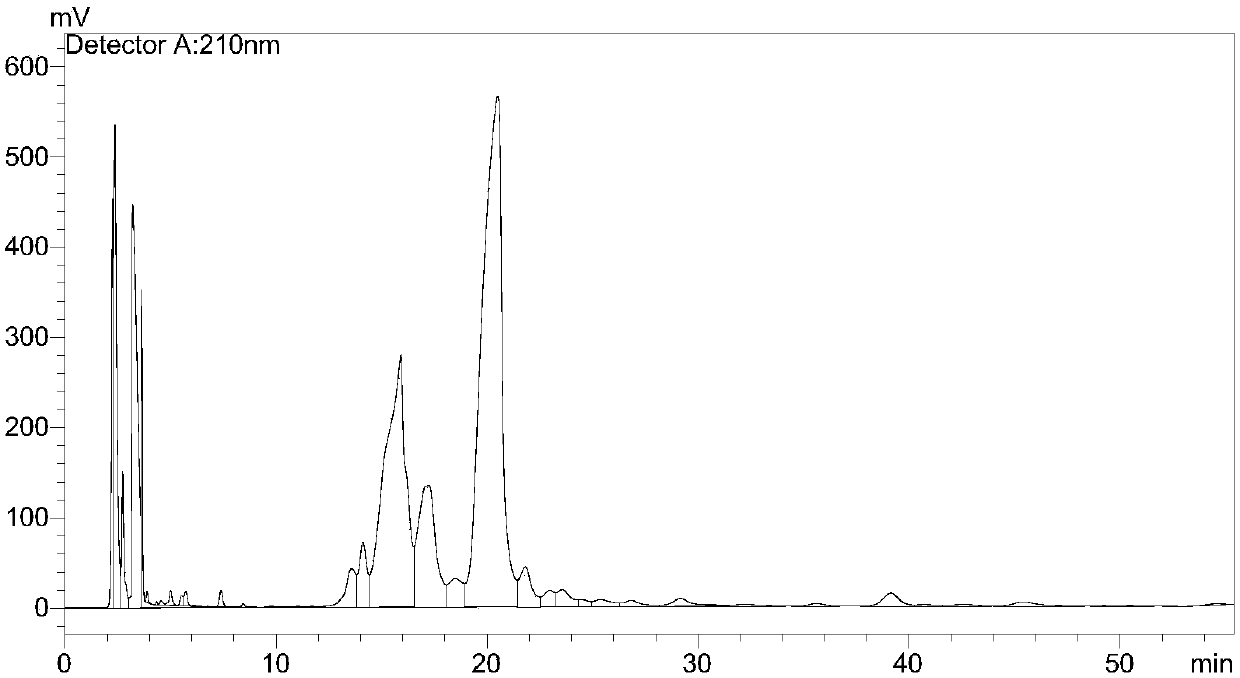
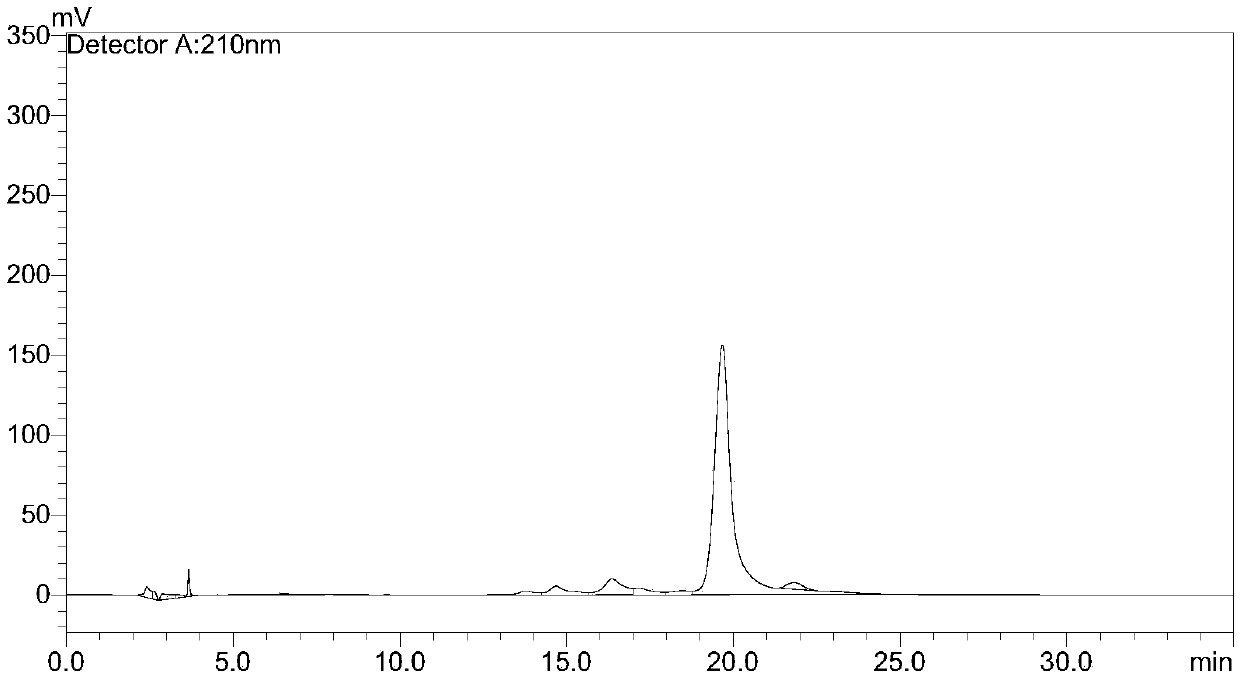
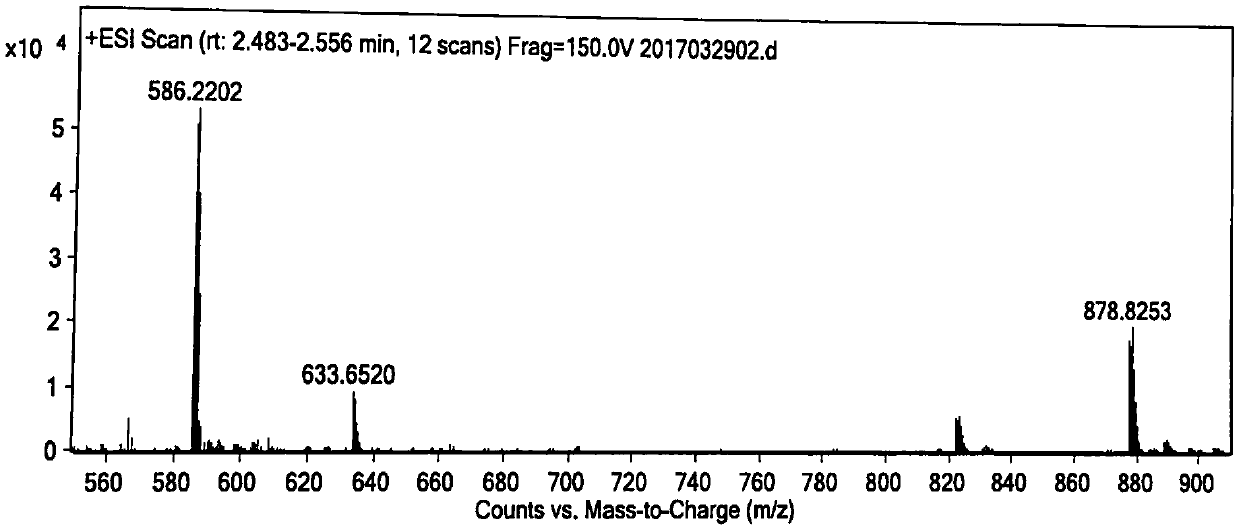
Method for purifying telavancin
A technology of telavancin and purification method, which can be used in the preparation methods of peptides, chemical instruments and methods, organic chemistry, etc., and can solve problems such as insufficient purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A method for purifying telavancin, comprising the steps of:
[0037] 1) Mix the reaction mixture containing telavancin with methanol-formic acid-water solution in a weight ratio of 1:5, dissolve the reaction mixture, filter out solid insolubles, and obtain the filtrate; the above-mentioned methanol-formic acid-water solution is in a volume ratio of 2: 1:1 configuration; the reaction mixture of telavancin is a reductive amination reaction between vancomycin hydrochloride and N-(9-fluorenylmethoxycarbonyl)-decylaminoacetaldehyde, and then removes 9- Telavancin crude product synthesized by Mannich reaction with fluorenylmethoxycarbonyl protecting group and aminomethylphosphonic acid;
[0038] 2) Pass the filtrate through the well-balanced chromatographic medium of the eluent, the weight ratio of the loading filtrate to the chromatographic medium is 1:50; the chromatographic medium is octadecyl bonded silica gel; the eluent is 3 column volumes The solvent A-phosphate buffer ...
Embodiment 2
[0041] A method for purifying telavancin, comprising the steps of:
[0042] 1) Mix the reaction mixture containing telavancin with methanol-formic acid-water solution in a weight ratio of 1:10, dissolve the reaction mixture, filter out solid insolubles, and obtain the filtrate; the above-mentioned methanol-formic acid-water solution is in a volume ratio of 2: 1:1 configuration; the reaction mixture of telavancin is a reductive amination reaction between vancomycin hydrochloride and N-(9-fluorenylmethoxycarbonyl)-decylaminoacetaldehyde, and then removes 9- Telavancin crude product synthesized by Mannich reaction with fluorenylmethoxycarbonyl protecting group and aminomethylphosphonic acid;
[0043] 2) Pass the filtrate through the well-balanced chromatographic medium of the eluent, the weight ratio of the loading filtrate to the chromatographic medium is 1:100; the chromatographic medium is octadecyl bonded silica gel; the eluent is 5 column volumes The solvent A-phosphate buf...
Embodiment 3
[0046] A method for purifying telavancin, comprising the steps of:
[0047] 1) Mix the reaction mixture containing telavancin with methanol-formic acid-water solution in a weight ratio of 1:7, dissolve the reaction mixture, filter out solid insolubles, and obtain the filtrate; the above-mentioned methanol-formic acid-water solution is in a volume ratio of 2: 1:1 configuration; the reaction mixture of telavancin is a reductive amination reaction between vancomycin hydrochloride and N-(9-fluorenylmethoxycarbonyl)-decylaminoacetaldehyde, and then removes 9- Telavancin crude product synthesized by Mannich reaction with fluorenylmethoxycarbonyl protecting group and aminomethylphosphonic acid;
[0048] 2) Pass the filtrate through the well-balanced chromatographic medium of the eluent, the weight ratio of the loading filtrate to the chromatographic medium is 1:70; the chromatographic medium is octadecyl bonded silica gel; the eluent is 4 column volumes The solvent A-phosphate buffe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com