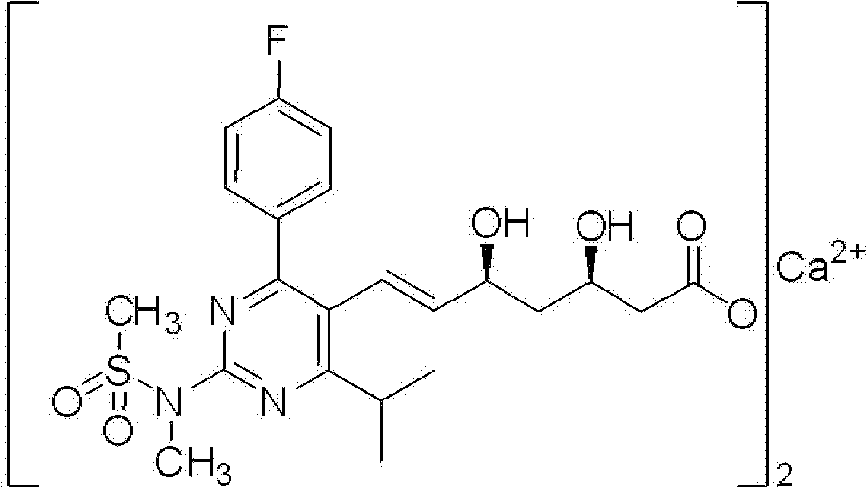
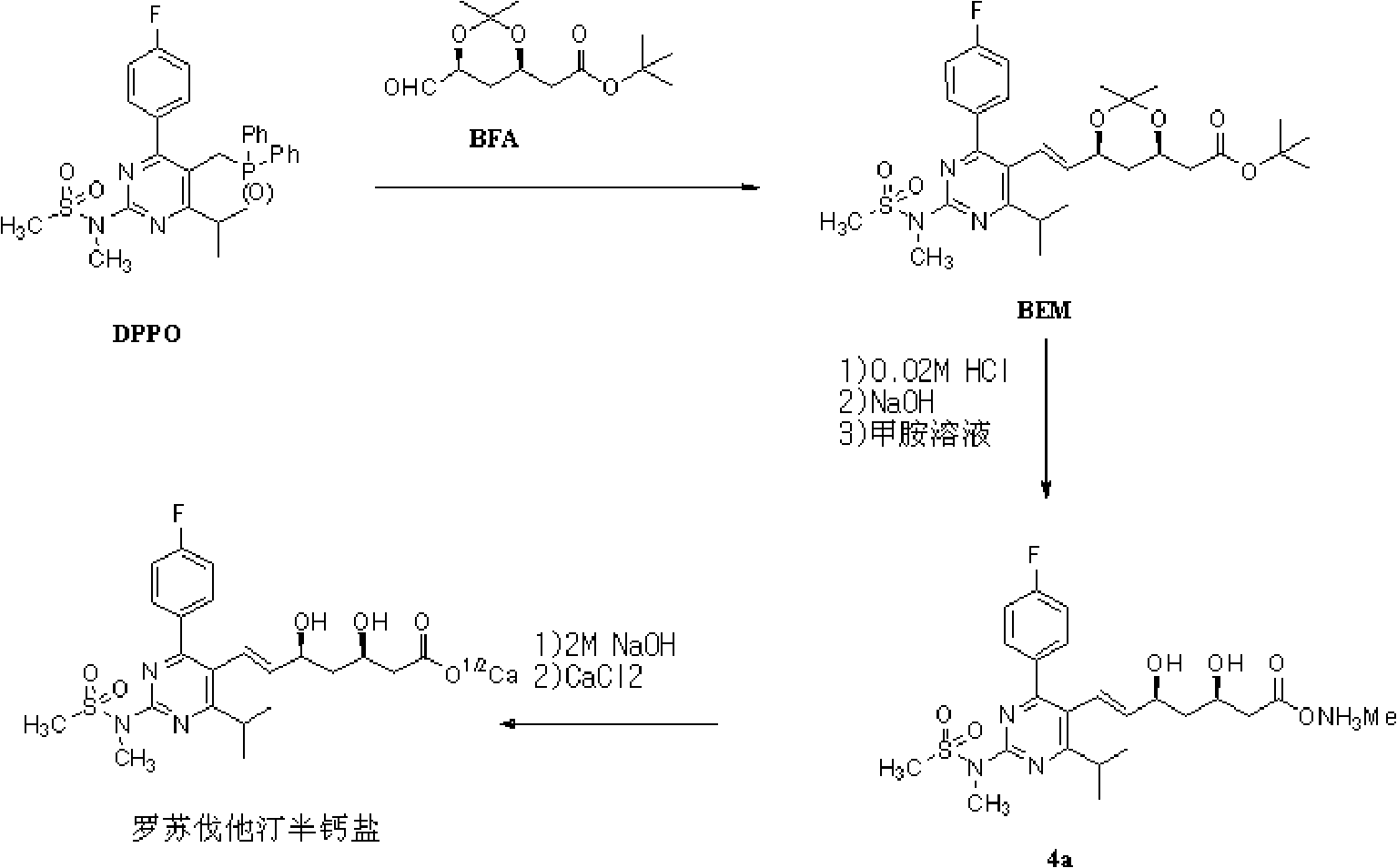
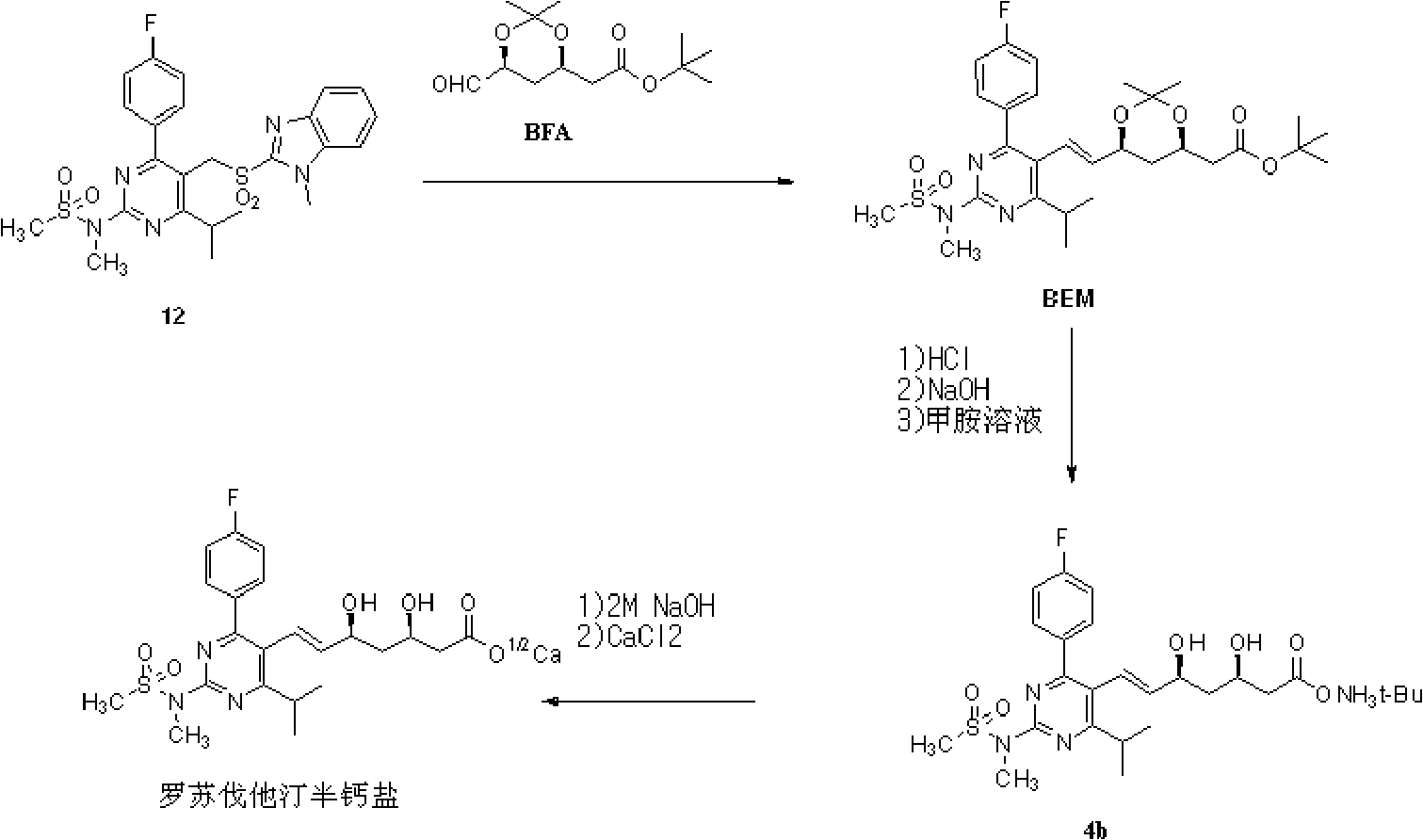
Novel method for preparing rosuvastatin, intermediate compounds useful for preparing same, and method for preparing same
A compound and reaction technology, applied in the field of preparation of medicinal rosuvastatin, can solve the problems of low purity and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Embodiment 1: Preparation of 6-chloro-5-hydroxyl-3-oxohexanoic acid (2-methyl-1-phenylpropan-2-yl) ester (7a) prepare
[0102]
[0103] Lithium bis(trimethylsilyl)amide (190.8 g) was dissolved in tetrahydrofuran (1400 ml) in the main reaction vessel before cooling to -75°C and flushing with nitrogen. (2-Methyl-1-phenylpropan-2-yl) acetate (219.2 g) was charged to a separate reaction vessel and dissolved by adding tetrahydrofuran (300 ml), and the solution was gradually was added dropwise to the main reaction vessel, followed by stirring for 1 hour. (S)-Ethyl 4-chloro-3-hydroxybutyrate (50 g) was added and dissolved in tetrahydrofuran (50 ml), and the resulting solution was gradually added dropwise to the main reaction vessel over 20 minutes, after which Stir for 5 hours. Thereafter, acetic acid (124 ml) was gradually added dropwise thereto, and the temperature was gradually raised to 0°C. Ethyl acetate (1500 ml) and purified water (1500 ml) were added thereto, ...
Embodiment 2
[0105] Embodiment 2: Preparation of 6-bromo-5-hydroxyl-3-oxohexanoic acid (2-methyl-1-phenylpropan-2-yl) ester (7b) prepare
[0106]
[0107] In the same manner as in Example 1, except that ethyl (S)-4-bromo-3-hydroxybutyrate (50 g) was used instead of ethyl (S)-4-chloro-3-hydroxybutyrate (50 g) Go through the process. The crude title compound (290.0 g) was obtained. A part of the crude compound was purified by a silica gel column (ethyl acetate:n-hexane=3:7 (v / v)) to obtain the following NMR data.
[0108] 1 H-NMR (400MHz, CDCl 3 )δ7.35-7.20 (m, 5H), 4.40-4.36 (m, 1H), 3.69-3.65 (m, 2H), 3.43 (s, 2H), 3.02 (m, 1H), 2.81-2.77 (m, 2H), 2.00(s, 2H), 1.50-1.46(m, 6H)
Embodiment 3
[0109] Example 3: (3R,5S)-6-Chloro-3,5-dihydroxy-hexanoic acid (2-methyl-1-phenylpropan-2-yl) ester (8a) preparation of
[0110]
[0111] The crude compound 6-chloro-5-hydroxy-3-oxohexanoic acid (2-methyl-1-phenylpropan-2-yl) ester (7a, 298g) prepared in Example 1 was dissolved in tetrahydrofuran (2000ml ) and methanol (1000ml) in a mixed solvent, and the solution was cooled to -75°C. At the same temperature, methoxydiethylborane (78.8 ml) was gradually added dropwise to the solution over 20 minutes, followed by stirring for 40 minutes. Thereafter, sodium borohydride (25.0 g) was added in 5 portions, followed by stirring for 5 hours, and acetic acid (96 ml) was gradually added dropwise thereto. The mixture was warmed to room temperature and kept there. Ethyl acetate (1800 ml) and 3% aqueous hydrogen peroxide solution (1500 ml) were charged into the reaction zone, followed by stirring for 30 minutes and extraction. The aqueous layer was back extracted with ethyl aceta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com