Recombinant human Claudin18.2 tumor vaccine and preparation method thereof
A rhhis-tt-claudin18.2, tumor vaccine technology, applied in the field of medicine and biology, can solve the problems of high recurrence rate of surgical resection, strong toxic and side effects of chemotherapy and radiotherapy, and high cost of monoclonal antibody treatment, and achieves easy and safe preparation method. high sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0042] In order to understand the present invention more clearly, the present invention will be further described in detail below in conjunction with the embodiments completed by the inventor.
[0043] The present invention will be described in further detail below in conjunction with the accompanying drawings and embodiments.
[0044] 1. The recombinant human Claudin18.2 tumor vaccine (rhHis-TT-Claudin18.2) provided by the present invention has an amino acid sequence of:
[0045] HMKSSQYIKANSKFIGEFDQWSTQDLYNNPVTAVFNYQGLWRSCVRESSGFTECRGYFTLLGLPAMLQAV
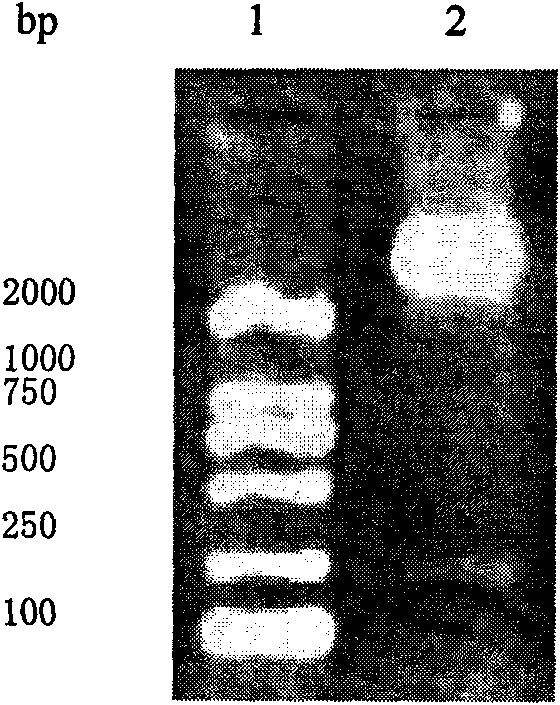
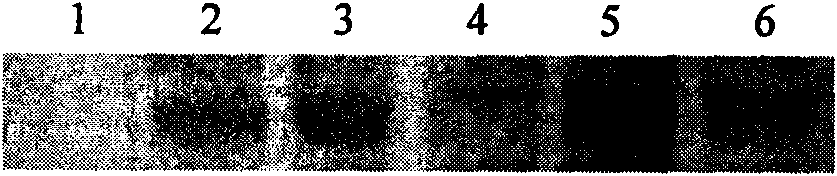
[0046] 2. The above-mentioned recombinant human Claudin18.2 tumor vaccine (rhHis-TT-Claudin18.2) preparation method comprises the following steps in turn:
[0047] (1) Cloning of rhHis-TT-Claudin18.2 gene and construction of prokaryotic expression vector:
[0048] According to the gene sequence of the first extracellular region of human Claudin18.2 in GeneBank, the conventional RT-PCR method was used to introduce the EcoRI res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com