Method for evaluating stable state of flora in excrement sample and application of method in colorectal cancer screening
An evaluation method, a technology of colorectal cancer, applied to the indicator bacteria combination and its calculation indicating the relationship between flora balance in individual stool samples, non-invasive diagnosis and screening of colorectal cancer, can solve the problem of out-of-control flora balance, etc. Achieve the effect of low cost, good intestinal health screening and promotion value of clinical auxiliary diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0022] Implementation Case 1: Total DNA Extraction from Stool Samples
[0023] 1. Sample collection: The stool samples collected in this study included 215 cases of CRC, 100 cases of NGC, and 156 cases of HS. 178 cases of BCD. Among the 215 CRC patients, 97 were early stage patients: 38 cases of stage I CRC and 59 cases of stage II CRC. Before collecting the specimens and background information of each patient, an informed consent was signed with the patient to obtain permission to use their information as research.
[0024] The average age of 215 CRC patients was 61.2 years old, ranging from 22 to 93 years old, including 118 males and 97 females; the average age of 178 BCD patients was 54.2 years old, ranging from 31 to 81 years old, including 86 males and 92 females ; The average age of 100 NGC patients was 58.4 years old, ranging from 34 to 73 years old, including 48 males and 52 females; the average age of 156 HS patients was 48.6 years old, ranging from 20 to 78 years o...
Embodiment example 2
[0028] Implementation Case 2: Quantitative Analysis of Microbial Indicator Bacteria
[0029] 1. Production of standard products: The bacterial DNA of Fn standard strain ATCC25586, Fp standard strain ATCC27766, and Bb standard strain BB-12 was used as standard products for conventional PCR reactions. PCR reaction system 25 μL: 10×PCR Buffer 2.5 μL, dNTP Mixture 1 μL, 5 μmol / L upstream and downstream primers 2 μL each, DNA template 2 μL, DNA Taq enzyme (5U / μL) 0.2 L, H 2 O15.3 μL. Amplification program: pre-denaturation at 95°C for 5 min, denaturation at 95°C for 1 min, annealing at 56°C for 1 min, extension at 72°C for 1 min, cycled 35 times.
[0030] The PCR amplification products of the above three kinds of bacteria were purified and recovered as the target DNA fragments respectively, and connected to the pGEM-T vector by the conventional cloning method, and the recombinant plasmid was transformed into competent bacteria DH5a, and the positive clones were obtained by blue-wh...
Embodiment example 3
[0034] Example 3: Evaluation of Fecal Microbial Markers
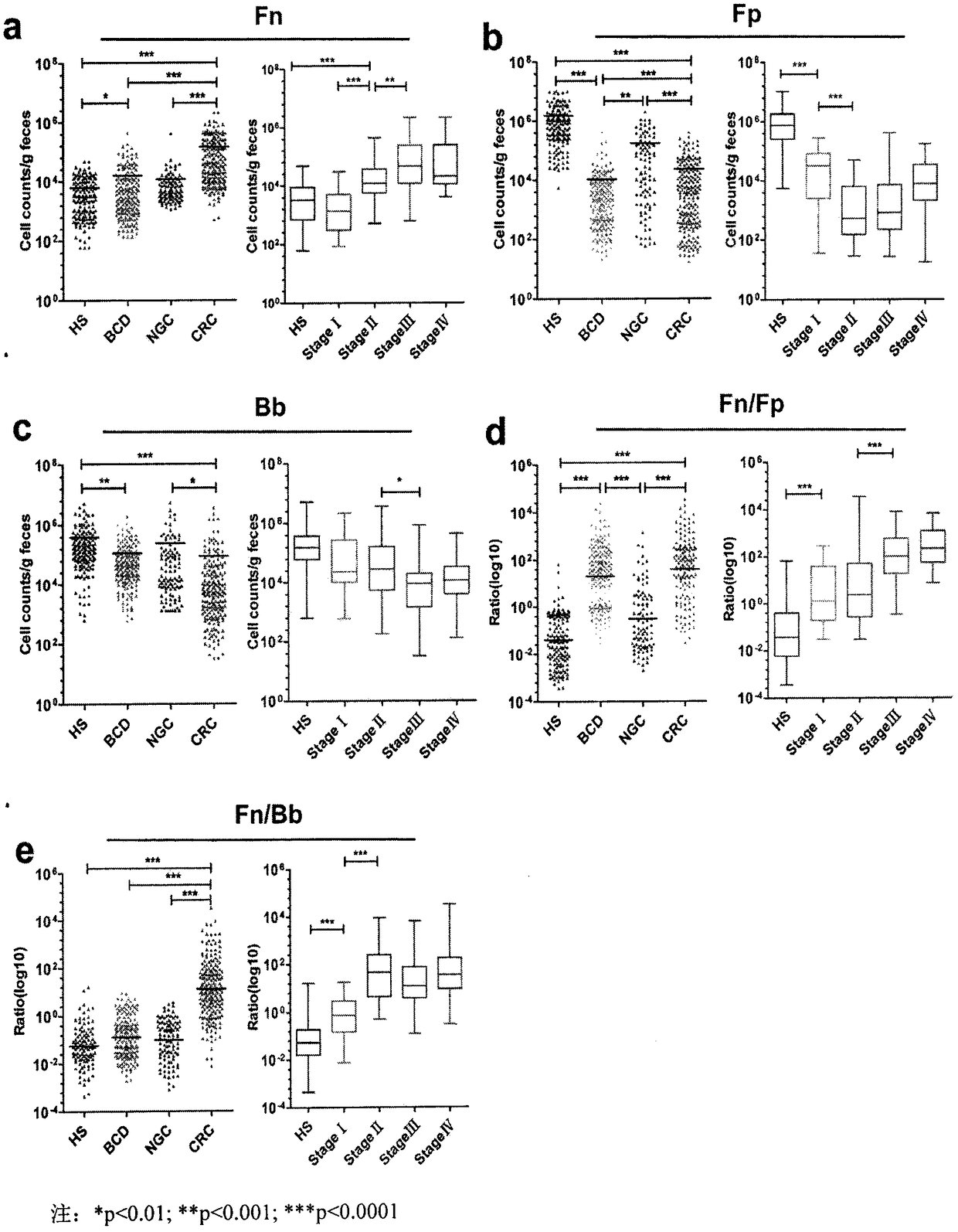
[0035] 1. The amount of Fn in the feces of the CRC group was significantly higher than that of the three control groups: HS, NGC, and BCD groups (pfigure 1 a.
[0036] 2. Compared with the HS group and NGC group, the amount of Bb and Fp in the feces of the CRC group was significantly reduced (HS group; pfigure 1 b, 1c).
[0037] 3. The ratio Fn / Fp of bacteria in feces in CRC and BCD groups was significantly higher than that in HS and NGC groups (pfigure 1 d.
[0038] 4. Compared with HS, BCD and NGC groups, the ratio Fn / Bb of the number of bacteria in feces in CRC group was significantly higher (pfigure 1 e.
[0039] 5. The Fn / Fp and Fn / Bb in feces of stage I CRC were significantly higher than those of HS group (all pfigure 1 d, 1e.
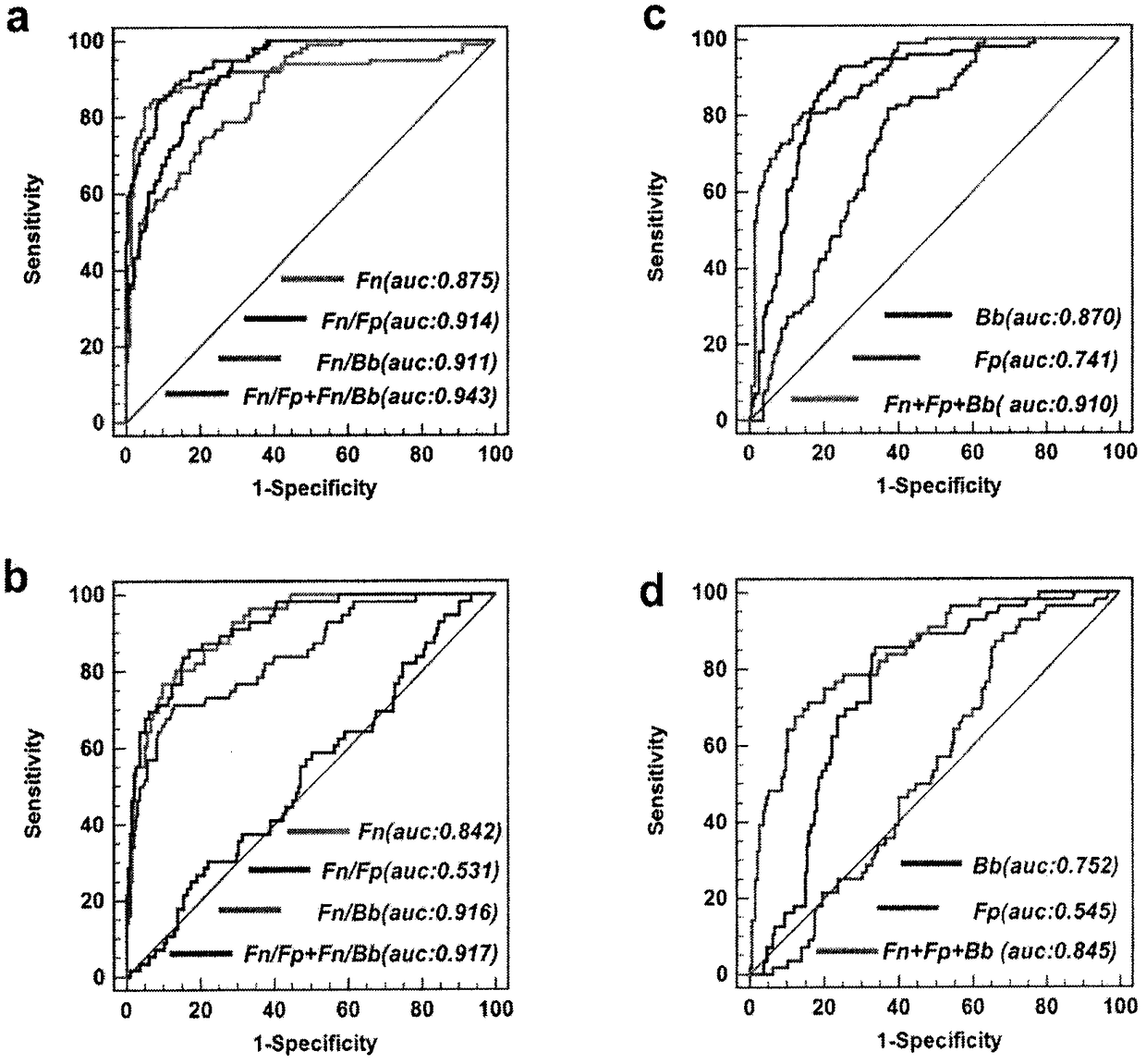
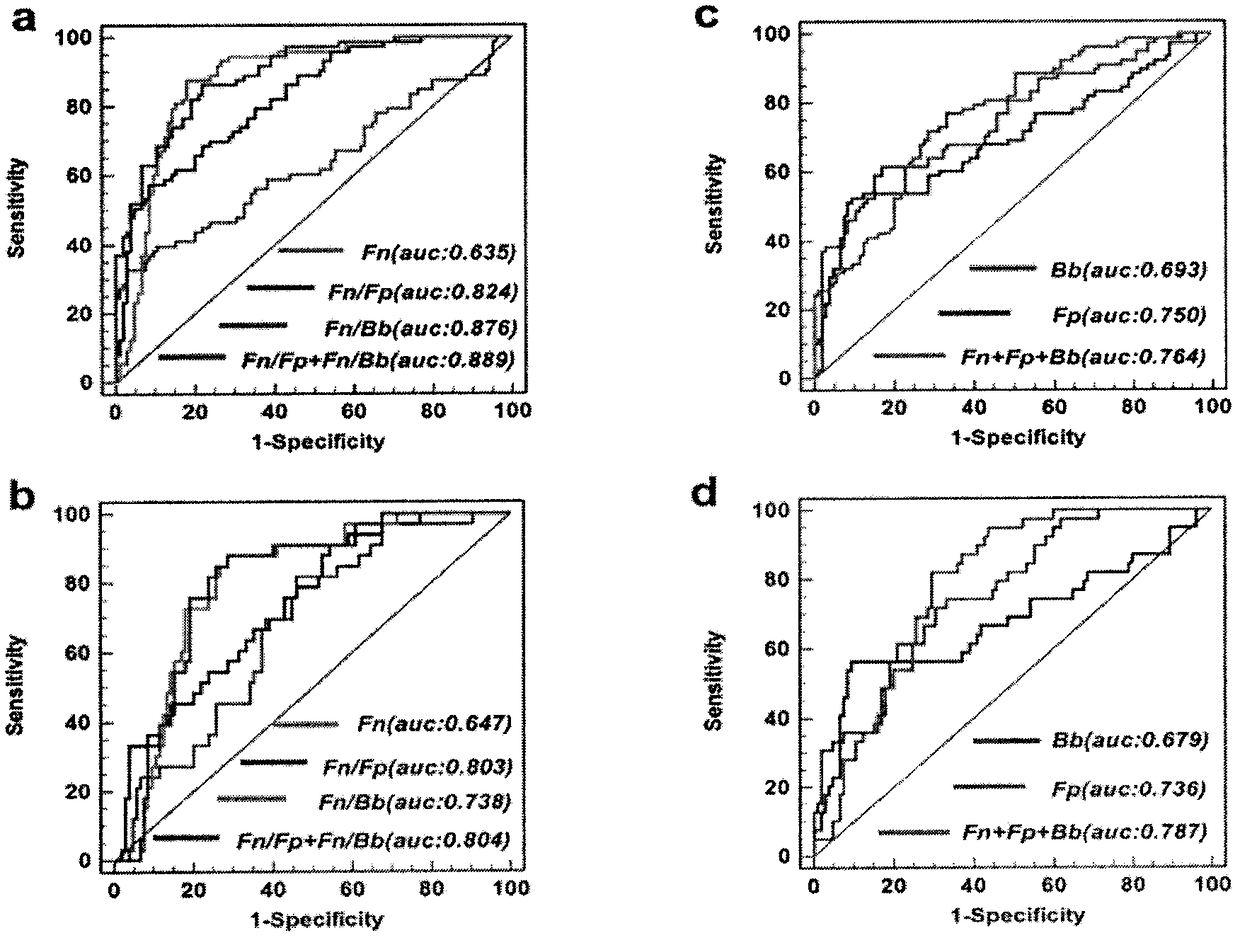
[0040] 6. These results indicate that the Fn / Bb ratio in feces has the ability to evaluate CRC; the Fn / Fp ratio has the ability to evaluate CRC and BCD
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com