Mutant fibroblast growth factor and use thereof in treating endocrine diseases
A technology for fibroblasts and endocrine diseases, applied in the directions of fibroblast growth factors, growth factors/inducing factors, metabolic diseases, etc. With the effect of development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Cloning of FGF-21 Gene
[0025] According to the nucleotide sequence of the FGF-21 gene with the signal peptide removed, primers were designed, and the FGF-21 gene with the signal peptide removed was obtained by complementary extension. Primers for FGF-21 mutants were designed using the online software Primer5.0.
[0026] Using extracted human liver total RNA as a template, Oligo(dT) 15 As primers, the first strand of cDNA was synthesized according to the instructions of M-MLV reverse transcriptase M-MLVRT. The reaction system and specific operations were as follows:
[0027]
[0028] Water bath at 70°C for 5 minutes, place on ice for 5 minutes, add in sequence:
[0029]
[0030] 37 ℃ water bath for 2 h, 70 ℃ water bath for 15 min, take 2 μl for PCR amplification reaction. The mature polypeptide cDNA of FGF-21 mutant was amplified by conventional PCR method, and the PCR reaction (50 μl system) was as follows:
[0031]
[0032] P1: 5' GGTCT...
Embodiment 2
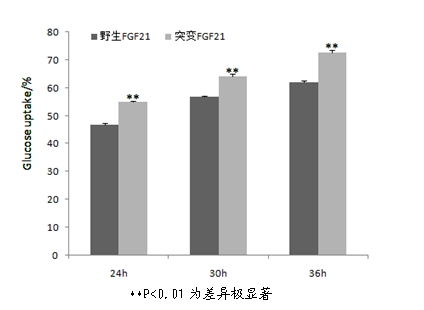
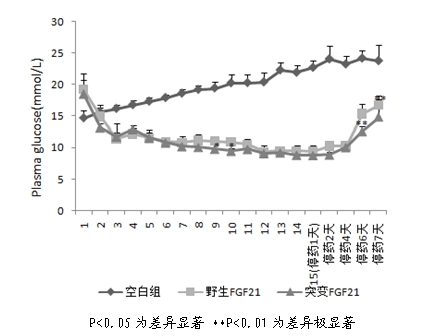
[0036] Example 2 Preparation and Activity Detection of Mutant Fibroblast Growth Factor-21
[0037] 1. Construction of mutant FGF-21 gene expression vector
[0038] Ligate the target fragment of the FGF-21 mutant recovered in Example 1 with the prokaryotic expression vector pET30a (+), and the ligation reaction system (10 μl) is as follows:
[0039]
[0040] A total of 10 μl of the system was mixed, and ligated at 4°C overnight. After enzyme digestion and identification, the recombinant plasmid pET-30a-FGF-21 was constructed;
[0041] 2. Obtaining of mutant FGF-21 protein
[0042] (1), induced expression
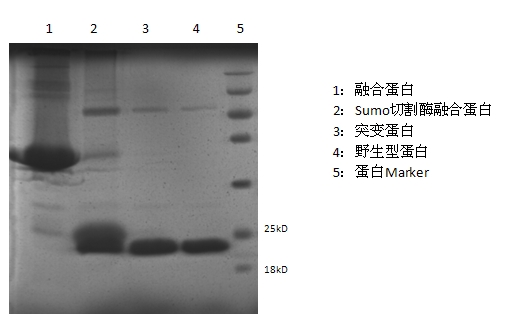
[0043] Transform the recombinant plasmid pET-30a-FGF-21 containing the correct sequence into the expression strain Trans etta (DE3) (Beijing Quanshijin Biotechnology Co., Ltd., catalog number: CD801). Transformed single colonies were inoculated into 5 mL of LB medium, cultured at 37°C for 10 h, inoculated in 500 mL of LB medium containing penicillin (50 mg / mL) at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com