Magnetic material for efficiently detecting circulating tumor cell and preparation method of magnetic material
A technology for tumor cells and magnetic materials, which is applied in the field of magnetic materials for efficient detection of circulating tumor cells and its preparation, can solve the problems of functionalization strategy limitations, expression differences, etc., and achieve narrow particle size distribution, high saturation magnetization, and magnetic properties. Good responsiveness and biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Synthesis of rhodamine (RhB)-labeled HA-SH
[0039]Dissolve hyaluronic acid HA (Mw=100KD, 806mg, 2mmol) in 200mL PBS buffer (0.01mol / L, pH=7.4). After the HA is completely dissolved, add 1-(3-dimethylaminopropyl )-3-ethylcarbodiimide EDC (1.15g, 6mmol) and 1-hydroxybenzotriazole HOBt (0.81g, 6mmol), then placed on a magnetic stirrer for magnetic stirring for 2h, then weighed cystamine salt Acid acid (1.35 g, 6 mmol) was added to the reaction system, and the reaction was carried out overnight with magnetic stirring. The obtained reaction solution was dialyzed to remove unreacted reactants, and dialyzed against deionized water for 24 hours. Add dithiothreitol DTT (1.54 g, 10 mmol) into the dialyzed reaction solution to continue the reaction for 24 hours, then transfer the reaction solution to a dialysis bag, and dialyze with deionized water for 2 days to remove unreacted DTT to obtain HA -SH.
[0040] RhB-labeled HA-SH can be obtained by grafting RhB on HA-SH ...
Embodiment 2
[0042] Example 2 Preparation of Superparamagnetic Fluorescent HA-SH Capsules (magneticcapsules, MCs)
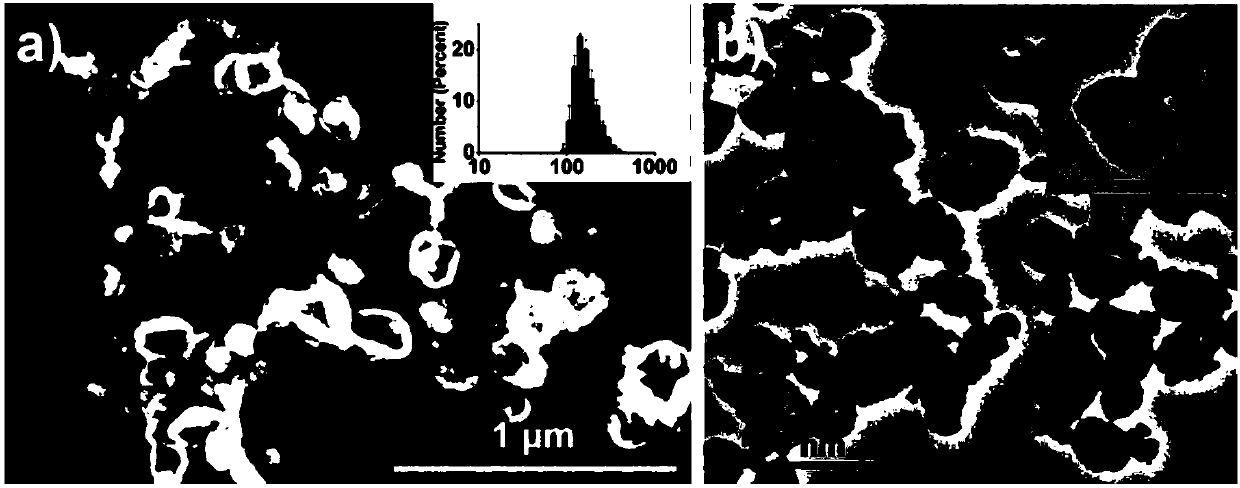
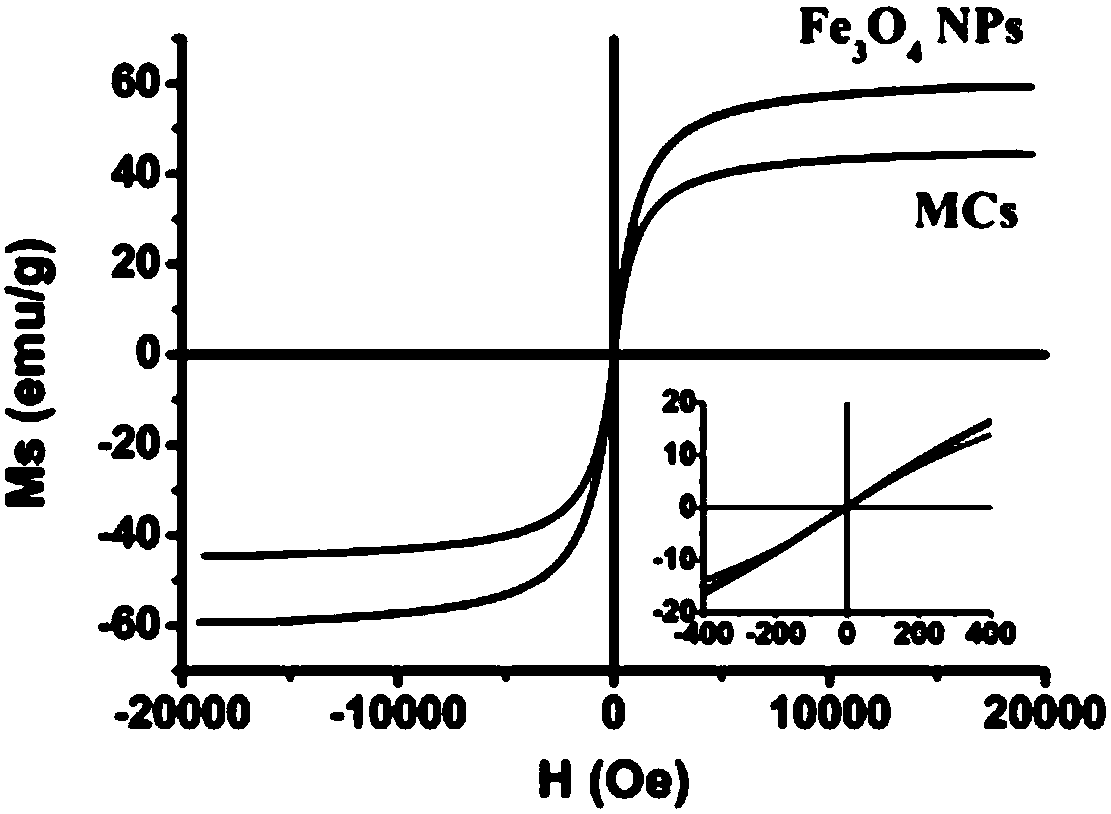
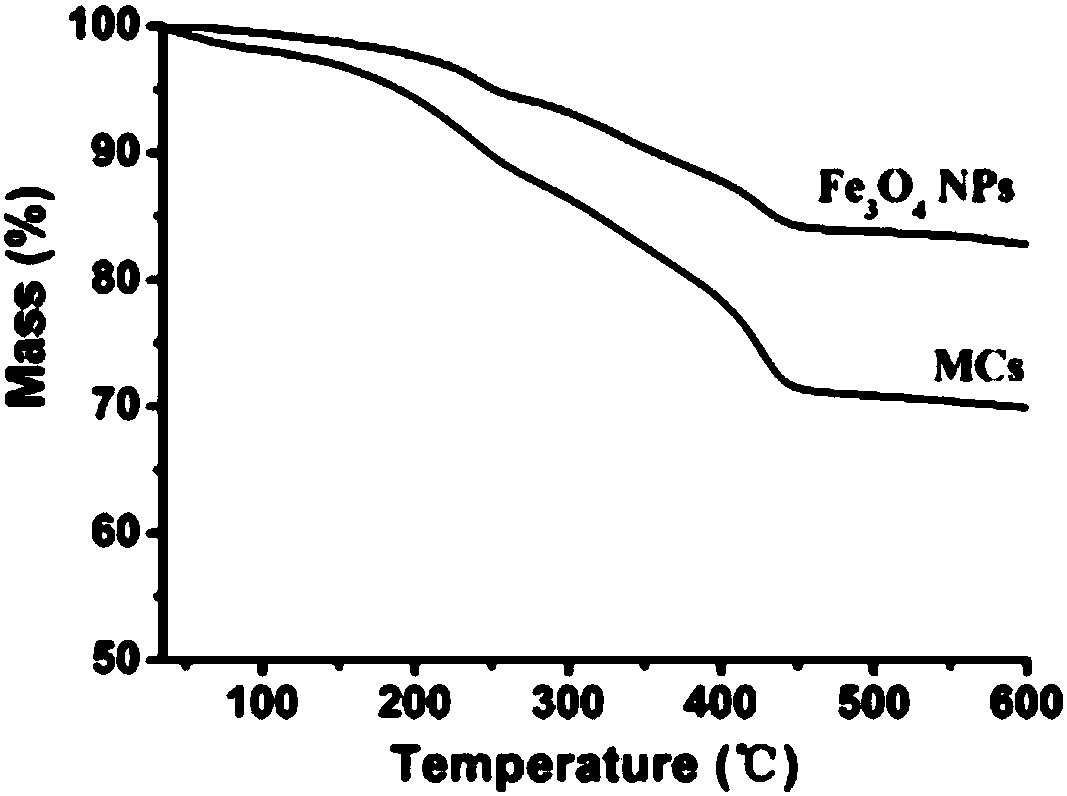
[0043] Take 10mg Fe 3 o 4 Nanoparticles (Fe 3 o 4 NPs) were dispersed in 1 mL of chloroform, 10 mg of RhB-HA-SH was dissolved in 25 mL of deionized water, and the two were mixed and placed in an ice bath. Use a cell disruptor to sonicate 3 times, each treatment for 5min (570W), add 1mL H for the third treatment 2 o 2 To promote the cross-linking of sulfhydryl groups, the resultant is subjected to magnetic separation (magnetic separation is to enrich the sample on a magnet), discard the supernatant after the magnetic separation is completed, and wash several times with PBS buffer solution, and finally adjust the concentration to 1 mg / mL to prepare superparamagnetic fluorescent HA-SH capsules.
[0044] Among them, Fe 3 o 4 Nanoparticles are prepared by pyrolysis method, the specific process is as follows: after removing water and oxygen from the system, iron acetylace...
Embodiment 3
[0050] Example 3 Preparation of targeted functional superparamagnetic fluorescent HA-SH capsules (TMCs)
[0051] The synthesis process of TMCs is as follows Figure 4 shown. Take about 5 mL of MCs suspension, add 2.5 mL of PBS solution containing 1MEDC and 1M HOBt, shake slowly at 4 °C for 4 h to activate MCs, then discard the supernatant by magnetic separation and redisperse the activated MCs in PBS. 10mg PEG-FA was added and reacted at room temperature for 4h, then transferred to 4°C, and about 50μg anti-EpCAM antibody was added to continue the reaction for 6h. The obtained product was collected by magnetic separation, washed several times with PBS buffer to remove unreacted reactants, and stored at 4°C for future use.
[0052] The above reaction products were verified, and the verification process was as follows: an appropriate amount of TMCs and an appropriate amount of Goat Anti-Rabbit IgG H&L (FITC) were incubated at 4°C for 30 min. After washing three times with PBS ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Saturation magnetization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com