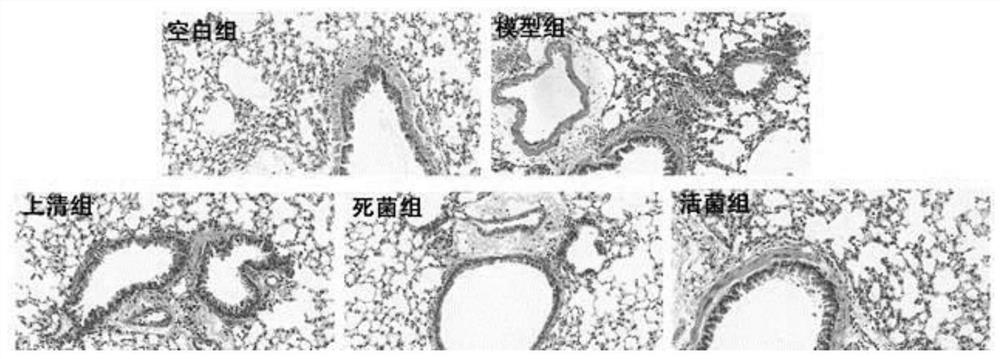
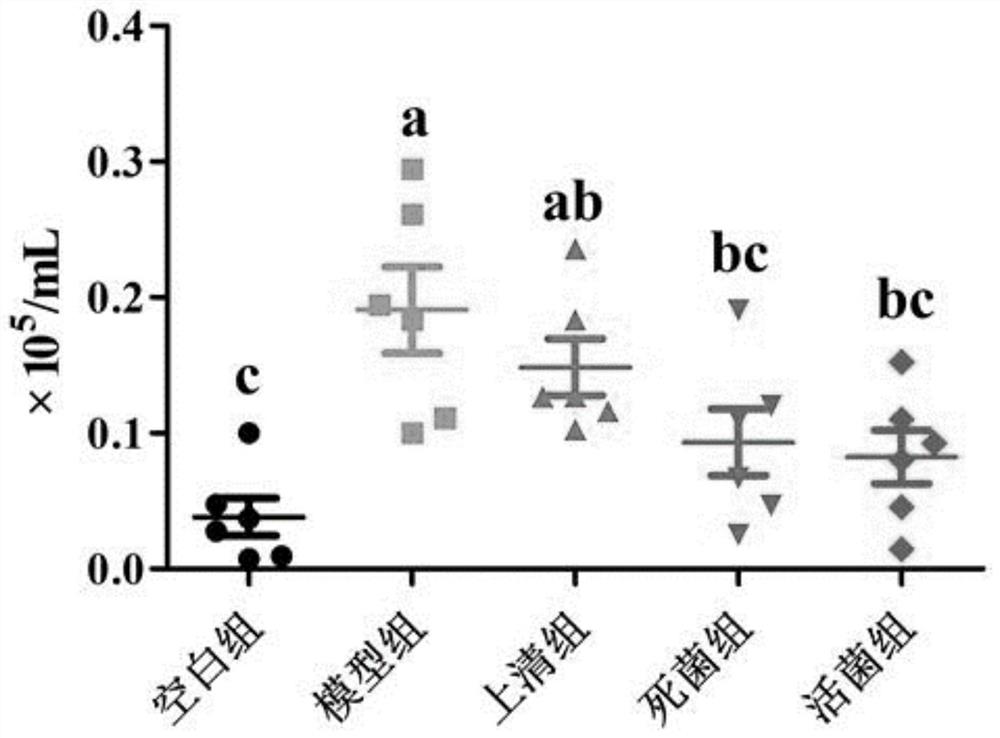
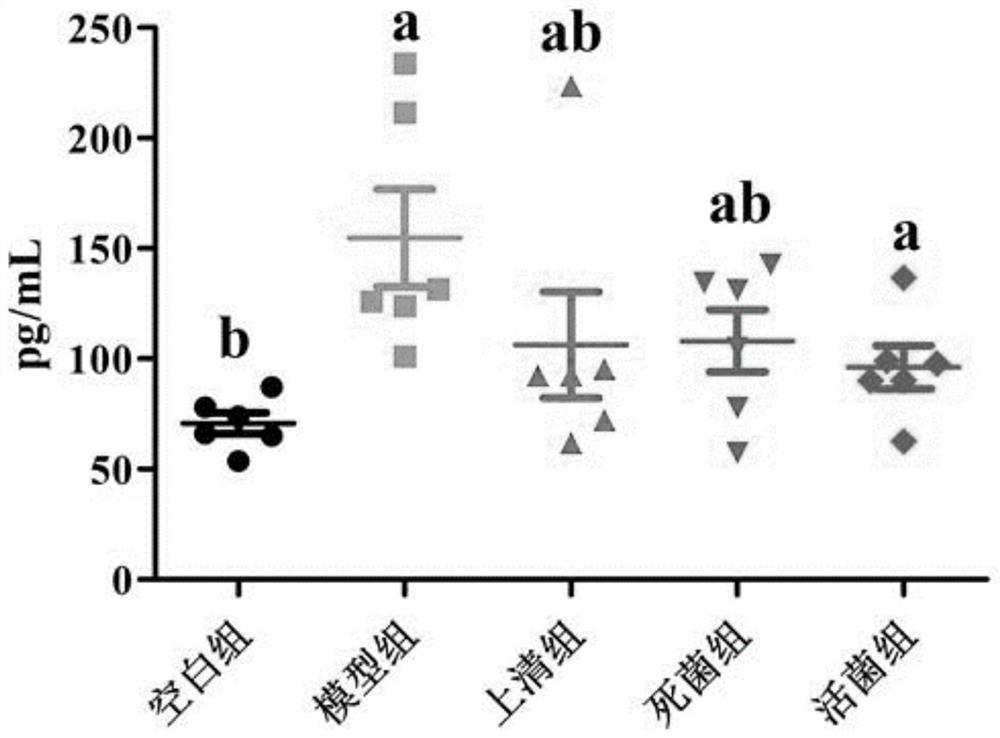
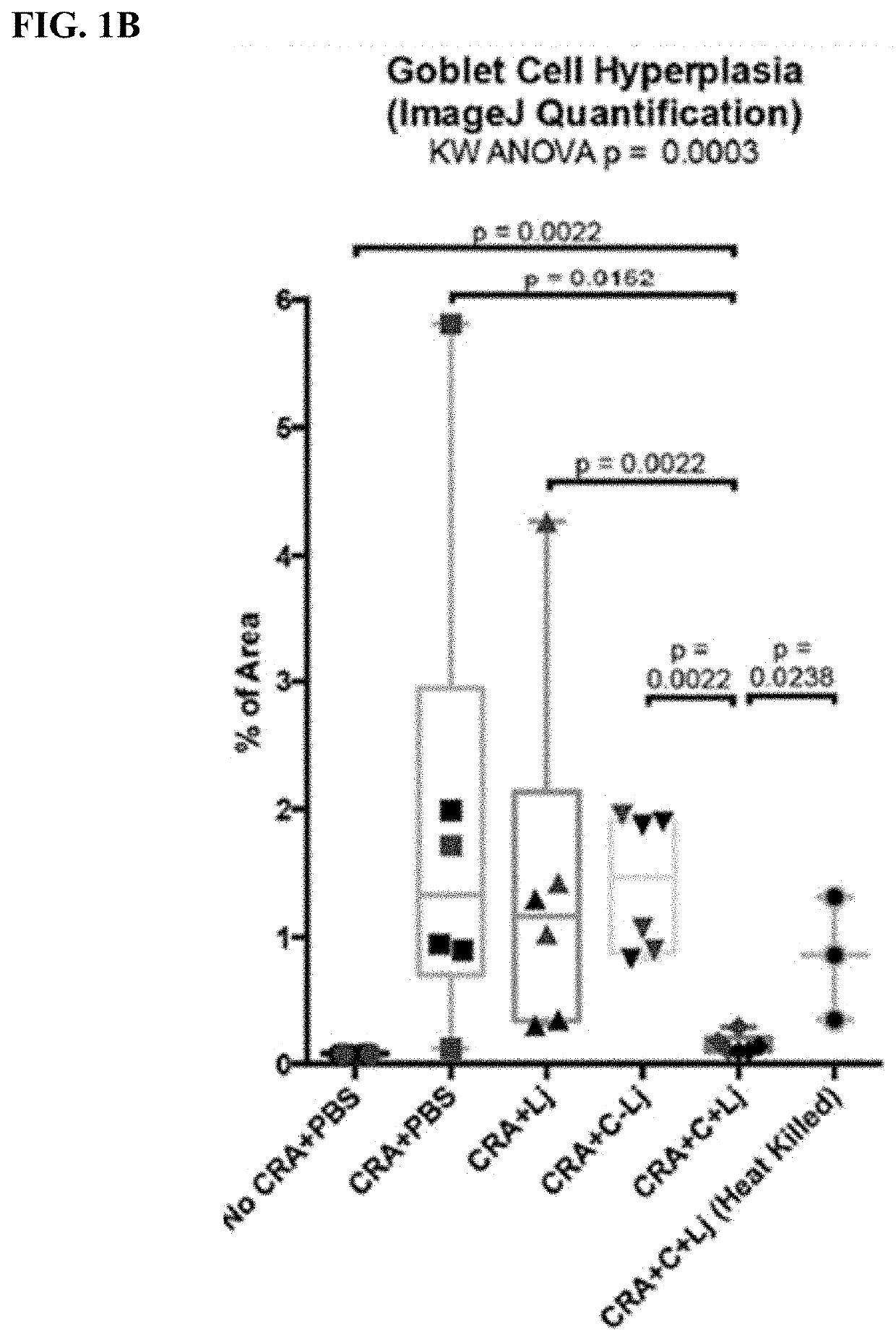
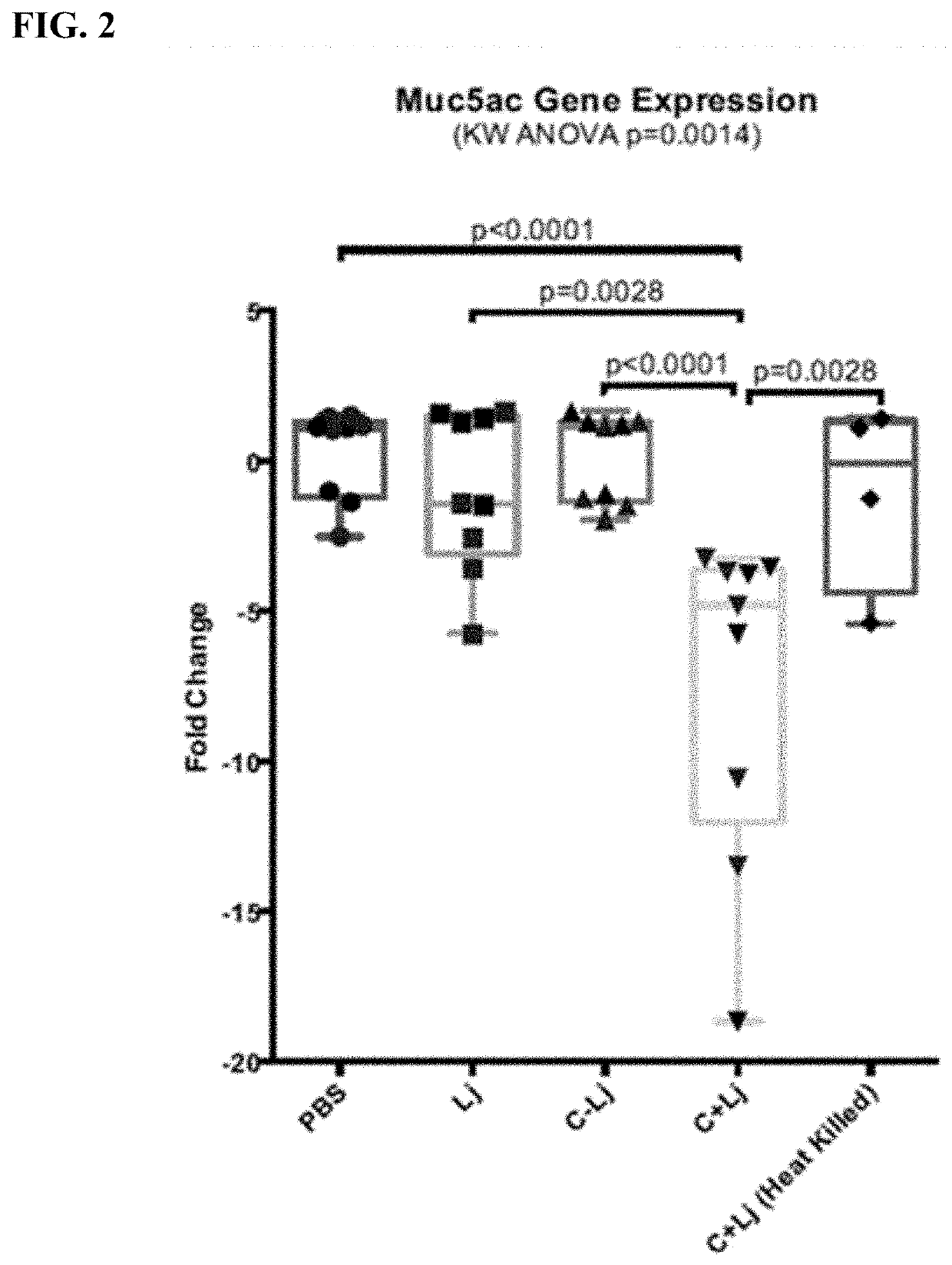
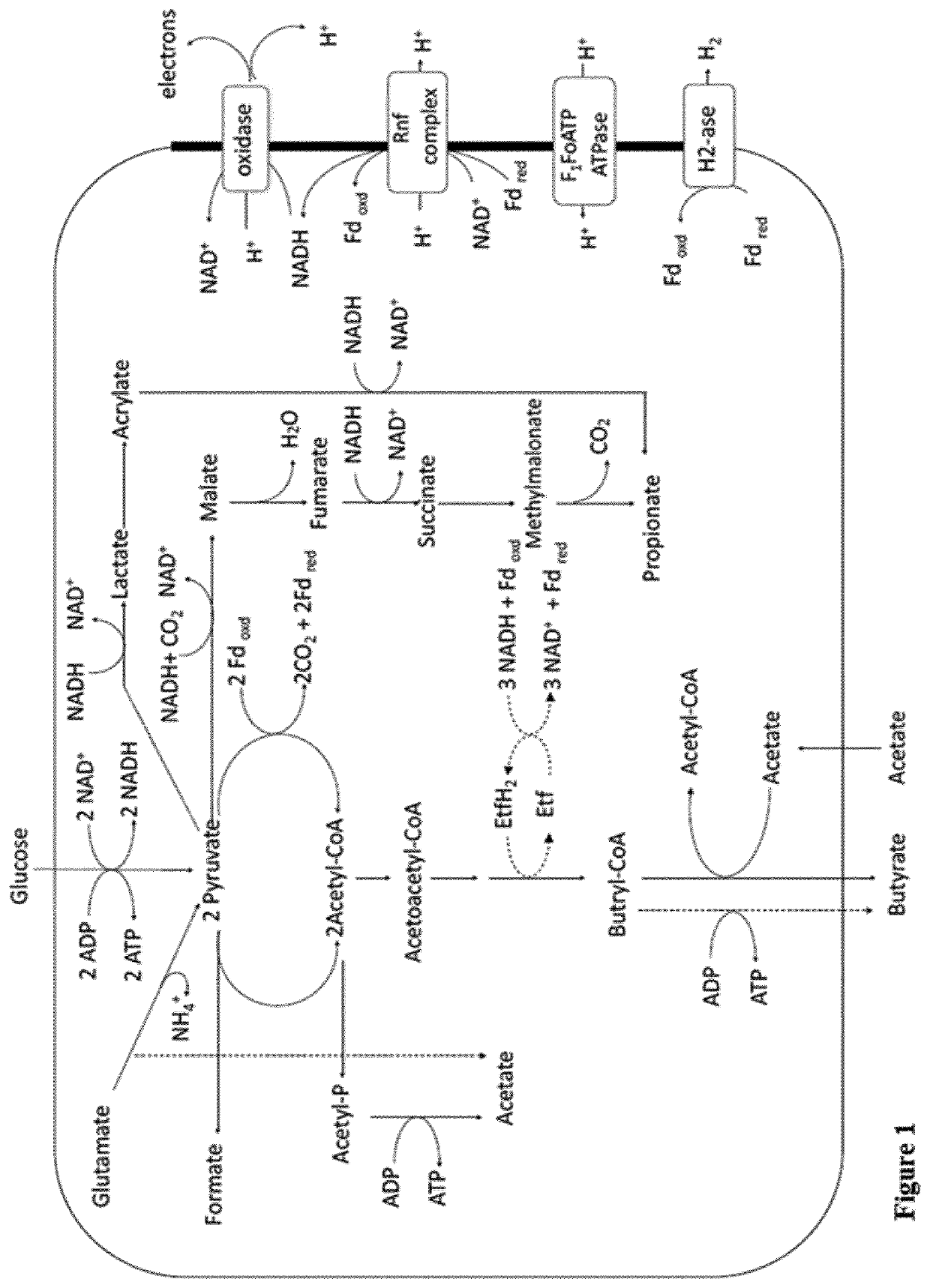
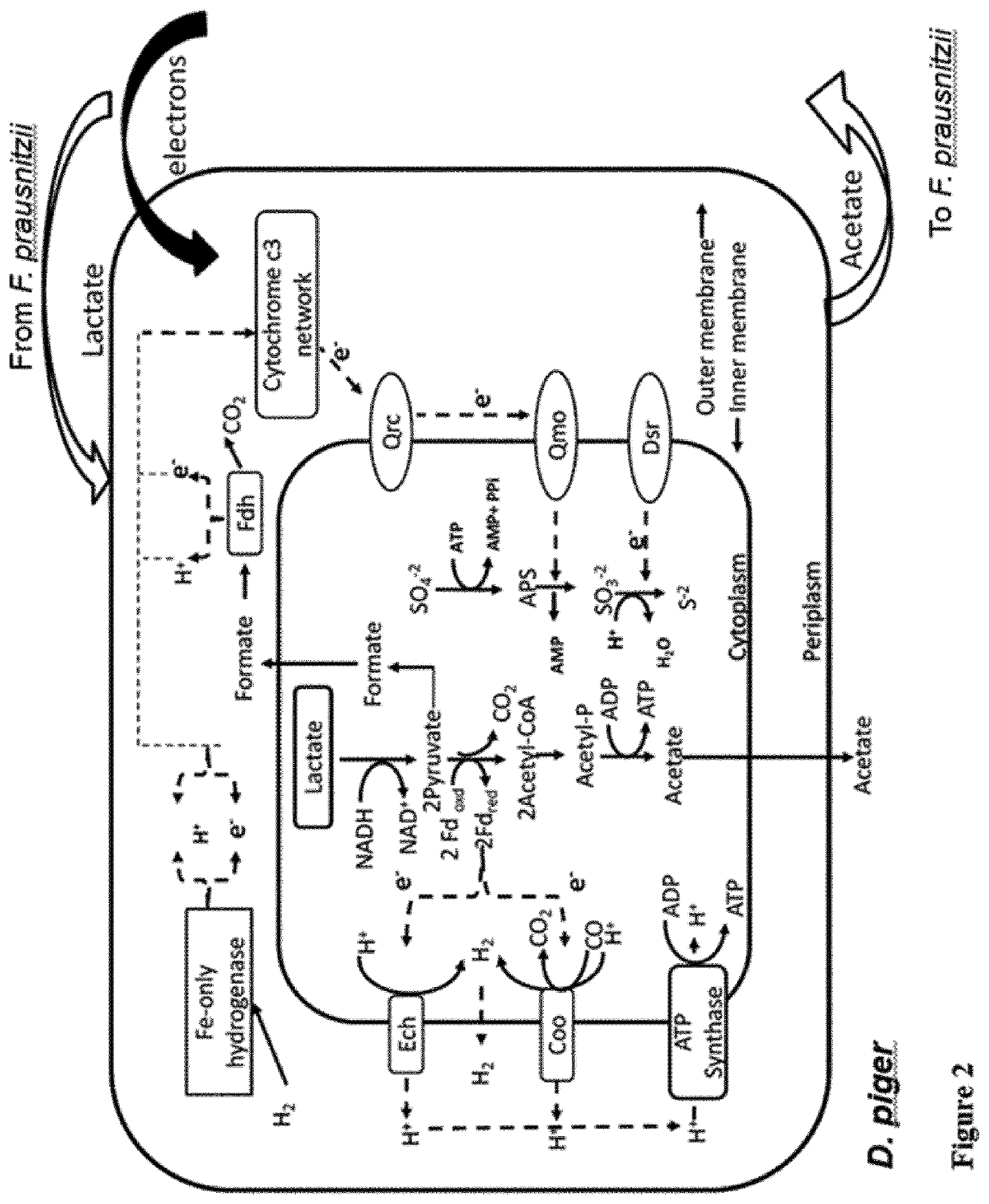
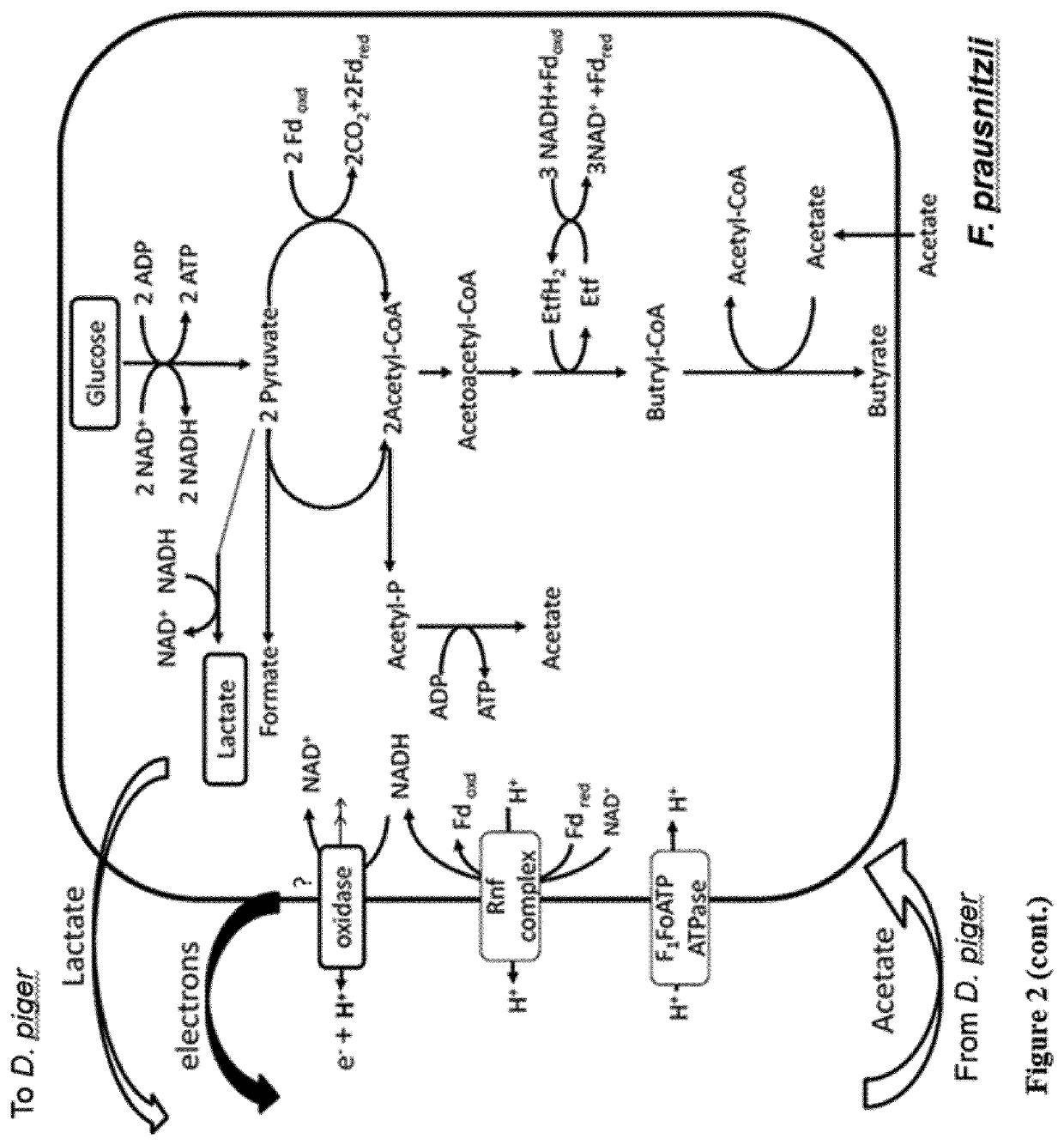
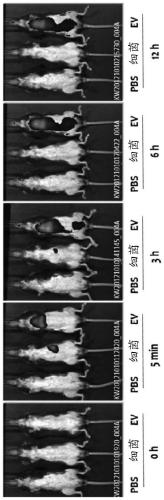
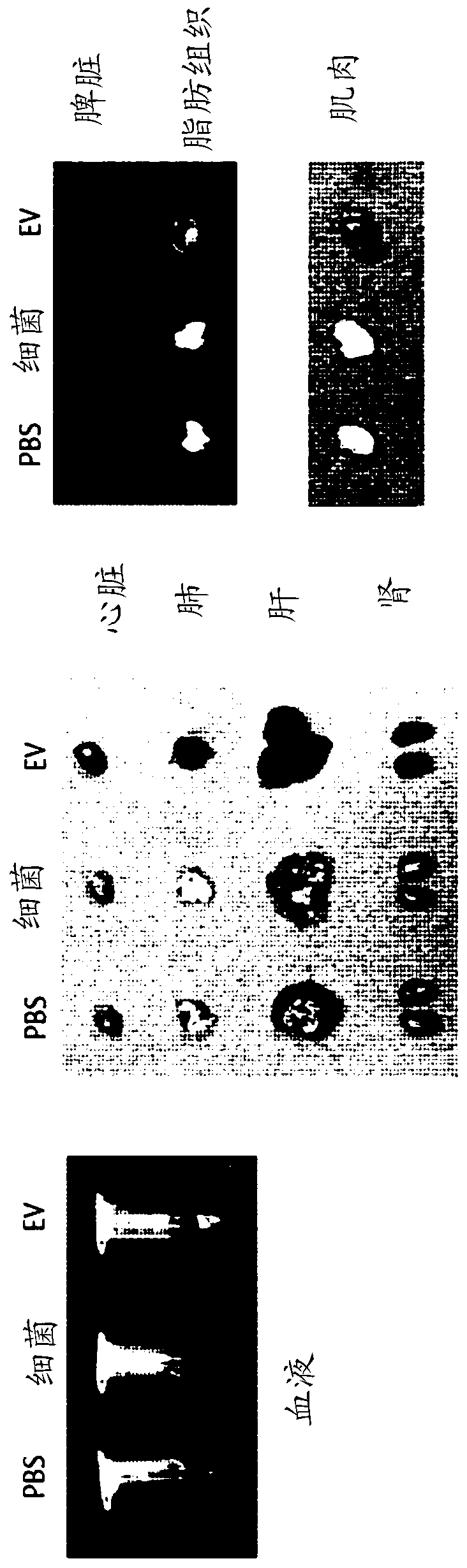
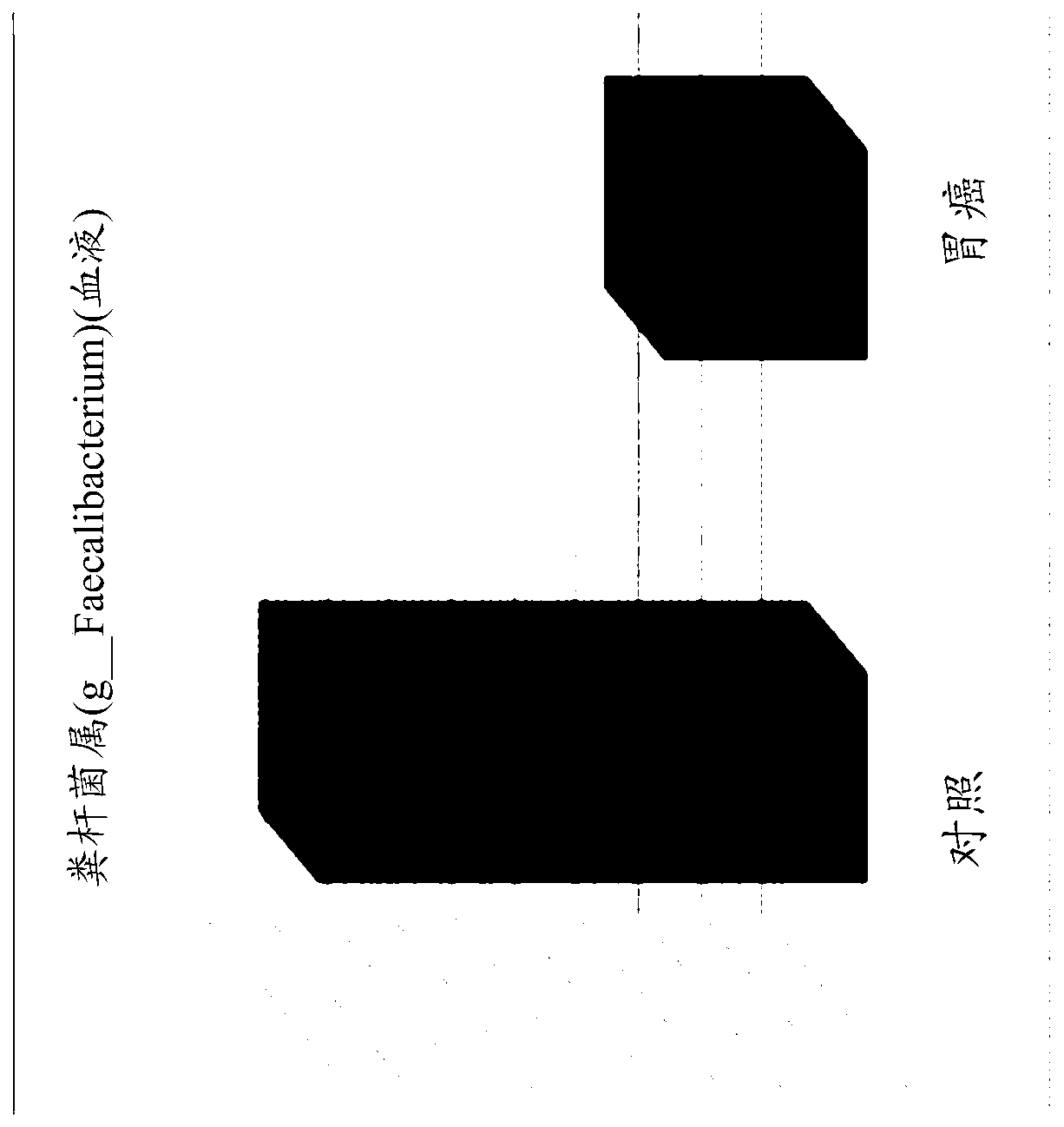
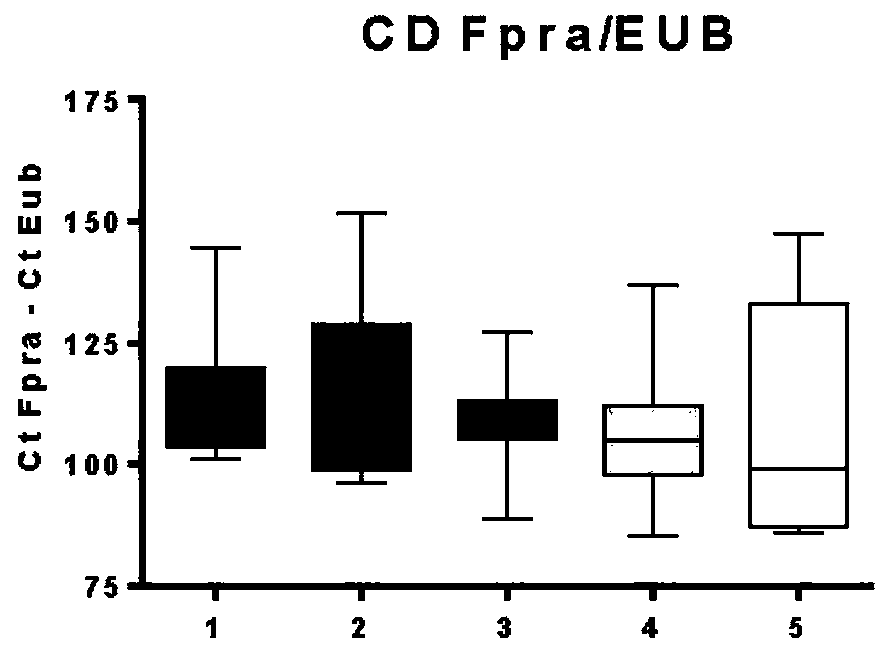
Patents
Literature
43 results about "Faecalibacterium prausnitzii" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
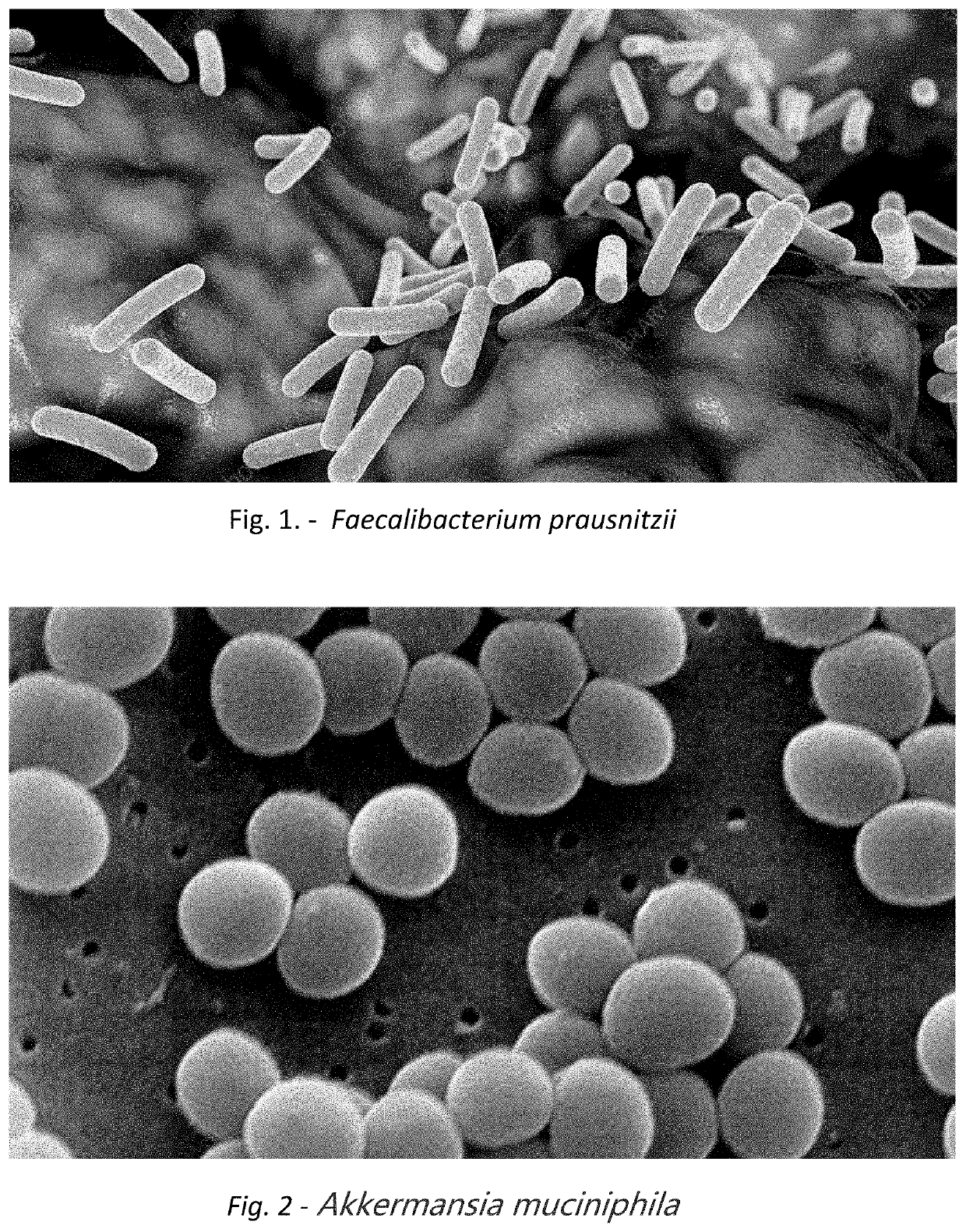
Faecalibacterium is a genus of bacteria. Its sole known species, Faecalibacterium prausnitzii is gram-positive, mesophilic, rod-shaped, anaerobic and is one of the most abundant and important commensal bacteria of the human gut microbiota. It is non-spore forming and non-motile. These bacteria produce butyrate and other short-chain fatty acids through the fermentation of dietary fiber.
Probiotics with methods for growth and use separately and in combination
InactiveUS20150246081A1Strengthening the immune systemBiocideUnknown materialsDietary supplementDiverticulitis
A dietary supplement that may contain probiotics from the genera Akkermansia, Bacteriodes, Faecalibacterium, Eubacterium, Escherichia, Collinsella, Desulfovibrio, Clostridium, Mycobacterium, Pediococcus, and Bifidobacterium. The dietary supplement may provide a variety of benefits including weight management, blood sugar management, treatment of irritable bowel syndrome, treatment of Crohn's disease, treatment of diverticulitis, treatment for inflammatory bowel, treatment for dysbiosis, and strengthening the immune system.
Owner:MORRIS SHAYNE KENNETH
Microbial marker of colorectal cancer and application of marker
ActiveCN109943636AReduce the prevalenceHigh detection specificityMicrobiological testing/measurementMicroorganism based processesIntestinal microorganismsFaecalibacterium prausnitzii
The invention provides a microbial marker of colorectal cancer and application of the marker. The microbial marker comprises Faecalibacterium, Streptococcus and Fusobacterium. The microbial marker ofcolorectal cancer has high precision of predicting the risk of colorectal cancer and high sensitivity, only the abundance of the microbial marker of colorectal cancer needs to be obtained, a risk early warning is given out through model calculation, and the possibility of getting colorectal cancer is evaluated. The microbial marker can be used for preventing colorectal cancer, warning a subject and giving the subject a prompt about whether it is necessary to further conduct diagnosis confirmation or not, and is also conductive for individuals to improve the intestinal microbial environment byadjusting diet or medical treatment, so that the risk of getting colorectal cancer is reduced for the individuals.
Owner:SHANGHAI BIOTECAN PHARMA +1
Method for evaluating stable state of flora in excrement sample and application of method in colorectal cancer screening
PendingCN108690864ALow costGood Gut Health ScreeningMicrobiological testing/measurementMicroorganism based processesClostridium leptumFeces
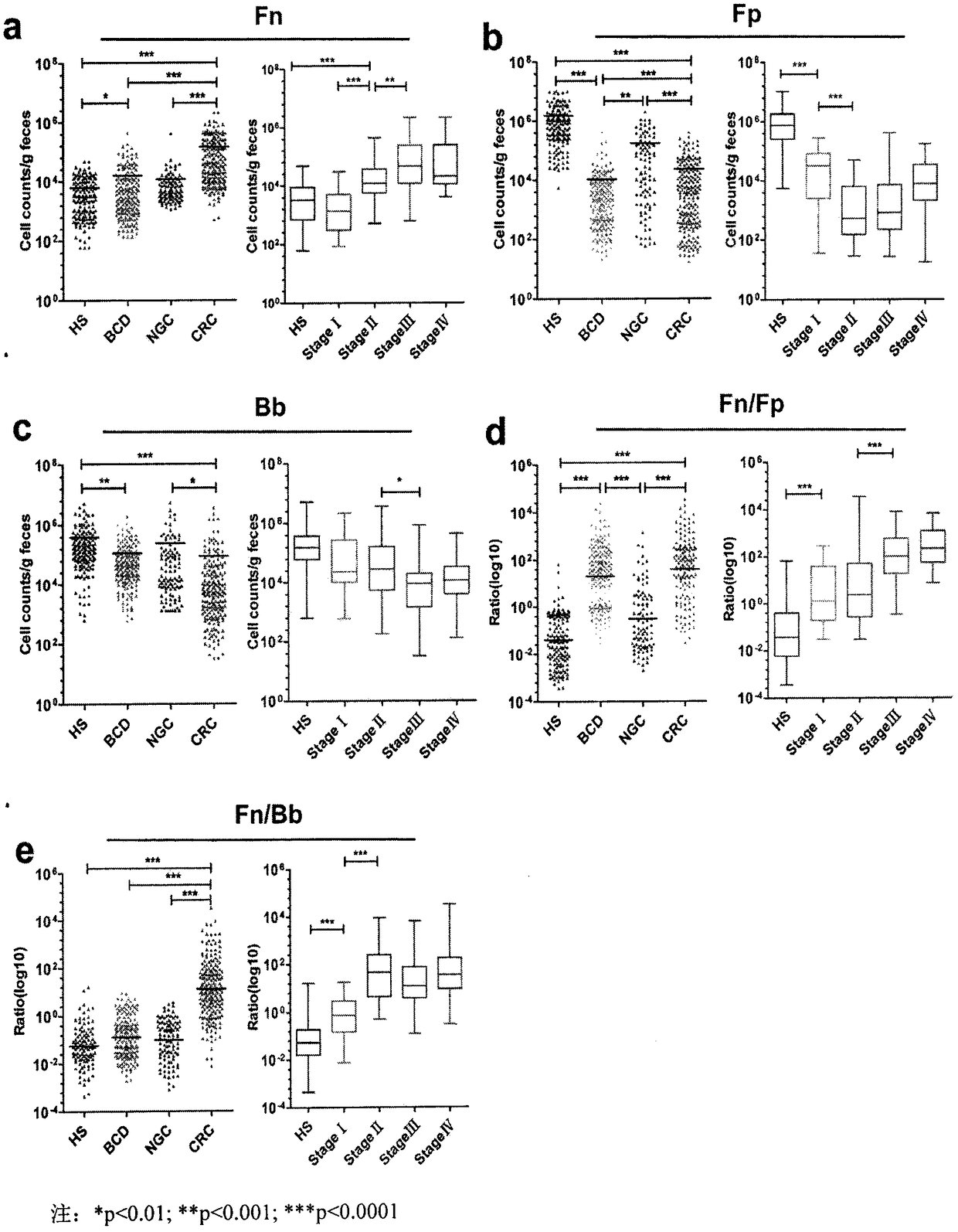
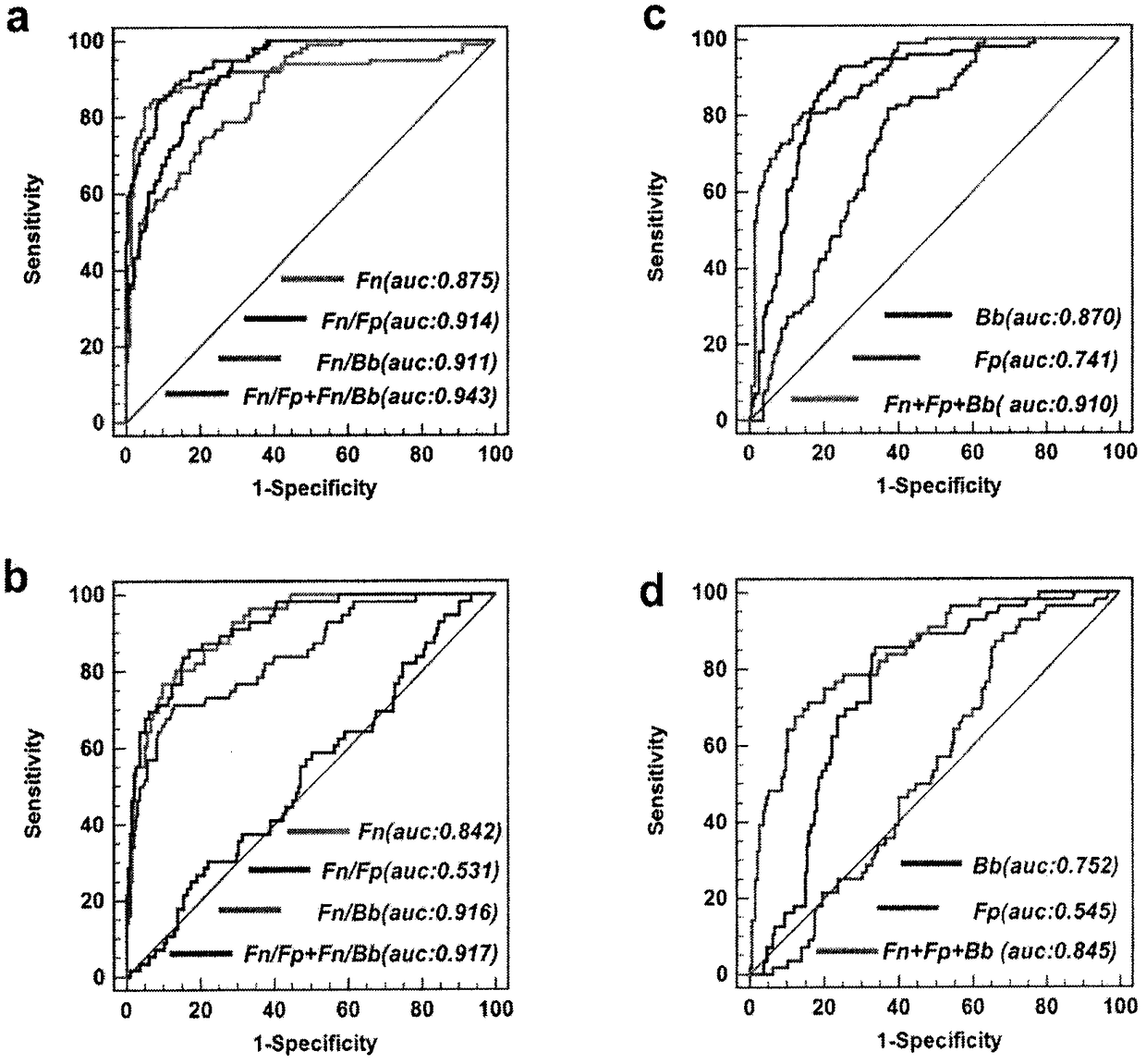
The invention relates to a method for calculating a flora balanced relation index in an individual excrement sample, and an application of the method in screening, diagnosis or auxiliary diagnosis incolorectal cancer (CRC). By extracting bacteria in excrement during DNA sequencing, the types and quantity characteristics of the bacteria can be obtained, and a CRC diagnosis by taking quantity ratiocharacteristic of a plurality of bacteria as a base is carried out. Compared with the methods used in clinical diagnosis or noninvasive screening of CRC with an applied patent, the method is completely noninvasive, and can realize accurate diagnosis of CRC. The analysis result displays that a ratio of fusobacterium nucleatum Fn to bifidobacteria Bb quantity (Fn / Bb) has high susceptibility and specificity on CRC screening, which can respectively reach 84.6% and 92.3% (AUC=0.911). the ratio of fusobacterium nucleatum Fn to clostridium leptum Fp (Fn / Fp) quantity is combined to increase the diagnosis value on CRC, and the Area Under Curve (AUC) of a subject work characteristic curve can reach 0.943. In addition, combination of Fn / Bb and Fn / Fp quantity ratio for screening I-stage CRC has 60% of specificity and 90% of sensitivity.
Owner:SUN YAT SEN UNIV
Method for reducing the likelihood of developing bladder or colorectal cancer in an individual human being
ActiveUS11026982B2Treatment complicationsAvoiding undesiredAntibacterial agentsOrganic active ingredientsPrevotellaMogibacterium diversum
A method for treating an individual suffering from one of bladder cancer and colorectal cancer employs a CRISPR system to selectively kill or reduce the numbers of pathogenic bacteria within the individual and the individual is then administered an immune checkpoint inhibitor. In particular embodiments, the pathogenic bacteria is one of E. coli, Pseudomonas aeruginosa and Klebsiella bacteria, and the checkpoint inhibitor is selected from the group consisting of nivolumab, pembrolizumab, pidilizumab, AMP-224, AMP-514, STI-A1110, TSR-042, RG-7446, BMS-936559, MEDI-4736, MSB-0020718C, AUR-012 and STI-A1010. Further embodiments include enhancing the growth of a second bacteria in the individual, such bacteria including Akkermansia, Bacteroides, Bifidobacterium, Clostridium, Enterococcus, Fusobacterium, Lactobacillus, Propionibacterium, Ruminococcus, Veillonella, Prevotella, Escherichia and Streptococcus. Still other embodiments include increasing the levels of Roseburia and / or Faecalibacterium prausnitzii, in the individual's gut microbiome.
Owner:SEED HEALTH INC
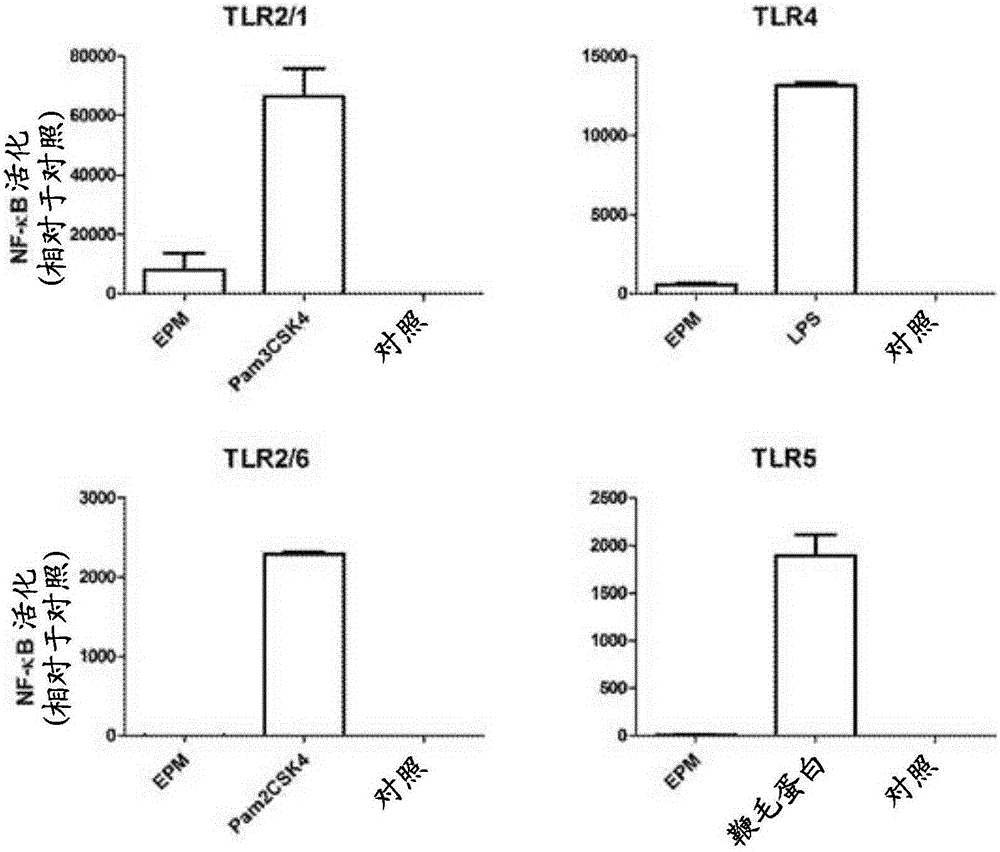
Use of faecali bacterium prausnitzii htf-f (dsm 26943) to suppress inflammation.
InactiveCN105228635AAntipyreticBacteria material medical ingredientsFaecalibacterium prausnitziiBULK ACTIVE INGREDIENT
The present invention relates to medicine, particularly immunology and gastroenterology. Specifically, it relates to probiotic bacteria and extracts thereof for therapeutic use for the treatment of inflammatory disorders such as inflammatory bowel disease. Provided is a composition comprising as active ingredient Faecalibacterium prausnitzii strain HTF-F (DSM 26943) or an extract thereof comprising extracellular polymeric matrix (EPM), and an acceptable carrier, diluent or excipient. Also provided is an anti-inflammatory composition comprising EPM extracted from F. prausnitzii strain HTF-F, and a method for preparing the same.
Owner:UNIVERSITY OF GRONINGEN +3
Application of Faecalibacterium prausnitzii in relieving allergic asthma and rhinitis Th2 reaction
ActiveCN112402459AImprove responseReduce the numberAnimal feeding stuffUnknown materialsFaecalibacterium prausnitziiIntravenous gammaglobulin
The invention relates to application of Faecalibacterium prausnitzii in relieving allergic asthma and rhinitis Th2 reaction, and belongs to the technical field of microorganisms and medicines. According to the application disclosed by the invention, the Faecalibacterium prausnitzii A2-165 is applied to relieving of allergic asthma and rhinitis, and the application is specifically embodied in that(1) the infiltration effect of memory inflammatory cells with lung pathological symptoms of mice with allergic asthma and rhinitis is remarkably improved; (2) the contents of IL-4, IL-5 and IL-13 in alveolar lavage fluid for the mice with allergic asthma and rhinitis are remarkably reduced; (3) the generation of dust mite specific immunoglobulin IgG1 in serum of the mice with allergic asthma and rhinitis is remarkably inhibited; and (4) the ratio of Tregs in the spleen is remarkably increased, so that the Faecalibacterium prausnitzii A2-165 has a huge application prospect in the preparation ofa product for prevention and / or treatment.
Owner:JIANGNAN UNIV
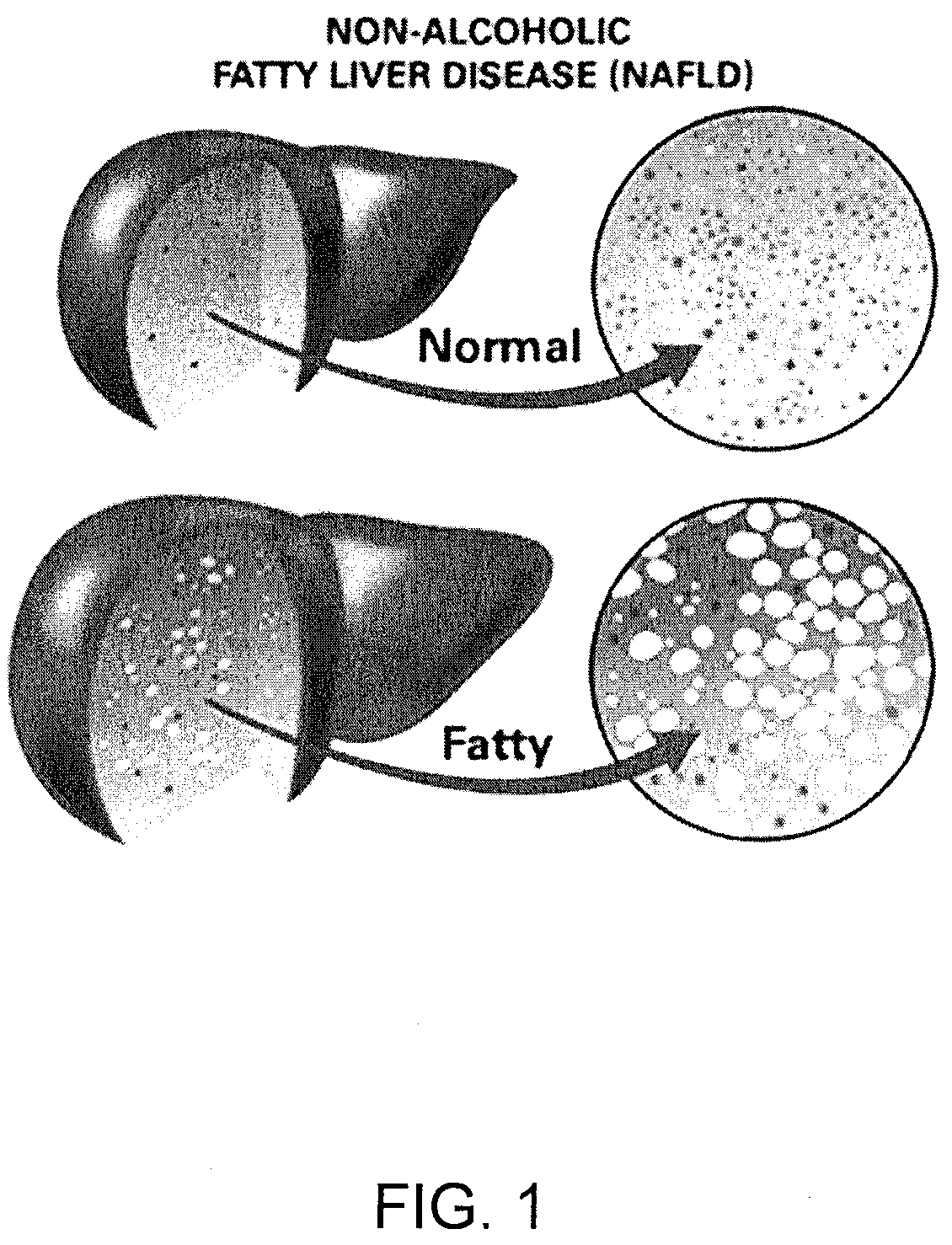
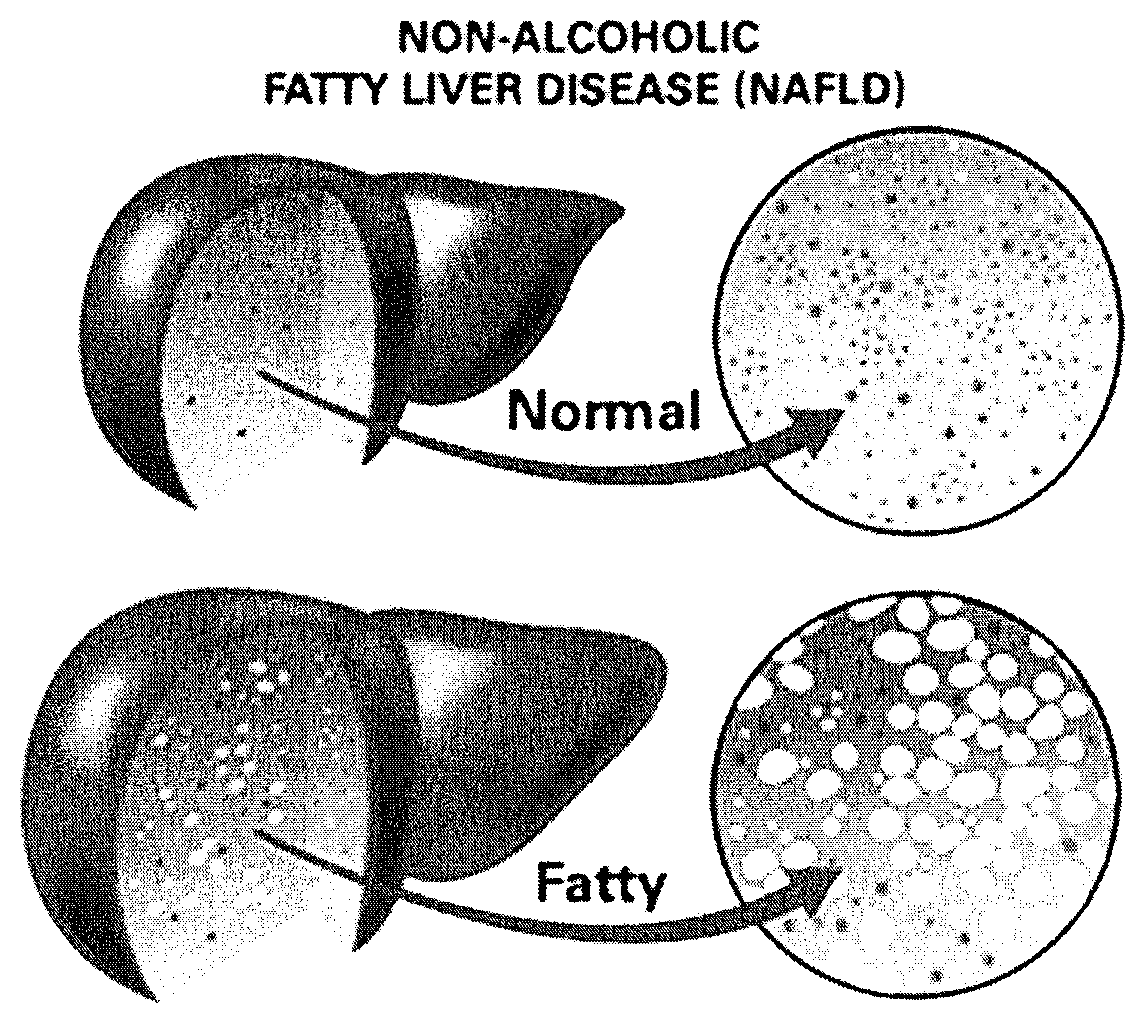
Method and system for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease
ActiveUS20200121743A1Inhibit progressEarly detectionOrganic active ingredientsPharmaceutical delivery mechanismDiseaseIntestinal microorganisms
A method for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease involves providing in the gut of an individual a population of beneficial bacteria selected from the group consisting of Lactobacillus species, and administering fiber to the individual to maintain a therapeutically effective amount of the beneficial bacteria in the gut of the individual. In certain embodiments, monoacylglycerolacyltransferase-3 (MGAT3) synthesis is inhibited to lower triacylglycerol (TAG) production, while in others, expression of diacylglycerolacyltransferase-2 (DGAT-2) is inhibited. The beneficial bacteria are preferably modified to produce increased amounts of butyrate and may also be encapsulated in a frangible enclosure. Levels of Roseburia are preferably increased while the levels of Akkermansia spp. in the individual's gut microbiome are reduced. In other embodiments, a therapeutically effective amount of a bacterial formulation comprising Faecalibacterium prausnitzii is administered, or a composition comprising modified L. reuteri bacteria having the ability to survive conditions in the duodenum or jejunum of the individual's small intestine. Other embodiments include the administration of a bacterial formulation comprising at least one of Coprococcus, Veillonella, Roseburia, Bifidobacterium, Faecalibacterium prausnitzii and Prevotella.
Owner:SEED HEALTH INC
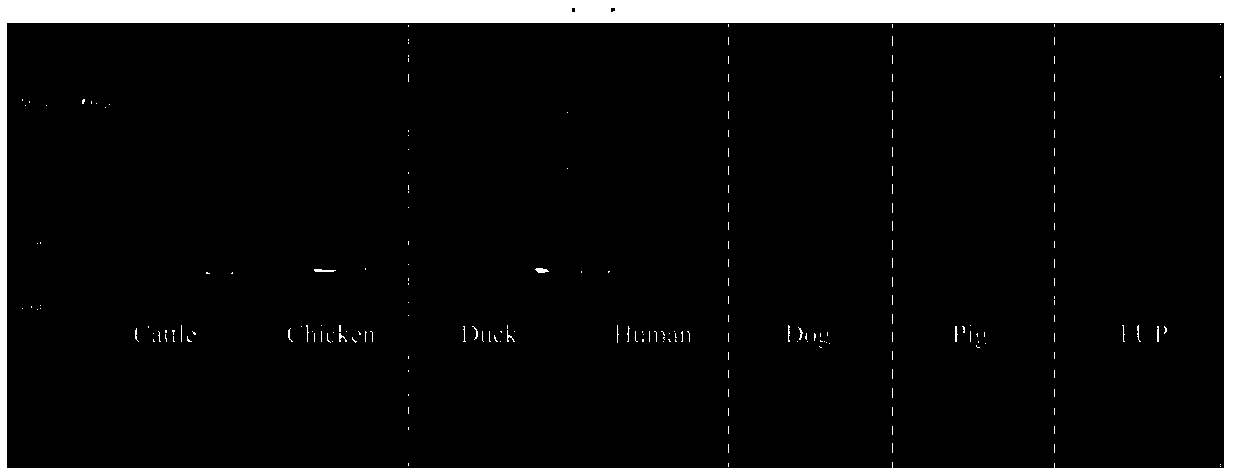
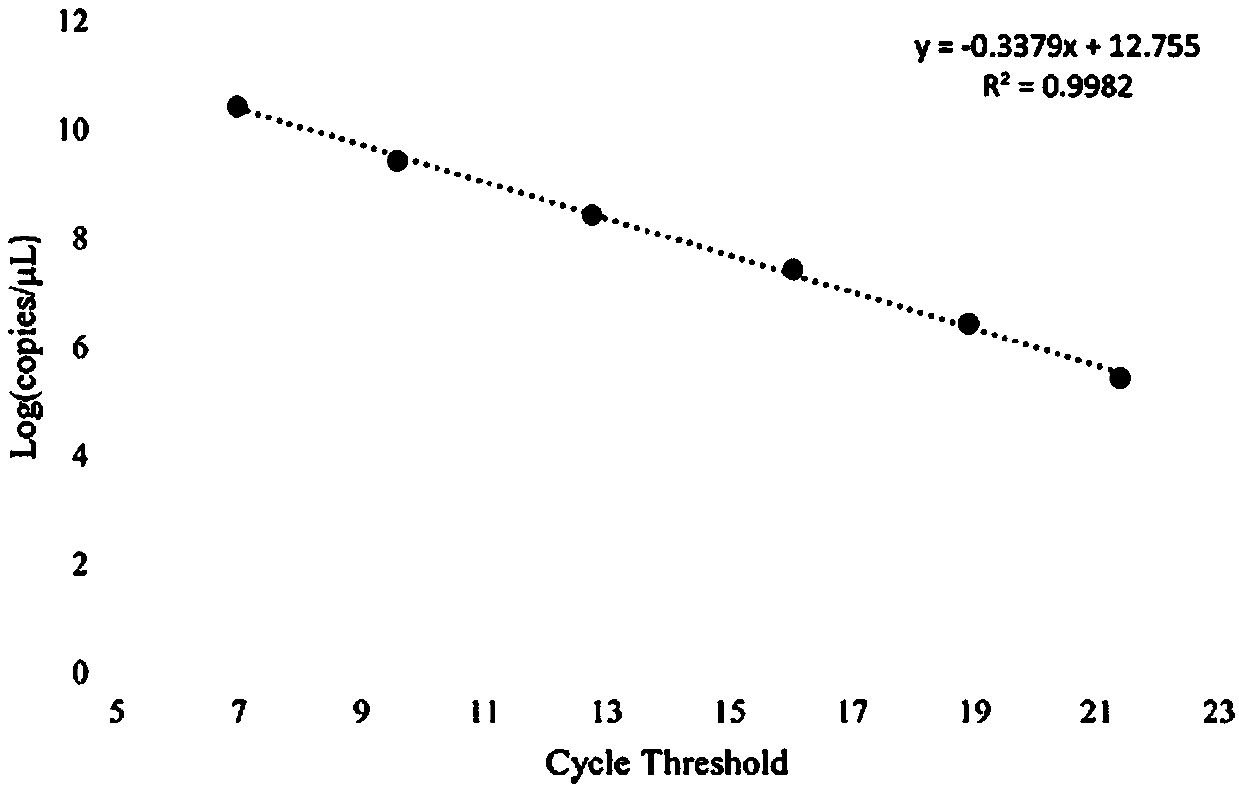
Method for detecting cowpat and detection kit
InactiveCN108676901AStrong specificityImprove stabilityMicrobiological testing/measurementDNA/RNA fragmentationFaecalibacterium prausnitziiCanis lupus familiaris
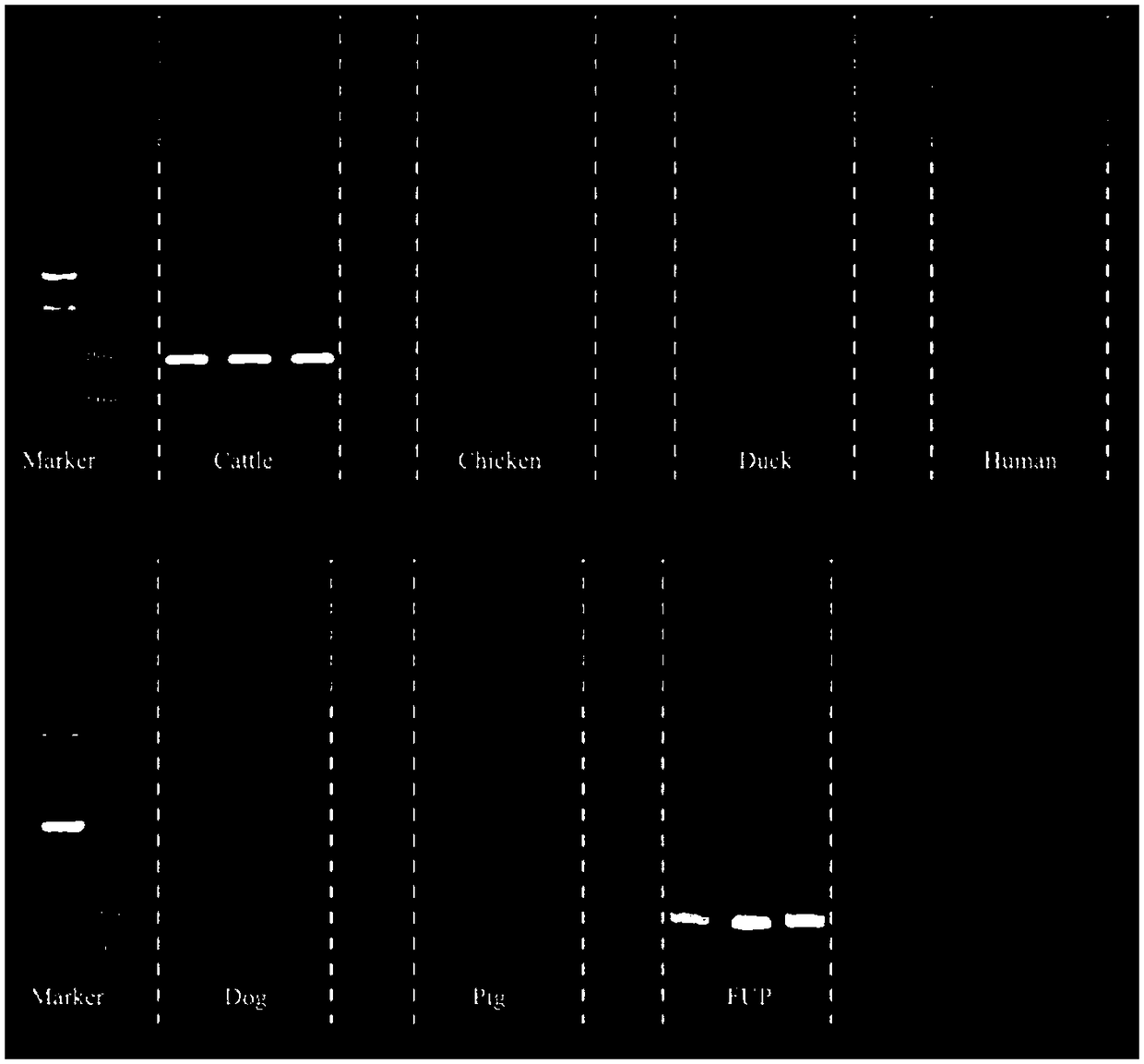
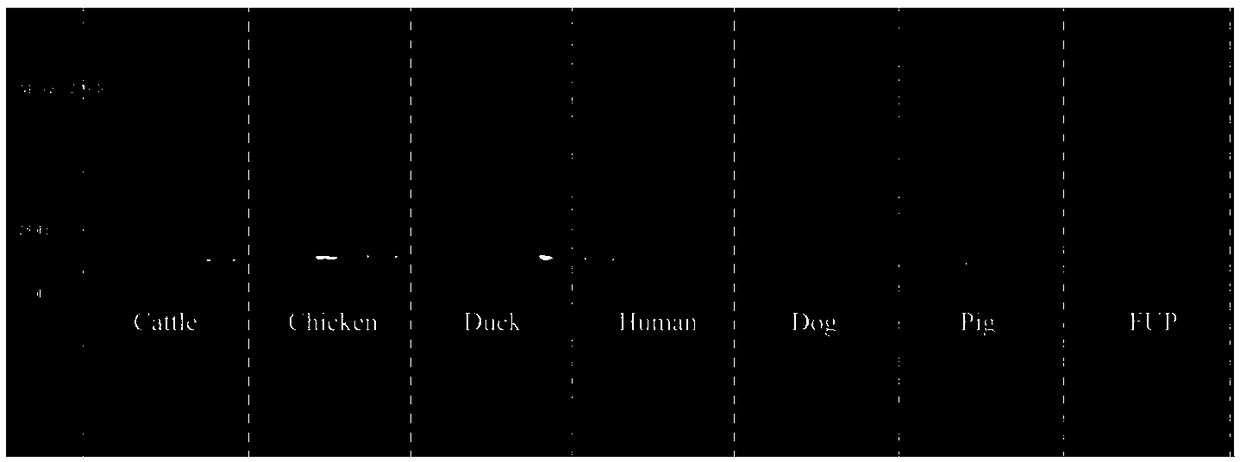
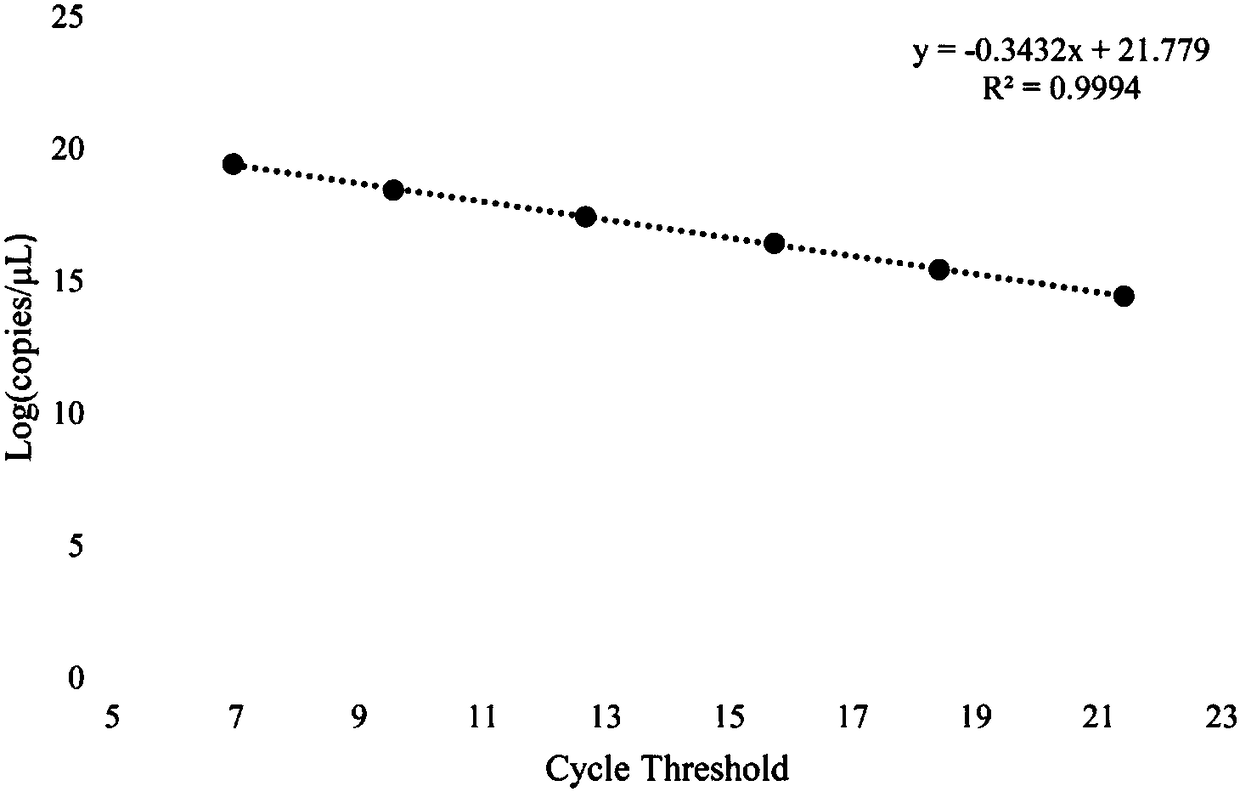
The invention belongs to the field of biotechnology detection and particularly relates to a method for detecting cowpat and a detection kit. Firstly, the invention verifies that the cowpat can be detected by detecting Faecalibacterium of cows; secondly, the invention provides detection primers and the detection kit for the cowpat. The detection primers can specifically react with the Faecalibacterium of the cows to generate a band while cannot react with the Faecalibacterium of chickens, ducks, human beings, dogs and pigs; in 6 specifies, the specificity of the primers reaches 100 percent. When the primers disclosed by the invention are used for detecting the Faecalibacterium of the cows, a lowest detectable limit (LOQs) is 3273 copies / [mu]l, and the sensitivity effect is excellent; when the primers are used for detecting interspecies of a cowpat sample, positive rat reaches 95.8 percent (46 / 48), and the stability is better. When the detection primers and the detection kit are used fordetecting water body pollution, whether cowpat pollution exists in the water body or not can be quickly judged and a pollution source is located.
Owner:WENZHOU UNIVERSITY
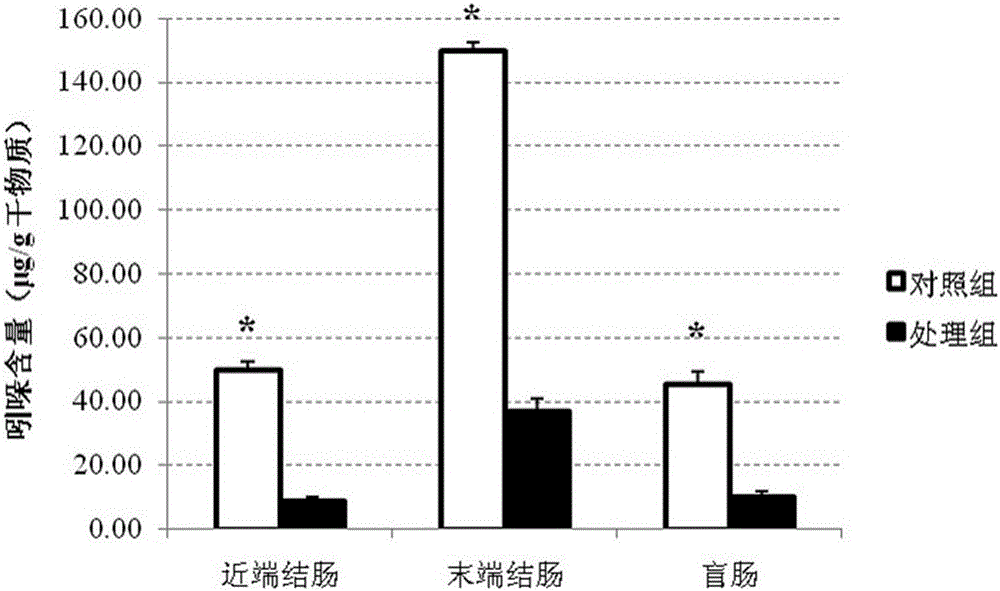
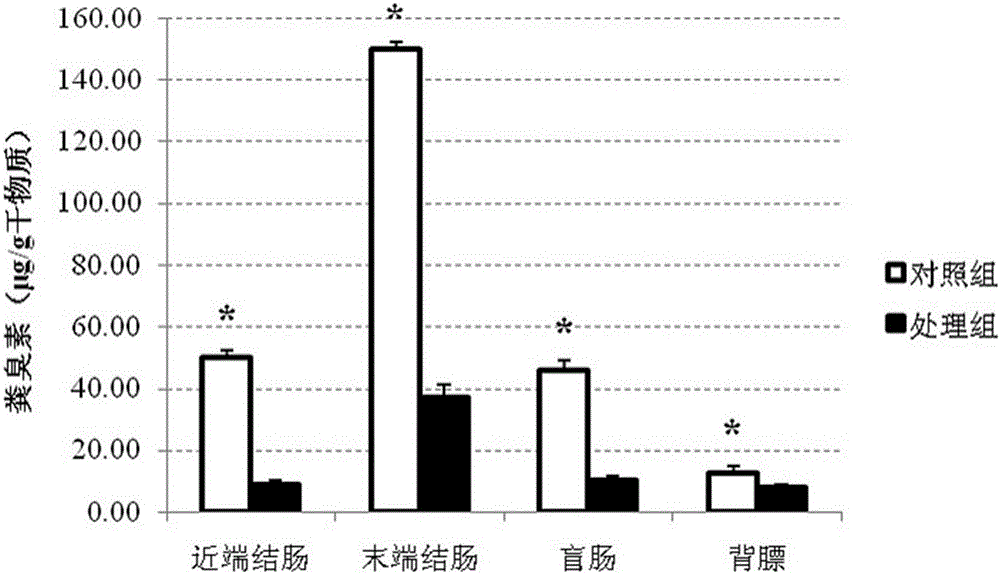
Composite fiber additive capable of improving quality of pork as well as preparation method and application of composite fiber additive
PendingCN106036085AImprove growth performanceImprove qualityFood processingAnimal feeding stuffStreptococcus pyogenesFiber
The invention relates to the technical field of animal feeds, in particular to a composite fiber additive capable of improving the quality of pork as well as a preparation method and application of the composite fiber additive. The composite fiber additive is prepared from oat bran, wheat bran, microcrystalline cellulose, beet residues, pea fibers, lentinan, fructo-oligosaccharide, inulin, konjac gum, sodium alginate and corn starch. In the composite fiber additive disclosed by the invention, the total quantity of dietary fibers is 12-60%, so that indole and skatole in caeca and colons of pigs can be effectively reduced, and the level of the skatole in back fat is reduced; the growing performance and the meat quality of the pigs are obviously improved; the content of pathogenic bacteria such as escherichia coli, staphylococcus aureus and streptococcus pyogenes in chyme in colons and caeca of the pigs is reduced, the content of probiotics such as lactobacilli and faecalibacterium prausnitzii is increased, the growth and the health of the pigs are promoted, and besides, the composite fiber additive has the characteristic of being convenient to prepare and use.
Owner:SICHUAN AGRI UNIV
Method for Reducing the Likelihood of Developing Cancer in an Individual Human Being
ActiveUS20200330526A1Reduce the possibilityPromote resultsOrganic active ingredientsPeptide/protein ingredientsFaecalibacterium prausnitziiProbiotic bacterium
A person's intestinal (gut), oral or skin microbiota is modified using specific combinations of pre-biotics, pro-biotics and / or anti-biotics to establish a defined microbiota that can treat and / or reduce the likelihood that individuals will experience various diseases, including cancer. The employment of various bacteria, whether in particular combinations or after being modified using CRISPR-type systems, leads to improved outcomes when checkpoint inhibitors are used to treat various forms of cancer. One embodiment is directed to a method for reducing the likelihood of developing cancer by providing in the gut of an individual a population of beneficial bacteria selected from the group consisting of Lactobacillus species. The level of Roseburia and Faecalibacterium prausnitzii, and / or Akkermansia muciniphila bacteria are increased in the individual's gut microbiome such that when an individual is administered an immune checkpoint inhibitor, its function is enhanced due to the presence of the bacterial population.
Owner:SEED HEALTH INC
Compositions for treating inflammation and uses thereof
ActiveUS20200121742A1Improve actionReduce releaseBacterial antigen ingredientsBacteriaBiotechnologyDisease
Provided herein are, inter alia, microbial compositions and methods of using the same. The microbial compositions provided include, inter alia, therapeutically effective amounts of Lactobacillus johnsonii, Faecalibacterium prausnitzii, Akkermansia muciniphila, Myxococcus xanthus and Pediococcus pentosaceus and are particularly useful for methods of treating and preventing inflammatory diseases.
Owner:RGT UNIV OF CALIFORNIA
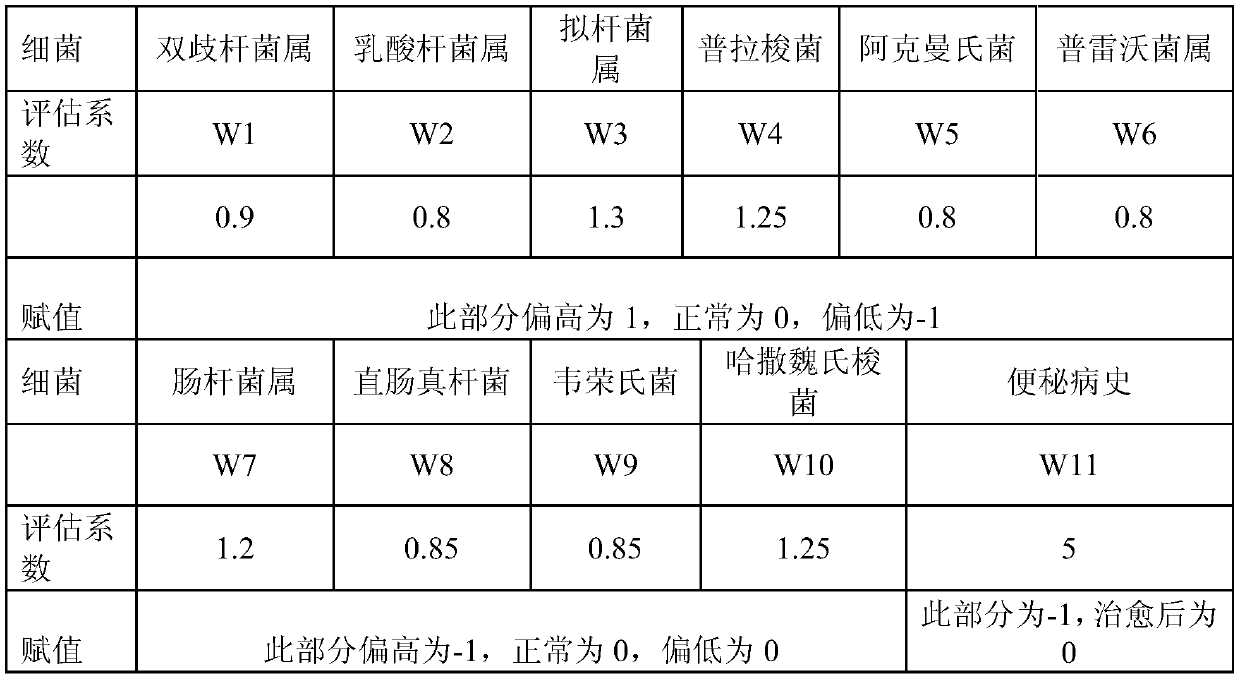
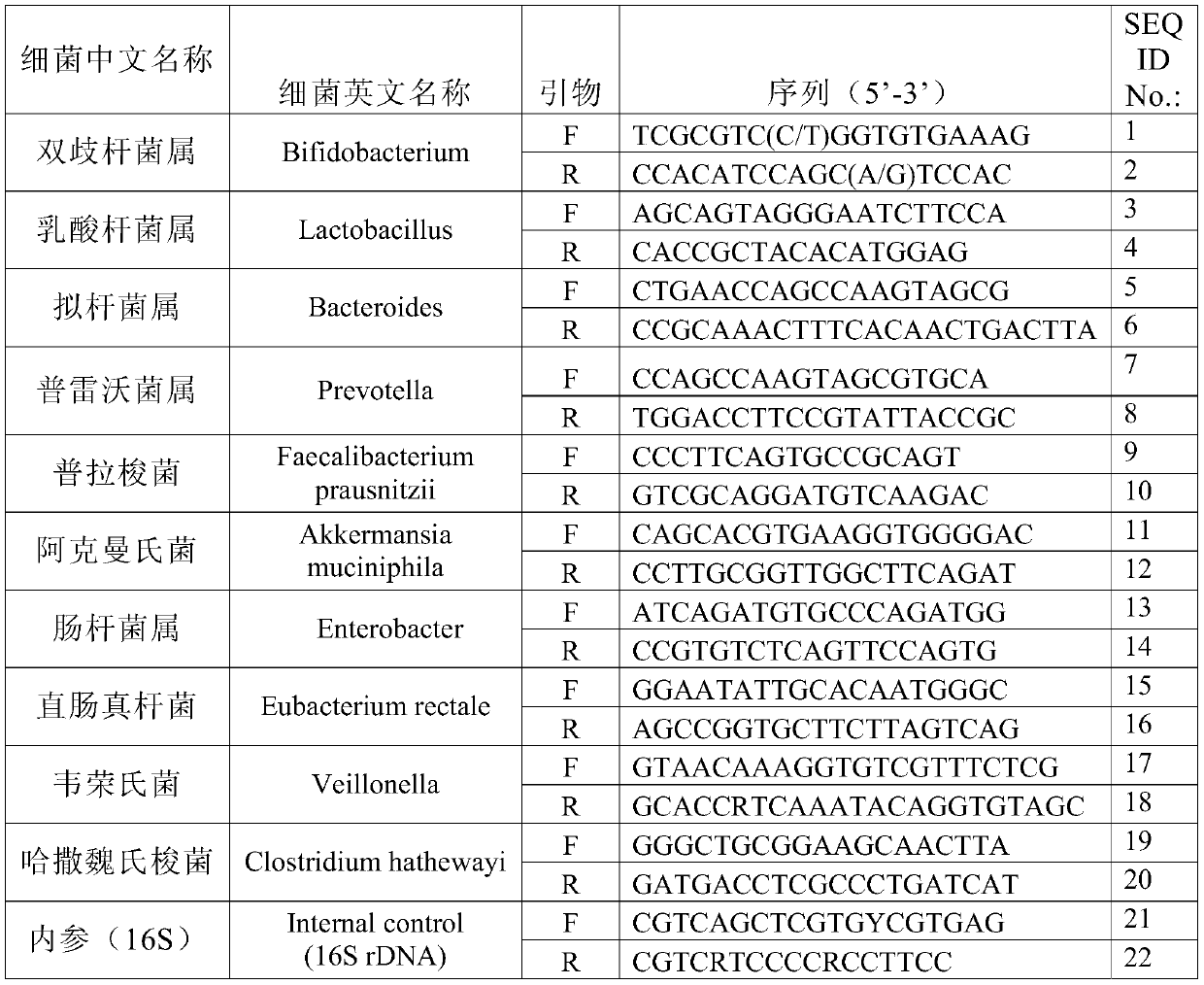
Method and device for evaluating constipation risk and constipation degree based on various intestinal bacteria contents
ActiveCN109749960ABacteriaMicrobiological testing/measurementFluorescenceFaecalibacterium prausnitzii
The invention provides a method and device for evaluating constipation risk and constipation degree based on various intestinal bacteria contents, particularly provides a set of biomarkers comprisingtwo or more biomarkers selected from the group consisting of bacteroides sp., faecalibacterium prausnitzii, akkermansia muciniphila, enterobacter sp., clostridium hathewayi, or a combination thereof.According to the method and device, bacteria related to constipation in the intestinal tract are taken as targets, the relative content of bacteria is rapidly and efficiently detected by adopting a fluorescence PCR technology, the method has the advantages of objectivity, timeliness, rapidness, accuracy and the like in clinical diagnosis of constipation, which can assist clinicians to select and formulate the best treatment scheme according to specific needs, the treatment period of the patient is greatly shortened, and the intestinal tract health is recovered as soon as possible.
Owner:SHANGHAI BIOTECAN PHARMA +1
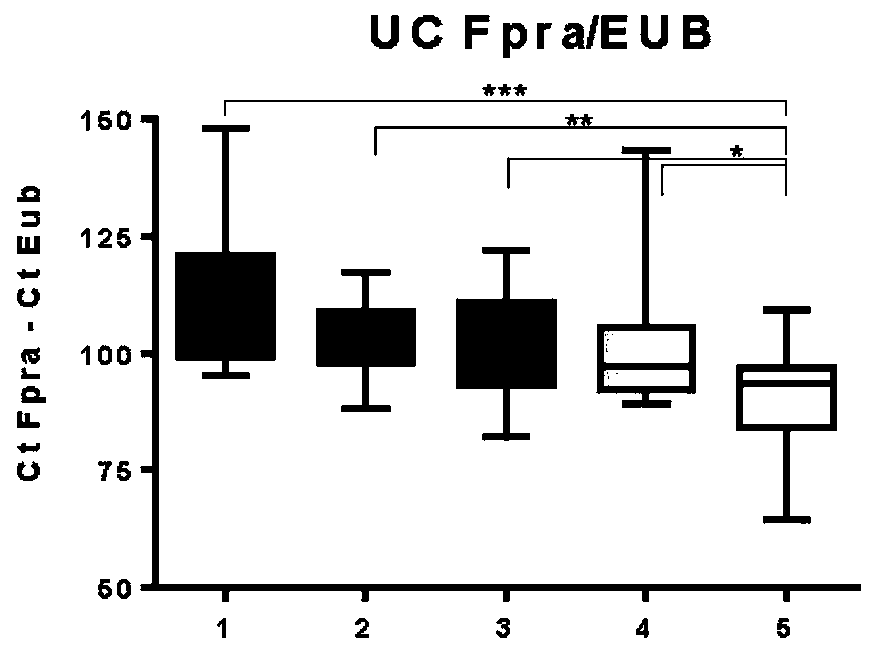
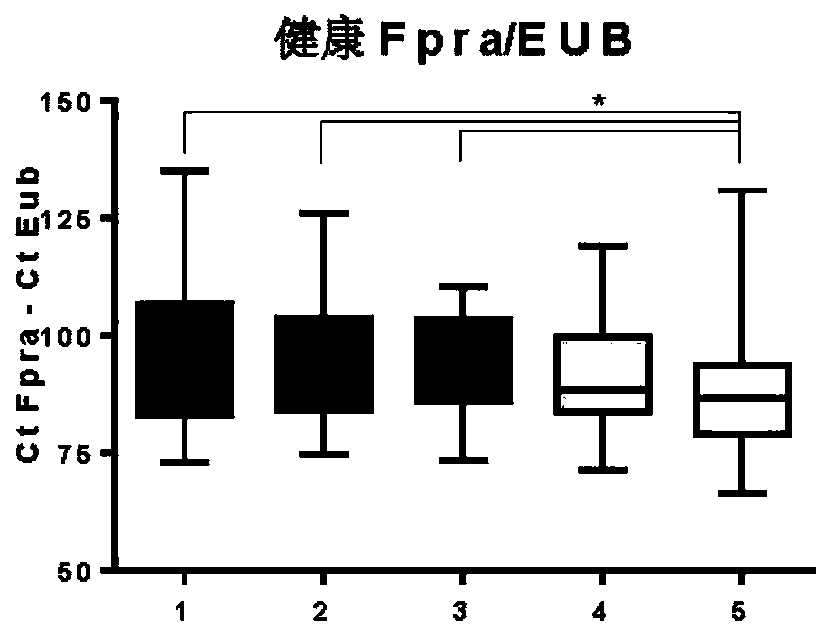
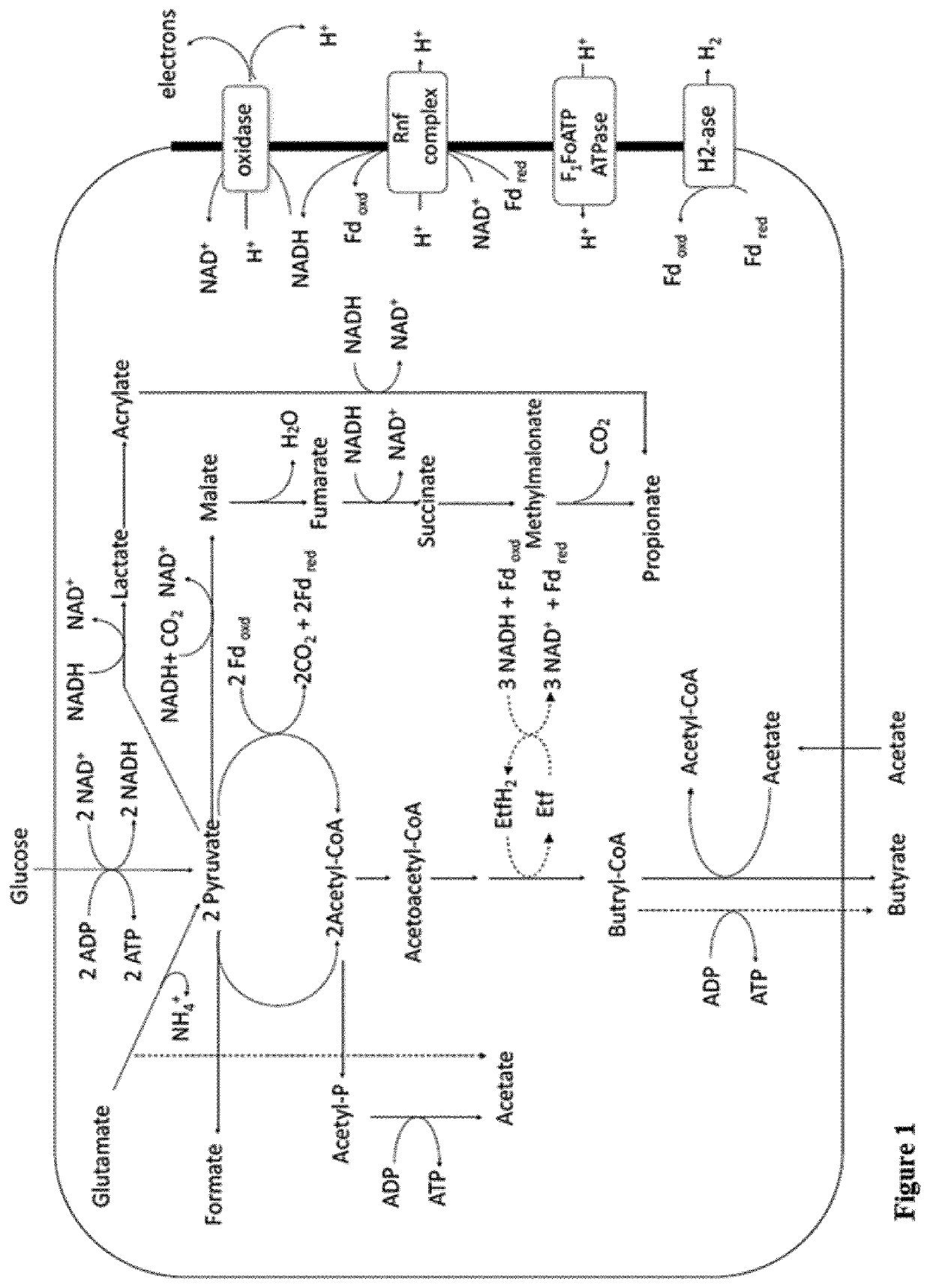
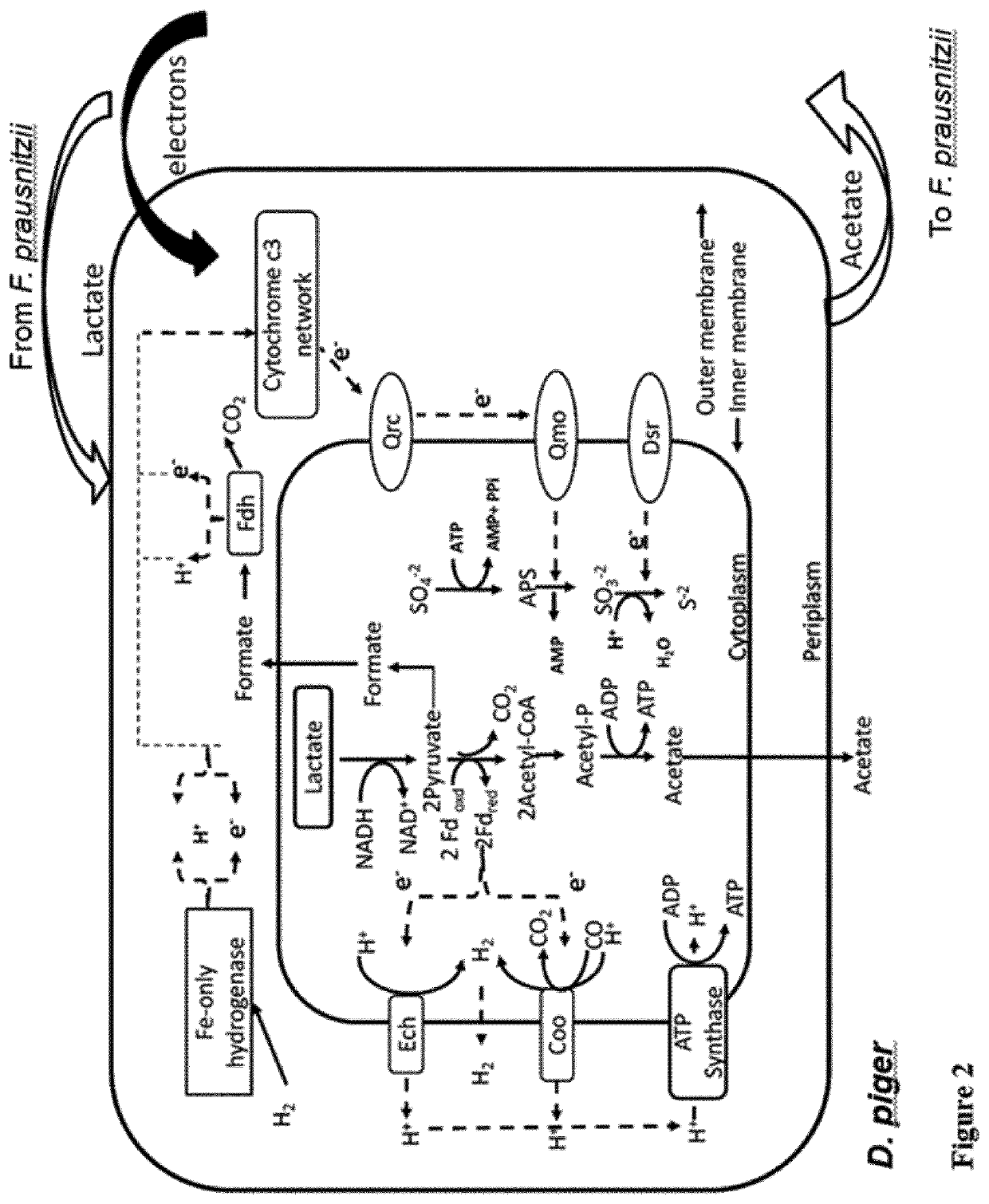
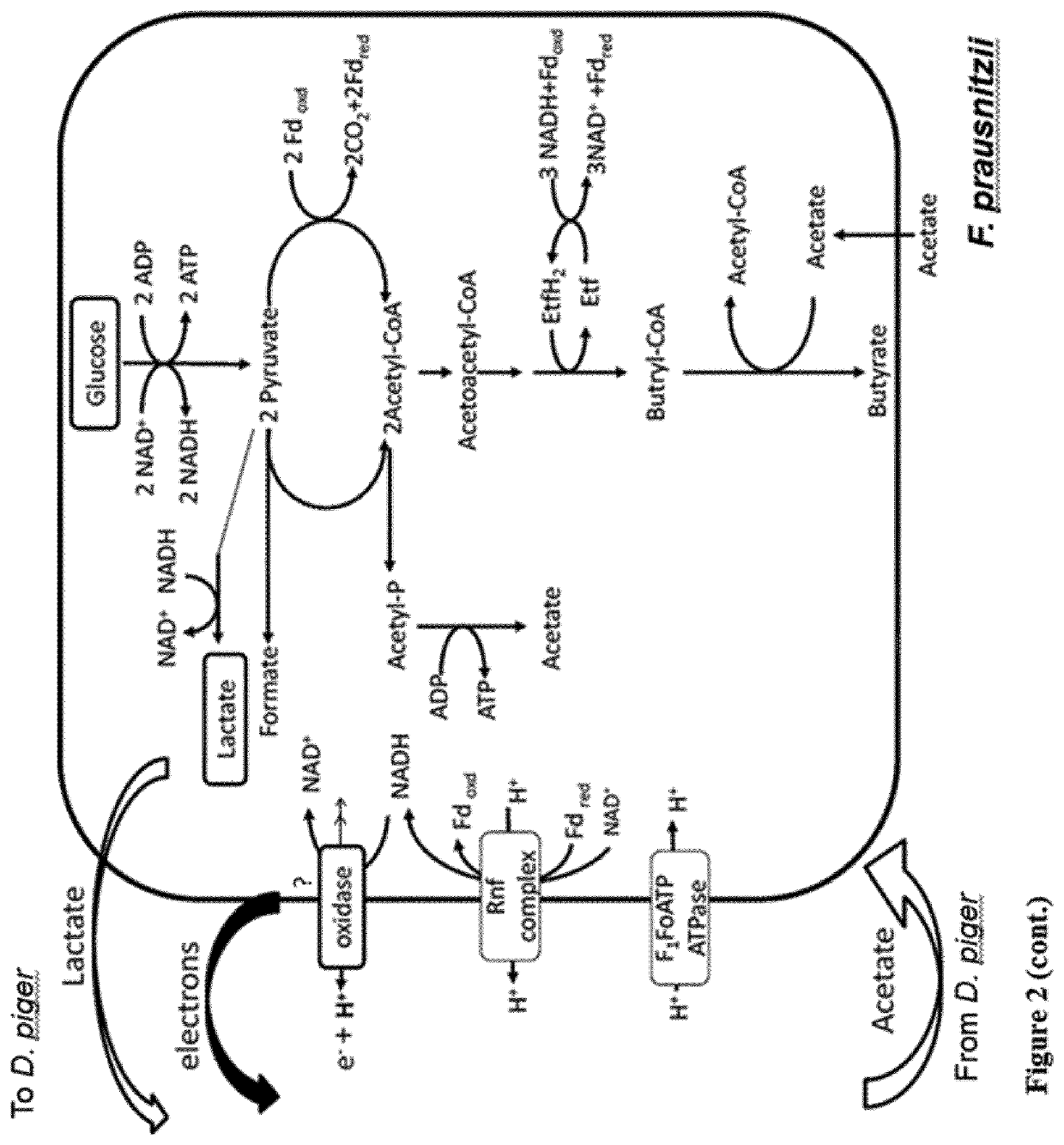
Faecalibacterium prausnitzii and desulfovibrio piger for use in the treatment or prevention of diabetes and bowel diseases
ActiveUS20200405777A1Increase productionIncrease the number ofBacteriaMetabolism disorderDisease irritable bowelDisease
The present invention relates generally to medicine. More specifically the invention relates to the use of synergistic probiotic bacteria as intervention for health. In particular, the present invention provides a strain of Faecalibacterium prausnitzii and a bacterial strain which has one or more of the characteristics of: (i) being acetate producing, (ii) being lactate consuming and (iii) having the ability to be an electron acceptor, for use in the treatment or prevention of a disease associated with reduced butyrate levels or a disease associated with reduced or low numbers of Faecalibacterium prausnitzii bacteria.
Owner:METABOGEN
Method for reducing the likelihood of developing cancer in an individual human being
ActiveUS10940169B2Reduce the possibilityPromote resultsOrganic active ingredientsPeptide/protein ingredientsFaecalibacterium prausnitziiProbiotic bacterium
A person's intestinal (gut), oral or skin microbiota is modified using specific combinations of pre-biotics, pro-biotics and / or anti-biotics to establish a defined microbiota that can treat and / or reduce the likelihood that individuals will experience various diseases, including cancer. The employment of various bacteria, whether in particular combinations or after being modified using CRISPR-type systems, leads to improved outcomes when checkpoint inhibitors are used to treat various forms of cancer. One embodiment is directed to a method for reducing the likelihood of developing cancer by providing in the gut of an individual a population of beneficial bacteria selected from the group consisting of Lactobacillus species. The level of Roseburia and Faecalibacterium prausnitzii, and / or Akkermansia muciniphila bacteria are increased in the individual's gut microbiome such that when an individual is administered an immune checkpoint inhibitor, its function is enhanced due to the presence of the bacterial population.
Owner:SEED HEALTH INC
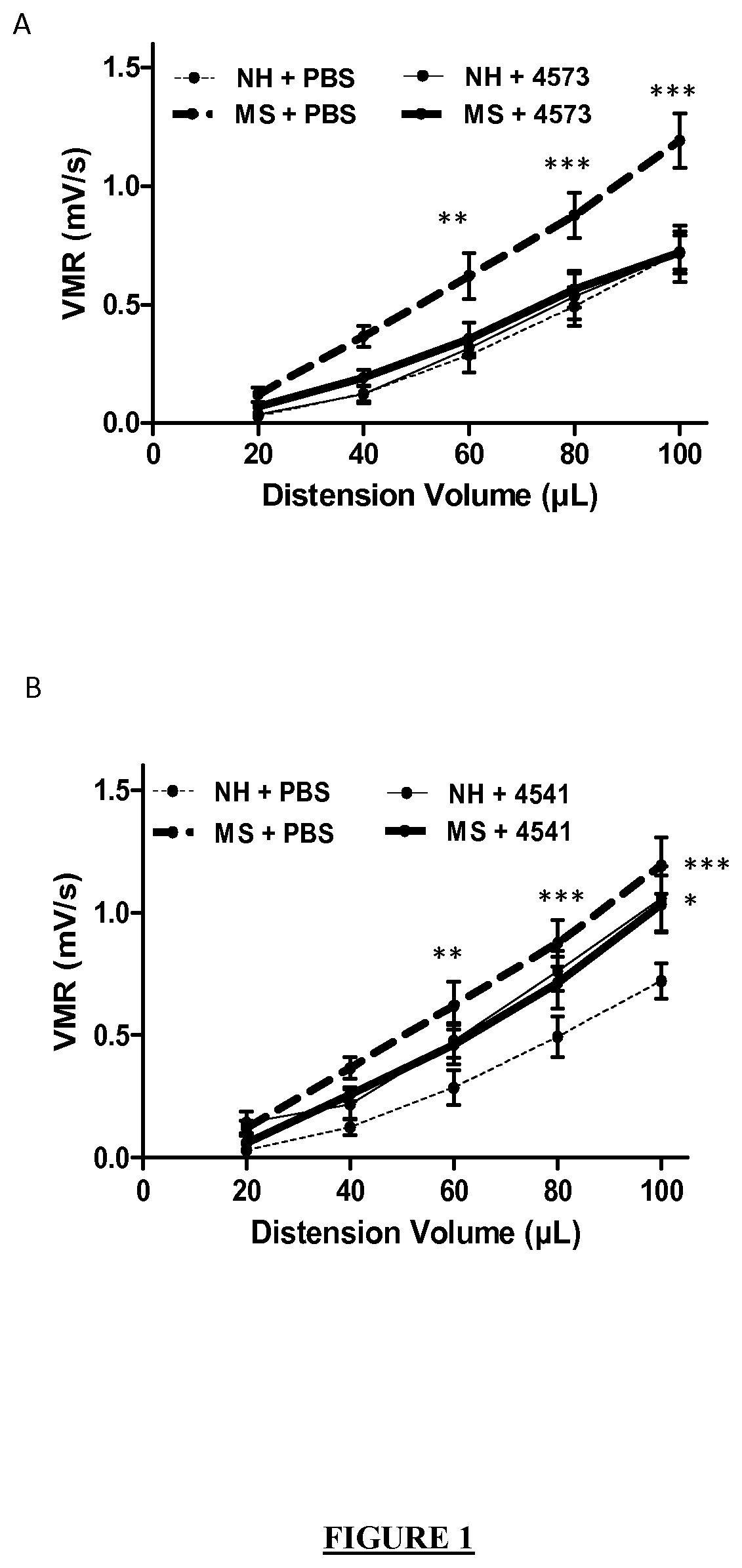
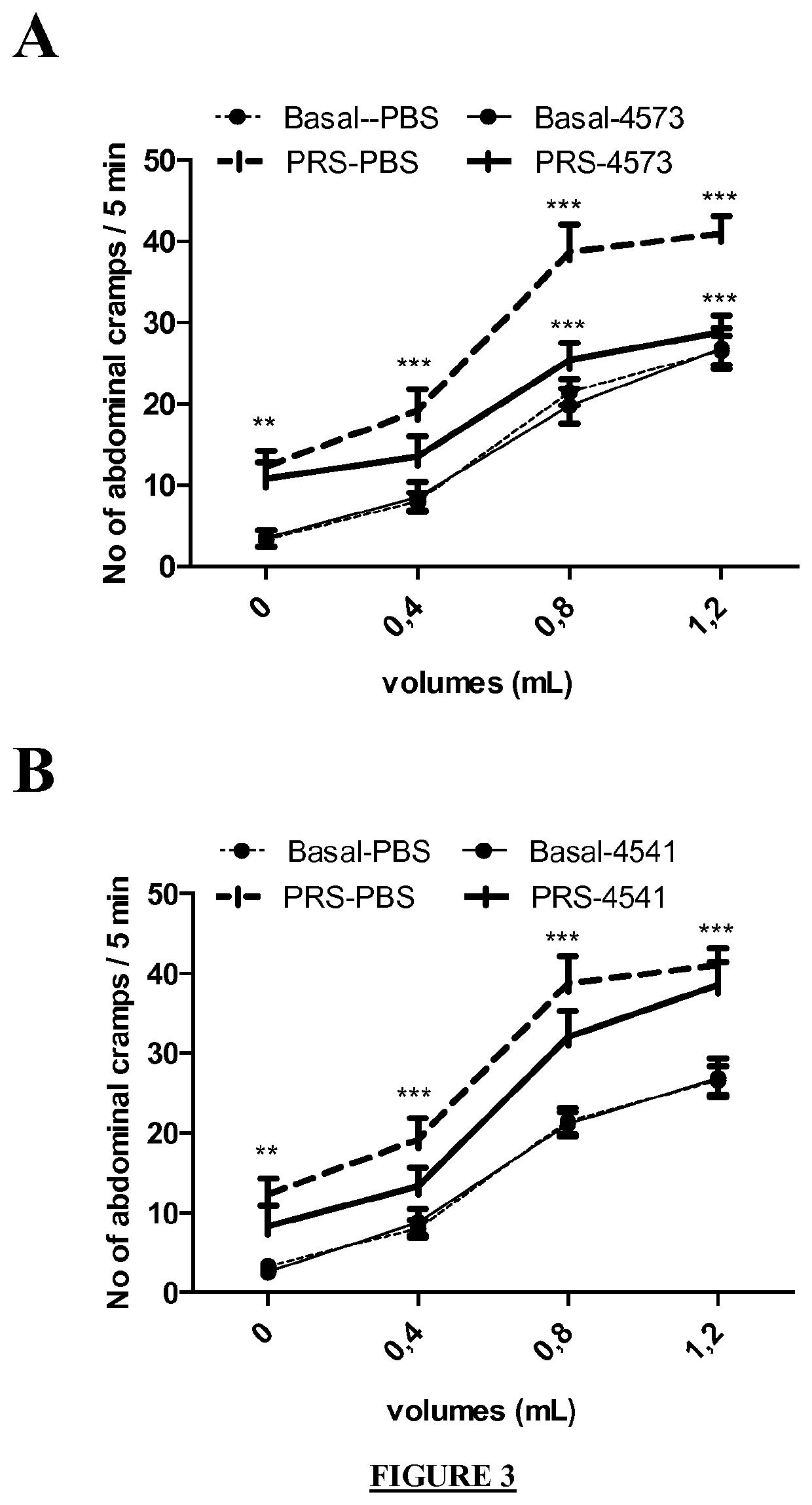
Faecalibacterium prausnitzii strain cncm 1-4573 for the treatment and prevention of gastrointestinal inflammation
The invention relates to a bacterial strain of species Faecalibacterium prausnitzii deposited at the CNCM under access number CNCM I-4573, for use in the treatment and / or prevention of an inflammatorygastrointestinal illness in an individual.
Owner:INST NAT DE RECH POUR LAGRICULTURE LALIMENTATION & LENVIRONNEMENT +2
Method and System for Reducing the Likelihood of Developing Depression in an Individual
ActiveUS20200397832A1Inhibition effectDesired effectPeptide/protein ingredientsBacteria material medical ingredientsBiotechnologyIntestinal microorganisms
A method for reducing the likelihood of developing depression in an individual involves providing in the gut of an individual a population of beneficial bacteria selected from the group consisting of bacterial species able to make small chain fatty acids, and preferably butyrate, and administering fiber to the individual to maintain a therapeutically effective amount of the beneficial bacteria in the gut of the individual. The individual's gut microbiome is modified to reduce the number of undesired bacteria and to increase the number of beneficial bacteria. Bacteria are preferably modified to remove one or more virulence facts or alternatively to produce increased amounts of SCFA's, such as butyrate. Beneficial bacteria may be encapsulated in a frangible enclosure to ensure they arrive in an individual's body while still viable, e.g. such as being first released in the lower gut rather than being exposed to the harsh conditions of an individual's stomach. In other embodiments, a therapeutically effective amount of a bacterial formulation comprising Faecalibacterium prausnitzii is administered. Other embodiments include the administration of a bacterial formulation comprising at least one of Coprococcus, Roseburia, Bifidobacterium, Faecalibacterium prausnitzii and L. casei to treat depression.
Owner:SEED HEALTH INC
Long chain dicarboxylic fatty acid (LCDFA) producing microbes and uses thereof
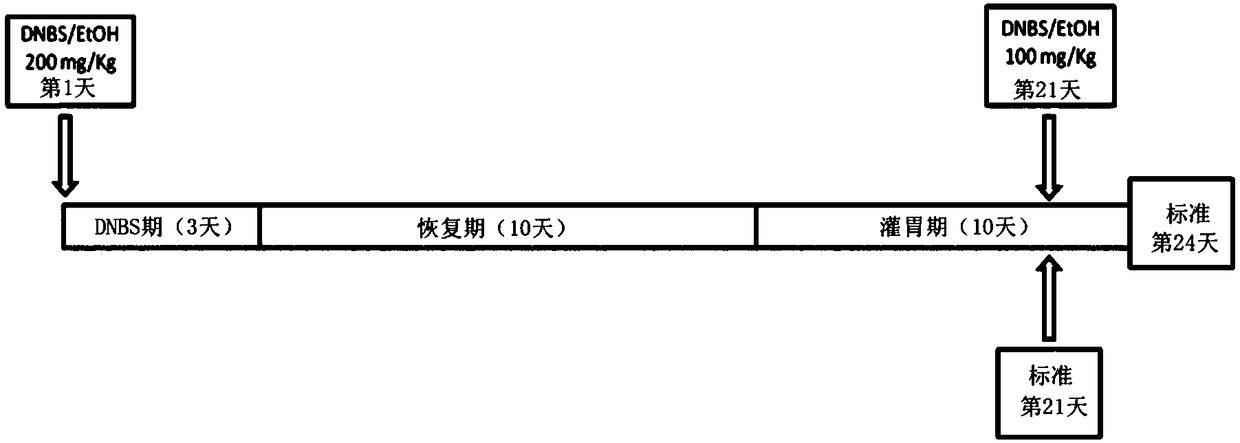
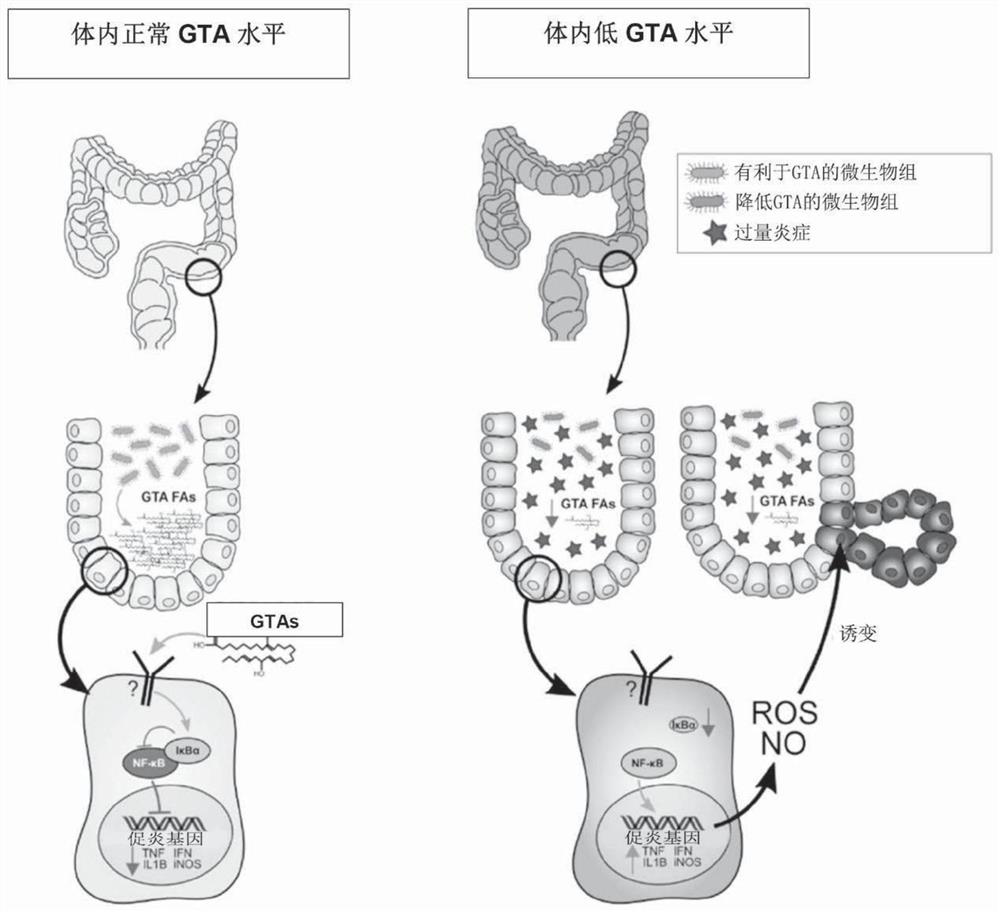
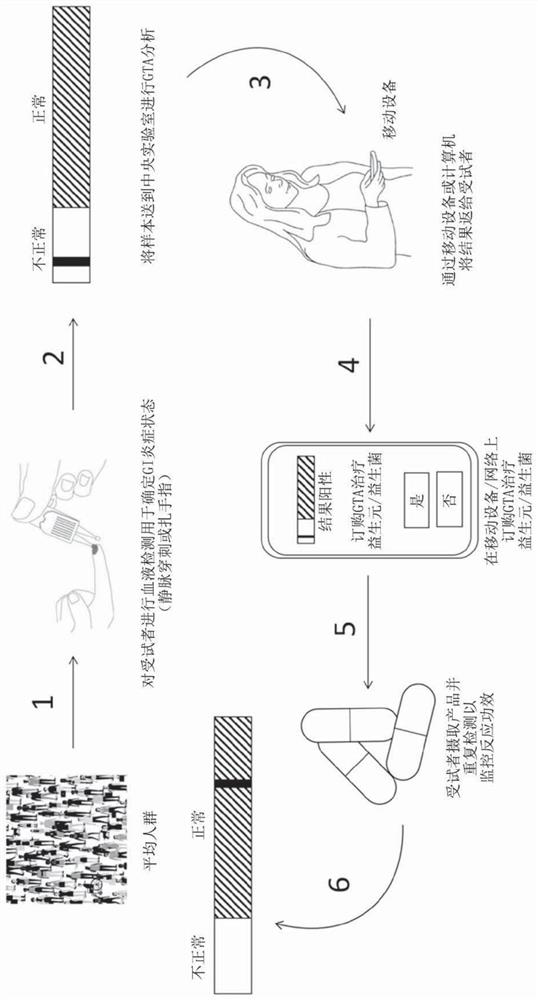
A method for increasing gastric tract acid (GTA) production in a mammalian subject. The method comprises administering a therapeutically-effective amount of a composition comprising at least one liveor attenuated culture of a microbial species selected from the genus Blautia, species Faecalibacterium prausnitzii, genus Bacteroides, family Ruminococcaceae, family Lachnospiraceae, genus Coprococcus, genus Roseburia, genus Oscillospira, species Ruminococcus bromii, genus Ruminococcus, family Costridiaceae, species Dorea formicigenerans, species Bacteroides uniformis, genus Dorea, genus Streptococcus, order Clostridiales, genus Anaerostipes, genus Dialister, species Bifidobacterium adolescentis, family Coriobacteriaceae, genus Faecalibacterium, genus Sutterella, species Bacteroides ovatus, genus Parabacteroides, genus Ruminococcus, species Bacteroides faecis, species Eubacterium biforme, genus Phascolartobacterium, and family Enterobacteriaceae; or a prebiotic composition which increasesgrowth and / or viability of said microbial species in the gut. Administering the composition increases the synthesis of at least one GTA dicarboxylic fatty acid metabolite in said subject. Also described are method for determining gastrointestinal inflammation status and kits for detecting and treating a gastric tract acid (GTA) insufficiency.
Owner:医学生命探索有限公司
Primers to detect cow dung contamination and detection kit
InactiveCN108411015AStrong specificityImprove stabilityMicrobiological testing/measurementDNA/RNA fragmentationFaecalibacterium prausnitziiMicrobiology
The invention belongs to the field of biological technology and particularly relates to primers to detect cow dung contamination and a detection kit. The detection primers can specifically react withcattle Faecalibacterium to generate a strip; chicken, duck, human, dog and pig Faecalibacterium cannot react; in the six species, the primer specificity reaches 100%. The primers applied to the detection of cattle Faecalibacterium has lowest detection limits (LOQ)s reaching 2490 c / mu l, and the sensitivity is excellent; same-species cow dung samples in detection reach 93.8% (45 / 48), and good stability is provided. The detection primers and kit can be used to detect water pollution so as to judge whether cow dung contamination is present in water, and to position a pollution source.
Owner:WENZHOU UNIVERSITY
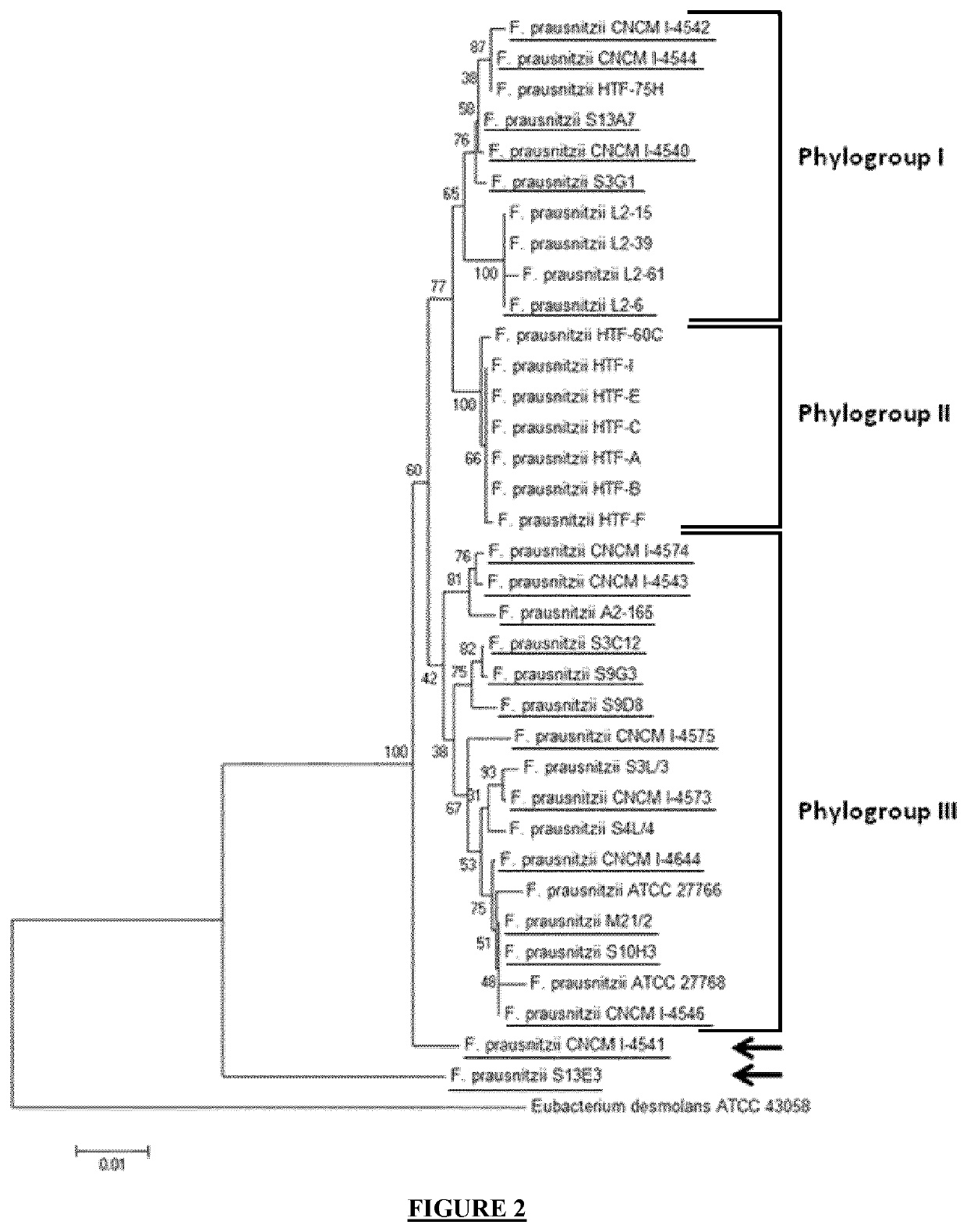
Faecalibacterium prausnitzii strains for treating and preventing gastrointestinal pain
InactiveUS20190381115A1Reduce sensitivityPrevent and reduce hypersensitivityAntipyreticMicroorganismsBacteroidesVisceral abdominal pain
The present invention relates to a bacterial strain of the Faecalibacterium prausnitzii species selected from a bacterial strain belonging to one of the phylogroups I, II and III, for use in the treatment and / or prevention of visceral abdominal pain in an individual. The present invention also concerns compositions comprising said bacterial strains as well as specific strains as such.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
Human pulmonary infection assessment method based on intestinal flora in excrement sample and application of human pulmonary infection assessment method
PendingCN112342280ARelieve painImproved prognosisMicrobiological testing/measurementMicroorganism based processesPulmonary infectionDisease
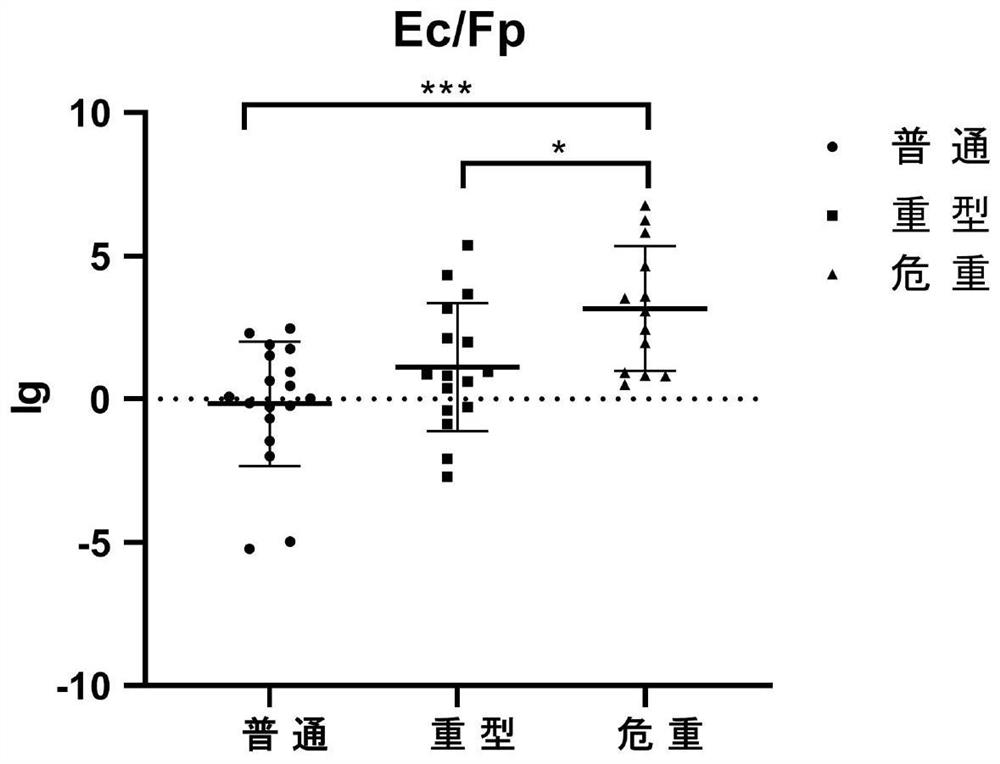
The invention discloses a human pulmonary infection assessment method based on intestinal flora in an excrement sample. The method comprises the following steps: collecting the excrement sample of a tested person and extracting DNA; configuring a PCR reaction system and performing PCR amplification; establishing a standard curve; performing quantitative analysis to obtain the quantity of the intestinal flora in the excrement sample; and calculating the ratio of enterococcus to faecalibacterium prausnitzii, and assessing the illness degree and prognosis condition of the tested person by combining the ratio with the value of the bifidobacterium content (Ec / Fp-B). According to the faecalibacterium prausnitzii method, the ratio of the enterococcus to the faecalibacterium prausnitzii in combination with the bifidobacterium content (Ec / Fp-B) can be used for assessing the disease degree and prognosis condition of pulmonary infection patients. The method has the advantages that the ratio of enterococcus to faecalibacterium prausnitzii in the intestinal flora is combined with bifidobacteria to effectively assess and judge the disease degree and prognosis condition of the pulmonary infectionpatients, the method is noninvasive and non-interventional, the pain of the patients is relieved, the detection efficiency is high, the detection cost is low and the like.
Owner:中诺(杭州)基因科技有限责任公司
Application of sodium diacetate in improving diversity of intestinal flora of animals
PendingCN110613057AIncrease diversityFast growthBacteriaMicroorganism based processesFaecalibacterium prausnitziiEnterobacter
The invention discloses an application of sodium diacetate in improving diversity of intestinal flora of animals. Humanized mouse model intervene experiment study of intestinal flora finds that the diversity of intestinal flora of model mice can be remarkably improved by adding a proper amount of sodium diacetate, wherein alpha diversity indexes (Chaol, Shannon and Simpson) are notably increased,the OUT index is increased by 2-4 times, and sodium diacetate has a remarkable reduction function on harmful bacteria such as Enterobacter, Escherichia-Shigella, Proteus, Faecalibacterium, Enterococcus, Streptococcus and Helicobacter and can remarkably reduce the inflammation level of intestines and bodies, relieve constipation, reduce diarrhea of the animals, increase the growth speed of animalsand enhance the disease-resistant capability of the animals.
Owner:GUANGDONG OCEAN UNIVERSITY
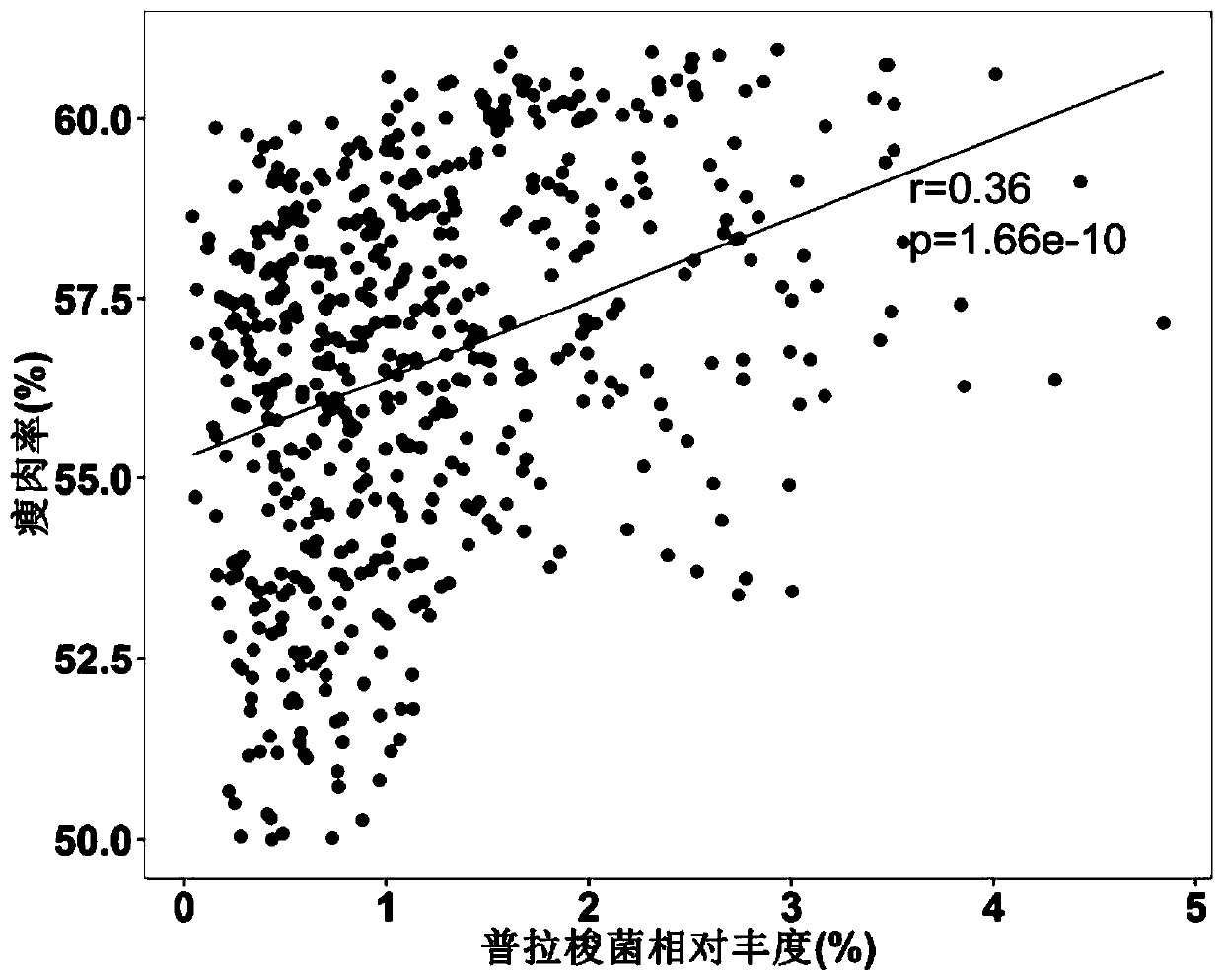
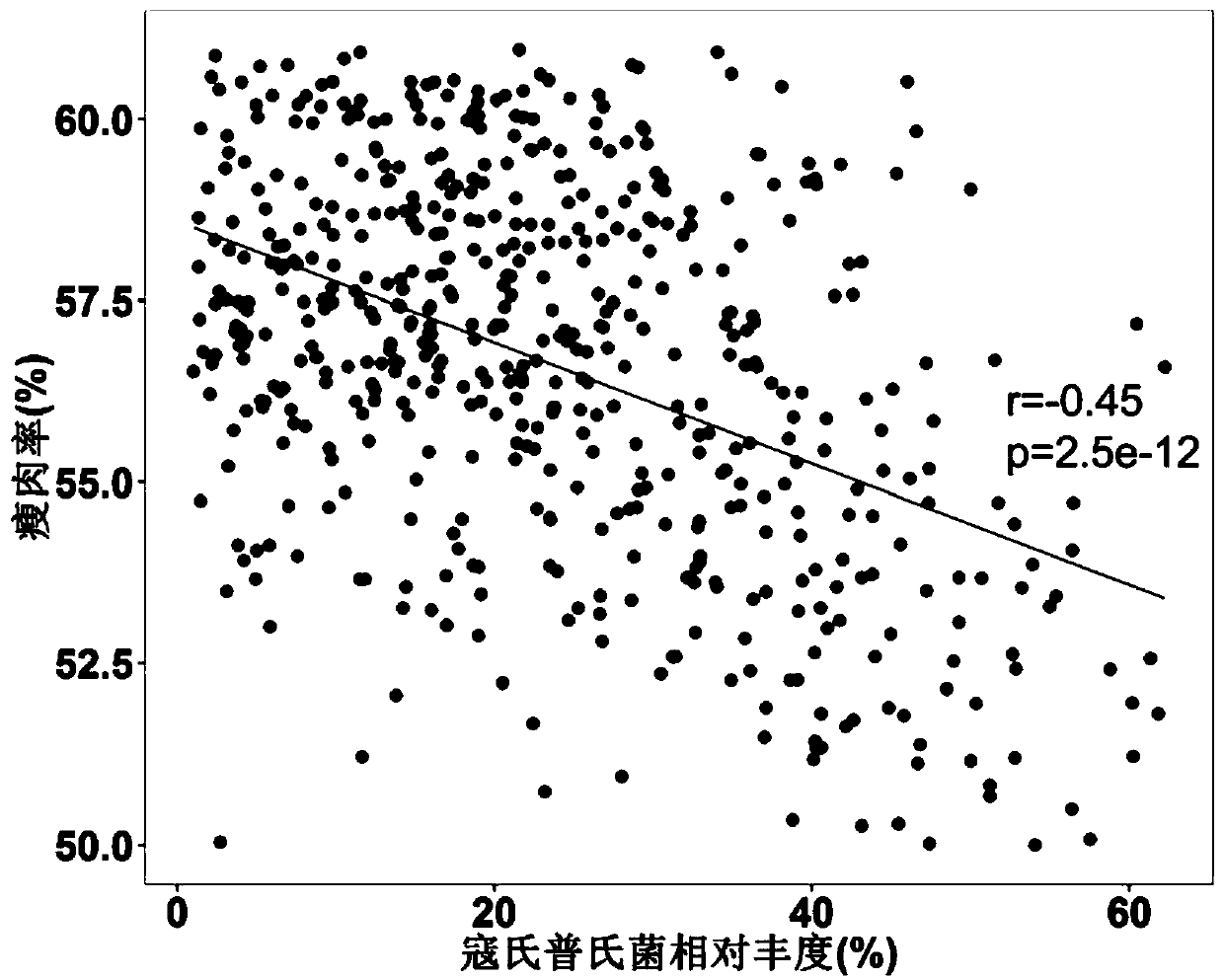
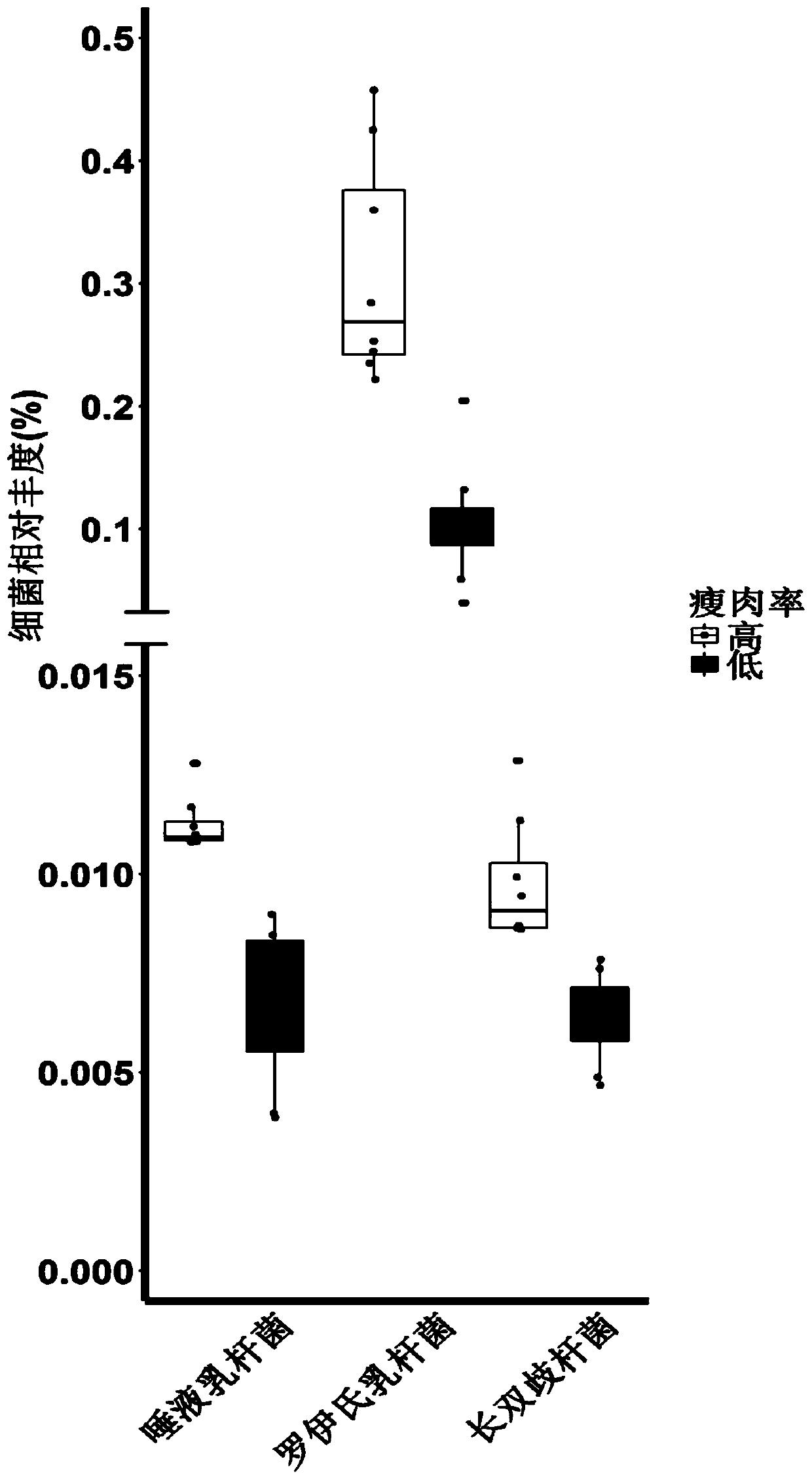
Application of bacteria to influencing lean meat percentage and/or fat content of animals
ActiveCN110447763AImprove lean meat percentageLow in fatBacteriaMicrobiological testing/measurementBiotechnologyBacteroides
The invention provides application of bacteria to influencing the lean meat percentage of animals. The bacteria include prevotella bacteria (at least one of Prevotella copri, Prevotella buccalis, Prevotella intermedia and Prevotella multiformis) with the function of reducing the lean meat percentage of animals, and lactobacillus bacteria (at least one of Lactobacillus reuteri, Lactobacillussalivarius, Bifidobacterium longum and Faecalibacterium prausnitzii) with the function of increasing the lean meat percentage of animals.
Owner:JIANGXI AGRICULTURAL UNIVERSITY
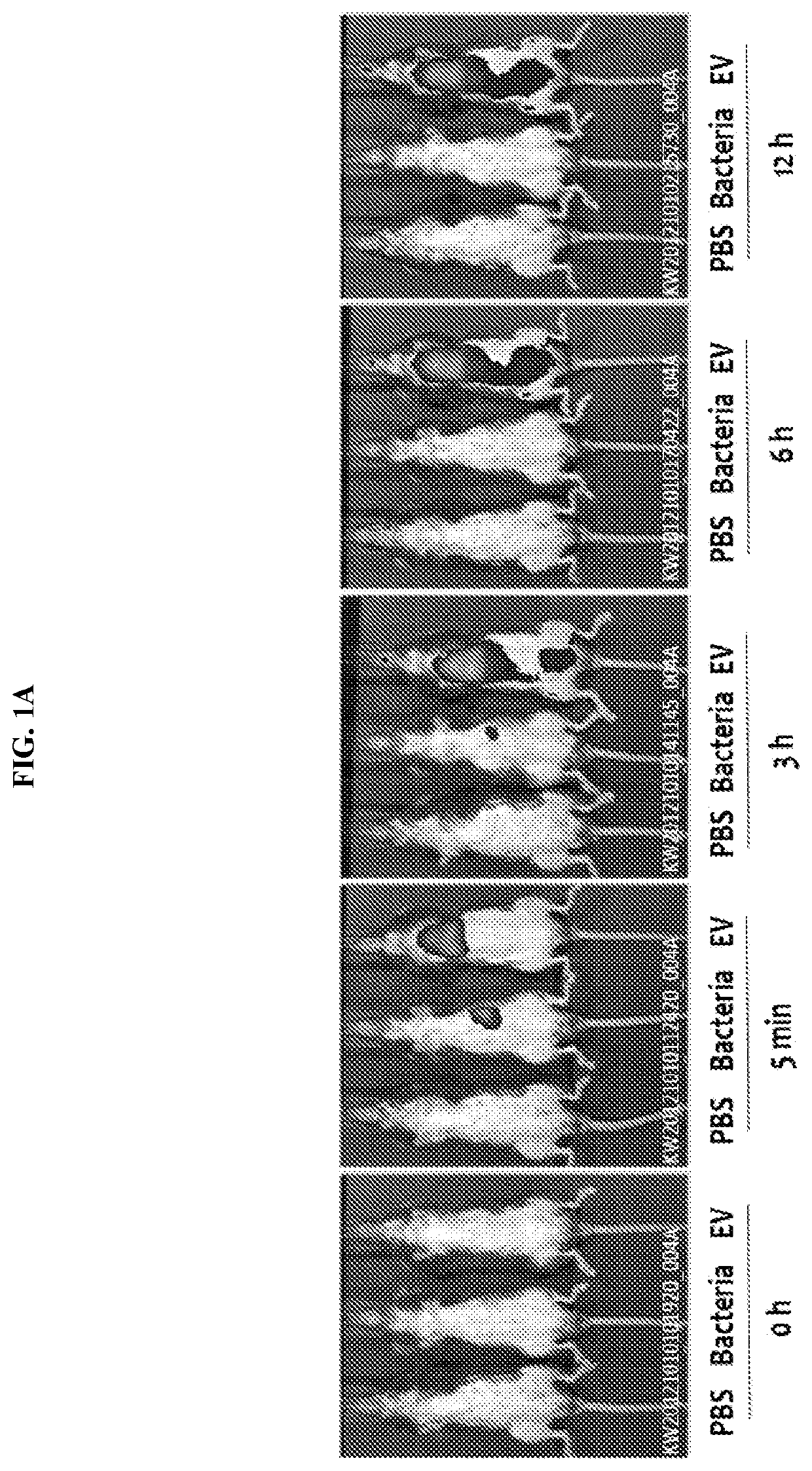
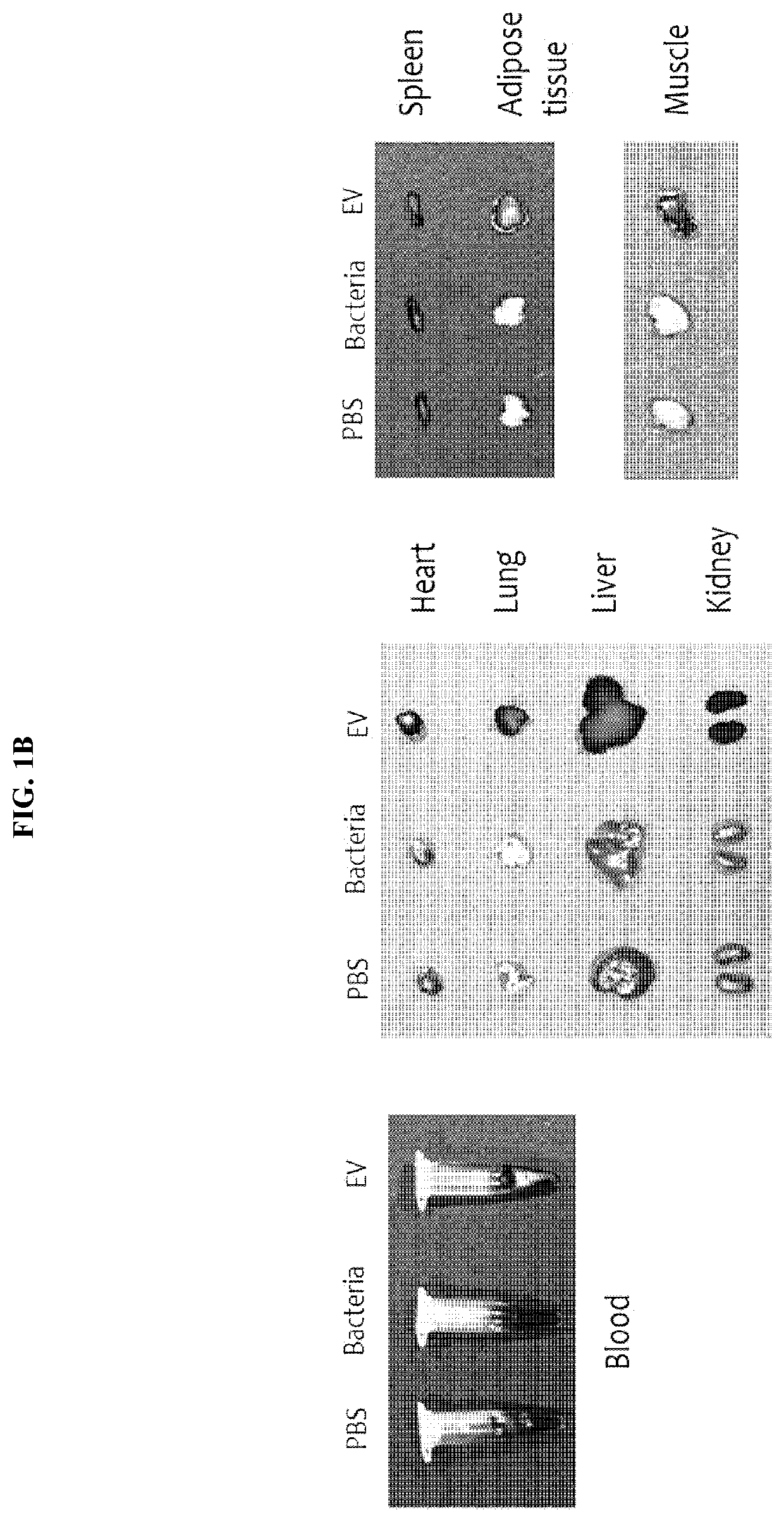
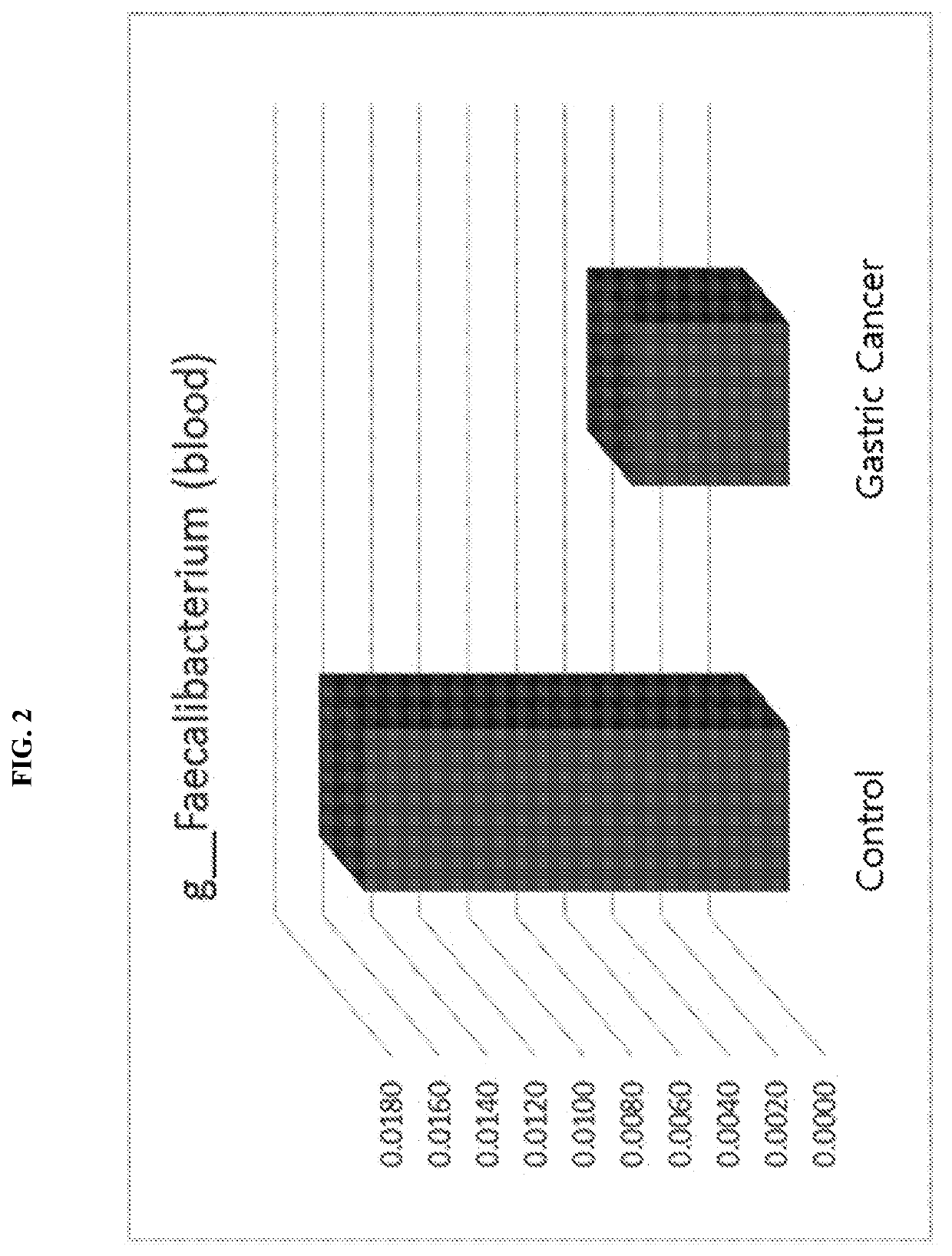
Nanovesicles derived from faecalibacterium prausnitzii, and uses thereof
PendingCN111587295AReduce contentInhibition of secretionNervous disorderBacteria material medical ingredientsDiseaseFaecalibacterium prausnitzii
The present invention relates to vesicles derived from Faecalibacterium prausnitzii and to uses thereof. It has been experimentally confirmed by the present inventors that the vesicles in the clinicalsamples of patients with gastric cancer, colorectal cancer, liver cancer, pancreatic cancer, cholangiocarcinoma, ovarian cancer, bladder cancer, lymphoma, myocardial infarction, atrial fibrillation,and Parkinson's disease were significantly reduced in comparison with a normal person and that when vesicles separated from the strain were administered, the secretion of inflammatory mediators causedby pathogenic vesicles, such as vesicles derived from colon bacilli, was significantly inhibited. The vesicles derived from Faecalibacterium prausnitzii according to the present invention are expected to be usefully employed for the purposes of developing a method for diagnosing gastric cancer, colorectal cancer, liver cancer, pancreatic cancer, cholangiocarcinoma, ovarian cancer, bladder cancer,lymphoma, myocardial infarction, atrial fibrillation, and / or Parkinson's disease, and a composition for preventing, alleviating, or treating said diseases.
Owner:MD HEALTHCARE INC
Grape skin for use in treatment of dysbiosis
ActiveCN111246870AMicrobiological testing/measurementDigestive systemBiotechnologyFaecalibacterium prausnitzii
The present invention relates to the use of grape skin or a composition comprising it as prebiotic for increasing the intestinal levels and / or activity of butyrate-producing bacteria, such as Faecalibacterium prausnitzii, F. prausnitzii phylogroup I (PHGI), F. prausnitzii phylogroup II (PHGII), Roseburia hominis and Subdoligranulum variabile, in a subject. More specifically it refers to grape skinor a composition comprising it for use in a method of treating intestinal dysbiosis in a subject, wherein intestinal dysbiosis is characterized by a decrease of butyrate- producing bacteria levels and / or activity. In addition, it pertains to grape skin or a composition comprising it for use in a method of treating an inflammatory or non-inflammatory intestinal disease in a subject having reducedlevels and / or reduced activity of butyrate-producing bacteria.
Owner:GOODGUT SL
Faecalibacterium prausnitzii and Desulfovibrio piger for use in the treatment or prevention of diabetes and bowel diseases
ActiveUS11260082B2Increase the number ofEnhanced colonizationBacteriaMetabolism disorderDisease irritable bowelDisease
The present invention relates generally to medicine. More specifically the invention relates to the use of synergistic probiotic bacteria as intervention for health. In particular, the present invention provides a strain of Faecalibacterium prausnitzii and a bacterial strain which has one or more of the characteristics of: (i) being acetate producing, (ii) being lactate consuming and (iii) having the ability to be an electron acceptor, for use in the treatment or prevention of a disease associated with reduced butyrate levels or a disease associated with reduced or low numbers of Faecalibacterium prausnitzii bacteria.
Owner:METABOGEN
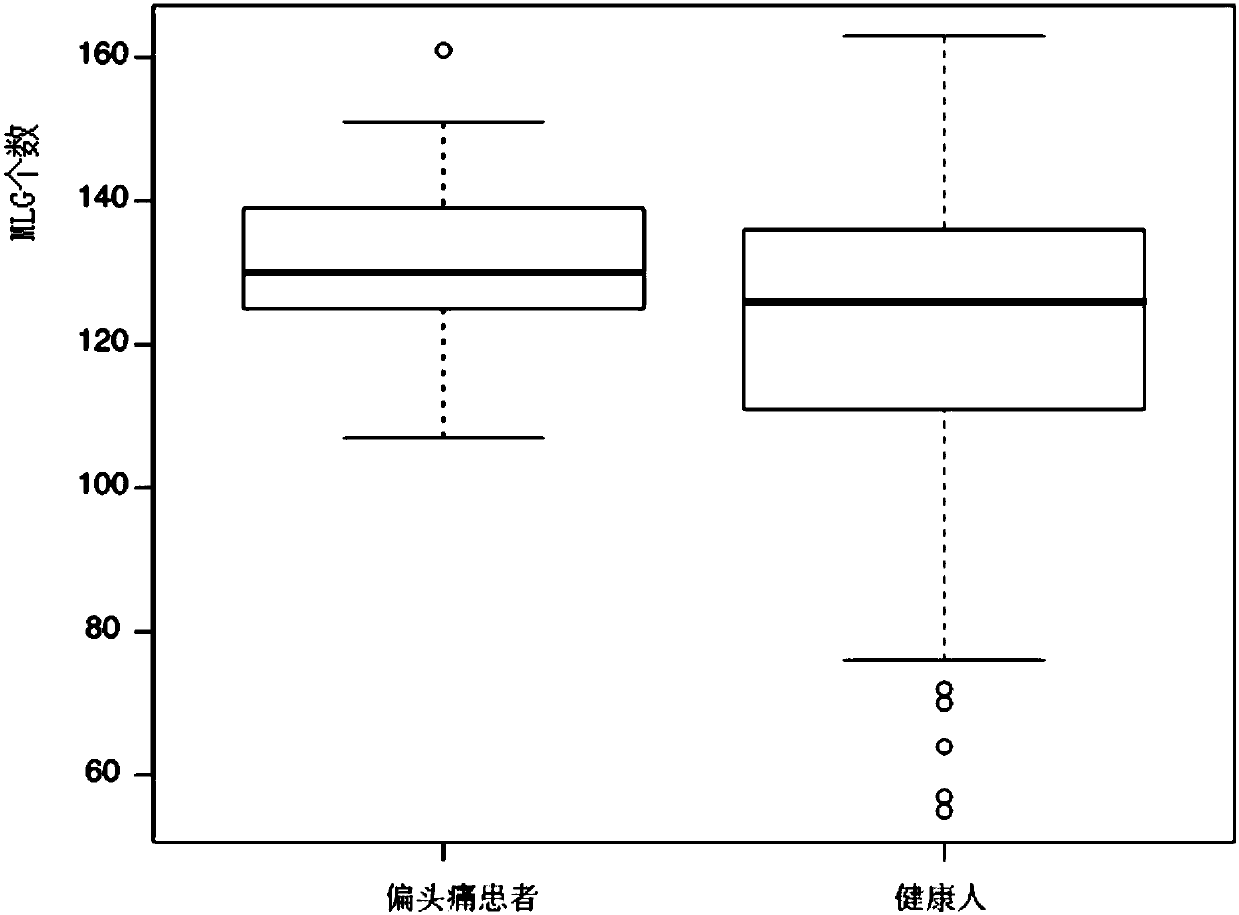
Migraine biomarker and use thereof
ActiveCN110396538AWith characteristicsSensitiveMicrobiological testing/measurementDisease riskDiagnosis methods
The present invention relates to the field of biomedicines and particularly relates to a migraine biomarker and an application thereof. The provided migraine biomarker comprises at least one selectedfrom the following: bacteroides thetaiotaomicron and / or analogues thereof, faecalibacterium prausnitzii and / or analogues thereof, bacteroides intestinalis and / or analogues thereof and anaeroridus colihominis and / or analogues thereof. The present invention provides the biomarker for early diagnosis of the migraine, and a diagnosis and disease risk assessment method of the migraine, and can solve problems that the existing migraine diagnosis method cannot conduct early warn, cannot predict migraine attack, etc.
Owner:SHENZHEN HUADA GENE INST
Nanovesicles derived from faecalibacterium prausnitzii and uses thereof
ActiveUS20210093677A1Inhibition of secretionNervous disorderBacteria material medical ingredientsEscherichia coliFaecalibacterium prausnitzii
Provided are vesicles derived from Faecalibacterium prausnitzii and to uses thereof. It has been experimentally confirmed by the present inventors that the vesicles in the clinical samples of patients with gastric cancer, colorectal cancer, liver cancer, pancreatic cancer, cholangiocarcinoma, ovarian cancer, bladder cancer, lymphoma, myocardial infarction, atrial fibrillation, and Parkinson's disease were significantly reduced in comparison with a normal person and that when vesicles isolated from the strain were administered, the secretion of inflammatory mediators caused by pathogenic vesicles, such as E. coli-derived vesicles, was significantly inhibited. The vesicles derived from Faecalibacterium prausnitzii according to the subject matter are expected to be usefully employed for the purposes of developing a method for diagnosing gastric cancer, colorectal cancer, liver cancer, pancreatic cancer, cholangiocarcinoma, ovarian cancer, bladder cancer, lymphoma, myocardial infarction, atrial fibrillation, and / or Parkinson's disease, and a composition for preventing, alleviating, or treating said diseases.
Owner:MD HEALTHCARE INC
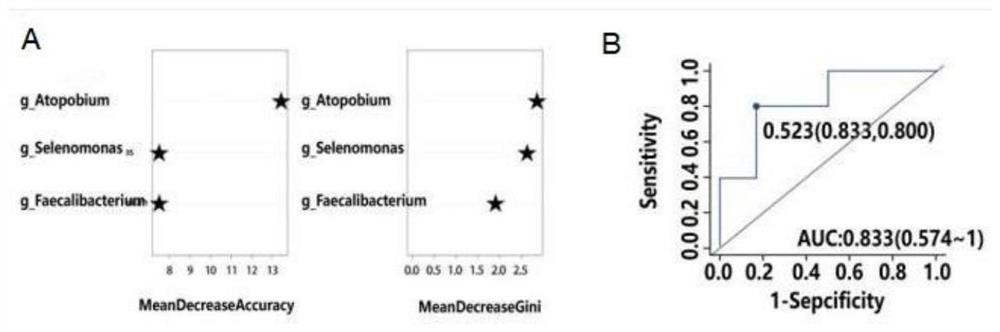
Kit for diagnosing malnutrition condition through flora detection of colon cancer tumor patient
PendingCN114592077AAccurate detectionMicrobiological testing/measurementMicroorganism based processesNutritionFaecalibacterium prausnitzii
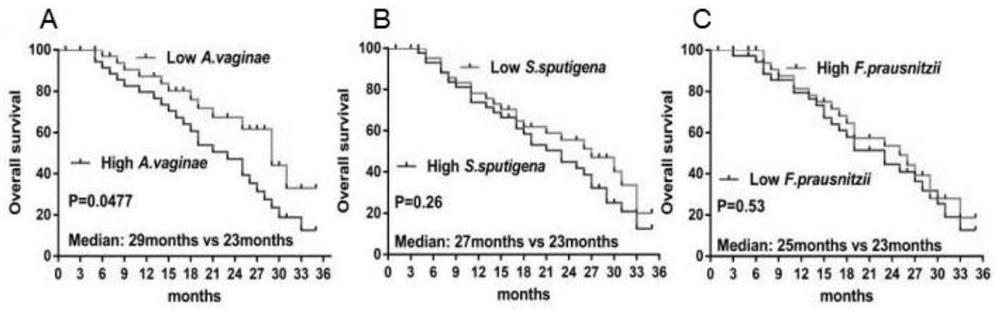
The invention discloses a kit for detecting and diagnosing malnutrition conditions of colon cancer tumor patients by flora, which comprises DNA (deoxyribonucleic acid) primer sequences of strains, DNA primer sequences of the strains, DNA primer sequences of the strains, DNA primer sequences of the strains, DNA primer sequences of the strains, DNA primer sequences of the strains, DNA primer sequences of the strains, DNA primer sequences of the strains, DNA primer sequences of the strains, DNA primer sequences of the strains, DNA primer sequences of the strains, DNA primer sequences of the strains, DNA primer sequences of the strains and DNA primer sequences of the strains, the kit further comprises an excrement sample preservation reagent, a PCR reaction reagent, a PCR product purification reagent and a sequencing reaction reagent. The method comprises the following steps: carrying out DNA extraction and PCR amplification on feces of a patient suffering from the colon cancer, then carrying out sequencing by using DNA sequencing primers of Atobium.Vaginae, Selenomonas sputigena and Faecalibacterium prausnitzii to obtain DNA sequence abundance of three strains in a sample, and diagnosing the nutritional status of the patient suffering from the colon cancer according to the correlation between the abundance of the three strains and the nutritional evaluation condition. The kit diagnosis method provided by the invention can noninvasively and accurately diagnose and evaluate the nutrition status of the colon cancer.
Owner:ZHEJIANG CANCER HOSPITAL
Method and system for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease
ActiveUS10842834B2Improvements in intestinal dysbiosisReduce intestinal permeabilityOrganic active ingredientsPharmaceutical delivery mechanismPrevotellaLactobacillus reuteri
A method for reducing the likelihood of developing liver cancer in an individual diagnosed with non-alcoholic fatty liver disease involves providing in the gut of an individual a population of beneficial bacteria selected from the group consisting of Lactobacillus species, and administering fiber to the individual to maintain a therapeutically effective amount of the beneficial bacteria in the gut of the individual. In certain embodiments, monoacylglycerolacyltransferase-3 (MGAT3) synthesis is inhibited to lower triacylglycerol (TAG) production, while in others, expression of diacylglycerolacyltransferase-2 (DGAT-2) is inhibited. The beneficial bacteria are preferably modified to produce increased amounts of butyrate and may also be encapsulated in a frangible enclosure. Levels of Roseburia are preferably increased while the levels of Akkermansia spp. in the individual's gut microbiome are reduced. In other embodiments, a therapeutically effective amount of a bacterial formulation comprising Faecalibacterium prausnitzii is administered, or a composition comprising modified L. reuteri bacteria having the ability to survive conditions in the duodenum or jejunum of the individual's small intestine. Other embodiments include the administration of a bacterial formulation comprising at least one of Coprococcus, Veillonella, Roseburia, Bifidobacterium, Faecalibacterium prausnitzii and Prevotella.
Owner:SEED HEALTH INC
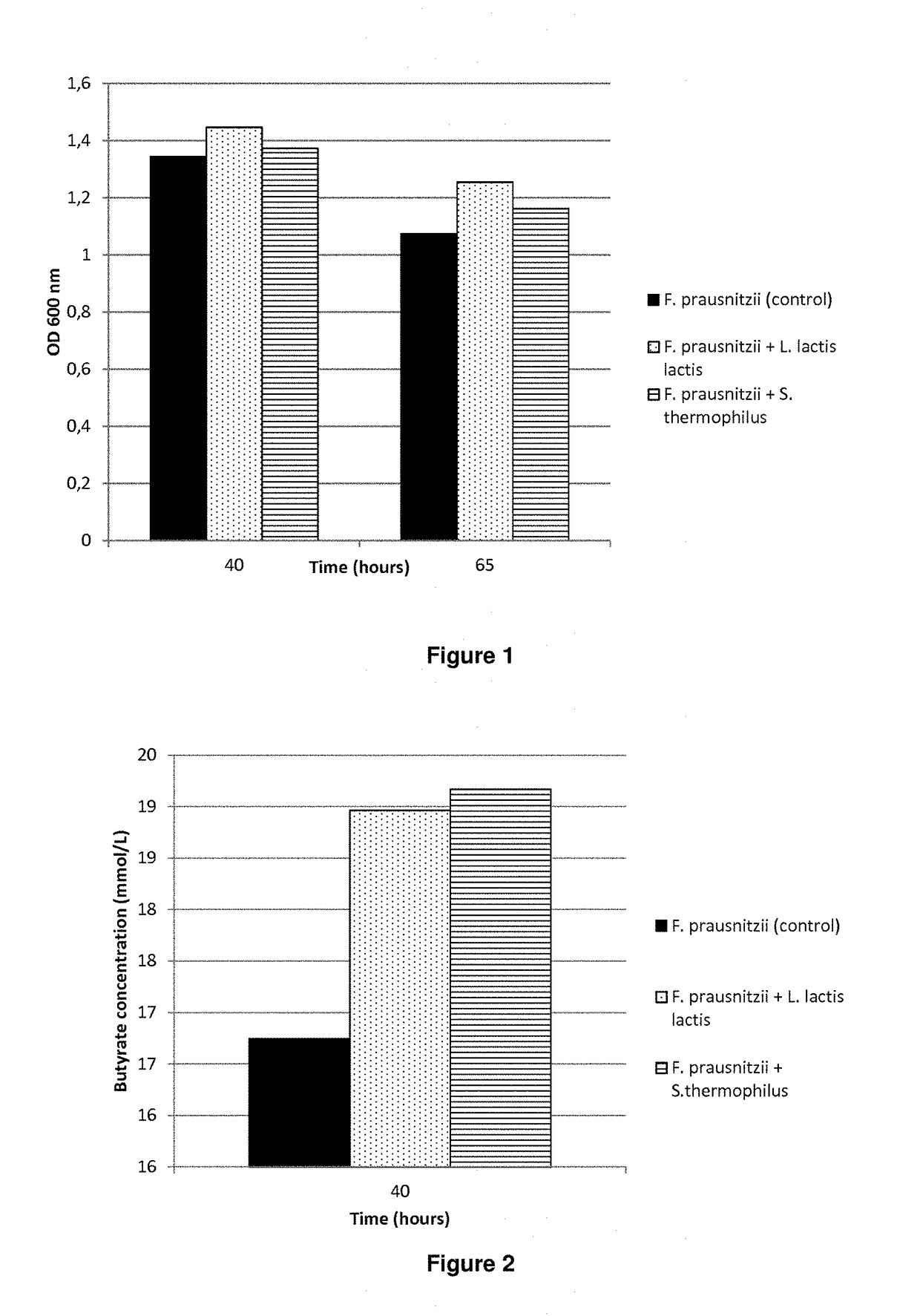
Compositions and methods for increasing or maintaining faecalibacterium prausnitzii populations
ActiveUS20190000893A1Promote growthIncrease productionMilk preparationBacteriaLactic acid bacteriumFaecalibacterium prausnitzii
The present invention relates to the use of at least one lactic acid bacterium, or a composition comprising thereof or conditioned thereby, for increasing or maintaining a Faecalibacterium prausnitzii population.
Owner:DANONE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com