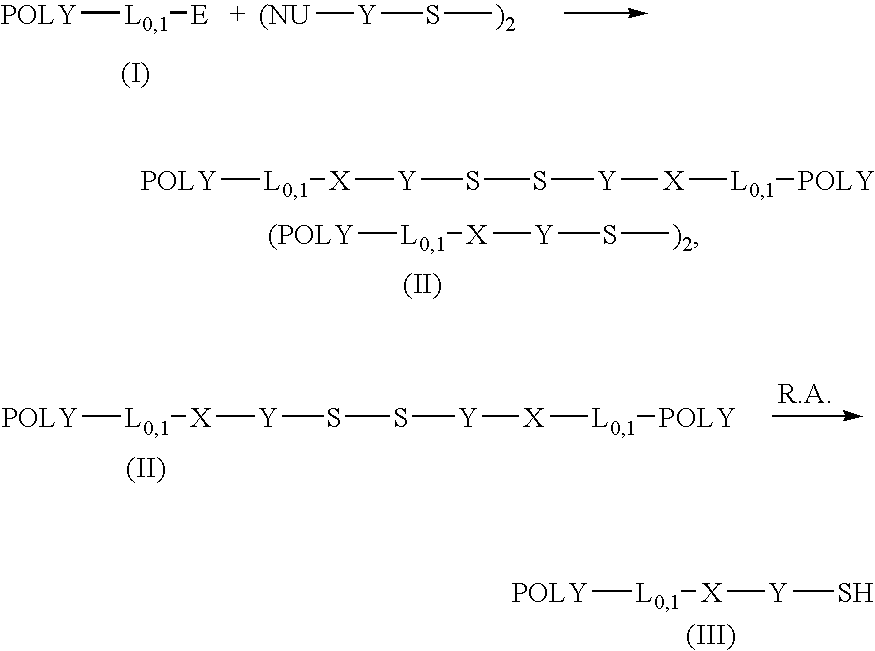Patents
Literature
5667 results about "Cysteine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cysteine (symbol Cys or C; /ˈsɪstiiːn/) is a semiessential proteinogenic amino acid with the formula HO₂CCH(NH₂)CH₂SH. The thiol side chain in cysteine often participates in enzymatic reactions, as a nucleophile. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. When used as a food additive, it has the E number E920. It is encoded by the codons UGU and UGC.
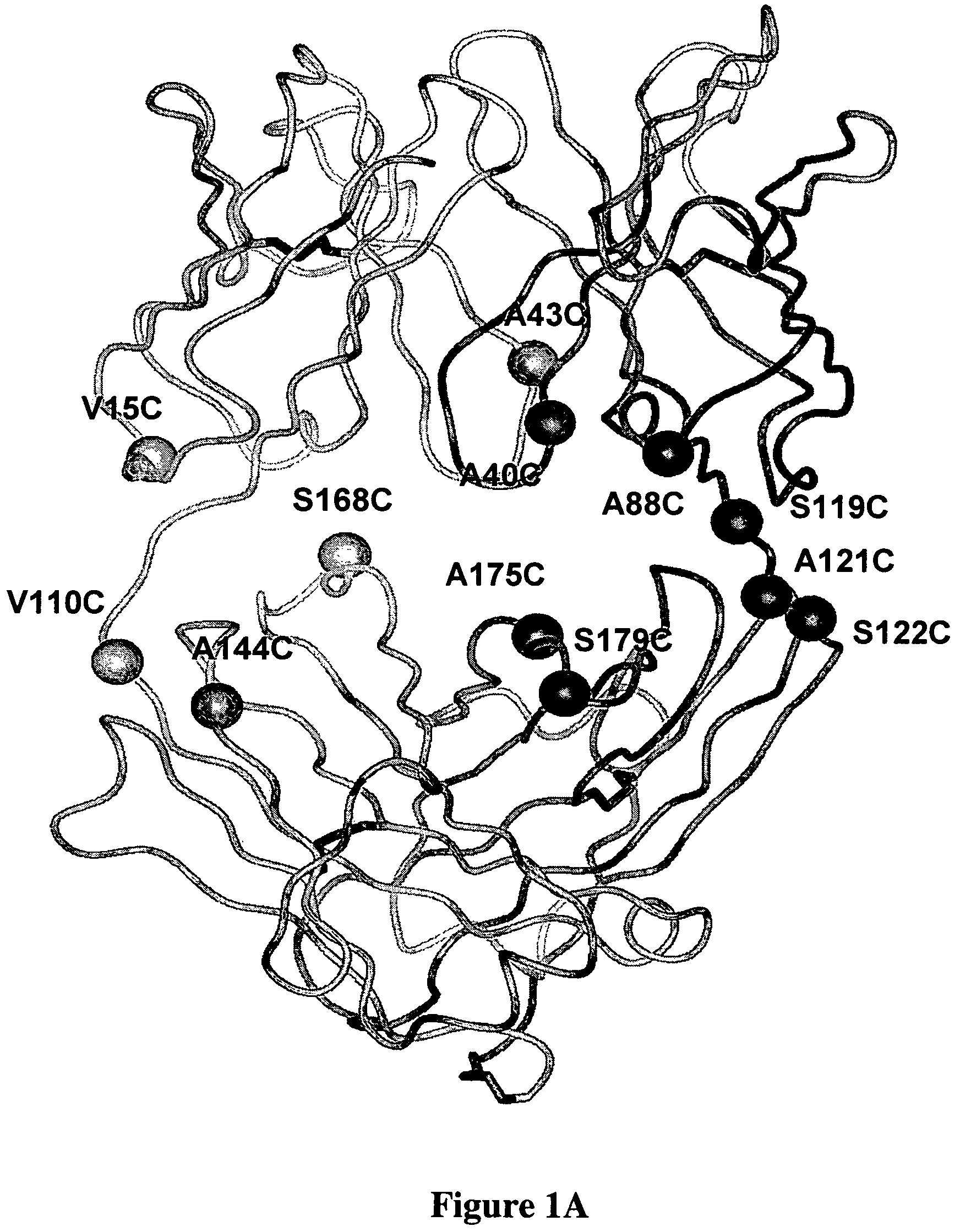
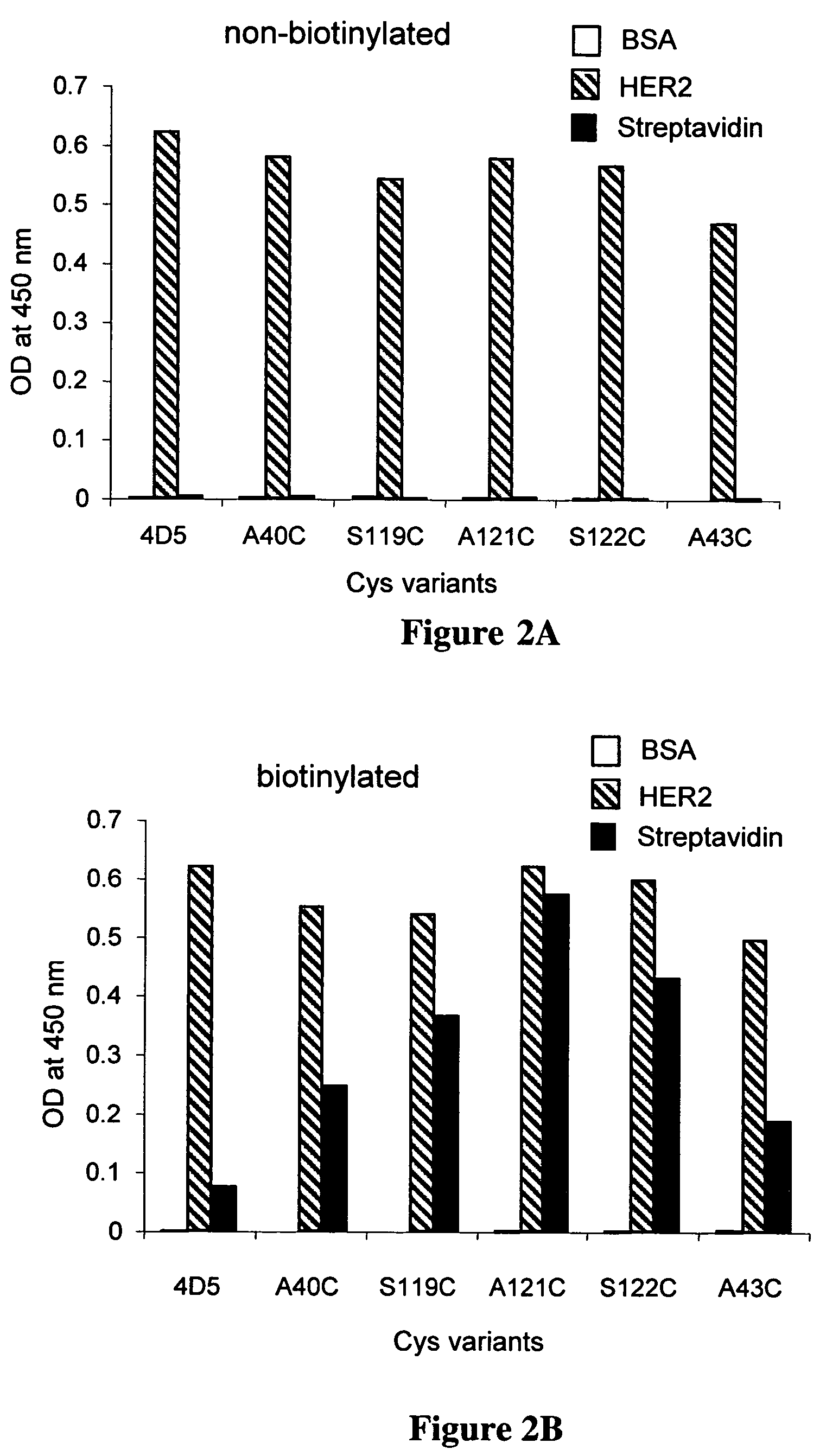
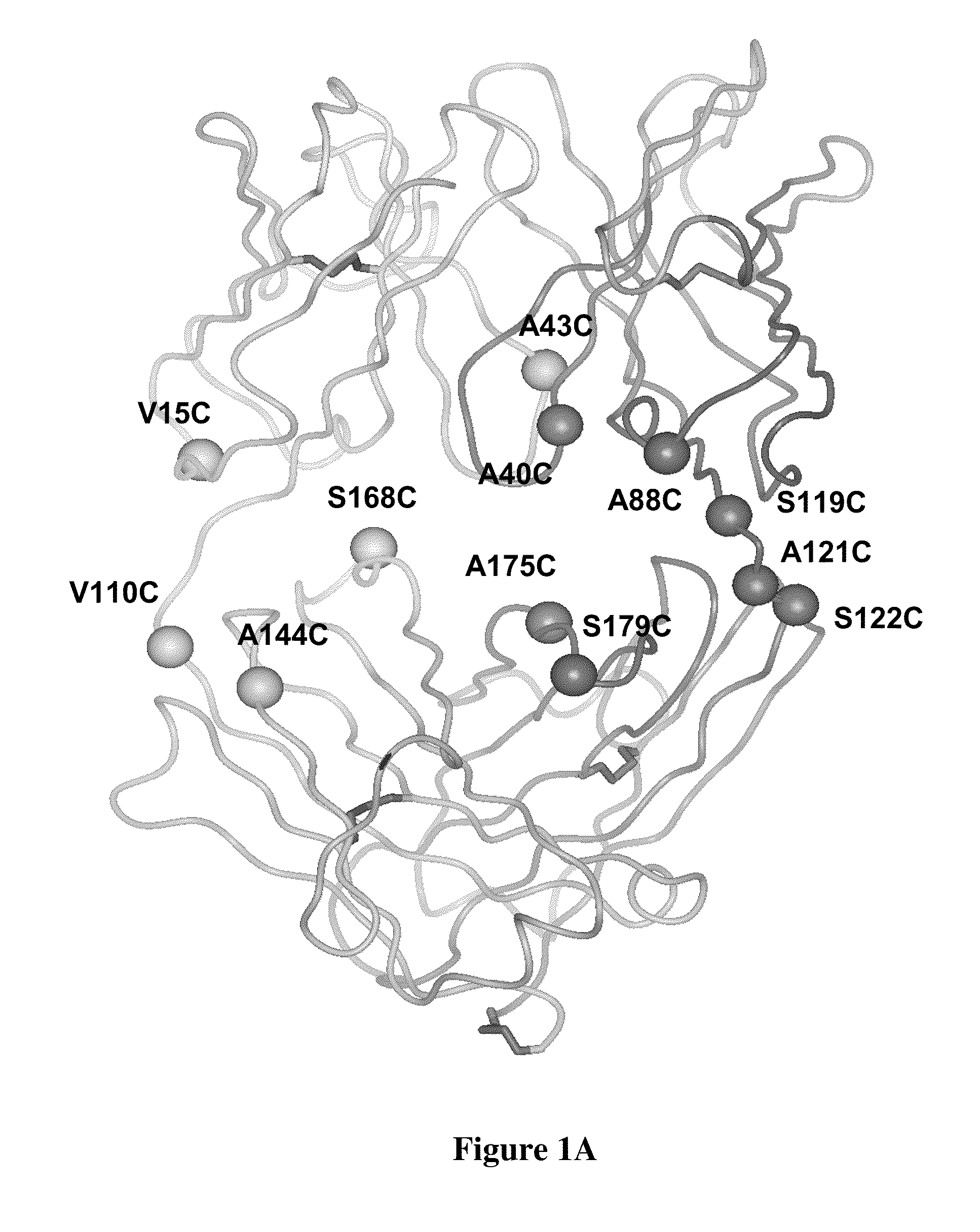
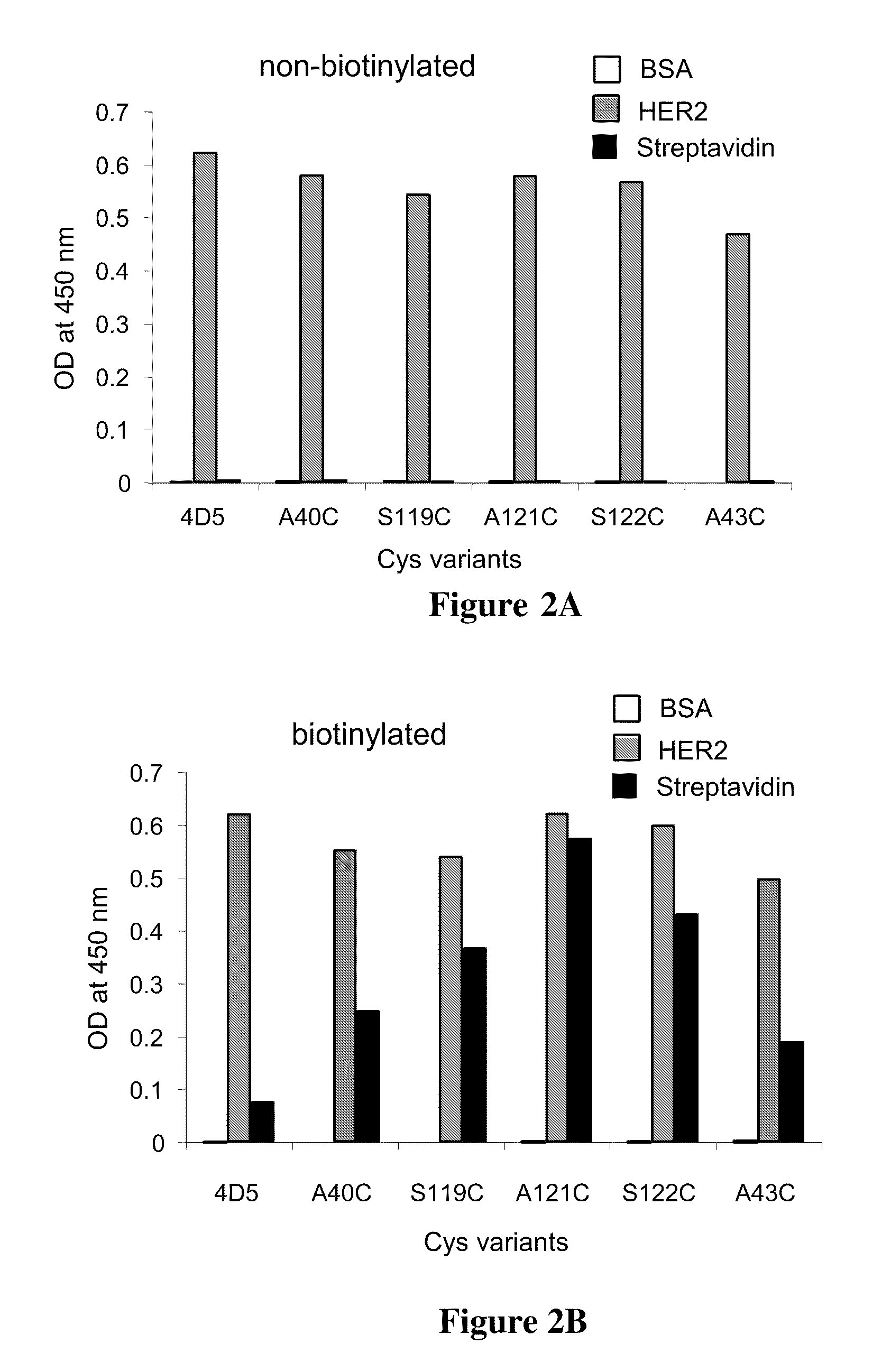
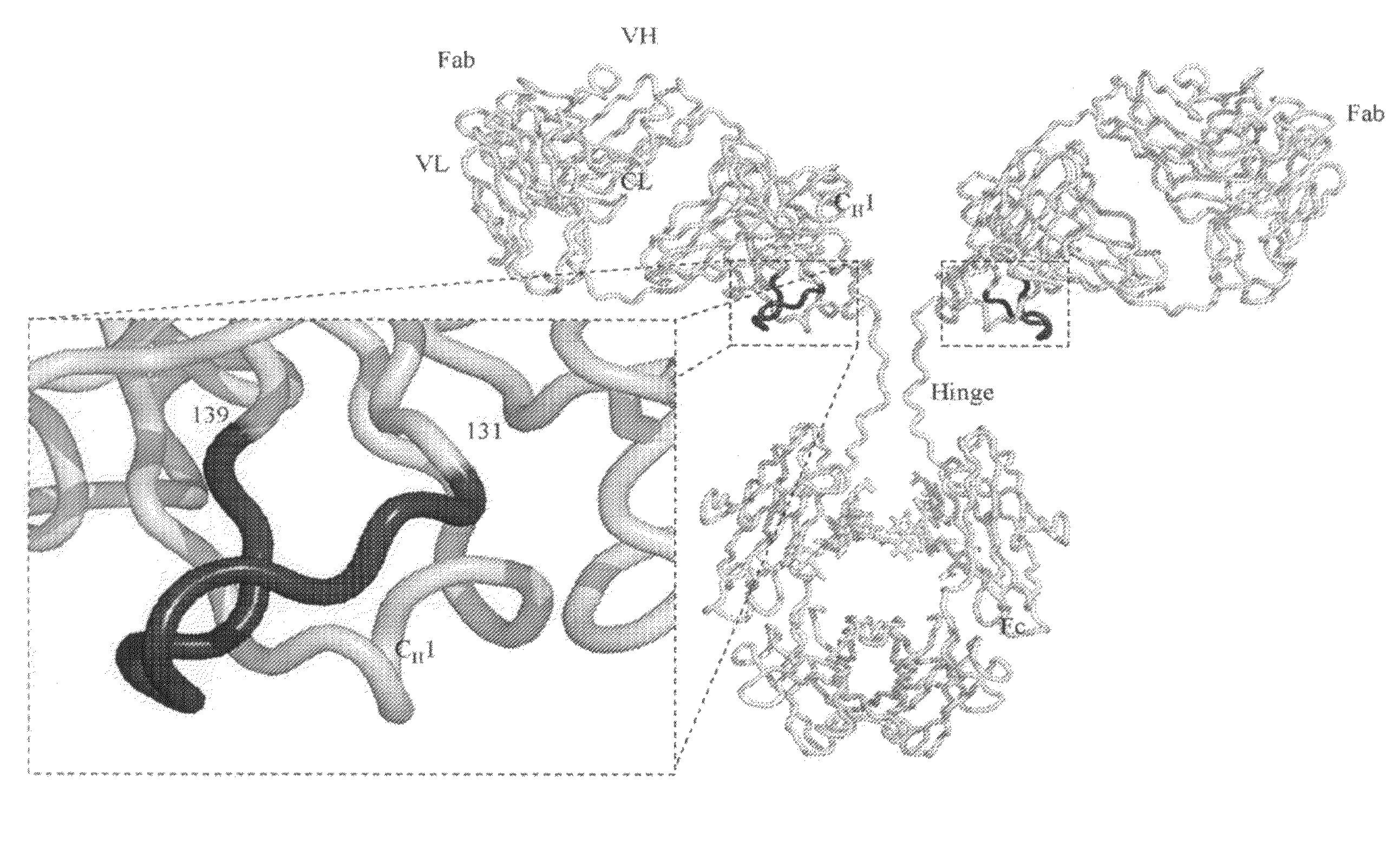
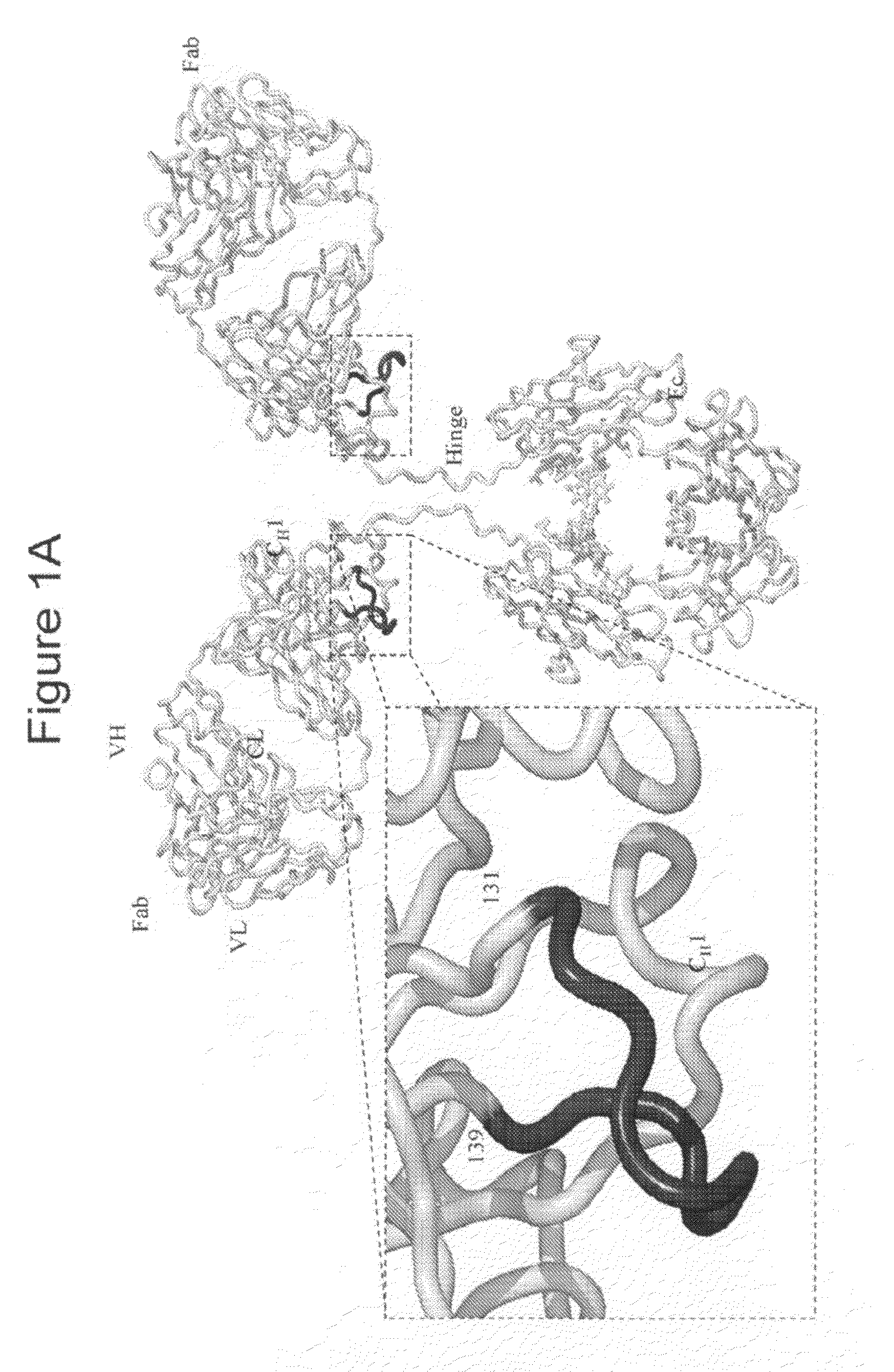
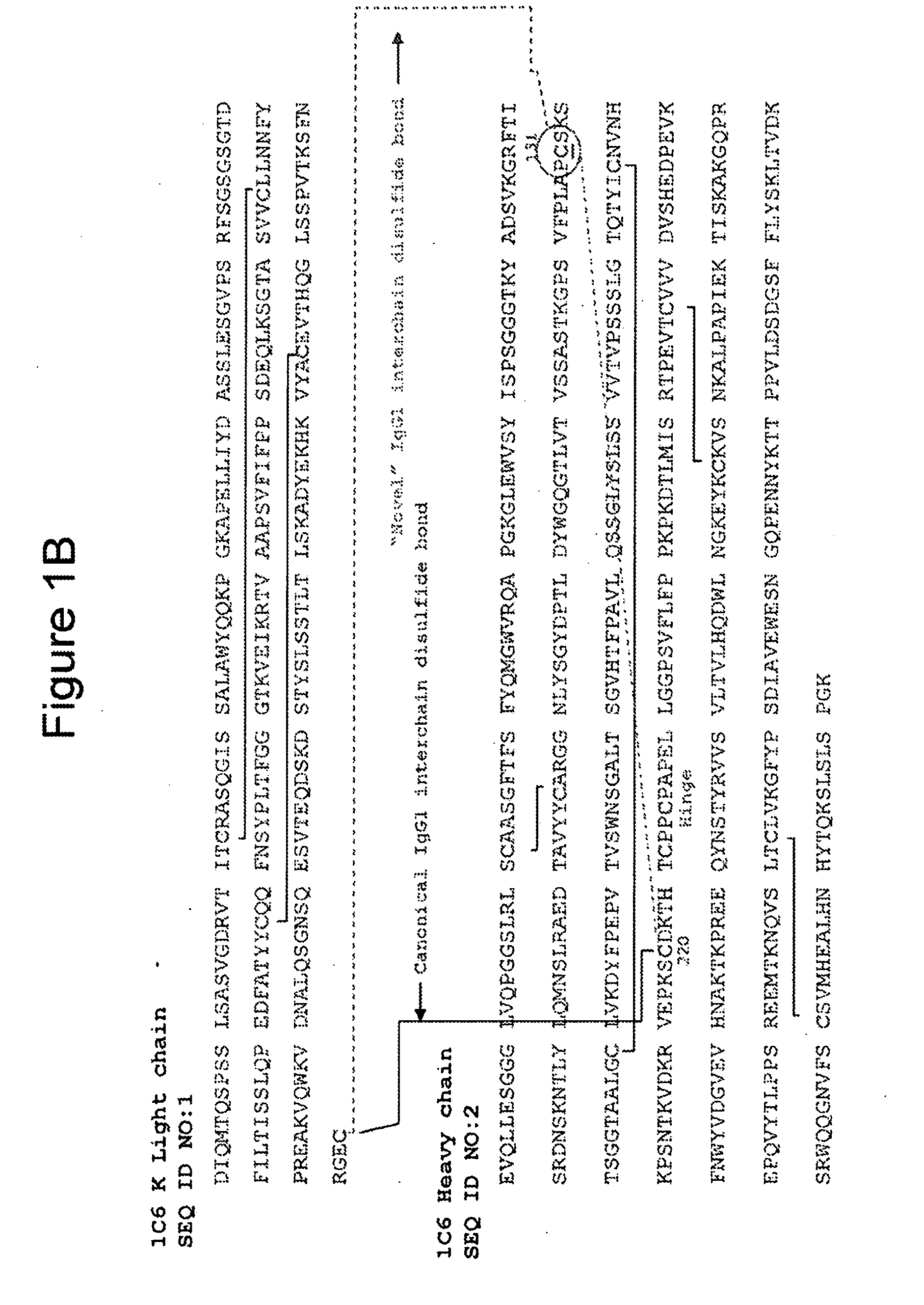
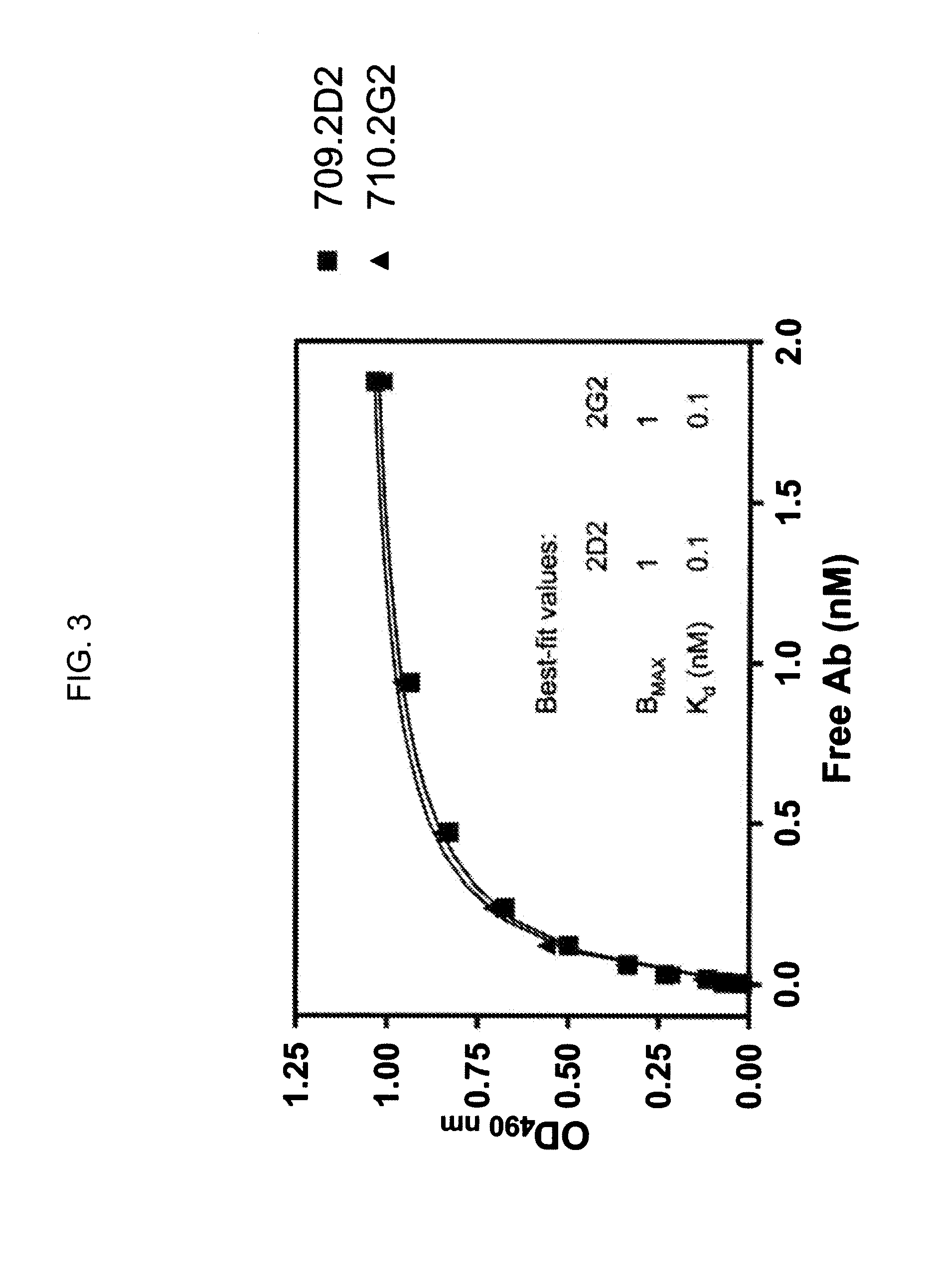
Cysteine engineered antibodies and conjugates
Owner:GENENTECH INC
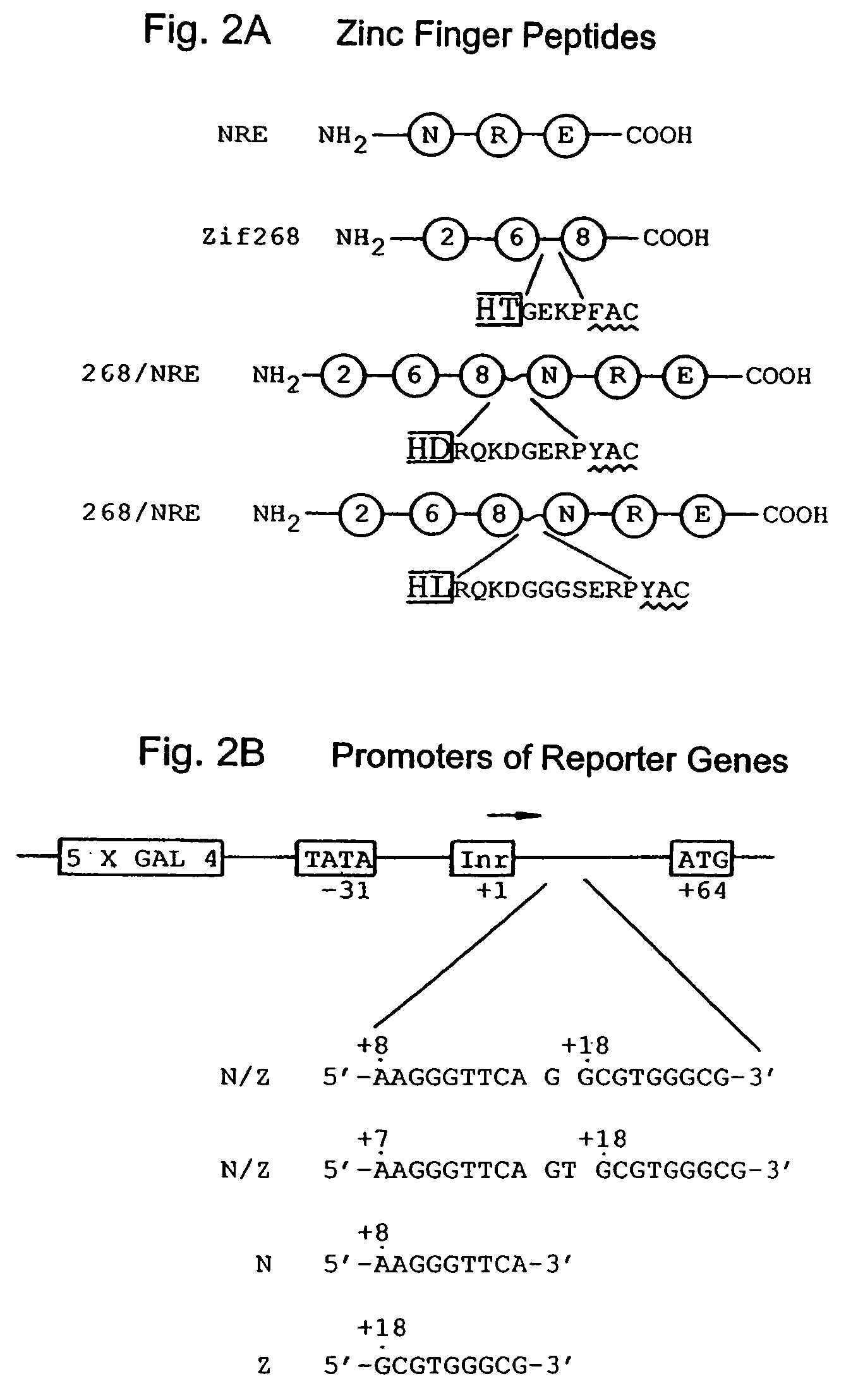
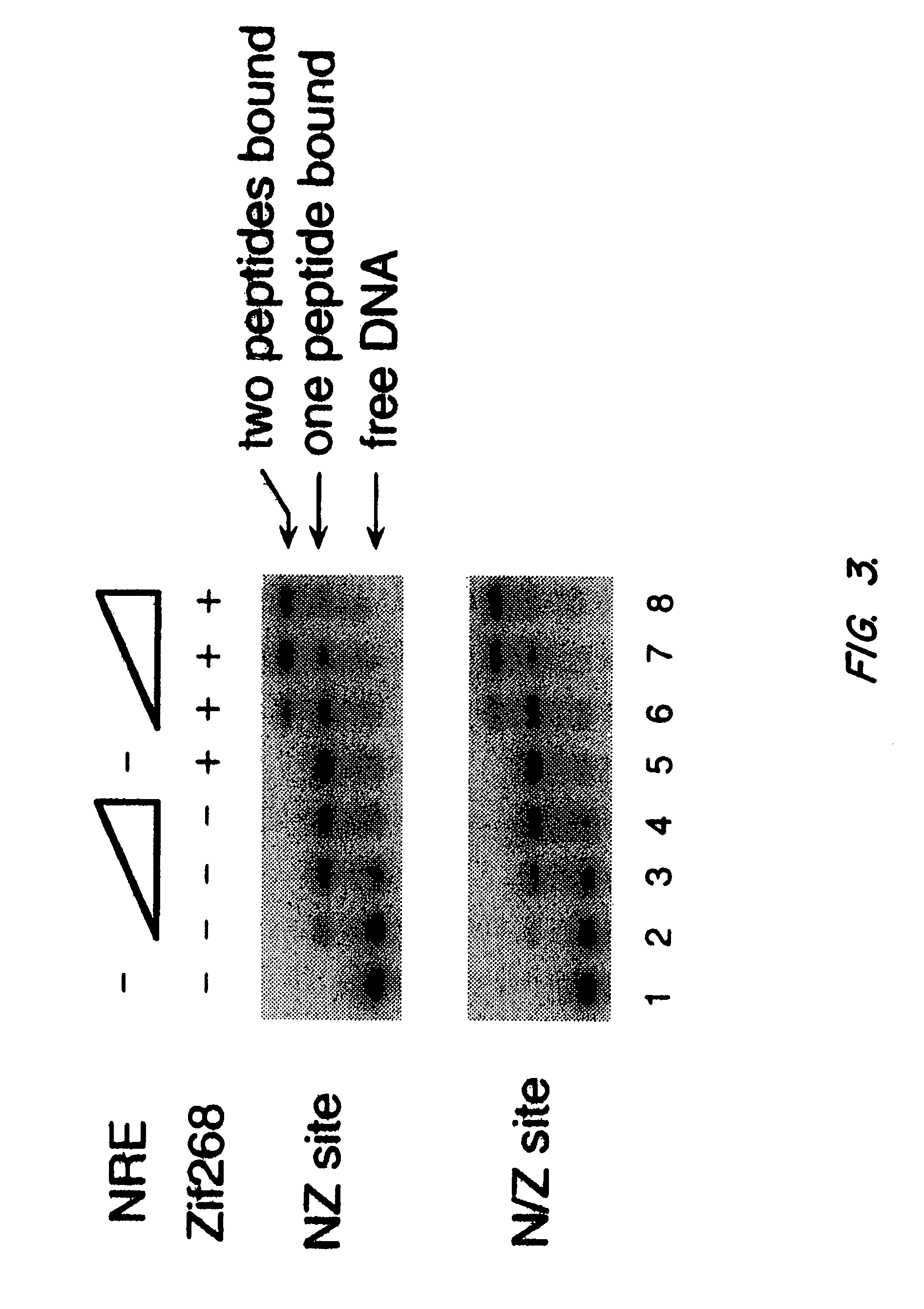
Nucleic acid encoding poly-zinc finger proteins with improved linkers
InactiveUS7153949B2Enhanced affinity and specificityImprove the level ofPeptide/protein ingredientsAntibody mimetics/scaffoldsDNA-binding domainNucleotide
Polynucleotides encoding chimeric proteins, and methods for their production and use are disclosed. The chimeric proteins comprise a flexible linker between two zinc finger DNA-binding domains, wherein the linker contains eight or more amino acids between the second conserved histidine residue of the carboxy-terminal zinc finger of the first domain and the first conserved cysteine residue of the amino-terminal zinc finger of the second domain.
Owner:MASSACHUSETTS INST OF TECH
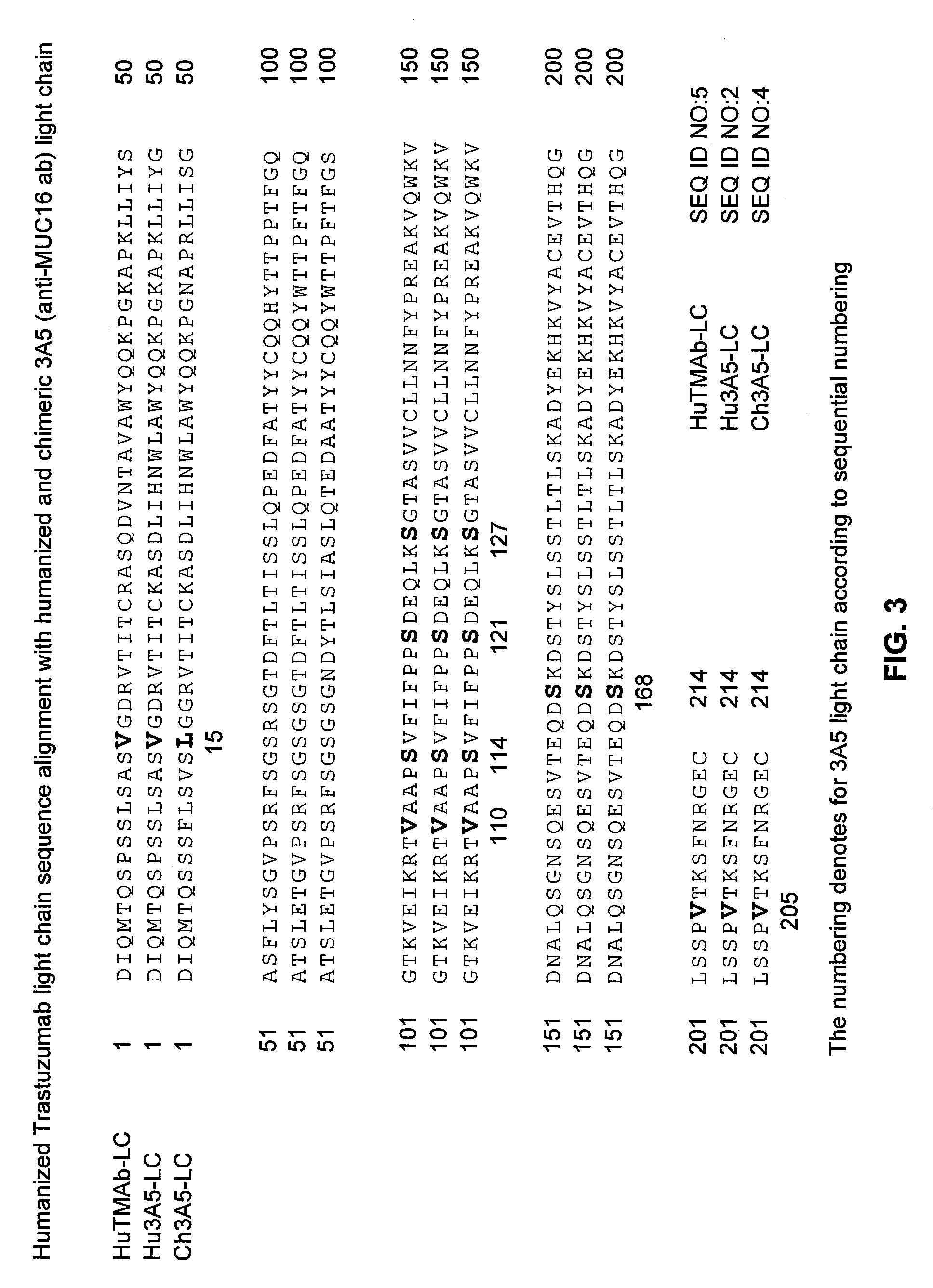
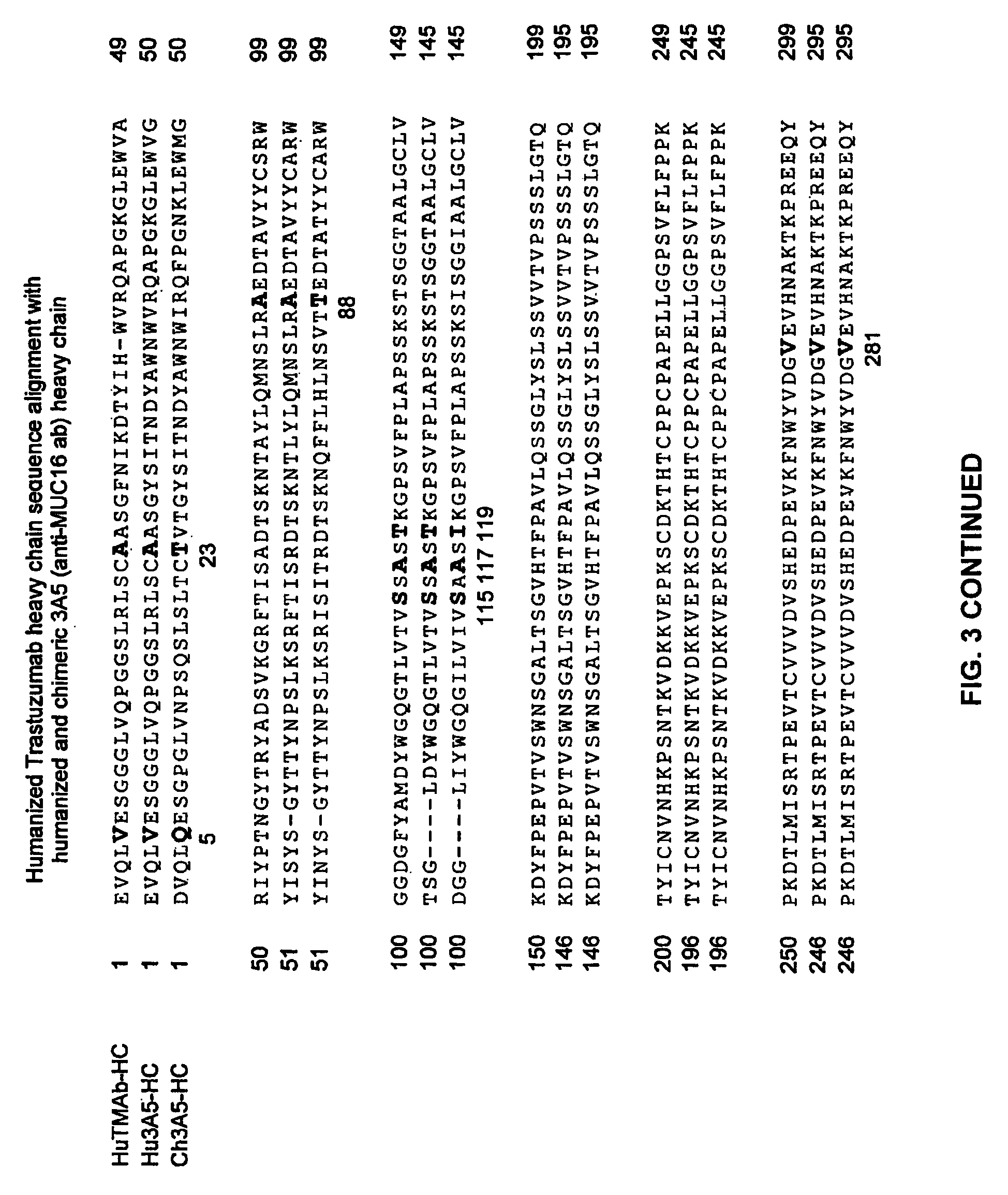
Cysteine engineered anti-MUC16 antibodies and antibody drug conjugates
ActiveUS7723485B2In-vivo radioactive preparationsImmunoglobulins against cell receptors/antigens/surface-determinantsCross-linkDrug compound
Cysteine engineered anti-MUC16 antibodies are engineered by replacing one or more amino acids of a parent anti-MUC16 antibody with non cross-linked, reactive cysteine amino acids. Methods of design, preparation, screening, and selection of the cysteine engineered anti-MUC16 antibodies are provided. Cysteine engineered anti-MUC16 antibodies (Ab) are conjugated with one or more drug moieties (D) through a linker (L) to form cysteine engineered anti-MUC16 antibody-drug conjugates having Formula I:Ab-(L-D)p Iwhere p is 1 to 4. Diagnostic and therapeutic uses for cysteine engineered antibody drug compounds and compositions are disclosed.
Owner:GENENTECH INC
Binding domain-immunoglobulin fusion proteins
InactiveUS20050175614A1Reduced ability to dimerizeHybrid immunoglobulinsAntipyreticCrystallographyAntigen
The invention relates to novel binding domain-immunoglobulin fusion proteins that feature a binding domain for a cognate structure such as an antigen, a counterreceptor or the like, a hinge region polypeptide having either zero or one cysteine residue, and immunoglobulin CH2 and CH3 domains, and that are capable of ADCC and / or CDC while occurring predominantly as monomeric polypeptides. The fusion proteins can be recombinantly produced at high expression levels. Also provided are related compositions and methods, including immunotherapeutic applications.
Owner:TRUBION PHARM INC
Novel recombinant proteins with N-terminal free thiol
InactiveUS20050170457A1Extended half-lifeIncreases circulating serum half-lifePeptide/protein ingredientsTissue cultureCysteine thiolateHalf-life
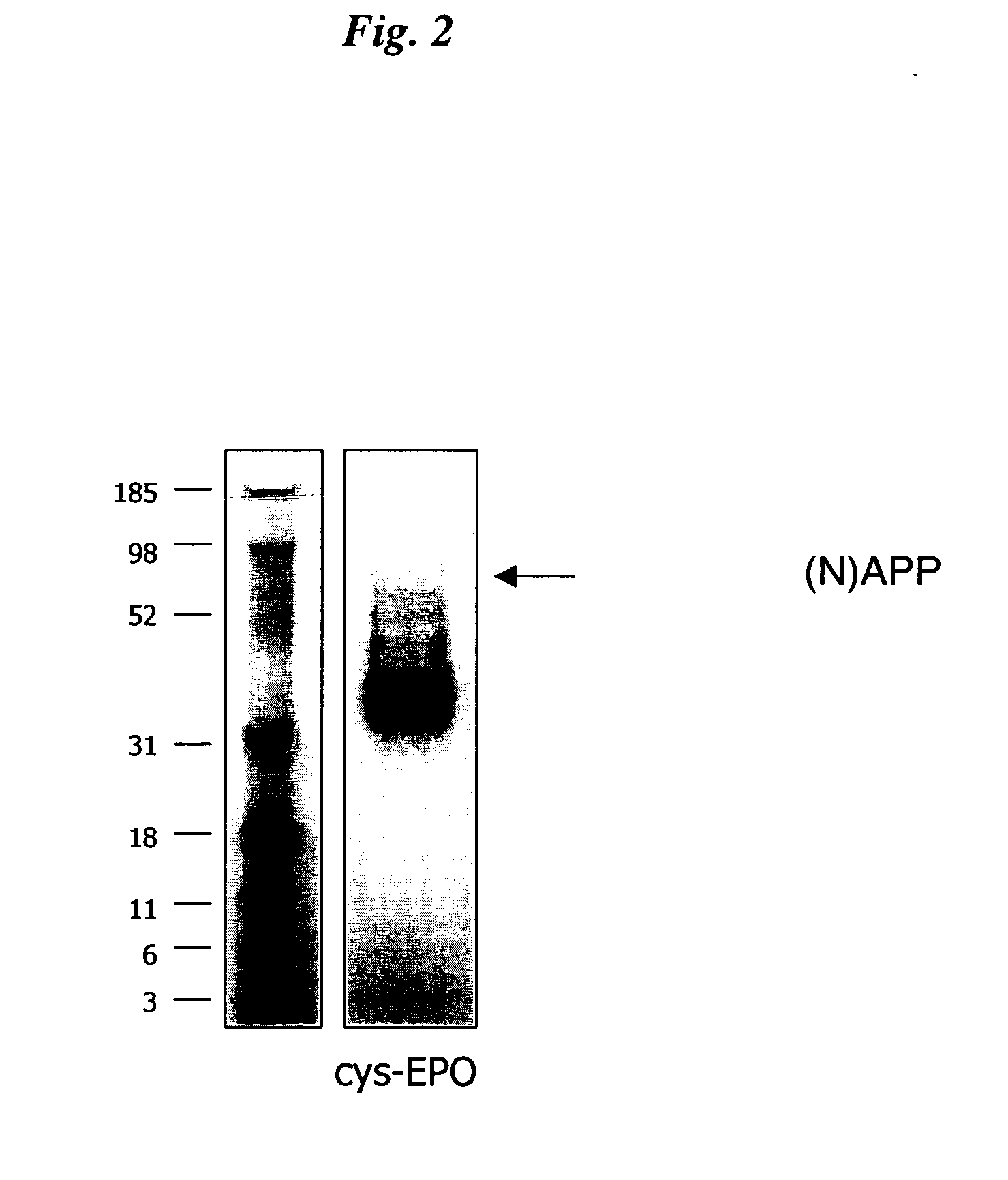
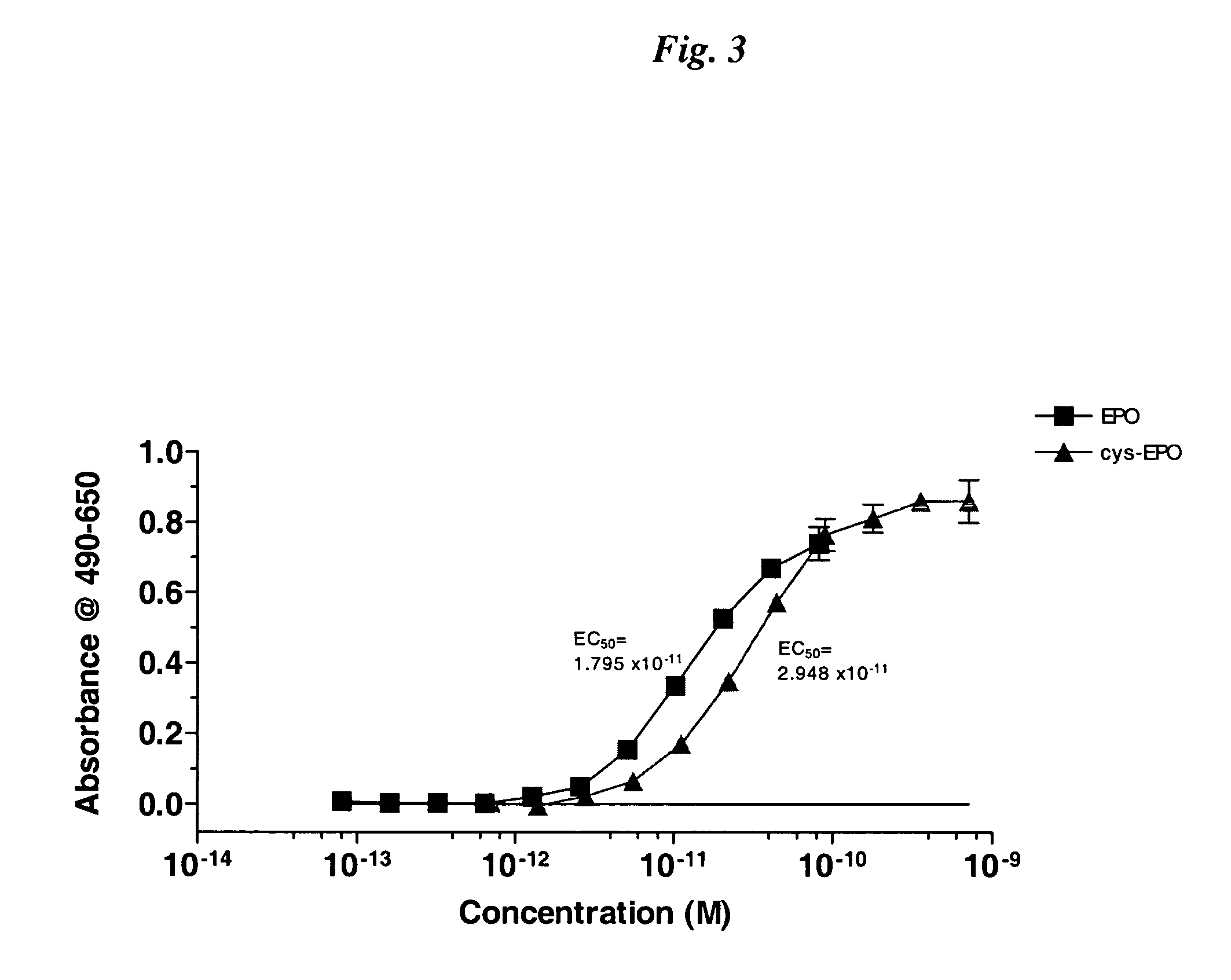
The present invention relates to novel modified proteins having N-terminal free thiols that can be produced by recombinant methods and are ready for further chemical derivatization. In particular, the invention relates to erythropoietin conjugate compounds having altered biochemical, physiochemical and pharmacokinetic properties. More particularly, one embodiment of the invention relates to erythropoietin conjugate compounds of the formula: (M)n-X-A-cys-EPO (I) where EPO is an erythropoeitin moiety selected from erythropoietin or an erythropoietin variant having at least one amino acid different from the wild-type human EPO, or any pharmaceutical acceptable derivatives thereof having biological properties of causing bone marrow cells to increase production of red blood cells; cys represents the amino acid cysteine and occurs at position −1 relative to the amino acid sequence of the erythropoietin moiety; A indicates the structure of the residual moiety used to chemically attach X to the thiol group of −1Cys; X is a water soluble polymer such as a polyalkylene glycol or other polymer; M is an organic molecule (including peptides and proteins) that increases the circulating half-life of the construct; and N is an integer from 0 to 15.
Owner:CENTOCOR
IGG separation medium
A separation medium having a base matrix and matrix-bound groups which exhibit recombinant Protein A containing a cysteine. The groups are of formula:where B is a bridge which binds to the base matrix and X includes a heteroatom N or S from rProtein A-cys. In a preferred embodiment X is a thioether sulphur and / or a secondary amine (-NH-). An alternative embodiment features a variant of Protein A in which the C-terminal residue is cysteine.
Owner:GE HEALTHCARE BIOPROCESS R&D
Specific kinase inhibitors
Resorcylic acid lactones having a C5-C6 cis double bond and a ketone at C7 and other compounds capable of Michael adduct formation are potent and stable inhibitors of a subset of protein kinases having a specific cysteine residue in the ATP binding site.
Owner:KOSAN BIOSCI
Zirconium-radiolabeled, cysteine engineered antibody conjugates
InactiveUS20100111856A1Peptide/protein ingredientsGenetic material ingredientsAntibody conjugateAntibody fragments
Antibodies are engineered by replacing one or more amino acids of a parent antibody with non cross-linked, highly reactive cysteine amino acids. Antibody fragments may also be engineered with one or more cysteine amino acids to form cysteine engineered antibody fragments (ThioFab). Methods of design, preparation, screening, and selection of the cysteine engineered antibodies are provided. Cysteine engineered antibodies (Ab) are conjugated with one or more zirconium complex (Z) labels through a linker (L) to form cysteine engineered zirconium-labeled antibody conjugates having Formula I:Ab-(L-Z)p Iwhere p is 1 to 4. Imaging methods and diagnostic uses for zirconium-radiolabeled, cysteine engineered antibody conjugate compositions are disclosed.
Owner:F HOFFMANN LA ROCHE & CO AG
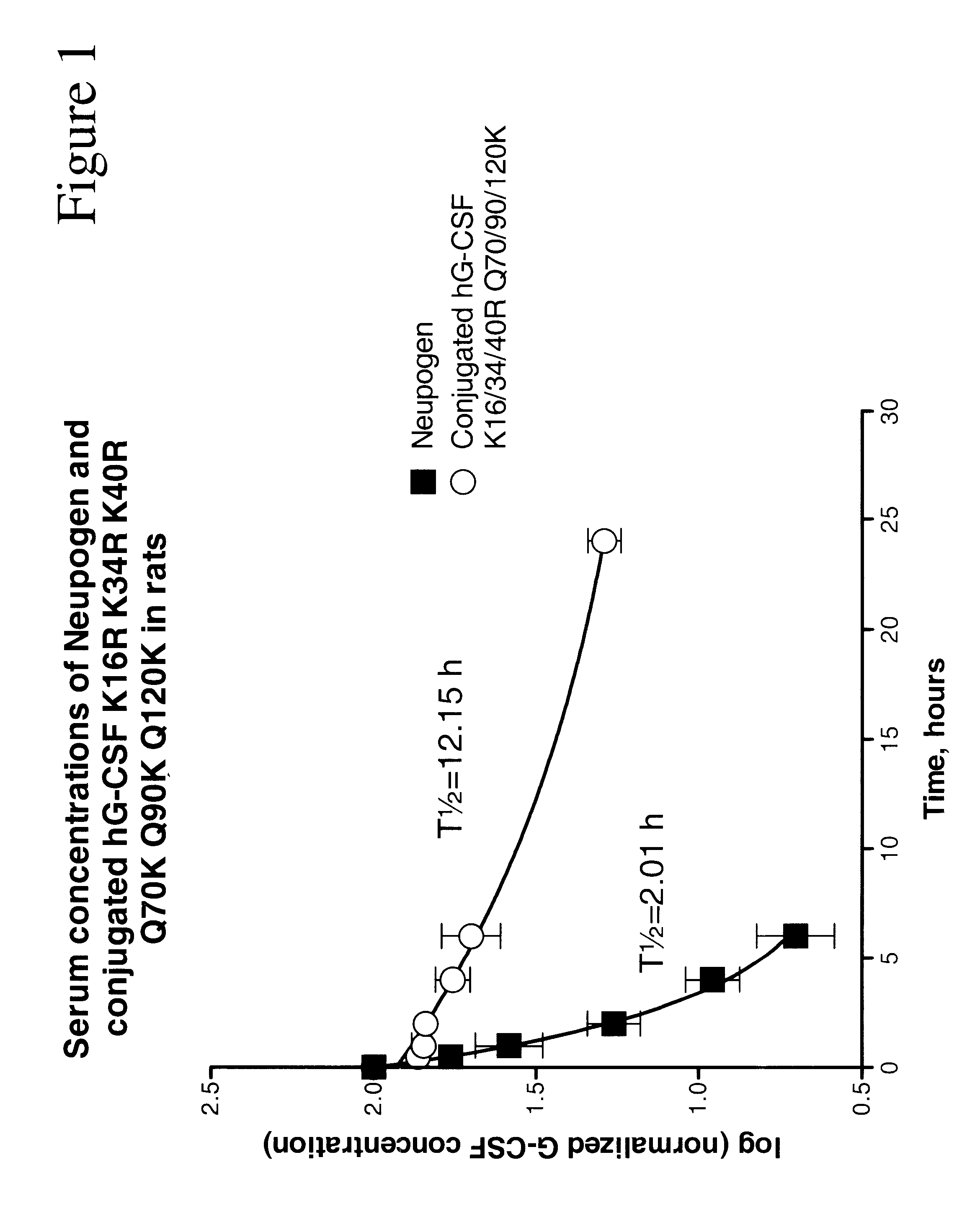
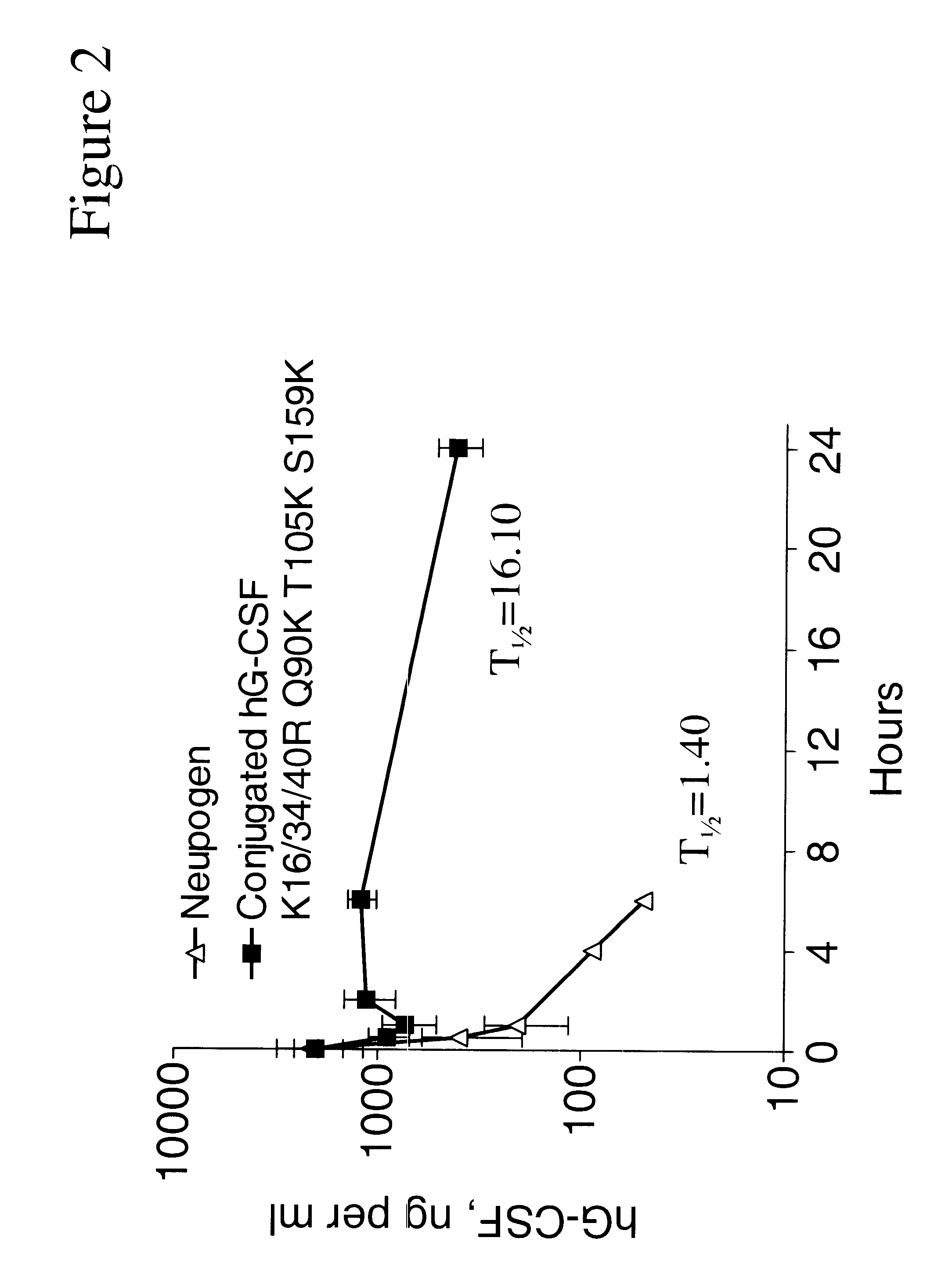
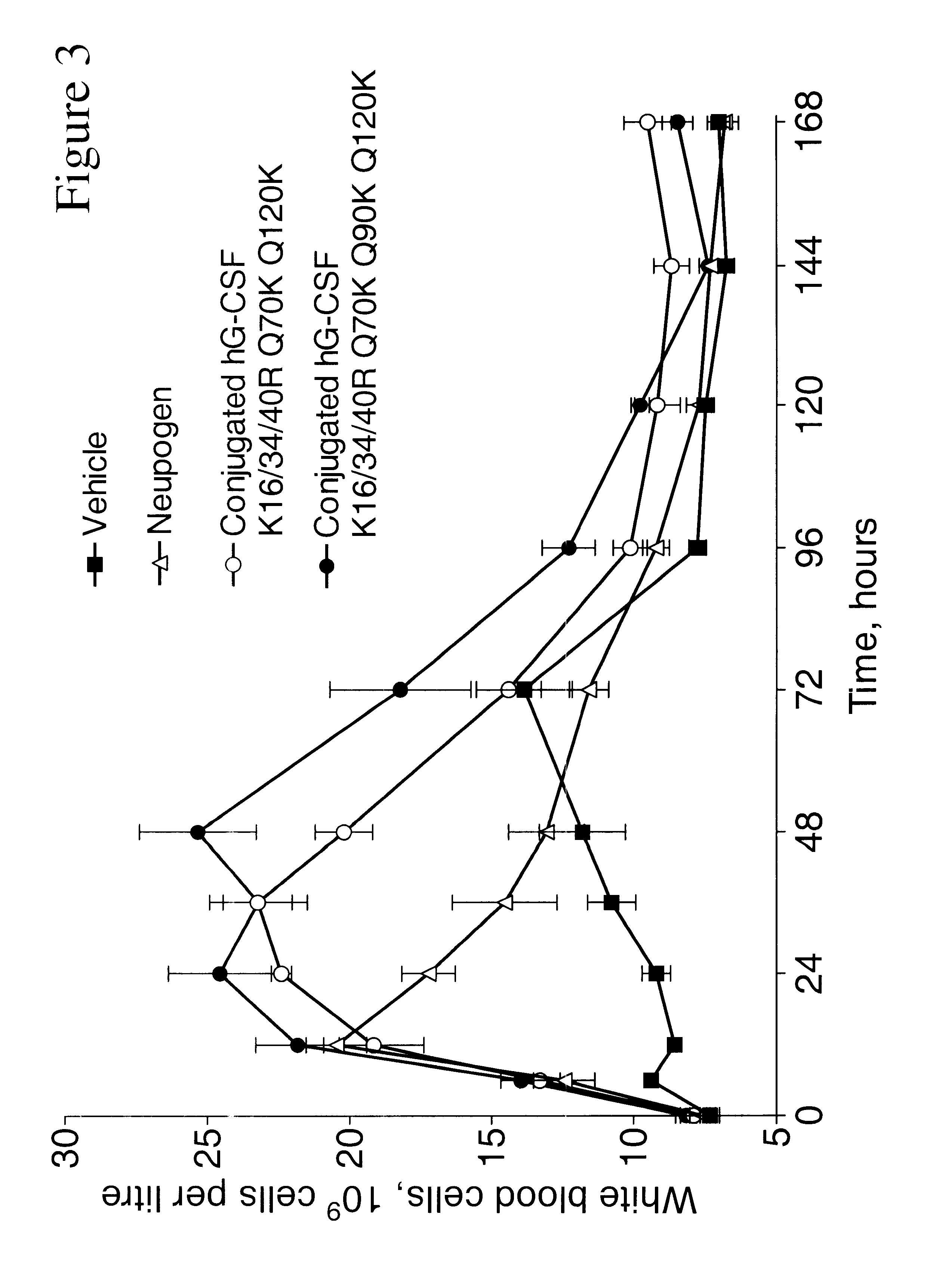
G-CSF conjugates
InactiveUS6555660B2Improved propertyReduced in vitroBiocidePeptide/protein ingredientsHalf-lifePolyethylene glycol
The invention relates to polypeptide conjugates comprising a polypeptide exhibiting G-CSF activity and having an amino acid sequence that differs from the amino acid sequence of human G-CSF in at least one specified introduced and / or removed amino acid residue comprising an attachment group for a non-polypeptide moiety, and having at least one non-polypeptide moiety attached to an attachment group of the polypeptide. The attachment group may e.g. be a lysine, cysteine, aspartic acid or glutamic acid residue or a glycosylation site, and the non-polypeptide moiety may e.g. be a polymer such as polyethylene glycol or an oligosaccharide. The conjugate, which has a reduced in vitro bioactivity compared to hG-CSF, has one or more improved properties such as increased biological half-life and increased stimulation of neutrophils.
Owner:MAXYGEN
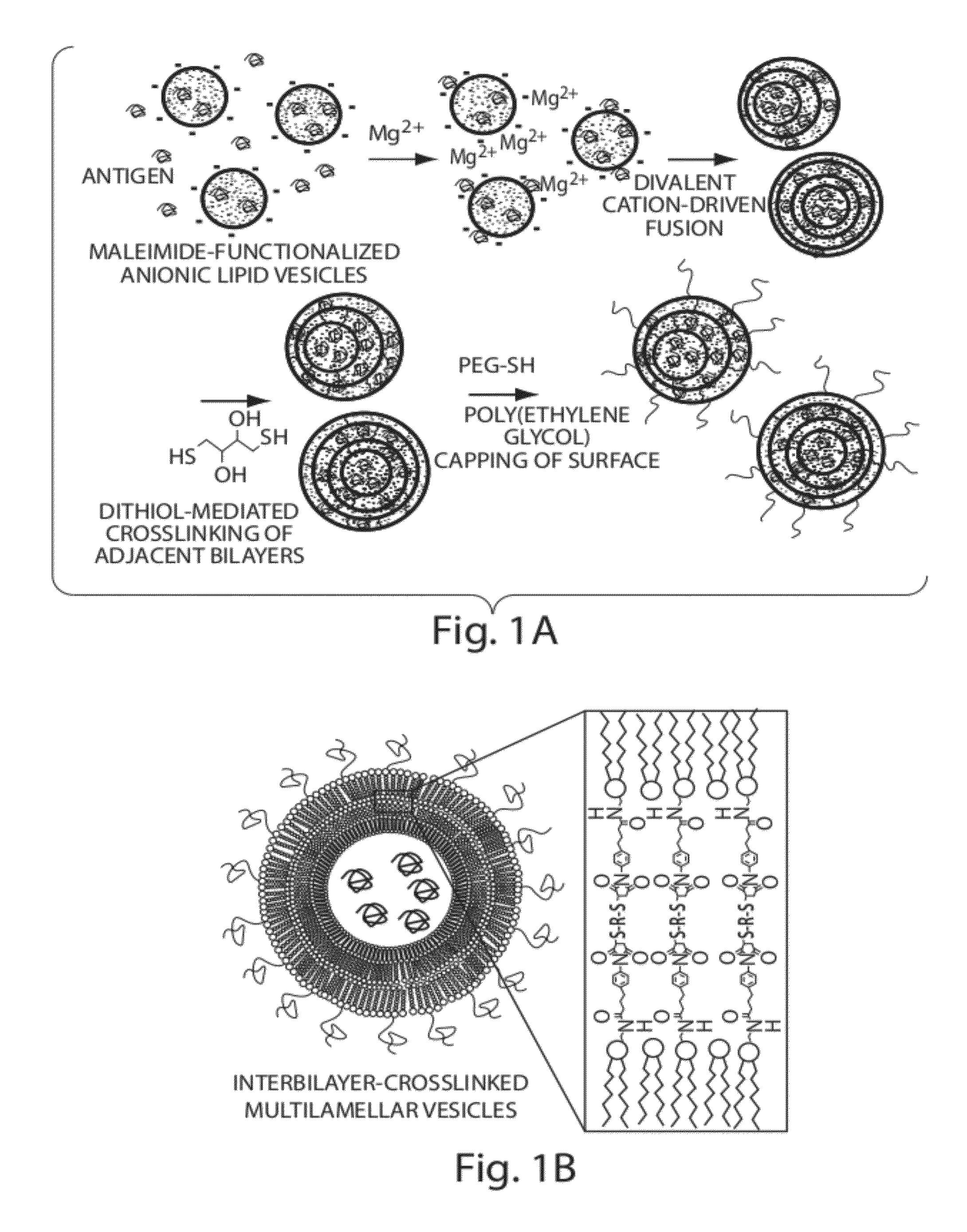
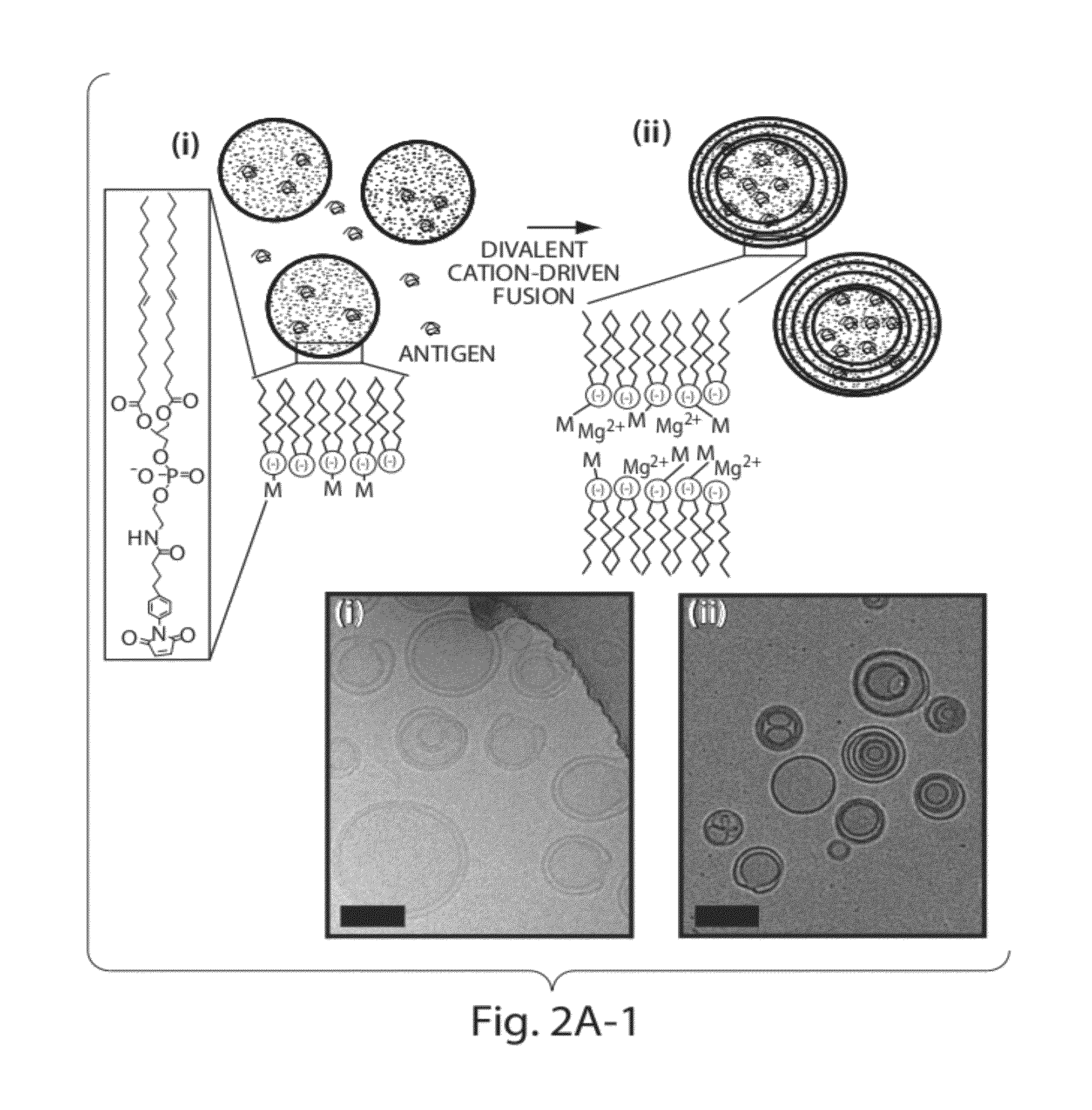
Lipid vesicle compositions and methods of use
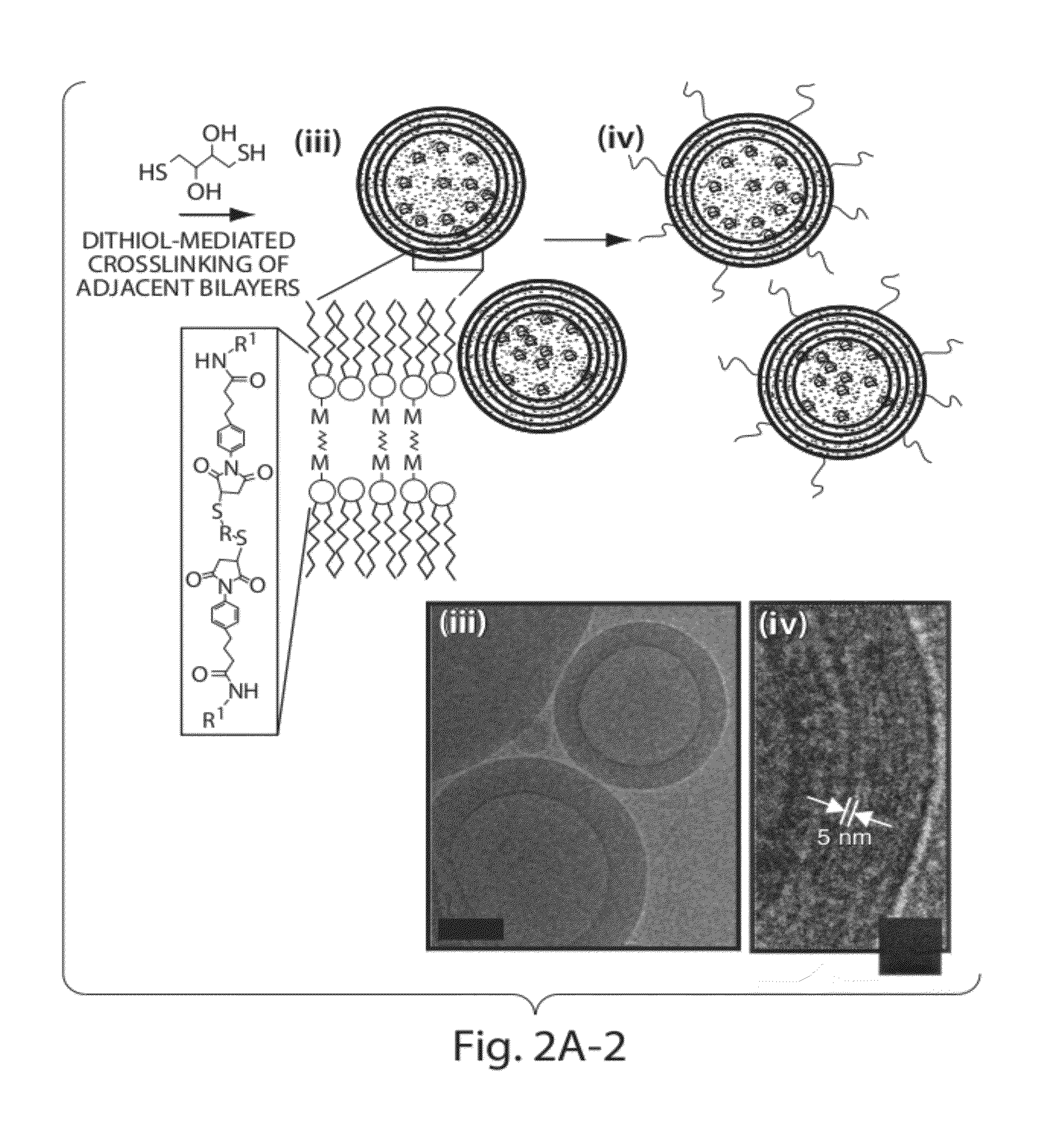
ActiveUS20120177724A1Increase load capacityImprove the level ofPeptide/protein ingredientsMicroencapsulation basedAntigenVesicle/vacuole
The invention provides delivery systems comprised of stabilized multilamellar vesicles, as well as compositions, methods of synthesis, and methods of use thereof. The stabilized multilamellar vesicles comprise terminal-cysteine-bearing antigens or cysteine-modified antigens, at their surface and / or internally.
Owner:MASSACHUSETTS INST OF TECH +1
Cysteine engineered antibodies and conjugates
Cysteine engineered antibodies comprising a free cysteine amino acid in the heavy chain or light chain are prepared by mutagenizing a nucleic acid sequence of a parent antibody and replacing one or more amino acid residues by cysteine to encode the cysteine engineered antibody; expressing the cysteine engineered antibody; and isolating the cysteine engineered antibody. Certain highly reactive cysteine engineered antibodies were identified by the PHESELECTOR assay. Isolated cysteine engineered antibodies may be covalently attached to a capture label, a detection label, a drug moiety, or a solid support.
Owner:GENENTECH INC
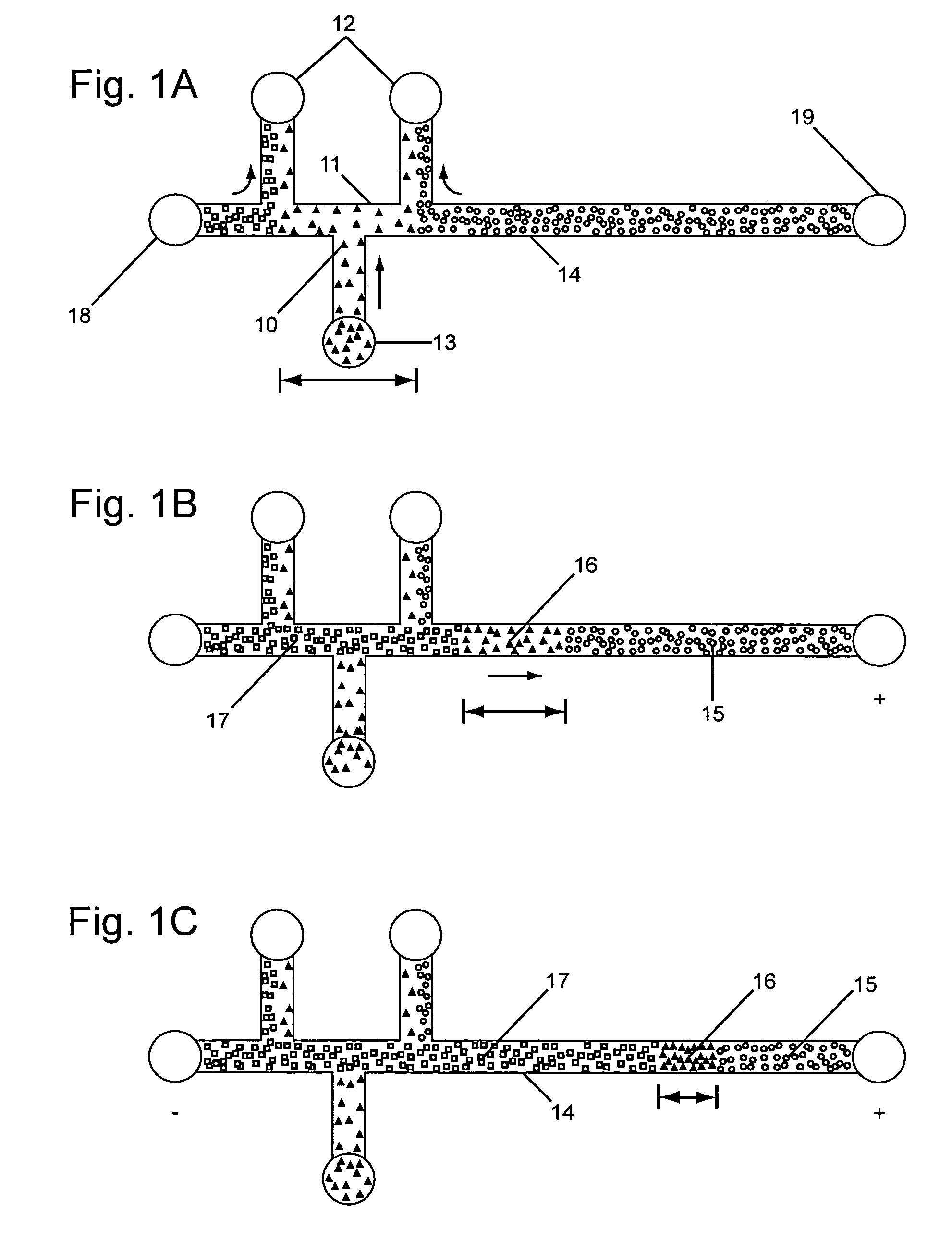
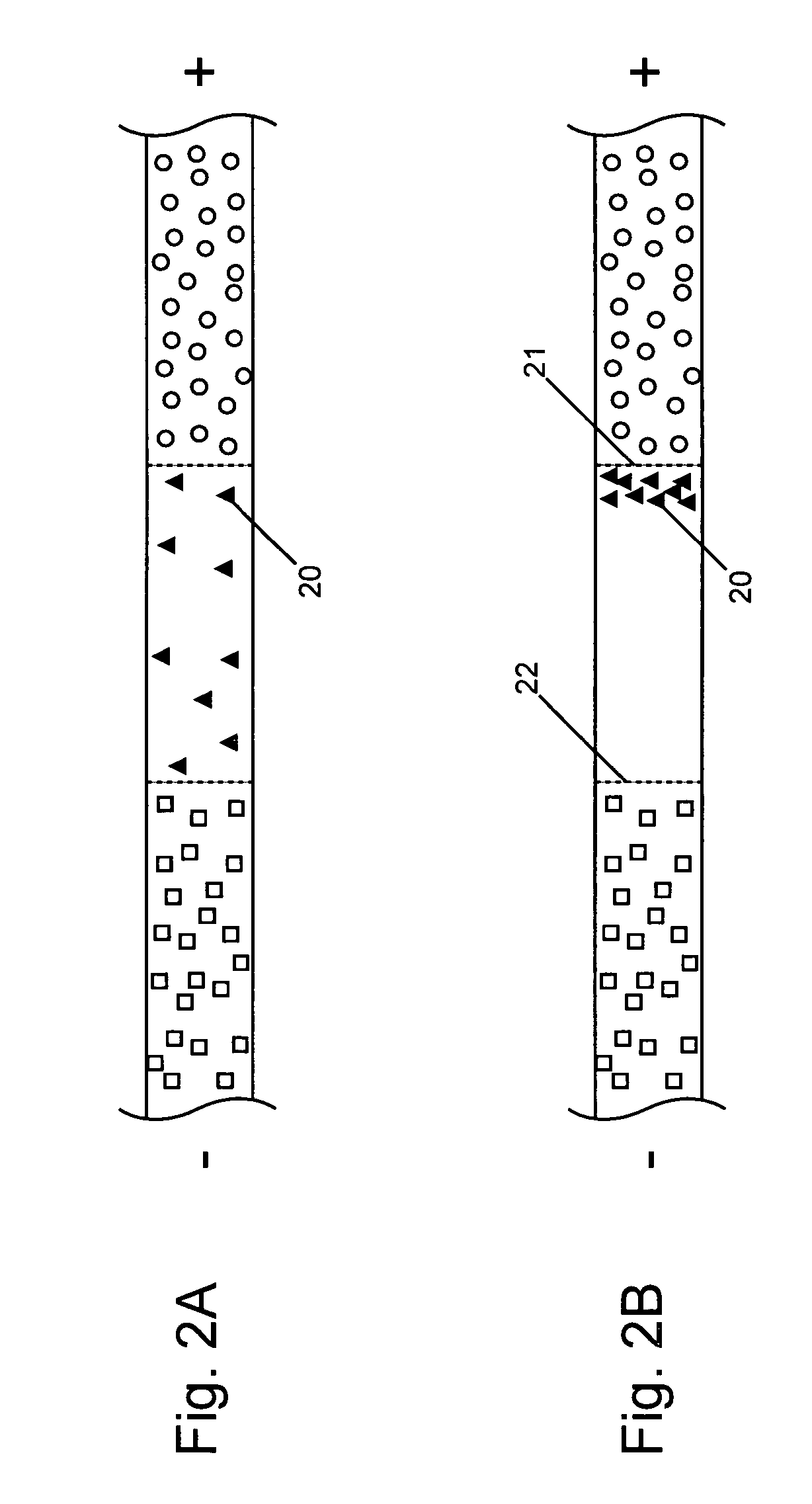
Analyte injection system
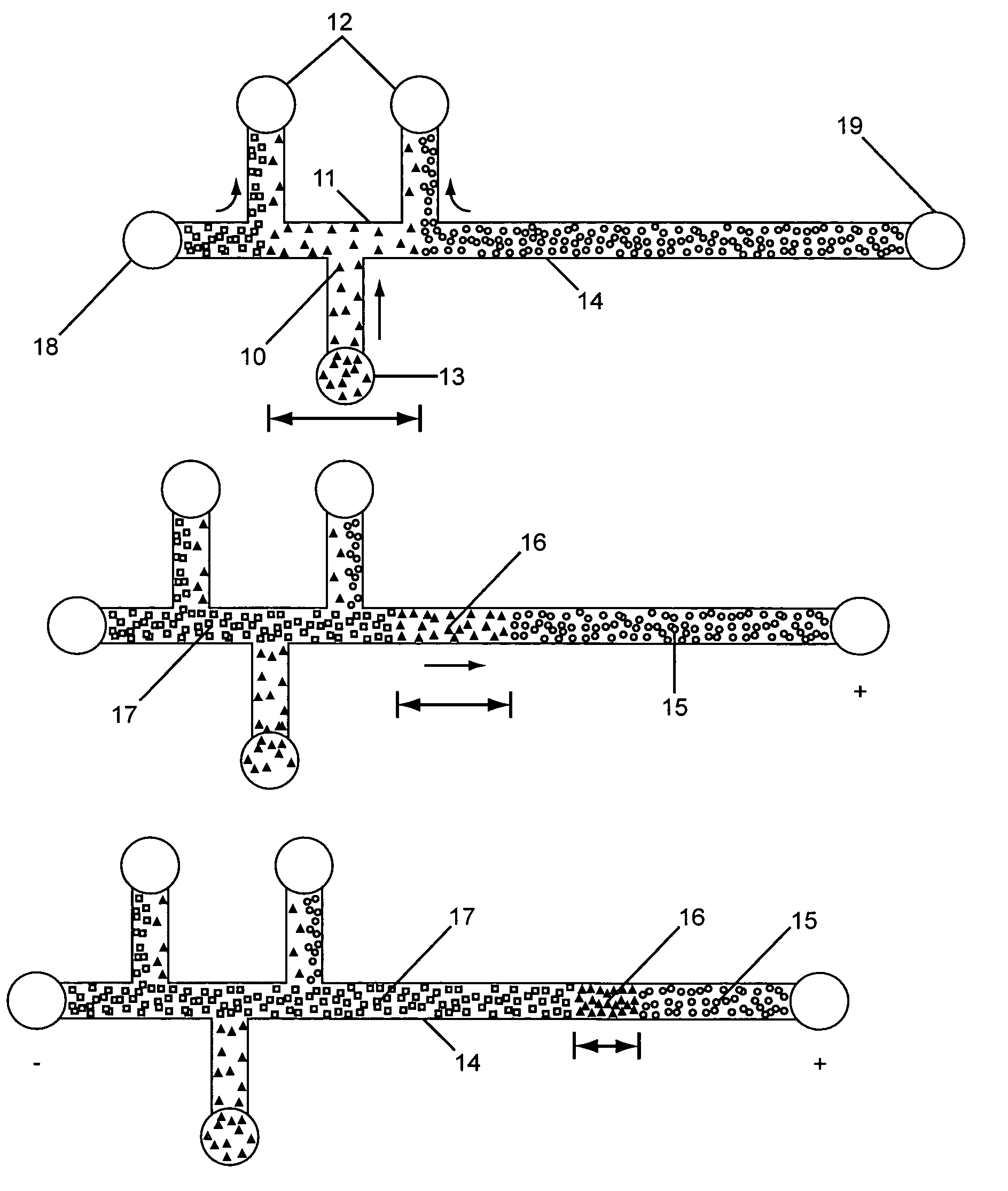
InactiveUS20050133370A1Increase in sizeShort amount of timeSludge treatmentVolume/mass flow measurementGlutaric acidAntibody conjugate
This invention provides methods and devices for spatially separating at least first and second components in a sample which in one exemplary embodiment comprises introducing the first and second components into a first microfluidic channel of a microfluidic device in a carrier fluid comprising a spacer electrolyte solution and stacking the first and second components by isotachophoresis between a leading electrolyte solution and a trailing electrolyte solution, wherein the spacer electrolyte solution comprises ions which have an intermediate mobility in an electric field between the mobility of the ions present in the leading and trailing electrolyte solutions and wherein the spacer electrolyte solution comprises at least one of the following spacer ions MOPS, MES, Nonanoic acid, D-Glucuronic acid, Acetylsalicyclic acid, 4-Ethoxybenzoic acid, Glutaric acid, 3-Phenylpropionic acid, Phenoxyacetic acid, Cysteine, hippuric acid, p-hydroxyphenylacetic acid, isopropylmalonic acid, itaconic acid, citraconic acid, 3,5-dimethylbenzoic acid, 2,3-dimethylbenzoic acid, p-hydroxycinnamic acid, and 5-br-2,4-dihydroxybenzoic acid, and wherein the first component comprises a DNA-antibody conjugate and the second component comprises a complex of the DNA-antibody conjugate and an analyte.
Owner:WAKO PURE CHEMICAL INDUSTRIES +1
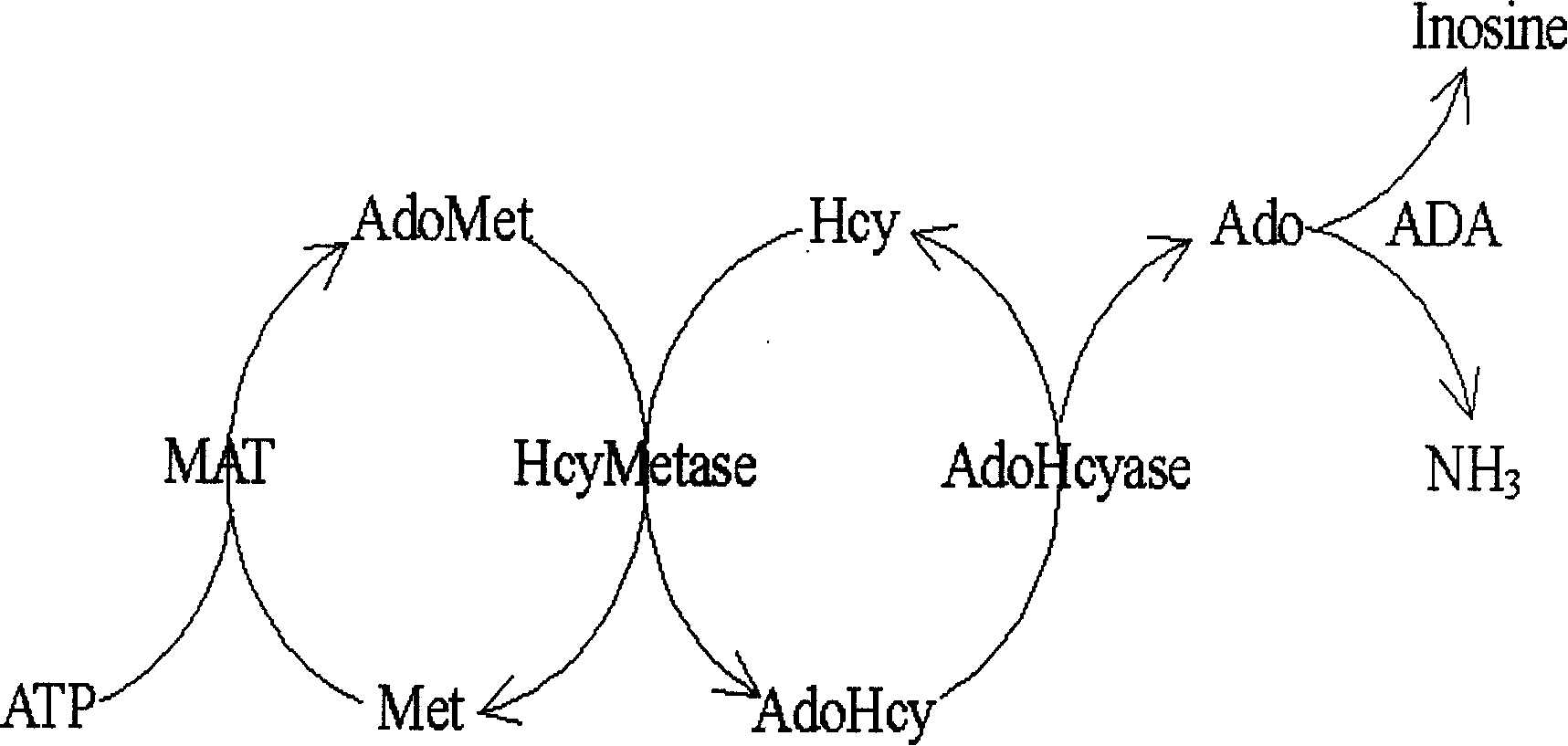
Homotypic cysteine measuring method and its reagent
ActiveCN1560610AAbsorbance value dropsHigh sensitivityMaterial analysis by observing effect on chemical indicatorBiological testingAdenosineHomocysteine measurement
The invention relates to an enzymology measuring method and reagent with circular increment technology, applied to measure the content of homeotypic cysteine in the measured sample. The character is: with a circular increment technology, increases the measurement sensitivity, thus it can carries on automatic measurement to the homeotypic cysteine in the sample liquid like normal enzymology diagnosing agent. The homeotypic cysteine in the sample liquid reacts with the S-adenosine-L-methionine repeatedly and circularly under the effect of homeotypic cysteine methyl kinase and adenosine homeotypic cysteine, generates the adenosine, the speed of the adenosine generation is in direct proportion to the homeotypic cysteine in the sample, through the generation speed, the aim to measure the content of the homeotypic cysteine in sample can be achieved.
Owner:北京安百胜诊断科技有限公司 +1
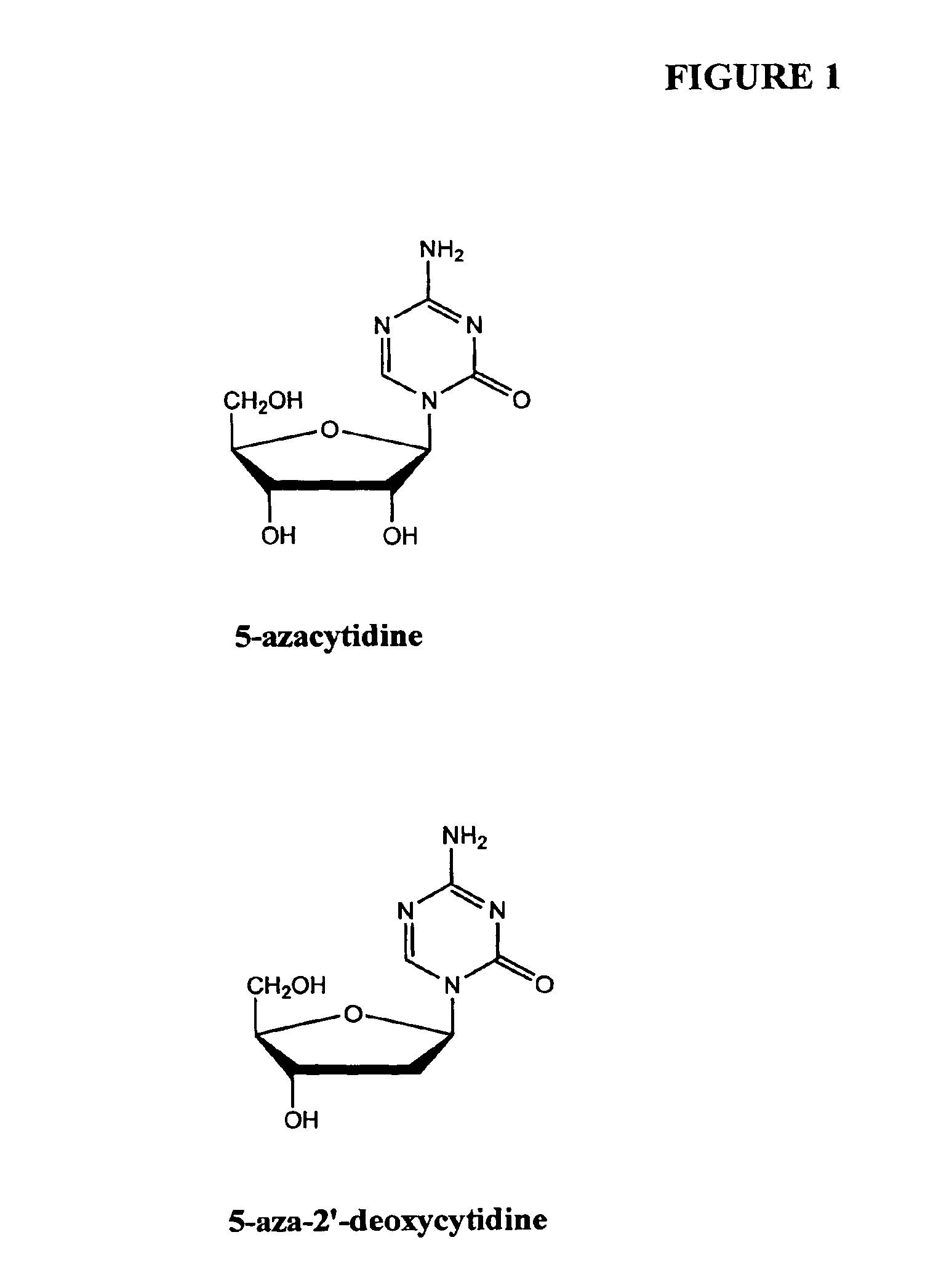
Compositions and methods for reestablishing gene transcription through inhibition of DNA methylation and histone deacetylase
InactiveUS6905669B2Better clinical outcomeReduce dosageBiocideCell receptors/surface-antigens/surface-determinantsCyclic peptideDisease
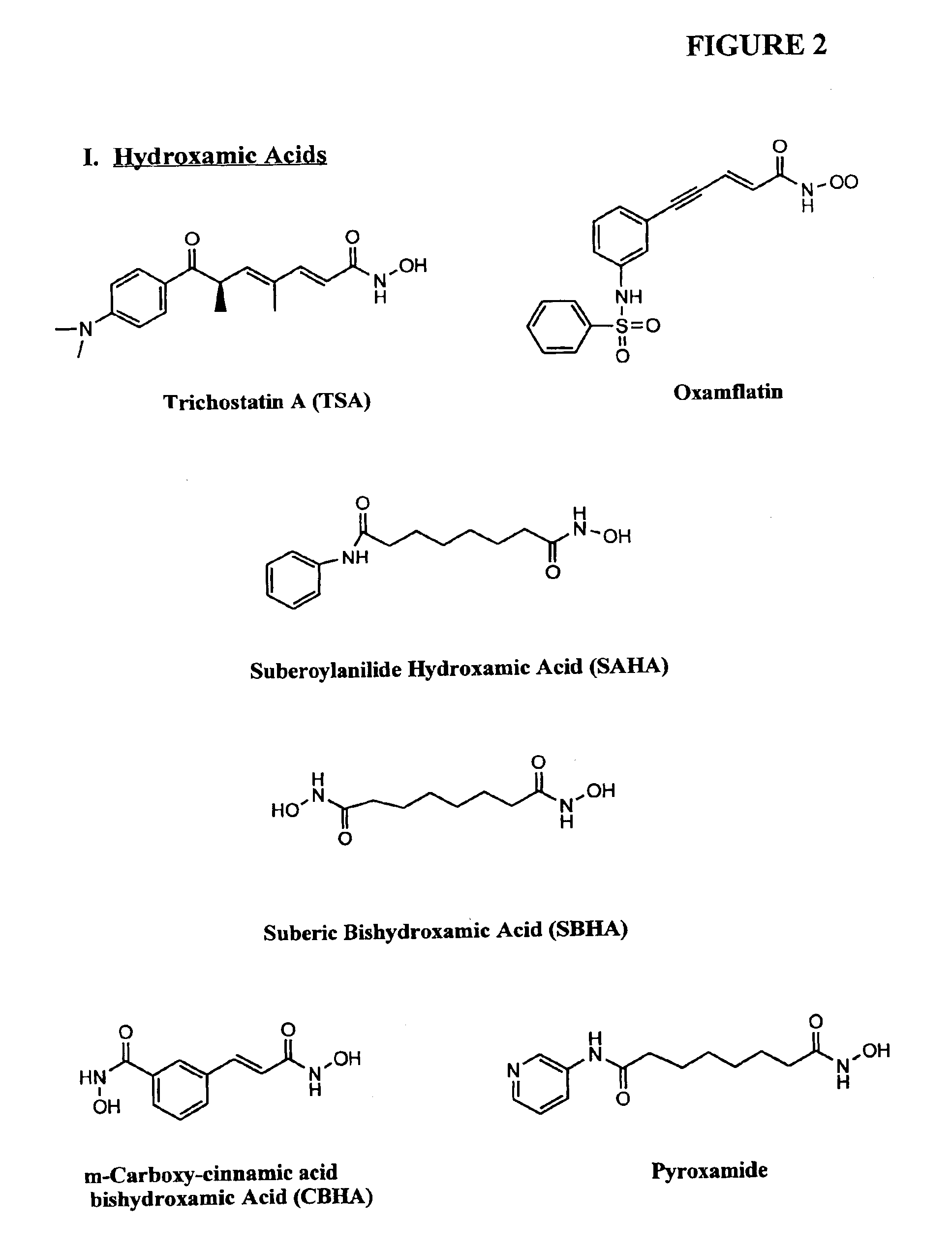
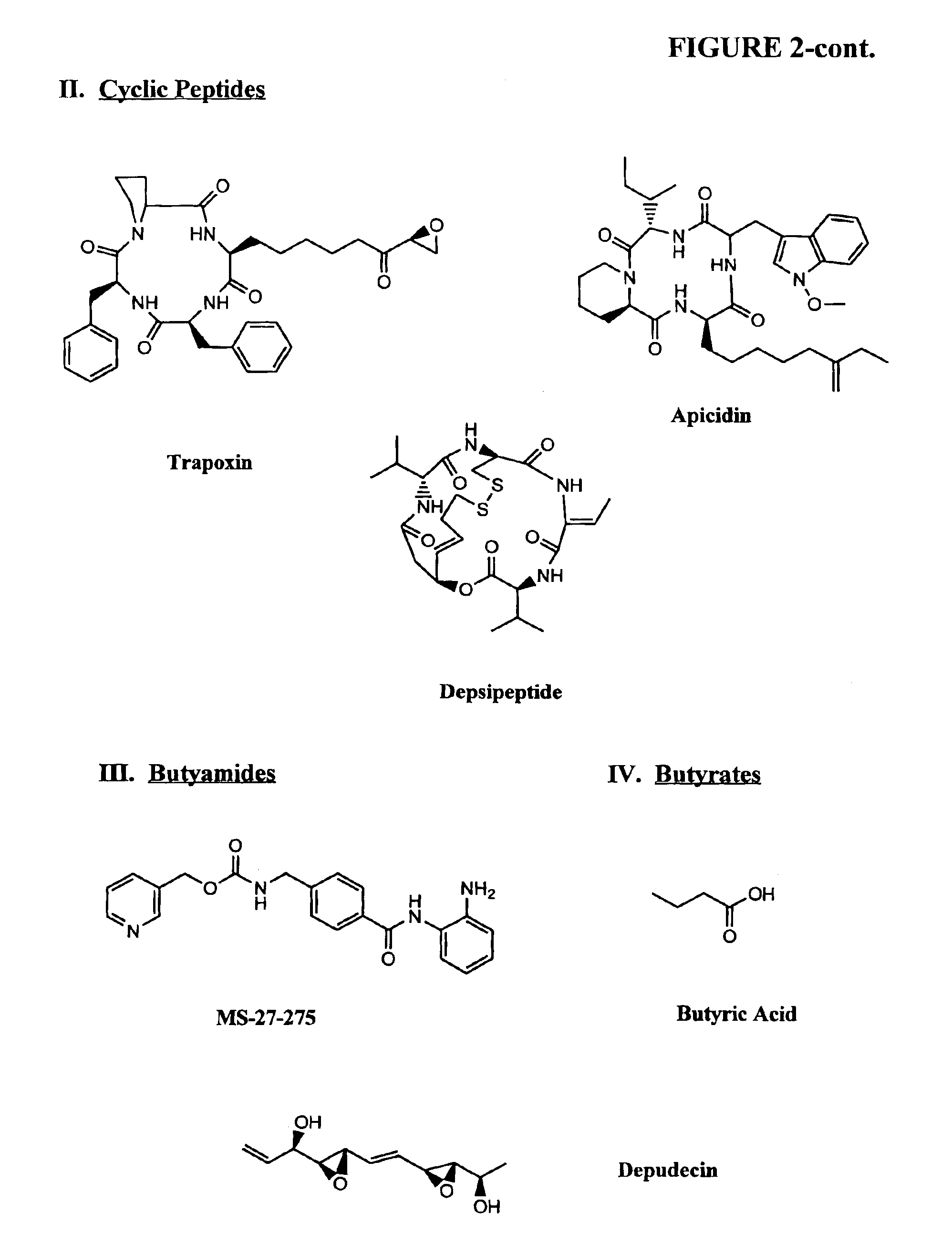
Compositions and methods are provided for treating diseases associated with aberrant silencing of gene expression such as cancer by reestablishing the gene expression through inhibition of DNA hypomethylation and histone deacetylase. The method comprises: administering to a patient suffering from the disease a therapeutically effective amount of a DNA methylation inhibitor such as a cysteine analog such as decitabine, in combination with an effective amount of histone deacetylase inhibitor such as hydroxamic acid, cyclic peptide, benzamide, butyrate, and depudecin.
Owner:SUPERGEN
Method of using hydroxycarboxylic acids or related compounds for treating skin changes associated with intrinsic and extrinsic aging
A composition comprising an amphoteric or pseudo-amphoteric agent and a polyhydroxy alpha hydroxyacid existing as a free acid, lactone, or salt, and isomeric or non-isomeric forms thereof is provided. The amphoteric or pseudo-amphoteric agent can be selected from amino acids, dipeptides, aminoaldonic acid, aminouronic acid, lauryl aminoproplyglycine, aminoaldaric acid, neuraminic acid desulfated heparin, deacetylated hyaluronic acid, hyalobiuronic acid, chondrosine, deacetylated chondroitin, creatine, creatinine, hydroxyproline, homocysteine, homocystine, homoserine, ornithine, citrulline, phosphatidylserine, and sphingomyelin. The composition may contain other additives, including cosmetic or pharmaceutical agents for topical treatment of dermatological disorders.
Owner:TRISTRATA TECH
Method and kit for investigating humotype semi-cystinol by enzyme biochemical reaction
InactiveCN1693879AMicrobiological testing/measurementColor/spectral properties measurementsChemical reactionFluorescence
The invention is an enzyme biologic and chemical reaction measure method to mensurate homotype cysteine in a biologic sample. Cysteine can lose its ammonia and be changed into alpha-4-ketone acid, ammonia and sulfureted hydrogen by L-ovi-ammonia acid and Y-dispeling enzyme. Sulfureted hydrogen and fluorescence cpd.DMPD2HCL can create blue product-sub-armour blue whose degree of absorbing light is 670nm, in the situation of Fe3+ and acid. Thus, people can mensurate the chroma of homotype cysteine in a biologic sample by knowing this degree. This method can measure the chroma of homotype cysteine(3-1000 uMs). The invention also involves a reagent box used for implementing the method mentioned above. The box uses liquid double reagents, and needs less quantity of samples(25 microlitre or serum or plasm);In addition, the respond time is short, and the operation is easy. Thus, this method is suitable for a great deal of examinations. The reagent box costs less than other types.
Owner:ZHEJIANG YAKE SCI & TECH +1
Homogeneous preparations of IL-28 and IL-29
ActiveUS7157559B2Improve expression levelIncrease productionPeptide/protein ingredientsAntipyreticMutated proteinPolynucleotide
Homogeneous preparations of IL-28A, IL-28B, and IL-29 have been produced by mutating one or more of the cysteine residues in the polynucleotide sequences encoding the mature proteins. The cysteine mutant proteins can be shown to either bind to their cognate receptor or exhibit biological activity. One type of biological activity that is shown is an antiviral activity.
Owner:ZYMOGENETICS INC
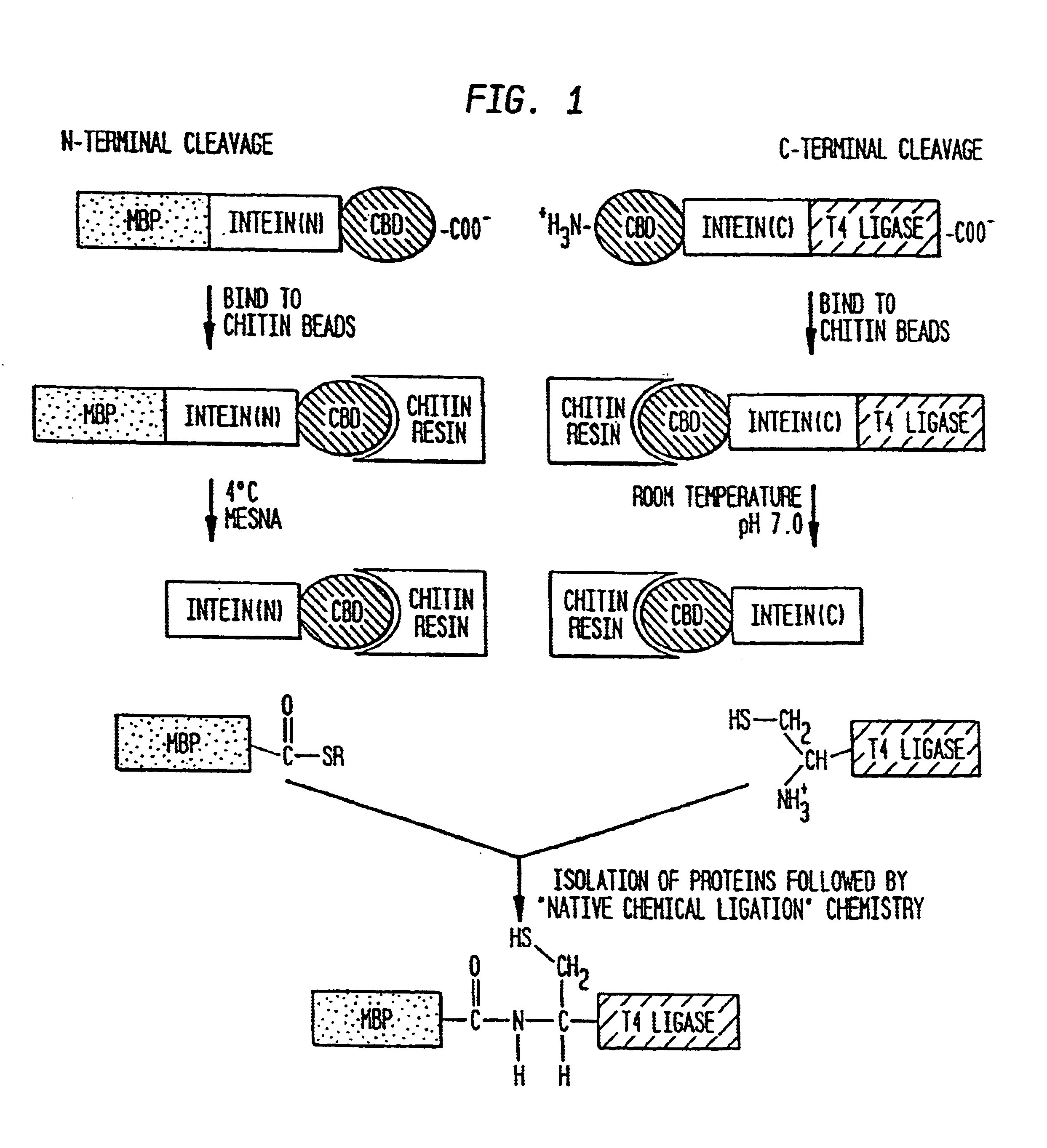
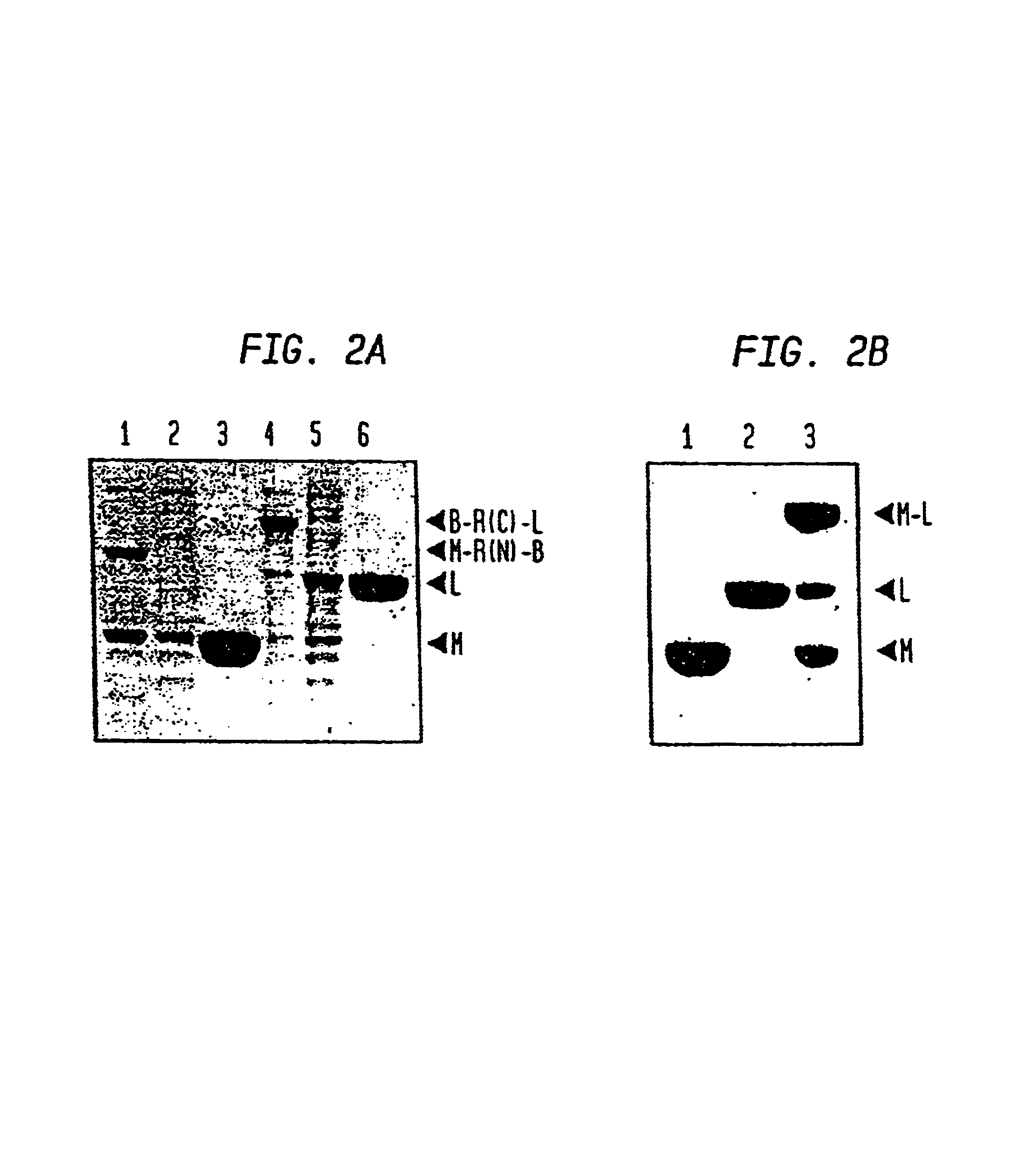
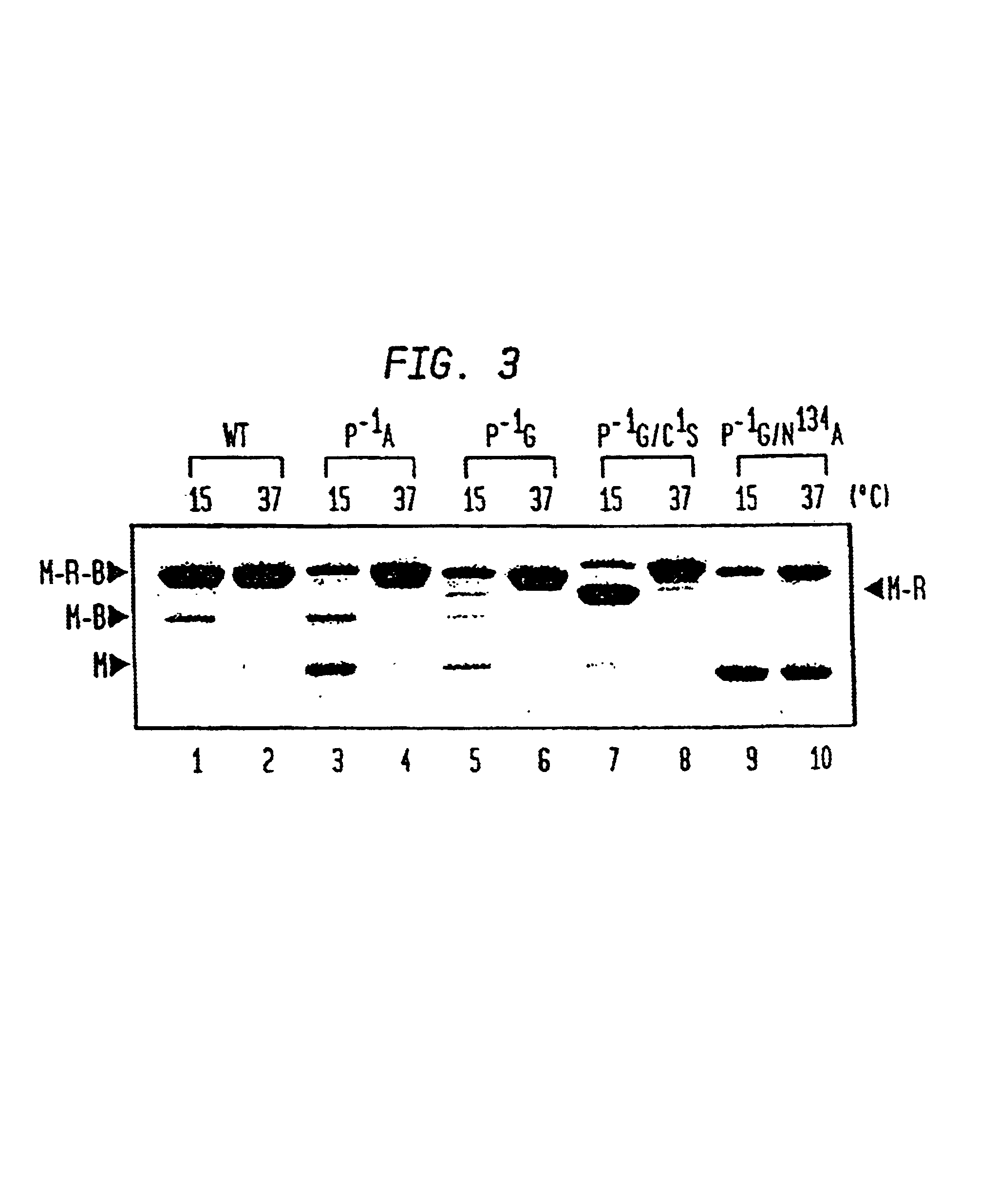
Intein-mediated protein ligation of expressed proteins
InactiveUS6849428B1Eliminate needBacteriaFusion with post-translational modification motifProtein targetIntein
A method for the ligation of expressed proteins which utilizes inteins, for example the RIR1 intein from Methanobacterium thermotrophicum, is provided. Constructs of the Mth RIR1 intein in which either the C-terminal asparagine or N-terminal cysteine of the intein are replaced with alanine enable the facile isolation of a protein with a specified N-terminal, for example, cysteine for use in the fusion of two or more expressed proteins. The method involves the steps of generating a C-terminal thioester-tagged target protein and a second target protein having a specified N-terminal via inteins, such as the modified Mth RIR1 intein, and ligating these proteins. A similar method for producing a cyclic or polymerized protein is provided. Modified inteins engineered to cleave at their C-terminus or N-terminus, respectively, and DNA and plasmids encoding these modified inteins are also provided.
Owner:NEW ENGLAND BIOLABS
Thiol selective water soluble polymer derivatives
ActiveUS20050014903A1Improve responseThiol preparationPharmaceutical non-active ingredientsCouplingWater soluble
Owner:NEKTAR THERAPEUTICS INC
L-cysteine producing microorganism and method for producing L-cysteine
InactiveUS20050221453A1High expressionBacteriaRecombinant DNA-technologyMicroorganismCysteine thiolate
L-Cysteine is produced by culturing a microorganism having an ability to produce L-cysteine and modified so that expression of emrAB, emrKY, yojIH, acrEF, bcr, or cusA gene should be enhanced in a medium to produce and accumulate L-cysteine in the medium and collecting the L-cysteine from the medium. Genes coding for novel L-cysteine-excreting proteins are identified, and utilized for breeding of L-cysteine-producing microorganism to provide a novel method of producing L-cysteine.
Owner:AJINOMOTO CO INC
Methods and compositions for kinase inhibition
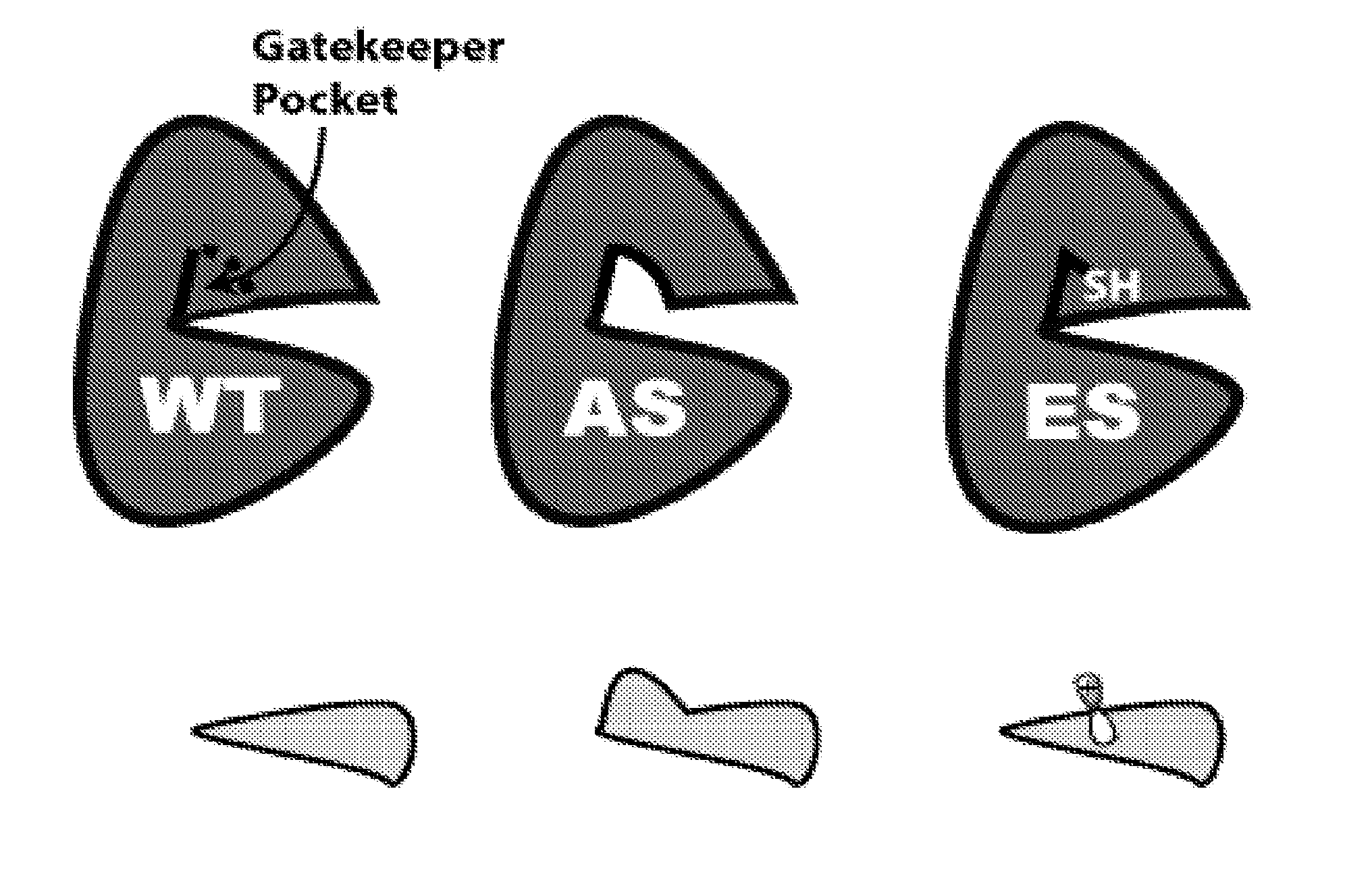
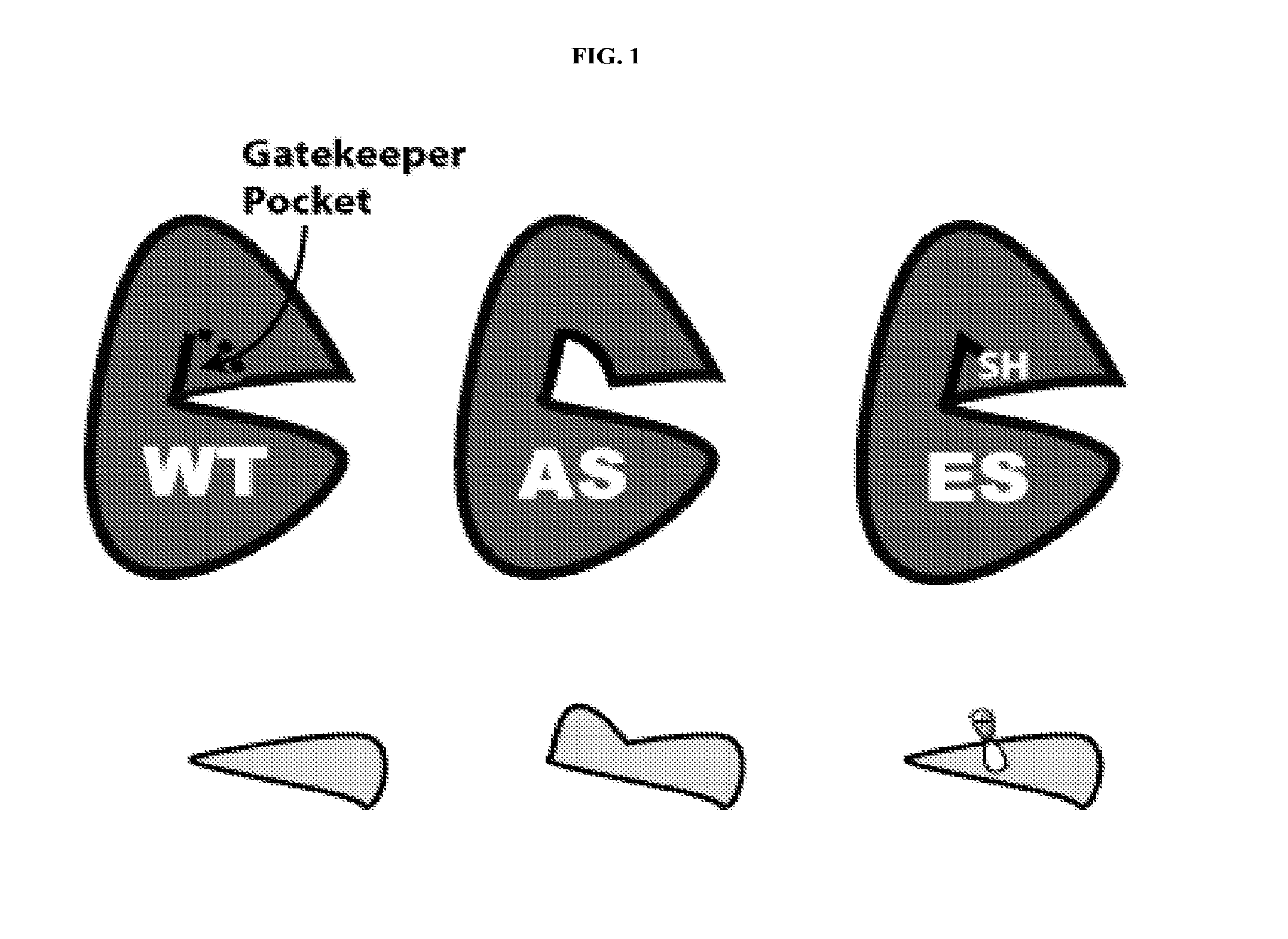
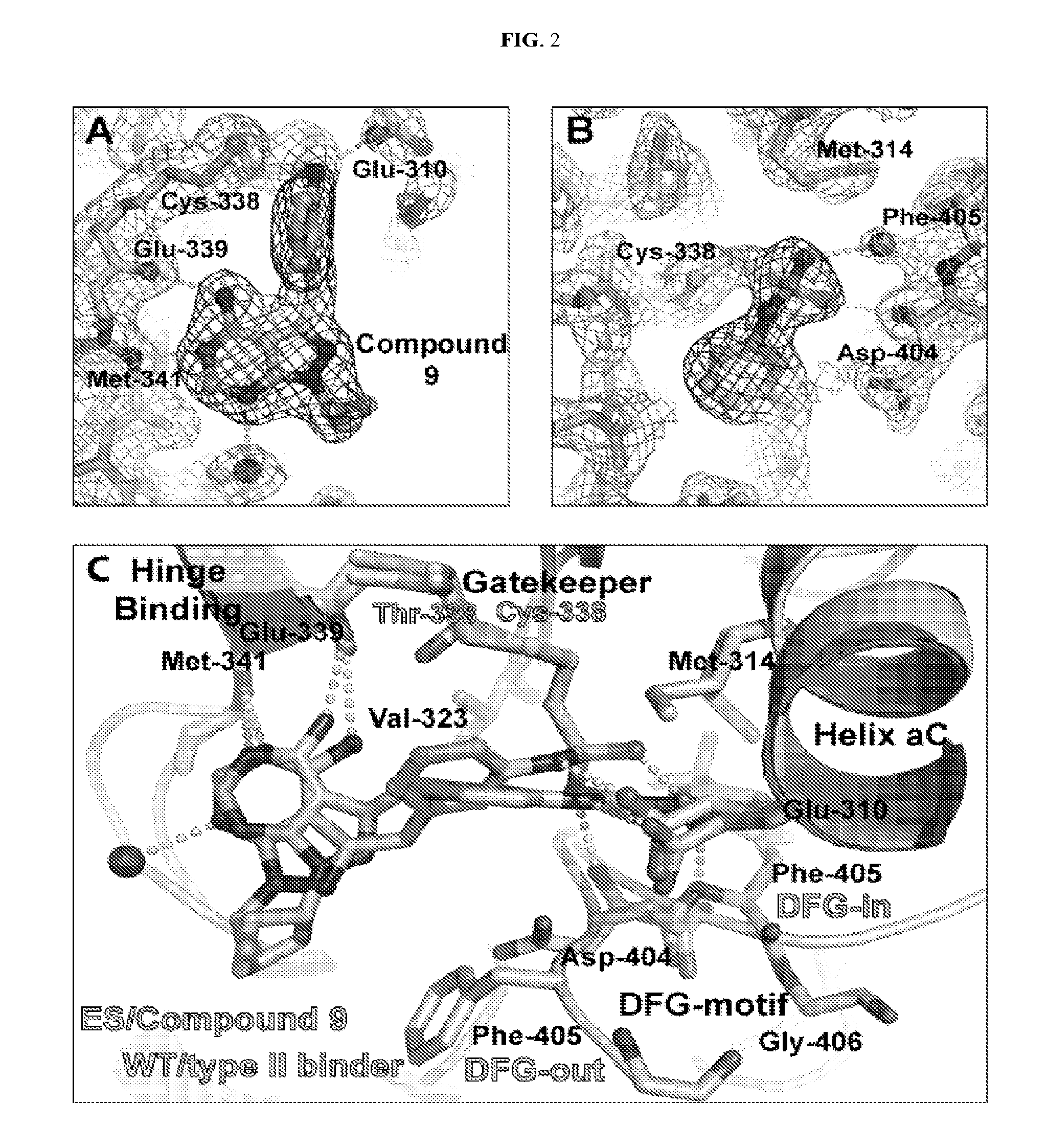
The present invention sets forth a new chemical genetic approach for engineering kinase enzymes with a cysteine gatekeeper residue as well as for developing electrophilic inhibitors thereto. The present invention also provides a Src proto-oncogenic tyrosine kinase with a cysteine gatekeeper that recapitulates wild type activity and can be irreversibly inhibited both in vitro and in cells. The present invention also provides methods and compositions for modulating kinases and for treating kinase-associated diseases.
Owner:RGT UNIV OF CALIFORNIA
Multi-vitamin and mineral nutritional supplements
InactiveUS20050214383A1Adequate intakeLoss of protectionHeavy metal active ingredientsBiocidePlant sterolLow density lipoprotein cholesterol
The invention provides a nutritional supplement which includes micronutrients to facilitate reduction of cholesterol, and / or reduction of homocystein and / or reduction of low-density lipoprotein-cholesterol (LDL-C) oxidation in humans. In one embodiment the supplement is a multi-vitamin, a mineral supplement which includes at least one component known to reduce cholesterol. The invention further provides a method for tableting one fourth to one half of the daily effective dosage of a phytosterol containing nutritional supplement in a practical sized tablet and a method for reducing blood cholesterol in humans.
Owner:WYETH LLC
Use of phosphoketolase for producing useful metabolites
Owner:AJINOMOTO CO INC
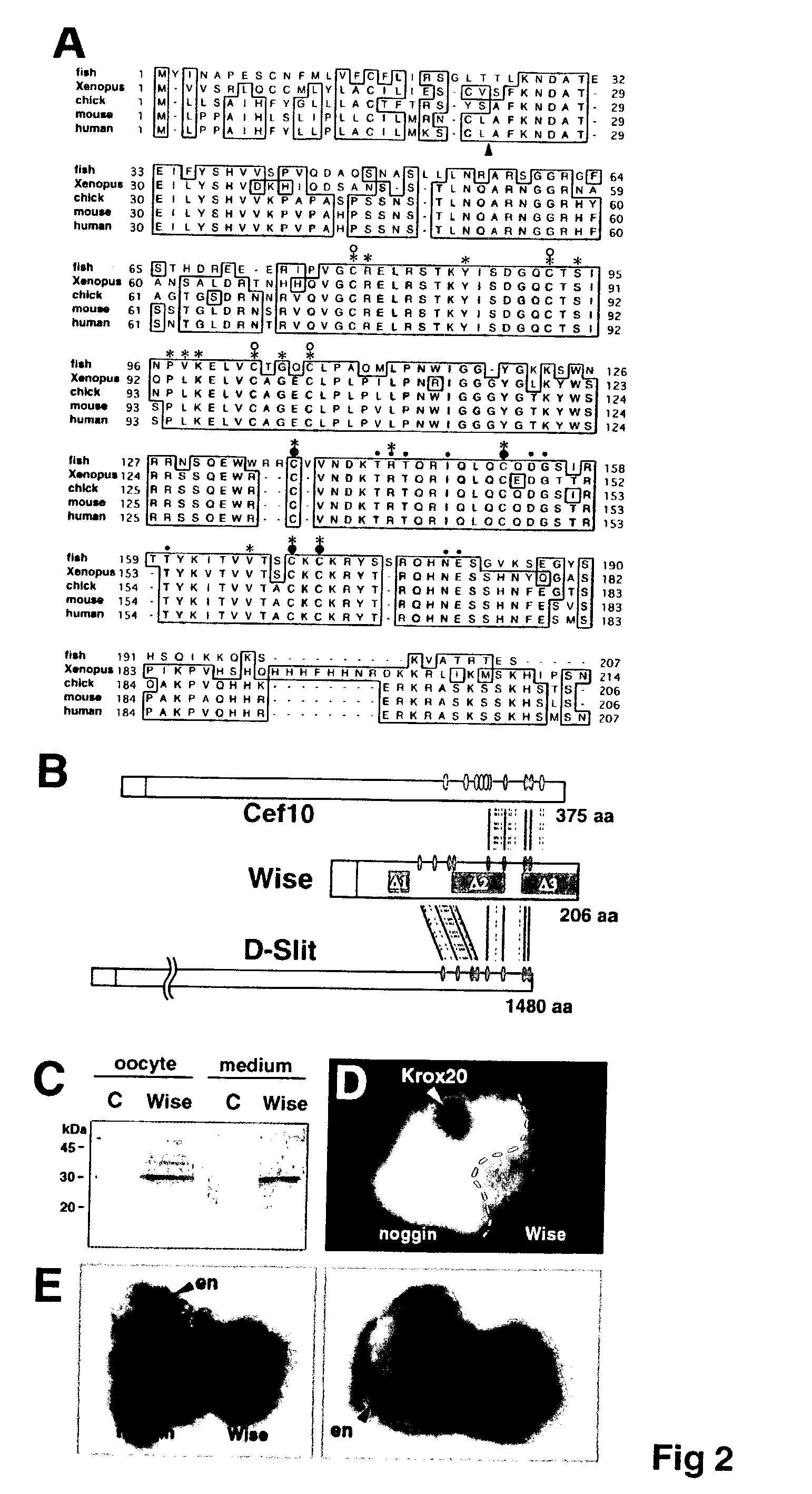
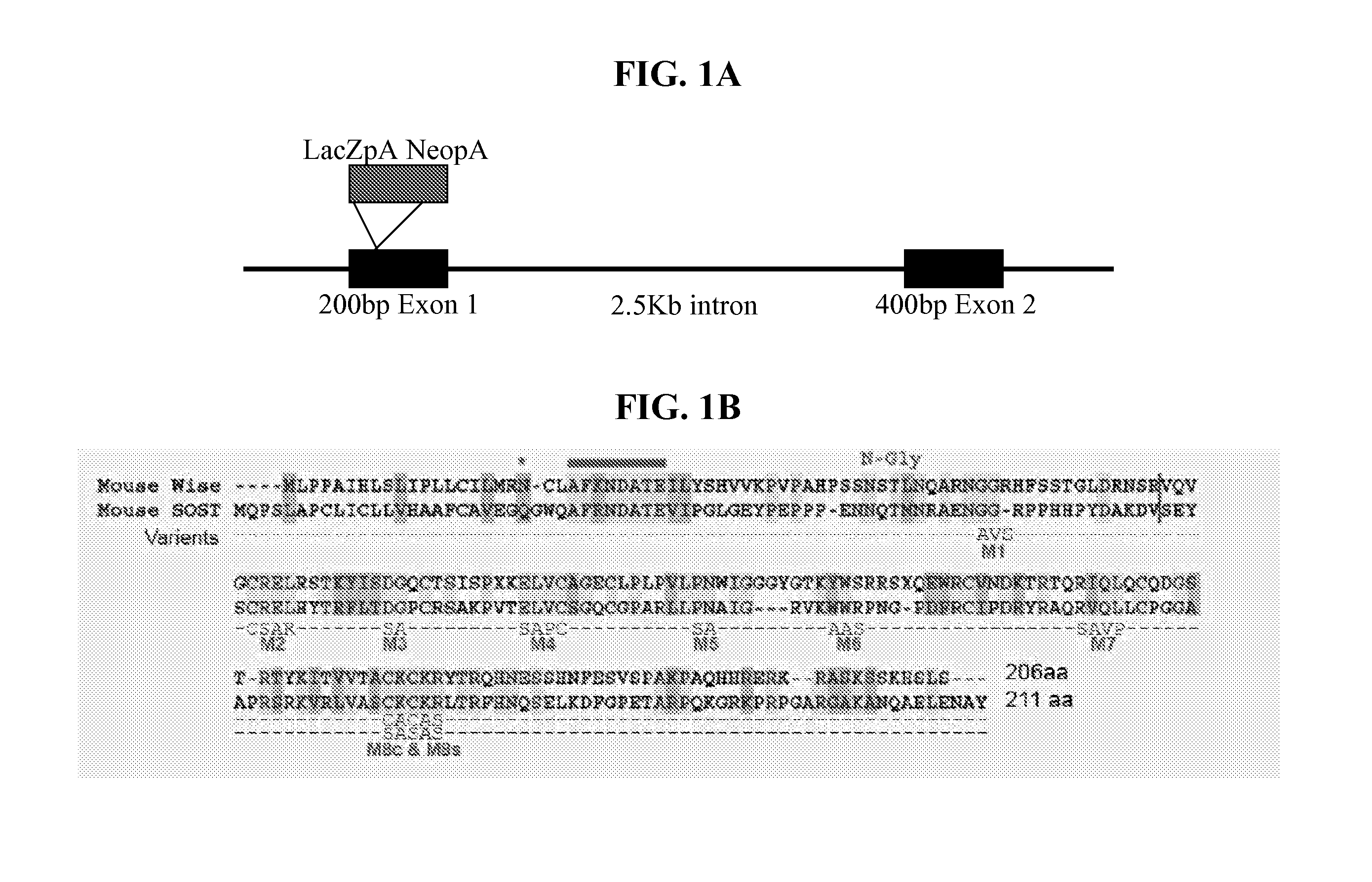
Wise/Sost nucleic acid sequences and amino acid sequences
InactiveUS20040023356A1Increased bone depositionIncrease depositionHydrolasesPeptide/protein ingredientsNucleic acid sequencingFab Fragments
The present invention relates to nucleic acid sequences and amino acid sequences which influence bone deposition, the Wnt pathway, ocular development, tooth development, and may bind to LRP. The nucleic acid sequence and polypeptides include Wise and Sost as well as a family of molecules which express a cysteine knot polypeptide. Additionally, the present invention relates to various molecular tools derived from the nucleic acids and polypeptides including vectors, transfected host cells, monochronal antibodies, Fab fragments, and methods for impacting the pathways.
Owner:STOWERS INST FOR MEDICAL RES
Cysteine Engineered Antibodies For Site-Specific Conjugation
Cysteine engineered antibodies useful for the site-specific conjugation to a variety of agents are provided. Methods for the design, preparation, screening, selection and use of such antibodies are also provided.
Owner:MEDIMMUNE LLC
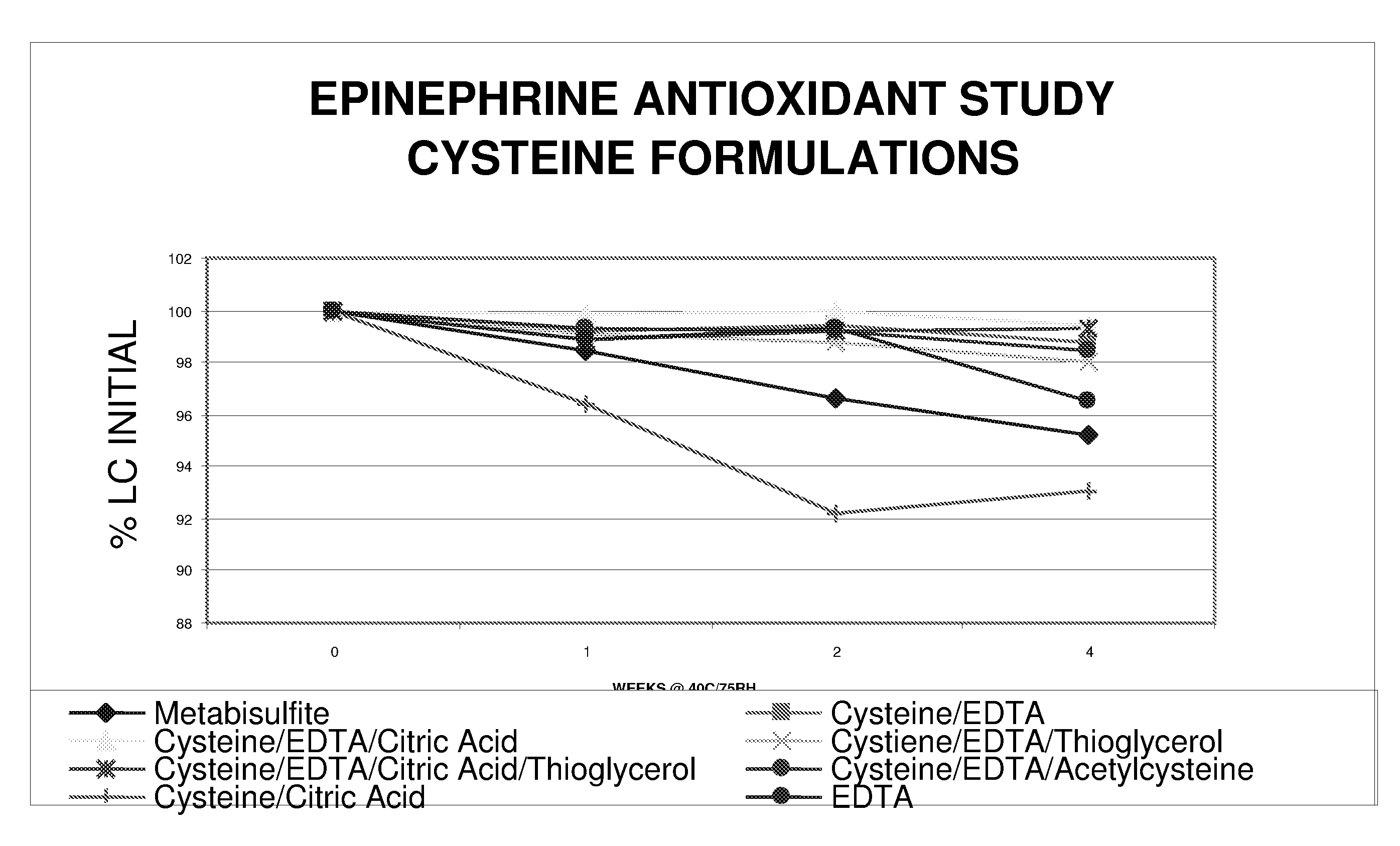
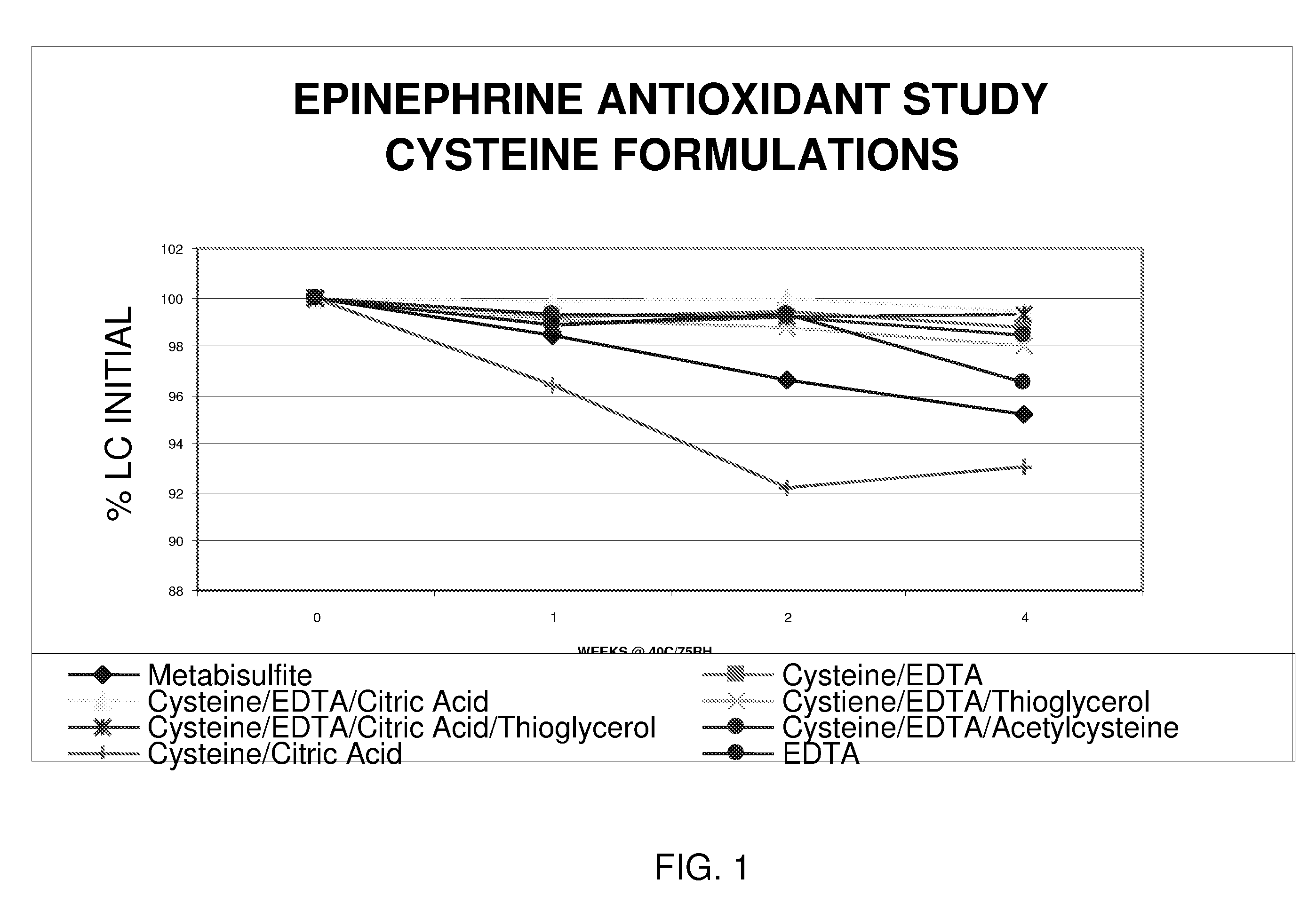
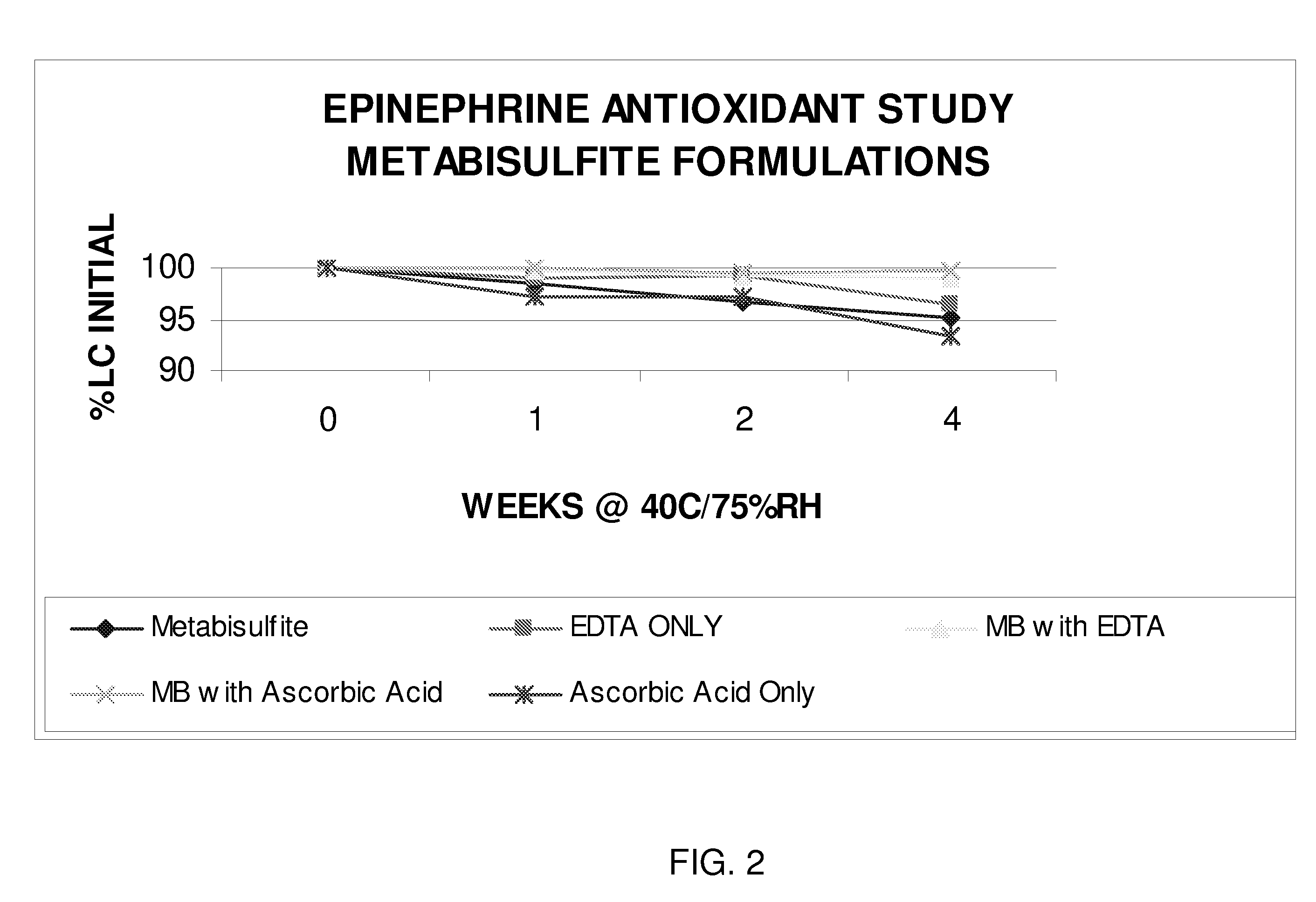
Epinephrine formulations
InactiveUS20080269347A1Improve stabilityBiocideOrganic active ingredientsCardiorespiratory arrestAntioxidant
The present invention generally concerns an epinephrine formulation that has enhanced stability. In particular embodiments, the formulation is an injectable formulation. In specific aspects, the formulation comprises epinephrine, EDTA, and one or more of an antioxidant such as cysteine, citric acid, acetylcysteine, or thioglycerol. The formulations are suitable for any medical condition that is in need of epinephrine, although in specific embodiments the medical condition is anaphylaxis, asthma, or cardiac arrest.
Owner:UNION SPRINGS PHARMA
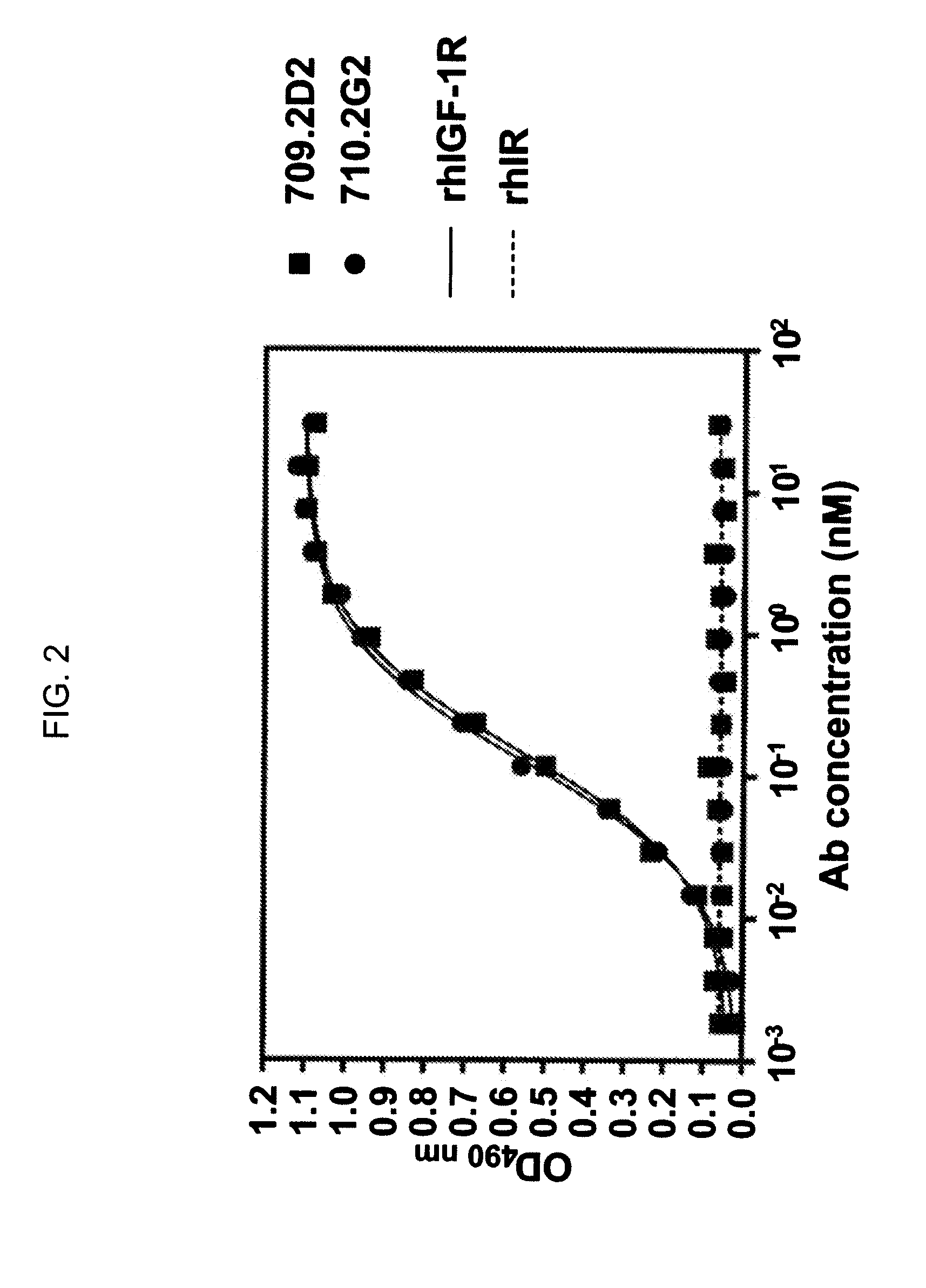
Novel Class of Monospecific and Bispecific Humanized Antibodies that Target the Insulin-like Growth Factor Type I Receptor (IGF-1R)
InactiveUS20100226884A1Organic active ingredientsPeptide/protein ingredientsDiseaseAntiendomysial antibodies
The present invention provides compositions and methods of use of anti-IGF-1R antibodies or antibody fragments. Preferably the antibodies bind to IGF-1R but not IR; are not agonists for IGF-1R; do not block binding of IGF-1 or IGF-2 to isolated IGF-1R, but effectively neutralize activation of IGF-1R by IGF-1 in intact cells; and block binding of an R1 antibody to IGF-1R. The antibodies may be murine, chimeric, humanized or human R1 antibodies comprising the heavy chain CDR sequences DYYMY (SEQ ID NO:1), YITNYGGSTYYPDTVKG (SEQ ID NO:2) and QSNYDYDGWFAY (SEQ ID NO:3) and the light chain CDR sequences KASQEVGTAVA (SEQ ID NO:4), WASTRHT (SEQ ID NO:5) and QQYSNYPLT (SEQ ID NO:6). Preferably the antibodies bind to an epitope of IGF-1R comprising the first half of the cysteine-rich domain of IGF-1R (residues 151-222). The anti-IGF-1R antibodies may be used for diagnosis or therapy of various diseases such as cancer.
Owner:IMMUNOMEDICS INC
Composition and method for reducing the risk or progression of cardiovascular, glaucoma, tardive dyskinesia and other diseases
InactiveUS20040087479A1Reduce riskShorten the progressBiocideOrganic active ingredientsBeta-CaroteneAdditive ingredient
Elevated levels of homocysteine have been implicated as an important risk factor for cardiovascular and other diseases. A composition for decreasing levels of plasma homocysteine and a method for administering the composition are provided, the composition containing dextromethorphan (DM), folic acid and vitamins B6 and B12. The composition provides a synergistic therapeutic effect so that lower amounts of the above ingredients may be employed to minimize any undesirable side effects caused by the use of high levels of a component such as DM. Preferred compositions for cardiovascular diseases further include lecithin, vitamin E, betacarotene, procyanidins / flavonoids, trimethylglycine, garlic oil and minerals. Other compositions for treating glaucoma include bilberry, bioflavonoids and beta-carotene and for treating tardive dyskinesia include an antioxidant such a grape seed extract and pine bark extract, lecithin and oligomeric proanthocyanidins. The compositions may be administered using any suitable means such as orally or intravenous.
Owner:SOSNOWSKI ROBERT E +1
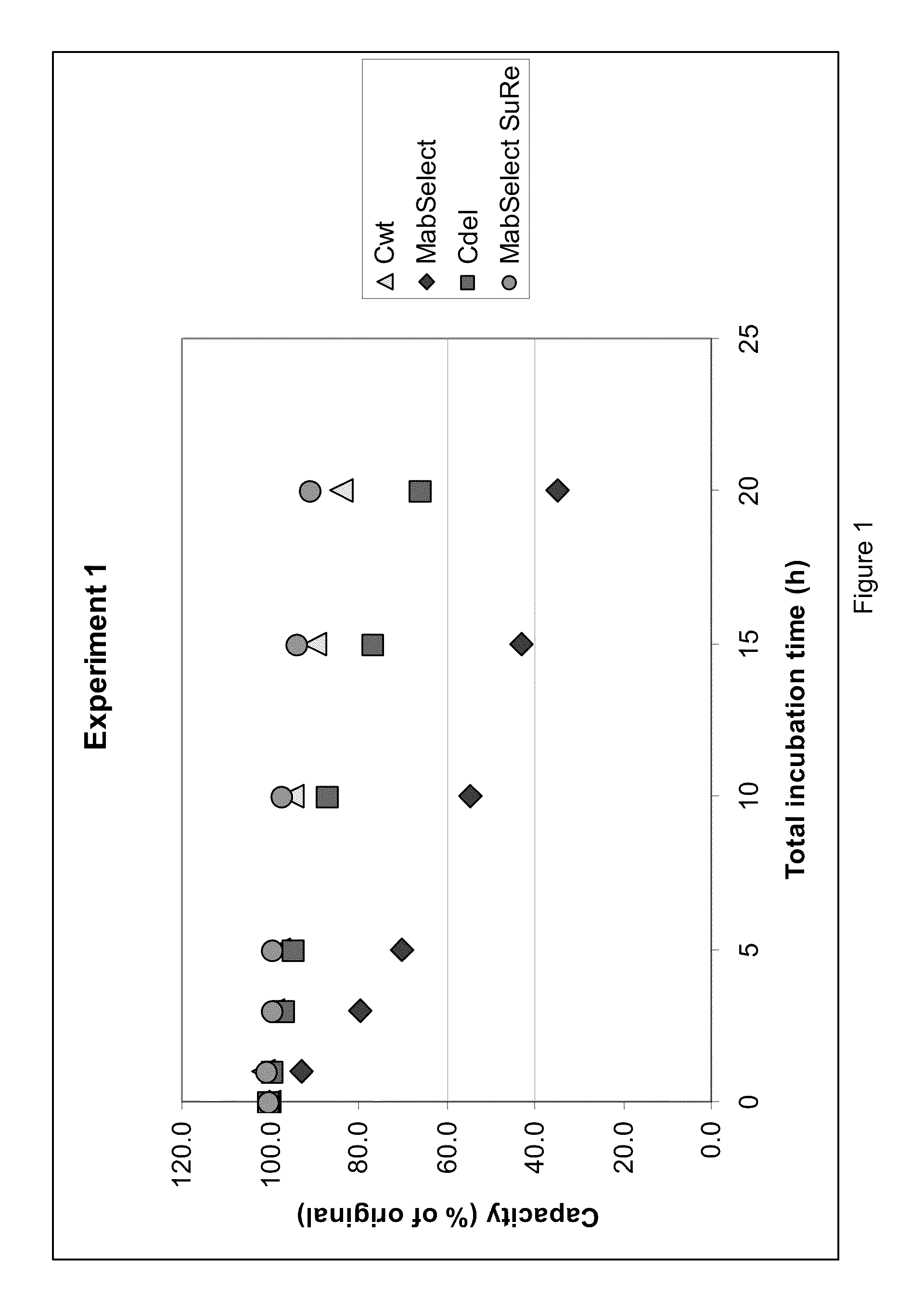
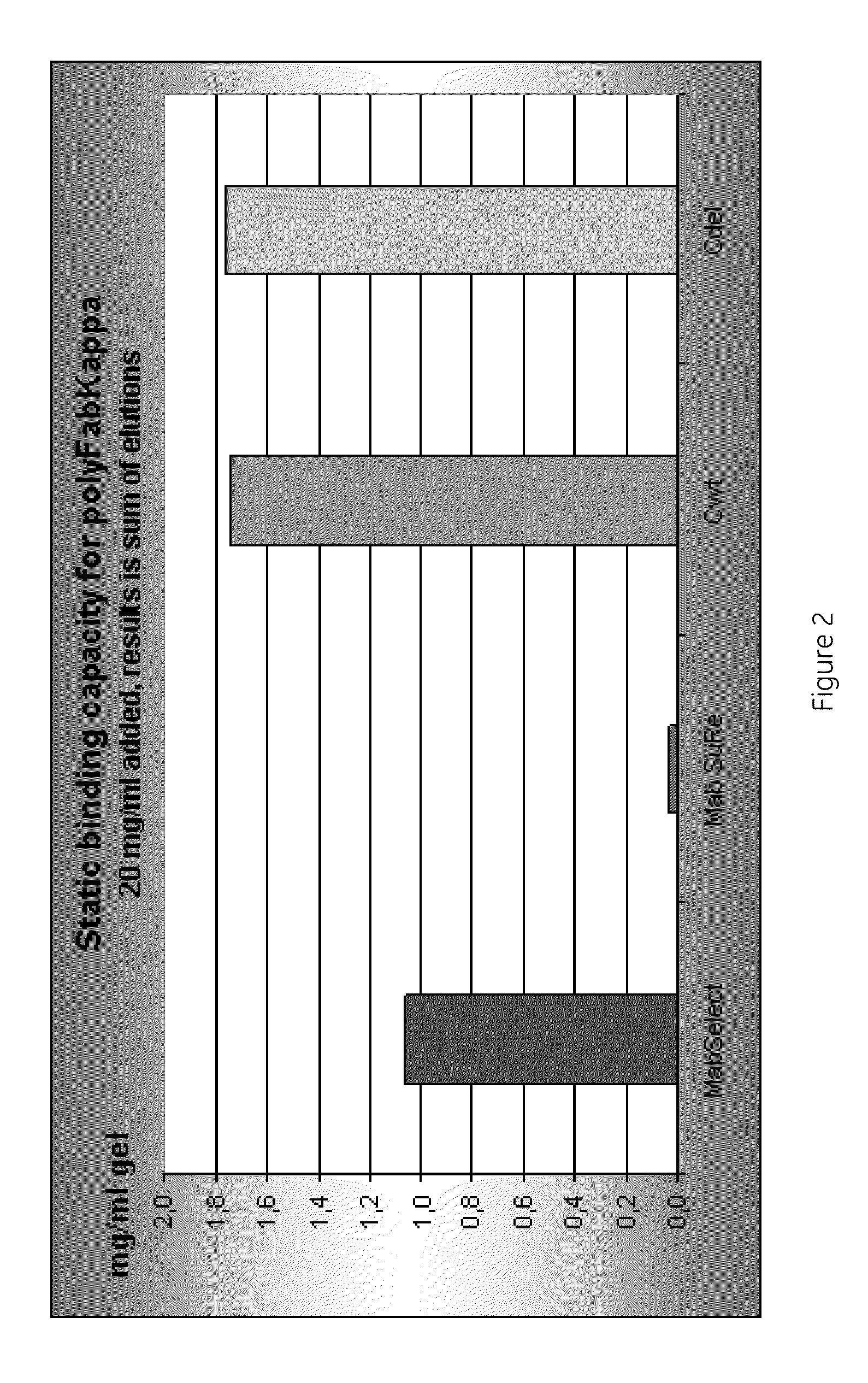
Chromatography ligand comprising domain C from Staphylococcus aureus protein A for antibody isolation
ActiveUS8329860B2Process economyPeptide/protein ingredientsSolid sorbent liquid separationArginineCoupling
The present invention relates to a chromatography ligand, which comprises Domain C from Staphylococcus protein A (SpA), or a functional fragment or variant thereof. The chromatography ligand presents an advantageous capability of withstanding harsh cleaning in place (CIP) conditions, and is capable of binding Fab fragments of antibodies. The ligand may be provided with a terminal coupling group, such as arginine or cysteine, to facilitate its coupling to an insoluble carrier such as beads or a membrane. The invention also relates to a process of using the ligand in isolation of antibodies, and to a purification protocol which may include washing steps and / or regeneration with alkali.
Owner:CYTIVA BIOPROCESS R&D AB
Peptides for treatment and diagnosis of bone diseases
ActiveUS20070292444A1Peptide/protein ingredientsGenetic material ingredientsCystine knotCysteine thiolate
The present invention is directed to isolated polypeptides and antibodies suitable for producing therapeutic preparations, methods, and kits relating to bone deposition. One objective of the present invention is to provide compositions that improve bone deposition. Yet another objective of the present invention is to provide methods and compositions to be utilized in diagnosing bone dysregulation. The therapeutic compositions and methods of the present invention are related to the regulation of Wise, Sost, and closely related sequences. In particular, the nucleic acid sequences and polypeptides include Wise and Sost as well as a family of molecules that express a cysteine knot polypeptide.
Owner:STOWERS INST FOR MEDICAL RES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com