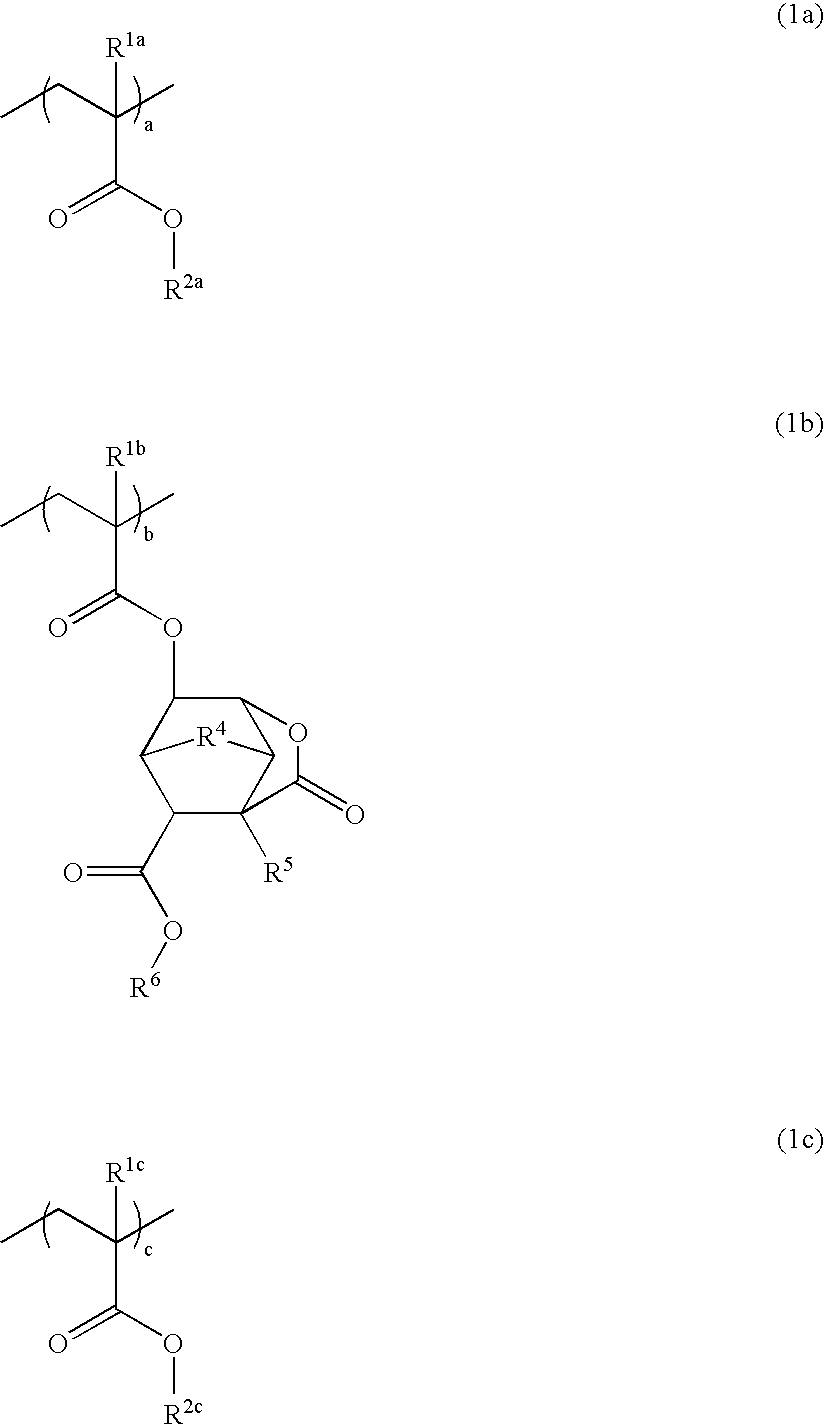
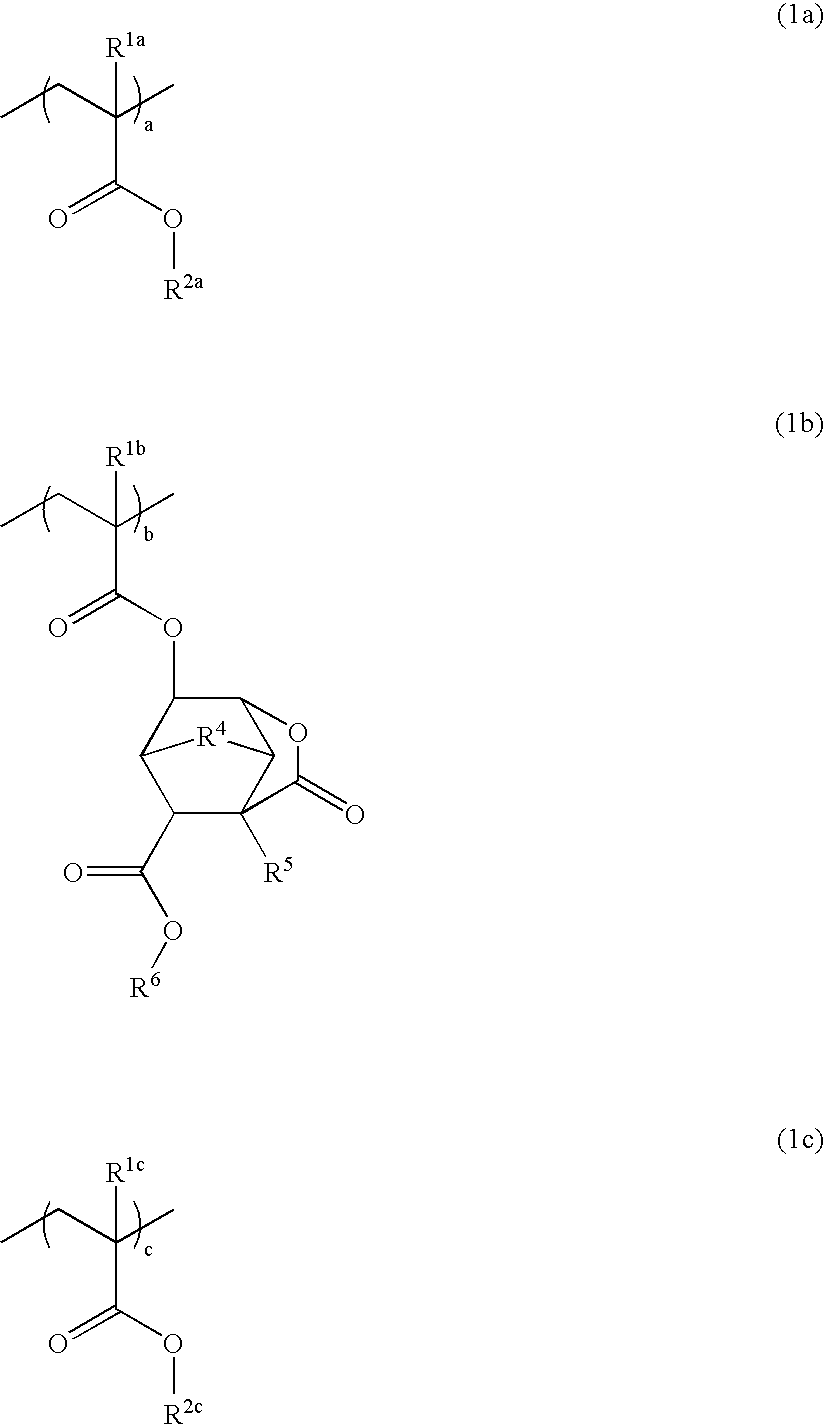
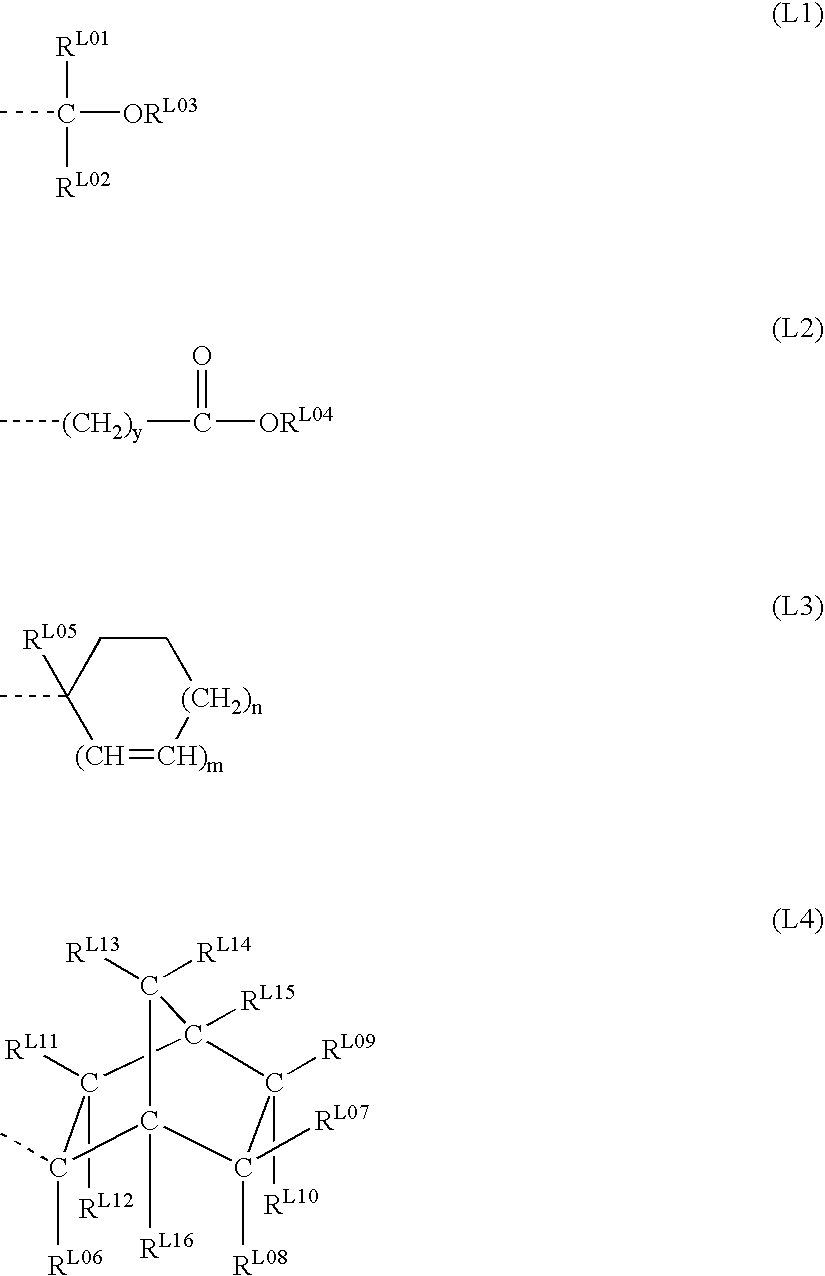
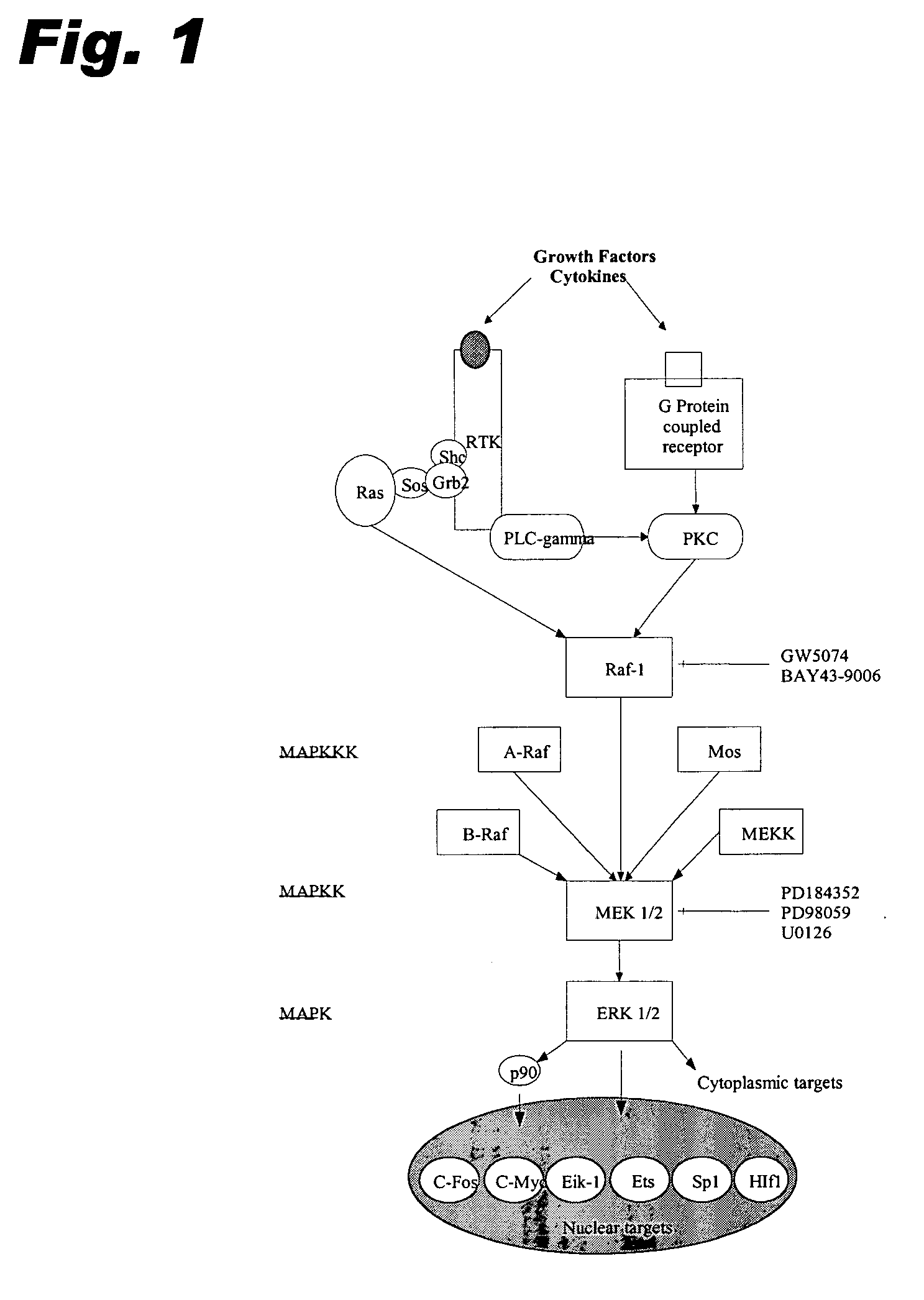
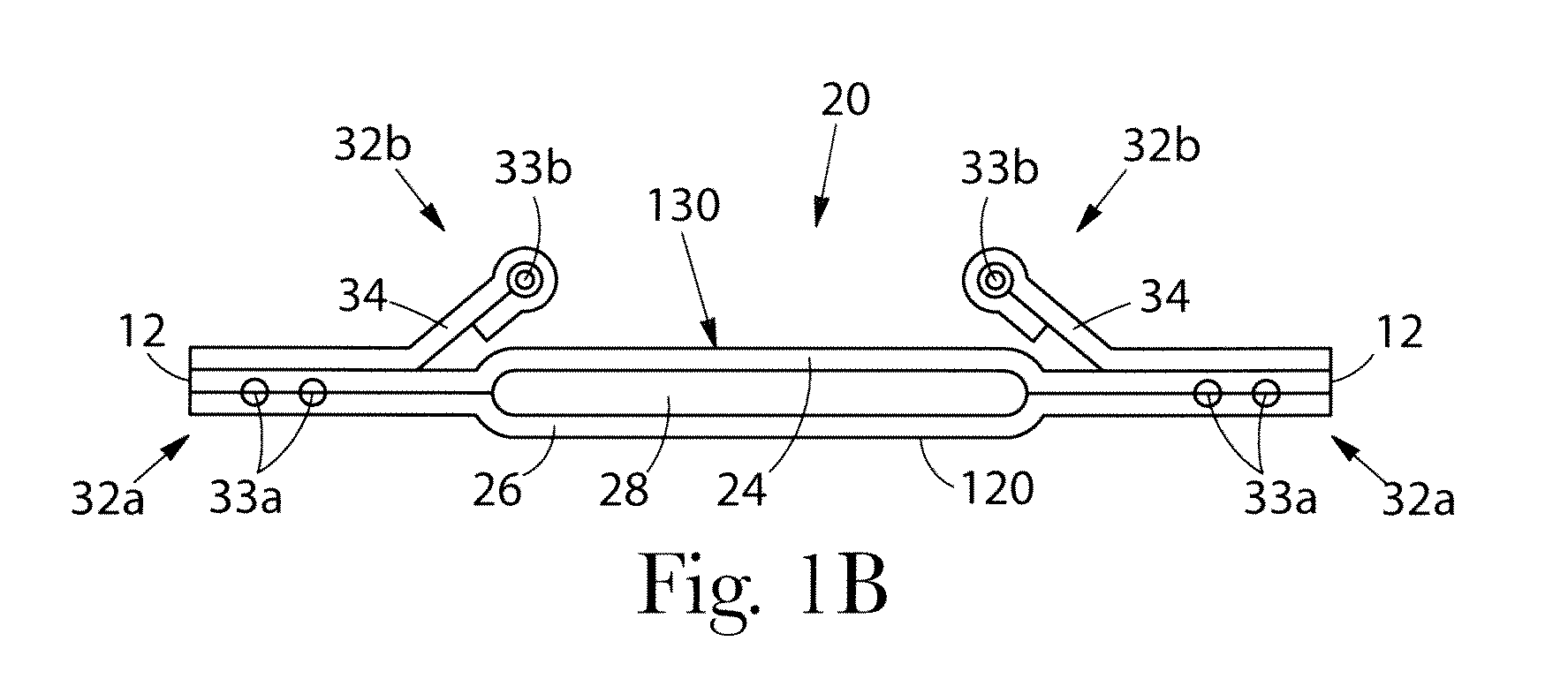
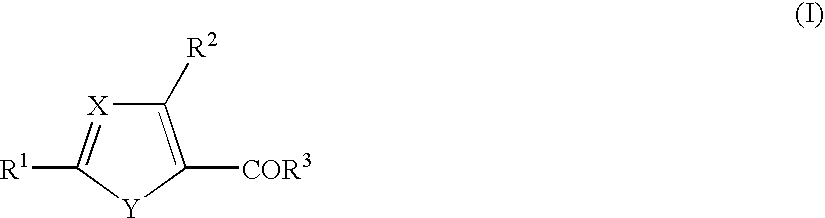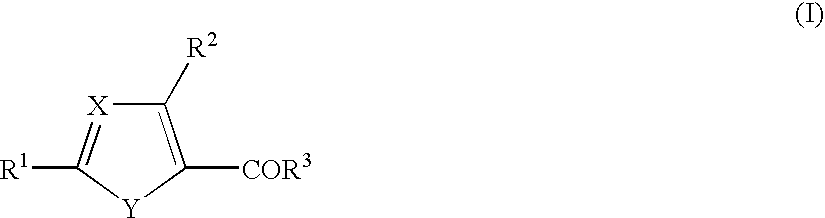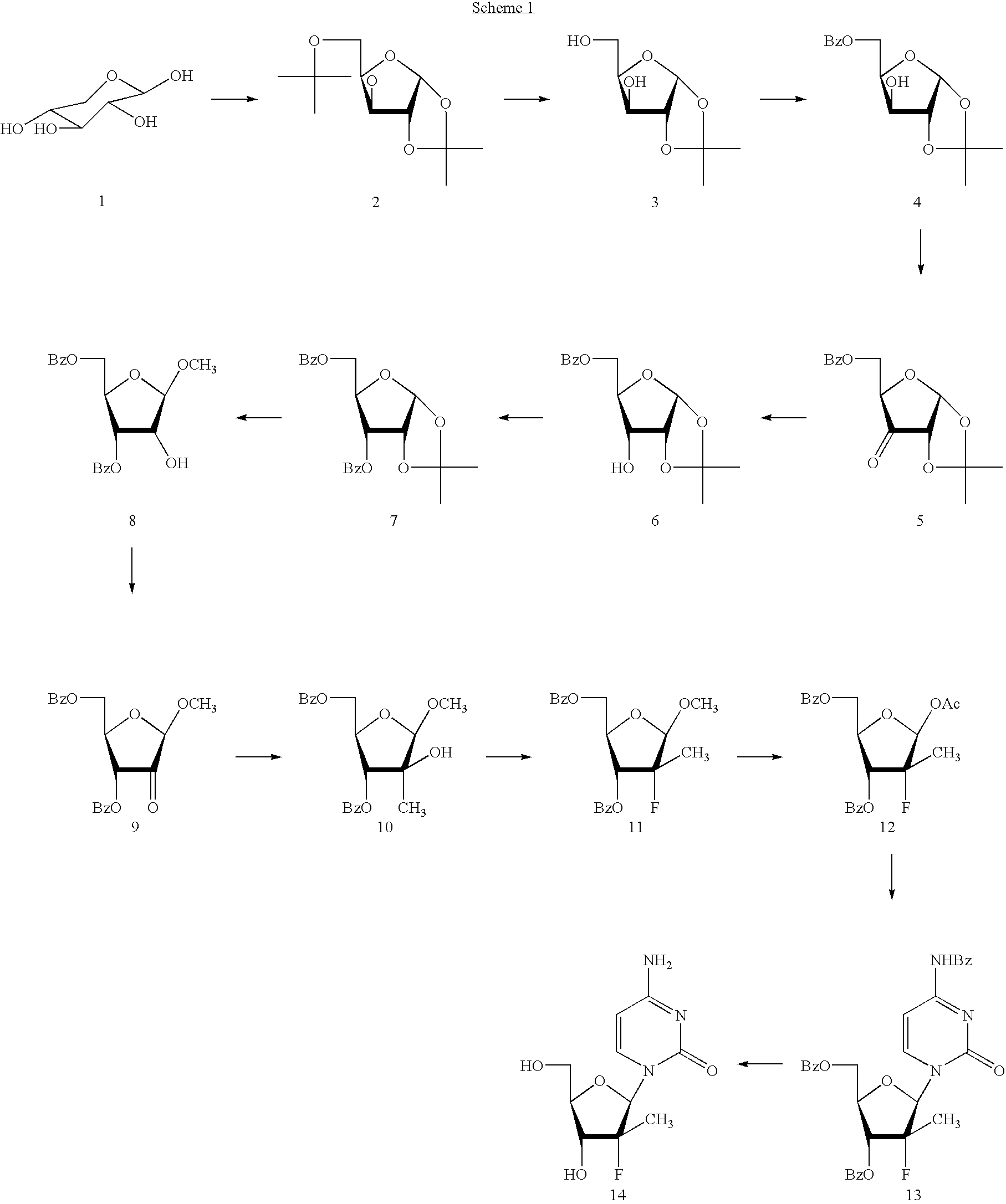Patents
Literature
5424 results about "Lactone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (–(C=O)–O–), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring. Lactones are formed by intramolecular esterification of the corresponding hydroxycarboxylic acids, which takes place spontaneously when the ring that is formed is five- or six-membered. Lactones with three- or four-membered rings (α-lactones and β-lactones) are very reactive, making their isolation difficult. Special methods are normally required for the laboratory synthesis of small-ring lactones as well as those that contain rings larger than six-membered.
Polymer, resist composition, and patterning process
To a resist composition, an alkali-soluble polymer having fluorinated ester-containing lactone units incorporated therein is included as an additive. The resist composition forms a resist film having a reduced contact angle after development. The resist film prevents water penetration during immersion lithography.
Owner:SHIN ETSU CHEM IND CO LTD
Specific kinase inhibitors
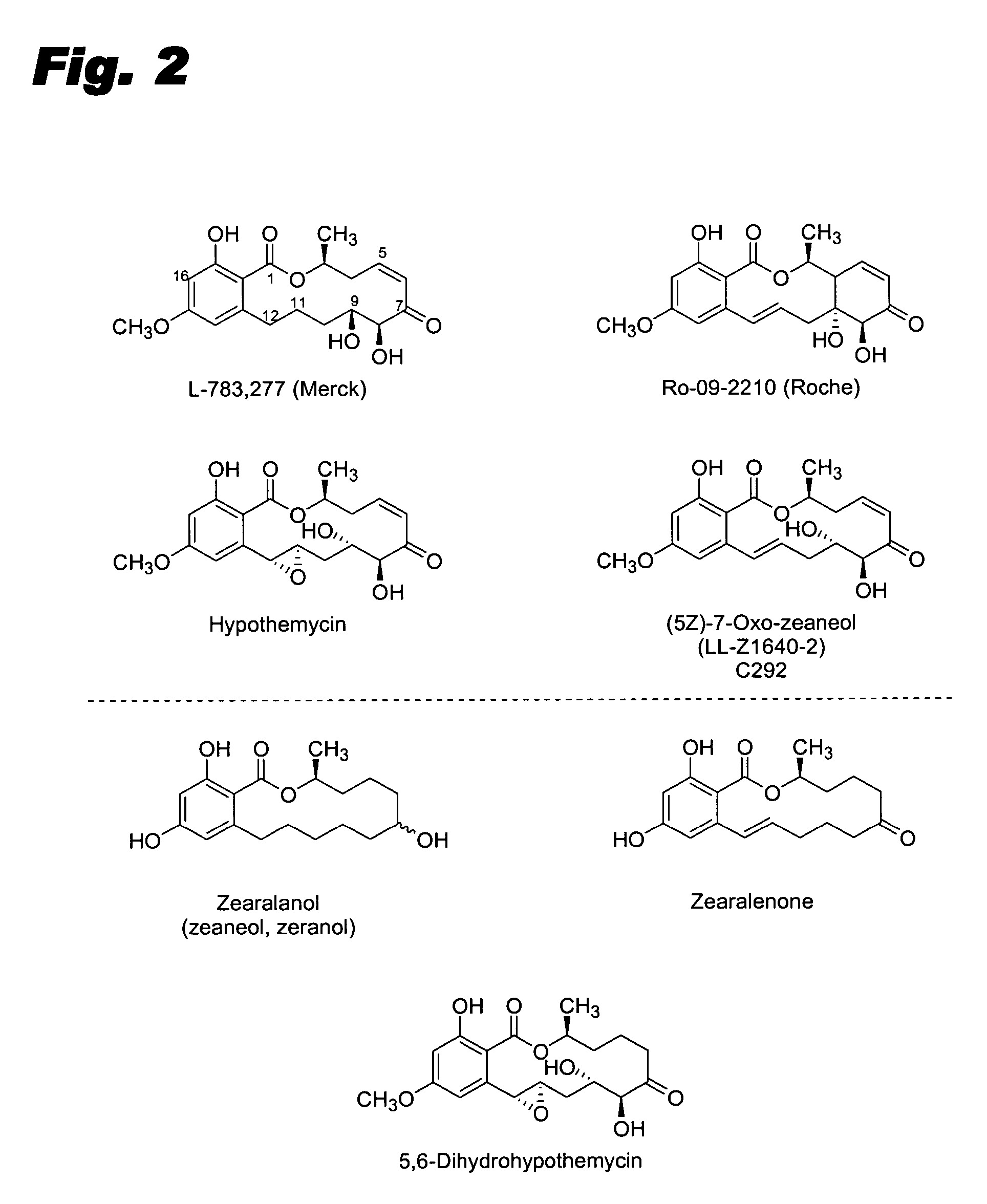
Resorcylic acid lactones having a C5-C6 cis double bond and a ketone at C7 and other compounds capable of Michael adduct formation are potent and stable inhibitors of a subset of protein kinases having a specific cysteine residue in the ATP binding site.
Owner:KOSAN BIOSCI
Wound healing polymer compositions and methods for use thereof
The present invention provides bioactive polymer compositions that can be formulated to release a wound healing agent at a controlled rate by adjusting the various components of the composition. The composition can be used in an external wound dressing, as a polymer implant for delivery of the wound healing agent to an internal body site, or as a coating on the surface of an implantable surgical device to deliver wound healing agents that are covalently attached to a biocompatible, biodegradable polymer and / or embedded within a hydrogel. Methods of using the invention bioactive polymer compositions to promote natural healing of wounds, especially chronic wounds, are also provided. Examples of biodegradable copolymer polyesters useful in forming the blood-compatible, hydrophilic layer or coating include copolyester amides, copolyester urethanes, glycolide-lactide copolymers, glycolide-caprolactone copolymers, poly-3-hydroxy butyrate-valerate copolymers, and copolymers of the cyclic diester monomer, 3-(S)[(alkyloxycarbonyl)methyl]-1,4-dioxane-2,5-dione, with L-lactide. The glycolide-lactide copolymers include poly(glycolide-L-lactide) copolymers formed utilizing a monomer mole ratio of glycolic acid to L-lactic acid ranging from 5:95 to 95:5 and preferably a monomer mole ratio of glycolic acid to L-lactic acid ranging from 45:65 to 95:5. The glycolide-caprolactone copolymers include glycolide and ε-caprolactone block copolymer, e.g., Monocryl or Poliglecaprone.
Owner:MEDIVAS LLC
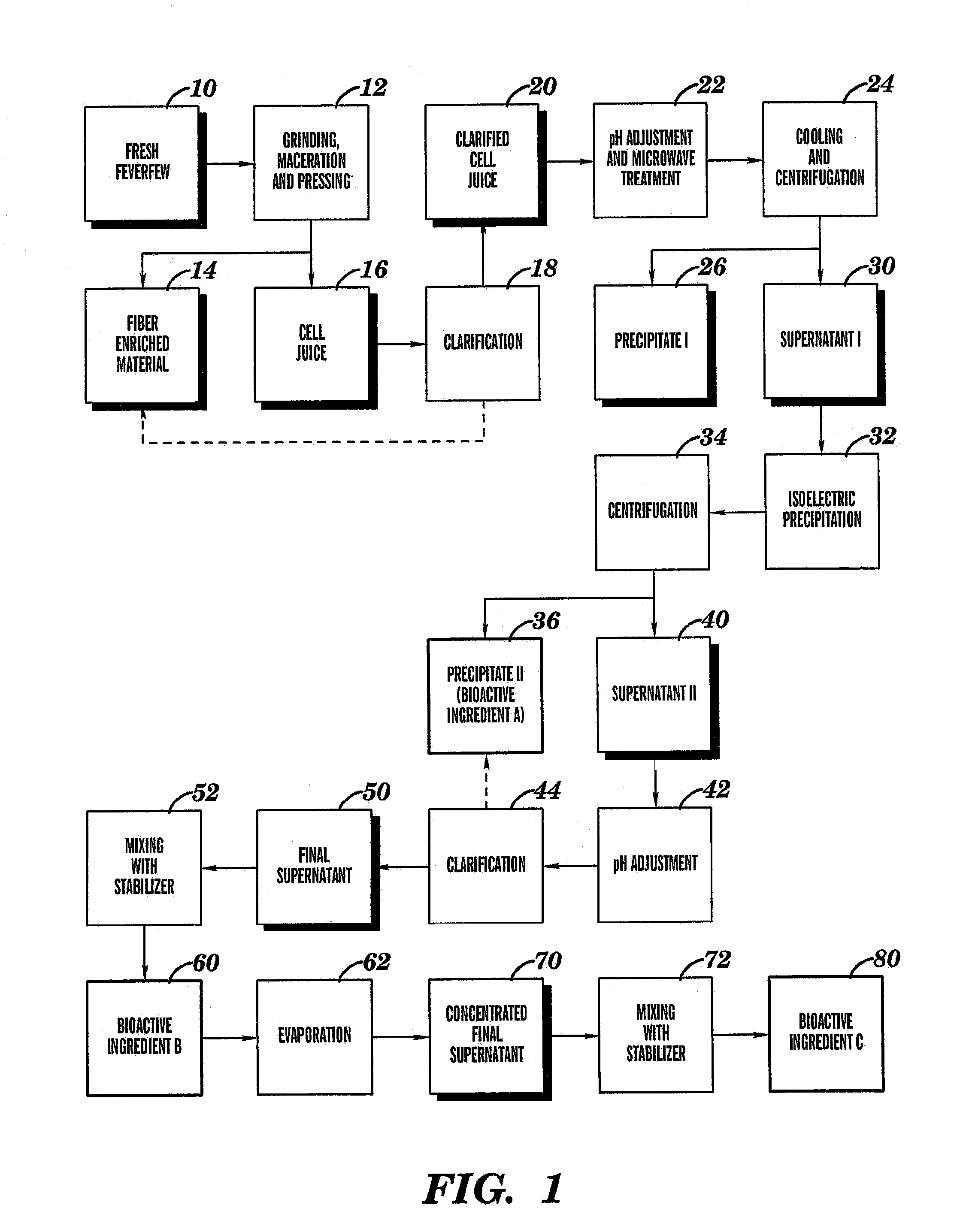
Parthenolide free bioactive ingredients from feverfew (Tanacetum parthenium) and processes for their production
ActiveUS7537791B2Inhibitory activityProtection from damageAntibacterial agentsBiocideTanacetum partheniumAdditive ingredient
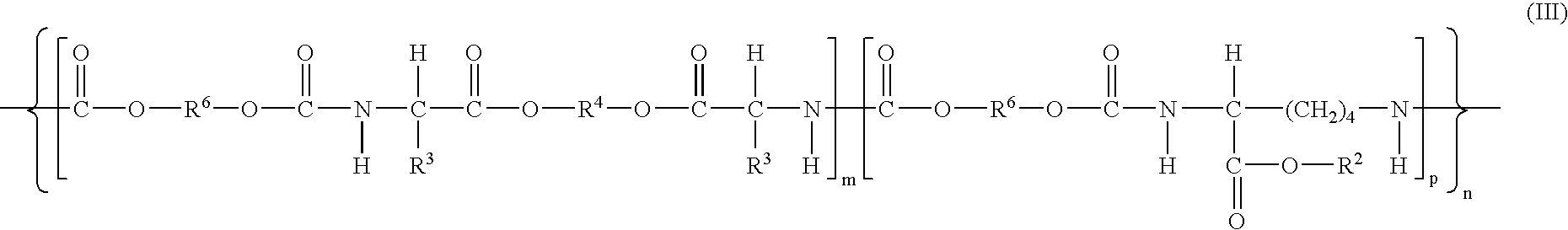
The present invention relates to bioactive ingredients that include isolated bioactive fractions derived from cell juice of fresh biomass of a feverfew (Tanacetum parthenium) plant. The bioactive fractions are either free of or substantially free of α-unsaturated γ-lactones (e.g., parthenolide). Further, the bioactive fractions have anti-inflammatory and antioxidant activity. The present invention also relates to a method for isolating a bioactive fraction that is derived from cell juice of fresh biomass of a feverfew (Tanacetum parthenium) plant and that is at least substantially free of α-unsaturated γ-lactones (e.g., parthenolide). The present invention also relates to a method for preparing a stabilized cell juice serum fraction and a stabilized concentrated cell juice serum fraction that are free of α-unsaturated γ-lactones (e.g., parthenolide). The present invention also relates to a bioactive composition that includes a mixture of one or more of the isolated bioactive fractions of the present invention.
Owner:ISP INVESTMENTS LLC
Method of using hydroxycarboxylic acids or related compounds for treating skin changes associated with intrinsic and extrinsic aging
A composition comprising an amphoteric or pseudo-amphoteric agent and a polyhydroxy alpha hydroxyacid existing as a free acid, lactone, or salt, and isomeric or non-isomeric forms thereof is provided. The amphoteric or pseudo-amphoteric agent can be selected from amino acids, dipeptides, aminoaldonic acid, aminouronic acid, lauryl aminoproplyglycine, aminoaldaric acid, neuraminic acid desulfated heparin, deacetylated hyaluronic acid, hyalobiuronic acid, chondrosine, deacetylated chondroitin, creatine, creatinine, hydroxyproline, homocysteine, homocystine, homoserine, ornithine, citrulline, phosphatidylserine, and sphingomyelin. The composition may contain other additives, including cosmetic or pharmaceutical agents for topical treatment of dermatological disorders.
Owner:TRISTRATA TECH
Bioavailability and improved delivery of alkaline pharmaceutical drugs
InactiveUS20050196418A1Easy to optimizeCosmetic preparationsPowder deliveryBioavailabilityDermatology
Embodiments of the invention relate to a composition, a process of making the composition, and to the use of the composition. The compositions include a molecular complex formed between an alkaline pharmaceutical drug and at least one selected from a hydroxyacid, a polyhydroxy acid, a related acid, a lactone, or combinations thereof. The compositions provide improved bioavailability and improved delivery of the drug into the cutaneous tissues.
Owner:YU RUEY J +1
Bioabsorbable and biocompatible polyurethanes and polyamides for medical devices
ActiveUS20060188547A1Low toxicitySuture equipmentsOrganic active ingredientsAbsorbable polymersPolyester
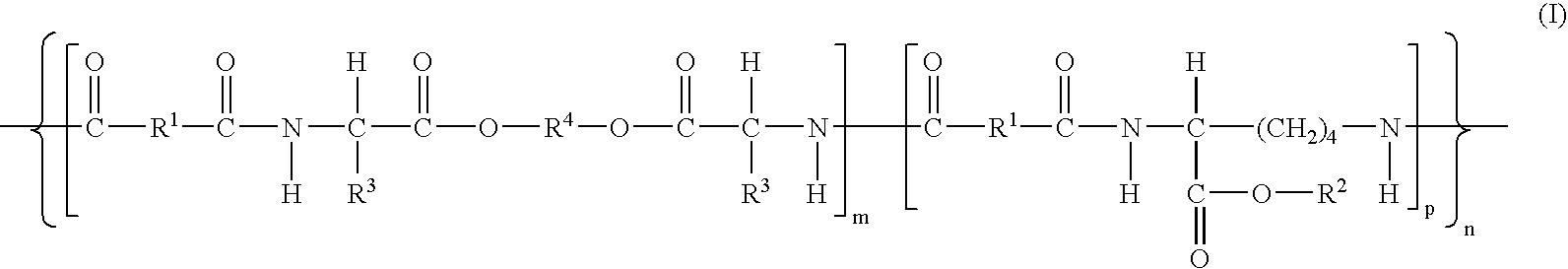
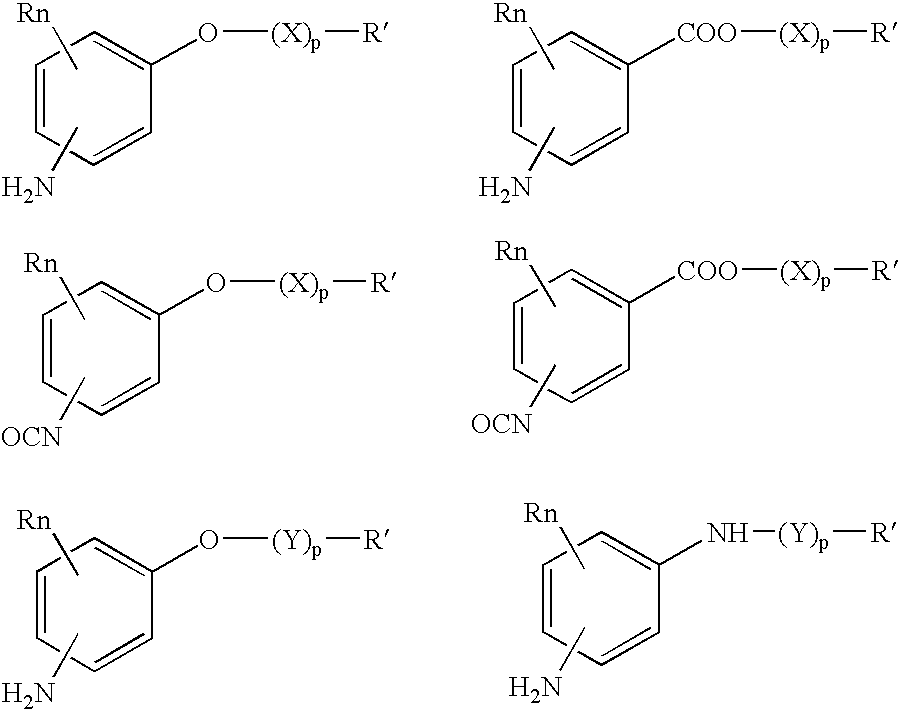
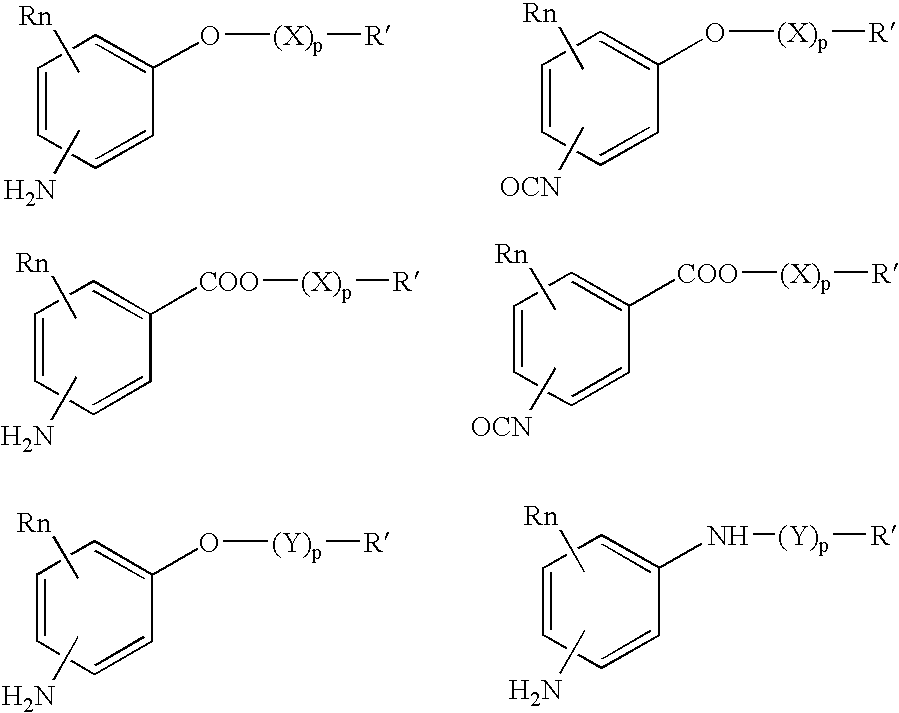
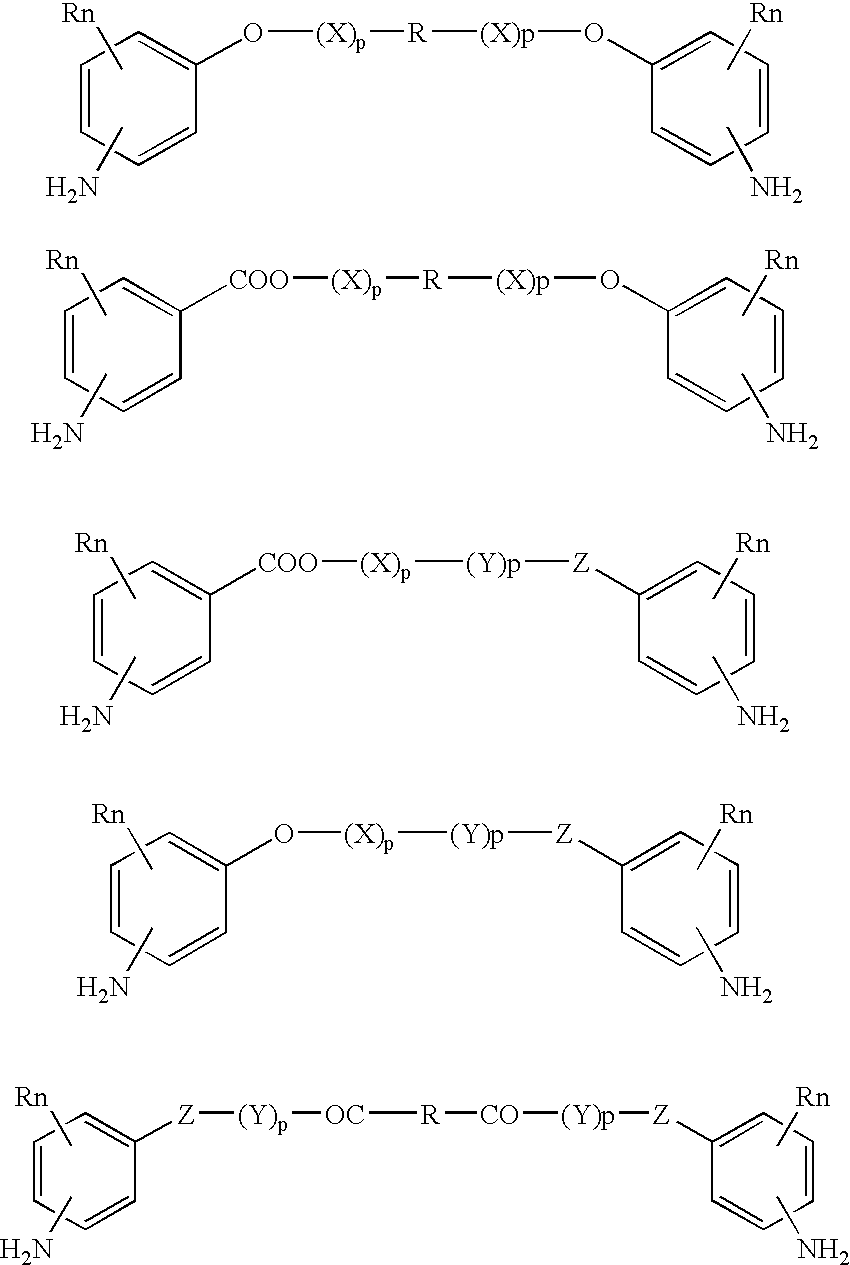
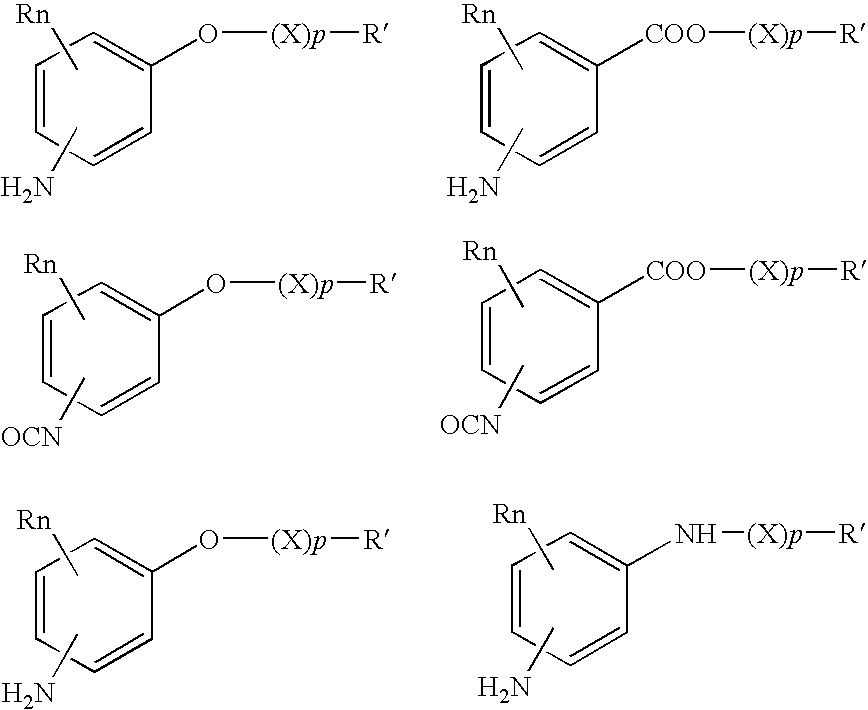
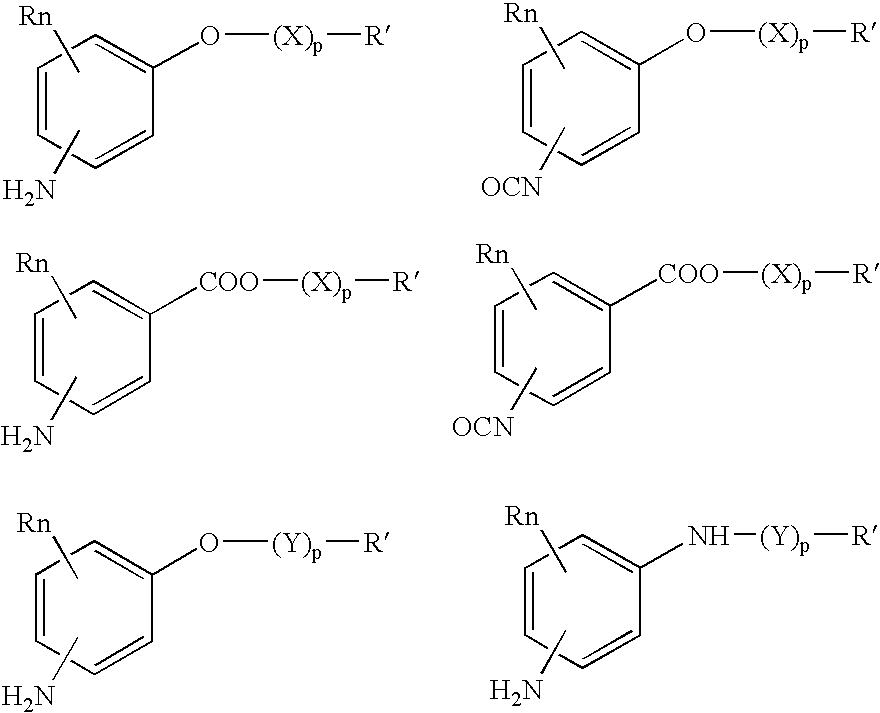
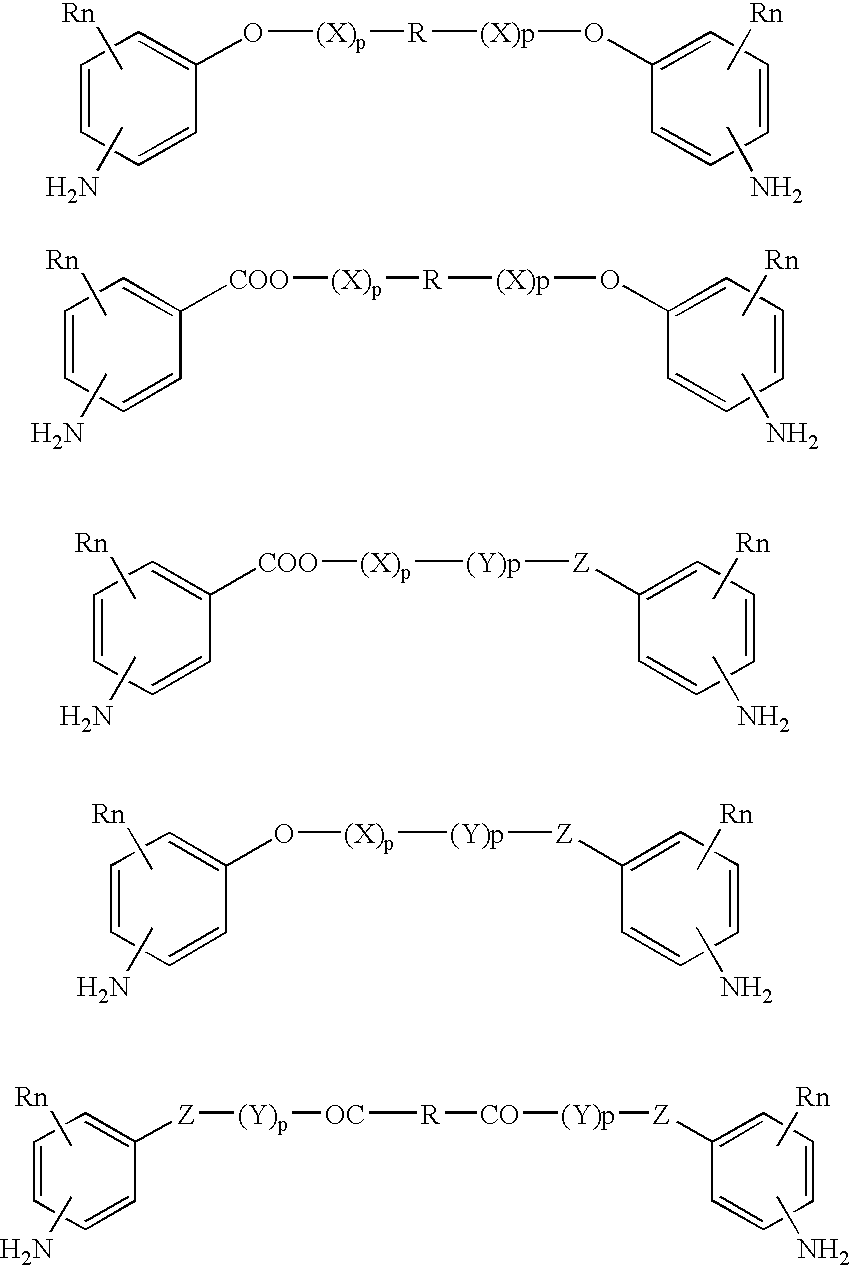
Absorbable polyurethanes, polyamides and polyester urethanes prepared from at least one compound selected from: or the diamines and diisocyanates thereof, wherein each X represents a member independently selected from —CH2COO— (glycolic acid moiety), —CH(CH3)COO— (lactic acid moiety), —CH2CH2OCH2COO— (dioxanone), —CH2CH2CH2CH2CH2COO— (caprolactone moiety), —(CH2)yCOO— where y is one of the numbers 2, 3, 4 or 6-24 inclusive, and —(CH2CH2O)z′CH2COO— where z′ is an integer between 2 and 24, inclusive; each Y represents a member independently selected from —COCH2O— (glycolic ester moiety), —COCH(CH3)O— (lactic ester moiety), —COCH2OCH2CH2O— (dioxanone ester), —COCH2CH2CH2CH2CH2O— (caprolactone ester), —CO(CH2)mO— where m is an integer between 2, 3, 4 or 6-24 inclusive, —COCH2O(CH2CH2O)n— where n is an integer between 2 and 24, inclusive; R′ is hydrogen, benzyl or an alkyl group, the alkyl group being either straight-chained or branched; p is an integer between 1 and 4, inclusive; and Rn represents one or more members selected from H, alkoxy, benzyloxy, aldehyde, halogen, carboxylic acid and —NO2, which is attached directly to an aromatic ring or attached through an aliphatic chain. Absorbable polymers prepared from these compounds are useful for drug delivery, tissue engineering, tissue adhesives, adhesion prevention and other implantable medical devices.
Owner:BEZWADA BIOMEDICAL LLC
Bioabsorbable and biocompatible polyurethanes and polyamides for medical devices
Absorbable polyurethanes, polyamides and polyester urethanes prepared from at least one compound selected from:or the diamines and diisocyanates thereof, wherein each X represents a member independently selected from —CH2COO— (glycolic acid moiety), —CH(CH3)COO— (lactic acid moiety), —CH2CH2OCH2COO— (dioxanone), —CH2CH2CH2CH2CH2COO— (caprolactone moiety), —(CH2)yCOO— where y is one of the numbers 2, 3, 4 or 6-24 inclusive, and —(CH2CH2O)z′CH2COO— where z′ is an integer between 2 and 24, inclusive; each Y represents a member independently selected from —COCH2O— (glycolic ester moiety), —COCH(CH3)O— (lactic ester moiety), —COCH2OCH2CH2O— (dioxanone ester), —COCH2CH2CH2CH2CH2O— (caprolactone ester), —CO(CH2)mO— where m is an integer between 2, 3, 4 or 6-24 inclusive, —COCH2O(CH2CH2O)n— where n is an integer between 2 and 24, inclusive; R′ is hydrogen, benzyl or an alkyl group, the alkyl group being either straight-chained or branched; p is an integer between 1 and 4, inclusive; and Rn represents one or more members selected from H, alkoxy, benzyloxy, aldehyde, halogen, carboxylic acid and —NO2, which is attached directly to an aromatic ring or attached through an aliphatic chain. Absorbable polymers prepared from these compounds are useful for drug delivery, tissue engineering, tissue adhesives, adhesion prevention and other implantable medical devices.
Owner:BEZWADA BIOMEDICAL LLC
Absorbent article comprising a synthetic polymer derived from a renewable resource and methods of producing said article
An element of an absorbent article is provided. The element has a bio-based content of at least about 50% based on the total weight of the element, and comprises a synthetic polymer derived from a renewable resource via a first intermediate compound selected from the group consisting of crotonic acid, propiolactone, ethylene oxide, i-propanol, butanol, butyric acid, propionic acid, 2-acetoxypropanoic acid, methyl 2-acetoxypropanoate, methyl lactate, ethyl lactate, polyhydroxybutyrate, and a polyhydroxyalkanoate comprising 3-hydroxypropionate monomers. An absorbent article comprising the element and a method of making an element for an absorbent article also are provided.
Owner:THE PROCTER & GAMBLE COMPANY
Plant growth regulating composition and its application
InactiveCN102613246AGood regulationPromote growthBiocidePlant growth regulatorsNitrohumic acidEthephon
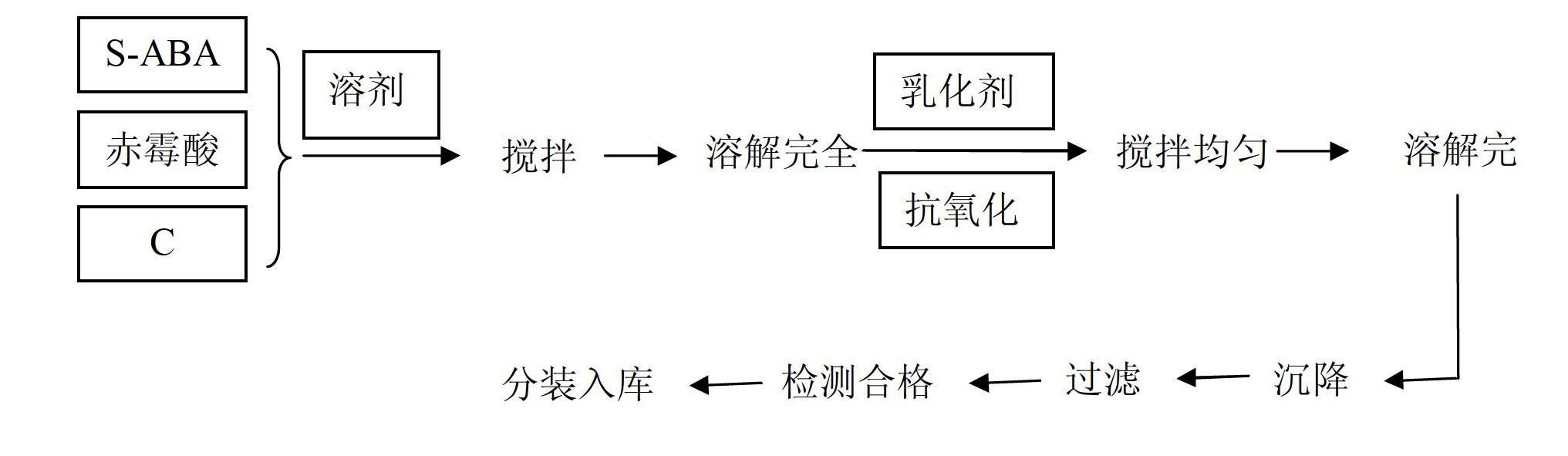
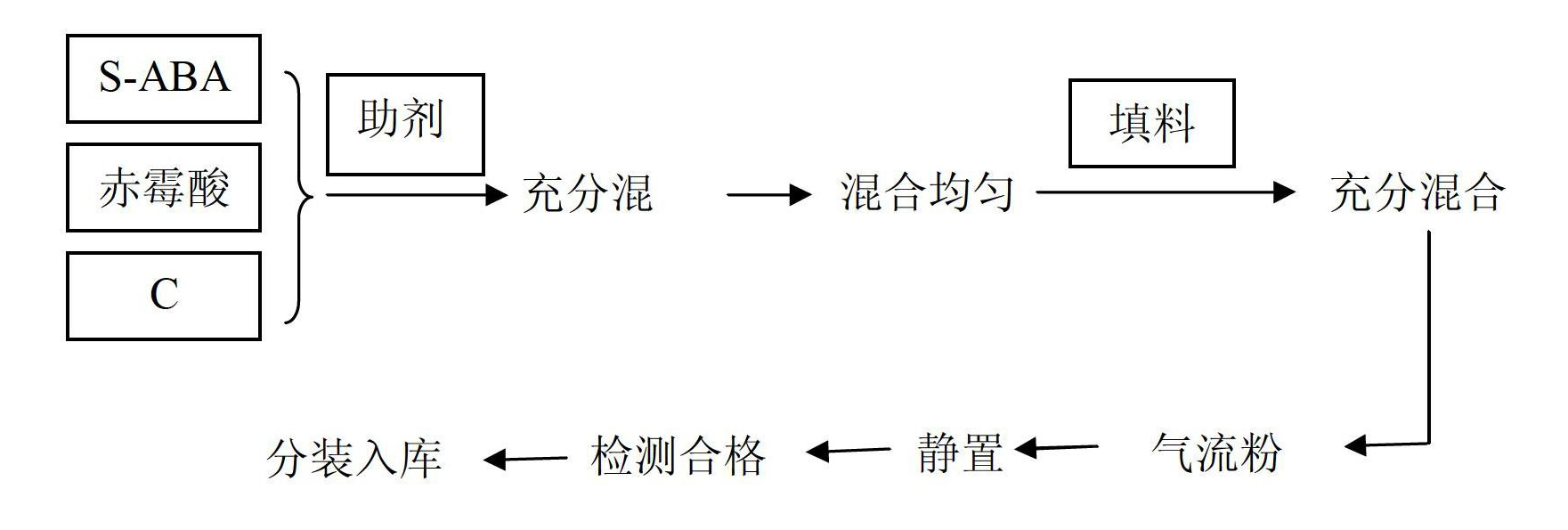
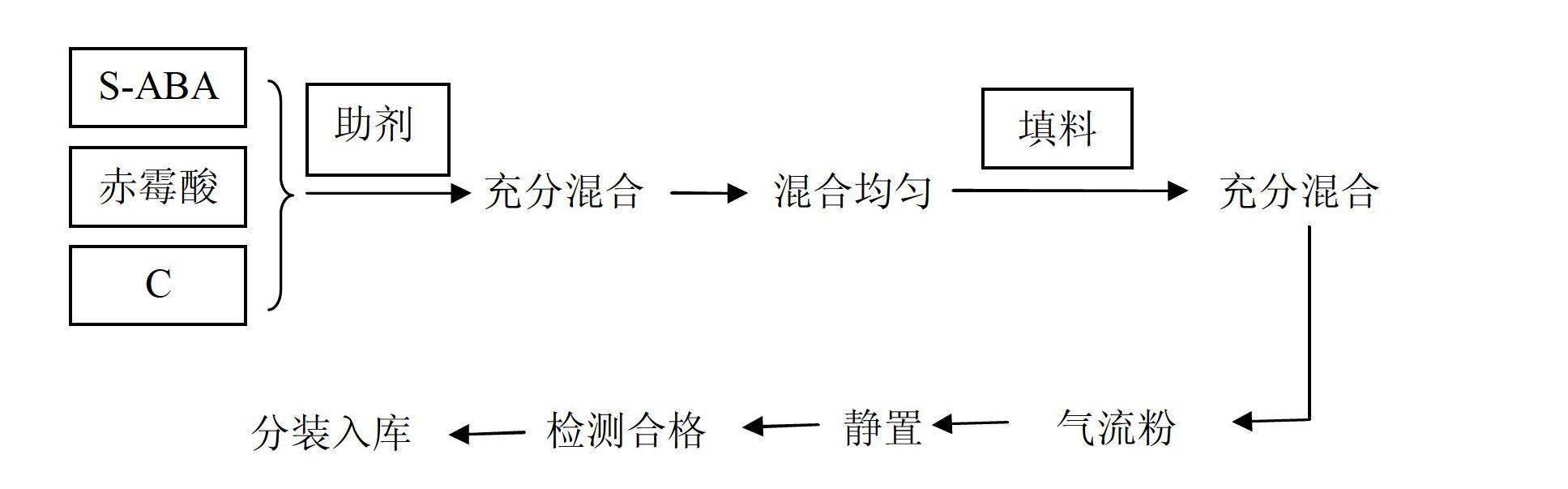
The invention discloses a plant growth regulating composition and its application. The composition comprises the following components: A) natural abscisic acid, B) gibberellic acid (GA3, or of GA4 +7), and C) an existing plant growth regulator. The existing plant growth regulator is selected from one or more than two of 6-BA (6-benzylaminopurine), thidiazuron, brassinolide, sodium nitrophenolate, potassium nitrophenolate, chlormequat chloride, triacontanol, naphthlcetic acid and its salt, indolebutyric acid and its salt, indoleacetic acid and its salt, ethephon, diethyl aminoethyl hexanoate, paclobutrazol, uniconazole, fulvic acid, Mepiquat chloride, nitrohumic acid, jasmonic acid and triapenthenol. Specifically, the components A), B) and C) are in a weight ratio of 1:0.001-500:0.001-500. The S-ABA (S-abscisic acid) added in the invention can expand the prevention and treatment objects, reduce or prevent phytotoxicity, reduce dosage, and lower the use cost.
Owner:SICHUAN LOMON BIO TECH CO LTD
Absorbable implants and methods for their use in hemostasis and in the treatment of osseous defects
ActiveUS20050065214A1Stimulate bone healing processLower potentialBiocidePowder deliveryBarium saltTG - Triglyceride
Two (or more), -component, body-implantable, absorbable, biocompatible, putty, and non-putty hemostatic tamponades for use in surgery. Component 1 is a finely powdered bulking material, preferably less than 50 microns, e.g. the calcium, magnesium, aluminum, or barium salts of saturated or unsaturated carboxylic acids containing about 6 to 22 carbon atoms, hydroxyapatite, DBM, polyglycolide, polylactide, poldioxinones, polycaprolactones, absorbable glasses, gelatin, collagens, mono, and polysaccharides starches. Component 2, a dispersing vehicle, may be esters of C8-C18 monohydric alcohols with C2-C6 aliphatic monocarboxylic acids; C2-C18 monohydric alcohols with polycarboxylic acids; C8-C30 monohydric alcohols; tocopherol and esters thereof with C2-C10 aliphatic monocarboxylic acids or polycarboxylic acids; absorbable 10-14C hydrocarbons; free carboxylic acids such as oleic, capric, and lauric; dialkyl ethers and ketones; alkyl aryl ethers and ketones, polyhydroxy compounds and esters and ethers thereof; (ethylene oxide / propylene oxide copolymers), oils e.g. olive oil, castor oil and triglycerides.
Owner:ABYRX
Adhesion-Preventive Film
InactiveUS20080090936A1Good flexibilityProvide effectSuture equipmentsCosmetic preparationsLactideCaprolactone
An adhesion-preventive film is provided that is excellent in flexibility and can prevent cracks from occurring. The adhesion-preventive film contains a copolymer of lactide and caprolactone. The lactide and the caprolactone of the copolymer has a mole ratio in the range of 65:35 to 80:20. Even when this adhesion-preventive film is used in a curved state in vivo or is wound around an affected part such as a tendon, for example, it can provide an adhesion-preventive function for a sufficiently long period without cracking.
Owner:JMS CO LTD
Cyanoacrylate-capped heterochain polymers and tissue adhesives and sealants therefrom
The present invention is directed toward cyanoacrylate-based tissue adhesive or sealant compositions comprising cyanoacrylate-capped heterochain polymers, such as those comprising one or more oxyalkylene, alkylene carbonate, and ester-units derived from cyclic lactones. Such compositions can be radiochemically sterilized and used in repairing internal organs or tissue blocking body conduits.
Owner:POLY MED
Ternary mixture of biodegradable polyesters and products obtained therefrom
InactiveUS6841597B2RapidityImproves UV stabilitySynthetic resin layered productsPaper coatingPolymer scienceBiodegradable polyester
The invention relates to a mixture of biodegradable polyesters comprising: (A) a polyhydroxy acid of the poly-ε-caprolactone type and its copolymers, (B) aliphatic polyester, and (C) a polymer of polylactic acid, in which the concentration of (A) varies with respect to (A+B) in the range between 40 and 70% by weight, and the concentration of (C) with respects to (A+B+C) lies between 2 and 30%.
Owner:NOVAMONT SPA
Process for preparing GAMM-hexalactone, products produced therefrom dan organoleptic uses of said products
A process for producing high yields of gamma -hexalactone and 2-pentanone from the corresponding hexanoic acid starting material is carried out with high amounts of oxygen and sugar in the presence of a mold microorganism. Fragrance compositions and foodstuff compositions are augmented and enhanced by the presence of the product compounds.
Owner:INTERNATIONAL FLAVORS & FRAGRANCES
Preparation method and application of polycaprolactone/natural polymer composite porous scaffold
InactiveCN102277737AImprove hydrophilicityEasy to spreadLayered productsFibre typesFiberPolymer science
Preparation method and application of polycaprolactone / natural polymer composite porous scaffold, method: including preparation of polycaprolactone electrospun nanofiber membrane; (1) placing polycaprolactone electrospun nanofiber membrane in alkaline solution reaction, soaking and rinsing in deionized water; (2) putting the fiber membrane obtained in (1) into DMTMM or natural polymer solution of DTMMM to react, immersing and rinsing in deionized water, and then immersing in natural polymer for room temperature reaction, to remove Ionized water soaking and rinsing; (3) Evenly coating and casting the mixed solution of natural polymer materials on the fiber membrane obtained in (2), freeze-drying, and extracting the solvent; (4) Applying the double-layer scaffold obtained in (3) After soaking in the cross-linking room temperature reaction, soaking in deionized water, rinsing, and freeze-drying. The porous scaffold prepared by the invention is stable, has suitable pore diameter, good biocompatibility and fast degradation rate, and can be used for skin and clinical tissue and organ defect repair, reconstruction or wound dressing.
Owner:NANCHANG UNIV
Method for preparing caprolactone from cyclohexanone by catalytic oxidation
InactiveCN101307045AHigh yieldHigh selectivityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsCyclohexanoneHalogen
The invention relates to a method for preparing caprolactone through cyclohexanone catalytic oxidation. The method comprises the following steps that: raw materials, namely cyclohexanone, oxidant, solvent and catalyst, are respectively added to a reaction vessel; the reaction vessel is heated to at a temperature between 40 and 100 DEG C; constant temperature reaction is carried out for 1-15 hours; after the reaction is finished, the raw materials are cooled and separated, and then a finished product of caprolactone can be obtained, wherein the catalyst contains 50-100 percent of zinc oxide and 50-0 percent of other metallic oxide in weight percentage; the solvent is nitrile; the oxidant is hydrogen peroxide or peracetic acid. High caprolactone yield and selectivity can be obtained by adopting the method provided by the invention; meanwhile, the catalyst used in the method is low in price, easy to get and simple to prepare, contains no halogen element, has high stability, and can be used repeatedly.
Owner:HUNAN UNIV
Natural insect and arthropod repellent
InactiveUS6306415B1Avoid damageSafe, long-lasting, effective and pleasantBiocideCosmetic preparationsCarboxylic acidStable fly
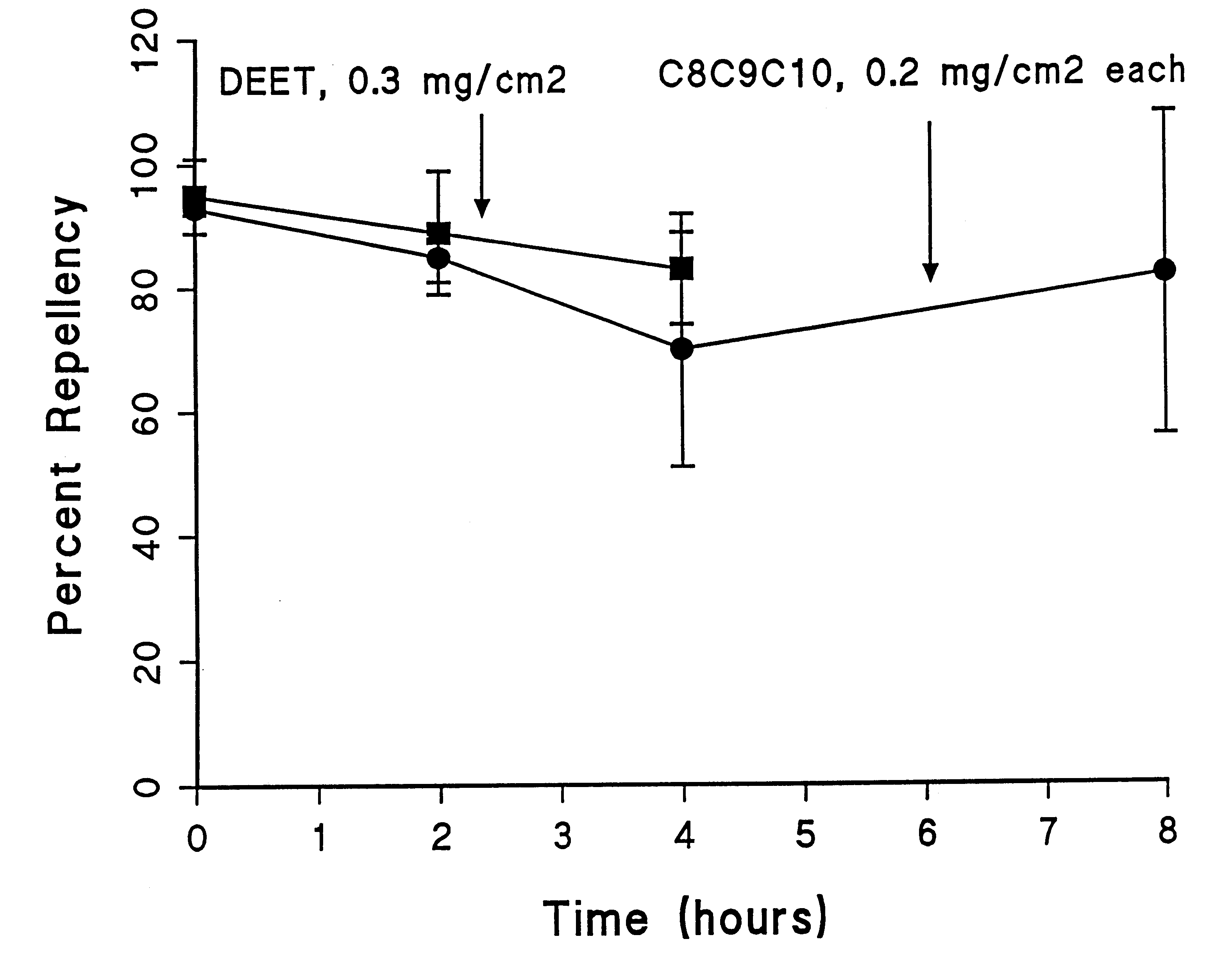
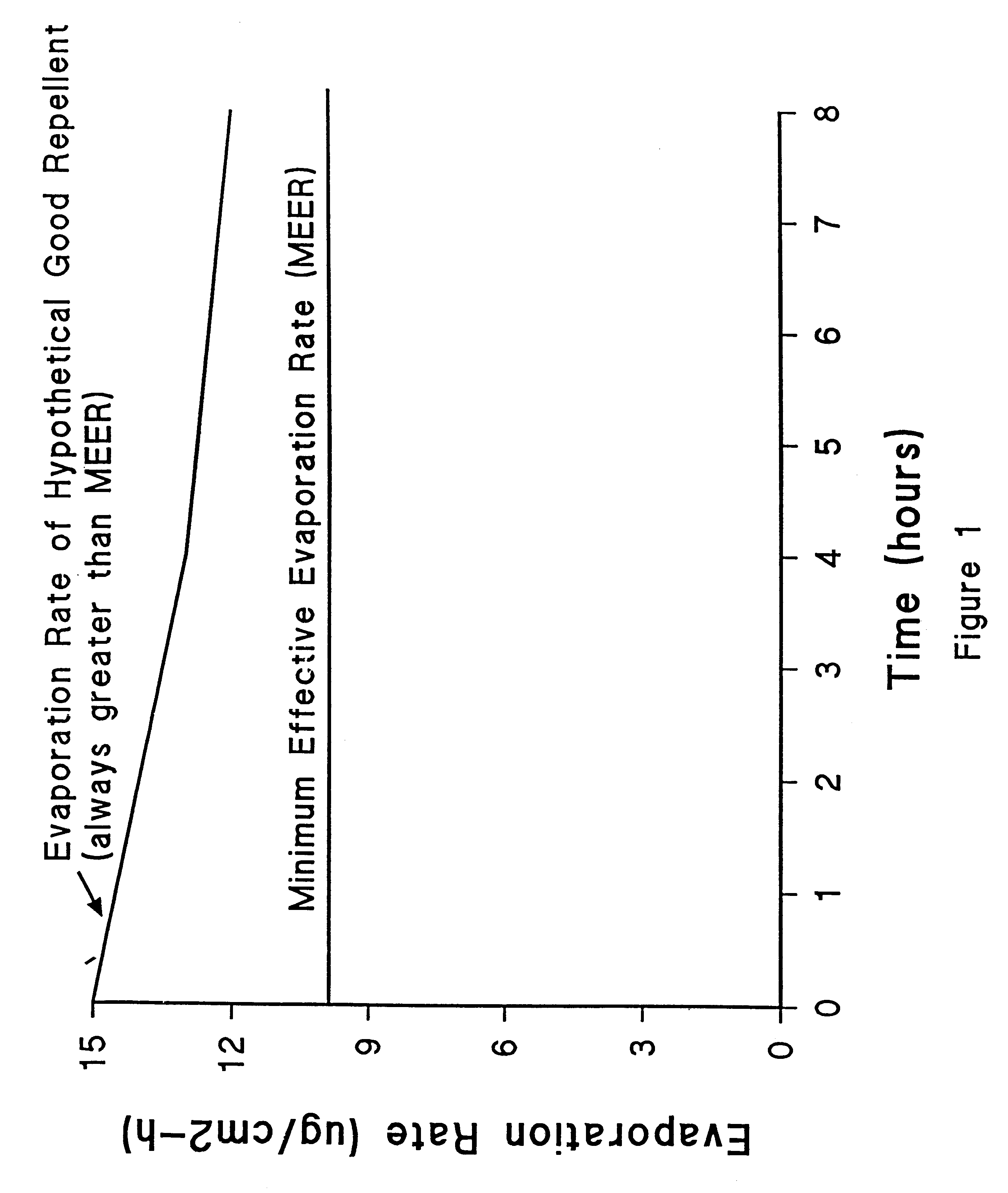
A topical insect repellent with extended duration of protection was obtained from mixtures of molecules based on two or more volatile repellent organic molecular species occurring naturally on the human skin surface. The novel repellent comprises mixtures of lower, intermediate, and higher volatility organic molecules. Active ingredients for formulations are obtained from homologous series of carboxylic acids, alcohols, ketones, and lactones which span a similar range of volatility and which occur naturally on the skin surface. Volatile silicone fluid imparts mildness and water repellency to the repellent formulations. The new natural repellent exhibits the longevity and repellency that is comparable to N,N-diethyl-m-toluamide (DEET), a synthetic compound employed in almost all commercial formulations, but the inventive natural repellent is more acceptable than DEET, which has an unpleasant odor and imparts a greasy feel to the skin. The inventive insect repellent, formulated in a volatile silicone fluid, was shown to repel and incapacitate stable flies. This finding demonstrated that repellency was not limited to mosquitoes, but extends to other biting flies or insects, thus demonstrating the utility of the novel insect repellent for protecting pets and livestock as well as humans.
Owner:STRATACOR
Cyclic compounds
InactiveUS20040142930A1Strong inhibitory activityLess side effectsBiocideGroup 5/15 element organic compoundsArylAlkoxy group
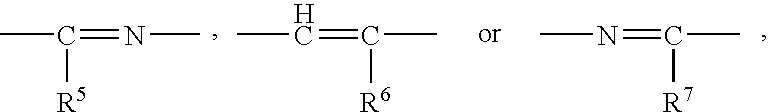
1. A cyclic compound of the formula (I) or a pharmacologically acceptable salt thereof, wherein X is =CH- or =N-, Y is -NH-, -NR<4>-, -S-, -O-, -CH=N-, -N=CH-, -N=N-, -CH=CH-, etc., R<1 >is a lower alkoxy group, an amino group, a heterocyclic ring containing N atom(s), or a hydroxy group substituted by a heterocyclic ring containing N atom(s) (each of which is optionally substituted), R<2 >is a lower alkylamino group which is optionally substituted by an aryl group, a lower alkoxy group which is optionally substituted by an aryl group, a lower alkoxy group substituted by an aromatic heterocyclic ring containing N atom(s), R<3 >is an aryl group, a heterocyclic ring containing N atom(s), a lower alkyl group, a lower alkoxy group, a cyclo lower alkoxy group, a hydroxy group substituted by a heterocyclic ring containing N atom(s), or an amino group (each of which is optionally substituted), and R<3 >and a substituent in Y may be combined to form a lactone ring. The compound of the present invention has excellent selective PDE V inhibitory activity and therefore, is useful as a therapeutic or prophylactic drug for treating various diseases due to functional disorders on cGMP-signaling.
Owner:MITSUBISHI TANABE PHARMA CORP
Melt-processible polymer composition comprising fluoropolymer having long chain branches
A polymer melt additive that is suitable for use as a processing aid in the extrusion of a non-fluorinated polymer. The polymer melt additive composition includes a fluoropolymer that has a long chain branching index (LCBI) of at least 0.2 and a zero shear rate viscosity at 265° C. of not more than 107 Pa's. The polymer melt additive may additionally include other compounds such as, polyoxyalkylene polymer or polycaprolactone.
Owner:3M INNOVATIVE PROPERTIES CO
Ternary mixture of biodegradable polyesters and products obtained therefrom
InactiveUS20040092672A1RapidityMaintain transparencyFlexible coversWrappersPolymer scienceBiodegradable polyester
The invention relates to a mixture of biodegradable polyesters comprising: (A) a polyhydroxy acid of the poly-epsilon-caprolactone type and its copolymers, (B) aliphatic polyester, and (C) a polymer of polylactic acid, in which the concentration of (A) varies with respect to (A+B) in the range between 40 and 70% by weight, and the concentration of (C) with respects to (A+B+C) lies between 2 and 30%.
Owner:NOVAMONT SPA
Trace elements
The invention discloses a trace element solution, which comprises at least one metal selected from the group comprising selenium, copper, zinc, manganese and chromium; and at least one component selected from the group comprising a vitamin, a vaccine, a growth stimulant, a dewormer, iron dextran, an antibiotic and a synchronization preparation. The synchronization preparation is a combination of injectable hormonal preparations, inplantable hormonal preparations, intravaginal hormonal preparation and other slow release hormonal preparation. The antibiotics include oral, injectable and implantable theurapeutic remedies. The vaccine includes antigens and a combination of antigens and adjuvents. The growth stimulants include zeranol, estradiol, testosterone, progesterone and trenbolone acetate. The dewormer includes macrocydic lactones, leramizoles, benzimidazoles and salicylanilides. The macrocydic lactones include doramectin, ivermectin, abamectin and moxidectin.
Owner:WARBURTON TECH
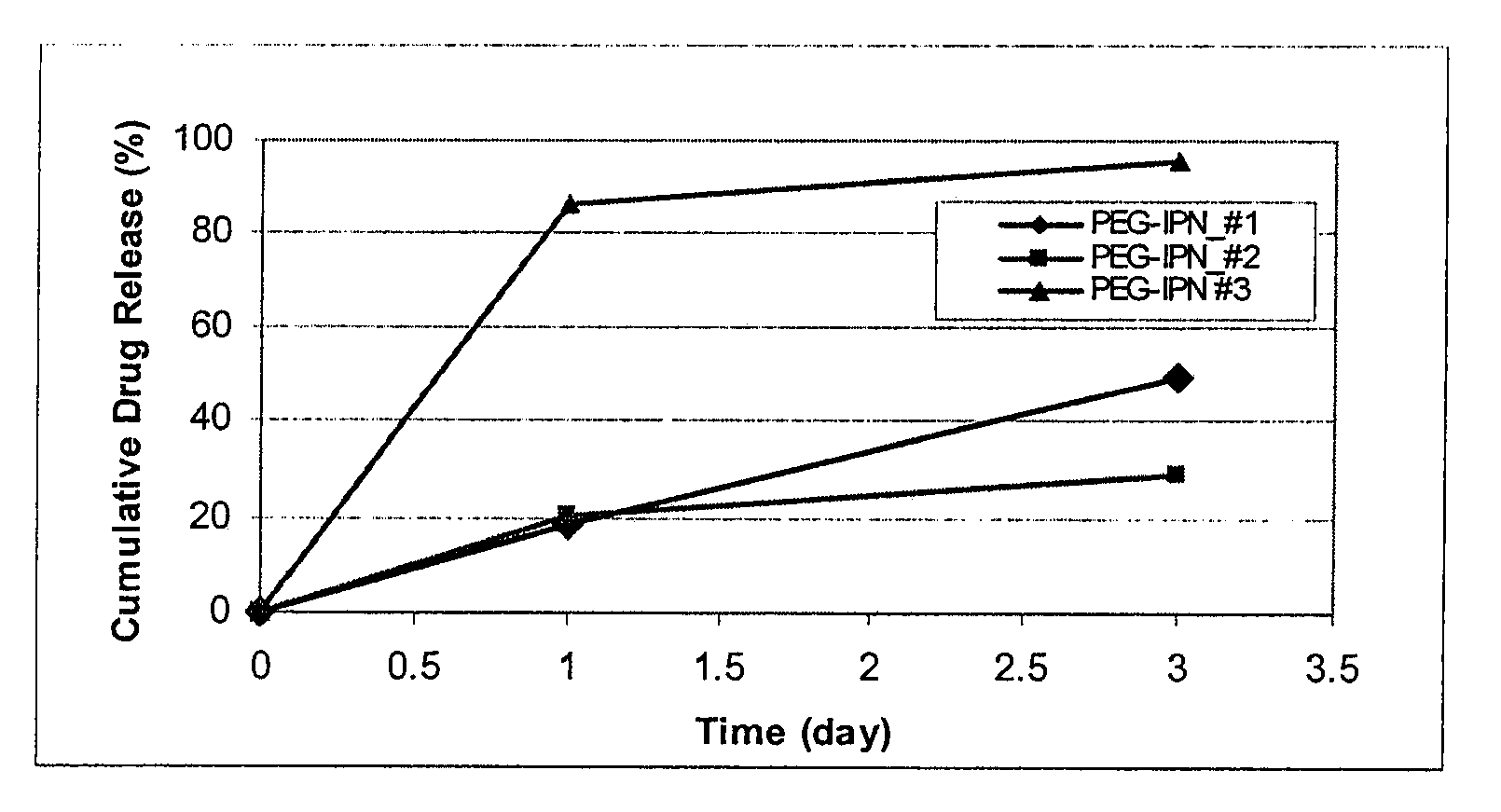
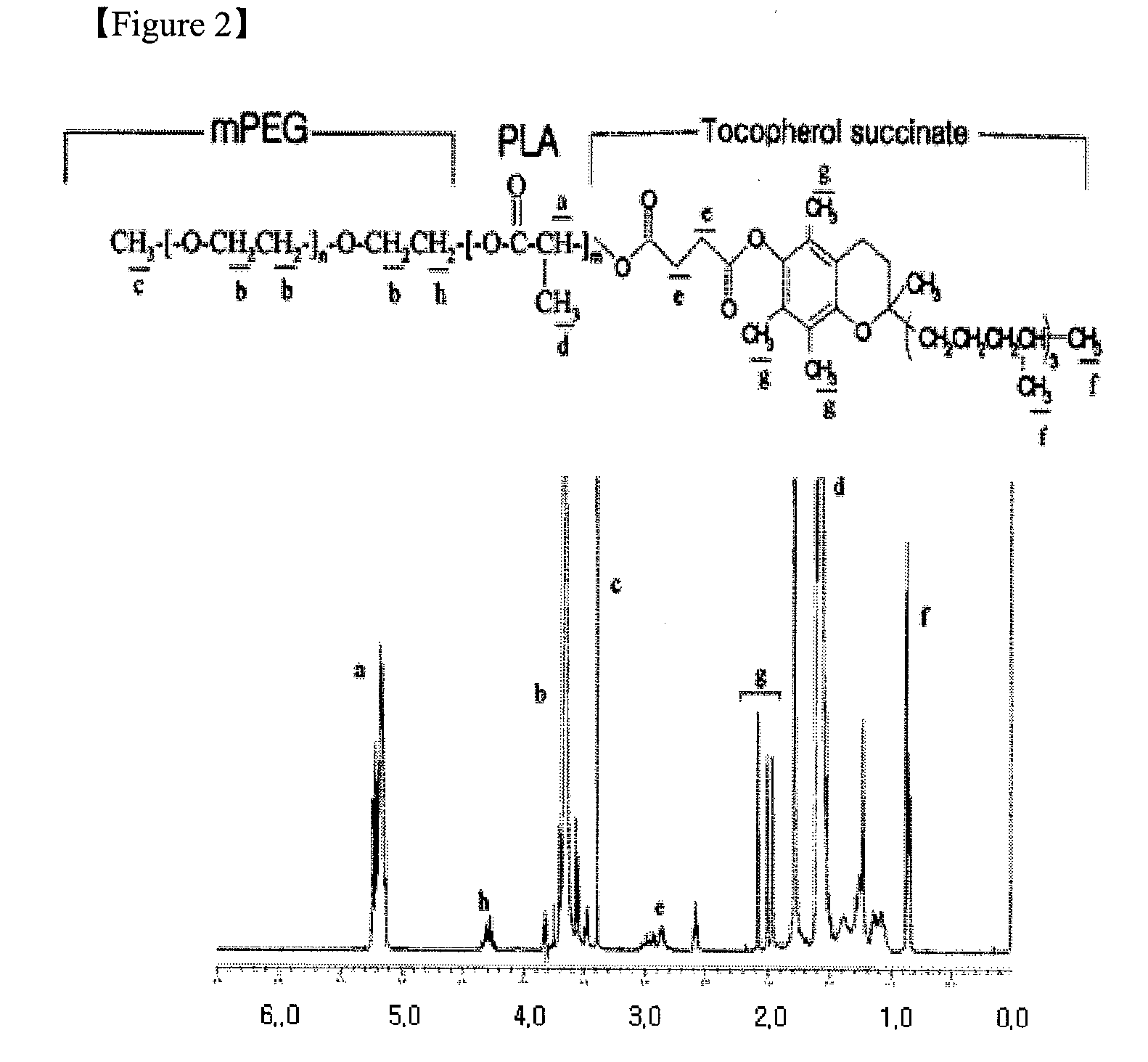
Coating comprising poly (ethylene glycol)-poly (lactide-glycolide-caprolactone) interpenetrating network
Methods for fabricating coatings for implantable medical devices are disclosed. The method comprises forming a coating on an implantable device comprising an interpenetrating network or semi-interpenetrating network. The interpenetrating network or semi-interpenetrating network comprises poly(ethylene glycol) and an aliphatic polyester copolymer. It is also provided an implantable device and a method of using the implantable device.
Owner:ABBOTT CARDIOVASCULAR
Nonaqueous electroulyte secondary battery and electrolyte used in battery
The present invention provides a safe non-aqueous electrolyte secondary battery with characteristics analogous to those of a conventional battery by minimizing battery expansion that causes damage to a device during high temperature exposure or storage. The non-aqueous electrolyte secondary battery comprises: (a) a chargeable and dischargeable positive electrode; (b) a negative electrode capable of absorbing and desorbing lithium; (c) a separator for preventing direct electron transfer between the positive electrode and the negative electrode; and (d) an non-aqueous electrolyte; the non-aqueous electrolyte comprising a non-aqueous solvent and a solute, the non-aqueous solvent comprising a lactone, the solute comprising lithium bis(fluorosulfonyl)imide represented by the formula (1): (F-O2S-N-SO2-F)Li.
Owner:PANASONIC CORP
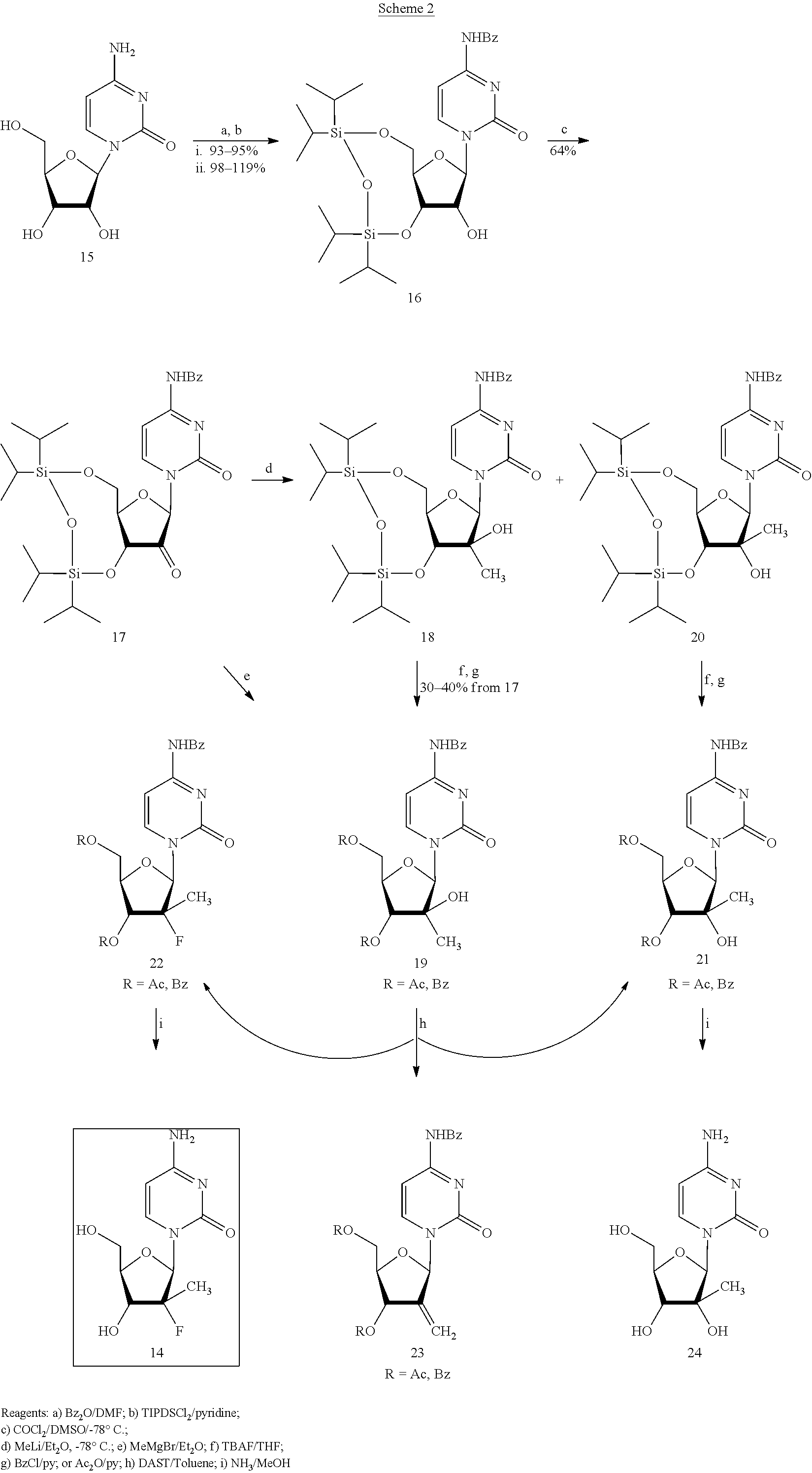
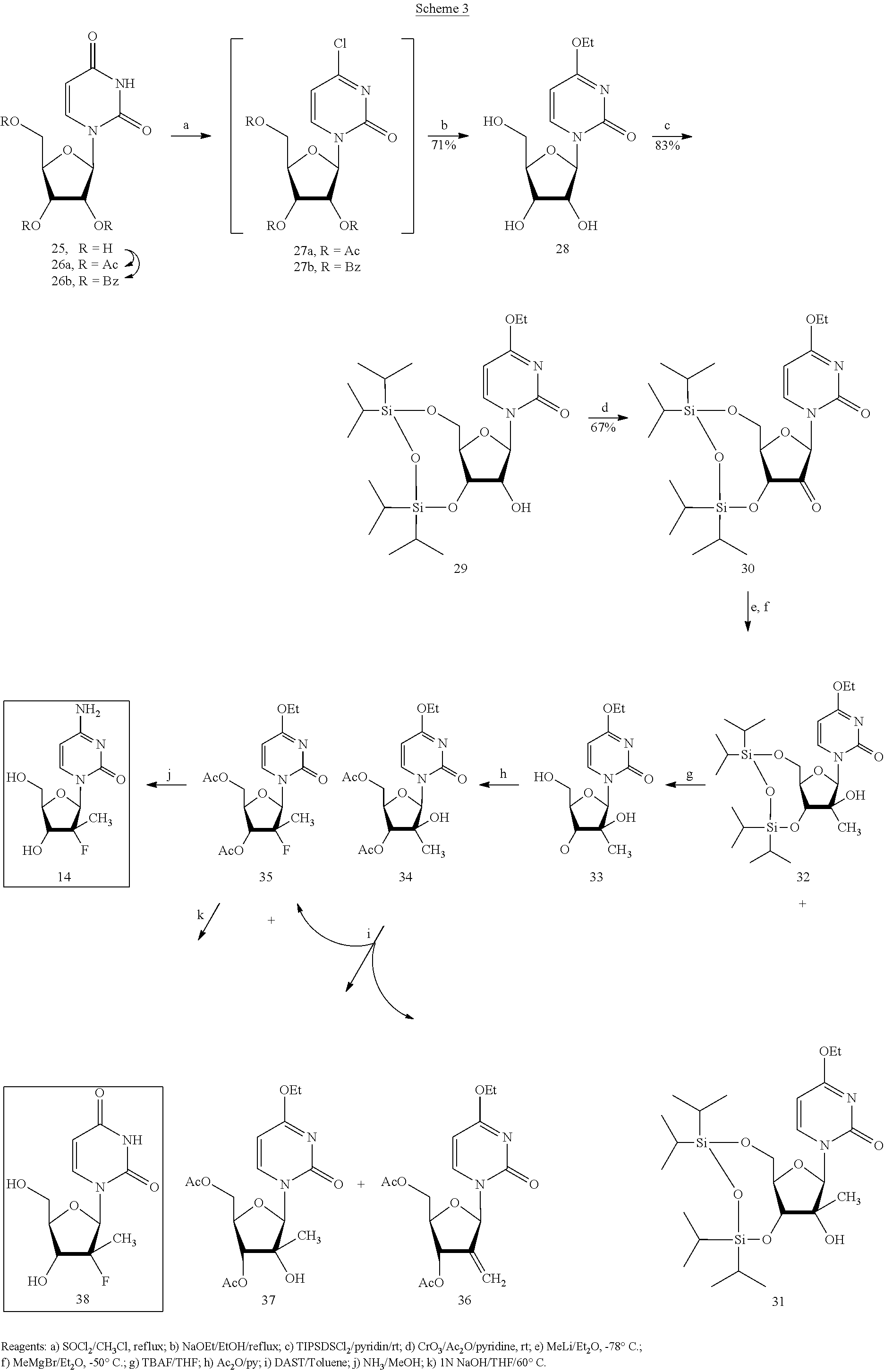
Preparation of 2'-fluoro-2'-alkyl-substituted or other optionally substituted ribofuranosyl pyrimidines and purines and their derivatives
The present invention provides (i) processes for preparing a 2′-deoxy-2′-fluoro-2′-methyl-D-ribonolactone derivatives, (ii) conversion of intermediate lactones to nucleosides with potent anti-HCV activity, and their analogues, and (iii) methods to prepare the anti-HCV nucleosides containing the 2′-deoxy-2′-fluoro-2′-C-methyl-β-D-ribofuranosyl nucleosides from a preformed, preferably naturally-occurring, nucleoside.
Owner:GILEAD SCI INC
Powder composition, a powder dispersion in oil and a cosmetic composition containing said powder composition and a powder dispersion in oil
InactiveUS6342239B1Less cohesivenessGood dispersionCosmetic preparationsPowder deliveryDispersion stabilityNitrogen
A powder composition comprising, a copolymer containing (A) an organopolysiloxane monomer, one or more kinds of monomer selected from a group composed by (B) a monomer containing nitrogen group, a monomer possessing a polyoxyalkylene group, a monomer possessing a polylactone group, a monomer possessing a hydroxyl group and a monomer possessing an anionic group and a powder. Further, a powder dispersion in oil comprising said copolymer, powder and oil, and a cosmetic composition containing them. Said powder composition and a powder dispersion in oil have a less cohesion of powder particles and is superior in a dispersing ability and a dispersion stability, and the cosmetic composition which contains said powder composition has a good stability and gives an excellent sensation at the actual use.
Owner:KOBAYASHI KOSE CO LTD
Block Copolymerized Polyimide Ink Composition for Printing
InactiveUS20080275181A1Strong adhesionLow modulusPrinted circuit aspectsSemiconductor/solid-state device manufacturingBenzoic acidSolvent
The object is to provide a polyimide ink composition having good printing properties and good continuous printing properties, which composition can be dried at a low temperature of not higher than 220° C., and which composition gives a coating film, after being dried, having excellent dimensional stability, heat resistance, low modulus of elasticity, flexibility, resistance to warping, chemical resistance, adhesiveness with substrates, and plating resistance. This object is accomplished by a polyimide ink composition for printing, comprising a mixed solvent containing an benzoic acid ester solvent and a glyme solvent, and a polyimide soluble in the mixed solvent; wherein the polyimide is obtained by polycondensing a polyimide oligomer with a tetracarboxylic dianhydride component(s) and / or a diamine component(s) having no siloxane bond in molecular skeleton thereof the polyimide oligomer being prepared by polycondensing a tetracarboxylic dianhydride component(s) and a diamine component(s) having siloxane bonds in molecular skeleton thereof in the presence of a base catalyst(s), or a mixed catalyst including a lactone(s) and / or an acidic compound(s) and a base(s); the content of the diamine component(s) having siloxane bonds based on the total diamine components being 15 to 85% by weight.
Owner:PI R & D +1
Submicron nanoparticle of poorly water soluble camptothecin derivatives and process for preparation thereof
InactiveUS20100008998A1Efficient productionExcellent lactone stabilityBiocidePowder deliveryPolymer scienceNanoparticle
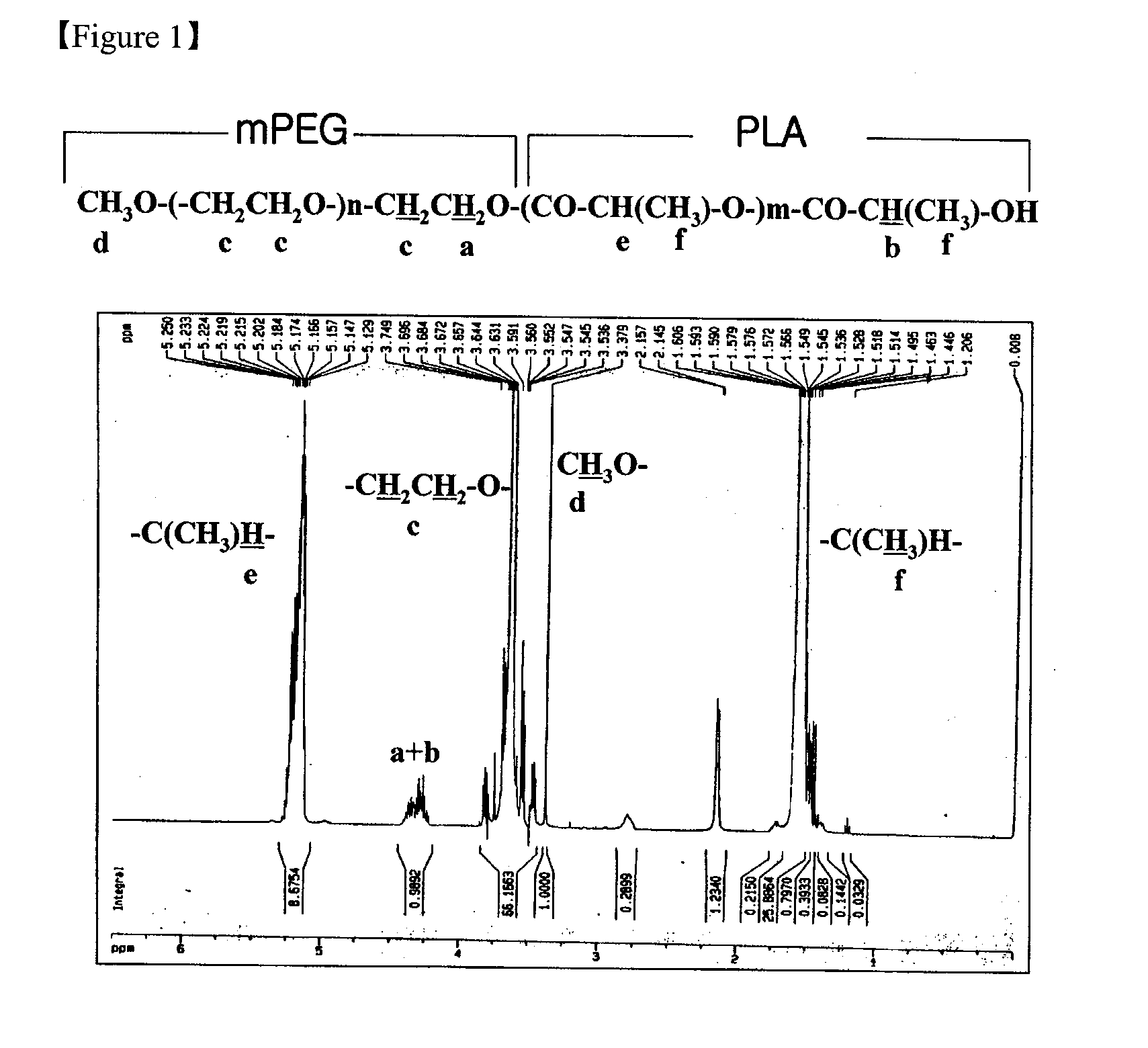
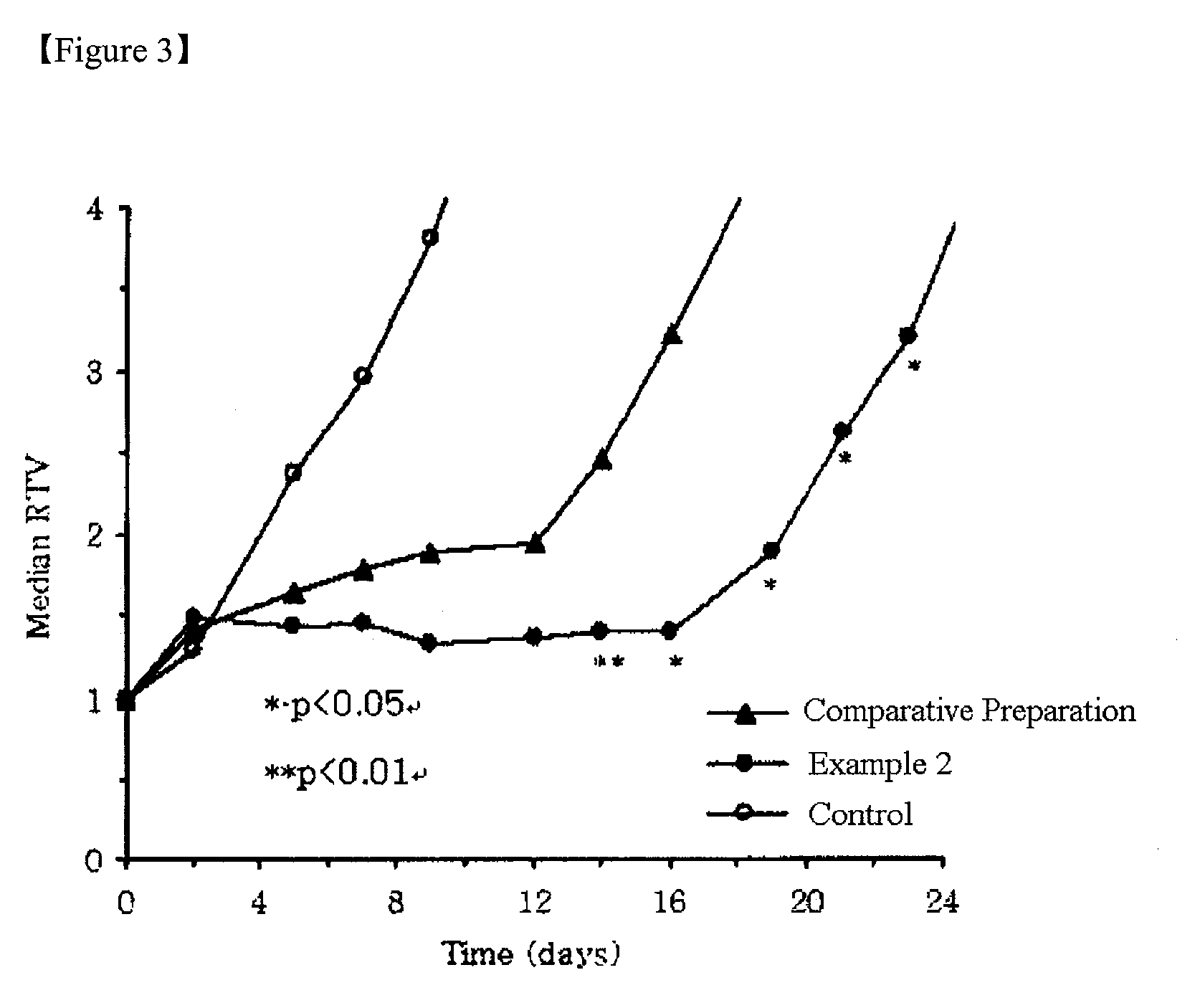
The present invention relates to a nanoparticle composition comprising a camptothecin derivative, solid polyethyleneglycol and an anti-associative agent, and the process for preparing the same. Specifically, the present invention provides a composition comprising a nanoparticle of the camptothecin derivative, which is prepared by solid-dispersing the poorly water soluble camptothecin derivative in polyethyleneglycol and dissolving the solid dispersions in an aqueous solution containing an anti-associative agent. The composition of the present invention stabilizes the camptothecin derivative lactone form in body fluid for effective anticancer activity.
Owner:SAMYANG BIOPHARMLS CORP
Systems and processes for polymer production
ActiveUS20180094100A1Higher and smoother conversion to acrylic acidHigh degreePreparation from carboxylic acid esters/lactonesPolymer sciencePolymer
Owner:NOVOMER INC
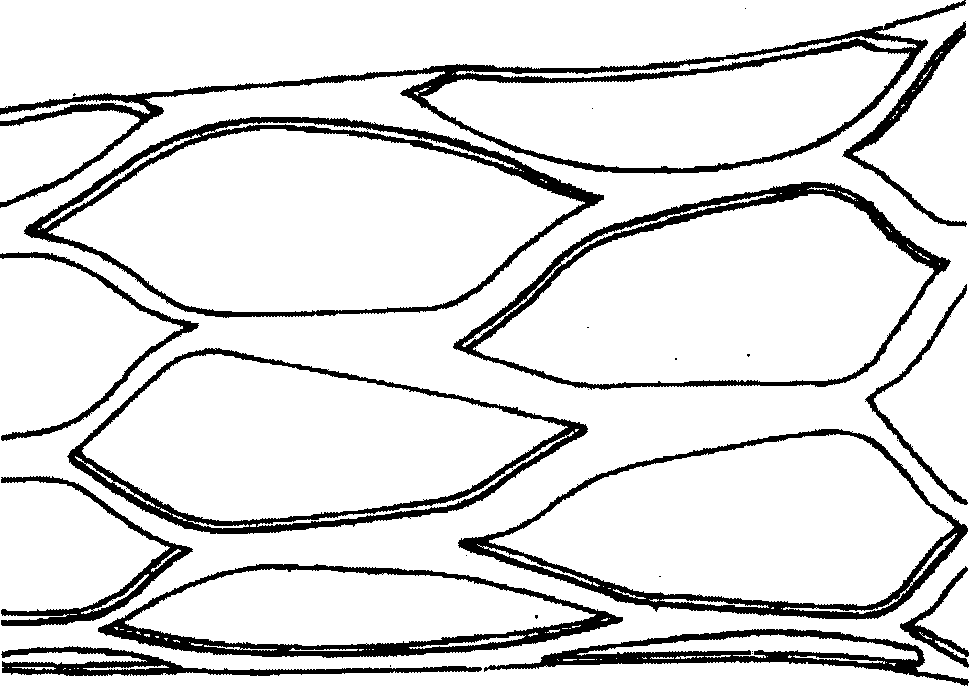
Medicine eluted cardiovascular frame and its preparing process
InactiveCN1355005AContinuous and stable releasePromote healingStentsCoatingsCardiovascular stentEthylene Homopolymers
A medicine eluted cardiovascular frame is composed of expandible support and the medicine coated biodegradable layer coated on the said frame. The said biodegradable material contains one of the homopolymer and copolymer of glycollide, L-lactide or epsilon-caprolactone, and the copolymer of multi-group amino acids. The medicine can resist the cardiovascular narrowness. The process for preparing the said support includes such steps as preparing the said expandible support, immersing it in the mixture of the said medicine, biodegradable material and solvent, and drying. It can prevent thrombosis.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com