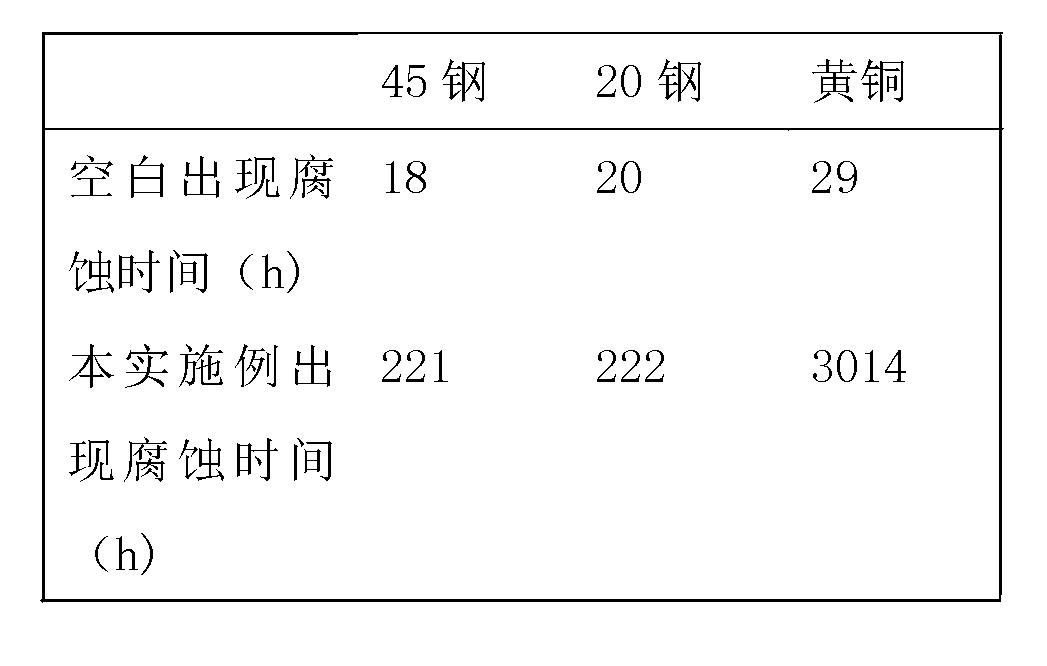
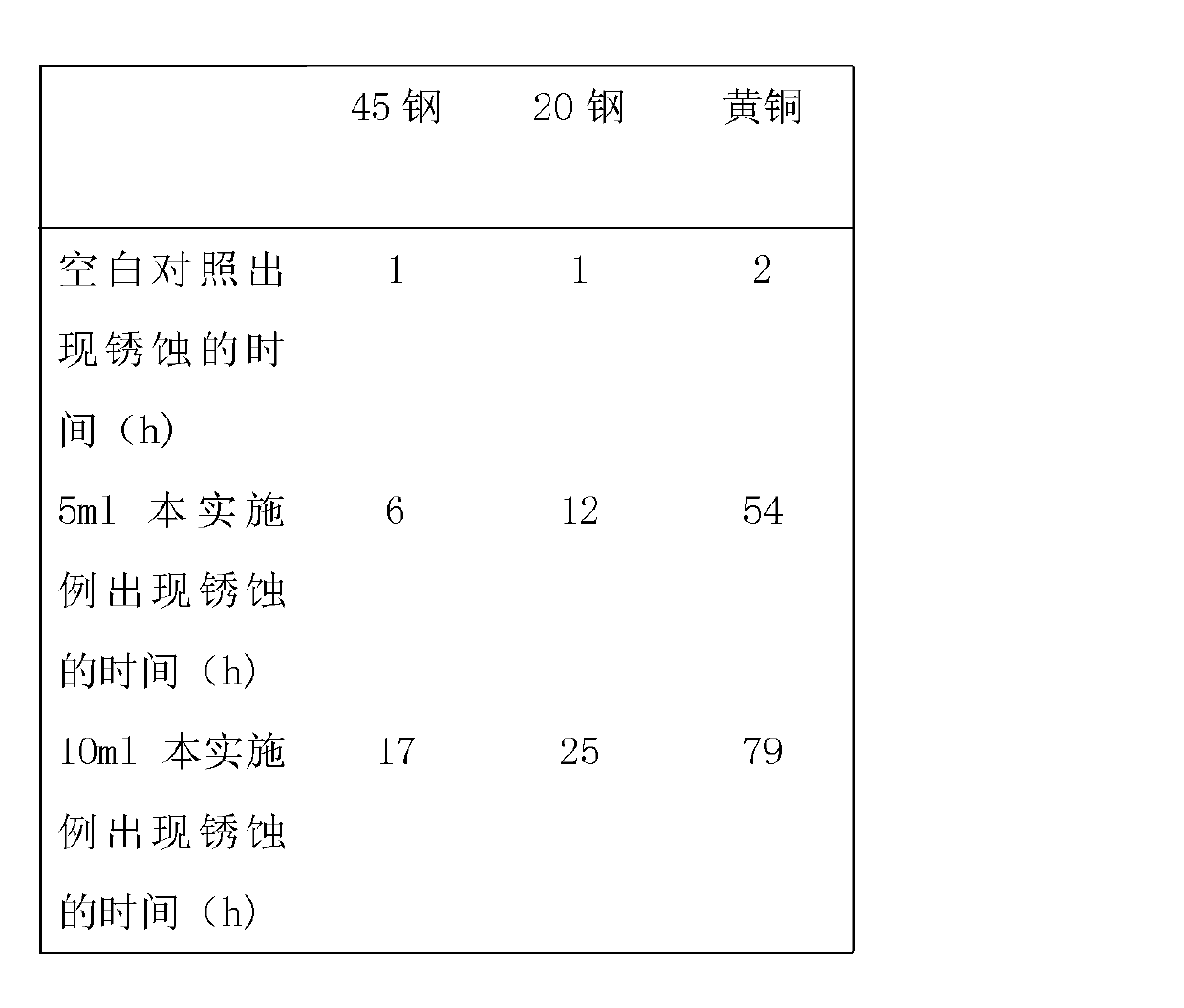
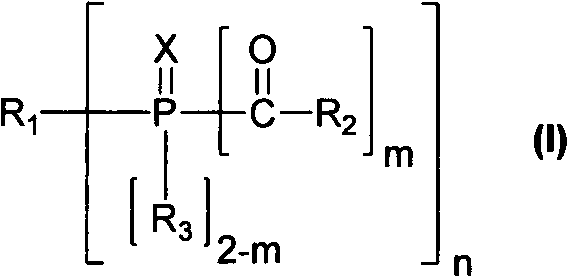
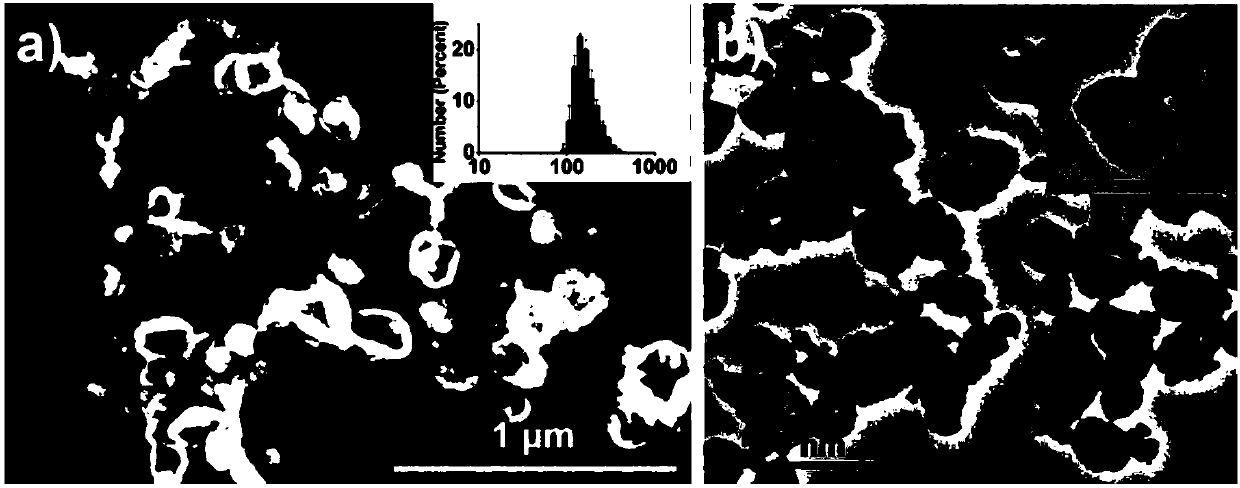
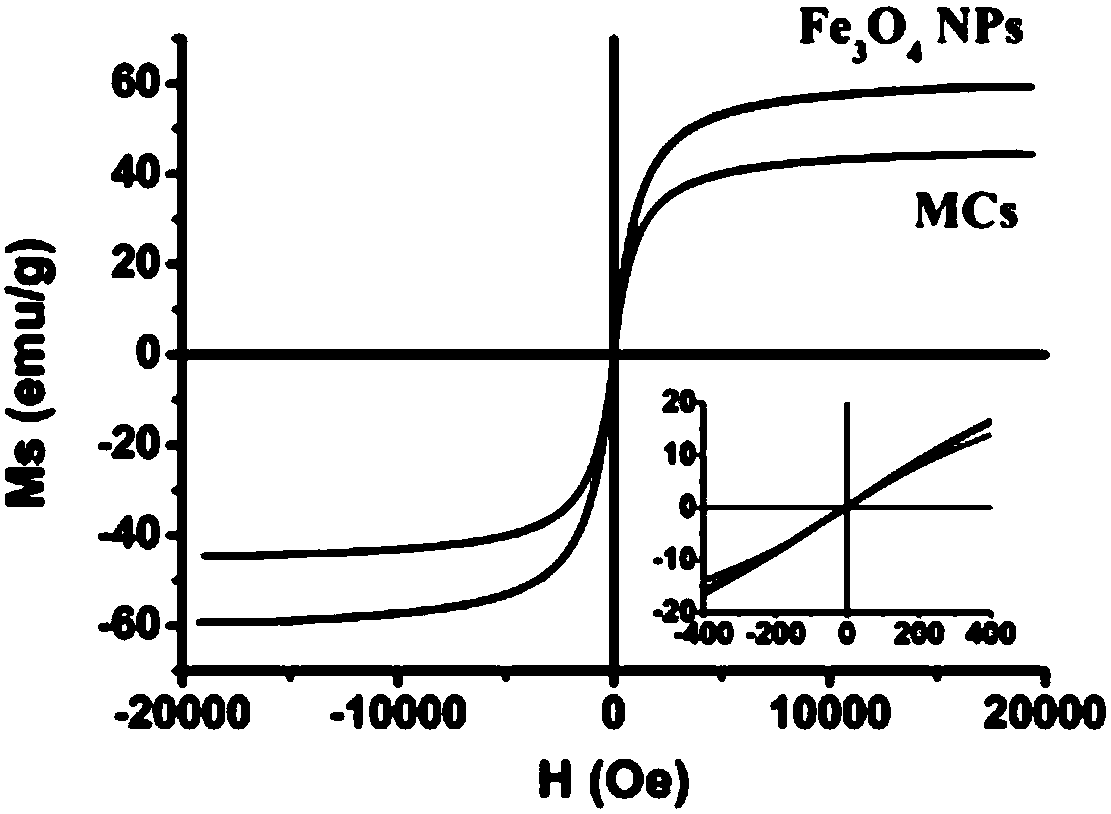
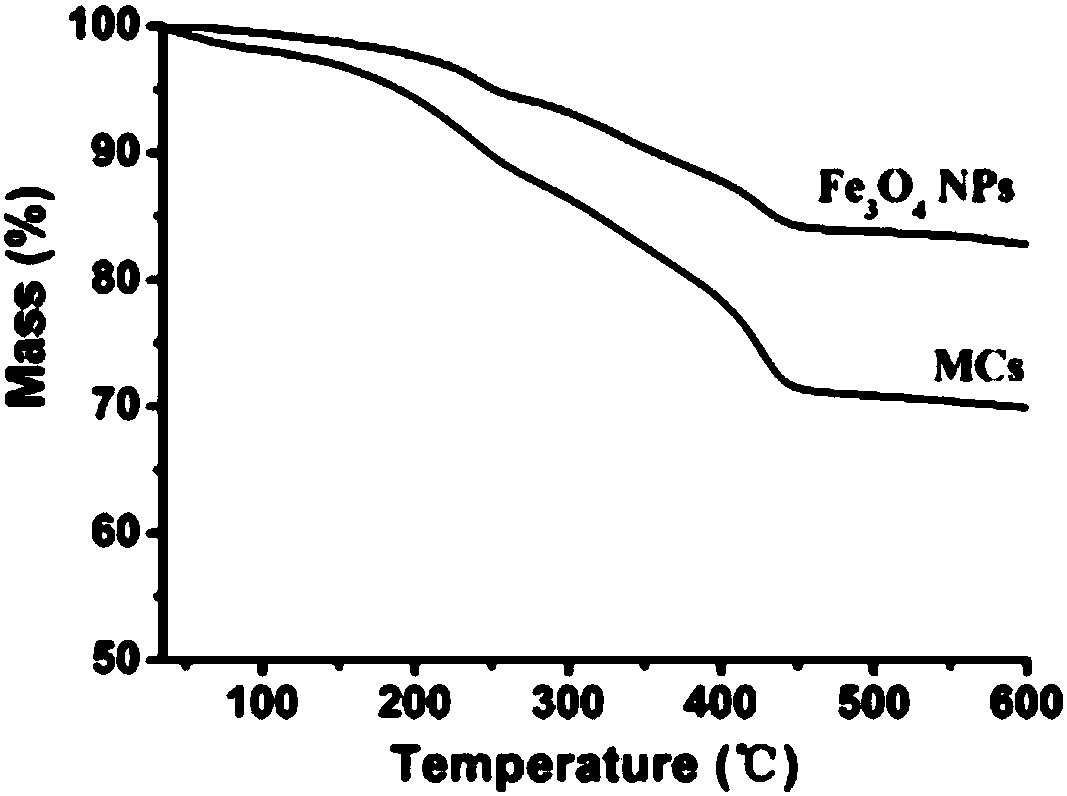
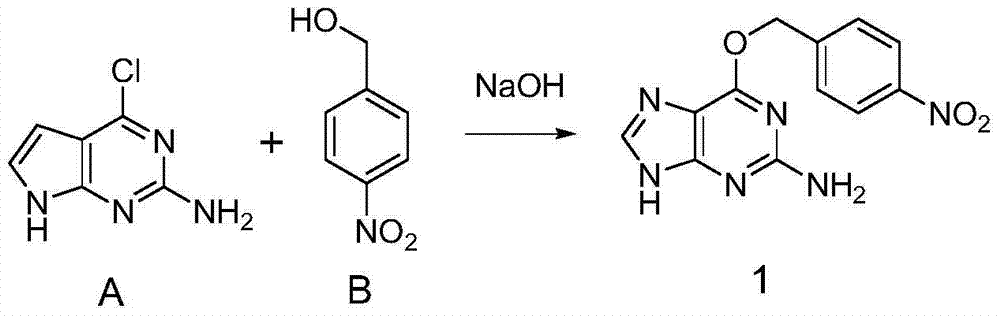
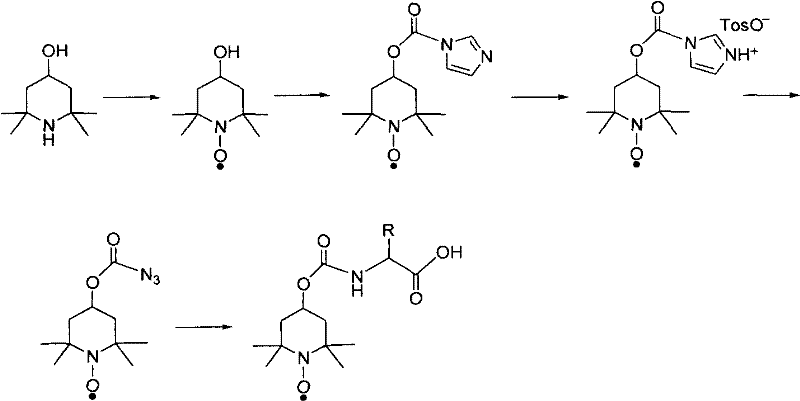
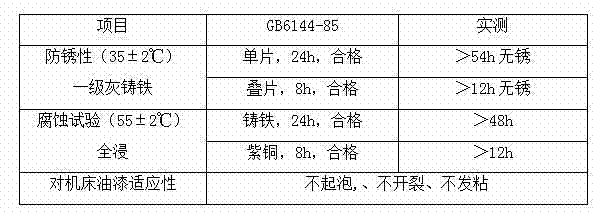
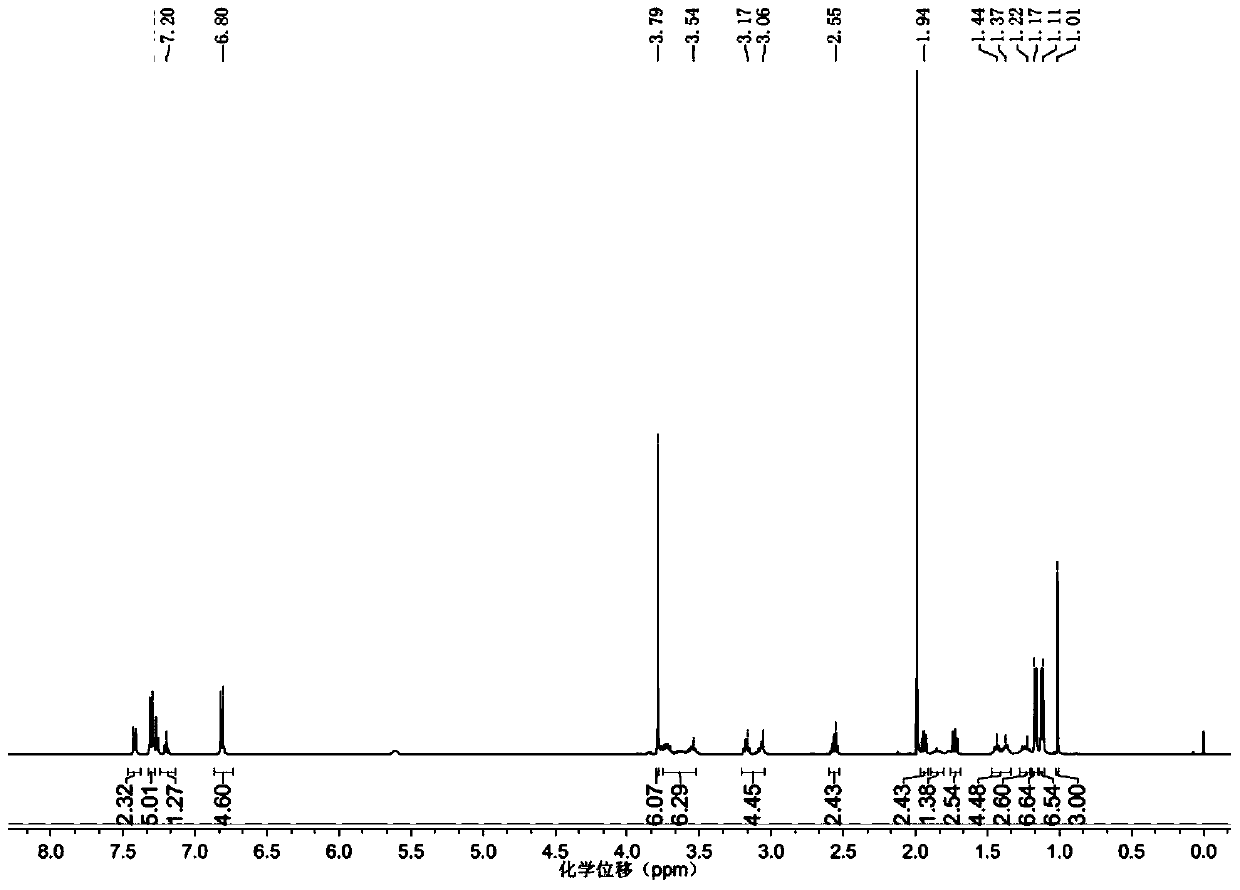
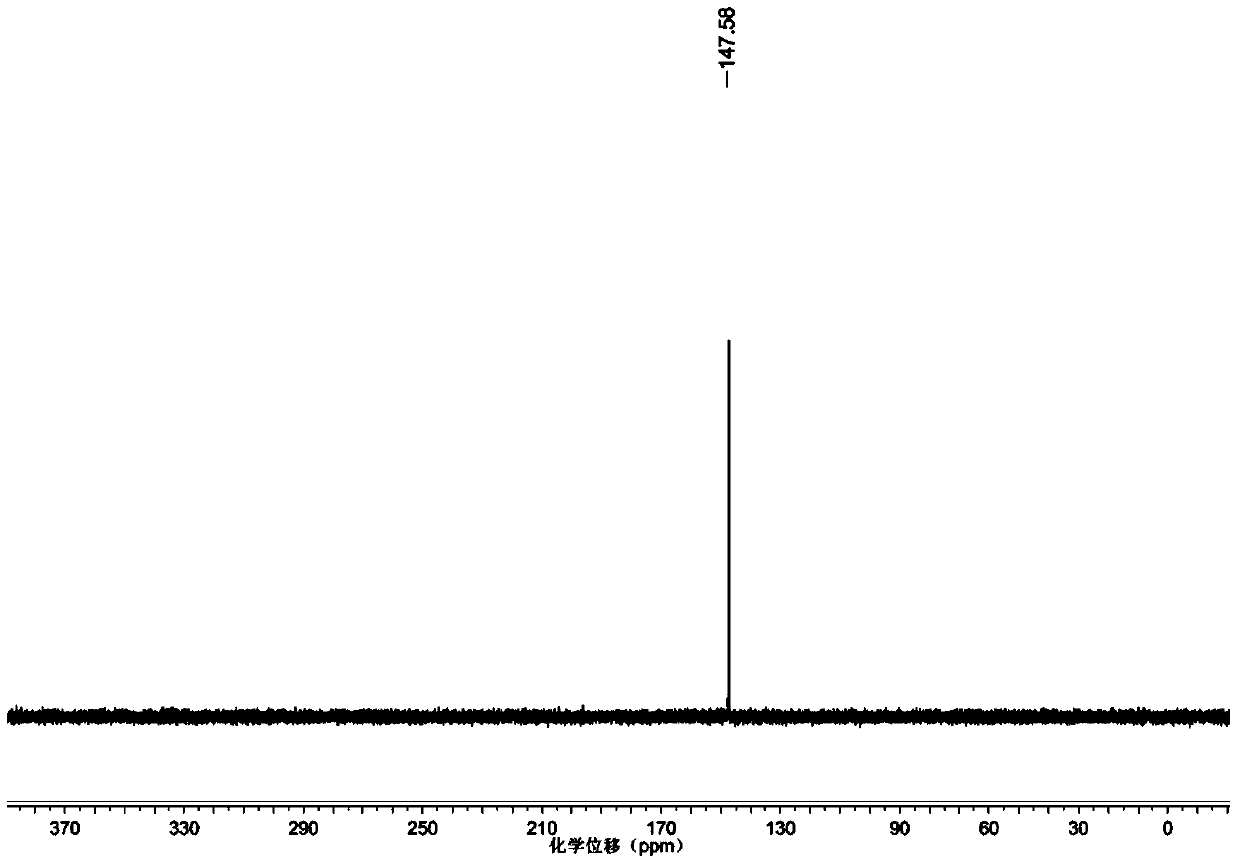
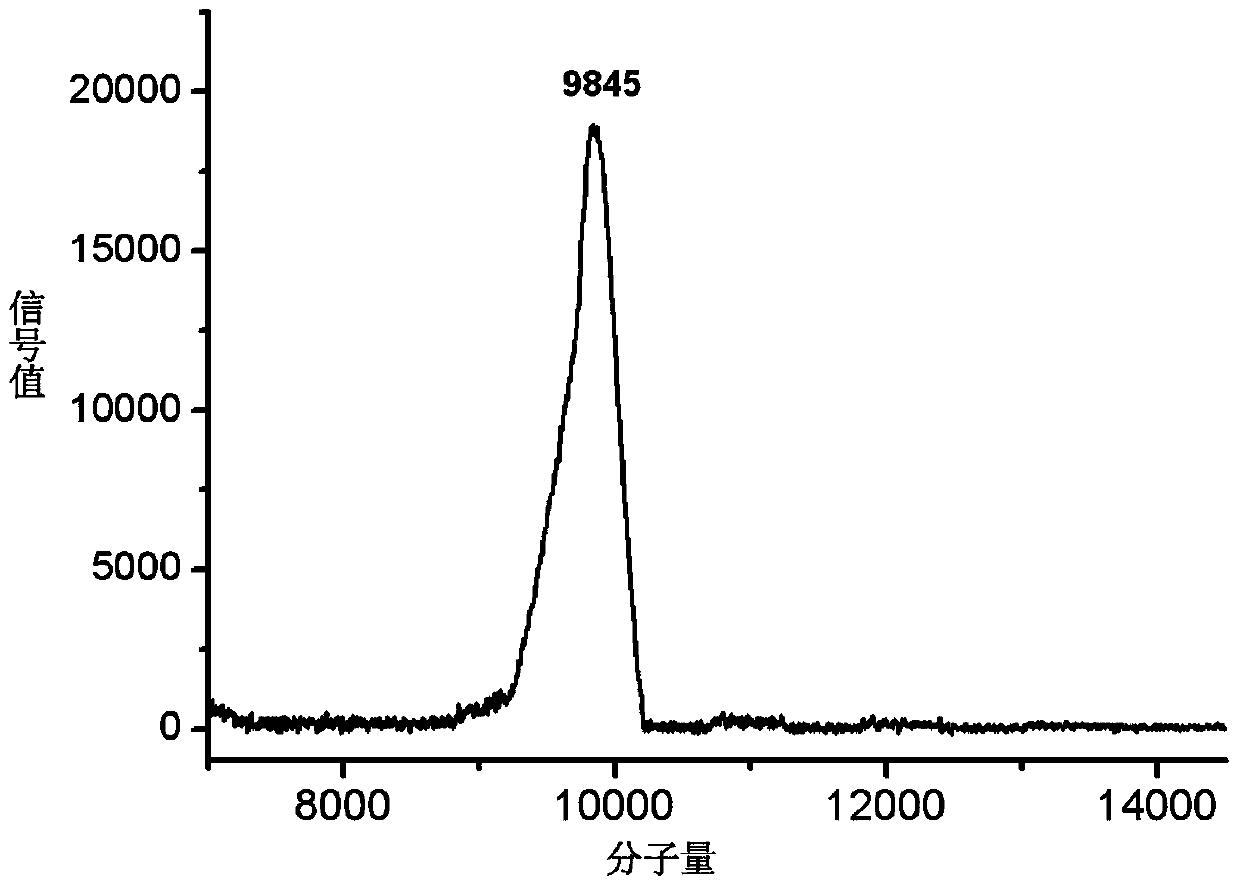
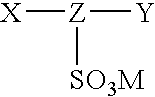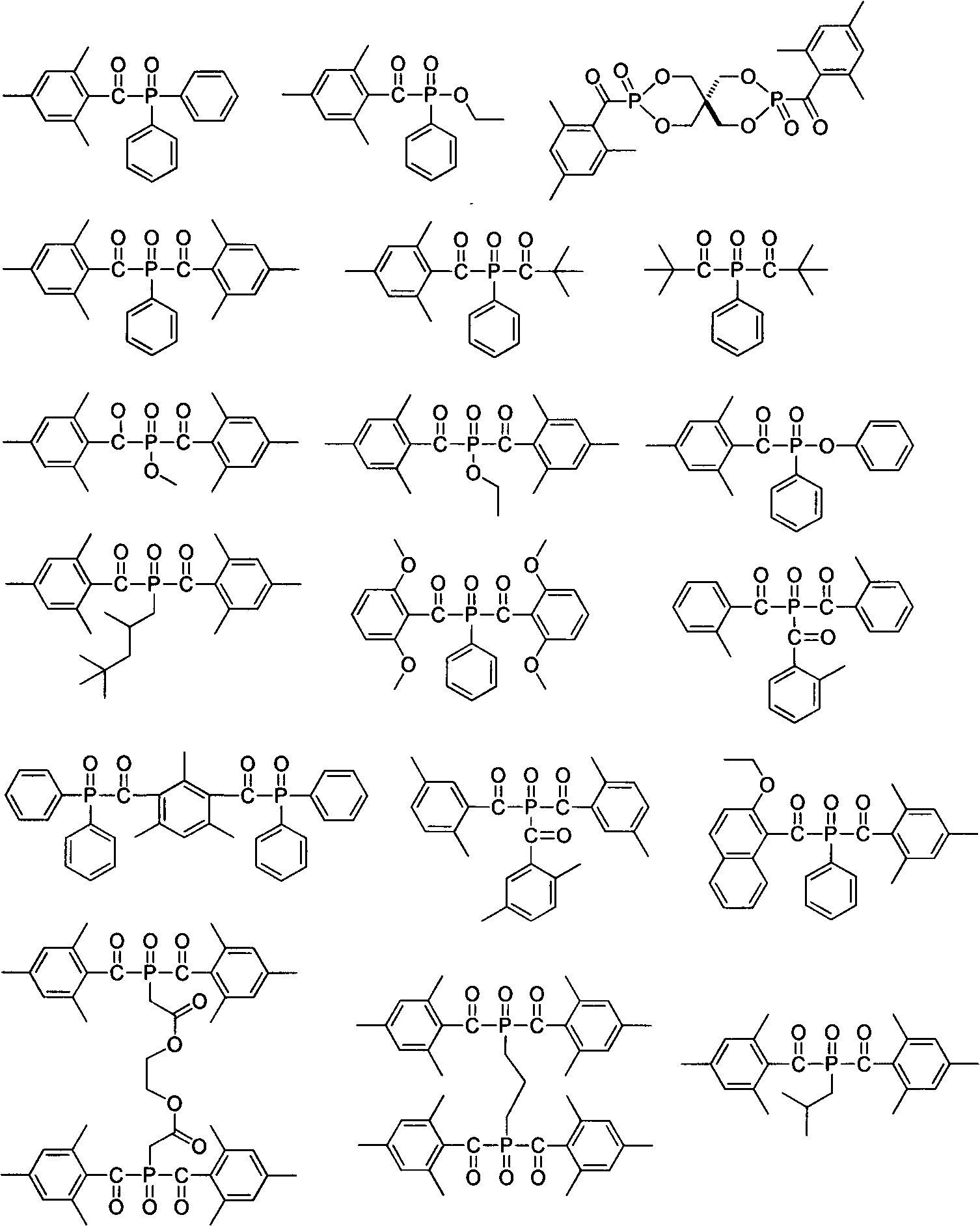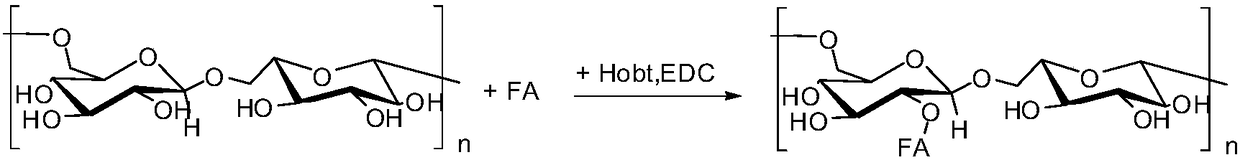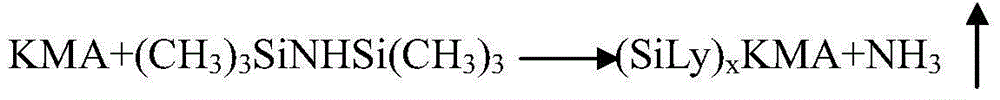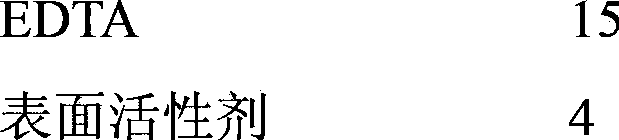Patents
Literature
242 results about "Hydroxybenzotriazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
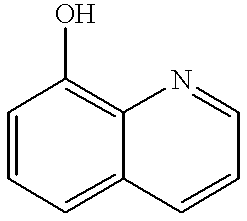
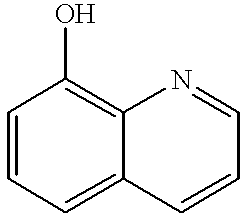
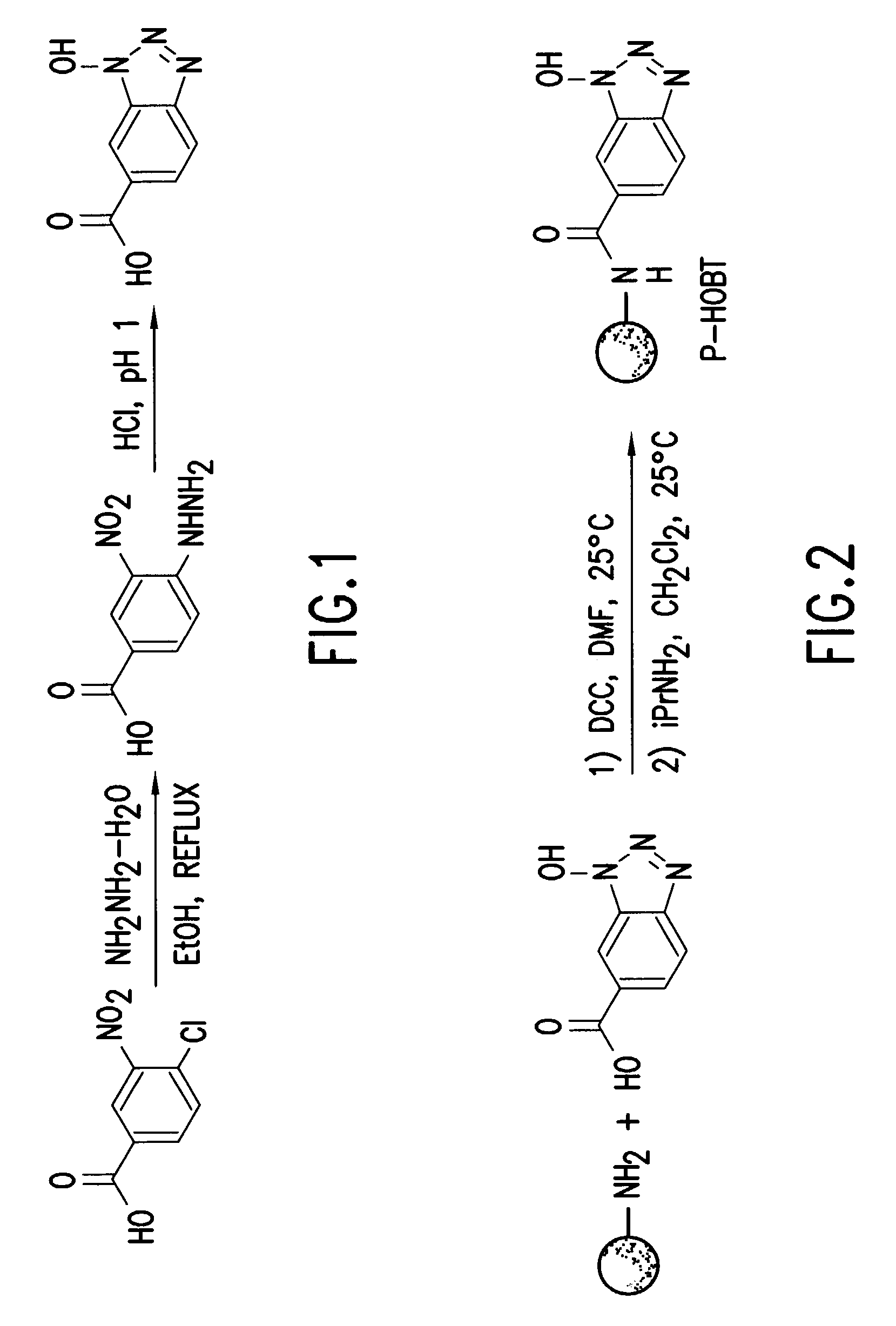
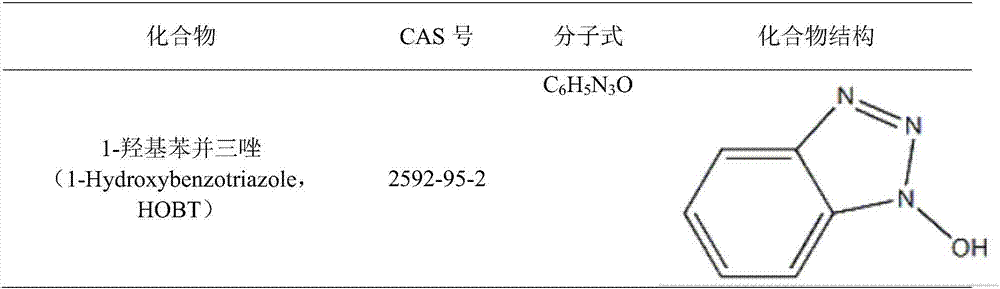
Hydroxybenzotriazole (abbreviated HOBt) is an organic compound that is a derivative of benzotriazole. It is a white crystalline powder, which as a commercial product contains some water (~11.7% wt as the HOBt monohydrate crystal). Anhydrous HOBt is explosive.
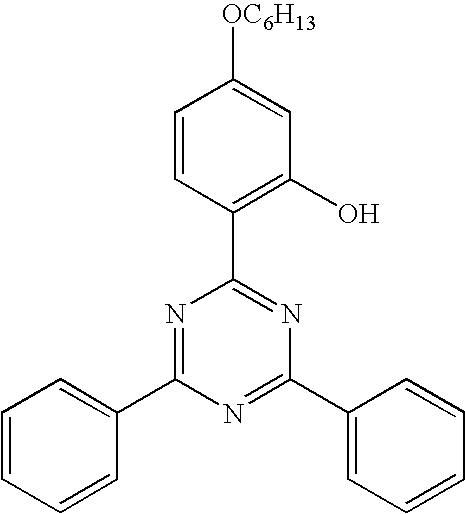
Matt, UV-stable, Thermoformable, co-extruded polyester film, a method for the production thereof and the use of the same
The invention relates to biaxially oriented, co-extruded polyester films. Said films comprise a base layer which consists of at least 70% by weight of a thermoplastic polyester, preferably polyethylene terephthalate (PET) with a diethylene glycol and / or polyethylene glycol content greater than 1.3% by weight and have at least one matt outer layer and optionally additional intermediate layers. The films also contain at least one UV-absorber, preferably hydroxy benzotriazoles and triazines. The films are characterized by high UV-stability, no embrittlement after exposure to temperature, a matt surface devoid of unwanted clouding and excellent thermoforming properties and are, together with the molded bodies produced therefrom, suitable for numerous interior and exterior applications. The (matt) outer layers can be identical or different and contain a mixture or a blend of a component I which consists of PET homopolymers and / or copolymers and a component II which is a copolymer resulting from the condensation product of isophthalic acid, an aliphatic dicarboxylic acid and a sulfomonomer with a copolymerizable aliphatic or cycloaliphatic glycol.
Owner:MITSUBISHI POLYESTER FILM
Computer-to-plate by ink jet
InactiveUS20020043171A1Simple and inexpensive methodProduce littleDuplicating/marking methodsHand compositionComputer to plateHydroxybenzotriazole
A method for preparing a lithographic printing plate by means of ink jet is disclosed. The ink jet fluid contains an oleophilizing compound as defined in the description. Preferred compounds are 8-hydroxyquinolines, 7-hydroxybenzimidazoles, and 7-hydroxybenztriazoles.
Owner:AGFA NV
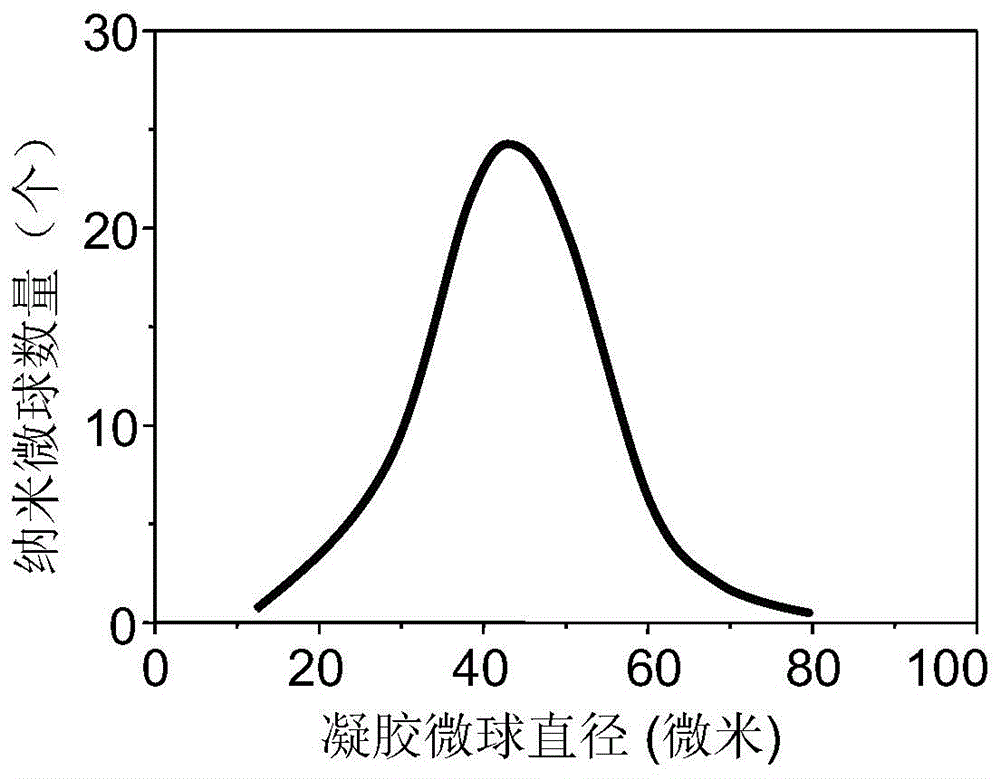
Biological consistent natural polymer hydrogel and preparation method thereof
The invention discloses a biocompatible natural polymer hydrogel and a preparation method thereof. The hydrogel is produced by the following steps: chitosan or glucan or gelatin and 1-Hydroxy benzotriazole are dispersed in the water, and stirred until transparent solution is formed; N-Acetyl-L-Cysteine or 2,3-Dimercaptosuccinic acid is added, then dripped into the water solution prepared with 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide, the pH is adjusted, stirred for 2-4h; dialyzed with 5mmol / L hydrochloric acid solution containing 2Mu mol / L EDTA under lucifugal condition within 2-4 days, dialyzed with 5mmol / L hydrochloric acid solution containing 1g / L NaCl, and then dialyzed with 1mmol / L hydrochloric acid solution to achieve the desired product. The hydrogel has excellent hydrophilicity, moisture retaining property, permeability and flexibility, the degradation product has no toxic or adverse effect to the body, and can meet the requirements of modern medical science on dressing to the largest extent.
Owner:NANKAI UNIV
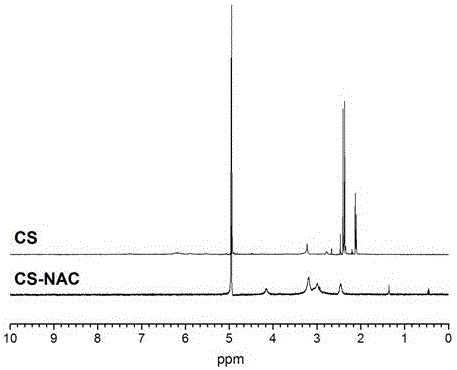
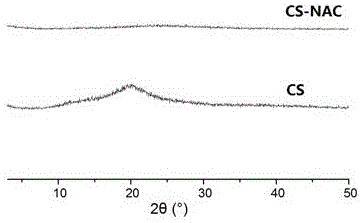
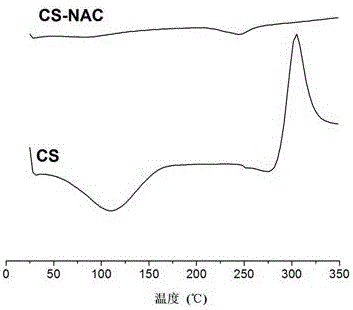
Novel bioadhesive thiolated chitosan synthesis method
The invention relates to a novel bioadhesive thiolated chitosan synthesis method.By taking a sulfydryl donor and chitosan as initial materials, taking 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 1-hydroxybenzotriazole as condensing agents and selecting a proper organic solvent as an inert solvent for NAC activation, free amino groups of the sulfydryl donor and the chitosan are connected to obtain thiolated chitosan.Compared with the chitosan, the thiolated chitosan has the advantages of excellent water solubility, high electropositivity, remarkably improved bioadhesiveness and biocompatibility and wide applicability to fields of biomedicine, food, chemical engineering and the like.In a sulfydryl donor activation process, a reaction solvent is changed into the inert organic solvent from deionized water, and accordingly hydrolysis of the activated sulfydryl donor is effectively avoided, the free sulfydryl content of products is increased, and biopotency is guaranteed.The novel bioadhesive thiolated chitosan synthesis method is mild in preparation condition, controllable in reaction, simple in preparation process and suitable for large-scale continuous production.
Owner:SHENYANG PHARMA UNIVERSITY
Computer-to-plate by ink jet
InactiveUS6457413B1Simple and inexpensive methodProduce littleDuplicating/marking methodsPrinting pre-treatmentComputer to plateEngineering
A method for preparing a lithographic printing plate by means of ink jet is disclosed. The ink jet fluid contains an oleophilizing compound as defined in the description. Preferred compounds are 8-hydroxyquinolines, 7-hydroxybenzimidazoles, and 7-hydroxybenztriazoles.
Owner:AGFA NV
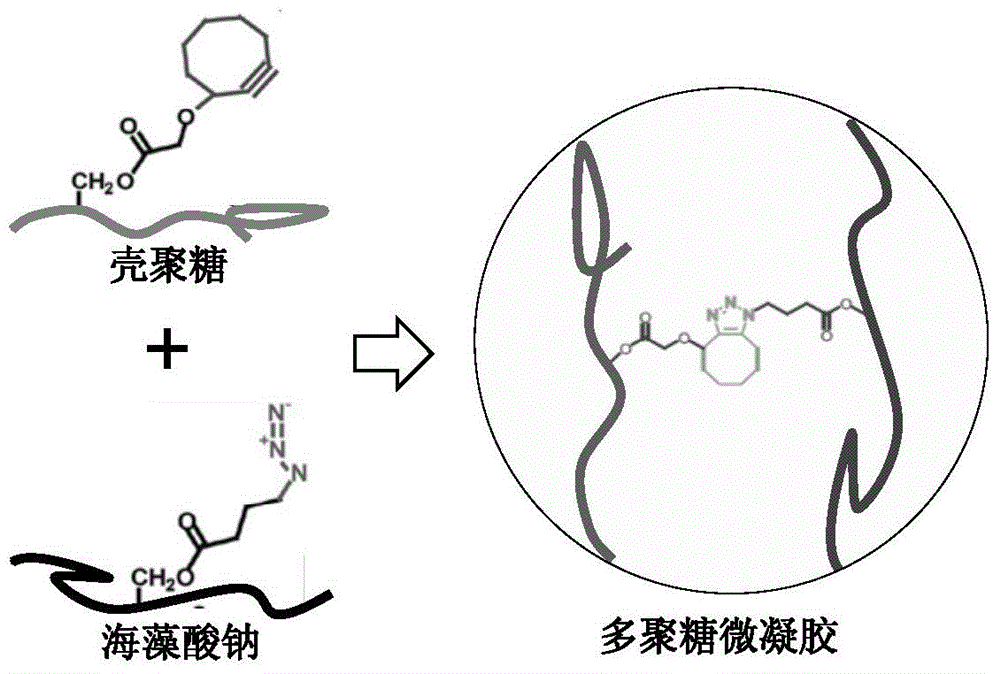
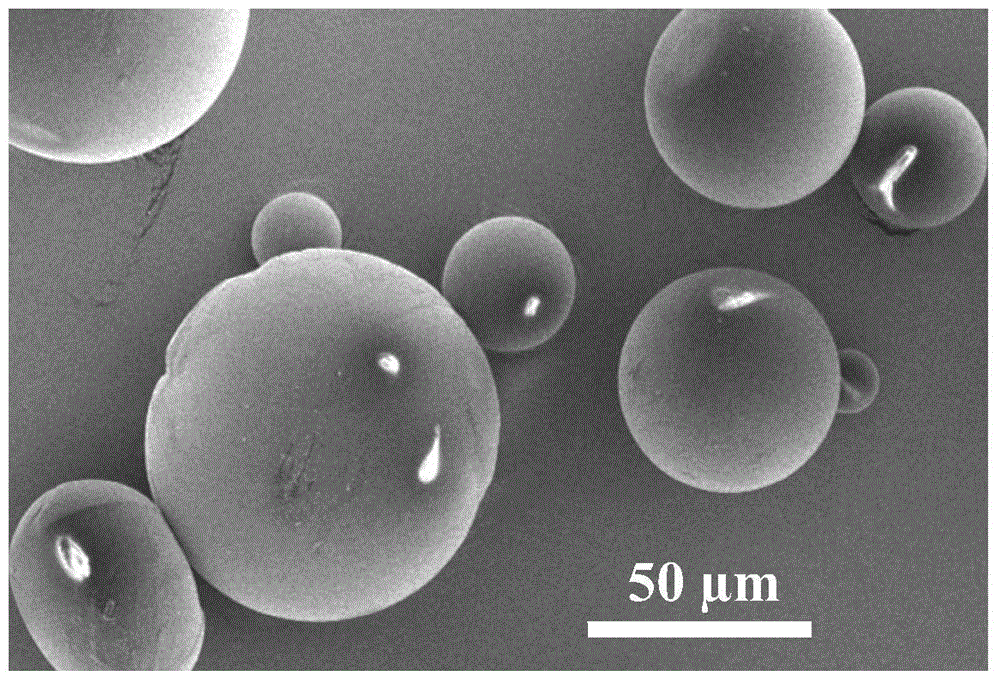
Preparation method of copper-free click crosslinking polysaccharide microspheres
InactiveCN106140040AReduce usageGuaranteed CompatibilityMicroballoon preparationMicrocapsule preparationUltrasonic emulsificationFreeze-drying
The invention discloses a preparation method of copper-free click crosslinking polysaccharide microspheres. The preparation method comprises the following steps that chitosan and 1-hydroxyl benzotriazole are dissolved in water at room temperature, and cyclooctyne-3-glycolic acid is dissolved in a tetrahydrofuran / water mixed solvent; after the two solutions are mixed, diisopropylethylamine is added, stirring reaction is performed, and cyclooctyne chitosan is obtained through dialysis and freeze-drying; sodium alginate and carbodiimide are dissolved in water, 11-azido-3,6,9-triethyl ether-1-amine is added, stirring reaction is performed, and azido sodium alginate is obtained through dialysis and freeze-drying; water solutions of the cyclooctyne chitosan and the azido sodium alginate are respectively prepared, after mixing according to the volume ratio, emulsifier Span-80 containing paraffin oil is added, and an emulsification emulsion is obtained through ultrasonic emulsification; the emulsification emulsion volatilizes and stays overnight and is poured into isopropanol for precipitation of microspheres, and the crosslinking polysaccharide microspheres are obtained through freeze-drying after cleaning. The process is simple, makes products safe and non-toxic and is suitable for the medical fields of drug release, gene therapy, tissue engineering and the like.
Owner:NANJING UNIV OF SCI & TECH
Transparent, sealable, UV-resistant polyester film, its use and process for its production
InactiveUS6863954B2Synthetic resin layered productsRecord information storagePolymer scienceUltraviolet
Biaxially oriented, coextruded polyester films with a base layer at least 90% by weight of which is composed of a thermoplastic polyester, preferably polyethylene terephthalate (PET), and with at least one sealable outer layer and a second nonsealable outer layer and, if desired, with other intermediate layers, and comprising at least one UV absorber, preferably hydroxybenzotriazoles and triazines, have high UV resistance, do not embrittle when exposed to high temperatures, have a surface without undesirable haze and are suitable for numerous indoor and outdoor applications. The outer layers comprise antiblocking agents, such as silica with an average particle diameter preferably below 50 nm and / or above 2 μm, and the sealable outer layer is preferably composed of a copolyester which has been built up from ethylene terephthalate units and ethylene isophthalate units.
Owner:MITSUBISHI POLYESTER FILM
Gas-phase antirust agent containing modified nanometer bentonite
The invention discloses a gas-phase antirust agent containing modified nanometer bentonite. The antirust agent is prepared from the following raw materials in parts by weight: 90-95 parts of castor oil, 0.9-1.2 parts of ferrocene, 0.9-1.8 parts of polyisobutene, 1-2 parts of 2-aminoethyl heptadecenyl imidazoline, 0.8-1.3 parts of N-phenyl-2-naphthylamine, 1.1-1.7 parts of 1-hydroxybenzotriazole, 0.9-1.1 parts of urotropine, 1-2 parts of zinc dialkyl dithiophosphate, 0.4-0.8 part of sodium dodecyl benzene sulfonate, 4.4-4.8 parts of film-forming resins and 0.8-1.4 parts of modified nanometer bentonite. The gas-phase antirust agent has excellent contact antirusting performance and good gas-phase antirusting effect and has good gas-phase antirusting effect and contact antirusting effect on steels and brasses.
Owner:湖南三创富泰环保材料股份有限公司
Photoinitiator mixture of novel aromatic hydroxyl ketone and acylphosphine oxide, and composite system of photoinitiator mixture and photoabsorber
The invention discloses a novel photoinitiator system compounded by using aromatic hydroxyl ketone and acylphosphine oxide compounds, and an alkene-containing unsaturated system which can be cured through light (ultraviolet light or visible light or equivalent light sources) irradiation. The alkene-containing unsaturated system is obtained by compounding the photoinitiator system and an appropriate hydroxybenzotriazole photoabsorber with a red-shift light absorption characteristic.
Owner:SHENZHEN UV-CHEMTECH CO LTD
Chitosan/polylysine dendritic macromolecular core-shell nanoparticles and preparation method thereof
InactiveCN106589391AGood biocompatibilityImprove biological characteristicsGenetic material ingredientsNanotechnologyNitrogen gasPolylysine
The invention discloses chitosan / polylysine dendritic macromolecular core-shell nanoparticles and a preparation method thereof. The chitosan / polylysine dendritic macromolecular core-shell nanoparticles are characterized in that the chitosan / polylysine dendritic macromolecular core-shell nanoparticles are of a chitosan derivative formed by carrying out an amidation reaction on an amino on chitosan with the weight average molecular weight of 5*10<4> to 2*10<5> and the deacetylation degree of 85 percent to 100 percent, and carboxyl on a polylysine dendritic macromolecule. The method disclosed by the invention comprises the following steps: preparing the chitosan into a chitosan water solution with the concentration of 0.5mg / ml to 5mg / ml and marking the chitosan water solution as a solution A; stirring at the speed of 500rpm to 1000rpm at 20 DEG C to 35 DEG C under the protection of nitrogen gas, and dropwise adding the solution A into a DMF (Dimethyl Formamide) solution of the polylysine dendritic macromolecule, EDC (Dichloroethane) and HOBt (Hydroxybenzotriazole); continually stirring and reacting for 2 days to 5 days; dialyzing for 2 days to 4 days, and freezing and drying to obtain a product. The praparation method is simple, has moderate reaction conditions and is easy to control.
Owner:TIANJIN UNIV OF COMMERCE
Liquid-phase synthesis method of dipeptide diaminobutyroyl benzylamide diacetate
InactiveCN107936108ASimple processLow costPeptide preparation methodsAnimals/human peptidesSolubilitySynthesis methods
The invention discloses a liquid-phase synthesis method of dipeptide diaminobutyroyl benzylamide diacetate. The method comprises the following steps of enabling Boc-Beta-Ala-OH, N-ethyl-5-phenylisoxazole-3'-sulfonic acid inner salt and H-Pro-OMe.HCl to react to obtain Boc-Beta-Ala-Pro-OMe, and enabling the Boc-Beta-Ala-Pro-OMe to react with LiOH to obtain Boc-Beta-Ala-Pro-OH; afterwards, synthesizing Boc-Beta-Ala-Pro-DAB(Boc)-OH with the Boc-Beta-Ala-Pro-OH and H-DAB(Boc)-OMe.HCl by adopting the same method; next, enabling the Boc-Beta-Ala-Pro-DAB(Boc)-OH, 1-hydroxybenzotriazole, N,N-diisopropylcarbodiimide and benzylamine to react to obtain Boc-Beta-Ala-Pro-DAB(Boc)-NH-Bzl, finally, removing a Boc protecting group by using trifluoroacetic acid, separating and purifying, so as to obtain the dipeptide diaminobutyroyl benzylamide diacetate with purity being more than 95 percent. According to the liquid-phase synthesis method, amino acid methyl ester which is low-cost and is easily obtained is used as a raw material; a by-product generated and synthesized by a peptide bond in each step has water solubility and is easily separated, and the liquid-phase synthesis method which has a simple and convenient process and lower cost is provided for the synthesis of the dipeptide diaminobutyroyl benzylamide diacetate.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
Magnetic material for efficiently detecting circulating tumor cell and preparation method of magnetic material
ActiveCN107858144ANarrow particle size distributionHigh saturation magnetizationOrganic/organic-metallic materials magnetismTumor/cancer cellsBiocompatibility TestingSuperparamagnetism
The invention provides a magnetic material for efficiently detecting a circulating tumor cell and a preparation method of the magnetic material. The preparation method comprises the steps of: (1) allowing hyaluronic acid, 1-(3-dimethylamino propyl)-3-ethyl-carbodiimide hydrochloride, 1-hydroxybenzotriazole, cysteamine hydrochloride and dithiothreitol to react to prepare HA-SH, (2) grafting rhodamine onto HA-SH to prepare RhB-HA-SH, (3) allowing an Fe3O4 nanoparticle, RhB-HA-SH and H2O2 to react to prepare superparamagnetic fluorescent HA-SH, and (4) allowing superparamagnetic fluorescent HA-SH, 1-(3-dimethylamino propyl)-3-ethyl-carbodiimide hydrochloride, 1-hydroxybenzotriazole, PEG-FA and anti-EpCAM (epithelial cell adhesion molecule) to perform antibody reaction to prepare the magneticmaterial. The magnetic material is high in saturation magnetization, and good in magnetic response and biocompatibility, can achieve specific binding with the circulating tumor cell and has certain universality.
Owner:SICHUAN UNIV
Preparation method and applications of oriented immobilized PEGA composite resin
ActiveCN104844710ADoes not affect conformationDoes not affect activityCarrier-bound/immobilised peptides4-nitrobenzyl alcoholTert-Butyloxycarbonyl protecting group
The invention discloses a preparation method and applications of an oriented immobilized PEGA composite resin. The preparation method comprises following steps: 2-amino-6-chloropurine and 4-nitrobenzyl alcohol are taken as raw materials for Williamson ether synthesis so as to obtain 4-nitryl-O6-benzylguanine; 4-nitryl- O6-benzylguanine is subjected to reduction reaction with protection of t-butyloxycarboryl; coupling reaction of an obtained compound with gamma-aminobutyric acid protected by carbobenzoxy chloride is carried out in the presence of ethyl dimethyl carbodiimide and 1-hydroxybenzotriazole; O<6>-benzylguanine derivative modified PEGA resin is obtained via reduction, separation and purification, reaction with PEGA, and deprotection; and transalkylation reaction of the O<6>-benzylguanine derivative modified PEGA resin with a protein containing MGMT label is carried out, so that oriented immobilization on PEGA resin surface via thioether covalent bonds is realized. Reaction of the preparation method is stable; the steps are simple; a stable uniform protein coating with uniform orientation can be formed on solid material surface by a prepared product; and the preparation method is used for preparing reagent kit with specific recognition effects.
Owner:NORTHWEST UNIV
Combretastatin compound and preparation method and application thereof
InactiveCN102249987ANovel structureThe synthesis process is simpleOrganic chemistryAntineoplastic agentsDistillationEthyl acetate
The invention discloses a combretastatin compound and a preparation method. The preparation method of the combretastatin compound of the invention comprises the following steps: dissolving 3'-amino combretastatin and N-(1-oxyl-2,2,6,6,-tetramethyl-oxygen-carbonyl)-L-amino acid in dried dichloromethane, uniformly stirring under argon protection, adding dicyclohexylcarbodiimide and 1-hydroxybenzotriazole, stirring and reacting under argon protection, filtering and removing white precipitates after the reaction, removing the solvent by distillation to obtain a crude product, purifying the crude product by column chromatography, and eluting the product by petroleum ether and ethyl acetate liquid with a volume ratio of 10:1-5:1 to obtain the target product. The combretastatin compound of the invention is applicable to the preparation of anticancer medicaments, and is especially applicable to the preparation of medicaments for treating leukemia, liver cancer, gastric cancer, and cervical cancer.
Owner:LANZHOU UNIVERSITY
Auto cooling fluid
InactiveCN104087255AImprove stabilityGuaranteed cycleHeat-exchange elementsSilver carbonateSilver phosphate
The invention discloses auto cooling fluid, comprising the following raw materials in parts: 10-18 parts of diethylene glycol, 11-14 parts of oxalic acid, 13-15 parts of 1,2-propylene glycol, 12-15 parts of ethylene glycol ethyl ether acetate, 8-12 parts of triethyl phosphonoacetate, 8-12 parts of glufosinate-ammonium, 5-8 parts of N-(phosphonomethyl)iminodiacetic acid, 6-8 parts of styrene-acrylic-triazole, 6-7 parts of 1-hydroxybenzotriazole, 6-9 parts of benzotriazole, 2-3 parts of dimeticone, 1-3 parts of hydroxyl silicone oil, 3-4 parts of a dimethyl silicone polymer, 3-5 parts of butanol, 1-3 parts of sodium hydroxide, 2-3 parts of sodium peroxide, 2-4 parts of potassium chlorate, 1-3 parts of silver carbonate, 2-5 parts of silver phosphate and 150-160 parts of purified water. The auto cooling fluid has good stability, the microbial flora can be inhibited and killed, floccules, flotage, sediments and the like are prevented, effective circulation of the cooling liquid is ensured, the fault of a cooling liquid circulating system is relieved and prevented, the anti-corrosion effect is good, corrosion to the cooling liquid circulation system can be reduced and delayed, the fault of the cooling liquid circulating system is reduced, and the service life of the auto cooling liquid is prolonged.
Owner:梁胜光
High-property water-based cutting fluid for metal working and preparation method thereof
The invention discloses a high-property water-based cutting fluid for metal working. The invention is characterized in that the cutting fluid is prepared from the following raw materials in parts by weight: 0.5-1 part of 1-hydroxybenztriazole, 2-3 parts of zinc sulfate, 2-4 parts of polysodium phosphate, 2-3.5 parts of hydraulic oil, 3.5-5 parts of sodium dodecyl benzene sulfonate, 2-3 parts of sulfurized haco oil, 1.5-2.5 parts of EDTA disodium, 0.8-1.5 parts of cuprous oxide, 5-7 parts of assistant and 200 parts of deionized water. By mixing the oily lubricating substance and surfactant, the cutting fluid has favorable lubricating property and enhances the product quality stability; by adding the 1-hydroxybenztriazole, sulfurized haco oil, hydraulic oil and the like, the extreme pressure abrasion resistance and rust resistance are enhanced, and the metal surface can not be easily abraded; and by adding the assistant, the cutting fluid has favorable abrasion resistance, dispersity, lubricating property and film formation property. Due to the adoption of the water-based formula, the cutting fluid has the advantages of favorable cleaning property, favorable cooling property, stable quality and low tendency to deterioration, and is easy to store and suitable for metal working.
Owner:绩溪县徽洋车桥有限责任公司
Deoxyribonucleic acid (DNA) base analogue with photo-crosslinking group biaziridine and synthetic method thereof
ActiveCN103435648ASimple stepsRoutine operation is stableGroup 5/15 element organic compoundsMonomerN,N-Diisopropylethylamine
The invention discloses a deoxyribonucleic acid (DNA) base analogue with a photo-crosslinking group biaziridine and a synthetic method thereof, and relates to the DNA base analogue and the synthetic method thereof. The DNA base analogue with the photo-crosslinking group biaziridine is a biaziridine phosphoramidite monomer; an intermediate product with two hydroxyl groups is connected on the basis of a compound 1; a material 1-amino-hexylene glycol is led; an amido bond is formed under condensation of dicyclohexylcarbodiimide and 1-hydroxybenzotriazole, so as to obtain an immediate product 2; the material 4-4'-dimethoxy triphenyl methyl chloride is led; pyridine and dichloromethane are taken as solvents; an immediate product 3 is obtained under catalytic action of 4-dimethylamino-pyridine; the material 2-cyanoethyl N, N-diisopropyl chlorinated phosphoramide is led; N,N-diisopropylethylamine is taken as alkali and a catalyst; dichloromethane is taken as a solvent, so as to obtain the final product under the condition of anaerobic reaction without water.
Owner:XIAMEN UNIV
Transparent, low-flammability, UV-resistant film made from a crystallizable thermoplastic, its use and process for its production
InactiveUS7138176B2Good orientationMaintain good propertiesGain controlSynthetic resin layered productsThermoplastic2-hydroxybenzophenone
The present invention relates to a transparent, low-flammability, UV-resistant, oriented film made from a crystallizable thermoplastic and having a thickness of from 5 to 300 μm. The film comprises at least one UV stabilizer and at least one flame retardant, and at least the flame retardant, and preferably also the UV stabilizer, is fed directly as a masterbatch to the crystallizable thermoplastic during production of the film. The film may have one or more layers, and the UV stabilizer may have been selected from the group consisting of the 2-hydroxybenzophenones, the 2-hydroxybenzotriazoles, the organonickel compounds, the salicylic esters, the cinnamic ester derivatives, the resorcinol monobenzoates, the oxanilides, the hydroxybenzoic esters, the sterically hindered amines and triazines, and the flame retardant may be an organic phosphorus compound, in particular an organic phosphorus compound soluble in polyethylene terephthalate.
Owner:MITSUBISHI POLYESTER FILM
Antirust coating with high acid resistance and preparation method thereof
The invention discloses an antirust coating with high acid resistance, which comprises the following components by weight: 70-80 parts of styrene-acrylic emulsion, 24-30 parts of pure acrylic emulsion, 6-8 parts of ethanol, 1-3 parts of ethylene glycol monobutyl ether, 2-3 parts of triethanolamine, 1-3 parts of hydroquinone, 1-3 parts of 2,6-butylated hydroxytoluene, 3-4 parts of sodium ethylenediamine tetramethylene phosphonate, 2-3 parts of tartaric acid, 1-2 parts of citric acid, 1-2 parts of modified adhesive, 0.6-0.9 part of 1-hydroxybenzotriazole, 0.3-0.5 part of sodium polyacrylate, and a proper amount of deionized water. The coating is excellent in comprehensive performance of adhesion to a substrate, corrosion resistance and adhesion, completely replaces the sand blasting and shot-blasting coating pretreatment process, protects the surface of steel which is rusted and not pre-treated efficiently for a long time, and not only prolongs the service life of anti-corrosion finish, but also is simpler, more efficient, more economic, and more environmental-friendly in the anti-corrosion coating process.
Owner:ANHUI CHAOHU SOUTH MEMBRANE IND
Preparation method of acetic acid redfish calcitonin
InactiveCN103254305AImprove coupling efficiencyHigh yieldCalcitoninsPeptide preparation methodsFreeze-dryingRedfish
The invention discloses a preparation method of acetic acid redfish calcitonin. The preparation method comprises the following steps of: deprotecting Rink Amide MBHA resin by using a deprotection reagent and removing a Fmoc protecting group; sequentially coupling the deprotected Rink Amide MBHA resin serving as a starting material with Fmoc-protected amino acids serving as monomers to obtain acetic acid redfish calcitonin peptide resin, wherein a condensing agent is N,N-diisopropyl carbodiimide (DIC) / 1-hydroxybenzotriazole (HOBt) or benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP) / HOBt; splitting the acetic acid redfish calcitonin peptide resin, adding diethyl ether and precipitating the acetic acid redfish calcitonin peptide resin to obtain reduction type acetic acid redfish calcitonin crude peptide; cyclizing the reduction type acetic acid redfish calcitonin crude peptide to obtain oxidation type acetic acid redfish calcitonin crude peptide; and performing purification, salt conversion, concentration and freeze drying on the oxidation type acetic acid redfish calcitonin crude peptide to obtain the acetic acid redfish calcitonin. According to the preparation method, the yield of the acetic acid redfish calcitonin reaches over 20 percent.
Owner:QINGDAO GUODA BIOLOGICAL PHARMA
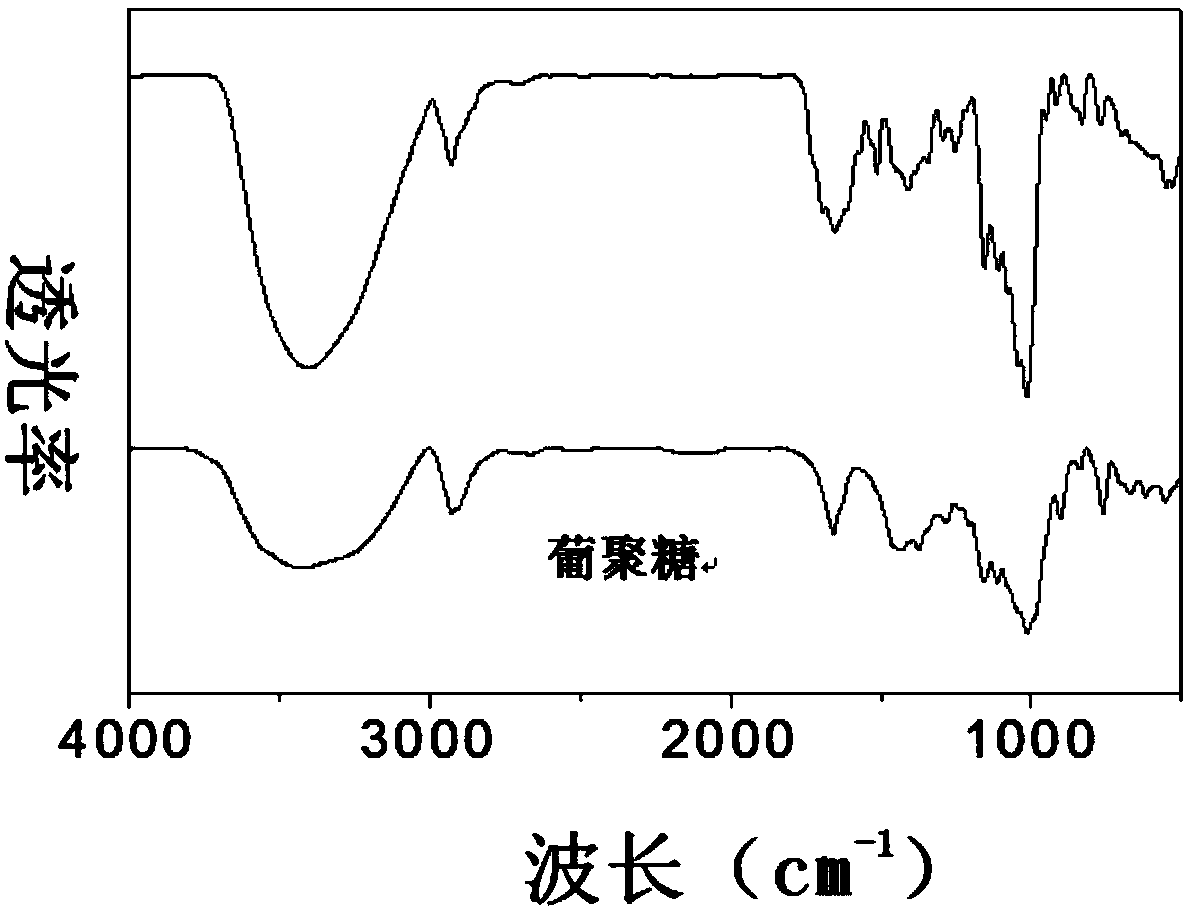
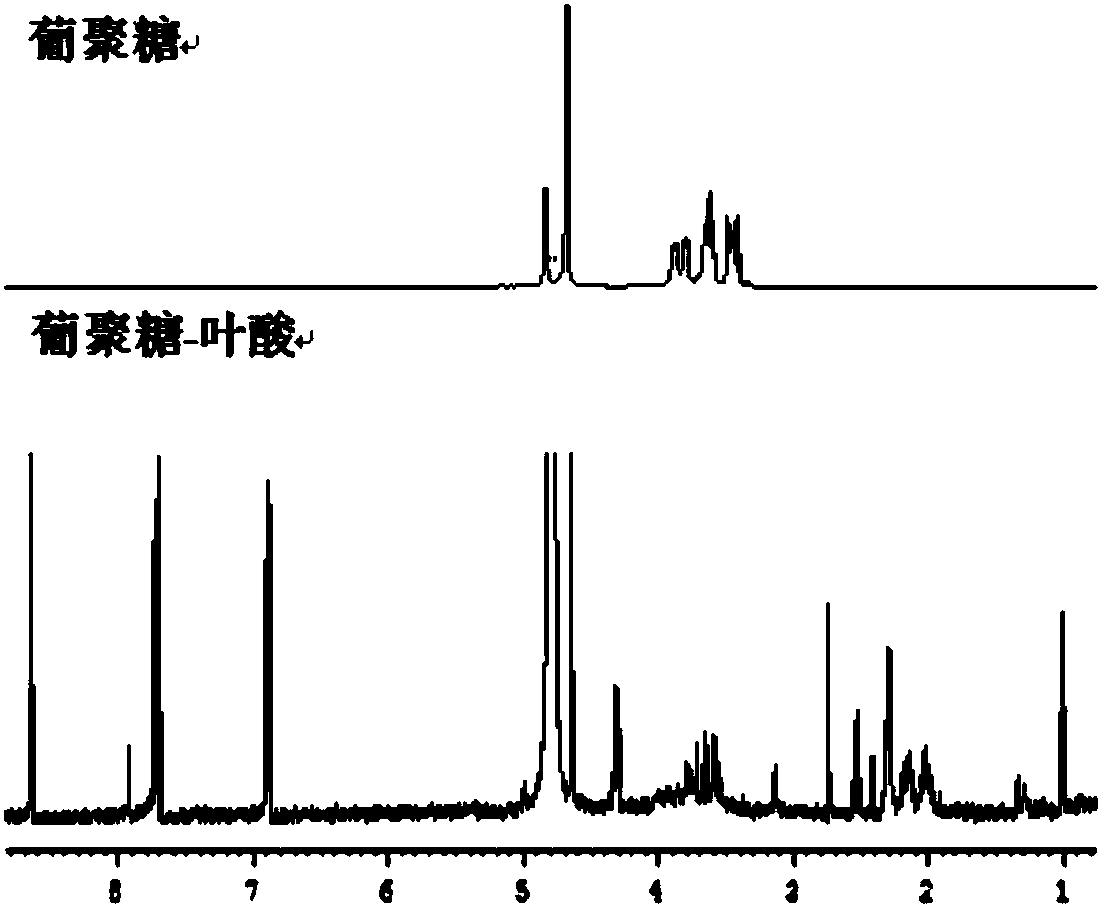
Preparation method of polysaccharide grafted folic acid copolymer and nanoparticle thereof
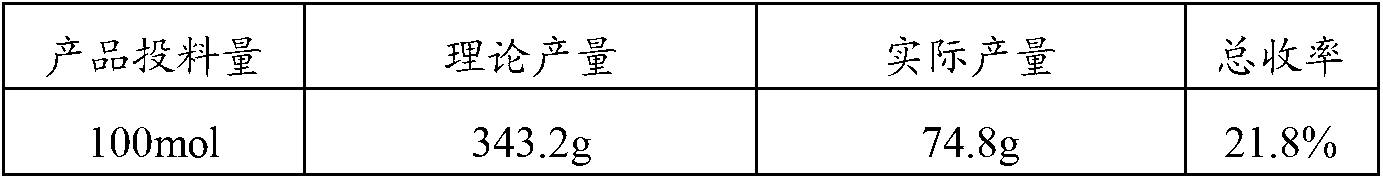
ActiveCN108264578AThe synthesis steps are simpleHigh biosecurityPharmaceutical non-active ingredientsAntineoplastic agentsTumor targetNitrogen gas
The invention discloses a preparation method of a polysaccharide grafted folic acid copolymer and a nanoparticle thereof. The method includes: using anhydrous dimethylsulfoxide to dissolve folic acid,then adding 1-hydroxybenzotriazole and N-N'-dicyclohexylcarbodiimide for carboxyl activating reaction, mixing a carboxyl activated folic acid solution with a dimethylsulfoxide solution of polysaccharide, carrying out esterification reaction for 24-72h under nitrogen protection and at a temperature of 40-80DEG C, and performing purification to obtain a copolymer mixture; conducting freezing dryingto obtain the polysaccharide grafted folic acid copolymer lyophilized powder, i.e. the polysaccharide grafted folic acid copolymer. The polysaccharide grafted folic acid copolymer can be applied as acarrier in preparation of a nanoparticle preparation with folic acid receptor targeting function, and the nanoparticle preparation can be used for tumor targeted drug delivery.
Owner:HUAZHONG UNIV OF SCI & TECH
Volatile rust preventive oil
The invention discloses a volatile rust preventive oil. The volatile rust preventive oil comprises, by weight, 62-70 parts of No.50 machine oil, 1-2 parts of sodium benzoate, 3-4 parts of zinc butyl octyl dithiophosphate t202, 3-4 parts of polyether, 1-2 parts of alkyl amine oxide polyoxyethylene ether, 2-3 parts of amino silicone oil, 0.2-1 parts of polyethylene glycol 1000, 0.1-0.2 parts of potassium triborate, 5-7 parts of neutral barium dinonynalphthalene sulfonate, 2-3 parts of 1-hydroxybenzotriazole and 10-13 parts of a film forming assistant. The rust preventive oil has the advantages of safe use of raw materials, and good environmental protection property, is especially suitable for being used on workpieces with complex structures, and has a very good slow release effect on the fine seams of parts or components with fine apertures.
Owner:TIANCHANG RUNDA METAL ANTIRUST AUX
Industrial dewaxing agent
The invention provides an industrial dewaxing agent. The industrial dewaxing agent is prepared by dissolving oleic acid diethanolamine ester, a surfactant, a corrosion inhibiter and a wetting agent in deionized water and then uniformly mixing. The industrial dewaxing agent consists of the following ingredients in parts by mass: 10-15 parts of oleic acid diethanolamine ester, 5-8 parts of the surfactant, 3-4 parts of the corrosion inhibiter and 40-50 parts of deionized water, wherein the oleic acid diethanolamine ester is a reaction product of oleic acid and diethanolamine according to the molar ratio of 1:2; the surfactant comprises span 20, span 60, span 80, tween 40 and tween 60; the corrosion inhibiter is one of benzotriazole, petroleum sulfonate and alpha-hydroxybenzotriazole.
Owner:季爱英
A synthetic method of amikacin
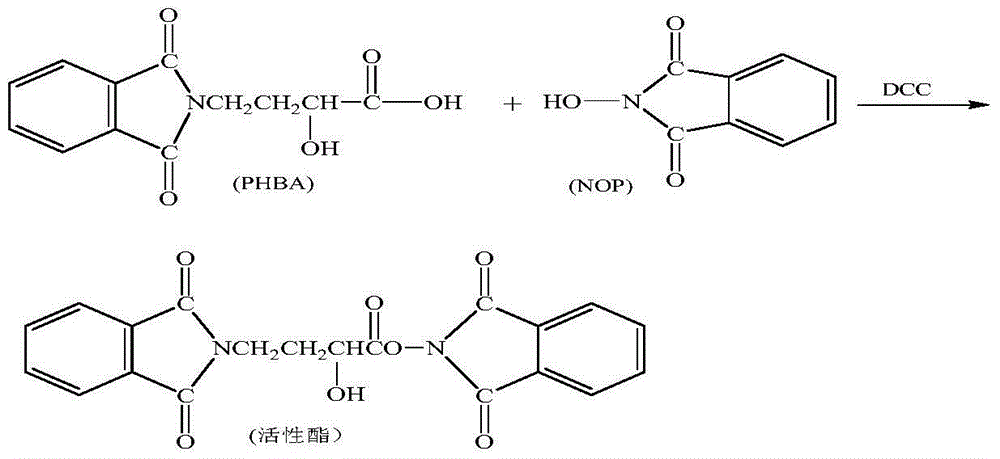
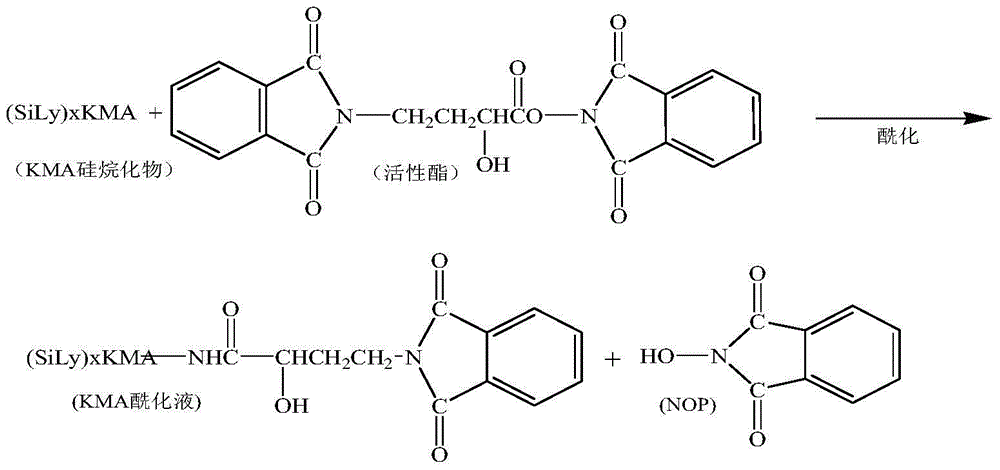
InactiveCN105440090AReduce manufacturing costEasy to operateSugar derivativesSugar derivatives preparationAmikacinSilylene
A synthetic method of amikacin is disclosed. Gamma-4-phthalimido-2-hydroxy butyric acid is adopted as a raw material for direct acylation. 4-dimethylaminopyridine (DMAP) or 1-hydroxybenzotriazole (HOBT) is adopted as a catalyst. N,N'-dicyclohexylcarbodiimide is adopted as a condensing agent. Silyl kanamycin A is directly acylated to obtain an acylation product and the acylation product is subjected to acidolysis and hydrazinolysis to obtain the amikacin. A production operation for specially preparing active ester for acylation is not needed in the method, thus simplifying operation steps and production equipment. N-hydroxyphthalimide for preparing the active ester is not used in the direct acylation, thus reducing the production cost. By optimization of acylation conditions, selectivity of the acylation is improved, the synthesis yield is ensured, contents of impurities is lowered, and beneficial conditions for subsequent purification of the amikacin are provided.
Owner:CHONGQING DAXIN PHARMA +2
Rust removal cleaning agent
The invention discloses a rust removal cleaning agent, which is prepared by uniformly mixing the following components in parts by mass: 10 to 15 parts of EDTA (ethylene diamine tetraacetic acid), 4 to 10 parts of a surfactant, 1 to 3 parts of bentonite and 3 to 4 parts of a corrosion inhibitor, wherein the surfactant is one of polyoxyethylene stearate and diethylene glycol fatty acid ester; the corrosion inhibitor is one of benzotriazole, petroleum sulfonate, alpha-hydroxybenzotriazole, hexamethylenetetramine, tallow amine and 8-hydroxyquinoline.
Owner:季爱英
Preparation method of water-soluble magnolol derivative, honokiol derivative and intermediate of magnolol derivative and honokiol derivative and related monohydroxyl protection intermediate
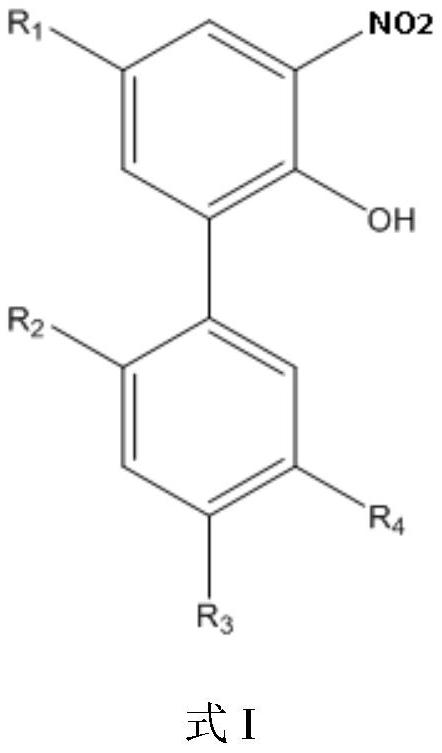
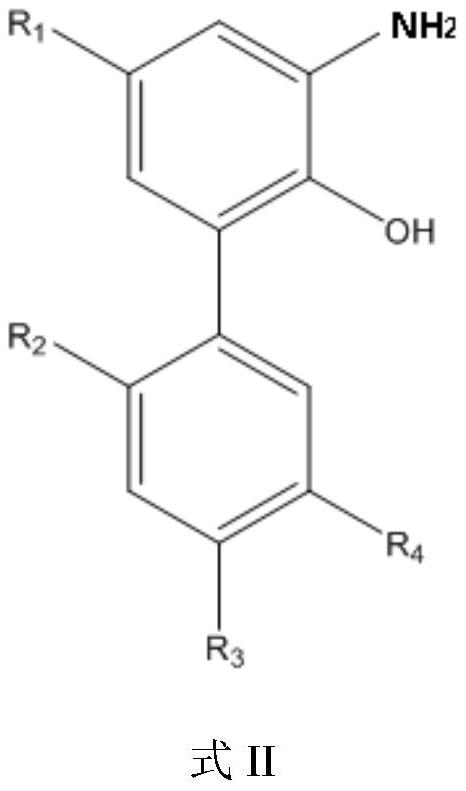
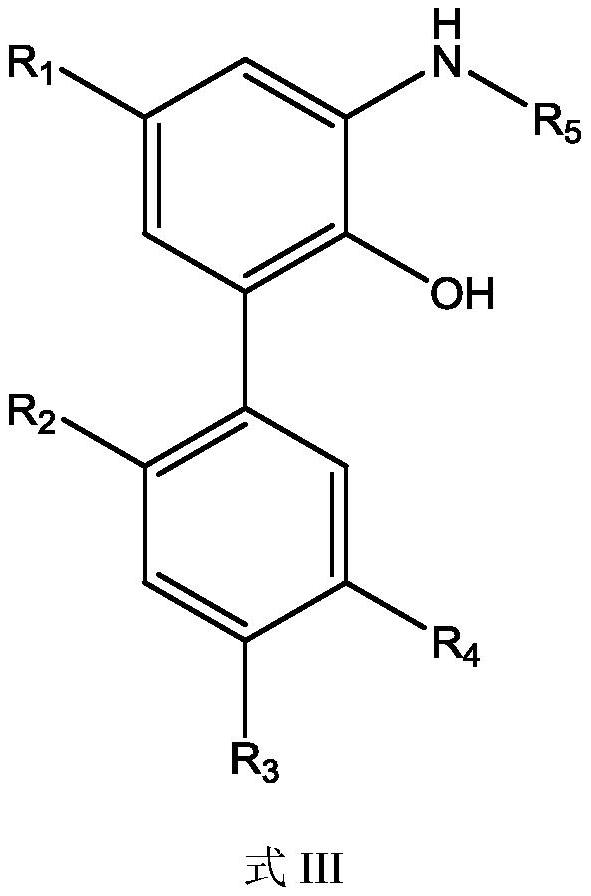
ActiveCN112125805AHigh selectivityHigh synthesis efficiencyCarbamic acid derivatives preparationOrganic compound preparationMagnololNitration
The invention provides a preparation of a water-soluble magnolol derivative, a honokiol derivative and an intermediate of the magnolol derivative and the honokiol derivative and a related monohydroxylprotection intermediate. The nitration intermediate has a structure as shown in a formula I which is described in the specification, wherein in the formula I, R2 is hydroxyl, and R3 is H; or R2 is H,and R3 is hydroxyl; and R1 and R4 are respectively and independently selected from electron donating groups of C1-C12. The preparation method comprises the steps of carrying out monohydroxyl protection on a compound A and a hydroxyl protection reagent in the presence of an acid-binding agent to form a monohydroxyl protection compound, wherein R1, R2, R3 and R4 in the compound A have the same definition as the former, and the hydroxyl protection reagent is p-toluenesulfonyl chloride and 1-hydroxybenzotriazole; and sequentially carrying out nitration reaction and deprotection reaction on the monohydroxyl protection compound to obtain a nitration intermediate. The synthesis efficiency of the water-soluble magnolol derivative and the honokiol derivative is effectively improved.
Owner:BEIJING HONGHUI MEDITECH CO LTD
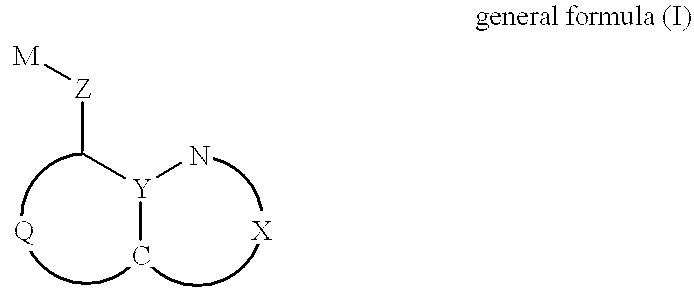
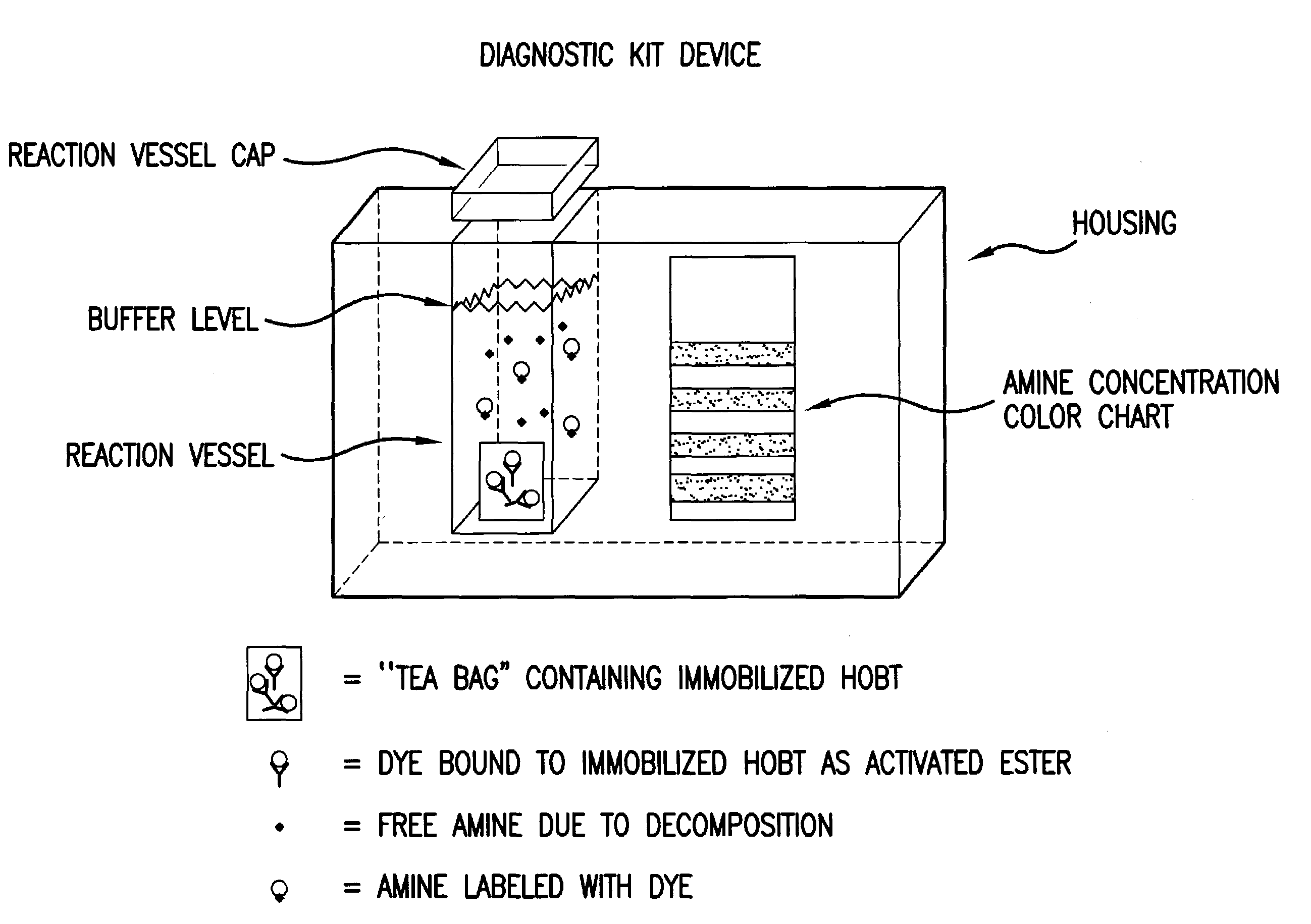
Amine detection method and materials
InactiveUS7229835B2Bioreactor/fermenter combinationsBiological substance pretreatmentsCarboxylic acidDerivatization
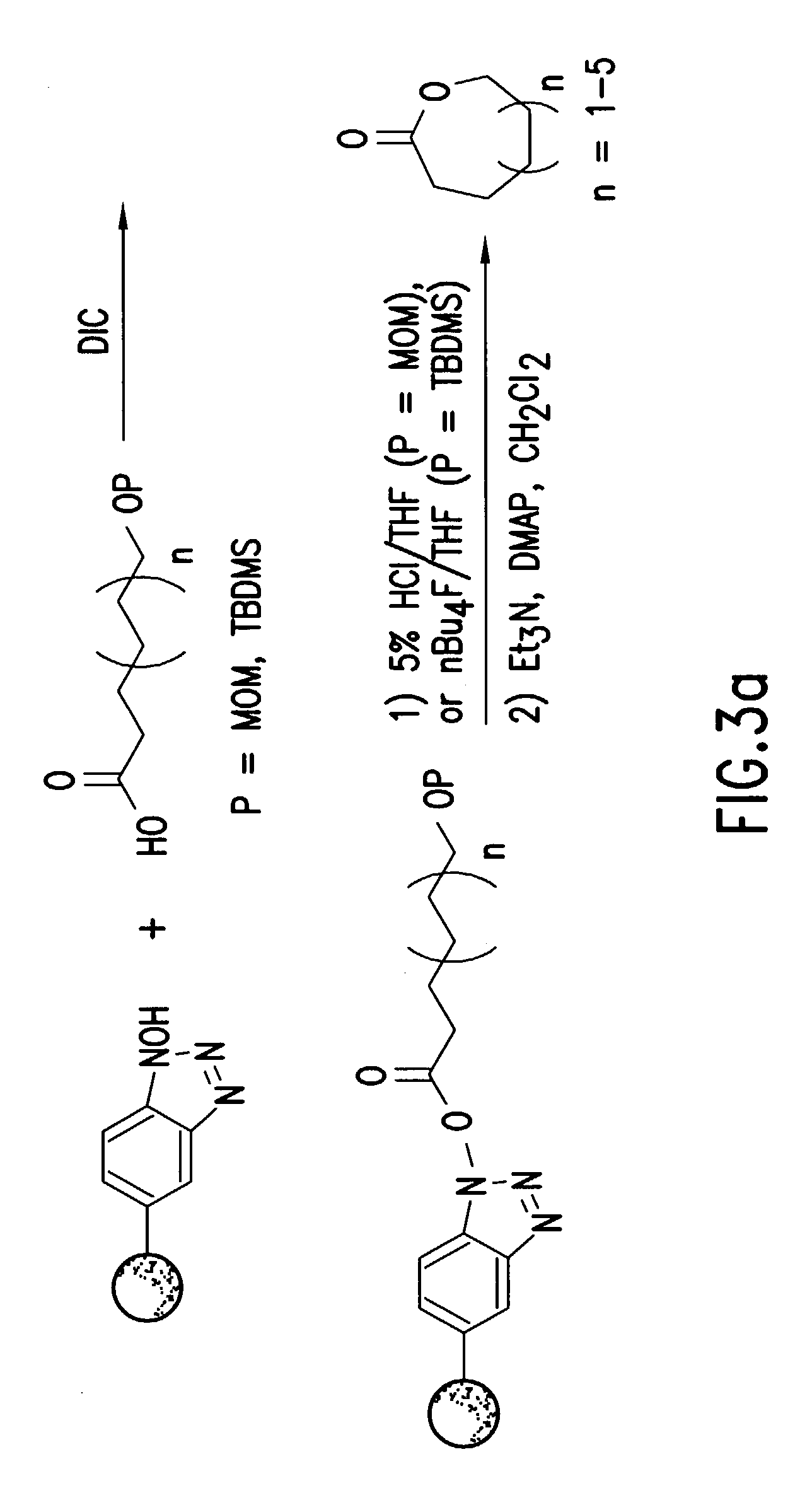
A compound linked to a solid support (R) through a divalent linker moiety (X) and which is represented by the following formula:is disclosed. In particular, the 1-hydroxybenzotriazole-6-carboxylic acid is directly linked to the support under mild conditions (i.e., in aqueous or organic solvents at neutral pH and at room temperature). The polymer bound 1-hydroxybenzotriazole-6-carboxylic acid can be used for the derivatization of amines as well as for single step amino group modification of proteins, peptides, and amines via acylation or sulfonylation reactions. A flow through device and method for the single step amino group modifications of proteins, peptides, and amines is disclosed. Also disclosed is a flow through device for the detection of amines in a sample. Additionally, a device and method for the detection of amines in a sample using 1-hydroxybenzotriazole-6-carboxylic acid is disclosed. In a preferred embodiment, the device is used to detect the presence of amines in a spoiled meat product. Diagnostic kits for detecting the presence of amines is also disclosed.
Owner:UNIV OF MARYLAND BALTIMORE COUNTY
Method for detecting 1-hydroxybenzotriazole in soil and plants
ActiveCN106908539AReduce usageEasy to operateComponent separationHydroxybenzotriazoleSolid phase extraction
The invention discloses a method for detecting 1-hydroxybenzotriazole in soil and plants. The method comprises following steps: pretreatment of a sample; accelerated solvent extraction; preparation of a standard working solution; detection with an HPLC-MS (high performance liquid chromatography-mass spectrometer). According to the method, a rapid and efficient extraction method with high recovery rate is established with an accelerated solvent extraction technology, after synchronous purification or solid-phase extraction purification, detection is performed with HPLC-MS, so that matrix interference is effectively eliminated, the recovery rate of 1-hydroxybenzotriazole in a soil sample is 92.6%, standard deviation is 2.4%, the matrix effect is 90.7%, detection limit is 0.15 ng / g, and limit of quantitation is 0.51 ng / g; the recovery rate of 1-hydroxybenzotriazole in a plant sample is 89.8%, standard deviation is 1.6%, the matrix effect is 90.3%, detection limit is 0.69 ng / g, and limit of quantitation is 2.31 ng / g.
Owner:中科检测技术服务(广州)股份有限公司
Synthesis technique of benzotriazole
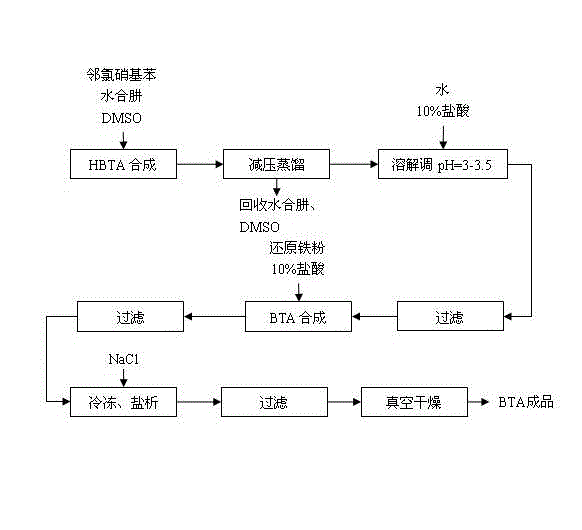
The invention relates to a synthesis technique of benzotriazole, which comprises the following two reaction steps: 1. reacting o-chloronitrobenzene and hydrazine hydrate to generate 1-hydroxybenzotriazole (HBTA), wherein DMSO (dimethyl sulfoxide) is used as a solvent to implement synthetic reaction of HBTA under homogeneous conditions; and 2. reacting the HBTA and reduced iron powder to generate the benzotriazole product. The DMSO with strong polarity promotes the reaction, so that the hydrazine hydrate can be added at one time, and the consumption of the hydrazine hydrate is reduced as compared with the existing technique; and meanwhile, the DMSO and hydrazine hydrate can be conveniently recovered. In the after-treatment process of preparing benzotriazole by reducing the HBTA, the salting-out mode is utilized to separate the product, thereby greatly enhancing the production efficiency and lowering the production cost as compared with the existing extraction method.
Owner:陈守文
Synthesis method of 2-amino-5-chloro-N,3-dimethylbenzamide
InactiveCN105859574APromote oxidationReduce usageOrganic compound preparationCarboxylic acid amides preparationChemical synthesisBenzoic acid
The invention discloses a synthesis method of 2-amino-5-chloro-N,3-dimethylbenzamide, belonging to the technical field of organic chemical synthesis. The method comprises the following steps: carrying out oxidation under the catalytic actions of N-hydroxyphthalimide and cobalt acetylacetonate to generate benzoic acid, carrying out substitution reaction with chlorine gas to generate 3,5-dichlorobenzoic acid, shielding off 5- chlorine by using a shielding reagent, carrying out methyl substitution on the 5- chlorine by using a Grignard reagent to generate 3-methyl-5-chlorobenzoic acid, carrying out nitro-substitution on the 3-methyl-5-chlorobenzoic acid and nitric acid under the catalytic action of concentrated sulfuric acid to generate 2-nitro-3-methyl-5-chlorobenzoic acid, carrying out catalytic hydrogenation to reduce the nitro group into amino group, carrying out reaction under the actions of N,N'-diisopropylcarbodiimide and 1-hydroxybenztriazole to obtain an intermediate, and carrying out reaction on the intermediate and methylamine to obtain the 2-amino-5-chloro-N,3-dimethylbenzamide. The method is simple to operate, and obviously enhances the synthesis yield to 92% or above.
Owner:CHANGZHOU AMANTE CHEM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com