Patents
Literature
55results about How to "Reduce intestinal permeability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
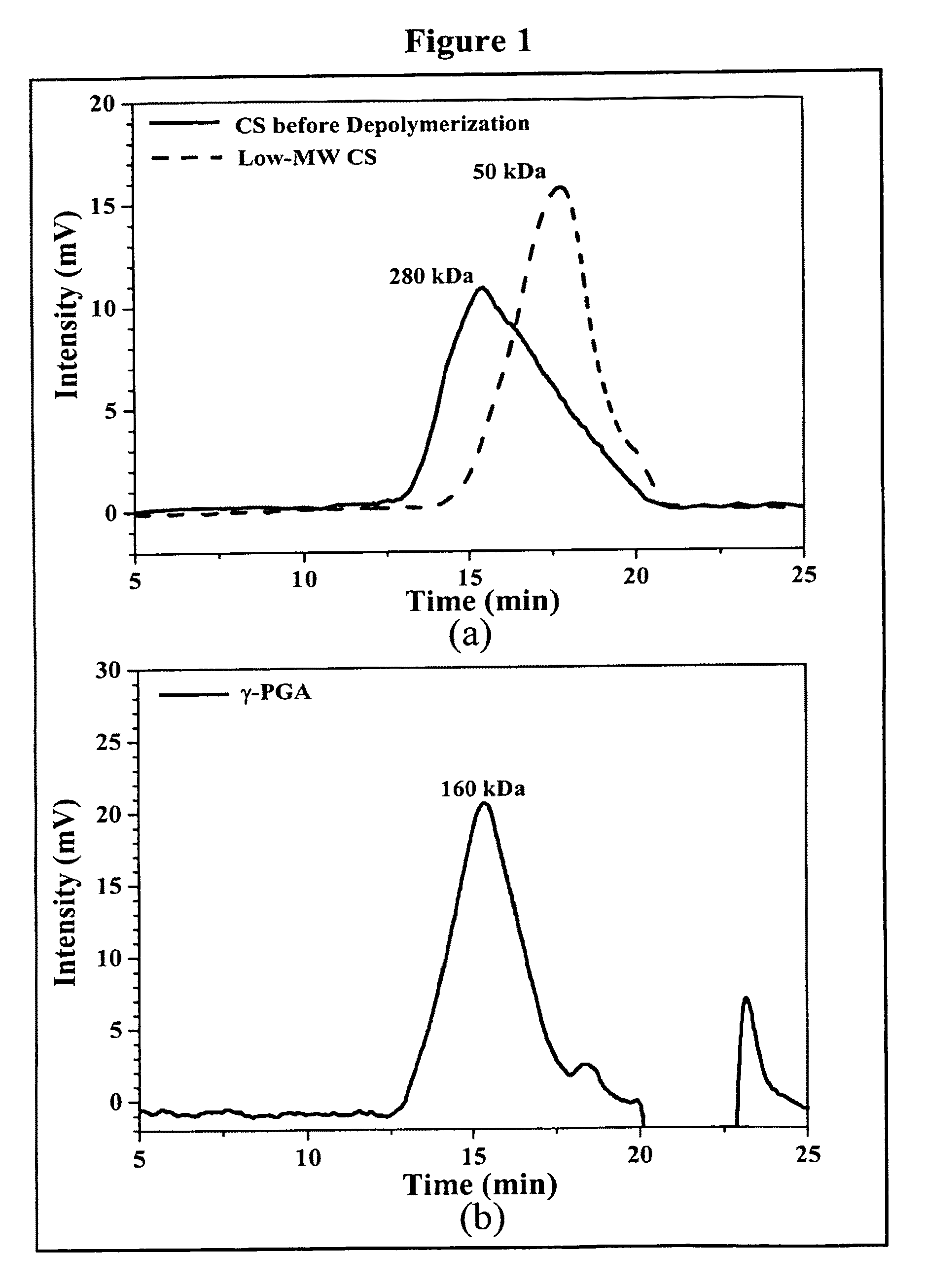
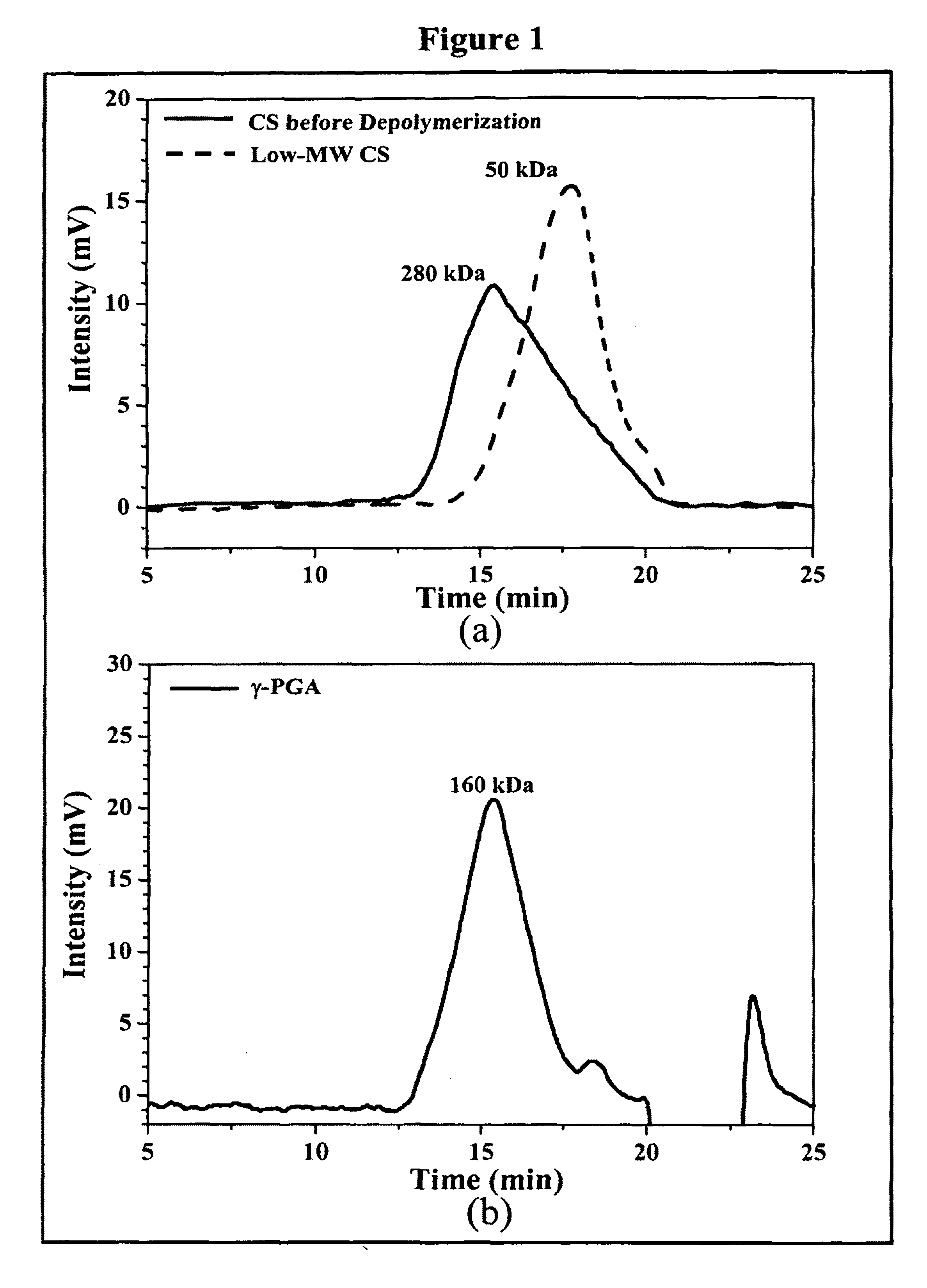
Pharmaceutical composition of nanoparticles
InactiveUS8257740B1Enhances intestinal epithelial barrier functionReduce intestinal permeabilityPowder deliveryPharmaceutical non-active ingredientsNanoparticleMedicine
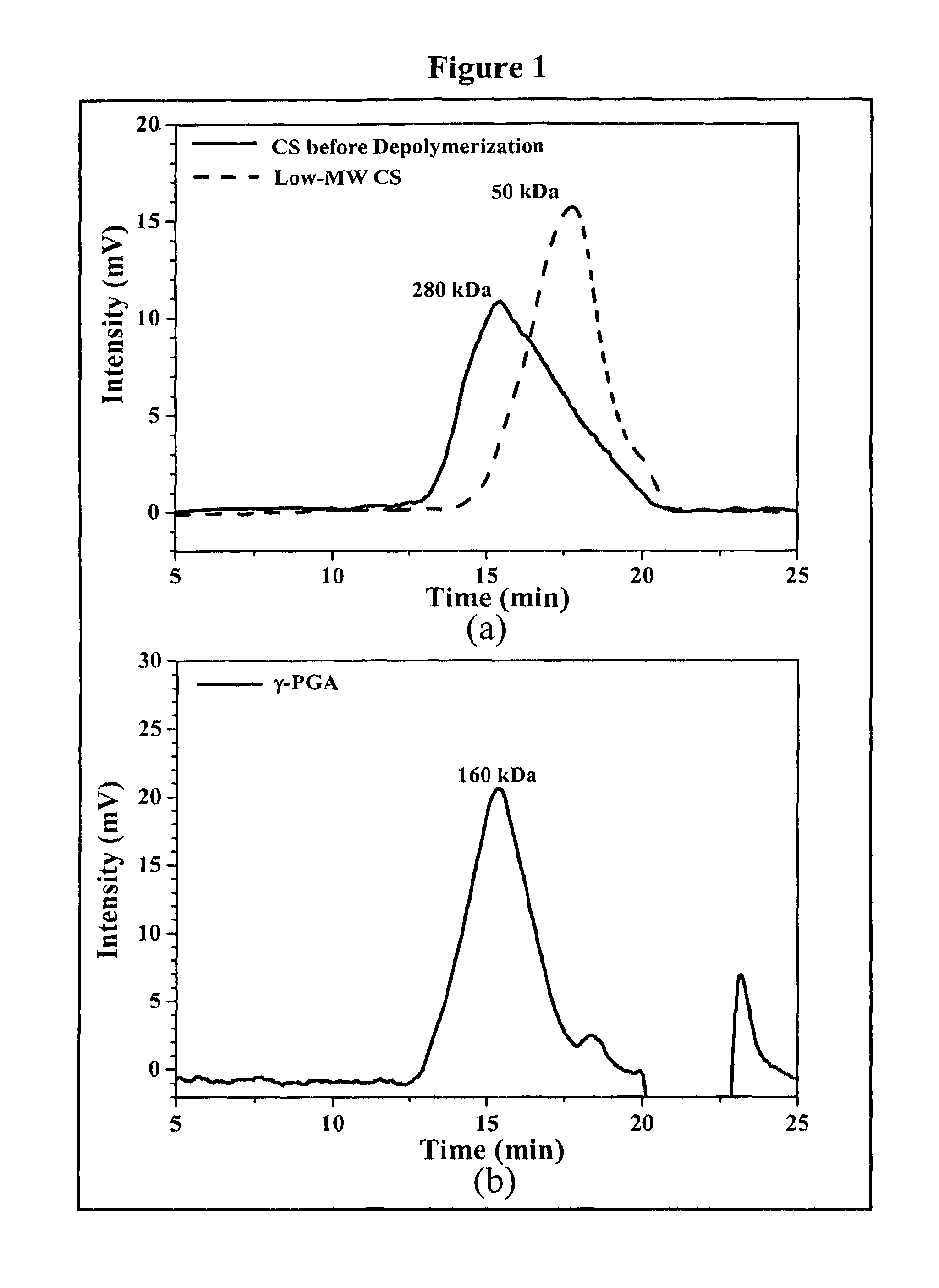
The invention discloses a composition of chitosan-shelled nanoparticles and methods of manufacturing. The chitosan-shelled nanoparticles are characterized with a positive surface charge and enhanced epithelial ermeability for oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
Diagnosis and treatment of autism spectrum disorder
ActiveUS20140065132A1Improve behaviorImprove performanceBiocideNervous disorderClinical psychologyAutism spectrum disorder
Disclosed herein are compositions, systems, and methods for diagnosing and treatment of subjects suffering from anxiety, autism spectrum disorder (ASD), or a pathological condition with one or more of the symptoms of ASD.
Owner:CALIFORNIA INST OF TECH
Composition with docosapentaenoic acid
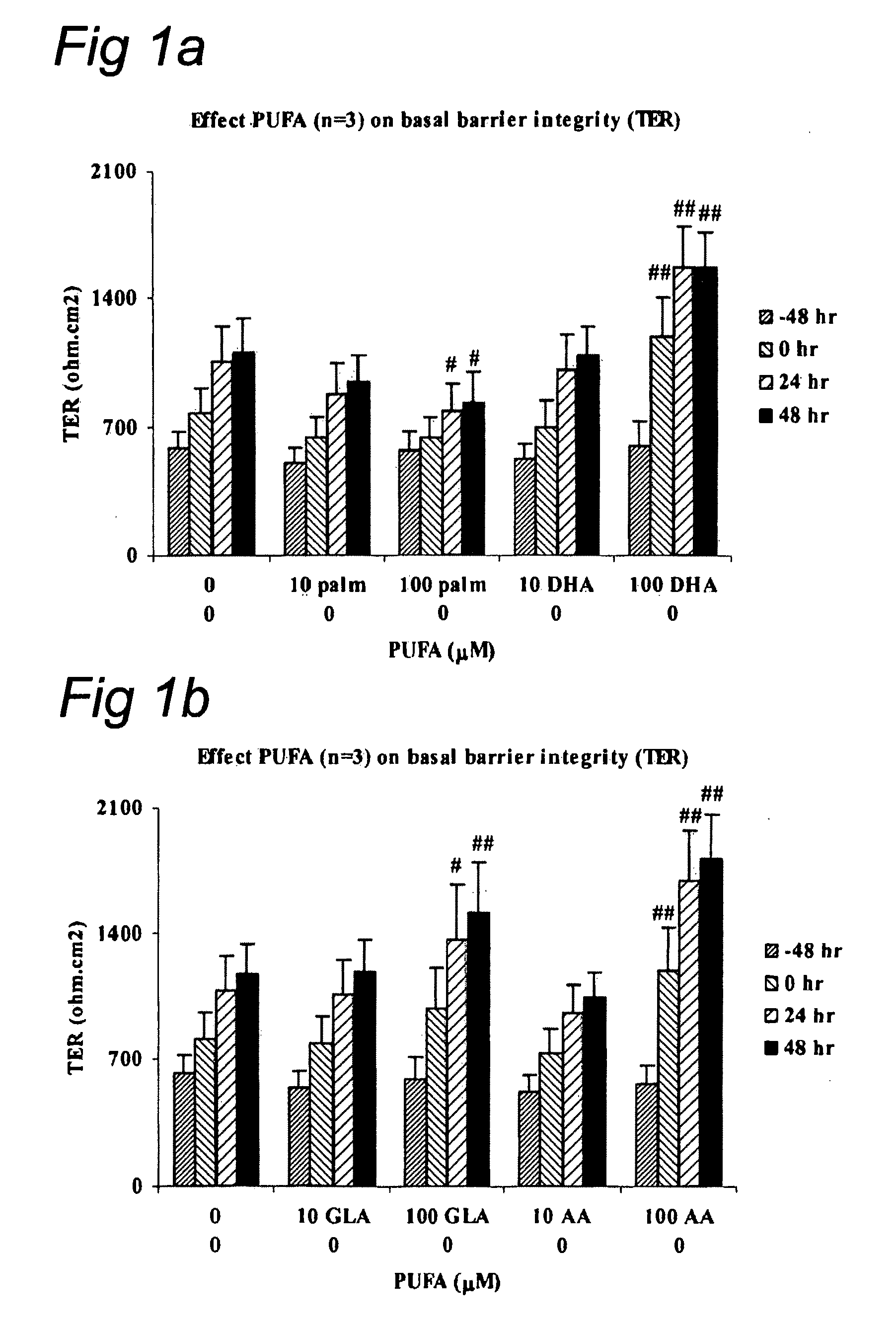
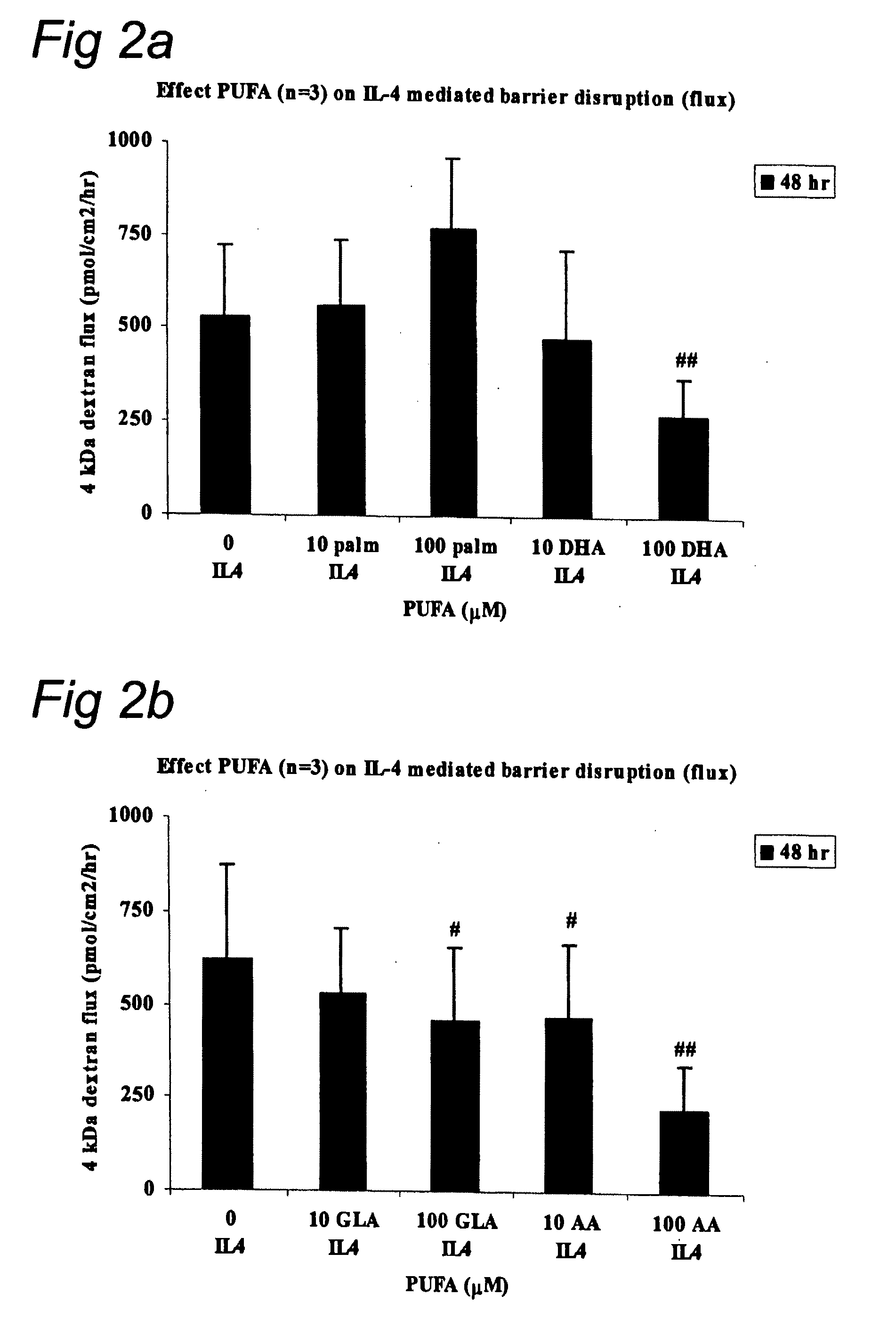
InactiveUS20090054329A1Stimulate barrier integrityImproving barrier integrityBiocidePeptide/protein ingredientsEPA - Eicosapentaenoic acidBarrier integrity
This invention concerns a method for stimulating barrier integrity in a subject by administering to a subject a composition comprising docosapentaenoic acid (22:5 n3; DPA). Also the invention concerns a composition comprising DPA and eicosapentaenoic acid (EPA).
Owner:NUTRICIA
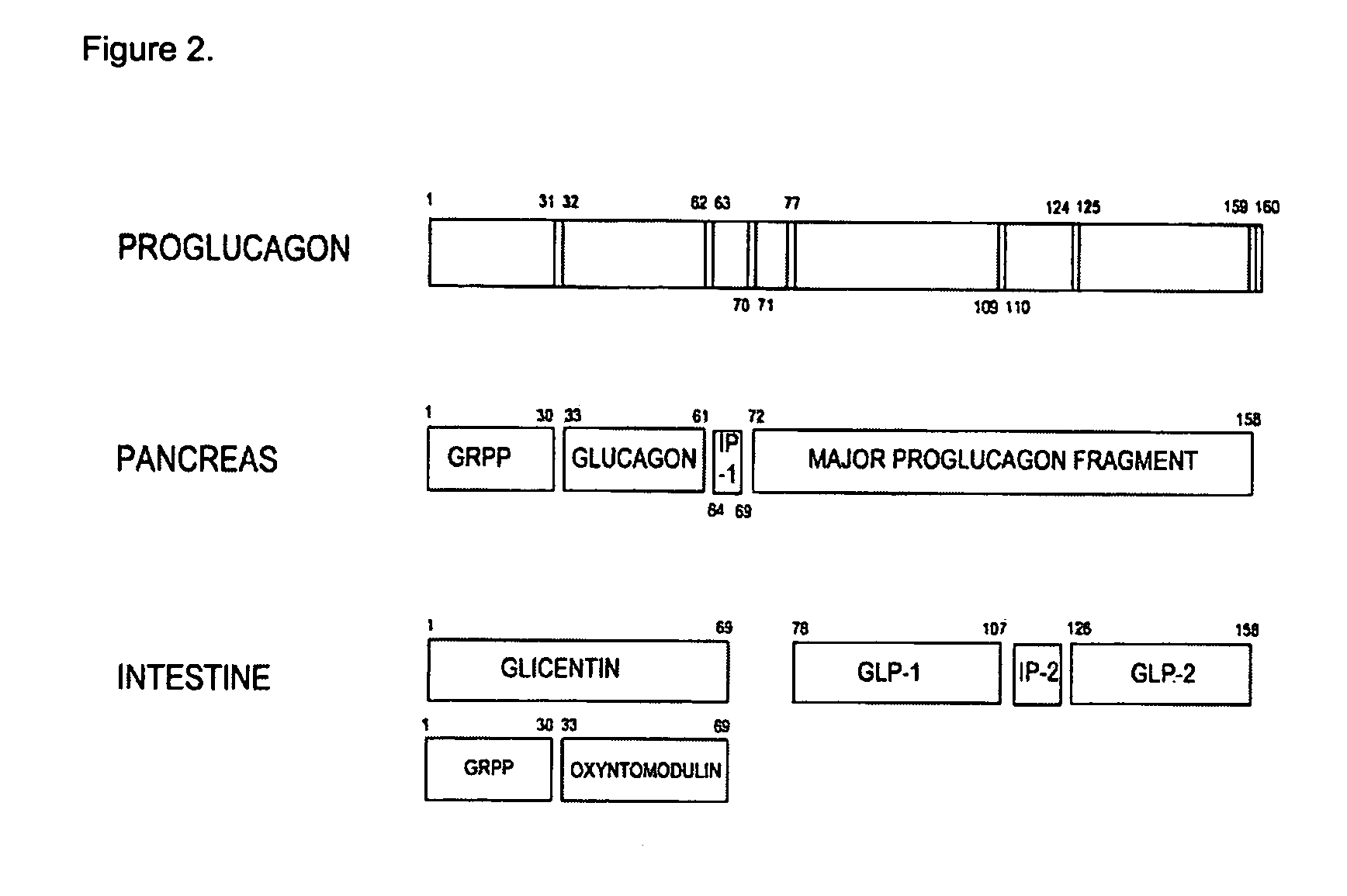
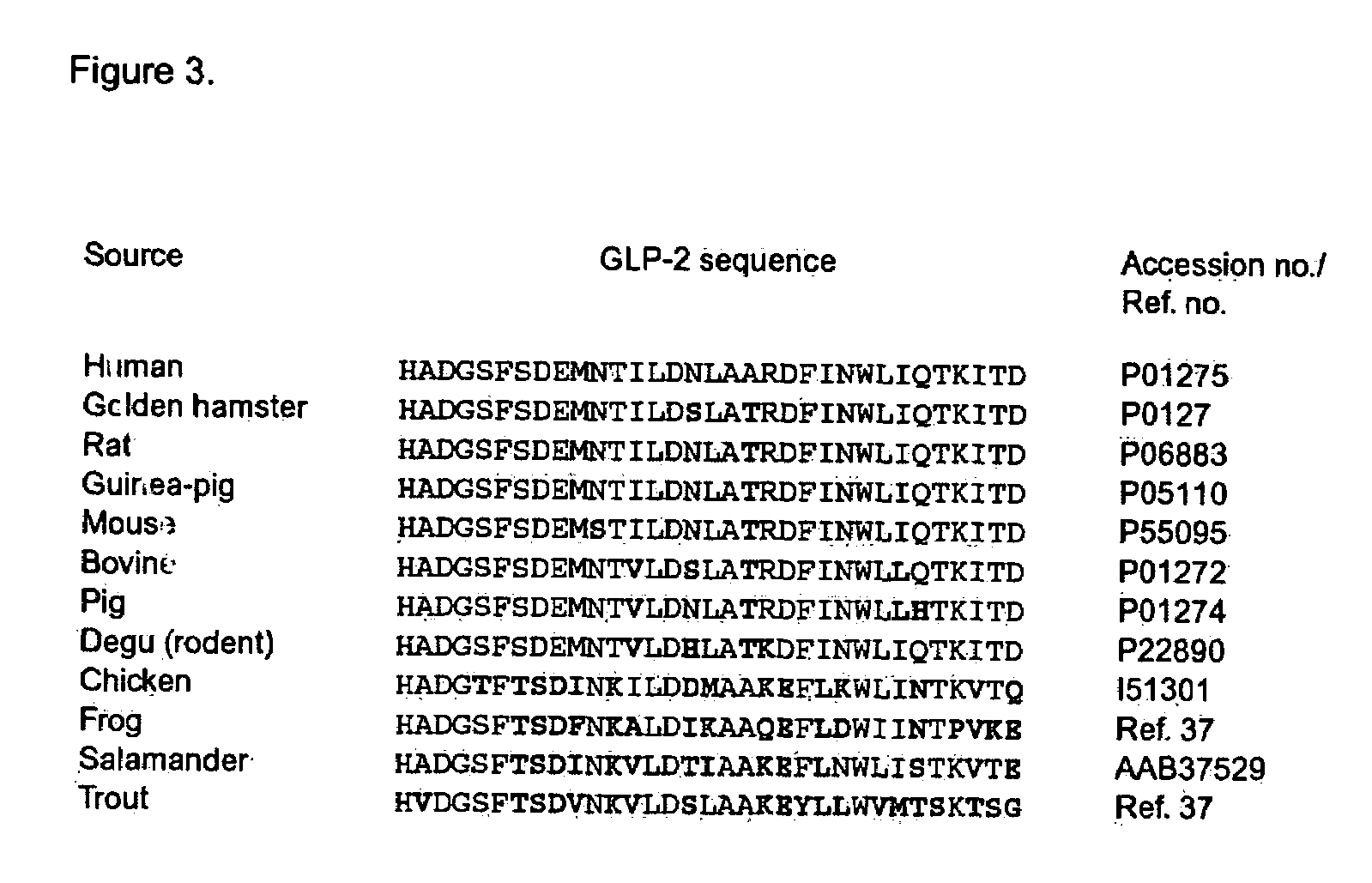
GLP-2 compounds, formulations, and uses thereof
InactiveUS7411039B2Reduces intestinal permeabilityInhibit gastric emptying and gastric acid secretionPeptide/protein ingredientsImmunoglobulinsPeptideChemistry
The present invention relates to novel human glucagon-like peptide-2 (GLP-2) peptides and human glucagon-like peptide-2 derivatives which have a protracted profile of action as well as polynucleotide constructs encoding such peptides, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions, uses and methods of treatment.
Owner:NOVO NORDISK AS
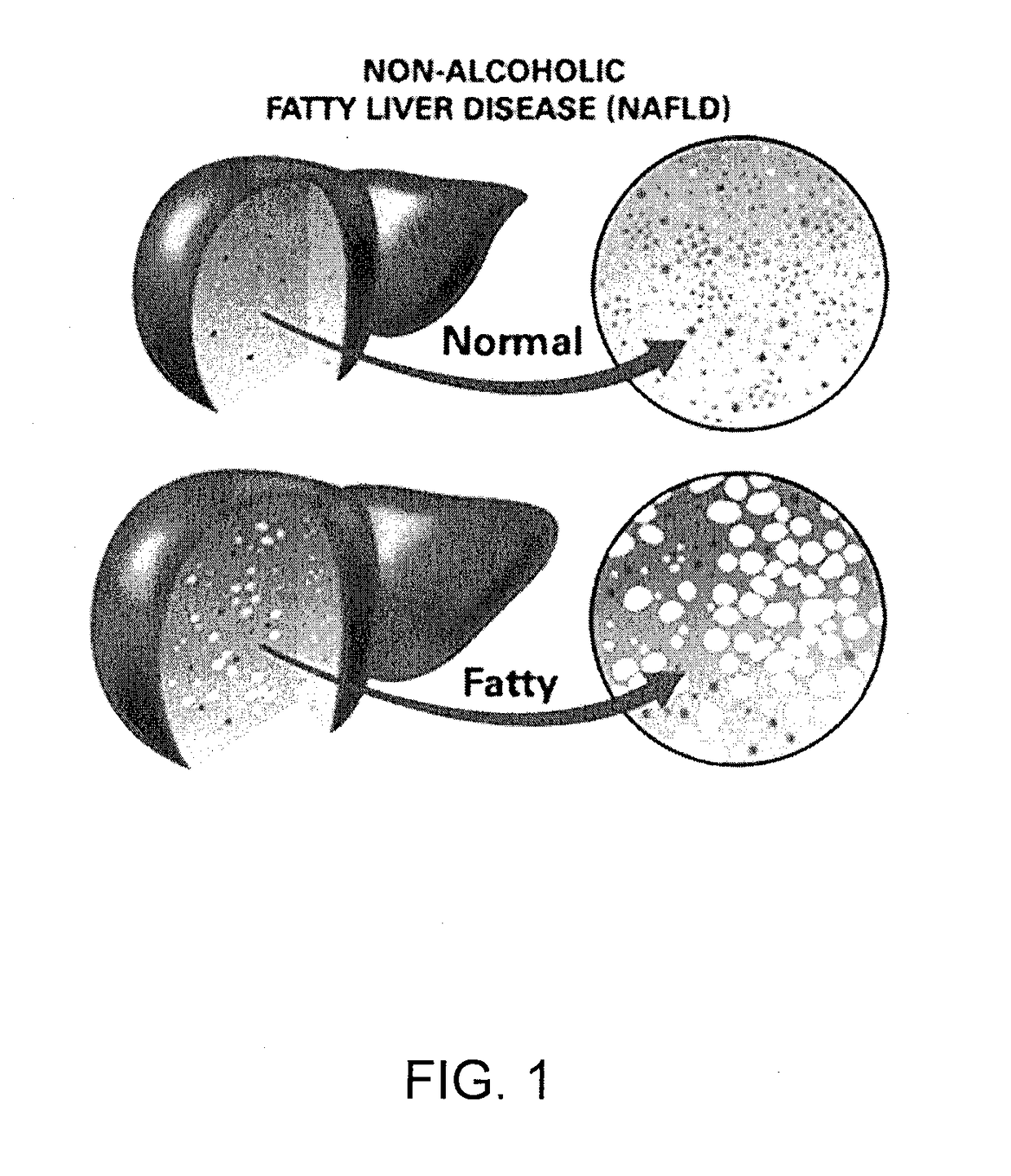
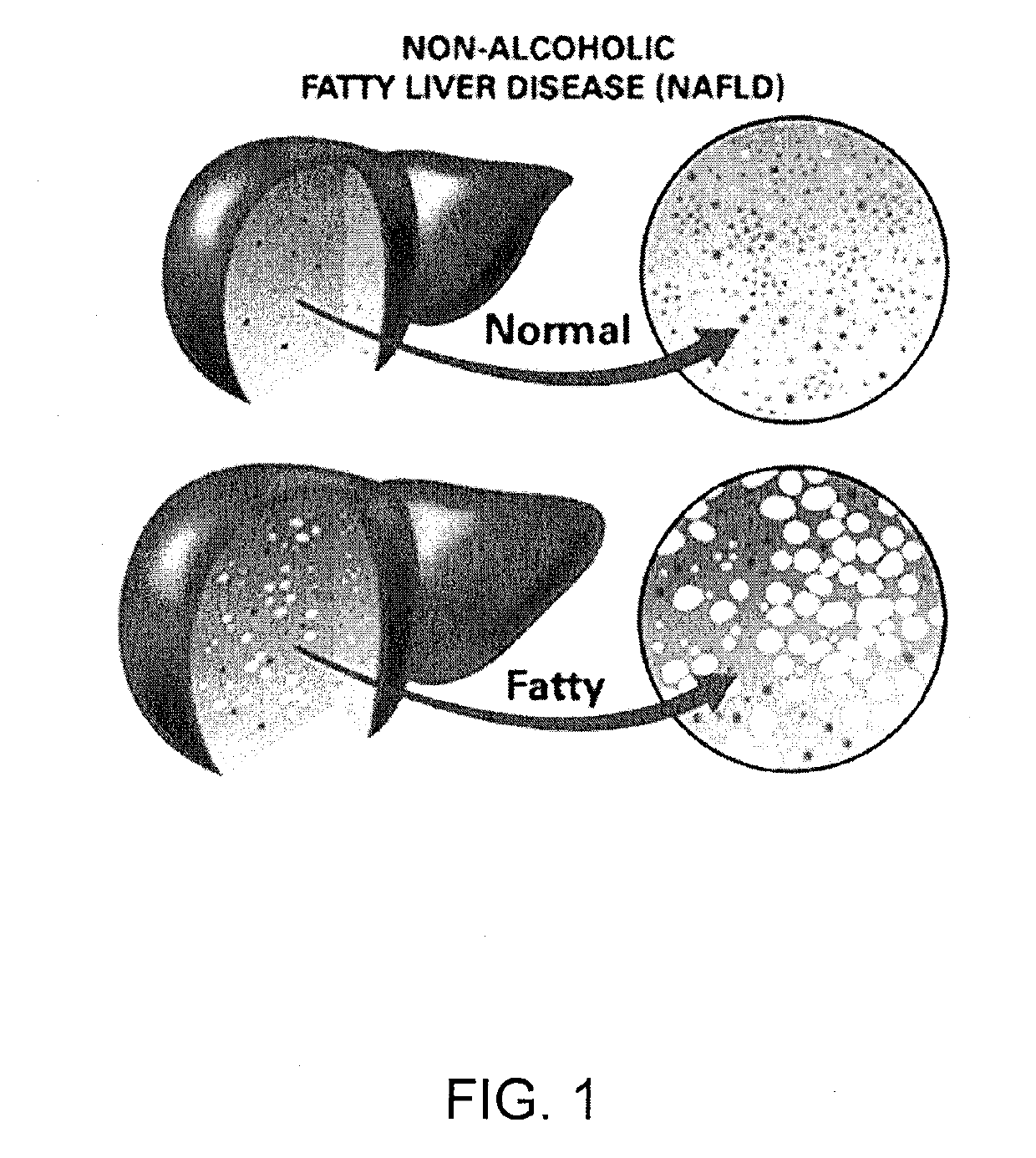
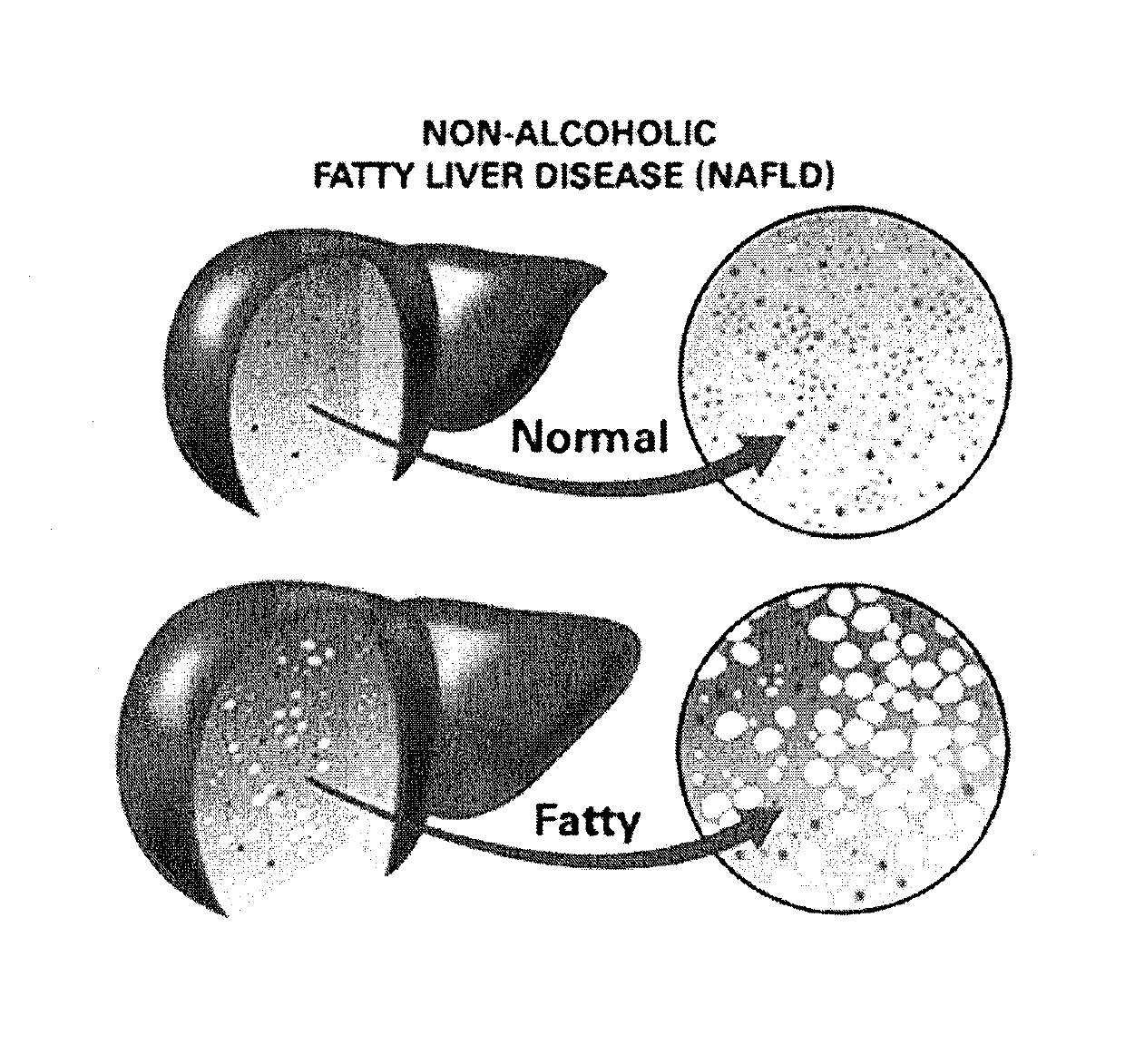
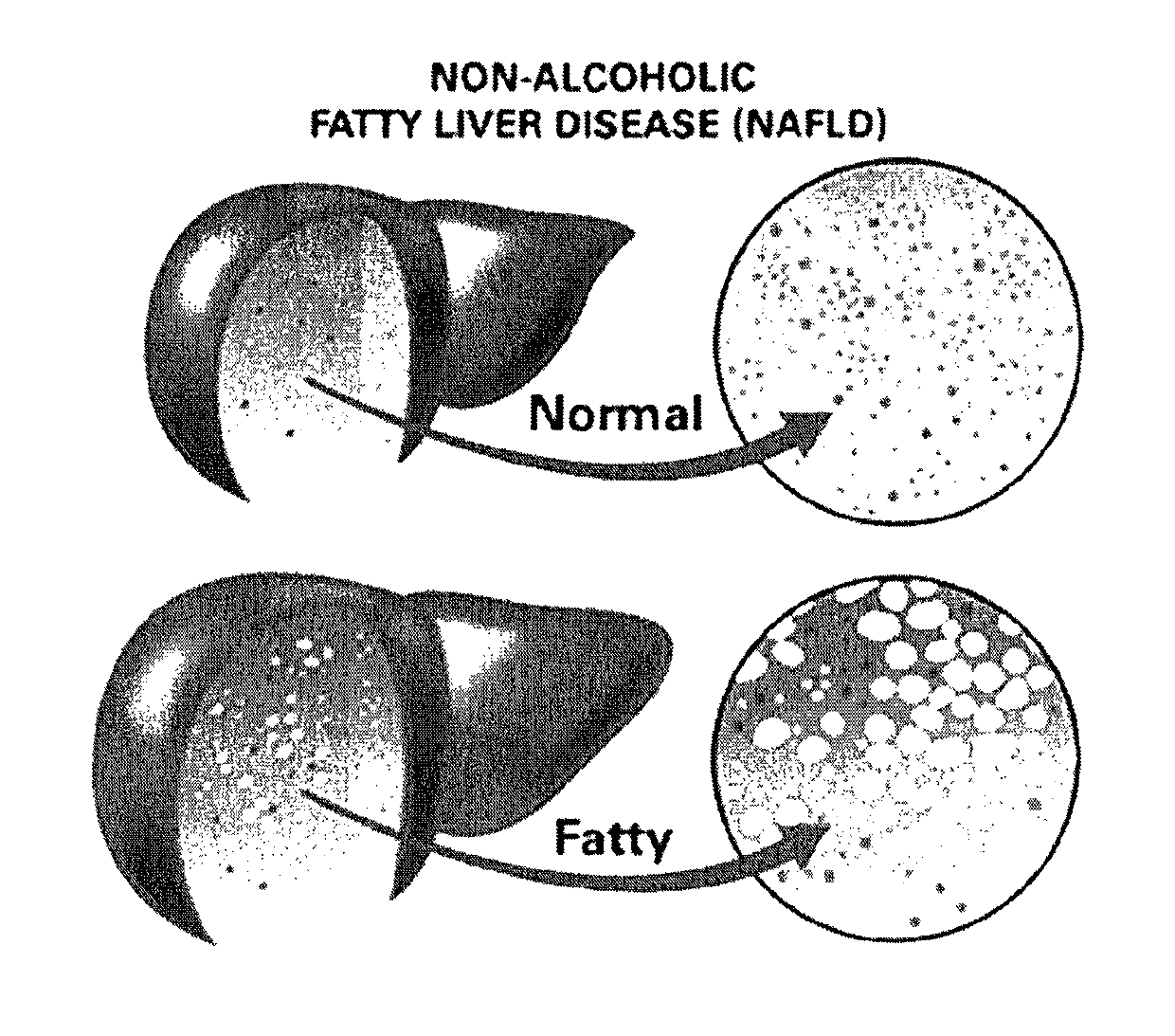
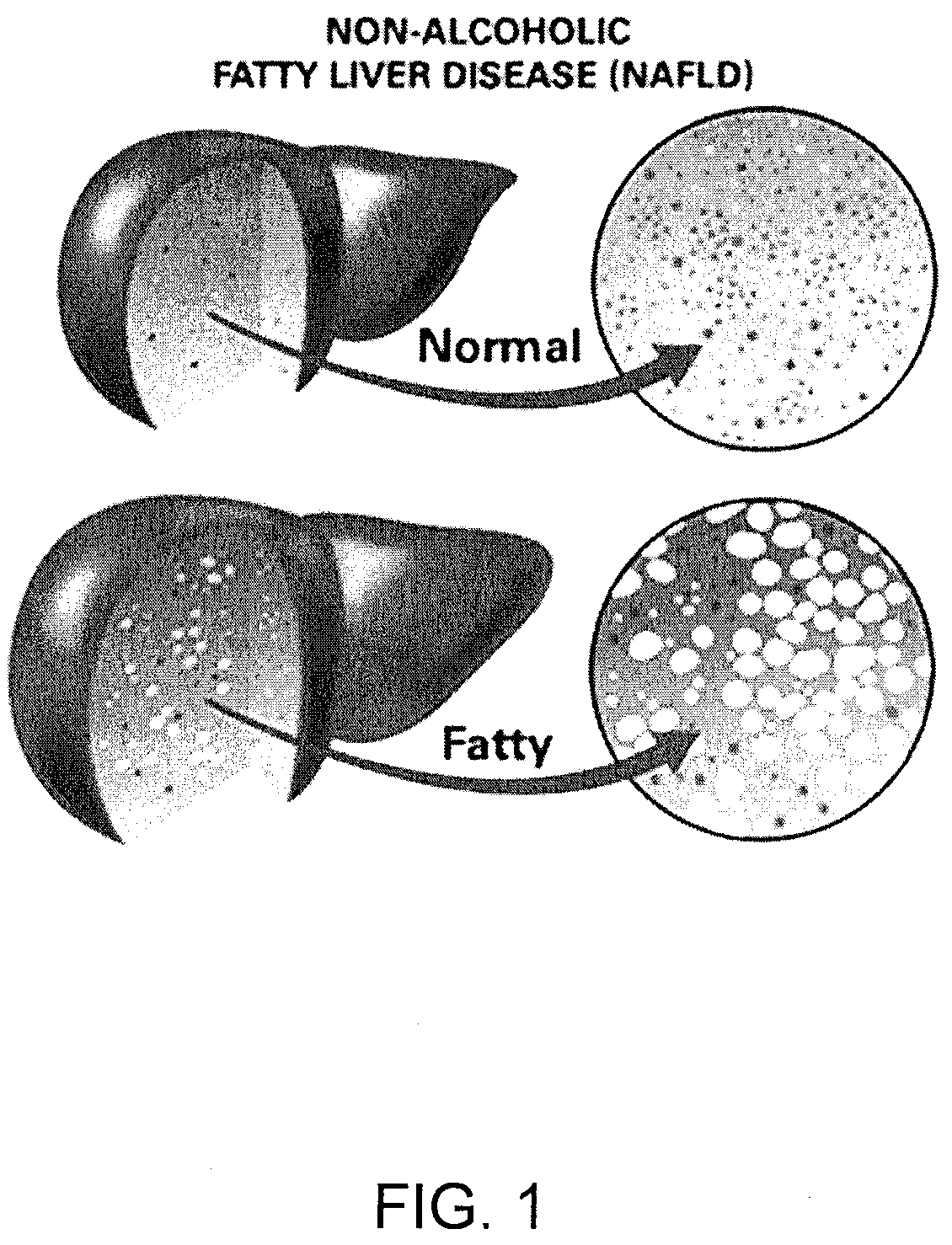
Method and System for Treating Non-Alcoholic Fatty Liver Disease
ActiveUS20170106026A1Inhibit progressEarly detectionUnknown materialsObesity treatmentIntestinal microorganismsInsulin resistance
A method and system for treating non-alcoholic fatty liver disease (NAFLD) involves the modulation of the gut microbial of a person suffering from NAFLD, and in particular the provision of a probiotic therapy configured to reduce liver aminotransferases, total-cholesterol, TNF-α and to improve insulin resistance in NAFLD patients.
Owner:SEED HEALTH INC
Method and system for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease
ActiveUS20190117709A1Inhibit progressEarly detectionOrganic active ingredientsDigestive systemFiberMonoacylglycerol acyltransferase
A method for reducing the likelihood of developing non-alcoholic steatohepatitis (NASH) in an individual diagnosed with non-alcoholic fatty liver disease involves providing in the gut of an individual a population of beneficial bacteria selected from the group consisting of Lactobacillus species, and at least 6 grams per day of fiber to the individual to maintain a therapeutically effective amount of the beneficial bacteria in the gut of the individual. In certain embodiments, monoacylglycerolacyltransferase-3 (MGAT3) synthesis is inhibited to lower triacylglycerol (TAG) production, while in others, expression of diacylglycerolacyltransferase-2 (DGAT-2) is inhibited. The beneficial bacteria are preferably modified to produce increased amounts of butyrate and are also encapsulated in a frangible enclosure. Levels of Roseburia are preferably increased while the levels of Akkermansia spp. in the individual's gut microbiome are reduced.
Owner:SEED HEALTH INC
Pharmaceutical composition of nanoparticles
InactiveUS8535640B1Sufficient solubilityEnhances intestinal epithelial barrier functionOrganic active ingredientsPowder deliveryNanoparticleSurface charges
The invention discloses a composition of nanoparticles composed of positively charged chitosan, one negatively charged substrate, and optionally a zero-charge compound or at least one tumor necrosis factor inhibitor for oral delivery. The chitosan-based nanoparticles are characterized with a positive surface charge and enhanced permeability for oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
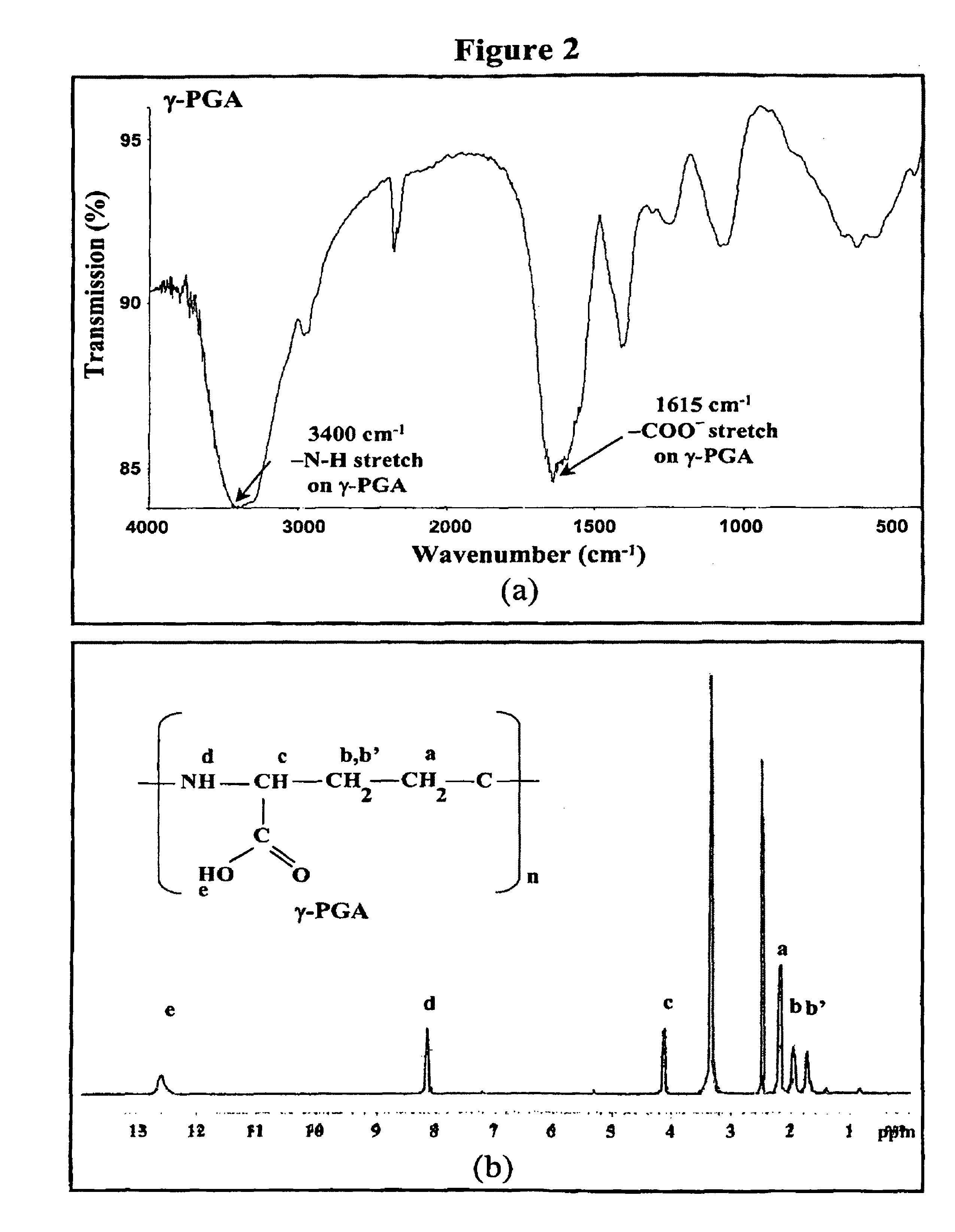
Pharmaceutical composition of nanoparticles
InactiveUS8361439B1Sufficient solubilityEnhances intestinal epithelial barrier functionPowder deliveryOrganic active ingredientsNanoparticleActive agent
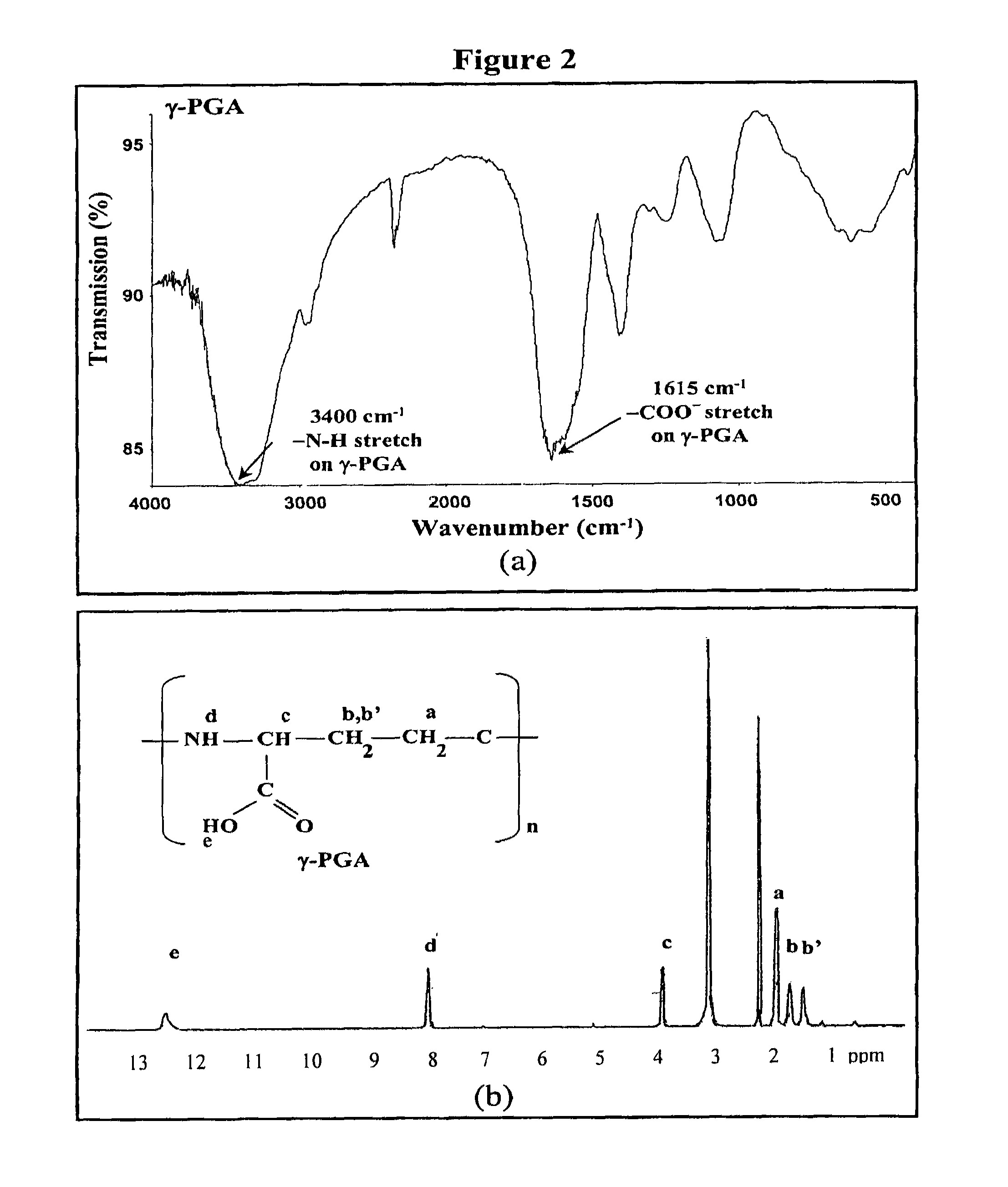
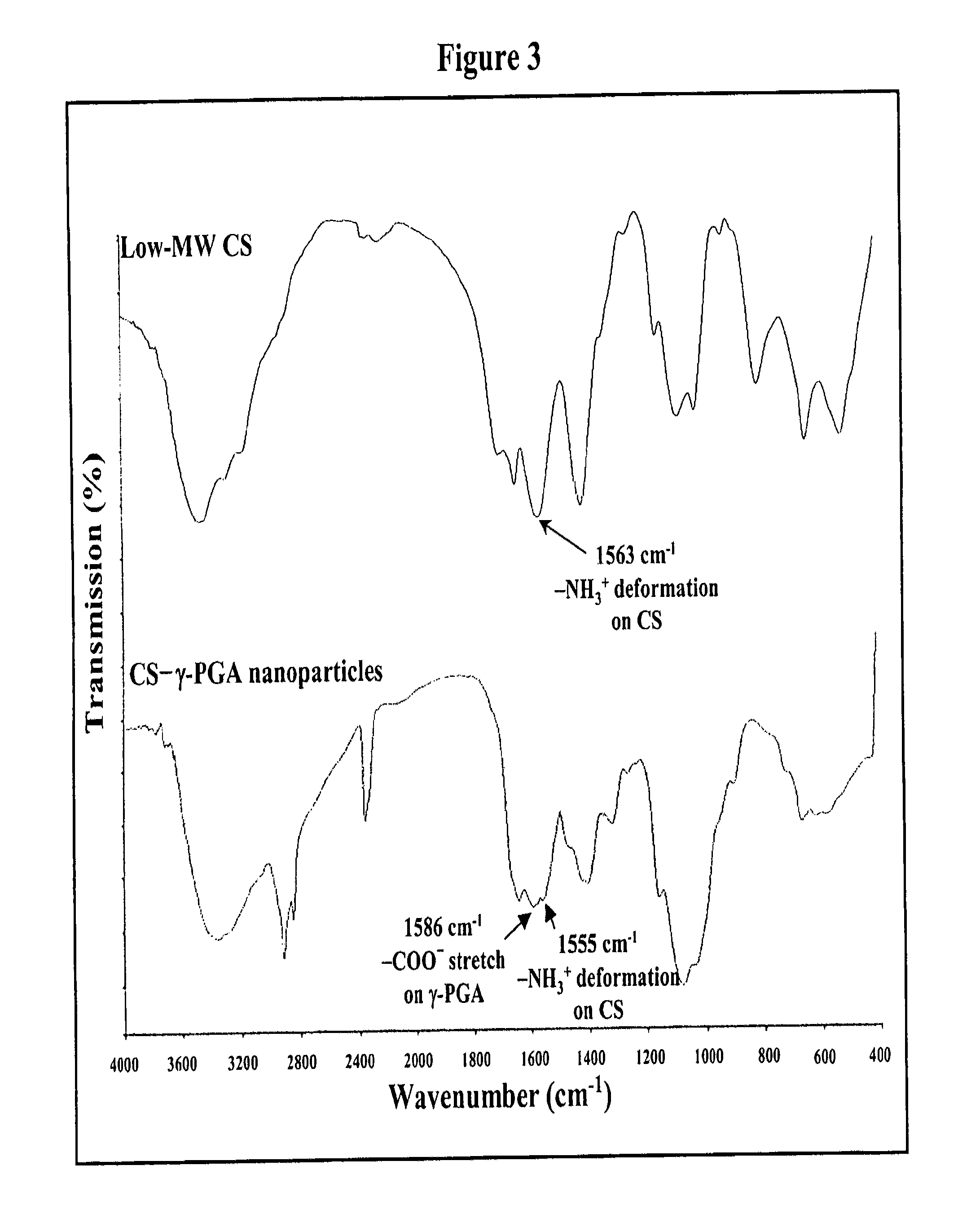
The invention discloses a pharmaceutical composition of bioactive nanoparticles composed of chitosan, poly-glutamic acid, and a bioactive agent for oral delivery. The chitosan-based nanoparticles are characterized with a positive surface charge and enhanced permeability for oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
Pharmaceutical composition of nanoparticles for protein drug delivery
InactiveUS8048404B1Enhances intestinal epithelial barrier functionReduce intestinal permeabilityPowder deliveryIn-vivo radioactive preparationsActive agentNanoparticle
The invention discloses a pharmaceutical composition of bioactive nanoparticles composed of chitosan, poly-glutamic acid, and a bioactive agent for oral delivery. The chitosan-based nanoparticles are characterized with a positive surface charge and enhanced permeability for oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
Barrier integrity in HIV patients
InactiveUS20100167982A1Reducing mucosal productionImproving intestinal integrityBiocidePeptide/protein ingredientsDocosahexaenoic acidEicosapentaenoic acid
The invention concerns a method for stimulating intestinal barrier integrity in a patient infected with HIV by administering to said patient composition comprising: eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and arachidonic acid (ARA), and at least two distinct oligosaccharides.
Owner:NV NUTRICIA
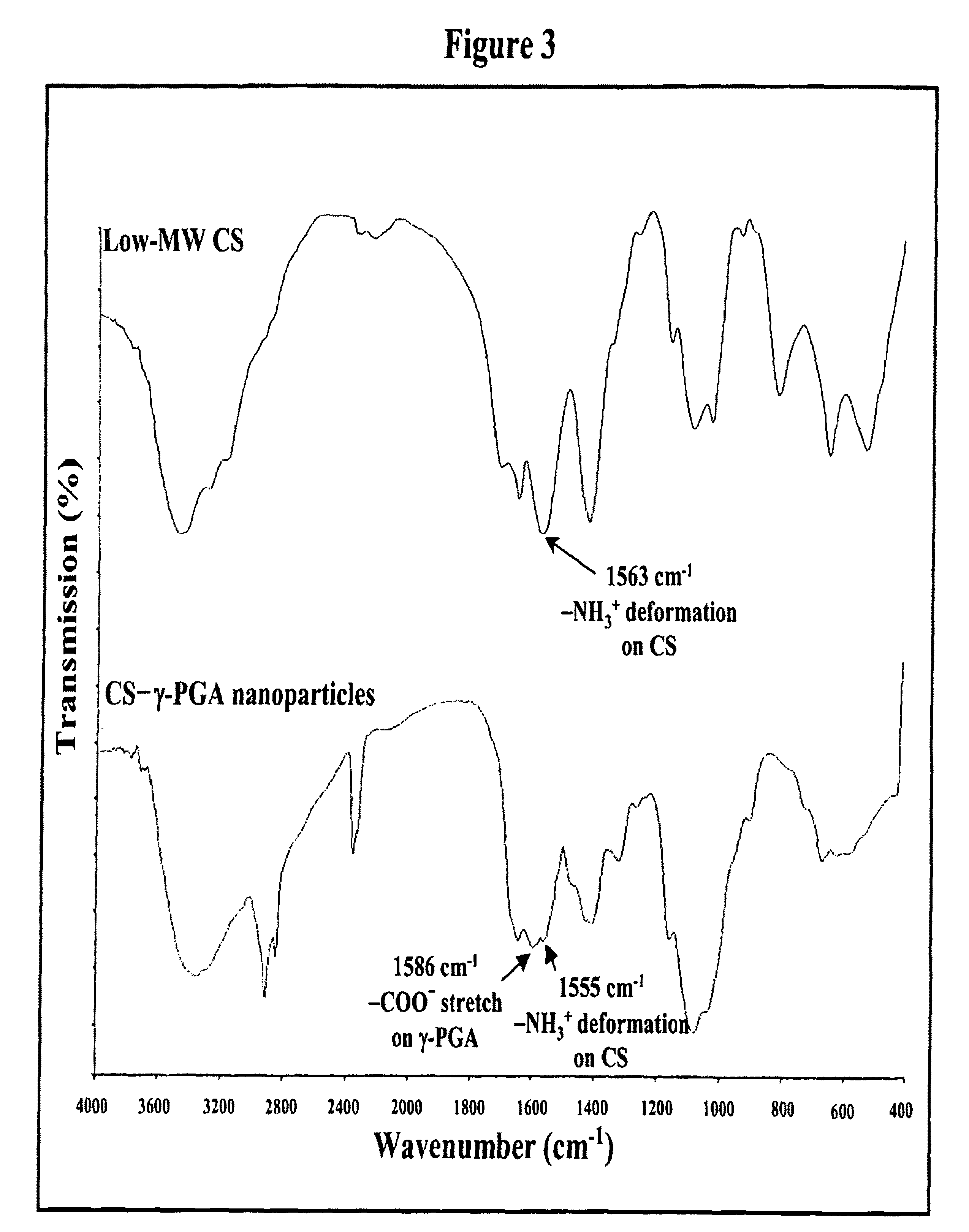
Nanoparticles for protein drug delivery
InactiveUS7998458B2Reduce resistanceEasy to transportIn-vivo radioactive preparationsPeptide/protein ingredientsMedicineNanoparticle
The invention discloses the nanoparticles composed of chitosan, poly-glutamic acid, and at least one anti-hemophilic factor or bioactive agent characterized with a positive surface charge and their enhanced permeability in oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
Pharmaceutical composition of nanoparticles for protein drug delivery
InactiveUS20120003306A1Enhances intestinal epithelial barrier functionReduce intestinal permeabilityPowder deliveryBiocideActive agentNanoparticle
The invention discloses a pharmaceutical composition of bioactive nanoparticles composed of chitosan, poly-glutamic acid, and a bioactive agent for oral delivery. The chitosan-based nanoparticles are characterized with a positive surface charge and enhanced permeability for oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
Pharmaceutical composition of nanoparticles
InactiveUS8202839B1Sufficient solubilityEnhances intestinal epithelial barrier functionPowder deliveryOrganic active ingredientsNanoparticleSurface charges
The invention discloses a pharmaceutical composition of bioactive nanoparticles composed of chitosan, poly-glutamic acid, and a bioactive agent for oral delivery. The chitosan-based nanoparticles are characterized with a positive surface charge and enhanced permeability for oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
Pharmaceutical composition of nanoparticles
InactiveUS8337811B1Sufficient solubilityEnhances intestinal epithelial barrier functionOrganic active ingredientsPowder deliveryNanoparticleSurface charges
The invention discloses a pharmaceutical composition of bioactive nanoparticles composed of chitosan, poly-glutamic acid, and a bioactive agent for oral delivery. The chitosan-based nanoparticles are characterized with a positive surface charge and enhanced permeability for oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
Method and system for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease
ActiveUS10512661B2Improvements in intestinal dysbiosisReduce intestinal permeabilityOrganic active ingredientsDigestive systemDiseaseTriacylglycerol VLDL
A method for reducing the likelihood of developing non-alcoholic steatohepatitis (NASH) in an individual diagnosed with non-alcoholic fatty liver disease involves providing in the gut of an individual a population of beneficial bacteria selected from the group consisting of Lactobacillus species, and at least 6 grams per day of fiber to the individual to maintain a therapeutically effective amount of the beneficial bacteria in the gut of the individual. In certain embodiments, monoacylglycerolacyltransferase-3 (MGAT3) synthesis is inhibited to lower triacylglycerol (TAG) production, while in others, expression of diacylglycerolacyltransferase-2 (DGAT-2) is inhibited. The beneficial bacteria are preferably modified to produce increased amounts of butyrate and are also encapsulated in a frangible enclosure. Levels of Roseburia are preferably increased while the levels of Akkermansia spp. in the individual's gut microbiome are reduced.
Owner:SEED HEALTH INC
Pharmaceutical composition of nanoparticles
InactiveUS7993625B1Sufficient solubilityEnhances intestinal epithelial barrier functionPowder deliveryIn-vivo radioactive preparationsNanoparticleMedicine
The invention discloses a pharmaceutical composition of bioactive nanoparticles composed of chitosan, poly-glutamic acid, and a pegylated bioactive agent for oral delivery. The chitosan-based nanoparticles are characterized with a positive surface charge and enhanced permeability for oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
Pharmaceutical composition of nanoparticles
InactiveUS8354094B1Sufficient solubilityEnhances intestinal epithelial barrier functionOrganic active ingredientsPowder deliveryNanoparticleActive agent
The invention discloses a pharmaceutical composition of bioactive nanoparticles composed of chitosan, poly-glutamic acid, and a bioactive agent for oral delivery. The chitosan-based nanoparticles are characterized with a positive surface charge and enhanced permeability for oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
Nanoparticles for protein drug delivery
InactiveUS7910086B1Reduce inflammatory responseEnhances intestinal epithelial barrier functionPowder deliveryIn-vivo radioactive preparationsDrug deliveryActive agent
The invention discloses a pharmaceutical composition of bioactive nanoparticles composed of chitosan, poly-glutamic acid, and a bioactive agent for oral delivery. The chitosan-based nanoparticles are characterized with a positive surface charge and enhanced permeability for oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
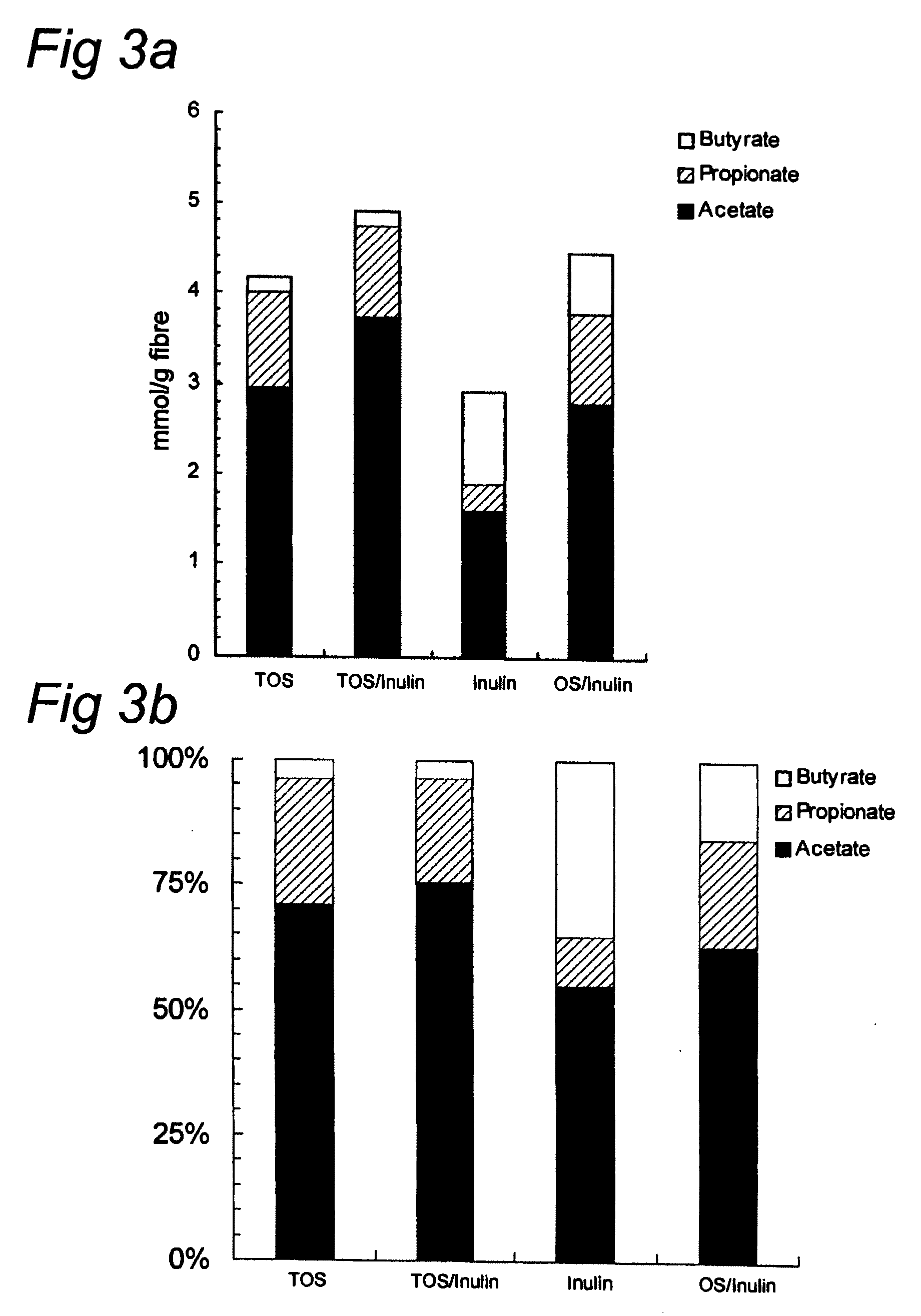
Synthetic composition for treating metabolic disorders
ActiveUS20160310514A1Reduce intestinal permeabilityReduce endotoxemiaOrganic active ingredientsMetabolism disorderOligosaccharideMetabolic disorder
The invention relates to a synthetic composition comprising one or more human milk monosaccharides and / or one or more human milk oligosaccharides for use in reducing intestinal permeability, endotoxemia and / or low-grade inflammation in patients with metabolic disorders.
Owner:GLYCOM AS
Method and system for reducing the likelihood of developing NASH in an individual diagnosed with non-alcoholic fatty liver disease
ActiveUS10245288B2Improvements in intestinal dysbiosisReduce intestinal permeabilityUnknown materialsNon-surgical orthopedic devicesDiseaseOxidized low density lipoprotein
A method for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease that involves providing an individual with an effective amount of a composition of bacteria modified using a Clustered Regularly Interspaced Short Palindromic Repeats-CRISPR-associated system (CRISPR-Cas) or Clustered Regularly Interspaced Short Palindromic Repeats from Prevotella and Francisella 1 (CRISPR / Cpf1) system so that the bacteria is able to produce a therapeutically effective amount of anti-bodies to oxidized low density lipoprotein, with the modified bacteria preferably being from the Lactobacillus, Bifidobacterium, and Streptococcus species; and most preferably including L. reuteri bacteria modified using CRISPR-Cas and / or Cpf1 systems so that it is able to survive the conditions in the duodenum and jejunum of the small intestine of a human.
Owner:SEED HEALTH INC
Nanoparticles for protein drug delivery
InactiveUS7919072B1Reduce inflammationEnhances intestinal epithelial barrier functionIn-vivo radioactive preparationsPharmaceutical non-active ingredientsNanoparticleDrug biological activity
The invention discloses the bioactive nanoparticles composed of chitosan, poly-glutamic acid, and at least one bioactive agent characterized with a positive surface charge and enhanced permeability in oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
Pharmaceutical composition of nanoparticles for protein drug delivery
InactiveUS8202508B1Enhances intestinal epithelial barrier functionReduce intestinal permeabilityIn-vivo radioactive preparationsPeptide/protein ingredientsActive agentSurface charges
The invention discloses a pharmaceutical composition of bioactive nanoparticles composed of chitosan, poly-glutamic acid, and a bioactive agent for oral delivery. The chitosan-based nanoparticles are characterized with a positive surface charge and enhanced permeability for oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
Method and system for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease
ActiveUS20200121743A1Inhibit progressEarly detectionOrganic active ingredientsPharmaceutical delivery mechanismDiseaseIntestinal microorganisms
A method for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease involves providing in the gut of an individual a population of beneficial bacteria selected from the group consisting of Lactobacillus species, and administering fiber to the individual to maintain a therapeutically effective amount of the beneficial bacteria in the gut of the individual. In certain embodiments, monoacylglycerolacyltransferase-3 (MGAT3) synthesis is inhibited to lower triacylglycerol (TAG) production, while in others, expression of diacylglycerolacyltransferase-2 (DGAT-2) is inhibited. The beneficial bacteria are preferably modified to produce increased amounts of butyrate and may also be encapsulated in a frangible enclosure. Levels of Roseburia are preferably increased while the levels of Akkermansia spp. in the individual's gut microbiome are reduced. In other embodiments, a therapeutically effective amount of a bacterial formulation comprising Faecalibacterium prausnitzii is administered, or a composition comprising modified L. reuteri bacteria having the ability to survive conditions in the duodenum or jejunum of the individual's small intestine. Other embodiments include the administration of a bacterial formulation comprising at least one of Coprococcus, Veillonella, Roseburia, Bifidobacterium, Faecalibacterium prausnitzii and Prevotella.
Owner:SEED HEALTH INC
Pharmaceutical composition of nanoparticles for protein drug delivery
InactiveUS8048453B1Enhances intestinal epithelial barrier functionReduce intestinal permeabilityPowder deliveryPharmaceutical non-active ingredientsNanoparticleDrug biological activity
The invention discloses a pharmaceutical composition of bioactive nanoparticles composed of chitosan, poly-glutamic acid, and a bioactive agent for oral delivery. The chitosan-based nanoparticles are characterized with a positive surface charge and enhanced permeability for oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
Nanoparticles for protein drug delivery
InactiveUS20110142927A1Reduce resistanceEasy to transportPeptide/protein ingredientsPharmaceutical non-active ingredientsMedicineActive agent
The invention discloses the nanoparticles composed of chitosan, poly-glutamic acid, and at least one anti-hemophilic factor or bioactive agent characterized with a positive surface charge and their enhanced permeability in oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
Pharmaceutical composition of nanoparticles
InactiveUS8119102B1Sufficient solubilityEnhances intestinal epithelial barrier functionOrganic active ingredientsIn-vivo radioactive preparationsActive agentNanoparticle
The invention discloses a pharmaceutical composition of bioactive nanoparticles composed of chitosan, poly-glutamic acid, and a bioactive agent for oral delivery. The chitosan-based nanoparticles are characterized with a positive surface charge and enhanced permeability for oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
Pharmaceutical composition of nanoparticles
InactiveUS8287905B1Enhances intestinal epithelial barrier functionReduce intestinal permeabilityPowder deliveryPeptide/protein ingredientsEpithelial permeabilityMedicine
The invention discloses a composition of chitosan-shelled nanoparticles and methods of manufacturing. The chitosan-shelled nanoparticles are characterized with a positive surface charge and enhanced epithelial permeability for oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
Nanoparticles for protein drug delivery
InactiveUS7988949B2Reduce resistanceEasy to transportNervous disorderIn-vivo radioactive preparationsNanoparticleMedicine
The invention discloses the nanoparticles composed of chitosan, poly-glutamic acid, and at least one antibiotics or equivalent bioactive agent characterized with a positive surface charge and their enhanced permeability in oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
Health feed
InactiveUS20050107303A1Good for healthPromote and maintain good quality fecesAntibacterial agentsBiocideHuman animalImmunoglobulin A
The present invention relates to a method of promoting immunoglobulin A secretion in the mucosal membranes of non-human animals. The method comprises administering a foodstuff comprising glutamine to the non-human animal.
Owner:MARS UK
Nanoparticles for protein drug delivery
InactiveUS7863259B1Reduce resistanceEasy to transportBiocidePeptide/protein ingredientsMedicineNanoparticle
The invention discloses the nanoparticles composed of chitosan, poly-glutamic acid, and at least one antibiotics or equivalent bioactive agent characterized with a positive surface charge and their enhanced permeability in oral drug delivery.
Owner:NANOMEGA MEDICAL CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com