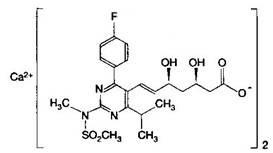
Rosuvastatin calcium oral drug composition
A technology of rosuvastatin calcium and its composition, which is applied in the field of rosuvastatin calcium oral pharmaceutical composition and its preparation, can solve the problems of stability of rosuvastatin calcium preparation, difficulty in configuring product operation, and the difficulty of pharmaceutical composition. Less than the requirements of the storage period, etc., to achieve the effect of improving safety, overcoming poor stability, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
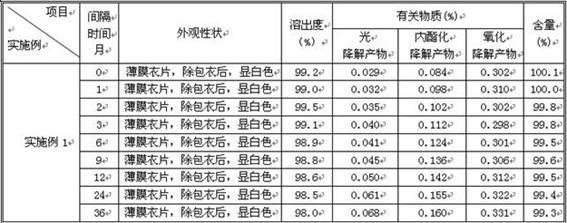
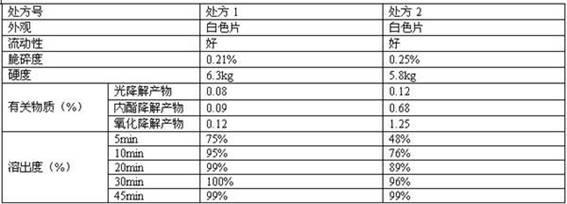
Examples
Embodiment 1
[0076] The second part of the present invention relates to the preparation method of preparing rosuvastatin calcium pharmaceutical composition, and its step comprises:
[0077] 1) Preparation and processing of raw and auxiliary materials: pass rosuvastatin calcium and auxiliary materials through a 100-mesh sieve for later use.
[0078] 2) Weighing: Weigh the above-mentioned raw and auxiliary materials according to the prescription amount and calculate the feeding amount after checking by two people.
[0079]3) Dissolve the prescribed amount of hydroxypropyl beta cyclodextrin, meglumine and sodium sulfite in an appropriate amount of 80% ethanol solution, then add the prescribed amount of rosuvastatin calcium and stir until completely dissolved, as a binder.
[0080] 4) Mix the prescription amount of lactose, microcrystalline cellulose, and croscarmellose sodium evenly, and set aside.
[0081] 5) Granulation: Add 3) to 4), and granulate with a 30-mesh nylon screen.
[0...
Embodiment 2
[0092] Its step is with embodiment 1.
[0093] Example 3
Embodiment 3
[0096] Its step is with embodiment 1.
[0097] Example 4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com