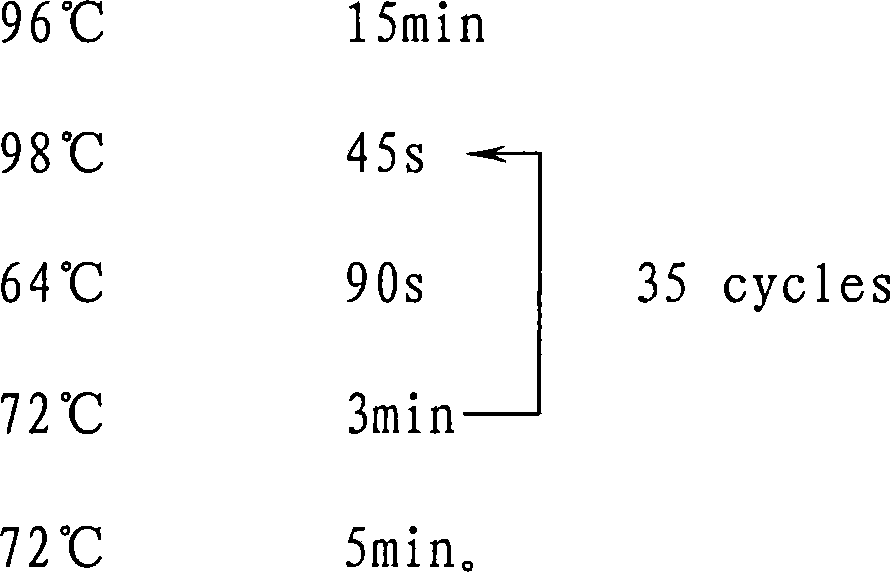
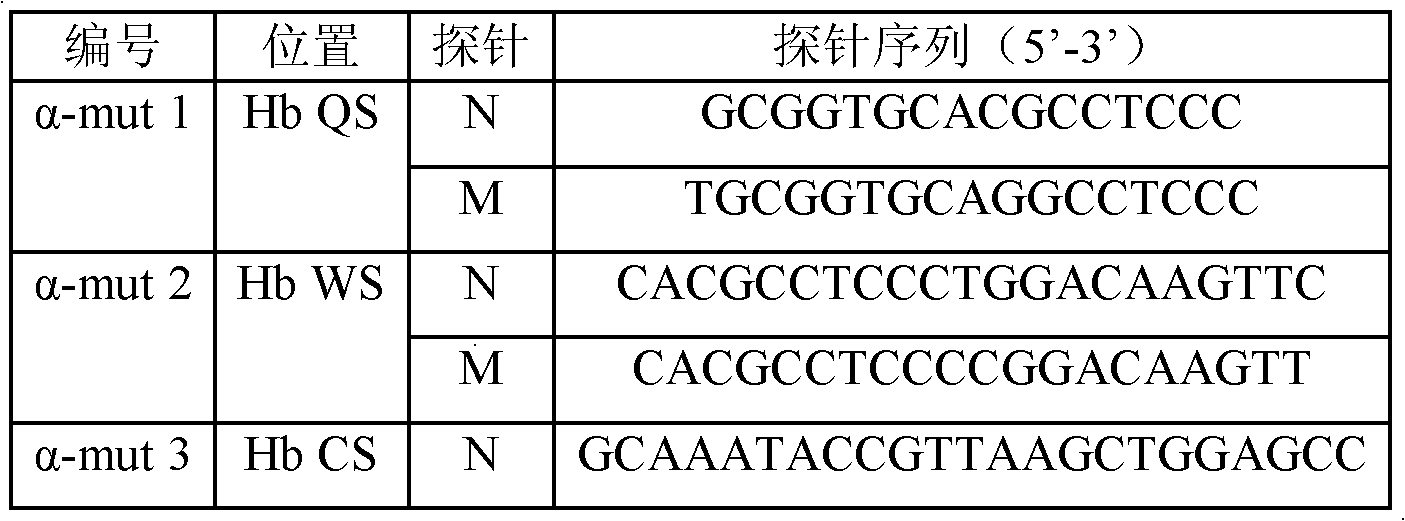
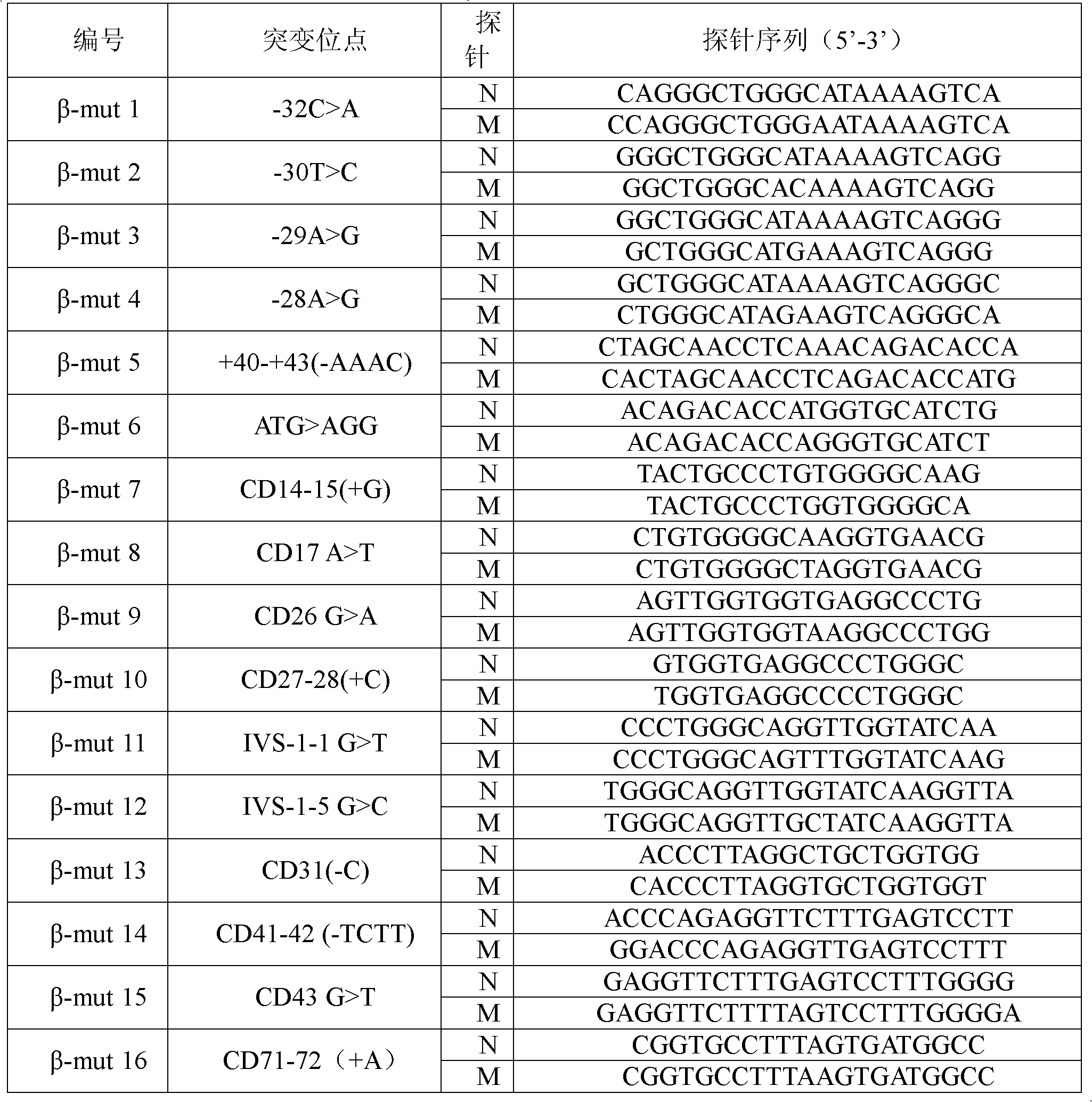
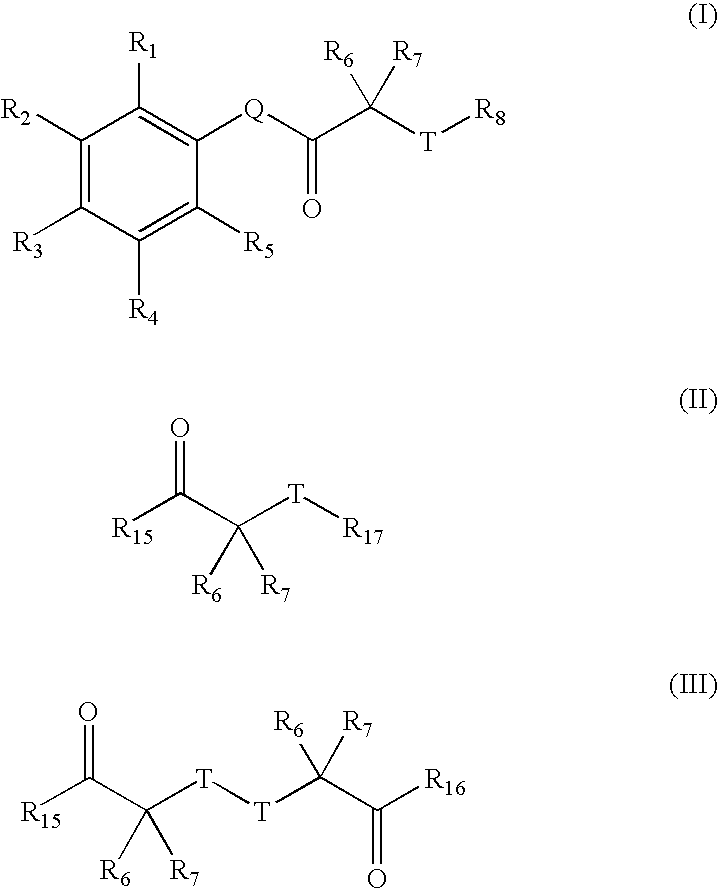
Patents
Literature
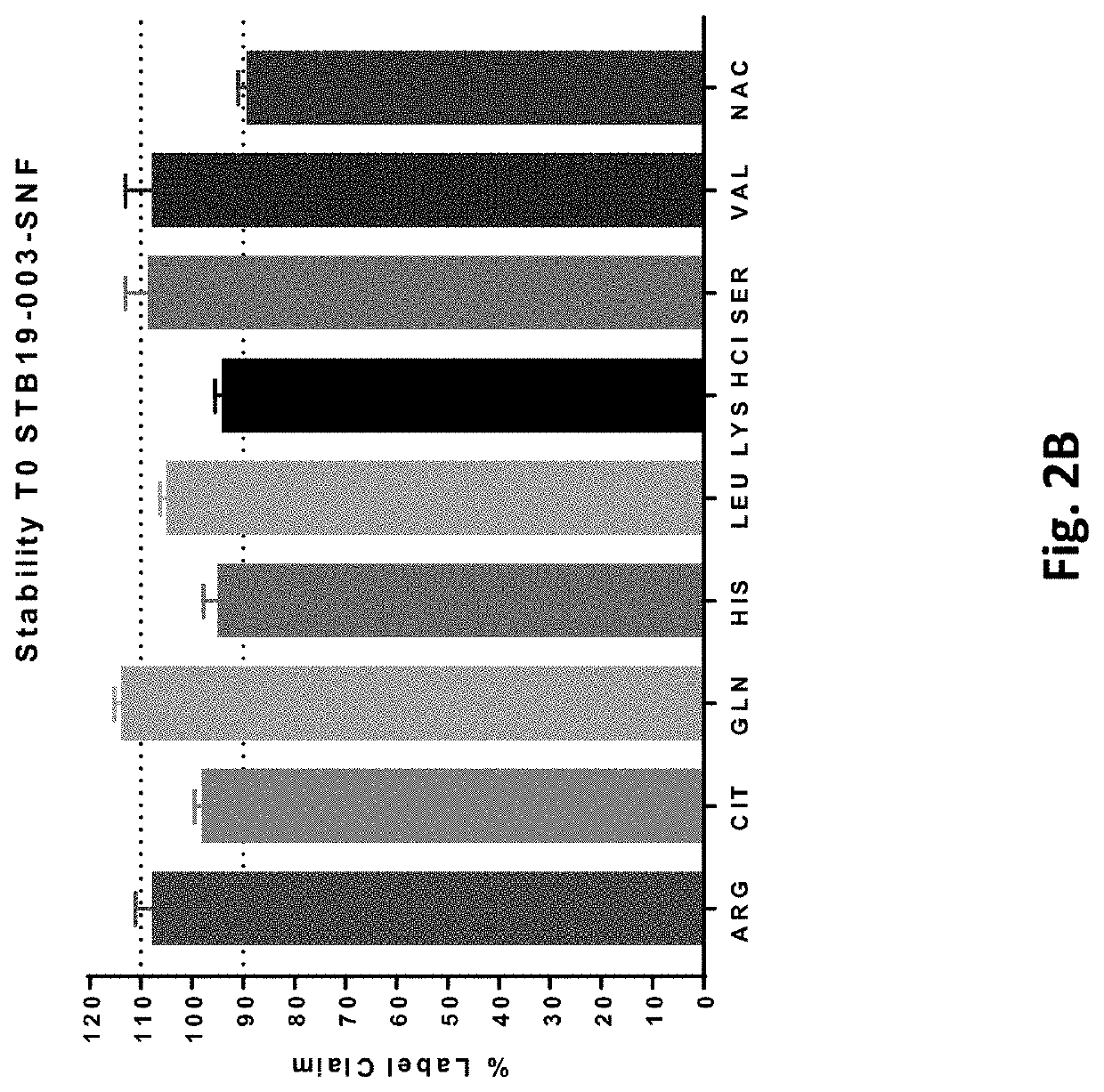
206 results about "Thalassemia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An inherited blood disorder characterized by the formation of abnormal form of hemoglobin.
Methods and compositions for the treatment and management of hemoglobinopathy and anemia
InactiveUS20050143420A1Good effectRelieve symptomsBiocidePeptide/protein ingredientsRed blood cellThalassemia
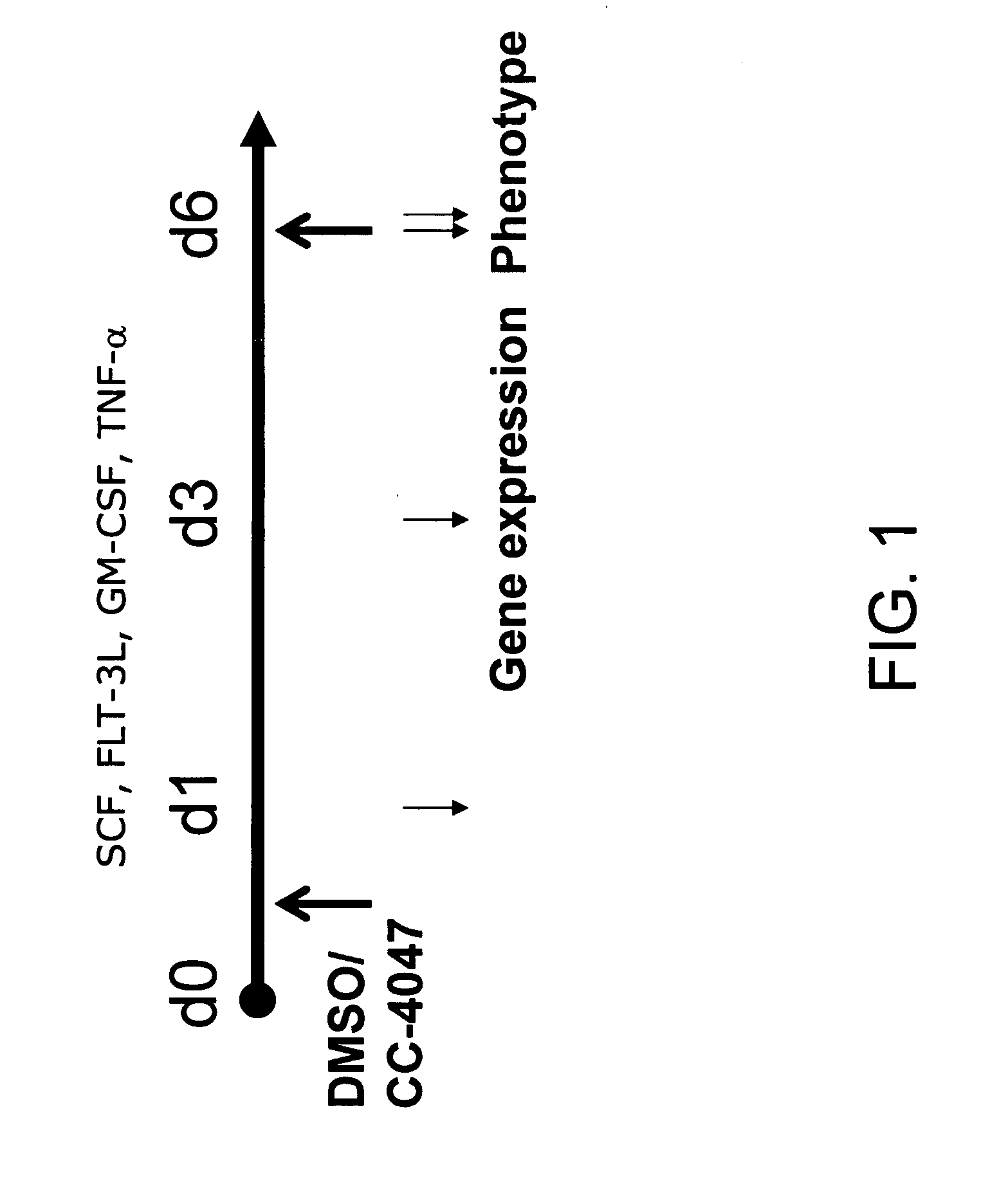
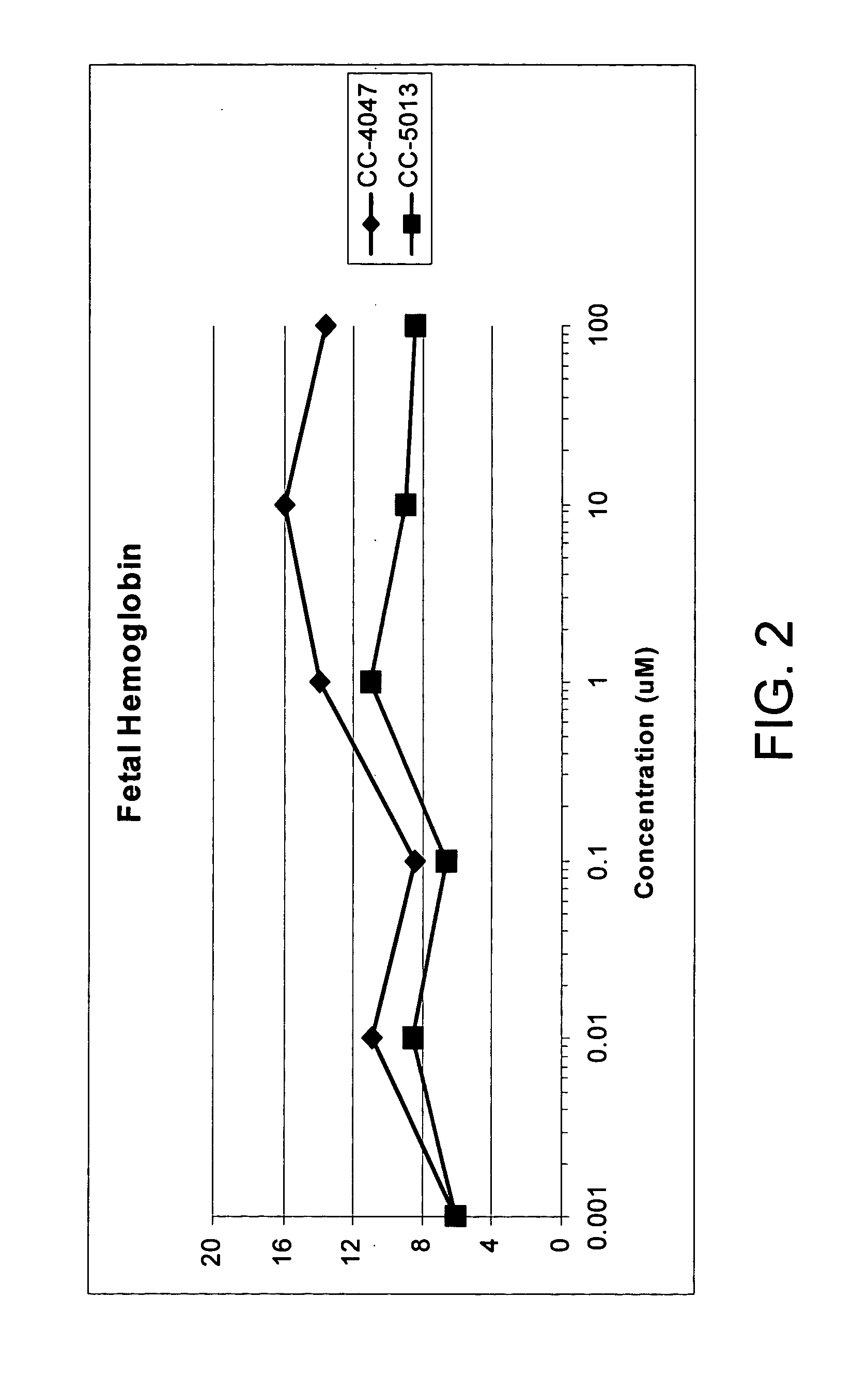
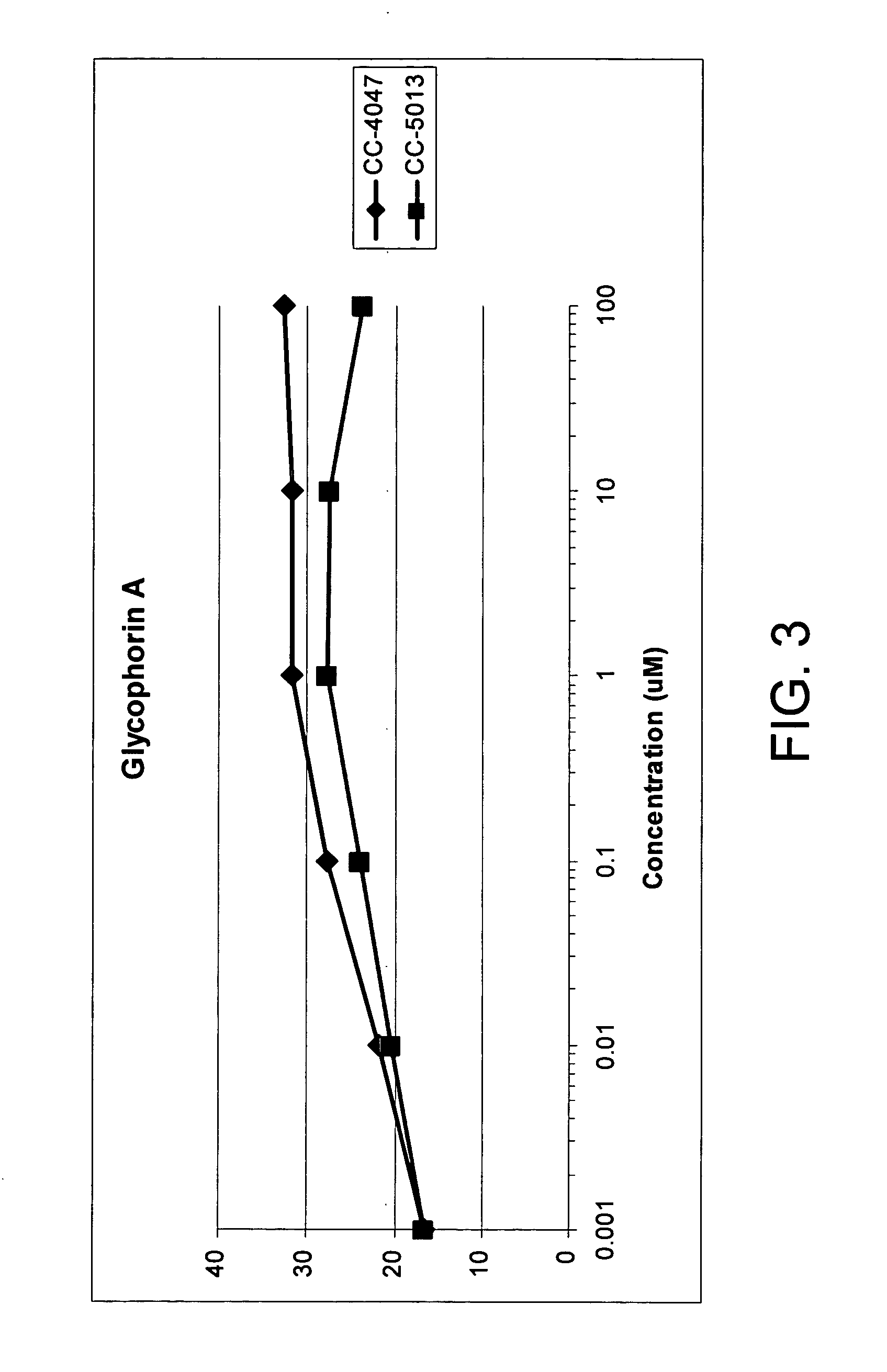
The present invention is directed to the use of immunomodulatory compounds, particularly members of the class of compounds known as IMiDs™, and more specifically the compounds 4-(Amino)-2-(2,6-dioxo(3-piperidyl))-isoindoline-1,3-dione and 3-(4-amino-1-oxo-1,3-dihydroisoindol-2-yl)-piperidine-2,6-dione, to induce the expression of fetal hemoglobin genes, genes essential for erythropoiesis, and genes encoding alpha hemoglobin stabilizing protein, within a population of CD34+ cells. These compounds are used to treat hemoglobinopathies such as sickle cell anemia or β-thalassemia, or anemias caused by disease, surgery, accident, or the introduction or ingestion of toxins, poisons or drugs.
Owner:SIGNAL PHARMA LLC
Compositions and methods for the treatment of hemoglobinopathies
InactiveUS20150166969A1Improve the level ofAvoid the insertion of vector sequencesFusion with DNA-binding domainSugar derivativesThalassemiaGlobin genes
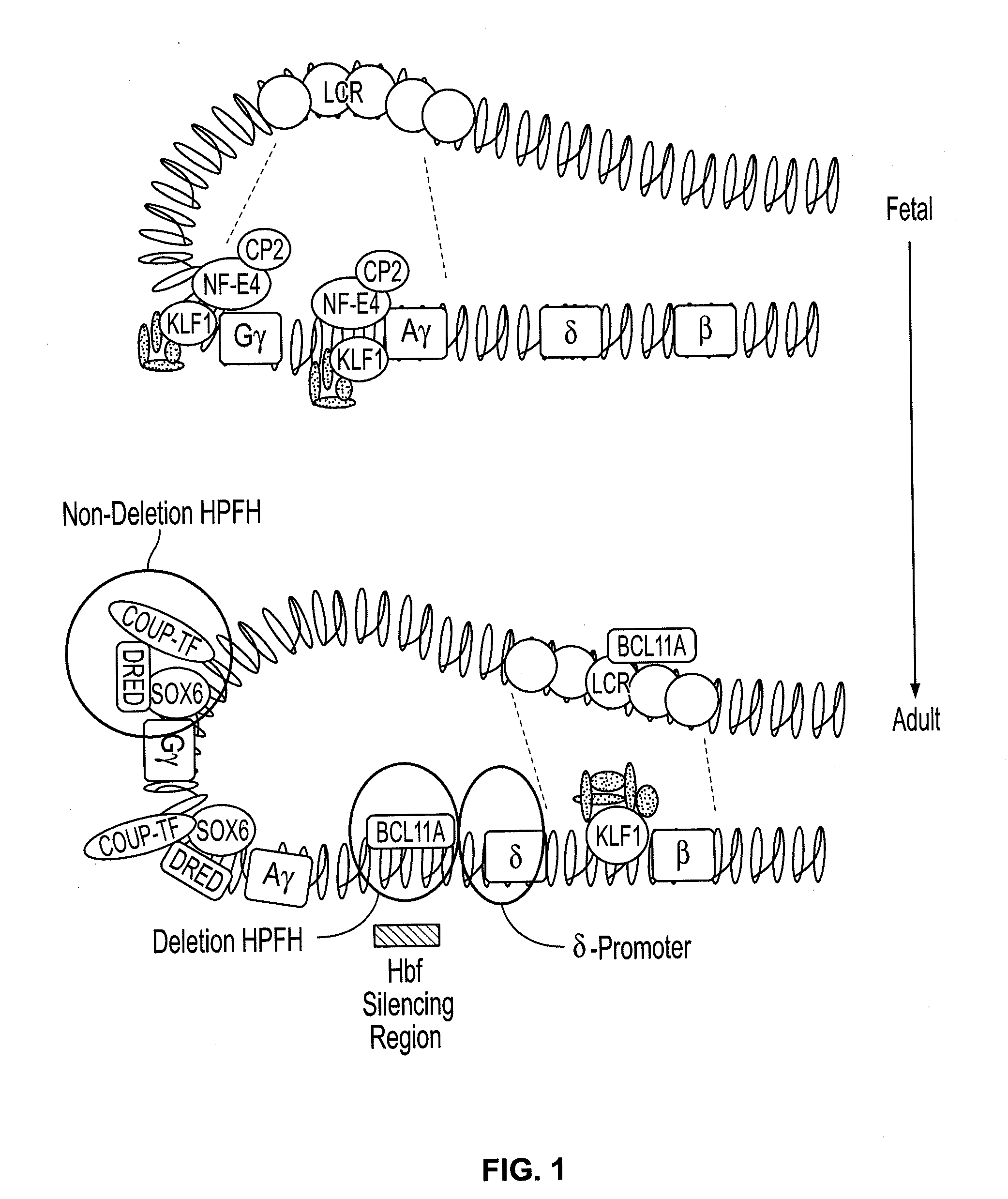
Provided are compositions and methods for the treatment of hemoglobinopathies such as thalassemias and sickle cell disease. Compositions and methods include one or more endonuclease(s) or endonuclease fusion protein(s), including one or more homing endonuclease(s) and / or homing endonuclease fusion protein(s) and / or CRISPR endonuclease(s) ad / or CRISPR endonuclease fusion protein(s): (a) to disrupt a Bcl11a coding region; (b) to disrupt a Bcl11a gene regulatory region; (c) to modify an adult human β-globin locus; (d) to disrupt a HbP silencing DNA regulatory element or pathway, such as a Bcl11a-regulated HbP silencing region; (e) to mutate one or more γ-globin gene promoter(s) to achieve increased expression of a γ-globin gene; (f) to mutate one or more δ-globin gene promoter(s) to achieve increased expression of a δ-globin gene; and / or (g) to correct one or more β-globin gene mutation(s).
Owner:NAT INST OF HEALTH DIRECTOR DEITR
Nucleic acid film tape and kit for diagnosing alpha mediterranean anemia
ActiveCN101092647AReduce financial burdenRich reference informationMicrobiological testing/measurementPrenatal diagnosisThalassemia
This invention relates to test kit and test paper with oligonucleotide probes for diagnosing alpha-thalassemia. The test paper comprises a base, and specific oligonucleotide probes immobilized on the base. The oligonucleotide probes comprise: specific oligonucleotide probes for detecting mutation of alpha-globin gene, specific oligonucleotide probes for detecting deletion of alpha-globin gene, and base sequence complementary with the above sequences. The test kit comprises specific primer for amplifying alpha-globin gene or transcript, and specific oligonucleotide probes for detecting mutation / deletion of alpha-globin gene. The test kit and the test paper have such advantages as low cost, rapid detection, and stable results. The test kit and the test paper can be used in detection of mutation / deletion of alpha-globin gene, and prenatal diagnosis of severe thalassemia (alphaTalpha) caused by point mutation, and do not have error diagnosis.
Owner:亚能生物技术(深圳)有限公司
Method, kit and application for diagnosing thalassemia based on liquid chip system
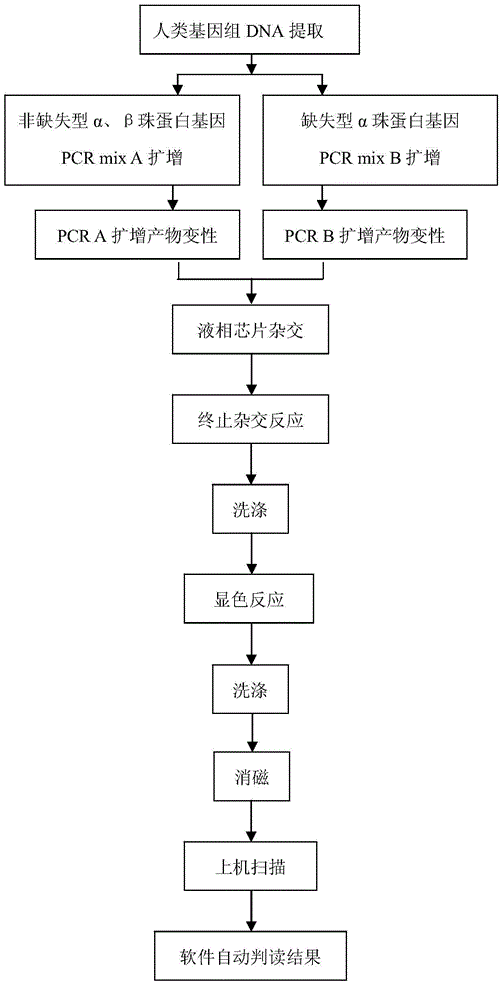
ActiveCN102277419ADetection time is shortEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceMicrosphere
Disclosed are a method for diagnosing thalassemia based on a liquid chip system, and a kit and an application. The present invention designs probes for detecting a, ß thalassemia genetic defect types. These probes are respectively cross-linked and mixed with fluorescent encoding microspheres of different colors to obtain liquid chips for diagnosing a, ß thalassemia. After PCR amplification is performed, by using a specific primer of the present invention, on a sample to be detected, a biotin-labeled PCR product is obtained, which is then hybridized with the liquid chip of the present invention, and further undergoes fluorescence labeling by use of phycoerythrin. Finally, a detection result is read out through a liquid chip detector. The kit of the present invention comprises PCR primers and specific probes, and can detect deletion or non-deletion point mutation a, ß thalassemia.
Owner:GUANGDONG WOMEN & CHILDREN HOSPITAL
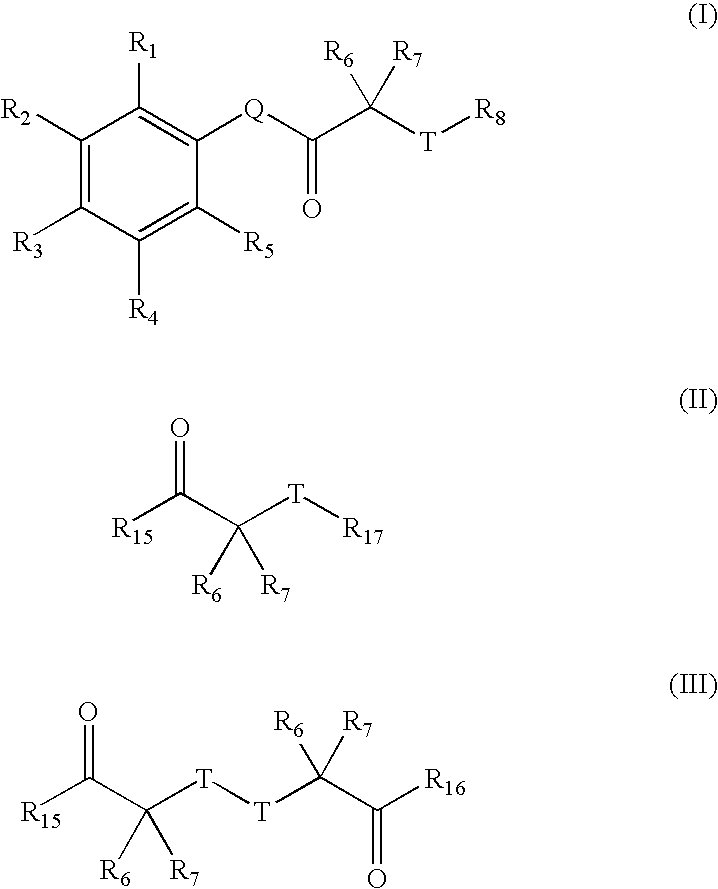
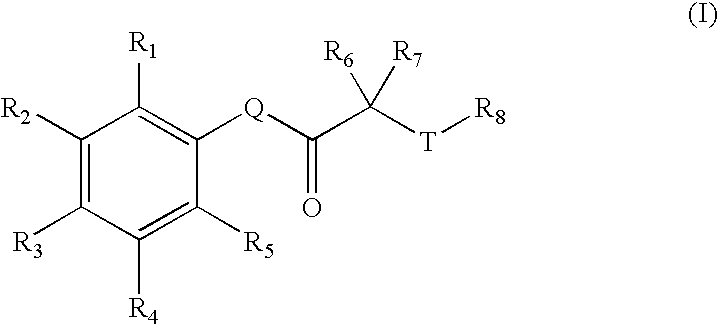
Carbonyl compounds as inhibitors of histone deacetylase for the treatment of disease
InactiveUS20050026907A1Inhibition of catalytic activityBiocideOrganic chemistryAutoimmune conditionHistone deacetylase
Disclosed herein are carbonyl compounds of Formula I, II, or III, and others as described herein. Also disclosed are methods of treating disease, such as cancer, neurological disorders, including polyglutamine-repeat disorders, anemias, thalassemias, inflammatory conditions, autoimmune diseases and cardiovascular conditions, using the compounds of the invention. In addition, methods of modulating the activity of histone deacetylase (HDAC) are also disclosed.
Owner:KALYPSYS INC
Thalassemia gene detection method based on fluorescence labeling quantitation PCR (Polymerase Chain Reaction) technology
InactiveCN103255225ARealize detectionEnables fluorescence detectionMicrobiological testing/measurementFluorescenceThalassemia
The invention discloses a thalassemia gene detection method based on a fluorescence labeling quantitation PCR (Polymerase Chain Reaction) technology, and the detection method mainly comprises the following steps of: firstly designing a general fluorescence labeling primer; then designing an amplification primer aiming at a detection site; when the amplification primer is designed, tailing one primer of an amplification primer pair, wherein the tailing sequence is consistent to the sequence of the general fluorescence labeling primer; when PCR reaction is carried out, mixing a tailed primer group and the general fluorescence labeling primer to realize the fluorescence labeling of an amplification product; and after the PCR reaction is finished, placing PCR products into a sequencer, and confirming the lengths of different PCR products through a series of internal references of known molecular weight of a result by utilizing the Genemapper function of the sequencer to judge the genetype of a detected sample. The detection method disclosed by the invention can realize the detection of different genotypes of alpha thalassemia through a single tube.
Owner:钦州市妇幼保健院
Multicyclic sulfonamide compounds as inhibitors of histone deacetylase for the treatment of disease
Owner:KALYPSYS INC
Group of probes, detection kit and detection method for detecting thalassemia gene point mutation based on liquid chip of locked nucleic acid sensibilization
ActiveCN104293937AIncrease the Tm valueMicrobiological testing/measurementDNA/RNA fragmentationMicrosphereThalassemia
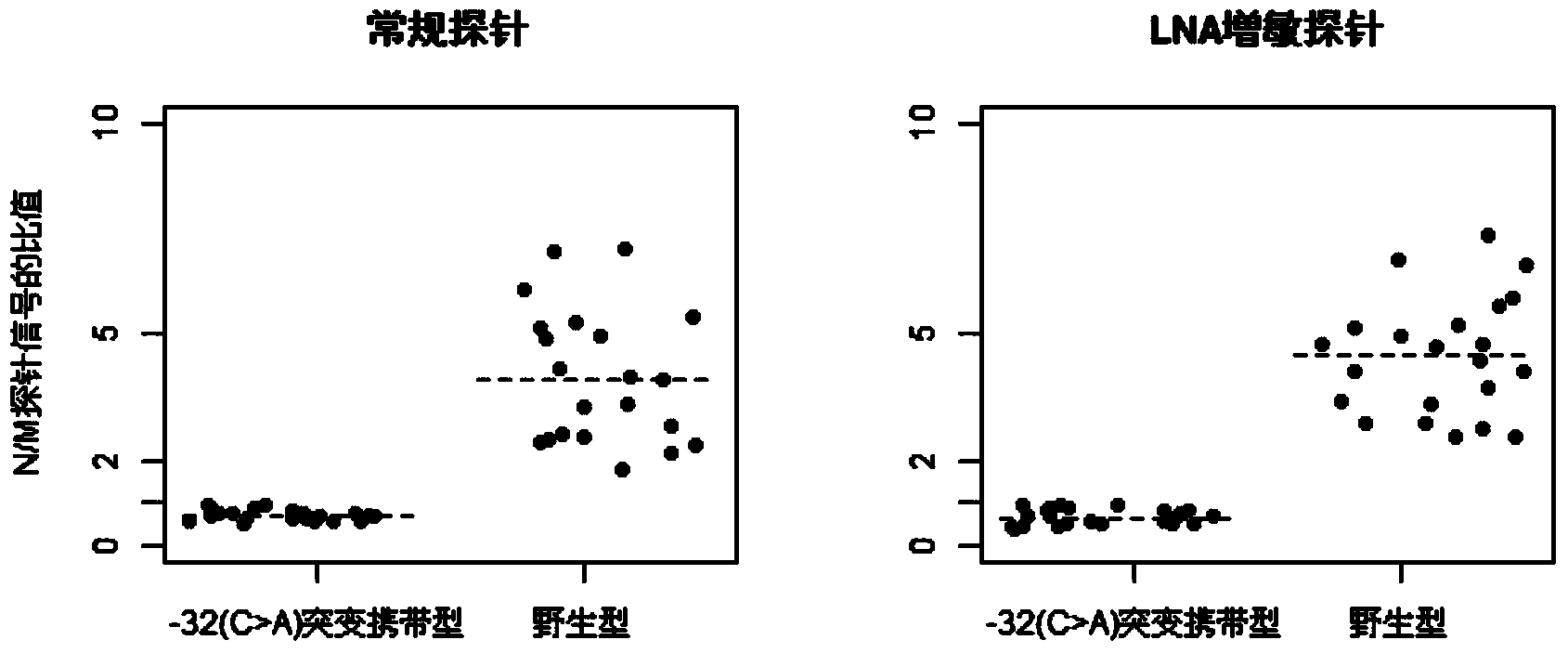
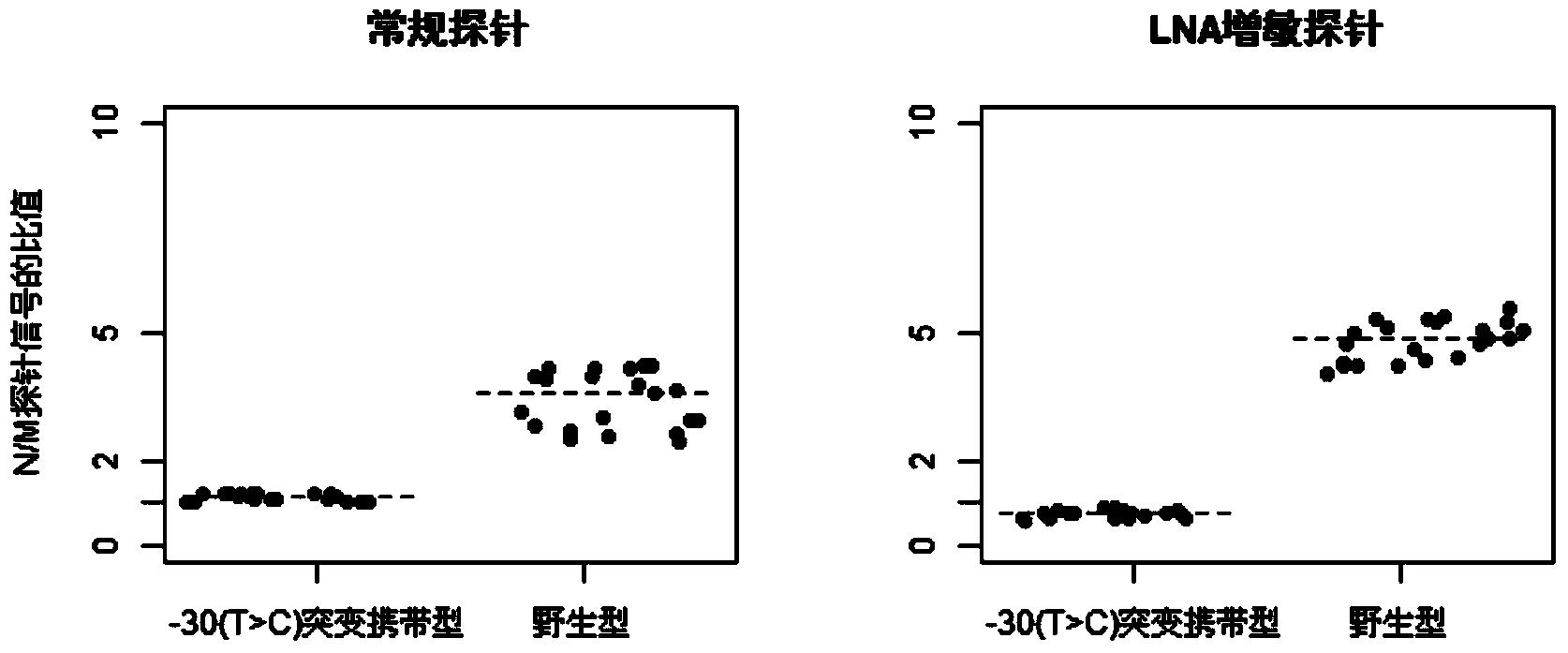
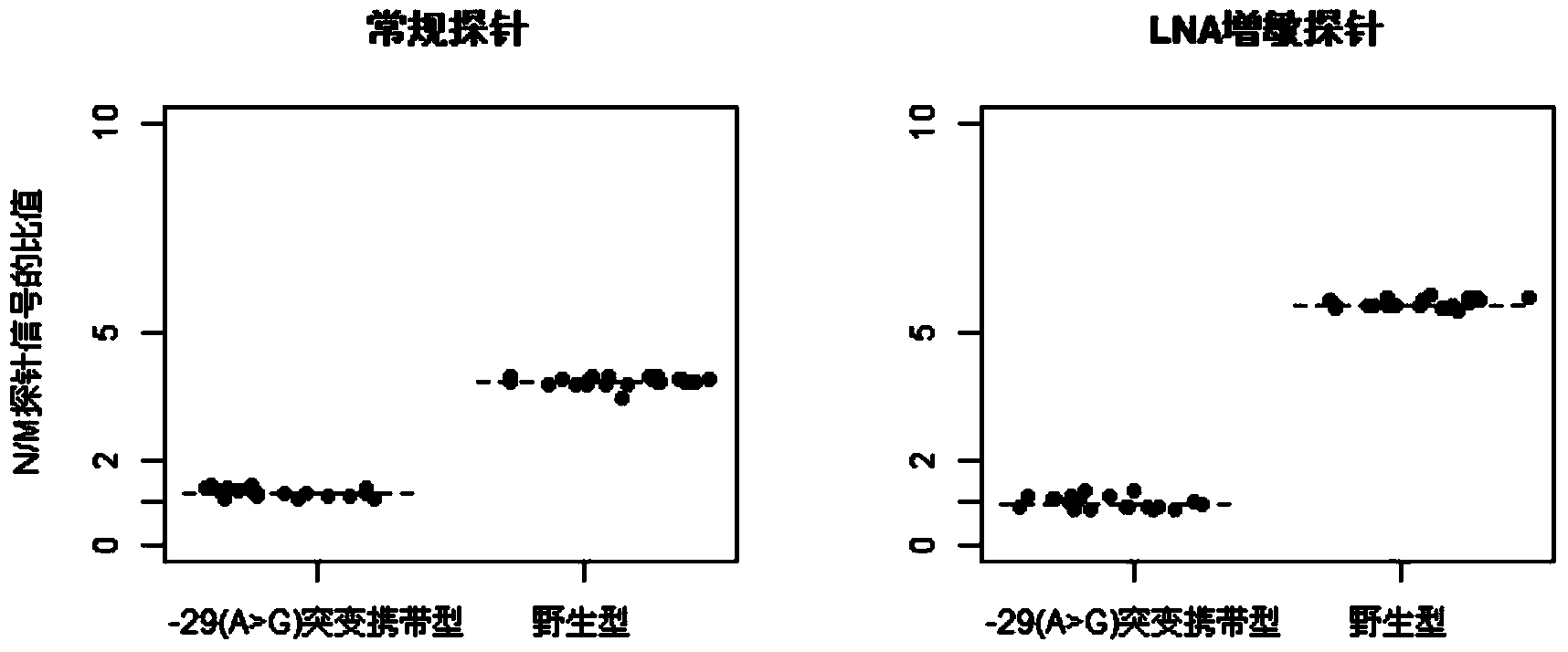
The invention discloses a group of probes, a detection kit and a detection method for detecting thalassemia gene point mutation based on a liquid chip of locked nucleic acid sensibilization. Due to the characteristics that locked nucleic acid can flexibly modify probes, probes for detecting thalassemia gene point mutation are designed; these probes are respectively coupled to different numbers of microspheres; a specific primer disclosed by the invention is designed to amplify mutation sites, so as to obtain a PCR product; the PCR product is hybridized with the microspheres coupled to the probes; fluorescence labeling is carried out by using a fluorescence labeling reagent; and finally detection on the mutation sites is carried out through a liquid chip detector. The probes based on LNA sensibilization are shortened in comparison with a conventional probe, and still can keep relatively high Tm values; so that the hybridization signal ratio of completely matched probes and mismatched probes is improved to over four times from below twice in the past, thus the probes are easy to distinguish.
Owner:GUANGDONG WOMEN & CHILDREN HOSPITAL
Inhibitors of 2-oxoglutarate dioxygenase as gamma globin inducers
InactiveUS20070042937A1Increase gene expressionInhibit hydroxylationBiocidePeptide/protein ingredientsDiseaseThalassemia
The present invention provides methods for increasing endogenous globin expression in a subject, specifically γ-globin expression. The invention also provides compounds and medicaments for use in the methods. The methods are particularly useful for increasing fetal hemoglobin production in a subject, and can be used to treat various disorders, e.g., β thalassemia and sickle cell disease.
Owner:ISIS INNOVATION LTD +1
Nucleic acid membrane strip and kit for alpha and beta mediterranean anemia gene detection
ActiveCN102943115ALow costIncreased risk of missed detectionMicrobiological testing/measurementDNA/RNA fragmentationThalassemiaNucleic Acid Probes
The invention relates to diagnostic reagent of mediterranean anemia, in particular to a nucleic acid membrane strip and kit for alpha and beta mediterranean anemia gene detection. The technical scheme is that the nucleic acid membrane strip for alpha and beta mediterranean anemia gene detection comprises a substrate and a specific oligonucleotide probe fixed on the substrate, wherein the specific oligonucleotide probe comprises 6 normal alpha-mediterranean anemia (3 deletion types and 3 mutant types) probes and 17 mutant type beta-mediterranean anemia probes. By means of the nucleic acid membrane strip and the kit, deflection type alpha-mediterranean anemia, non-deflection type alpha-mediterranean anemia and beta-mediterranean anemia can be detected in one step simultaneously. Compared with the existing like technology, 2 unreported beta-mediterranean anemia (-30 and -32) and 1 unreported non-deflection type alpha-mediterranean anemia (alpha WS alpha) are detected simultaneously, occurrence rate of the three mediterranean anemia in China is not low, the risk of leak detection in poor areas can be greatly increased without detection, and severe burden can be brought to families and the society.
Owner:亚能生物技术(深圳)有限公司
Fluorescent quantitative PCR kit for detecting alpha-globin gene deletion
InactiveCN102146476AImprove featuresGood repeatabilityMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceThalassemia
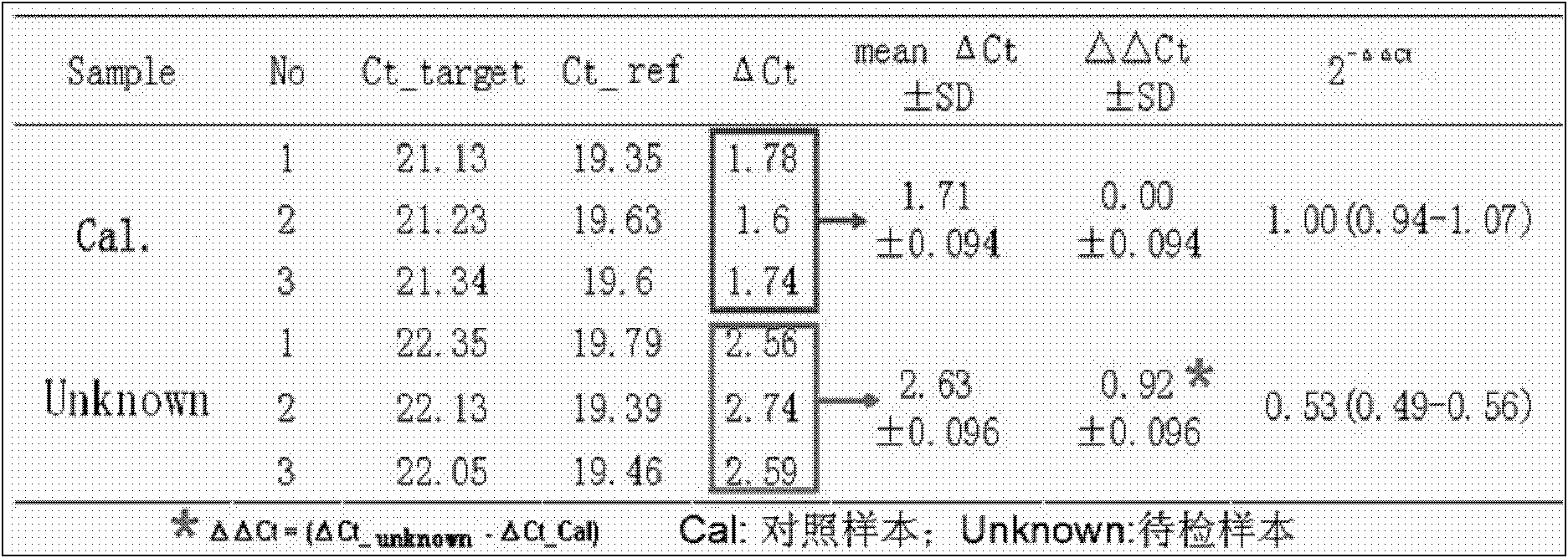
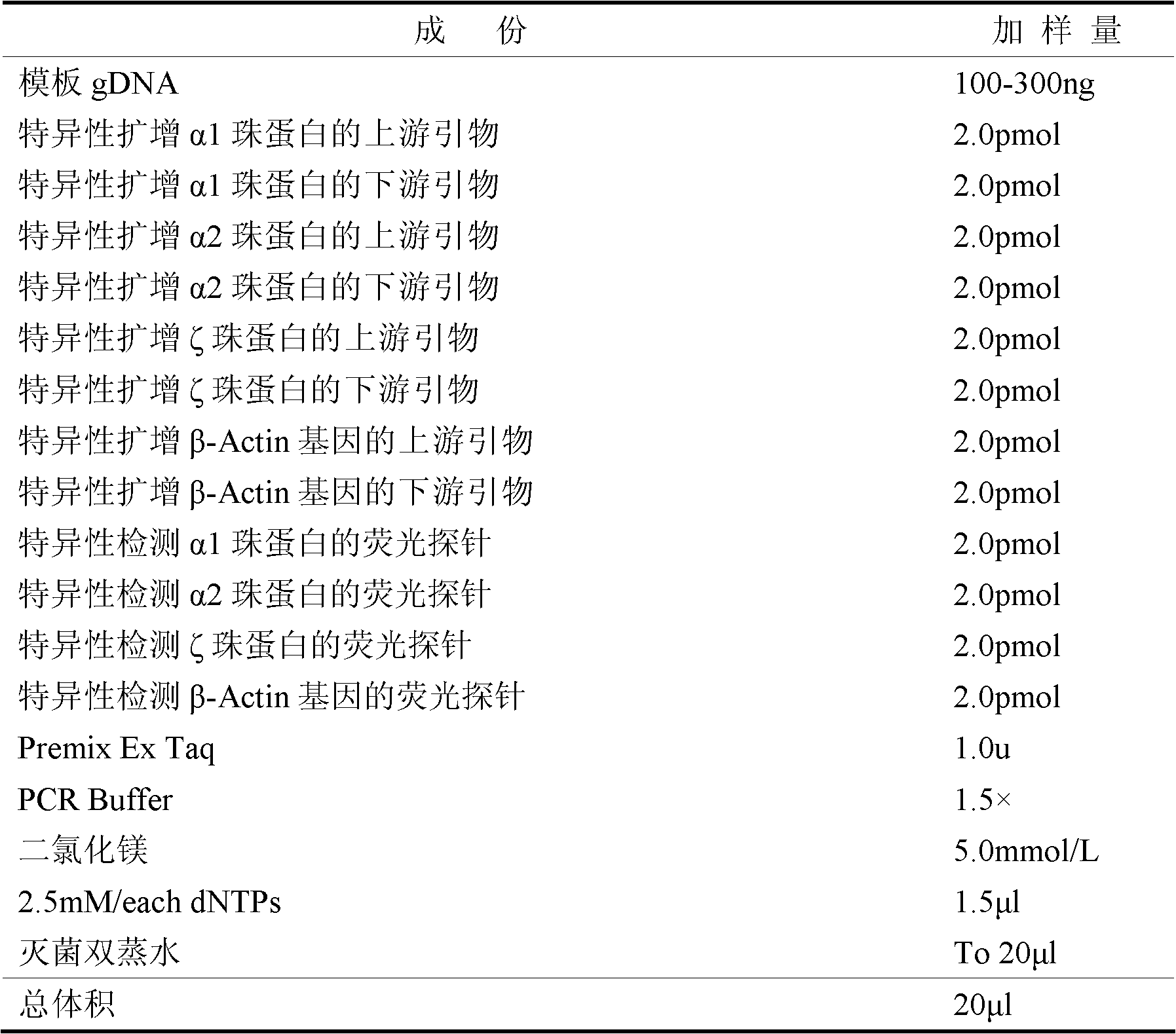
The invention belongs to the field of biochemistry, and provides a fluorescent quantitative polymerase chain reaction (PCR) kit for detecting alpha-globin gene deletion. The kit consists of a pair of specific amplified xi globin primers, a pair of specific amplified alpha1 globin primers, a pair of specific amplified alpha2 globin primers, a pair of specific amplified beta-Actin gene primers, a fluorescent probe for specifically detecting xi globin, a fluorescent probe for specifically detecting alpha1 globin, a fluorescent probe for specifically detecting alpha2 globin, a fluorescent probe for specifically detecting beta-Actin genes, a DNA polymerase and the like. The kit has good sensitivity and accuracy for detecting depletion alpha-thalassemia, has good repeatability and stability, and is totally suitable for clinical detection of alpha-thalassemia.
Owner:SOUTHERN MEDICAL UNIVERSITY
Method for detecting fetal thalassemia pathogenic gene and kit
ActiveCN108642160AEnable prenatal testingSmall sample sizeMicrobiological testing/measurementThalassemiaGenomic DNA
The invention discloses a method for detecting a fetal thalassemia pathogenic gene, which comprises the following steps of: (1) screening SNP sites, wherein the SNP sites are used for designing a primer pool of the thalassemia gene in an amplification genome and for capturing the probe of the thalassemia gene of the free DNA in the plasma of a pregnant woman; (2) extracting the free DNA in the plasma of the pregnant woman and the whole blood genomic DNA of the father, the mother and a born sibling and constructing a corresponding DNA library, and carrying out template preparation and enrichment; (3) sequencing the free DNA and the whole blood genomic DNA library in step (2); (4) constructing the haploid genotype of the SNP sites on the thalassemia gene, combining the sequencing informationof the free DNA and the whole blood genomic DNA library, analyzing the genetic condition of the parent source and the genetic condition of the parent source, so as to determine the corresponding genotype of the SNP sites of the fetal. According to the method, target area capture and high-throughput sequencing technology are used, so that the noninvasive antepartum detection of the thalassemia isrealized; the required sample amount is small; on the basis of detecting the mutation of the parent source, the method can realize the detection of the gene mutation of the maternal source of the fetal.
Owner:GUANGZHOU DARUI BIOTECH +1
Real-time fluorescence quantitative PCR(Polymerase Chain Reaction) detection primers and kit for thalassemia
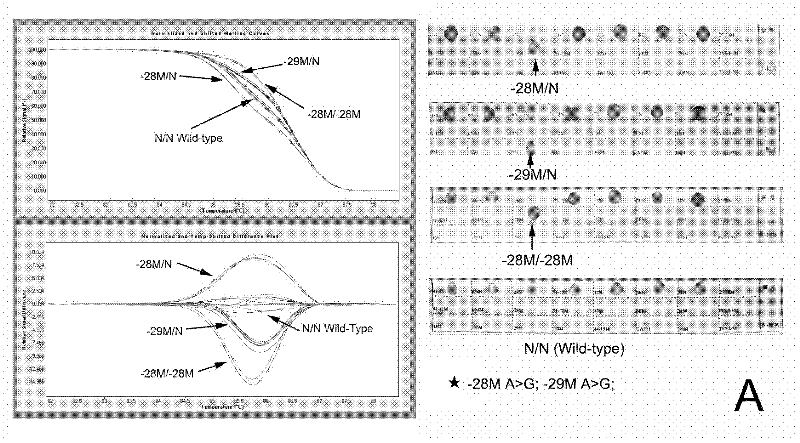
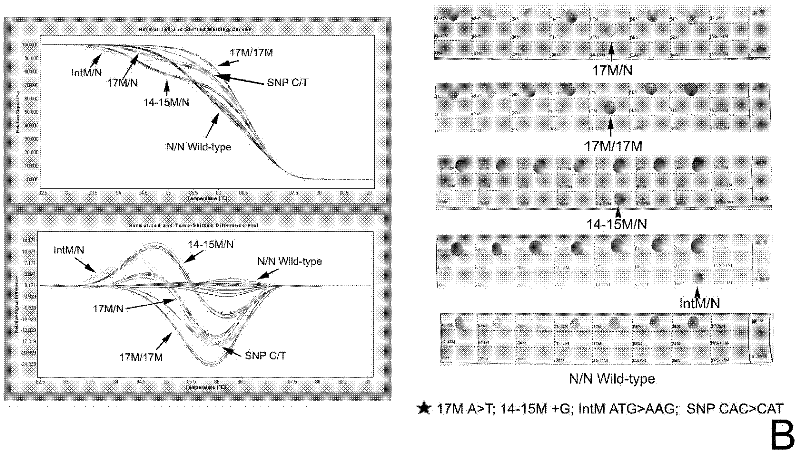
InactiveCN102409100AAvoid pollutionMeet the needs of rapid detectionMicrobiological testing/measurementDNA/RNA fragmentationStart codonCD43
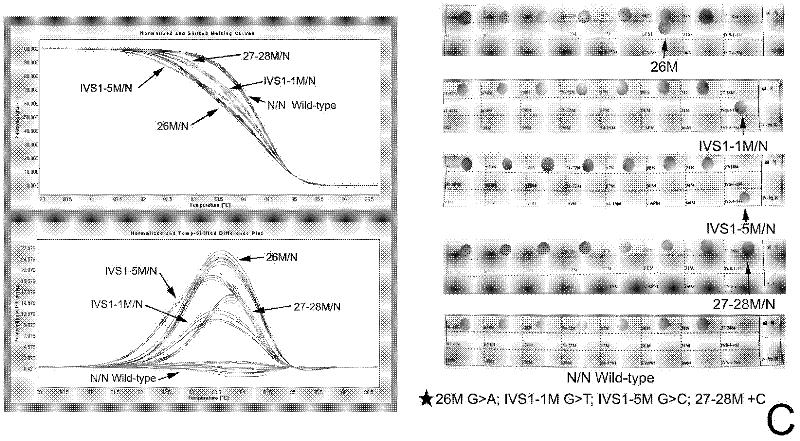
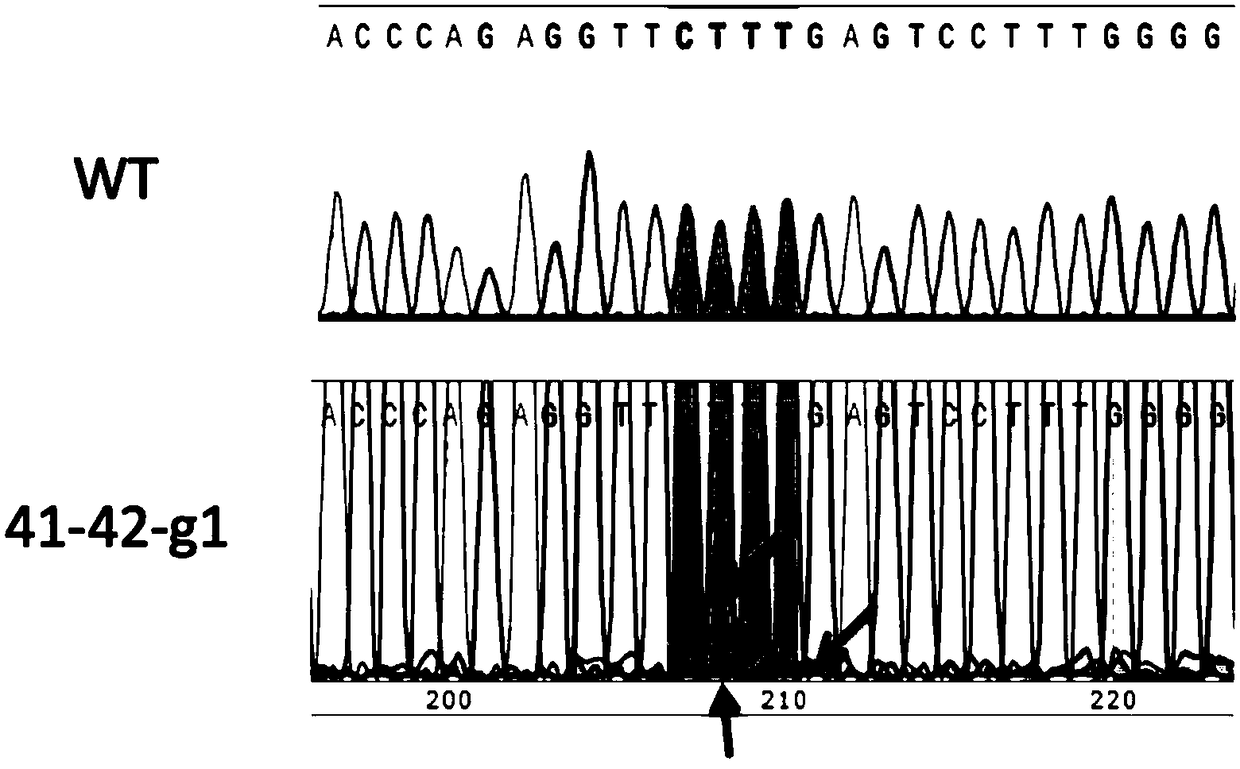
The invention discloses a real-time fluorescence quantitative PCR(Polymerase Chain Reaction) detection kit and related primers for thalassemia genes. The kit comprises more than one pair of the following primers: SEQ ID NO.1 and SEQ ID NO.2 aiming at TATA kits nt-28 (A->G) (-28M), -29 (A->G) (-29M) and CAP-40-43 (AACC) (CAPM) genotypes; SEQ ID NO.3 and SEQ ID NO.4 aiming at CD14-15 (+G) (14-15M), CD17 (A->T) (17M) and the initiation codon mutant ATG->AGG(IntM) genotypes; SEQ ID NO.5 and SEQ ID NO.6 aiming at CD26 (G->A)(betaEM) CDs27-28+C(27-28M), IVS1-1(G->T)(IVS1-1M), IVS1-5(G->C)(IVS1-5M) genotypes; SEQ ID NO.7 and SEQ ID NO.8 aiming at CDs41-42-TCTT (41-42M), CD43G->T(43M), 41-42M / 41-42M genotypes; SEQ ID NO.9 and SEQ ID NO.10 aiming at CD71-72(+A) (71-72M) genotypes; SEQ ID NO.11 and SEQ ID NO.12 aiming at IVS2-654C->T(654M) and 654M / 654M genotypes; and SEQ ID NO.13 and SEQ ID NO.14 aiming at constant spring (CS), Quong Sze (QS) and Westmead (WS) genotypes. The kit has the advantages of good detection specificity and high detection sensitivity.
Owner:潮州市中心医院
Method and kit for detecting southeast Asia deletion alpha-thalassemia
ActiveCN102146475AMicrobiological testing/measurementFluorescence/phosphorescenceSoutheast asiaThalassemia
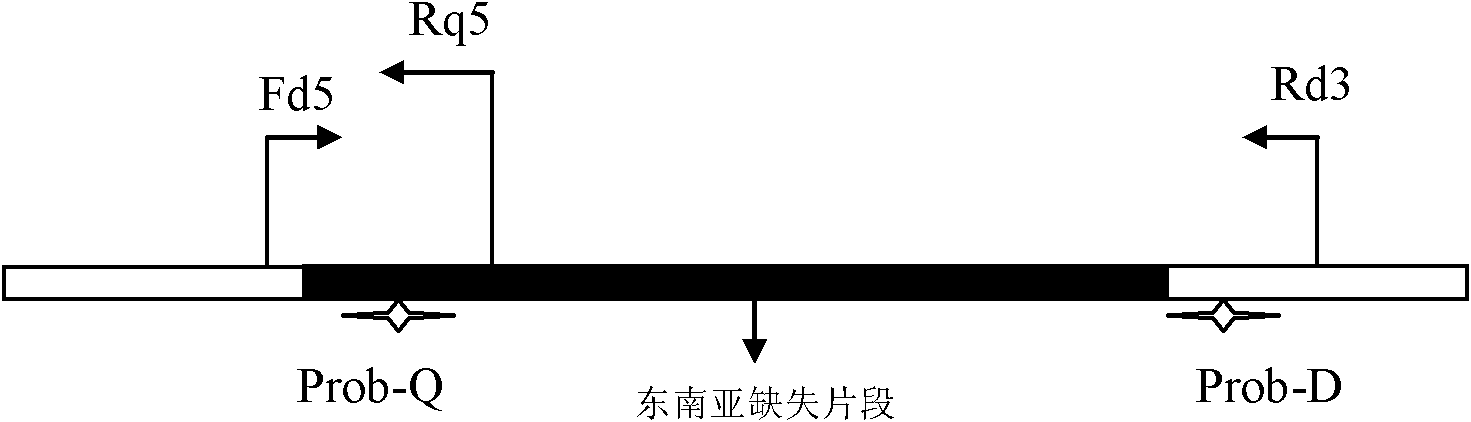
The invention relates to the field of biotechnology, and discloses a method and kit for detecting southeast Asia deletion alpha-thalassemia. The detection method comprises the following steps of: designing a pair of primers Fd5 and Rd3 and a probe Prob-D at two sides of the broken end of a southeast Asia deletion alpha-thalassemia deleted fragment, designing a reverse primer Rq5 and a probe Prob-Q at the 5' end of the deleted fragment, extracting the DNA of a sample to be detected, performing real-time fluorescence PCR (polymerase chain reaction) by taking the DNA of the sample to be detectedas a template, and then analyzing the sample to be detected according to the real-time fluorescence PCR amplification curve. The kit disclosed by the invention comprises the primers Fd5, Rd3 and Rq5 and the probes Prob-D and Prob-Q marked with different wavelength reporting fluorophores. According to the detection method disclosed by the invention, amplification and detection are simultaneously performed according to the fluorescence signal intensity, thus saving time, improving the sensitivity, and being a simple and quick detection method with high sensitivity and easiness in popularization.
Owner:SHENZHEN KANGMEI BIOTECH +1
Kit for detecting alpha-thalassemia genes
Owner:亚能生物技术(深圳)有限公司
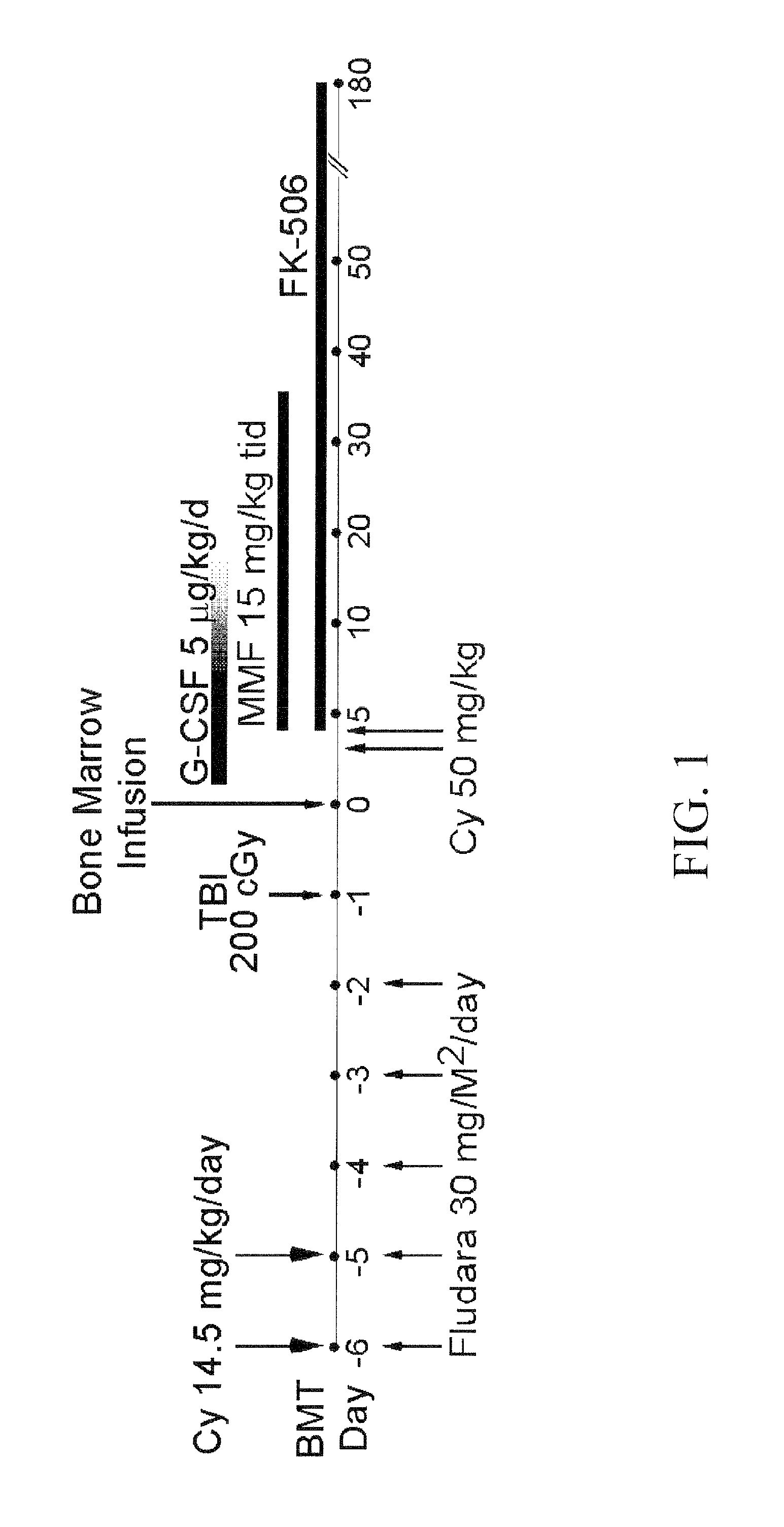
Use of high-dose, post-transplantation oxazaphosphorine drugs for reduction of transplant rejection
InactiveUS20120148577A1Reduce transplant rejectionReduce rejectionBiocidePeptide/protein ingredientsGackstroemiaSickle cell anemia
A lymphocytotoxic, but hematopoietic stem cell-sparing, high-dose amount of an oxazaphosphorine drug such as, for example, cyclophosphamide, administered post-transplantation can be used to reduce transplant rejection, including graft-versus-host-disease (GVHD). In some embodiments, the transplants are bone marrow transplants or hematopoietic stem cell transplants carried out for the treatment of hematologic disorders, including hematologic malignancies and non-malignant hematologic disorders. In some embodiments, the transplants are carried out for the treatment of hereditary hemoglobinopathies, such as sickle cell anemia and thalassemia.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Compositions and methods for the treatment of hemoglobinopathies and thalassemias
ActiveUS20190388376A1Function increaseIncrease synthesisOrganic active ingredientsDipeptide ingredientsHemoglobinopathyThalassemia
This disclosure provides compositions and methods for improving erythrocyte dysfunction or treating a hemoglobinopathy or a thalassemia (e.g., sickle cell disease or β-thalassemia).
Owner:AXCELLA HEALTH INC
Kit for simultaneously detecting thalassemia mutant type and deletion type and application thereof
PendingCN108796054AEfficient detectionLow costNucleotide librariesMicrobiological testing/measurementBeta globinThalassemia
Owner:BGI BIOTECH WUHAN CO LTD +1
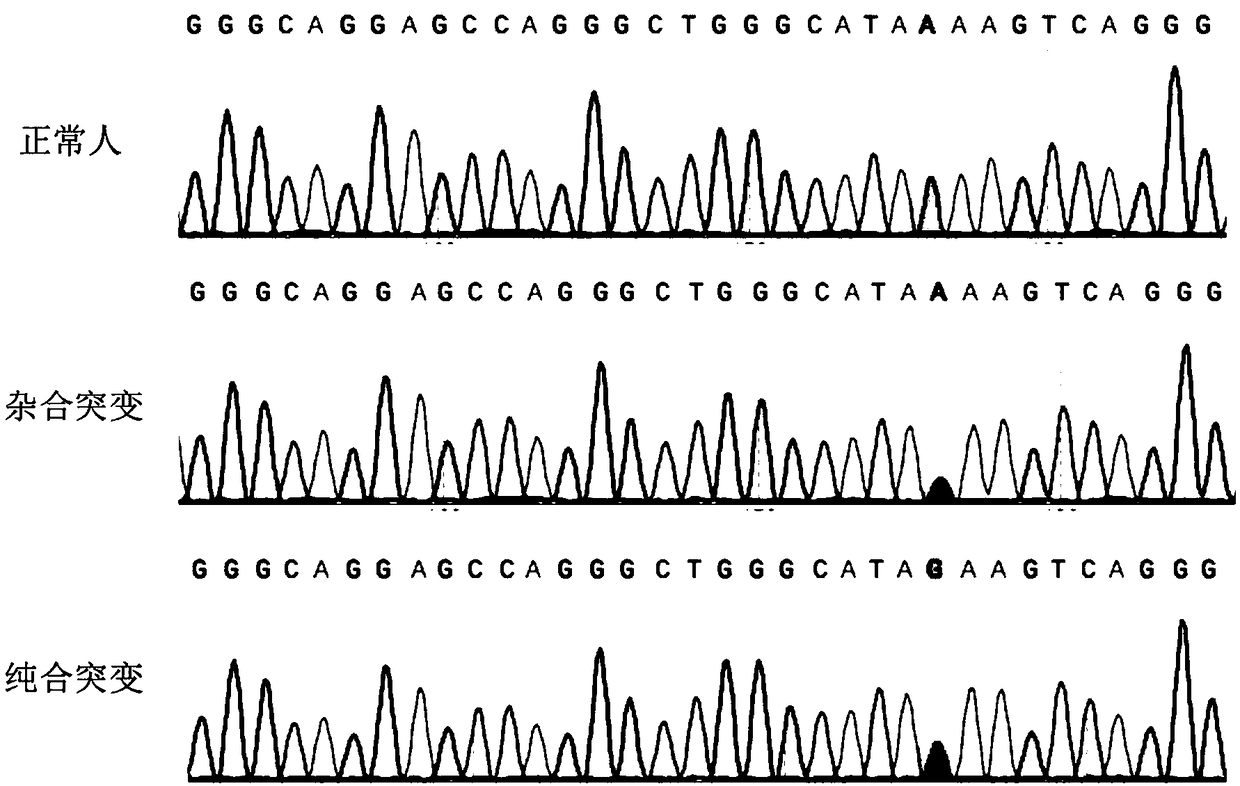
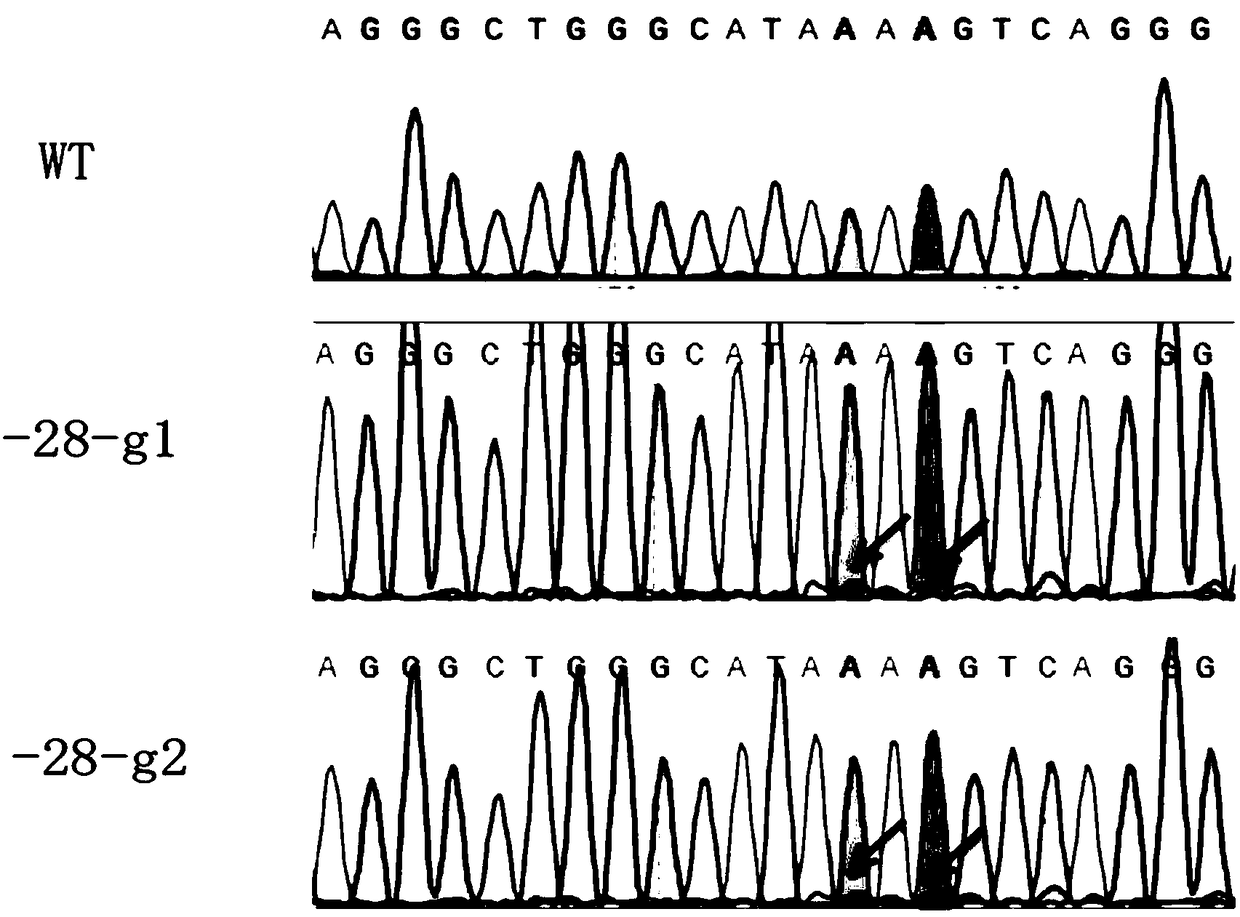
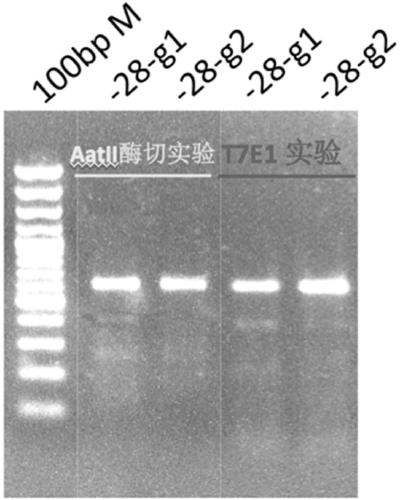
sgRNA editing HBB-28 mutation site based on CRISPR/Cas9 technology, vector and application thereof
InactiveCN109266652APrecision Repair ImprovementStable introduction of DNAVector-based foreign material introductionThalassemiaAllele
An sgRNA editing HBB-28 mutation site based on CRISPR / Cas9 technology is disclosed, the nucleotide sequence of which is shown in SEQ ID NO.1 or SEQ ID NO.2. The invention also provides a vector associated therewith, a cell, a gene editing kit and an application thereof, in particular a method for editing the HBB-28 mutation site based on the CRISPR / Cas9 technology, comprising constructing a CRISPR / Cas9 expression vector use sgRNA having a nucleotide sequence represented by SEQ ID NO.1 or SEQ ID NO.2. The invention can specifically induce HBB-28 Traceless modification of gene mutation sites inthalassemia can selectively introduce monoallelic and biallelic sequence changes with high efficiency and precision, which greatly promotes the precise repair of thalassemia.
Owner:广州鼓润医疗科技有限公司
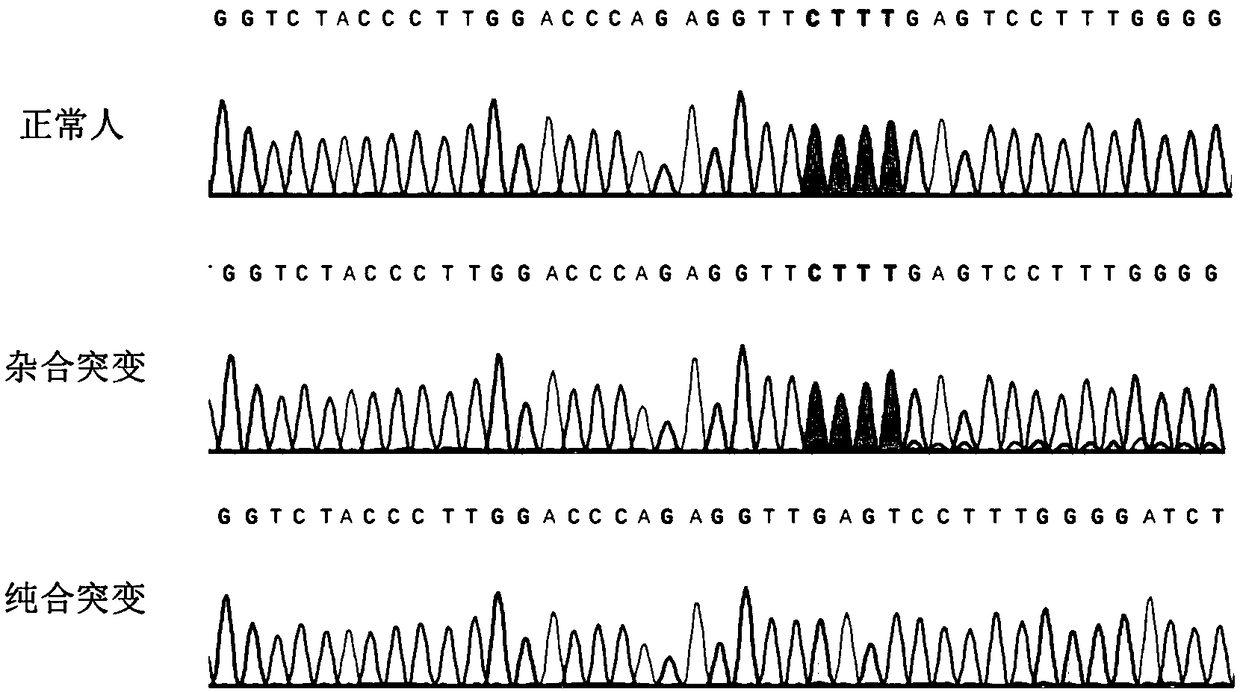
SgRNA editing HBB-41/42 deletion mutation site based on CRISPR/Cas9 technology
InactiveCN109266651APrecision Repair ImprovementGenetically modified cellsStable introduction of DNANucleotideThalassemia
Owner:广州鼓润医疗科技有限公司
Bar code magnetic bead liquid chip detection kit for thalassemia gene
ActiveCN104789672AEasy to operateRapid responseMicrobiological testing/measurementHybridization probeMagnetic bead
The invention provides a bar code magnetic bead liquid chip detection kit for a thalassemia gene. The detection kit comprises a PCR reagent for amplifying the thalassemia gene and a hybridization reagent for detecting the thalassemia gene; the hybridization reagent comprises a liquid chip; the liquid chip comprises a bar code magnetic bead coupled with a specificity hybridization probe, a blank bar code magnetic bead, a dissociative hybridization positive inverted-sequence probe and a hybridization buffer solution. The detection kit is simple to operate, quick in reaction, high in flux, and digitized.
Owner:BIOCHAIN BEIJING SCI & TECH
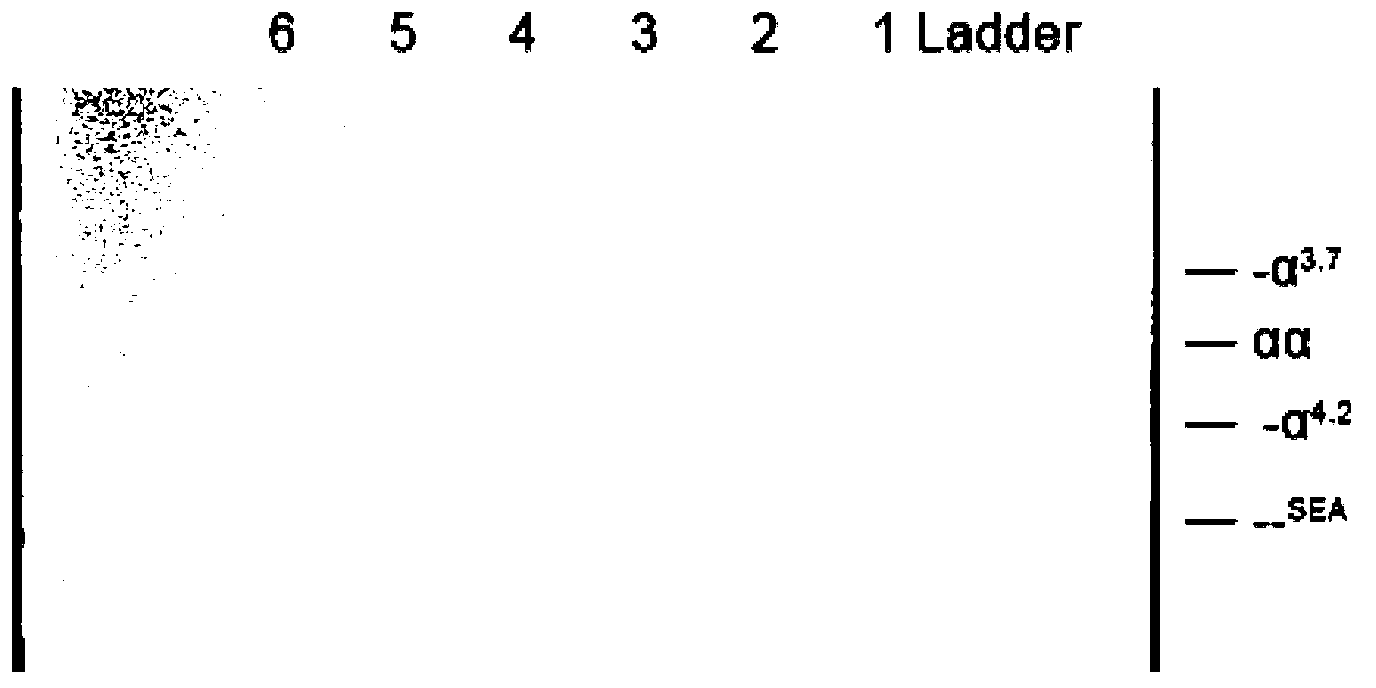
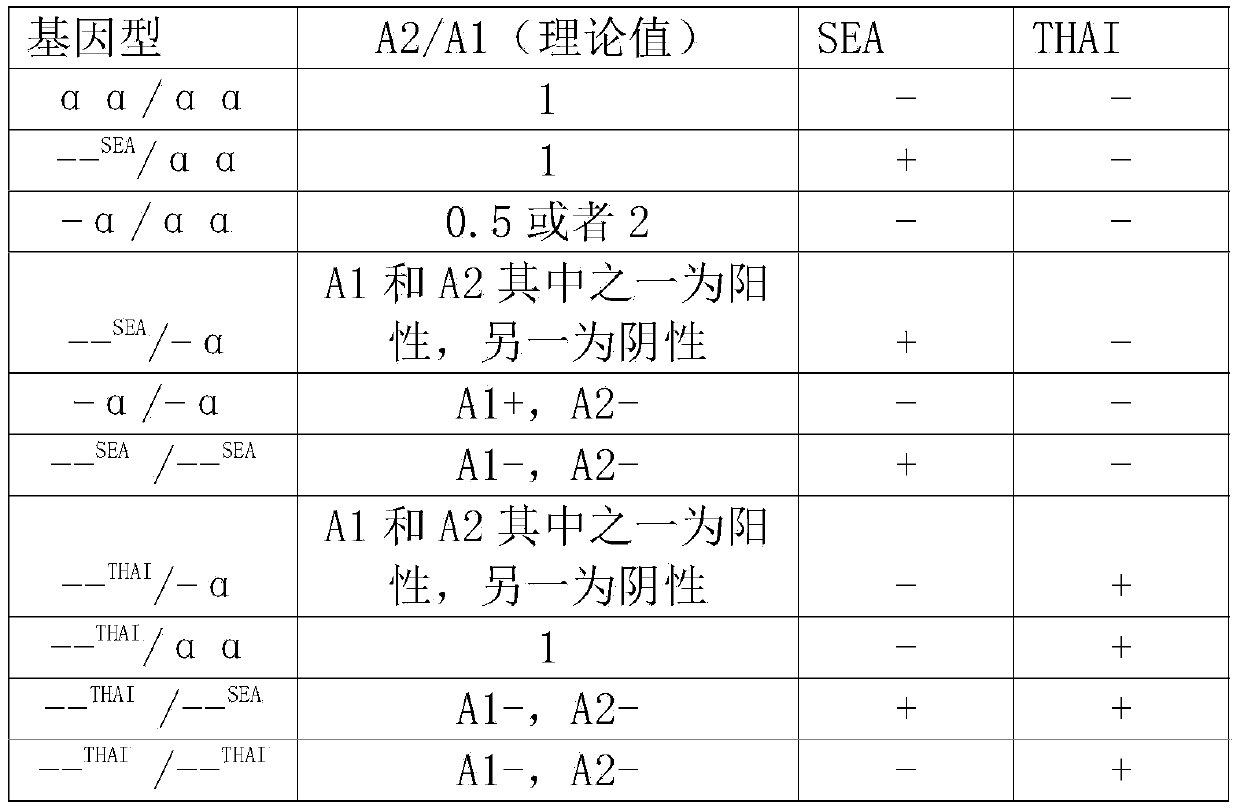
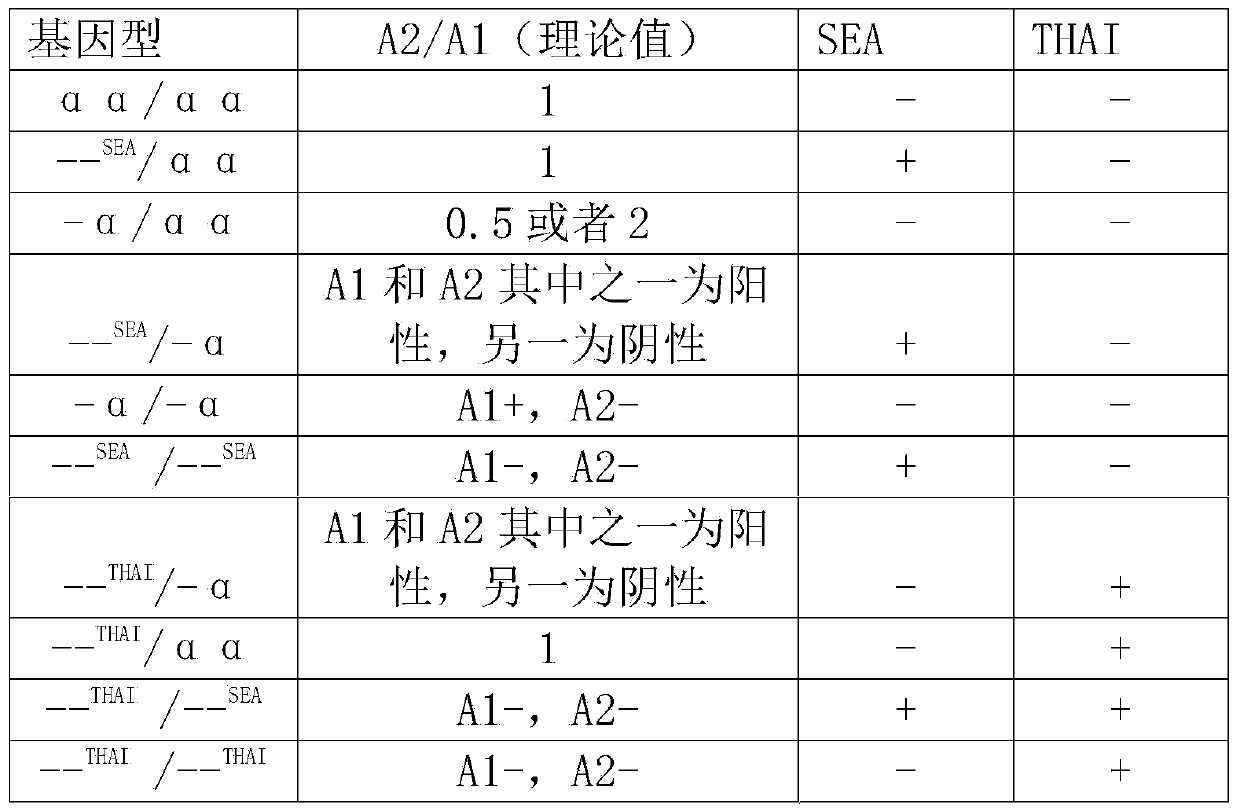
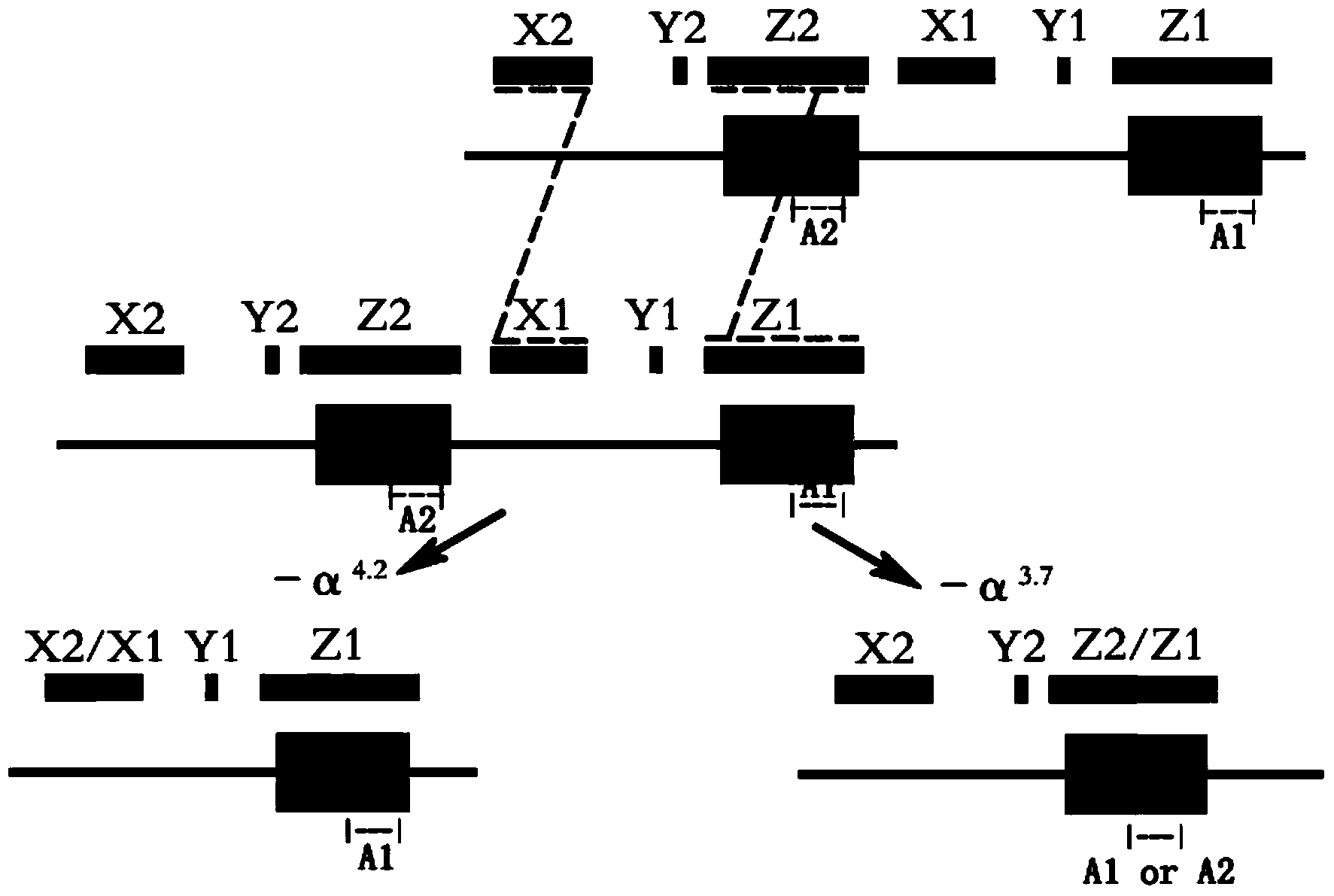
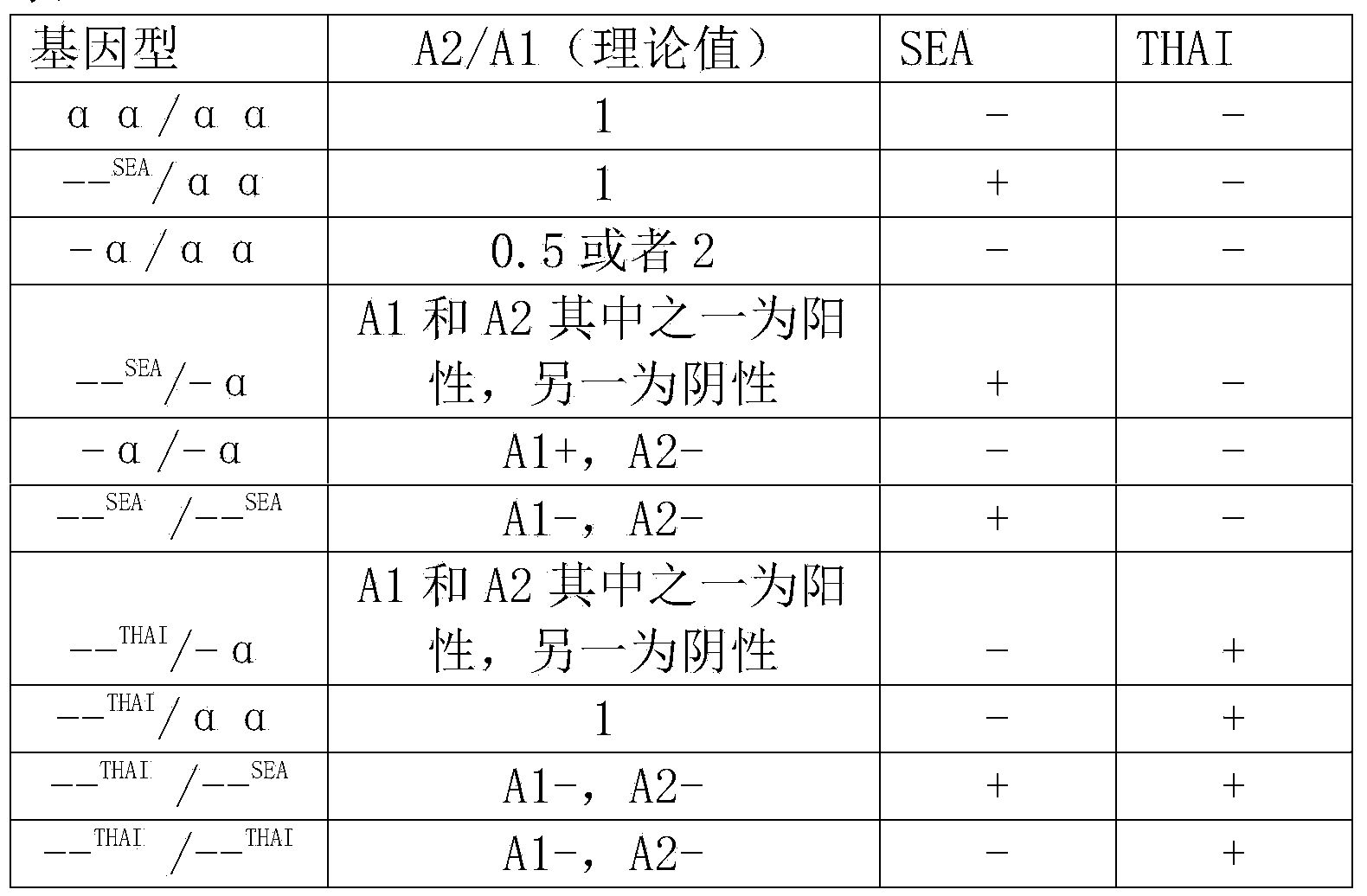
Alpha-thalassemia gene detecting kit
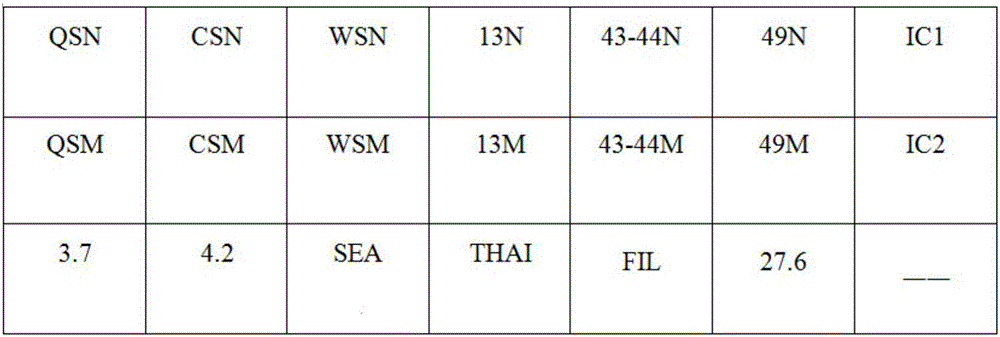
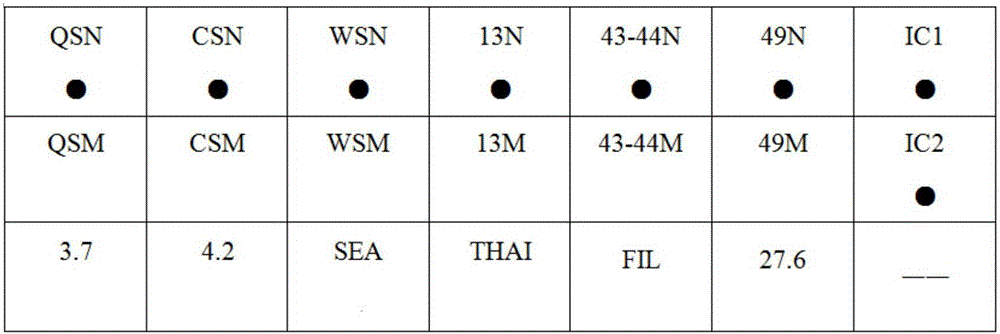
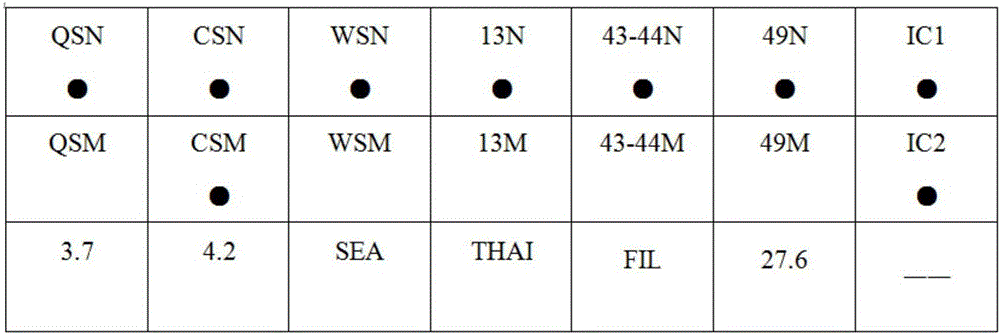
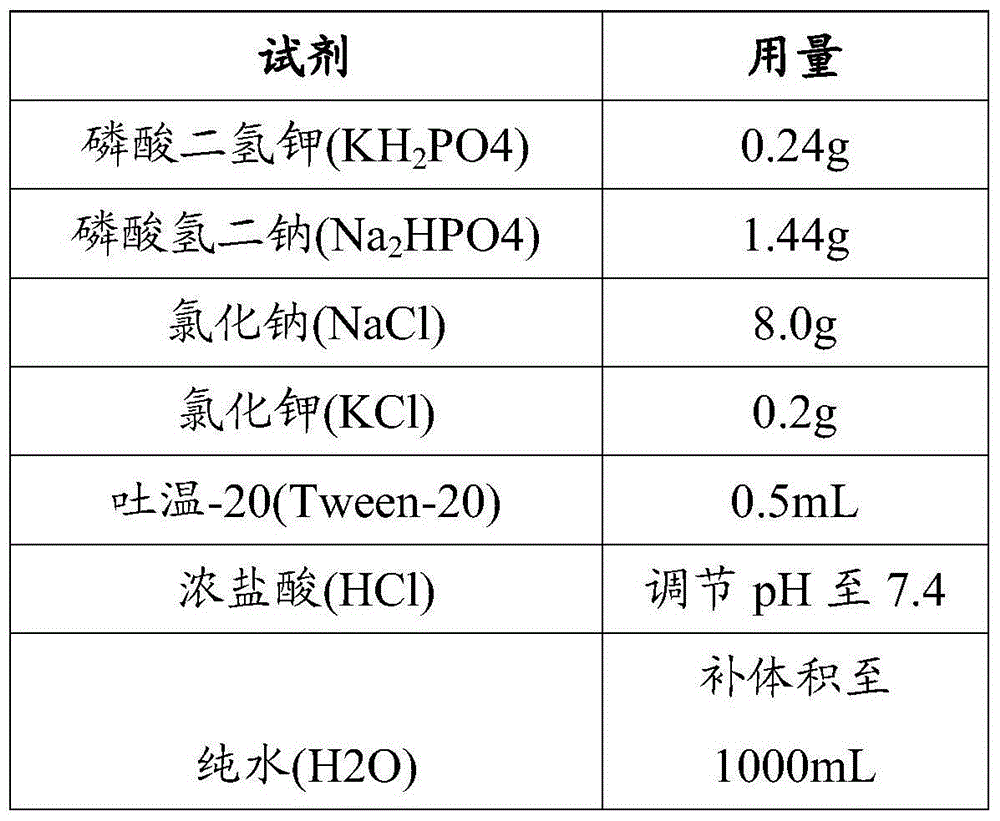
The invention provides a kit capable of detecting four deletion alpha-thalassemia genes (-alpha<3.7>, -alpha<4.2>, --<SEA> and --<THAI>) and three non-deletion alpha-thalassemia genes (alpha<CS>alpha, alpha<QS>alpha and alpha<WS>alpha) at the same time.The kit comprises a DNA chip, a PCR solution I and a PCR solution II.The DNA chip comprises a substrate and probes fixed to the substrate.The kit has the advantages that based on the PCR-reversal point hybridization detecting principle, corresponding amplification primers and probes are designed according to the mutation or deletion loci of genotypes, biotin is used for labeling the primers, amidogen is used for labeling the probes, the DNA chip (nylon membrane) is adopted as a substrate, the probes are fixed to the nylon membrane, and PCR products amplified by the specific primers and the probes fixed to the DNA chip are subjected to hybridization and signal developing box interpretation to diagnose thalassemia.
Owner:亚能生物技术(深圳)有限公司
Powder mixture composition of natural materials for controlling deficiencies in polypeptide alpha and beta hemoglobin chains
InactiveUS20140220163A1Reduce needReduces/eliminates side effectBiocideAnimal repellantsPowder mixtureBone marrow transplant
The embodiments herein provide a powdered composition of natural substances for controlling deficiencies in polypeptide alpha and beta hemoglobin chains and a diet plan for treating thalassemia patient. The composition comprises passargad powder, pars powder, purgative material, fumigation material, snuffing material, vinegar syrup and parsomash powder. The passargad powder is a stomach tonic and acts as anti-parasitic, anti-diarrheal, anti-dysentery, anti-bloating and anti-heartburn agents. The pars powder comprises sprouted grains for providing nutrition to cells. The purgative material is body purifier or detoxification agent and helps in motion and bowl movement. The vapor / fumigation material softens and reduces stickiness of sputum and removes sputum. The snuff helps in sneezing and removal of sputum. The vinegar syrup breaks down fat and removes toxins. The parsomash is an detoxification agent. The composition and dietary plan eliminates the need of bone marrow transplant, blood transfusion and defroxamin in thalassemia patients.
Owner:SOLEIMANI BABADI HAMZEH
Kit and method for detecting mutation of thalassemia-related gene and use thereof
The invention provides a kit and method for detecting mutation of a thalassemia-related gene and a use thereof and relates to the technical field of medical genetics. The kit for detection is used for detecting many types of patients such as people needing premarital checkup, people in gestation, newborns, people in a zone having high incidence of thalassemia and people having thalassemia family heredity history, can realize accurate, comprehensive, visual and simple detection of mutation of a thalassemia-related gene, has a detection mutation range comprising HBA1, HBA2 and HBB genes in the genome and neighbouring zones and has the characteristics of simpleness, accuracy, good repeatability and promotion and use easiness.
Owner:深圳市龙华区人民医院 +1
Kit for detecting common depletion alpha-thalassemia and use method thereof
InactiveCN104178573AEnsure consistencyAccurate judgmentMicrobiological testing/measurementSpecific detectionFluorescence
The invention discloses a kit for detecting common deletion form alpha-thalassemia and a using method of the kit. The kit comprises a pair of primers capable of performing simultaneous amplification of a signature sequence A1 in an alpha 1 section and a signature sequence A2 in an alpha 2 section in an alpha-globin gene cluster, a pair of primers capable of performing amplification of a --<SEA> genetype in the alpha-globin gene cluster and a pair of primers capable of performing amplification of a --<THAI> genetype in the alpha-globin gene cluster as well as a fluorescence probe for specific detection of the signature sequence A1 in the alpha 1 section, a fluorescence probe for specific detection of the signature sequence A2 in the alpha 2 section, a fluorescence probe for specific detection of a --<SEA> genetype amplicon and a fluorescence probe for specific detection of a --<THAI> genetype amplicon. As for the alpha-thalassemia globin gene deletion detection, the kit provided by the invention has high sensitivity, stability, accuracy and specificity.
Owner:龙驹
Kit for detecting common deletion form alpha-thalassemia and using method of kit
InactiveCN103966319AEnsure consistencyAccurate judgmentMicrobiological testing/measurementDNA/RNA fragmentationSpecific detectionFluorescence
The invention discloses a kit for detecting common deletion form alpha-thalassemia and a using method of the kit. The kit comprises a pair of primers capable of performing simultaneous amplification of a signature sequence A1 in an alpha 1 section and a signature sequence A2 in an alpha 2 section in an alpha-globin gene cluster, a pair of primers capable of performing amplification of a --<SEA> genetype in the alpha-globin gene cluster and a pair of primers capable of performing amplification of a --<THAI> genetype in the alpha-globin gene cluster as well as a fluorescence probe for specific detection of the signature sequence A1 in the alpha 1 section, a fluorescence probe for specific detection of the signature sequence A2 in the alpha 2 section, a fluorescence probe for specific detection of a --<SEA> genetype amplicon and a fluorescence probe for specific detection of a --<THAI> genetype amplicon. As for the alpha-thalassemia globin gene deletion detection, the kit provided by the invention has high sensitivity, stability, accuracy and specificity.
Owner:龙驹
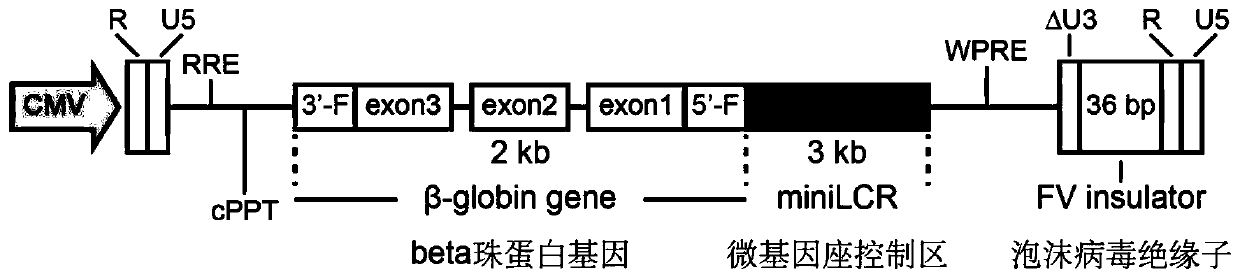
Lentiviral vector suitable for gene therapy of thalassemia and sickle anemia
ActiveCN110564770ALower titerInhibition of activationVectorsHaemoglobins/myoglobinsThalassemiaSickle cell anemia
The invention discloses a lentiviral vector suitable for gene therapy of thalassemia and sickle anemia. The lentiviral vector comprises a beta globin expression cassette; the expression cassette comprises a micro-locus control area, a gene sequence of a beta globin, a promoter sequence of a flank of the upstream of the gene sequence of the beta globin, and a flanking sequence of the downstream ofthe gene sequence of the beta globin; the micro-locus control area is a micro-control element which is screened out from the locus control area of beta globin 16kb and does not contain an HS1 area. Compared with the prior art, the screened beta globin expression cassette has both efficiency and specificity, and does not cause reduction of lentivirus titer; a screened insulator sequence which comesfrom foamy virus and is only 36 bp does not contain a hidden RNA splicing signal, and has the functions of maintaining gene expression and preventing gene activation in a region where the insulator sequence is located; the lentiviral vector can be used for gene therapy of thalassemia and sickle anemia.
Owner:SHANGHAI BDGENE TECH CO LTD
Ferroportin-inhibitor salts
ActiveUS11129820B2Low toxicityImprove bioavailabilityOrganic active ingredientsOrganic chemistryThalassemiaPharmaceutical Substances
The invention relates to novel salts of compounds of the general formula (I), pharmaceutical compositions comprising them and the use thereof as medicaments, in particular for the use as ferroportin inhibitors, more particularly for the use in the prophylaxis and / or treatment of diseases caused by a lack of hepcidin or iron metabolism disorders, such as particularly iron overload states such as in particular thalassemia, sickle cell disease and hemochromatosis.
Owner:VIFOR (INT) AG
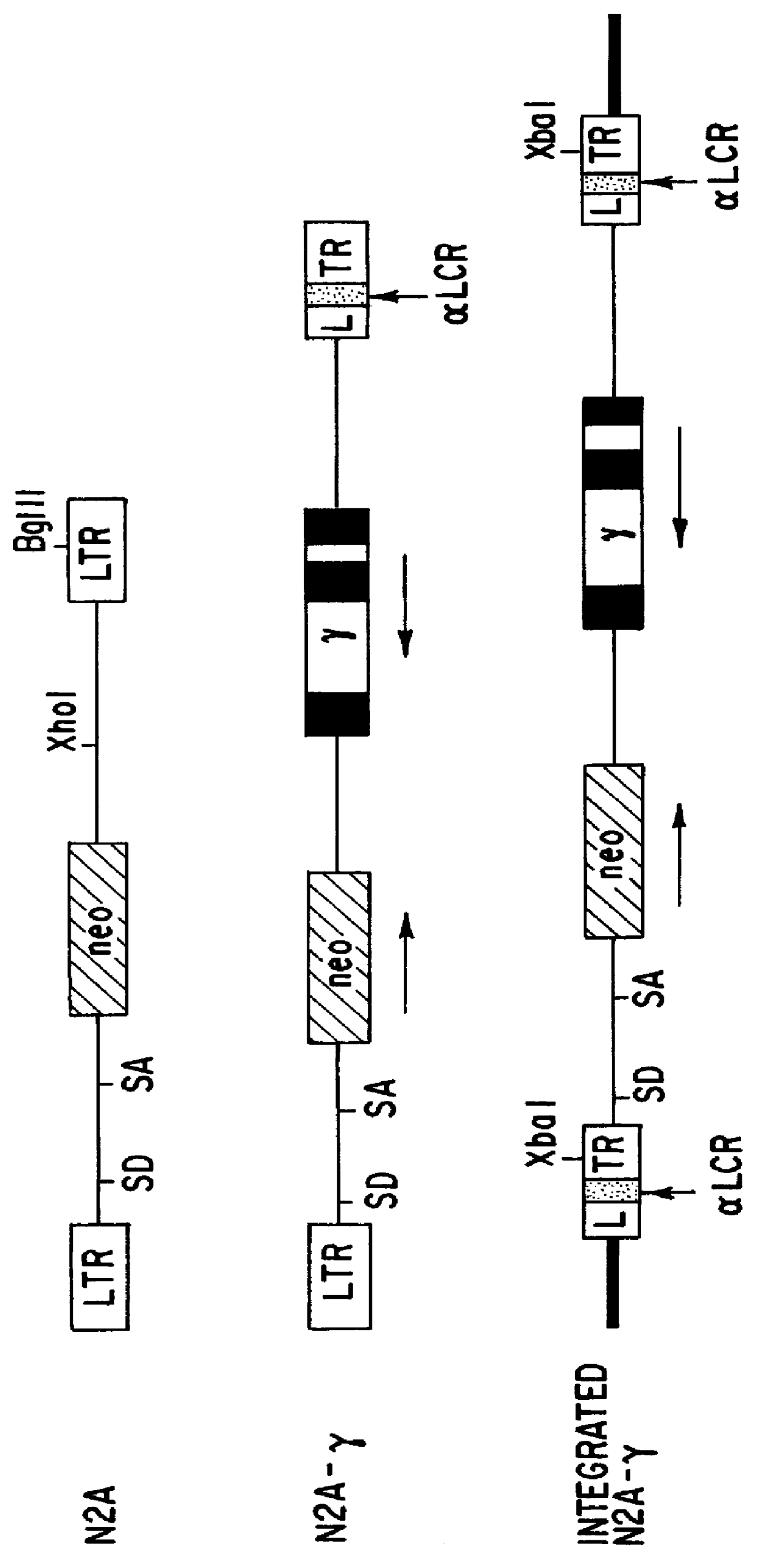
Vectors for expression of globin genes
InactiveUS6022738AImprove stabilityExcellent meanVirusesHaemoglobins/myoglobinsSickle cell anemiaThalassemia
The present invention relates to vectors comprising an alpha -globin locus control region ( alpha LCR) and a gene encoding an erythroid protein. In particular embodiments, a retroviral vector comprising an alpha LCR and a globin gene may be used to treat globin-based genetic disorders, including sickle cell anemia and beta -thalassemia.
Owner:MT SINAI SCHOOL OF MEDICINE
Method and system for determining fetus alpha thalassemia gene haplotype
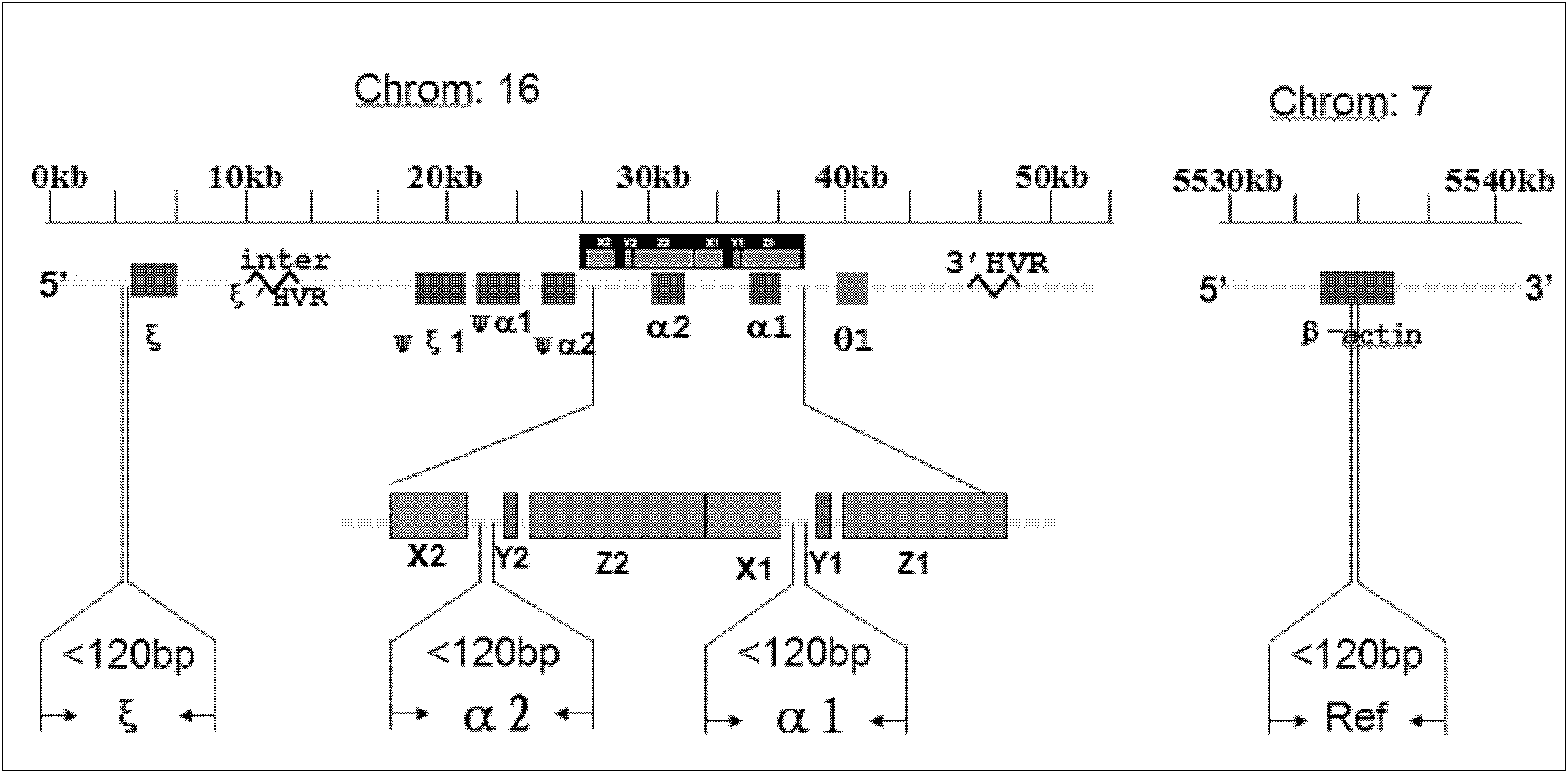
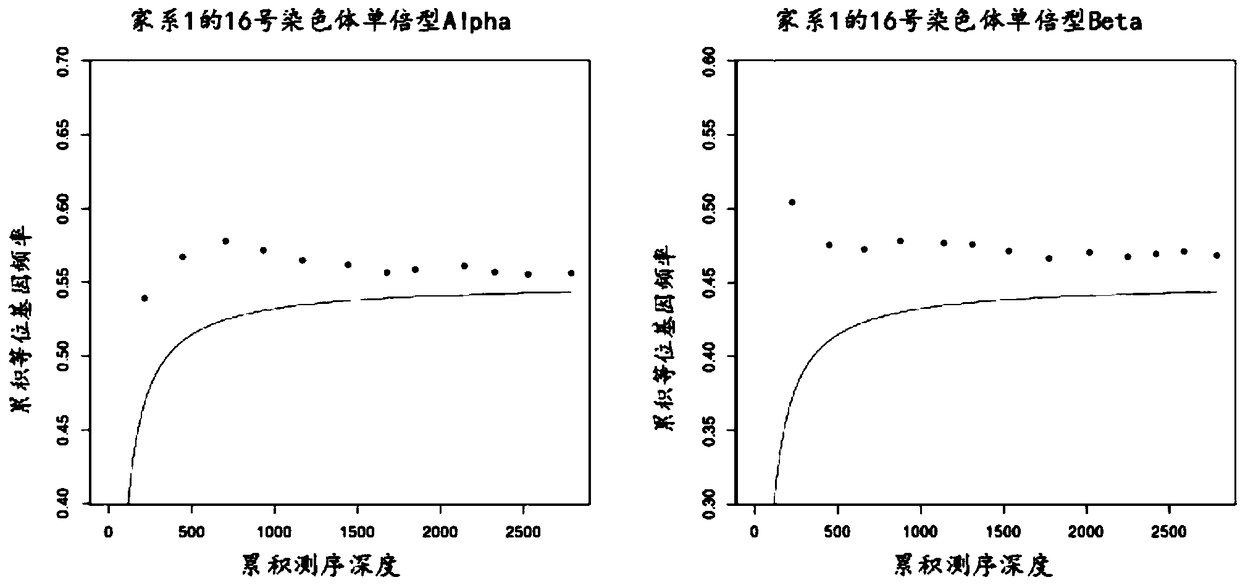
ActiveCN108048541AAvoid the risk of harming the fetusRelieve stressMicrobiological testing/measurementRelationship - FatherThalassemia
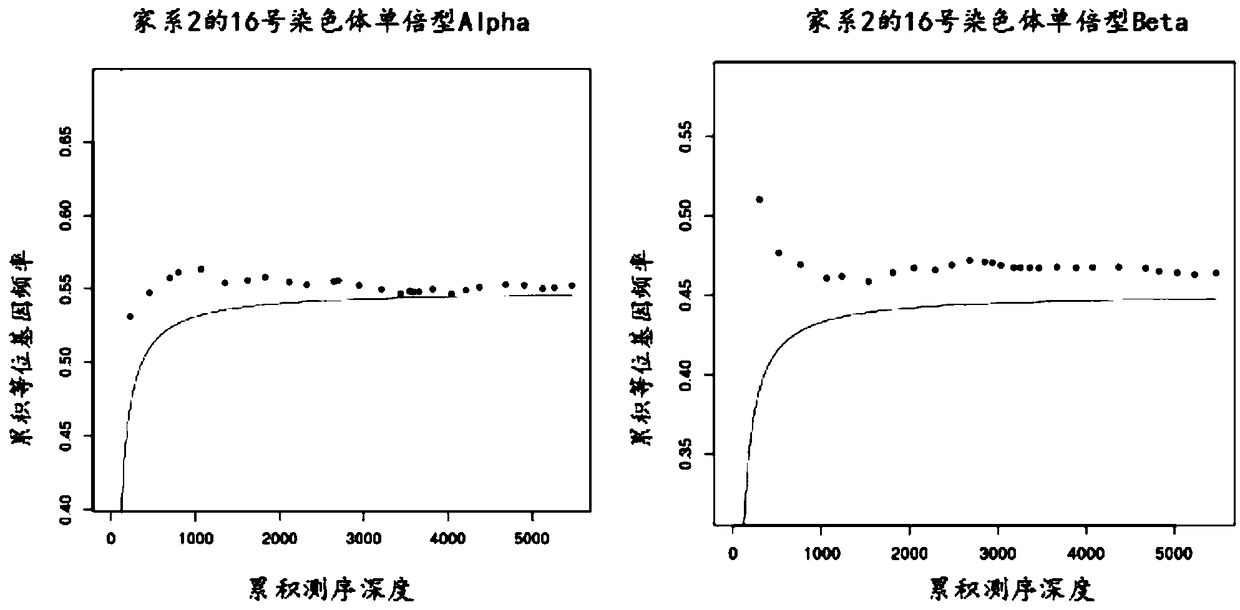
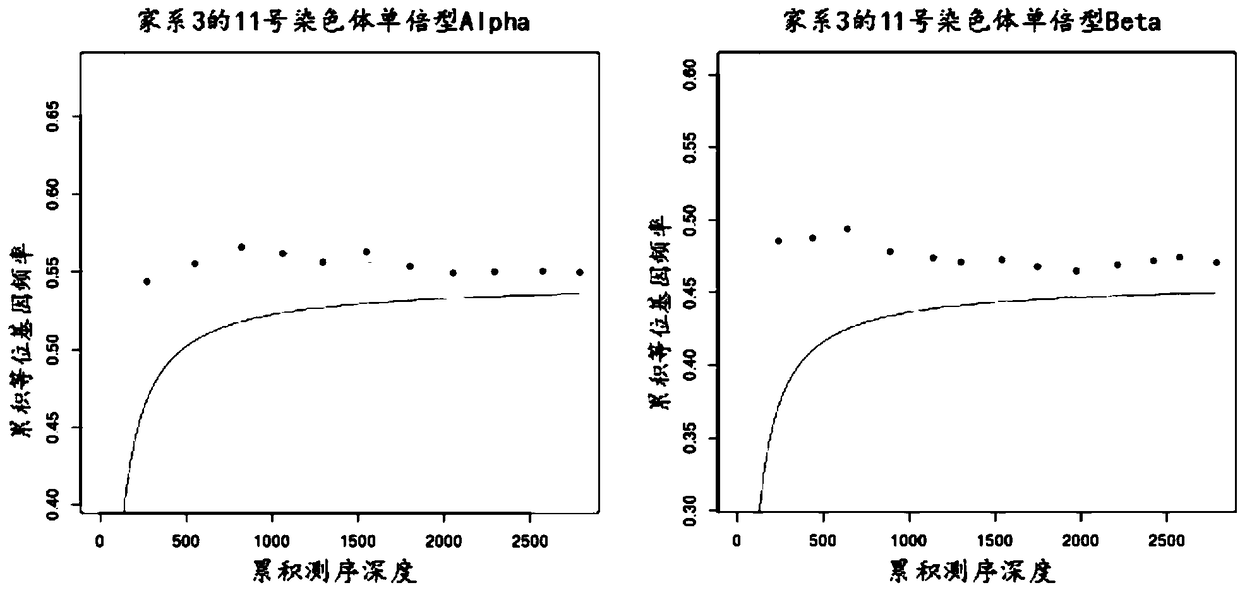
The invention relates to a method for determining a fetus alpha thalassemia gene haplotype. The method comprises the following steps: establishing a third-generation sequencing library according to ablood sample, wherein the blood sample is collected from blood samples of the father and mother of the fetus; performing the third-generation sequencing for the third-generation sequencing library, and obtaining a third-generation sequencing result; determining a father and mother alpha thalassemia gene haplotype according to the third-generation sequencing result; establishing a second-generationsequencing library according to a detection sample, wherein the detection sample is collected from a peripheral blood sample of a pregnant woman; performing the second-generation sequencing for the second-generation sequencing library, and obtaining a second-generation sequencing result; and determining the fetus alpha thalassemia gene haplotype according to the second-generation sequencing result as well as the father and mother alpha thalassemia gene haplotype.
Owner:GUANGZHOU JINGKE DX CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com