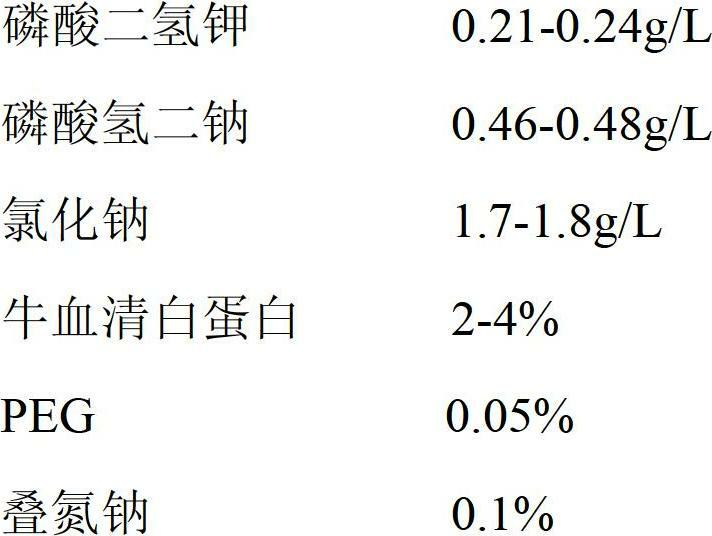
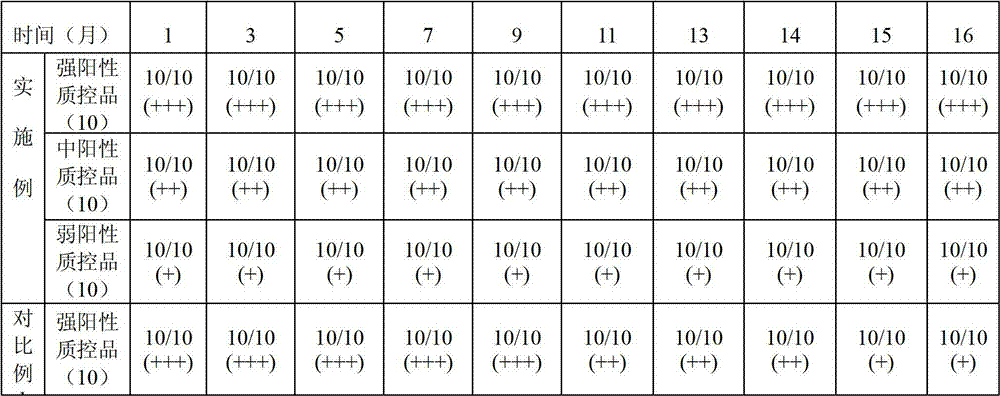
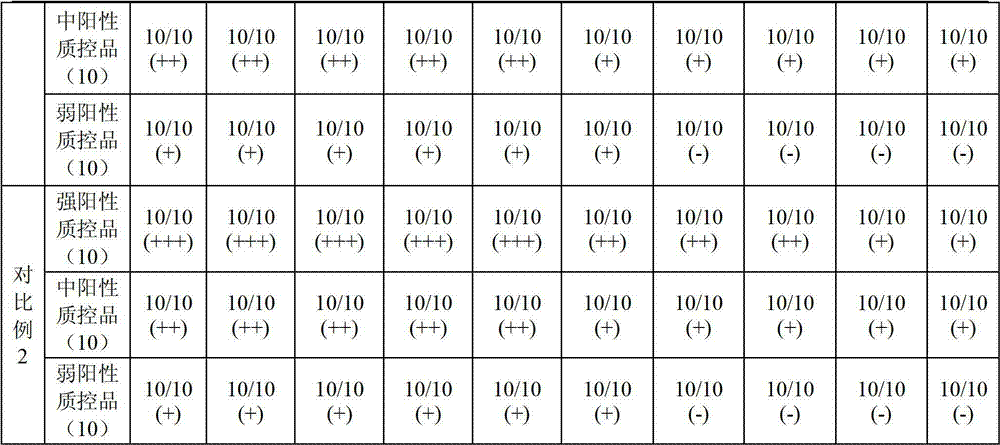
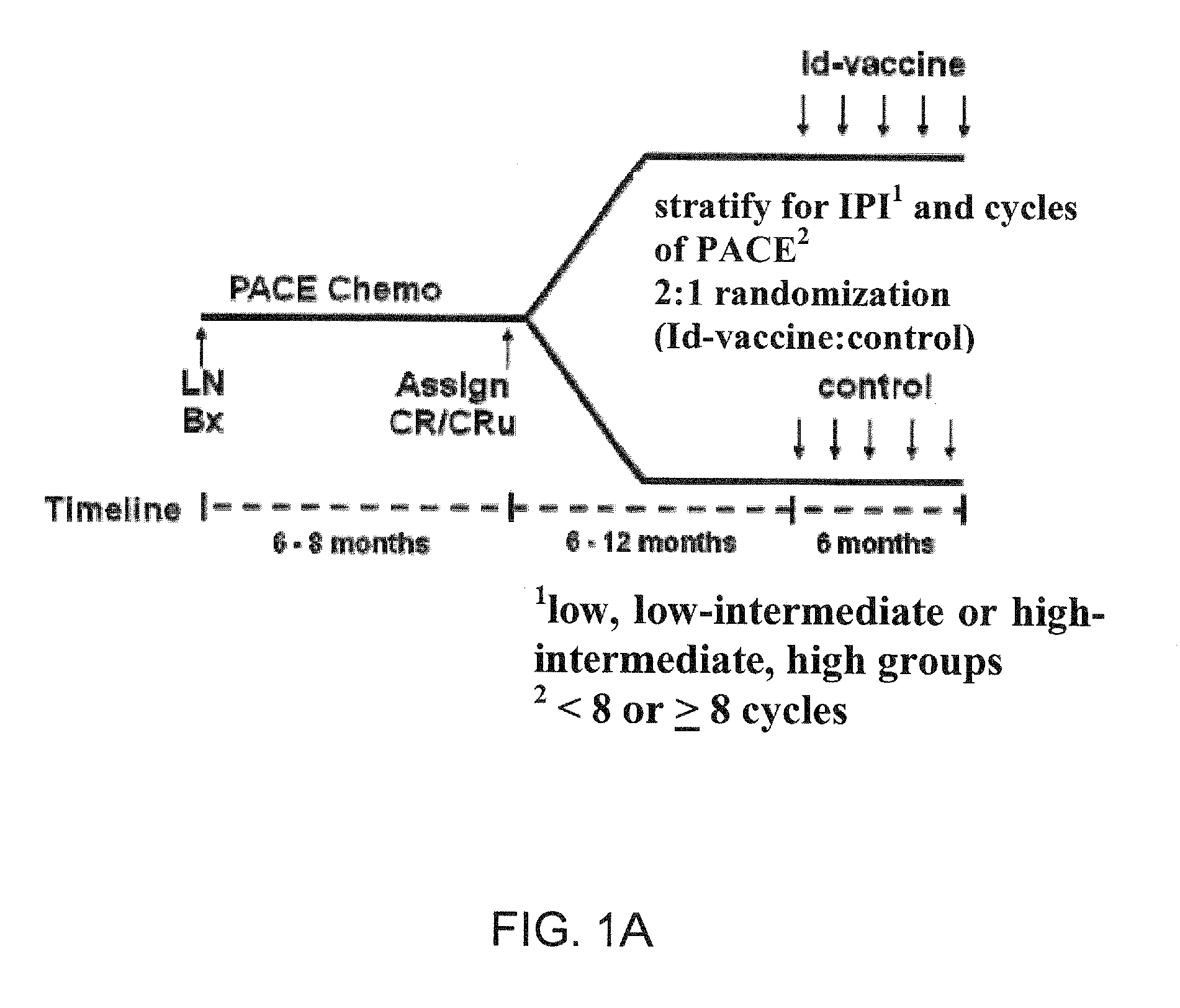
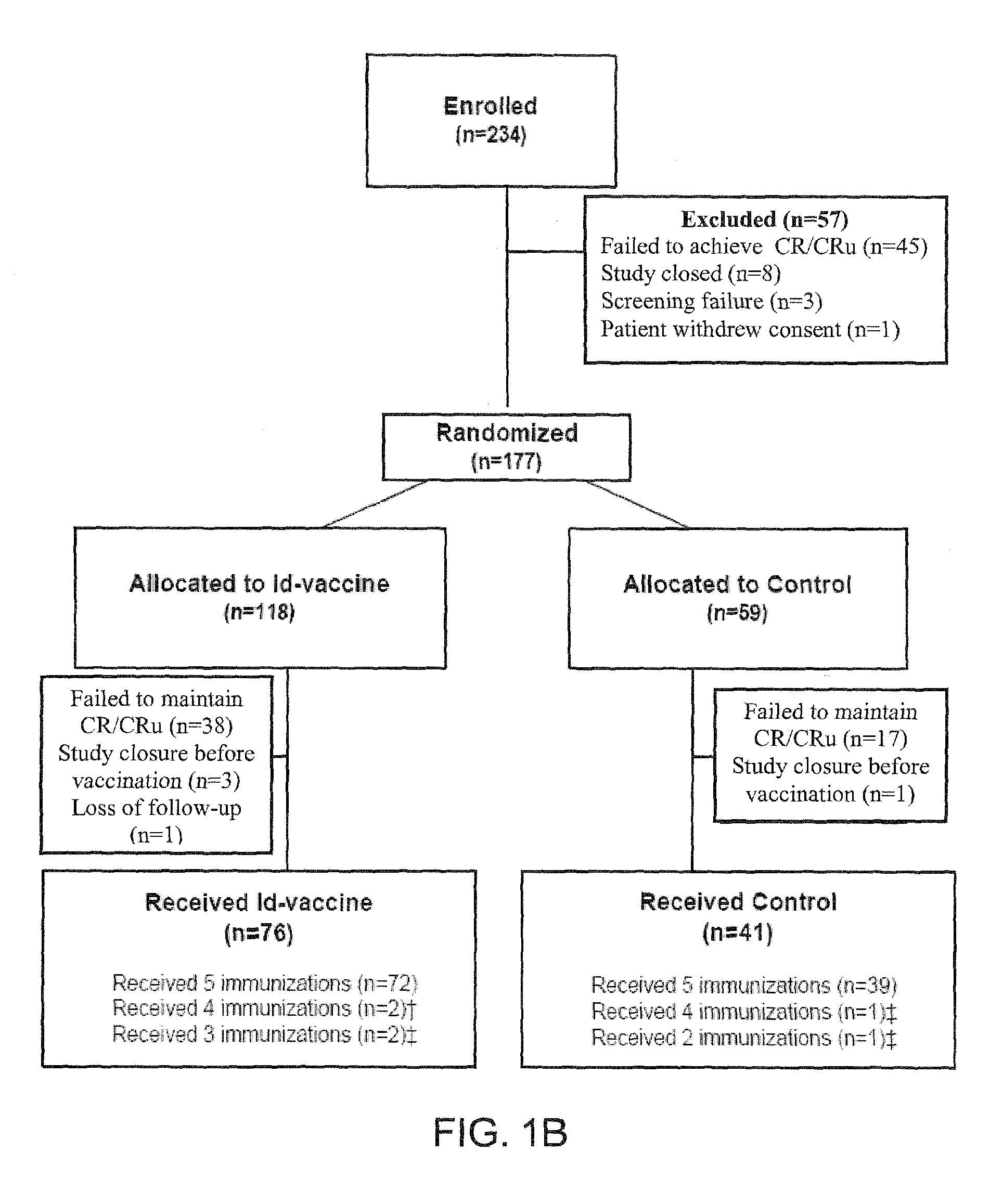
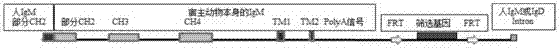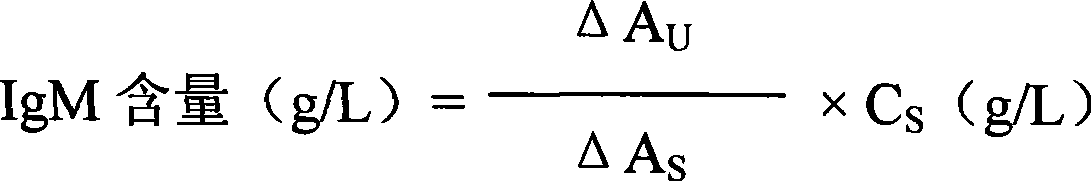Patents
Literature
81 results about "Immunoglobulin M" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
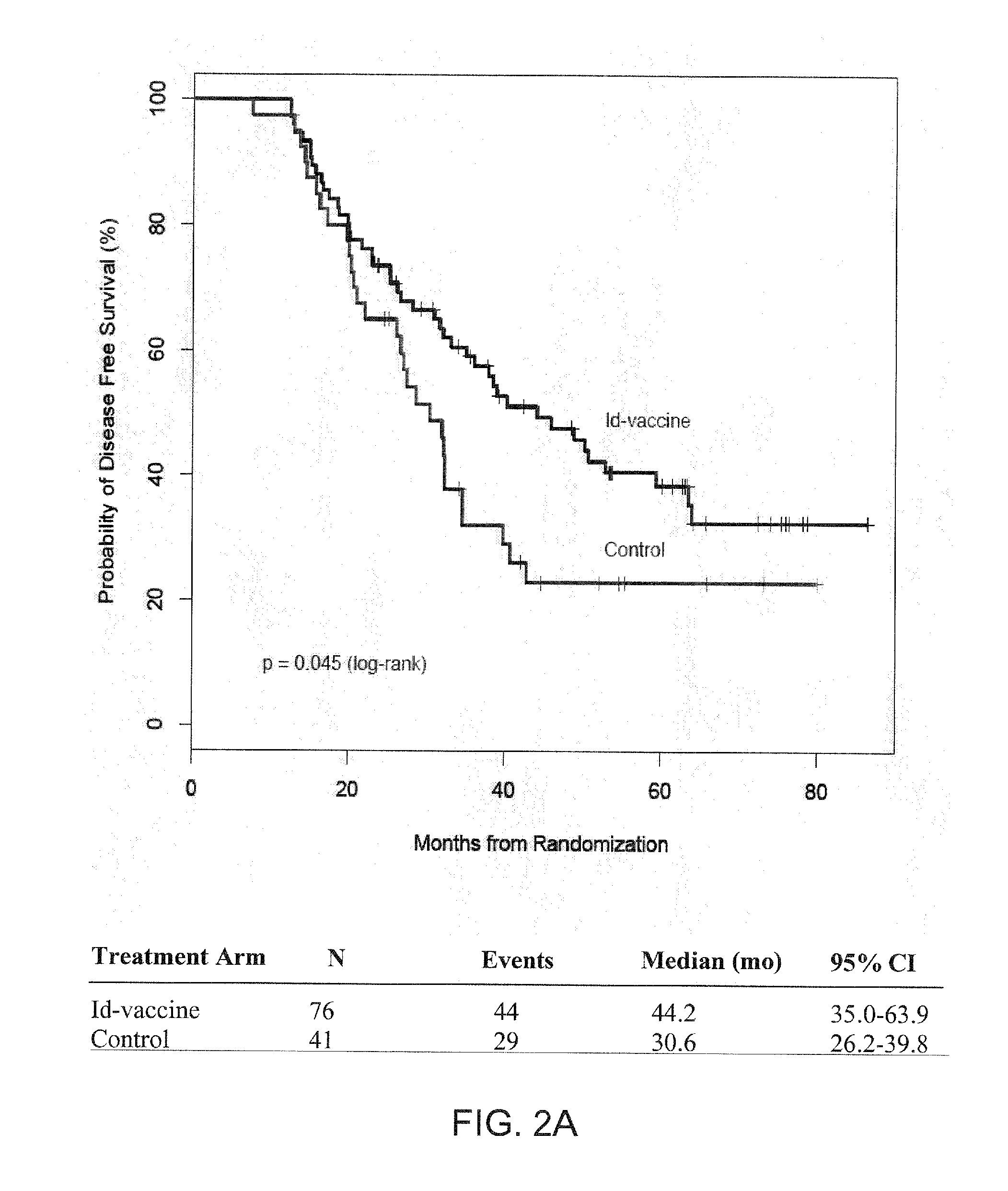
Immunoglobulin M (IgM) is one of several isotypes of antibody (also known as immunoglobulin) that are produced by vertebrates. IgM is the largest antibody, and it is the first antibody to appear in the response to initial exposure to an antigen. In the case of humans and other mammals that have been studied, the spleen, where plasmablasts responsible for antibody production reside, is the major site of specific IgM production.
Electrochemical immunosensor for detecting toxoplasma gondii IgM antibody and preparation method thereof
InactiveCN102914648AImprove conductivityEasy to passMaterial electrochemical variablesMedical diagnosisElectronics
The invention belongs to the technical field of analytical chemistry and chemical sensors and discloses an electrochemical immunosensor for detecting a toxoplasma gondii IgM (Immunoglobulin m) antibody (Tg-IgM) of a gravida and a preparation method of the electrochemical immunosensor. The immunosensor is prepared by sequentially modifying graphene, polythionine, gold nanoparticles and capture antigen to the surface of a glassy carbon electrode. An enzyme-functionalized nano-composite detection probe with an electrical signal amplifying function is prepared by assembling enzyme and a second antibody with high proportions on an Au-Fe3O4 surface. According to the sandwich immunoassay principle, the concentration of Tg-IgM is determined by using an electrochemical signal generated by catalysis of enzyme to a substrate. According to the electrochemical immunosensor, the specificity of immunoreaction is combined with the sensitivity of electrochemical detection; the transmission of electronics is promoted by using the graphene, the polythionine, the gold nanoparticles, Au-Fe3O4 and other material; and the sensitivity of the detection is improved. The electrochemical immunosensor has the advantages of simplicity and convenience for operation, favorable regeneration performance and detection cost reduction. The electrochemical immunosensor prepared on the basis can be also used for detecting other immunological markers and has favorable application prospect in medical diagnosis.
Owner:CHONGQING MEDICAL UNIVERSITY
Latex-enhanced immunoturbidimetry kit for measuring Helicobacter pylori antibody as well as preparation method and application thereof
ActiveCN102662059AHigh sensitivityWide linear rangeMaterial analysisHelicobacter pylori AntibodyBiology
The invention discloses a latex-enhanced immunoturbidimetry kit for measuring a Helicobacter pylori antibody as well as a preparation method and application thereof. The kit comprises a diluent, latex reagent and blank liquid of Helicobacter pylori antigen, a standard product and a quality control product, wherein the Helicobacter pylori antigen can be one or more of full-tropina antigen of Helicobacter pylori, urease antigen of Helicobacter pylori, cytotoxin-related protein A antigen of Helicobacter pylori or cell vacuole toxin A antigen of Helicobacter pylori; the latex reagent is a mixture of two latex granules which have different particle sizes and are adsorbed by Helicobacter pylori; and the Helicobacter pylori antibody for preparing the standard product and the quality control product is derived from IgM (immunoglobulin M) and / or IgG (immunoglobulin G) in serum of a patient infected by Helicobacter pylori. The kit is suitable for various full-automatic biochemical analyzers and semiautomatic biochemical analyzers, and has the advantages of rapid detection, high sensitivity, strong specifically, good accuracy and the like.
Owner:BEIJING MOKOBIO LIFE SCI CO LTD
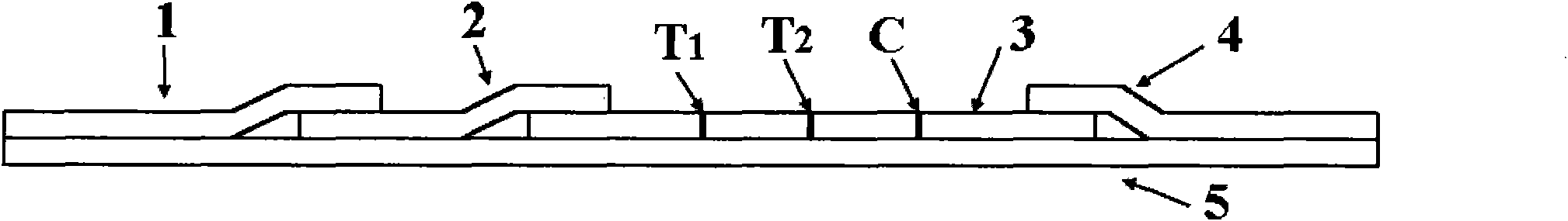
Rubella virus IgG and IgM antibody joint inspection kit and preparation method thereof
InactiveCN102109519AQuick screeningEasy to operateMaterial analysis by observing effect on chemical indicatorGlass fiberNitrocellulose
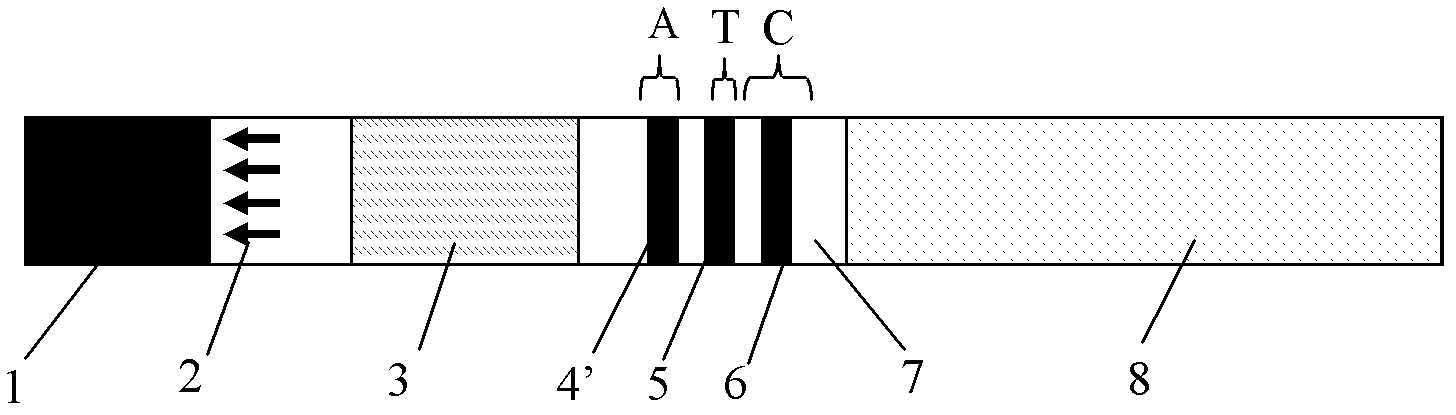
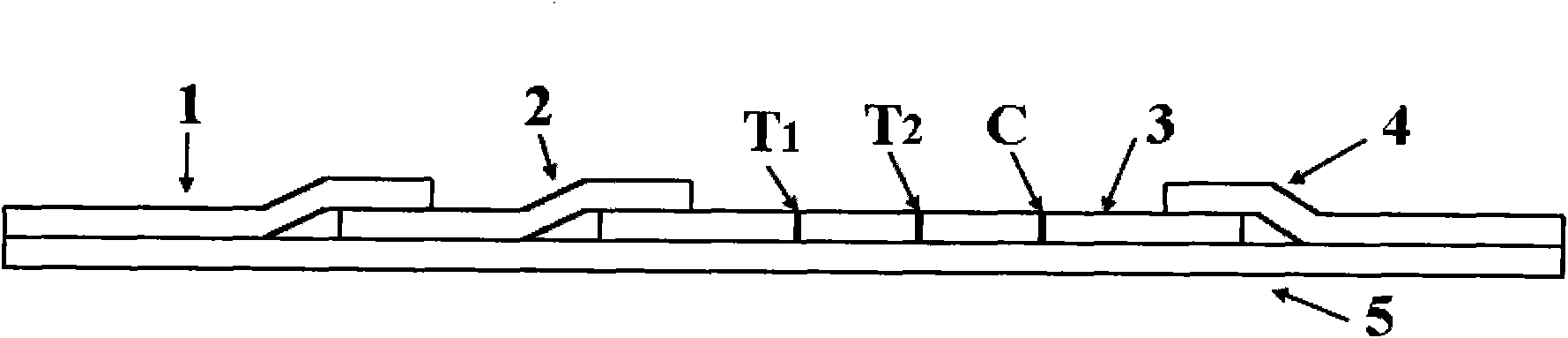
The invention provides a rubella virus immunoglobulin G and immunoglobulin M (IgG and IgM) antibody joint inspection kit and a preparation method thereof. The kit comprises a sample pad (1), a colloidal gold pad (2), a nitrocellulose membrane (3), a sample absorption pad (4) and a bottom plate (5), wherein the colloidal gold pad is a colloidal gold-labeled rubella virus antigen glass fiber (or a non-woven fabric); and the nitrocellulose membrane is coated with a mouse anti-human IgM antibody and a rubella virus antigen serving as detection lines, and goat anti-mouse IgG serving as a quality control line in turn. Rubella virus IgG and IgM antibodies are detected by specific antigen antibody reaction and a colloidal gold immunochromatography technology and can be jointly detected through one-time operation, so that the operation process is simplified, and the kit has the characteristics of quick response, high sensitivity and the like, is easy to operate and is economical and practical.
Owner:北京库尔科技有限公司
Mycoplasma pneumonia antigen, preparation method and immunodetection kit
ActiveCN103059109AAchieve recombinant expressionHigh sensitivityDepsipeptidesBiological testingMycoplasma antibodyIgm antibody
The invention provides a mycoplasma pneumonia (MP) antigen. The antigen fragment of the antigen is MP371 or MP661, wherein the locus of MP 371 is at an N terminal 371-480aa of MP adhesion protein P1, and the locus of MP661 is at an N terminal 661-772aa of MP adhesion protein P1. The invention also relates to a kit comprising any antigen or antigen composition and the application of the kit in detection of a mycoplasma pneumonia antibody. The mycoplasma pneumonia antigen is a recombinant antigen cloned and expressed by a codon-optimized gene and has stronger antigen specificity than that of the antigen cultivated and extracted by MP. The kit can be used for specific detection of an anti-MP-IgM (immunoglobulin m) antibody in clinical samples, thus identifying and diagnosing the infection of mycoplasma pneumonia at early stage.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Method for detecting hepatitis A virus antibodies, kit for detection through method and preparation method for kit
ActiveCN103018446AHigh activityTolerates a wide pH rangeMaterial analysisAgainst vector-borne diseasesHepatitis A AntigensHepatitis A Virus Antibody
The invention relates to the technical field of biology, in particular to a method for detecting hepatitis A virus antibodies, a kit for detection through the method and a preparation method for the kit. Antihuman IgM (immunoglobulin m) and antihuman IgG (immunoglobulin g) are fixed on a membrane respectively; blood serum to be detected are added; after the hepatitis A antibodies in the blood serum to be detected are combined with the antihuman IgM and the antihuman IgG fixed on the membrane, enzyme-labeled hepatitis A antigen solution is added; and finally, color development solution is added for color development so as to display the result. The invention has the advantages as follows: false positive interference in the result caused by rheumatoid disease factors in a sample can be eliminated, the process that enzyme labels hepatitis A antigen is enabled to be quicker and more efficient, and meanwhile, the antibody cost of the kit is lowered.
Owner:QINGDAO HIGHTOP BIOTECH
IgM (immunoglobulin M) antibody detection test strip
The invention relates to an IgM (immunoglobulin M) antibody detection test strip, comprising an IgM antibody detection line, wherein a matter capable of absorbing human IgM and rheumatoid factors is coated in front of the IgM antibody detection line. Preferably, the matter is an anti-huamn IgG antibody, Protein G and human IgG aptamer; both the IgM antibody detection line and the matter are both on a nitrocellulose membrane; or the matter is on an absorption pad in front of the IgM antibody detection line, and the absorption pad can be arranged between the nitrocellulose membrane and a combined pad, and also can be arranged between the combined pad and a sample pad. The IgM antibody detection test strip provided by the invention can simply and quickly detect the IgM antibody, obviously improve the detection accuracy, provide a powerful support for the next accurate treatment and is suitable for large-scale promotion and application.
Owner:无锡博慧斯生物医药科技有限公司
ABO/RhD blood group antigen detection reagent card and preparation method thereof
InactiveCN102680716AAdded Negative Control AssayAvoid misjudgment of resultsBiological testingMonoclonal antibodyGroup A - blood
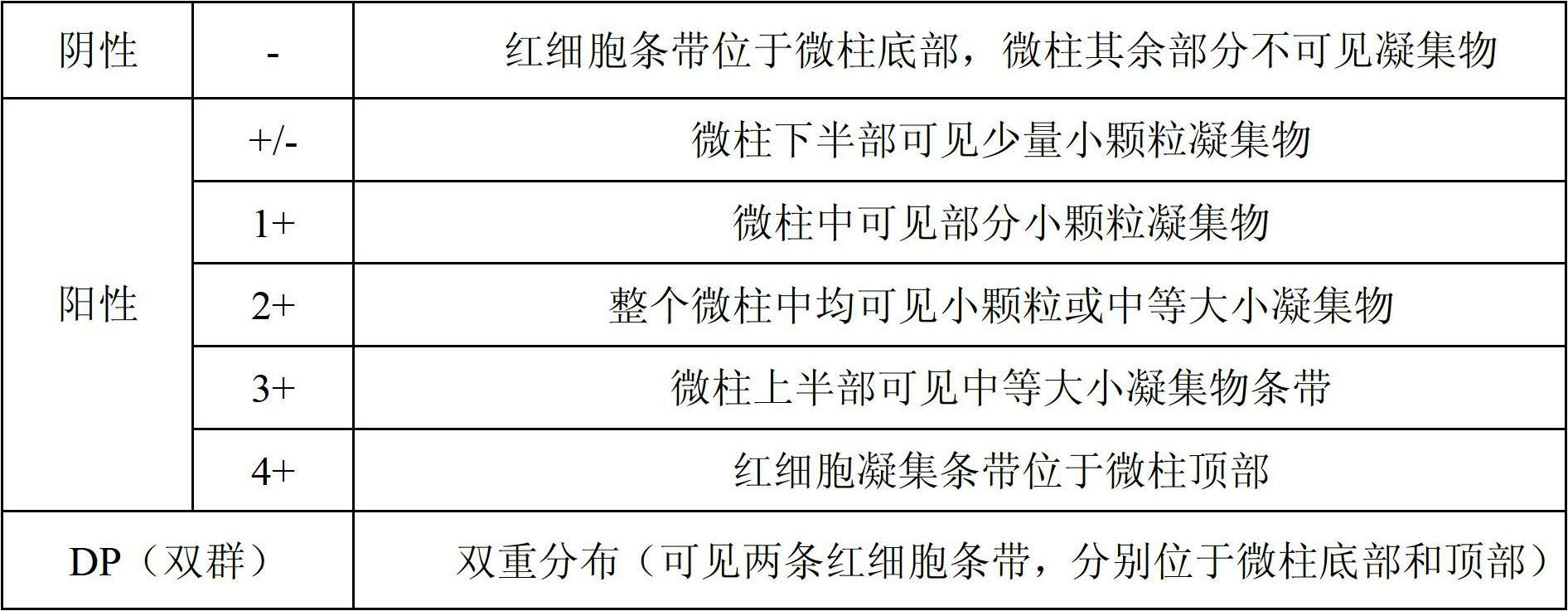
The invention relates to a preparation method of an ABO / RhD blood group antigen detection reagent card. Eight microcolumn gel tubes are arranged on the detection reagent card, wherein two gel tubes contain gel and anti-A monoclonal antibodies with an immunoglobulin m (IgM) property, two gel tubes contain gel and anti-B monoclonal antibodies with the IgM property, two gel tubes contain gel and anti-D monoclonal antibodies of RhD blood type with the IgM property, and two gel tubes contain a gel suspending medium buffer solution and gel.
Owner:BEIJING KINGHAWK PHARMA
Chimeric nucleic acid molecule and application thereof to humanized antibody preparation
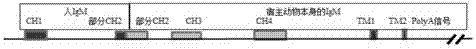
ActiveCN105441455AHigh affinityImprove rearrangement efficiencyHybrid immunoglobulinsFermentationHumanized antibodyImmunoglobulin M
The invention provides a nucleic acid molecule. The nucleic acid molecule comprises human immunoglobulin genes or segments thereof and is characterized by also comprising gene segments in a host animal IgM (immunoglobulin M) constant region. The nucleic acid molecule can be used for efficiently preparing full humanized antibodies and has the effect of solving the problem of incompatibility of the interactions between BCRs (B cell receptors) of different species and Ig alpha and Ig beta. Meanwhile, the humanized antibodies expressed by the nucleic acid molecule are unnecessary to undergo second modification.
Owner:CHONGQING JINMAIBO BIOTEC CO LTD
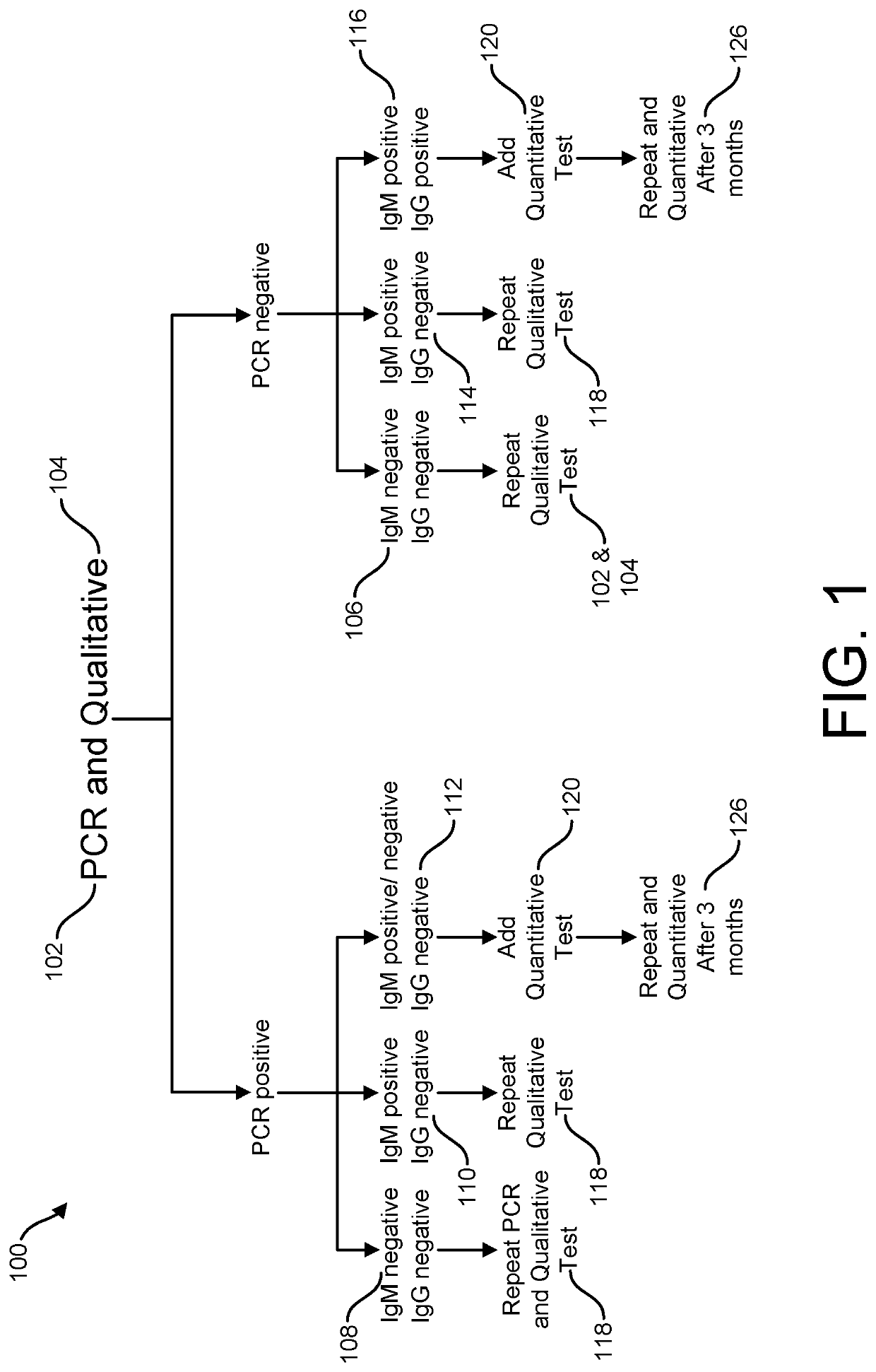
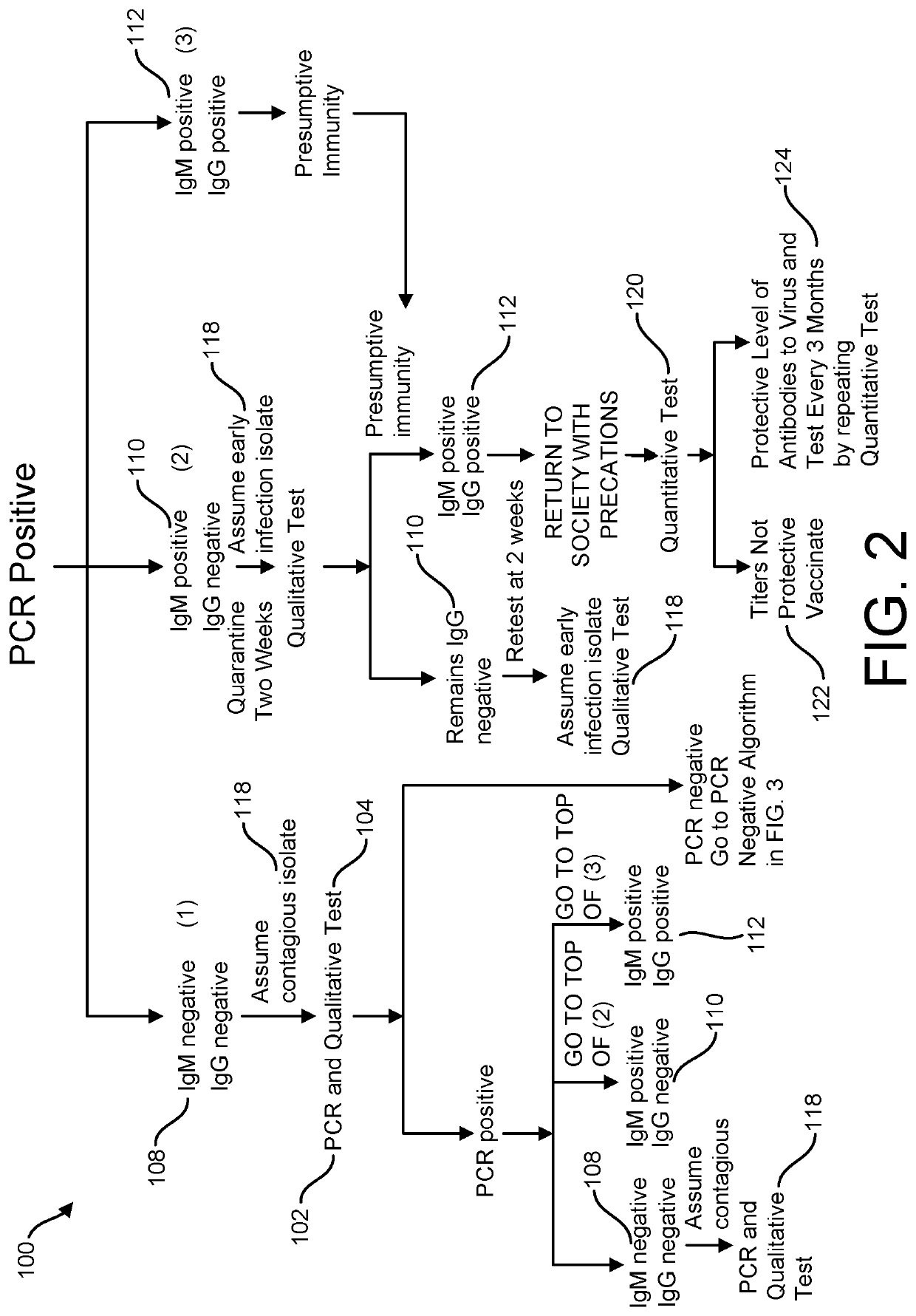
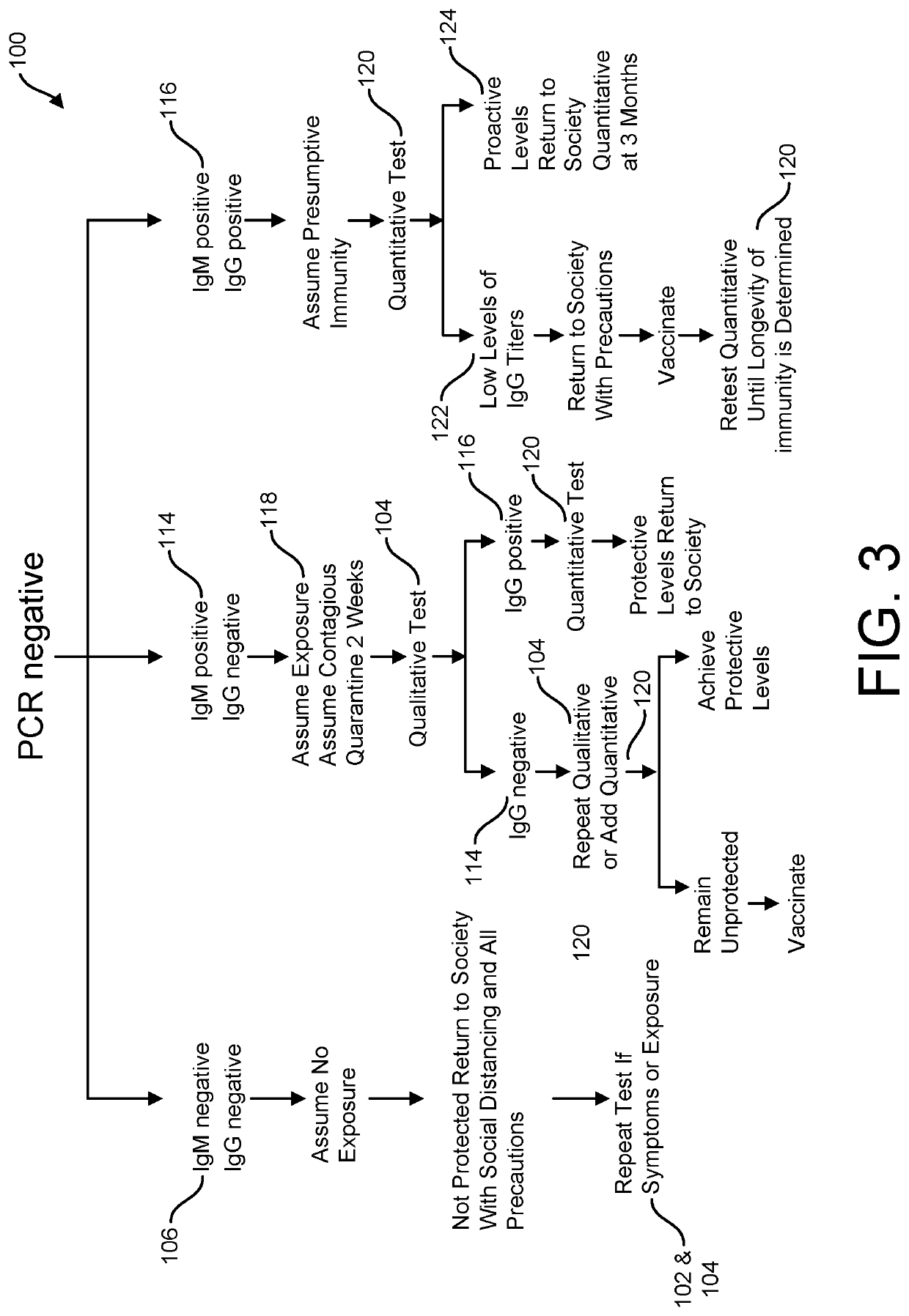
Risk Stratification for Contagious Disease
InactiveUS20200291490A1Intuitive imageHealth-index calculationMicrobiological testing/measurementReverse transcriptaseIntravenous gammaglobulin
A method of risk stratification for contagious disease is provided. The method performs a reverse transcriptase polymerase chain reaction (RT-PCR) test on a patient to determine the presence of a viral infection. The method next performs tests for the presence of antibody Immunoglobulin M (IgM) and Immunoglobulin G (IgG) assays. The method assigns the patient a level of readiness to return to society corresponding to the combination of the results of the qualitative RT-PCR, IgM, and IgG tests and quantitative IgG+ antibody testing. The levels of readiness to return to society may be verified by a QR code on a mobile device.
Owner:SENSIVA HEALTH LLC
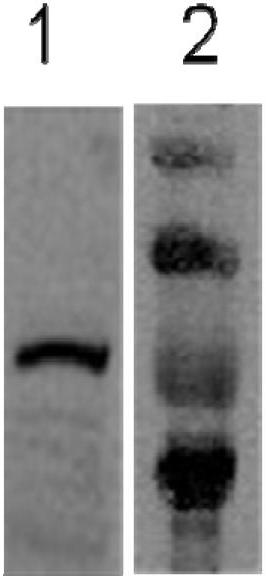
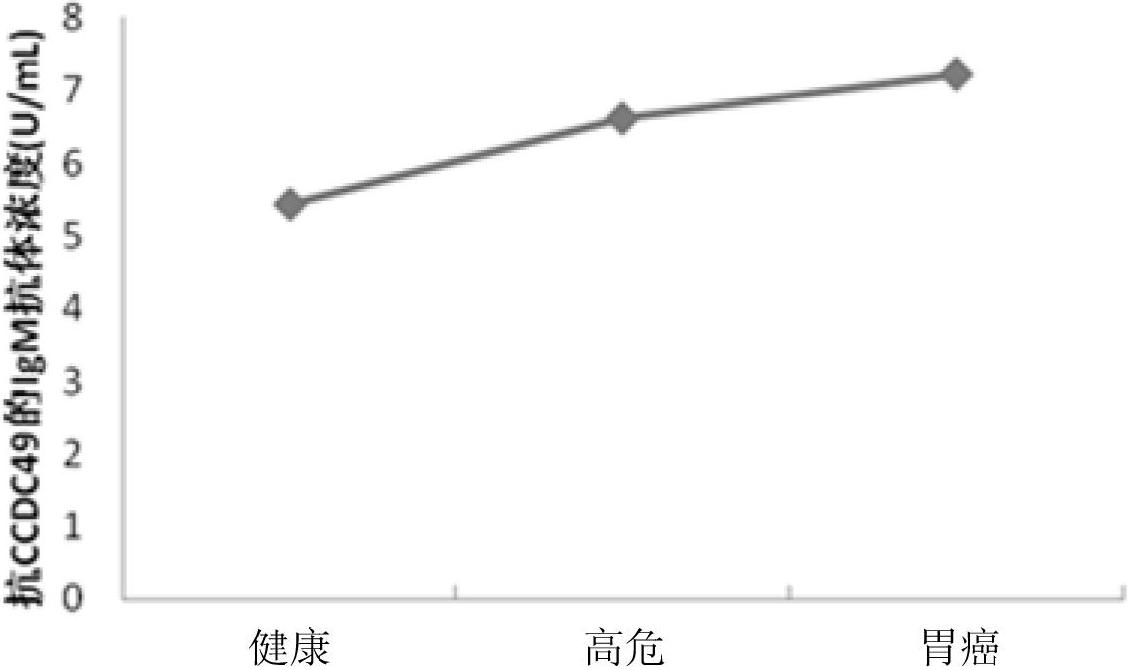
Diagnostic kit and application of coiled-coil domain containing protein 49 (CCDC49) in preparing gastric cancer early-stage diagnostic reagent
The invention relates to the field of the biological science, in particular to a diagnostic kit and an application of coiled-coil domain containing protein 49 (CCDC49) in preparing a gastric cancer early-stage diagnostic reagent. The diagnostic kit is used for diagnosing the early-stage gastric cancer. The diagnostic kit comprises an elisa plate, human protein CCDC49, standard serum, an enzyme labeled reagent, enzyme substrate solution, confining liquid, sample diluent, detergent liquid and termination liquid, wherein the human protein CCDC49 is wrapped on the elisa plate. Compared with the prior art, the diagnostic kit has advantages that 1, a commodity diagnostic kit which is sensitive, safe, reliable and easy to operate is provided, the immunoglobulin m (IgM) antibody for resisting the protein CCDC49 in the human serum can be qualitatively determined, and the early diagnosis of the gastric cancer can be assisted; and 2, the specificity of the provided serum biological labeled protein CCDC49 is 81 percent, the sensitivity is 82 percent, and the characteristics of high specificity and high sensitivity can be realized.
Owner:SHANGHAI JIAO TONG UNIV
Colloidal gold test strip for rapid detection of Human enterovirus 71 (EV71) IgM (immunoglobulin m)
The invention discloses a colloidal gold test strip for rapid detection of Human enterovirus 71 (EV71) IgM (immunoglobulin m), belonging to the field of medical detection consumables. The colloidal gold test strip is characterized in that a colloidal gold-labeled cellulose pad is coated with colloidal gold-labeled EV71 antibody; a control line C is coated with anti-mouse IgG polyclonal antibody; a test line T is coated with anti-Mu chain monoclonal antibody; and a sample cellulose pad is coated with EV71 recombinant antigen. The inventive test strip adopts antibody capture principle and colloidal gold technology to detect EV71 IgM in serum / plasma / whole blood samples simply, sensitively, precisely and rapidly; and can be used for the rapid detection of EV71 IgM and diagnosis of hand-foot-mouth disease.
Owner:蓝十字生物药业(北京)有限公司 +1
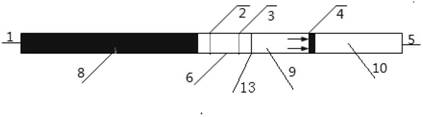
Detection reagent of treponema pallidum IgM (Immunoglobulin M) antigen colloidal gold method and preparation method thereof
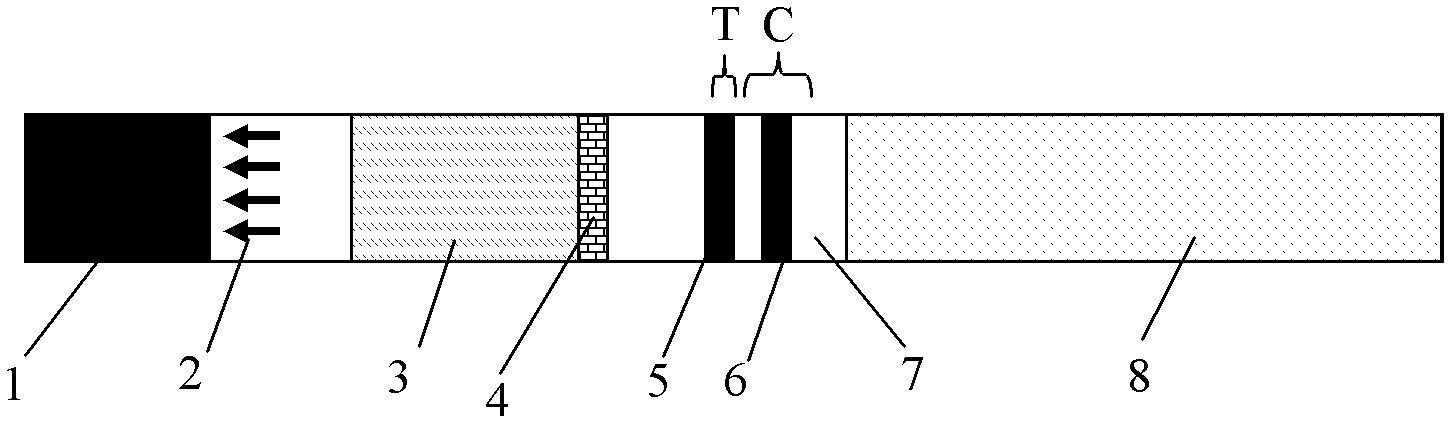
The invention discloses a detection reagent of a treponema pallidum IgM (Immunoglobulin M) antigen colloidal gold method and a preparation method thereof. The reagent comprises a gold conjugate pad (3), a cellulose nitrate reaction membrane (4), a sample pad (2), water absorbing paper (5) and a PVC (Polyvinyl Chloride) back lining (1), wherein the sample pad, the gold conjugate pad, the cellulose nitrate reaction membrane and the water absorbing paper are sequentially and mutually laminated and adhered on the PVC back lining; the sample pad is laminated on the gold conjugate pad for 2-3 mm, the gold conjugate pad is laminated on the cellulose nitrate reaction membrane for 2-3 mm, and the water absorbing paper is laminated on the cellulose nitrate reaction membrane for 2-3 mm; the gold conjugate pad is coated with an anti-human IgM monoclonal antibody-colloidal gold conjugate; and positions of a quality control region (7) and a detection region (6) of the cellulose nitrate reaction membrane are coated with goat anti-mouse IgG antibody or rabbit anti-mouse IgG antibody and specific gene recombinant treponema pallidum antigen respectively. A production process of a product provided by the invention is simple and easy to control; the detection reagent is easy, convenient and quick in detection; and a result can be read in 25 minutes and is not affected by the IgG antibody and is accurate and reliable.
Owner:BEIJING BIONEOVAN
Method and kit for performing quick co-detection on anti-human Hi (Haemophilus influenzae) IgM (Immunoglobulin M) and IgG (Immunoglobulin G) antibodies based on magnetic separation and multi-color quantum dot labeling
ActiveCN104181301AFast separationIt has the effect of synergistic amplification of multiple signalsFluorescence/phosphorescenceSerum igeMagnetic bead
The invention discloses a method and a kit for performing quick co-detection on anti-human Hi (Haemophilus influenzae) IgM (Immunoglobulin M) and IgG (Immunoglobulin G) antibodies based on magnetic separation and multi-color quantum dot labeling. The kit consists of anti-human Hi antibody capturing nano magnetic beads with an anti-human Hi IgM and IgG antibody gathering function, anti-human IgM and IgG antibody nano probes labeled by multi-color quantum dots, quality control substances and a PBST buffering solution, wherein the quality control substances comprise a positive quality control substance and a negative quality control substance; the positive quality control substance is serum, in which anti-human Hi IgM and IgG antibodies of human Hi infected people are respectively positive; the negative quality control substance is serum, in which anti-human Hi IgM and IgG are respectively negative. The kit and the method have the advantages of simplicity, quickness and high sensitivity, and can be used for carrying out synchronous detection on the anti-human Hi IgM and IgG antibodies.
Owner:HUBEI UNIV OF TECH +1
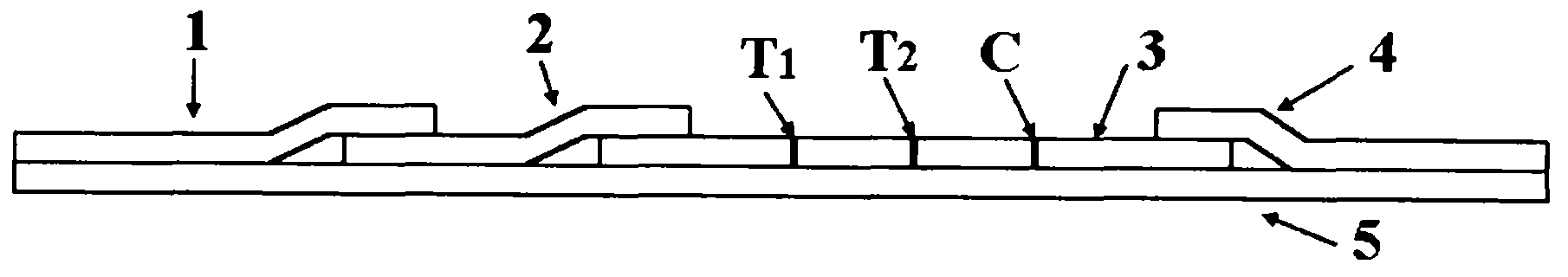
HSV-I and HSV-II IgM antibody joint inspection kit and preparation method thereof
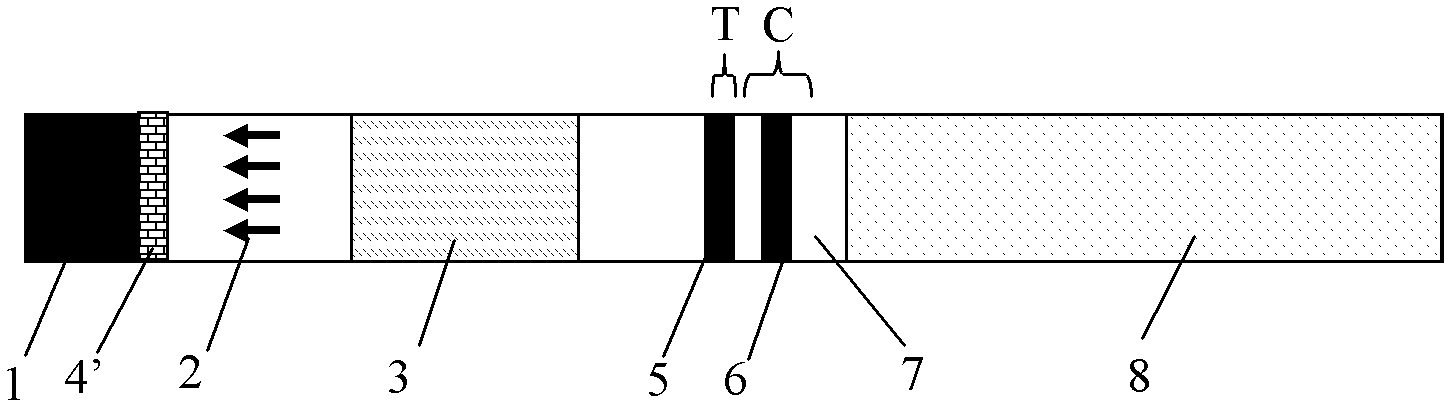
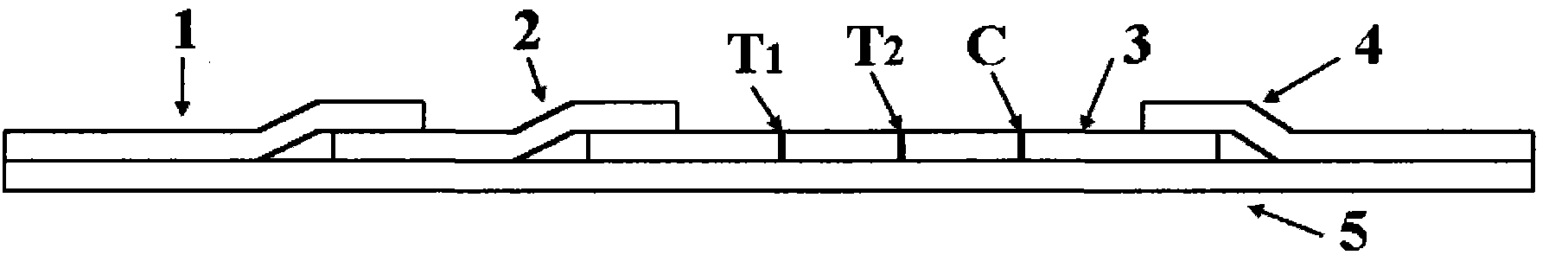
The invention relates to a herpes simplex virus I and II (HSV-I and HSV-II) immunoglobulin M (IgM) antibody joint inspection kit and a preparation method thereof. The kit comprises a sample pad (1), a colloidal gold pad (2), a nitrocellulose membrane (3), a sample absorption pad (4) and a bottom plate (5), wherein the colloidal gold pad is a colloidal gold-labeled mouse anti-human IgM monoclonal antibody glass fiber or a non-woven fabric; and the nitrocellulose membrane is coated with an HSV-I antigen and an HSV-II antigen serving as detection lines, and a goat anti-mouse IgM antibody serving as a quality control line respectively. HSV-I and HSV-II IgM antibodies are detected by a colloidal gold immunochromatography technological principle and can be jointly detected through one-time operation, so that the operation process is simplified, and the kit has the characteristics of quick response, high sensitivity and the like, is easy to operate and is economical and practical.
Owner:北京库尔科技有限公司
Homogeneous immunodetection kit for detecting target immunoglobulin M (IgM) antibody in sample as well as using method and application of homogeneous immunodetection kit
ActiveCN107976535AEfficient detectionEliminate distractionsBiological testingComplement C1qExcited state
The invention relates to a homogeneous immunodetection kit for detecting a target immunoglobulin M (IgM) antibody in a sample as well as a using method and application of the homogeneous immunodetection kit. The kit comprises a reagent a, a reagent b and a reagent c, wherein the reagent a comprises a known antigen, and the known antigen can be specifically bonded to the target IgM antibody in thesample to be detected so as to form a first complex; the reagent b comprises a complement C1q, the complement C1q can be specifically bonded to the first complex so as to form a second complex, and the complement C1q is not enabled to be bonded to a free IgM antibody; any of the known antigen and the complement C1q is connected with a receptor; the receptor can react with received singlet oxygen so as to produce detectable chemiluminescence signals; the reagent c comprises a donor, and the donor can generate the singlet oxygen in an excited state. A method for detecting the target IgM antibodyin the sample by using the kit overcomes the effect of a non-specific IgM antibody on detection.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Preparation method and applications of biotin marked rabbit anti-tilapia IgM (immunoglobulin m) polyclonal antibody
The invention discloses a preparation method of a biotin marked rabbit anti-tilapia IgM (immunoglobulin m) polyclonal antibody. The preparation method comprises the following steps: collecting tilapia serum, and purifying to obtain purified tilapia IgM; immunizing a rabbit by using the tilapia IgM to obtain a rabbit anti-tilapia IgM polyclonal antibody, and detecting the titer of anti-tilapia serum of the rabbit anti-tilapia IgM polyclonal antibody by adopting ELISA (enzyme linked immunosorbent assay); strengthening the immunity of the purified tilapia IgM, then collecting and separating to obtain serum containing the rabbit anti-tilapia IgM polyclonal antibody, purifying, adding biotin and mixing uniformly to obtain the biotin marked rabbit anti-tilapia IgM polyclonal antibody. The biotin marked rabbit anti-tilapia IgM polyclonal antibody prepared by the method disclosed by the invention is good in specificity and high in purity and titer, and can have a specific binding reaction with the tilapia IgM. The invention also discloses applications of the biotin marked rabbit anti-tilapia IgM polyclonal antibody prepared by the method in ELISA and Western-Blot.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
Chlamydia pneumoniae IgM (immunoglobulin M) colloidal golden method kit and preparation method thereof
InactiveCN102749446AObvious detectabilityReduce the effectMaterial analysisMurine antibodyChlamydiae
The invention discloses a chlamydia pneumoniae IgM (immunoglobulin M) colloidal golden method kit which comprises recombinant chlamydia pneumoniae antigens enveloped on a nitrocellulose membrane detection line, goat anti-rat IgG (immunoglobulin G) antibodies enveloped on a quality control line and rat anti-human IgM monoclonal antibodies which have colloidal gold labels and are enveloped on a gold label pad, the concentration of the chlamydia pneumoniae antigens ranges from 1mg / ml to 2mg / ml and is measured by SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis), the concentration of the goat anti-rat IgG (immunoglobulin G) antibodies ranges from 1mg / ml to 3mg / ml, and the concentration of the rat anti-human IgM monoclonal antibodies ranges from 5g / mL to 30g / mL and is measured by the SDS-PAGE. The chlamydia pneumoniae IgM colloidal golden method kit has the advantages that the kid is speedy, simple, convenient and accurate and is high in sensitivity, and a judgment result can be read after integral operation time of dozens of minutes; and a colloidal gold quick detecting paper strip is made of the multi-epitope recombinant antigens and is simple and convenient to operate, low in cost, good in specificity and high in sensitivity, can be used for single-component detection and is popularized easily, and detection and control effects for chlamydia pneumoniae IgM are obvious.
Owner:北京中检安泰诊断科技有限公司
Detection method of hemorrhagic fever with renal syndrome IgM antibodies and reagent kit
ActiveCN103033616AReduce the impact of stabilityExtended shelf lifeMaterial analysisSerum igeIgm antibody
The invention relates to the technical field of biological detection, in particular to a detection method of hemorrhagic fever with renal syndrome IgM (immunoglobulin M) antibodies and a reagent kit. The detection method comprises the steps that hemorrhagic fever with renal syndrome antigens are fixed on a membrane; to-be-detected serum is added; the hemorrhagic fever with renal syndrome IgM antibodies in the to-be-detected serum are combined with the antigens fixed on the membrane; gold labeled operating fluid containing mouse anti-human IgM monoclonal antibodies is added; the mouse anti-human IgM monoclonal antibodies are combinec with the hemorrhagic fever with renal syndrome IgM antibodies in the to-be-detected serum; a red color is shown; scrubbing liquid is added finally; and the redundant gold labeled operating fluid and other impurities are washed out. The reagent kit has the characteristics of longer quality guarantee period, less cross reaction, better color rendering performance and the like.
Owner:山东康华生物医疗科技股份有限公司
Materials and methods for directing an immune response to an epitope
InactiveUS20140140986A1Prolonged remission durationEvaluating effectImmunoglobulins against animals/humansAntibody ingredientsEpitopeImmunoglobulin M
The present invention relates to compositions, kits, and methods useful for directing an immune response to an epitope of an antigen in a subject, by sensitizing the subject to the epitope and / or by tolerizing the subject to the epitope. The sensitizing method comprises co-administering to the subject the epitope and an immunoglobulin M (IgM) constant region (IgM Fc region). The tolerizing method comprises co-administering to the subject the epitope and an immunoglobulin G (IgG) constant region (IgG Fc region) to the subject.
Owner:BIOVEST INT
Immune globulin M detection reagent
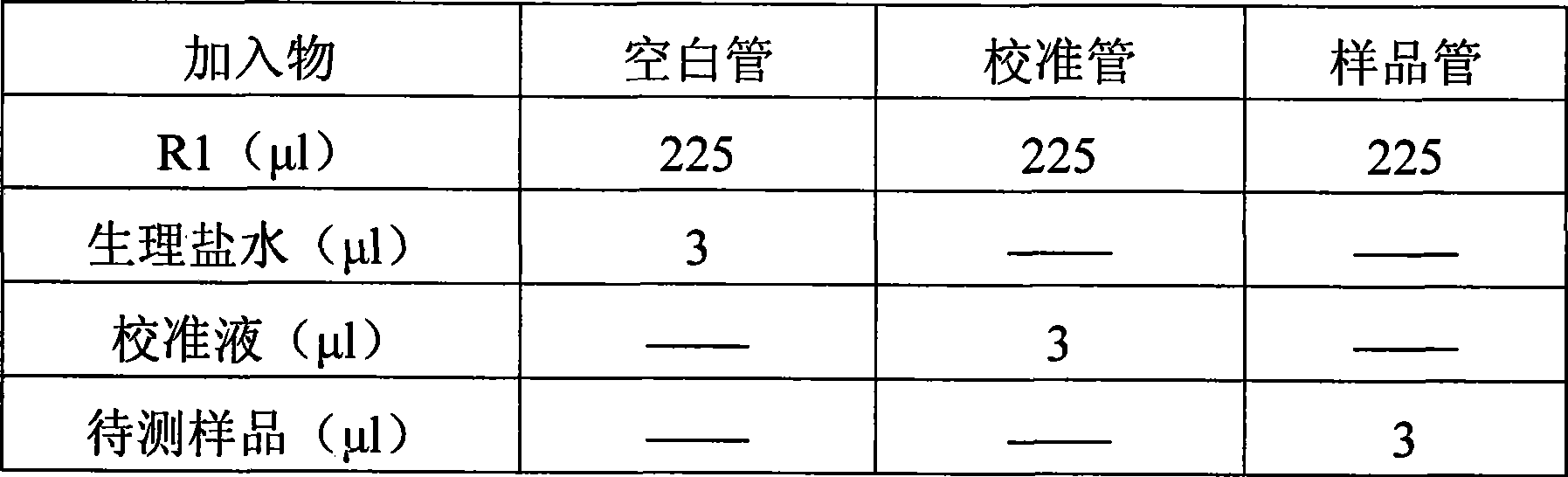
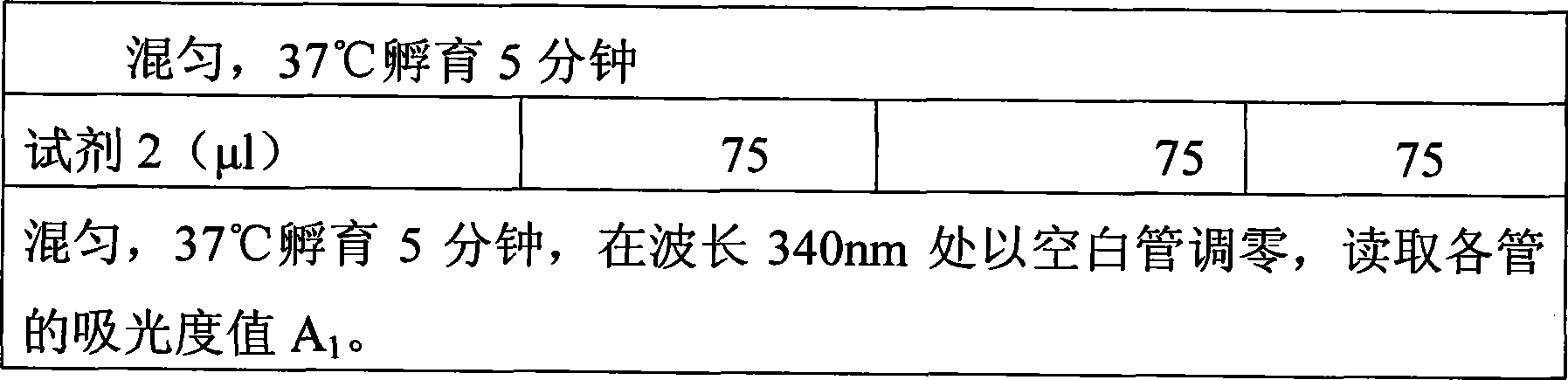
The invention discloses an immunoglobulin M detection reagent, which is provided with simple operation, high accuracy and good reproducibility and strong anti-interference ability; the sample is free from dilution and the detection reagent can be applicable to the immunoglobulin M (IgM) detection reagents of various automatic biochemical analyzers. The immunoglobulin M detection reagent comprises an IgA reagent, an anti-IgA antibody reagent and a liquid serotype constant value calibration liquid; wherein, the IgA reagent enables the IgA antigenic sites in the sample to be fully exposed so as to facilitate the full combination with the anti-IgA antibody reagent; the anti-IgA antibody reagent has high idiosyncrasy with the IgA antigens in human serum; and the liquid serotype constant value calibration liquid is compared with the sample for result calculation.
Owner:王贤理
Detection test paper for quickly diagnosing Lyme disease, and preparation method thereof
InactiveCN109596824AShort detection timeMeet the needs of on-site testingMaterial analysisAgainst vector-borne diseasesBiologyLyme disease
The invention discloses detection test paper for quickly diagnosing the Lyme disease, and a preparation method thereof. A method for detecting the Lyme disease by ELISA (Enzyme Linked Immunosorbent Assay) is long in detection time and is not suitable for substrate detection. The colloidal gold immunochromatography detection test paper for quickly diagnosing the Lyme disease comprises a bottom plate, a sample cushion, a Jinbiao cushion, a nitrocellulose membrane and a water adsorption cushion, wherein the sample cushion, the Jinbiao cushion, the nitrocellulose membrane and the water adsorptioncushion are arranged and connected in sequence and are all arranged on the bottom plate; the nitrocellulose membrane is provided with a first detection line, a second detection line and a quality control line, wherein the first detection line is provided with a mouse anti-human IgM (Immunoglobulin M) monoclonal antibody, the second detection line is provided with a mouse anti-human IgG (Immunoglobulin G) monoclonal antibody, and the quality control line is provided with a goat anti-mouse IgG polyclonal antibody; and the Jinbiao cushion is provided with Lyme disease recombinant antigen-colloidal gold conjugate. The detection test paper has the advantages of short detection time, high detection accuracy, high specificity and convenience in operation, and does not need the help of other equipment instruments.
Owner:HANGZHOU ALLTEST BIOTECH
Colloidal gold test strip for fast detecting IgG and IgM antibodies of dengue fever virus
InactiveCN102707057AFast locationFast color developmentMaterial analysisAgainst vector-borne diseasesFiberSmall sample
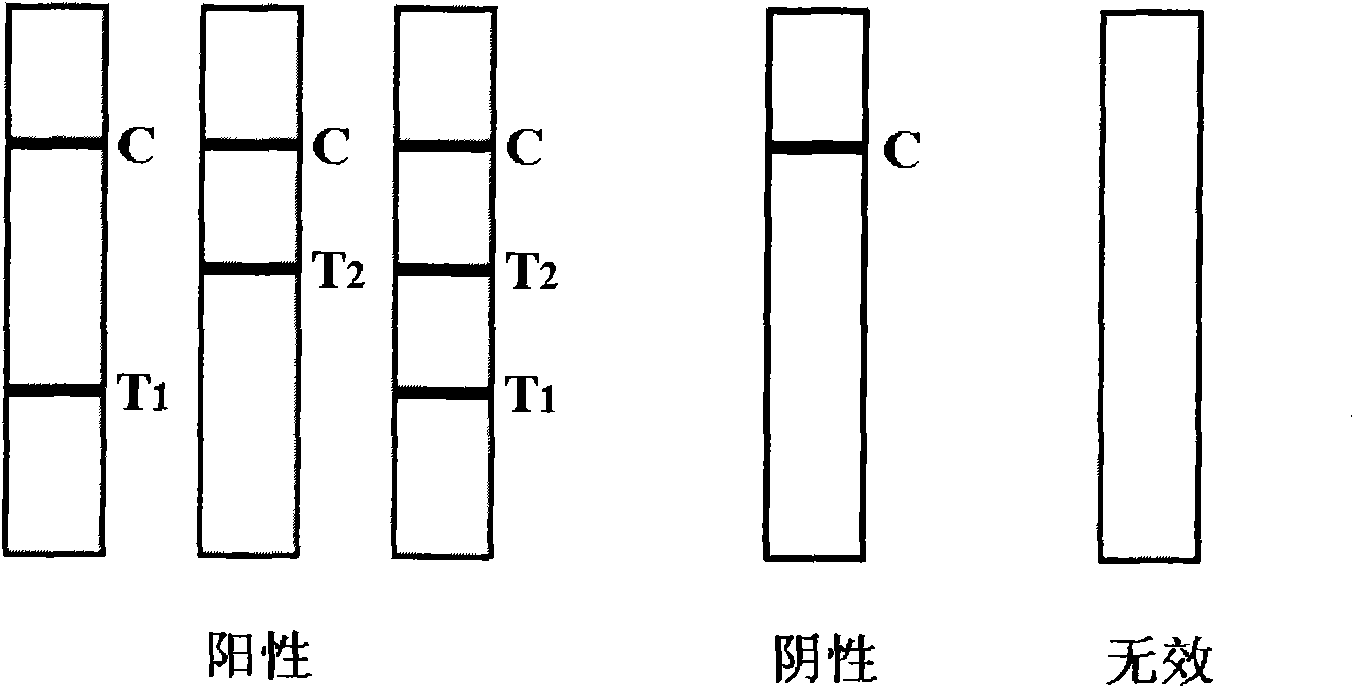
The invention discloses a colloidal gold test strip for fast detecting IgG (immunoglobulin G) and IgM (immunoglobulin M) antibodies of dengue fever virus and belongs to medical test consumables. The colloidal gold test strip is characterized in that 1-4 kinds of antigens are enveloped on a gold labeled protein-adsorbed fiber combination pad; the antigens are selected from four subtype antigens of the dengue fever virus labeld by colloidal gold; a polyclonal antibody of the antigens for resisting the dengue fever virus is enveloped on a quality control line C; an anti-mu chain monoclonal antibody is enveloped on a detection line T1, and an anti-human IgG monoclonal antibody is enveloped on a detection line T2. The test strip disclosed by the invention can fast detect serotype 1, 2, 3 and 4 type IgG and IgM antibodies of the dengue fever virus by adopting an antibody capture method combined with an immune colloidal gold technique and has the advantages of simplicity, sensitivity, specificity and accuracy. The test strip disclosed by the invention has the advantages of small sample quantity and wide storage condition, can save resources and can judge the positive result of the IgG or IgM antibody of the dengue fever virus once at the same time.
Owner:蓝十字生物药业(北京)有限公司 +1
Test paper and method for detecting IgG (immunoglobulin G) and IgM (immunoglobulin m) in liquid sample simultaneously
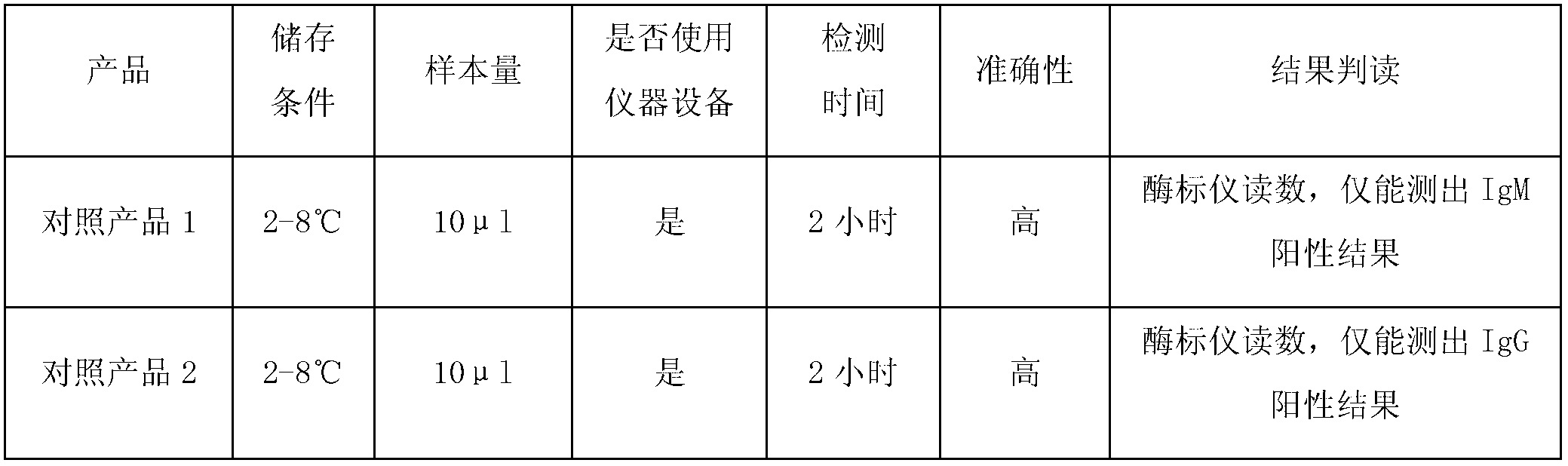
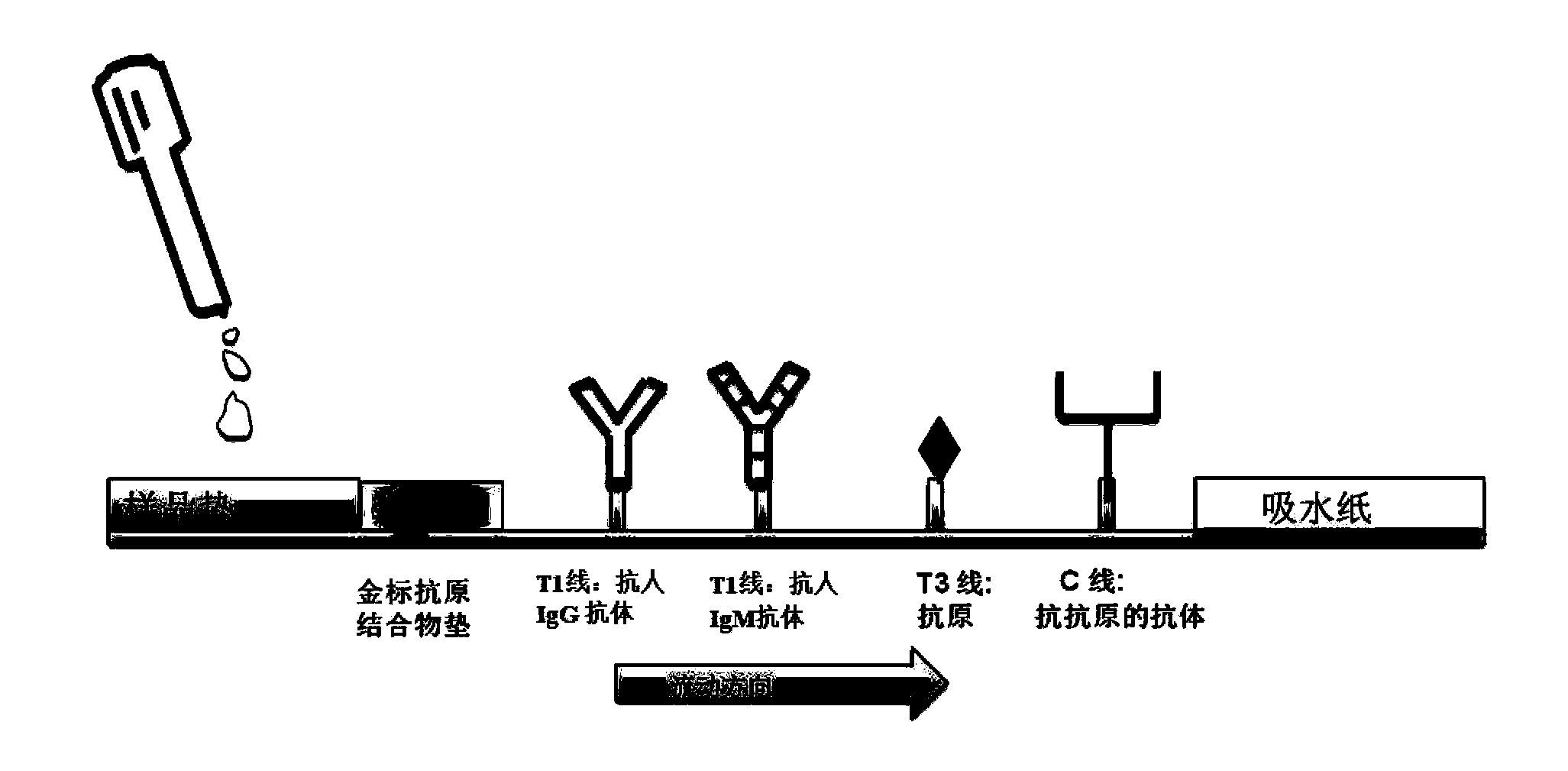
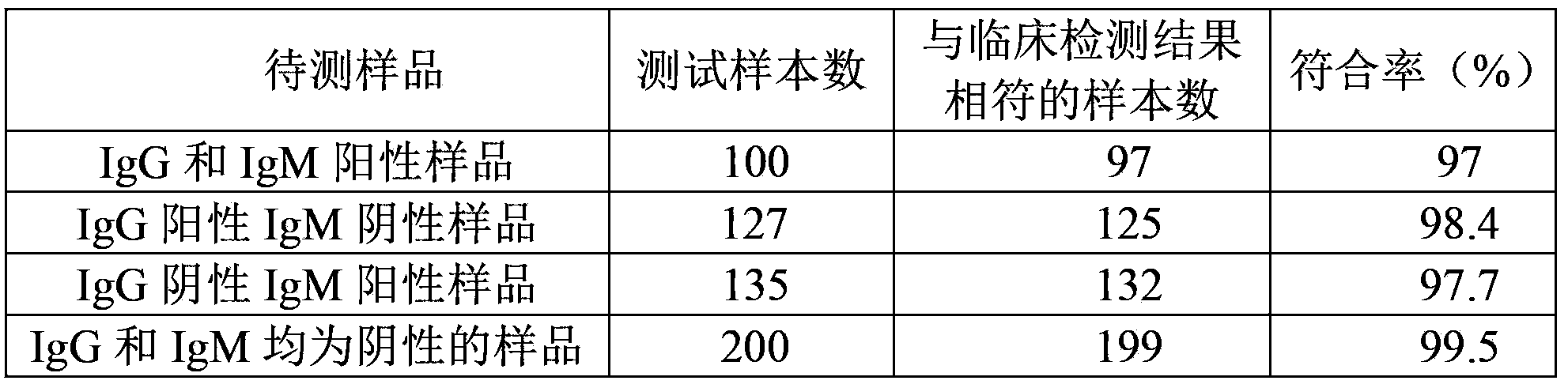
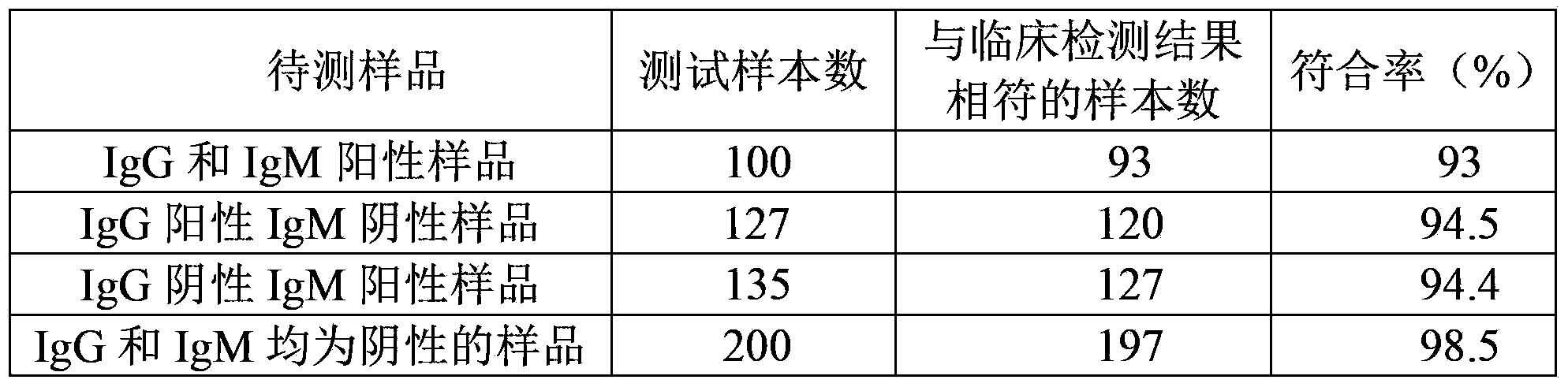
The invention discloses test paper. The test paper comprises a sample adding region, a binder region, an observation region and a water absorption region, which are sequentially arranged, wherein the binder region contains antigen combined colloidal gold which can be eluted by a liquid sample; the observation region comprises antibody lines, an antigen line T3 and a quality control line C in sequence from the binder region to the water absorption region; the antibody lines comprise a T1 line and a T2 line; the antibody line T1 is fixedly provided with an anti-human IgG antibody, the antibody line T2 is fixedly provided with an anti-human IgM antibody, the antigen line T3 is fixedly provided with by an antigen, and the quality control line C is fixedly provided with an anti-antigen antibody; and the IgG and the IgM can be specifically combined with the antigen. The test paper can detect whether the tested sample contains IgG and IgM simultaneously one time, can prevent leaked detection, shorten the detection time and reduce the detection expense and can be convenient for field detection.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Natural anti-oxidation low-density lipoprotein antibody for suppressing atherosclerosis
InactiveCN101654481AReduce areaInhibition formationImmunoglobulins against animals/humansAntibody ingredientsAntigenOxidized low density lipoprotein
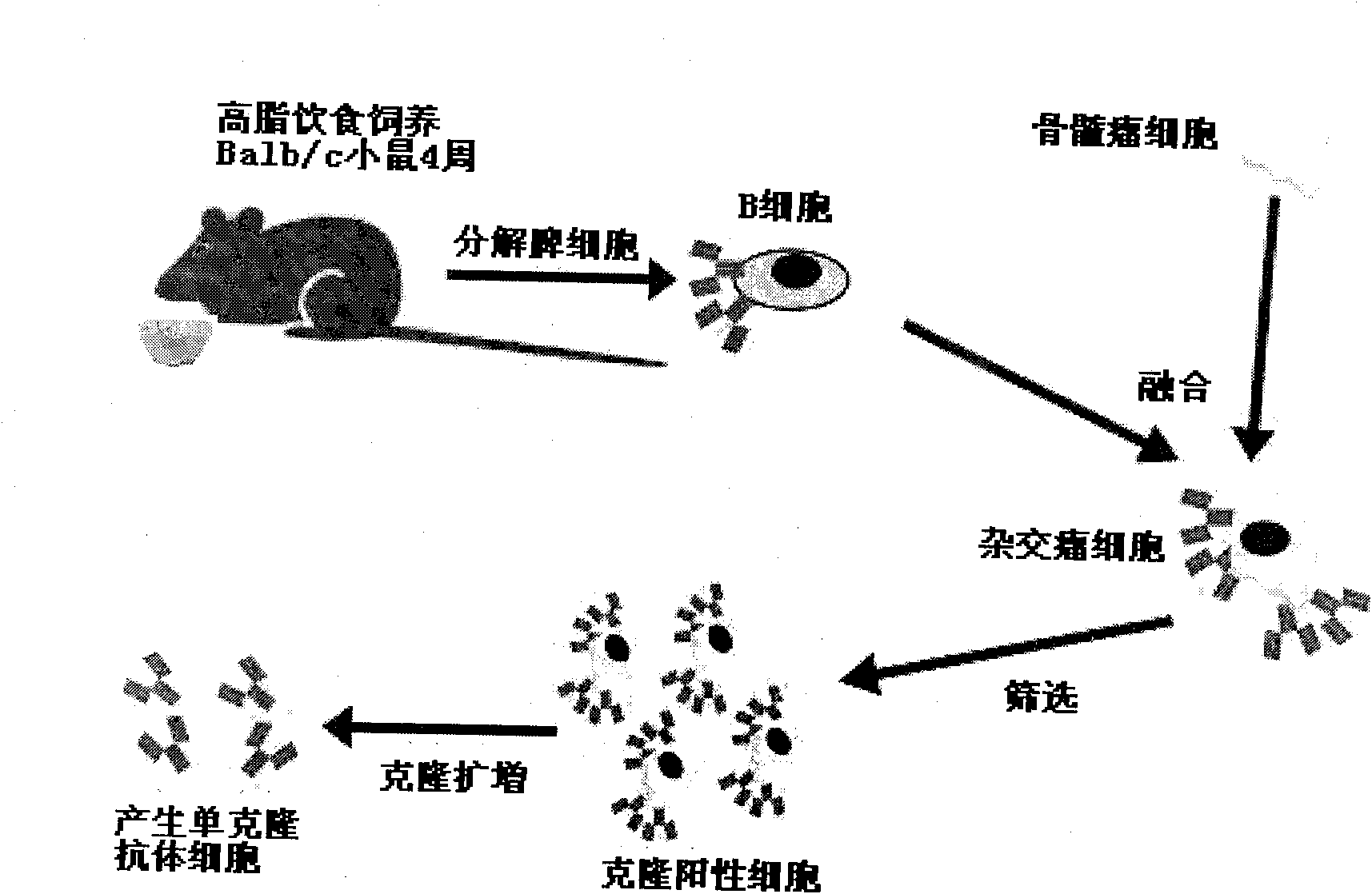
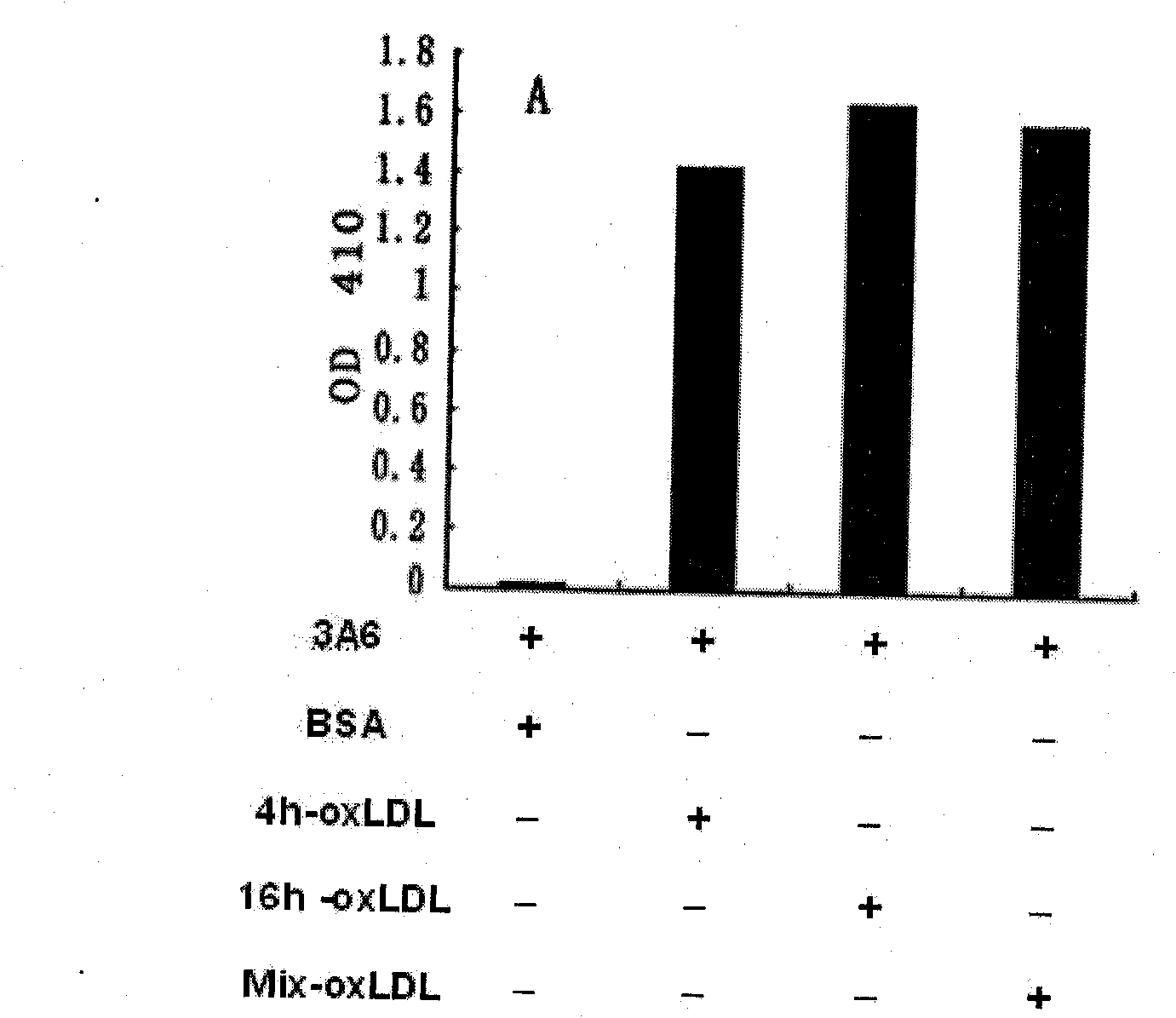
The invention belongs to the technical field of biomedicine, in particular to a natural anti-oxidation low-density lipoprotein (oxLDL) IgM subclass antibody for suppressing atherosclerosis. The natural anti-oxidation low-density lipoprotein (oxLDL) IgM subclass antibody is characterized in that a Babl / c rat is bred with a high-cholesterol diet for 4 weeks under a condition of no special pathogen;splenic cells (B cells primarily) are separated, the B cells and myeloma cells are combined by a chemical method, and hybridoma cells are obtained; and oxidized low-density lipoprotein (oxLDL) is usedas an antigen, an indirect enzyme-linked immunosorbent assay (ELISA) experiment is carried out on the growth holes of the positive hybridoma cells, positive cloning holes are determined, and the required cloning positive cells are screened out by a limiting dilution method. The obtained cloning positive cells are cloned and multiplied, cells generating a monoclonal antibody are obtained, and thenatural oxLDL-resisting immunoglobulin M (IgM) subclass antibody 3A6 is obtained. The antibody can be used for lowering the formation of the atherosclerosis of the rat and provides a novel idea and anovel method for researching the generation, the development and the treatment of the atherosclerosis.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Method and kit for performing quick co-detection on anti-Mc (Moraxella catarrhalis) IgM (Immunoglobulin M) and IgG (Immunoglobulin G) antibodies based on magnetic separation and multi-color quantum dot labeling
ActiveCN104181306AFast separationIt has the effect of synergistic amplification of multiple signalsBiological material analysisBiological testingSerum igeCo detection
The invention discloses a method and a kit for performing quick co-detection on anti-Mc (Moraxella catarrhalis) IgM (Immunoglobulin M) and IgG (Immunoglobulin G) antibodies based on magnetic separation and multi-color quantum dot labeling. The kit consists of anti-Mc antibody capturing nano magnetic beads with an anti-Mc IgM and IgG antibody gathering function, anti-human IgM and IgG antibody nano probes labeled by multi-color quantum dots, quality control substances and a PBST buffering solution, wherein the quality control substances comprise a positive quality control substance and a negative quality control substance; the positive quality control substance is serum, in which anti-Mc IgM and IgG antibodies of Mc infected people are respectively positive; the negative quality control substance is serum, in which anti-Mc IgM and IgG are respectively negative. The kit and the method have the advantages of simplicity, quickness and high sensitivity, and can be used for carrying out synchronous detection on the anti-Mc IgM and IgG antibodies.
Owner:HUBEI UNIV OF TECH +1
Reagent kit for detecting immunoglobulin m (IgM) AlphaLISA for resisting enterovirus71 capsid protein1
The invention provides a reagent kit for detecting immunoglobulin m (IgM) AlphaLISA for resisting enterovirus71 capsid protein1, which comprises donor microbeads, receptor microbeads and biotinylated enterovirus71 capsid protein1 antibodies. Optimal dose of biotinylated vitamin P (VP)1 is fished out to be 37.5ng through the enzyme-linked immuno sorbent assay (ELISA), and a detecting method is built based on the IgM AlphaLISA for resisting enterovirus71 (EV71) VP1 through optimization of the donor microbeads, the receptor microbeads, blood serum and other test reaction conditions. The reagent kit is used for detecting the enterovirus71, is good in specificity, high in sensitivity and small in blood serum dosage, does not need to be washed, and cannot be influenced by hemolysis.
Owner:中国疾病预防控制中心病毒病预防控制所
ELISA (enzyme-linked immuno sorbent assay) detection kit for porcine parvovirus IgM (immunoglobulin m) antibodies as well as preparation method and application of ELISA detection kit
InactiveCN103364552AOvercoming defects such as low sensitivityHigh sensitivityMaterial analysisAntigenHorseradish peroxidase
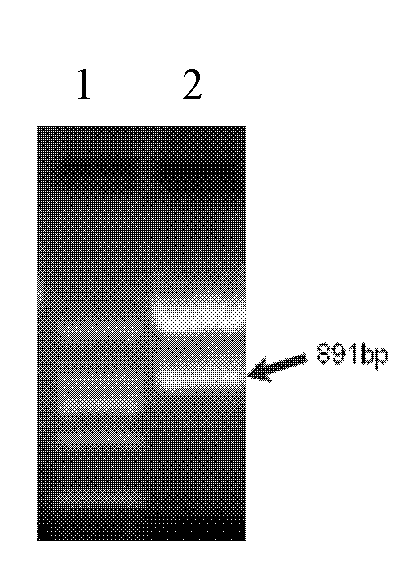
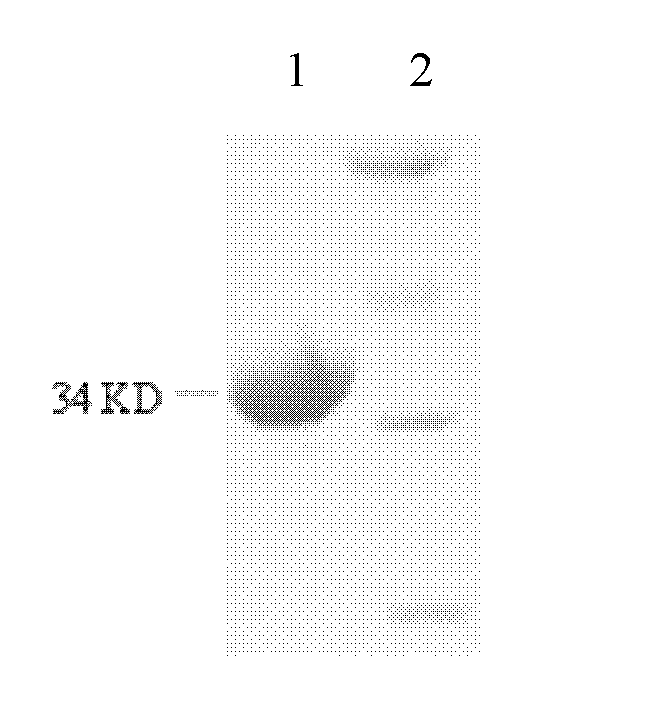
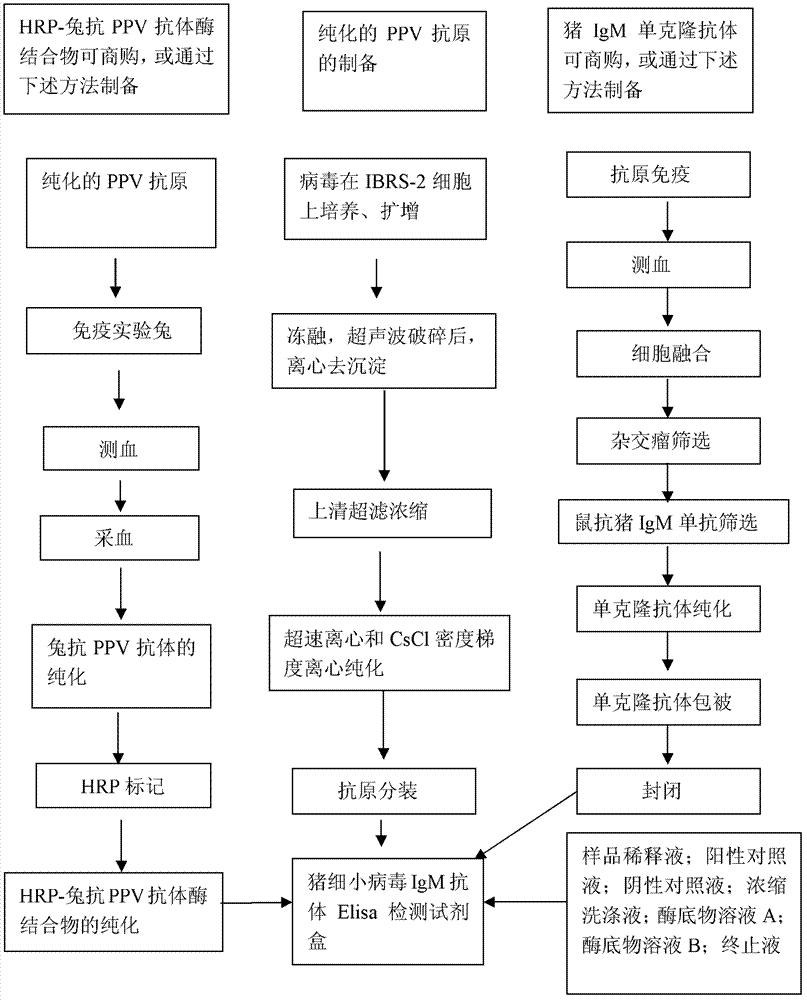
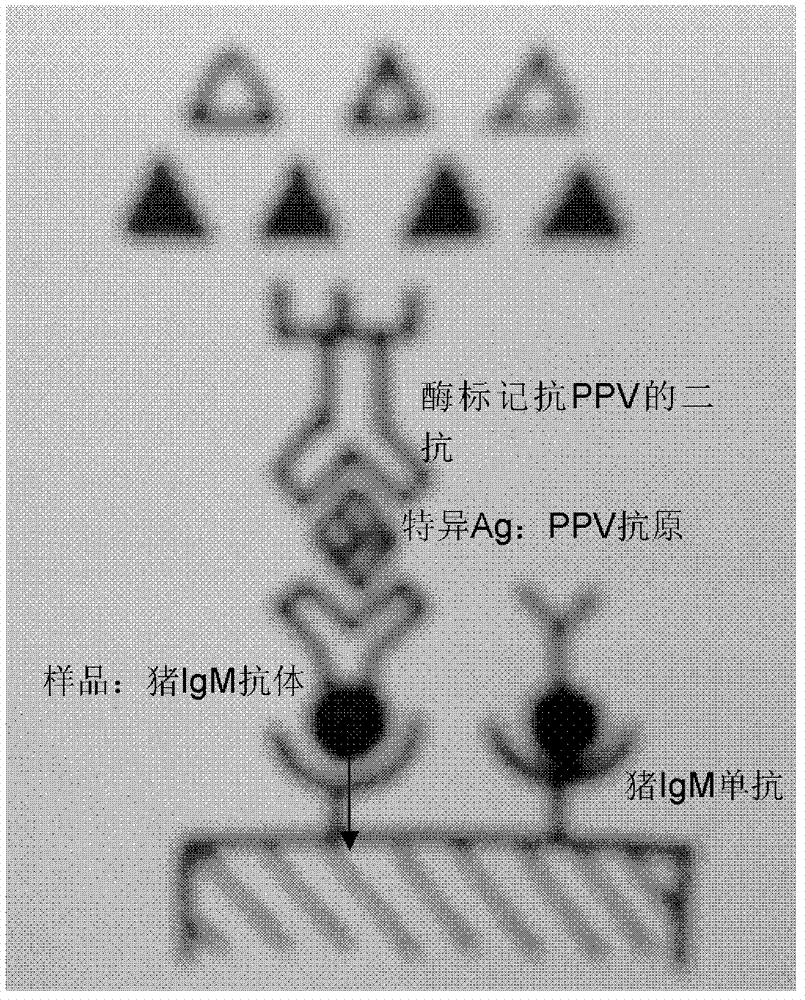
The invention relates to an ELISA (enzyme-linked immuno sorbent assay) detection kit for porcine parvovirus IgM (immunoglobulin m) antibodies as well as a preparation method and application of the ELISA detection kit. The ELISA detection kit for the porcine parvovirus IgM antibodies comprises an ELISA plate coating a porcine IgM monoclonal antibody, sealing fluid, a sample diluent, a detection antigen, an enzyme conjugate, a concentrated cleaning solution, a zymolyte solution A, a zymolyte solution B and a stop solution, wherein the detection antigen is a purified porcine parvovirus, and the enzyme conjugate is a horse-radish peroxide enzyme-anti-PPV (porcine parvovirus) antibody enzyme conjugate. The specificity of the ELISA detection kit provided by the invention reaches 100% and the sensitivity of the ELISA detection kit reaches up to 1:800, so that the ELISA detection kit can be used for performing early diagnosis on PPV infection of a swinery.
Owner:WUHAN CHOPPER BIOLOGY
Purification method of human immunoglobulin for intravenous injection
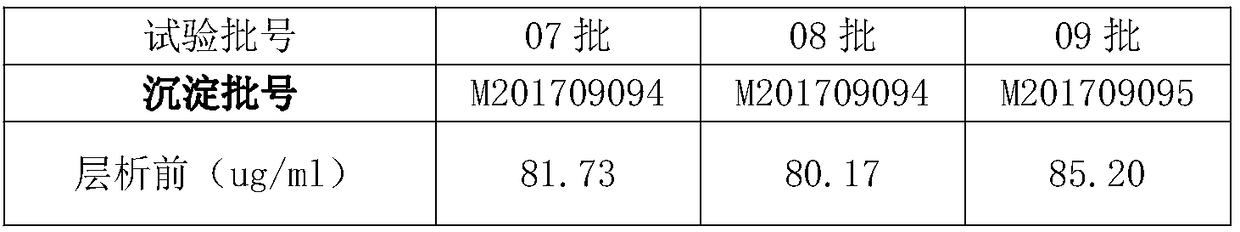
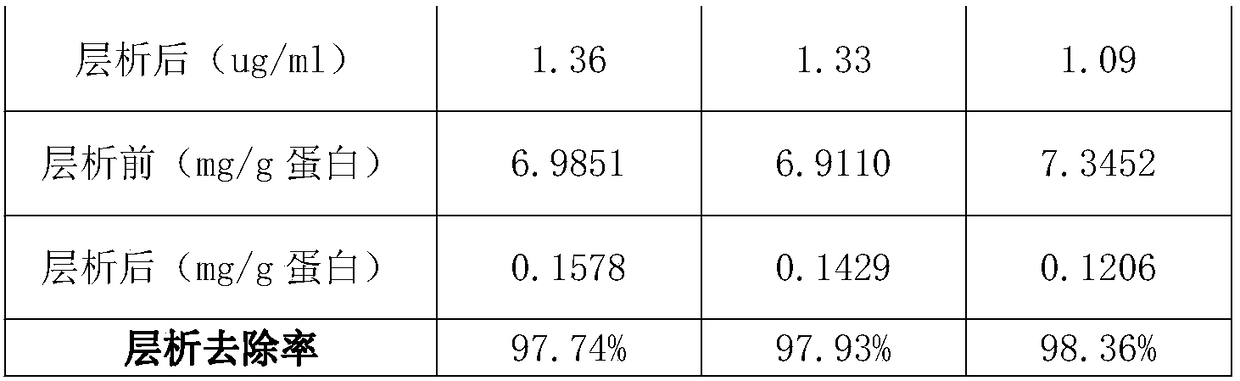
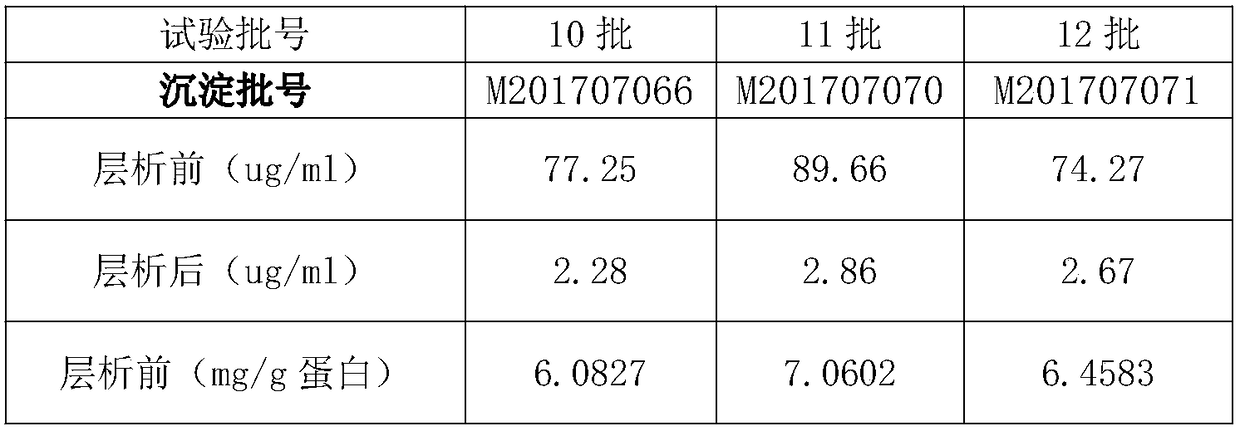
InactiveCN108623677AAccurately control pHGood removal effectPeptide preparation methodsImmunoglobulinsPurification methodsChromatography liquid
The invention discloses a purification method of human immunoglobulin for intravenous injection. The purification method is used for purification of a secondary sedimentation ingredient. The purification method of the human immunoglobulin for the intravenous injection is characterized by comprising the following steps of: S1, dissolve: dissolving the secondary sedimentation ingredient with water for injection, and stirring for 2-4h at 2.0-8.0 DEG C to form a dissolve liquid, S2, filtration: filtering the dissolve liquid with a 0.45 micrometers filter membrane and then with a 0.2 micrometers filter membrane to form a filtrate, S3: filtrate adjustment: adjusting a pH (potential of hydrogen) of the filtrate to 5.60-6.00, a protein concentration to 10-13g / L, and conductivity to 0.2-1.90ms / cm to form a pre-chromatography liquid, S4, chromatography: performing chromatography with strong anion exchange gel, flushing the gel before the chromatography for balancing to allow a difference betweena pH of the gel and a pH of the liquid before the chromatography to be from -0.10 to 0.10, performing gel chromatography sample loading at a linear flow rate of 0.5-1.5cm / min and chromatography loading capacity of not exceeding 600g / L, and collecting a liquid after the chromatography. The method is simple and controllable, and greatly reduces a content of IgA (immunoglobulin A) and IgM (immunoglobulin M) in the human immunoglobulin for the intravenous injection.
Owner:HUALAN BIOLOGICAL ENG CHONGQING +1
Fluorescent quantitative PCR (Polymerase Chain Reaction) primers for detecting mycoplasma pneumoniae (MP) and application thereof
ActiveCN103740836AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationTest sampleIgm antibody
The invention discloses fluorescent quantitative PCR (Polymerase Chain Reaction) primers for detecting mycoplasma pneumoniae (MP) and application thereof. A primer pair provided by the invention is specific to a 16SrRNA gene or ATPase gene of MP and can be a primer pair 1 (shown by a sequence 1 and a sequence 2) or a primer pair 2 (shown by a sequence 3 and a sequence 4) or a primer pair 3 (shown by a sequence 5 and a sequence 6). Shown by experiments, compared with other commercialized detection primers, IgM (Immunoglobulin M) antibody detection methods and culture methods, a method in which the primer pair provided by the invention can be used for carrying out fluorescent quantitative PCR detection on test samples has the advantage that the MP can be specifically detected without being interfered by four kinds of common pathogenic mycoplasmas which have a relatively close genetic relationship with the MP, as well as common respiratory tract bacteria and fungal pathogenic bacteria. The method can be used for qualitatively detecting the MP and detecting the MP quantitatively very well, is a quantitative PCR detection technology which is quick, is strong in specificity and high in sensitivity and is suitable for clinically detecting the MP of all strains, and has good detection effect and application value in clinical detection.
Owner:BEIJING CHAOYANG HOSPITAL CAPITAL MEDICAL UNIV
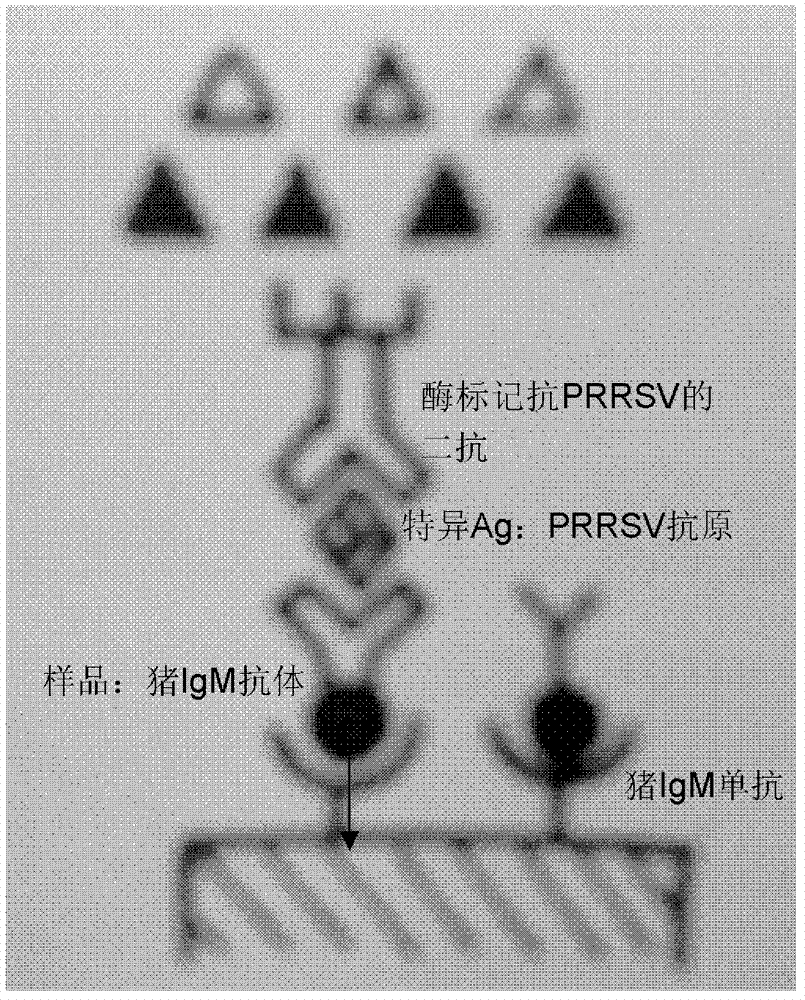
ELISA (enzyme-linked immuno sorbent assay) detection kit for porcine reproductive and respiratory syndrome virus IgM (immunoglobulin m) antibodies as well as preparation method and application of ELISA detection kit
InactiveCN103364551AOvercoming defects such as low sensitivityHigh sensitivityRecovery/purificationMaterial analysisAntigenSorbent
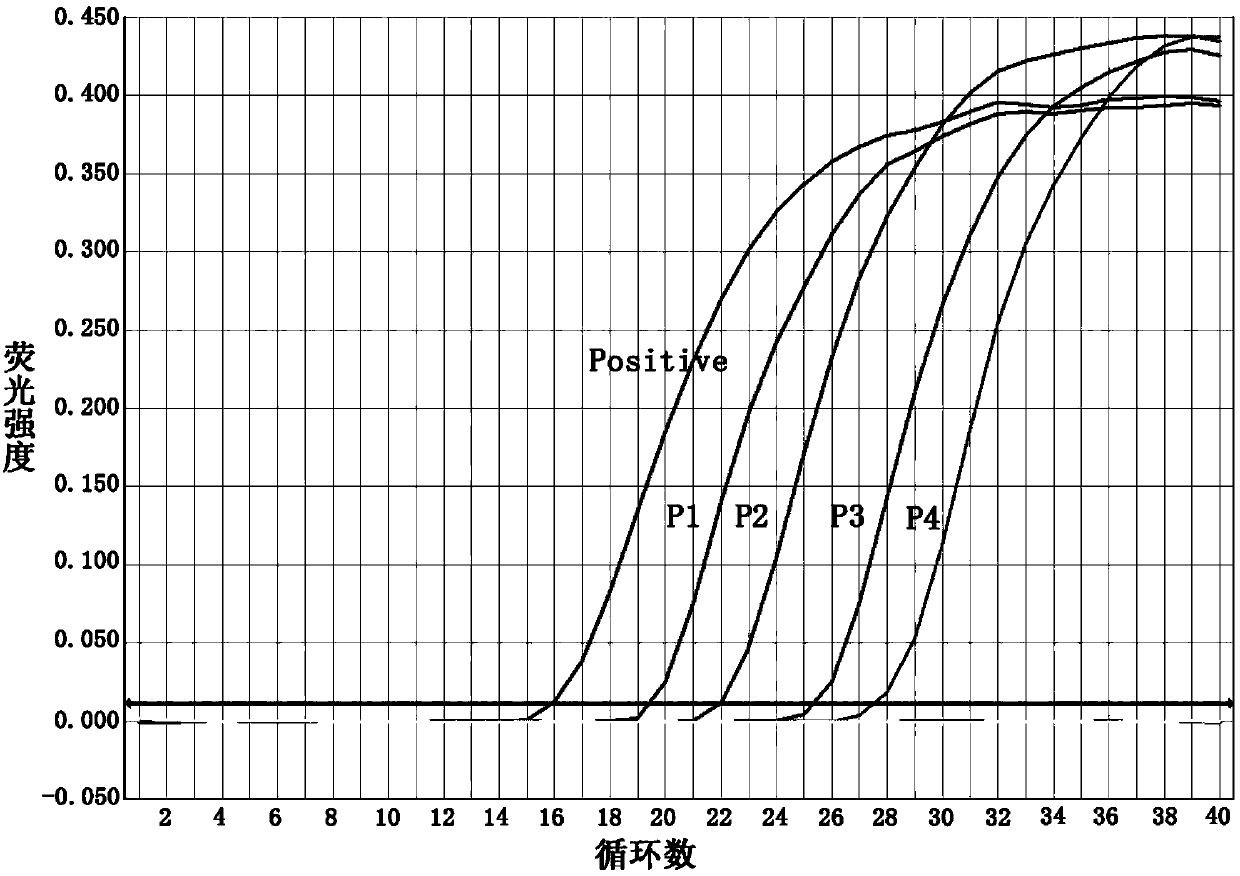
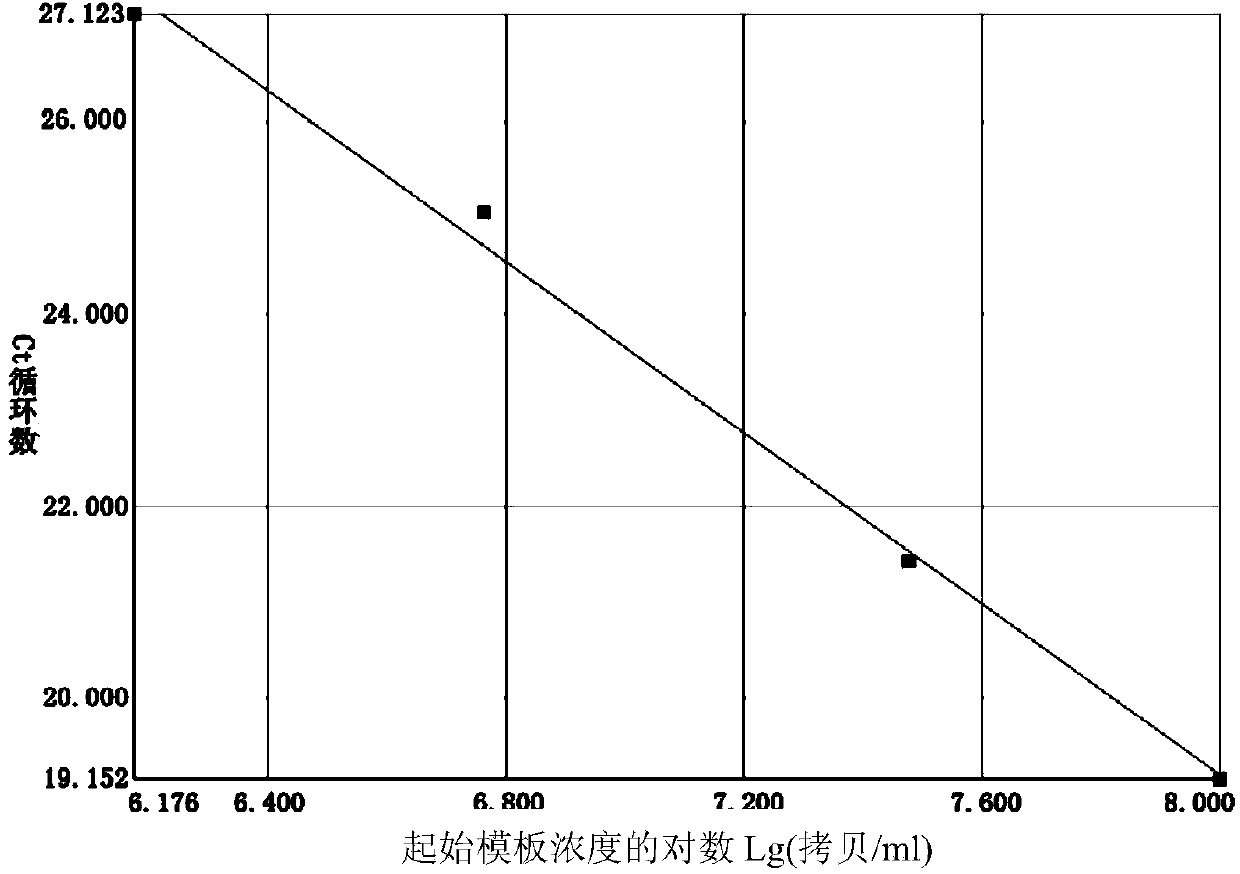
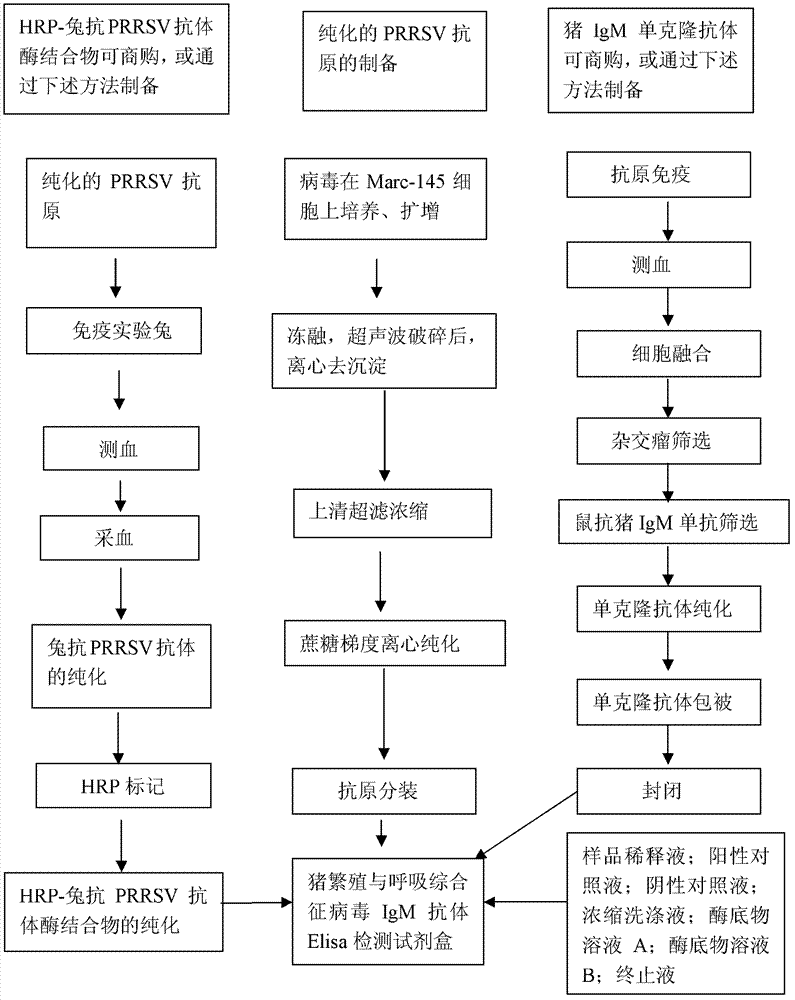
The invention relates to an ELISA (enzyme-linked immuno sorbent assay) detection kit for porcine reproductive and respiratory syndrome virus IgM (immunoglobulin m) antibodies as well as a preparation method and application of the ELISA detection kit. The ELISA detection kit for the porcine reproductive and respiratory syndrome virus IgM antibodies comprises an ELISA plate coating a porcine IgM monoclonal antibody, sealing fluid, a sample diluent, a detection antigen, an enzyme conjugate, a concentrated cleaning solution, a zymolyte solution A, a zymolyte solution B and a stop solution, wherein the detection antigen is a purified porcine reproductive and respiratory syndrome virus, and the enzyme conjugate is a horse-radish peroxide enzyme-anti-PRRSV (porcine reproductive and respiratory syndrome virus) antibody enzyme conjugate. The specificity of the ELISA detection kit provided by the invention reaches 100% and the sensitivity of the ELISA detection kit reaches up to 1:800, so that the ELISA detection kit can be used for performing early diagnosis on PRRSV infection of a swinery.
Owner:WUHAN CHOPPER BIOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com