Patents
Literature
469 results about "Determinant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
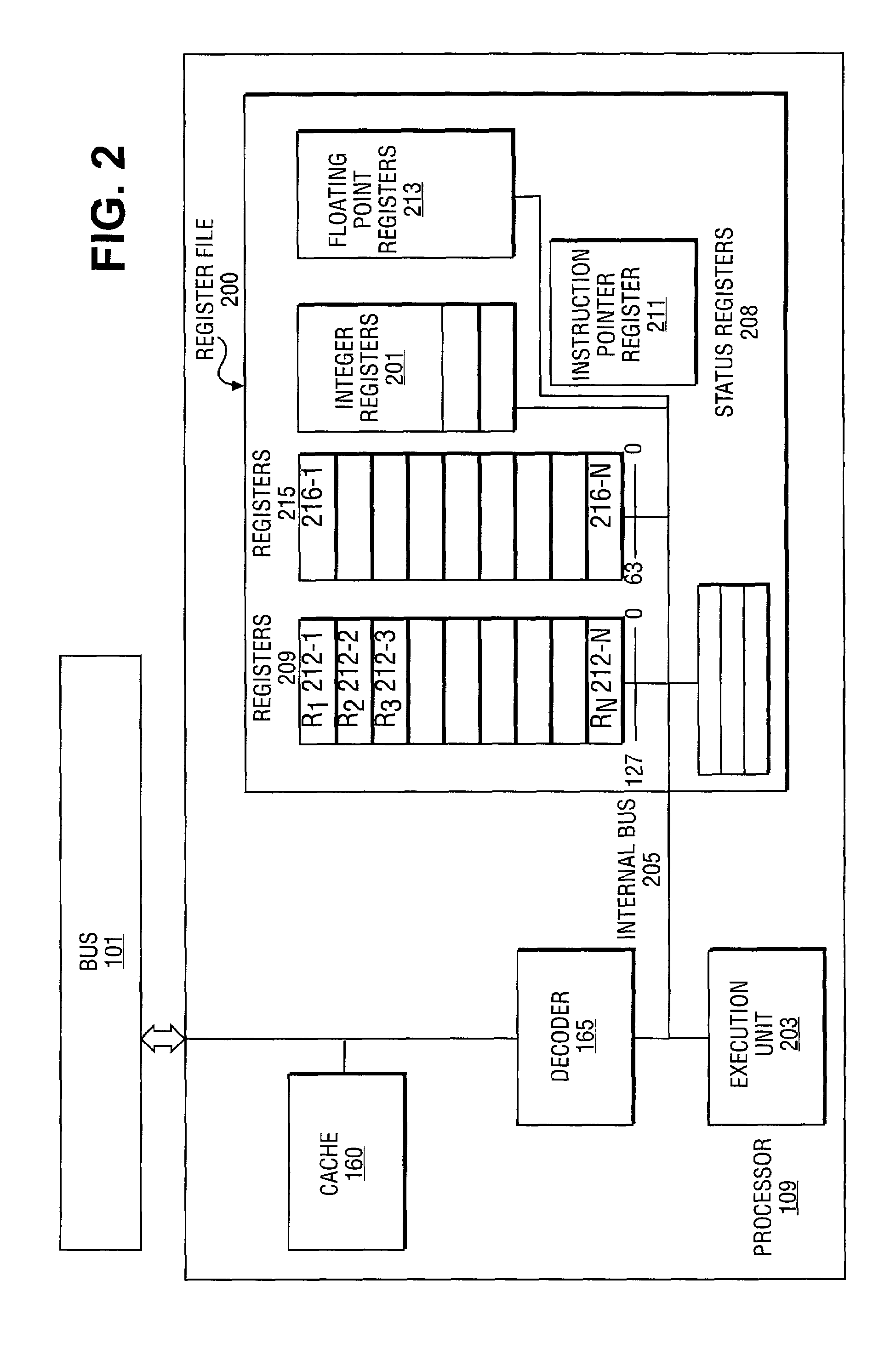
In linear algebra, the determinant is a scalar value that can be computed from the elements of a square matrix and encodes certain properties of the linear transformation described by the matrix. The determinant of a matrix A is denoted det(A), det A, or |A|. Geometrically, it can be viewed as the volume scaling factor of the linear transformation described by the matrix. This is also the signed volume of the n-dimensional parallelepiped spanned by the column or row vectors of the matrix. The determinant is positive or negative according to whether the linear mapping preserves or reverses the orientation of n-space.
Antibody specificity transfer using minimal essential binding determinants
The present invention provides methods of making antibodies having the binding specificity of a reference antibody. Antibodies generated by the methods of the inventions have at least one minimal essential binding specificity determinant from a heavy chain or light chain CDR3 from the reference antibody. The method can be used, e.g., in humanization procedures. The invention also provides libraries and antibodies made in accordance with the methods.
Owner:HUMANIGEN INC
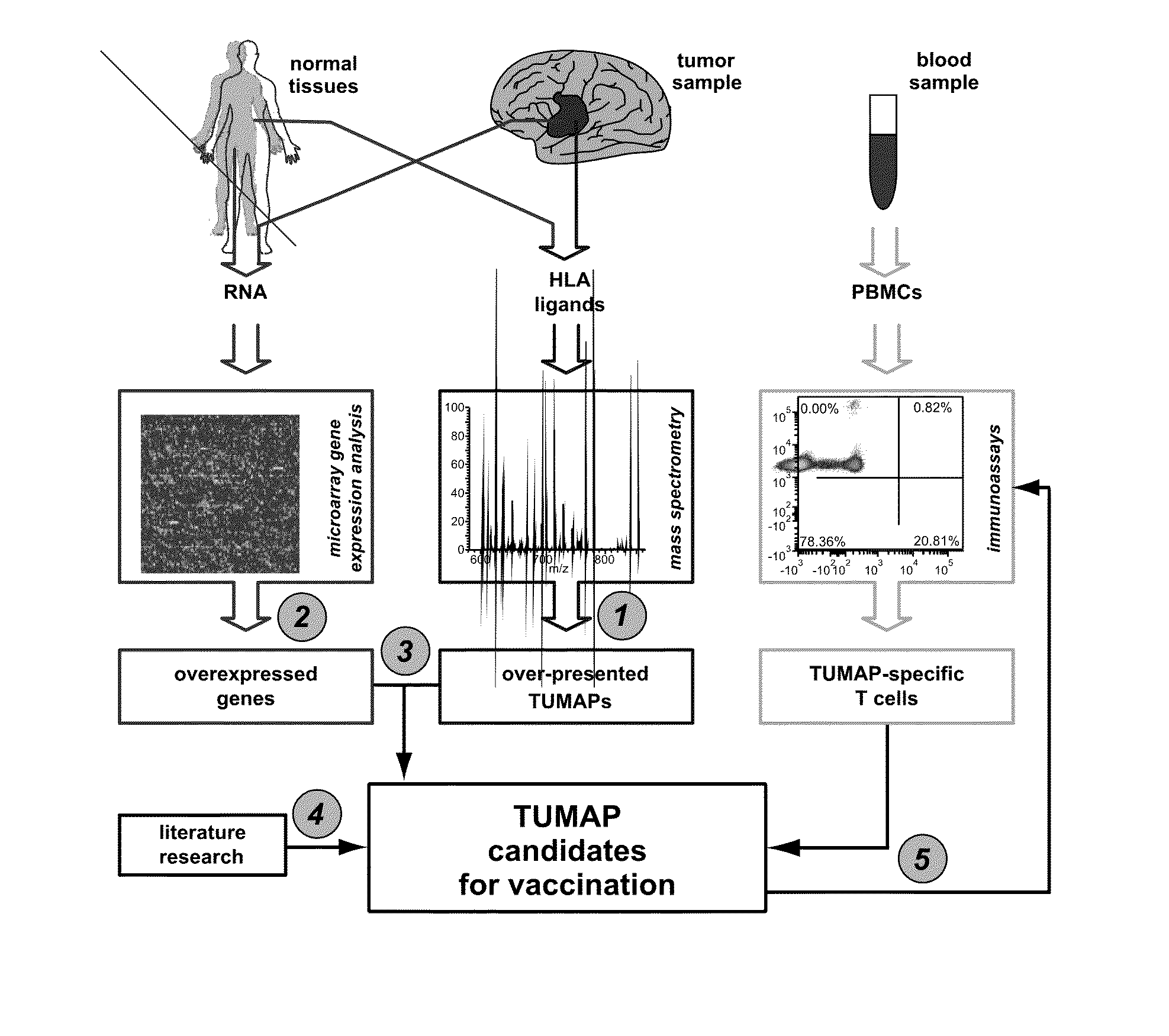
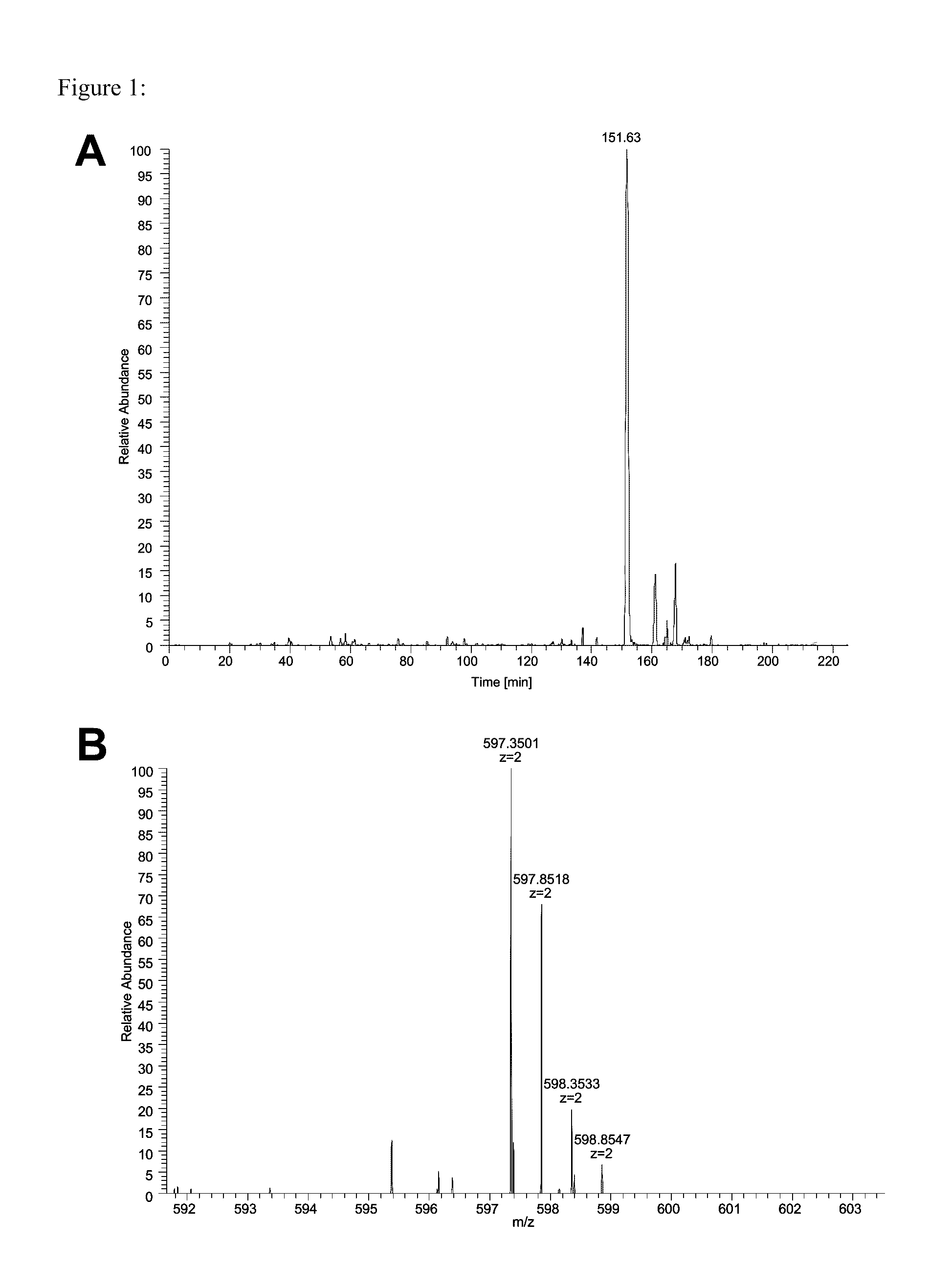
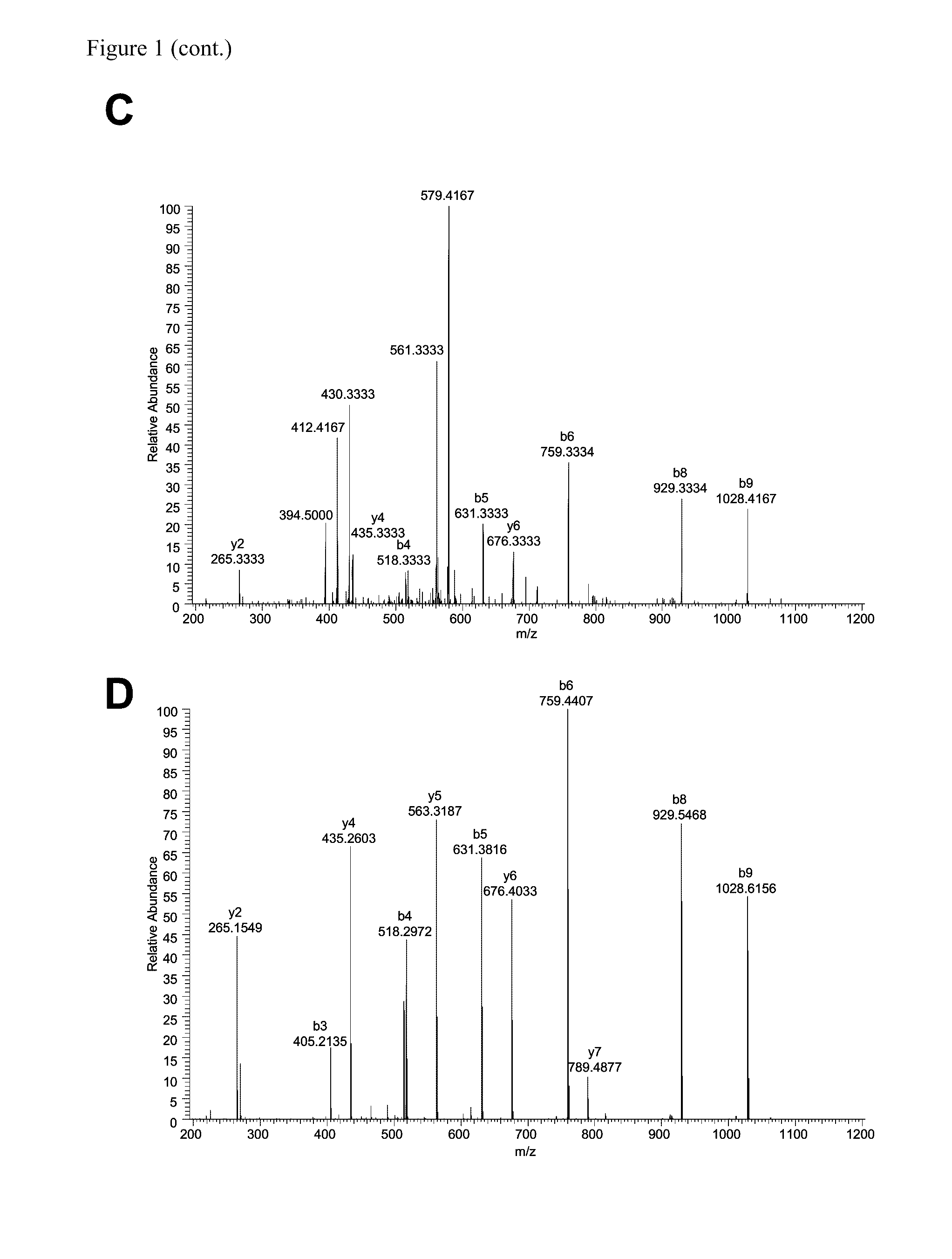
Method for differentially quantifying naturally processed hla-restricted peptides for cancer, autoimmune and infectious diseases immunotherapy development
ActiveUS20130096016A1Efficient use ofBiological material analysisLibrary member identificationDiseaseAntigen
The invention relates to a method for quantitatively identifying relevant HLA-bound peptide antigens from primary tissue specimens on a large scale without labeling approaches. This method can not only be used for the development of peptide vaccines, but is also highly valuable for a molecularly defined immunomonitoring and the identification of new antigens for any immunotherapeutic strategy in which HLA-restricted antigenic determinants function as targets, such as a variety of subunit vaccines or adoptive T-cell transfer approaches in cancer, or infectious and autoimmune diseases.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
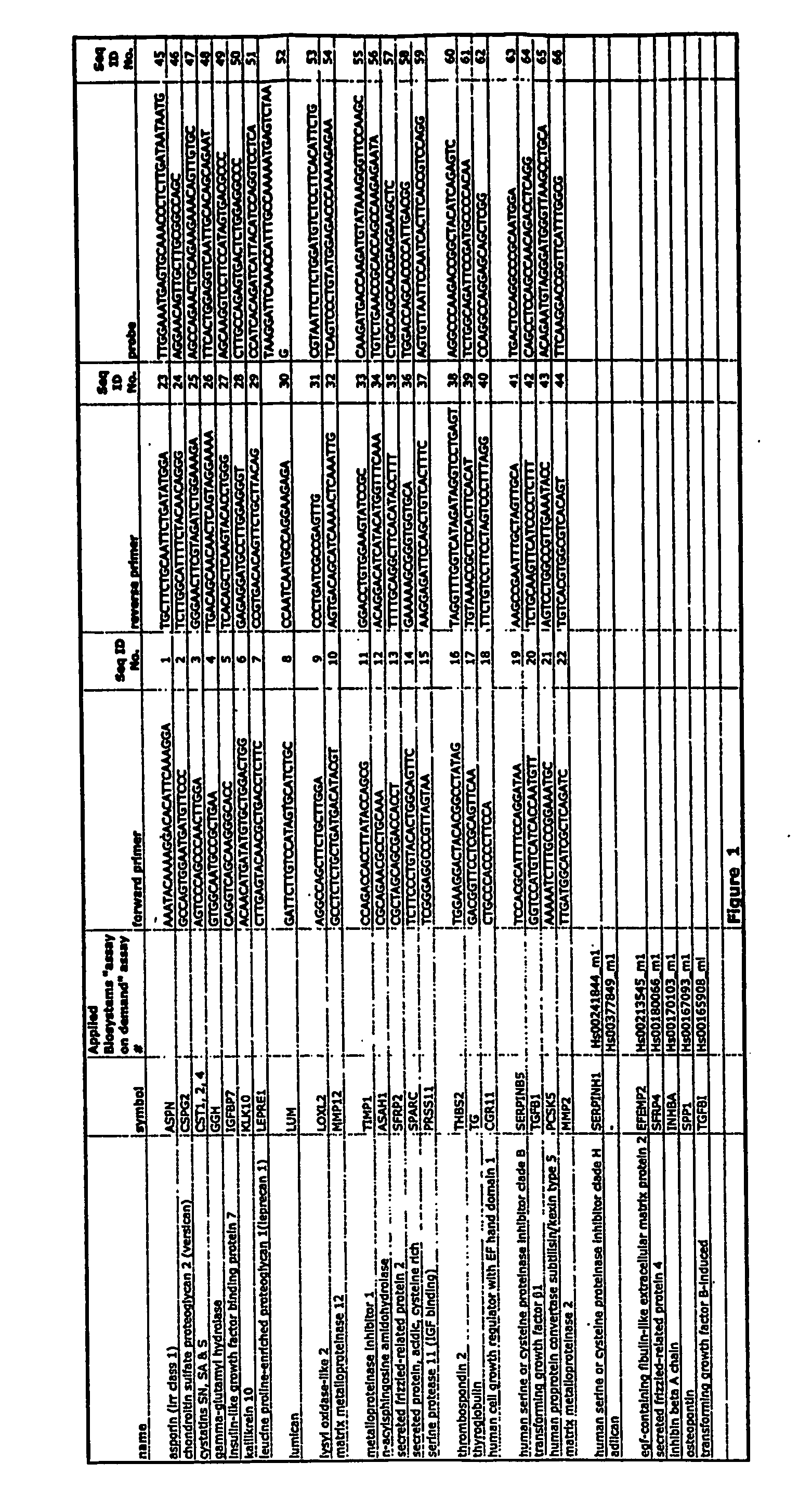
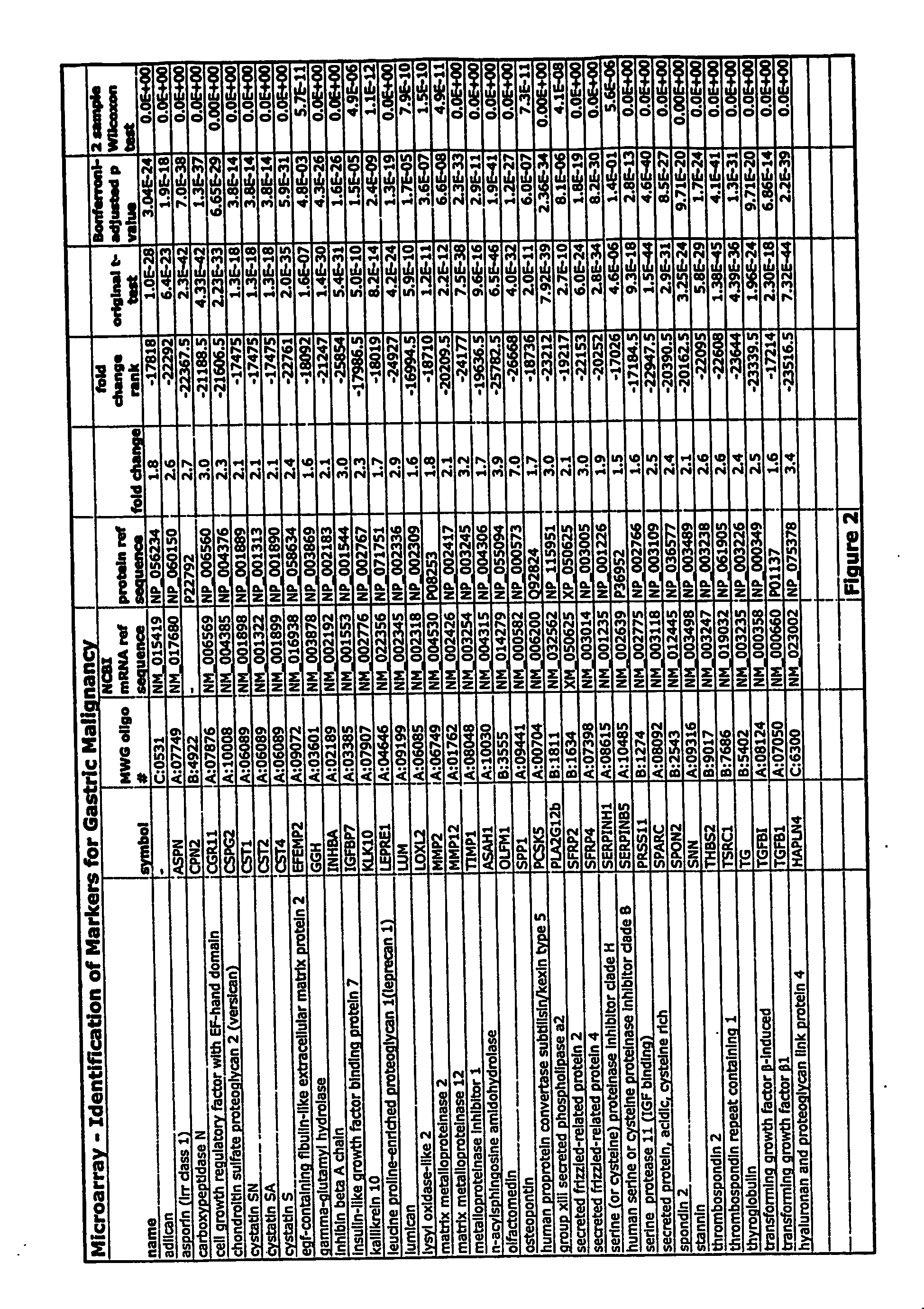
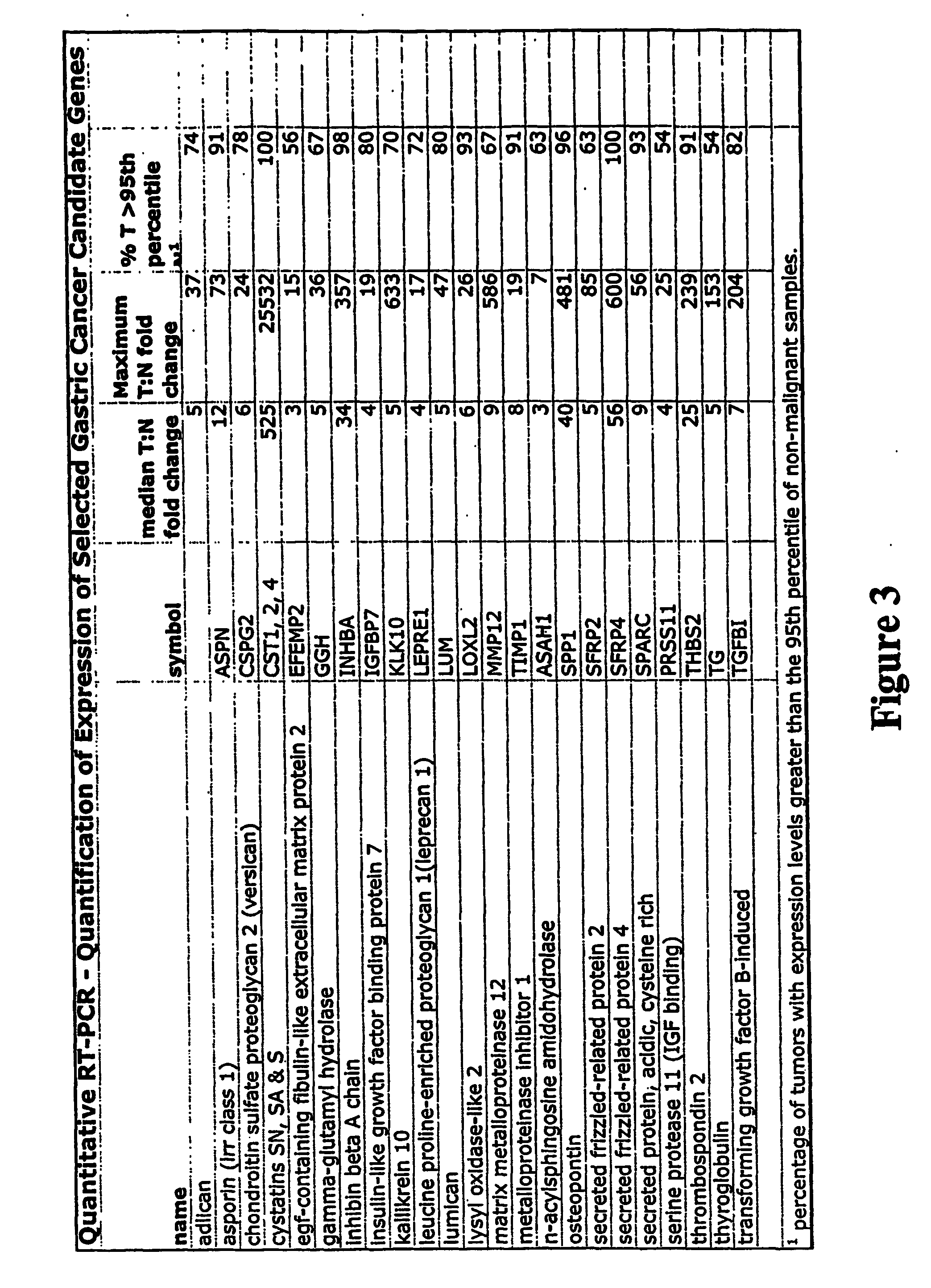
Markers for detection of gastric cancer
InactiveUS20070184439A1Reduce frequencyHigh expressionMicrobiological testing/measurementEnzymology/microbiology apparatusAbnormal tissue growthHigh concentration
Early detection of tumors is a major determinant of survival of patients suffering from tumors, including gastric tumors. Members of the GTM gene family can be over-expressed in gastric tumor tissue and other tumor tissue, and thus can be used as markers for gastric and other types of cancer. GTM proteins can be released from cancer cells, and can reach sufficiently high concentrations in the serum and / or other fluids to permit their detection. Thus, methods and test kits for detection and quantification of GTM can provide a valuable tool for diagnosis of gastric cancer.
Owner:PACIFIC EDGE
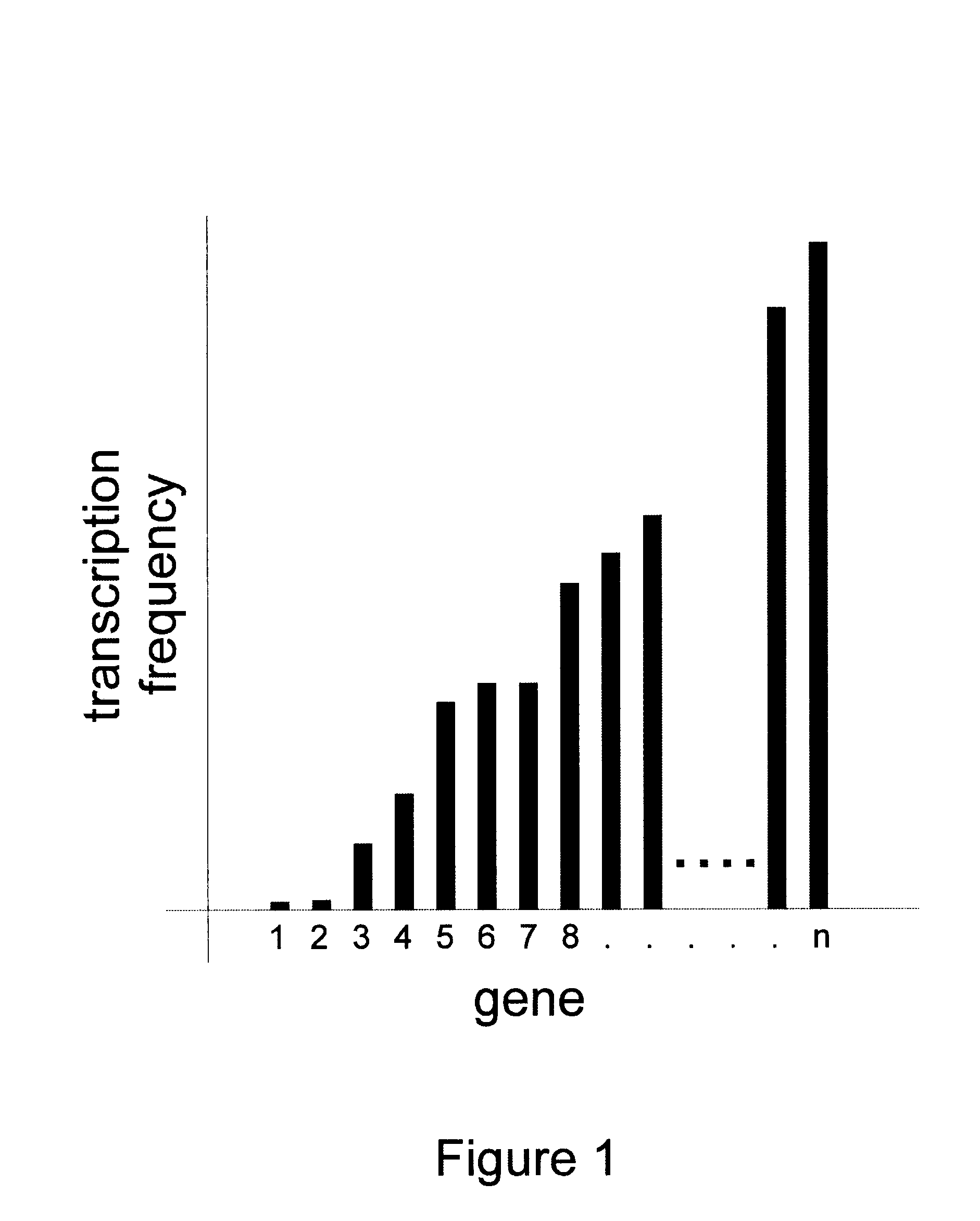
Methods for gene array analysis of nuclear runoff transcripts
InactiveUS6617112B2Rapid and efficient and extensive analysisAccurate predictionSugar derivativesMicrobiological testing/measurementStructure functionOrganism
Methods for determining transcription rate of mRNA in eukaryotic cells using nuclear runoff transcription where labeled RNA molecules are hybridized against an array of at least 500 nucleic acid molecule probes representing at least part of the genome of the native eukaryotic organism to identify the quantity of nascent mRNA transcripts in said cells. The method can be used to simultaneously identify the quantity of a large number of mRNA transcripts. A rate of degradation for distinct mRNA in a eukaryotic cell rate is determined by comparing a steady state mRNA with nuclear runoff mRNA. Steady state to nuclear runoff ratios are used to determine gene and mRNA structure function relations that leads to gene expression and mRNA stability, predict structural determinants for mRNA stability and predict regulatory motifs for transcription rates. Methods of constructing recombinant organisms with enhanced stability for mRNA expressed from a gene of interest comprise introducing into the genome of an organism a gene containing one or more sequence elements that confer structural stability on mRNA transcribed from said gene.
Owner:MONSANTO TECH LLC
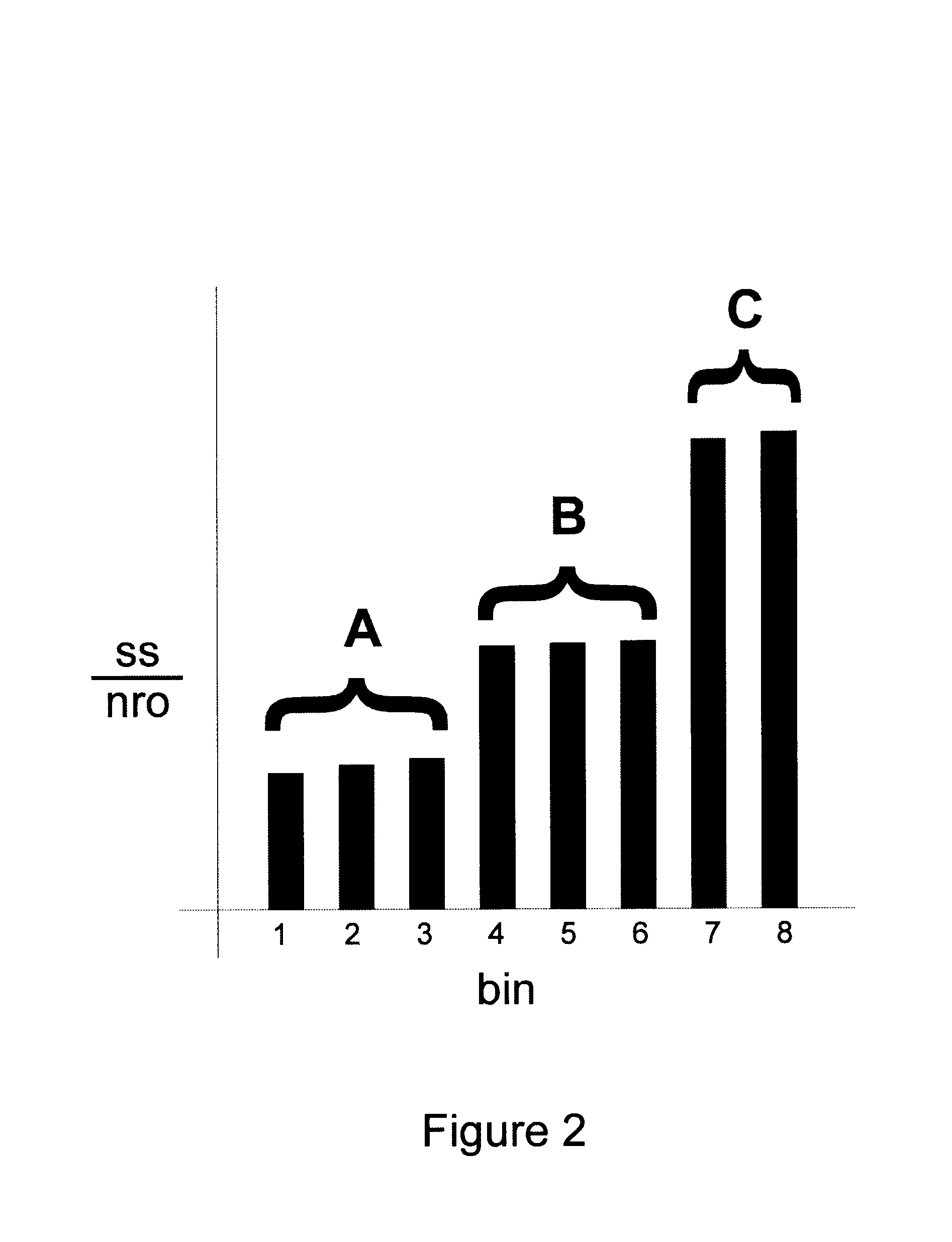
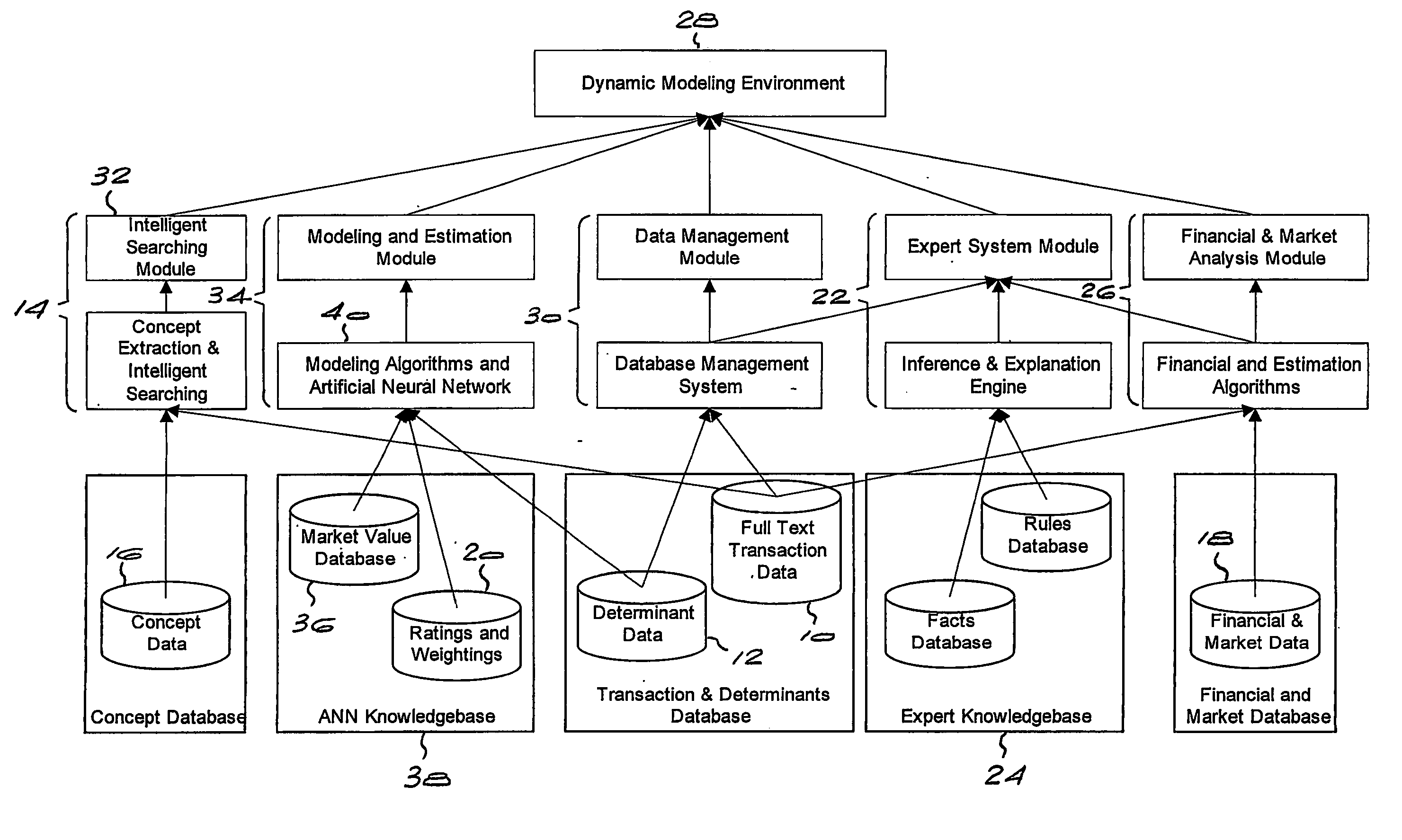
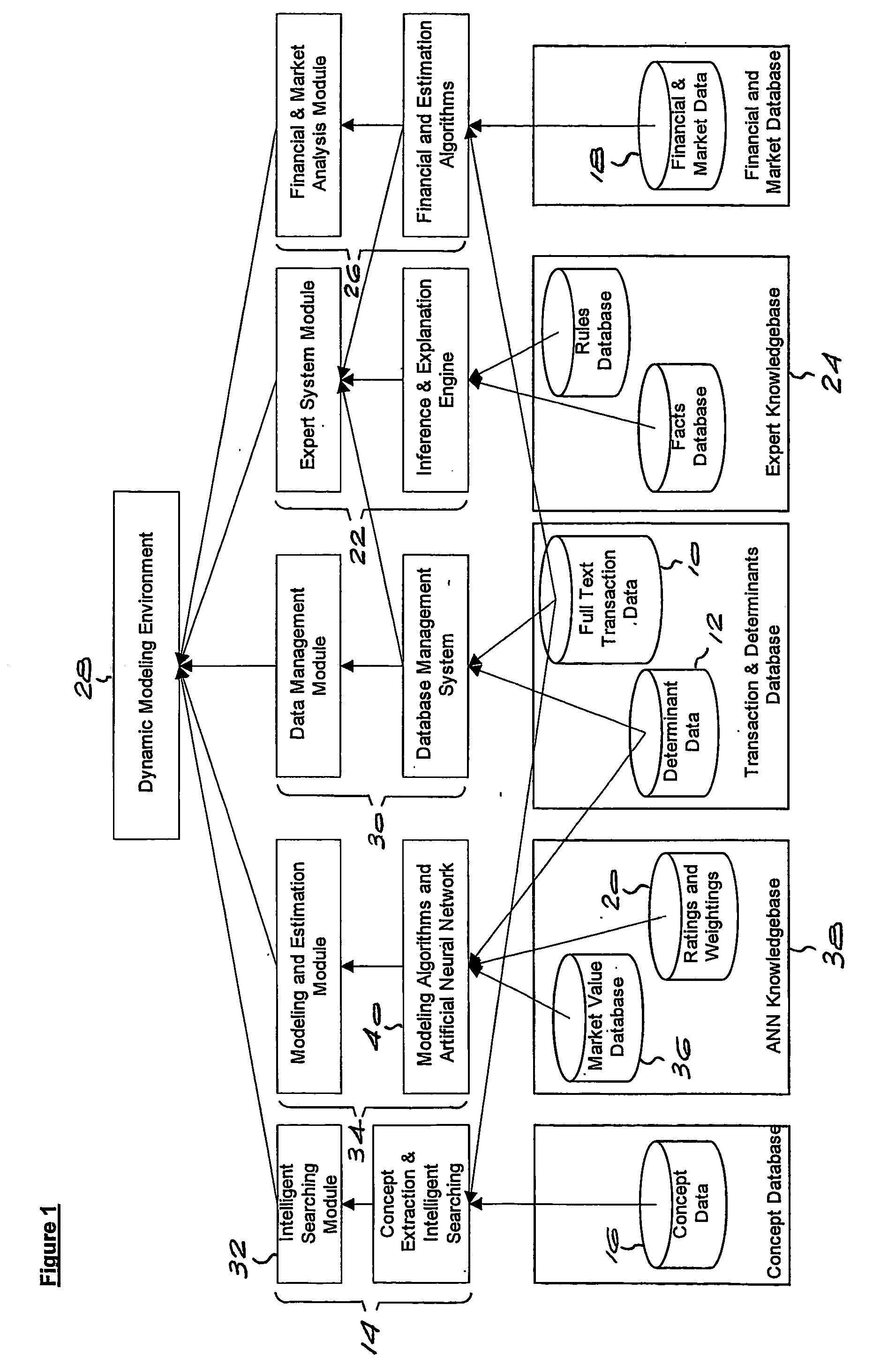
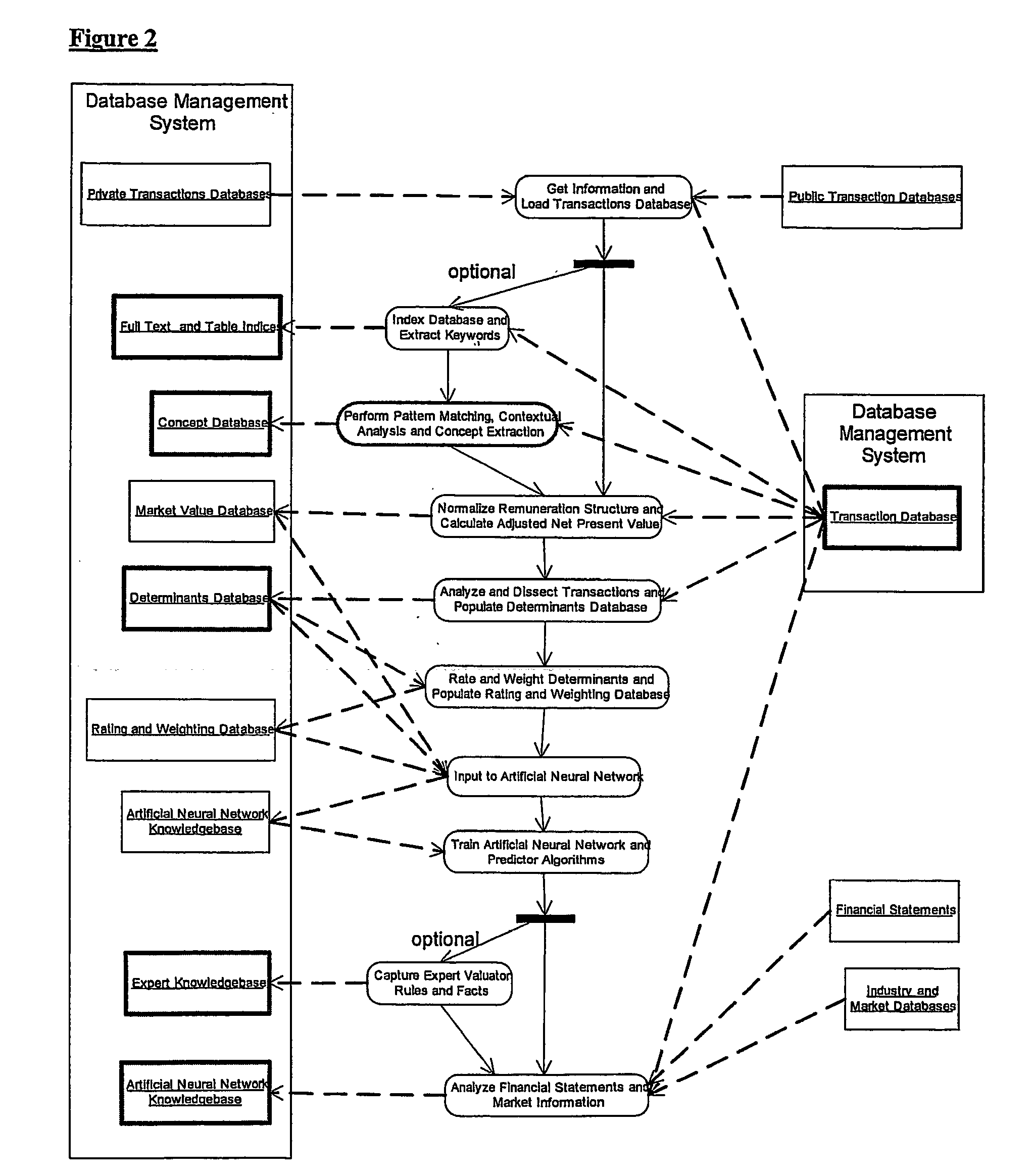
Method and system for valuing intellectual property
InactiveUS20050071174A1Accurately determineFinanceOffice automationPrediction algorithmsIntellectual property
Owner:LEIBOWITZ MARK HAROLD
Method and device for performing caching of dynamically generated objects in a data communication network
ActiveUS20070156965A1Improve abilitiesEasy to handleDigital data information retrievalTransmissionObject storeClient-side
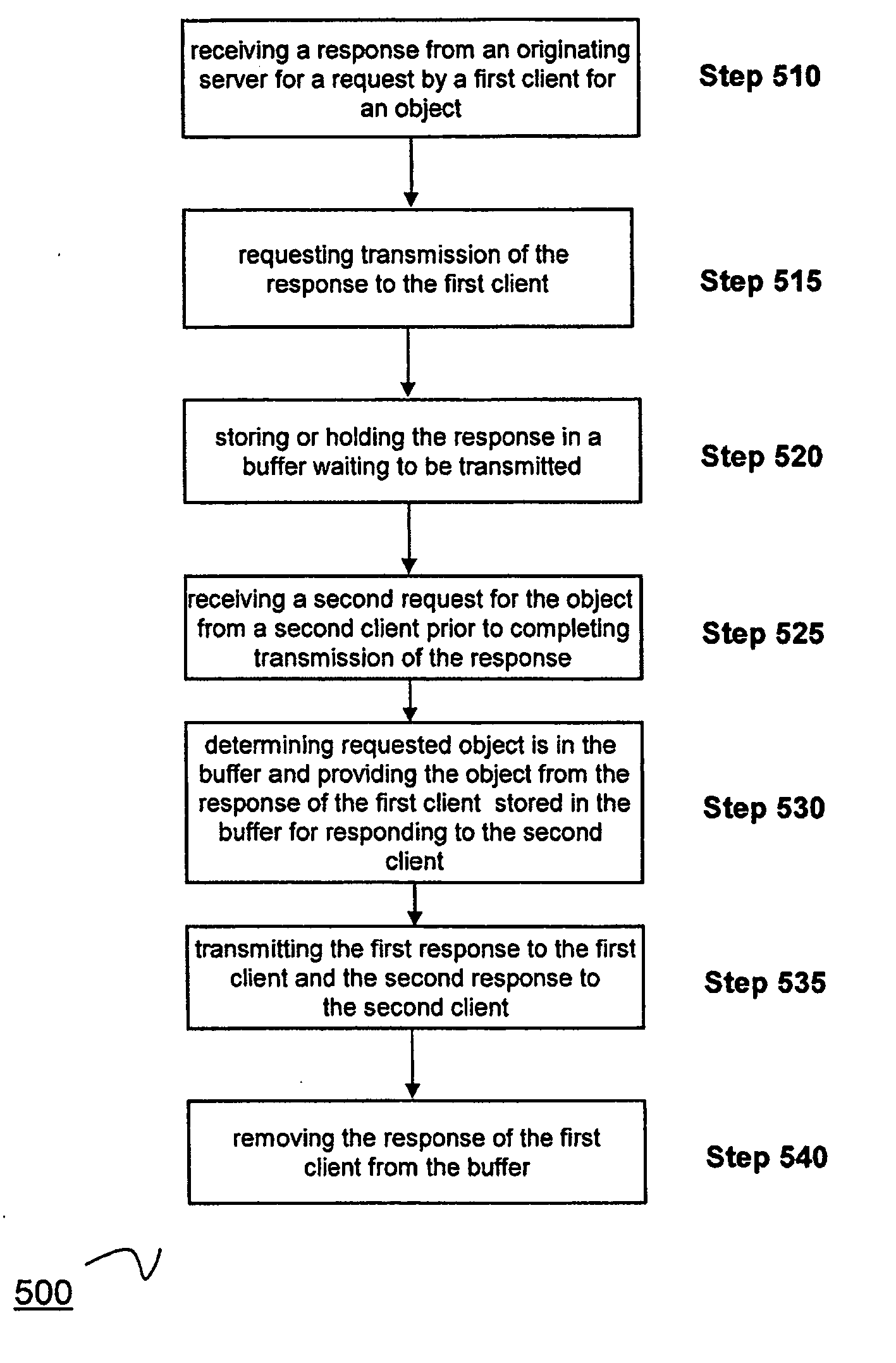
A method for maintaining a cache of dynamically generated objects. The method includes storing in the cache dynamically generated objects previously served from an originating server to a client. A communication between the client and server is intercepted by the cache. The cache parses the communication to identify an object determinant and to determine whether the object determinant indicates whether a change has occurred or will occur in an object at the originating server. The cache marks the object stored in the cache as invalid if the object determinant so indicates. If the object has been marked as invalid, the cache retrieves the object from the originating server.
Owner:CITRIX SYST INC
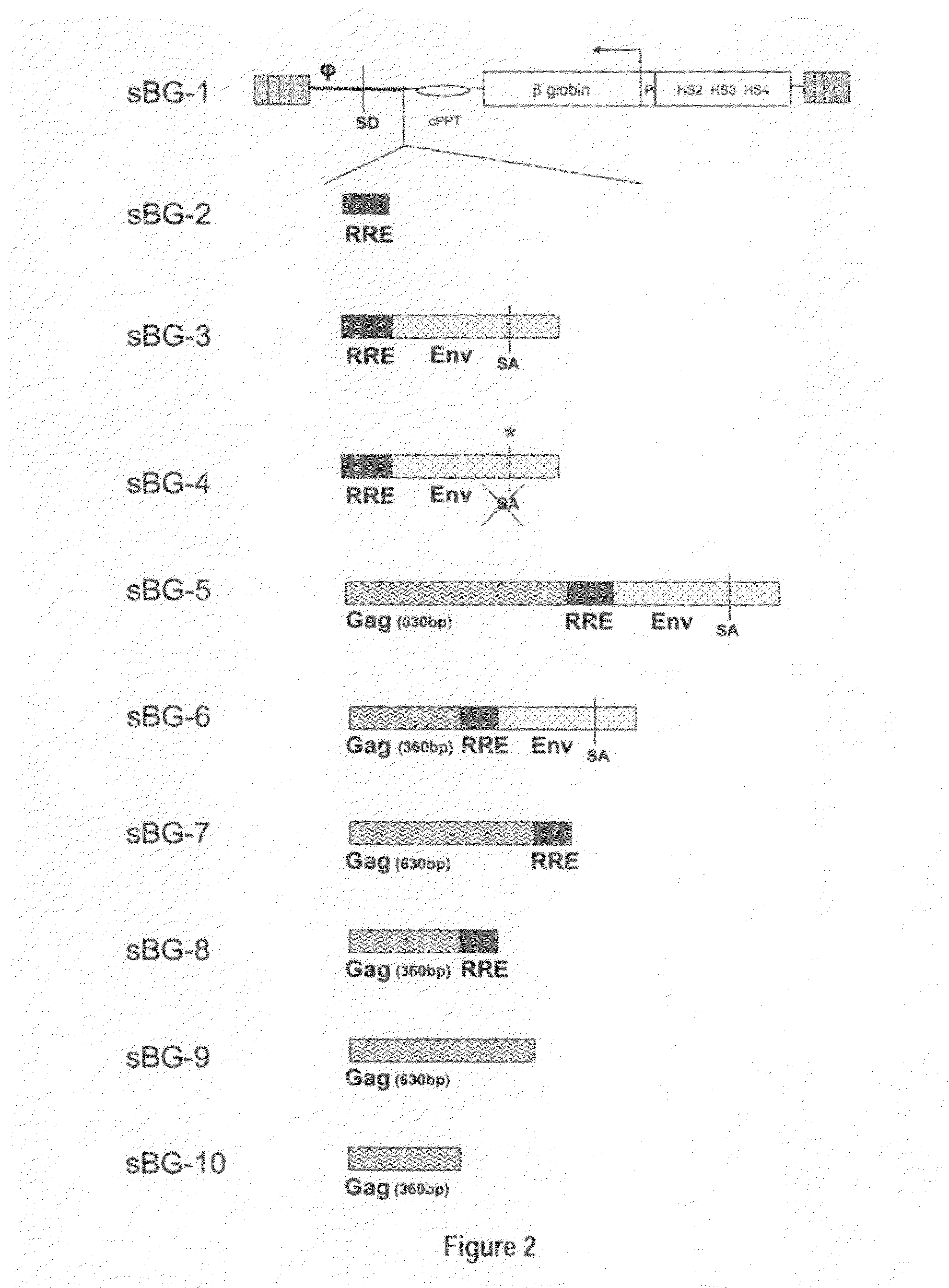
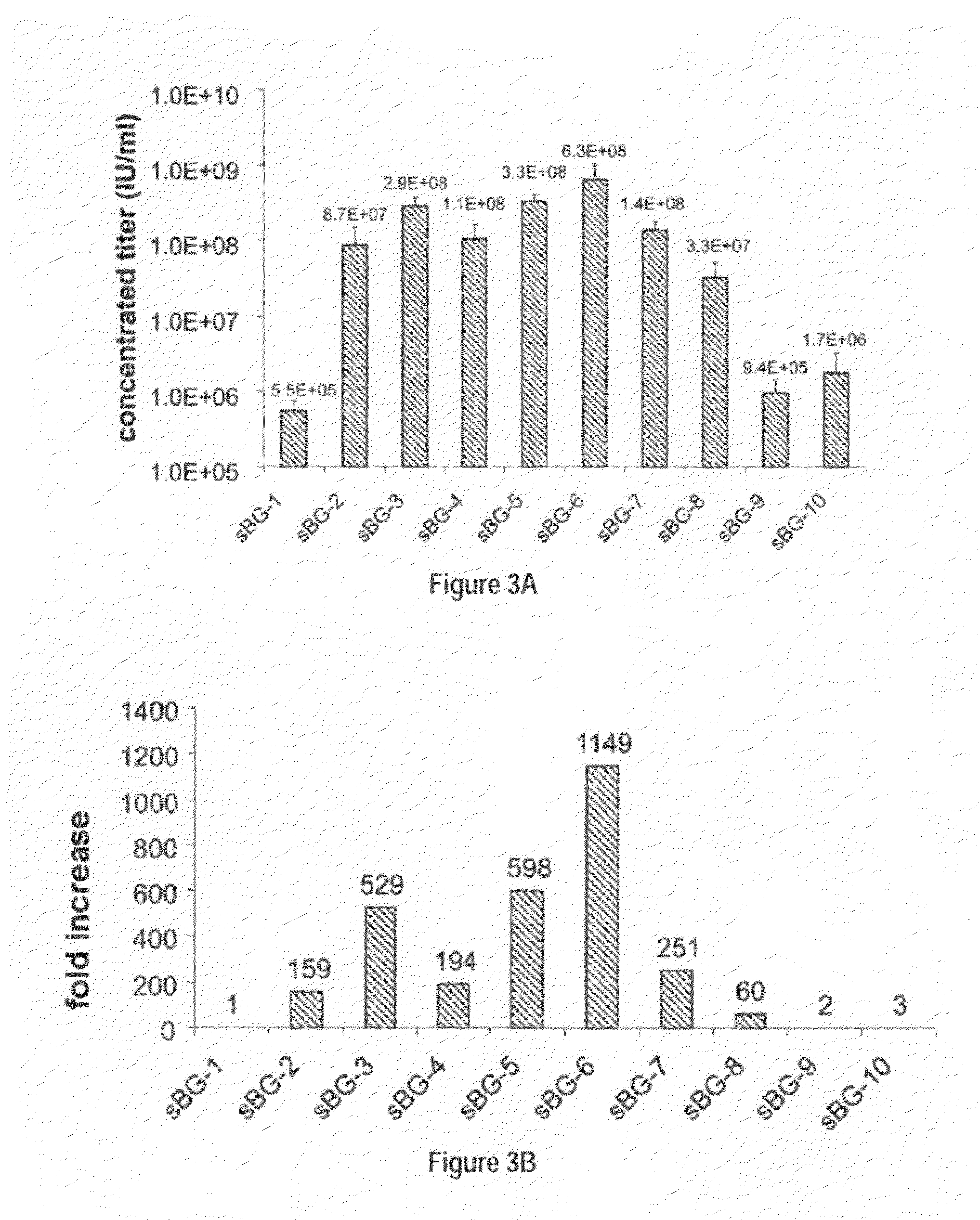
Optimization of determinants for successful genetic correction of diseases, mediated by hematopoietic stem cells
InactiveUS20110294114A1Improve stabilityImprove securityVectorsSugar derivativesNervous systemSickle cell anemia
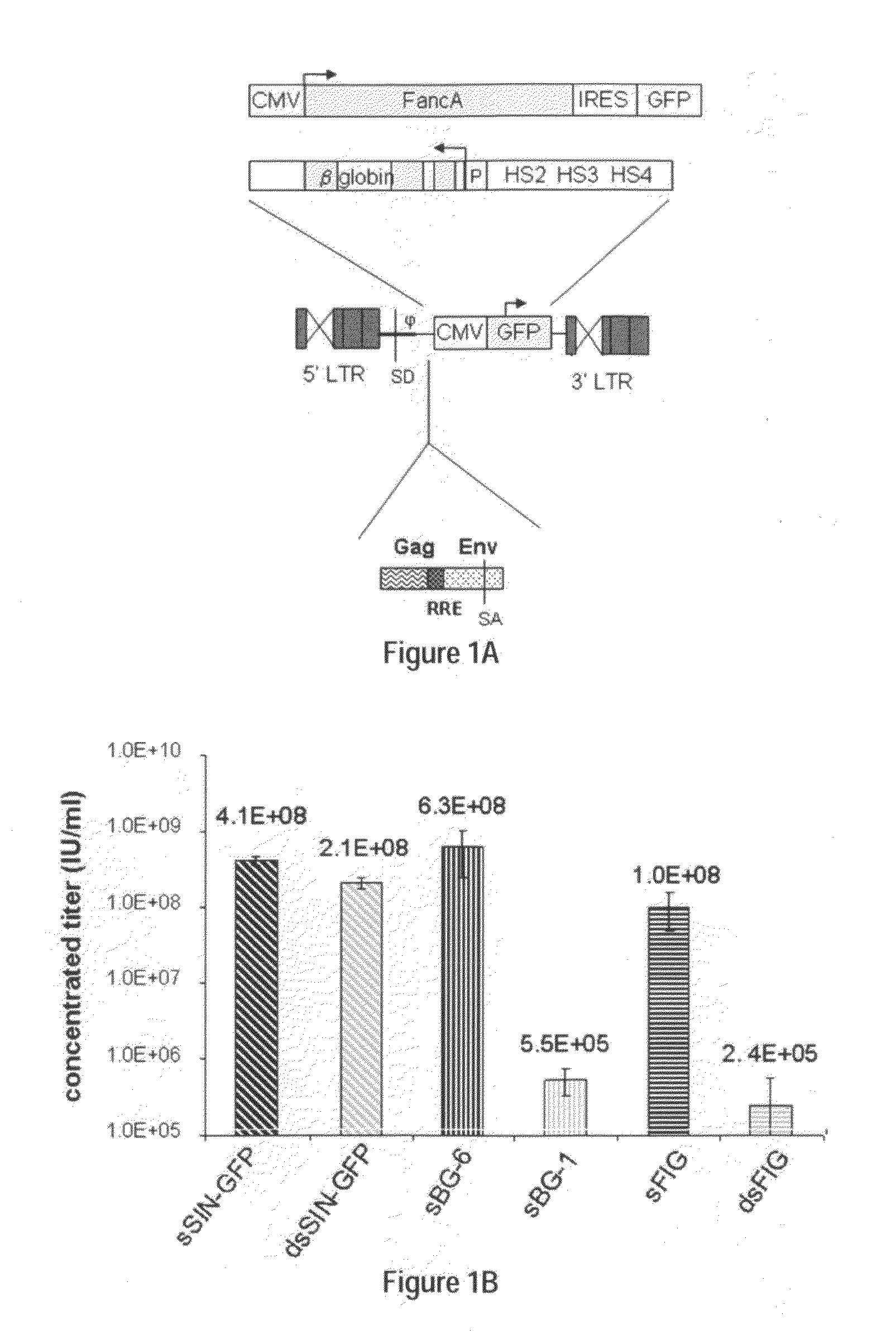
Methods and compositions disclosed herein generally relates to methods of determining minimum hematopoietic stem cell (HSC) chimerism and gene dosage for correction of a hematopoietic disease; in particular, in in vivo models. The invention also relates to modified lentiviral expression vectors for increase a viral titer and various methods for increasing such titers as well as expression vectors capable of enhancing such titers. The invention also relates to CHS4 chromatin insulator-derived functional insulator sequences. The invention further relates to methods for genetic correction of diseases or reducing symptoms thereof, such as sickle cell anemia, a lysosomal storage disease. The invention further relates to a method of improving and / or correcting one or more central nervous system (CNS) abnormalities caused by one or more lysosomal storage disease. The invention further relates to methods of improving titer in transfection-based bioreactor culture production or transfection-based production systems using eukaryotic cells.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
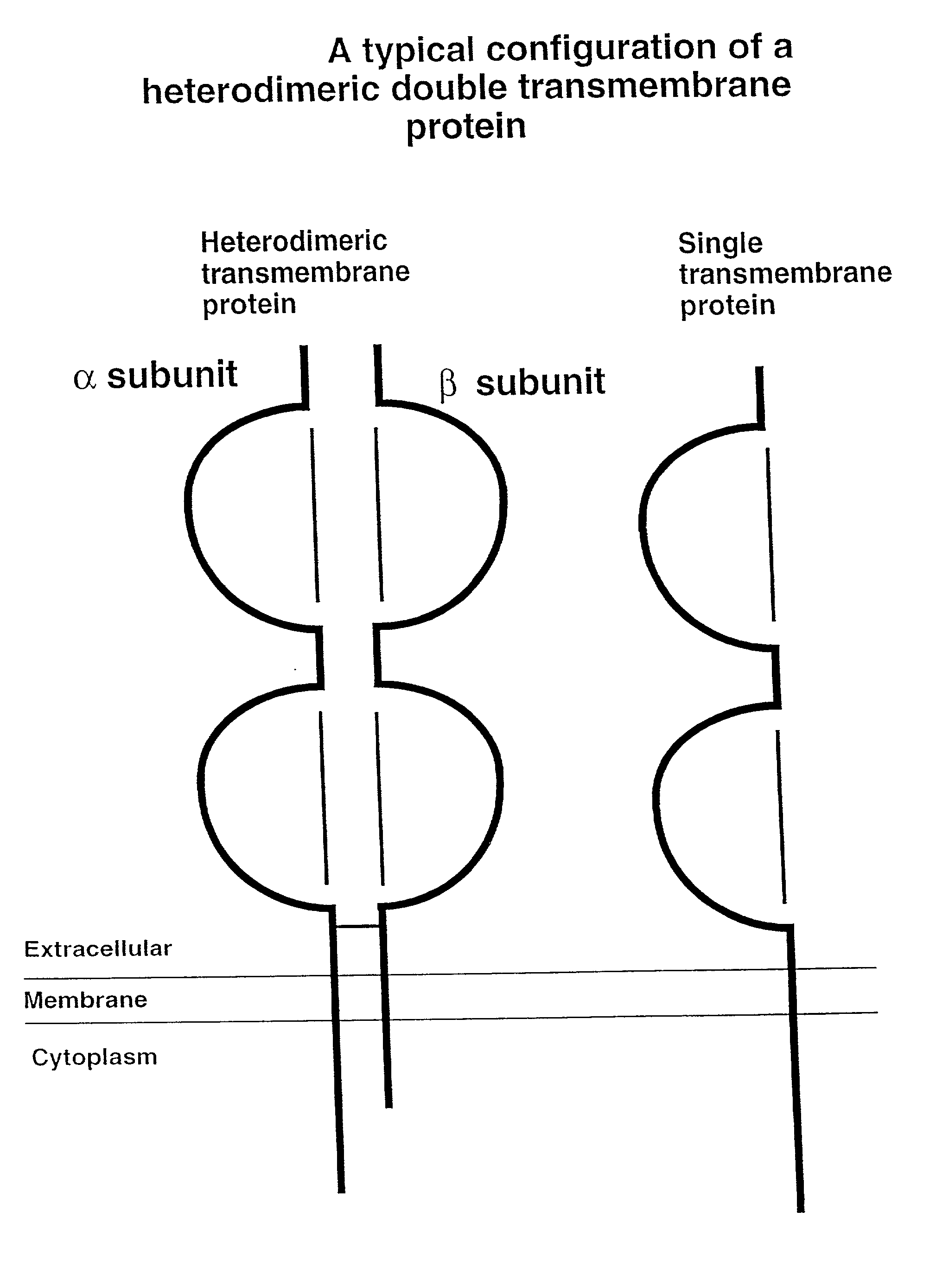
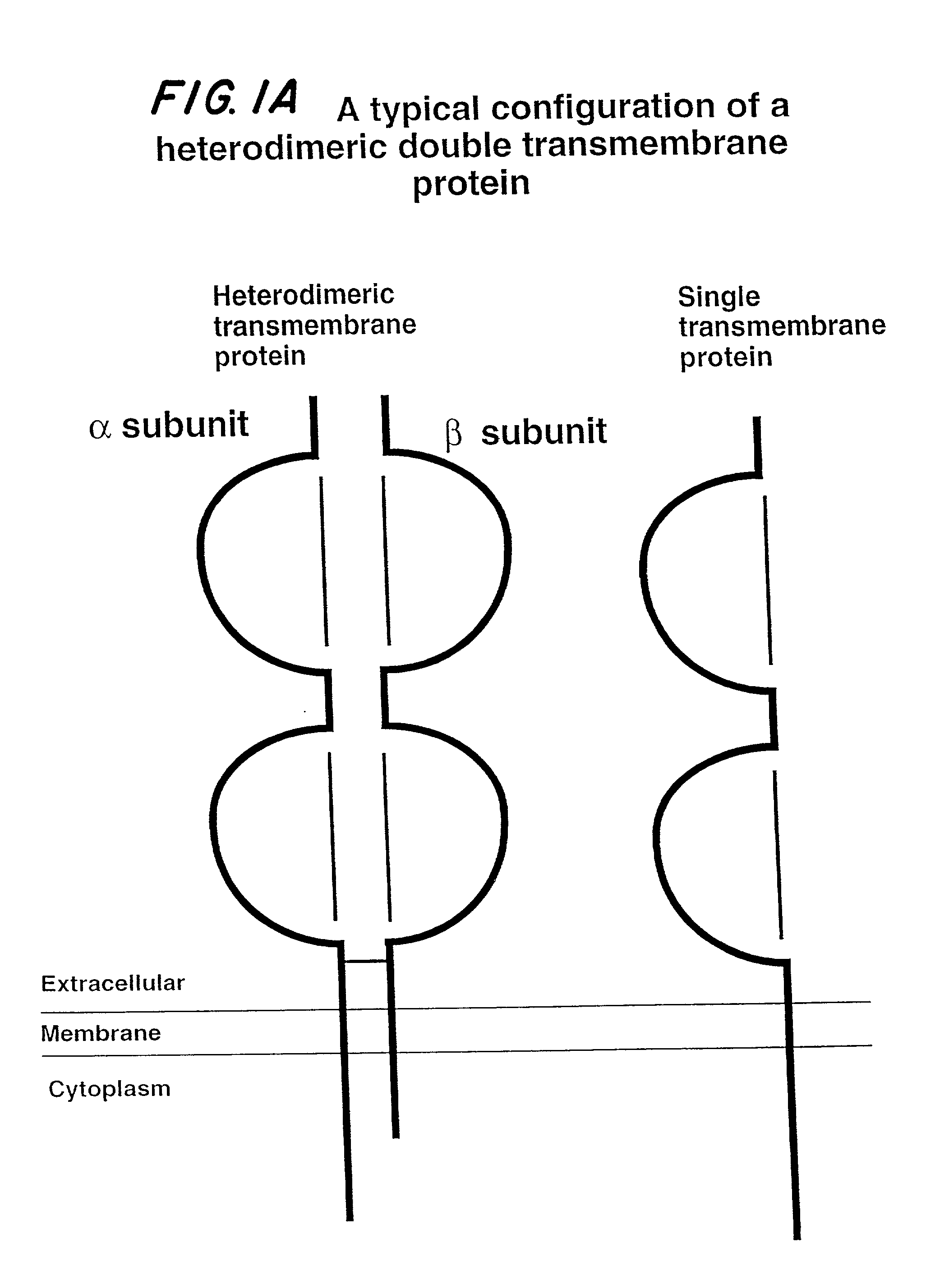
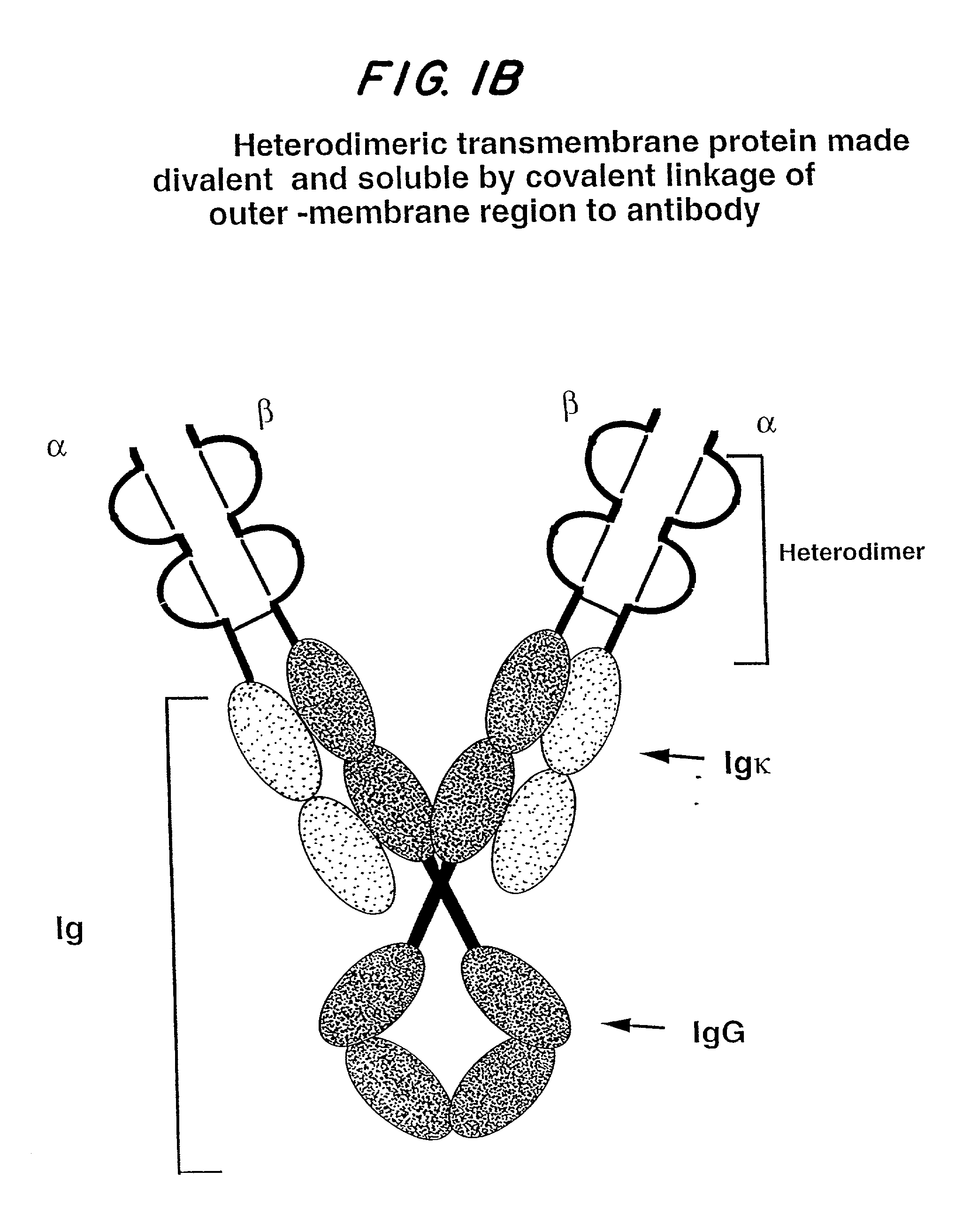
Soluble divalent and multivalent heterodimeric analogs of proteins
InactiveUS20020127231A1Well representedHigh affinityVirusesPeptide/protein ingredientsADAMTS ProteinsSpecific immunity
Specificity in immune responses is in part controlled by the selective interaction of T cell receptors with their cognate ligands, peptide / MHC molecules. The discriminating nature of this interaction makes these molecules, in soluble form, good candidates for selectively regulating immune responses. Attempts to exploit soluble analogs of these proteins has been hampered by the intrinsic low avidity of these molecules for their ligands. To increase the avidity of soluble analogs for their cognates to biologically relevant levels, divalent peptide / MHC complexes or T cell receptors (superdimers) were constructed. Using a recombinant DNA strategy, DNA encoding either the MHC class II / peptide or TCR heterodimers was ligated to DNA coding for murine Ig heavy and light chains. These constructs were subsequently expressed in a baculovirus expression system. Enzyme-linked immunosorbant assays (ELISA) specific for the Ig and polymorphic determinants of either the TCR or MHC fraction of the molecule indicated that infected insect cells secreted approximately 1 .mu.g / ml of soluble, conformnationally intact chimeric superdimers. SDS PAGE gel analysis of purified protein showed that expected molecular weight species. The results of flow cytometry demonstrated that the TCR and class II chimeras bound specifically with high avidity to cells bearing their cognate receptors. These superdimers will be useful for studying TCR / MHC interactions, lymphocyte tracking, identifying new antigens, and have possible uses as specific regulators of immune responses.
Owner:SCHNECK JONATHAN +1
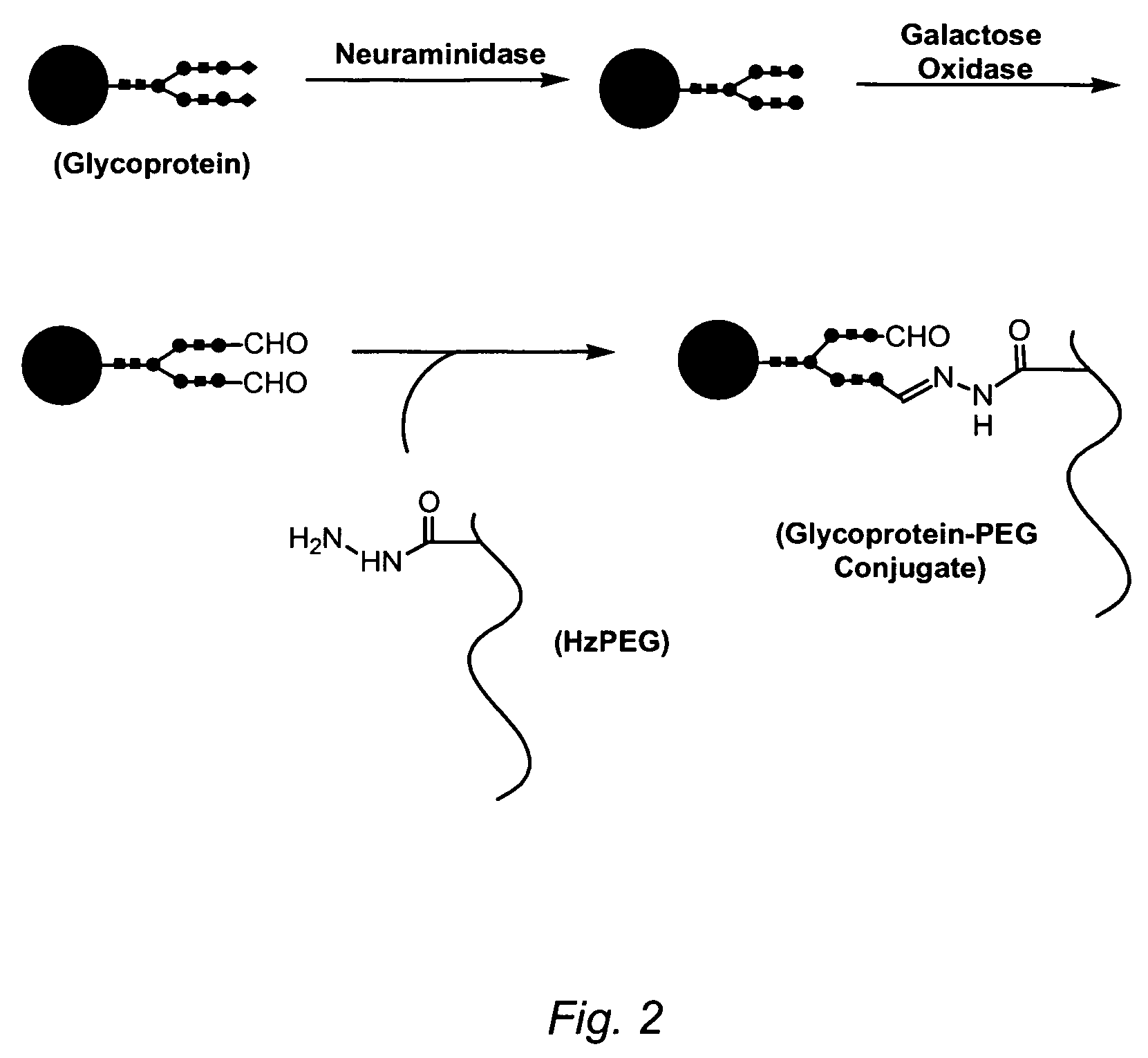
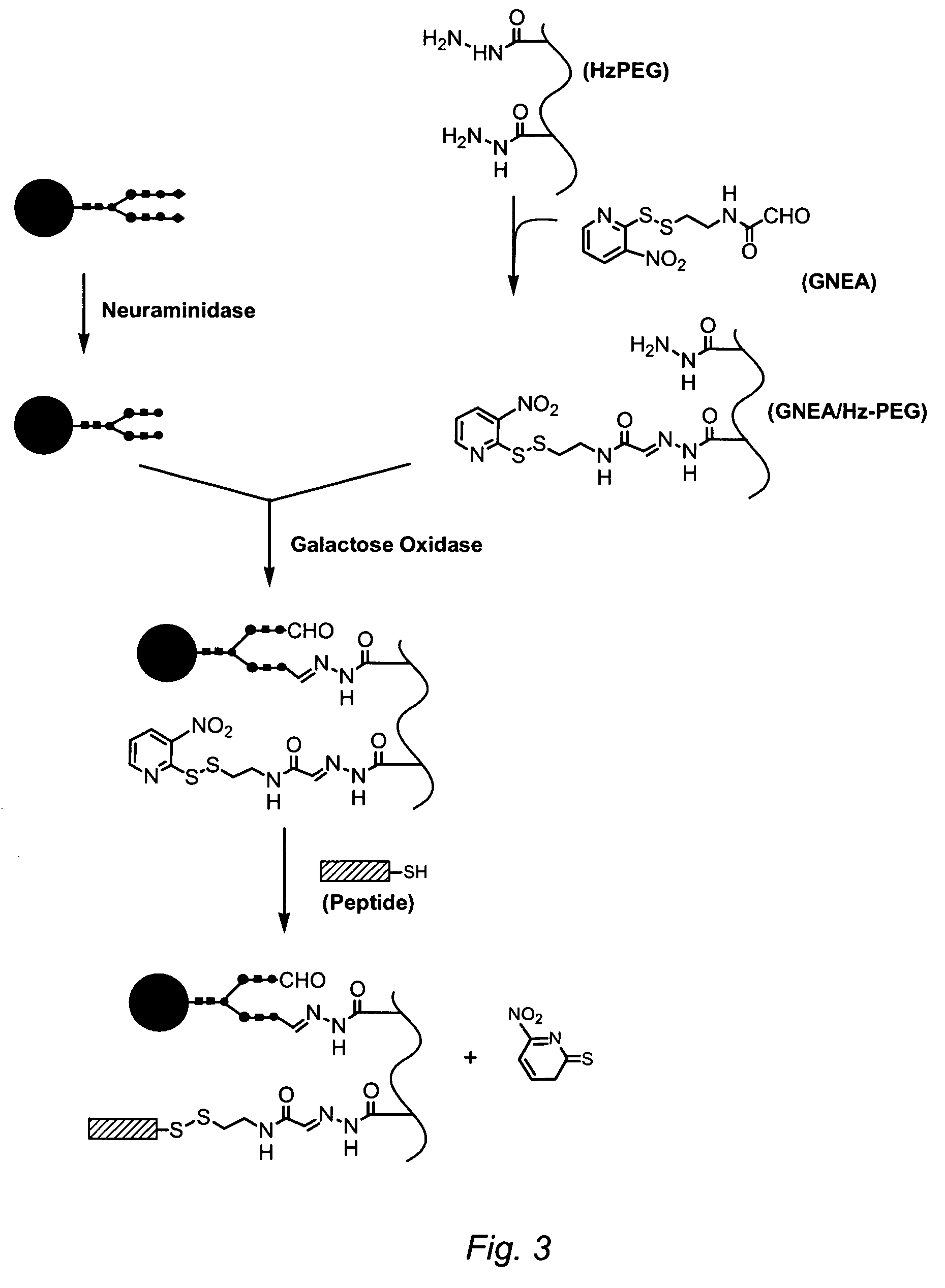
Targeting of glycoprotein therapeutics
Methods of making ligand-decorated polymer conjugates of therapeutic glycoproteins are described. Improved targeting of glycoproteins to specific tissues is achieved by masking the natural carbohydrate and other surface determinants with high molecular weight polymers, such as, e.g., PEG, polysialic acid, etc., which in turn are decorated with target-specific ligands. In some embodiments, acid-labile linkages in such conjugates or rapidly degradable masking groups allow for the intracellular release of the polymer from the glycoprotein, for example, under conditions found in lysosomes.
Owner:GENZYME CORP
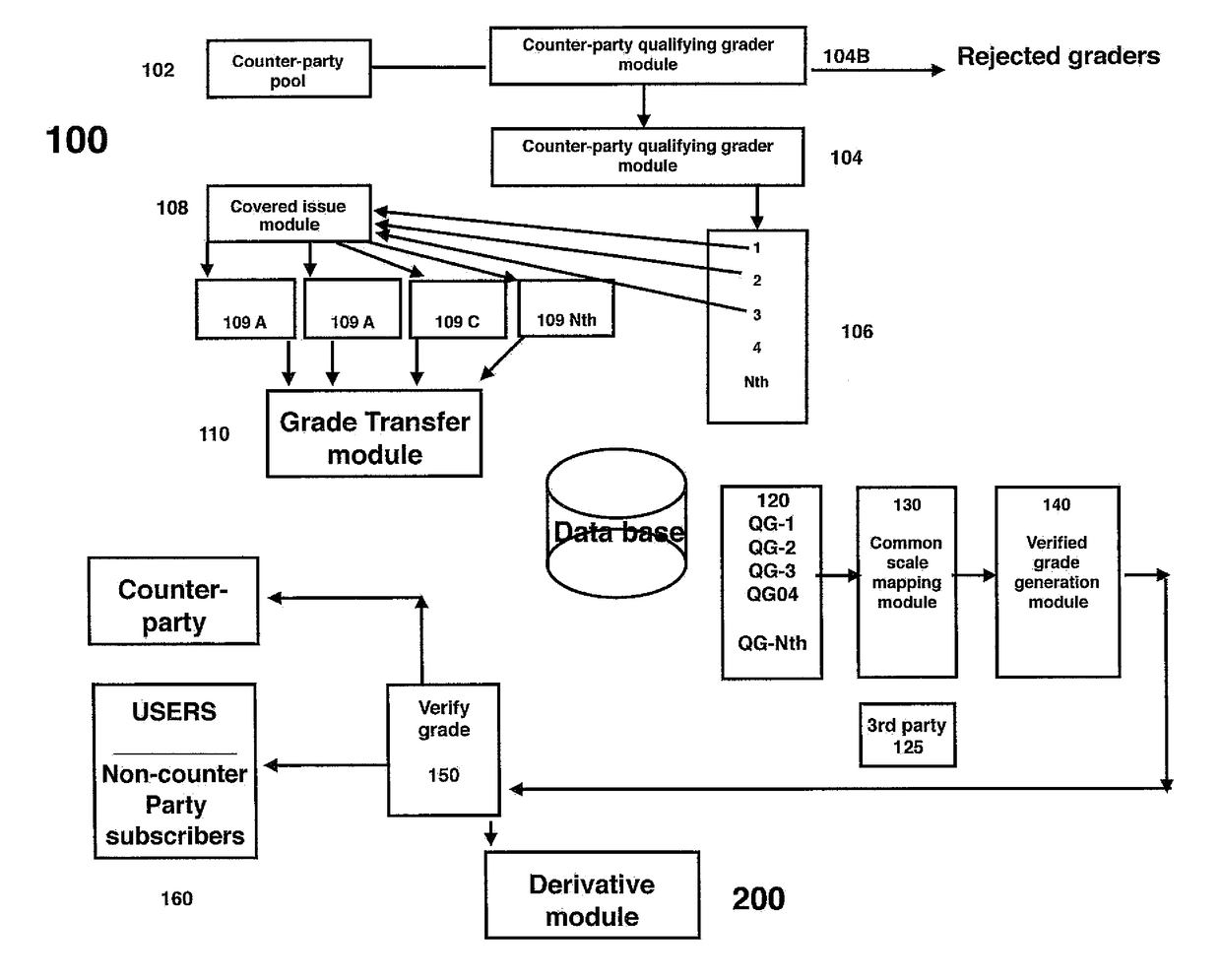
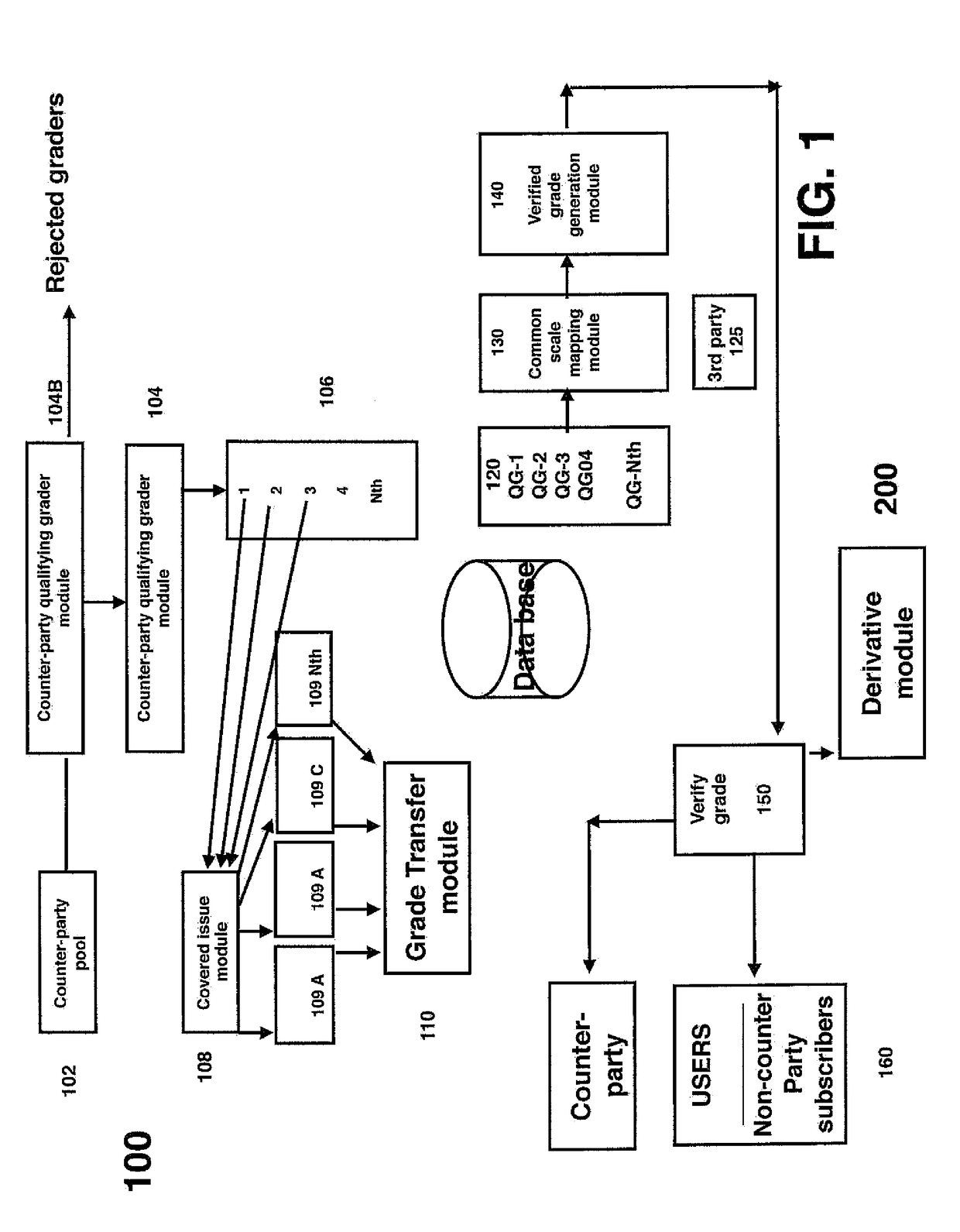
Select group crowdsource enabled system, method and analytical structure to perform securities valuations and valuation adjustments and generate derivatives thereform
A select group, crowd-sourced enabled system, method and analytical structure to perform securities valuations and valuation adjustments thereof and generate derivatives therefrom is disclosed. The system is supplied with data generated by and from selected crowd-sourced entities. A computer retrieves and processes the data to perform an analysis according to a series of mapping points and criteria as a function of risk, asset class, issue name, coupons, maturity data, identification numbers, issuer data and other analytical determinants to generate a relative and absolute risk grade for each security. The system may then generate risk grades for baskets of securities and permit the establishment of derivatives (contracts) based upon an aggregate risk grade, which derivatives may be traded separate from the underlying securities.
Owner:HEIMOWITZ DANIEL
Drug Delivery Formulations For Targeted Delivery
The size and location of microsphere uptake / delivery are important determinants of the final biodistribution of oral microsphere systems. Formulations, kits, methods of administering the formulations, and using the kits are described herein. The formulations are oral dosage formulations. In one embodiment, the formulations contain microparticles and / or nanoparticles having a homogenous size range selected to optimize uptake in a specific region of the GI tract and target drug delivery to specific organs. In some embodiments, the dosage formulation contains an enteric coating and / or a magnetic material. In a preferred embodiment, the formulation contains a magnetic material and an active agent to be delivered, optionally the active agent is in the form of micro- or nano-particles. In some embodiments metallomucoadhesive materials and / or magnetic materials are employed as magnetic and / or mucoadhesive sources. Formulations containing magnetic materials can be localized using the kits and methods disclosed herein. In one embodiment, the method includes orally administering the formulation and applying an extracorporeal magnet to a site on the outside surface of the patient's body in an area that closely apposes the location in the gastrointestinal tract to which delivery of the formulation is desired. The extracorporeal magnet is applied for a suitable time period to allow for the drug to be released from the formulation and / or to allow for the formulation to adhere to the site. Both magnetic and mucoadhesive forces may be utilized to site-direct and retain the dosage form in the region of the gastrointestinal (GI) tract most suitable for the desired delivery.
Owner:PEROSPHERE INC
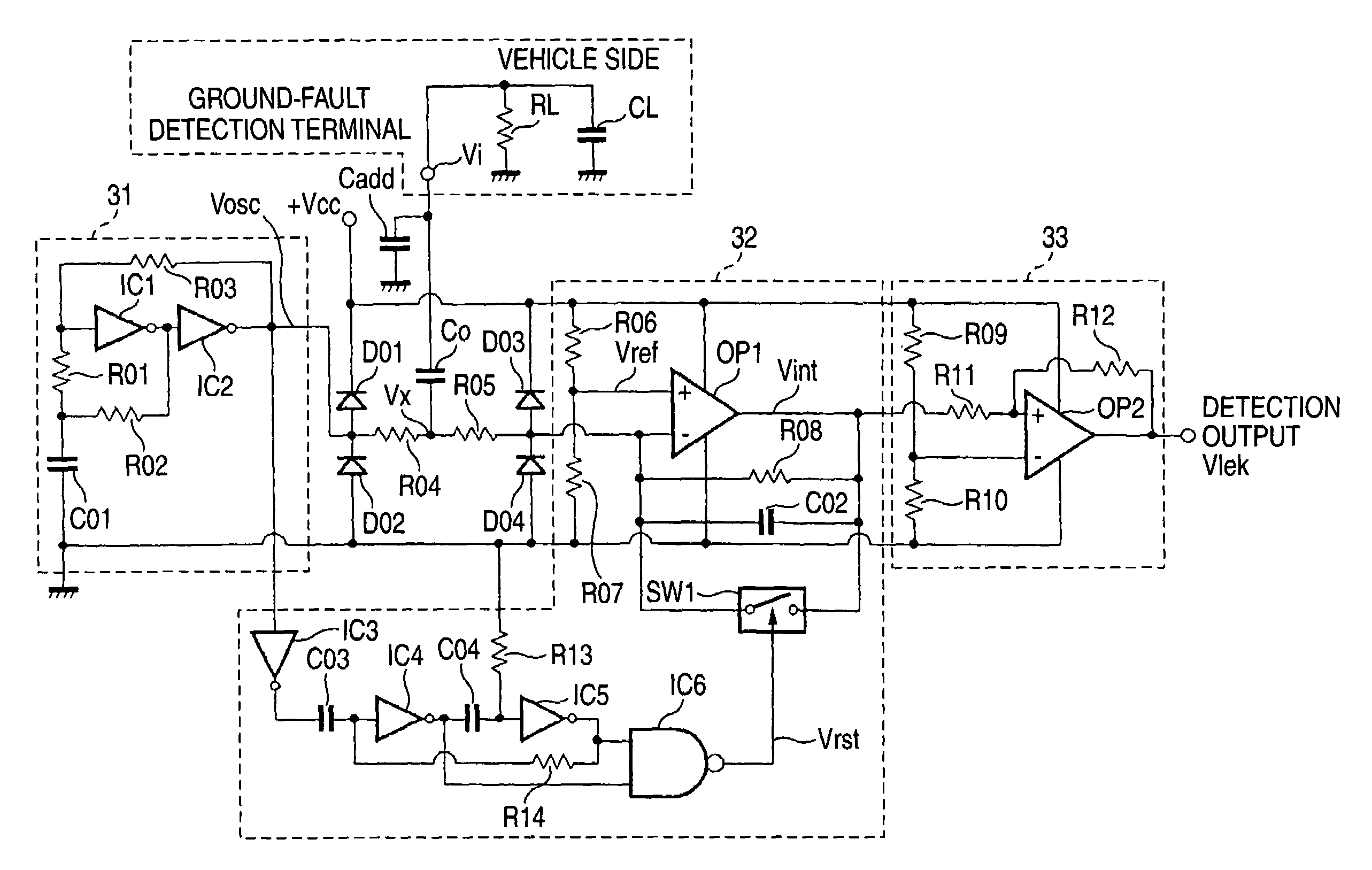
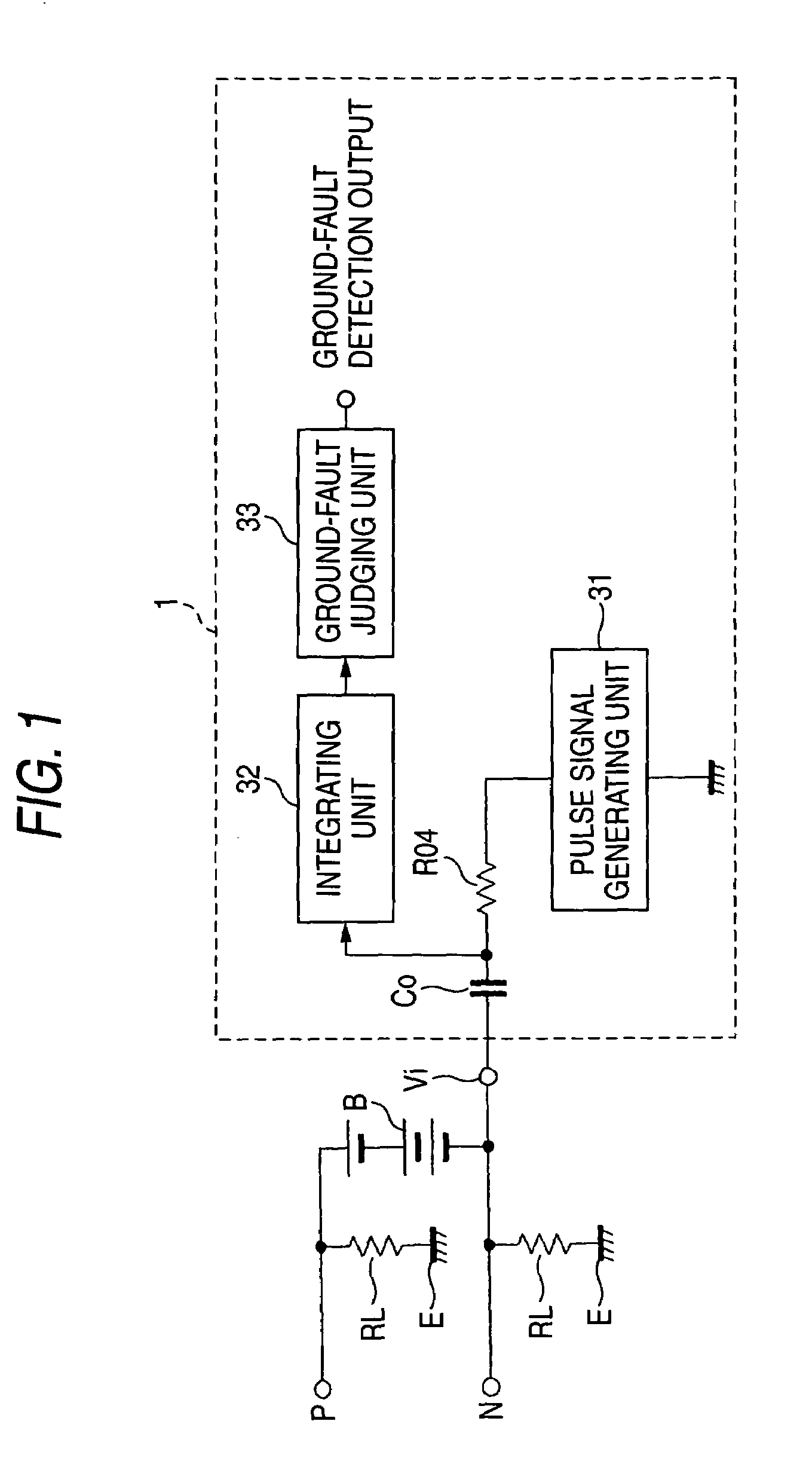
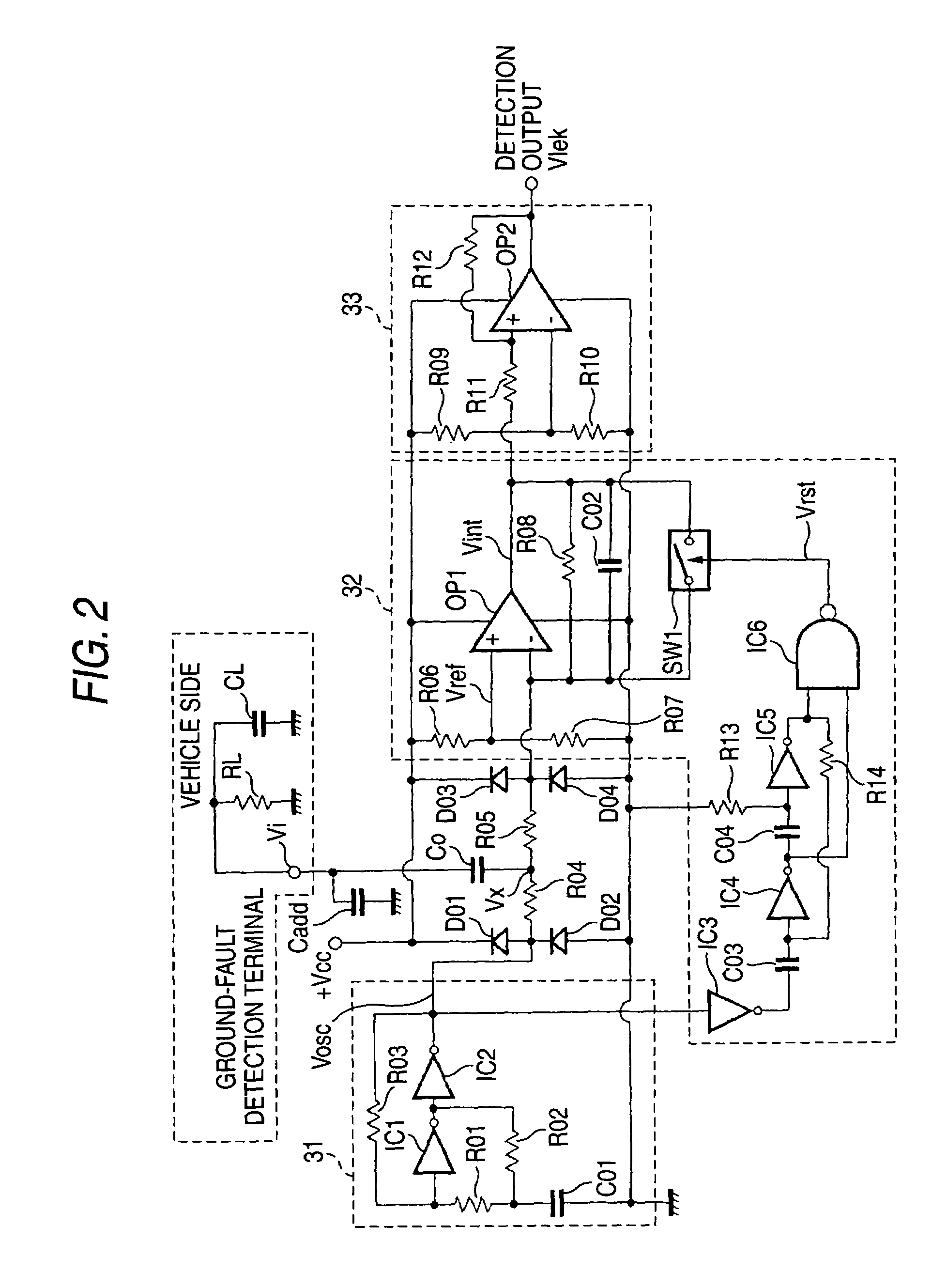
Ground-fault detecting device and insulation resistance measuring device
ActiveUS6984988B2Improve accuracyHigh sensitivityElectric devicesImpedence measurementsElectrical resistance and conductanceElectricity
A ground-fault detecting device includes a power source which is electrically insulated from a vehicle body, a pulse signal generator which generates a pulse signal having a high level and a low level which appear repeatedly in a prescribed cycle, a detection resistor which is connected to the pulse signal generator and the power source, a coupling capacitor which is connected to the detecting resistor in series, an integrator which integrates a difference between a first reference voltage and a detection voltage of the pulse signal at a connecting point of the detection resistor and the coupling capacitor over an integration interval, and a ground-fault determinant which judges whether a ground fault has occurred on the basis of an output of the integrator. The integration interval is at least part of a high-level interval and a low-level interval of the pulse signal.
Owner:YAZAKI CORP
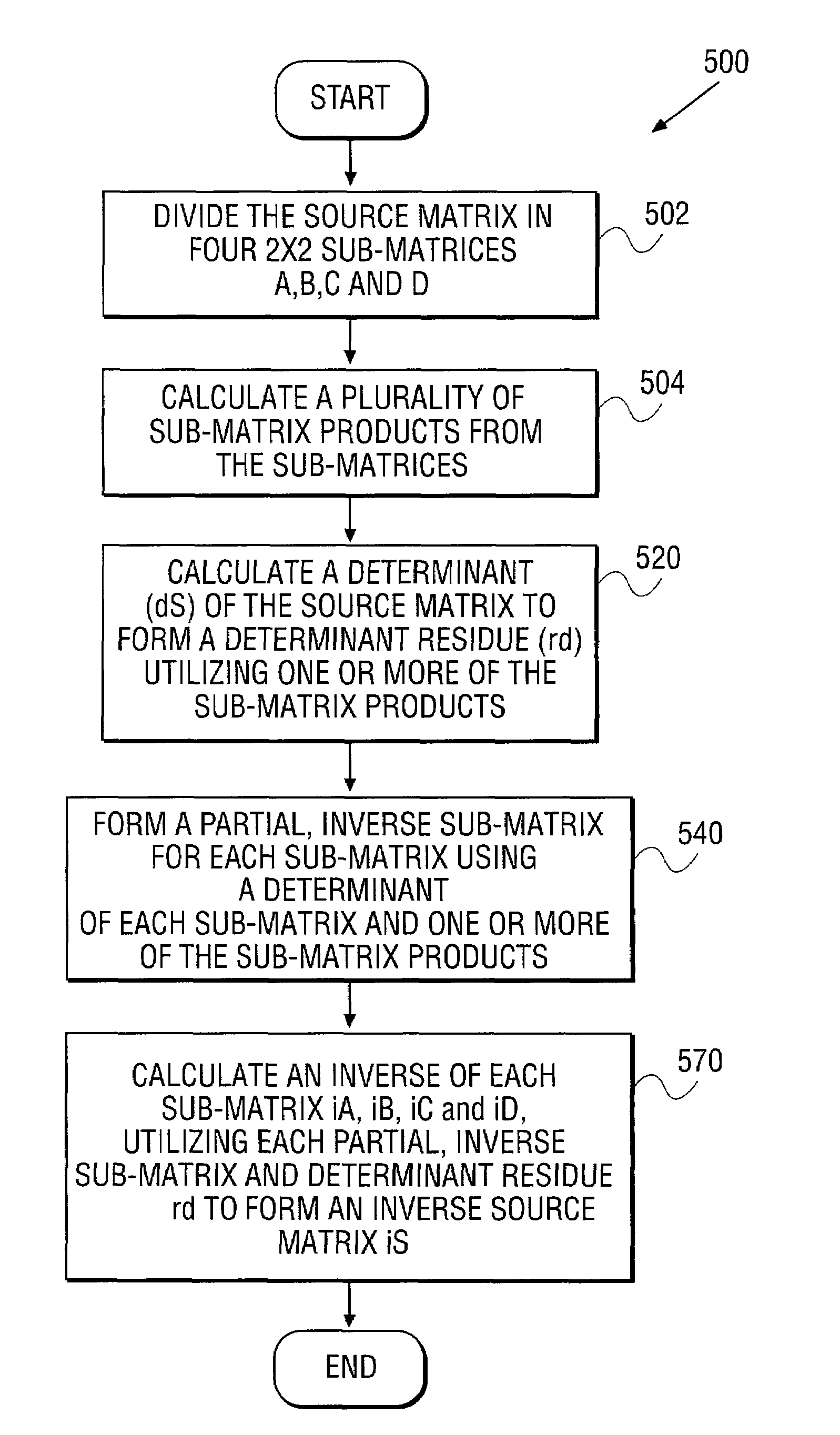
Apparatus and method for inverting a 4x4 matrix
An apparatus and method for inverting a 4×4 source matrix. A source matrix is divided into four 2×2 sub-matrices. A plurality of sub-matrix products are subsequently calculated from the sub-matrices. Next, a determinant of the source matrix is calculated to form a determinant residue utilizing the previously computed sub-matrix products. Calculation of partial inverse for each sub-matrix is next performed, using the sub-matrix products and determinants of the sub-matrices. Finally, an inverse of each sub-matrix is calculated, utilizing the partial inverse sub-matrices and the determinant residue to form an inverse of the 4×4 source matrix. The article allows processors to store two floating-point elements within a Single Instruction Multiple Data (SIMD) register. Accordingly, a sub-matrix is represented using two SIMD registers, resulting in improved computational locality and efficiency. Other embodiments are described and claimed.
Owner:INTEL CORP
Differentially weighted modifiable prescribed history reporting apparatus, systems, and methods for decision support and health
ActiveUS20160379511A1Facilitate communicationAdd supportMedical automated diagnosisElectrical appliancesGraphicsMedical record
Apparatus, systems, and methods of health reporting are disclosed that enhance decision support and provide alerts when health deterioration or the need for intervention has been detected. Embodiments utilize combined pictorial representations (including physical, mental, and social health determinants) as an avatar or pictogram, comparing health status to a general or target population, and using different methods to represent and reinforce health status. The apparatus, systems, and methods further enable individuals to access their own medical records in a way that can be understood by them and therefore is more widely accessible. The invention enhances health reporting, improving utility, usability and desirability of health reporting tools.
Owner:RESCON
Molecular determinants of myeloma bone disease and uses thereof
InactiveUS7642238B2Prevent and reverse bone lossPrevent bone lossPeptide/protein ingredientsMicrobiological testing/measurementNormal boneNewly diagnosed
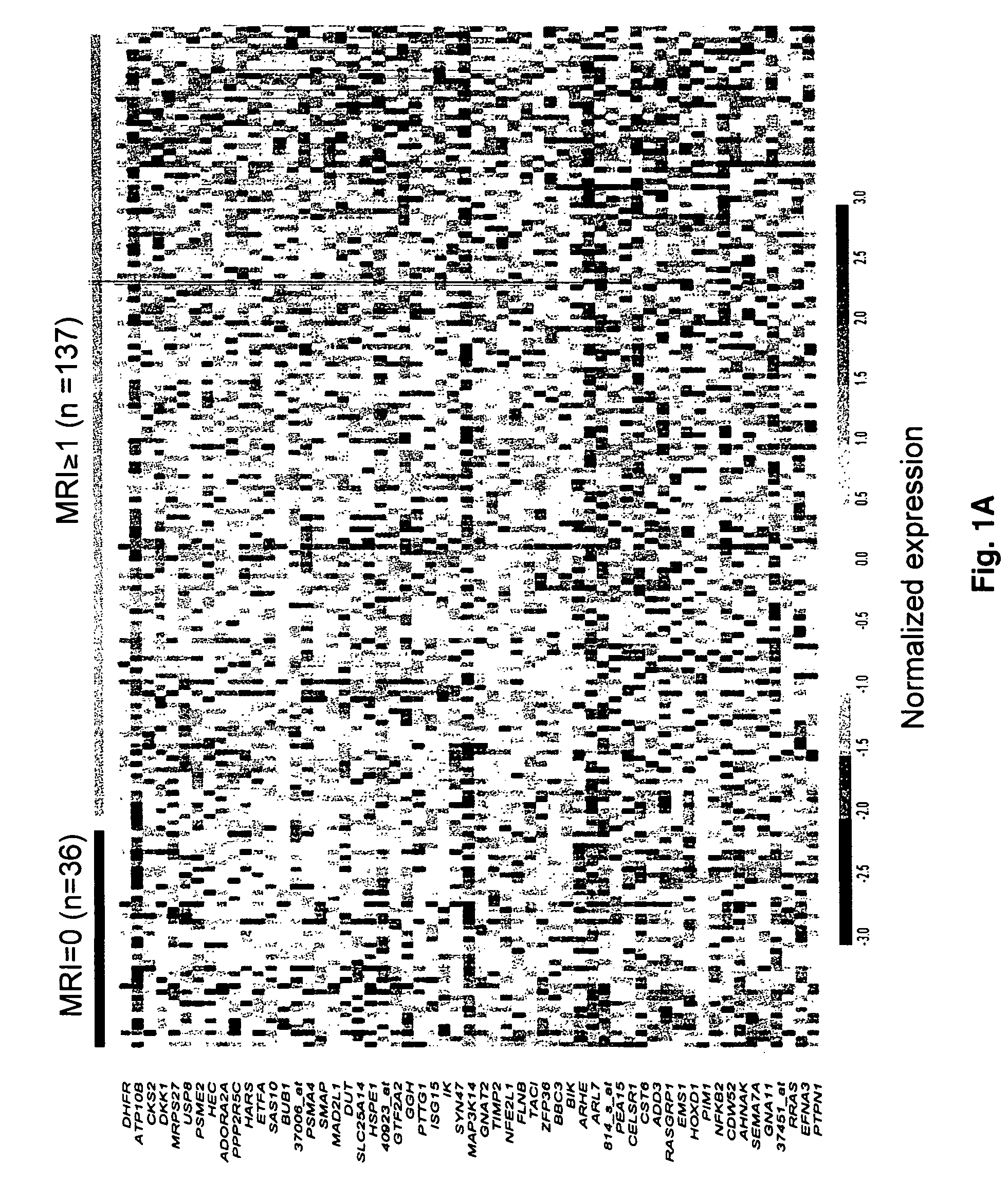
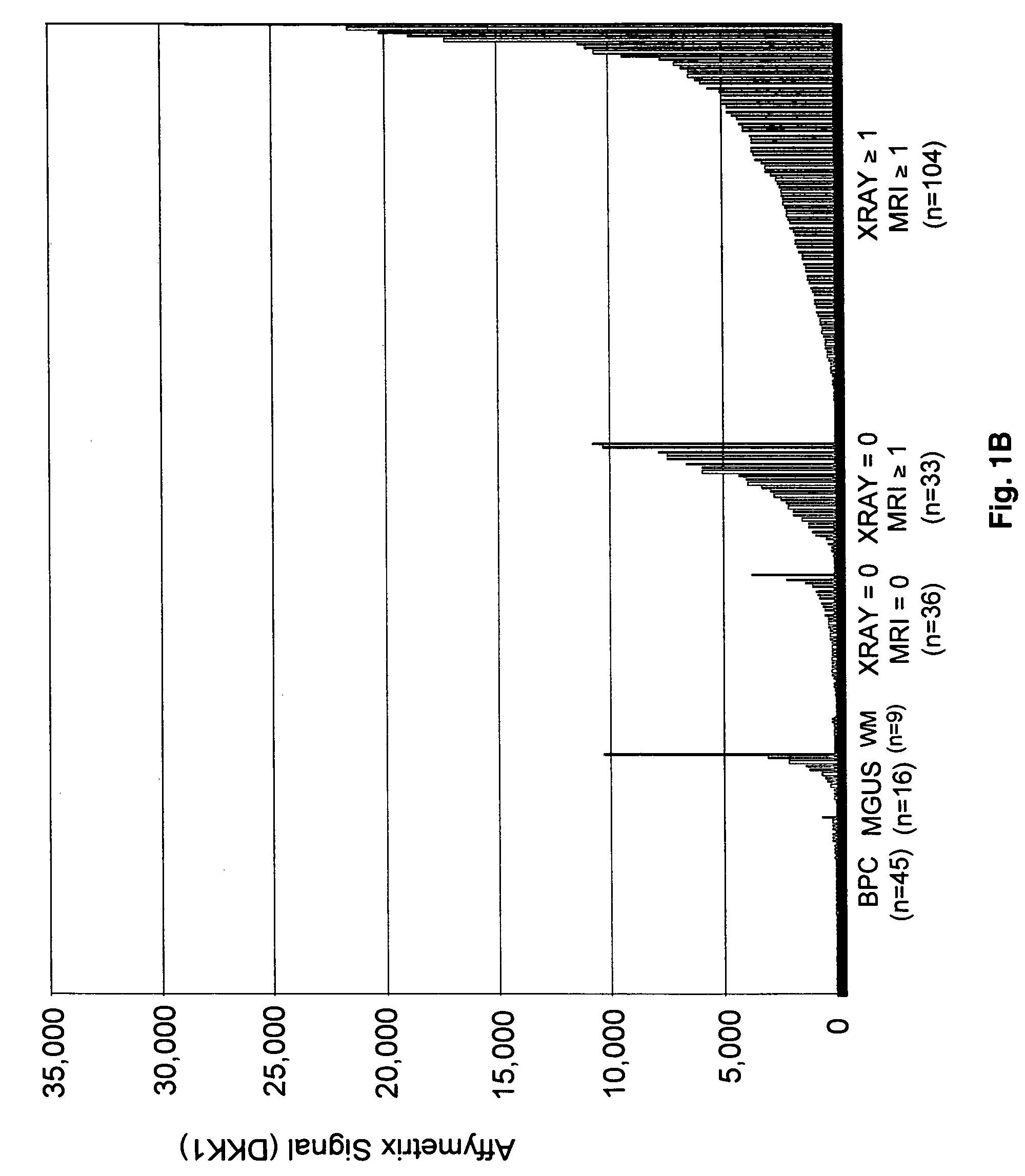
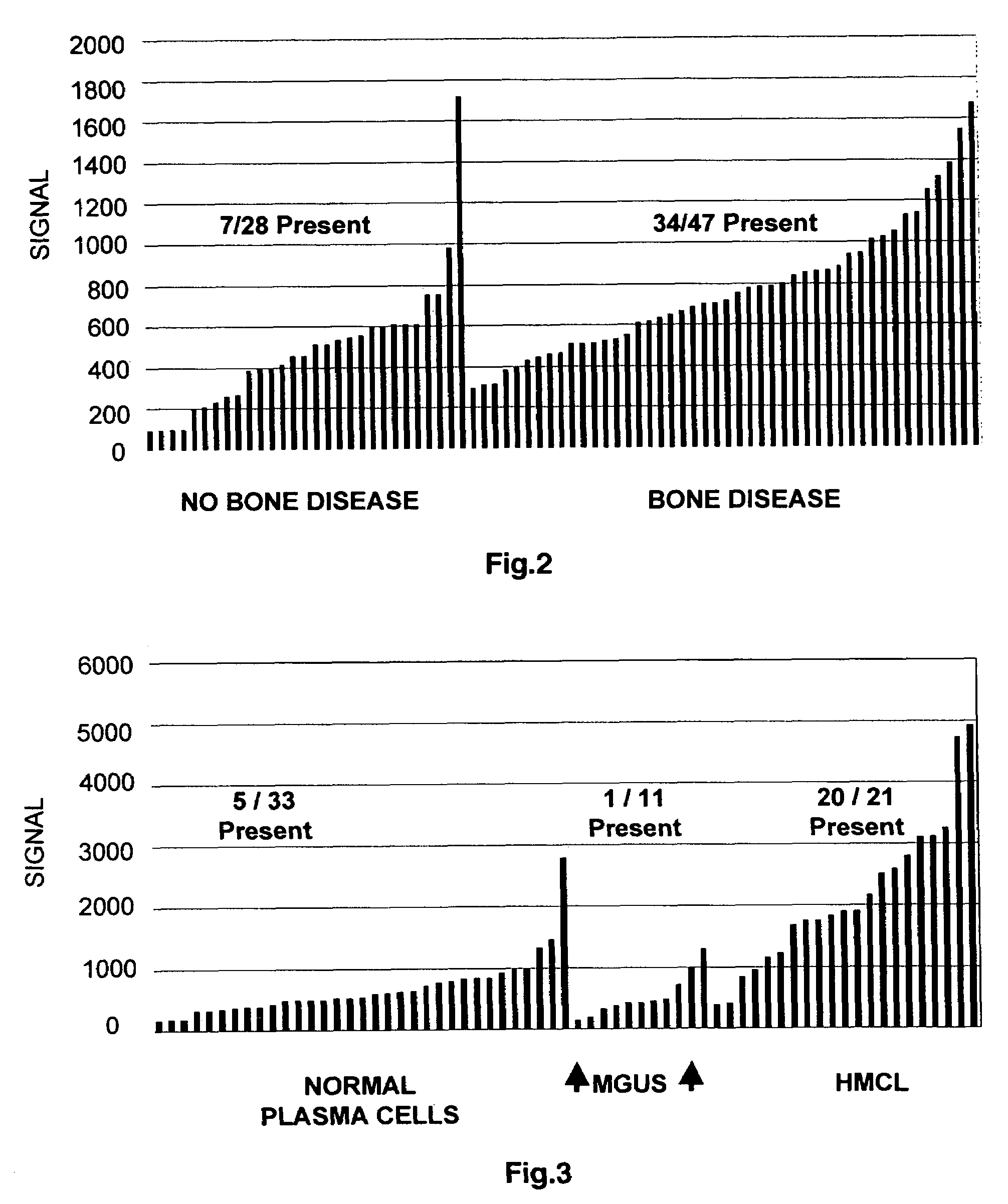
To identify molecular determinants of lytic bone disease in multiple myeloma, the expression profiles of ˜12,000 genes in CD138-enriched plasma cells from newly diagnosed multiple myeloma patients exhibiting no radiological evidence of lytic lesions (n=28) were compared to those with ≧3 lytic lesions (n=47). Two secreted WNT signaling antagonists, soluble frizzled related protein 3 (SFRP-3 / FRZB) and the human homologue of Dickkopf-1 (DKK1), were expressed in 40 of 47 with lytic bone lesions, but only 16 of 28 lacking bone lesions (P<0.05). DKK1 and FRZB were not expressed in plasma cells from 45 normal bone marrow donors or 10 Waldenstrom's macroglobulinemia, a related plasma cells malignancy that lacks bone disease. These data indicate that these factors are important mediators of multiple myeloma bone disease, and inhibitors of these proteins may be used to block bone disease.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Antibody specificity transfer using minimal essential binding determinants
The present invention provides methods of making antibodies having the binding specificity of a reference antibody. Antibodies generated by the methods of the inventions have at least one minimal essential binding specificity determinant from a heavy chain or light chain CDR3 from the reference antibody. The method can be used, e.g., in humanization procedures. The invention also provides libraries and antibodies made in accordance with the methods.
Owner:HUMANIGEN INC
Methods for the production of non-covalently complexed and multivalent proteosome sub-unit vaccines
InactiveUS6476201B1Shorten the timeIncrease temperatureAntibacterial agentsOrganic active ingredientsContinuous monitoringContamination
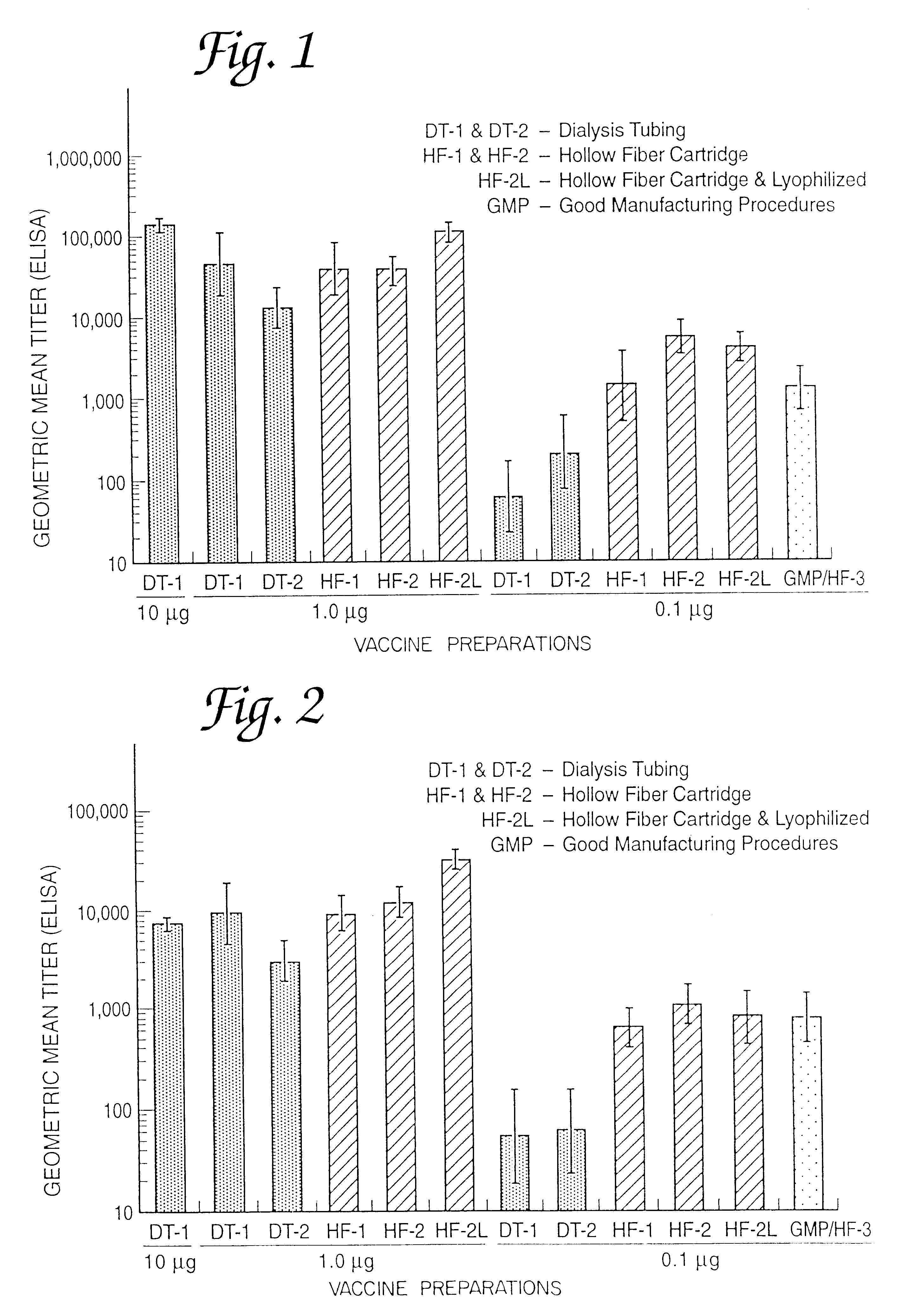
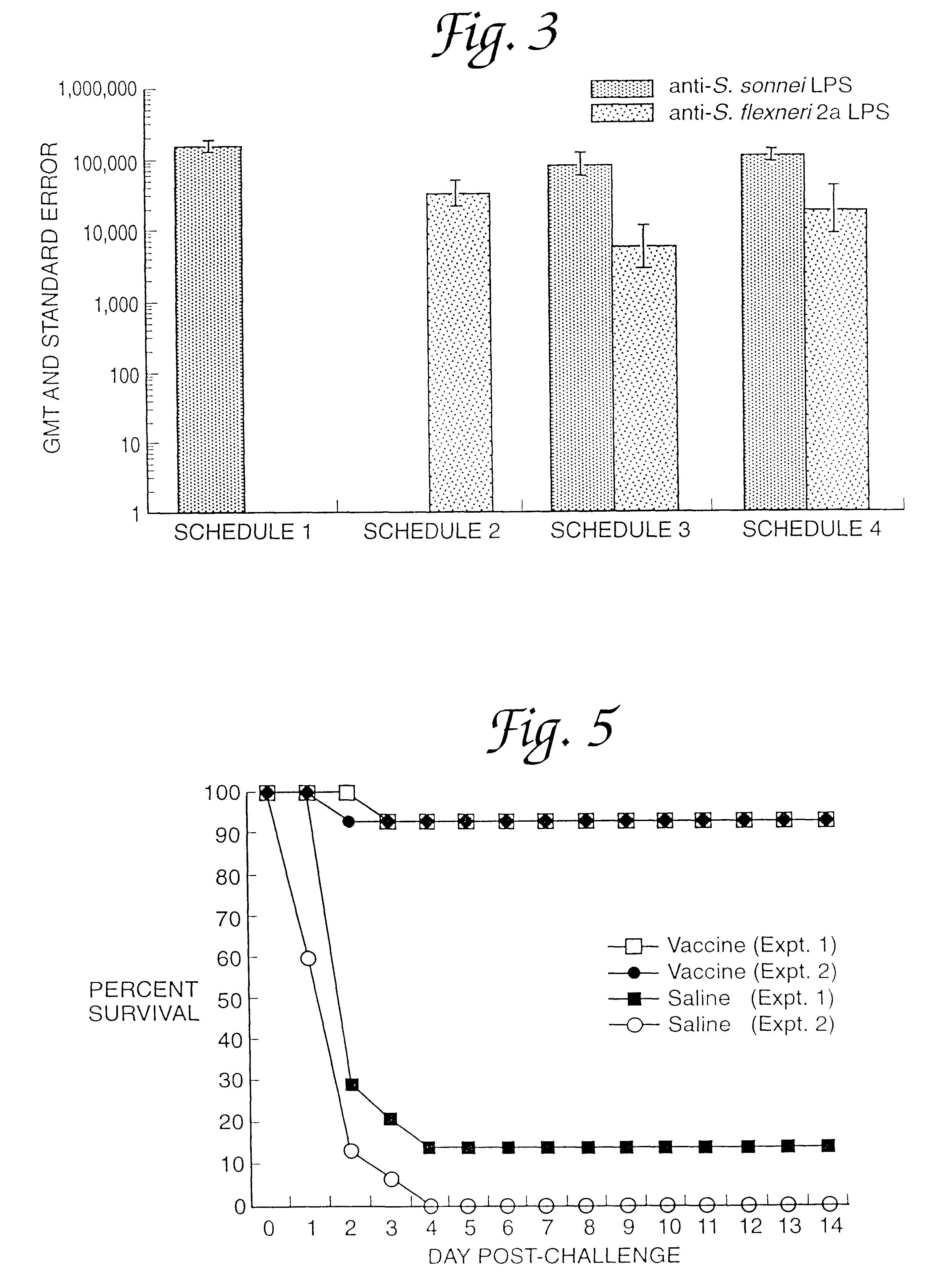
A continuous method for preparing proteosome-amphiphilic determinant vaccines for parenteral or mucosal administration using diafiltration or ultrafiltration technology. The amphiphilic determinants include lipopolysaccharides from gram negative bacteria, e.g. S. flexneri, P. shigelloides and S. sonnei. Proteosomes are obtained from group B type 2b meningococci. The active proteosome-amphiphilic determinant complexes (non-covalent complexes) of the vaccine are formed using diafiltration or ultrafiltration to remove the detergent under non-static conditions. The use of diafiltration or ultrafiltration decreases processing time and the opportunity for contamination and further permits the use of ambient temperature and efficient scale-up. In addition, the process permits the reliable and continuous monitoring of the dializate which enhances the efficiency of the entire process. The time of dialysis for the production of a lot of vaccine is reduced from 7-10 days to less than 72 hours and usually less than 48 or 24 hours. The use of the process optimizes the presence of each antigenic component in the preparation of multivalent vaccines.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY +1
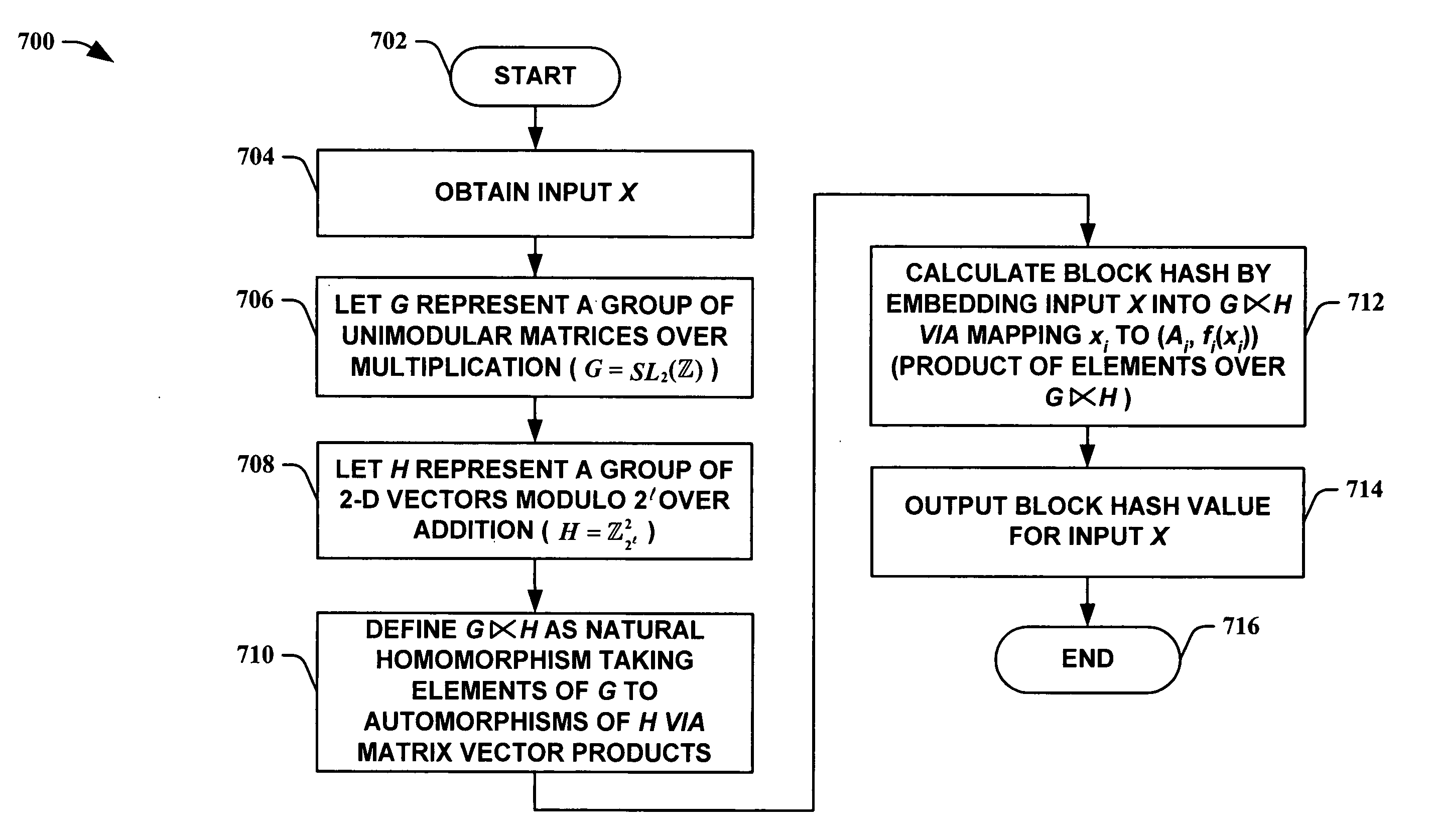
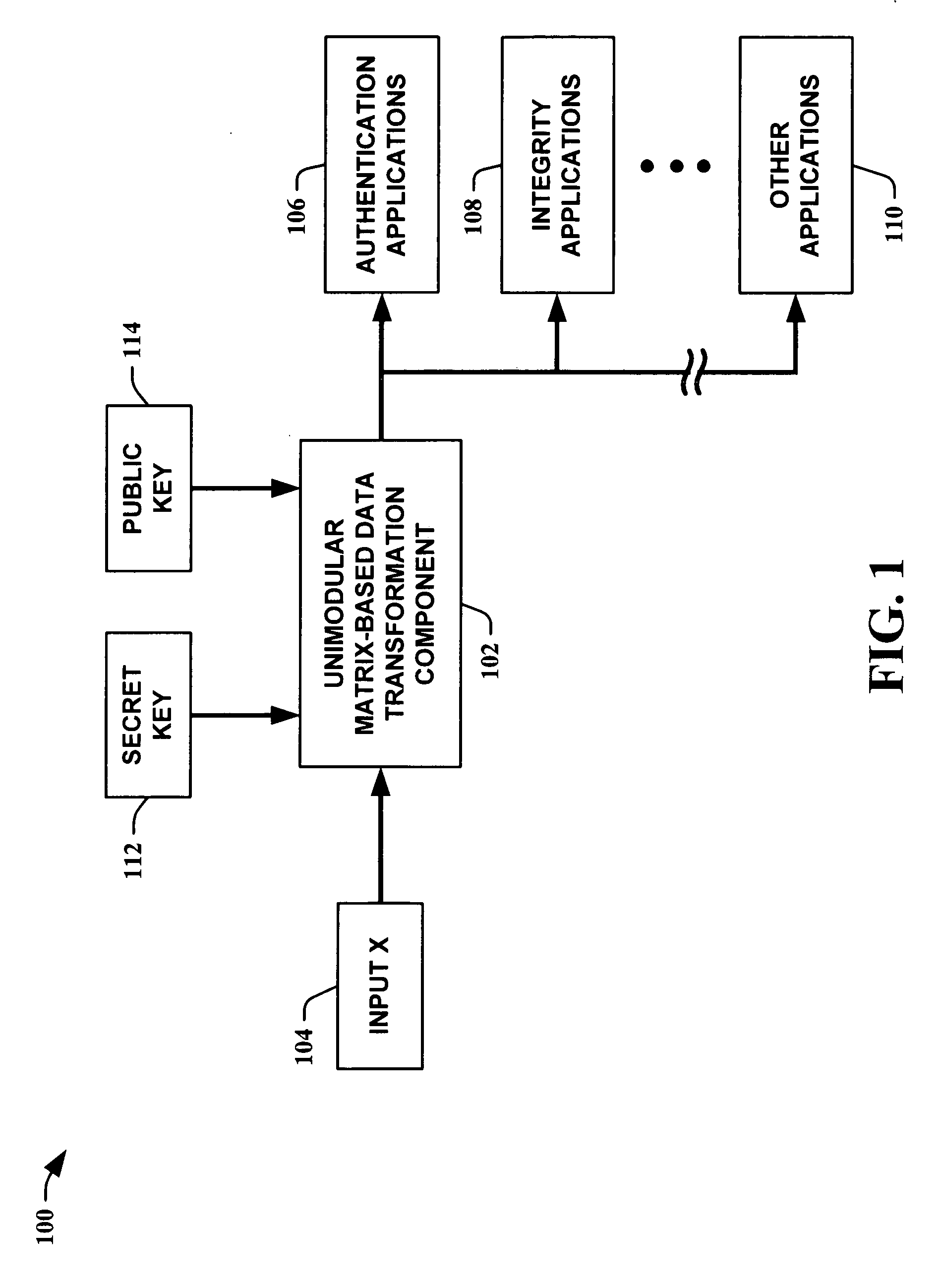
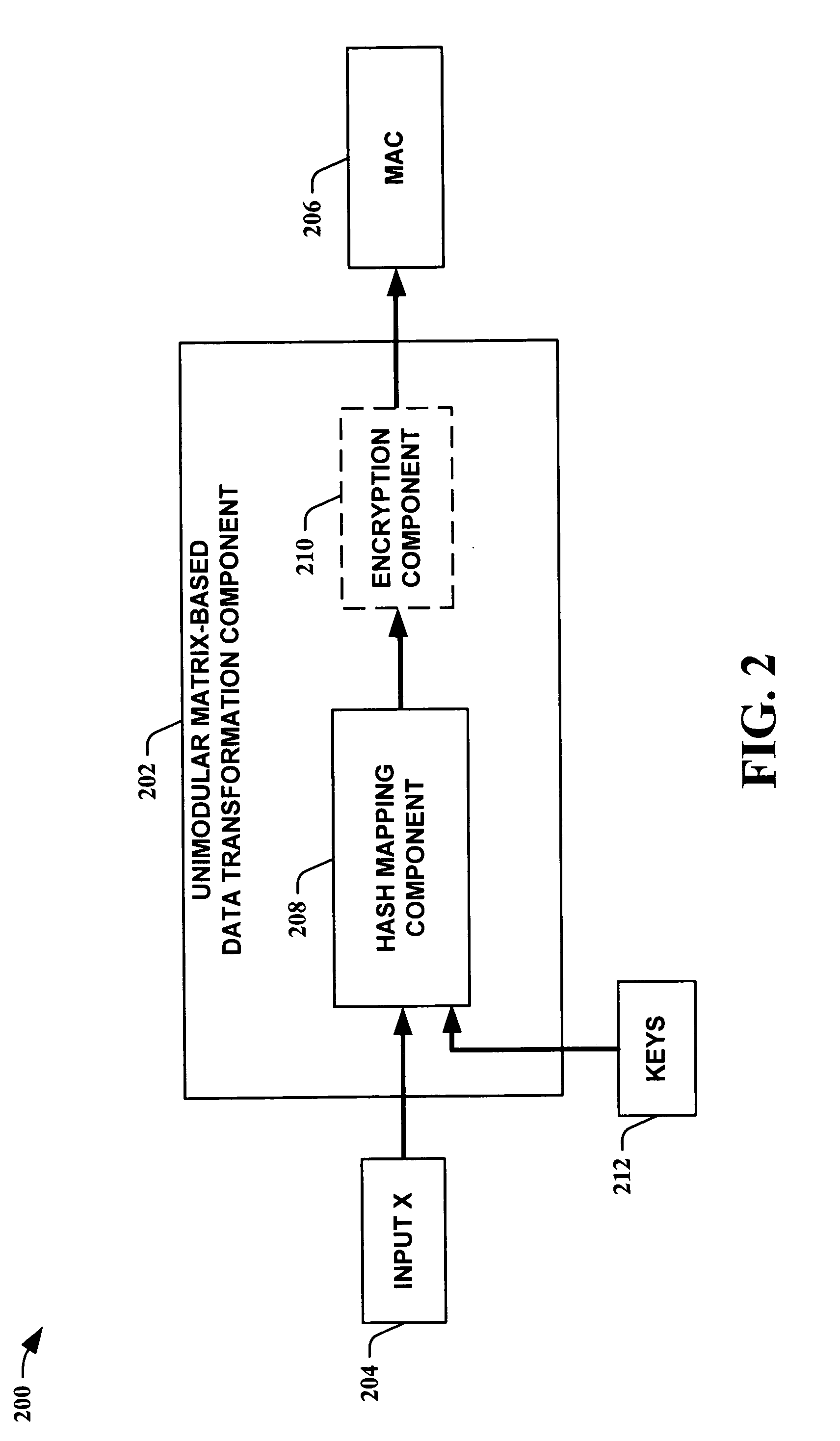
Unimodular matrix-based message authentication codes (MAC)
InactiveUS20050210260A1Improve speed performanceHigh performance hash value generationUser identity/authority verificationMemory systemsKey sizeUniversal hashing
The present invention leverages the invertibility of determinants of unimodular matrices to provide a universal hash function means with reversible properties and high speed performance. This provides, in one instance of the present invention, length controllable hash values comprised of vector pairs that can be processed as one instruction in a SIMD (single instruction, multiple data) equipped computational processor, where the vector pair is treated as a double word. The characteristics of the present invention permit its utilization in streaming cipher applications by providing key data to seed the ciphering process. Additionally, the present invention can utilize smaller key lengths than comparable mechanisms via inter-block chaining, can be utilized to double hash values via performing independent hash processes in parallel, and can be employed in applications, such as data integrity schemes, that require its unique processing characteristics.
Owner:MICROSOFT TECH LICENSING LLC
Gaming machine
InactiveUS6916243B2Enhance interestIncrease entertainmentRoulette gamesApparatus for meter-controlled dispensingAlgorithmDisplay device
Owner:KONAMI GAMING
Method for Differentially Quantifying Naturally Processed HLA-Restricted Peptides for Cancer, Autoimmune and Infectious Diseases Immunotherapy Development
The invention relates to a method for quantitatively identifying relevant HLA-bound peptide antigens from primary tissue specimens on a large scale without labeling approaches. This method can not only be used for the development of peptide vaccines, but is also highly valuable for a molecularly defined immunomonitoring and the identification of new antigens for any immunotherapeutic strategy in which HLA-restricted antigenic determinants function as targets, such as a variety of subunit vaccines or adoptive T-cell transfer approaches in cancer, or infectious and autoimmune diseases.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
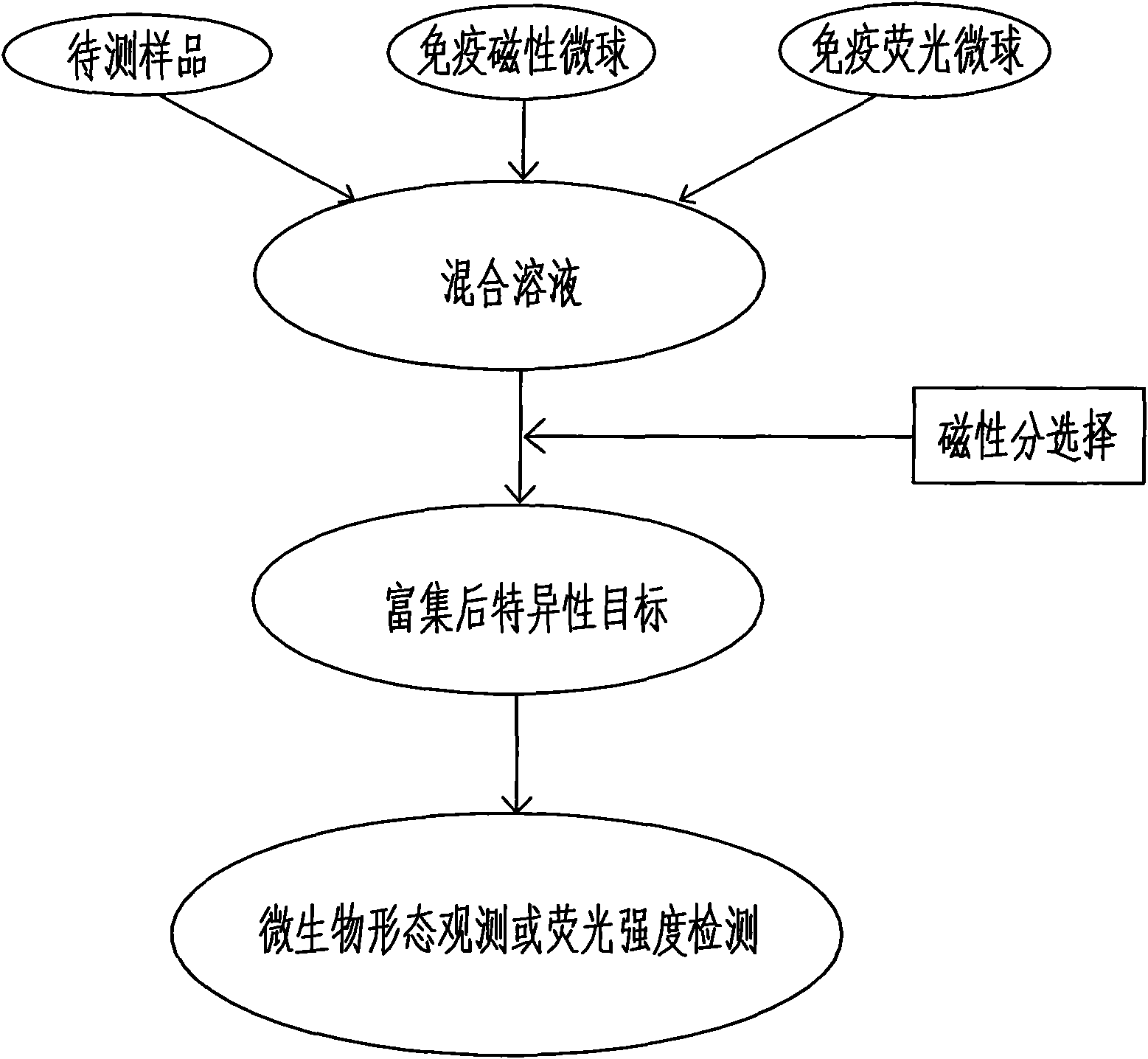
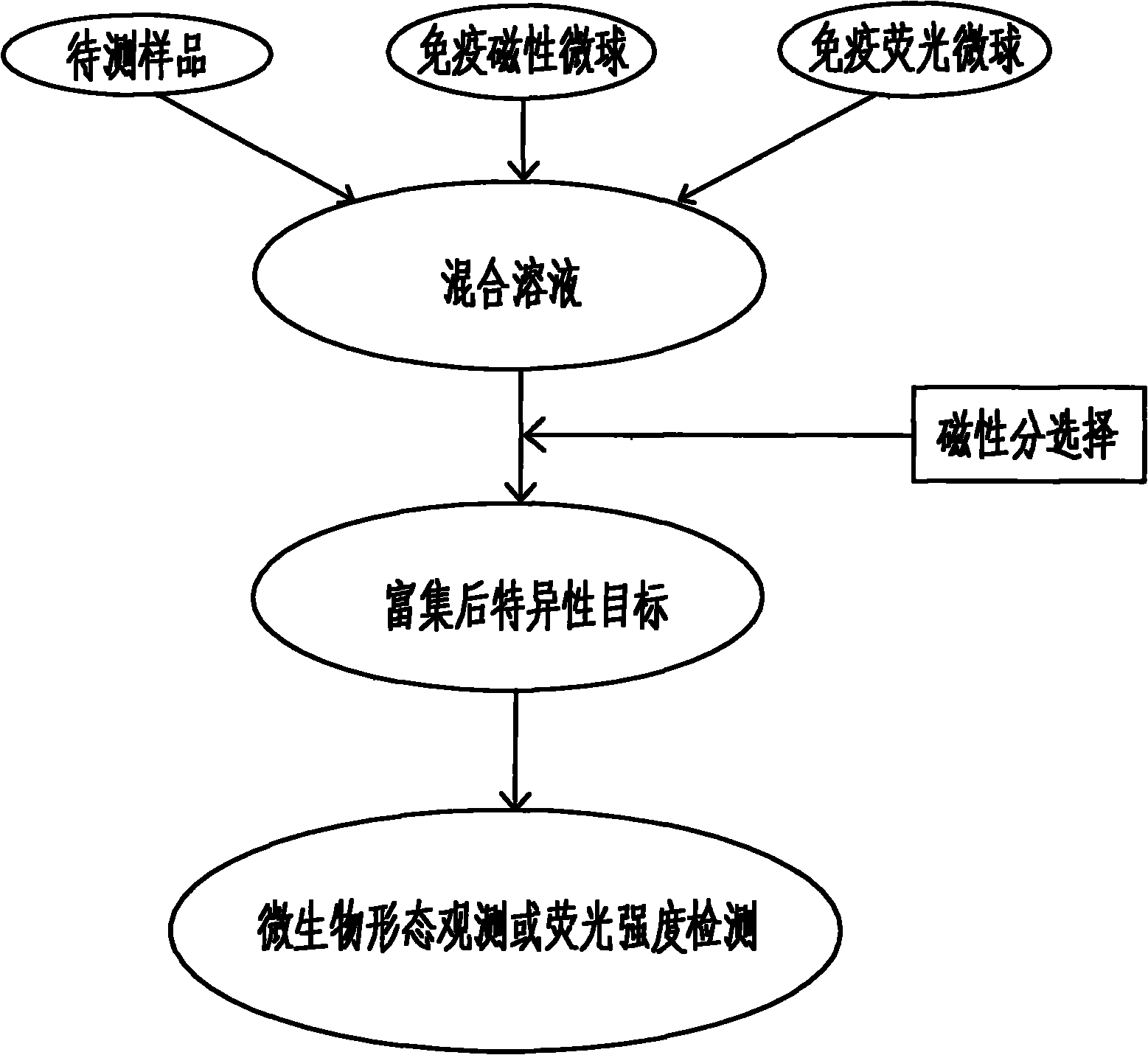
Magnetic fluorescent kit for rapidly detecting microbes as well as preparation method and use method thereof
InactiveCN102253193AEfficient separationRapid Quantitative AnalysisBiological testingFluorescence/phosphorescenceImmunofluorescenceMicrosphere
The invention discloses a magnetic fluorescent kit for rapidly detecting microbes as well as a preparation method and a use method thereof. The kit comprises two components: 1) immunomagnetic microspheres specifically bound with the microbes to be detected; and 2) immunofluorescent microspheres specifically bound with the microbes to be detected. The preparation method comprises the following steps: (1) preparation of the immunomagnetic microspheres; and (2) preparation of the immunofluorescent microspheres. The use method comprises the following steps: (1) adding the sample to be detected, lyophilized powder of the immunomagnetic microspheres and the immunofluorescent microspheres to a buffer solution; (2) ensuring the surfaces of the identified microbes to have antigenic determinants simultaneously bound with the immunomagnetic microspheres and the immunofluorescent microspheres; (3) enriching the microbes bound with the immunomagnetic microspheres through magnetic separation of the immunomagnetic microspheres; and (4) qualitatively and quantitatively judging the microbes by measuring the fluorescence intensity of the immunofluorescent microspheres bound with the separated and enriched microbes. The kit, the preparation method and the use method have the advantages of rapidness, quantitative property and wide scope of application.
Owner:EMERGING THERAPEUTICS SHANGHAI CO LTD
Method of targeted gene delivery using viral vectors
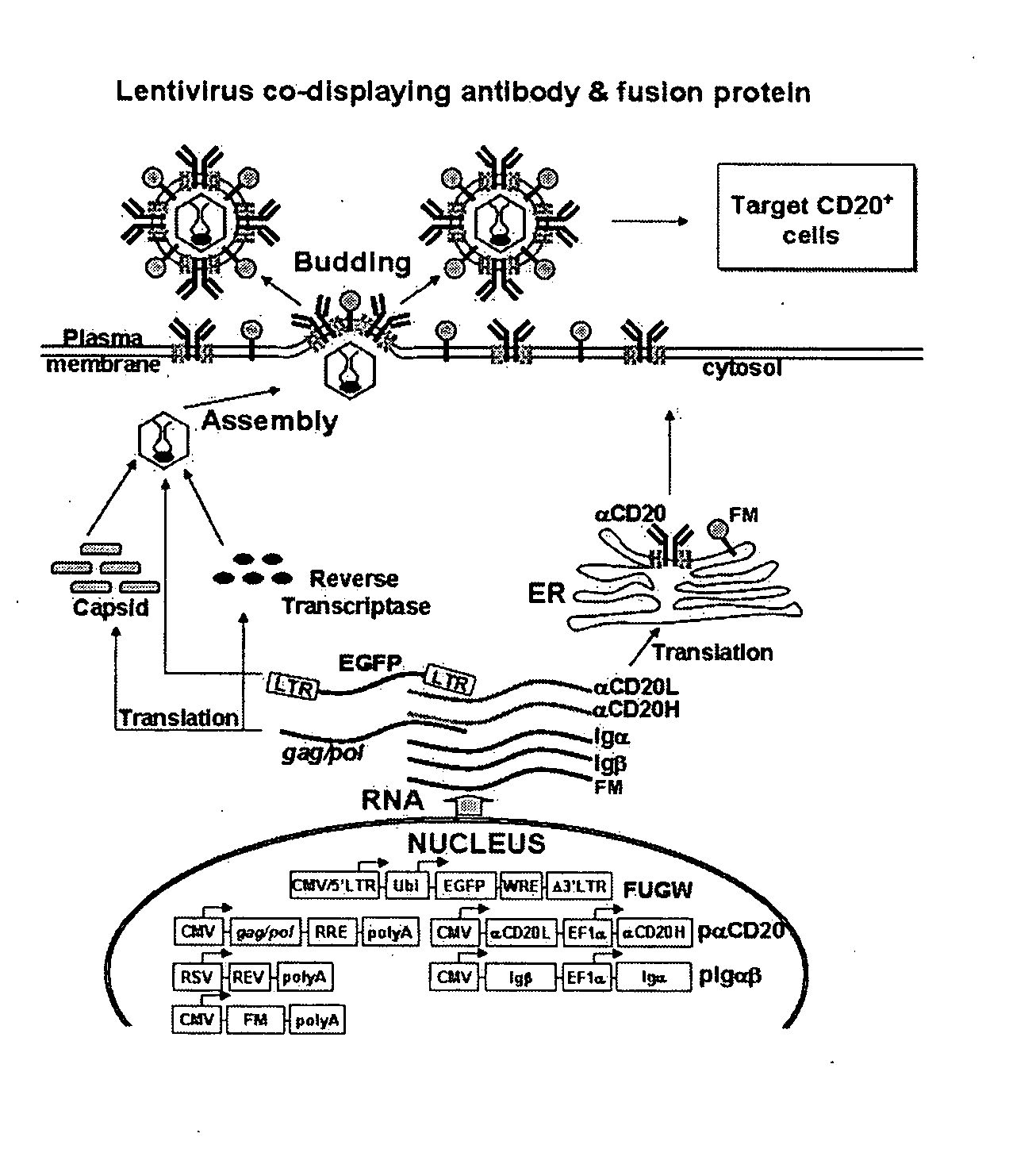
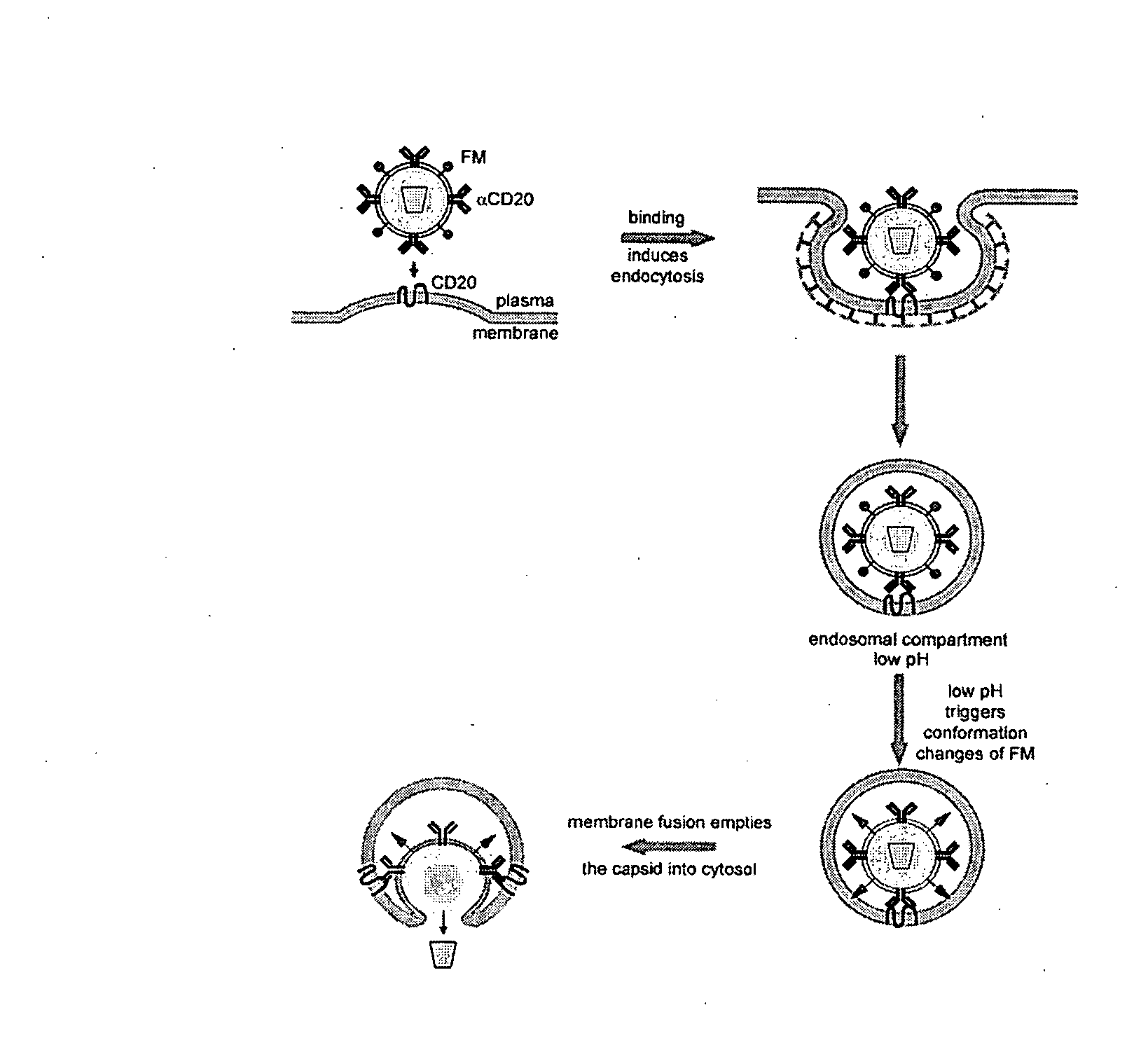
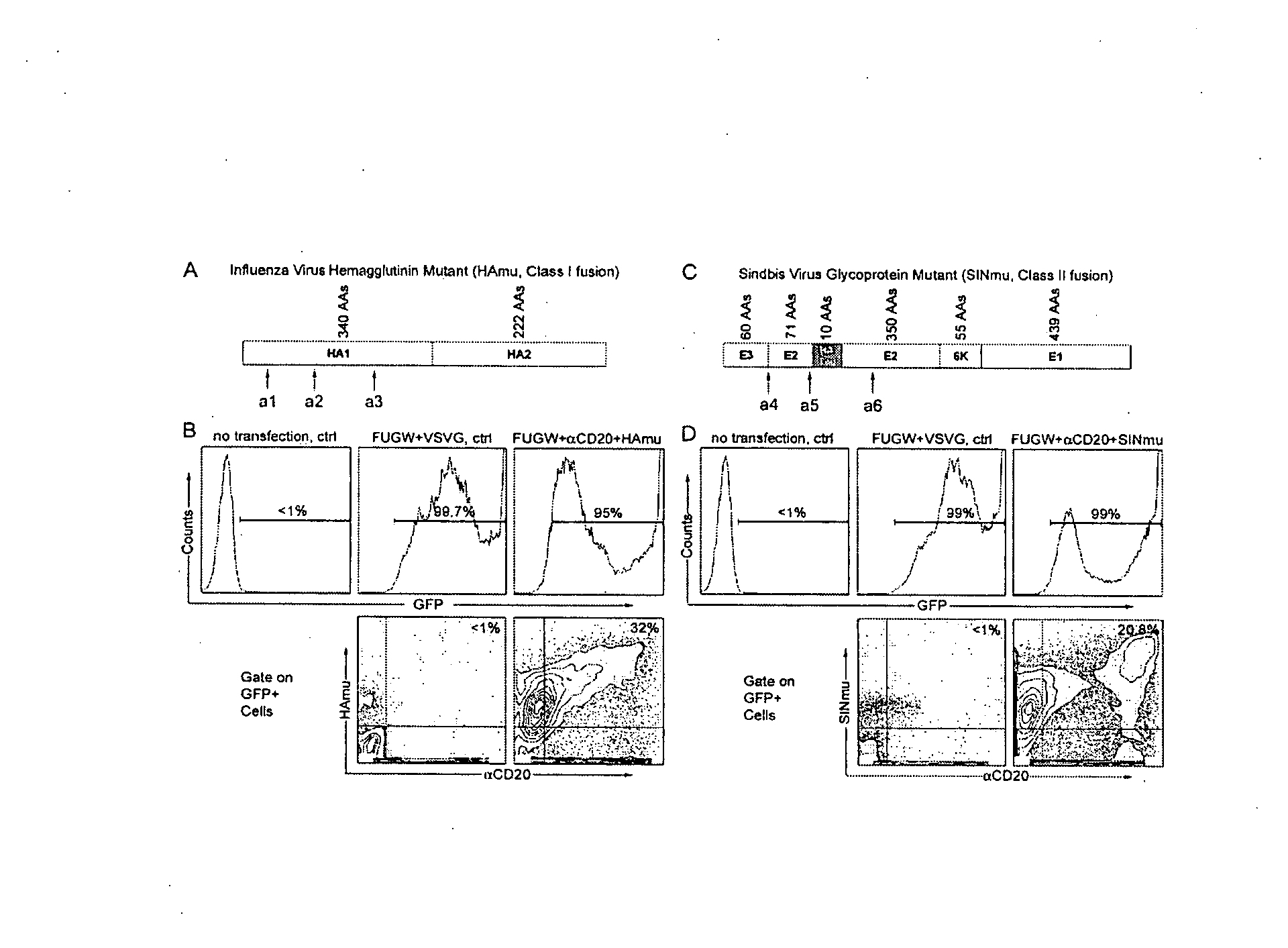
Methods and compositions are provided for delivering a polynucleotide encoding a gene of interest to a target cell using a virus. The virus envelope comprises a cell-specific binding determinant that recognizes and binds to a component on the target cell surface, leading to endocytosis of the virus. A separate fusogenic molecule is also present on the envelope and facilitates delivery of the polynucleotide across the membrane and into the cytosol of the target cell. The methods and related compositions can be used for treating patients having suffering from a wide range of conditions, including infection, such as HIV; cancers, such as non-Hodgkin's lymphoma and breast cancer; and hematological disorders, such as severe combined immunodeficiency.
Owner:CALIFORNIA INST OF TECH
Inverse kinematics solution method for six-degree-of-freedom serial robot
ActiveCN102637158AAvoid problems with rank less than orderIngenious ideaComplex mathematical operationsRobot kinematicsTabu search
The invention discloses an inverse kinematics solution method for a six-degree-of-freedom serial robot. The inverse kinematics solution method comprises the steps of: establishing a connecting rod coordinate system and setting variables theta 1, theta 2, theta 3, theta 4, theta 5 and theta 6; setting an initial configuration; solving theta 4, theta 5 and theta 6 by utilizing a geometric method; and eliminating theta 1, theta 2 and theta 3 by utilizing an algebra elimination method and introducing a tabu search algorithm when solving a non-orthogonal spheroid or the terminal structure of the non-orthogonal spheroid, thereby solving out corresponding numerical solutions. The inverse kinematics solution method is smart in conception and utilizes the geometric method and the algebra elimination method for comprehensive solution, thereby avoiding the problem that the rank of an equation determinant of coefficient is smaller than order caused by arbitrary establishing of equations and correctly obtaining the analytic solutions of six axes efficiently; and for complex-structure trigonometric function relationship, a linear equation in two unknowns can be effectively transformed to a linear equation with one unknown by the elimination method in the use of the geometric method, and therefore a unique corresponding analytic solution is obtained.
Owner:CHENGDU CRP ROBOT TECH CO LTD
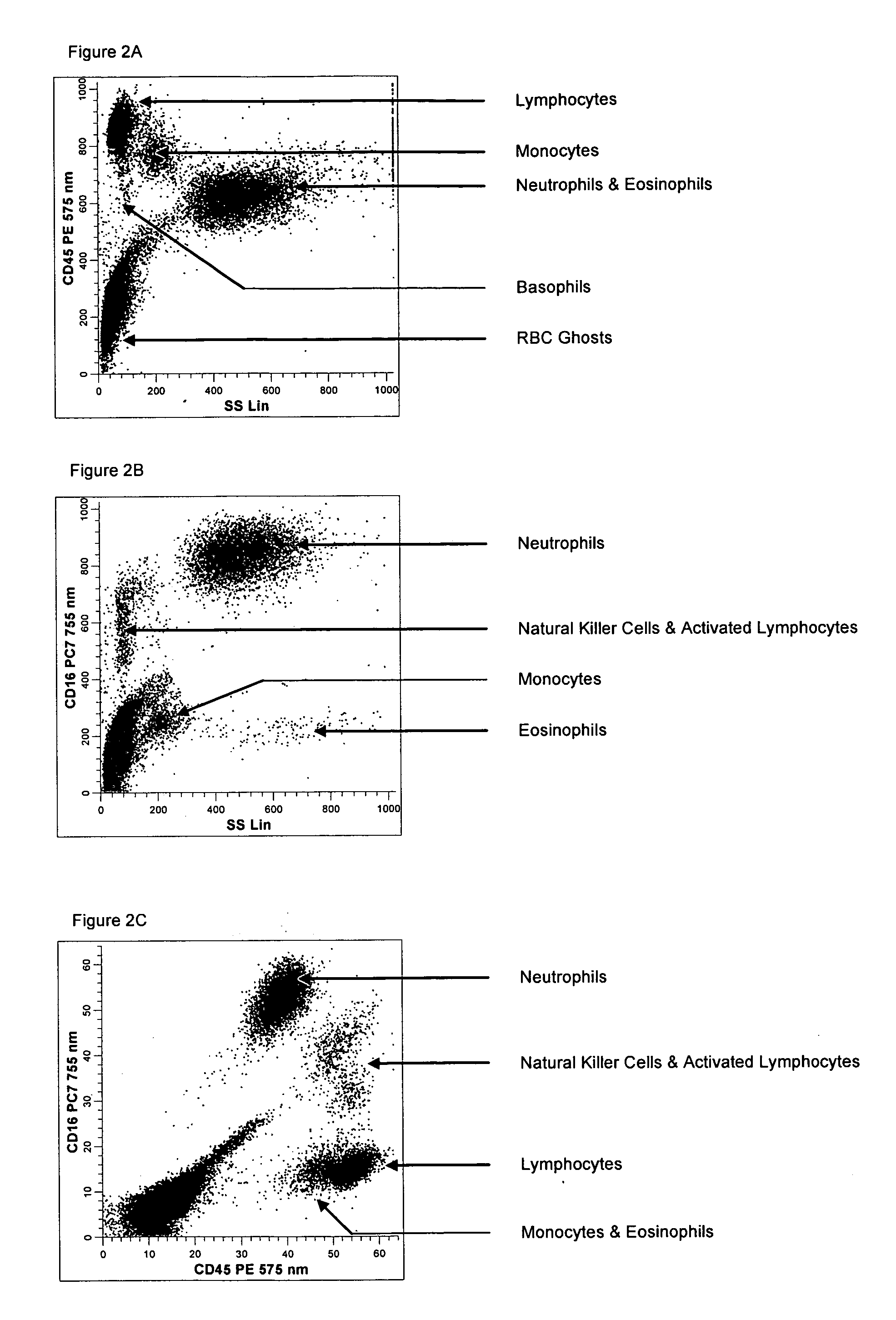
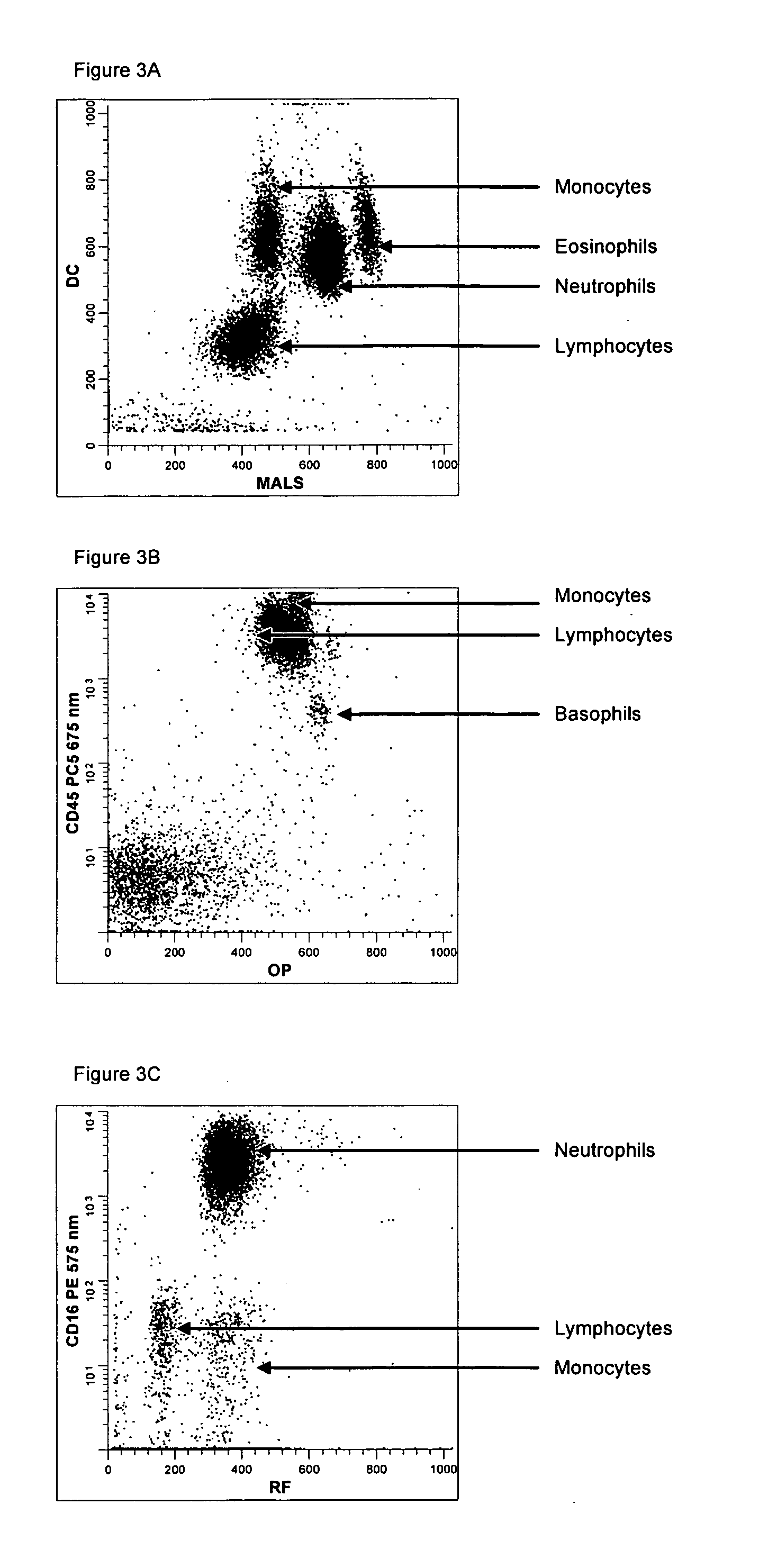
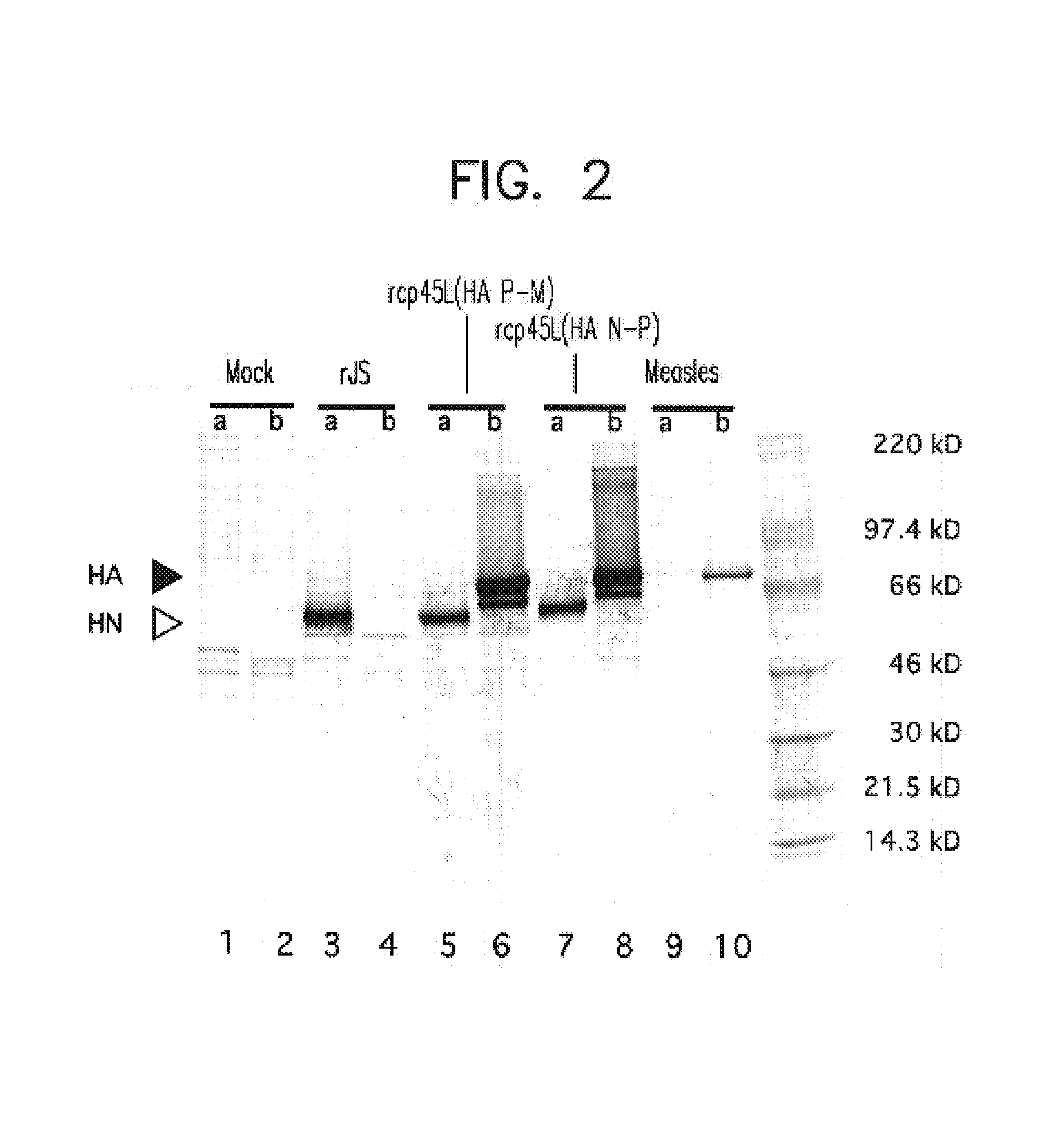
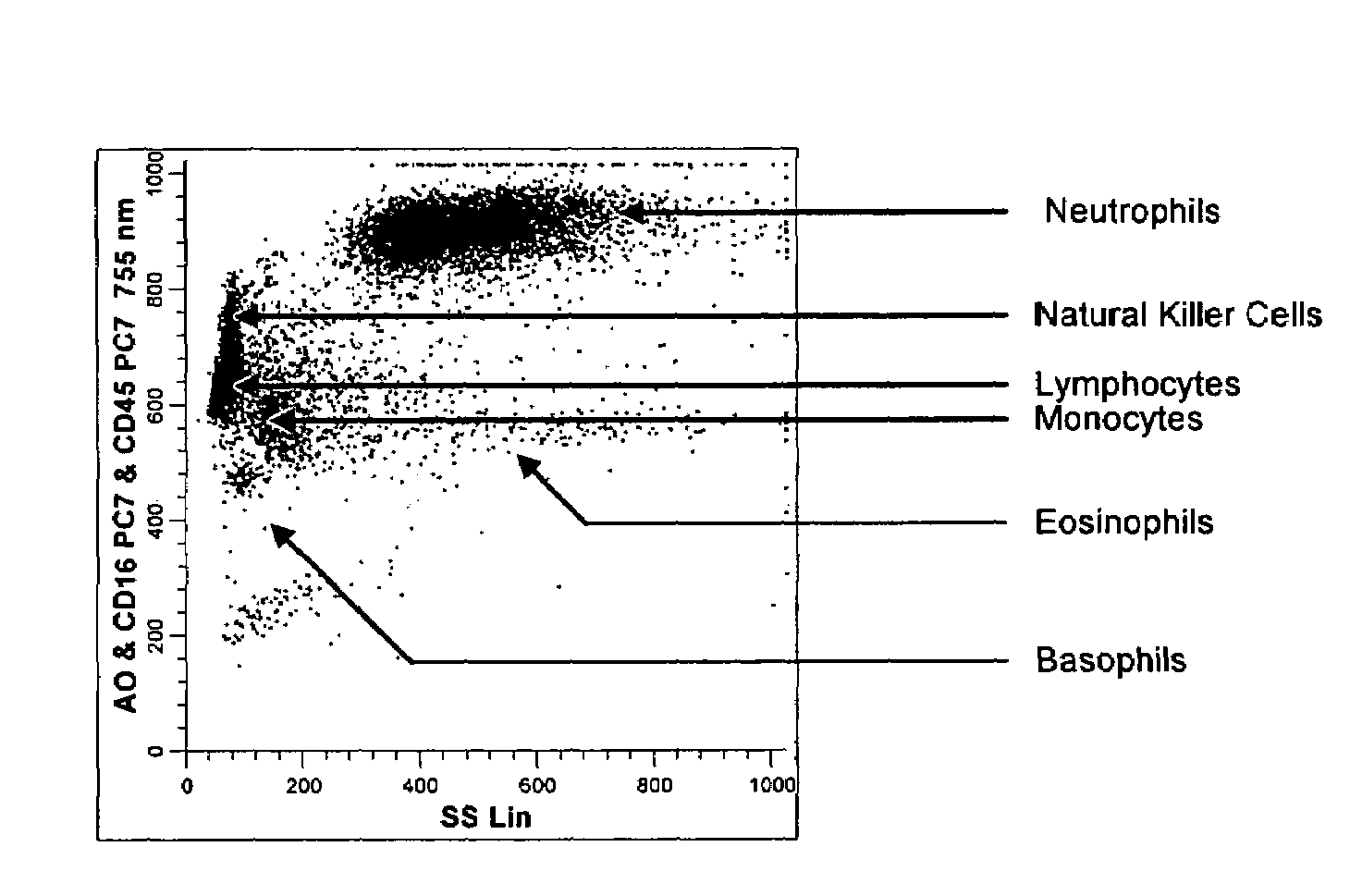
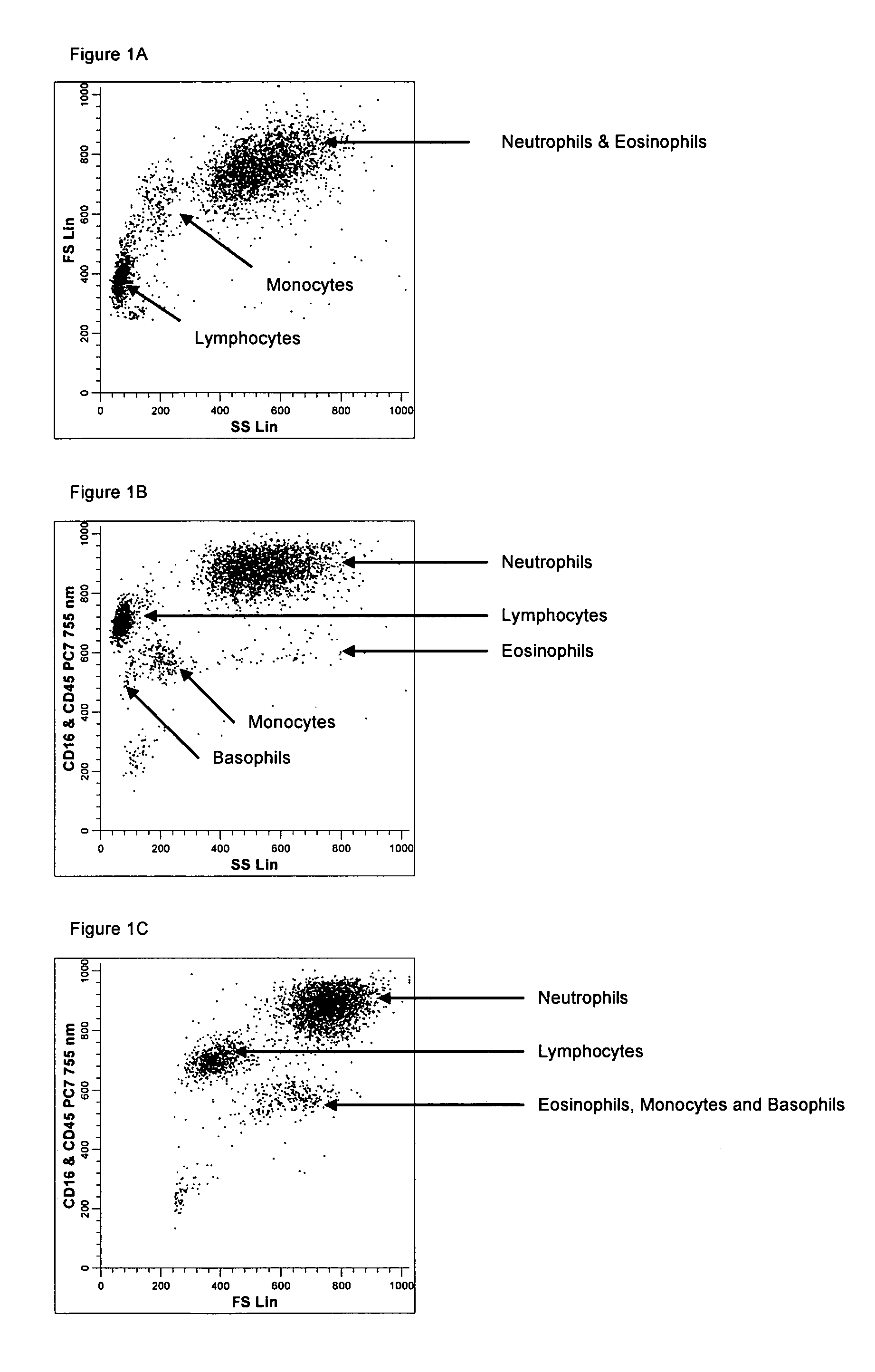
Method for a fully automated monoclonal antibody-based extended differential
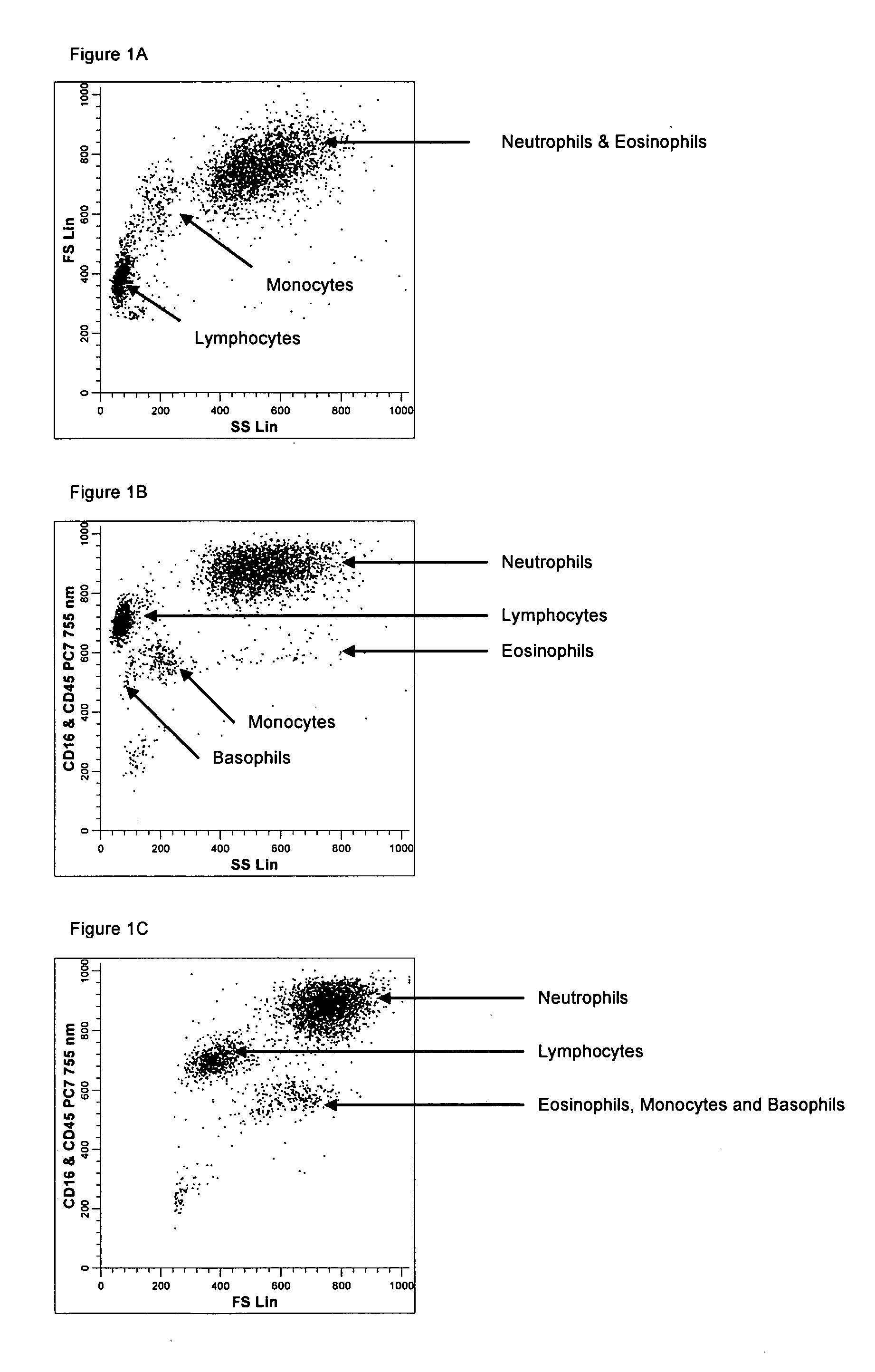
ActiveUS20050260766A1Great advanceHigh degree of sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenWhite blood cell
A method useful for the enumeration of cell populations in a biological sample includes the steps of reacting in a single reaction mixture a sample, a first antibody labeled with a fluorochrome having a first emission spectrum and an additional antibody. The first antibody binds to an antigenic determinant differentially expressed on leukocytes and non-leukocytes. The additional antibody binds to an antigenic determinant differentially expressed on mature and immature granulocytes or myeloid cells, and is labeled either with the first fluorochrome or an additional fluorochrome having an emission spectrum distinguishable from the first emission spectrum. The reaction mixture can be mixed with a nucleic acid dye having an emission spectrum that overlaps with one of the first or additional emission spectra. The reaction mixture may be treated with a lytic system that differentially lyses non-nucleated red blood cells and conserves leukocytes. Populations of hematological cells are detected and enumerated using at least two parameters (fluorescence, optical, and electrical) for each population.
Owner:BECKMAN COULTER INC
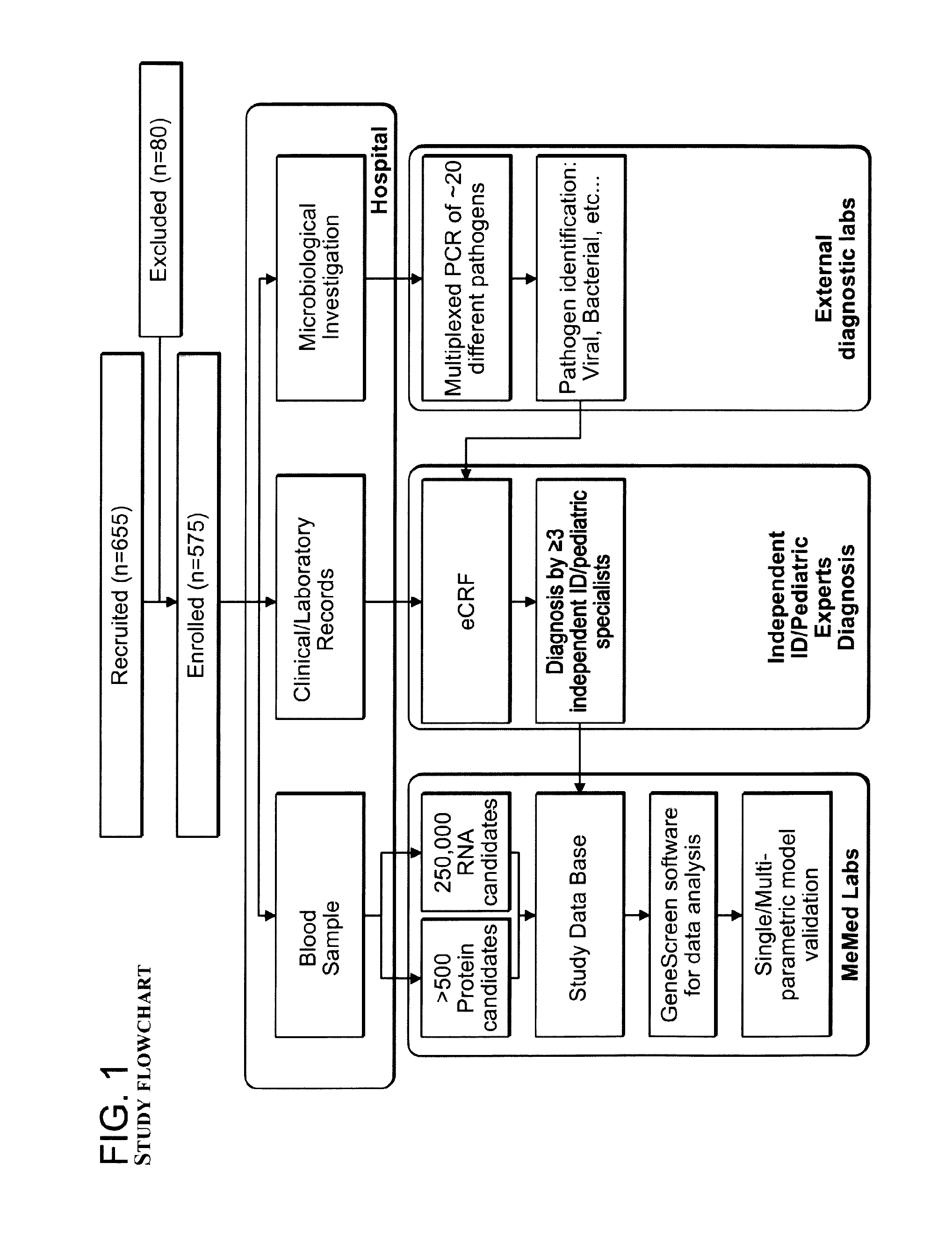
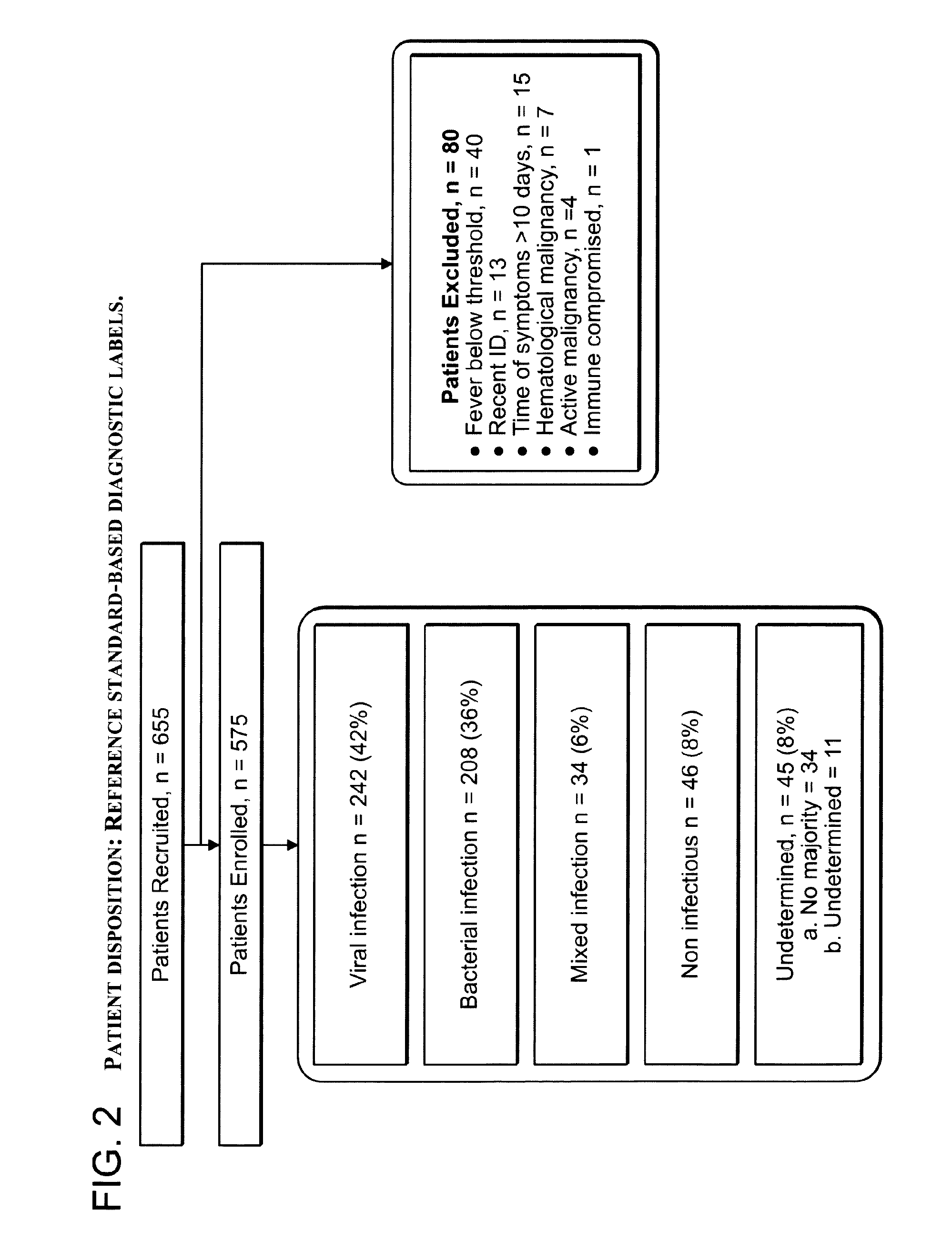
Signatures and determinants for diagnosing infections and methods of use thereof
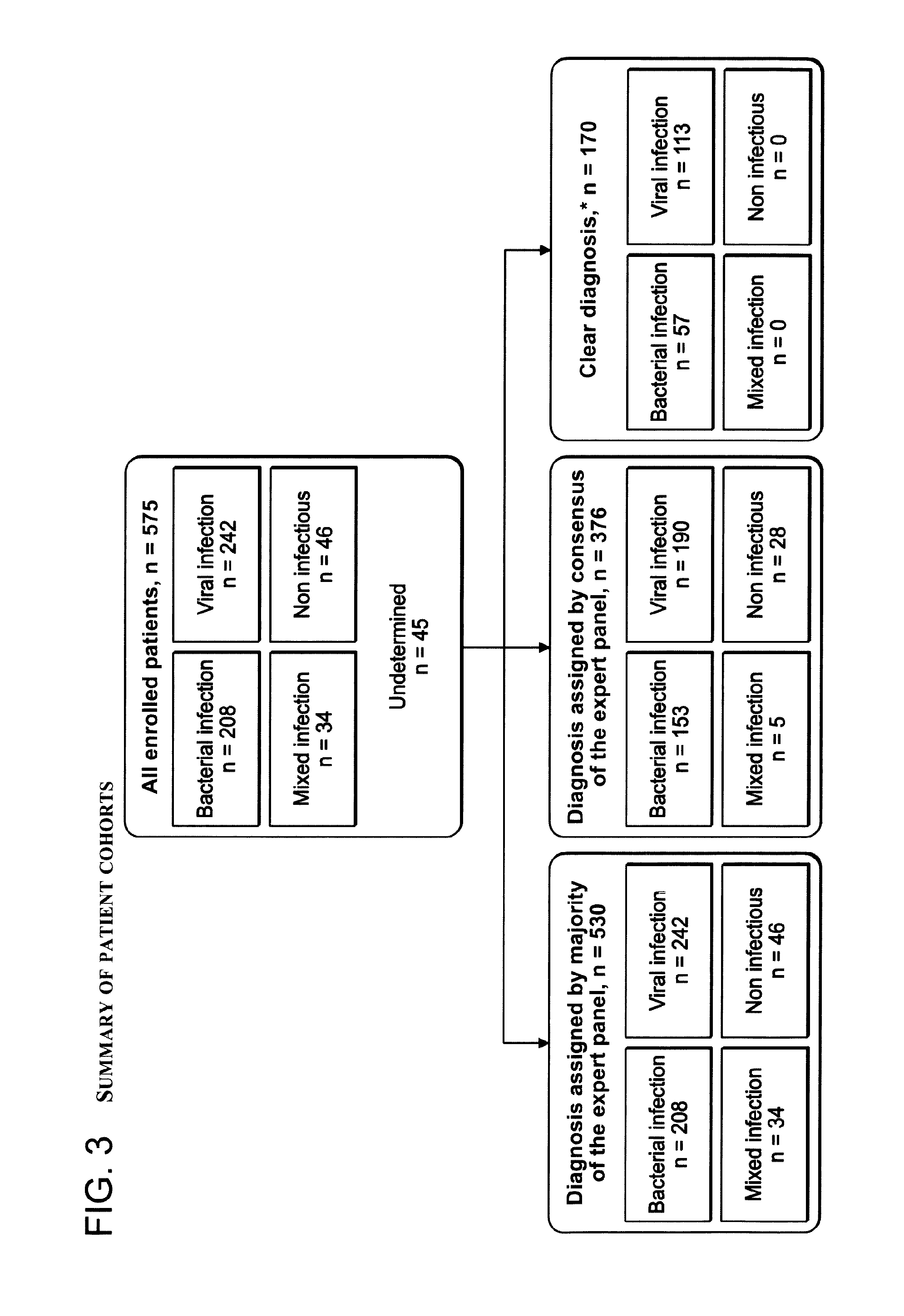
ActiveUS20150017630A1Prevent unnecessary antibiotic treatmentRapid diagnosisElectrolysis componentsVolume/mass flow measurementPoint of careSide effect
Antibiotics (Abx) are the world's most misused drugs. Antibiotics misuse occurs when the drug is administered in case of a non-bacterial infection (such as a viral infection) for which it is ineffective. Overall, it is estimated that 40-70% of the worldwide Abx courses are mis-prescribed. The financial and health consequences of Abx over-prescription include the direct cost of the drugs, as well as the indirect costs of their side effects, which are estimated at >$15 billion annually. Furthermore, over-prescription directly causes the emergence of Abx-resistant strains of bacteria, which are recognized as one of the major threats to public health today. This generates an immediate need for reliable diagnostics to assist physicians in correct Abx prescription, especially at the point-of-care (POC) where most Abx are prescribed. Accordingly, some aspects of the present invention provide methods using biomarkers for rapidly detecting the source of infection and administrating the appropriate treatment.
Owner:MEMED DIAGNOSTICS
High affinity anti-human IgE antibodies
The invention relates to high affinity human monoclonal antibodies, particularly those directed against isotypic determinants of immunoglobulin E (IgE), as well as direct equivalents and derivatives of these antibodies. These antibodies bind to their respective target with an affinity at least 100 fold greater than the original parent antibody. These antibodies are useful for diagnostics, prophylaxis and treatment of disease.
Owner:TANOX
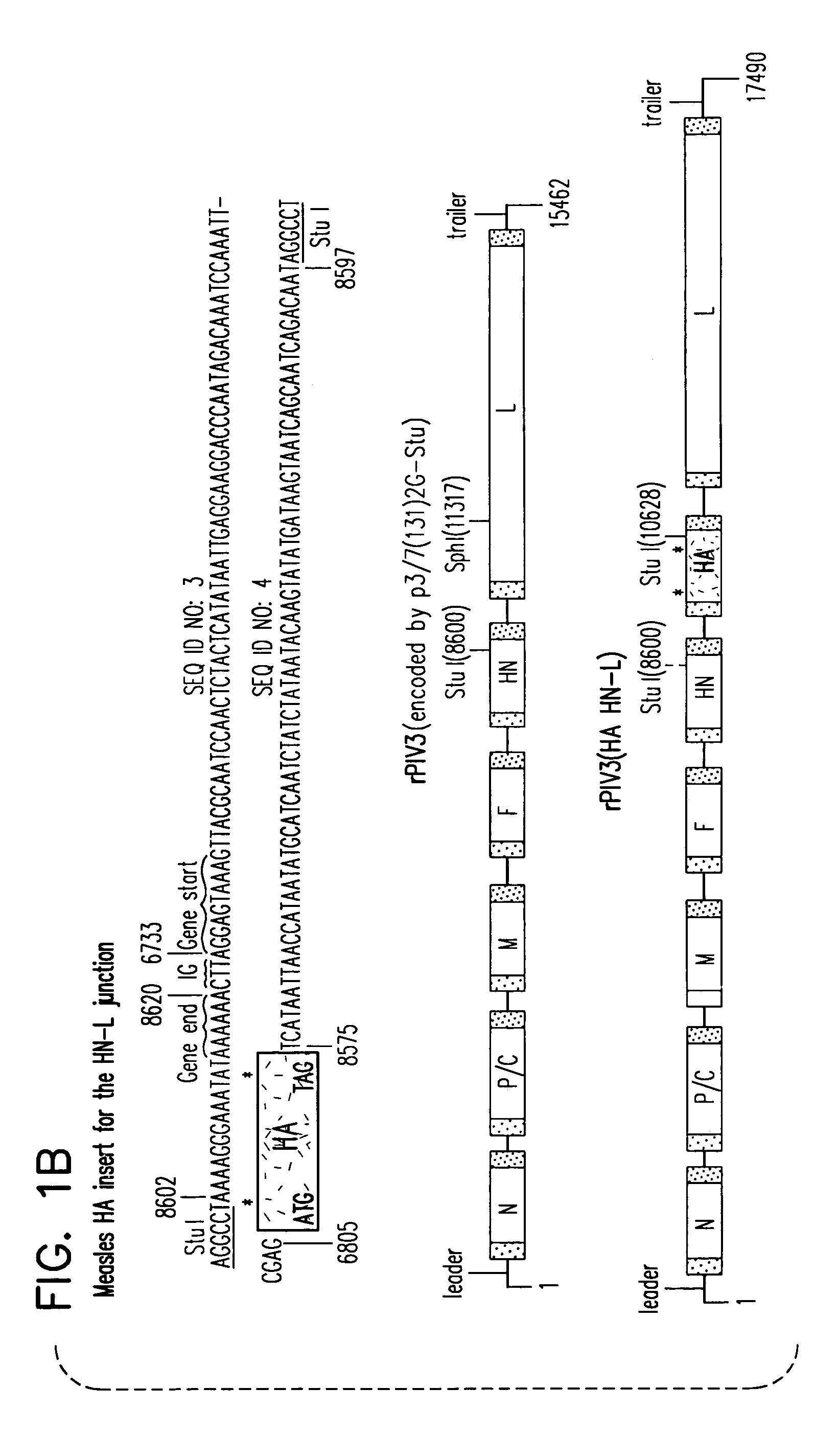
Construction and use of recombinant parainfluenza viruses expressing a chimeric glycoprotein
InactiveUS7250171B1Prone to infectionHigh titerSsRNA viruses negative-senseOrganic active ingredientsHeterologousEpitope
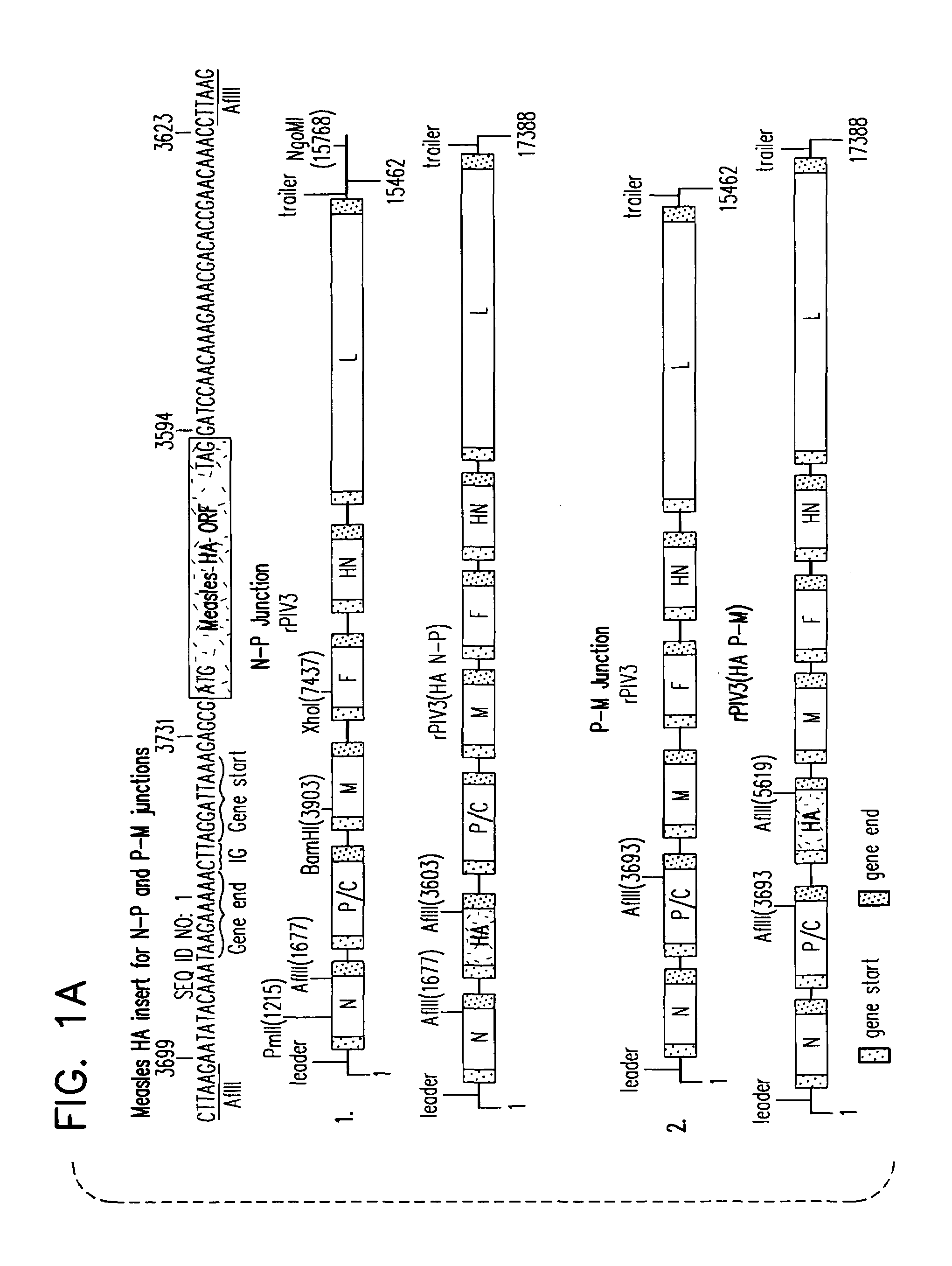
Chimeric parainfluenza viruses (PIVs) are provided that incorporate a PIV vector genome or antigenome modified to encode a chimeric glycoprotein incorporating one or more heterologous antigenic domains, fragments, or epitopes of a second, antigenically distinct HPIV. These chimeric viruses are infectious and attenuated in humans and other mammals and are useful in vaccine formulations for eliciting an immune responses against one or more PIVs, and, optionally against respiratory syncytial virus (RSV). Also provided are isolated polynucleotide molecules and vectors incorporating a chimeric PIV genome or antigenome which includes a HPIV vector genome or antigenome combined or integrated with one or more heterologous genome segment(s) encoding one or more antigenic determinant(s) of a heterologous PIV to encode a chimeric glycoprotein. In preferred aspects of the invention, the chimeric virus is attenuated for use as a vaccine agent by additional mutations or nucleotide modifications introduced into the chimeric genome or antigenome.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE
Method for a fully automated monoclonal antibody-based extended differential
ActiveUS7625712B2Bioreactor/fermenter combinationsBiological substance pretreatmentsFluorescenceWhite blood cell
A method useful for the enumeration of cell populations in a biological sample includes the steps of reacting in a single reaction mixture a sample, a first antibody labeled with a fluorochrome having a first emission spectrum and an additional antibody. The first antibody binds to an antigenic determinant differentially expressed on leukocytes and non-leukocytes. The additional antibody binds to an antigenic determinant differentially expressed on mature and immature granulocytes or myeloid cells, and is labeled either with the first fluorochrome or an additional fluorochrome having an emission spectrum distinguishable from the first emission spectrum. The reaction mixture can be mixed with a nucleic acid dye having an emission spectrum that overlaps with one of the first or additional emission spectra. The reaction mixture may be treated with a lytic system that differentially lyses non-nucleated red blood cells and conserves leukocytes. Populations of hematological cells are detected and enumerated using at least two parameters (fluorescence, optical, and electrical) for each population.
Owner:BECKMAN COULTER INC
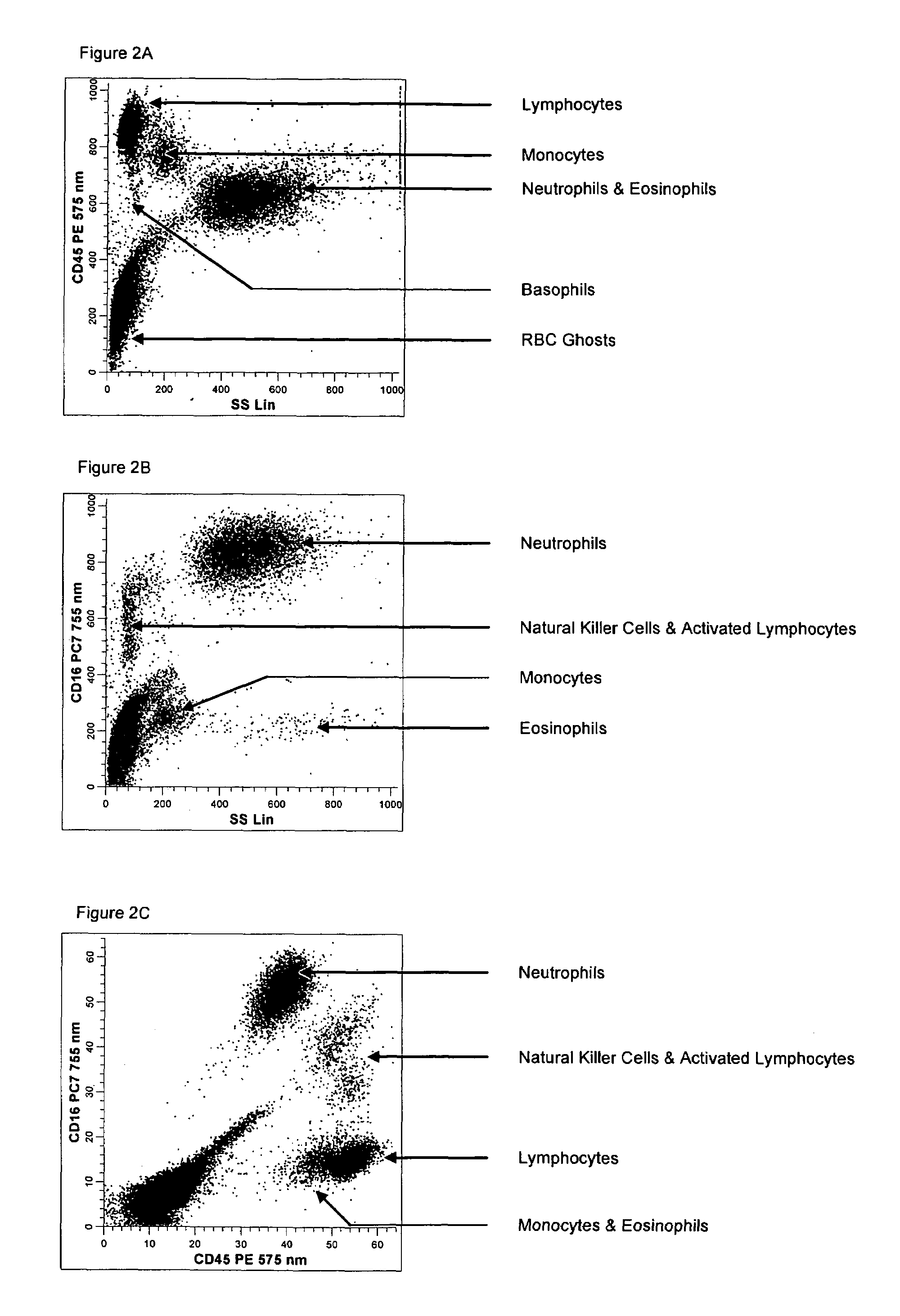
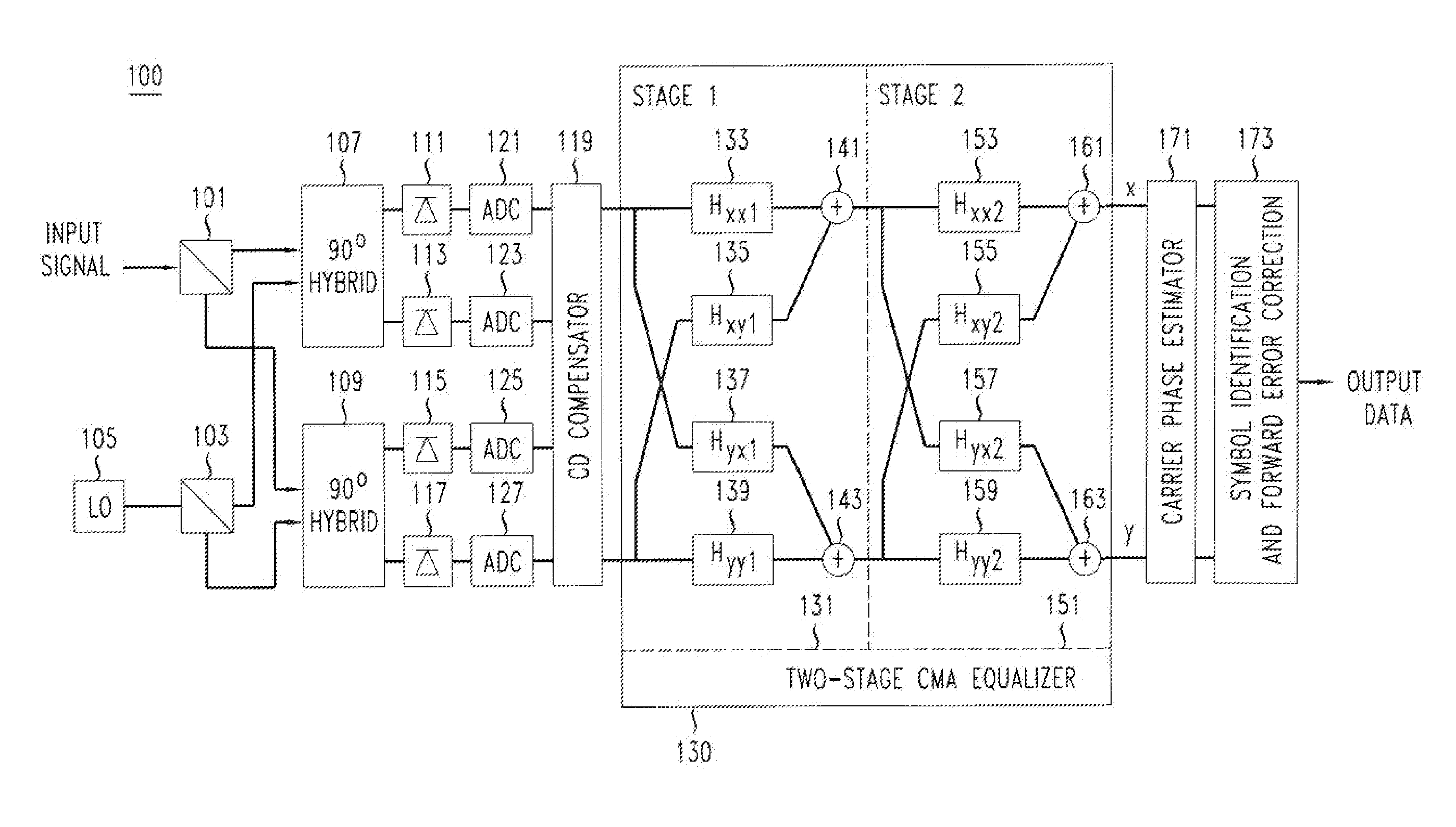
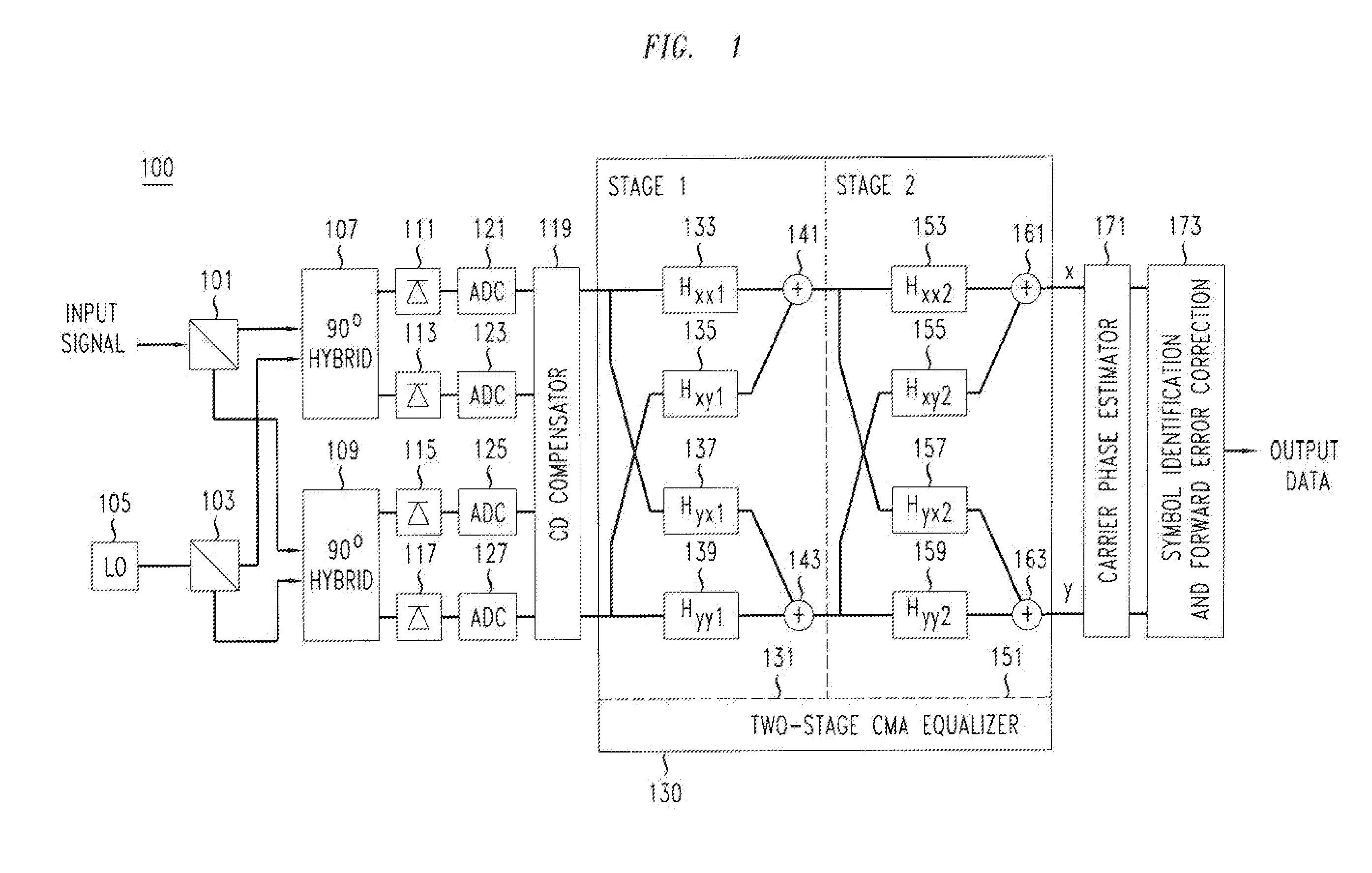
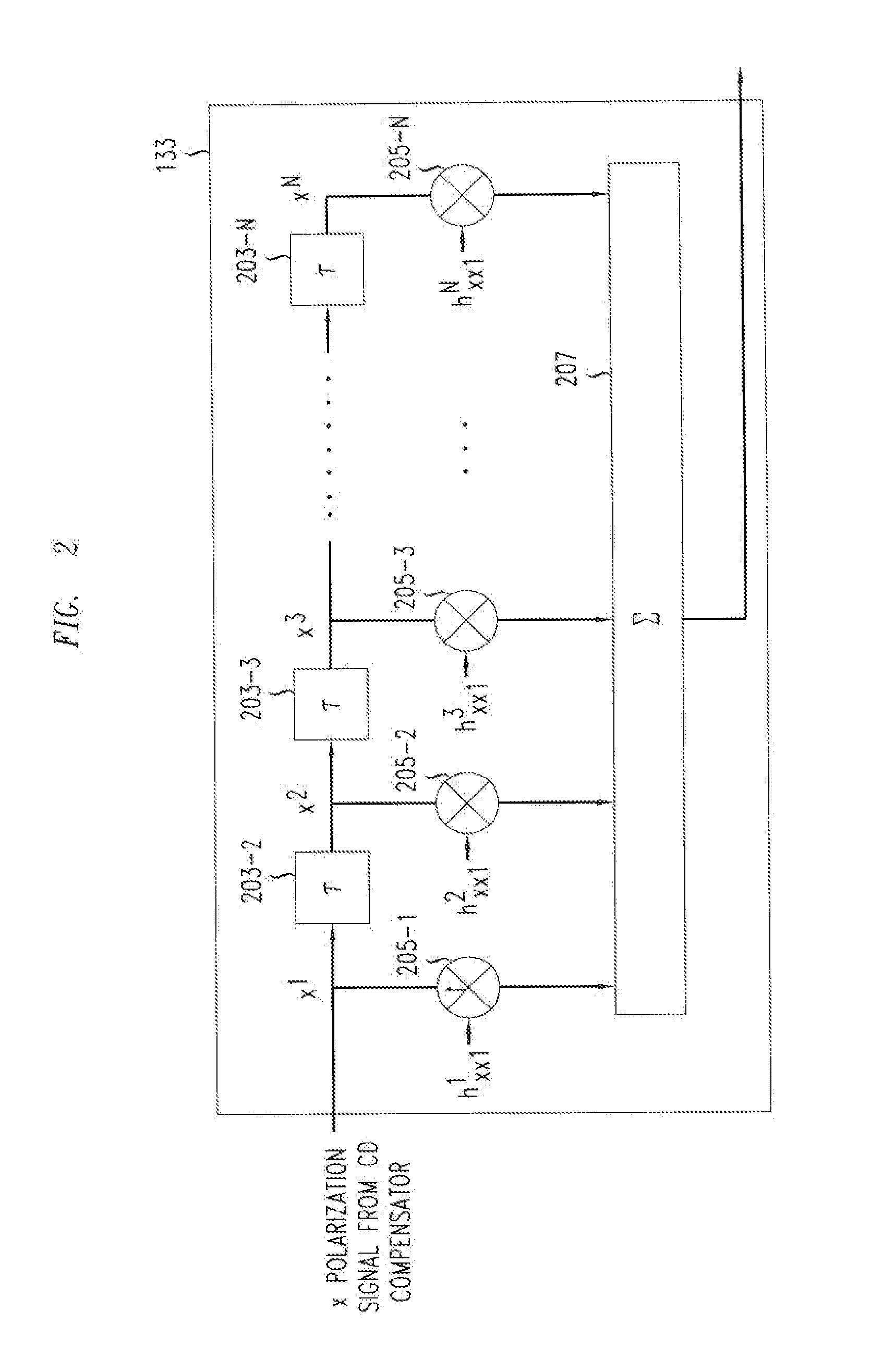
Method And Apparatus For Polarization-Division-Multiplexed Optical Coherent Receivers
ActiveUS20110142449A1Avoid problemsPolarisation multiplex systemsElectromagnetic receiversSingle stageEngineering
The singularity problem of the constant modulus algorithm (CMA) equalizer may be overcome by implementing the CMA equalizer as a two-stage equalizer, with the first stage being a modified version of a CMA equalizer and the second stage being a conventional CMA equalizer. The first stage may be made up of four sub-equalizers, of which only two of the sub-equalizers are independent, i.e., uncorrelated to each other. This first stage equalizer may compensate for PMD. The second stage equalizer is a conventional CMA equalizer made up of four sub-equalizers that are adjusted independently. This second stage equalizer may compensate for polarization-dependent loss (PDL) and any residual CD that is not fully compensated for by a CD compensator before the two-stage equalizer. Advantageously, as the determinant of the first stage never approaches zero, the singularity problem of a conventional CMA single-stage-only equalizer is avoided by the two-stage equalizer.
Owner:ALCATEL LUCENT SAS
Molecular antigen array
InactiveUS7264810B2Efficient inductionHighly ordered and repetitive antigenVirusesPeptide/protein ingredientsSpecific immunityAllergy
The present invention is related to the fields of molecular biology, virology, immunology and medicine. The invention provides a composition comprising an ordered and repetitive antigen or antigenic determinant array. The invention also provides a process for producing an antigen or antigenic determinant in an ordered and repetitive array. The ordered and repetitive antigen or antigenic determinant is useful in the production of vaccines for the treatment of infectious diseases, the treatment of allergies and as a pharmaccine to prevent or cure cancer and to efficiently induce self-specific immune responses, in particular antibody responses.
Owner:KUROS BIOSCIENCES AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com