Thrombopoietin peptide analogue freeze-dried preparation
A technology for thrombopoietin and freeze-dried preparations, which can be used in freeze-dried delivery, medical preparations containing active ingredients, blood diseases, etc., and can solve problems such as loss of activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Effect of Buffer System on the Stability of Thrombopoietin Peptoids
[0019] pH in injectable pharmaceutical formulations is an important factor affecting the stability of protein products. The pH of the formulation is maintained by adding suitable buffer salts. Such as adding phosphate, acetate, citrate and so on. In order to investigate the stability of the preparation under different pH conditions, the experiment of the influence of different pH conditions on the stability of the preparation was carried out according to the following conditions:
[0020] Prepare acetic acid-sodium acetate buffer system, citrate, phosphate buffer system, 1ml / bottle (protein concentration is 0.5mg / ml), pH design is 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0 The concentration is 20mmol / L, (see Table 1 below). By using the SUPERDEX G-25 desalting column, the buffer system of the thrombopoietin peptidomimetic stock solution was replaced with the design group, and the ...
Embodiment 2
[0026] Example 2: Effects of Adding Different Preparation Excipients on the Stability of Thrombopoietin Peptoids
[0027] Protein lyoprotectants have always been the focus of biopharmaceutical research, and it is also a problem that must be solved before protein is used as a drug in clinical practice. The excipients we use as protective agents commonly used in protein drugs include albumin, sugars, amino acids, and surfactants. In the present invention, some auxiliary materials suitable for human body application are selected and screened. Although human albumin has a good protein protection effect, it has not been investigated due to its limited source and potential safety hazards.
[0028] The polyols suitable for the present invention can choose mannitol and sorbitol, preferably mannitol as excipients.
[0029] Polypeptides and amino acids suitable for the present invention can be selected from the following group of substances: glycine, arginine, alanine and proline, ...
Embodiment 3
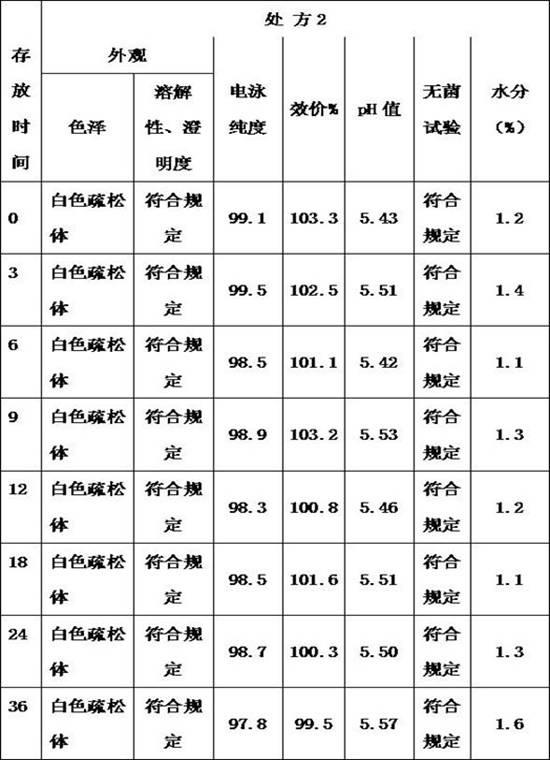
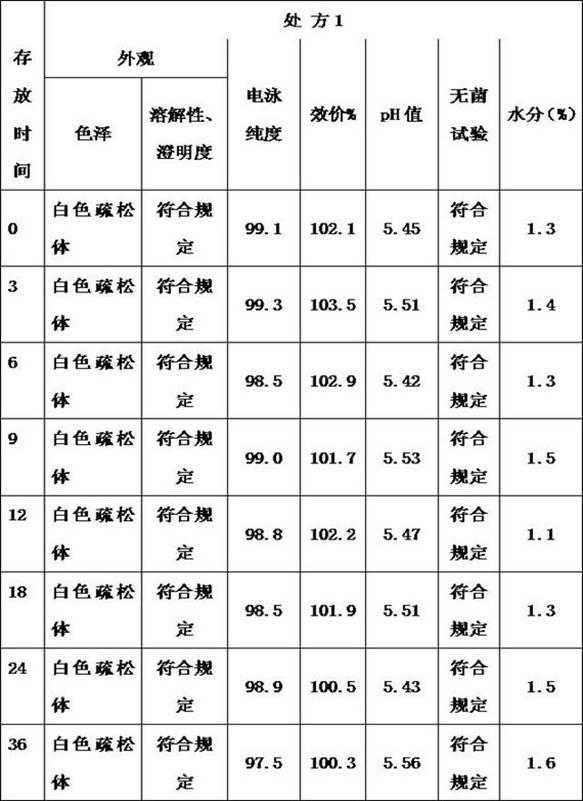
[0056] Example 3: Preparation of lyophilized formulations containing thrombopoietin peptoids
[0057] Take 500ml of thrombopoietin peptide stock solution, the protein concentration is 2mg / ml, and contains 20mmol acetic acid-sodium acetate buffer solution (pH 5.5), weigh 25g of glycine and add it to the stock solution to dissolve completely, and finally add an appropriate amount of 20mmol acetic acid- Sodium acetate buffer (pH 5.5) was diluted to make the final volume of the preparation 1000ml, after mixing evenly, the preparation was sterile filtered with a 0.22 micron filter membrane and distributed into vials. The composition of the final preparation is as follows: thrombopoietin peptidomimetic is 1 mg / ml, the concentration of acetic acid-sodium acetate buffer is 20 mmol / L (pH 5.5), the content of glycoxylic acid is 2.5% (weight ratio), and the content of Tween 80 0.005% (volume ratio).
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com