Patents
Literature
34 results about "Area under curve" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phishing webpage detection method based on Hungary matching algorithm
InactiveCN101826105AImprove scalabilityThe detection process is fastSpecial data processing applicationsPattern recognitionRegression analysis
A phishing webpage detection method based on Hungary matching algorithm is characterized by firstly extracting the text feature signatures, image feature signatures and general webpage feature signatures of the rendered webpages and more comprehensively depicting the features after access to webpages; and then computing the optimal matching of bipartite graphs by Hungary algorithm to search for the matched feature pairs among different webpage signatures and more objectively measuring the similarity among the webpages on the basis, thereby improving the phishing webpage detection efficiency. The method is also characterized by determining the inside weights of the text features, image features and global image features by utilizing the area under curve and determining the relative weightsamong the text similarity, image similarity and global image similarity during webpage similarity computation by utilizing logarithmic regression analysis. The precision and the recall rate are greatly improved in the method provided by the invention.
Owner:NANJING UNIV OF POSTS & TELECOMM
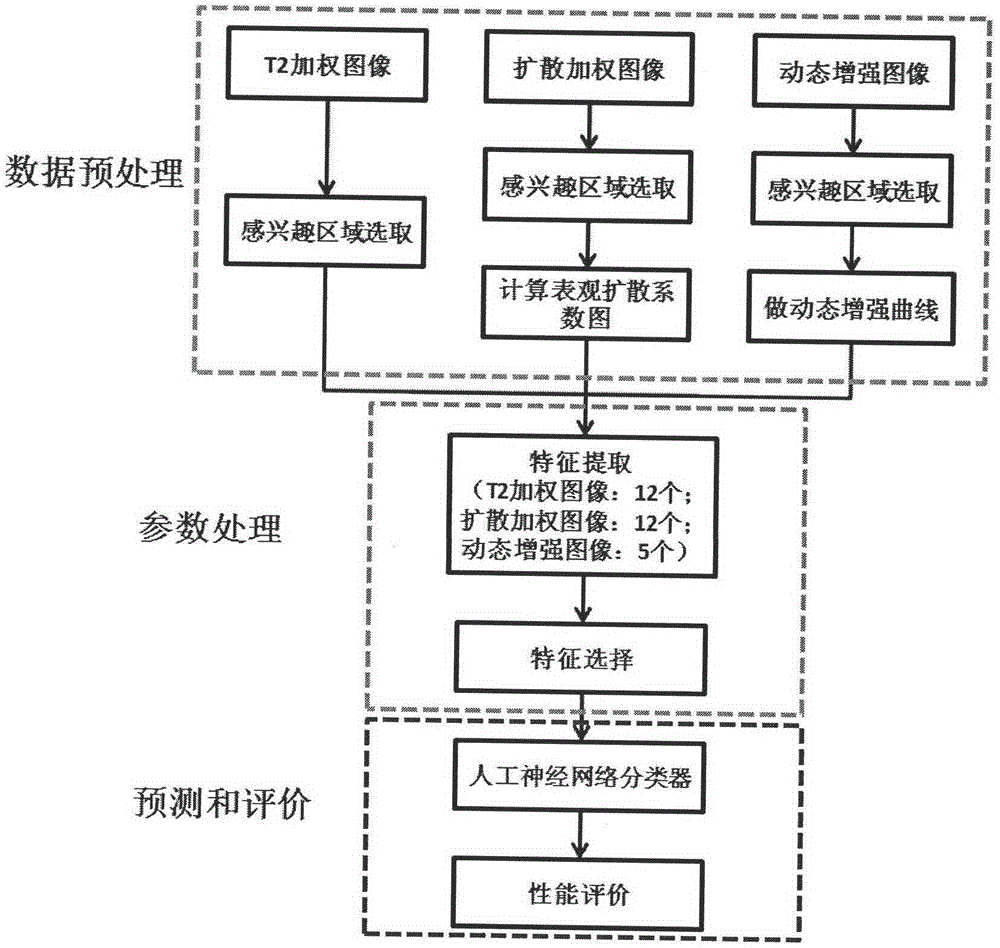
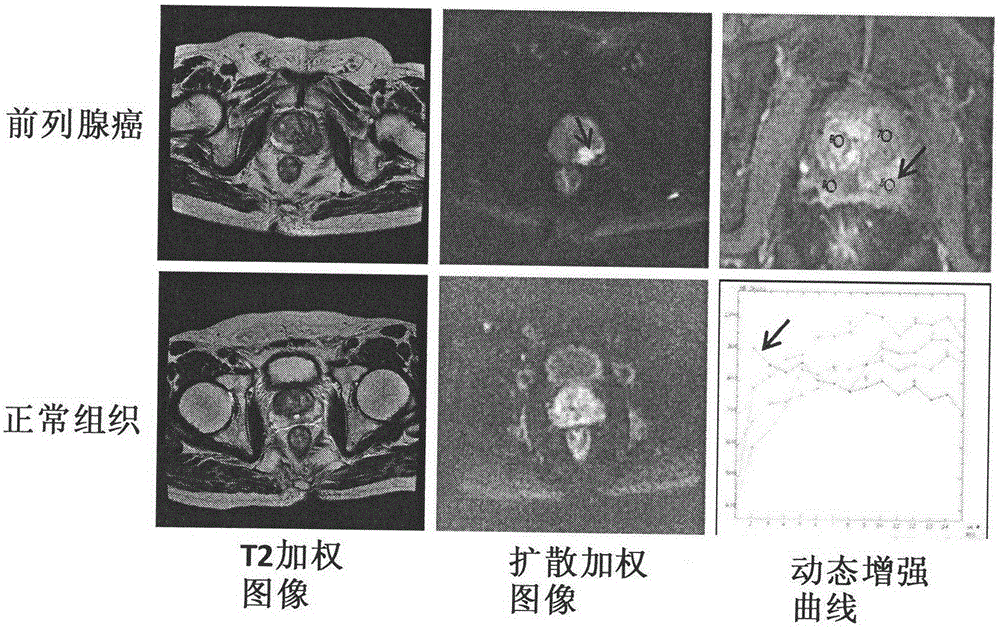
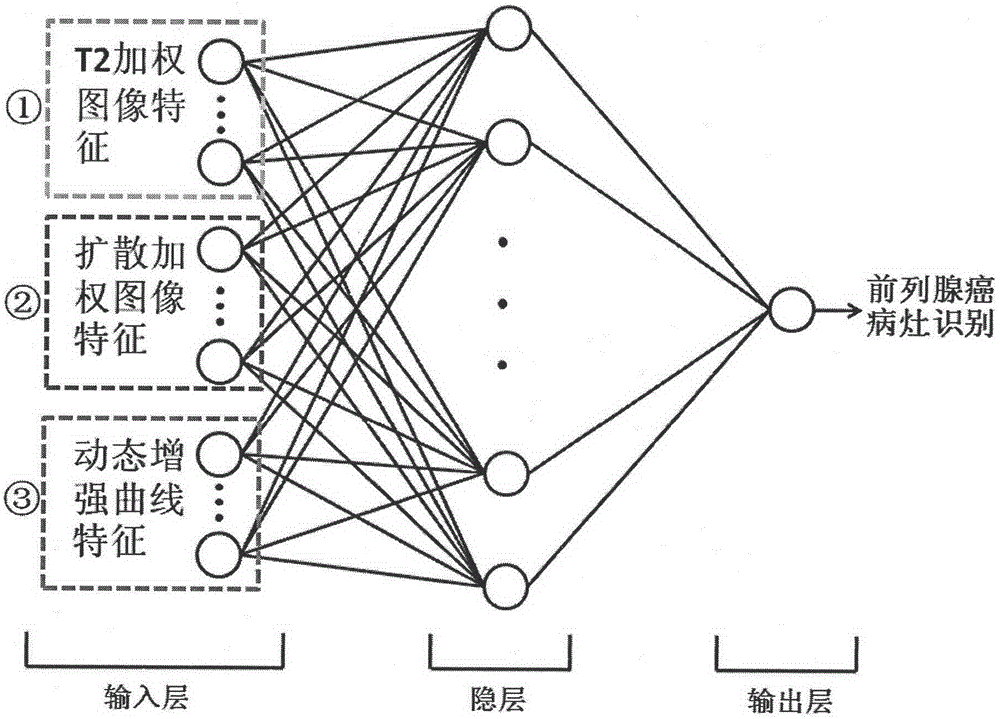
Multi-parameter magnetic resonance image based prostate cancer computer auxiliary identification system
InactiveCN104424386AImprove accuracyIncreased sensitivityDiagnostic recording/measuringSensorsReceiver operating characteristicImage pre processing
The invention discloses a multi-parameter magnetic resonance image based prostate cancer computer auxiliary identification system and method. The multi-parameter magnetic resonance image based prostate cancer computer auxiliary identification system comprises three portions of an image preprocessing module, a parameter processing module and a prediction and evaluation module. According to the multi-parameter magnetic resonance image based prostate cancer computer auxiliary identification system, the characteristics of a T2 weighted image, a diffusion weighted image and a dynamic enhanced image are comprehensively utilized and the purpose of the identification of the prostate cancer focus is achieved through an artificial neural network structure; the ROC (Receiver Operating Characteristic) area under curve of the peripheral zone of prostate of the system is 0.931 and the identification accuracy is 0.887 and the ROC area under curve in the central gland is 0.909 and the identification accuracy is 0.915 through a test; the image information obtained through the conventional magnetic resonance imaging scanning sequence scanning sequence can be well integrated, quantitative parameters in the images are utilized in a maximum mode, and the identification result of the prostate cancer is objectively provided; the operation is simple and convenient, the reference can be intuitively provided for doctors, and the important basis is provided for the subsequent diagnosis scheme.
Owner:PEKING UNIV
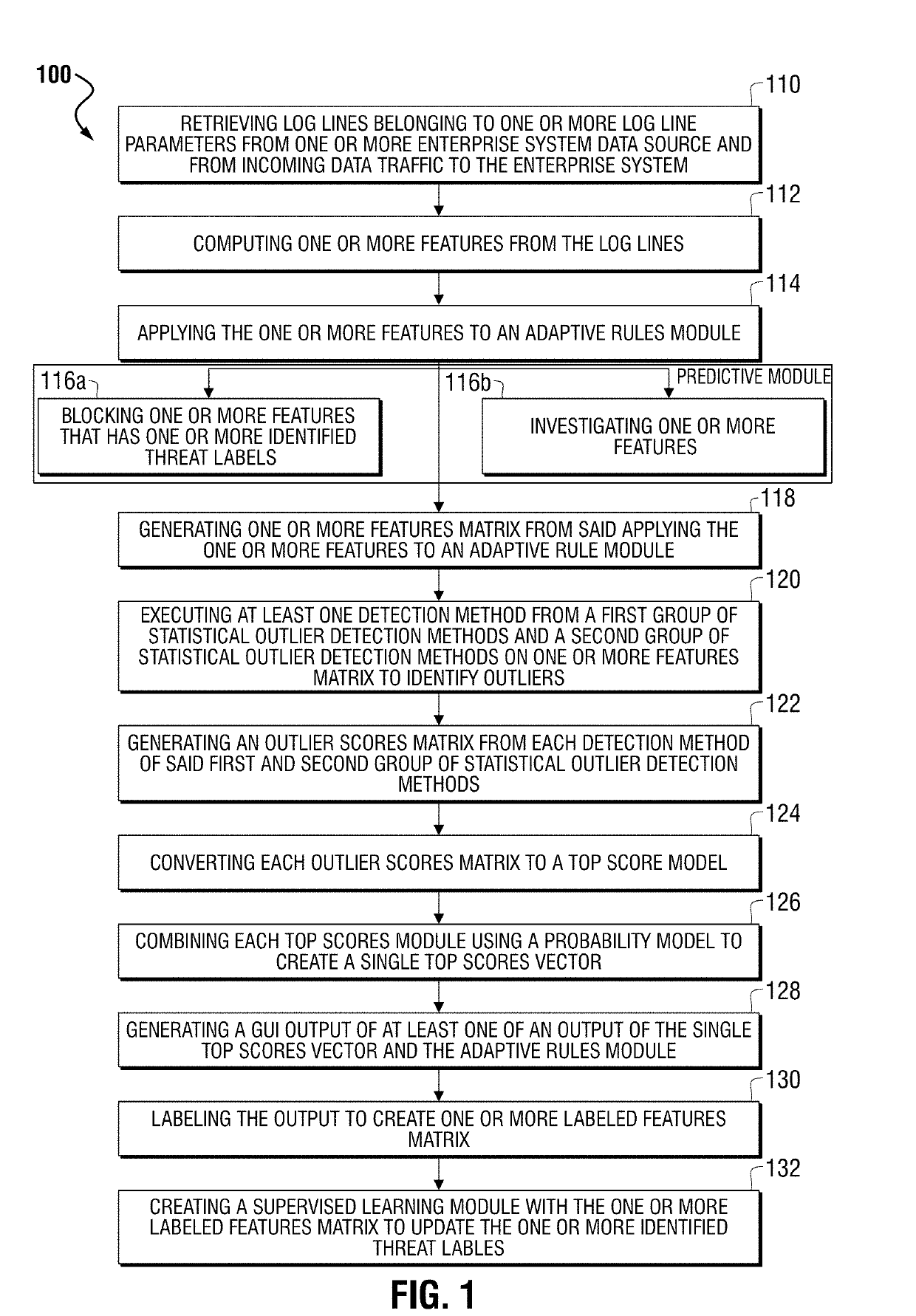
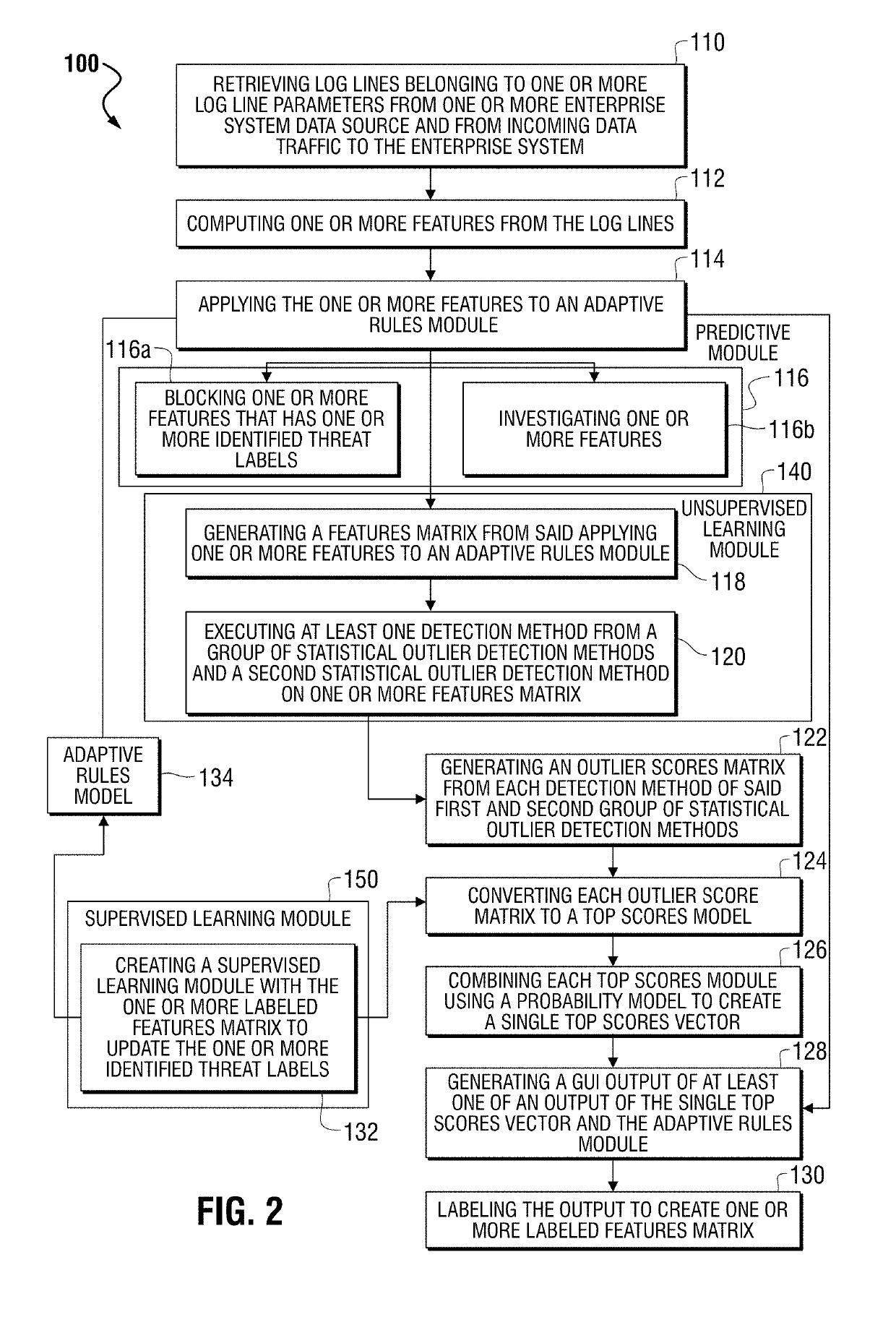
Method and system for generating synthetic feature vectors from real, labelled feature vectors in artificial intelligence training of a big data machine to defend
ActiveUS20190132343A1Lower the volumeProblem can be addressedMathematical modelsCharacter and pattern recognitionFeature vectorSynthetic data
Identifying and detecting threats to an enterprise system groups log lines from enterprise data sources and / or from incoming data traffic. The process applies artificial intelligence processing to the statistical outlier in the event of the statistical outliers comprises a sparsely labelled real data set, by receiving the sparsely labelled real data set for identifying malicious data and comprising real labelled feature vectors and generating a synthetic data set comprising a plurality of synthetic feature vectors derived from the real, labelled feature vectors. The process further identifies the sparsely labelled real data set as a local data set and the synthetic data set as a global set. The process further applies a transfer learning framework for mixing the global data set with the local data set for increasing the precision recall area under curve (PR AUC) for reducing false positive indications occurring in analysis of the threats to the enterprise.
Owner:CORELIGHT INC
Link prediction method in large-scale microblog heterogeneous information network
InactiveCN105893637AThe maximum K accuracy rate is stableSpecial data processing applicationsWeb data retrieval using information identifiersMicrobloggingInformation networks
The invention relates to the field of Internet technology, and provides a link prediction method in a large-scale microblog heterogeneous information network. The link prediction method comprises the following steps: filtering users according to preset strategy; extracting a plurality of links from the network, wherein a positive example set is ET, and a negative example set is EF; calculating the characteristics of all nodes in ETUEF and the characteristics of the links in an E-ET-EF network, and converting the characteristics of the nodes into the characteristics of link relations; dividing the EFUET into a training set, a verification set and a test set, training models on the training set, selecting model hyper-parameters with the optimal prediction result on the verification set to obtain a final model h theta (x) and a threshold value theta; putting any link relation in the test set into the model, so that the probability P generated by the link relation can be obtained. Experiments show that the area under curve and F value of the method provided by the invention are obviously improved compared with a method based on local information similarity and path similarity, and the method has higher maximum K accuracy rate stability.
Owner:SICHUAN UNIV
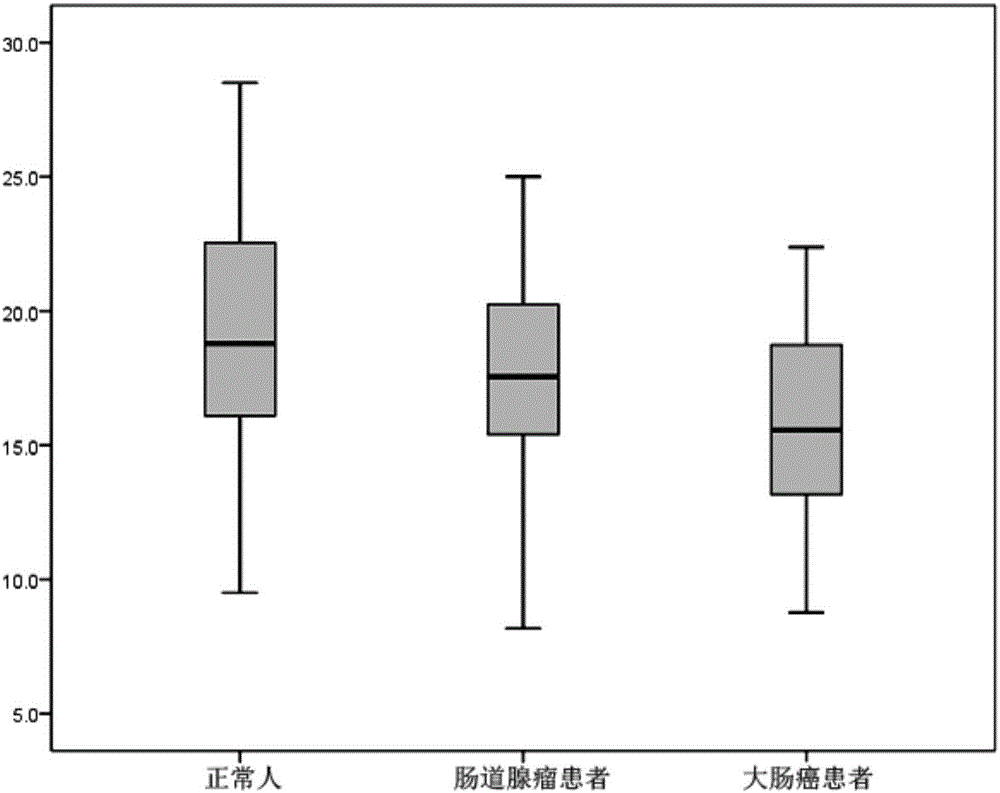
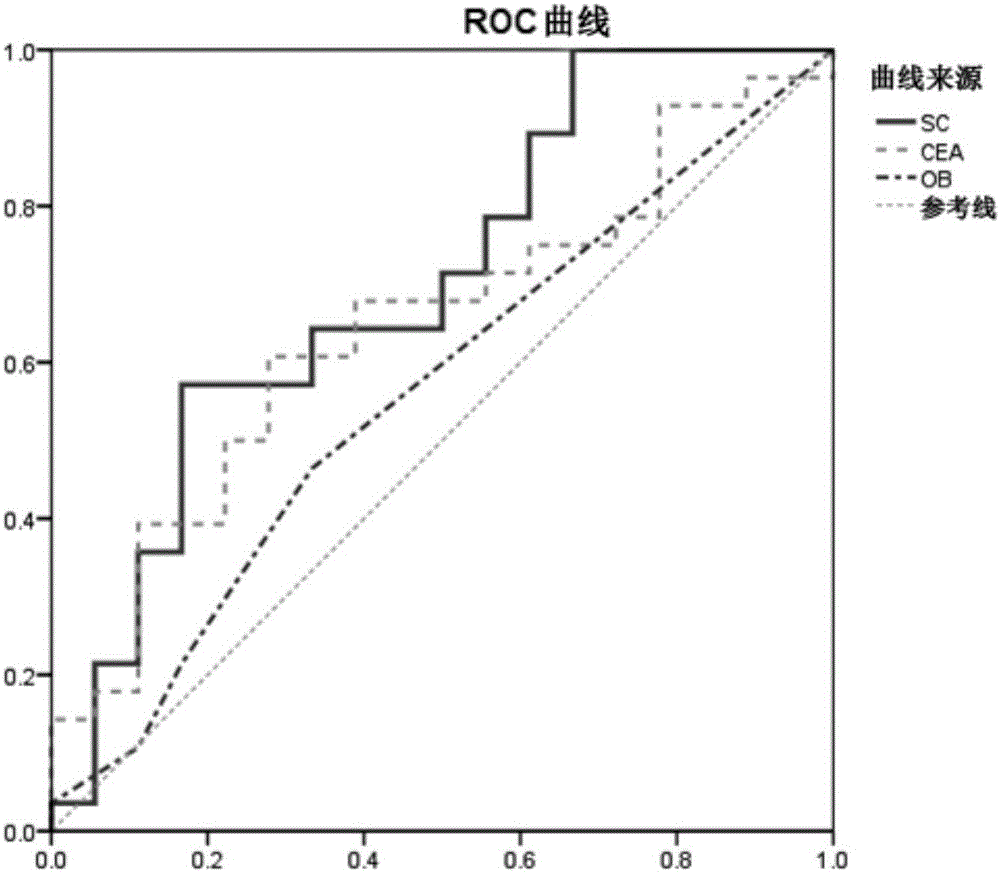
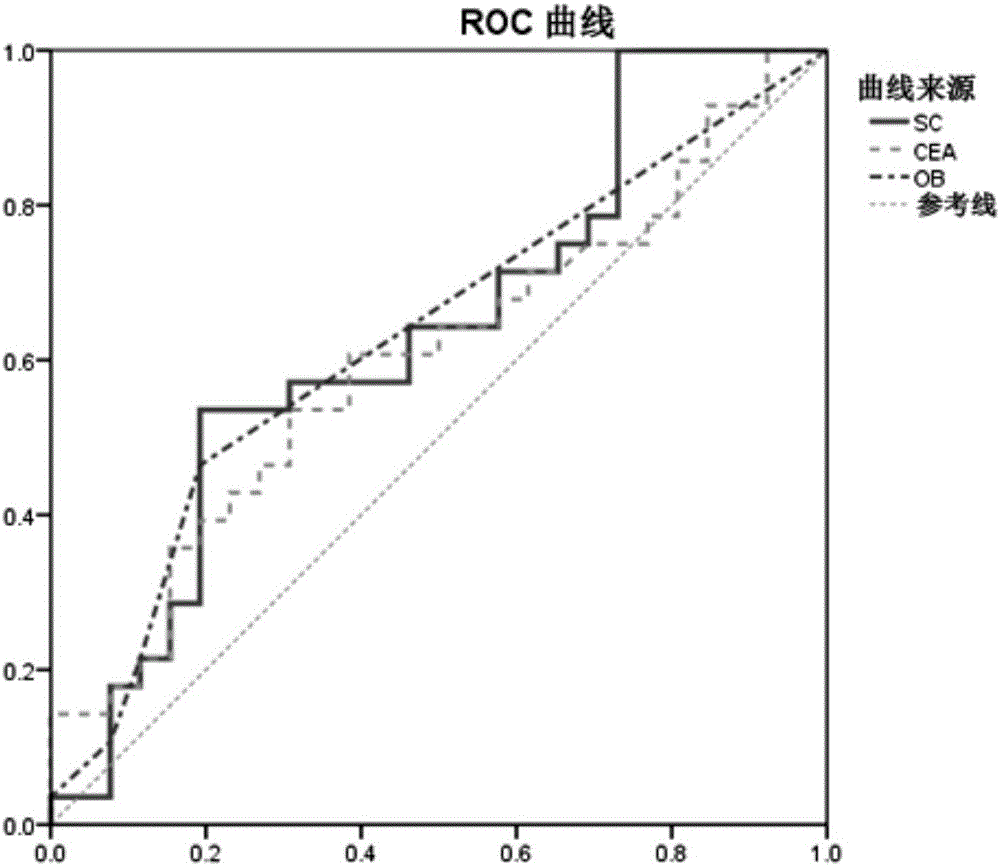
Method for evaluating stable state of flora in excrement sample and application of method in colorectal cancer screening
PendingCN108690864ALow costGood Gut Health ScreeningMicrobiological testing/measurementMicroorganism based processesClostridium leptumFeces
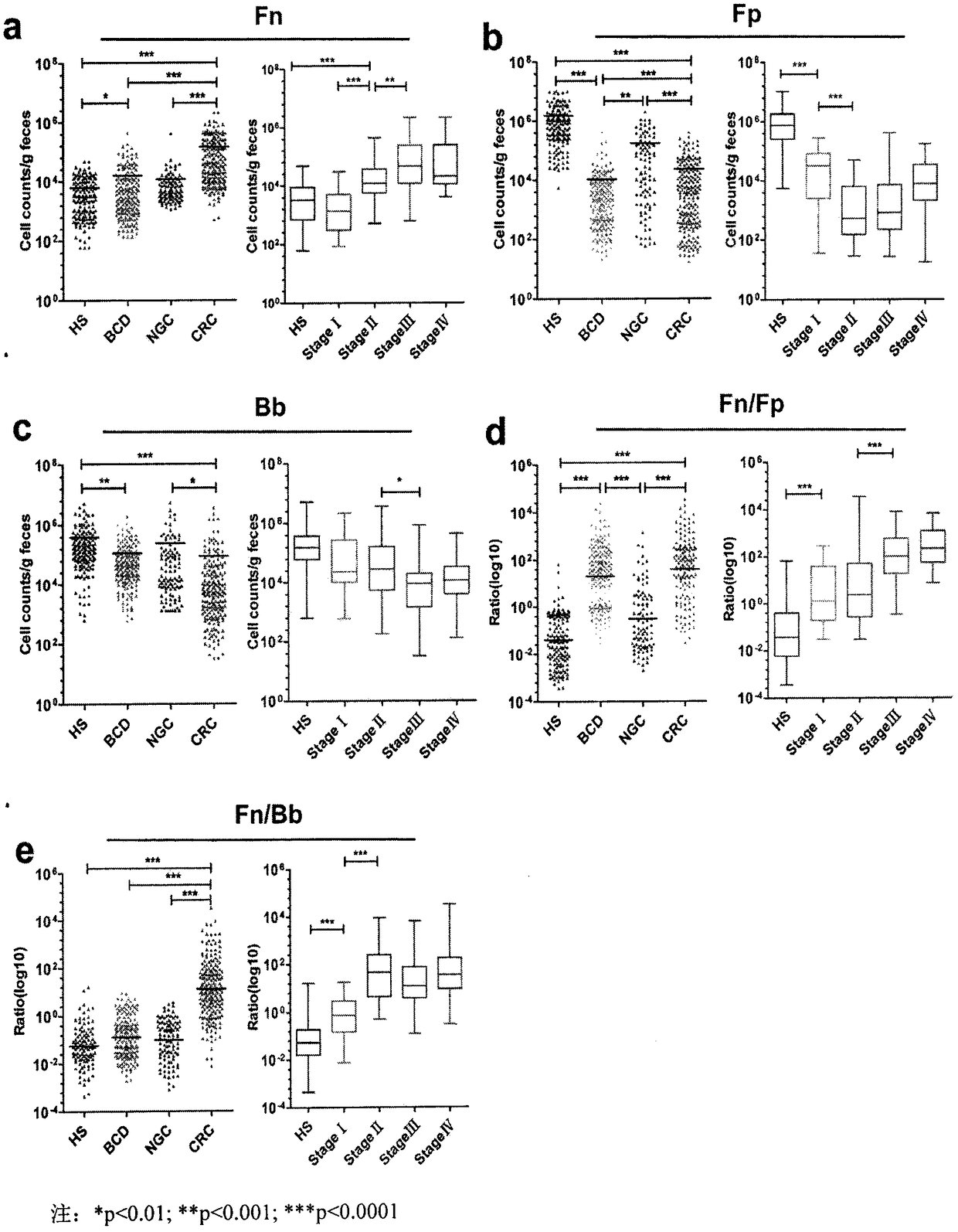
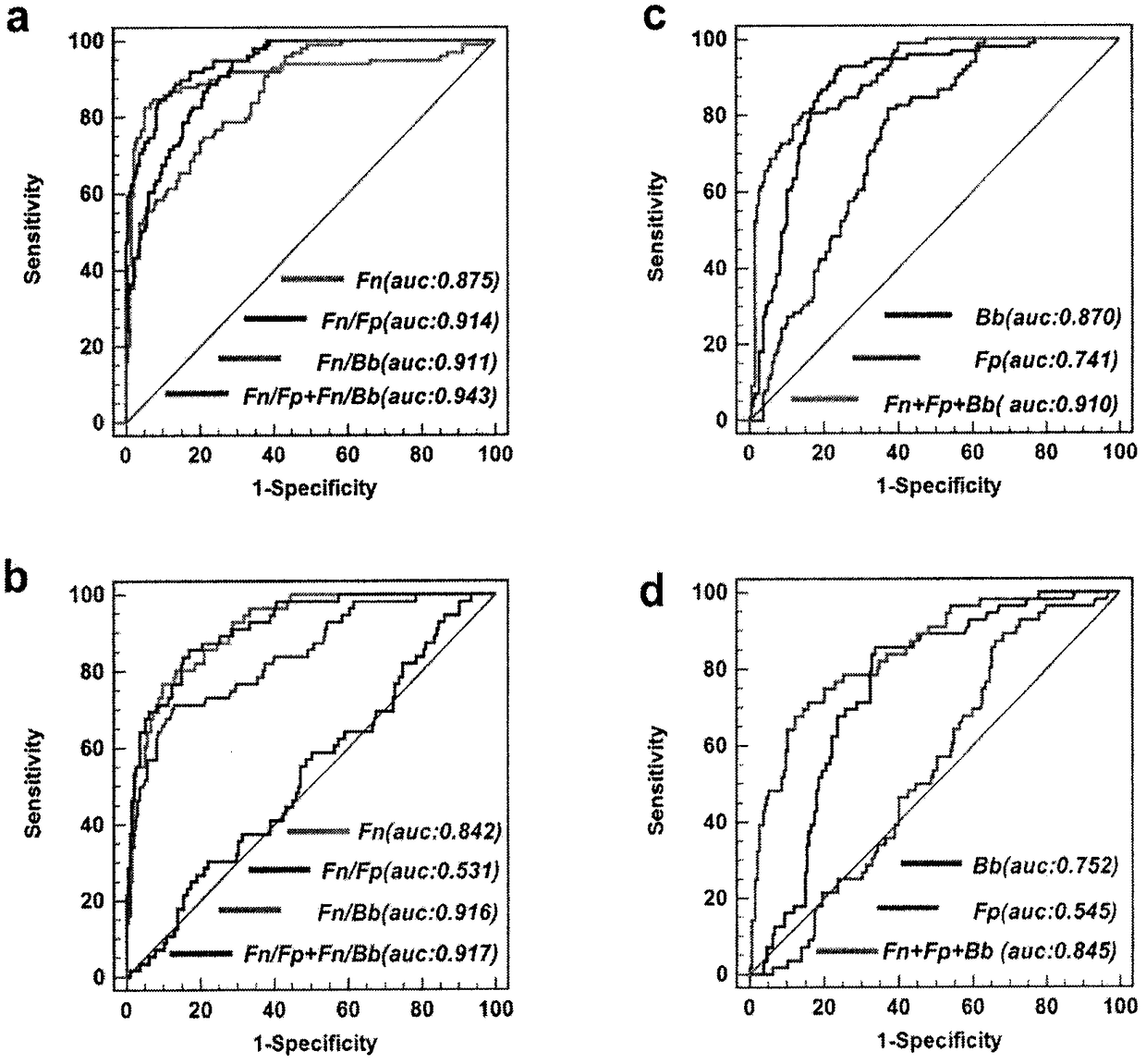
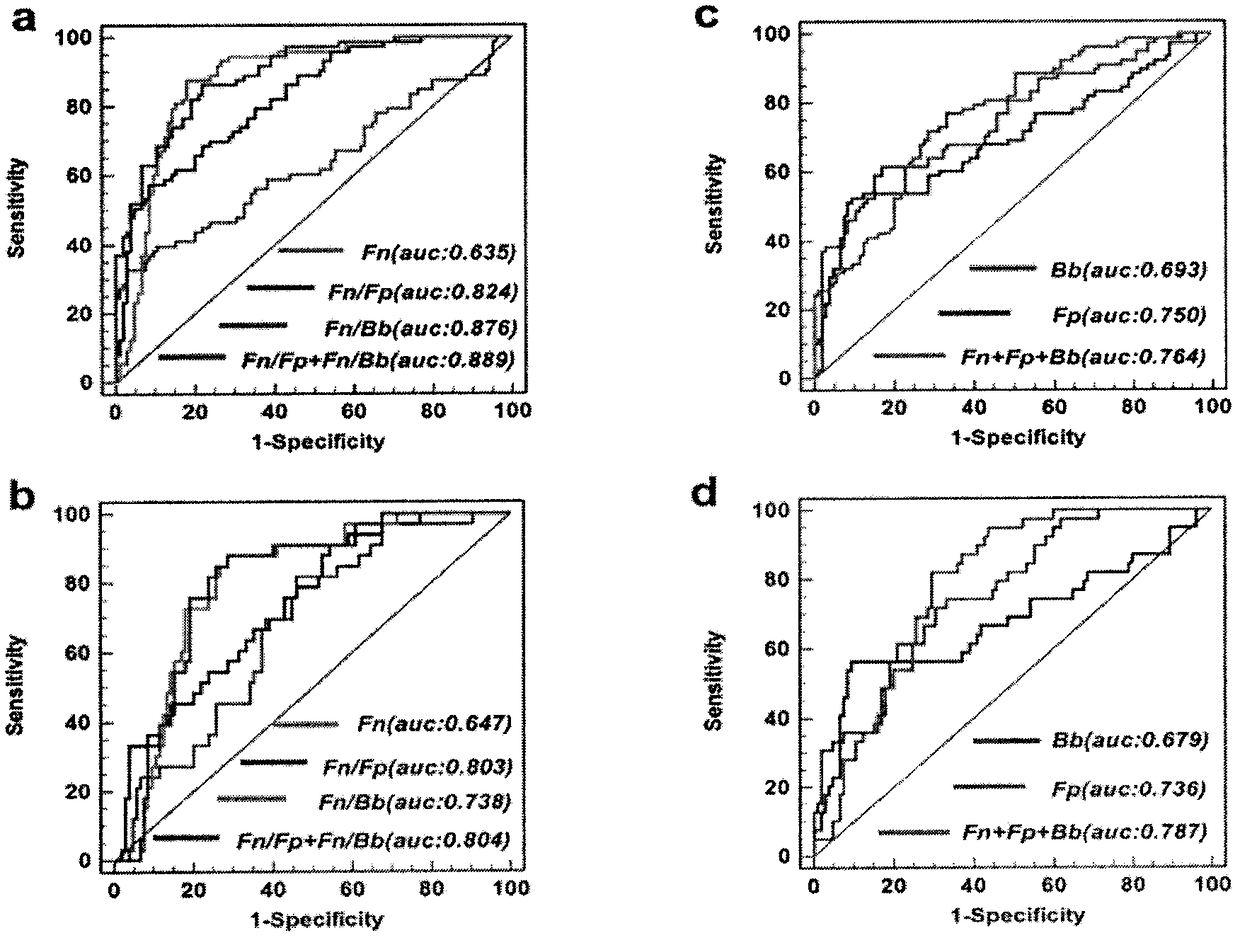
The invention relates to a method for calculating a flora balanced relation index in an individual excrement sample, and an application of the method in screening, diagnosis or auxiliary diagnosis incolorectal cancer (CRC). By extracting bacteria in excrement during DNA sequencing, the types and quantity characteristics of the bacteria can be obtained, and a CRC diagnosis by taking quantity ratiocharacteristic of a plurality of bacteria as a base is carried out. Compared with the methods used in clinical diagnosis or noninvasive screening of CRC with an applied patent, the method is completely noninvasive, and can realize accurate diagnosis of CRC. The analysis result displays that a ratio of fusobacterium nucleatum Fn to bifidobacteria Bb quantity (Fn / Bb) has high susceptibility and specificity on CRC screening, which can respectively reach 84.6% and 92.3% (AUC=0.911). the ratio of fusobacterium nucleatum Fn to clostridium leptum Fp (Fn / Fp) quantity is combined to increase the diagnosis value on CRC, and the Area Under Curve (AUC) of a subject work characteristic curve can reach 0.943. In addition, combination of Fn / Bb and Fn / Fp quantity ratio for screening I-stage CRC has 60% of specificity and 90% of sensitivity.
Owner:SUN YAT SEN UNIV
Systems and methods for detection and localization of image and document forgery
ActiveUS20180101751A1Data processing applicationsDigital data information retrievalPattern recognitionData set
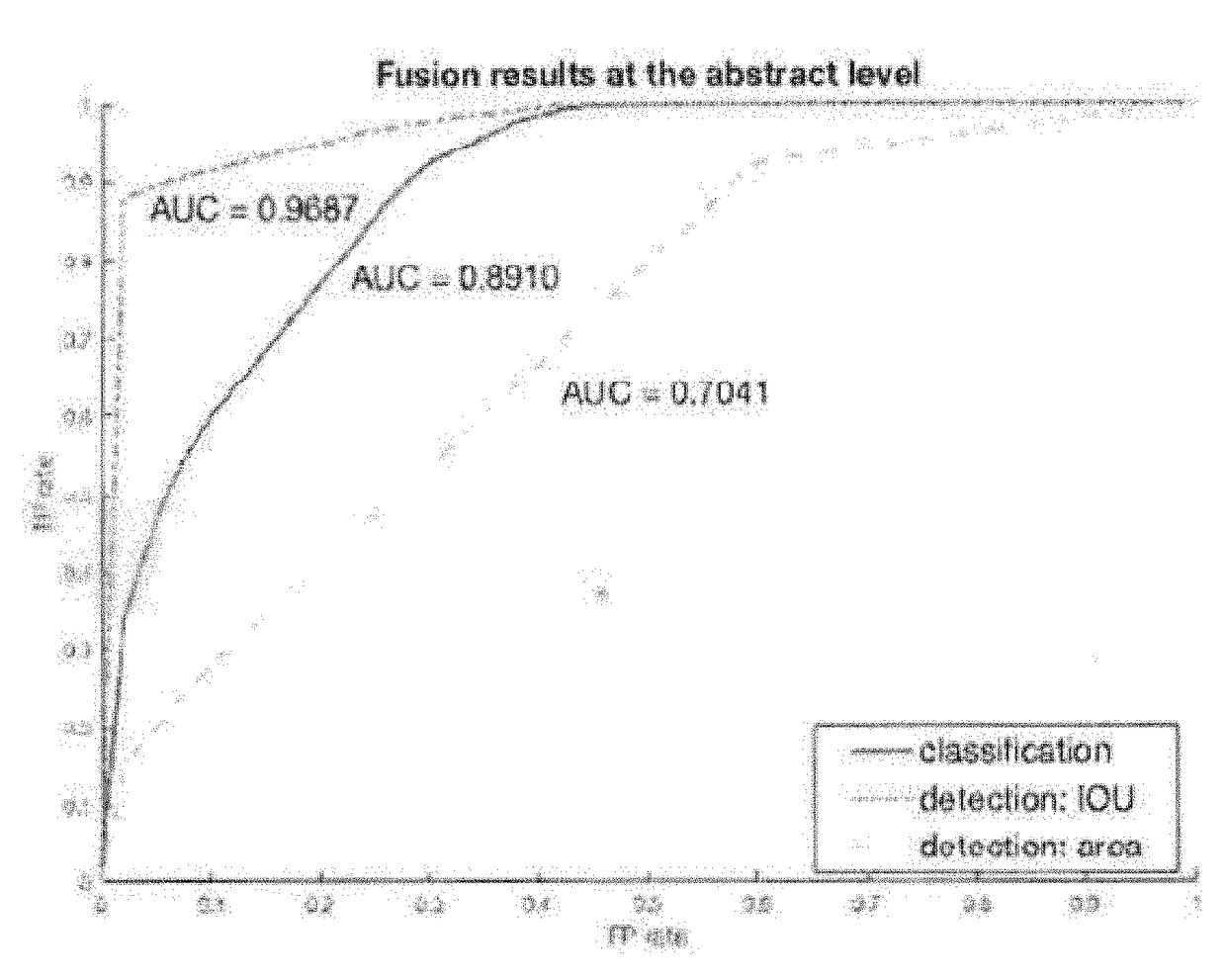
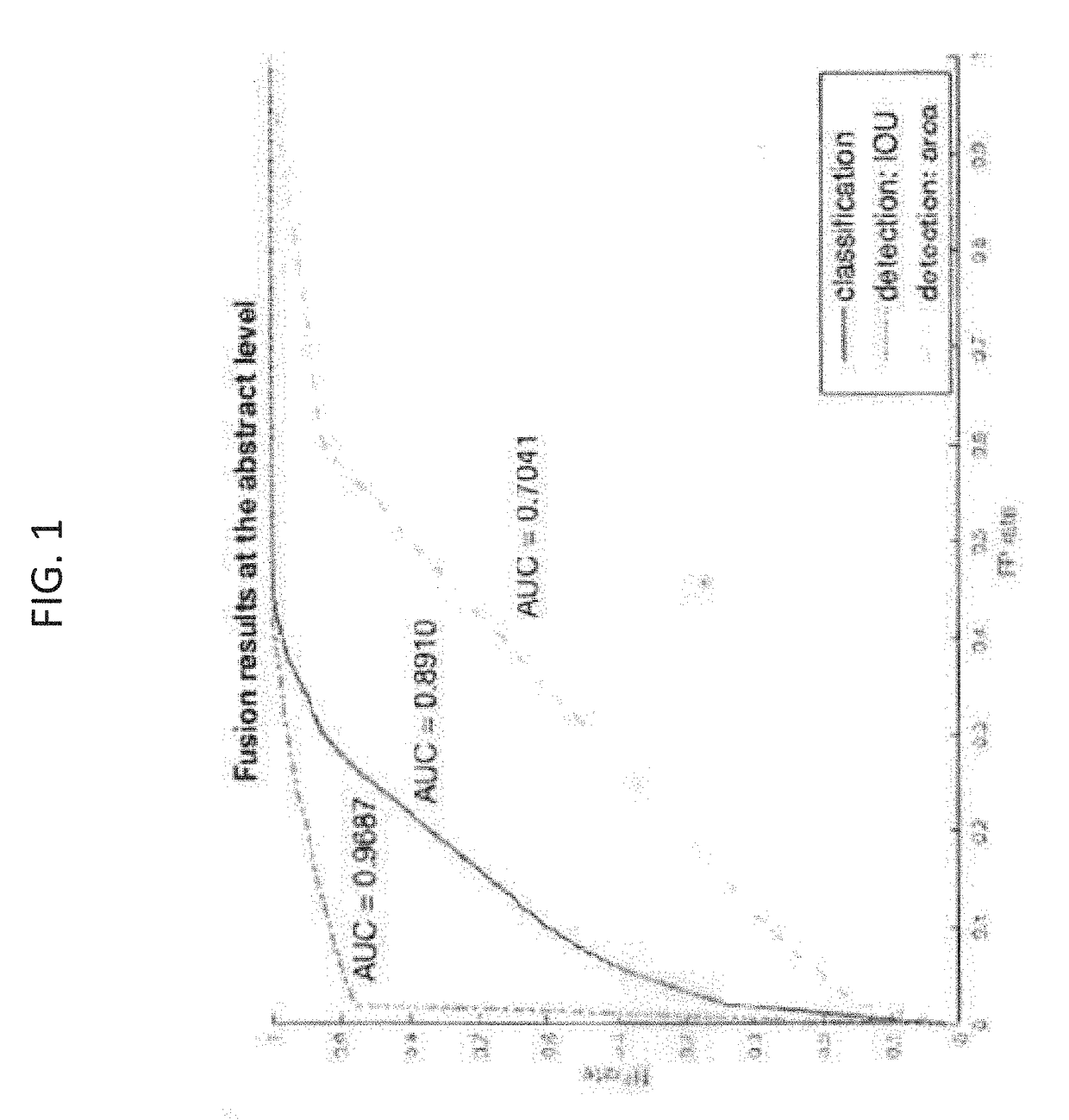
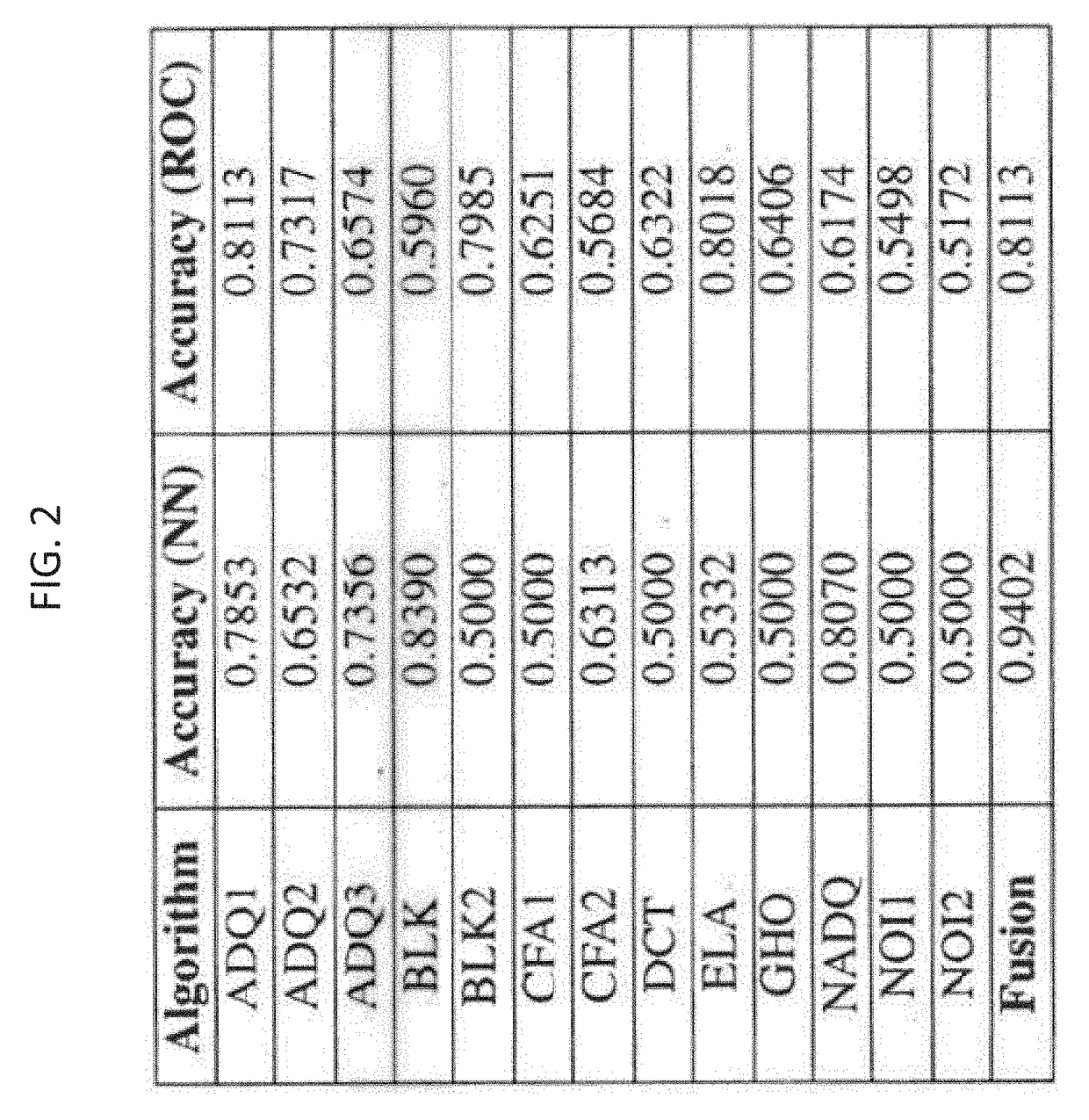
Systems and methods for detection and localization of image and document forgery. The method can include the step of receiving a dataset having a plurality of authentic images and a plurality of manipulated images. The method can also include the step of benchmarking a plurality of image forgery algorithms using the dataset. The method can further include the step of generating a plurality of receiver operating characteristic (ROC) curves for each of the plurality of image forgery algorithms. The method also includes the step of calculating a plurality of area under curve metrics for each of the plurality of ROC curves. The method further includes the step of training a neural network for image forgery based on the plurality of area under curve metrics.
Owner:INSURANCE SERVICES OFFICE INC
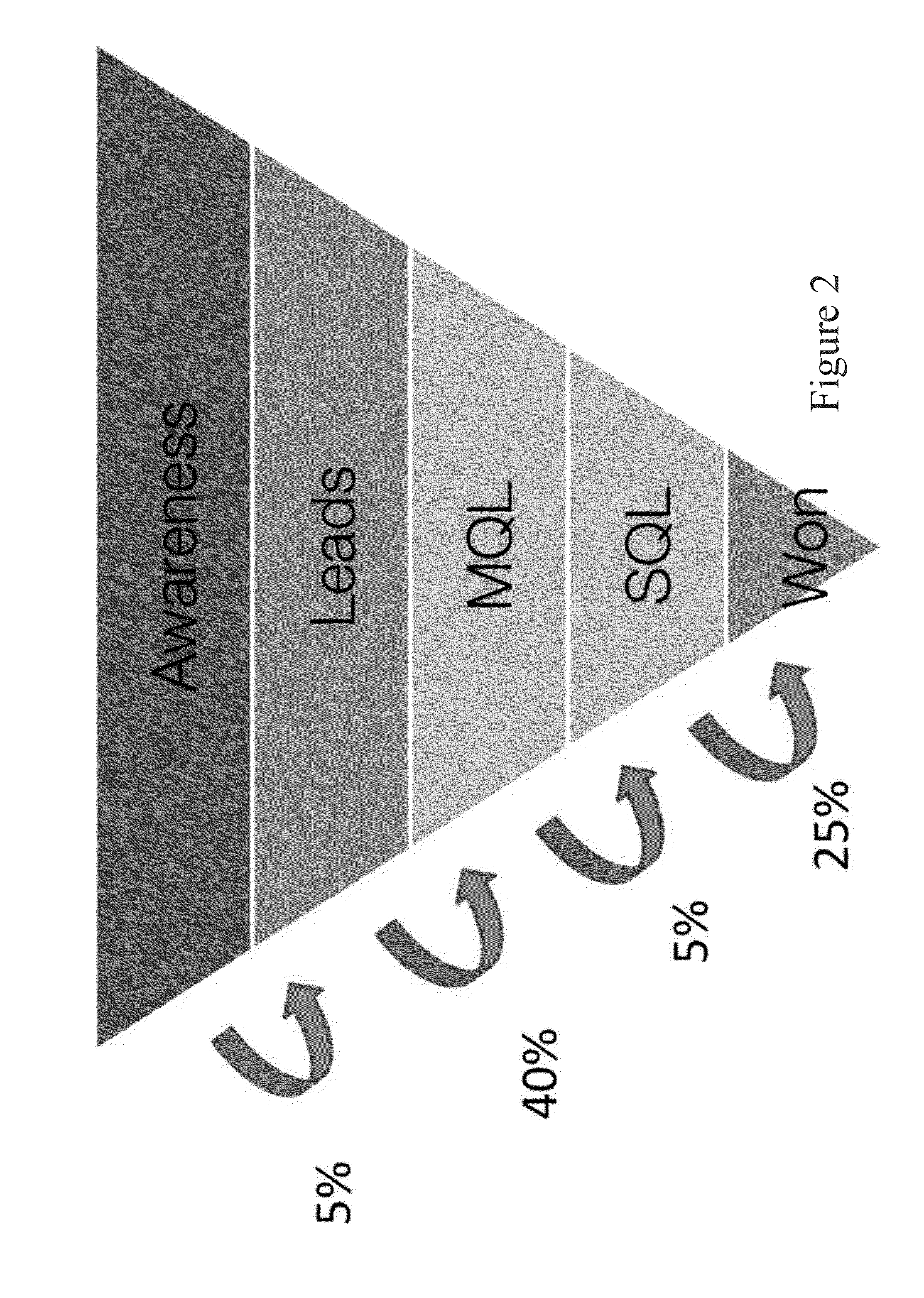
System and method for full funnel modeling for sales lead prioritization
A system and method for full funnel modeling for sales lead prioritization are disclosed. A particular embodiment includes two models, DQM (direct qualification model) and FFM (full funnel model), which can be used to rank sales leads based on probability of conversion to a sales opportunity, probability of successful sale, or expected revenue. These models can replace traditional, manually created lead scoring systems, which use hand-tuned scores and are therefore error-prone and non-probabilistic. The disclosed methods achieve high AUC (Area Under Curve) scores in our experiments, and we show that they can result in a substantial increase in conversion rate, a substantial increase in successful sale rate, as well as dramatic increases in total revenue. Unlike traditional lead-scoring, our methods provide an intuitive probabilistic score, and focus more on features that measure customer fit than customer behavior, meaning quality leads can be found earlier on in the sales process.
Owner:MICROSOFT TECH LICENSING LLC
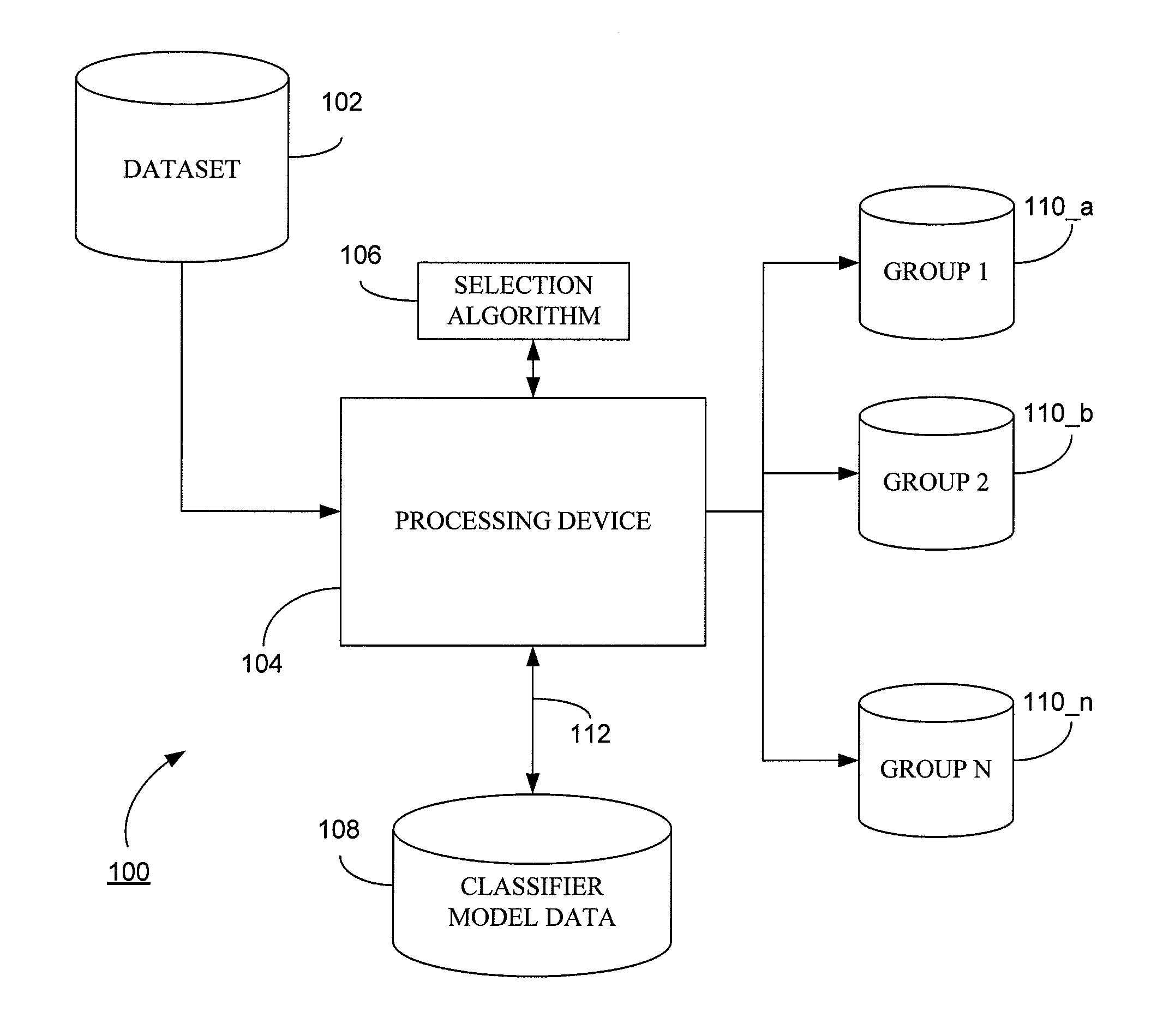
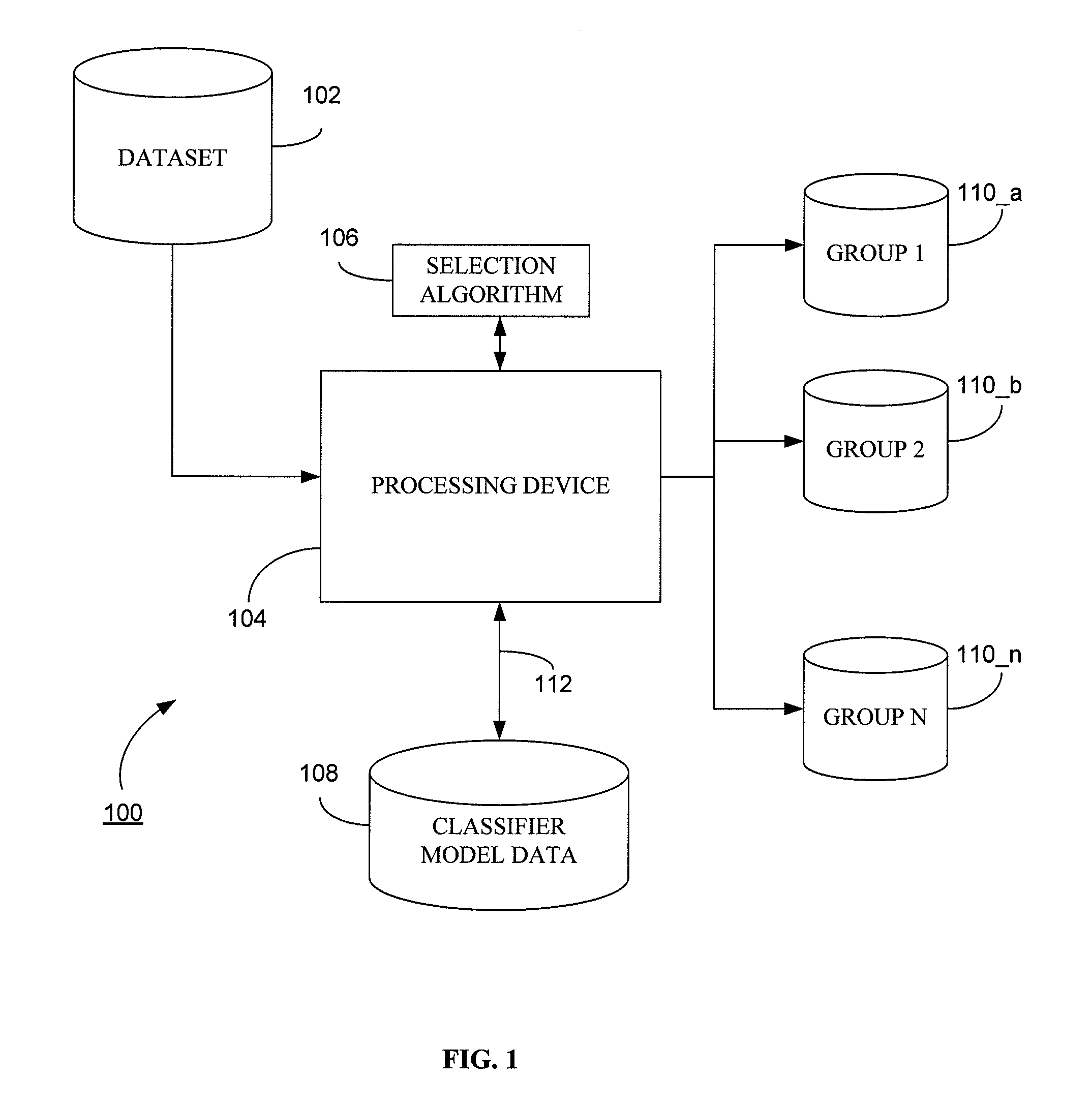
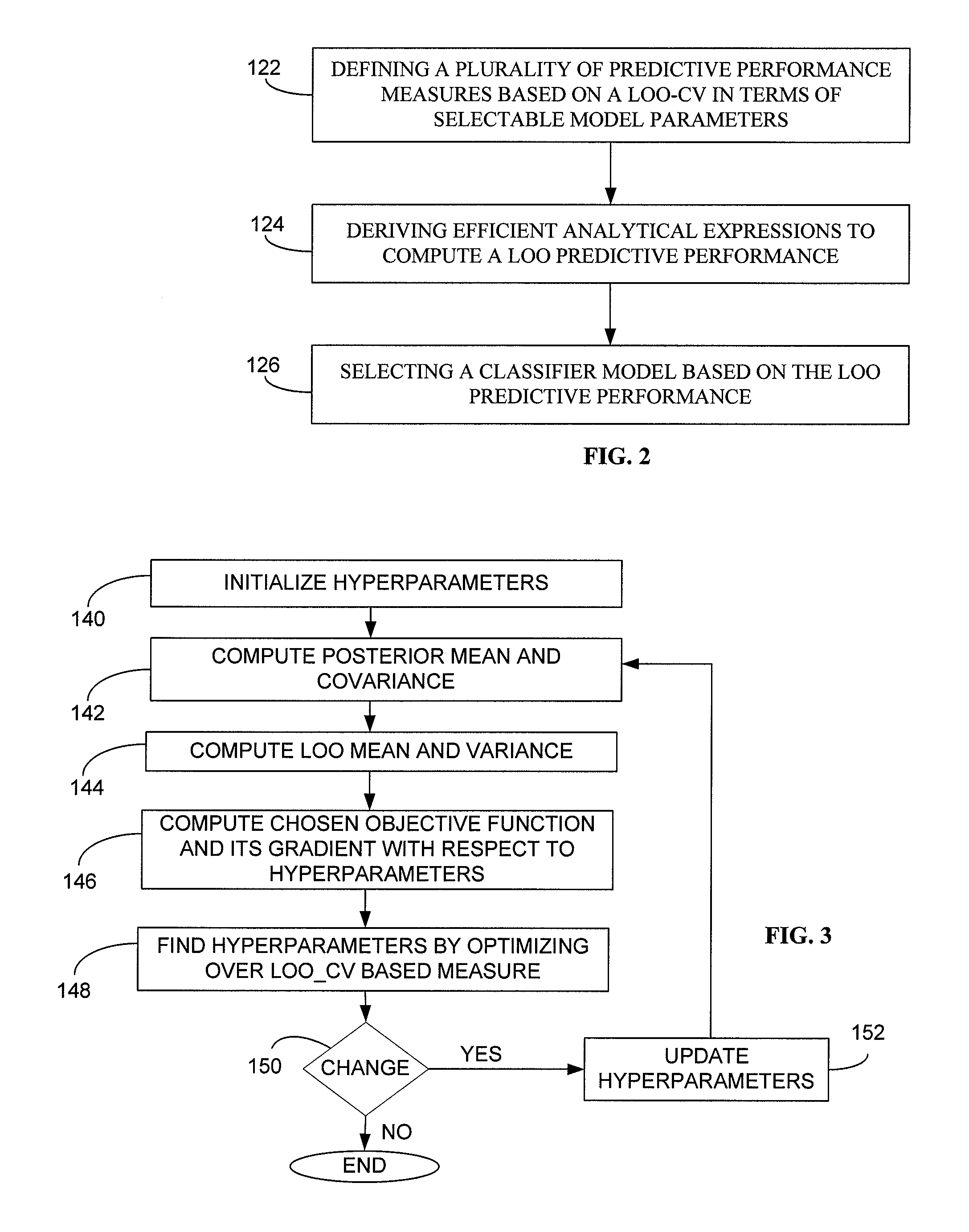
System and method for generating a classifier model for classifying web content
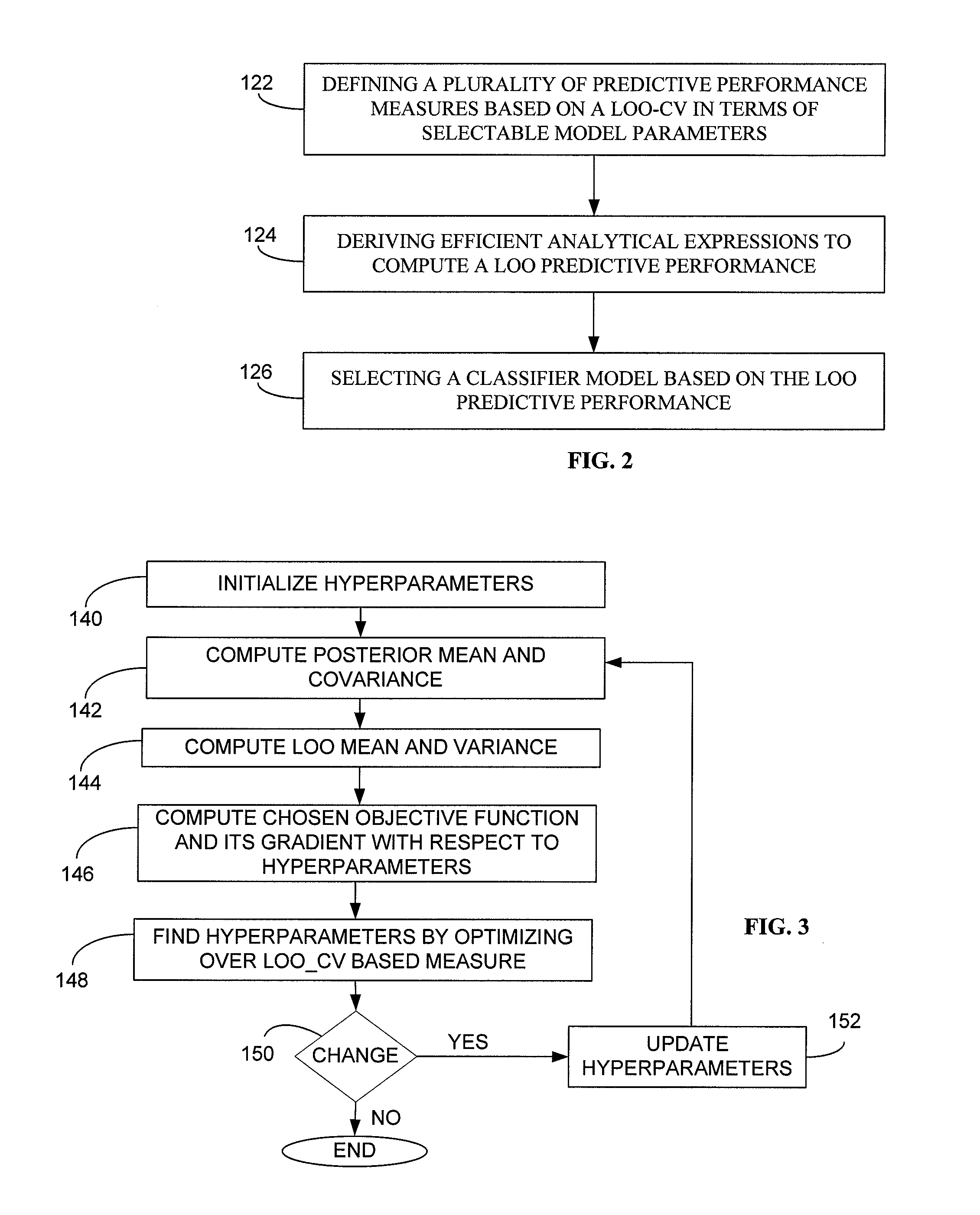
Generally, the present invention provides a method and computerized system for generating a classifier model, wherein the classifier model is operative to classify web content. The method and computerized system includes a first step of defining a plurality of predictive performance measures based on a leave one out (LOO) cross validation in terms of selectable model parameters. Exemplary predictive performance measures includes smoothened predictive measures such as F-measure, weighted error rate measure, area under curve measure, by way of example. The method and computerized system further includes deriving efficient analytical expressions for predictive performance measures to compute the LOO predictive performance and their derivatives. The next step is thereupon selecting a classifier model based on the LOO predictive performance.
Owner:R2 SOLUTIONS
CircRNA marker used for breast cancer diagnosis and application of marker
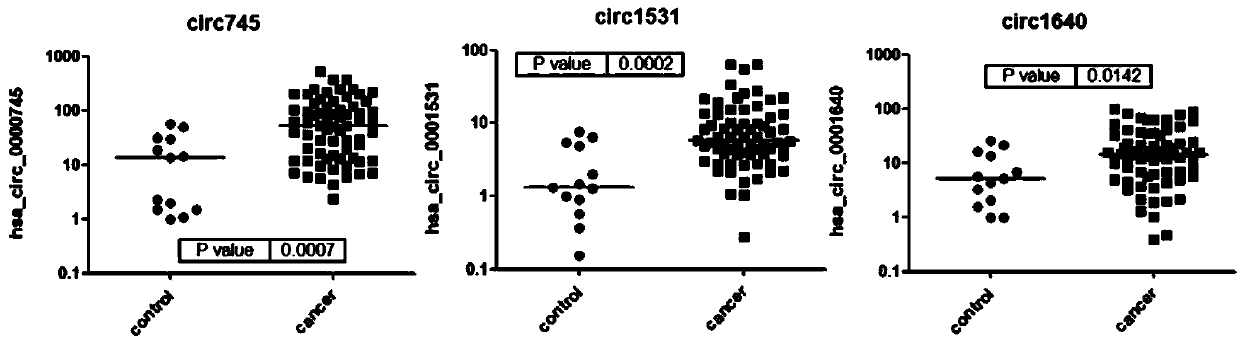
ActiveCN109097477AEasy to distinguishMicrobiological testing/measurementDNA/RNA fragmentationMedicineCurve analysis
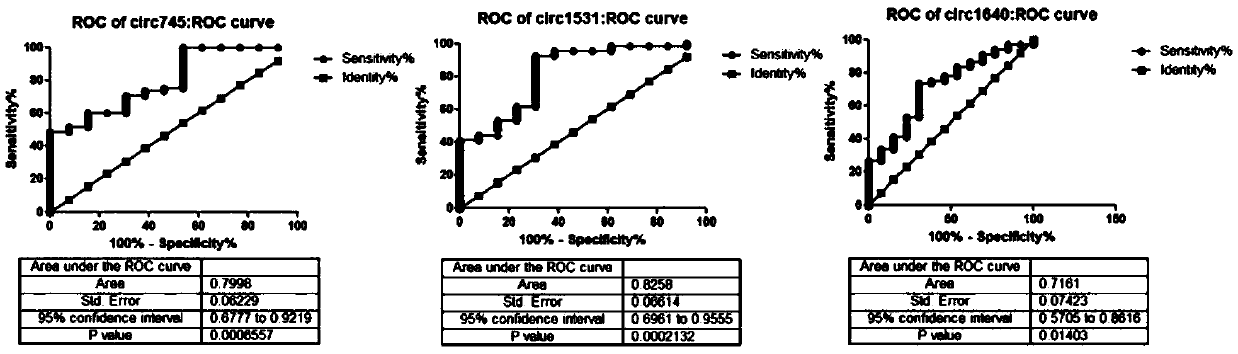
The invention relates to a circRNA marker used for breast cancer diagnosis and an application of the circRNA marker. Compared with healthy control groups, it is found that expression of 3 kinds of circRNA (hsa_circ_0000745, hsa_circ_0001531 and hsa_circ_0001640) in whole blood of breast cancer is up-regulated. ROC curve analysis displays that the marker can better distinguish breast cancer patients and healthy people, and the 3 kinds of circRNA have the AUCs (area under curve) of 0.7998, 0.8253 and 0.7161 respectively, the sensitivity of 48.53%, 92.65% and 73.53% respectively and the specificity of 100%, 69.23% and 69.23% respectively.
Owner:SHANDONG UNIV QILU HOSPITAL
System and method for generating a classifier model
Generally, the present invention provides a method and computerized system for generating a classifier model, wherein the classifier model is operative to classify web content. The method and computerized system includes a first step of defining a plurality of predictive performance measures based on a leave one out (LOO) cross validation in terms of selectable model parameters. Exemplary predictive performance measures includes smoothened predictive measures such as F-measure, weighted error rate measure, area under curve measure, by way of example. The method and computerized system further includes deriving efficient analytical expressions for predictive performance measures to compute the LOO predictive performance and their derivatives. The next step is thereupon selecting a classifier model based on the LOO predictive performance.
Owner:R2 SOLUTIONS
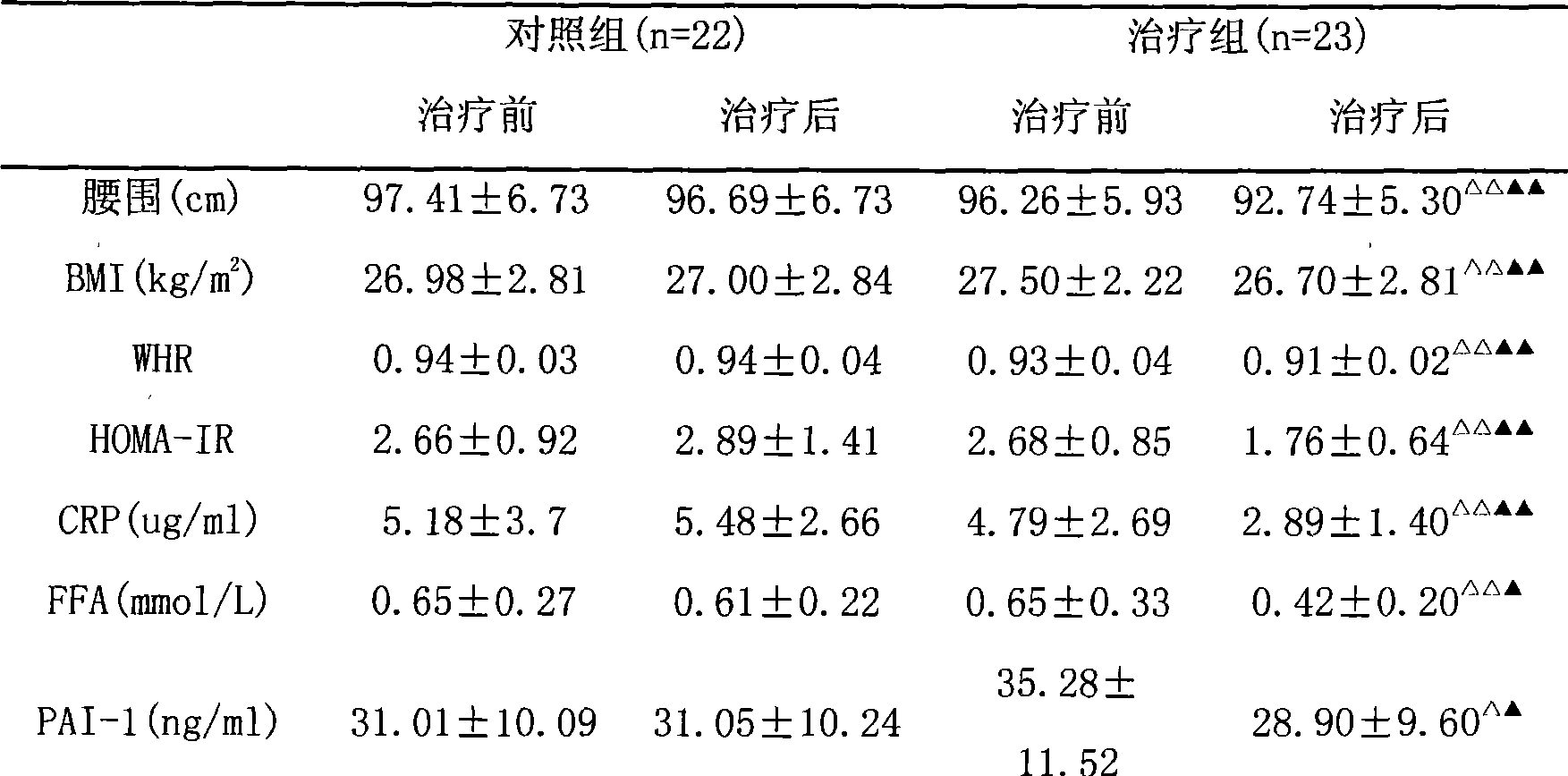
Traditional Chinese compound for curing central obesity and nonalcoholic fatty liver disease and preparation method thereof
InactiveCN101530517AReduce abdominal circumferenceReduced CT ratioMetabolism disorderDigestive systemInflammatory factorsTreatment effect
The invention discloses a traditional Chinese compound preparation for curing nonalcoholic fatty liver and central obesity and is characterized in that the compound preparation is prepared by adding medicinal excipient to the extractives of milk veteh, rhizoma coptidis, prepared rhubarb, raw cattail pollen and herba artemisiae capillaris, wherein the weight mixture ratio of milk veteh, rhizoma coptidis, prepared rhubarb, raw cattail pollen and herba artemisiae capillaris is 1-4:1:1-3:1-3:1-3. Proved by clinical trial, the drug combination of the invention can reduce the abdominal perimeter of patients, lower the AUC (area under curve) of triglyceride after dinner, improve the CT specific value of liver and spleen and bring down the transaminase level of liver with evident treatment effect. Verified by clinical research, the compound can obviously increase the insulin sensitivity of patients, lower inflammatory factor level and improve fibrinolysis-blood clotting function. Shown by experimental research, the drug of the invention can enhance the sensitivity of fat and muscle cells to insulin and has significant value in treating nonalcoholic fatty liver and central obesity which take insulin resistance as fundamental pathological link.
Owner:王文健
Reagent for detecting clostridium symbiosum and application thereof
InactiveCN105803061AEasy to judgeIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesBacteroidesFeces
The invention discloses an application of the reagent for detecting clostridium symbiosum in preparing an early colorectal cancer diagnostic kit. According to the diagnostic kit, DNA of bacteria in excrement is extracted and a quantitative polymerase chain reaction is conducted so that the feature of relative abundance of the clostridium symbiosum can be acquired, and diagnosis of early colorectal cancers (limited to the colon cancer and the rectal cancer of submucosa) is conducted with the feature as a basis. Compared with noninvasive colorectal cancer screening methods applied to clinics at present and applied for a patent, the regent is completely noninvasive, operation is relatively easy and low in price, the colorectal cancers can be predicted more accurately, and the area under curve (AUC value) of an ROC curve is 0.70 or above.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
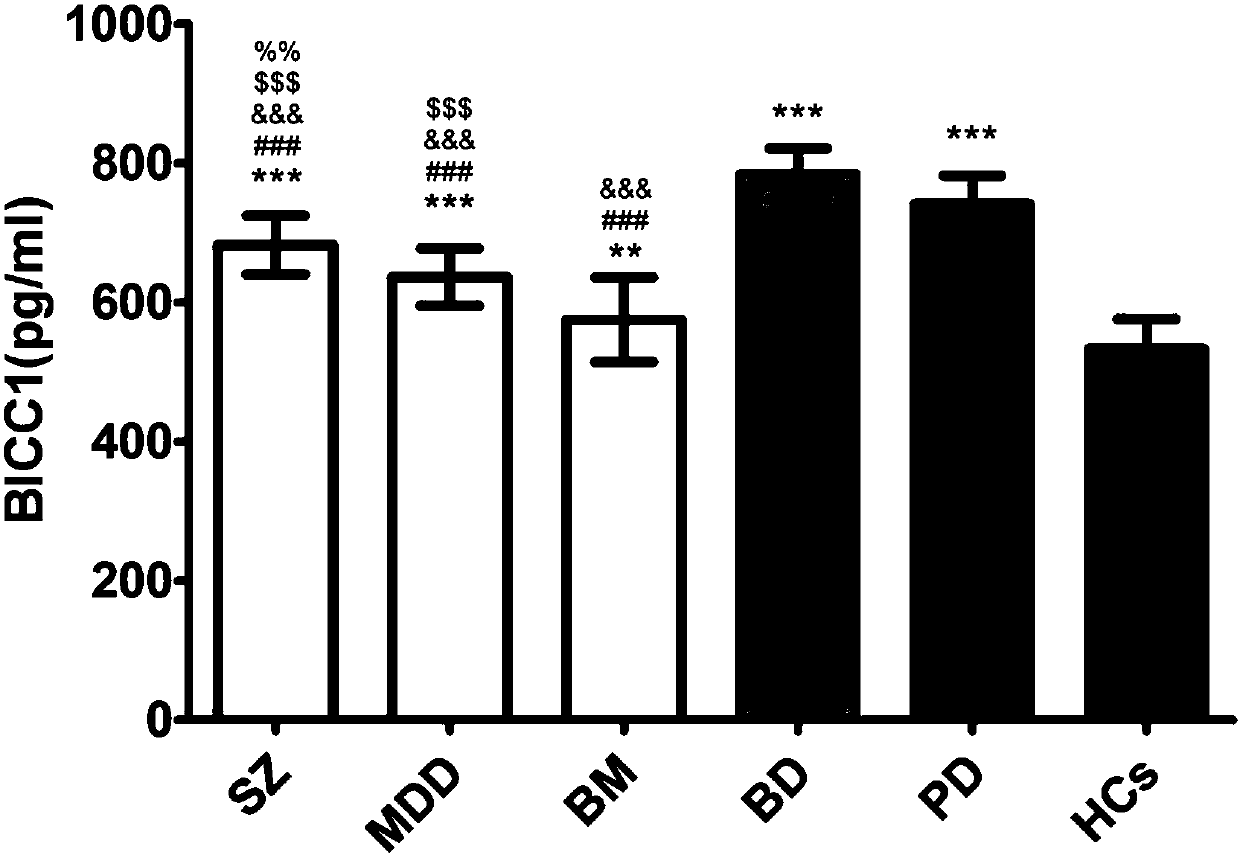
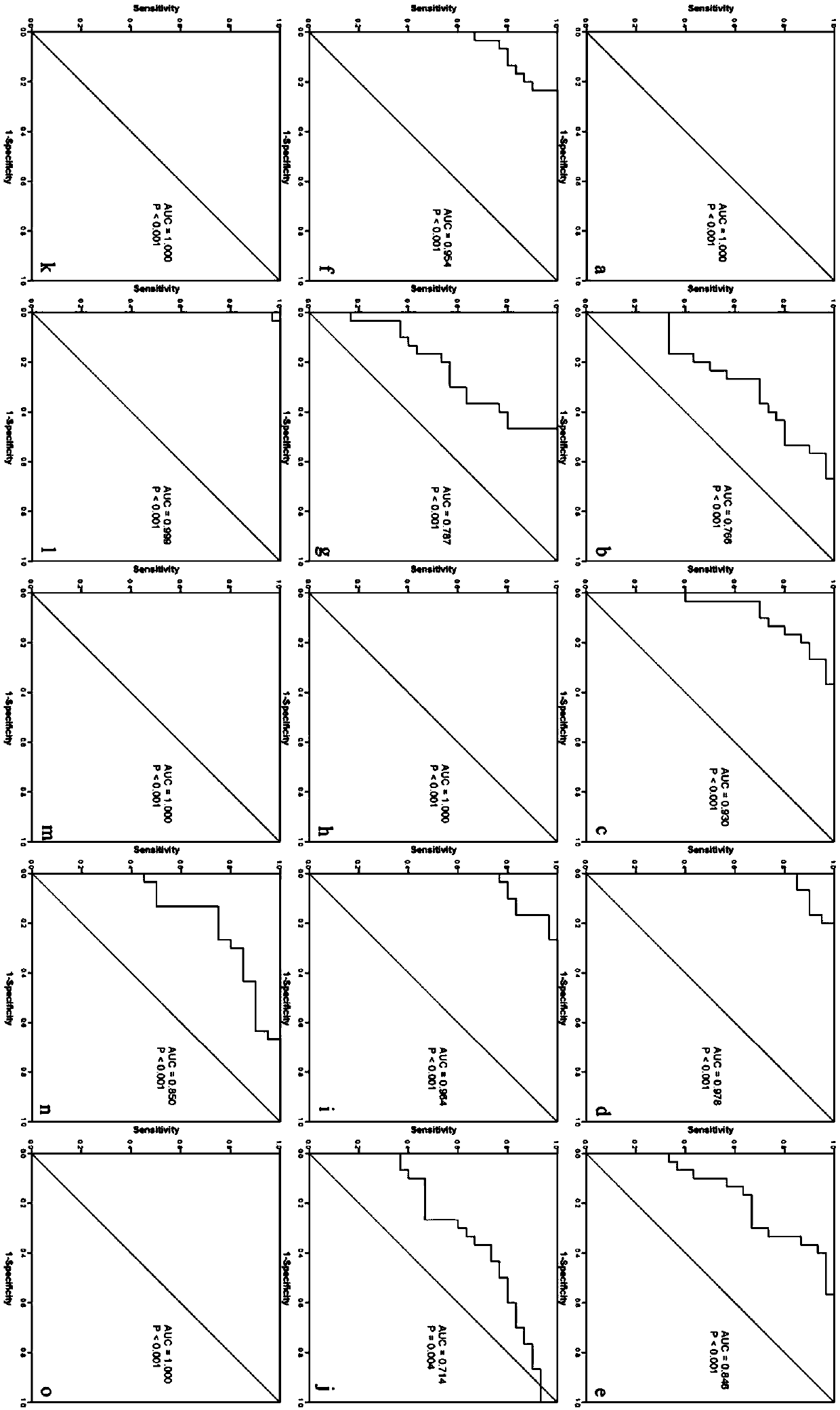
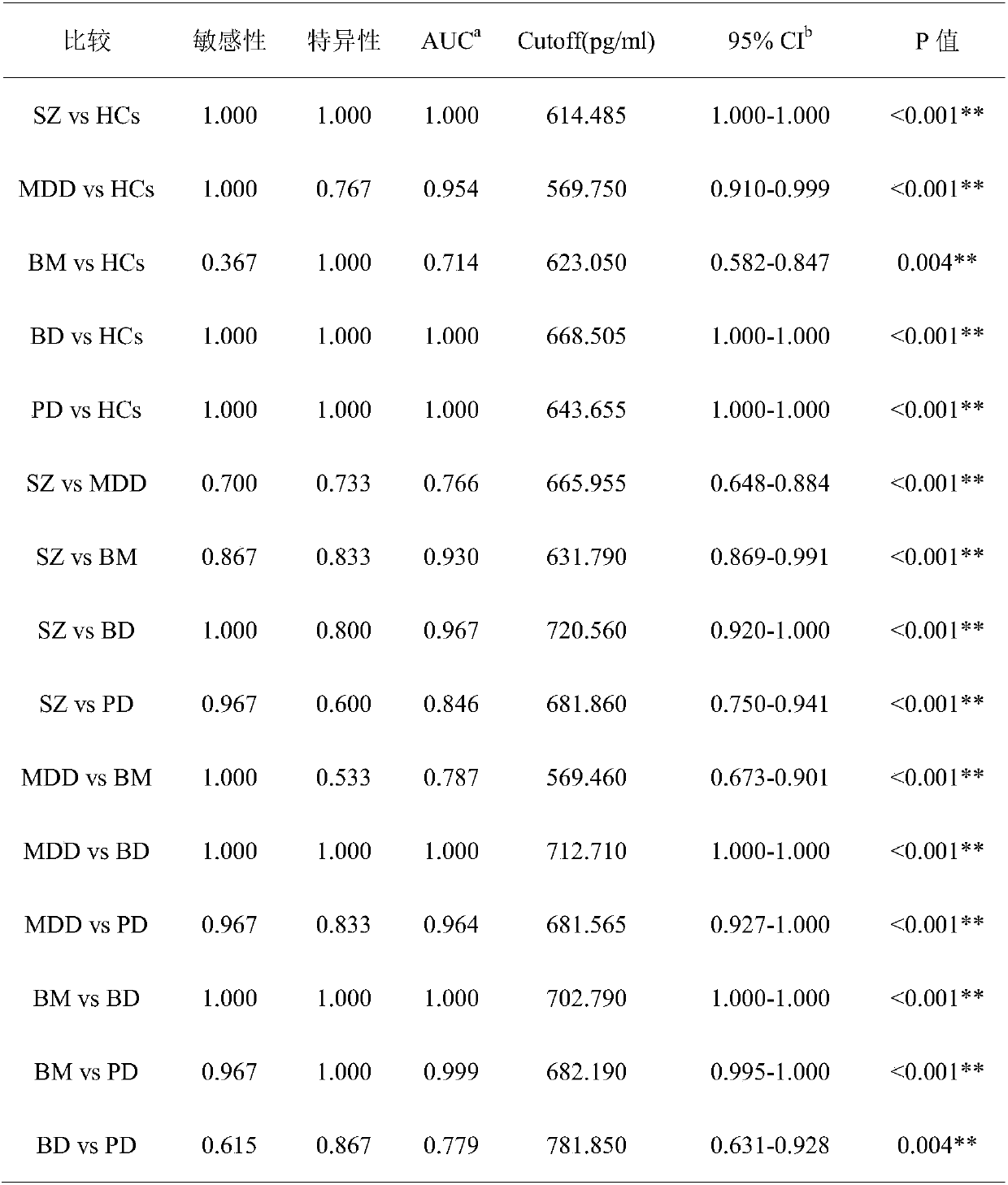
New application of BICC1 protein in mental disease diagnosis
The invention discloses application of reagents for detecting BICC1 protein content in the preparation of mental disease diagnosis products. Researches discover that BICC1 protein has differential expression in the serum of people with schizophrenia, unipolar depressive disorder, bipolar mania, bipolar depression and panic disorder and in the serum of people, without mental diseases, in a controlgroup (the expression of the BICC1 protein is increased in the serum of the people with the schizophrenia, the unipolar depressive disorder, the bipolar mania, the bipolar depression and the panic disorder). By using the BICC1 protein as the index, a brand new approach for detecting the schizophrenia, the unipolar depressive disorder, the bipolar mania, the bipolar depression and the panic disorder is provided, AUC (area under curve) under an ROC curve is 0.714-1.0, and a high diagnosis value is achieved.
Owner:江苏集萃医工交叉技术研究所有限公司
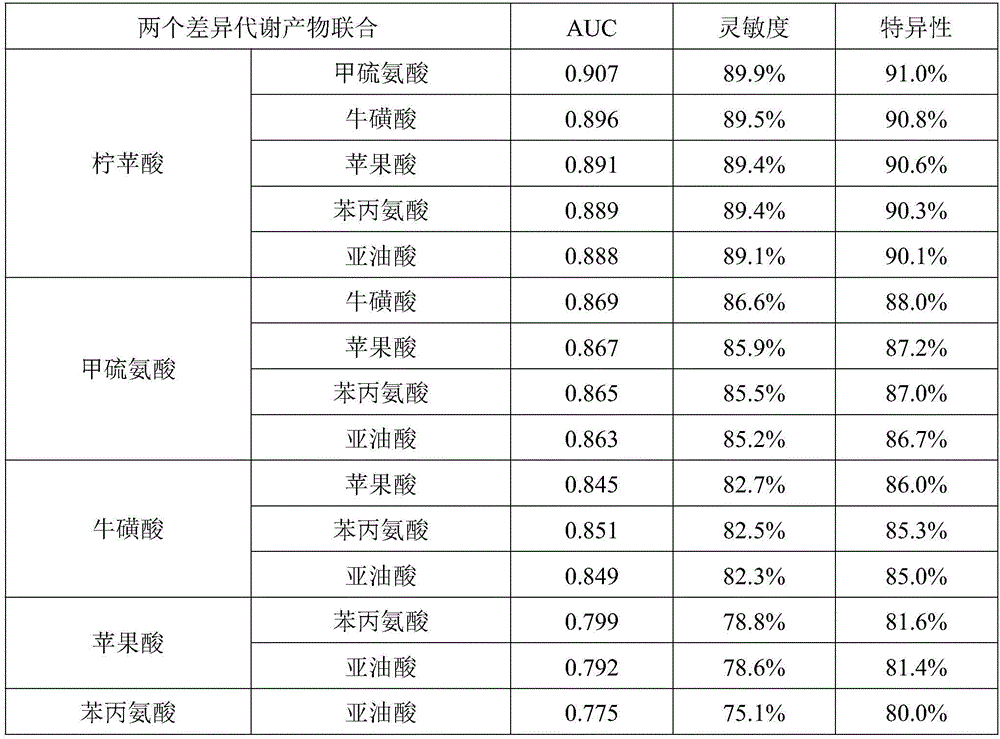
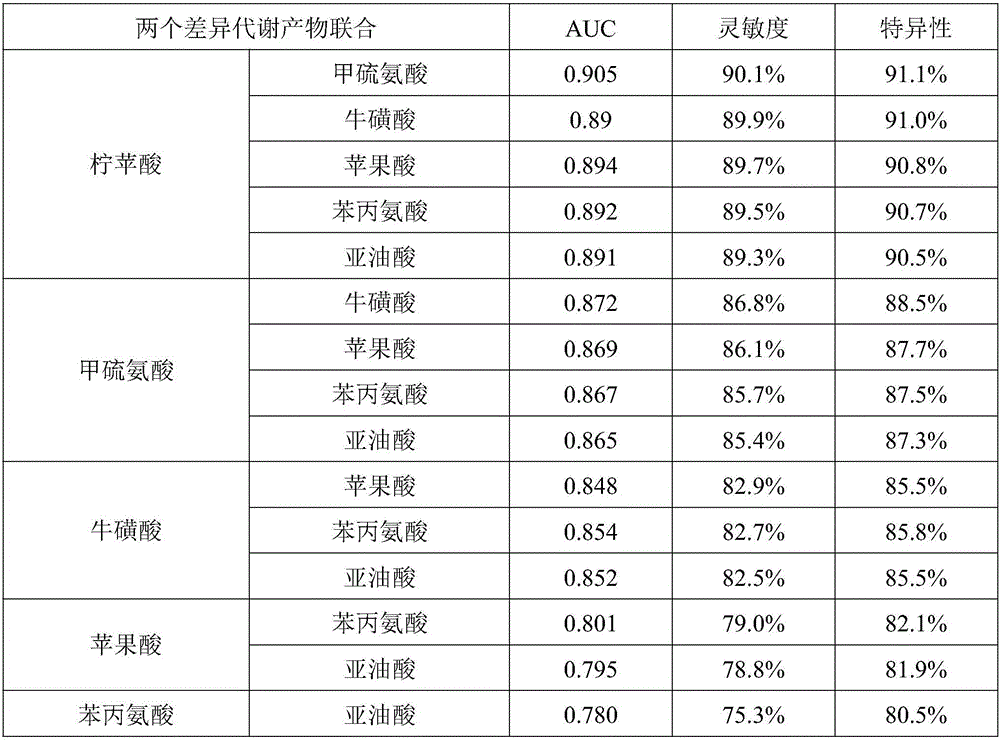
Metabolic marker group for diagnosing coronary heart disease
ActiveCN105758966AEasy diagnosisImprove accuracyComponent separationDisease diagnosisCoronary heart diseaseTaurine
The invention discloses a metabolic marker group for diagnosing a coronary heart disease. The metabolic marker group comprises one or more of citramalic acid, methionine, taurine, malic acid, phenylalanine and linoleic acid. In a ROC (receiver operating characteristic) curve evaluation method, under the condition that the AUC (area under curve) is larger than 0.5, the closer the AUC is to 1, the better the diagnosis effect is. A metabolic marker has lower accuracy when the AUC is 0.5-0.7, has certain accuracy when the AUC is 0.7-0.9 and has higher accuracy when the AUC is 0.9 or larger. Verification proves that when the single metabolic marker in the metabolic marker group is used for diagnosing and distinguishing patients with the coronary heart disease from subjects with normal coronary arteries, or diagnosing and distinguishing the patients with the coronary heart disease from patients with coronary atherosclerosis, the AUCs are all 0.7 or larger; when a plurality of the metabolic markers are used in combination, the AUC is closer to 1 than that when the single metabolic marker is used, so that the diagnosis effect is better; when six metabolic markers are used in combination, the AUC is closest to 1, and diagnosing and distinguishing effects are the best.
Owner:苏州帕诺米克生物科技有限公司
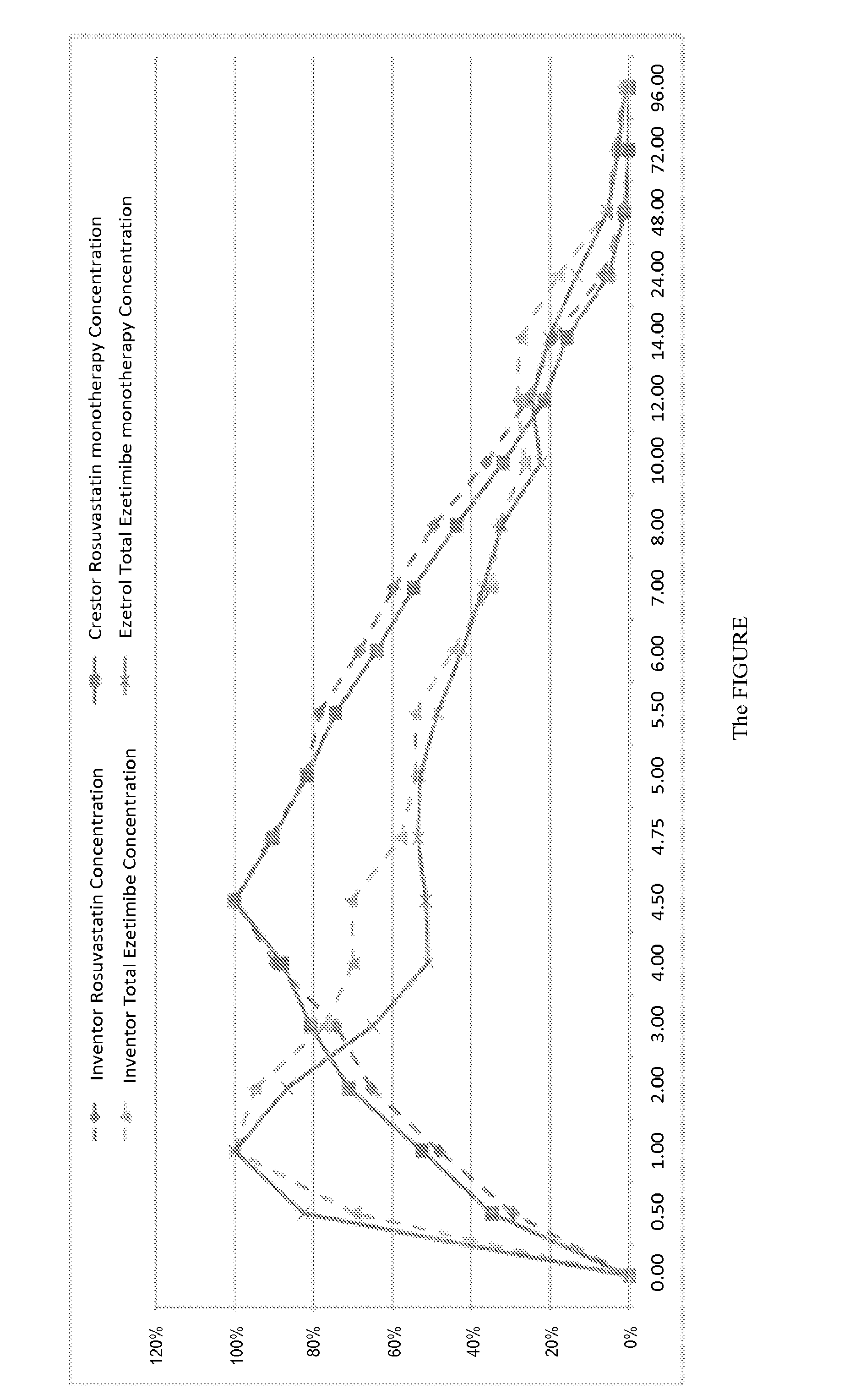
Oral Tablet Formulation Consisting Of Fixed Combination Of Rosuvastatin And Ezetimibe For Treatment Of Hyperlipidemia And Cardiovascular Diseases
ActiveUS20140287042A1Improve solubilityLower cholesterol levelsBiocideOrganic active ingredientsSecondary hyperlipidemiaEzetimibe
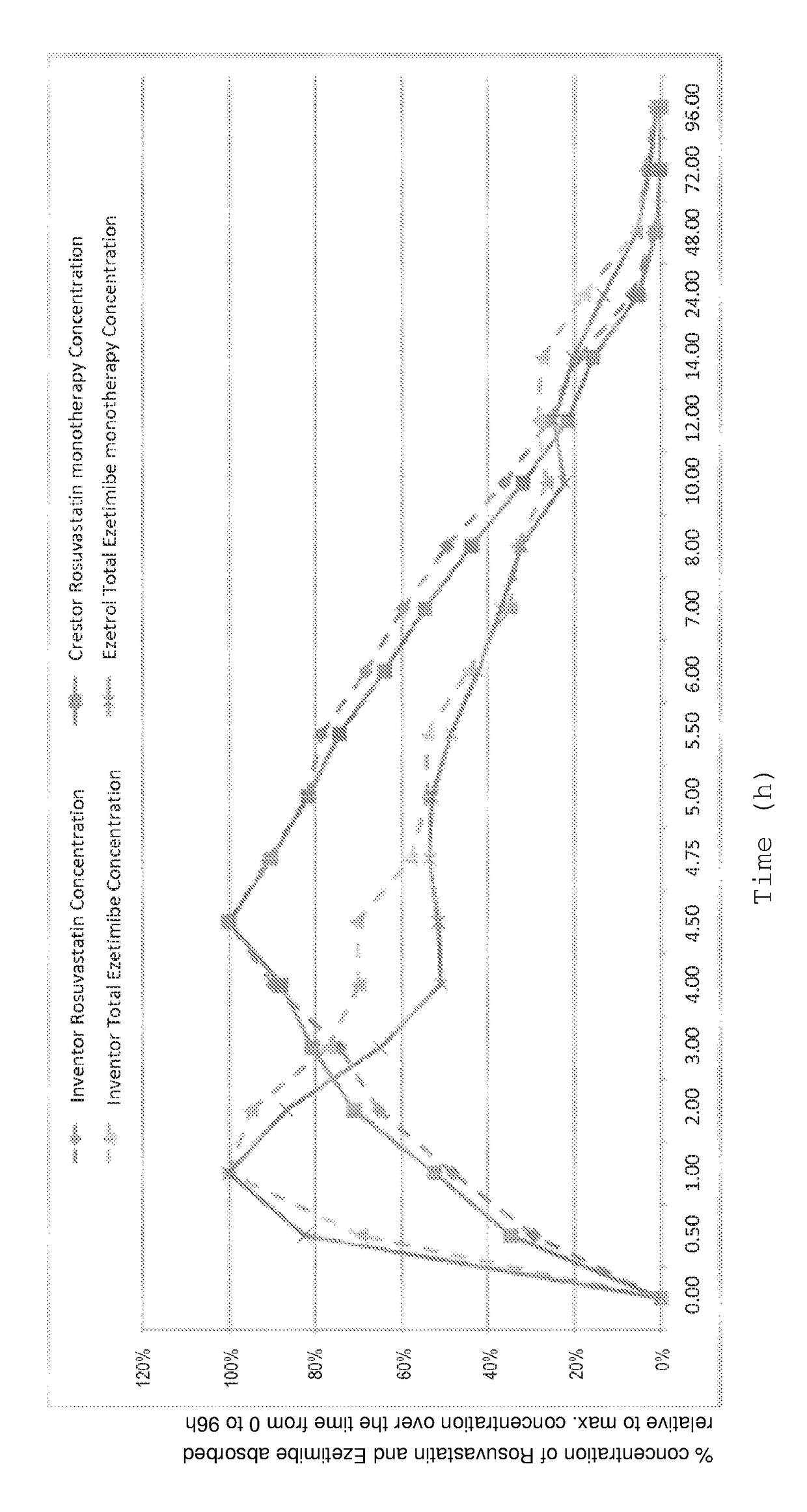
The present invention is an orally consumed fixed combination formulation of both rosuvastatin and ezetimibe in one tablet that is expected to have the same Area Under Curve as two active ingredients taken together individually orally, and pharmaceutically acceptable additives suitable for the preparation. In preferred embodiments of this invention, the rosuvastatin is in the form of rosuvastatin calcium and the pharmaceutically acceptable additives are selected from diluents, disintegrants, glidants, lubric ants, colorants and combinations thereof.
Owner:ALTHERA LIFE SCI
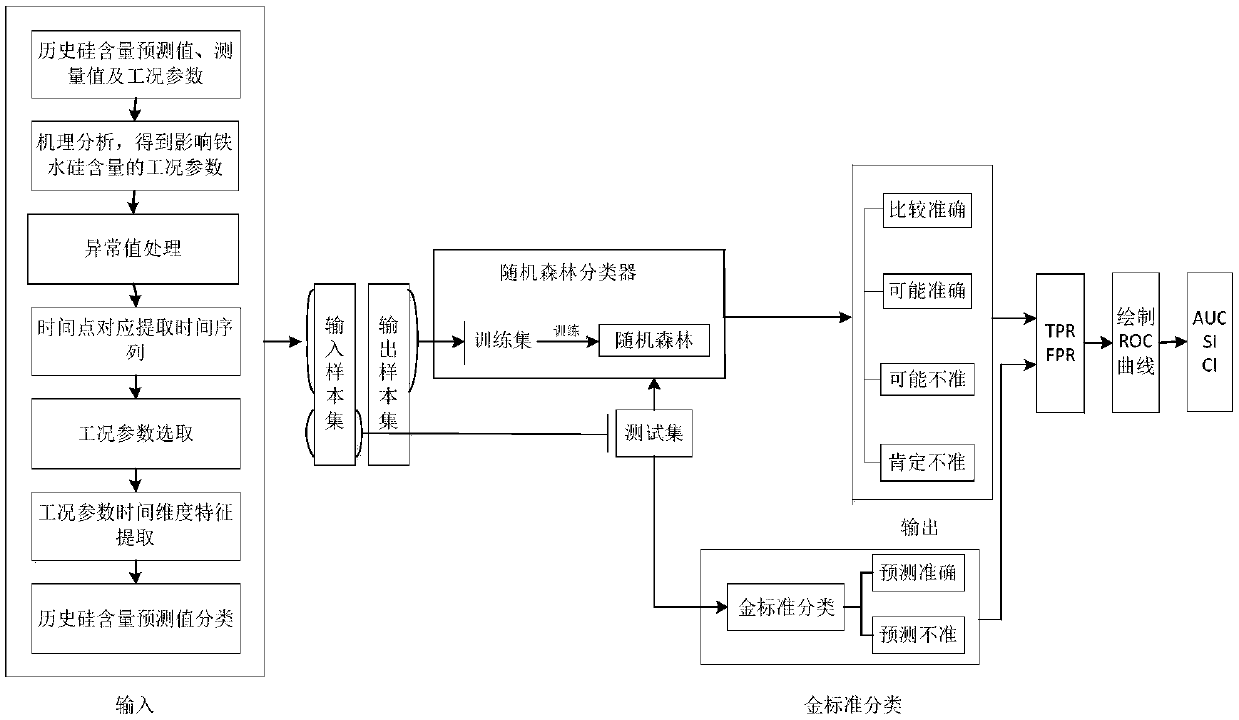
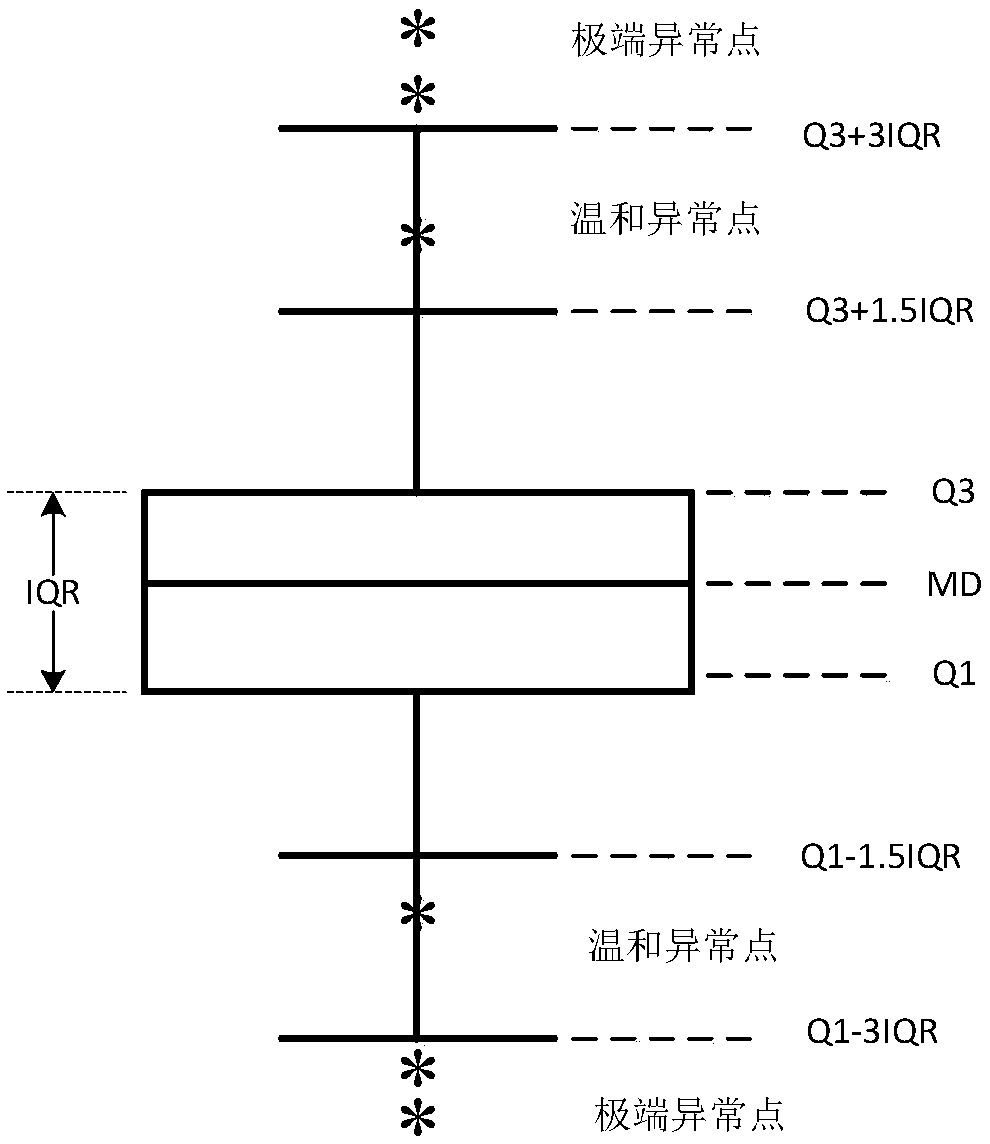
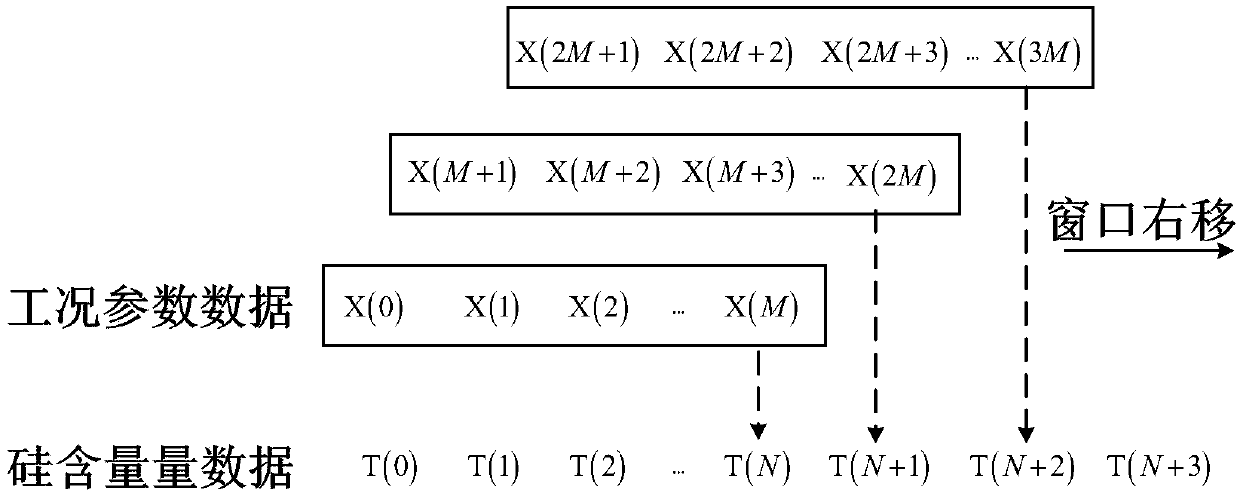
Method and device for evaluating accuracy of model for predicting silicon content in hot metal of blast furnace
ActiveCN108875118ASteel manufacturing process aspectsBlast furnace detailsReceiver operating characteristicTrue positive rate
The invention provides a method and device for evaluating the accuracy of a model for predicting the silicon content in hot metal of a blast furnace. The method comprises the steps of: obtaining working condition parameters influencing the silicon content in the hot metal of the blast furnace in historical data, a silicon content measurement value and a silicon content prediction value of a silicon content prediction model to be evaluated, and, according to a trained accuracy prediction model, classifying the prediction result accuracy of the silicon content prediction value, so that a first evaluation classification result is obtained; classifying the accuracy of the silicon content prediction value according to the silicon content measurement value, so that a second evaluation classification result is obtained; and, obtaining a true positive rate TPR and a false positive rate FPR based on the first evaluation result and the second evaluation result, and evaluating the reliability ofthe silicon content prediction model through a receiver operating characteristic curve ROC. A ROC is drawn through a prediction result; the performance of the prediction model can be overall judged through the indexes, such as an AUC (Area Under Curve); and thus, production can be guided by selection of a proper prediction model for a site.
Owner:CENT SOUTH UNIV
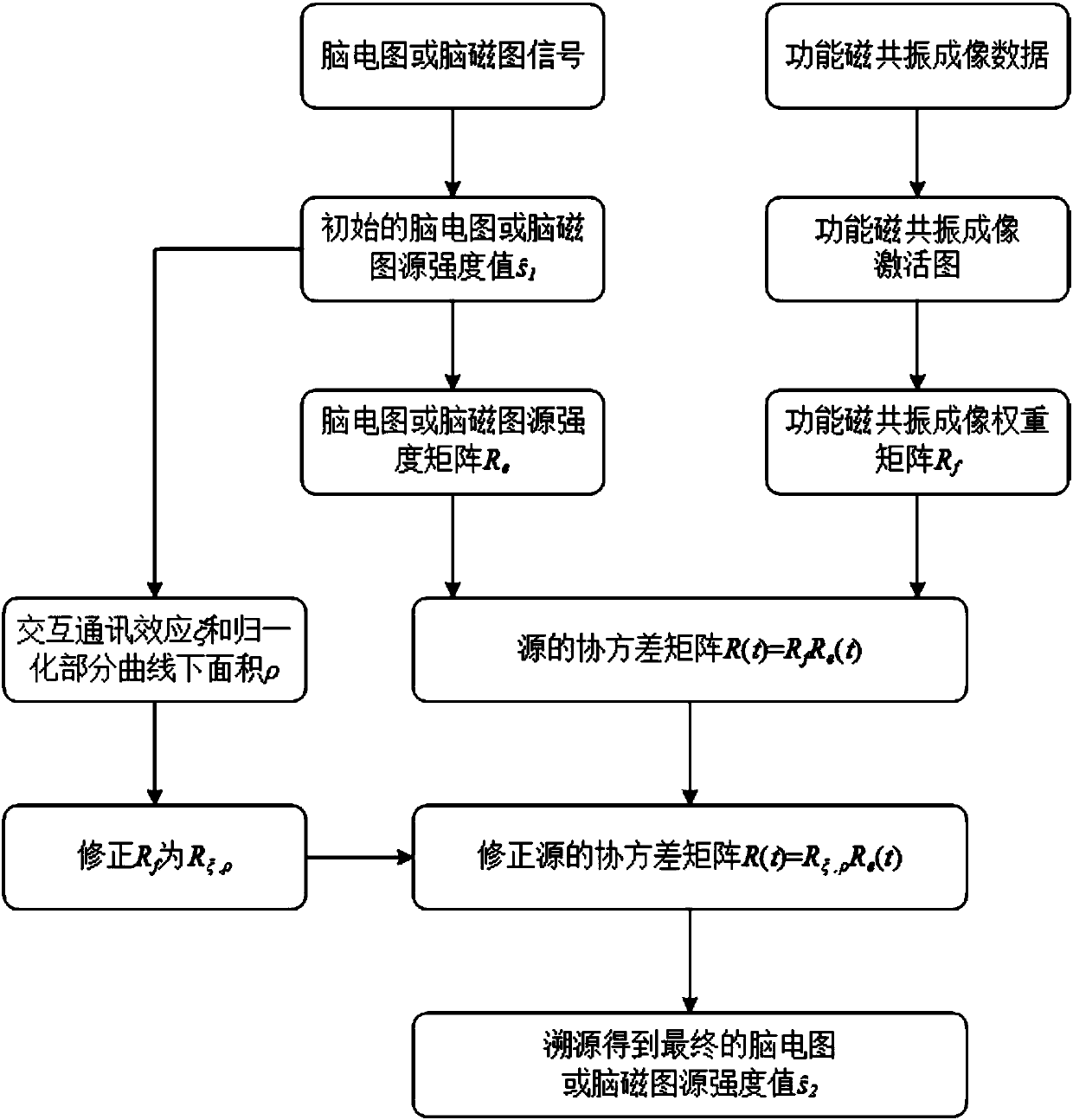
Time-varying constraint electroencephalogram or magnetoencephalogram source tracing method based on functional magnetic resonance imaging
ActiveCN107550493APrecise positioningDiagnostic recording/measuringSensorsTemporal changeElectroencephalography
The invention provides a time-varying constraint electroencephalogram or magnetoencephalogram source tracing method based on functional magnetic resonance imaging. The method comprises the steps thatan electroencephalogram or magnetoencephalogram signal is recorded and traced, an initial electroencephalogram or magnetoencephalogram source intensity value is obtained, and an electroencephalogram or magnetoencephalogram source intensity matrix is obtained on the basis; functional magnetic resonance imaging data is recorded, a functional magnetic resonance imaging activation image is obtained onthe basis of the functional magnetic resonance imaging data, and then a functional magnetic resonance imaging weight matrix is obtained; a covariance matrix of a source is obtained according to the functional magnetic resonance imaging weight matrix and the electroencephalogram or magnetoencephalogram source intensity matrix, and the covariance matrix of the source is set to be changed along withtime; the interactive communication effect and the area-under-curve of a normalization part are calculated according to the initial electroencephalogram or magnetoencephalogram source intensity value, and the covariance matrix of the source is corrected on the basis; source tracing is carried out according to the corrected covariance matrix of the source, a final electroencephalogram or magnetoencephalogram source intensity value is obtained, and source tracing is completed.
Owner:PEKING UNIV
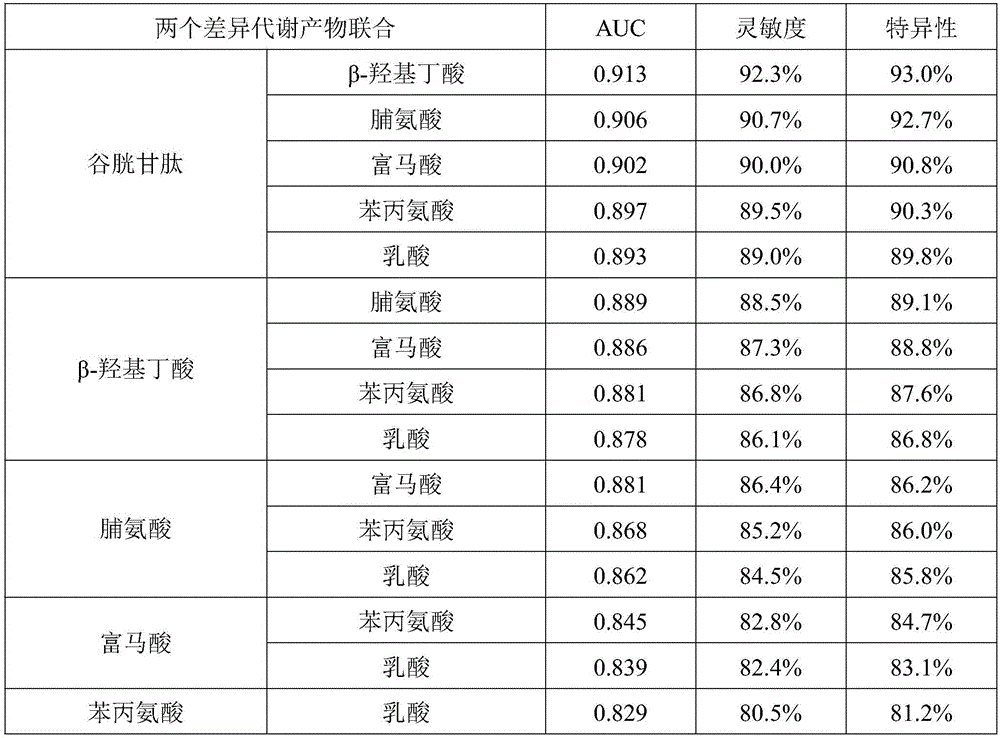
Marker group for diagnosing and distinguishing coronary arterial atherosclerosis from stable angina pectoris
ActiveCN105758967AEasy diagnosisImprove accuracyComponent separationDisease diagnosisBeta-Hydroxybutyric acidReceiver operating characteristic
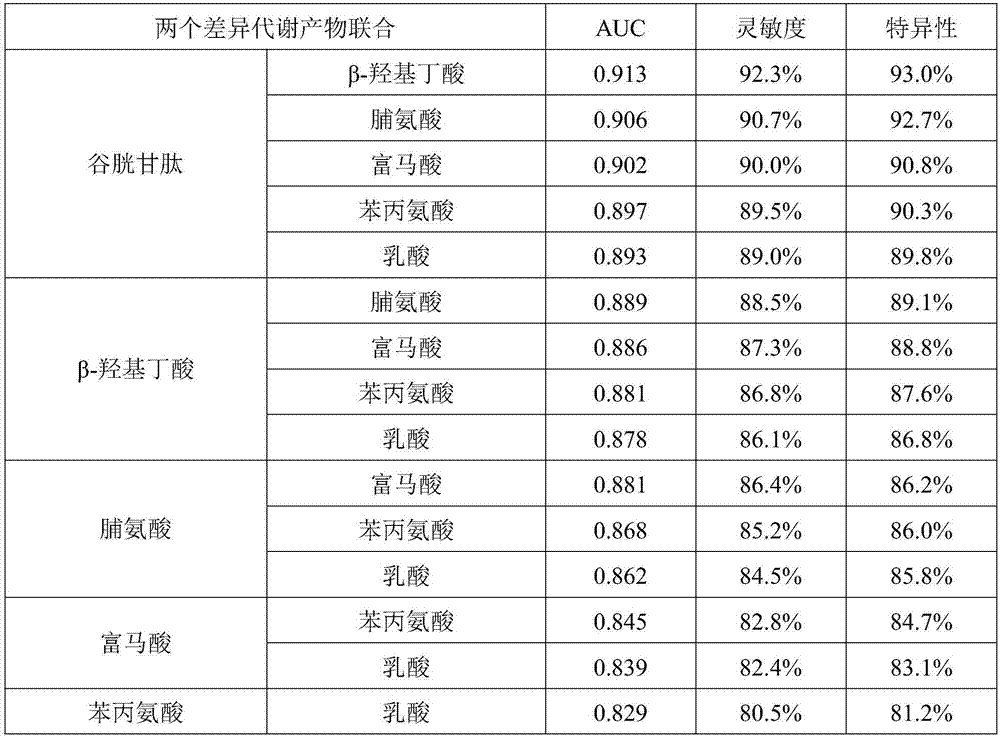
The invention discloses a marker group for diagnosing and distinguishing coronary arterial atherosclerosis from stable angina pectoris. The marker group comprises one or more of glutathione, beta-hydroxybutyric acid, proline, fumaric acid, phenylalanine and lactic acid. When a single marker is used for diagnosing and distinguishing patients with the stable angina pectoris from patients with the coronary arterial atherosclerosis, the AUCs (areas under curve) of a ROC (receiver operating characteristic) are all 0.7 or larger, and the marker has a clinical diagnosis significance; when the markers are used for diagnosis in combination, with the increase of the combination number, the AUC is further increased and reaches 0.988 when 6 markers are combined, and the sensitivity and the specificity are 98.5% and 98.3% respectively under the optimum cutoff value. The metabolic marker group can accurately diagnose and distinguish the coronary arterial atherosclerosis from the stable angina pectoris, and is high in sensitivity and specificity.
Owner:CHINA PHARM UNIV
Health-care food with anti-fatigue effect and preparation method thereof
The invention discloses health-care food with an anti-fatigue effect and a preparation method thereof. The health-care food is prepared from the following raw materials in part by weight: 300 to 450 parts of lophatherum gracile Brongn and 250 to 400 parts of American ginseng. A health-care product preparation provided by the invention has the advantages of obviously prolonging the burden swimming time of mice, reducing the production of serum urea nitrogen of the mice, reducing area under curve of blood lactate after the motion of the mice along with the anti-fatigue health-care effect. The health-care food adopts medicinal and edible natural plants as main raw materials, so that the preparation process and the product quality is stable and controllable; the curative effect is obvious; the provided formulation is convenient to administrate; and the health-care food is suitable for all kinds of people to eat.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
Novel application of tetrahydrocurcumin
InactiveCN102552225AClear efficacyCorrect clinical disturbanceMetabolism disorderKetone active ingredientsReduced subcutaneous fatTG - Triglyceride
The invention provides application of tetrahydrocurcumin to a medicine for preventing and / or treating obese type II diabetes, and a medicinal composition for preventing and / or treating obese type II diabetes. The tetrahydrocurcumin has the effects of reducing body weight, fasting blood glucose, triglyceride, total cholesterol and low-density lipopmtein cholesterol, raising high-density lipopmtein cholesterol, reducing area under curve (AUC) of glucose tolerance, and reducing subcutaneous fat and abdominal fat on spontaneous obese type II diabetic mice.
Owner:SICHUAN ACAD OF CHINESE MEDICINE SCI
Methods for administering Anti-il-5 antibodies
The present invention relates generally to the methods for the treatment and diagnosis of conditions mediated by IL-5 and excess eosinophil production, and more specifically to mAbs, Fabs, chimeric and humanized antibodies. More particularly, methods are provided for reducing eosinophils in a human in need thereof, which method comprises administering to said human a composition comprising at least one anti-IL-5 antibody, wherein at least one anti-IL-5 antibody provides a mean maximum plasma concentration of said anti-IL-5 antibody of at least about 1.03±0.21 μg / mL, an Area Under the Curve value of at least about 15.5±2.7 μg / day / mL and a serum half-life of about 16.2±2.1 days to about 21.7±2.8 days.
Owner:GLAXO SMITHKLINE LLC
Application of the system for detecting the expression of mir-30c-5p in predicting the curative effect of aspirin on patients with cardiovascular diseases
The invention discloses an application of a system for detecting miR-30c-5p expression quantity in prediction of a curative effect of aspirin for treating cardiovascular disease patients. In the application disclosed by the invention, an ROC curve analysis is carried out on miR-30c-5p of platelet hyper-reactivity patients by taking non-platelet hyper-reactivity patients a control, the area under curve is 0.720, the sensitivity is 54.84%, and the specificity is 82.25%. The result shows that the expression quality of miR-30c-5p in venous blood of detected objects can be used for judging the clinical curative effect of aspirin for treating the cardiovascular disease patients.
Owner:PEKING UNIV FIRST HOSPITAL
Intravital residual activity insulin algorithm
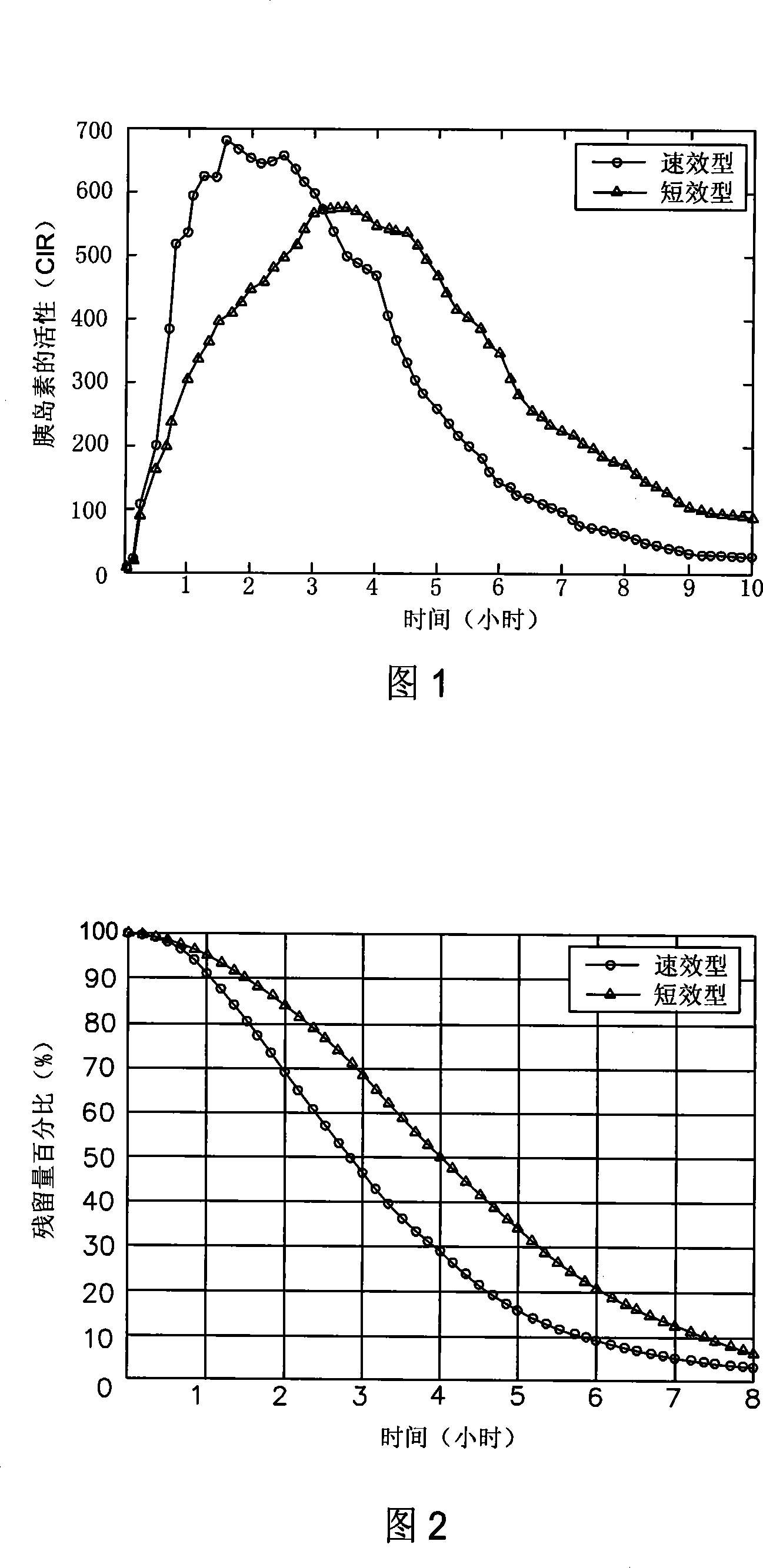
InactiveCN101194827AImprove compatibilityCalculations are reliableData processing applicationsDiagnostic recording/measuringHuman bodyCurrent meter
The invention relates to an algorism of residual active insulin in a human body, which enables the algorism to share higher compatibility through building up a quick-effective or short-lived insulin pharmacodynamics curve diagram data base, and to contain more crowds and have more reliable numerical result through the detailed classification of the data base. The invention is quantitatively described through the gross area under the total antihypelipidemic activity PD (pharmacodynamics) curve diagram of extrinsic insulin, the residual antihypelipidemic activity of one time point after dosing can be quantitatively described by the rest area under curve after the time point, which is more convenient and direct, and then the relationship curve of residual quick-effective or short-lived active insulin and time is calculated, namely the percentage curve of residual quantity on each time point, and the algorism is more scientific and accurate. Doctors or patients can easily receive the residual quantity of active insulin in patients' body in current time according to the interval time between the current time and last administration time, matching with the relationship curve of residual quick-effective or fugitive active insulin and time.
Owner:珠海福尼亚医疗设备有限公司
A biomarker group for diagnosis of coronary atherosclerosis and stable angina
ActiveCN105758967BEasy diagnosisImprove accuracyComponent separationDisease diagnosisHydroxybutyric acidAngina
A marker group for making diagnosis to distinguish coronary arterial atherosclerosis from stable angina pectoris. The marker group comprises one or more of glutathione, β-hydroxybutyric acid, proline, fumaric acid, phenylalanine and lactic acid. When a single marker is used for making diagnosis to distinguish patients with the stable angina pectoris from patients with the coronary arterial atherosclerosis, the areas under curve (AUC) of a ROC curve are all 0.7 or larger, and the marker has a clinical diagnosis significance; when the marker is used for diagnosis in combination, with the increase of the combination number, the AUC is further increased and reaches 0.988 when 6 markers are combined, and the sensitivity and the specificity are 98.5% and 98.3% respectively under the optimum cutoff value. The metabolic marker group can be used to accurately making diagnosis to distinguish the coronary arterial atherosclerosis from the stable angina pectoris, and has high sensitivity and specificity.
Owner:CHINA PHARM UNIV
Bioequivalence evaluating method for medicines for reducing blood sugar by using pharmacodynamics as index
InactiveCN109529055AImprove review consistencyLow reliabilityCompounds screening/testingConfidence intervalConcentrations glucose
The invention provides a bioequivalence evaluating method for medicines for reducing blood sugar by using pharmacodynamics as an index, and relates to a three-cycle three-cross bioequivalence evaluating method by using pharmacodynamics parameters of blood sugar concentration as an evaluating index, wherein the medicines are mainly alpha-glycosidase inhibitor type medicines. Examines are divided into three groups randomly, and are respectively subjected to medicine test of reference medicines, contrast medicines and vacancy medicines at the three-cycle experiment stage, and then are subjected to glucose load experiment, and besides, blood samples within 4 hours are collected, and concentration determination results of glucose in serum are recorded as the blood sugar concentration. Evaluating parameters include the largest blood sugar concentration difference (delta Cmax) of the examinees under the condition of being subjected to medicine test of the reference medicines or the contrast medicines and the vacancy medicines; and the 2-4-hoursblood sugar concentration-time area under curve difference (delta AUC0-n) of the examinee under the condition of being subjected to medicine test of reference medicines or contrast medicines or vacancy medicine. The judgment standard lies in that the equivalence standard lies in that the 90% confidence interval of the group geometric mean valueratio of the delta Cmax to the delta AUC0-n in the evaluating parameters of a test preparation and the contrast medicines needs to fall in the range of 80%-125%.
Owner:金日制药(中国)有限公司
Oral tablet formulation consisting of fixed combination of rosuvastatin and ezetimibe for treatment of hyperlipidemia and cardiovascular diseases
ActiveUS9763885B2Improve solubilityLower cholesterol levelsOrganic active ingredientsPill deliverySecondary hyperlipidemiaEzetimibe
The present invention is an orally consumed fixed combination formulation of both rosuvastatin and ezetimibe in one tablet that is expected to have the same Area Under Curve as two active ingredients taken together individually orally, and pharmaceutically acceptable additives suitable for the preparation. In preferred embodiments of this invention, the rosuvastatin is in the form of rosuvastatin calcium and the pharmaceutically acceptable additives are selected from diluents, disintegrants, glidants, lubricants, colorants and combinations thereof.
Owner:ALTHERA LIFE SCI
Application of system for detecting miR-30c-5p expression quantity in prediction of curative effect of aspirin for treating cardiovascular disease patients
The invention discloses an application of a system for detecting miR-30c-5p expression quantity in prediction of a curative effect of aspirin for treating cardiovascular disease patients. In the application disclosed by the invention, an ROC curve analysis is carried out on miR-30c-5p of platelet hyper-reactivity patients by taking non-platelet hyper-reactivity patients a control, the area under curve is 0.720, the sensitivity is 54.84%, and the specificity is 82.25%. The result shows that the expression quality of miR-30c-5p in venous blood of detected objects can be used for judging the clinical curative effect of aspirin for treating the cardiovascular disease patients.
Owner:PEKING UNIV FIRST HOSPITAL
Compositions of diclofenac acid
The present invention provides solid oral pharmaceutical compositions comprising wet milled Diclofenac acid and one or more pharmaceutically acceptable excipients and process for preparation thereof. The present invention particularly relates to solid oral pharmaceutical compositions comprising wet milled diclofenac acid with median particle size of less than 1000 nm. In addition the compositions of present invention have the comparable dissolution profiles with marketed composition of diclofenac acid. Maximum Plasma Concentration (Cmax) and Area Under Curve (AUC) values of compositions of present invention are within the limit of 80% to 125% of Cmax and AUC of the marketed composition of diclofenac acid capsules respectively.
Owner:LUPIN LTD
Traditional Chinese compound for curing central obesity and nonalcoholic fatty liver disease and preparation method thereof
InactiveCN101530517BReduce abdominal circumferenceReduced CT ratioMetabolism disorderDigestive systemInflammatory factorsClinical trial
The invention discloses a traditional Chinese compound preparation for curing nonalcoholic fatty liver and central obesity and is characterized in that the compound preparation is prepared by adding medicinal excipient to the extractives of milk veteh, rhizoma coptidis, prepared rhubarb, raw cattail pollen and herba artemisiae capillaris, wherein the weight mixture ratio of milk veteh, rhizoma coptidis, prepared rhubarb, raw cattail pollen and herba artemisiae capillaris is 1-4:1:1-3:1-3:1-3. Proved by clinical trial, the drug combination of the invention can reduce the abdominal perimeter ofpatients, lower the AUC (area under curve) of triglyceride after dinner, improve the CT specific value of liver and spleen and bring down the transaminase level of liver with evident treatment effect. Verified by clinical research, the compound can obviously increase the insulin sensitivity of patients, lower inflammatory factor level and improve fibrinolysis-blood clotting function. Shown by experimental research, the drug of the invention can enhance the sensitivity of fat and muscle cells to insulin and has significant value in treating nonalcoholic fatty liver and central obesity which take insulin resistance as fundamental pathological link.
Owner:王文健
Medicinal composition for preventing and treating diabetes
The invention discloses a medicinal composition for preventing and treating diabetes. The medicinal composition contains 60 to 85 percent by weight of white mulberry root-bark and 7 to 11 percent by weight of mulberry serving as effective components. As proved by animal experiments, the medicinal composition for preventing and treating diabetes in an intervening way can effectively lower the blood glucose levels of diabetic rats, and reduce the glucose tolerance experimental area under curve.
Owner:NINGBO UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com