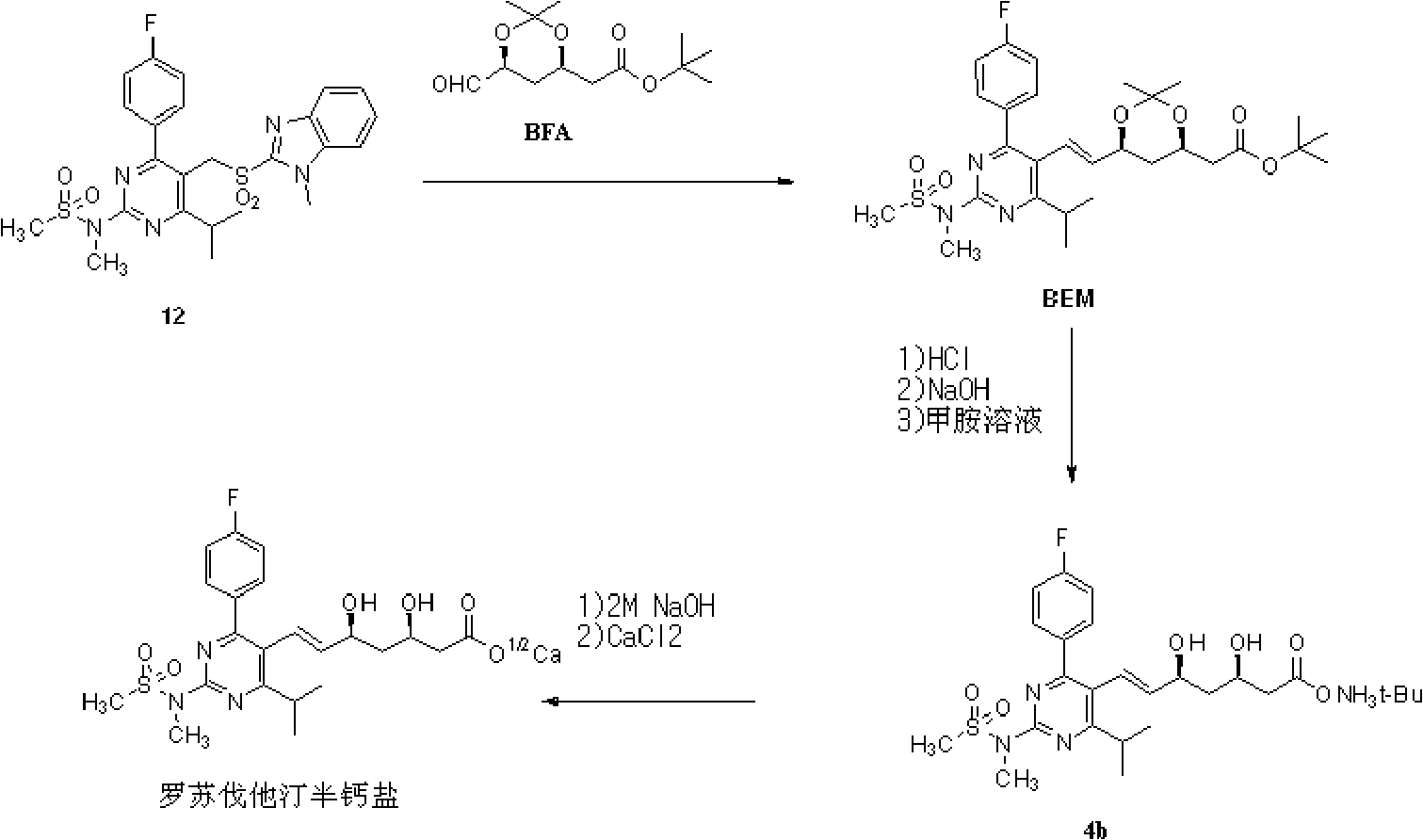
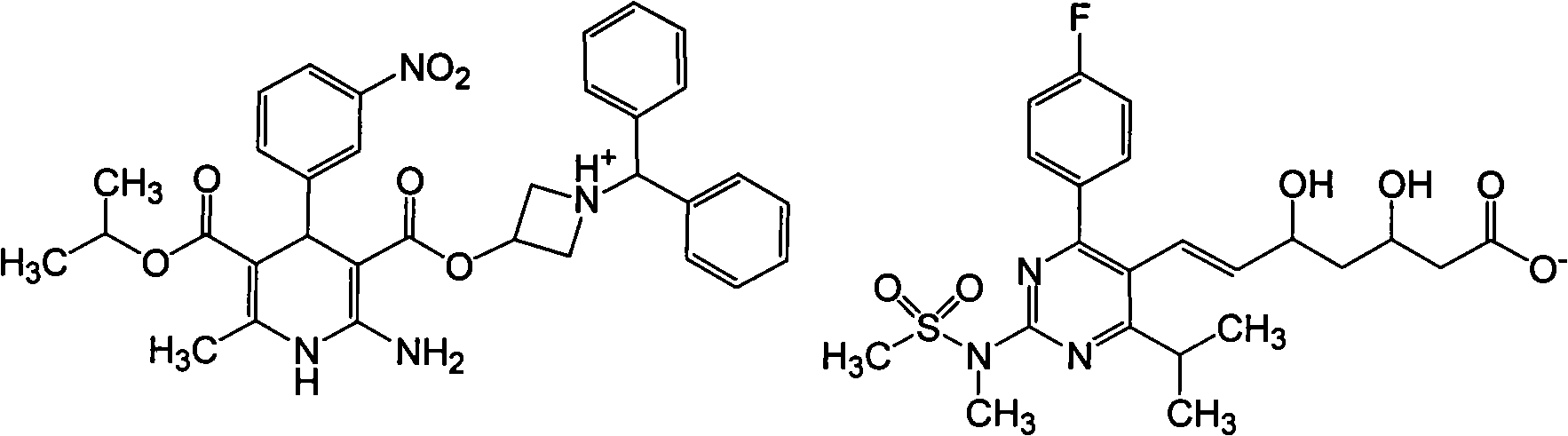
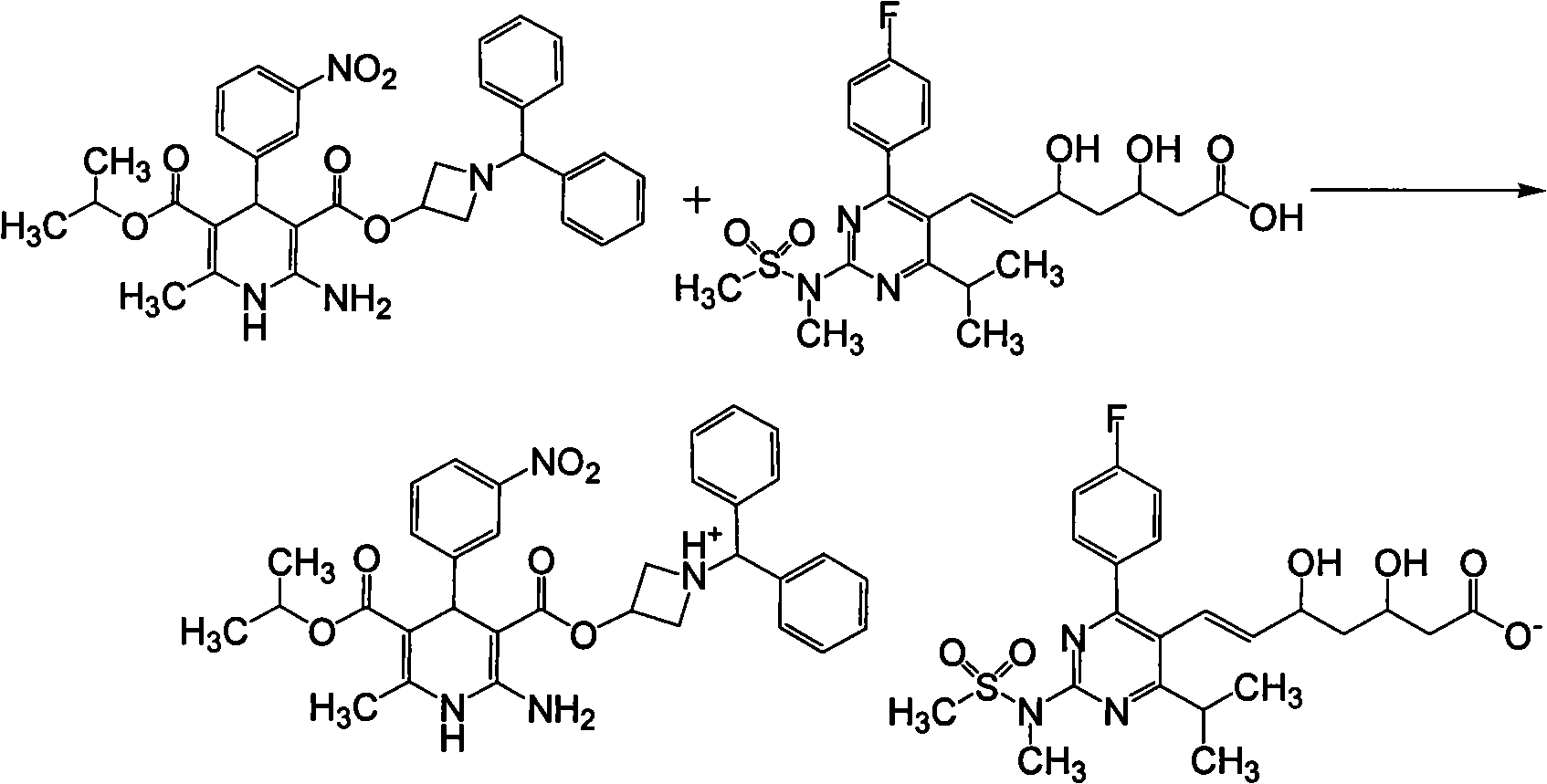
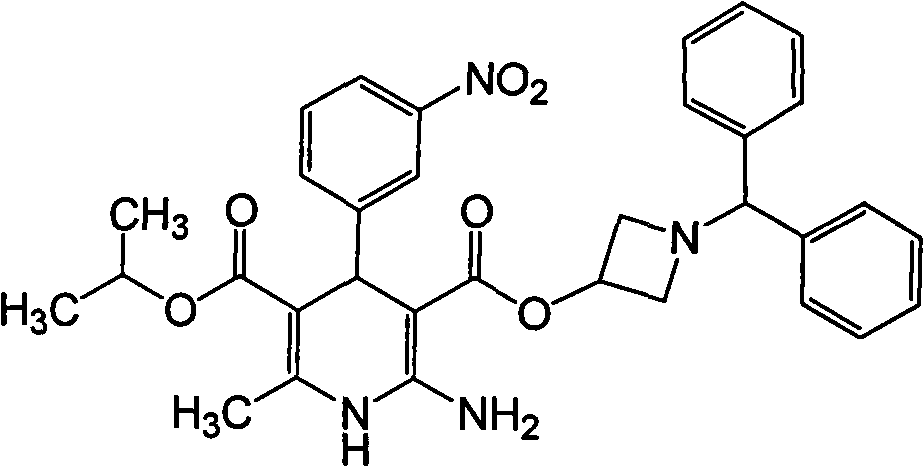
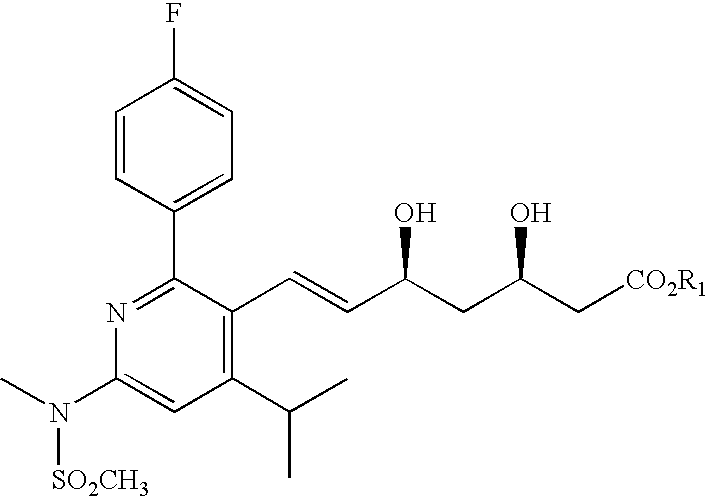
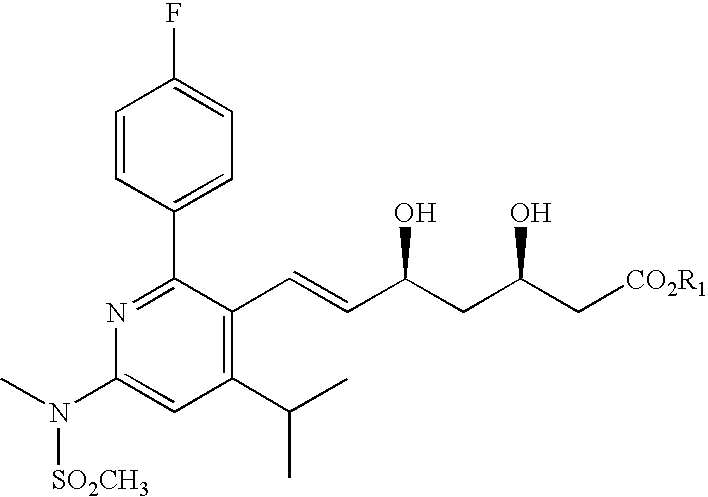
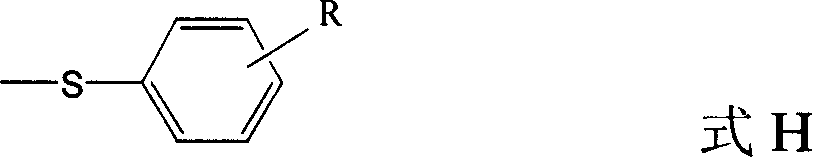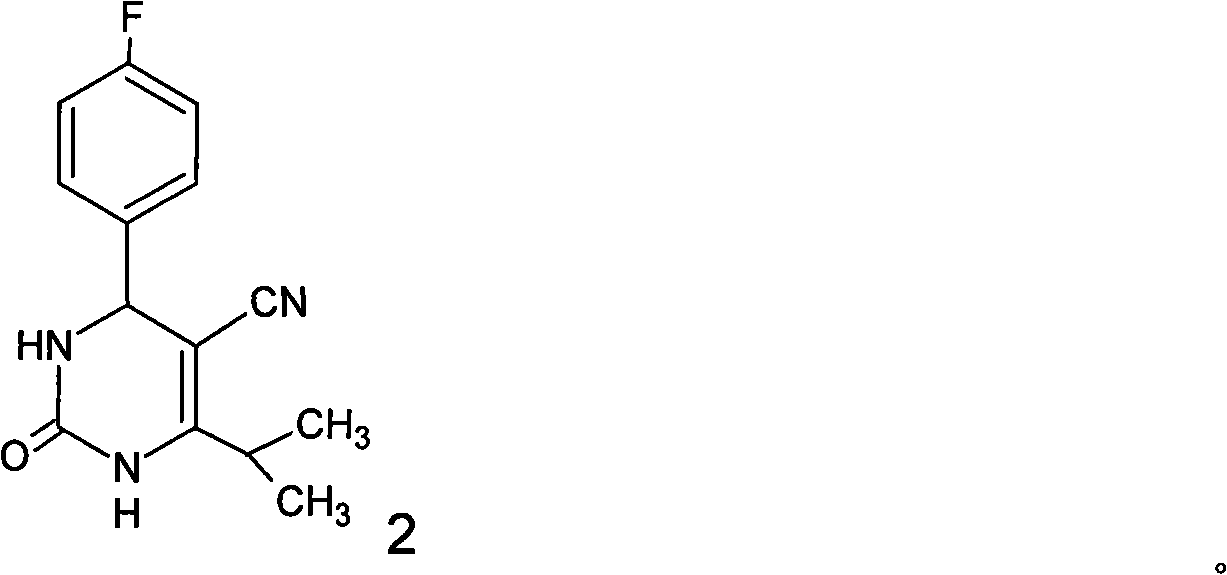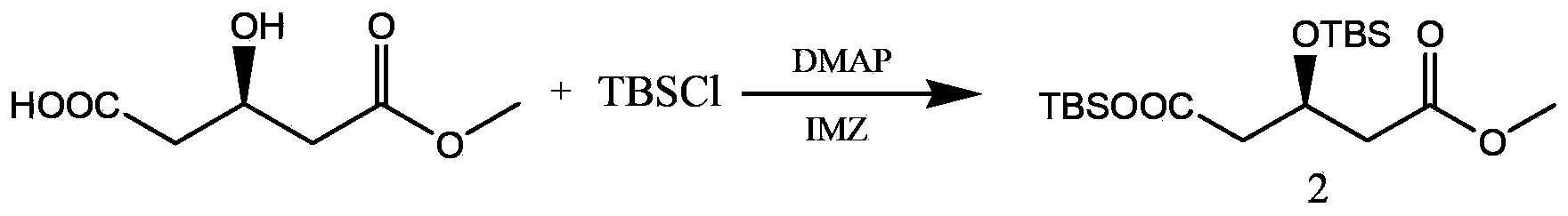Patents
Literature
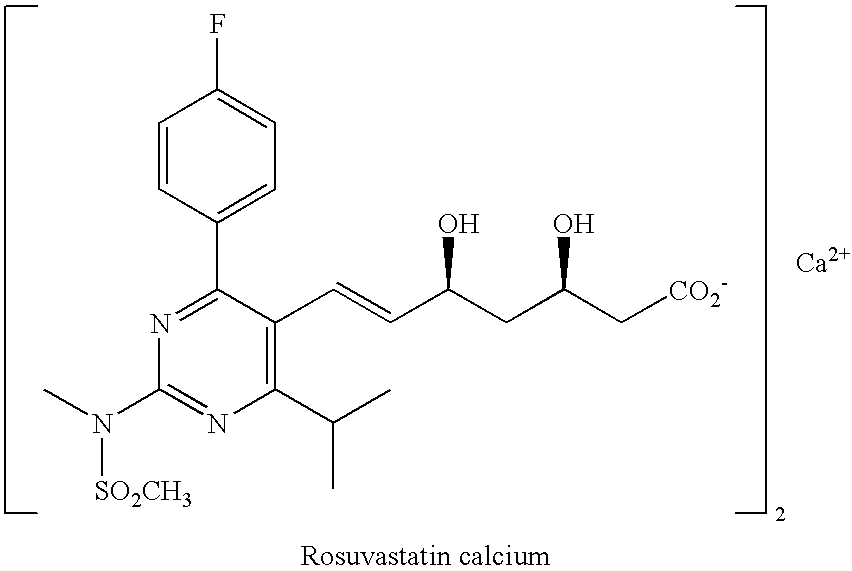
244 results about "Rosuvastatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
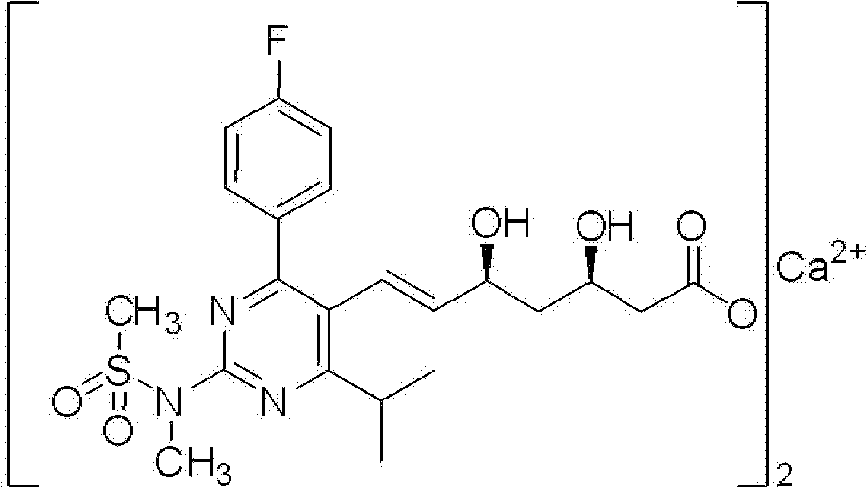
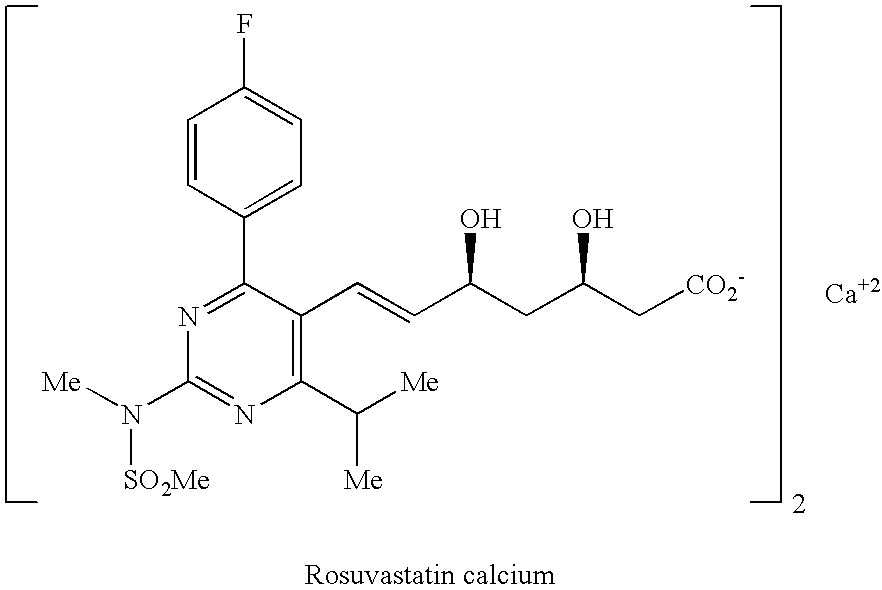
Rosuvastatin is used along with a proper diet to help lower "bad" cholesterol and fats (such as LDL, triglycerides) and raise "good" cholesterol (HDL) in the blood.
Omega-3 fatty acid self-emulsifying composition
InactiveUS20180015038A1Reduce the amount requiredImprove compatibilityNervous disorderAntipyreticPolyoxyethylene castor oilEmulsion
A pharmaceutical composition comprising, in relation to 100% by weight of a total amount of a self-emulsifying composition, 70 to 90% by weight of eicosapentaenoic acid ethyl ester as a first medicinal component, 0.5 to 6% by weight of water, 1 to 29% by weight of polyoxyethylene sorbitan fatty acid ester (optionally further comprising polyoxyethylene castor oil) as an emulsifier, 1 to 25 parts by weight of lecithin in relation to 100 parts by weight of the eicosapentaenoic acid ethyl ester, and pitavastatin, rosuvastatin, or a salt thereof as a second medicinal component. The composition is excellent in any one of self-emulsifying property, dispersibility of the composition, emulsion stability, absorbability, and storage stability of the medicinal components and a preparation.
Owner:MOCHIDA PHARM CO LTD
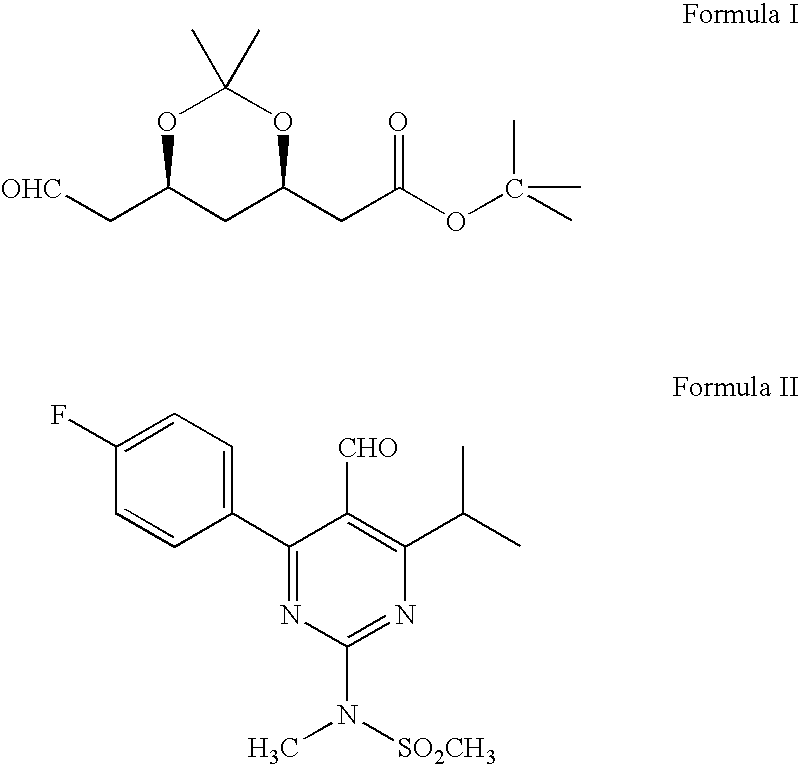
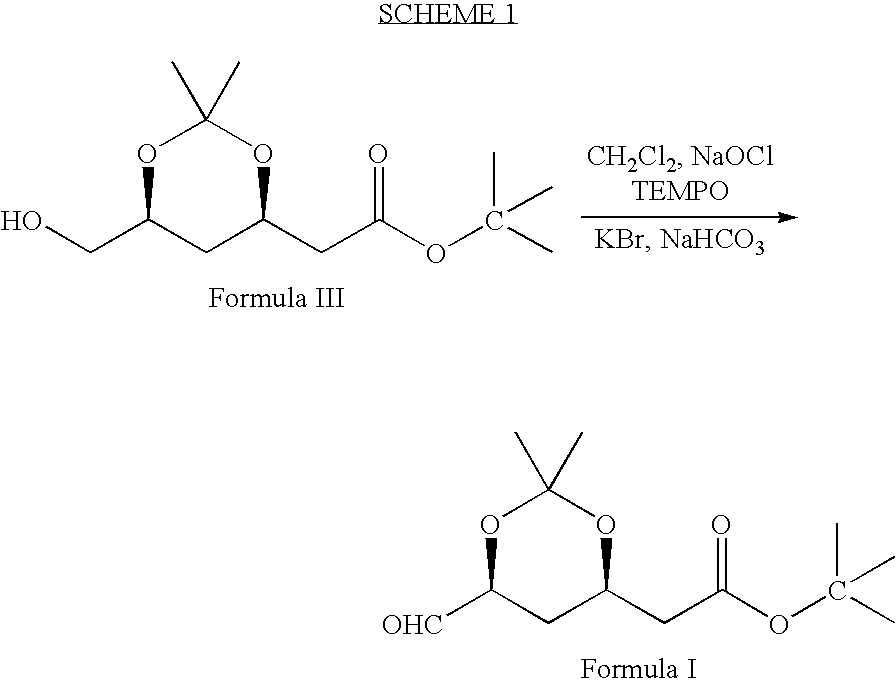
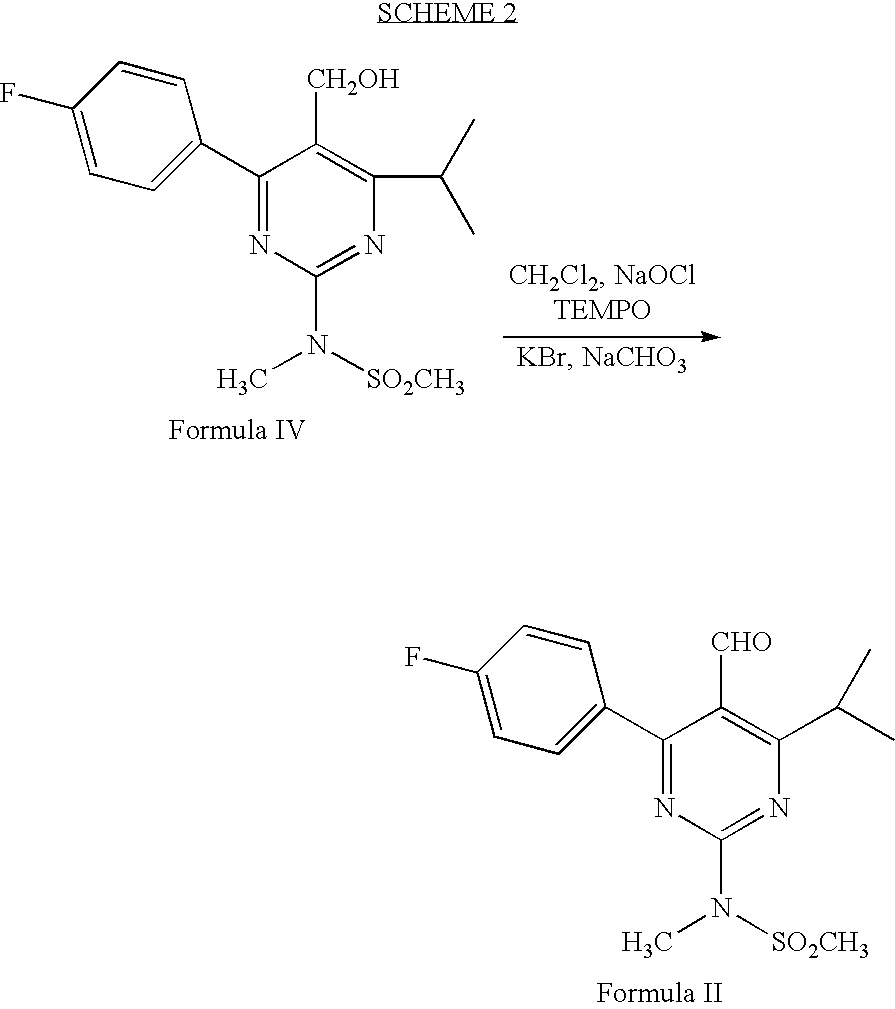
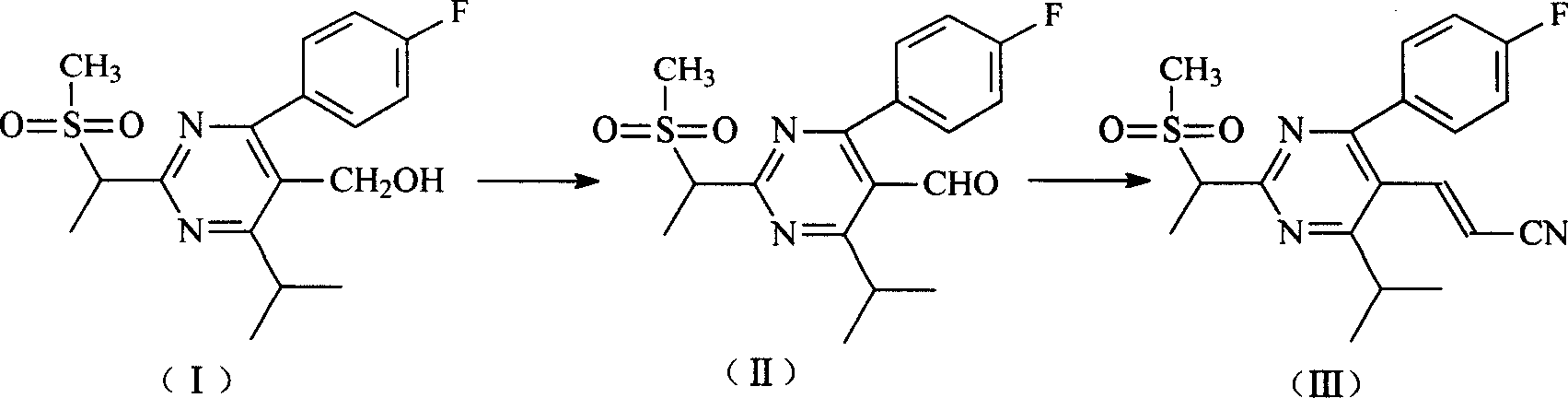
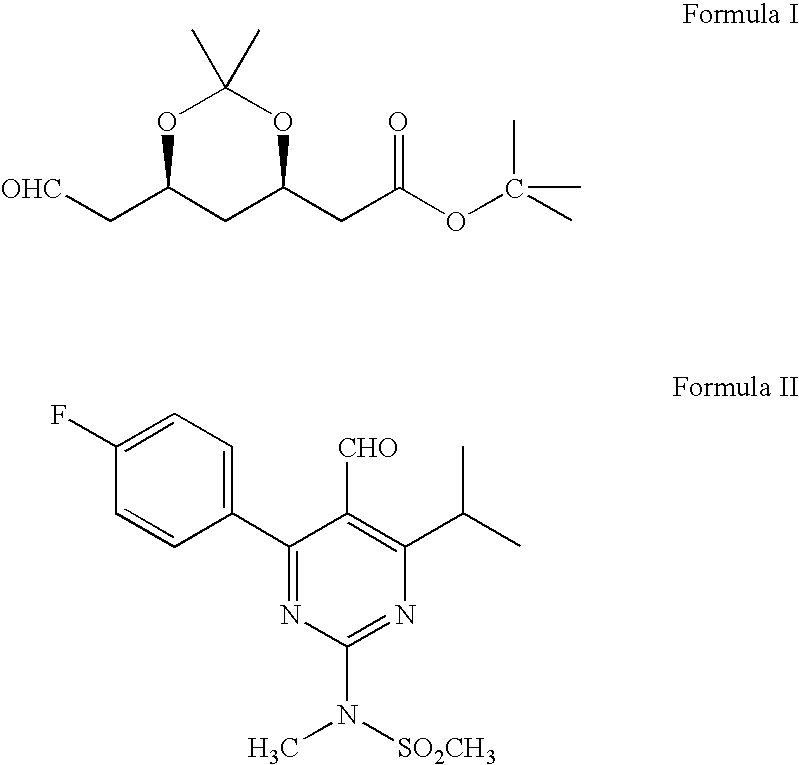
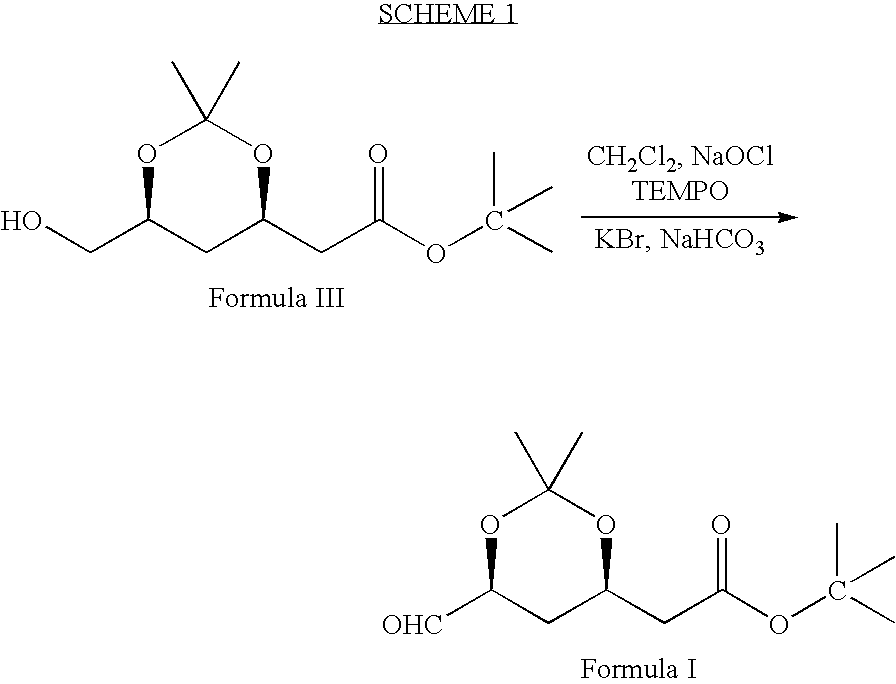
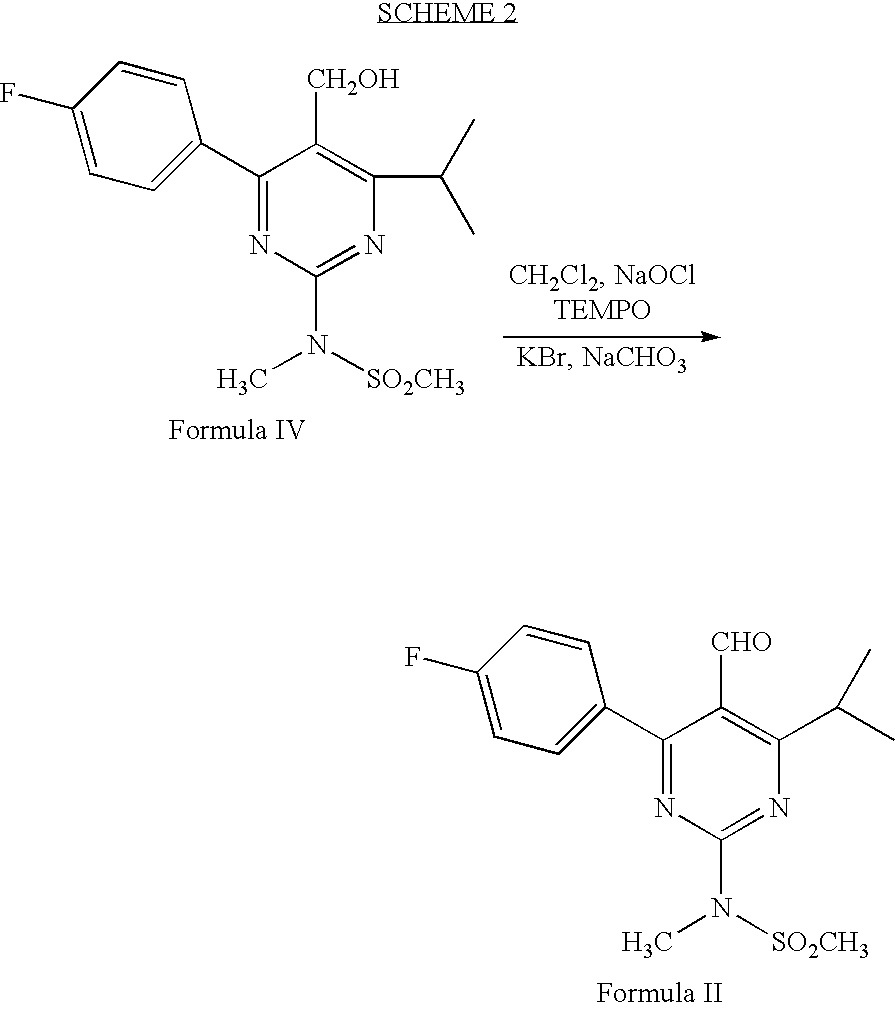
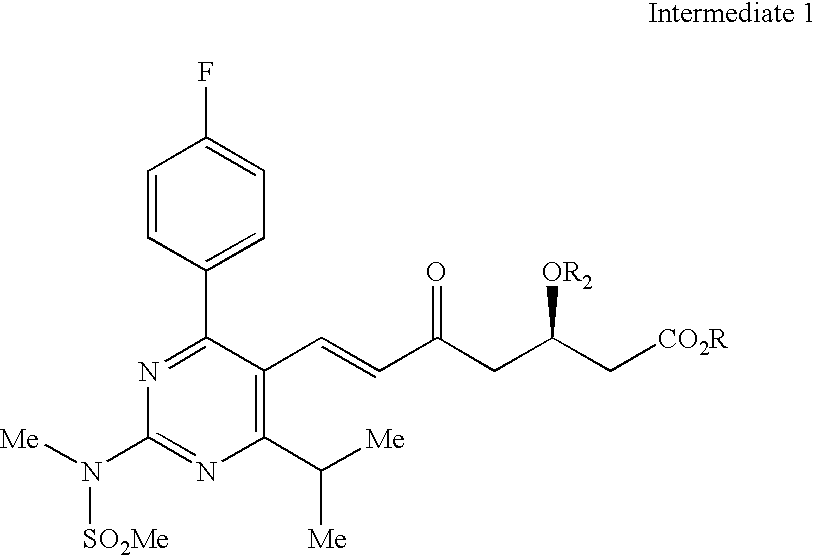
Processes to produce intermediates for rosuvastatin
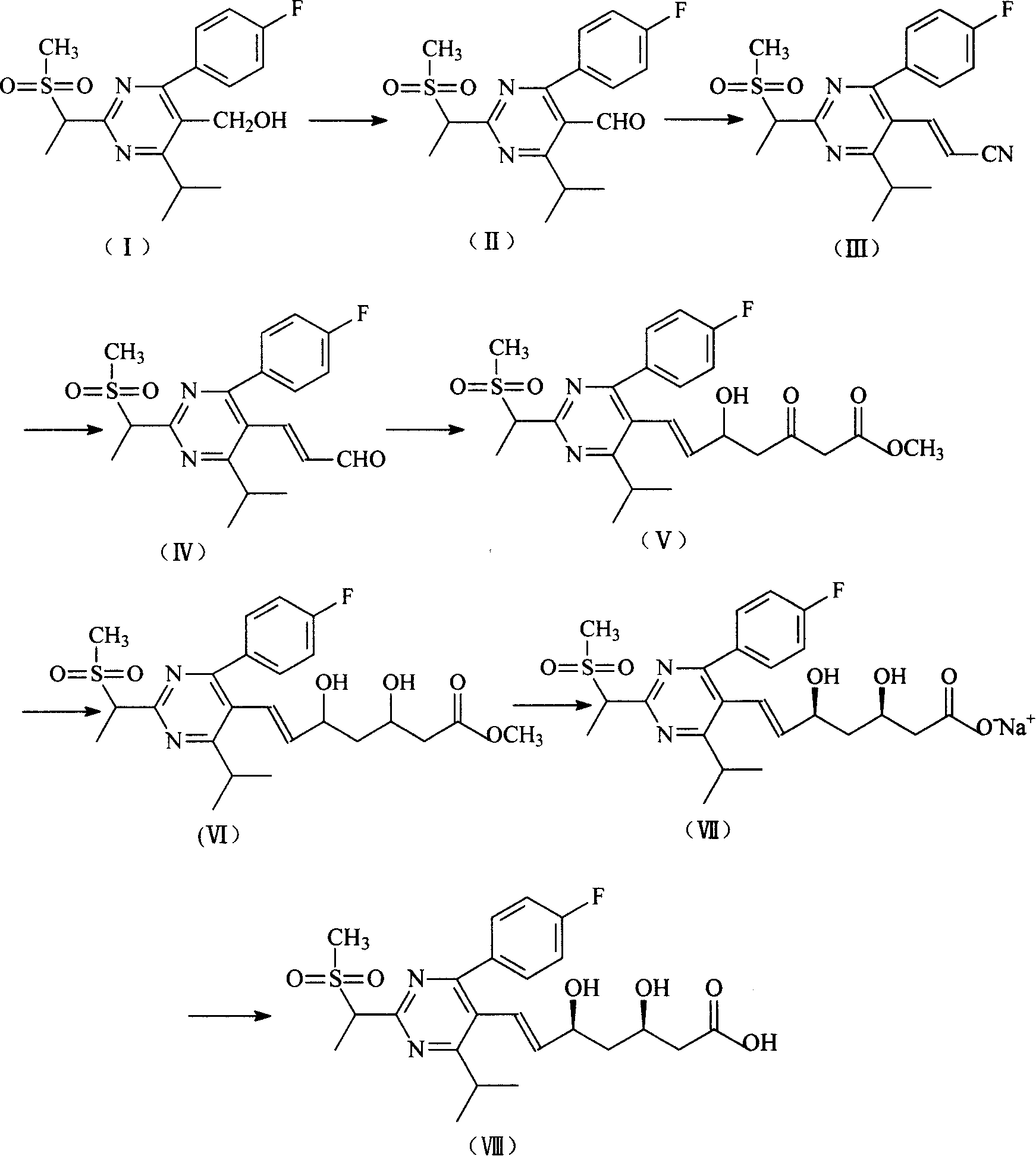
Intermediate compounds for preparing rosuvastatin are prepared by a process comprising oxidizing hydroxy groups to aldehyde groups, using sodium hypochlorite and 2,2,6,6-tetramethyl piperidinyl oxy free radical (TEMPO) as a catalyst.
Owner:DR REDDYS LAB LTD +1
Compositions comprising fenofibrate and rosuvastatin
InactiveUS20050096391A1Improve bioavailability in vivoSubstance may accumulateBiocidePill deliveryHMG-CoA reductasePharmaceutical medicine
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of fenofibrate and the HMG CoA reductase inhibitor rosuvastatin or a pharmaceutically active salt thereof, which upon oral administration provides a relative AUC0-24 value (AUCfibric acid / AUCrosuvastatin) of between about 150 and about 12,000. The solid compositions are manufactured without any need of addition of water or aqueous medium and comprise at least 80% of the active substances fenofibrate and rosuvastatin in dissolved form, or, optionally, atorvastatin in micronized form, in order to ensure suitable bioavailability.
Owner:LIFECYCLE PHARMA AS
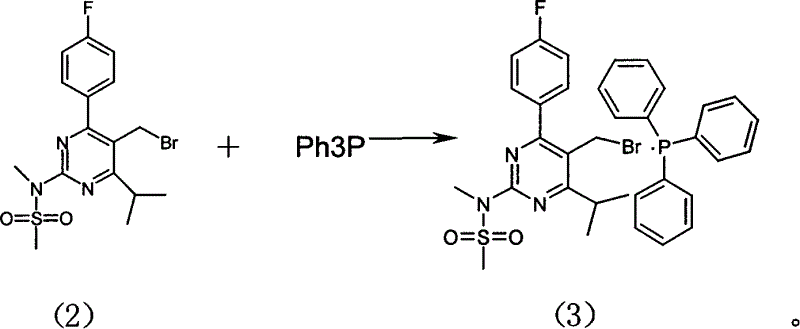
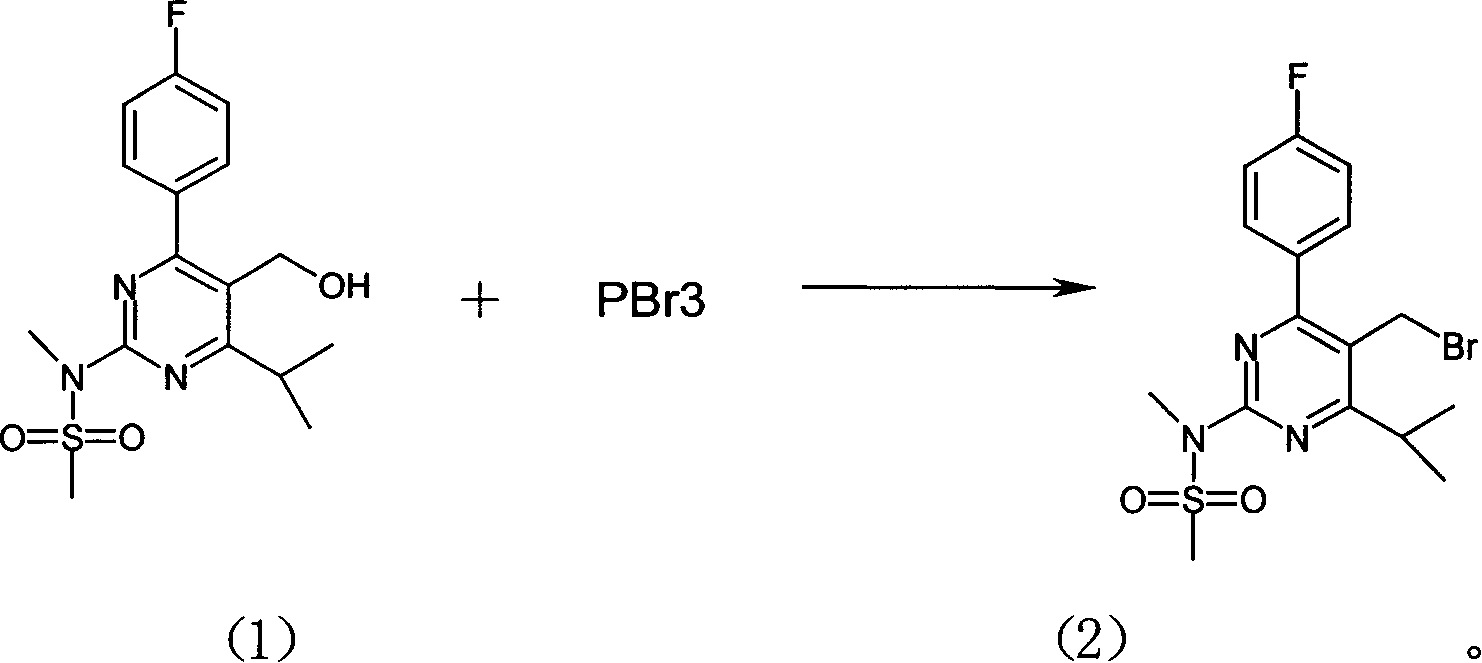
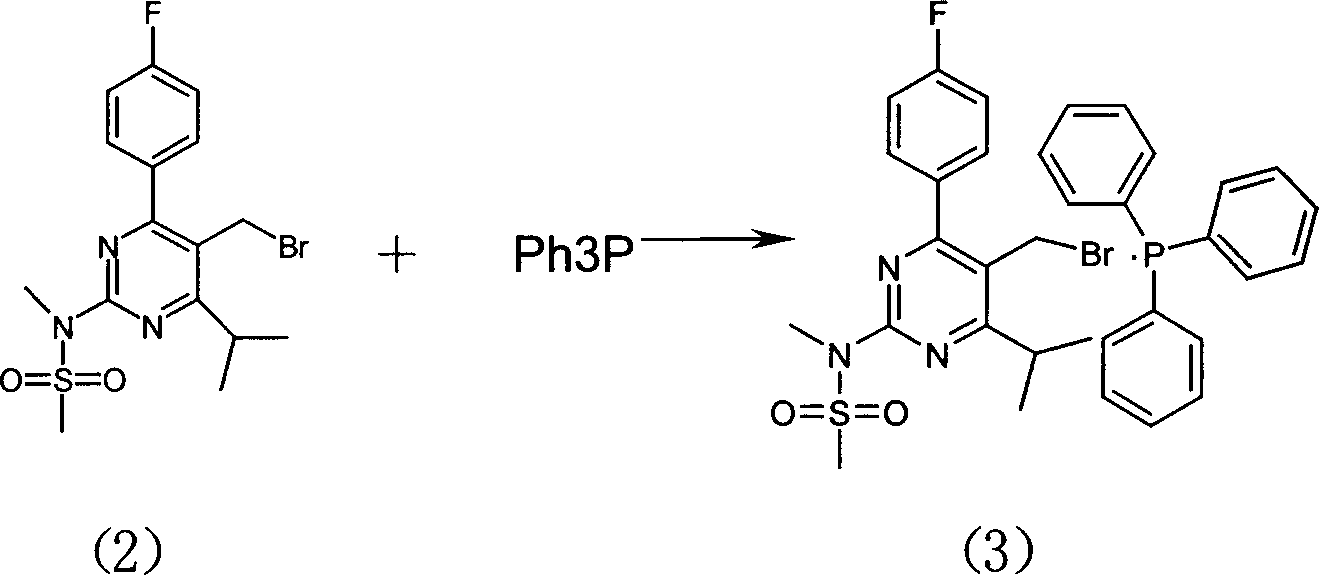
Method for preparing Rosuvastain and its intermediate
The present invention relates to a preparation method of medicine for reducing blood-lipid-Reshufatadine compound. Said method uses side chain aldehyde compound and triphenyl phosphorus ylide reagent compound and makes them undergo the condensation reaction so as to obtain Reshufatadine compound with high yield. Said invention also provides its key intermediate compound.
Owner:ZHEJIANG HISUN PHARMA CO LTD
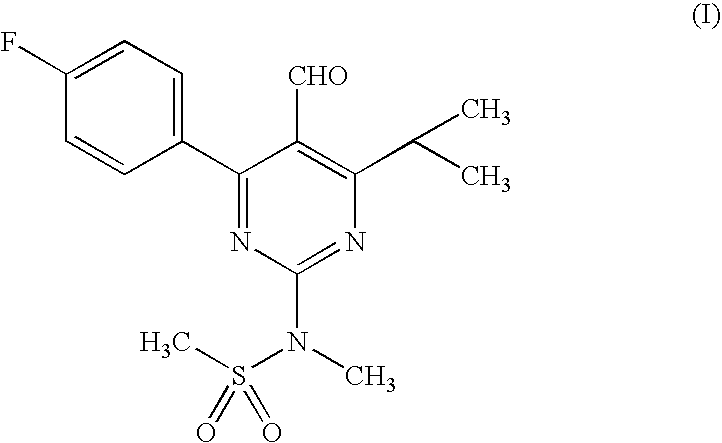
Process for the preparation of rosuvastatin
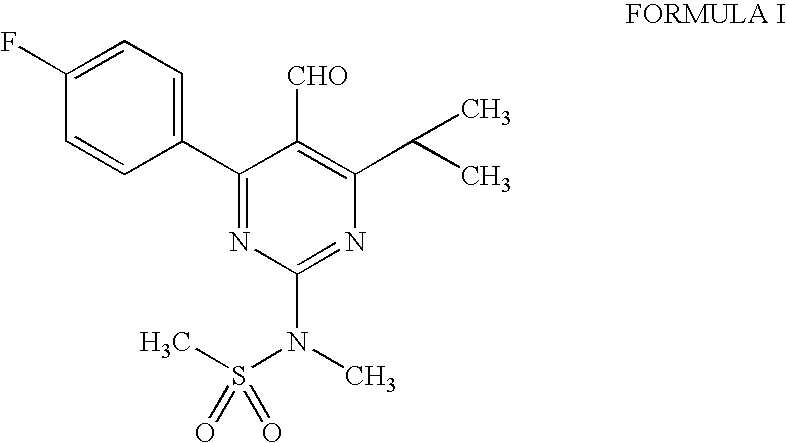
The present invention relates to a cost effective and industrially advantageous process for the preparation of 4-4(fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulphonylamino)-5-pyrimidinecarboxaldehyde, referred to here as pyrimidine aldehyde of structural Formula I and to the use of this compound as intermediate for the preparation of rosuvastatin.
Owner:RANBAXY LAB LTD
Preparation method and intermediate of rosuvastatin and its pharmaceutical salts
The present invention discloses new preparation process of (E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfothio) amino] pyrimidyl-5-radical] -(3R.5S)-3,5-dihydroxy heptyl-6-olefine acid (Rosuvastatin) and its medicinal salt, and new intermediate. The preparation process has short period, less reaction steps, high yield and low cost.
Owner:四川抗菌素工业研究所有限公司
Pharmaceutical formulations for carrier-mediated transport statins and uses thereof
InactiveUS20050119331A1Improve bioavailabilityMechanism is preventedBiocideOrganic chemistryLipid formationCholesterol
The present invention relates to formulations comprising therapeutically effective amounts of at least one acid-stable, carrier-mediated transport statin, at least one poorly water-soluble, carrier-mediated transport statin, or at least one large molecular weight, carrier-mediated transport statin, such as atorvastatin and rosuvastatin, or a pharmaceutically acceptable salt thereof, and methods of their use. The present formulations and methods are designed to exhibit a controlled-release of a therapeutic amount of the statin in the small intestine, thereby limiting systemic exposure of the statin and maximizing liver-specific absorption of the drug. The formulations and methods of the present invention are particularly useful for treating and / or preventing conditions that are benefited by decreasing levels of lipids and / or cholesterol in the body.
Owner:CIRC PHARM RES & DEV LTD
Kit for simultaneously detecting SLCO1B1, APOE and LDLR gene multisite mutation
The invention belongs to the technical field of gene mutation detection, and concretely discloses a kit for simultaneously detecting SLCO1B1, APOE and LDLR gene multisite mutation. Through meticulous design, multi-time verification, screening and optimization, specific primers and probes based on a Taqman allelic gene resolution analysis method are obtained; eight functional variation of the SLCO1B1, APOE and LDLR genes can be detected; the time from DNA (Deoxyribonucleic Acid) extraction to fluorescent PCR (Polymerase Chain Reaction) to result obtaining is less than four hours; and the manual operation time is less than two hours. The kit comprising the primer pairs and the probe pairs has the advantages that the time is saved; convenience is realized; the sensitivity is high; and both the positive conformity rate and the negative conformity rate of a sample are higher than 99 percent, and the like. A detection method provided by the invention is mainly used for the personalized medication auxiliary diagnosis of statins such as simvastatin, atorvastatin, fluvastatin and rosuvastatin.
Owner:钟诗龙
Solid preparation containing rosuvastain calcium liposome
InactiveCN102028658AImprove efficacyImprove bioavailabilityOrganic active ingredientsPill deliveryCholesterolSOY LECITHIN
The invention discloses a solid preparation containing rosuvastain calcium liposome and particularly relates to the solid preparation containing rosuvastain calcium liposome and a preparation method thereof. The rosuvastain calcium liposome mainly comprises 1 part of rosuvastain calcium, 2-25 parts of soyabean lecithin, 0.5-10 parts of cholesterol, 0.5-12 parts of tween 80 and 0.05-10 parts of sodium desoxycholate. The rosuvastain calcium solid preparation prepared from the liposome not only increases the dissolution, but also improves the stability and the bioavilability. The quality of preparation products is improved and the toxic or side effects are reduced.
Owner:HAINAN MEIDA PHARMA
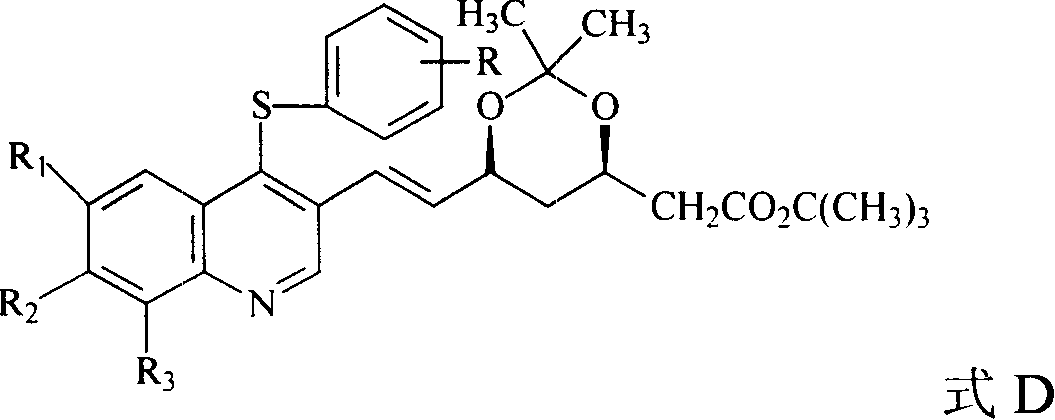
Quinolines compounds and their intermediates, preparation method and application
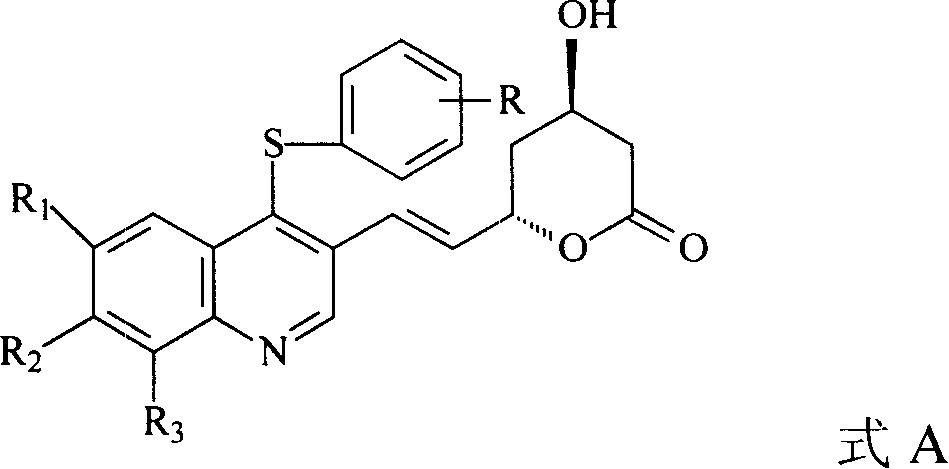
The invention discloses a quinolinic compound showed in the formula A and pharmacy acceptable solvate, optical isomers or polymorphic substance and a reaction intermediate compound showed in the formula D; wherein, R1, R2 and R3 are respective and independent H, halogen or group showed in the formula H; wherein, the R is H, the halogen, alkyl of C1-C4 or alkoxide of C1-C4. The invention further discloses a preparation method thereof and application for preparing medicines for inhibiting HMG CoA reductase and treating hyperlipemia related diseases. Compared with the existing fuvastatin, rosuvastatin and pitavastatin in the prior art, the quinolinic compound of the invention can better inhibiting the activity of the HMG CoA reductase and can be used for treating hyperlipemia related diseases.
Owner:SHANGHAI INST OF PHARMA IND
Novel boronate esters
The present invention relates to optically active boronate derivatives which are useful as intermediates for the synthesis of HMG-CoA enzyme inhibitors such as atorvastatin, cerivastatin, rosuvastatin, pitavastatin, and fluvastatin.
Owner:BIOCON LTD
Rosuvastatin intermediates and process for the preparation of rosuvastatin
Owner:TEVA PHARM USA INC
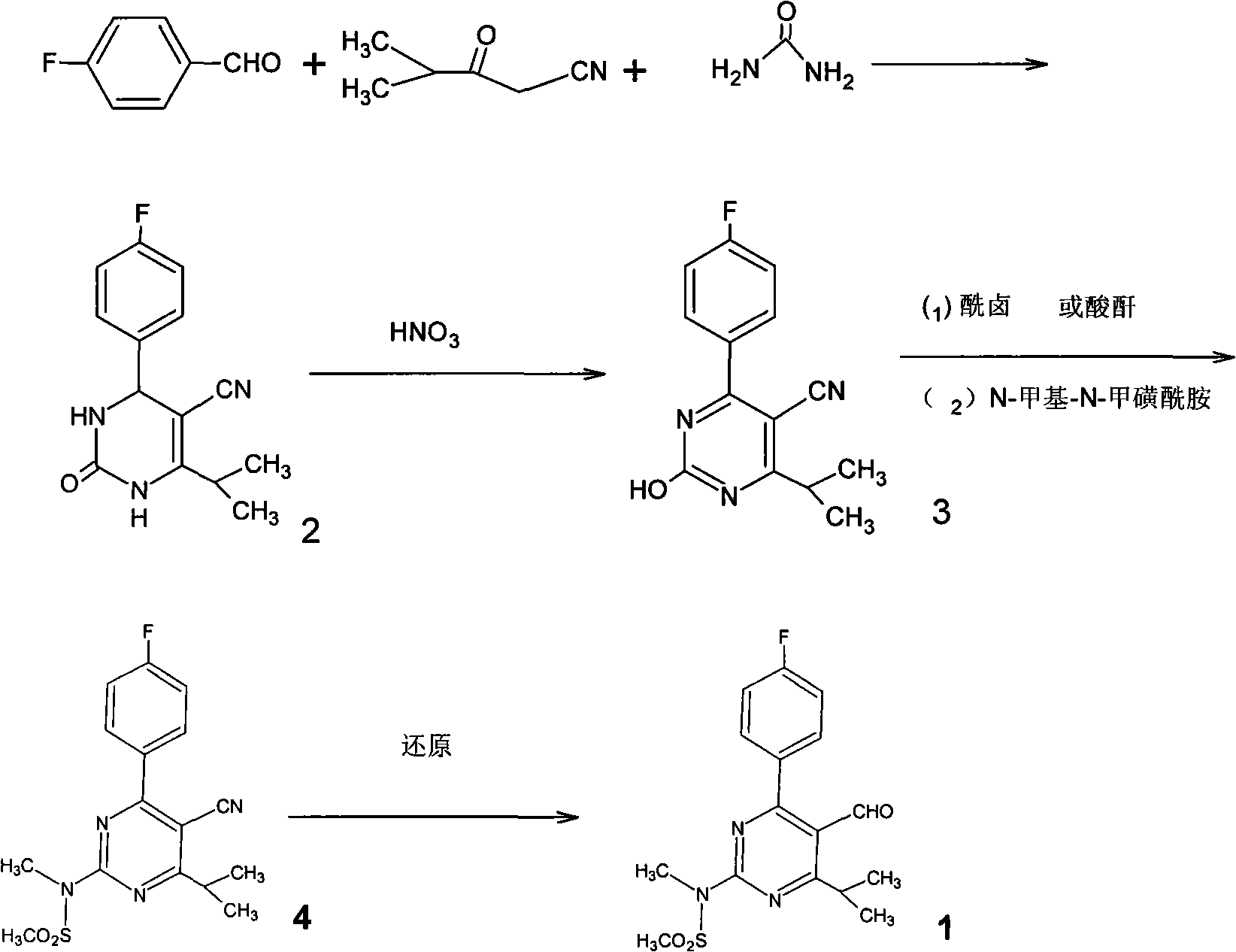
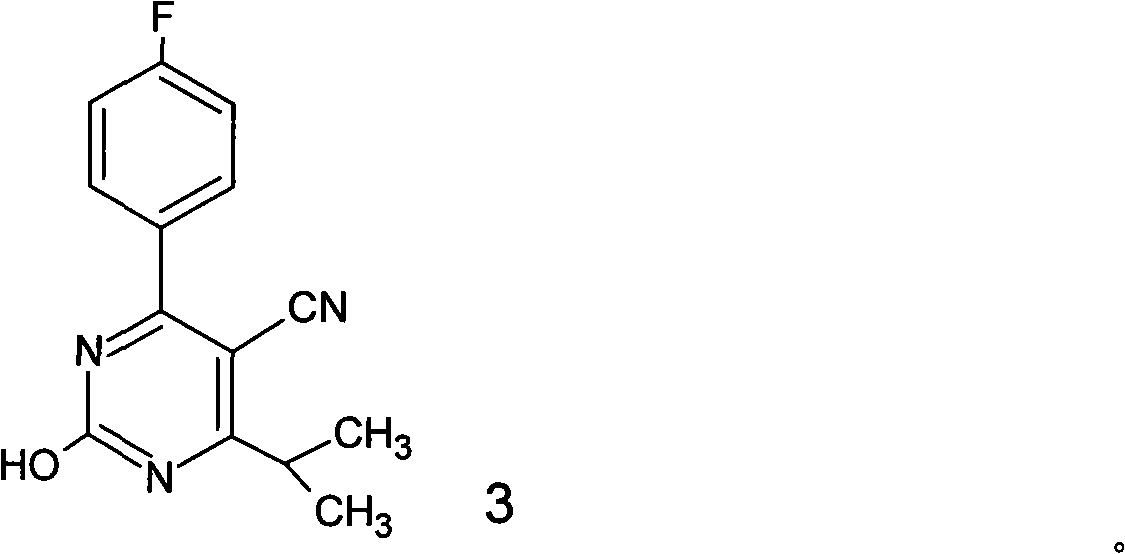
Preparation of 4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonyl amido) pyrimidine-5-formaldehyde
The invention provides a method for preparing a Rosuvastatin intermediate, 4-Fluorophenyl-6-isopropyl-2-(N-methyl-N-methylsulfonyl amino) pyrimidinyl-5-formyl. The method uses isobutyryl acetonitrile, 4-Fluorobenzaldehyde and carbamide as raw materials and is obtained by steps of cyclization, oxidation, substitution and reduction etc. The method of the invention dose not need expensive raw materials and has the advantages of low technological cost, simple reaction, high product yield and being applicable to industrialized production.
Owner:滁州市庆云医药有限公司
Composite formulation comprising a film coating layer containing rosuvastatin or a pharmaceutically acceptable salt thereof
InactiveUS20160045444A1Avoid high breakageReduce tensionOrganic active ingredientsBiocidePolyethylene glycolPVA - Polyvinyl alcohol
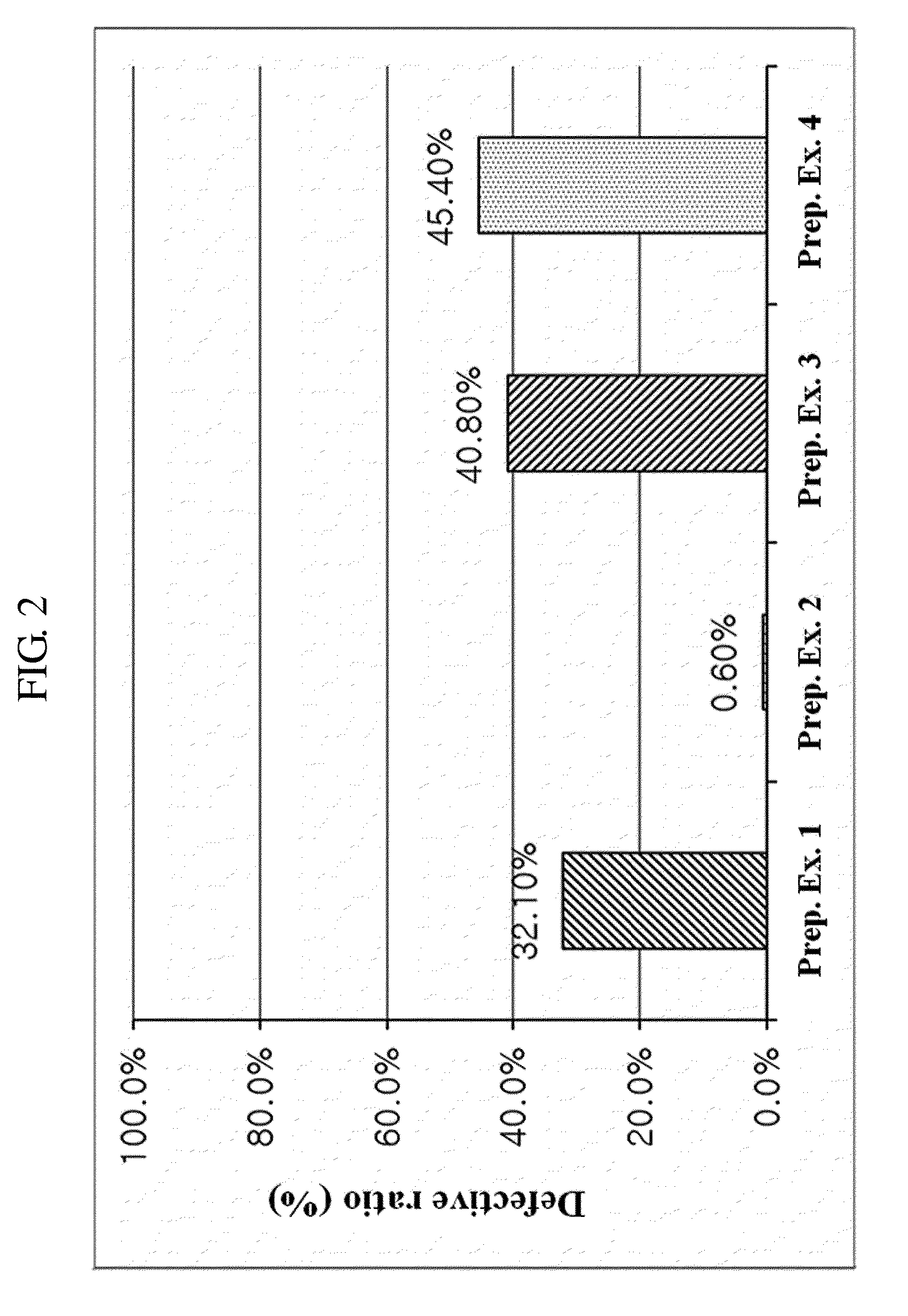
Provided is a composite formulation comprising: (i) a core including a first pharmacological component; and (ii) a film coating layer formed on a surface of the core, which contains rosuvastatin or a pharmaceutically acceptable salt thereof as a second pharmacological component and a polyvinyl alcohol-polyethylene glycol graft copolymer and polyvinyl alcohol as a coating material. In the composite formulation of the present invention, tension and fluidity of the film coating layer are excellent, and thus breakage and a defective ratio are low. Accordingly, a composite agent containing rosuvastatin effective to relieve and treat a hyperlipemia symptom, or its pharmaceutically acceptable salt can be provided with high efficiency, and is present in a composite formulation form, and thus compliance of a patient can be improved.
Owner:HANMI PHARMA
Pharmaceutical composition and purpose thereof
ActiveCN102485228AImprove protectionReduce incidenceOrganic active ingredientsPill deliverySide effectActive component
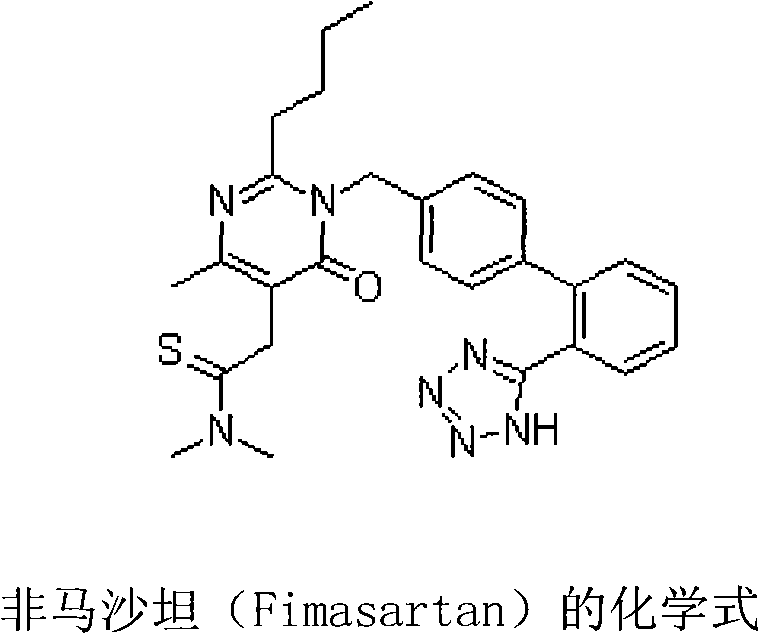
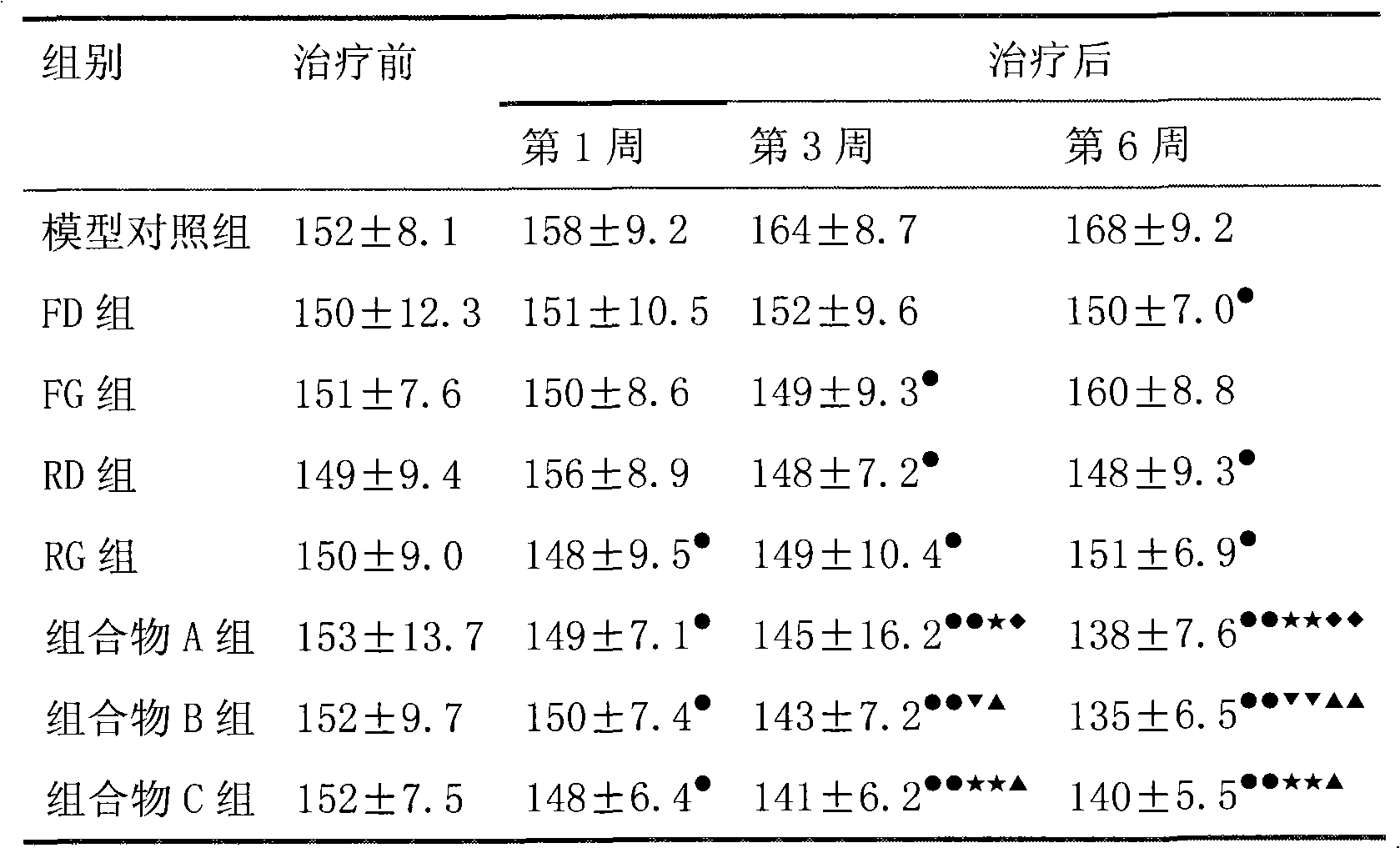
The invention belongs to the filed of medicine and discloses a pharmaceutical composition using fimasartan and rosuvastatin or medicinal salt thereof as active components, wherein a mass ratio of rosuvastatin or medicinal salt thereof to fimasartan is 1:0.25-250 and preferably 1:10-100. The pharmaceutical composition containing rosuvastatin medicinal salt thereof and fimasartan has obvious synergism on treating hypertension and complications thereof, low treatment cost, little side-effect and good clinic application prospect.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for preparing rosuvastatin intermediate
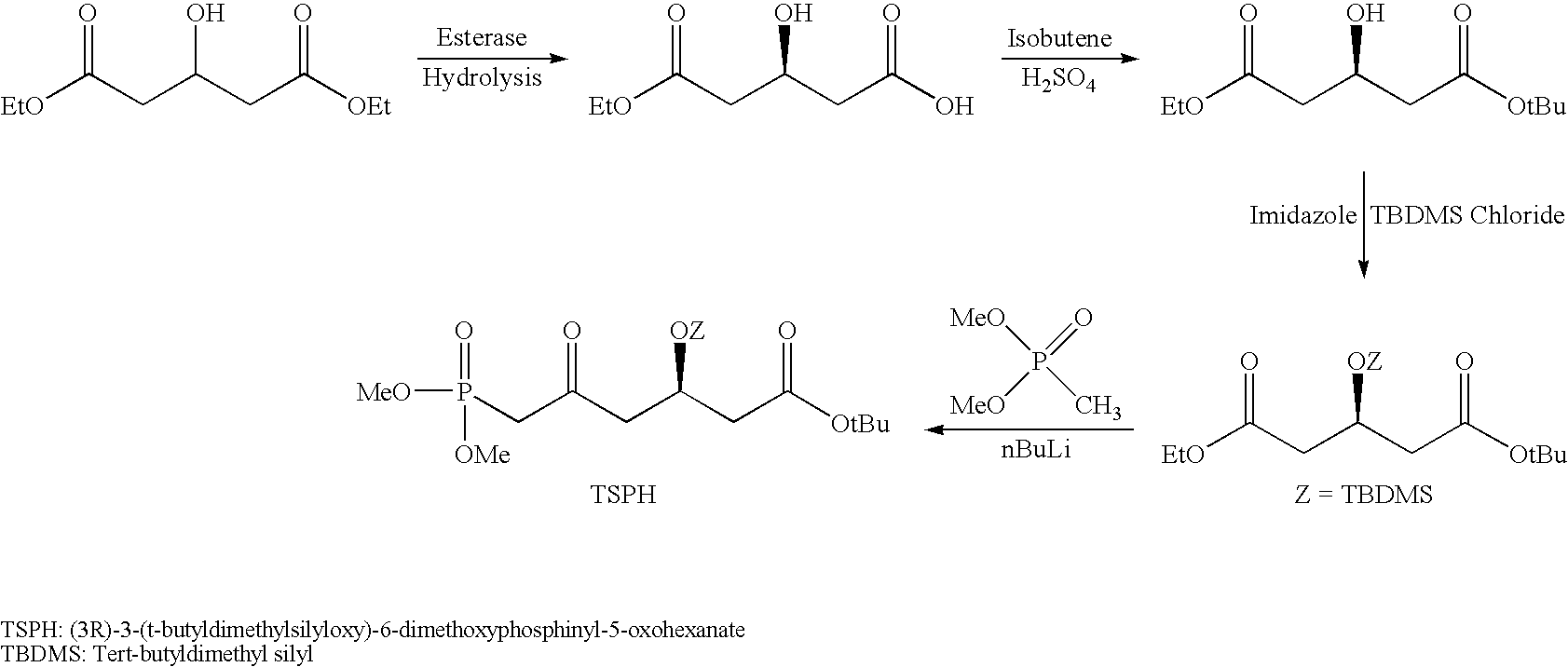
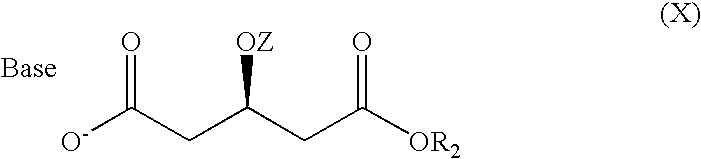
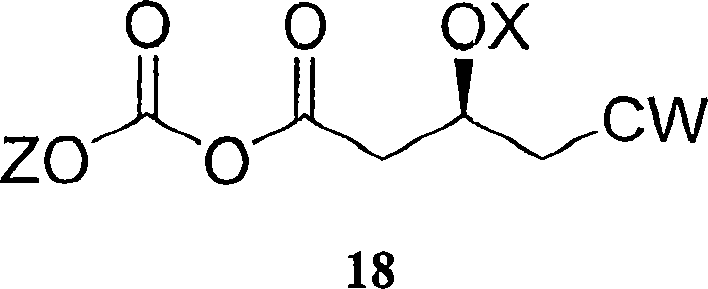
ActiveCN103361386ASolve the problem of low concentration and low conversion rateIncrease concentrationHydrolasesMicroorganism based processesHydroxybutyric acidSubstrate concentration
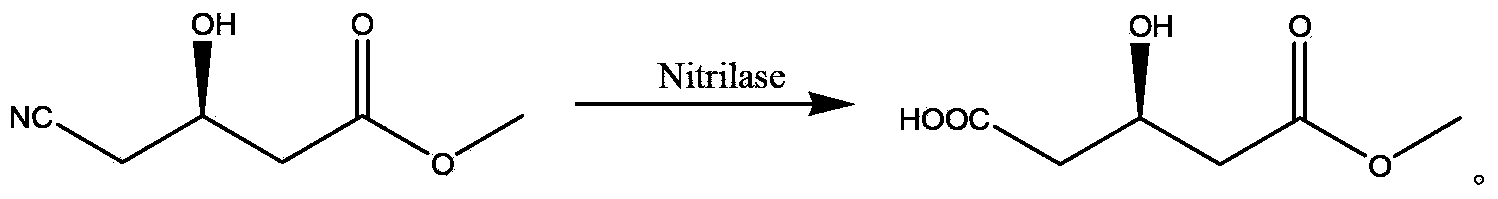
The invention relates to a method for preparing a rosuvastatin intermediate which is (R)-3-hydroxy-monomethyl glutarate and hydroxyl-protected (R)-3-hydroxy- monomethyl glutarate. According to the method, (R)-4-cyano-3-hydroxy-methyl butyrate which servers as a substrate is subjected to reaction under the action of an enzyme to generate the (R)-3-hydroxy- monomethyl glutarate, and particularly the enzyme is the recombinant nitrilase, and the reaction is carried out in a aqueous-phase buffering solution with the pH value of 7.0-9.0 at the temperature of 25-35 DEG C. Because the specific recombinant nitrilase is adopted by the method, the problems of ultralow concentration of the substrate and low conversion rate in the existing method for preparing the rosuvastatin intermediate through biological catalysis are solved. The method has the characteristics of mild reaction conditions and high reaction efficiency, is easy and convenient to operate and has important industrial application value.
Owner:CODEXIS INC
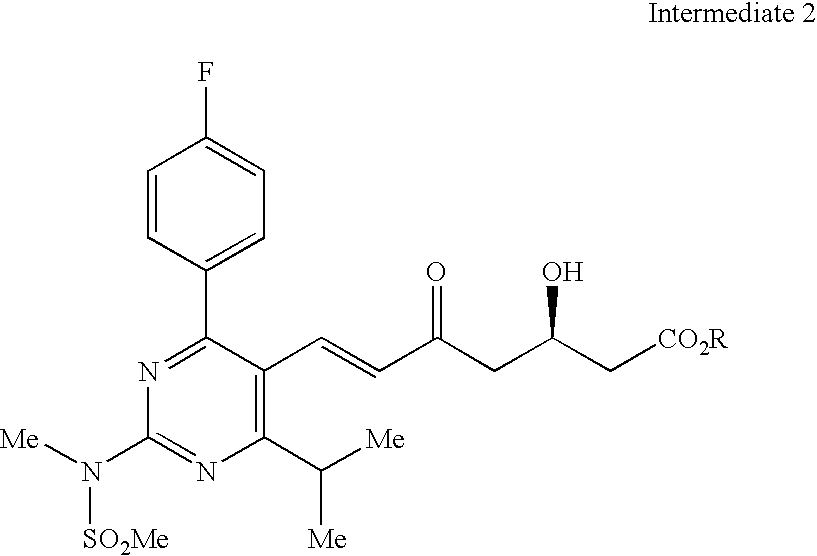
Processes to produce intermediates for rosuvastatin
Intermediate compounds for preparing rosuvastatin are prepared by a process comprising oxidizing hydroxy groups to aldehyde groups, using sodium hypochlorite and 2,2,6,6-tetramethyl piperidinyl oxy free radical (TEMPO) as a catalyst.
Owner:DR REDDYS LAB LTD +1
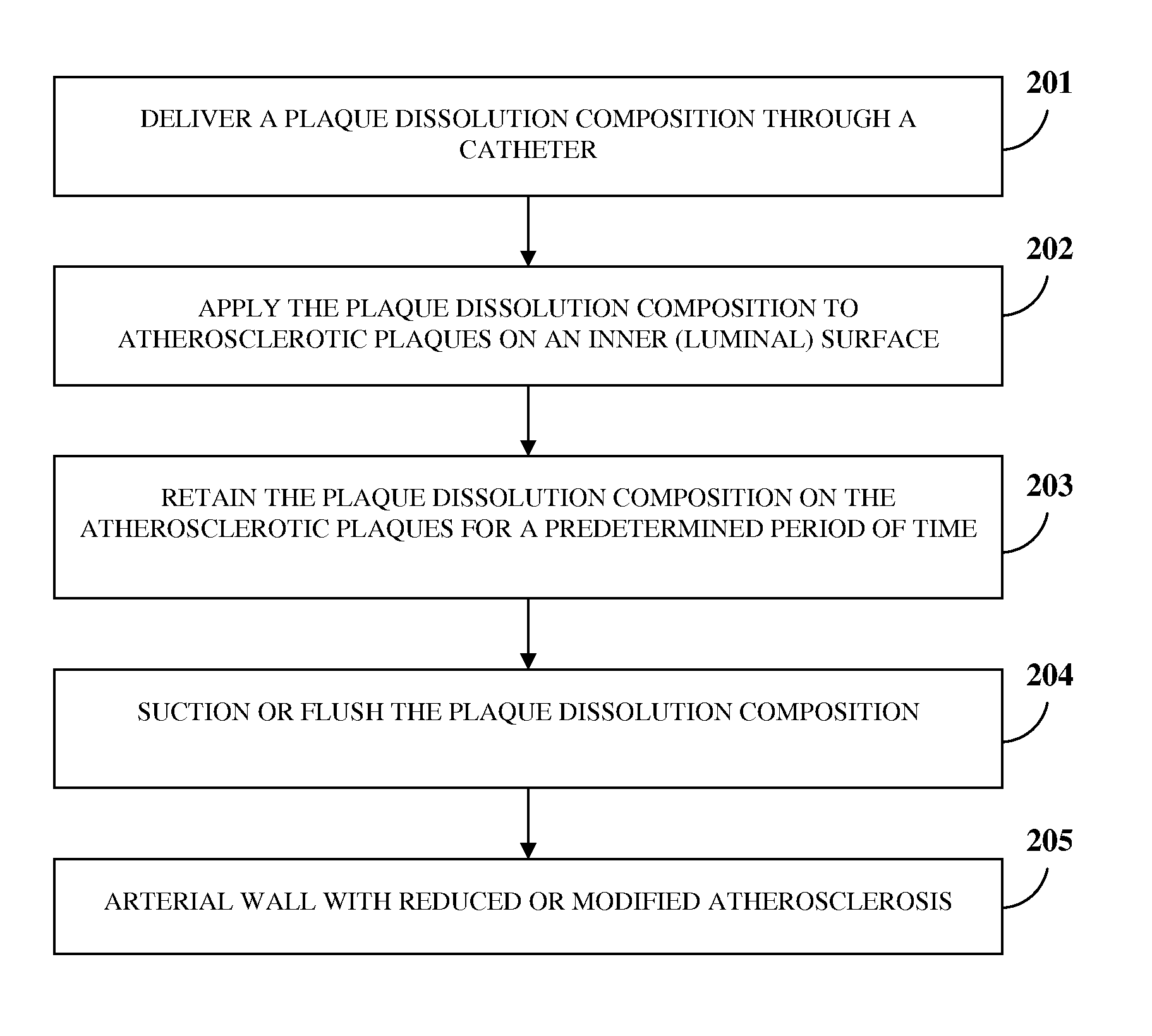
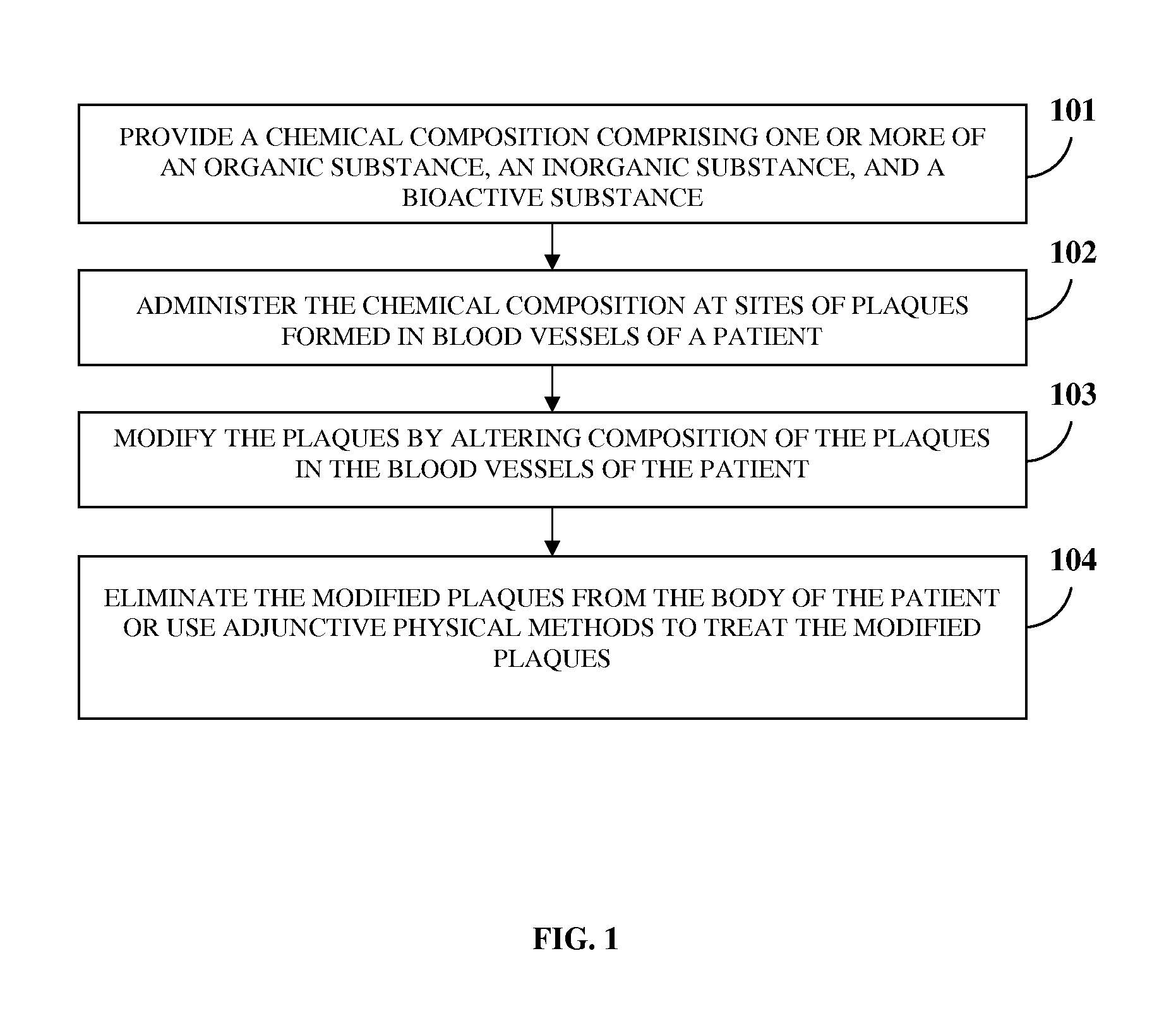
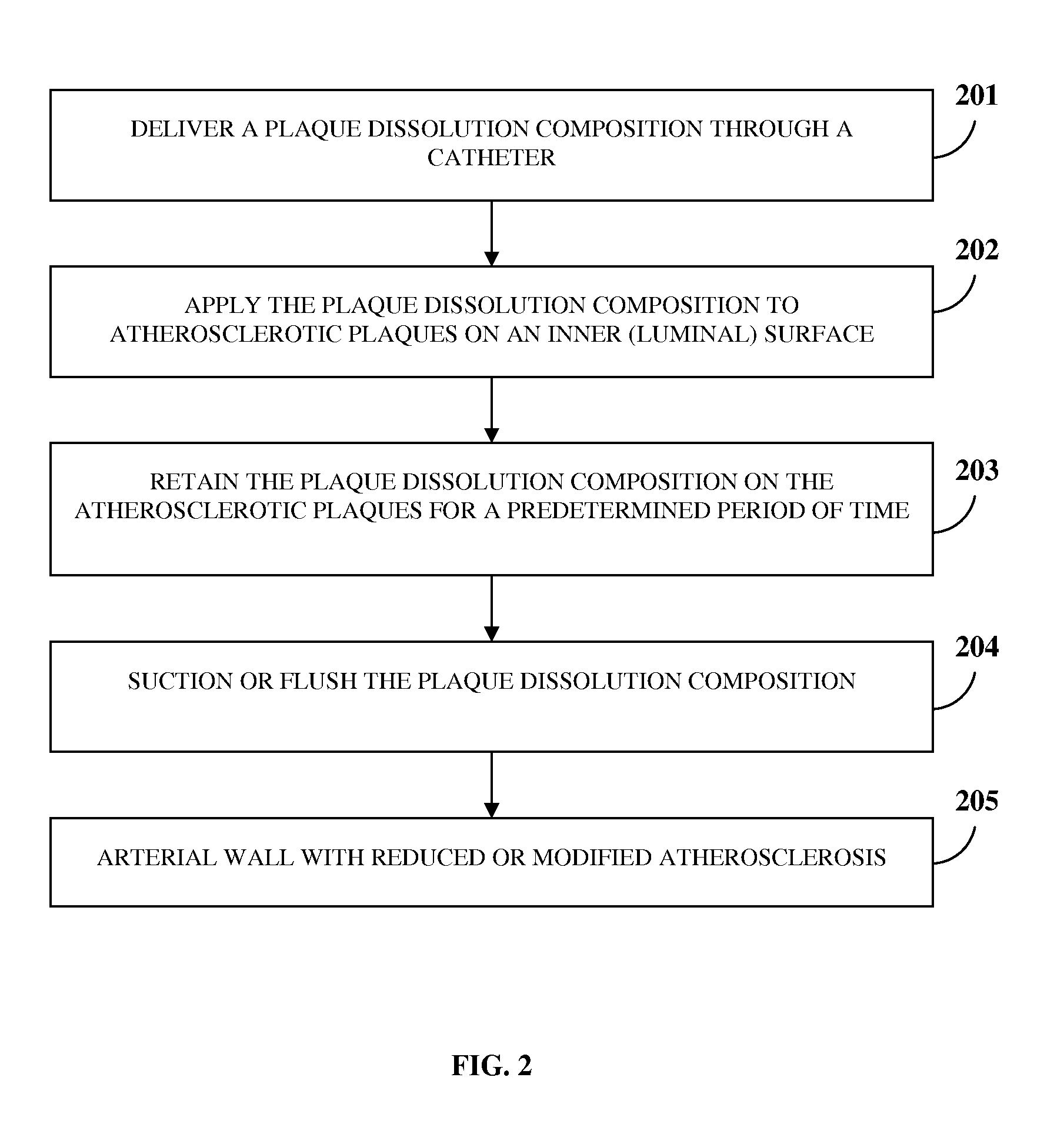
Atherosclerotic Plaque Dissolution Composition
InactiveUS20110196383A1High densityEasy to disassembleBiocideHalogenated hydrocarbon active ingredientsOctanoic AcidsBioactive substance
A method and chemical composition for modifying and dissolving atherosclerotic plaques formed in a patient's blood vessels is provided. A chemical composition comprising one or more of an organic substance, an inorganic substance, and a bioactive substance is administered at sites of the plaques. The chemical composition comprises one or more of d-limonene, propylene glycol, octanoic acid, 2-octane, glycerine, acetylsalicylic acid, acetic acid, omega-3 fatty acids, ethanol, methanol, ezetimibe, rosuvastatin, resveratrol, lactic acid, gluconic acid, chloroform, carbon disulfide, dichloromethane, toluene, lauryldimethyl hydroxysultaine, and any combination thereof. The chemical composition enables modification of plaques by altering their composition. The modification comprises partial dissolution, complete dissolution, or elimination of the plaques, and makes the plaques amenable to different forms of plaque treatment. The modified plaques are eliminated from the patient's body. The modification or elimination of the plaques facilitates treatment of cardiovascular diseases caused due to plaques formed in the blood vessels.
Owner:ATHEROLYSIS MEDICAL
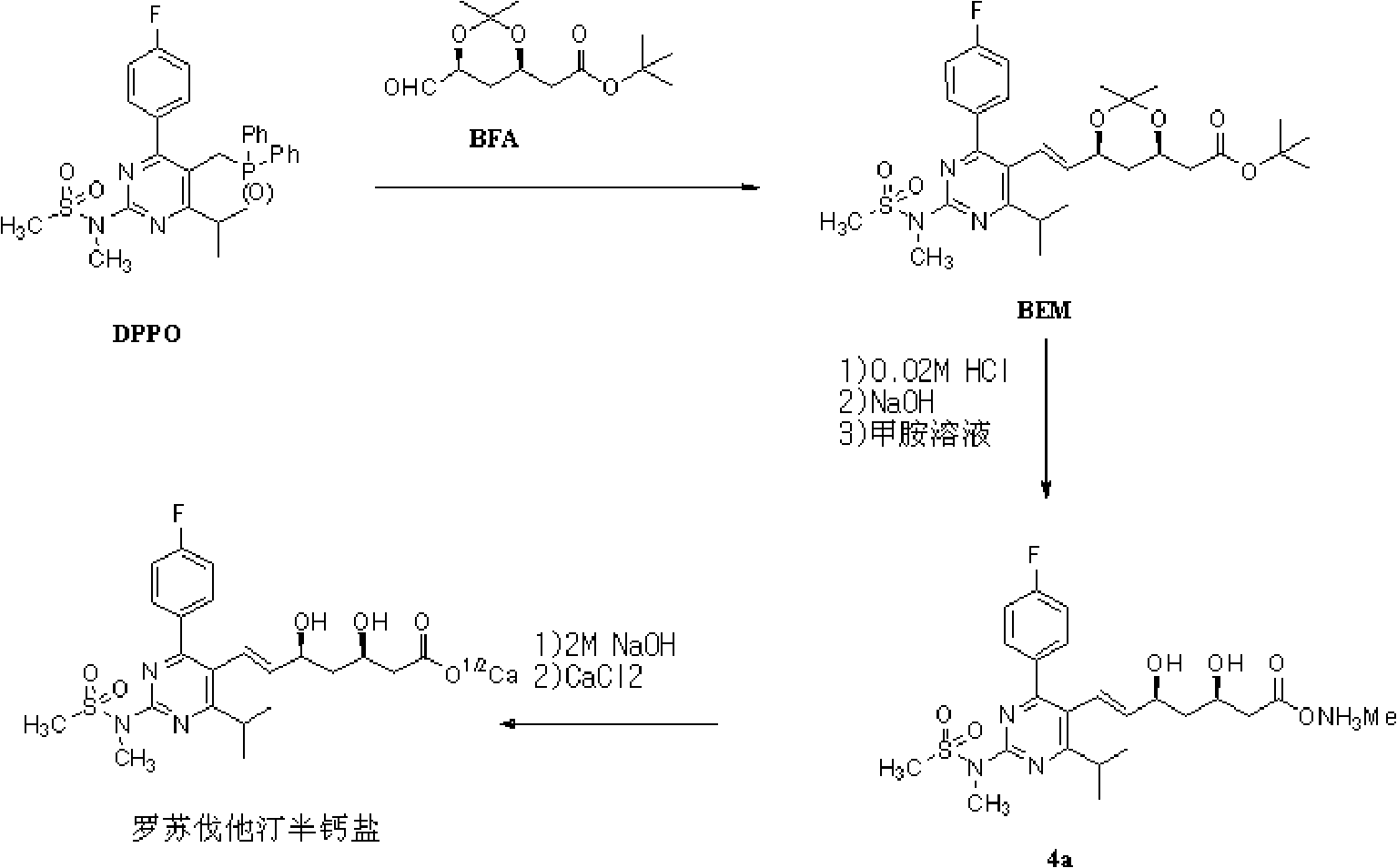
Novel method for preparing rosuvastatin, intermediate compounds useful for preparing same, and method for preparing same
InactiveCN102459196AHigh yieldOrganic active ingredientsMetabolism disorderMedicinal chemistryCalcium salts
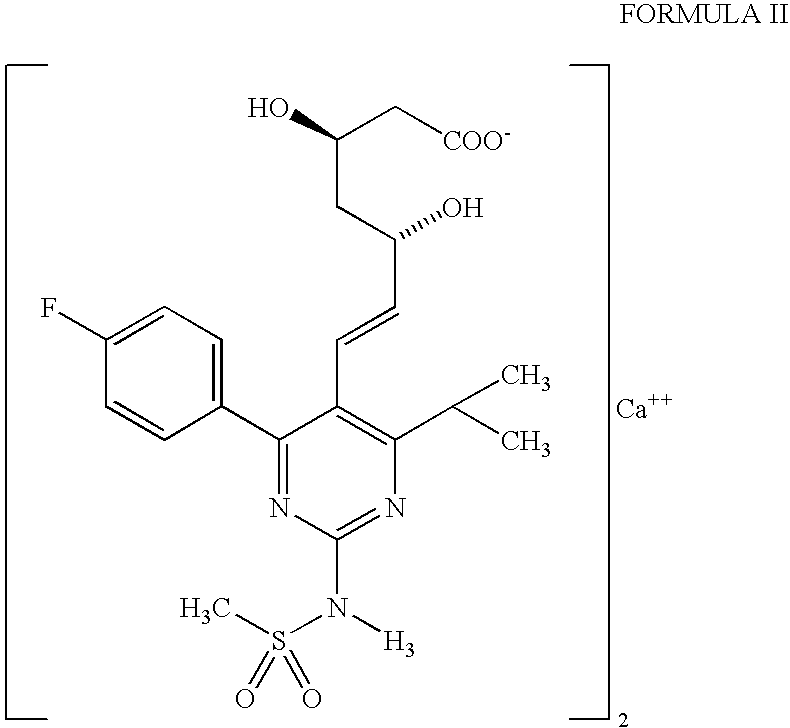
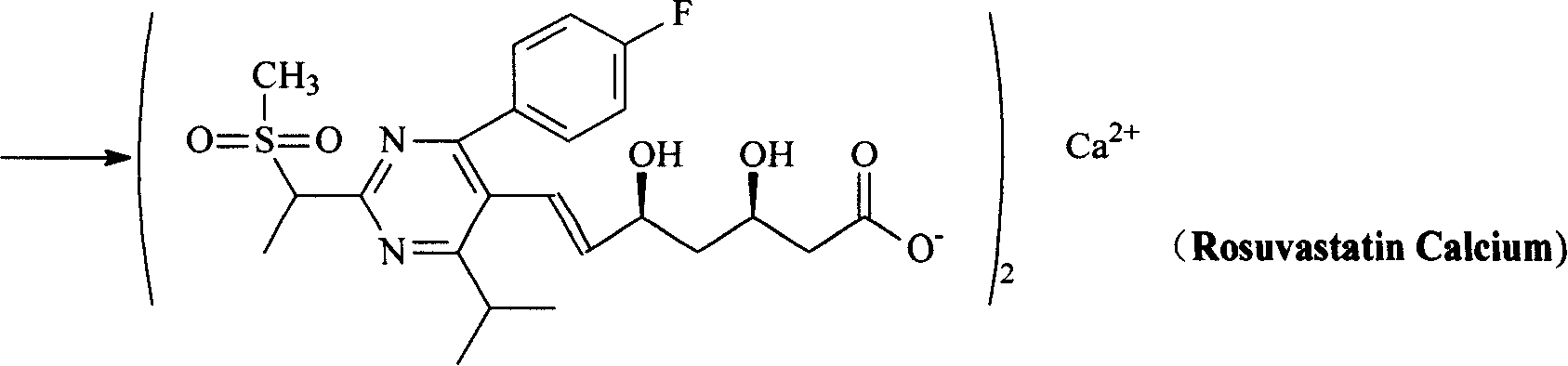
The present invention relates to novel intermediate compounds used in preparing Rosuvastatin or the pharmaceutically acceptable salts thereof, to a method for preparing same, and to a method for preparing Rosuvastatin or the pharmaceutically acceptable salts thereof from the intermediates. The preparation method of the present invention has the effect of providing Rosuvastatin hemi-calcium salts with an excellent yield rate.
Owner:CHONG KUN DONG CORP
Rosuvastatin azelnidipine composition
InactiveCN101336921ALower blood pressureLower blood lipid levelsOrganic active ingredientsMetabolism disorderOrganic acidOrganic base
A pharmaceutical composition is an organic salt formed by an organic acid rosuvastatin and an organic base azelnidipine with a molar ratio of 1:1. The pharmaceutical composition can be made into any dosage forms together with a pharmaceutically-acceptable carrier for reducing blood pressure and blood lipid, reducing myocardial infarction and treating cerebral apoplexy. The inventive pharmaceutical composition has the advantages of stable physiochemical indexes, controllable product quality, and convenient administration.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Double-layer tablet containing ezetimibe and rosuvastatin and preparation method thereof
ActiveCN103585157AImprove the quality of lifeReduce adverse reactionsOrganic active ingredientsMetabolism disorderSolubilityAdditive ingredient
The invention relates to a double-layer tablet which contains ezetimibe and rosuvastatin serving as effective components and has a lipid-lowering effect and a preparation method thereof. Aiming at the problems poor water solubility of ezetimibe, instability of rosuvastatin to acid and oxygen and the like, the method adopts a micronization technology to improve the dissolution rate of ezetimibe and adopts an anti-oxidizing agent and a double-layer tabletting technology to improve the stability of rosuvastatin in a human body, further, ingredients of a medicine is enabled to take effect sufficiently, and the best synergistic effect is achieved; and a compound preparation agent is mainly applied to treatment of diseases relative to hypercholesteremia.
Owner:WUHAN WUYAO SCI & TECH
Pharmaceutical composition containing calcium blocker, AII receptor blocker and statins
InactiveCN101618215AReduce morbidityImprove complianceSenses disorderMetabolism disorderCandesartanLacidipine
The invention relates to a pharmaceutical composition containing a calcium channel blocker (CCB) or the mixture thereof, an angiotonin 3II receptor blocker (ARB) or the mixture thereof, statins or the mixture thereof and a pharmaceutically acceptable carrier, wherein the CCB is selected from l-amlodipine, amlodipine, lacidipine, nitrendipine or the mixture thereof; the angiotonin II receptor blocker is selected from telmisartan, losartan, irbesartan, candesartan or the mixture thereof; and the statins are selected from atorvastatin, simvastatin, ruishufatadine, fluvastatin or the mixture thereof. The pharmaceutical composition is used for treating various high blood pressures and preventing or treating cardiovascular and cerebrovascular diseases relevant to the hypertension, reduces the disease rate and / or mortality rate of the cardiovascular and cerebrovascular diseases and also improves the adaptability for a sufferer taking medicine.
Owner:王丽燕
Preparation method of rosuvastatin intermediate and intermediate compound
InactiveCN104059024AAvoid it happening againReduce difficulty of reactionOrganic chemistryThioureaBenzaldehyde
The invention discloses a preparation method of a rosuvastatin intermediate: 4-(4-fluorobenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonyl amino) pyrimidine-5-formonitrile. The method comprises the following steps: carrying out a reaction on ethyl isobutyrate and acetonitrile to generate isobutyryl acetonitrile; and then carrying out cyclization, methylation, reduction, oxidization and substitution on isobutyryl acetonitrile and p-fluoro benzaldehyde as well as thiourea to prepare the intermediate. The 4-(4-fluorobenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonyl amino) pyrimidine-5-formonitrile prepared by the method is low in cost, simple to react, high in yield and easy to realize industrialized production.
Owner:ZHEJIANG UNIV
Therapeutic compositions containing amlodipine niacin and rosuvastatin medicament
The invention relates to a pharmaceutical composition containing amlodipine besylate, and a statins compound or a pharmaceutically acceptable salt thereof, a preparation method and a medicine box for drug combination of the medicine composition. The composition comprises the following components: a) a certain amount of the amlodipine besylate; b) a certain amount of one or more statins compounds or pharmaceutically acceptable salts thereof; and c) a pharmaceutically acceptable carrier or a diluting agent. The composition or the medicine box can be used for treating patients suffering from angina pectoris, atherosclerosis and / or accompanied hypertension and hyperlipoidemia, and treating patients (including human beings) having heart risky symptoms.
Owner:BEIJING ROCK PHARMA
Rosuvastatin and salts thereof free of rosuvastatin alkylether and a process for the preparation thereof
InactiveUS20060258882A1Lower Level RequirementsGroup 4/14 element organic compoundsOrganic active ingredientsImpurityStereochemistry
The present invention provides rosuvastatin and intermediates thereof having a low level of alkylether impurity and processes for the preparation thereof.
Owner:TEVA PHARM USA INC
Preparation of rosuvastatin
InactiveCN101119975AGroup 4/14 element organic compoundsOrganic active ingredientsImpurityStereochemistry
The present invention provides rosuvastatin and intermediates thereof having a low level of alkylether impurity and processes for the preparation thereof.
Owner:TEVA PHARMA IND LTD
Medicinal composition containing rosuvastatin or pitavastatin and B clan vitamin
ActiveCN1891219AGood curative effectImprove compliancePill deliveryGranular deliveryDiseasePitavastatin
The present invention relates to a medicine composition conlaining resulvatatine or pivatatine compound and vitamins B, in which the resulvatatine or pivatatine content is 1-40 mg and vitamins B content is 0.1-50 mg. Said invention also provides the application of said medicine composition in preparation of medicine for preventing and curing the diseases of atherosclerosis, angiocardiopathy and cerebrovascular disease.
Owner:SHENZHEN AUSA PHARM CO LTD
New method of biologically synthesizing (R)-3-hydroxylglutarate monoester
The invention relates to a new method of biologically synthesizing (R)-3-hydroxylglutarate monoester and more particularly provides a method of preparing the (R)-3-hydroxylglutarate monoester from (S)-4-chloro-3-hydroxybutyrate through halogenohydrin dehalogenase recombinant bacteria and nitrilase recombinant bacteria, or double-enzyme co-expression recombinant bacteria, in a one-pot manner. The (R)-3-hydroxylglutarate monoester is a key intermediate of statin medicines such as fluvastatin, rosuvastatin, pitavastatin and the like. The method is mild in reaction conditions, is free of pollution and is simple in process route.
Owner:TIANJIN INST OF IND BIOTECH CHINESE ACADEMY OF SCI
Process for preparation of rosuvastatin
InactiveUS20090124803A1Commercially viableOrganic active ingredientsBiocideSide chainRegioselectivity
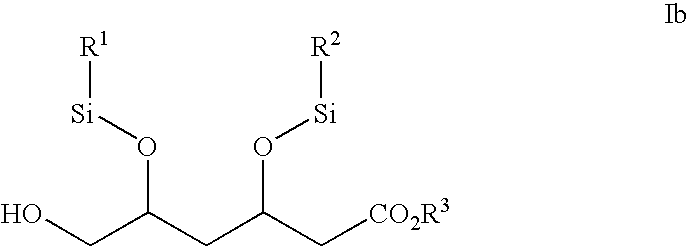
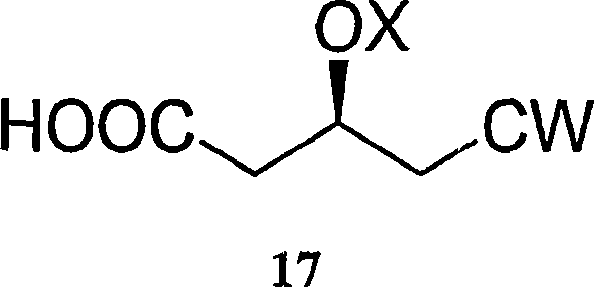
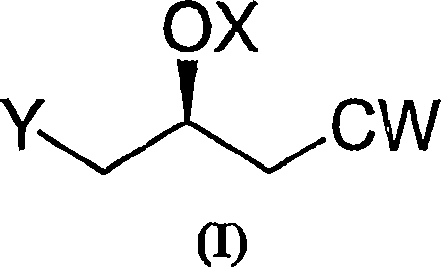
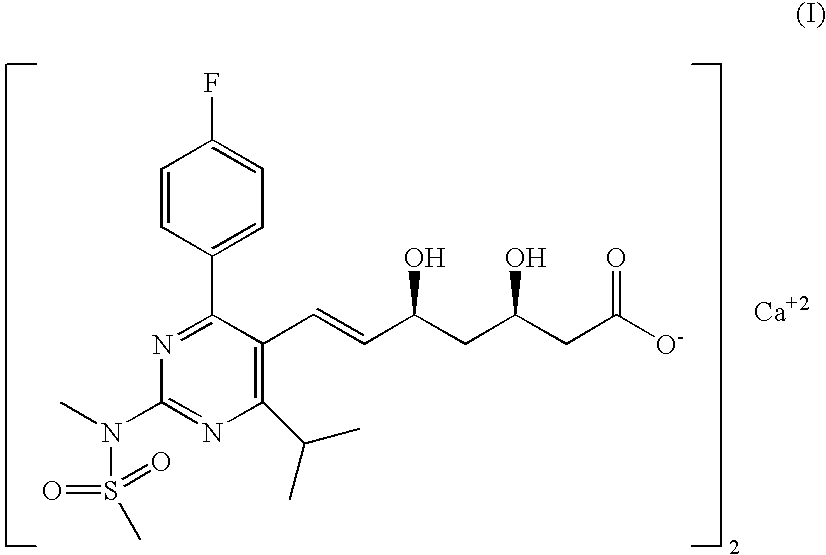
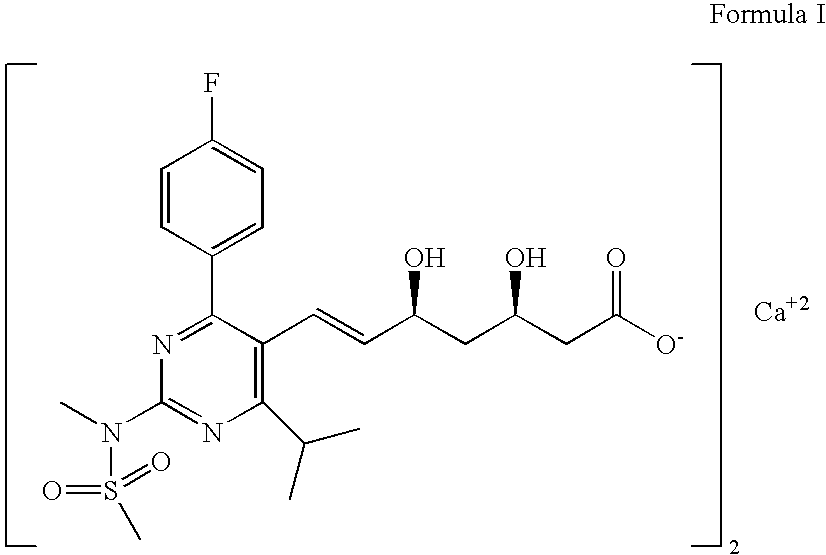
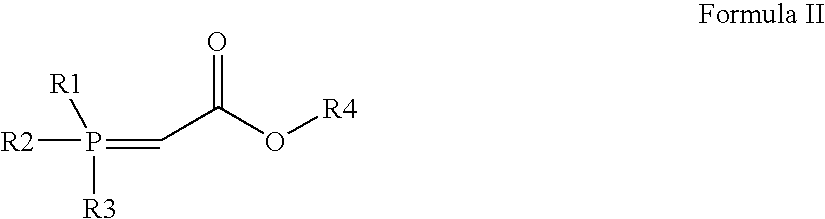
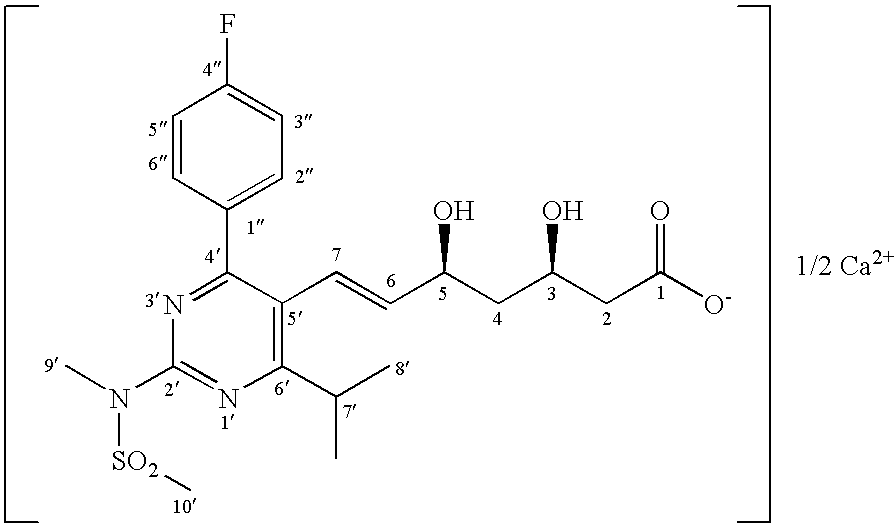
The invention relates to commercially viable process for the preparation of Rosuvastatin by an early introduction of the correct absolute stereochemistry at C-5 (S) of Rosuvastatin side chain followed by regioselective chain extension using novel side chain building blocks. It is yet another object of the invention is to provide novel intermediates that may be used for the preparation of Rosuvastatin. Formula (I).
Owner:UNICHEM LAB LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com