Optimization of determinants for successful genetic correction of diseases, mediated by hematopoietic stem cells
a technology of hematopoietic stem cells and determinants, which is applied in the field of optimizing determinants for successful genetic correction of diseases, can solve the problems of reducing titers, limited treatment options for lsds, and little to address cns pathologies for this group of disorders, so as to enhance the stability and safety of gene expression and enhance the stability and safety of transgene expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Lentivirus Cis Elements Required for Efficient Packaging of Large Transgenes Cassettes Like β-Globin
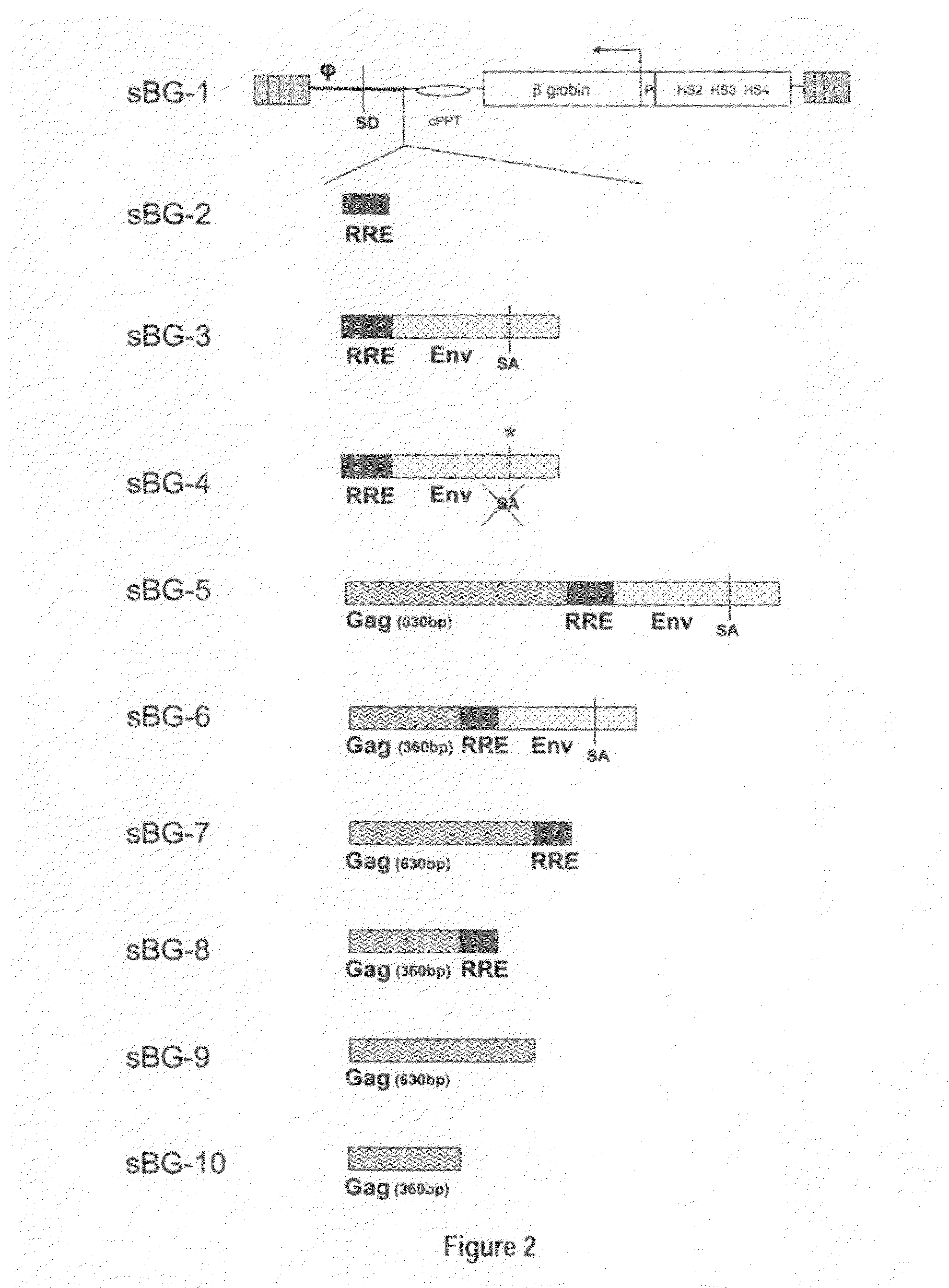
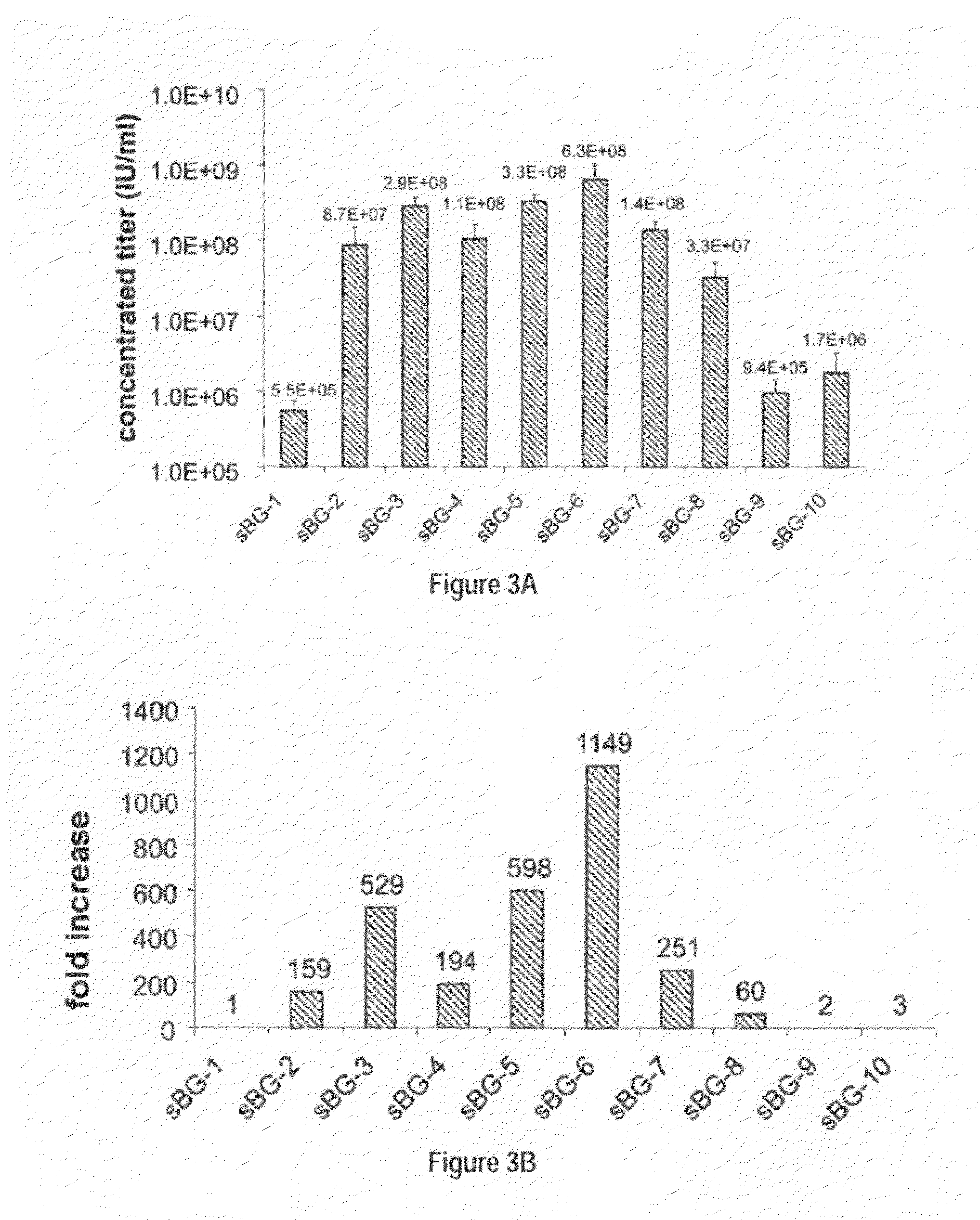
[0118]This study investigated whether lentivirus non-coding cis-sequences played a specific role in the RNA export, packaging or expression of β-globin. The vector life-cycle was studied in self-inactivating (SIN)-lentiviruses, carrying the β-globin gene and locus control region (BG), or GFP cDNA. Systematic analysis started with a completely ‘gutted’ minimal SIN-lentivirus carrying only the packaging region; and SIN-lentiviruses containing increasing HIV cis-elements, along with a SIN-gamma-retrovirus. It was discovered that (i) SIN-gamma-retrovirus or a gutted / minimal SIN-lentivirus encoding GFP generated high titers and mediated high GFP expression. (ii) However, SIN-gamma-retrovirus or the gutted SIN-lentivirus encoding either BG or a similar sized large transgene had barely detectable titers compared to the SIN-lentivirus carrying cis-elements. (iii) Systematic addition of cis-el...
example 2
BG Expression from Gutted SIN-γRV
[0119]It has been postulated that γRV are unable to successfully express hβ-globin due to transcriptional interference between the strong γRV LTR promoter / enhancer elements and the internal LCR enhancer. SRS11.SF is a SIN-γRV that encodes the GFP cDNA under control of an internal Spleen Focus-Forming Virus (SFFV) promoter / enhancer. The SFFV-GFP in SRS11.SF was replaced with BG, an expression cassette that was successfully utilized in a standard SIN-LV to achieve therapeutic h β-globin expression in thalassemia, to generate SRS11.BG. The rationale for using SRS.11, despite the notoriety of β-globin γRV was: (i) it contains the minimal packaging region (ψ), lacks gag sequences and can carry a larger vector payload, yet retains extremely high titers; (ii) it carries a large 400 bp U3 deletion of the 3′LTR, comparable to the deletion in SIN-LV. (iii) Large LCR elements have never been tested in γRV due to restrictions on vector payload.
[0120]Infectious t...
example 3
Expression of Large / Small Transgenes from Standard or Gutted / Minimal LV
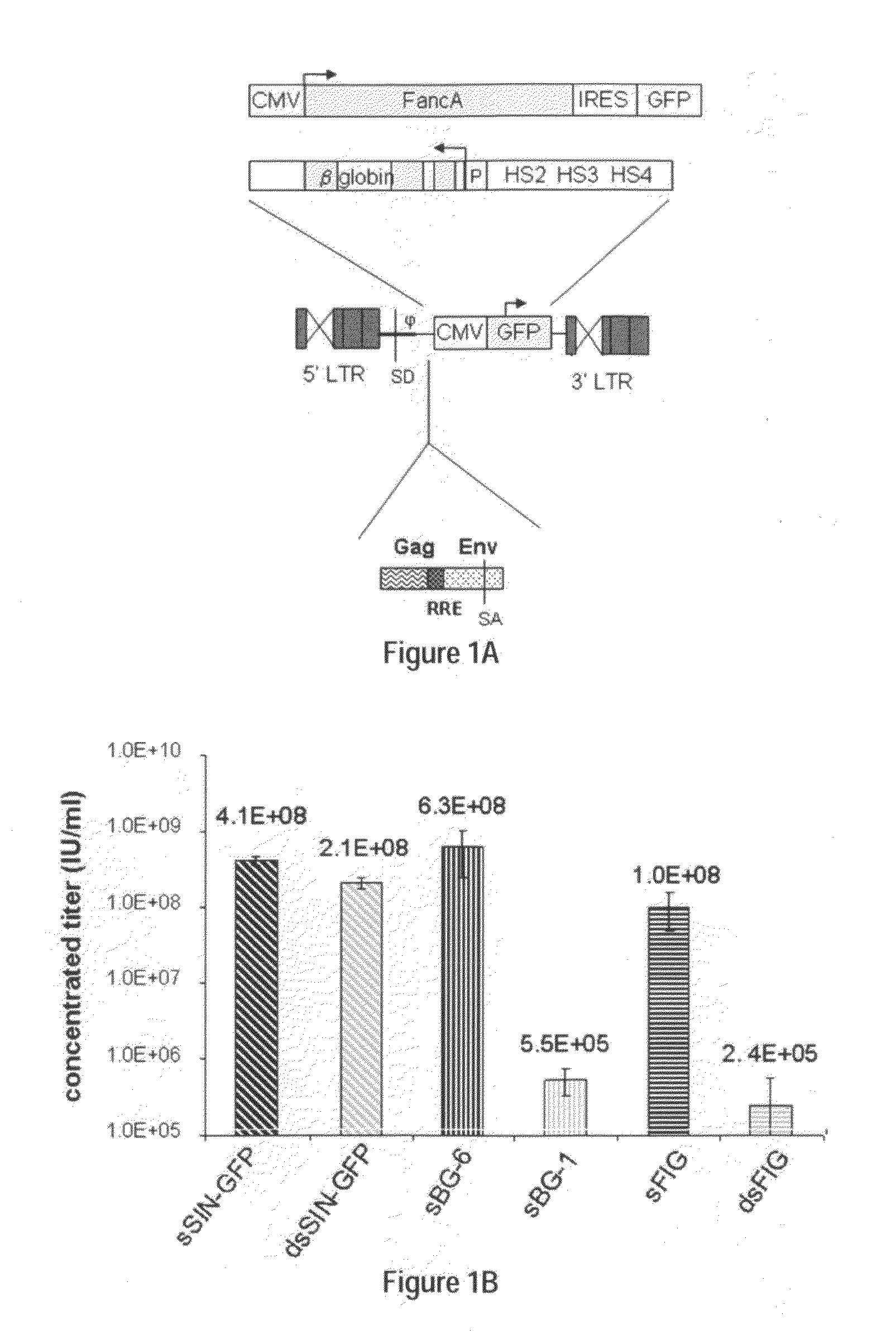
[0121]In contrast to the SIN-γRV used herein, the “standard” SIN-LV commonly used retains relatively large portions of viral sequences amounting to about 20-25% of the HIV genome. These cis elements are: the LTR (634 bp for wt HIV LTR or 235 bp for SIN-LV LTR), the packaging signal ψ(150 bp), 5′ portion of the gag gene (300 or 600 bp), env sequences including the rev response element (RRE, 840 bp) and the central flap / polypurine tract (cPPT) from the pol gene (120 bp).
[0122]To examine the requirement of cis-sequences for GFP versus BG, the CMV-GFP cassette was cloned in a) the “standard” SIN-LV containing cis sequences listed above (sSIN-GFP), and b) a ‘gutted’ minimal SIN-LV where the gag, RRE and the rest of the env sequences were deleted and only the ψ region was retained (dsSIN-GFP; FIG. 1A). The titers of the minimal dsSIN-GFP LV were only 2-times lower than the titers of the “standard” LV sSIN-GFP FIG. 1B; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com