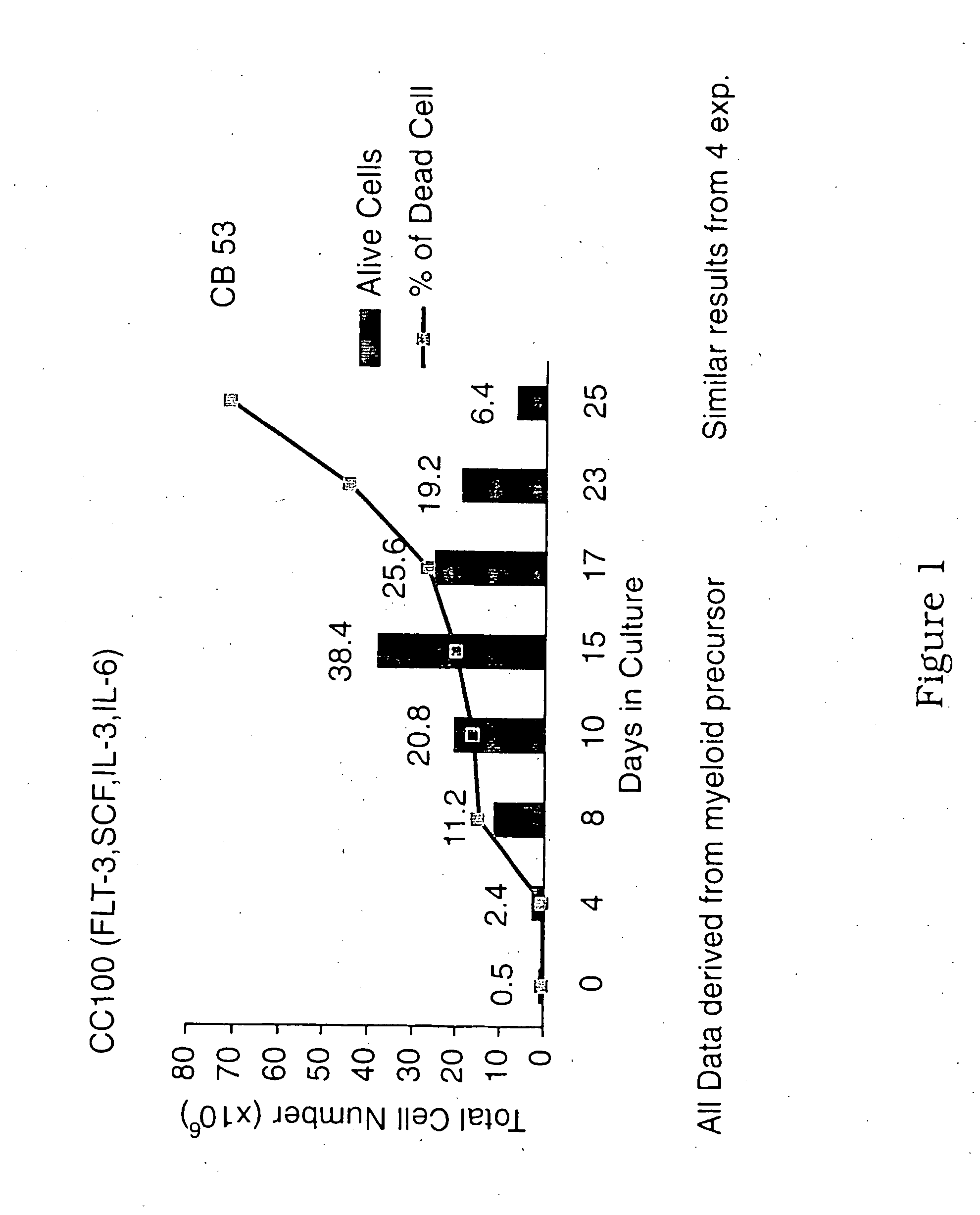
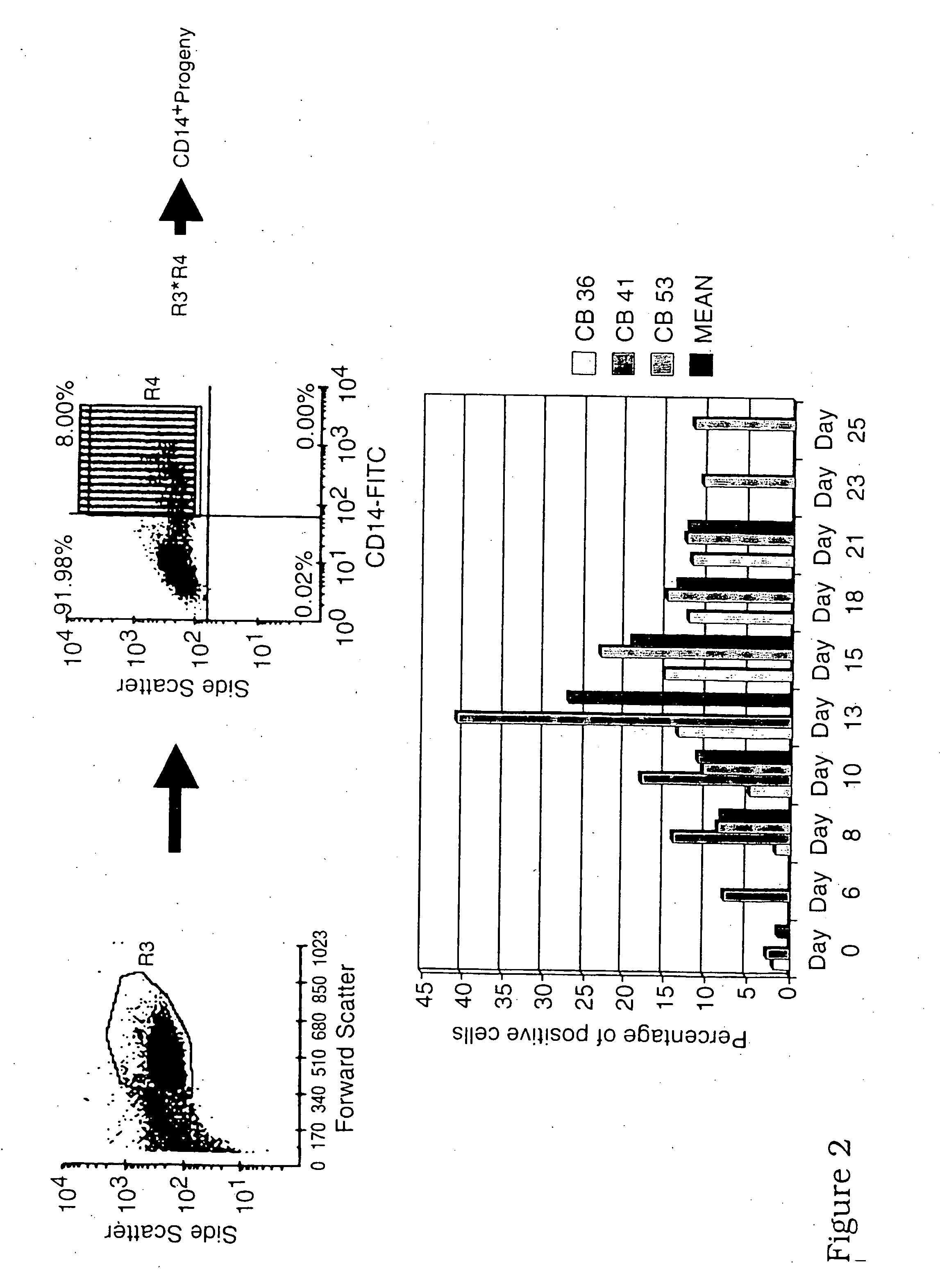
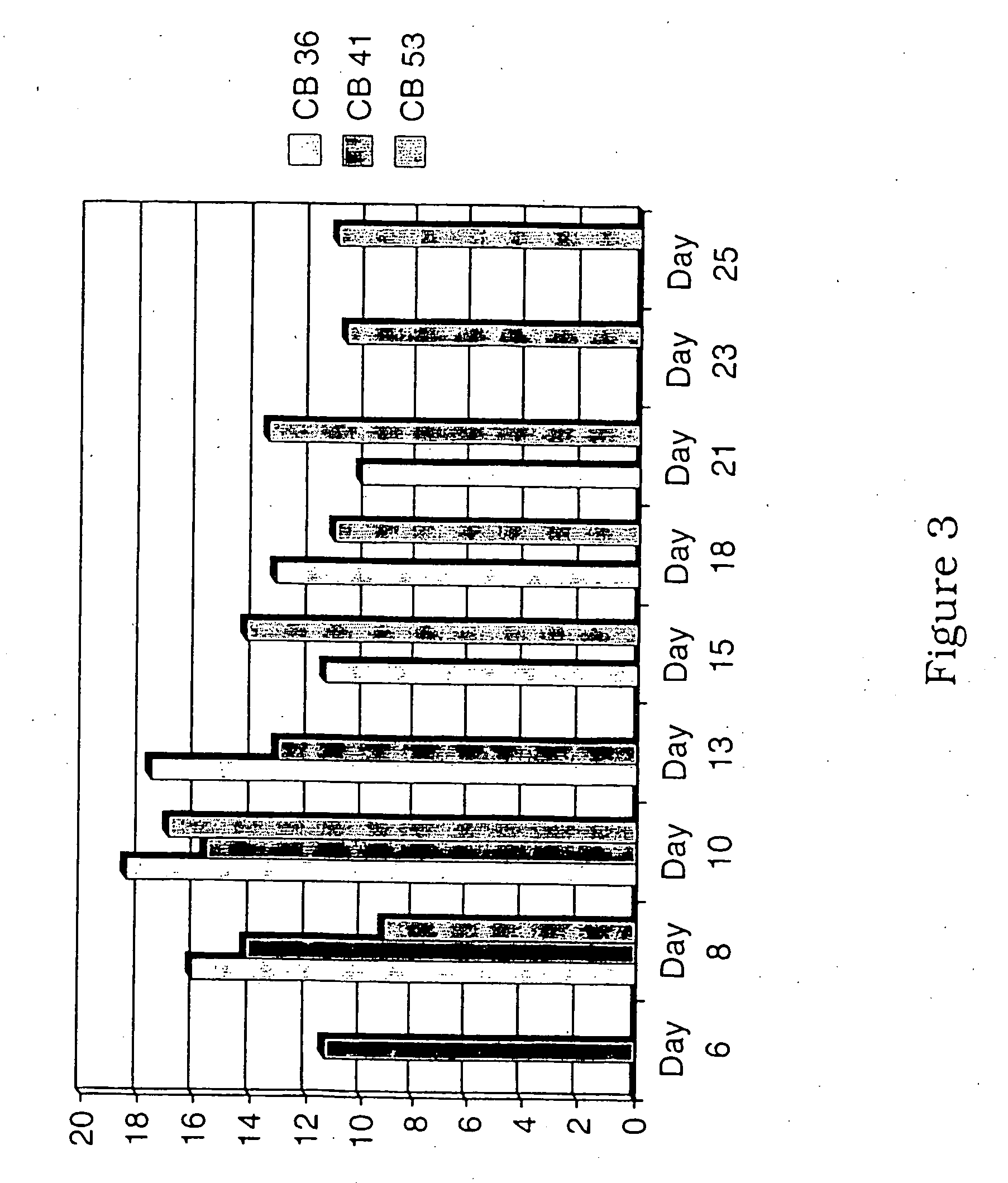
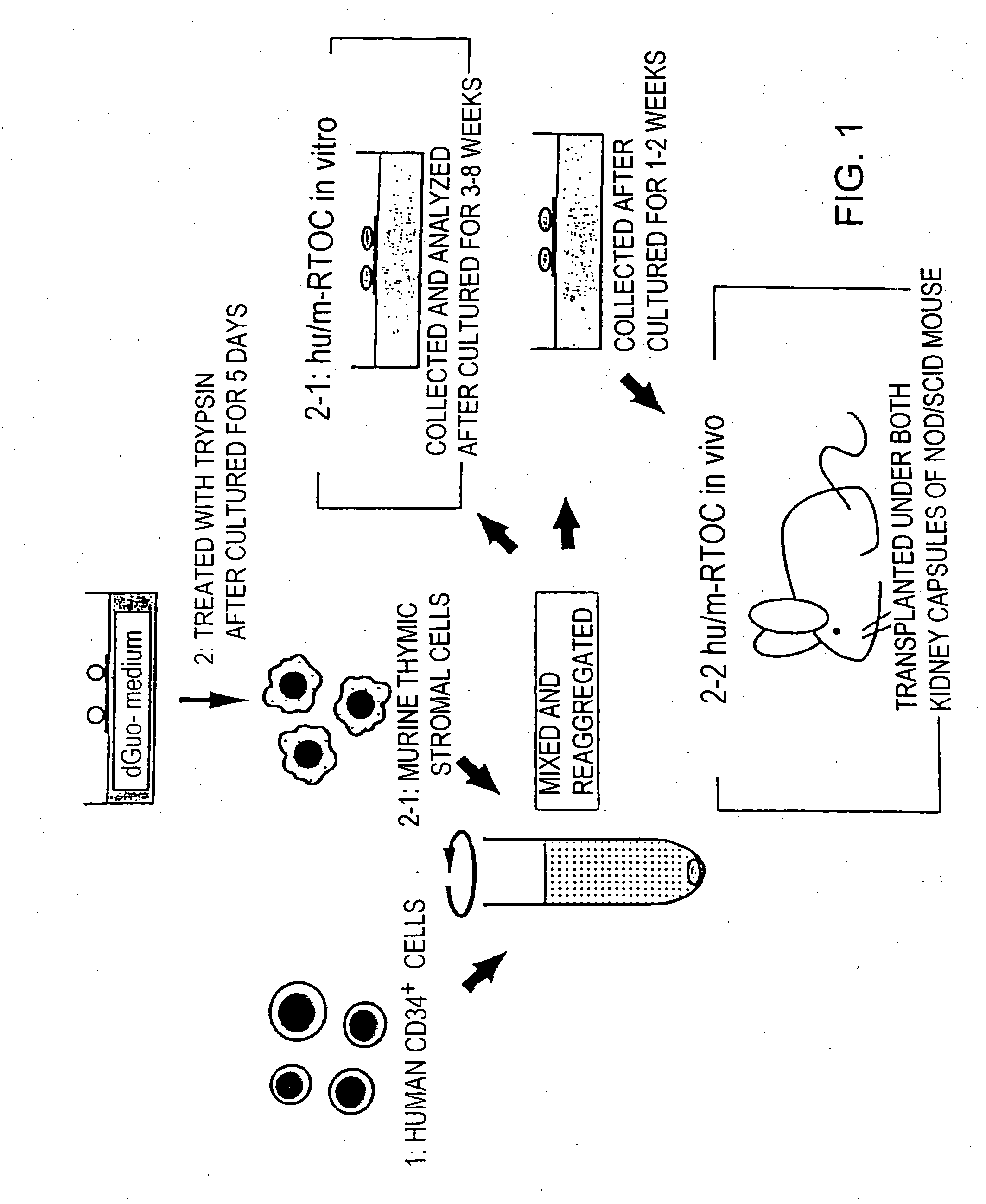
Patents
Literature
388 results about "CD34" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
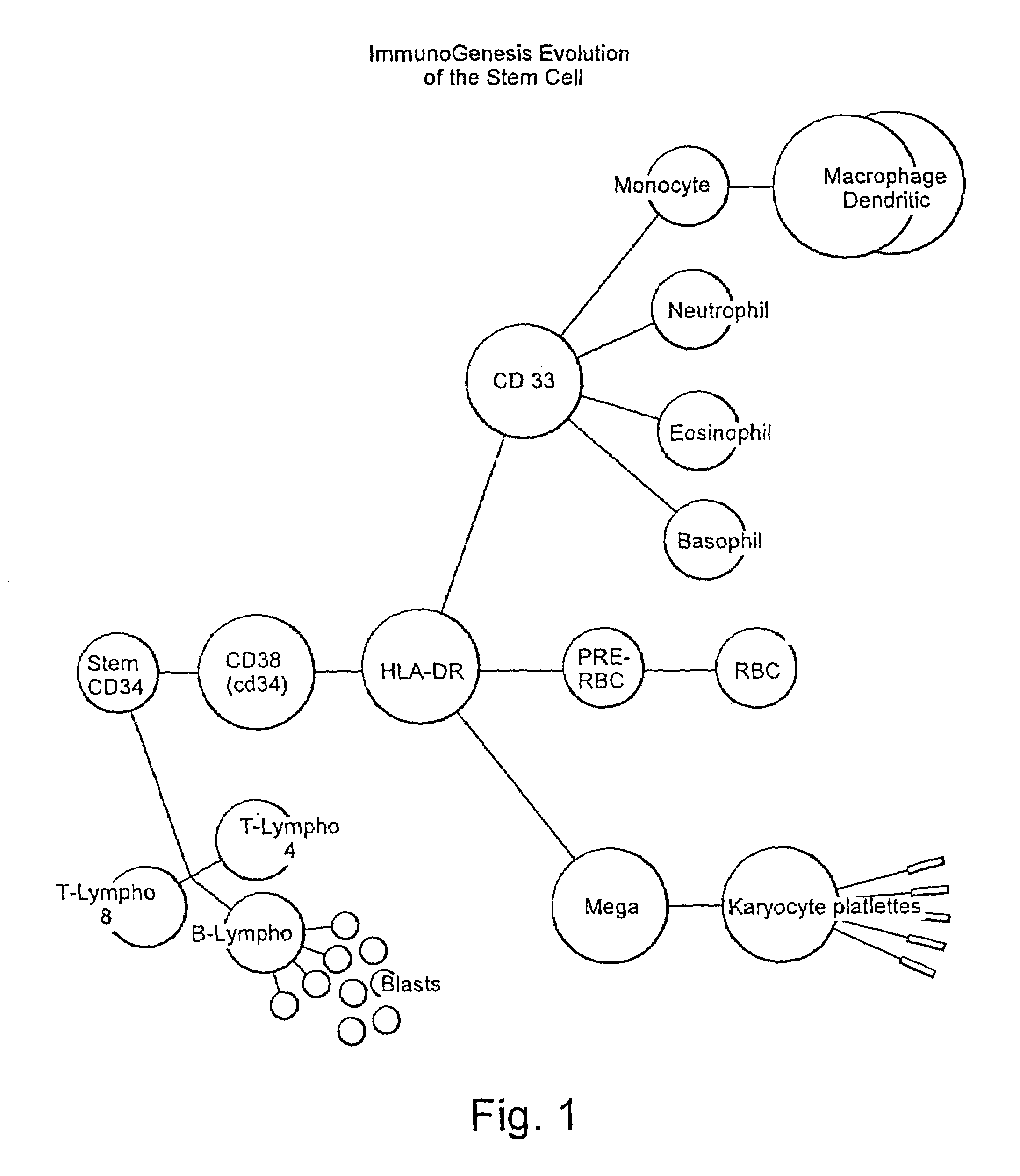
CD34 is a transmembrane phosphoglycoprotein protein encoded by the CD34 gene in humans, mice, rats and other species. CD34 derives its name from the cluster of differentiation protocol that identifies cell surface antigens. CD34 was first described on hematopoietic stem cells independently by Civin et al. and Tindle et al. as a cell surface glycoprotein and functions as a cell-cell adhesion factor. It may also mediate the attachment of hematopoietic stem cells to bone marrow extracellular matrix or directly to stromal cells. Clinically, it is associated with the selection and enrichment of hematopoietic stem cells for bone marrow transplants. Due to these historical and clinical associations, CD34 expression is almost ubiquitously related to hematopoietic cells however it is actually found on many other cell types as well.
Multipotent amniotic fetal stem cells
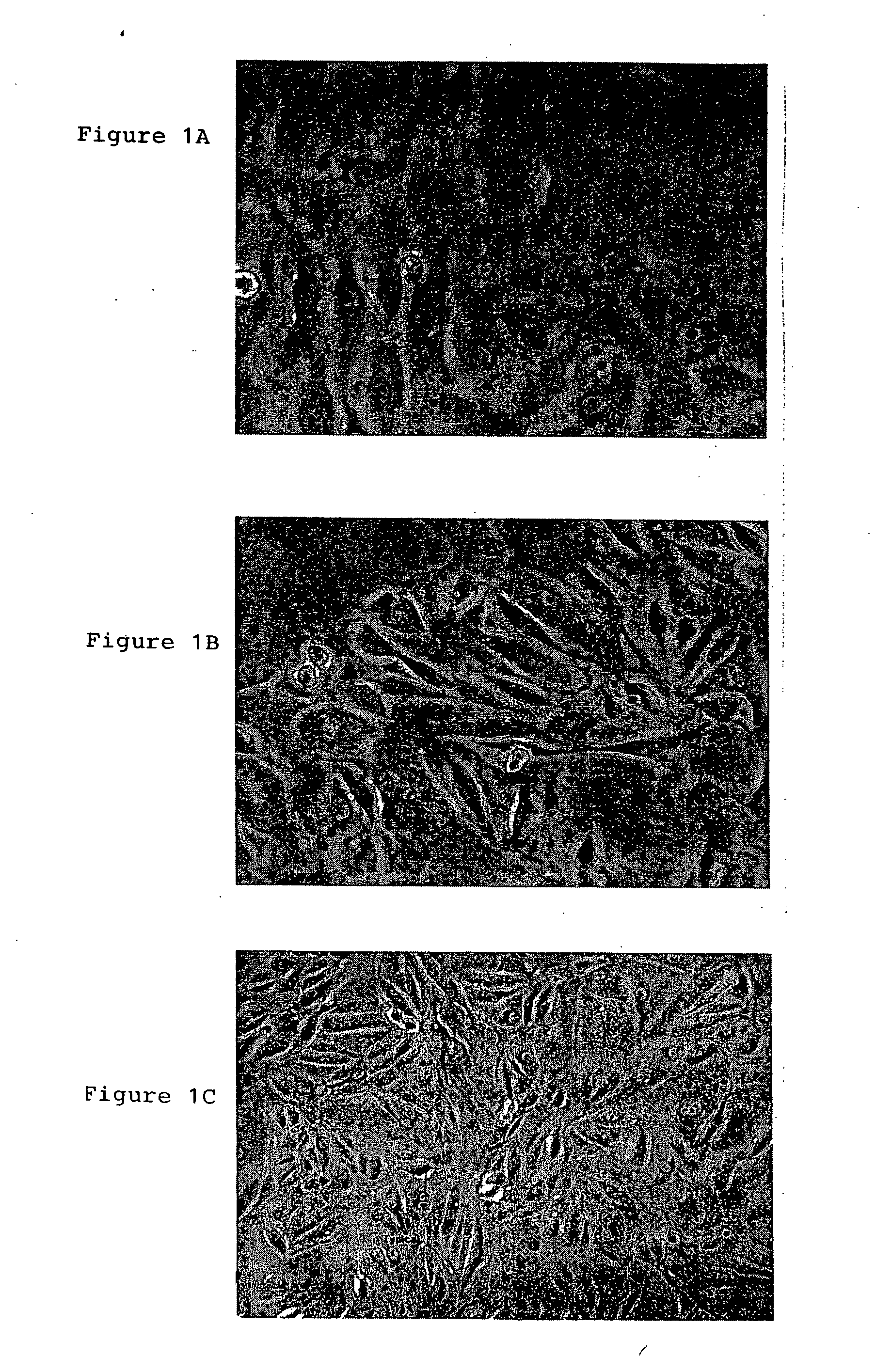
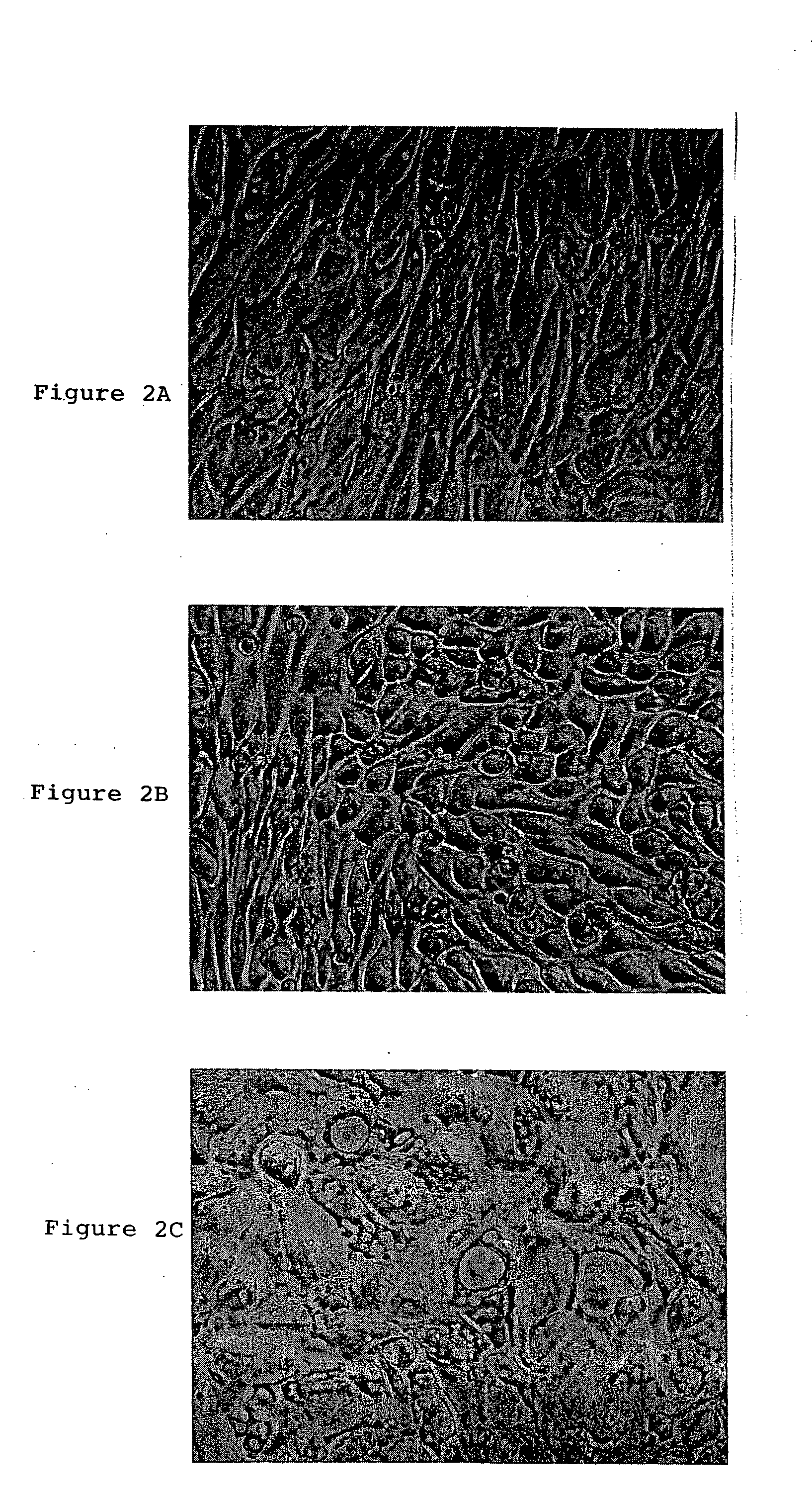
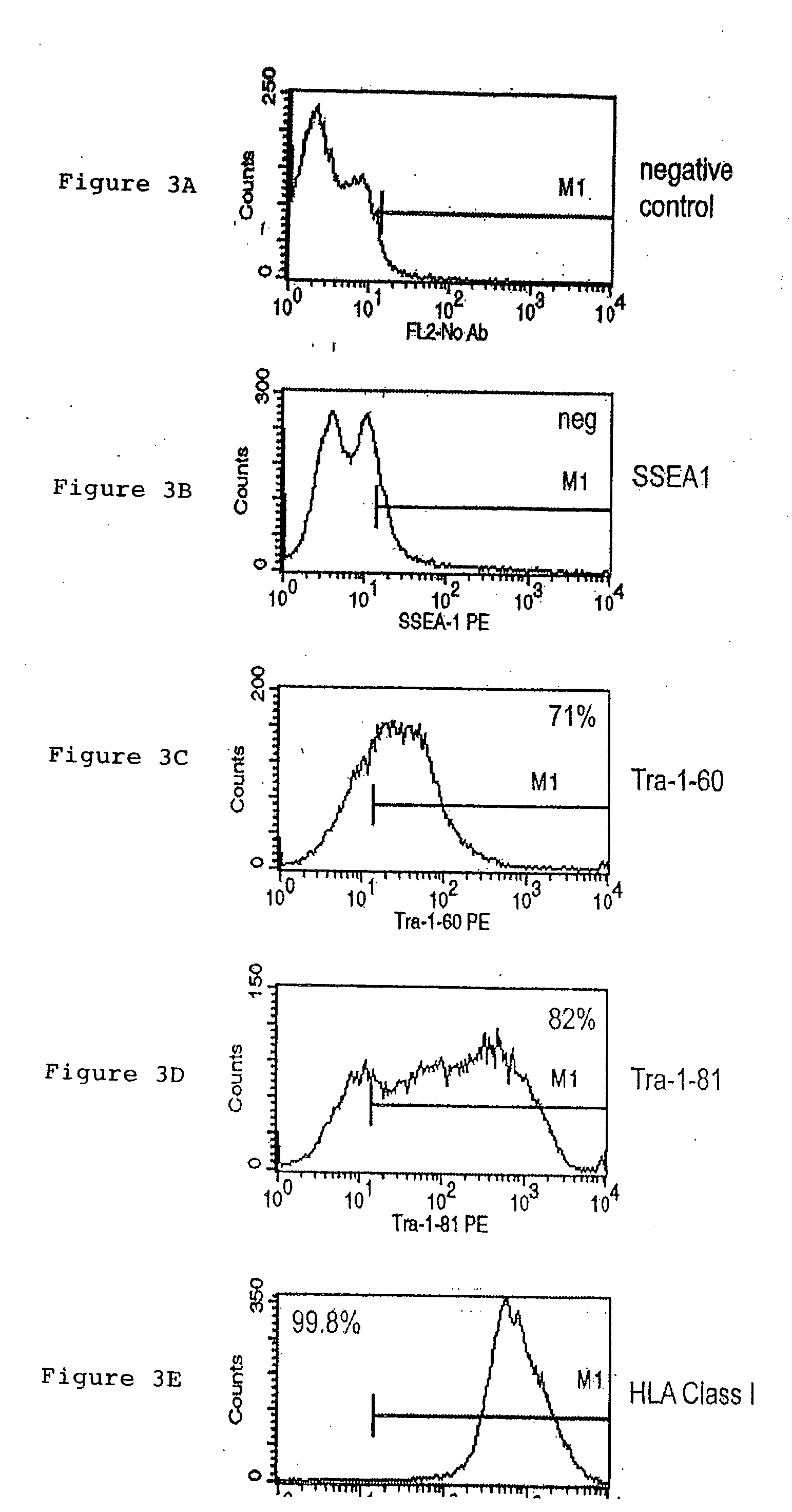
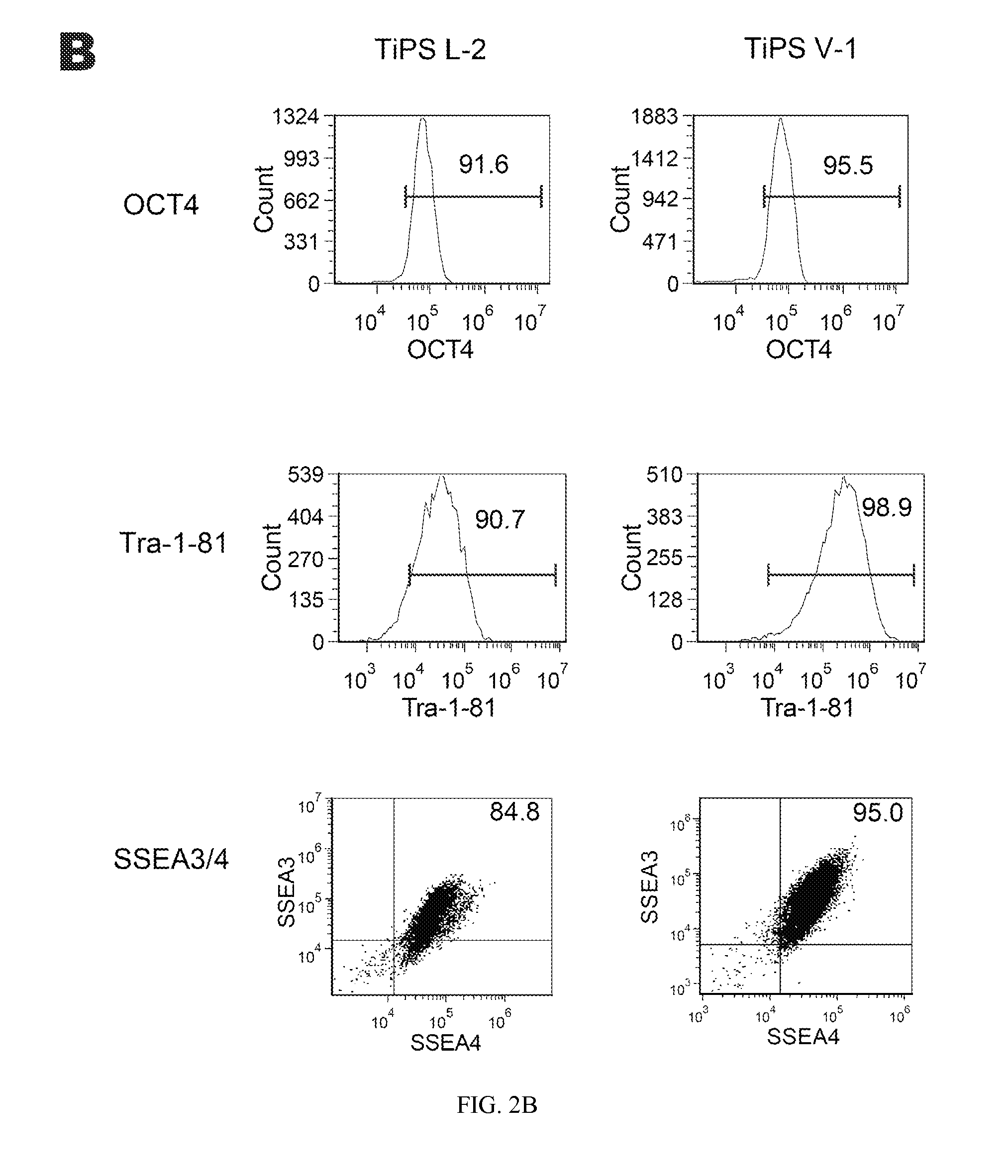
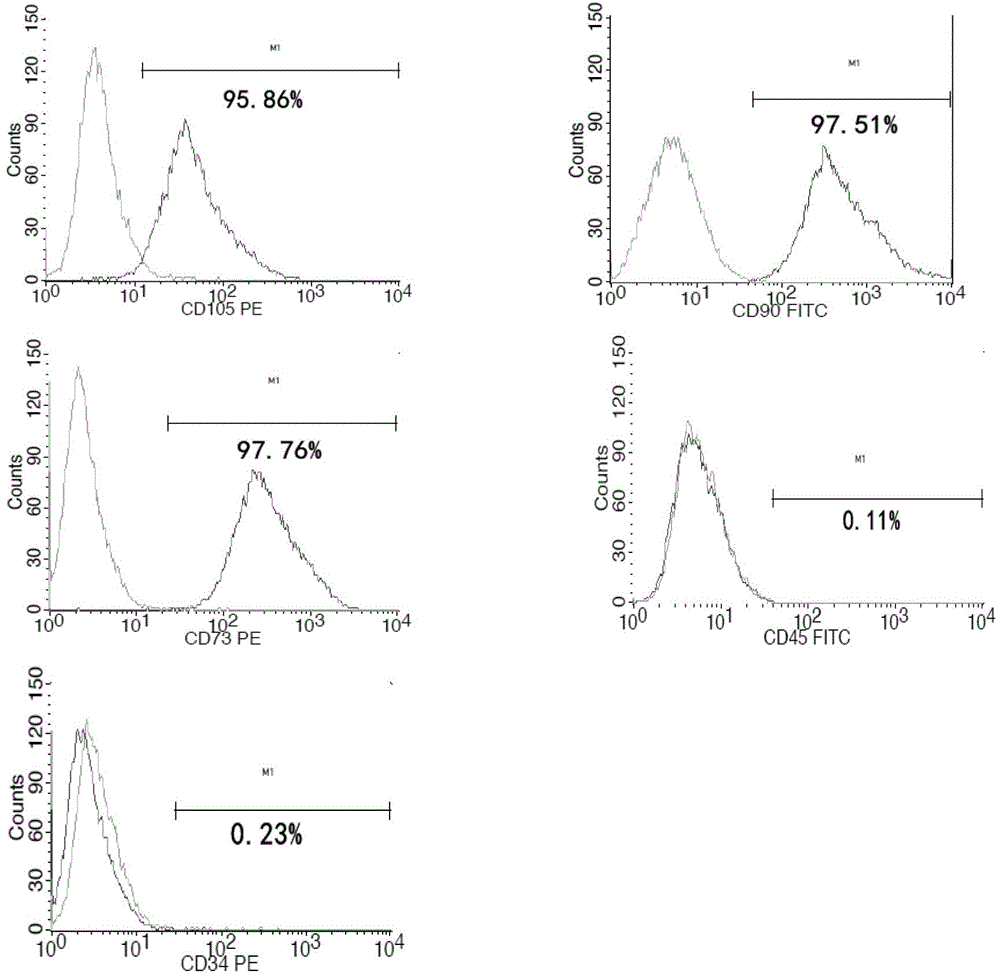
A source of multipotent amniotic fluid / fetal stem cells (MAFSCs) is disclosed. MAFSC are of fetal origin and have a normal diploid karyotype. These cells are characterized by the following cell surface markers: SSEA3, SSEA4, Tra-1-60, Tra-1-81, Tra-2-54, HLA class I, CD13, CD44, CD49b, CD105 and are distinguished by the absence of the antigen markers CD34, CD45, and HLA Class II, but are distinguished from mouse embryonic stem cells in that these cells do not express the cell surface marker SSEA1. MAFSC express the stem cell transcription factor Oct-4. MAFSC cells can be propagated for an indefinite period of time in continuous culture in an undifferentiated state. The MAFSCs have the ability to differentiate in culture in a regulated manner, into three or more subphenotypes. Cells can then be differentiated into endodermal, mesodermal and ectodermal derived tissues in vitro and in vivo. A method for isolating, identifying, expanding and differentiating MAFSCs is disclosed.
Owner:RGT UNIV OF CALIFORNIA
Methods and compositions for the treatment and management of hemoglobinopathy and anemia
InactiveUS20050143420A1Good effectRelieve symptomsBiocidePeptide/protein ingredientsRed blood cellThalassemia
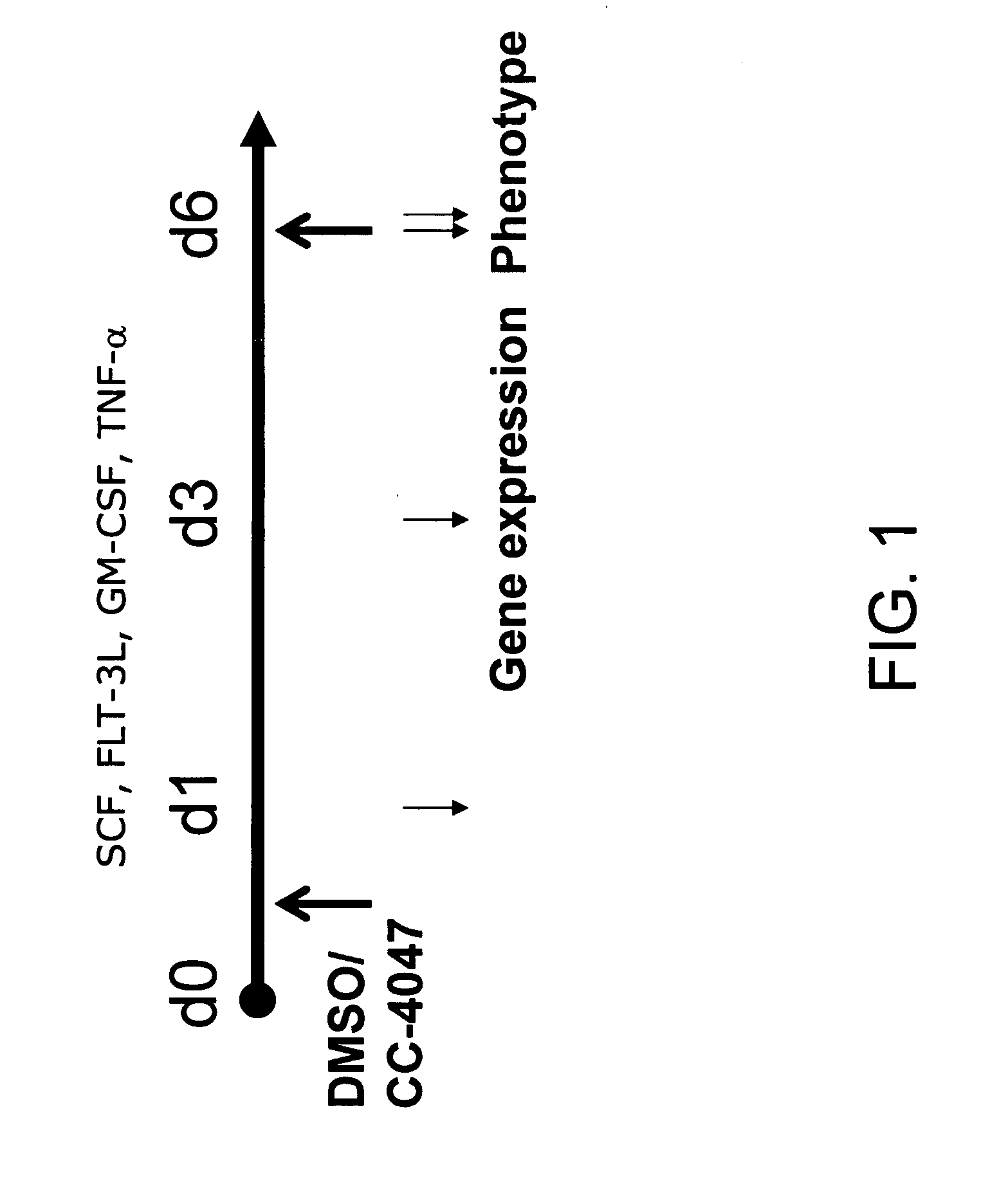
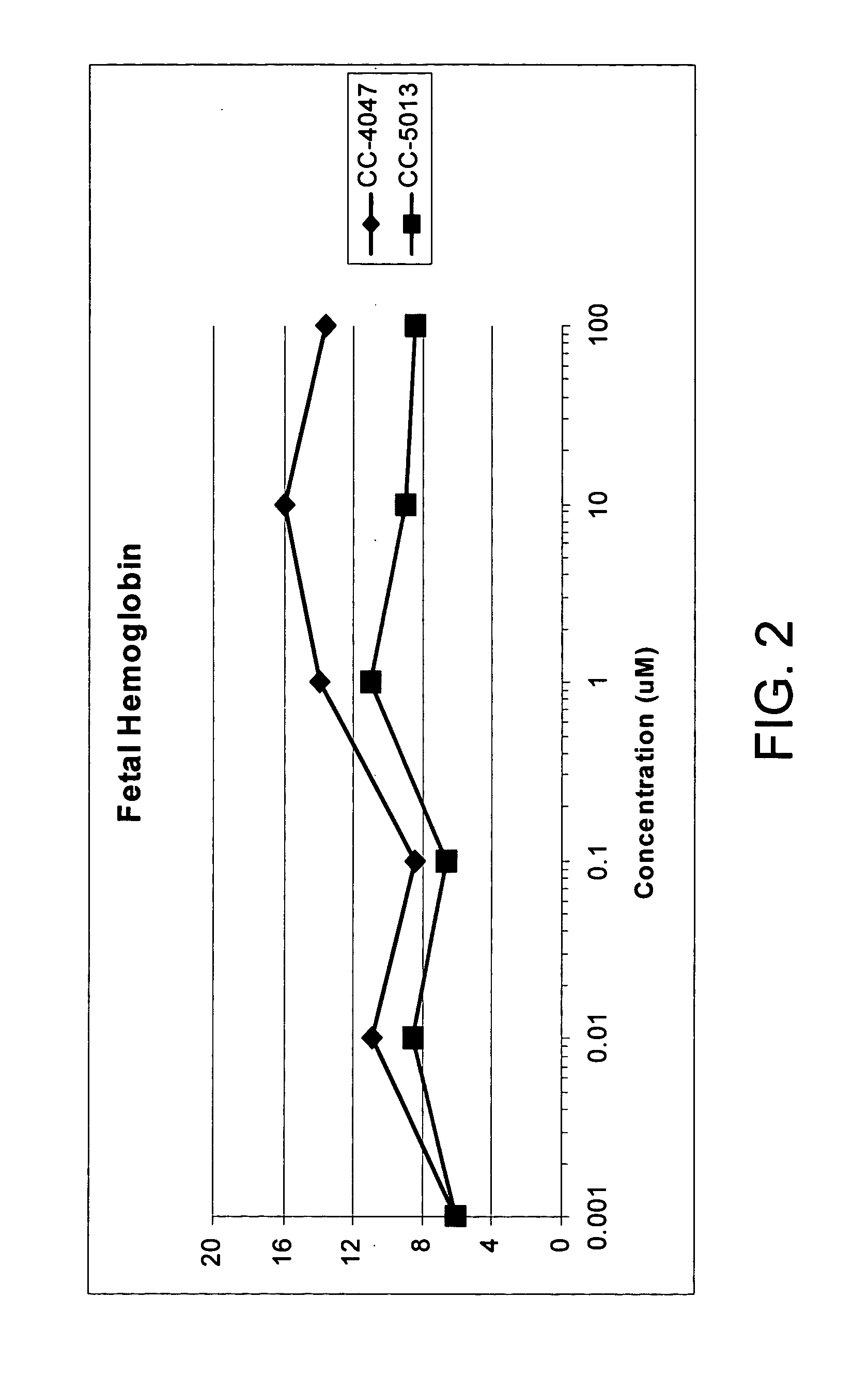
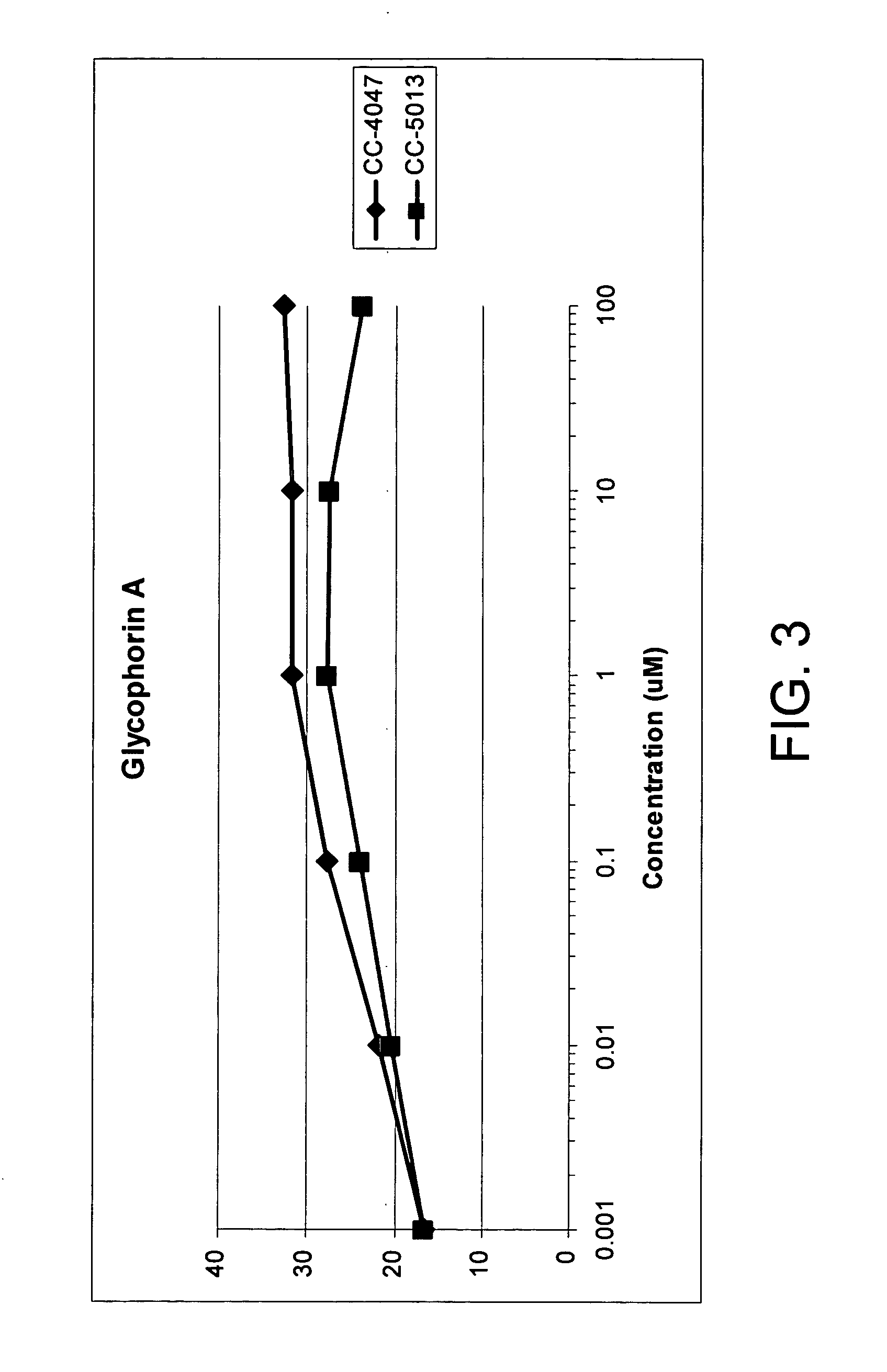
The present invention is directed to the use of immunomodulatory compounds, particularly members of the class of compounds known as IMiDs™, and more specifically the compounds 4-(Amino)-2-(2,6-dioxo(3-piperidyl))-isoindoline-1,3-dione and 3-(4-amino-1-oxo-1,3-dihydroisoindol-2-yl)-piperidine-2,6-dione, to induce the expression of fetal hemoglobin genes, genes essential for erythropoiesis, and genes encoding alpha hemoglobin stabilizing protein, within a population of CD34+ cells. These compounds are used to treat hemoglobinopathies such as sickle cell anemia or β-thalassemia, or anemias caused by disease, surgery, accident, or the introduction or ingestion of toxins, poisons or drugs.
Owner:SIGNAL PHARMA LLC
Method for stimulating an immune response
InactiveUS6977073B1Stimulating directed immune responseStimulate immune responseBiocideArtificial cell constructsAbnormal tissue growthHematopoietic cell
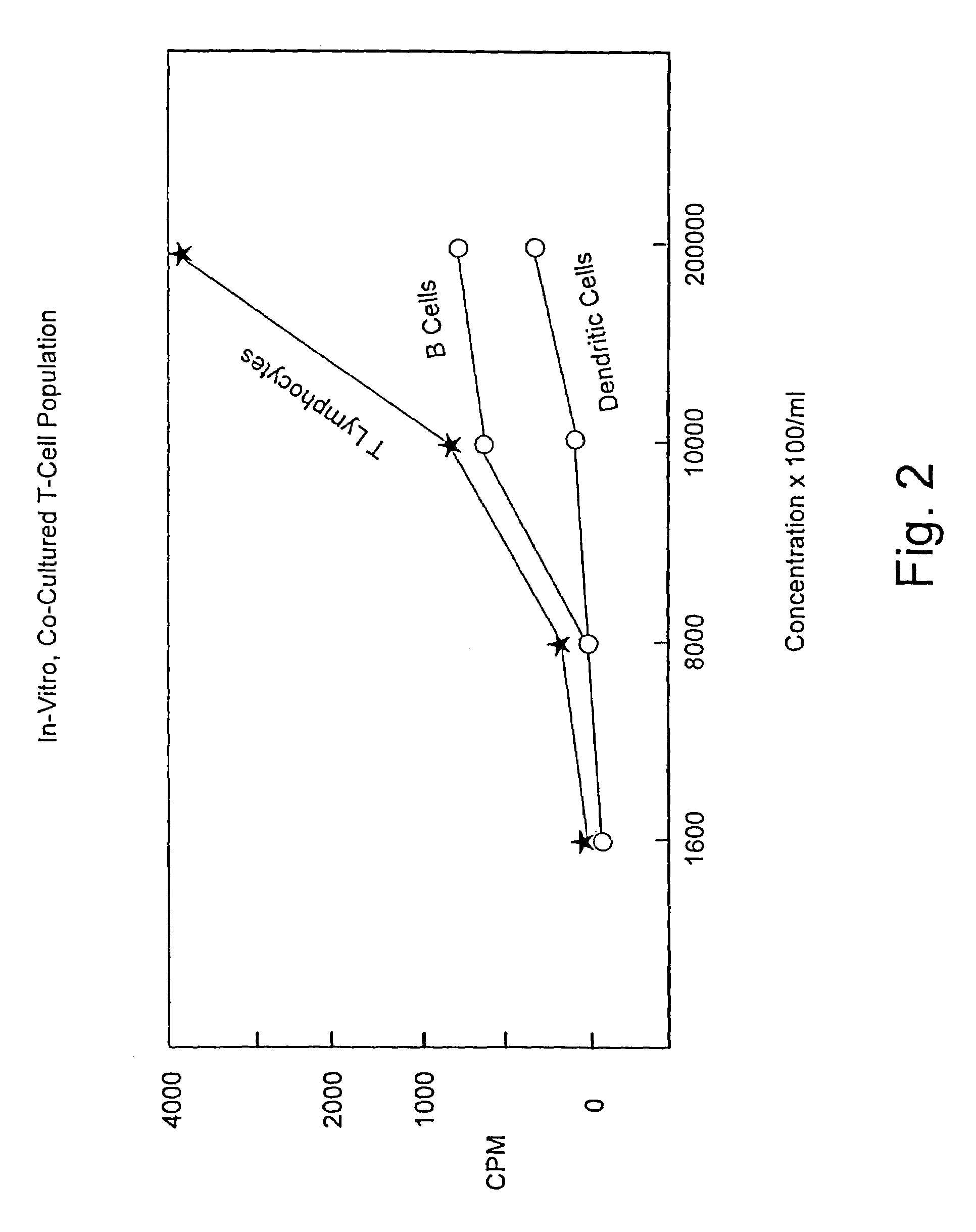
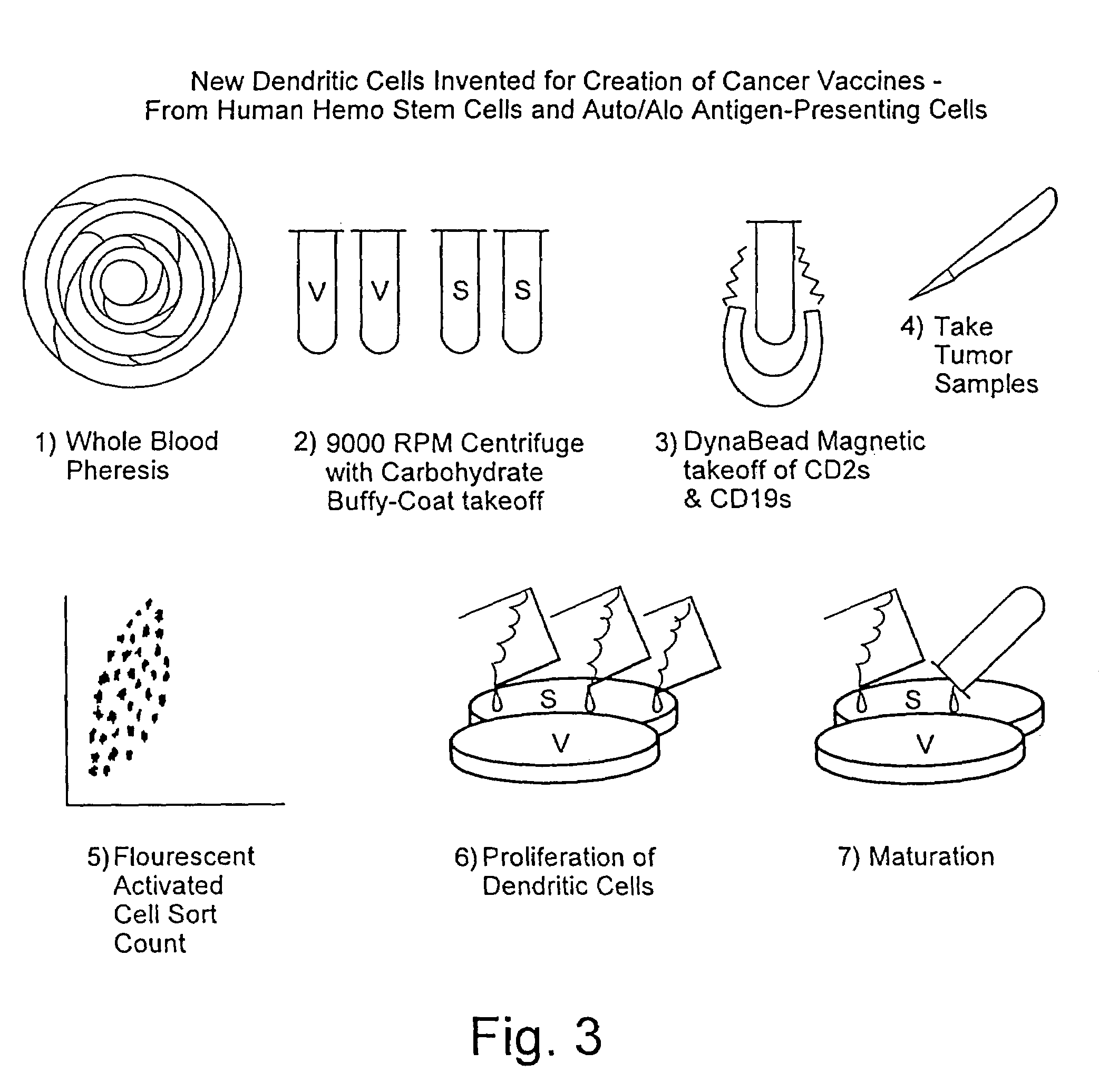
A method is described whereby dendritic cells derived from the CD34+ and CD 34−hematopoietic cell lineages are directed to become programmable antigen presenting cells. The programmed cells may be pulsed with tumor cell RNA or tumor cell RNA expression products. The protocol provides for directing the maturation of dendritic cells to become antigen presenting cells. The protocol further provides for isolating tumor cell RNA from biopsy material that has been prepared in paraffin block storage. The directed dendritic cell is provided with a plurality of tumor markers by using tumor RNA in toto, the poly A+RNA fraction or the expression product of such RNA. Once activated the dendritic cells are incubated with T4 and T8 lymphocytes to stimulate and sensitize the T lymphocytes which upon introduction either into a donor host or a nondonor recipient will provide immune response protection.
Owner:CEZAYIRLI CEM
Method for placenta mesenchyma stem cell separation and in vitro amplify cultivation
InactiveCN101270349AImprove sorting effectImprove purification rateSkeletal/connective tissue cellsBiological testingCD34Decidual tissue
The invention provides a method for separation of placenta-derived mesenchymal stem cells, and for amplification and culture in vitro. After decidual tissue cells on the side of a placenta matrix are adopted and collected, primary culture cells are amplified by adhering to wall; the placenta-derived mesenchymal stem cells are purified by adopting positive and negative immunological sorting and combination method to get CD34<->CD105<+> cells which are secondarily amplified and cultured in a serum-free culture system in vitro. By adopting the method provided by the invention, just few primary culture cells (1X10<5> cells) are needed every time to produce a good sorting result; and the purification rate of the placenta-derived mesenchymal stem cells is further improved. Not only the culture system used has significant advantage of amplification over placenta-derived mesenchymal stem cells, but also the amplified cells have multiplex differentiation potential. The method can be applied for purification of placenta-derived mesenchymal stem cells and amplification and culture in vitro.
Owner:ZHEJIANG UNIV
Optimizing culture medium for CD34<+> hematopoietic cell expansion
InactiveUS20050032122A1Simple compositionCulture processBlood/immune system cellsHematopoietic cellSerum free
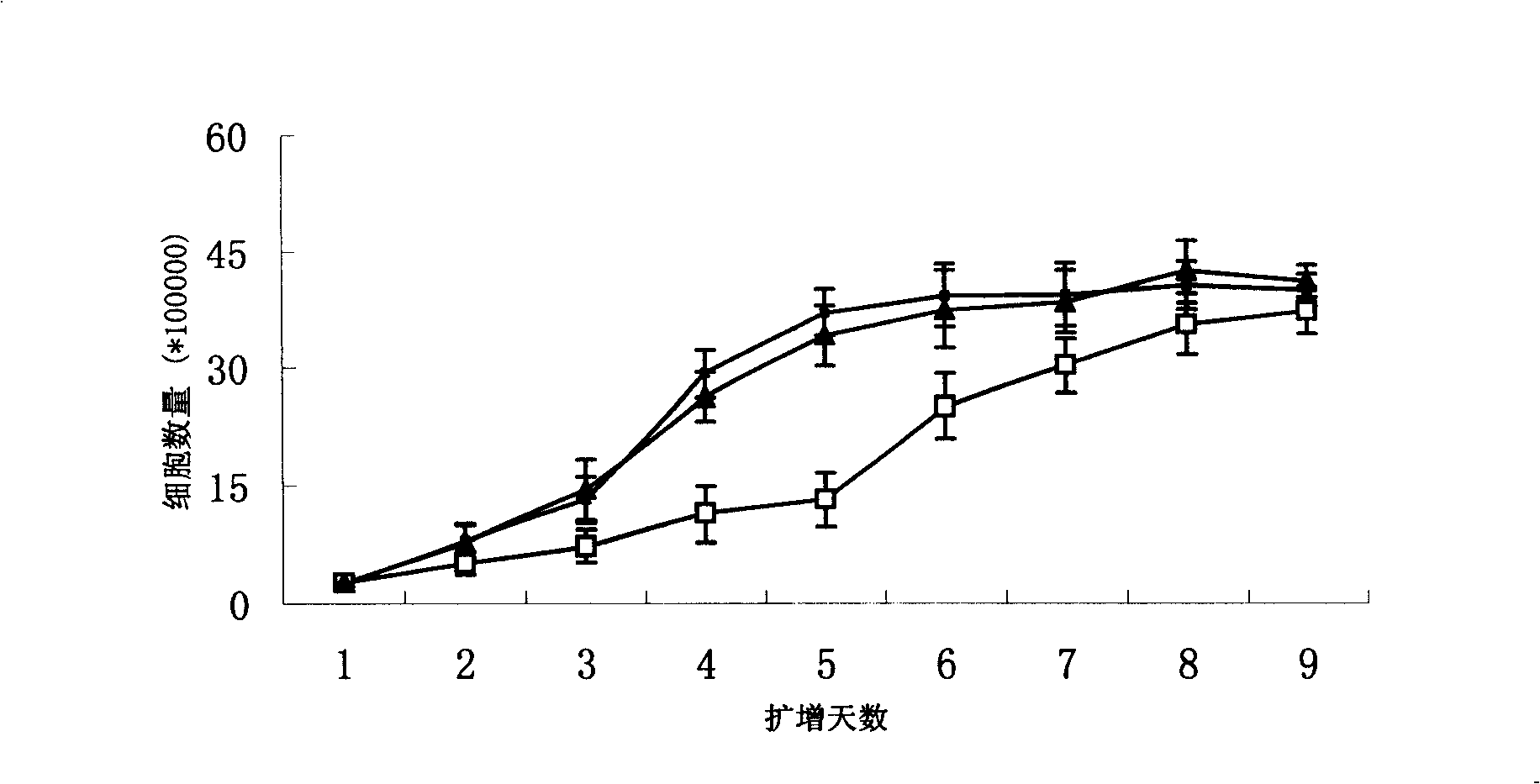
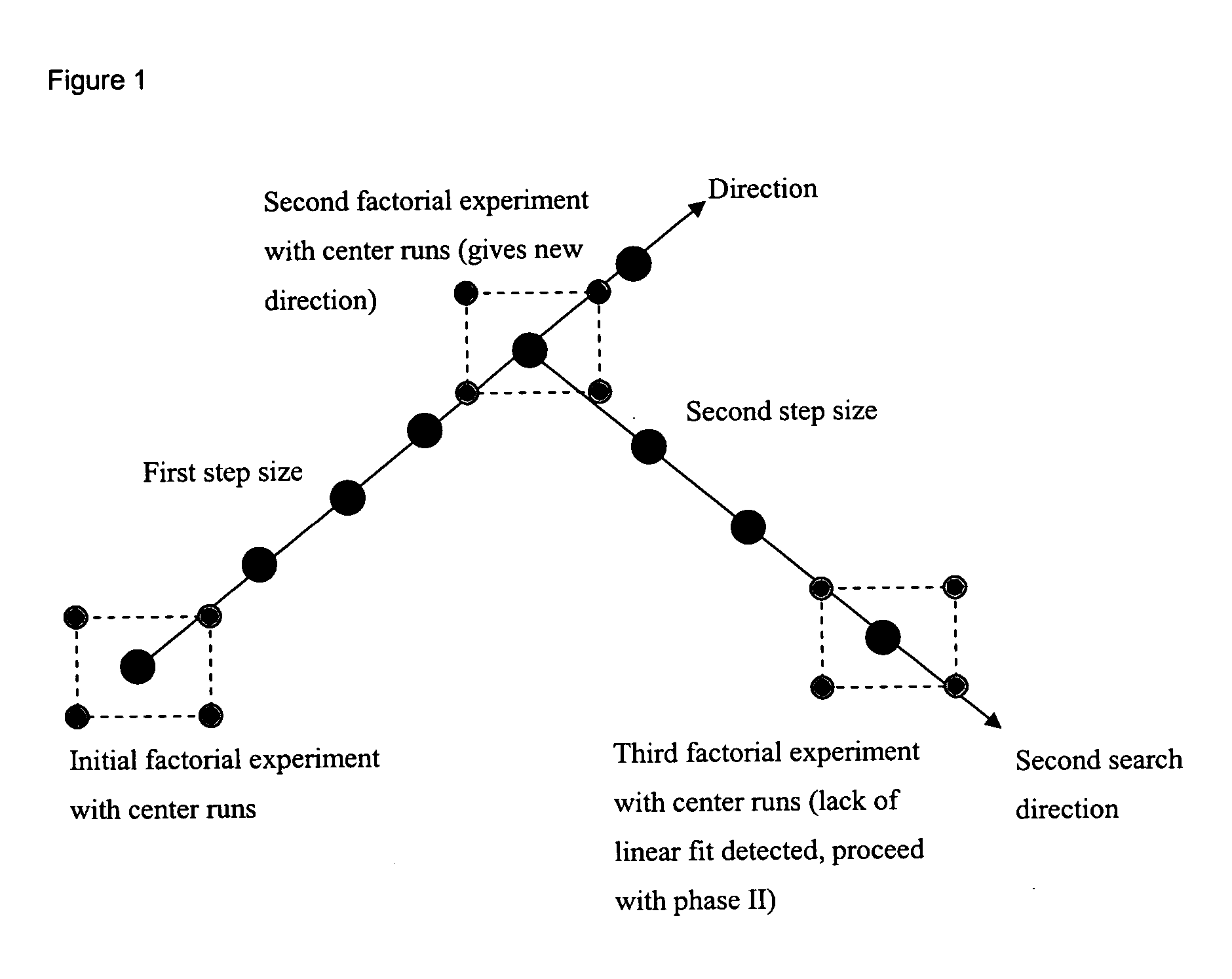
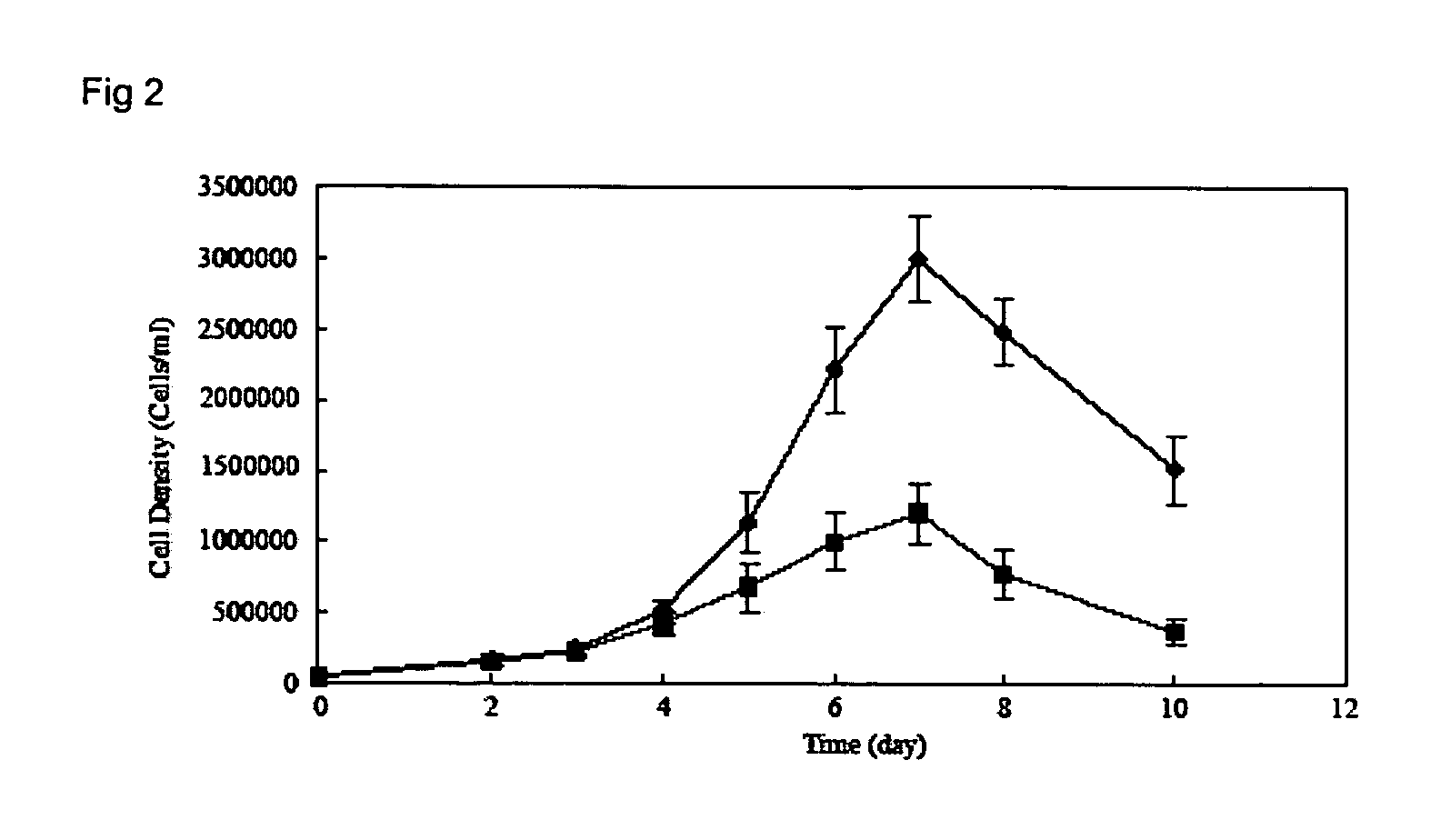
The present invention provides a method of determining the optimal composition of a serum-free, eukaryotic cell culture medium supplement, using 2-level factorial design and the deepest ascent method. The invention further provides a method of making a serum-free eukaryotic cell culture medium supplement and the generated thereof. The invention further provides a method of making a serum-free, eukaryotic cell culture medium and the medium generated thereof. The invention further provides a kit containing the medium of the invention. The invention also provides a method of expanding CD34<+> hematopoietic cells and a composition comprising CD34<+> hematopoietic cells in a serum-free, eukaryotic cell culture medium of the invention.
Owner:SINO CELL TECH
Reprogramming T cells and hematopoietic cells
ActiveUS8741648B2Increase capacityGenetically modified cellsArtificial cell constructsProgenitorHematopoietic cell
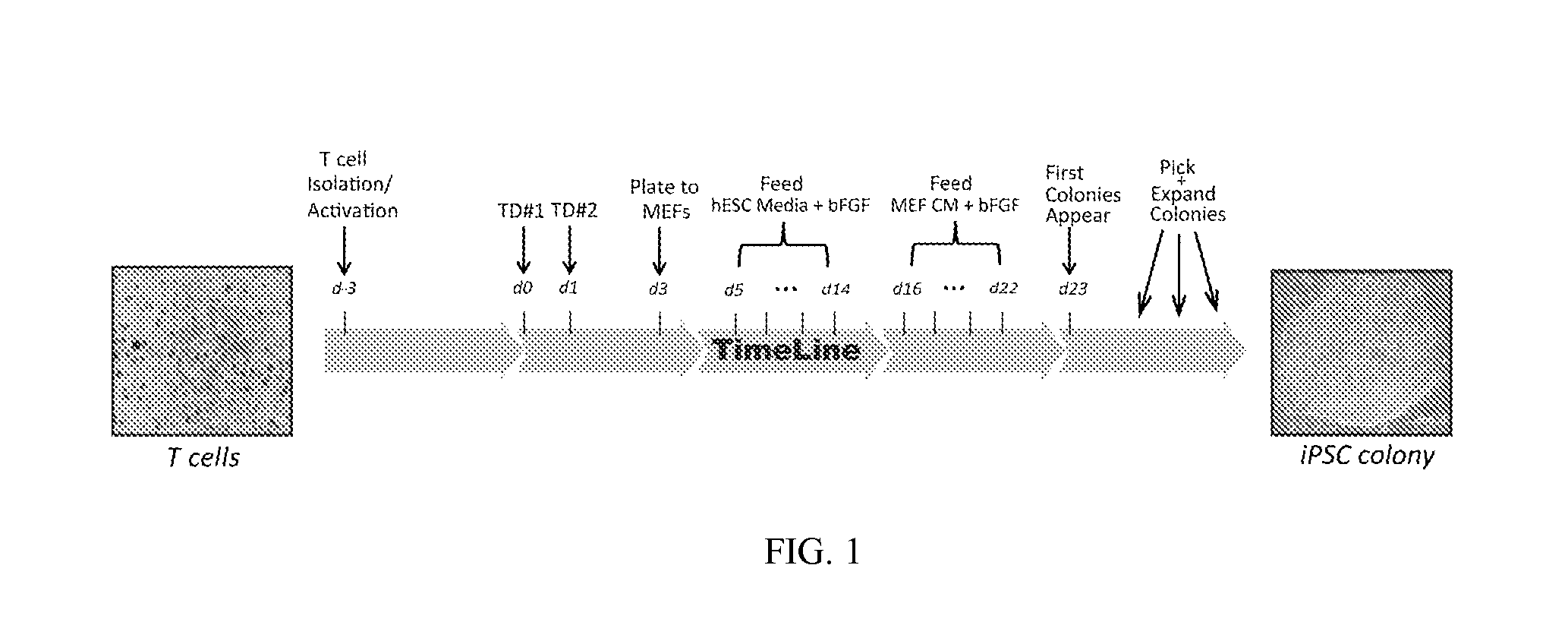
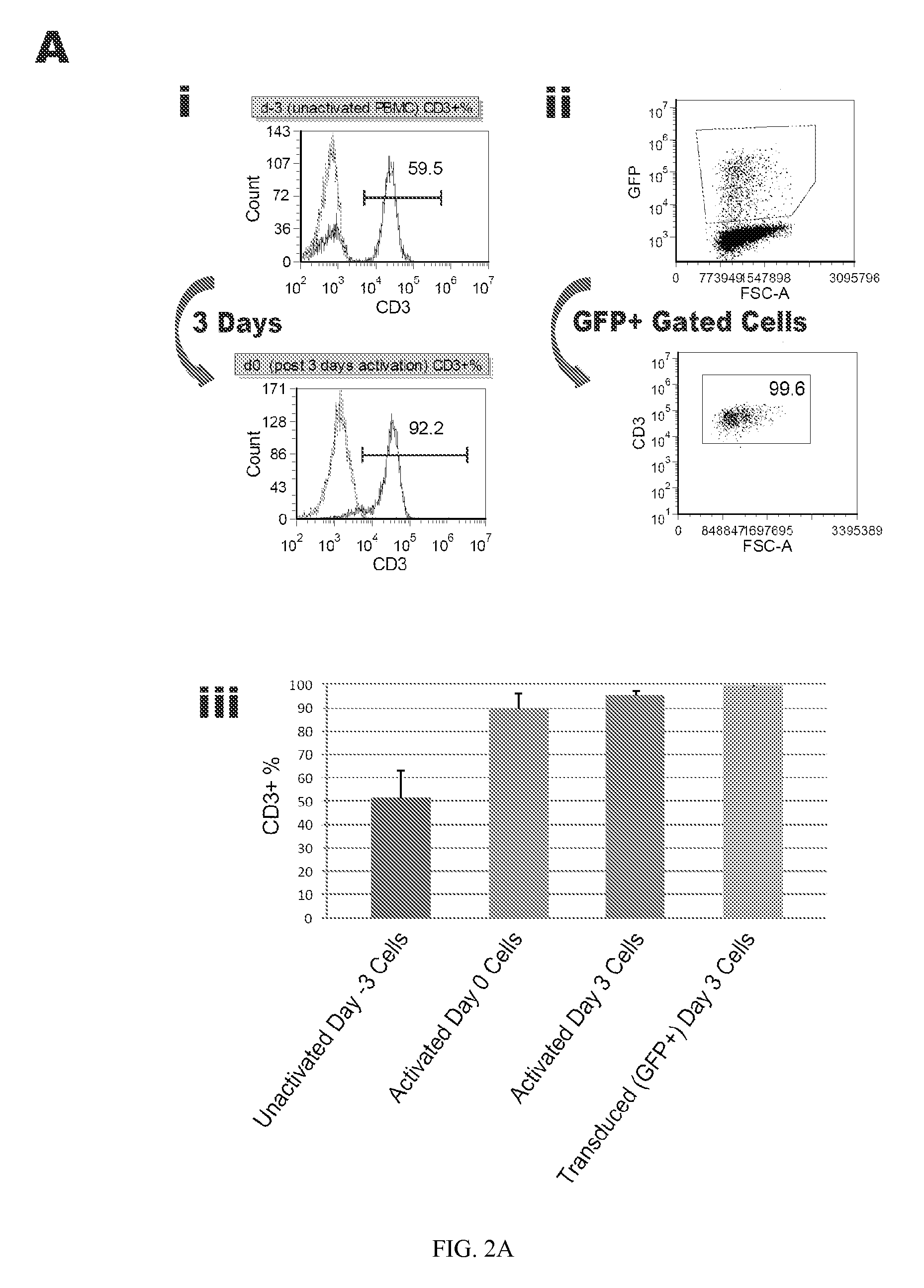
Methods and compositions relating to the production of induced pluripotent stem cells (iPS cells) are disclosed. For example, induced pluripotent stem cells may be generated from CD34+ hematopoietic cells, such as human CD34+ blood progenitor cells, or T cells. Various iPS cell lines are also provided. In certain embodiments, the invention provides novel induced pluripotent stem cells with a genome comprising genetic rearrangement of T cell receptors.
Owner:FUJIFILM CELLULAR DYNAMICS INC
Cellular compositions which facilitate engraftment of hematopoietic stem cells while minimizing the risk of gvhd
The present invention provides for cellular compositions which facilitate engraftment of hematopoietic stem cells from a syngeneic, allogeneic or xenogeneic donor. The cellular compositions of the invention facilitate engraftment while minimizing the risk of graft versus host disease in the graft recipient. According to a preferred embodiment of the invention, a cell composition is provided, which cell composition comprises hematopoietic stem cells, such as CD34+ cells and / or facilitating cells, in combination with αβ TCR+ T cells. The invention also relates to methods of using the cellular compositions of the invention to induce donor specific tolerance in a recipient, thus allowing the transplantation of donor organs, cells and tissues. Also disclosed are methods of treating leukemia and cancer as well as infectious diseases caused by viruses.
Owner:JEWISH HOSPITAL HEALTHCARE SERVICES
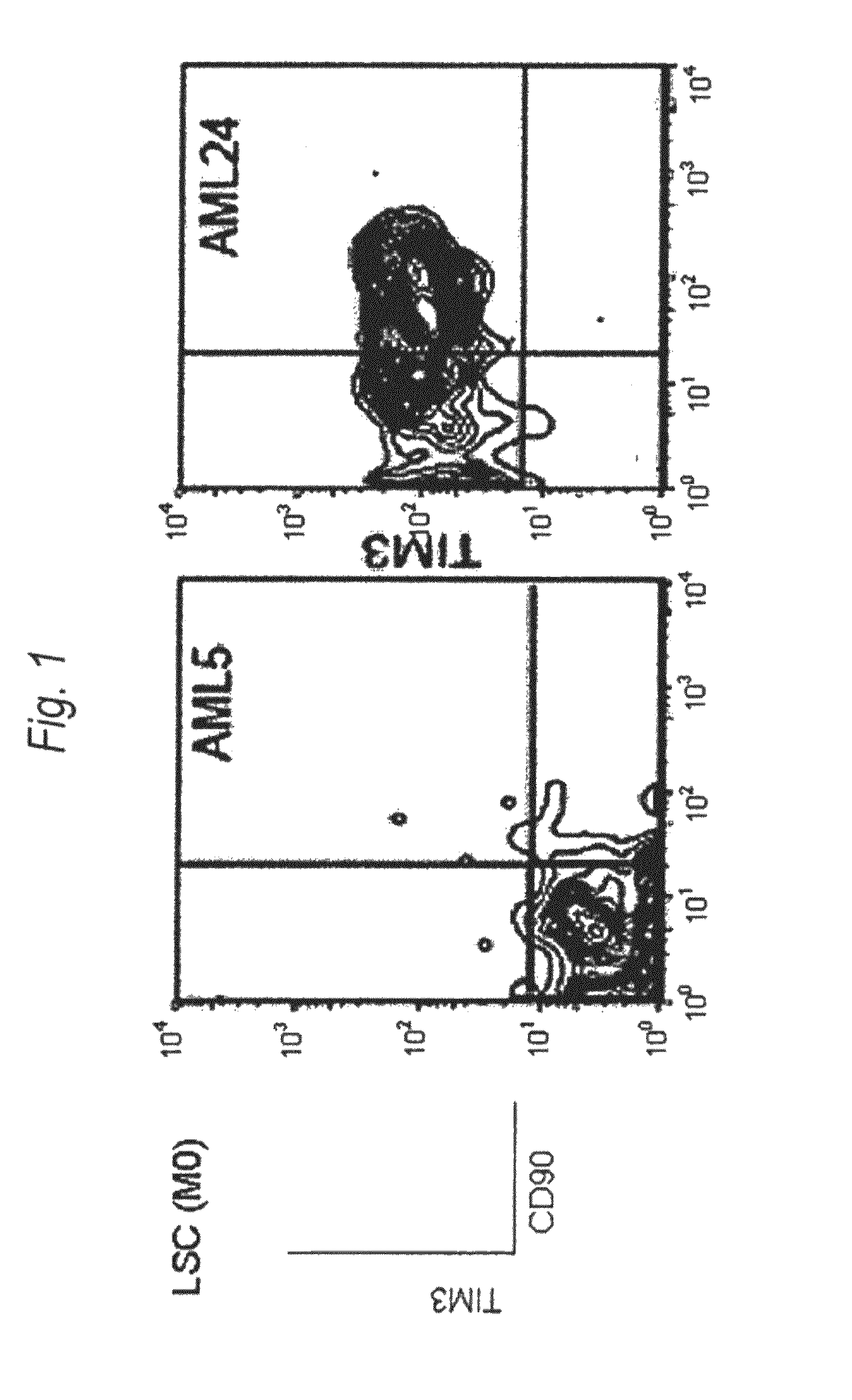
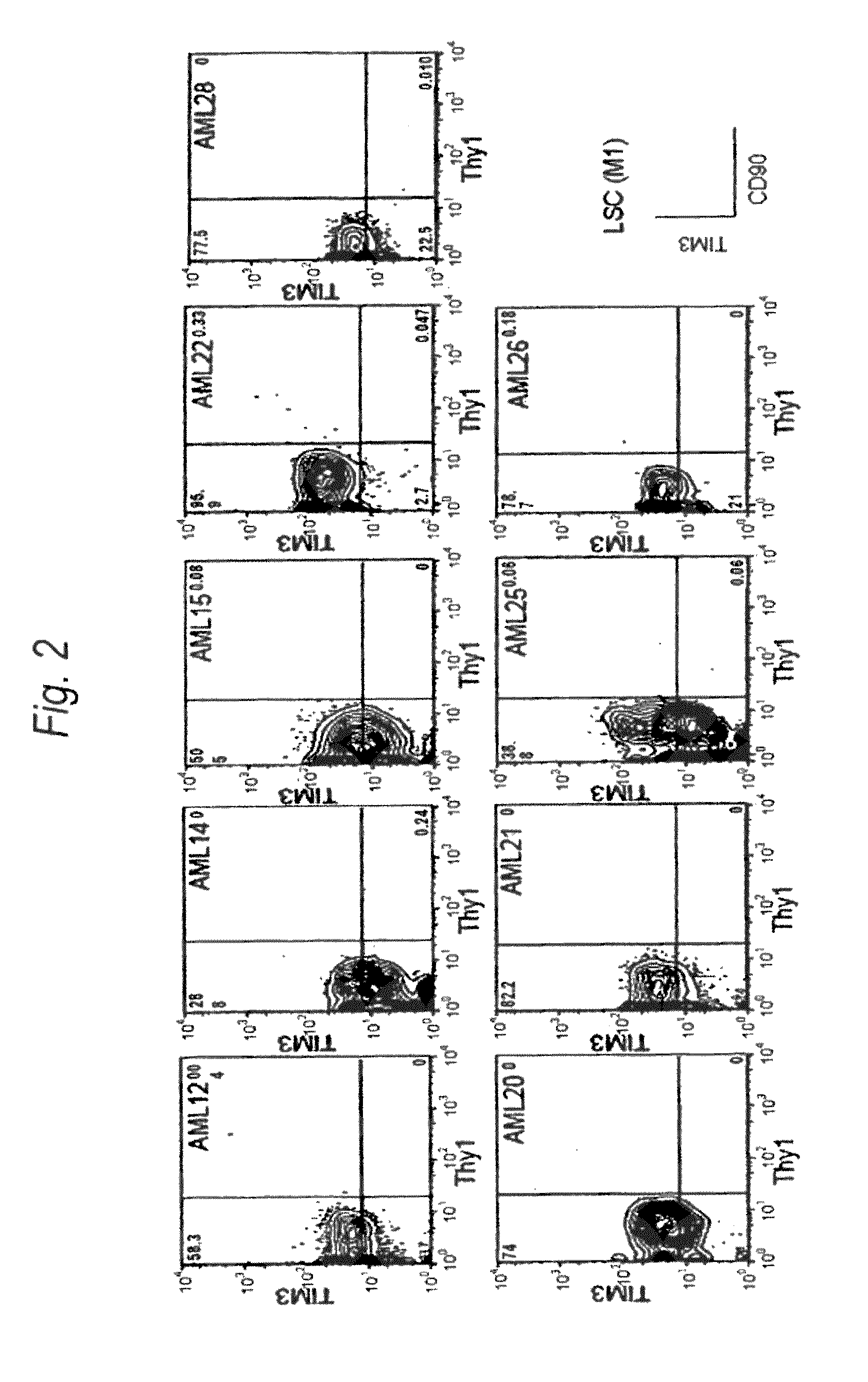
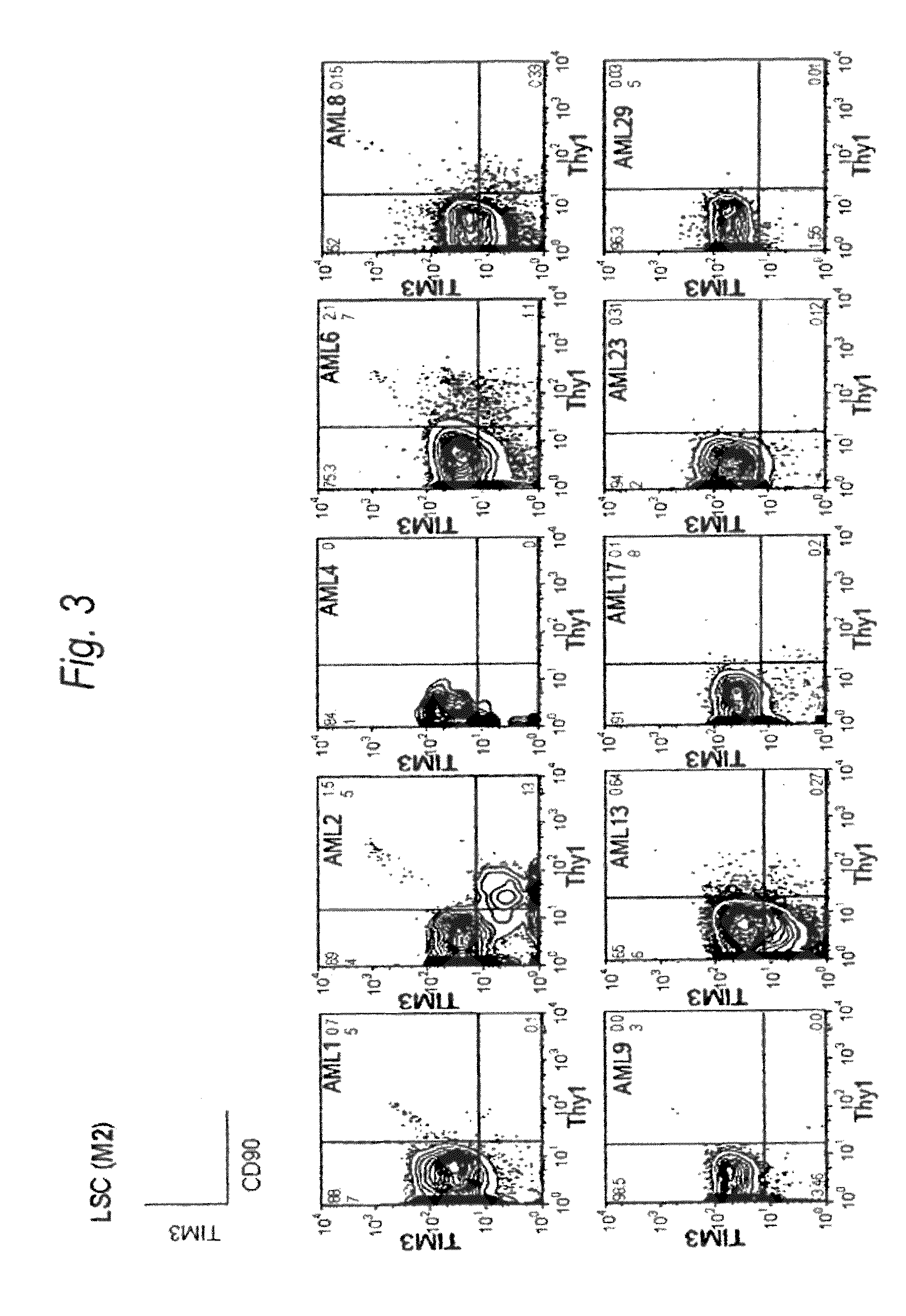
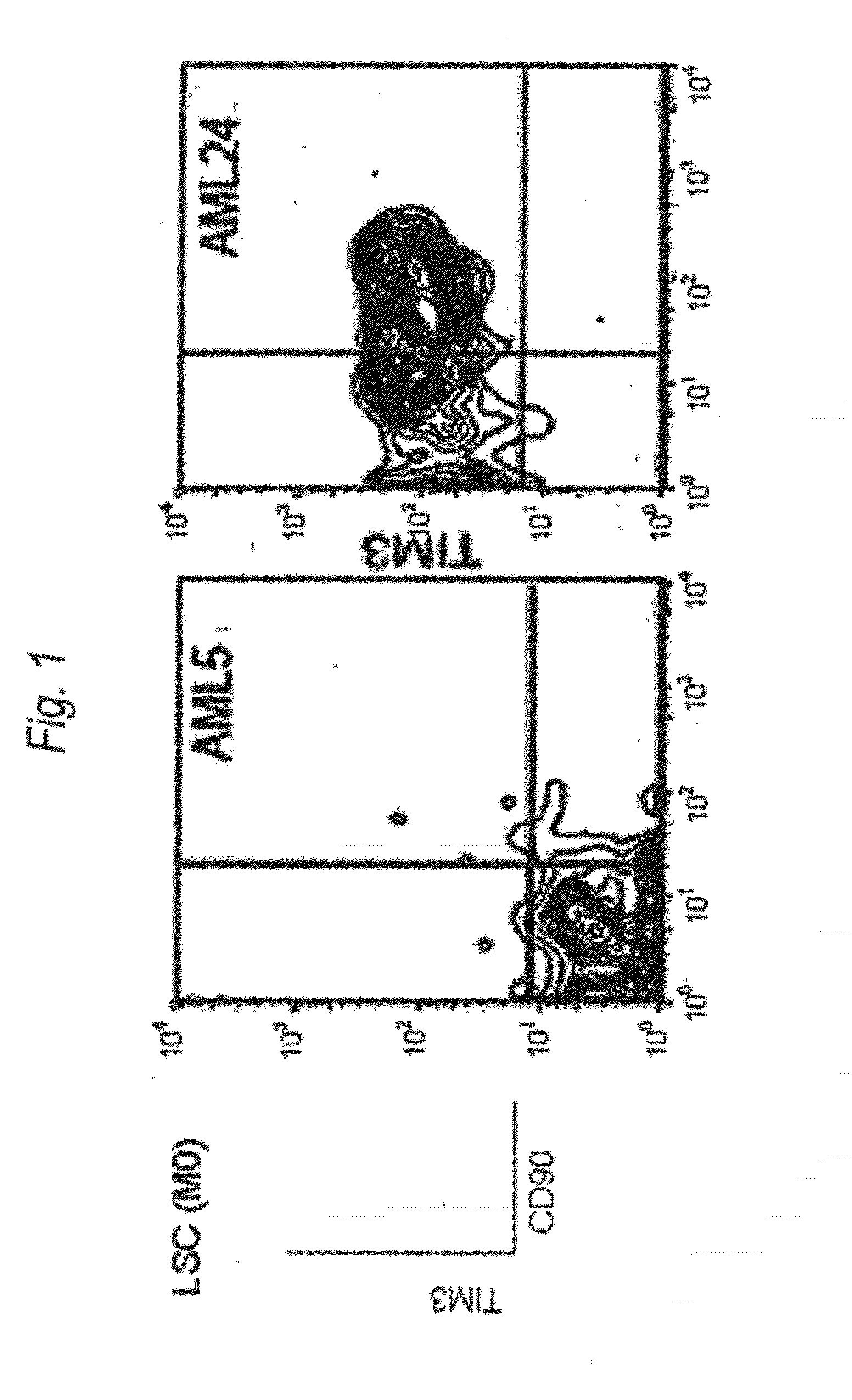
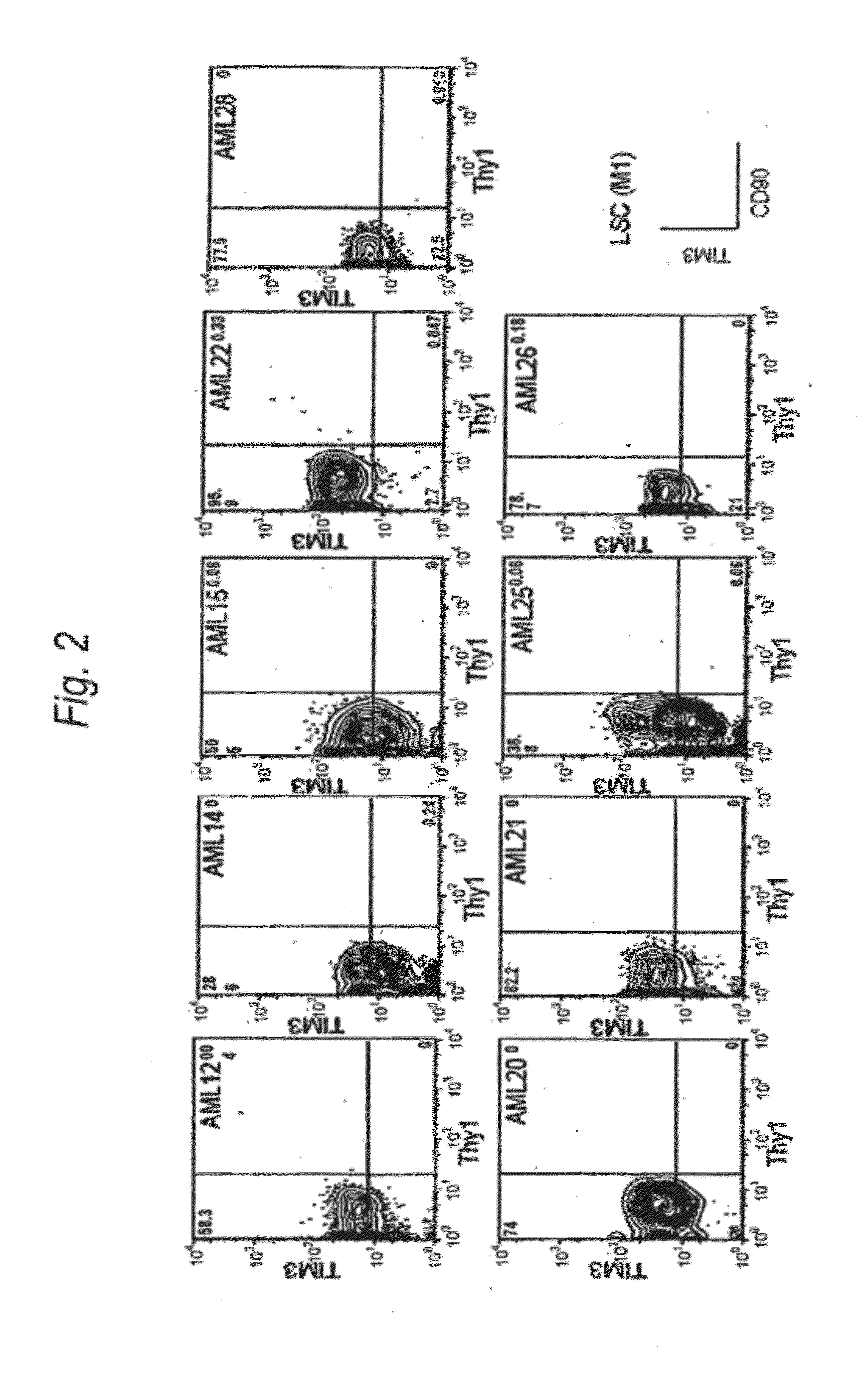
Method for treatment of blood tumor using anti-TIM-3 antibody
Disclosed is a therapeutic method comprising administering a TIM-3 antibody to a subject who is suspected to be suffering from blood tumor and in whom TIM-3 has been expressed in a Lin(−)CD34(+)CD38(−) cell fraction of bone marrow or peripheral blood or a subject who has been received any treatment for blood tumor. Also disclosed is a composition for preventing or treating blood tumor, which comprises a TIM-3 antibody as an active ingredient. Conceived diseases include those diseases which can be treated through the binding or targeting of the TIM-3 antibody to blood tumor cells (AML cells, CML cells, MDS cells, ALL cells, CLL cells, multiple myeloma cells, etc.), helper T cell (e.g., Th1 cells, Th17 cells), and antigen-presenting cells (e.g., dendritic cells, monocytes, macrophages, and cells resembling to the aforementioned cells (hepatic stellate cells, osteoclasts, microglial cells, intraepidermal macrophages, dust cells (alveolar macrophages), etc)), all of which are capable of expressing TIM-3. The diseases for which the therapeutic use is to be examined include blood diseases in which the expression of TIM-3 is observed in bone marrow or peripheral blood, particularly blood tumor.
Owner:KYOWA HAKKO KIRIN CO LTD +1
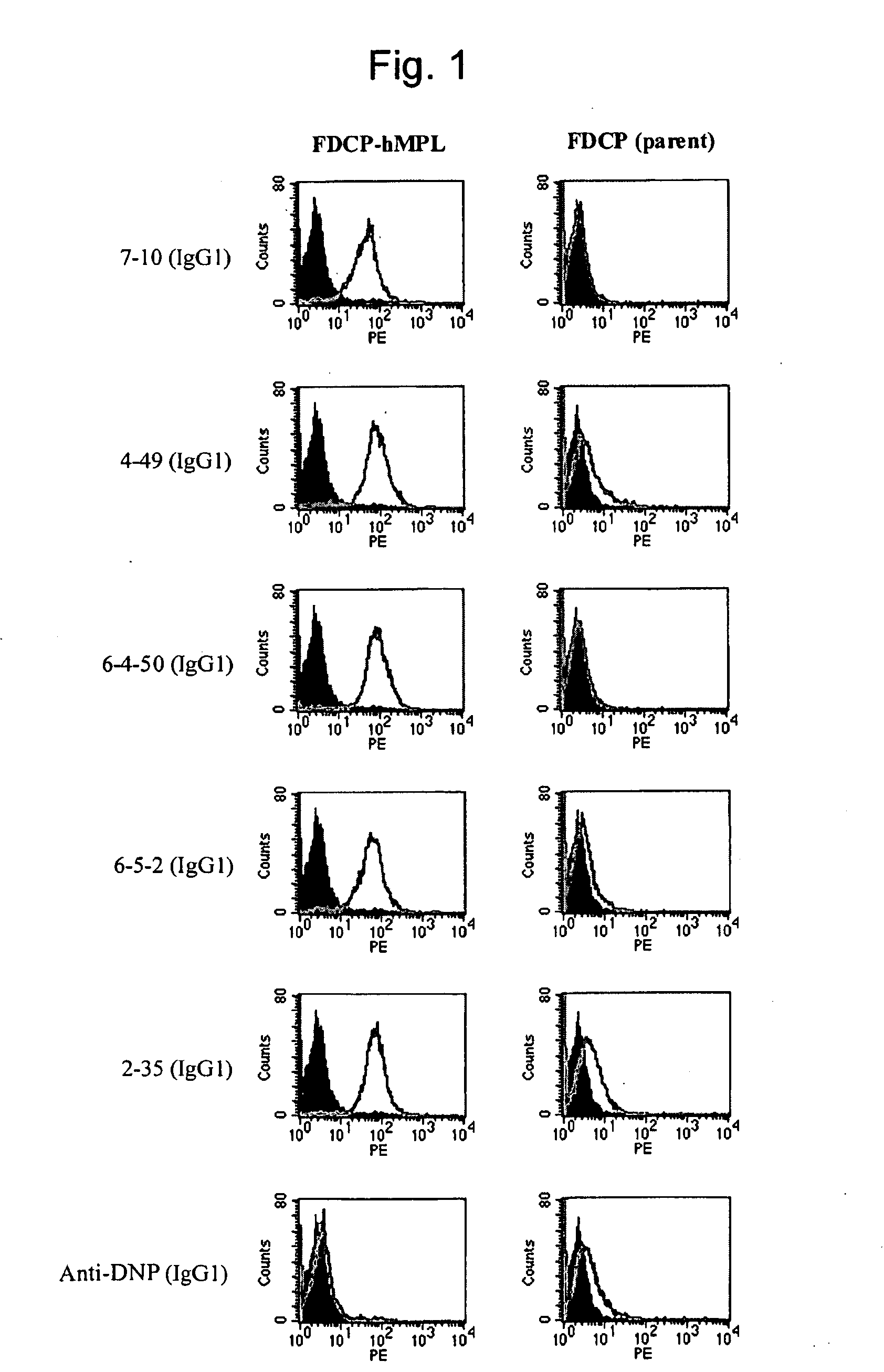
Agonist antibody to human thrombopoietin receptor
InactiveUS20100004429A1High activityLow antigenicityThrombopoietinHybrid immunoglobulinsHuman plateletUmbilical cord
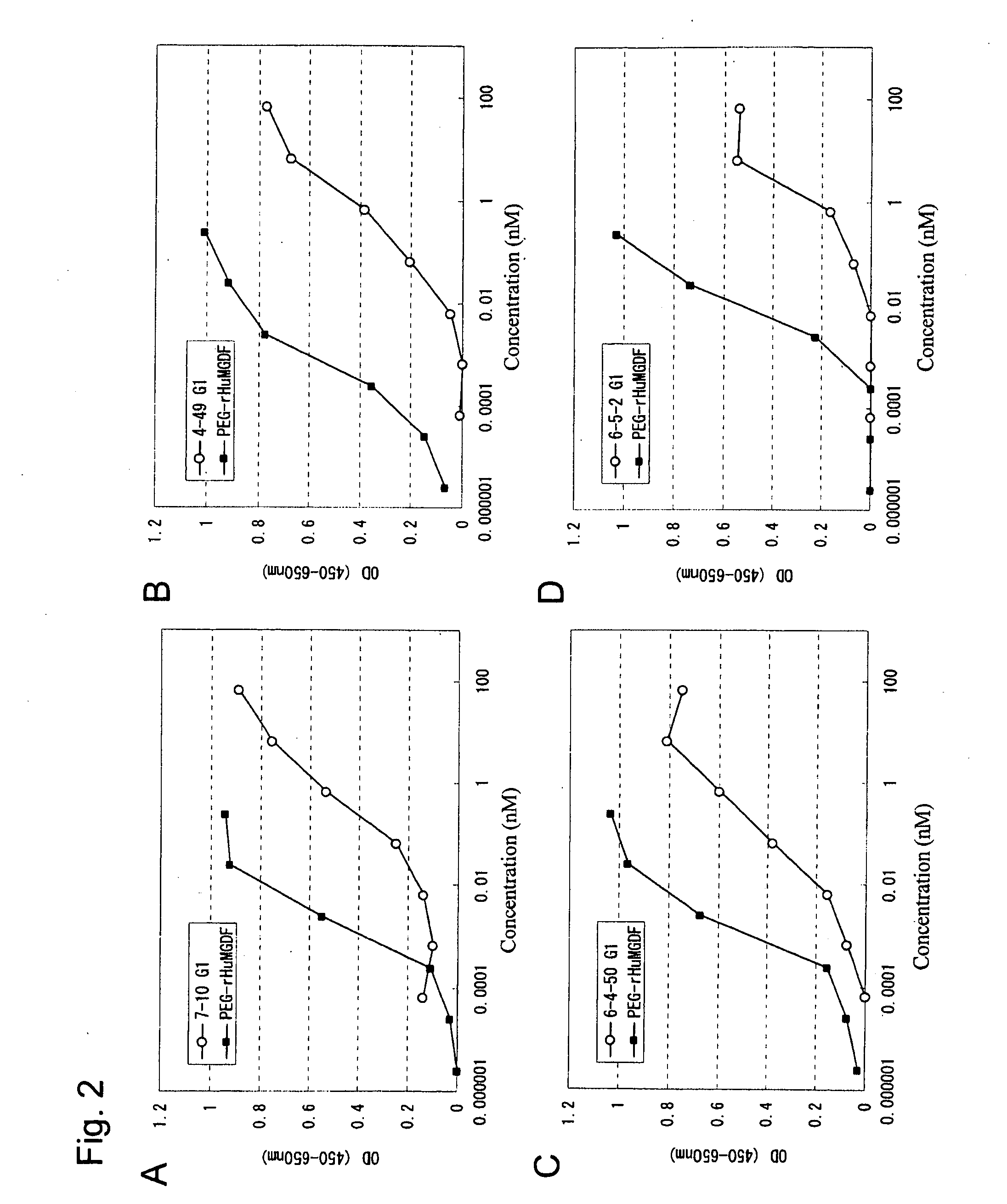
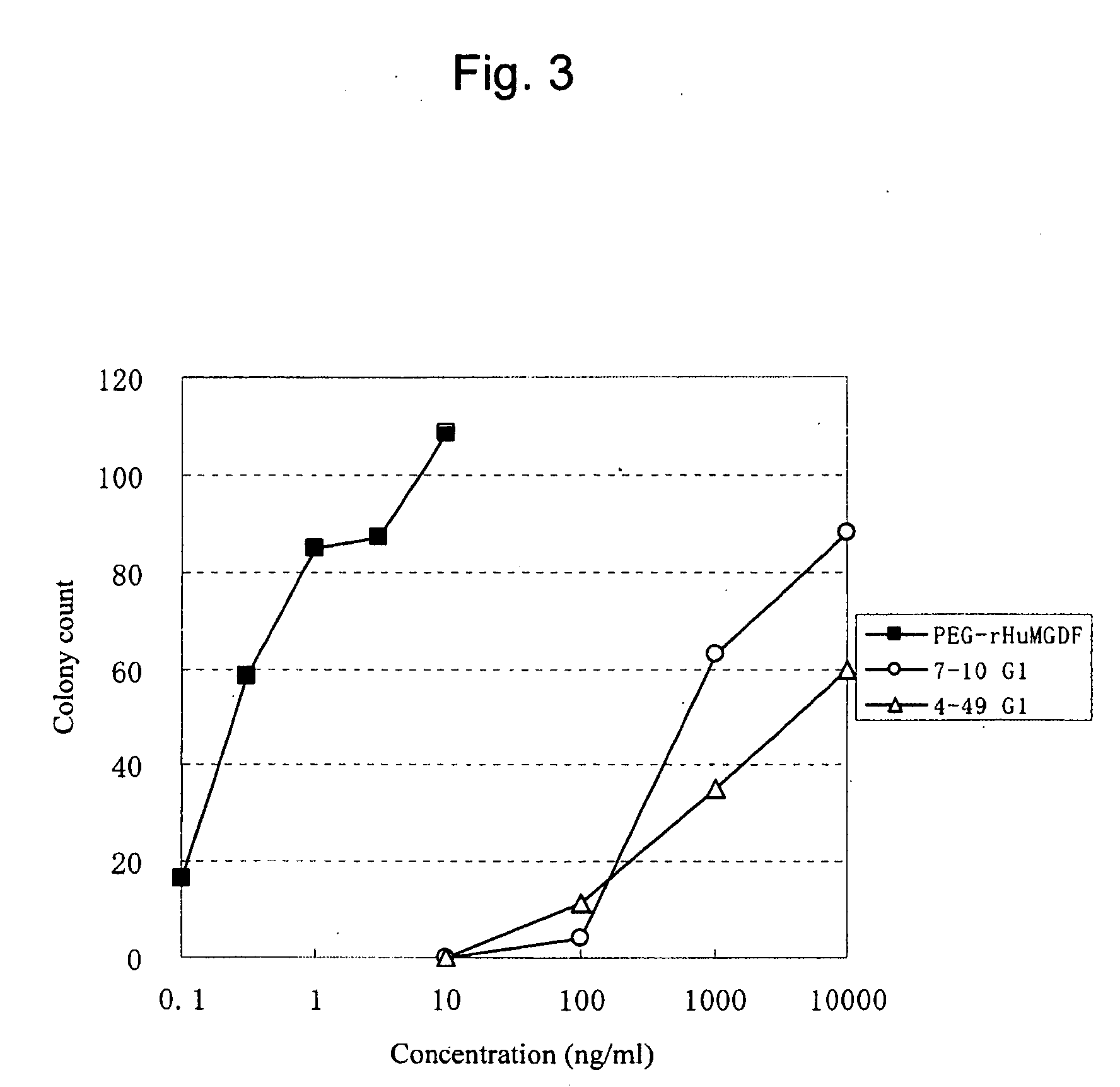
This invention provides an agonist antibody to a human thrombopoietin receptor (alias: human c-Mpl). More particularly, this invention provides an agonist antibody to a human thrombopoietin receptor, wherein the agonist antibody comprises: antibody constant regions comprising (1) amino acid sequences in a heavy chain constant region and a light chain constant region of a human antibody, (2) an amino acid sequence of a heavy chain constant region with a domain substituted between human antibody subclasses, and an amino acid sequence of a light chain constant region of a human antibody, or (3) amino acid sequences comprising a deletion(s), substitution(s), addition(s), or insertion(s) of one or several amino acid residues in the amino acid sequences of (1) or (2) above; and antibody variable regions capable of binding to and activating a human thrombopoietin receptor; and wherein the agonist antibody has the properties: (a) that the antibody induces colony formation at a concentration of 10,000 ng / ml or lower as determined by the CFU-MK colony formation assay using human umbilical-cord-blood-derived CD34+ cells; and (b) that the antibody has a maximal activity at least 50% higher than that of PEG-rHuMGDF and an 50% effective concentration (EC50) of 100 nM or less in the cell proliferation assay using UT7 / TPO cell. Also provided is a pharmaceutical composition for treating thrombocytopenia comprising said antibody.
Owner:KYOWA HAKKO KIRIN CO LTD
Stem cell populations and methods of use
InactiveUS20050272152A1Improve scalabilityImprove the level ofCulture processSkeletal disorderCulture mediumsCD34
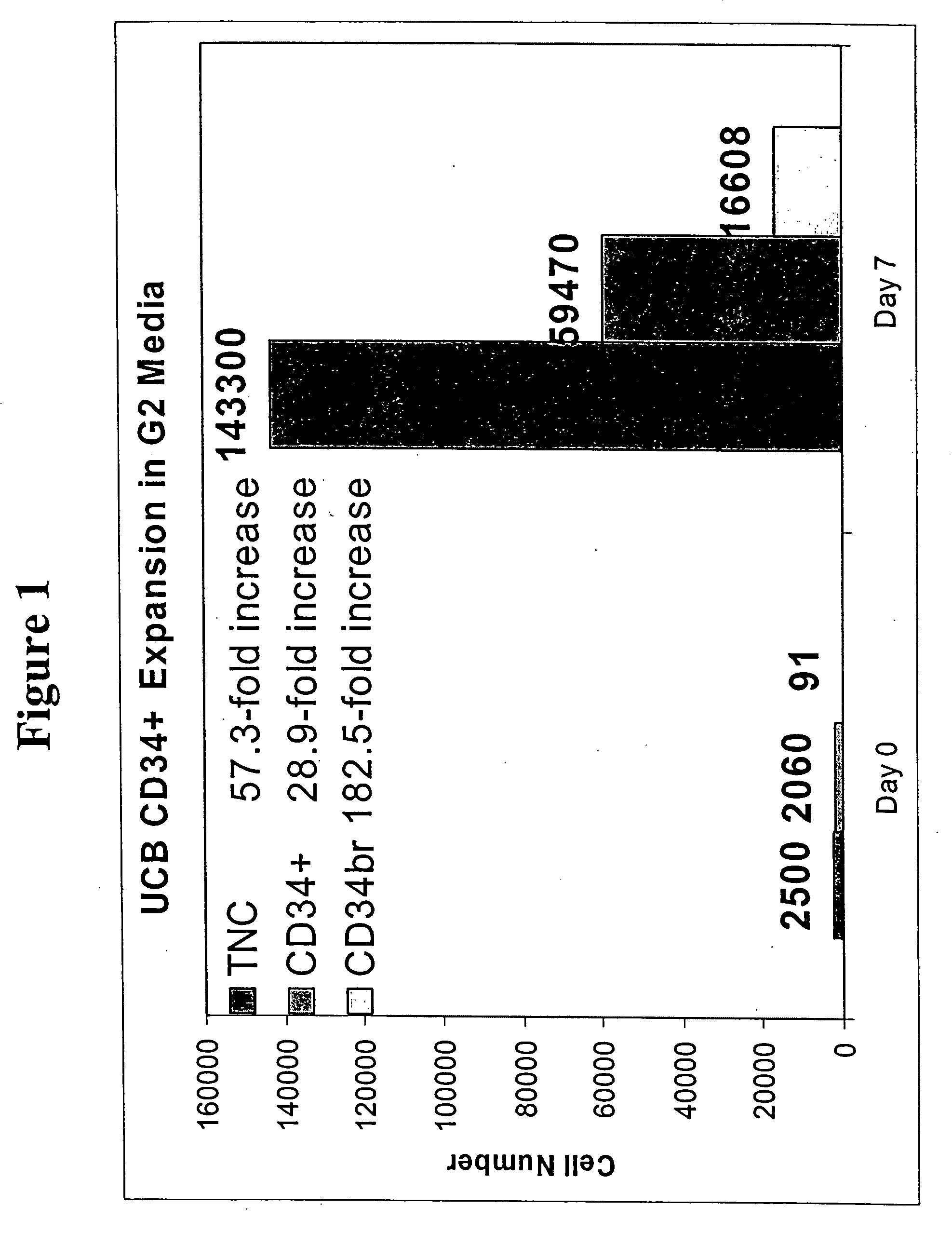
The invention provides populations of expanded CD34-expressing cells and methods of use. Particular embodiments provide for defined culture media useful for growing these cells, and grafts comprising these cells. The invention finds use in methods for reconstituting, repairing, and regenerating tissue damage.
Owner:BECTON DICKINSON & CO
Hemangioblast progenitor cells
InactiveUS20040052771A1Reduce bleedingAvoid adjustmentBiocideArtificial cell constructsP-selectinProgenitor
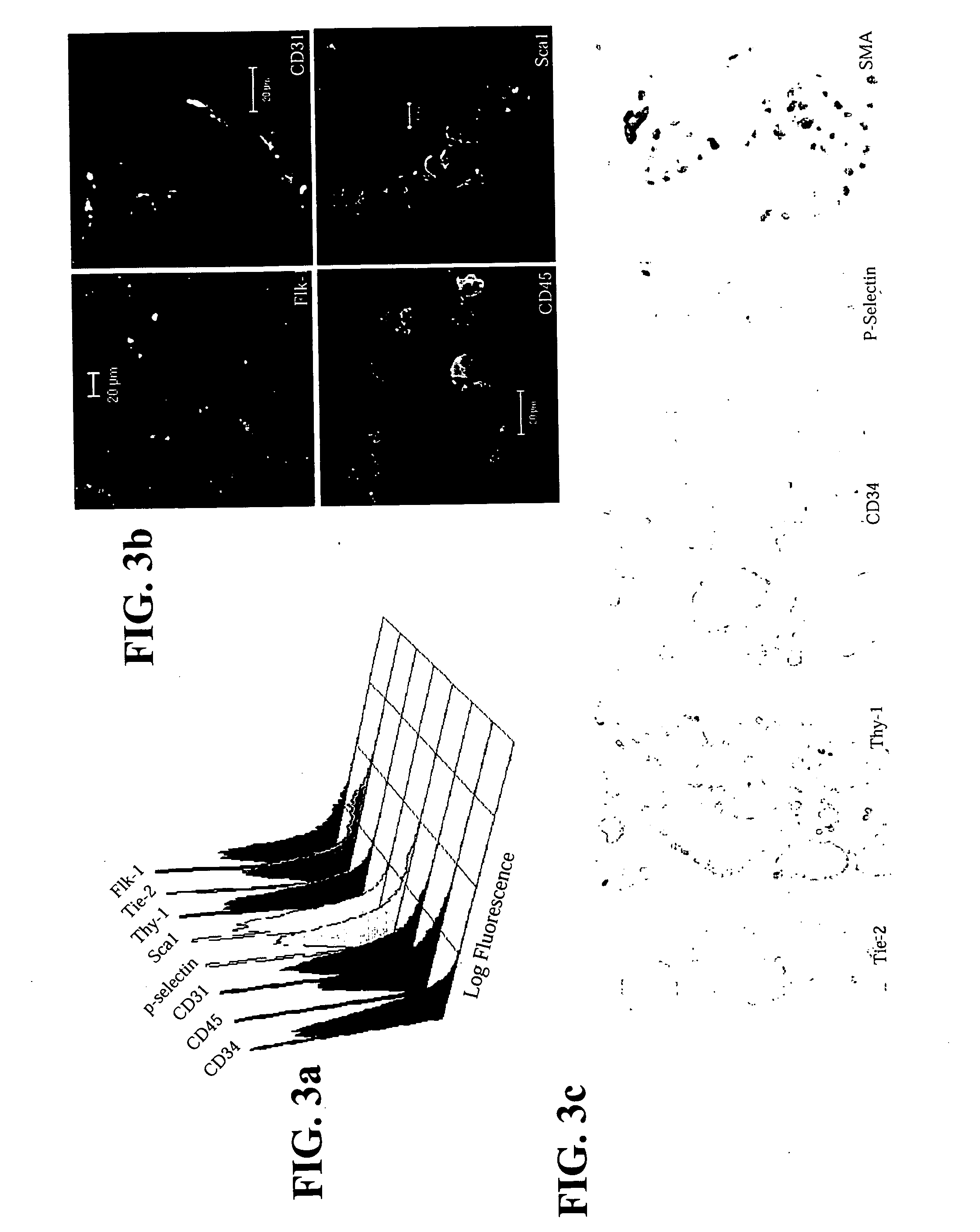
The invention relates to isolated hemangioblast cells. Hematopoietic and endothelial cells are postulated to be derived from a common progenitor, hemangioblast. While hemangioblast has been isolated retrospectively during embryonic stem cell differentiation, it has not been isolated from embryos or from bone marrow. Prospectively stable clonal cell lines have been isolated from mammalian embryos, from embryonic stem cells and from mammalian bone marrow that can differentiate in vitro into tubular structures with both endothelial and hematopoietic markers such as CD34, CD31, Flk-1, TIE2, P-selectin, Sca-1, thy-1, CD45, and smooth muscle actin. Gene expression profiles in the undifferentiated and differentiated cells were consistent with endothelial and hematopoietic differentiation potential. Transplantation studies in isogenic or immunodeficient mice demonstrated that these cells were not tumorigenic. In an appropriate microenvironment, the cells incorporate into the vasculature and participate in hematopoiesis.
Owner:NAT UNIV OF SINGAPORE +1
Growth of neural precursor cells using umbilical cord blood serum and a process for the preparation thereof for therapeutic purposes
InactiveUS20040203142A1Promote growthArtificial cell constructsBlood/immune system cellsCord blood stem cellCell culture media
This invention is concerned with stem cells derived from umbilical cord blood serum and a method for growing human embryonic stem cells and adult cells comprising sera separated from clotted umbilical cord blood, including growing and differentiating cord blood stem cells into neural precursors, comprising transdifferentiating CD34+ stem cells from mononuclear cells derived from umbilical cord blood to neural precursors. The stem cells obtained from the umbilical cord include pluripotent stem and progenitor cell population of mononuclear cells, and separating pluripotent stem and progenitor cell population of mononuclear cells obtained from the umbilical cord blood. A magnetic cell separator is used to separate out cells which contain a CD marker and then expanding the cells in a growth medium containing retinoic acid and one or more growth factors BDNF, GDNF, NGF and FGF as a differentiating agent. The invention is also concerned with the transplantation and repair of nerve damage, stokes, spinal injury, Parkinson's and Alzheimer's, prepared in accordance with the aforesaid method and a media for culturing umbilical cord blood stem calls consisting essentially of cord blood stem cells derived from umbilical cord blood serum.
Owner:RELIANCE LIFE SCI PYT
Multipotent lymphohematopoietic progenitor cells
Owner:WISCONSIN ALUMNI RES FOUND
Generation of dendritic cells from cd34+precursors
The present invention relates to a method for inducing development of dendritic cells from CD34+ precursor cells. More particularly, the present invention relates to a method of differentiating CD34+ precursor cells into myeloid- and or lymphoid-dendritic cells. The present invention provides a protocol for the development of dendritic cells from a biological sample inter alia cord blood, bone marrow or peripheral blood CD34+ stem cells. The dendritic cells of the present invention are useful as therapeutic cellular agents such as in the development of vaccines and in modulating immunological responsiveness. More particularly, the present invention provides compositions which can be used for inducing a protective immune response in a subject against inter alia pathogenic infections, autoimmune diseases and cancer.
Owner:THE OF THE TRUSTEES OF THE SISTERS OF MERCY & QUEENSLAND
Chimeric mouse having an immune system constructed with human CD34+ cells and use thereof
InactiveUS20070067854A1Maintaining immunityReduced activityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenScid mice
Owner:HABU SONOKO
Production from blood of cells of neural lineage
InactiveUS20080318314A1Easy diagnosisNervous system cellsBlood/immune system cellsIn vitro stimulationPrecursor cell
A method including in vitro stimulating a core cell population (CCP) of at least 5 million cells that have a density of less than 1.072 g / ml, and at least 1.5% of which are CD34+CD45− / dim, to differentiate into a neural progenitor / precursor cell population (NPCP). Other embodiments are also described.
Owner:KWALATA TRADING
Hematopoietic stem cell gene therapy
InactiveUS20050020524A1Avoid infectionHigh activityBiocideUnknown materialsLhrh antagonistCell resistance
The present disclosure provides methods for gene therapy utilizing hematopoietic stem cells, lymphoid progenitor cells, and / or myeloid progenitor cells. The cells are genetically modified to provide a gene that is expressed in these cells and their progeny after differentiation. In one embodiment the cells contain a gene or gene fragment that confers to the cells resistance to HIV infection and / or replication. The cells are administered to a patient in conjunction with treatment to reactivate the patient's thymus. The cells may be autologous, syngeneic, allogeneic or xenogeneic, as tolerance to foreign cells is created in the patient during reactivation of the thymus. In one embodiment the hematopoietic stem cells are CD34+. The patient's thymus is reactivated by disruption of sex steroid mediated signaling to the thymus. In another embodiment, this disruption is created by administration of LHRH agonists, LHRH antagonists, anti-LHRH receptor antibodies, anti-LHRH vaccines or combinations thereof.
Owner:NORWOOD IMMUNOLOGY
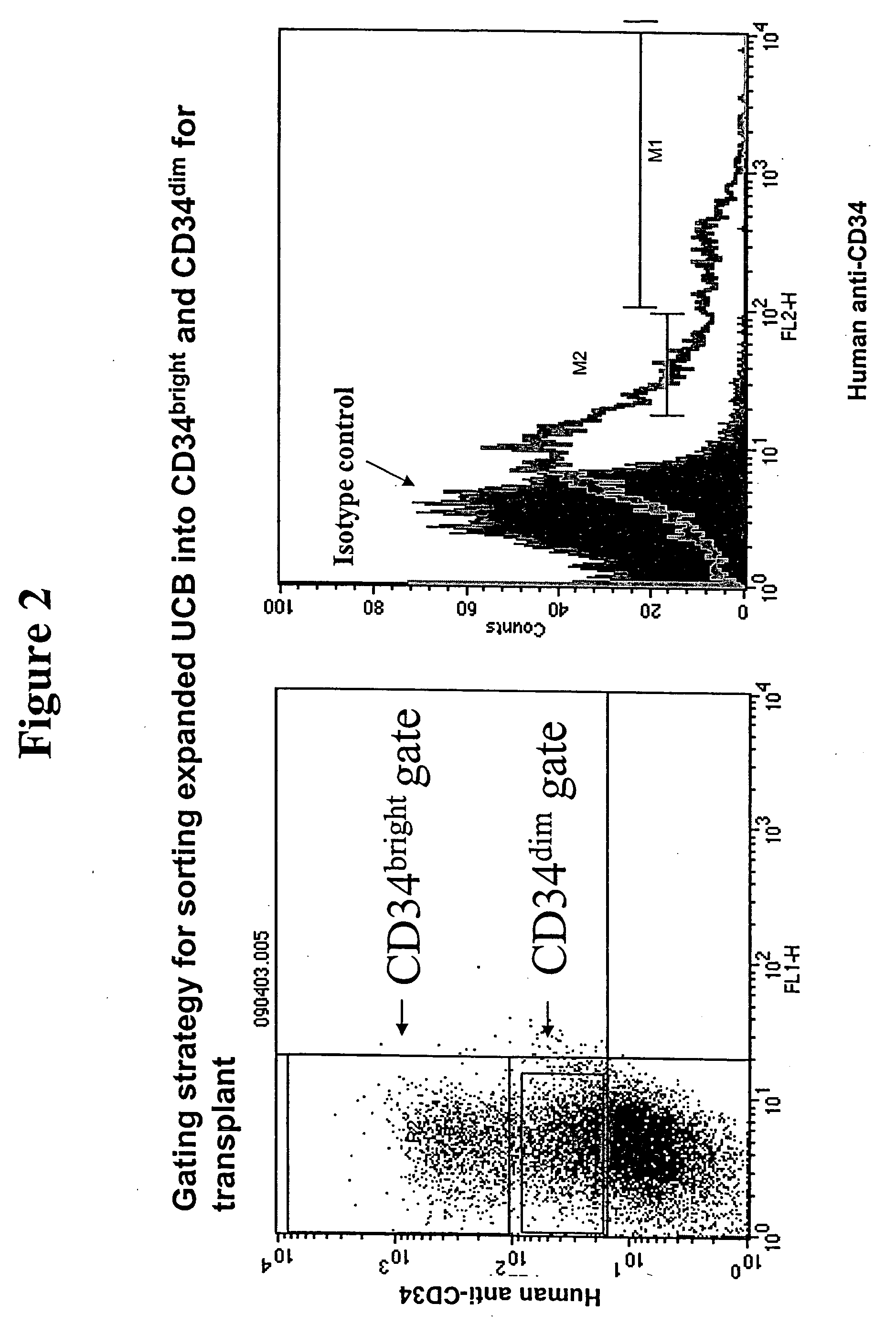
Stem and progenitor cell compositions recovered from bone marrow or cord blood; system and method for preparation thereof
InactiveUS20070269887A1Not labor intensiveEasy to implementOther blood circulation devicesBiomass after-treatmentProgenitorMicrocontroller
The invention includes compositions of stem and progenitor cells recovered from bone marrow or cord blood containing most of the viable CD34+ cells and substantially depleted of red blood cells resident in the original sample, without any xenobiotic additives to aid cell separation. The invention also includes a system and method for preparing the compositions. The system includes a bag set and a processing device, which utilizes an optical sensor, microcontroller, servo motor, accelerometer, load cell, and battery. The system and method utilize centrifugation to stratify the cells into layers and then separate and transfer the stem cells into a stem cell bag. The processing device's microcontroller receives input from the device's accelerometer, load cell and optical sensor to direct the metering valve in the bag set to open and close to permit the transfer of as many stems cells as possible with as few red cells as possible.
Owner:THERMOGENESIS
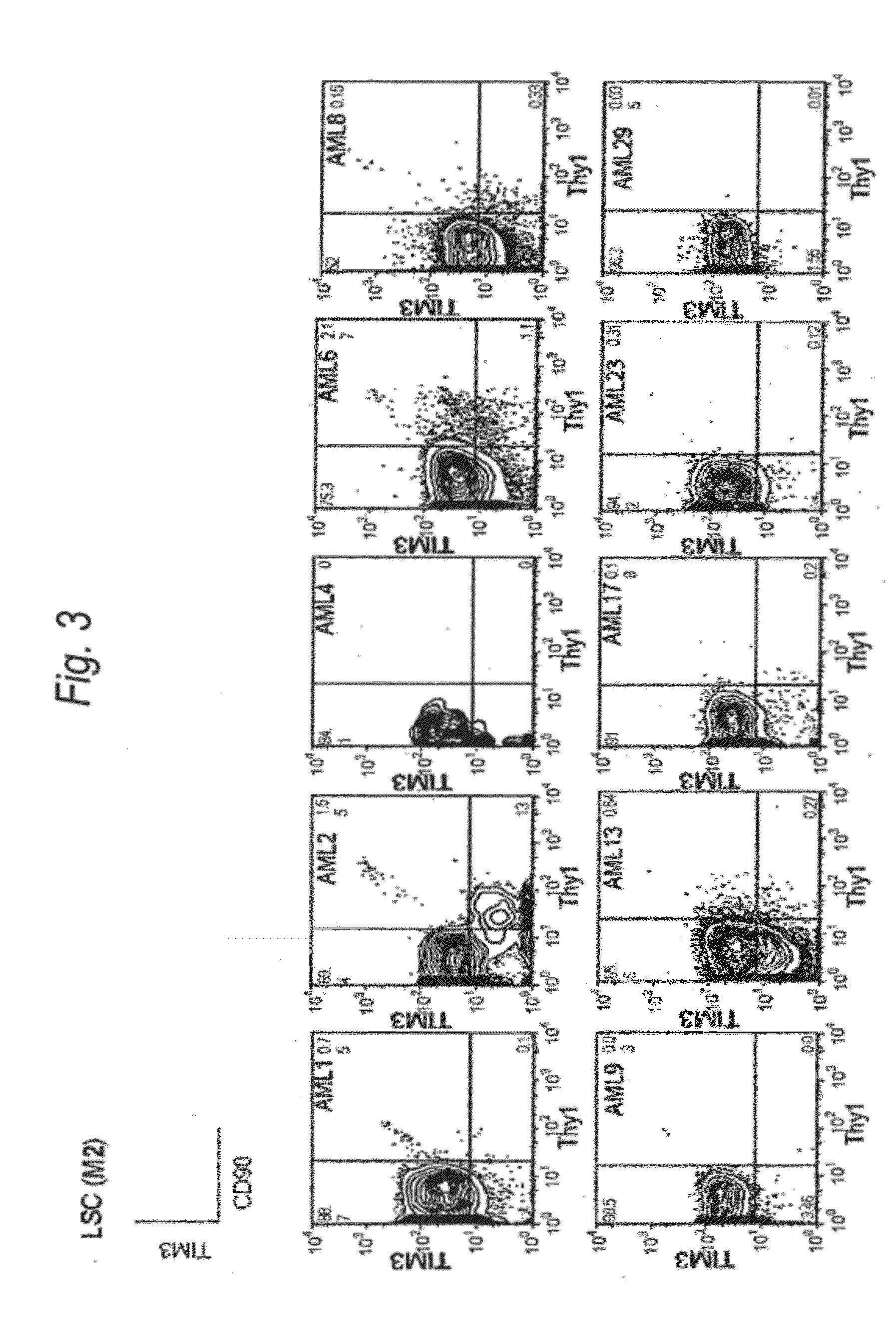
Method for treatment of blood tumor using Anti-tim-3 antibody
ActiveUS20120100131A1Use to establishBiological material analysisAntibody ingredientsDiseaseCell Fraction
Disclosed is a therapeutic method comprising administering a TIM-3 antibody to a subject who is suspected to be suffering from blood tumor and in whom TIM-3 has been expressed in a Lin(−)CD34(+)CD38(−) cell fraction of bone marrow or peripheral blood or a subject who has been received any treatment for blood tumor. Also disclosed is a composition for preventing or treating blood tumor, which comprises a TIM-3 antibody as an active ingredient. Conceived diseases include those diseases which can be treated through the binding or targeting of the TIM-3 antibody to blood tumor cells (AML cells, CML cells, MDS cells, ALL cells, CLL cells, multiple myeloma cells, etc.), helper T cell (e.g., Th1 cells, Th17 cells), and antigen-presenting cells (e.g., dendritic cells, monocytes, macrophages, and cells resembling to the aforementioned cells (hepatic stellate cells, osteoclasts, microglial cells, intraepidermal macrophages, dust cells (alveolar macrophages), etc)), all of which are capable of expressing TIM-3. The diseases for which the therapeutic use is to be examined include blood diseases in which the expression of TIM-3 is observed in bone marrow or peripheral blood, particularly blood tumor.
Owner:KYOWA HAKKO KIRIN CO LTD +1
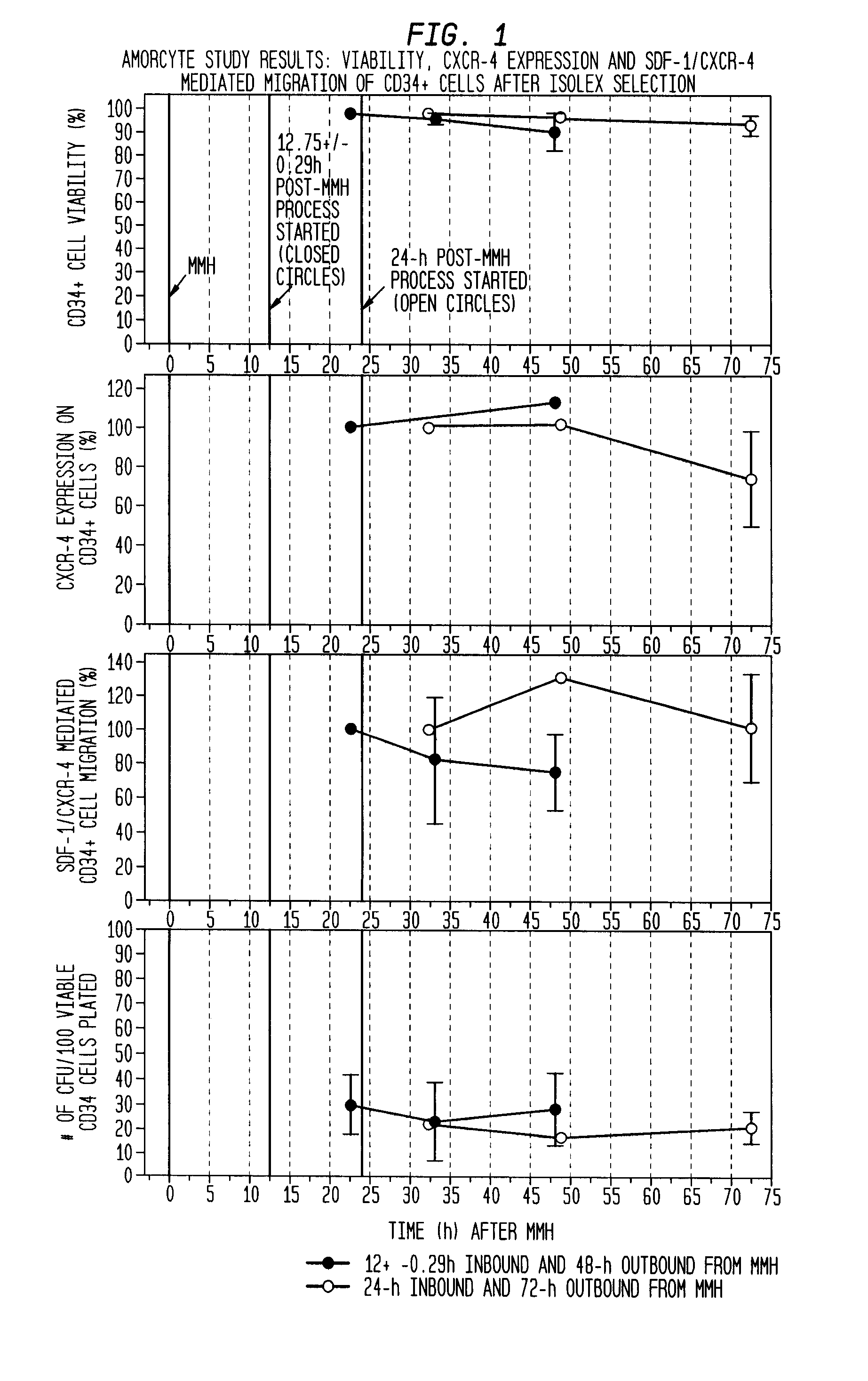
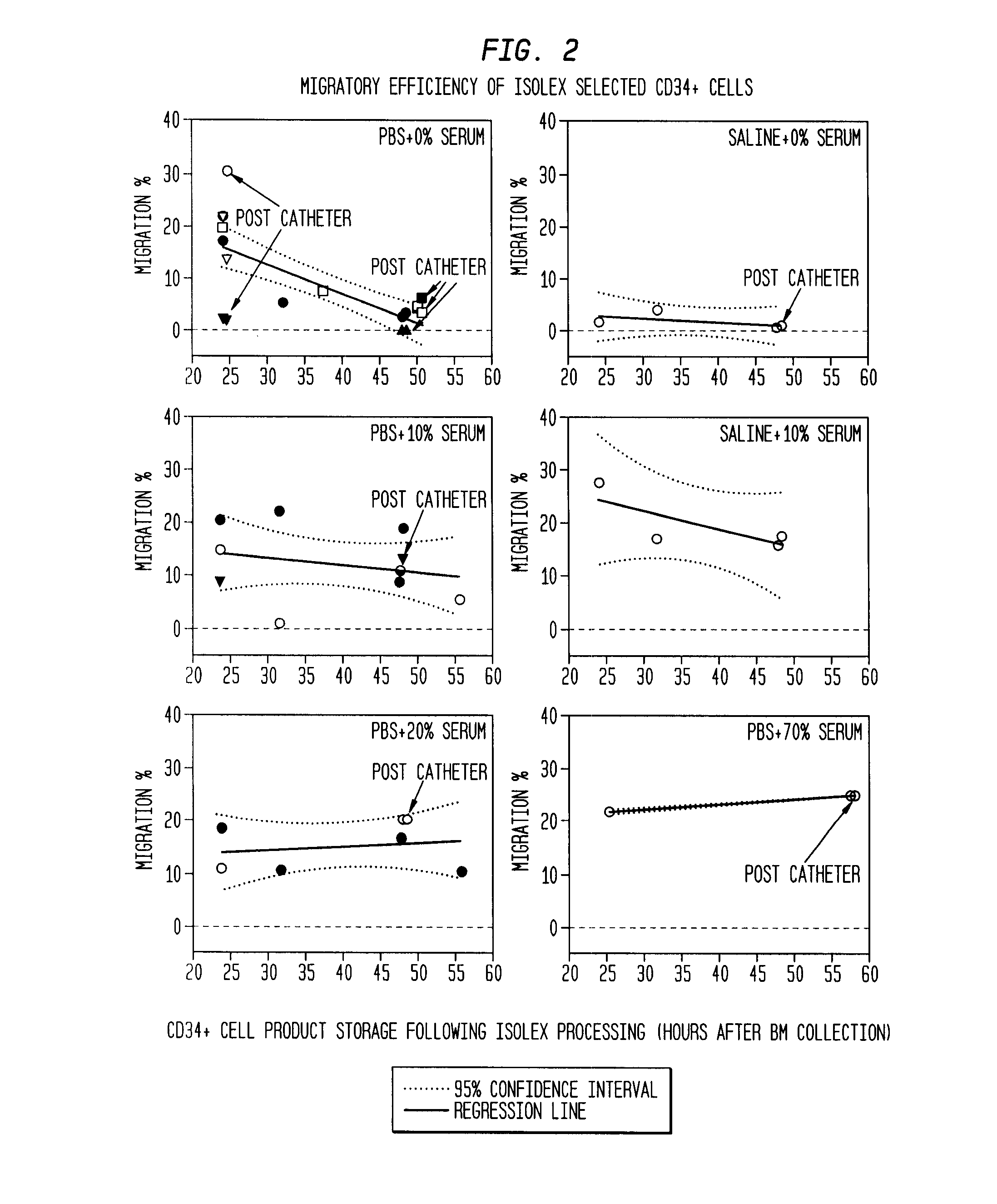
Infarct area perfusion-improving compositions and methods of vascular injury repair
ActiveUS20100143317A1Increase perfusionDecline in muscle functionBiocidePeptide/protein ingredientsBlood vessel injuryPerfusion
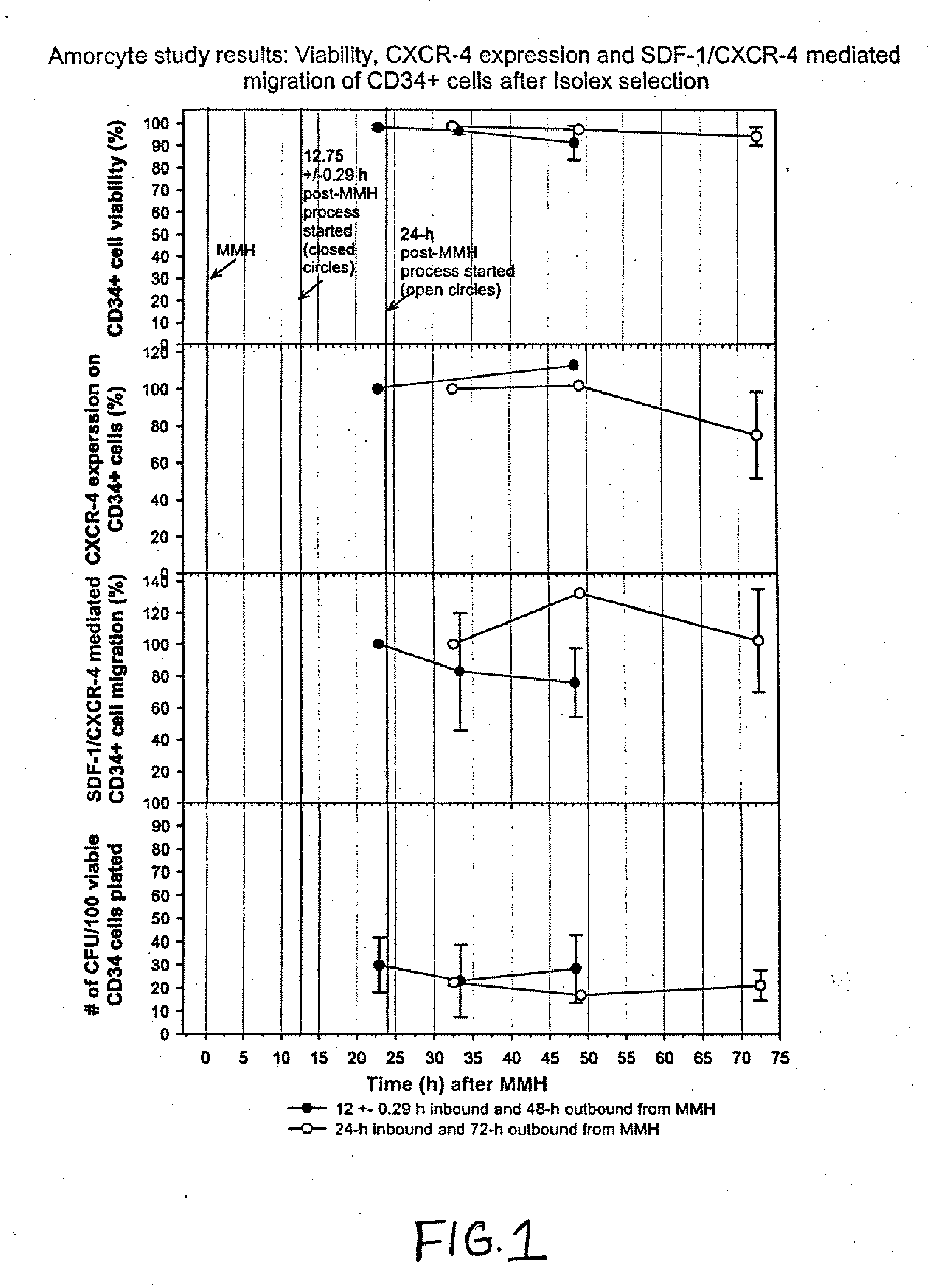
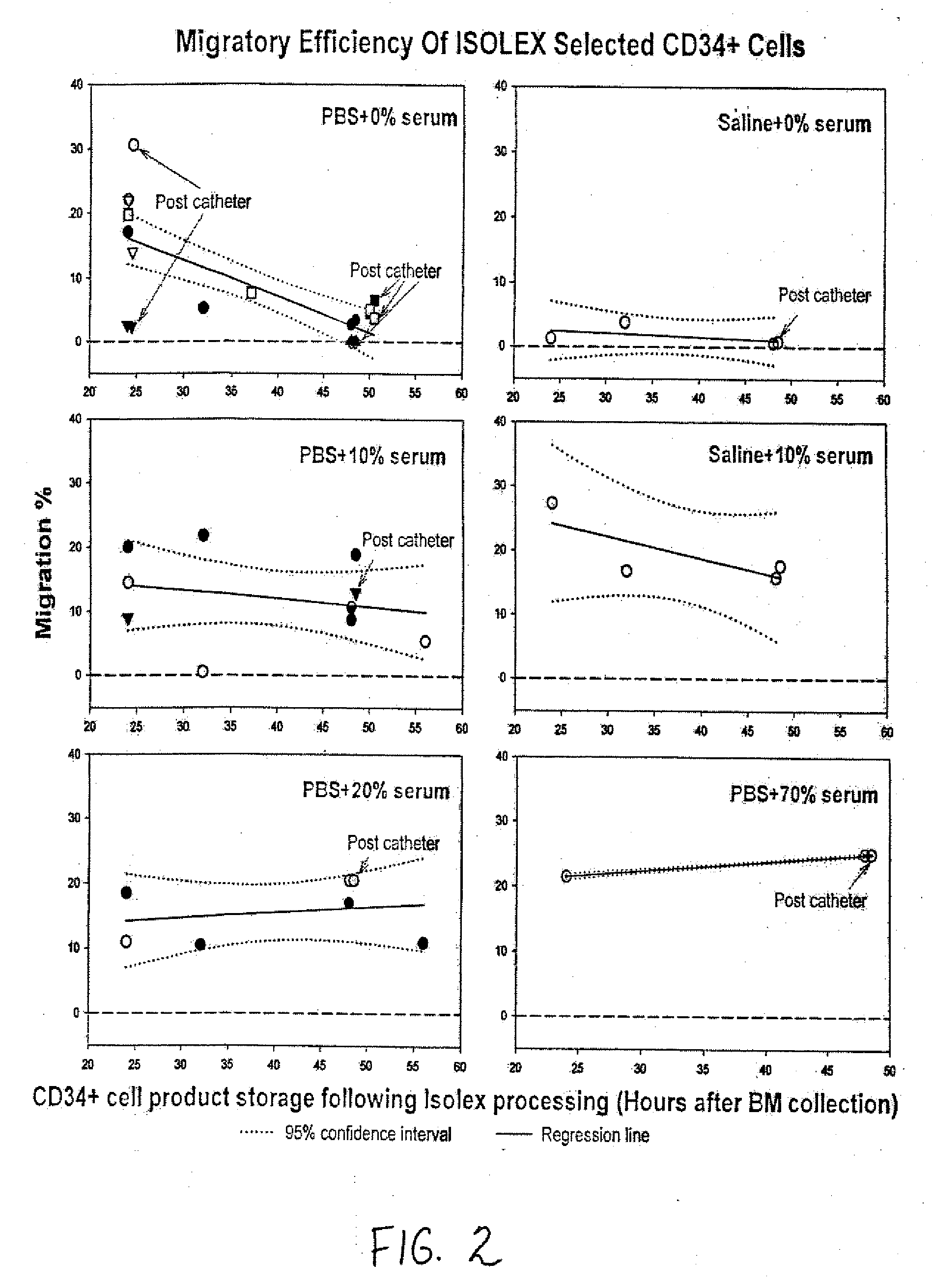
The described invention provides pharmaceutical compositions for treating an infarct area injury and methods of treating or repairing the infarct area injury in a revascularized subject in the aftermath of an acute myocardial infarction resulting from a natural disease process by administering to the subject parenterally through a catheter a sterile pharmaceutical composition containing a therapeutically effective amount of a nonexpanded sterile isolated chemotactic hematopoietic stem cell product as a first therapeutic agent and optionally a therapeutically effective amount of at least one compatible second therapeutic agent. The infarct area-improving amount of the sterile isolated chemotactic hematopoietic stem cell product comprises an enriched population of isolated autologous CD34+ cells containing a subpopulation of potent cells expressing CXCR-4 and having CXCR-4-mediated chemotactic activity such that the enriched population of isolated autologous CD34+ hematopoietic stem cells provides at least 0.5×106 potent CD34+ cells expressing CXCR-4 and having CXCR-4 mediated chemotactic activity.
Owner:CALADRIUS BIOSCI
Device and method for in vitro high-density cultivation of erythrocyte
ActiveCN103194389AAchieve high density cultureVersatileBlood/immune system cellsTissue/virus culture apparatusHollow fibreHigh density
The invention discloses a device for in vitro high-density cultivation of erythrocyte. The device comprises a stirred tank bioreactor and a hollow fiber cell intercepting device, wherein the structure of the hollow fiber cell intercepting device includes a cylindrical sleeve formed by 4-10 hollow fiber tubes which are parallel to one another; an elastic valve, a piston and a vacuum pump are orderly arranged at the bottom of the cylindrical sleeve; and the upper part of the cylindrical sleeve is connected with the stirred tank bioreactor through a connecting pipe. A method for in vitro high-density cultivation of erythrocyte is also disclosed by the invention. The method comprises the following steps of (1) separating a mononuclear cell; (1) separating CD34+ hematopoietic stem cell; and (3) differentiating the hematopoietic stem cell into the erythrocyte. By using the device and the method, hematopoietic stem cells with different sources can be utilized to carry out in vitro differentiation, so as to form a lot of erythrocytes with complete functions. High-density cultivation of the erythrocytes can be achieved; and the density is improved by about 200 times in comparison with a common cell cultivation method.
Owner:厦门三一造血技术有限公司
Sub totipotential stem cell and preparation method and application thereof
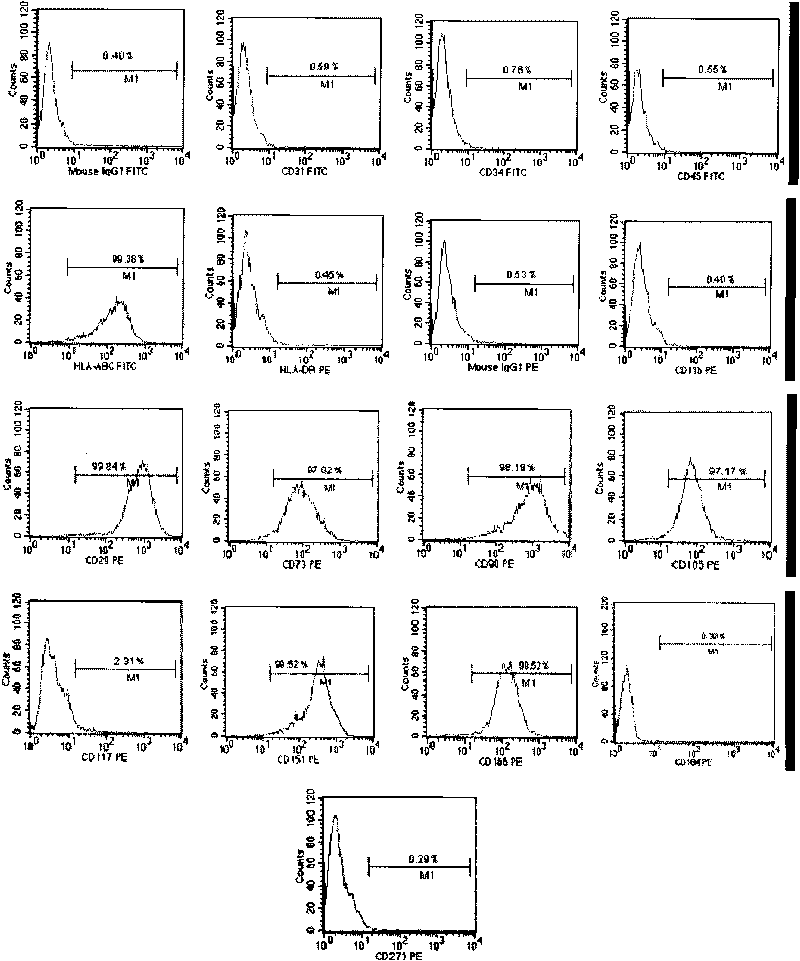
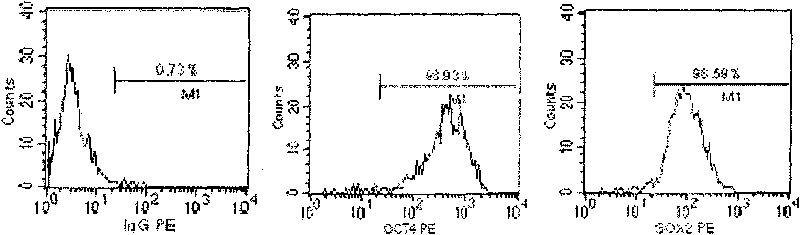
The invention discloses a method for preparing a population of?human pluripotent stem cells and the application thereof. The preparation of stem cells is characterized by comprising the following steps: CD151<+>, CD31<->, Sox<2+> pluripotent stem cells are separated and collected from human umbilical cord and or placenta tissues; the cells adhere to grow in a culture vessel under a predetermined condition and expand through passage 20 or above to be still stable in gene expression. The population of cells of this invention do not form teratoma after injection into animals. The human pluripotent stem cells highly express CD151, OCT4 and Sox-2 as specific markers of embryonic stem cells, as well as specific markers of epidermic cells, endothelial cells, thrombocytes, dendritic cells, while lack expression of CD31, CD34, CD45 and HLA-II. The pluripotent stem cells are also characterized as being able to adhere to tissue culture plastic and having the potential to differentiate into three germ layers: endoderm, mesoderm and ectoderm. These pluripotent stem cells are able to be used as carrier cells of gene therapy and for the treatment of diseases caused by cell damage or cell aging. The present invention provides a method of isolating, purifying and culturally expanding of a population of human pluripotent stem cell for preparing the high purity injection preparation. The preparation of stem cells has a good therapeutic effect on the treatment of diseases caused by cell damage or cell aging in animal and human clinical trials. The preparation also has no toxic side effect and no immune rejection.
Owner:BEIJING HEALTH & BIOTECH (H&B) CO LTD
Method of prodcing megacaryocyle using umbilical blood CD 344+cell in vitro induction method
InactiveCN1556197AIncrease multipleImprove production efficiencyTissue cultureHuman plateletWhite blood cell
A process for generating megacaryocyte by in vitro induction to umbilical blood cell CD34+ includes in vitro amplifying the CD34+ in the culture medium containing fetal calf serum, human SCF, human TPO and / or ligand flt-3 and inducing the generation of megacaryocytes in the non-serum culture medium containing human TPO, human interleukin 3(IL-3) and / or human GM-CSF. Its advantage is high inducing efficiency.
Owner:上海伯瑞生物技术发展有限公司 +1
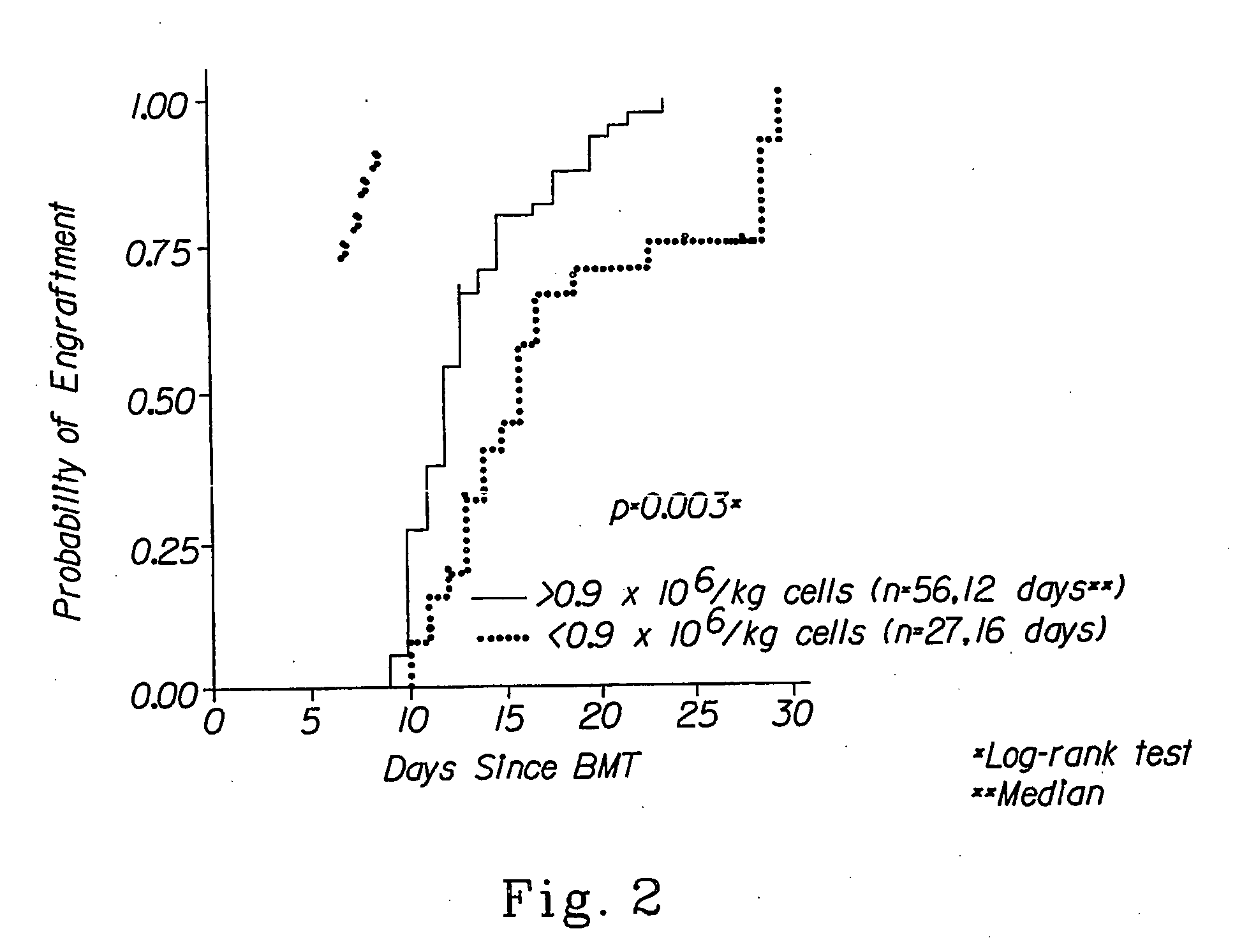
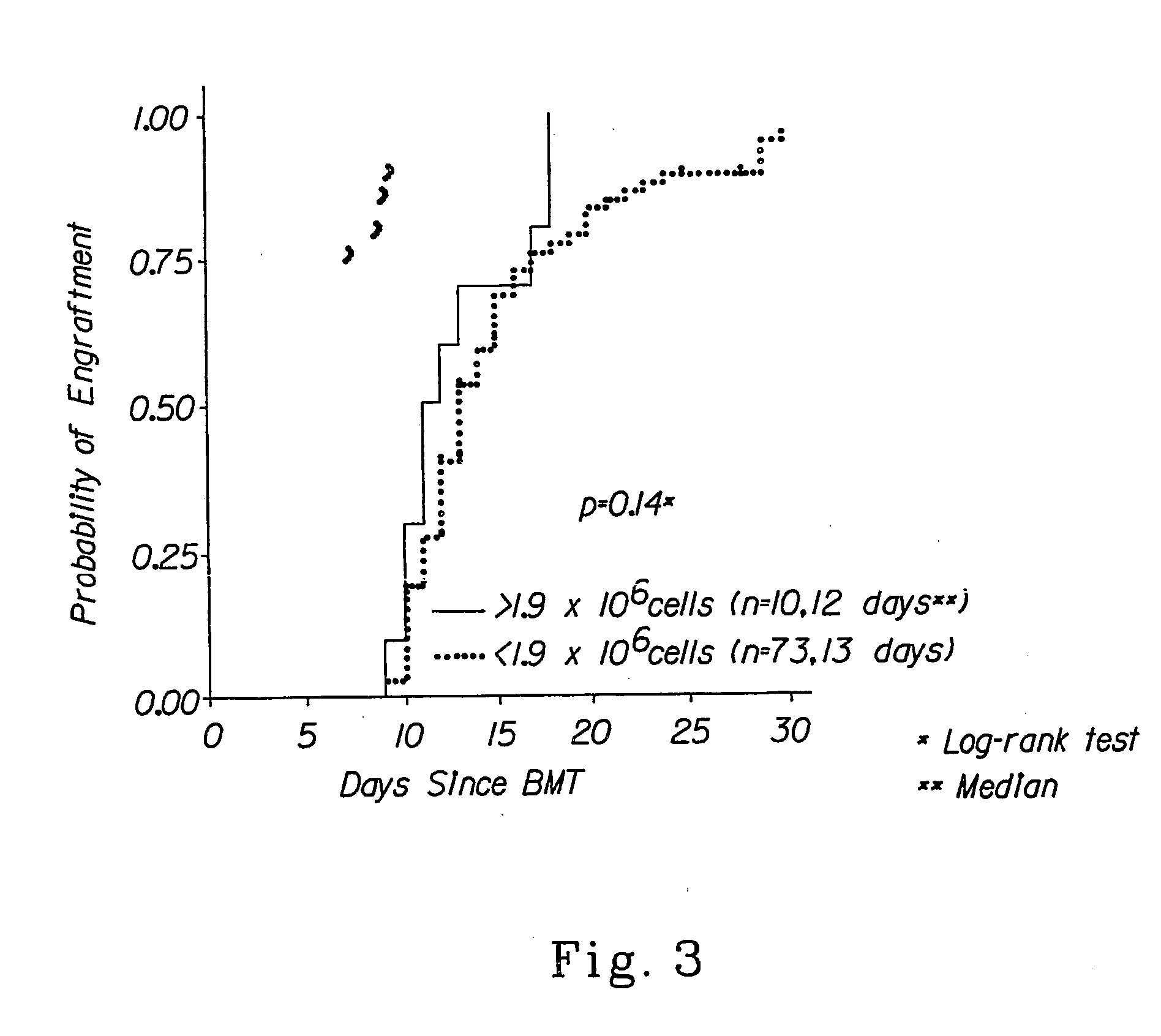
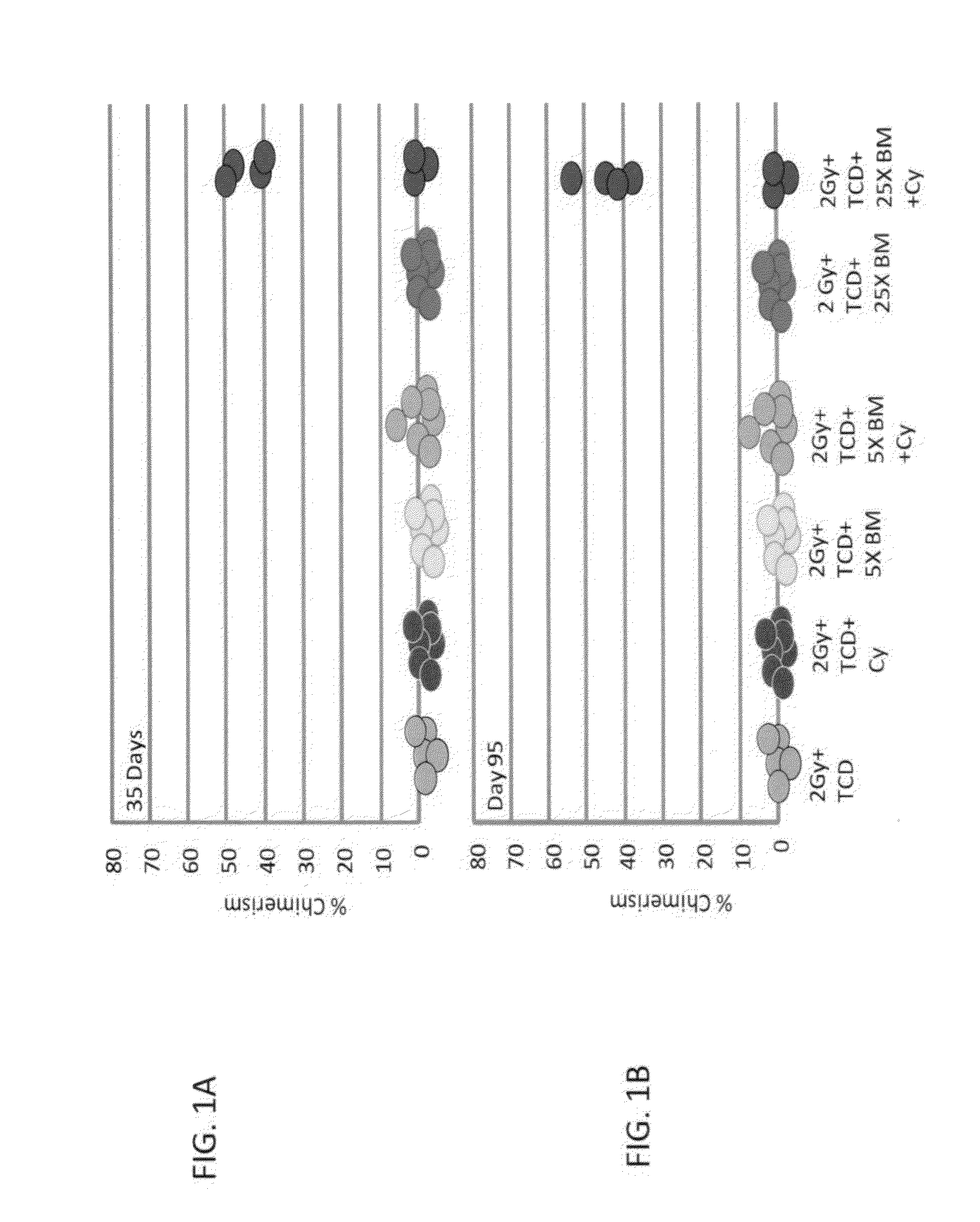
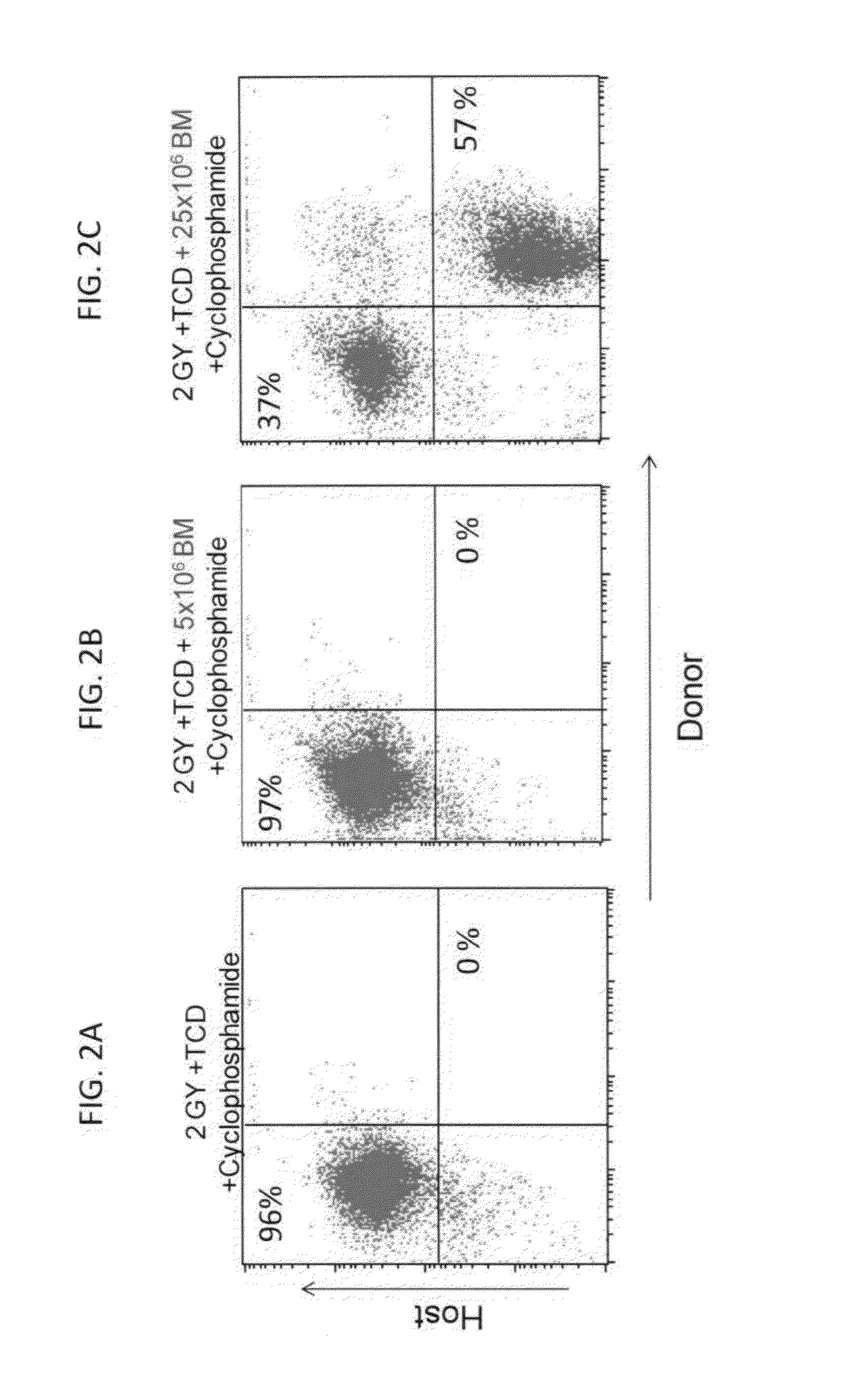
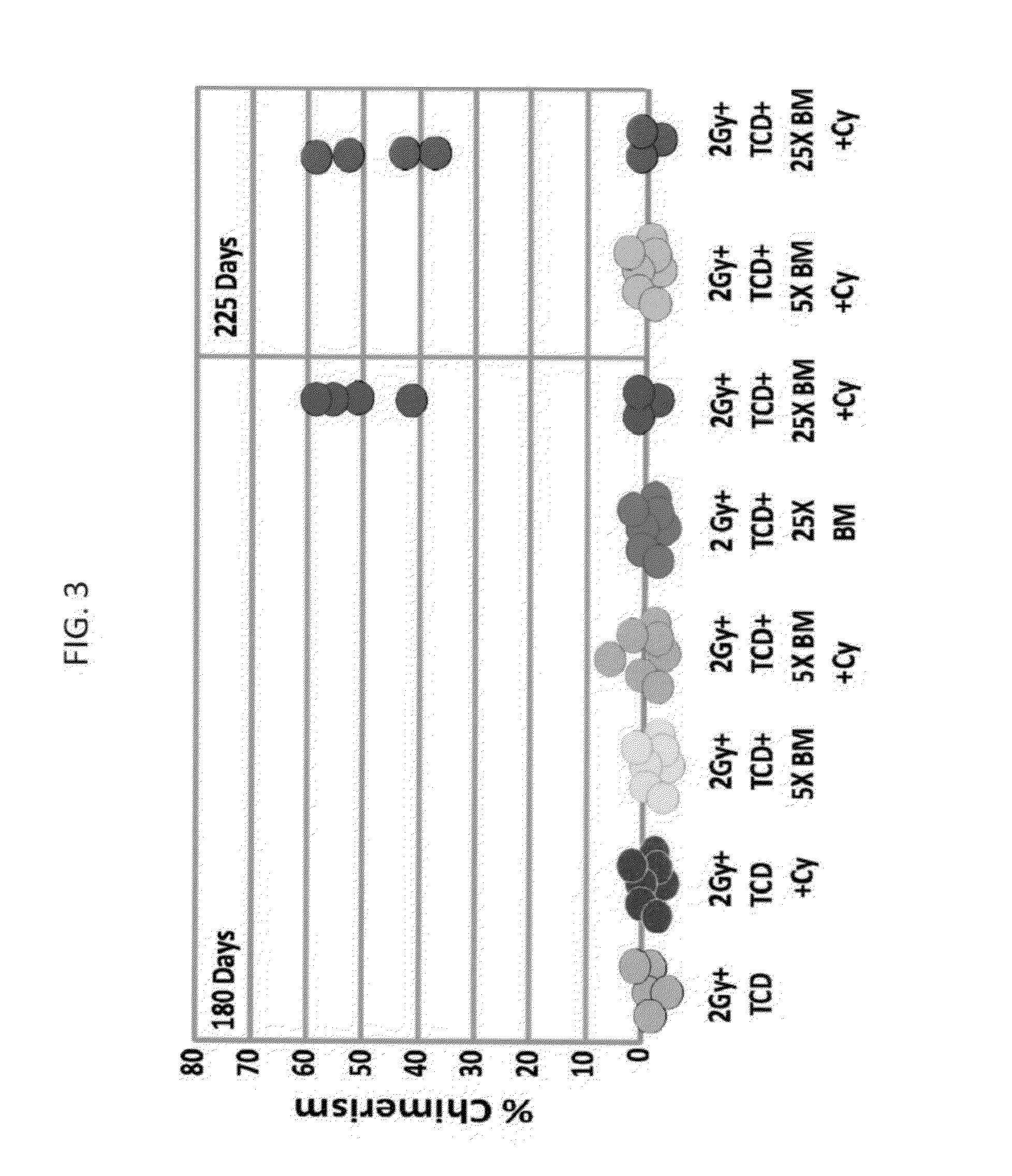
Combination therapy for a stable and long term engraftment using specific protocols for t/b cell depletion
A method of treating a subject in need of a non-syngeneic cell or tissue graft is disclosed. The methos comprising: (a) transplanting into a subject a dose of T cell depleted immature hematopoetic cells, wherein the T cell depleted immature hematopoetic cells comprise less than 5×105 CD3+ T cells per kilogram body weight of the subject, and wherein the dose comprises at least about 5×106 CD34+ cells per kilogram body weight of the subject, and wherein the T cell depleted immature hematopoetic cells are obtained by separating the T cells from the immature hematopoetic cells by magnetic cell sorting, and (b) administering to the subject a therapeutically effective amount of cyclophosphamide, wherein the therapeutically effective amount comprises 25-200 mg per body weight, thereby treating the subject.
Owner:YEDA RES & DEV CO LTD
Cd34-derived recombinant adeno-associated vectors for stem cell transduction and systemic therapeutic gene transfer
Novel adeno-associated virus (AAV) isolates in nucleotide and amino acid forms and uses thereof are provided. The isolates show tropism for certain target tissues, such as blood stem cells, liver, heart and joint tissue, and may be used to transduce stem cells for introduction of genes of interest into the target tissues. Discrete modified portions of the cap gene, VP1, VP2, and VP3, may be used alone or in combination in the present methods.
Owner:CITY OF HOPE
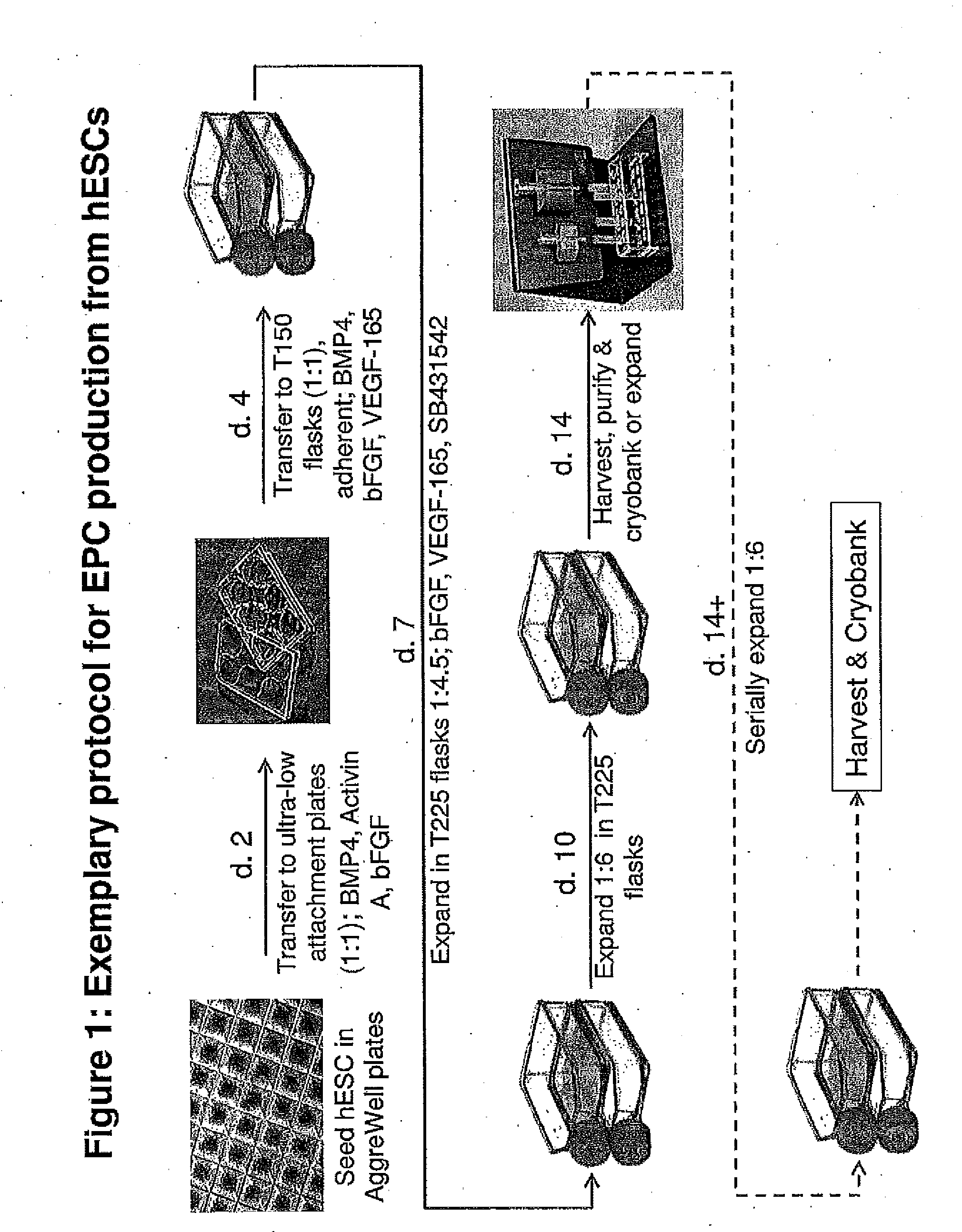
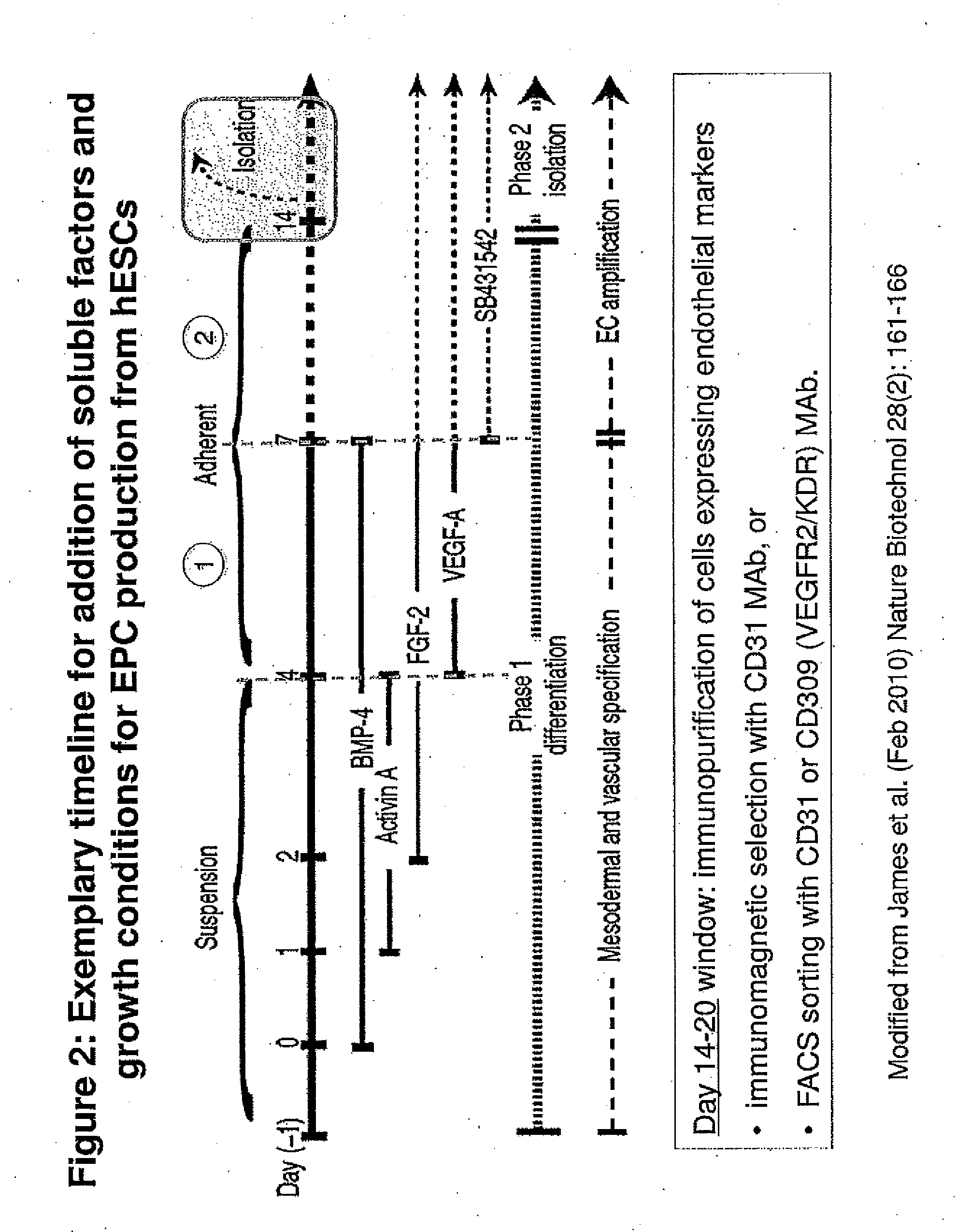
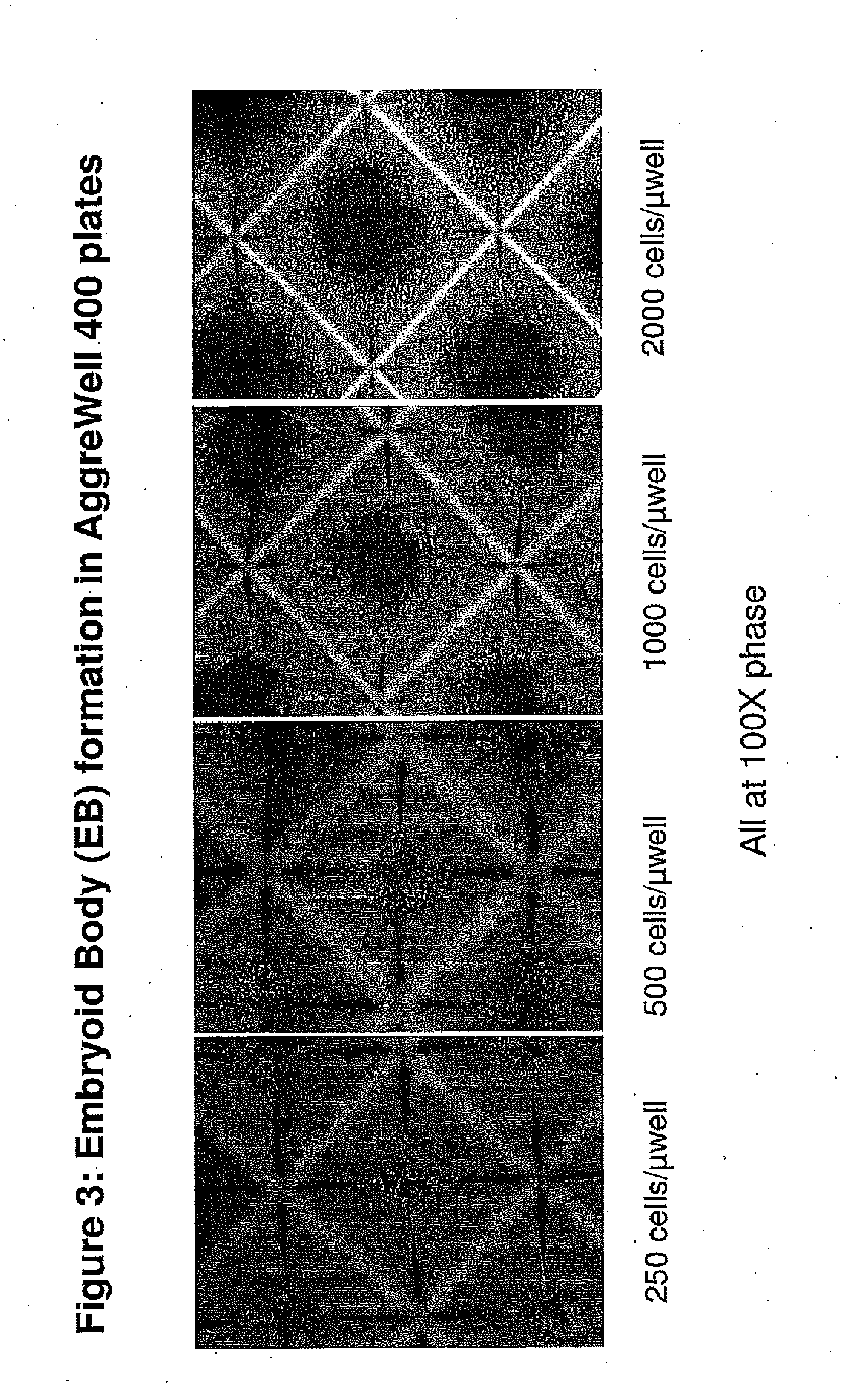
Methods and Compositions for Producing Endothelial Progenitor Cells from Pluripotent Stem Cells
InactiveUS20120295347A1Artificial cell constructsCell culture supports/coatingProgenitorLymphatic vessel
Aspects of the present invention are drawn to methods and compositions for producing endothelial progenitor cells (EPCs) in vitro from pluripotent stem cells and compositions containing such EPCs. The methods produce sufficient EPCs to use in therapeutic applications. In certain embodiments the EPCs are bipotent, giving rise to both vascular and lymphatic endothelial cells. In certain embodiments, EPCs express one or more of the following gene products: LYVE-1, PV-1 / PAL-E, CD31, and CD34.
Owner:RECYTE THERAPEUITCS
Mixing stem cell injection and preparation method thereof
ActiveCN103330720AImprove securityProliferation effect is goodAntinoxious agentsMammal material medical ingredientsProgenitorMesenchymal stem cell
The invention discloses a mixing stem cell injection and a preparation method thereof, and belongs to the field of cell preparation agents. The injection of the invention is mixture of mesenchymal stem cells from umbilical cord source and hematopoietic stem / progenitor cells from the umbilical cord blood source; the method of the mixing stem cell injection comprises the following steps: CD34+ hematopoietic stem cells are separated from the fresh umbilical cord blood and purified, and are subjected to mixed culture and amplification together with the mesenchymal stem cells from the umbilical cord source, during the amplification, the culture system is culture medium including AB umbilical cord blood plasma the volume fraction of which is 15 percent and the cell factor, 6*104 mesenchymal stem cells and CD34+ hematopoietic stem cells with the concentration of 5*104 / ml are inoculated into a culture flask and are cultured for about 10 days, and two kinds of cells are obtained at the same time, so that the mixed stem cell injection including 5*105 / ml of hematopoietic stem / progenitor cells and 2*105 / ml of mesenchymal stem cells and containing normal saline with the volume fraction of AB umbilical cord blood plasma as 15 percent is obtained.
Owner:广州市天河诺亚生物工程有限公司
Compositions and methods of vascular injury repair
The present invention relates to pharmaceutical compositions comprising a chemotactic hematopoietic stem cell product comprising an enriched population of CD34+ cells containing a subpopulation of cells having chemotactic activity, methods of preparing these compositions and use of these compositions to treat or repair vascular injury, including infarcted myocardium.
Owner:CALADRIUS BIOSCI
CD123+ dendritic cells in blood and lymphoid tissues
InactiveUS6294381B1Artificial cell constructsImmunoglobulins against cell receptors/antigens/surface-determinantsCell lineageDendritic cell
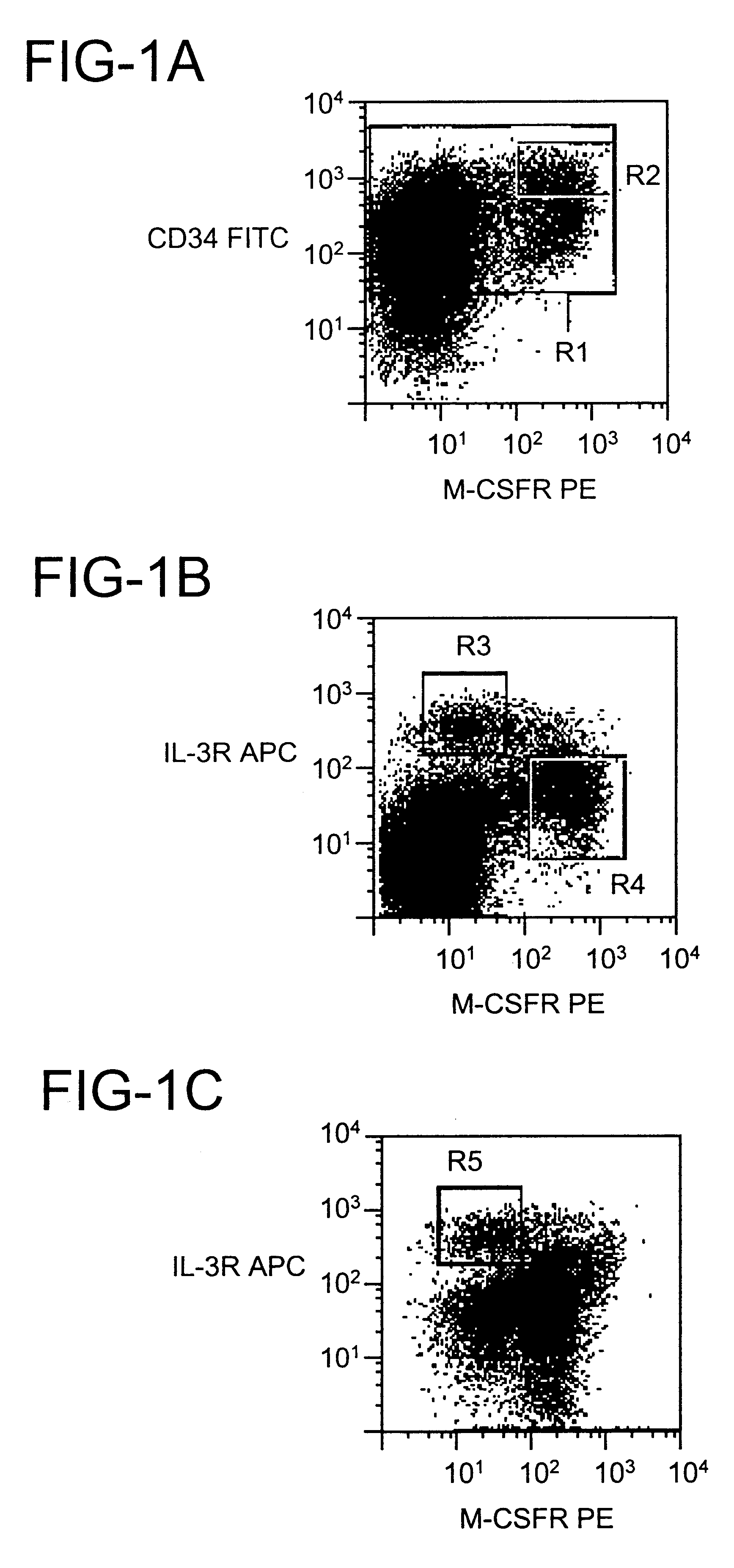
Dendritic cells (DCs) are the primary antigen presenting cells during the initiation of T cell-dependent immune responses. The cells originate from the bone marrow and have been suggested to represent a distinct cell lineage. However, distinct DC precursors have not been identified in bone marrow, and mature monocytes can also give rise to DCs. The instant invention presents a distinct DC precursor among bone marrow CD34+ cells. The cells express high levels of the interleukin-3 receptor alpha chain and CD4 and can be uniquely identified also in blood and lymphoid tissues by this phenotype.
Owner:OLWEUS JOHANNA +1
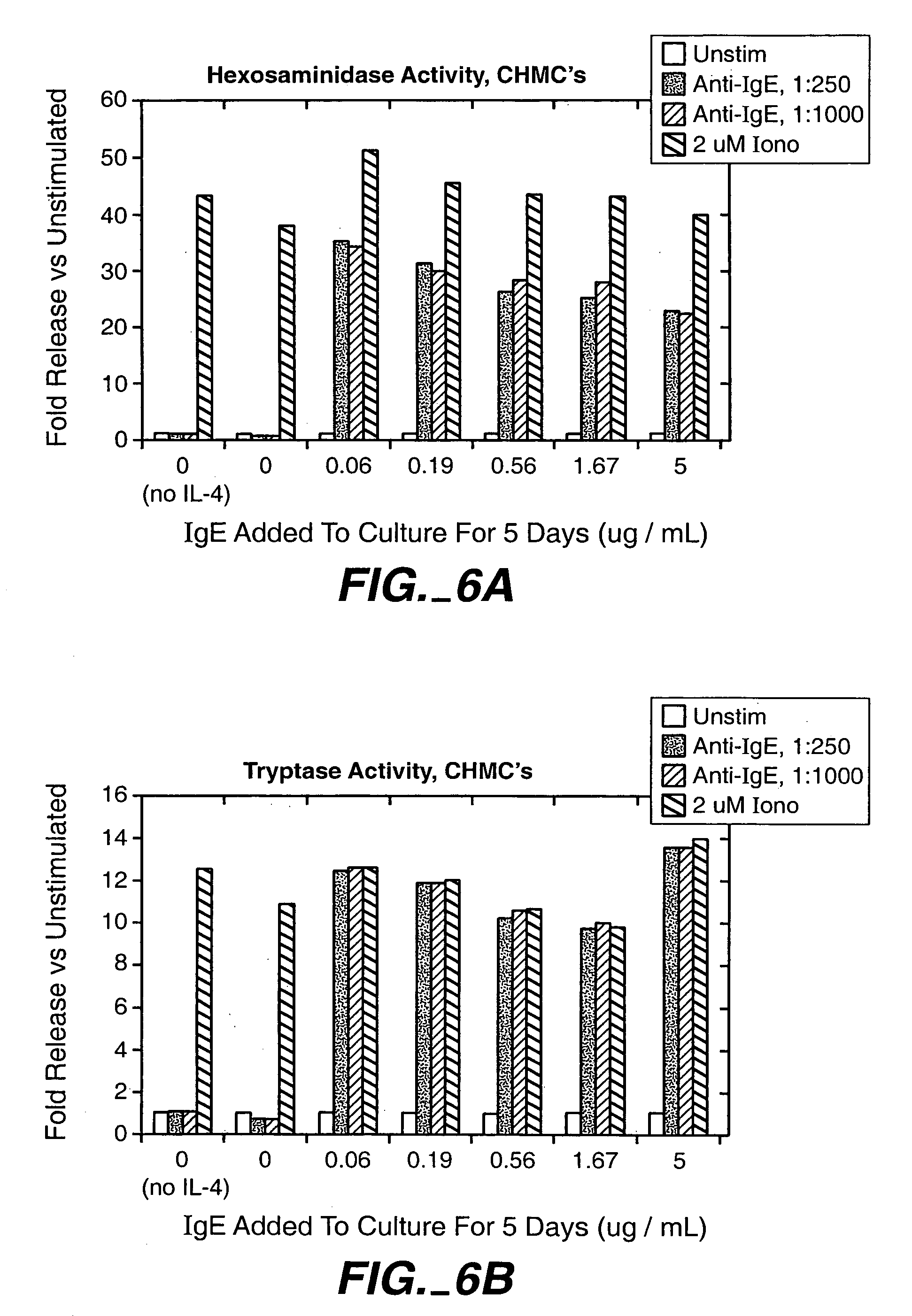
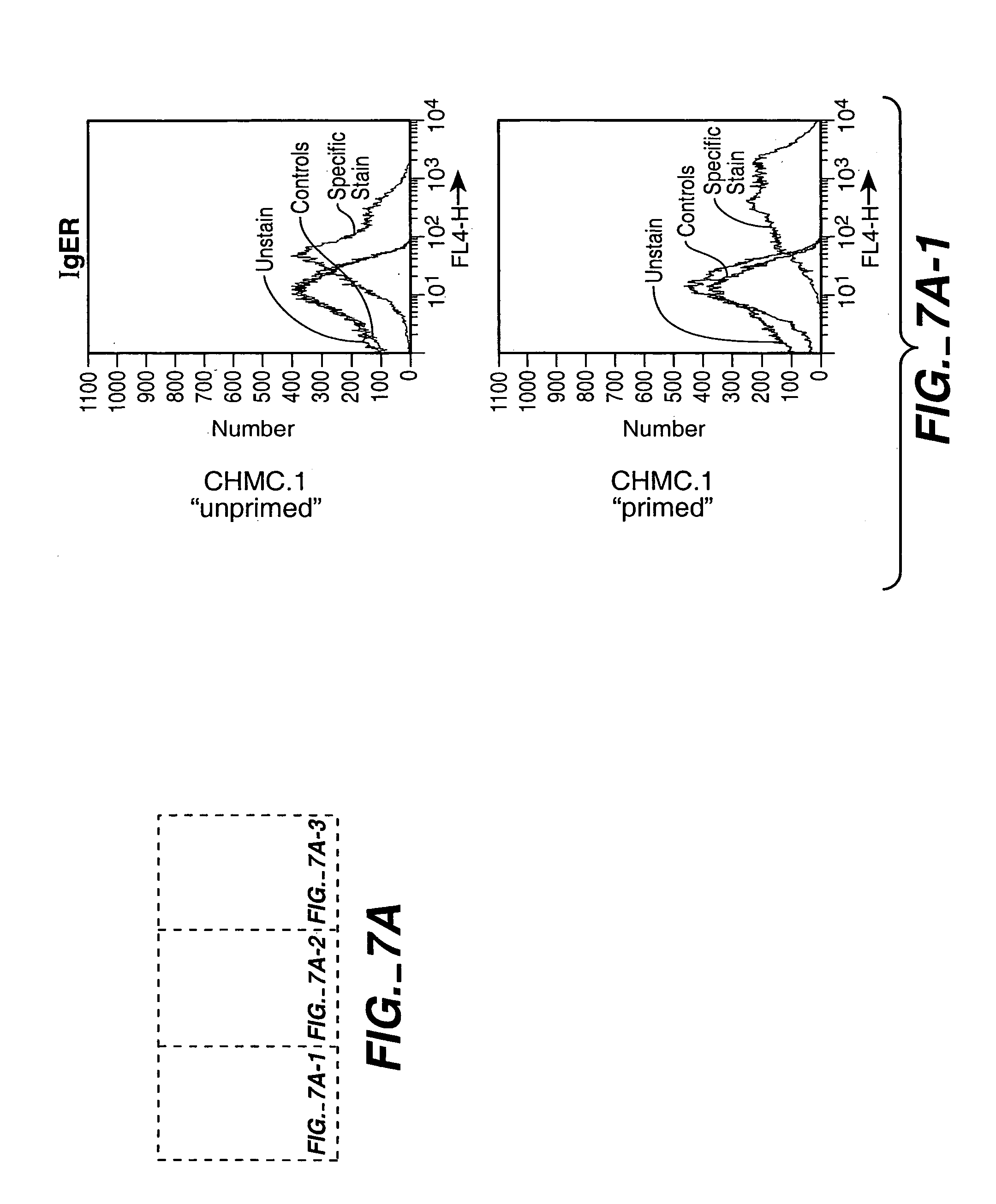
Production of cultured human mast cells and basophils for high throughput small molecule drug discovery
InactiveUS7070996B2BiocideMicrobiological testing/measurementProgenitorHigh-Throughput Screening Methods
Provided are methods for producing and screening proliferated populations of CD34-negative progenitor cells, mucosal mast cells, connective tissue-type mast cells and basophil cells. The methods generate uniform proliferated populations of cells. The proliferated populations contain a uniform population of a size suitable for use in high throughput screening methods, for example, screening for agents that alter exocytosis. The invention includes screening the proliferated populations with at least one candidate bioactive agent, and evaluating the cells to detect a cell with an altered phenotype. The invention also includes isolating a candidate bioactive agent that causes the altered phenotype. Additionally, cells formed according to the described methods are also encompassed by the invention.
Owner:RIGEL PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com