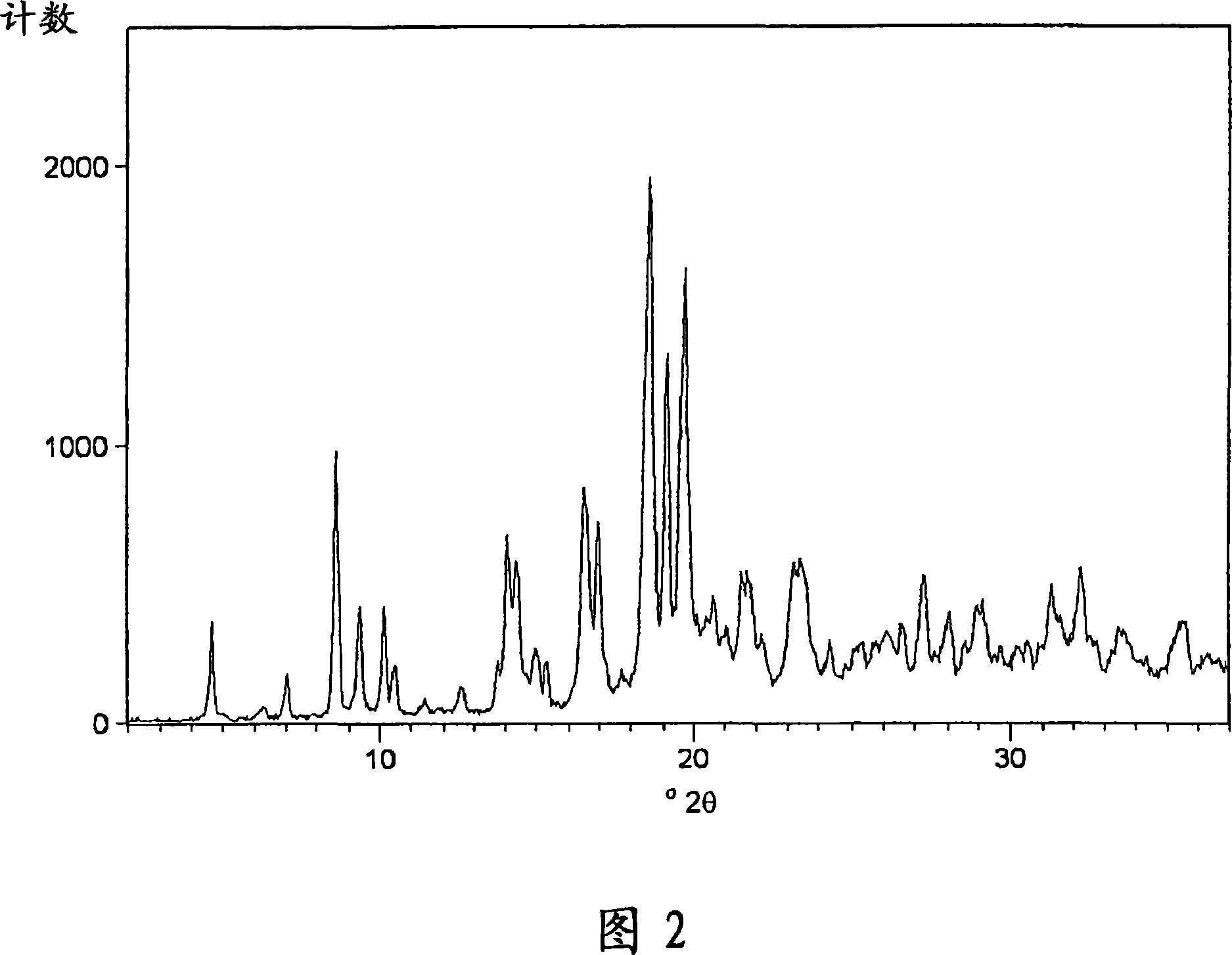
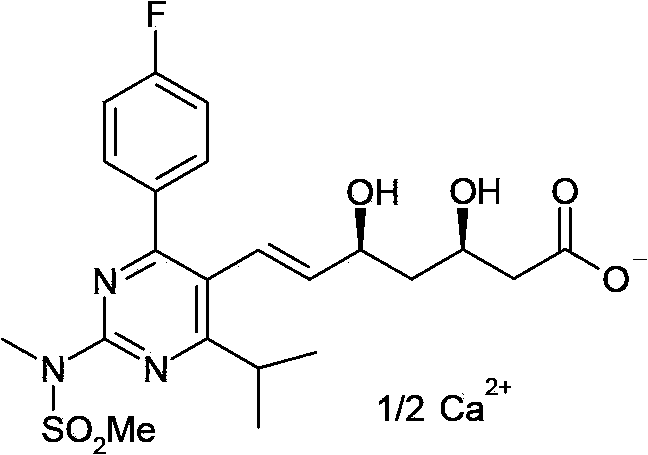
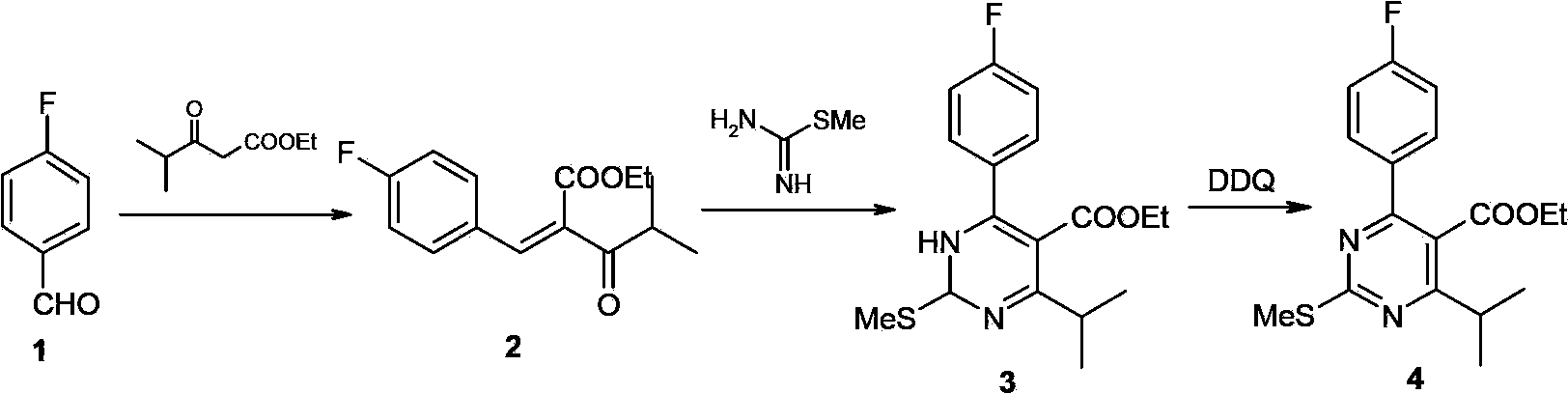
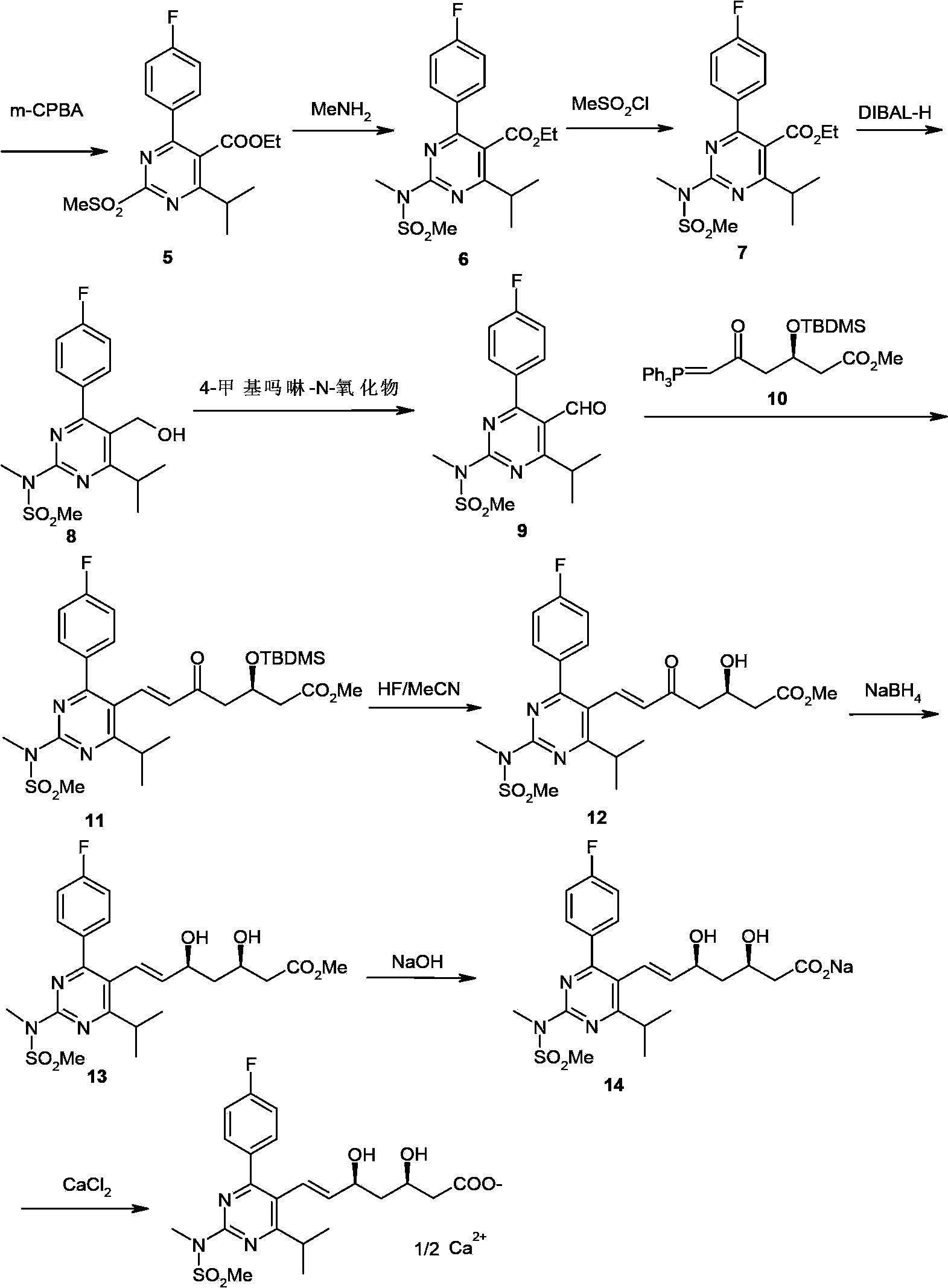
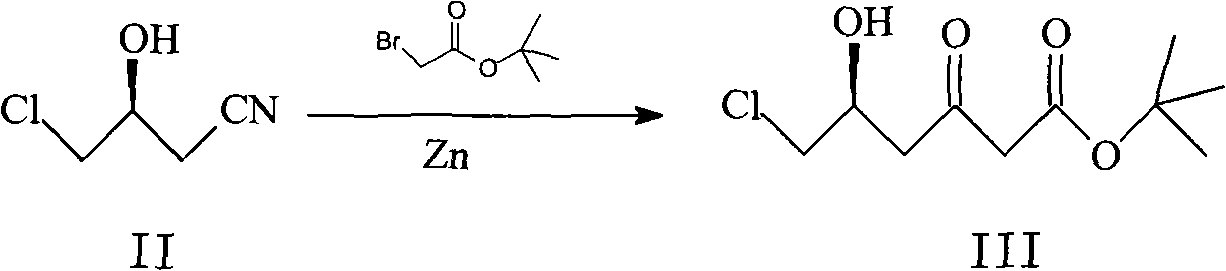Patents
Literature
280 results about "Rosuvastatin Calcium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
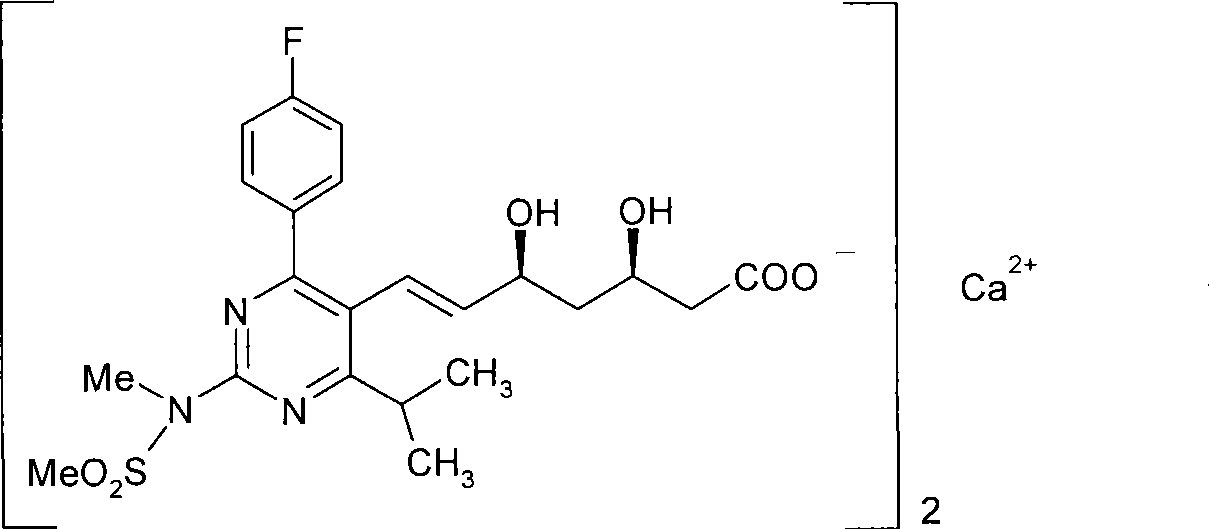
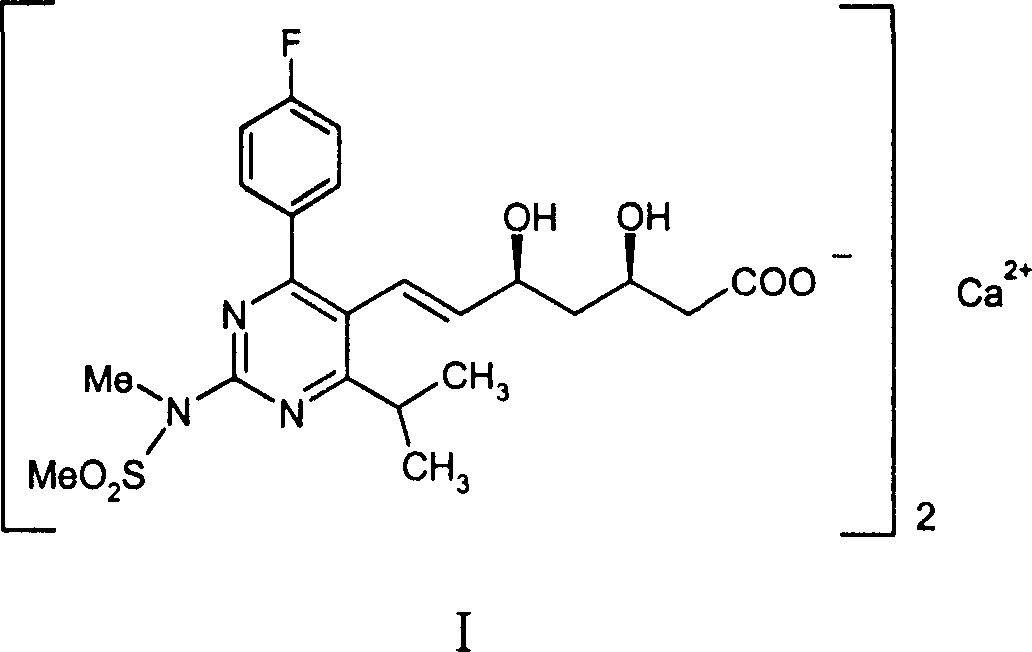
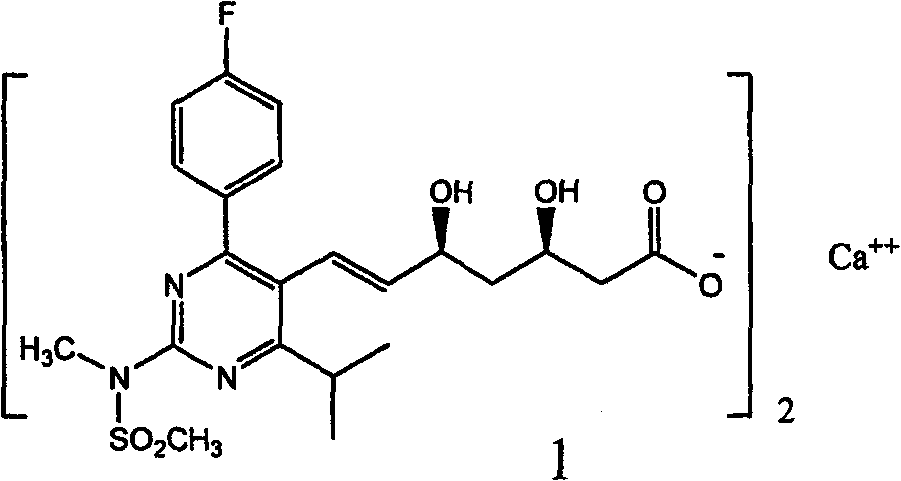
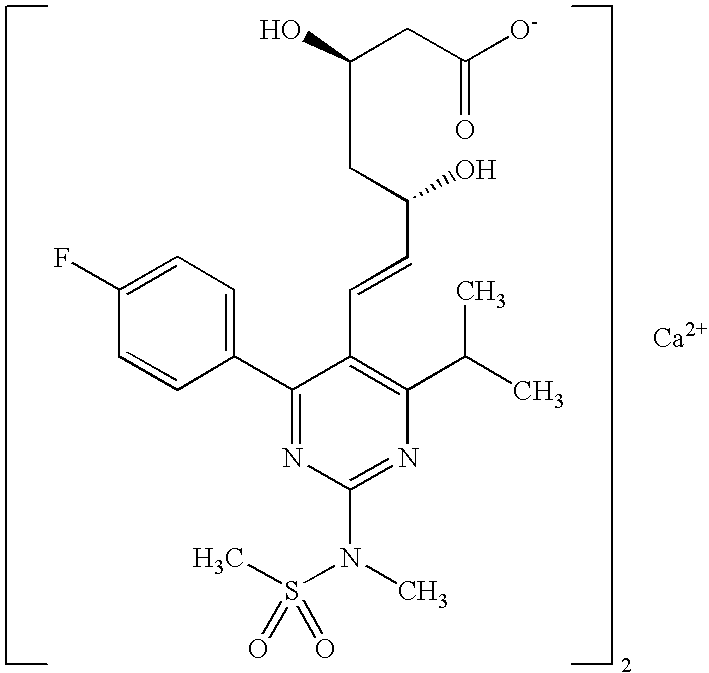
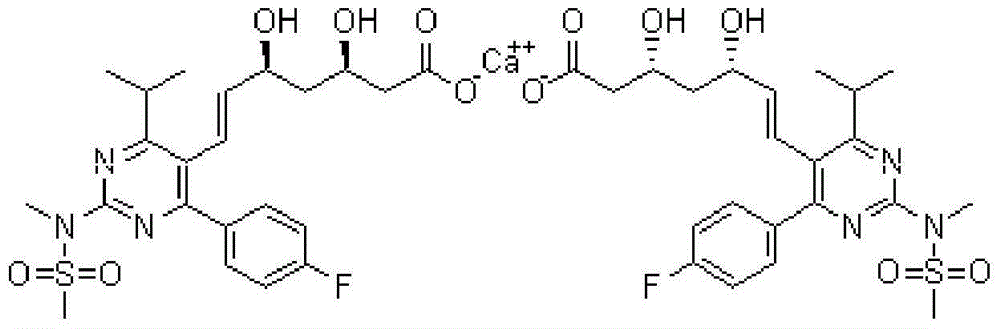
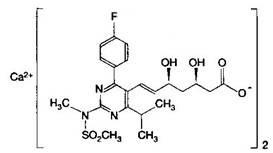
A HYDROXYMETHYLGLUTARYL-COA-REDUCTASE INHIBITOR, or statin, that reduces the plasma concentrations of LDL-CHOLESTEROL; APOLIPOPROTEIN B, and TRIGLYCERIDES while increasing HDL-CHOLESTEROL levels in patients with HYPERCHOLESTEROLEMIA and those at risk for CARDIOVASCULAR DISEASES.
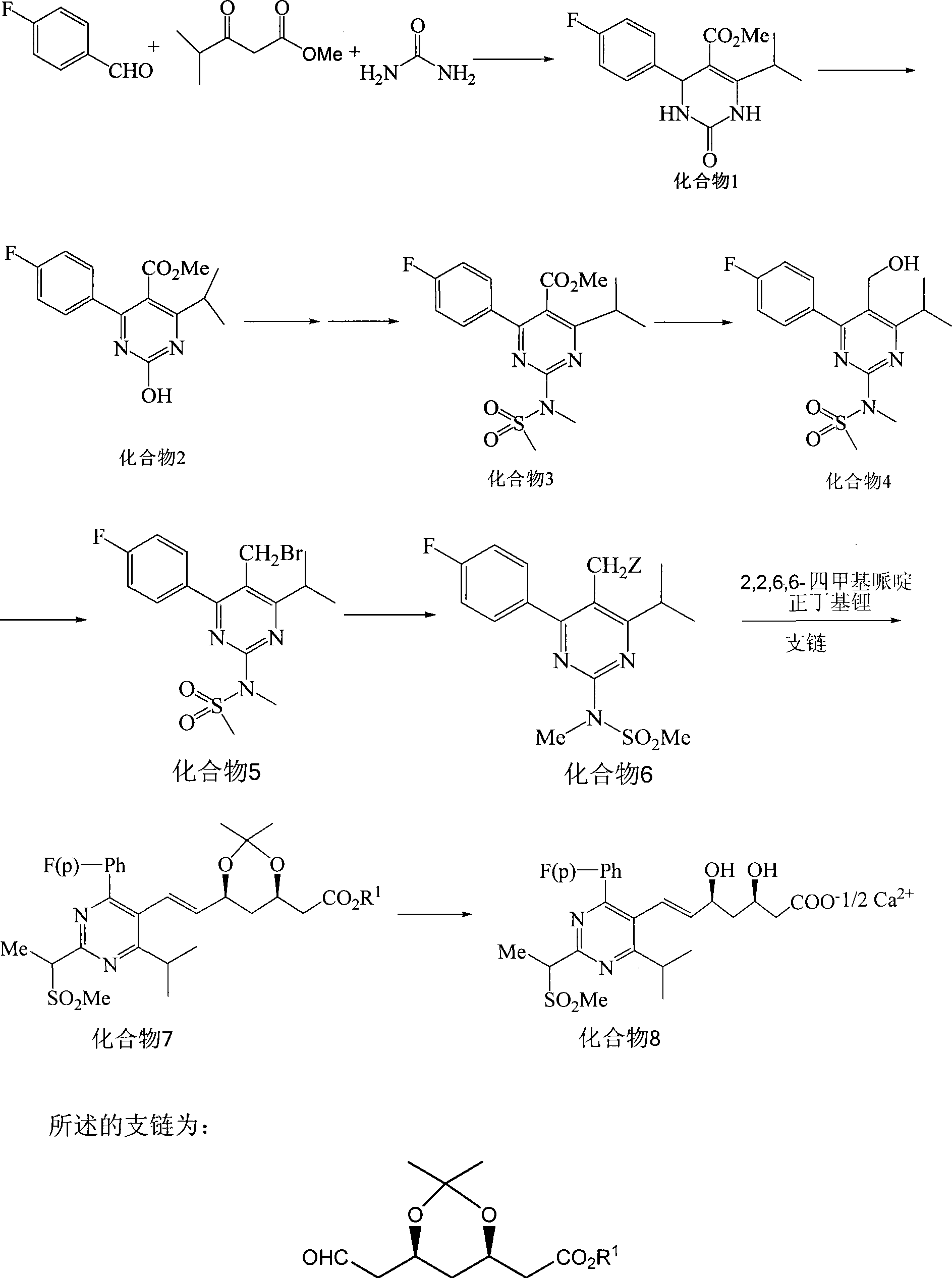
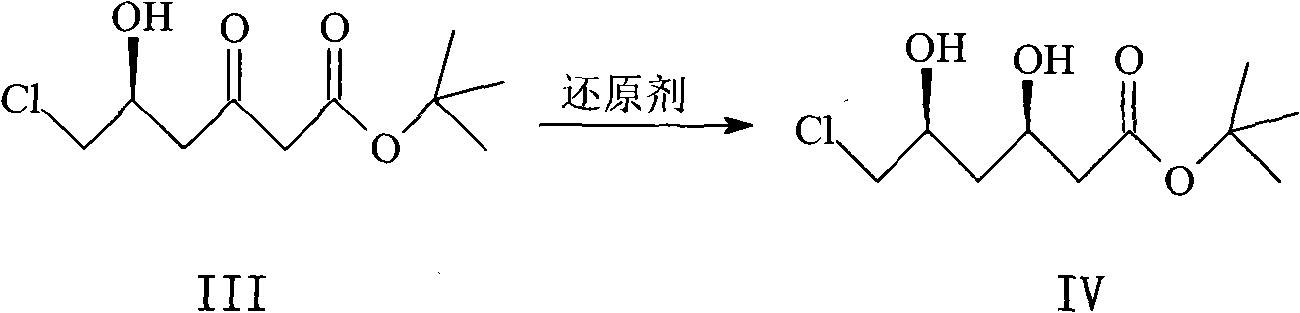
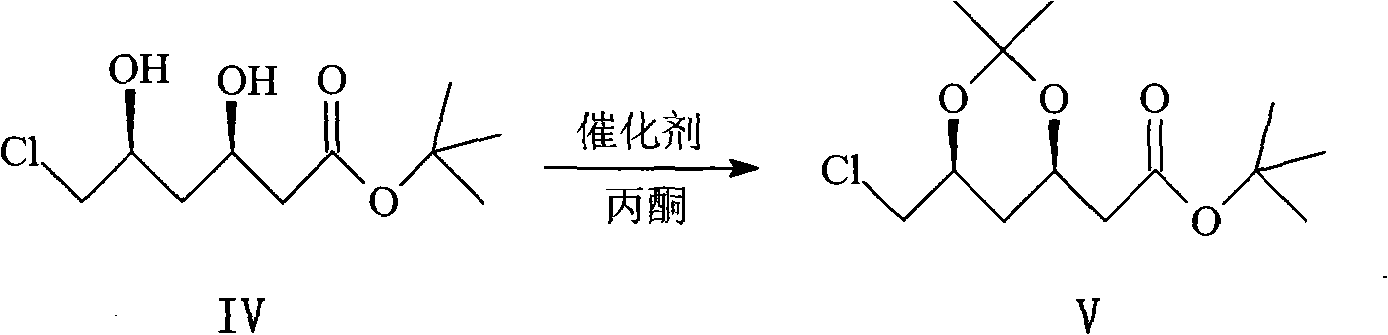
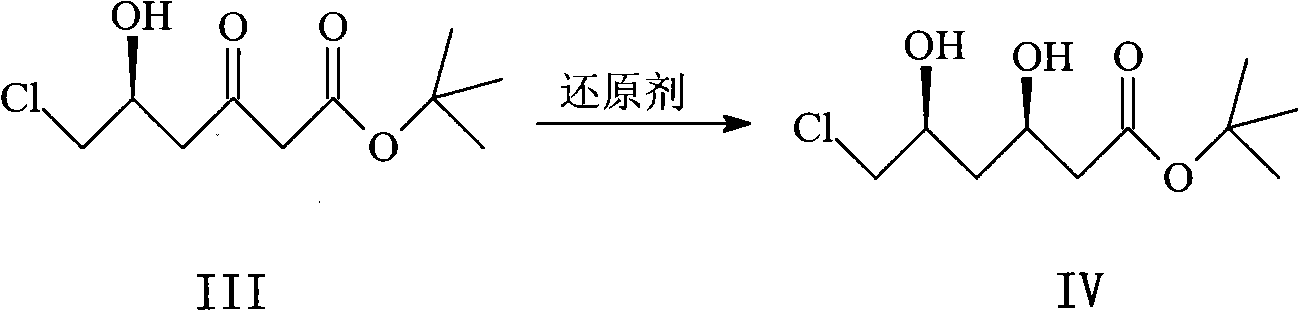
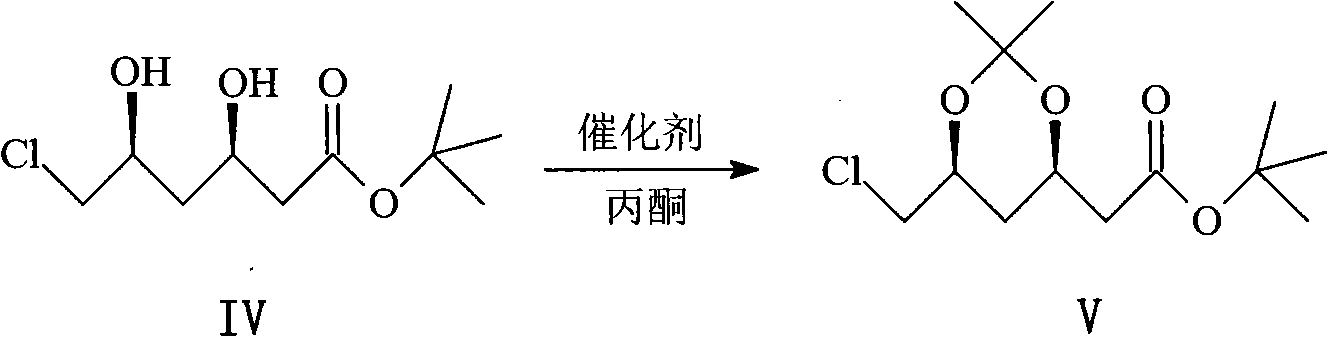
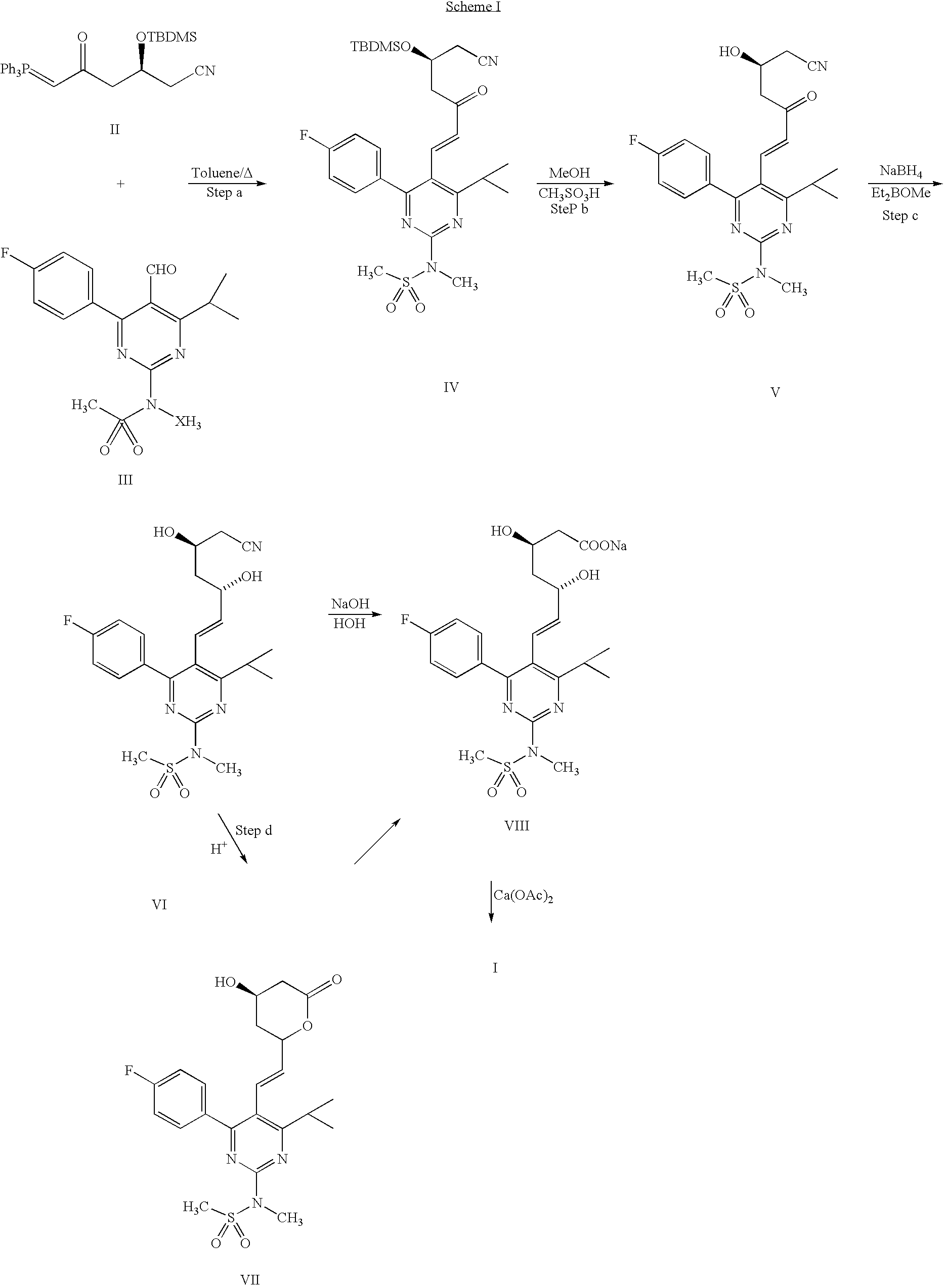
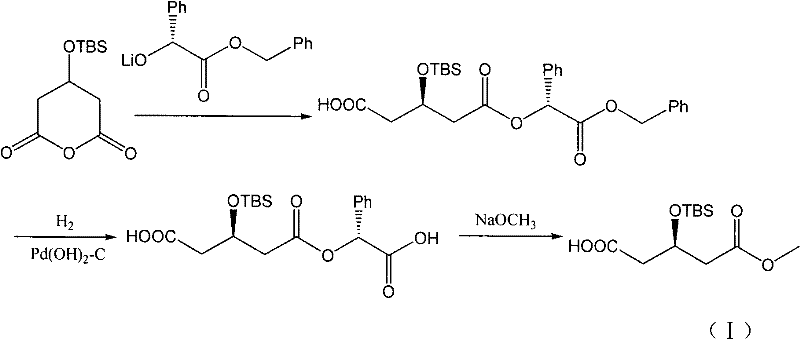
Method for synthesizing rosuvastatin intermediate and rosuvastatin
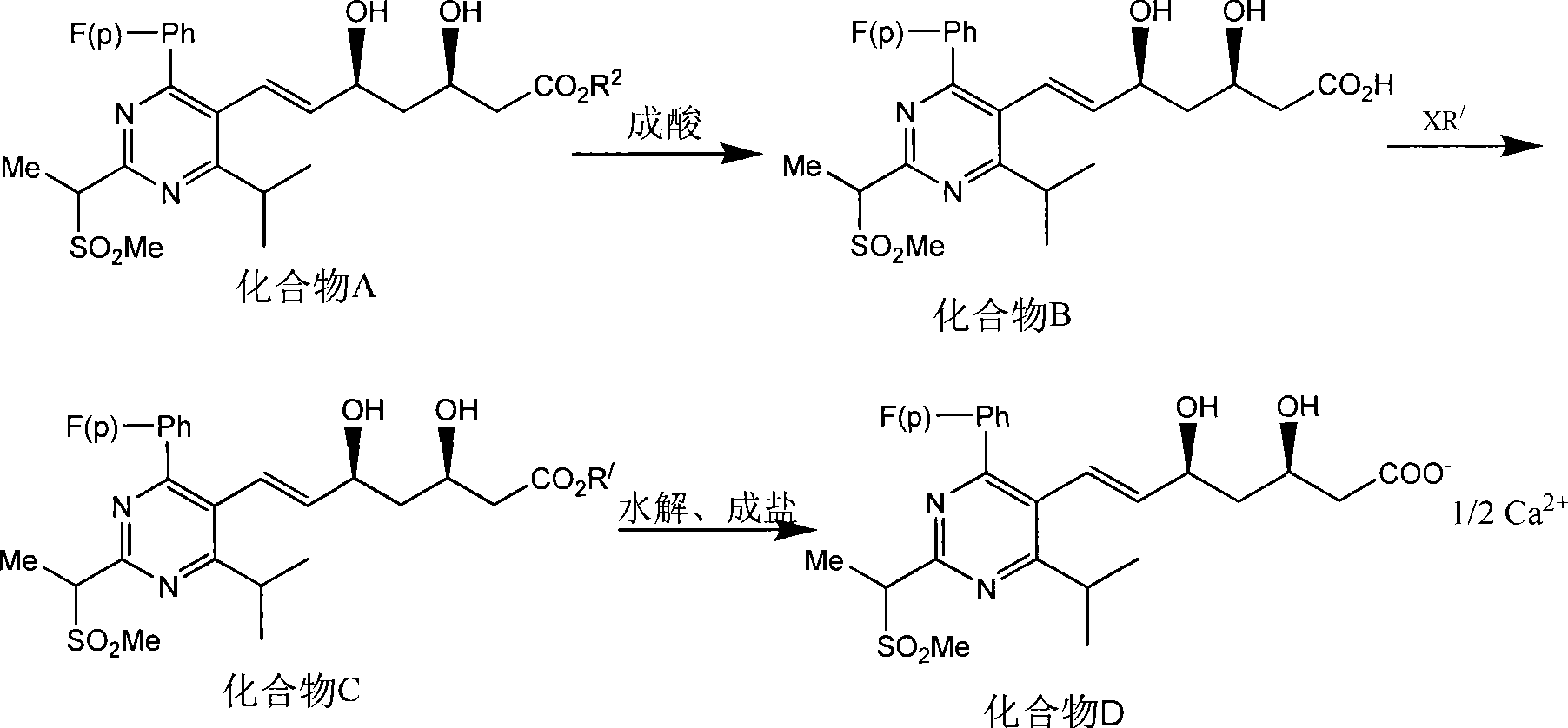
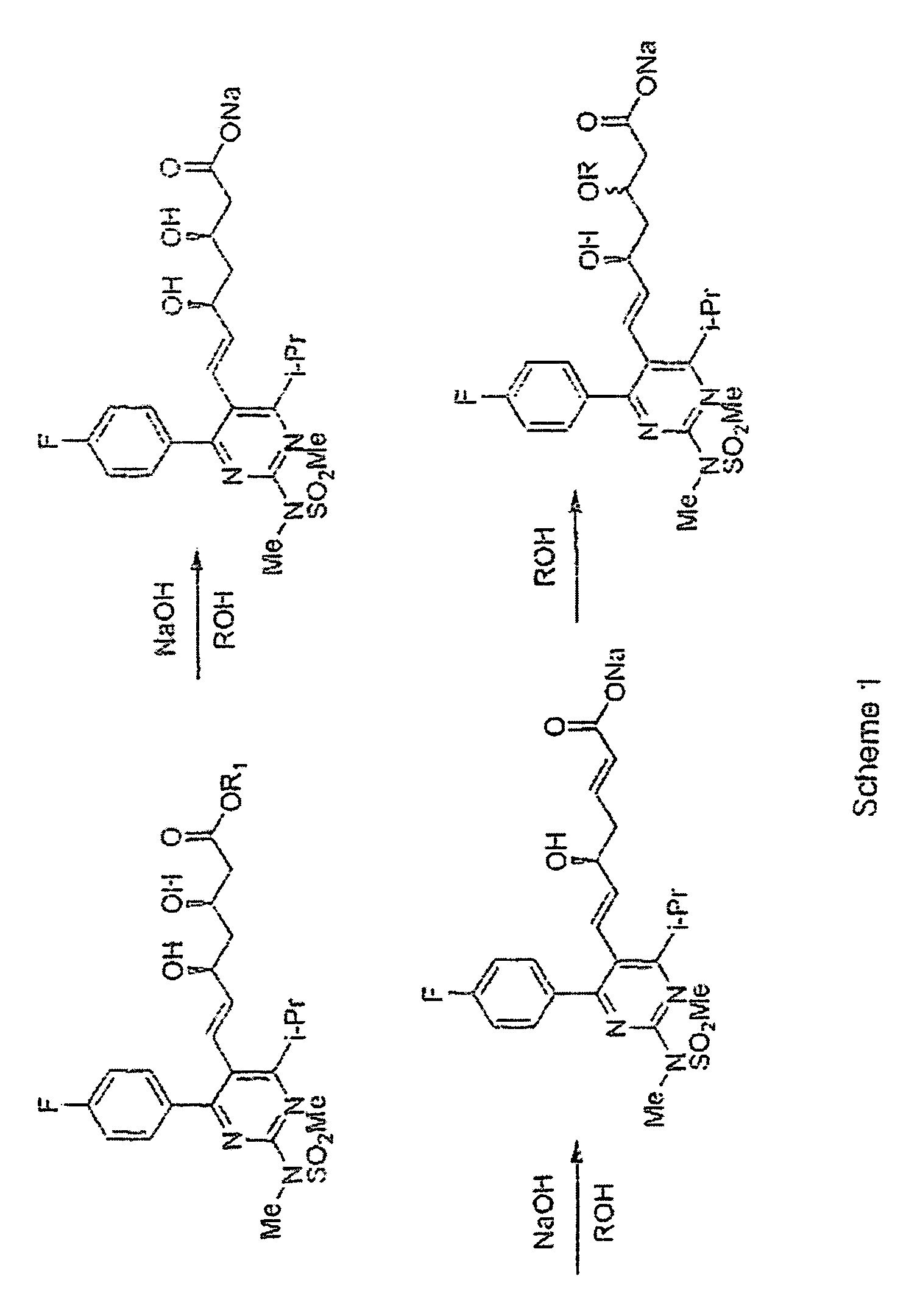
The invention provides a synthetic method of rosuvastatin calcium, which is characterized in that a compound 6 and branched chain react under the action of 2,2,6,6,-tetramethylpiperidine and n-butyl lithium to generate a compound 7, the reaction temperature is increased to minus 30 DEG C and the aspects such as reaction solvent and reaction reagent are improved so that the total reaction route has the advantages of mild condition, low device requirement and suitability for industrial production. Meanwhile, another improved synthetic route of rosuvastatin calcium comprises the following steps: converting esters into acids, synthesizing esters again, and synthesizing the rosuvastatin calcium. The rosuvastatin calcium purity is as high as 99.8%.
Owner:ENANTIOTECH CORP
Novel anhydrous amorphous forms of rosuvastatin calcium, pitavastatin calcium and fluvastatin sodium
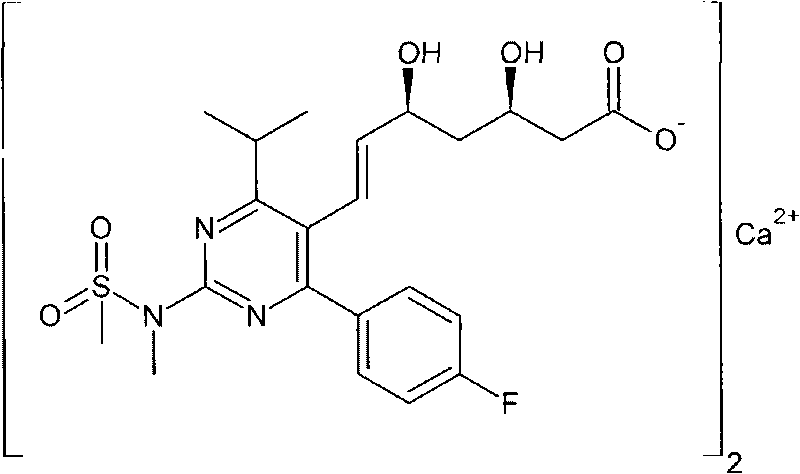
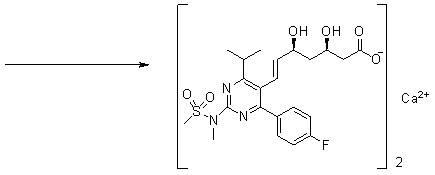
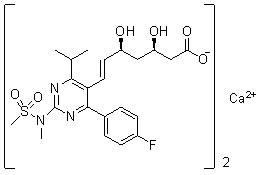
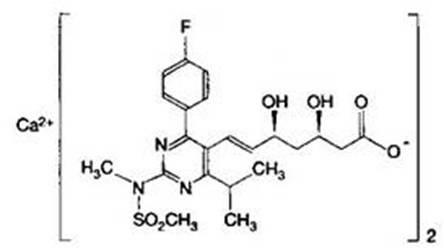
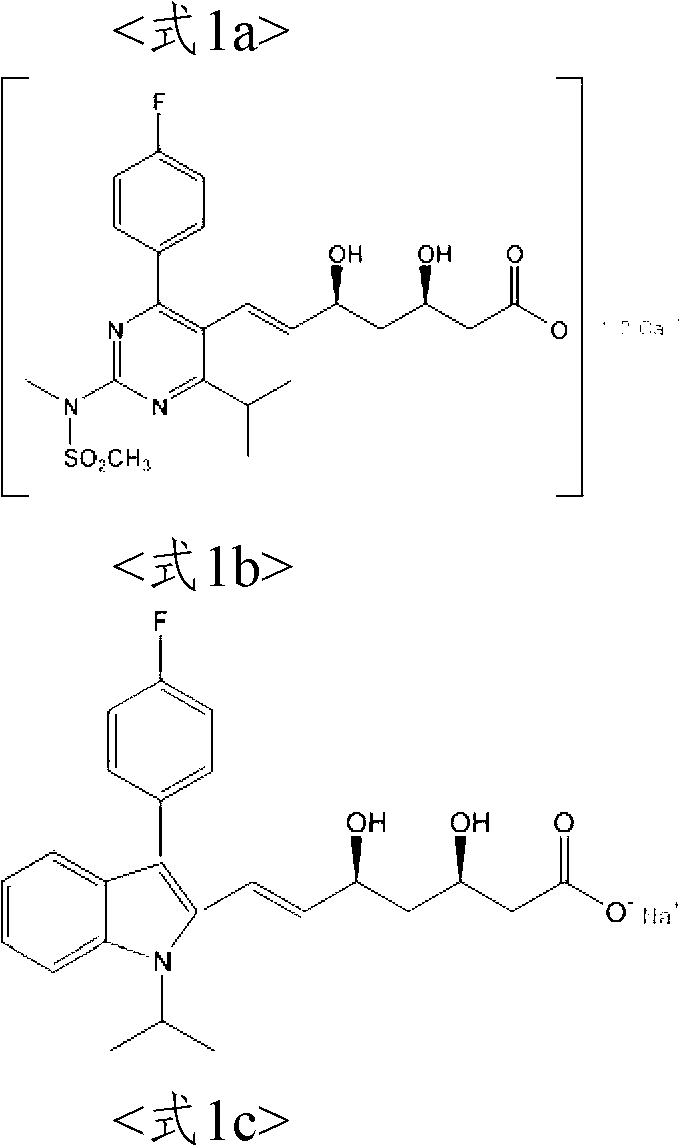
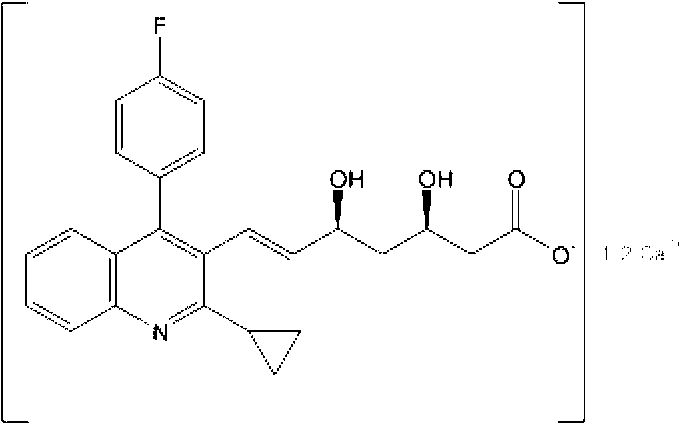
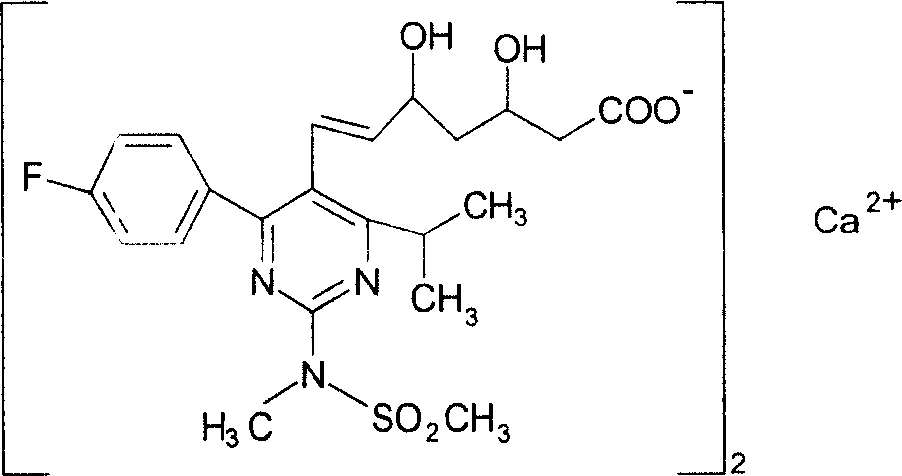
The present invention relates to novel anhydrous amorphous forms of bis[(E)[4-(4-fluorophenyl)isopropyl[methyl(methylsulfonyl)amino]pyrimidinyl](3R,5S)-3,5-dihydroxyhept enoic acid]calcium salt (rosuvastatin calcium), (±)7-(3-(4-fluorophenyl)-1-(1-methylethyl)-1H-indol-2-yl)3,5-dihydroxy heptenoic acid monosodium salt (fluvastatin sodium) and bis[(E)-3,5-dihydroxy-7-[4′-(4″-fluorophenyl)-2′-cyclopropyl-quinolin-3′-hept-6-enoic acid]calcium salt (pitavastatin calcium), to processes for their preparation, to pharmaceutical compositions containing them and to methods of treatment using the same. The rosuvastatin calcium, pitavastatin calcium and fluvastatin sodium obtained are known valuable agents useful in treating hyperlipidemia and hypercholestrolemia.
Owner:MAI DE
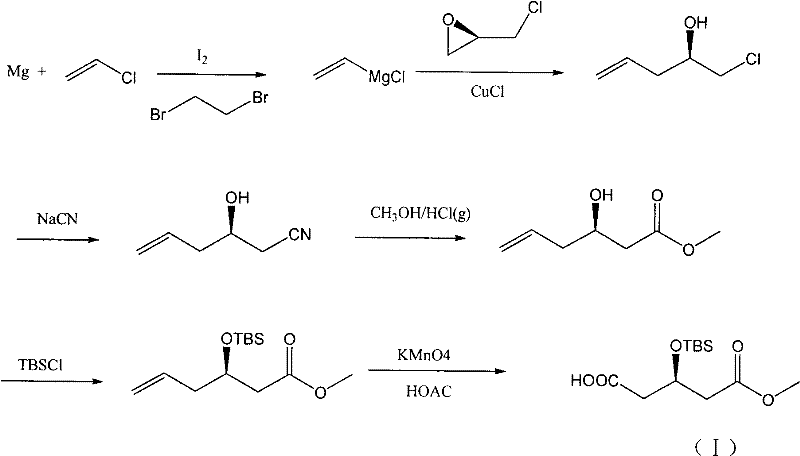
Method for preparing rosuvastatin calcium midbody
ActiveCN101735272AHigh yieldReduce pollutionGroup 5/15 element organic compoundsBulk chemical productionWittig reactionGrignard reaction
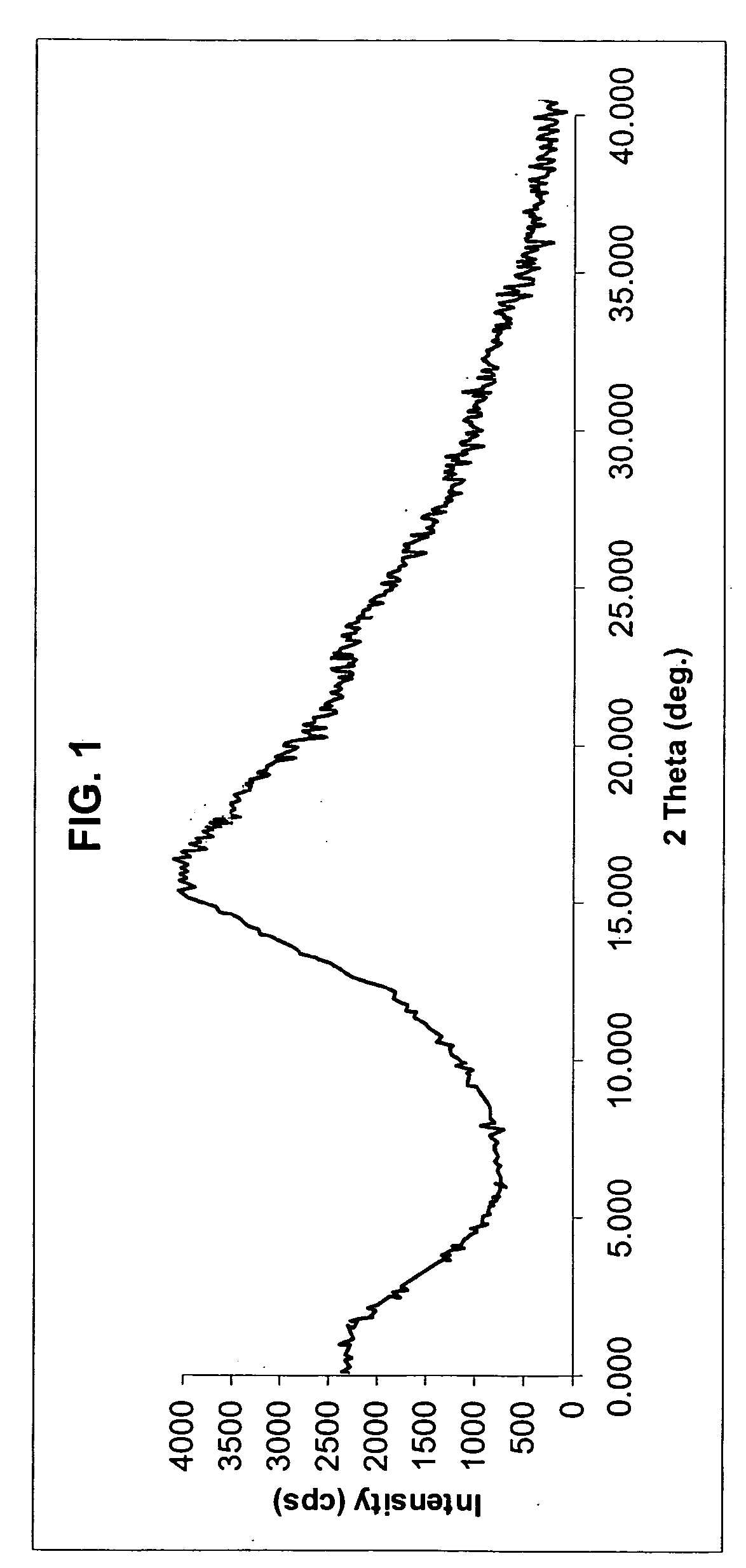
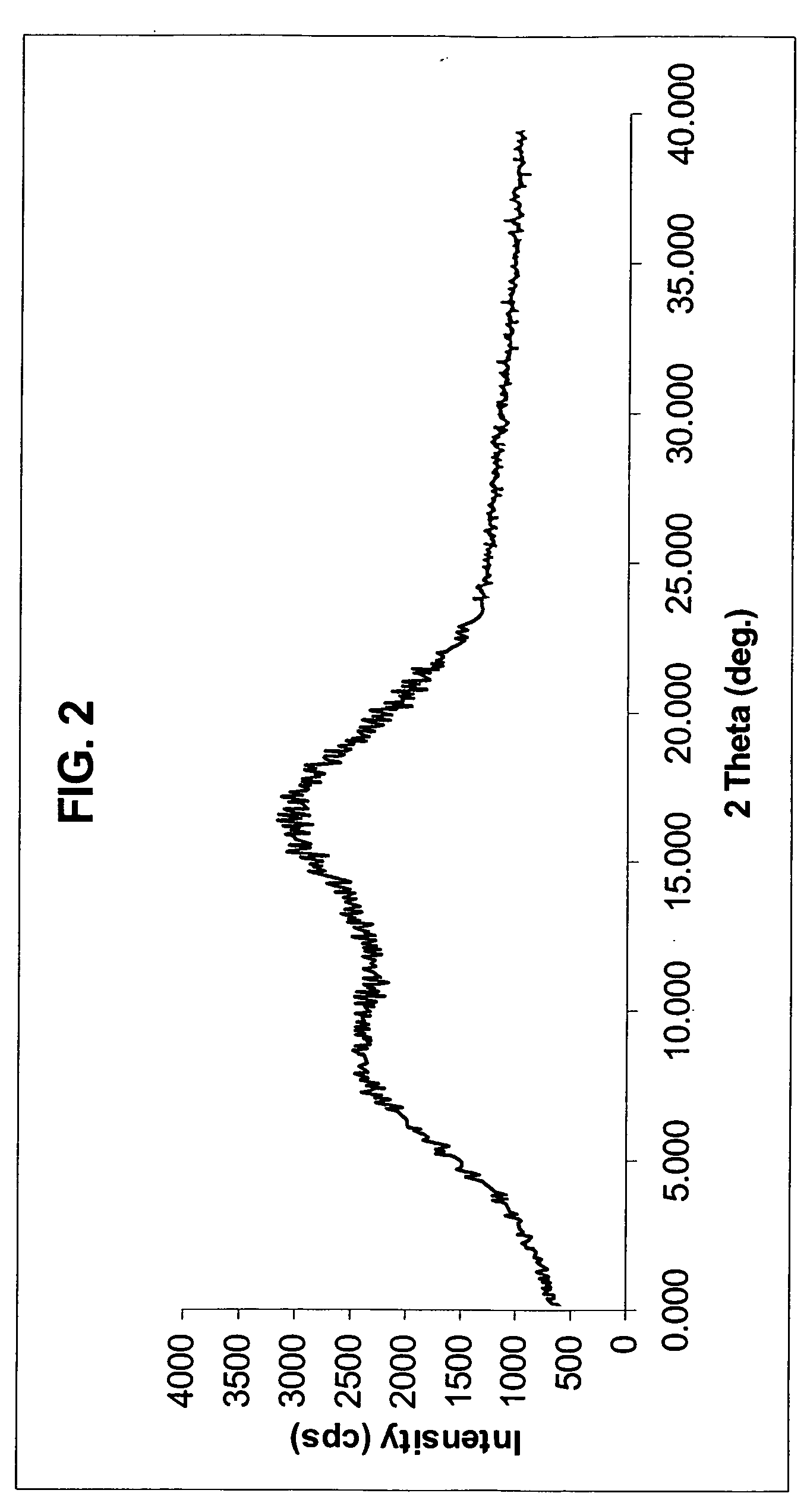
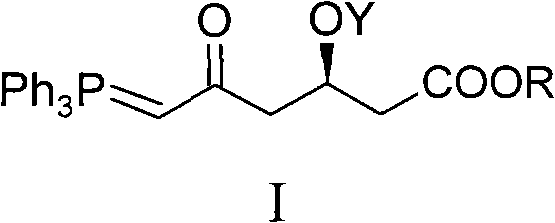
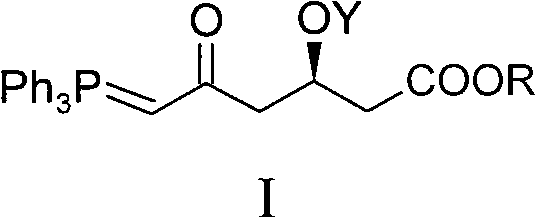
The invention discloses a method for preparing a rosuvastatin calcium midbody, namely a compound (R is C1-C10 alkyl, and Y is a hydroxyl protecting group) shown as the general formula I. Chloroethylene and R-epoxy chloropropane as initial raw materials are carried out seven steps of reaction, such as Grignard reaction, sodium cyanide nucleophilic substitution reaction, alcoholysis reaction, hydroxyl protection, oxidizing reaction, methylchloroformate acylation reaction and Wittig reaction to prepare the compound shown as the general formula I. The method has mild condition, simple and convenient operation, stable process, low cost and easy acquisition of raw materials, high product yield, easy disposal of the three wastes, less environmental pollution, low preparation cost and suitability for industrialized large-scale production.
Owner:JIANGXI DONGBANG PHARMA
Method for preparing Rosuvastatin Calcium and key intermediate
InactiveCN1872841AGuaranteed pureComplete stereoselectivityOrganic chemistryCombinatorial chemistryRosuvastatin Calcium
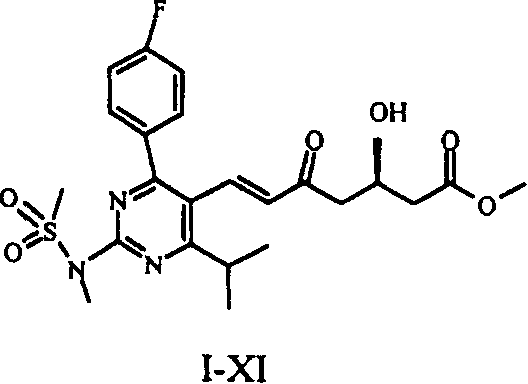
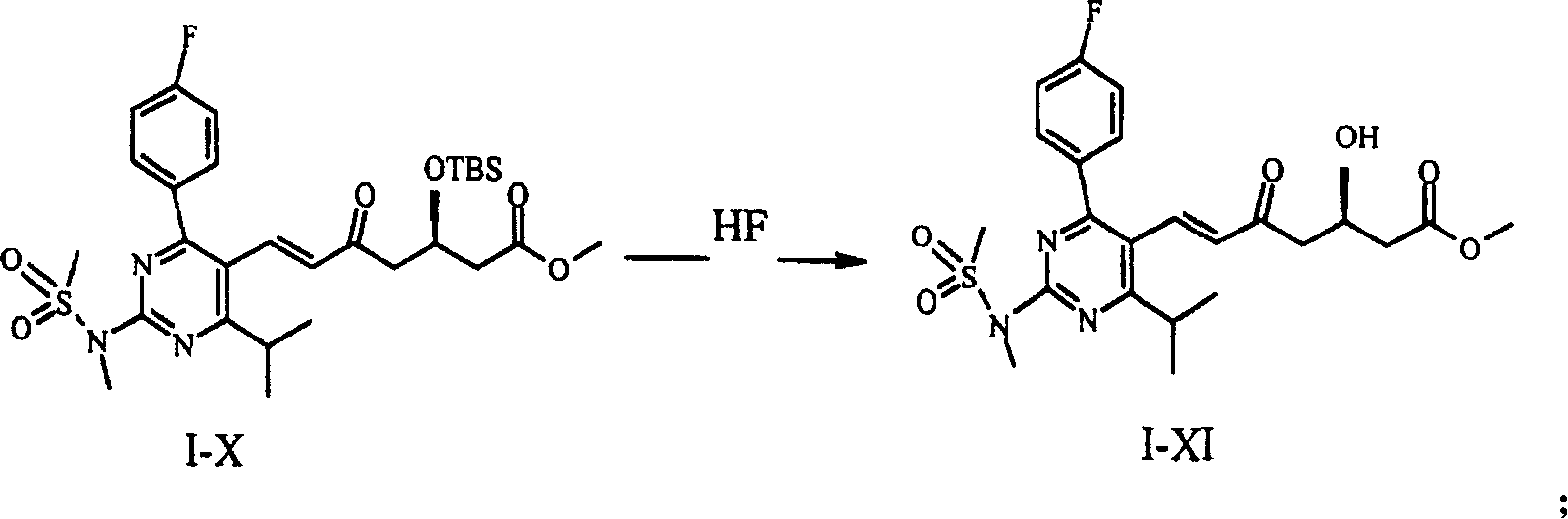
This invention provides a method for preparing rosuvastatin intermediate compound I-XI. The method can ensure a high purity of I-XI, thus ensuring the completeness of the stereoselectivity in subsequent reactions. Although the yield of I-XII from I-X in this invention is a little lower than that in the invention EP 0,521,471 A1, the purification of I-XII only needs a low-efficiency chromatographic column. The method thus has such advantages as low cost and high efficiency.
Owner:SHANGHAI SINE PHARMA LAB
Synthetic method of key intermediate of rosuvastatin calcium side chain
ActiveCN101613341AEase of industrial productionSimple and fast operationOrganic compound preparationCarboxylic acid esters preparationSide chainRosuvastatin Calcium
The invention discloses a preparation method of a key intermediate of rosuvastatin calcium side chains. (S)-3-hydroxy-4-chlorine-butyronitrile is taken as a starting material and subject to four steps condensation, reduction, hydroxyl protection and condensation reactions to obtain the intermediate. The method has simple operation during the reaction, products in the steps are easily separated and purified, no silica gel column is needed for purification and separation, the yield is above 80%, an intermediate with high chemical purity and optical purity can be obtained, and conclusion of GC determination is that the chemical purity is not less than 99.4%, and the optical purity is not less than 99.3%ee.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for preparing intermediate of synthesizing rosuvastatin calcium
ActiveCN1958593AReduce usageEliminate cryogenic operationGroup 4/14 element organic compoundsGroup 5/15 element organic compoundsRosuvastatin CalciumCarbonate
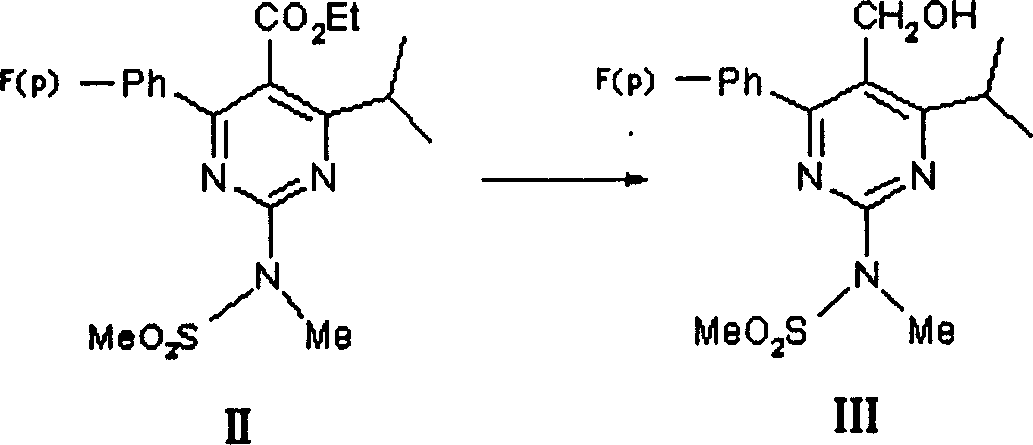
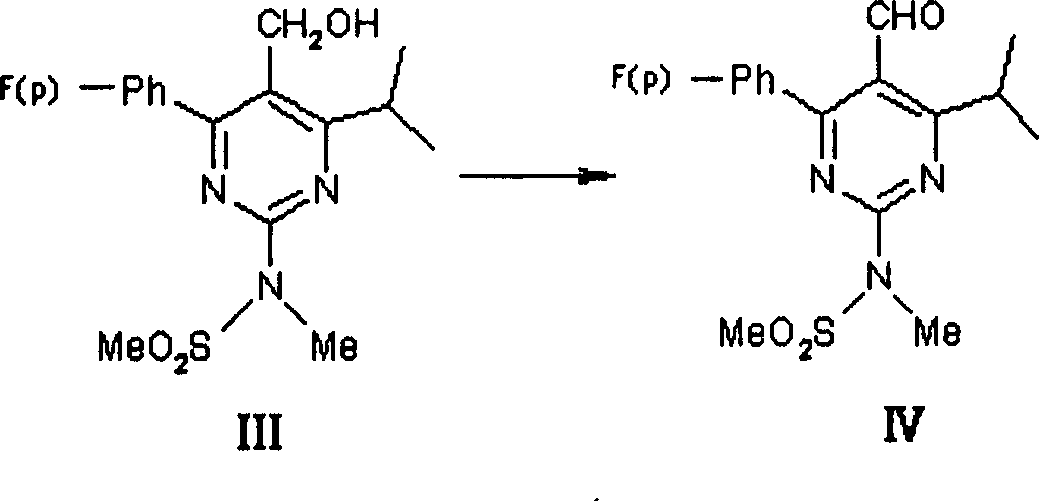
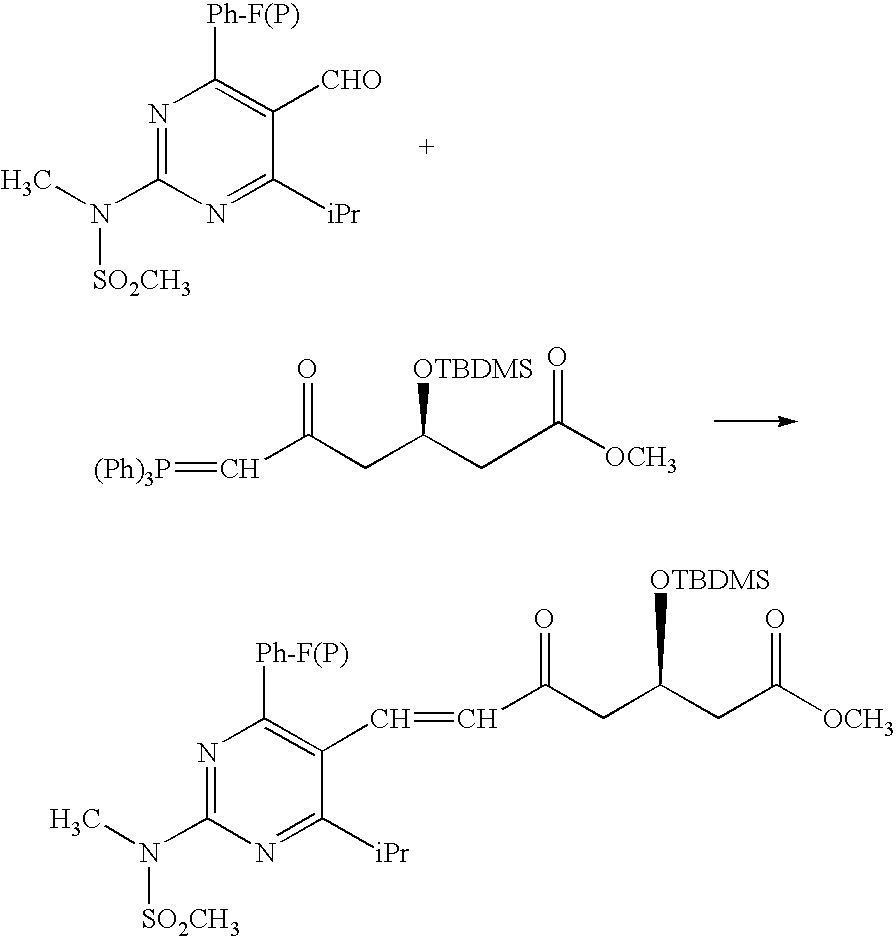
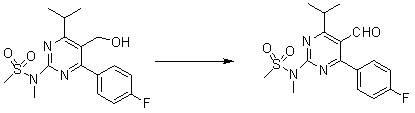
This invention discloses a method for preparing methyl (R)-7-[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-mesylamino)pyrimidin-5-yl]-3-tert-butyldimethylsiloxyl-5-carbonyl-6(E)-hepetnate as the intermediate for synthesizing rosuvastatin calcium. The method comprises: reducing pyrimidine carbonate (II) by borohydride / chloride to obtain pyrimidine methanol (III), oxidizing by potassium dichromate to obtain pyrimidine formaldehyde, and condensing in the presence of chiral phosphonate to obtain methyl (R)-7-[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-mesylamino)pyrimidin-5-yl]-3-tert-butyldimethylsiloxyl-5-carbonyl-6(E)-hepetnate (VI). The method has such advantages as abundant raw materials, low cost, mild reaction conditions, short time and high yield. The byproduct is water-soluble.
Owner:SHANGHAI INST OF PHARMA IND
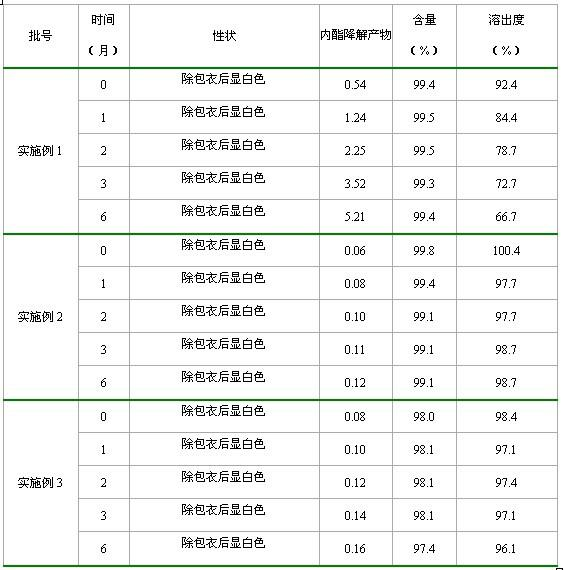
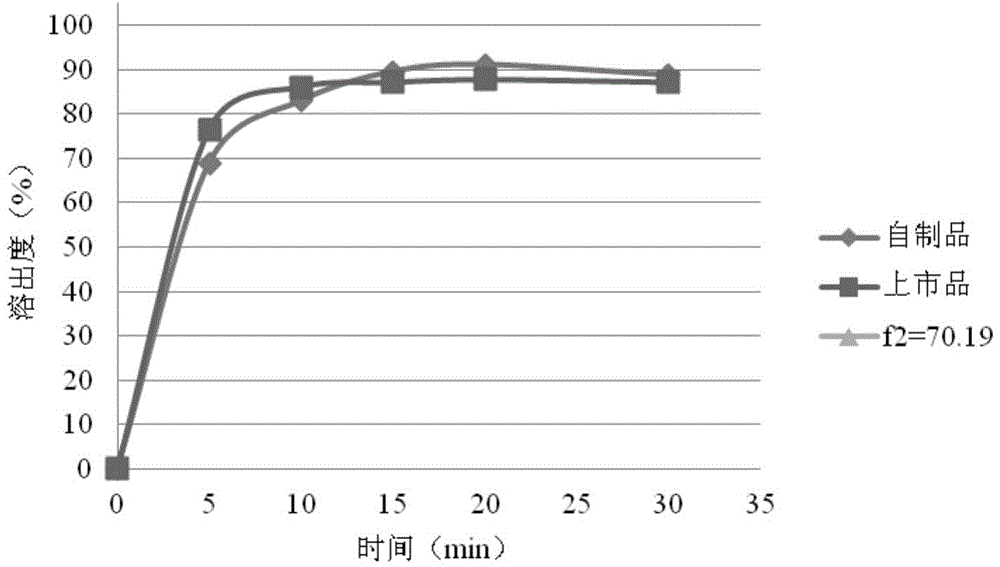
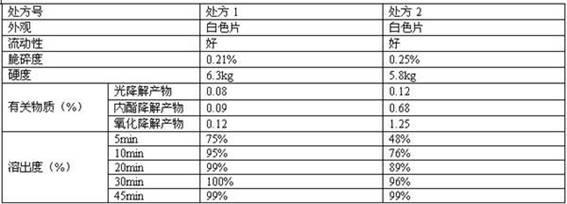
Rosuvastatin calcium tablet and preparation method
ActiveCN102860994AImprove liquidityGood compressibilityOrganic active ingredientsMetabolism disorderDissolutionLactose
The invention relates to a rosuvastatin calcium tablet and a preparation method, the tablet is composed of a tablet core and a coating layer, the tablet core is composed of rosuvastatin calcium, lactose, microcrystalline cellulose, a disintegrating agent and a lubricant, the lactose is spray dried lactose or particle lactose, and the microcrystalline cellulose is flowing steam dried microcrystalline cellulose or spray dried microcrystalline cellulose. The rosuvastatin calcium tablet is not required for adding a stabilizing agent or aerosil or preparing to a special preparation, the rosuvastatin calcium preparation with good stability, excellent dissolution and content uniformity can be obtained by a direct tabletting technology, and the method of the invention has the advantages of simple technology and low cost, and is suitable for large scale production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Preparation method of key intermediate of rosuvastatin calcium side chain
ActiveCN101624390AEase of industrial productionSimple and fast operationOrganic compound preparationCarboxylic acid esters preparationSide chainRosuvastatin Calcium
The invention provides a preparation method of a key intermediate of a rosuvastatin calcium side chain, comprising the following steps: using (S)-4-chlorine-3-hydroxybutanoate as an initial raw material; and preparing the key intermediate through four-step reactions of condensation, reduction, hydroxy group protection and condensation. The reaction process is simple to operate, the products in each step are easy to separate and purify, the purification and separation step is carried out without a silicagel column, and the yield is more than 80 percent, therefore, the intermediate with higher chemical purity and optical purity can be obtained. The GC determination shows that the chemical purity is more than or equal to 99.5 percent and the optical purity is more than or equal to 99.2 percent ee.
Owner:LUNAN PHARMA GROUP CORPORATION
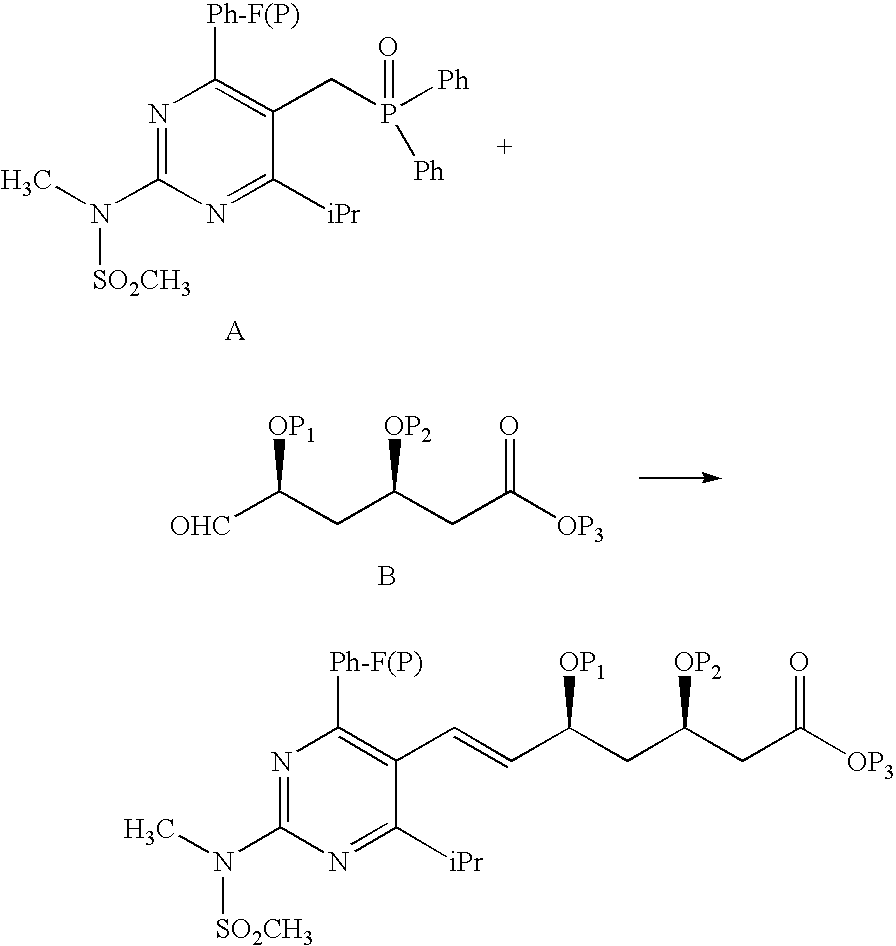
Synthetic Method and Intermediates of Rosuvastatin Calcium and Preparation Methods of Intermediates
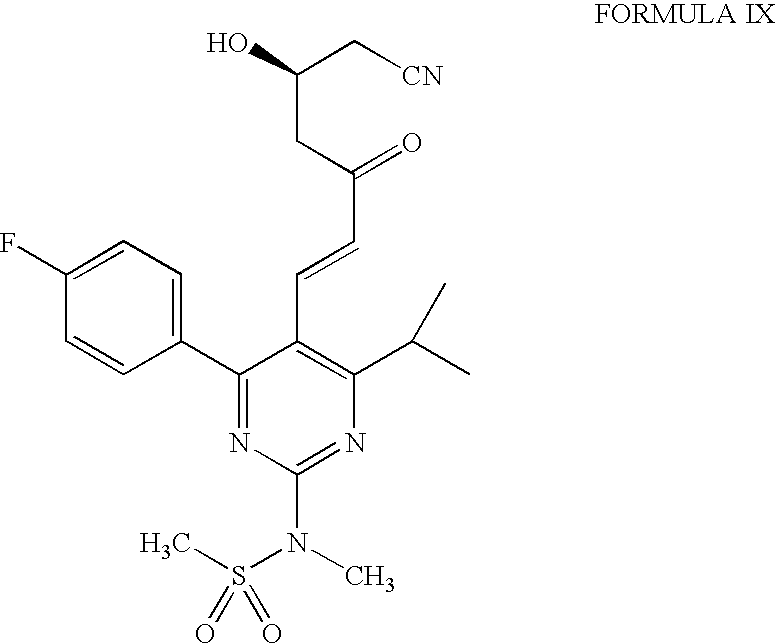
The present invention publicly discloses a synthetic method and intermediates of rosuvastatin calcium and synthetic methods of the intermediates. The synthetic method uses 4-4′-fluorophenyl-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyridine-5-formaldehyde as the raw material, includes 4-4′-fluorophenyl-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyridine-5-acrylonitrile (intermediate I) from a nitrilized reaction, and 4-4′-fluorophenyl-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyridine-5-acraldehyde (intermediate II) from an aldehydized reaction of the intermediate I, and further goes through such unit processes as side-chain extension, ketone-group reduction, ethyl-group hydrolysis and neutralization reaction or decomposition reaction to obtain rosuvastatin calcium. The nitrilized reagent can be phosphate diethylacetonitrile, acetonitrile, etc.; the aldehyde reductant can be diisobutyl aluminum hydride, red aluminum, etc.; and the ketone-group reductant can be diethylmethoxyborane, NaBH4, KBH4, etc.
Owner:ANHUI QINGYUN PHARMA & CHEM
Preparation method of rosuvastatin calcium
The invention discloses a preparation method of rosuvastatin calcium. The method comprises the following steps of: synthesizing an important intermediate, i.e., [4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-mesyl amino)pyrimidine-5-radical]methanol (compound IV); and preparing the rosuvastatin calcium (compound I). The method has the advantages of readily available raw materials, high stereoselectivity, high yield and suitability for industrial production.
Owner:湖南欧亚药业有限公司
Process for the preparation of amorphous rosuvastatin calcium
InactiveUS20070191318A1BiocideOrganic active ingredientsSecondary hyperlipidemiaRosuvastatin Calcium
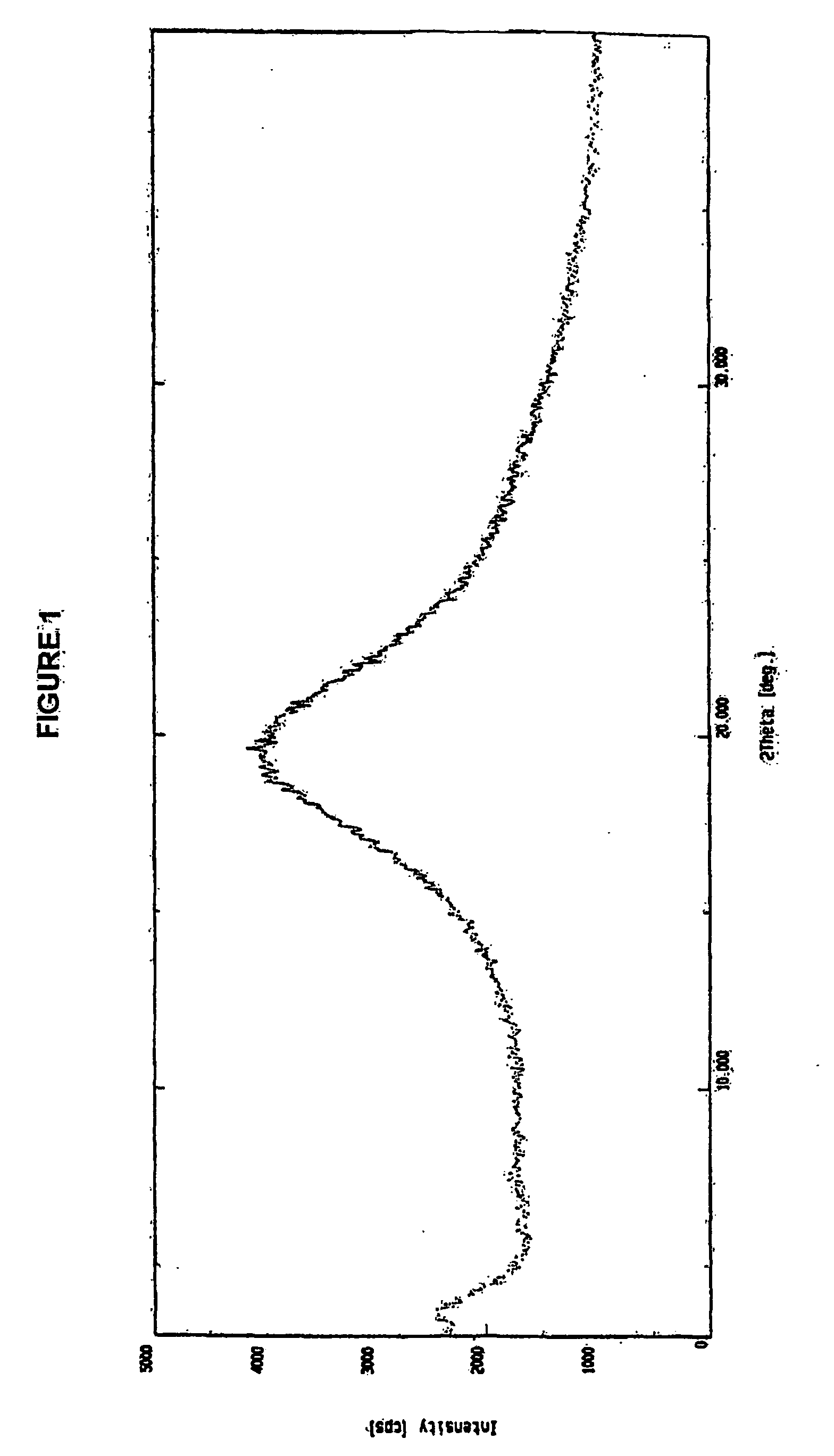
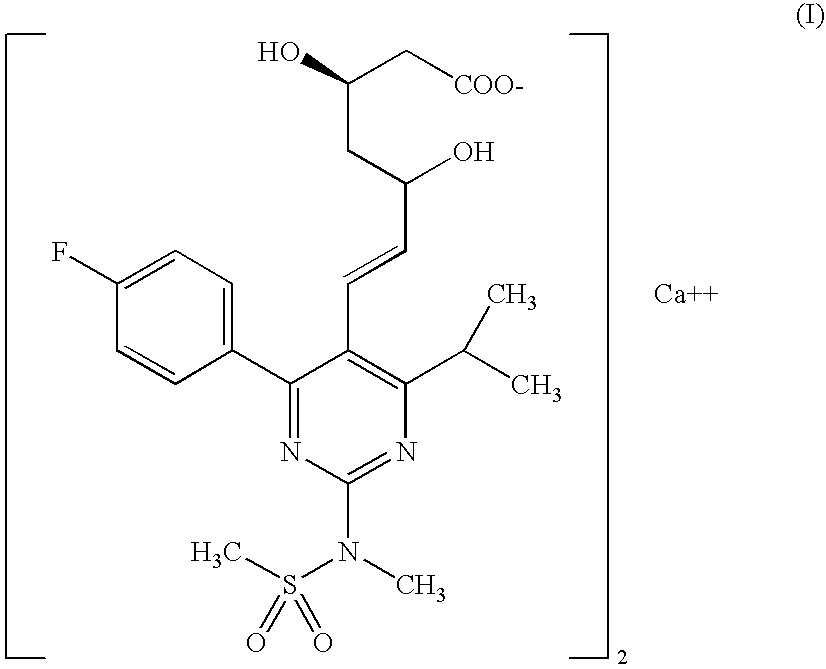
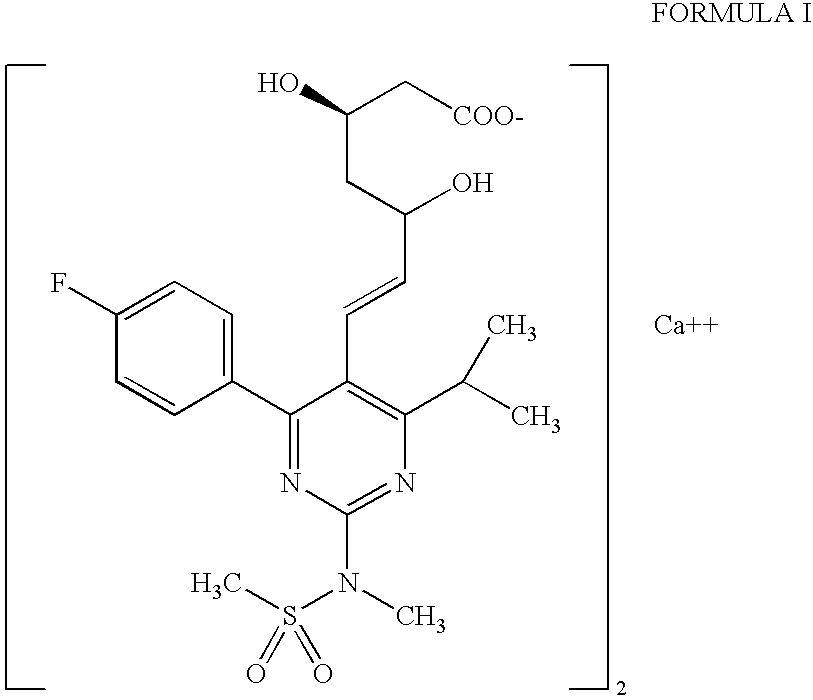
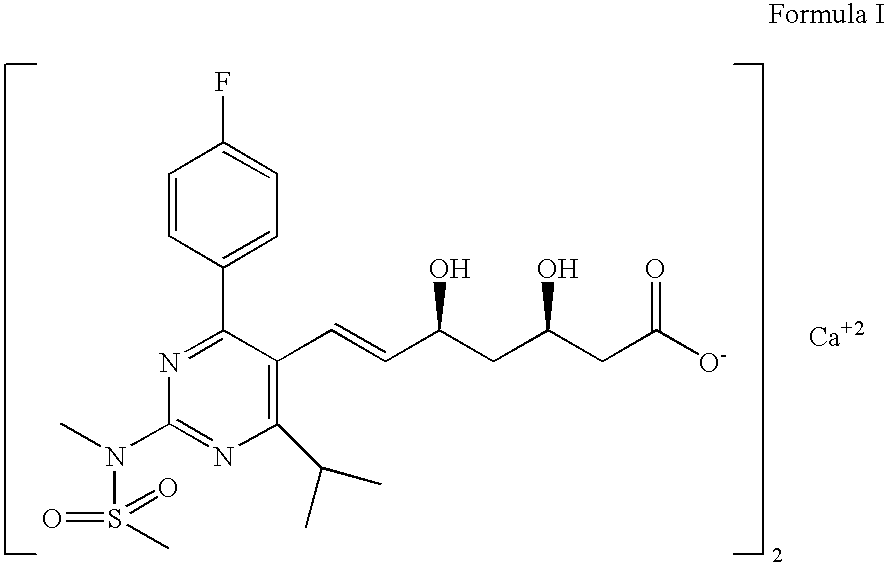
The invention relates to processes for the preparation of amorphous rosuvastatin calcium. More particularly, it relates to the preparation of pure amorphous rosuvastain calcium and pharmaceutical compositions that include the pure amorphous rosuvastatin calcium. The invention also relates to use of said compositions for treating hyperlipidemia, hypercholesterolemia, and atherosclerosis. Formula (I).
Owner:RANBAXY LAB LTD
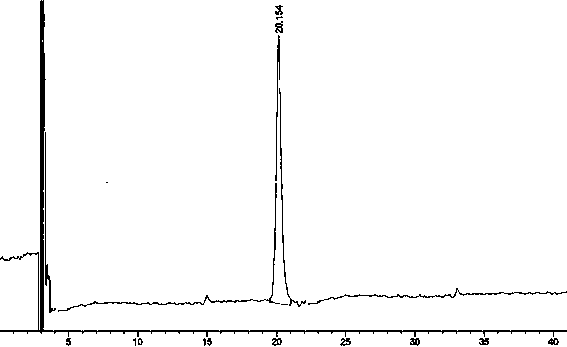
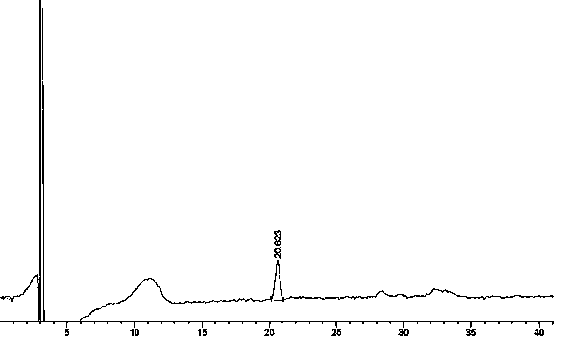
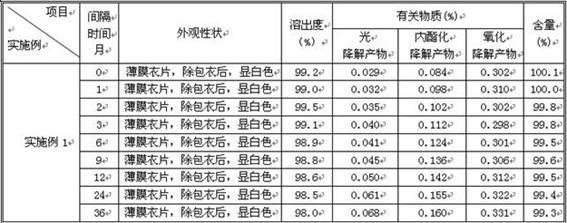
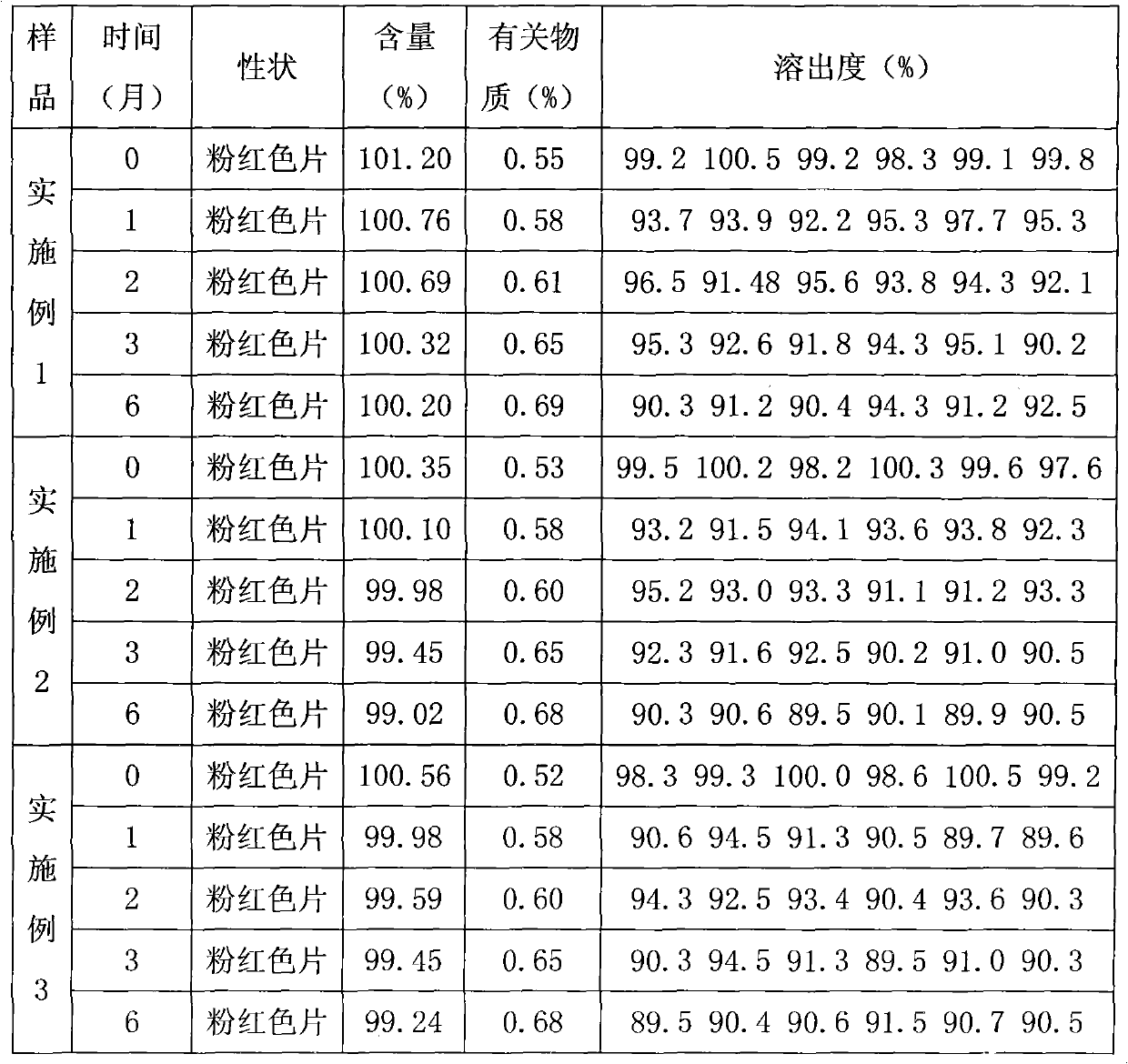
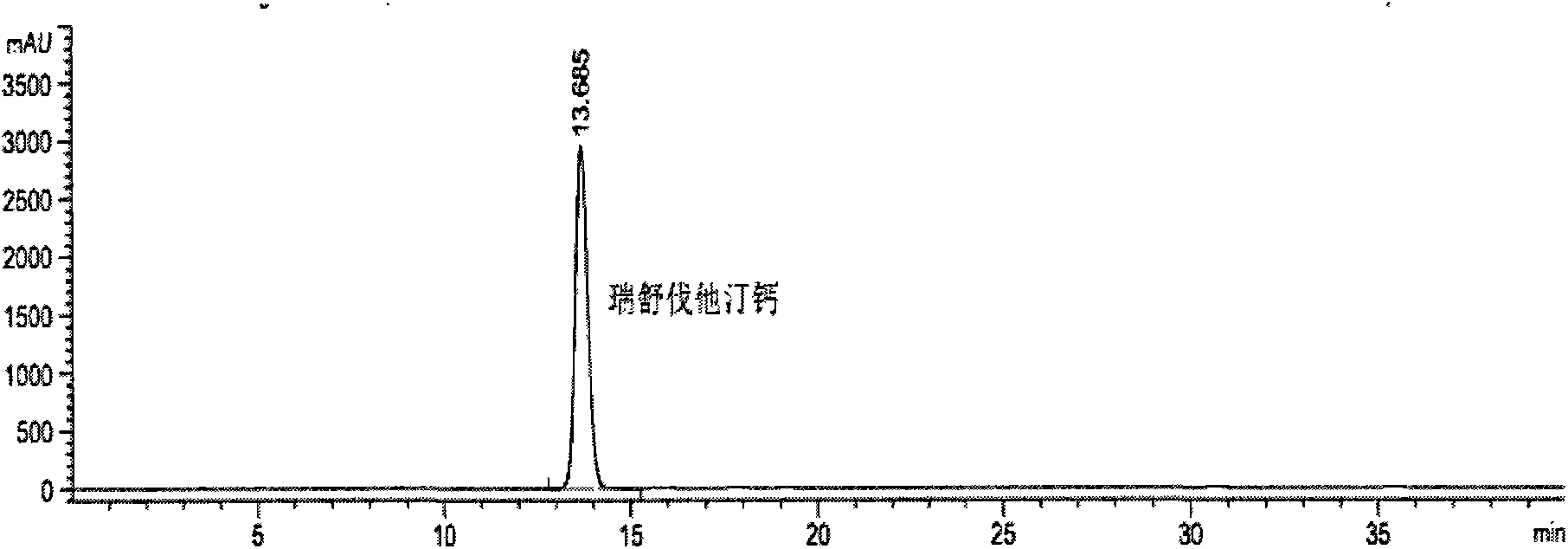
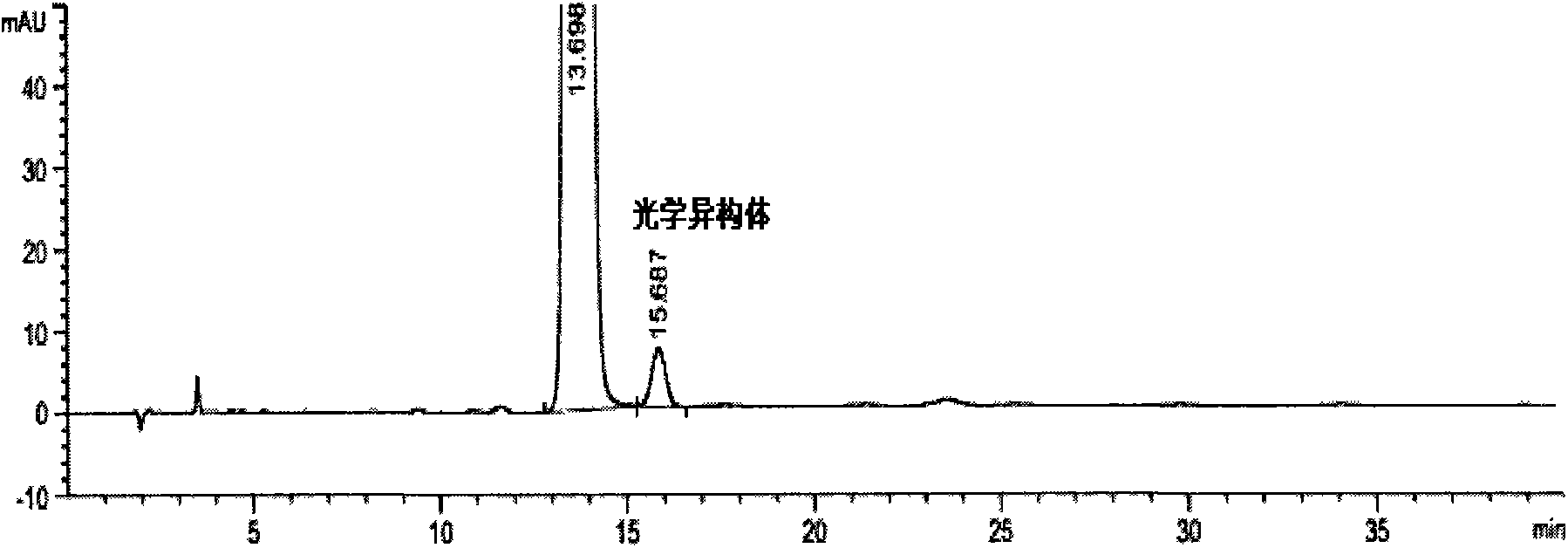
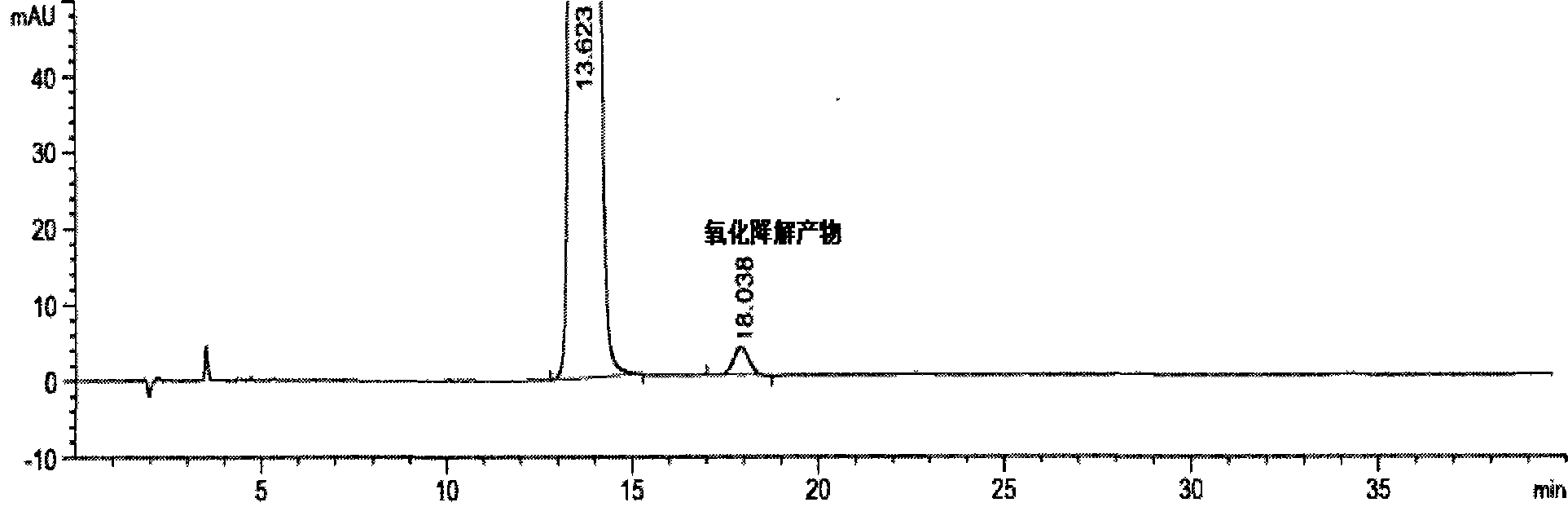
Preparation detection method for substances related to rosuvastatin calcium preparation
The invention relates to a preparation detection method for substances related to rosuvastatin calcium preparation. The preparation detection method comprises the following steps: preparing (3S,5S)-rosuvastatin calcium diastereoisomer, rosuvastatin-5S-lactone, rosuvastatin-5R-lactone, rosuvastatin-5-oxide, a rosuvastatin photodegradation product 1, a rosuvastatin photodegradation product 2, rosuvastatin photodegradation product 1-lactone and rosuvastatin photodegradation product 2-lactone, which are used as comparison products of the substances related the rosuvastatin calcium preparation; metering a test sample solution, a comparison product solution and a blank solution; and injecting the solutions into a liquid chromatograph respectively. The chromatographic conditions are as follows: a chromatographic column is a C18 chromatographic column; the column temperature is 30-40 DEG C, the flow speed is 0.6-1.0ml / min and the detection wavelength is 210-260nm. The content of the substances related to the rosuvastatin calcium preparation can be detected so as to be good for improving the quality standard of the rosuvastatin calcium preparation.
Owner:RUNZE PHARMACEUTICAL (SUZHOU) CO LTD
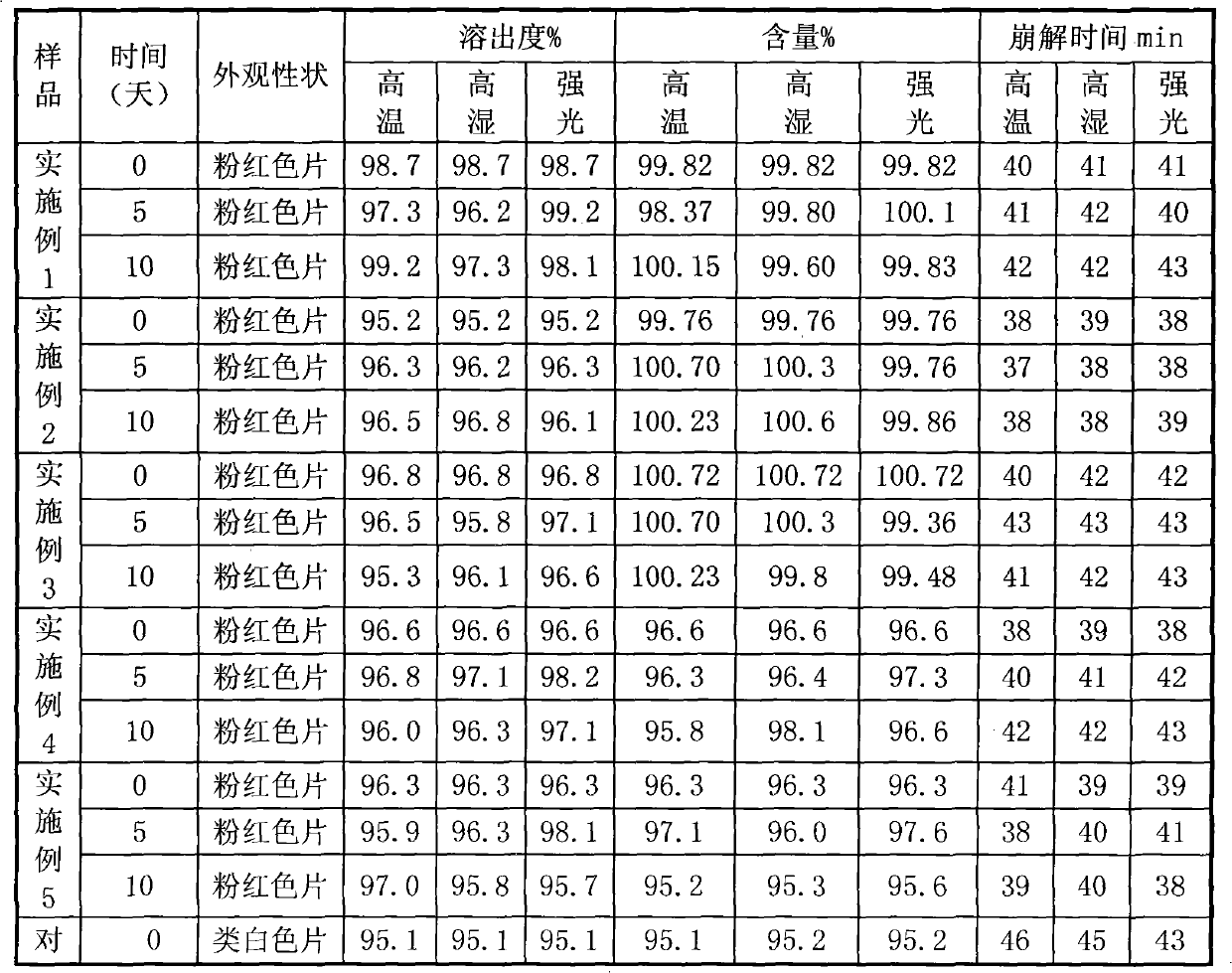
Rosuvastatin calcium tablet and preparation method thereof
ActiveCN104398484AHigh dissolution rateImprove stabilityOrganic active ingredientsMetabolism disorderDissolutionBULK ACTIVE INGREDIENT
The invention provides a rosuvastatin calcium pharmaceutical composition and a preparation method thereof. A rosuvastatin calcium tablet comprises a tablet core and a film coating, and is characterized in that the tablet core is formed by taking rosuvastatin calcium as a pharmaceutical active ingredient and a pharmaceutically auxiliary material; the pharmaceutically auxiliary material contains a microcrystalline cellulose-lactose compound, calcium hydrophosphate, polyvinylpolypyrrolidone and magnesium stearate. A direct powder compression method is adopted for tabletting to prepare a rosuvastatin calcium preparation, so that the stability, dissolution speed and content uniformity of the medicine are improved; the rosuvastatin calcium tablet is simple in technology and low in cost, and is suitable for large-scale production.
Owner:SHIJIAZHUANG NO 4 PHARMA
Method for preparing tablet drug composition containing Rosuvastatin calcium
InactiveCN102008477AThe monitoring indicators are qualifiedLow dissolution rateOrganic active ingredientsMetabolism disorderCyclodextrinRosuvastatin Calcium
The invention relates to a method for preparing a tablet drug composition containing Rosuvastatin calcium. The preparation forms of the drug composition can be administered to the patients safely and the stability of Rosuvastatin calcium and the absorbability in gastrointestinal tract can be improved. Specifically, the invention relates to a method for preparing the drug composition containing amorphous Rosuvastatin calcium and beta-cyclodextrin. The method is characterized by grinding Rosuvastatin calcium and beta-cyclodextrin under alkaline condition, drying and crashing to obtain (80-100)-mesh fine powder, mixing the fine powder with appropriate auxiliary materials, preparing granules, carrying out tabletting and carrying out coating, thus preparing the drug composition. The in vitro release of the composition is improved to a greater degree, thus improving the bioavailability; and the composition can effectively treat hyperlipidemia, is convenient to take orally, covers the bad taste and is fast to disintegrate and absorb and convenient to carry.
Owner:TIANJIN HANRUI PHARMA
Process for the preparation of rosuvastatin
InactiveUS20060149065A1Obvious benefitsSilicon organic compoundsMetabolism disorderHMG-CoA reductaseRosuvastatin Calcium
The present invention relates to a process for the preparation of rosuvastatin calcium, a promising new HMG-CoA reductase inhibitor.
Owner:SUN PHARMA INDS
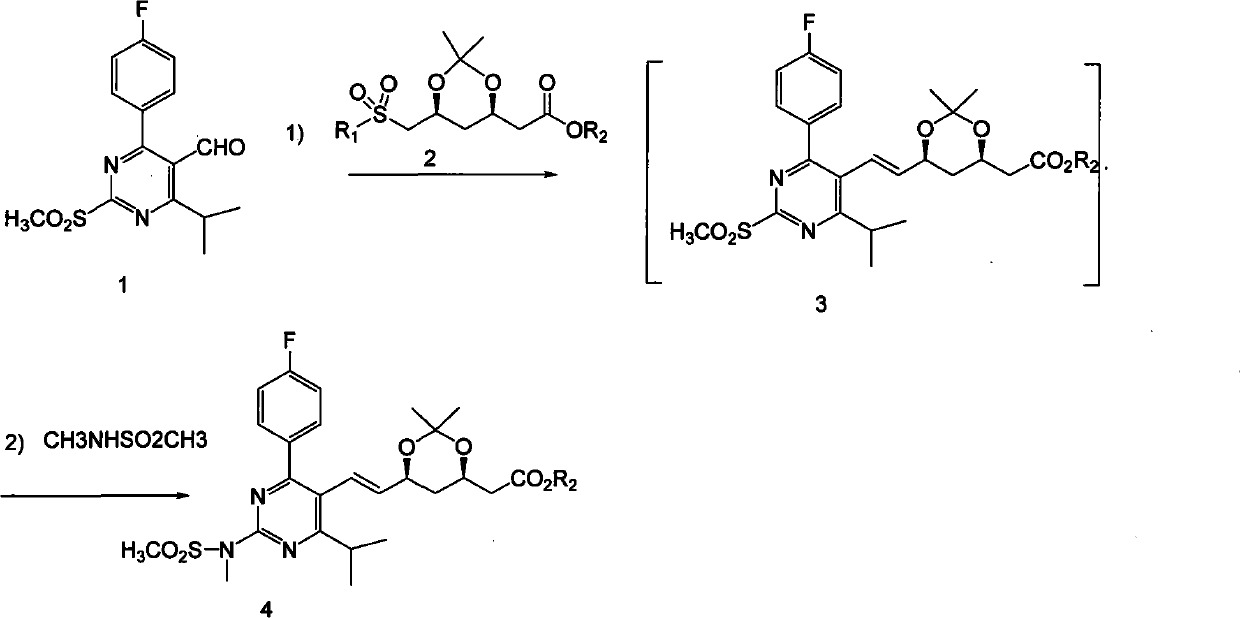
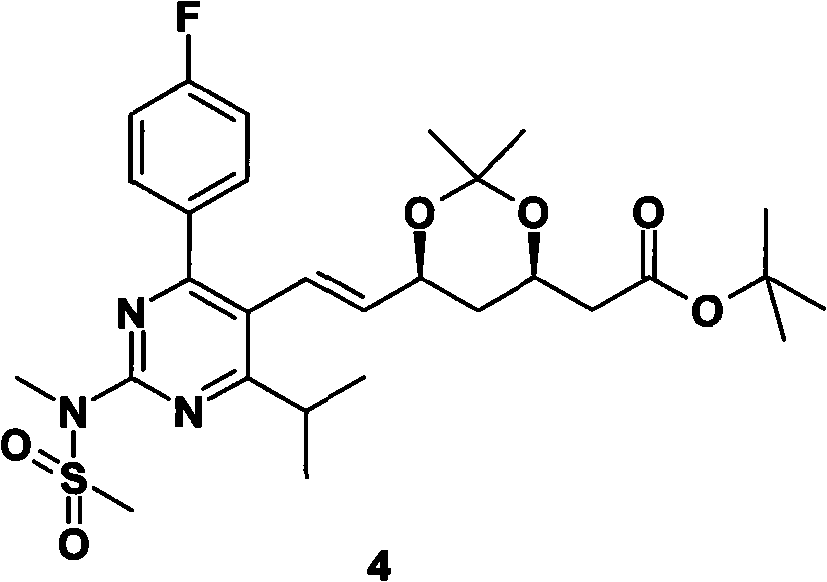
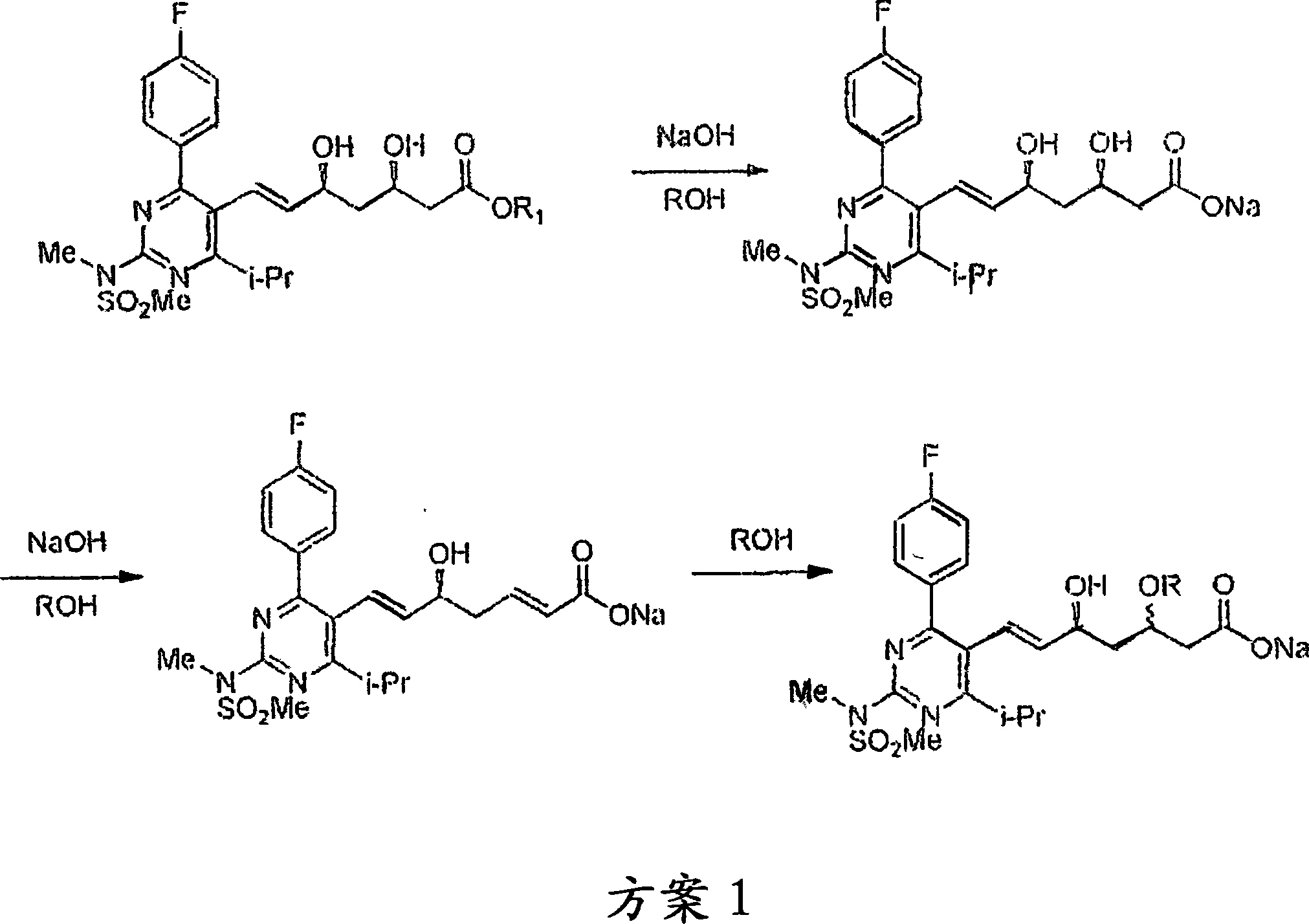
Method for preparing (3R, 5S, E)-7-{2-(N-methylsulphonylamino) -4-(4-fluorophenyl)-6-isopropyl-pyrimidine-5-yl}-2,2-dimethyl-3,5-dioxane-6-heptenoic acid
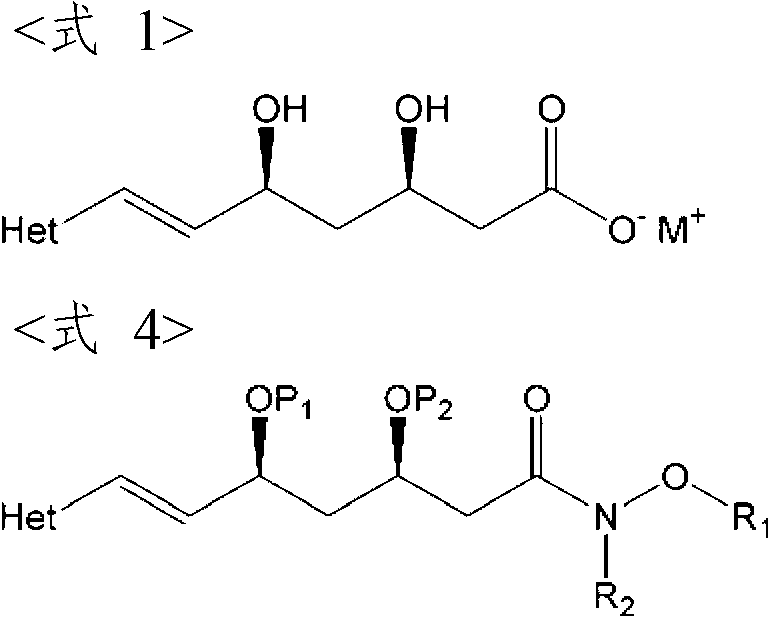
ActiveCN102219780AHigh yieldEasy to industrializeOrganic chemistryRoom temperatureRosuvastatin Calcium
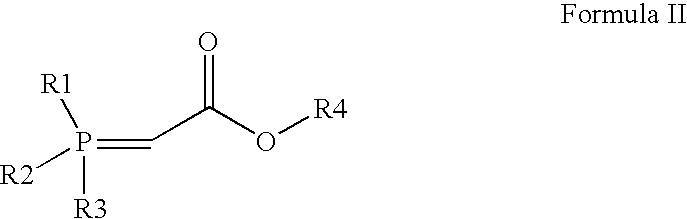
The invention relates to a method for preparing (3R, 5S, E)-7-{2-(N-methylsulphonylamino) -4-(4-fluorophenyl)-6-isopropyl-pyrimidine-5-yl}-2,2-dimethyl-3,5-dioxane-6-heptenoic acid (compound shown by a formula 4) which is an important intermediate of Rosuvastatin Calcium in a one-pot way. The method comprises the steps of adding a compound in the formula 1 and a compound in the formula 2 into a solvent under protection of dried inert gas, adding a certain amount of strong base at minus 60 DEG C, then slowly increasing temperature to room temperature, supplementing the strong base after a compound in the formula 3 is completely produced, adding N-methylsulphonylamino, and reacting to produce a compound in the formula 4 at the room temperature. The method is stable in yield and easy to operate, and provides a method for preparing Rosuvastatin, and the method has lower cost, is simplified for operation and easy for industrialization. The formula 1, 2, 3, and 4 are shown in the description, wherein R1 is shown in description, and R2 is alkyl, naphthenic base or benzyl, and preferably methyl, ethyl or tertiary butyl.
Owner:SHANGHAI JINGXIN BIOLOGICAL MEDICAL
Process for preparing rosuvastatin calcium
InactiveUS20100029940A1Simple, industrially applicable and economically viableOrganic chemistryRosuvastatin CalciumMedicinal chemistry
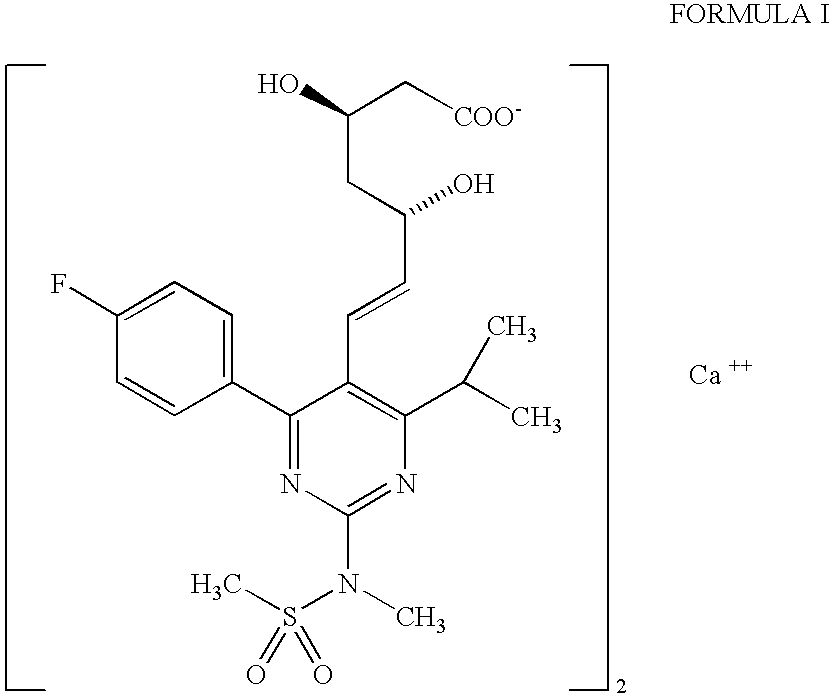
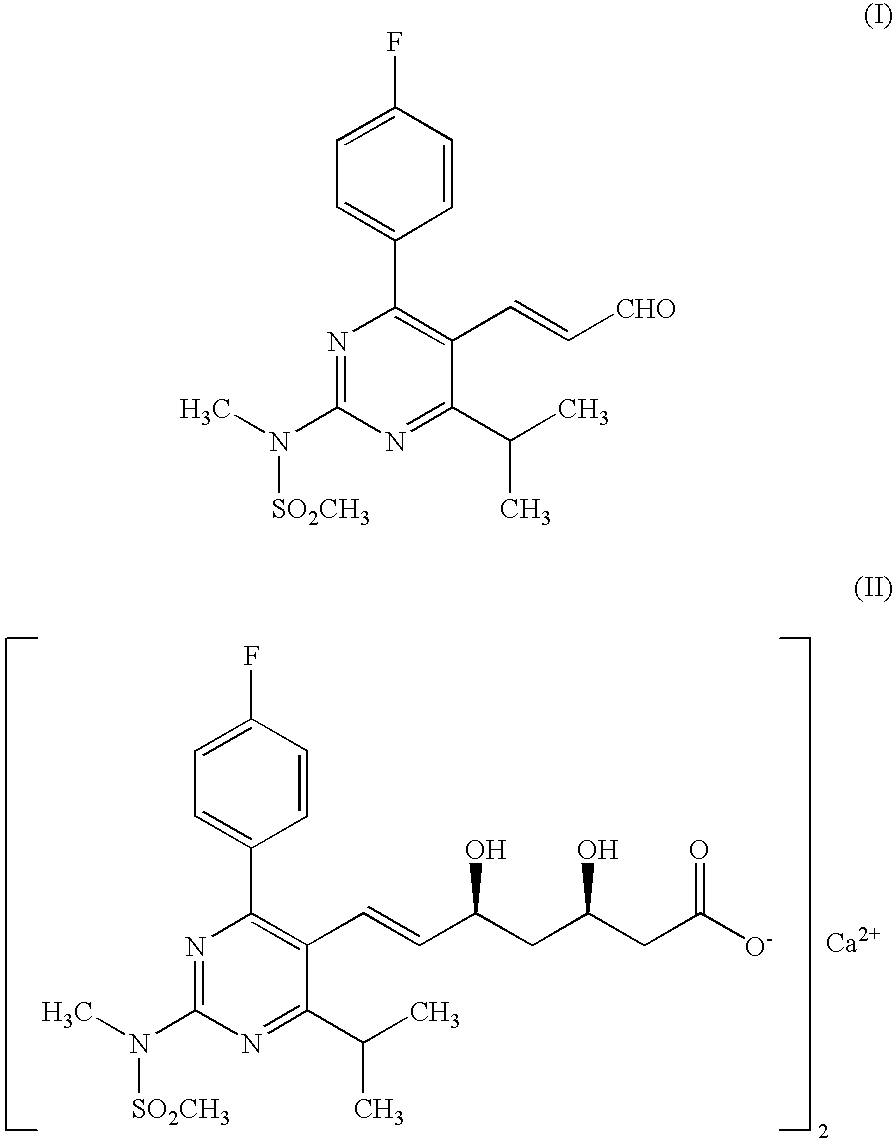
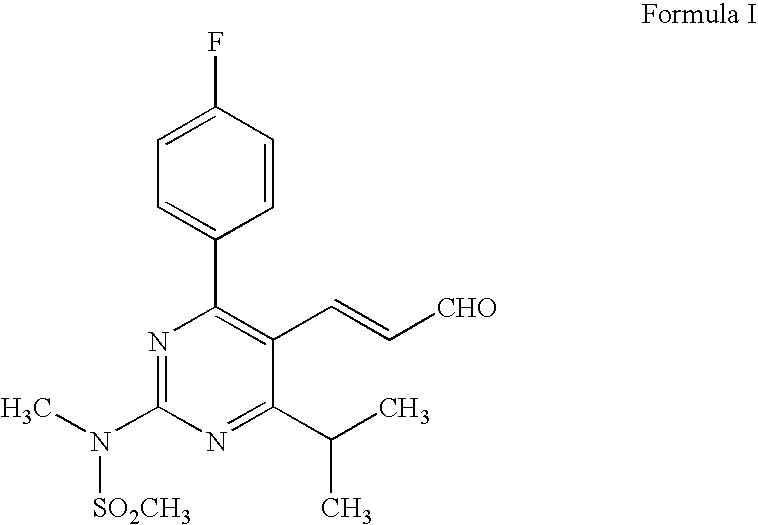
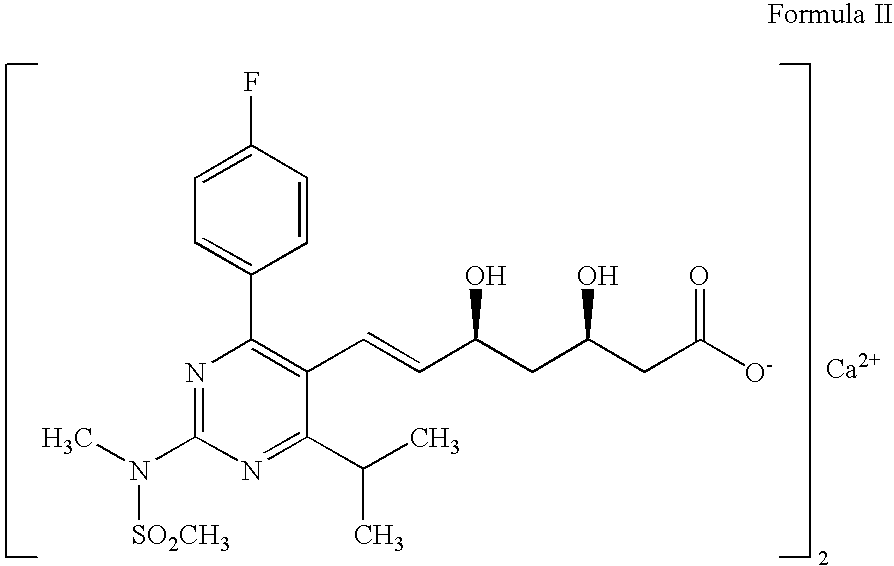
The present invention relates to an improved process for preparing (2E)-3-[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methylsulfonylamino)pyrimidin-5-yl]-propenal of Formula I which is an intermediate useful in the preparation of bis[(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methyl-sulfonyl)amino]pyrimidin-5-yl](3R,5S)-3,5-dihydroxyhept-6-enoicacid] calcium salt of Formula II
Owner:AUROBINDO PHARMA LTD
Rosuvastatin calcium tablet and preparation method thereof
ActiveCN104473899ADissolution rate is fastHigh dissolution rateOrganic active ingredientsMetabolism disorderDissolutionLactose
The invention discloses a rosuvastatin calcium tablet and a preparation method thereof. The rosuvastatin calcium tablet comprises components in parts by weight as follows: 5.0-10.5 parts of rosuvastatin calcium, 20-22 parts of microcrystalline cellulose, 73.5-78.8 parts of lactose, 40 parts of calcium carbonate, 4.5 parts of crosslinked polyvinylpyrrolidone and 1.5 parts of magnesium stearate. The rosuvastatin calcium tablet comprises the rosuvastatin calcium, the microcrystalline cellulose, the lactose, the calcium carbonate, the crosslinked polyvinylpyrrolidone and the magnesium stearate, dosages of all the components are strictly controlled, main medicines and auxiliary materials complement one another to realize a synergistic effect, and the prepared tablet is high in dissolution speed, high in dissolution rate and high in bioavailability; raw materials are easy to obtain, the cost is low, the product equality is controllable, the quality stability and the safety are high, and the rosuvastatin calcium tablet is suitable for popularization and application.
Owner:HENAN RUNHONG PHARMA
Process for Preparation of Calcium Salt of Rosuvastatin
The invention relates to commercially viable process for the preparation of Rosuvastatin by an early introduction of correct absolute stereochemistry at C-5 (S) of Rosuvastatin side chain followed by regioselective chain extension using novel side chain building blocks and less expensive reagents. It is yet another object of the invention is to provide novel intermediates that may be used for the preparation of Calcium salt of Rosuvastatin.Formula (I).
Owner:DESHPANDE PANDURANG BALWANT +3
Rosuvastatin calcium oral drug composition
The invention relates to a rosuvastatin calcium oral drug composition and a preparation method thereof. The rosuvastatin calcium oral drug composition is characterized by comprising the following components in proportion: 10-80mg of rosuvastatin calcium, 20-160mg of hydroxypropyl betadex, 5-40mg of meglumine, 2-16mg of sodium sulfite, 100-800mg of lactose, 50-400mg of microcrystalline cellulose, 20-160mg of croscarmellose sodium, 2-16mg of magnesium stearate and a proper amount of 80 percent ethanol solution. The preparation method comprises the following steps of: dissolving hydroxypropyl betadex, meglumine and magnesium stearate into a proper amount of 80% ethanol solution; adding rosuvastatin calcium and mixing until the rosuvastatin calcium is completely dissolved; evenly mixing lactose, microcrystalline cellulose and croscarmellose sodium for later use; quantitatively adding the solution into an evenly-mixed accessory for pelletization; and obtaining a finished product by drying, palletizing, totally mixing, checking an intermediate body, pressing into pieces, coating and packing. The prepared rosuvastatin calcium tablet has good stability and high bioavailability.
Owner:TIANJIN HANRUI PHARMA
Tablet containing Rosuvastatin calcium and preparation process thereof
ActiveCN101766578AImprove quality stabilityHigh dissolution rateOrganic active ingredientsMetabolism disorderCyclodextrinLactose
The invention belongs to the filed of medicine, in particular to a tablet containing Rosuvastatin calcium and a preparation process thereof. As for the tablet containing the Rosuvastatin calcium of the invention, lactose and hydroxypropyl cyclodextrin with the ratio ranging from 1:1 to 2.5:1 are selected to serve as fillers, and opacifier medicinal iron oxide red is added in production process. In the invention, a direct powder tabletting method is utilized for taletting, and the prepared tablet containing the Rosuvastatin calcium has the characteristics of stable long-term preservation, rapid disintegration rate, high dissolution and high bioavailability.
Owner:LUNAN BETTER PHARMA
Method for determining content of rosuvastatin calcium and related substances thereof by employing HPLC (high performance liquid chromatography) method
ActiveCN103454352ASimple and fast operationEfficient separationComponent separationFiller ExcipientTrifluoroacetic acid
The invention discloses a method for determining content of rosuvastatin calcium and related substances thereof by employing an HPLC (high performance liquid chromatography) method. By adopting the method, octadecylsilane chemically bonded silica is selected as a chromatographic column of a filler, and a mobile phase is formed by methanol and water (containing 0.1-0.8% of trifluoroacetic acid). The content of the rosuvastatin calcium and the related substances thereof can be accurately determined under a chromatographic condition, so that the quality of materials and preparation of the rosuvastatin calcium is well controlled.
Owner:GUANGDONG XIANQIANG PHARMA
Rosuvastatin calcium sustained-release preparation and preparation method thereof
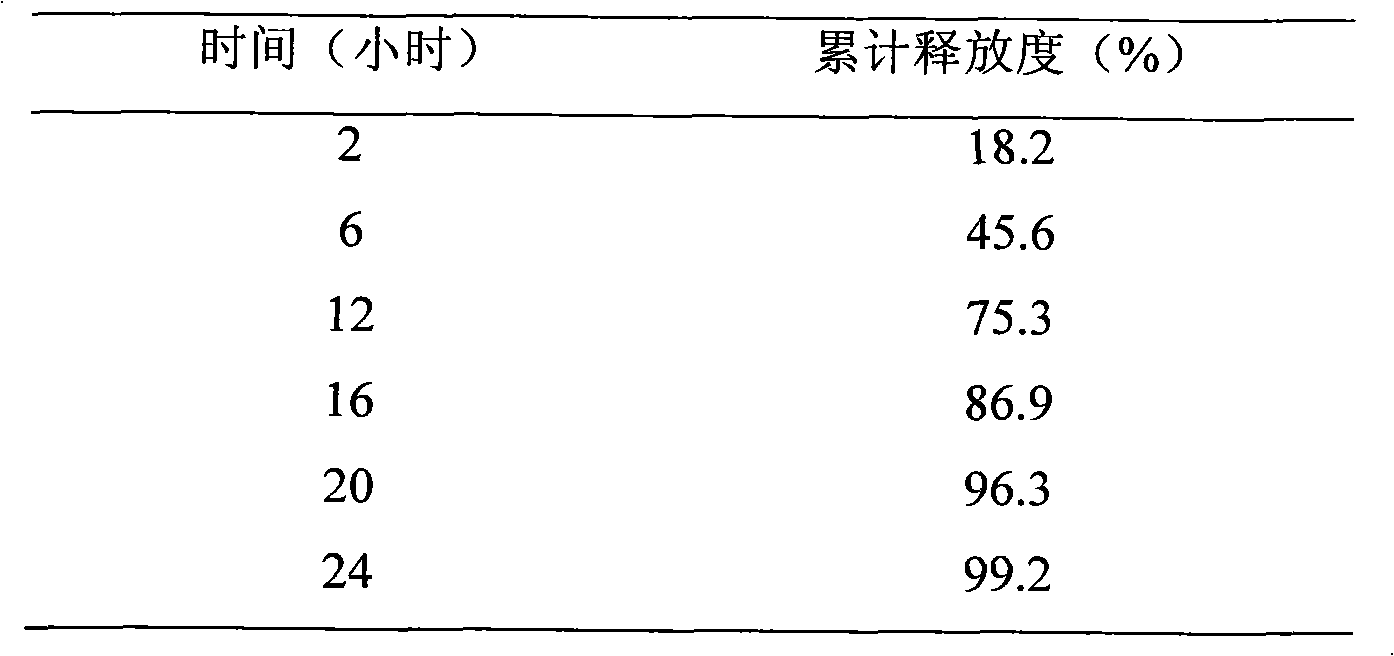
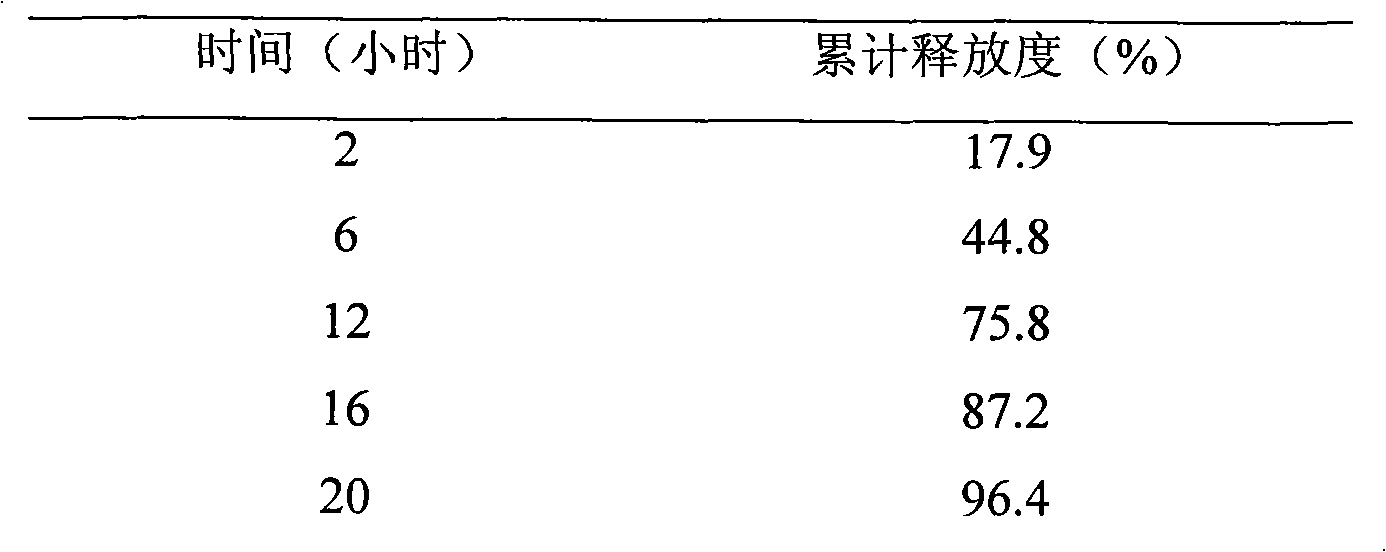
InactiveCN101889975AOvercome the defect of "peak valley" fluctuation in blood drug concentrationImprove securityOrganic active ingredientsMetabolism disorderRosuvastatin CalciumBlood drug
The invention relates to a rosuvastatin calcium sustained-release preparation and a preparation method thereof. The rosuvastatin calcium sustained-release preparation basically contains 5 to 10mg of rosuvastatin calcium, and the balance of sustained-release framework material and other pharmaceutical excipients. The preparation method is simple; and all the materials are proportioned and the preparation is prepared by the preparation method for common tablets, granules or capsules. The rosuvastatin calcium sustained-release preparation prepared by the method avoids adverse reactions such as rhabdomyolysis, proteinuria, nephrosis, kidney failure, hepatotoxicity and the like caused by overdosage of medicaments; meanwhile, due blood concentration and time for treating diseases after the medicaments are taken can be maintained, and the peak valley phenomenon of the blood concentration is effectively avoided.
Owner:BEIJING HONGWAN PHARMA TECH
Process For The Preparation Of HMG-COA reductase inhibitors and intermediates thereof
ActiveCN103025727AAvoid formingFew reaction stepsOrganic active ingredientsOrganic chemistryHMG-CoA reductaseRosuvastatin Calcium
Owner:YUHAN
Preparations containing rosuvastatin calcium and amlodipine and method for preparing the same
InactiveCN101095680AIt has the effect of lowering blood fat and blood pressureSynergisticOrganic active ingredientsMetabolism disorderLevamlodipineRosuvastatin Calcium
The invention provides a medical compound, which comprises effective amount of rosuvastatin calcium and amlodipine, which is characterized in that said two material are combined together and they can reduce blood fat and blood pressure, and they are cooperative.
Owner:SHANGHAI SINE PHARMA LAB
Preparation method of intermediate of rosuvastatin calcium side chain
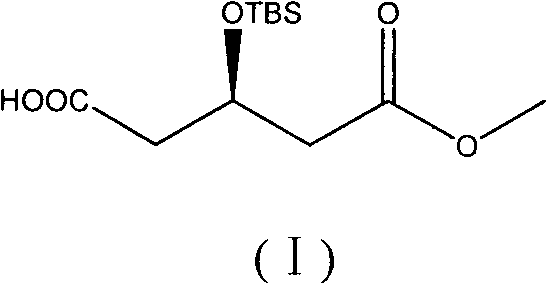
The invention discloses a preparation method of an intermediate of a rosuvastatin calcium side chain, which comprises the following steps: firstly allowing (R)-4-cyano-3- methyl hydroxybutyrate to react with tert-butyl-dimethylchlorosilane, performing nitrile group hydrolysis in water phase under the action of an enzyme with the obtained (R)-4-cyano-3-tert-butyl dimethylsiloxyl methyl butyrate (II) as a substrate to generate a target product with a structure (I). The invention has the characteristics of being mild in reaction, high in yield, environment friendly, low in cost, and high in chiral purity of the prepared product.
Owner:JIANGSU ALPHA PHARM CO LTD
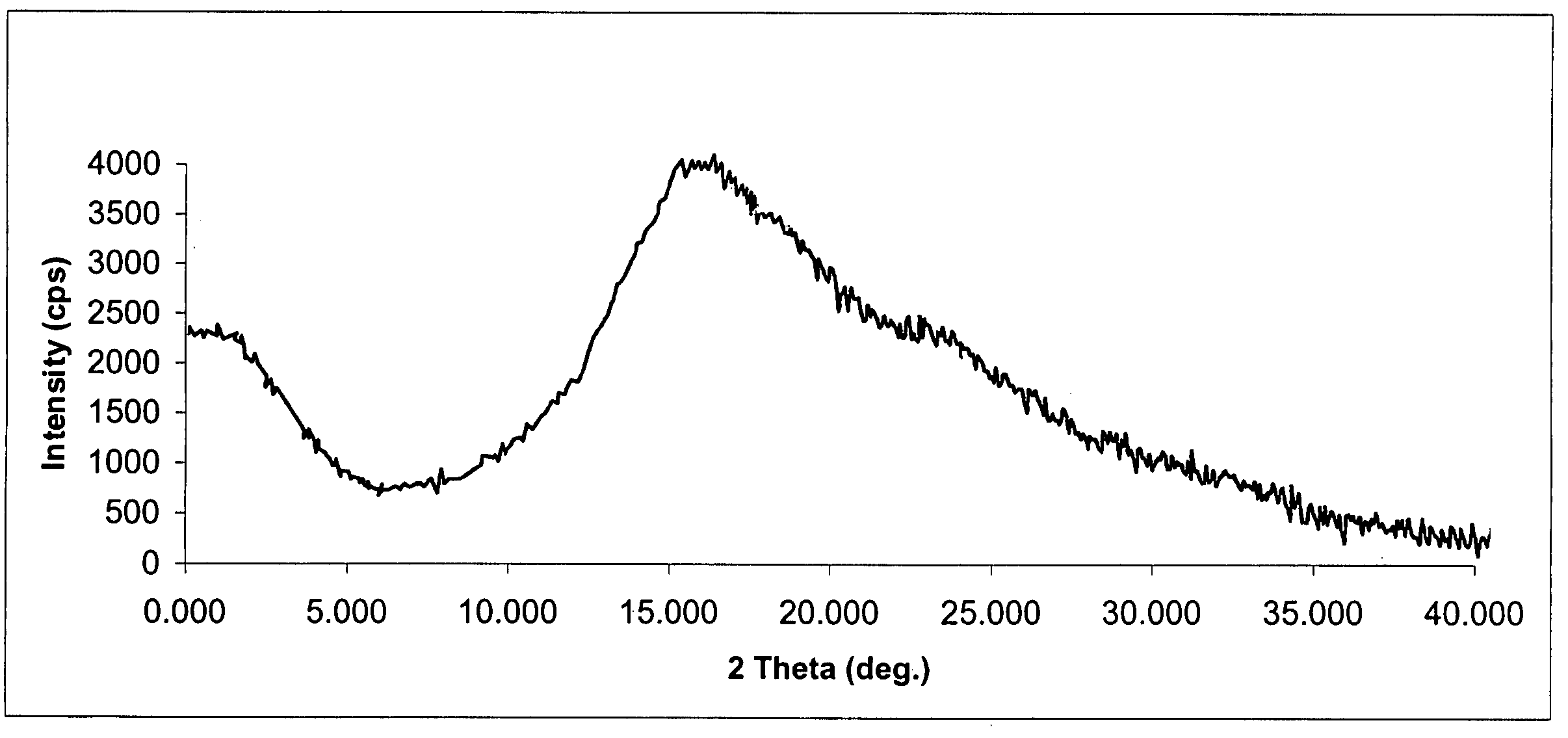
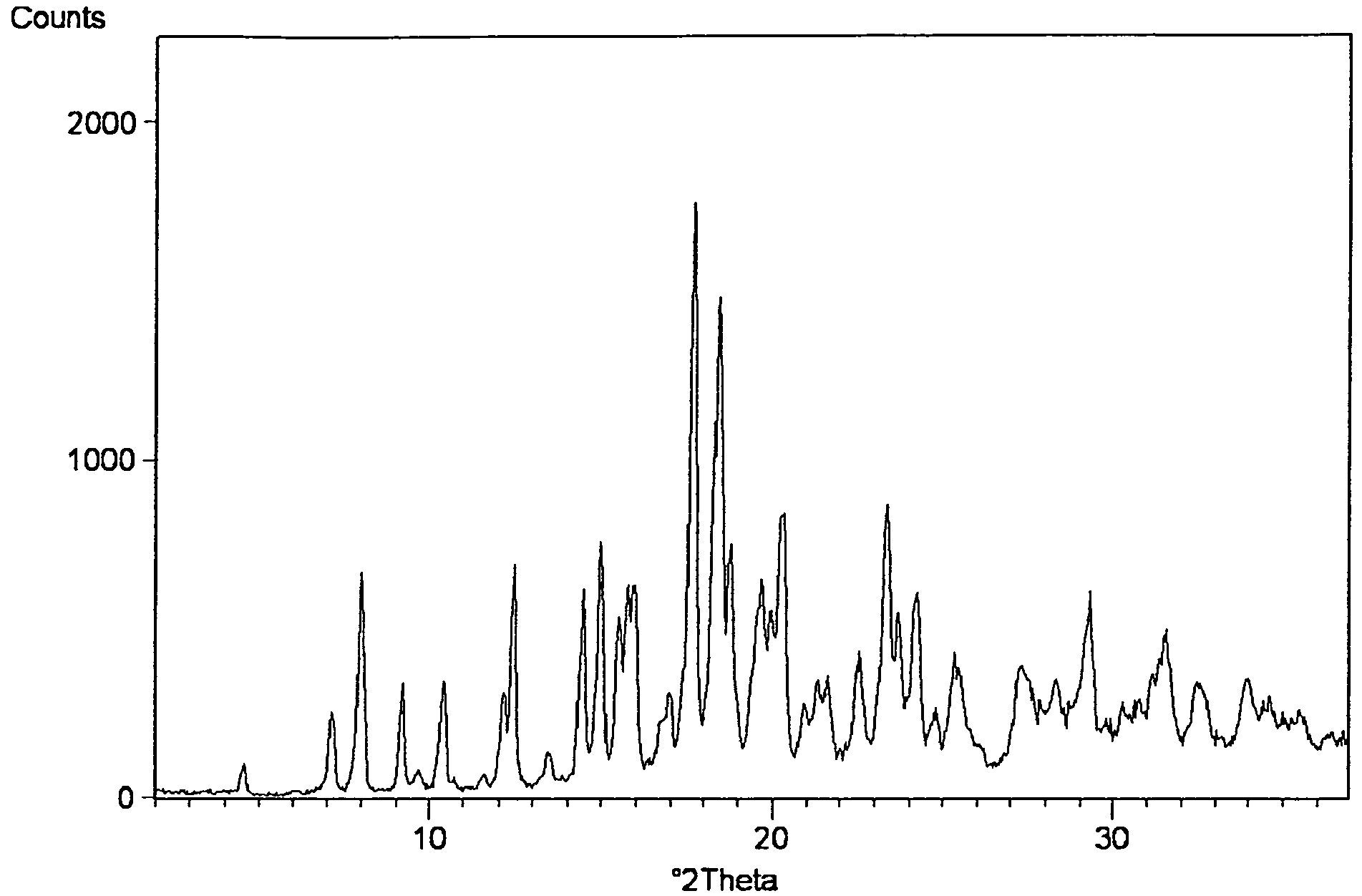
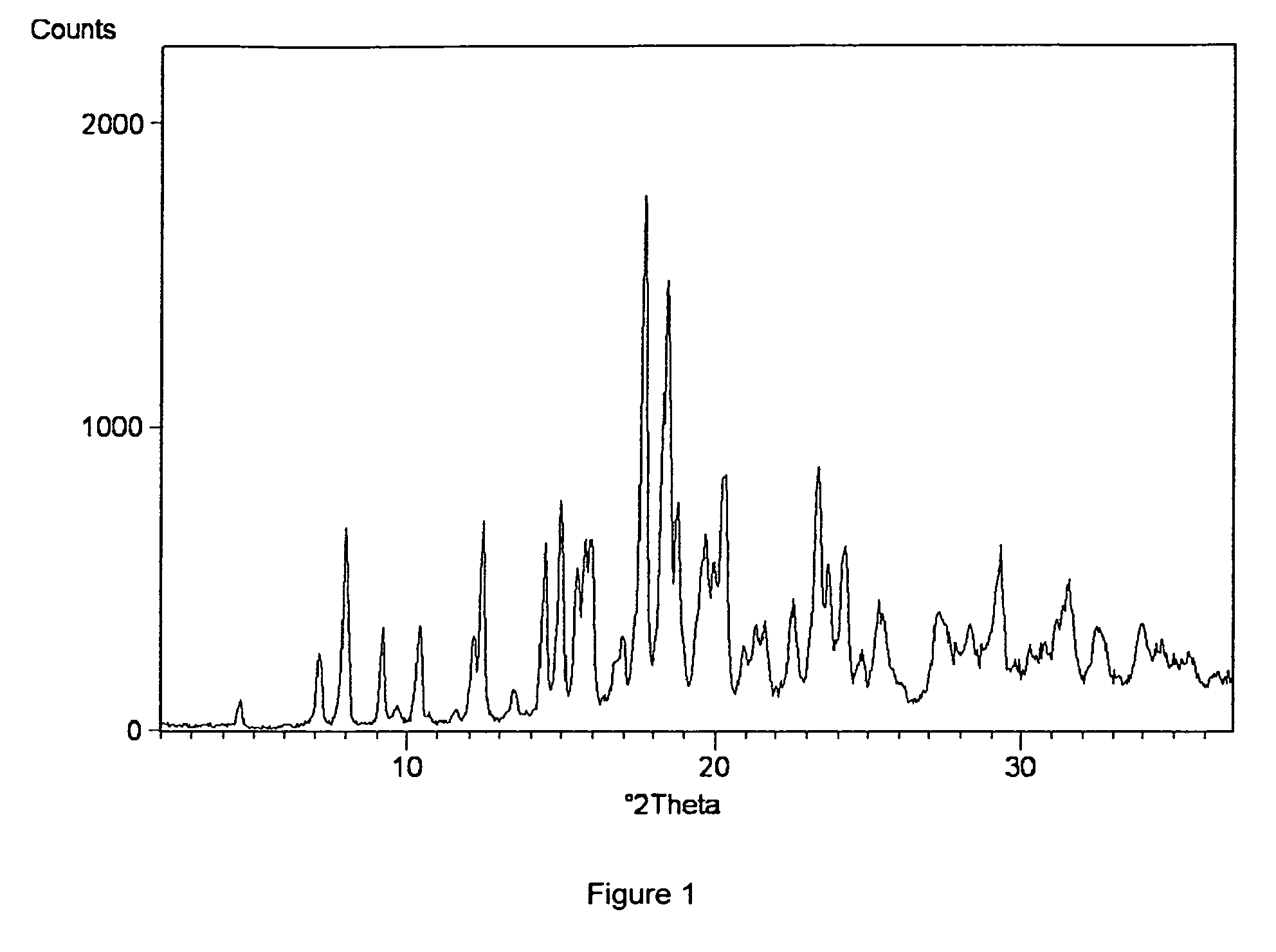
Process for preparing amorphous rosuvastatin calcium free of impurities
InactiveCN101208307AOrganic active ingredientsOrganic chemistryHMG-CoA reductaseRosuvastatin Calcium
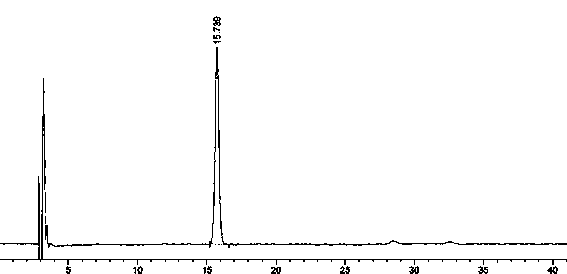
A pure amorphous form of rosuvastatin calcium having purity of more than 99.5%, preferably a purity of more than 99.8%, more preferably a purity of more than 99.9% as determined by HPLC area percentage, and free from any traces of alkali metal impurities is disclosed. A process of preparing said pure amorphous form of rosuvastatin calcium is disclosed, which comprises hydrolysis of C1-C5 alkyl esters of rosuvastatin, preferably terf-butyl ester of rosuvastatin, with an organic nitrogen base, e.g. guanidines, amidines, amines and quaternary ammonium hydroxides, in the presence of water, optionally containing aprotic solvent, following the conversion of thus obtained rosuvastain salt with a source of calcium to desired rosuvastatin calcium, which is then isolated. An alternative process is disclosed, which comprises the conversion of numerous novel ammonium salts of rosuvastatin, preferably tert-octylammonium salt of rosuvastatin, with the source of calcium to desired commercial rosuvastatin calcium. Rosuvastatin calcium is HMG CoA reductase, useful in the treatment of hyperlipidemia, hypercholesterolemia and atherosclerosis.
Owner:LEK PHARMA D D
Preparation methods for rosuvastatin calcium and intermediates thereof
ActiveCN103483269ANovel structureOperational securityOrganic chemistryRosuvastatin CalciumSafe operation
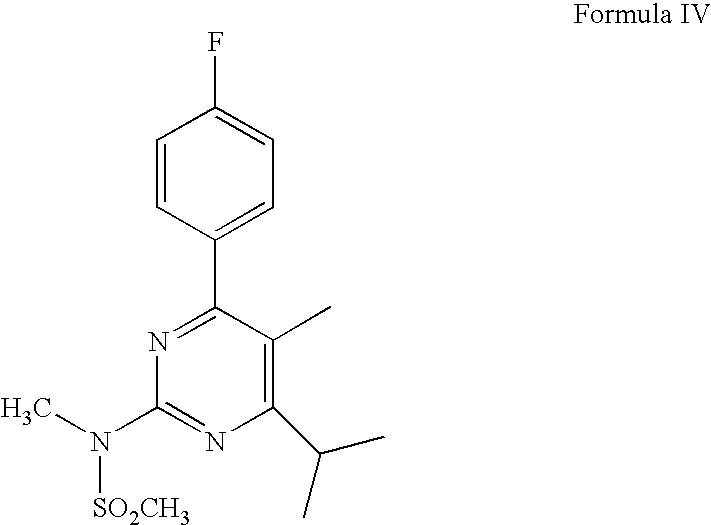
The present invention relates to preparation methods for rosuvastatin calcium and intermediates thereof, and specifically discloses intermediate compounds for preparing rosuvastatin calcium, and preparation methods thereof, wherein the structures of the intermediates are respectively represented by a formula II, a formula III, a formula IV, a formula VI and a formula VII in an instruction. The present invention further discloses a preparation method for rosuvastatain or a salt thereof, wherein the preparation method is based on the preceding five intermediate compounds and the preparation methods thereof, has characteristics of simple and safe operation and low production cost, and is suitable for industrial production.
Owner:SHANGHAI DESANO PHARMA INVESTMENT +1
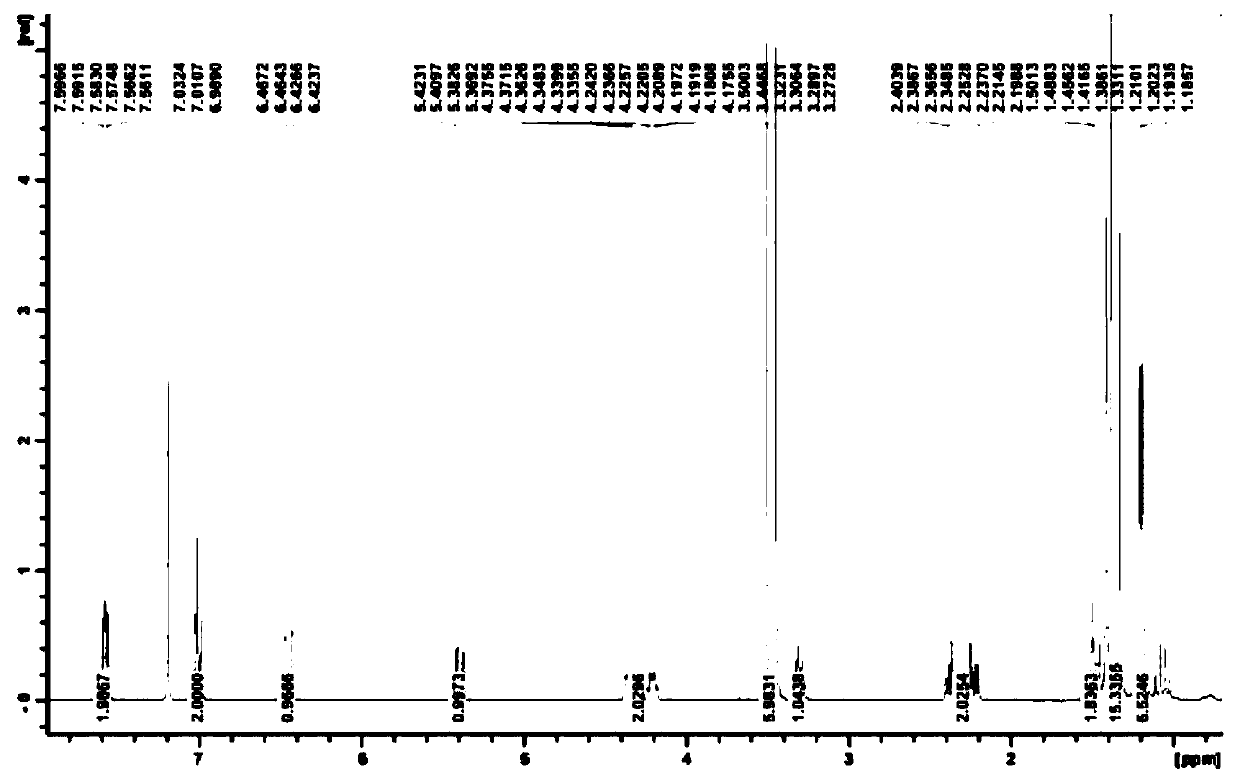
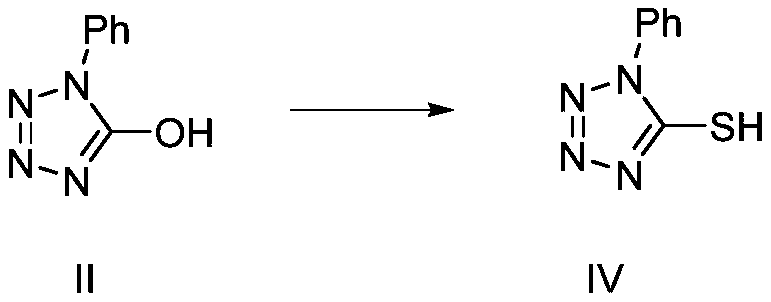
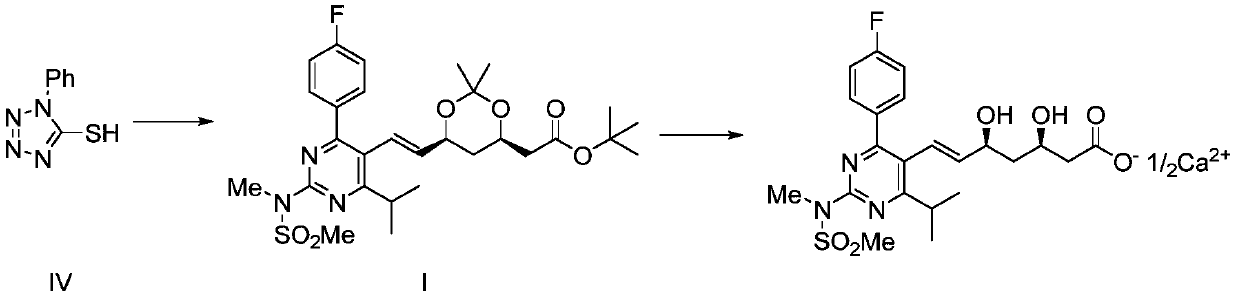
Recycling method of 1-phenyl-5-hydroxytetrazole
ActiveCN110627736ARealize reasonable recyclingReduce manufacturing costOrganic chemistryRosuvastatin Calcium1-phenyl-5-mercaptotetrazole
The invention discloses a recycling method of 1-phenyl-5-hydroxytetrazole, and belongs to the technical field of medicinal chemistry. The preparation method comprises the following steps: a byproduct1-phenyl-5-hydroxytetrazole generated in preparation process of a rosuvastatin calcium intermediate is converted into 1-phenyl-5-mercaptotetrazole; and meanwhile, the 1-phenyl-5-mercaptotetrazole is further used for preparing a rosuvastatin calcium intermediate or rosuvastatin calcium. According to the method disclosed by the invention, reasonable recycling of the byproduct 1-phenyl-5-hydroxytetrazole is realized, the raw materials are fully utilized, the production cost of rosuvastatin calcium and the intermediate thereof is reduced, and the emission of three wastes is reduced.
Owner:JIANGSU ALPHA PHARM CO LTD
Process for Preparing Amorphous Rosuvastatin Calcium of Impurities
A pure amorphous form of rosuvastatin calcium substantially free from alkali metal impurities is disclosed. A process of preparing a pure amorphous form of rosuvastatin calcium is disclosed, which comprises hydrolysis of C1-C5 alkyl esters of rosuvastatin, preferably tert-butyl ester of rosuvastatin, with an organic nitrogen base, e.g. guanidines, amidines, amines and quaternary ammonium hydroxides, in the presence of water, optionally containing aprotic solvent, following the conversion of thus obtained rosuvastatin salt with a source of calcium to obtain rosuvastatin calcium, which is then isolated. An alternative process is disclosed, which comprises the conversion of numerous novel ammonium salts of rosuvastatin, preferably tert-octylammonium salt of rosuvastatin, with the source of calcium to desired commercial rosuvastatin calcium. Rosuvastatin calcium is an inhibitor of HMG CoA reductase, useful in the treatment of hyperlipidemia, hypercholesterolemia and atherosclerosis.
Owner:LEK PHARMA D D
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com