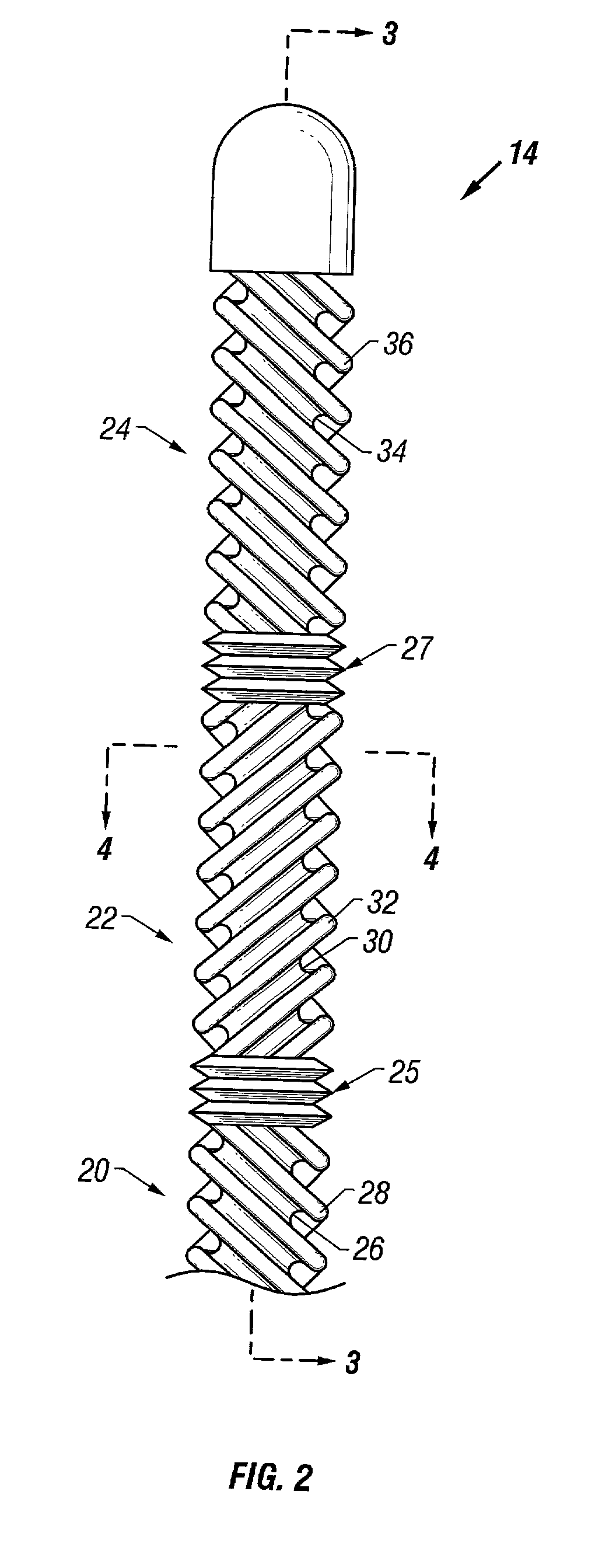
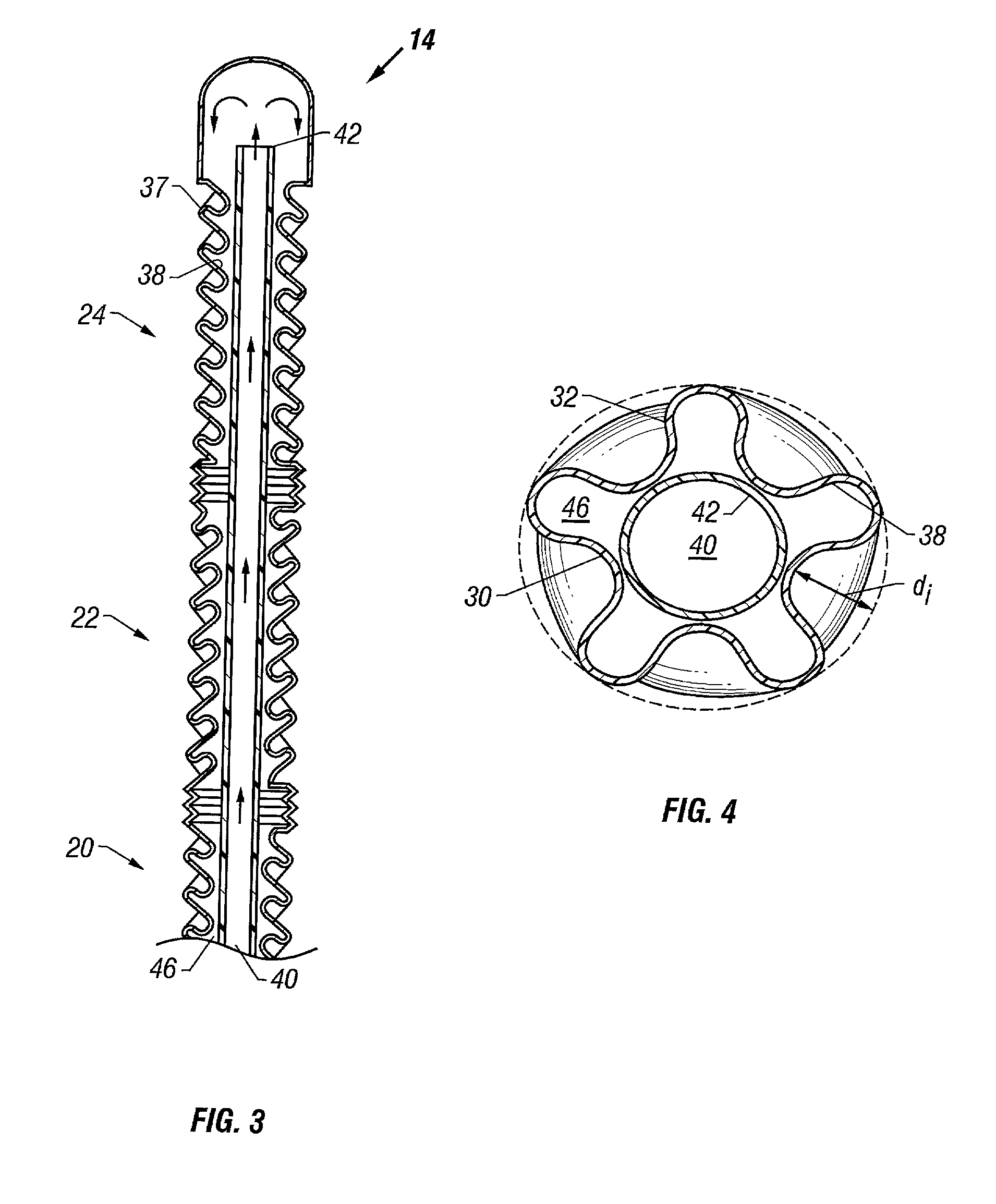
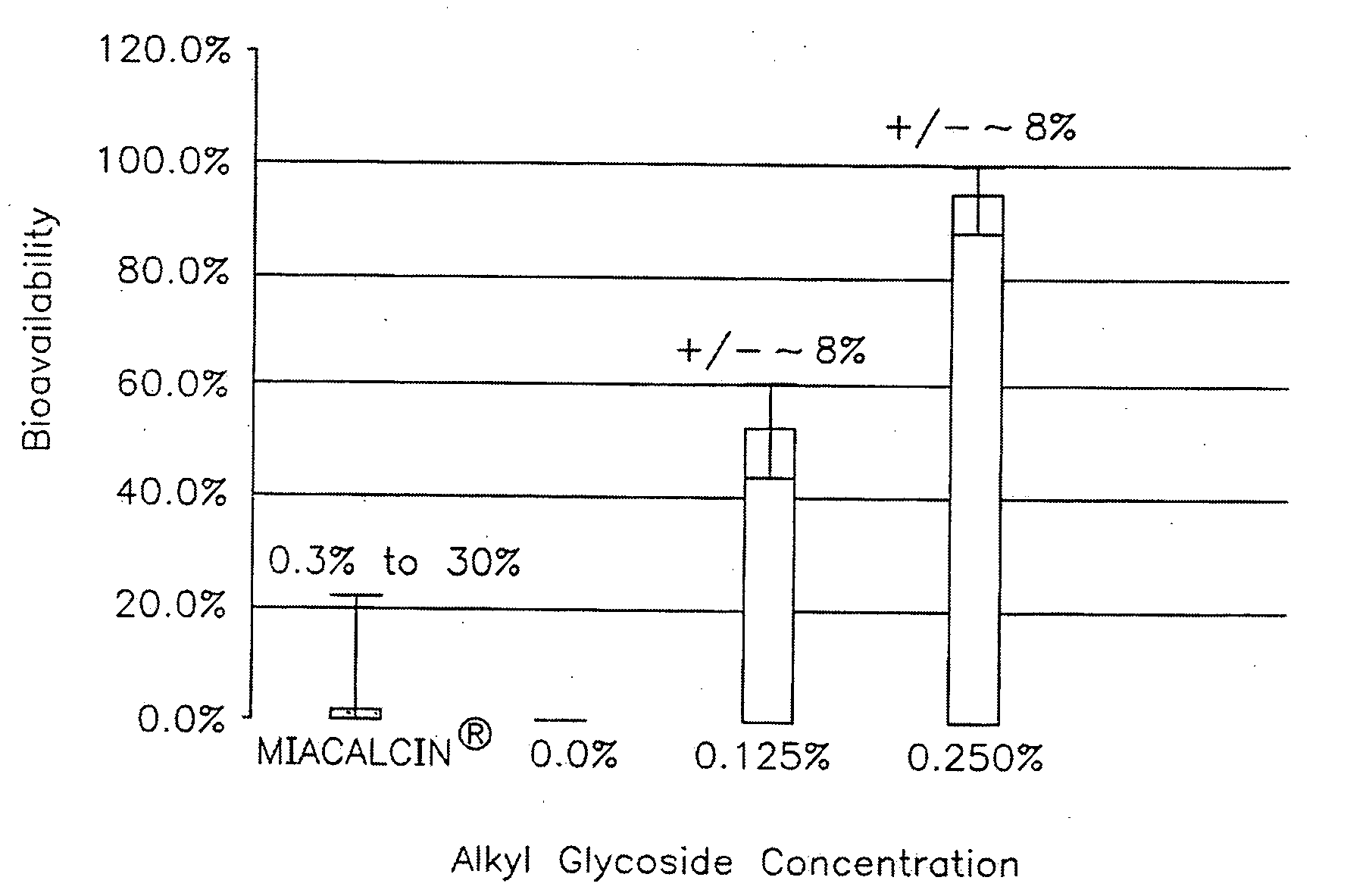
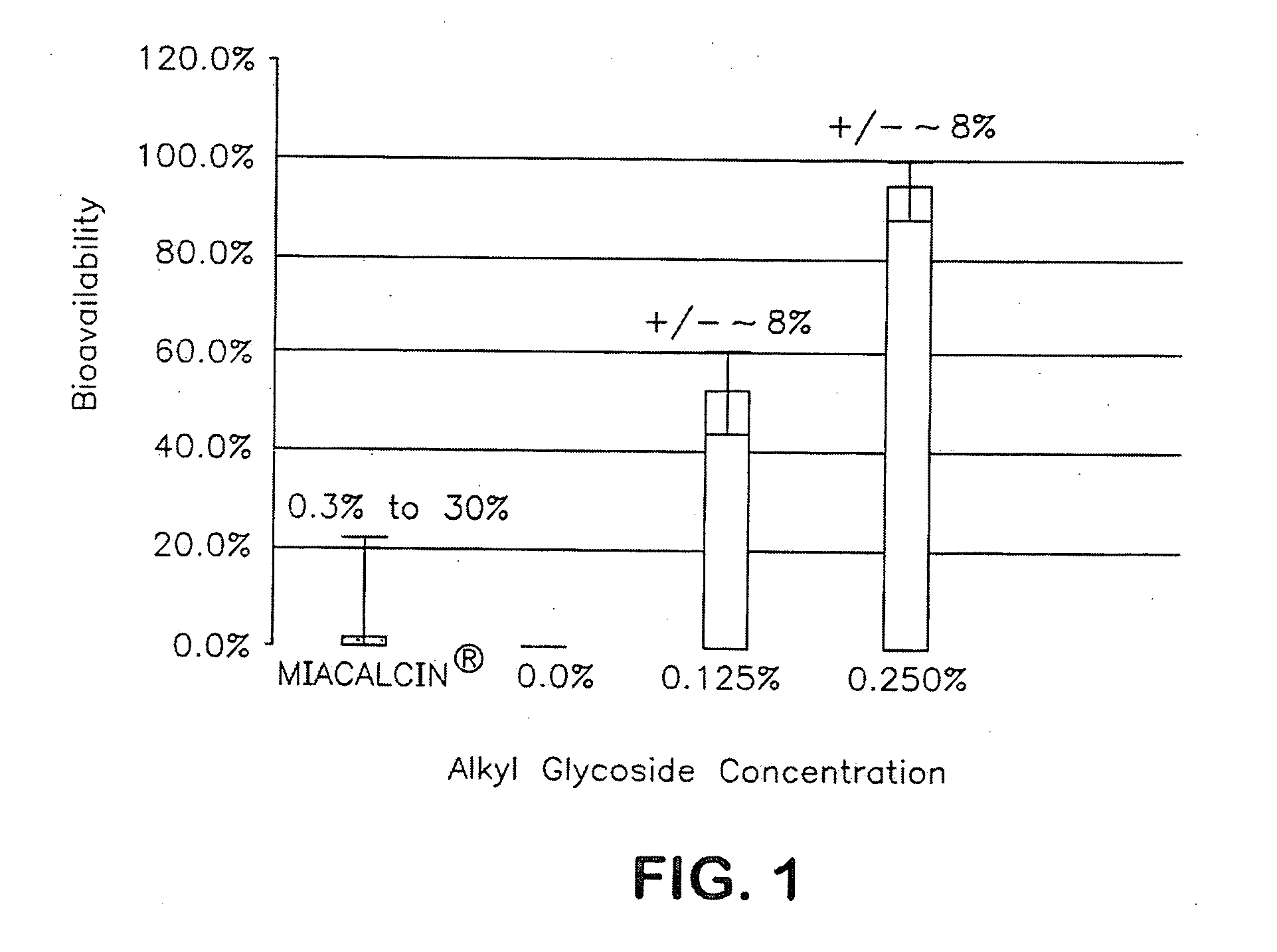
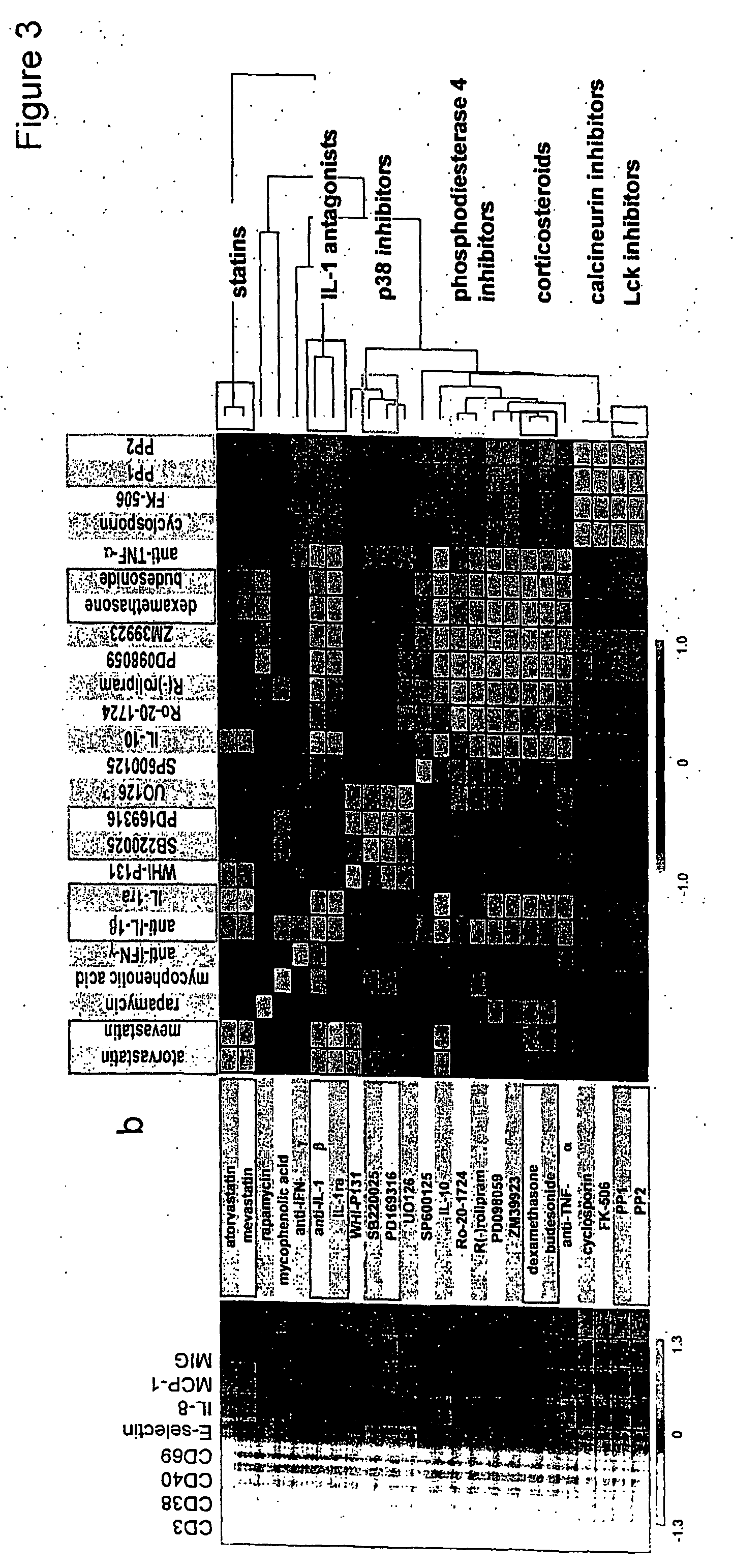
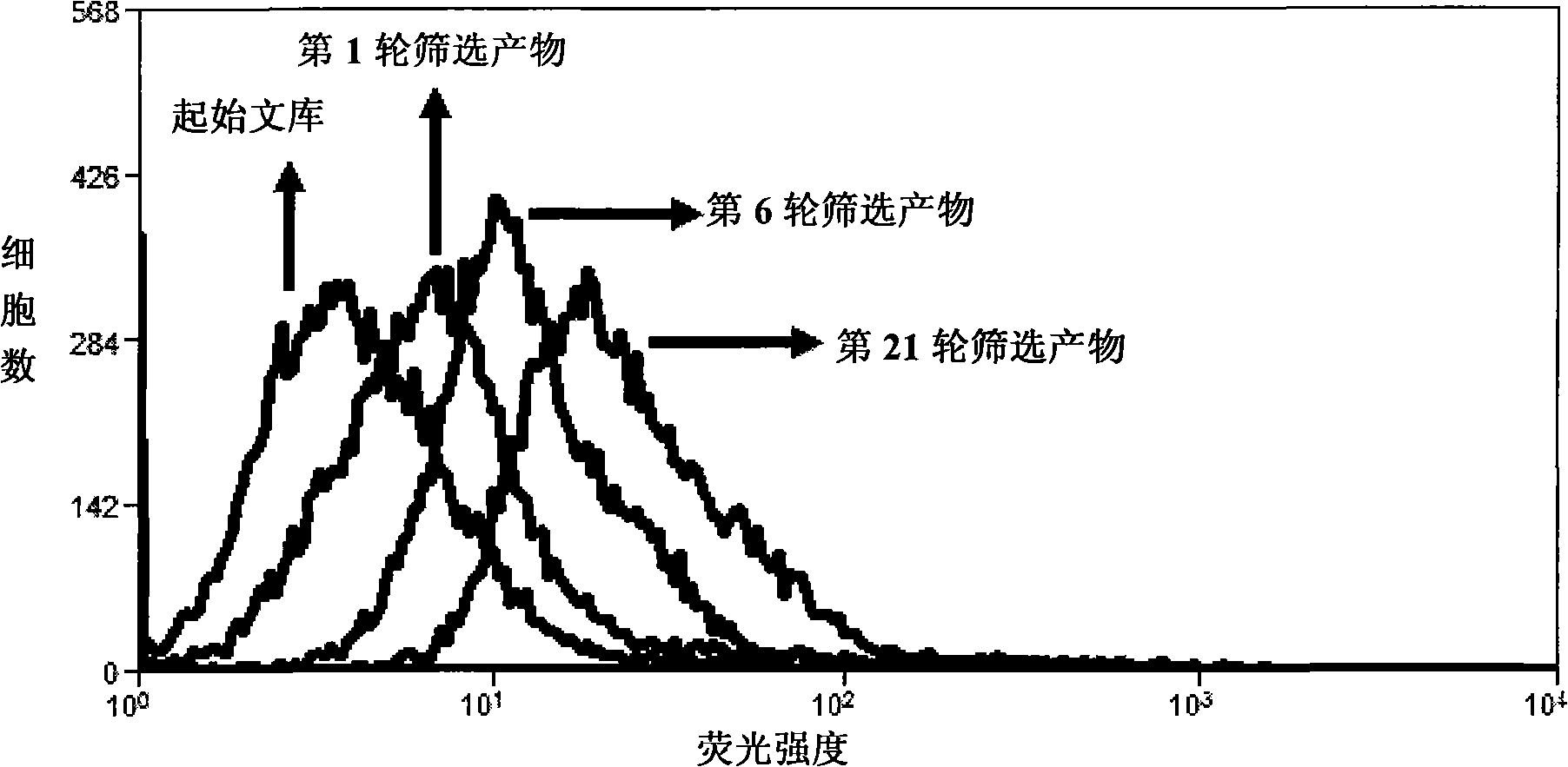
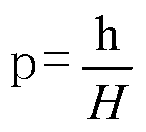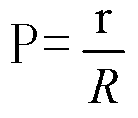Patents
Literature
643 results about "Drug action" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The action of drugs on the human body is called pharmacodynamics, and what the body does with the drug is called pharmacokinetics. The drugs that enter the human tend to stimulate certain receptors, ion channels, act on enzymes or transporter proteins. As a result, they cause the human body to react in a specific way.
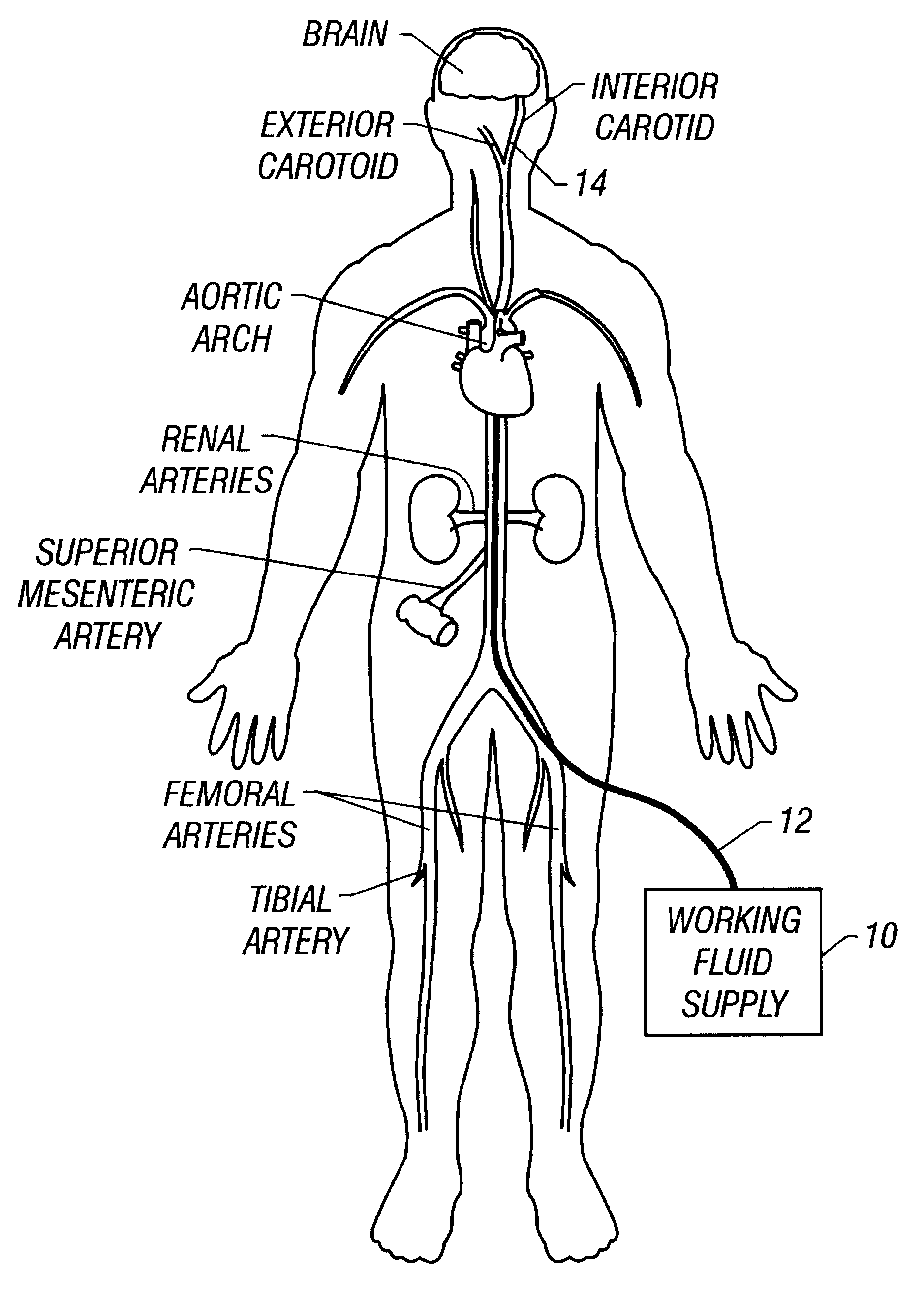
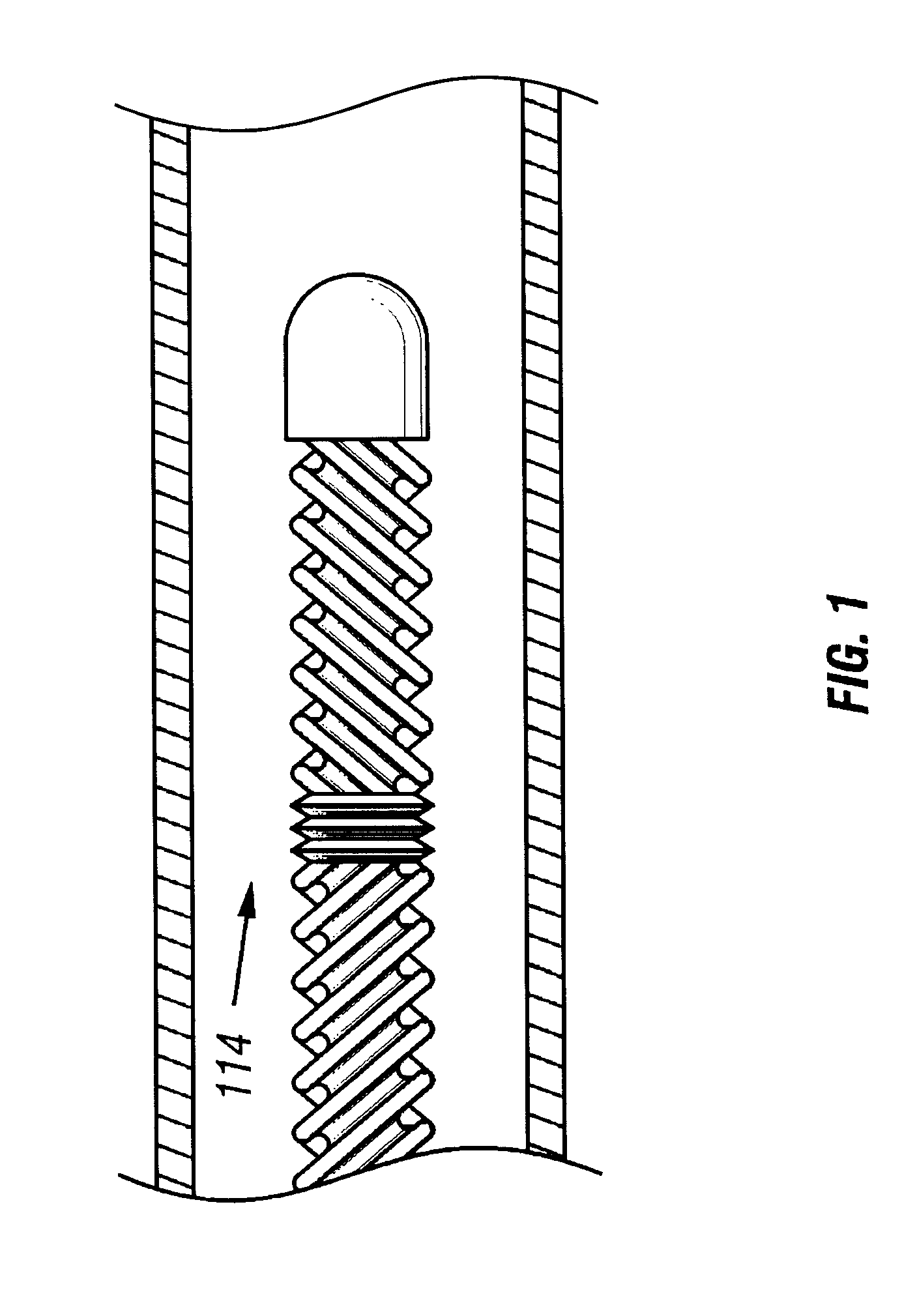
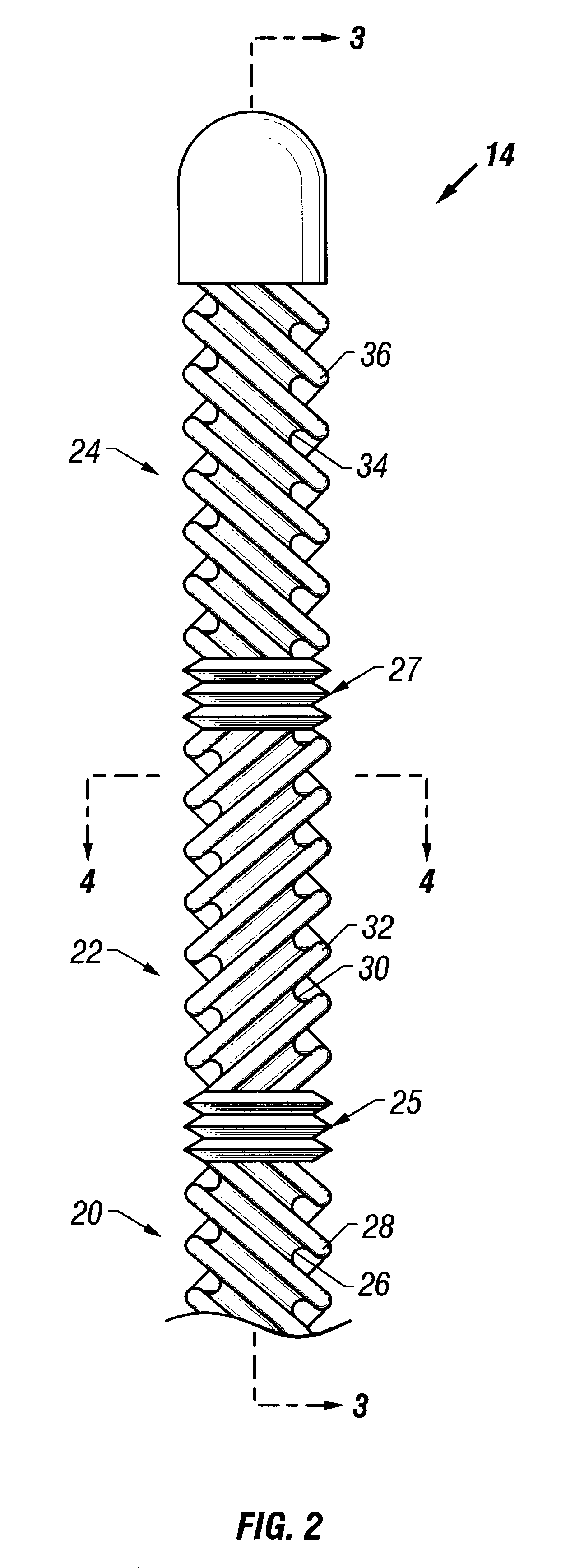
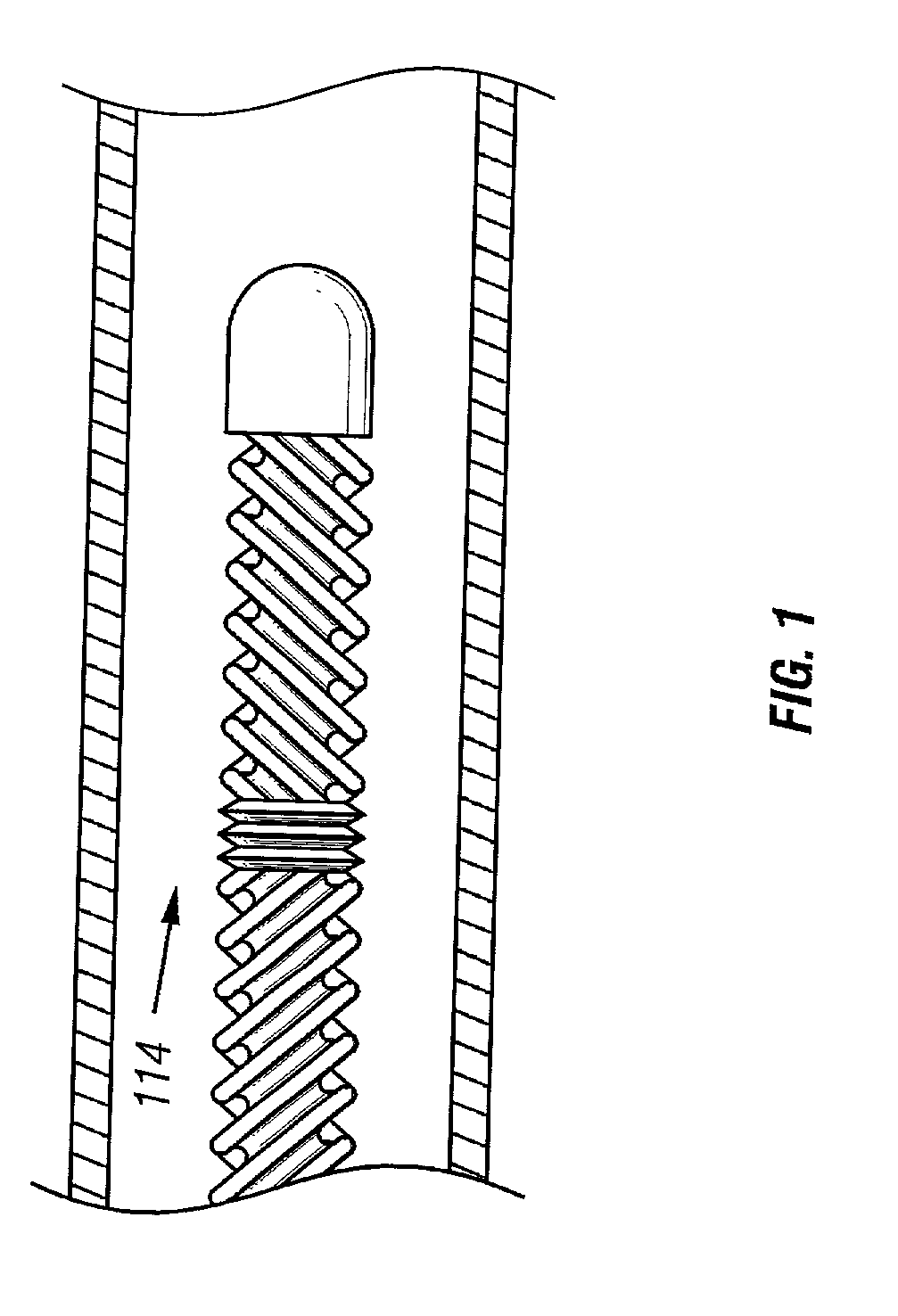
Method and apparatus for location and temperature specific drug action such as thrombolysis
A method is provided of localizing a drug action where the drug is present throughout a vascular system. The localization occurs to within a volume of blood in a blood vessel, the vascular system having an initial temperature substantially within a first temperature range. A temperature-specific enzyme is delivered throughout a vascular system including a volume of blood in a blood vessel, the temperature-specific enzyme having a working temperature within a prespecified temperature range that does not substantially overlap the first temperature range. A heat transfer element is delivered to a blood vessel in fluid communication with the volume of blood. The temperature of the heat transfer element is adjusted such that the volume of blood in the blood vessel is heated or cooled to the prespecified temperature range. In this way, the action of the temperature-specific enzyme is substantially limited to the volume of blood heated or cooled. In an alternative embodiment, the temperature-specific enzyme is localized to the volume of blood in the blood vessel, and the heat transfer element is disposed in fluid communication with the volume of blood in the blood vessel. The enzyme localization may occur by way of direct injection or by way of injection through a lumen of a catheter. The injection lumen of the catheter may be disposed at least partially adjacent or in combination with the heat transfer element and its associated inlet and outlet lumens.
Owner:ZOLL CIRCULATION
Method and apparatus for location and temperature specific drug action such as thrombolysis
Owner:INNERCOOL THERAPIES INC
Compositions for Drug Administration
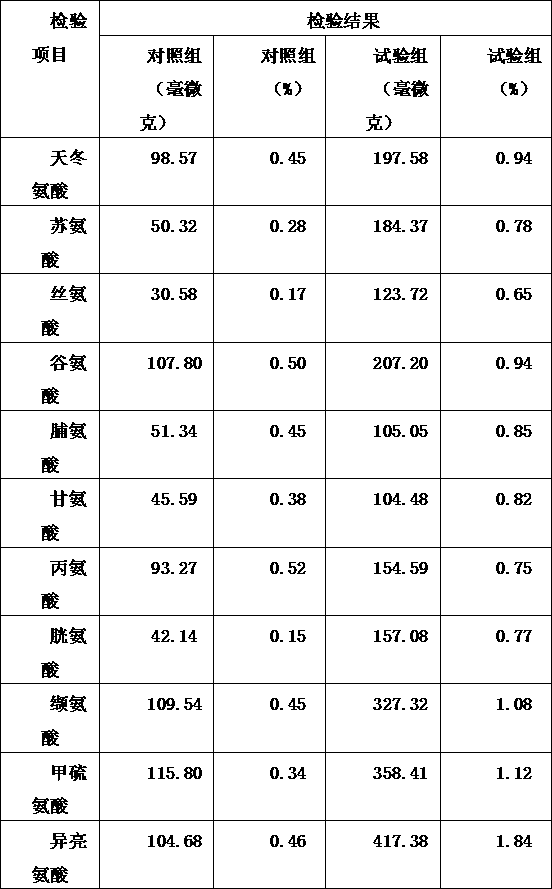
InactiveUS20090047347A1Improve absorption and bioavailabilityToxic effectsPowder deliveryBiocideDrug metabolismHepatic first pass effect
The present invention provides compositions and methods and for speeding the onset of drug action and reducing the first-pass effect drug metabolism in fast-dispersing drug formulations.
Owner:AEGIS THERAPEUTICS LLC
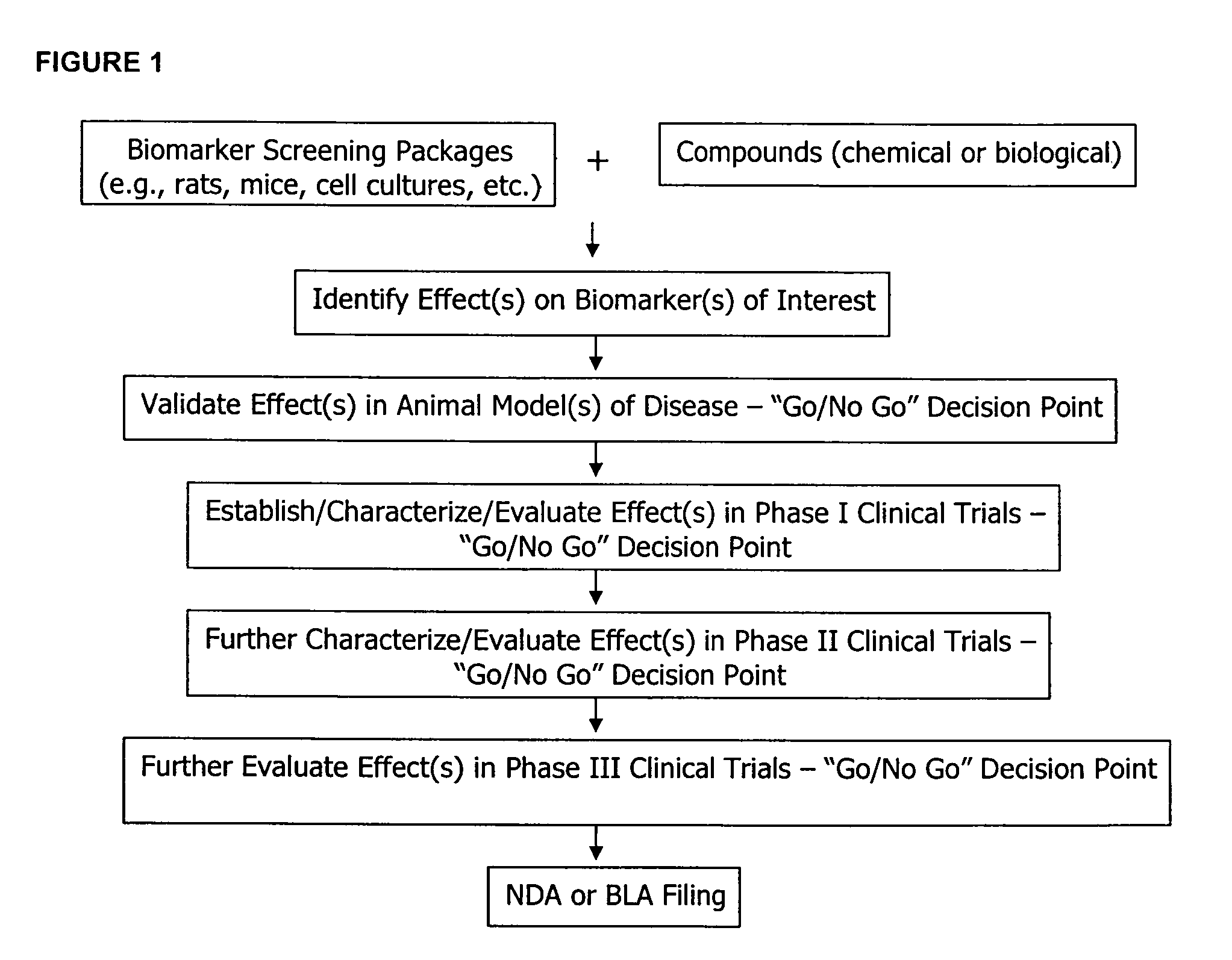
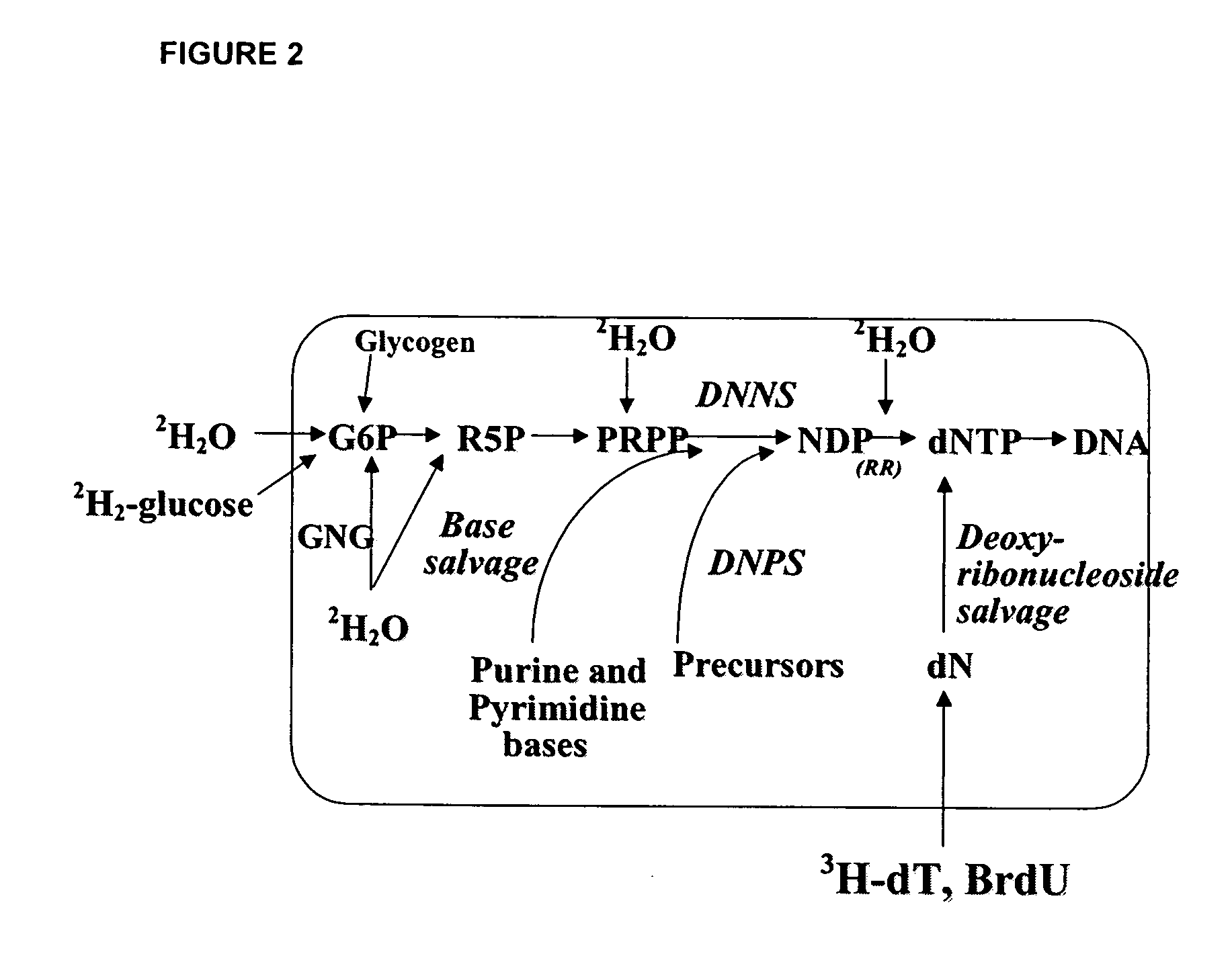
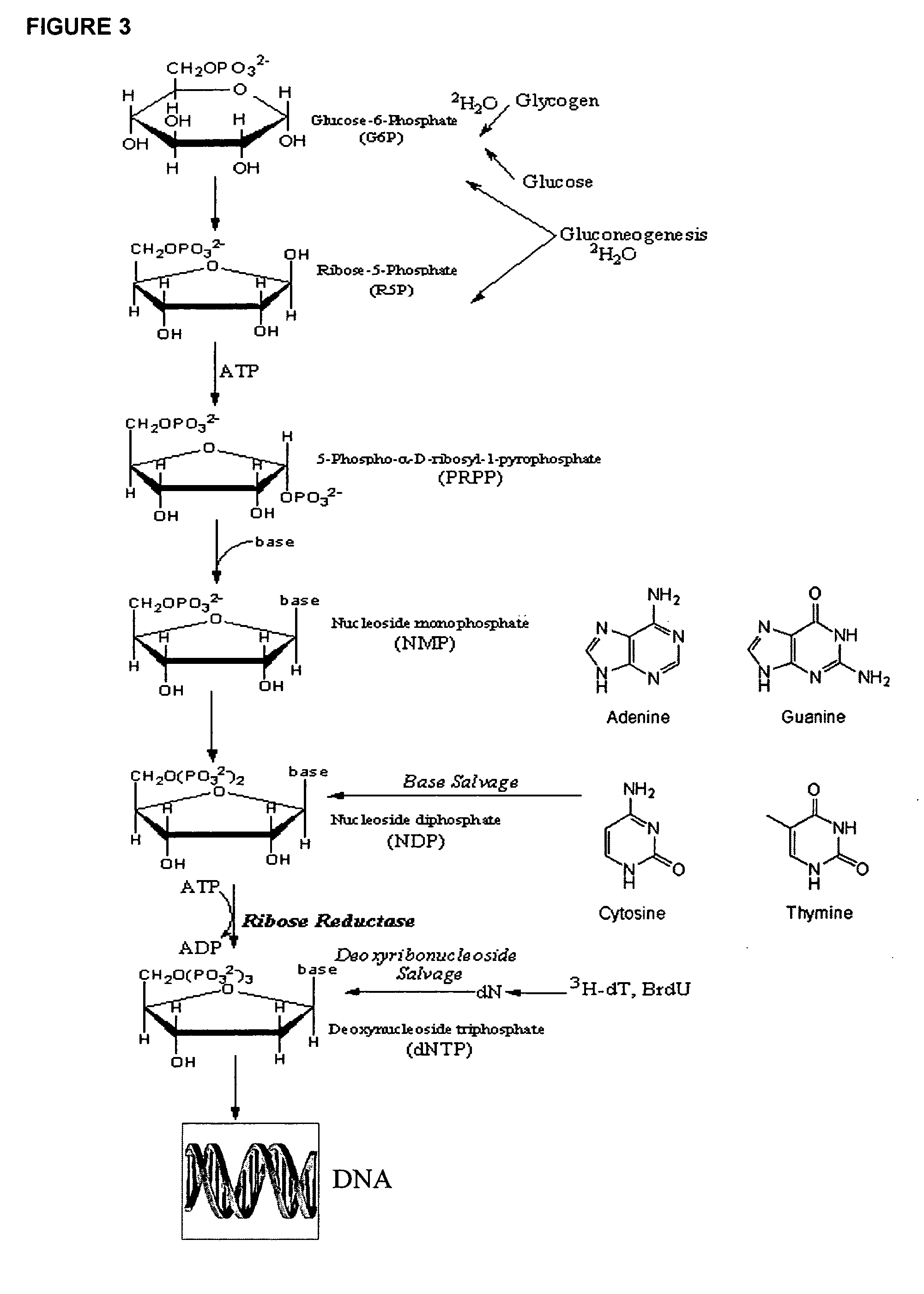
Molecular flux rates through critical pathways measured by stable isotope labeling in vivo, as biomarkers of drug action and disease activity
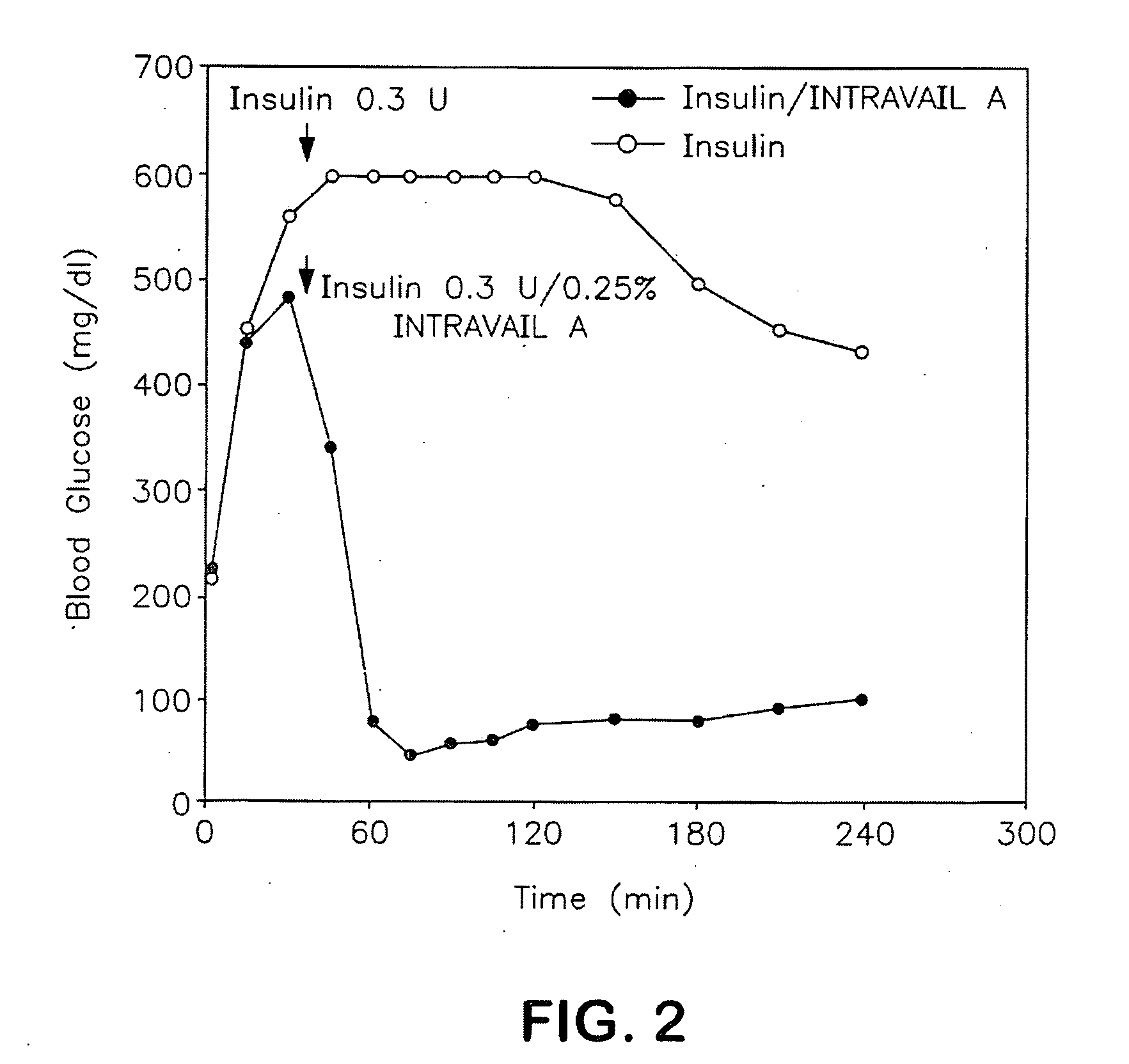
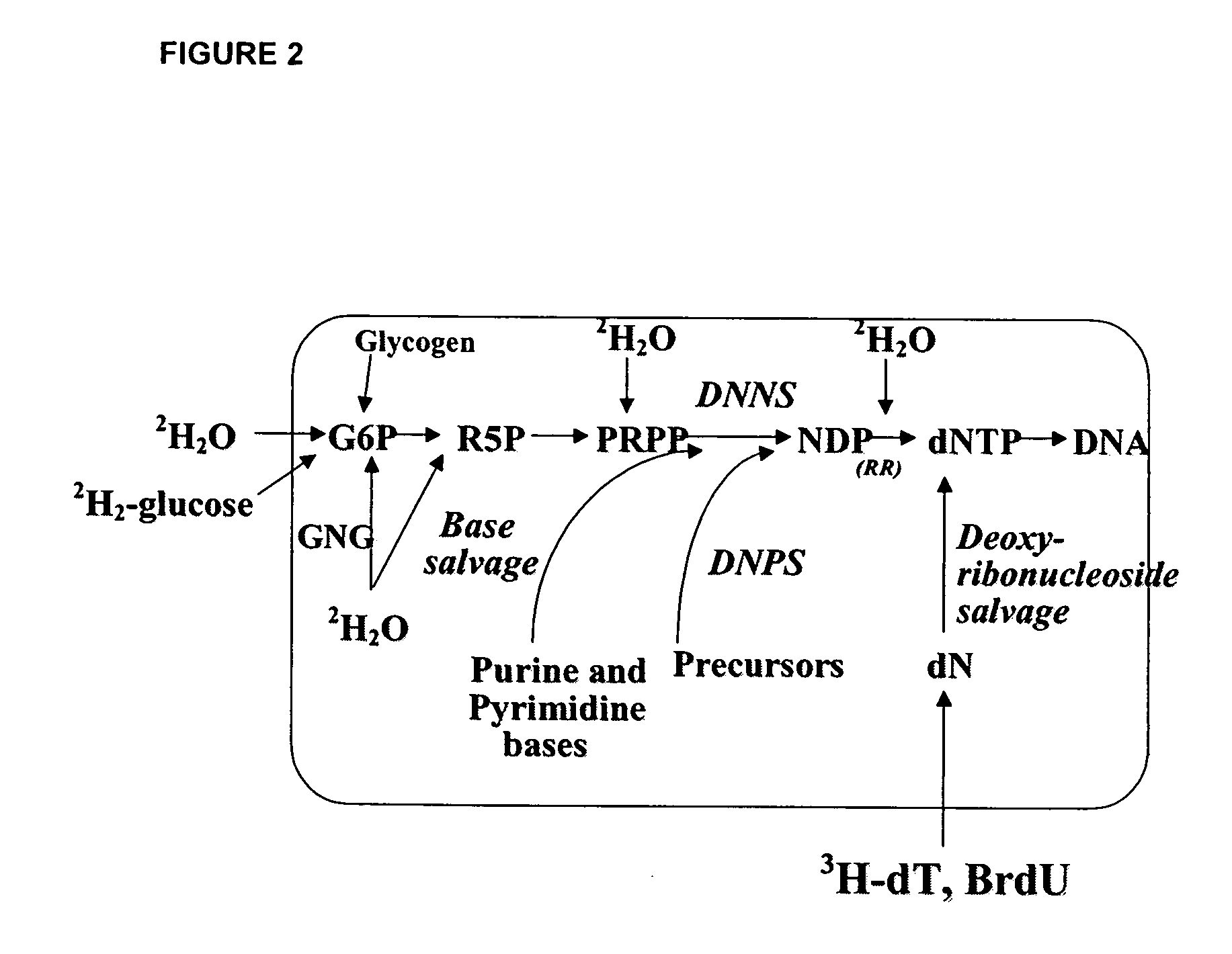
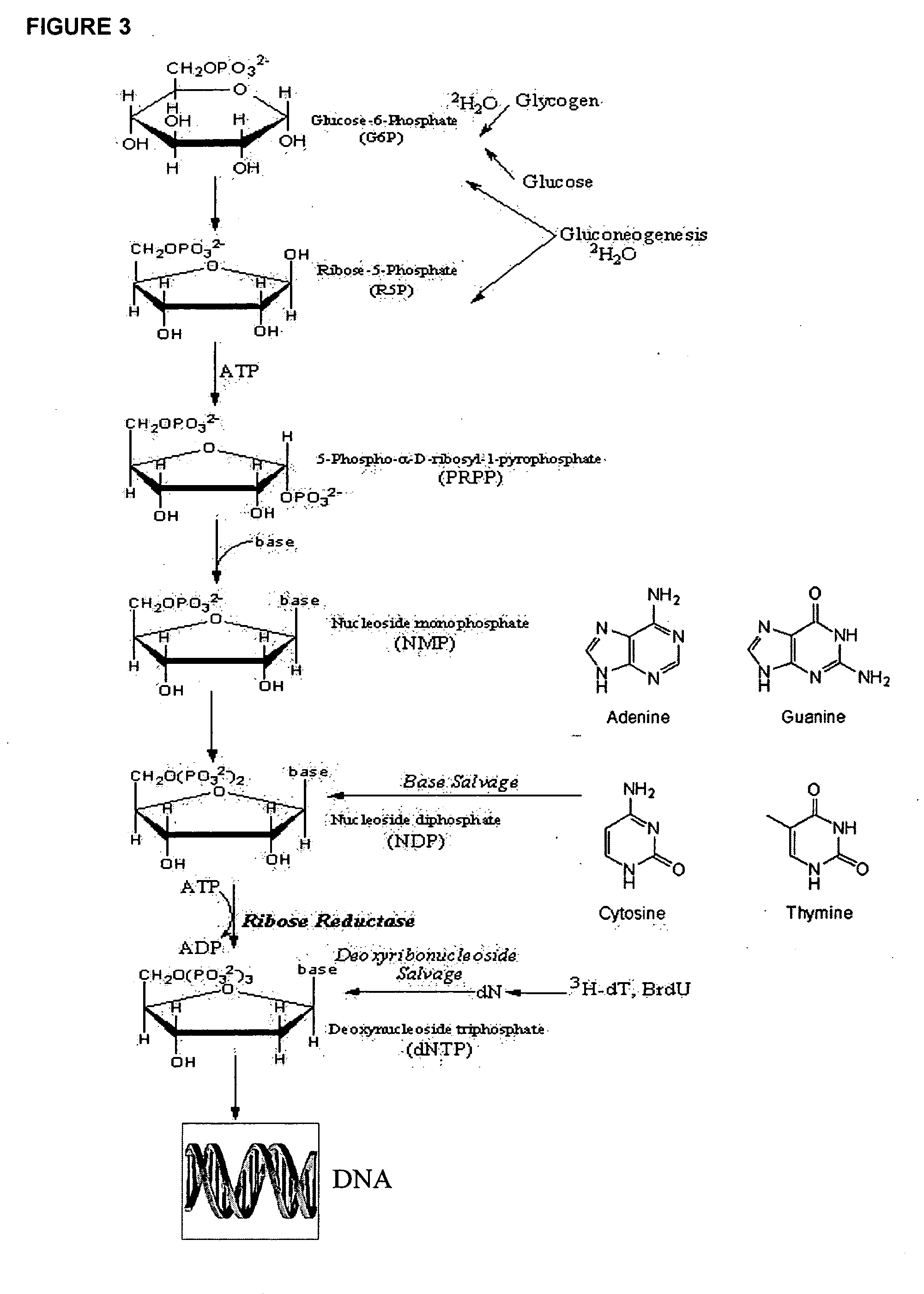
The methods described herein enable the evaluation of compounds on subjects to assess their therapeutic efficacy or toxic effects. The target of analysis is the underlying biochemical process or processes (i.e., metabolic process) thought to be involved in disease pathogenesis. Molecular flux rates within the one or more biochemical processes serve as biomarkers and are quantitated and compared with the molecular flux rates (i.e., biomarker) from control subjects (i.e., subjects not exposed to the compounds). Any change in the biomarker in the subject relative to the biomarker in the control subject provides the necessary information to evaluate therapeutic efficacy of an administered drug or a toxic effect and to develop the compound further if desired. In one aspect of the invention, stable isotope-labeled substrate molecules are administered to a subject and the label is incorporated into targeted molecules in a manner that reveals molecular flux rates through one or more metabolic pathways of interest. By this method, a comparison between subjects and control subjects reveals the effects of the chemical entity or entities on the biomarkers. This, in turn, allows for the identification of potential therapeutic uses or toxicities of the compound. Combinations of compounds can also be systematically evaluated for complementary, synergistic, or antagonistic actions on the metabolic pathways of interest, using the methods of the present invention as a strategy for identifying and confirming novel therapeutic or toxic combinations of compounds.
Owner:RGT UNIV OF CALIFORNIA
Oral medicine for treating cardio-cerebral vascular disease and preparation process thereof
InactiveCN1502337AReduce the number of daily dosesMedication convenienceOrganic active ingredientsPharmaceutical delivery mechanismOral medicineDisease
The present invention discloses a slowly-released oral medicine preparation which is made up by using breviscapine as main raw material and has the functions of promoting blood circulation and removing blood stasis, removing obstruction in the channels to relieve pain for curing angiocardiopathy and cerebrovascular disease with obvious therapeutic effect and its preparation method. Said slowly-released tablet (by one tablet) contains 60-120 mg of breviscapine, 20-75 mg of diluent, 50-150 mg of filling agent and 10-50 mg of slow release material. Said invention also provides its preparation method and concrete steps.
Owner:CHENGDU LIST PHARMA
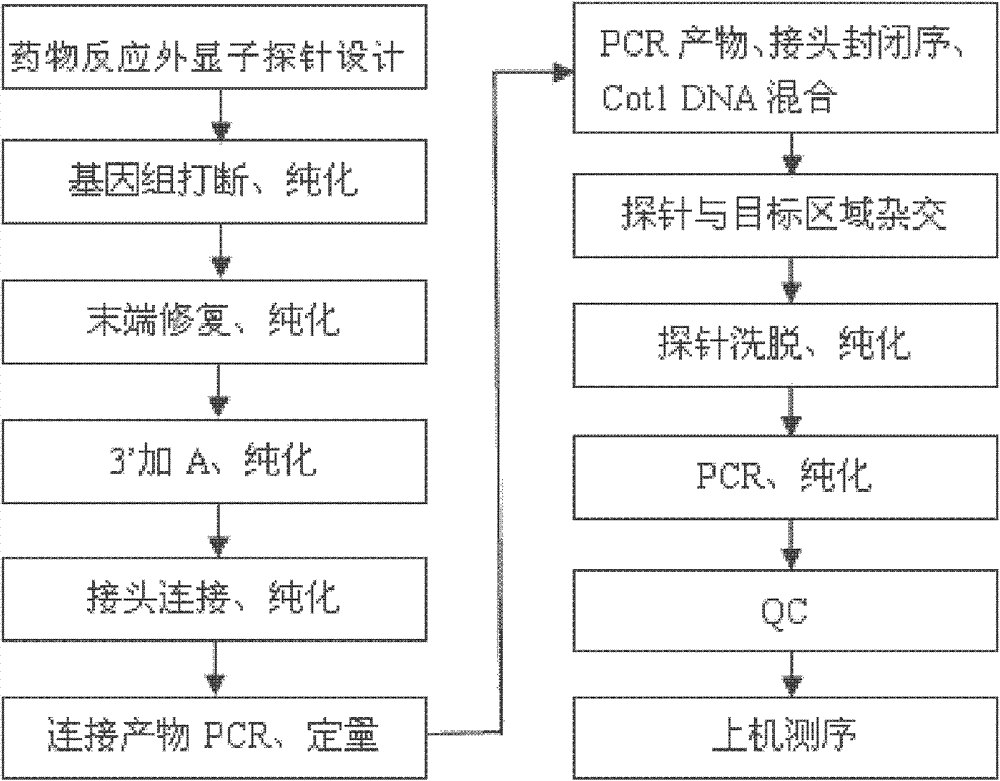
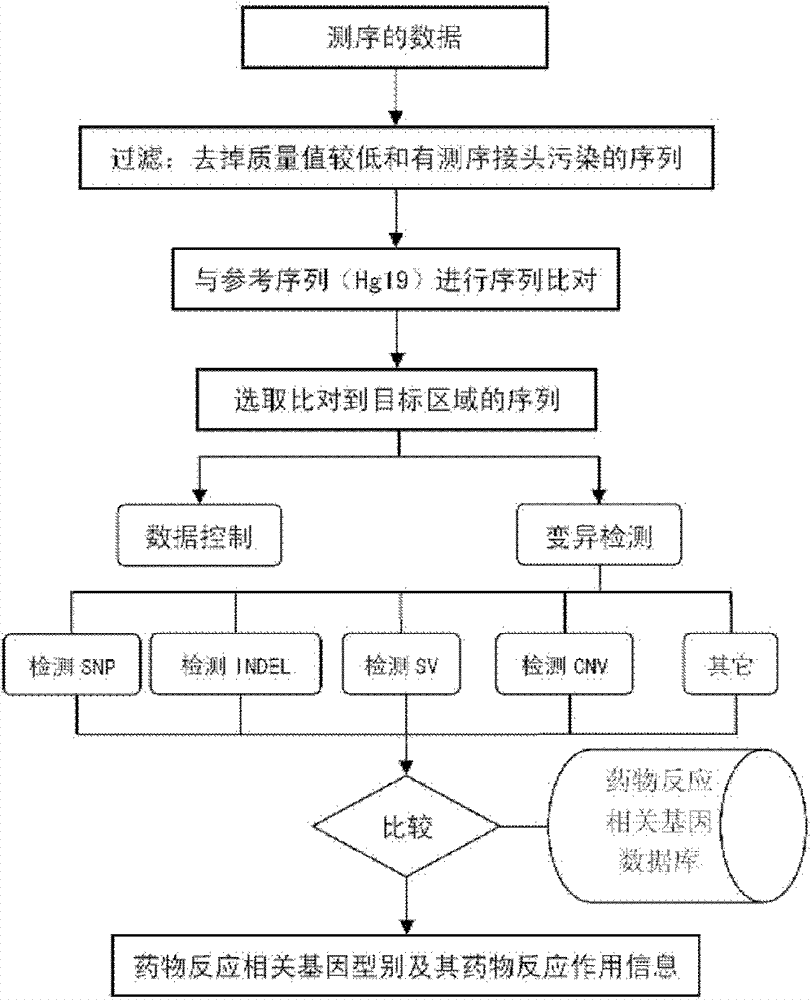
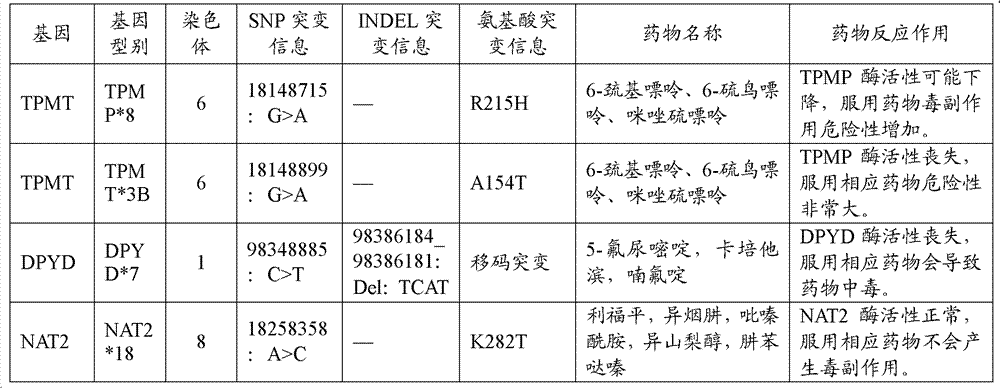
Drug related gene type database, gene typing and drug action detection method
ActiveCN103198238AGive quickly and accuratelyFast and accurate auxiliary basisMicrobiological testing/measurementProteomicsTyping methodsGenotype
The invention discloses a standard gene type database of drug action related genes and a construction method of the standard gene type database. The construction method comprises the following steps: comparing the corresponding special sequence of mutation information with the human whole genome group standard sequence to obtain the corresponding relation between the special sequence and the human whole genome group standard sequence; and according to the corresponding relation, converting the gene type into the standard gene type corresponding to the human whole genome group standard sequence. On the basis, the invention discloses a drug action related gene typing method and a drug action detection method. The database provides a unified standard for drug action related gene typing and a more accurate basis for clinical medication. The gene typing and drug action detection method covers 48 human drug action related genes and can carry out multi-sample detection on all the known mutation sites and the corresponding drug information at the same time. The gene typing method can detect unknown polymorphic sites, and lays the foundation of researching and finding new polymorphic sites affecting the drug action.
Owner:BGI GENOMICS CO LTD
Molecular flux rates through critical pathways measured by stable isotope labeling in vivo, as biomarkers of drug action and disease activity
The methods described herein enable the evaluation of compounds on subjects to assess their therapeutic efficacy or toxic effects. The target of analysis is the underlying biochemical process or processes (i.e., metabolic process) thought to be involved in disease pathogenesis. Molecular flux rates within the one or more biochemical processes serve as biomarkers and are quantitated and compared with the molecular flux rates (i.e., biomarker) from control subjects (i.e., subjects not exposed to the compounds). Any change in the biomarker in the subject relative to the biomarker in the control subject provides information to evaluate therapeutic efficacy of an administered drug or a toxic effect and to develop the compound further if desired. In one aspect of the invention, stable isotope-labeled substrate molecules are administered to a subject and the label is incorporated into targeted molecules in a manner that reveals molecular flux rates through metabolic pathways of interest.
Owner:RGT UNIV OF CALIFORNIA
Method for propagating Chinese medicine ducks
ActiveCN102696548AReduce drug residuesReduce odorFood processingAnimal feeding stuffBiotechnologyAnimal science
The invention discloses a method for propagating Chinese medicine ducks, which comprises the following steps: I, selecting a Chinese medicine formulation; II, fermenting and processing the Chinese medicine formulation to obtain zymotic fluid and fermentation body; and III, applying the zymotic fluid and the fermentation body in a method for propagating the Chinese medicine ducks in different stages. Owing to the method for propagating Chinese medicine ducks, the propagation of the Chinese medicine ducks no longer needs hormone and antibiotic, zymotic Chinese medicine formulation elaborates drug action to the maximum extent, and large-scale propagation is realized; the method has the characteristics of simple operation, safety and environment-protection, natural state, zero pollution, drugand food isogeny, pure, zero side effect, absence of no drug resistance and the like, and can effectively reduce drug residual of duck production; the physique of the ducks can be remarkably enhanced, the immunity and antibody level are improved, and generation of diseases is reduced; and the effects of health care and treatment are achieved, the smell of dejection of the ducks is radiated, and the ecological environment is ameliorated.
Owner:安徽笑果农牧产业科技有限公司
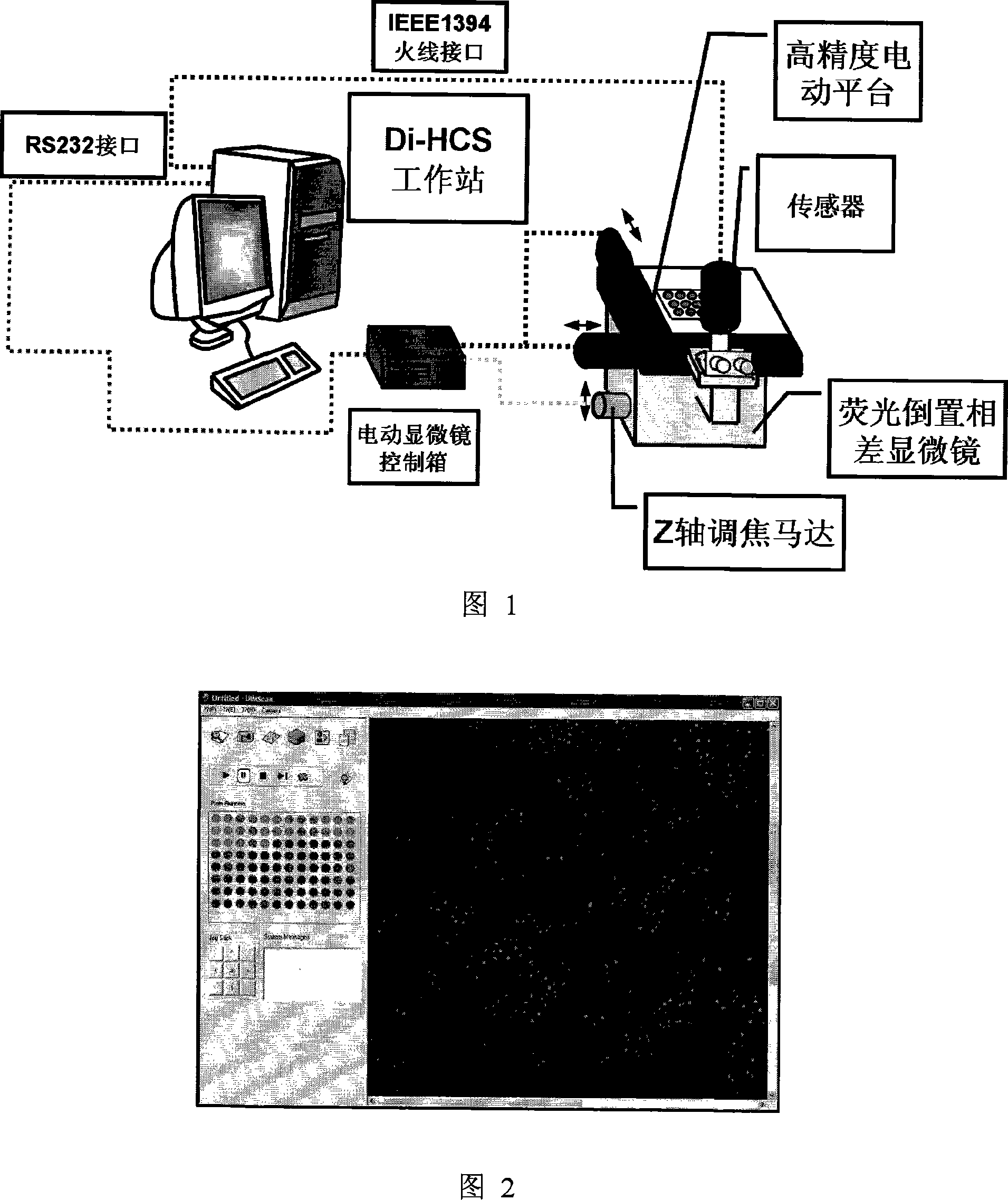
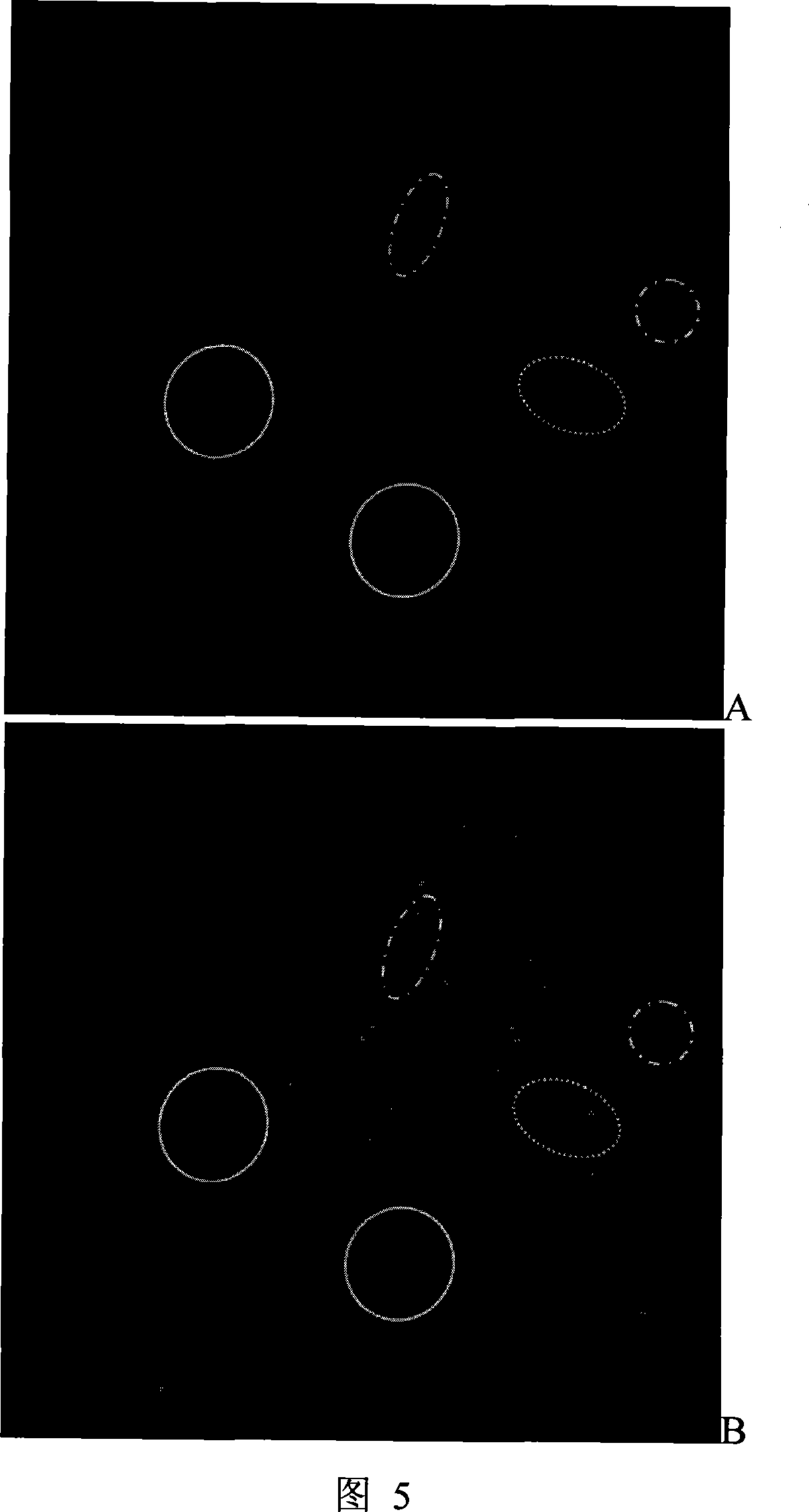
Antineoplastic drug evaluation and screening method based on cell microscopic image information
InactiveCN101149327ARealize program controlRealize control program controlMicrobiological testing/measurementIndividual particle analysisMicroscopic imageFluorescence
The invention provides the appraisal and selective method for the antineoplastic drug based on the cell micrograph information, which uses a sort of selection and appraisal hardware system to appraisal and select the antineoplastic drug by different fluorescence dye marking and measuring the multicellular parameter change in cell. The hardware system is made up of the high precision electric hydrous platform, the fluorescence vision system, the image collecting and processing system and working station. The diacetoxyl fluoresceine dyeing measures the active cell number; the double dyeing method of Hoechst33342 and iodized pyridine appraise the drug inducing the cell die; the three dyeing method of FDA, Hoechst33342 and PI analyzes the die mode induced by drug. The invention can measure at least two kinds of single cell or cell subgroup which expresses different drone cell organ. The method is in reason and can be used in study of drug action mechanism and selecting the high hedonic drug and toxicity analyzing, which can be used in selecting drug and appraising the drug toxicity.
Owner:ZHEJIANG UNIV
Kangfu anti-inflammatory vaginal expansion suppository as well as preparation method and detection method thereof
ActiveCN103301295AWork quicklyImprove stabilityWeighing by removing componentSuppositories deliverySecondary InfectionsSuppository
The invention relates to a Kangfu anti-inflammatory vaginal expansion suppository. The expansion suppository comprises active ingredients prepared from raw material medicines such as radix sophorae flavescentis, patrinia and Chinese violet, a matrix and an expansion carrier. The suppository is fully expanded, so that the medicine-containing matrix is fully contacted with the inner wall of the vagina, and external flow of the liquid medicine is avoided; the medicine-containing matrix in the expansion suppository also comprises a dispersing carrier, so that release of the active ingredients can be promoted, and the dissolution rate is improved. The invention also provides a preparation method and a detection method of the expansion suppository. The provided Kangfu anti-inflammatory vaginal expansion suppository employs seven ingenious advanced technologies and has the beneficial effects that the external flow of the liquid medicine is avoided, the stability is high, the effect is fast, the drug action time is long, and secondary infection is avoided.
Owner:哈尔滨田美药业股份有限公司
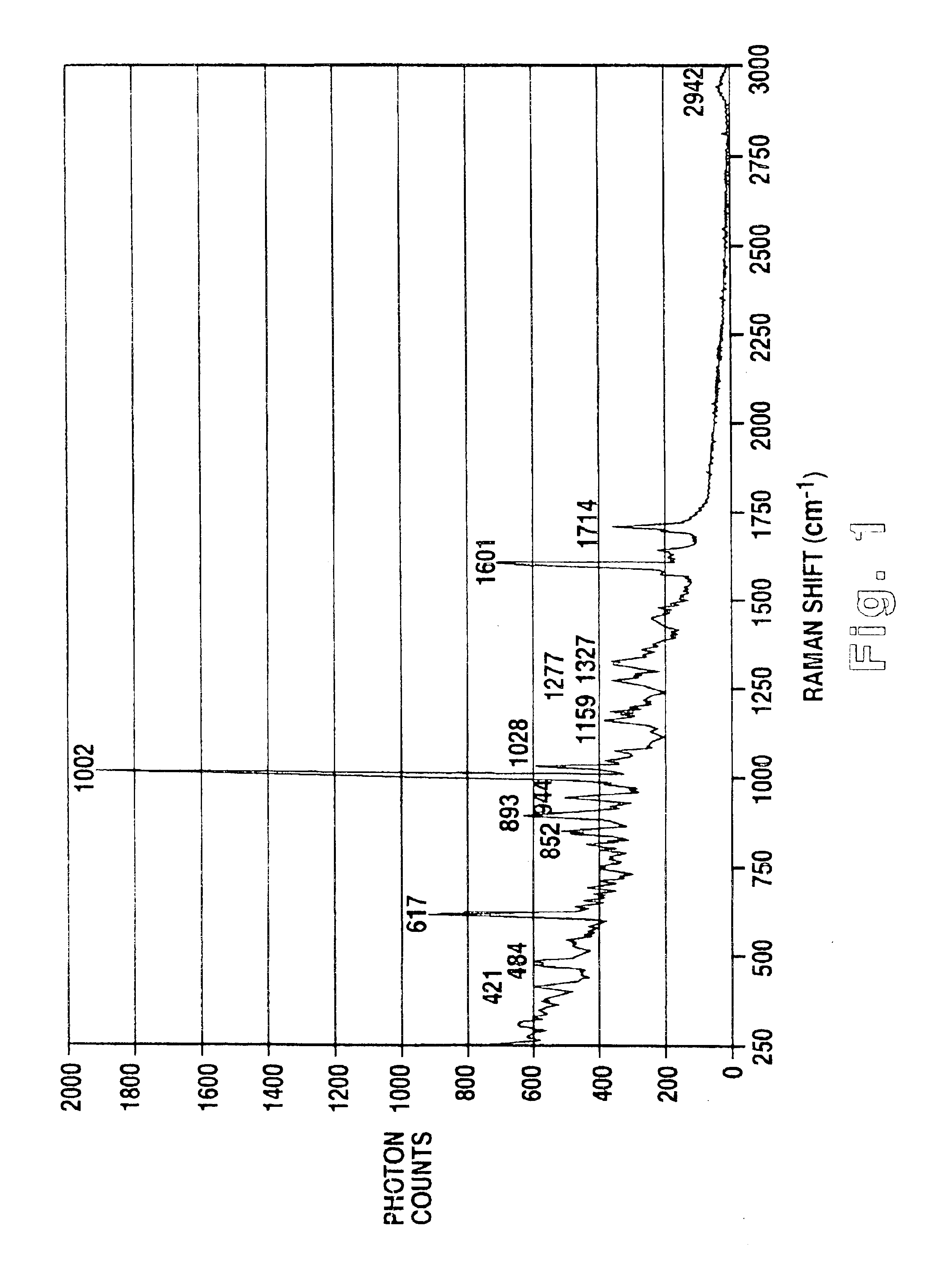
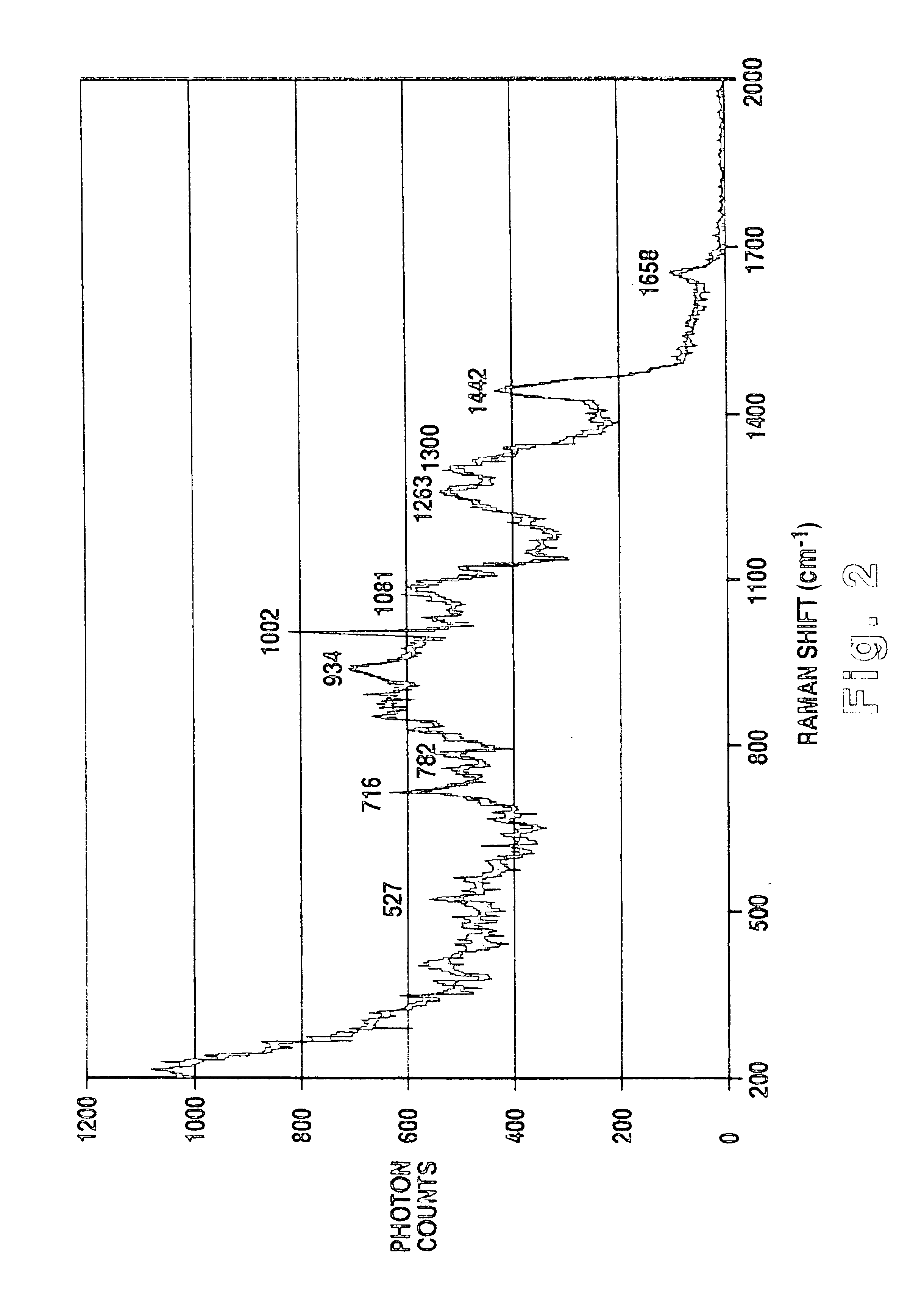
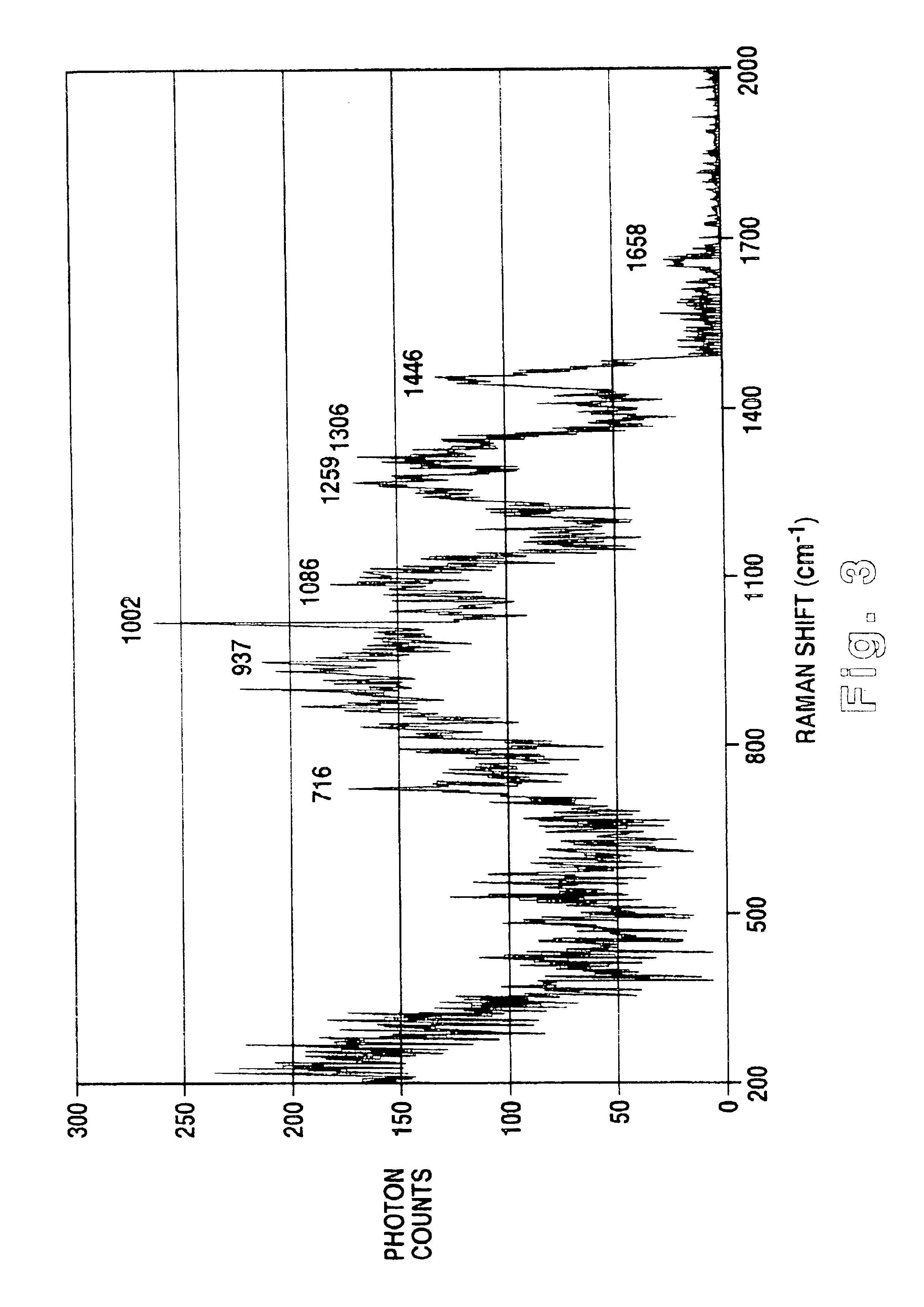
Methodology of using raman imaging microscopy for evaluating drug action within living cells
InactiveUS6939686B2Convenient and cost-effective methodRadiation pyrometryMicrobiological testing/measurementStudy drugRaman imaging
A method of using Raman imaging microscopy to evaluate drug actions in living cells is disclosed. Specifically the invention describes the methods of using Raman imaging microscopy to detect drug uptake, distribution, binding, and metabolism in a single cell, and to study drug pharmacokinetics at the cellular level. The method involves measuring the Raman image of both the drug and the cell. Control images and post-treatment images of the cell were studied. Ratio images were calculated and the requisite information was obtained from a study of the intensity of the bright areas in the ratio images.
Owner:SOUTHWEST RES INST
Xiaomi vagina expansion suppository, preparation method and detection method thereof
ActiveCN103285133AGuaranteed effective concentrationPrevent outflowWeighing by removing componentHydroxy compound active ingredientsSuppositoryDrug action
The present invention relates to a Xiaomi vagina expansion suppository, which comprises an active component, a matrix and an expansible expansion carrier. The present invention further relates to a preparation method and a detection method of the expansion suppository. According to the expansion suppository, seven original leading technologies are adopted; the expansion suppository is made into a hollow expansion suppository, and the matrix and the active component are completely separated, such that stability of the expansion suppository is increased compared with stability of the ordinary suppository; the active component, the dispersion carrier and the absorption blocking agent are combined so as to increase an active component dissolution rate, well release the active component, and prolong a drug action time; and the Xiaomi vagina expansion suppository has effects of rapid effect, prolonged action time, drug liquid outflow prevention, vagina cleaning, secondary infection prevention, and the like.
Owner:哈尔滨田美药业股份有限公司
Functional imaging of autoregulation
InactiveUS20090171195A1Diagnostics using fluorescence emissionCharacter and pattern recognitionDiseaseFunctional imaging
The present invention provides a method for detailed delineation of variation of autoregulation and more particularly tissue metabolism. These enhanced capabilities allow for new insights into factors impacting on body function, detection and monitoring of disease states, understanding of drug actions and other physiological effectors such as diet and physical exercise.
Owner:BARBOUR RANDALL L
Cell-based assays for determining drug action
InactiveUS20070072246A1Expand coverageHigh resolutionBiostatisticsVertebrate cellsActive agentSignaling network
Compositions and methods are provided for the classification of biologically active agents according to their effect on human biology, through the use of complex, primary human cell-based disease models in scalable assay formats. The systems of the invention utilize the simultaneous activation of multiple signaling pathways to generate and identify patterns of expression of physiologically important cell surface and secreted molecules. Combinations of multiple cell types may be utilized. Systems encompassing multiple cell types not only respond to perturbations of each cell type's intracellular signaling networks, but also to inhibition of pathways of communication between cells. Readout information may be combined in multi-system analysis, where the profiles obtained from multiple systems are combined in order to provide enhanced resolution for agent classification.
Owner:DISCOVERYX CORP
In situ gel preparation loaded with Kangfuxin and its preparation method and use
InactiveCN102784169AExtended stayAvoid pollutionAnthropod material medical ingredientsPharmaceutical delivery mechanismPatient complianceDuodenal ulcer
The invention belongs to the field of medicinal preparations and a novel medical technology and relates to an in situ gel preparation loaded with Kangfuxin and its preparation method and use. The in situ gel preparation loaded with Kangfuxin has temperature sensibility. The in situ gel preparation loaded with Kangfuxin is characterized in that Kangfuxin as a raw material and polymers are dissolved in a buffer solution or purified water according to a ratio. Because of polymer reverse-phase gel properties, the in situ gel preparation loaded with Kangfuxin is in a liquid state and can freely flow at a room temperature (of 20 DEG C) under storage conditions, and when the in situ gel preparation loaded with Kangfuxin is spread on a wound, under the action of a body temperature, the in situ gel preparation loaded with Kangfuxin forms fast semi-solid gel. The in situ gel preparation loaded with Kangfuxin is convenient for patient use, improves patient compliance, can effectively store drugs and can slowly release drugs so that drug action time is prolonged and drug efficacy is improved. Through oral administration, the in situ gel preparation loaded with Kangfuxin can be used for treating blood stasis, stomachache, gastrorrhagia, gastric ulcer and duodenal ulcer and assistantly treating Yin deficiency and pulmonary tuberculosis, and phthisis. Through external administration, the in situ gel preparation loaded with Kangfuxin can be used for treatment and anti-adhesion of metal-inflicted wounds, trauma wounds, ulcer wounds, fistula wounds, burn wounds, scald wounds, bedsore wounds and surgical wounds.
Owner:王成
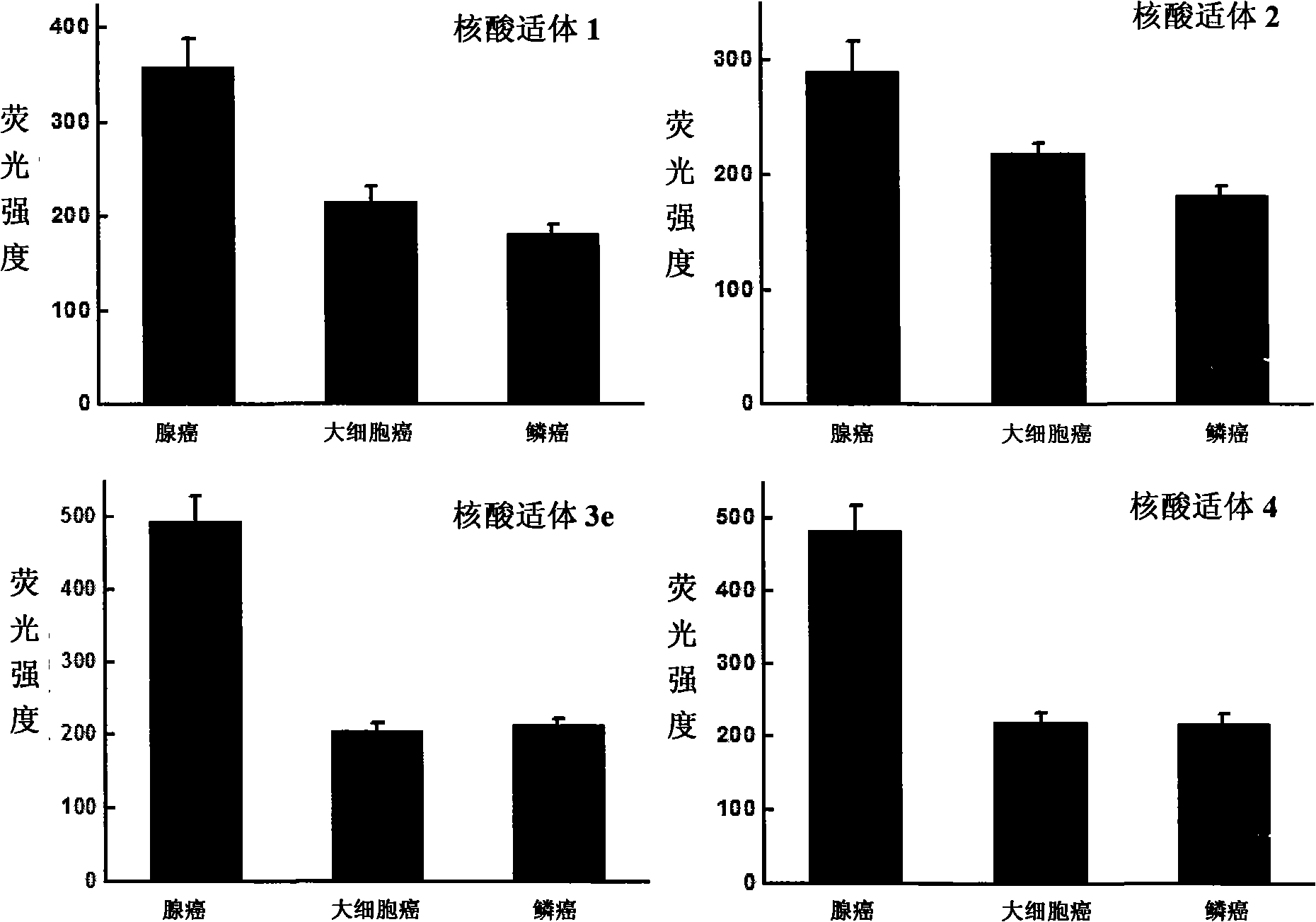
Aptamer for typing different subtypes of non-small cell lung cancer and method for screening the same
ActiveCN101538570AEarly diagnosisAccurate typingMicrobiological testing/measurementLibrary screeningTumor therapyScreening method
The invention discloses aptamer for typing different subtypes of non-small cell lung cancer and a method for screening the same. The aptamer provided by the invention is any DNA segment of nucleotide expressed by sequence 1 to sequence 9 in a sequence table. The aptamer is applied for typing different subtypes of non-small cell lung cancer. The aptamer of the invention can differentiate the different subtypes of non-small cell lung cancer on the molecule response signal under the condition of not knowing tumor markers of the non-small cell lung cancer. Using the aptamer of the invention for identifying the combined target is good for discovering the tumor markers of the different subtypes of non-small cell lung cancer, and earlier and exactly diagnosing and typing non-small cell lung cancer and discovering the tumor for treating new drug action targets.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Cyclosporine A micelle eye drop and preparing method thereof
ActiveCN103054796AImprove solubilityMicellar particle size is smallSenses disorderCyclic peptide ingredientsSolubilityReduced dose
The invention relates to a cyclosporine A micelle eye drop and a preparing method thereof. The cyclosporine A micelle eye drop comprises cyclosporine A used as a main drug, aseptic and isoosmotic adjusting agents, and is characterized by further comprising a copolymer of polythene caprolactam, polyvinyl acetate and polyethylene glycol used as drug auxiliary materials. The preparing method includes: preparing a cyclosporine A micelle through a solvent rotary evaporation film forming method; obtaining cyclosporine A micelle powder after filtering, freezing and drying the cyclosporine A micelle; and dispersing and dissolving the powder into a buffer solution to obtain the cyclosporine A micelle eye drop. According to the cyclosporine A micelle eye drop, the cyclosporine A has good solubility in a water solution, the micelle is small in particle diameter and uniform in distribution range and drug stability is good. The cyclosporine A micelle eye drop not only reduces drug irritation, but also improves drug absorption of a cornea, reduces drug concentration, prolongs drug action time, reduces dosing frequency, improves patient compliance, and has good economical efficiency.
Owner:SHANDONG EYE INST
Medicine for treating osteoporosis and preparation method of medicine
InactiveCN104435230AImprove immunityQuick resultsAntipyreticAnalgesicsSide effectAngelica Sinensis Root
The invention discloses a medicine for treating osteoporosis and a preparation method of the medicine, and belongs to the field of traditional Chinese medicines. The effective components of the medicine disclosed by the invention are prepared from the following raw materials: psychotria serpens, quail, membranaceous beautyleaf root, ventilago leiocarpa benth, thesium chinensis, mussel, sambucus adnata, philippine flemingia root, lichen of parmelia saxitilis, hoya lancilimba, angelica sinensis, false nettleleaf pepper herb, cabbage, cinnamomum burmanni peel, liquorice and herba pyrolae. The medicine is appropriate of compatibility, has the efficacies of building bodies, invigorating bones, invigorating the spleen, replenishing qi, relaxing spasm, relieving pain, tonifying kidneys and deficiency, eliminating dampness, dredging channels, coordinating the drug actions of a prescription by combination of a plurality of medicinal materials, has the advantages of being fast in acting, high in recovery rate, free of toxic or side effect, safe and convenient on the treatment of osteoporosis, and has relatively significant effects of lubricating joints, and strengthening tendons and bones; meanwhile, the immunity of a human body is obviously improved; and the medicine can be widely applied to clinical treatment of the osteoporosis.
Owner:鹿泽兵
Method for preparing sotalol hydrochloride of injection
InactiveCN1481786AOvercome the shortcomings of slow oral absorption and slow onset of actionImprove stabilityPowder deliveryAmide active ingredientsFreeze-dryingSotalol Hydrochloride
The freeze dried sotalol hydrochloride for injection contains sotalol hydrochloride 20-80 g and excipient 40-100 g in each 1000 ampules. As freeze dried preparation for injection, the present invention has the advantages of fast absorption and action and high stability in the preservation period. It may be administrated via intravenous transfusion to further raise the medicine stability. The present invention is suitable for ventricular fast arythmia, etc.
Owner:吉林市卓怡康纳制药有限公司
Controlled-release formulation containing tamsulosin hydrochloride
The present invention relates to a controlled-release preparation containing tamsulosin hydrochloride. The preparation of the present invention can release tamsulosin hydrochloride at zero-order rate regardless of ambient conditions of stomach and small intestine, and therefore can reduce several side effects and maintain the effect of the drug constantly. In addition, the tamsulosin hydrochloride-containing controlled-release preparation of the present invention is simple to manufacture and cost-effective, and can be made with conventional tabletting machine and coating machine used usually so that it does not need a specific equipment.
Owner:CTC BIO INC
Preparation method of chimonanthus nitens valid target, production method and use of formulation thereof
ActiveCN101357146AReduce dosageEasy to takeAntibacterial agentsAntimycoticsDiseaseAdditive ingredient
The invention relates to a preparation method of effective fractions of shining wintersweet leaf, a preparation method and a use of a preparation thereof, in particular to an application of the effective fractions of the shining wintersweet leaf and the preparation thereof in drugs used for preventing and treating respiratory system diseases as well as in anti-cold drugs, antimicrobial drugs, anti-inflammation drugs, antiviral drugs, antifungal drugs, etc. In the invention, the volatile effective fractions and non-volatile effective fractions are added to the crude drug of the shining wintersweet medicinal material with a weight ratio of the actual extraction amount of the shining wintersweet to the effective fractions being 1.0-2.6:0.5-3.5, thus the effective fractions of the shining wintersweet leaf are obtained. A preparation of the effective fractions of the shining wintersweet can be made into granules, soft capsules, capsules, drop pills, tablets, compound capsules, dispersible tablets, etc. In the invention, the preparation of the shining wintersweet is deeply developed, which ensures that the preparation is rich in active ingredients, has definite effective ingredients, obvious pharmacological action and significant improvement of drug action. Besides, the preparation has stable performance and controllable quality, is safe and reliable, has less dosage, and is favorable for transportation and storage of the medicine as well as for patients to take.
Owner:江西佑美制药有限公司 +1
Composite medicine coating balloon, preparation method thereof, and composite medicine coating balloon dilatation catheter
The invention discloses a composite medicine coating balloon. The composite medicine coating balloon comprises a balloon body and a composite medicine coating covering the surface of the balloon body,wherein the composite medicine coating comprises a bottom-layer coating, a middle-layer coating and an outer-layer coating; the bottom-layer coating is used for coating the surface of the balloon body, and consists of medicines A and a hydrophilic excipient; the middle-layer coating consists of a medicine B packed with a packing agent and hydrophilic excipients / lipophilic excipients / amphipathic excipients; and the outer-layer coating consists of a medicine C and lipophilic excipients / amphipathic excipients. The invention further discloses a preparation method of the composite medicine coatingballoon and a composite medicine coating balloon dilatation catheter. According to the composite medicine coating balloon, the loss of traditional Chinese medicines in the balloon transporting process can be reduced, besides, the vasculopathy position can have effective medicine concentration within a short term, vascular restenosis is restrained, long-time medicine release can be provided, durable medicine action time can be maintained, and the restenosis rate can be reduced.
Owner:KOSSEL MEDTECH SUZHOU
Somatotrophic Chinese medicinal herbal feed additive for pigs
InactiveCN102406082AFast growthImprove conversion rateFood processingAnimal feeding stuffChemical synthesisMedicinal herbs
The invention relates to a somatotrophic Chinese medicinal herbal feed additive for pigs. The additive is prepared by micronizing and mixing the following raw materials in parts by weight: 8 to 12 parts of malt, 4 to 8 parts of hawthorn, 4 to 6 parts of betel nut, 3 to 7 parts of dried tangerine peel, 3 to 7 parts of Chinese atractylodes, 3 to 5 parts of akebiaquinata, 2 to 4 parts of cyrtomium rhizome, and 2 to 4 parts of liquorice. The feed additive does not contain any antibiotics or chemical synthesized medicines, can remarkably increase the growing speed of pigs, reduce mortality and selection rate and improve feed conversion rate, and has no biological toxicity, low cost, economy and practicality. According to the micronization technology in the invention, the absorption utilization degree and drug action of effective components of medicines can be effectively improved, and the comprehensive utilization effect to raw materials is increased.
Owner:ZHENJIANG TIANHE BIOLOGICAL TECH
Schistosoma japonicum katsurada recombinant protein SjSAPLP4 as well as encoding gene and application thereof
ActiveCN105384803AImproving immunogenicityGood antigenicityBiological material analysisAntiparasitic agentsDIAGNOSTIC ANTIGENSFhit gene
The invention provides a schistosoma japonicum katsurada recombinant protein SjSAPLP4 as well as encoding gene and application thereof. The protein has an amino acid sequence shown as SEQ ID NO.2 or an amino acid sequence having a same function, formed by replacing, omitting and / or adding one or more amino acid residues for the amino acid sequence shown as SEQ ID No.2. The invention also provides a gene sequence for encoding the protein. The recombinant protein SjSAPLP4 is good in immunogenicity, can be used as an excellent diagnostic antigen, can be used for preparing a schistosoma japonicum katsurada diagnosis kit having high sensitivity and high specificity, also can be used for preparing an anti-schistosome vaccine and can be used as a potential drug acting target spot to screen the drug for treating the schistosoma japonicum katsurada.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
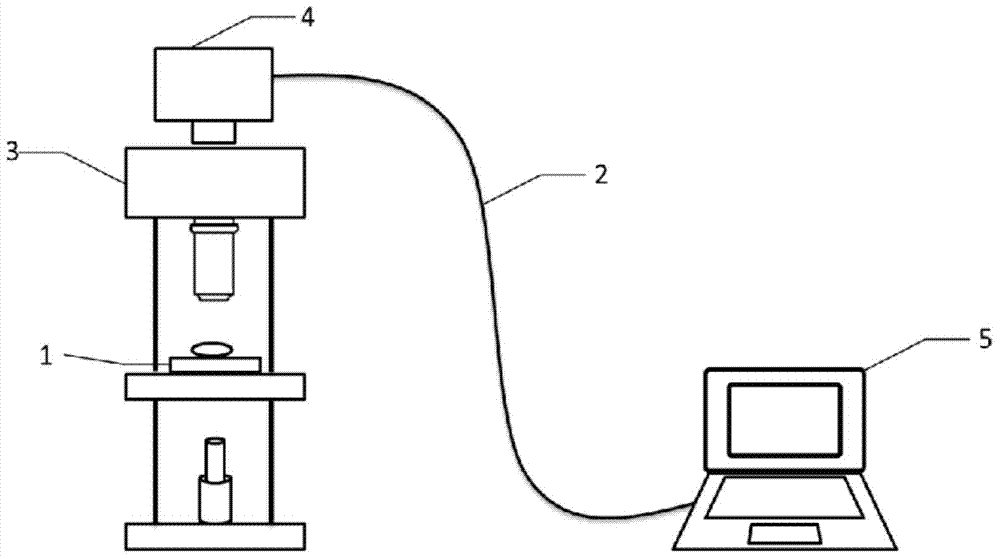
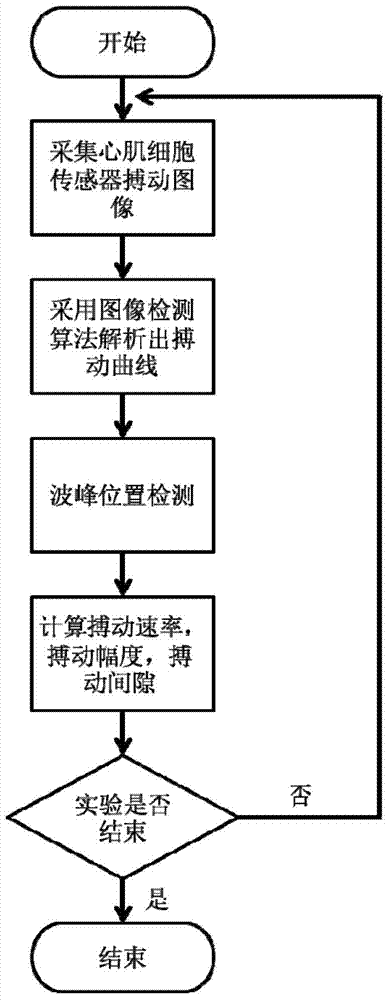
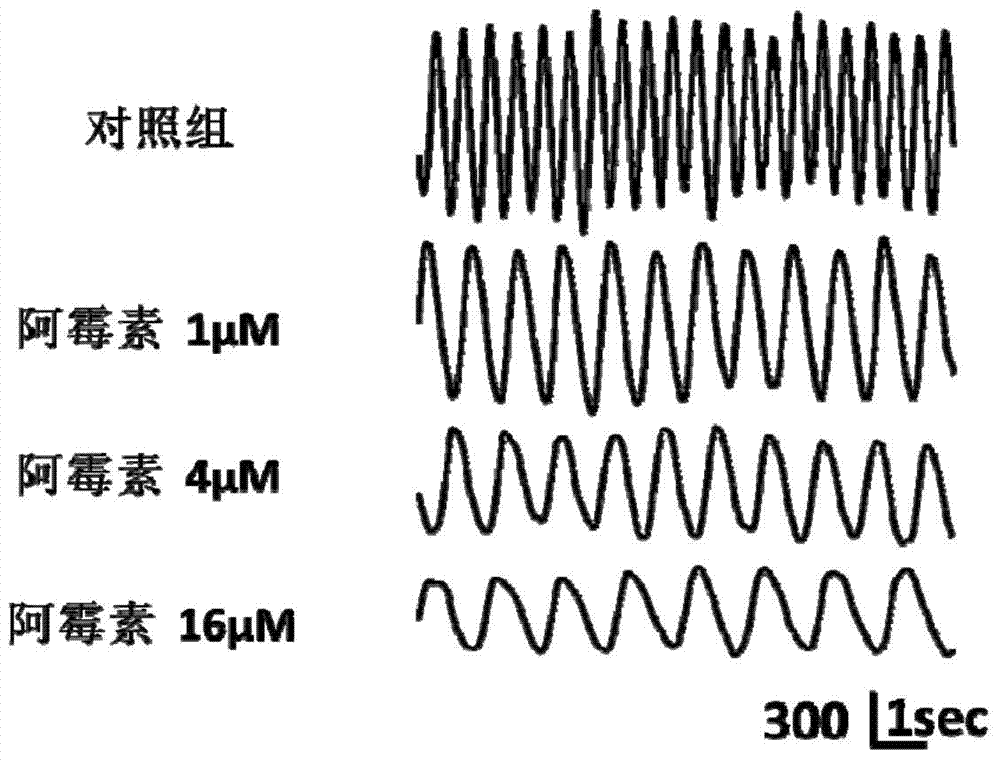
Drug cardiotoxicity detection analysis method based on myocardial cell sensor
InactiveCN104297249ALow costObserve intuitivelyMaterial analysis by optical meansTesting medicinal preparationsRgb imagePeak value
The invention discloses a drug cardiotoxicity detection analysis method based on a myocardial cell sensor. According to the drug cardiotoxicity detection analysis method, a high-performance and low-cost cardiac muscle cell sensor is constructed by adopting ex vivo myocardial cell culture; the change of pulsation images when myocardial cells mechanically pulsate is calculated and detected by adopting algorithms such as image acquisition, conversion of an RGB image into a gray level image, image binaryzation, image matrixing and peak detection, and the mechanical pulsation of the myocardial cells is quantized by using a difference value of the pulsation images, the detection on a rate, an amplitude and a pulsation interval of mechanical pulsation of the myocardial cells is realized; by analyzing the change of the mechanical pulsation state of the myocardial cells over time under the drug action, the cardiotoxicity of a drug is evaluated. Compared with an existing drug cardiotoxicity detection analysis method, the drug cardiotoxicity detection analysis method has the advantages of no marks, no loss, low cost, simple operating steps, and the like, and is capable of observing and evaluating the drug cardiotoxicity simply for a long time.
Owner:ZHEJIANG UNIV
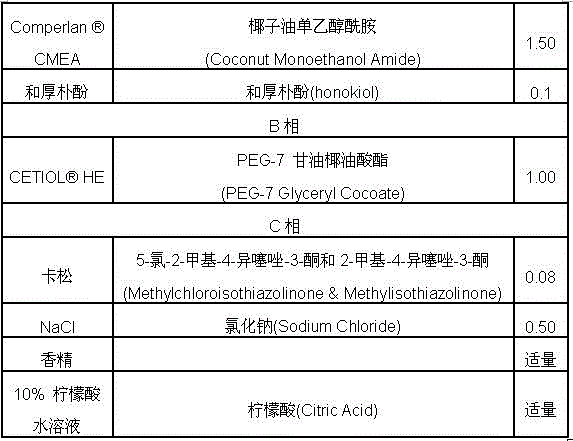
Product for treating skin diseases caused by Malassezia
ActiveCN102743363AImprove reproductive performanceInhibition of fertilityCosmetic preparationsHair cosmeticsDiseaseMalassezia
The invention provides a product for treating skin diseases caused by Malassezia, and medicines and daily chemical products of honokiol, wherein the product treats honokiol or its derivative as an active component; and the medicines and the daily chemical products are prepared through dispersing the honokiol or its derivative in medicine carriers. Above medicaments provided by the invention can effectively inhibit the breeding of the Malassezia, have the advantages of low toxicity and high medicine effect efficiency, and have wide application prospects.
Owner:晟薇药业(上海)有限公司
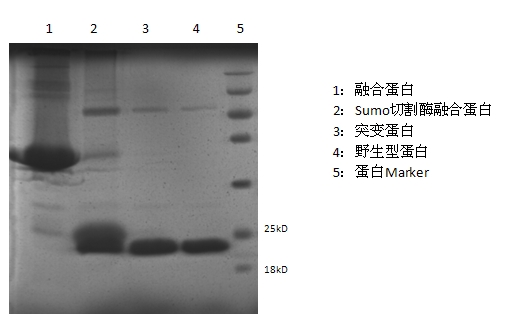
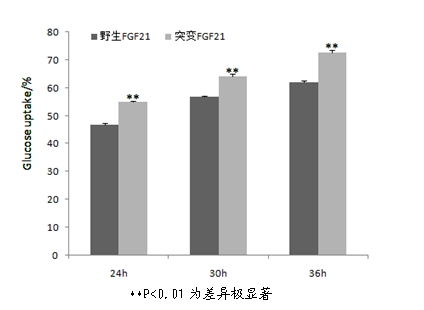
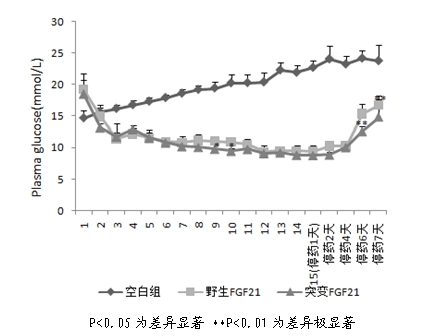
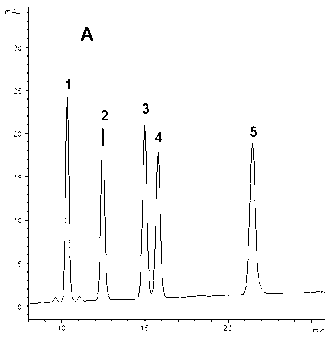
Mutant fibroblast growth factor and use thereof in treating endocrine diseases
ActiveCN102603886AImprove stabilityQuick effectBacteriaPeptide/protein ingredientsArginineCell membrane
The invention discloses a mutant fibroblast growth factor (FGF-21) and use of the mutant fibroblast growth factor in treating endocrine diseases. The amino acid sequence of the fibroblast growth factor-21 disclosed by the invention is shown as SEQ ID NO: 2, and the sequence of a gene for encoding the mutant fibroblast growth factor is shown as SEQ ID NO: 1. According to the invention, a wild typeFGF-21 is used as a template, two arginine (Arg) residues are introduced through a downstream primer, and a mutant is obtained through polymerase chain reaction (PCR). The strong basicity and positive charges in the physiological condition of Arg are mainly utilized so that the isoelectric point of FGF-21 is up-regulated, and FGF-21 binding to the surfaces of cell membranes is facilitated. The results of an animal experiment show that the mutant FGF-21 disclosed by the invention can more effectively reduce the blood glucose level in an animal, and furthermore, the mutant disclosed by the invention has the advantages of fast onset of drug action, lasting drug effect and the like in reducing the blood glucose level. The mutant GF-21 disclosed by the invention can be used as a medicine to treat endocrine diseases such as diabetes, metabolic syndrome, lipid metabolism disorder and the like.
Owner:TIANJIN TASLY PHARMA CO LTD
Application of panaxadiol saponins fraction in preparing medicine for preventing dermatitis and scar
ActiveCN102743402AHigh purityStable efficacyOrganic active ingredientsCosmetic preparationsMedicinal herbsGlucocorticoid
The invention provides application of a panaxadiol saponins fraction in preparing a medicine for preventing dermatitis and scar and a health-care cosmetic. The panaxadiol saponins fraction comprises the following main constituents: ginsenoside Rb1, ginsenoside Rb2, ginsenoside Rb3, ginsenoside Rc and ginsenoside Rd. The medicine or the cosmetic is prepared from active constituents in the single panaxadiol saponins fraction or / and other medicines together and a pharmaceutically acceptable or cosmetic acceptable carrier. The medicine or the cosmetic is prepared by utilizing raw materials: ginseng rhizome medicinal materials, American ginseng rhizome medicinal materials, ginseng stem leaf medicinal materials, American ginseng leaf medicinal materials and total extractives or total saponins of the ginseng rhizome medicinal materials, the American ginseng rhizome medicinal materials, the ginseng stem leaf medicinal materials and American ginseng leaf medicinal materials through a chromatographic separation and purification method combining macroporous resin column chromatography and octadecylsilane chemically bonded silica column chromatography. The scar can be prevented from forming while the tissue regeneration and repair are promoted by the medicine or the cosmetic. Compared with glucocorticoids, cellular immunity is integrally regulated, the drug action is stable after the medicine is suspended, and the drug action advantage is obvious. The medicine or the health-care cosmetic is highly safe. The structural formula of panaxadiol saponins is shown in the specification.
Owner:ZHEJIANG UNIV
Compound taxol and its derivative docetaxel fat emulsion and preparation method
InactiveCN101006997APro-apoptosisGood anticancer effectOrganic active ingredientsEmulsion deliverySolubilityVegetable oil
The invention relates to complex paclitaxel and its derivates docetaxel intralipid which includes the following ingredients: paclitaxel or docetaxel, vegetable oil, solubilizing agent, lecithin, glycerine, and water for injection at a ratio of 0.5-10:10-100:10-100:10-20:20-25:700-950. The preparing method includes the following steps: stirring with high speed homogenating machine or ultrasonic oscillating to get the protogala; preparing the complex paclitaxel intralipid with high pressure homogenizer. The preparation is intralipid in O / W type which packages paclitaxel or docetaxel into the compound oil phase. The compound oil phase has good solubility for paclitaxel or docetaxel which has prevented the phenomenon of precipitation after diluting the emulsion; the ingredients of compound oil has the function of coordinated antitumous effect; it can also release the injecting irritative response, haemolysis and hypersensitiveness; it has the function of targeting which has increased the drug action.
Owner:董英杰
Pharmaceutical composition for treating common cold or cough caused by common cold in children and preparation method thereof
The invention provides a pharmaceutical composition for treating common cold, especially for treating common cold or cough caused by common cold in children, and a preparation method of the pharmaceutical composition. So far, no special medicinal materials have been found for cough caused by retention of phlegm-heat in the lung and failure of the lung to disperse and descend in children; and no pharmaceutical compositions containing Radix Scutellariae Baicalensis, Rhizoma Fagopyri Dibotrydis, Folium Eriobotryae Japonicae (processed with honey), Bulbus Fritillariae Thunbergii, Rhizoma Polygoni Cuspidati and Radix Glycyrrhizae have been developed in the prior art. The pharmaceutical composition provided by the invention has the characteristics of rapid onset of drug actions, small use amounts, no adverse side effects, good stability, low cost, high safety and low toxicity; is suitable for large-scale production; and is especially suitable for children.
Owner:北京东方运嘉科技发展有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com