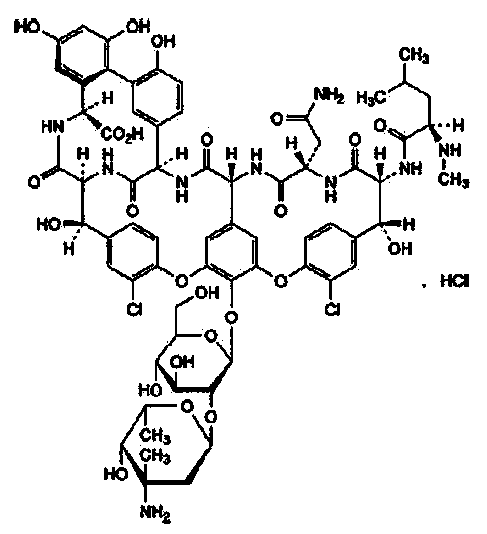
Refining method for vancomycin hydrochloride
A vancomycin hydrochloride and refining method technology, applied in the direction of peptides, etc., can solve the problems of difficult filtration, fine crystals, and inability to recycle, and achieve the effect of simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: Preparation of vancomycin hydrochloride solution
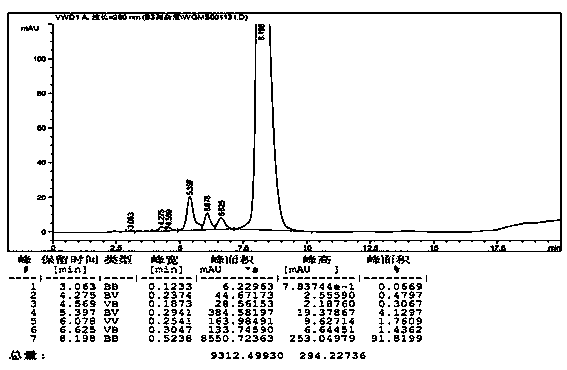
[0028]3.62 liters of vancomycin hydrochloride extract with a concentration of 87 g / L and a purity of 82% was passed through a chromatographic column equipped with 4 liters of PS / DVB reverse chromatographic filler. Then use a gradient top wash with 5% n-butanol aqueous solution, and the concentration of n-butanol aqueous solution rises to 10% at the end of the top wash. The eluted fractions were collected in sections with an automatic collector, and the purity of the fractions was detected by HPLC. Fractions with a purity of more than 90% were combined and concentrated by nanofiltration to obtain 3.45 liters of vancomycin hydrochloride solution. Concentration: 68.98 g / L, yield: 85.0%, purity: 91.82%, see figure 2 .
Embodiment 2
[0029] Embodiment 2: Preparation of vancomycin
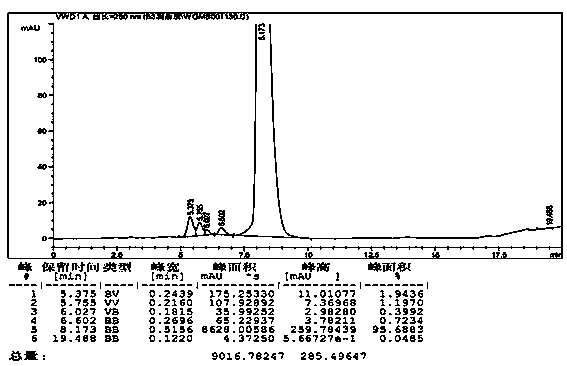
[0030] 3 liters of the vancomycin hydrochloride solution that example 1 makes is put in the cold water bath of 15 ℃. Add 30 grams of solid ammonium chloride under stirring, stir well to dissolve it completely, adjust the pH to 8.08 with 4N ammonia water under stirring, stir for 3 hours, during which a large amount of solids precipitate, and let it stand overnight. The solid was collected by suction filtration. The obtained solid was dried under high vacuum at 30°C for 4 hours, then pulverized, and then dried under high vacuum at 30°C for 20 hours. Get 197.4 grams of vancomycin solid. Potency: 868μg / mg, yield: 90.18%, purity: 95.68%, see image 3 .
Embodiment 3
[0031] Embodiment 3: Preparation of vancomycin hydrochloride
[0032] Suspend 180 g of the vancomycin solid obtained in Example 2 in 8 liters of purified water, cool to 5°C, adjust the pH to 2.8 with 4M hydrochloric acid under stirring, and fully dissolve it. The solution was placed in a nanofiltration system and concentrated to a volume of 4 liters. Add 4 liters of purified water and continue to concentrate to a volume of 4 liters. This washing operation was repeated 5 times. Finally the solution was concentrated to a volume of 1.3 liters.
[0033] Add 10 grams of activated carbon to the above concentrate, stir at room temperature for 30 minutes, filter, and then filter the filtrate through a 0.22 μm membrane, put it in a plate, put it in a freeze dryer, and freeze it at -45°C for 4 hours. Turn on the vacuum to below 15Pa for sublimation, and raise the temperature to -10°C, and keep the vacuum at this temperature for 10 hours. Then continue to raise the temperature to 0°C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com