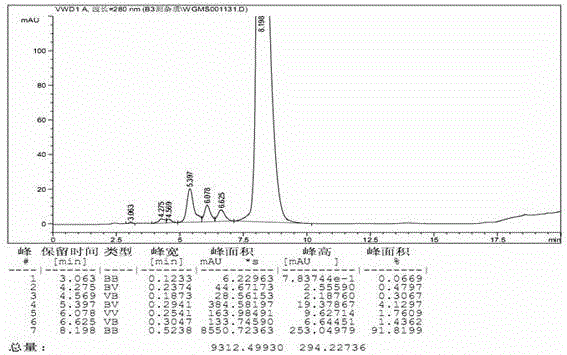
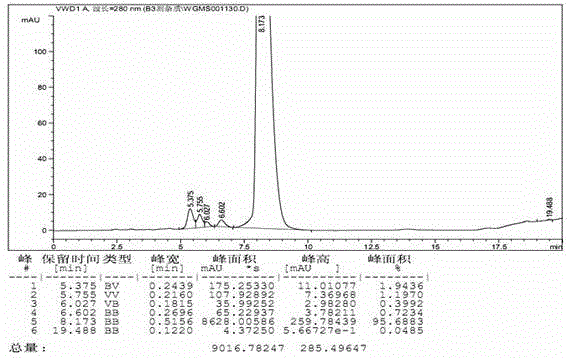
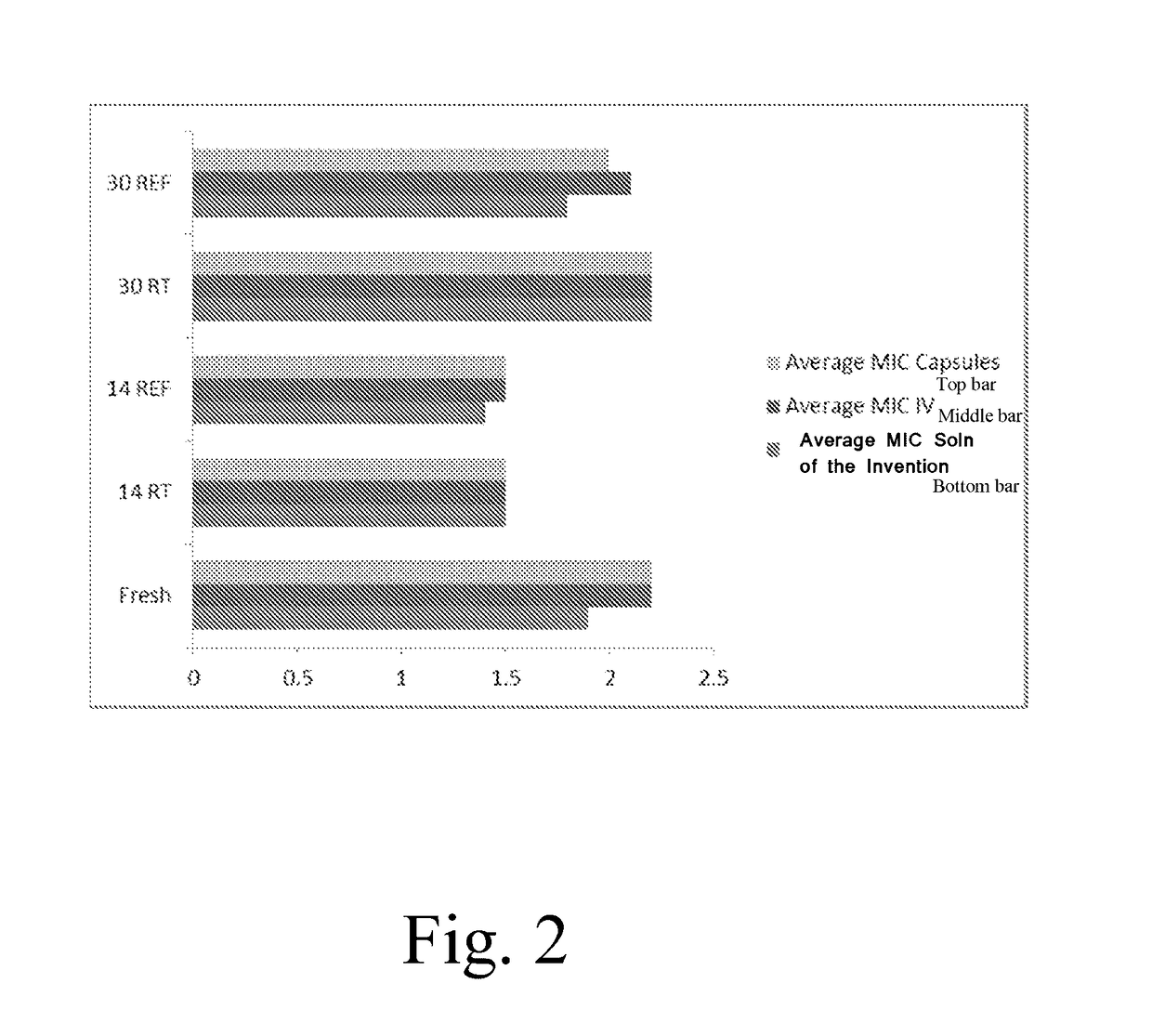
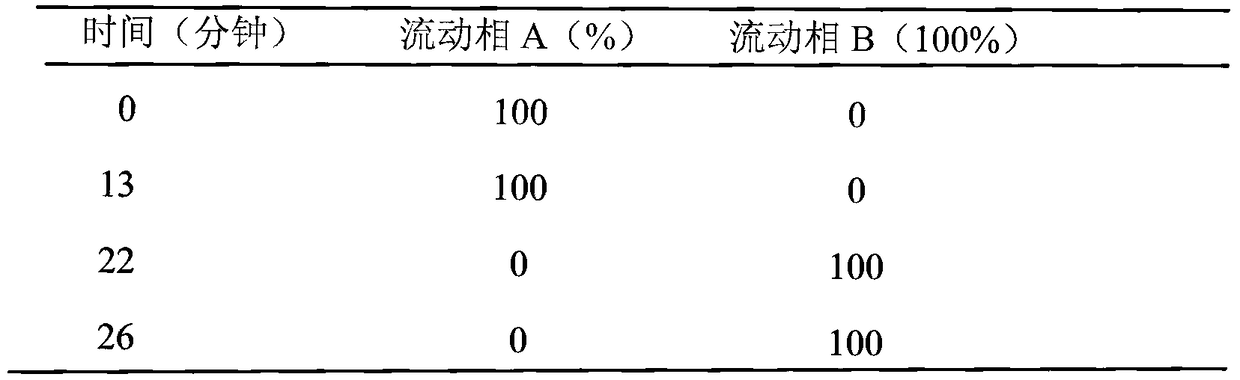
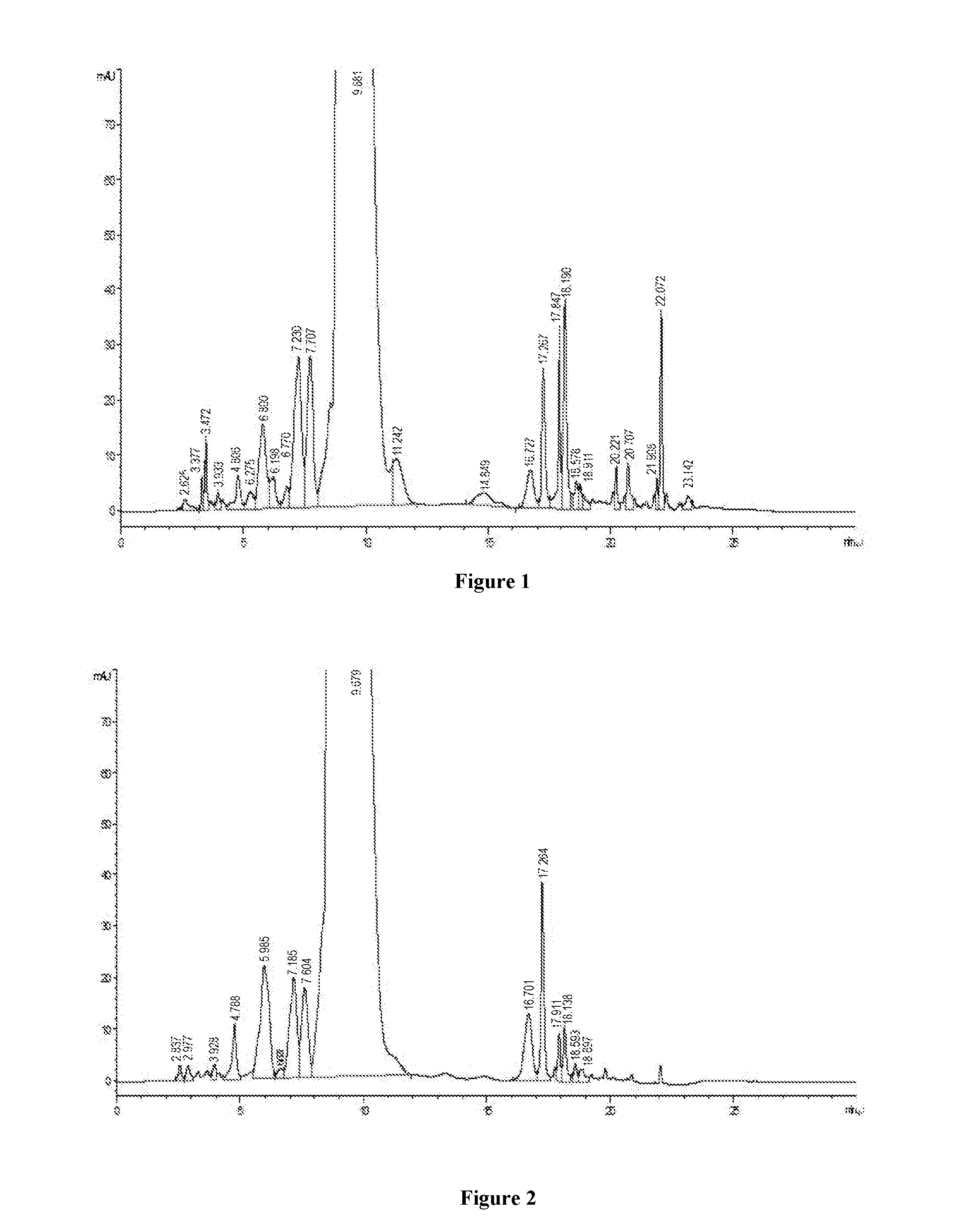
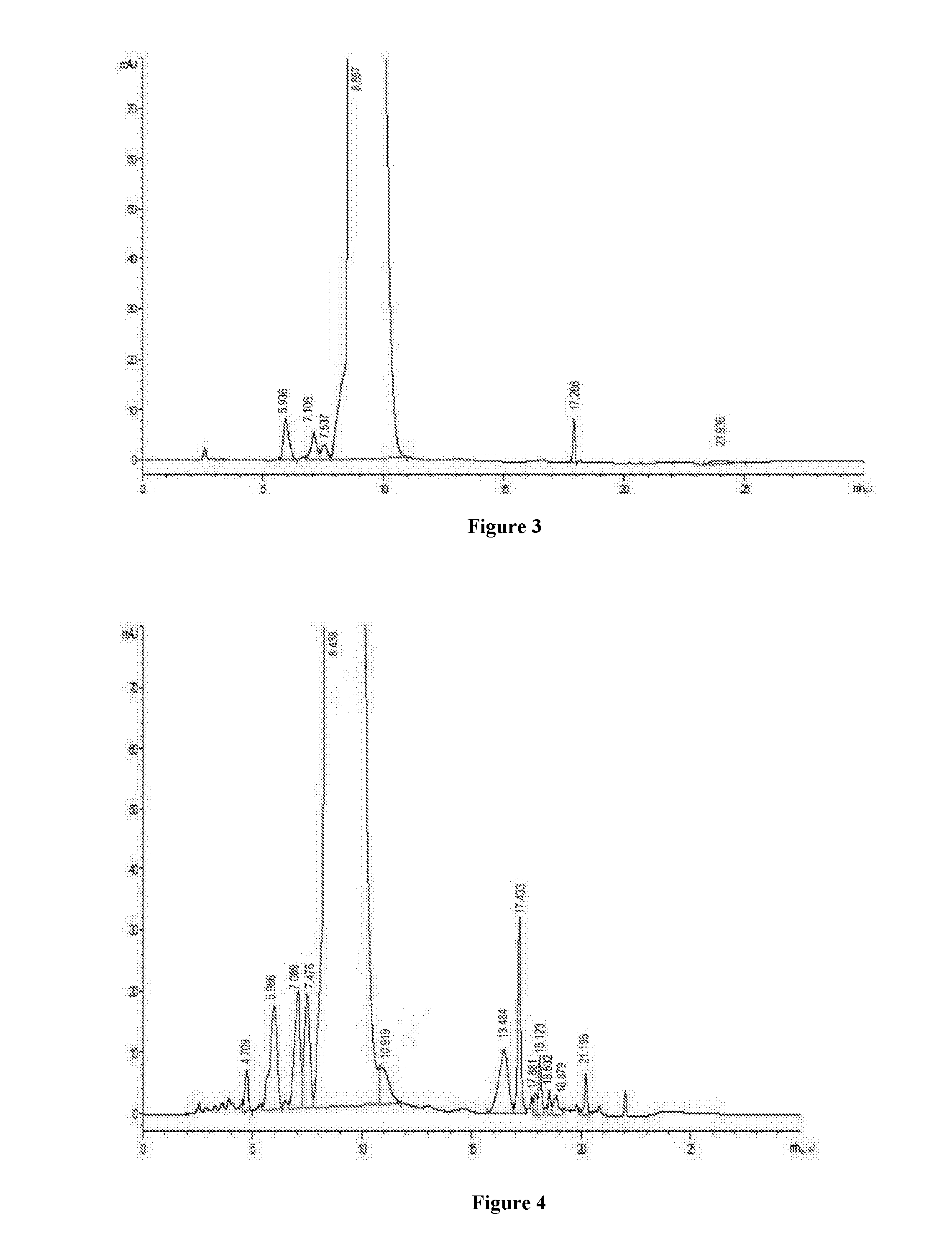
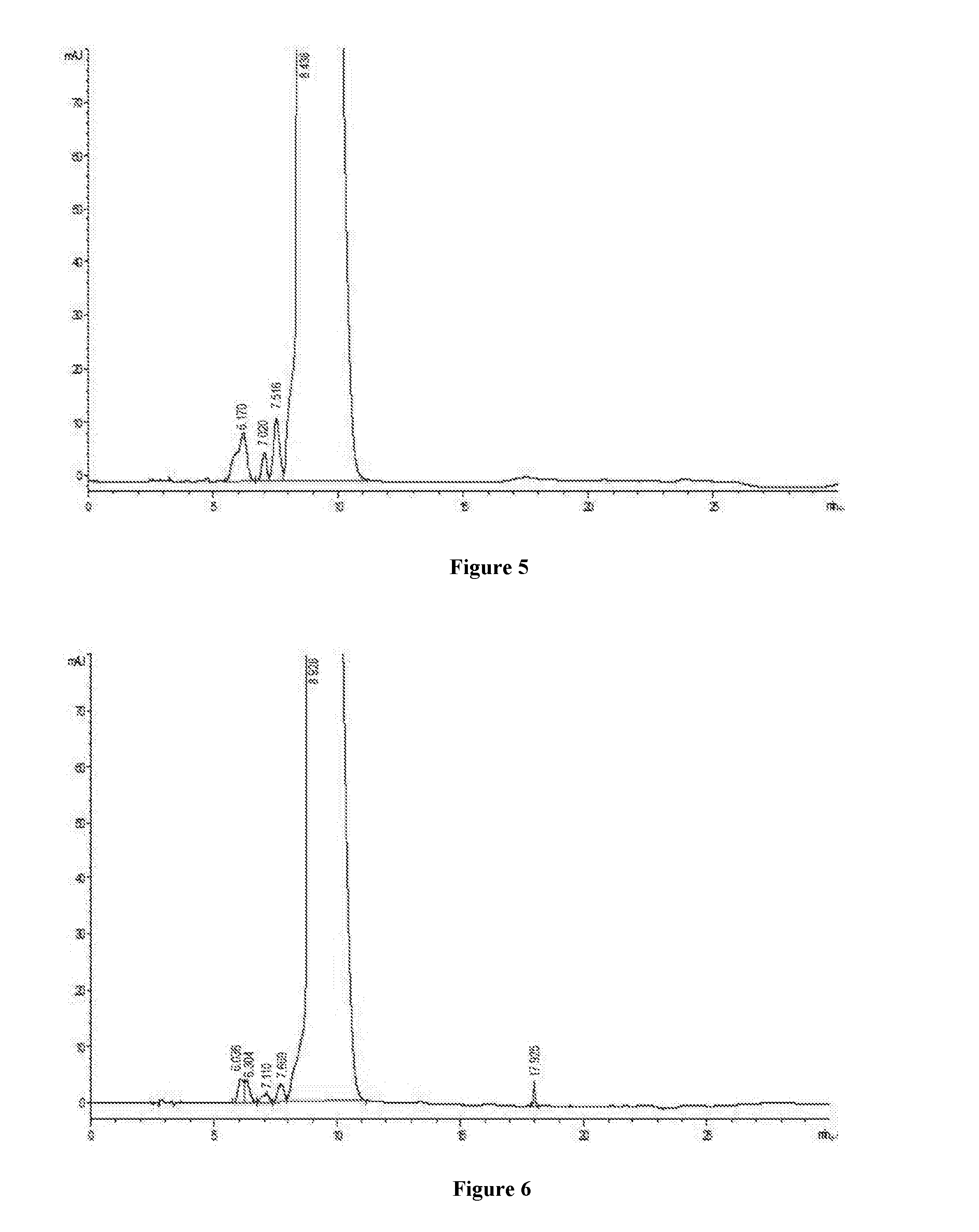
Patents
Literature
32 results about "Vancomycin Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
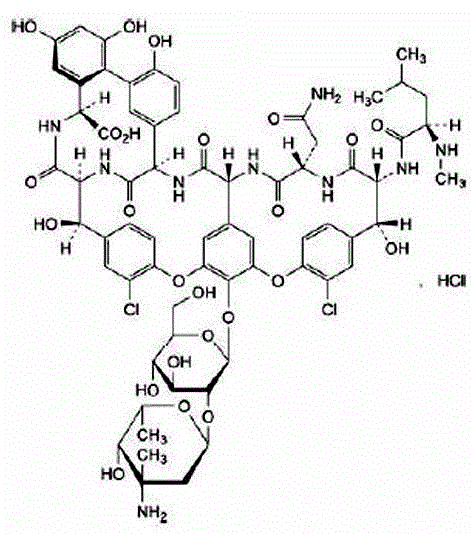
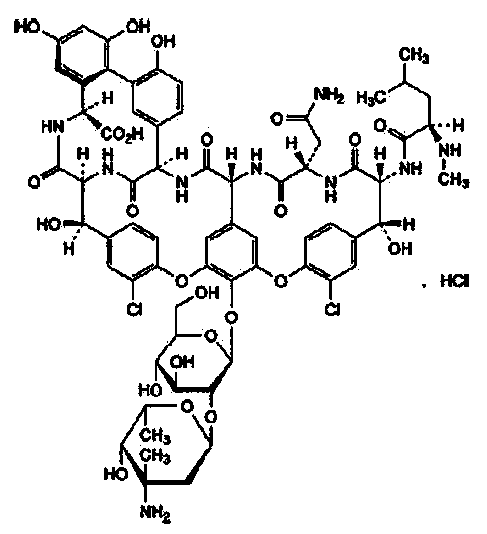
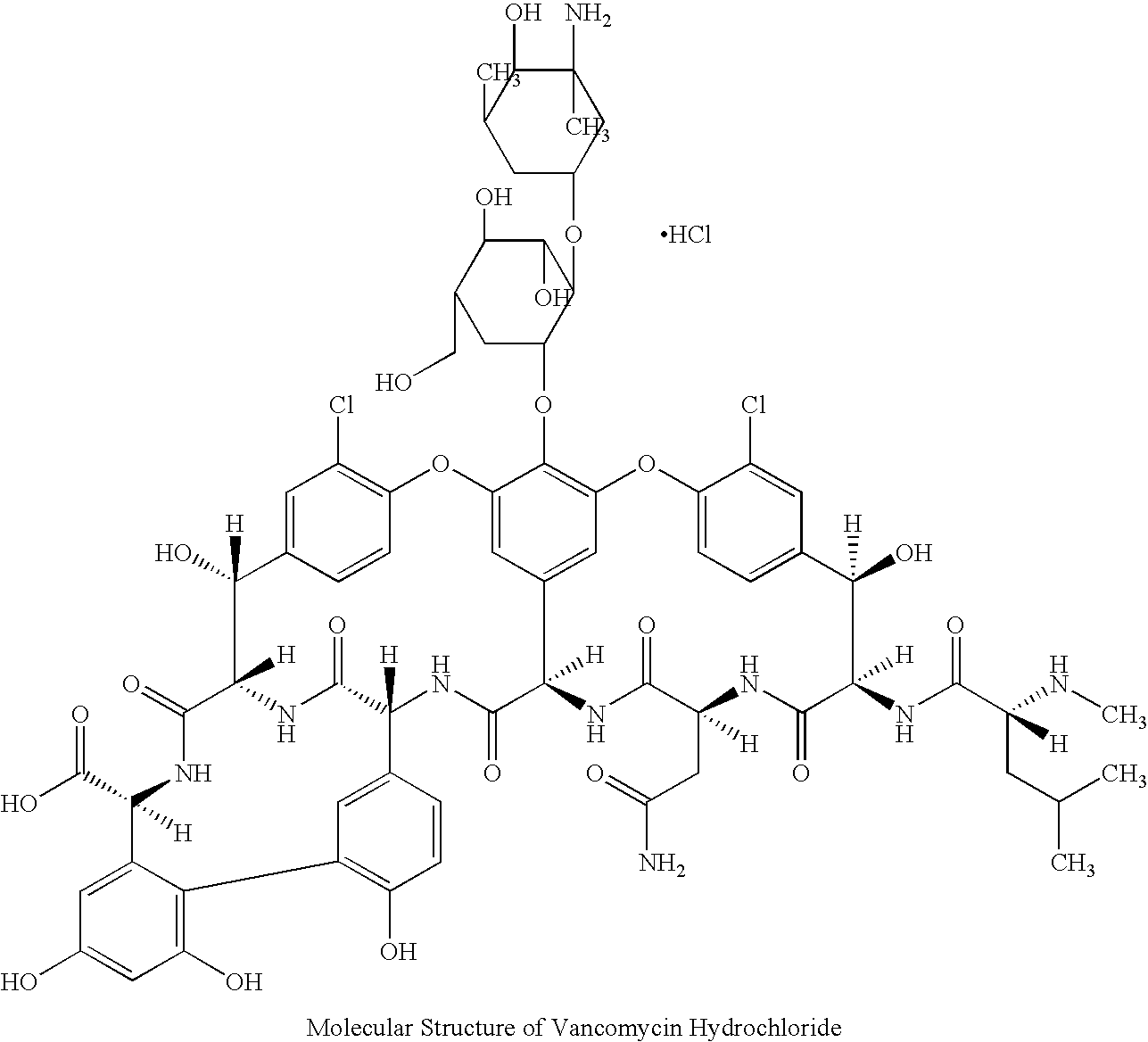
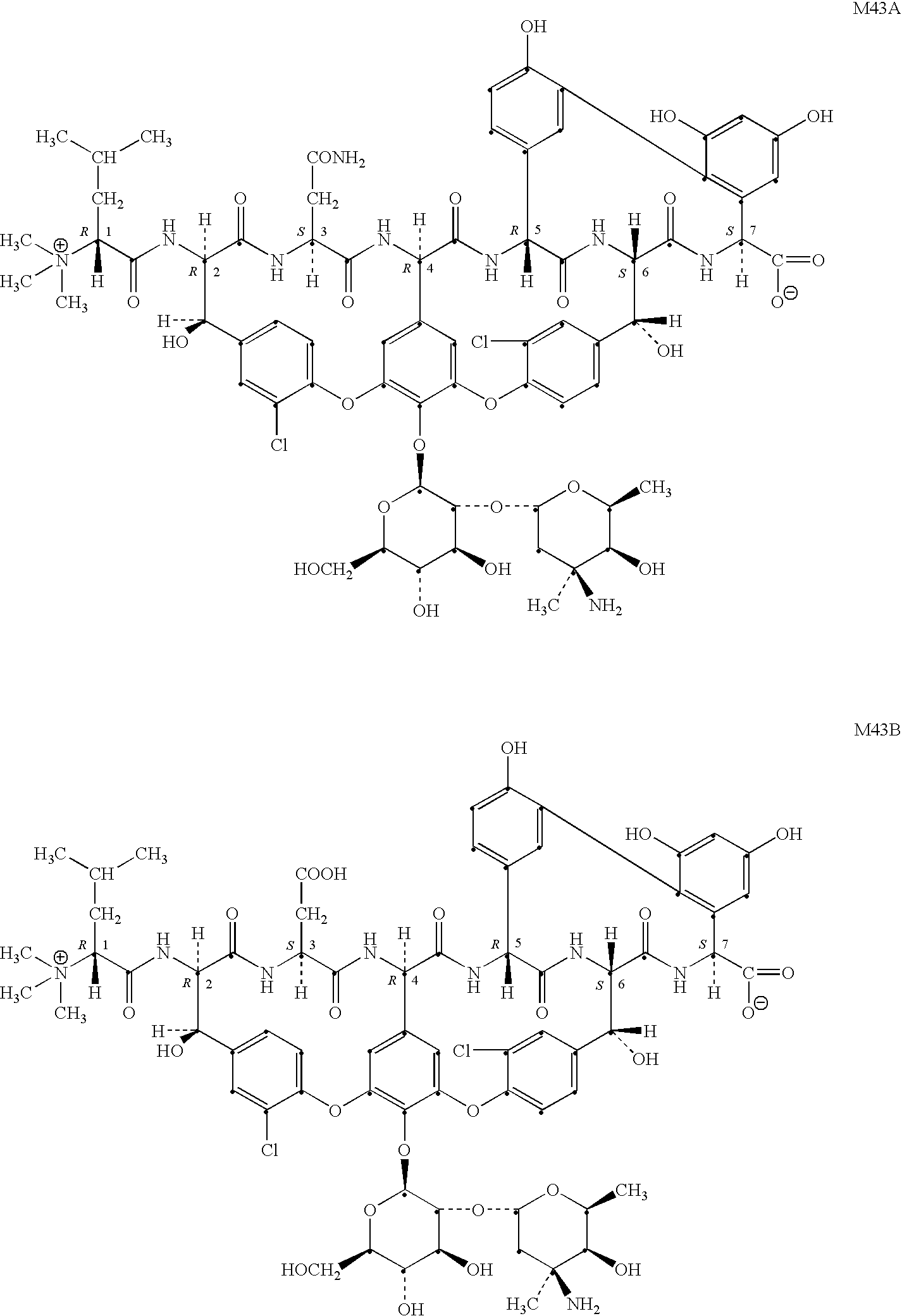
The hydrochloride salt of vancomycin, a branched tricyclic glycosylated peptide with bactericidal activity against most organisms and bacteriostatic effect on enterococci. At a site different from that of penicillins and cephalosporins, vancomycin binds tightly to the D-alanyl-D-alanine portion of cell wall precursors, thereby interfering with bacterial cell wall synthesis. This leads to activation of bacterial autolysins that destroy the cell wall by lysis. Vancomycin may also alter the permeability of bacterial cytoplasmic membranes and may selectively inhibit RNA synthesis.
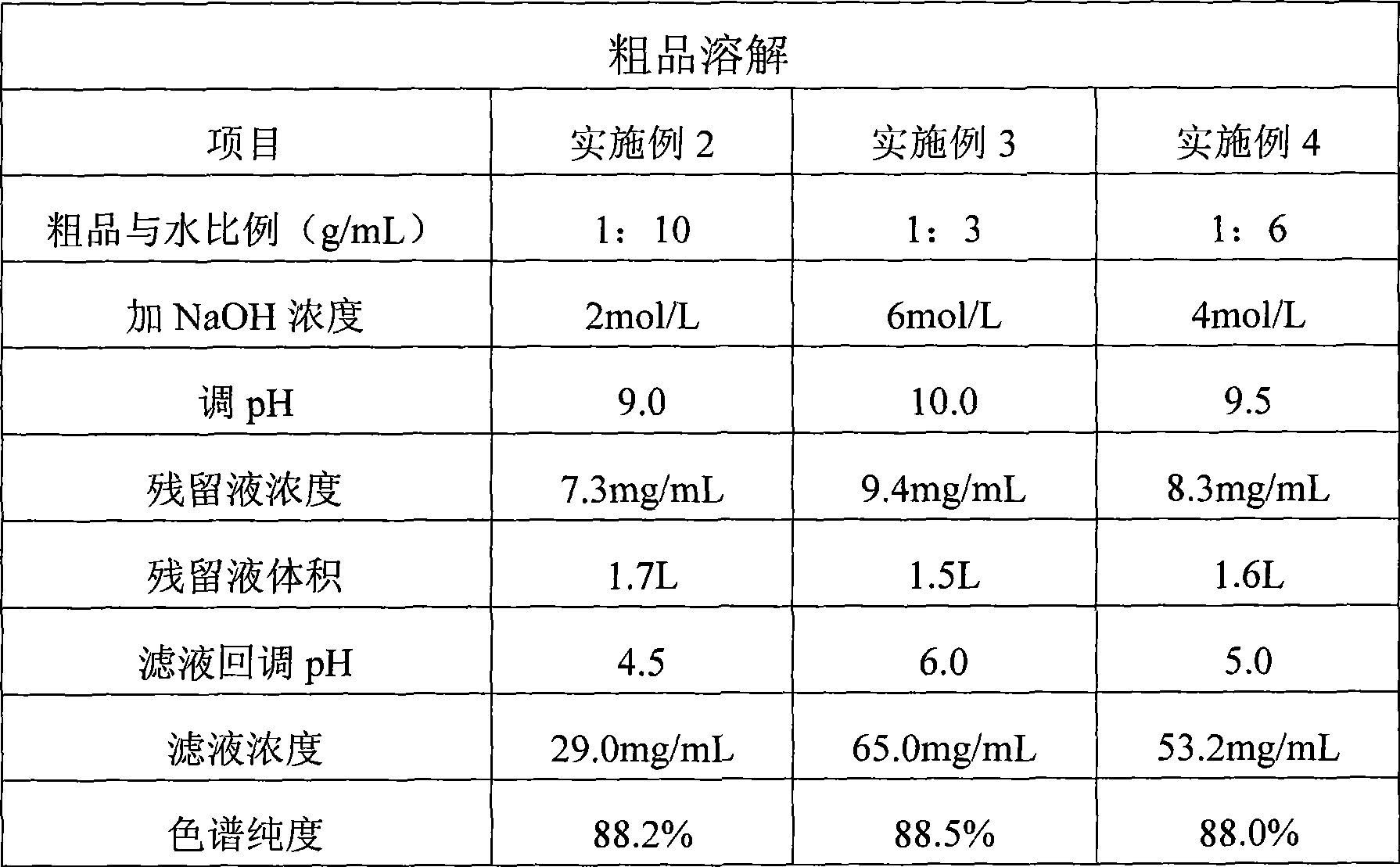
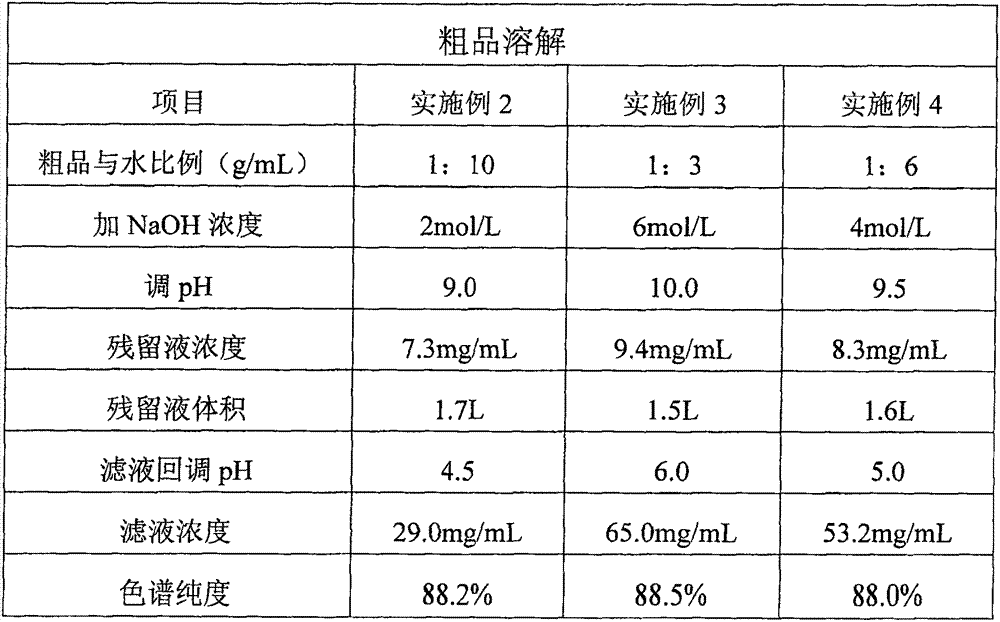
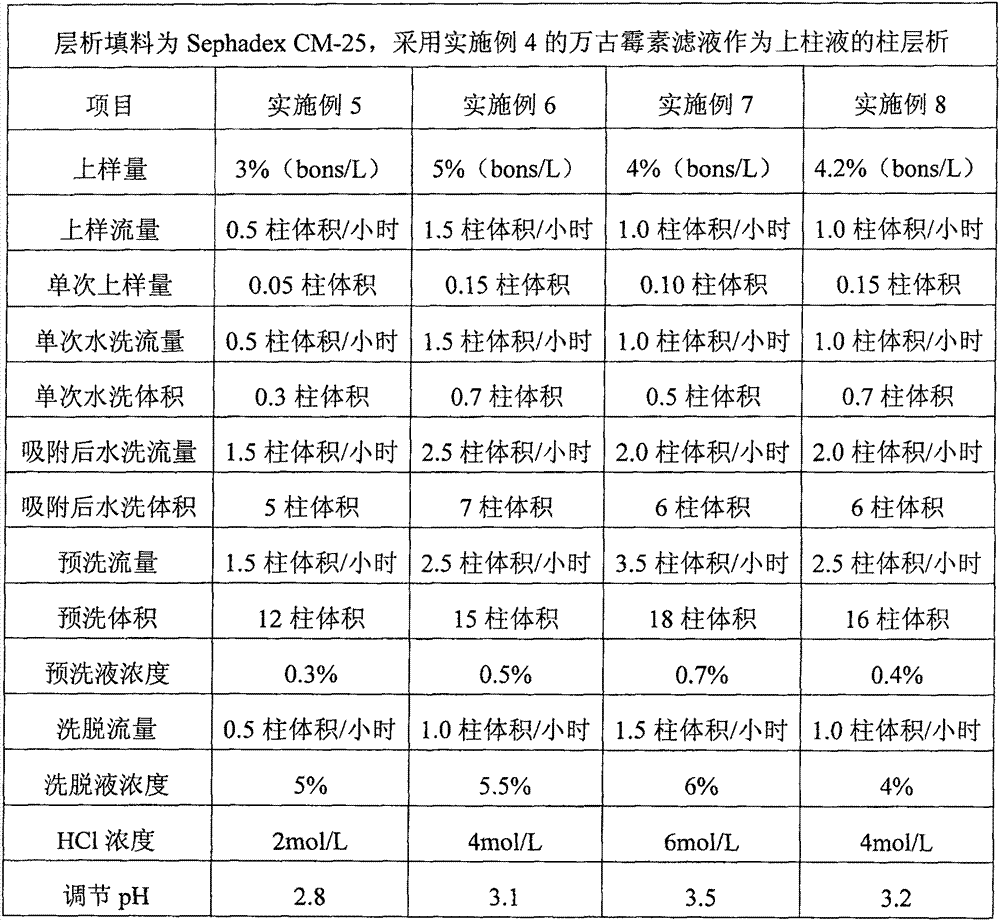
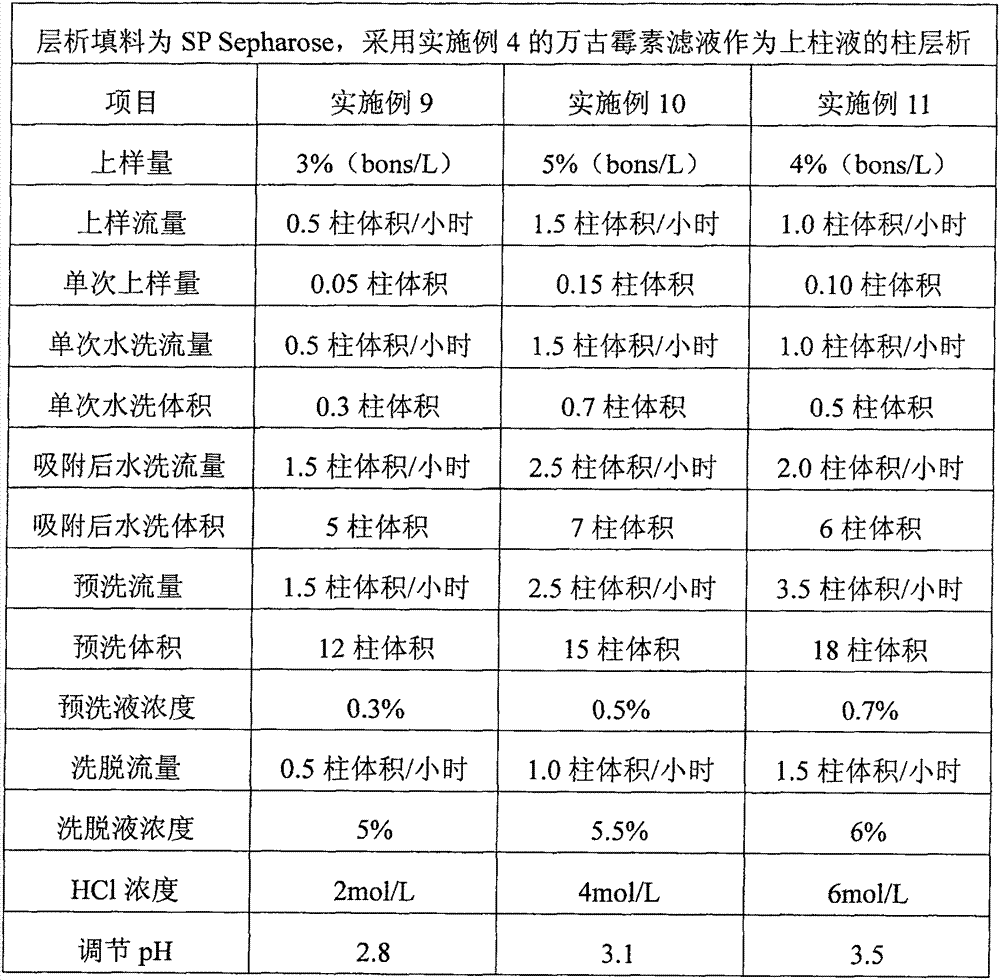
Preparation of high-purity vancomycin hydrochloride
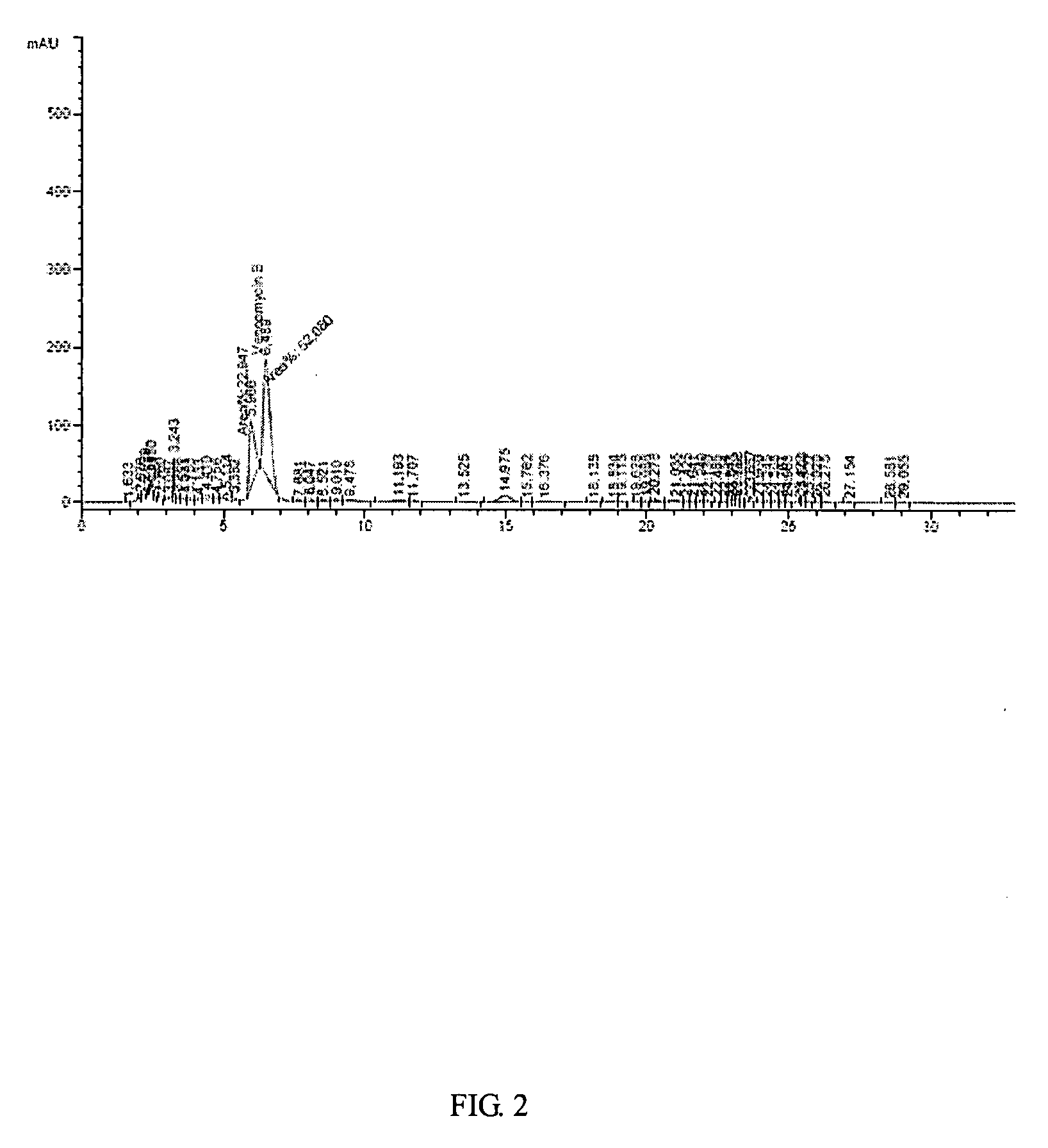
ActiveCN101440127AEffective components increaseHigh purityPeptide preparation methodsVancomycin HydrochlorideChromatography liquid
The invention relates to an industrial feasibly method for preparing a high-purity vancomycin hydrochloride. The method comprises the following steps of putting a crude vancomycin with the content of vancomycin not less than 80 percent in a dextran resin glass chromatography column containing the NH4HCO3 mobile phase for column chromatography to obtain an effective chromatography liquid; adding NaCl water solution into the effective chromatography liquid and stirring to produce a deposit; top cleaning by 90 percent ethanol water solution for a few times and drying to obtain the vancomycin hydrochloride. Effective components of the vancomycin hydrochloride obtained through the method are improved greatly, other impurities are reduced greatly, and the purity is very high; meanwhile, the overall color of the product is improved remarkably, and the product is suitable for oral or injection administration.
Owner:ZHEJIANG NOVUS PHARMA CO LTD
Method and system for producing high purity vancomycin hydrochloride
ActiveUS20140260098A1High purityLow impurity contentAntibacterial agentsSemi-permeable membranesVancomycin HydrochlorideExcipient
A method is provided for preparing spray dried powder containing vancomycin hydrochloride. The method comprises providing a vancomycin hydrochloride solution with a chromatographic purity of at least 95%, adding an excipient to the vancomycin hydrochloride solution to form a mixture solution of the vancomycin hydrochloride solution and the excipient, concentrating the mixed solution of the vancomycin hydrochloride solution and the excipient to form a 20% to 30% vancomycin concentrate, filtering the vancomycin concentrate to form a final filtrate, and spray drying the final filtrate to form a spray dried vancomycin hydrochloride powder with EP impurity B level of not more than 1.5%.
Owner:ZHEJIANG MEDICINE CO LTD
Vancomycin hydrochloride for injection and its preparing method
InactiveCN1857716AThe composition of the prescription is simpleThe method is reliable and practicalAntibacterial agentsPharmaceutical delivery mechanismVancomycin HydrochlorideGlycine
The vancomycin hydrochloride injection consists of medicine vancomycin hydrochloride in 90%-99% and supplementary material in 1%-10%, and is prepared into powder for injection with supplementary material of stabilizer and / or antioxidant. The medicine powder has potency higher than 900 IU / mg, and is dissolved in water to form 50 mg / ml solution with absorbance at 465 nm lower than 0.065 and pH 2.0-4.0. It is prepared through freeze drying process. The vancomycin hydrochloride injection of the present invention has simple preparation process, common medicinal supplementary material citric acid, glycine, L-cysteine etc, and high normal temperature storage stability.
Owner:ZHEJIANG UNIV +1
Process of purifying vancomycin hydrochloride
Provided is a method of purifying a vancomycin from a fermentation broth of a microorganism containing vancomycin, including passing the fermentation broth of a microorganism containing vancomycin through a strong acid cation exchange resin, a weak base anion exchange resin, alumina and a hydrophobic absorbent resin sequentially.
Owner:SAMYANG BIOPHARMLS CORP +1
Process of purifying vancomycin hydrochloride
InactiveUS20060003406A1Simple processAntibacterial agentsSaccharide peptide ingredientsVancomycin HydrochlorideMicroorganism
Provided is a method of purifying a vancomycin from a fermentation broth of a microorganism containing vancomycin, including passing the fermentation broth of a microorganism containing vancomycin through a strong acid cation exchange resin, a weak base anion exchange resin, alumina and a hydrophobic absorbent resin sequentially.
Owner:SAMYANG BIOPHARMLS CORP +1
Preparation method of vancomycin hydrochloride
InactiveCN104672310AAchieving the positive effect of crystallizationHigh purityPeptide preparation methodsVancomycin HydrochlorideOrganic solvent
The invention discloses a preparation method of vancomycin hydrochloride, which comprises the following steps: adding a vancomycin crude extracting solution into any other acid except hydrochloric acid for salification, adding a water-soluble organic solvent to crystallize, dissolving the crystal in water, and exchanging with an anion exchange resin to obtain the hydrochloride. The method can effectively crystallize the vancomycin out of the crude extracting solution, thereby enhancing the vancomycin purity, reducing the equipment corrosion by hydrochloric acid, saving the equipment investment and prolonging the service life of the equipment.
Owner:CHONGQING DAXIN PHARMA +2
Ophthalmic ointments for treating infective eye disease
InactiveUS6852311B1Good effectHighly effective therapeutic formulationAntibacterial agentsSenses disorderVancomycin HydrochlorideSide effect
Ophthalmic ointments for treating infective eye diseases which are particularly effective against infective eye diseases caused by methicillin-resistant Staphylococcus aureus (MRSA) or methicillin-resistant Staphylococcus epidermidis (MRSE) and contain as the active ingredient from 0.01 to 5.0% of vancomycin hydrochloride. Compared with intravenous administration, topical administration of these ophthalmic ointments is accompanied with no problem of the occurrence of side effects such as renal toxicity and thus enables the maintenance of a therapeutically effective concentration.
Owner:TOA PHARMA
Refining method for vancomycin hydrochloride
InactiveCN102863519AHigh yieldHigh purityPeptide preparation methodsVancomycin HydrochlorideOrganic solvent
The invention discloses a refining method for vancomycin hydrochloride. In the method, a vancomycin hydrochloride solution of which the purity is over 90 percent is refined into vancomycin hydrochloride of which the purity is over 95 percent; and in a refining process, solid-liquid separation is realized without using any organic solvent, and the yield is over 80 percent. Vancomycin in a mother solution obtained through solid-liquid separation can be returned to a previous separating process for recycling. The refining method is easy and convenient for operating, has high yield, and is a feasible industrial refining process.
Owner:FUJIAN BOMEI BIOLOGICAL TECH CO LTD
Composition and method for vancomycin oral liquid
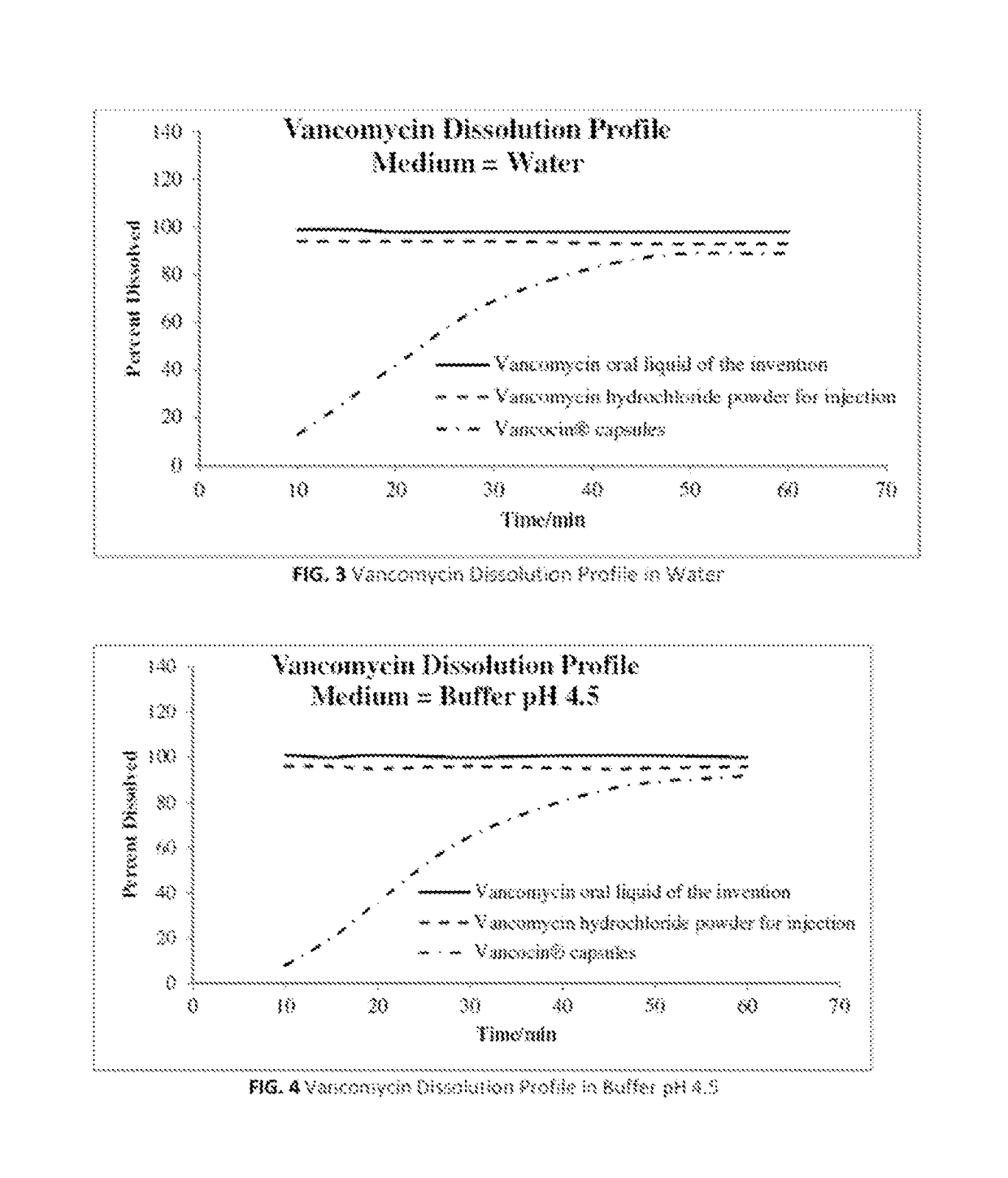
The invention relates to stable vancomycin hydrochloride powder for oral liquid formulations. Also provided herein are methods of using vancomycin oral liquid formulations for the treatment of certain diseases such as Clostridium difficile pseudomembranous colitis and Staphylococcal enterocolitis as well as kits and related products thereof.
Owner:AZURITY PHARMA INC
Metal implant with antibacterial and osteosynthesis-promoting functions and preparation method thereof
ActiveCN107648674AImprove drug loading capacityEfficient killingAnodisationPharmaceutical delivery mechanismVancomycin HydrochlorideMicro arc oxidation
The present invention relates to a metal implant having antibacterial and osteosynthesis-promoting functions and a preparation method thereof, and the method comprises (1) preparing a metal implant, (2) subjecting the metal implant to surface treatment by using a micro-arc oxidation method, and covering the surface of the metal implant with a micro-arc oxidation coating containing calcium and phosphorus elements; (3) immersing the metal implant treated in the step (2) into a dopamine hydrochloride solution with the concentration of 2 to 5 mg / mL, and shaking at constant temperature of 30 to 45DEG C for 20 to 24 hours; (4) using deionized water or distilled water as a solvent, adding heparin sodium to prepare a heparin sodium solution with the concentration of 1 to 5 mg / mL, using the heparin sodium solution as a solvent, and adding vancomycin hydrochloride for preparing a vancomycin hydrochloride solution with a concentration of 50-200 mug / mL; immersing the metal implant treated in thestep (3) in the vancomycin hydrochloride solution, and shaking at constant temperature of 30 to 45 DEG C for 3 to 12 hours to obtain the metal implant.
Owner:刘忠军 +1
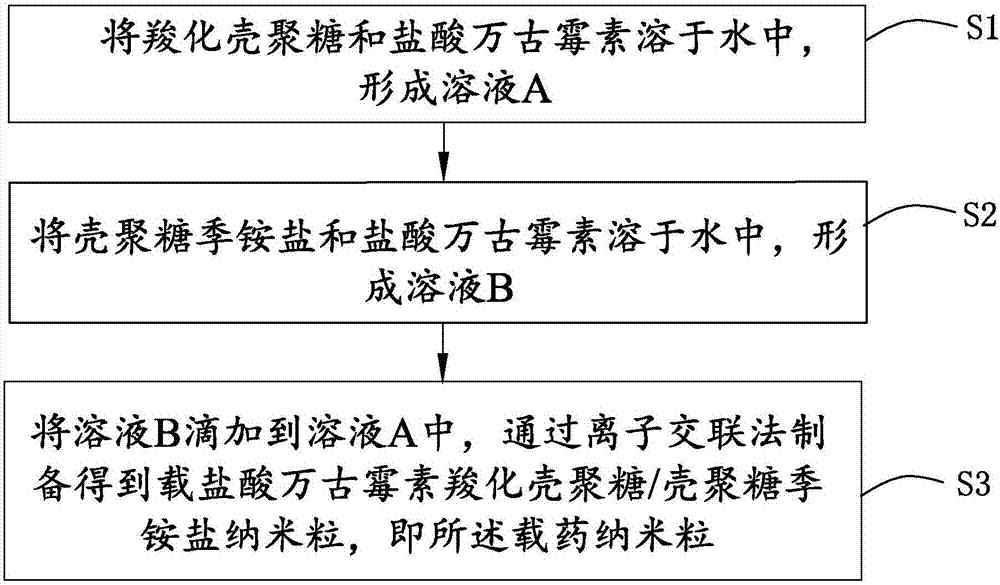
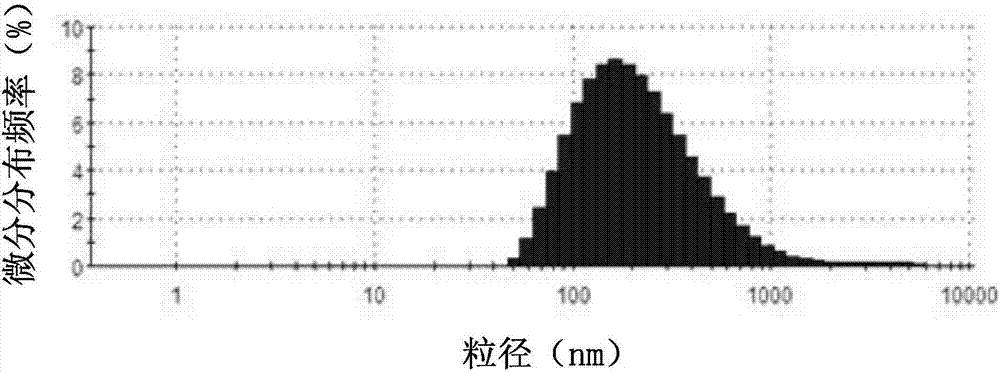
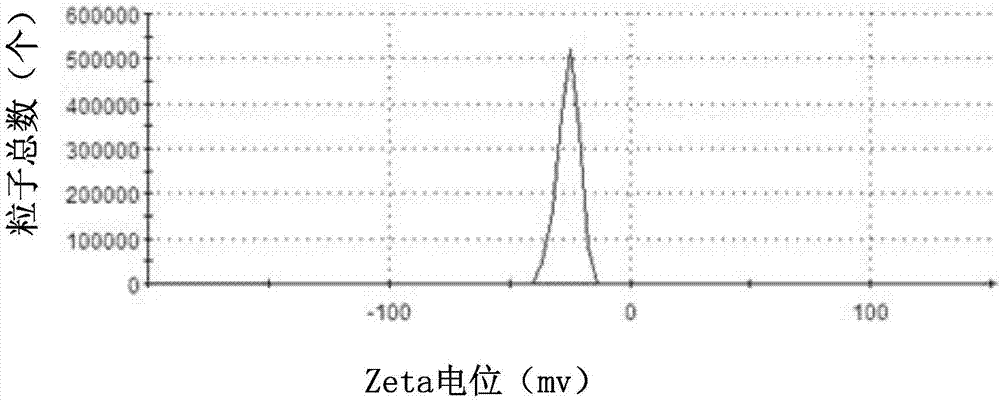
Drug-carried nanoparticle, aerogel and preparation method and application thereof
ActiveCN107049988AHigh drug loading rateAchieve biodegradabilityAntibacterial agentsSkeletal disorderVancomycin HydrochlorideDisease
The invention discloses a drug-carried nanoparticle. The active ingredient of the drug-carried nanoparticle is vancomycin hydrochloride; a carrier material is formed by compounding carboxylation chitosan and chitosan quaternary ammonium salt according to a mass ratio of 10:(1 to 5) through ion crosslinking. The drug-carried nanoparticle has the advantages that the drug carrying rate on the vancomycin is obviously increased, the biodegradability is realized, and the drug-carried nanoparticle can be used for treating osteomyelitis. The invention also provides a preparation method and application of the drug-carried nanoparticle, and also provides a preparation method and application of aerogel based on the drug-carried nanoparticle. By utilizing the temperature-sensitive characteristic of the aerogel, the drug is administrated to the disease focal part, and is slowly released at the disease focal part, so as to improve the disease focal part.
Owner:ZHEJIANG PHARMA COLLEGE
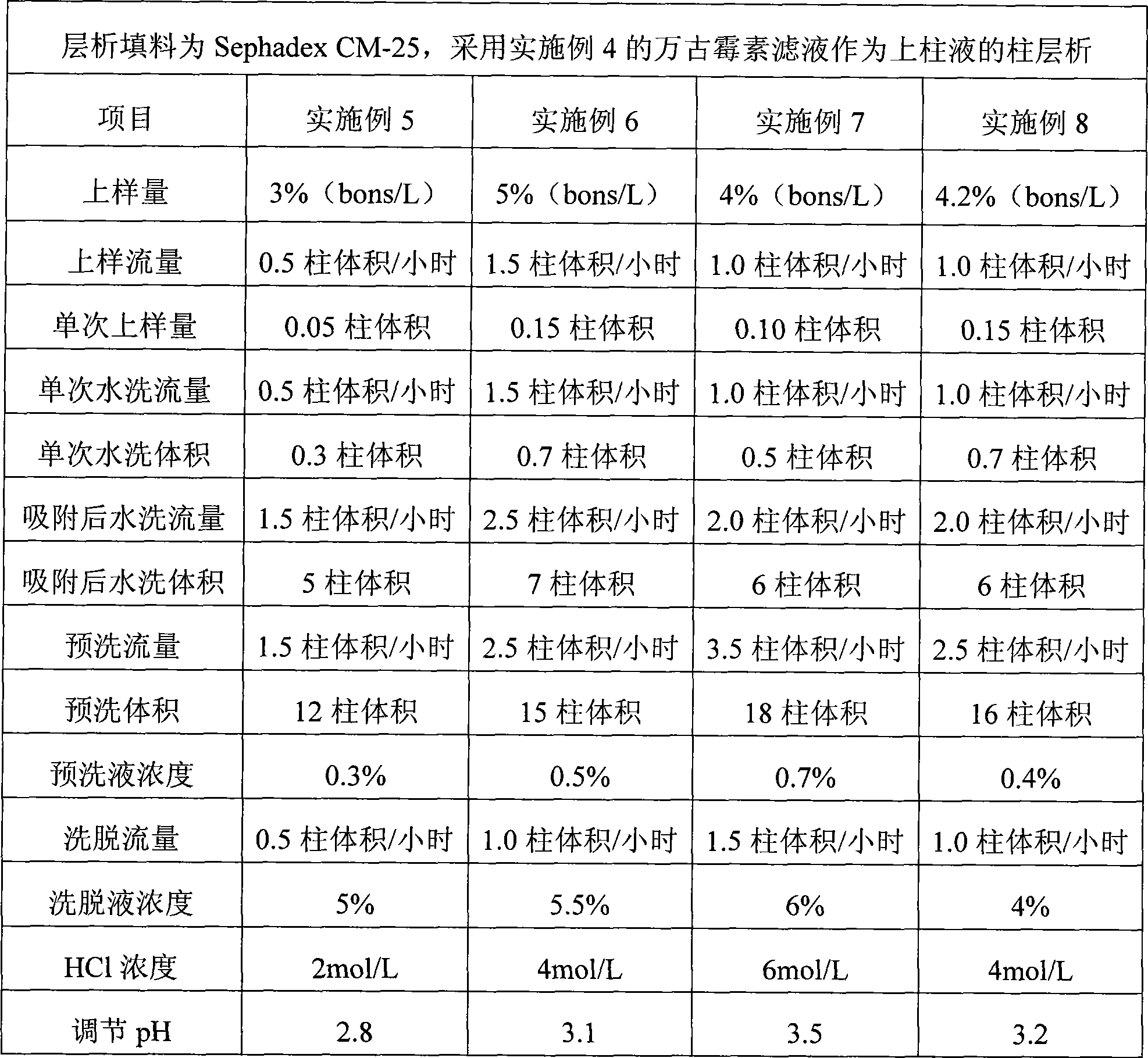
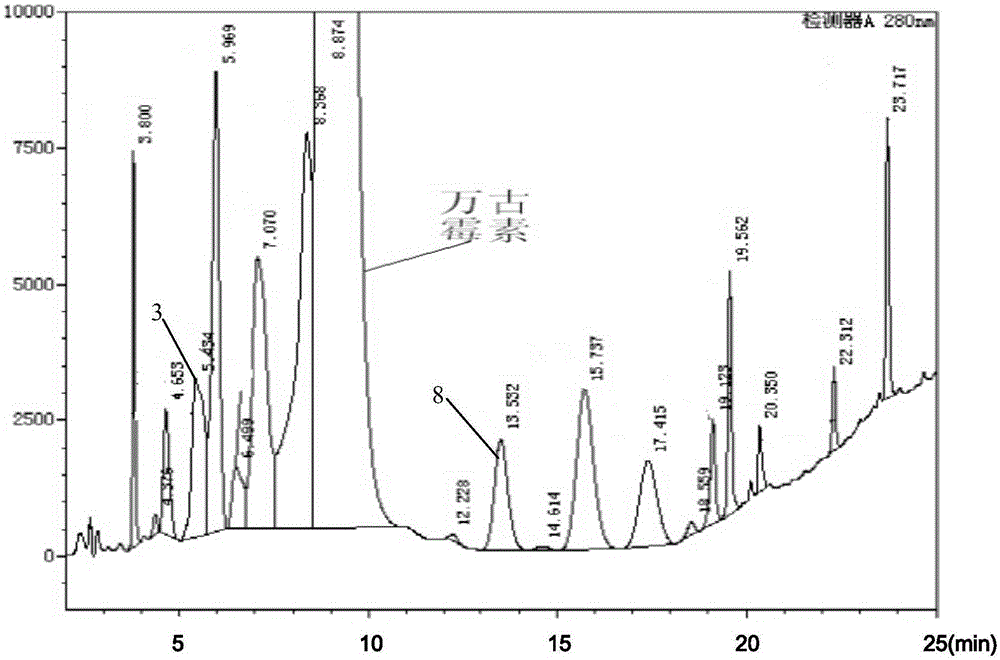
Method for preparing high-purity sample of vancomycin hydrochloride impurities 3 and 8
InactiveCN106565820ASimple processLow costPeptide preparation methodsVancomycin HydrochlorideWater baths
The invention discloses a method for preparing a high-purity sample of vancomycin hydrochloride impurities 3 and 8. The method comprises the following steps: taking vancomycin hydrochloride crystalline powder, preparing the vancomycin hydrochloride crystalline powder into a solution with concentration being 40-50g / L by use of a sodium chloride aqueous solution; preserving heat for 72-73 hours at a water bath ranging from 70 DEG C to 75 DEG C; cooling the solution to 4-6 DEG C, and carrying out nanofiltration desalination; carrying out resin chromatography enrichment on nanofiltration liquor, and separately collecting desorbed solutions of an impurity 3 and an impurity 8, purity of which is greater than 80%; sequentially carrying out ultrafiltration and nanofiltration on the desorbed solutions; and desalinizing the nanofiltration liquor with a high-pressure liquid-phase chromatography preparation column to obtain high-purity samples of the impurity 3 and the impurity 8, and the purity of the samples is greater than 97%. The method disclosed by the invention is simple in process, so that preparation cost is greatly reduced.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
Mutant strain of Amycolatopsis orientalis and process for preparing vancomycin hydrochloride
The present invention provides a process for preparing vancomycin hydrochloride using a mutant strain of Amycolatopsis orientalis (accession No. KCCM-10836P) comprising the steps of i) mutating and isolating a strain of Amycolatopsis orientalis (accession No. KCCM-10836P) for producing vancomycin from mother strain of Amycolatopsis orientalis (accession No. ATCC-19795) using NTG(N-methyl-N′-nitro-N-nitrosoguanidine) in the selection medium; ii) seed culturing the isolated mutant strain of Amycolatopsis orientalis (accession No. KCCM-10836P); iii) cultivating and fermenting said mutant strain of Amycolatopsis orientalis in the fermentation medium consisting of 12˜18 (w / v) % of dextrin, 2.2˜3.8 (w / v) % of bean powder, 1.9˜2.9 (w / v) % of potato protein, 0.10˜0.14 (w / v) % of sodium chloride and a small amount of minerals; iv) filtering vancomycin using microfilter in the cultivation broth by removing mycelia; v) purifying obtained vancomycin using column system compacted with cation exchange resin, anion exchange resin and absorbent resin; and vi) crystallizing the purified vancomycin with hydrochloric acid.
Owner:BIONGENE
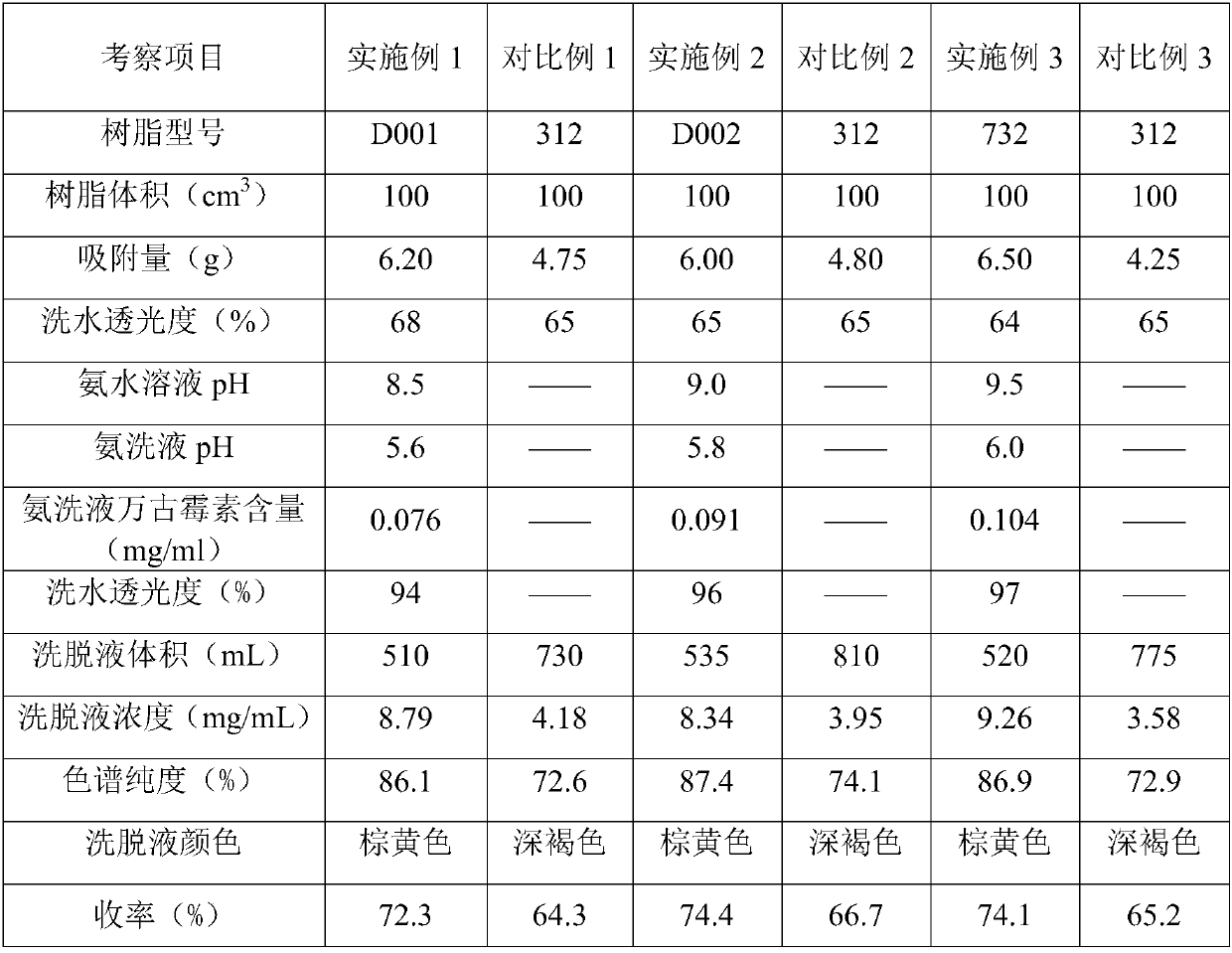
Method for improving purity of vancomycin hydrochloride through ion exchange resin
ActiveCN107641149AHigh purityHigh light transmittancePeptide preparation methodsVancomycin HydrochlorideDesalination
The invention provides a method for improving the purity of vancomycin hydrochloride through ion exchange resin. The method comprises the following steps: (a), filtering and then adsorbing a vancomycin fermentation liquor through macroporous strong acid cation exchange resin; (b), washing saturated resin through deionized water after the resin is saturated; (c), circularly washing the saturated resin through alkaline aqueous solution after step (b) is accomplished; (d), eluting the saturated resin through an eluent, so as to form vancomycin eluant; (e), performing concentration and desalination on the eluant through a nanofiltration membrane, and obtaining a vancomycin crude product after water washing. The method ensures that quality indexes, such as the purity, transparency and color degree, of a vancomycin intermediate are greatly improved through the specific macroporous strong acid cation exchange resin, a special washing technology and a membrane system, is simple in production technology, high in yield, easy to control, and suitable for large-scale industrial production.
Owner:华北制药华胜有限公司
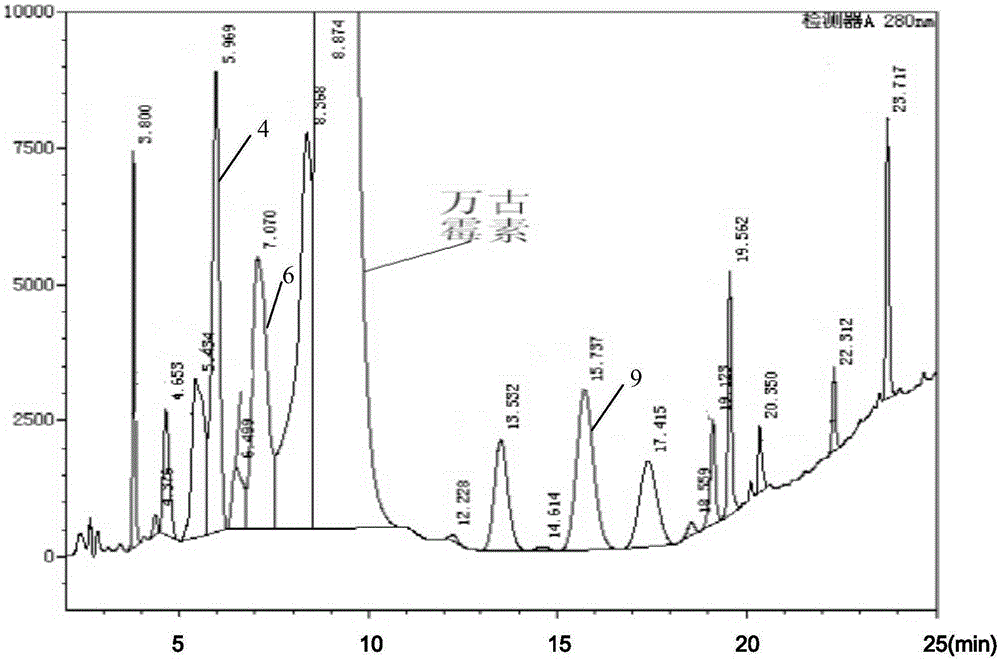
Method for preparing high-purity samples of impurities of vancomycin hydrochloride
InactiveCN106565818ASimple processLow costPeptide preparation methodsVancomycin HydrochlorideWater baths
The invention discloses a method for preparing high-purity samples of related impurities (4, 6 and 9) of vancomycin hydrochloride. The method comprises the steps: preparing an aqueous solution with the concentration of 10g / L to 15g / L from crystalline powder of vancomycin hydrochloride; carrying out water-bath heating to the temperature of 30 DEG C to 33 DEG C, adding ethylene glycol, and carrying out stirring and heat preservationfor 120 to 122 hours; then, cooling the solution to 20 DEG C to 21 DEG C, and dropwise adding anhydrous ethanol into the cooled solution slowly for crystallization; and carrying out crystal separation by using high performance liquid chromatography preparative columns, thereby obtaining the high-purity samples of the impurities (4, 6 and 9) in vancomycin hydrochloride. According to the method, the process is simple, and the preparation cost is reduced greatly.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
Method for preparation of high purity samples of 3 impurities in vancomycin hydrochloride
InactiveCN106565819ASimple processLow costPeptide preparation methodsWater bathsVancomycin Hydrochloride
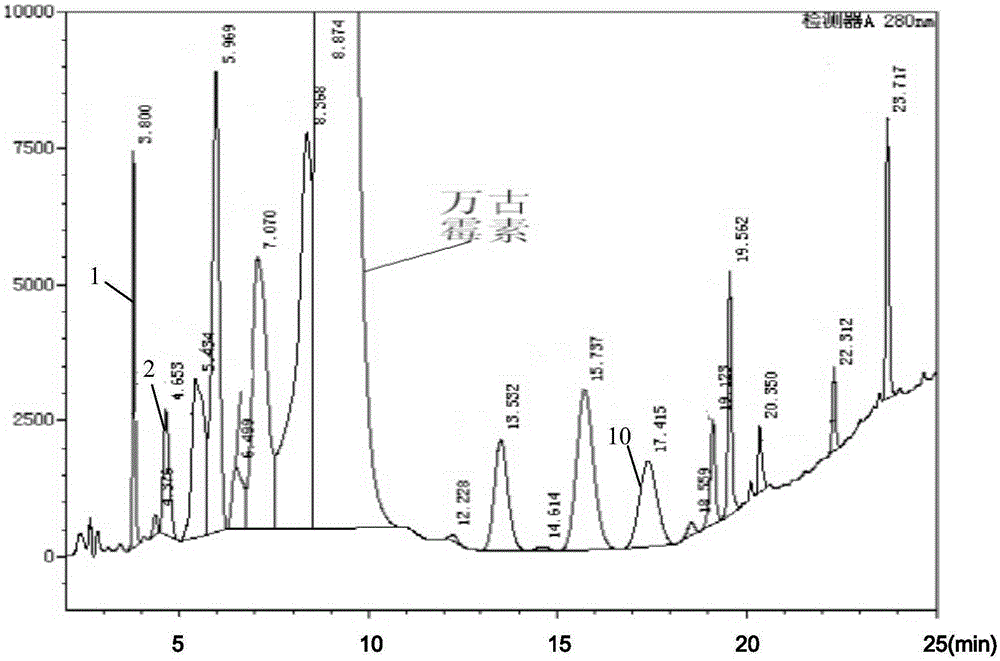
The invention discloses a method for preparation of high purity samples of an impurity 1, an impurity 2 and an impurity 10 in vancomycin hydrochloride. The method includes: taking vancomycin hydrochloride crystalline powder and preparing it into an aqueous solution with a concentration of 40-50g / L; conducting water bath heat preservation at 25-30DEG C, adding hydrogen peroxide, and performing stirring for 40-42h; then adding an oxalic acid aqueous solution slowly, stirring the substances evenly till no generation of bubbles, then stopping adding, and carrying out ultrafiltration and nanofiltration in order; slowly adding ethanol into nanofiltrate to conduct crystallization, and performing pumping filtration to obtain a crystallization mother solution; and employing a high pressure liquid chromatography preparation column to isolate the crystallization mother solution, thus obtaining high purity samples of the impurity 1, impurity 2 and impurity 10 in vancomycin hydrochloride. The method has simple process, and greatly lowers the preparation cost.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
Amorphous vancomycin hydrochloride as well as preparation method, application and pharmaceutical composition thereof
ActiveCN101613396AEffective components increaseHigh purityPeptidesSaccharide peptide ingredientsVancomycin HydrochlorideHigh density
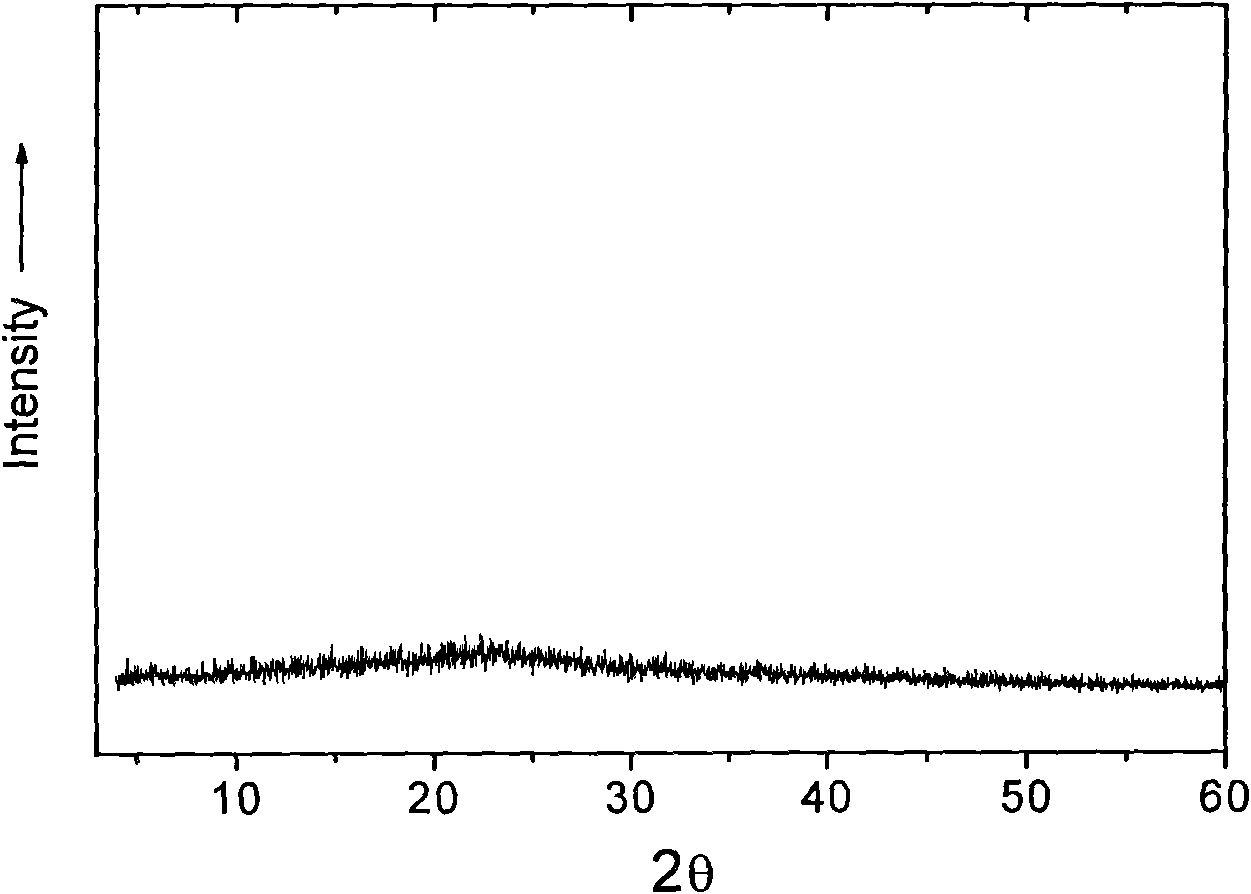
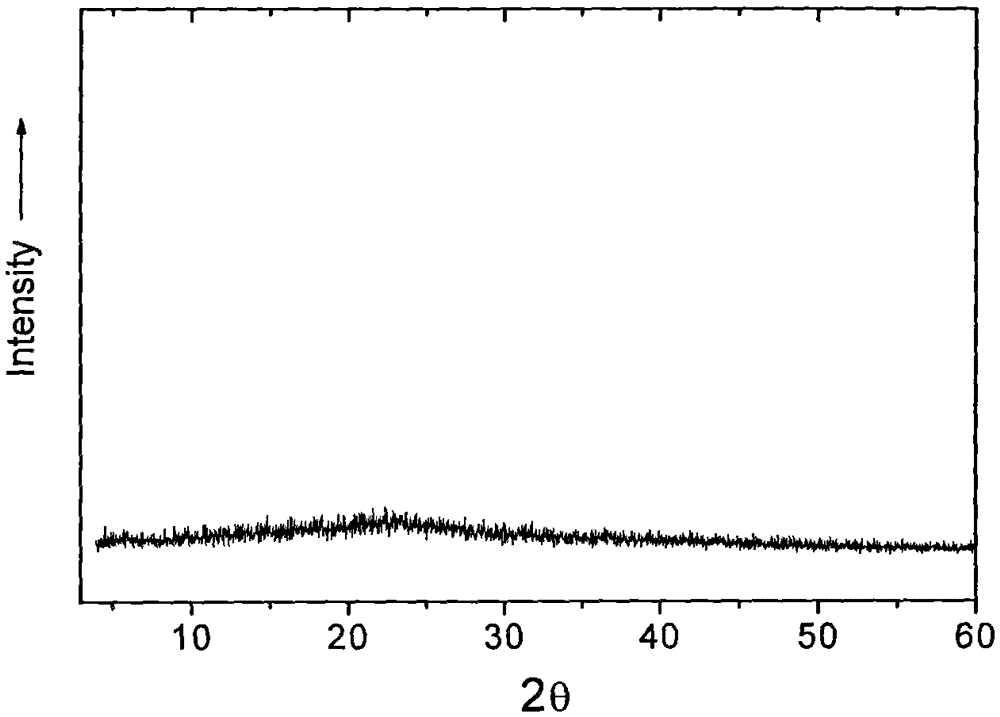
The invention provides a high-density amorphous vancomycin hydrochloride, a preparation method thereof, an application of the vancomycin hydrochloride for oral administration or injection administration and a pharmaceutical composition containing the vancomycin hydrochloride. The vancomycin hydrochloride in an amorphous state has the characteristics similar to the diffraction pattern of an X-ray powdery crystal shown in the attached drawing 1. The effective components of vancomycin hydrochloride prepared by the preparation method are enhanced greatly, other impurities are reduced to a large extent, the purity is high, and the chromatographic purity can reach more than 95 percent. The amorphous vancomycin hydrochloride not only can be used as injections, but also can be used as capsules and other solid preparations. Meantime, the appearance and the color of products are improved obviously, and the products are suitable for oral administration or injection administration.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Preparation method of high-purity samples of vancomycin hydrochloride impurities 11, 13 and 15
ActiveCN106568620BSimple processLow costPreparing sample for investigationPeptide preparation methodsWater bathsVancomycin Hydrochloride
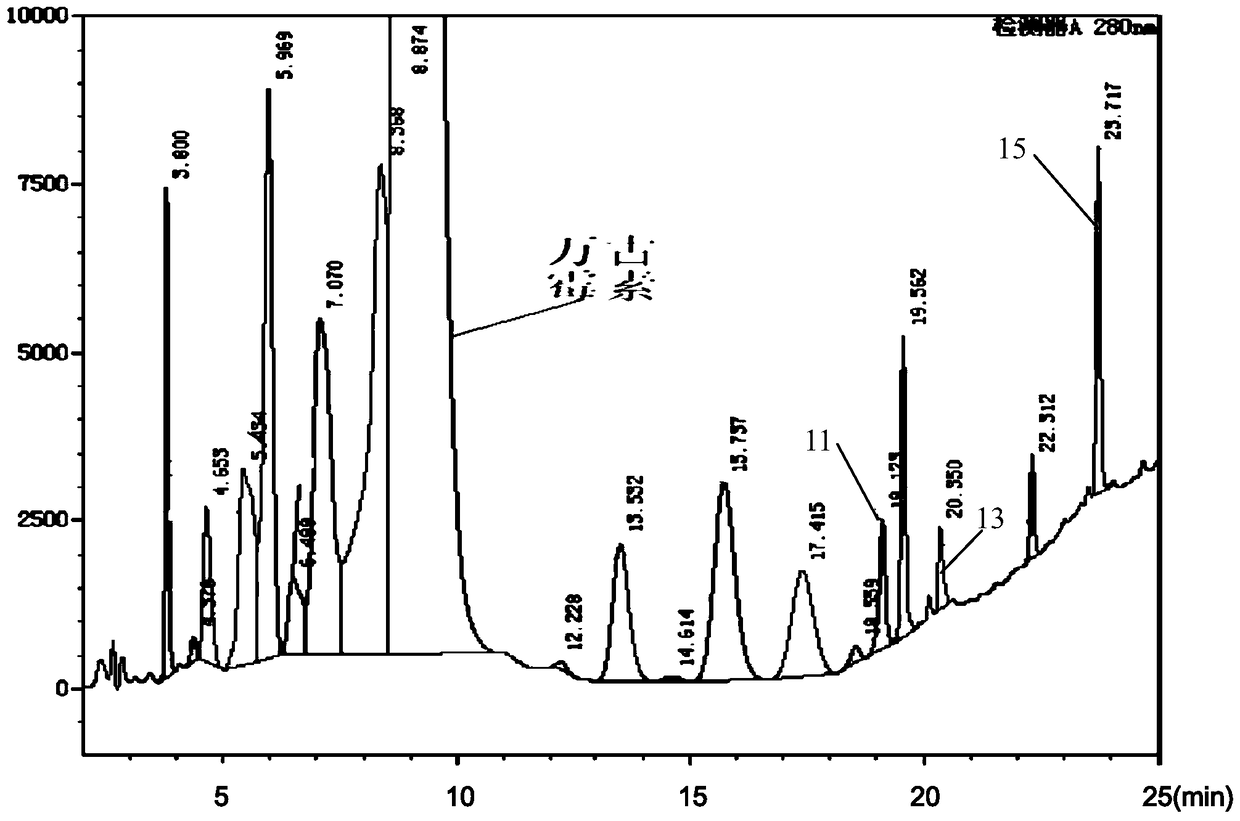
The invention discloses a preparation method of high purity samples of vancomycin hydrochloride impurities 11, 13, and 15. The preparation method comprises the following steps: preparing a vancomycin hydrochloride water solution with a concentration of 60 to 70 g / L from vancomycin hydrochloride crystallized powder, adjusting the pH to 4-6, maintaining a constant temperature of 40 to 42 DEG C for 8 to 9 hours in water bath, adjusting the pH to 6-7, carrying out macroporous resin chromatographic enrichment, collecting the coarse product liquids (with impurities from 40 to 60%) of impurities 11, 13, and 15, subjecting the coarse product liquids to ultrafiltration and nano filtration in sequence, and desalinating the nano filtrate by a high performance liquid chromatography (HPLC) column to obtain the high purity samples of vancomycin hydrochloride impurities 11, 13, and 15. The technology is simple, and the preparation cost is largely reduced.
Owner:NEW FOUNDER HLDG DEV LLC +2
Refining method for vancomycin hydrochloride
InactiveCN102863519BHigh yieldHigh purityPeptide preparation methodsVancomycin HydrochlorideOrganic solvent
The invention discloses a refining method for vancomycin hydrochloride. In the method, a vancomycin hydrochloride solution of which the purity is over 90 percent is refined into vancomycin hydrochloride of which the purity is over 95 percent; and in a refining process, solid-liquid separation is realized without using any organic solvent, and the yield is over 80 percent. Vancomycin in a mother solution obtained through solid-liquid separation can be returned to a previous separating process for recycling. The refining method is easy and convenient for operating, has high yield, and is a feasible industrial refining process.
Owner:FUJIAN BOMEI BIOLOGICAL TECH CO LTD
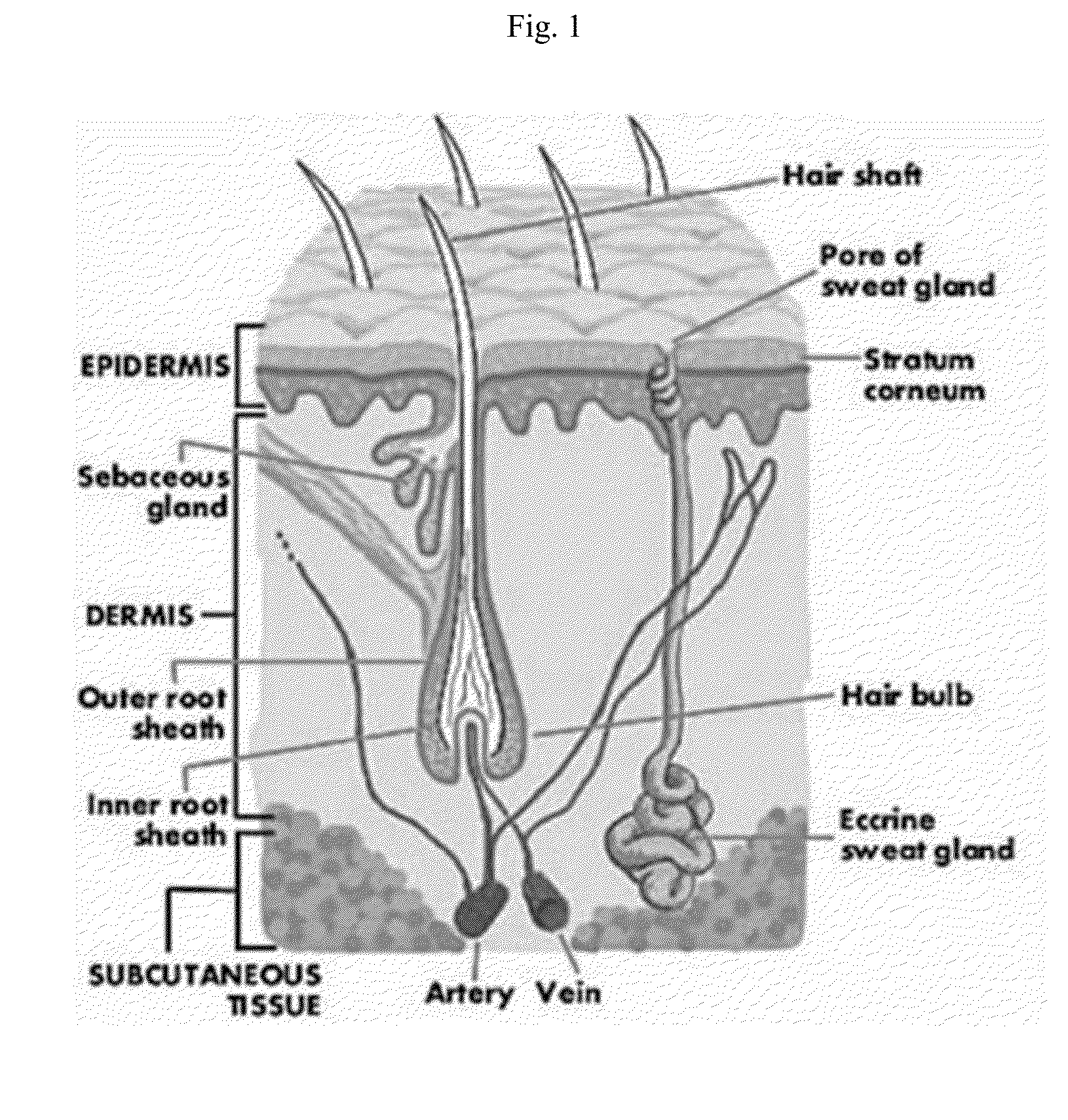
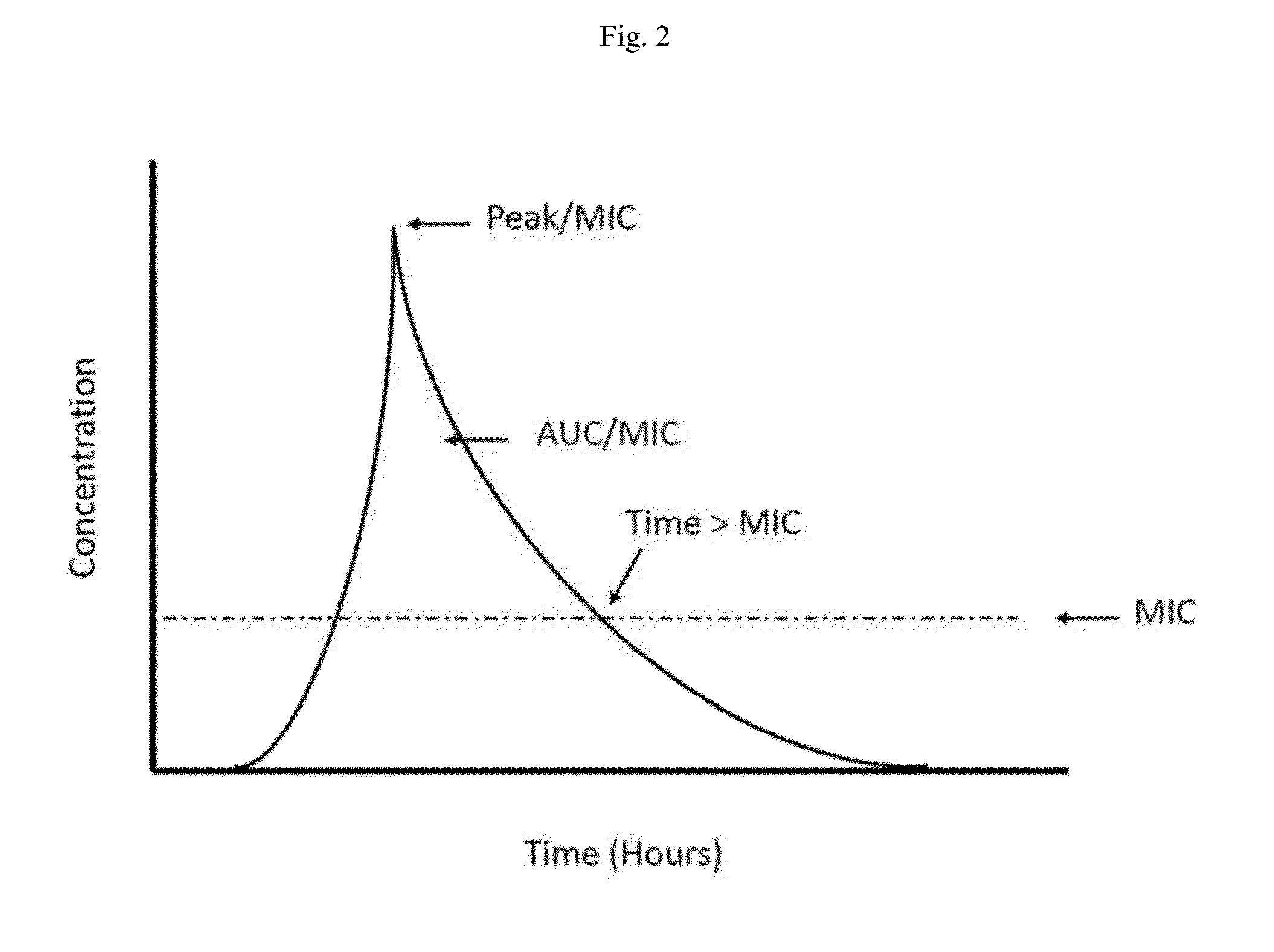
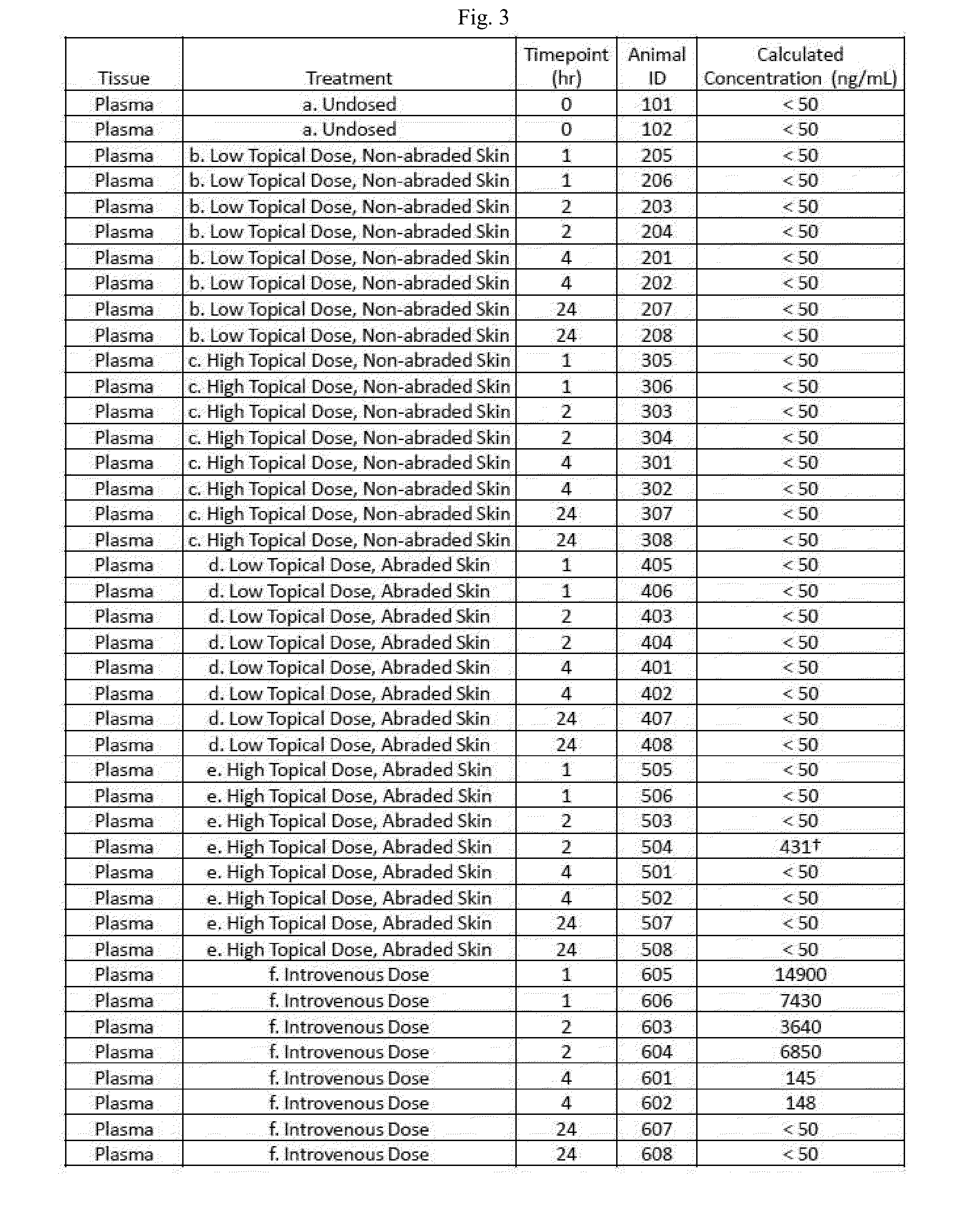
Topical vancomycin formulation and methods of use
ActiveUS20160015774A1Reduced systemic exposureReduce exposureAntibacterial agentsBiocideVancomycin HydrochlorideTopical ointment
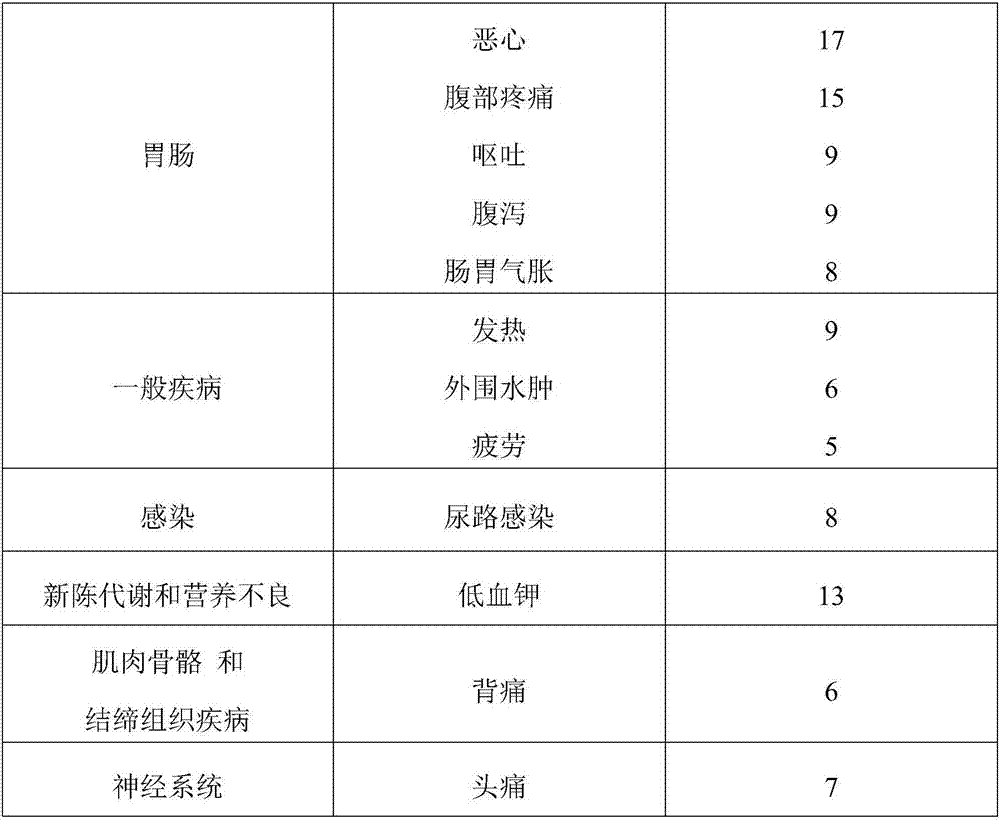
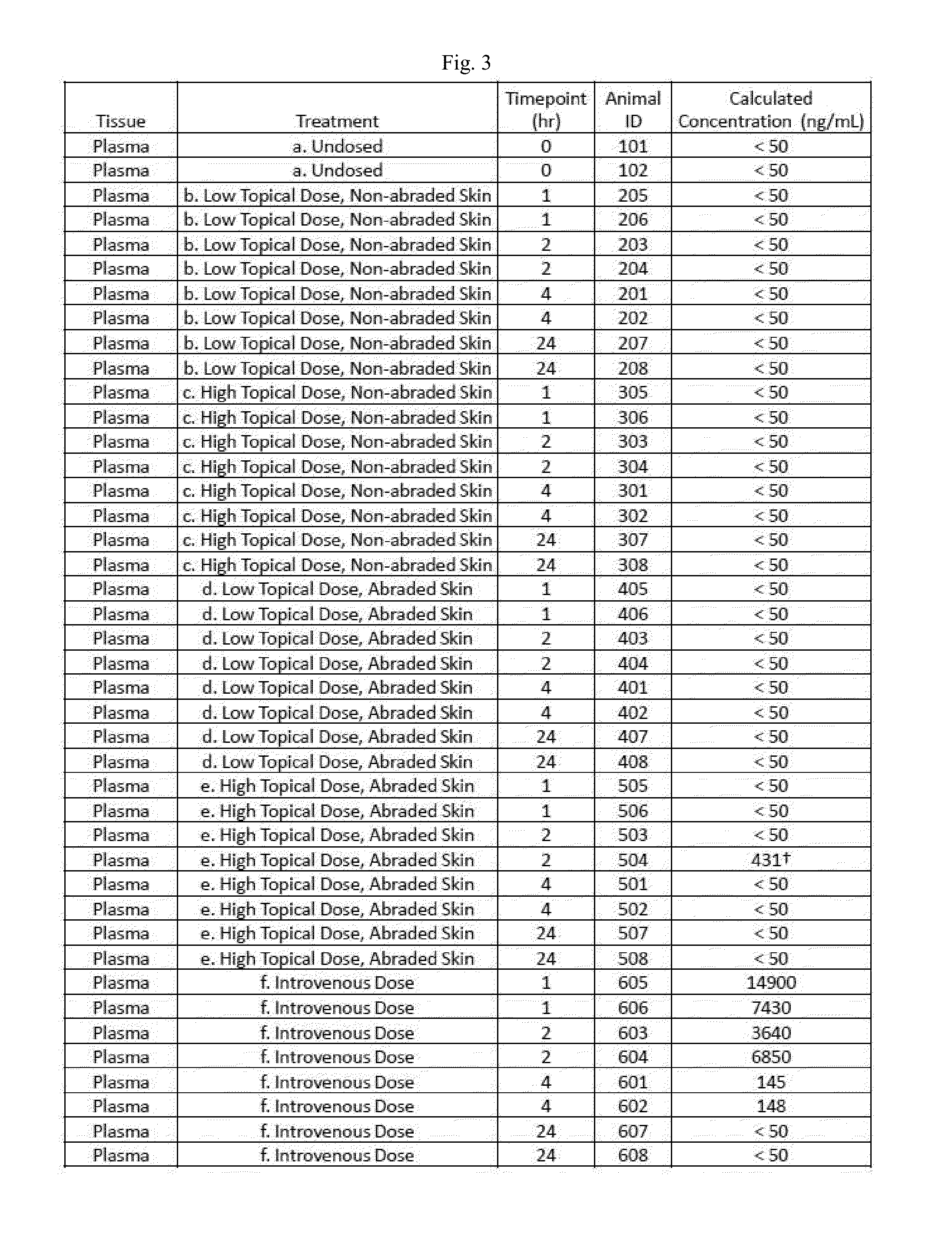
Disclosed herein are methods, compositions, and kits for treating a skin condition caused by a bacterial infection at a skin depth with a topical ointment comprising vancomycin hydrochloride. Also disclosed herein are methods, compositions, and kits for testing susceptibility of vancomycin for treating a bacterial infection at a skin depth, and of optimizing a topical ointment therapeutic regimen.
Owner:KUROBE
Preparation of high-purity vancomycin hydrochloride
ActiveCN101440127BEffective components increaseHigh purityPeptide preparation methodsVancomycin HydrochlorideChromatography liquid
The invention relates to an industrial feasible method for preparing a high-purity vancomycin hydrochloride. The method comprises the following steps of (1) putting a crude vancomycin with the content of vancomycin not less than 80% in a dextran resin glass chromatography column containing the NH4HCO3 mobile phase for column chromatography to obtain an effective chromatography liquid; (2) adding NaCl water solution into the effective chromatography liquid and stirring to produce a deposit; (4) top cleaning by 90% ethanol water solution for a few times and drying to obtain the vancomycin hydrochloride. Effective components of the vancomycin hydrochloride obtained through the method are improved greatly, other impurities are reduced greatly, and the purity is very high; meanwhile, the overall color of the product is improved remarkably, and the product is suitable for oral or injection administration.
Owner:ZHEJIANG NOVUS PHARMA CO LTD
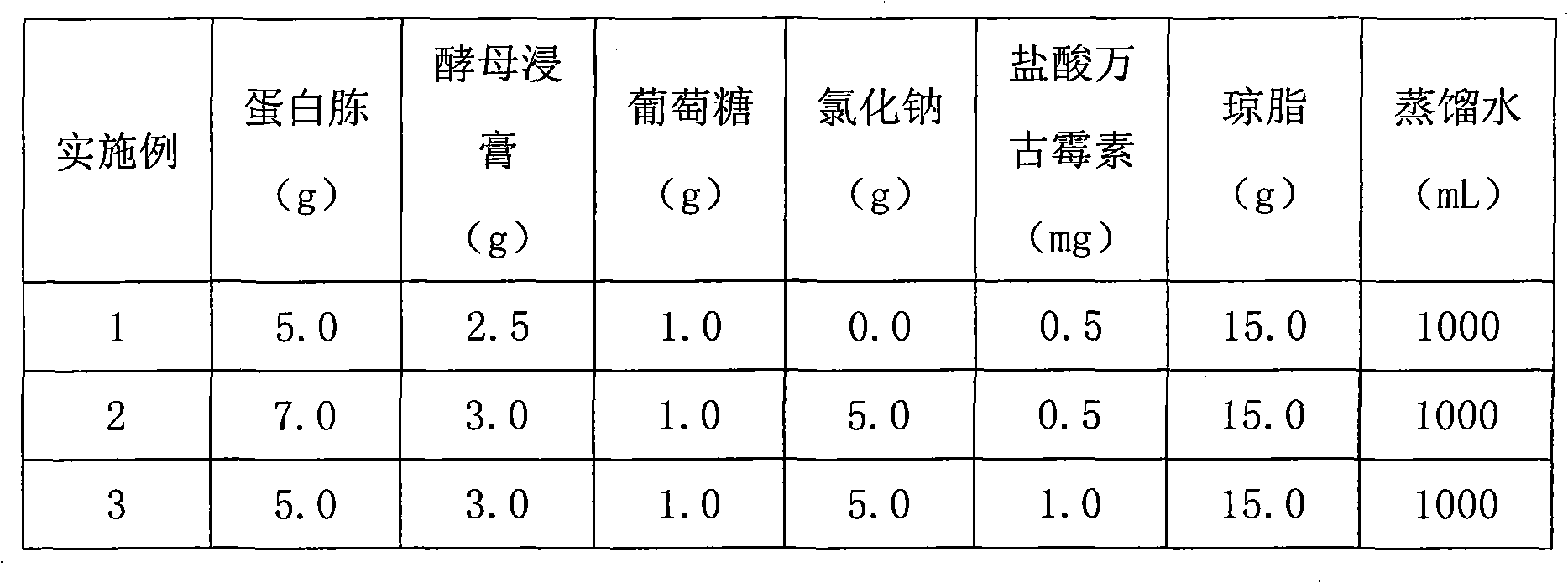
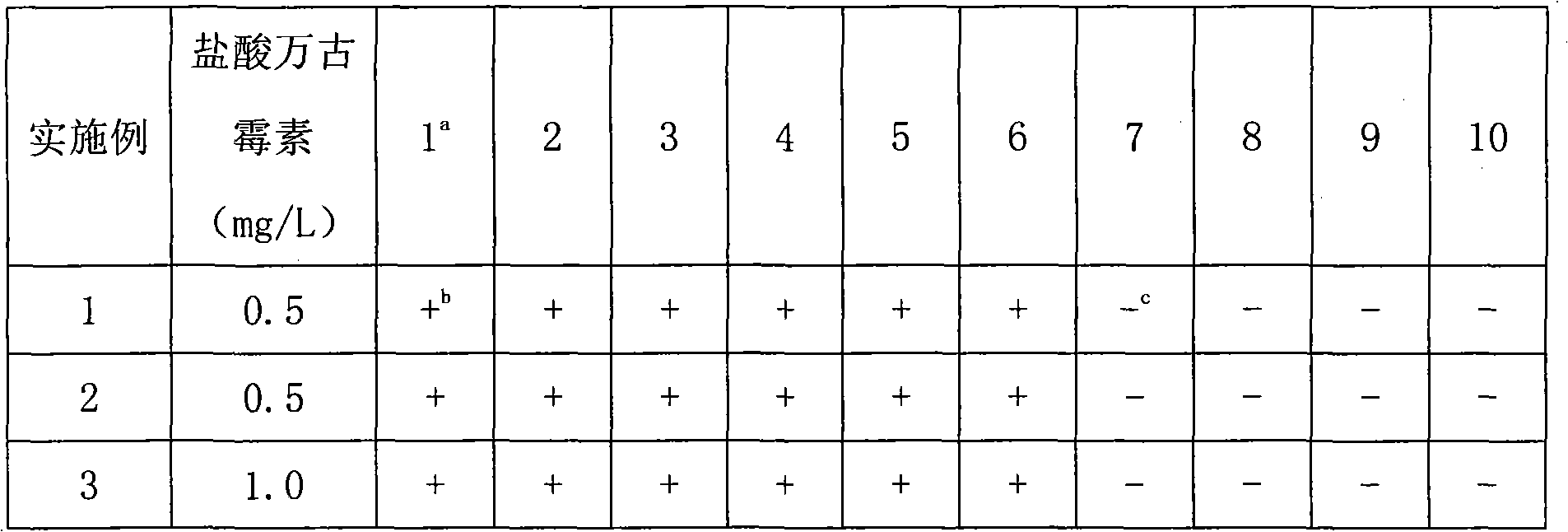
A universal detection medium for Gram-negative bacteria
InactiveCN102286605AHigh selectivityEasy to prepareMicrobiological testing/measurementVancomycin HydrochlorideDistilled water
A kind of Gram-negative bacteria universal detection culture medium of the present invention is composed of: peptone 5.0g~7.0g, yeast extract 2.5g~3.0g, glucose 1.0g, sodium chloride 0g~5.0g, agar 15.0g, Distilled water 1000mL, vancomycin hydrochloride 0.5mg / L~1.0mg / L. The culture medium of the present invention has high selectivity, and can specifically and selectively cultivate Gram-negative bacteria; the preparation is simple, and vancomycin is used alone as a selection agent, and the simplest formula even only needs to add 0.5 mg / L to the plate counting agar ~1.0mg / L of vancomycin hydrochloride is enough; it is friendly to human and environment, the concentration of vancomycin used is not more than 1.0mg / L; the work efficiency is improved, and it can directly count the colonies of Gram-negative bacteria.
Owner:南宁海关技术中心
Separation And Purification Method For Vancomycin Hydrochloride Of High Purity
ActiveUS20160264620A1Easy to subsequently processEasy to solvent recoveryAntibacterial agentsSaccharide peptide ingredientsVancomycin HydrochloridePurification methods
Provided is a separation and purification method for vancomycin hydrochloride of high purity. The method comprises the following steps: (1) obtaining a vancomycin hydrochloride solution from a crude vancomycin product by ion exchange chromatography and obtaining a concentrate by nanofiltration desalination and concentration; (2) adjusting the concentrate with a hydrochloric acid solution and then performing a column chromatography using a reverse chromatography column for the adjusted concentrate; (3) collecting the chromatographic solution of vancomycin to obtain a mixed chromatographic solution; (4) adjusting the mixed chromatographic solution, and separating the solution and the salts by nanofiltration desalination and concentration to obtain a concentrate; and (5) obtaining a vancomycin dry powder with a chromatographic purity of up to 99% and a pure white appearance by dehydrating and drying the concentrate of step (4), or by solvent crystallization or salting-out crystallization.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Composite material bionic bone and preparation method thereof
ActiveCN114010838AHigh hardnessAdjust PH valuePharmaceutical delivery mechanismCleaning using toolsVancomycinumVancomycin Hydrochloride
Owner:中国人民解放军总医院第八医学中心
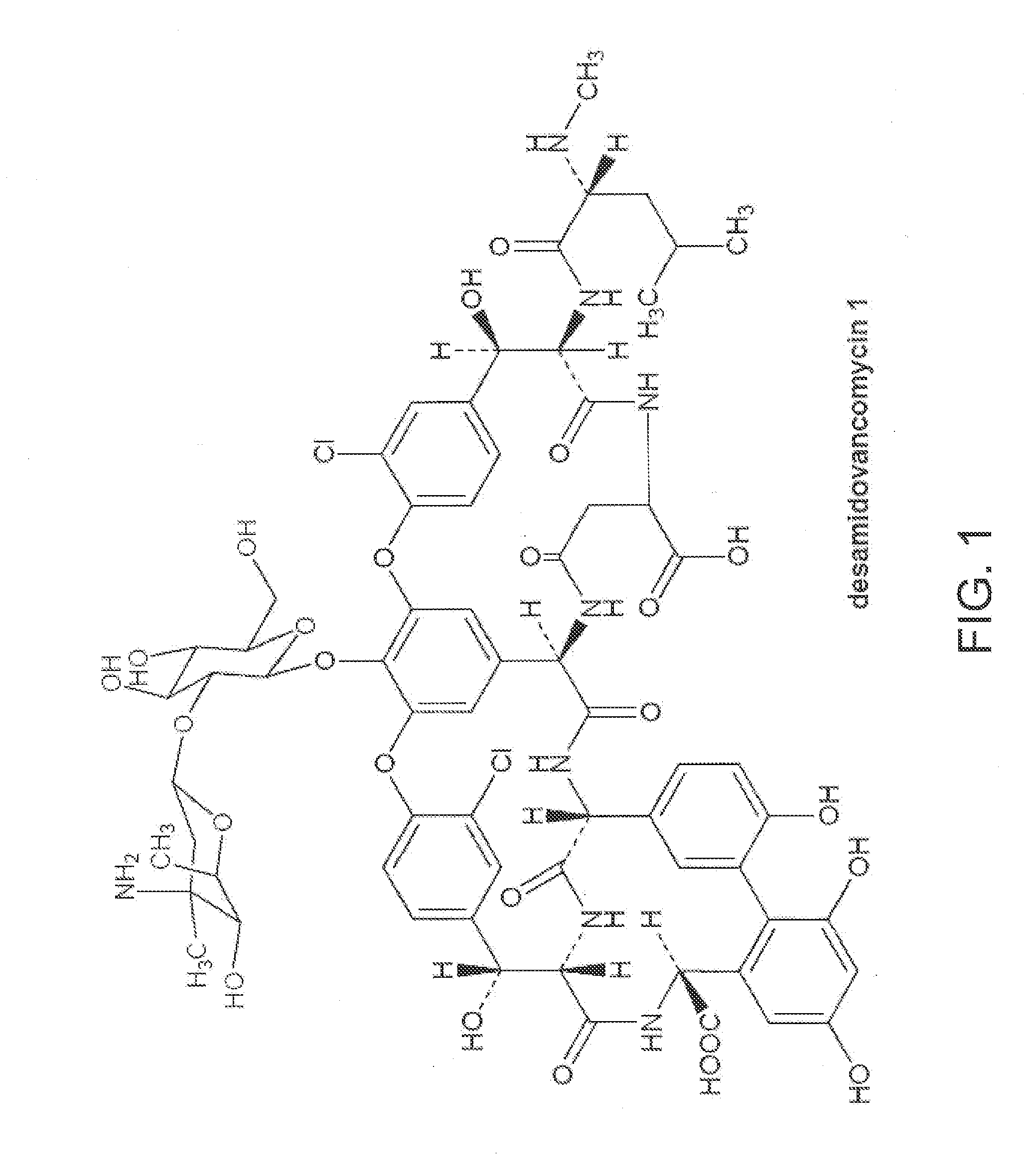
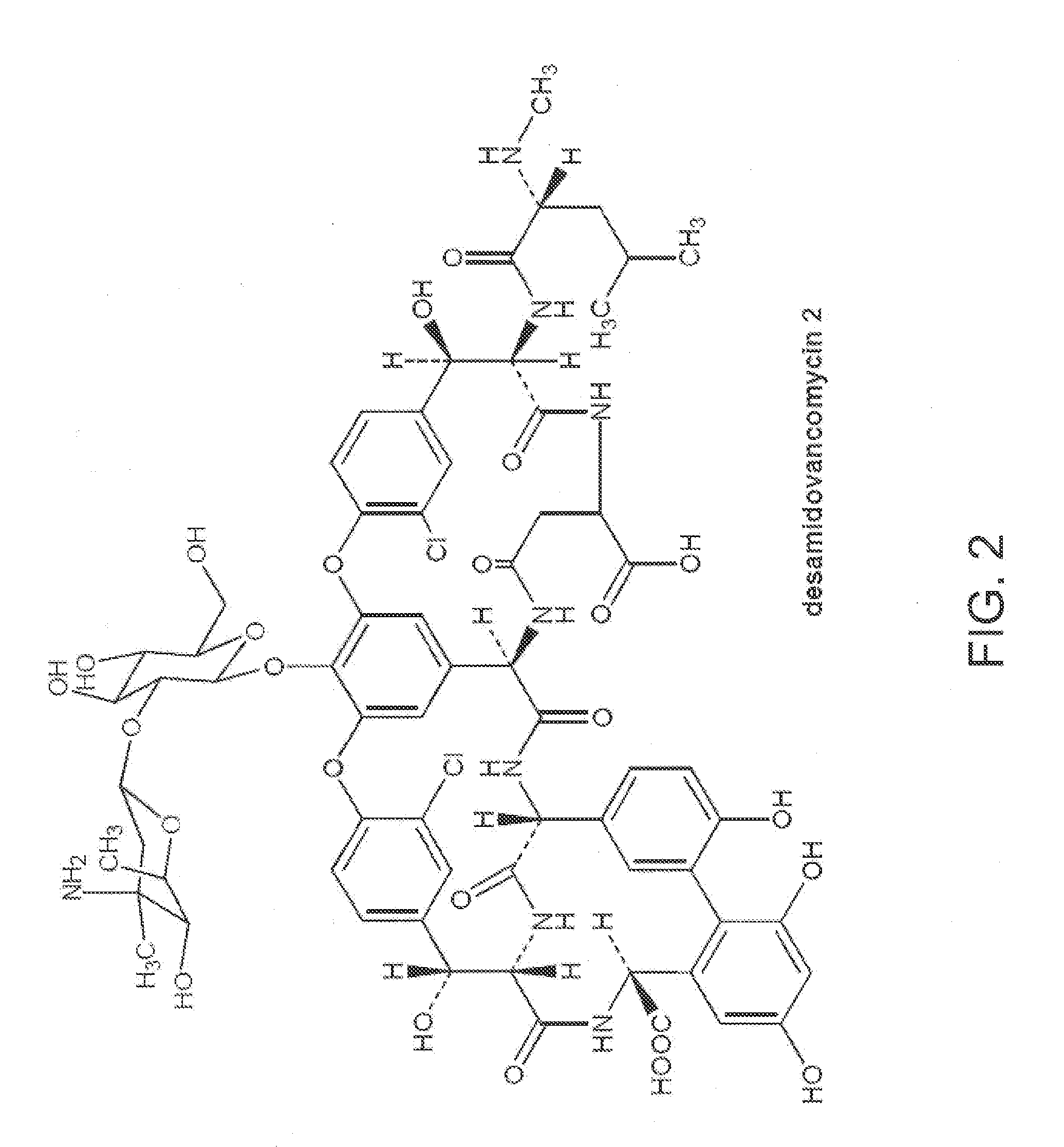
Deshydroxy vancomycin, the preparation, pharmaceutical composition and the use
ActiveUS20100222252A1The fermentation process is simpleQuality is obtainedAntibacterial agentsBiocideVancomycin HydrochlorideSalt water
The present invention provides a deshydroxy vancomycin compound,a method of its preparation and a pharmaceutical composition comprising a pharmaceutically effective amount of the deshydroxy vancomycin and the use of the said composition in the preparation of drugs for the treatment of susceptible bacteria infections. The method includes the following steps: (1) preparing a concentrated vancomycin solution containing the deshydroxy vancomycin by fermentations of Amycolatopsis Orientalis with Deposit No. CGMCCNO.1183; (2) separating and purifing the concentrated vancomycin solution to obtain a refined filtrate of vancomycin hydrochloride containing the deshydroxy vancomycin by column chromatography; and (3) further separating and purifing the refined filtrate to obtain the deshydroxy vancomycin by chromatography. Wherein, separation and purification is processed by column chromatography in a gel chromatography column containing a salt-water mobile phase, separation and purification is processed by chromatography in a macroporous adsorption resin chromatography column containing a buffer-methanol mobile phase.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Non-crystalline vancomycin hydrochloride, its preparation method and use, and its pharmaceutical composition
ActiveCN101613396BEffective components increaseHigh puritySaccharide peptide ingredientsPeptidesVancomycin HydrochlorideOral medication
The invention provides a high-density amorphous vancomycin hydrochloride, a preparation method thereof, an application of the vancomycin hydrochloride for oral administration or injection administration and a pharmaceutical composition containing the vancomycin hydrochloride. The vancomycin hydrochloride in an amorphous state has the characteristics similar to the diffraction pattern of an X-ray powdery crystal shown in the attached drawing 1. The effective components of vancomycin hydrochloride prepared by the preparation method are enhanced greatly, other impurities are reduced to a large extent, the purity is high, and the chromatographic purity can reach more than 95 percent. The amorphous vancomycin hydrochloride not only can be used as injections, but also can be used as capsules and other solid preparations. Meantime, the appearance and the color of products are improved obviously, and the products are suitable for oral administration or injection administration.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Separation and purification method for vancomycin hydrochloride of high purity
ActiveUS10131689B2High purityReduce the presence of impuritiesAntibacterial agentsSaccharide peptide ingredientsVancomycin HydrochloridePurification methods
Owner:ZHE JIANG MEDICINE CO LTD XINCHANG PHARMA FAB
Method and system for producing high purity vancomycin hydrochloride
ActiveUS9428291B2High purityImprove performanceAntibacterial agentsSemi-permeable membranesVancomycin HydrochlorideSpray dried
A method is provided for preparing spray dried powder containing vancomycin hydrochloride. The method comprises providing a vancomycin hydrochloride solution with a chromatographic purity of at least 95%, adding an excipient to the vancomycin hydrochloride solution to form a mixture solution of the vancomycin hydrochloride solution and the excipient, concentrating the mixed solution of the vancomycin hydrochloride solution and the excipient to form a 20% to 30% vancomycin concentrate, filtering the vancomycin concentrate to form a final filtrate, and spray drying the final filtrate to form a spray dried vancomycin hydrochloride powder with EP impurity B level of not more than 1.5%.
Owner:ZHEJIANG MEDICINE CO LTD
Vancomycin hydrochloride medicine composition and method for preparing vancomycin hydrochloride medicine composition
PendingCN106963939ABroaden the use of dosage formsImprove stabilityAntibacterial agentsDigestive systemVancomycin HydrochlorideHard Capsule
The invention discloses a vancomycin hydrochloride medicine composition and a method for preparing the same. The vancomycin hydrochloride medicine composition comprises 10-60% of vancomycin hydrochloride, 30-90% of matrixes and 0-10% of lubricants, and preferably comprises 30-40% of vancomycin hydrochloride, 50-70% of matrixes and 2-8% of lubricants. The matrixes are one or two types of PEG (polyethylene glycol) 1000-6000, stearic acid and poloxamer 188, and the lubricants are one or two types of micro-powder silica gel, talc powder, sodium stearyl fumarate, sodium dodecyl sulfate and magnesium stearate. The vancomycin hydrochloride medicine composition can be in one of dosage forms of soft capsules, hard capsules and tablets. The vancomycin hydrochloride medicine composition and the method have the advantages that components in the vancomycin hydrochloride medicine composition are easily matched with one another, and medicines are high in stability; hot melting technologies are adopted in the method for preparing the vancomycin hydrochloride medicine composition, processes for producing the vancomycin hydrochloride medicine composition are simple, can be implemented conveniently and quickly, are stable and can be scaled up advantageously, and service dosage forms of the vancomycin hydrochloride can be broadened.
Owner:YUNG SHIN PHARMA IND KUNSHAN
Topical vancomycin formulation and methods of use
ActiveUS9241971B1Reduce exposureAntibacterial agentsAerosol deliveryVancomycin HydrochlorideAugmented Ointment
Disclosed herein are methods, compositions, and kits for treating a skin condition caused by a bacterial infection at a skin depth with a topical ointment comprising vancomycin hydrochloride. Also disclosed herein are methods, compositions, and kits for testing susceptibility of vancomycin for treating a bacterial infection at a skin depth, and of optimizing a topical ointment therapeutic regimen.
Owner:KUROBE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com