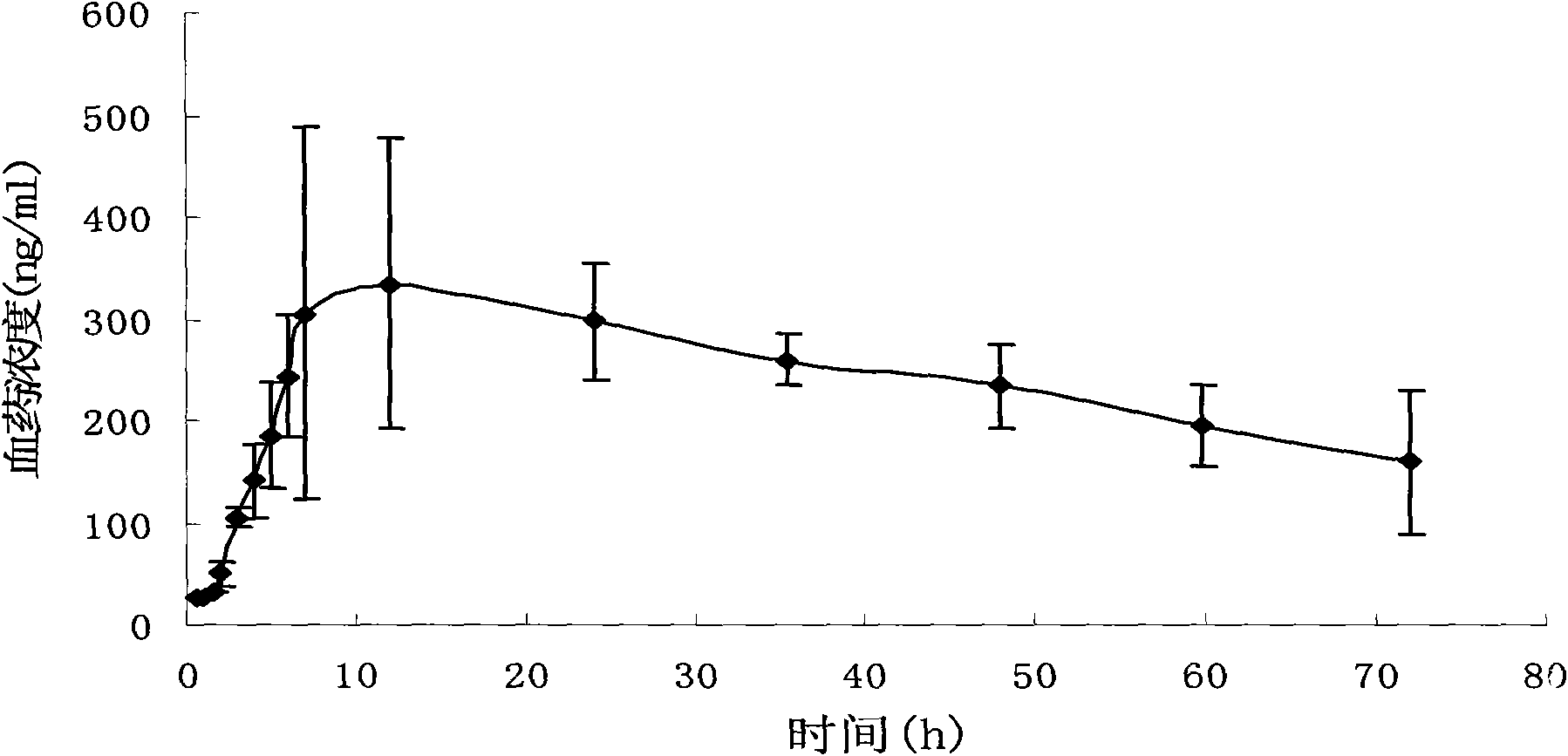
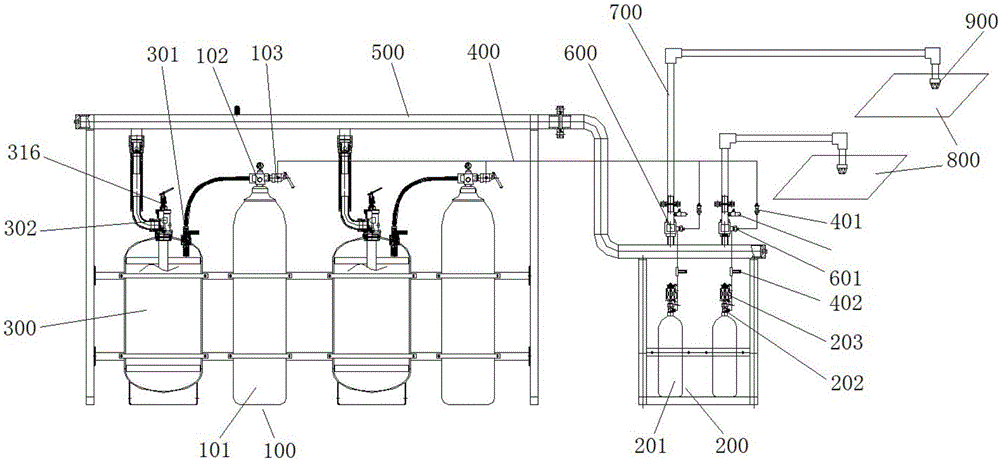
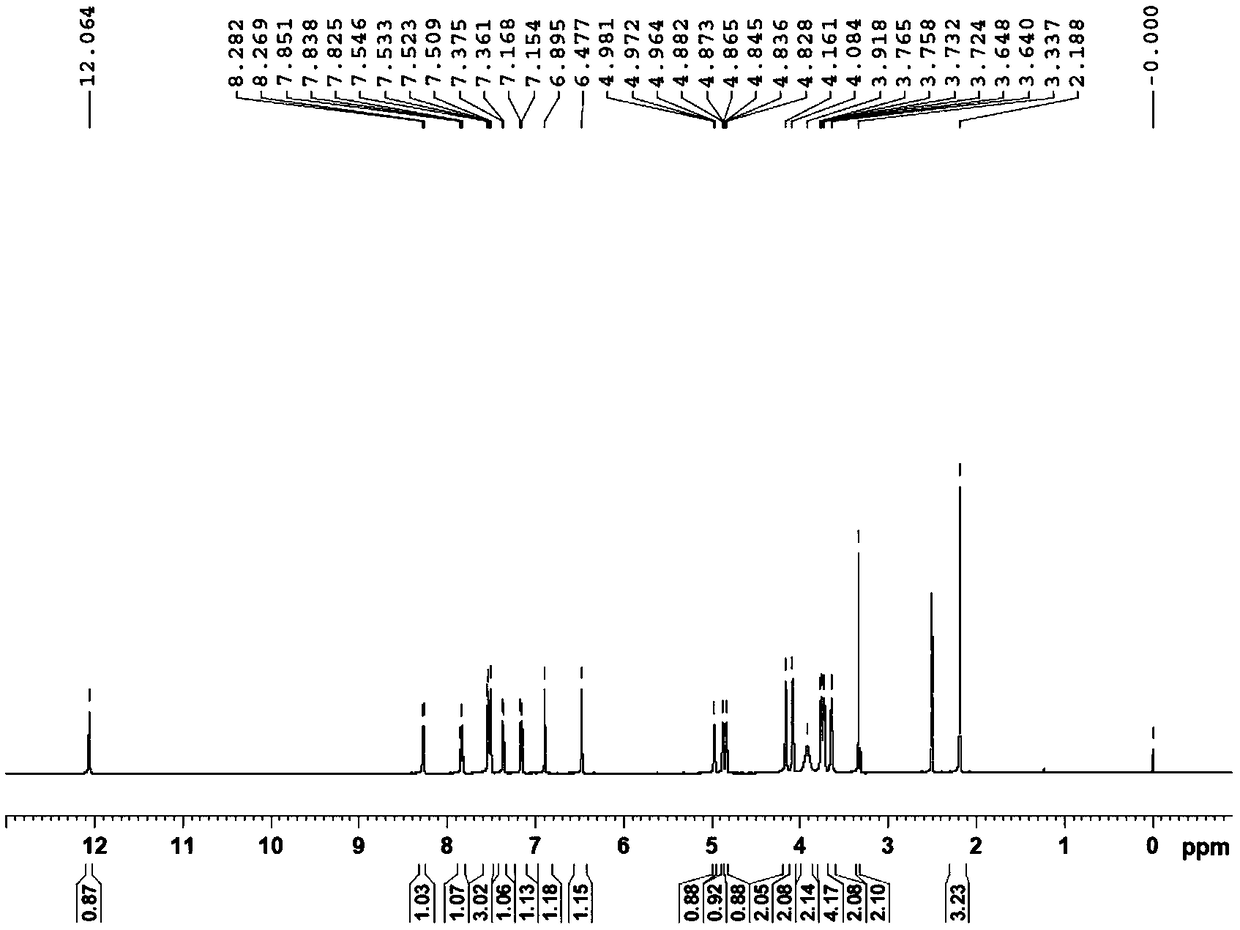
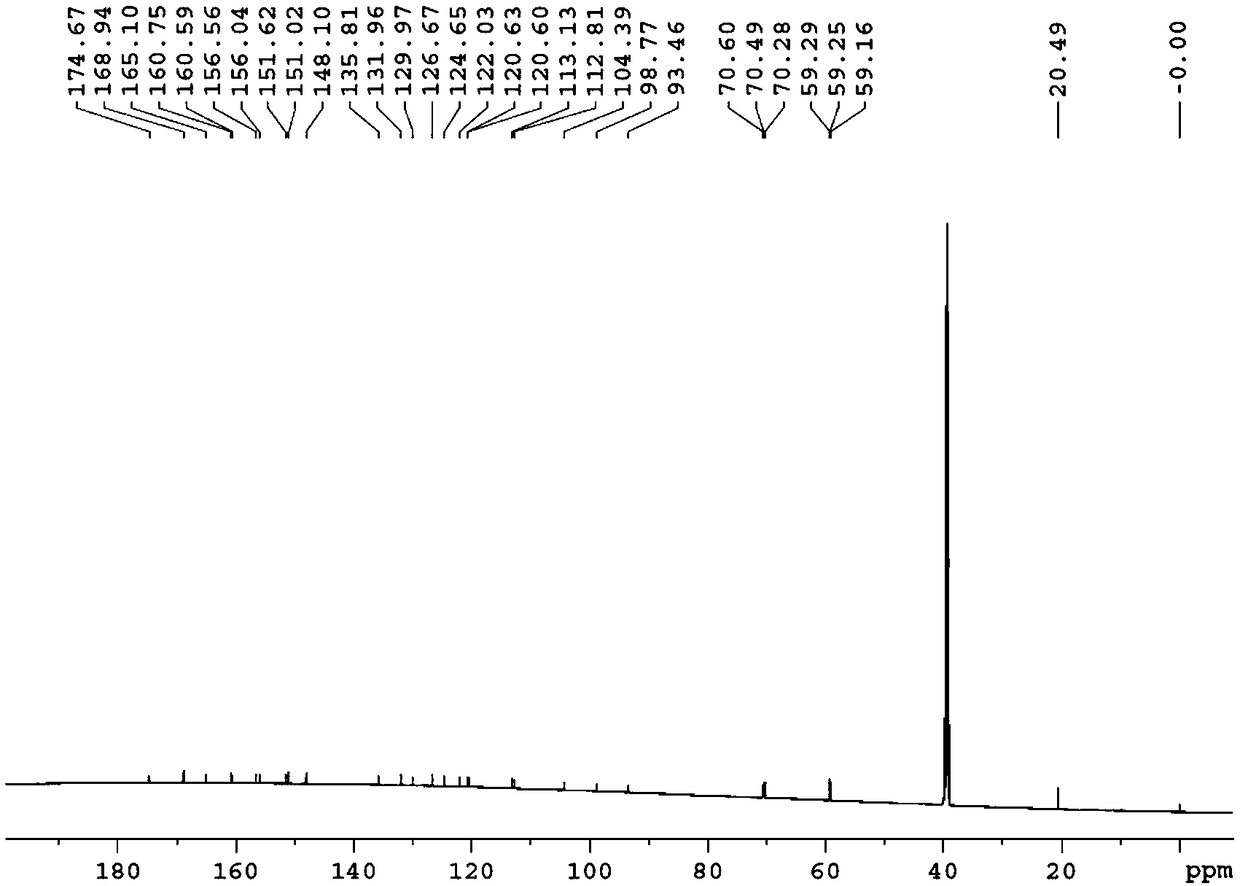
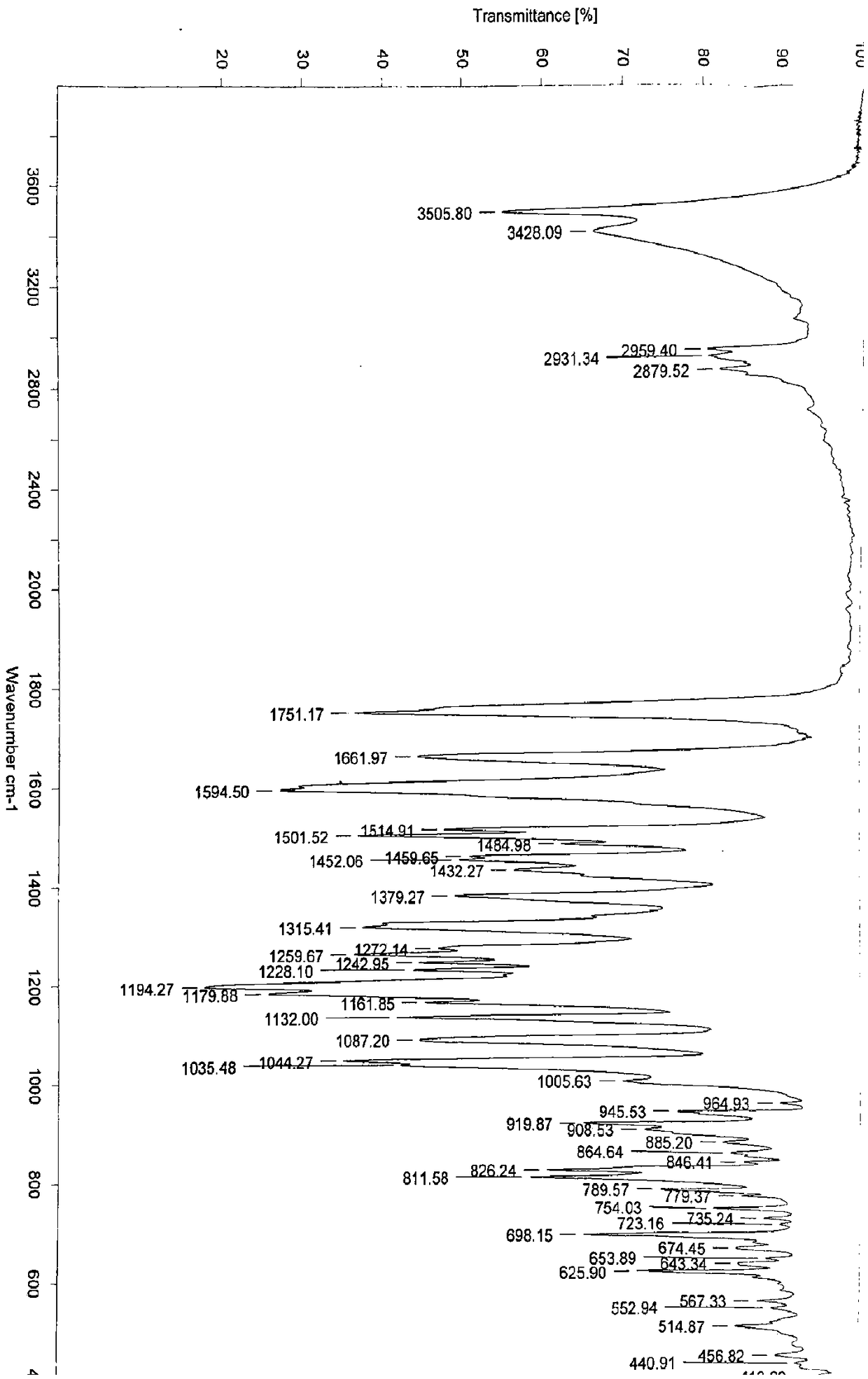
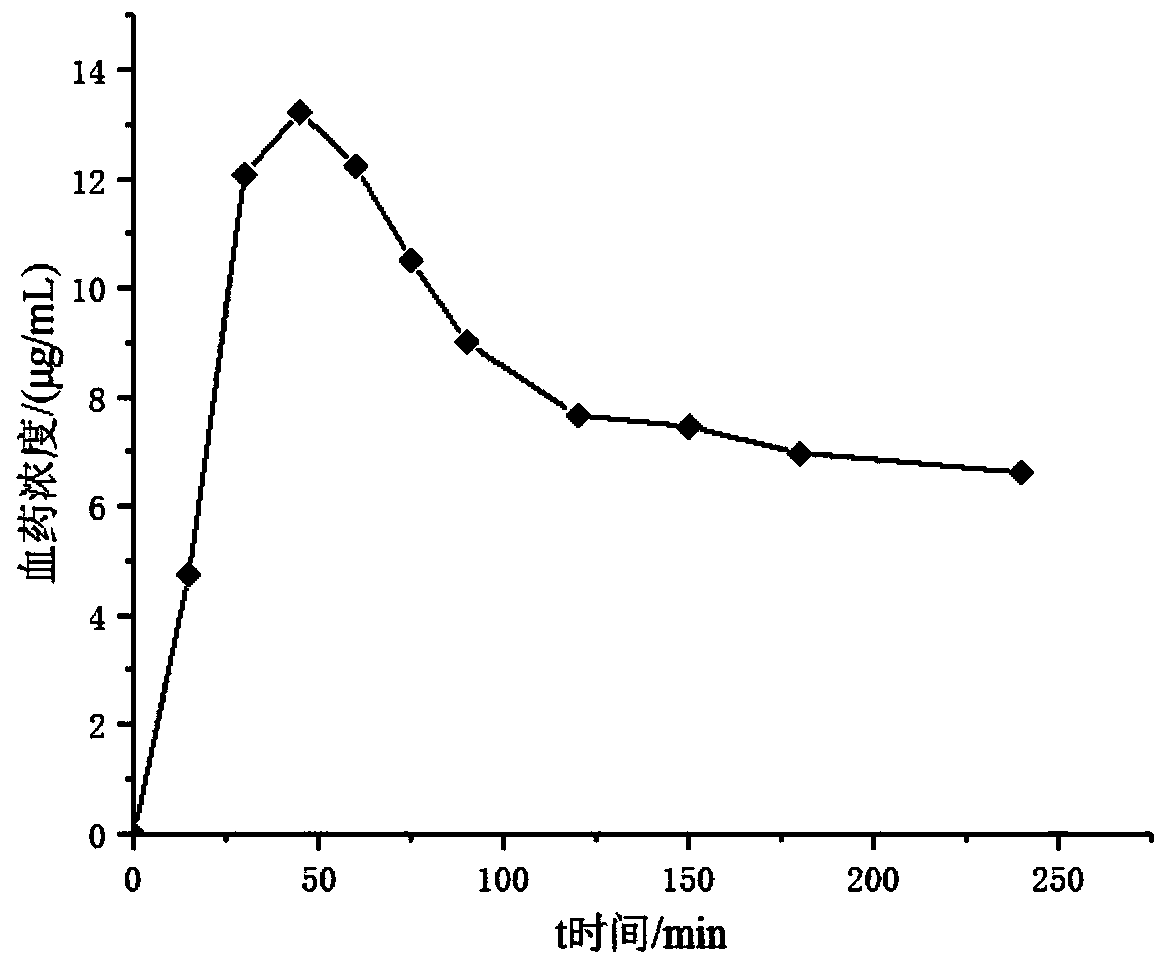
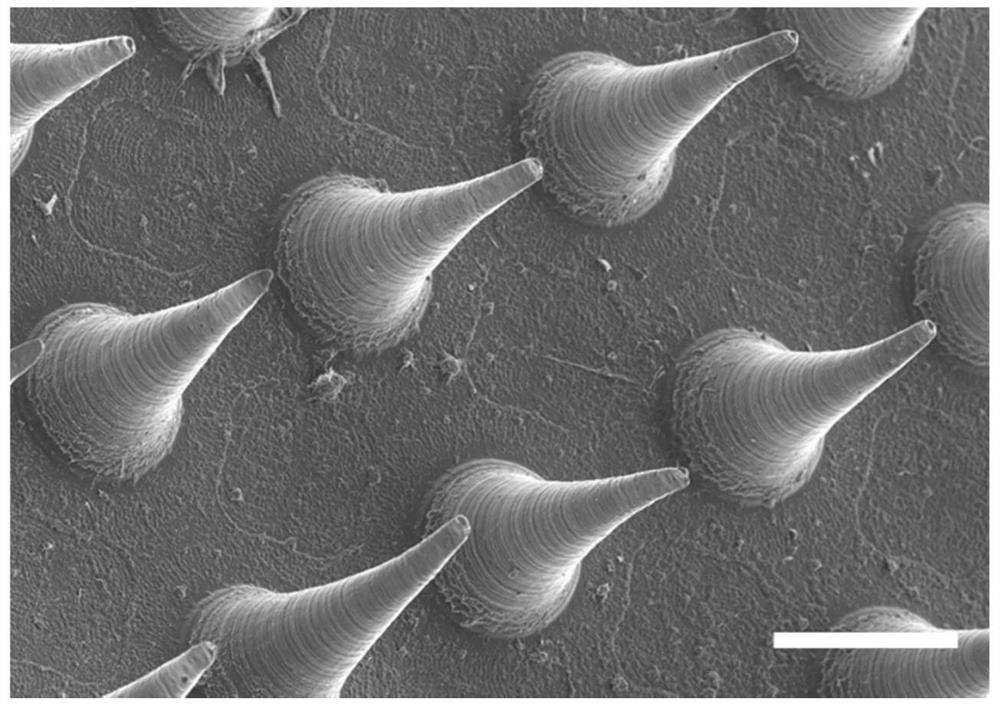
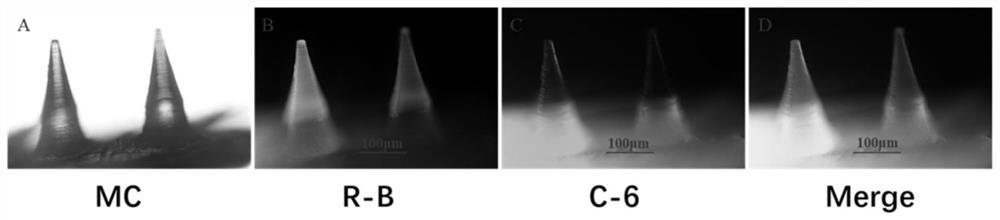
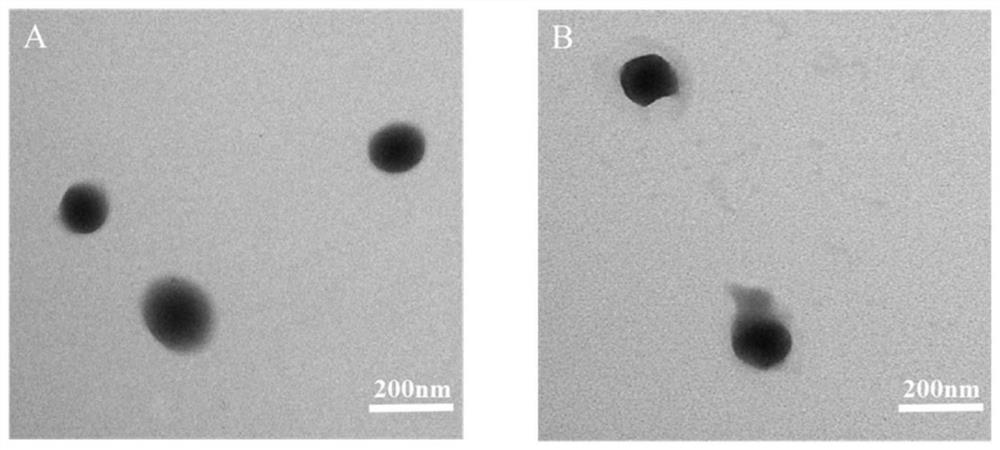
Patents
Literature
69results about How to "Continuous and stable release" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antibacterial nano fiber material and preparation method thereof
InactiveCN101358382ANo side effects on the human bodyContinuous and stable releaseFilament/thread formingMacromolecular non-active ingredientsFiberSide effect
The invention relates to an antibacterium nano fiber material and a preparation method thereof; the material comprises polymer superfine fiber and antibacterial agent, and the weight ratio is 60 to 98: 2 to 40; the preparation method comprises the following steps: (1) the antibacterial agent is dissolved in distilled water to prepare solution; the polymer superfine fiber is dissolved in methylene dichloride or chloroform organic solvent, emulsifier is added to be mixed evenly, to obtain solution which is dispersed evenly; (2) the two types of solution are mixed to obtain even water-in-oil W / O latex, and then electrostatic spinning is conducted to the latex, to obtain the antibacterium nano fiber material. The nano fiber material has good ventilation property and filterability, still has bacteriostasis and sterilization functions for a long time after stably releasing the antibacterial agent, has simple preparation method, adopts the biodegradable and bioabsorbable polymer as carrier materials, can be absorbed by the human body after fully releasing, is not left, does not need secondary operation and has no side effect.
Owner:DONGHUA UNIV
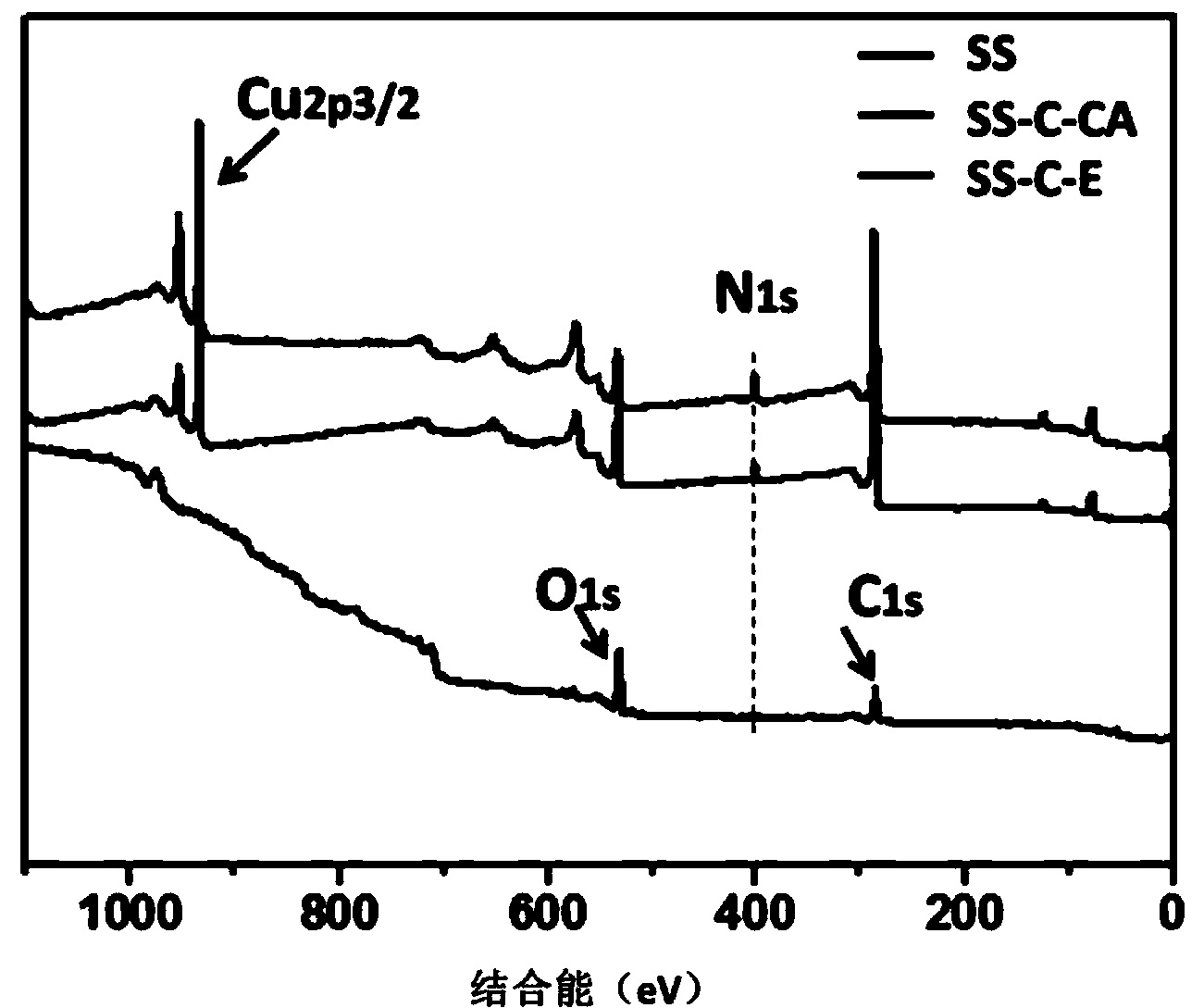
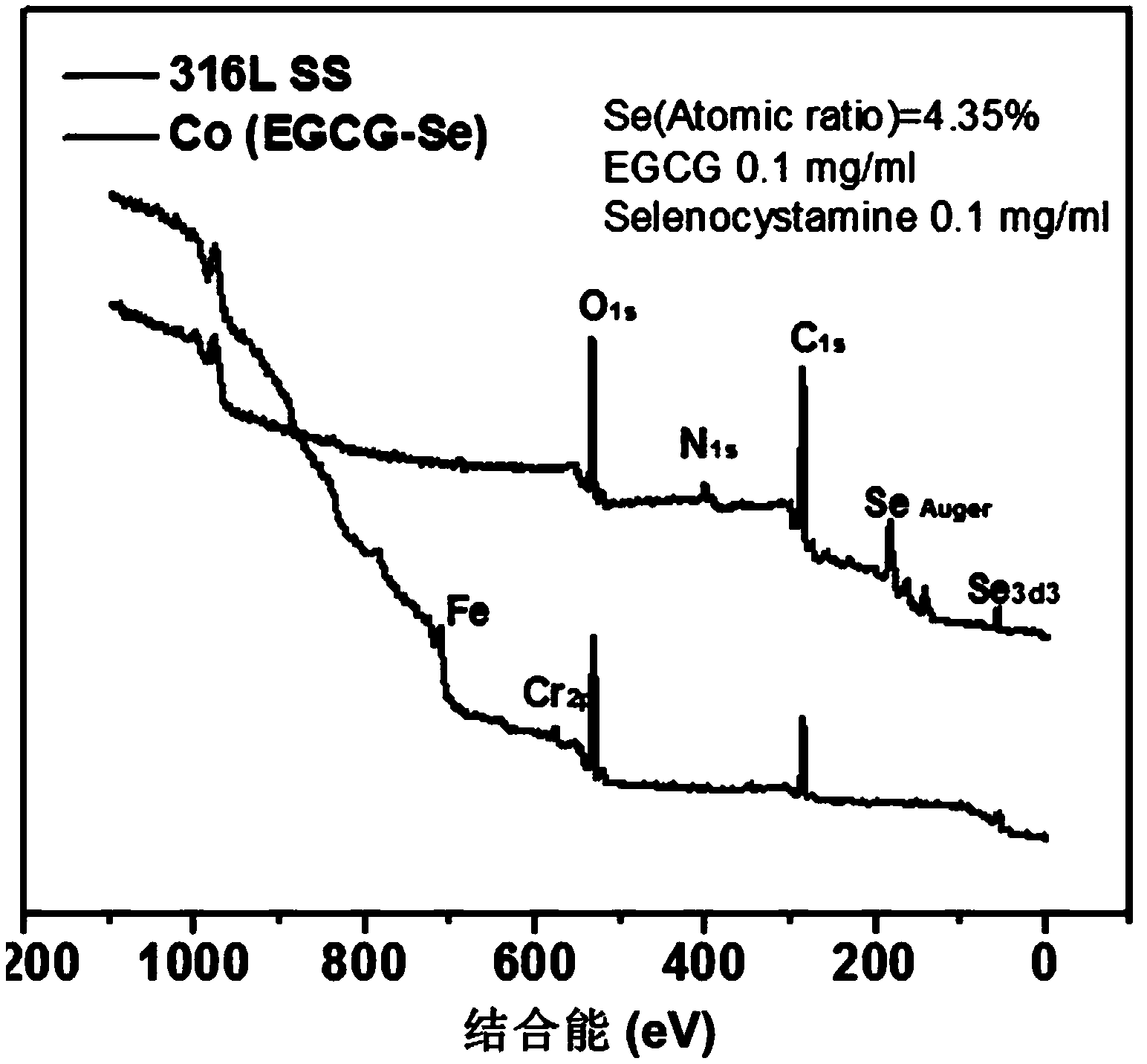
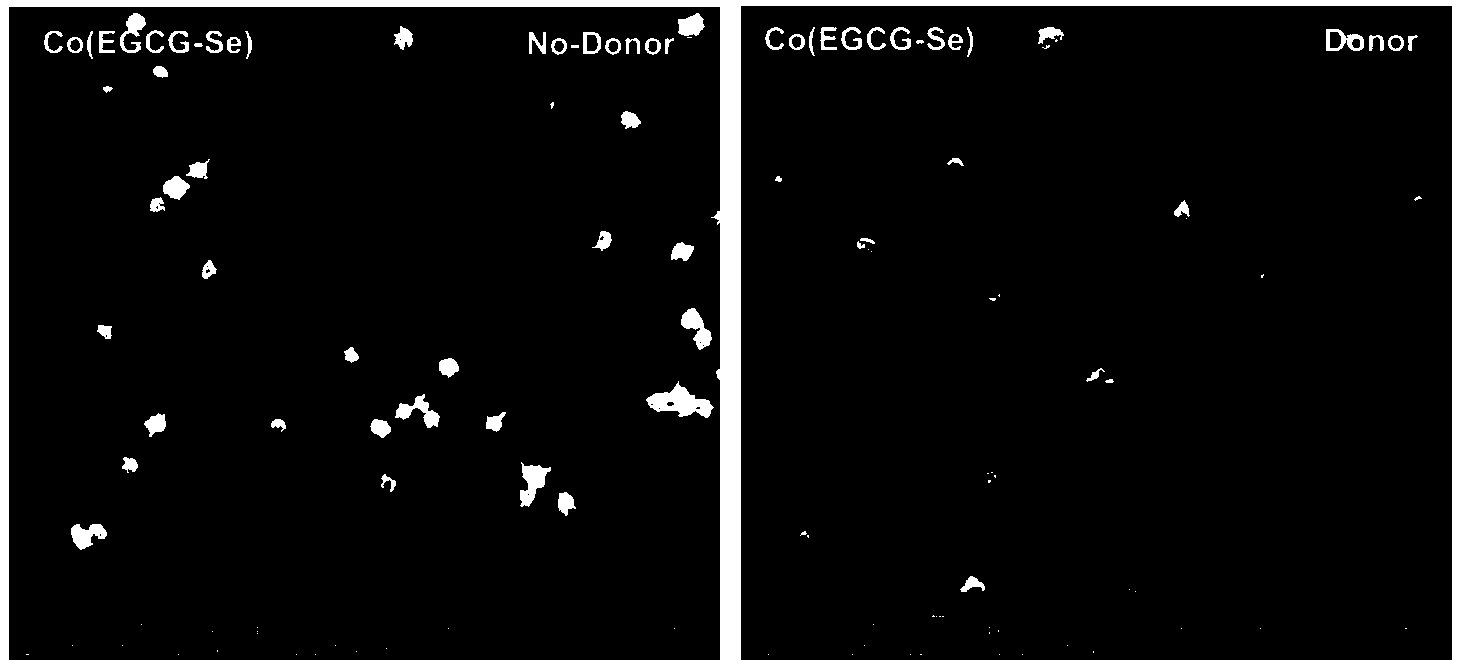
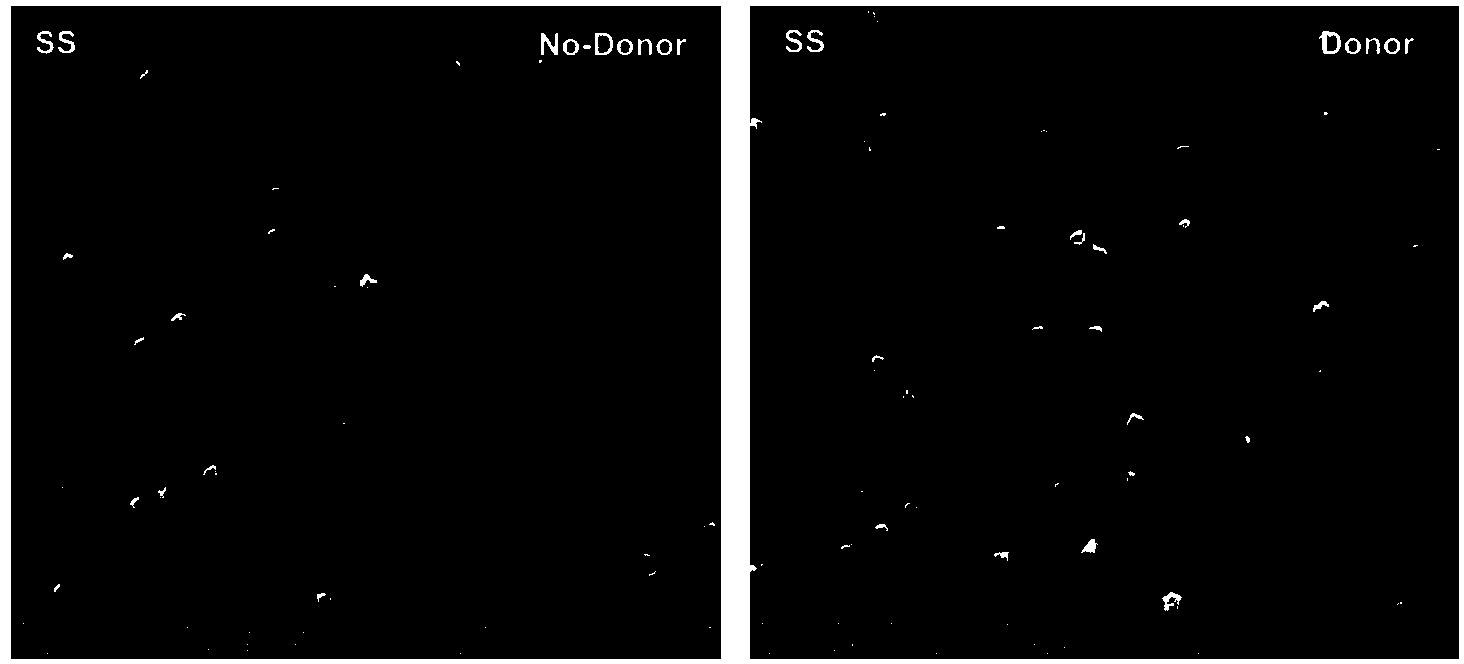
Preparation method of copper ion mediated anticoagulant coating with function of in situ catalysis of NO release
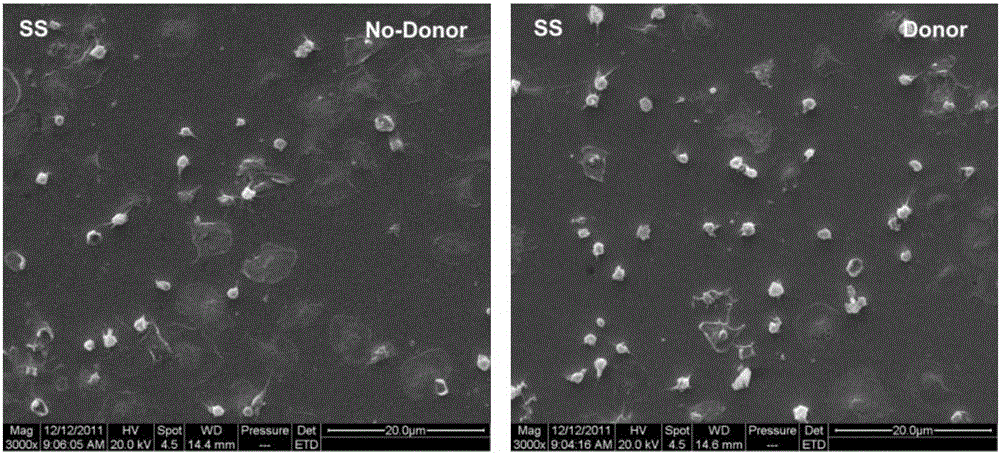
ActiveCN104208760ALong-acting sustained releaseImprove researchPharmaceutical containersMedical packagingNo donorsBlood vessel
The invention discloses a preparation method of a copper ion mediated anticoagulant coating with function of in situ catalysis of NO release. The method includes steps of preparing an acidic buffer solution, and adding a compound with pyrogallol structure and certain concentration, a compound with amino or thiol, and copper ion soluble salt. The method of the invention has the advantages of simple operation, mild reaction conditions and easiness. The prepared coating has the advantages of controllable content of the units containing multiple amino or thiol compounds, and easily controlled loading amount of copper ion. The modified coating prepared by the method has excellent adhesion with a base material, and through act of the copper ions in the coating with the blood, the coating can conduct in situ catalyze the NO donor molecules in blood to continuously decompose and release NO molecules, thus realizing the inhibition of platelet activation and aggregation, inhibiting smooth muscle cell proliferation and migration, and protecting vascular endothelial layer function.
Owner:GUANGZHOU NANCHUANG EVEREST MEDICAL TECH CO LTD
Preparation method of anticoagulant material with function of inducing and catalyzing release of endogenous NO
ActiveCN104208761AControl release speedLong releasePharmaceutical containersMedical packagingVascular endotheliumReaction temperature
The invention discloses a preparation method of an anticoagulant material with function of inducing and catalyzing release of endogenous NO. The method comprises the steps of: configuring an alkaline Tris-buffer buffer solution, and adding a compound with certain concentration and pyrogallol structure and a compound with disulfide bond or diselenium bond and containing amino or thiol at two ends; and placing the substrate materials in a reaction solution, controlling the reaction temperature, reacting for 1-24 h, removing the substrate, and cleaning and drying to obtain the objective material. The invention has the advantages of simple operation, mild reaction conditions and easy operation. The component unit of the compound with disulfide bond or diselenium bond and containing amino or thiol at two ends has controllable content; the modified coating prepared by the method has excellent adhesion, and catalyzes the blood NO donors to continuously release NO molecules through in situ catalysis, inhibits activation and aggregation of platelets, inhibits proliferation of smooth muscle cells, and protects vascular endothelium.
Owner:GUANGZHOU NANCHUANG EVEREST MEDICAL TECH CO LTD
Venlafaxine hydrochloride controlled release tablets and preparation method thereof
ActiveCN101953822AReduce manufacturing costStable blood concentrationOrganic active ingredientsNervous disorderSustained-Release PreparationsSide effect
The invention discloses venlafaxine hydrochloride controlled release tablets and a preparation method thereof. The venlafaxine hydrochloride controlled release tablets are elementary osmotic pump (EOP) controlled release tablets which comprise single-layer tablet cores, insoluble semi-permeable membranes and medicament release holes. In the invention, the high-molecular-weight ethylene oxide, microcrystalline cellulose and sodium chloride three auxiliary materials mixed in a proper ratio form a balance system for medicament dissolution and osmotic pressure generation in a pump chamber, and tablet weight is adjusted by using detrix as a filler, so that a high-medicament-capacity osmotic pump preparation can be obtained and the stable and lasting release can be kept. The preparation effectively overcome the shortcomings that the conventional sustained-release preparation is limited in the stability and controllability of the medicament release and can generate stable and constant-speed release, and the medicament release is protected from the influences of stomach and intestine environments, so that individual difference is reduced and the medicament controlled release design aim of giving play to curing effects and reducing side effects is further fulfilled.
Owner:HEFEI LIFEON PHARMA
Humic-acid-containing water-soluble fertilizer capable of increasing selenium content of grapes, and preparation method and application thereof
InactiveCN106631517ANo caking phenomenonAnti-acidAlkali orthophosphate fertiliserAmmonium orthophosphate fertilisersPhosphateAlcohol sugars
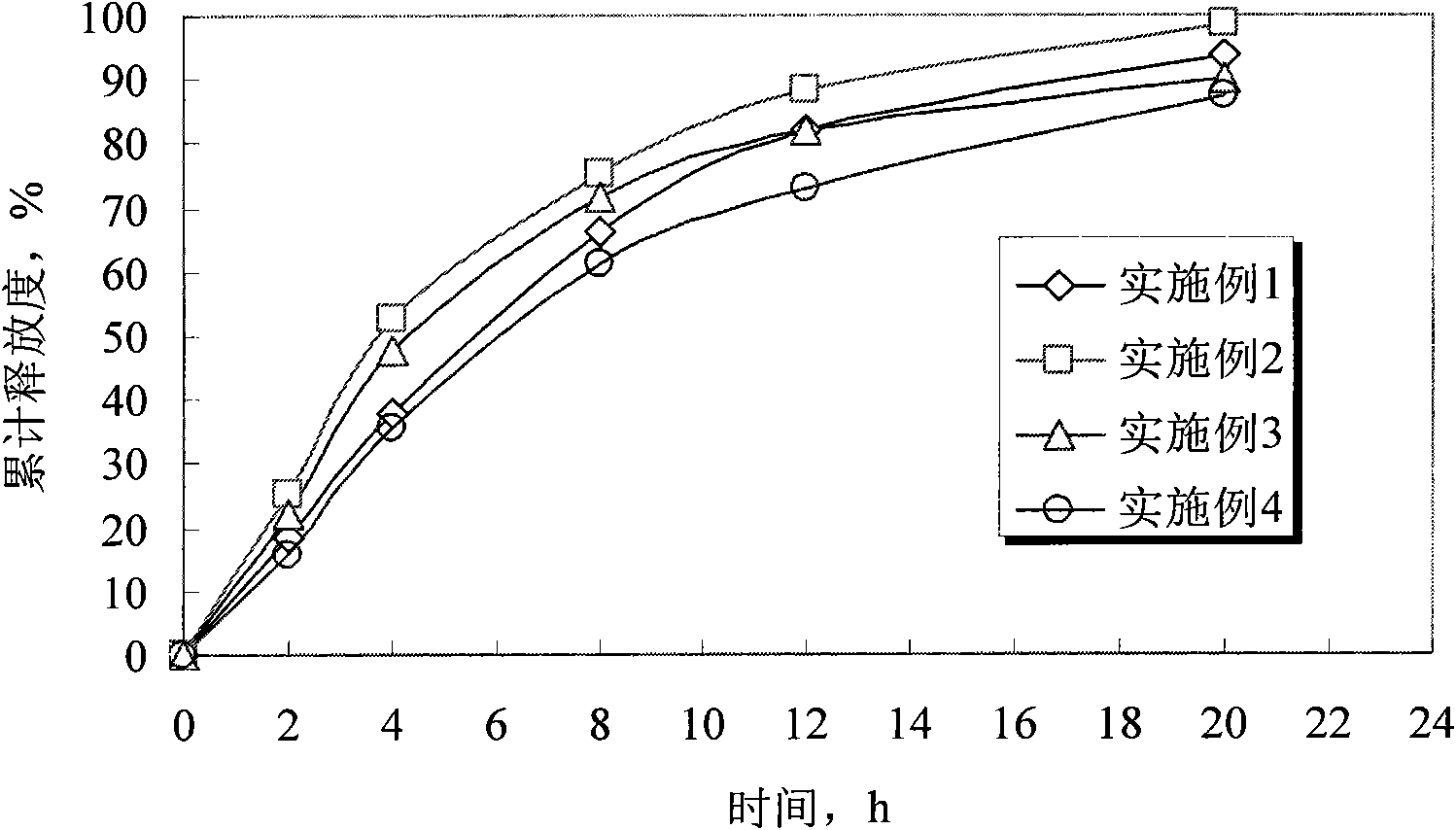
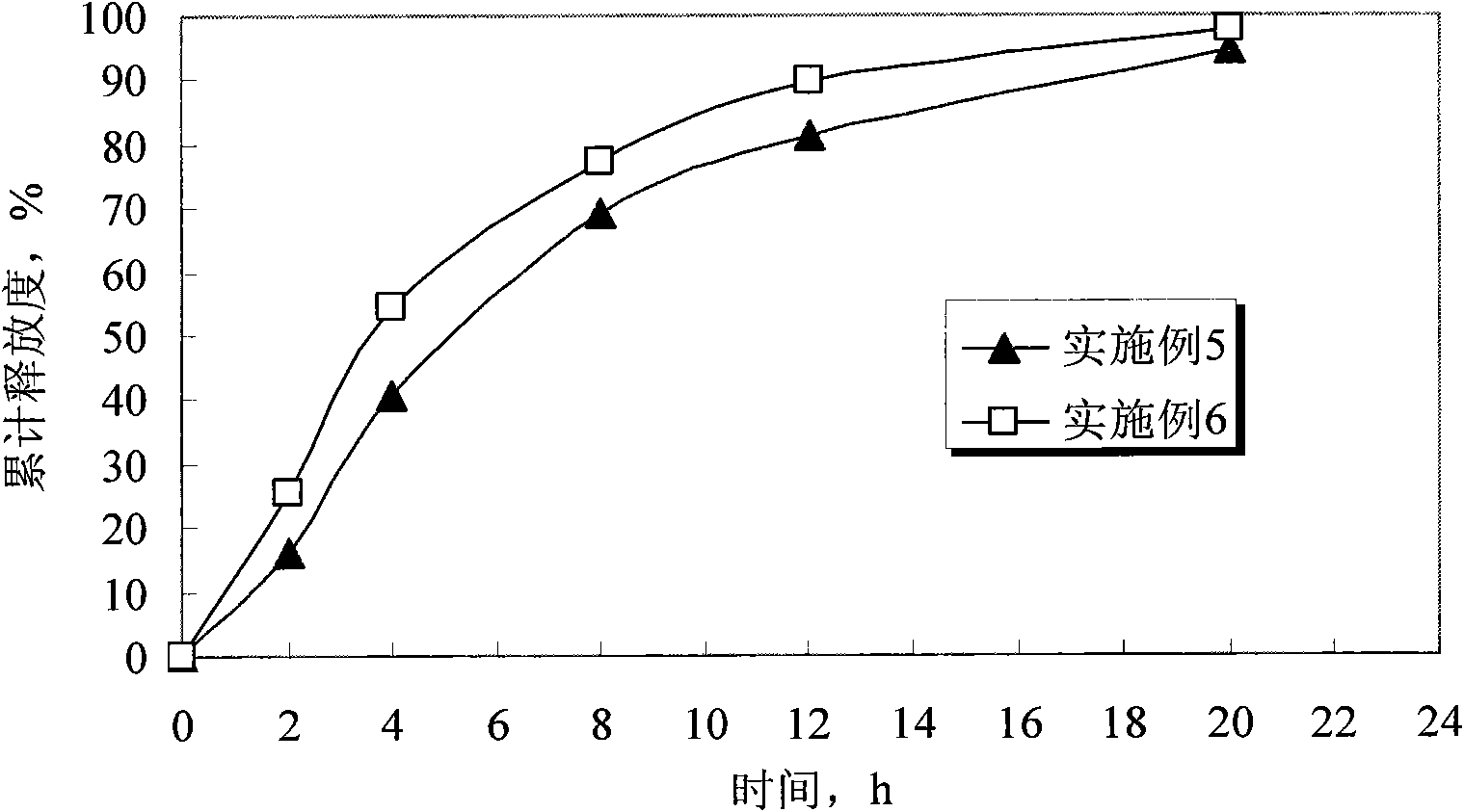
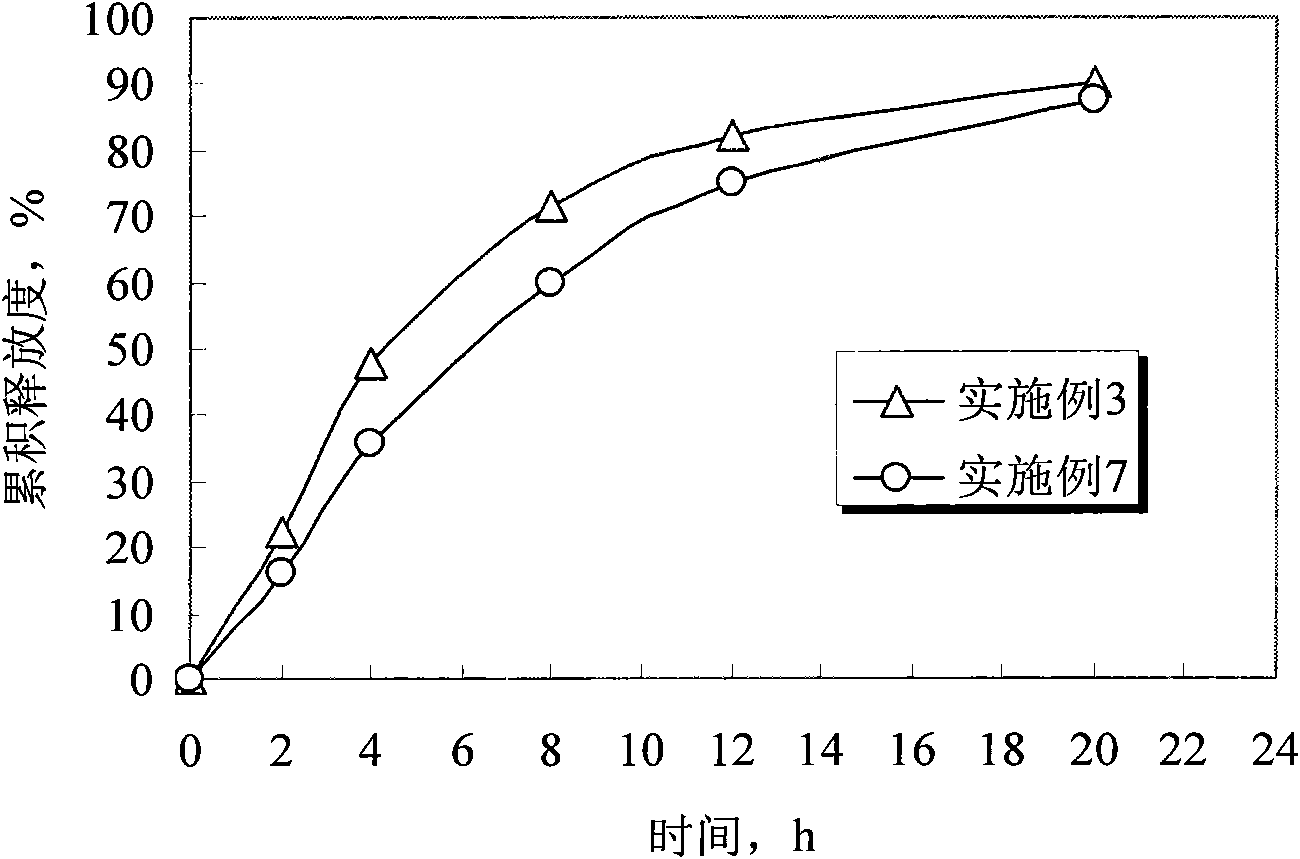
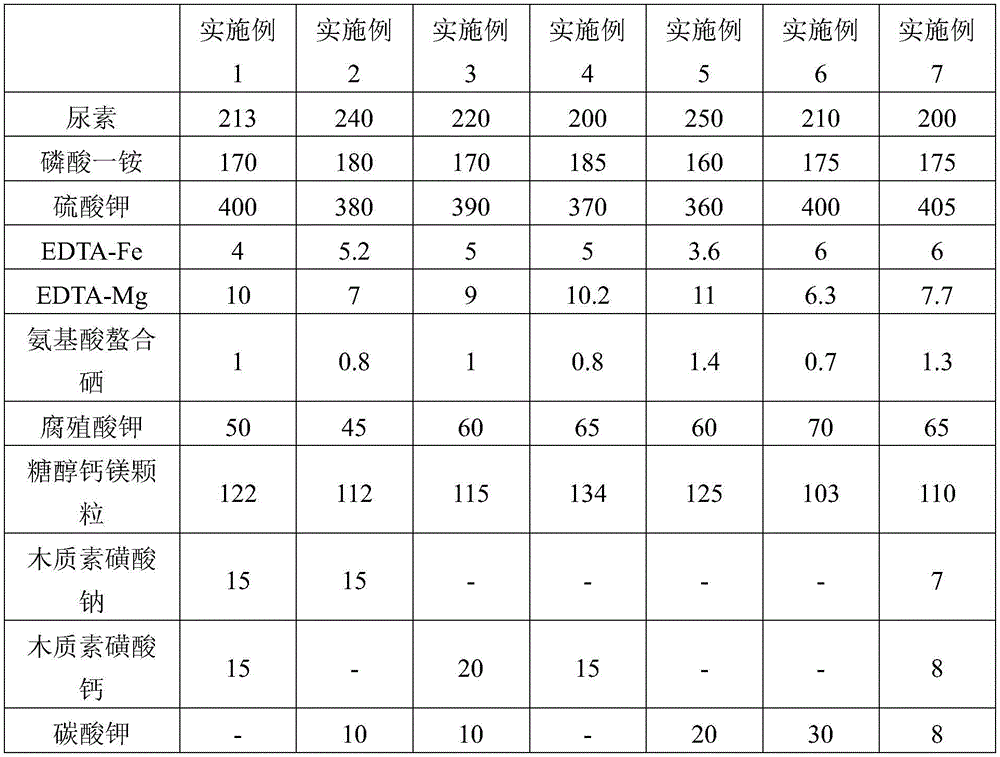
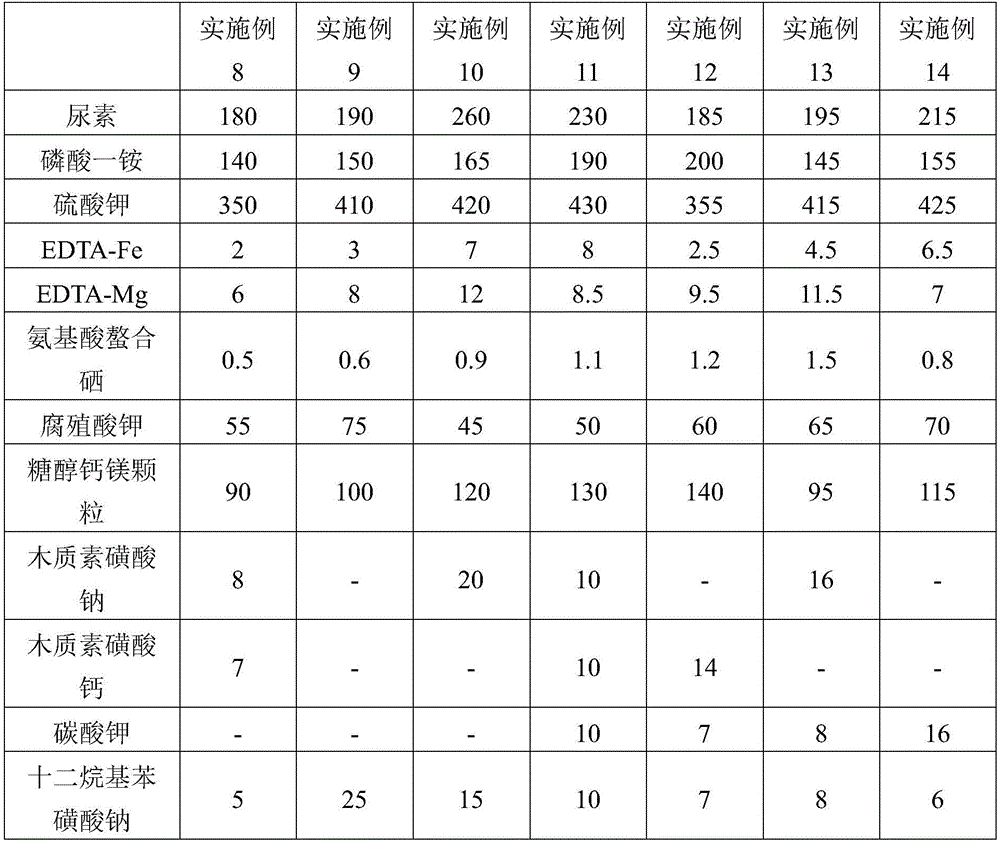
The invention discloses humic-acid-containing water-soluble fertilizer capable of increasing selenium content of grapes, and belongs to the technical field of fertilizer. The humic-acid-containing water-soluble fertilizer comprises, by weight, 180-260 parts of urea, 140-200 parts of monoammonium phosphate, 350-430 parts of potassium sulphate, 2-8 parts of EDTA (ethylene diamine tetraacetic acid) chelated iron, 6-12 parts of EDTA chelated magnesium, 0.5-1.5 parts of amino acid chelated selenium, 45-75 parts of potassium humate, 90-140 parts of sugar alcohol calcium magnesium particles and 20-40 parts of lignosulfonate and / or potassium carbonate and / or sodium dodecyl benzene sulfonate. The invention further provides a preparation method and application of the humic-acid-containing water-soluble fertilizer. The humic-acid-containing water-soluble fertilizer is particularly applied to selenium element absorption in growth period of the grapes, and sugar degree and yield are increased.
Owner:河北中仁化肥集团有限公司 +1
Sustained releasing huperzine preparation
InactiveCN1208054CContinuous and stable releaseRelease stabilityNervous disorderOil/fats/waxes non-active ingredientsChemical synthesisDrug release
A slowly releasing huperzine for treating sanile dementia by oral application features that its active components are huperzine A and its humolog, or their salts or ester derivatives. Its advantages are slow release (more than 12 hr), and high curative effect and safety.
Owner:解健博
Inductive agent for regeneration of periodontium tissue of temperature sensitivity and preparation method thereof
InactiveCN102133430ARebuild and restore growth activityOvercoming the disadvantage of short half-lifePeptide/protein ingredientsGenetic material ingredientsHalf-lifePhosphate
The invention provides an inductive agent for regeneration of periodontium tissue of temperature sensitivity, which is a temperature sensitivity hydrogel composite body taking chitosan, chitosan quaternary ammonium and Alpha, Beta-phosphate as carriers, and the carriers are loaded with plasmids containing bone tissue growth factor coding genes. The invention also provides a preparation method forthe regeneration of periodontium tissue of the temperature sensitivity. In the invention, the composite body is planted into a body, target DNA directly transfects target cells and continuously expresses coded growth factors, rebuilds and recovers the growth activity of osteoblast; along with the gradual degradation and absorption of biological support materials, tissues with the original specialstate and function are formed, so that the purpose of alveolar bone regeneration and function rebuilding are achieved. In the invention, the defect that the half-life period is short due to the direct application of an exogenous growth factor is overcome, and the barrier of immunological rejection as the exogenous cells are planted into the body is broken through, therefore, the clinical application value and the clinical application potential are exact.
Owner:HOSPITAL ATTACHED TO QINGDAO UNIV
Pravastatin transdermal administration preparation and preparation method thereof
ActiveCN101856342ALittle side effectsLower total cholesterolMetabolism disorderEster active ingredientsIrritationRelease time
The invention relates to a pravastatin transdermal administration preparation and a preparation method thereof. In the invention, pravastatin or a pharmaceutically acceptable salt of the pravastatin serves as a medicament active ingredient, and the medicament active ingredient is a filling closed transdermal preparation which consists of a protective layer, an adhesive layer, a controlled-release membrane layer, a medicament reservoir and a backing layer; the medicament reservoir comprises 1 to 20 percent of pravastatin, and the balance of reservoir substrate; the adhesive layer comprises 0 to 10 percent of pravastatin, and the balance of pressure-sensitive adhesive; the reservoir substrate comprises 5 to 50 percent of transdermal enhancer, while the adhesive layer comprises 0 to 20 percent of transdermal enhancer; and the medicament release area of the preparation is 1 to 100 cm<2>. The pravastatin transdermal administration preparation is convenient to use and carry; the first pass effect of a liver and a gastrointestinal tract is avoided; and an in-vivo experiment of an animal proves that the preparation has no irritation or sensitization to skin. The pravastatin transdermal administration preparation can keep stable and persistent blood concentration, the continuous medicament releasing time is 1 to 7 days, so that the preparation provides the more convenient and safer treatment method for a patient.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Spare pressure type heptafluoropropane fire extinguishing system
The invention provides a spare pressure type heptafluoropropane fire extinguishing system. The spare pressure type heptafluoropropane fire extinguishing system comprises a power gas cylinder set, a driving gas cylinder set, a heptafluoropropane storage cylinder, a fire extinguishing agent container valve, a pressure reducing device, a power gas container valve, a driving gas container valve, a first starter, a second starter and an electromagnetic starter. When a fire breaks out, the electromagnetic starter and the gas driving cylinder set act, released gas enters the first starter and the third starter respectively, the power gas container valve and a selection valve are opened, and gas released from the power gas cylinder set constantly enters the heptafluoropropane storage cylinder after being subjected to pressure reduction by virtue of the pressure reducing device and delayed; and when pressure of a fire extinguishing agent in the heptafluoropropane storage cylinder reaches a certain pressure, the fire extinguishing agent enters the second starter by virtue of a connecting pipe, the second starter acts to open the fire extinguishing agent container valve, and the fire extinguishing agent in the heptafluoropropane storage cylinder extinguishes a fire in a specified protection zone by virtue of a pipe network. By applying the spare pressure type heptafluoropropane fire extinguishing system provided by the invention, safety of the fire extinguishing system can be guaranteed, and constant pressure stable output of heptafluoropropane also can be guaranteed.
Owner:广东鹰穗消防设备有限公司
Bulley aconitne transdermal paster
InactiveCN1507866AStable blood concentrationEasy to useNervous disorderMedical devicesExternal applicationRelease time
The present invention relates to a medicine plaster for external application, in particular, it is a transdermal plaster containing caowujiasu. Said plaster has medicine storage unit, back-lining layer and protecting layer. Said medicine storage unit is formed from caowujiasu and storage matrix, every plaster contains 0.1mg-100 mg of caowujiasu, and the rest is storage matrix. The medicine-releasing area of said plaster is 1 sq.cm-100 sq.cm, and the transdermal speed rate is 0.05 microgram / h.sq.cm, and its continuous medicine-releasing time is 1-12 days. Its theapeutic effect is reliable, quality is stable and convenient in application.
Owner:KPC PHARM INC
Air purifying agent
InactiveCN105079843AImprove timelinessGood sterilization and decontamination effectGaseous substancesDeodrantsAir cleaningPollutant
The invention provides an air purifying agent, and relates to the technical field of indoor environmental protection. The air purifying agent is prepared from the following components in parts by weight: 10 to 20 parts of a stable chlorine dioxide solution at mass concentration of 3 to 6 percent, 0.5 to 1 part of a citric acid solution at mass concentration of 40 to 60 percent, 0.5 to 1 part of a monosodium phosphate at mass concentration of 40 to 60 percent, 1 to 2 parts of gel and 0.5 to 1 part of a sustained-release preparation. According to the air purifier, pollutants in indoor air can be removed for a long time without side effects and secondary damage.
Owner:BAOHUSAN ENVIRONMENTAL PROTECTION SCI & TECH CHENGDU
Medicine eluted cardiovascular frame and its preparing process
InactiveCN1303947CContinuous and stable releasePromote healingStentsCoatingsCardiovascular stentEthylene Homopolymers
A medicine eluted cardiovascular frame is composed of expandible support and the medicine coated biodegradable layer coated on the said frame. The said biodegradable material contains one of the homopolymer and copolymer of glycollide, L-lactide or epsilon-caprolactone, and the copolymer of multi-group amino acids. The medicine can resist the cardiovascular narrowness. The process for preparing the said support includes such steps as preparing the said expandible support, immersing it in the mixture of the said medicine, biodegradable material and solvent, and drying. It can prevent thrombosis.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Effective slurry thick and stable discharging device and method
PendingCN109260774ASettling thickening time shortenedSingle consumption saturation point dropSettling tanks feed/dischargeSedimentation settling tanksUltrasonic generatorEngineering
The invention provides an effective slurry thick and stable discharging device and method and belongs to the technical field of flocculation settling. The device comprises a cabin body, transducer carrier tubes, sound / ultrasonic transducers, a digital controller, a rotation driving device, a mixing helix tube, a flocculant tube, an overflow pipe, a barretter, a carrier tube stabilizer and a sand outlet, wherein the transducer carrier tubes are arranged inside the cabin body, the rotation drive device is arranged at the upper end of the cabin body, and thus a rotation blind-less scanning systemis formed; the sound / ultrasonic transducers are respectively arranged at a settling section, a thickening section and a discharging section in each transducer carrier tube to form a sound field destabilization granulating active region, a sound field resonant draining thickening active region and a sound field excitation liquefying discharge active region respectively, and are connected with thesound / ultrasonic transducers through transducer connecting wires; the mixing helix tube is connected with the flocculant tube and is communicated with the barretter. The device has simple structure and process, and can realize quickly thick and stable discharging of slurry.
Owner:UNIV OF SCI & TECH BEIJING
High-efficiency fertilizer for seedling cultivation and preparation method thereof
InactiveCN108516902ARich in nutrientsIncrease profitCalcareous fertilisersAlkali orthophosphate fertiliserLivestock manureMicrobial agent
The invention provides a high-efficiency fertilizer for seedling cultivation and a preparation method thereof. The high-efficiency fertilizer is prepared from livestock manure, grass ash, an EM microbial agent, polymer resin, nitrogen fertilizer, phosphate fertilizer, potassium fertilizer, modified calcium carbonate, soybean meal, distiller's grains, sugar residues, a dispersant, filler, compoundfertilizer, trace elements, composite gel carriers and an oxygen-increasing agent. The prepared fertilizer has complete nutrient elements, the soil can be effectively improved, the soil compaction caused by excessive use of chemical fertilizer is prevented, and the robust growth of seedlings is promoted; in addition, through one-time application of the fertilizer, the nutrients can be slowly released, the utilization rate of the fertilizer is increased, and the loss of fertilizer efficiency is reduced; meanwhile, the preparation method has the advantages that the material cost is low, the rawmaterials are easy to obtain, and the process is simple and brief; the preparation method has high use value and good application prospects.
Owner:来安县出尘茶业有限公司
Landscaping nursery stock humus soil and preparation method thereof
InactiveCN106747968AImprove water permeability and breathabilityStrong ability to retain water and fertilizerCalcareous fertilisersMagnesium fertilisersPorosityControl release
The invention relates to the field of agricultural planting and discloses landscaping nursery stock humus soil. The landscaping nursery stock humus soil is obtained from the following materials: 45-55wt% of garden soil, 5-10wt% of fruit peel, 5-10wt% of grass carbon, 5-10wt% of fallen leaves of broadleaf wood, 5-10wt% of fallen leaves of coniferous wood, 5-10wt% of bark, 0.5-1.5wt% of controlled-release oxygenation fertilizer and balance of food waste water. The landscaping nursery stock humus soil provided by the invention is applicable to nursery stock cultivation, full of nutrients and high in porosity, has a slow-release effect and can continuously and stably release nutrients and oxygen and promote growth of nursery stock for a long term. A preparation method of the landscaping nursery stock humus soil is simple and reasonable, and the prepared landscaping nursery stock humus soil is stable in quality, so that the landscaping nursery stock humus soil is applicable to mass production.
Owner:重庆市江津区森德家庭农场
Pramipexole dihydrochloride sustained release tablet and preparation method thereof
InactiveCN104146979AContinuous and stable releaseFacilitated releaseOrganic active ingredientsNervous disorderTolerabilitySide effect
The invention provides a pramipexole dihydrochloride sustained release tablet and a preparation method thereof. The pramipexole dihydrochloride sustained release tablet comprises a pramipexole dihydrochloride tablet core and a coating, and one side or two sides of the tablet are provided with holes. The tablet core comprises at least a water-soluble filler, or comprises at least one cationic polymer, which is not dissolved in almost neutral (pH 5-7) environment and is rapidly dissolved in a medium with the pH less than 5. Due to the cationic polymer, the final tablet can release in the medium with the pH less than 5 faster than in the medium with the pH of 5-7. The pramipexole dihydrochloride sustained release tablet can constantly and stably release in a body, is not affected by the change of pH of the gastrointestinal tract, only needs to be taken once a day, is easy to take, has small side effects, stable curative effect and good tolerance and compliance, is beneficial to treatment of patients of Parkinsonism, can be used for maintaining the blood concentration in effective therapeutic concentration range for long and reducing the dosing frequency, and has greater clinical application value. The preparation method is simple and is applicable to industrial production.
Owner:SHANGHAI SUNTECH PHARMA
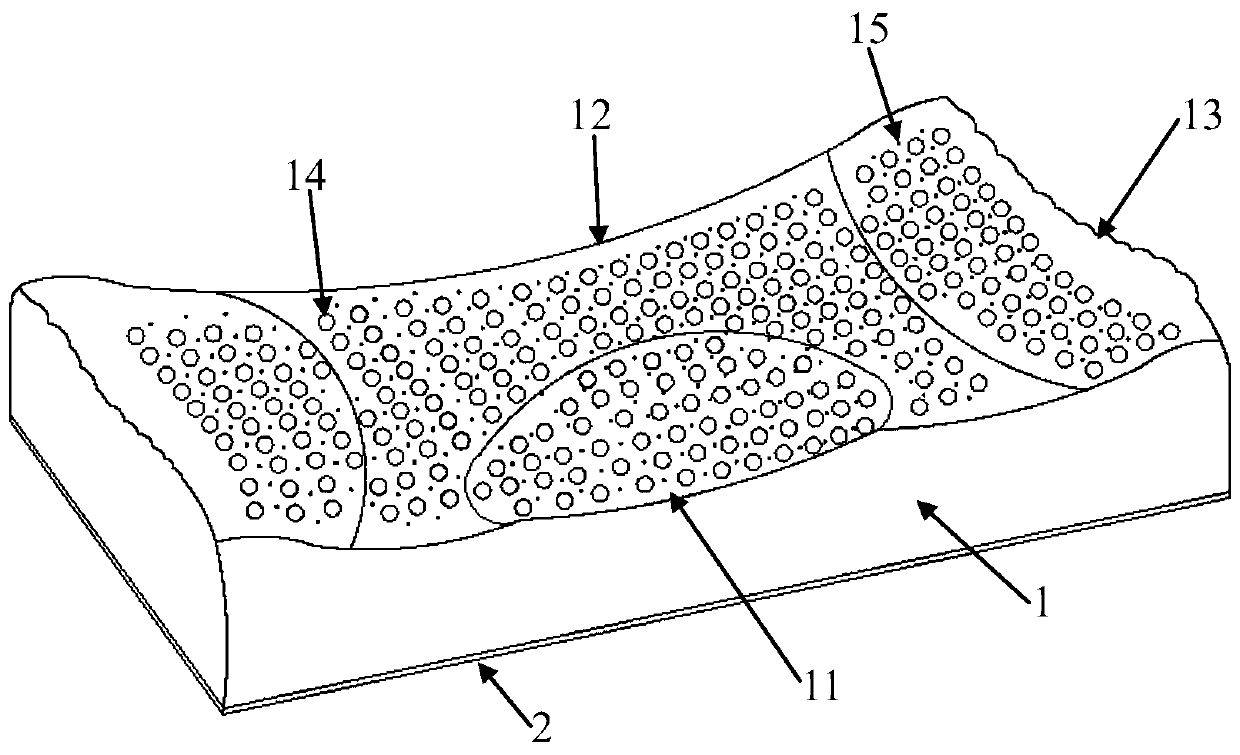
Anion latex pillow and preparation method thereof
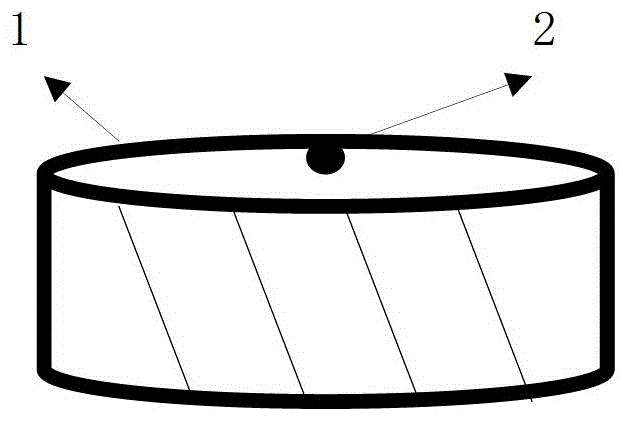
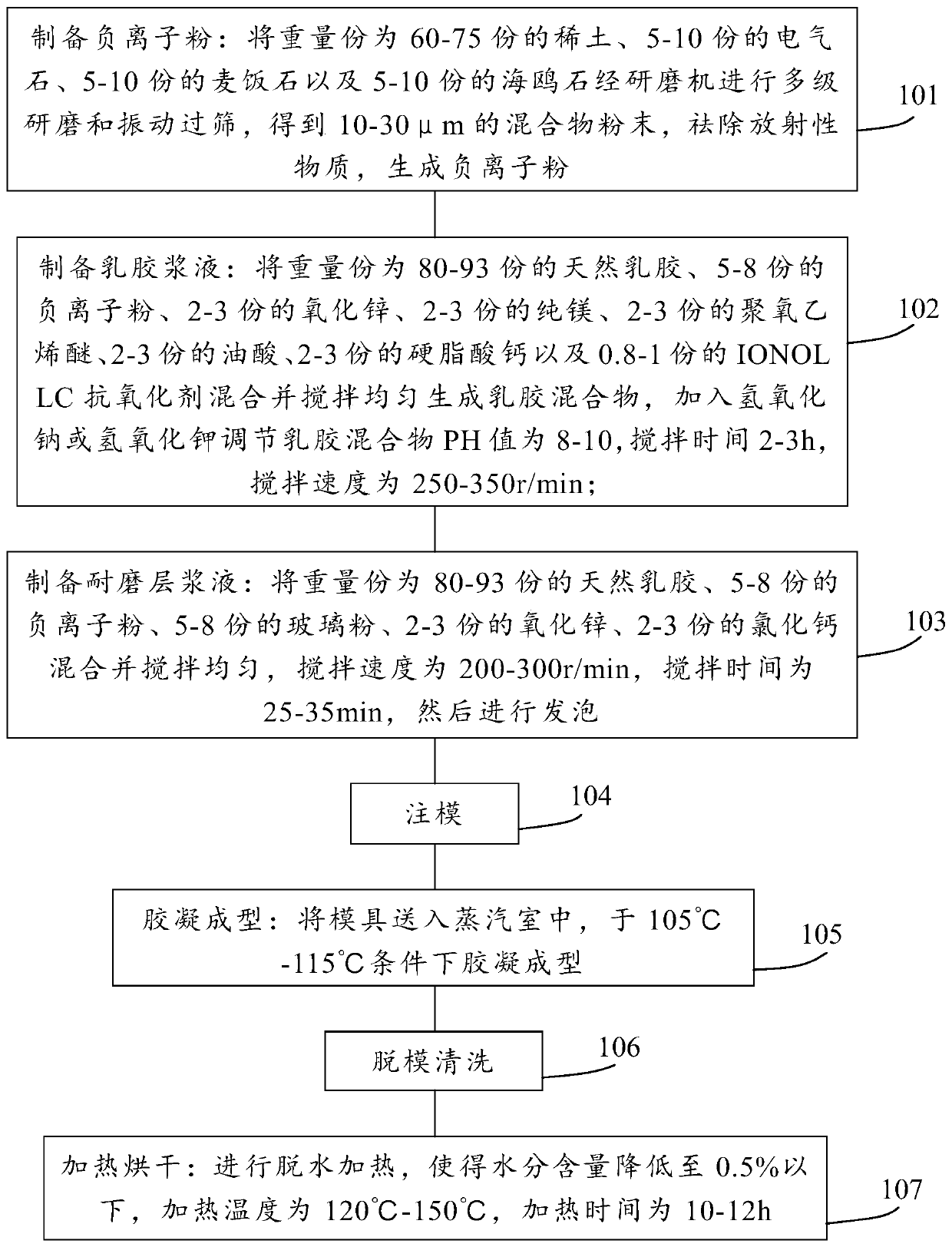
InactiveCN109832888AEasy to deodorizeImprove antibacterialPillowsBed-coversWear resistantAntioxidant
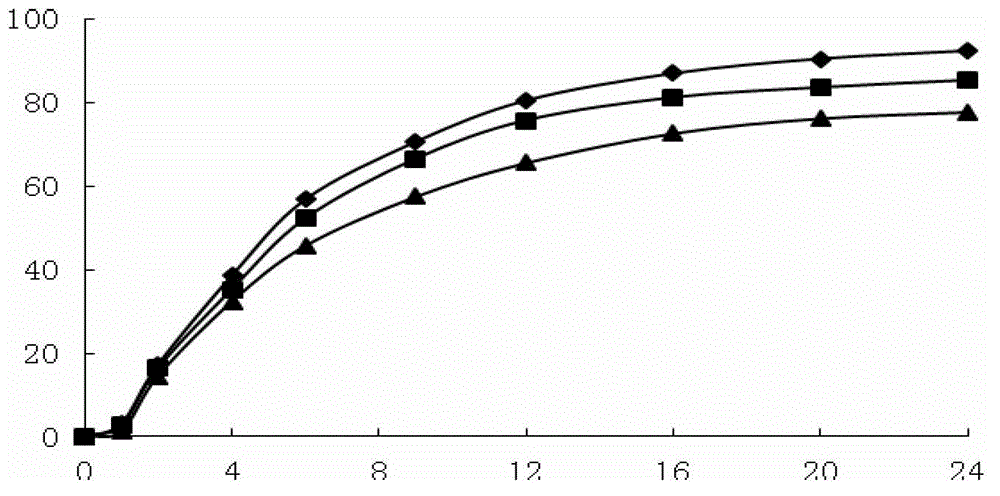
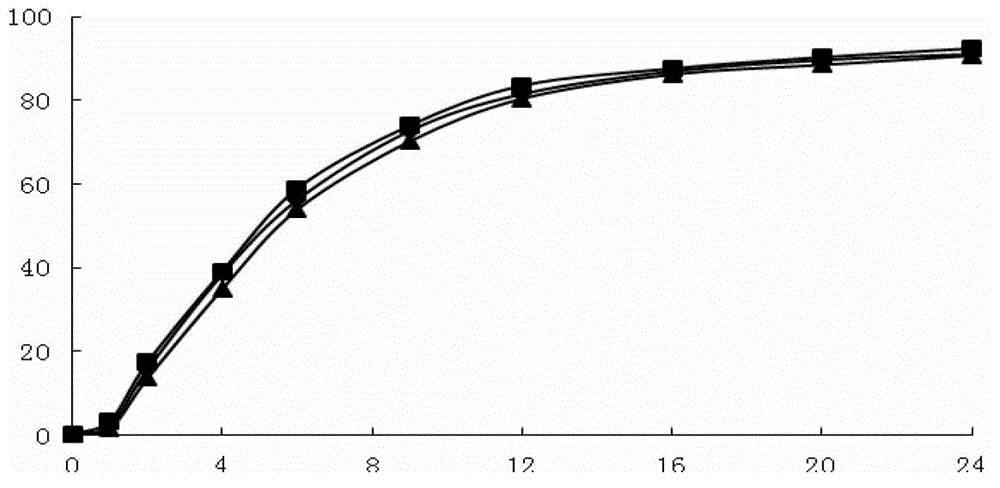
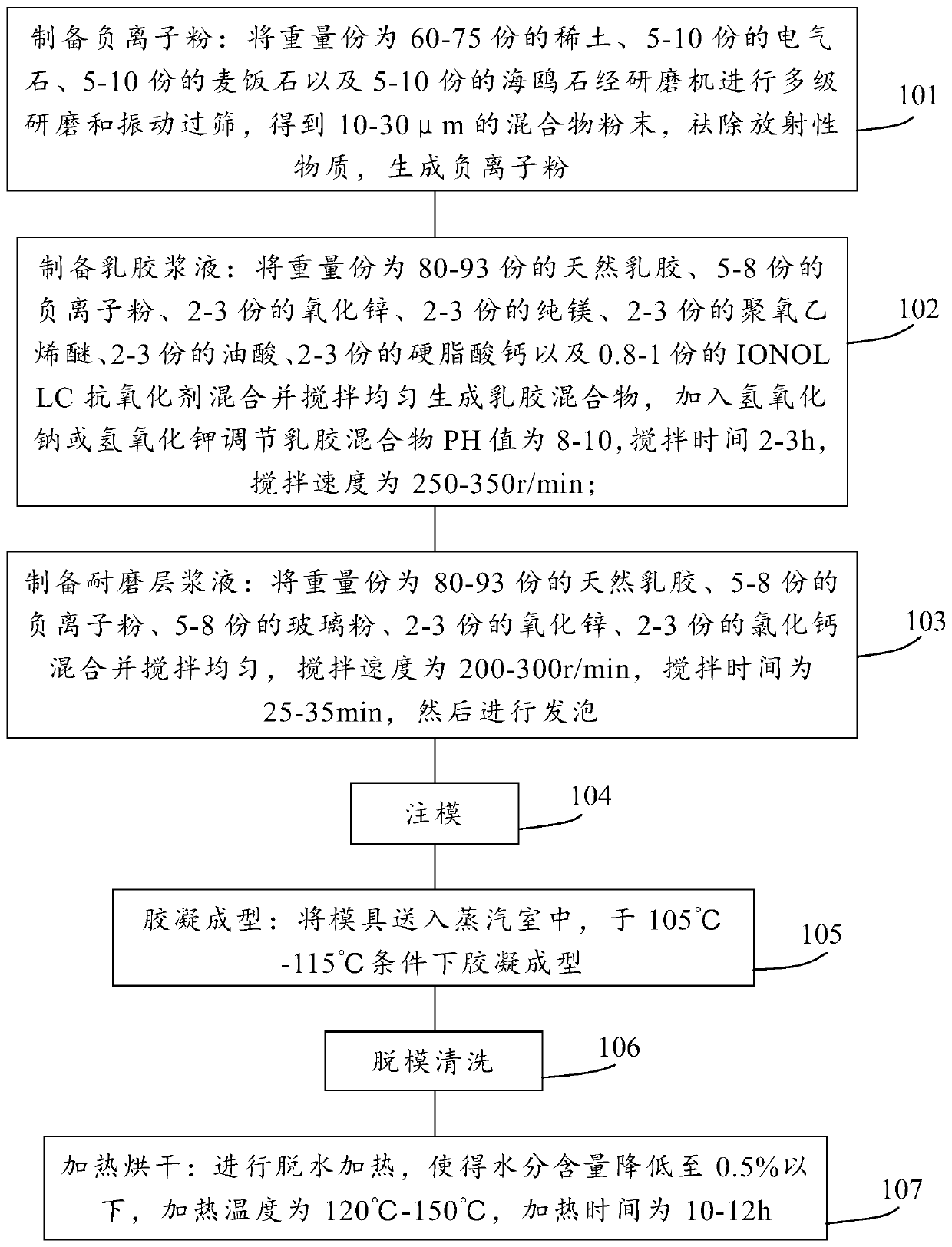
The invention provides an anion latex pillow and a preparation method of the anion latex pillow. The preparation method of the anion latex pillow comprises the following steps: preparing anion powder,grinding rare earth, tourmaline, medical stone and gull rock, and removing radioactive substance; preparing latex slurry, mixing natural emulsion, the anion powder, zinc oxide, pure magnesium, polyoxyethylene ether, oleic acid, calcium stearate and IONOLLC antioxidant, carrying out uniform stirring, and adding sodium hydroxide or potassium hydroxide for adjusting the pH to be 8-10, wherein duringthe stirring process, appropriate amount of air is introduced, so that sufficient foaming is realized; preparing wear-resistant layer slurry, mixing the natural emulsion, the anion powder, glass powder, zinc oxide, and calcium chloride, and carrying out uniform stirring; and after injection molding, carrying out gelatinizing forming under 105-115 DEG C, and finally, carrying out demolding washingand heating drying operation. For the preparation method provided by the invention, the forming quality and good health care effects of the anion latex pillow can be guaranteed, meanwhile, due to thesimplified preparation process, the good environmental protection property is obtained.
Owner:江苏沃荷乳胶科技股份有限公司
A preparation method of an anticoagulant material capable of inducing and catalyzing the release of endogenous NO
ActiveCN104208761BContinuous and stable releaseInhibitory activityPharmaceutical containersMedical packagingVascular endotheliumReaction temperature
The invention discloses a preparation method of an anticoagulant material with function of inducing and catalyzing release of endogenous NO. The method comprises the steps of: configuring an alkaline Tris-buffer buffer solution, and adding a compound with certain concentration and pyrogallol structure and a compound with disulfide bond or diselenium bond and containing amino or thiol at two ends; and placing the substrate materials in a reaction solution, controlling the reaction temperature, reacting for 1-24 h, removing the substrate, and cleaning and drying to obtain the objective material. The invention has the advantages of simple operation, mild reaction conditions and easy operation. The component unit of the compound with disulfide bond or diselenium bond and containing amino or thiol at two ends has controllable content; the modified coating prepared by the method has excellent adhesion, and catalyzes the blood NO donors to continuously release NO molecules through in situ catalysis, inhibits activation and aggregation of platelets, inhibits proliferation of smooth muscle cells, and protects vascular endothelium.
Owner:GUANGZHOU NANCHUANG EVEREST MEDICAL TECH CO LTD
Di'ao Xinxuekang sustained-release preparation and its preparation method and use
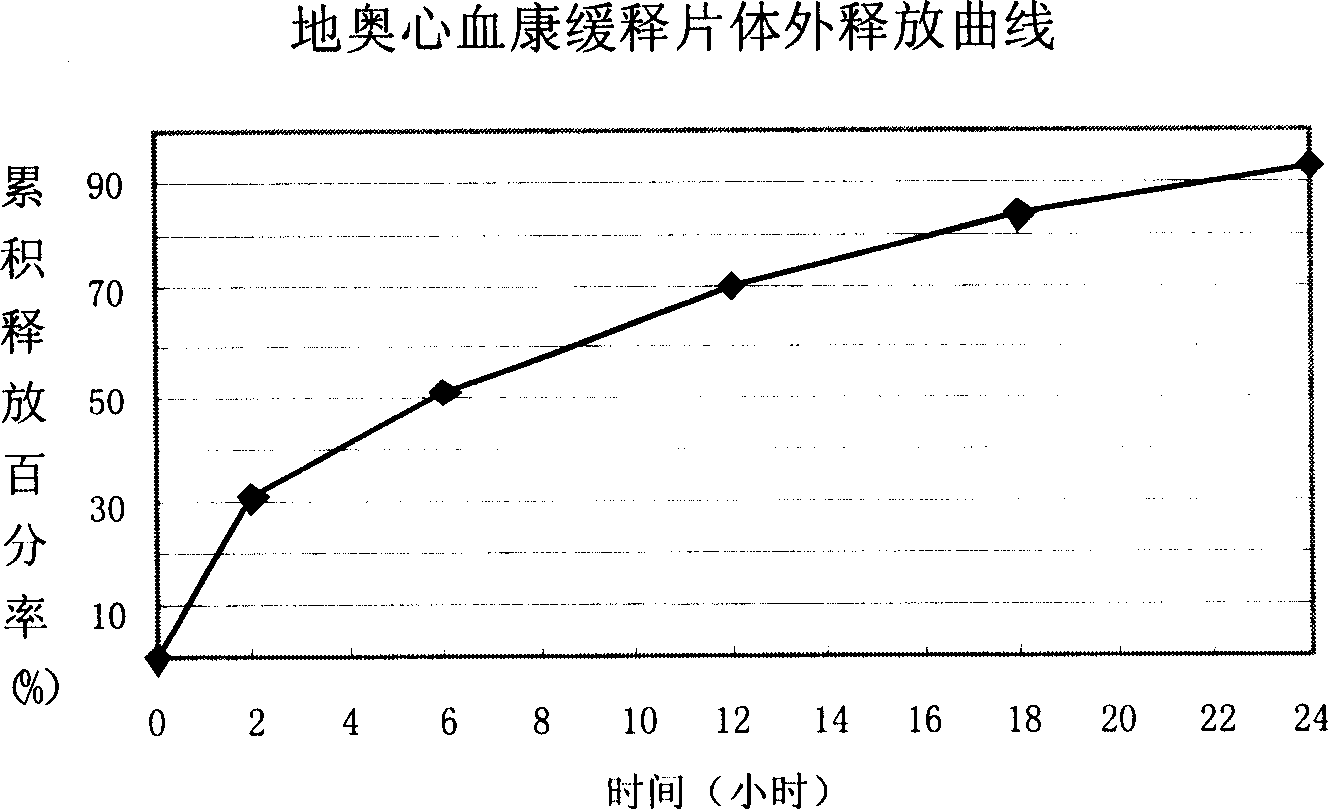
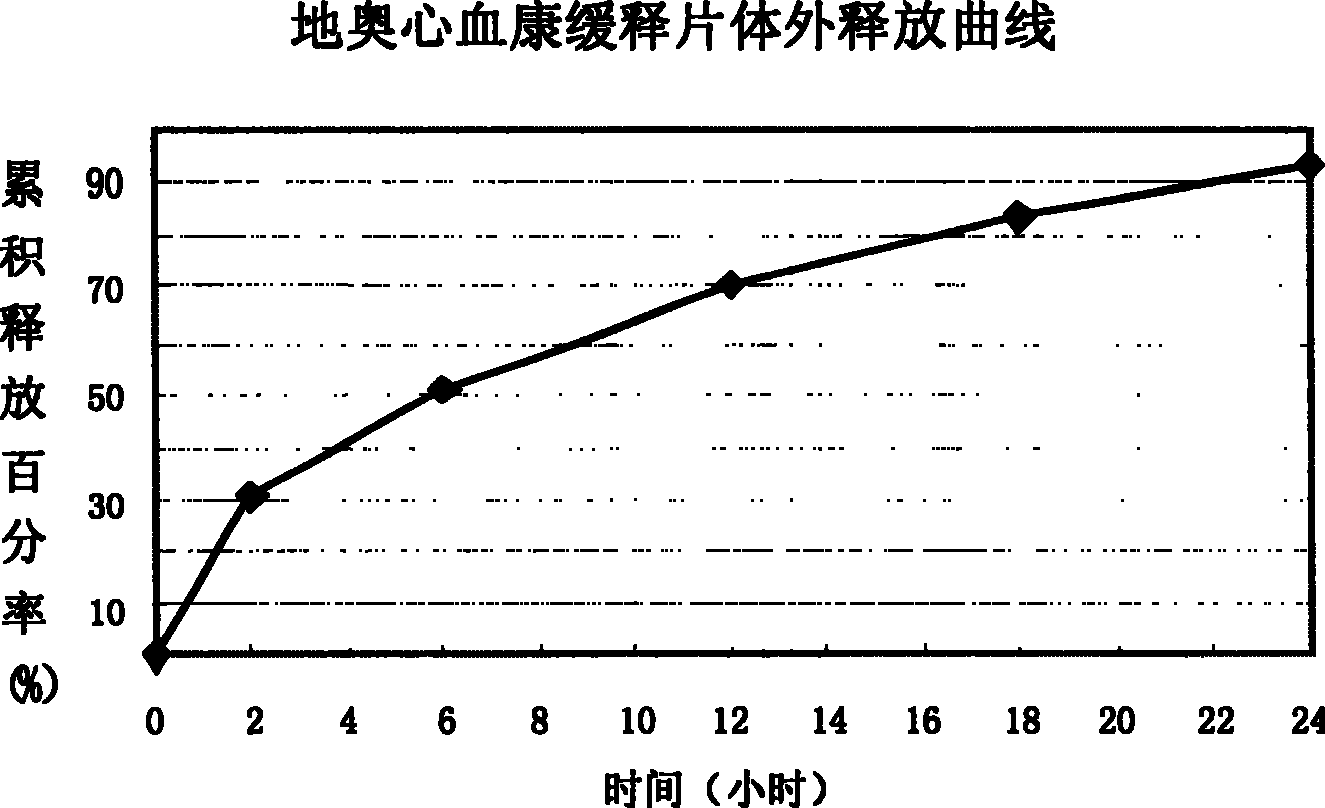
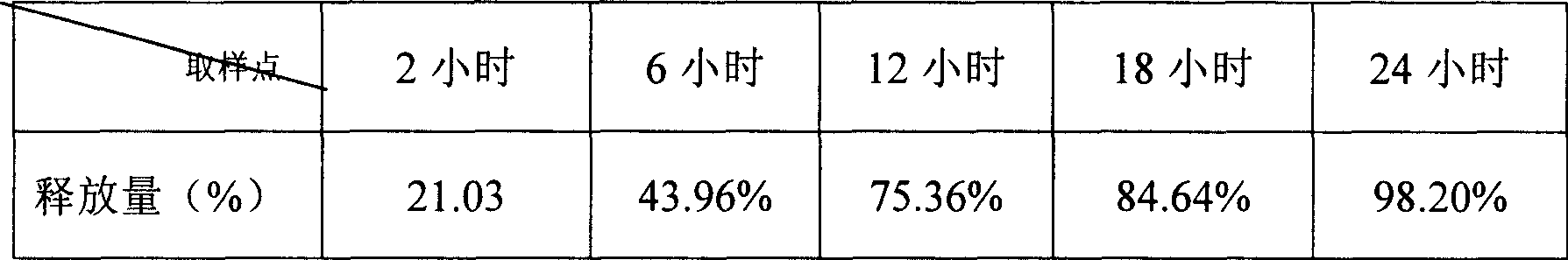
ActiveCN101108230BImprove solubilitySolve slow-release technical problemsPharmaceutical delivery mechanismDiseaseIn vitro test
The invention provides a Di'ao Xinxuekang sustained-release preparation, which is composed of steroidal total saponins extracted from the rhizome of Chinese yam or / and Dioscorea punctatus as active ingredients, combined with solid dispersion technology and melting granulation technology or slow-release coating technology , that is, firstly mix the total steroidal saponins with a hydrophilic carrier material or / and a solubilizer to prepare a solid dispersion with excellent solubility, and then add a slow-release material to prepare the preparation. In vitro tests show that the sustained-release preparation is released continuously and smoothly within 24 hours, which provides a new option for the prevention and treatment of cardiovascular and cerebrovascular diseases.
Owner:CHENGDU DIAO PHARMA GROUP
Pharmaceutical compound as well as preparation and application thereof
ActiveCN109384759AEfficient releaseReduced resistance effectAntibacterial agentsOrganic active ingredientsDrug compoundChemistry
The invention relates to a drug, in particular to a pharmaceutical compound as well as preparation and an application thereof. The compound, or pharmaceutically acceptable salt, or a 5-hydroxy complex and 4-keto complex, or a solvate of the compound has the structure shown in the description, wherein R1 is (CH2) n, R2 and R3 are selected from H or O-(CH2)n-OH, and R4 is selected from H or (CH2)n-OH, and n is an integer of 1-10.
Owner:SHANGHAI HUAIFENG BIOTECHNOLOGY CO LTD
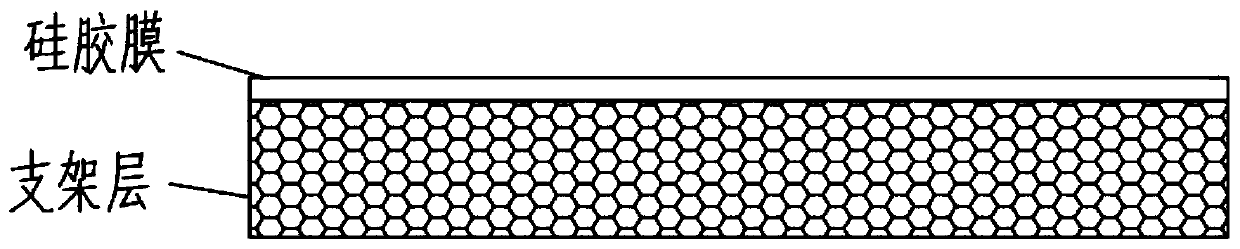
Slow-release and micro-organism-inhibition type artificial dermis model and construction method thereof
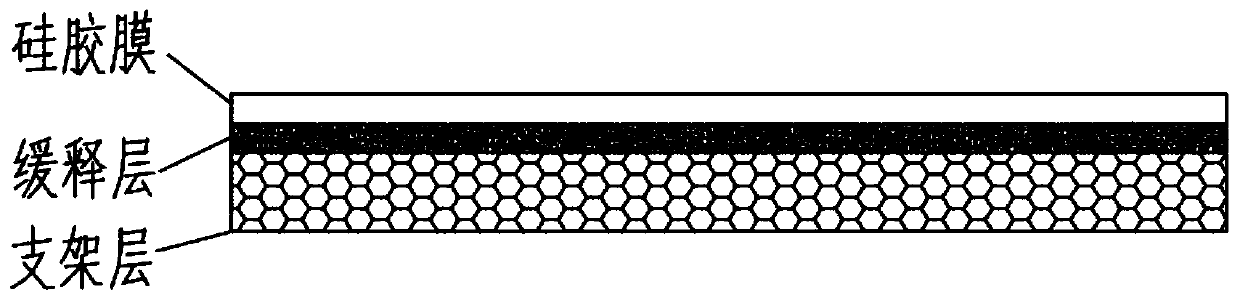
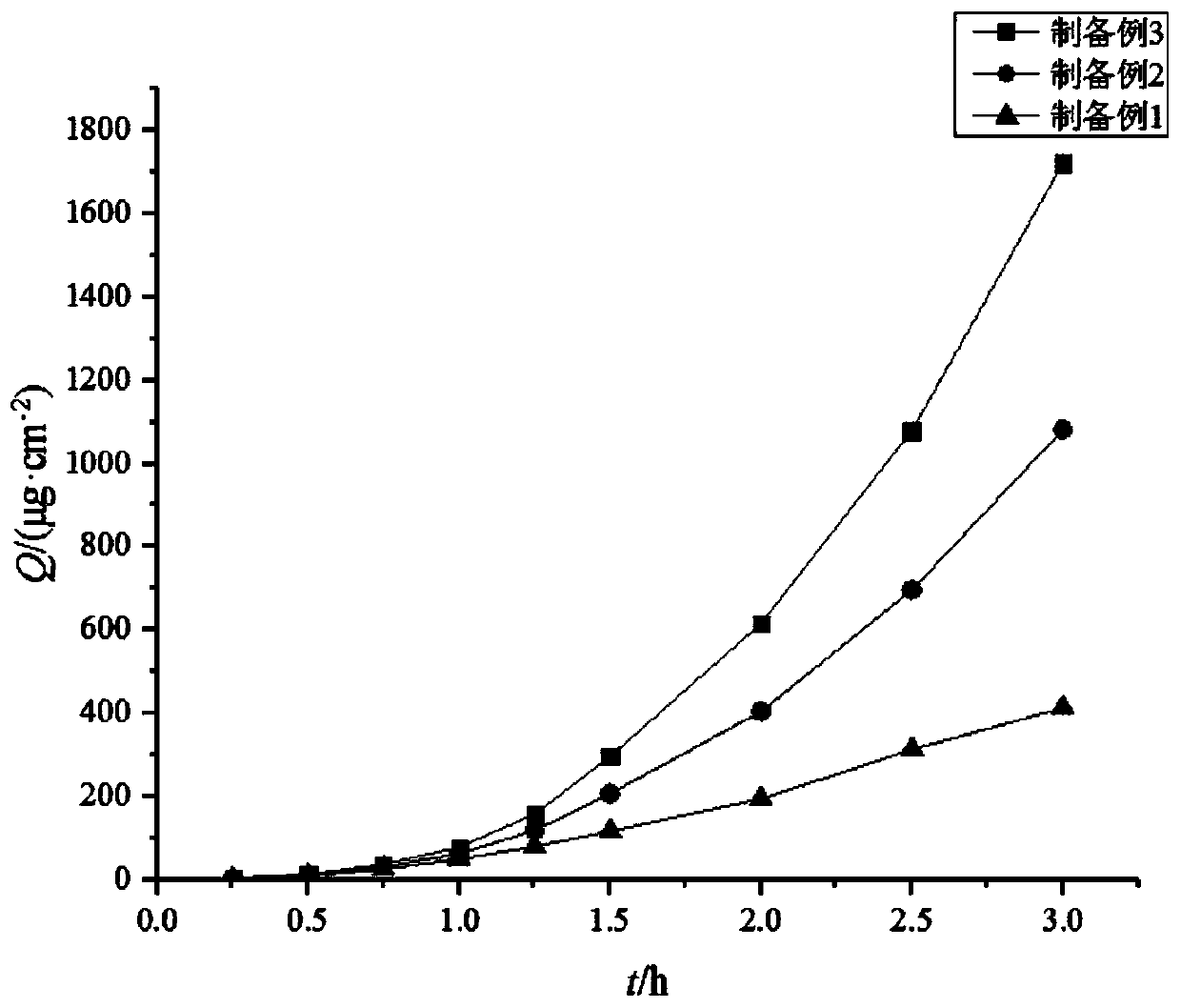
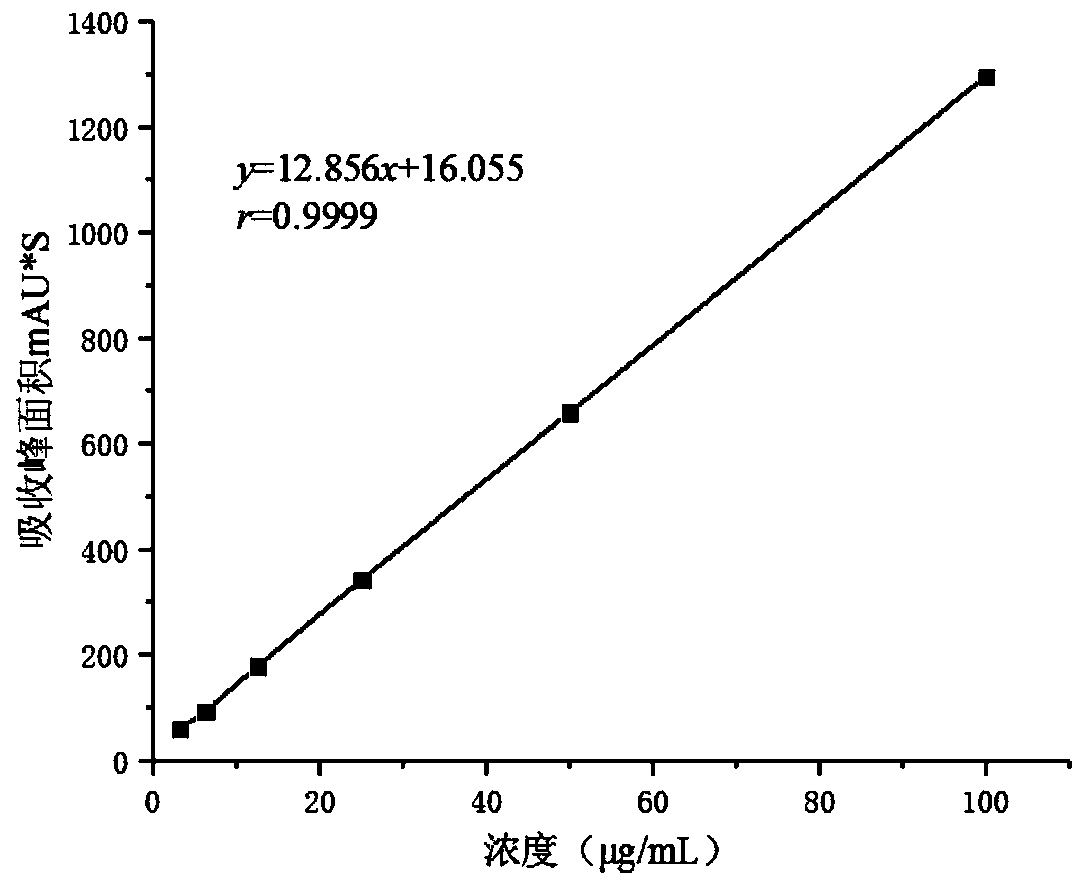
ActiveCN110025830AAdjustable release rateContinuous and stable releasePharmaceutical delivery mechanismCoatingsCelluloseFreeze-drying
The present invention discloses a slow-release and micro-organism-inhibition type artificial dermis model and a construction method thereof. The slow-release and micro-organism-inhibition type artificial dermis model comprises a silica gel membrane, a stent layer arranged under the silica gel membrane, and a slow-release layer arranged between the silica gel membrane and the stent layer. The construction method is as follows: the slow-release layer is selected from a bio-cellulose membrane, the bio-cellulose membrane in an expanded state is prepared into a dense bio-cellulose thin membrane byvacuum drying, at the same time, a micro-organism-inhibition agent is retained inside the bio-cellulose thin membrane, and then the bio-cellulose thin membrane is spread flatly on a bottom part of a mold and freeze-dried together with collagen and other stent components to be integrated together; finally, the integrated material is bonded with the silica gel membrane to form the final model; and adesign of the slow-release layer can load the micro-organism-inhibition agent, also can regulate and control a releasing speed of the micro-organism-inhibition agent, enables the micro-organism-inhibition agent to be stably and evenly released, and avoids infection or poisoning during use of artificial dermis.
Owner:ZHENDE MEDICAL CO LTD
Ligustrazine film coating agent and preparation method thereof
ActiveCN110787151AEffective fastLasting effectOrganic active ingredientsAntipyreticActive agentOrganosolv
The invention provides a ligustrazine film coating agent and a preparation method thereof, which belong to the technical field of film coating agent preparation, and the ligustrazine film coating agent comprises the following components in parts by weight: 3-20 parts of ligustrazine, 3-15 parts of a film forming material, 2-10 parts of a plasticizer, 5-25 parts of an organic solvent, 0.1-1 part ofa surfactant, 0.3-1.3 parts of a penetration enhancer, and 27.7-86.6 parts of water. The ligustrazine film coating agent provided by the invention has the advantages of quick effect, lasting effect,good spreading property, strong adhesive force, no irritation to skin, and convenience in administration; the ligustrazine film coating agent is administrated in a transdermal manner, has the characteristics of high safety, good compliance and large sample loading capacity, can continuously and stably release medicines within specified time, is stable in blood concentration, and fundamentally eliminates the defects of ligustrazine injections and tablets.
Owner:DALI UNIV
Drug-loaded nanofiber as well as preparation method and application thereof
ActiveCN110478521ATo achieve the effect of sustained releaseGood biocompatibilityConjugated cellulose/protein artificial filamentsFilament/thread formingFiberGram
The invention relates to topical skin products and in particular to a drug-loaded nanofiber as well as a preparation method and application thereof. The drug-loaded nanofiber contains nanofibers formed by auxiliary components and palmatine loaded on the nanofibers, wherein the content of the auxiliary components is 5-75g relative to the palmatine per gram. The preparation method of the drug-loadednanofiber comprises the following steps: performing electrostatic spinning on a spinning solution containing the palmatine and auxiliary components so as to obtain the drug-loaded nanofiber. The invention furthermore relates to application of the drug-loaded nanofiber in preparation of a topical skin product for inhibiting scars. The drug-loaded nanofiber takes the palmatine as an active ingredient, the drug action of the active ingredient is released in a sustained manner, and the drug-loaded nanofiber has effects of promoting healing, diminishing inflammation, regulating growth and proliferation behaviors of cells and further effectively inhibiting formation and growth of the scars, can be widely applied to the topical skin products, and is particularly used for dressings. The drug-loaded nanofiber disclosed by the invention is prepared by adopting a blend electrostatic spinning method, the preparation process is simple, and the prepared drug-loaded nanofiber is continuous and uniform.
Owner:GUILIN MEDICAL UNIVERSITY
Temperature-sensitive type slow-releasing pesticide for trapping and killing oriental fruit moths by utilizing phototaxis and preparation method thereof
InactiveCN106614559AContinuous and stable releaseReduce pollutionBiocideAnimal repellantsPhototaxisFluorescence
The invention discloses a temperature-sensitive type slow-releasing pesticide for trapping and killing oriental fruit moths by utilizing phototaxis. The temperature-sensitive type slow-releasing pesticide is prepared from the following raw materials in parts by weight: 2 to 5 parts of fumalic acid, 9 to 16 parts of starch, a proper amount of dimethyl sulfoxide, 1 to 4 parts of p-toluenesulfonic acid, 3 to 12 parts of sodium hydrogen carbonate, a proper amount of anhydrous ethyl ether, 16 to 25 parts of N-piperidineacrylamide, a proper amount of de-ionized water, 2 to 5 parts of sodium dodecyl sulfate, 19 to 28 parts of a pesticide active component, 2 to 6 parts of 2,2'-azodiisobutyronitrile, 1 to 2 parts of tetramethylethylenediamine, 2 to 6 parts of light-induced energy-storage fluorescent powder and 1 to 3 parts of a fluorescent dyestuff. According to the temperature-sensitive type slow-releasing pesticide disclosed by the invention, the fumalic acid and the starch are used as the raw materials for synthesizing starch ferulate; the starch ferulate and the N-piperidineacrylamide are subjected to a free radical polymerization reaction to prepare temperature-sensitive type hydrogel; the temperature-sensitive type hydrogel can be reversibly expanded and retracted along the changes of environment temperature, so that the pesticide active component can be continuously and stably released; the aims that the effect is lasting, the pollution is reduced and the oriental fruit moths are economically controlled are realized.
Owner:徐海燕
Layered microneedle, layered microneedle contraceptive system and preparation method and application of layered microneedle contraceptive system
PendingCN114272199AAvoid embarrassmentRealize the purpose of stealth drug deliveryPharmaceutical delivery mechanismPolystyrenePyrrolidinones
The invention discloses a layered microneedle, a layered microneedle contraception system and a preparation method and application of the layered microneedle, the layered microneedle comprises a polystyrene (PS) layer and a polyvinylpyrrolidone (PVP) layer, a needle body of the layered microneedle contraception system comprises nanospheres LNG (at) PCL / F68 and free levonorgestrel (LNG), and the nanospheres LNG (at) PCL / F68 are prepared by mixing the levonorgestrel (LNG), polycaprolactone (PCL) and poloxamer 188 (F68). A certain amount of medicine can be continuously and stably released by adjusting the dosage of F68 or the input amount of medicine LNG, and the requirements of different crowds for contraception duration are met. Meanwhile, due to the removable design of the layered microneedle substrate, the discomfort caused by the fact that other percutaneous preparations such as dressings stay on the skin surface for a long time is avoided, and the embarrassment that a patient uses contraceptive products is avoided.
Owner:CHINA PHARM UNIV
Nano photo-catalytic purification fabric and manufacturing method thereof
InactiveCN110528268ALong-lasting anti-mildewLong-lasting antibacterial functionBiochemical fibre treatmentArtifical filament manufactureSocial benefitsPhoto catalytic
The invention discloses a nano photo-catalytic purification fabric and a manufacturing method thereof. By a hydrothermal reaction of graphene-chitosan composite aerogel micropowder and a titanium source in an acidic solution, a nano purification finishing agent is prepared, and by impregnating a base fabric in a mixed solution of the nano light purification finishing agent and water, executing baking and then executing washing with water and drying, the nano photo-catalytic purification fabric is obtained. The nano photo-catalytic purification fabric has long-acting mildew-proof and antibacterial functions, has the effect of purifying air and removing formaldehyde, can continuously and stably release negative ions, meets the diversified demands of consumers in pursuit of comfort, green, environmental protection, health care and the like, can be popularized on a large scale, and has higher economic and social benefits.
Owner:成都良品家饰工程有限公司
Ilepcimide-containing controlled release tablet as well as preparation method and application thereof
ActiveCN113577037AContinuous and stable releaseReduce blood concentration fluctuationsOrganic active ingredientsNervous disorderIlepcimideBlood concentration
The invention discloses a controlled release tablet containing ilepcimide and a preparation method and application thereof. The controlled release tablet containing ilepcimide comprises a tablet core and a semi-permeable membrane layer coating the outer surface of the tablet core; the tablet core comprises a medicine-containing layer and a boosting layer; the medicine-containing layer and the boosting layer are arranged in a stacked mode. The drug-containing layer comprises ilepcimide and a solubilizing chelating agent; the boosting layer comprises a permeation promoting polymer; the semi-permeable membrane layer is made of a semi-permeable membrane material; and the semipermeable membrane layer is provided with small drug release holes. Compared with a common normal release preparation, the ilepcimide-containing controlled release tablet provided by the invention has the advantages that the taking times and dosage are reduced, the ilepcimide-containing controlled release tablet can be taken 1-2 times per day, the blood concentration fluctuation of the medicine in a human body can be reduced, the toxic and side effects are reduced, the medication safety is improved, and the curative effect of the ilepcimide on diseases such as senile dementia is enhanced.
Owner:北京斯利安健康科技有限公司
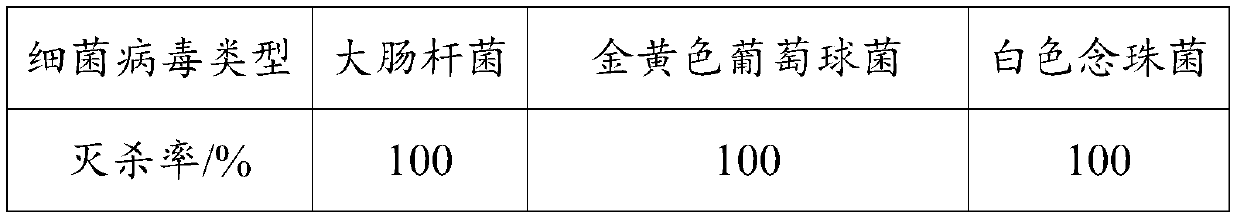
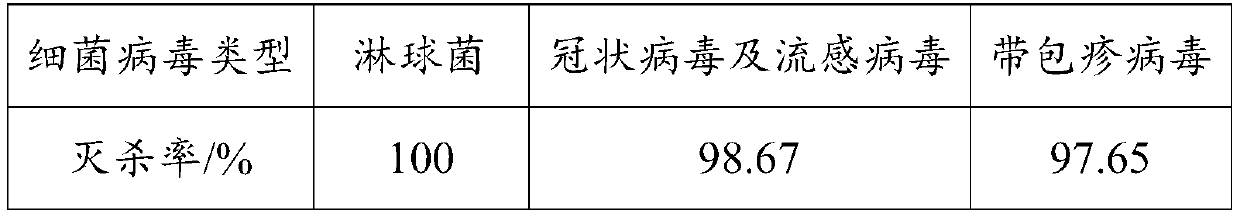
Bacterium and virus killing coating as well as preparation method and application thereof
InactiveCN111592804ALong-term stabilityImprove timelinessAntifouling/underwater paintsPaints with biocidesBacterial virusMeth-
The invention is applicable to the technical field of coating, and provides bacterium and virus killing coating as well as a preparation method and application thereof. The coating is prepared from the following components: waterborne acrylic emulsion, a thickening agent, a dispersing agent, a defoaming agent, a coalescing agent, pigment and filler, polyhexamethylene biguanidine, a metal chelatingagent and water. The coating provided by the invention is safe, non-toxic, long-acting and stable, is suitable for indoor coating, and can be used for killing various bacteria and viruses in air, sothat the current situation that the traditional sterilization coating only can be used for bacterium killing and cannot be used for efficiently killing the viruses is improved. According to the invention, the metal chelating agent is added to chelate cations in the polyhexamethylene biguanide; therefore, the polyhexamethylene biguanide can be promoted to kill various electronegative bacteria and viruses, and the bacterium and virus killing effects of the polyhexamethylene biguanide can be continuously and stably released, so that the bacterium and virus killing efficiency of the polyhexamethylene biguanide is improved, and the timeliness of the polyhexamethylene biguanide is enhanced.
Owner:河南彩虹建材科技有限公司
A jugular vein intubation drug delivery device for cerebral infarction
Owner:上海澎立生技医药研究有限公司
Clothes anion finishing liquid
InactiveCN107164946AStrong adhesionCerebral cortex function and mental activity enhancementFibre treatmentChemistrySeaweed food
The invention discloses a clothes anion finishing liquid which comprises the following components in parts by weight: 2-5 parts of seaweed carbon, 4-8 parts of shells, 1.4-2.7 parts of tourmaline superfine powder, 1-2.5 parts of sodium alkyl benzene sulfonate, 1-2.5 parts of a quaternary ammonium salt, 0.1-0.2 part of active polypeptide and 1000-1200 parts of water. The clothes anion finishing liquid disclosed by the invention has the beneficial effects of being good in adhesion property to clothes, good in washing resistance and capable of stably releasing anions after multiple times of washing; clothes soaked with the clothes anion finishing liquid disclosed by the invention can continuously and stably release anions, and the anion release concentration is greater than 5000 / cm<3>; and the softness and the comfort of clothes soaked with the clothes anion finishing liquid disclosed by the invention are not changed, and functional fabrics with functions such as ultraviolet resistance and bacterial resistance are not affected either.
Owner:海盐派特普科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com