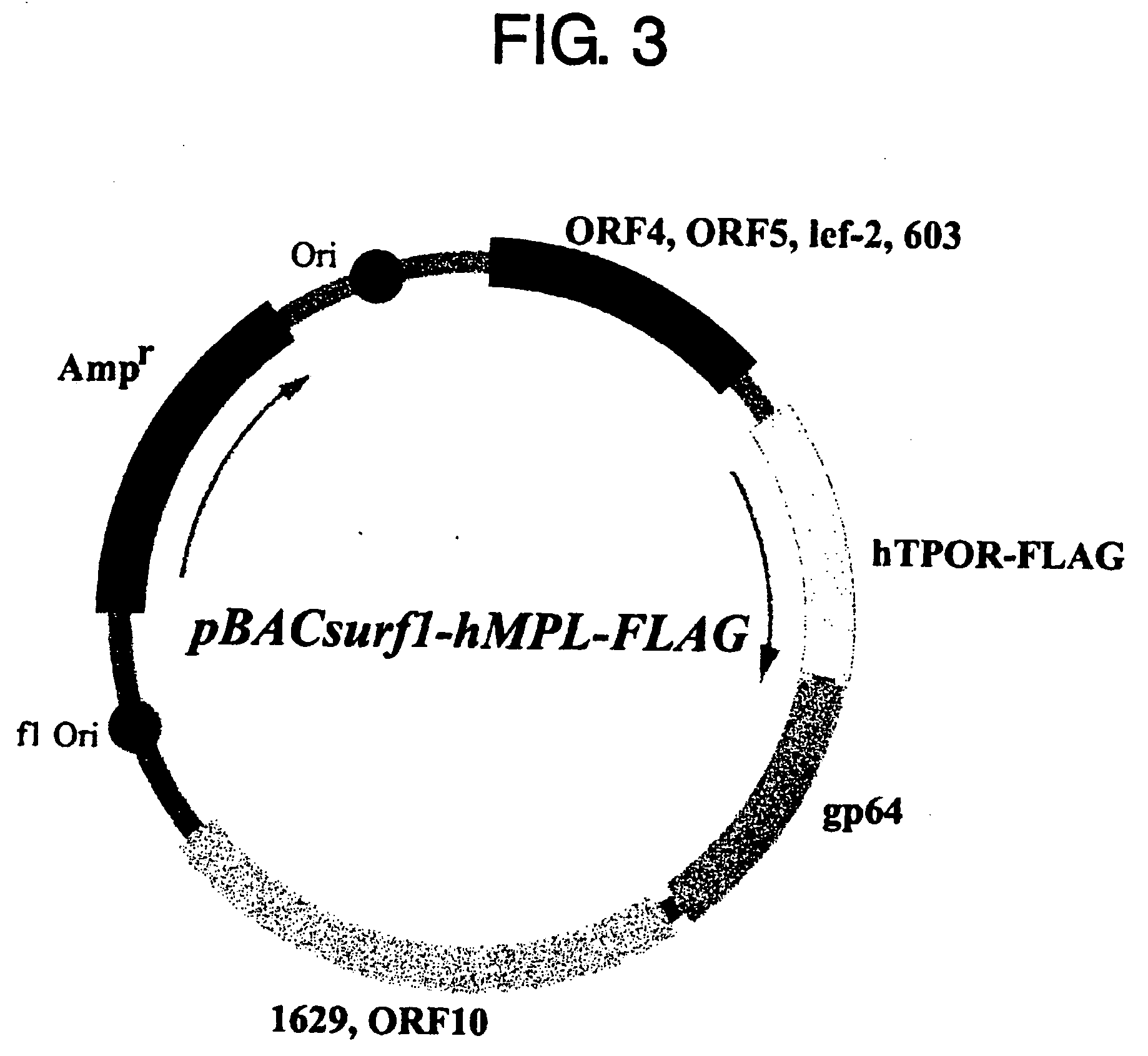
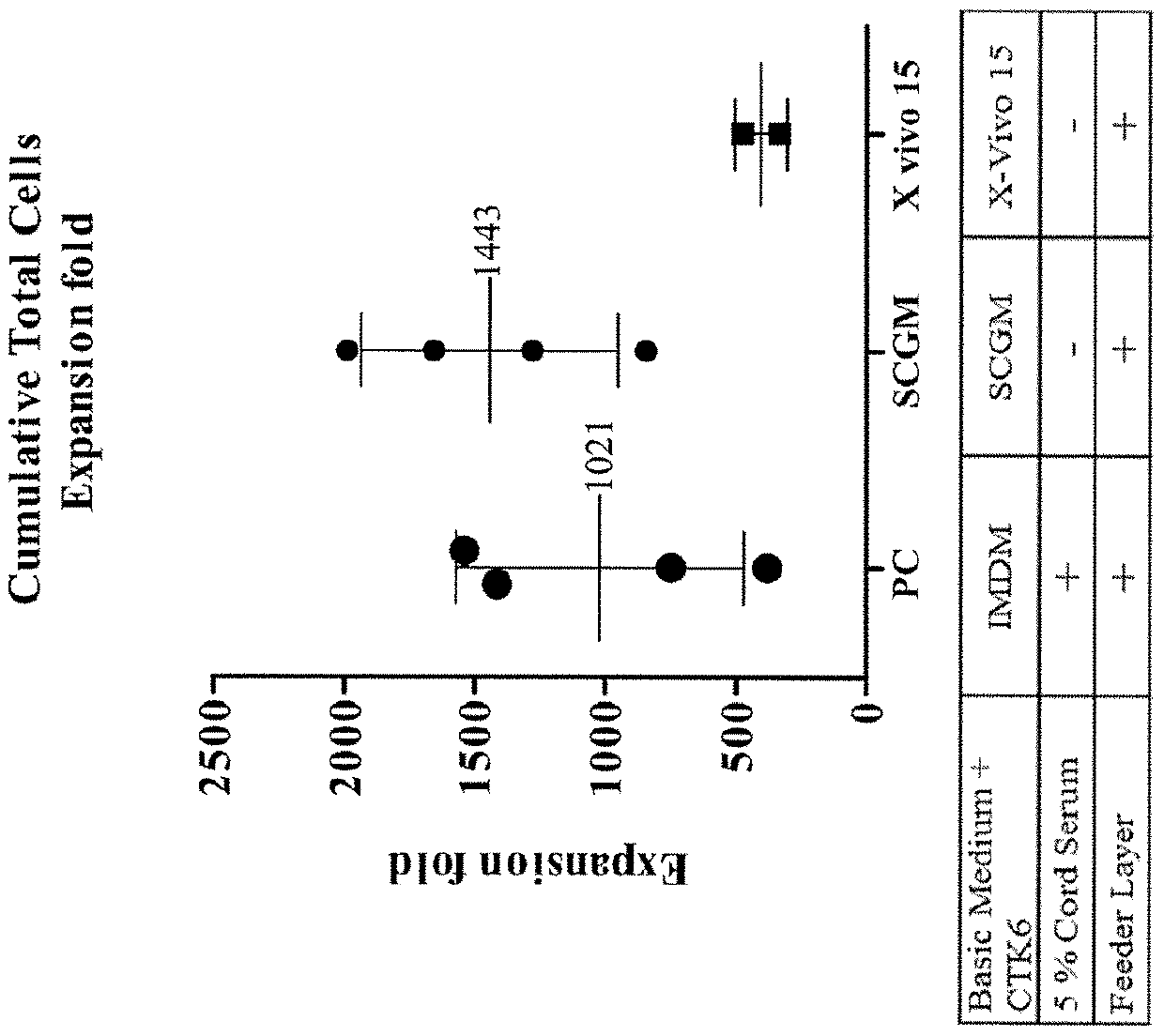
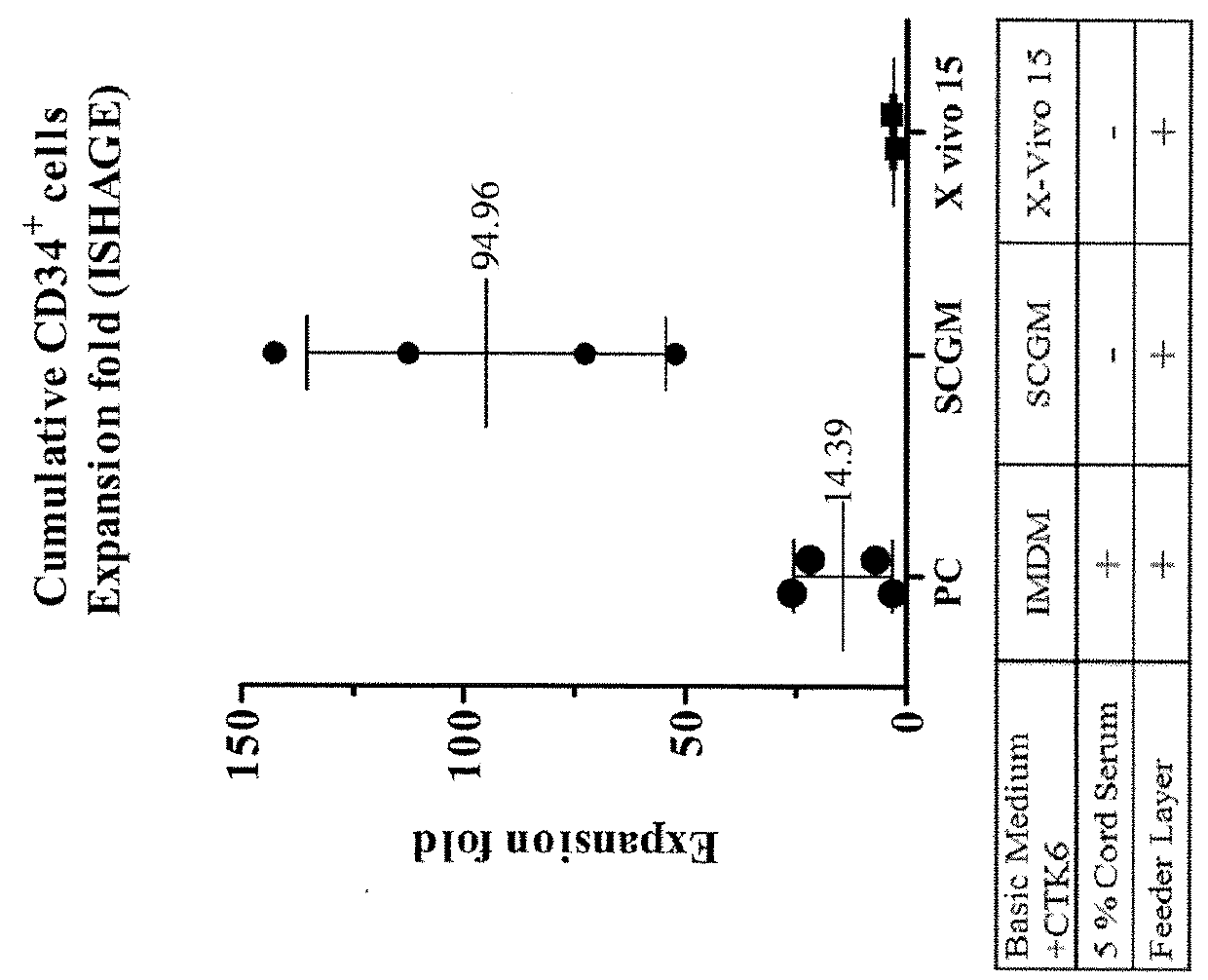
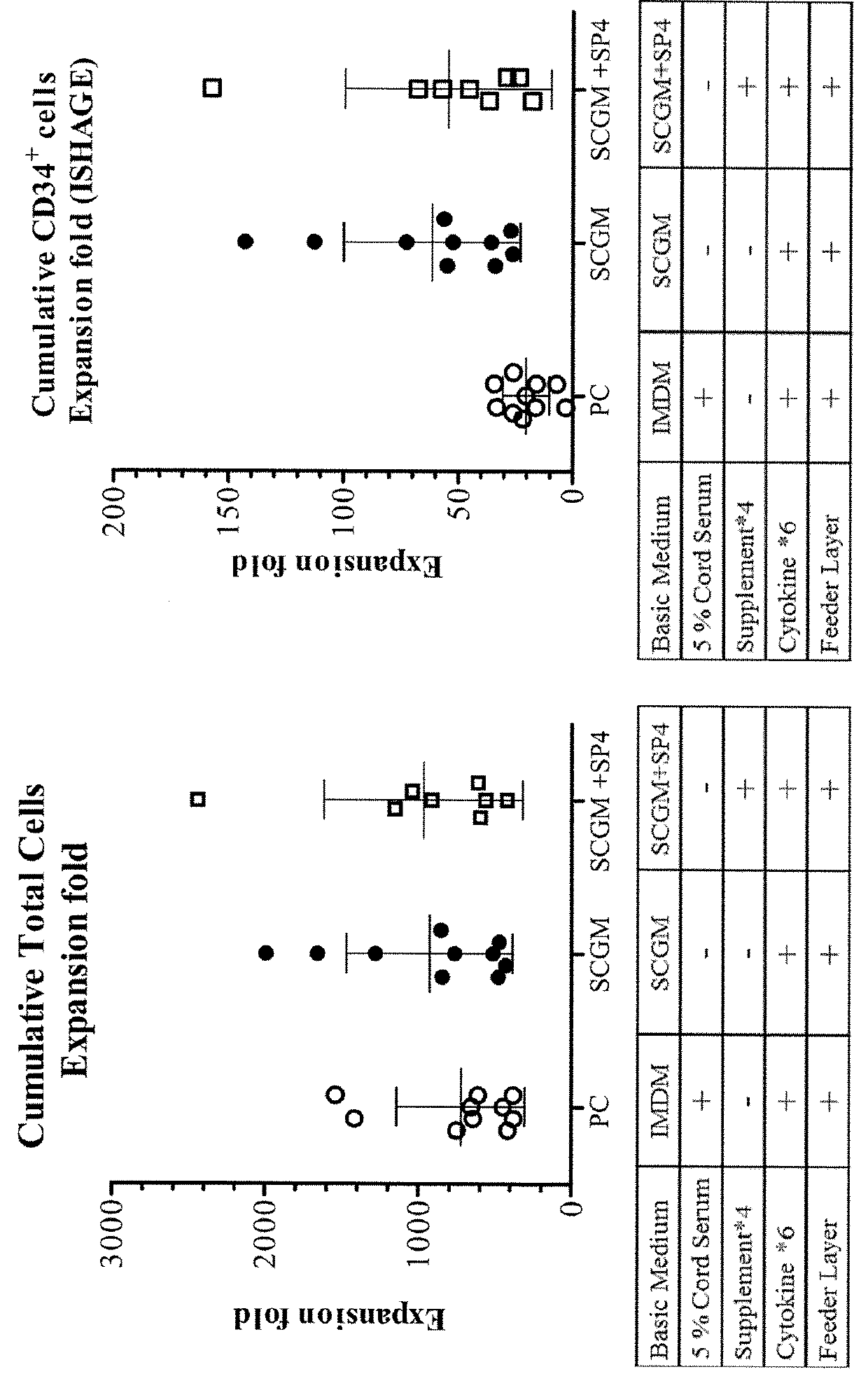
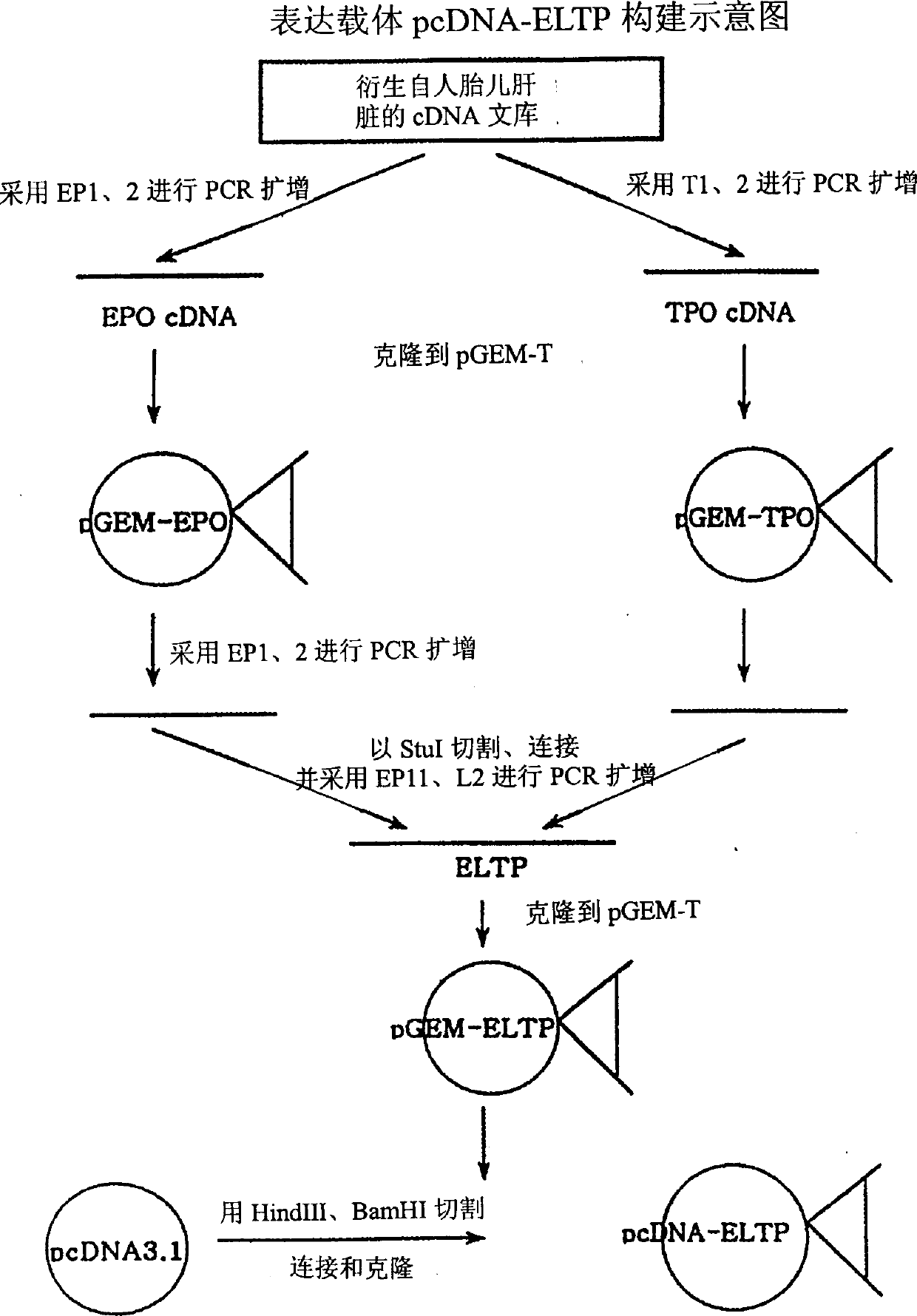
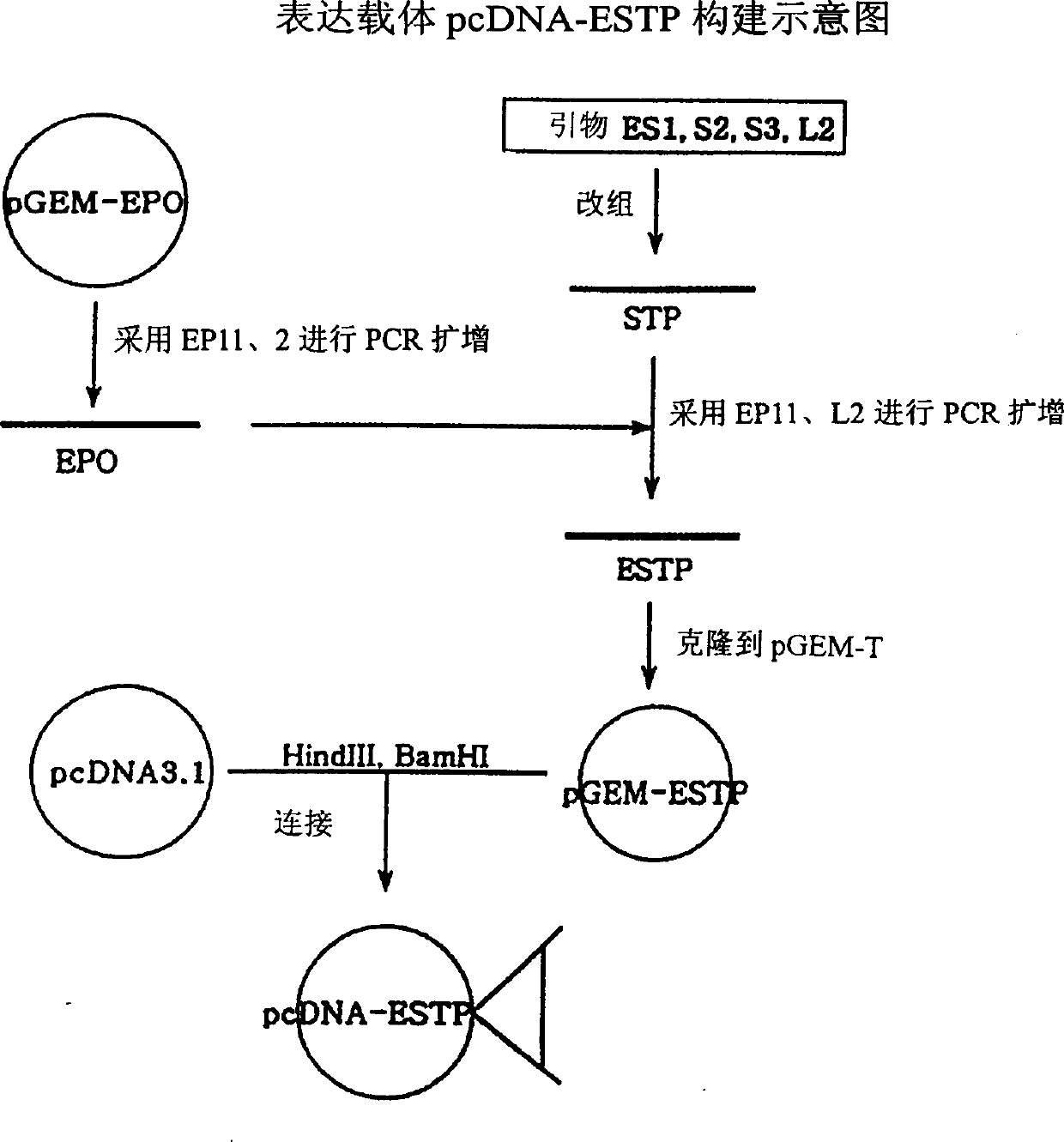
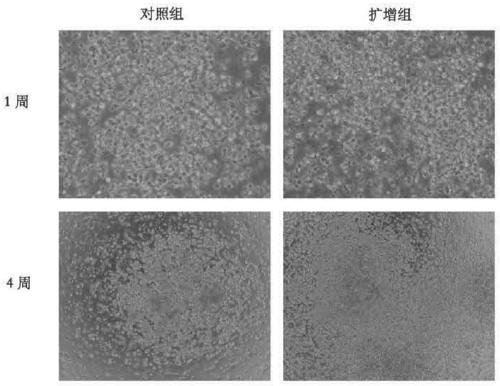
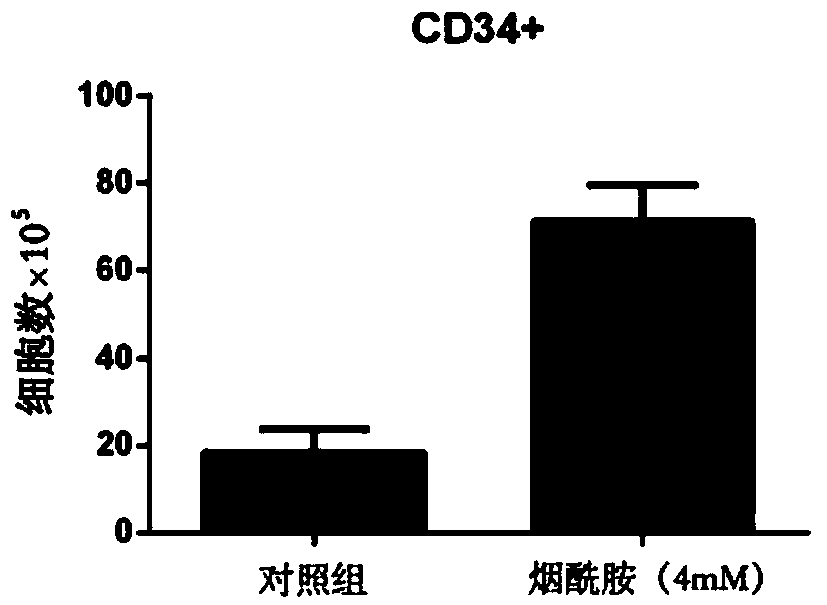
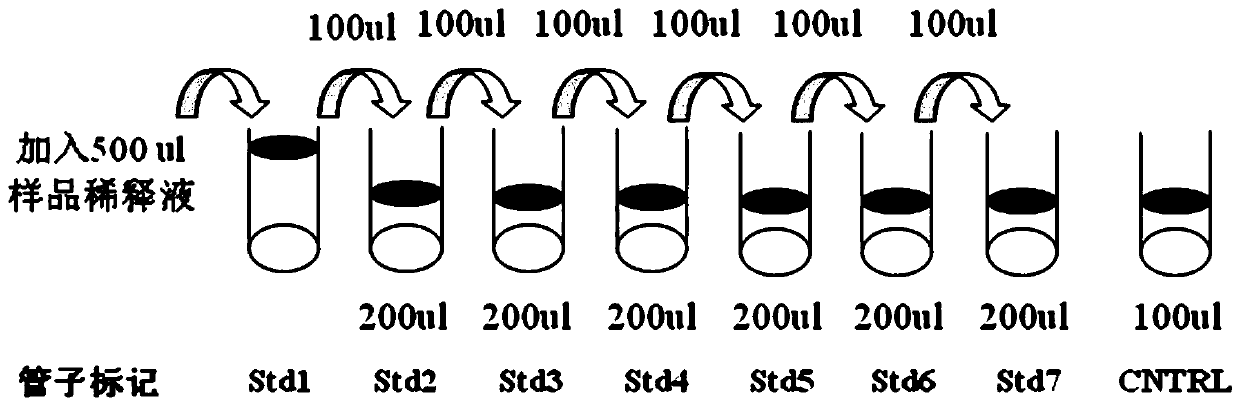
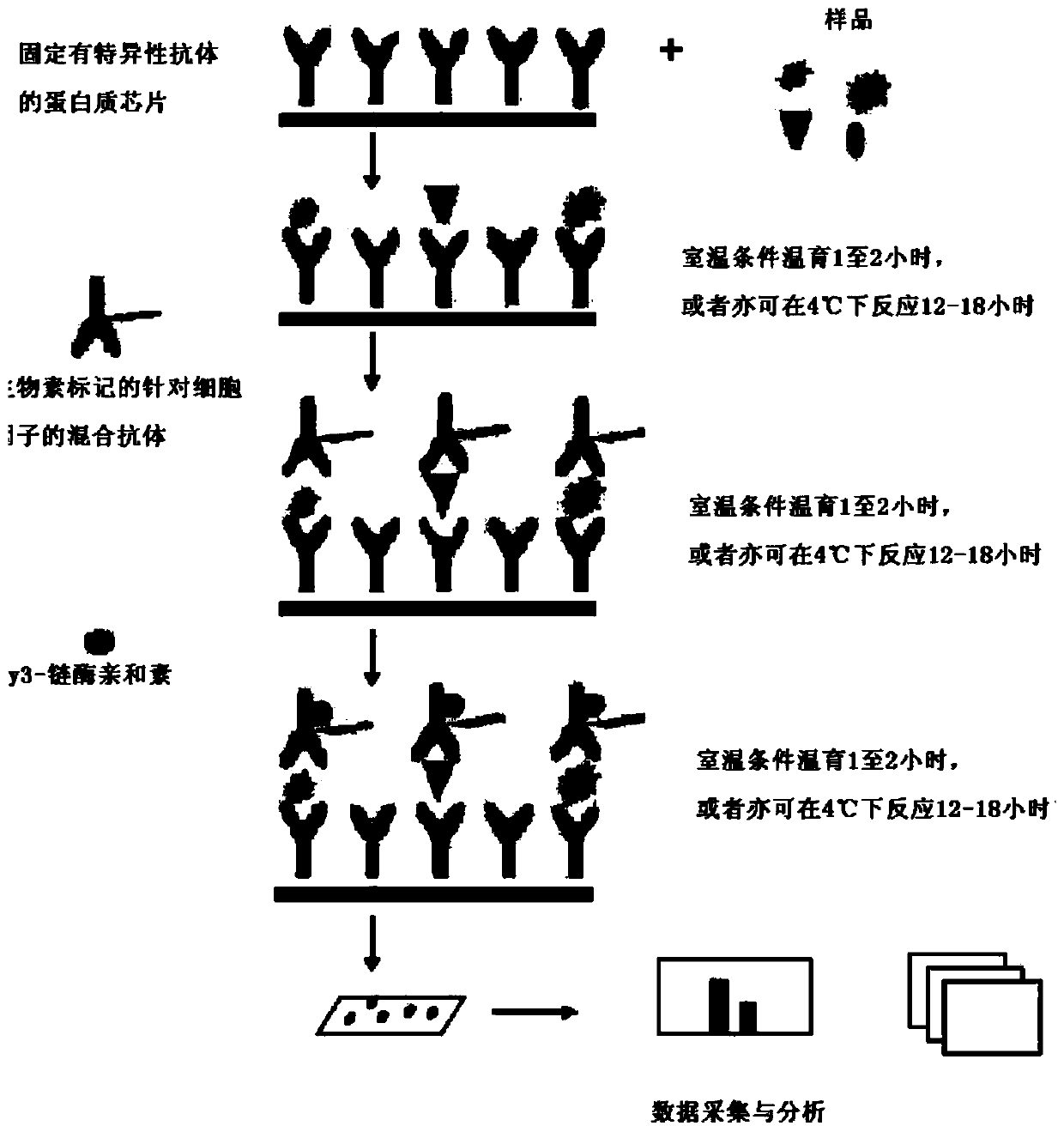
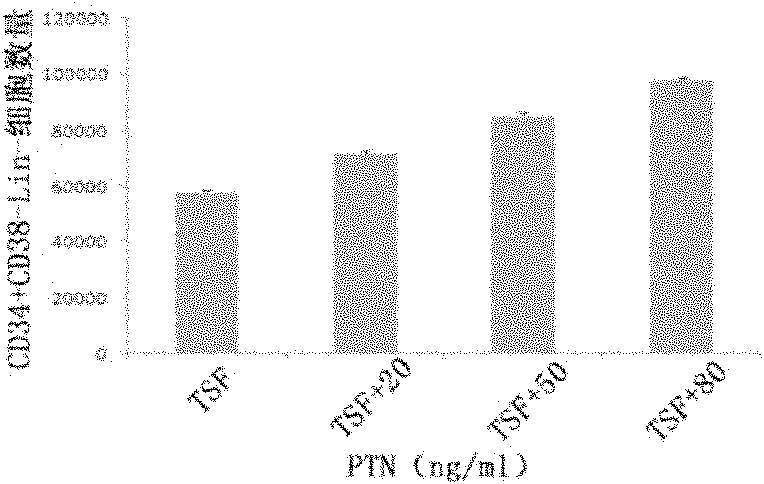Patents
Literature
88 results about "Thrombopoietin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
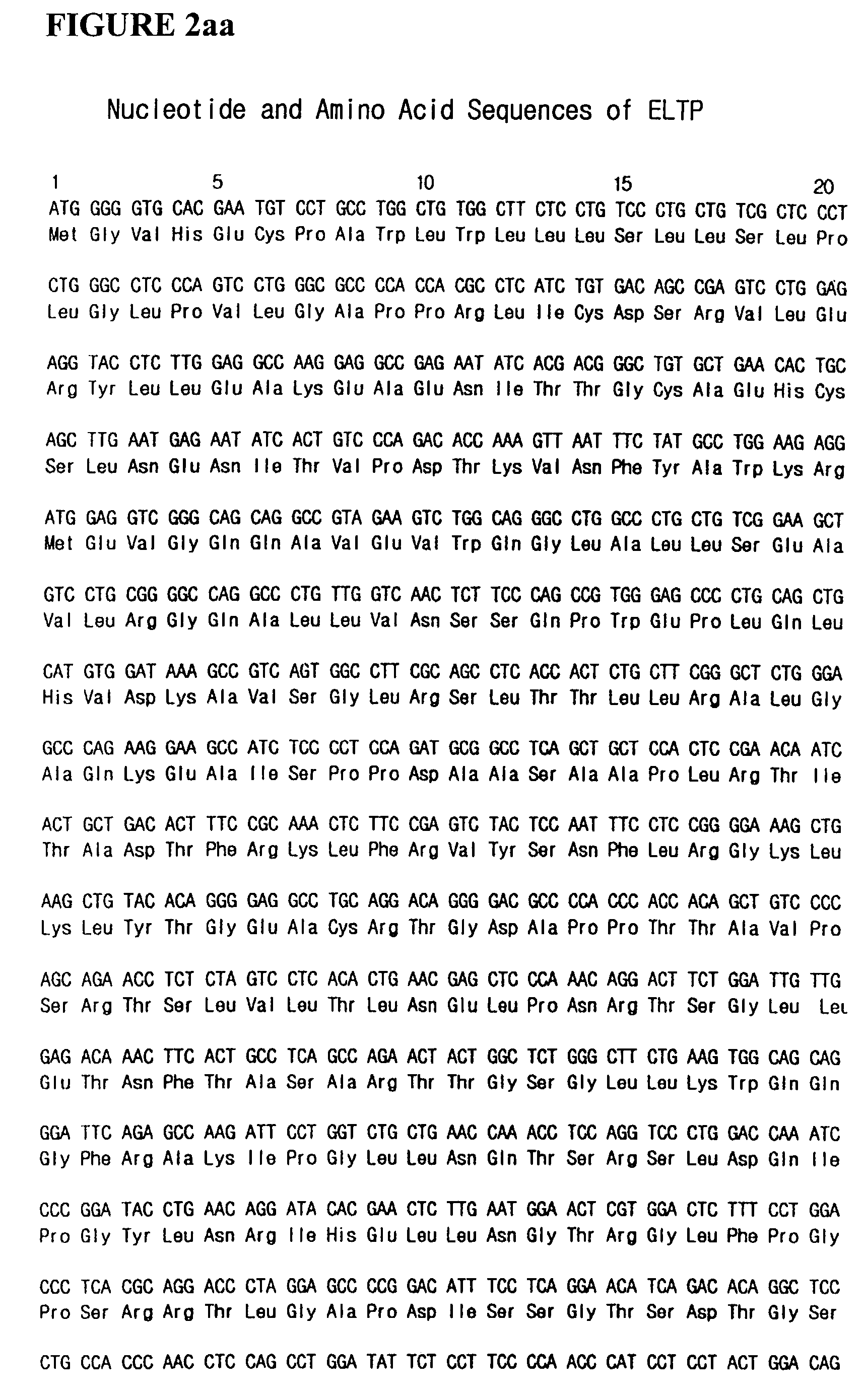
Thrombopoietin (THPO) also known as megakaryocyte growth and development factor (MGDF) is a protein that in humans is encoded by the THPO gene. Thrombopoietin is a glycoprotein hormone produced by the liver and kidney which regulates the production of platelets. It stimulates the production and differentiation of megakaryocytes, the bone marrow cells that bud off large numbers of platelets.
Treatment of respiratory chain disorders using compounds having erythropoietin or thrombopoietin activity
Methods of treating mitochondrial respiratory chain disorders using compounds having erythropoietin activity or thrombopoietin activity are disclosed. Indicators for assessing the efficacy of treatment are discussed.
Owner:EDISON PHARMA
Cultured hematopoietic stem cells and method for expansion and analysis thereof
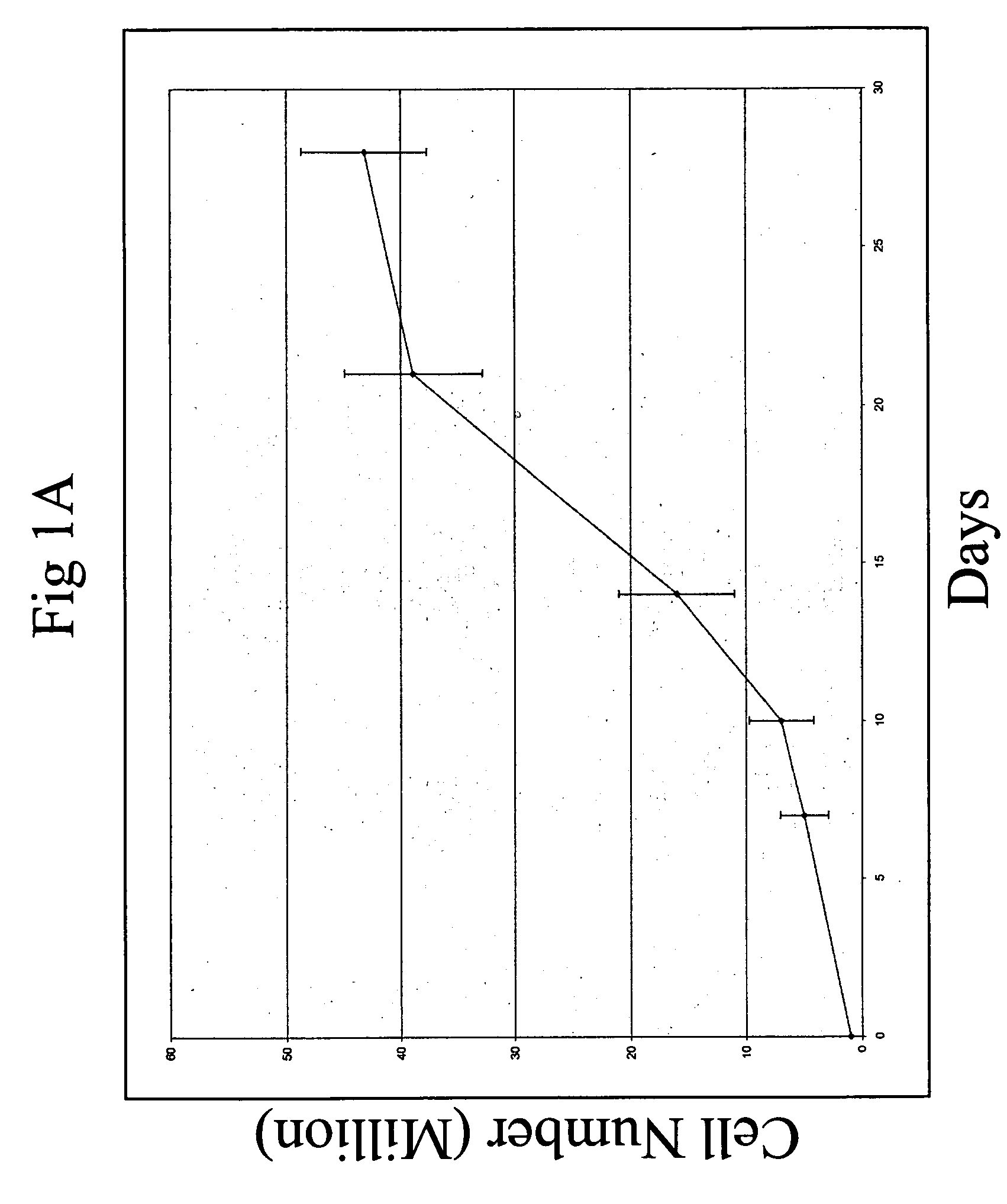
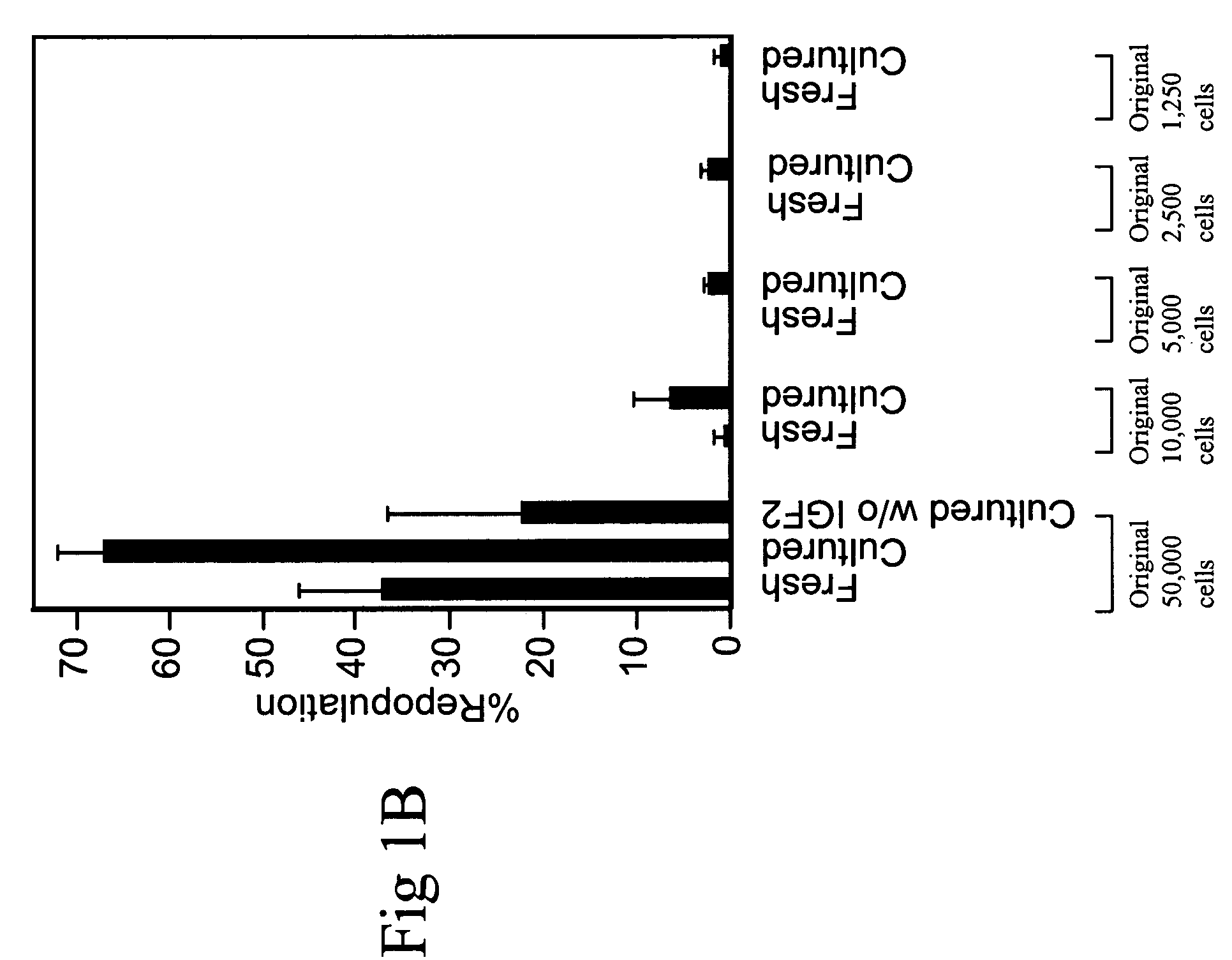
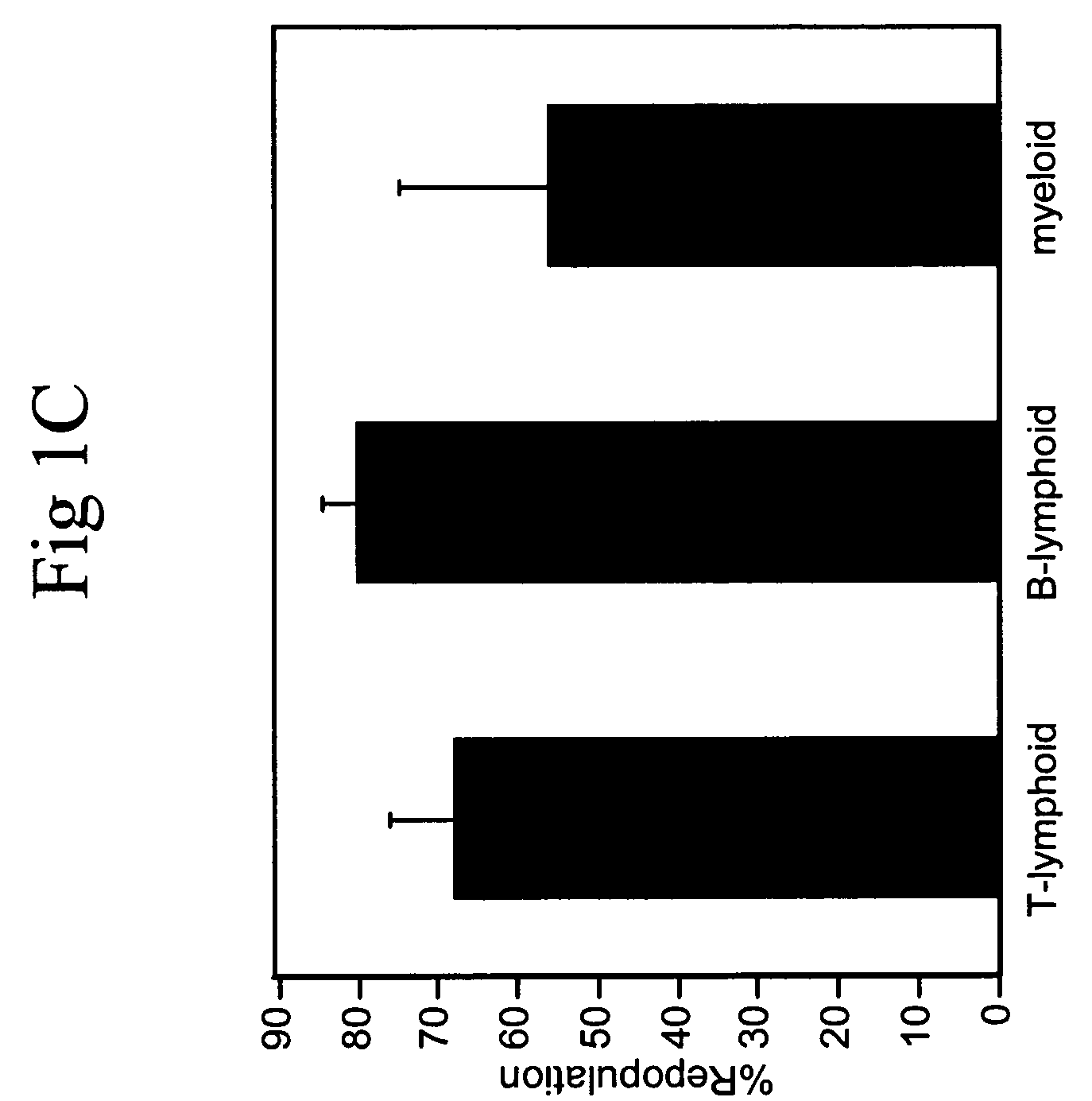
Hematopoietic stem cells and methods for ex vivo expansion of hematopoietic stem cells are provided. The methods comprise culturing the cells in a media containing an effective amount insulin-like growth factor (IGF), fibroblast growth factor (FGF), thrombopoietin (TPO), and stem cell factor (SCF), under conditions sufficient for expansion of said cells. Methods for identifying expanded hematopoeitc stem cells and kits for ex vivo expansion of hematopoietic stem cells are also provided.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES
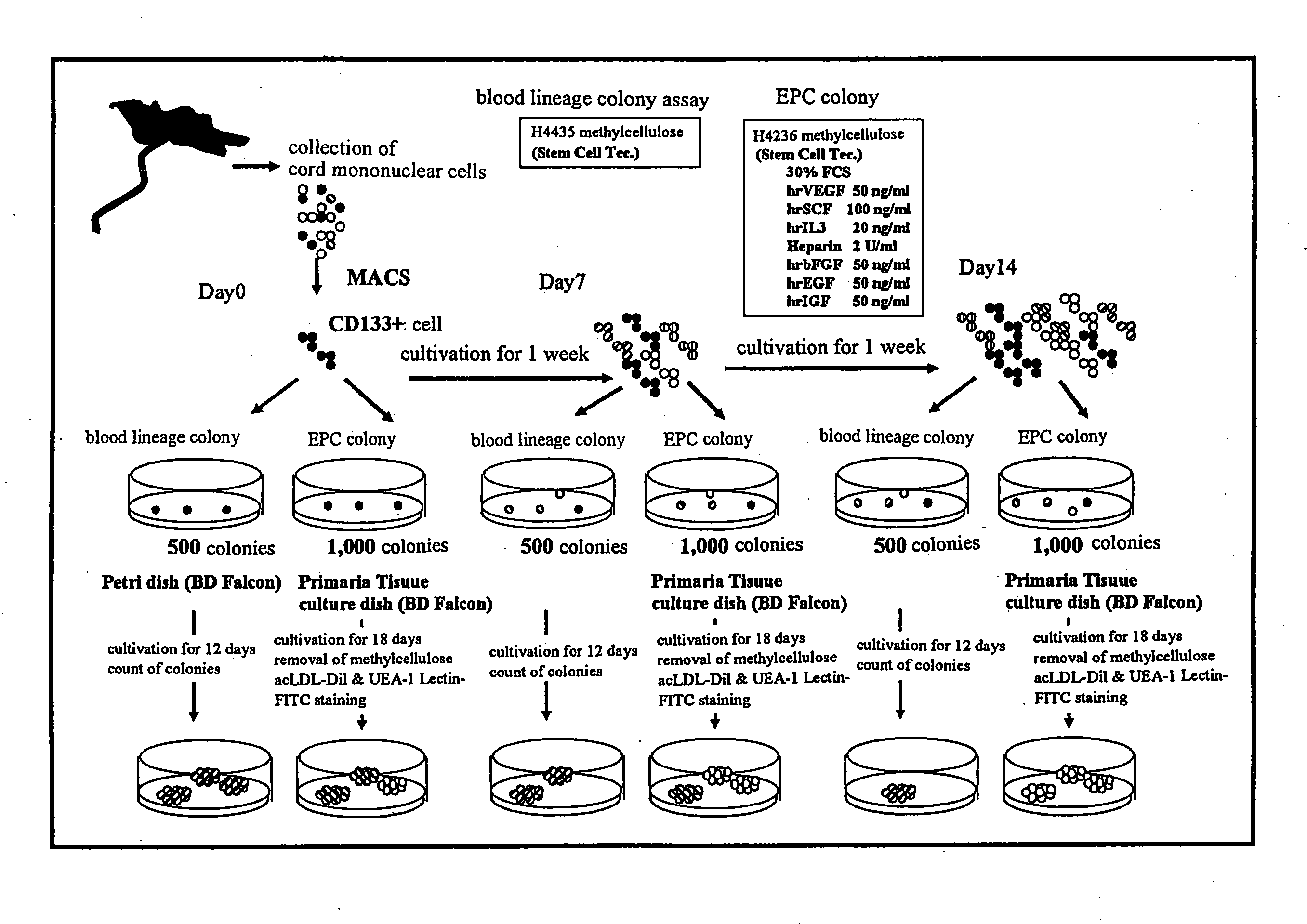
Method for Amplification of Endothelial Progenitor Cell in Vitro
ActiveUS20080166327A1Function increaseImprove heart functionBiocideMammal material medical ingredientsInterleukin 6Fms-Like Tyrosine Kinase 3
The present invention provides a method for expanding an endothelial progenitor cell in vitro. More particularly, the present invention provides a method for culturing a hemangioblast comprising incubating a hemangioblast in a serum-free culture medium containing one or more factors selected from the group consisting of stem cell growth factor, interleukin-6, FMS-like tyrosine kinase 3 and thrombopoietin, and a vascular endothelial cell produced by the method; and a serum-free culture medium containing one or more factors selected from the group consisting of stem cell growth factor, interleukin-6, FMS-like tyrosine kinase 3 and thrombopoietin, and a kit for the preparation of the serum-free culture medium and the like.
Owner:STEMMED +1
Ligand having agonistic activity to mutated receptor
InactiveUS20060189794A1X-ray/infra-red processesImmunoglobulins against cell receptors/antigens/surface-determinantsThrombopoiesisThrombopoietin
The present inventors used antibody engineering techniques to prepare functional antibodies that correspond to individual mutations in causative genes of diseases, and discovered that such antibodies enable the treatment of the diseases. Specifically, the inventors succeeded in preparing ligands, particularly minibodies, which have agonistic activity to receptors that have almost completely lost responsiveness to their natural ligands because of gene mutations (for example, a thrombopoietin (TPO) receptor whose reactivity to TPO has been markedly impaired), and which can transduce signals by interacting with these mutant receptors at levels comparable to normal.
Owner:CHUGAI PHARMA CO LTD
Serum-free culture medium and method for expanding hematopoietic stem cells
InactiveUS20180163177A1Culture processSkeletal/connective tissue cellsVitamin CHematopoietic growth factor
A serum-free culture medium for hematopoietic stem cell (HSC) expansion is provided. The serum-free culture medium includes a serum-free base medium, cytokines, an umbilical cord mesenchymal stem cell conditioned medium and supplemental components. The cytokines comprise stem cell factor, thrombopoietin and hematopoietic growth factor Flt3 ligand. The umbilical cord mesenchymal stern cell conditioned medium is derived from culturing human umbilical cord mesenchymal stem cells. The supplemental components comprise vitamin C, vitamin E or a combination of vitamin C and vitamin E.
Owner:HEALTHBANKS BIOTECH
Thrombopoietin activity modulating compounds and methods
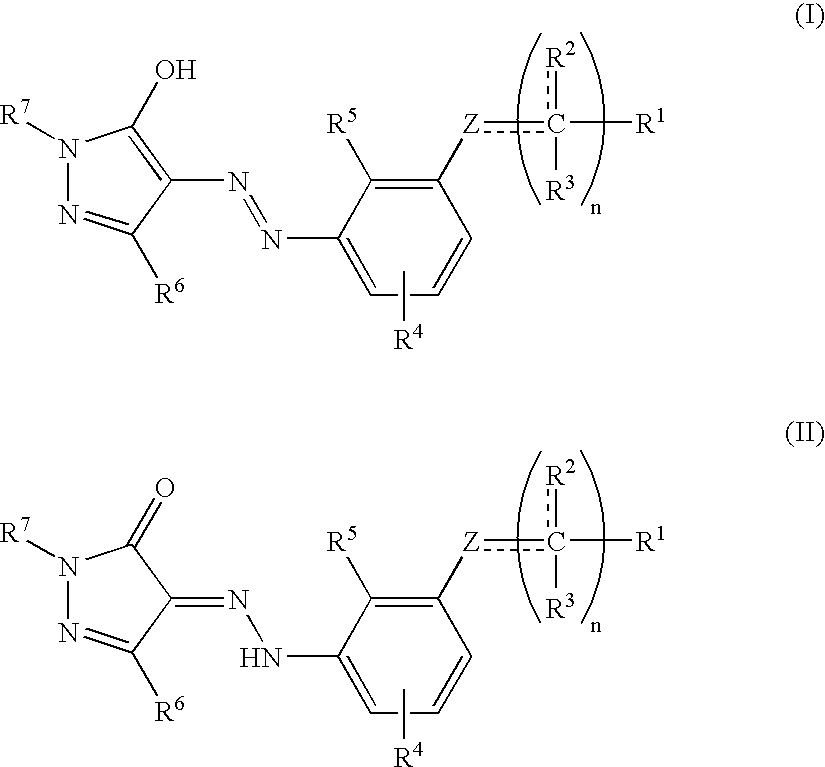
Disclosed herein are novel diazenyl pyrazole compounds and related compounds. Also disclosed herein are methods of using these compounds for the treatment of diseases and conditions associated with modulating a thrombopoietin activity.
Owner:LIGAND PHARMA INC
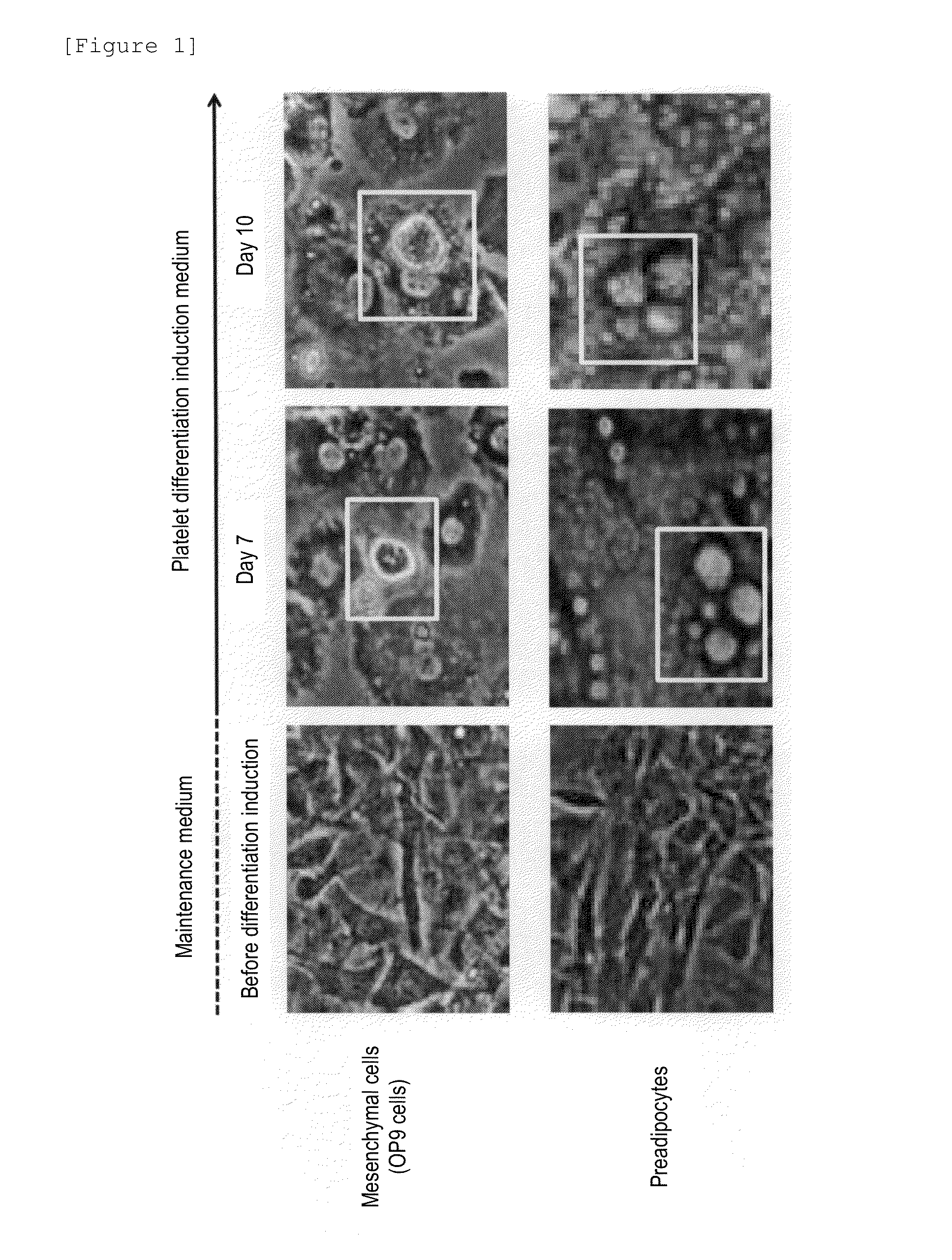
Method for producing megakaryocytes, platelets and/or thrombopoietin using mesenchymal cells
ActiveUS20160177265A1Improve efficiencyLow costCulture processSkeletal/connective tissue cellsMethyl xanthineMegakaryocyte
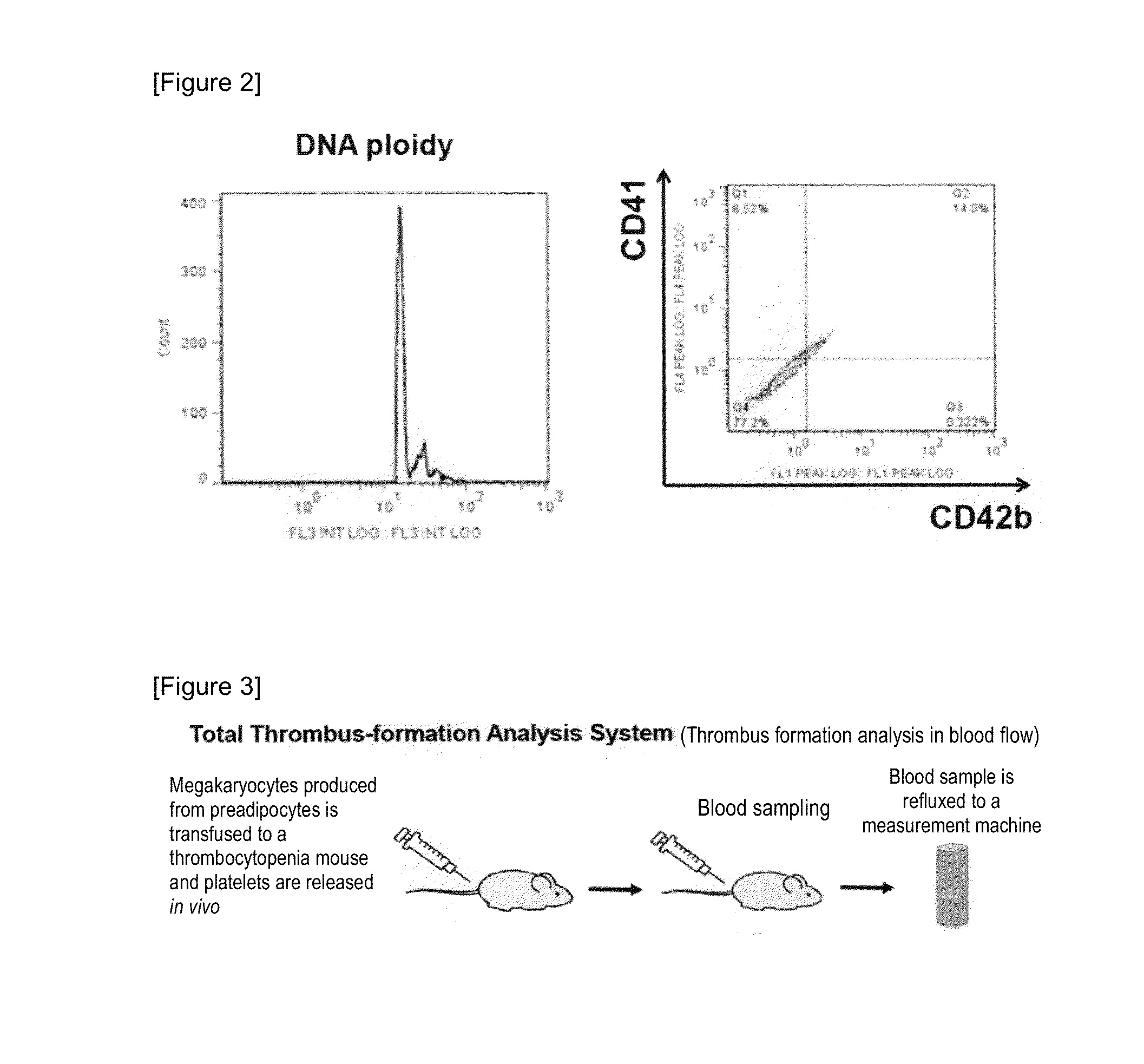
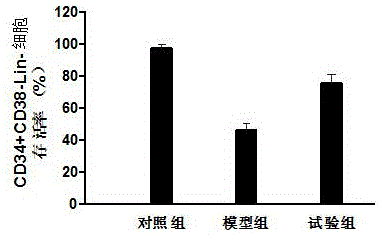
Provided is a megakaryocyte and / or platelet production method, enabling to produce a megakaryocyte and / or platelet from mesenchymal cells such as preadipocytes in a relatively short period of time, simply, in a large amount and at lower cost or more efficiently in vitro and a method for producing TPO simply and in a larger amount. A first invention is a method for producing a megakaryocyte and / or platelet, comprising culturing a mesenchymal cell in a mesenchymal cell culturing basic medium containing an iron ion and an iron transporter and collecting megakaryocytes and / or platelets from a culture. A second invention is a method for producing thrombopoietin, comprising culturing a mesenchymal cell or mesenchymal cell-derived megakaryocyte in a mesenchymal cell culturing basic medium containing an iron ion and an iron transporter and collecting thrombopoietin from a culture. A third invention is a method for producing thrombopoietin, comprising culturing a preadipocyte in a preadipocyte culturing basic medium containing dexamethasone, 3-isobutyl-1-methylxanthine and insulin and collecting thrombopoietin from a culture.
Owner:ADIPOSEEDS INC
Ex-vivo expansion culture medium of umbilical cord blood hematopoietic stem cells and application thereof
ActiveCN105112374AMaintain self-renewal abilityHigh proliferation rateBlood/immune system cellsReceptorUmbilical cord
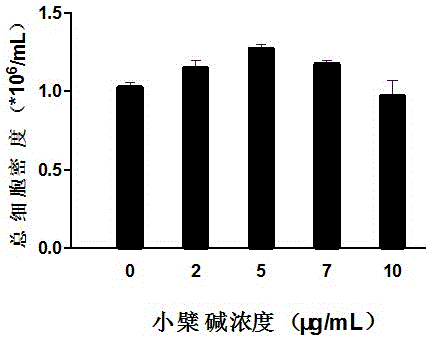
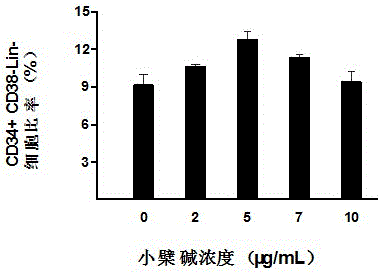
The invention belongs to the technical field of stem cells, and relates to an ex-vivo expansion culture medium of umbilical cord blood hematopoietic stem cells and an application thereof. The ex-vivo expansion culture medium comprises an IMEM basal culture medium, serum, thrombopoietin, stem cell factors, FMS-liketyrosinc kinase 3 ligand, interleukin-1, interleukin-6, stem cell growth factors and berberine. The ex-vivo expansion culture medium of the umbilical cord blood hematopoietic stem cells has the advantages of being high in hematopoietic stem cell proliferation rate, capable of significantly improving the implantation capacity of the hematopoietic stem cells transplanted into a receptor and the reconstruction capacity of a hematopoietic system and capable of well maintaining the properties of the hematopoietic stem cells and is an ideal ex-vivo expansion culture medium of the umbilical cord blood hematopoietic stem cells.
Owner:广东美赛尔细胞生物科技有限公司
Cytokines and cytokine receptors with reduced immunogenicity
InactiveUS20050220800A1Low immunogenicityReduced immunogenic responsePeptide/protein ingredientsTissue cultureTumor necrosis factor receptorBiology
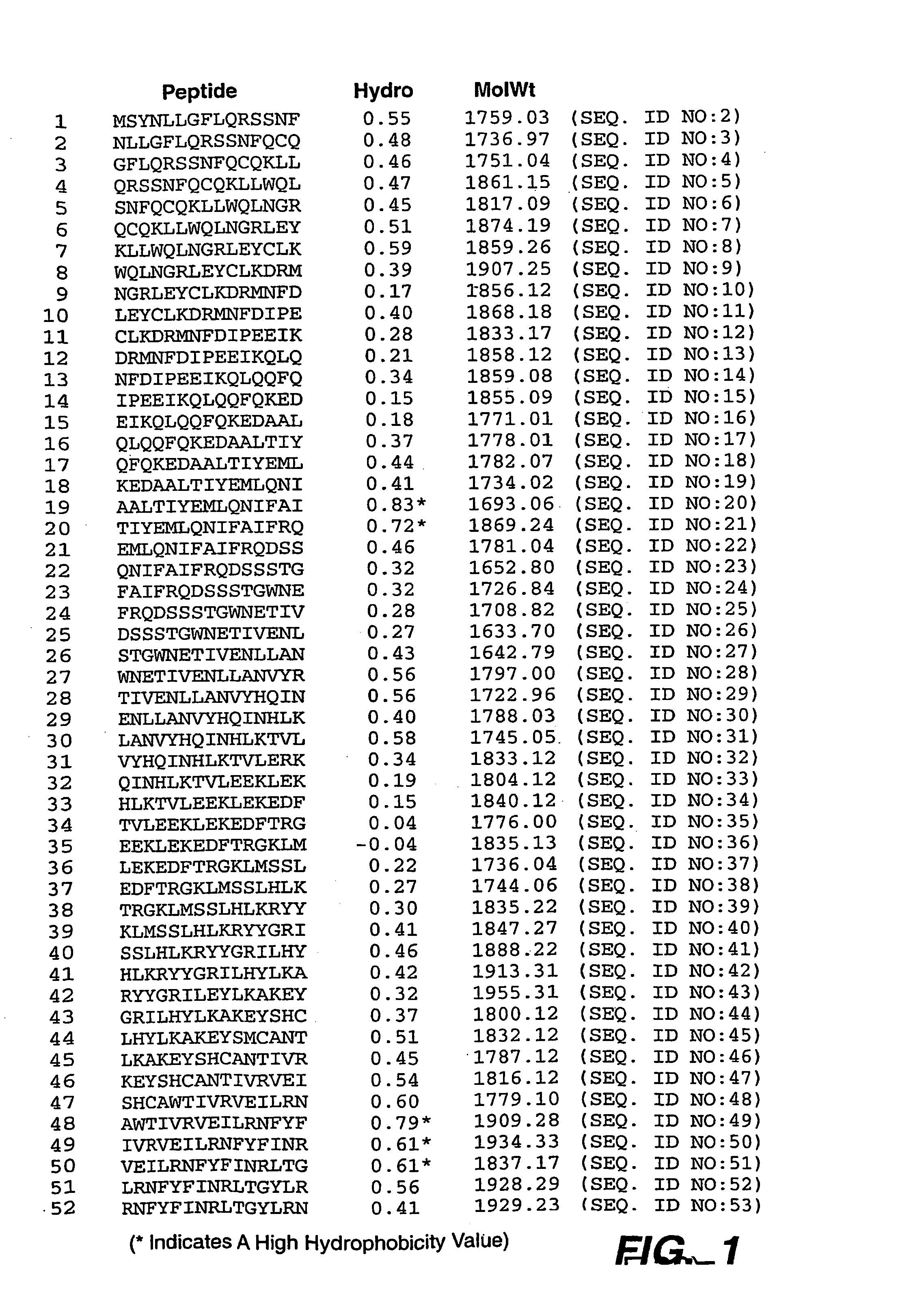
The present invention provides methods for the identification of CD4+ T-cell epitopes in the sequences of various proteins, namely, human cytokines and cytokine receptors, as well as the production of peptides which when incorporated into the protein sequence, are no longer capable of initiating the CD4+ T-cell response. In some embodiments, the present invention provides means and compositions suitable for reducing the immunogenicity of cytokines and cytokines receptors such as interferon-β, soluble tumor necrosis factor receptor-1, erythropoietin, and thrombopoietin.
Owner:SCOTT POWER D +1
Culture system for amplification of hematopoietic stem cells of cord blood and applications thereof
InactiveCN102080065AImproving the effect of implantationImprove proliferative abilityBlood/immune system cellsCord blood stem cellHematopoietic cell
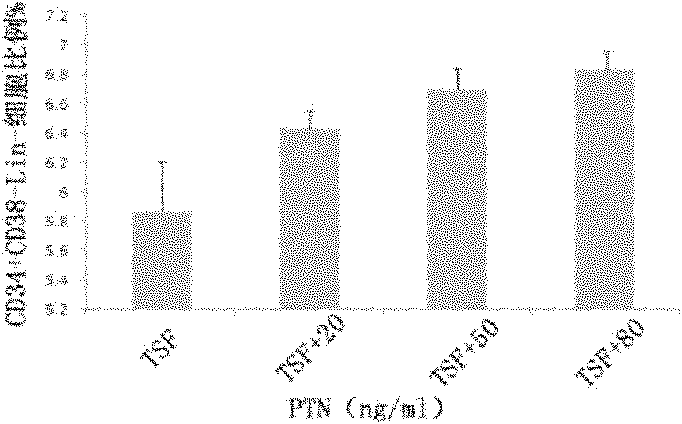
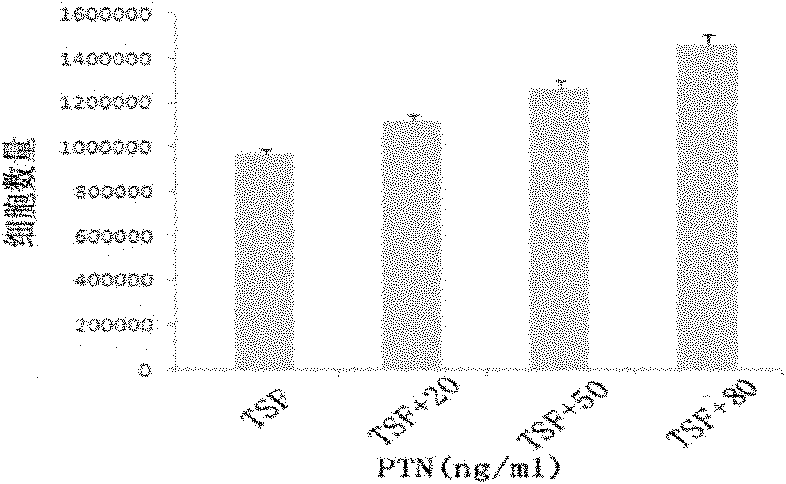
The invention discloses a culture system for the amplification of hematopoietic stem cells of cord blood and applications thereof. The culture system contains a 1640 culture medium, fetal bovine serum (FBS), thrombopoietin, stem cell factor (SCF) and human FMS tyrosine kinase 3 ligand Flt-3L and also contains pleiotrophin PTN. A proper concentration of PTN is added in the existing hematopoietic stem cell medium to prepare the culture system which can support the growth and amplification of the hematopoietic stem cells of cord blood more effectively in vitro and particularly increase the implanting capability of hematopoietic stem cells after being implanted in a receptor obviously and the reconstruction capability of the hematopoietic system. Therefore, new application prospects are opened up for the clinical applications of the stem cells of cord blood.
Owner:UNION STEMCELL & GENE ENG
Compounds exhibiting thrombopoietin-like activities
InactiveUS6887890B2Excellent thrombopoietic actionLow antigenicityBiocidePeptide/protein ingredientsArylHydrogen atom
The compounds of the invention are compounds represented by the following general formula (1): wherein E represents one selected from the group consisting of a methylidyne group and a nitrilo group, R1 represents one selected from the group consisting of optionally substituted aryl groups and optionally substituted heteroaryl groups, R2 represents one selected from the group consisting of a hydrogen atom and alkyl groups, W1 represents an amino acid residue, A represents one selected from the group consisting of a carbonyl group and a sulfonyl group, X1 represents one selected from the group consisting of optionally substituted alkylene groups and optionally substituted alkenylene groups, and p represents 0 or 1;and their pharmacologically acceptable salts, which exhibit thrombopoietin-like activity.
Owner:CHUGAI PHARMA CO LTD
Preparation and application of dimerized fusion protein
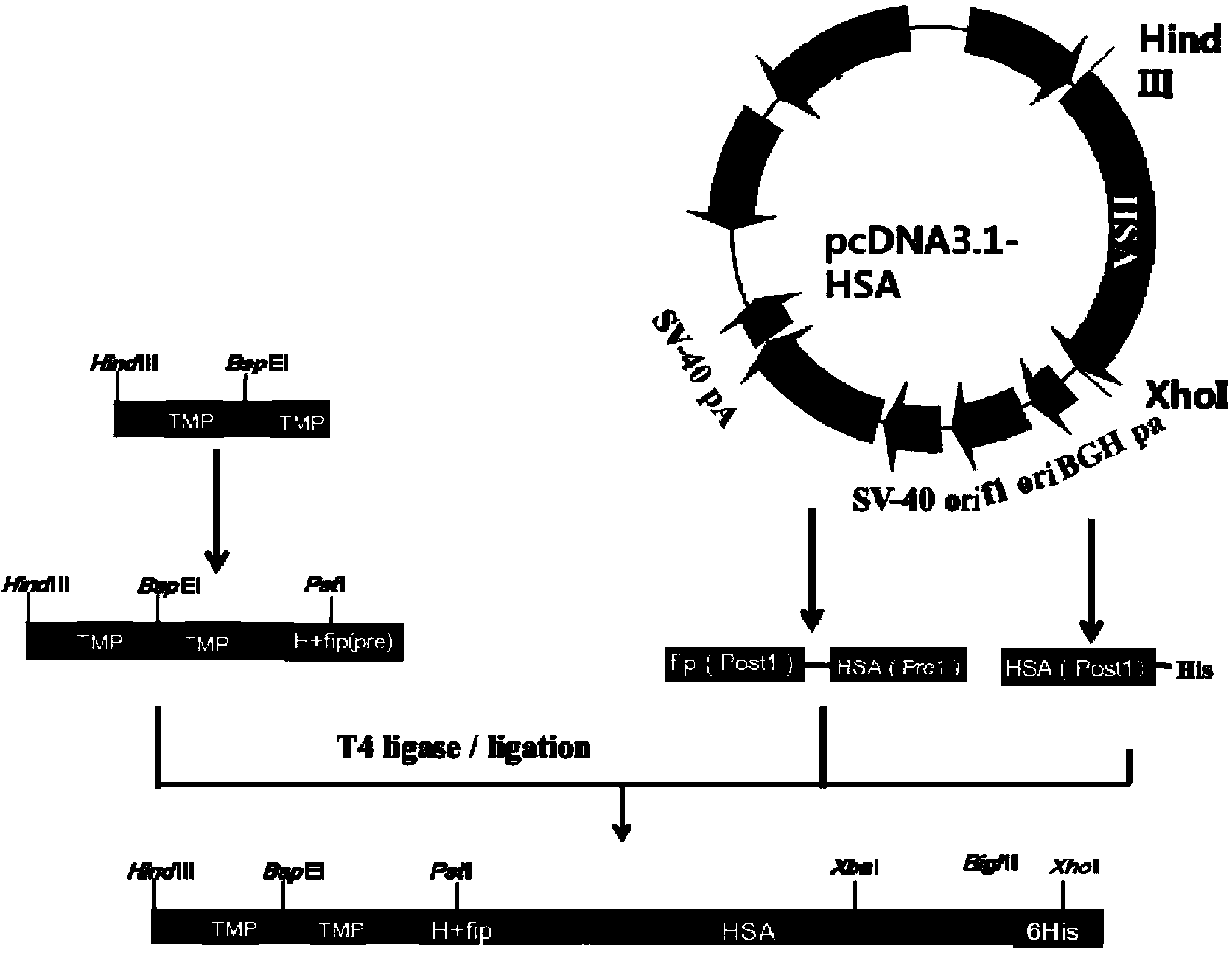
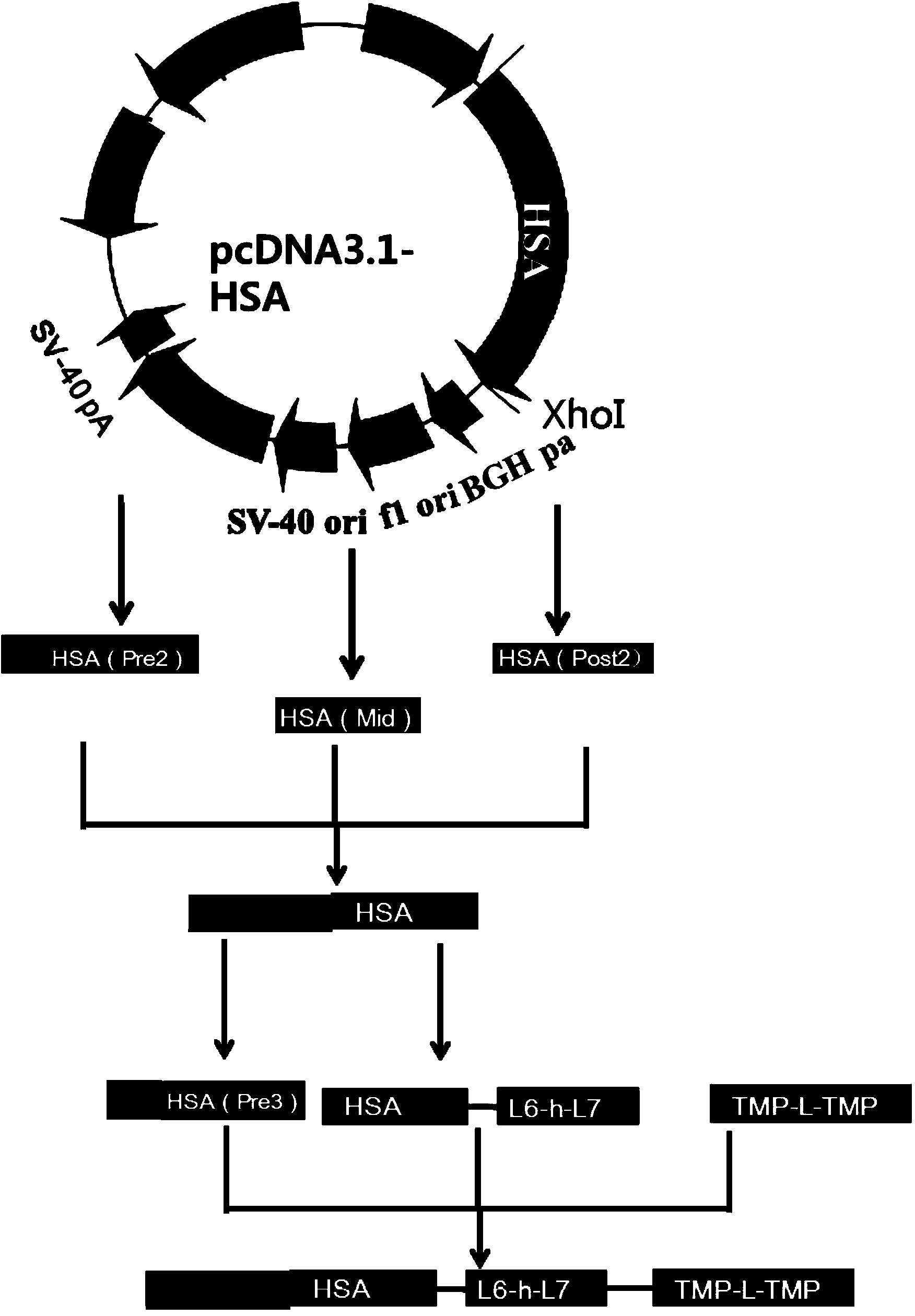
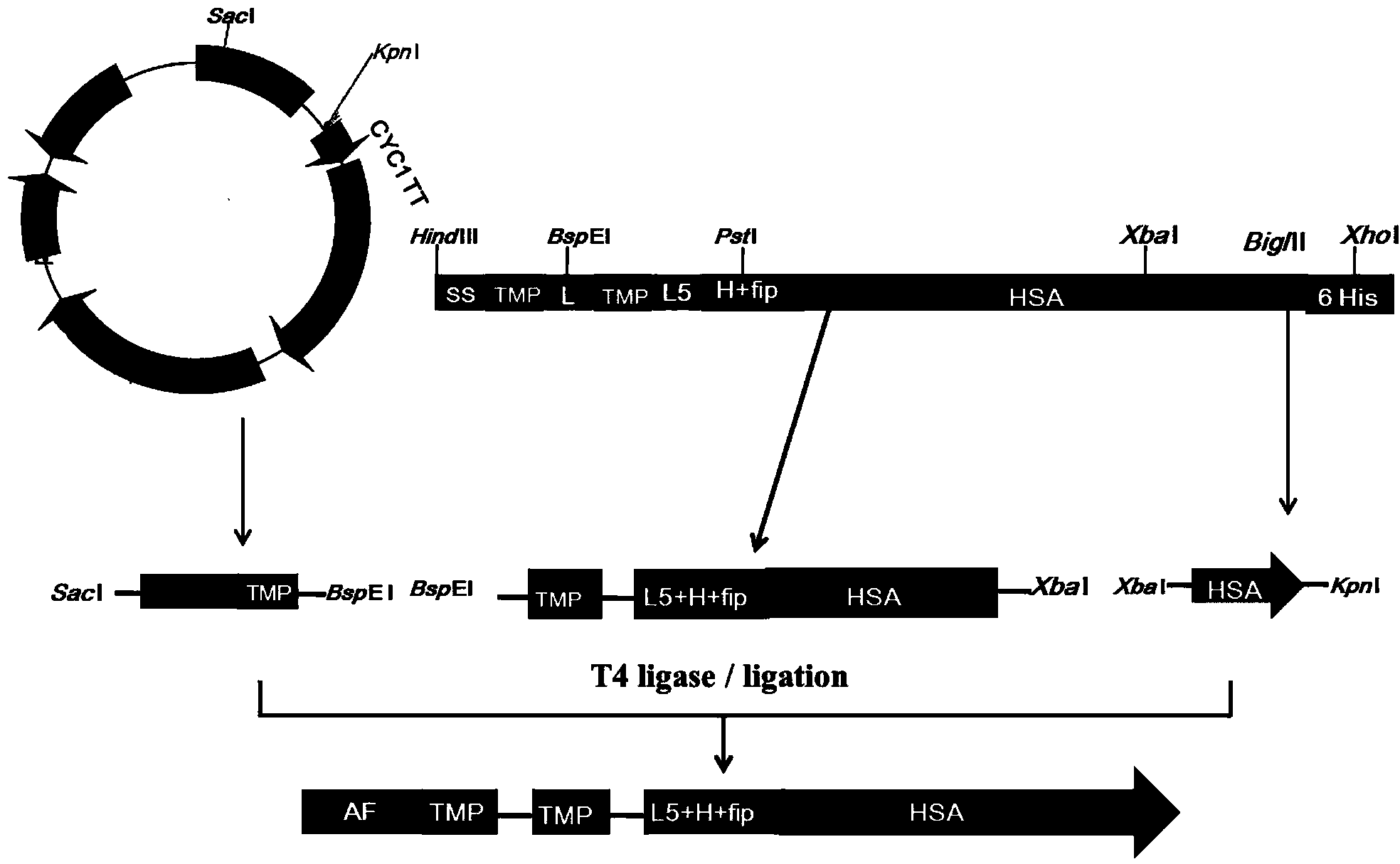
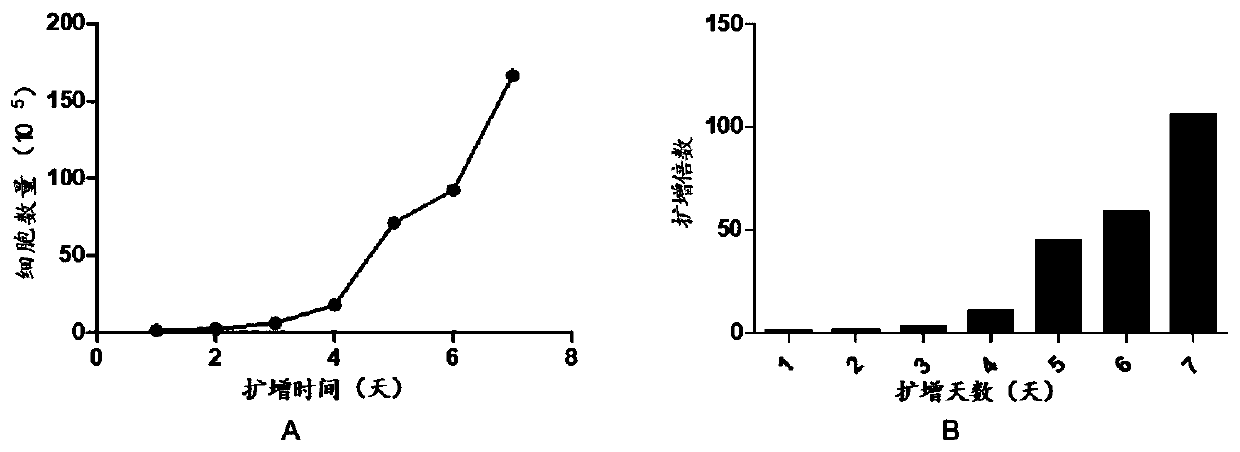
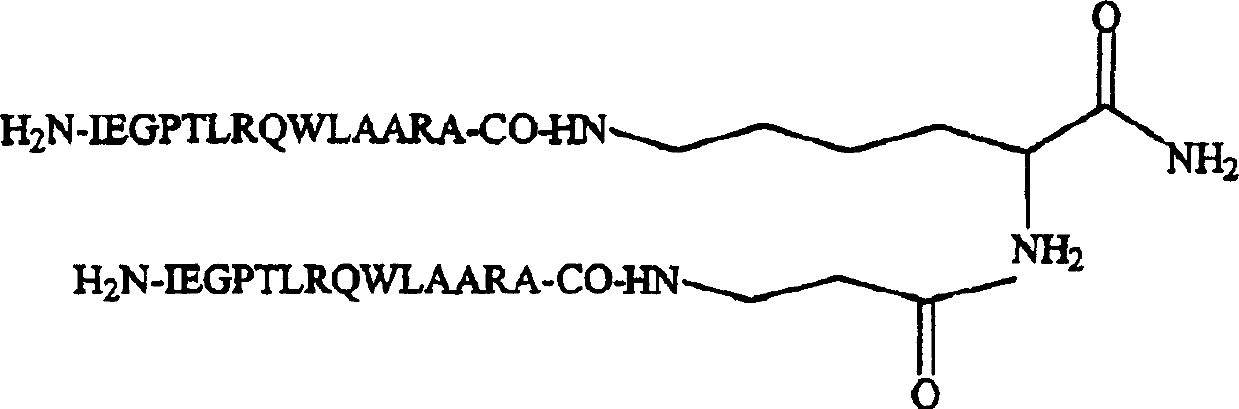
The invention discloses preparation and application of a dimerized thrombopoietin (TPO) mimic peptide TMP diad-human serum albumin fusion protein. The fusion protein contains an HAS and two TPO mimic peptides which are connected through a linkage oligopeptide and a dimerization domain so as to ensure the fusion protein is dimerized correctly when expression. The two TPO mimic peptides can be connected with N terminal or C terminal of HAS. The dimerized HAS protein carrier and the TPO mimic peptide fusion protein provided by the invention have a significant activity of promoting thrombopoiesis in vivo and can be applied in preparation of drugs for treating primary and secondary thrombocytopenia diseases.
Owner:LANZHOU UNIVERSITY
Stem cell amplifying culture medium and stem cell culture method
InactiveCN110628718AGood amplification effectUse low concentrationBlood/immune system cellsCell culture active agentsInterleukin 6Cord blood stem cell
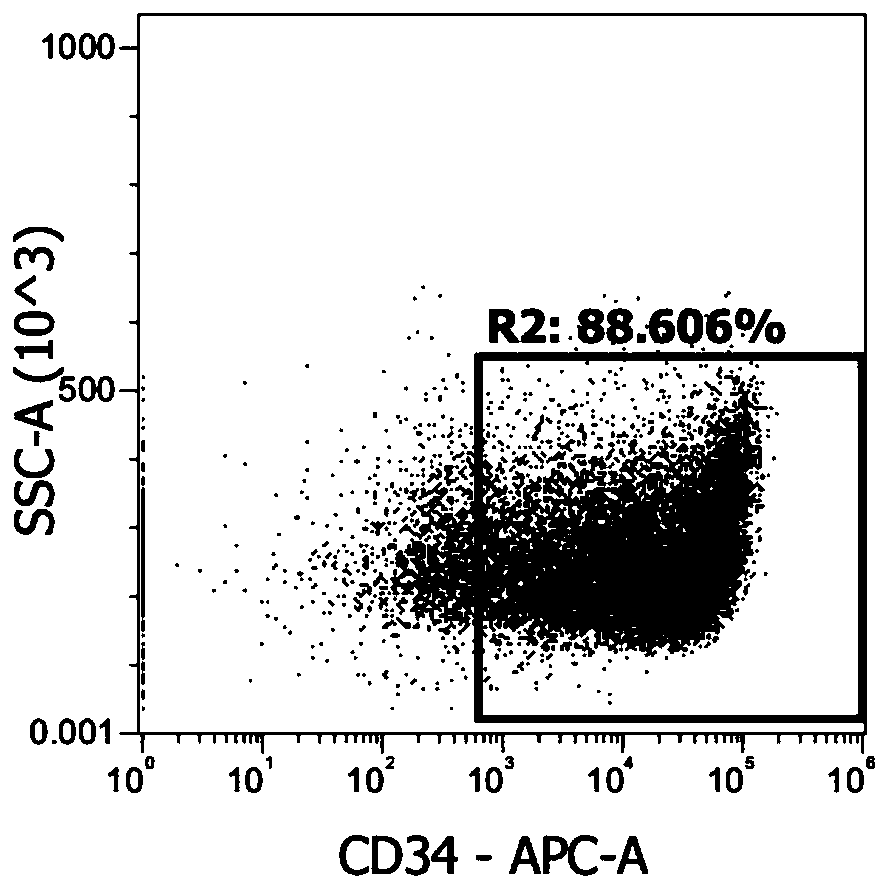
The invention relates to a stem cell amplifying culture medium and a stem cell culture method. The ingredients of the stem cell amplifying culture medium comprise a basic culture medium, a stem cell factor, thrombopoietin, an FMS like tyrosine kinase 3 ligand and interleukin-6, wherein the final concentration of the stem cell factor is 90ng / mL-110ng / mL, the final concentration of the thrombopoietin is 10ng / mL-30ng / mL, the final concentration of the FMS like tyrosine kinase 3 ligand is 90ng / mL-110ng / mL, and the final concentration of the interleukin-6 is 10ng / mL-30ng / mL. When the stem cell amplifying culture medium is used for culturing cord blood hematopoietic stem cells, obvious cell amplifying effects can be presented, CD34+ on cell surfaces is also notably increased, during cell amplification, the cell state is stable, the multi-directional differentiation capacity is maintained, and the culture medium is an economic efficient culture system.
Owner:THE THIRD AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIVERSITY
Dimeric thrombopoietin peptide mimetics binding to MP1 receptor and having thrombopoietic activity
Owner:AMGEN K A INC
Modified thrombopoietin with reduced immunogenicity
InactiveUS20040071688A1Improve accuracyIncrease the number ofPeptide/protein ingredientsHydrolasesHuman plateletThrombopoietin
The present invention relates to polypeptides to be administered especially to humans and in particular for therapeutic use. The polypeptides are modified polypeptides whereby the modification results in a reduced propensity for the polypeptide to elicit an immune response upon administration to the human subject. The invention in particular relates to the modification of human thrombopoietin (TPO) to result in TPO proteins that are substantially non-immunogenic or less immunogenic than any non-modified counterpart when used in vivo.
Owner:MERCK PATENT GMBH
Culture medium of umbilical cord mesenchymal stem cells
InactiveCN108865989APromote growthImprove securityCulture processSkeletal/connective tissue cellsArginineUmbilical cord
The invention provides a culture medium of umbilical cord mesenchymal stem cells. The culture medium comprises a DMEM (Dulbecco's Modified Eagle Medium) / F12 culture medium, an adding component added into the DMEM / F12 culture medium and ivy extract, wherein the adding component is prepared from the following components: human serum albumin, transferrin, a vascular endothelial growth factor, a basicfibroblast growth factor, arginine, a leukemia inhibitory factor, a stem cell factor, beta cytokine, parathyroid hormone, a platelet-derived growth factor, a tumor necrosis factor alpha, interleukin,erythropoietin, thrombopoietin, alpha-D-glucose, vitamin C, resveratrol, NaHCO3 and ganglioside; the ivy extract is liquid and is extracted from leaves of ivy through a conventional method; the finalconcentration is 20 to 50mg / L after the ivy extract is added. By adopting the culture medium, the problems in the prior art are solved, and the cells can keep good state and proliferation speed.
Owner:QINGDAO RESTORE BIOTECHNOLOGY CO LTD
Method For Amplification And Functional Enhancment Of Blood Derived Progenitor Cells Using A Closed Culture System
InactiveUS20120003738A1Safer and more feasible and cost-effectiveEfficient expansionCulture processArtificial cell constructsProgenitorInterleukin 6
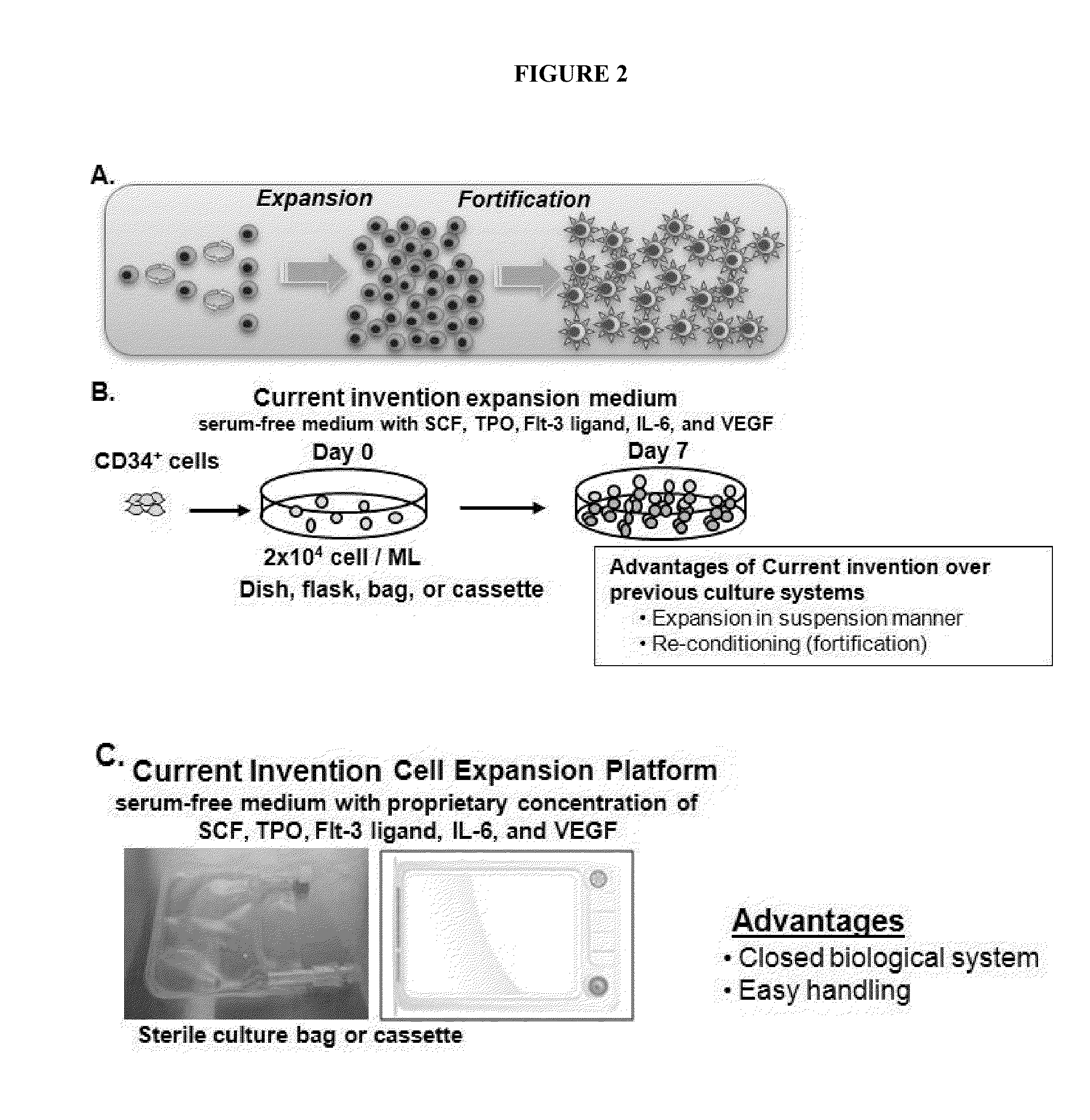
The present invention provides a method for expanding and improving functional capacity of human adult-derived progenitor cells in vitro using a closed culture system. The present invention provides a favorable condition for cell therapy to promote tissue repair and organogenesis via vasculogenesis and angiogenesis in clinical settings. The proposed closed bag culture system for culturing hemangioblast comprises of, in one embodiment, a serum-free culture medium containing one or more factors selected from the group consisting of stem cell growth factor, interleukin-6, FMS-like tyrosine kinase 3, thrombopoietin, and vascular endothelial growth factor and a kit for the preparation of the serum-free culture medium and the like.
Owner:UNIVERSITY HOSPITALS OF CLEVELAND CLEVELAND
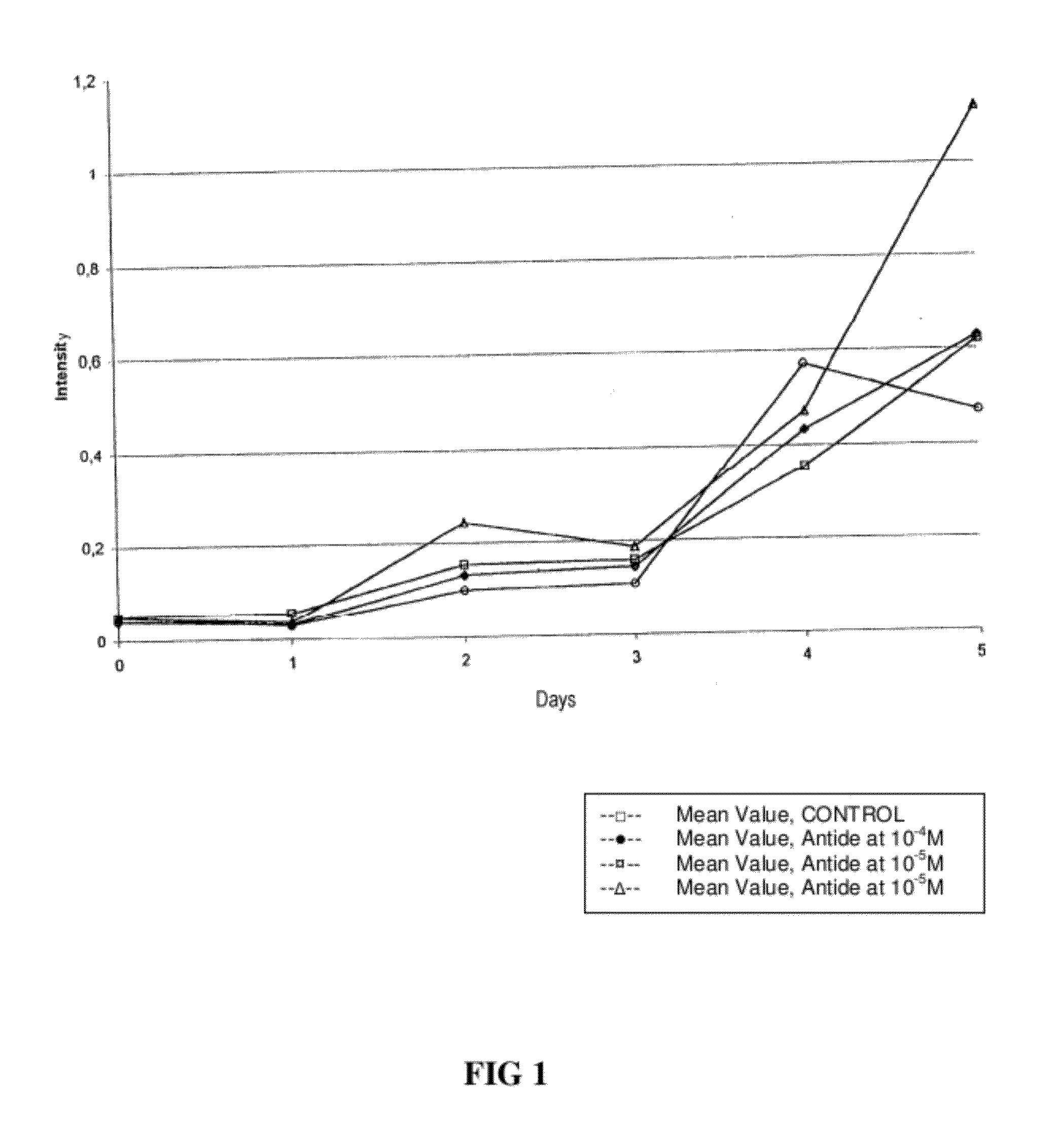
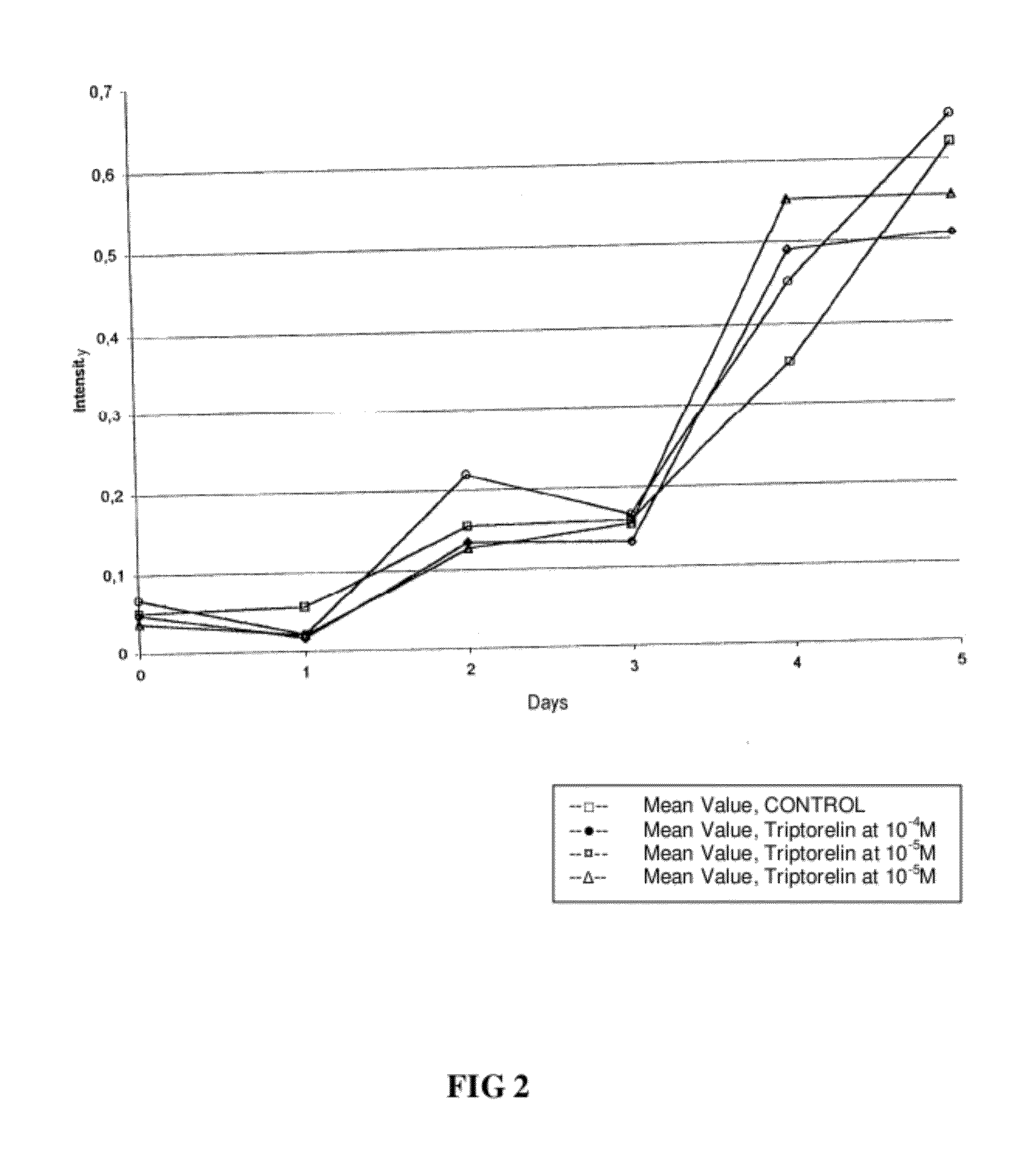
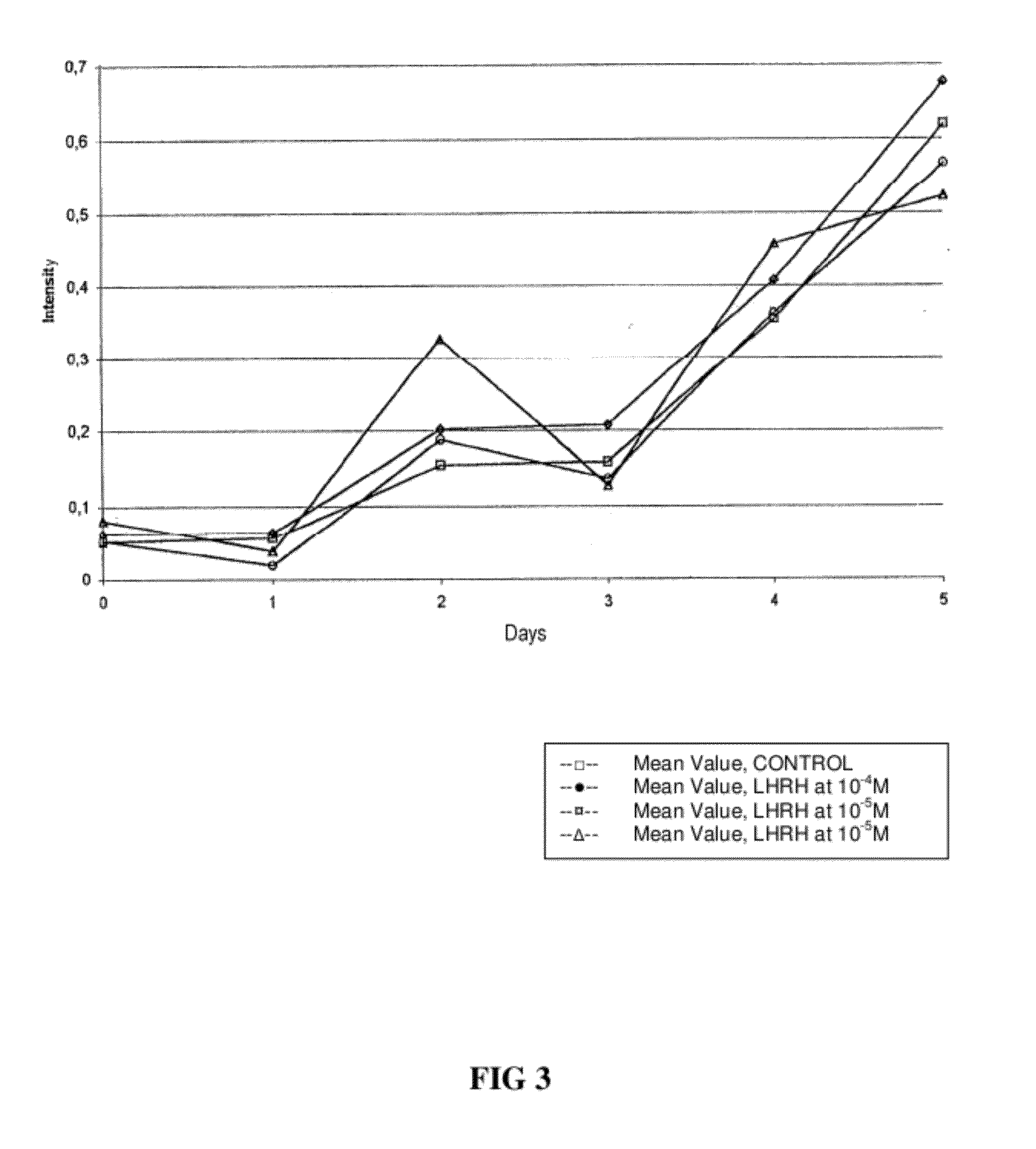
Methods for reducing gnrh-positive tumor cell proliferation
InactiveUS20100190692A1Reduce time delayPromote formationCompounds screening/testingBiocideGerm layerNervous system
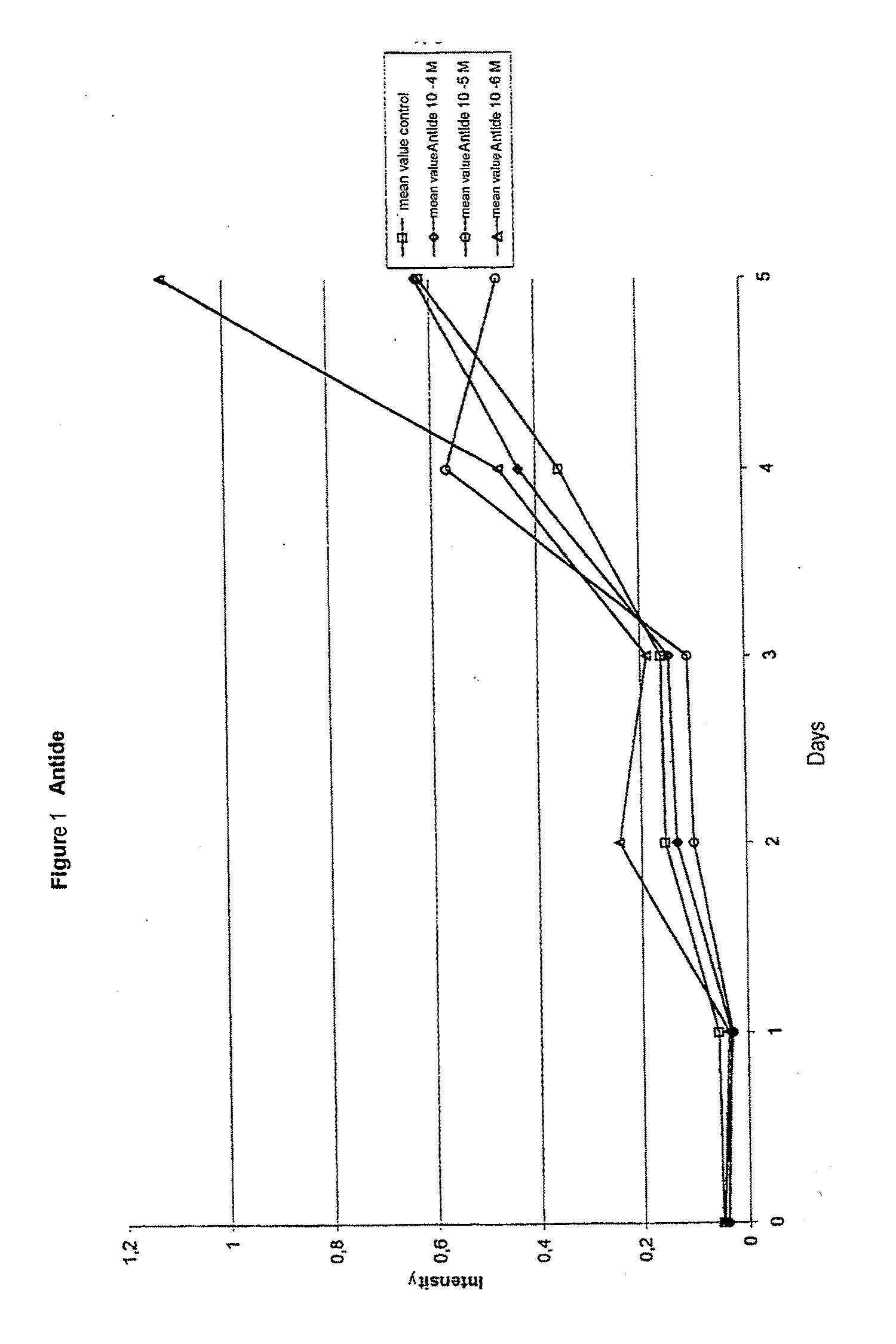
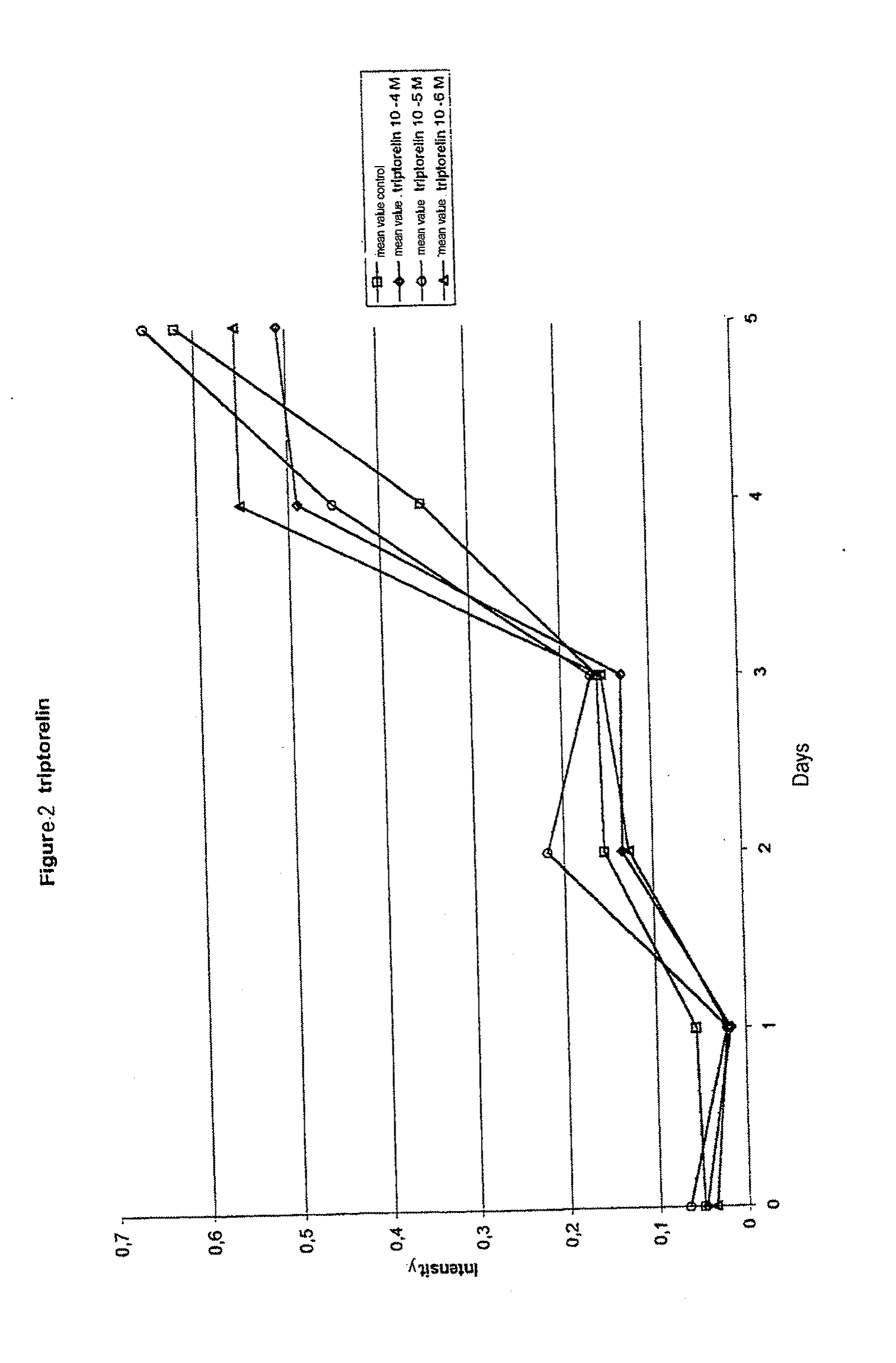
A method for recognizing and evaluating the presence and function of GnRH receptors on tumor cells originating in the brain and / or nervous system and / or the meninges and / or reactive neuroglia cells and / or primitive neuroectodermal tumor cells and / or on Kaposi sarcoma is provided. Furthermore a method for reducing degenerate GnRH-positive tumor cells and / or for decreasing cellular replication of the above GnRH-positive tumor cells comprising administering to a cell or to a subject a replication decreasing amount of a GnRH agonist and / or GnRH antagonist and / or an erythropoietin agonist, and / or a thrombopoietin agonist, and / or a endothelin antagonist and / or a gonadotropin inhibiting hormone agonist is also provided. Furthermore, a diagnostic kit for detecting GnRH receptors on tumor cells according to the present methods is disclosed.
Owner:VAN GROENINGHEN JOHANNES C
Fusion protein with reinforced erythrocytin activity in vivo
InactiveCN1421461AImprove in vivo activityThrombopoietinPeptide/protein ingredientsIn vivoC-terminus
Owner:CJ HEALTHCARE CORP
Stem cell in-vitro multiplication culture system and method
InactiveCN109370988AProlonged proliferation cycleIncrease profitCulture processBlood/immune system cellsCytokineNicotinamide
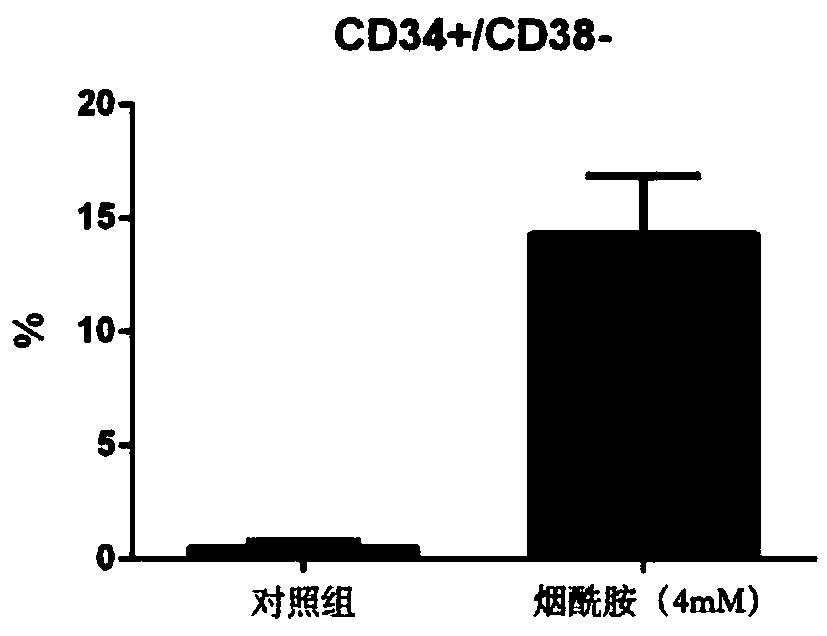
The invention relates to a stem cell in-vitro multiplication method. A multiplication culture system containing niacinamide substances is adopted, a serum-free culture medium is used as a basic culture medium, and 8%-12% of fetal bovine serum, a cytokine composition and niacinamide substances are added. The cytokine composition includes a stem cell factor, thrombopoietin, an FLt3 ligand, G-CSF, IL-3 and IL-6. Compared with conventional culture systems, the culture system can effectively increase the number of hematopoietic stem cells and the cell proportion of CD34+ / Lin- and CD34+ / CD38- when culturing CD34+ cells for 1-12 weeks and is helpful to solving of the problem of insufficient cells in hematopoietic stem cell transplantation.
Owner:章毅 +7
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
InactiveUS20130230871A1Easy to adaptDisease diagnosisBiological testingAbnormal macrophageInterleukin 5
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using a one or more assays configured to detect a kidney injury marker selected from the group consisting of Interleukin-5, Interleukin-6 receptor subunit beta, Tissue factor, Sex hormone-binding globulin, Alpha-2-macroglobulin, Apolipoprotein A-I, Calcitonin, Thrombopoietin, C-reactive protein, Intercellular adhesion molecule 3, Macrophage metalloelastase, Apolipoprotein B-100, and Fibrinogen as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
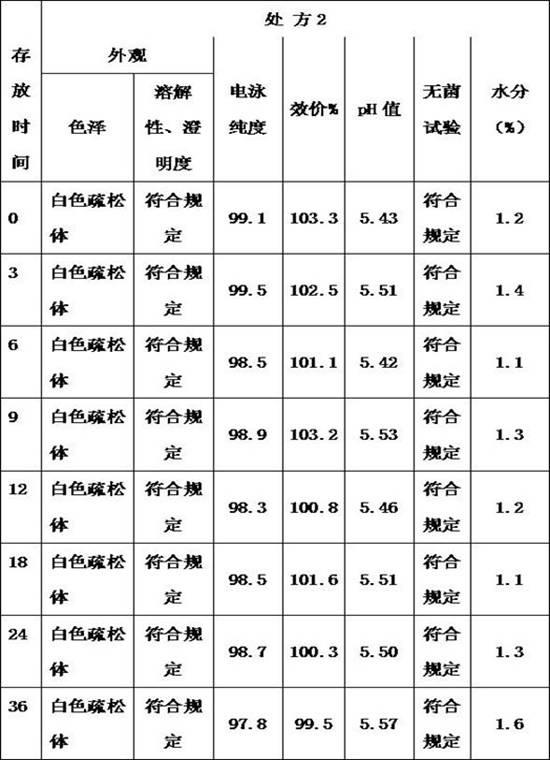
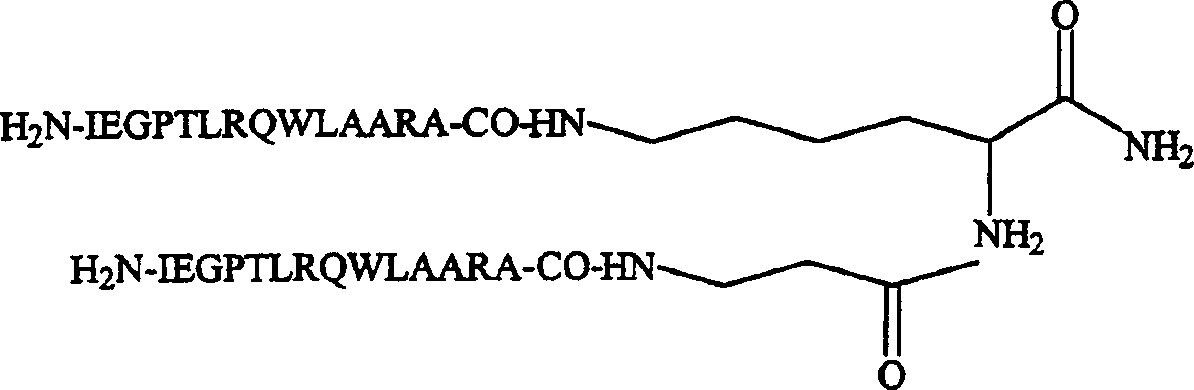
Thrombopoietin peptide analogue freeze-dried preparation
InactiveCN102552184AStable storageSuitable for industrial productionPowder deliveryPeptide/protein ingredientsGlycineMANNITOL/SORBITOL
The invention provides a thrombopoietin peptide analogue freeze-dried preparation. The freeze-dried preparation is obtained by dissolving thrombopoietin and medicinal auxiliary materials into a medicinal buffering liquid of which the pH is 5-6.0, wherein the protein concentration of the thrombopoietin is 0.1-1 mg / ml; the medicinal auxiliary materials include mannite, glycine and tween 80; the mass concentration of the mannite is 2-8 percent; the mass concentration of the glycine is 1-5 percent; and the volume concentration of the tween 80 is 0.001-0.01 percent. The freeze-dried preparation containing a thrombopoietin peptide analogue has the advantages of high efficiency, easiness, convenience, low cost and suitability for industrial production.
Owner:山东泉港药业有限公司
Methods for reducing gnrh-positive tumor cell proliferation
InactiveUS20120238494A1Reduce time delayPromote formationBiocidePeptide/protein ingredientsGerm layerPresent method
A method for recognizing and evaluating the presence and function of GnRH receptors on tumor cells including those originating in the brain and / or nervous system and / or the meninges and / or reactive neuroglia cells and / or primitive neuroectodermal tumor cells and / or on Kaposi sarcoma is provided. Furthermore, a method for reducing degenerate GnRH-positive tumor cells and / or for decreasing cellular replication of the above GnRH-positive tumor cells comprising administering to a cell or to a subject a replication decreasing amount of a GnRH agonist and / or GnRH antagonist and / or an erythropoietin agonist, and / or a thrombopoietin agonist, and / or a endothelin antagonist and / or a gonadotropin inhibiting hormone agonist is also provided. Furthermore, a diagnostic kit for detecting GnRH receptors on tumor cells according to the present methods is disclosed.
Owner:VAN GROENINGHEN JOHANNES C
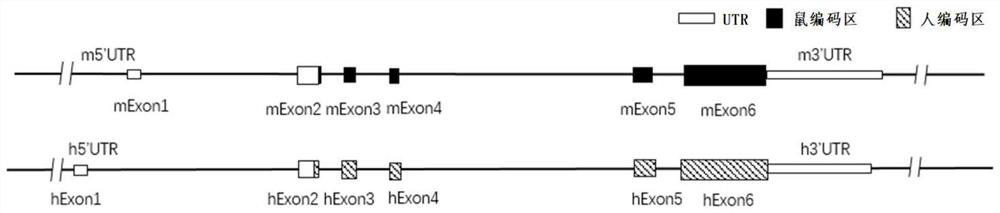
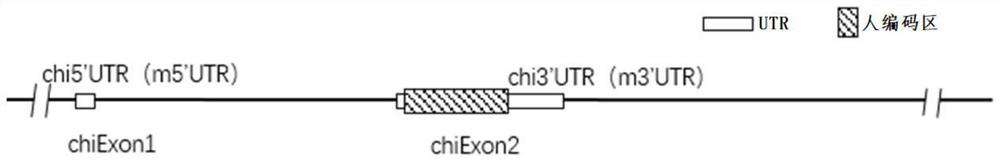
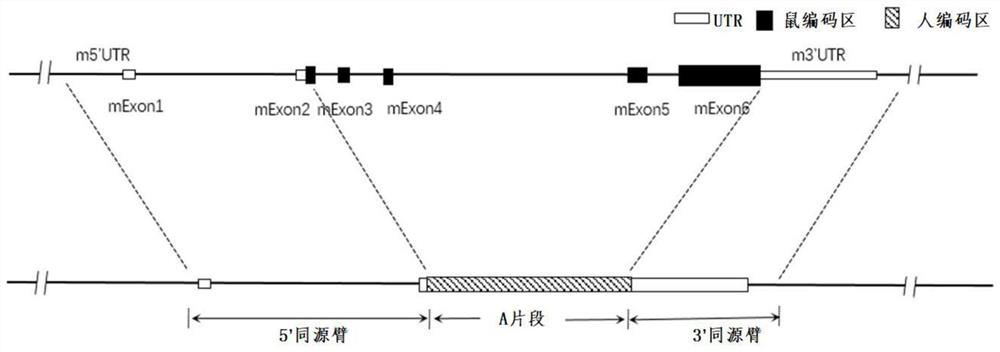
Construction method and application of thrombopoietin (THPO) gene humanized non-human animal
The invention provides a construction method and application of a thrombopoietin (THPO) gene humanized non-human animal. The method comprises the following steps: introducing a sequence for encoding human THPO protein into an animal genome by using a homologous recombination manner; the animal can normally express the human or humanized THPO protein in vivo, to promote the development of human T cells and NK cells; the non-human animal can be used as an animal model for human THPO signal mechanism research and drug screening for diseases including tumors and the like, and has important application value for new drug research and development of immune targets. The invention further provides a targeting vector of a THPO gene, sgRNA of the target THPO gene and application of the targeting vector and the sgRNA to preparation of a humanized THPO gene.
Owner:BIOCYTOGEN PHARMACEUTICALS (BEIJING) CO LTD
Dimeric thrombopoietin peptide mimetics binding to MP1 receptor and having thrombopoietic activity
Owner:AMGEN INC
Fusion protein having enhanced in vivo activity of erythropoietin
ActiveUS7098318B2Prolong half-life in vivoHigh activityPeptide/protein ingredientsAntibody mimetics/scaffoldsHuman bodyHalf-life
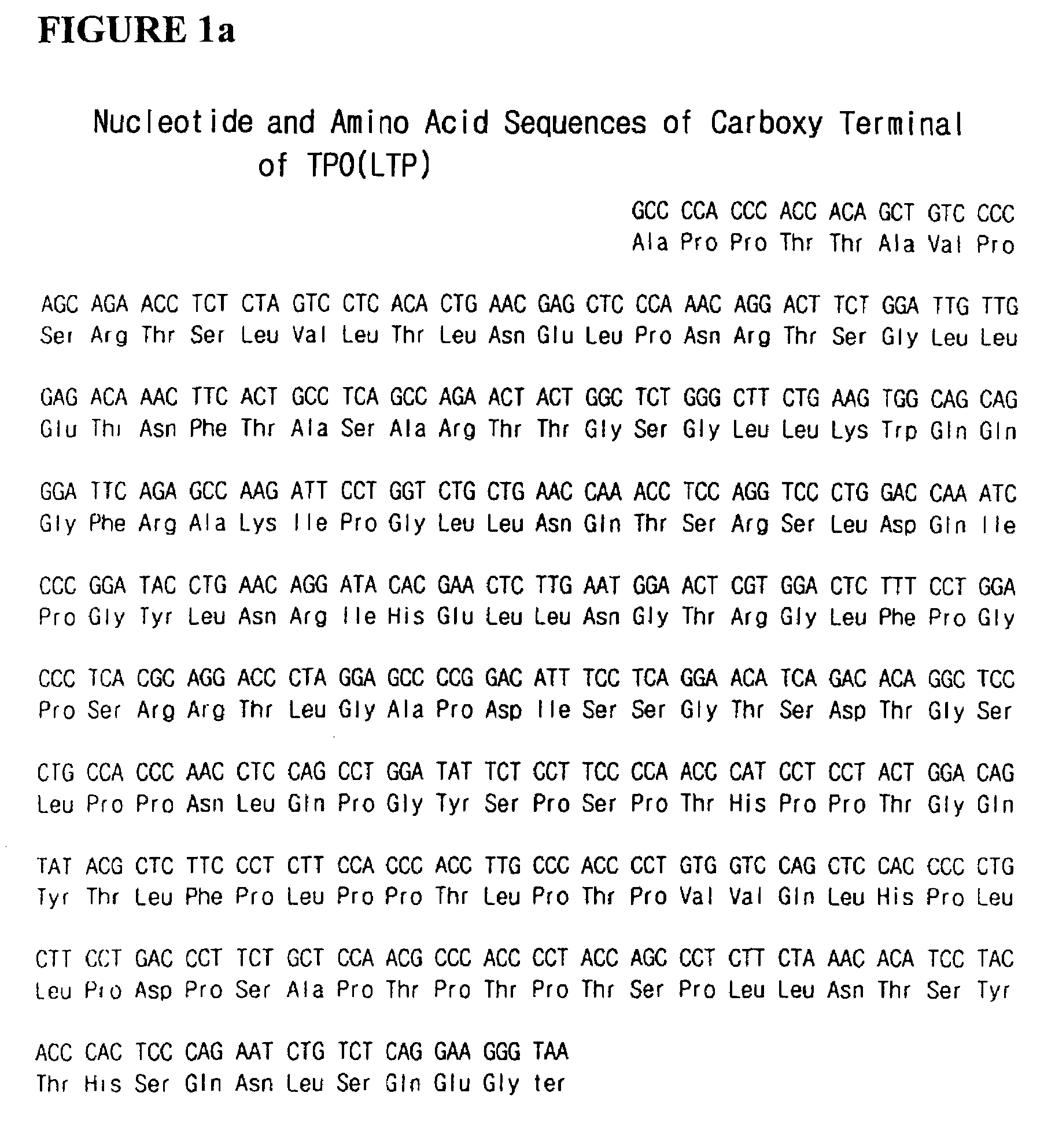
The present invention relates to a fusion protein having enhanced in vivo activity of erythropoietin wherein a carboxy terminal peptide fragment of thrombopoietin is fused with the carboxy terminal of human erythropoietin. This fusion protein has highly enhanced in vivo half-life due to increased carbohydrate content without loss of the inherent activity of erythropoietin, and does not cause any antigenicity when applied to the human body.
Owner:CJ HEALTHCARE CORP
Method for preparing recombinant glycoproteins with high sialic acid content
ActiveCN102482674AIncreased sialic acid contentThrombopoietinCell receptors/surface-antigens/surface-determinantsArginineTert-leucine
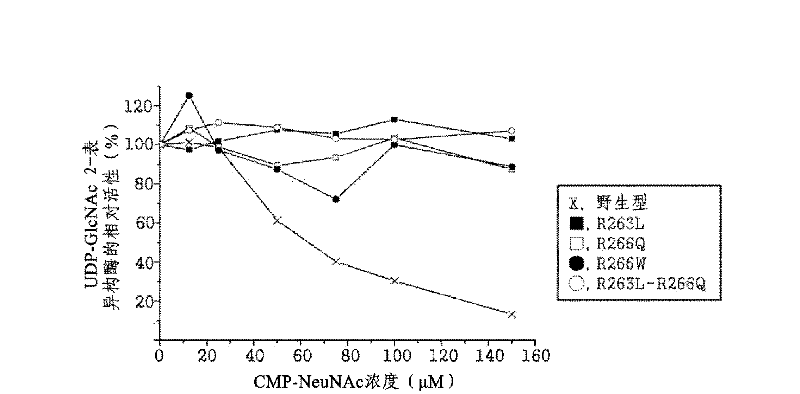
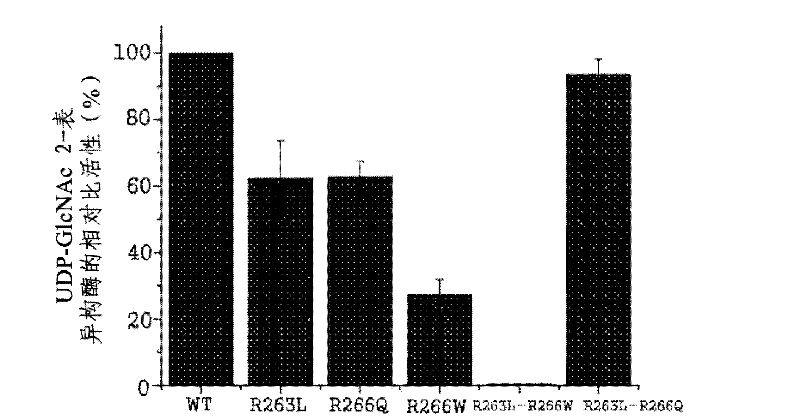
The present disclosure relates to a method for preparing recombinant glycoproteins with high sialic acid content. More specifically, for UDP-GlcNAc 2-epimerase / ManNAc kinase (GNE / MNK) enzyme where point mutation was induced by substituting arginine at position 263 by leucine only or by further substituting arginine at position 266 by glutamine, epimerase activity is constantly maintained, and overexpressed cells thereof experience an increase in intracellular cytidine monophosphate (CMP)-sialic acid content, irrespective of CMP-sialic acid concentration.,Particularly, since in an glycoprotein(such as, erythropoietin and thrombopoietin)-producing host cell where point mutationinduced GNE / MNK, human alpha-2,3-sialyltransferase and a CMP-sialic acid transporter gene are simultaneously overexpressed, intracellular content of CMP-sialic acid and sialic acid in glycoprotein increases in cells, overexpression in a host cell producing a sialylated recombinant glycoprotein the three genes above may be useful for preparing glycoprotein with increased sialic acid content.
Owner:KOREA ADVANCED INST OF SCI & TECH
Recombinant targeted infuse protein for promoting thrombocytopoiesis and its preparation method
ActiveCN1737012AThrombocytopeniaImprove bioavailabilityPeptide/protein ingredientsHybrid peptidesDiseaseSecondary thrombocytopenia
The invention provides a fusion protein produced through restructuring targeted thrombopoietin (TPO), which comprises a TPO simulated peptide region and a human growth hormone polypeptide region, bridge joint peptides are arranged between the two regions. The invention also provides the preparing method and use of the fusion protein.
Owner:ARMY MEDICAL UNIV +1
Ophthalmic neuromyelitis spectrum disease biomarker group, application thereof, protein chip and kit
ActiveCN111521812AOvercome operabilityOvercoming detectionDisease diagnosisBiological testingHerpesvirus entry mediatorPancreatic hormone
The invention belongs to the technical field of biological medicines and particularly relates to a biological marker group for optic neuromyelitis spectrum diseases, application of the biological marker group, a protein chip and a kit. The invention provides an optic neuromyelitis spectrum disease biomarker group which is characterized by comprising monocyte chemotactic protein-3, LIGHT, macrophage inflammatory protein-1 delta, insulin-like growth factor-2, a glucocorticoid induced tumor necrosis factor receptor, thrombopoietin and a herpes virus entry medium, wherein the LIGHT is a lymphotoxin analogue which can be induced and expressed on a T cell and competes with glycoprotein D of HSV to be combined with HVEM. The application fills the blank that no reliable and accurate product and method for diagnosing and identifying optic neuromyelitis lineage diseases exist clinically at present.
Owner:RAYBIOTECH INC GUANGZHOU
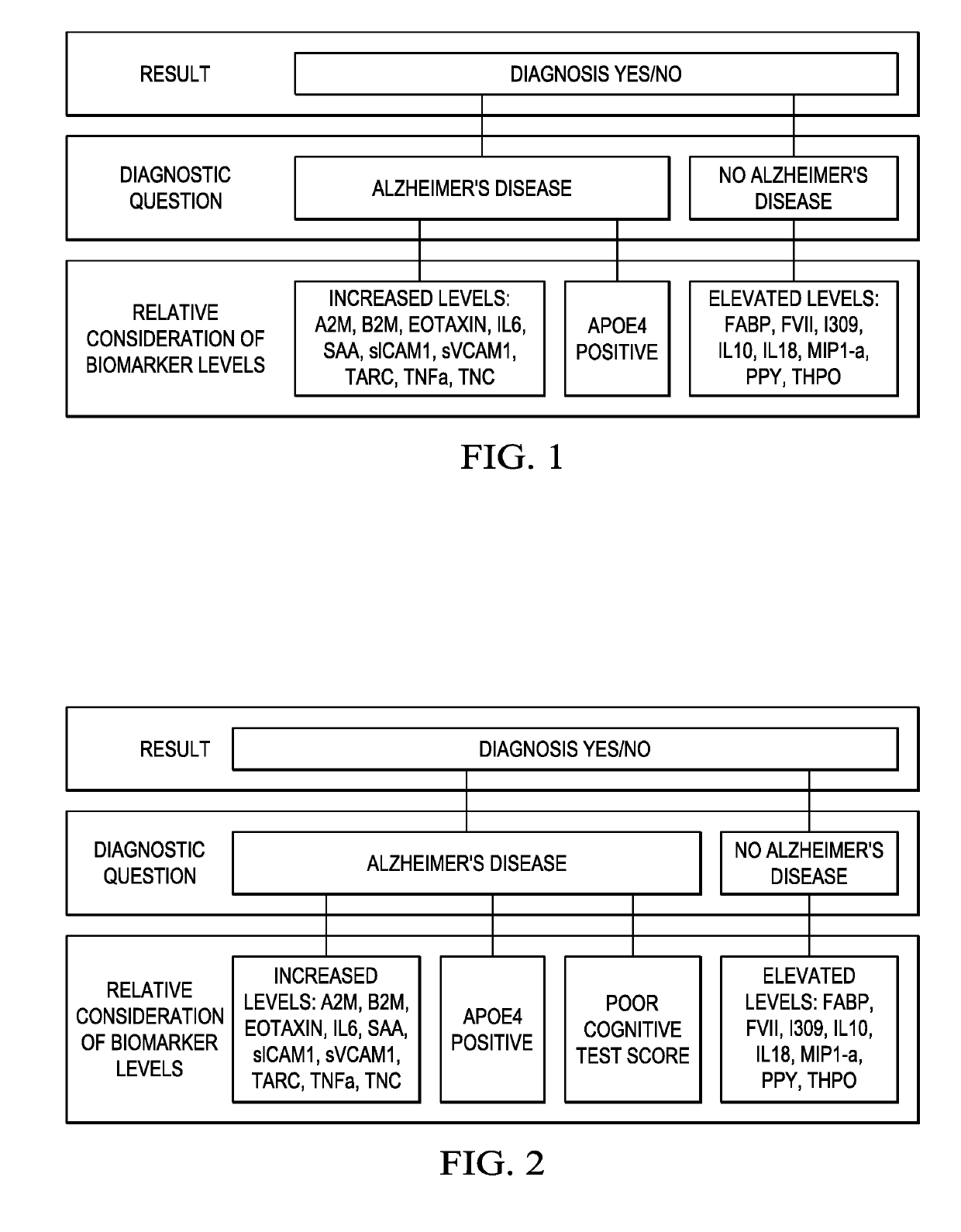
Blood Test for Screening Out Amyloid and Alzheimers Disease Presence
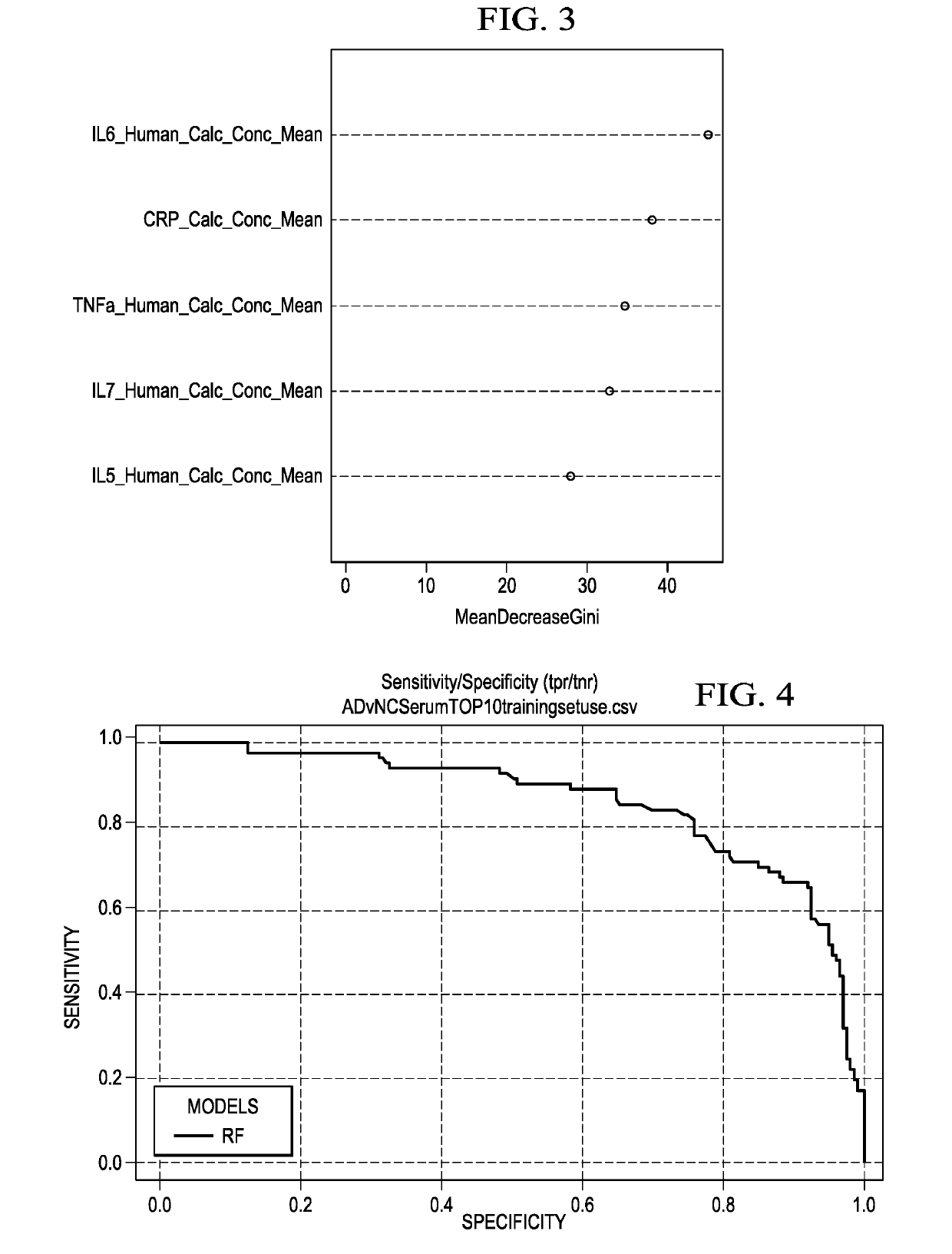
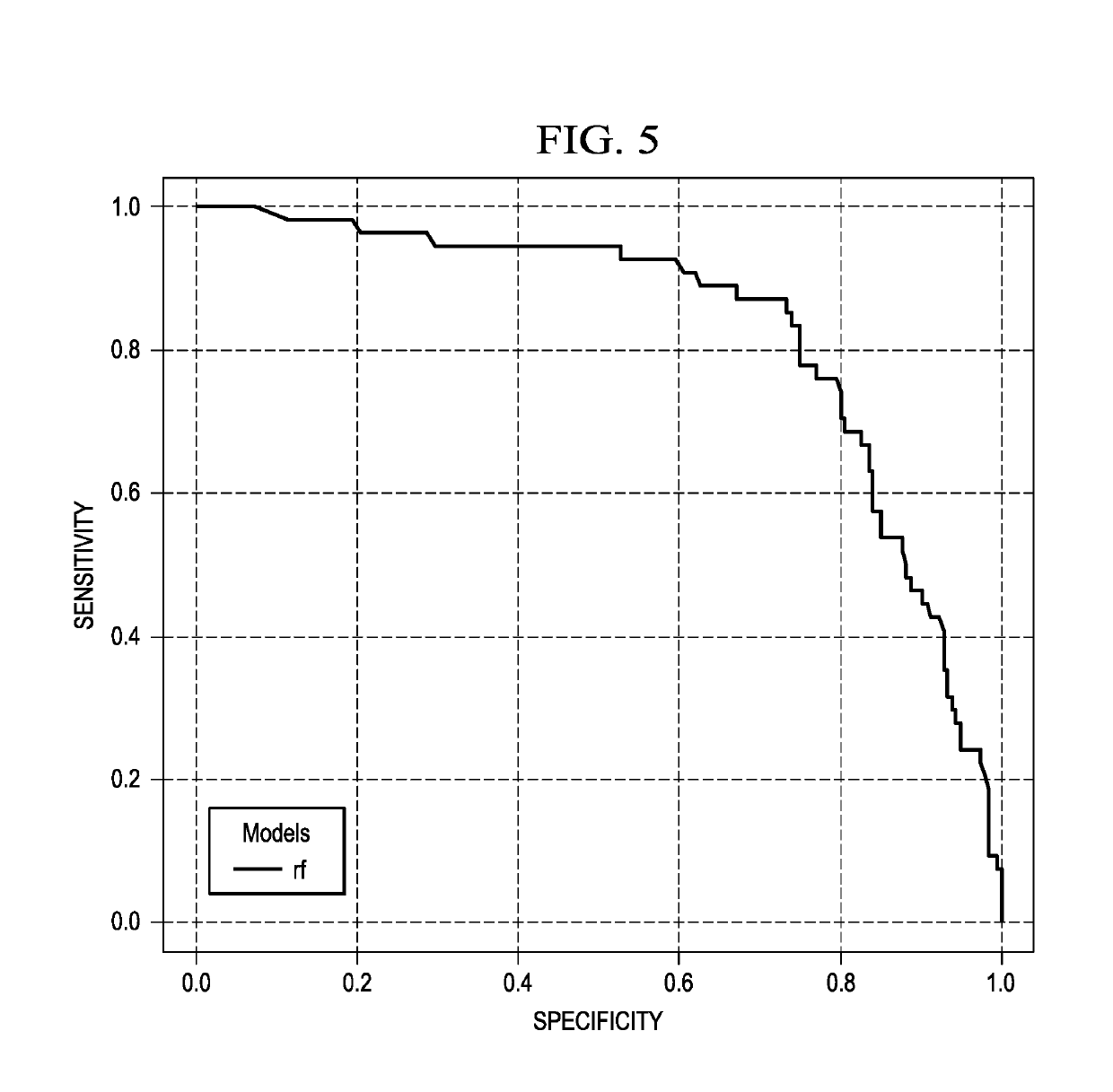
The present invention includes a method for excluding patients from the need for further analysis of Alzheimer's Disease comprising: obtaining a blood or serum sample from a patient in a primary care setting; determining the expression levels of at least 4 of the following proteins: FABP, beta 2 microglobulin, PPY, soluble tumor necrosis factor receptor 1 (sTNFR1), CRP, VCAM-1, thrombopoietin, α2 macroglobulin, eotaxin 3, tumor necrosis factor-alpha (TNF-α), tenascin C (TNC), IL-5, IL-6, IL-7, IL-10, IL-18, 1309, Factor VII, thymus and activation-regulated chemokine (TARC), serum amyloid A (SAA), and intercellular cell-adhesion molecule-1 (ICAM-1); comparing the level of expression from the sample with a statistically locked-down, multi-ethnic, broad age spectrum statistical sample; and determining if the patient is excluded from further testing for Alzheimer's Disease, thereby eliminating the need for further testing of the patient.
Owner:UNIV OF NORTH TEXAS HEALTH SCI CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com