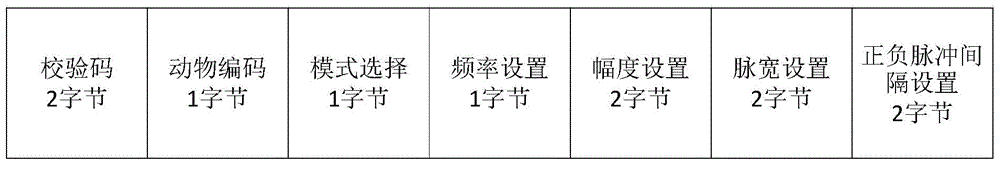
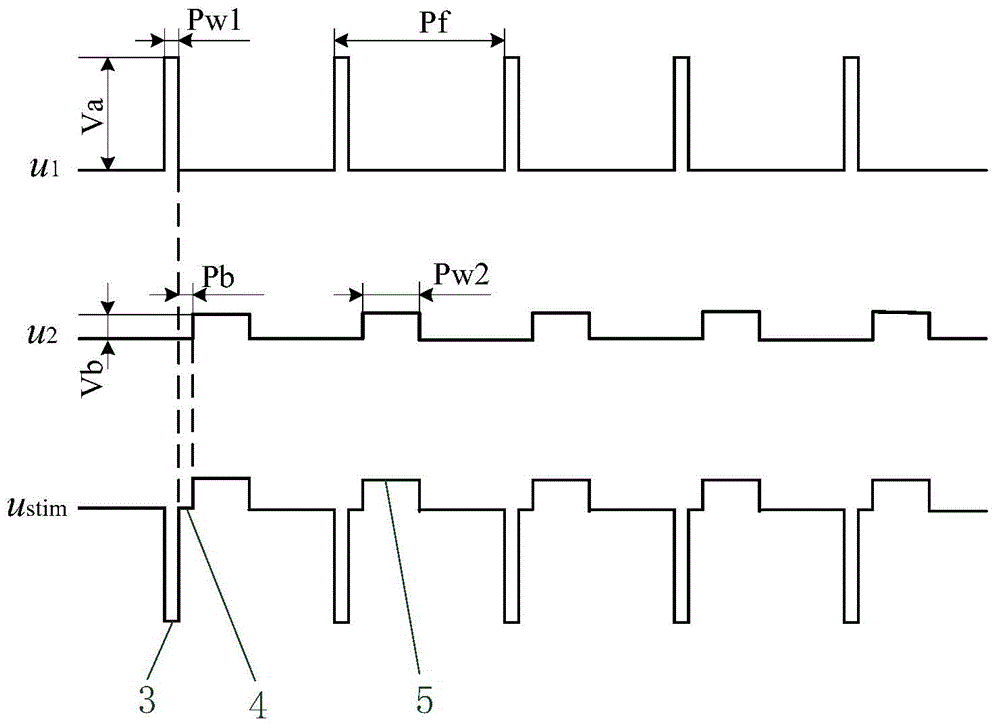
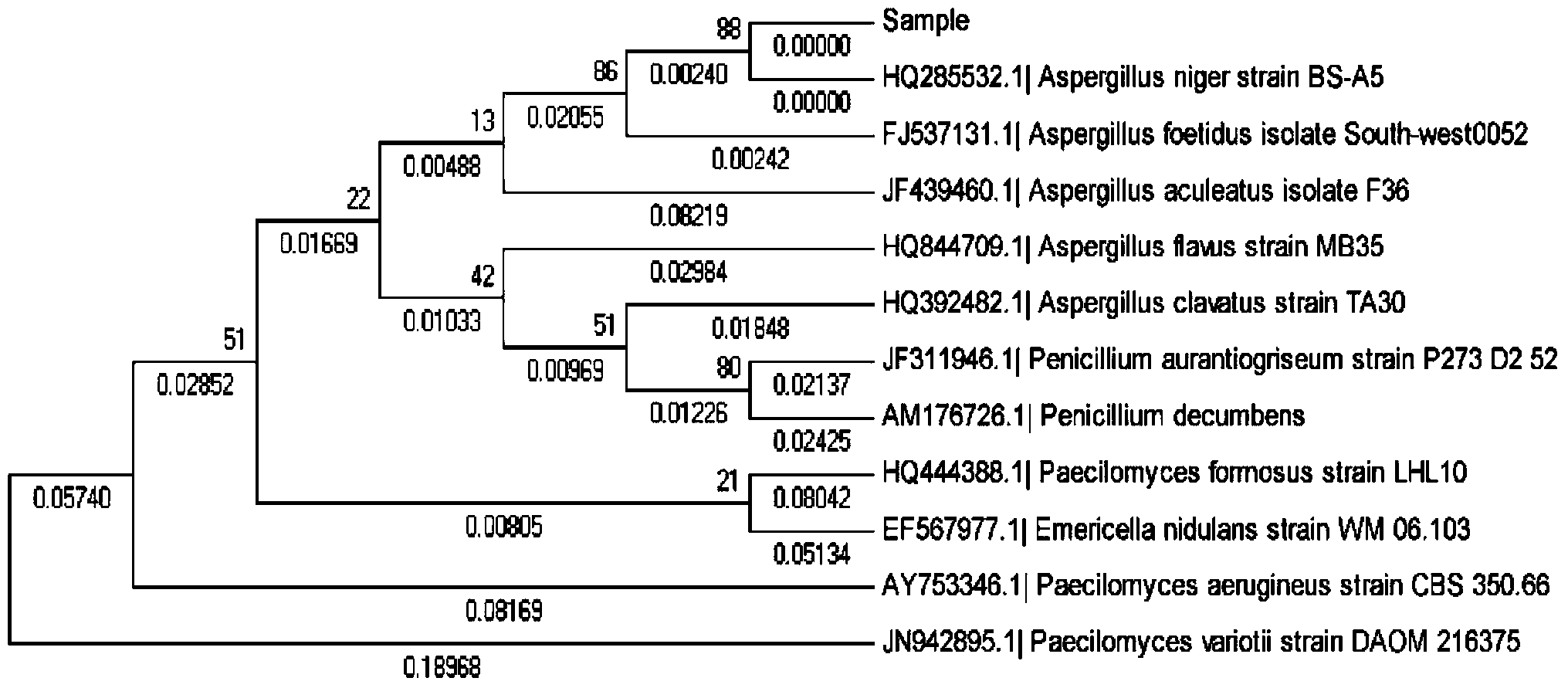
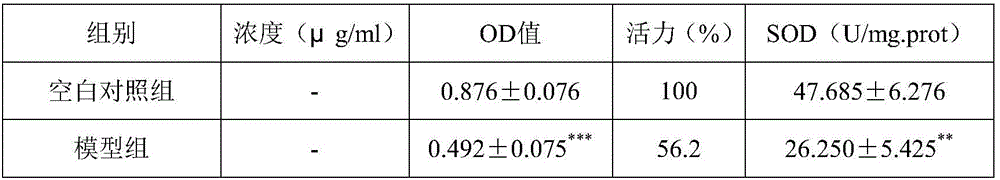
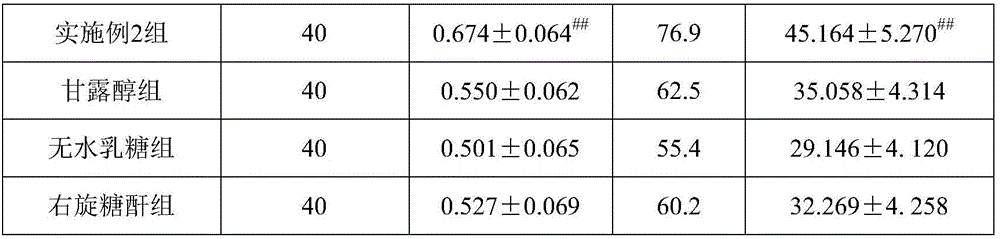
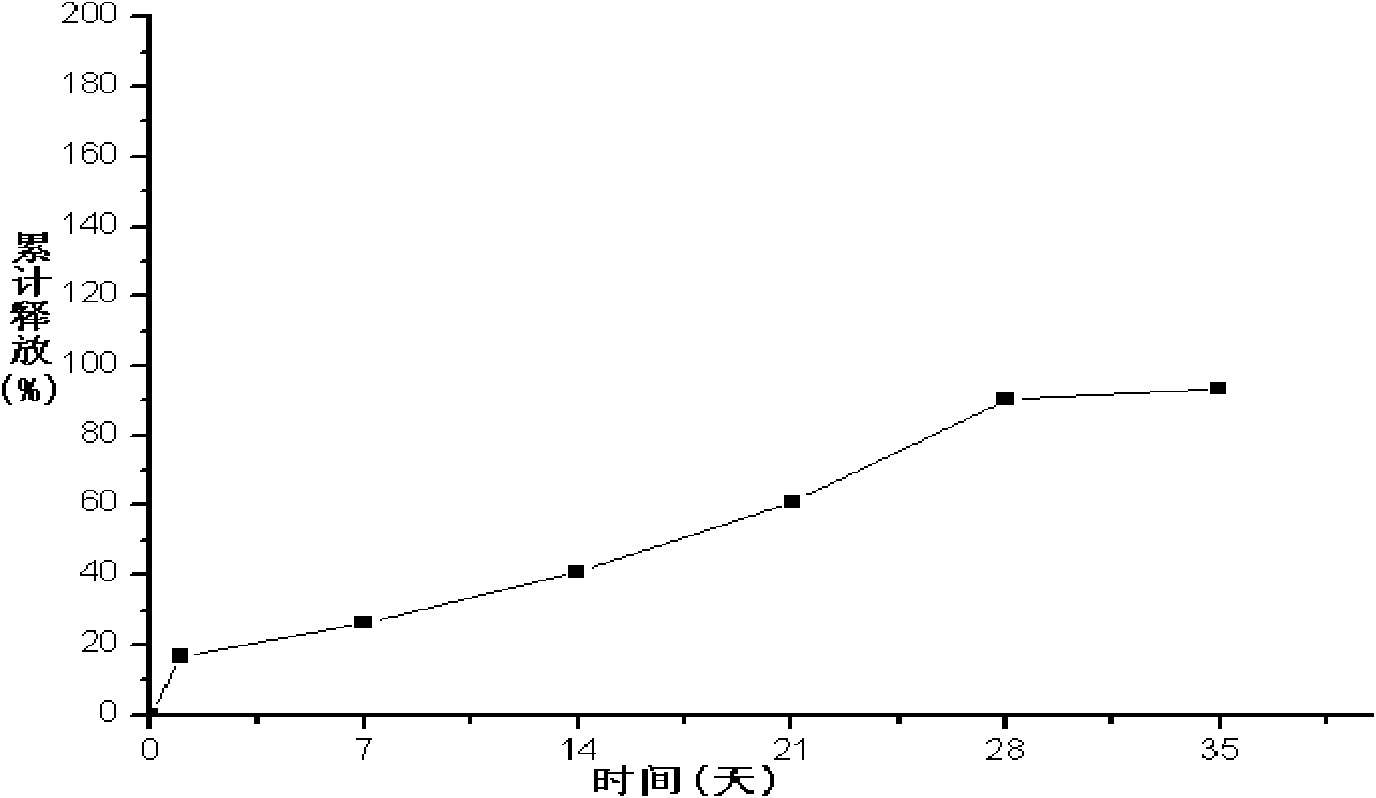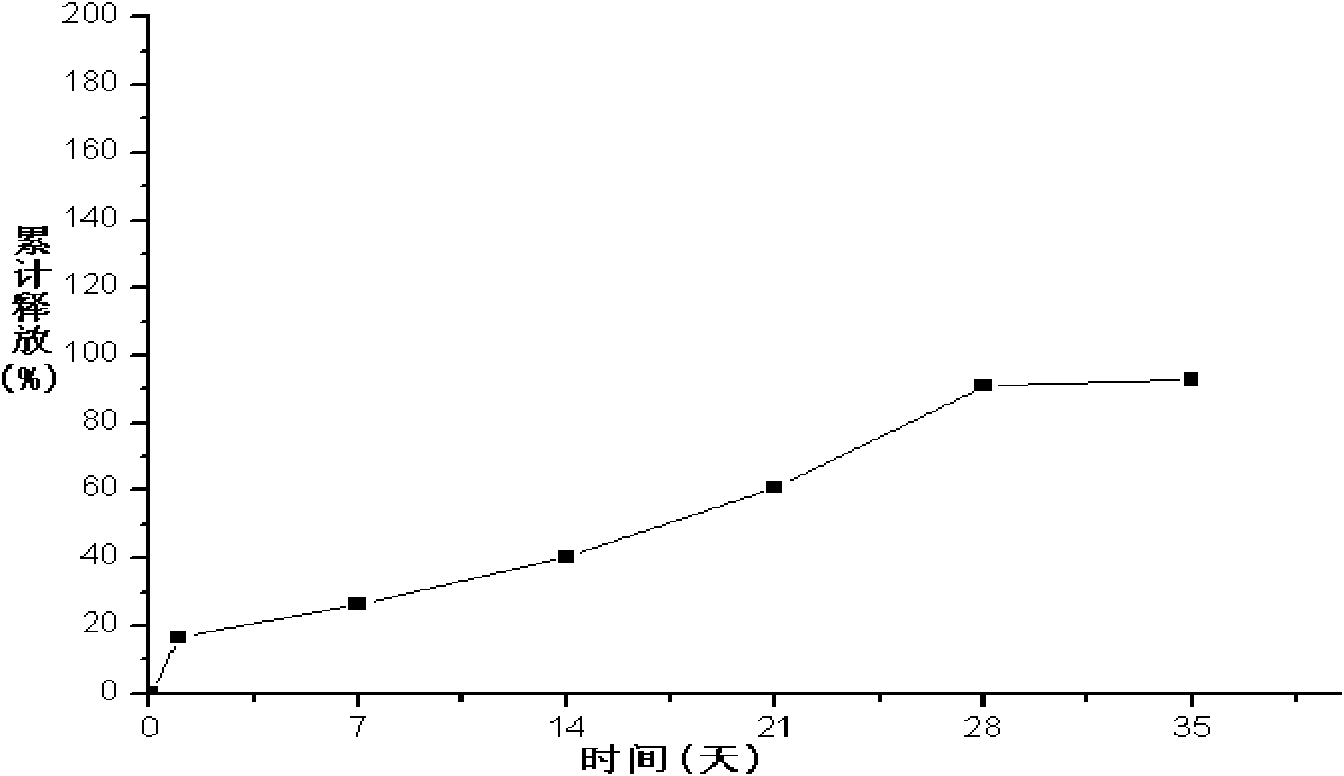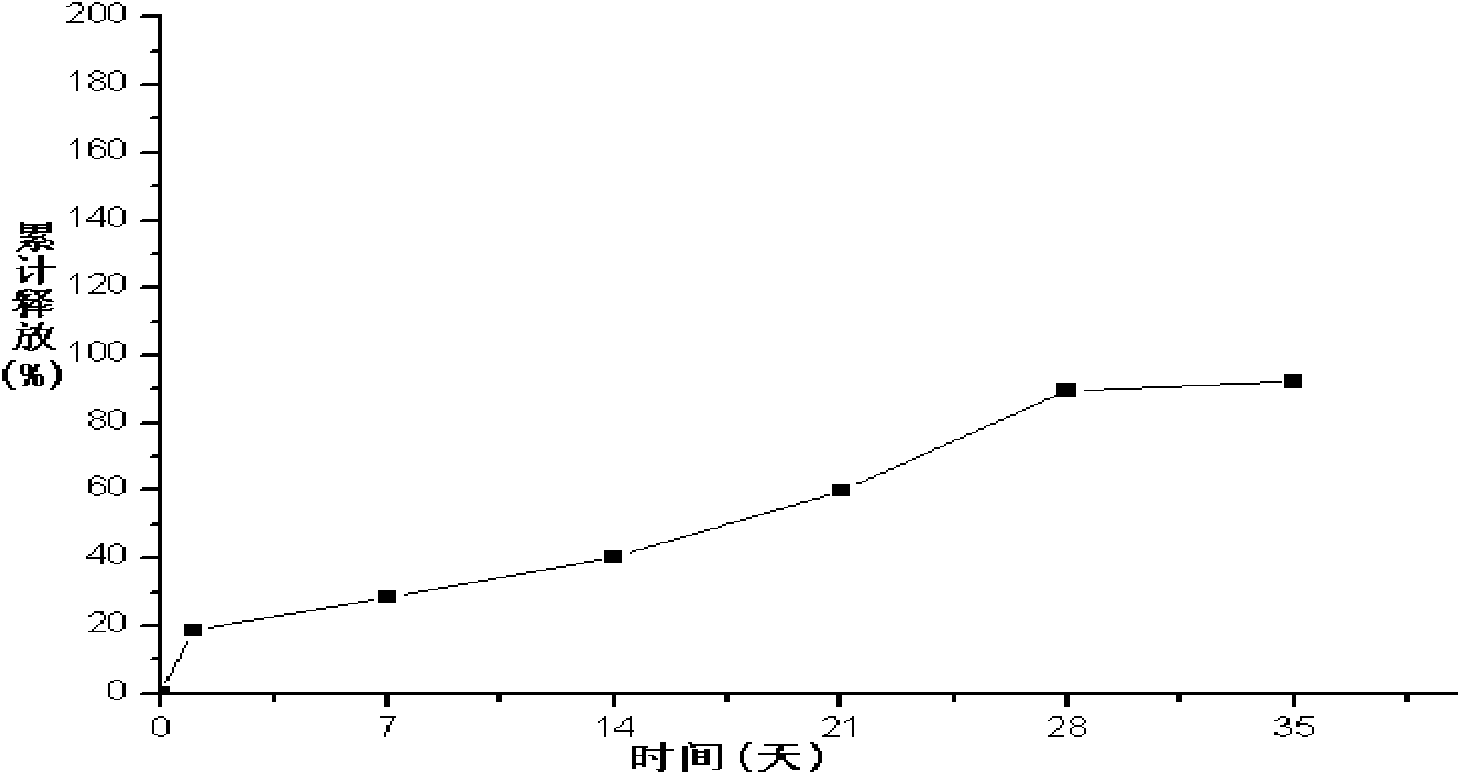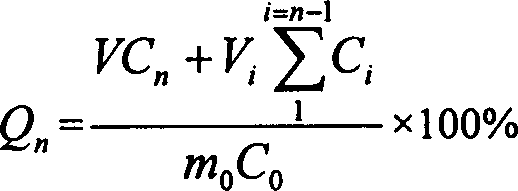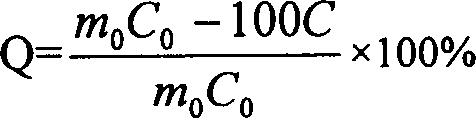Patents
Literature
2666 results about "Animals experiments" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
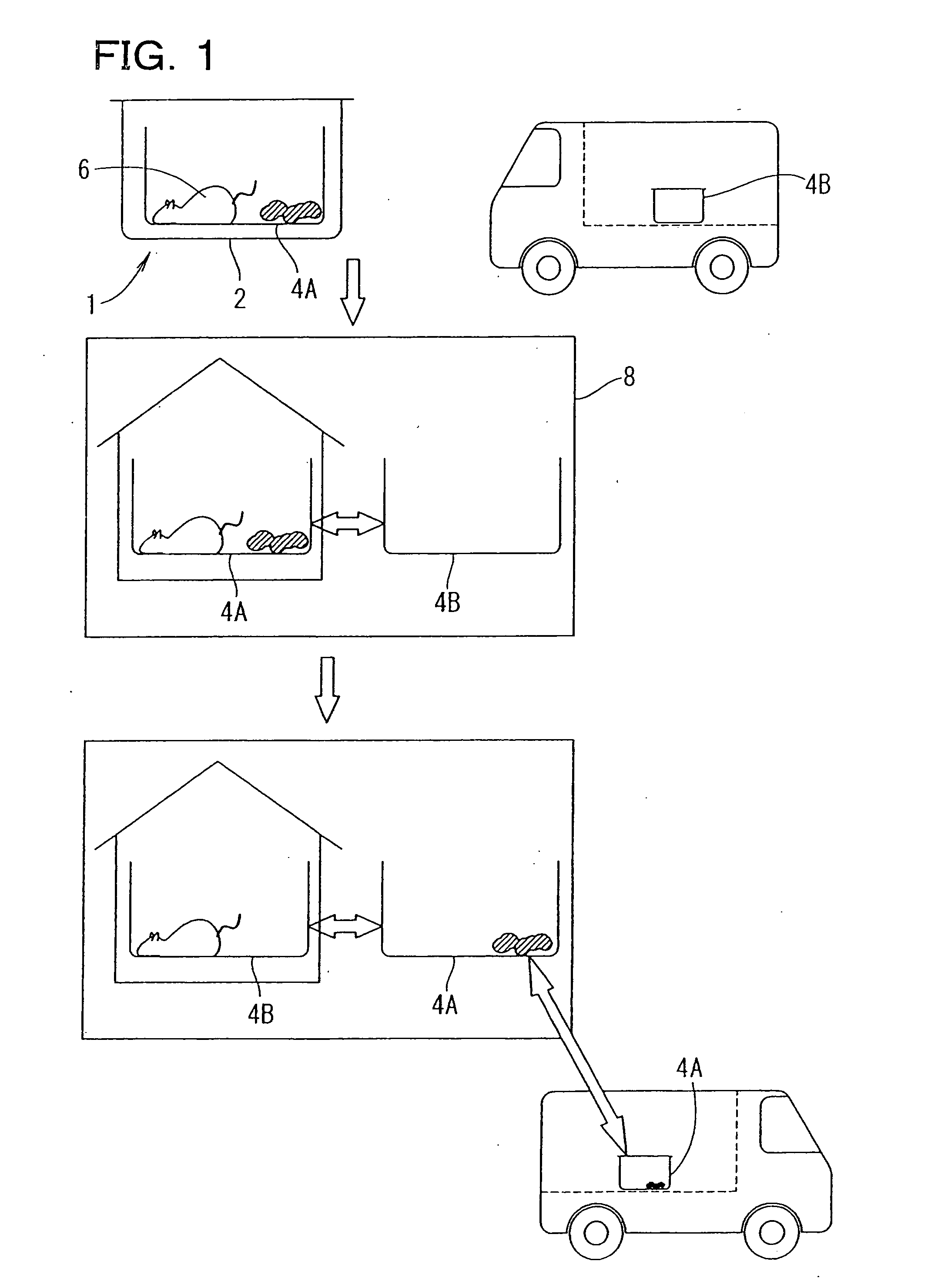
Animal breeding system and utilization of the system
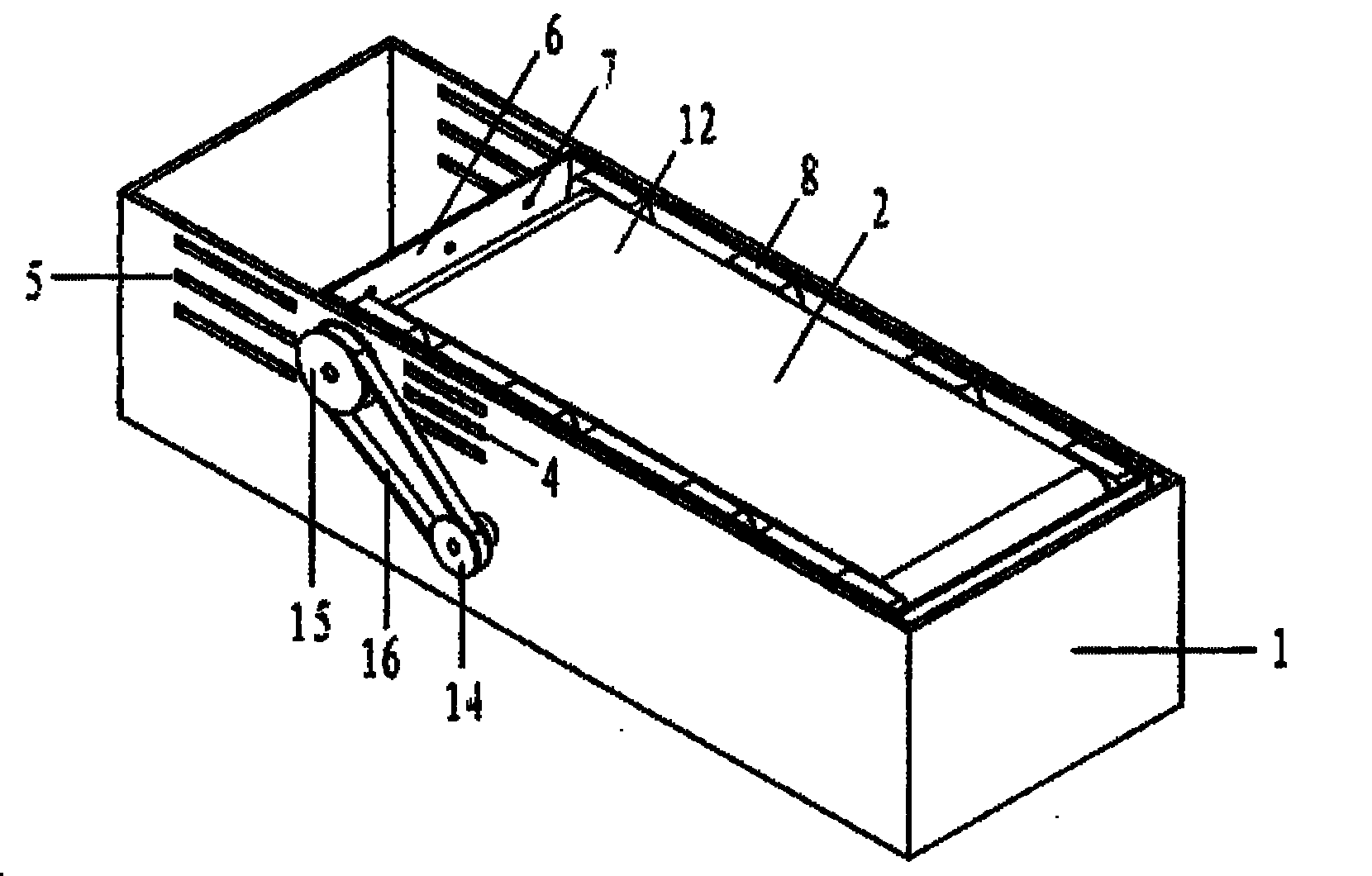
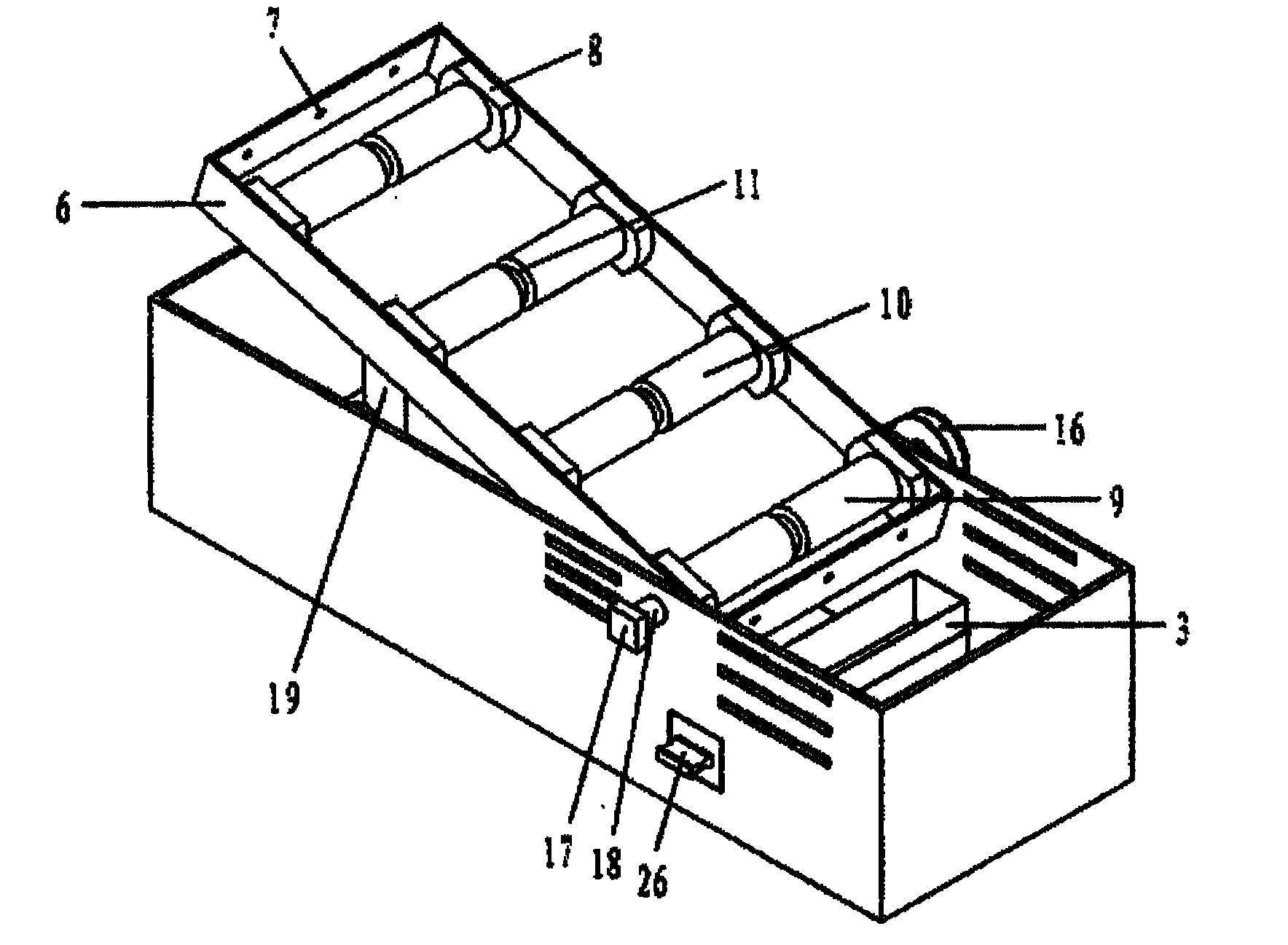
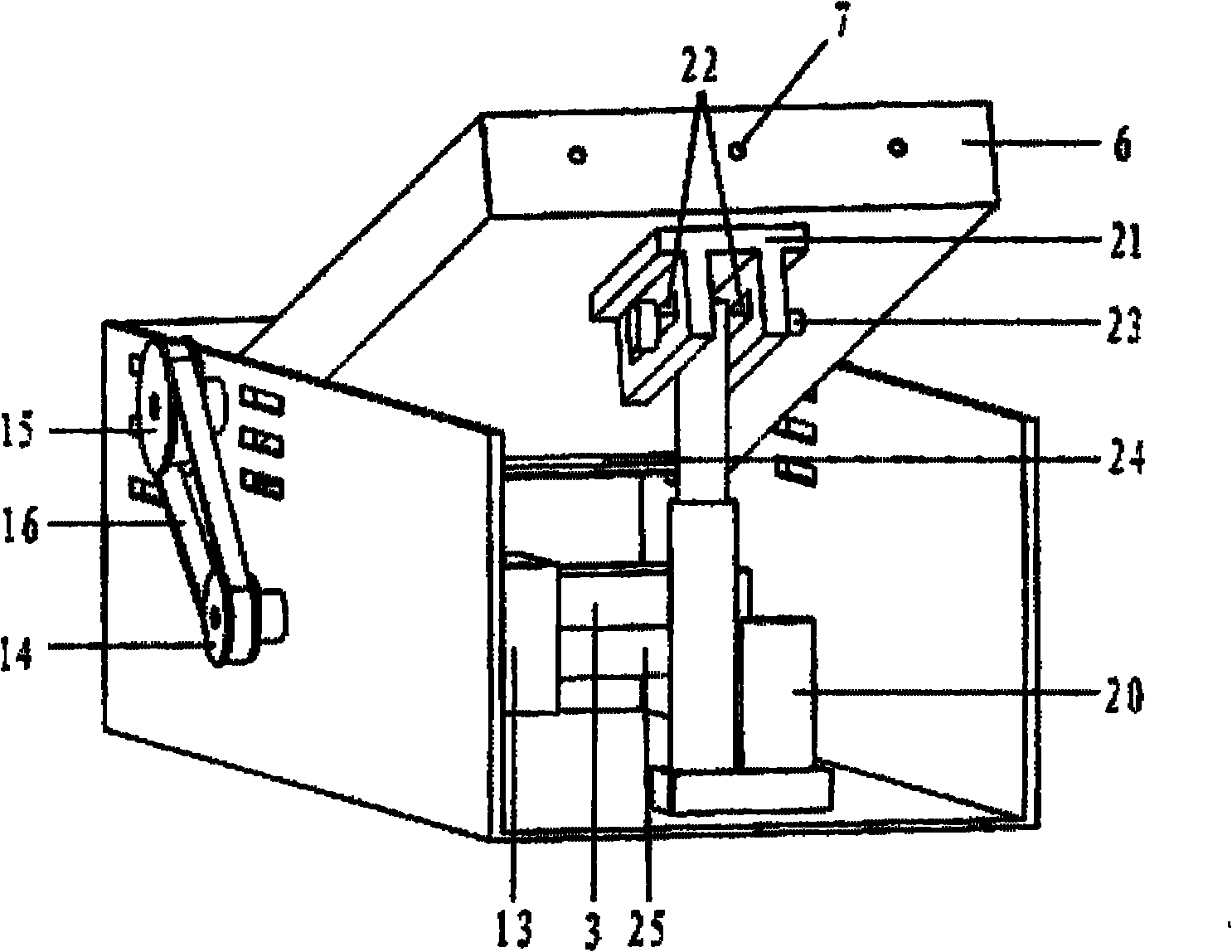
The present invention has objectives of providing a low-cost, easy-to-handle individual isolation animal breeding unit and a system for using the same, significantly reducing costs of facilities and maintenance for animal experiments and saving on labor, and providing a new animal transportation form (breeding cage having a transportation function). For achieving the objectives, the present invention provides a system for maintaining an animal breeding environment, comprising A) an animal breeding unit capable of comprising an internal tray having a sufficient space for breeding an animal of interest, comprising an external tray capable of accommodating the internal tray, and capable of keeping a predetermined cleanliness degree; B) means for providing the internal tray having the predetermined cleanliness degree; C) an internal tray exchange unit capable of accommodating the animal breeding unit and exchanging the internal tray while keeping the predetermined cleanliness degree; and D) means for recovering the exchanged internal tray.
Owner:OSAKA UNIV
Immobilized and activity-stabilized complexes of LHRH antagonists and processes for their preparation
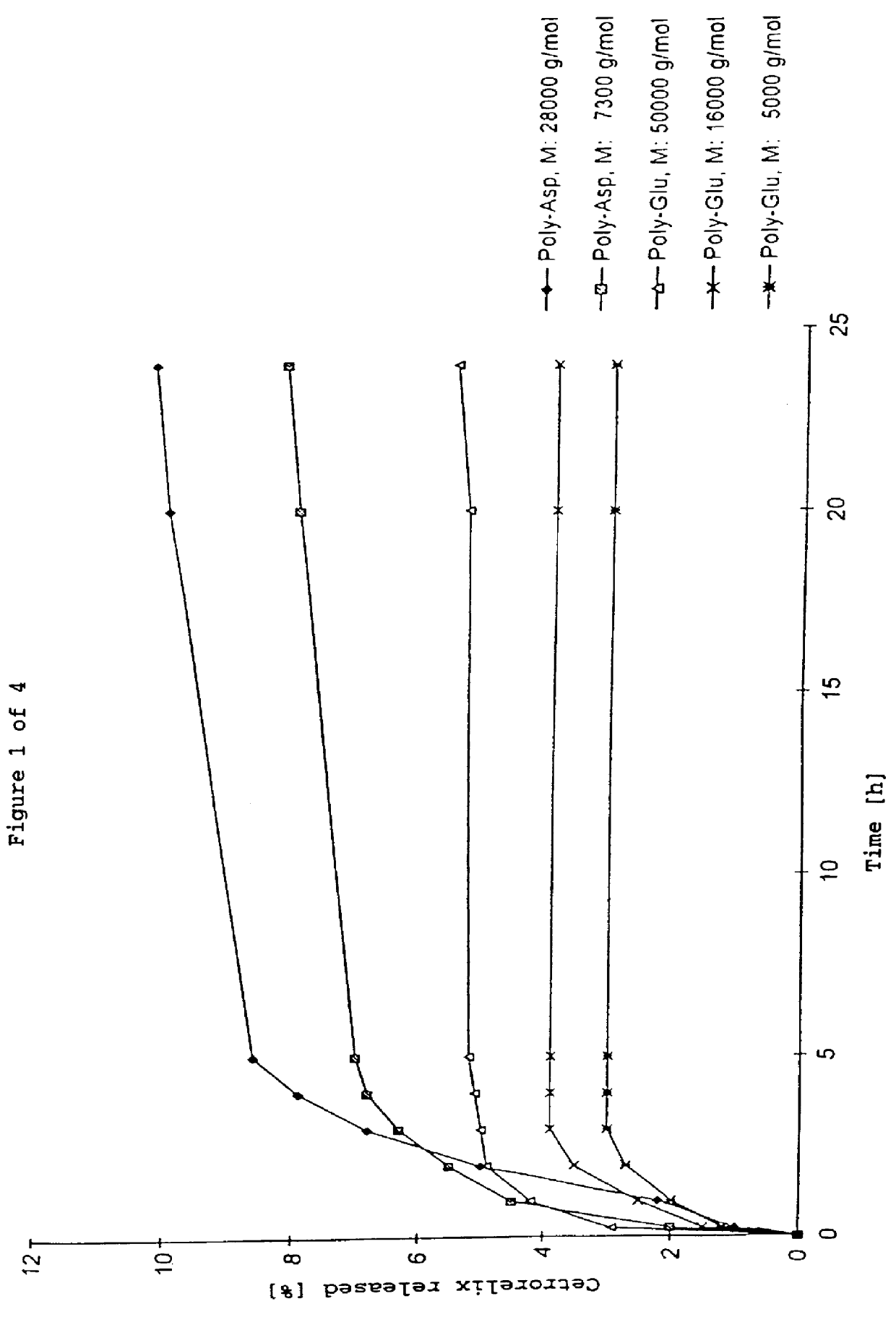
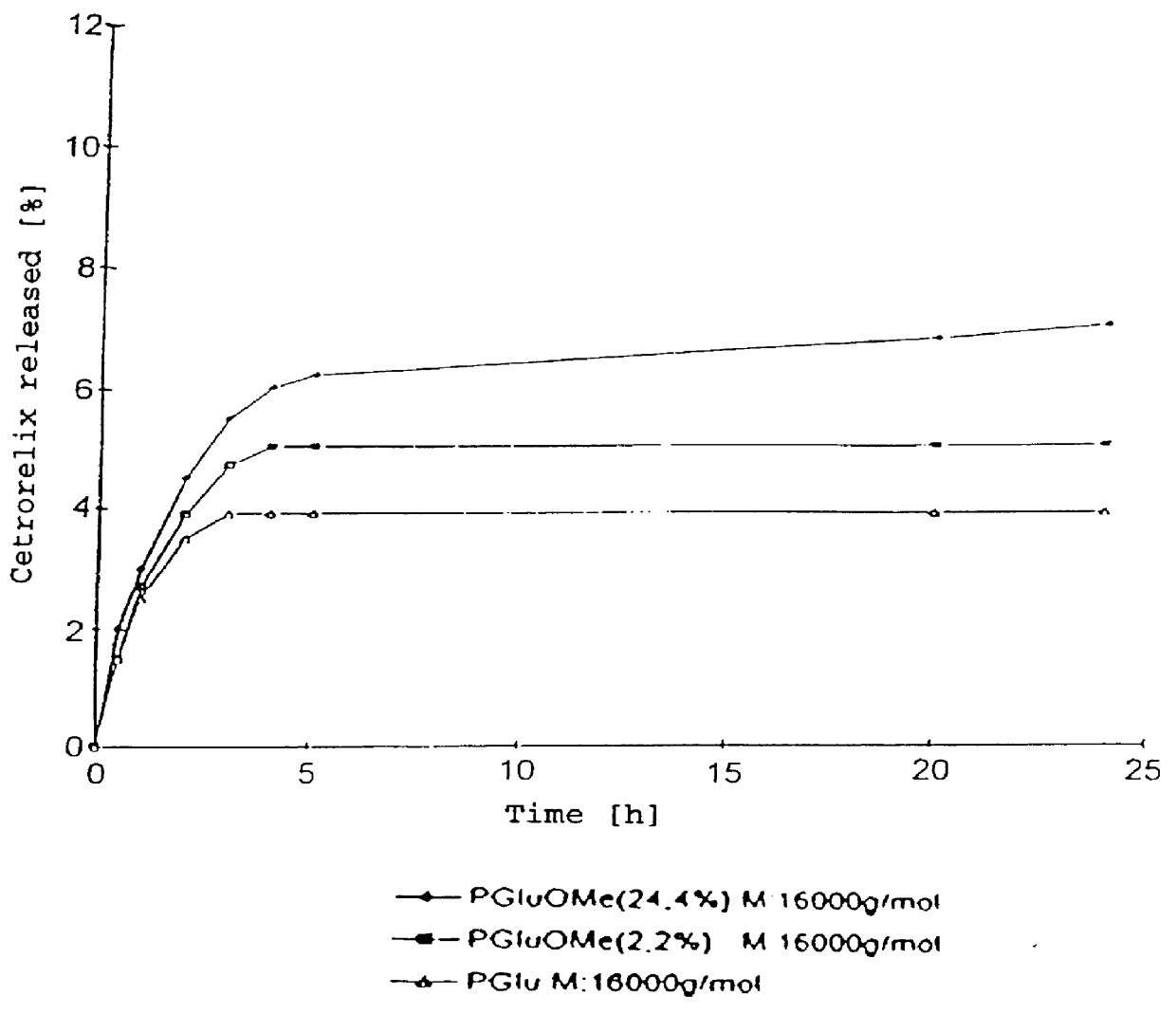
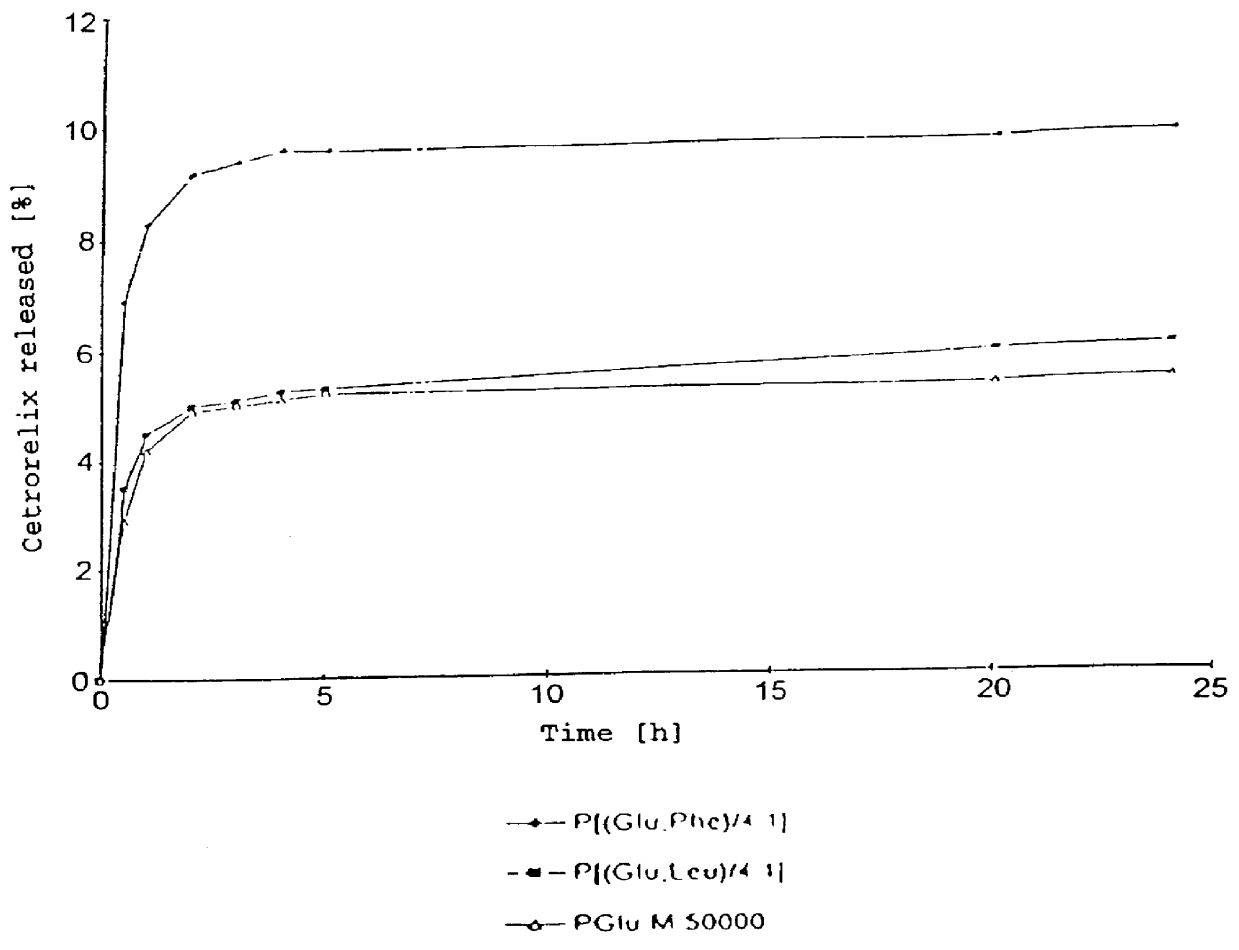
In this invention, a release-delaying system is to be developed for LHRH antagonists, in particular for cetrorelix, which allows the active compound to be released in a controlled manner over several weeks by complexation with suitable biophilic carriers. The acidic polyamino acids polyglutamic acid and polyaspartic acid were selected for complexation with cetrorelix. The cetrorelix polyamino acid complexes are prepared from aqueous solutions by combination of the solutions and precipitation of the complexes, which are subsequently centrifuged off and dried over P2O5 in vacuo. If complexes having a defined composition are to be obtained, lyophilization proves to be a suitable method. The cetrorelix-carboxylic acid complexes were also prepared from the aqueous solutions. In the random liberation system, the acidic polyamino acids poly-Glu and poly-Asp showed good release-delaying properties as a function of the hydrophobicity and the molecular mass of the polyamino acid. In animal experiments, it was possible to confirm the activity of the cetrorelix-polyamino acid complexes as a depot system in principle. It is thus possible by complexation of cetrorelix with polyamino acids to achieve testosterone suppression in male rats over 600 hours. The release of active compound here can be controlled by the nature and the molecular mass of the polymers.
Owner:ZENTARIS GMBH
Process of extracting 1-deoxy nojirimycin
InactiveCN101020655AGood hypoglycemic effectGuaranteed to bring inOrganic chemistryMetabolism disorderDiabetes modelYerba santa extract
The present invention is process of extracting 1-deoxy nojirimycin (DNJ) from mulberry leaf. Mulberry leaf extract is treated with inorganic acid, cooled to eliminate impurity and column chromatographically separated to obtain DNJ. Animal experiment of mouse shows that the mulberry leaf extract has obvious blood sugar reducing effect, so that the present invention may be used in preparing blood sugar reducing health product. The present invention has low preparation cost, and is suitable for industrial production.
Owner:ZHEJIANG UNIV
System for automating animal testing protocols
ActiveUS20120180731A1Improve throughputEasy to completeAnimal housingOther apparatusSmall animalAnimal testing
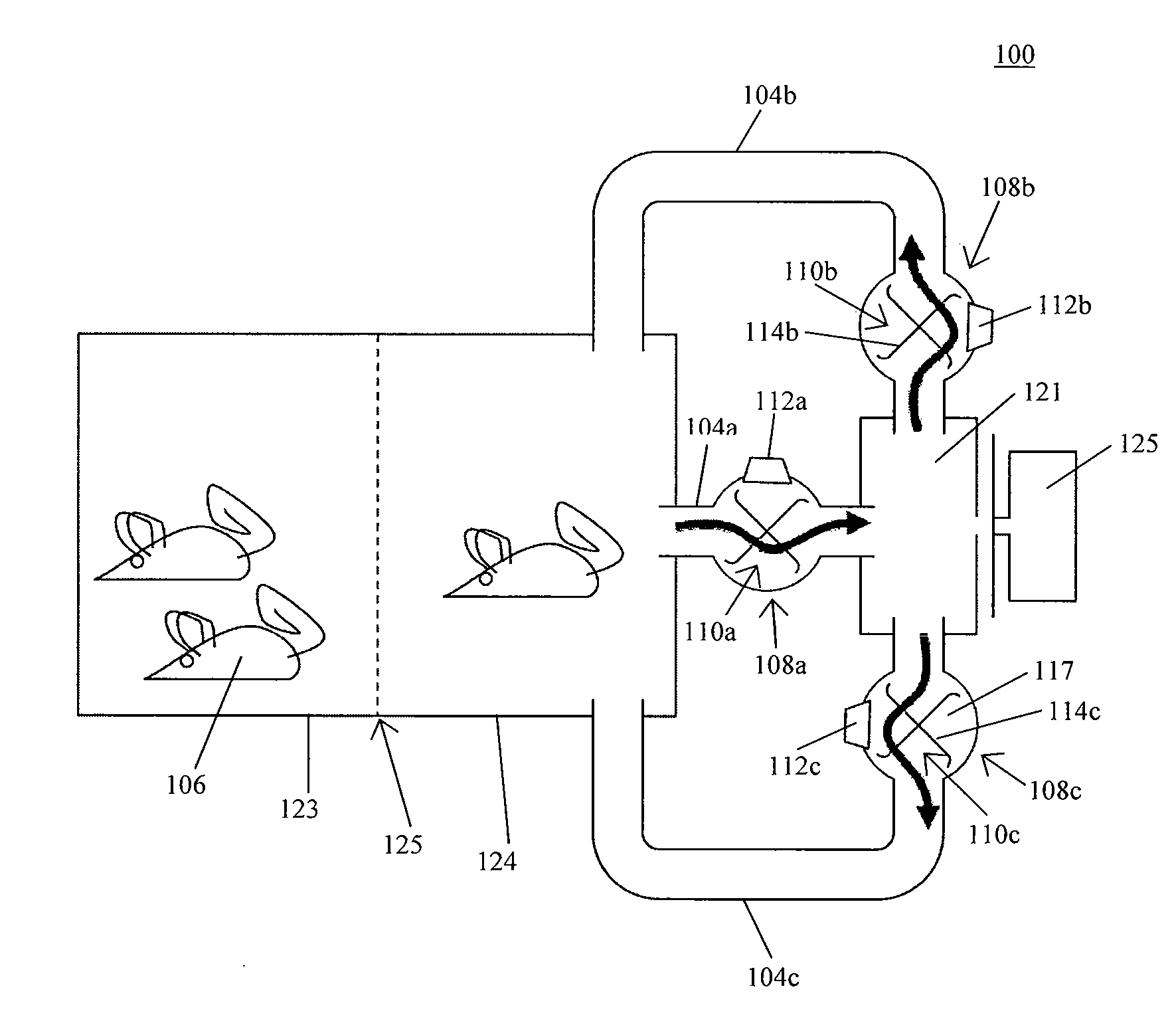
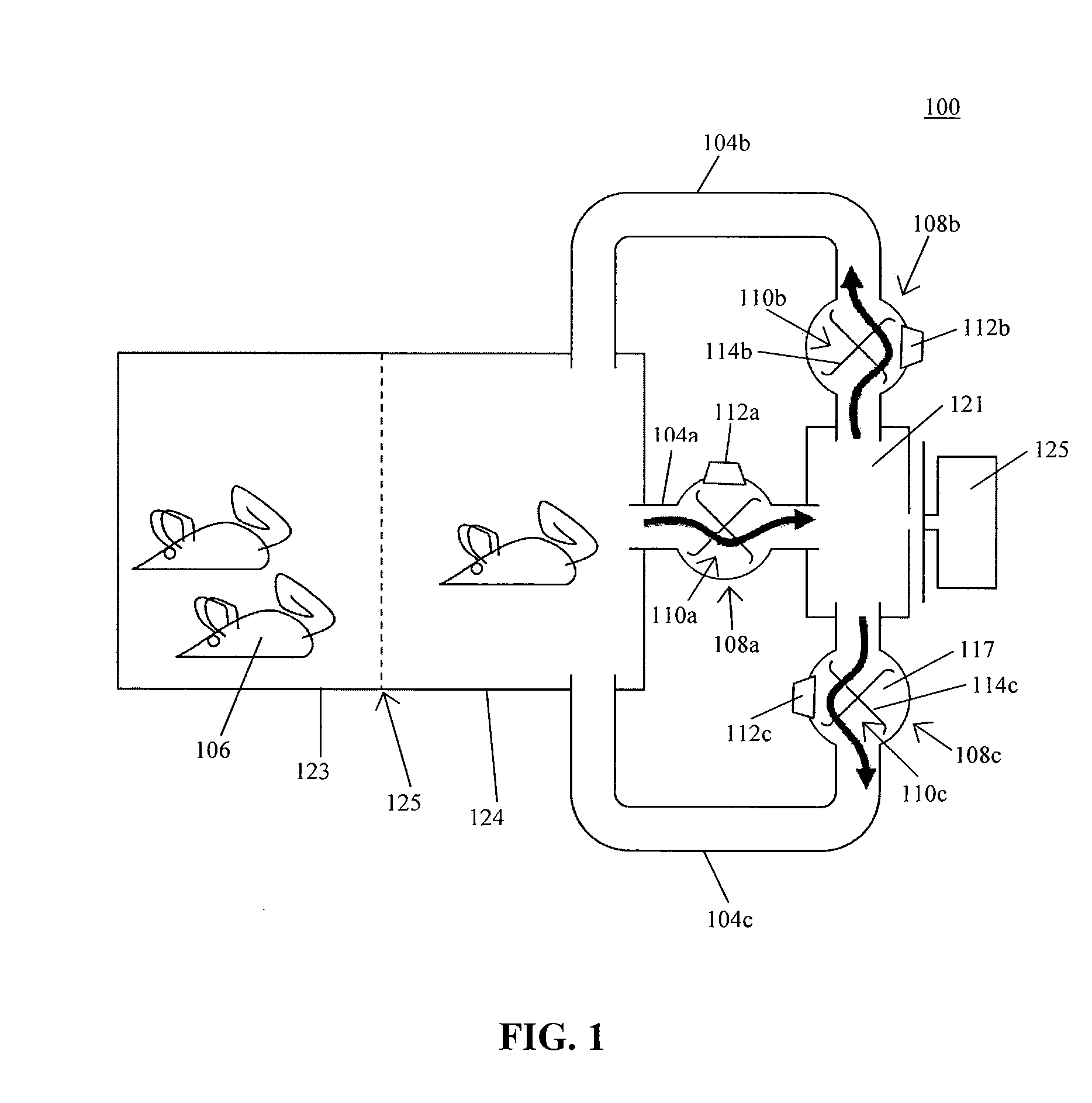
In one aspect, the present invention provides a housing system for conducting high throughput animal experiments. The housing system includes a home cage, at least one rotatable turnstile enclosed by housing to form two or more isolation chambers, a means for animal identification, and one or more action stations functionally coupled to one or more isolation chambers. The turnstile includes a plurality of one or more separation members rotatable about a vertical axis, each isolation chamber bounded by one or more separation members. The action stations contain one or more devices facilitating completion of at least one animal-directed or experimentor-initiated action. In a preferred embodiment, the home cage is sufficiently sized to house a plurality of small animals, such as mice. Tunnel passageways may be connected to the home cage, including one or more tunnel passageways containing a rotatable turnstile. Additional embodiments include rotatable turnstiles, rotatable turnstile assemblies, and methods of conducting high throughput animal experiments using the devices and systems described herein.
Owner:PURDUE RES FOUND INC
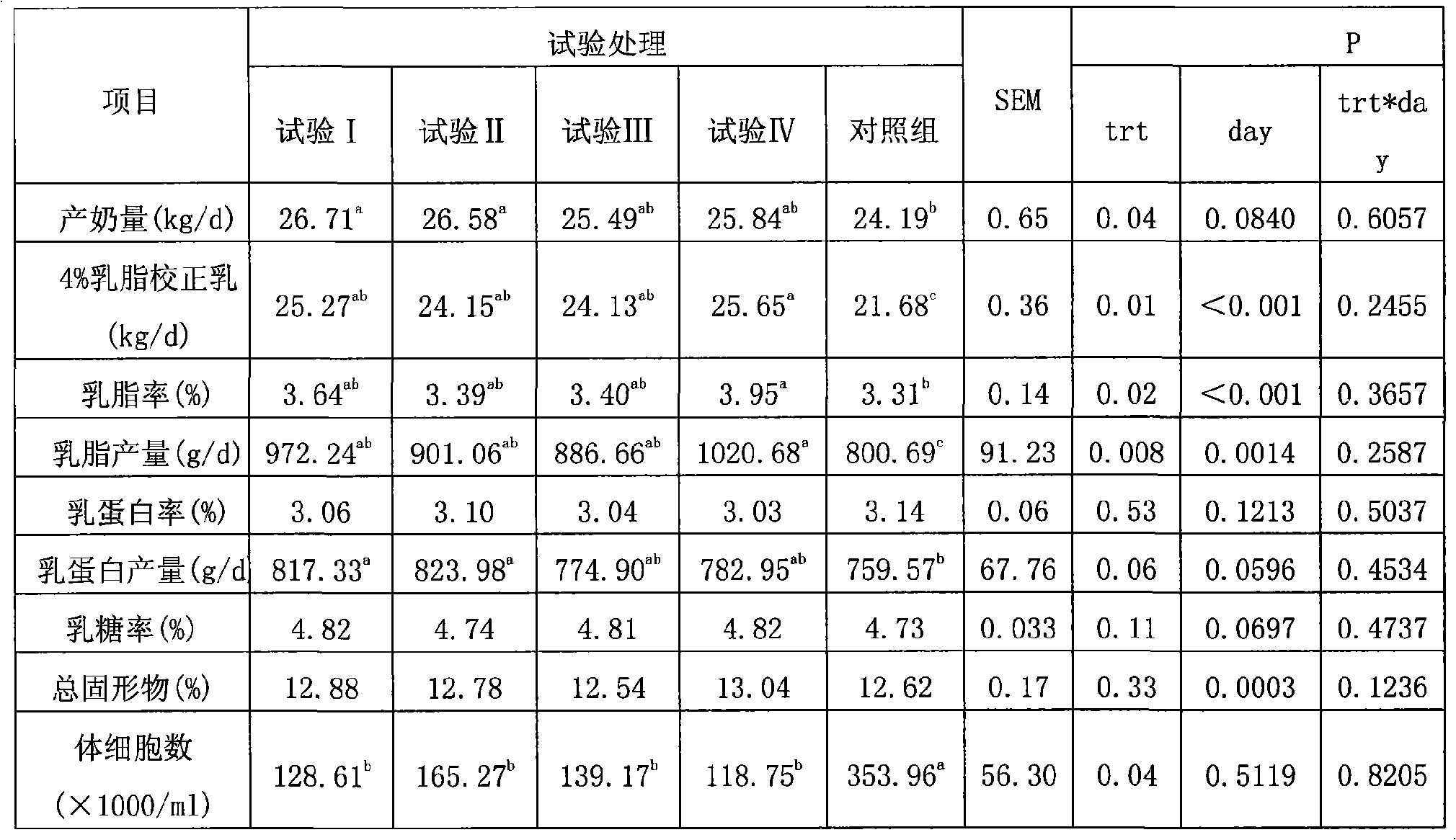
Composite microorganism additive agent for milk cattle feed stuff and method of preparing the same
InactiveCN101253934AReduce somatic cell countIncrease milk productionFungiBacteriaDiseaseBiotechnology
The invention discloses a compound microorganism additive for milk cow feed. The microorganism additive consists of fermentation product mixture of natto bacillus subtilis, enterococcus faecium and saccharomyces cerevisiae, wherein the total number of bacteria is more than 10<9>cfu / g. Long-term repeating animal experiments show that three microorganisms in the compound microorganism additive can develop synergy, thereby significantly improving the milk yield of the milk cow, fat percentage in the milk and milk protein yield, significantly reducing the somatic number of the milk cow in lactation period and the disease probability of mastitis and subclinical mastitis, and significantly enhancing the autoimmunity of the milk cow organism. The compound microorganism additive has the advantages of low cost, significant effect and good application and market prospects.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
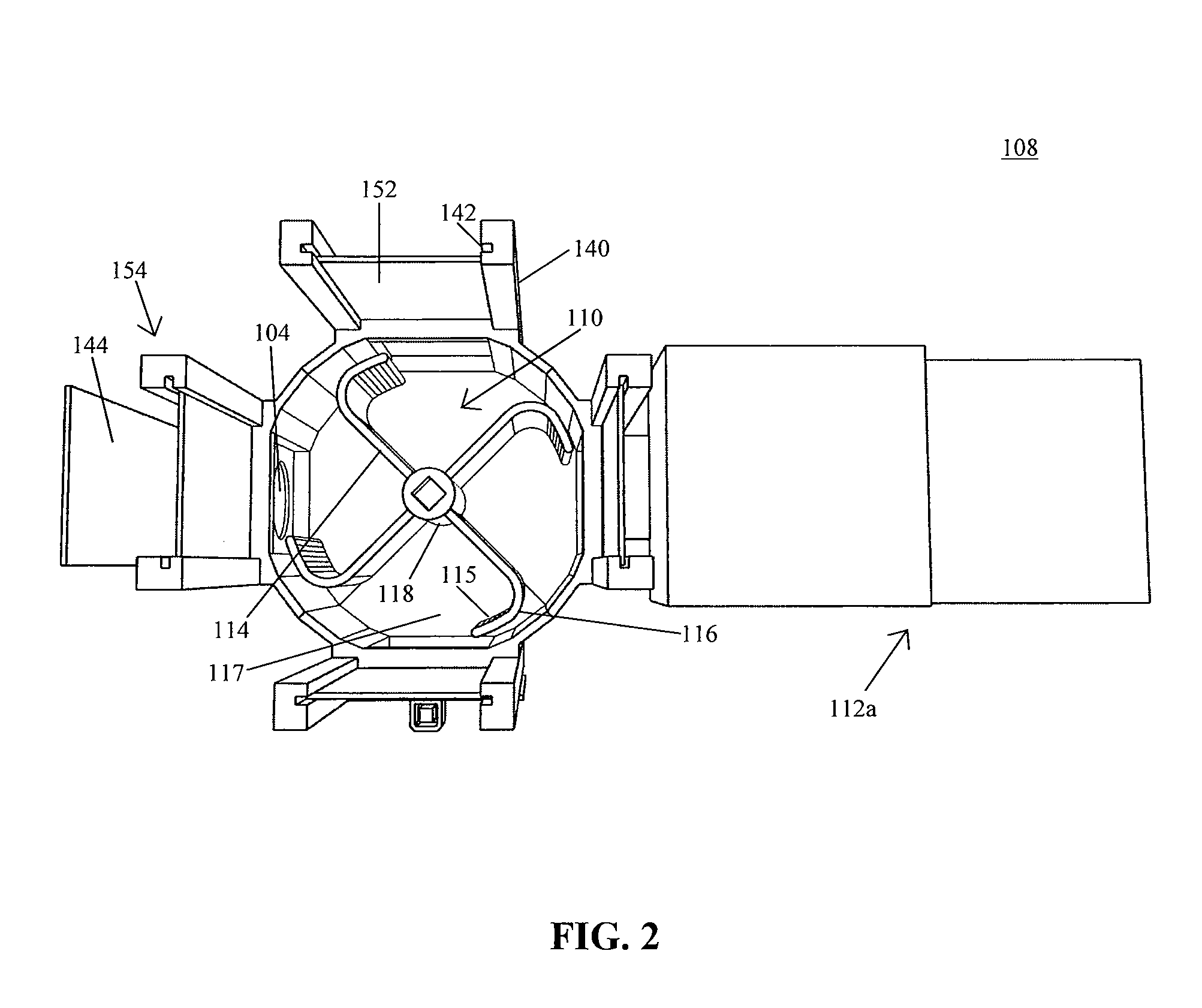
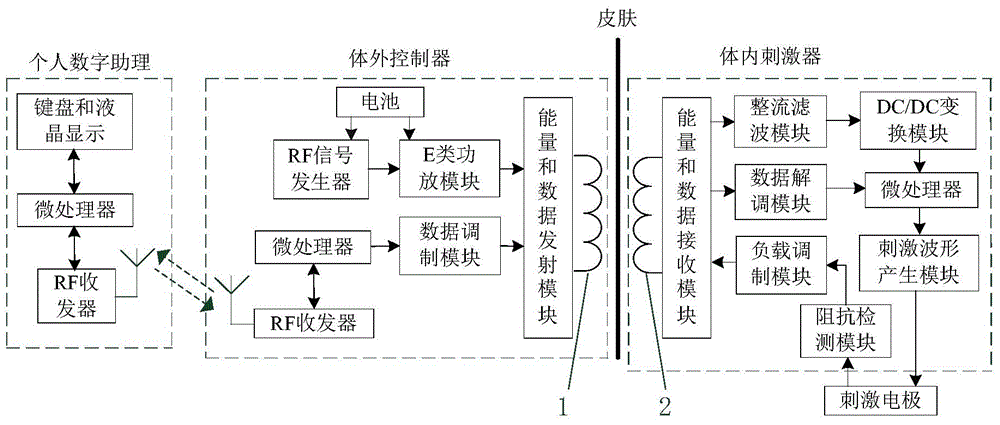
Implantable nerve electrical stimulation device and system
ActiveCN104096313ASimultaneous transmissionAvoid the Risk of Surgical ReplacementTransmission systemsArtificial respirationDiseaseNervous system
The invention discloses an implantable nerve electrical stimulation device which comprises a personal digital assistant (PDA), an in vitro controller, an in vivo stimulator and a stimulation electrode. A user uses the PDA to record the information of an experiment animal, and the working condition of the in vivo stimulator and the stimulation electrode. Stimulation parameters are programmed and are transmitted to the in vitro controller through bidirectional wireless radio frequency communication. Data and energy percutaneous wireless transmission is carried out between the in vitro controller and the in vivo stimulator through in vitro and in vivo coil coupling. The in vivo stimulator is sealed by a biology compatible silicone material, produces a stimulation pulse with specific parameters, and outputs the stimulation pulse to the stimulation electrode which is implanted into an epidural space through operation, so as to carry out spinal cord nerve electrical stimulation. The stimulation electrode is a multi-contact electrode based on a flexible circuit board technology. Insulation package is carried out on a number of gold-plated electrode contacts through polyimide. The device and the system, which are provided by the invention, are used for analgesia and promoting the mechanism study of movement function recovery after spinal cord injury, and can be used for the animal experiment study of the electrical stimulation therapy of Parkinson and other neurological diseases.
Owner:HUAZHONG UNIV OF SCI & TECH
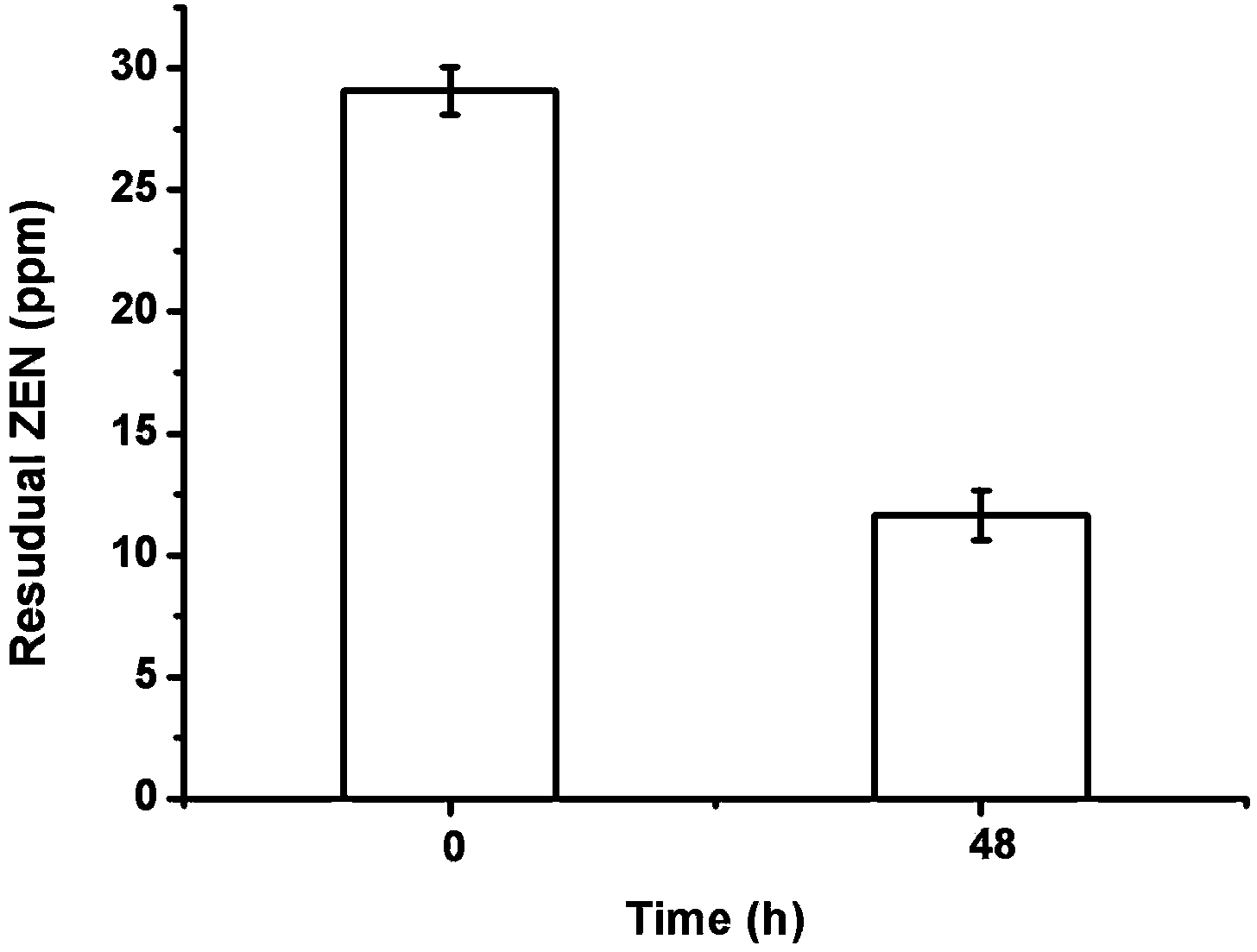
Food-grade aspergillus niger strain and application of strain in zearalenone degradation
ActiveCN103937681AReduce contentPromote degradationFungiMicroorganism based processesToxinAspergillus niger
The invention discloses a food-grade aspergillus niger strain FS-Z1 which is separated out from sauce grains and capable of preventing and treating zearalenone toxin. The collection number of the food-grade aspergillus niger strain FS-Z1 is CCTCC NO: M 2013703. The strain has excellent degradation effect on the zearalenone and the degradation rate reaches 89.56%. Besides, animal experiments in rats verify that the zearalenone in the corn steep liquor degraded by using an aspergillus niger fermentation liquor has no toxicity.
Owner:JIANGNAN UNIV
Liraglutide long-acting microsphere injection and preparation method thereof
ActiveCN102085355AReduce the burden of treatmentImprove Medication AdherencePowder deliveryPeptide/protein ingredientsTreatment burdenMicrosphere
The invention provides a long-acting microsphere injection as an antidiabetic medicament, the long-acting microsphere injection comprises liraglutide, PLGA (poly(lactic-co-glycolic acid)), excipients and a surfactant, and the invention simultaneously relates to a preparation method of the injection. Liraglutide long-acting sustained-release microspheres provided by the invention are designed to perform subcutaneous injection once every 28 days, thereby greatly reducing treatment burden on a patient, improving medication compliance and reducing treatment cost; simultaneously, results of in vitro release studies, animal experiments and the like prove that the obtained sustained-release microspheres can slowly release a medicament for a long time in vitro and in vivo.
Owner:蚌埠丰原涂山制药有限公司
Berbamine derivative and application of salt thereof
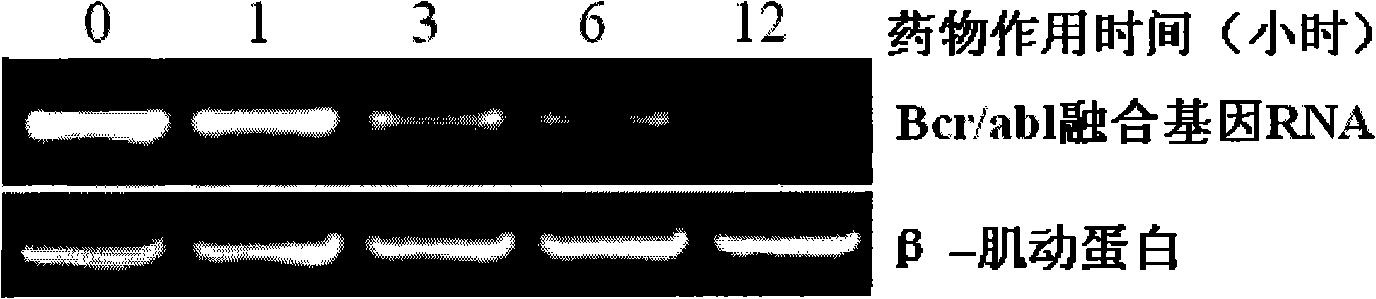
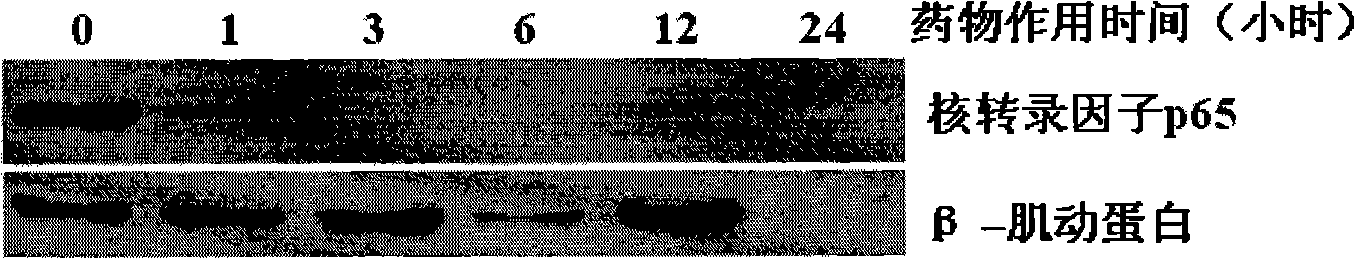
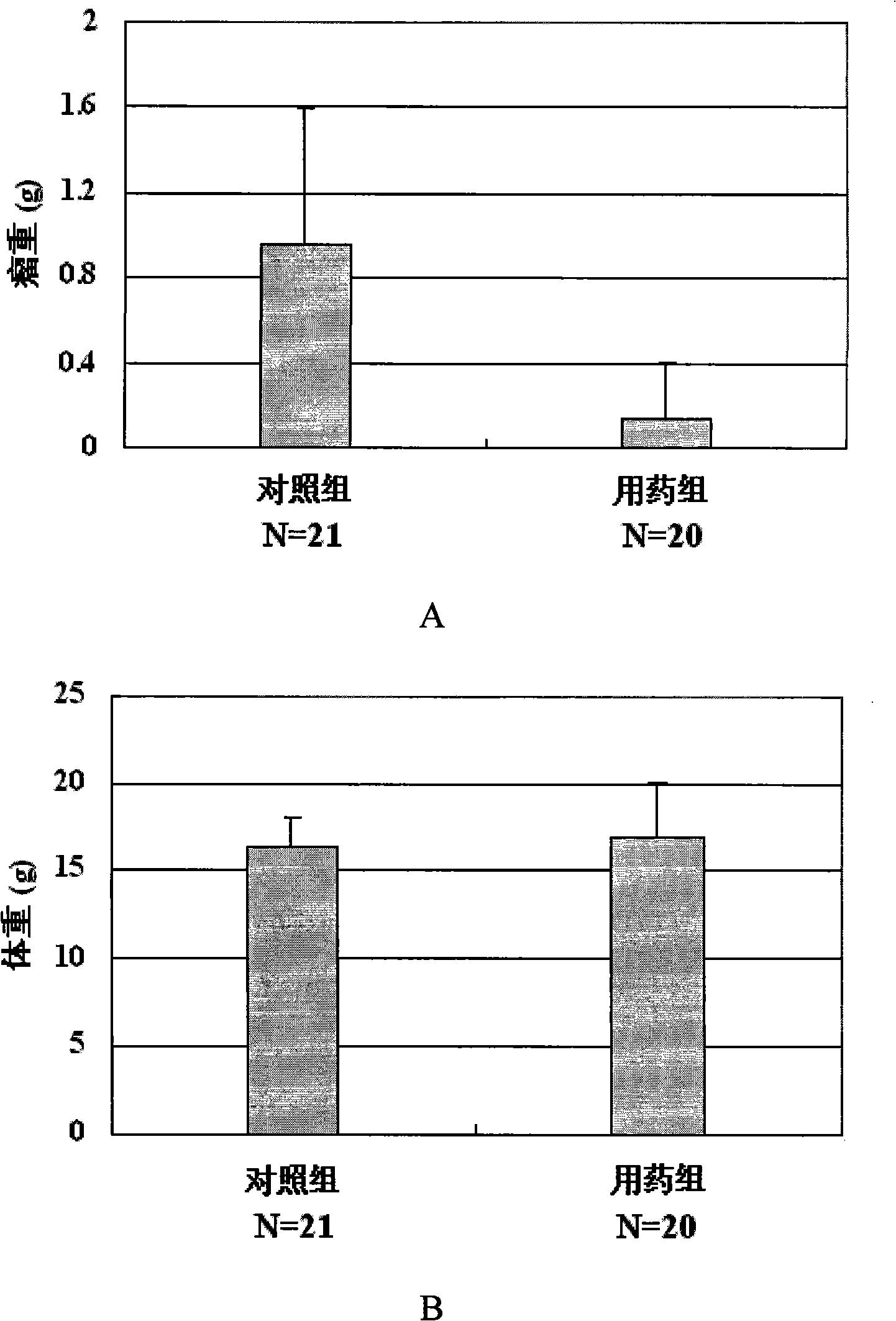
InactiveCN101273989AHigh antagonistic activityStrong against leukemiaOrganic active ingredientsAntineoplastic agentsDiseaseSide effect
The invention provides an application of a type of berbamine derivatives and salts thereof in the preparation of drugs for the treatment of tumors, which is mainly applied in the preparation of the drugs for the prevention and treatment of nuclear transcription factor NF-kBp65 activity-related diseases and BCR / ABL transcription activity-related diseases. The drugs are combined and prepared by the compounds of the invention and one or more pharmaceutically acceptable excipients. The preparation forms comprise solid preparations, semi-solid preparations or liquid preparations. The type of berbamine derivatives and the salts thereof provided by the invention have broader and stronger anti-leukemia and anti-solid-tumor activity, the tumors proved to be sensitive are leukemia, multiple myeloma, liver cancer, osteosarcoma and breast cancer; the toxicity and the side effects are lighter. An in vitro cell culture system and animal experiments confirm that the berbamine derivatives and the salts thereof have no significant toxicity or side effects to the growth of normal human hematopoietic cells and experimental animals under the anti-tumor dosage, which are superior to the commonly used chemotherapy drugs.
Owner:HANGZHOU BENSHENG PHARMA
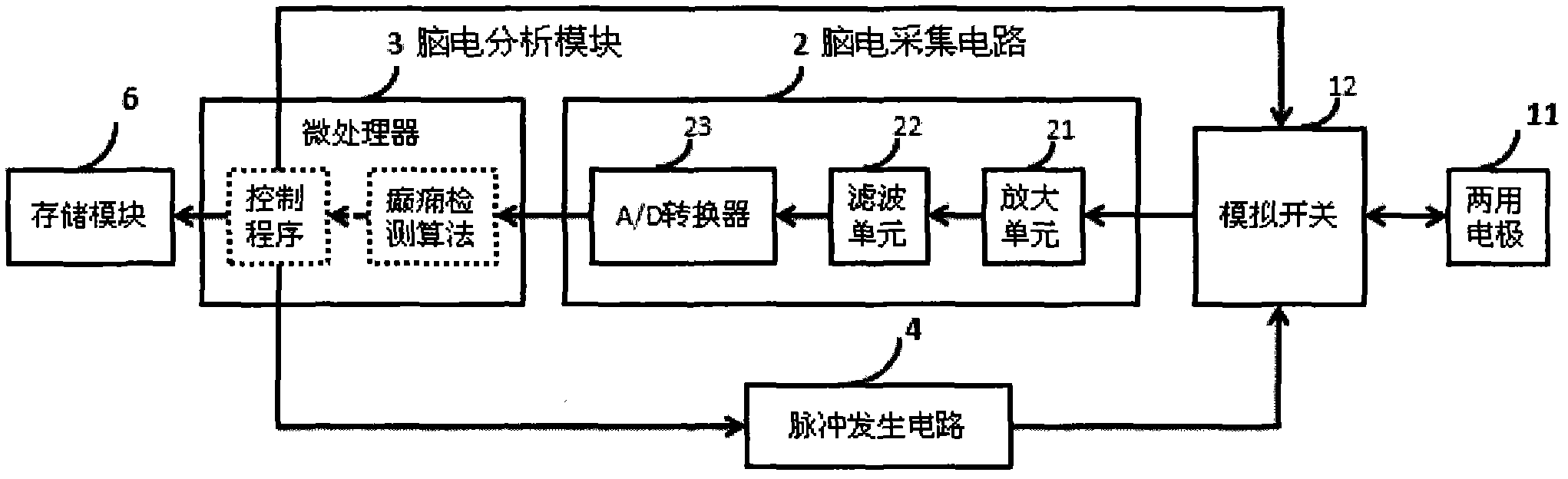
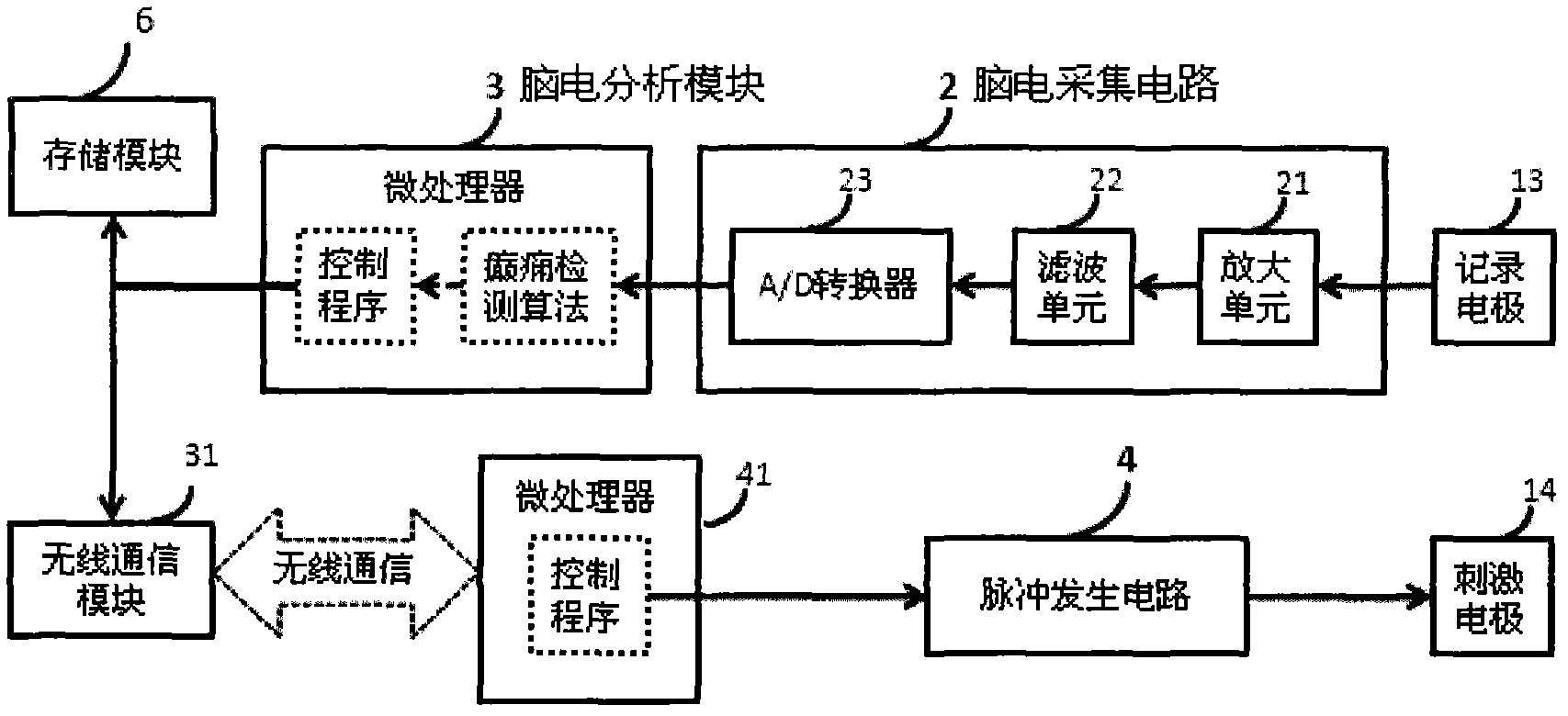
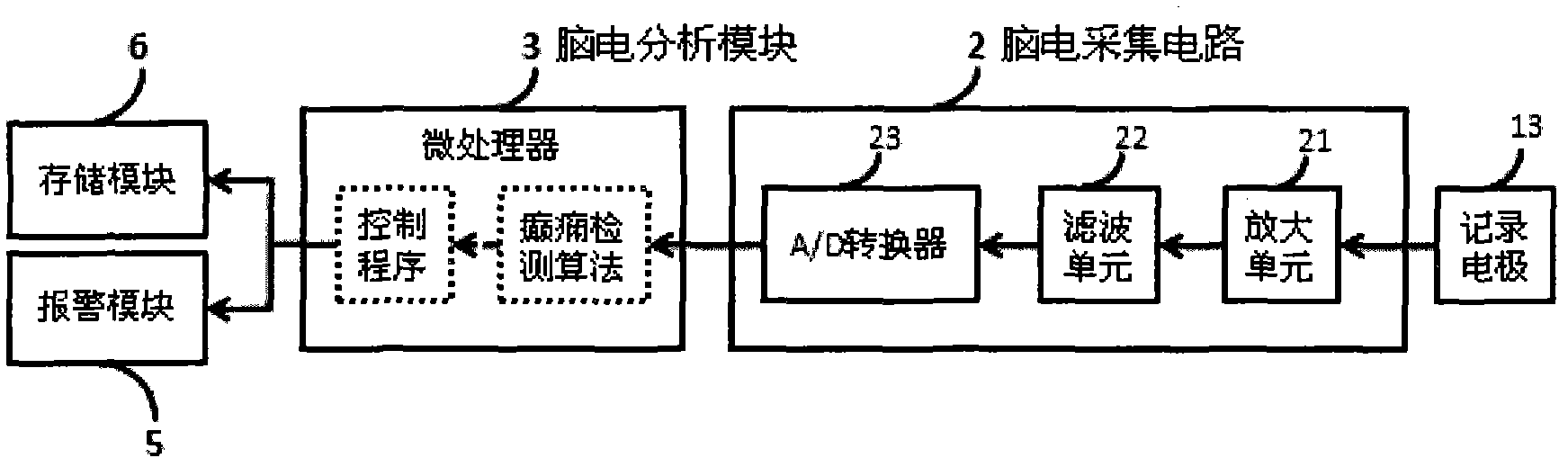
Electroencephalograph (EEG)-based epilepsy detection and intervention device
ActiveCN102613971AReduce the risk of side effectsImprove the quality of lifeDiagnostic recording/measuringSensorsElectricityMedicine
The invention provides an electroencephalograph (EEG)-based epilepsy detection and intervention device, which has the functions of recording EEG (scalp EEG, electrocorticogram (ECoG) and deep local field potential (LFP) EEG), analyzing the recorded EEG, detecting epilepsy electrograph onset (EO), forecasting epilepsy behavior onset and giving an alarm about the forecast epilepsy onset or implementing intervention means such as electrical stimulation. The device can also be used for research of corresponding animal experiments.
Owner:BEIJING PINS MEDICAL +1
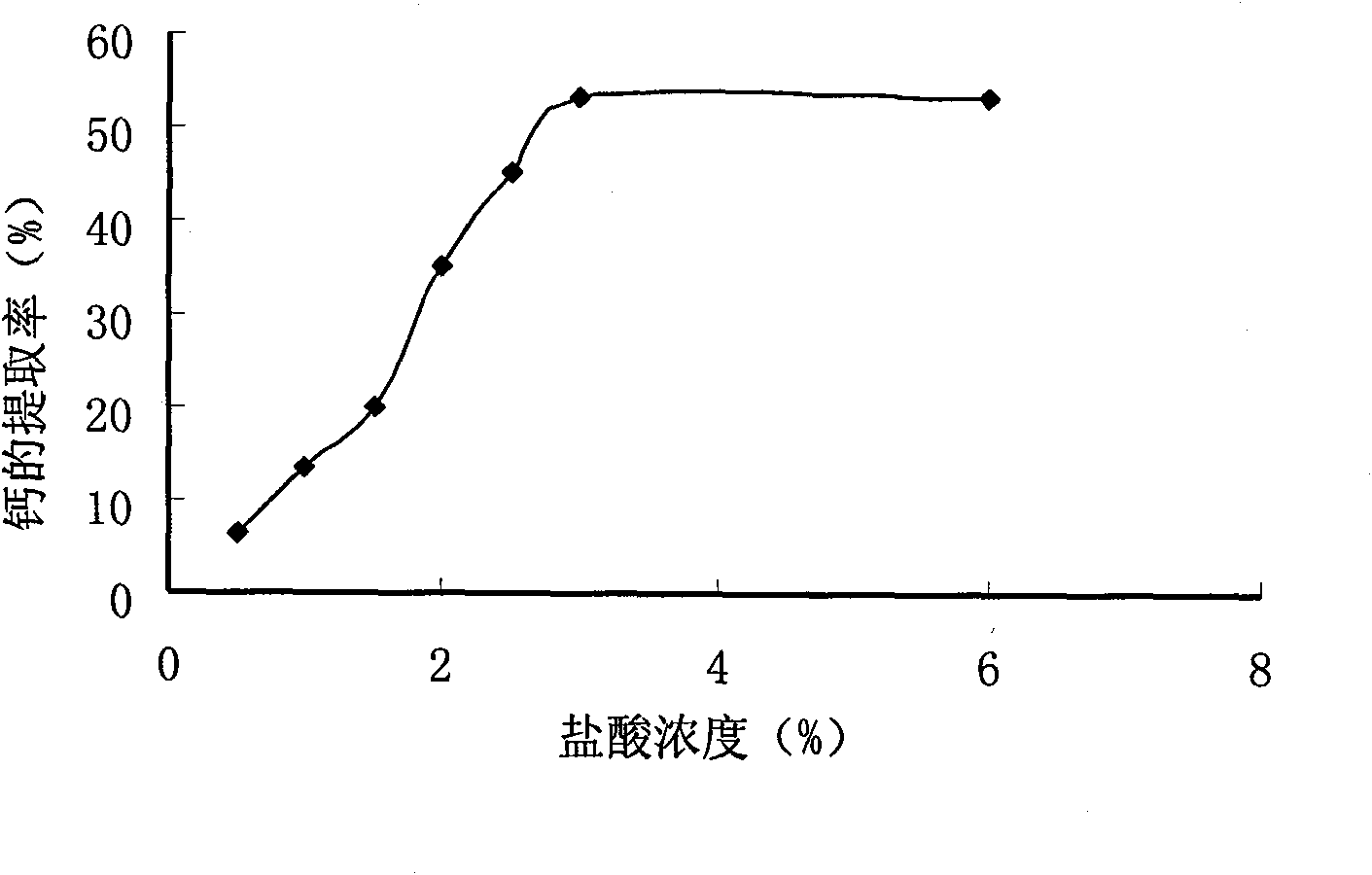
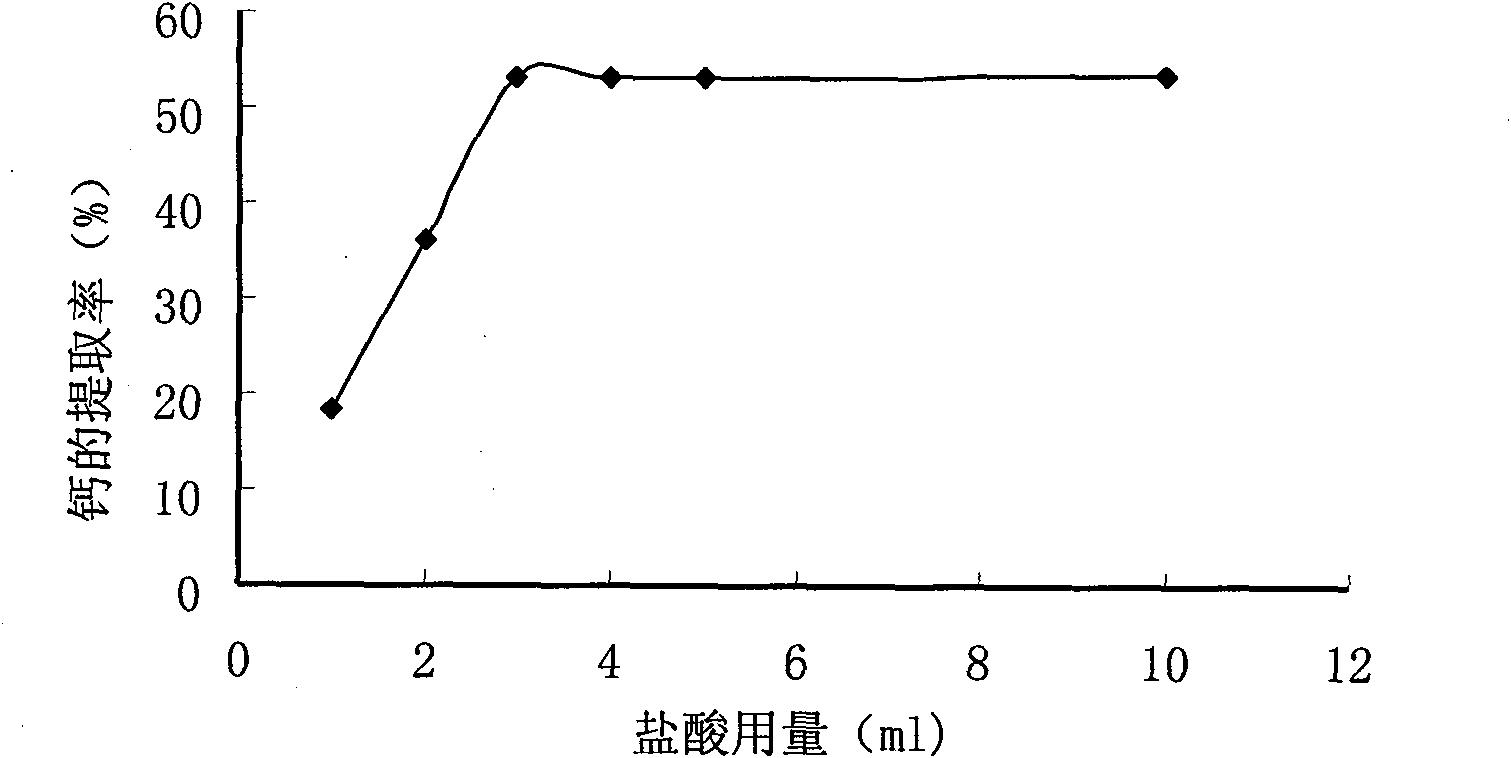
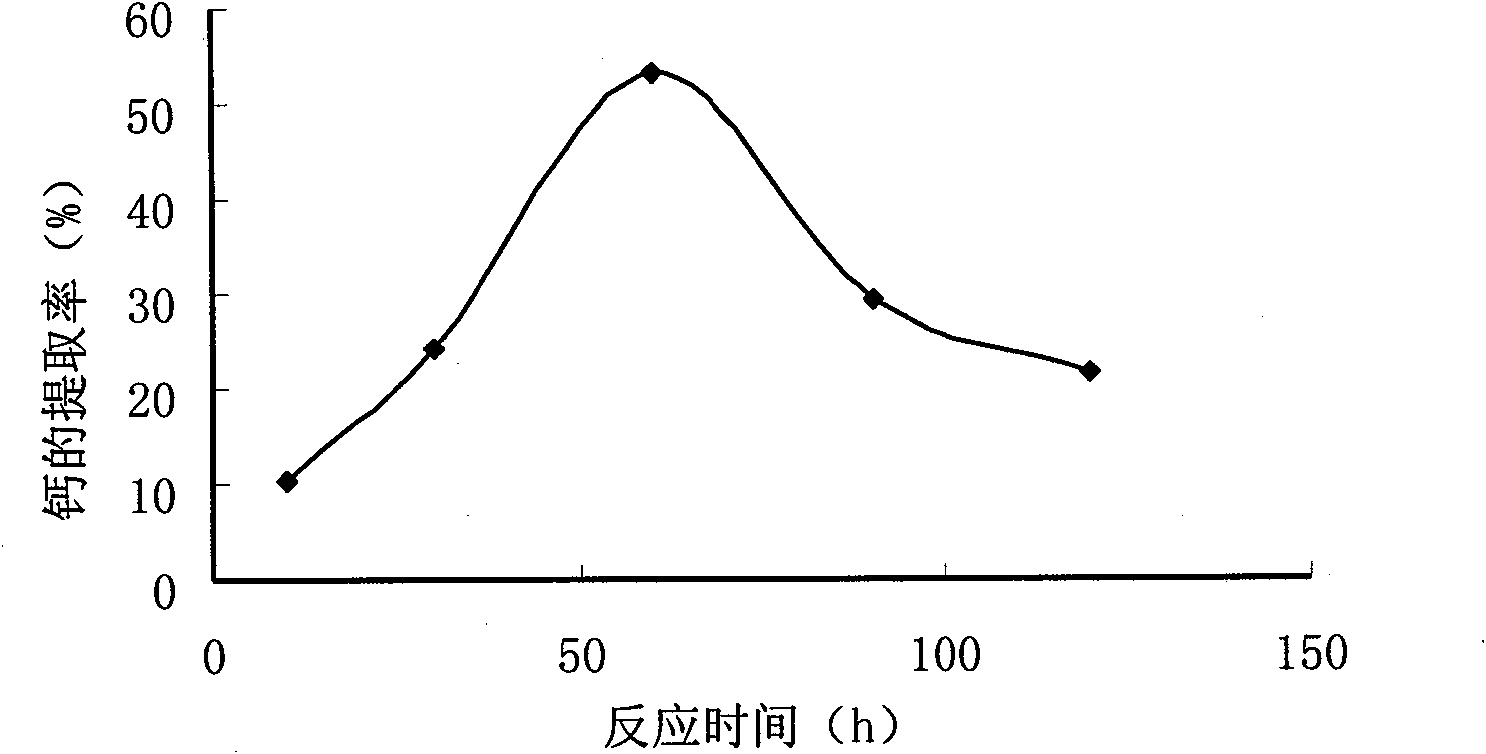
Method for preparing compound amino acid chelate calcium from low-value freshwater fish bones
InactiveCN101648884ATake advantage ofIncrease added valueOrganic compound preparationAmino-carboxyl compound preparationCalcium borogluconateSodium hydroxide
The invention relates to a method for preparing compound amino acid chelate calcium from low-value freshwater fish bones. The method comprises the following steps: washing and steaming fish bones andthen soaking the fish bones in sodium hydroxide; then washing the fish bones to be neutral; drying and pulverizing the fish bones; extracting calcium in fish bone powder by an acidolysis method; chelating calcium ions in the fish bone powder by using compound amino acid; carrying out centrifugation to obtain clear liquid; washing an obtained product twice by absolute alcohol and then carrying outcentrifugation to obtain precipitates; and drying and pulverizing the precipitates to obtain a calcium acid chelate capsule product. The invention firstly adopts the fish bones which are by-products of processing low-value freshwater fishes to prepare the compound amino acid chelate calcium, thereby greatly improving the additional value of the by-products of the freshwater fishes. Proved by an animal experiment, the compound amino acid chelate calcium has thighbones calcium content of a rat of 32.81-43.41 and thighbones calcium retention rate of 5.30-16.30. If the compound amino acid chelatecalcium is matched with VD3, the bioavailability can be increased more and the effect of calcium supplement is improved. The calcium supplement effect of the compound amino acid chelate calcium is equivalent to the calcium supplement effect of calcium gluconate and higher than the calcium supplement effect of calcium carbonate, but the calcium retention rate of the compound amino acid chelate calcium is obviously higher than that of the calcium gluconate group and the calcium carbonate group.
Owner:FARM PROD PROCESSING & NUCLEAR AGRI TECH INST HUBEI ACAD OF AGRI SCI
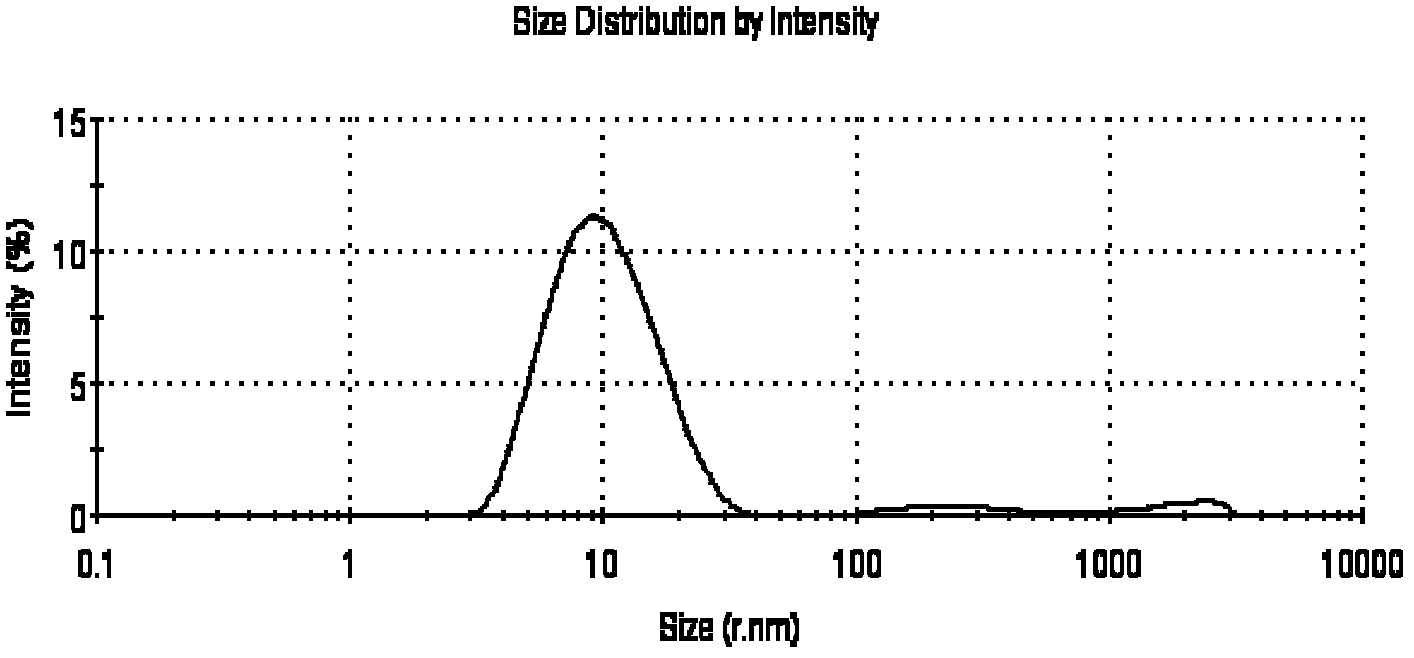
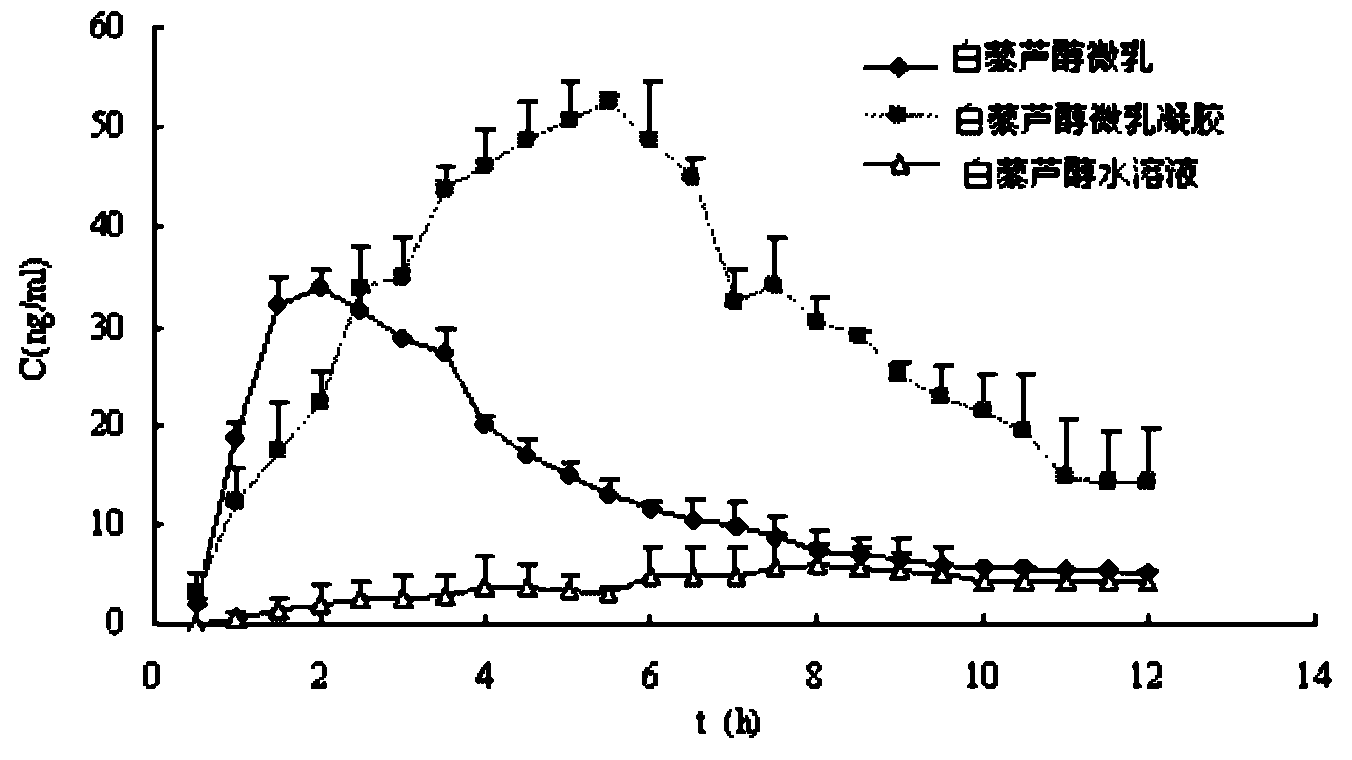
Micro-emulsion thermosensitive gel for skin external use and preparation method thereof
InactiveCN102614107AIncreased percutaneous penetration rateIncrease drug concentrationHydroxy compound active ingredientsPharmaceutical delivery mechanismRetention timeIrritation
The invention relates to the technical field of medicines, and in particular relates to a micro-emulsion thermosensitive gel for skin wound healing and a preparation method thereof. The micro-emulsion thermosensitive gel disclosed by the invention is composed of medicines, oil phase, a surfactant, a cosurfactant, a gel matrix and water, wherein the medicines are selected from resveratrol, paeonol and matrine. Proved by animal experiments, the prepared micro-emulsion thermosensitive gel has the characteristics of nearly no irritation to skin, safety in use, large percutaneous infiltration quantity, high percutaneous infiltration rate, long retention time of the micro-emulsion gel in the skin, high medicine concentration in the skin, good slow-release effect and good effect of promoting the healing of skin wound injury. The micro-emulsion thermosensitive gel disclosed by the invention has the advantages of simpleness in preparation method, low cost, convenience and safety in use and capability of effectively promoting the skin wound healing. According to the invention, a novel dosage form is provided for treating the skin wound.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Multi-function animal weight-losing running table and control system thereof
InactiveCN101822223ATaming and training devicesProgramme total factory controlSpinal cordEngineering
The invention discloses a multi-function animal weight-losing running table and a control system thereof. The running table comprises a running machine, a weight-losing support device and a recovery robot. Running training or combination training of running training and electric stimulus is performed on the experimental animal by arranging organic glass and a stimulating electrode on the running machine; and the weight-losing running table training combining running table training and weight-losing support is performed on the experimental animal by arranging the weight-losing support device on the running machine. In the process of weight-losing running table training, the recovery robot can assist hind legs of the experimental animal in doing exercise training, so that a correct or preset training task is provided for a passive training model, the movement locus of the hind legs in the positive training model is recorded and the record is used for quantitatively evaluating the exercising function. The invention also provides a medicinal animal experiment device which is mainly used for walking function recovery of the experimental animal with hurt spinal cord and providing the animal running table training needed by sports medicine.
Owner:HUAZHONG UNIV OF SCI & TECH
Recombinant human Claudin18.2 tumor vaccine and preparation method thereof
InactiveCN101584860AImprove securitySimple manufacturing methodMicroorganism based processesAntibody medical ingredientsSide effectMouse Stomach
The invention belongs to the field of medicinal biotechnology, and in particular relates to a recombinant human Claudin18.2 tumor vaccine and a preparation method thereof. The invention aims to solve the problems of immunotherapy for stomach cancer, pancreatic cancer, esophagus cancer and metastatic and non-metastatic ovarian cancer, such as high after-excision recurrence rate, strong chemo-treatment and radiation treatment toxic side effect and high monoclonal antibody therapy cost. The invention adopts a technical proposal that the recombinant human Claudin18.2 tumor vaccine has a sequence of HMKSSQYIKANSKFIGEFDQWSTQDLYNNPVTAVFNYQGLWRSCVRESSGFTECRGYFTLLGLPAMLQAV. Animal experiments prove that rhClaudin18.2 fusion protein can induce high-titre neutralizing antibody in the bodies of tumor-bearing mice by over 1:10,000; the antibody can be combined with human KATOIII and PANC-1 tumor cells and mouse stomach cancer MFC and pancreatic cancer MPC-83 cells; and the protein serving as a tumor vaccine can suppress the growth of the stomach cancer MFC and pancreatic cancer MPC-83 cells in the bodies of the mice.
Owner:西安杰诺瓦生物科技有限公司
Phosphorylcholine Conjugates and Corresponding Antibodies
ActiveUS20070286868A1Increased and decreased riskSignificant positive effectImmunoglobulins against animals/humansAntibody ingredientsPhosphorylcholineIgm antibody
IgG and IgM autoantibody levels against phosphorylcholine in subjects with hypertension (diastolic pressure>95 mmHg) were determined at baseline in order to determine the importance of antibodies for the development of atherosclerosis. The results show that increases in intima-media thickness (IMT) at a follow-up four years after baseline were significantly less prevalent in subjects having high IgM autoantibodies to phosphorylcholine. The presence or absence of IgM autoantibodies against phosphorylcholine is thus related to an increased or decreased risk of developing ischemic cardiovascular diseases. A method to determine IgM antibodies toward phosphorylcholine is proposed in this invention to identify subjects at risk of developing ischemic cardiovascular diseases. Animal experiments show that medium to high levels of IgM antibodies can be detected in plasma after active immunization with a keyhole limpet hemocyanin (KLH)-phosphorylcholine conjugate. A pharmaceutical composition comprising a phosphorylcholine conjugate (active immunization) or a monoclonal antibody with specificity to a phosphorylcholine conjugate (passive immunization) is proposed and the use of these compositions as active or passive immunogens in the treatment or prevention of atherosclerosis.
Owner:ATHERA BIOTECH
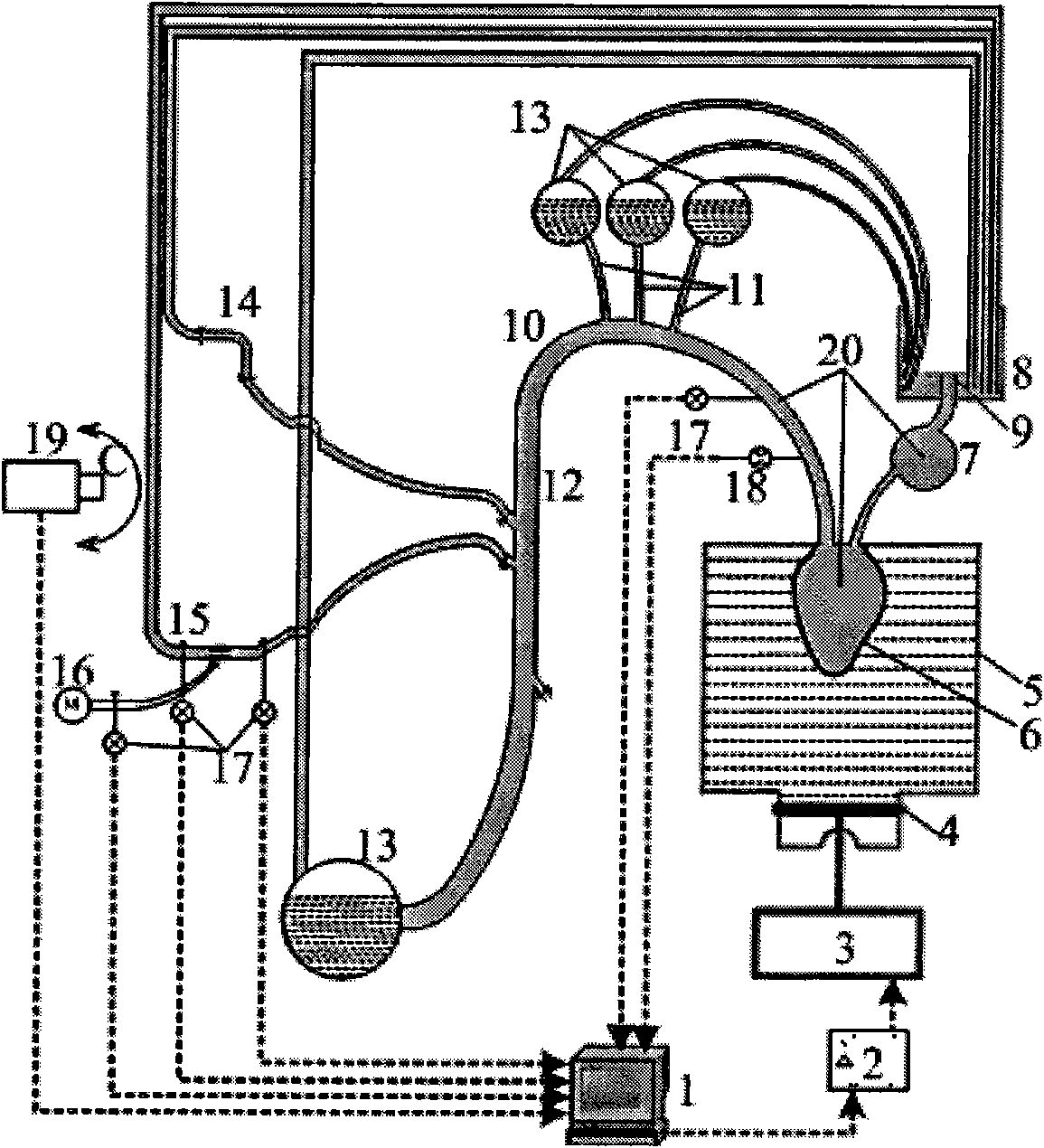
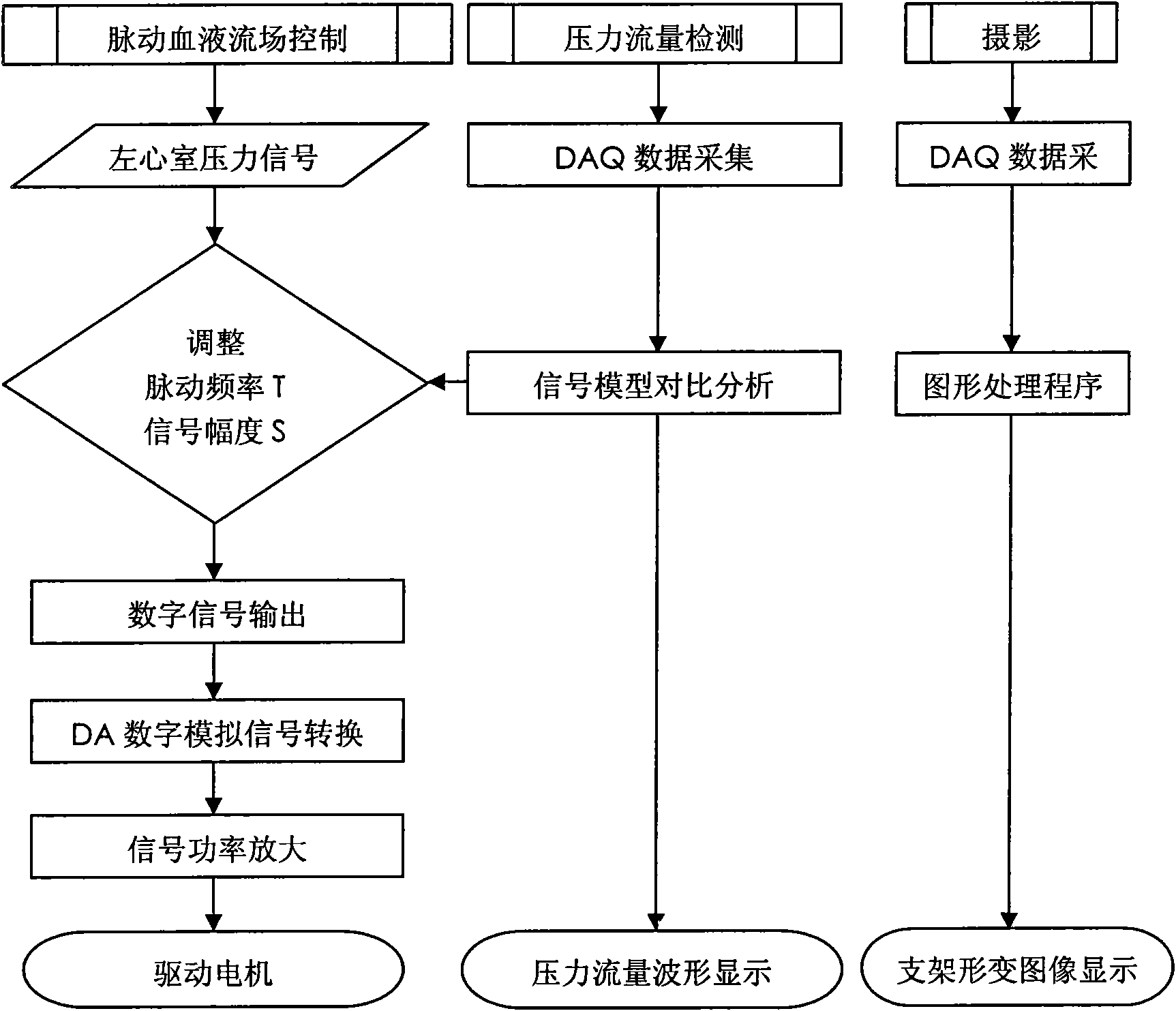
Biomechanical experiment simulation device for implantation of intravascular stent
The invention discloses a biomechanical experiment simulation device for implantation of intravascular stent, which consists of an artificial cardiovascular system, an artificial blood and pulsatile blood flow field control system and a mechanical experiment observation device. The upper part of a liquid storage box of the artificial cardiovascular system is soaked in a silica gel ventriculus sinister model geometrically similar to the ventriculus sinister; the inlet end of the silica gel ventriculus sinister model is connected with the open liquid storage box through a silica gel hollow round ball simulating the right atrium; the outlet end of the silica gel ventriculus sinister model is connected with an aortic arch model; a microcomputer of the artificial blood and pulsatile blood flow field control system is connected with a power amplifier, a linear step motor and a piston in turn; and artificial blood with similar blood viscosity and specific gravity and containing simulated thrombus particles polystyrene microspheres is arranged in a closed-loop blood flow field model. Through the device, a simulated operation for stent implantation can be effectively and visually preformed between the clinical experiments and animal experiments on the intravascular stent, and effective biochemical evaluation on the flexibility, strength and stability of the stent can be realized.
Owner:SOUTH CHINA UNIV OF TECH
Functional skin care product containing purslane extractive and preparation method thereof
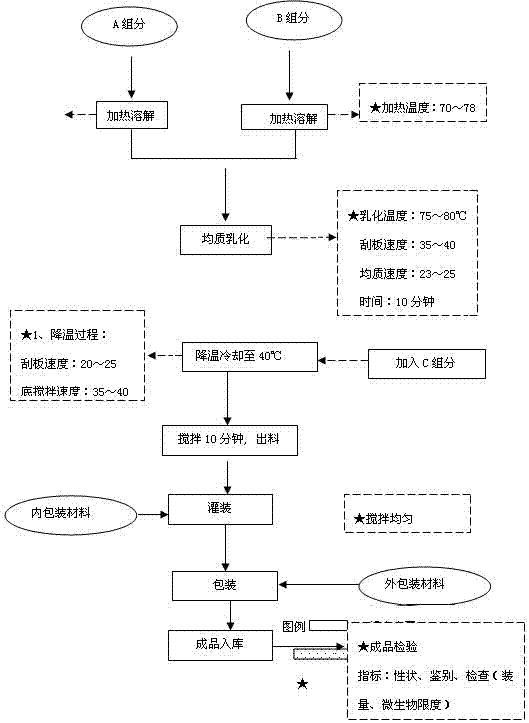
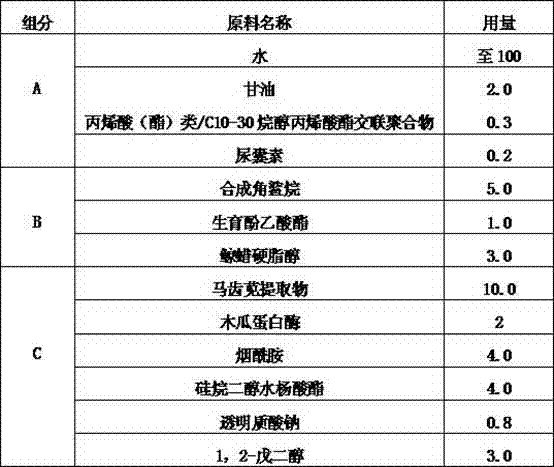
ActiveCN102764217AMild textureImprove securityCosmetic preparationsAntipyreticGlycerolPurslane extract
The invention is a functional skin care product containing purslane extractive. Raw materials of the functional skin care product include, by weight, 10 parts of purslane extractive, 1-2 parts of Prinsepia utilis rogle oil, 2-5 parts of glycerol, 0.3-0.5 parts of crylic acid (ester) / C10-30 alkanol acrylate cross-linked polymer, 2-3 parts of 1,2-pentanediol, 1-5 parts of synthesized squalane, 1-2 parts of tocopheryl acetate, 2-3 parts of cetostearyl alcohol, 0.1-0.2 part of allantoin, 0.3-0.8 part of sodium hyaluronate, 3-5 parts of Butyrospermum parkii butter, and 100 parts of deionized water. Or the Prinsepia utilis rogle oil and the Butyrospermum parkii butter are not added. By the aid of animal experiments, namely, mouse auricle swelling models due to dimethlbenzene, the functional skin care product containing purslane extractive is fine in anti-inflammation and anti-allergy effects, mild in nature and fine in safety, and is free of toxic reaction and anaphylaxis. The functional skin care product has an adjuvant therapy function to skin barrier recovery of common discosmetic dermatosis such as hormone dependent dermatitis and acne, and is fine in curative effect and safety.
Owner:YUNNAN BOTANEE BIO TECH GRP CO LTD
Efficient and green diarrhea-preventing feed additive composite for weaned pigs
ActiveCN102228154AReduce usageAvoid the problem of drug resistance caused by abuseAnimal feeding stuffAccessory food factorsAnimal scienceAnimal testing
Owner:辽宁九州生物科技有限公司
Magnesium iso-glycyrrhetate gel and its preparing method and use
ActiveCN1615886AFine and even textureSafe and effective treatmentOrganic active ingredientsPharmaceutical delivery mechanismMedicineContact dermatitis
The present invention relates to the field of medicine preparation, and discloses a kind gel containing magnesium isoglycyrrhetate and its preparation process and application. The prepared gel has stable characteristic and convenient use. Animal experiment shows that the gel of the present invention can treat psoriasis, chronic eczema and dermatitis, contact dermatitis and other allergic dermatosis.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Method for preparing macromolecule resin type bilirubin sorbent
InactiveCN1557538AImprove mechanical propertiesAvoid the danger of blood pressure dropsOther chemical processesCross-linkSorbent
The present invention features that the bilirubin adsorbent is synthesized by selecting well biocompatible polystyrene-divinylbenzene copolymer as carrier, PHEMA as coating agent, epichlorohydrin for activating the functional hydroxy radical of PHEMA, and glutaraldehyde as cross-linking agent for cross-linking functional cyclodextrin molecule. The bilirubin adsorbent has biocompatibility and adsorption capacity superior to active carbon and cationic resin adsorbing material, and has no adsorbent molecule falling resulting in immunological dysfunction. Animal experiment shows that the novel bilirubin adsorbent may be used to eliminate bilirubin from blood, blood plasma and albumin solution effectively.
Owner:杭州科锐净化工程有限公司
Human umbilical cord mesenchymal stem cell extract freeze-dried powder and preparation method
ActiveCN106109496AIncrease SOD activityAgainst oxidative damagePowder deliveryAntinoxious agentsIrritationFreeze-drying
The invention relates to human umbilical cord mesenchymal stem cell extract freeze-dried powder and a preparation method. The preparation method of the human umbilical cord mesenchymal stem cell extract freeze-dried powder comprises the following steps: obtaining human umbilical cord mesenchymal primary stem cells, which are relatively high in purity, by virtue of density gradient centrifugation, conducting subculture, collecting a culture supernatant of the 3rd-18th generations of cells, and mixing the supernatant with trehalose, so that the freeze-dried powder is prepared. The human umbilical cord mesenchymal stem cell extract freeze-dried powder prepared by the invention has good appearance characteristics; the freeze-dried powder, when redissolved, is relatively rapid to dissolve and is completely dissolved; and animal experiments prove that the freeze-dried powder is low in irritation, high in safety and reliable to use. In addition, the freeze-dried powder disclosed by the invention can effectively preserve various bioactive cell factors in the human umbilical cord mesenchymal stem cell culture supernatant, protect skin from ultraviolet damage and promote the synthesis of skin collagen, and the freeze-dried powder has functions of whitening the skin and delaying aging.
Owner:广东省华桑丽皙生物技术有限公司
High compound fiber rectification food additive
The invention relates to food, in particular to a food additive and particularly discloses a high compound fiber rectification food additive, solving the problems of simple composition and poor edible effect of the prior compound rectification food. The high compound fiber rectification food additive comprises the following components of wheat fibers, corn fibers, soybean lecithin, small red beans, perilla seeds, perilla leaves, coix seeds, almond, jujube kernels, mulberry leaves, chrysanthemum, medlar, papaya, dried gingers, licorices, parched white hyaciath beans, parched hawthorn fruit, fried malts, radish seeds, galli stomachichum corium, yams, tangerine peels, Tuckahoe, finger citron, fructus cannabis, lily, grosvenor momordicae and plum seeds. The high compound fiber rectification food additive is developed according to the principles of homology of medicine and food and modern nutrition and aiming to regulate a dietary structure and nutrition rectification. Animal experiments and human body taking experiments show that the product has the remarkable functions of regulating a human body health state such as reducing lipid and blood sugar, losing weight, relaxing bowels, improving sleep and the like.
Owner:山西纠偏古膳要道食品开发有限公司
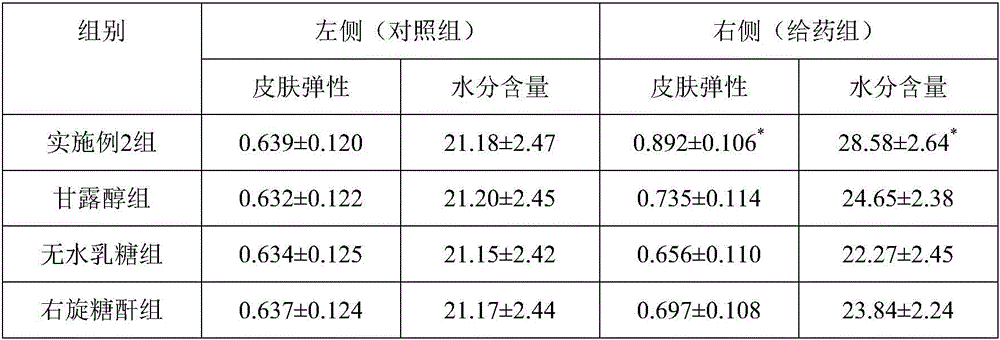
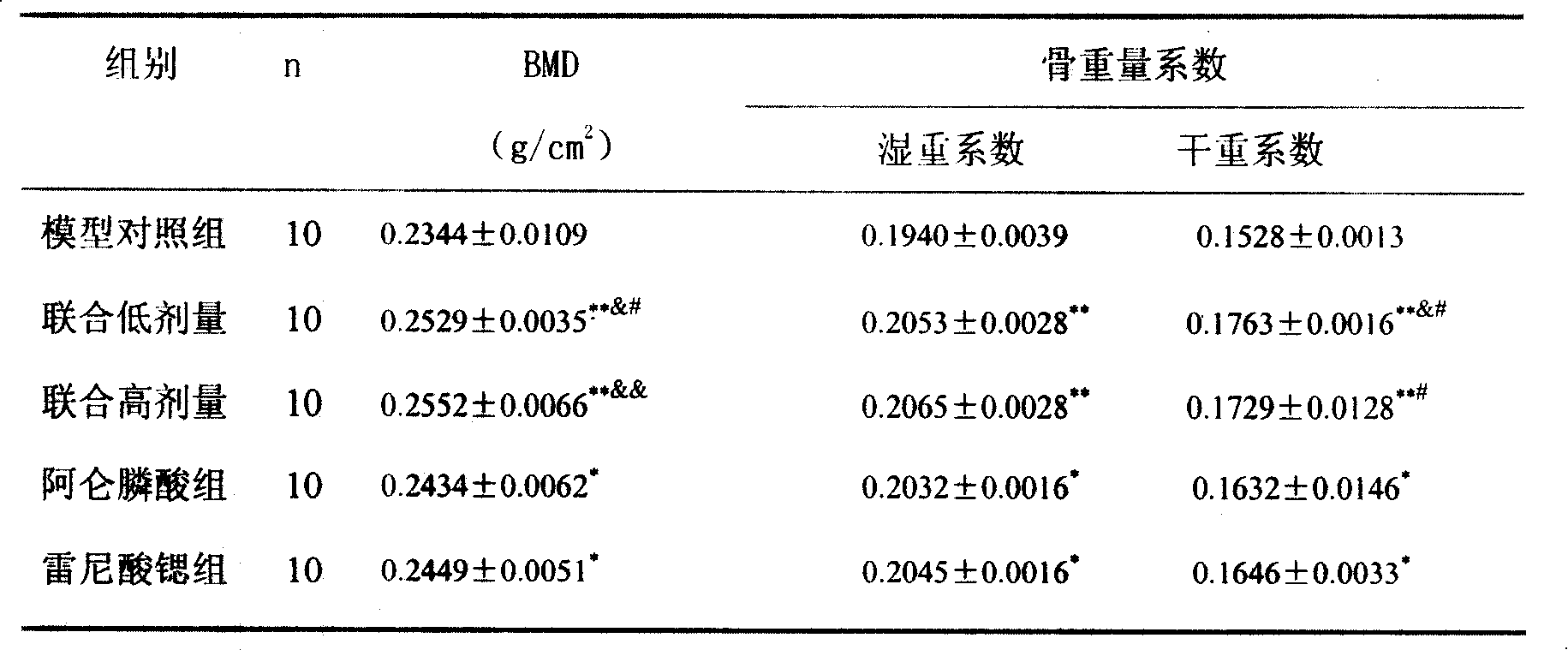
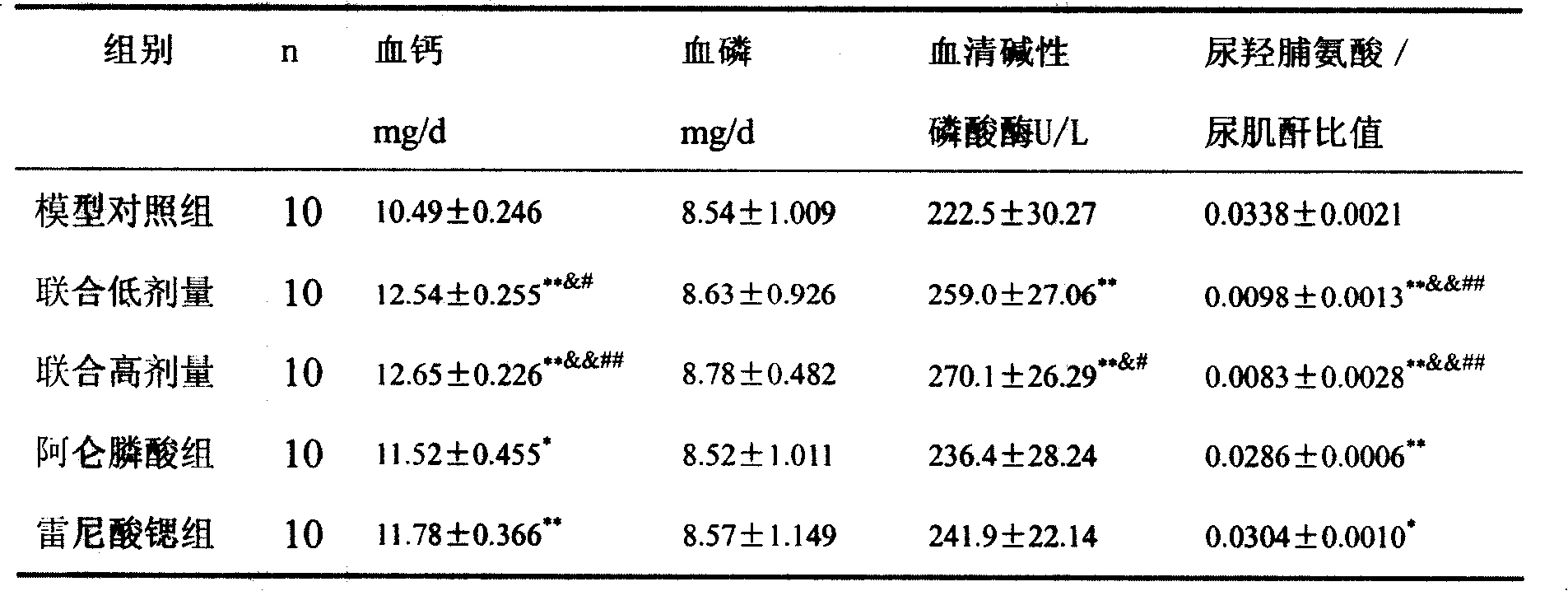
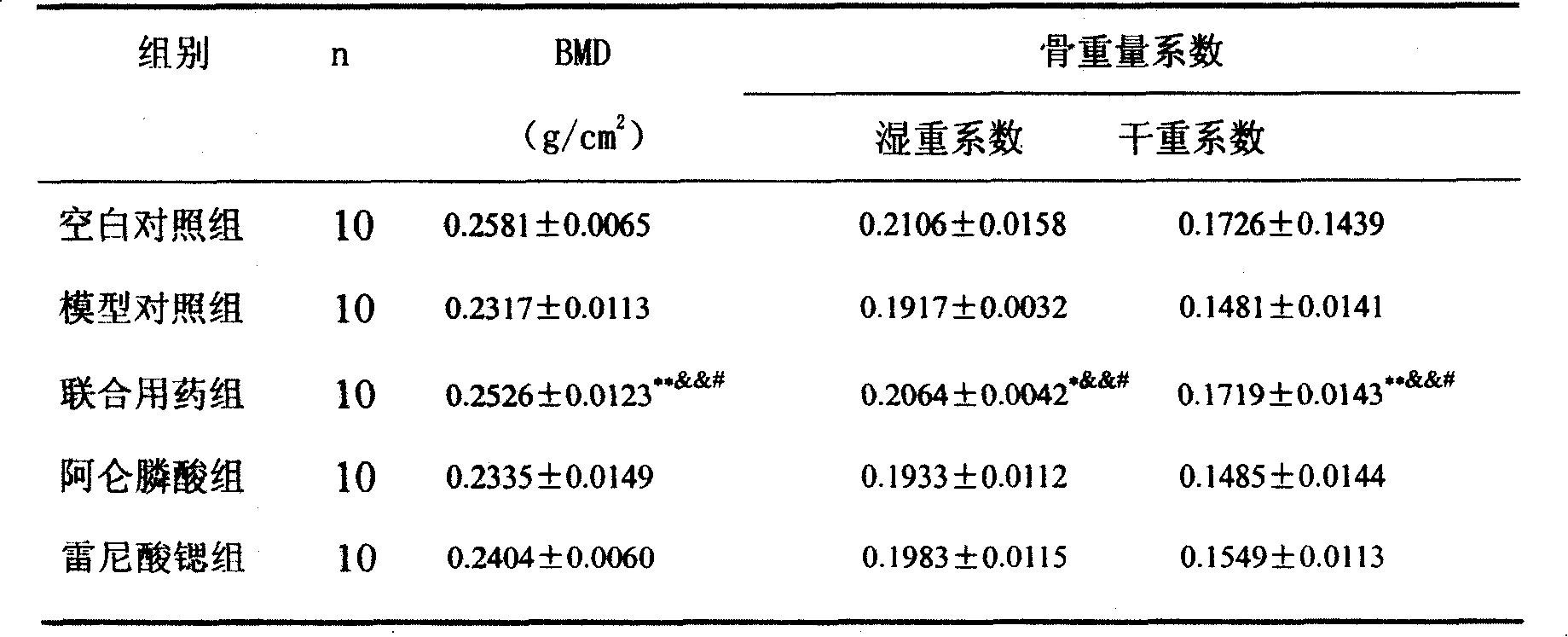
Medicine compounds for treating osteoporosis
ActiveCN101229177AReduce dosageSignificant effectOrganic active ingredientsSkeletal disorderMicro structureSide effect
The invention provides a medical compound for treating osteoporosis which is characterized in that the invention contains strontium ranelate and bisphosphonate. Animal experiments indicate that the invention achieves the unexpected effect for treating the osteoporosis. The osteoporosis is a bone disease of the whole body characterized by the low bone mass and the degeneration of the micro structure of the bone organization, companying with the enhancement of the bone fragility and easy happened bone broken for which no ideal treatment medicine exists in the clinic. The bisphosphonate of the invention comprises alendronate, risedronate sodium, ibandronate, pamidronate, Etidronate, disodium clodronate and zoledronic acid, etc. In the invention, the dosage of the bisphosphonate is greatly reduced, which can effectively reduce the happening of side effects and is convenient to use the medicine.
Owner:LUNAN PHARMA GROUP CORPORATION
Phentolamine external-applied preparation and its preparation method
InactiveCN101143127AGuaranteed validityImprove transdermal drug absorptionOrganic active ingredientsSexual disorderWestern medicineSide effect
The invention belongs to the western medicine preparation field and concretely relates to an efficient and quick effective external preparation of phentolamine which considers the volatile oil of natural plant and the extracted finished product of the volatile oil of the natural plant as a percutaneous sorbefacient and the preparation method of the external preparation. The invention selects the percutaneous sorbefacient of the natural volatile oil to prepare an external percutaneous preparation of the phentolamine of the invention by considering the phentolamine as the only curative active matter of the preparation and adding the necessary medicinal auxiliary materials. The animal experiment testifies that the invention can keep the drug usability and synchronously reduce the medicament dosage greatly and reduce the side effect to the whole body and the use cost. Compared with the prior art, the preparation can improve the percutaneous absorption of the drug dosage of the phentolamine very obviously and shortens the latent period of effecting, and the preparation has good medicament safety and can provide the patient with more satisfying curative effect.
Owner:FUDAN UNIV
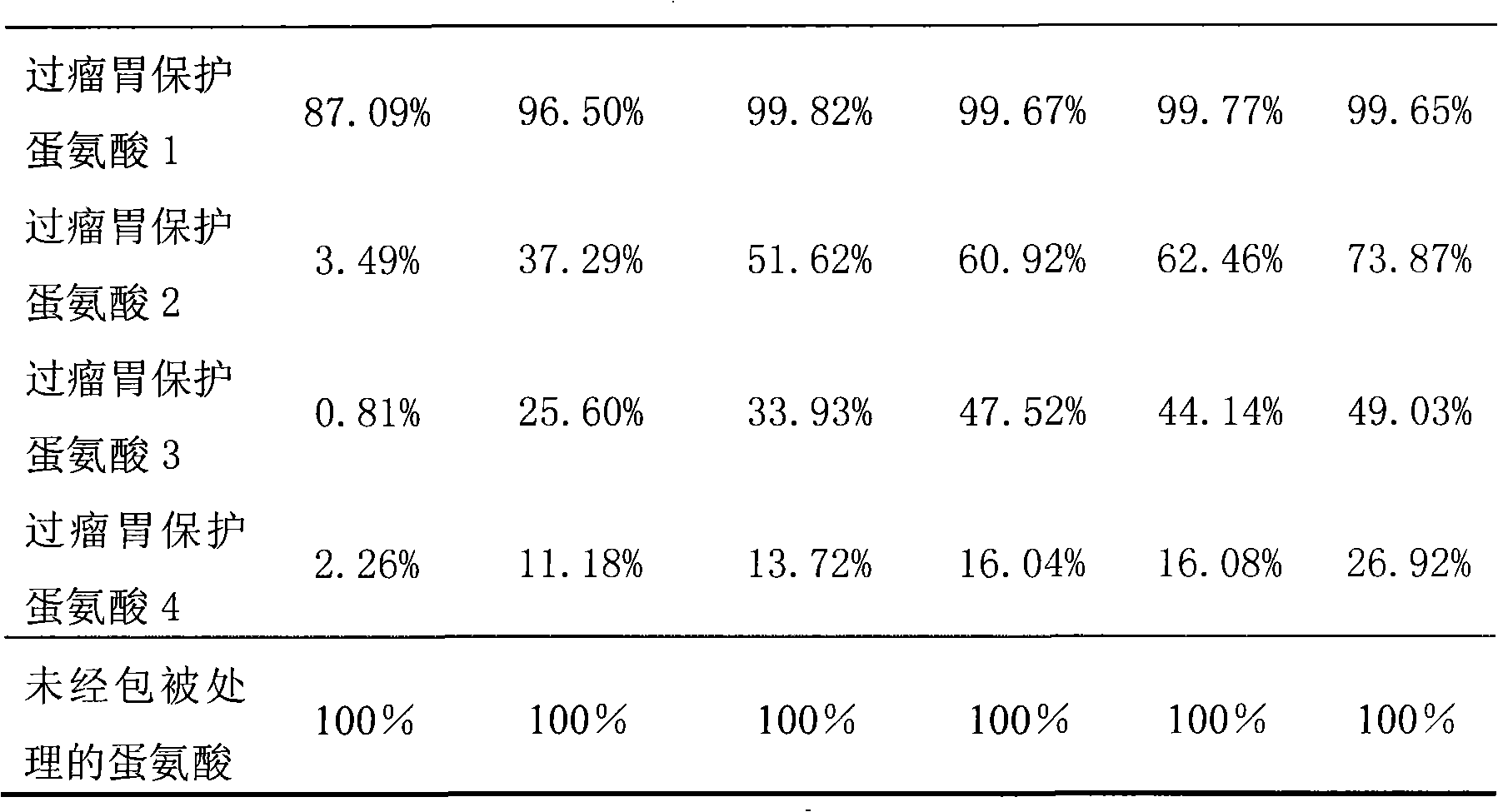
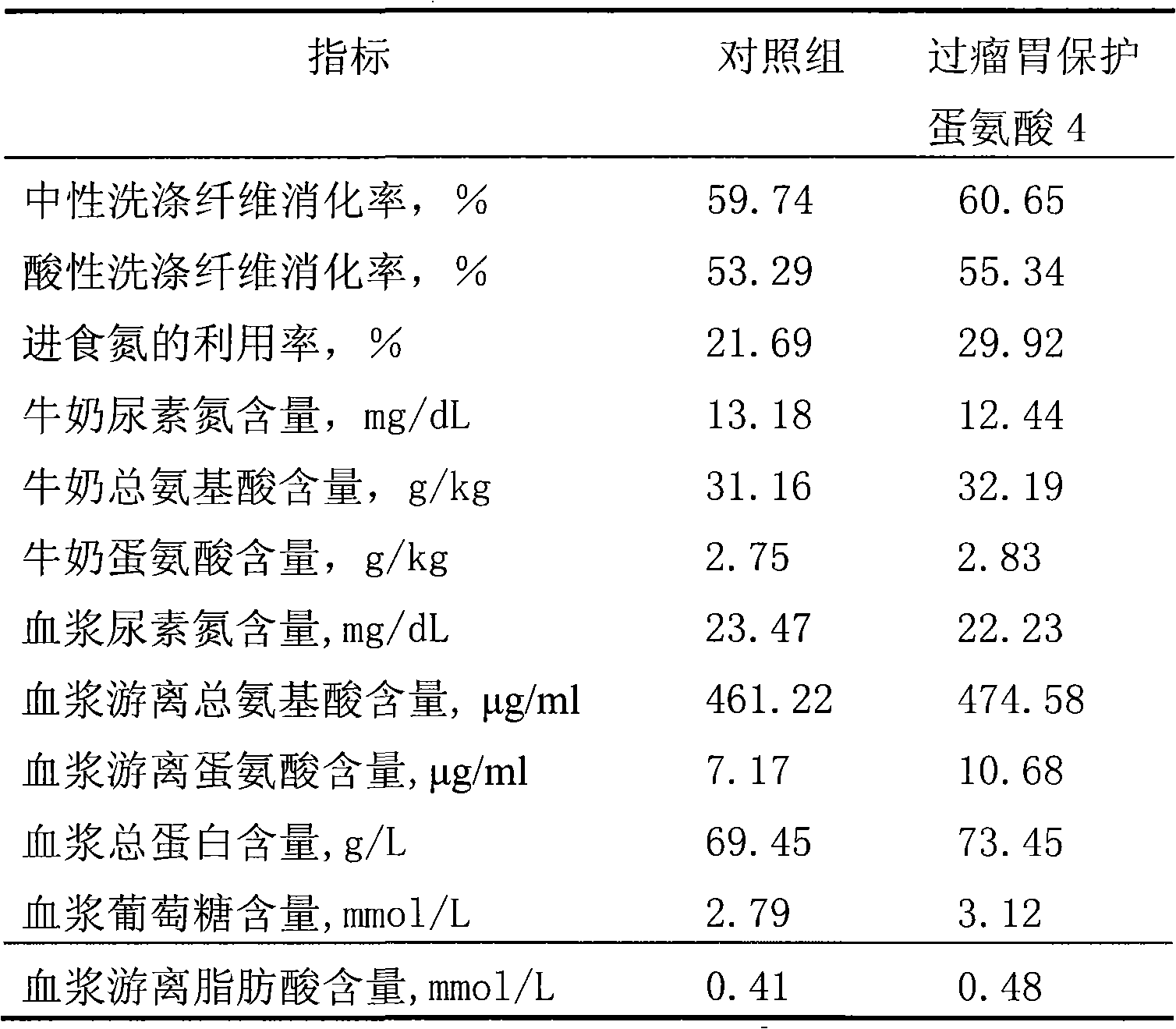
Rumen protected methionine and production method thereof
ActiveCN101664108APromote nitrogen metabolismIncrease milk productionAnimal feeding stuffAccessory food factorsAdditive ingredientBULK ACTIVE INGREDIENT
The invention discloses rumen protected methionine and a production method thereof. The rumen protected methionine consists of a core and a shell, wherein the shell is rumen pass fat powder, the active ingredient of the core is methionine; and the mass ratio of the methionine to the rumen pass fat powder is (35-65):(30-60). Animal experiments prove that: the rumen protected methionine has good stability and high small intestine digestibility, can obviously improve the nitrogen metabolism of milk cows, can improve the milk yield and the milk quality of the milk cows during the production, and obtain considerable economic benefit.
Owner:CHINA AGRI UNIV +1
Tissue engineered skin with basilar membrane and construction method thereof
InactiveCN101954124ASimple methodEasy to get materialsArtificial cell constructsVertebrate cellsEpidermis structureManufacturing technology
The invention relates to the technical fields of tissue engineering and medical wound repair. At present, a living skin substitute constructed by using the materials of polylactic acid, polyglycolic acid, collagen, hyaluronic acid, and the like as a dermic bracket has the defects that on one hand, host materials are difficult to extract, the living skin substitute has complicated manufacturing technology and is expensive in cost and difficult to widely popularize and apply clinically; and on the other hand, the living skin substitute does not have a skin basilar membrane structure so that healed skin does not resist pressure and wear, and the living skin has unfirm adhesion to the epidermis and is easy to shed and break or form water blisters so that the structural and morphological development of the normal epidermis is influenced; and allogeneic acellular dermis is taken from cadaver skin and is limited in sources and expensive in cost, and thus clinical application is limited. The invention aims at providing a skin substitute which uses surface-finished and modified amnion as the basilar membrane and blood plasma as stroma, which has the advantages of wide material sources, low cost and simple preparation method. An animal experiment proves that the complete basilar membrane and hemidesmosomes can be retained in in-vivo transplantation, and the formation of an epidermal structural form is accelerated and promoted.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
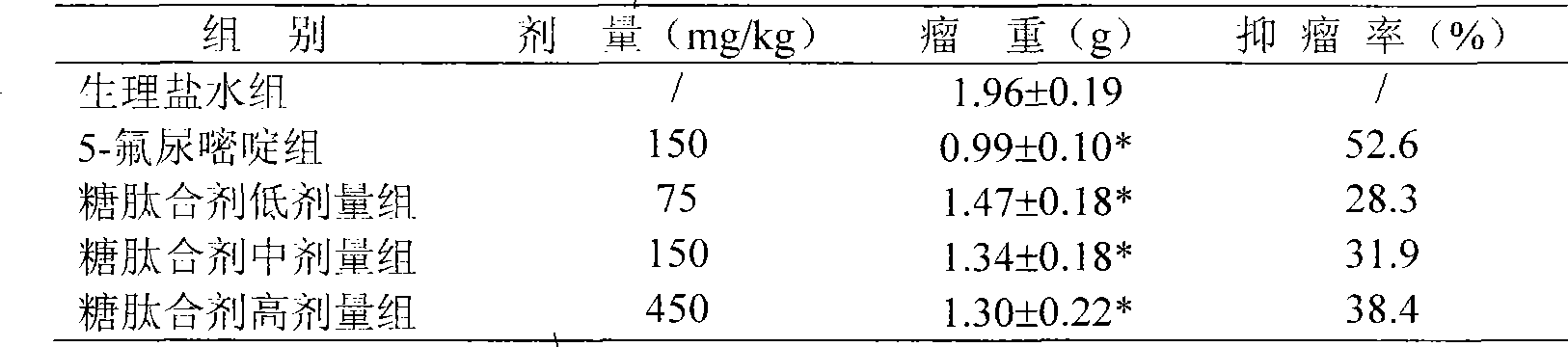
Application of glycopeptide mixture in preparing adjuvant therapy medicaments of tumor and health food
The invention relates to a glycopeptide mixture product and application thereof in preparing a medicament for improving the nutritional status and immunologic function of tumor patients receiving radiotherapy and chemotherapy and health food or food. A glycopeptide mixture is prepared by mixing marine oligopeptide and bioactive polysaccharide according to certain proportion. The marine oligopeptide is a group of oligopeptide mixtures with low molecular weights which are produced by taking fish skin, fish meat or fish bone of marine fishes as main raw materials through enzymatic hydrolysis, can be dissolved by trichloroacetic acid, and have relative molecular mass less than 1,000, and has the characteristics of easy absorption, quick absorption, low viscosity, good water solubility, and the like. The bioactive polysaccharide comprises lentinan, ganoderan, grifola polysaccharide, panaxan, lycium bararum polysaccharide and algal polysaccharide. Animal experiments and clinical observation prove that the glycopeptide mixture prepared by mixing the marine oligopeptide and the bioactive polysaccharide has the effect of improving the immunologic function of tumor-bearing mice and the nutritional status of the tumor patients receiving radiotherapy and chemotherapy.
Owner:李勇 +1
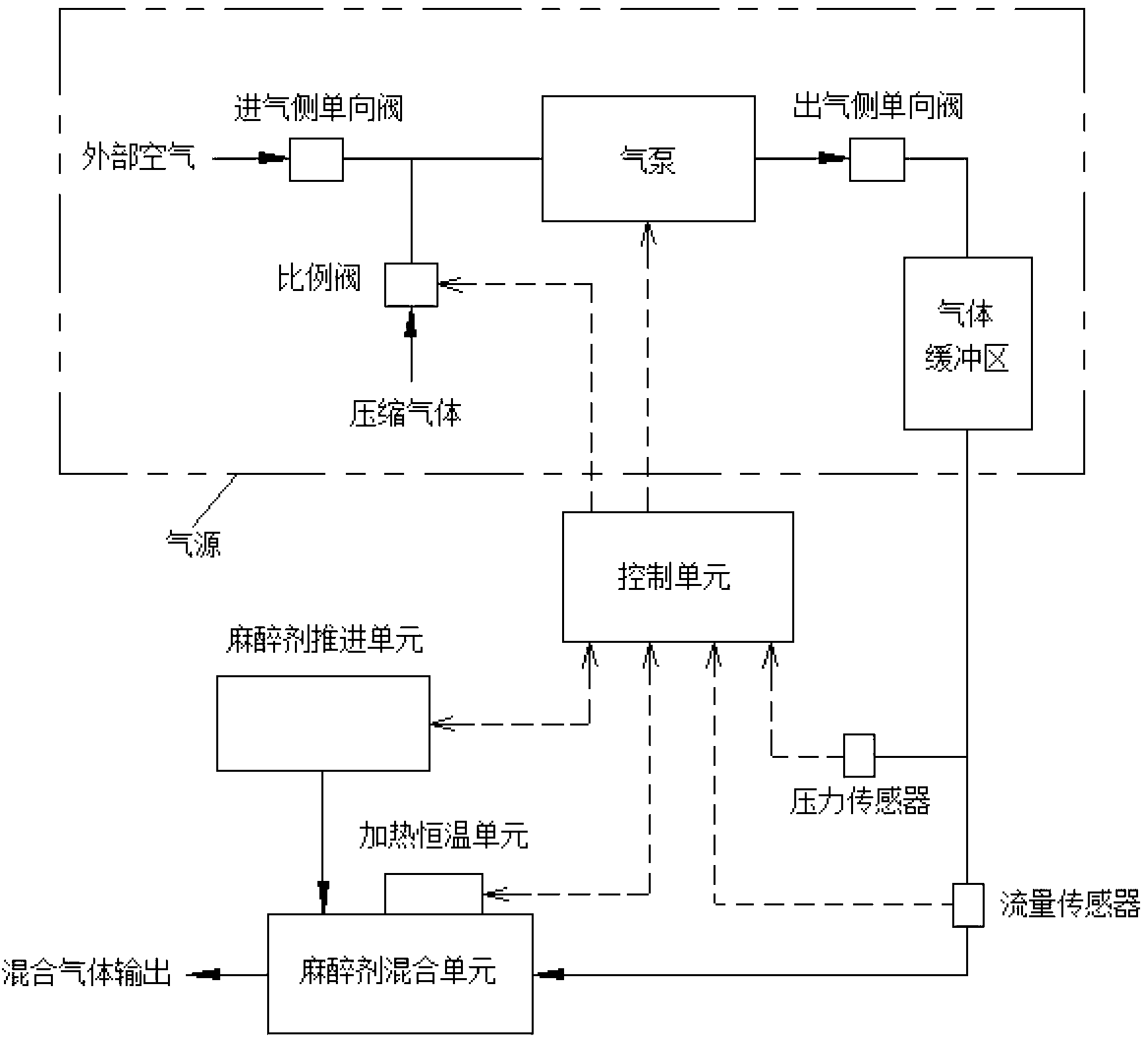
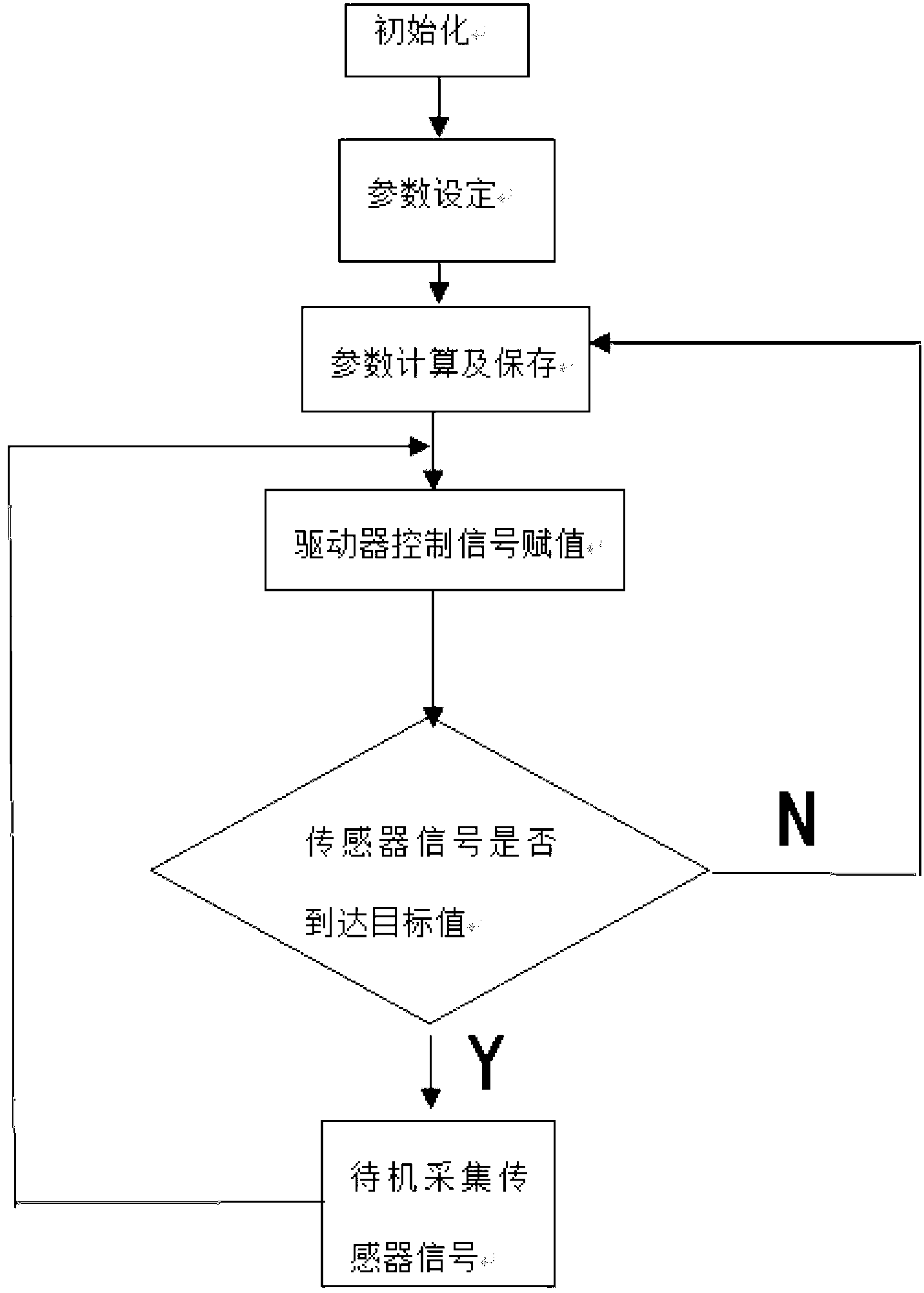
Precise propelling type electronic anesthesia machine
The invention provides a precise propelling type electronic anesthesia machine which is characterized by being provided with an anesthetic propelling unit, a pressure and flow testing unit and a control unit. An anesthetic mixing unit is provided with a heating constant temperature unit. The anesthetic propelling unit is a precise injection unit and comprises an injection pump, a propelling controller and a stepping motor with a reduction gearbox. The pressure and flow testing unit comprises a pressure sensor and a flow sensor. The control unit comprises a signal processing module, a motion execution drive module and a single-chip microcomputer. The heating constant temperature unit is a PID intelligent temperature controller provided with a temperature sensor. According to the precise propelling type electronic anesthesia machine, anesthetic is directly injected into an anesthetic mixing and volatilizing cavity, heated in real time and controlled accurately, and therefore control of the vaporizing and volatilizing process of the anesthetic is economical, precise, accurate and stable. A volatilization core does not need to be adopted, requirements for the initial amount of the anesthetic can be avoided, the range of the applicable anesthetic is wide, generality is strong, a user can switch different kinds of the anesthetic conveniently, and convenience is brought to application in the animal experiment field.
Owner:RWD LIFE SCI
Broccoli seed extractive and preparing method thereof
InactiveCN101229211AEasy to carry and useEasy to storePill deliveryAntineoplastic agentsCancer preventionAdditive ingredient
The invention discloses a seed extract of Broccoli and relevant preparation method. The preparation method is to receive the extract of the Broccoli seed hydrolyzing, degreasing, inactivating, and condensing the seed of the Broccoli and to receive a health product by adding auxiliary materials. The main components of the Broccoli extract of the invention is compounds of isothiocyanate; wherein, sulforaphane is the most activated compound with maximal content, and sulforaphane is also the isothiocyanate compound with maximal cancer-prevention activity. The health product containing the Broccoli extracts can effectively help human body improve the resistance to toxicants after the proof of animal experiment, thereby effectively reducing the formation of tumor induced by chemical matters.
Owner:GUANGDONG PHARMA UNIV
Application of penehyliclidine hydrochloride in preparing medicine
InactiveCN1739511AEffective for abdominal painEffective for biliary colicOrganic active ingredientsAntiinfectivesCurative effectPharmacology
The present invention relates to the application of penehyclidine hydrochloride in preparing medicine, and is especially the application of penehyclidine hydrochloride in preparing medicine for resisting infectious shock. Animal experiment and clinical observation prove the curative effect of penehyclidine hydrochloride in resisting infectious shock and improving micro circulation. The present invention expands the medicinal use of penehyclidine hydrochloride.
Owner:CHENGDU LIST PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com