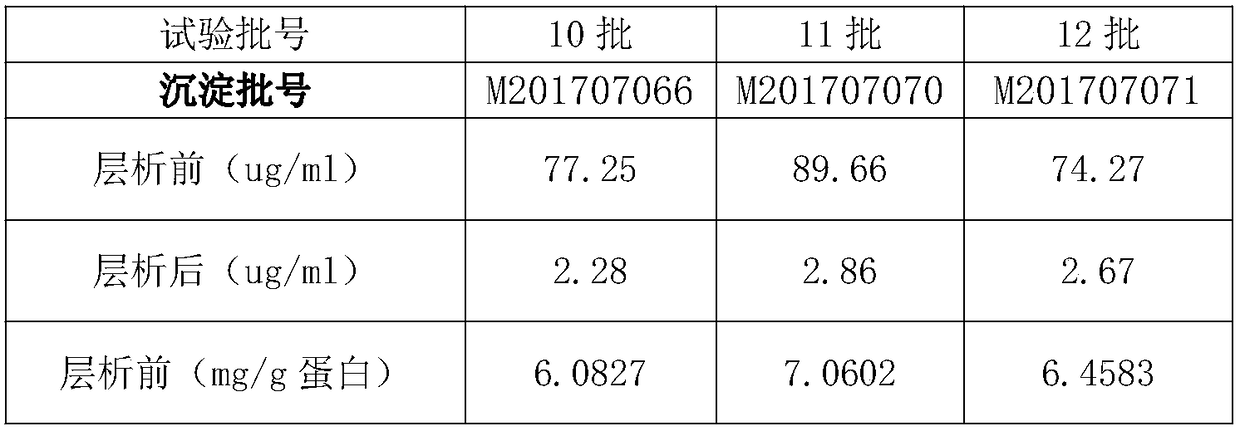
Patents
Literature
57 results about "Chromatography liquid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Liquid chromatography (LC) refers to any chromatographic procedure in which the moving phase is a liquid, in contrast to the moving gas phase of gas chromatography. Traditional column chromatography (whether adsorption, partition, or ion-exchange), thin-layer and paper chromatography, and modern LC are each examples of liquid chromatography.
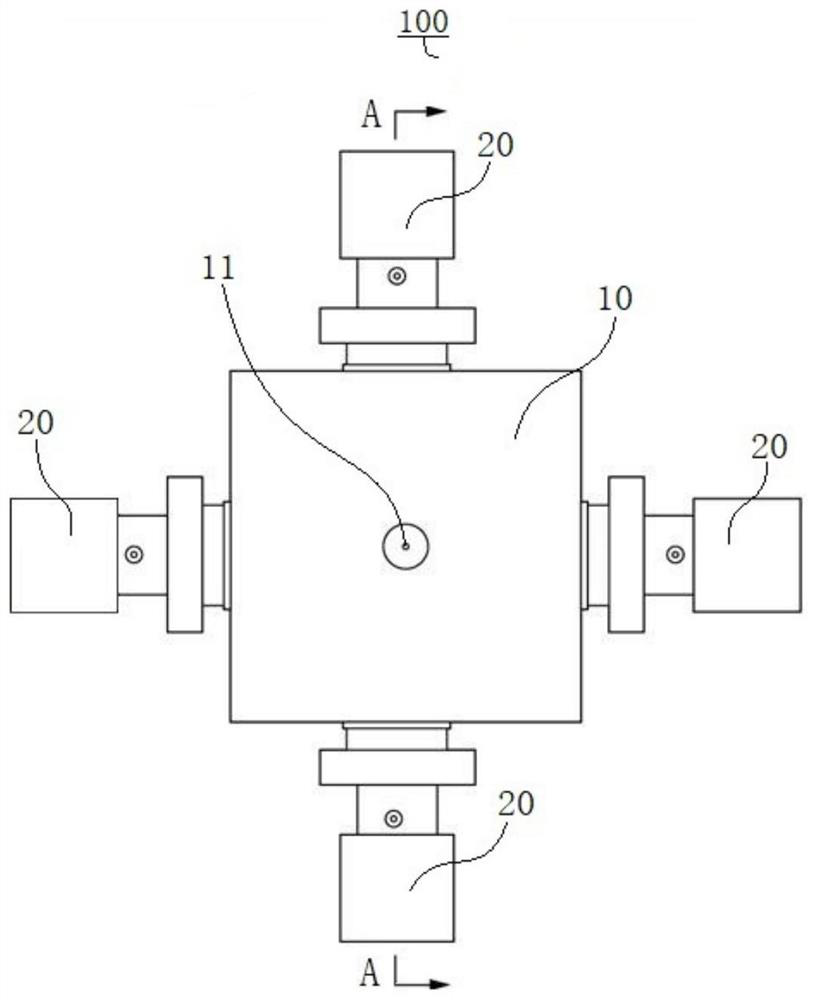
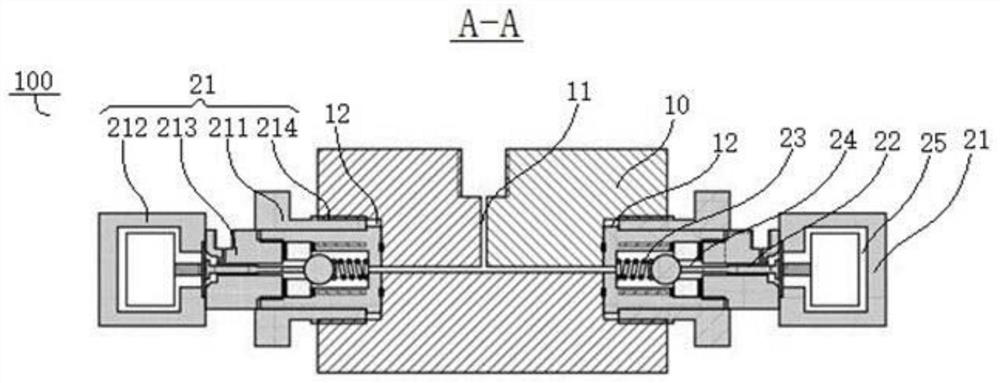
Liquid Chromatography Apparatus
InactiveUS20080135484A1Efficiently definedEfficient deliveryIon-exchange process apparatusComponent separationStationary phaseChromatography liquid
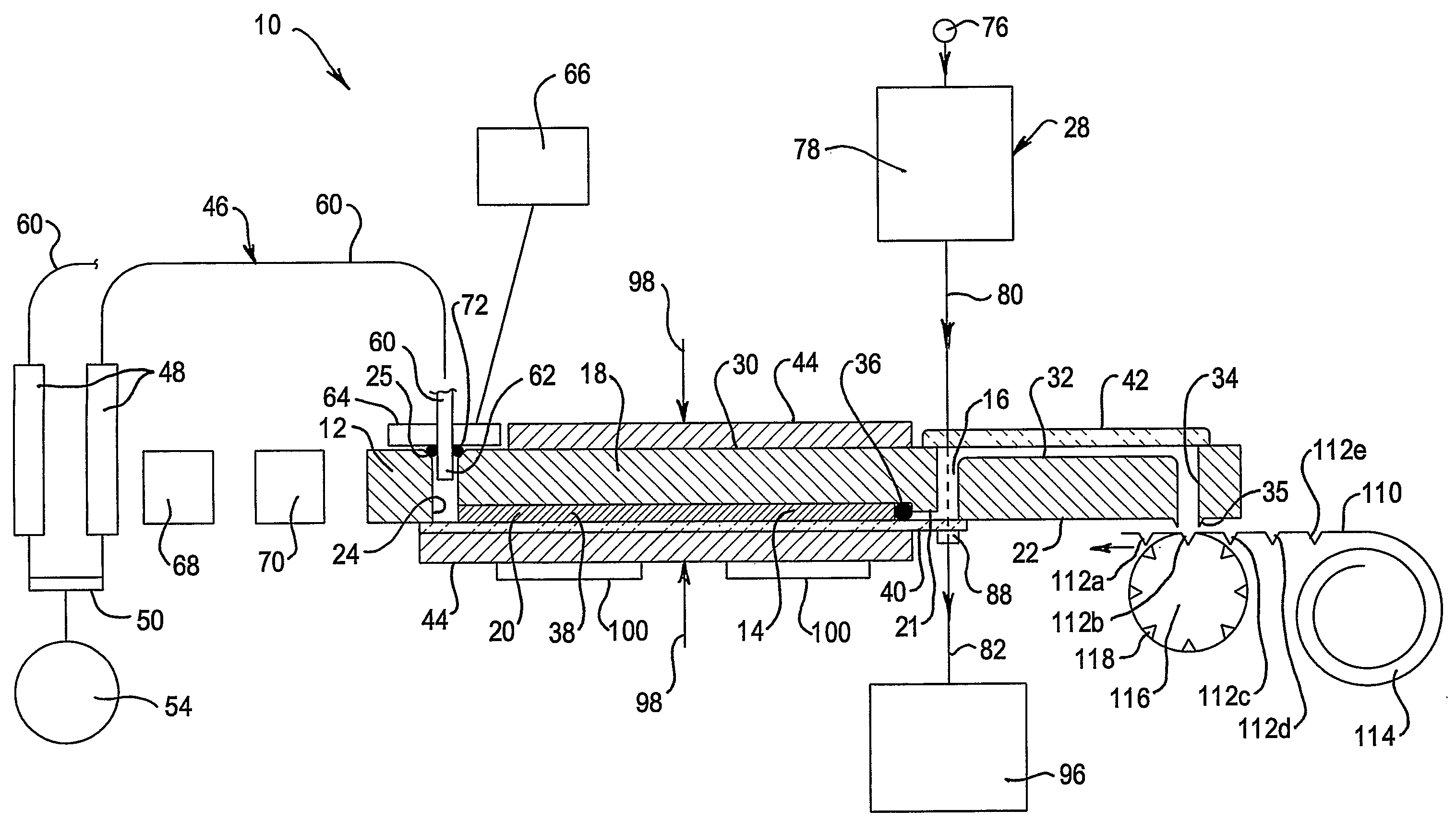
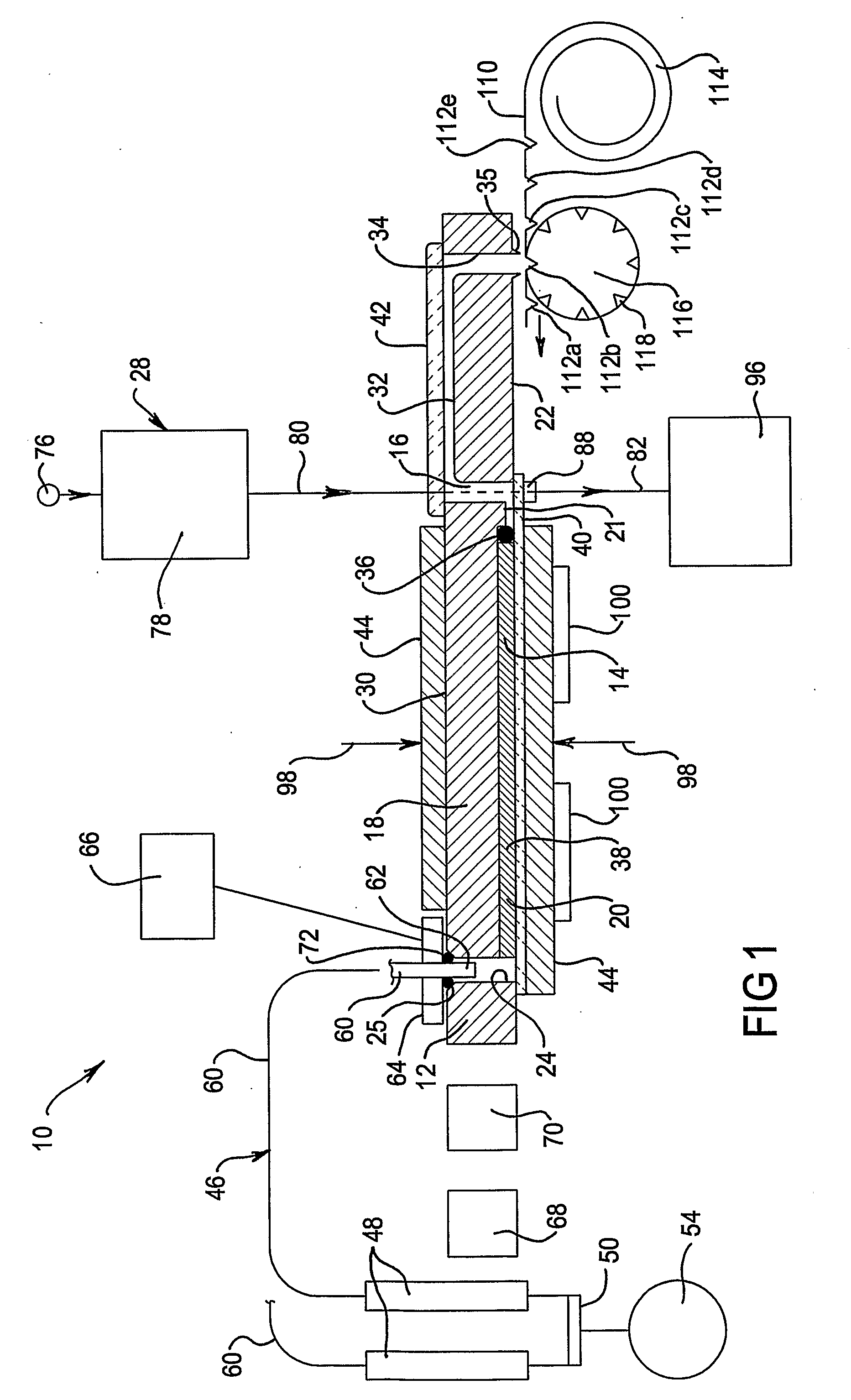
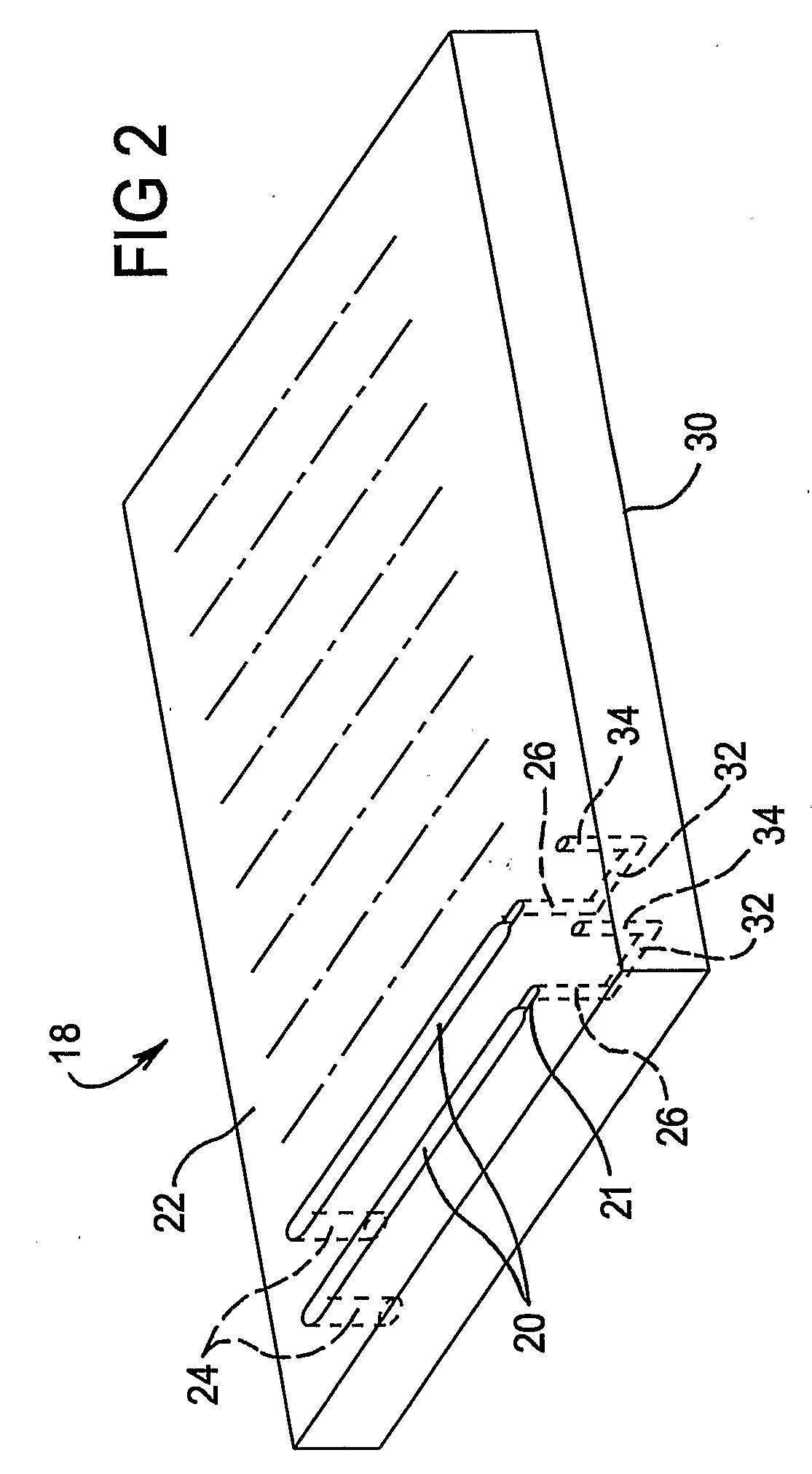
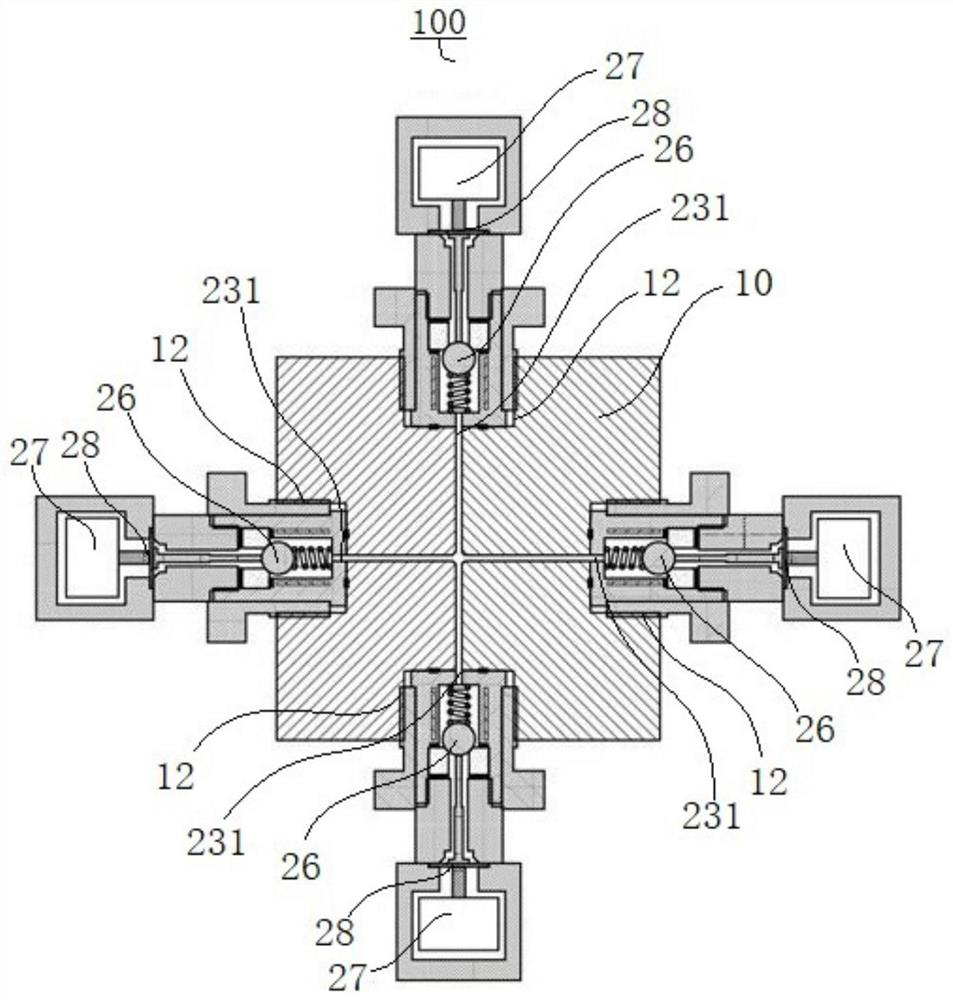
A multi-column liquid chromatography system (10) for performing a plurality of liquid chromatography separations in parallel is based on a column plate structure (12) having parallel grooves (20) formed in a surface (22) of a plate (18), a cover sheet (40) bonded to the surface (22) to cover the grooves (20) and a stationary phase (38) contained in each covered groove (20). Through holes (24, 26) in the plate (18) define respective inlets (24) for the chromatography columns (14) and flow cells (16) at outlets, with the cover sheet (40) providing an optically transparent end wall for the flow cells (16) and another cover sheet (42) bonded to the opposite surface (30) of the plate (18) providing the other optically transparent end wall for the flow cells (16). Thus merely three parts need be provided for a structure for providing the chromatography columns, that is, a plate having grooves and through holes plus two cover sheets. The chromatography system (10) additionally includes a pumping system (46) comprising a syringe pump (48) for each column (14), an optical system (28) for transmitting analytical radiation through the flow cells (16) and a fraction collection sheet (110) containing wells (112) which is fed past outlets (34-35) from the column plate structure (12).
Owner:VARIAN AUSTRALIA PTY LTD
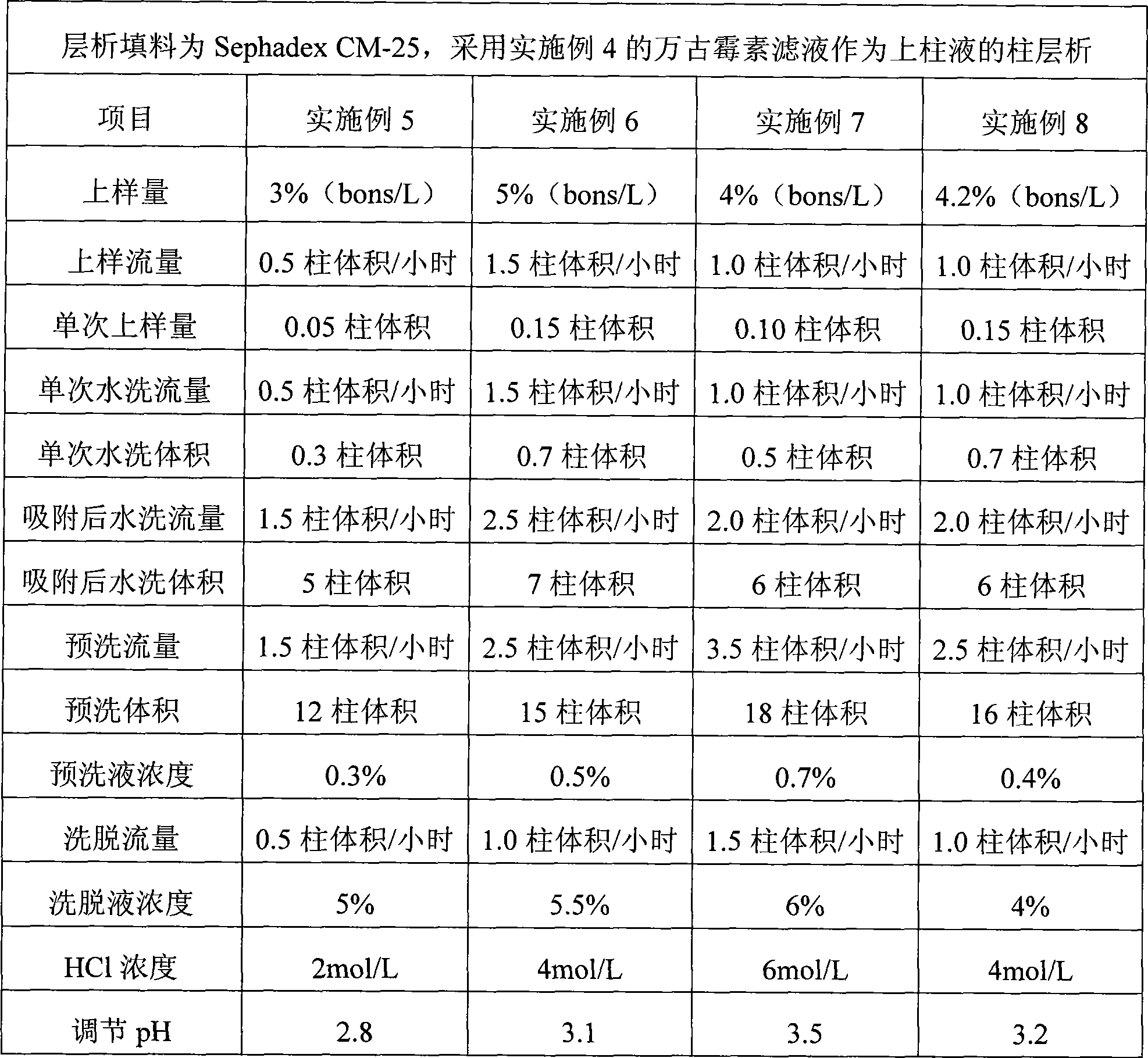
Preparation of high-purity vancomycin hydrochloride
ActiveCN101440127AEffective components increaseHigh purityPeptide preparation methodsVancomycin HydrochlorideChromatography liquid
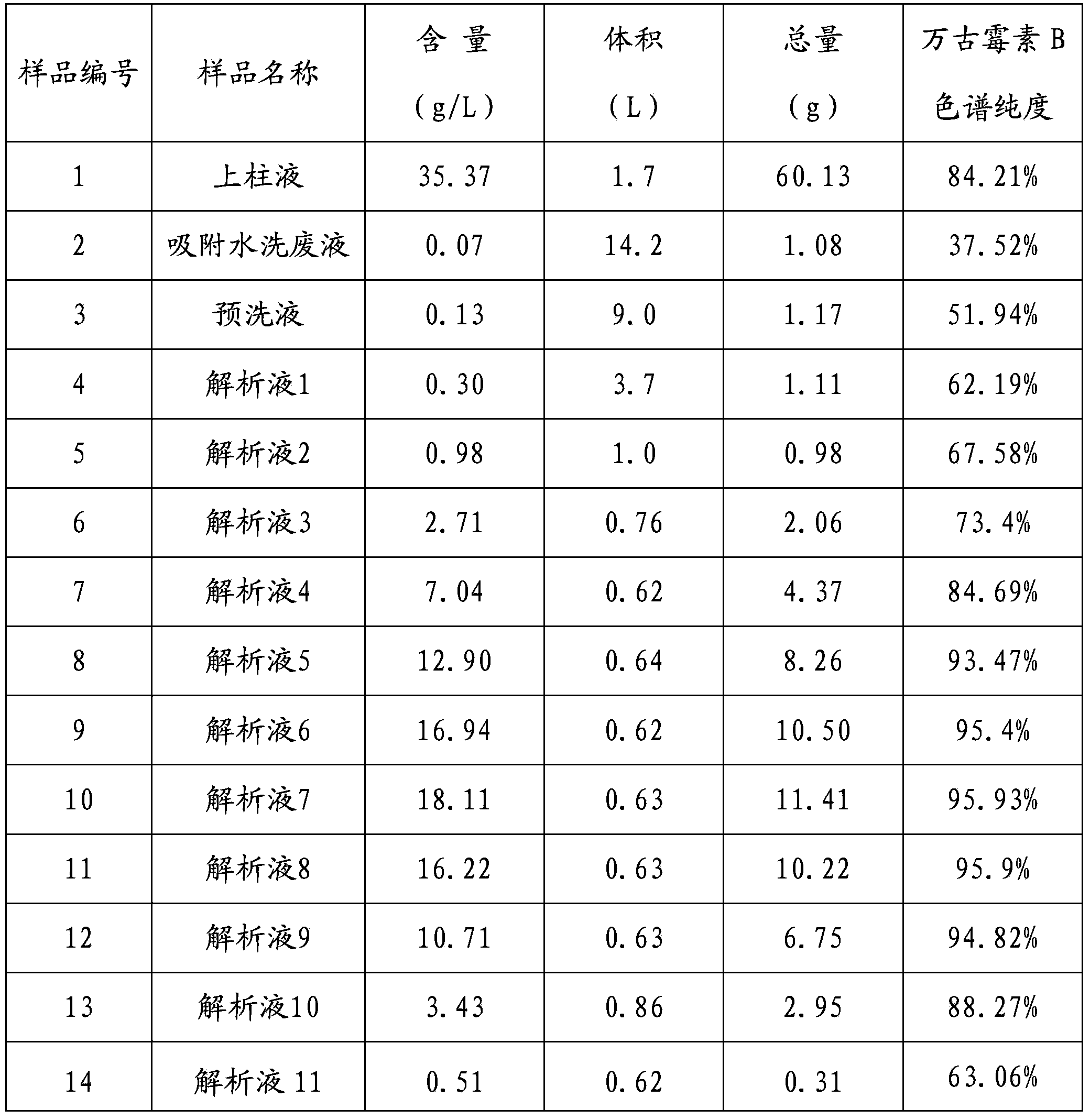
The invention relates to an industrial feasibly method for preparing a high-purity vancomycin hydrochloride. The method comprises the following steps of putting a crude vancomycin with the content of vancomycin not less than 80 percent in a dextran resin glass chromatography column containing the NH4HCO3 mobile phase for column chromatography to obtain an effective chromatography liquid; adding NaCl water solution into the effective chromatography liquid and stirring to produce a deposit; top cleaning by 90 percent ethanol water solution for a few times and drying to obtain the vancomycin hydrochloride. Effective components of the vancomycin hydrochloride obtained through the method are improved greatly, other impurities are reduced greatly, and the purity is very high; meanwhile, the overall color of the product is improved remarkably, and the product is suitable for oral or injection administration.
Owner:ZHEJIANG NOVUS PHARMA CO LTD
Preparation method of vancomycin with high purity
ActiveCN103408639ASmall granularityLow swelling ratePeptide preparation methodsChromatography liquidVancomycin
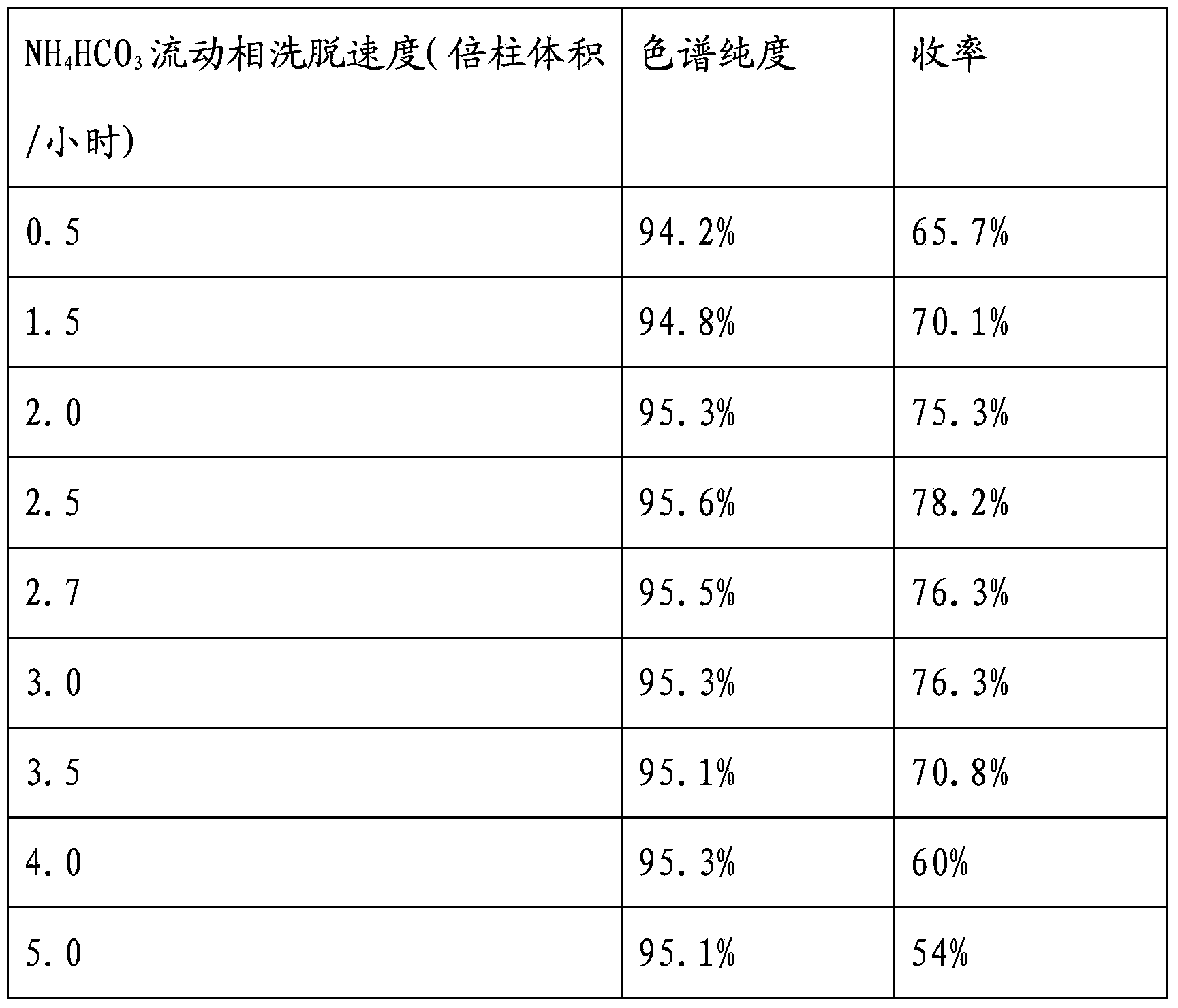
The invention belongs to the field of antibiotic preparation technologies, and more specifically relates to a preparation method of vancomycin with high purity. The preparation method comprises following steps: a destaining solution of vancomycin is subjected to column chromatography in a chromatography media, wherein the chromatography media is UniPMM50CAR, the NH4HCO3 mass concentration of a mobile phase of column chromatography is 0.2 to 0.7%; when absorbance rises to 40 at a detection wavelength of 280nm, chromatography liquids are collected segment by segment, the pH value of the chromatography liquids is adjusted to 3.0 to 3.5, the chromatography liquids are preserved under 4 DEG C, and the collected chromatography liquids are mixed. Bubbles in the chromatographic column caused by degradation of carbonate are not likely to generate by using the preparation method. The preparation method is capable of increasing stage number and separation efficiency. The appearance of the obtained vancomycin is improved significantly, purity is as high as 99%, and the vancomycin can be taken orally or by injection.
Owner:LIVZON GROUP FUZHOU FUXING PHARMACEUTICAL CO LTD
Human fibrinogen preparation method
ActiveCN107827974AEfficient removalHigh purityFibrinogenPeptide preparation methodsFiberChromatography liquid
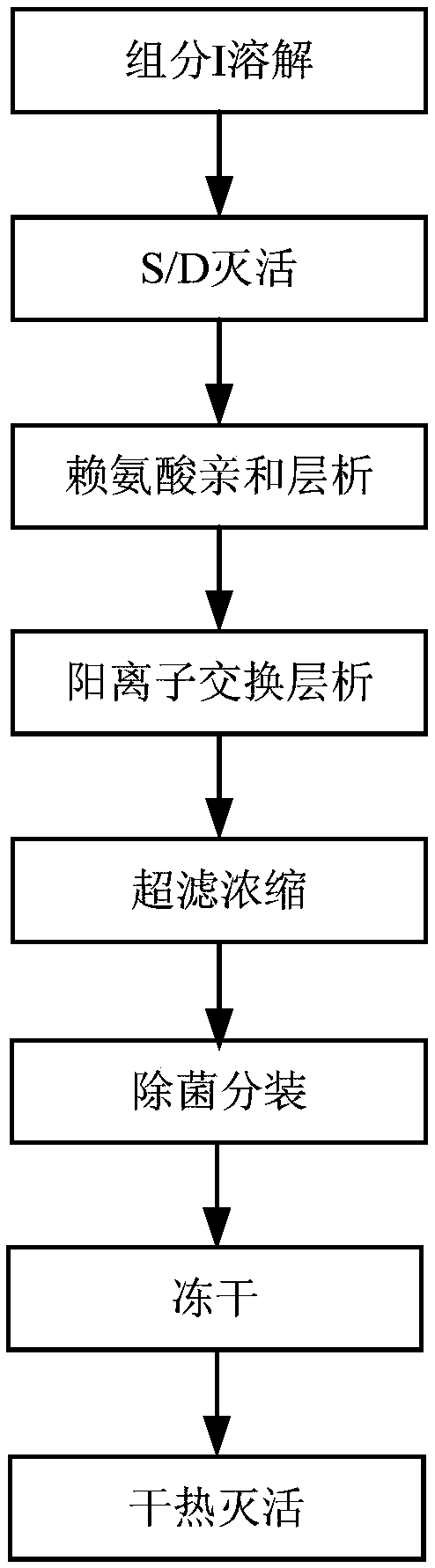
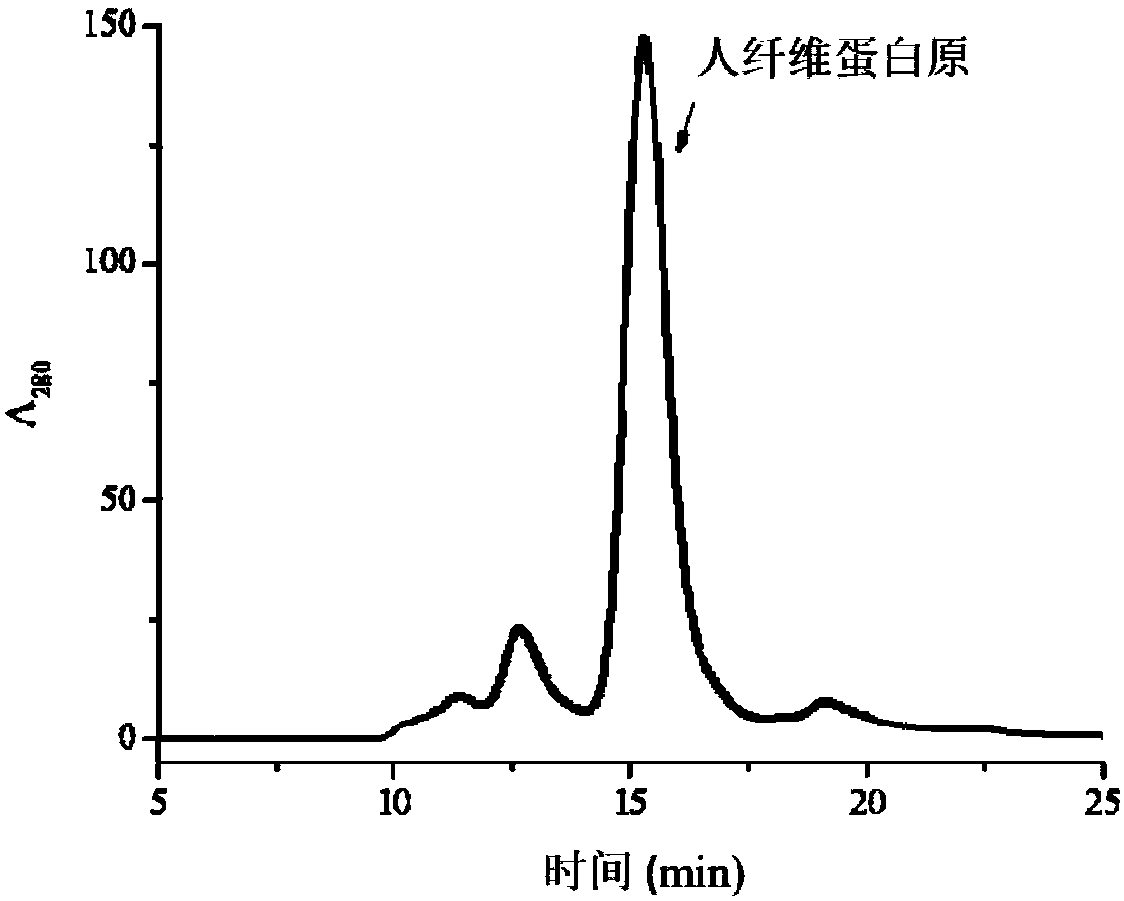
The invention provides a human fibrinogen preparation method which comprises the following steps: (1) dissolving a human plasma low-temperature ethanol precipitation component I into an extracting solution to obtain a component I solution; (2) performing S / D inactivation on the component I solution obtained in the step (1); (3) balancing a lysine affinity chromatography medium by equilibrium liquid, then performing column chromatography on the solution obtained in the step (2) and collecting a penetration peak; (4) balancing a cation exchange chromatography medium by the equilibrium liquid, then performing column chromatography on the penetration peak obtained in the step (3), leaching a chromatography column by leachate after chromatography finishes, finally eluting by an eluant and collecting an elution peak to obtain human fibrinogen. The method disclosed by the invention has simpleness in operation and can effectively remove plasminogen; a prepared human fibrinogen product has theadvantages of higher purity and stability.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +2
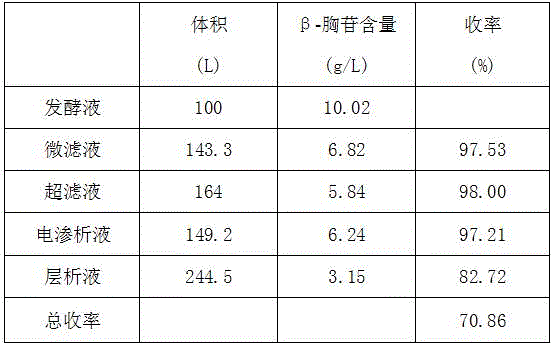
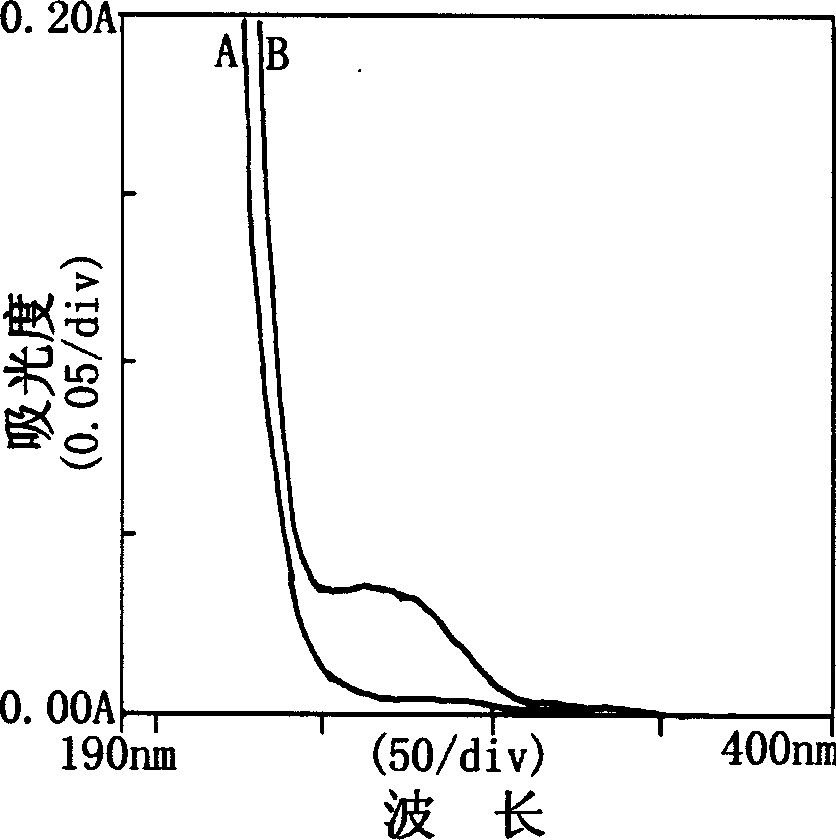
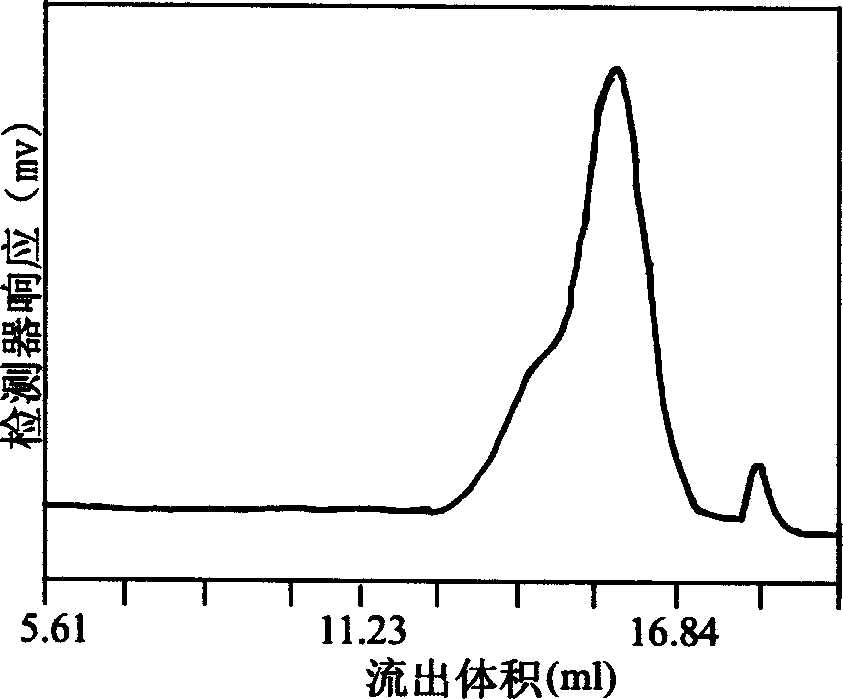
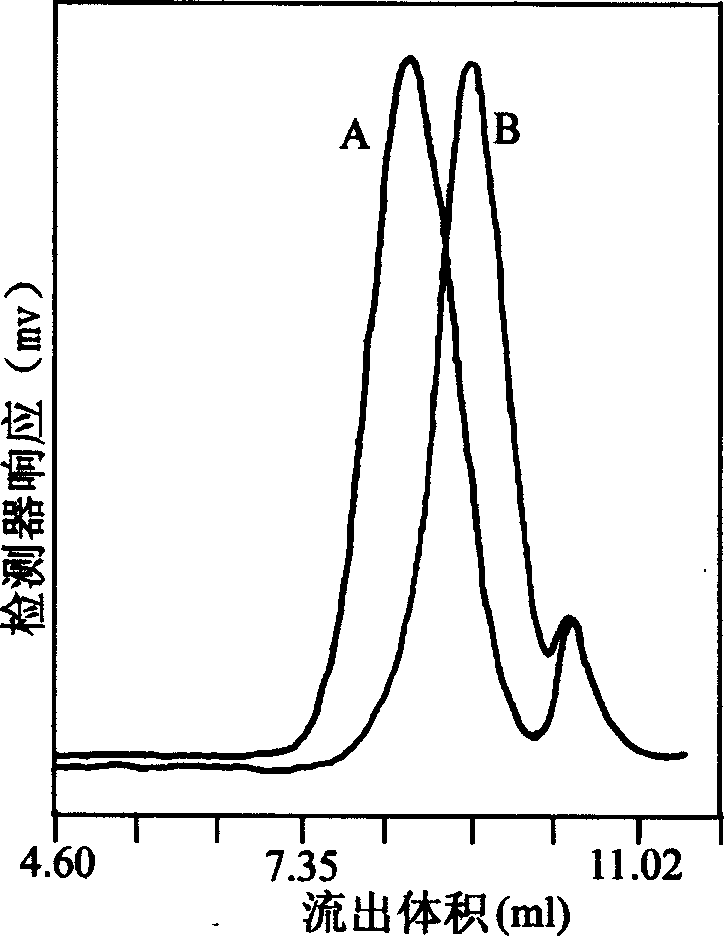
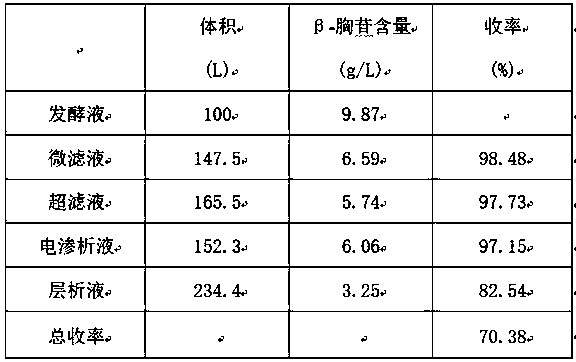
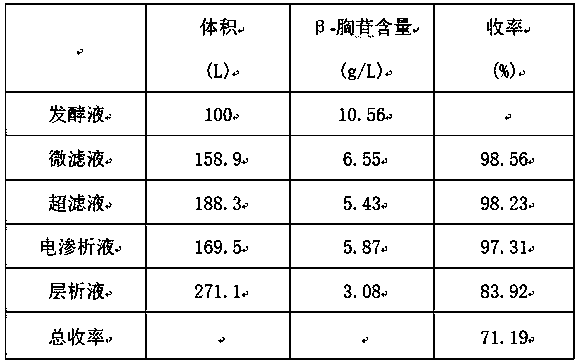
Method for extracting beta-thymidine from fermentation liquid
InactiveCN105859809AHigh extraction yieldQuality assuranceSugar derivativesSugar derivatives preparationChromatography liquidEconomic benefits
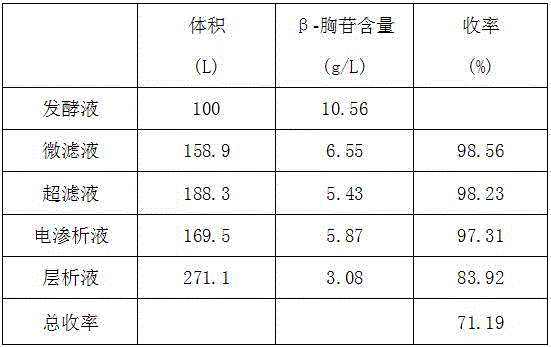
The invention discloses a method for extracting beta-thymidine from fermentation liquid. The method comprises the following steps: (1) preheating the fermentation liquid; (2) performing microfiltration on the fermentation liquid; (3) performing ultrafiltration on the microfiltration liquid; (4) performing electrodialysis on the ultrafiltration liquid; (5) concentrating the electrodialysis liquid; (6) performing chromatography on the concentrated liquid; and (7) concentrating and crystallizing the chromatography liquid. According to the method, a membrane separation technique, an electrodialysis technique and a chromatography separation technique are implemented in the extraction process of the beta-thymidine fermentation liquid, so that a good impurity removal effect can be achieved, and the process is simple, convenient and environment-friendly and high in automation degree. By adopting the extraction process disclosed by the invention, the purity of the liquid phase of a beta-thymidine product is greater than or equal to 98.5%, the external standard content is greater than or equal to 98.5, the total yield is greater than or equal to 70%, industrial application of extracting beta-thymidine from the fermentation liquid is achieved, and good economic benefits and industrial popularization prospects can be achieved.
Owner:NANCHANG UNIV
Urushi polysaccharide extracting method
The invention discloses a process for extracting natural CPS-M having tumor inhibiting function comprising, preparing powder from natural lacquer liquid by propanone or xylene, lixiviating the powder with leach, removing glucoprotein and enzyme through salting out, centrifugal filtration, desalinizing the filter liquor through ultrafiltration or dialysis, column chromatography, neutralizing the chromatography liquid, concentrating, alcohol deposition, vacuum drying. The polysaccharide obtained has evident biological activity for inhibiting S-180 tumor.
Owner:WUHAN UNIV
Mixed Bed Ion Exchange Adsorber
InactiveUS20170298091A1Good purification resultImprove performanceSemi-permeable membranesMembranesFiberBiological pump
The present invention refers to new species of an ion exchange adsorber which is suitable for the separation of host cell proteins (HCPs), antibody fragments and low molecular weight substances from solutions containing antibodies. The invention especially refers to a process for purifying biological samples by separating biomolecules of interest and impurities, comprising steps of contacting a sample with said chromatography media consisting of fibers, said fibers having imparted thereon functionality enabling ion exchange chromatography and / or hydrophobic interaction.
Owner:MILLIPORE CORP
Fluid distribution unit for a chromatography column
ActiveUS20170173495A1Overcomes drawbackSimple designComponent separationDispersed particle separationChromatography liquidAudio power amplifier
A read circuit for an electrical signal produced by a POSFET device is disclosed which comprises a transconductance amplifier (4) connected at its inverting input to the output of the POSFET device (1), at least one neuron (8a, 8b) connected to the output of said transconductance amplifier, said amplifier being adapted to receive a signal from the POSFET device representative of a force or pressure exerted on it and to produce at its output at least one current signal (I0−, I0+) representative of such force or pressure, the at least one neuron being adapted to receive said current signal and to produce an output signal (12a, 12b) being a pulse train with frequency proportional to said current signal.
Owner:NOVASEP PROCESS SOLUTIONS
Purification method of human immunoglobulin for intravenous injection
InactiveCN108623677AAccurately control pHGood removal effectPeptide preparation methodsImmunoglobulinsPurification methodsChromatography liquid
The invention discloses a purification method of human immunoglobulin for intravenous injection. The purification method is used for purification of a secondary sedimentation ingredient. The purification method of the human immunoglobulin for the intravenous injection is characterized by comprising the following steps of: S1, dissolve: dissolving the secondary sedimentation ingredient with water for injection, and stirring for 2-4h at 2.0-8.0 DEG C to form a dissolve liquid, S2, filtration: filtering the dissolve liquid with a 0.45 micrometers filter membrane and then with a 0.2 micrometers filter membrane to form a filtrate, S3: filtrate adjustment: adjusting a pH (potential of hydrogen) of the filtrate to 5.60-6.00, a protein concentration to 10-13g / L, and conductivity to 0.2-1.90ms / cm to form a pre-chromatography liquid, S4, chromatography: performing chromatography with strong anion exchange gel, flushing the gel before the chromatography for balancing to allow a difference betweena pH of the gel and a pH of the liquid before the chromatography to be from -0.10 to 0.10, performing gel chromatography sample loading at a linear flow rate of 0.5-1.5cm / min and chromatography loading capacity of not exceeding 600g / L, and collecting a liquid after the chromatography. The method is simple and controllable, and greatly reduces a content of IgA (immunoglobulin A) and IgM (immunoglobulin M) in the human immunoglobulin for the intravenous injection.
Owner:HUALAN BIOLOGICAL ENG CHONGQING +1
New SMB Process
ActiveUS20140128627A1High yieldHigh purityFatty oils/acids recovery from wasteSampled-variable control systemsChromatographic separationOrganic solvent
The present invention provides a chromatographic separation process for recovering a polyunsaturated fatty acid (PUFA) product from a feed mixture, which process comprises the steps of: (i) purifying the feed mixture in a first separation step in a simulated or actual moving bed chromatography apparatus having a plurality of linked chromatography columns containing, as eluent, an aqueous organic solvent, to obtain an intermediate product; and (ii) purifying the intermediate product obtained in (i) in a second separation step using a simulated or actual moving bed chromatography apparatus having a plurality of linked chromatography columns containing, as eluent, an aqueous organic solvent, to obtain the PUFA product; wherein (a) the first and second separation steps are carried out sequentially on the same chromatography apparatus, the intermediate product being recovered between the first and second separation steps and the process conditions in the chromatography apparatus being adjusted between the first and second separation steps such that the PUFA product is separated from different components of the feed mixture in each separation step; or (b) the first and second separation steps are carried out on separate first and second chromatography apparatuses respectively, the intermediate product obtained from the first separation step being introduced into the second chromatography apparatus, and the PUFA product being separated from different components of the feed mixture in each separation step.
Owner:BASF PHARMA CALLANISH
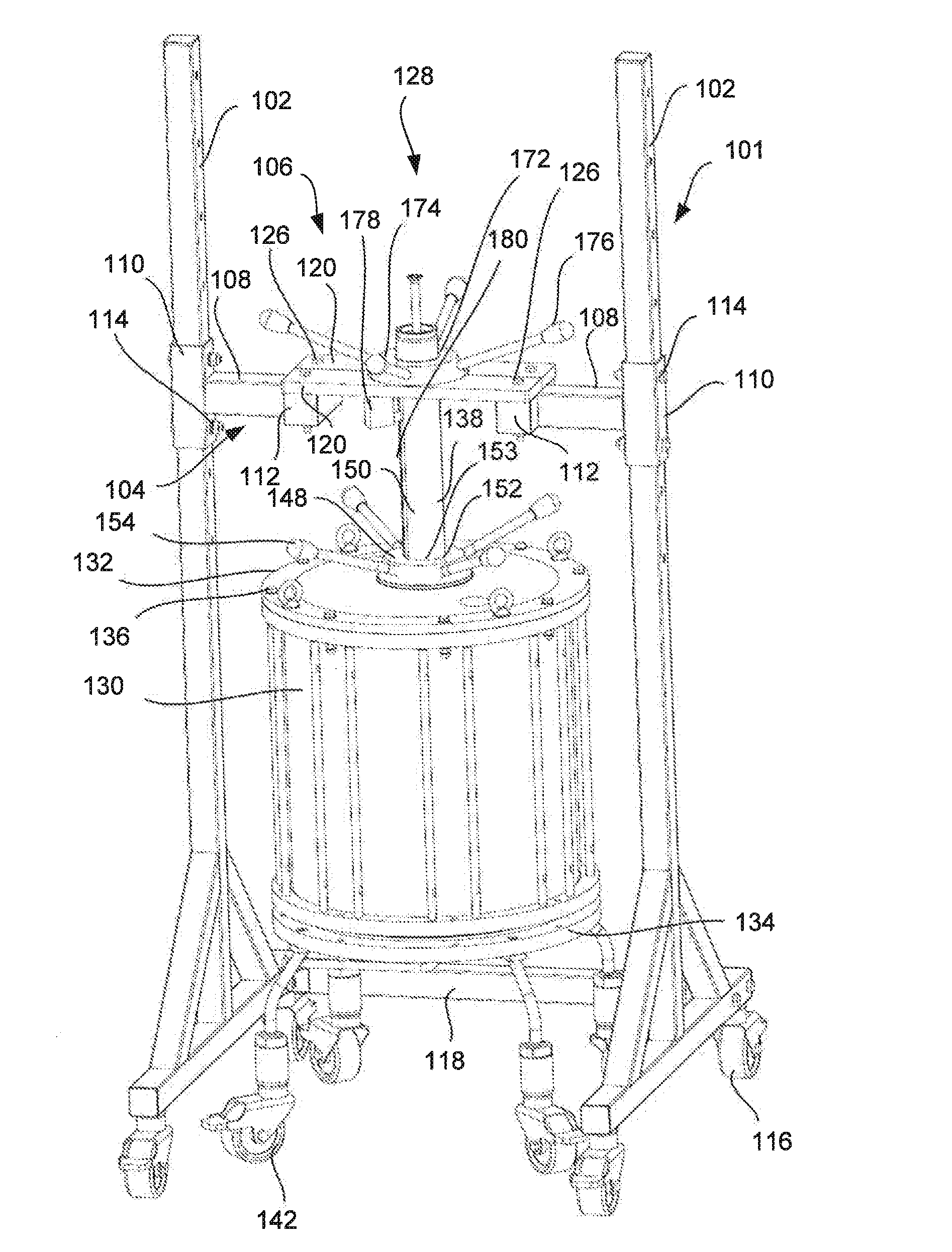
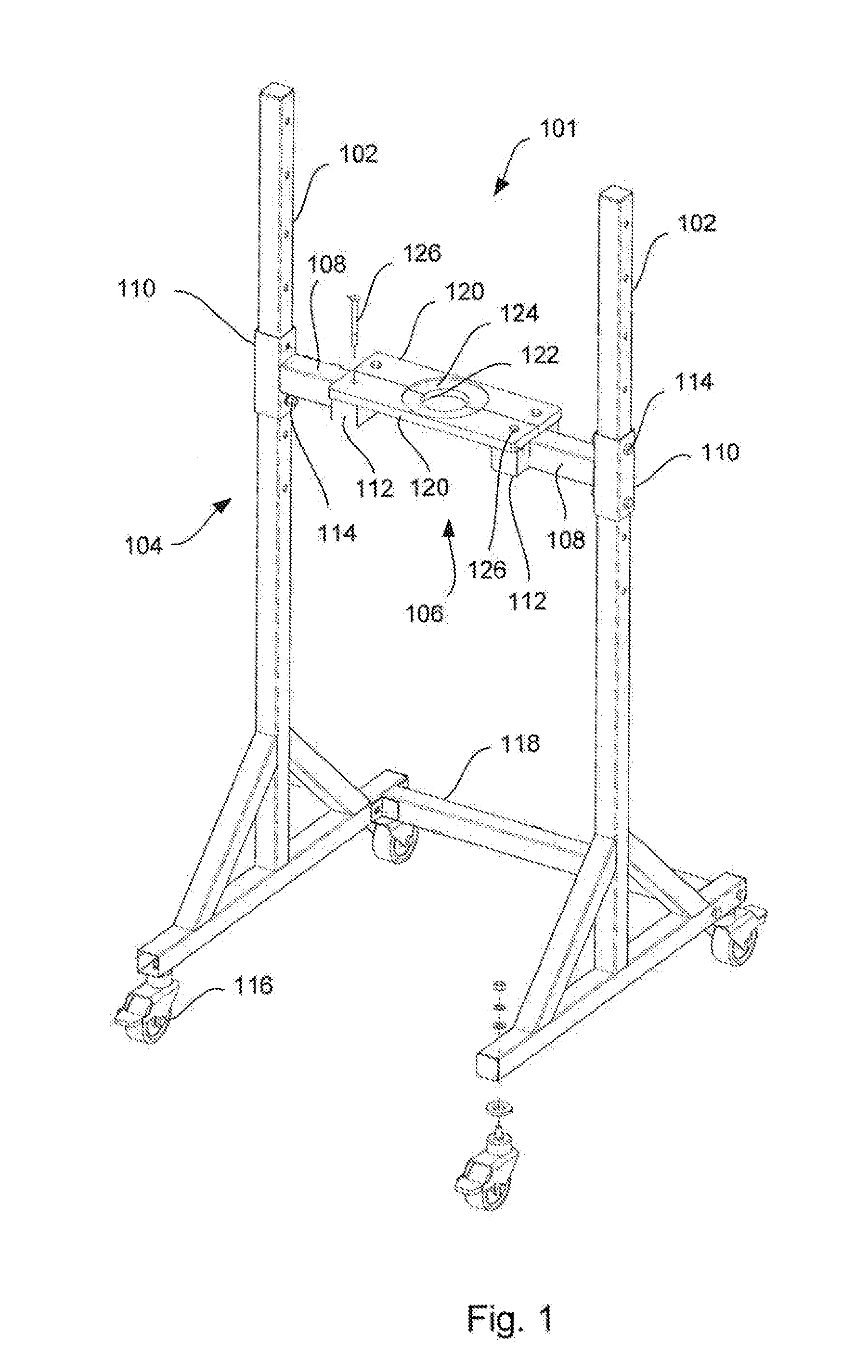
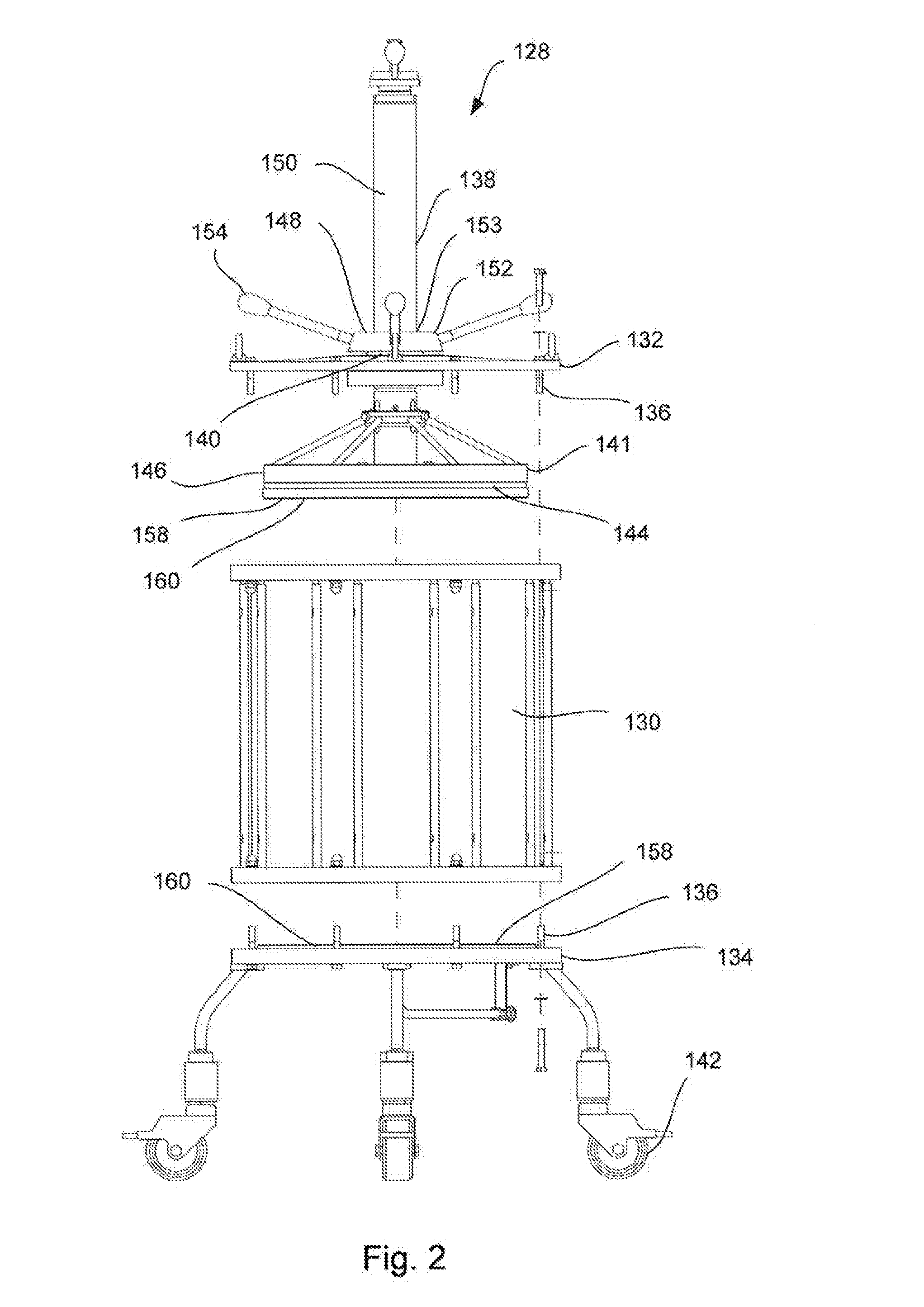
Chromatography column frame and method of conducting maintenance on and packing of a chromatography column
ActiveUS20150198568A1Low costShorten the timeComponent separationSolid sorbent liquid separationChromatography liquidEngineering
A chromatography column frame comprising at least two legs, a support arrangement connected to said at least two legs, and a holder connected to said support arrangement. Said holder is arranged to releasably hold an adaptor rod of a chromatography column, so that the adaptor rod is prevented to move in a horizontal direction and so the adaptor rod is allowed to move in a vertical direction.
Owner:CYTIVA SWEDEN AB
Method for purifying telavancin
ActiveCN107629115AHigh purityStrong hydrophobic interactionPeptide preparation methodsBulk chemical productionChromatography liquidSilanes
The invention provides a method for purifying telavancin. The method comprises the following steps: 1) a reaction mixture contain telavancin is mixed and dissolved by a methanol-formic acid-aqueous solution according to weight ratio being 1: 5 to 1: 10; 2) a filtrate is purified by a chromatography media balanced by an eluate, and the weight ratio of a sample filtrate to the chromatography media is 1: 50 to 1: 100; the chromatography media is octadecyl silane; and 3) the above chromatography liquid is concentrated through a nanofiltration membrane to more than 1 / 10 original volume, a hydrochloric acid aqueous solution with a pH value being 4-5 is supplemented when the chromatography liquid is concentrated to 1 / 5 original volume during the above process, buffer salt is removed after repetition is carried out for 3-5 times, and hydrochloric acid is added for adjusting the pH value to 0.5-2 to obtain a concentrate. The selected chromatography media octadecyl silane combines a proper acidsolution or a salt solvent system for separating the high-purity hydrochloric acid telavancin during a separating process of a telavancin compound due to tight performance effect with a material medium.
Owner:FUJIAN INST OF MICROBIOLOGY
Separation purification method for natural abscisic acid
InactiveCN105439847ASimple methodExtracted abscisic acid with high purityCarboxylic compound separation/purificationChromatography liquidUltrafiltration
A separation purification method for natural abscisic acid belongs to the field of biological fermentation and production of biochemical pesticides. The method comprises performing plate-frame filtration on an abscisic acid fermentation liquid to obtain a plate-frame filtrate, then processing the filtrate through an ultrafiltration membrane and a condensation membrane, so as to obtain a concentrate, acidifying the condensate, then performing extraction, pretreating the extraction liquid, then adding an eluent, adsorbing impurities through adsorption column chromatography, performing reduced-pressure concentration on the chromatography liquid, adding a non-polar solvent into the concentrate, filtering and drying, so as to obtain the abscisic acid product. The method is simple and convenient, abscisic acid extracted from the fermentation liquid is high in purity and high in yield, and product residue in the mother liquid is less.
Owner:江西新瑞丰生化股份有限公司
Method for recovering 16-dehydropregnenolone acetate from 16-dehydropregnenolone acetate mother liquor
InactiveCN103554208AApplication utilization rate is highLow priceSteroidsDehydropregnenolone acetateChromatography liquid
The invention relates to a method for recovering 16-dehydropregnenolone acetate from a 16-dehydropregnenolone acetate mother liquor, wherein the method is intended to provide the method with advantages of simple technology, high recovery rate and good product quality. The method for recovering 16-dehydropregnenolone acetate from the 16-dehydropregnenolone acetate mother liquor comprises the following steps of: mother liquor condensation, solvent extraction, chromatography to remove impurities, condensation of a chromatography liquid, refining, crystallization, centrifugation and drying, to obtain 16-dehydropregnenolone acetate. The method for recovering 16-dehydropregnenolone acetate from the 16-dehydropregnenolone acetate mother liquor can make an overall yield of the 16-dehydropregnenolone acetate reach 72%, raising 4-7% compared with a traditional technology; a used reagent belongs to a common chemical product, and has advantages of easy availability, low cost, simple technology operation and convenient industrialization; and the method is used for recovering the 16-dehydropregnenolone acetate from the 16-dehydropregnenolone acetate mother liquor, wherein the purity of the 16-dehydropregnenolone acetate obtained after refining is larger than 99%, the obtained 16-dehydropregnenolone acetate can be directly used as a product, and a secondary mother liquor can be reused into the 16-dehydropregnenolone acetate production.
Owner:XIANGXI HUALI PHARMA
Preparation method of ginkgo fruit protein peptide capable of reducing blood pressure
ActiveCN110183516AStrong inhibitory activitySimple preparation processPeptide preparation methodsFermentationChromatographic separationChromatography liquid
The invention discloses a preparation method of ginkgo fruit protein peptide capable of reducing blood pressure. The preparation method comprises the following specific steps of performing shell removal on fresh ginkgo fruits, performing crushing treatment, and performing extraction to obtain crude protein; performing enzymolysis on the crude protein through proteases; performing ultrafiltration on enzymatic hydrolysate; performing dextran gel chromatography on ultrafiltration permeation substances, and performing liquid-phase chromatographic separation on chromatography liquid, so as to obtain the ginkgo protein peptide having the potential of reducing blood pressure, wherein the amino acid sequence of the ginkgo protein peptide is threonine-asparagine-leucine-aspartic acid-tryptophan-tyrosine (TNLDWY). Small peptide prepared through the preparation method is high in angiotensin converting enzyme (ACE) restraining activity, cysteine residues do not exist in the amino acid sequence, anadditive for protecting bioactive peptide does not need to be added, and the ginkgo fruit protein peptide is simple in preparation technology, low in cost, high in product quality and suitable for industrialized production.
Owner:HEFEI UNIV OF TECH
Detection method of new psychoactive substance MDBZP
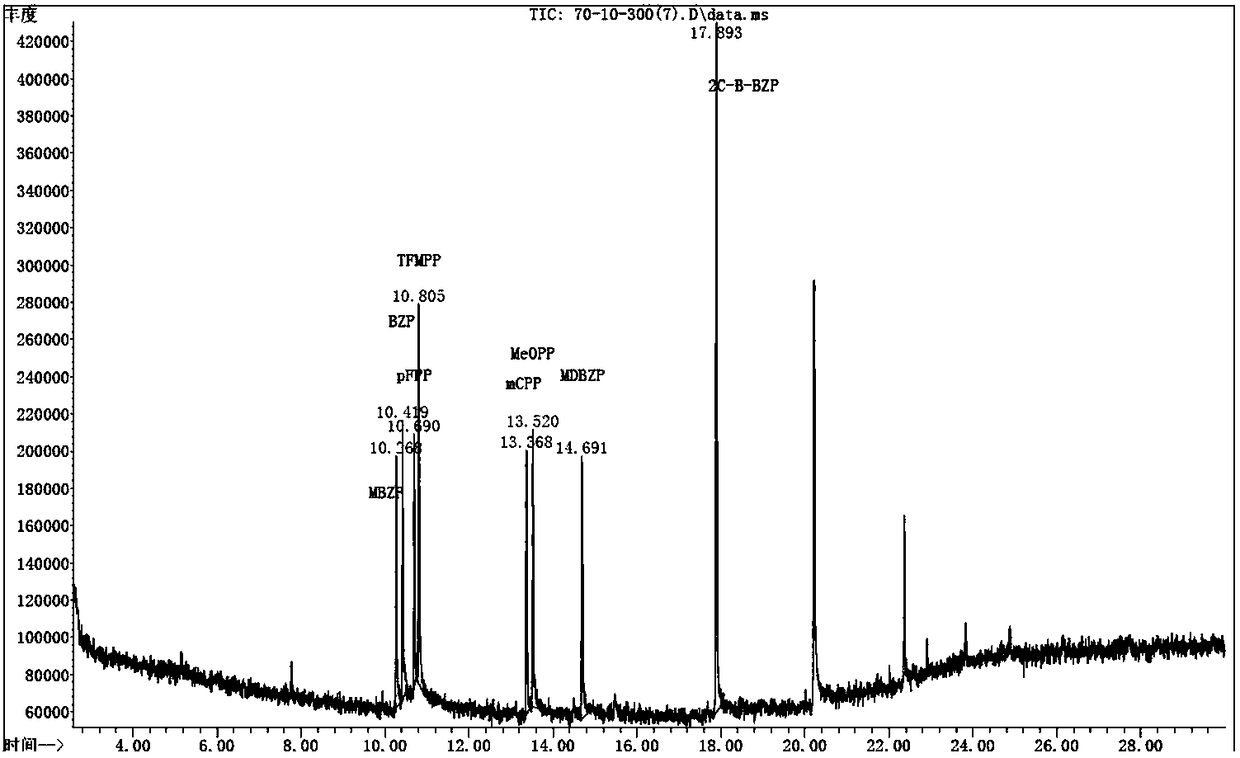
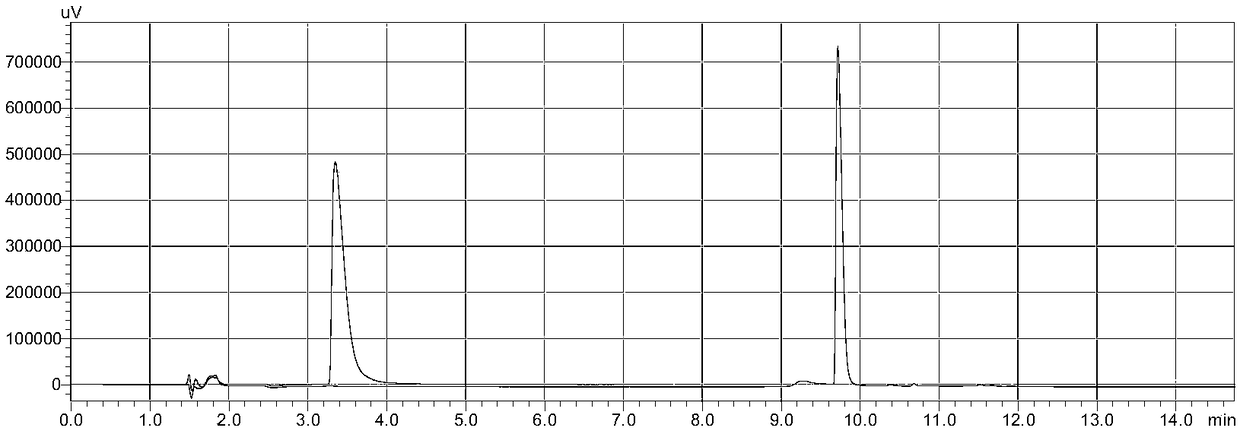
PendingCN108535380AEfficient extractionFully extractedComponent separationPsychoactive substanceGas chromatography–mass spectrometry
The invention discloses a detection method of a new psychoactive substance MDBZP. The method, provided by the invention, for accurately and efficiently determining the MDBZP in biological samples comprises qualitative and quantitative analysis methods including gas chromatography-mass spectrometry, gas chromatography, liquid chromatography, liquid chromatography-mass spectrometry, and the like; the biological samples can be at least one of blood, urine, saliva and hair. Due to the large number of interfering factors in the biological samples, the pretreatment process of the tested materials isvery important. By means of a great number of experiments, the detection method effectively and fully extracts MDBZP in the biological samples by using a solid phase extraction process; the high-efficiency qualitative and quantitative analysis is realized; the analysis methods are high in sensitivity and wide in linear range. A detector is low in cost and beneficial to automatic analysis operation.
Owner:INST OF FORENSIC SCI OF MIN OF PUBLIC SECURITY
Separation and purification method of pneumocandin B0 serine analog
PendingCN110655557AGood effectReduce adverse reactionsPeptide preparation methodsChromatography liquidIsocratic elution
The invention relates to a separation and purification method of a pneumocandin B0 serine analog. The separation and purification method comprises the following steps of (a) performing chromatographyenrichment for the first time through polymer substrate chromatogram fillings, performing isocratic elution, and collecting parts of which the purity is greater than 30%; (b) performing chromatographyenrichment for the second time through chromatogram fillings having hydrophilic effects, performing isocratic elution, and collecting parts of which the purity is greater than 60%; (c) performing chromatography enrichment for the third time through antiphase silica gel substrate chromatogram fillings, performing isocratic elution, and collecting parts of which the purity is greater than 80%; and(d) after purifying for the third time, performing decompressed concentration on chromatography liquid of which the pneumocandin B0 serine analog purity is greater than 80%, removing a solvent, and performing freeze-drying so as to obtain the pneumocandin B0 serine analog. Through the adoption of the preparation method disclosed by the invention, the pneumocandin B0 serine analog of which the purity is greater than 80% is subjected to structure identification, and a base is established for further performing biological activity screening or performing biological activity screening after structure modification.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
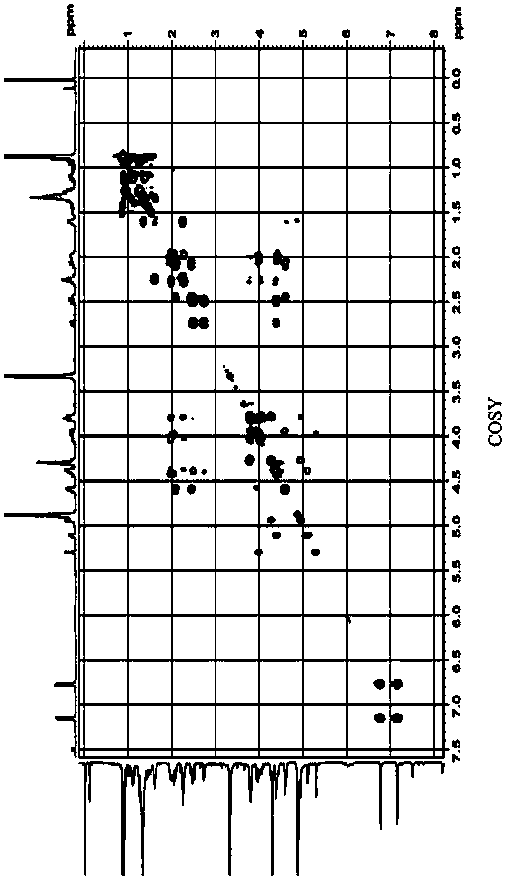
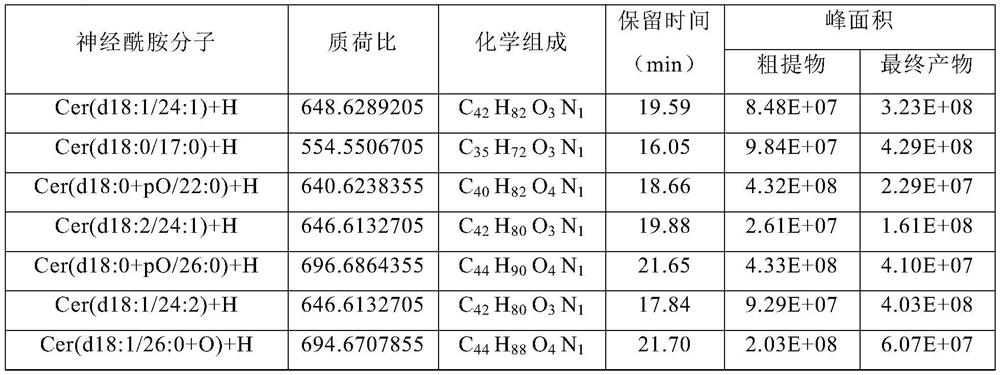
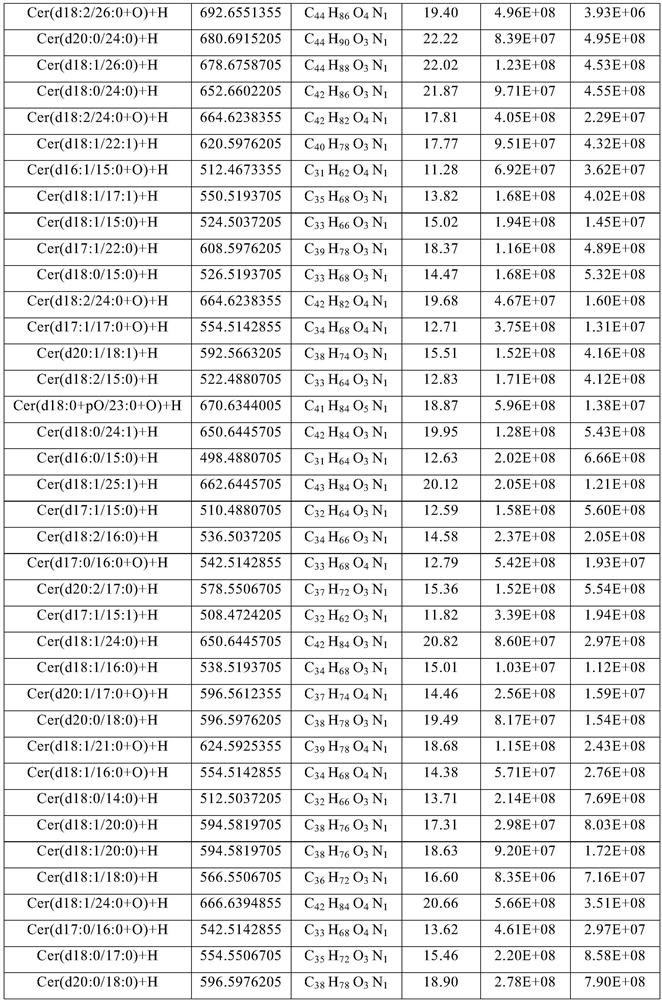
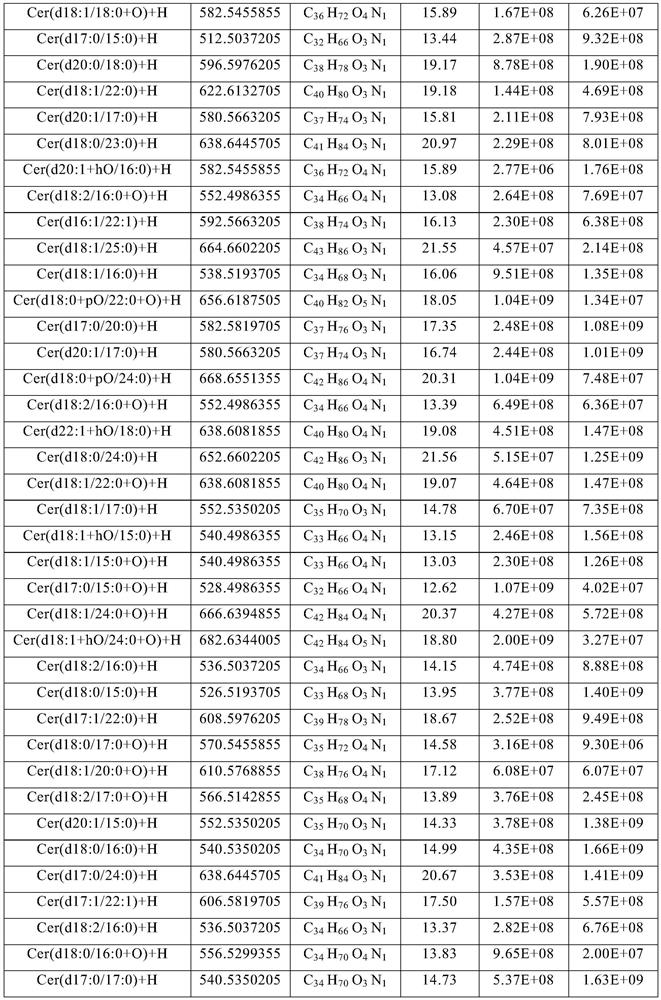
Method for extracting ceramide from urban sludge
ActiveCN111977918AAvoid pollutionExpand access channelsSludge treatmentOrganic compound preparationChromatography liquidSludge
The invention provides a method for extracting ceramide from urban sludge. The method comprises the following steps: (1) conducting extraction treatment of the urban sludge, and sequentially filteringand concentrating the extracting solution to obtain a crude extract; and (2) conducting silica gel column chromatography purification treatment of the crude extract, collecting the chromatography liquid containing the ceramide, and concentrating the chromatography liquid to obtain the ceramide. In the method, the extraction solvent in step (1) and the eluent in step (2) are a mixed solution of ethanol and n-hexane. Ceramide can be extracted from urban sludge by the method, a new path of resource utilization of urban sludge is opened up, and higher economic benefits of urban sludge can be produced.
Owner:RENMIN UNIVERSITY OF CHINA
Packing system and method for chromatography columns
ActiveUS9597610B2Improve bed stabilityHigh column efficiencyIon-exchange process apparatusComponent separationChromatography liquidChromatography column
The invention relates to a system for packing chromatography columns with a chromatography medium and packing method for use in such columns. In particular, the invention relates to a method and system for packing chromatography columns where the column and chromatography media are pre-sterilized.
Owner:CYTIVA BIOPROCESS R&D AB
Liquid chromatography high-pressure resistant gradient valve and liquid chromatography liquid inlet system
ActiveCN109444310ANot easy to produceEasy liquid controlComponent separationChromatography liquidEngineering
The invention discloses a liquid chromatography high-pressure resistant gradient valve and a liquid chromatography liquid inlet system. The liquid chromatography high-pressure resistant gradient valvecomprises a valve body and at least two high-pressure valves, wherein a mixing outlet and at least two interfaces are formed in the valve body, and each the interface is communicated with the mixingoutlet; each the interface is respectively connected with one of the high-pressure valves, each the high-pressure valve comprises an outer shell, a valve core, an elastic holding piece and an electriccontrol driving piece, a liquid inlet cavity, a liquid outlet cavity and a communication cavity which are sequentially communicated are formed in each the outer shell, a liquid inlet is formed in each the liquid inlet cavity, a liquid outlet is formed in each the liquid outlet cavity, and each the liquid outlet is connected with each the interface; the valve cores are arranged in the liquid outlet cavities in a movable manner; the elastic holding pieces are arranged in the liquid outlet cavities, and the elastic holding pieces are abutted against the valve cores to block the valve cores at the communication cavities; and the electric control driving pieces are arranged on the outer shells and are matched with the valve cores to drive the valve cores to move, and the electric control driving pieces drive the valve cores to open the communication cavities when receiving liquid inlet information. The liquid chromatography high-pressure resistant gradient valve has the characteristics ofhigh pressure resistance and gradient accuracy.
Owner:ANHUI WAYEE SCI & TECH CO LTD
Preparation method of high-purity daptomycin lactone hydrolysate
InactiveCN106866791ASimple methodLow costPeptide preparation methodsChromatography liquidHydrolysate
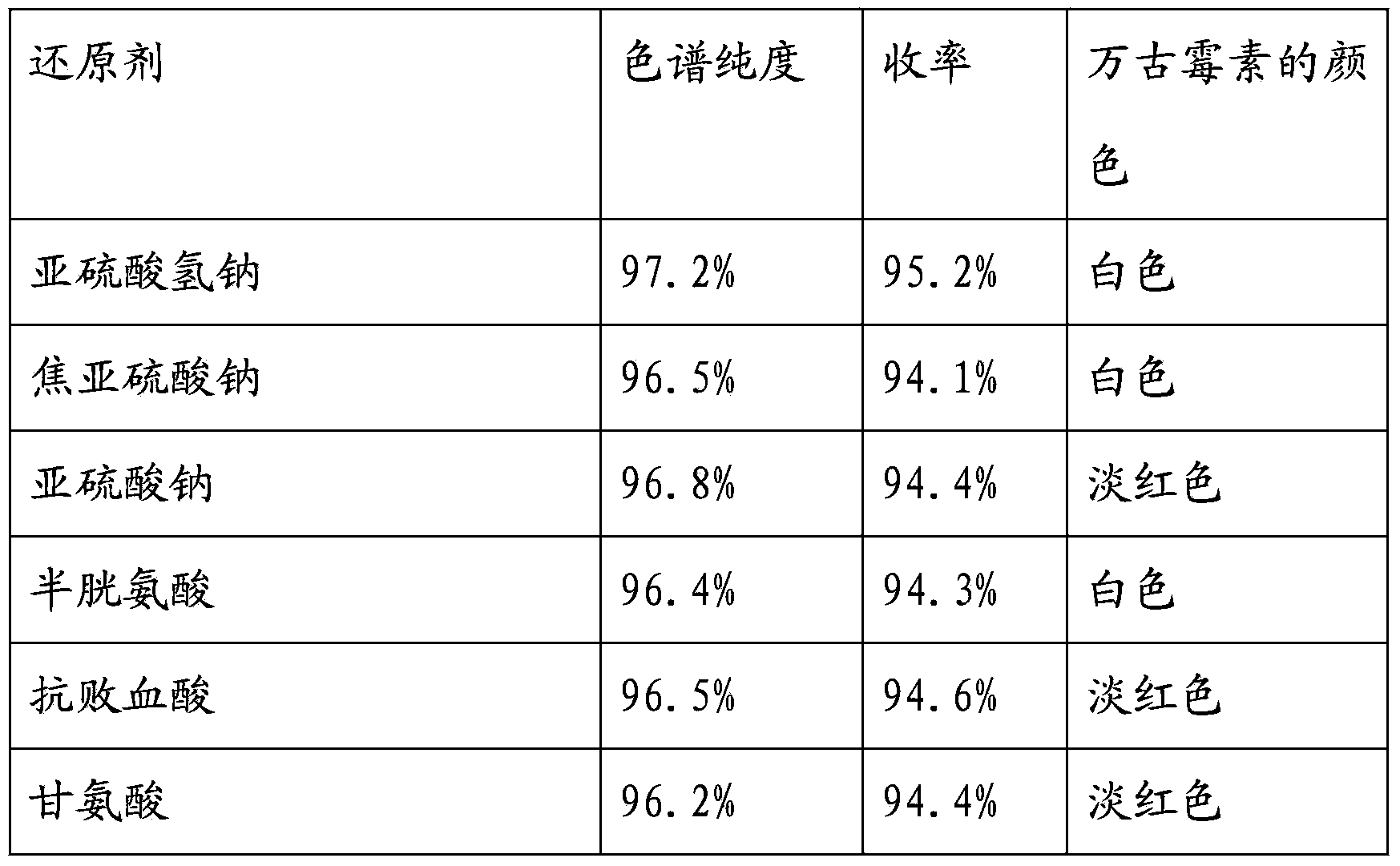
The invention discloses a preparation method of high-purity daptomycin lactone hydrolysate; according to the method, a daptomycin resin chromatography liquid has the pH adjusted and is converted into a liquid containing a relatively-high-purity daptomycin lactone hydrolysate, then by preparative chromatography, the high-purity daptomycin lactone hydrolysate with the chromatographic purity of more than 96% is obtained. The method is simple and easy to operate and low in cost.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
Preparation of high-purity vancomycin hydrochloride
ActiveCN101440127BEffective components increaseHigh purityPeptide preparation methodsVancomycin HydrochlorideChromatography liquid
The invention relates to an industrial feasible method for preparing a high-purity vancomycin hydrochloride. The method comprises the following steps of (1) putting a crude vancomycin with the content of vancomycin not less than 80% in a dextran resin glass chromatography column containing the NH4HCO3 mobile phase for column chromatography to obtain an effective chromatography liquid; (2) adding NaCl water solution into the effective chromatography liquid and stirring to produce a deposit; (4) top cleaning by 90% ethanol water solution for a few times and drying to obtain the vancomycin hydrochloride. Effective components of the vancomycin hydrochloride obtained through the method are improved greatly, other impurities are reduced greatly, and the purity is very high; meanwhile, the overall color of the product is improved remarkably, and the product is suitable for oral or injection administration.
Owner:ZHEJIANG NOVUS PHARMA CO LTD
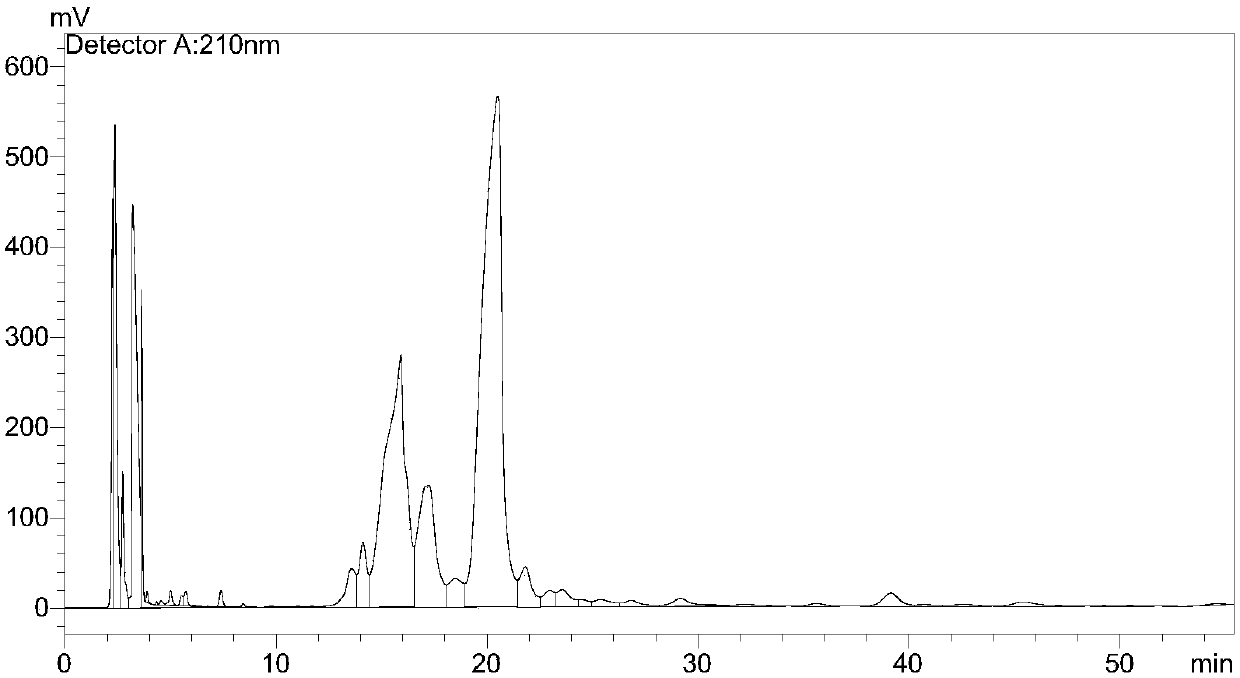
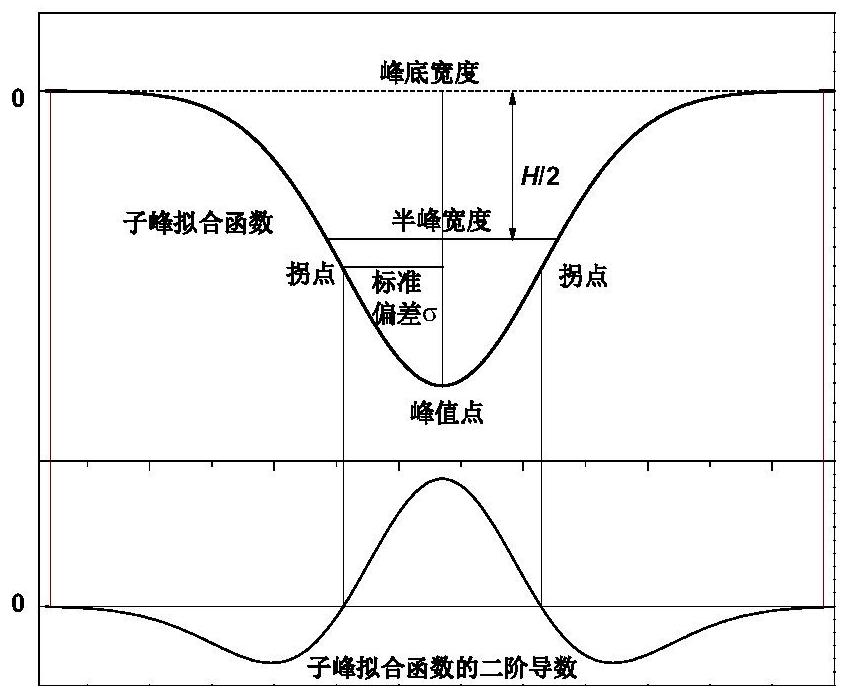
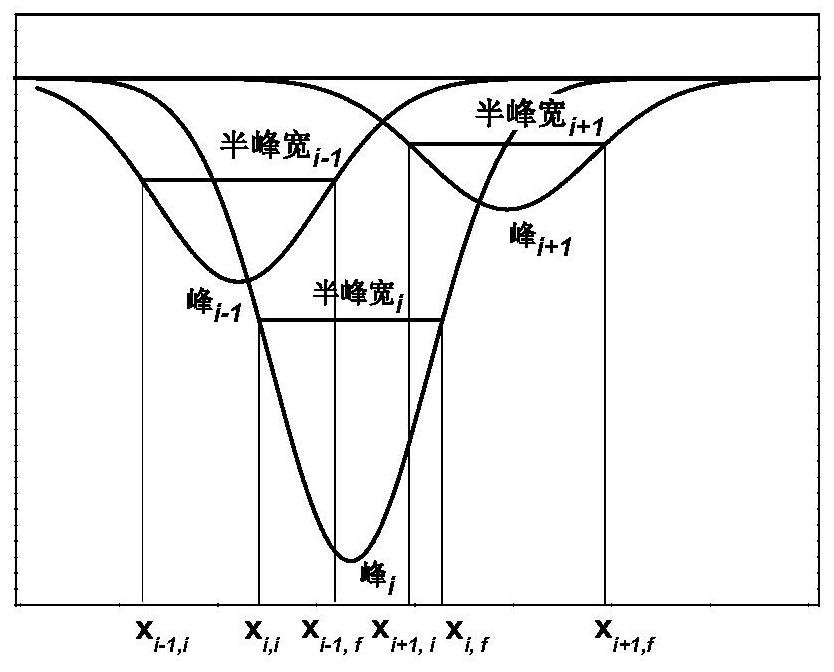
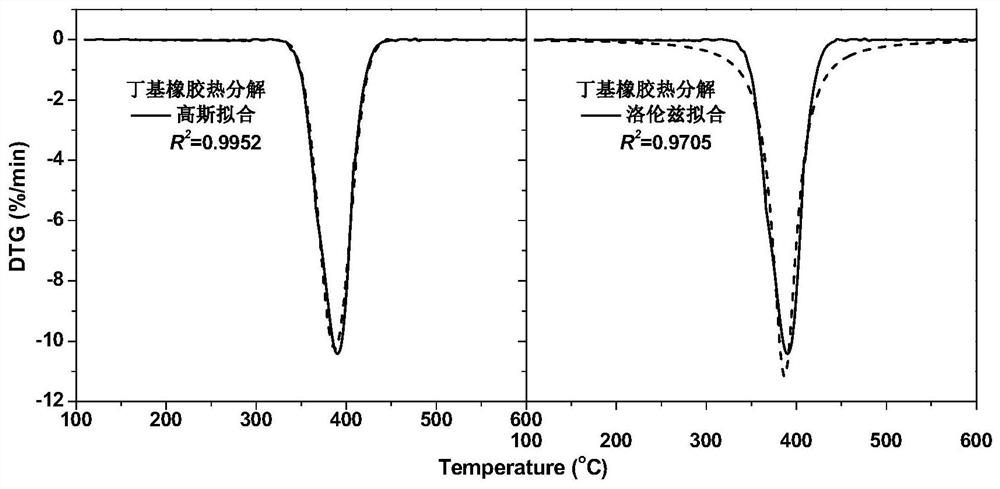
Method for peak-splitting decoupling of chemical reaction process or chemical structure of substance
PendingCN111707574AWeighing by removing componentMaterial analysis using wave/particle radiationChemical structureChromatography liquid
The invention discloses a method for peak-splitting decoupling of a chemical reaction process or a chemical structure of a substance. According to the method, a peak division function is selected according to the regular characteristics of a chemical reaction or the chemical structure characteristics of substances, peak division fitting limiting conditions are set, and the number of sub-peaks is judged jointly according to the fitting degree R2 of a peak division result and the overlapping degree between the sub-peaks. The method provided by the invention is suitable for analyzing overlapped complex chemical reaction processes or complex substance structures. The method has the characteristics of simplicity and feasibility, and can be used for analyzing various curves or maps including thermogravimetry, mass spectrometry, nuclear magnetic resonance, paramagnetic resonance, infrared spectroscopy, Raman spectroscopy, X-ray photoelectron spectroscopy, X-ray diffraction, gas chromatography, liquid chromatography and the like.
Owner:BEIJING UNIV OF CHEM TECH
Method for purifying loropetalum chinense flower red pigment by using aluminum oxide-calcium carbonate composite adsorbent
InactiveCN104448918AEasy to separateLow priceOther chemical processesChemical industryChromatography liquidPollen
The invention discloses a method for purifying loropetalum chinense flower red pigment by using an aluminum oxide-calcium carbonate composite adsorbent. The method comprises the following steps: 1) preparing a loropetalum chinense flower red pigment concentrated solution, namely, firstly, extracting a loropetalum chinense flower red pigment extract from loropetalum chinense pollen powder, and further concentrating the loropetalum chinense flower red pigment extract, thereby obtaining the loropetalum chinense flower red pigment concentrated solution; and 2) purifying the loropetalum chinense flower red pigment, namely, by taking aluminum oxide and calcium carbonate as a composite adsorbent, feeding the loropetalum chinense flower red pigment concentrated solution onto a column for adsorption, and eluting by using an eluent, thereby obtaining the a purified loropetalum chinense flower red pigment chromatography liquid. As activated neutral aluminum oxide and calcium carbonate with the mass ratio of 2:1 are adopted as the composite adsorbent, the separation effect on loropetalum chinense flower red pigment is good; the price is low, and the production period is short, and meanwhile the production cost is low, the production efficiency is high and the equipment investment is small as no complex equipment is needed.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
Split type immunochromatographic detection device and immunochromatographic detection method
PendingCN112305217AHigh sensitivityImprove accuracyMaterial analysisChromatography liquidMechanical engineering
The invention provides a split type immunochromatography detection device and an immunochromatography detection method. The split type immunochromatography detection device comprises a first part anda second part. The first part comprises a first shell and a sample pad which is arranged in the first shell, one part of the sample pad is exposed out of the first shell, the first shell is provided with a first liquid adding part, and the first liquid adding part is at least used for adding chromatographic liquid. The second part comprises a second shell and a test strip arranged in the second shell. The first part is independent of the second part and can be detachably connected with the second part; when the first part is connected with the second part, the split type immunochromatography detection device can absorb chromatography liquid through the first liquid adding part, and the sample pad is in contact with the test strip. According to the device and the method disclosed in the scheme, the functions of the sample pad are utilized to the maximum extent, the steps of the sample pretreatment process and used consumables are simplified, and the sensitivity and accuracy of immunochromatographic detection are improved.
Owner:SHENZHEN BIOEASY BIOTECHNOLOGY CO LTD
Immunochromatographic analysis method
ActiveUS20170023559A1Avoid it happening againHigh sensitivitySolid sorbent liquid separationMaterial analysisGlass fiberAnalyte
An object is to provide an immunochromatographic analysis method capable of shortening the developing time without decreasing the detection sensitivity, and also capable of reducing the return of the liquid of a developed component, and a method for detecting a detection target contained in an analyte using an immunochromatographic analysis device including an absorption part composed of glass fiber, wherein the analyte and a labeling substance are developed in a chromatography medium part as a mobile phase in the presence of a nonionic surfactant, and the detection target is detected in a detection part is provided.
Owner:TANAKA PRECIOUS METAL IND
A method for extracting β-thymidine from fermentation broth
InactiveCN105859809BHigh extraction yieldQuality assuranceSugar derivativesSugar derivatives preparationChromatography liquidEconomic benefits
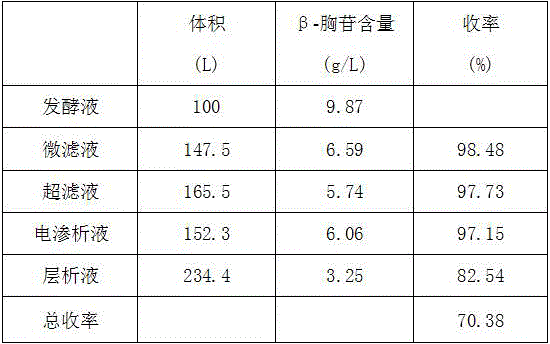
The invention discloses a method for extracting beta-thymidine from fermentation liquid. The method comprises the following steps: (1) preheating the fermentation liquid; (2) performing microfiltration on the fermentation liquid; (3) performing ultrafiltration on the microfiltration liquid; (4) performing electrodialysis on the ultrafiltration liquid; (5) concentrating the electrodialysis liquid; (6) performing chromatography on the concentrated liquid; and (7) concentrating and crystallizing the chromatography liquid. According to the method, a membrane separation technique, an electrodialysis technique and a chromatography separation technique are implemented in the extraction process of the beta-thymidine fermentation liquid, so that a good impurity removal effect can be achieved, and the process is simple, convenient and environment-friendly and high in automation degree. By adopting the extraction process disclosed by the invention, the purity of the liquid phase of a beta-thymidine product is greater than or equal to 98.5%, the external standard content is greater than or equal to 98.5, the total yield is greater than or equal to 70%, industrial application of extracting beta-thymidine from the fermentation liquid is achieved, and good economic benefits and industrial popularization prospects can be achieved.
Owner:NANCHANG UNIV
Two-step resin method for preparing salidrose
InactiveCN1880326BSimple processSuitable for industrial productionSugar derivativesSugar derivatives preparationChromatography liquidFiltration
This invention relates to a method for separating and preparing high-purity rhodiola root glycosides from natural rhodiola root crude extract using two-step resin method, comprising: dissolve the rhodiola root crude extract in the 20-90Def C water with stirring followed by filtration, adjust the filtrate pH to 1-5 then load on the absorption resin column at room temperature, elute with organic solvent like acetone, low-carbon alcohol or acetate, concentrate the eluent, and load on the chromatography column at room temperature, concentrate and evaporate the chromatography liquid to dryness, then add in organic solvent like low-carbon alcohol or acetate and heat to re-dissolve, filtrate and concentrate the filtrate to precipitate the crystals, then obtain the high-purity rhodiola root glycosides after filtration. By using one resin absorption and one resin chromatography, this invention can prepare high-purity rhodiola root glycosides with 90%-98% purity from low-content(1%-5%) natural rhodiola root crude extract, and the absorption and chromatography process is done at room temperature. Besides, the process is easy and qualified for industrial production.
Owner:常州朗诣节能技术有限公司
Liquid chromatography high pressure gradient valve and liquid chromatography inlet system
ActiveCN109444310BExacerbated by negative pressureImprove the mixing effectComponent separationChromatography liquidElectric control
The invention discloses a liquid chromatography high pressure resistant gradient valve and a liquid chromatography liquid inlet system, wherein the liquid chromatography high pressure resistant gradient valve comprises: a valve body and at least two high pressure valves; the valve body is provided with a mixing outlet and at least two Each interface is connected to the mixing outlet; each interface is connected to a high-pressure valve, and the high-pressure valve includes: a shell, a valve core, an elastic retainer, and an electric control drive. The liquid inlet chamber has a liquid inlet, the liquid outlet chamber has a liquid outlet, and the liquid outlet is connected to the interface; the valve core is movably arranged in the liquid outlet chamber; the elastic holder is arranged in the liquid outlet chamber, The elastic retainer stops against the spool so that the spool is blocked in the communication cavity; the electric control drive is arranged on the casing and cooperates with the spool to drive the spool to move, and the electric control drive drives the spool when receiving the liquid inlet information. The spool opens the communication cavity. The liquid chromatography high pressure resistant gradient valve of the invention has the characteristics of high pressure resistance and gradient accuracy.
Owner:ANHUI WAYEE SCI & TECH CO LTD
Furopyridine compound as well as preparation method and application thereof
PendingCN114539271AImprove efficacyOrganic active ingredientsOrganic chemistryFuranChromatographic separation
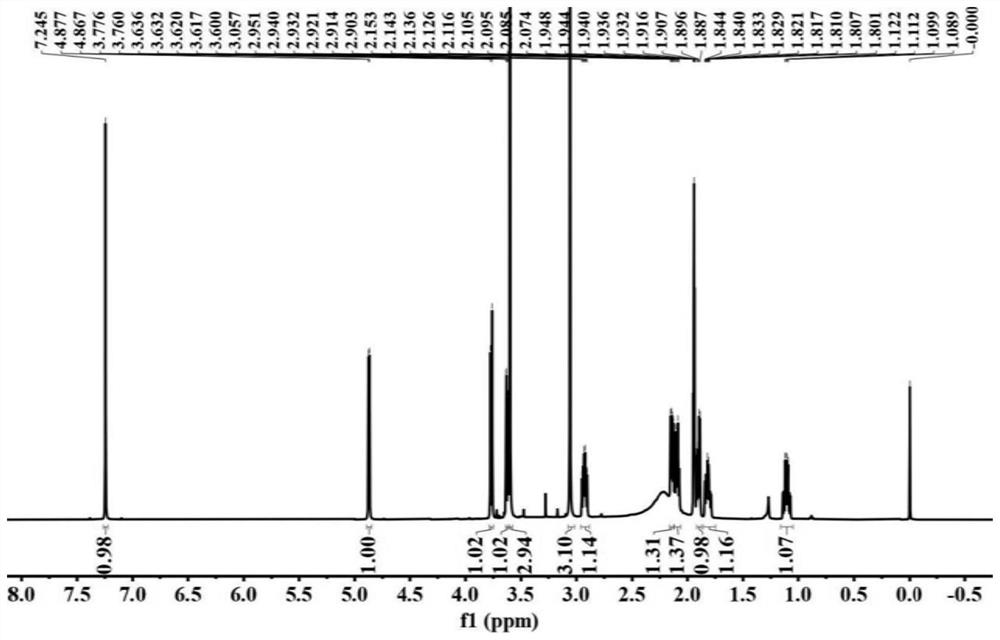
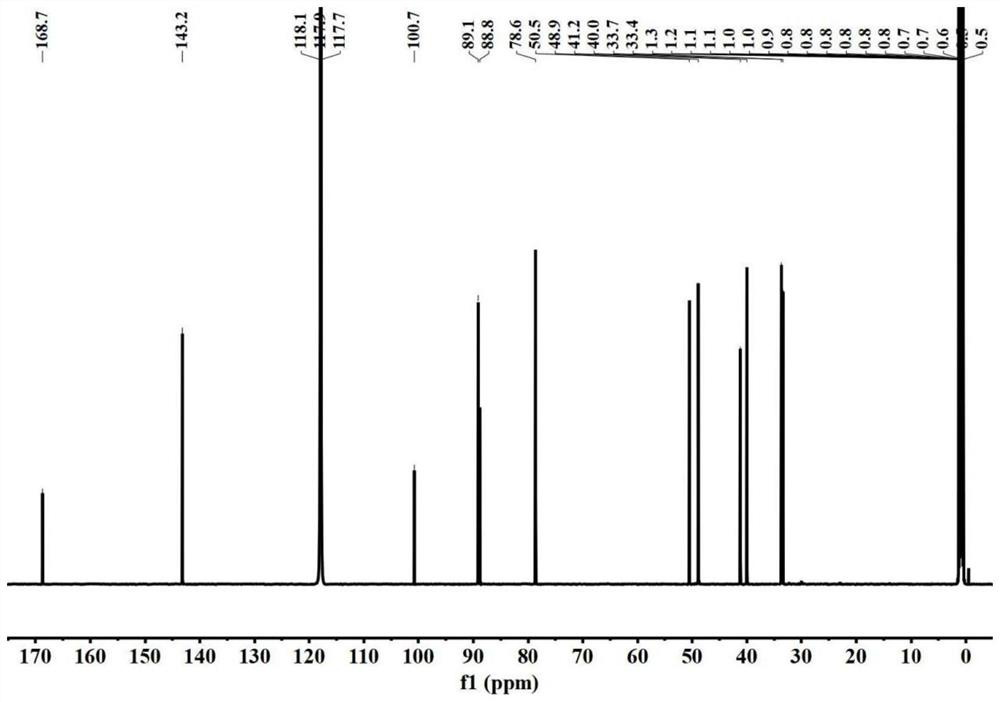
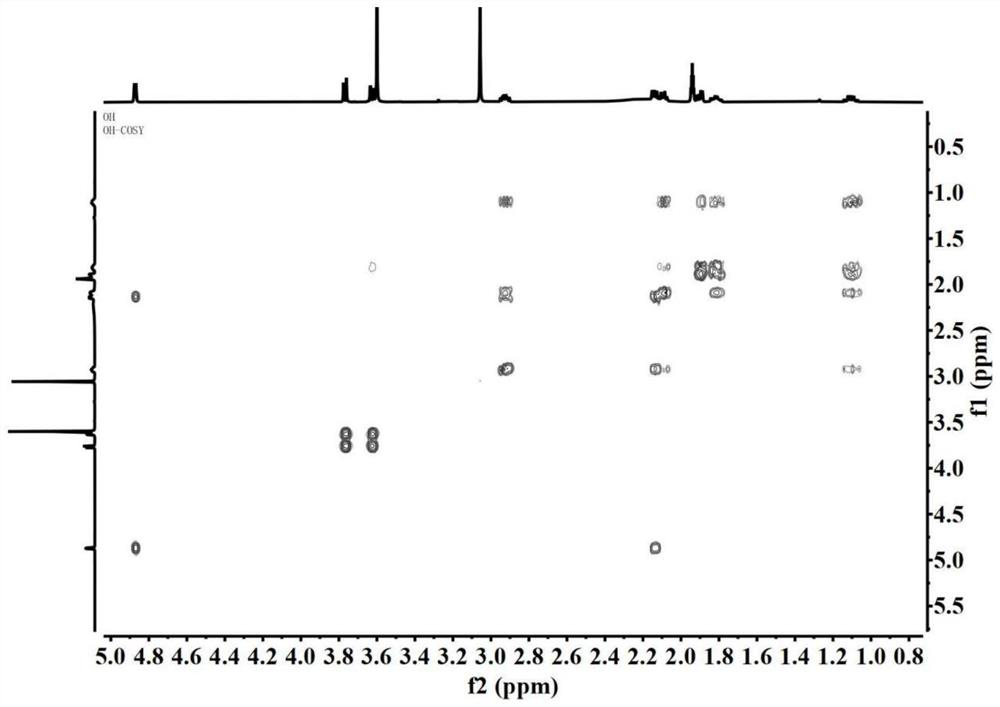
The invention belongs to the technical field of chemical medicines, and particularly relates to a furo-pyridine compound as well as a preparation method and application thereof. The furo-pyridine compound is obtained by taking genipin and methylamine as raw materials, reacting under specific conditions and then performing column chromatography and liquid chromatography separation. Experiments prove that the compound has a remarkable inhibiting effect on proliferation of colon cancer cells and can be used for preparing anti-tumor drugs.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com