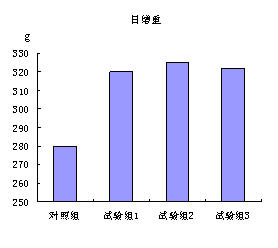
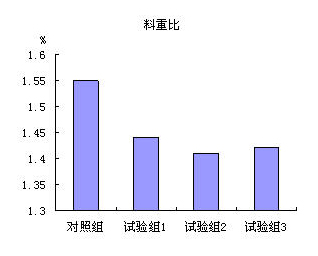
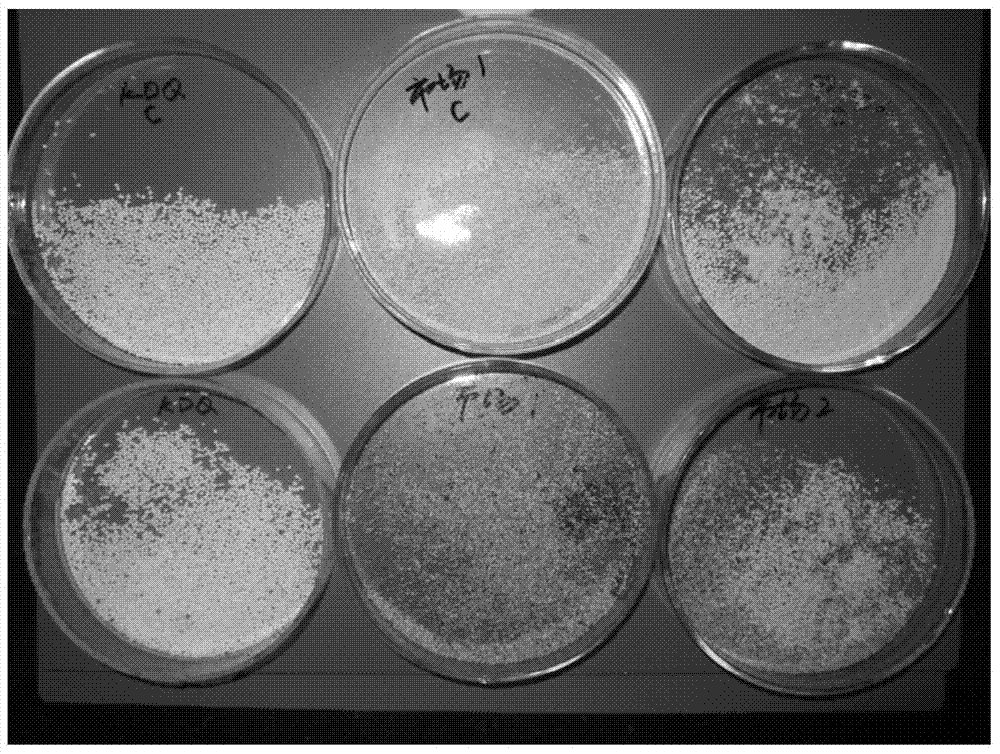
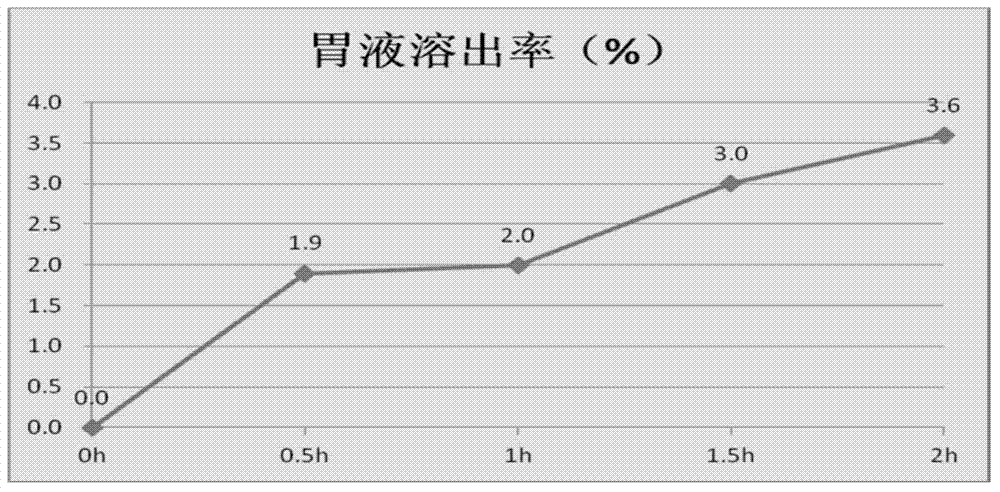
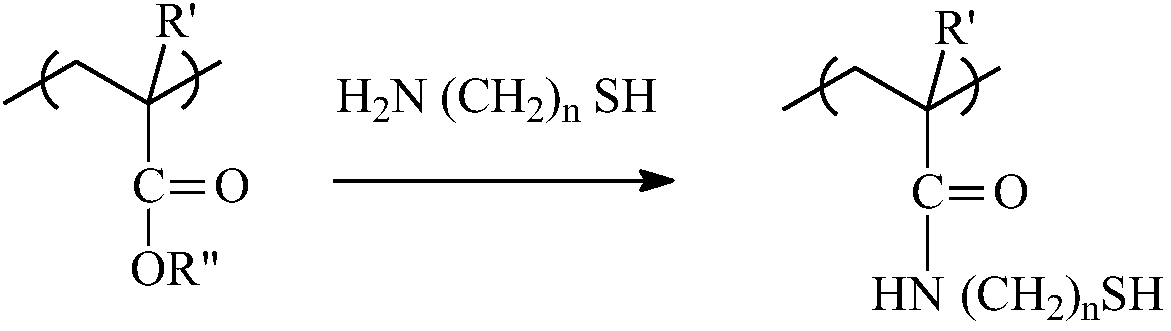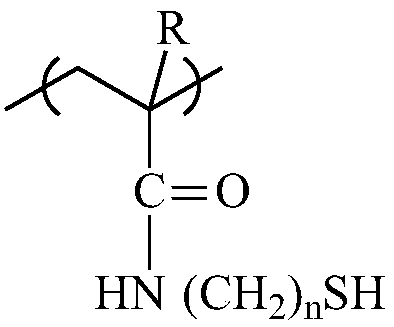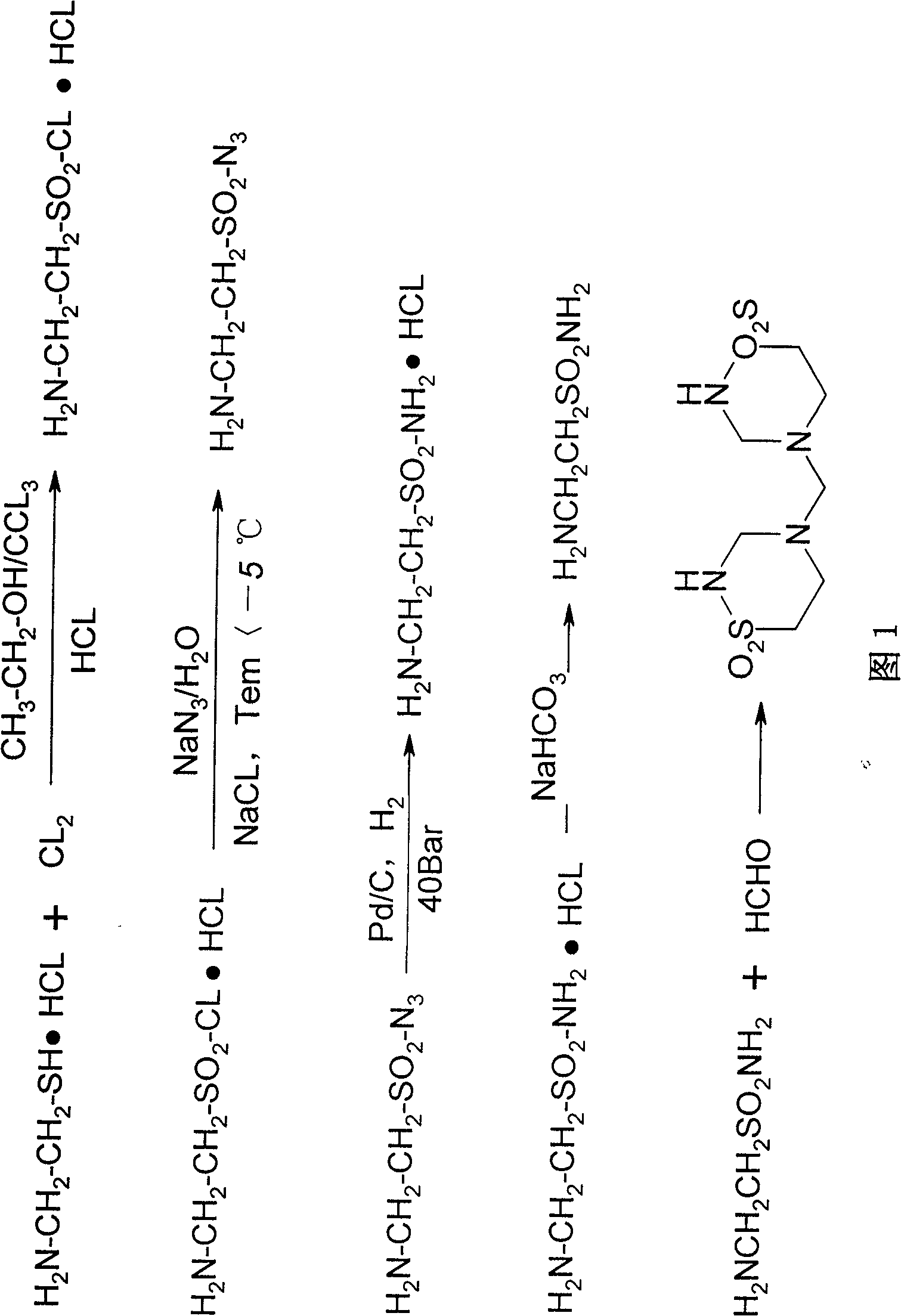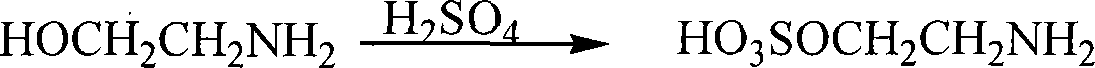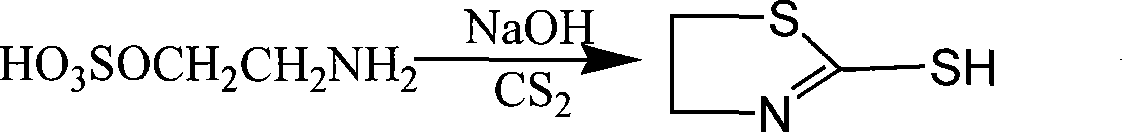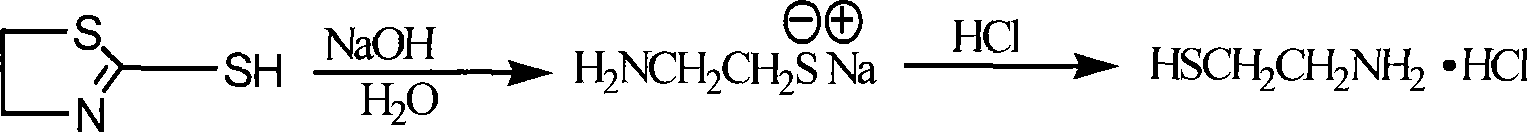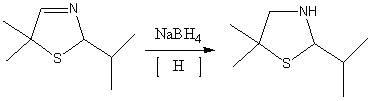Patents
Literature
147 results about "CYSTEAMINE HYDROCHLORIDE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
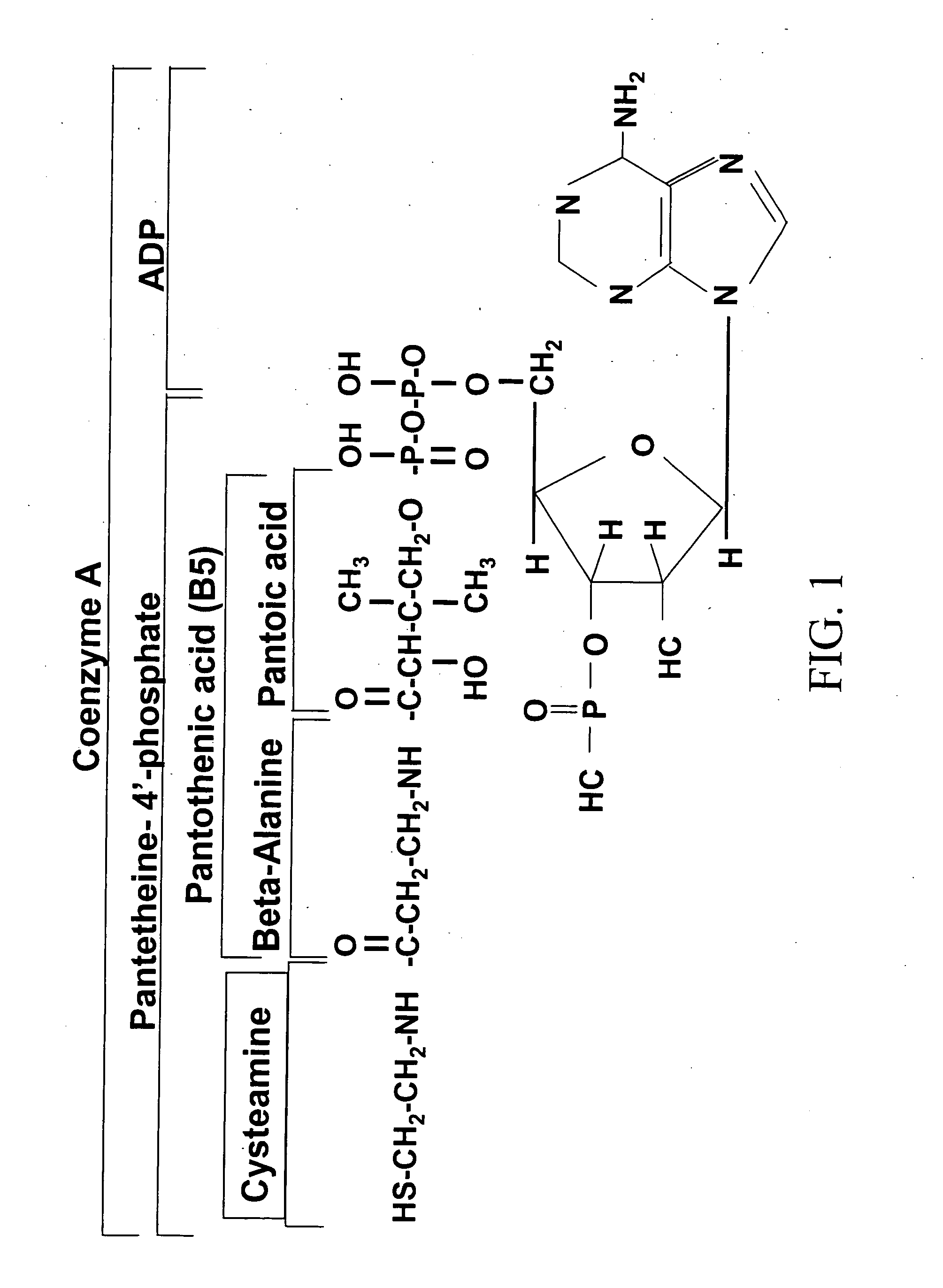
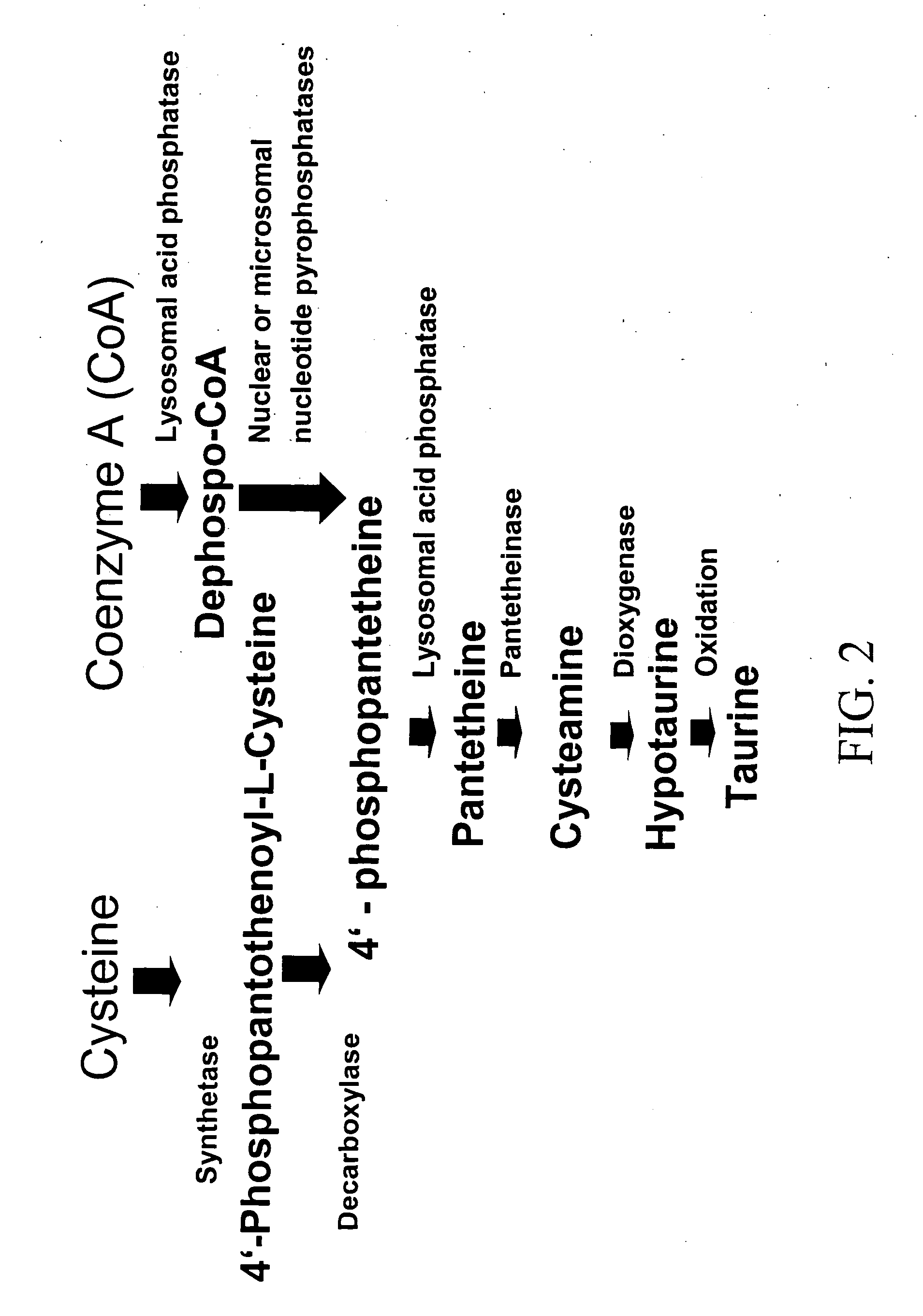
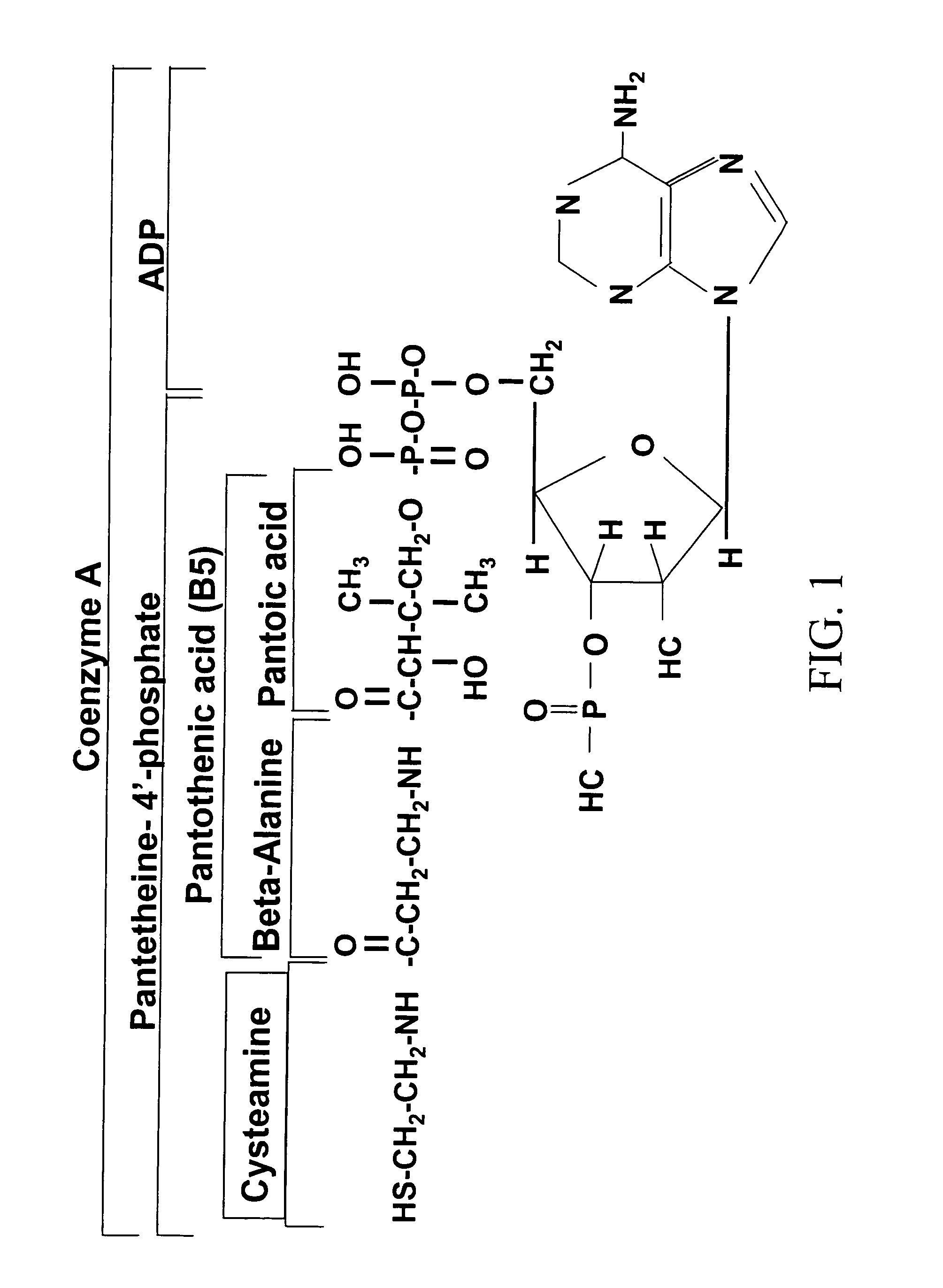
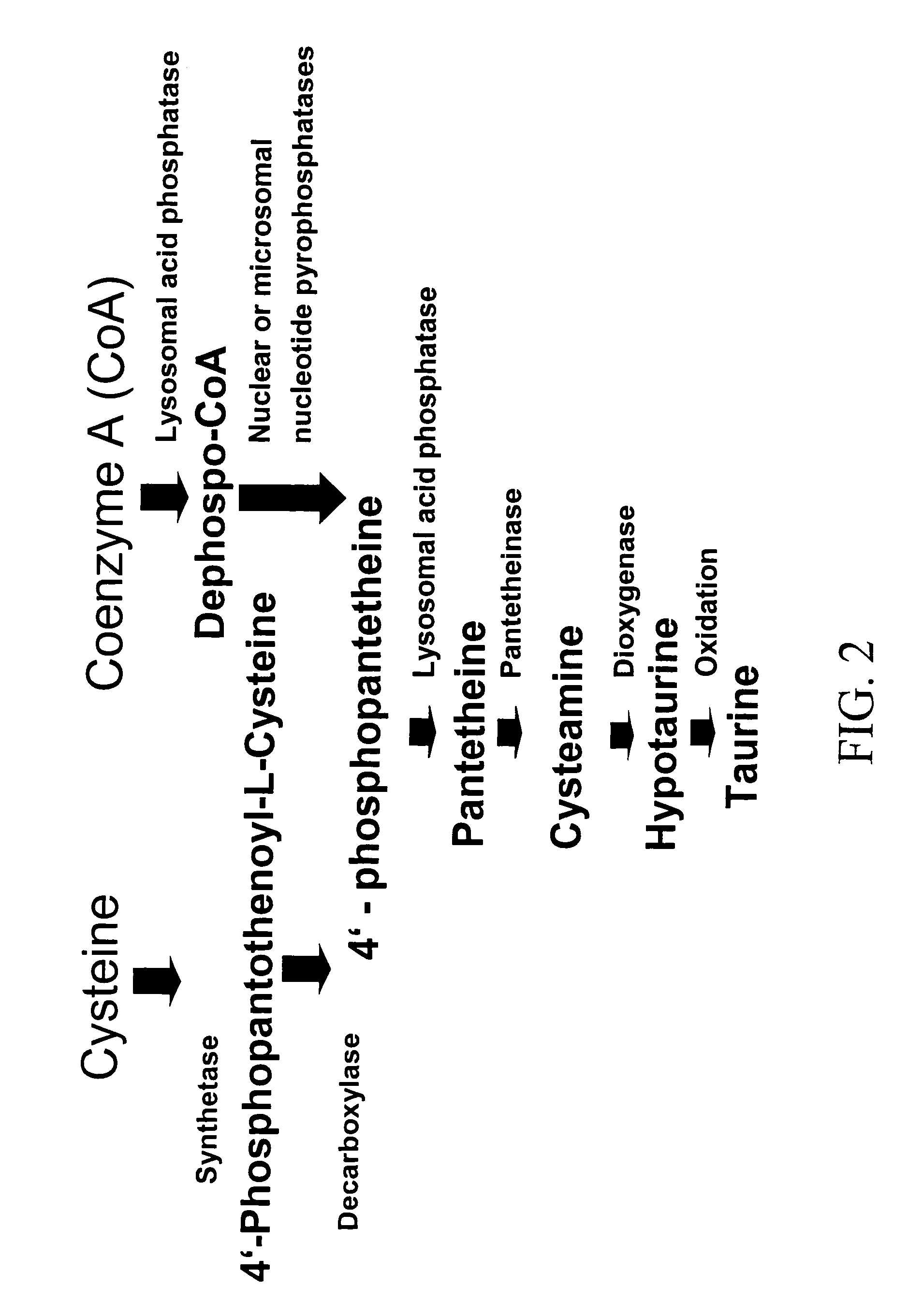
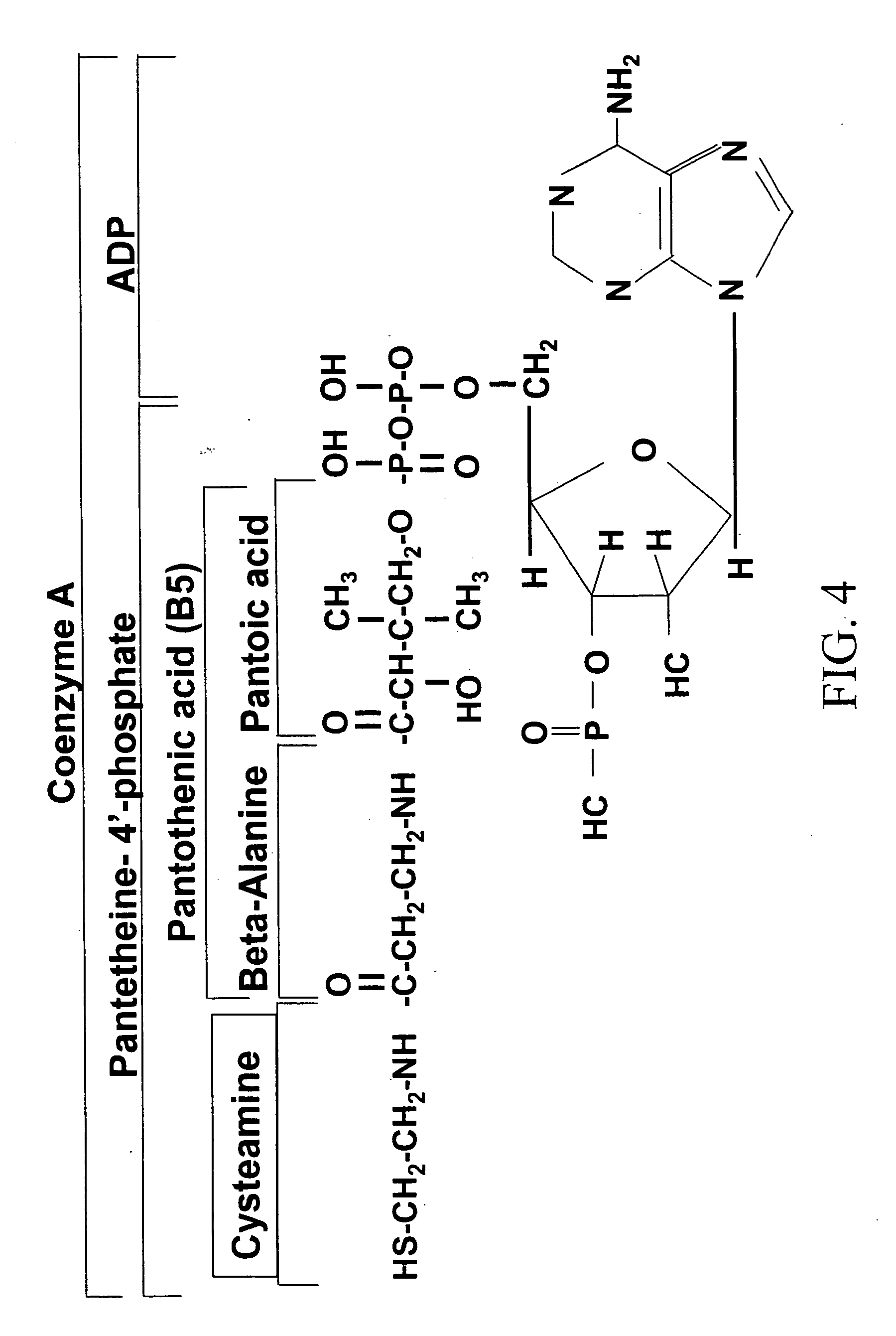
Cysteamine Hydrochloride is the salt formulation of the decarboxylated form of the amino acid cysteine. Clinically, cysteamine hydrochloride is used in the treatment of radiation sickness and disorders of cysteine excretion.
Compositions and methods for treating diabetes
InactiveUS20050137125A1Reducing and eliminating severityReducing and eliminating and intensityBiocideSenses disorderOral medicationGlucose polymers
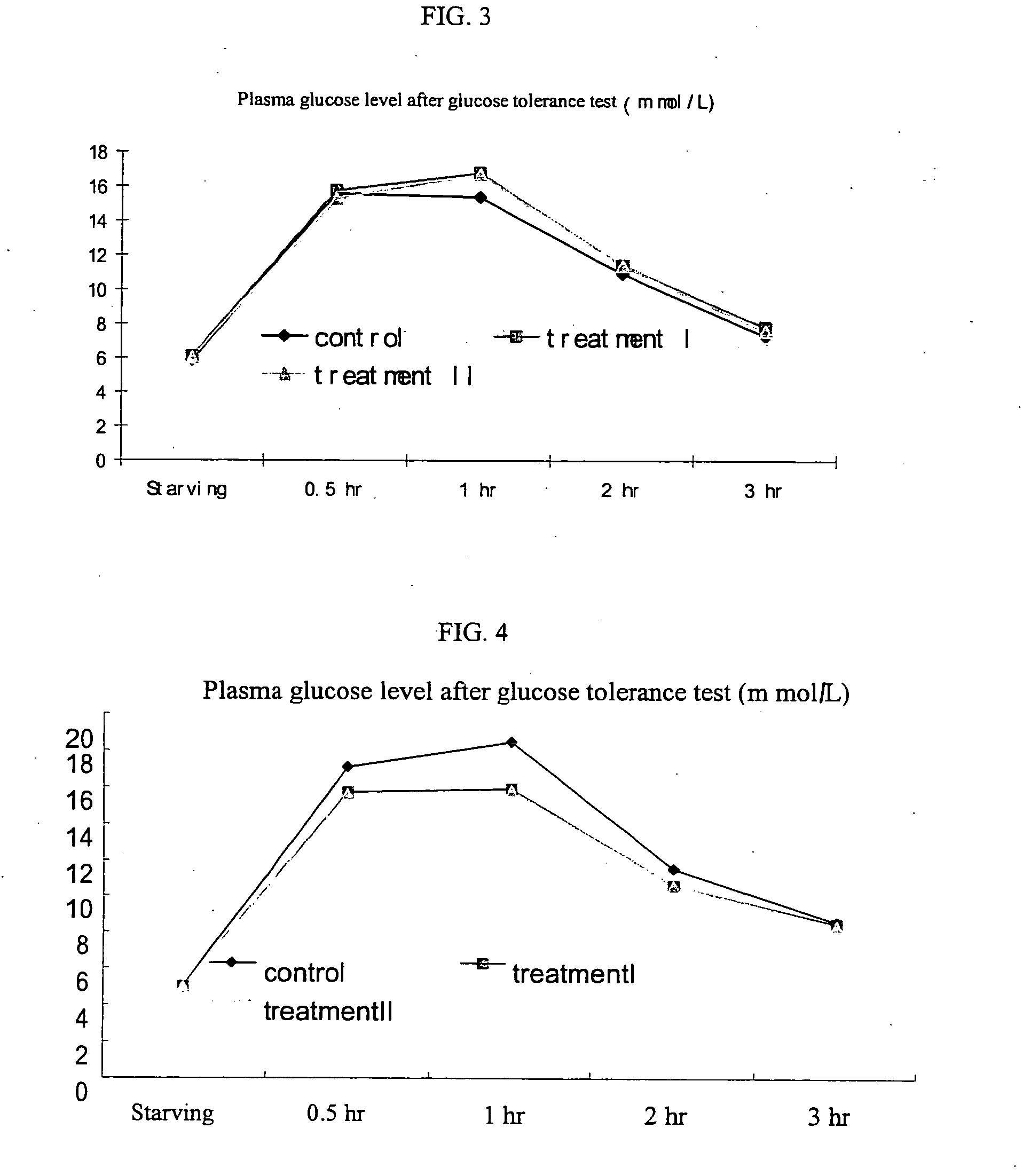
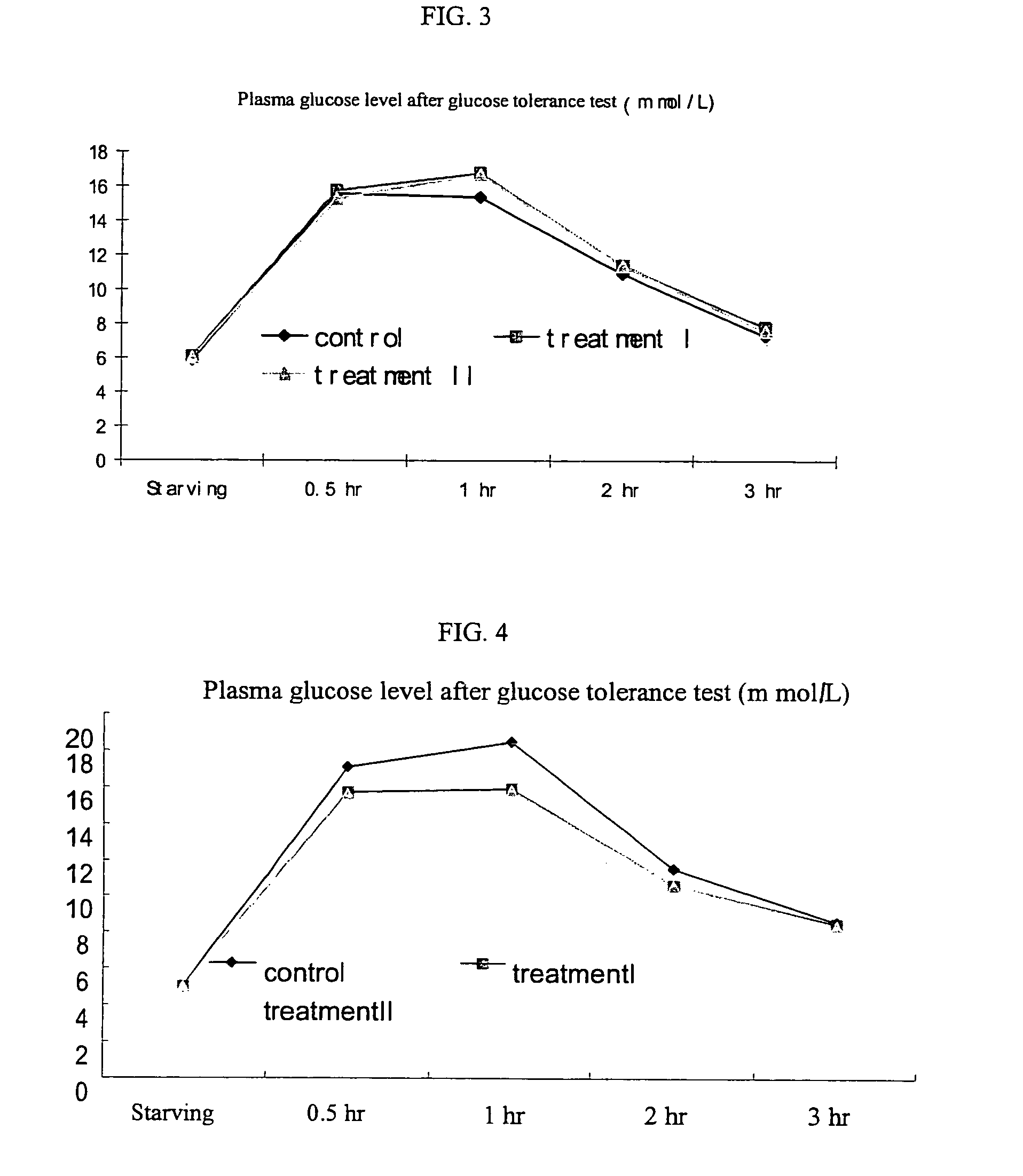
The subject invention provides compositions and methods for treating diabetes in patients. In a preferred embodiment, the invention provides compositions methods for treating diabetes and / or preventing or alleviating complications associated with diabetes. Specifically exemplified herein is the concurrent administration of a cysteamine compound with at least one additional therapeutic agent to prevent and / or treat diabetes as well as prevent and / or treat complications associated with diabetes. In a preferred embodiment, oral administration of cysteamine hydrochloride with Metformin to a patient diagnosed with diabetes can substantially regulate the patient's glucose metabolism and insulin sensitivity.
Owner:OMEGA BIO PHARMA I P 3
Compositions and methods for treating diabetes
InactiveUS7442720B2Efficacious in lowering blood glucose levelReduce and eliminate and and durationAntibacterial agentsBiocideOral medicationGlucose polymers
The subject invention provides compositions and methods for treating diabetes in patients. In a preferred embodiment, the invention provides compositions methods for treating diabetes and / or preventing or alleviating complications associated with diabetes. Specifically exemplified herein is the concurrent administration of a cysteamine compound with at least one additional therapeutic agent to prevent and / or treat diabetes as well as prevent and / or treat complications associated with diabetes. In a preferred embodiment, oral administration of cysteamine hydrochloride with Metformin to a patient diagnosed with diabetes can substantially regulate the patient's glucose metabolism and insulin sensitivity.
Owner:OMEGA BIO PHARMA I P 3
Slow release pellet of high-stability type cysteamine hydrochloride and preparation method thereof
InactiveCN101653426AAdd lessIncrease return rateOrganic active ingredientsPharmaceutical delivery mechanismSilicon dioxideWall material
The invention relates to a slow release pellet of high-stability type cysteamine hydrochloride and a preparation method thereof. The slow release pellet comprises the following components in portionsby weight: 30-70 portions of cysteamine hydrochloride, 20-40 portions of silicon dioxide and 5-25 portions of polymer material as coating wall material. The preparation method comprises the followingsteps: firstly, using silicon dioxide pellets to absorb liquid cysteamine hydrochloride in proportion to form cysteamine hydrochloride particles; then, using the coating wall material to encapsulate the cysteamine hydrochloride particles to form a membrane cavity control structure; and drying the structure to obtain slow release pellets of 50% cysteamine hydrochloride. The slow release pellet hasthe characteristics of stable product quality, high content, reasonable preparation method, easy batch production and simple using method and can be added into the premix.
Owner:冯利萍
Vesicles consisting of amphiphilic polymer and application of vesicles
InactiveCN102657873AEasy to packEfficient packagingGenetic material ingredientsPharmaceutical non-active ingredientsBackbone chainDouble bond
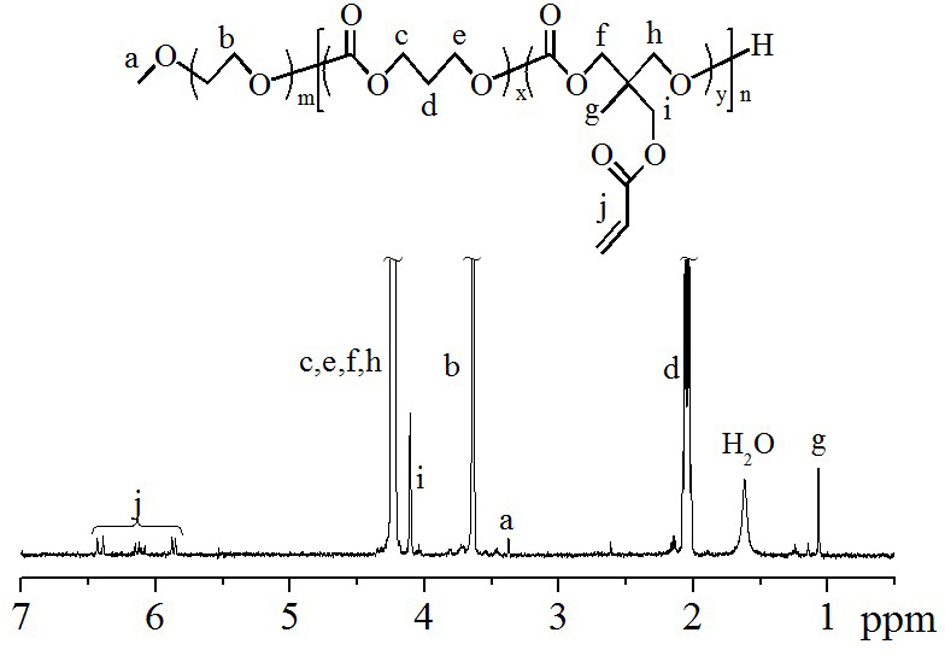
The invention discloses vesicles consisting of amphiphilic polymer and application of the vesicles. A main chain of the amphiphilic polymer consists of a hydrophilic chain segment and a biodegradable hydrophobic chain segment, wherein the molecular weight of the hydrophilic chain segment is 4 to 6kDa; the molecular weight of the hydrophobic chain segment is 3 to 5 times that of the hydrophilic chain segment; the hydrophobic chain segment is formed by performing random copolymerization on a monomer A and a monomer B in a molar ratio of (5-20):1; the monomer A is trimethylene carbonate or cyclic carbonate; the monomer B is acrylate-based carbonate or vinyl sulfone-based carbonate; the hydrophobic chain segment is grafted with a short branched chain; the grafting position is a double bond of the monomer B; a monomer forming the short branched chain is 3-mercaptopropionic acid, cysteamine hydrochloride or cysteine; and the grafting rate is 0.3 to 1. The vesicles are directly prepared from the amphiphilic polymer in an aqueous solution and used as a carrier and a release system of a protein medicine, the encapsulating efficiency and bioavailability of the protein medicine can be improved, and the stability of the encapsulated protein is enhanced.
Owner:SUZHOU UNIV
Materials and methods for improving alcohol metabolism and alleviating the effects of hangovers
InactiveUS20050148674A1Promote alcohol metabolismImprove abilitiesBiocideNervous disorderMedicineCompound (substance)
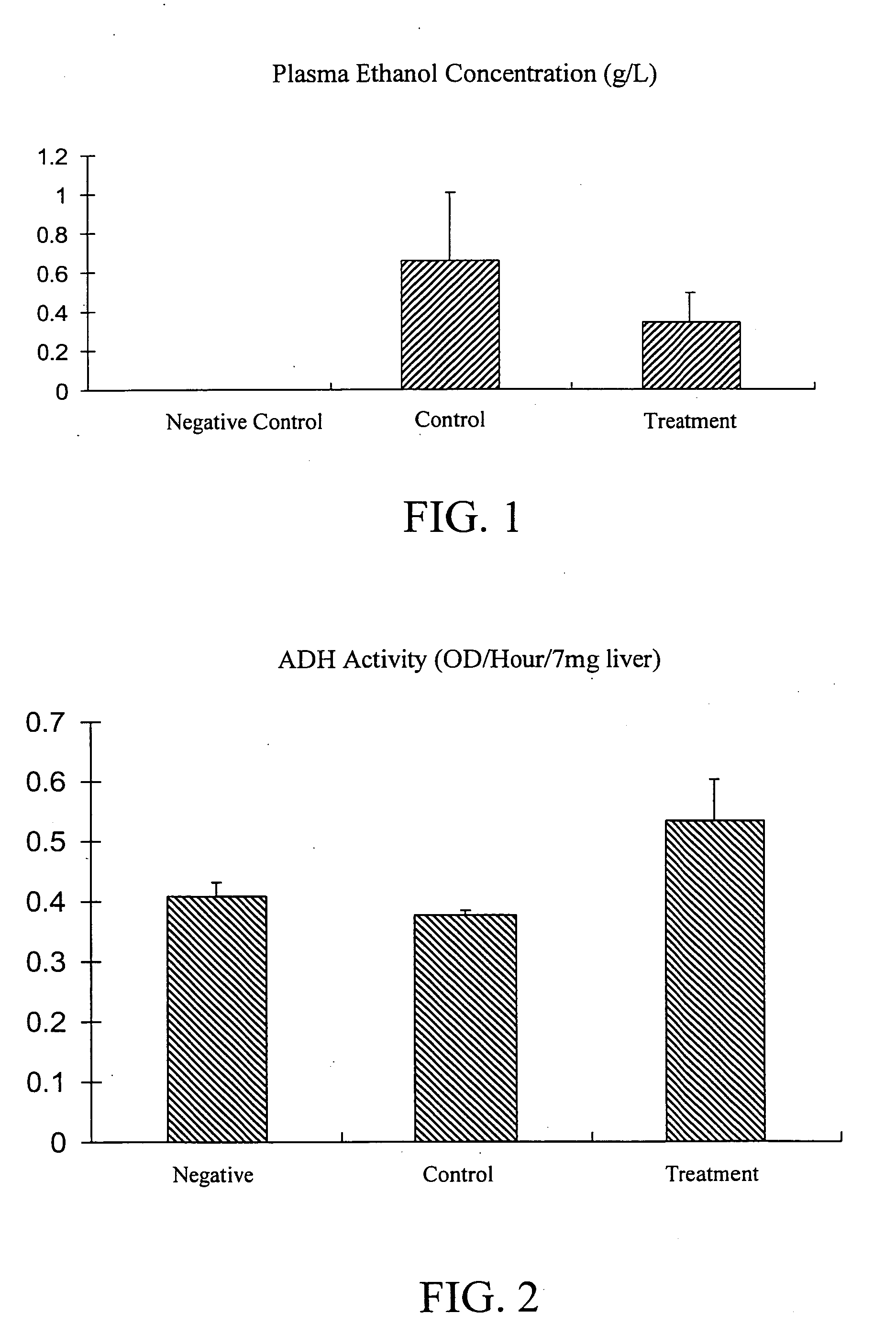
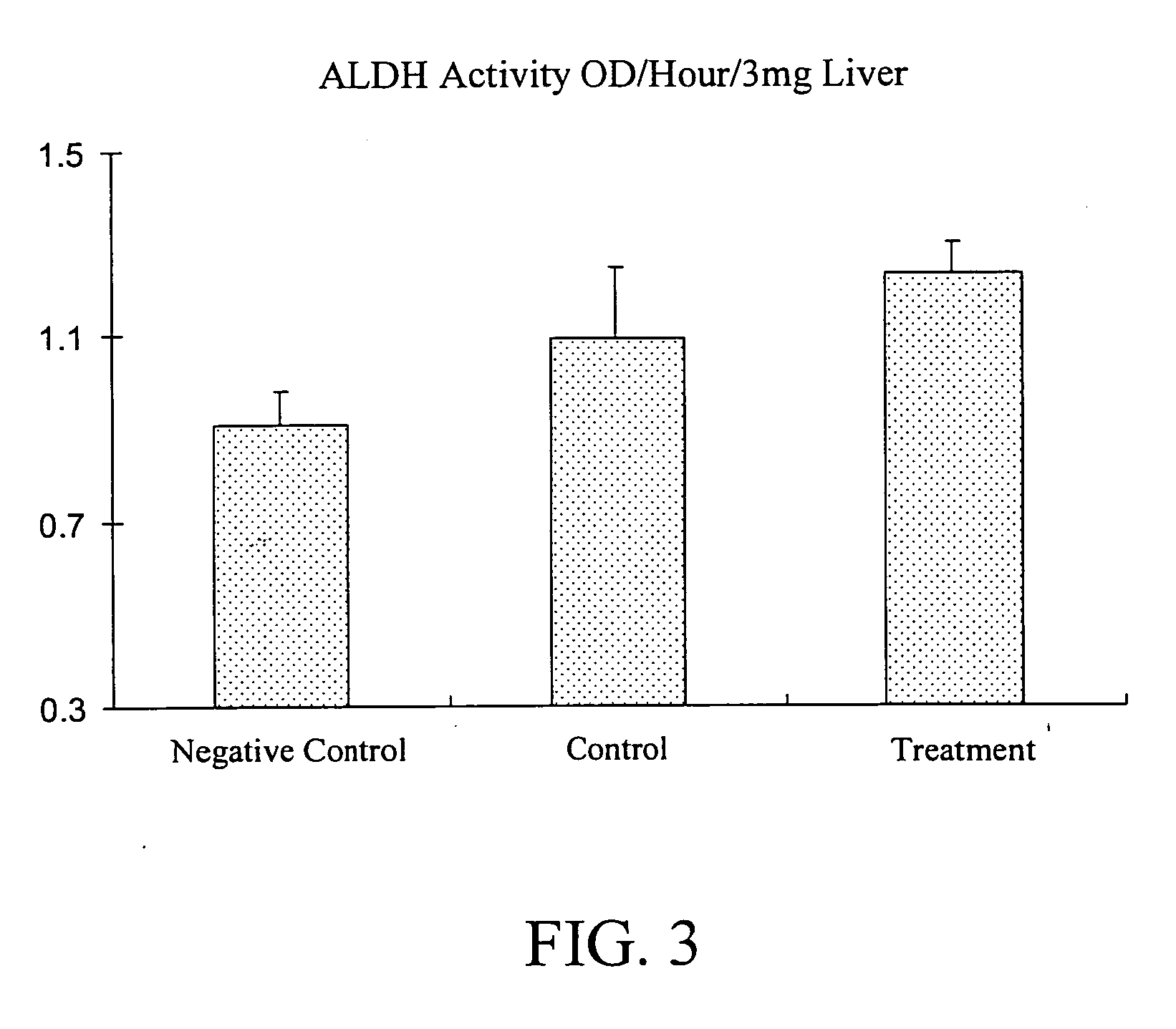
The subject invention provides materials and methods for improving alcohol metabolism in animals. In a preferred embodiment, the invention provides methods for increasing the ability of people to consume alcohol while reducing hangovers or other effects of intoxication. Specifically exemplified herein is the use of a cysteamine compound to reduce the adverse effects of alcohol consumption. For example, the undesirable and unpleasant symptoms association with hangovers can be reduced through consumption, according to the subject invention, of cysteamine hydrochloride.
Owner:OMEGA BIO PHARMA I P 1 +1
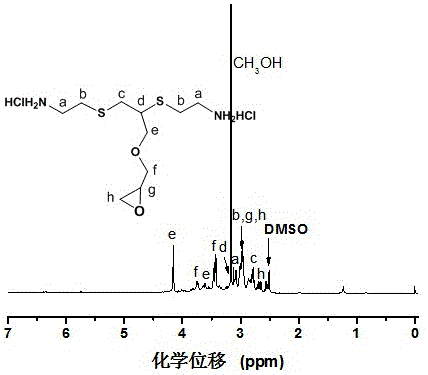
Hyperbranched polythioether polyamine, and preparation method and use thereof
ActiveCN106243354AIncrease the degree of branchingGood fluorescent effectBiocideOther chemical processesGlycidyl ethersFluorescence
The invention relates to the field of preparation methods of polyetheramine, and especially relates to a hyperbranched polythioether polyamine, and a preparation method and a use thereof. The preparation method is simple, raw materials are nontoxic and are easy to obtain, cysteamine hydrochloride can be prepared through hydrolyzing human and animal hair, alkynyl glycidyl ether can be directly purchased, and the method also has the advantages of low requirements of devices involved in the preparation process, no special or strict conditions, and mild reaction conditions. The molecular periphery of the hyperbranched polythioether polyamine has a plurality of primary amino groups, so the hyperbranched polythioether polyamine can be used as an epoxy resin flexible curing agent; and hyperbranched polythioether polyamine hydrochloride which is the precursor of the hyperbranched polythioether polyamine has a strong fluorescence effect, a good antibacterial performance and a good sterilizing performance.
Owner:YANTAI UNIV
Digestion promoting mandarin fish feed with disease resisting effect
InactiveCN105994962AHigh in nutrientsIncrease productionFood processingClimate change adaptationDiseaseSynbiotics
The invention discloses digestion promoting mandarin fish feed with a disease resisting effect. The feed is prepared from fish meal, soybean meal, rapeseed meal, cottonseed meal, peanut meal, middling flour, prawn powder, blood worm powder, zeolite powder, alfalfa meal, bone meal, monopotassium phosphate, table salt, shrimp bran powder, cod-liver oil, palm oil, modified soybean phosphate, Vc-polyphosphoester, yeast powder, shrimp shell meal, an adhesive, choline chloride, mercaptamine, citric acid, taurine, flavomycin, saccharicterpenin, Chinese herbal medicine disease prevention additives, phagostimulant, a complex enzyme preparation and synbiotics. The mandarin fish feed can promote digestion of mandarin fish and improve the disease resistance of the mandarin fish.
Owner:ANQING WANYIJI CATTLE AQUATIC PROD CULTURE
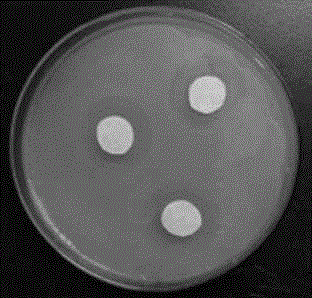
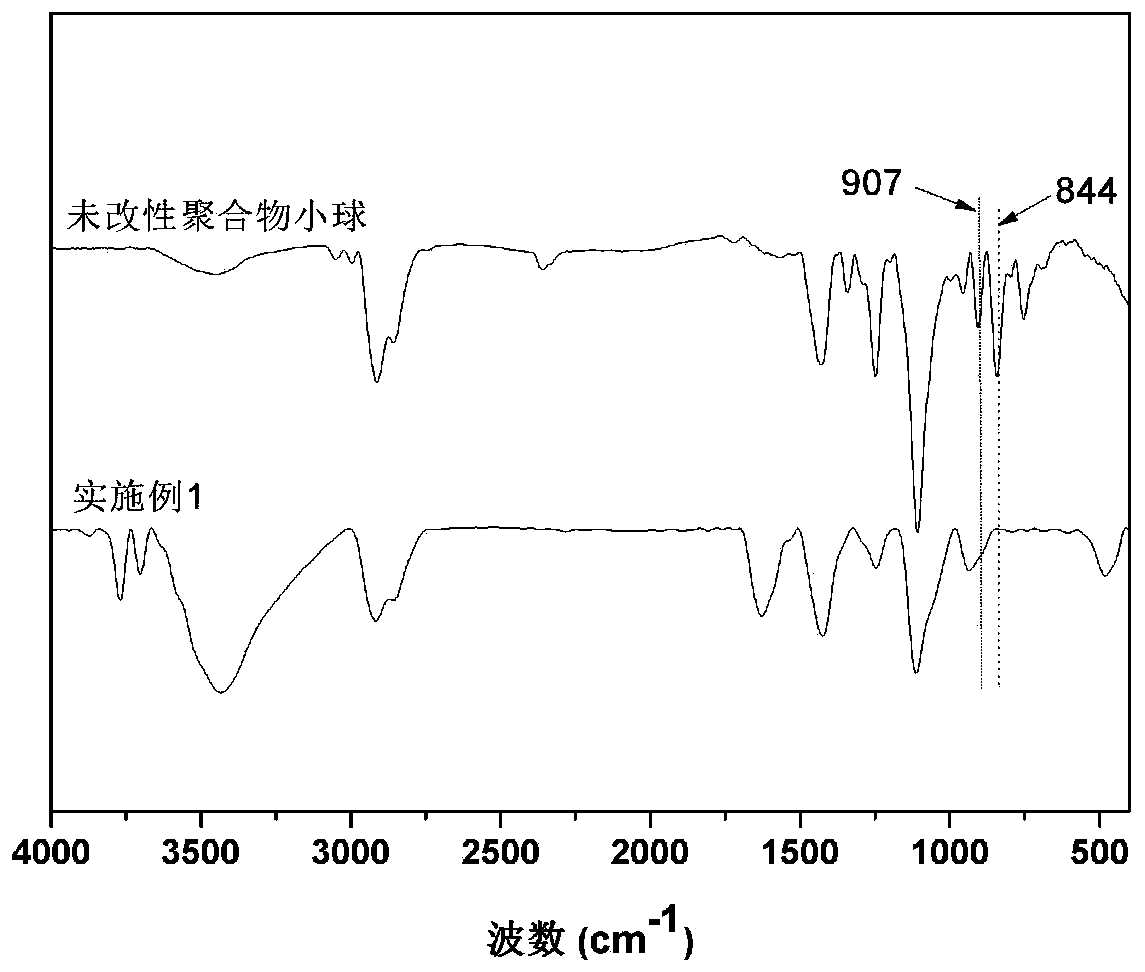
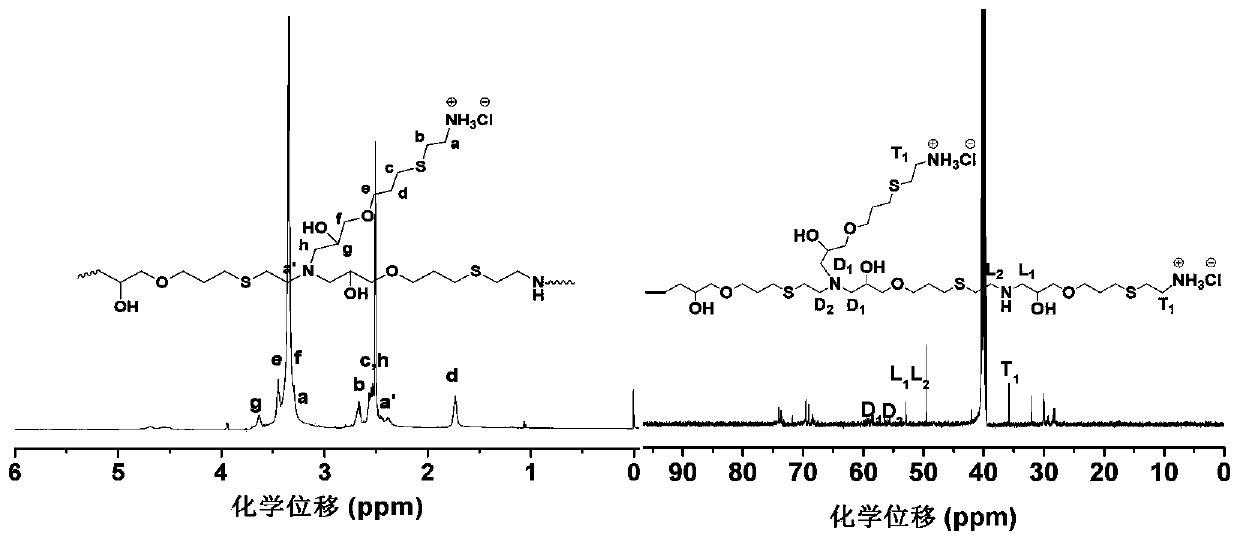
Quaternary ammonium salt type hyperbranched polythioether modified polymer microspheres and preparation method thereof
ActiveCN110918014ANot easy to fall offPoor solubilityBiocideFungicidesSodium methoxidePolymer science
The invention discloses quaternary ammonium salt type hyperbranched polythioether modified polymer microspheres and a preparation method thereof. The preparation method comprises the following steps of: under a photo-initiation condition, taking polyethylene glycol as a pore-foaming agent, taking alkyl dithiol, propargyl glycidyl ether and 1,7-octadiyne as raw materials, and preparing epoxy groupmicroporous polymer spheres by using a sulfydryl-alkyne addition suspension polymerization method; then, under the condition of 0 DEG C, taking methanol as a solvent, and initiating allyl glycidyl ether and a cysteamine compound by ultraviolet light to react to synthesize an [alpha]-epoxy-[omega]-ammonium-based intermediate; and, in a solvent and under the condition of 60 DEG C, synthesizing the hyperbranched ammonium polythioether modified polymer microspheres from the epoxy group microporous polymer spheres, the [alpha]-epoxy-[omega]-ammonium-based intermediate and sodium methoxide in one step. The obtained product has a good killing effect on escherichia coli, and the modified polymer microspheres can be recycled through simple filtration.
Owner:YANTAI UNIV
Method for preparing superoxide dismutase (SOD)-loaded gamma-poly glutamic acid hydrogel
InactiveCN103977447AImprove activity retention performanceEvenly dispersedCosmetic preparationsToilet preparationsCarboxyl radicalSide chain
The invention discloses a method for preparing superoxide dismutase (SOD)-loaded gamma-poly glutamic acid hydrogel. The method comprises the following steps: adopting a 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride / N-hydroxysuccinimide (EDC / NHS) activation system, and forming an SOD-gamma-polyglutamic acid (PGA) immobilized product by using lysine residues and poly glutamic acid side chain carboxyl of SOD; crosslinking the SOD-gamma-PGA obtained by the reaction by using a diamine crosslinking agent cysteamine hydrochloride, so as to form an SOD-gamma-PGA gel system. The gel system can be applied to wound dressing or mask, and can play the antioxidant damage action, and the skin wound is quickly repaired.
Owner:TIANJIN AROLYN BIOTECH
Preparation method of mercury ion adsorption resin
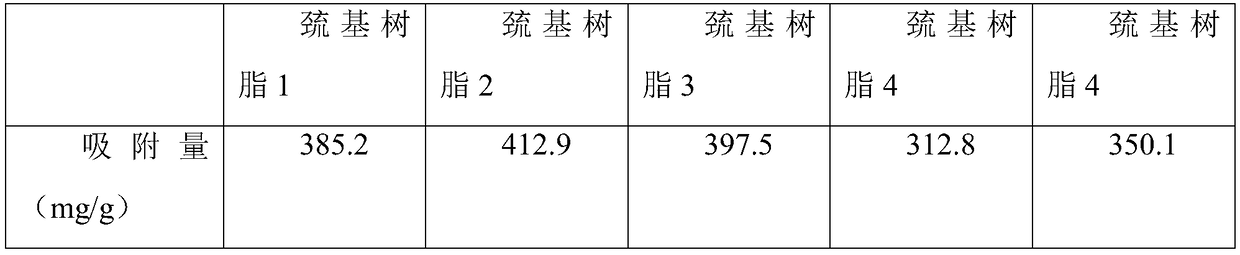
InactiveCN109289807ASimple preparation processLow costOther chemical processesAlkali metal oxides/hydroxidesCross-linkIon exchange
Belonging to the technical field of ion exchange resin, the invention specifically relates to a preparation method of a mercury ion adsorption resin. The steps include: mixing a functional group monomer containing double bonds and ester group, a cross-linking agent, a pore-forming agent and an initiator, and performing dissolution to obtain an oil phase; then dissolving a dispersant and sodium chloride in water to obtain an aqueous phase, finally dispersing an oil phase in the aqueous phase, performing stirring to disperse the oil phase into oil droplets in the aqueous phase, performing stirring, controlling the diameters of the oil droplets, conducting heating for polymerization reaction to get obtain a post-polymerization product, and performing cleaning with hot water; then conducting extraction in a Soxhlet extractor, and performing cooling and drying to obtain white spheres; under the protection of nitrogen, adding cysteamine hydrochloride into a solvent for dissolution, conducting stirring, adding triethylamine or a sodium hydroxide solution, then adding the white spheres, carrying out heating reflux reaction, and performing cooling, filtering and washing to obtain the mercury ion adsorption resin. The mercury ion adsorption resin prepared by the method provided by the invention can achieve a mercury ion adsorption capacity up to 412.9 mg / g, the adsorption effect is outstanding, and the application prospects are broad.
Owner:扬州金珠树脂有限公司
Feed for promoting growth of river crab
InactiveCN105410462AReduce secretionReduce digestion and absorptionClimate change adaptationAnimal feeding stuffBiotechnologyMonocalcium phosphate
The invention discloses feed for promoting growth of a river crab. The feed comprises the following raw materials: wheat, corn, dextrin, potato powder, wheat bran, fish meal, shrimp meal, bean pulp, peanut meal, rapeseed meal, cellulose, sodium carboxymethylcellulose, monocalcium phosphate, vitamin B1, vitamin B2, vitamin B3, vitamin B5, vitamin B6, vitamin A, vitamin C, pantothenic acid, folic acid, inositol, choline, mineral pre-mixing powder, unshelling element, oil used for feed, garlic essential oil, cinnamon leaf oil and additive, wherein the additive comprises the following raw materials: vitamin E, vitamin B12, bacillus licheniformis, bacillus subtilis, mannan oligosaccharide and cysteamine hydrochloride. The feed has the beneficial effects of promoting the growth of the river crab, enhancing immunity of the river crab, being environmentally friendly due to no drug residue and providing comprehensive nutrients and sufficient energy.
Owner:QUANJIAO TIANRUN ECOLOGICAL BREEDING PROFESSIONAL COOP
Composition and method for long-lasting non-permanent straightening of human hair
InactiveUS20120121526A1Minimal damageStructuredCosmetic preparationsHair removalPhysiologyPre treatment
A mild reducing composition and method for straightening human hair are described. The composition contains cysteamine hydrochloride in the range of 5-10% by weight at a pH range of 7.0-9.0. The composition is applied to hair and processed for 15-45 minutes. The hair is rinsed with water, dried with a blow-dryer and ironed with a straightening iron. The hair is allowed to air-oxidize for 24-72 hours. The resultant straightening lasts 2 to 4 months, and the hair gradually reverts to the pre-treatment state with no obvious re-growth. The treatment may be re-applied every 2 to 4 months without significant damage.
Owner:AWARE PROD
Antibacterial, disease-resisting and anti-hypoxia growth-promoting feed of mandarin fish
InactiveCN105851714AImprove disease resistanceIncrease nutritionClimate change adaptationAnimal feeding stuffPhytaseAstaxanthin
The invention discloses antibacterial, disease-resisting and anti-hypoxia growth-promoting feed of mandarin fish. The antibacterial, disease-resisting and anti-hypoxia growth-promoting feed is prepared from the following raw materials including fishmeal, alpha-starch, tenebrio molitor powder, mung bean flour, rice bran powder, fermented soybean meal, carotene, vitamin E, lecithin, brown sugar, soybean phospholipid, mixed oil, citric acid, streptococcus lactis, a traditional Chinese medicine complex agent, phytase, dihydropyridine, glucocyamine, phenylglycin, cysteamine hydrochloride, L-carnitine, soybean isoflavone, chitosan, malto oligosaccharides, probiotics, astaxanthin, lysozyme, nano-collagen, an emulsifying agent, novasil and flavomycin. With the adoption of the feed of the mandarin fish, disclosed by the invention, the antibacterial, disease-resisting and anti-hypoxia capability of the mandarin fish can be effectively improved and the growth of the mandarin fish is promoted.
Owner:ANQING WANYIJI CATTLE AQUATIC PROD CULTURE
Cysteamine-modified copper nanocluster solution fluorescent probe as well as preparation and application
ActiveCN108329904AEasy to operateThe detection process is fastFluorescence/phosphorescenceLuminescent compositionsCopperFluorescence spectrometer
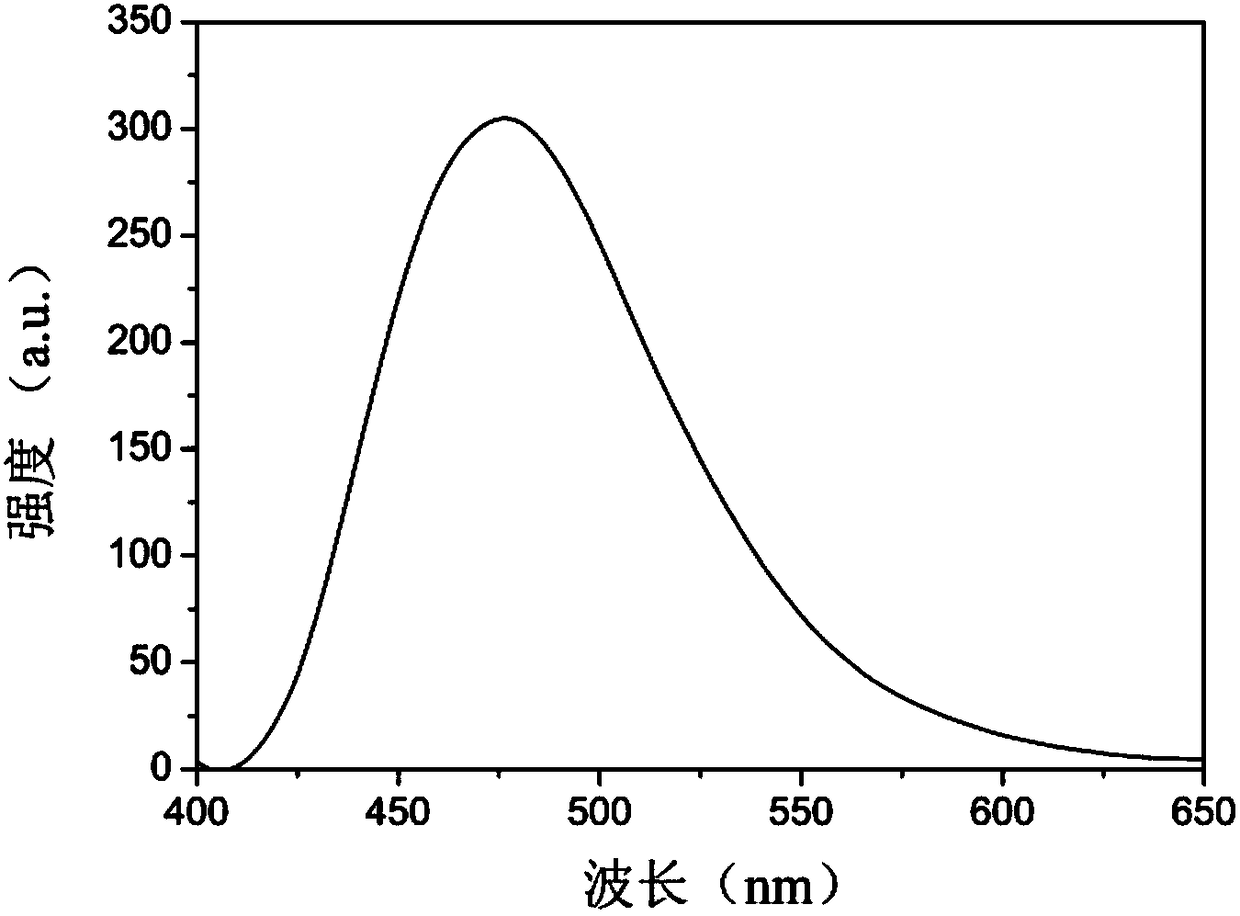
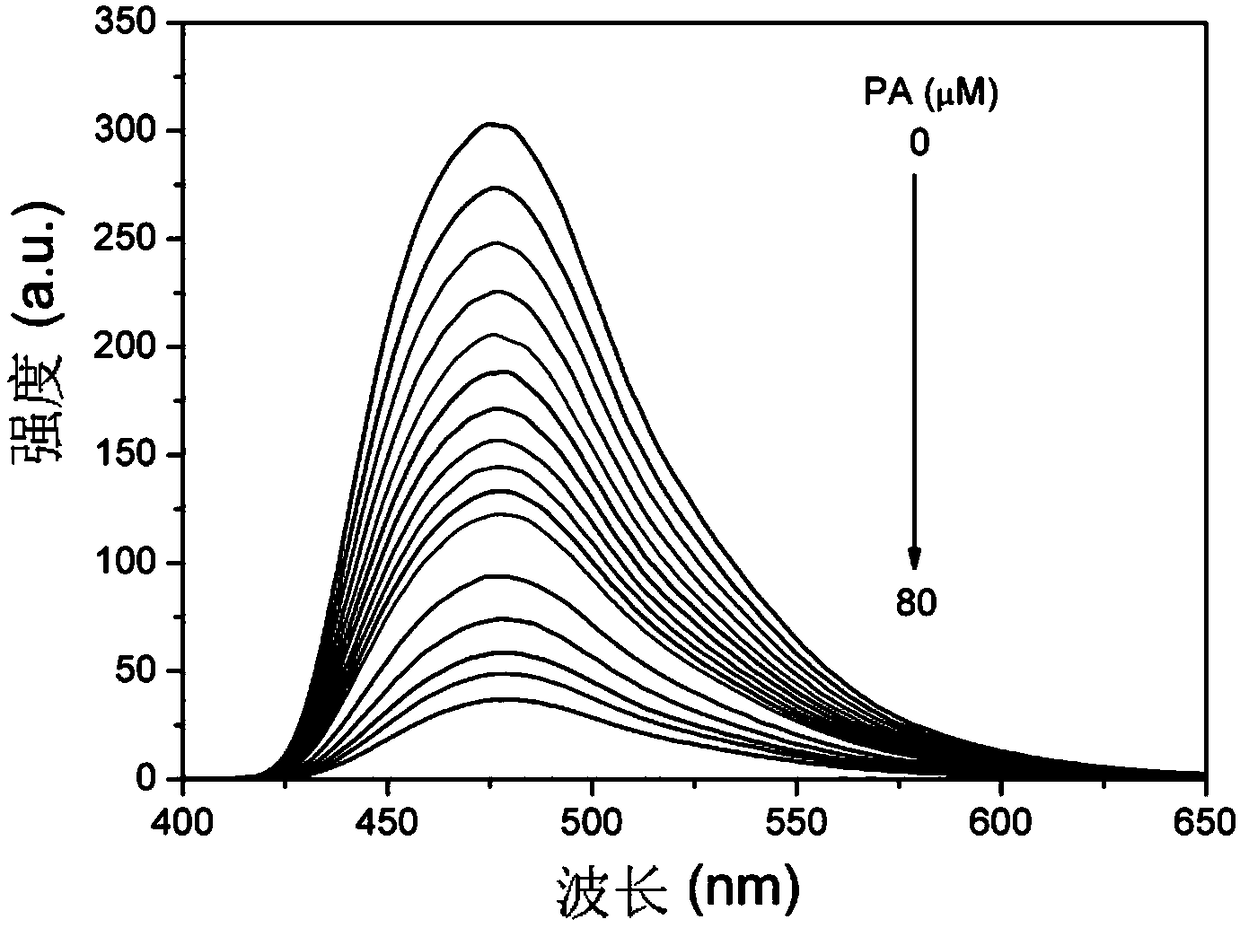
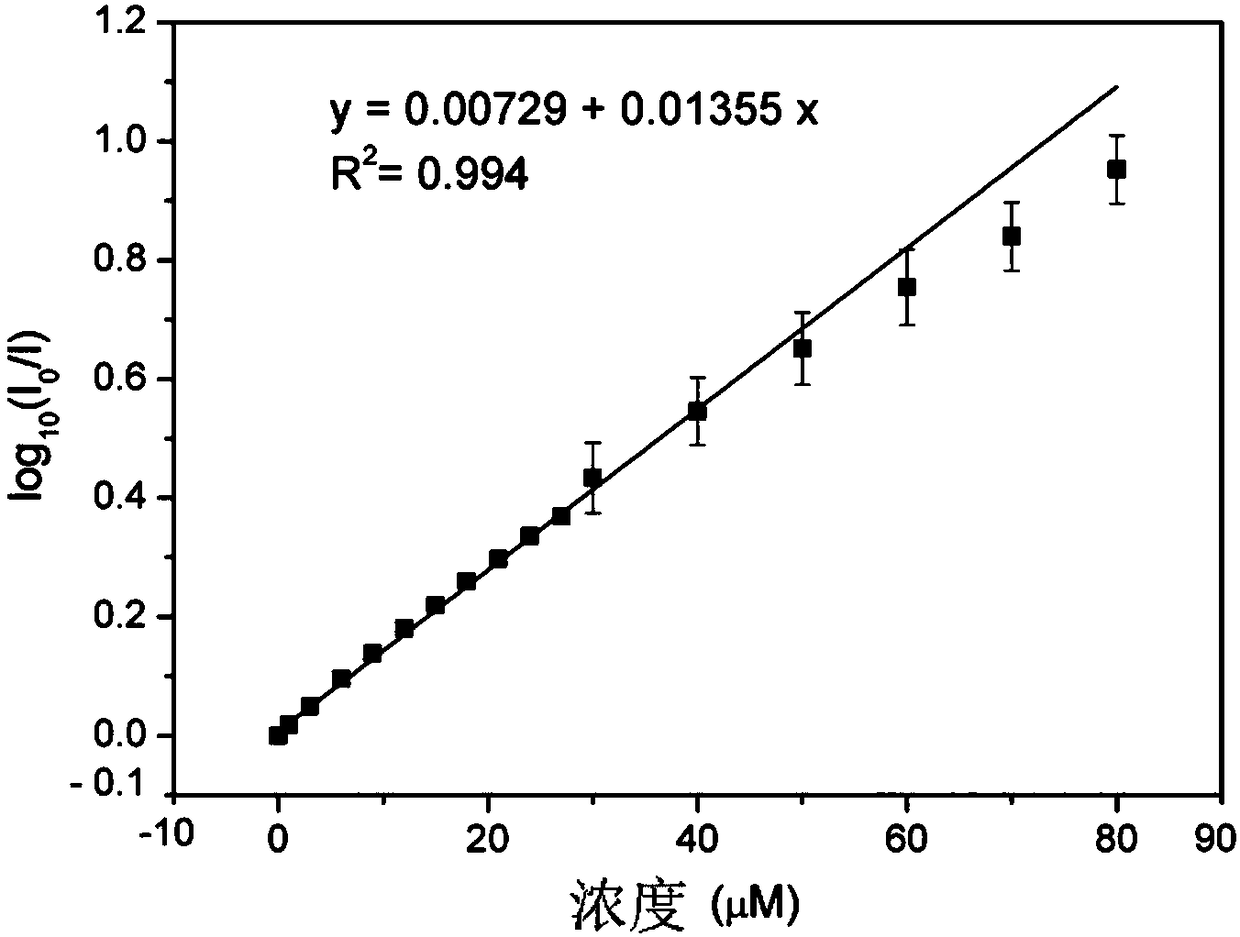
The invention belongs to the technical field of analysis and detection and discloses a cysteamine-modified copper nanocluster solution fluorescent probe as well as preparation and an application. Ascorbic acid is dissolved in ultrapure water, a copper ion solution and a cysteamine hydrochloride solution are sequentially added, the mixture is uniformly stirred and mixed, and the cysteamine-modifiedcopper nanocluster solution fluorescent probe is obtained. The cysteamine-modified copper nanocluster solution fluorescent probe is added to ultrapure water to be diluted, a to-be-detected picric acid solution is added, a fluorescence spectrometer is used for detection, and the concentration of picric acid in the to-be-detected solution is analyzed according to the relation between the fluorescence intensity and the concentration of picric acid. The cysteamine-modified copper nanocluster solution is used for detecting picric acid for the first time, and the fluorescence quenching probe is designed to perform fluorescence detection on picric acid, has the advantages of being simple to operate, high in detection speed, good in selectivity and high in sensitivity and has the linear range of0-80 mu mol / L and the detection limit of 0.139 mu mol / L.
Owner:JINAN UNIVERSITY
Magnetic material for efficiently detecting circulating tumor cell and preparation method of magnetic material
ActiveCN107858144ANarrow particle size distributionHigh saturation magnetizationOrganic/organic-metallic materials magnetismTumor/cancer cellsBiocompatibility TestingSuperparamagnetism
The invention provides a magnetic material for efficiently detecting a circulating tumor cell and a preparation method of the magnetic material. The preparation method comprises the steps of: (1) allowing hyaluronic acid, 1-(3-dimethylamino propyl)-3-ethyl-carbodiimide hydrochloride, 1-hydroxybenzotriazole, cysteamine hydrochloride and dithiothreitol to react to prepare HA-SH, (2) grafting rhodamine onto HA-SH to prepare RhB-HA-SH, (3) allowing an Fe3O4 nanoparticle, RhB-HA-SH and H2O2 to react to prepare superparamagnetic fluorescent HA-SH, and (4) allowing superparamagnetic fluorescent HA-SH, 1-(3-dimethylamino propyl)-3-ethyl-carbodiimide hydrochloride, 1-hydroxybenzotriazole, PEG-FA and anti-EpCAM (epithelial cell adhesion molecule) to perform antibody reaction to prepare the magneticmaterial. The magnetic material is high in saturation magnetization, and good in magnetic response and biocompatibility, can achieve specific binding with the circulating tumor cell and has certain universality.
Owner:SICHUAN UNIV
Coated cysteamine hydrochloride and preparation method thereof
InactiveCN102302088APromote growthSimple processAnimal feeding stuffAccessory food factorsCelluloseStearic acid
The invention relates to a coated cysteamine hydrochloride in the technical field of the coating of the cysteamine hydrochloride. The coated cysteamine hydrochloride comprises the following components in parts by weight: 29-35 parts of cysteamine hydrochloride, 13-17 parts of stearic acid, 12-14 parts of cellulose aqueous solution with methylcellulose content of 5 wt%, 4.5-5.5 parts of citric acid and 32-38 parts of white carbon black. The preparation method comprises the following steps of: weighing raw materials; adding the stearic acid, the cellulose aqueous solution, the cysteamine hydrochloride and the citric acid to a colloid mill in sequence to be ground into milk-white liquid; adding the liquid to the white carbon black in a stirring state so as to obtain white bulk granular powder; and screening the powder by using a screen of 0.3 mm. The coated cysteamine hydrochloride and the preparation method thereof, provided by the invention, have the advantages of simple process, cheapraw material, low production cost, high finished product rate and no generation of substances like waste gas, wastes and the like; therefore, no environment pollution is caused, the stability problemof the cysteamine hydrochloride is solved, and no great irritation can be caused to the stomach while the coated cysteamine hydrochloride is taken for a long time.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Synthetic method for taurolidine and pharmaceutical preparations
ActiveCN101274921AHigh purityAvoid the disadvantages of many and complex, prone to side reactionsAntibacterial agentsOrganic chemistrySide effectCurative effect
Owner:CHANGCHUN MAILING BIOLOGICAL ENG CO LTD
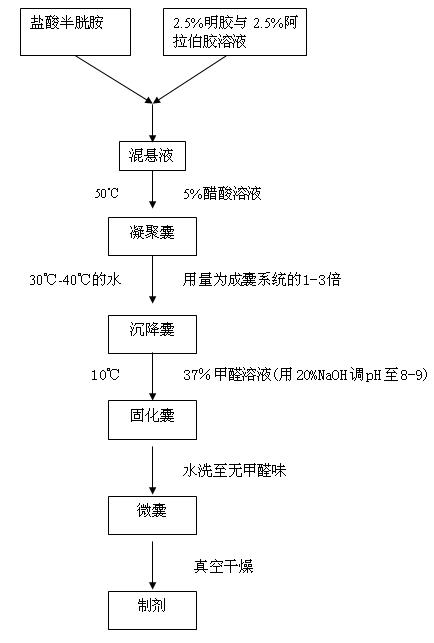
Method for producing enteric cysteamine hydrochloride coated granules
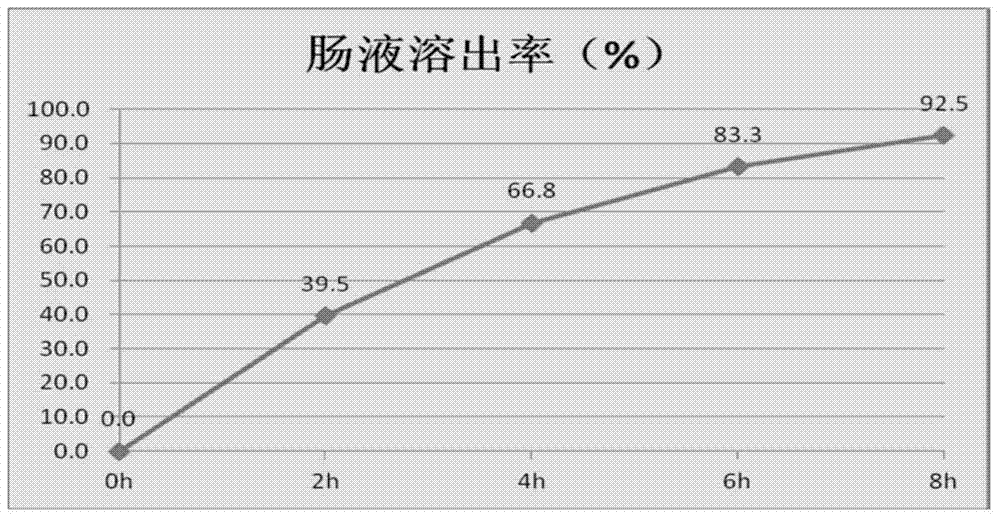
ActiveCN102077905AAvoid stimulationEffectively Regulate Growth Hormone LevelsAnimal feeding stuffAccessory food factorsLarge doseCanker
The invention relates to a method for producing enteric cysteamine hydrochloride coated granules, which comprises the following process steps: 1, combining cysteamine hydrochloride and a capsule material to prepare mixed suspension; 2, adding the mixed suspension into 5-percent acetic acid solution, mixing at 50 DEG C to form coacervated capsules, adding water at 30 to 40 DEG C in a volume which is 1 to 3 times that of the coacervated capsules to form suck capsules, adding 37-percent formaldehyde solution and mixing at 10 DEG C to form solidified capsules; 3, washing the solidified capsules till no formaldehyde smell is given off to form microcapsules; and 4, dying under vacuum to obtain cysteamine hydrochloride coated granule preparation. In the method, the cysteamine hydrochloride coated with an imported coating material is prevented from being oxidized in any environment and the properties of the cysteamine hydrochloride coated granules are stable, the dissolution rate of the cysteamine hydrochloride coated granules in stomach is smaller than 2 percent, and the use of the cysteamine hydrochloride coated granules in a large dose does not cause canker; the cysteamine hydrochloride is released in intestinal tract accurately and can dissolve and be absorbed completely; and thus, the bioavailability is improved greatly. The product can be added into an auxiliary material as a raw material to form the low-content cysteamine hydrochloride coated granules.
Owner:WUXI ZHENGDA POULTRY
Stable mercaptamine enveloped by microcapsules and preparation method thereof
ActiveCN103652366ASolving activitySolve the disadvantage of discolorationAnimal feeding stuffCYSTEAMINE HYDROCHLORIDECorn starch
The invention relates to stable mercaptamine enveloped by microcapsules. The stable mercaptamine is mainly prepared from the following materials in parts by weight: 20-55 parts of mercaptamine, 15-35 parts of corn starch, 0.1-10 parts of sodium carboxymethylcellulose, 1-10 parts of sodium alginate, 0.1-10 parts of ethyecellulose, 10-15 parts of beta-cyclodextrin and 10-50 parts of carnauba wax. The stable mercaptamine and the preparation process provided by the invention have the advantages that the problems of easy oxidation and easy moisture absorption are effectively solved, so that the purpose of protecting the mercaptamine is achieved; simultaneously, the release of the mercaptamine in the stomach is furthest limited, the occurrence of the gastric ulcer is avoided, and the full release in the intestinal tract is achieved, so that more effective utilization by animals is achieved, the growth of the animals is promoted and the application prospect is better.
Owner:HANGZHOU KINGTECHINA FEED CO LTD
Composite premix and feed for later stage of growing-fattening pigs
InactiveCN107259148AEasy to shapeStay in shapeFood processingAnimal feeding stuffCynomorium songaricumLate stage
The invention discloses a composite premix for the later stage of growing-fattening pigs. The composite premix comprises cynomorium songaricum extract and preferably comprises cysteamine hydrochloride, guanidinoacetic acid, licorice root extract, acanthopanax root extract, peppermint extract, dried tangerine peel extract, Chinese magnoliavine fruit extract, capsanthin extract and rosemary extract. The invention discloses composite premix feed for the late stage of growing-fattening pigs and the feed is prepared from the composite premix. The composite premix and the composite premix feed for the later stage of growing-fattening pigs can prevent intestinal and respiratory diseases in the later stage of growing-fattening pigs, improve human immunity, prevent diarrhea in the later stage of growing-fattening pigs, increase a yield and a survival rate in the later stage of growing-fattening pigs and improve pig carcass quality, feed conversion and a meat production cost.
Owner:BEIJING SIFANGGHONG FEED TECH CO LTD
Method for preparing cysteamine hydrochloride by basic hydrolysis
The invention relates to a method using alkaline hydrolysis to prepare cysteamine hydrochloride, in particular to synthesizing cysteamine hydrochloride in alkali surroundings, which is characterized in that: ethanolamine solution and sulfate solution are used as raw material; 2- amino ethyl sulfate is firstly synthesized before made into ring in alkaline solution by 2-amino ethyl sulfate and carbon disulfide, thereby getting Alpha -mercaptothiazoline; alkaline hydrolysis is made upon Alpha -mercaptothiazoline to produce cysteamine hydrochloride. The preparation method for cysteamine hydrochloride by using alkaline hydrolysis has the advantages of simple method, short production period and low production cost. The recovery rate of synthesizing Alpha -mercaptothiazoline reaches more than 90%. The cysteamine hydrochloride can be widely applied in manufacturing anti-ulcer drugs such as ranitidine and cimetidine, manufacturing animal fodder additive, manufacturing cosmetic and hair wave-setting agent, manufacturing biochemical reagent and heavy metal ion complexion agent and manufacturing agent treating radiation syndrome and acute tetraethyl lead poisoning and in other areas.
Owner:CHONGQING UNIV
Granulating coated slow release product of cysteamine hydrochloride and preparation method thereof
ActiveCN101703472AEnhance the relaxation effectNot easily oxidizedOrganic active ingredientsPharmaceutical non-active ingredientsVitamin CPhosphoric acid
The invention discloses a granulating coated slow release product of cysteamine hydrochloride and a preparation method thereof. The product is obtained by taking cysteamine hydrochloride, corn starch, vitamin C, powdered glucose, stearic acid and 85wt percent phosphoric acid solution as raw materials through the processes of heating for dissolving, millard reaction, stabilization treatment, atomized granulation, hardening waterproof coating, enteric coating, drying, sieving and the like, wherein the content of the cysteamine hydrochloride in the product is more than or equal to 30.50 percent and the water content ranges from 0 percent to 8.0 percent. The method thoroughly eradicates the odor of the cysteamine hydrochloride, the product has the fragrance of bulked corn, and the glucose added in the millard reaction can conduct physical coverage and cause the cysteamine hydrochloride to be slowly released, thus increasing the palatability of products. The addition of glucose in full-price materials not only does not affect feed intake, but also can increase the feed intake. In the process of technological treatment and storage of the products, the content of cysteamine hydrochloride is stable.
Owner:上海邦成生物工程有限公司
Meat quality and flavor improver for tilapia mossambica and preparation method thereof
The invention relates to a feed additive in aquaculture industry, in particular to a meat quality and flavor improver for tilapia mossambica and a preparation method thereof. Each kilogram of improver comprises 50-100 g of ulva powder, 20-30 g of carnitine, 30-50 g of dimethylpropiothetin, 15-25 g of cysteamine HCl, 200-300 g of sodium chloride and the balance of zeolite powder. The preparation method comprises the following steps of: firstly, pulverizing the ulva powder, the carnitine, the dimethylpropiothetin, the cysteamine HCl and the sodium chloride, and sieving with a 100-mesh sieve; secondly, mixing the pulverized solid materials according to the proportions; and finally, mixing and stirring the mixed solid materials and the zeolite powder so that the coefficient of variation (CV) of uniformity of the mixture is less than 5%. The improver can efficiently increase the content of inosinic acid as a delicate flavor substance in tilapia mossambica meat, can efficiently increase the content of proteins, the content of essential amino acids and the content of taste amino acids in the tilapia mossambica meat, can improve the taste and the flavor of the tilapia mossambica meat, and can eliminate fishy smell.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Magnetic nanometer material, and preparation method and application thereof in enrichment analysis for glycosylation peptide fragment
ActiveCN106732474AThe synthesis method is simpleEasy to separateOther chemical processesMaterial analysis by electric/magnetic meansCYSTEAMINE HYDROCHLORIDEChemistry
The invention belongs to the technical field of biotechnology and nanometer material and specifically relates to a ferroferric oxide magnetic nanometer material modified with cysteamine hydrochloride, and a preparation method and an application thereof in enrichment analysis for a glycosylation peptide fragment. According to the invention, the ferroferric oxide magnetic nanometer material modified with cysteamine hydrochloride and a glycosylation peptide fragment solution are mixed in a mixed solution of acetonitrile, water and trifluoroacetic acid; the mixture is hatched in an enzymolysis instrument; the nanometer material is separated and the supernate is removed under an action of an external magnetic field; the nanometer material is washed with the mixed solution of acetonitrile, water and trifluoroacetic acid; the glycosylation peptide fragment enriched on the material is eluted with the mixed solution of acetonitrile, water and formic acid; and the mass spectrometry is combined for identifying. According to the invention, the operation is simple and quick; the material can be reused; the cost is low; the sensitivity and the selectivity are higher; the ferroferric oxide magnetic nanometer material is suitable for the enrichment analysis for the glycosylation peptide fragment and has excellent application prospect.
Owner:FUDAN UNIV
Preparation method of cysteamine hydrochloride controlled-release feed additive
InactiveCN102132768AReduce the temperatureHigh strengthAnimal feeding stuffAccessory food factorsMaillard reactionStearic acid
The invention relates to a preparation method of a cysteamine hydrochloride controlled-release feed additive, which comprises the following steps: weighing 30-70 percent of cysteamine hydrochloride, 25-60 percent of glucose and 1-10 percent of water in percentage by weight, heating and dissolving the compositions into solution, adding alkaline contents in the solution to lead the pH value to be 3.5-6.0 at the temperature of 85-89 DEG C, stirring the solution at the speed of 50-100 turns per minute, and reacting for 60-80min, thereby obtaining a liquid finished product of the cysteamine hydrochloride controlled-release feed additive; adsorbing the liquid finished product with white carbon black for 5-20min to prepare a solid granule finished product; and coating the surface of the solid granule finished product with fatty acid mixture formed by stearic acid, palmitic acid and oleic acid, so as to prepare a coated solid finished product of the cysteamine hydrochloride controlled-release feed additive. The preparation method can more effectively improve the intensity and the reaction completion degree of Maillard reaction, shorten the time of the Maillard reaction, and more effectively remove the smelly egg and bitter taste of cysteamine hydrochloride, so that the mouthfeeling of animals to the product is better, and the stability of the product is excellent.
Owner:JIANGNAN UNIV
Stabilized cysteamine growth promoting agent used for aquatic feeds and production method thereof
The invention relates to a growth agent for aquatic animals and a production method thereof, in particular to a stabilized cysteamine growth promoting agent used for aquatic feeds and a production method thereof, belonging to the technical field of feed additive production. The stabilized cysteamine growth promoting agent used for the aquatic feeds comprises raw materials of cysteamine hydrochloride and monohydrate zinc sulphate or heptahydrate zinc sulphate with the mass ratio of 1:1-1:3. The production method comprises the following steps of: mixing the cysteamine hydrochloride, the monohydrate zinc sulphate or the heptahydrate zinc sulphate, and water based on the mass ratio, and enabling the temperature of the mixed solution to be constantly at 40-50 DEG C; after the cysteamine hydrochloride and the zinc sulphate dissolve in the water, adding silicon dioxide and stirring for 20-90 minutes; and drying the mixed solution to be with 5-12 percent of moisture. The product of the invention has superfine product particle-size while ensuring the stability of the cysteamine, and overcomes the difficulty that traditional encapsulated type stabilized cysteamine agent is not suitable for aquatic feeds industry, wherein average particle size is smaller than 100 mesh standard sieve.
Owner:GUANGDONG HAID GROUP
Preparation method of 2-[[[5-(dimethylamino)methyl-2-furanyl]methyl]thio]ethylamine
The invention provides a preparation method of 2-[[[5-(dimethylamino)methyl-2-furanyl]methyl]thio]ethylamine. The preparation method comprises mixing a dimethylamine hydrochloride solution, polyformaldehyde and a quaternary ammonium salt, heating the mixture to 50 to 70 DEG C, adding furfuryl alcohol into the reaction system, carrying out a first condensation reaction process to obtain an intermediate, mixing the intermediate, cysteamine hydrochloride, concentrated hydrochloric acid and perchloric acid, carrying out a second condensation reaction process at the system temperature of 15 to 25 DEG C to obtain a mixed solution of 2-[[[5-(dimethylamino)methyl-2-furanyl]methyl]thio]ethylamine hydrochloride, alkalifying the mixed solution, extracting the solution through dichloromethane and distilling the extract to obtain 2-[[[5-(dimethylamino)methyl-2-furanyl]methyl]thio]ethylamine. The preparation method has the advantages of high yield, high product purity, simple processes and industrialization easiness.
Owner:HEBEI HAILI FRAGRANCES CO LTD
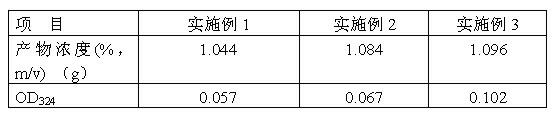
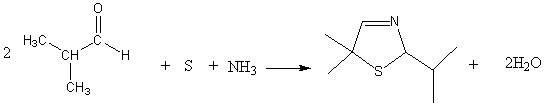
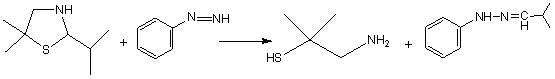
Synthetic method of dimethyl cysteamine hydrochloride
ActiveCN102432510AHigh purityReaction raw materials are readily availableThiol preparationThiazolineCYSTEAMINE HYDROCHLORIDE
The invention discloses a synthetic method of dimethyl cysteamine hydrochloride. The method comprises the following steps of: synthesizing 5,5-dimethyl-2-isopropyl thiazoline by taking isobutylaldehyde, element sulfur, ammonia gas and triethylamine as raw materials; reducing the 5,5-dimethyl-2-isopropyl thiazoline into 5,5-dimethyl-2-isopropyl thiazolidine under the actions of sodium borohydride and acid; and reacting the 5,5-dimethyl-2-isopropyl thiazolidine and phenyl hydrazine under the air insulating situation to obtain dimethyl cysteamine hydrochloride. The method has the advantages of readily-available reaction raw materials, easiness for operating the reaction process, low requirement on the reaction equipment, relatively mild reaction conditions, feeding of the product of every step of reaction into a next step reaction after refining and purifying, high yield and high purity, and the purity of the finally-obtained dimethyl cysteamine hydrochloride is close to 100 percent.
Owner:山东胜利生物工程有限公司
Antibiotic-free premix feed for breeding sows
InactiveCN106509441APromote secretionEasy feedingAnimal feeding stuffAccessory food factorsAntibiotic YFeed additive
The invention relates to an antibiotic-free premix feed for breeding sows. The antibiotic-free premix feed is prepared from the following components of table salt, lysine, bone meal, compound enzymes, choline chloride, chromium nicotinate, radix codonopsis, radix astragali, Chinese wolfberry fruits, radix scutellariae, methionine, threonine, vitamin A, vitamin D, vitamin E, vitamin B, folic acid, selenium yeast, cysteamine hydrochloride and soybean flavone, and besides, yucca, radix angelicae sinensis, radix rehmanniae preparata and pine needles are particularly added to be used as pig feed additives for replacing Western medicines and antibiotic drugs, so that the immunity of sows can also be enhanced, and the propagation performance of the sows can also be effectively improved. The antibiotic-free premix feed disclosed by the invention can supply adequate nutrients for the growth of the sows, complements various vitamins and minerals, and does not contain antibiotics, so that the antibiotics are avoided from being enriched in bodies of the breeding sows to influence the properties of piggies, and the antibiotic-free premix feed conforms to the health concept of modern people.
Owner:XINJIN BANGDE TECH
Feed formula capable of improving immune performance of animal and processing method
InactiveCN106879828AFull of nutritionImprove immunityFood processingAnimal feeding stuffAnimal scienceImmunocompetence
The invention discloses a feed formula capable of improving the immune performance of an animal. The feed formula is prepared from the following raw materials: corn, bean cakes, peanut meal, extruded soybeans, sweet potatoes, brown rice, cactus powder, wheat bran, hawthorn, calcium hydrophosphate, radix rehmanniae, astragalus membranaceus, radix sophorae flavescentis, honeysuckle, manyprickle acanthopanax roots, semen cuscutae, glossy privet fruits, angelica sinensis, andelion, perilla leaves, flos carthami, radix bupleuri, wolfberry fruits, polygonum multiflorum, liquorice, radix isatidis, mercaptamine and a salt. The invention further discloses a processing method of the feed formula capable of improving the immune performance of the animal. The prepared feed is abundant in nutrient, the immunocompetence of the animal can be effectively improved, and the feed does not contain hormone and antibiotic, does not generate a residue in the animal and meets the green and healthy requirements.
Owner:上海华扩达生化科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com