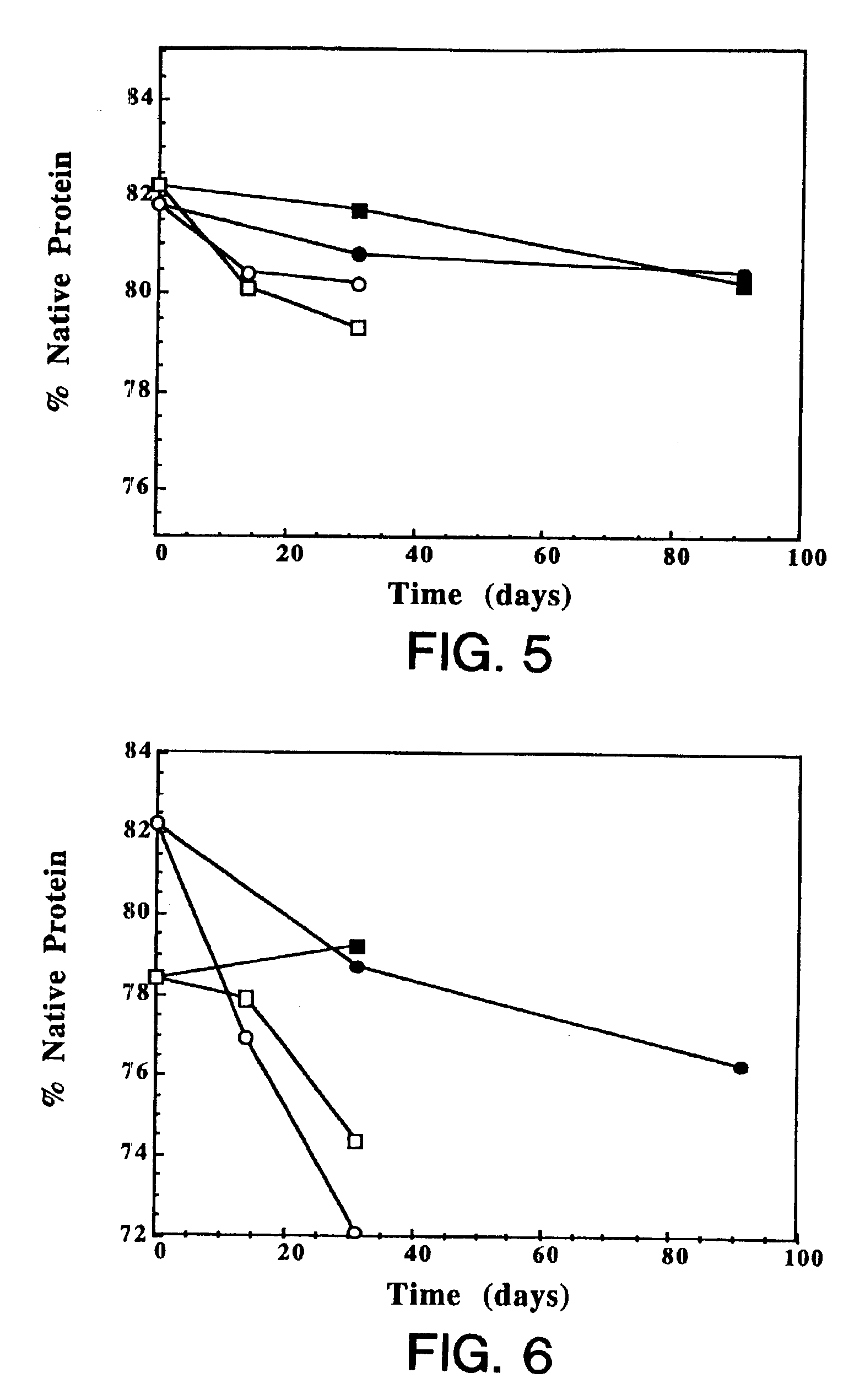
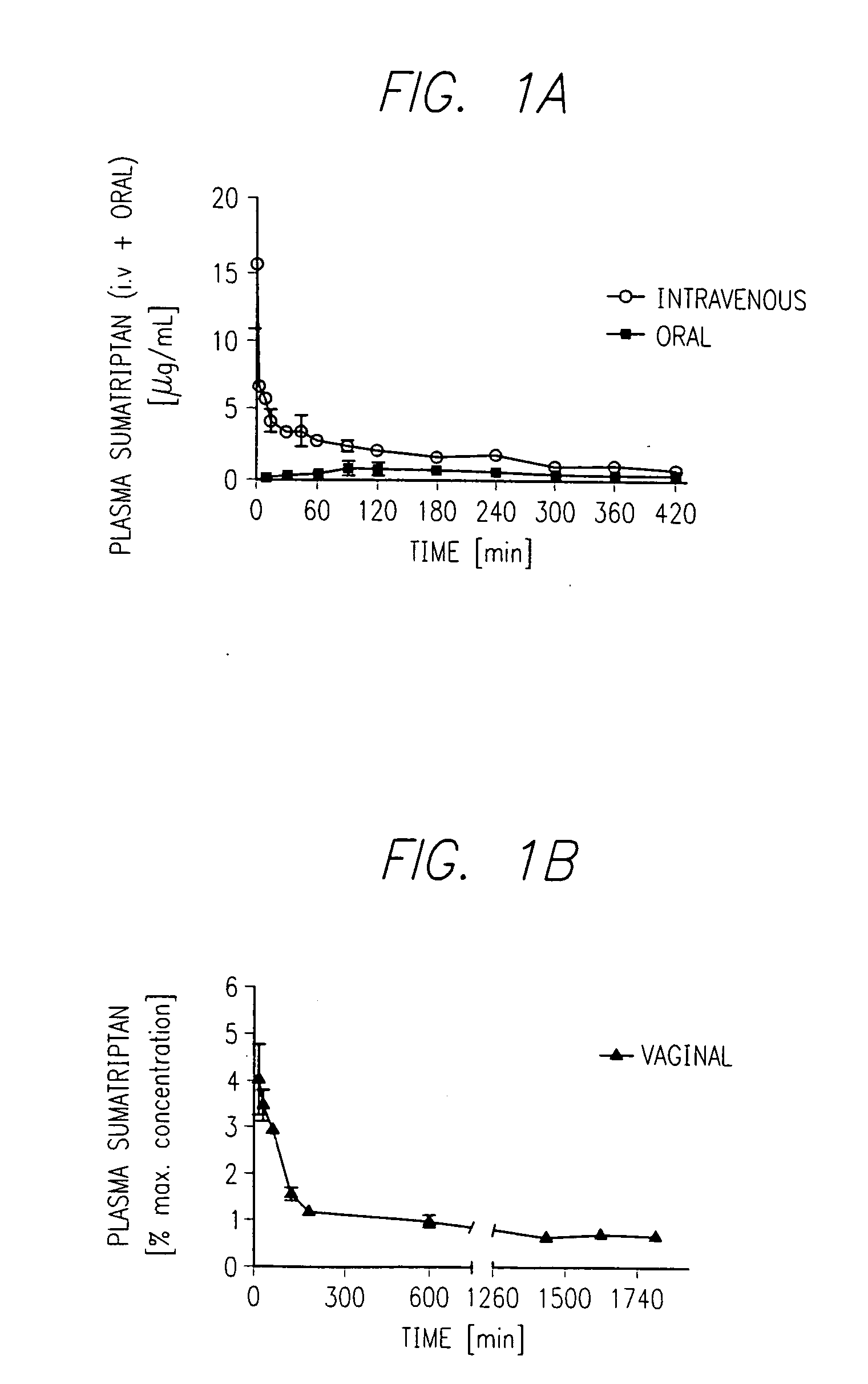
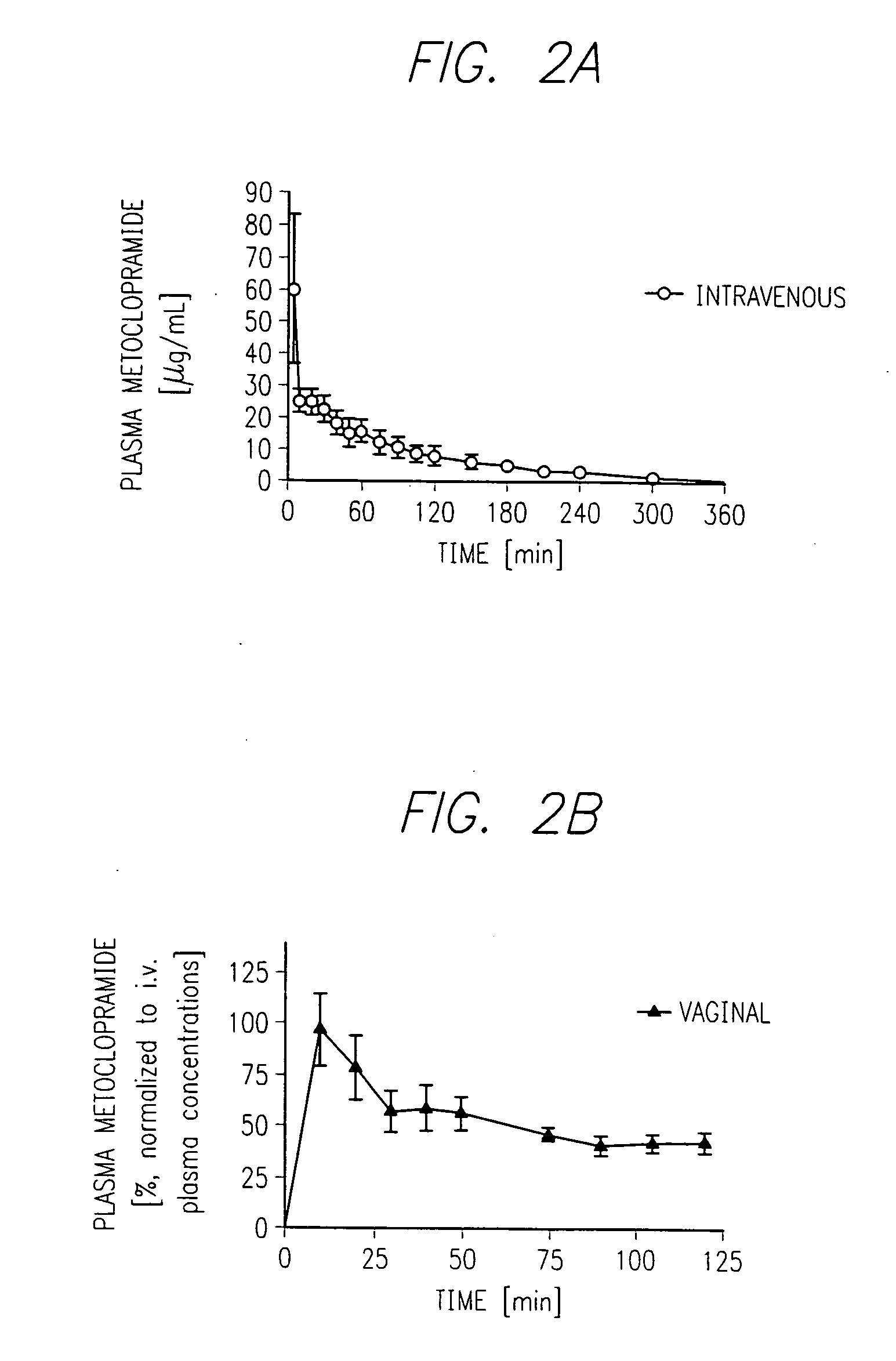
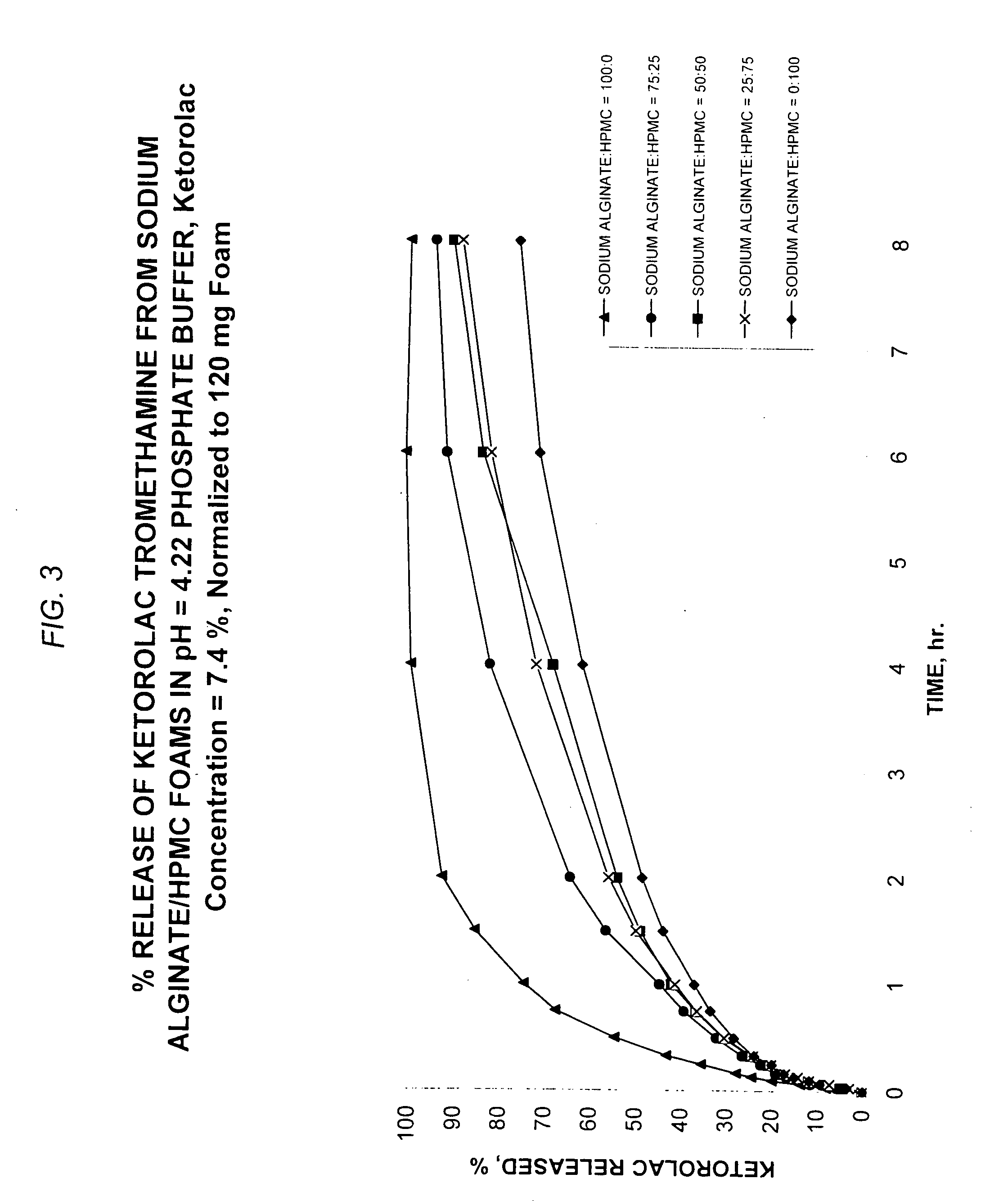
Patents
Literature
10620results about "Lyophilised delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
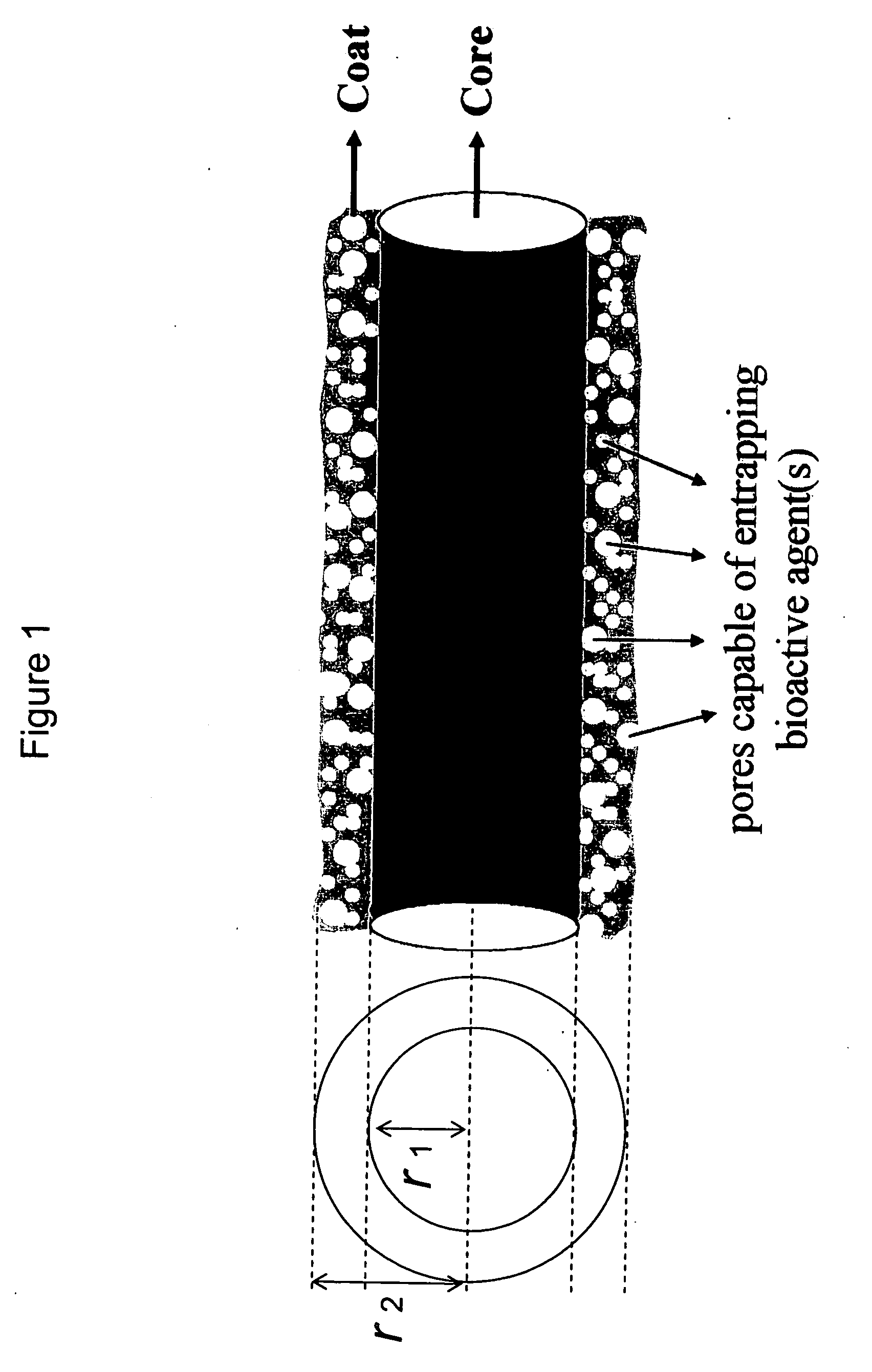
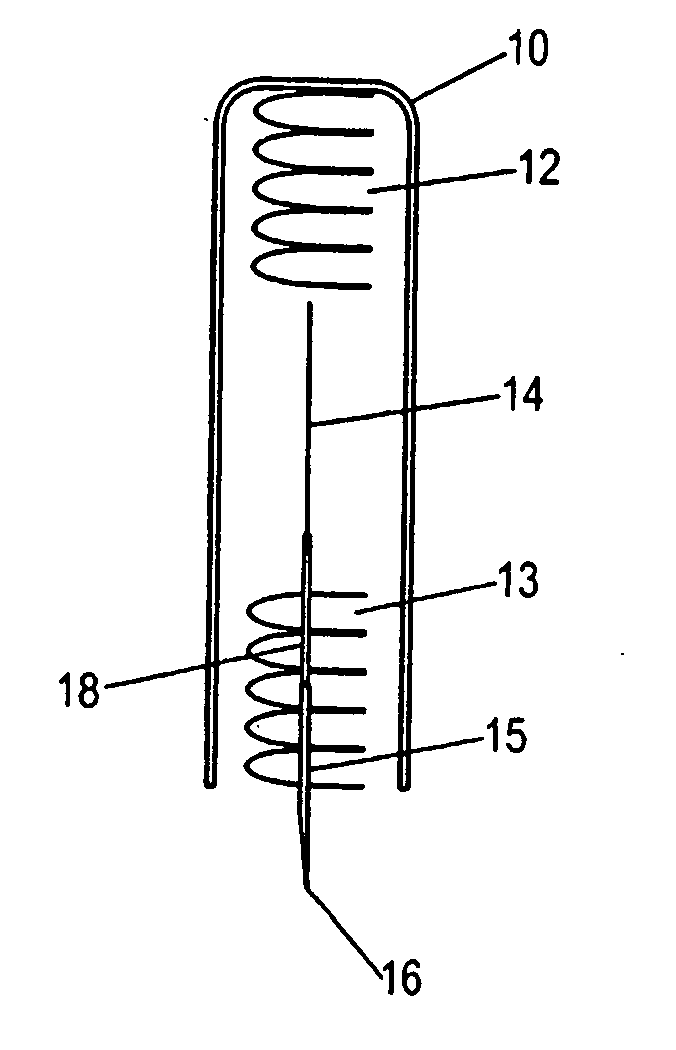
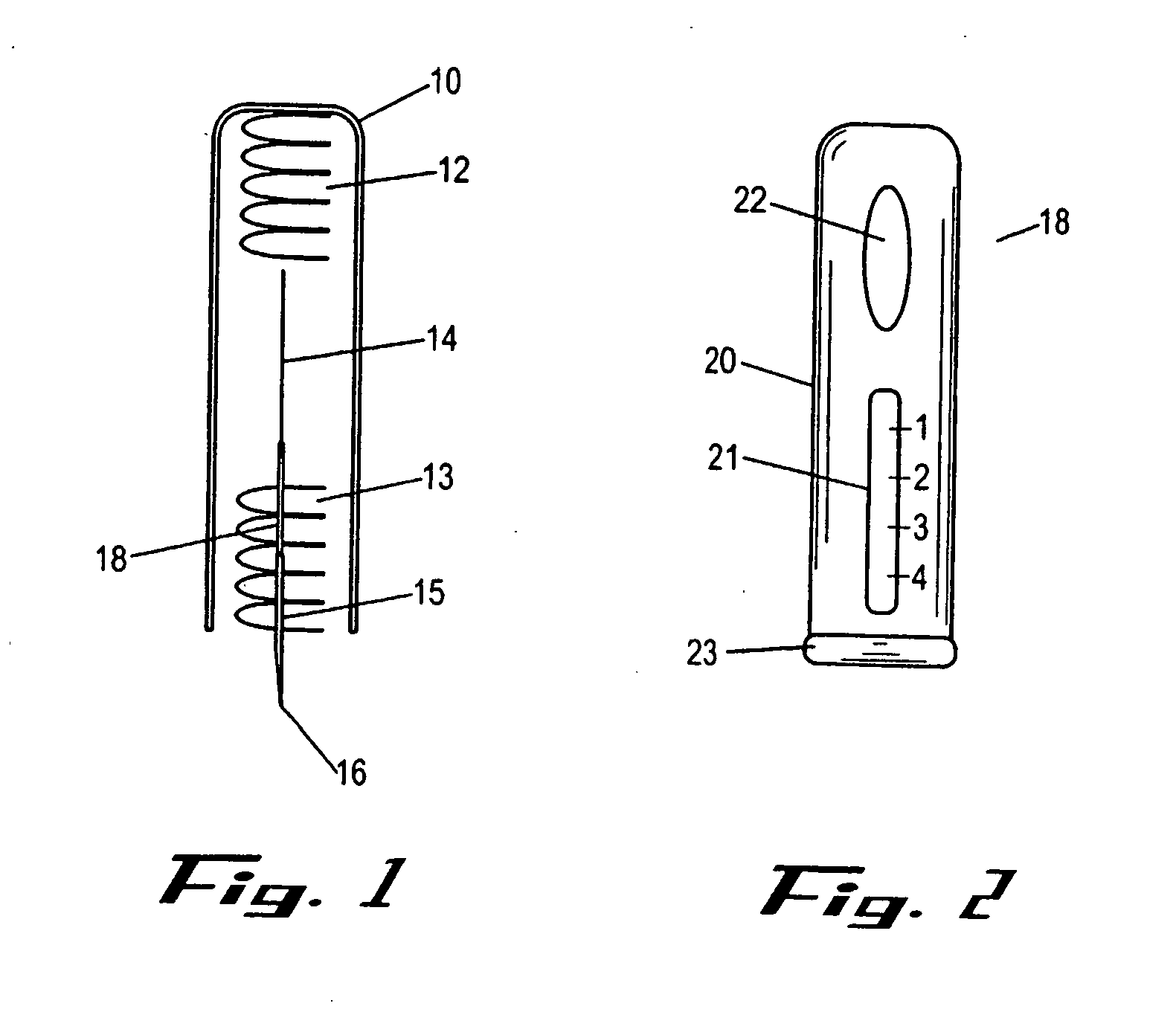
Drug-delivering composite structures
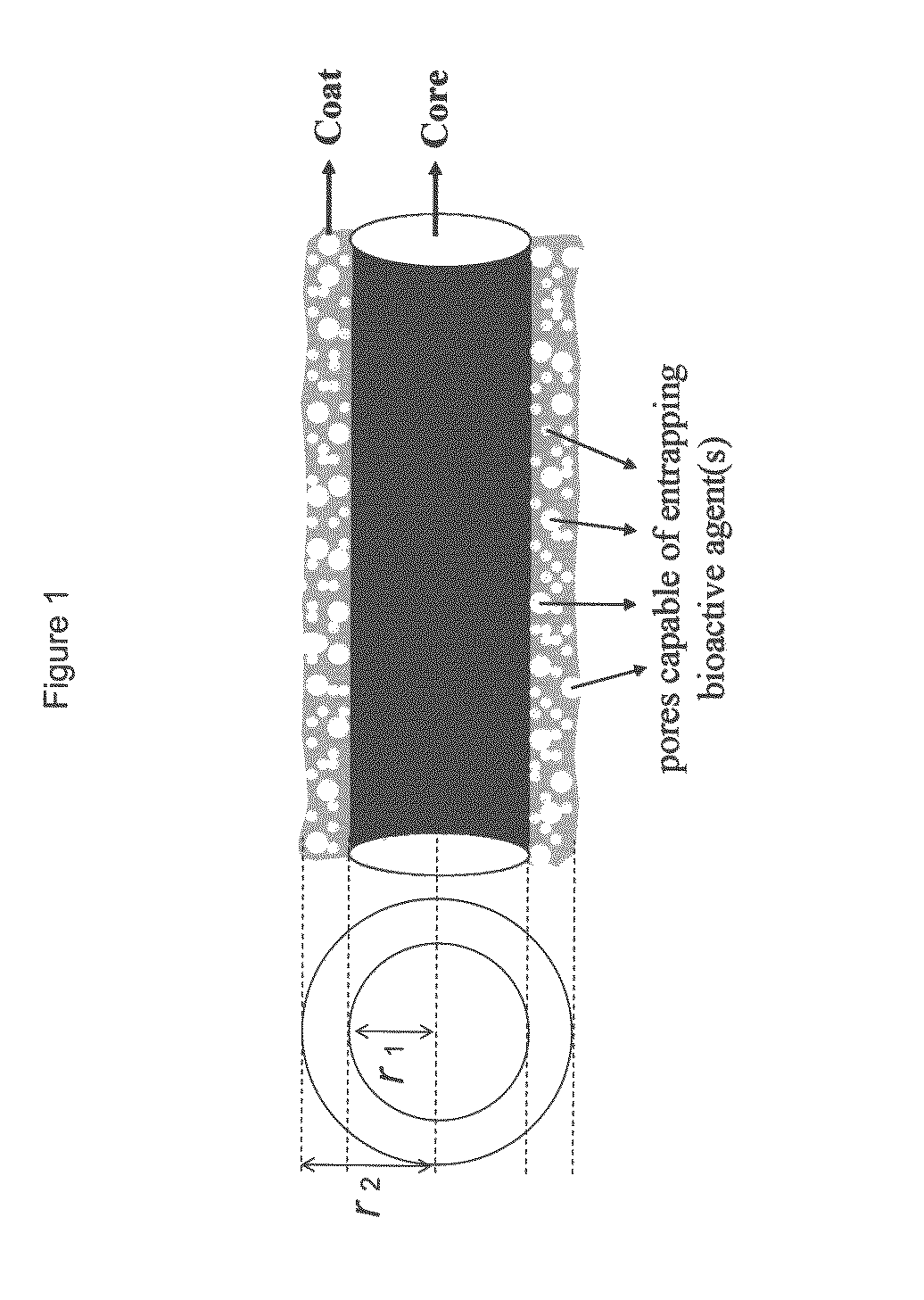
Composite structures composed of a fibril core and a polymeric coat and designed capable of encapsulating both hydrophobic and hydrophilic bioactive agents while retaining the activity of these agents are disclosed. Further disclosed are processes of preparing such composite structures, and medical devices and disposable articles made therefrom.
Owner:ZILBERMAN MEITAL
Methods and compositions useful for administration of chemotherapeutic agents
InactiveUS6096331AReduce morbidityLow toxicityPowder deliveryEchographic/ultrasound-imaging preparationsActive agentIn vivo
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of a pharmaceutically active agent, wherein the agent is associated with a polymeric biocompatible material.
Owner:ABRAXIS BIOSCI LLC
Nucleic Acid-Lipopolymer Compositions
InactiveUS20090042829A1Increase efficiency and dosing flexibilityEfficiently be lyophilizedSpecial deliveryPeptide/protein ingredientsCholesterolFiller Excipient
Compositions, methods, and applications that increase the efficiency of nucleic acid transfection are provided. In one aspect, a pharmaceutical composition may include at least about 0.5 mg / ml concentration of a nucleic acid condensed with a cationic lipopolymer suspended in an isotonic solution, where the cationic lipopolymer includes a cationic polymer backbone having cholesterol and polyethylene glycol covalently attached thereto, and wherein the molar ratio of cholesterol to cationic polymer backbone is within a range of from about 0.1 to about 10, and the molar ratio of polyethylene glycol to cationic polymer backbone is within a range of from about 0.1 to about 10. The composition further may include a filler excipient.
Owner:CLSN LAB
Apolipoprotein A-I agonists and their use to treat dyslipidemic disorders
The present invention provides peptides and peptide analogues that mimic the structural and pharmacological properties of human ApoA-I. The peptides and peptide analogues are useful to treat a variety of disorders associated with dyslipidemia.
Owner:DASSEUX JEAN LOUIS +5
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Protein formulations and methods of making same
ActiveUS20090291062A1Improve consistencyAccurate concentrationPeptide/protein ingredientsAntipyreticOsmolar ConcentrationExcipient
The invention provides an aqueous formulation comprising water and a protein, and methods of making the same. The aqueous formulation of the invention may be a high protein formulation and / or may have low levels of conductivity resulting from the low levels of ionic excipients. Also included in the invention are formulations comprising water and proteins having low osmolality.
Owner:ABBVIE BIOTECHNOLOGY LTD
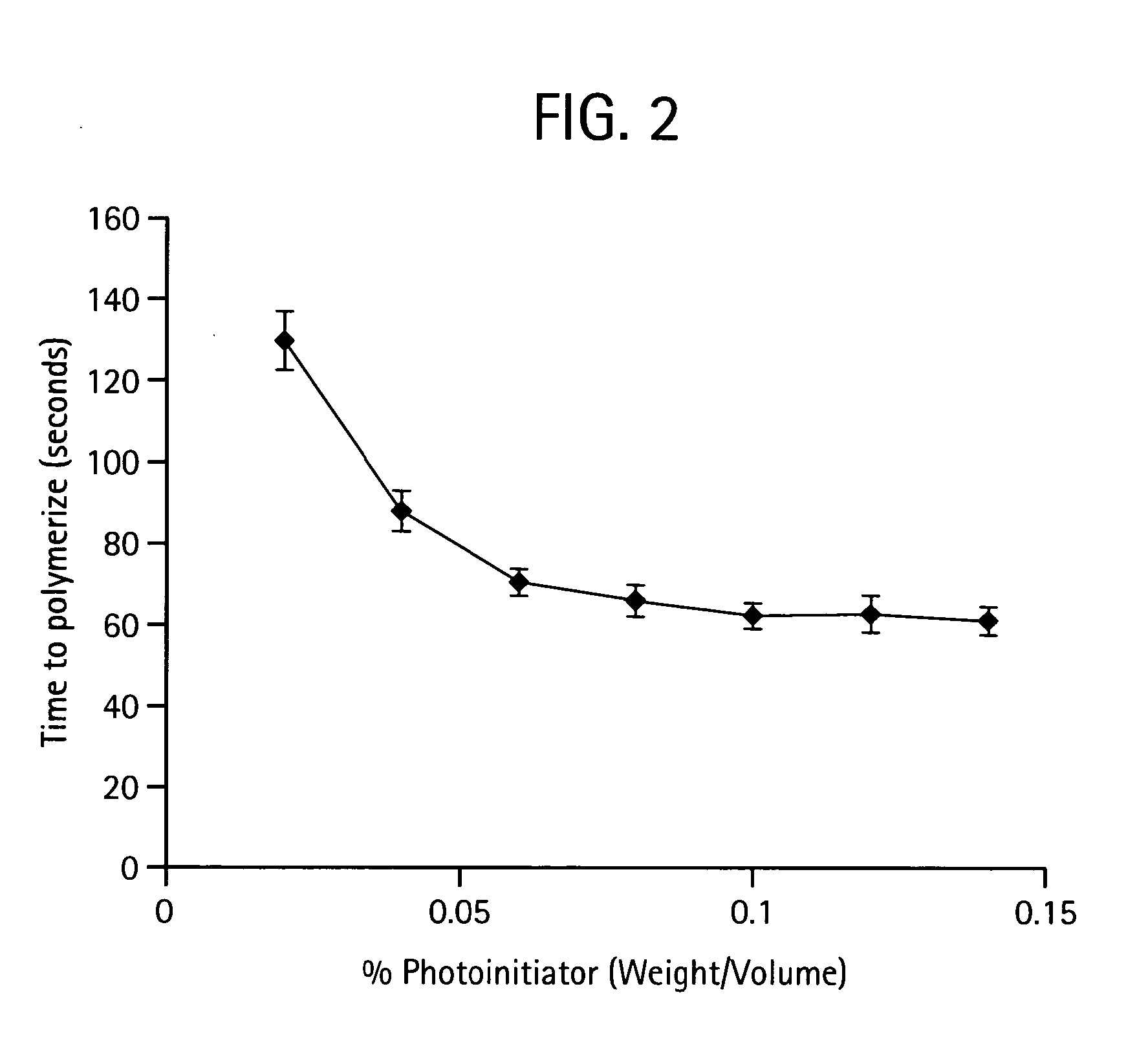
Lyophilization process and products obtained thereby
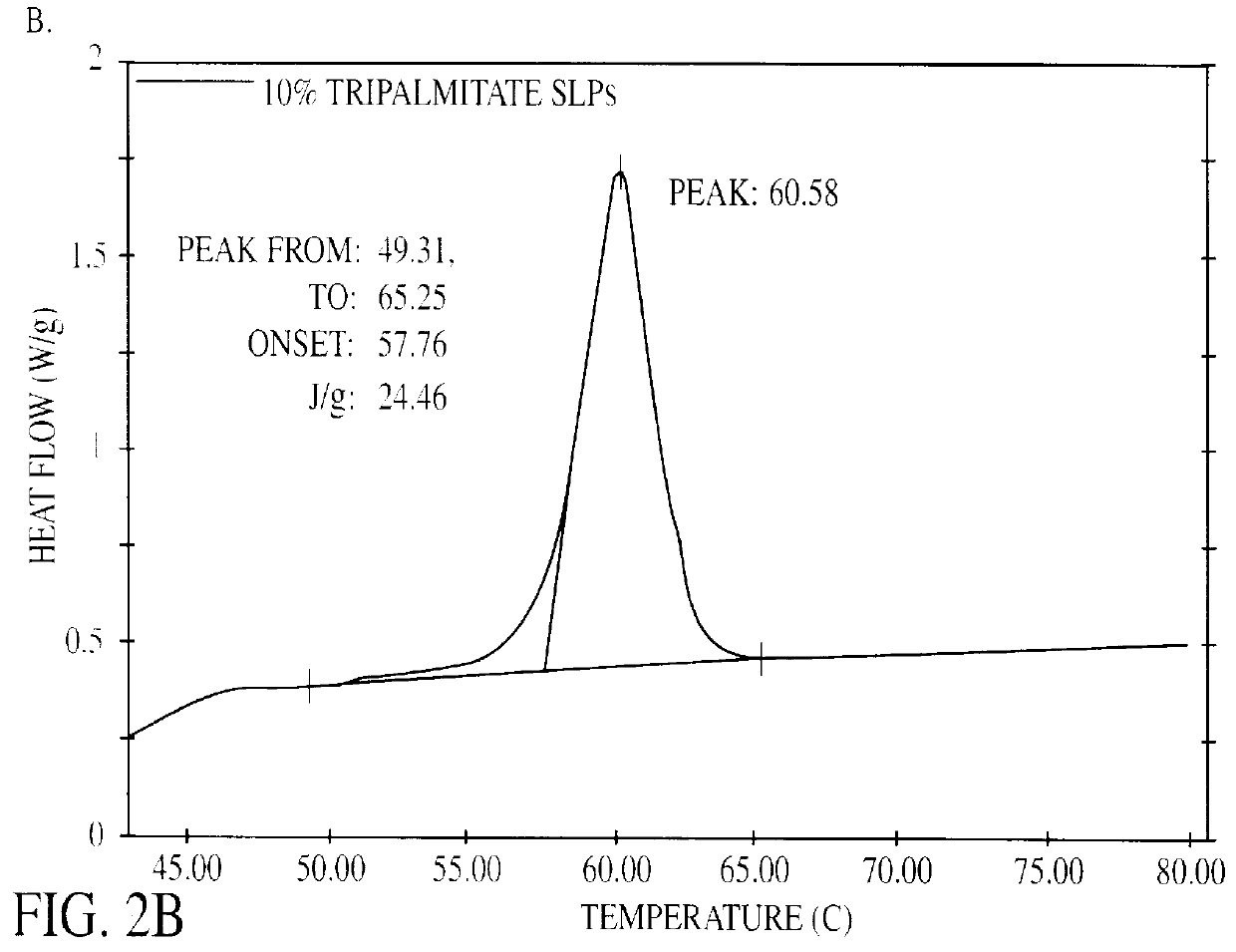
ActiveUS20070116729A1Dissolve fastSuitable for useBiocidePowder deliveryHigh concentrationFreeze-drying
A lyophilization process which comprises dissolving a material in one or more solvents for said material to form a solution; forcing said material at least partially out of solution by combining the solution and a non-solvent for the material, which non-solvent is miscible with the solvent or solvents used and wherein said non-solvent is volatilizable under freeze-drying conditions. In addition, for hydrophobic and / or lipophilic materials, the anti-solvent can be omitted, and the solution of the material in the solvent can be subjected directly to freeze drying. The lyophilizates can then be reconstituted with typical aqueous diluent in the case of hydrophilic materials. Hydrophobic and or lipophilic materials can be initially reconstituted with propylene glycol and / or polyethyleneglycol to form a high concentration solution therein and this is further diluted for use with a diluent of Intralipid, plasma, serum, or even whole blood.
Owner:SCIDOSE PHARMA +1
Compositions and methods of delivery of pharmacological agents
InactiveUS20050004002A1Reducing one or more side effectsInhibiting oxidation in the pharmaceutical compositionAntibacterial agentsOrganic active ingredientsSide effectPharmaceutical formulation
The present invention relates to a pharmaceutical composition comprising a pharmaceutical agent and a pharmaceutically acceptable carrier, which carrier comprises a protein, for example, human serum albumin and / or deferoxamine. The human serum albumin is present in an amount effective to reduce one or more side effects associated with administration of the pharmaceutical composition. The invention also provides methods for reducing one or more side effects of administration of the pharmaceutical composition, methods for inhibiting microbial growth and oxidation in the pharmaceutical composition, and methods for enhancing transport and binding of a pharmaceutical agent to a cell.
Owner:ABRAXIS BIOSCI LLC
Probiotic recolonisation therapy
The present invention relates to pharmaceutical compositions suitable for the treatment of chronic diseases associated with the presence of abnormal or an abnormal distribution of microflora in the gastrointestinal tract of a mammalian host, which compositions comprise viable non-pathogenic or attenuated pathogenic Clostridia. The compositions further comprise one or more additional viable non-pathogenic or attenuated pathogenic microorganisms selected from the group consisting of Bacteroides, Eubacteria, Fusobacteria, Propionibacteria, Lactobacilli, anaerobic cocci, Ruminococcus, E.Coli, Gemmiger, Desullomonas, Peptostreptococcus, and fungi. The present invention also provides pharmaceutical compositions suitable for the treatment of the same chronic diseases comprising viable non-pathogenic or attenuated pathogenic Escherichia coli, at least one strain of viable non-pathogenic or attenuated pathoenic Bacteroides and at least one strain of viable non-pathogenic or attenuated pathogenic microorganism.
Owner:FINCH THERAPEUTICS HLDG LLC
Protein formulation
InactiveUS7060268B2Reduce aggregationReduce formation of particulateBiocideOrganic active ingredientsDiluentHigh protein
A stable lyophilized protein formulation is described which can be reconstituted with a suitable diluent to generate a high protein concentration reconstituted formulation which is suitable for subcutaneous administration. For example, anti-IgE and anti-HER2 antibody formulations have been prepared by lyophilizing these antibodies in the presence of a lyoprotectant. The lyophilized mixture thus formed is reconstituted to a high protein concentration without apparent loss of stability of the protein.
Owner:GENENTECH INC
Coated vaginal devices for vaginal delivery of therapeutically effective and/or health-promoting agents
A vaginal device for delivering therapeutical and / or health-promoting agents. The vaginal device partly or completely coated by, covered by or combined with a coating or covering comprising a film, foam, strip, cap, cup or particles. The coating of the device comprises a mucoadhesive composition comprising a therapeutical and / or health-promoting agent.
Owner:UNIVERSITY OF MINNESOTA DULUTH
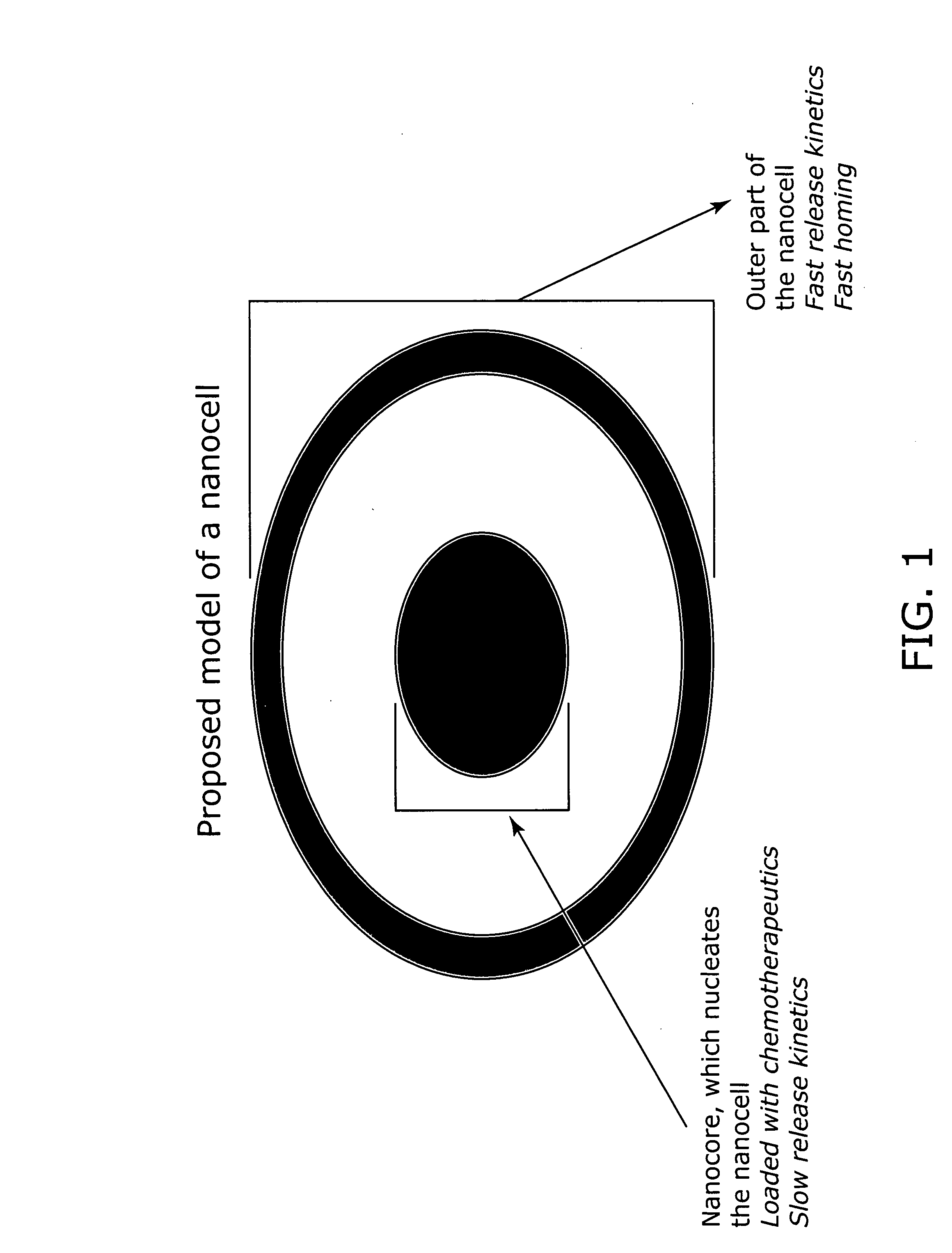
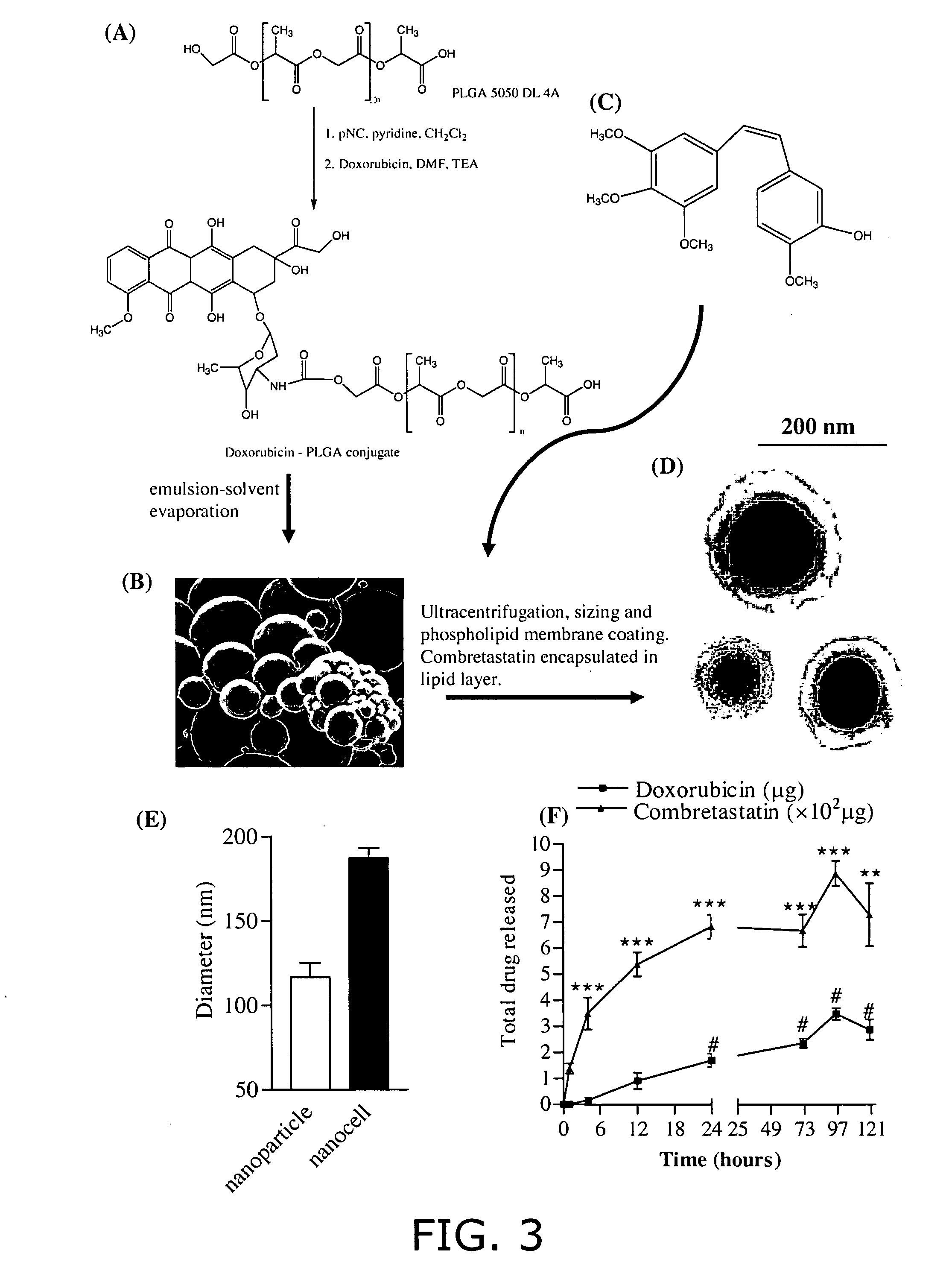
Nanocell drug delivery system
InactiveUS20050266067A1Avoid flowIncreased toxicityAntibacterial agentsOrganic active ingredientsLipid formationAntigen
Nanocells allow the sequential delivery of two different therapeutic agents with different modes of action or different pharmacokinetics. A nanocell is formed by encapsulating a nanocore with a first agent inside a lipid vesicle containing a second agent. The agent in the outer lipid compartment is released first and may exert its effect before the agent in the nanocore is released. The nanocell delivery system may be formulated in pharmaceutical composition for delivery to patients suffering from diseases such as cancer, inflammatory diseases such as asthma, autoimmune diseases such as rheumatoid arthritis, infectious diseases, and neurological diseases such as epilepsy. In treating cancer, a traditional antineoplastic agent is contained in the outer lipid vesicle of the nanocell, and an antiangiogenic agent is loaded into the nanocore. This arrangement allows the antineoplastic agent to be released first and delivered to the tumor before the tumor's blood supply is cut off by the antianiogenic agent.
Owner:MASSACHUSETTS INST OF TECH
Probiotic recolonisation therapy
The present invention relates to pharmaceutical compositions suitable for the treatment of chronic diseases associated with the presence of abnormal or an abnormal distribution of microflora in the gastrointestinal tract of a mammalian host, which compositions comprise viable non-pathogenic or attenuated pathogenic Clostridia. The compositions further comprise one or more additional viable non-pathogenic or attenuated pathogenic microorganisms selected from the group consisting of Bacteroides, Eubacteria, Fusobacteria, Propionibacteria, Lactobacilli, anaerobic cocci, Ruminococcus, E.Coli, Gemmiger, Desullomonas, Peptostreptococcus, and fungi. The present invention also provides pharmaceutical compositions suitable for the treatment of the same chronic diseases comprising viable non-pathogenic or attenuated pathogenic Escherichia coli, at least one strain of viable non-pathogenic or attenuated pathoenic Bacteroides and at least one strain of viable non-pathogenic or attenuated pathogenic microorganism.
Owner:FINCH THERAPEUTICS HLDG LLC
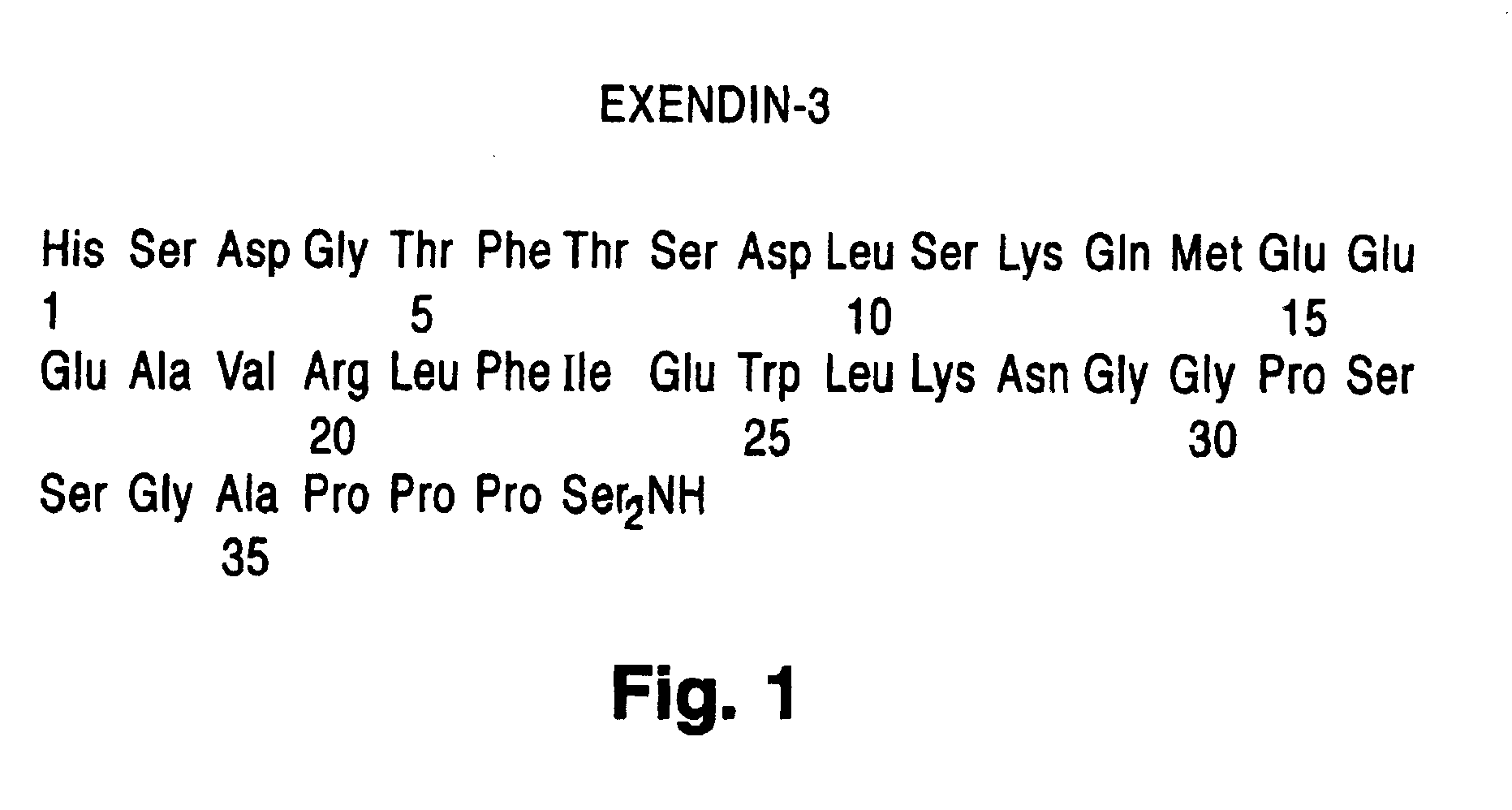
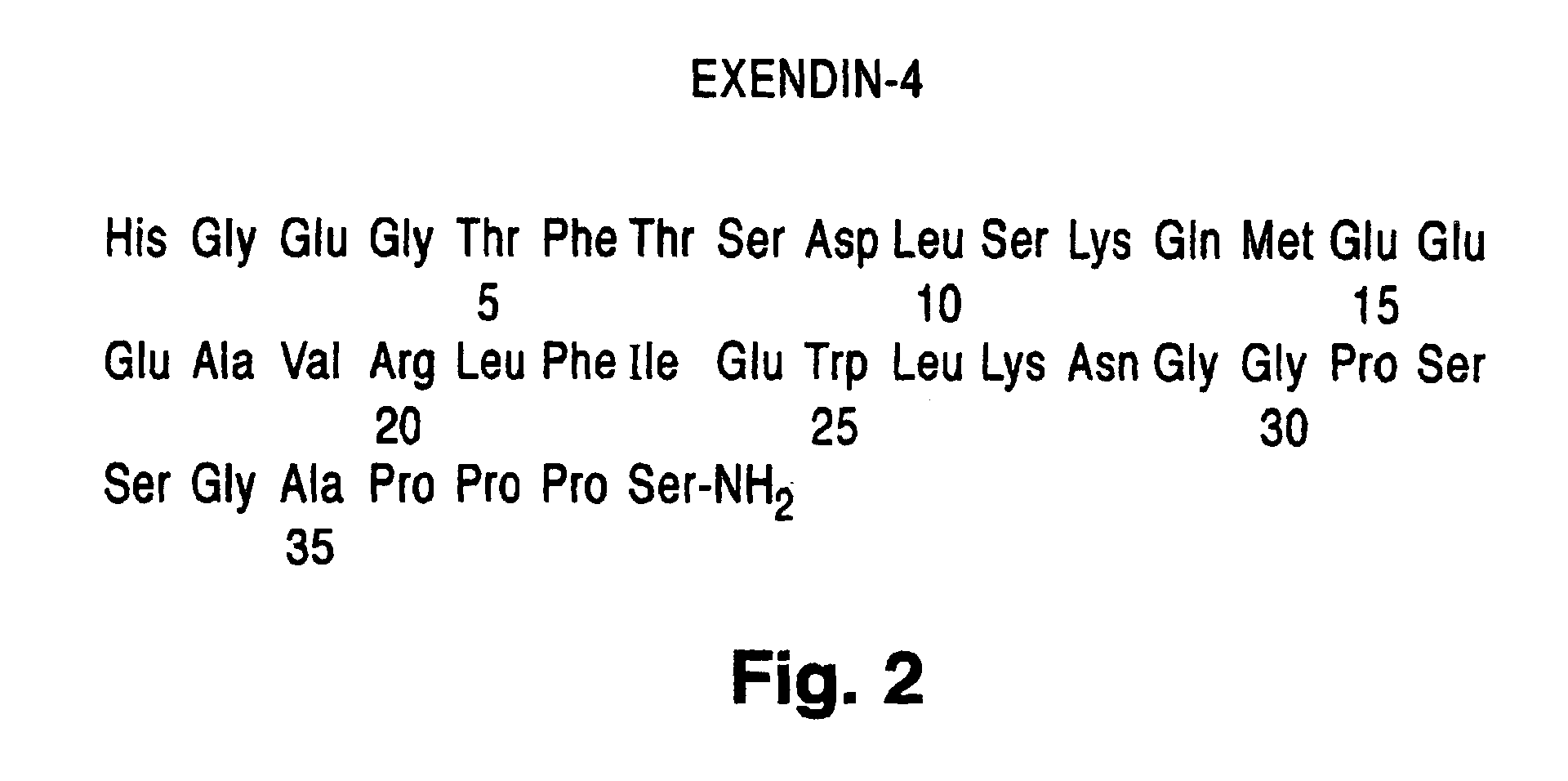
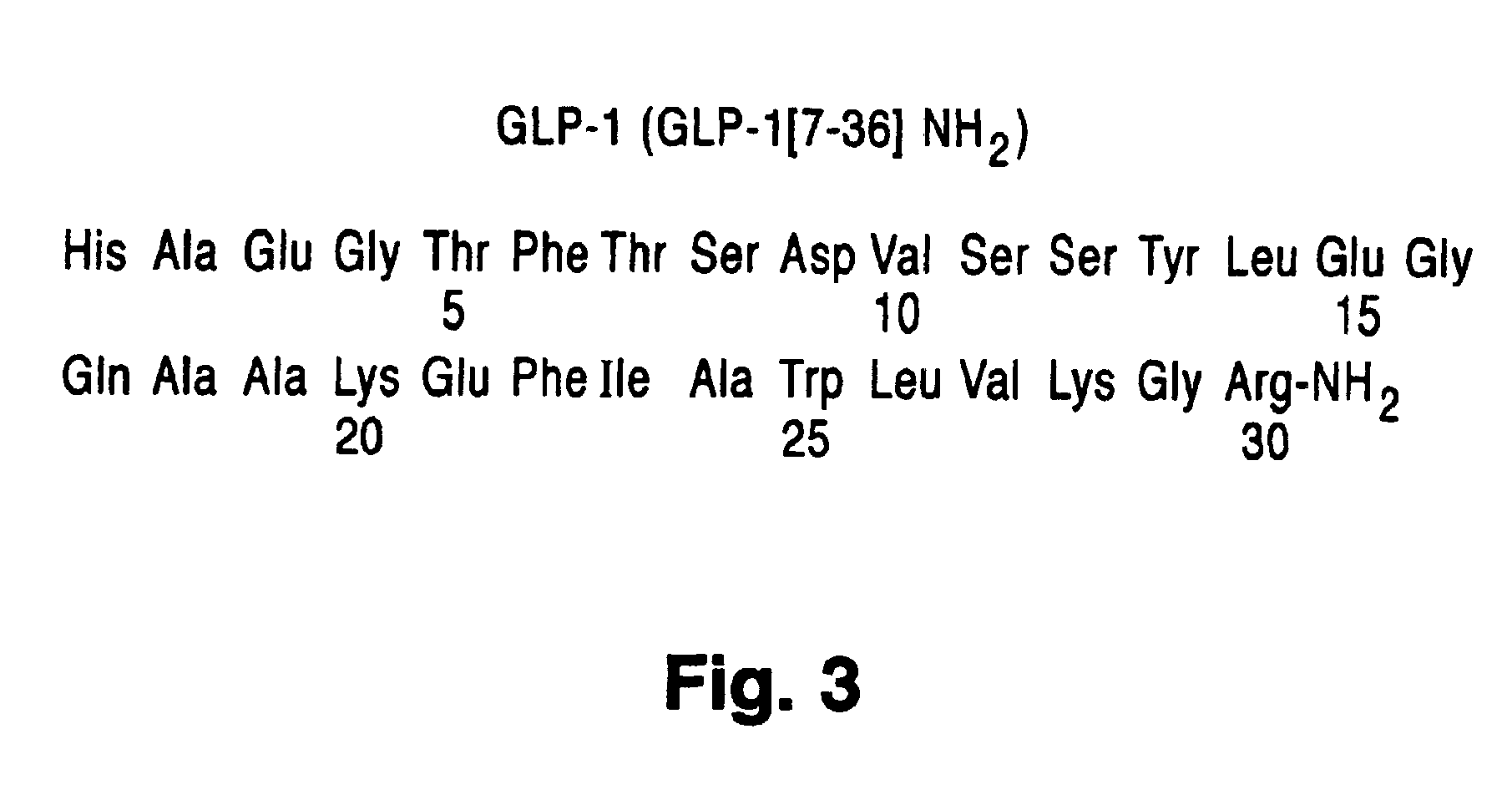
Exendin agonist formulations and methods of administration thereof
InactiveUS6902744B1Slow gastric emptyingLowering plasma glucose levelPowder deliveryPeptide/protein ingredientsGastric emptyingPlasma glucose
Novel exendin and exendin agonist compound formulations and dosages and methods of administration thereof are provided. These compositions and methods are useful in treating diabetes and conditions that would be benefited by lowering plasma glucose or delaying and / or slowing gastric emptying or inhibiting food intake.
Owner:ASTRAZENECA PHARMA LP
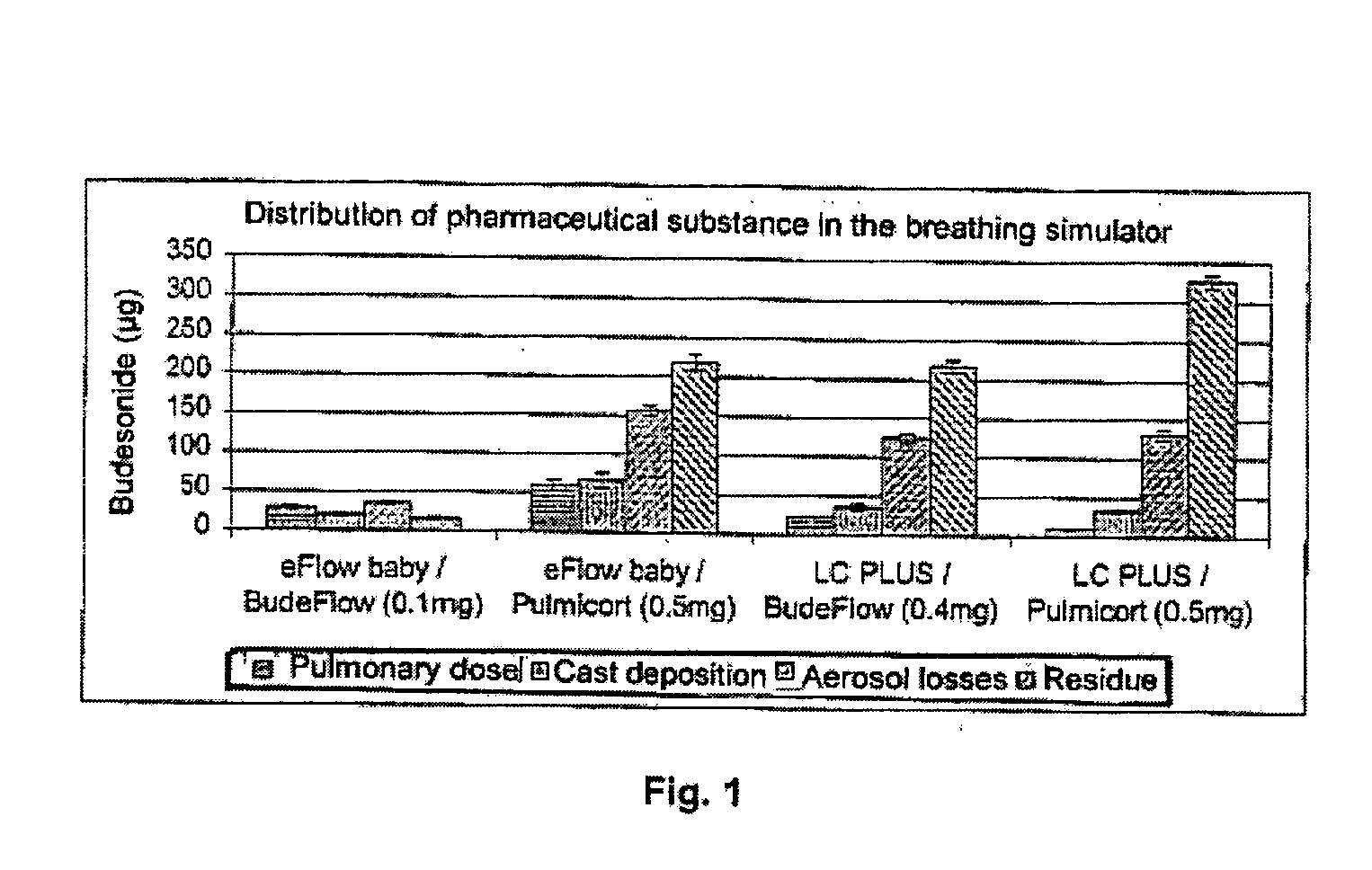
Pharmaceutical aerosol composition
Sterile compositions for administration as aerosols are described. They contain an active agent which is poorly water-soluble, a non-ionic surfactant acomponent and a phospholipid component. The compositions are suitable for oral or nasal inhalation, but also for topical or oromucosal administration. They are particulary useful for the efficient pulmonary administration of poorly soluble corticosteroids and can be aerosolized with common nebulizers.
Owner:PARI PHARMA GMBH
Antibody formulations and methods of making same
ActiveUS8420081B2Minimal aggregationLow levelPeptide/protein ingredientsAntipyreticExcipientNuclear chemistry
Owner:ABBVIE BIOTECHNOLOGY LTD
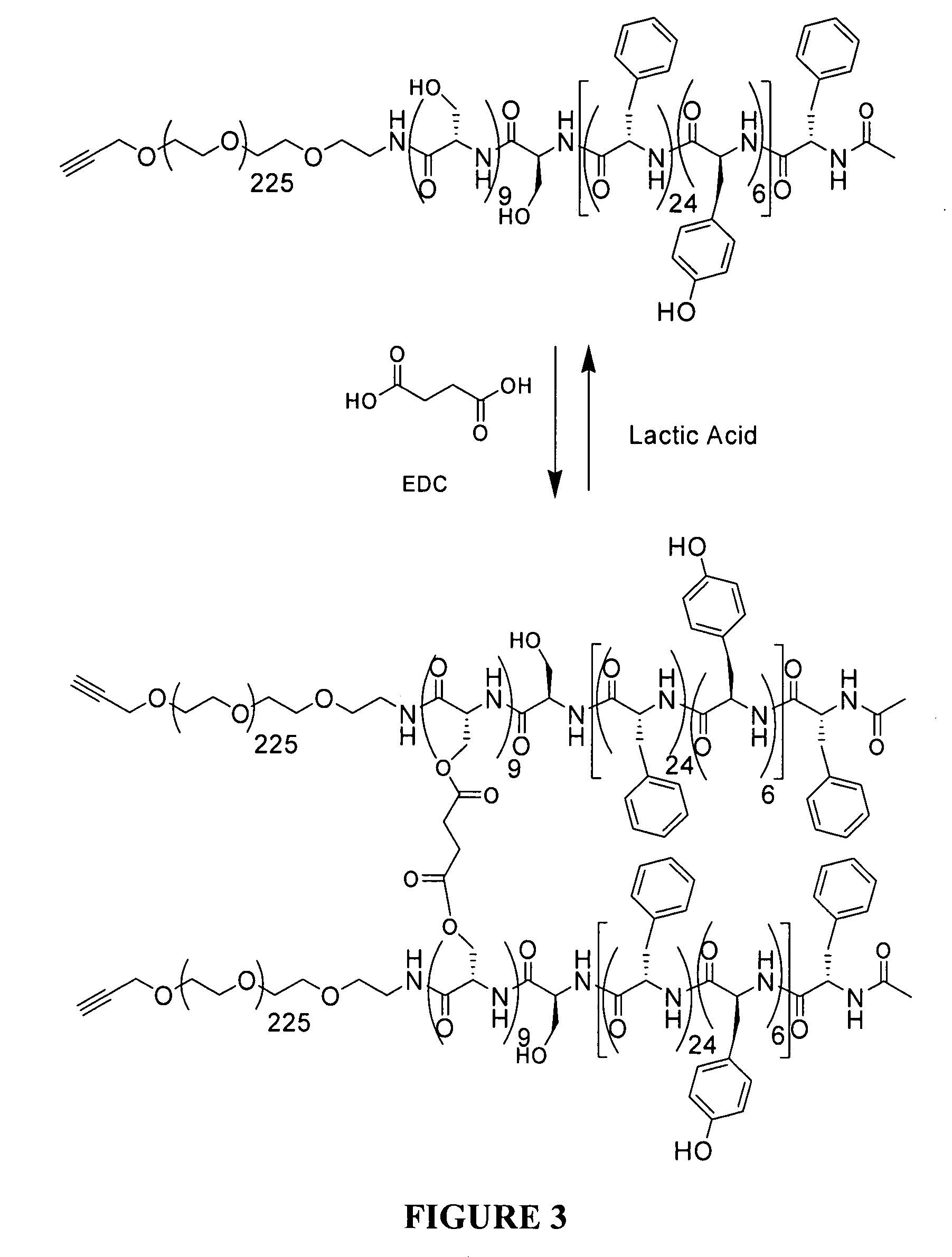
Drug-delivering composite structures
Composite structures composed of a fibril core and a polymeric coat and designed capable of encapsulating both hydrophobic and hydrophilic bioactive agents while retaining the activity of these agents are disclosed. Further disclosed are processes of preparing such composite structures, and medical devices and disposable articles made therefrom.
Owner:ZILBERMAN MEITAL
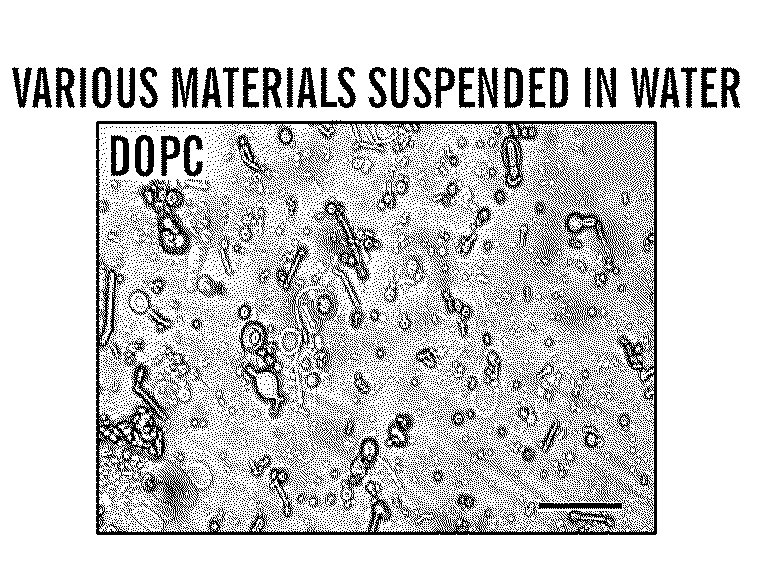
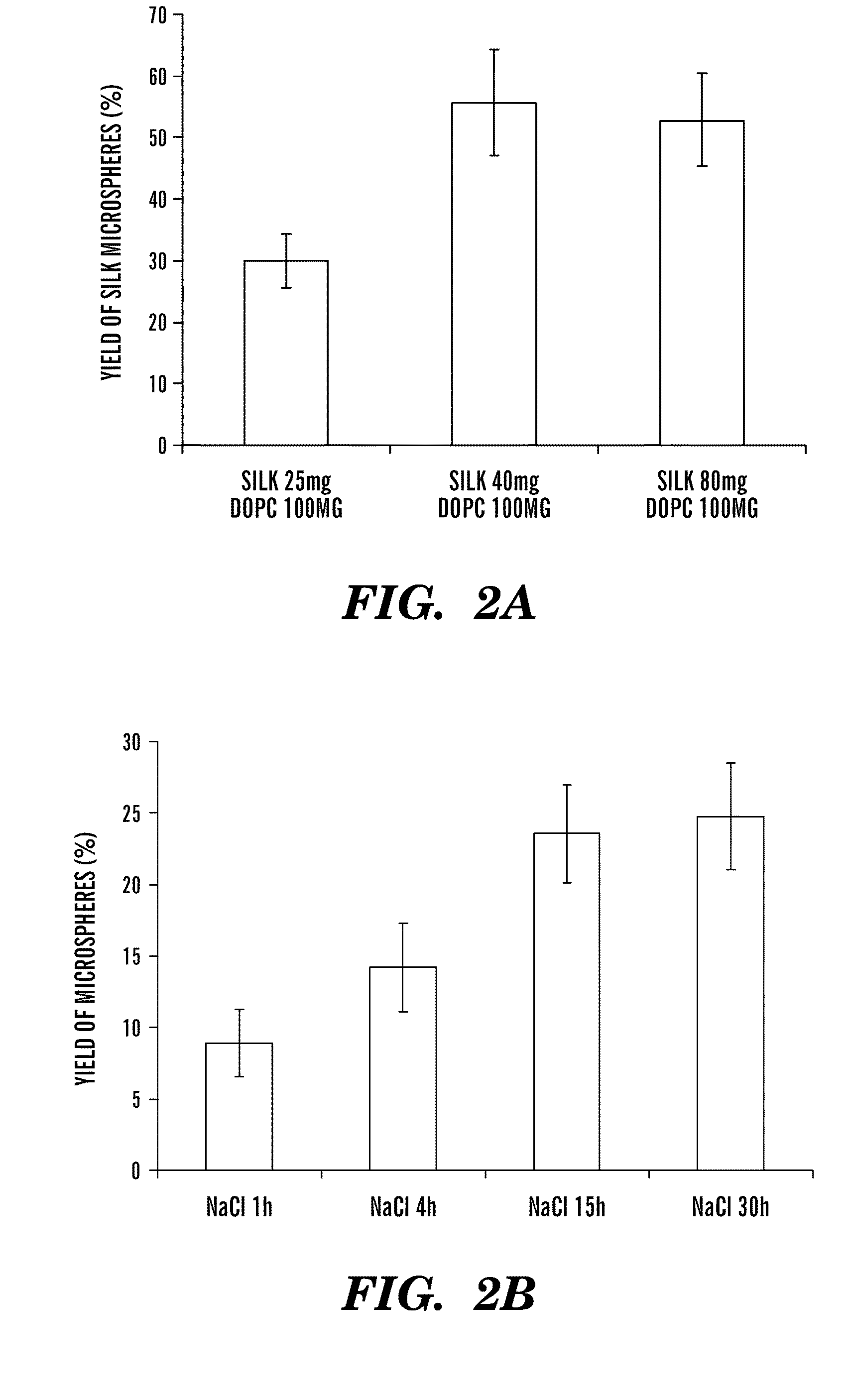
Silk microspheres for encapsulation and controlled release
A method was developed to prepare silk fibroin microspheres using lipid vesicles as templates to efficiently load therapeutic agents in active form for controlled release. The lipids are subsequently removed through the use of a dehydration agent, such as methanol or sodium chloride, resulting in β-sheet structure dominant silk microsphere structures having about 2 μm in diameter. The therapeutic agent can be entrapped in the silk microspheres and used in pharmaceutical formulations for controlled-release treatments.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Methods of synthesis and use of chemospheres
InactiveUS20110104052A1Prevents immunoclearanceExtended half-lifePowder deliveryBiocideDiagnostic agentComputed tomography
The present invention provides, in general, compositions comprising a hydrogel and an agent, for example a therapeutic agent or an imaging agent, for locoregional delivery. In certain preferred embodiments of the invention, the hydrogel compositions are detectable by Magnetic Responance and CT Scan and are used for locoregional delivery of therapeutic agents, for example chemotherapeutic agents. The invention also features polymer matrix compositions comprising nanoparticles that can be loaded after polymerization with bioactive agents, for example a diagnostic agent or therapeutic agent.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Microparticles for Oral Delivery
The invention provides microbeads containing oil-associated biologically active compounds and methods for their manufacture and use. The microbeads consist of a soluble complex of non-digestible polymer and emulsifier with oil-associated biologically active compounds embedded in a matrix of digestible polymer. The disclosed microbead complex protects the biologically active compounds, such as vitamins, fish oil and carotenoids, from oxidation, taste and odor degradation. The disclosed microbeads also provide protection from the stomach digestive distraction, and allows for the delivery of the biologically active compounds in the intestine.
Owner:INTERVET INC
Formulation of human antibodies for treating TNF-α associated disorders
ActiveUS8216583B2Improve stabilityAntibacterial agentsPowder deliveryADAMTS ProteinsPharmaceutical formulation
A liquid aqueous pharmaceutical formulation is described which has a high protein concentration, a pH of between about 4 and about 8, and enhanced stability.
Owner:ABBVIE BIOTECHNOLOGY LTD
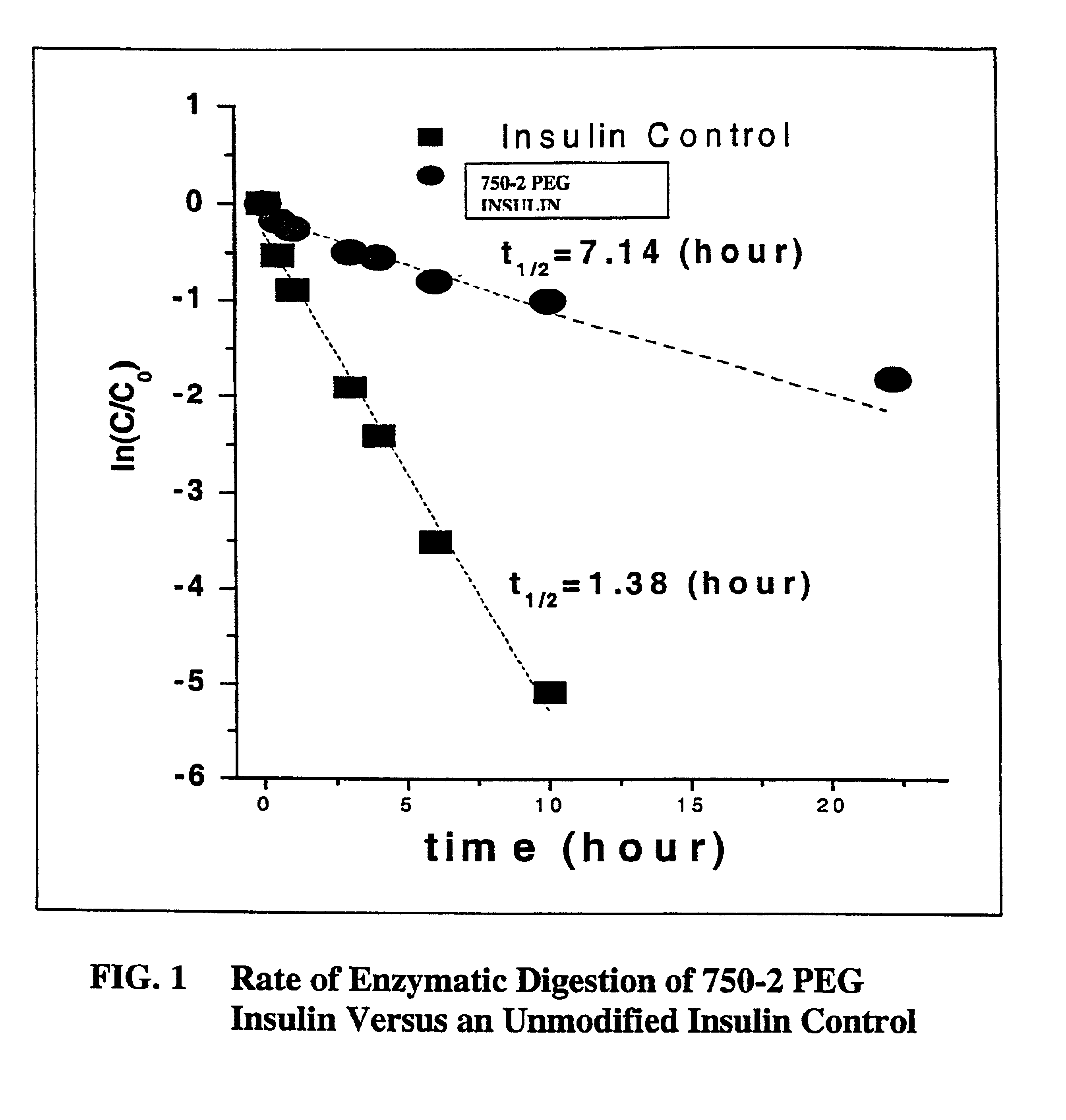
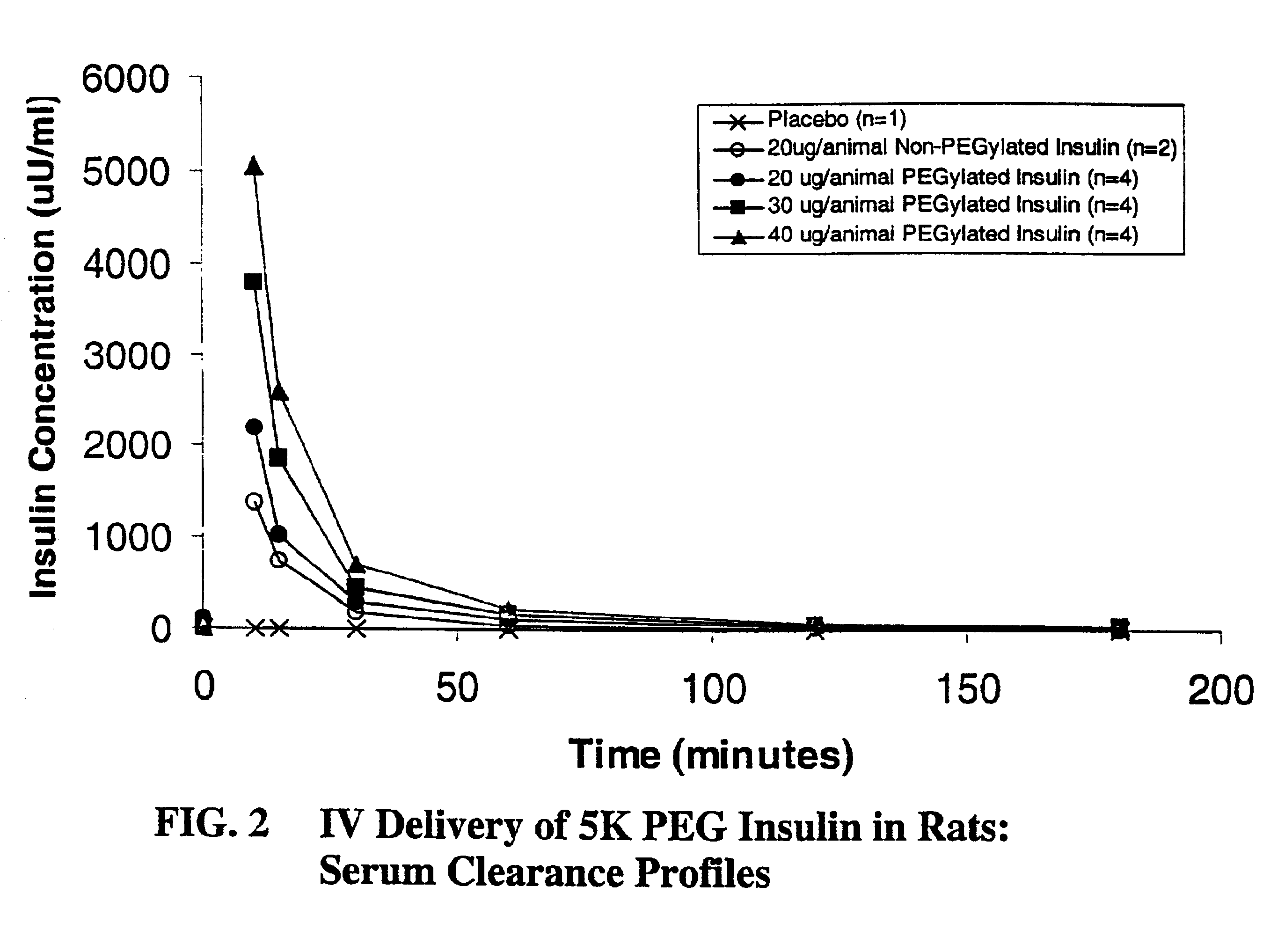
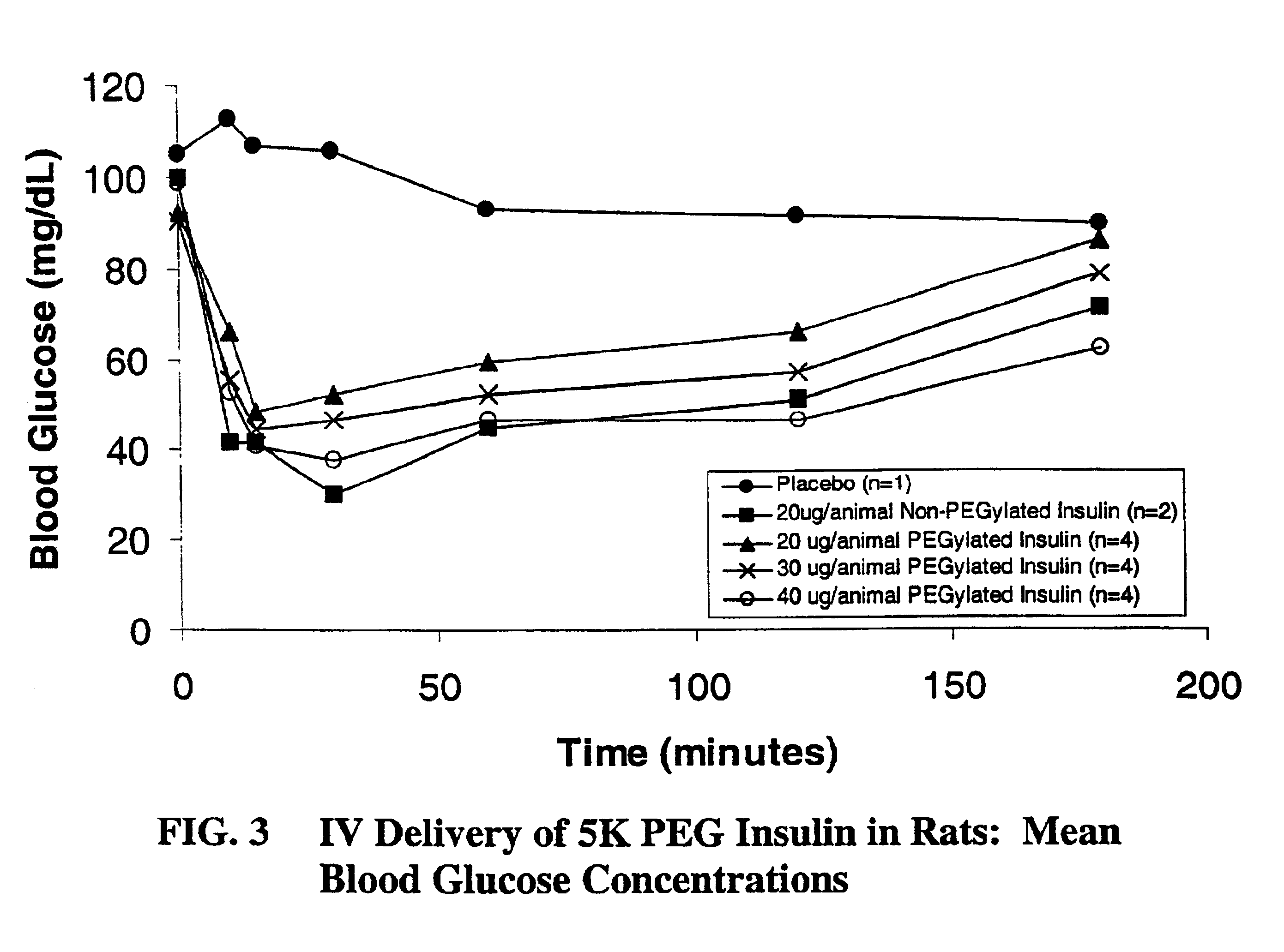
Pulmonary administration of chemically modified insulin
InactiveUS6838076B2Reduce probabilityPowder deliveryPeptide/protein ingredientsPolymer modifiedEndocrinology
The present invention provides active, hydrophilic polymer-modified derivatives of insulin. The insulin derivatives of the invention are, in one aspect, suitable for delivery to the lung and exhibit pharmakokinetic and / or pharmacodynamic properties that are significantly improved over native insulin.
Owner:SHEARWATER CORP
Methods for glucagon suppression using modified exendins
InactiveUS7153825B2Saccharide peptide ingredientsVasoactive intestinal peptideDiseaseBiological half-life
We claim a method of lowering plasma glucagon in a subject in need thereof comprising administering to the subject a composition comprising a modified exendin or modified exendin analog, wherein said modification comprises one or more molecule linked to an exendin or the exendin analog wherein said molecule is selected from the group consisiting of polyethylene glycol, gelatin and / or albumin. The modified exendin or the modified exendin analog has activity of suppressing glucagon secretion and / or lowering glucagon levels in the subject and possesses increased biological half-life compared to unmodified exendin or unmodified exendin analog. The method is useful in treating hyperglucagonemia and other disorders that would be benefited by lowering plasma glucagon or suppressing glucagon secretion.
Owner:AMYLIN PHARMA INC
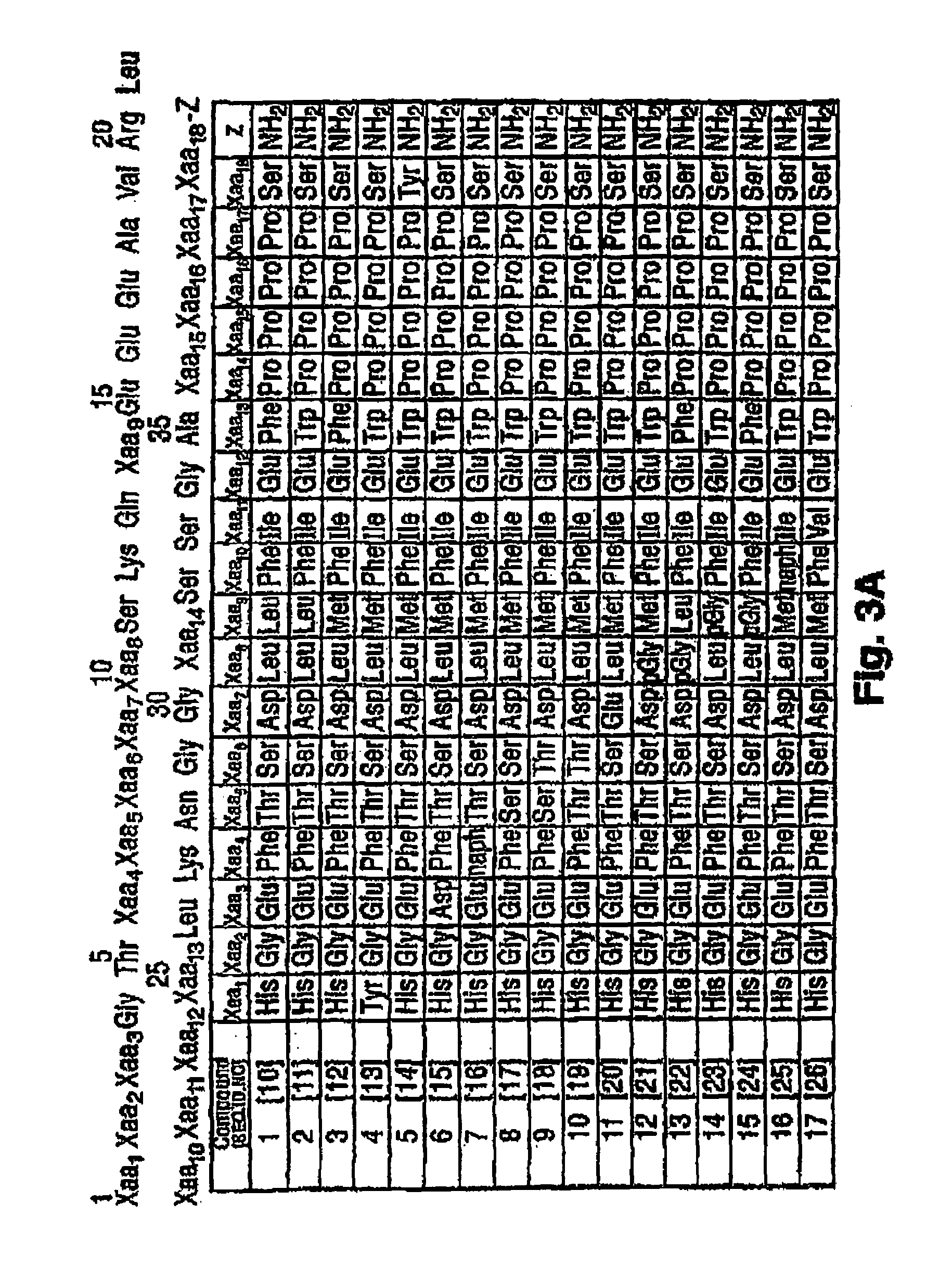
Peptide/lipid complex formation by co-lyophilization
The invention relates to the formation of peptide / lipid vesicles and complexes through the co-lyophilization of peptides, preferably that are able to adopt an amphipathic alphahelical conformation, and one or more lipids. A single solution which solubilizes both the peptides and lipids or two separate solutions may be lyophilized.
Owner:ESPERION THERAPEUTICS
Pharmaceutical composition of nanoparticles
InactiveUS8257740B1Enhances intestinal epithelial barrier functionReduce intestinal permeabilityPowder deliveryPharmaceutical non-active ingredientsNanoparticleMedicine
The invention discloses a composition of chitosan-shelled nanoparticles and methods of manufacturing. The chitosan-shelled nanoparticles are characterized with a positive surface charge and enhanced epithelial ermeability for oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
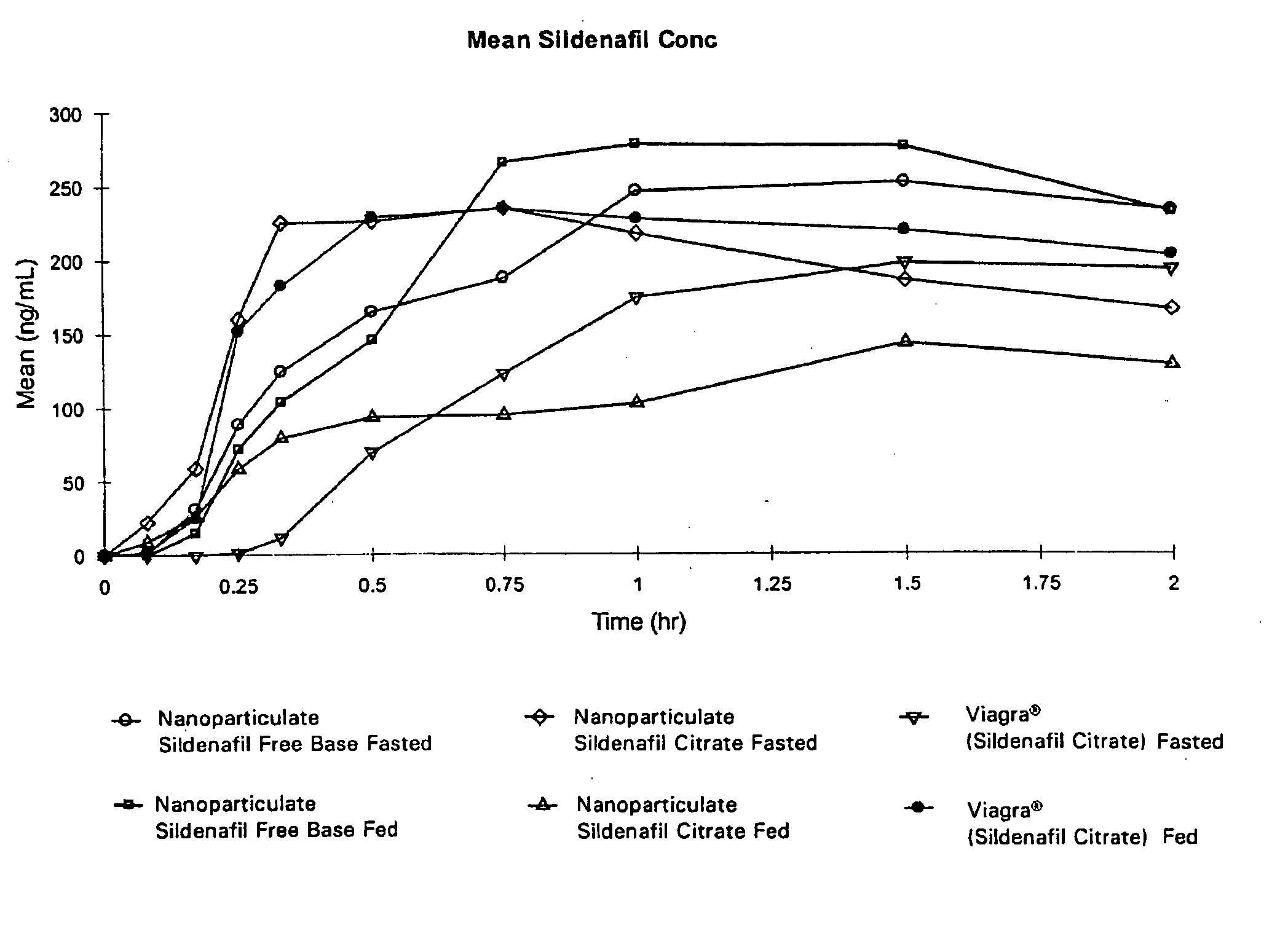
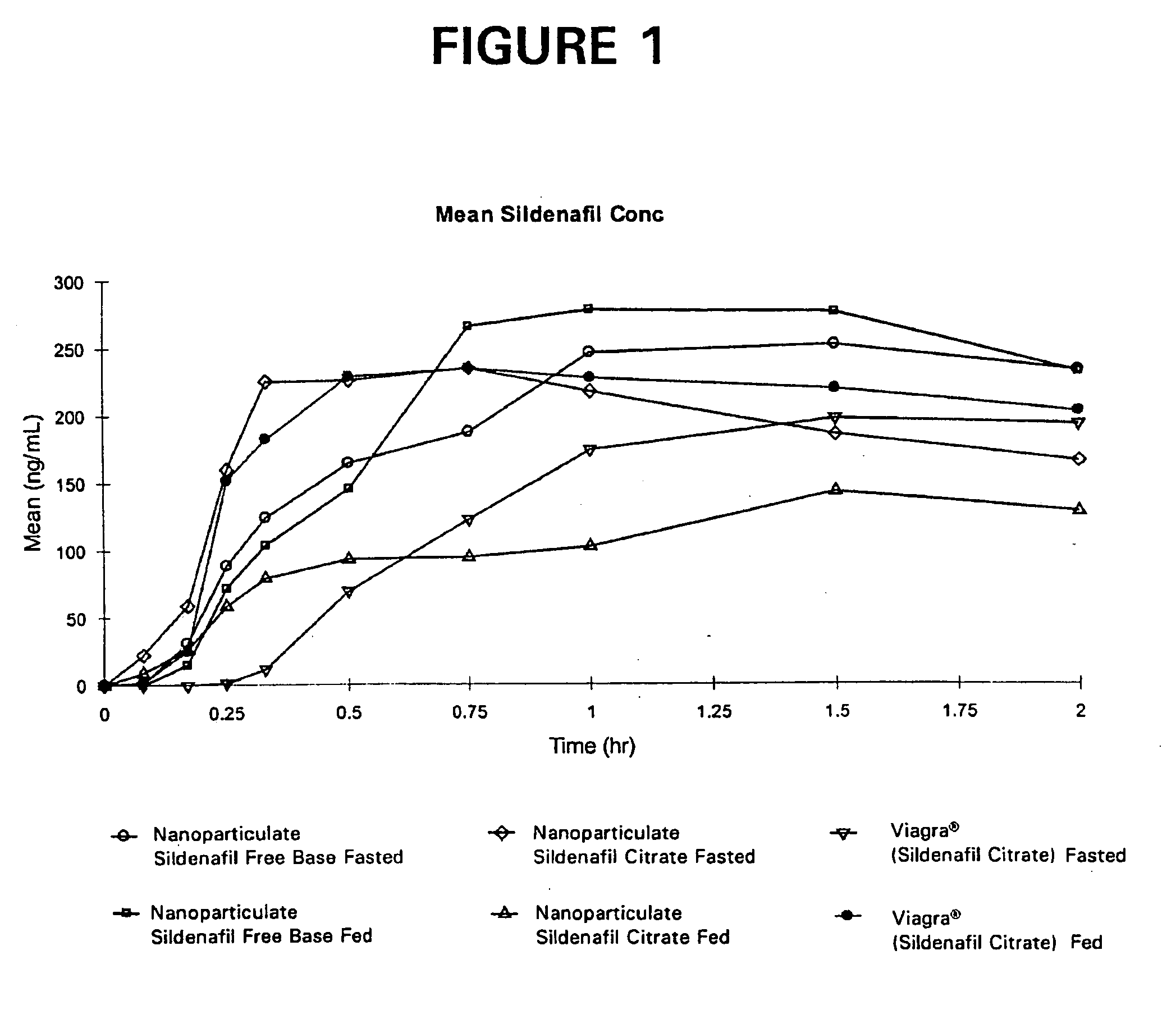
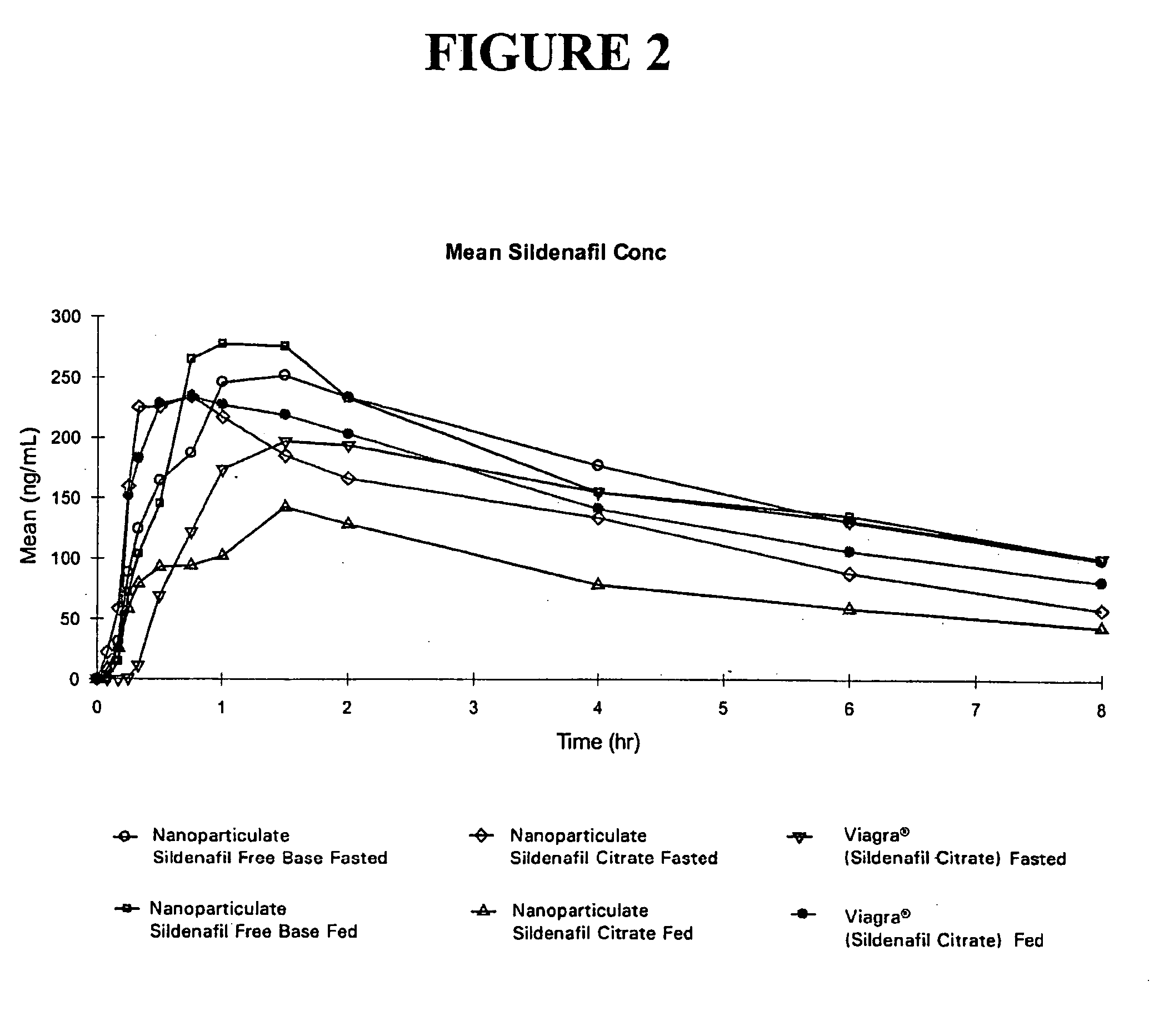
Novel compositions of sildenafil free base
InactiveUS20050042177A1Eliminate the effects ofOrganic active ingredientsPowder deliveryMicroparticleMedicinal chemistry
The present invention is directed to nanoparticulate compositions comprising sildenafil free base. The sildenafil free base particles of the composition have an effective average particle size of less than about 2000 nm.
Owner:ALKERMES PHARMA IRELAND LTD
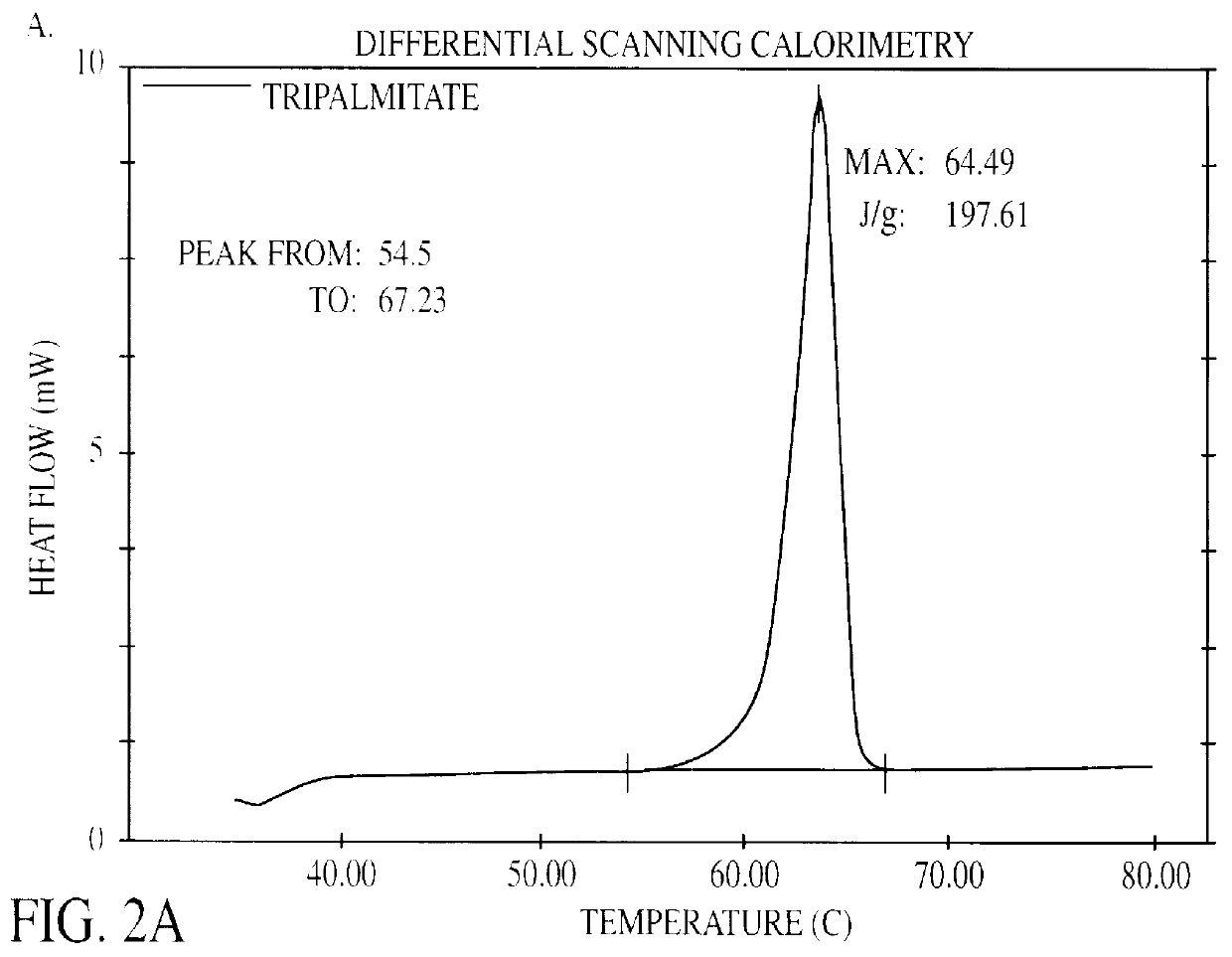
Solid lipid particles, particles of bioactive agents and methods for the manufacture and use thereof
InactiveUS6207178B1Suppresses decrease in specific surface areaImprove bioavailabilityBiocideCosmetic preparationsLipid formationLipid particle
The present invention is in the area of administration forms and delivery systems for drugs, vaccines and other biologically active agents. More specifically the invention is related to the preparation of suspensions of colloidal solid lipid particles (SLPs) of predominantly anisometrical shape with the lipid matrix being in a stable polymorphic modification and of suspensions of micron and submicron particles of bioactive agents (PBAs); as well as to the use of such suspensions or the lyophilizates thereof as delivery systems primarily for the parenteral administration of preferably poorly water-soluble bioactive substances, particularly drugs, and to their use in cosmetic, food and agricultural products. SLPs and PBAs are prepared by the following emulsification process: (1) A solid lipid or bioactive agent or a mixture of solid lipids or bioactive agents is melted. (2) Stabilizers are added either to the lipid or bioactive agent and to the aqueous phase or to the aqueous phase only depending on their physicochemical characteristics. Stabilizers may also be added or exchanged after homogenization. (3) Drugs or other bioactive substances to be incorporated into the SLPs may be melted together with the lipids if the physicochemical characteristics of the substance permit or may be dissolved, solubilized or dispersed in the lipid melt before homogenization. (4) The aqueous phase is heated to the temperature of the melt before mixing and may contain for example stabilizers, isotonicity agents, buffering substances, cryoprotectants and / or preservatives. (5) The molten lipid compounds and the bioactive agents are emulsified in an aqueous phase preferably by high-pressure homogenization.
Owner:PHARMACIA AB
Composition, method of preparation & application of concentrated formulations of condensed nucleic acids with a cationic lipopolymer
UndeterminedUS20090042825A1Increase efficiency and dosing flexibilitySpecial deliveryPeptide/protein ingredientsFiller ExcipientCholesterol
Compositions, methods, and applications that increase the efficiency of nucleic acid transfection are provided. In one aspect, a pharmaceutical composition may include at least about 0.5 mg / ml concentration of a nucleic acid condensed with a cationic lipopolymer suspended in an isotonic solution, where the cationic lipopolymer includes a cationic polymer backbone having cholesterol and polyethylene glycol covalently attached thereto, and wherein the molar ratio of cholesterol to cationic polymer backbone is within a range of from about 0.1 to about 10, and the molar ratio of polyethylene glycol to cationic polymer backbone is within a range of from about 0.1 to about 10. The composition further may include a filler excipient.
Owner:EXPRESSION GENETICS INC
Intracutaneous injection
ActiveUS20060211982A1Minimize injectionMinimize medicationPeptide/protein ingredientsAutomatic syringesSlurryIntracutaneous injection
The delivery of biopharmaceutical and other therapeutic agents parenterally to an animal via a minimally invasive, low pain administration is provided. The agents are delivered to the patient via, e.g., the epidermal, dermal, or subcutaneous layer of the skin in a concentrated form of injectable paste of slurry.
Owner:XERIS PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com