Method for detecting hepatitis A virus antibodies, kit for detection through method and preparation method for kit
A hepatitis A virus and kit technology, applied in the field of biology, can solve the problems of difficult storage, decreased activity of markers, cumbersome operation, etc., and achieve the effects of wide pH range tolerance, elimination of false positive interference, and fast and efficient process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
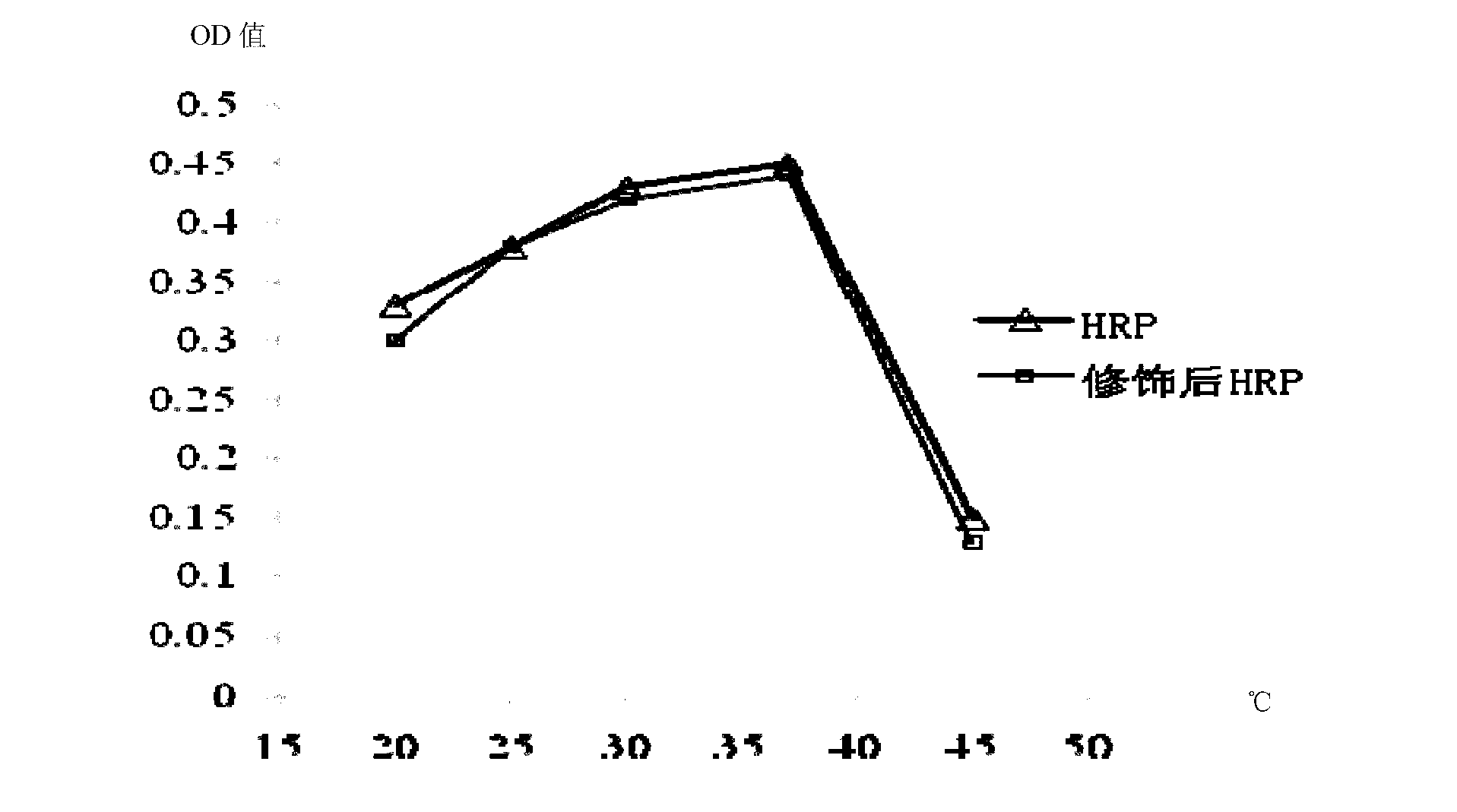
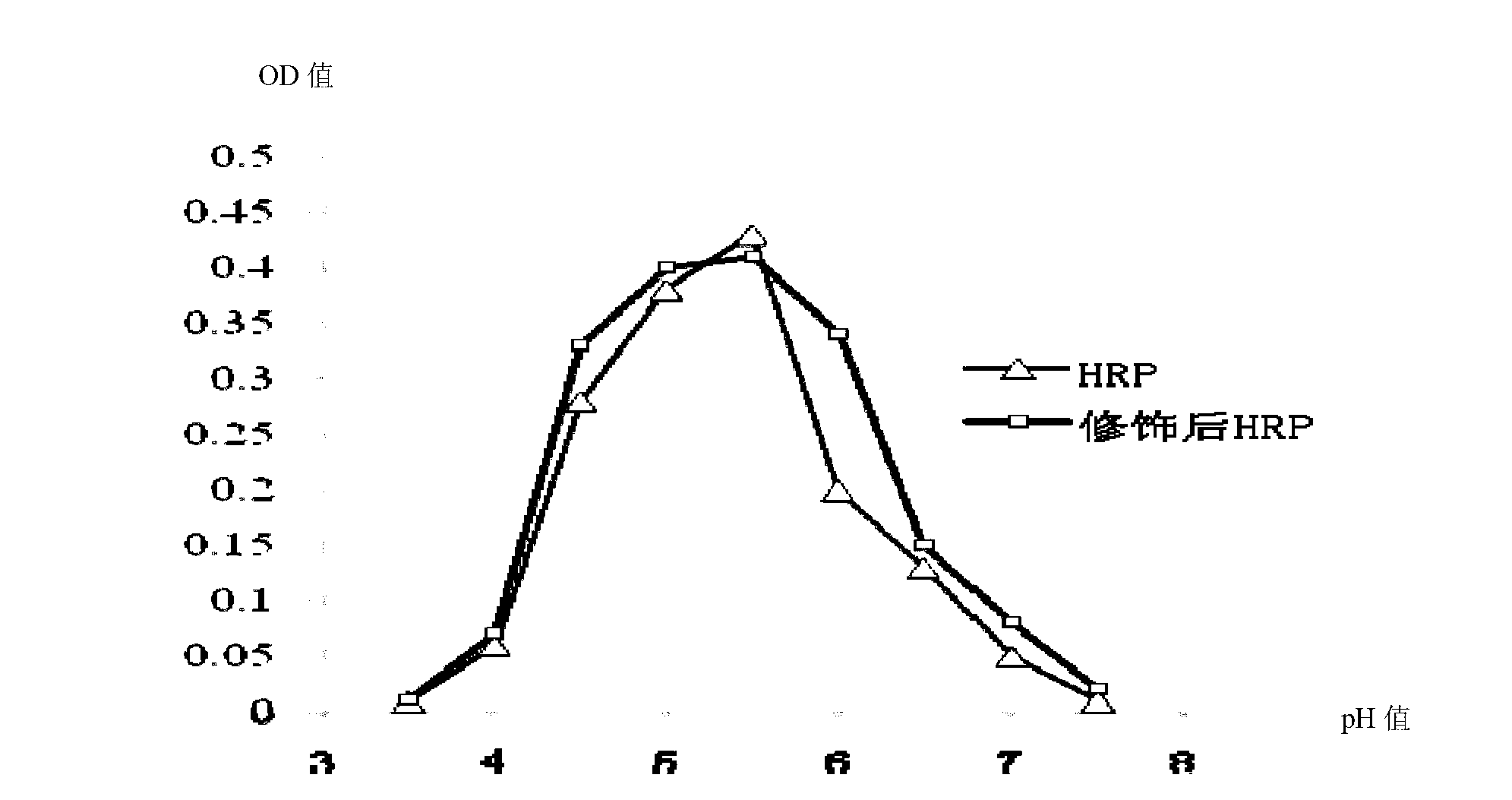
Image
Examples
Embodiment 1
[0051] Amination Modification of Horseradish Peroxidase
[0052] 1. Main raw materials: horseradish peroxidase HRP; ethylenediamine, EDC-HCl (1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride); sodium dihydrogen phosphate , disodium hydrogen phosphate, sodium chloride, benzoic acid, glycerin; trehalose.
[0053] 2. Main instruments: precision electronic balance; refrigerator; pH meter; oscillator; magnetic stirrer; freeze dryer.
[0054] 3. The steps are as follows:
[0055] Dissolve 1.0g of horseradish peroxidase in 25ml of 0.01M pH7.4 phosphate buffer, add 80.0mg of ethylenediamine, adjust the pH to 5.0 with HCl, mix the resulting solution with 120.0mg of EDC, and shake at room temperature for 1h , and then loaded into a dialysis bag and dialyzed with pre-prepared PBS for 2 hours.
[0056] Add 10% glycerin by volume, 3% trehalose by mass and 0.05% benzoic acid to the solution prepared above, and then store it in refrigeration for immediate use or after concentrat...
Embodiment 2
[0068] Preparation of enzyme-labeled hepatitis A antigen
[0069] 1. Raw materials: 1mg / ml hepatitis A antigen; 4.8mg / ml Sulfo-SMCC (4-(N-maleimidomethyl)cyclohexane-1-carboxylic acid sulfosuccinimidyl ester sodium salt) ;Ultrafiltration tube with molecular weight cut-off MWCO of 10K; Aminated HRP self-made or commercialized natural HRP; Sodium dihydrogen phosphate, disodium hydrogen phosphate, sodium chloride.
[0070] 2. Main instruments: high-speed centrifuge; refrigerator; pH meter.
[0071] 3. The operation steps are as follows:
[0072] (1) Dissolve a certain amount of amino-horseradish peroxidase in 1ml double-distilled water to make a 5mg / ml solution; at the same time, prepare 0.01M phosphate buffered saline (hereinafter referred to as PBS) for later use.
[0073] (2) Add 70 μl of cross-linking agent Sulfo-SMCC to 1 ml of newly prepared 5 mg / ml aminated HRP solution, mix well and react at room temperature for 30 min.
[0074] (3) Add the reactant to a MWCO 10K ultra...
Embodiment 3~5
[0101] Preparation of chromogenic solution
[0102] 1. Main raw materials: sodium acetate trihydrate, tetramethylbenzidine TMB, carbamide peroxide, ascorbic acid, chlorogenic acid; 2-cyano-3,3-diphenylisooctyl acrylate (octocrylene); Phosphoglycine.
[0103] 2. Preparation method:
[0104] (1) Weigh 8.3g of sodium acetate trihydrate crystals and add it to 600ml of ultrapure water, shake and mix well, adjust the pH of the solution to 5.0 with 0.1mol / L hydrochloric acid, and then prepare 0.05mol / L acetic acid-sodium acetate buffer liquid.
[0105] (2) Weigh 0.4-1.0 g of tetramethylbenzidine (hereinafter referred to as TMB), dissolve it in 3 ml of absolute ethanol, then add the dissolved TMB solution to the above-mentioned acetic acid-sodium acetate buffer solution, shake and mix well, spare.
[0106] (3) Weigh 80 mg of chlorogenic acid and 80 mg of ascorbic acid, add them to the buffer, shake and mix.
[0107] (4) Measure 10ml of isooctyl 2-cyano-3,3-diphenylacrylate (octoc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com