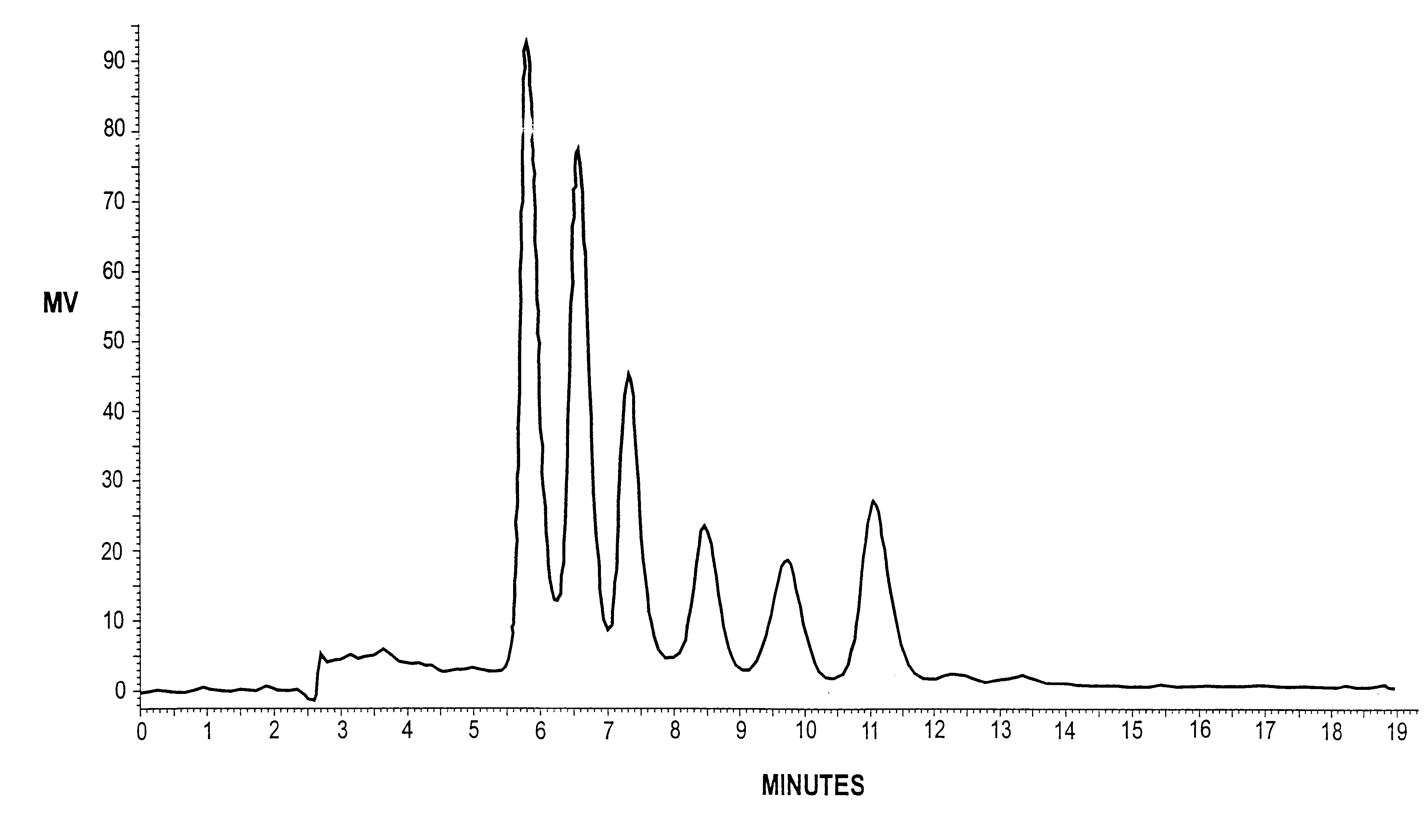
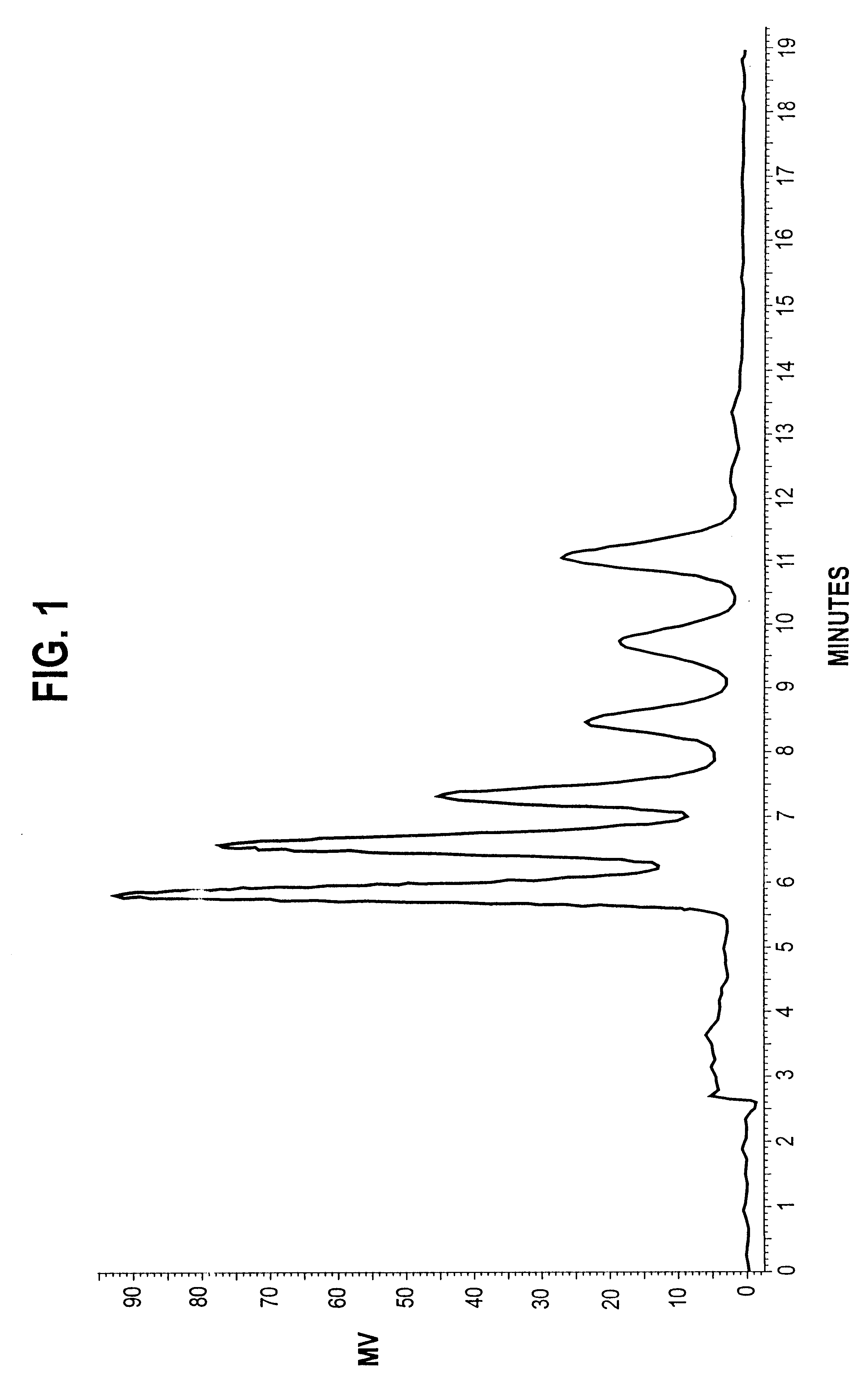
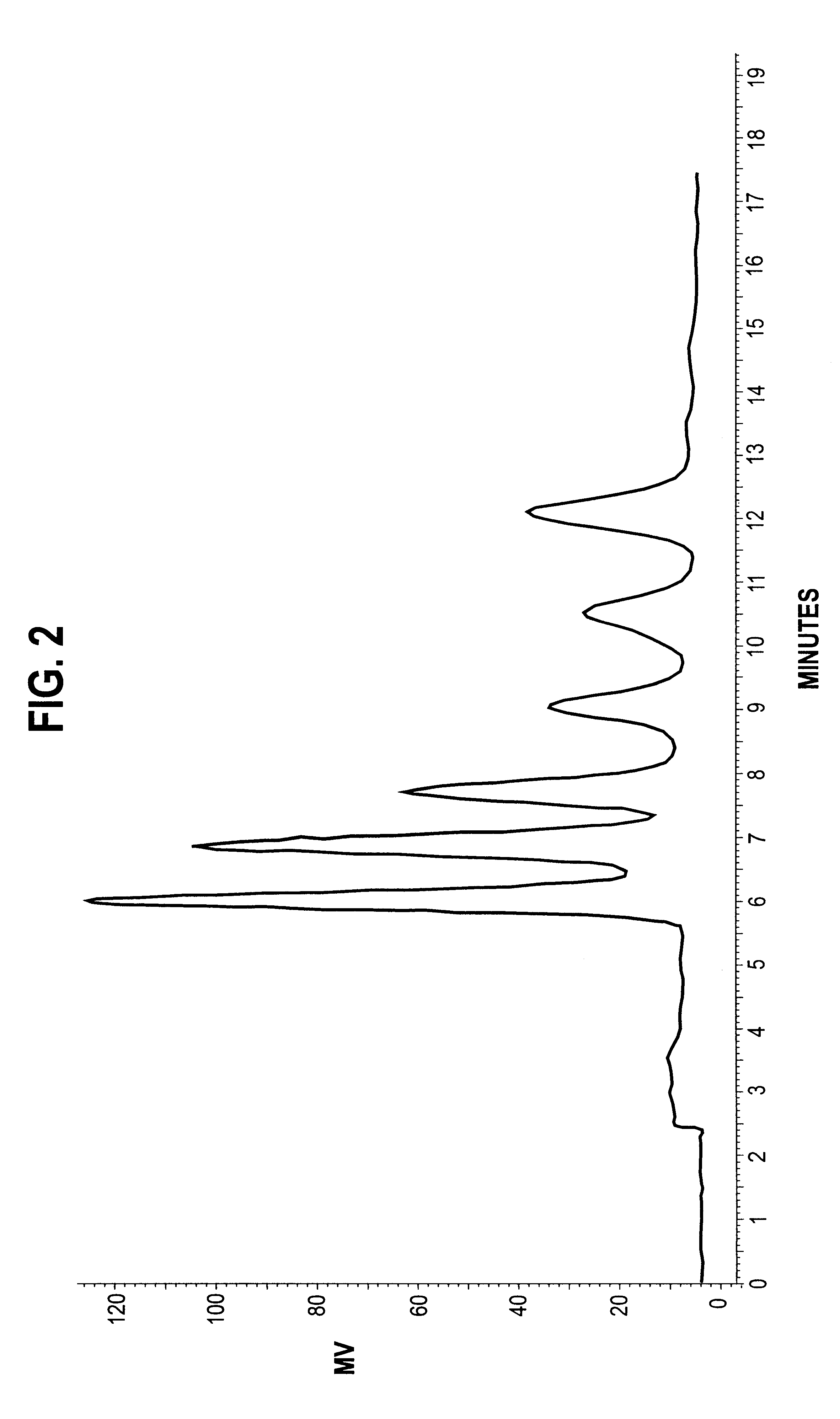
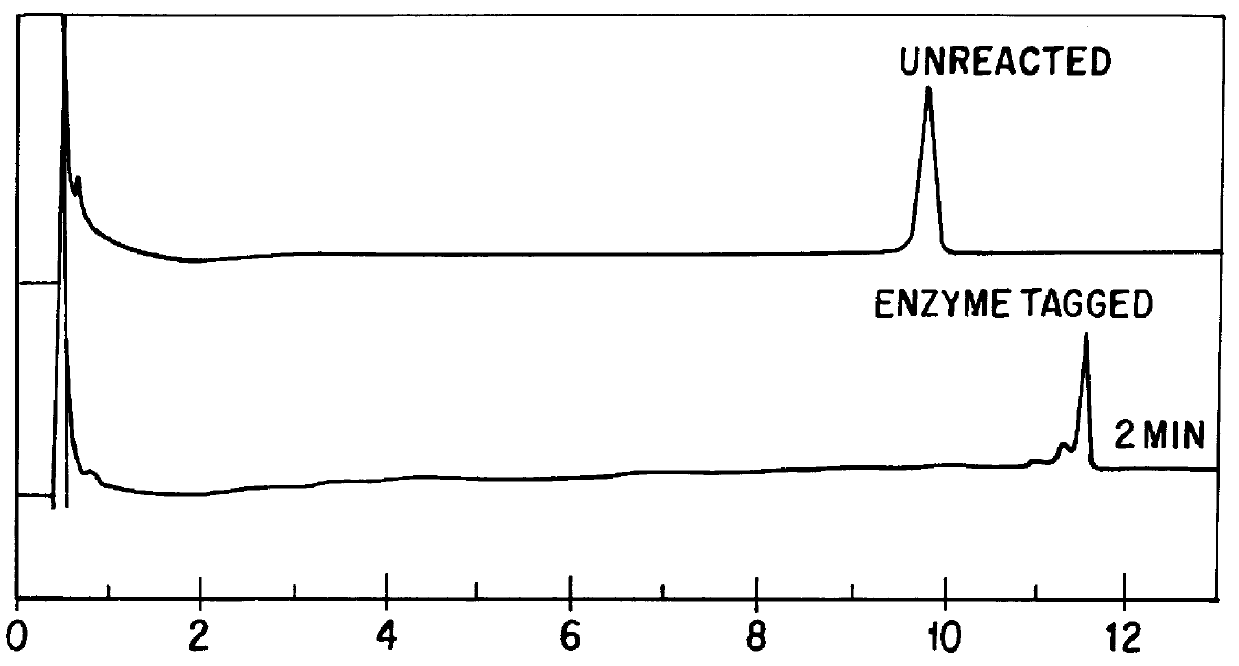
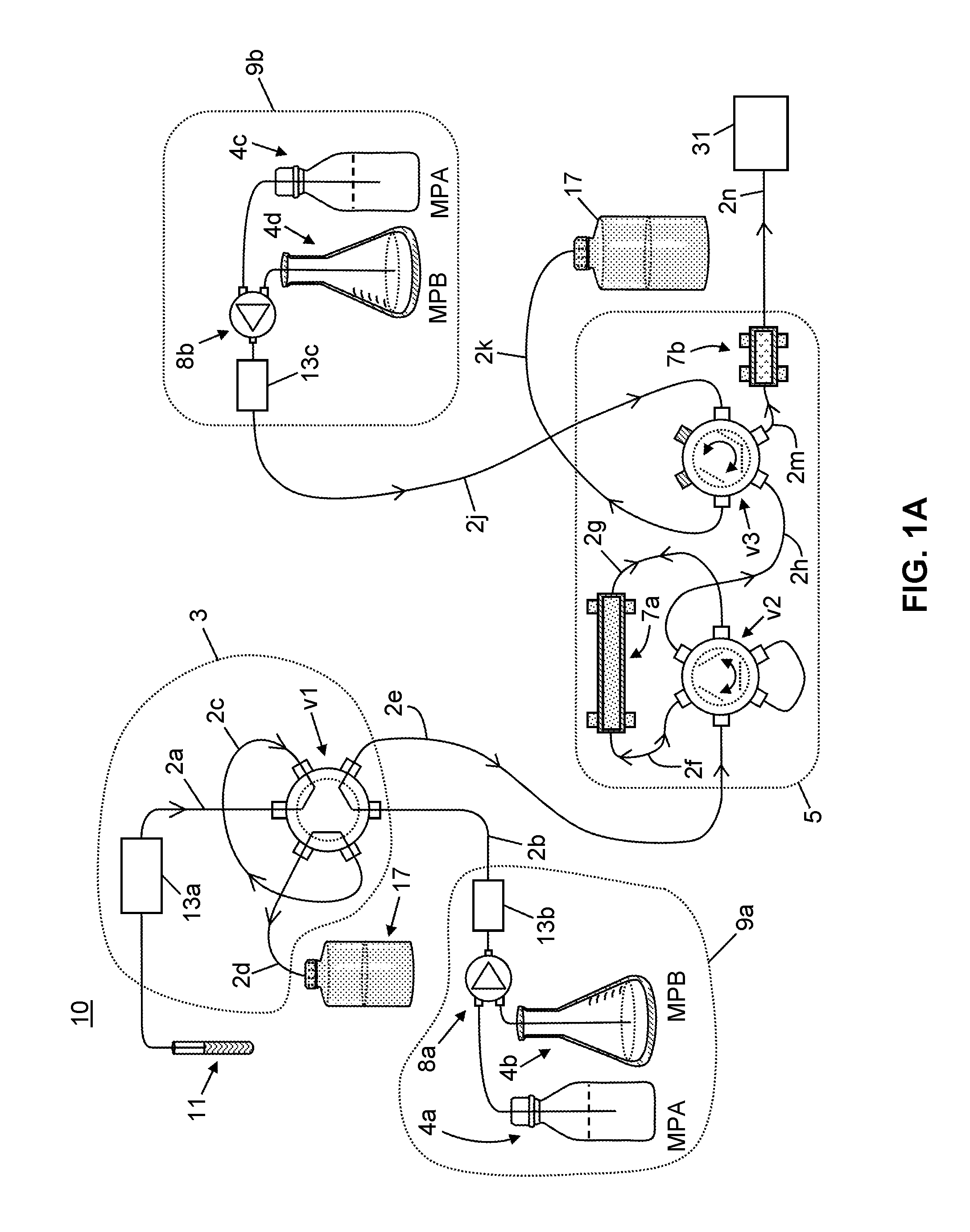
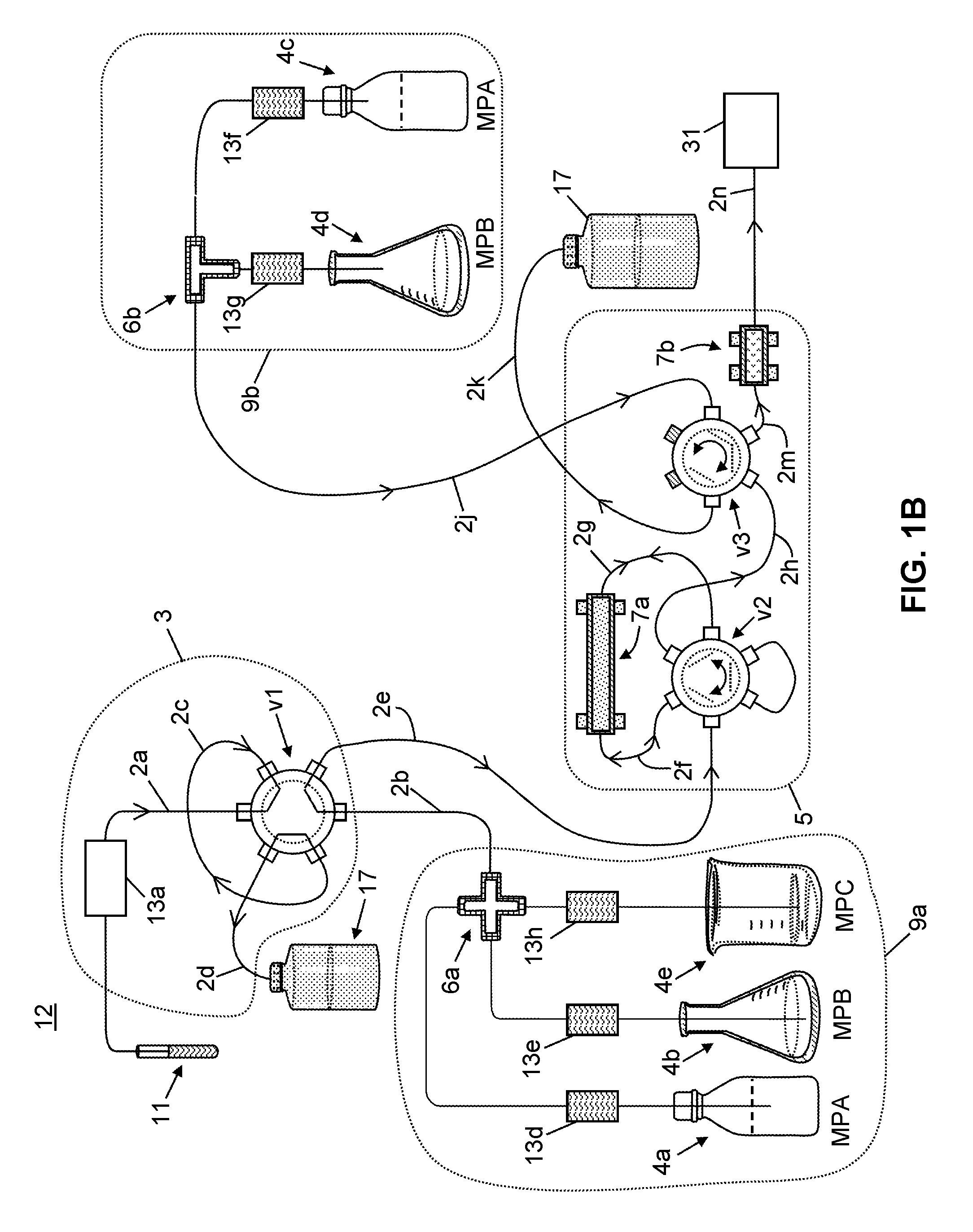
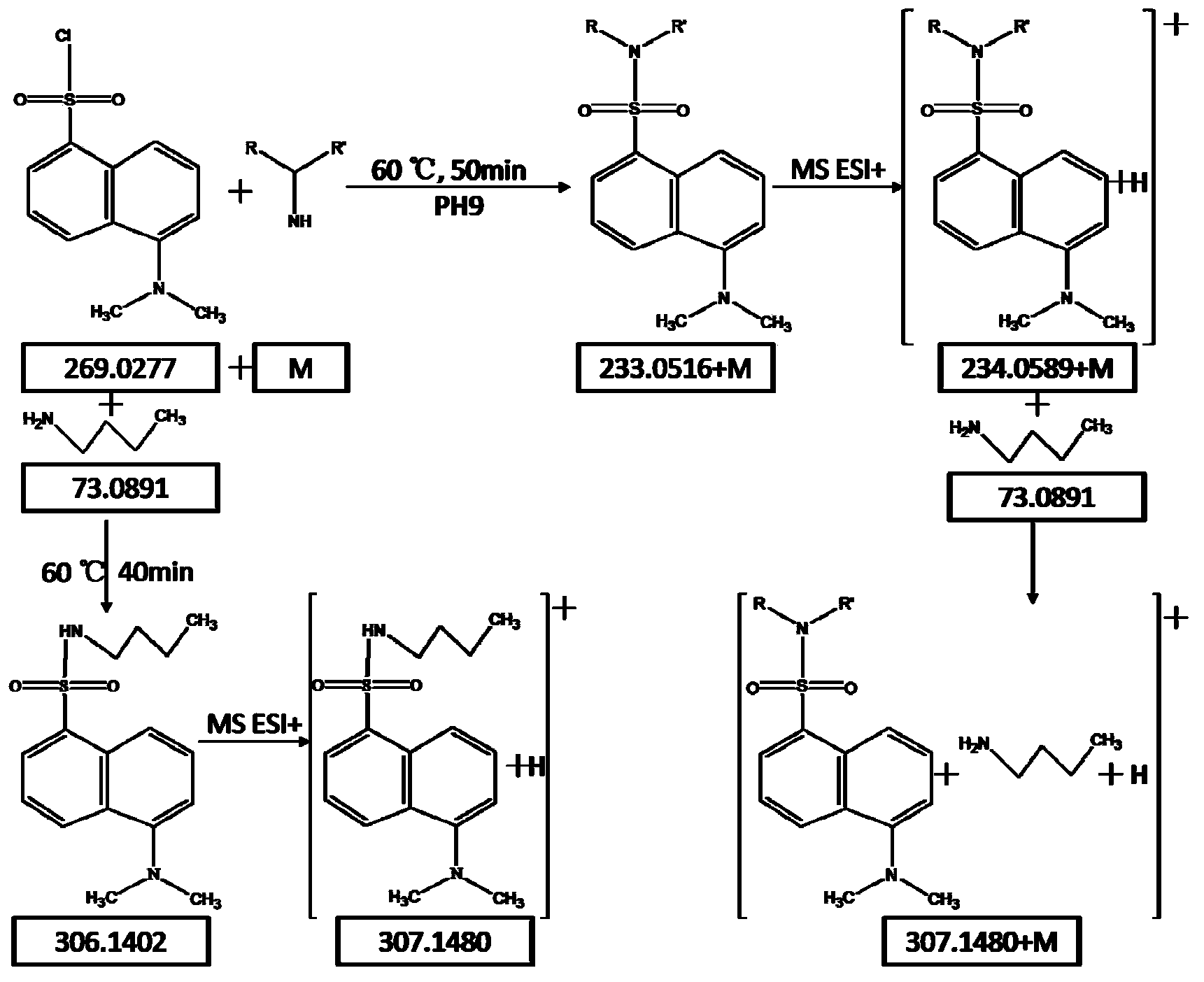
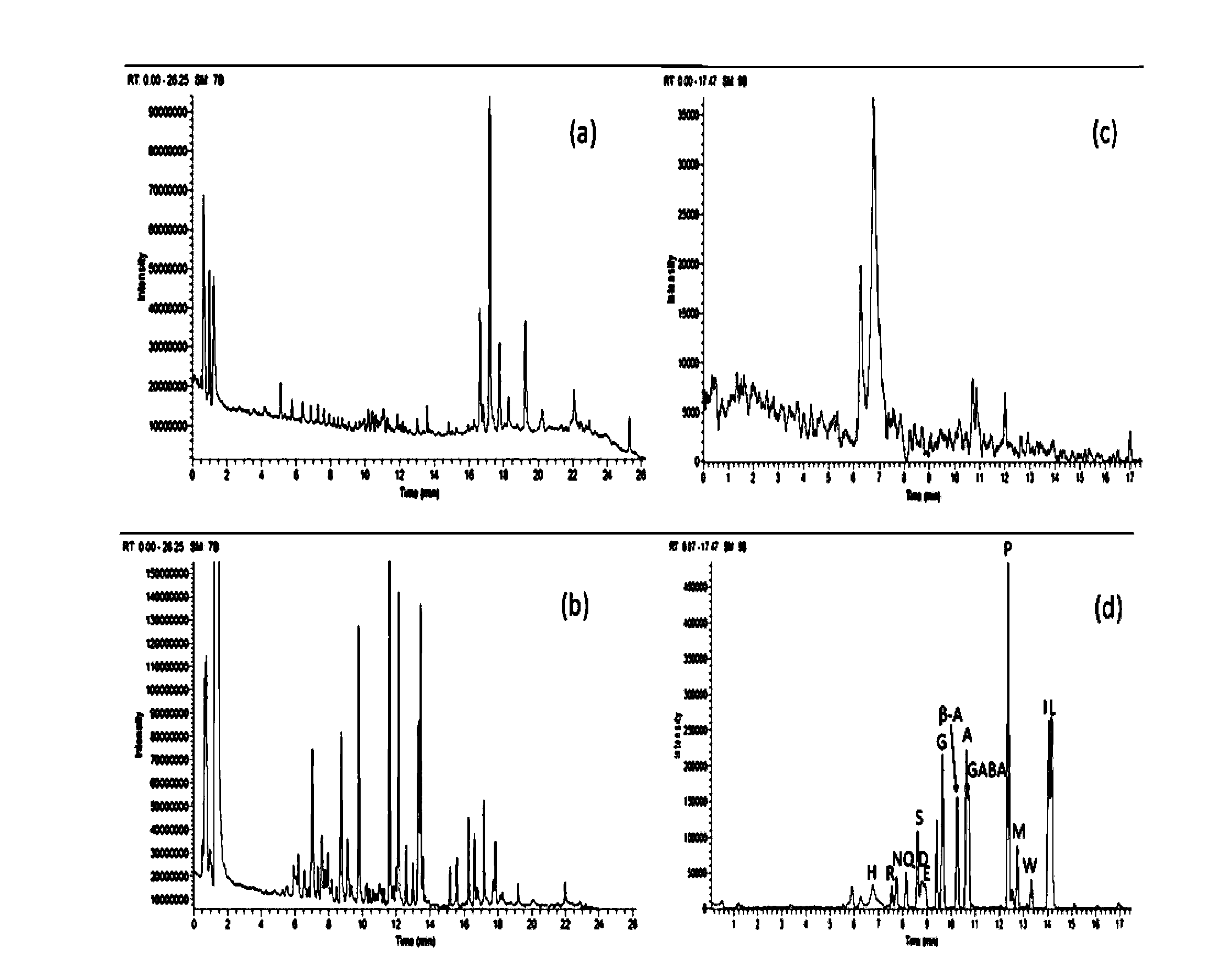
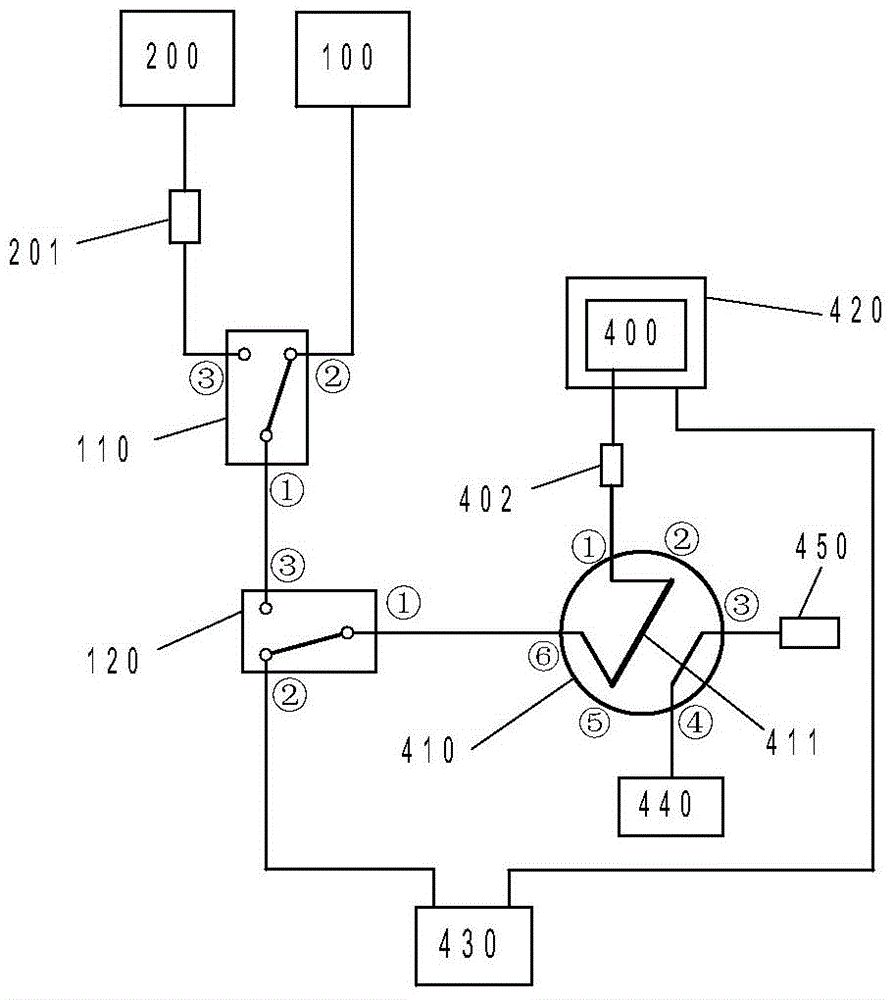
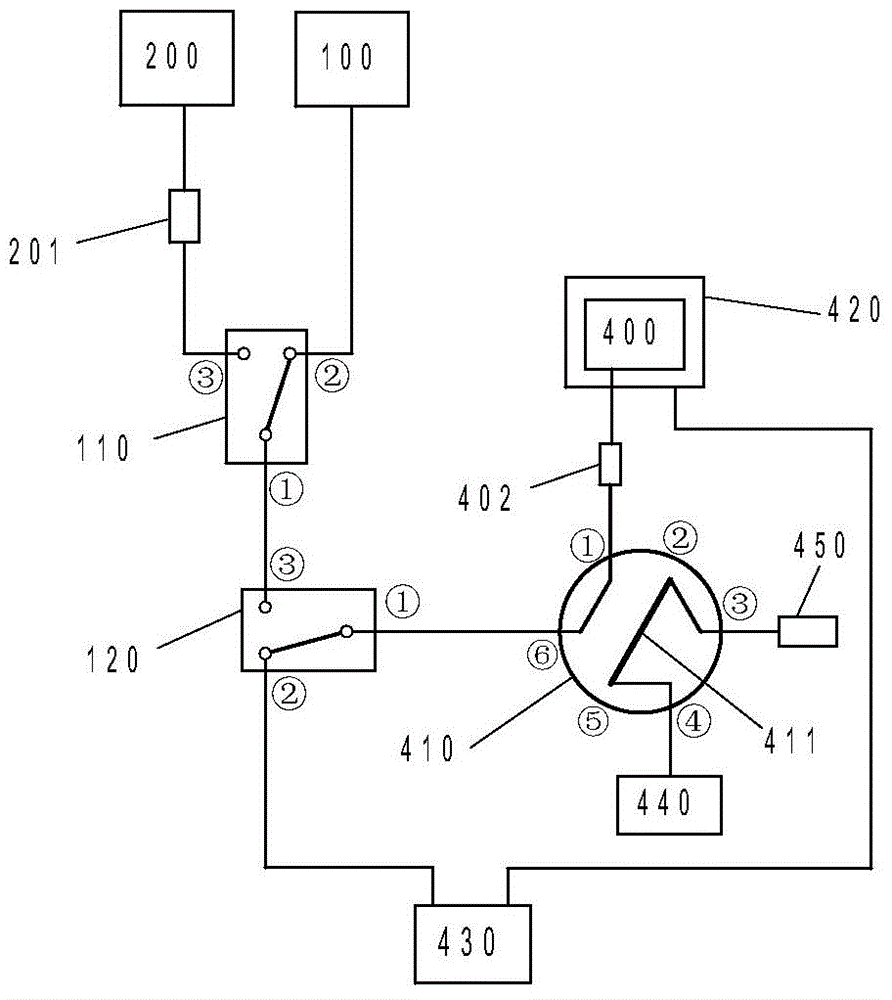
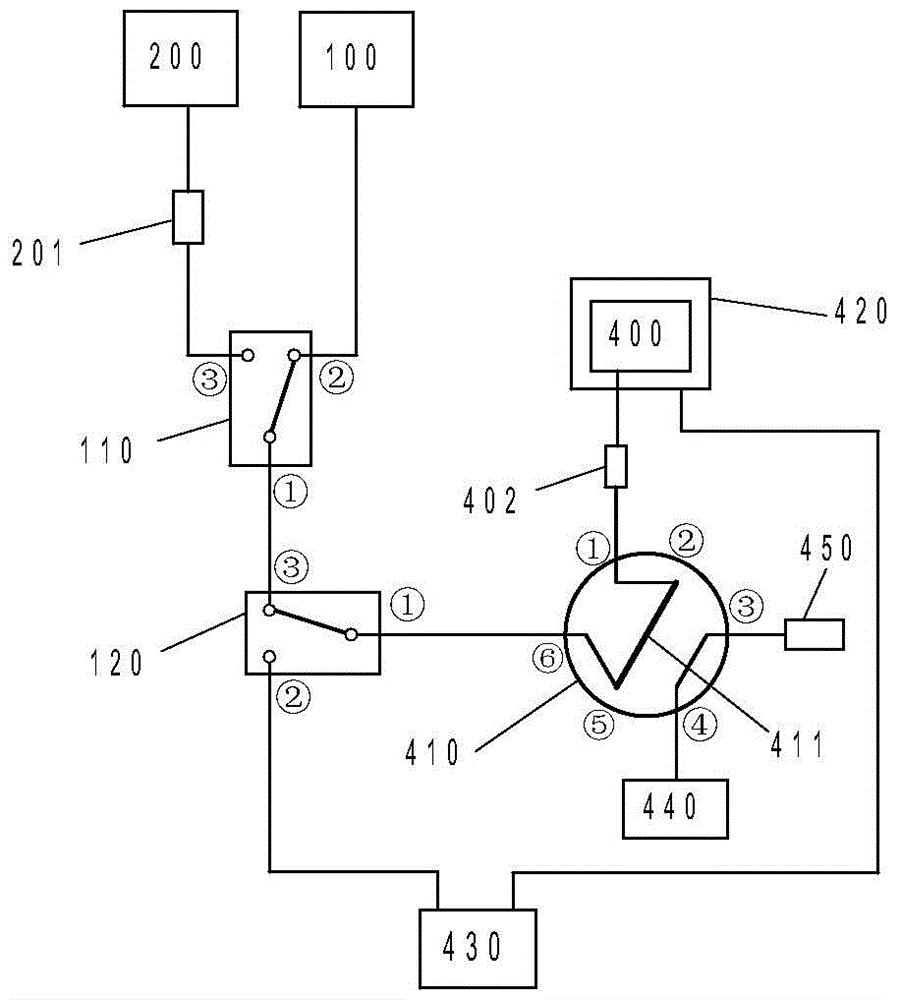
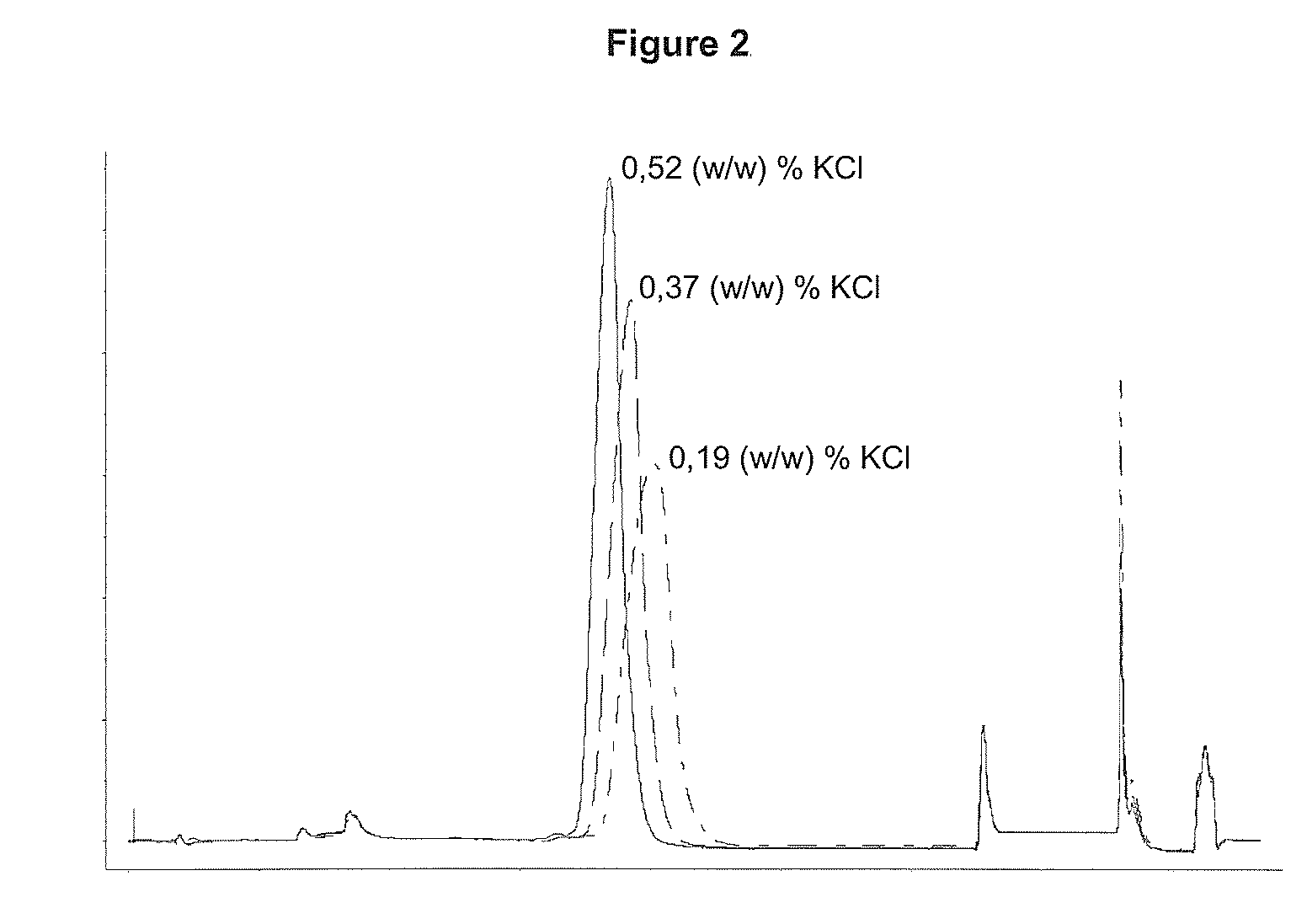
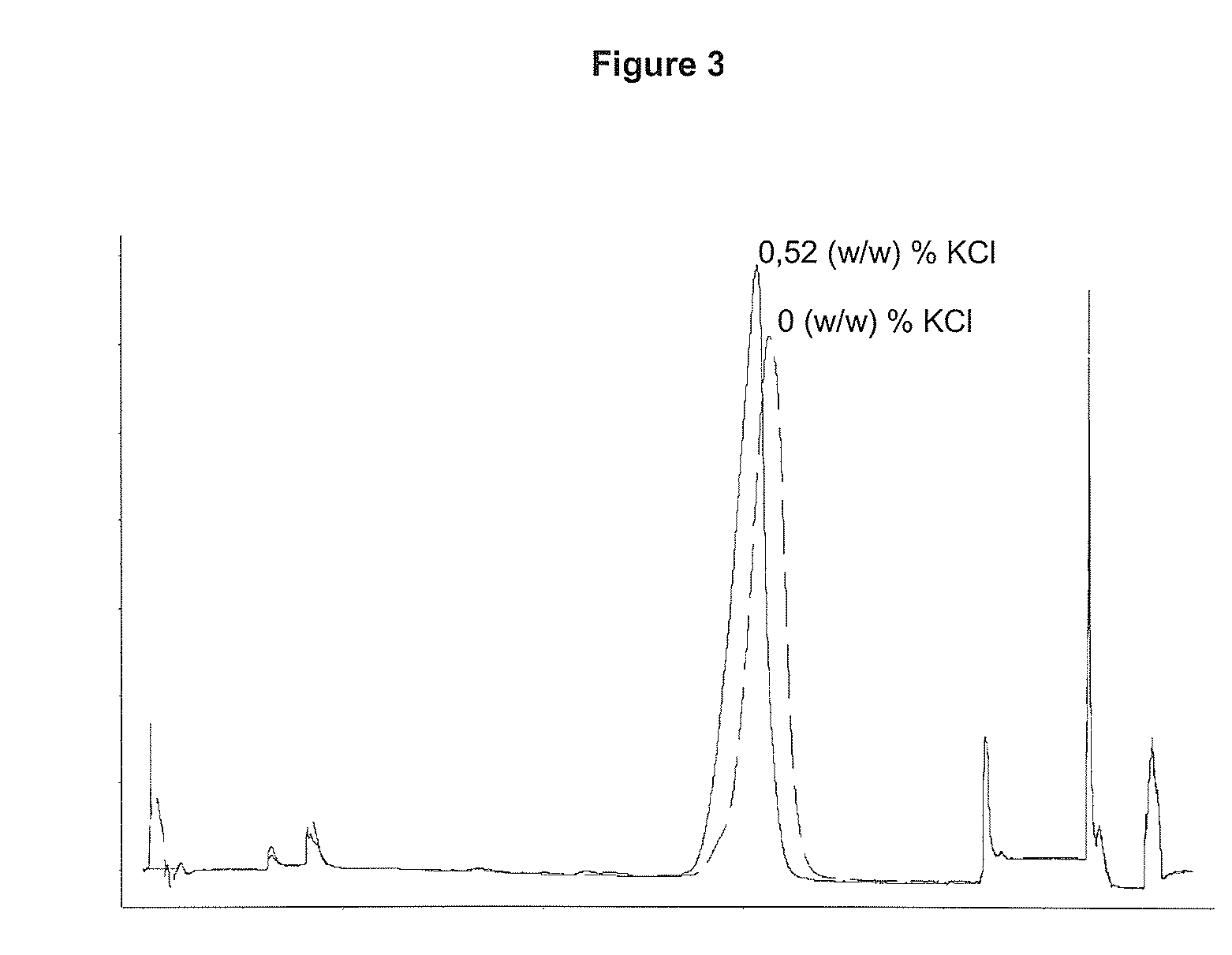
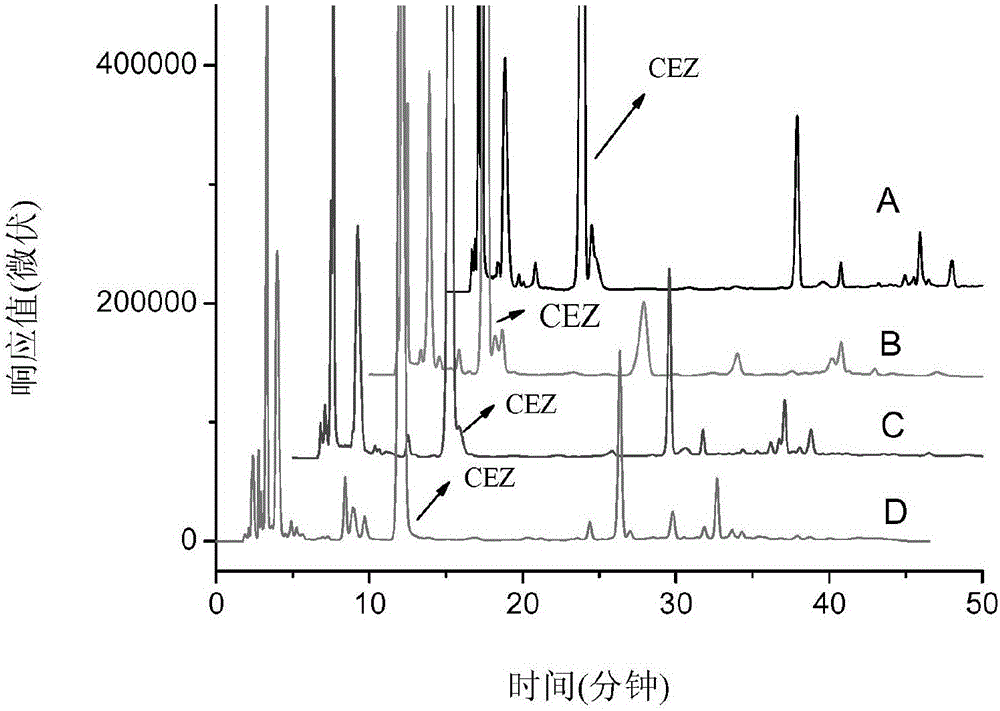
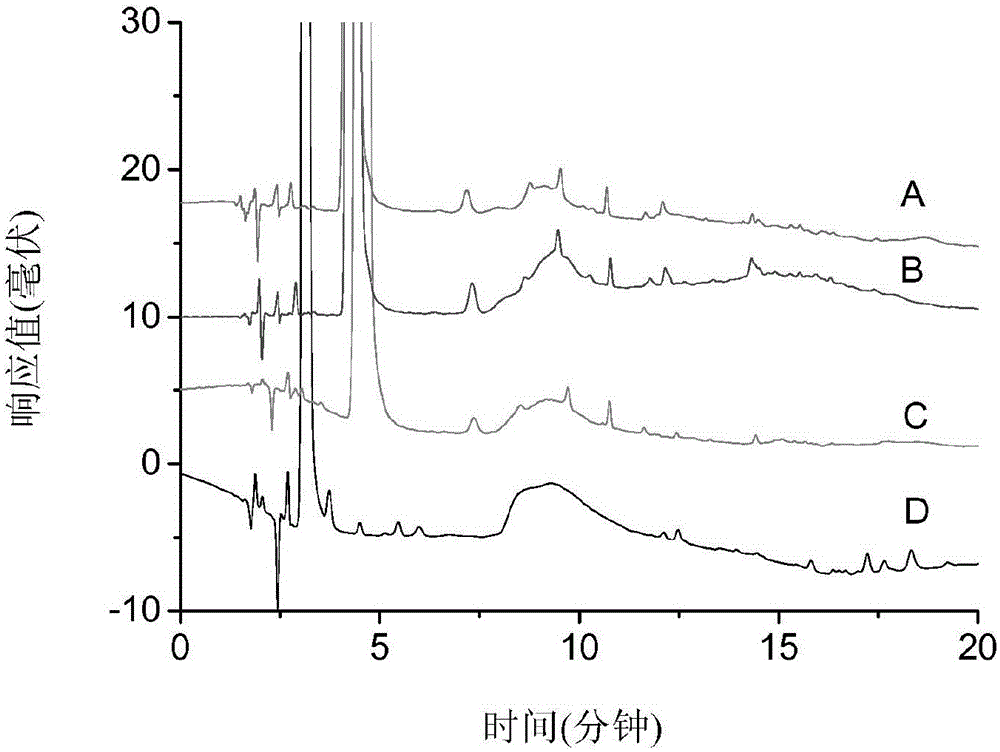
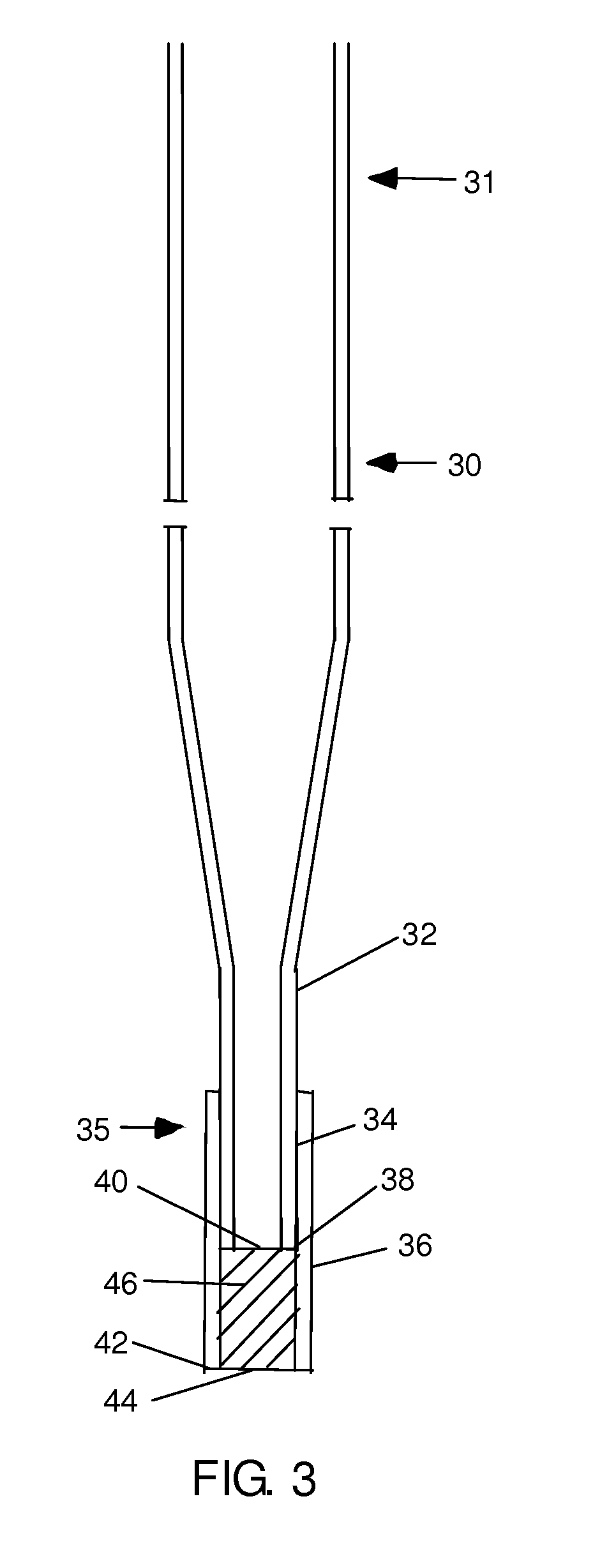
Patents
Literature
471 results about "Reversed-phase chromatography" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Reversed-phase chromatography (also called RPC, reverse-phase chromatography, or hydrophobic chromatography) includes any chromatographic method that uses a hydrophobic stationary phase. RPC refers to liquid (rather than gas) chromatography.
Method for producing purified tocotrienols and tocopherols using liquid chromatography
InactiveUS6395915B1Fatty oils/acids recovery from wasteOrganic compound preparationStationary phaseSolvent
A method separating tocotrienols from tocopherols and the isomers thereof on a large-scale or commercially feasible basis. Separation is accomplished using reverse phase partition liquid chromatography. The chromatography column is prepared using a stationary phase comprising a hydrophobic or aromatic reverse phase chromatography media. The mobile phase consists of a solvent which is capable of solubilizing the crude stock, tocotrienols and tocopherols. The chromatography column containing the stationary and mobile phases is loaded with a crude feed stock and the tocotrienols, tocopherols and / or isomers thereof are eluted at a predetermined linear velocity.
Owner:ASAHI KASEI TECHNROM
Method for separating and purifying sea-mussel mucin by using salting out and dialyzing
ActiveCN101585874ALow costIncrease concentrationPeptide preparation methodsAnimals/human peptidesHigh concentrationPurification methods
The invention relates to a method for separating and purifying the sea-mussel mucin by using salting out and dialyzing, the salting out and the dialyzing utilizes the proteinaceous physicochemical character to purify by a non- chromatograph method, and solves the problem of high apparatus and material cost in the chromatograph method for purifying the sea-mussel mucin, a quick, low-cost and high-concentration method for separating and purifying the sea-mussel mucin is provided. The strong acid is used for extracting, the salting out and the dialyzing are used for purifying, the sea-mussel mucin is determined by adopting the acetic acid-carbamide- polyacrylamide gel electrophoresis via the blue tetrazolium specificity colour development, and the purity is determined by the inversed phase chromatography quantitatively.
Owner:JIANGYIN USUN BIOCHEMICAL TECH CO LTD
Chromatographic method for mutation detection using mutation site specifically acting enzymes and chemicals
InactiveUS6027898AHighly reproducibleEasy to implementSugar derivativesMicrobiological testing/measurementChromatographic separationRetention time
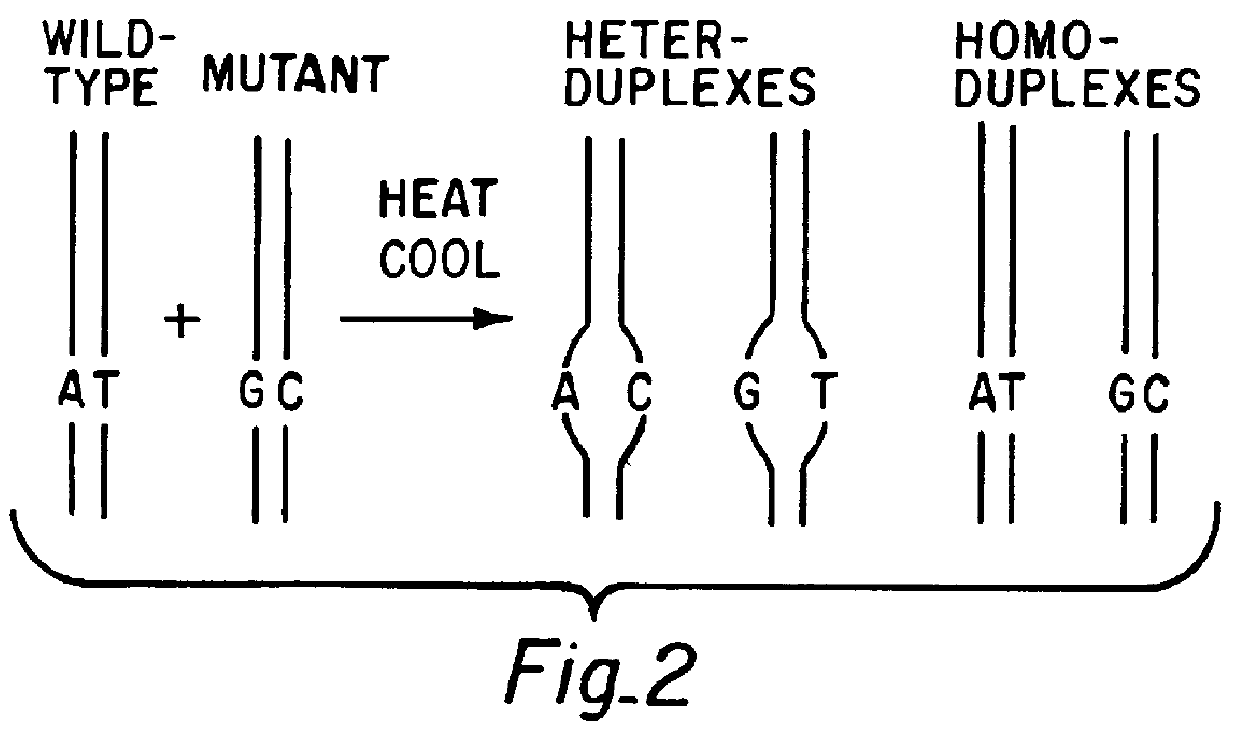
A method for analyzing a sample of double stranded DNA to determine the presence of a mutation therein comprises contacting the sample with a mutation site binding reagent, and chromatographically separating and detecting the product. The chromatographic separation can be performed using Matched Ion Polynucleotide Chromatography, size exclusion chromatography, ion exchange chromatography, or reverse phase chromatography. The mutation site binding reagent can be an enzyme or a non-proteinaceous chemical reagent. In one embodiment, a mutation site binding reagent binds to the site of mutation and alters the chromatographic retention time. In another embodiment, a mutation site binding reagent cleaves at the site of mutation, resulting in an increase in the number of fragments.
Owner:ADS BIOTEC INC
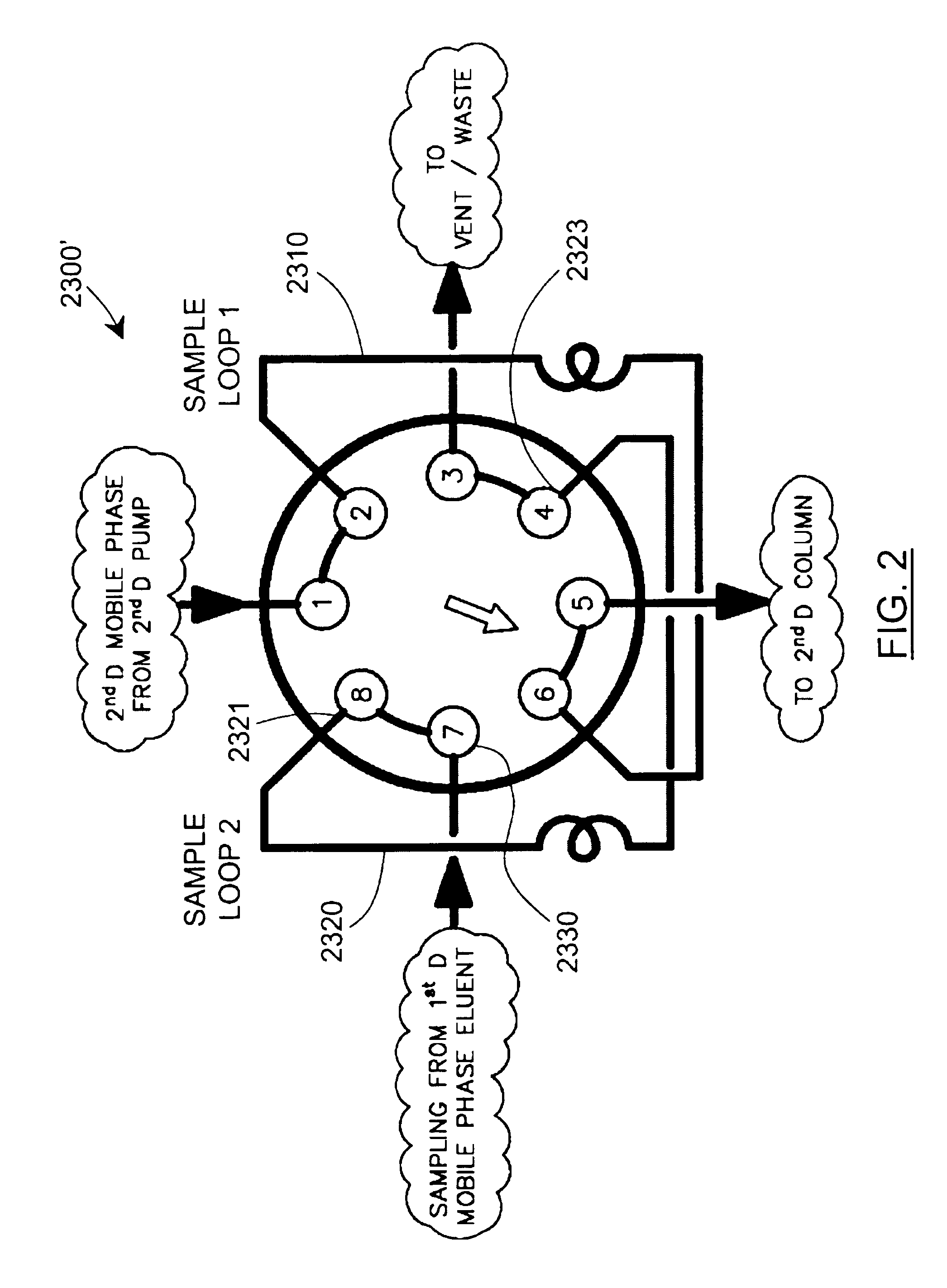
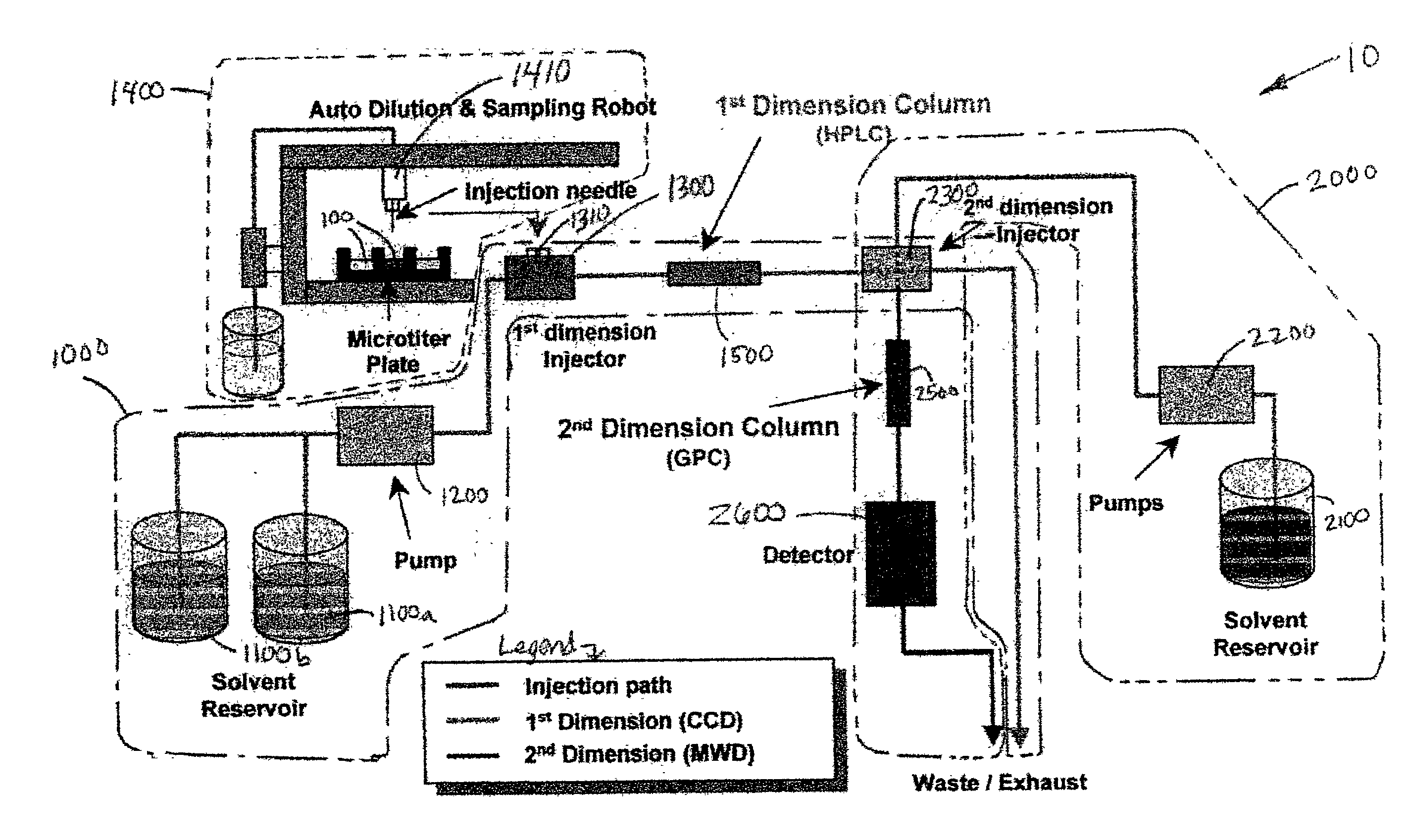
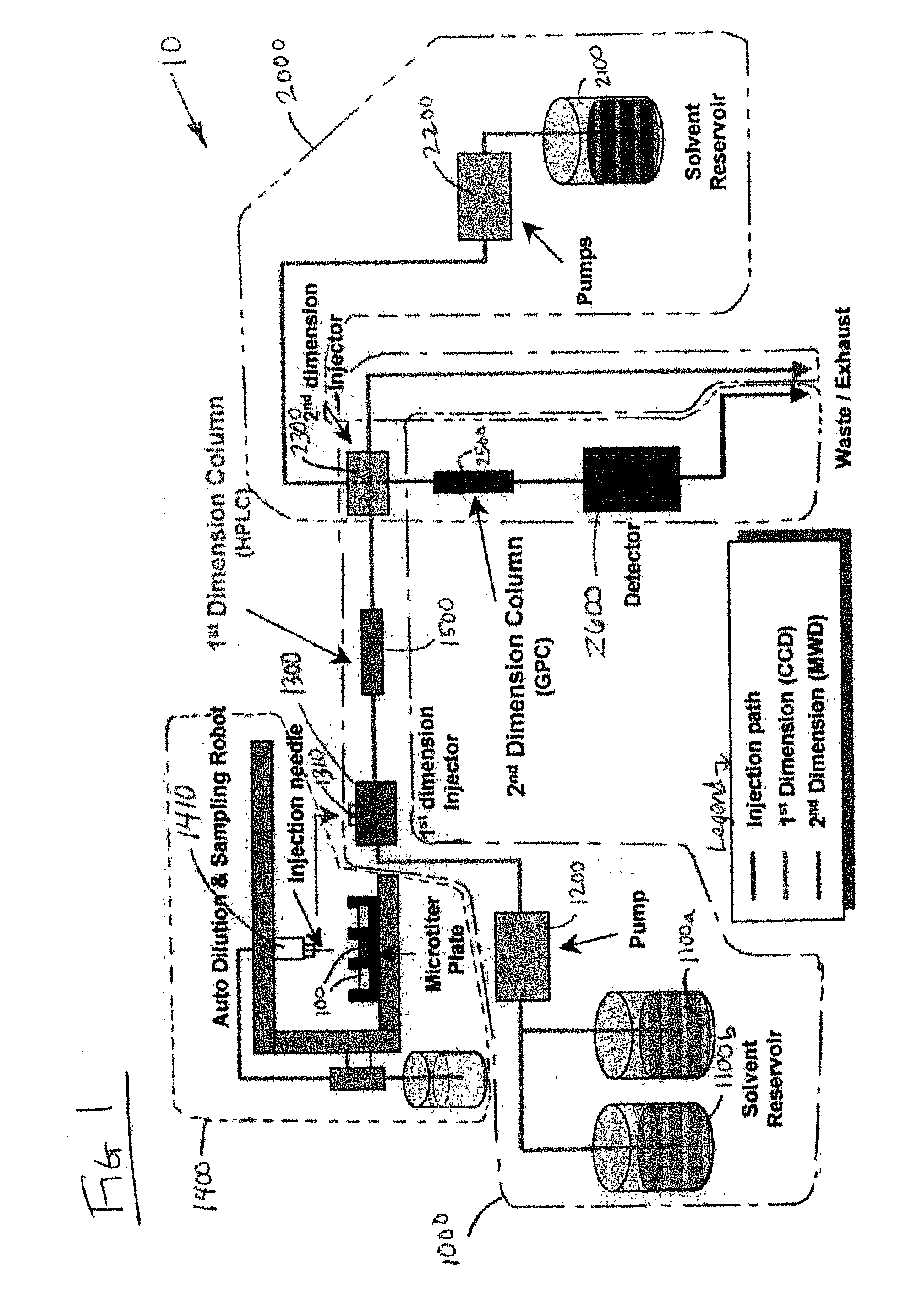
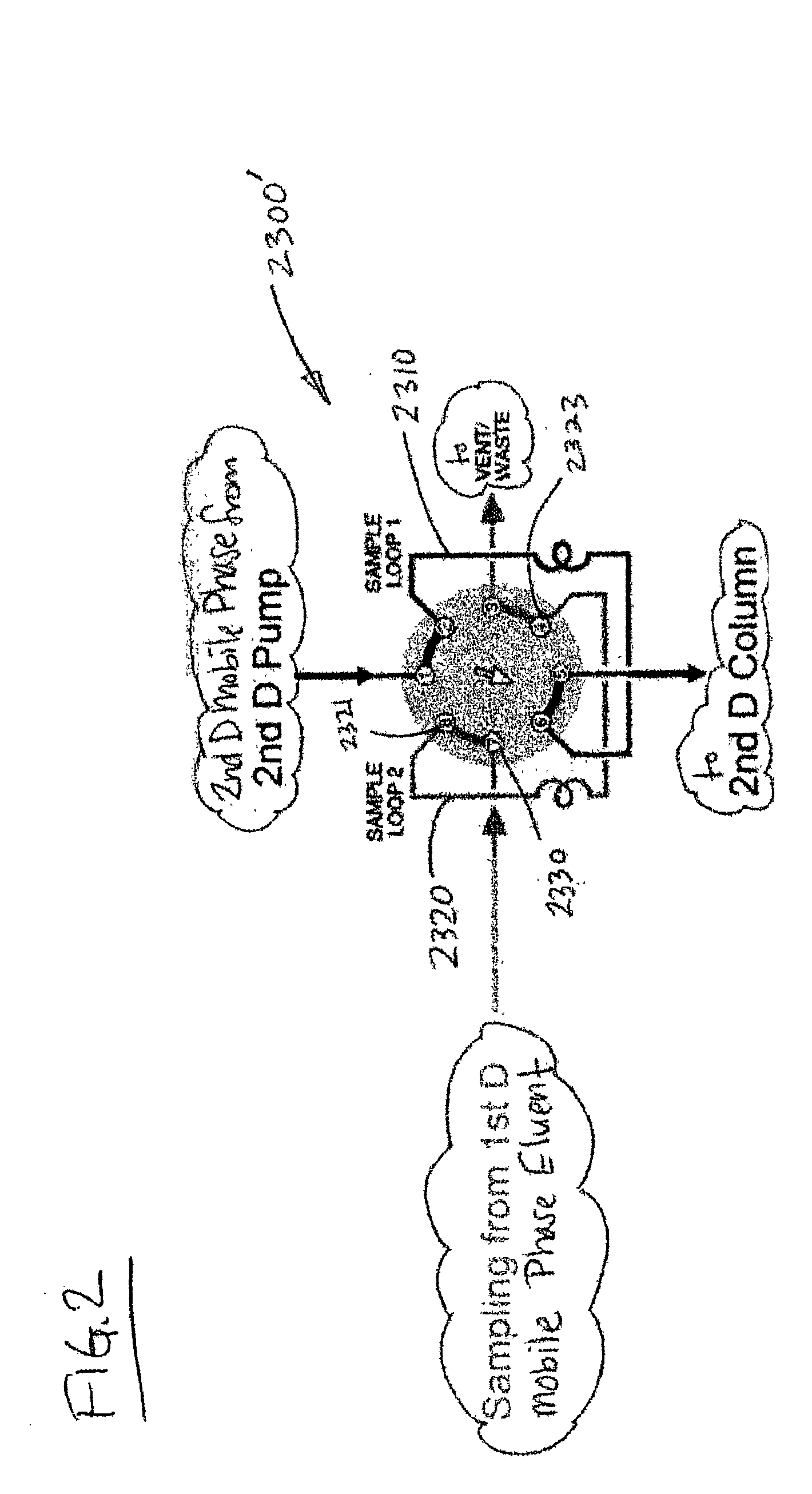
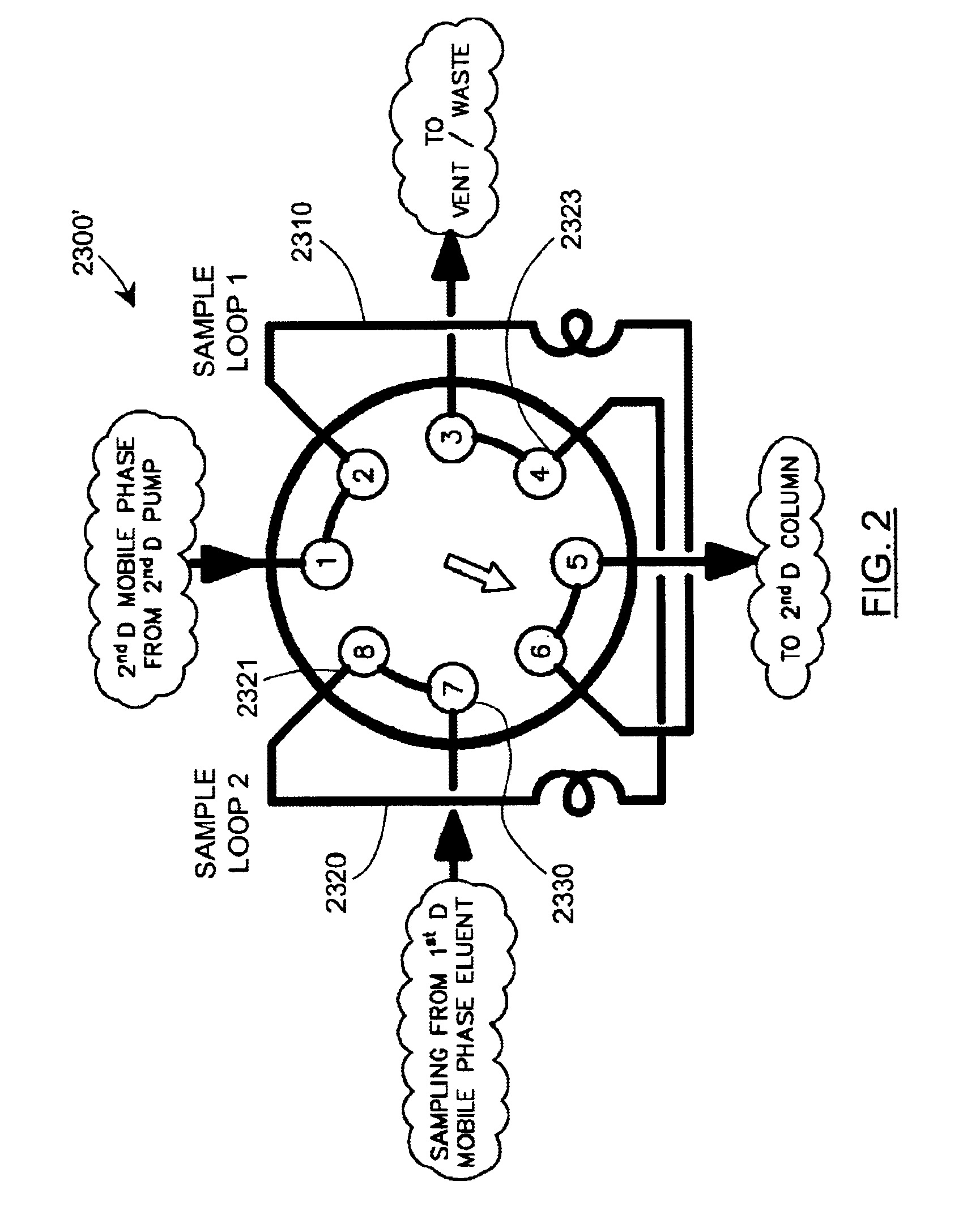
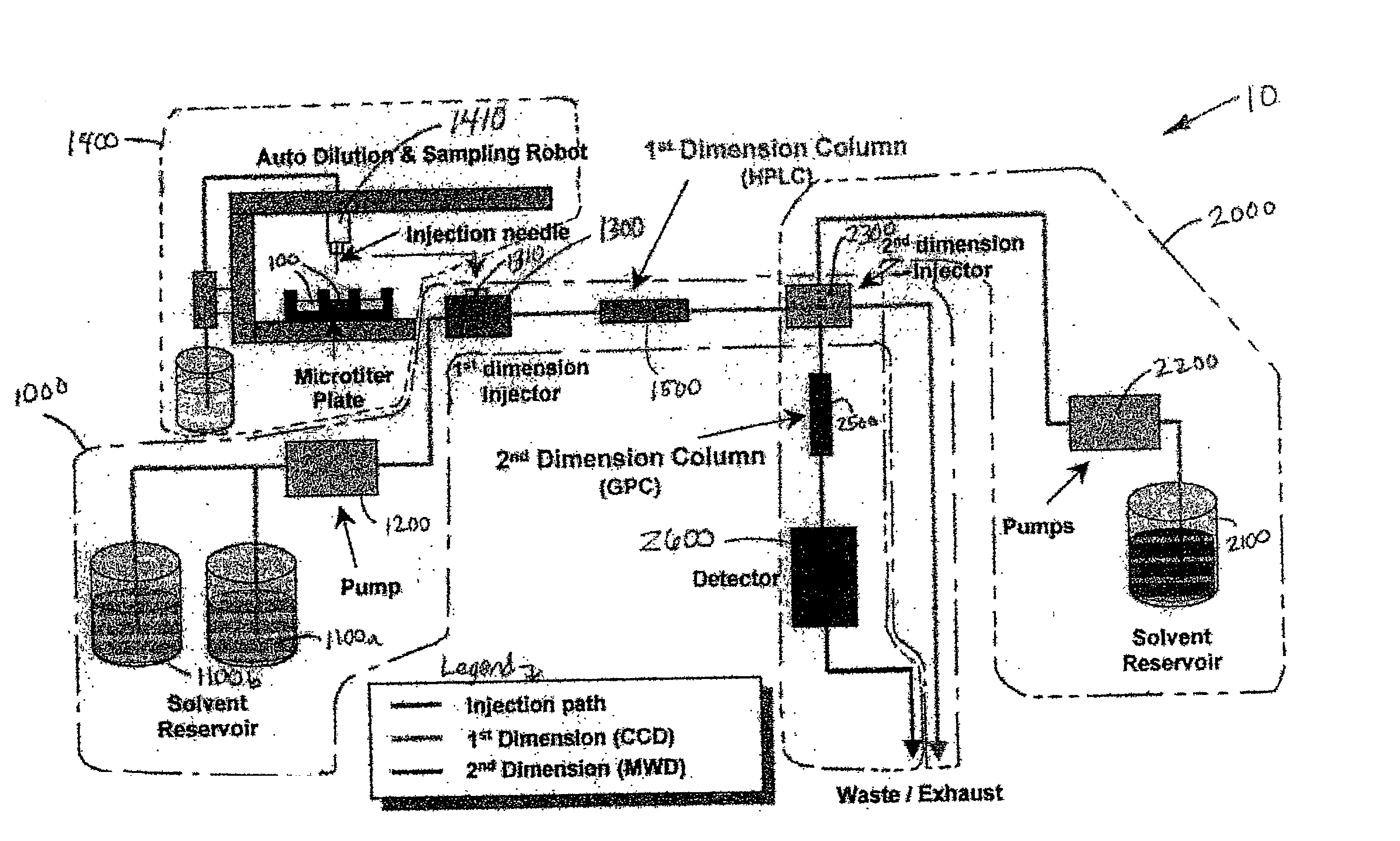
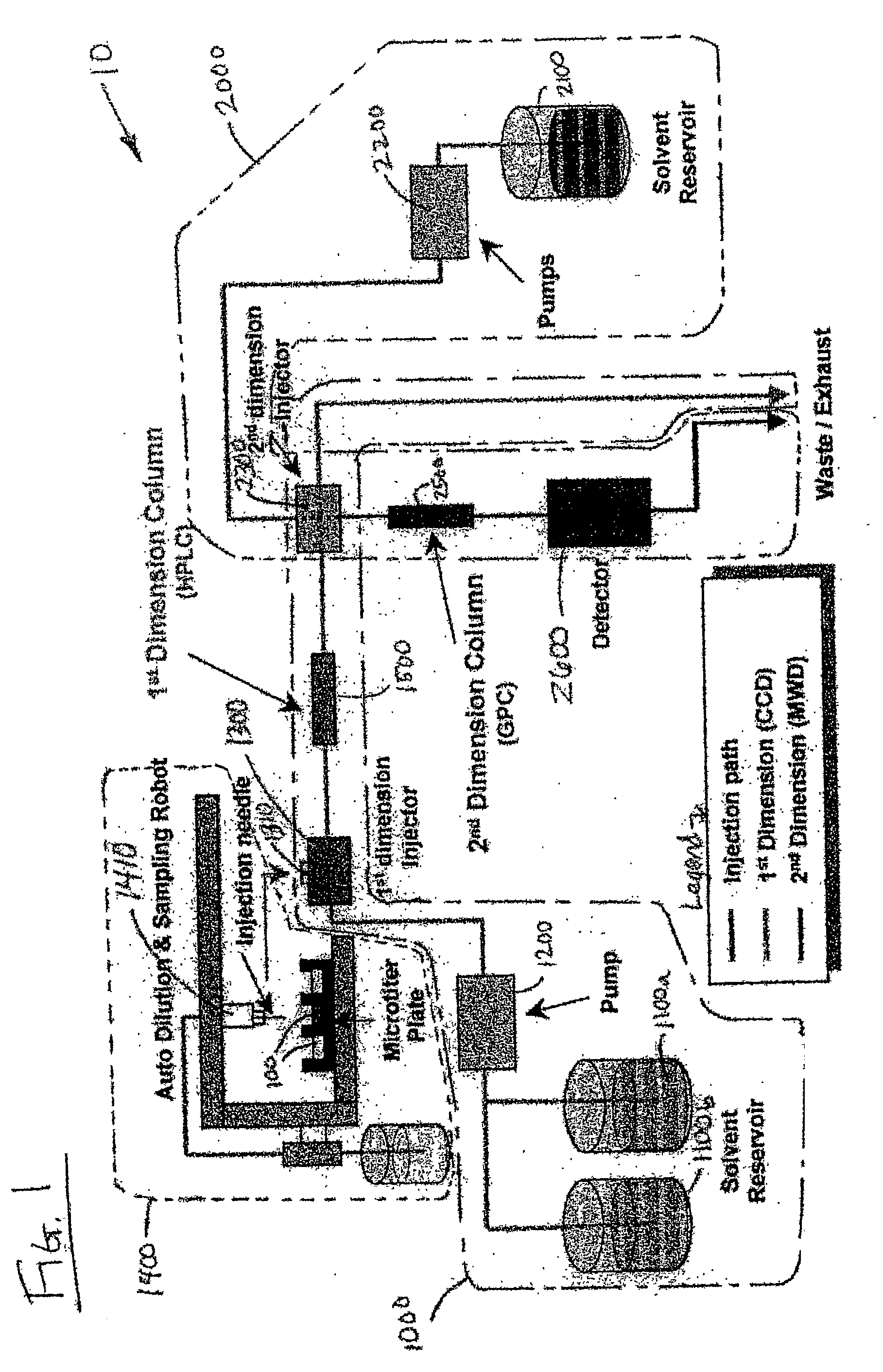
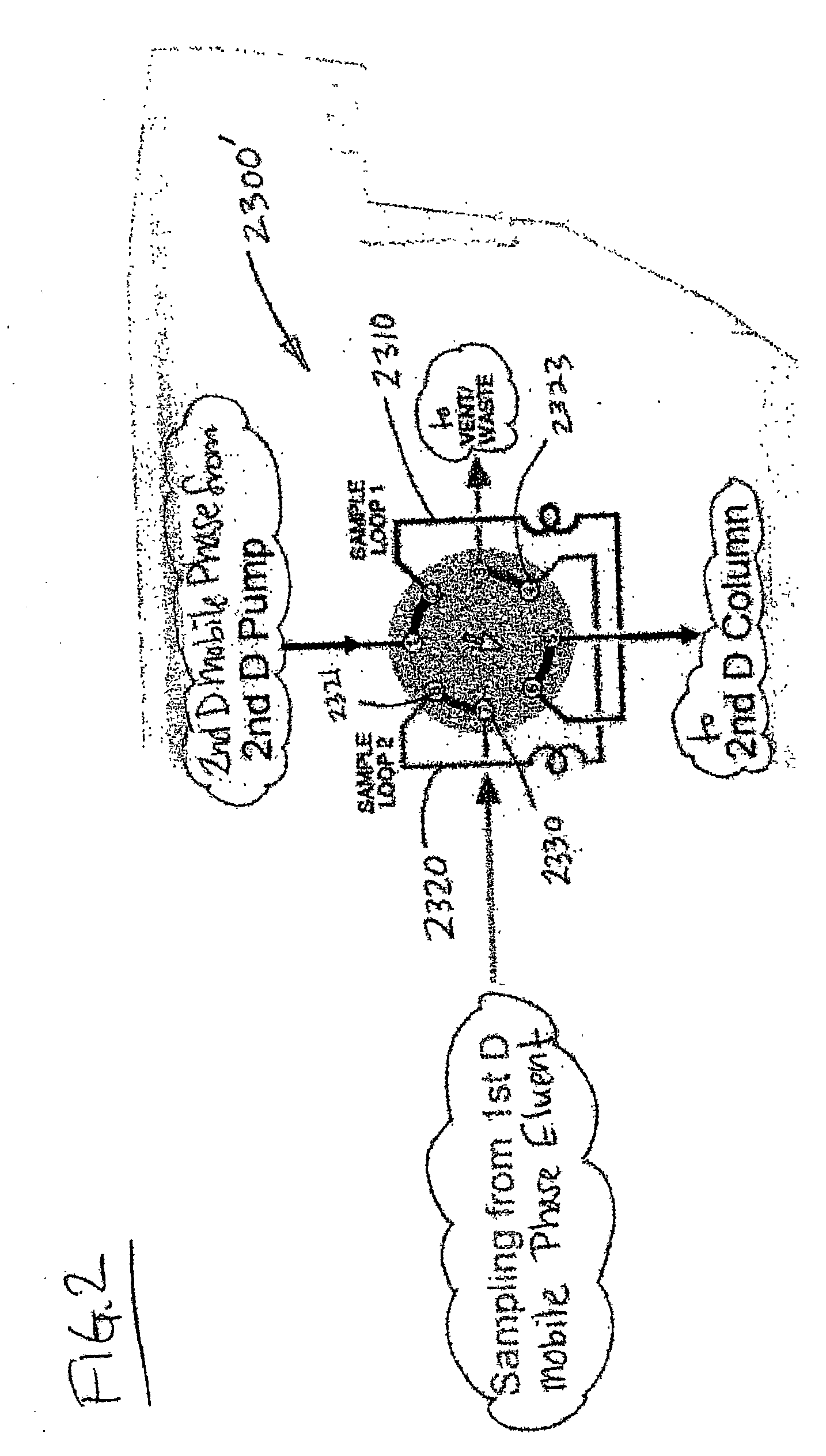
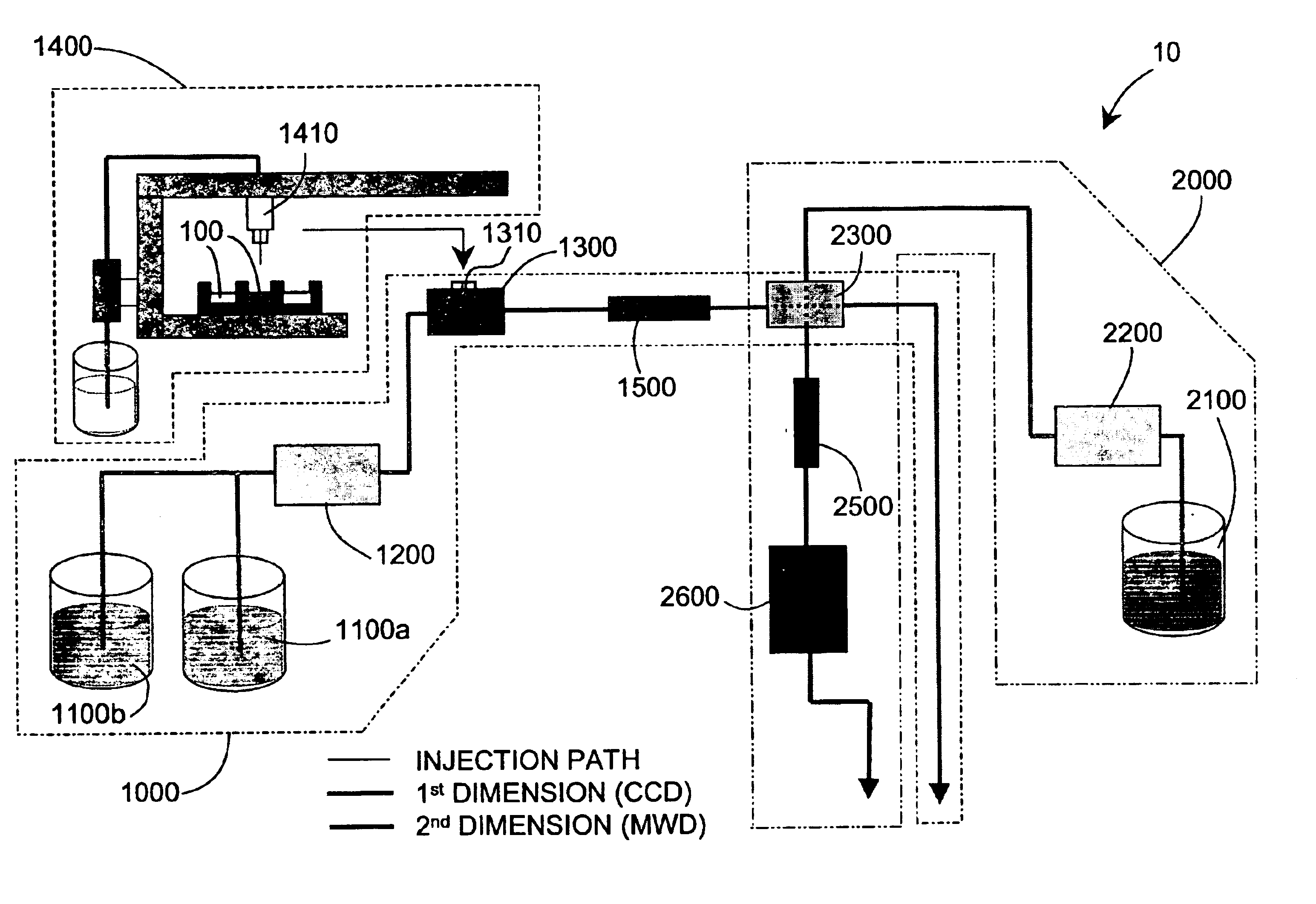
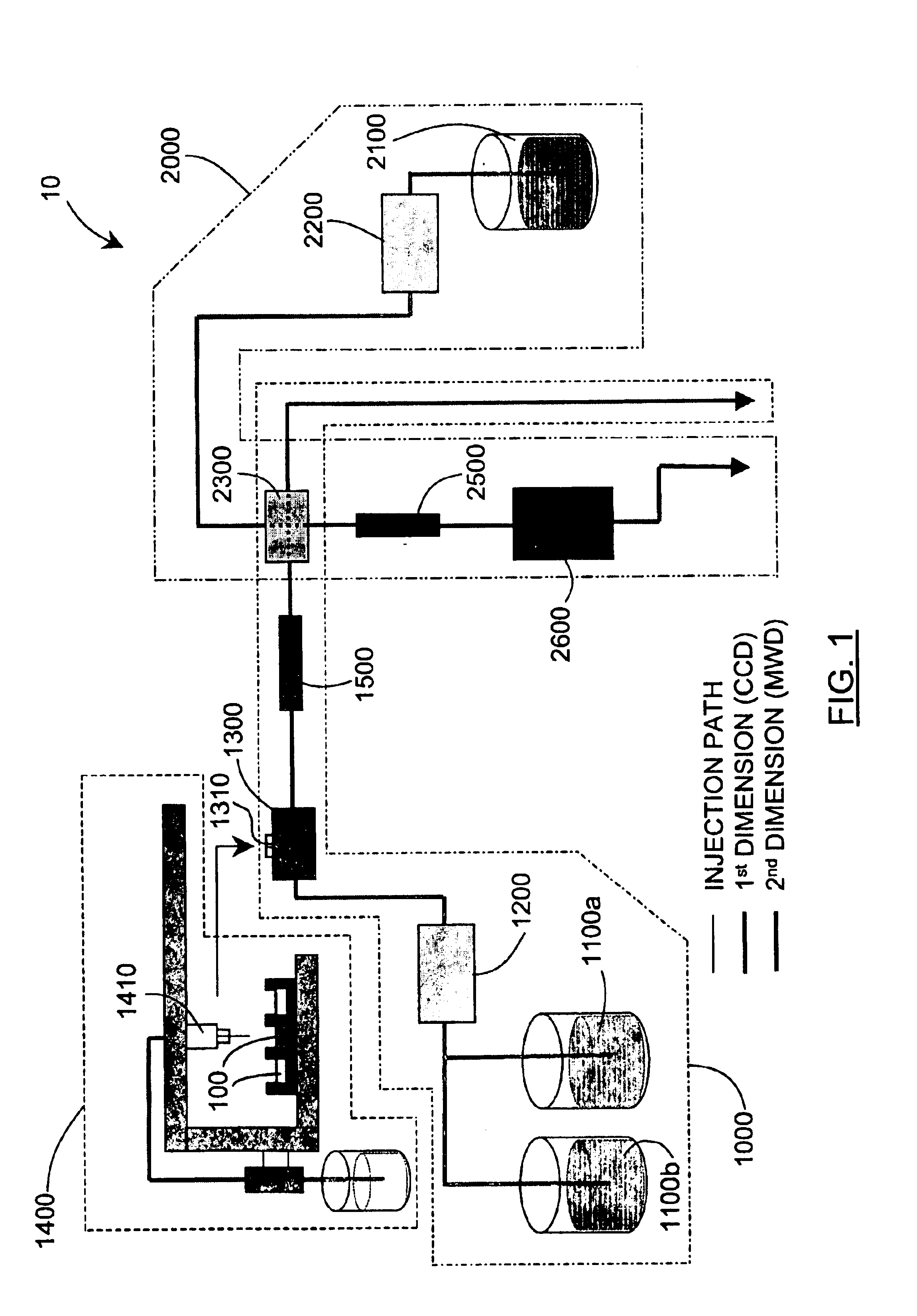
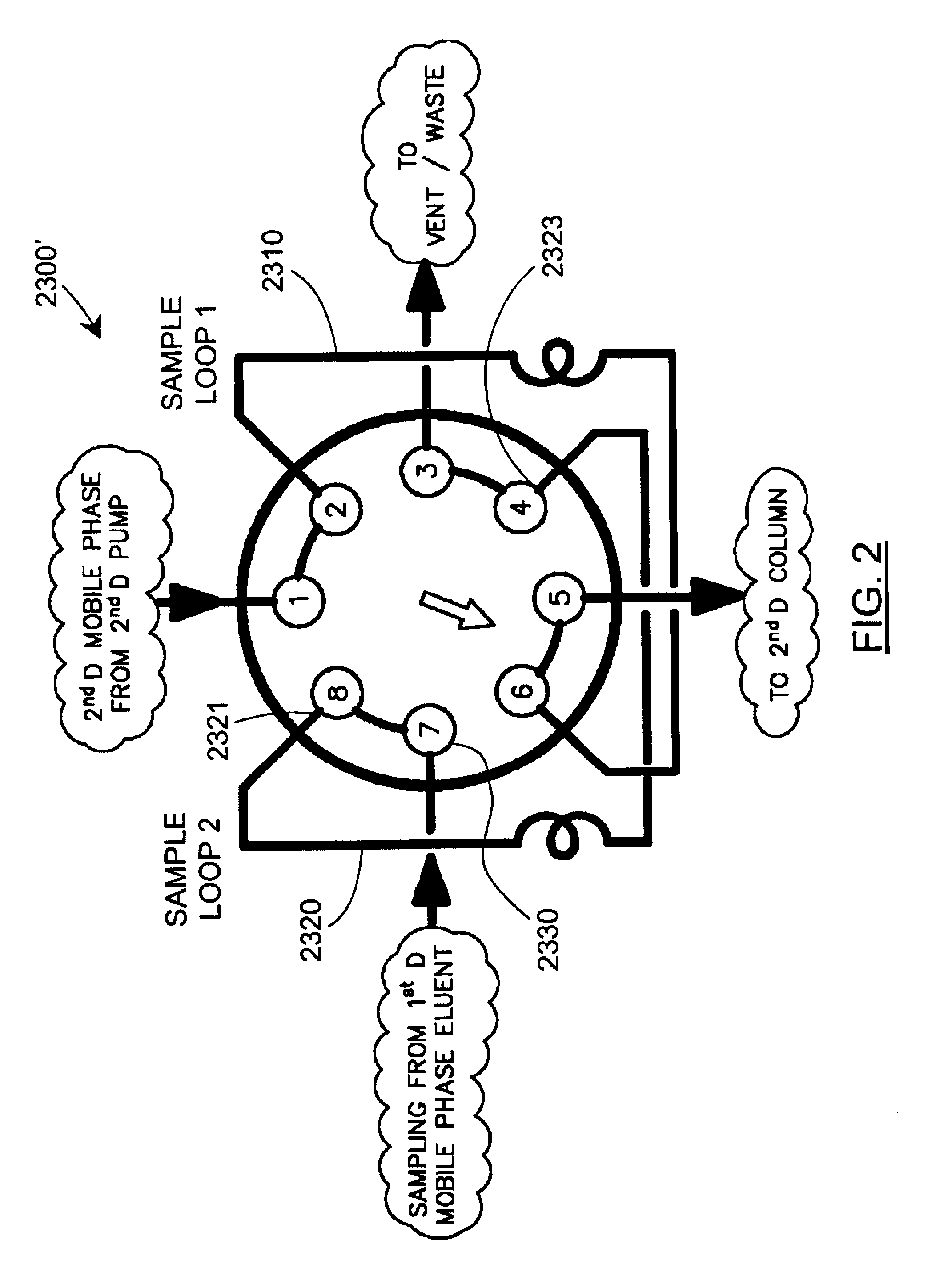
Methods and apparatus for characterization of polymers using multi-dimensional liquid chromatography with regular second-dimension sampling
InactiveUS6730228B2Less complicatedUniversal applicabilityIon-exchange process apparatusSequential/parallel process reactionsGradient elutionPhase gradient
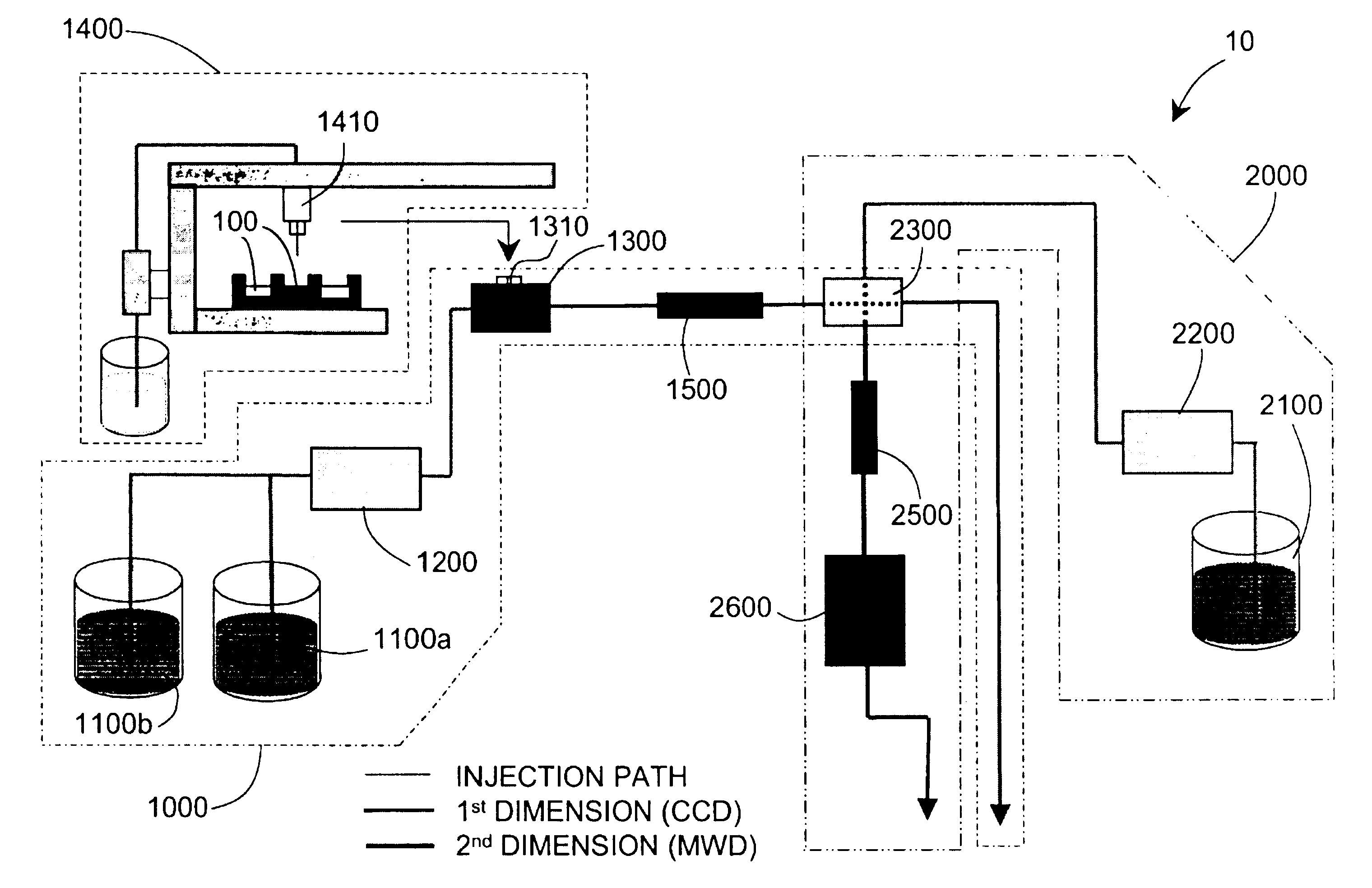
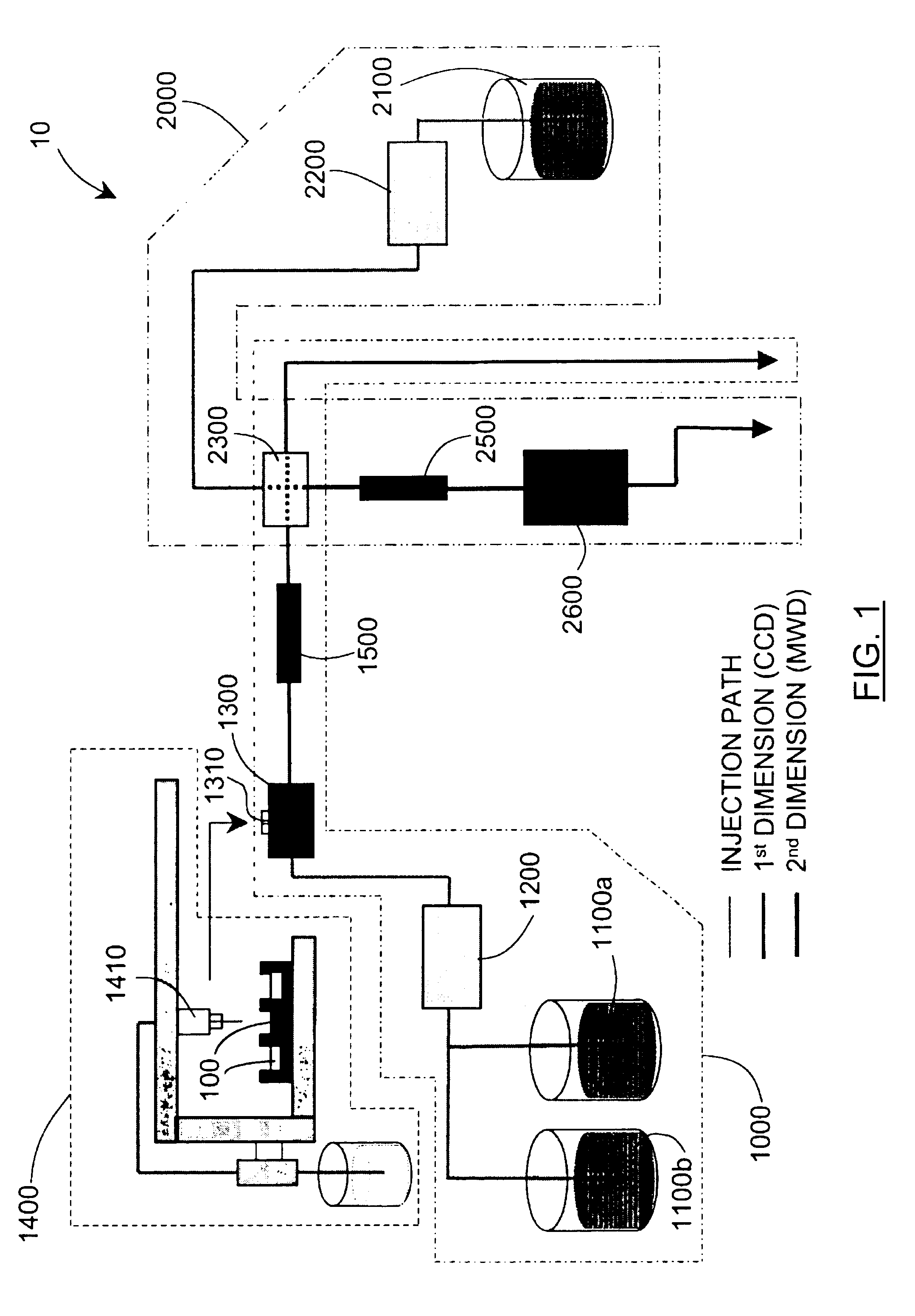
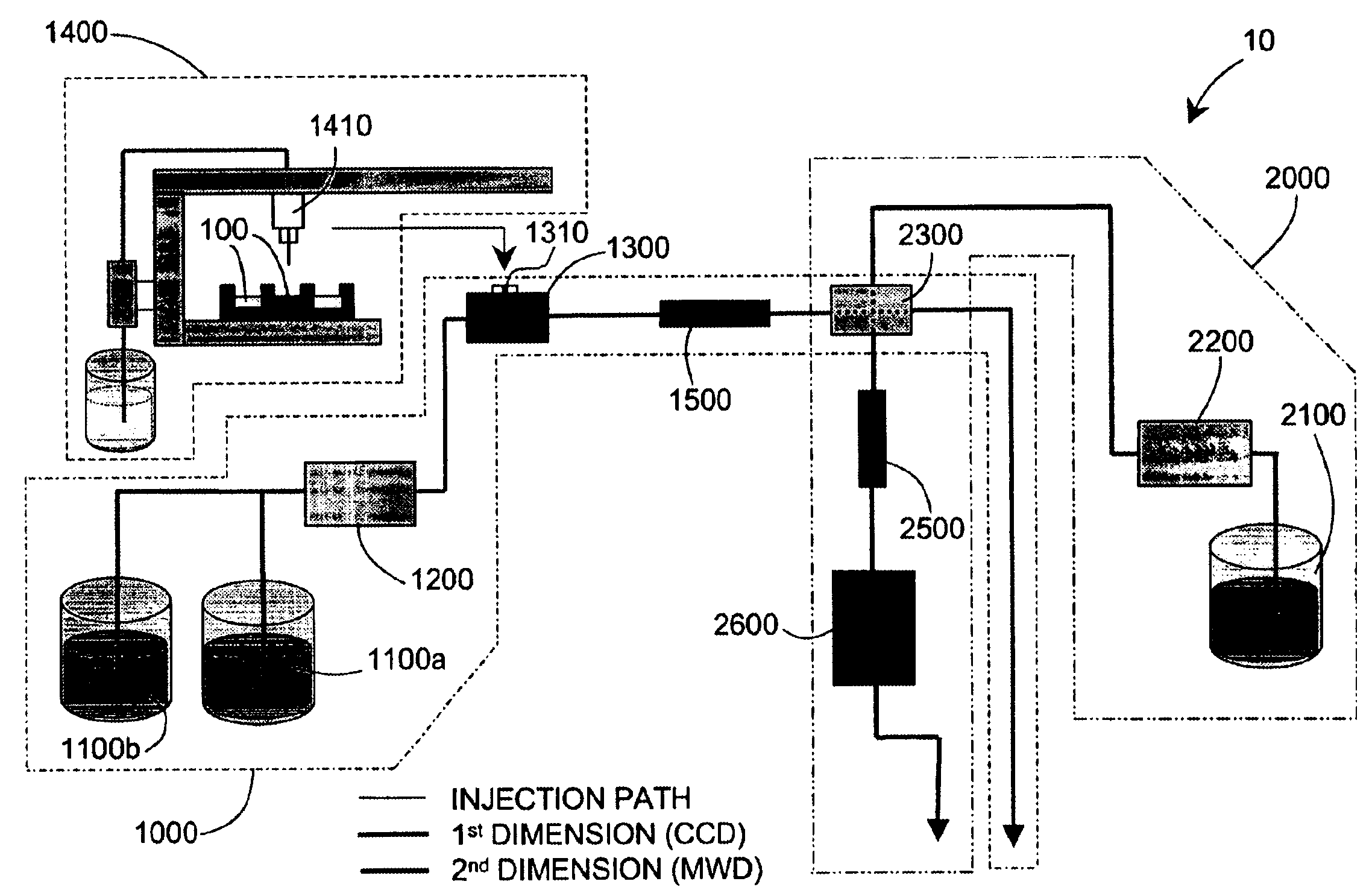
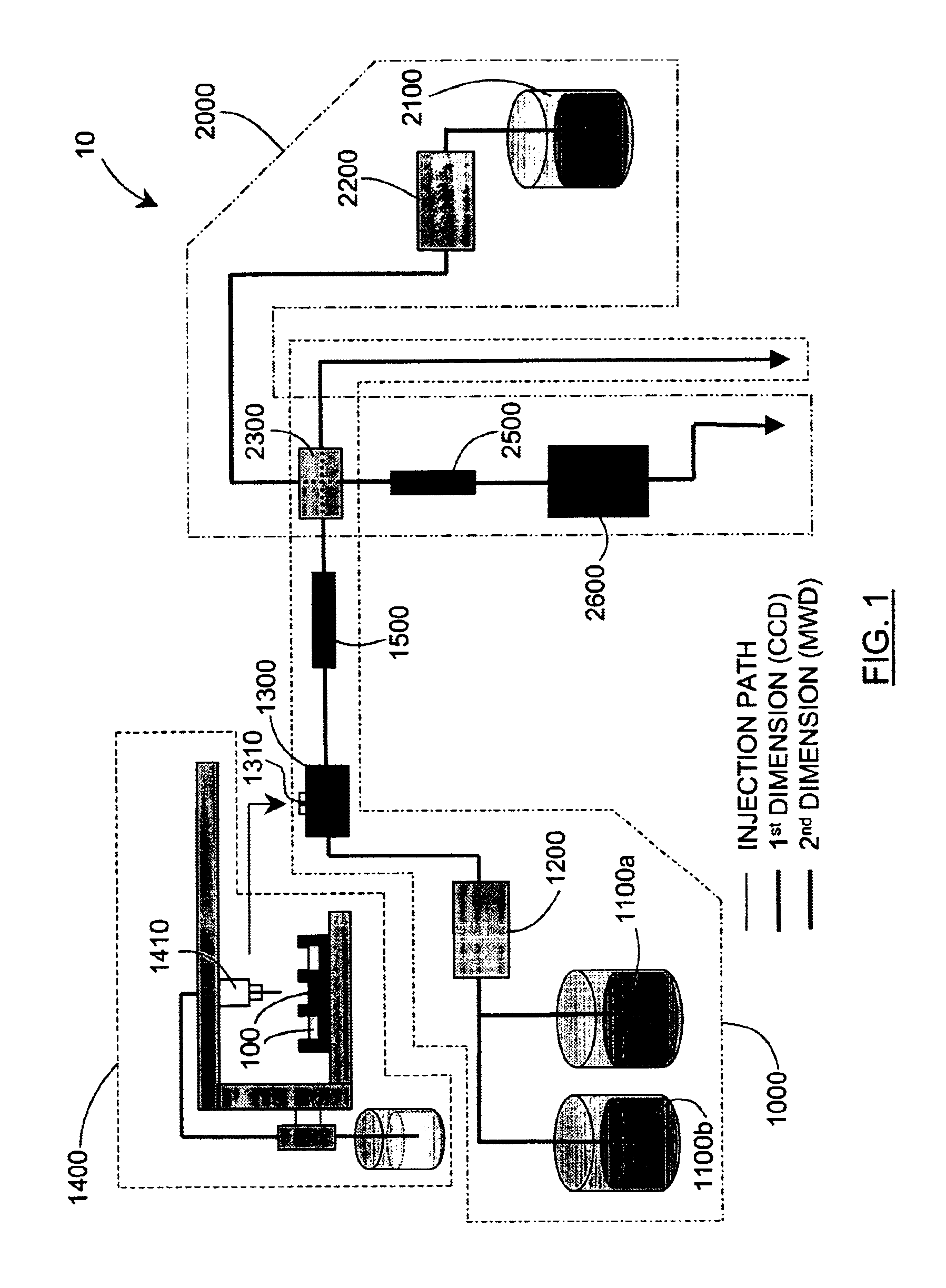
Methods and apparatus for characterizing a polymer sample and in preferred embodiments, libraries of polymer samples, in a comprehensive, directly-coupled multi-dimensional liquid chromatography system are disclosed. The first and second dimensions are preferably high-performance liquid chromatography dimensions, such as for example, a first dimension adapted for determining composition (e.g. adapted for mobile-phase gradient elution chromatography, including reverse phase chromatography, adsorption chromatography and the like), and a second dimension adapted for determining molecular weight or particle size (e.g., adapted for size exclusion chromatography, including gel permeation chromatography).
Owner:FREESLATE
Methods and apparatus for characterization of polymers using multi-dimensional liquid chromatography with parallel second-dimension sampling
InactiveUS20030089663A1Less complicatedUniversal applicabilityIon-exchange process apparatusSamplingGradient elutionPhase gradient
Methods and apparatus for characterizing a polymer sample and in preferred embodiments, libraries of polymer samples, in a comprehensive, directly-coupled multi-dimensional liquid chromatography system are disclosed. The first and second dimensions are preferably high-performance liquid chromatography dimensions, such as for example, a first dimension adapted for determining composition (e.g. adapted for mobile-phase gradient elution chromatography, including reverse phase chromatography, adsorption chromatography and the like), and a second dimension adapted for determining molecular weight or particle size (e.g., adapted for size exclusion chromatography, including gel permeation chromatography).
Owner:FREESLATE
Reversed chromatographic immunoassay
InactiveUS20040235189A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAnalyteOrder of reaction
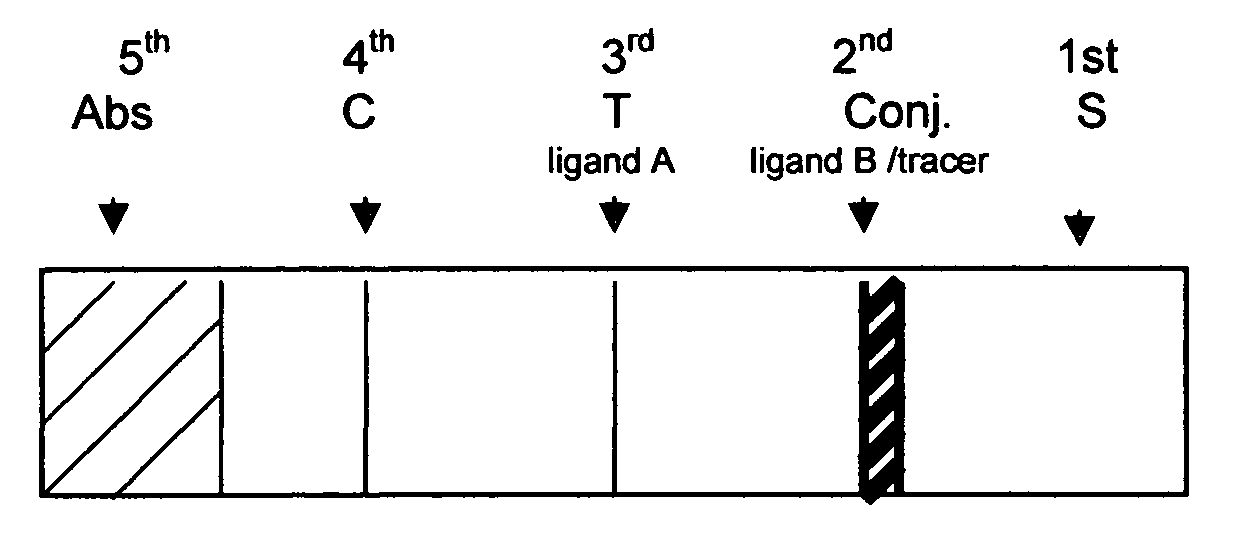
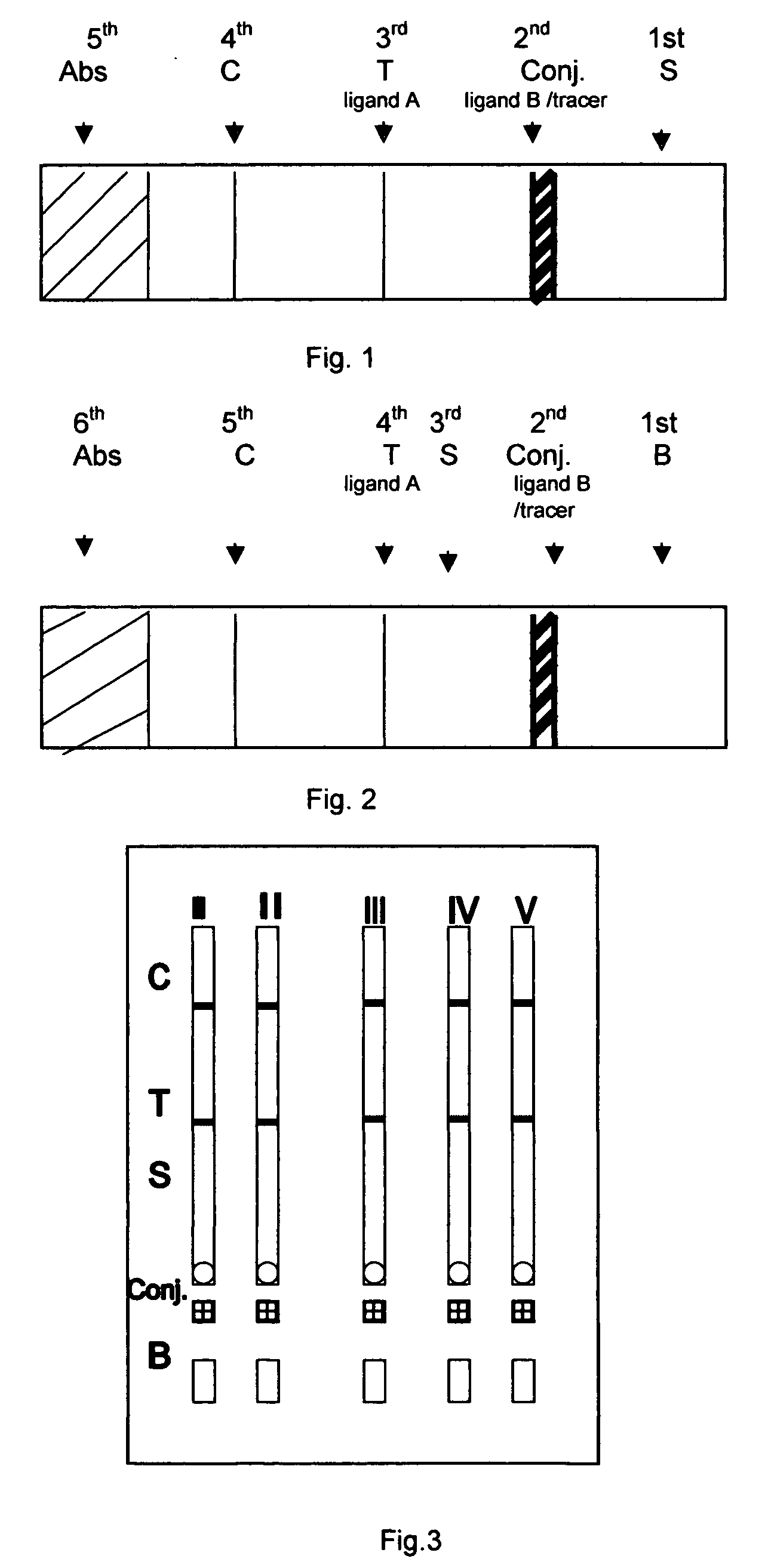
A chromatographic immunoassay test strip comprising of a solid support having the portions with said portions being in a strip so as to permit capillary flow communication with each other based on the help of adding a Buffer. This chromatographic immunoassay test strip herein changes the reaction order of the conventional chromatographic immunoassay solid test strip, wherein the analyte in the sample reacts with the specific binder (ligand A) immobilized on test zone prior to reacting with the ligand B / tracer. The reaction communication herein is achieved by a capillary flow aided by a Buffer. This solid chromatographic immunoassay test strip is useful in a variety of immunoassays.
Owner:LU WEI ZHAO
Methods for characterization of polymers using multi-dimensional liquid chromatography with parallel second-dimension sampling
InactiveUS6855258B2Less complicatedUniversal applicabilityIon-exchange process apparatusSamplingGradient elutionPhase gradient
Methods and apparatus for characterizing a polymer sample and in preferred embodiments, libraries of polymer samples, in a comprehensive, directly-coupled multi-dimensional liquid chromatography system are disclosed. The first and second dimensions are preferably high-performance liquid chromatography dimensions, such as for example, a first dimension adapted for determining composition (e.g. adapted for mobile-phase gradient elution chromatography, including reverse phase chromatography, adsorption chromatography and the like), and a second dimension adapted for determining molecular weight or particle size (e.g., adapted for size exclusion chromatography, including gel permeation chromatography).
Owner:FREESLATE
Compound with protein kinase inhibiting activity as well as preparation method and application of compound
ActiveCN106831898AConducive to industrial mass productionNervous disorderSugar derivativesDiseaseEthyl acetate
The invention discloses a compound with protein kinase inhibiting activity as well as a preparation method and application of the compound. The compound has a structure shown as a formula I, a formula II and a formula III. The compound provided by the invention can be used for medicines for treating cancers related with kinase inhibition, such as acute and chronic leukemia, lymphoma, breast cancer, lung cancer and sarcoma, and diseases including acquired immunodeficiency syndrome and Alzheimer's disease and the like. The invention further discloses the preparation method of the compound with the protein kinase inhibiting activity; the compound is produced through solid fermentation on rice by utilizing actinomyces; a fermented product is extracted through ethyl acetate and then is subjected to silica-gel column chromatography, gel column chromatography and reversed-phase chromatography separation and purification to obtain a product; the preparation method is easy to operate and implement. (The formula I, the formula II and the formula III are shown in the description.).
Owner:浙江美新控股有限公司
Methods and apparatus for characterization of polymers using multi-dimensional liquid chromatography with regular second-dimension sampling
InactiveUS20030080062A1Less complicatedUniversal applicabilityIon-exchange process apparatusSequential/parallel process reactionsGradient elutionPhase gradient
Methods and apparatus for characterizing a polymer sample and in preferred embodiments, libraries of polymer samples, in a comprehensive, directly-coupled multi-dimensional liquid chromatography system are disclosed. The first and second dimensions are preferably high-performance liquid chromatography dimensions, such as for example, a first dimension adapted for determining composition (e.g. adapted for mobile-phase gradient elution chromatography, including reverse phase chromatography, adsorption chromatography and the like), and a second dimension adapted for determining molecular weight or particle size (e.g., adapted for size exclusion chromatography, including gel permeation chromatography).
Owner:FREESLATE
Methods and apparatus for improved immunosuppressant drug monitoring
ActiveUS8759753B1Ensure sufficient separationEasy to separateIon-exchange process apparatusSugar derivativesStationary phaseESI mass spectrometry
A method for assaying one or more immunosuppressant drug analytes in a sample derived from whole blood comprises: (a) passing the sample dissolved in a mobile phase through a length of 30 mm or less of a stationary phase of a reversed-phase chromatographic column; (b) eluting the separated analytes from the reversed-phase chromatographic column; (c) ionizing molecules of the eluted separated analytes by a heated electrospray ionization source of a mass spectrometer so as to generate a plurality of precursor ion species; (d) isolating, for each analyte, a respective one of the precursor ion species; (e) fragmenting ions of each of the isolated precursor ion species in a fragmentation cell of the mass spectrometer so as to generate a plurality of product ions therefrom; and (f) detecting, for each analyte, the presence and quantity of a respective one of the product ion species using a detector of the mass spectrometer.
Owner:THERMO FINNIGAN
Method for purifying Carbetocin
The invention provides a technological method suitable for the industrialized purification of Carbetocin, a reversed-phase efficient liquid chromatography is used to purify the Carbetocin to result in high purity and good yield in order to meet industrialized demands. A crude peptide solution of the Carbetocin is filled with materials by a reversed-phase chromatographic column to be a stationary phase, a phosphate buffer solution is regarded as phase A and acetonitrile is regarded as phase B to implement gradient elution purification, wherein, the gradient: B%:20-40%, the pH of the phase A is 2.5-3.5; an anion exchange method is employed to convert the phosphate and trifluoroacetate to acetate. The invention employs one-step reversed-phase efficient liquid chromatography to purify followed by the acetate conversion by an anion exchange column in one step, thus obtaining high-purity acetate Carbetocin at high yield and offering an efficient purification technology for the massive purification and preparation of the peptide raw drugs.
Owner:HYBIO PHARMA
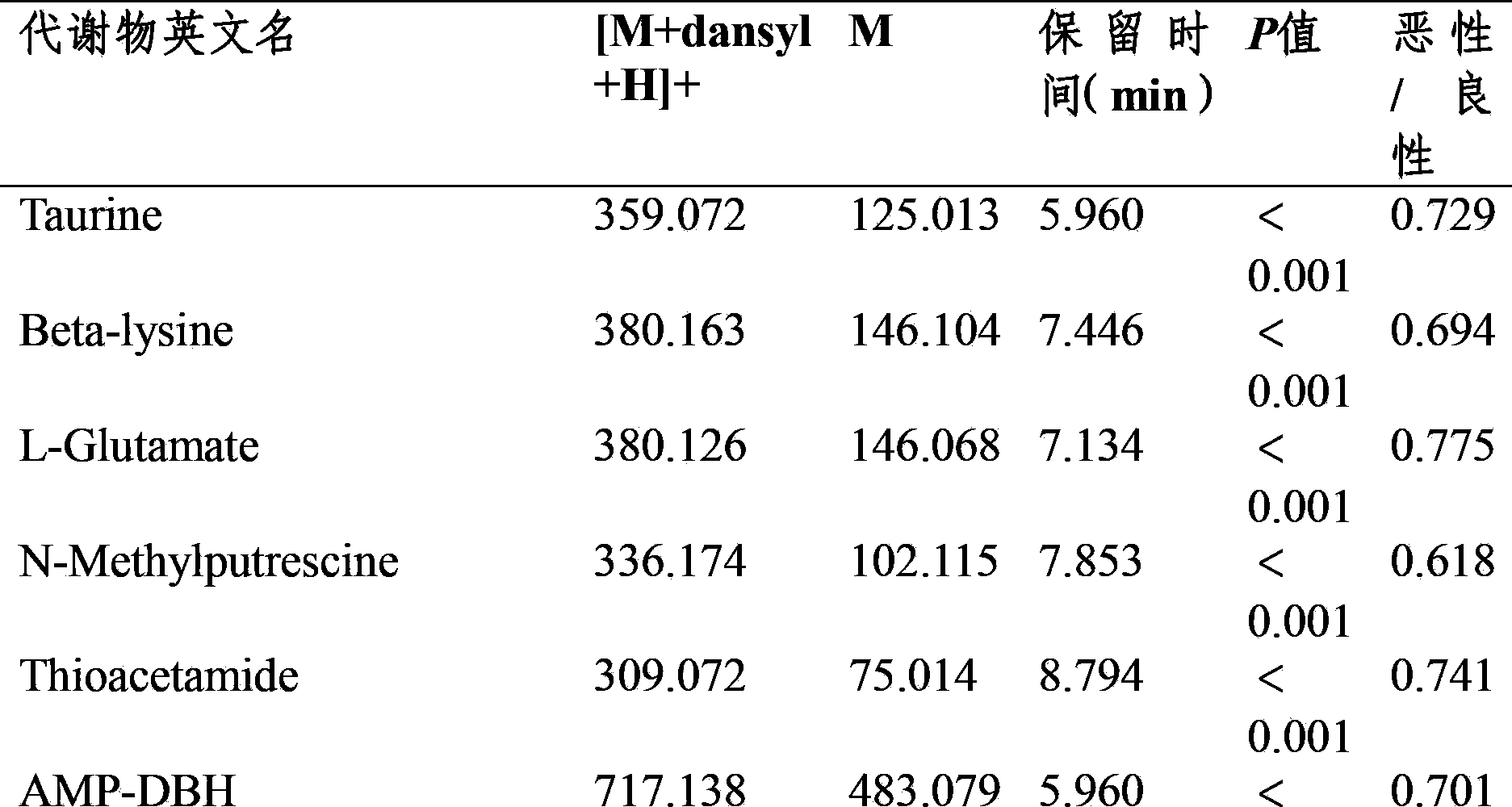
Method for analyzing amine substances in dansyl chloride derived-plasma based on liquid chromatography mass spectrometry
InactiveCN103822998AReduce consumptionStrong specificityComponent separationDipeptideGas chromatography–mass spectrometry
The invention discloses a method for analyzing amine substances in dansyl chloride derived-plasma based on liquid chromatography mass spectrometry. The method is characterized in that dansyl chloride is used as a derivatization reagent to perform derivatization on a metabolite containing primary amine and secondary amine, therefore the retention capability of the amine substances in reversion phase chromatography is increased, the ionization efficiency in mass spectrometric detection is increased, and the purpose of increasing the quantitative accuracy and detection sensitivity of the amine substances is achieved. The method has wide coverage for amine metabolites (comprising amino acid, methylation products, acetylation products, dipeptide and the like), can utilize 100 [mu]L of plasma samples to detect 113 amine substances, supplies the outline information of metabolome of the amine substances, and has the characteristics of good specificity, high accuracy and repeatability, less reagent consumption and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Rapid characterization of polymers
InactiveUS6866786B2Less complicatedUniversal applicabilitySequential/parallel process reactionsComponent separationGradient elutionPhase gradient
Methods and apparatus for characterizing a polymer sample and in preferred embodiments, libraries of polymer samples, in a comprehensive, directly-coupled multi-dimensional liquid chromatography system are disclosed. The first and second dimensions are preferably high-performance liquid chromatography dimensions, such as for example, a first dimension adapted for determining composition (e.g. adapted for mobile-phase gradient elution chromatography, including reverse phase chromatography, adsorption chromatography and the like), and a second dimension adapted for determining molecular weight or particle size (e.g., adapted for size exclusion chromatography, including gel permeation chromatography).
Owner:FREESLATE
Separation and purification method of high-purity vancomycin hydrochloride
ActiveCN104610434AEasy to recycleColor Appearance EnhancementAntibacterial agentsSaccharide peptide ingredientsPurification methodsIon exchange
Provided is a separation and purification method for vancomycin hydrochloride of a high purity. The method comprises the following steps: (1) obtaining a vancomycin hydrochloride solution from a crude vancomycin product by ion exchange chromatography and obtaining a concentrate by nanofiltration desalination and concentration; (2) adjusting the concentrate with a hydrochloric acid solution and then performing a column chromatography using a reverse chromatography column for the adjusted concentrate; (3) collecting the chromatographic solution of vancomycin to obtain a mixed chromatographic solution; (4) adjusting the mixed chromatographic solution, and separating the solution and the salts by nanofiltration desalination and concentration to obtain a concentrate; and (5) obtaining a vancomycin dry powder with a chromatographic purity of up to 99% and a pure white appearance by dehydrating and drying the concentrate of step (4), or by solvent crystallization or salting-out crystallization.
Owner:ZHE JIANG MEDICINE CO LTD XINCHANG PHARMA FAB
Method for extracting and separating paclitaxel and taxones compounds from yew
InactiveCN101397284AIncreased overall extraction rateEasy to separateOrganic chemistryConiferophyta medical ingredientsTaxane CompoundMonomer
The invention discloses a method for extracting and separating taxol and other taxane compounds from yew. The method includes the steps of extraction, degreasing, crude separation to an organic phase and an aqueous phase respectively, refined separation with reverse-phase chromatography, and crystallization. The invention sequentially adopts the ethanol of 80 to 95 percent, 60 to 75 percent and 20 to 55 percent to carry out gradient extraction to the yew material and also to fully extract taxane compounds with different polarities so as to lead the total extraction rate of the taxane compounds to be raised to more than 90 percent from 70 percent of the traditional art; the simultaneous extracting of 8 different tazane compound monomers from one set of the yew material is realized by the invention and the purity of each monomer can reach more than 98 percent; an aqueous phase substance which is generated in the extraction process abandoned by the traditional art is fully utilized to raise the utilization rate of the taxane compounds in the yew by 10 to 20 times, therefore, the bottleneck problem of industrial production of the lack of yew material is effectively solved.
Owner:桂林市振达生物制药有限公司
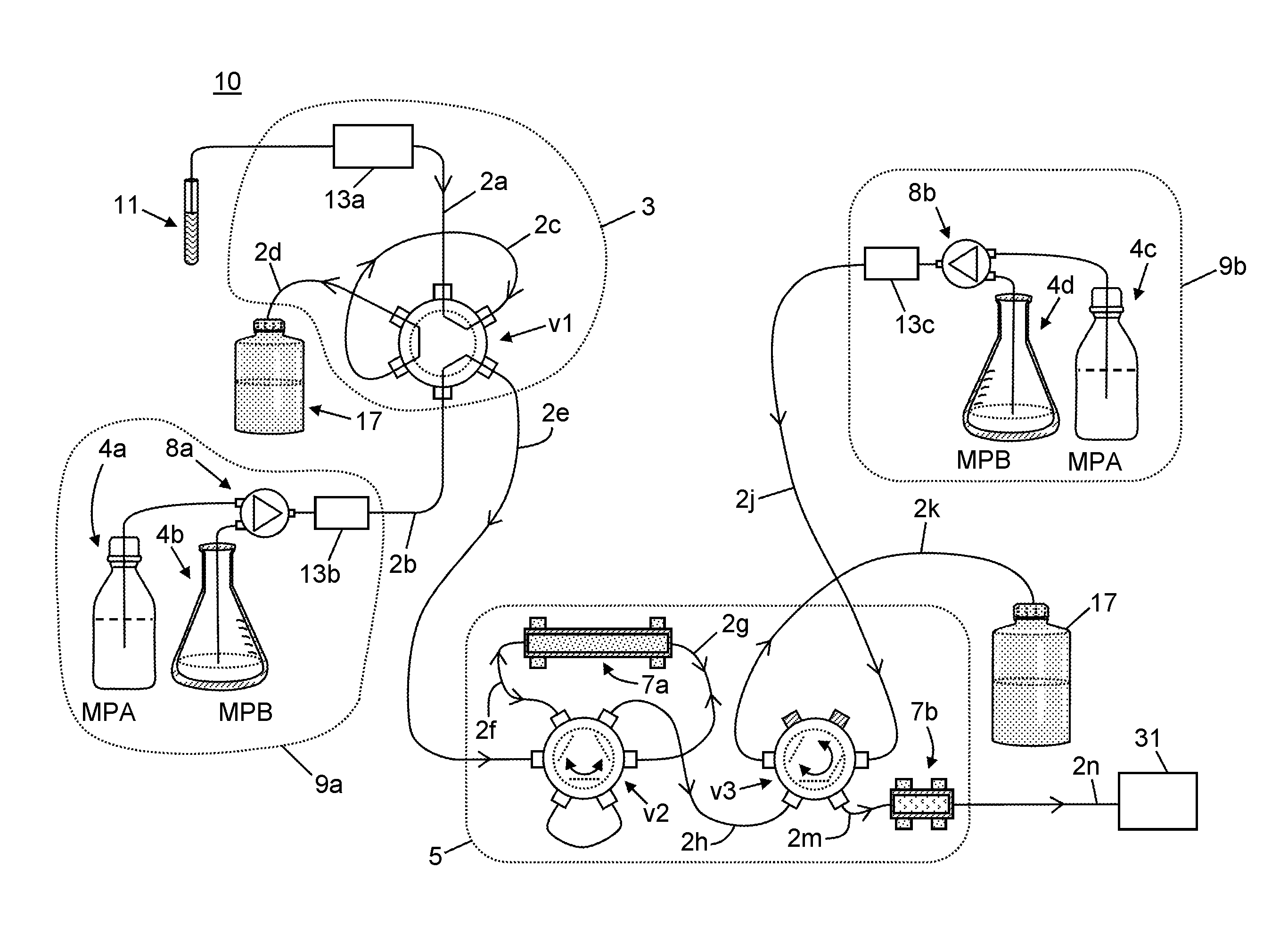
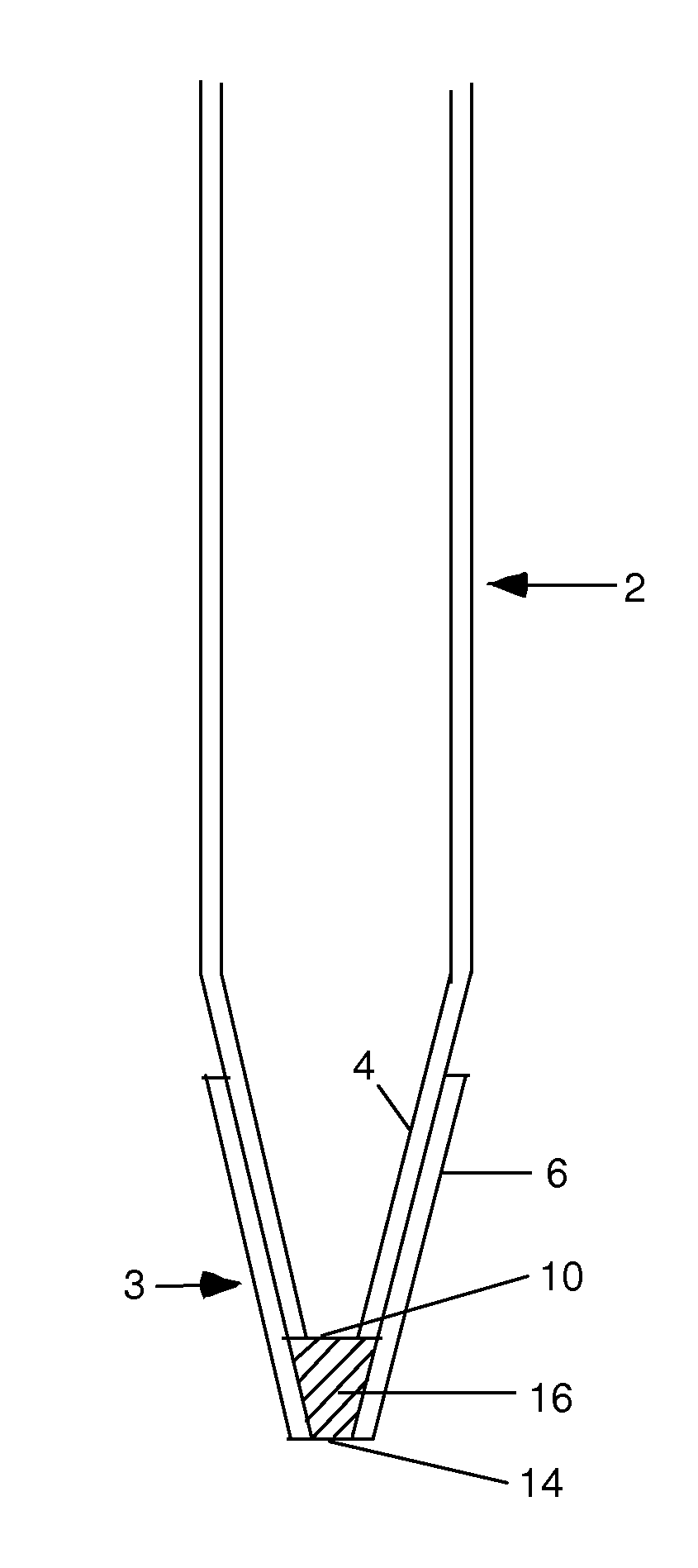
Method and Device for Gravity Flow Chromatography
InactiveUS20100170852A1Small diameterAvoid mixingIon-exchange process apparatusSamplingFiltrationIon exchange
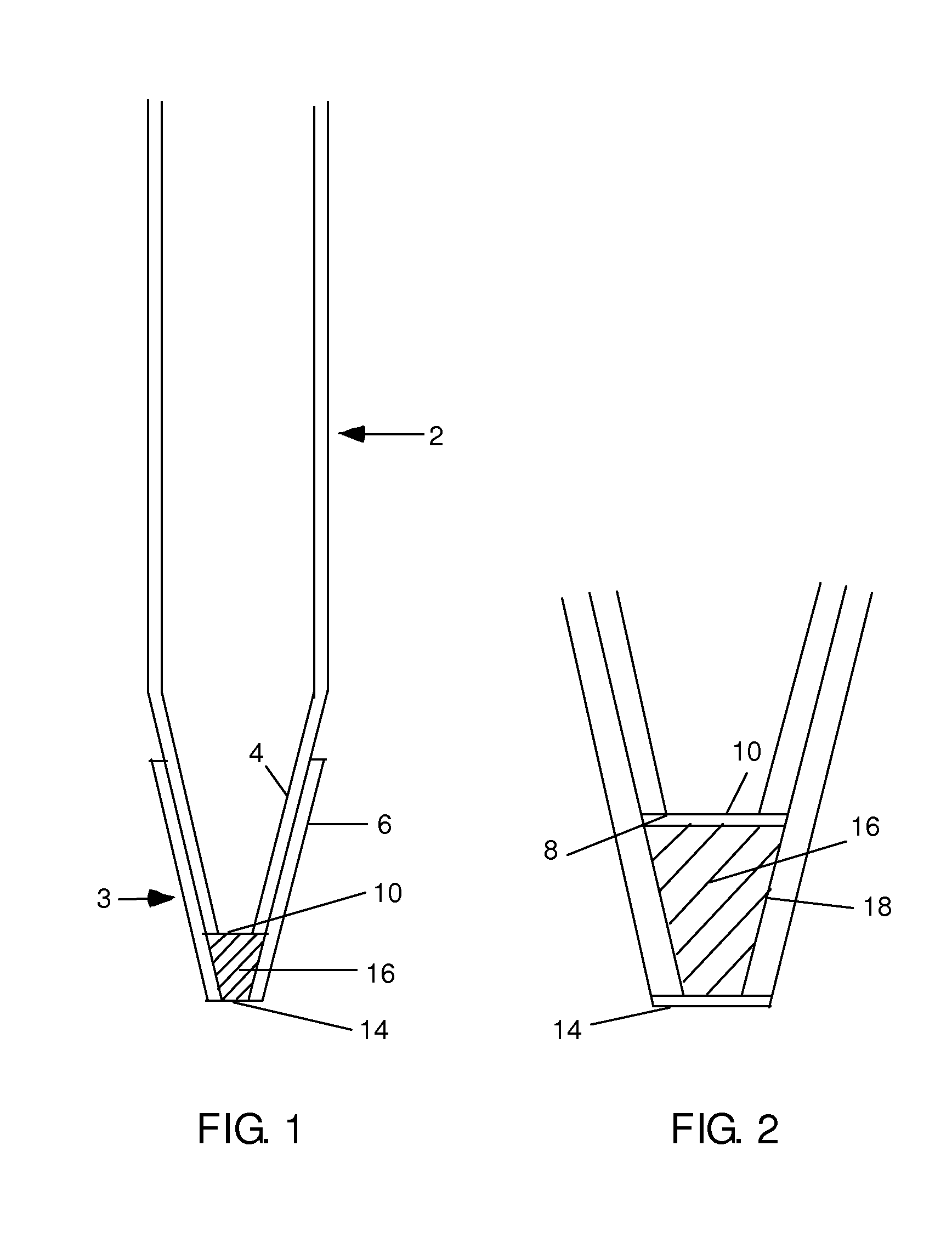
The invention provides gravity chromatographic columns for the purification of a material (e.g., a biological macromolecule, such as a peptide, protein or nucleic acid) from a sample solution, as well as methods for making and using such columns. The columns typically include a bed of media positioned above a bottom frit or between a bottom and top frit. In some embodiments, the columns employ modified pipette tips as column bodies. In some embodiments, the columns employ modified plates or racks as column bodies. In some embodiments, the invention provides methods and devices for gel filtration, desalting, buffer exchange, ion exchange, ion-pairing, normal phase and reverse phase chromatography. In some embodiments, the invention provides multiplexing gravity flow chromatography on a liquid handling robotic system.
Owner:SUH CHRIS +2
Preparation method of organic-inorganic hybridized monolithic column
ActiveCN103170161AAffect uniformitySlow down aggregationOther chemical processesComponent separationChromatographic separationSilica gel
The invention discloses an organic-inorganic hybridized monolithic column as well as a preparation method and an application thereof. The monolithic column comprises a silica gel framework and has a typical reversed phase chromatographic mechanism through introducing octyl. According to the monolithic column, tetraethoxysilane is served as an inorganic monomer, octyltriethoxysilane is served as an organic-inorganic hybridized monomer, a binary porogenic agent Pluronic F127 / lauryl amine is used for substituting for the conventional single porogenic agent-lauryl amine, methanol / water is served as a solvent, hydrochloric acid and lauryl amine are used as acid-base regulators, and the monolithic column is synthesized by adopting a double-step acid-base sol-gel method. The monolithic column obtained by the method is of an ideal double-pore structure, a preparation process is simple and easy to operate, and high column efficiency is obtained. Through utilizing the synthesizing method of the monolithic column, naphthalene is served as an analyte, and the height of the obtained column plate is reduced to 20-30 micrometers from 70-80 micrometers. The hybridized monolithic column can be used for the reversed phase chromatographic separation and enrichment of various hydrophobic substances.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Liquid chromatographic sampling passage cleaning device
ActiveCN104690047AEasy to cleanExtended service lifeHollow article cleaningDrying gas arrangementsParticulatesIon chromatography
The invention provides a liquid chromatographic sampling passage cleaning device. The liquid chromatographic sampling passage cleaning device mainly comprises a microfluidic pump, a compressed air source, a capillary flow limiter, a liquid-gas switching valve, a sampling-cleaning switching valve, a waste liquid collecting cup, a waste liquid bottle, a sampling valve, a sampling port and a program controller. After sampling, a cleaning solution is used for reversely rinsing a liquid chromatographic sampling passage to reversely rinse particles left on a filter in the sampling process and discharge the particles out of a sampling pipeline first and then microflow gas is used for reversely purging the sampling passage to expel liquid left inside the pipeline so as to avoid sample dilution during sampling once again. The liquid chromatographic sampling passage cleaning device is suitable for various analytical-grade liquid chromatographs, comprising positive chromatography, reverse chromatography, ion chromatography and the like, as well as suitable for cleaning a sampling loop for flow injection analysis, so that cross pollution of samples is avoided, the sampling repeating precision is improved, and the service life of the sampling filter can be greatly prolonged.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
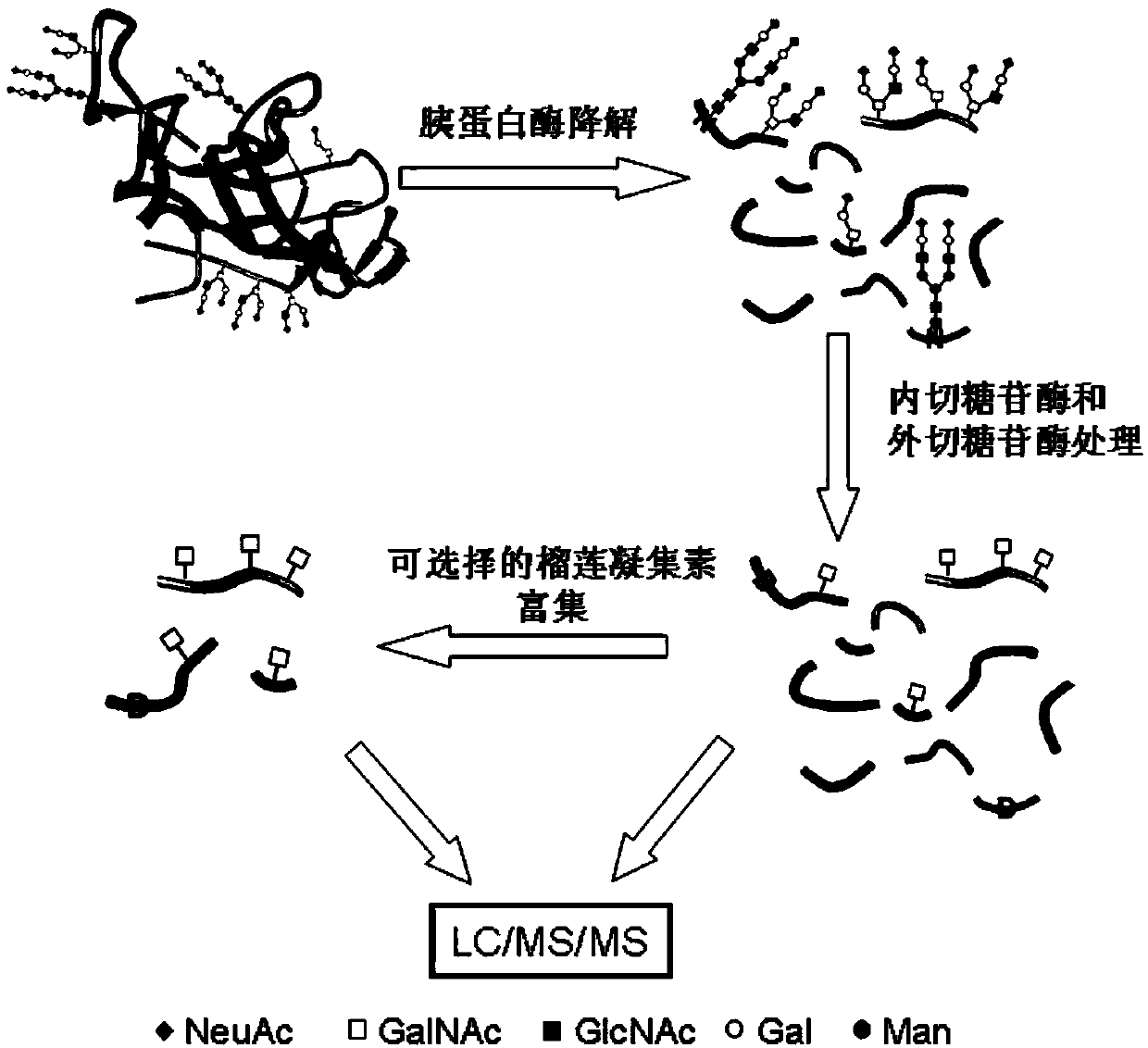
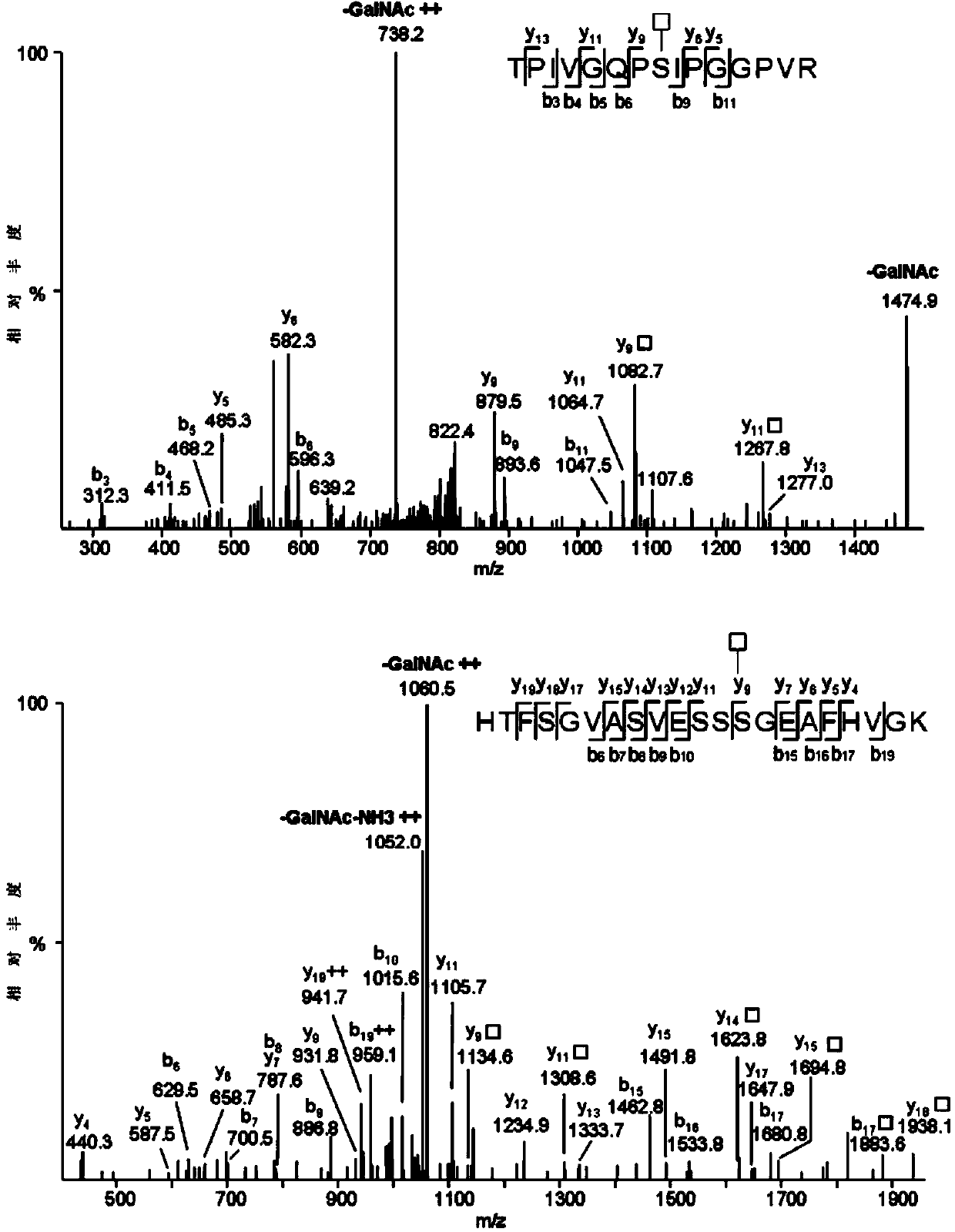
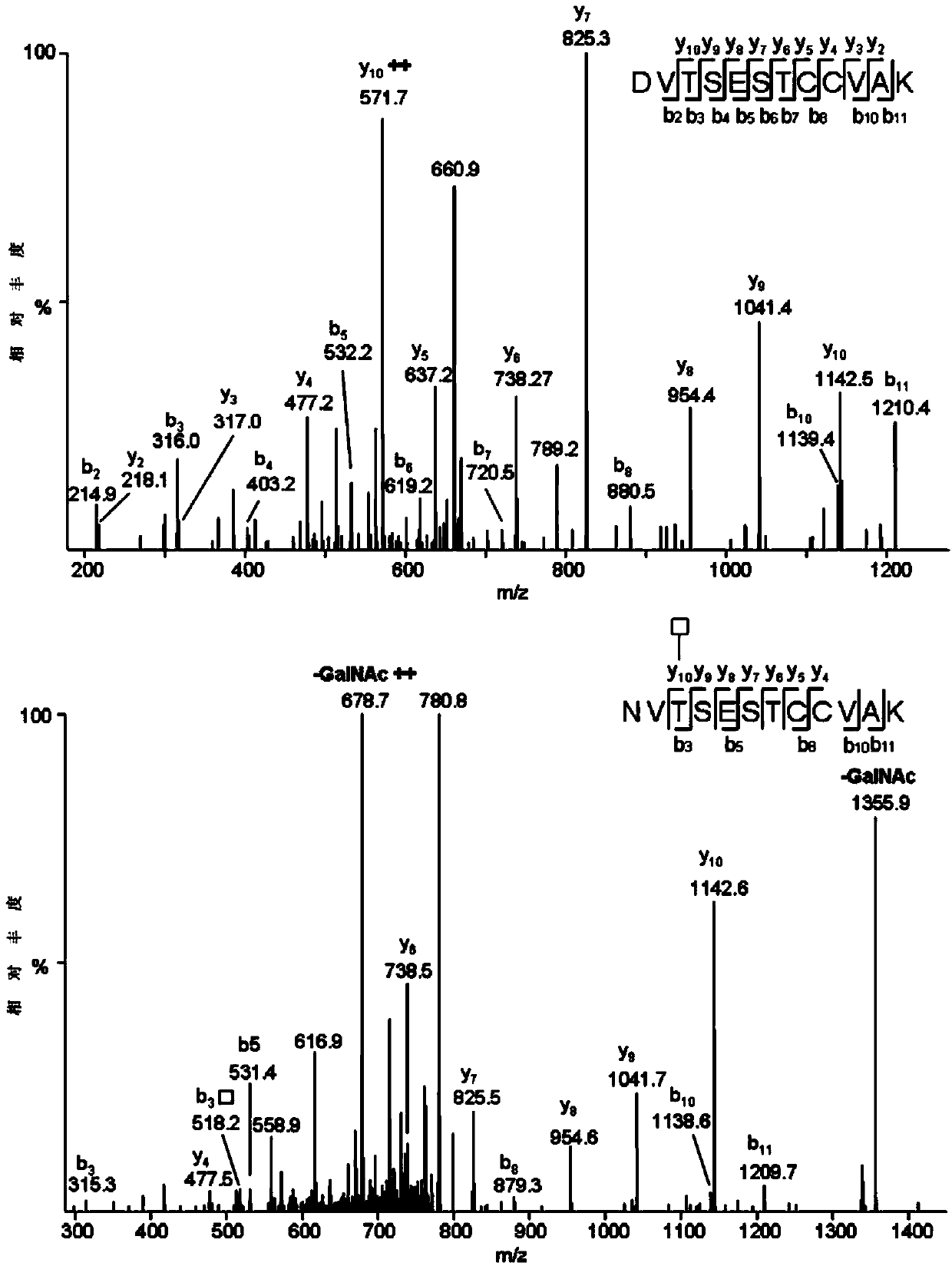
Method for analyzing protein O-glycosylation sites
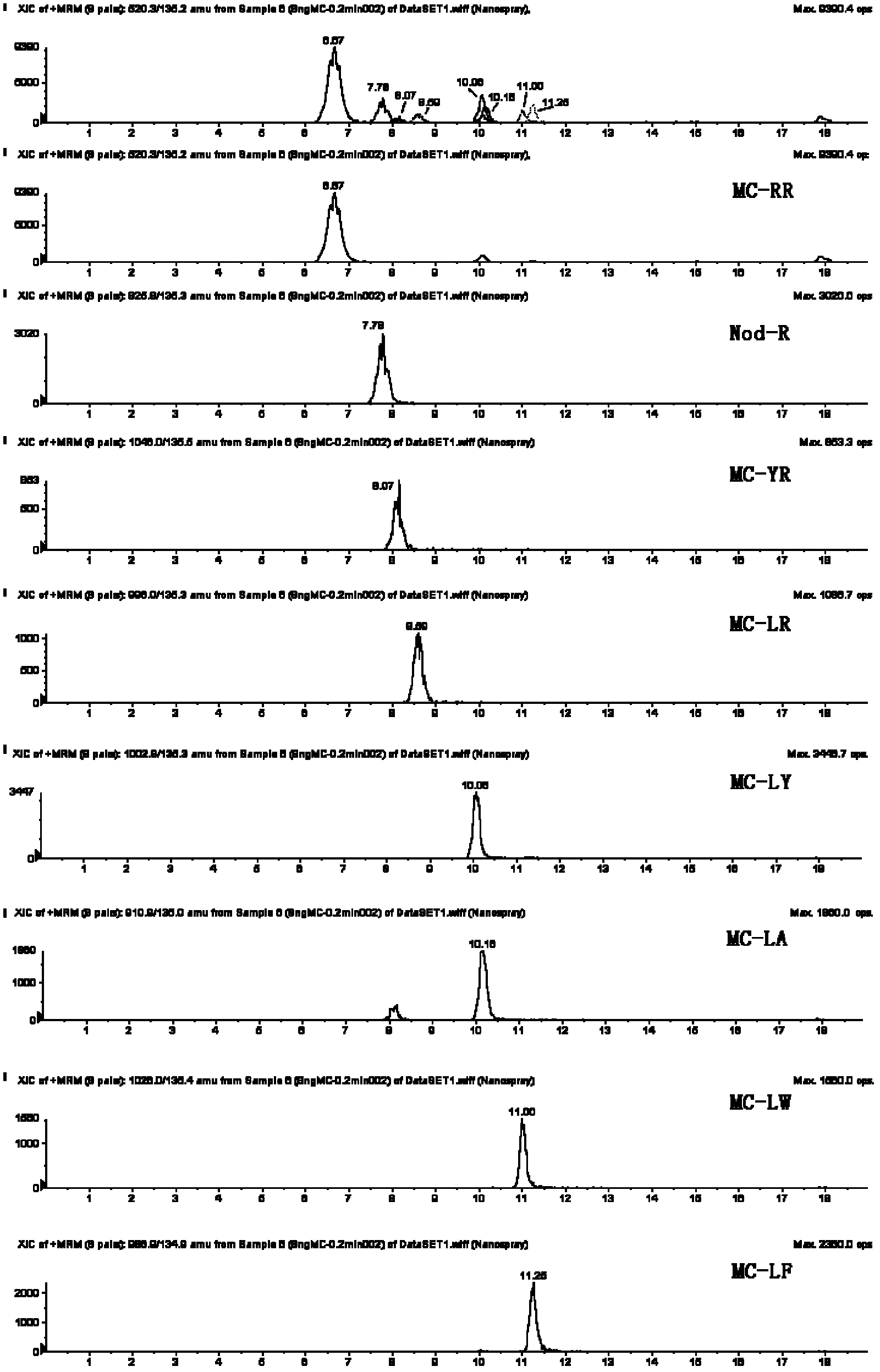
The invention relates to a method for analyzing protein O-glycosylation sites. The method comprises the following steps: (1), sequentially carrying out endonuclease digestion, endoglycosidase and exoglycosidase digestion on a proteome sample or a single protein sample to prepare a peptide and non-glycopeptide protein sample or single protein sample with a GalNAc label; (2) loading the sample on a durian agglutinin chromatographic column and separating to prepare a proteomic sample; (3) separating the single protein sample or the proteomic sample through with a C18 reversed-phase chromatographic column, and then detecting by a high-definition mass spectrometer in a cation mode to obtain a high-definition mass spectrogram; (4) processing the obtained mass spectrometric data and obtaining protein glycosylation site information though the searched result. The site information can be more rapidly, more accurately and more comprehensively obtained through establishing the method for detecting the O-glycosylation sites of glycoproteins in the single protein sample and the proteomic sample.
Owner:SHANDONG UNIV
Method for purifying oxidized beta-nicotinamide adenine dinucleotide
ActiveCN104876994AHigh purityHigh yieldSugar derivativesSugar derivatives preparationFreeze-dryingGradient elution
The invention discloses a method for purifying oxidized beta-nicotinamide adenine dinucleotide, which comprises the following steps: carrying out microfiltration and nanofiltration on a reaction solution obtained by enzyme catalysis reaction by using filter membranes, and collecting the concentrated solution for later use; adding acid into the concentrated filtrate to regulate the pH value of the concentrated solution, and carrying out gradient elution purification by using an inversed phase chromatographic column as a stationary phase, a buffer salt solution as a phase A and ethanol as a phase B; and carrying out nanofiltration concentration on the purified solution through a filter membrane, and carrying out freeze-drying with a vacuum freeze drier. By using the inversed phase high performance liquid chromatography to purify the oxidized beta-nicotinamide adenine dinucleotide, the oxidized beta-nicotinamide adenine dinucleotide has high purity and high yield, and achieves the industrialization requirements.
Owner:BONTAC INVITROLIFE BIO TECH SHENZHEN CO LTD
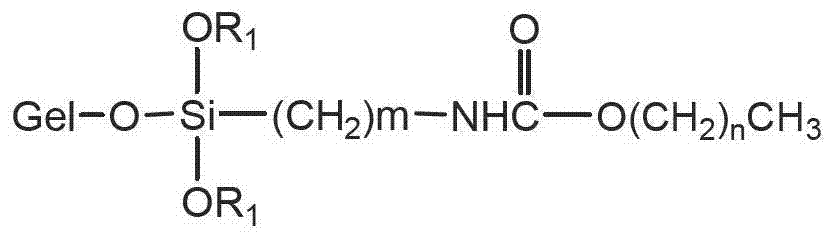
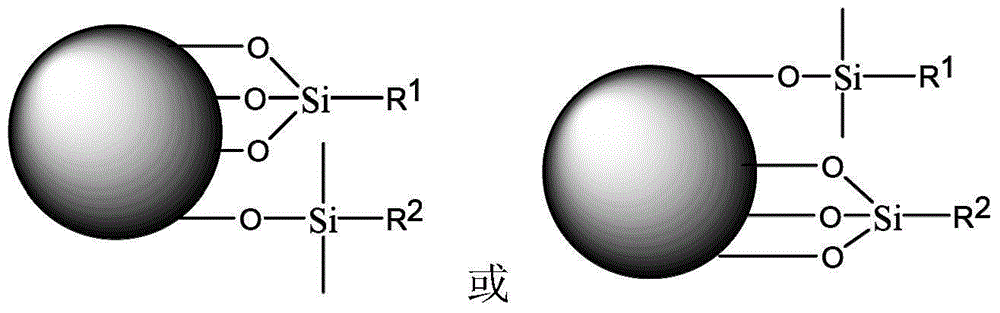
Carbamic acid ester type liquid phase chromatogram stationary phase and preparation method thereof
The invention relates to an efficient liquid phase chromatogram stationary phase which is characterized in that gel in the structure is a particle with hydroxyl, R1 is methyl or ethyl, m is equal to 3-8 and n is equal to 3-29. The liquid phase chromatogram stationary phase has the following advantages: 1, the structure is novel, a polar carbamic acid ester structure is embedded in a hydrophobic carbon chain, wherein an alkyl is at the tail end of an ester bond of the carbamic acid ester (O-alkyl), so that the effect of silicon hydroxyl on the surface of filler on separation can be benefited to being eliminated due to the principle of electrostatic screening, and the compatibility of chromatograph filler and pure water moving phase can be improved; 2, the preparation process for the stationary phase is simple and reliable, and the raw material is easy to obtain, thus being beneficial to batch production of the stationary phase; and 3, the liquid phase chromatogram stationary phase has wide application range. The stationary phase is a pervasive reversed phase chromatography stationary phase, has good analysis property to most polar and nonpolar compound, and has good market application prospect. The invention discloses a preparation method thereof.
Owner:CHANGZHOU HIGH TECH RES INST OF NANJING UNIV
Method for extracting and separating huperzine A and huperzine B from huperzia serrata
The invention relates to a method for extracting and separating huperzine A and huperzine B from huperzia serrata, which comprises total huperzine extraction and purification, and huperzine A / huperzine B separation and crystallization. The invention has the following advantages: acid soaking and ultrasonic repeated extraction can sufficiently precipitate total huperzines in the huperzia serrata; activated carbon and cation exchange resin are utilized to remove impurities; reversion phase chromatography columns are utilized to separate huperzine A and huperzine B; and concentration crystallization and recrystallization are respectively utilized to enhance the purity of the huperzine A and huperzine B. The invention effectively utilizes natural resources, enhances the recovery rate and extract content, and improves the economic benefit.
Owner:重庆市秀山红星中药材开发有限公司
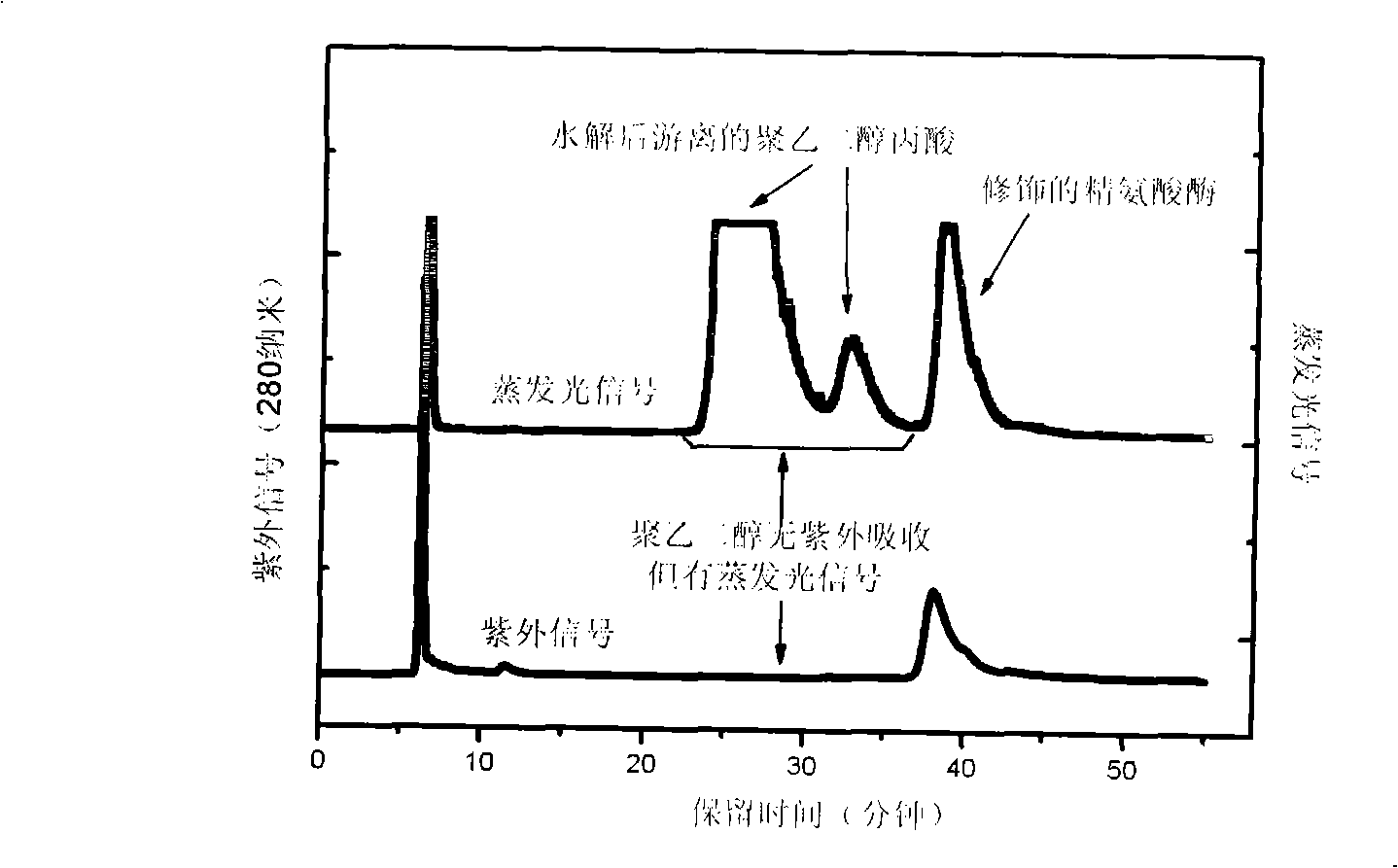
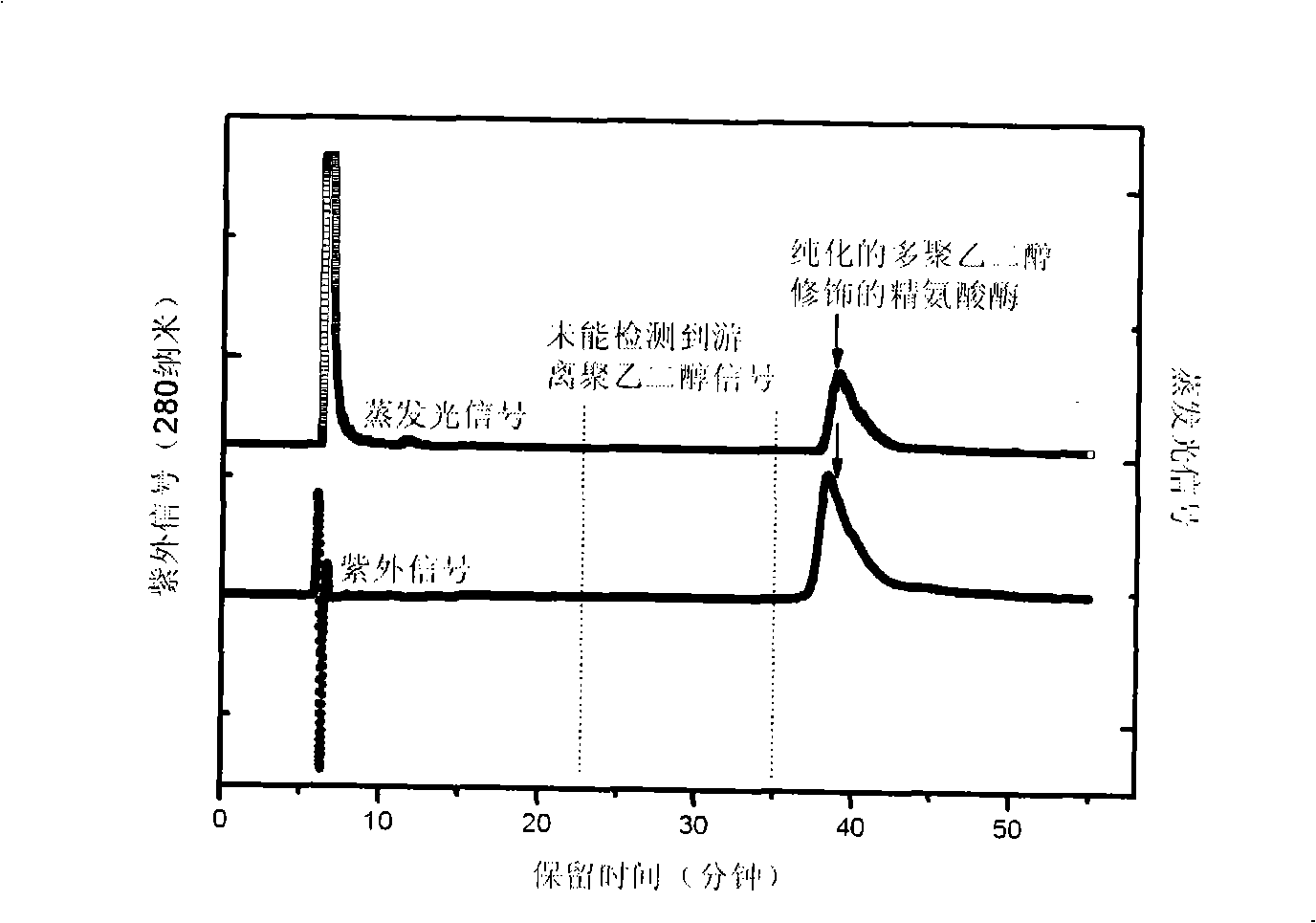
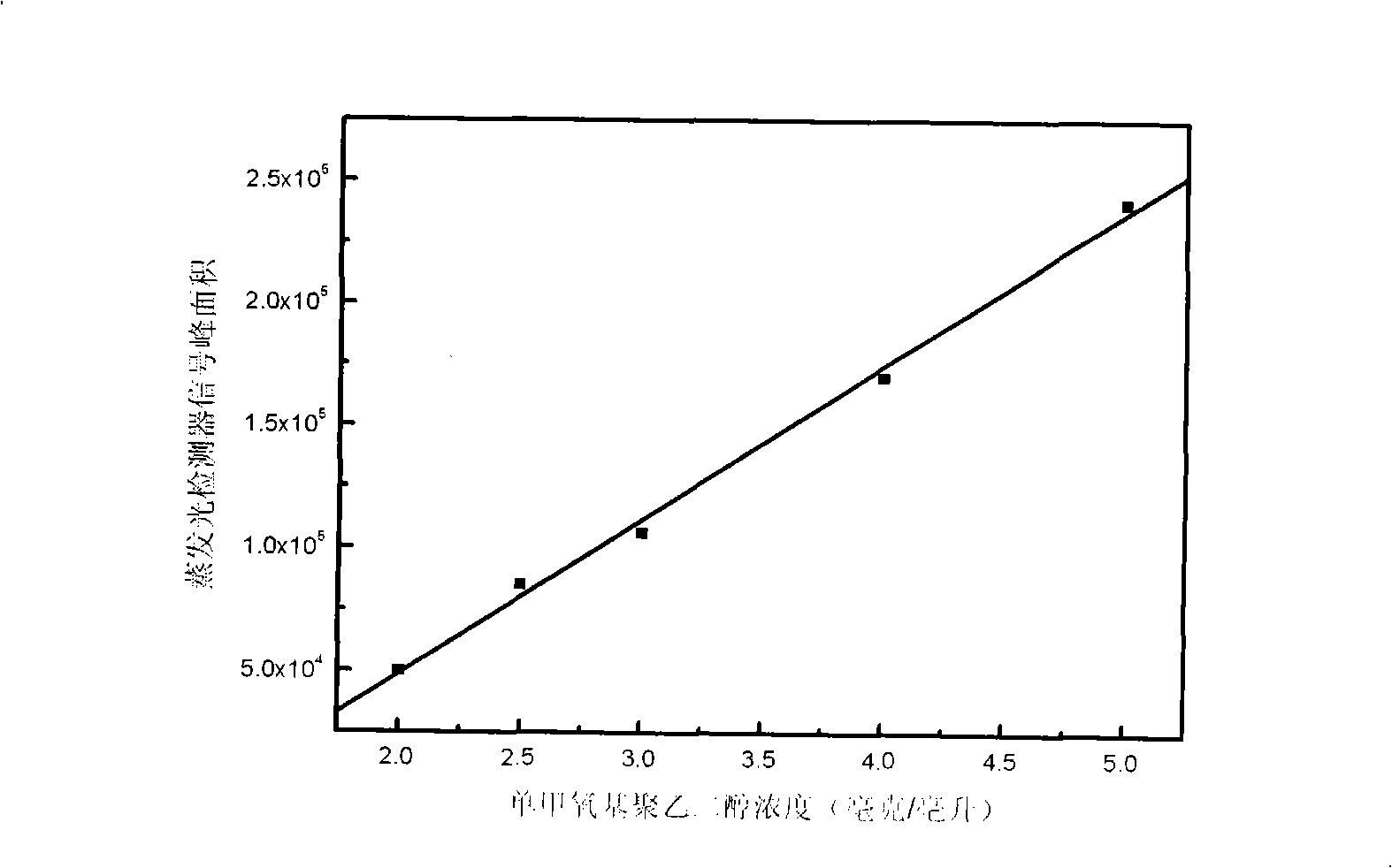
Method for measuring free polyethyleneglycol content in sample or products
The invention discloses an RP-HPLC detection method for the content of free polyethylene glycol in samples or products, which is characterized by making use of the non-polar difference of polyethylene glycol from other substances on the reversed-phase chromatography column and using UV detector combining evaporative light detector or diode array detector combining evaporative light detector signal contrast to screen signal peaks of free polyethylene glycol and to determine the content thereof by means of the external standard method. Based on the method, the invention also can be used for detecting the purity of polyethylene glycol modified products and the modification degree of modified products. The analysis method is highly sensitive, good in repetitiveness and selectivity, fast, efficient and simple, and is suitable for being used in the samples which have more complex components and are interfered in the polyethylene glycol-barium compound colorimetric determination provided by pharmacopoeia, especially suitable for being used in the polyethylene glycol modified samples or products.
Owner:HAINAN SIMCERE PHARMA CO LTD
Purification of proteins using preparative reverse phase chromatography (RPC)
InactiveUS20090036652A1Increase separation forceColony-stimulating factorDepsipeptidesIndustrial scaleReversed-phase chromatography
The present invention provides a method for industrial-scale protein separation by reverse phase chromatography by use of a buffer system and an additional salt.
Owner:NOVO NORDISK AS
Silica gel chromatographic packing for separation of alkaline compound and preparation method of silica gel chromatographic packing
ActiveCN105080492AHigh separation selectivityImprove stabilityOther chemical processesSolid sorbent liquid separationHydrogenIon exchange
The invention provides silica gel chromatographic packing for separation of an alkaline compound and a preparation method of the silica gel chromatographic packing. The method comprises the following step: reacting silica gel with a mixture of a nonpolar silane coupling agent and a polar silane coupling agent to prepare the silica gel chromatographic packing. The chromatographic packing prepared by the preparation method of the silica gel chromatographic packing for separation of the alkaline compound provided by the invention can provide acting forces in various forms such as hydrophobic effect, hydrogen bonding effect, electrostatic attraction and ionic exchange, and has better separation selectivity compared with conventional reversed-phase chromatographic packing.
Owner:浙江月旭材料科技有限公司
Preparation method of high-purity oritavancin key intermediate A82846B
The invention provides a preparation method of high-purity oritavancin key intermediate A82846B. The preparation method comprises the following steps: separating the oritavancin key intermediate A82846B from a fermented solution, first adjusting a pH, purifying by virtue of macroporous resin, then purifying by virtue of inversed phase chromatography, and finally crystallizing to obtain the high-purity A82846B. The method adopted by the invention is simple in operation, less in consumption of organic solvent, and capable of greatly reducing the generation of waste liquid; and moreover, the defects in the prior art that the adsorption efficiency of cation macroporous resin is low and the product yield is reduced due to the leaked adsorption can be overcome, and the preparation method is suitable for the industrialized production.
Owner:CHONGQING QIANTAI BIOLOGICAL MEDICINE
Crocin finger spectrum analysis method
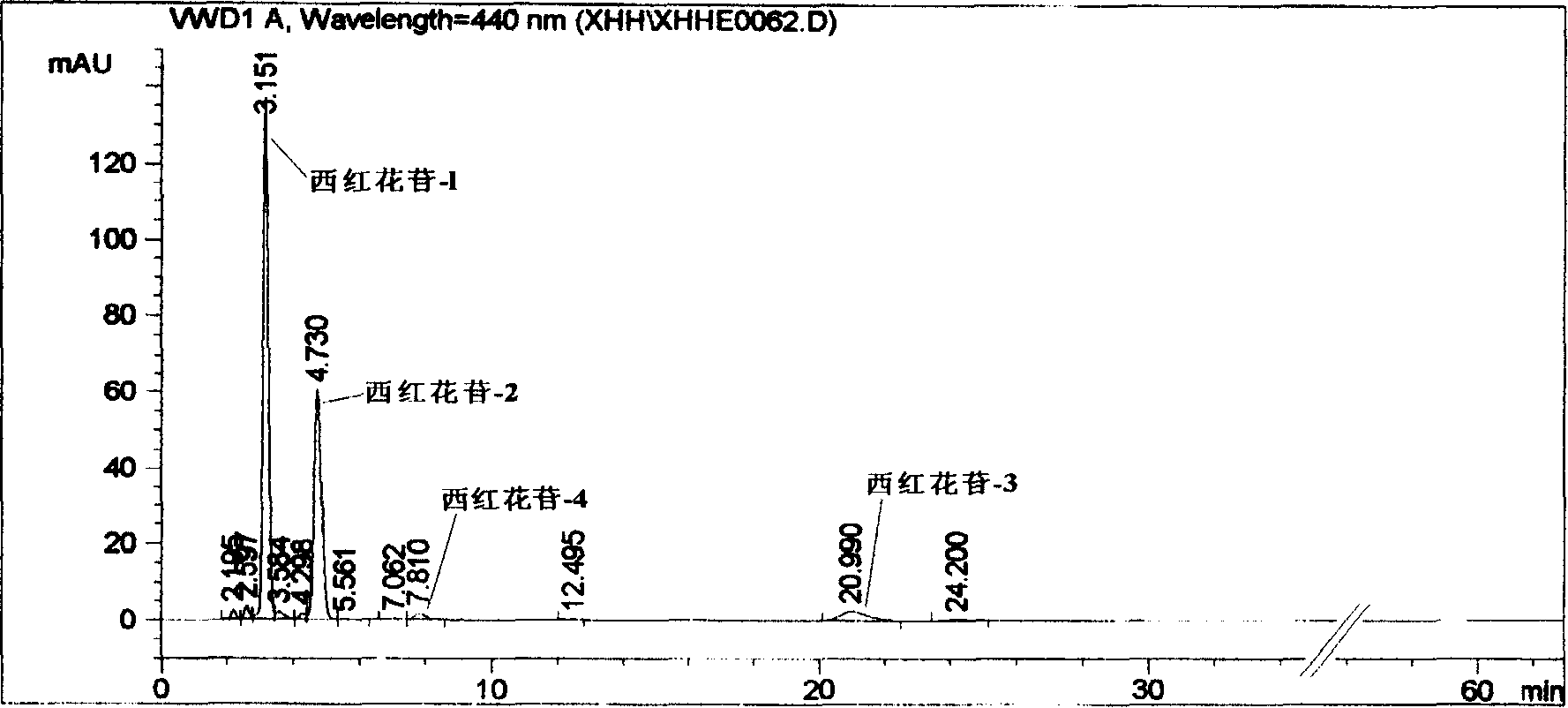
The invention discloses a fingerprint atlas analyzing method; which is characterized by the following: adding buffer in the inversed-phase chromatogram system for west carthamin; possessing stable chemical property for west carthamin-1, west carthamin-2, west carthamin-4 in the flow phase; displaying precise quantitative property and stable reserving time for west carthamin-3; satisfying the request of fingerprint atlas analysis.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Method and system for separation and purification of at least one narcotic alkaloid using reverse phase preparative chromatography
InactiveUS20050182257A1Improve throughputOptimized diameterOrganic chemistryComponent separationStationary phaseChromatographic column
An apparatus and method for separating at least one narcotic alkaloid that includes loading a stationary phase media into a high performance preparative liquid chromatography column, feeding a crude narcotic alkaloid solution into the chromatographic column, applying a mobile phase to the chromatographic column, and recovering a narcotic alkaloid eluate from the chromatographic column. The narcotic eluates are collected and treated separately from each other. Each separated alkaloid, however, has sufficient recovery and purity.
Owner:MALLINCKRODT INC
Method and Device for Gravity Flow Chromatography
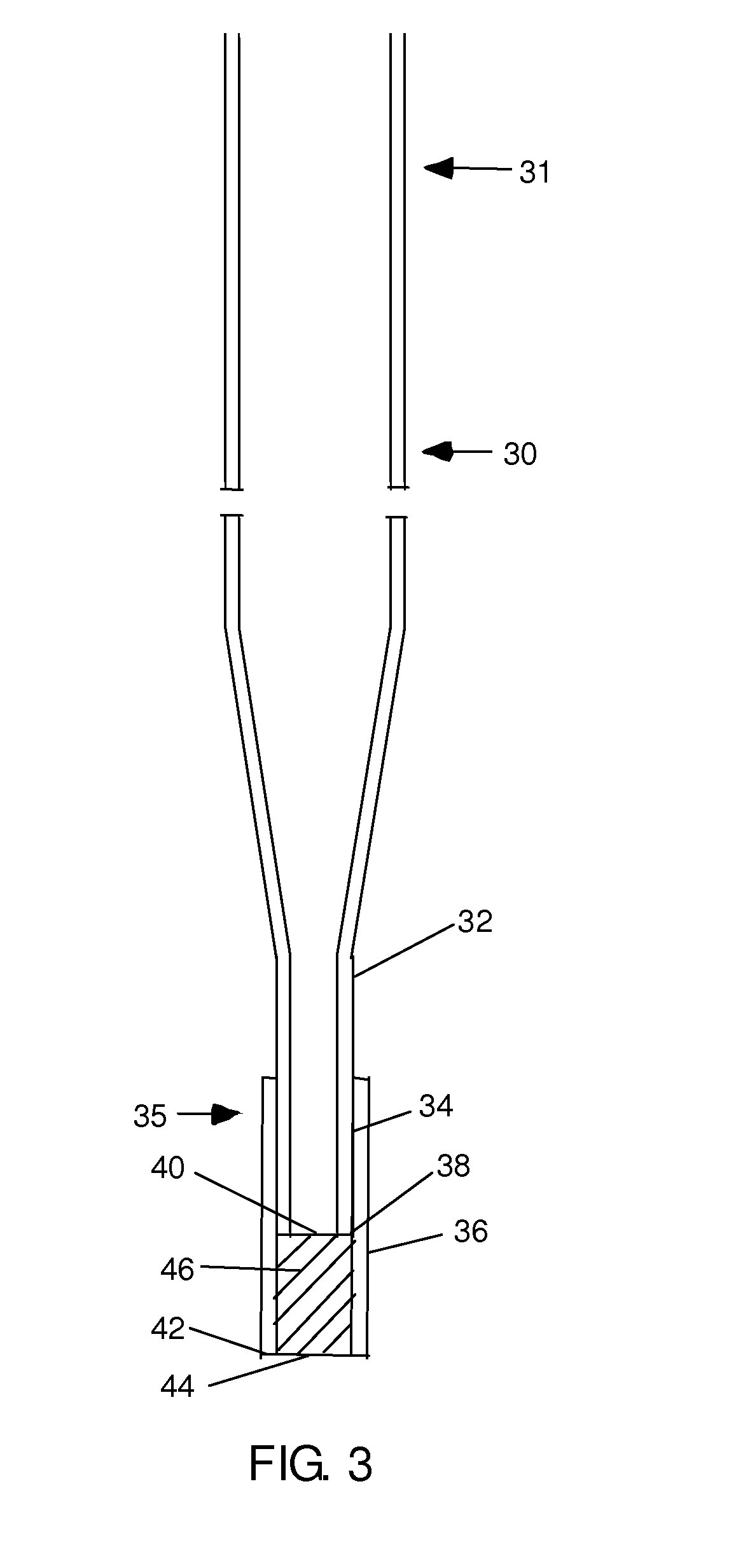
The invention provides gravity chromatographic columns for the automated purification of a material (e.g., a biological macromolecule, such as a peptide, protein or nucleic acid) from a sample solution, as well as methods for making and using such columns. The columns typically include a bed of media positioned above a bottom frit or between a bottom and top frit. In some embodiments, the columns employ modified pipette tips as column bodies. In some embodiments, the columns employ modified plates or racks as column bodies. In some embodiments, the invention provides methods and devices for gel filtration, desalting, buffer exchange, ion exchange, ion-pairing, normal phase and reverse phase chromatography. In some embodiments, the invention provides multiplexing gravity flow chromatography on a liquid handling robotic system.
Owner:SUH CHRIS +2
Dexrazoxane analytical method
ActiveUS20200003737A1Effective and accurateIncrease the number ofOrganic chemistryComponent separationOrganic solventFluid phase
A high performance liquid chromatography method used for dexrazoxane-related substances is provided, and in the method, a low-density bonding reversed-phase C18 chromatographic column resistant to pure water is employed; a gradient elution is carried out with mobile phase A and mobile phase B as eluents, the mobile phase A being a buffer, and the mobile phase B being an organic solvent; the volume percent of mobile phase A in eluents in a first stage of the gradient elution is not lower than 90%, and the duration of the first stage of the gradient elution ranges from 15˜30 minutes. By means of the analytical method, dexrazoxane is effectively separated from main impurities, and the qualities of the active pharmaceutical ingredients of dexrazoxane and the preparations thereof could be better controlled.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com