Method for producing enteric cysteamine hydrochloride coated granules
A cysteamine-coated and cysteamine hydrochloride technology is applied in the production field of enteric-coated cysteamine hydrochloride-coated particles, and can solve the problem that large-scale industrial production cannot be applied, large-scale industrial production is not easy, and disulfide is ineffective. and other problems to achieve the best absorption effect, improve bioavailability, and avoid adverse reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
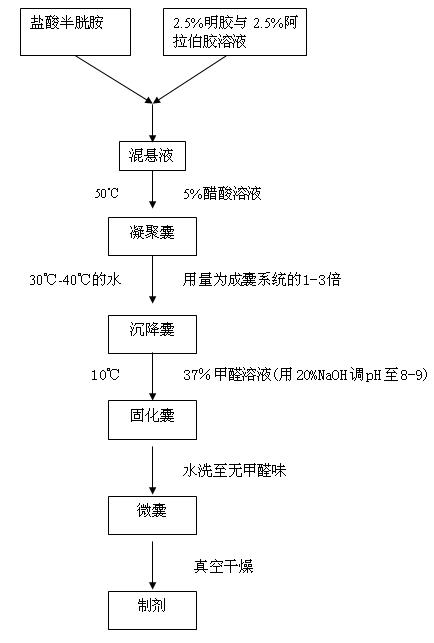
[0029] Mix and dissolve 1 part of cysteamine hydrochloride and 0.2 parts of capsule material (gelatin solution with mass concentration of 2.5% and gum arabic solution with mass concentration of 2.5%, weight ratio 1:1) to form a suspension. Add an appropriate amount of 5% acetic acid solution to the suspension, mix at 50°C, adjust the pH of the suspension to 4.0, and form coacervates. Then add 1 volume of water at 30°C to form a settling pocket. Then add 2.5 mL of formaldehyde solution with a mass concentration of 37%, mix at 10°C, and adjust the pH to 8.0 with an appropriate amount of 20% NaOH to form a solidified capsule. The obtained cured capsules are washed with water until there is no formaldehyde smell to form microcapsules. The obtained microcapsules are vacuum-dried to obtain the finished product.
Embodiment 2
[0031] Mix and dissolve 1 part of cysteamine hydrochloride and 0.3 parts of capsule material (gelatin solution with mass concentration of 4.0% and gum arabic solution with mass concentration of 4.0%, weight ratio 1:1) to form a suspension. Add an appropriate amount of 5% acetic acid solution to the suspension, mix at 50°C, adjust the pH of the suspension to 4.2, and form coacervates. Then add 2 times the volume of water at 35°C to form a settling pocket. Then add 2.5 mL of formaldehyde solution with a mass concentration of 37%, mix at 10°C, and adjust the pH to 8.5 with an appropriate amount of 20% NaOH to form a solidified capsule. The obtained cured capsules are washed with water until there is no formaldehyde smell to form microcapsules. The obtained microcapsules are vacuum-dried to obtain the finished product.
Embodiment 3
[0033] Mix and dissolve 1 part of cysteamine hydrochloride and 0.5 part of capsule material (gelatin solution with mass concentration of 5.0% and gum arabic solution with mass concentration of 5.0%, weight ratio 1:1) to form a suspension. Add an appropriate amount of 5% acetic acid solution to the suspension, mix at 50°C, adjust the pH of the suspension to 4.5, and form coacervates. Then add 3 times the volume of water at 40°C to form a sedimentation pocket. Then add 2.5 mL of formaldehyde solution with a mass concentration of 37%, mix at 10°C, and adjust the pH to 9.0 with an appropriate amount of 20% NaOH to form a solidified capsule. The obtained cured capsules are washed with water until there is no formaldehyde smell to form microcapsules. The obtained microcapsules are vacuum-dried to obtain the finished product.
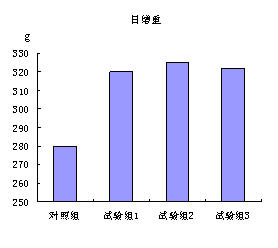
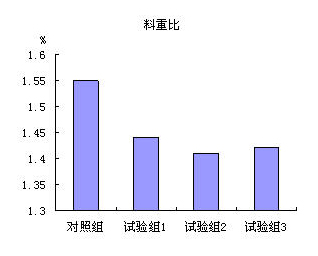
[0034] Illustrate effect of the present invention below by contrast test:
[0035] Add the cysteamine hydrochloride products (test groups 1-3) and the exis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com