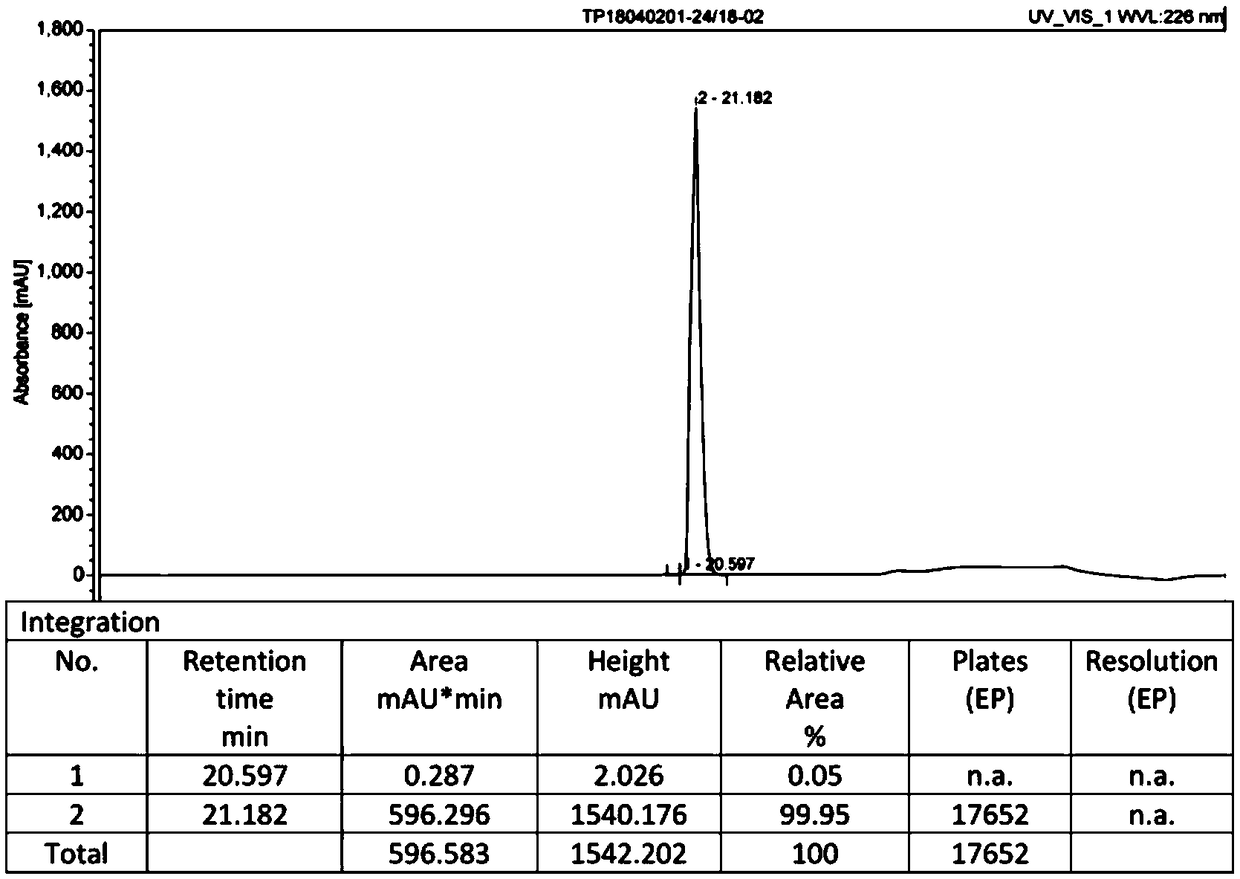
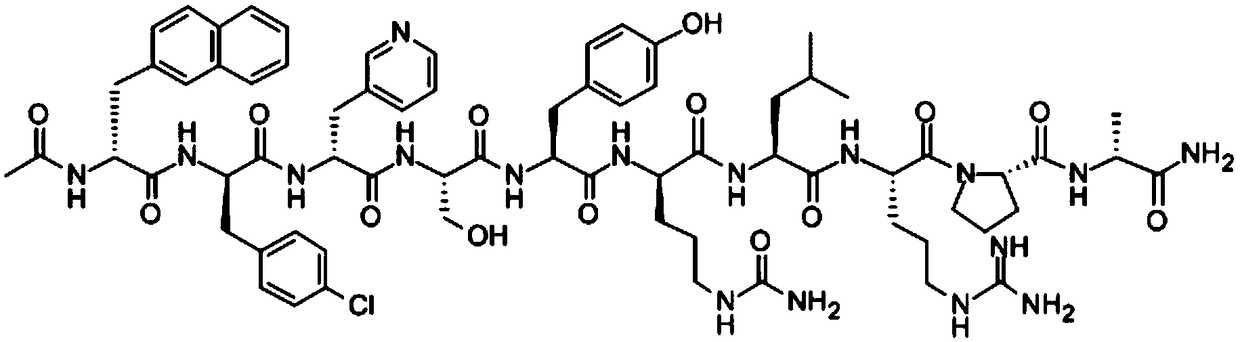
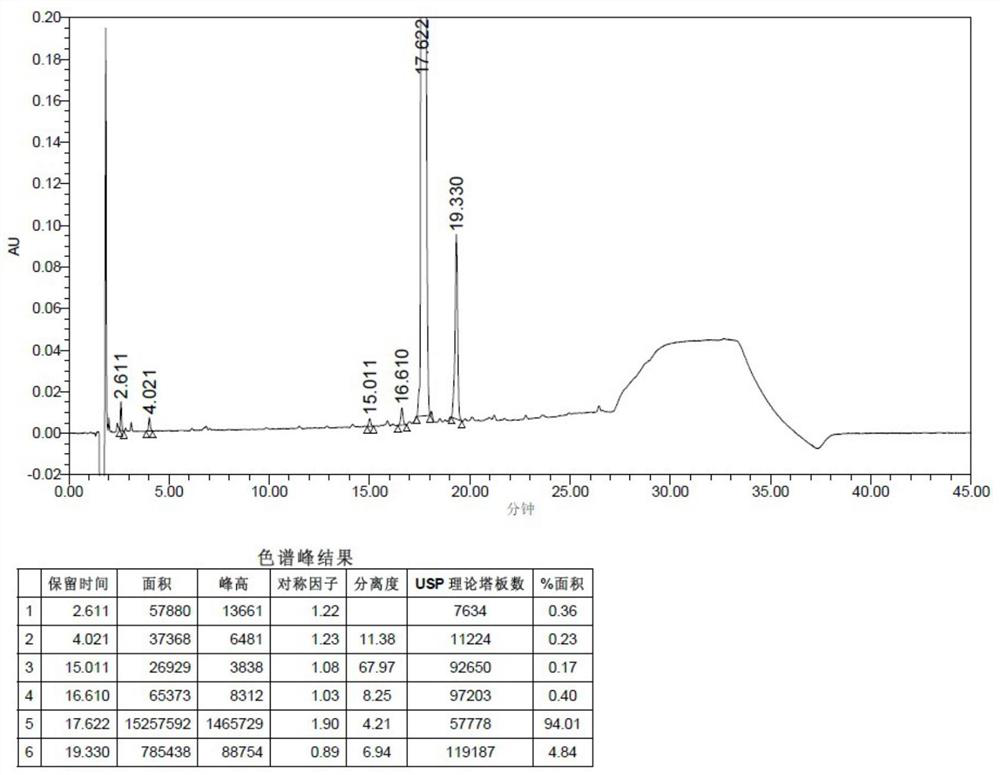
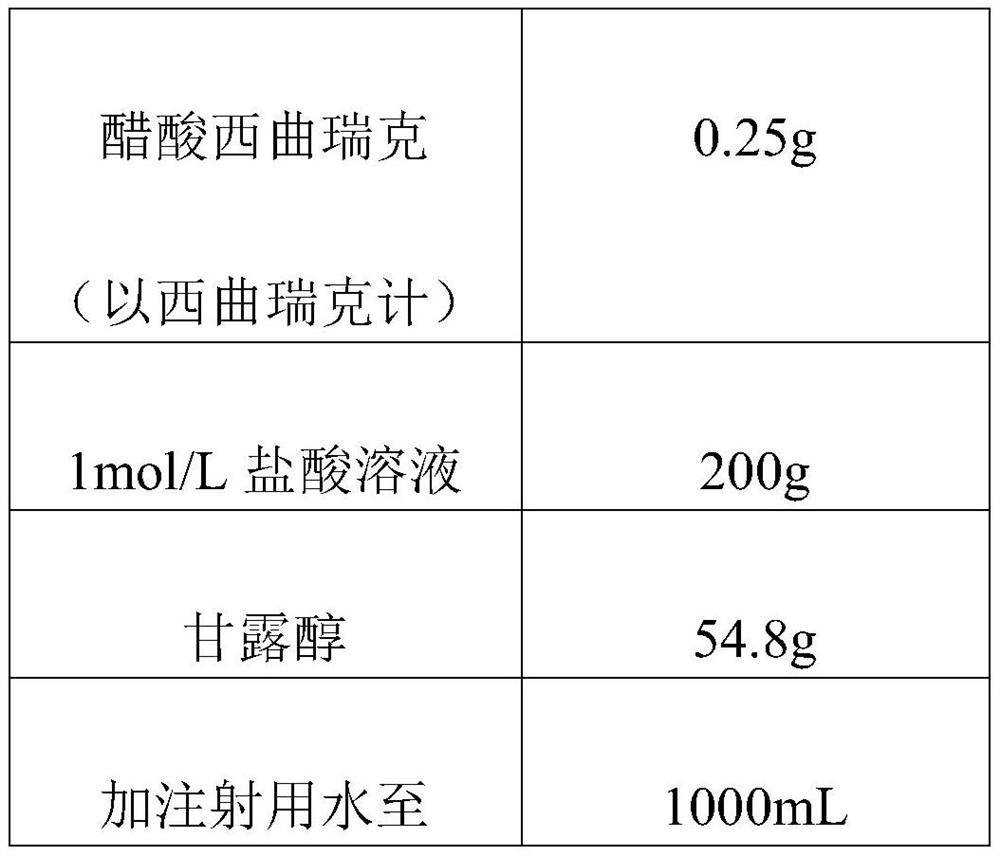
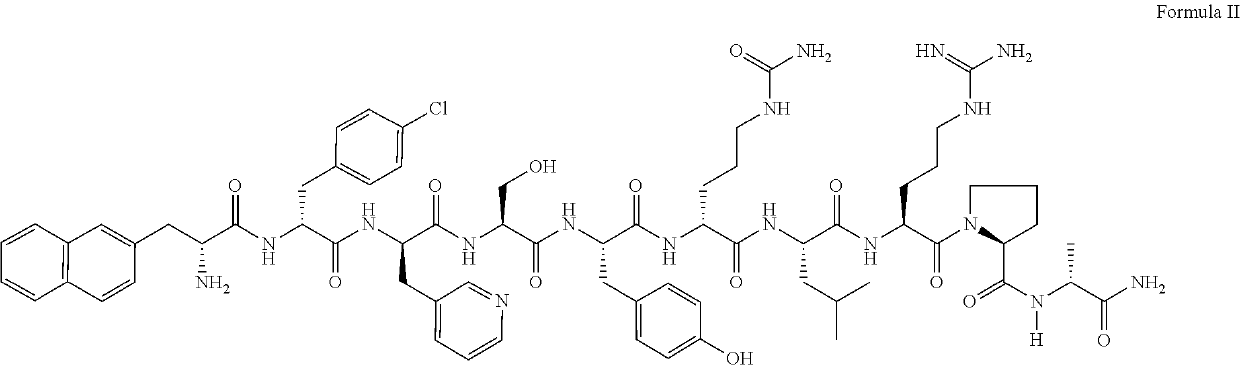
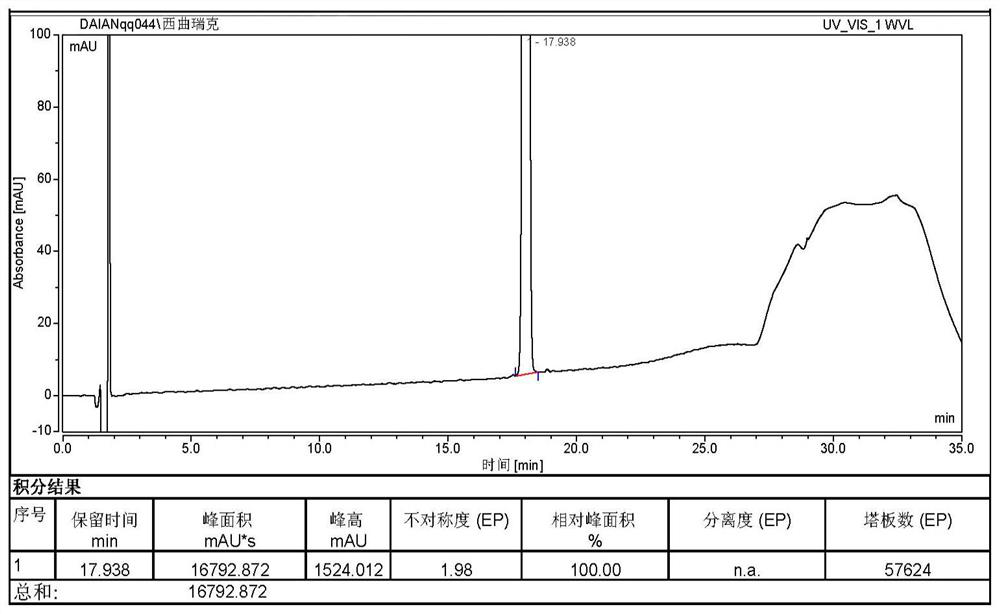
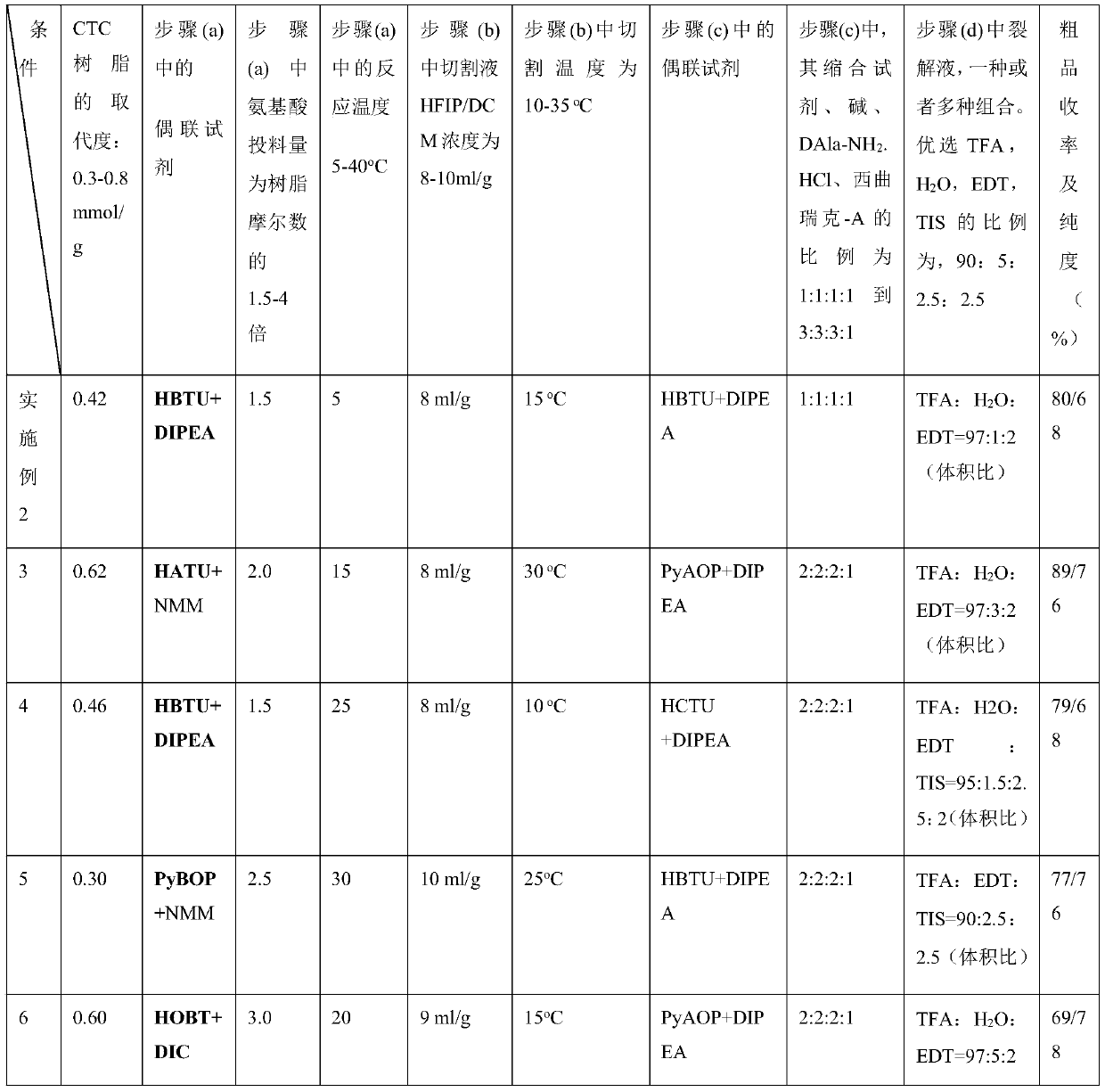
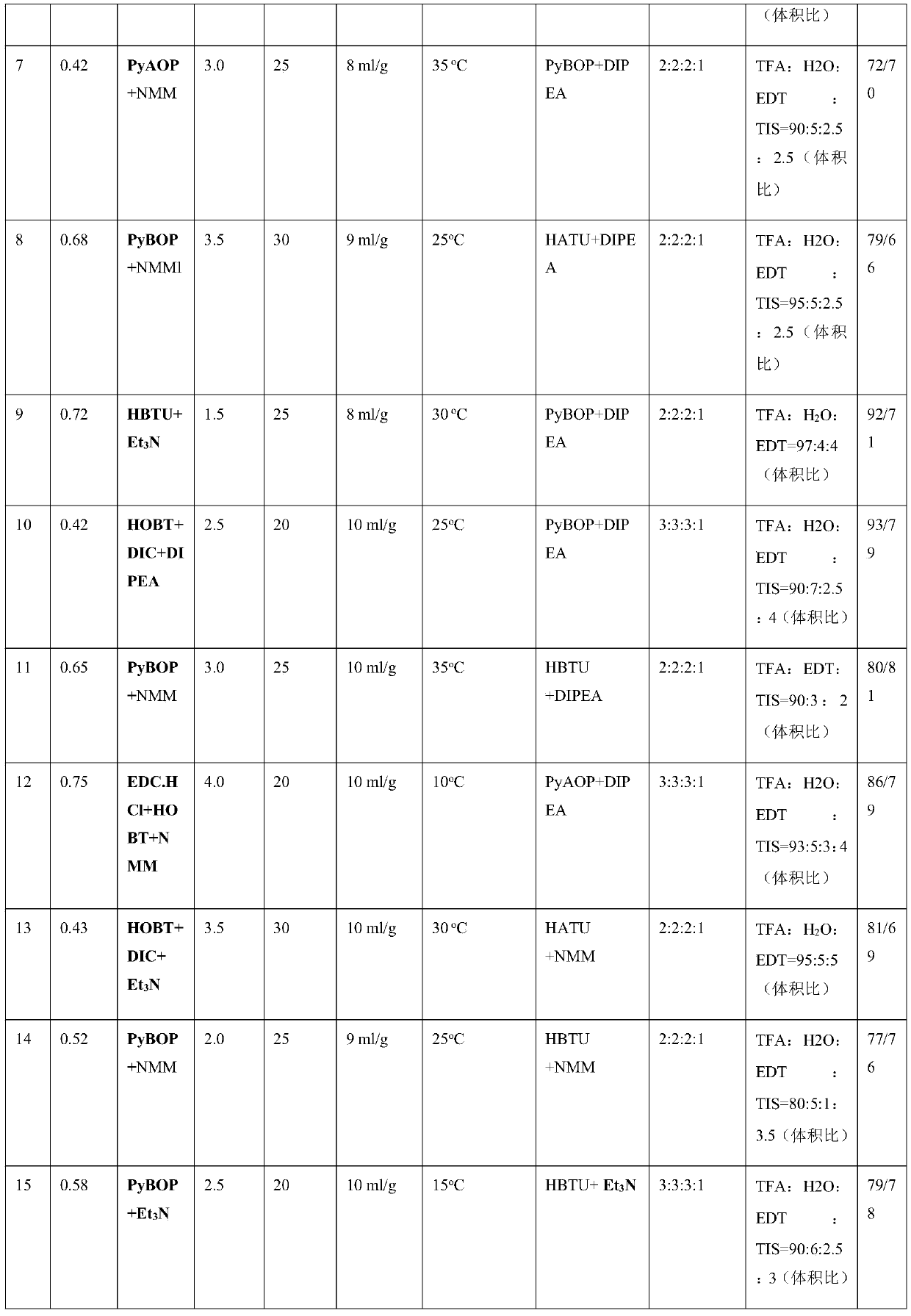
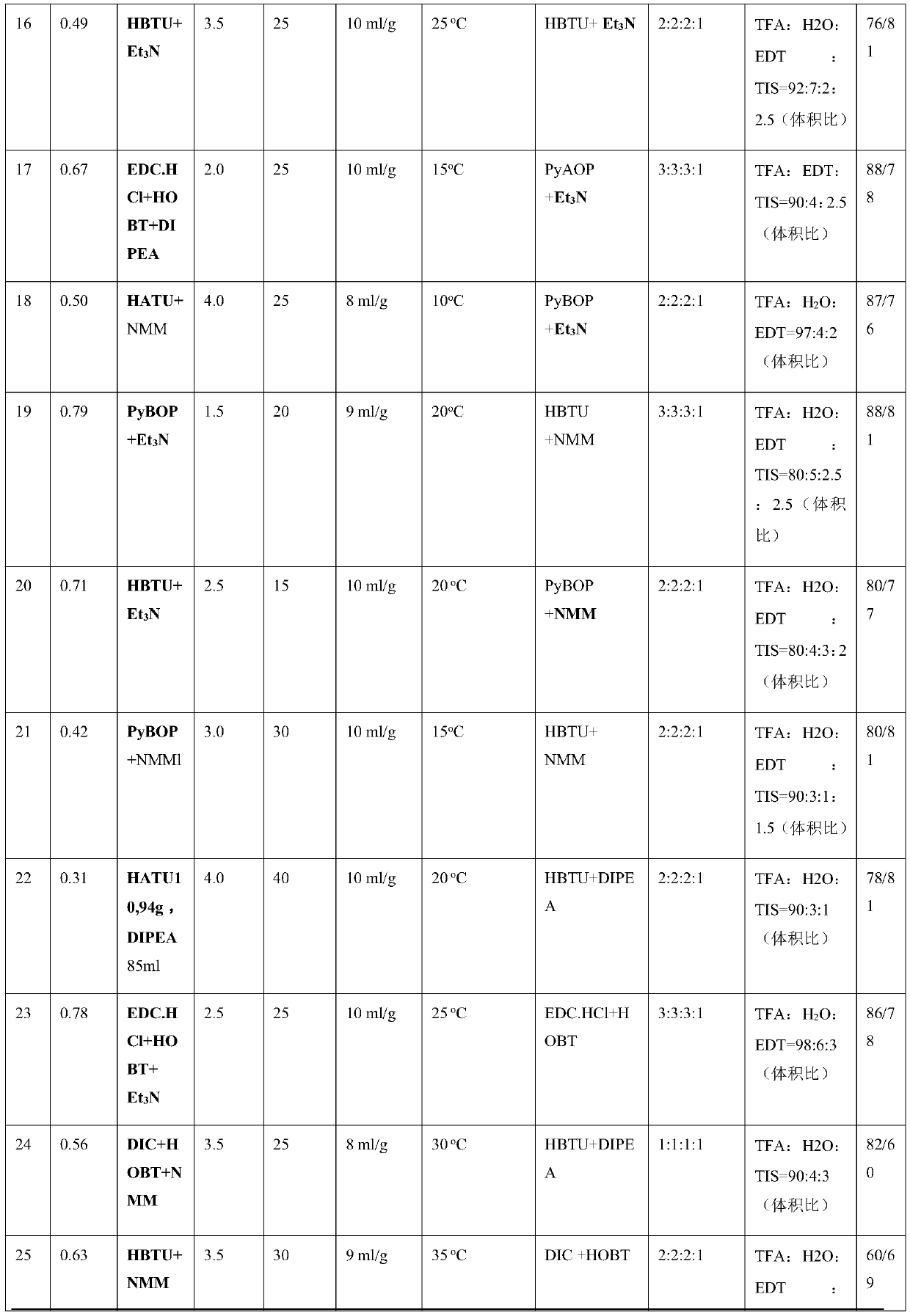
Patents
Literature
36 results about "Cetrorelix" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
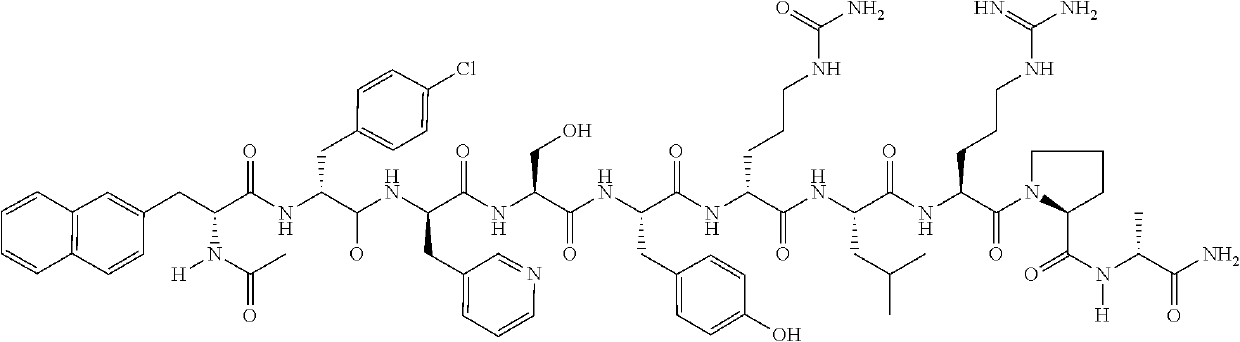
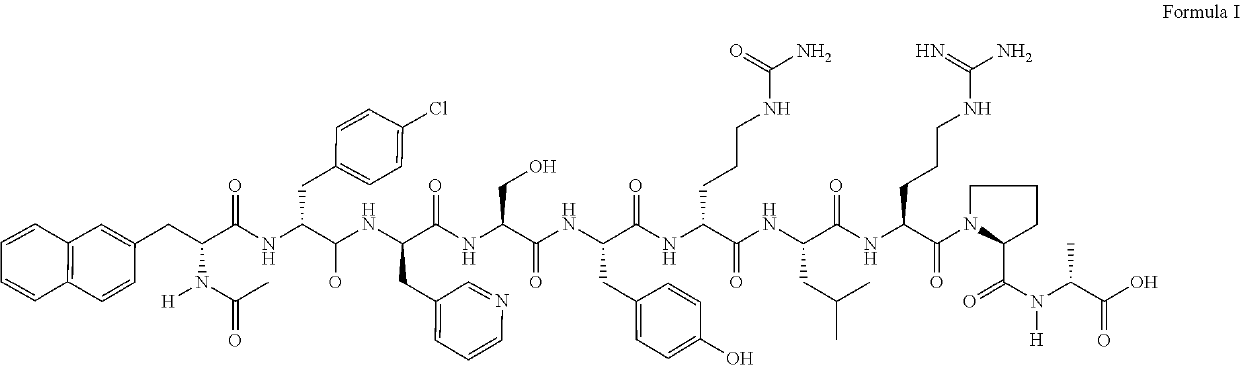
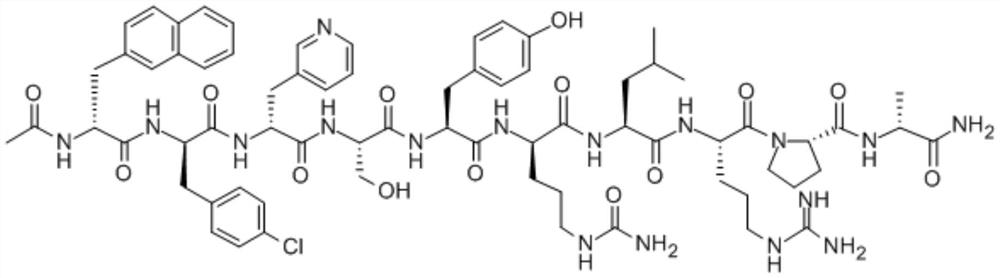
This medication is used by women having certain fertility treatments (controlled ovarian stimulation). Cetrorelix is usually used in combination with other hormones (FSH and hCG).
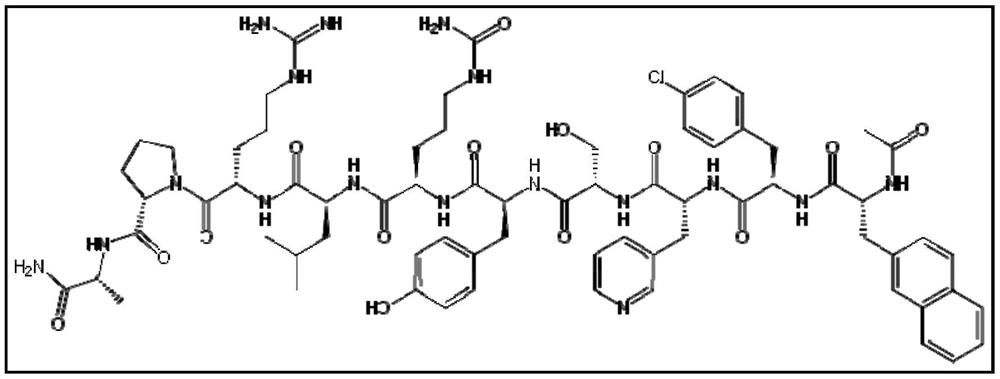
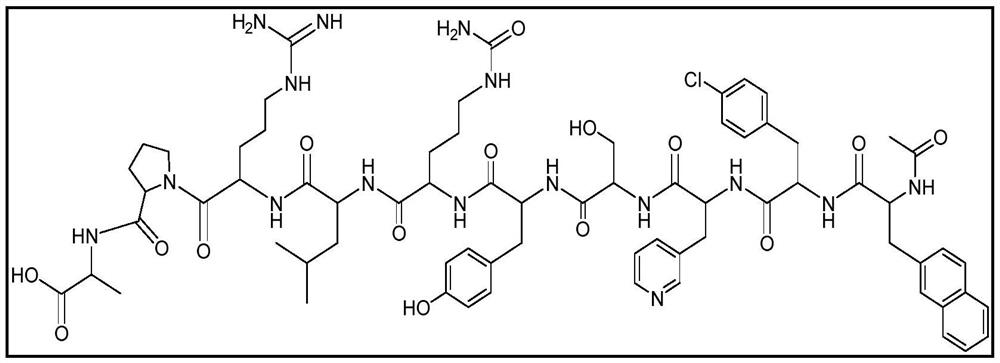
Immobilized and activity-stabilized complexes of LHRH antagonists and processes for their preparation
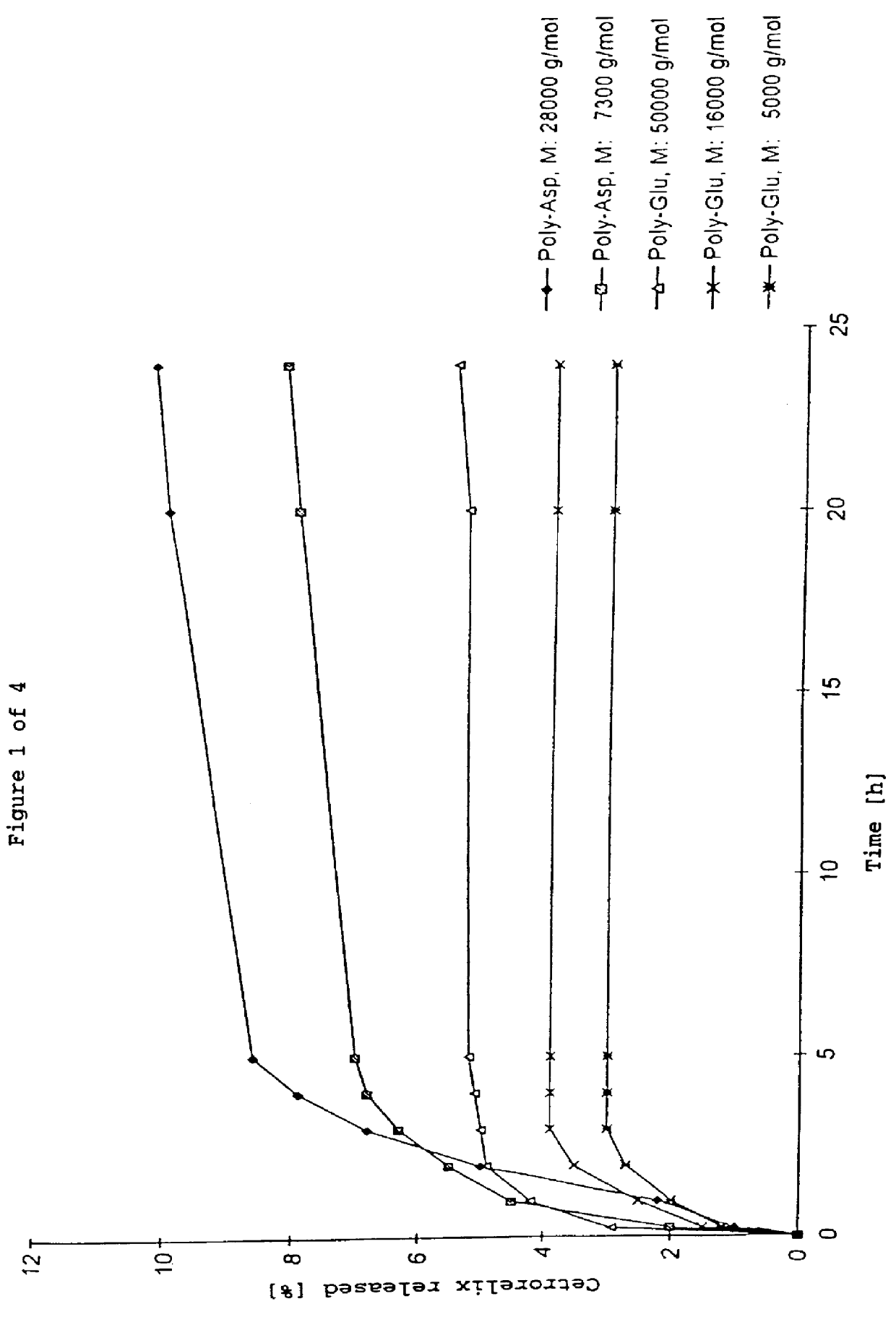
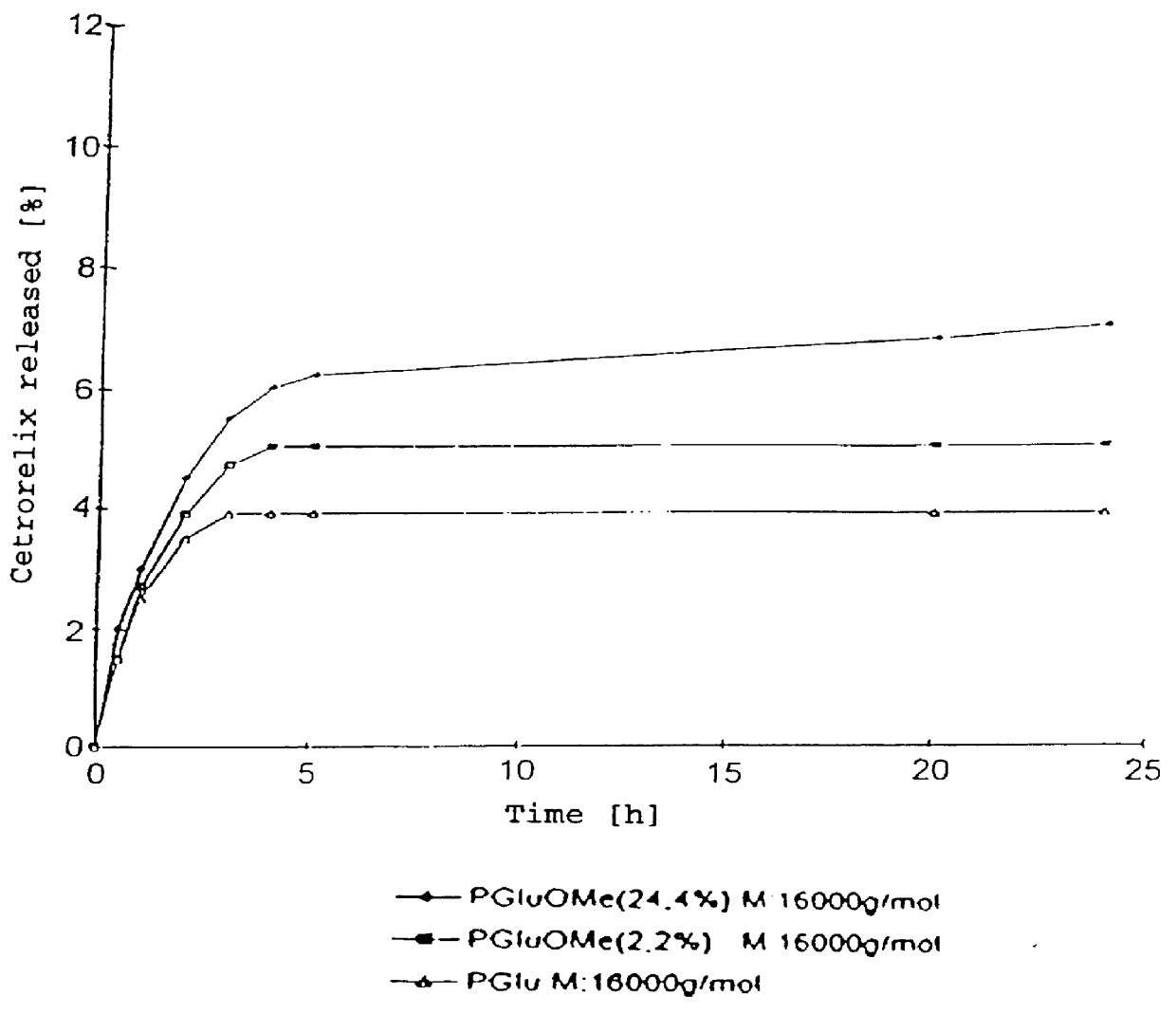
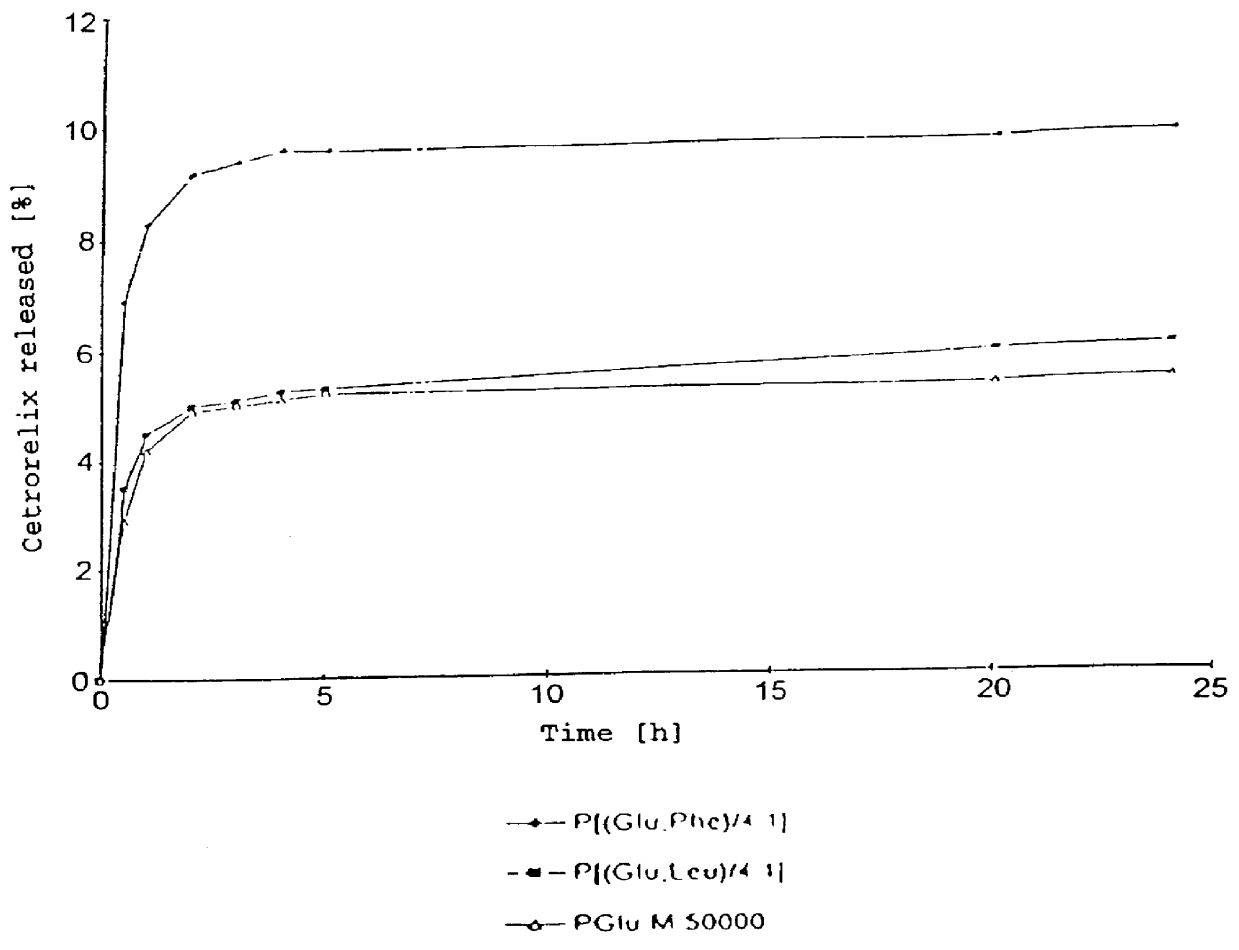
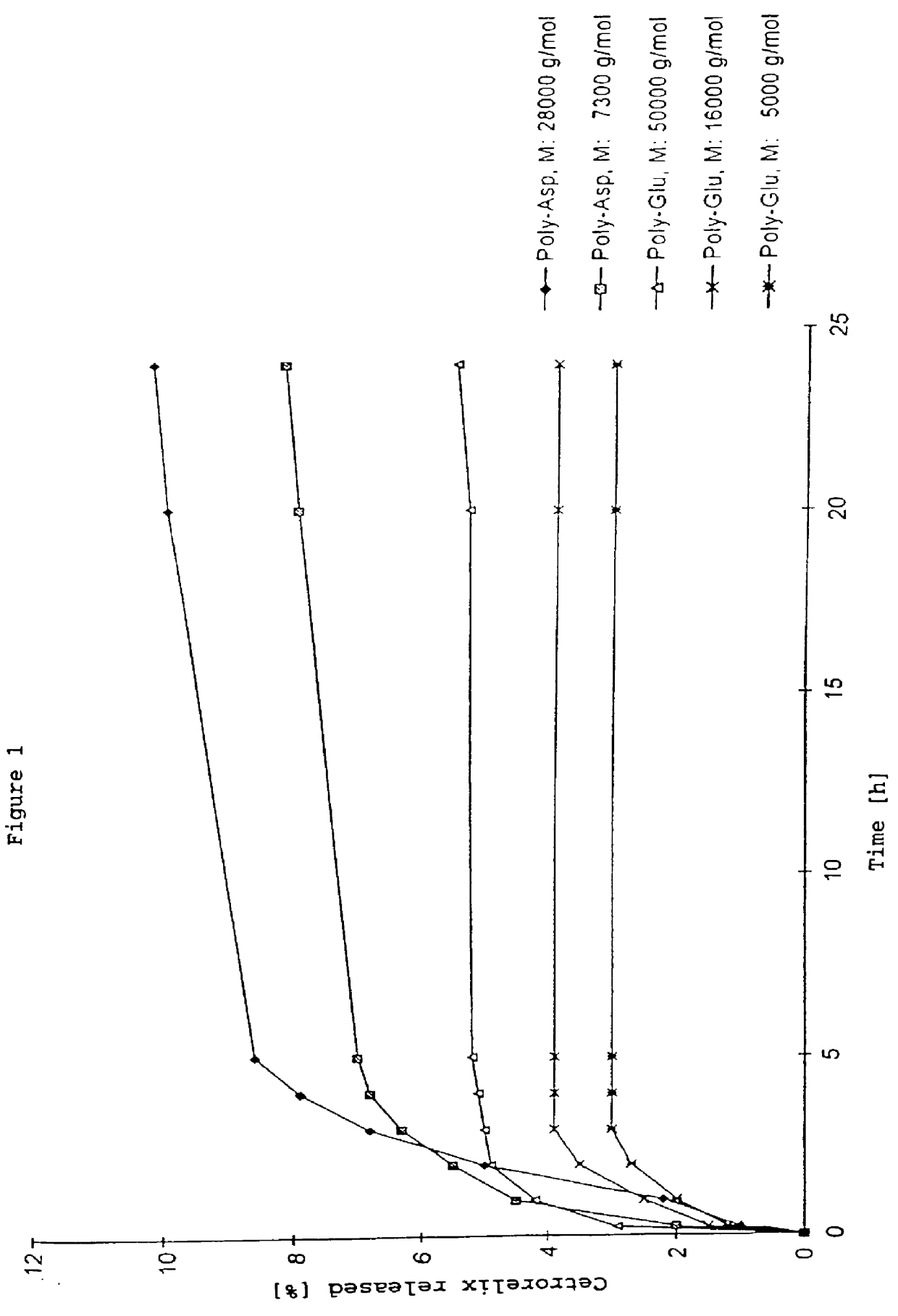
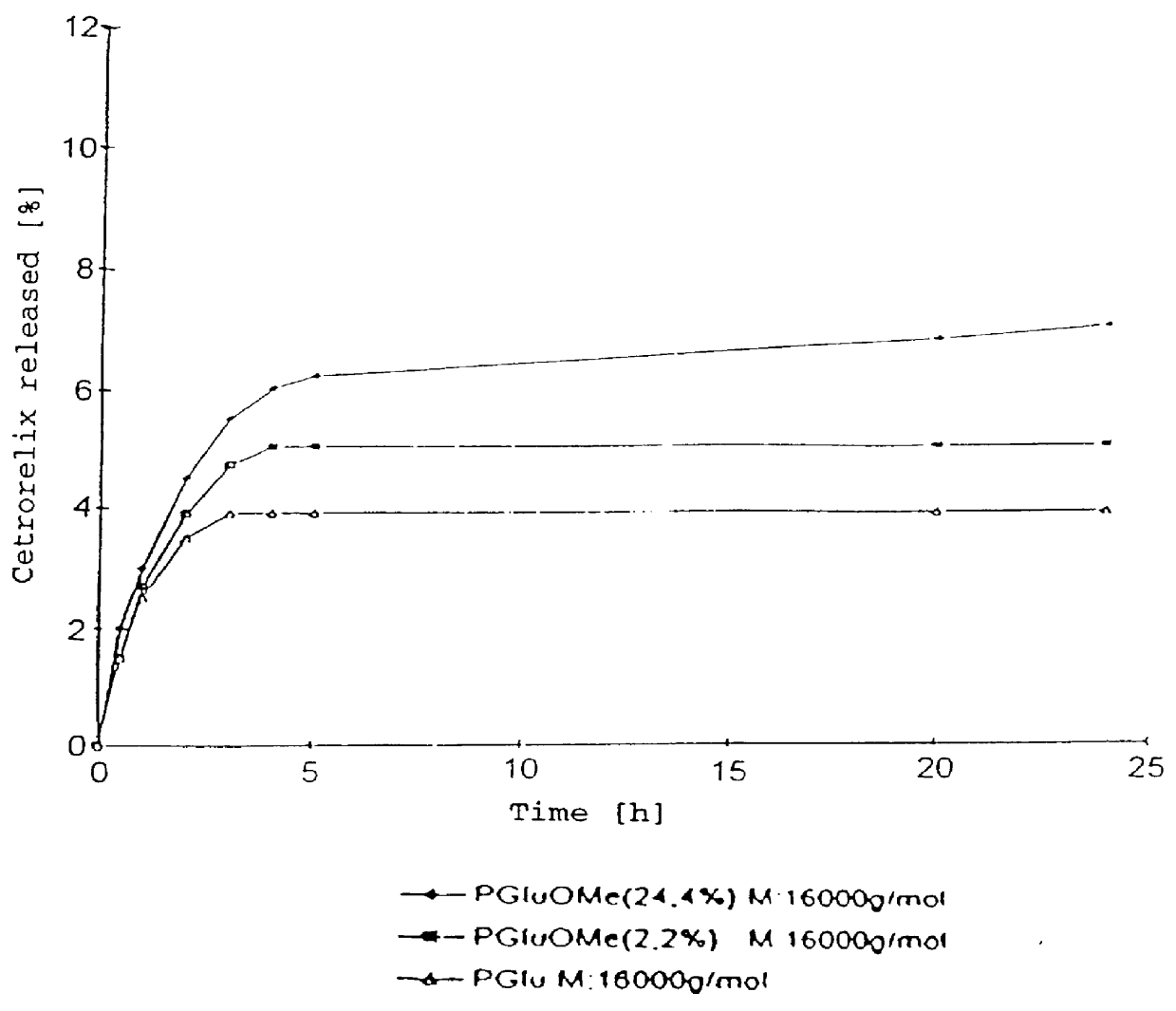
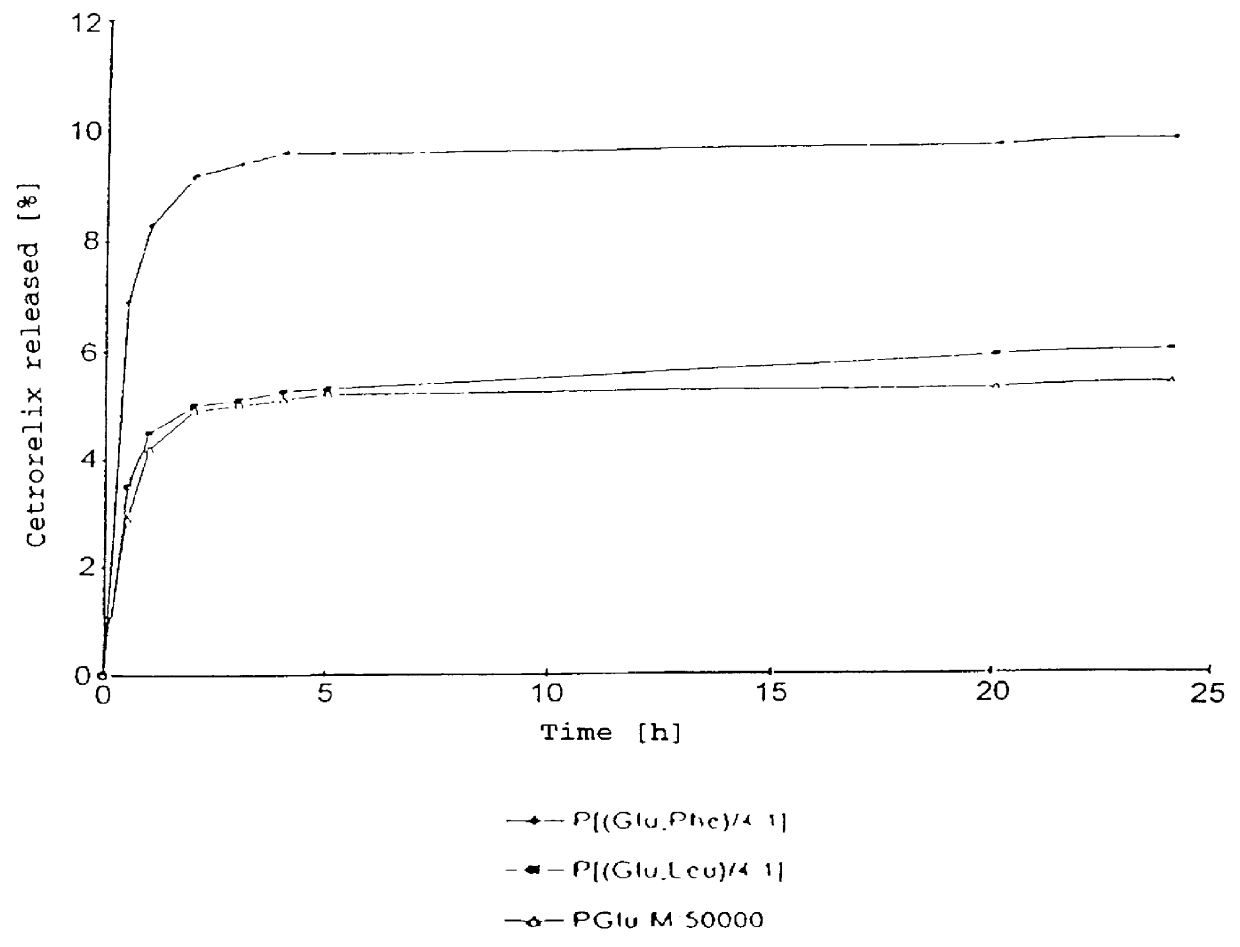
In this invention, a release-delaying system is to be developed for LHRH antagonists, in particular for cetrorelix, which allows the active compound to be released in a controlled manner over several weeks by complexation with suitable biophilic carriers. The acidic polyamino acids polyglutamic acid and polyaspartic acid were selected for complexation with cetrorelix. The cetrorelix polyamino acid complexes are prepared from aqueous solutions by combination of the solutions and precipitation of the complexes, which are subsequently centrifuged off and dried over P2O5 in vacuo. If complexes having a defined composition are to be obtained, lyophilization proves to be a suitable method. The cetrorelix-carboxylic acid complexes were also prepared from the aqueous solutions. In the random liberation system, the acidic polyamino acids poly-Glu and poly-Asp showed good release-delaying properties as a function of the hydrophobicity and the molecular mass of the polyamino acid. In animal experiments, it was possible to confirm the activity of the cetrorelix-polyamino acid complexes as a depot system in principle. It is thus possible by complexation of cetrorelix with polyamino acids to achieve testosterone suppression in male rats over 600 hours. The release of active compound here can be controlled by the nature and the molecular mass of the polymers.
Owner:ZENTARIS GMBH
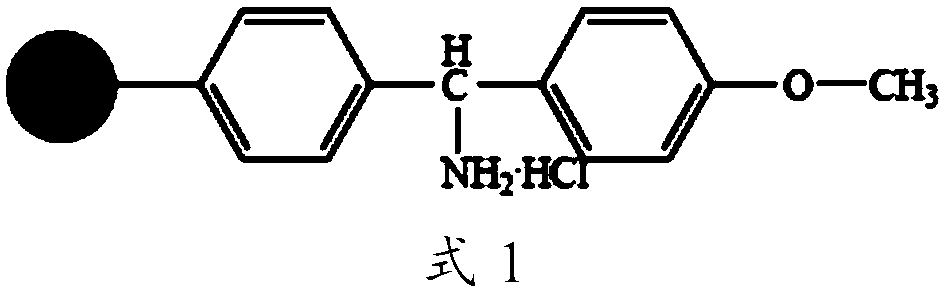
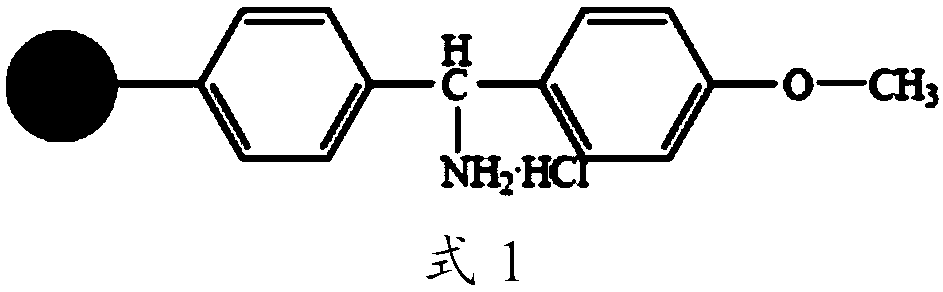
Preparation method of solid phase synthesis cetrorelix
ActiveCN101284863AConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionFreeze-dryingSide chain
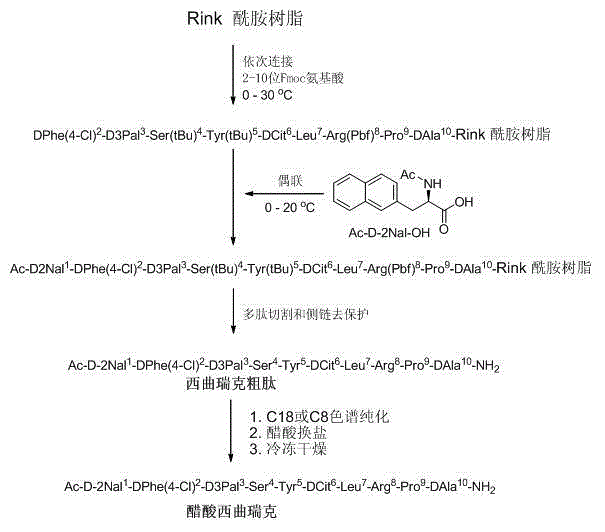
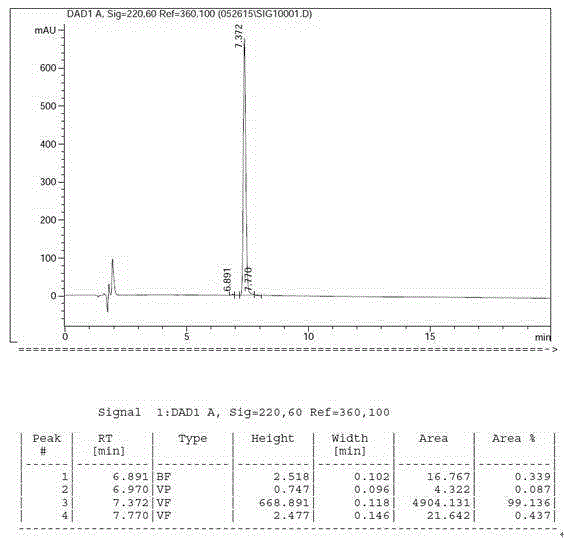
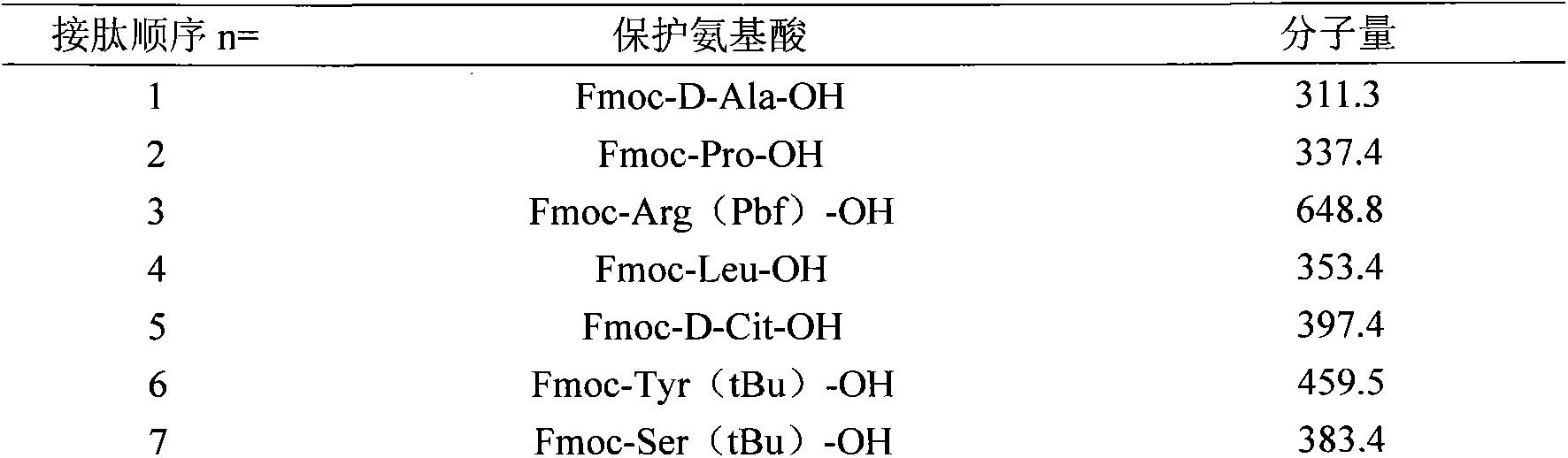
A method for realizing the solid phase synthesis of cetrorelix comprises the following steps that: Fmoc-Linker-MBHA-Resin is used as a starting raw material and is connected in turn with amino acid with Fmoc-protecting groups according to a solid phase synthesis method, so as to obtain protective decapeptide resin; meanwhile, the Fmoc-protecting groups are divested in turn; one sort of HBTU / HOBT or DIC / HOBT is used as condensing agent to carry out synthesized peptide, thereby obtaining protective decapeptide resin; acetylation reaction is carried out, and then protecting group side chain divesting and peptide cutting are carried out synchronously to obtain cetrorelix acetate which is separated and purified through C18 or C8 chromatographic column; finally, cetrorelix acetate acetate or trifluoroacetate is obtained after freeze drying.
Owner:滨海吉尔多肽有限公司
Process for the preparation of immobilized and activity-stabilized complexes of LHRH antagonists
In this invention, a release-delaying system is to be developed for LHRH antagonists, in particular for cetrorelix, which allows the active compound to be released in a controlled manner over several weeks by complexation with suitable biophilic carriers. The acidic polyamino acids polyglutamic acid and polyaspartic acid were selected for complexation with cetrorelix. The cetrorelix polyamino acid complexes are prepared from aqueous solutions by combination of the solutions and precipitation of the complexes, which are subsequently centrifuged off and dried over P2O5 in vacuo. If complexes having a defined composition are to be obtained, lyophilization proves to be a suitable method. The cetrorelix-carboxylic acid complexes were also prepared from the aqueous solutions. In the random liberation system, the acidic polyamino acids poly-Glu and poly-Asp showed good release-delaying properties as a function of the hydrophobicity and the molecular mass of the polyamino acid. In animal experiments, it was possible to confirm the activity of the cetrorelix-polyamino acid complexes as a depot system in principle. It is thus possible by complexation of cetrorelix with polyamino acids to achieve testosterone suppression in male rats over 600 hours. The release of active compound here can be controlled by the nature and the molecular mass of the polymers.
Owner:ZENTARIS GMBH
Preparation method of cetrorelix
InactiveCN104892732ADoes not affect yield issuesReduce pollutionLuteinising hormone-releasing hormonePeptide preparation methodsFluid phaseSide chain
The invention discloses a solid-phase synthesis method of cetrorelix. The method completely avoids toxic [D-Cit(Ac)]-cetrorelix impurities of D-citrulline side chain acetylation. According to the method, amino resin is used as an initial resin carrier, corresponding Fmoc-amino acids in a cetrorelix sequence are sequentially connected with the solid-phase synthesis method, and all-protected cetrorelix peptide resin is obtained through a condensation reaction and a deprotection reaction; then acidolysis cutting and sedimentation by the aid of diethyl ether are performed, and crude cetrorelix peptides are obtained; cetrorelix acetate is obtained through chromatographic purification, acetate conversion and freeze-drying. According to the method, Ac-D-2Nal-OH is selected as connection of the last amino acid, so that acetylation termination is avoided. Both a solid-phase synthesis technology and a liquid-phase synthesis technology can be adopted, few technological byproducts are produced, and separation and purification are easy. The purity of the finally obtained cetrorelix acetate product is up to 99%, the yield is up to 40%, and the method has the considerable economical application value and the broad application prospect.
Owner:CHINESE PEPTIDE CO
Method for preparing cetrorelix
InactiveCN104086632ADoes not affect yieldLow synthetic purityLuteinising hormone-releasing hormonePeptide preparation methodsPolymer scienceBackbone chain
The invention discloses a preparation method of cetrorelix. The method has the specific steps that: (A) AM resin is adopted as initial resin; according to cetrorelix main-chain peptide sequence, amino acids with N-terminal Fmoc protection and side-chain protection are coupled sequentially, wherein peptide sequence 6-site amino acid coupling adopts Fmoc-D-Orn(Dde)-OH; (B) when coupling is finished, Fmoc protecting group is removed, and an acetylation reaction is carried out on the N terminal; (C) a mixed solution with hydrazine hydrate and DMF with a volume ratio of 3:97 is used for removing D-ornithine side-chain Dde protecting group; tert-butyl isocyanate is added, such that D-ornithine side-chain in decapeptide resin is subjected to a modification reaction, and fully protected cetrorelix peptide resin is obtained; and (D) peptide resin is subjected to cracking, purification, desalination, and lyophilization, such that cetrorelix can be obtained. The content of a process impurity [D-Cit(Ac)<6>]-cetrorelix is less than 0.1%. The preparation process provided by the invention has the advantages of high product purity and low cost. The preparation method is suitable for large-scale cetrorelix production. With the process, the content of the impurity [D-Cit(Ac)<6>]-cetrorelix can be effectively controlled without influencing the yield of cetrorelix.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Cetrorelix purification and separation method
InactiveCN107312073AEfficient separationHigh yieldLuteinising hormone-releasing hormonePeptide preparation methodsFreeze-dryingEvaporation
The invention discloses a cetrorelix purification and separation method. The method includes steps: dissolving a crude product of cetrorelix in acetonitrile water solution, and filtering through a filter membrane to obtain crude solution for standby application; adopting a mobile phase A for balancing a reversed phase column, loading the crude solution into the reversed phase column, performing gradient eluting for separation and purification, wherein the mobile phase A refers to sodium dihydrogen phosphate aqueous solution, and a mobile phase B refers to acetonitrile; subjecting target peptide solution with purity higher than 99.5% to vacuum rotary evaporation and concentration at a water temperature not higher than 38 DEG C; adopting acetic acid aqueous solution for balancing the reversed phase column, loading a sample of concentrated liquid into the reversed phase column, and adopting an acetic acid aqueous solution / acetonitrile system for salt conversion; subjecting the converted acetate and the target peptide solution with purity higher than 99.5% to vacuum rotary evaporation and concentration at a water temperature not higher than 38 DEG C, and performing freeze drying to obtain powdery cetrorelix. The obtained cetrorelix is high in purity and yield, meets industrial production requirements and has a high economic value and a promising application prospect.
Owner:ZHEJIANG PEPTITES BIOTECH CO LTD
Method for preparing cetrorelix acetate through specific microwave synthesis
InactiveCN104861042AMild reaction conditionsImprove reaction efficiencyLuteinising hormone-releasing hormonePeptide preparation methodsAcetic acidInternational standard
The invention belongs to the technical field of synthesis and preparation methods of polypeptide drugs, and particularly relates to a synthesis and preparation method for cetrorelix acetate. The method is unique and exclusive. Rink Amide-AM resin is taken as a carrier, a low-cost HBTU / DIEA (hexafluorophosphate / diisopropylethylamine) is taken as a condensing agent, and a specific microwave synthesis technology is adopted, so that the condensation efficiency is improved and the crude yield reaches 92%; obtained crude cetrorelix acetate is purified with a reversed-phase high-performance liquid chromatography (rHPLC) method and subjected to acetate transformation treatment by adopting strong anion exchange resin, the yield of obtained pure cetrorelix acetate reaches more than 40%, and the acetic acid content of cetrorelix acetate reaches international standard requirements. The method greatly shortens reaction time and increases final product yield, thereby having considerable economic and applicable values and wide application prospects.
Owner:合肥国肽生物科技有限公司
Method for preparing cetrorelix acetate by taking Rink Amide-AM Resin as carrier
InactiveCN104277093AMild reaction conditionsImprove reaction efficiencyPeptide preparation methodsMicrowaveTrifluoroacetic acid
The invention belongs to the technical field of preparation methods of polypeptide medicaments and particularly relates to a preparation method of cetrorelix acetate. According to the preparation method provided by the invention, Rink Amide-AM Resin is taken as a carrier, low-cost DIC / HOBt is taken as a condensation agent, and a unique microwave reaction technology is adopted, so that the reaction time is shortened, the condensation efficiency is improved, and the yield of a crude product is more than 90%. The obtained cetrorelix acetate crude product is subjected to reversed-phase high performance liquid chromatography purification and treated by a unique technology for converting acetate to salt, so that the yield of an obtained pure product of cetrorelix acetate is more than 30%, and the toxicity of trifluoroacetic acid to an organism is thoroughly removed. The preparation method provided by the invention optimizes the condensation reaction step, a polypeptide synthesis process is simple and easy to operate, the production cost is low, and the product yield is high, so that the preparation method has considerable economic applicable values and extensive application prospects.
Owner:BIOTECH CO LTD QINGDAO BEI TAIKE
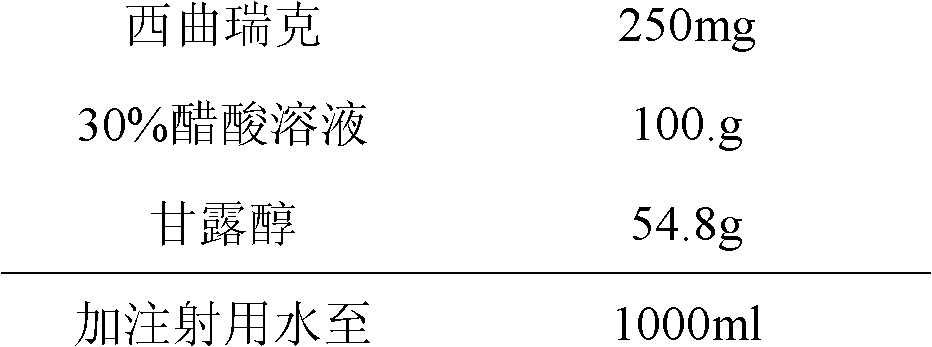
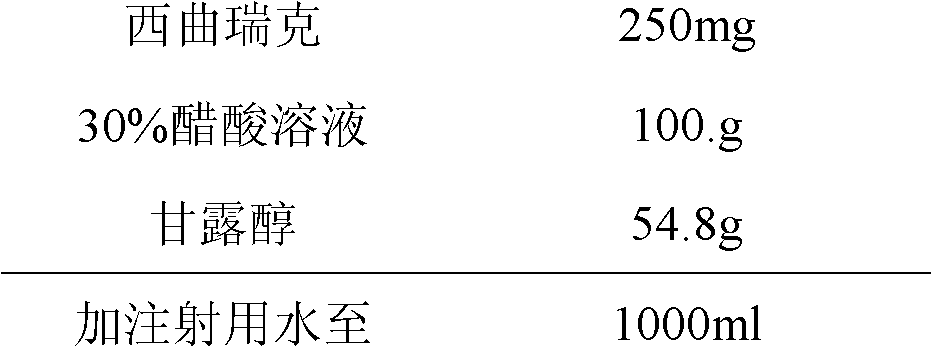
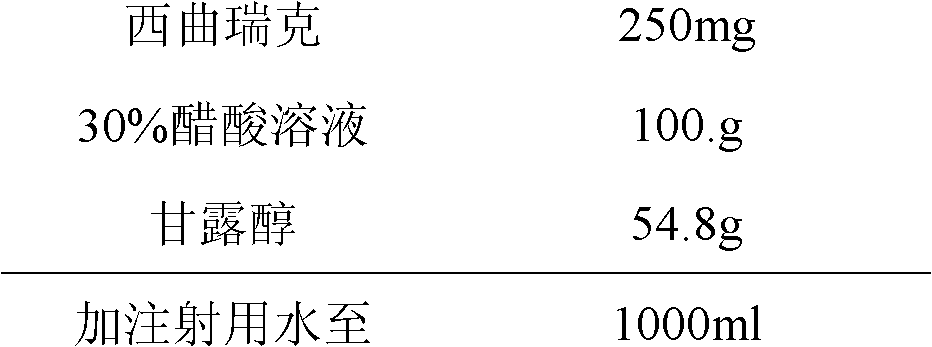
Stable cetrorelix medicinal composition and preparation method thereof
ActiveCN102423484ASteady injectionGuaranteed curative effectPowder deliveryPeptide/protein ingredientsMedicineCurative effect
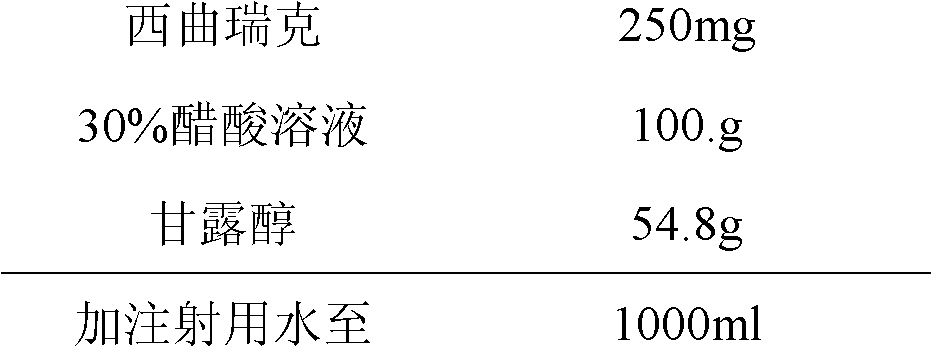
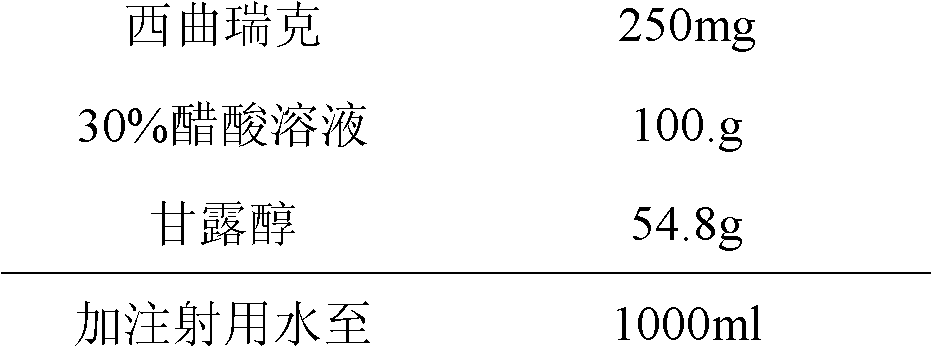
The invention relates to a stable cetrorelix medicinal composition and a preparation method thereof. The medicinal composition is obtained through the preparation method which comprises the following steps: 1, preparing a solution of mannitol and cetrorelix acetate, and filtering the solution; 2, packaging the solution obtained in step 1 to a container, and cooling step by step according to a case which comprises the following stages: a, cooling a plate layer to -25--40DEG C according to a cooling rate of not greater than 3DEG C / min and keeping the temperature for not less than 1h, and b, continuously cooling to -40DEG C or below and keeping the temperature of not less than 1h; and 3, drying to obtain the lyophilized medicinal composition. The stability of the medicinal composition obtained through the preparation method is good, so the medicinal composition still has definite curative effects when the medicinal composition is preserved for a long time.
Owner:辉凌制药中国有限公司
Separation and purification method of cetrorelix
ActiveCN107759667AHigh purityHigh and stable yieldLuteinising hormone-releasing hormonePeptide preparation methodsPurification methodsOrganic solvent
The invention provides a method for efficiently separating and purifying cetrorelix. The method comprises the following steps: dissolving and filtering a crude cetrorelix extract; applying a filteredsolution to a chromatographic column with uniform-particle reverse-phase silica gel, and performing chromatography; eluting a target product by using an aqueous acetic acid solution and an organic solvent as a mobile phase; sectionally collecting solutions with target peaks, and combining component solutions which meet requirements. The method is used for deeply purifying the cetrorelix; by only one chromatographic purification step, a requirement that the any individual impurity content of the cetrorelix is lower than 0.1% can be met; the purification yield is high and stable; moreover, the separation method is simple and convenient and can be applied to large-scale production, and the production cost is greatly reduced.
Owner:SUZHOU NANOMICRO TECH CO LTD
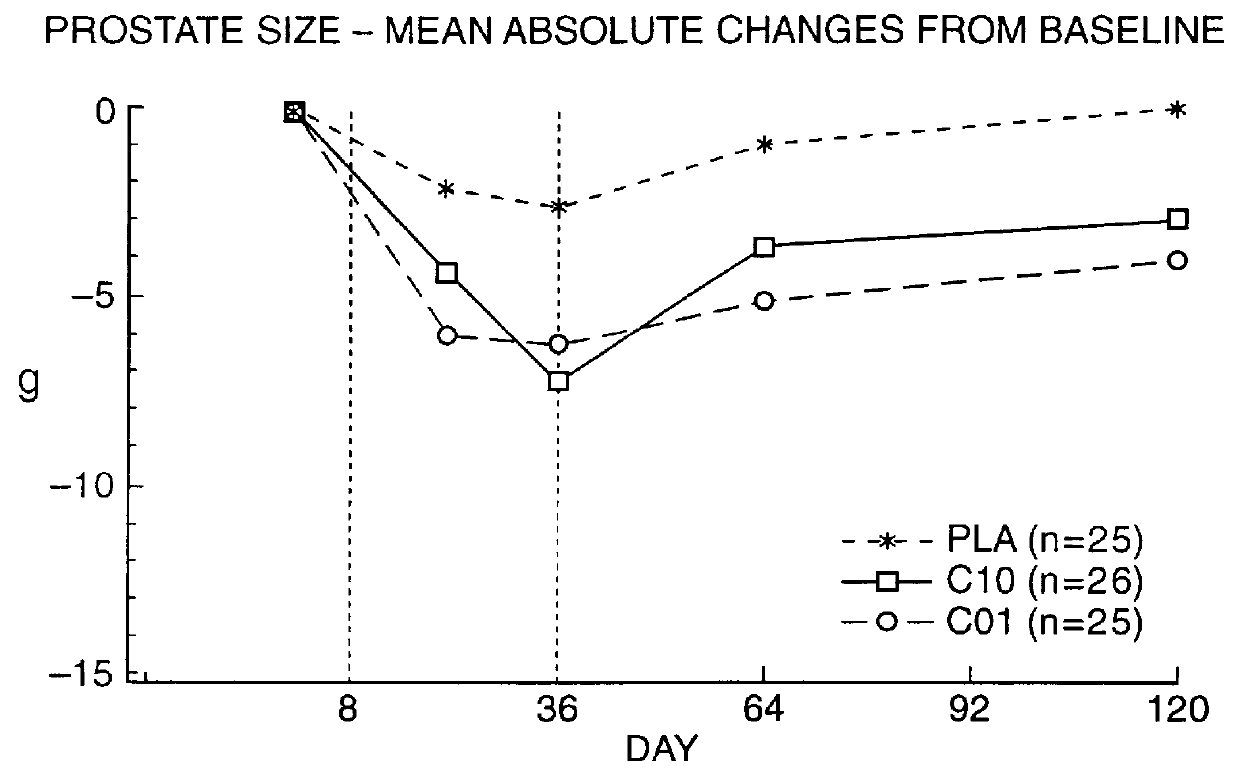
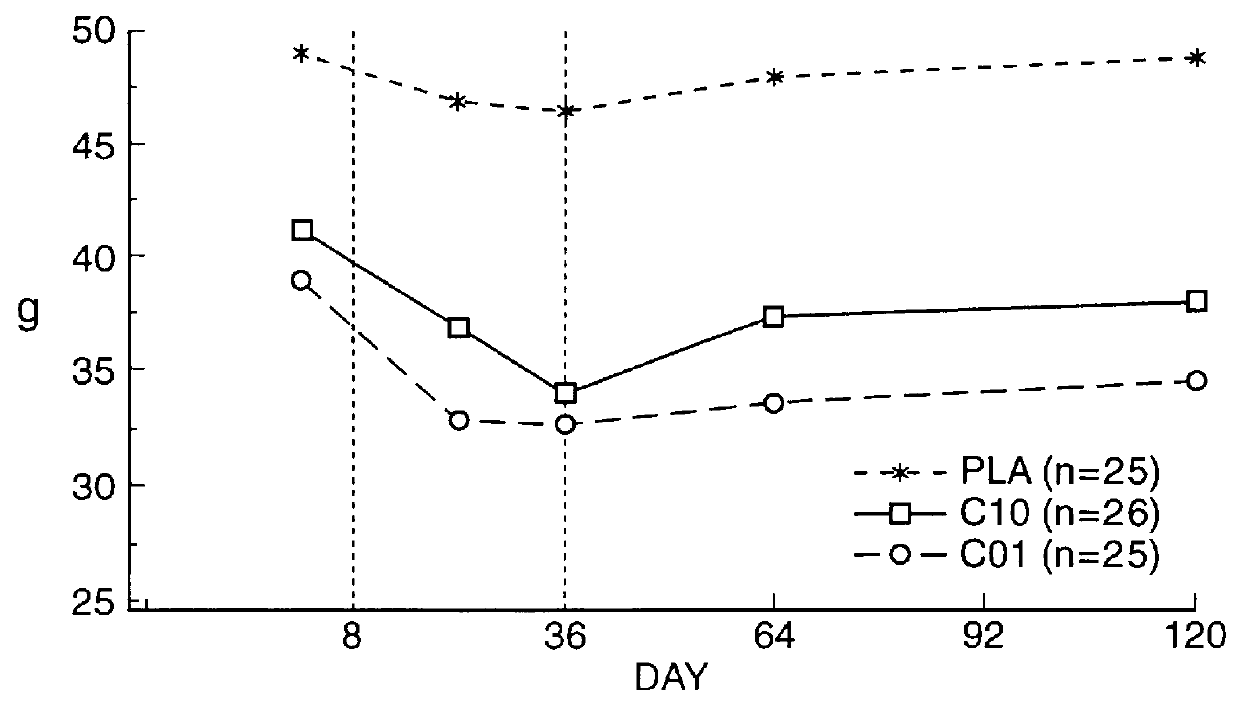
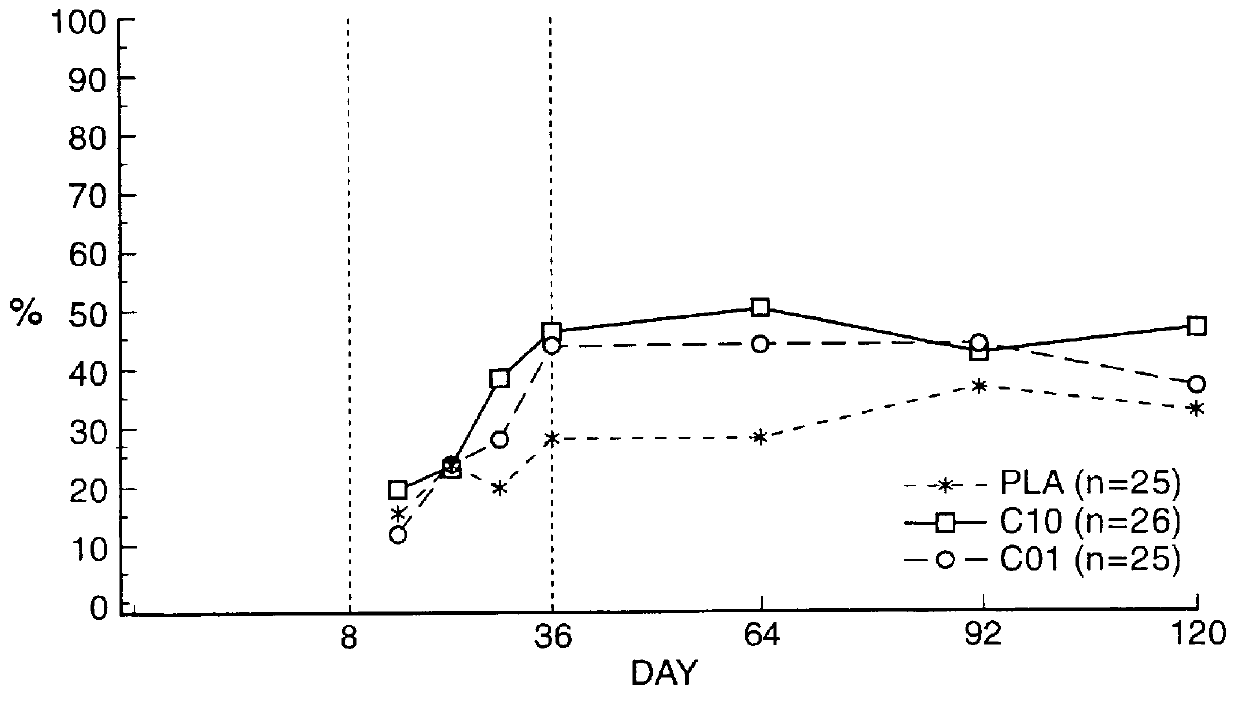
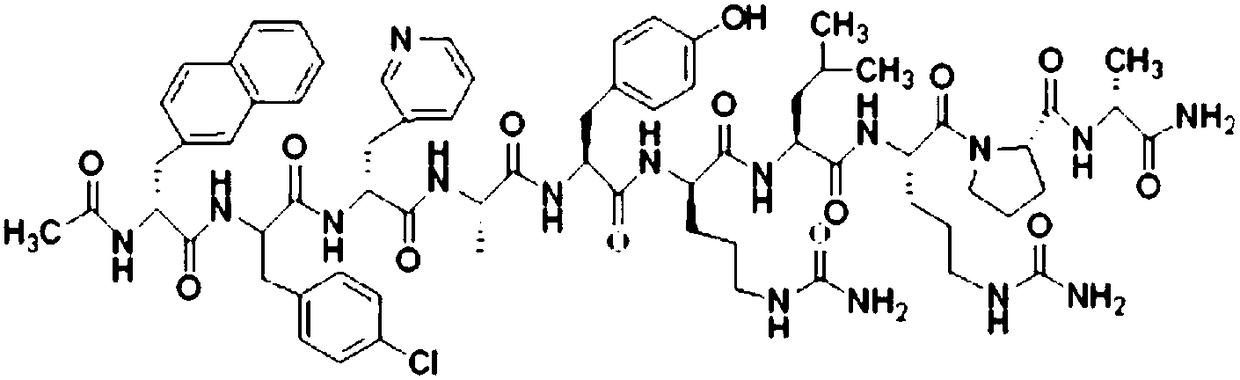
Means for treating prostate hyperplasia and prostate cancer
A regime for therapeutic management of a benign prostatic hyperplasia and prostatic cancer employs Cetrorelix alone or in combination with alpha -reductase inhibitors or alpha -receptor blocking agents. The regimen reduces the volume of the prostate and avoids the side effects associated with testosterone levels being in a castration range. Cetrorelix is administered at dosages between 0.5 mg / day and 20 mg / week or about 0.014 mg / kg body weight per day to 0.30 mg / kg body weight per week or at levels of about 25 to 120 mg of Cetrorelix per month or 0.376 mg / kg to 1.71 mg / kg per month. Cetrorelix can be administered with alpha -reductase inhibitors or alpha -receptor blocking agents.
Owner:ZENTARIS GMBH
Preparing method for cetrorelix
InactiveCN108264540AAvoid it happening againReduce difficultyLuteinising hormone-releasing hormonePeptide preparation methodsSynthesis methodsSide chain
The invention discloses a preparing method for cetrorelix. The preparing method for the cetrorelix includes the following steps that (1) resin is used as a carrier, the resin and the C-terminus aminoacid of the cetrorelix are esterified, and an amino acid-resin compound is prepared; (2) a protective cetrorelix-resin compound is prepared with the Fmoc strategy solid-phase polypeptide synthesis method; (3) a side-chain protecting group is removed with TFA, and a de-protection cetrorelix-resin compound is prepared; (4) a cetrorelix crude product is obtained with the ammonia analytical method; (5) the cetrorelix crude product is purified with the high performance liquid chromatography, and a cetrorelix competitive product is obtained. The preparing method is suitable for simply, convenientlyand efficiently preparing the cetrorelix; the problems that an existing method is numerous in side reaction, low in yield and high in cost are solved.
Owner:JIANGSU GENSCRIPT BIOTECH CO LTD
Preparation and use of oligopeptide lyophilisate for gonad protection
InactiveUS6867191B2Reduce physical and mental burdenEasy to usePowder deliveryBiocideAcetic acidFreeze-drying
A lyophilizate, method of preparation, and use of the lyophilizate for gonad protection is described. The lyophilizate comprises cetrorelix dissolved in 30% (v / v) acetic acid, transferred to water, and freeze-dried.
Owner:ZENTARIS IVF
Preparation method of solid phase synthesis cetrorelix
ActiveCN101284863BConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionFreeze-dryingSide chain
Owner:滨海吉尔多肽有限公司
Oligopeptide lyophilisate, their preparation and use
InactiveUS6863891B2Successful treatmentHigh activityPowder deliveryOrganic active ingredientsAcetic acidFreeze-drying
A novel lyophilizate and method of preparation as well as the use of the lyophilizate to treat female infertility and for gonad protection. Cetrorelix is dissolved in acetic acid 30% v / v, the solution is transferred to water and freeze dried.
Owner:ZENTARIS IVF
Cetrorelix acetate injection and preparation method thereof
PendingCN113616775ASafety and Effectiveness GuaranteeSolve the appearancePowder deliveryPeptide/protein ingredientsPhysical chemistryEthylic acid
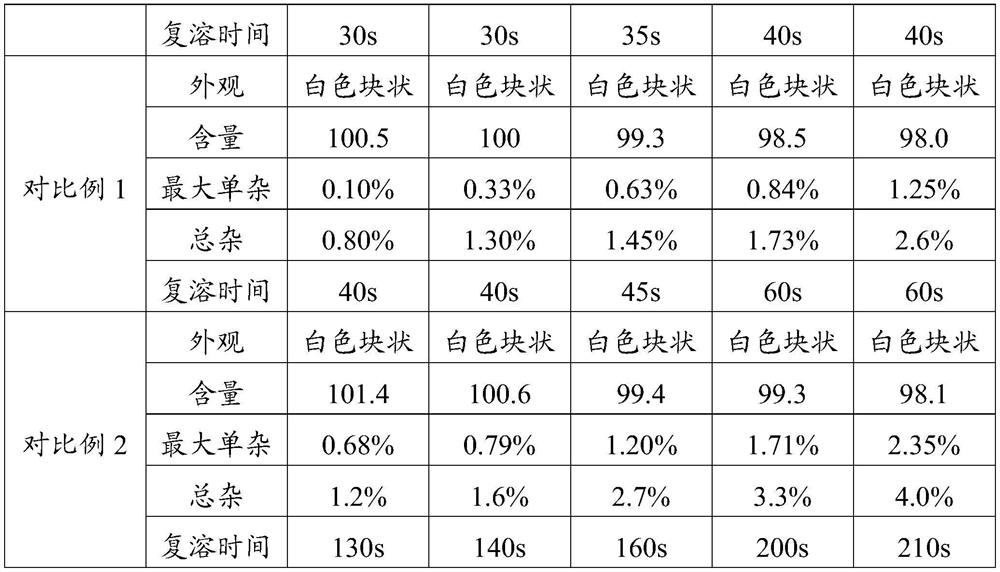
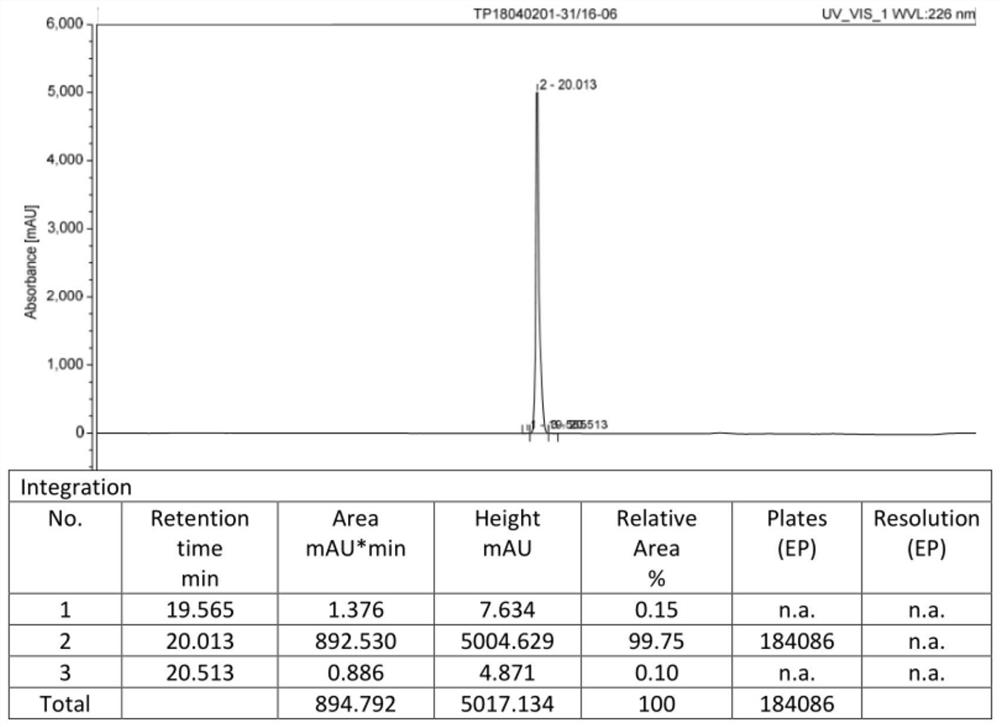
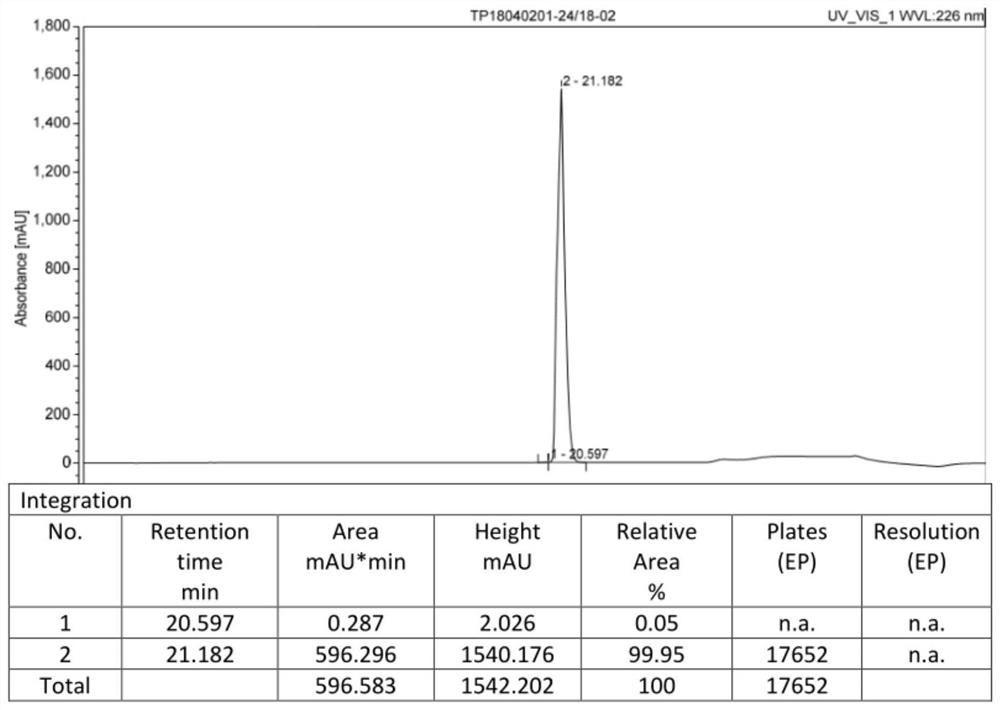
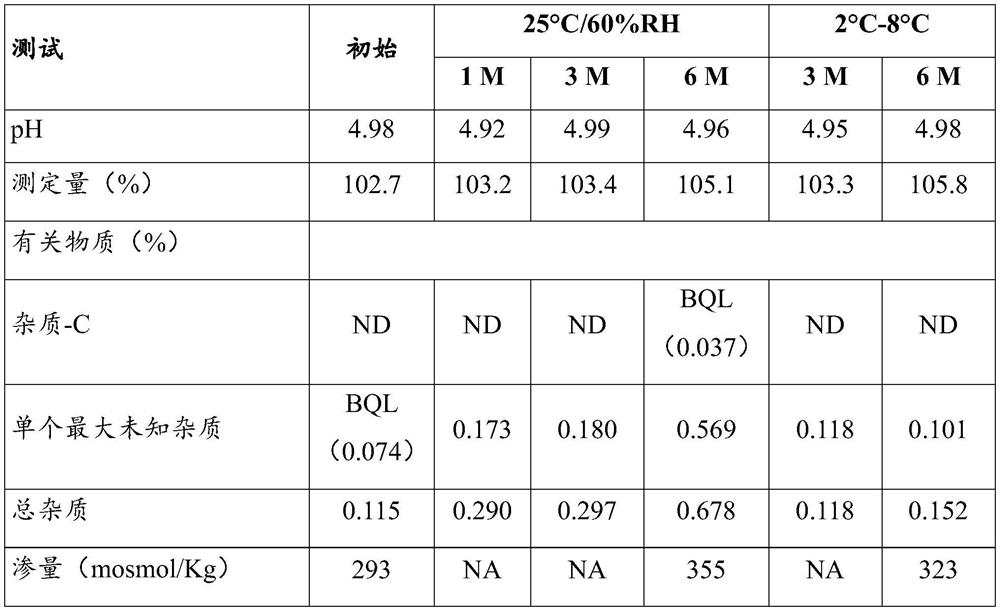
The invention provides a cetrorelix acetate injection and a preparation method thereof. By controlling the pre-freezing cooling rate and the primary drying vacuum degree, the obtained freeze-dried product is a blocky solid, the phenomenon of active ingredient aggregation after redissolution is avoided, and the impurity level of the freeze-dried product is reduced. The product disclosed by the invention is low in initial moisture and controllable in key quality attribute such as moisture, turbidity, pH, purity and the like in the storage process, and effectiveness and safety are better guaranteed.
Owner:南京锐志生物医药有限公司
Cetrorelix synthesis method
ActiveCN107778355AHigh purityHigh yieldLuteinising hormone-releasing hormonePeptide preparation methodsSynthesis methodsSide chain
The invention relates to the field of synthesis of medicines and discloses a cetrorelix synthesis method. A brand new amino resin is used as a carrier, protected D-Orn is taken as a precursor of D-Cit, a side chain protecting group is removed, the D-Orn is reacted with tert-butyl isocyanate to generate the D-Cit (tBu), a cetrorelix resin is dissolved by a special trifluoroacetic acid solution comprising hydrogen bromide, moreover, the side chain protecting group tBu of the generated D-Cit (tBu) is maximally removed, the finally obtained cetrorelix is higher in purity and total yield, the production of the toxic impurity [D-Cit(Ac)]-cetrorelix is avoided, the whole method is simple, convenient and easy to operate, the conditions are mild, and the product quality is relatively high.
Owner:CHENGDU SHENGNUO BIOPHARM
Method for purifying cetrorelix
ActiveCN109467591AReduce manufacturing costHigh yieldLuteinising hormone-releasing hormonePeptide preparation methodsOrganic solventPurification methods
The invention discloses a method for purifying cetrorelix and belongs to the technical field of separation and purification. The method disclosed by the invention mainly comprises the following steps:dissolving, stirring and filtering crude cetrorelix peptides; loading the filtered solution into a reverse phase silica gel column filled with polyoctadecane for performing chromatography; eluting the target product by taking a mixed solution of sodium sulfate and trifluoroacetic acid as a mobile phase A and taking an organic solvent as a mobile phase B; collecting the elution solution accordingto a target peak order, analyzing and detecting various collected solutions, gathering all the collected solutions meeting requirements, and inspecting. According to the method for efficiently and rapidly separating and purifying cetrorelix disclosed by the invention, [D-Arg8]-cetrorelix and [beta-Ala-D-Cit6]-cetrorelix can be effectively separated, the single purity content can be less than 0.1%by virtue of one-step purification, the total purity is 99.9% or higher, and the purification yield reaches 80%. The production process is simple and can be applied to enlarged and large-scale production, and the production cost of enterprises is reduced.
Owner:苏州天马医药集团天吉生物制药有限公司
Synthetic method of cetrorelix
ActiveCN112159461AEasy to synthesizeHigh yieldLuteinising hormone-releasing hormonePeptide preparation methodsPolymer scienceSide chain
The invention discloses a synthetic method of cetrorelix. According to the method, a Knorr-2-Cl-Resin resin is used as a starting raw material, and ten different amino acid resins are sequentially connected according to a solid-phase synthesis method to obtain a protected decapeptide resin. Fluorenylmethoxycarbonyl is sequentially removed during synthesis, then an N,N-dimethylformamide solution ofbenzotriazole-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate and N,N-diisopropylethylamine is used as a condensation agent for carrying out a condensation reaction to obtain the protected decapeptide resin, an acetylation reaction is carried out after the condensation reaction, side chain protecting groups are removed, a cetrorelix crude product is obtained after cracking, and the obtainedcetrorelix crude product is purified to obtain a finished product. According to the synthetic method, the Knorr-2-Cl-Resin resin is used as the starting raw material, and PyBoP / DIPEA is used as the condensation reagent, so that the polypeptide sequence is easier to synthesize, and the purity of the prepared cetrorelix crude peptide is relatively high.
Owner:KAIFENG MINGREN PHARMA
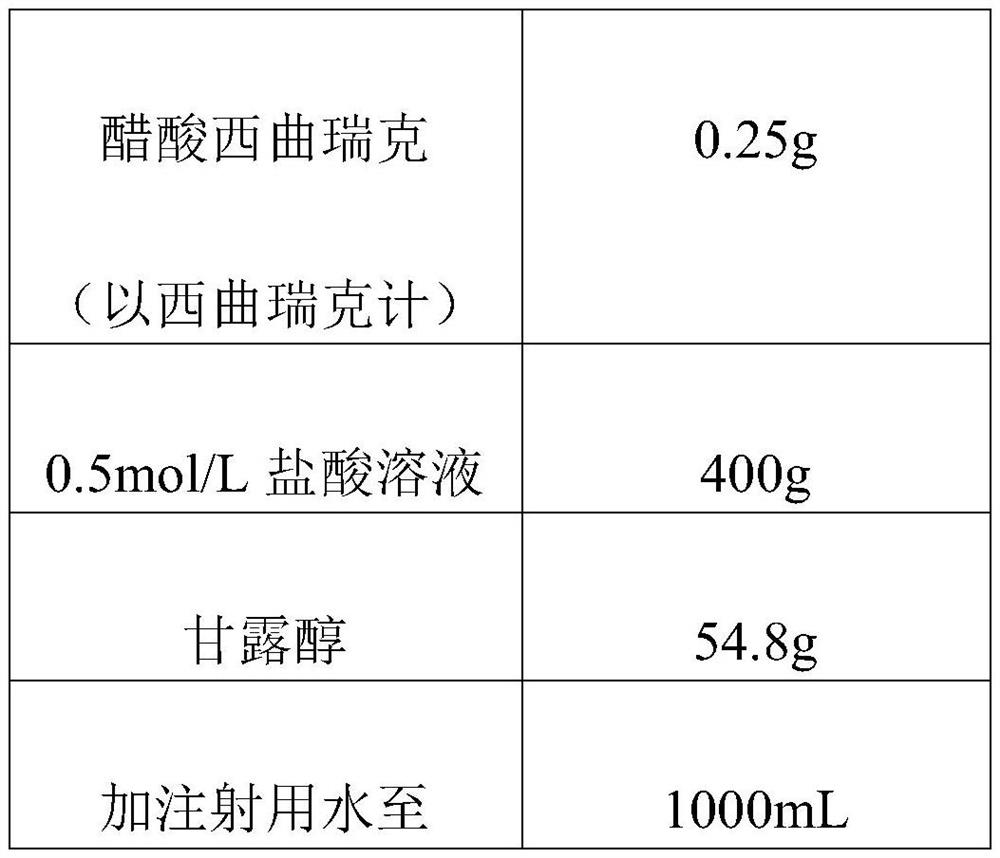
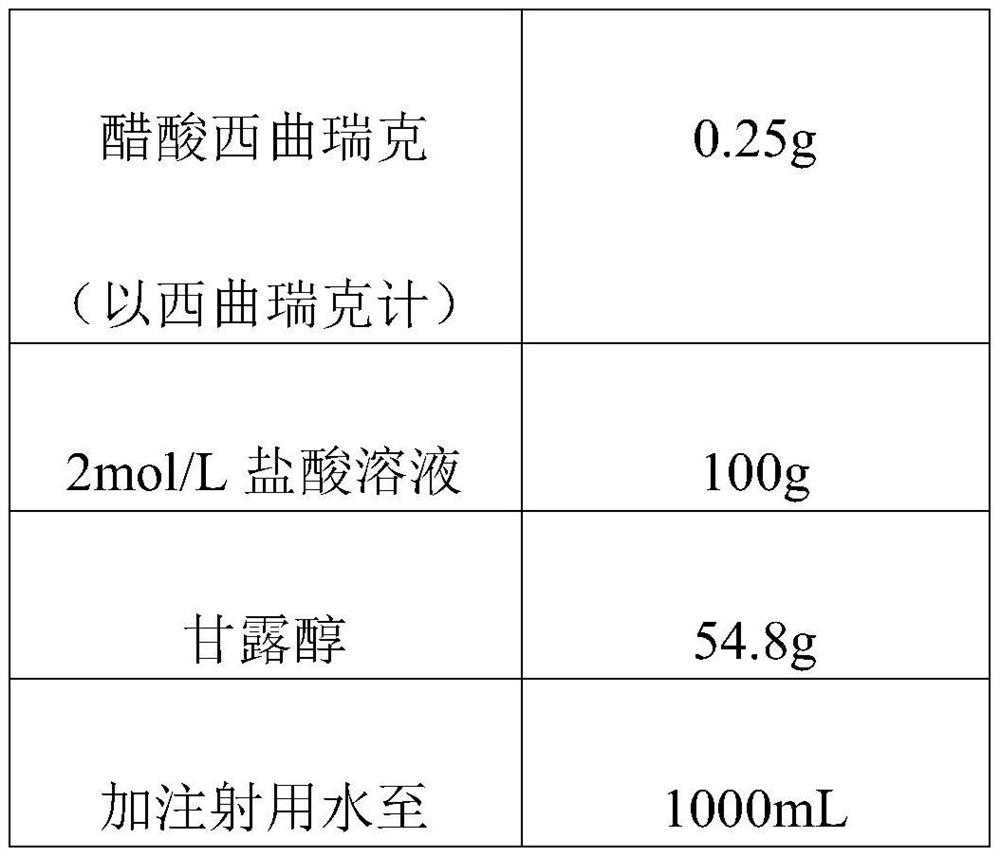
Cetrorelix pharmaceutical composition and preparation method thereof
PendingCN112717119AImprove stabilityShort reconstitution timePeptide/protein ingredientsInorganic non-active ingredientsUse medicationMedicine
The invention discloses a cetrorelix pharmaceutical composition and a preparation method thereof, and relates to the technical field of biological medicine. The preparation method comprises the steps that cetrorelix acetate is dissolved with hydrochloric acid, and a cetrorelix acetate hydrochloric acid solution is obtained; wherein in the cetrorelix acetate hydrochloric acid solution, the final concentration of hydrochloric acid is 0.05-3 mol / L. The cetrorelix medicine prepared by the preparation method provided by the invention has higher stability, can be stored for a long time at room temperature or ambient temperature, obviously shortens the redissolution time, and improves the safety during clinical medication.
Owner:南京康舟医药科技有限公司
Stable parenteral dosage form of cetrorelix acetate
PendingUS20210121517A1Improve performanceTrend downPeptide/protein ingredientsComponent separationParenteral Dosage FormCetrorelix
The present invention relates to a stable parenteral dosage form with a ready-to-inject sterile stable aqueous solution of Cetrorelix acetate. The invention also relates to an injection device prefilled with the ready-to-inject sterile stable aqueous solution of Cetrorelix acetate. The present invention relates a method of inhibiting premature luteinizing hormone surges in women undergoing controlled ovarian stimulation comprising a stable parenteral dosage form with a ready-to-inject sterile stable aqueous solution of Cetrorelix acetate.
Owner:SUN PHARMA INDS
Stable cetrorelix medicinal composition and preparation method thereof
ActiveCN102423484BGuaranteed curative effectSimple processPowder deliveryPeptide/protein ingredientsMedicineCurative effect
The invention relates to a stable cetrorelix medicinal composition and a preparation method thereof. The medicinal composition is obtained through the preparation method which comprises the following steps: 1, preparing a solution of mannitol and cetrorelix acetate, and filtering the solution; 2, packaging the solution obtained in step 1 to a container, and cooling step by step according to a case which comprises the following stages: a, cooling a plate layer to -25--40DEG C according to a cooling rate of not greater than 3DEG C / min and keeping the temperature for not less than 1h, and b, continuously cooling to -40DEG C or below and keeping the temperature of not less than 1h; and 3, drying to obtain the lyophilized medicinal composition. The stability of the medicinal composition obtained through the preparation method is good, so the medicinal composition still has definite curative effects when the medicinal composition is preserved for a long time.
Owner:辉凌制药中国有限公司
Salt conversion method of cetrorelix
ActiveCN112250735AAvoid degradationReduce the temperatureLuteinising hormone-releasing hormonePeptide preparation methodsReverse osmosisReversed-Phase Liquid Chromatography
The invention discloses a salt conversion method of cetrorelix. The method comprises the following steps of: firstly, preparing a crude peptide solution from cetrorelix crude peptide, filtering by adopting a water-based filter membrane, and collecting filtrate; carrying out reversed phase chromatography purification on the obtained filtrate, and collecting target peak eluent to obtain a cetrorelixsolution; concentrating the obtained solution by adopting a reverse osmosis membrane to obtain a concentrated solution; carrying out salt conversion on the obtained concentrated solution by adoptinga C18 reversed phase chromatographic column, replacing by adopting two systems in sequence in the salt conversion process, eluting by adopting an acetic acid solution methanol system, starting to collect the eluent after a main peak appears during elution, and stopping collecting after the main peak is completely ended to obtain a cetrorelix solution; and concentrating the obtained solution by adopting the reverse osmosis membrane, transferring the concentrated mixed solution into a freeze-drying disc, and putting the freeze-drying disc into a freeze dryer for freeze-drying to obtain a cetrorelix acetate finished product. By utilizing the method to carry out salt conversion on cetrorelix, the replacement efficiency is relatively high, and the obtained product is free of trifluoroacetic acid residues.
Owner:KAIFENG MINGREN PHARMA
Preparation method of cetrorelix
InactiveCN110903352ANo racemization reactionHigh purityLuteinising hormone-releasing hormonePeptide preparation methodsFluid phaseCetrorelix
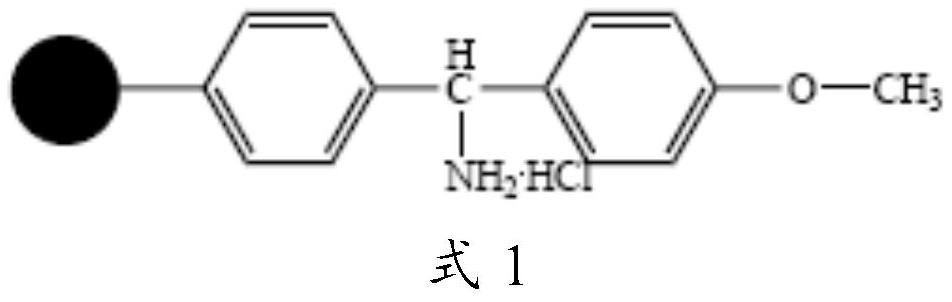
The invention relates to a preparation method of cetrorelix, specifically to a synthesis method combining solid and liquid phases. According to the order of cetrorelix sequence from C terminal to N terminal, 1-9 fragments are connected one by one by the solid-phase synthesis method under the action of a coupling reagent, and then the 1-9 fragments undergo fully-protected cut and then are subjectedto fragment condensation with DAla-NH2.HCl in a liquid phase so as to obtain fully-protected cetrorelix; the cetrorelix is cut by a lysis buffer; a cutting fluid is precipitated by ice ether to obtain cetrorelix crude peptide; and finally the cetrorelix crude peptide is purified by reverse-phase high-performance liquid chromatography to obtain a cetrorelix finished product. The preparation methodis simple, is suitable for industrial production, and has high production efficiency.
Owner:CHINESE PEPTIDE CO
A kind of method of synthesizing cetrorelix
ActiveCN107778355BHigh purityHigh yieldLuteinising hormone-releasing hormonePeptide preparation methodsSide chainTrifluoroacetic acid
The invention relates to the field of medicine synthesis and discloses a method for synthesizing cetrorelix. The present invention adopts a brand-new amino resin as a carrier, and adopts protected D-Orn as the precursor of D-Cit, then removes the side chain protecting group and reacts with tert-butyl isocyanate to generate D-Cit (tBu), through the unique containing Hydrogen bromide trifluoroacetic acid solution to acid hydrolyze the cetrorelix peptide resin, and simultaneously remove the side chain protecting group tBu of the generated D-Cit(tBu) to the greatest extent, and the finally obtained cetrorelix has a higher The purity and total yield avoid the generation of toxic impurity [D-Cit(Ac)]-cetrorelix, the whole method is simple and easy to operate, the conditions are mild, and the product quality is high.
Owner:CHENGDU SHENGNUO BIOPHARM
Cetrorelix acetate for injection and preparation method thereof
PendingCN114159544AGood dispersionImprove temperature resistancePowder deliveryPeptide/protein ingredientsHydroxyethyl starchMedicine
The invention belongs to the technical field of biological medicine, and particularly relates to cetrorelix acetate for injection and a preparation method thereof.The preparation method specifically comprises the steps that hydroxyethyl starch and mannitol are added into a cetrorelix acetate solution and mixed through ultrasonic waves, and a solution A is obtained; and performing spray freeze-drying treatment on the solution A to obtain cetrorelix acetate for injection. The cetrorelix acetate for injection is prepared by the preparation method. The cetrorelix acetate for injection prepared by the preparation method of the cetrorelix acetate for injection provided by the invention can be effectively prevented from being hydrolyzed after being unsealed, and the temperature resistance of the cetrorelix acetate for injection can be effectively improved after the cetrorelix acetate for injection is unsealed.
Owner:福州华为医药技术开发有限公司
A kind of purification method of cetrorelix
ActiveCN109467591BReduce manufacturing costHigh yieldLuteinising hormone-releasing hormonePeptide preparation methodsFluoroacetic acidTrifluoroacetic acid
The invention discloses a method for purifying cetrorelix and belongs to the technical field of separation and purification. The method disclosed by the invention mainly comprises the following steps:dissolving, stirring and filtering crude cetrorelix peptides; loading the filtered solution into a reverse phase silica gel column filled with polyoctadecane for performing chromatography; eluting the target product by taking a mixed solution of sodium sulfate and trifluoroacetic acid as a mobile phase A and taking an organic solvent as a mobile phase B; collecting the elution solution accordingto a target peak order, analyzing and detecting various collected solutions, gathering all the collected solutions meeting requirements, and inspecting. According to the method for efficiently and rapidly separating and purifying cetrorelix disclosed by the invention, [D-Arg8]-cetrorelix and [beta-Ala-D-Cit6]-cetrorelix can be effectively separated, the single purity content can be less than 0.1%by virtue of one-step purification, the total purity is 99.9% or higher, and the purification yield reaches 80%. The production process is simple and can be applied to enlarged and large-scale production, and the production cost of enterprises is reduced.
Owner:苏州天马医药集团天吉生物制药有限公司
Stable formulation of cetrorelix
InactiveCN114096266AOrganic active ingredientsPeptide/protein ingredientsPatient complianceCetrorelix
Owner:INTAS PHARM LTD
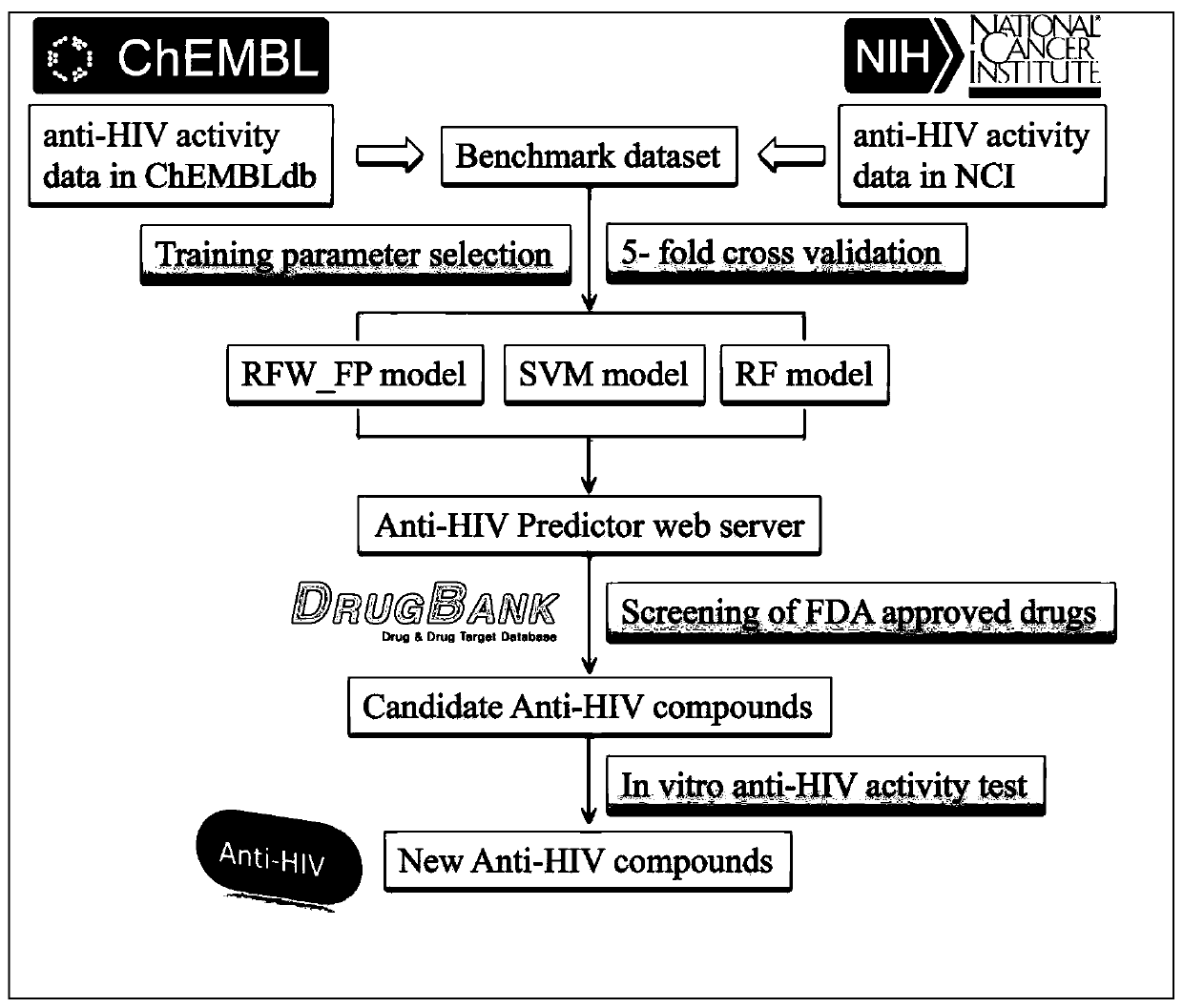
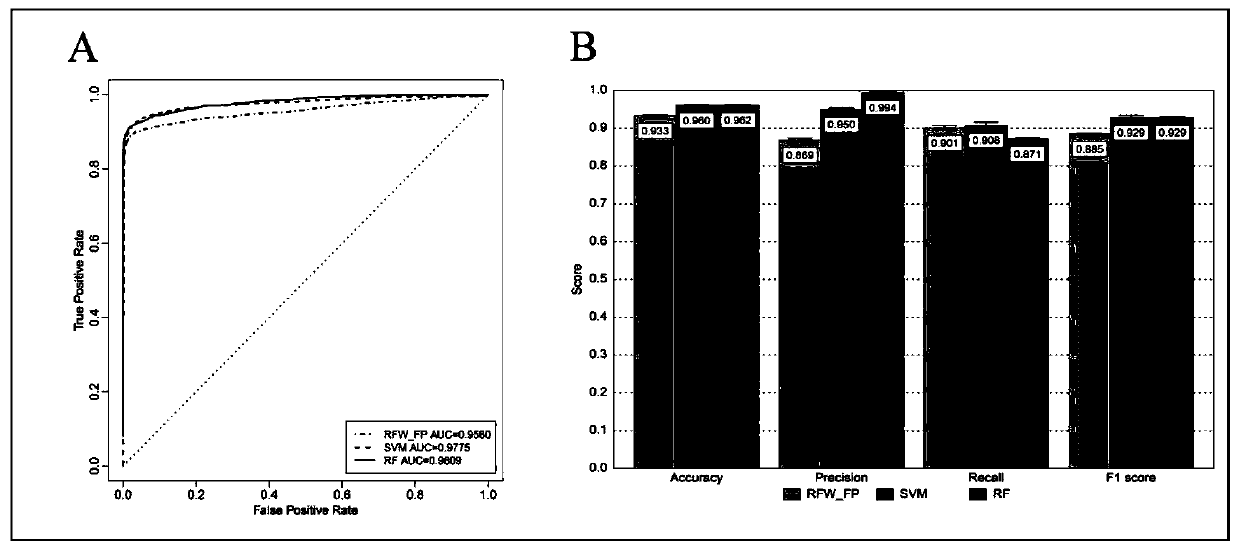
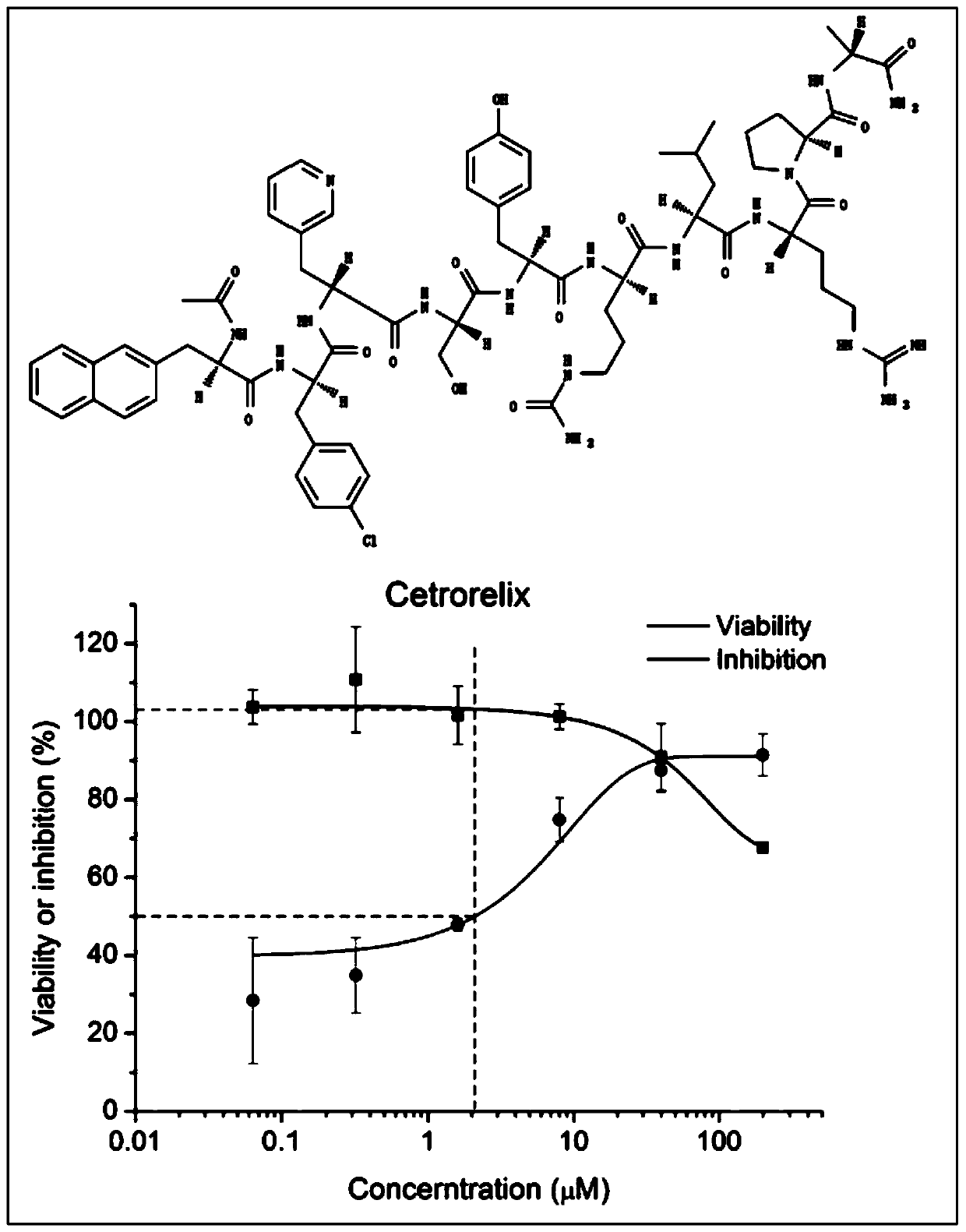
Application of Cetrorelix in the Preparation of Drugs for Treating AIDS
ActiveCN107007814BObvious anti-HIV effectPeptide/protein ingredientsAntiviralsAntigenHuman lymphocyte
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
Application of Cetrorelix to preparation of medicine for treating AIDs
ActiveCN107007814AObvious anti-HIV effectPeptide/protein ingredientsAntiviralsAntigenAnti hiv activity
The invention relates to application of Cetrorelix to preparation of medicine for treating AIDs. The invention provides a compound of Cetrorelix and a pharmaceutically acceptable salt and application thereof to preparation of medicine for treating AIDs. The human T lymphoid cell series C8166 and HIV-1 experiment strain HIV-1<NL4-3> are used for performing in-vitro cytotoxicity and anti-HIV activity detection; the cytotoxicity is detected by an MTT colorimetric method; the anti-HIV activity is detected by two methods including a synplasm inhibition experiment method and an HIV-1p24 antigen quantitative method. The result proves that the cytotoxicity of the Cetrorelix is very low; CC<50> is higher than 200 muM; when the concentration is EC<5>0(1.788 muM), the Cetrorelix completely has no toxicity on the cell C8166. Therefore the Cetrorelix can be used as the anti-HIV medicine.
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com