Separation and purification method of high-purity vancomycin hydrochloride
一种盐酸万古霉素、分离纯化的技术,应用在肽的制备方法、化学仪器和方法、有机化学等方向,能够解决色谱纯度不能一次性达到99%以上、残留溶剂不能有效去除、达不到残留溶剂要求等问题,达到易于处理和溶剂的回收、产品颜色外观提升、简单浓缩处理的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: Preparation of vancomycin hydrochloride solution with vancomycin B content not less than 95%
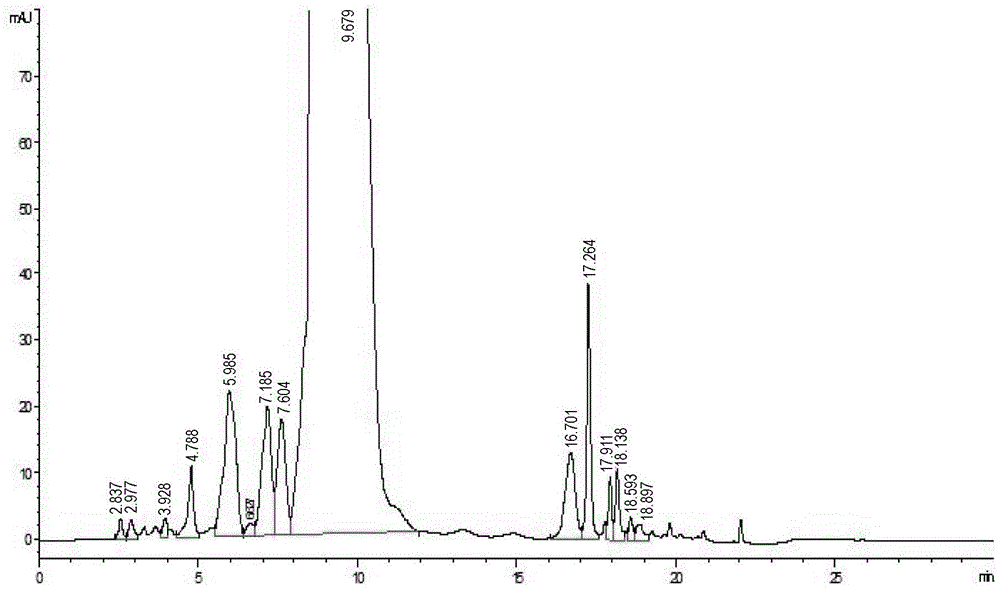
[0028] Dissolve 250 g of vancomycin crude product in 2.0 L of purified water in a beaker, stir well, and filter it with a filter membrane with a pore size of 0.2 μm after completely dissolving, then add water to dilute, and finally obtain 3.0 L of vancomycin hydrochloride crude product solution, The concentration is 43.6mg / ml, the chromatographic purity is 90.2%, see figure 1 .
[0029] Take 3000ml of the above-mentioned vancomycin hydrochloride crude product solution, pass it through the 8cm*60cm glass chromatography column equipped with dextran Sephadex CM-25, the column volume is about 4% of the column volume, and the adsorption method is to put the column volume liquid and 1 / 5 of the chromatography filler, and then put the mixture directly on the column, and then wash it with purified water at a flow rate of 1.5 times the column volume / hour. After washing 6 ...
Embodiment 2
[0031] Embodiment 2: Preparation of high-purity vancomycin hydrochloride finished product
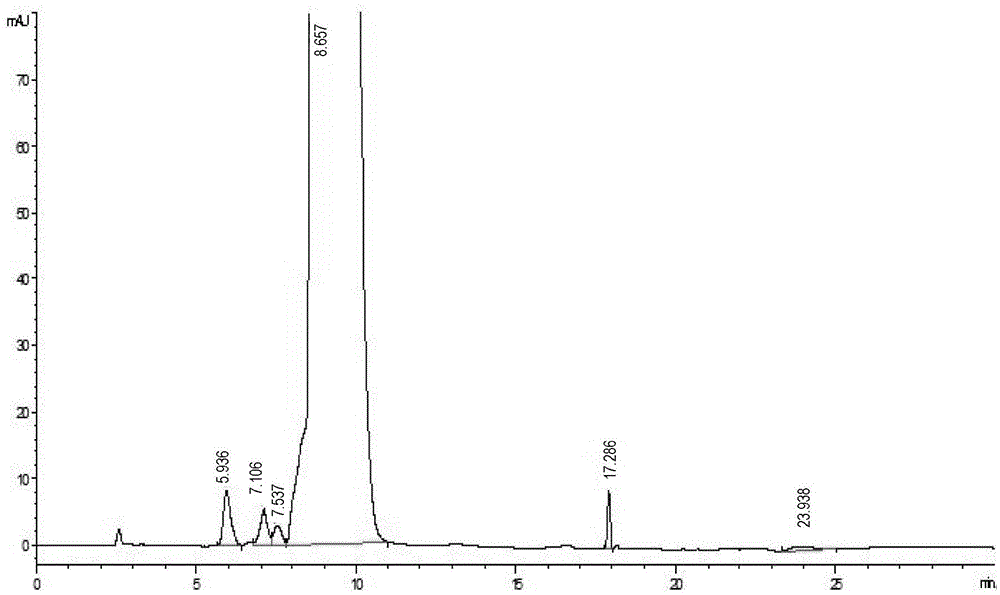
[0032] Take 680ml of the concentrated solution prepared in Example 1, pass through a preparative column (15cm*30cm) equipped with a C18 silica gel filler with a particle size of 30 μm, adjust pH=4.0 with hydrochloric acid, and contain 0.2% NH 4 Cl(W / V) and 8% methanol aqueous solution (V / V) were used as the mobile phase for pre-washing 100mim, the flow rate was 5BV / h, and then the proportion of methanol in the mobile phase was increased to 12% for elution, and the flow rate was 5BV / h h, online detection wavelength λ=280, when the absorption value starts to rise rapidly, start to collect the eluate, collect one bottle per 2.5L, collect about 10 bottles in total, detect the vancomycin B content of each bottle, the chromatographic purity is greater than 98.5% were mixed to obtain a total of 12.5 L of mixed chromatography solution with a concentration of 6.8 mg / ml, and the pH was adjusted t...
Embodiment 3
[0034] Example 3: Concentration effect comparison of nanofiltration membranes with different apertures
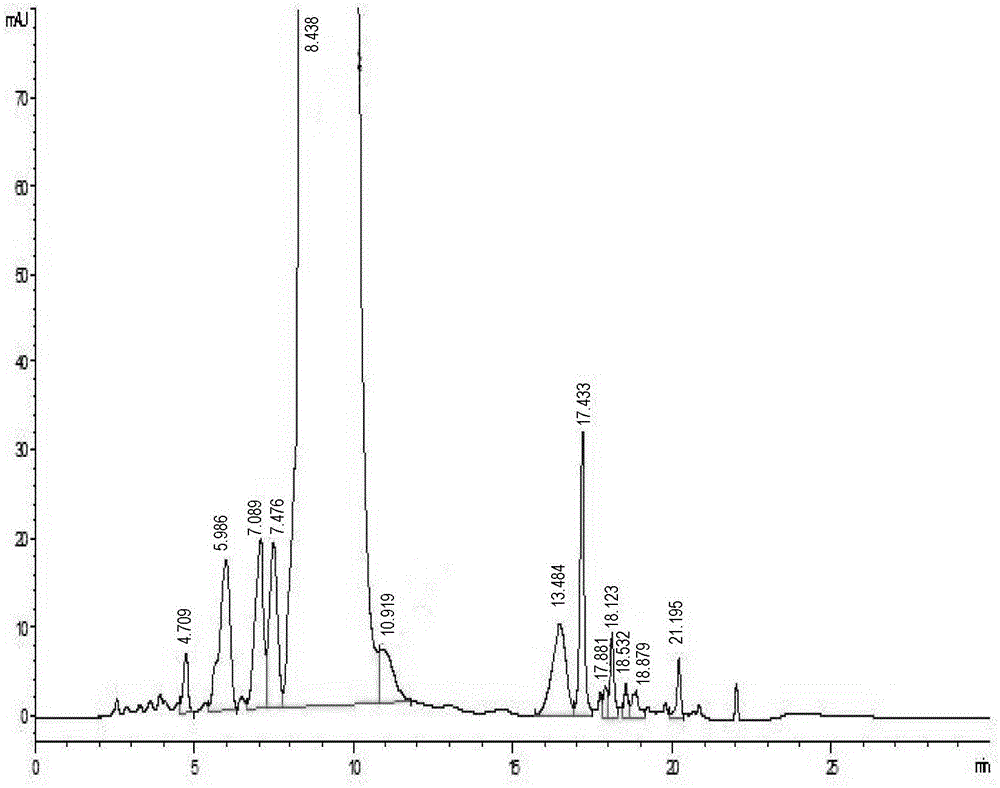
[0035] Select nanofiltration membrane tubes with different pore diameters, the pore diameters are 100Da, 200Da, 400Da and 800Da respectively, and the filtration area is 0.32m 2 , installed in turn on the small nanofiltration membrane equipment in the laboratory (model LNG-NF-101), take 8000ml of concentrated solution containing 10% vancomycin hydrochloride, divide it into 4 parts, first take one part for nanofiltration concentration, and the circulation pump pressure Control at 10bar. At the beginning of concentration, take a dialysis sample for potency testing, and record the flow rate. When the volume of the circulating fluid is 1000ml, take a dialysis sample for potency testing, and record the dialysis flow rate. After each use Clean the equipment and replace the next nanofiltration membrane tube with another concentrated solution for testing. The test results are as fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com