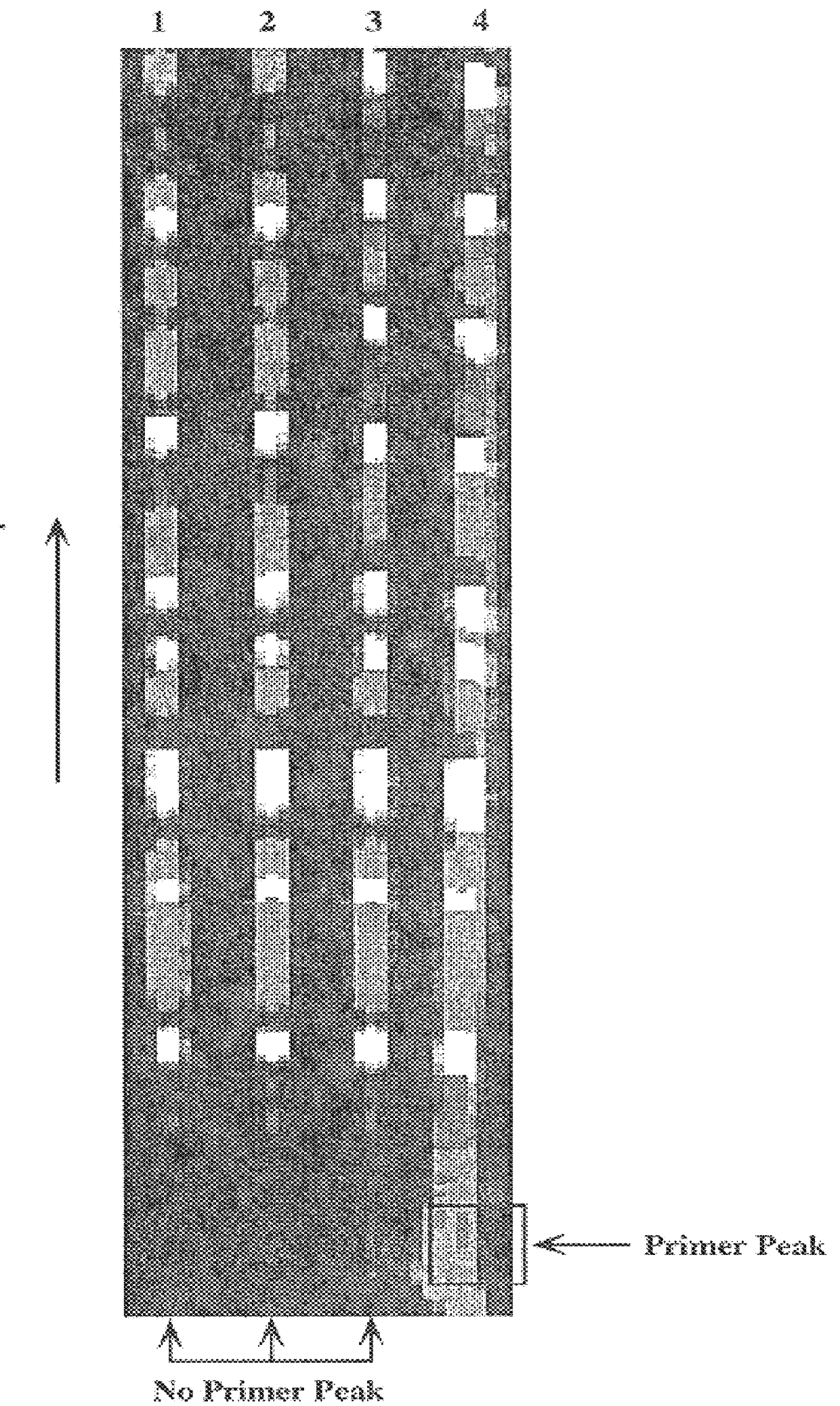
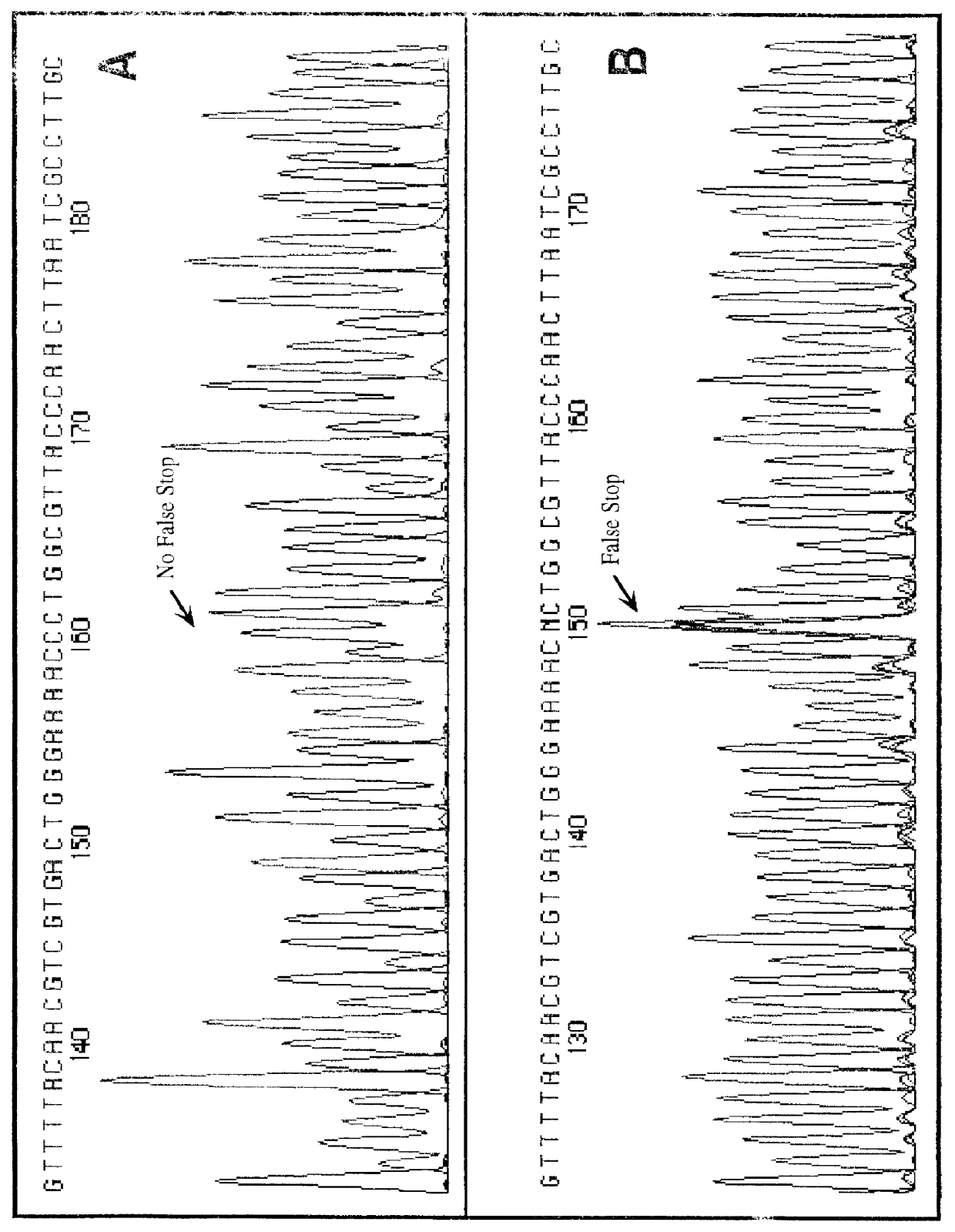
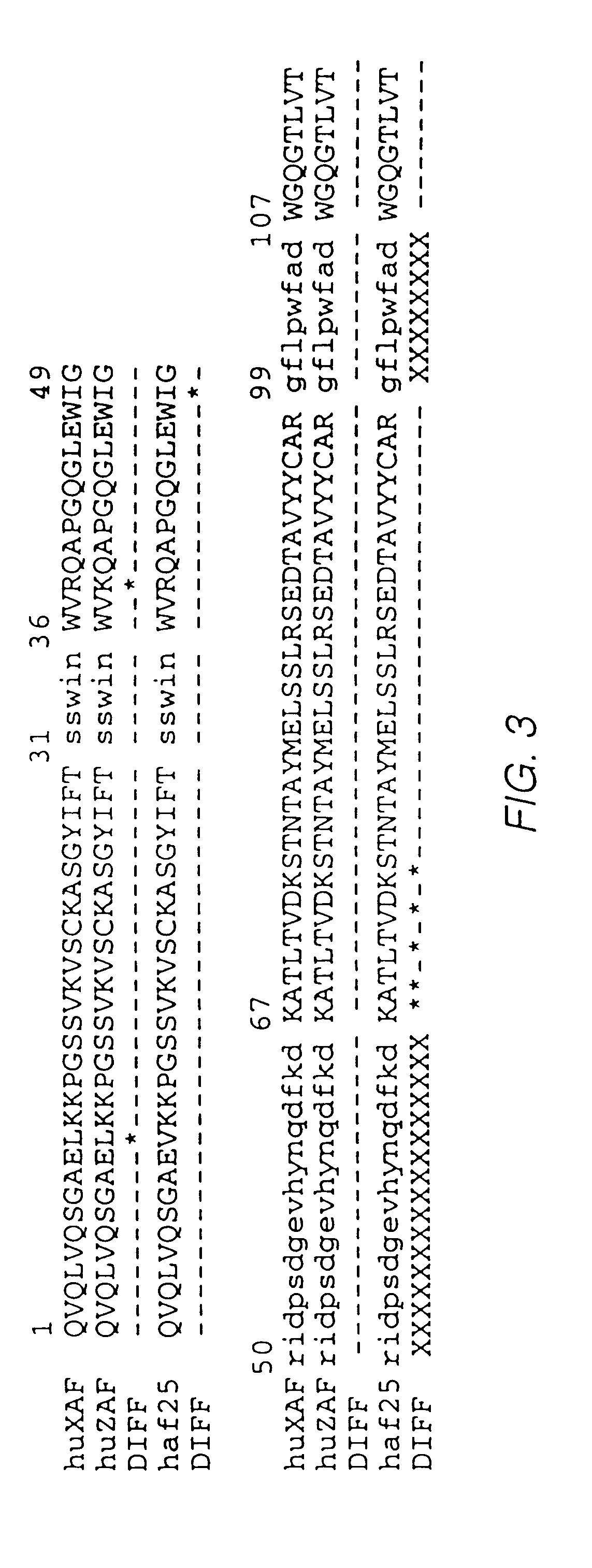
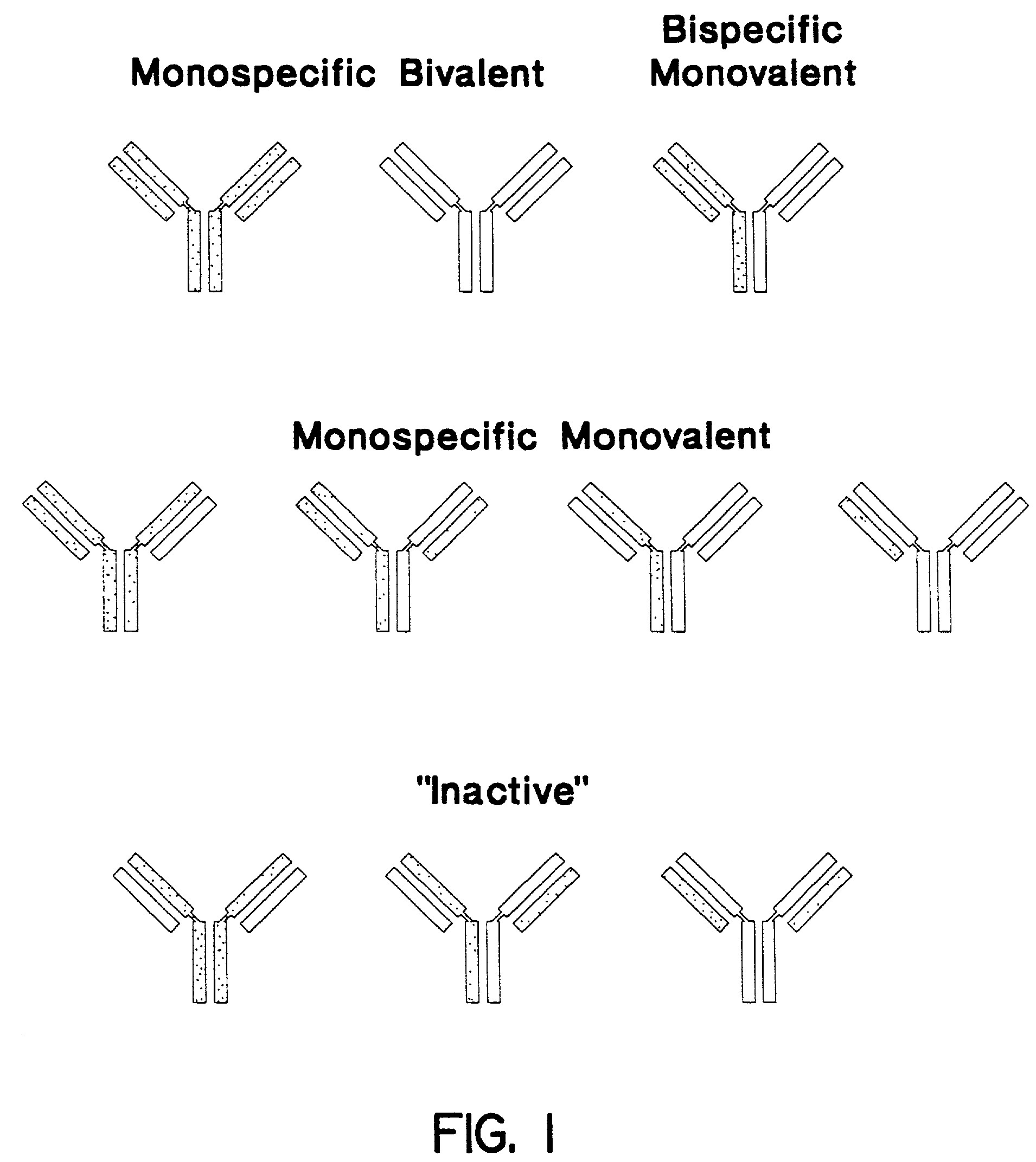
Patents
Literature
8219results about "Recombinant DNA-technology" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
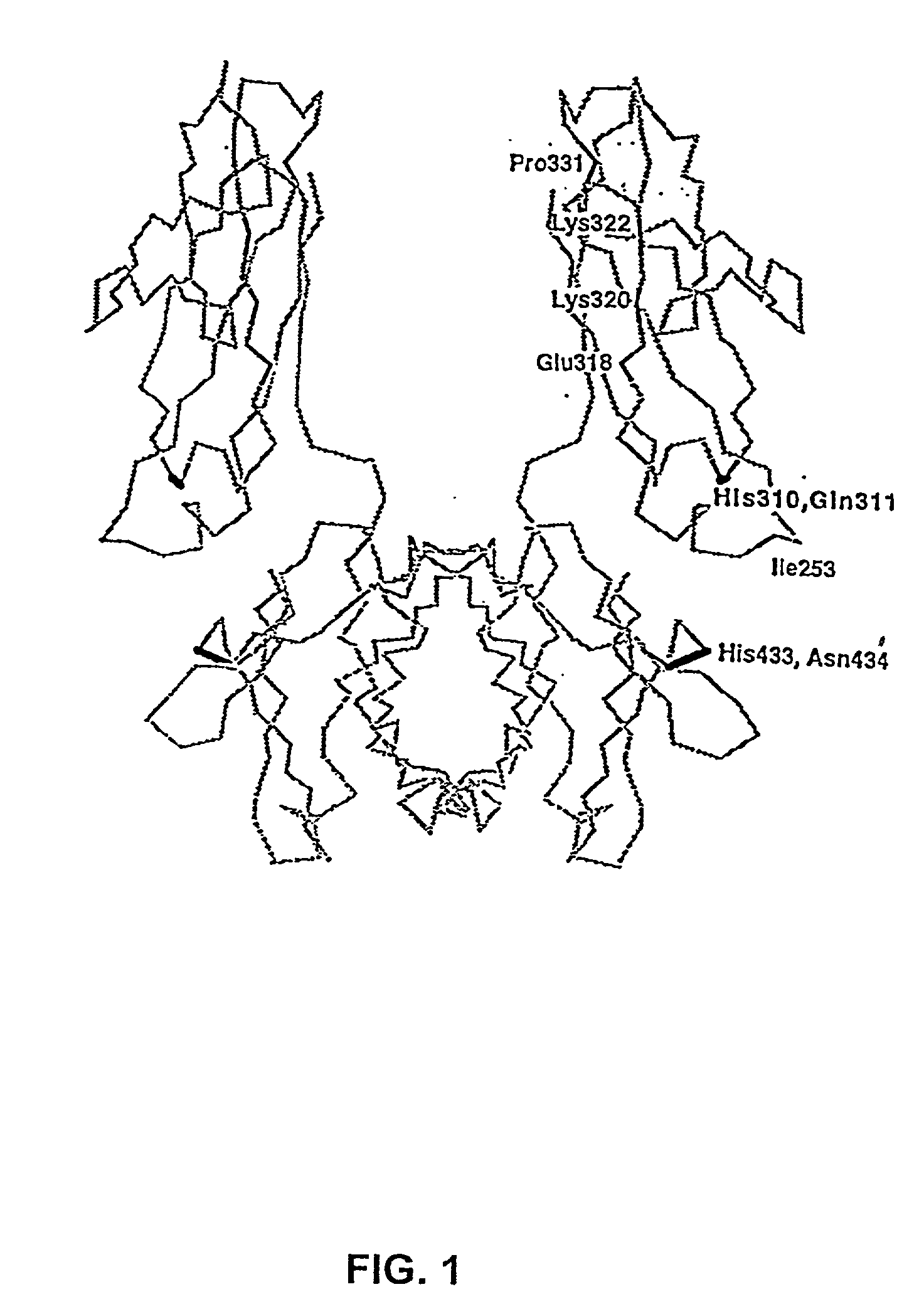
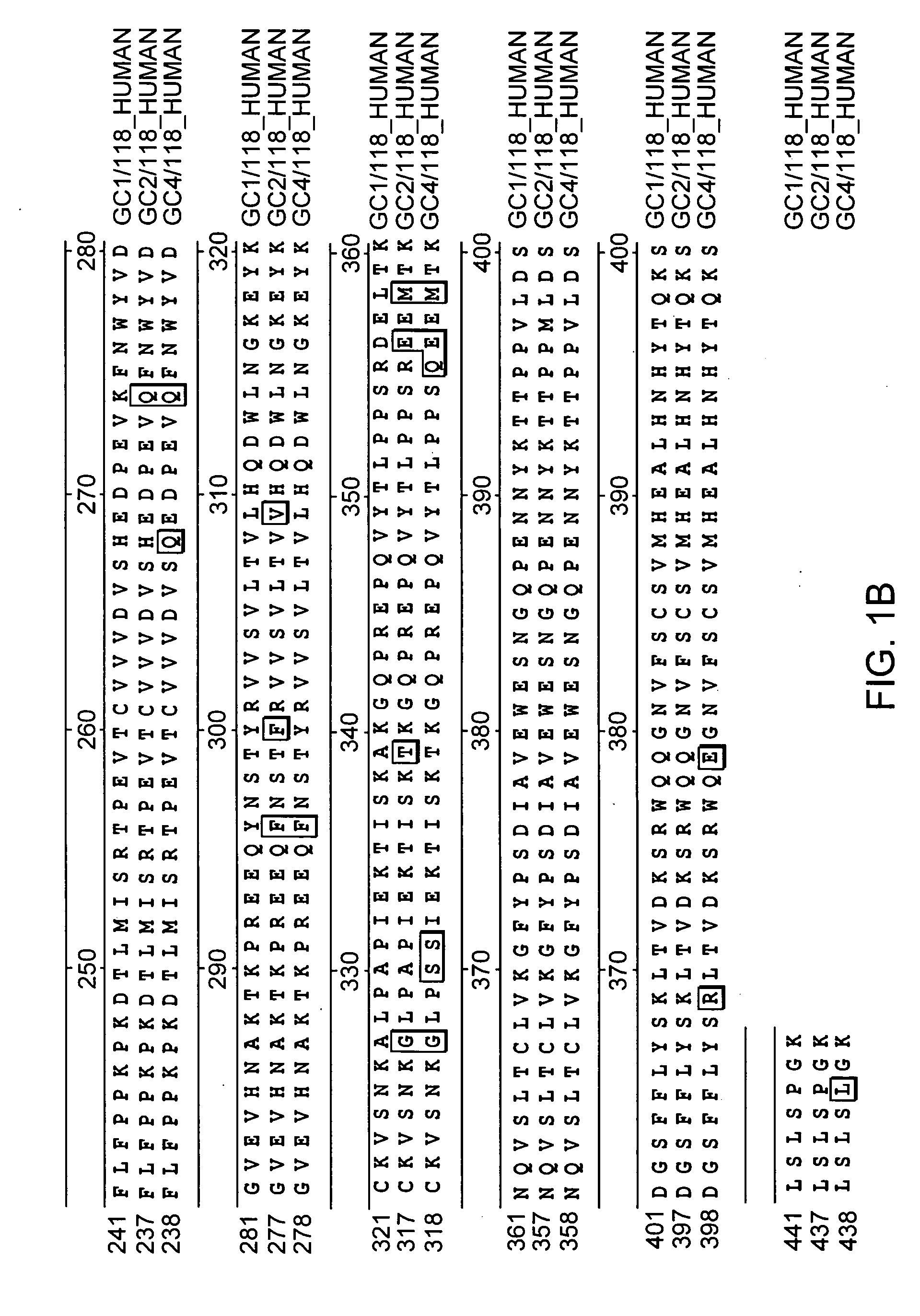
Polypeptide variants with altered effector function
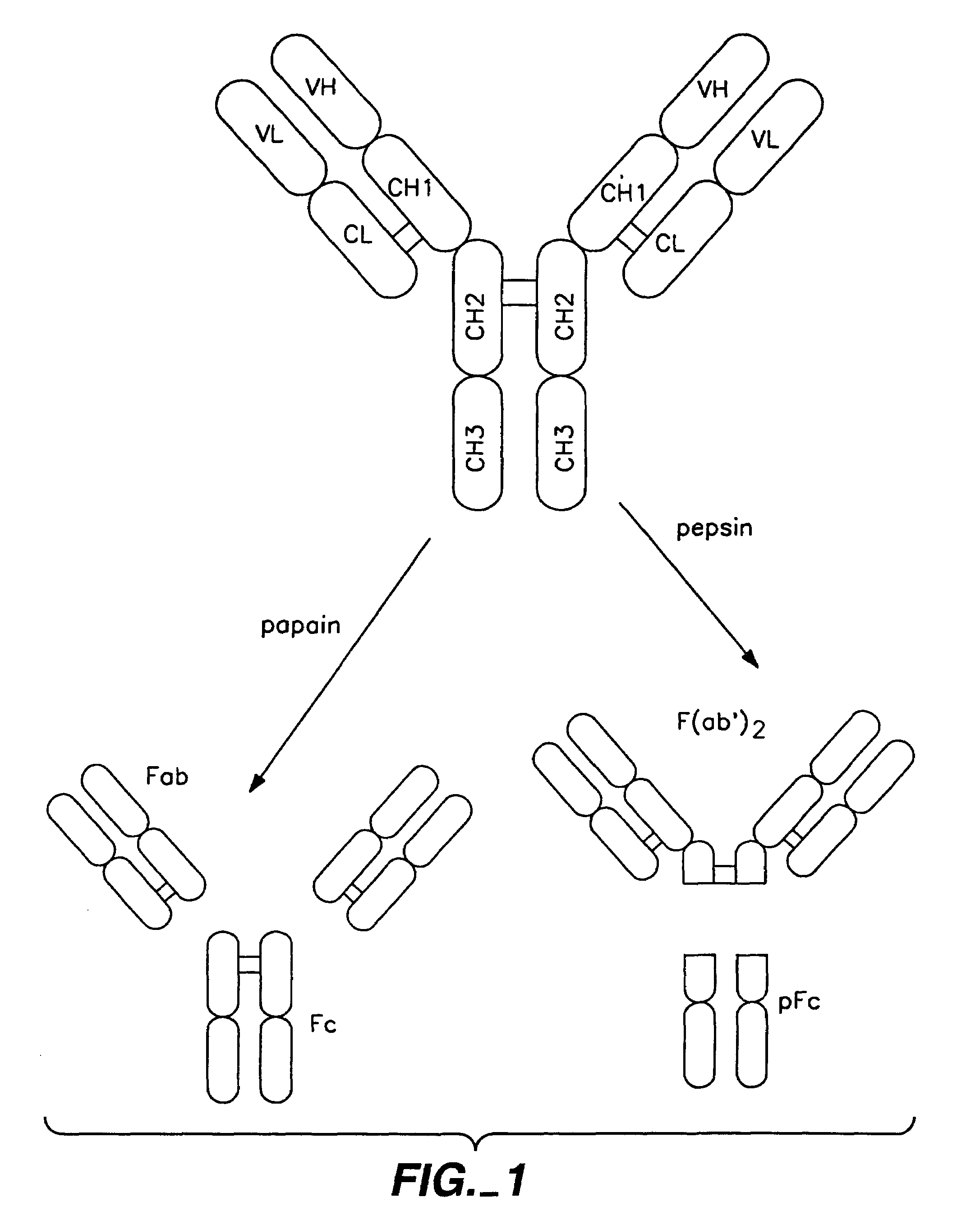
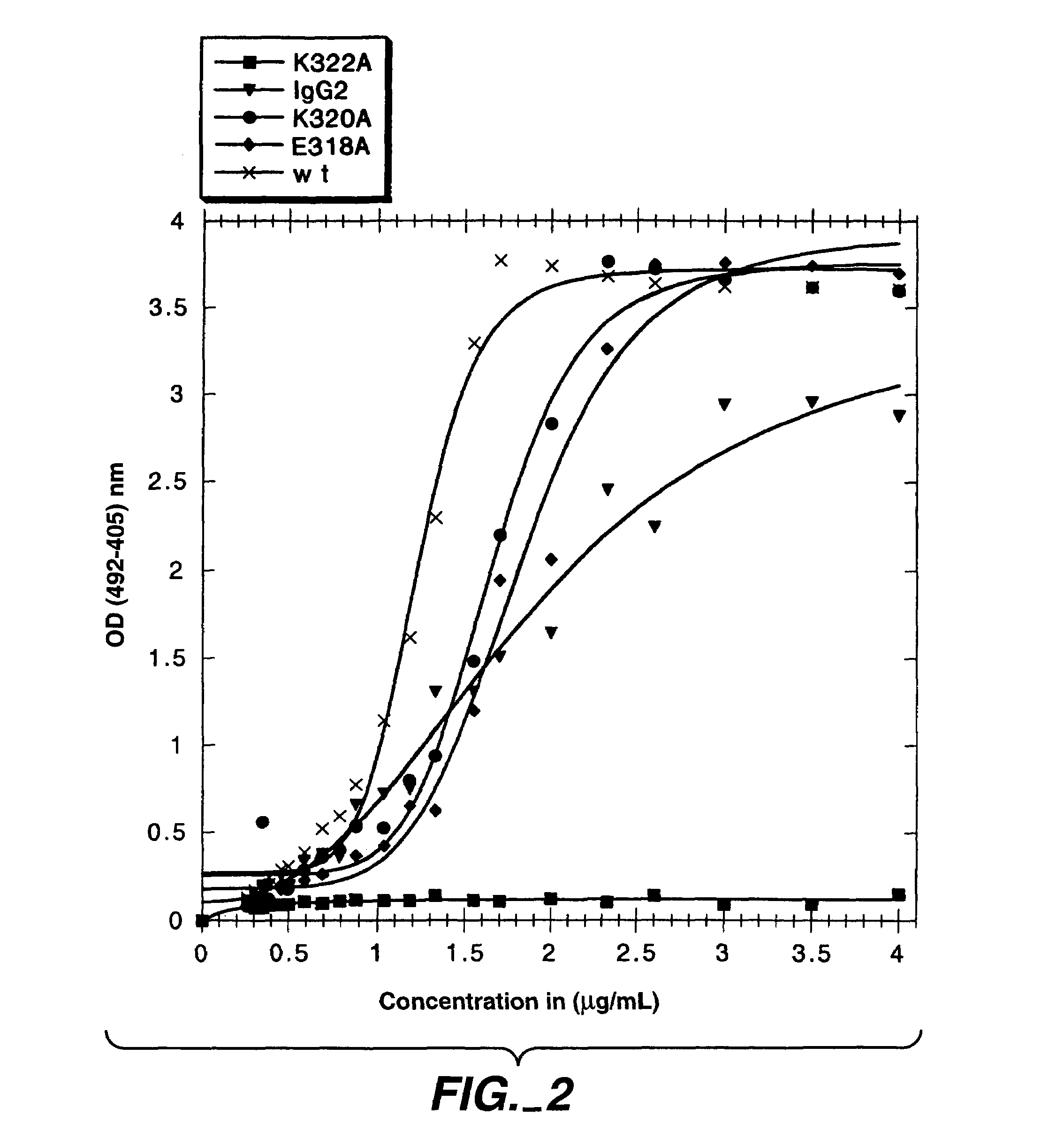
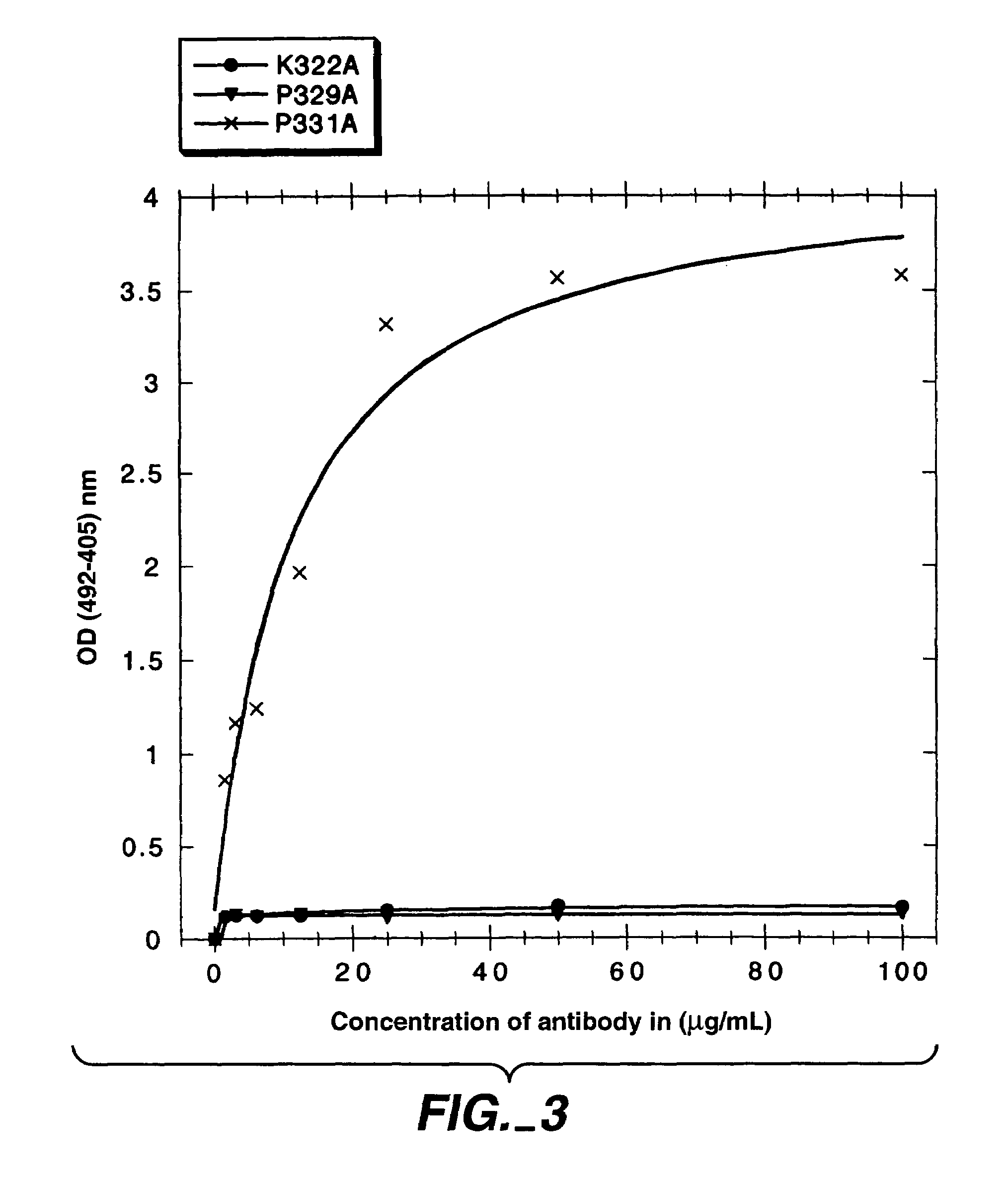
The present invention concerns polypeptides comprising a variant Fc region. More particularly, the present invention concerns Fc region-containing polypeptides that have altered effector function as a consequence of one or more amino acid modifications in the Fc region thereof.
Owner:GENENTECH INC
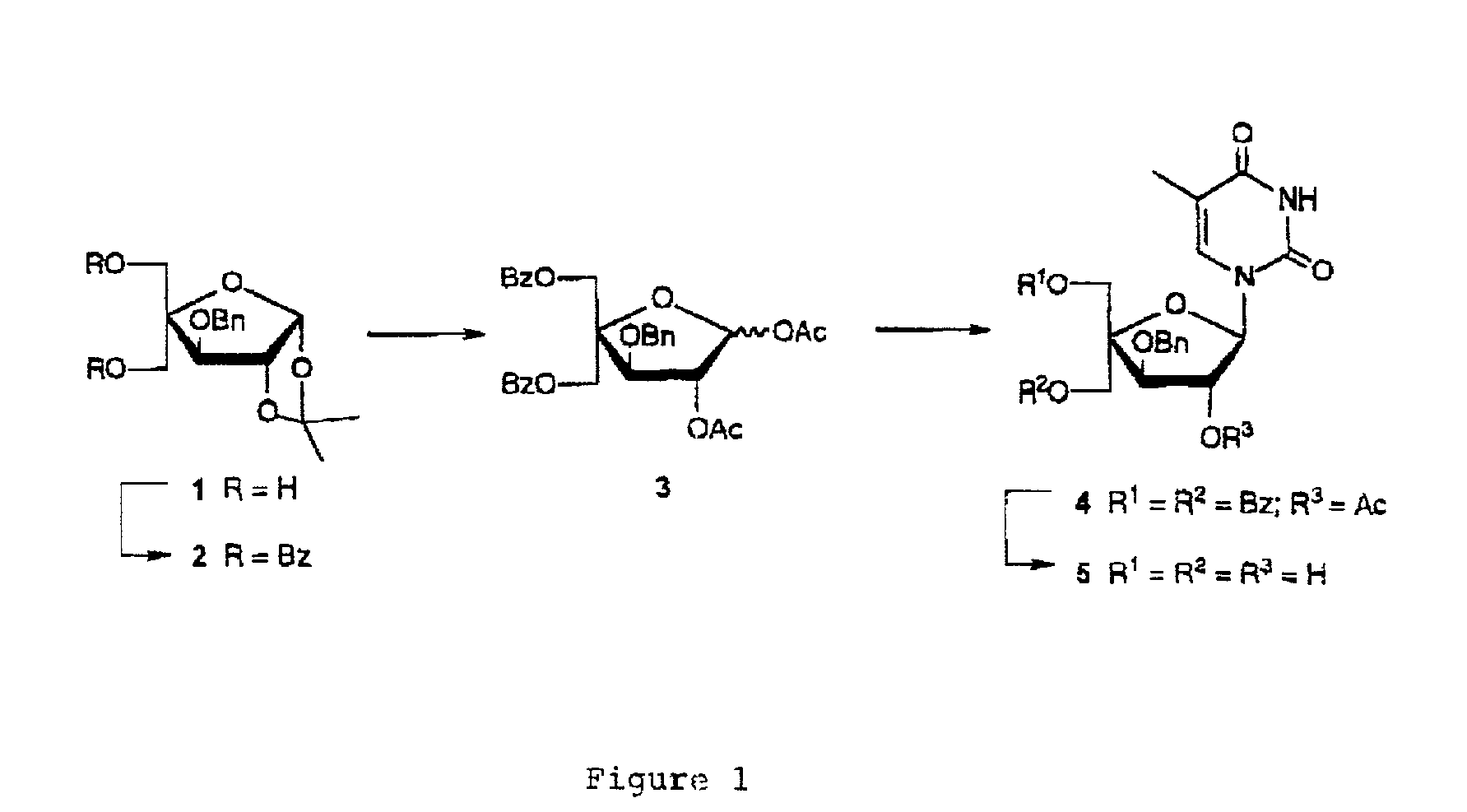
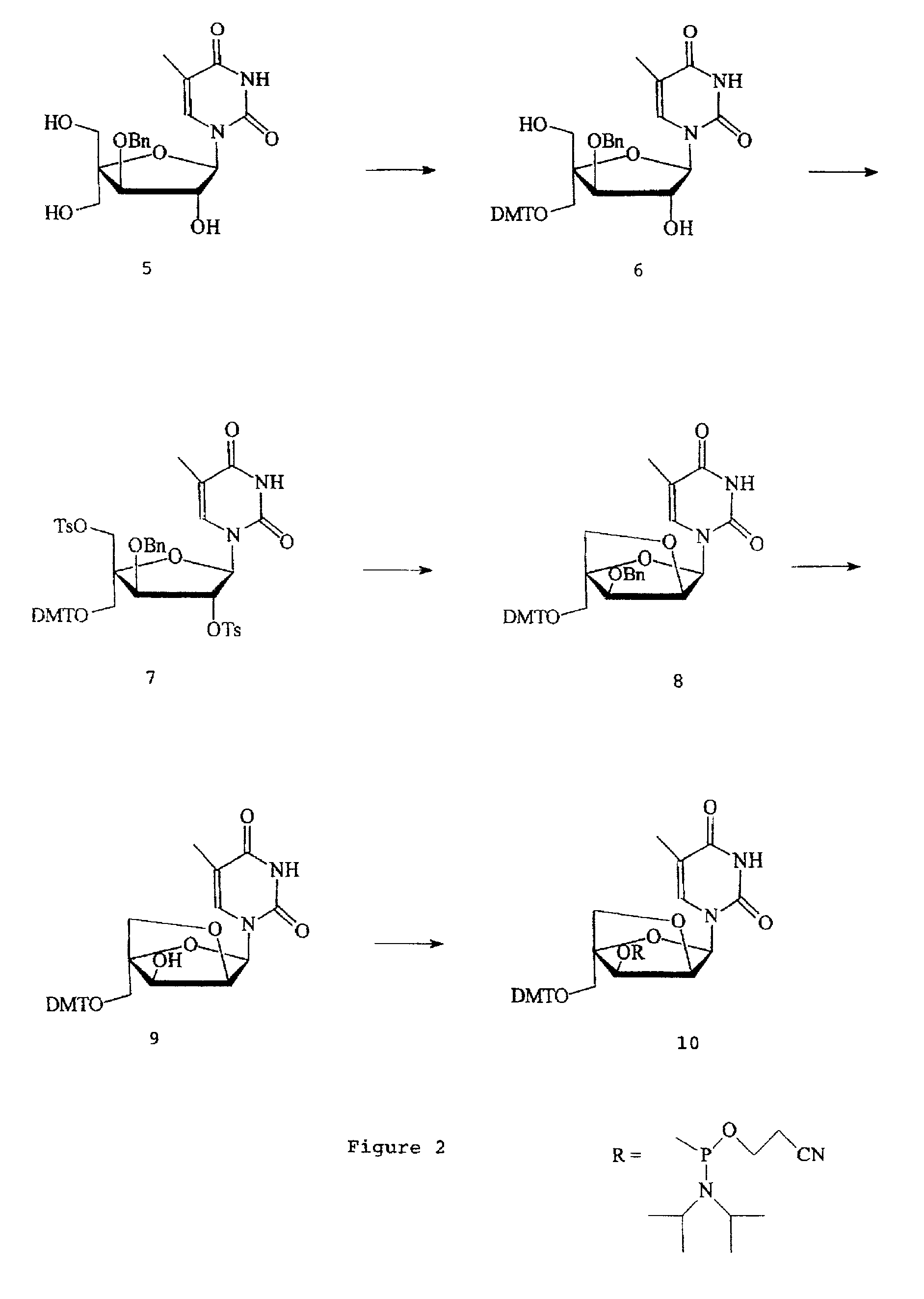
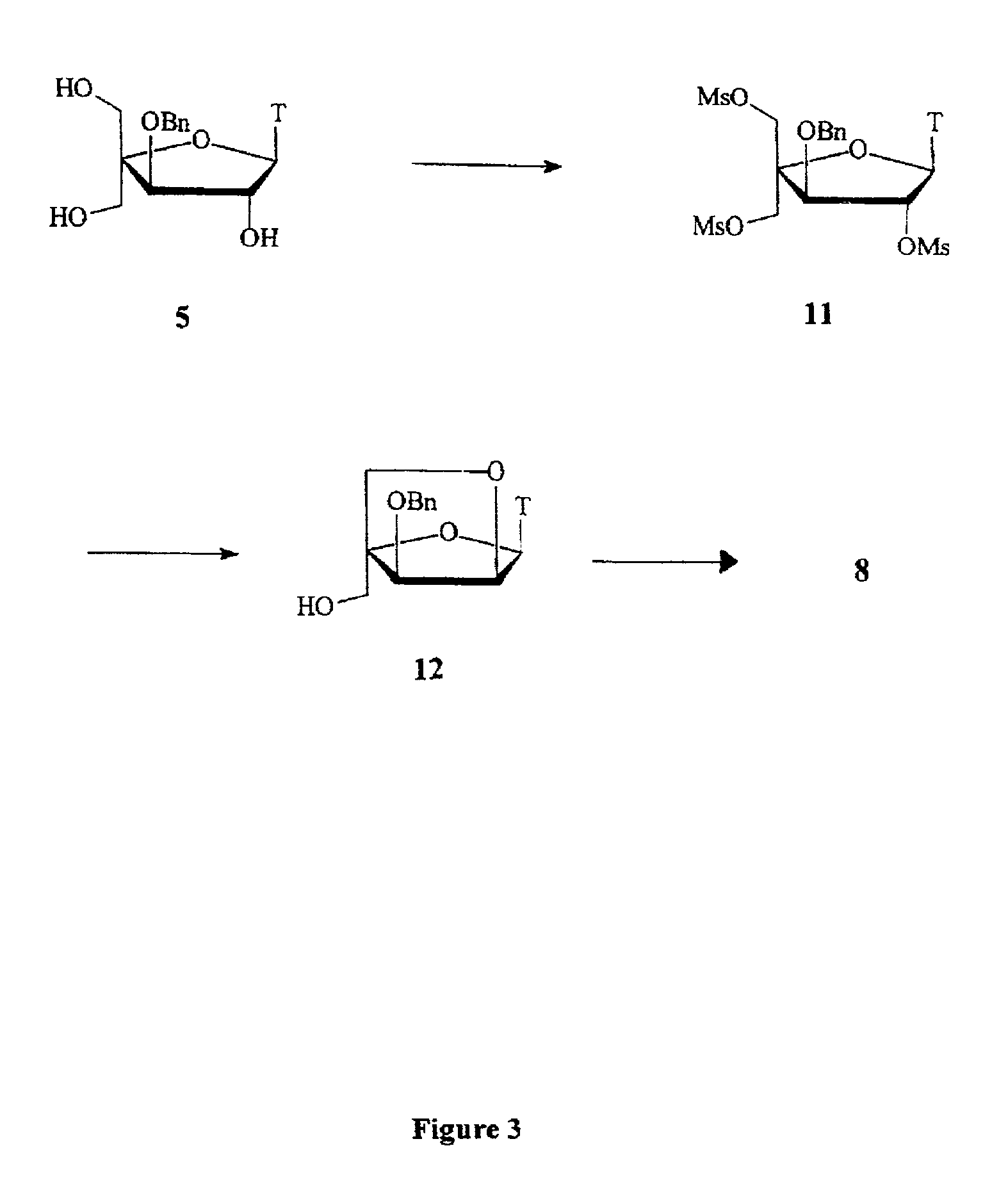
L-ribo-LNA analogues
Provided are L-ribo bicyclic nucleotide compounds as well as syntheses of such compounds. The nucleoside compounds of the invention are useful in forming oligonucleotides that can produce nucleobase specific duplexes with complementary single stranded and double stranded nucleic acids.
Owner:SANTARIS PHARMA AS
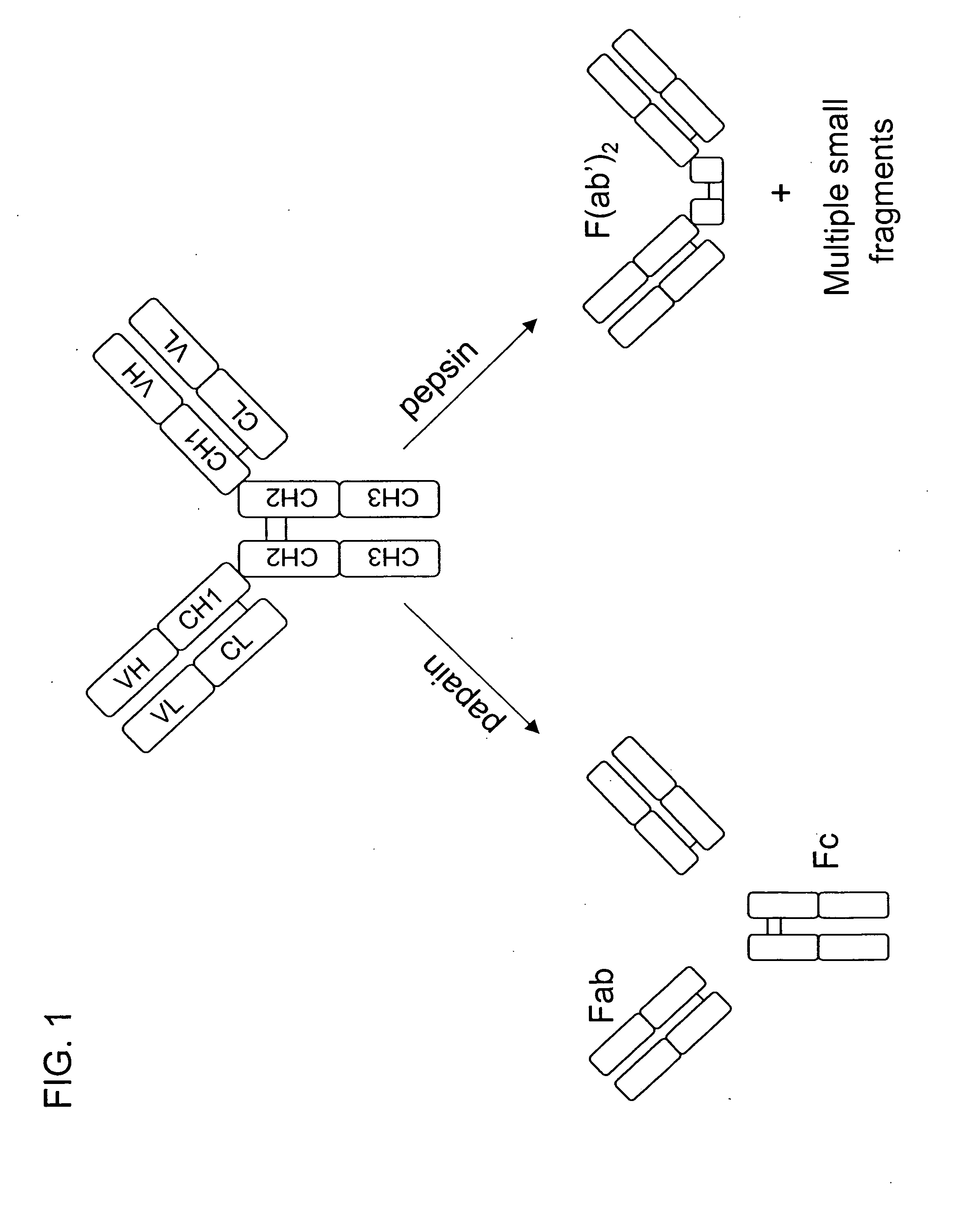
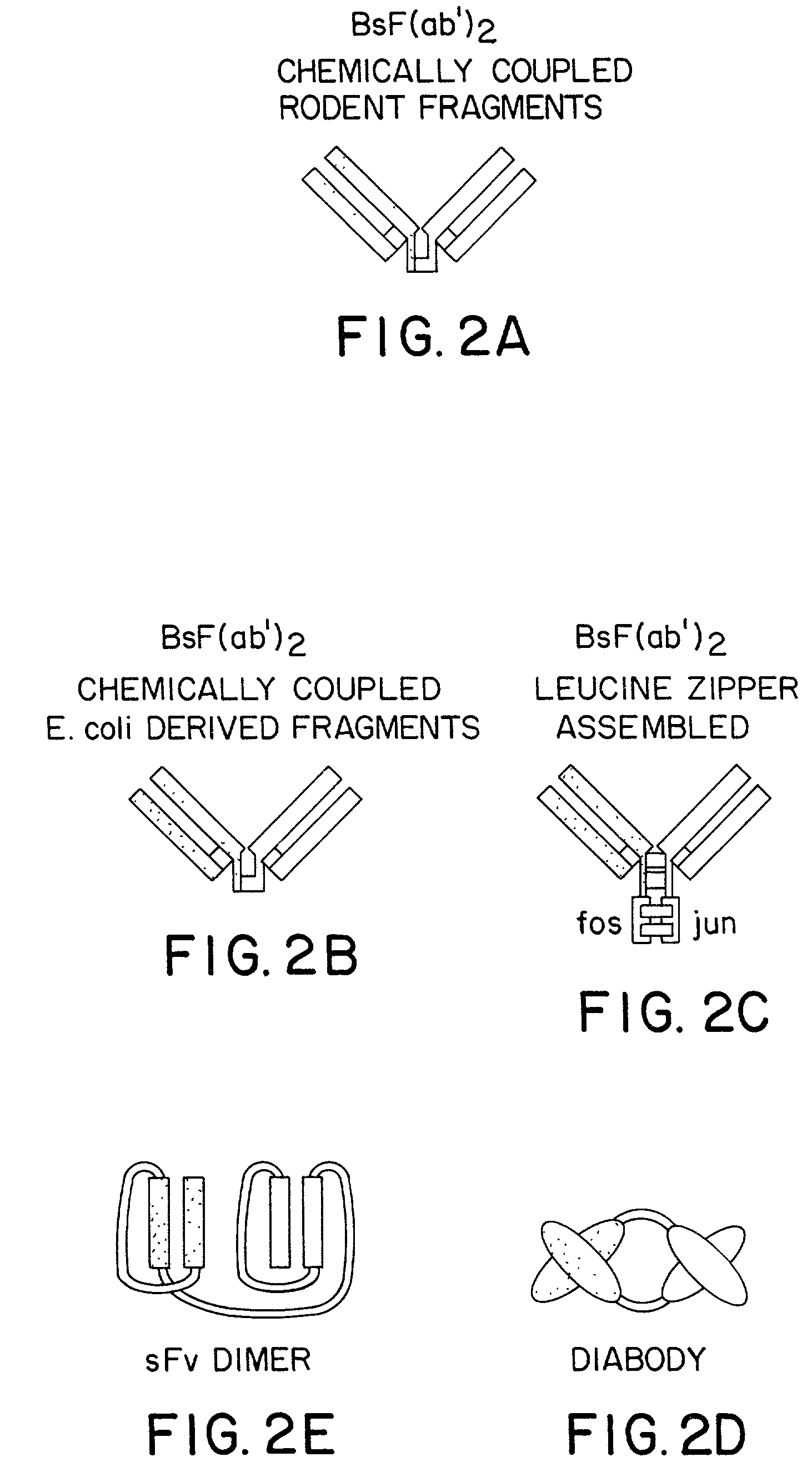
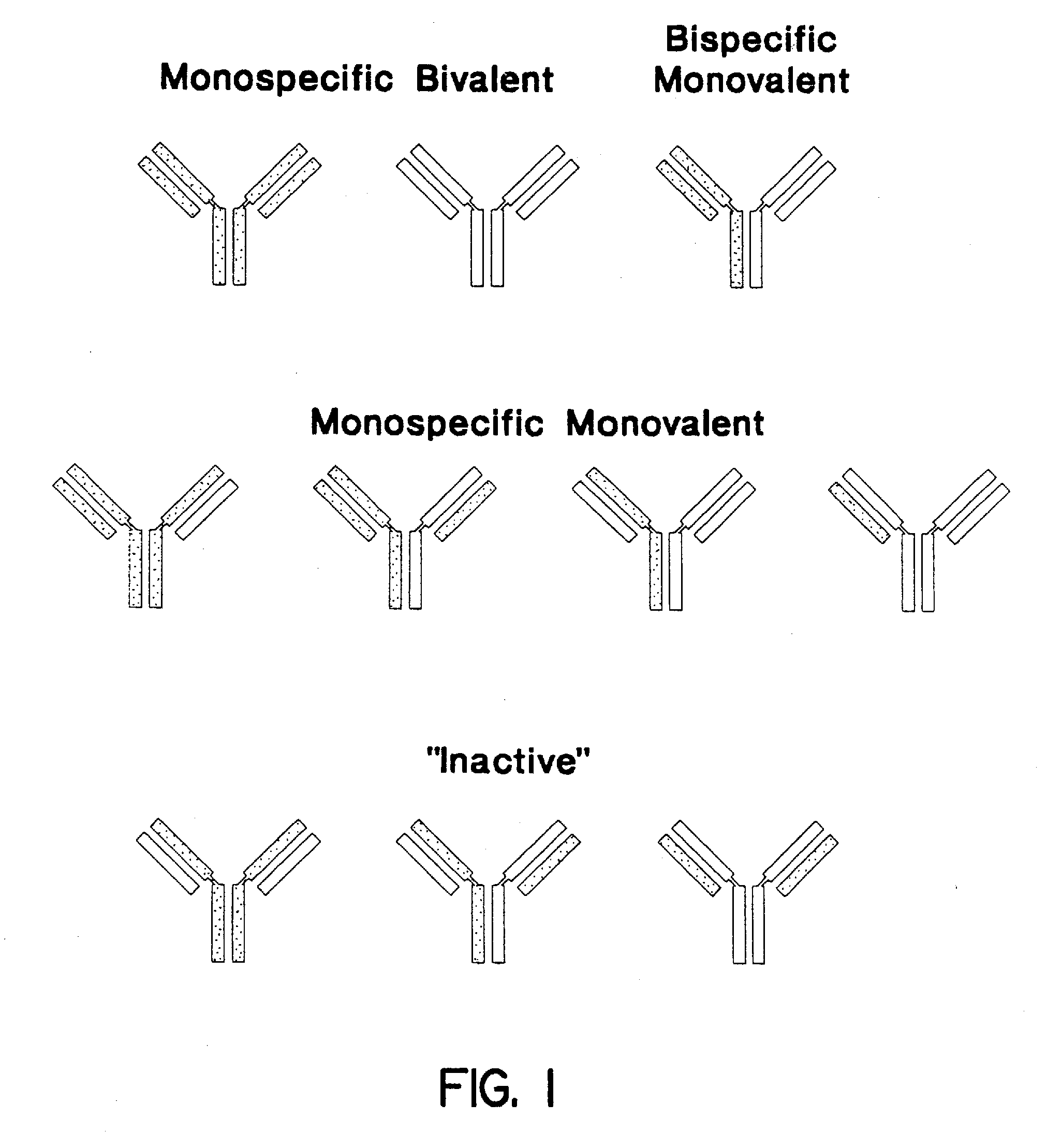
Multivalent antibodies and uses therefor
The present application describes engineered antibodies, with three or more functional antigen binding sites, and uses, such as therapeutic applications, for such engineered antibodies.
Owner:GENENTECH INC
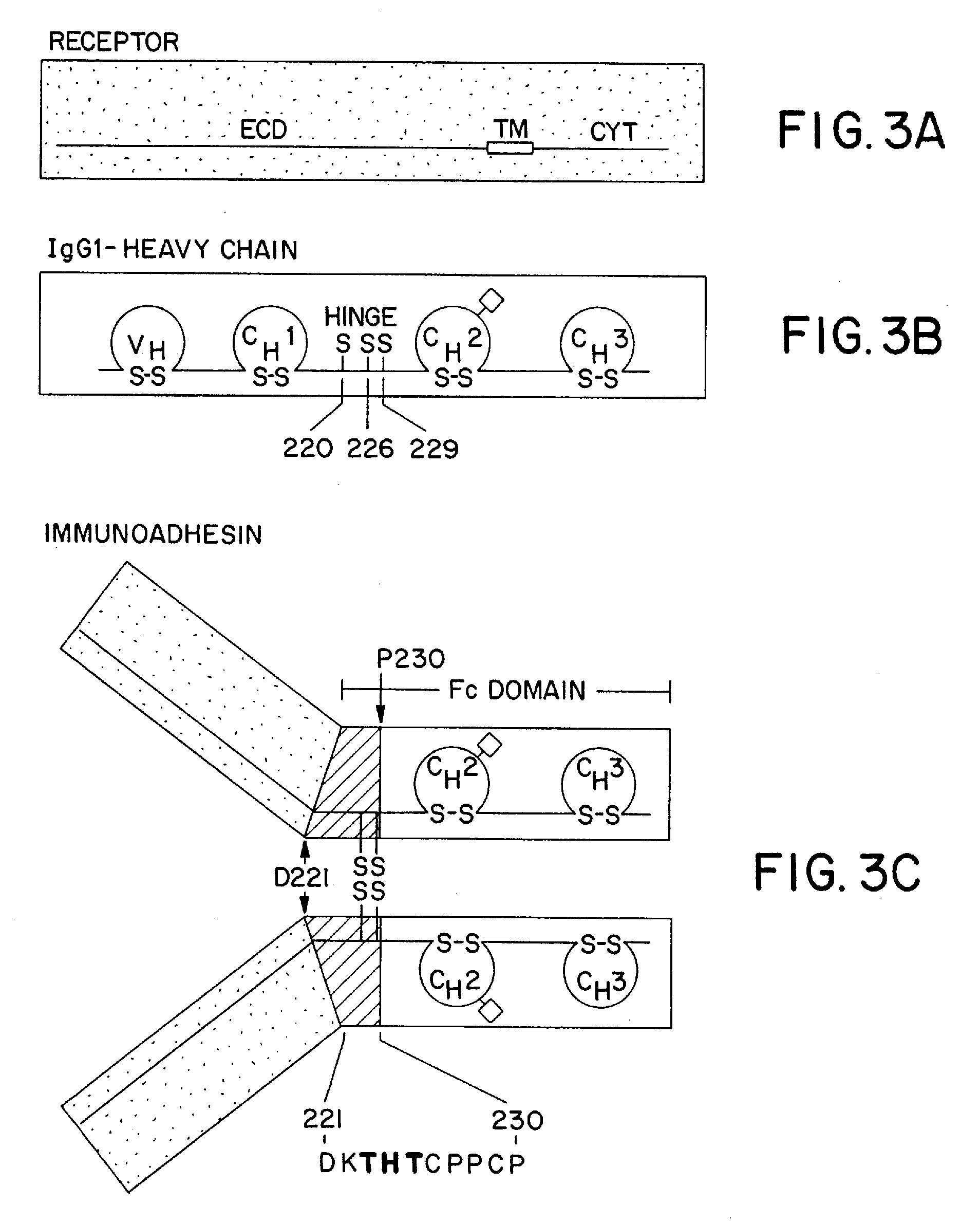
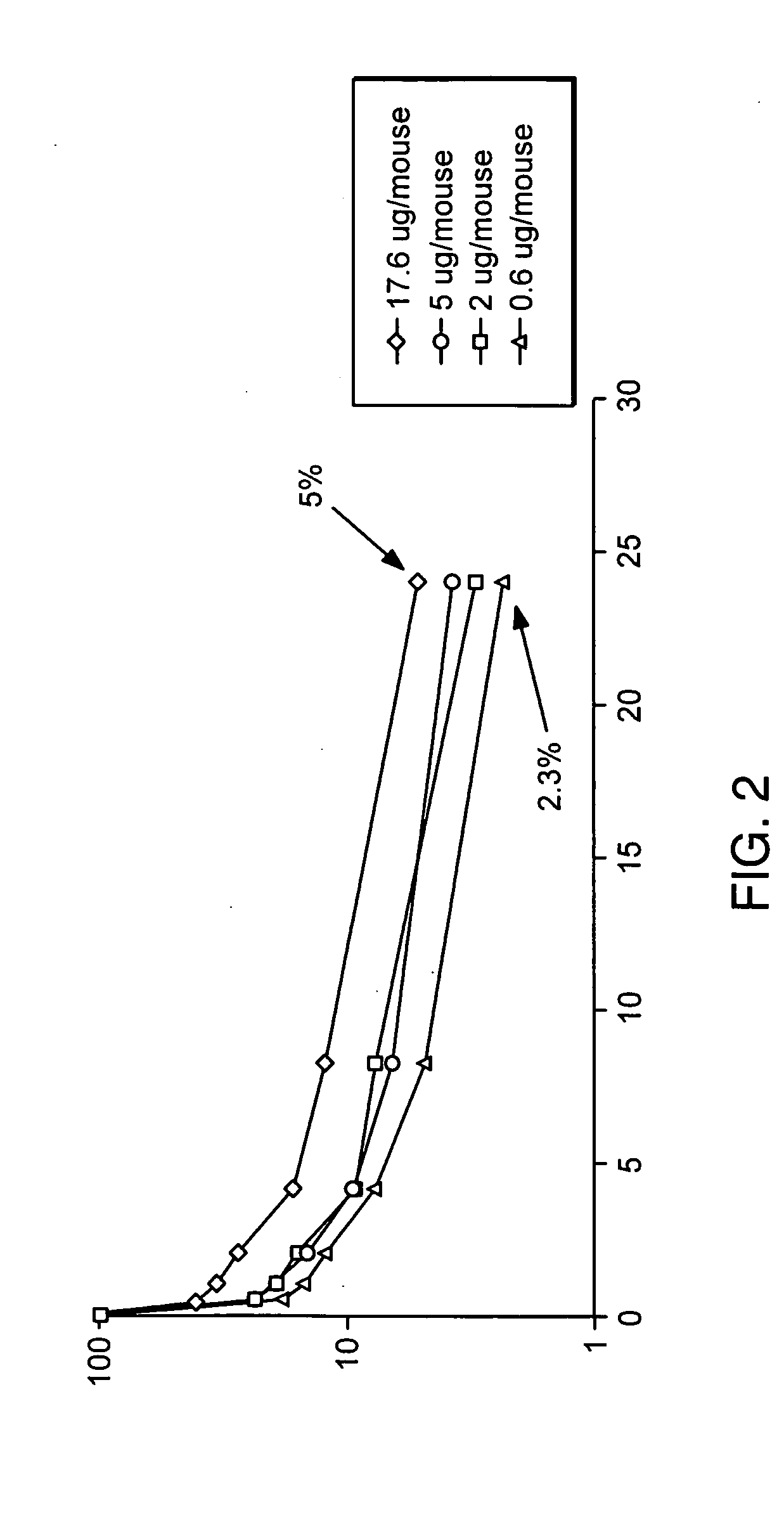
Molecules with extended half-lives, compositions and uses thereof
The present invention provides molecules, including IgGs, non-IgG immunoglobulin, proteins and non-protein agents, that have increased in vivo half-lives due to the presence of an IgG constant domain, or a portion thereof that binds the FcRn, having one or more amino acid modifications that increase the affinity of the constant domain or fragment for FcRn. Such proteins and molecules with increased half-lives have the advantage that smaller amounts and or less frequent dosing is required in the therapeutic, prophylactic or diagnostic use of such molecules.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Method of sequencing a nucleic acid
InactiveUS7244559B2Bioreactor/fermenter combinationsMaterial nanotechnologyNucleic acid detectionOligonucleotide
Owner:454 LIFE SCIENCES CORP
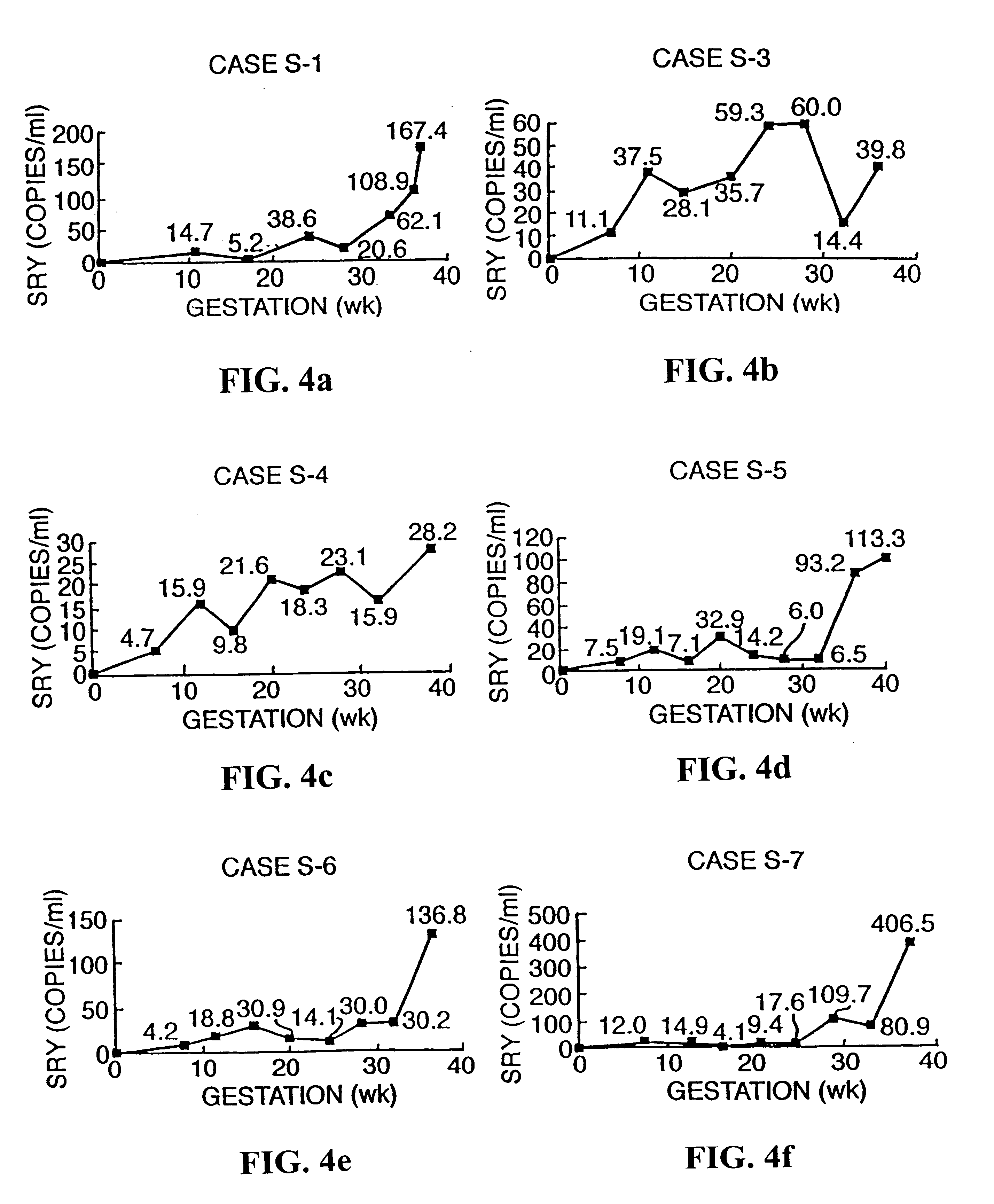
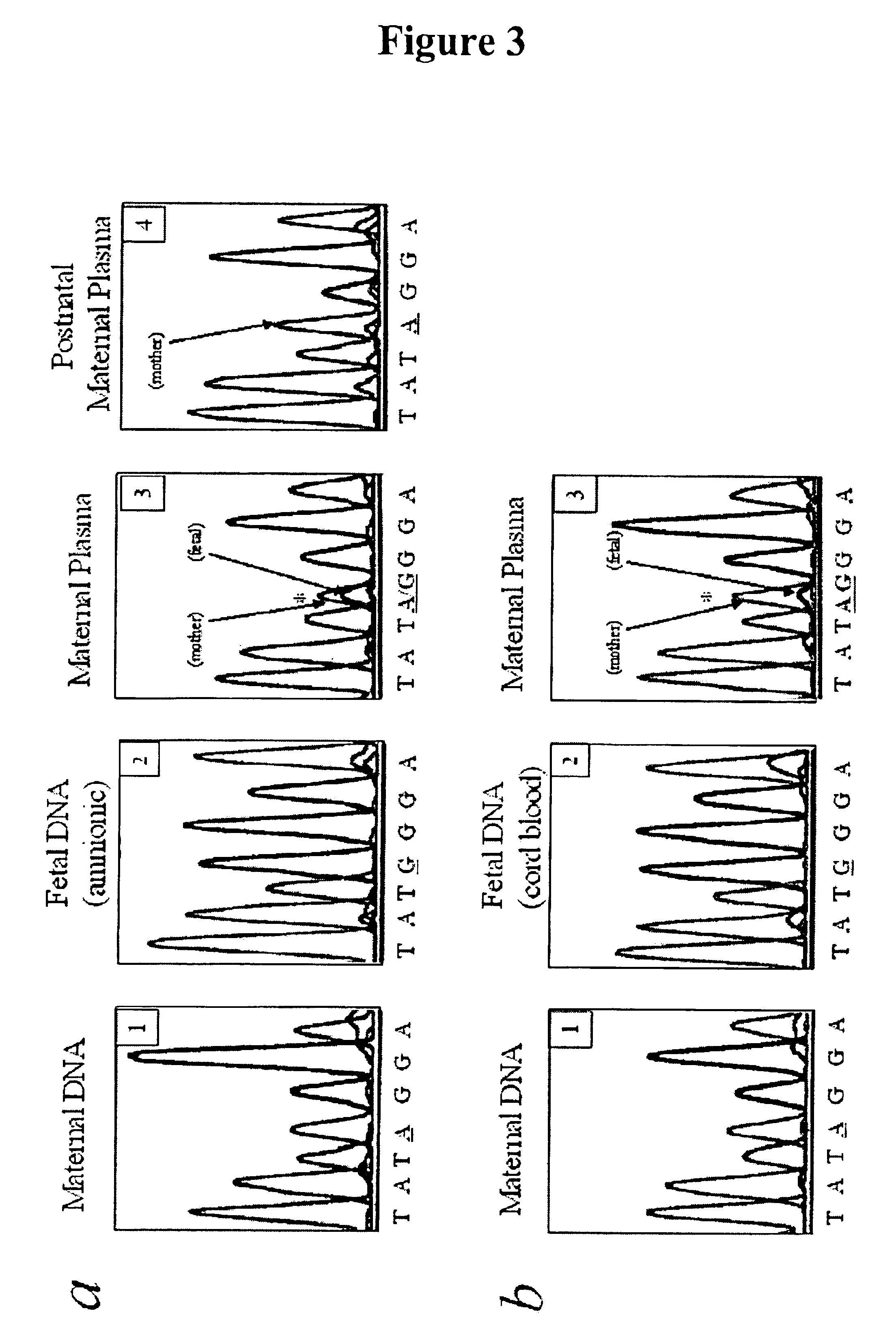
Non-invasive prenatal diagnosis
InactiveUS6258540B1% accurate detection rateIncrease the amount of foetal nucleic acid materialMicrobiological testing/measurementRecombinant DNA-technologyPrenatal diagnosisBlood typing
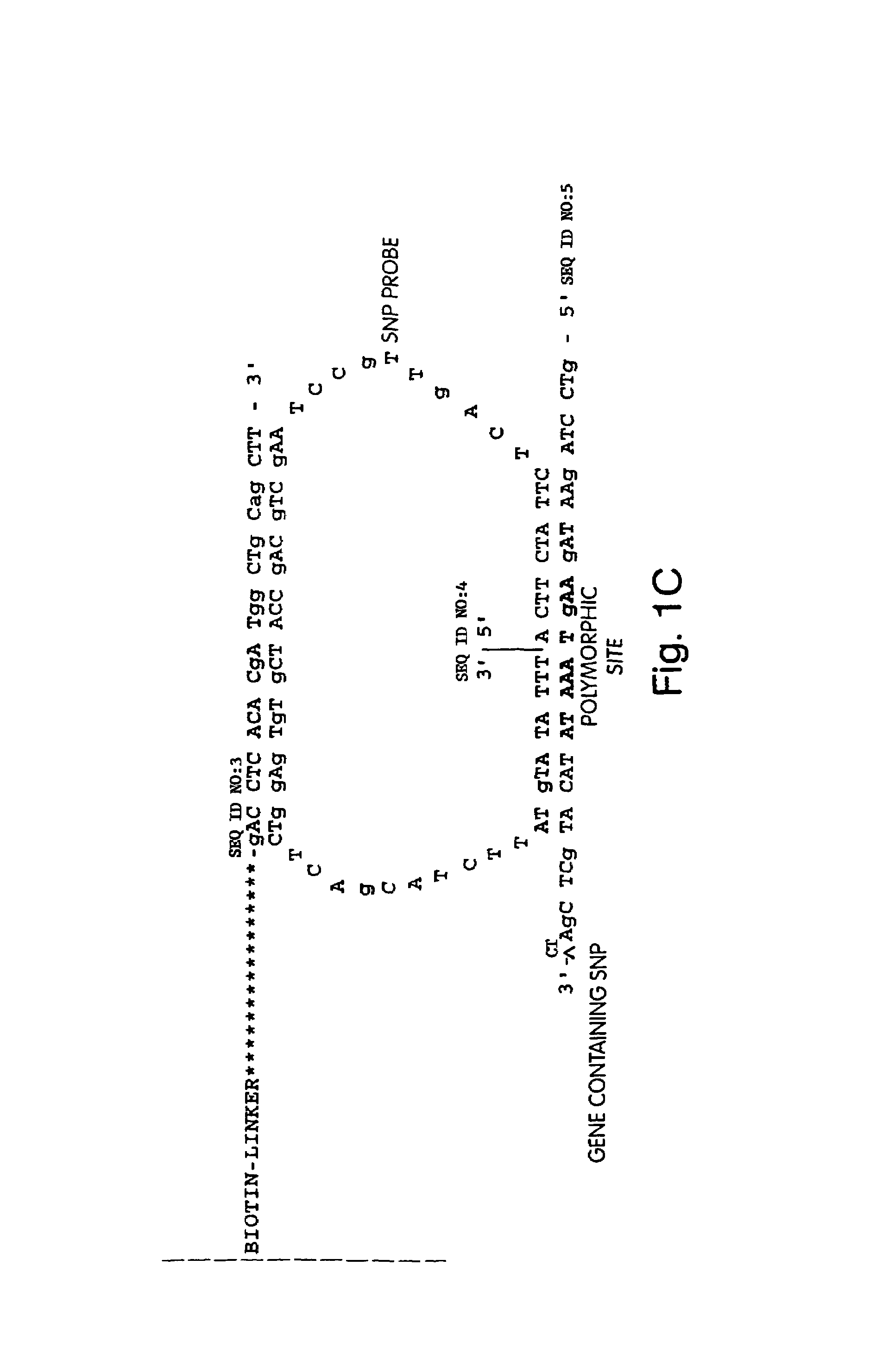
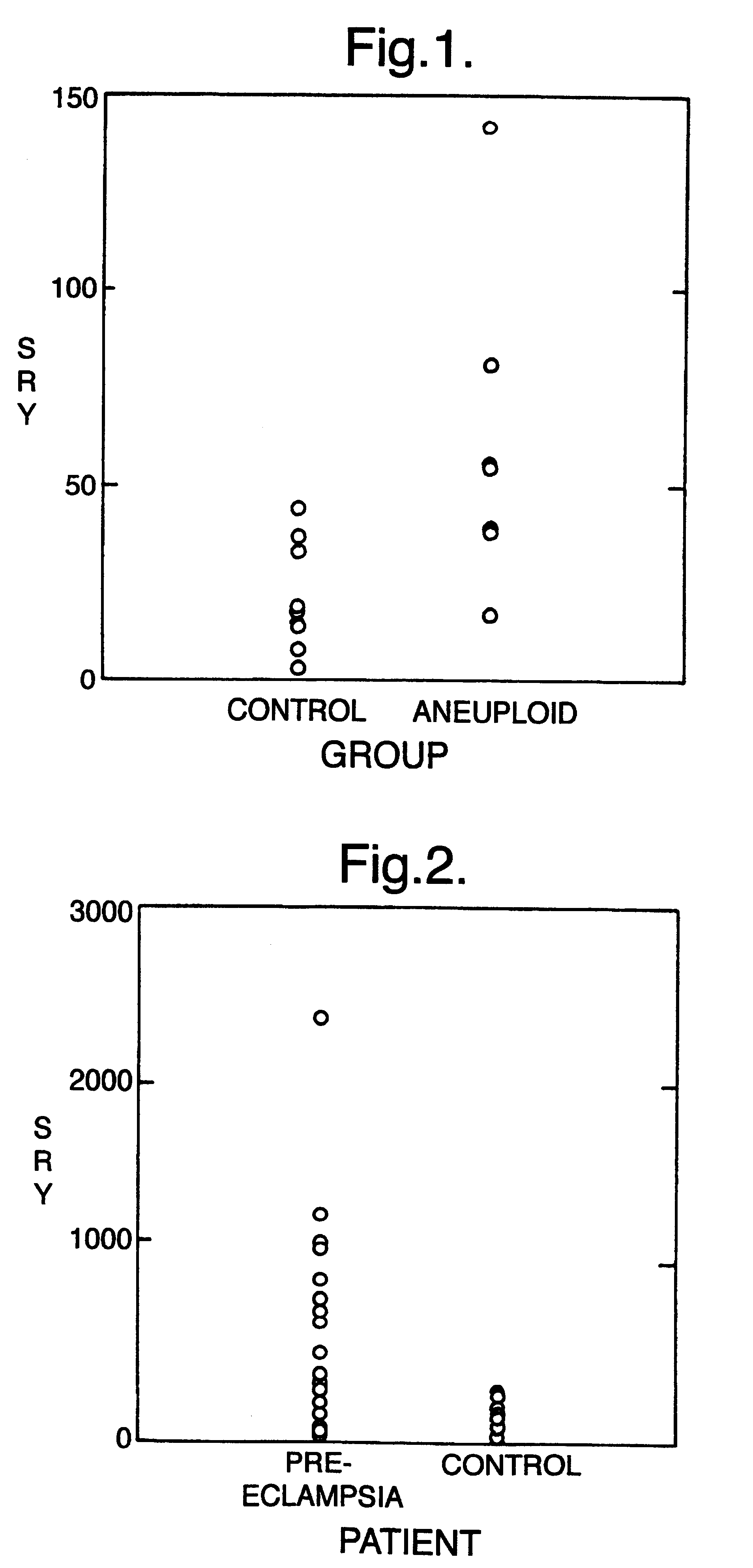
The invention relates to a detection method performed on a maternal serum or plasma sample from a pregnant female, which method comprises detecting the presence of a nucleic acid of foetal origin in the sample. The invention enables non-invasive prenatal diagnosis including for example sex determination, blood typing and other genotyping, and detection of pre-eclampsia in the mother.
Owner:SEQUENOM INC
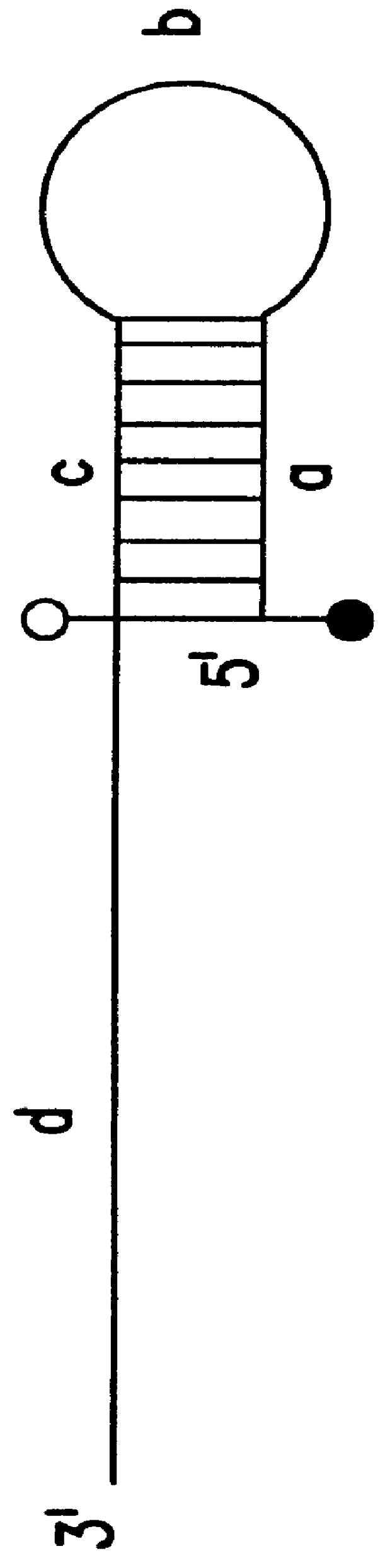
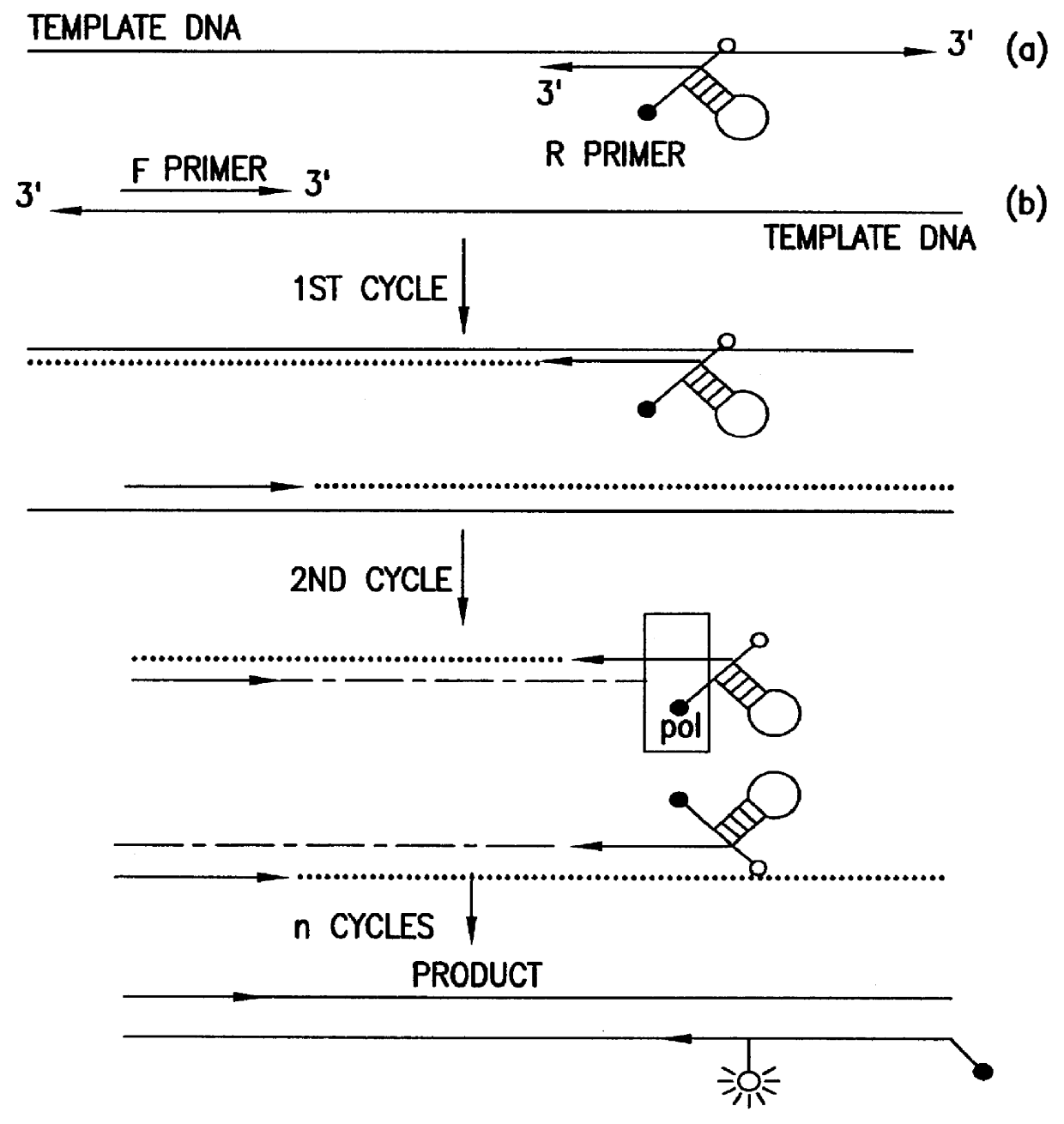
Nucleic acid amplification oligonucleotides with molecular energy transfer labels and methods based thereon
InactiveUS6117635AReduce the possibilityRapid and sensitive and reliableSugar derivativesMicrobiological testing/measurementEnergy transferFluorophore
The present invention provides labeled nucleic acid amplification oligonucleotides, which can be linear or hairpin primers or blocking oligonucleotides. The oligonucleotides of the invention are labeled with donor and / or acceptor moieties of molecular energy transfer pairs. The moieties can be fluorophores, such that fluorescent energy emitted by the donor is absorbed by the acceptor. The acceptor may be a fluorophore that fluoresces at a wavelength different from the donor moiety, or it may be a quencher. The oligonucleotides of the invention are configured so that a donor moiety and an acceptor moiety are incorporated into the amplification product. The invention also provides methods and kits for directly detecting amplification products employing the nucleic acid amplification primers. When labeled linear primers are used, treatment with exonuclease or by using specific temperature eliminates the need for separation of unincorporated primers. This "closed-tube" format greatly reduces the possibility of carryover contamination with amplification products, provides for high throughput of samples, and may be totally automated.
Owner:MILLIPORE CORP
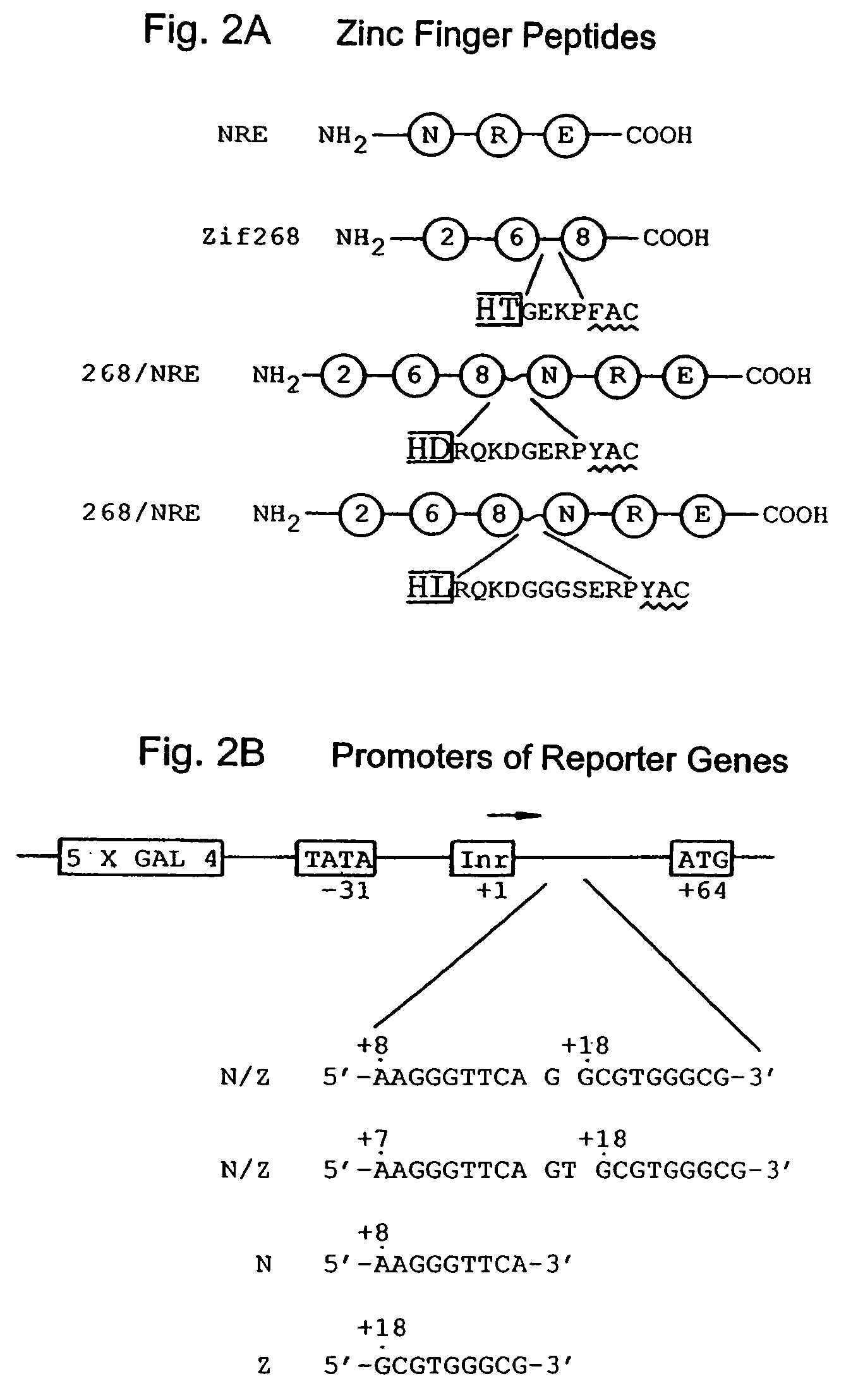
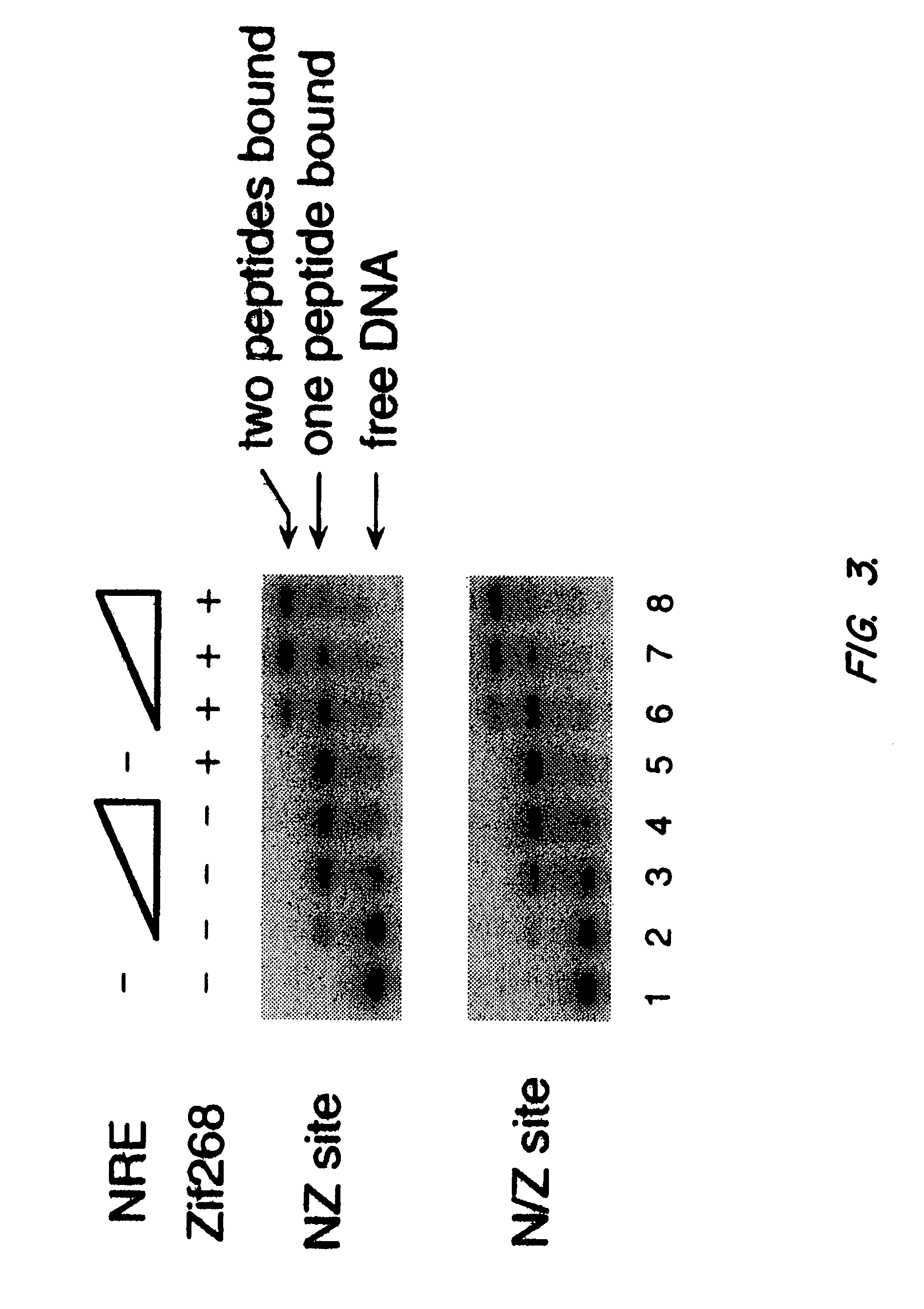
Nucleic acid encoding poly-zinc finger proteins with improved linkers
InactiveUS7153949B2Enhanced affinity and specificityImprove the level ofPeptide/protein ingredientsAntibody mimetics/scaffoldsDNA-binding domainNucleotide
Polynucleotides encoding chimeric proteins, and methods for their production and use are disclosed. The chimeric proteins comprise a flexible linker between two zinc finger DNA-binding domains, wherein the linker contains eight or more amino acids between the second conserved histidine residue of the carboxy-terminal zinc finger of the first domain and the first conserved cysteine residue of the amino-terminal zinc finger of the second domain.
Owner:MASSACHUSETTS INST OF TECH
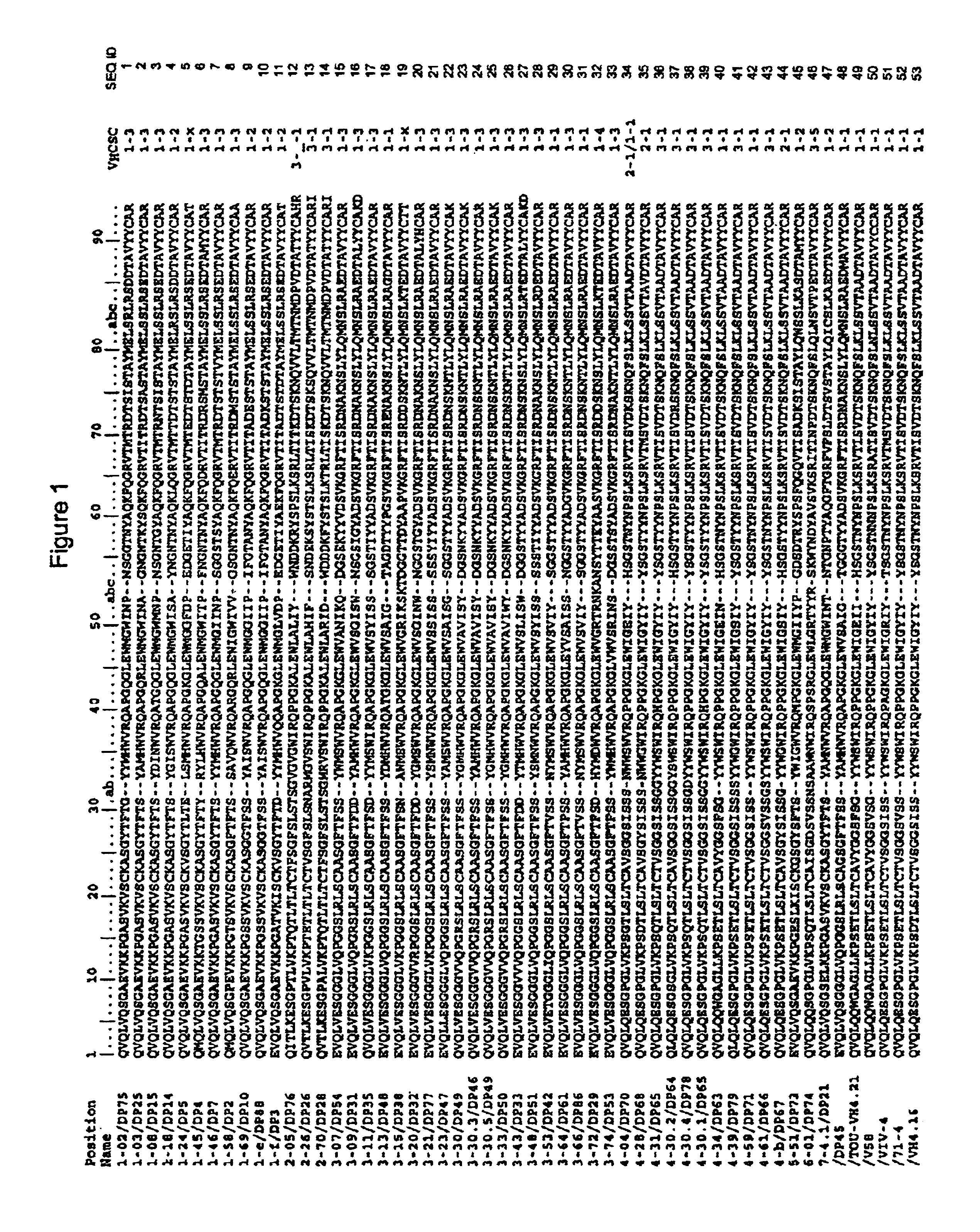
Super humanized antibodies
InactiveUS6881557B2Antibody mimetics/scaffoldsAnalogue computers for chemical processesHuman sequenceHumanized antibody
Disclosed herein are methods for humanizing antibodies based on selecting variable region framework sequences from human antibody genes by comparing canonical CDR structure types for CDR sequences of the variable region of a non-human antibody to canonical CDR structure types for corresponding CDRs from a library of human antibody sequences, preferably germline antibody gene segments. Human antibody variable regions having similar canonical CDR structure types to the non-human CDRs form a subset of member human antibody sequences from which to select human framework sequences. The subset members may be further ranked by amino acid similarity between the human and the non-human CDR sequences. Top ranking human sequences are selected to provide the framework sequences for constructing a chimeric antibody that functionally replaces human CDR sequences with the non-human CDR counterparts using the selected subset member human frameworks, thereby providing a humanized antibody of high affinity and low immunogenicity without need for comparing framework sequences between the non-human and human antibodies. Chimeric antibodies made according to the method are also disclosed.
Owner:ARROWSMITH TECH
Integrated active flux microfluidic devices and methods
InactiveUS6767706B2Rapid and complete exposureQuick and accurate and inexpensive analysisBioreactor/fermenter combinationsFlow mixersAntigenHybridization probe
The invention relates to a microfabricated device for the rapid detection of DNA, proteins or other molecules associated with a particular disease. The devices and methods of the invention can be used for the simultaneous diagnosis of multiple diseases by detecting molecules (e.g. amounts of molecules), such as polynucleotides (e.g., DNA) or proteins (e.g., antibodies), by measuring the signal of a detectable reporter associated with hybridized polynucleotides or antigen / antibody complex. In the microfabricated device according to the invention, detection of the presence of molecules (i.e., polynucleotides, proteins, or antigen / antibody complexes) are correlated to a hybridization signal from an optically-detectable (e.g. fluorescent) reporter associated with the bound molecules. These hybridization signals can be detected by any suitable means, for example optical, and can be stored for example in a computer as a representation of the presence of a particular gene. Hybridization probes can be immobilized on a substrate that forms part of or is exposed to a channel or channels of the device that form a closed loop, for circulation of sample to actively contact complementary probes. Universal chips according to the invention can be fabricated not only with DNA but also with other molecules such as RNA, proteins, peptide nucleic acid (PNA) and polyamide molecules.
Owner:CALIFORNIA INST OF TECH
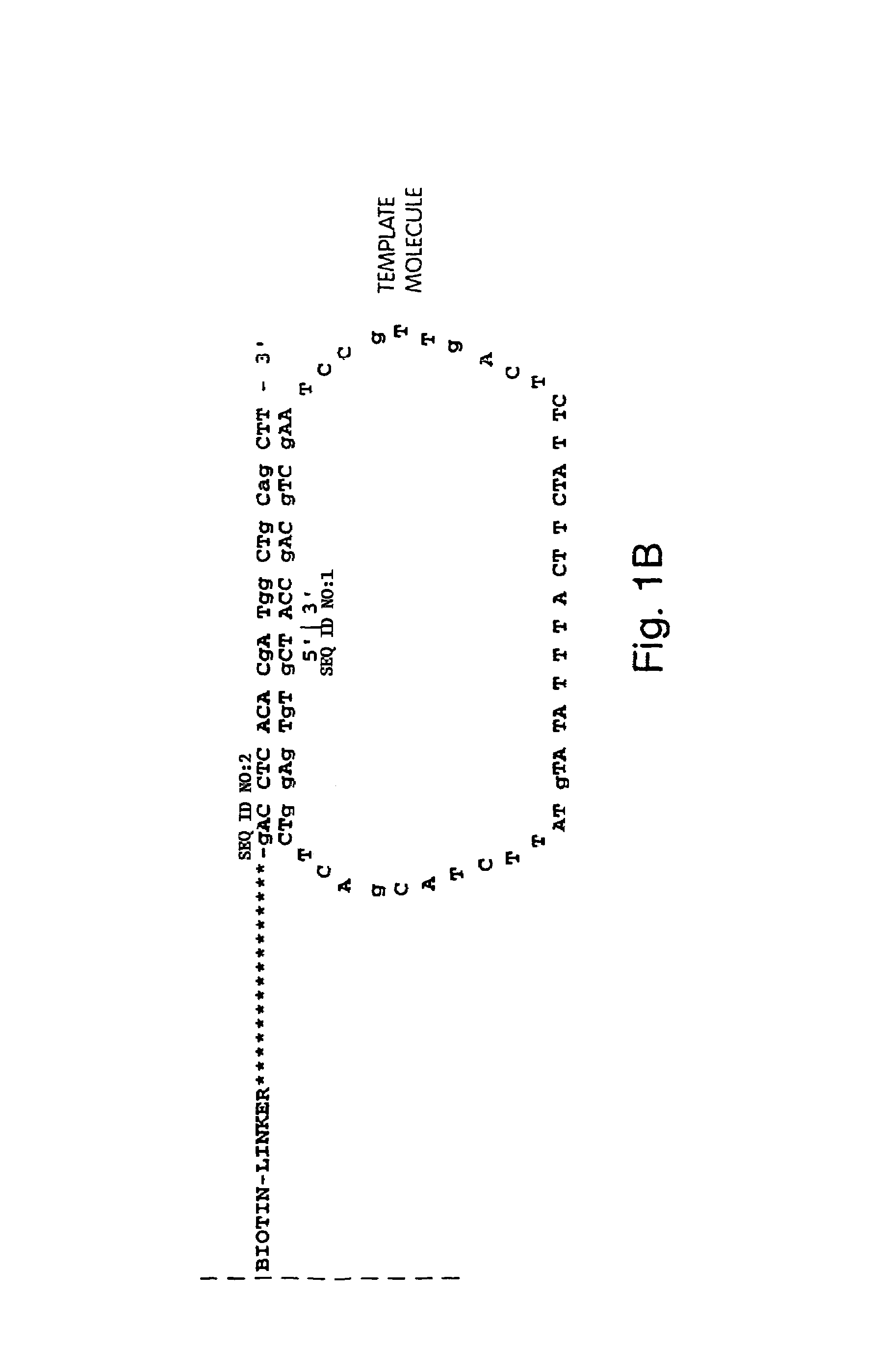
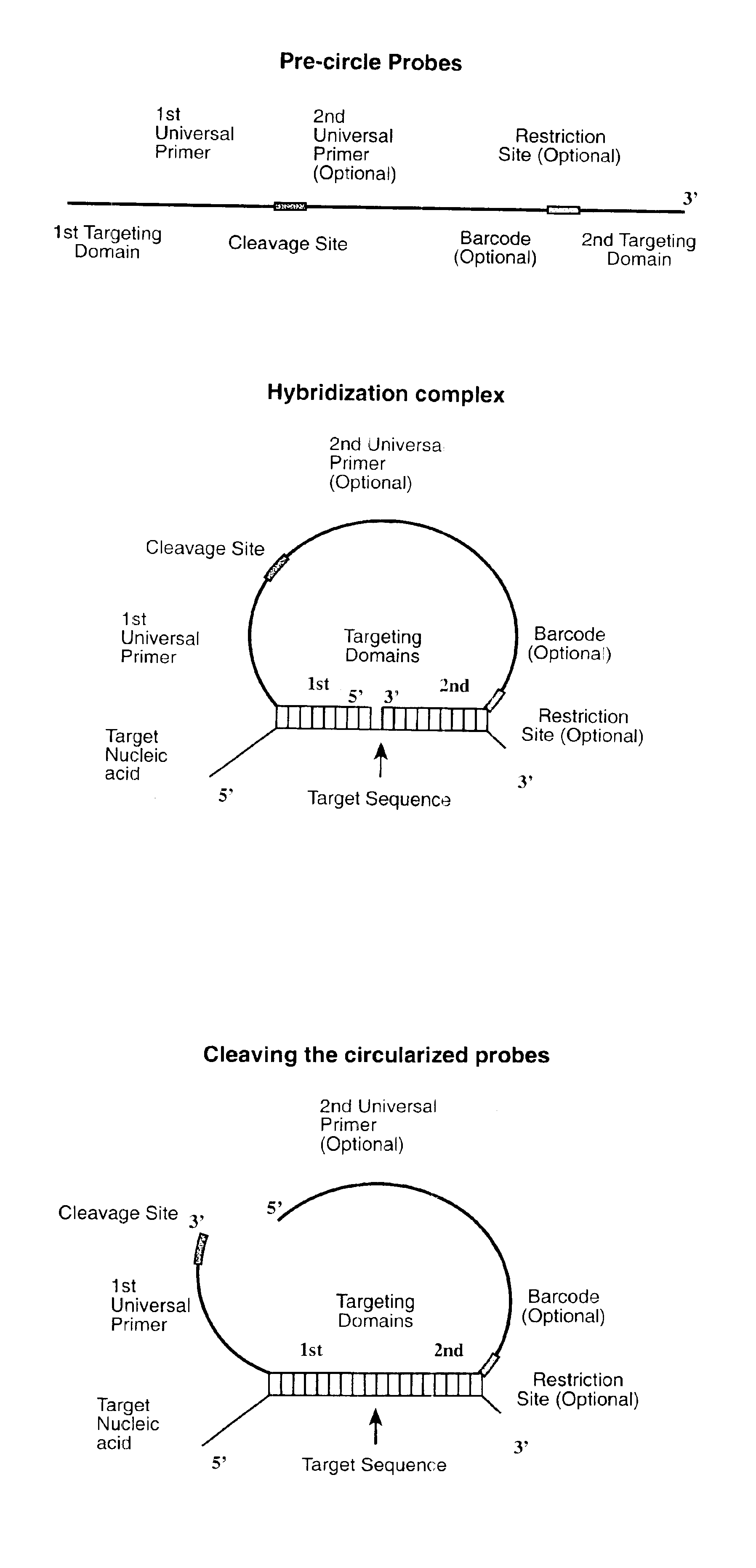
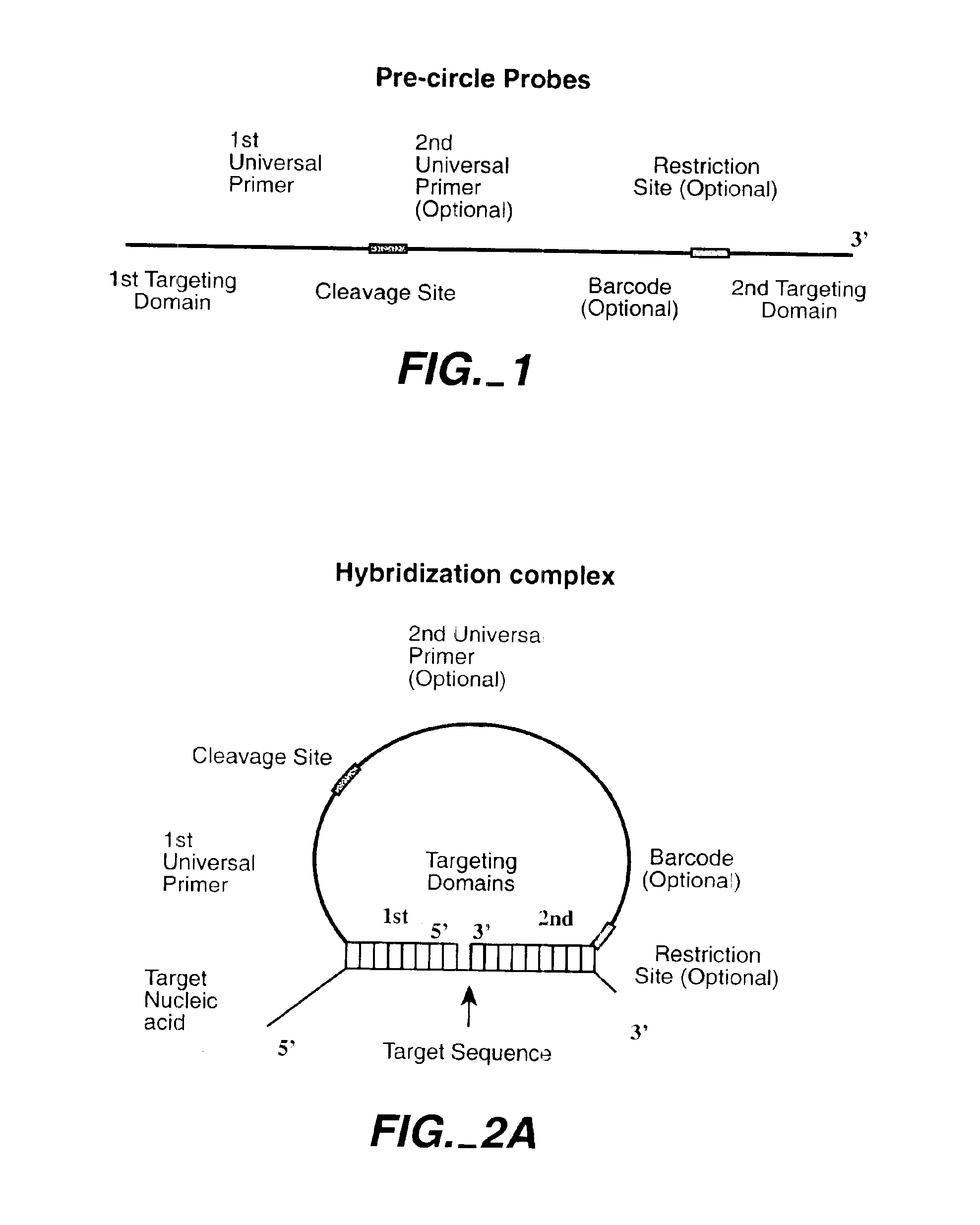
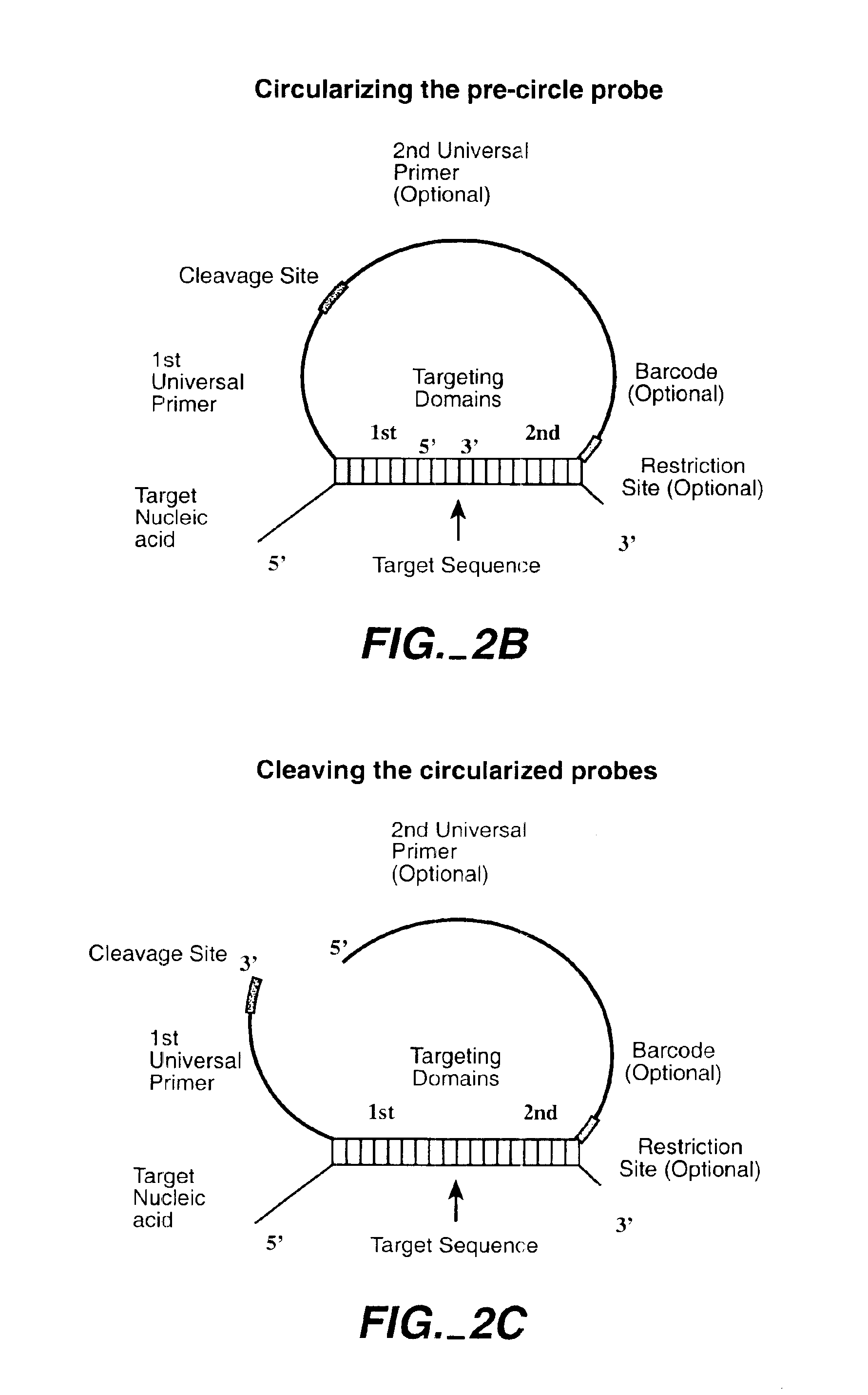
Direct multiplex characterization of genomic DNA
The invention is directed to novel methods of multiplexing nucleic acid reactions, including amplification, detection and genotyping. The invention relies on the use of precircle probes that are circularized in the presence of the corresponding target nucleic acids, cleaved, and then amplified.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Multiplexed detection of analytes
InactiveUS6890741B2Assure specificityReduce complexitySugar derivativesMicrobiological testing/measurementAnalyteAssay
The present invention is directed to sensitive and accurate multiplexed assays for target analyte detection.
Owner:ILLUMINA INC
Nucleic acid sequencing with solid phase capturable terminators comprising a cleavable linking group
InactiveUS6046005ABioreactor/fermenter combinationsBiological substance pretreatmentsNucleic acid sequencingNucleic acid sequence
Methods of enzymatic nucleic acid sequencing are provided in which solid-phase capturable chain terminators are employed. In the subject methods, sequencing fragments are generated, where the fragments comprise capturable chain terminators. The fragments are then captured on a solid phase and separated from the remaining components of the sequencing reaction. The fragments are then released from the solid phase, size separated and detected to yield sequencing data from which the sequence of the nucleic acid is determined.
Owner:INCYTE PHARMA INC
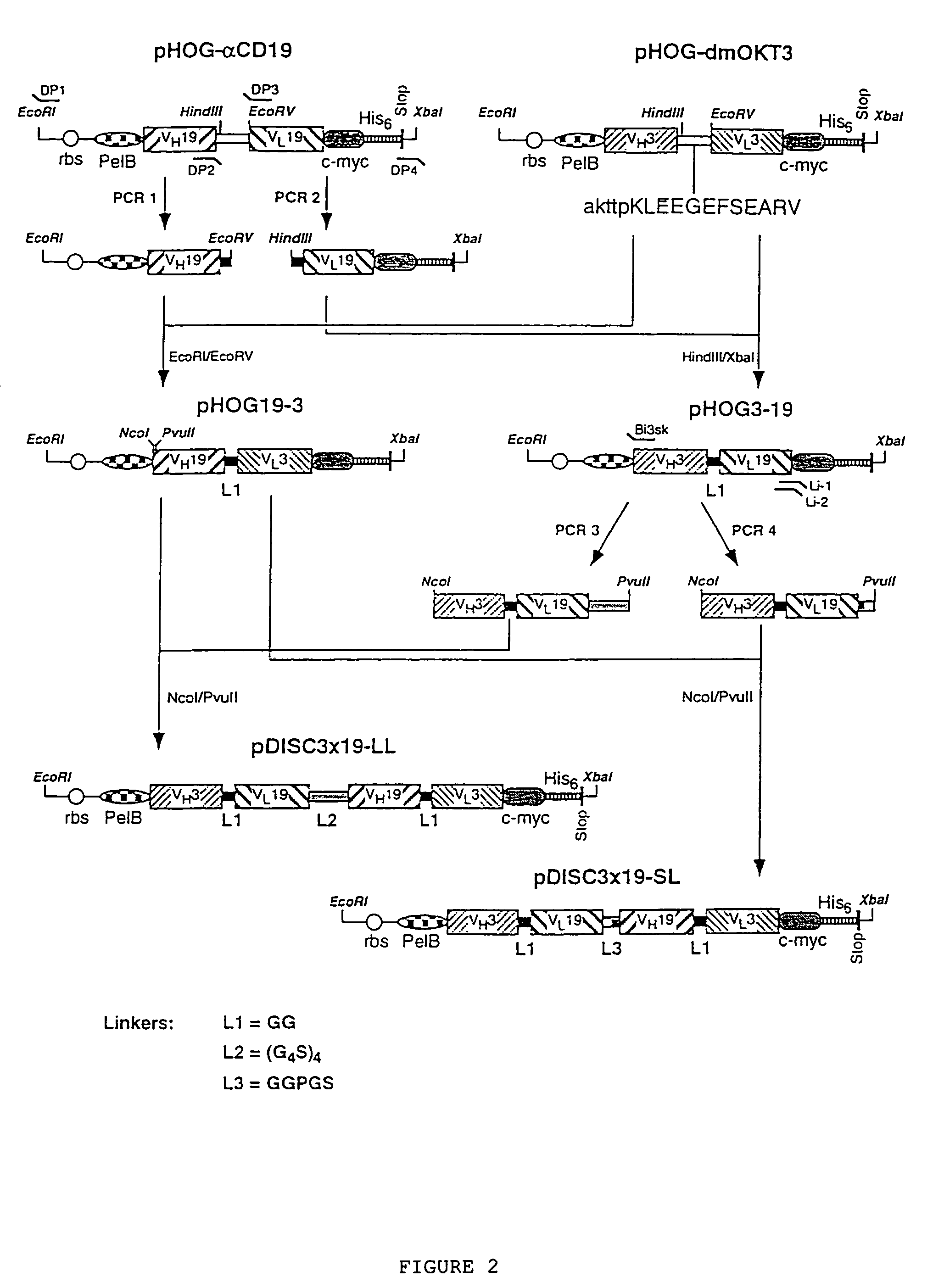
CD19-specific chimeric T cell receptor
InactiveUS7446179B2Peptide/protein ingredientsAntibody mimetics/scaffoldsIntracellular signallingTransmembrane domain
The present invention relates to a genetically engineered, CD19-specific chimeric T cell receptor and to immune cells expressing the chimeric receptor The present invention also relates to the use of such cells for cellular immunotherapy of CD9+ malignancies and for abrogating any untoward B cell function. The chimeric receptor is a single chain scFvFc:ζ receptor where scFvFc designates the extracellular domain, scFv designates the VH and VL chains of a single chain monoclonal antibody to CD19, Fc represents at least part of a constant region of an IgG1, and ζ represents the intracellular signaling domain of the zeta chain of human CD3. The extracellular domain scFvFc and the intracellular domain ζ are linked by a transmembrane domain such as the transmembrane domain of CD4. In one aspect, the chimeric receptor comprises amino acids 23-634 of SEQ I DNO:2. The present invention further relates to a method of making a redirected T cell expressing a chimeric T cell receptor by electroporation using naked DNA encoding the receptor.
Owner:CITY OF HOPE
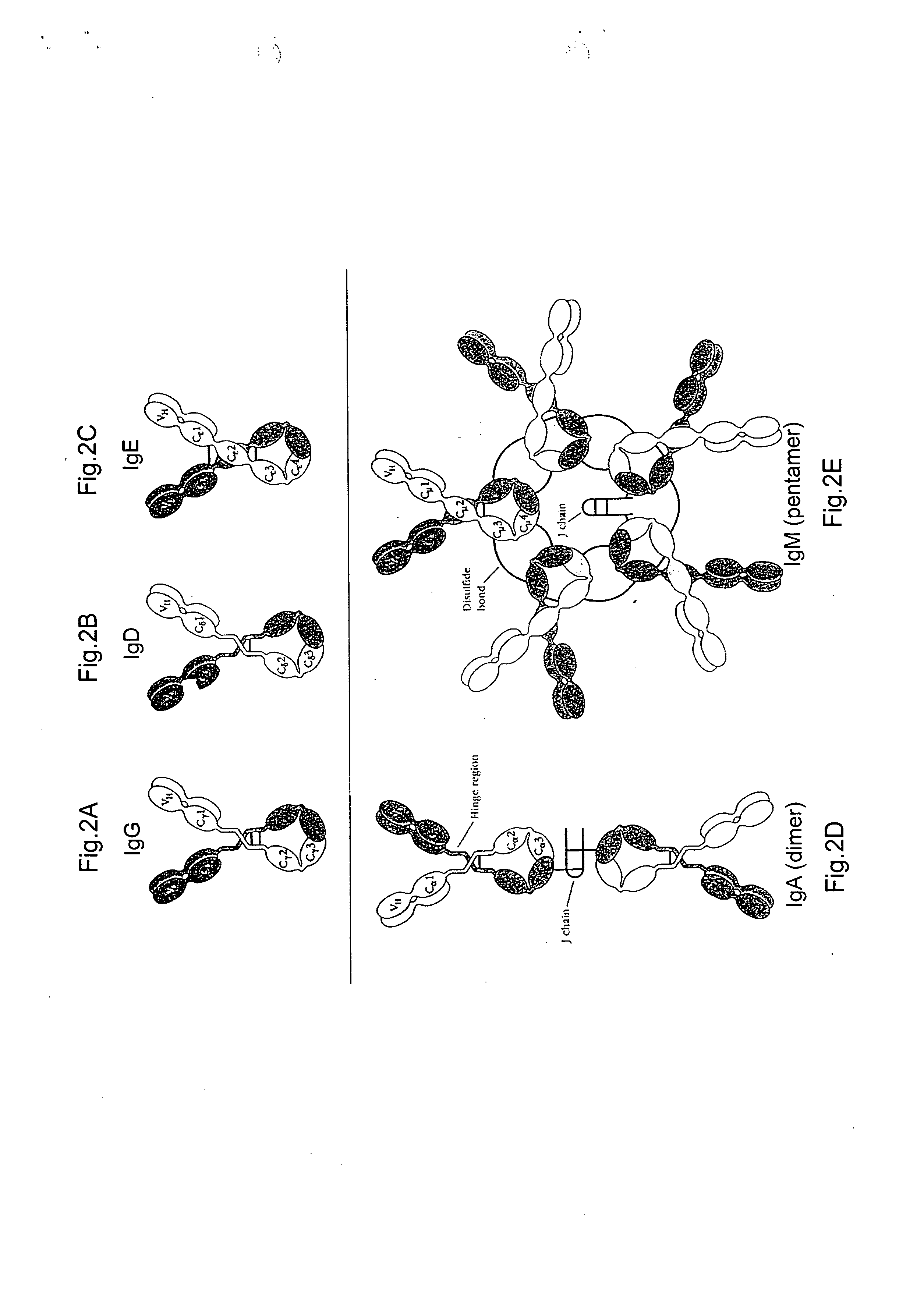
Humanized antibodies to gamma-interferon
The invention provides humanized immunoglobulins that bind to and neutralize gamma-interferon. The antibodies are useful for treatment of diseases of the immune system, particularly autoimmune diseases.
Owner:ABBOTT BIOTHERAPEUTICS CORP
Methods and materials for the growth of primate-derived primordial stem cells in feeder-free culture
Methods and materials for culturing primate-derived primordial stem cells are described. In one embodiment, a cell culture medium for growing primate-derived primordial stem cells in a substantially undifferentiated state is provided which includes a low osmotic pressure, low endotoxin basic medium that is effective to support the growth of primate-derived primordial stem cells. The basic medium is combined with a nutrient serum effective to support the growth of primate-derived primordial stem cells and a substrate selected from the group consisting of feeder cells and an extracellular matrix component derived from feeder cells. The medium further includes non-essential amino acids, an anti-oxidant, and a first growth factor selected from the group consisting of nucleosides and a pyruvate salt.
Owner:ASTERIAS BIOTHERAPEUTICS INC
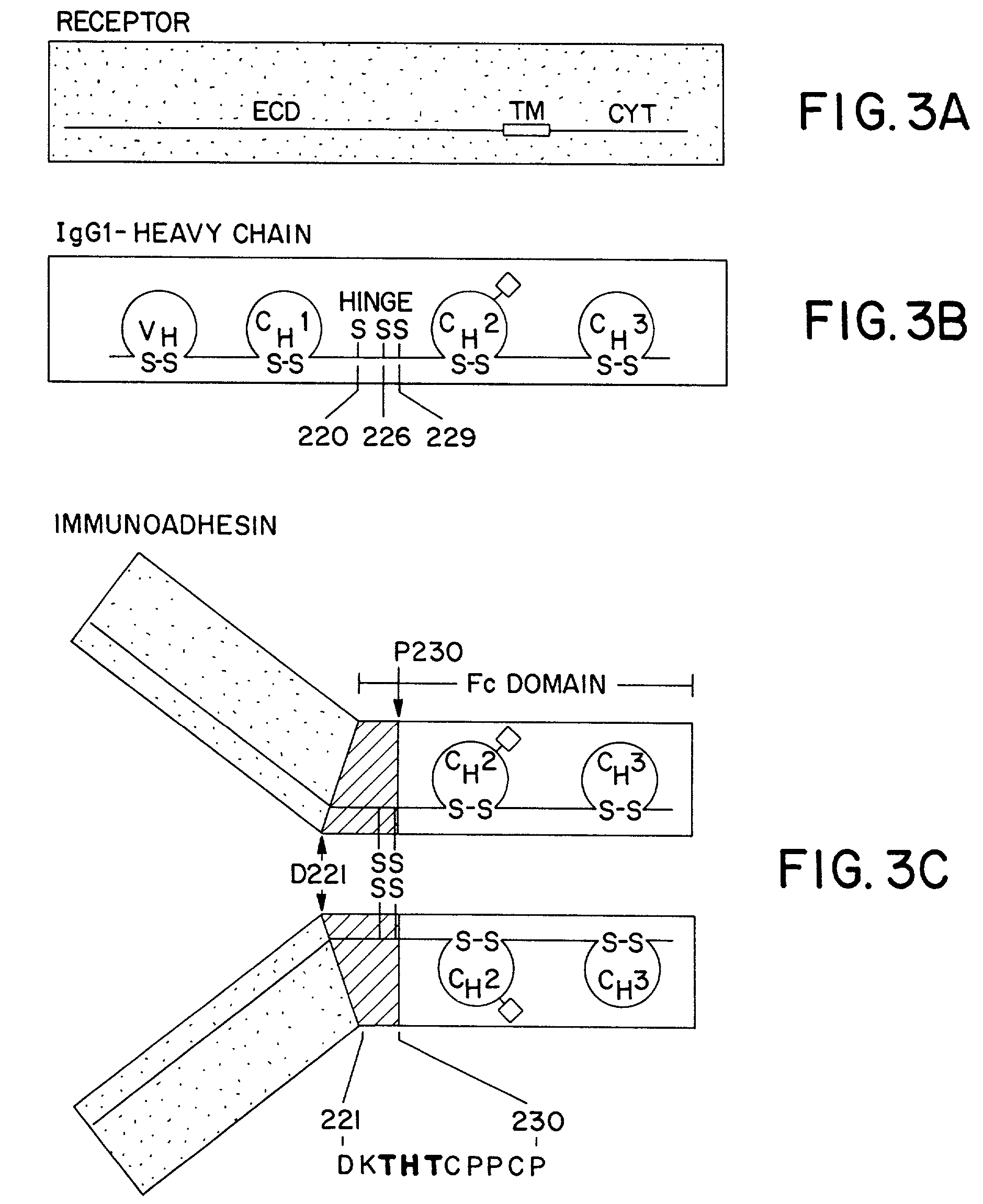
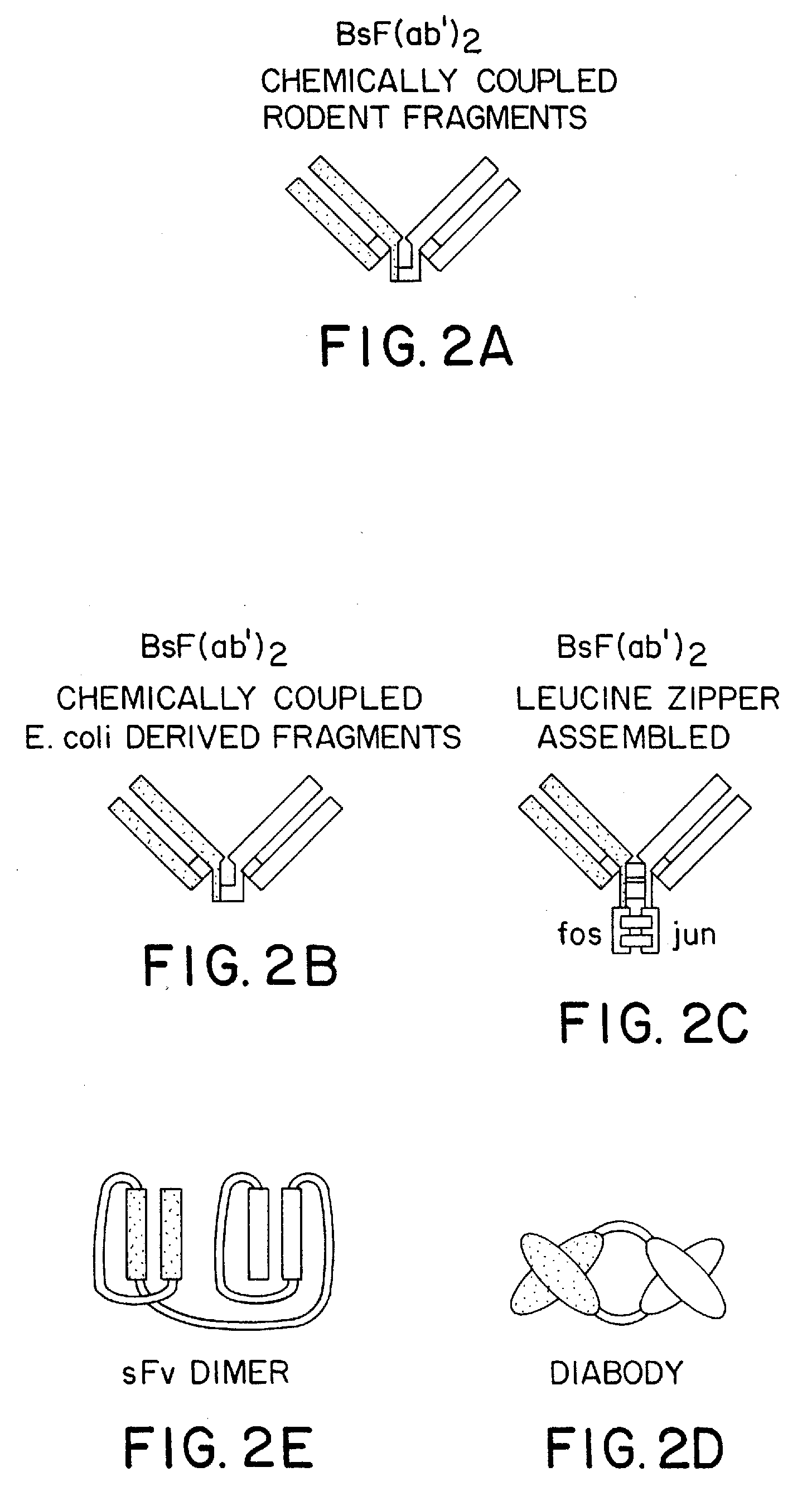
Method for making heteromultimeric polypeptides
InactiveUS7642228B2Increase productionAntibacterial agentsPeptide/protein ingredientsCrystallographyAmino acid side chain
The invention relates to a method of preparing heteromultimeric polypeptides such as bispecific antibodies, bispecific immunoadhesins and antibody-immunoadhesin chimeras. The invention also relates to the heteromultimers prepared using the method. Generally, the method involves introducing a protuberance at the interface of a first polypeptide and a corresponding cavity in the interface of a second polypeptide, such that the protuberance can be positioned in the cavity so as to promote heteromultimer formation and hinder homomultimer formation. “Protuberances” are constructed by replacing small amino acid side chains from the interface of the first polypeptide with larger side chains (e.g. tyrosine or tryptophan). Compensatory “cavities” of identical or similar size to the protuberances are created in the interface of the second polypeptide by replacing large amino acid side chains with smaller ones (e.g. alanine or threonine). The protuberance and cavity can be made by synthetic means such as altering the nucleic acid encoding the polypeptides or by peptide synthesis.
Owner:GENENTECH INC
Molecules with extended half-lives, compositions and uses thereof
InactiveUS20030190311A1High affinityExtended half-lifeCompounds screening/testingFungiIntravenous gammaglobulinIn vivo
The present invention provides molecules, including IgGs, non-IgG immunoglobulin, proteins and non-protein agents, that have increased in vivo half-lives due to the presence of an IgG constant domain, or a portion thereof that binds the FcRn, having one or more amino acid modifications that increase the affinity of the constant domain or fragment for FcRn. Such proteins and molecules with increased half-lives have the advantage that smaller amounts and or less frequent dosing is required in the therapeutic, prophylactic or diagnostic use of such molecules.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Preparation of phosphorothioate and boranophosphate oligomers
InactiveUS6160109AHigh diastereomeric excessLow costSugar derivativesOrganic-compounds/hydrides/coordination-complexes catalystsOligomerDiastereomer
Owner:MCGILL UNIV +1
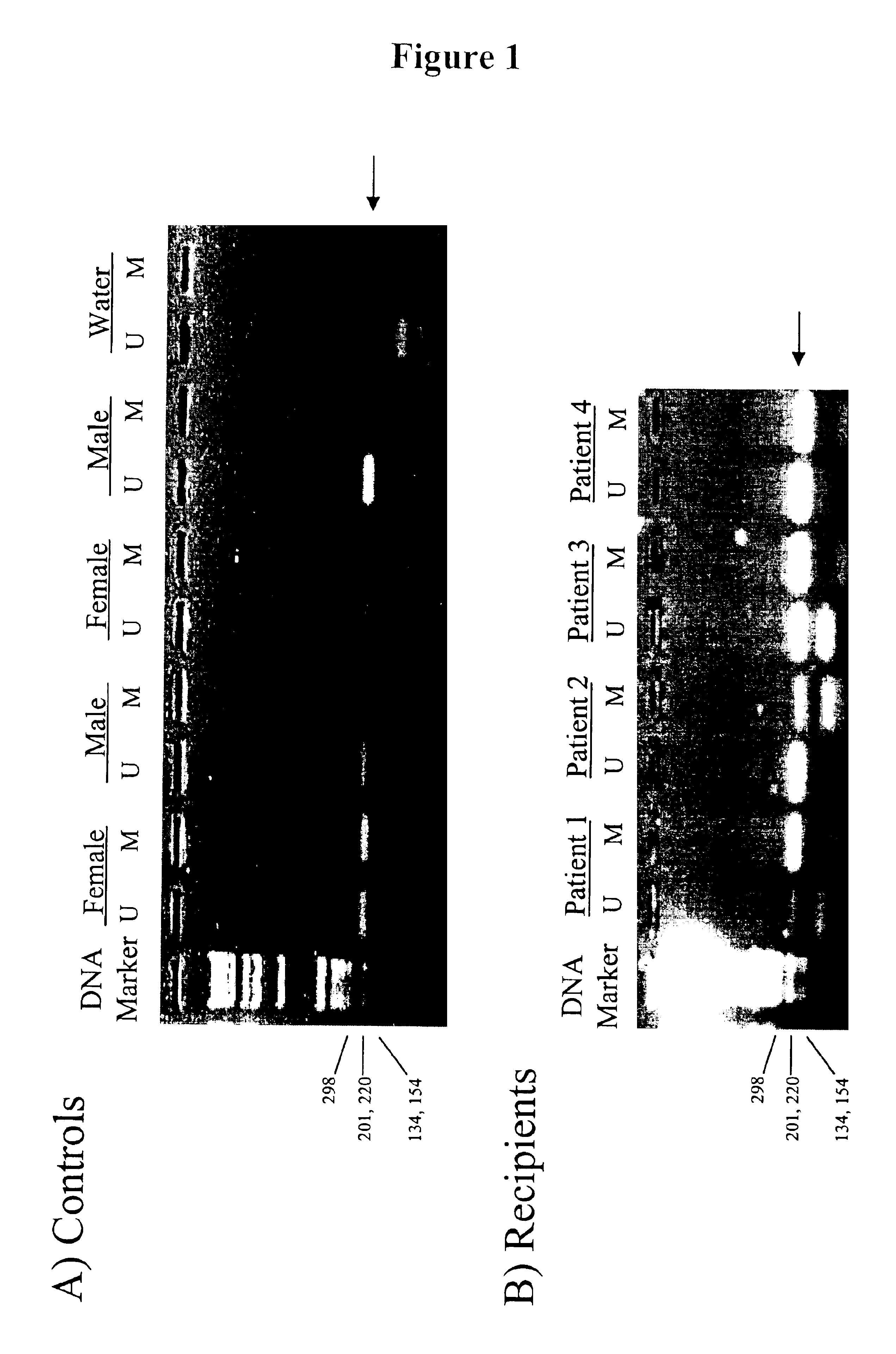
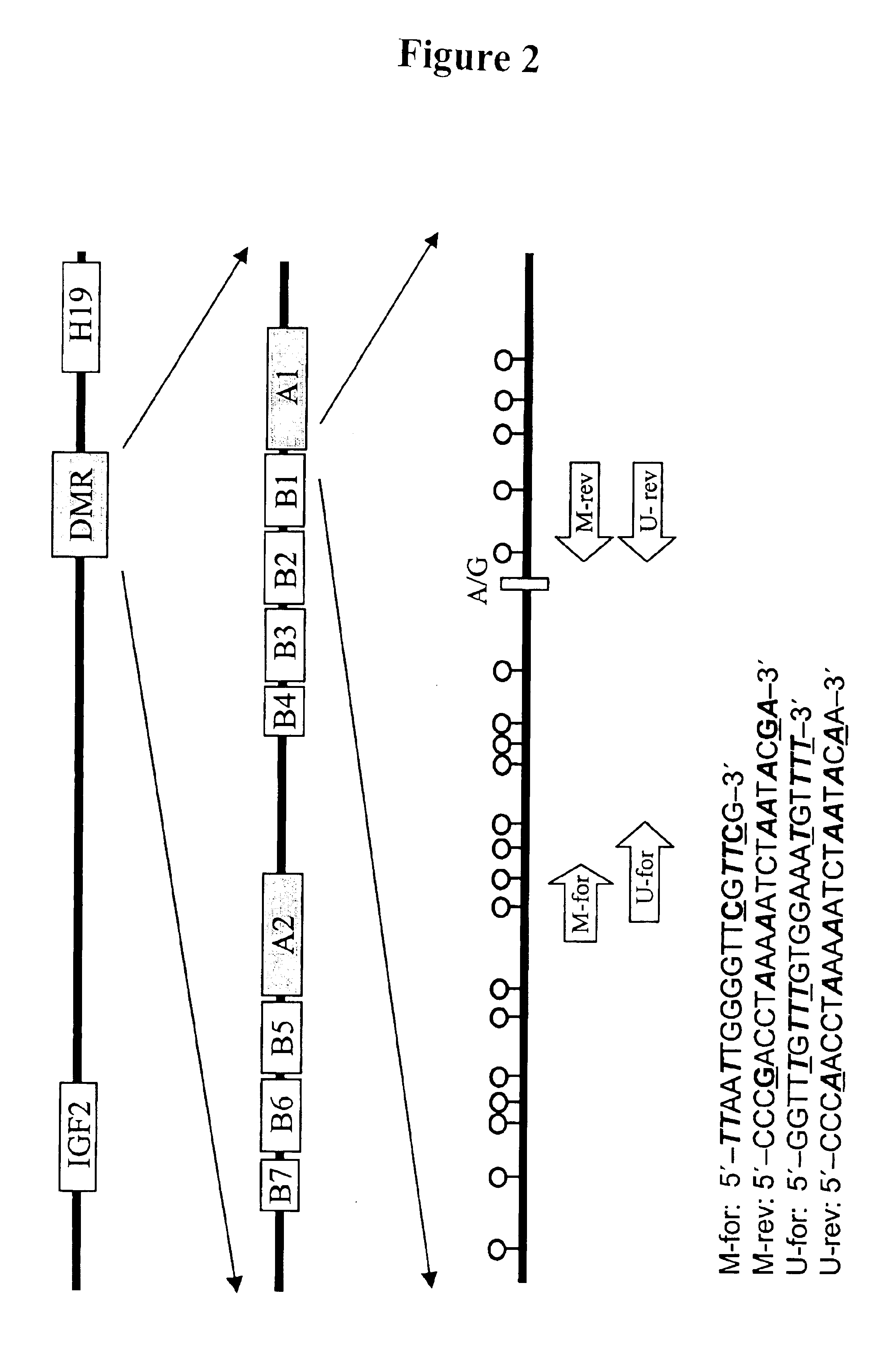
Non-invasive methods for detecting non-host DNA in a host using epigenetic differences between the host and non-host DNA
In a first aspect, the present invention features methods for differentiating DNA species originating from different individuals in a biological sample. These methods may be used to differentiate or detect fetal DNA in a maternal sample or to differentiate DNA of an organ donor from DNA of an organ recipient. In preferred embodiments, the DNA species are differentiated by observing epigenetic differences in the DNA species such as differences in DNA methylation. In a second aspect, the present invention features methods of detecting genetic abnormalities in a fetus by detecting fetal DNA in a biological sample obtained from a mother. In a third aspect, the present invention features methods for differentiating DNA species originating from an organ donor from those of an organ recipient. In a fourth aspect, the present invention features kits for differentiating DNA species originating from different individuals in a biological sample.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Non-invasive detection of fetal genetic traits
InactiveUS20050164241A1Facilitates non-invasive detectionComponent separationOther chemical processesPregnancyNon invasive
Blood plasma of pregnant women contains fetal and (generally>90%) maternal circulatory extracellular DNA. Most of said fetal DNA contains ≦500 base pairs, said maternal DNA having a greater size. Separation of circulatory extracellular DNA of <500 base pairs results in separation of fetal from maternal DNA. A fraction of a blood plasma or serum sample of a pregnant woman containing, due to size separation (e.g. by chromatography, density gradient centrifugation or nanotechnological methods), extracellular DNA substantially comprising ≦500 base pairs is useful for non-invasive detection of fetal genetic traits (including the fetal RhD gene in pregnancies at risk for HDN; fetal Y chromosome-specific sequences in pregnancies at risk for X chromosome-linked disorders; chromosomal aberrations; hereditary Mendelian genetic disorders and corresponding genetic markers; and traits decisive for paternity determination) by e.g. PCR, ligand chain reaction or probe hybridization techniques, or nucleic acid arrays.
Owner:SEQUENOM INC
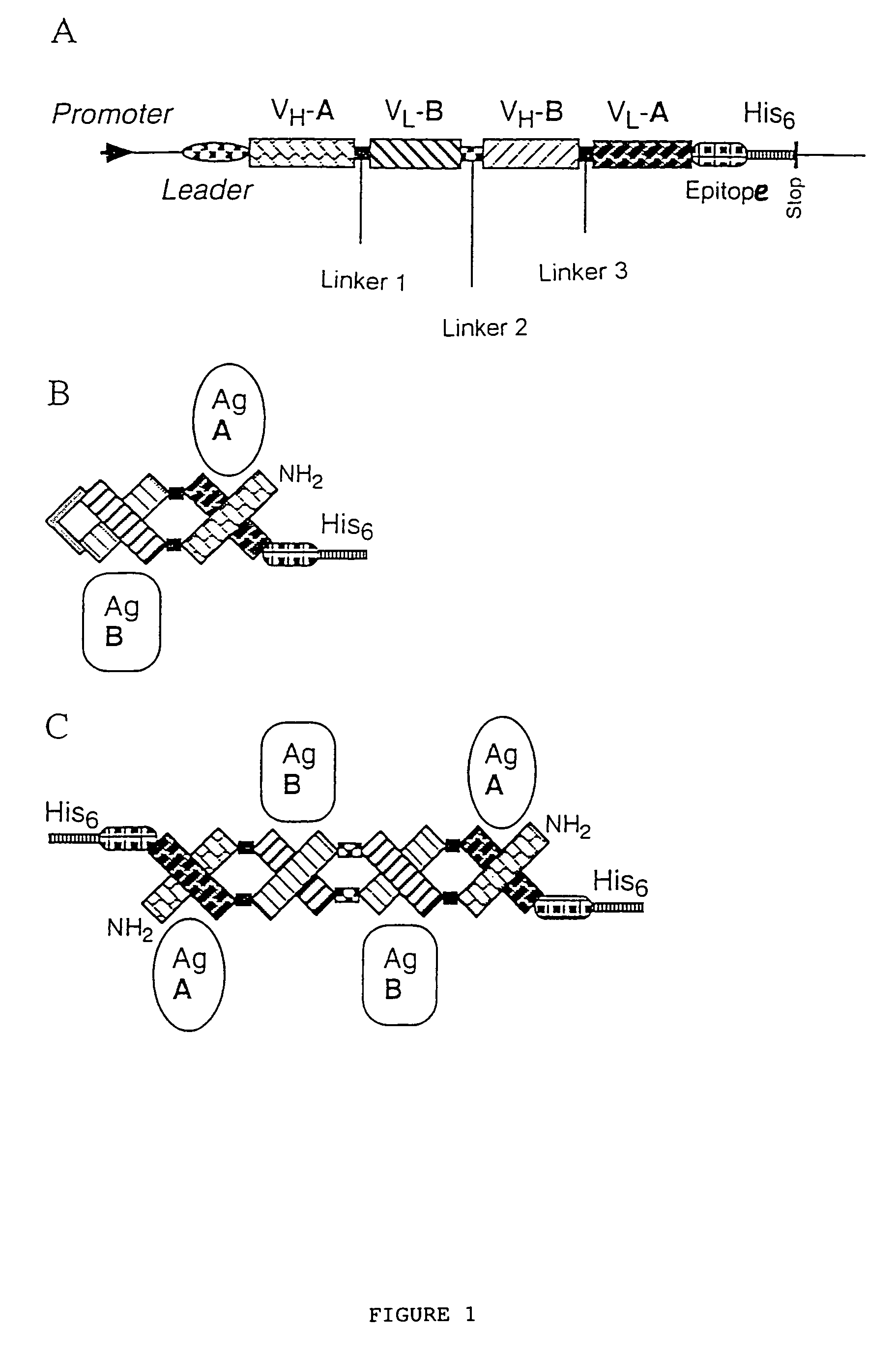
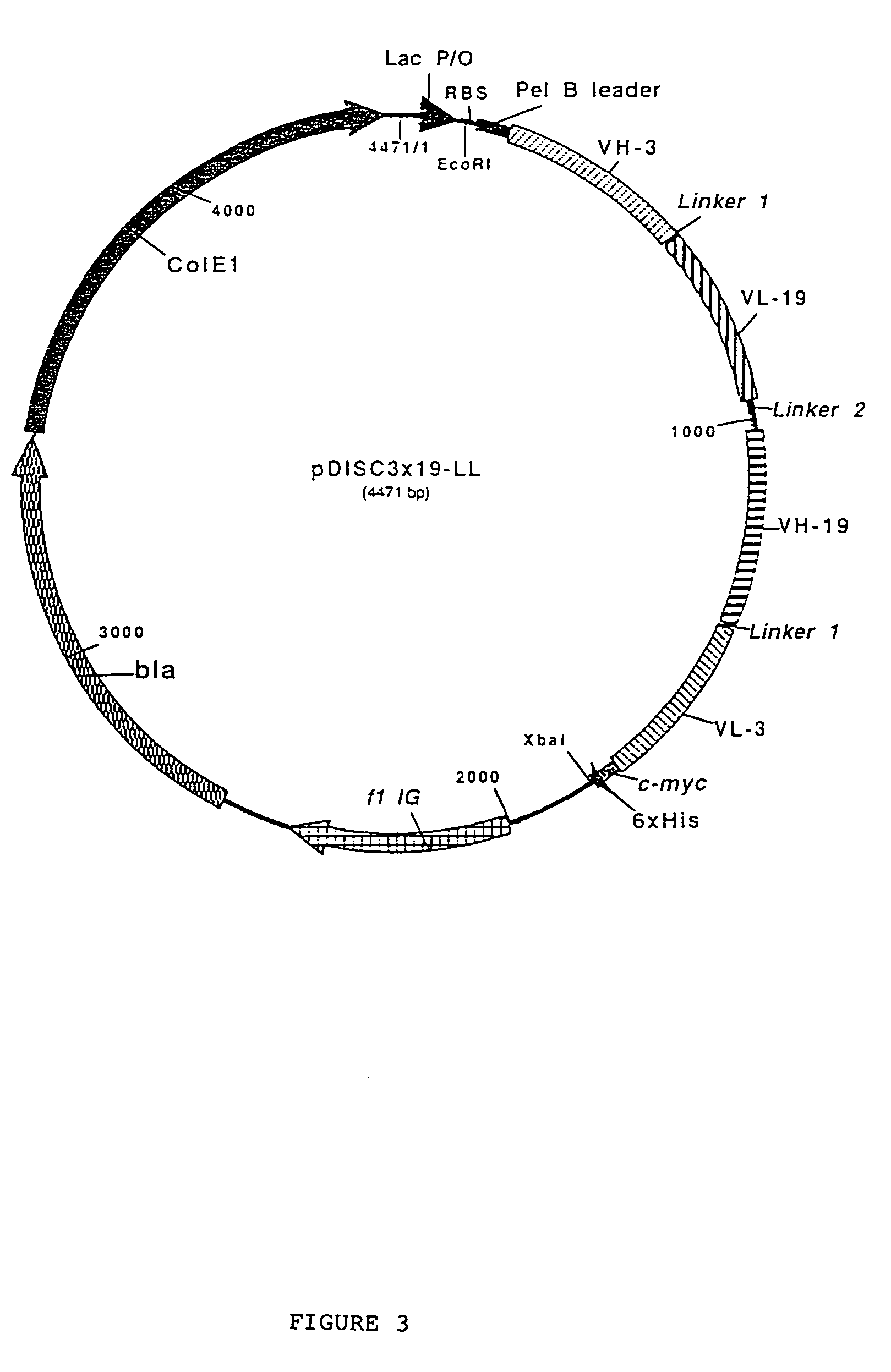
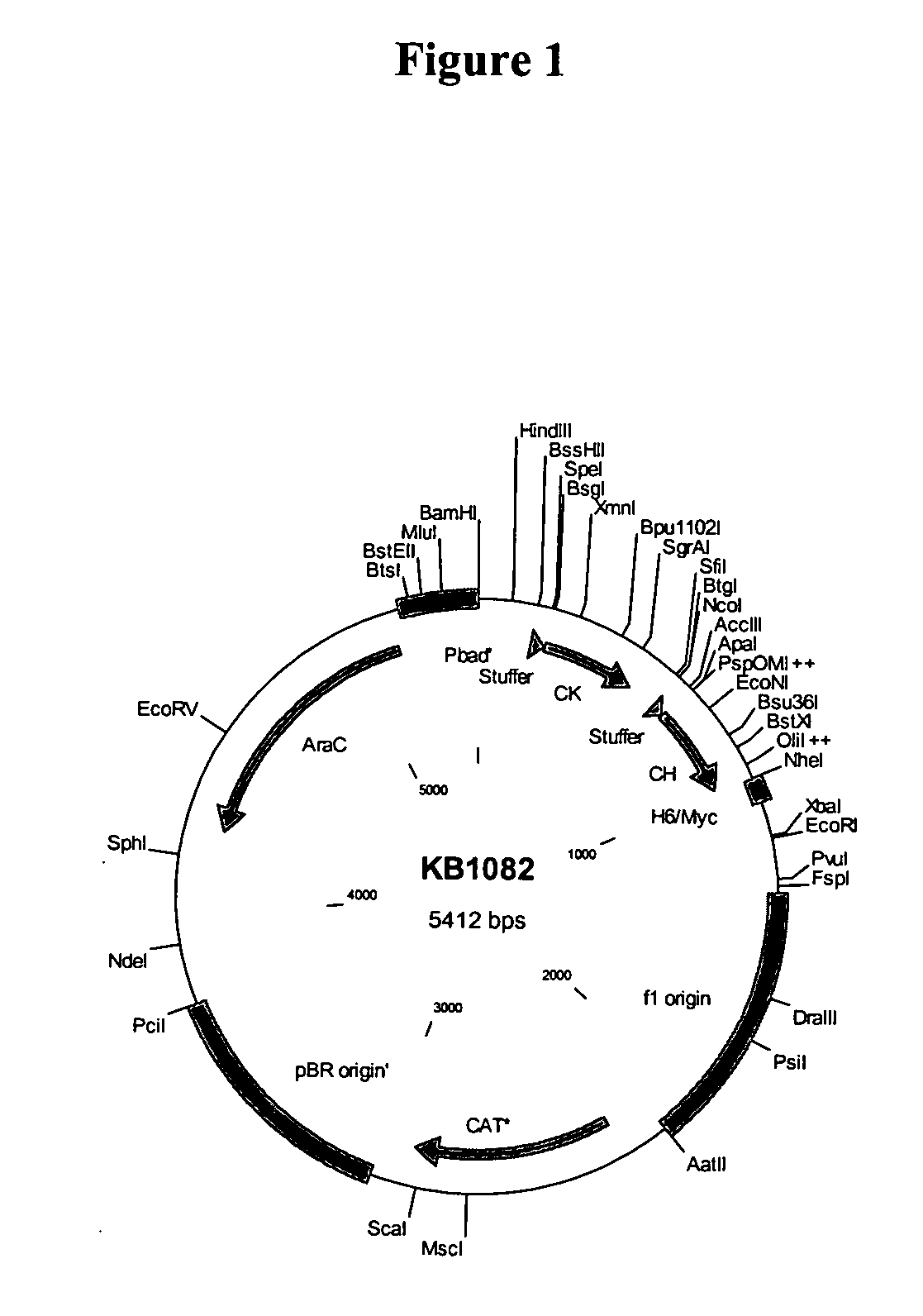
Multivalent antibody constructs
InactiveUS7129330B1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsVariable domainPlasmid
The present invention relates to multivalent Fvantibody construct having at least four variable domains which are linked with each over via the peptide linkers 1, 2 and 3. The invention also concerns expression plasmids which code for such an Fvantibody construct and a method of producing the Fvantibody constructs as well as their use.
Owner:DEUTES KREBSFORSCHUNGSZENT STIFTUNG DES OFFENTLICHEN RECHTS
Method for making heteromultimeric polypeptides
InactiveUS20070014794A1Increase productionAntibacterial agentsPeptide/protein ingredientsCrystallographyAmino acid side chain
The invention relates to a method of preparing heteromultimeric polypeptides such as bispecific antibodies, bispecific immunoadhesins and antibody-immunoadhesin chimeras. The invention also relates to the heteromultimers prepared using the method. Generally, the method involves introducing a protuberance at the interface of a first polypeptide and a corresponding cavity in the interface of a second polypeptide, such that the protuberance can be positioned in the cavity so as to promote heteromultimer formation and hinder homomultimer formation. “Protuberances” are constructed by replacing small amino acid side chains from the interface of the first polypeptide with larger side chains (e.g. tyrosine or tryptophan). Compensatory “cavities” of identical or similar size to the protuberances are created in the interface of the second polypeptide by replacing large amino acid side chains with smaller ones (e.g. alanine or threonine). The protuberance and cavity can be made by synthetic means such as altering the nucleic acid encoding the polypeptides or by peptide synthesis.
Owner:GENENTECH INC
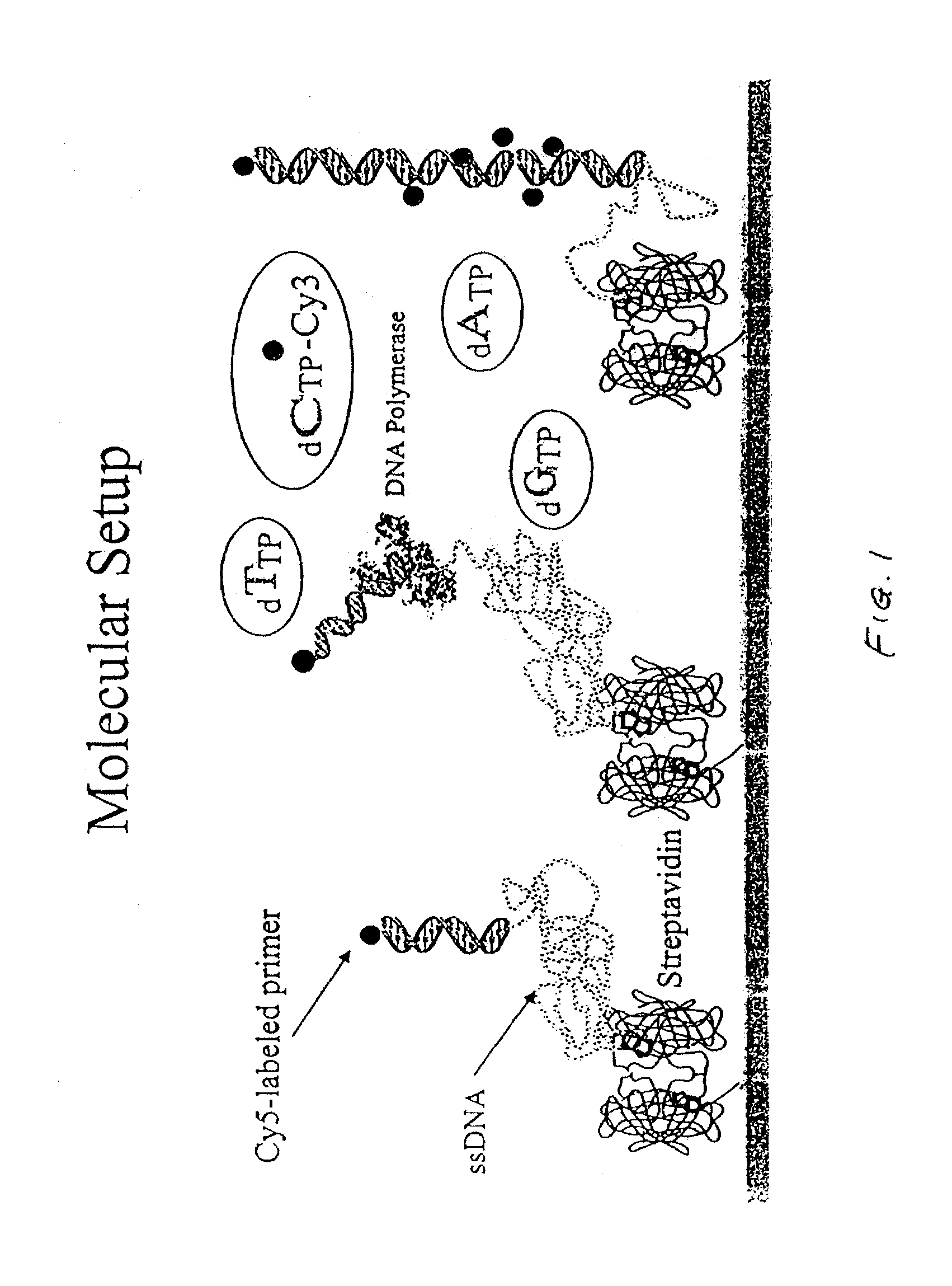
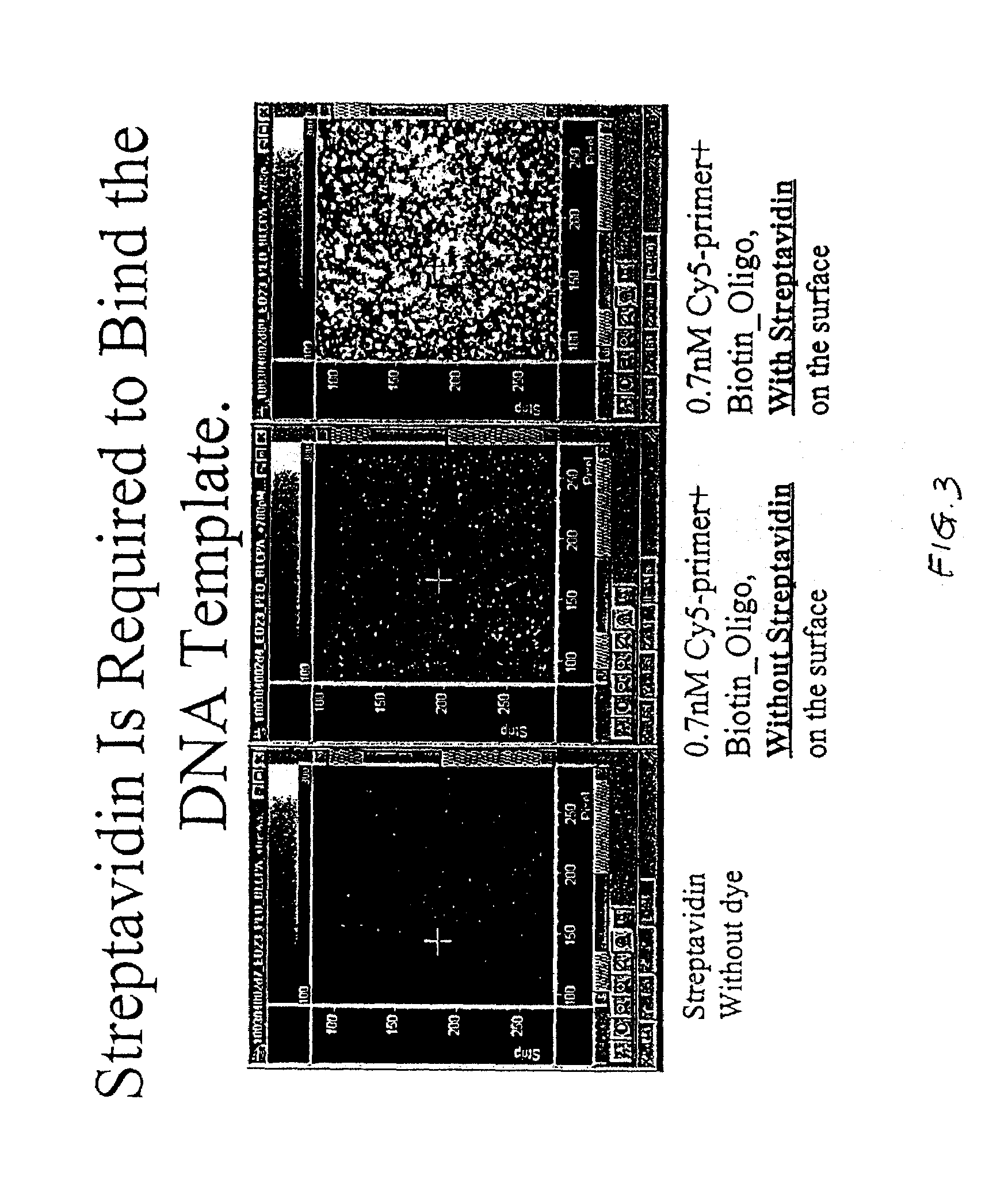
Method of determining the nucleotide sequence of oligonucleotides and DNA molecules
InactiveUS7037687B2Eliminate needBioreactor/fermenter combinationsBiological substance pretreatmentsOligonucleotide primersThermopile
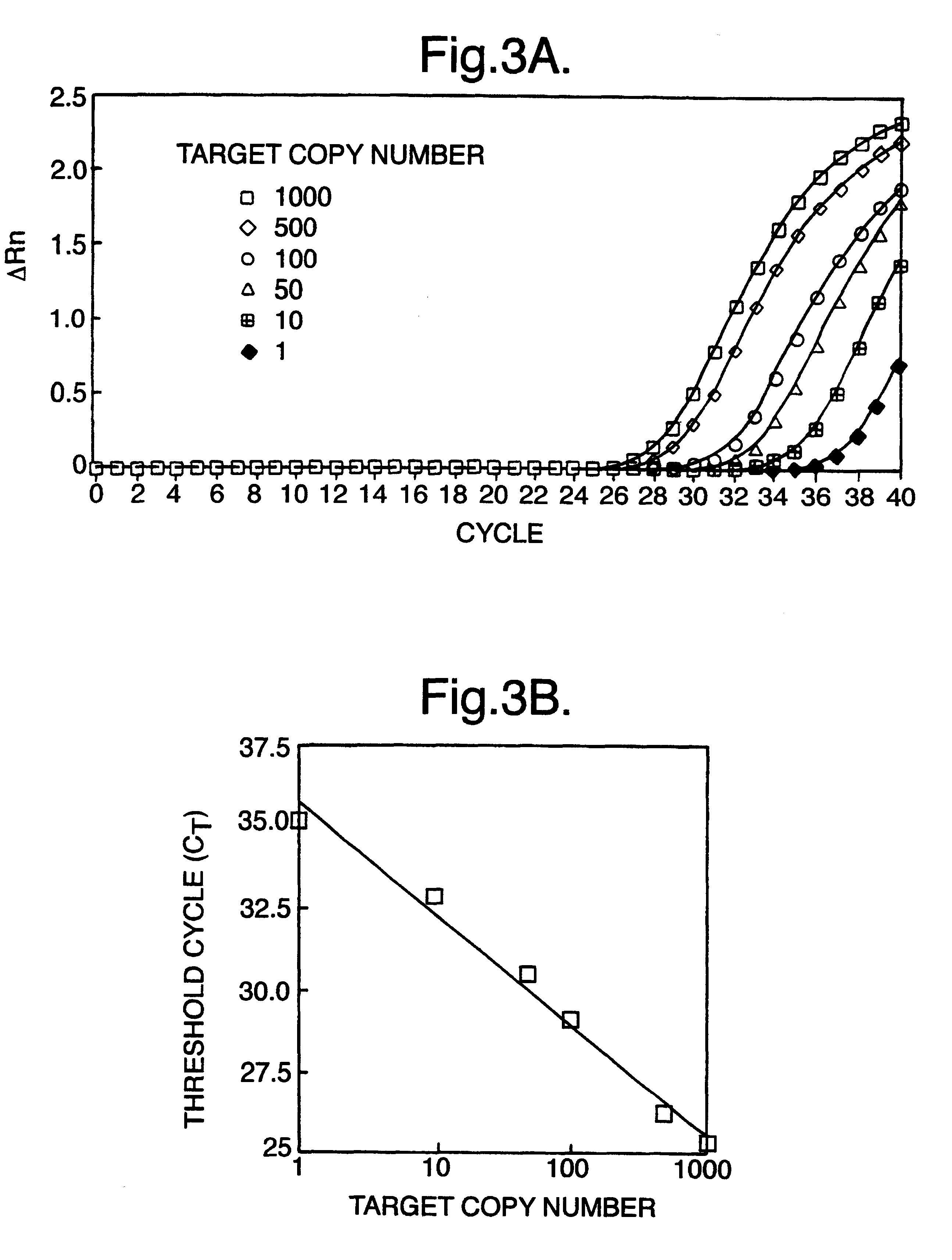
The present invention relates to a novel method for analyzing nucleic acid sequences based on real-time detection of DNA polymerase-catalyzed incorporation of each of the four nucleotide bases, supplied individually and serially in a microfluidic system, to a reaction cell containing a template system comprising a DNA fragment of unknown sequence and an oligonucleotide primer. Incorporation of a nucleotide base into the template system can be detected by any of a variety of methods including but not limited to fluorescence and chemiluminescence detection. Alternatively, microcalorimetic detection of the heat generated by the incorporation of a nucleotide into the extending template system using thermopile, thermistor and refractive index measurements can be used to detect extension reactions.
Owner:ALBERTA UNIV OF +1
Combinatorial libraries of monomer domains
InactiveUS20050221384A1Specificity of bindingEasy screeningPeptide librariesMicrobiological testing/measurementMonomerChemistry
Methods for identifying discrete monomer domains and immuno-domains with a desired property are provided. Methods for generating multimers from two or more selected discrete monomer domains are also provided, along with methods for identifying multimers possessing a desired property. Presentation systems are also provided which present the discrete monomer and / or immuno-domains, selected monomer and / or immuno-domains, multimers and / or selected multimers to allow their selection. Compositions, libraries and cells that express one or more library member, along with kits and integrated systems, are also included in the present invention.
Owner:AMGEN MOUNTAIN VIEW
Method of determining the nucleotide sequence of oligonucleotides and DNA molecules
InactiveUS6780591B2Eliminate needHigh purityMicrobiological testing/measurementRecombinant DNA-technologyFluorescenceOligonucleotide primers
The present invention relates to a novel method for analyzing nucleic acid sequences based on real-time detection of DNA polymerase-catalyzed incorporation of each of the four nucleotide bases, supplied individually and serially in a microfluidic system, to a reaction cell containing a template system comprising a DNA fragment of unknown sequence and an oligonucleotide primer. Incorporation of a nucleotide base into the template system can be detected by any of a variety of methods including but not limited to fluorescence and chemiluminescence detection. Alternatively, microcalorimetic detection of the heat generated by the incorporation of a nucleotide into the extending template system using thermopile, thermistor and refractive index measurements can be used to detect extension reactions.
Owner:LIFE TECH CORP
Labeled nucleoside polyphosphates
InactiveUS7041812B2Sugar derivativesMaterial analysis by observing effect on chemical indicatorNucleic acid detectionFluorescence
The present invention describes new compositions of matter in the form of labeled nucleoside polyphosphates with four or more phosphates. In addition compositions of nucleoside polyphosphates with four or more phosphates that are substrates for nucleic acid polymerases with enhanced substrate properties and methods of using these nucleoside polyphosphates for nucleic acid detection, characterization and quantification are described. The compositions provided by this invention include nucleoside polyphosphate, dideoxynucleoside polyphosphate, or deoxynucleoside polyphosphate analogues which have colorimetric, chemiluminescent, or fluorescent moieties, mass tags or an electrochemical tags attached to the terminal-phosphate. When a nucleic acid polymerase uses this analogue as a substrate, an enzyme-activatable label would be present on the inorganic polyphosphate by-product of phosphoryl transfer. Removal of the polyphosphate product of phosphoryl transfer via phosphate or polyphosphate transferring enzyme leads to a detectable change in the label attached thereon. When the polymerase assay is performed in the presence of a phosphatase, there is provided a convenient method for real-time monitoring of DNA or RNA synthesis and detection of a target nucleic acid.
Owner:GLOBAL LIFE SCI SOLUTIONS USA LLC
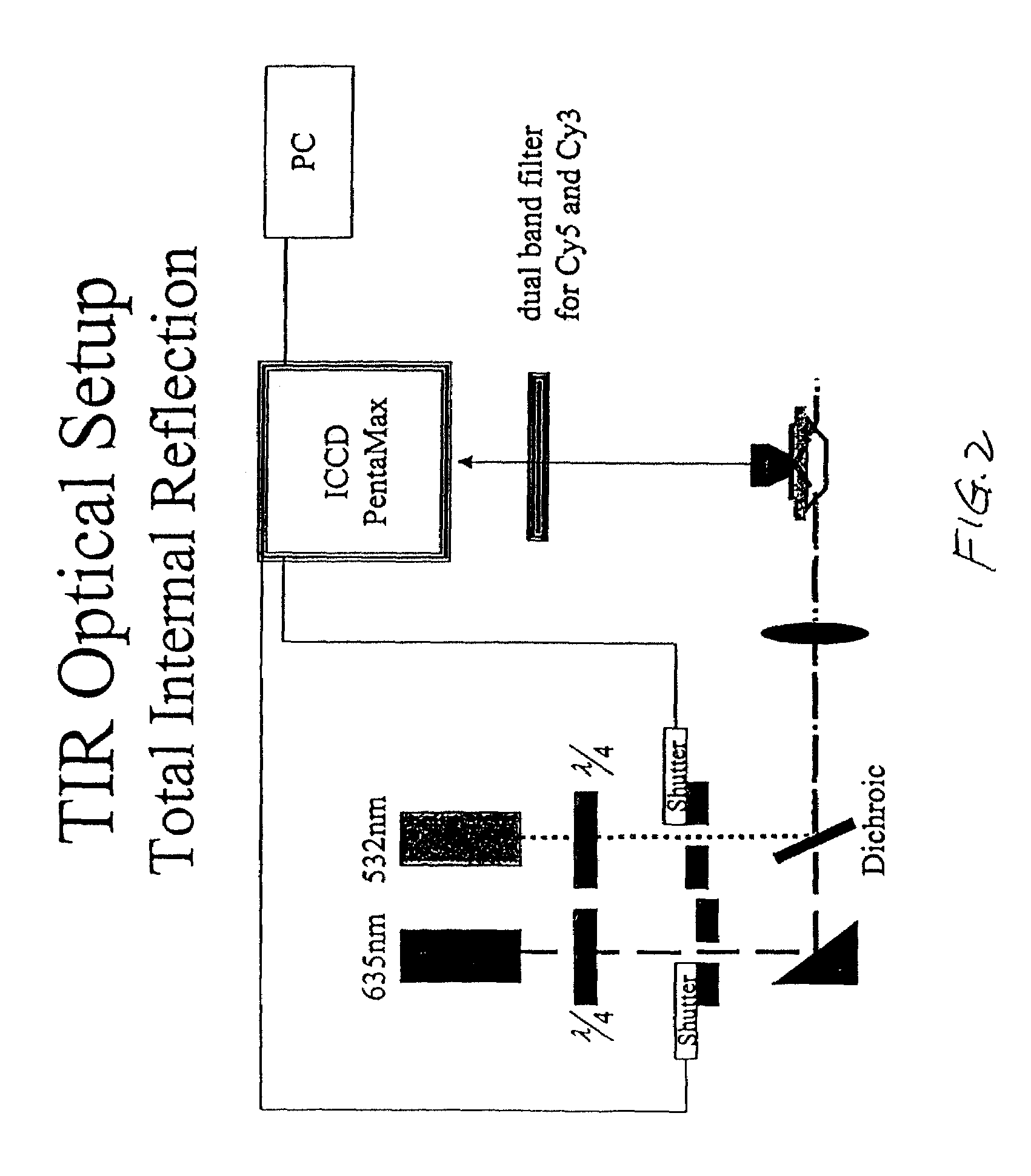
Methods and apparatus for analyzing polynucleotide sequences by asynchronous base extension
InactiveUS7297518B2Continues monitoring of the incorporation is facilitatedBioreactor/fermenter combinationsBiological substance pretreatmentsTotal internal reflectionFluorescence
The invention provides methods and apparatus for analyzing polynucleotide sequences by asynchronous base extension. Some applications of the invention utilize total internal reflection fluorescence microscopy to image polynucleotide molecules at single molecule resolution.
Owner:CALIFORNIA INST OF TECH
Fc-erythropoietin fusion protein with improved pharmacokinetics
InactiveUS20050202538A1Improve pharmacokineticsSimplify erythropoietin therapyPeptide/protein ingredientsAntibody mimetics/scaffoldsErythropoietinNucleic acid
Owner:MERCK PATENT GMBH
Antibody specificity transfer using minimal essential binding determinants
The present invention provides methods of making antibodies having the binding specificity of a reference antibody. Antibodies generated by the methods of the inventions have at least one minimal essential binding specificity determinant from a heavy chain or light chain CDR3 from the reference antibody. The method can be used, e.g., in humanization procedures. The invention also provides libraries and antibodies made in accordance with the methods.
Owner:HUMANIGEN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com