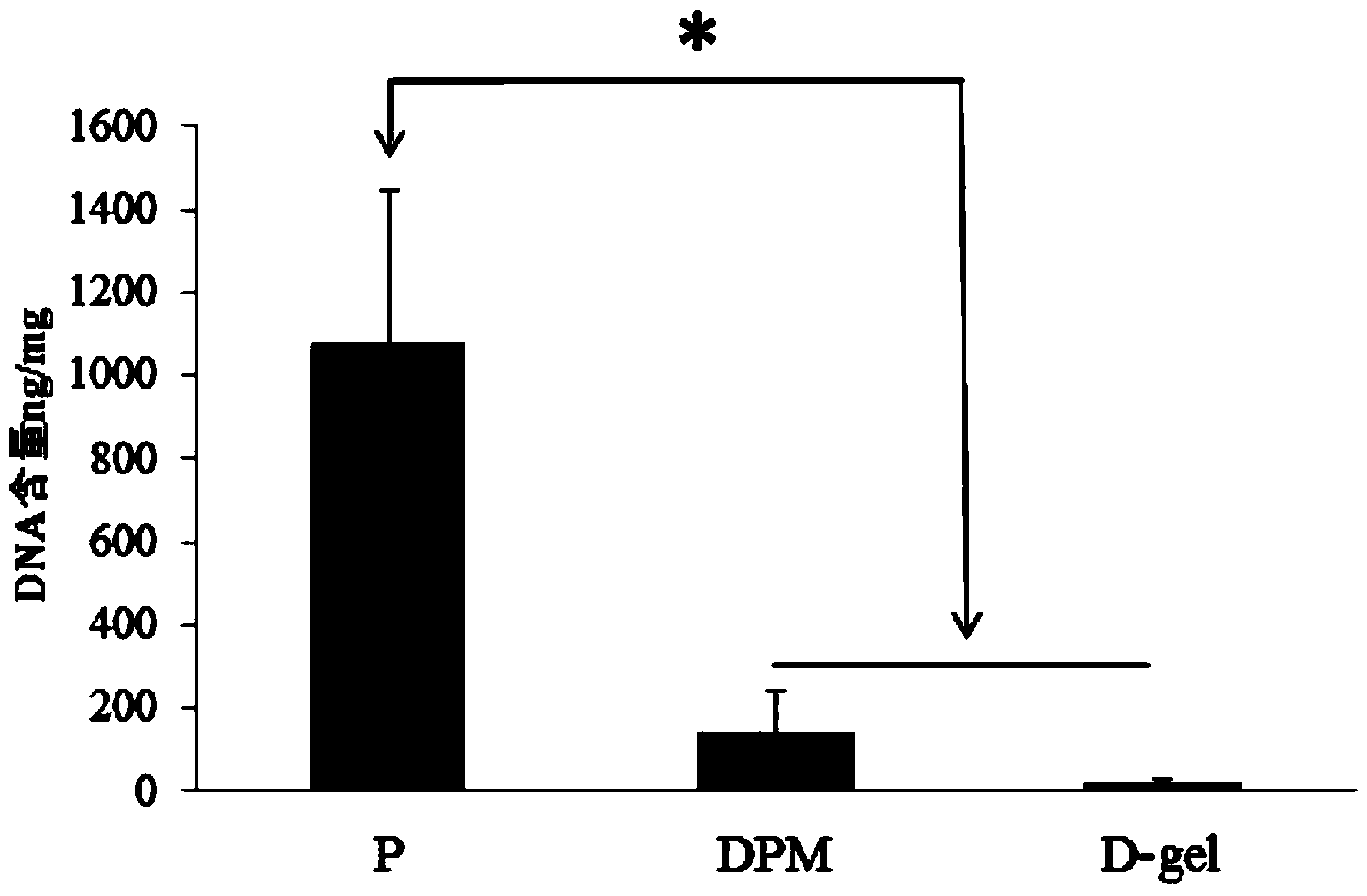
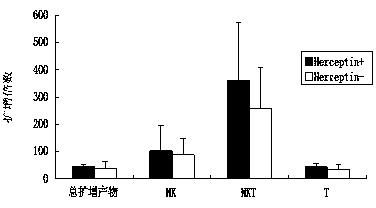Patents
Literature
296 results about "Phosphate buffered saline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phosphate-buffered saline (abbreviated PBS) is a buffer solution commonly used in biological research. It is a water-based salt solution containing disodium hydrogen phosphate, sodium chloride and, in some formulations, potassium chloride and potassium dihydrogen phosphate. The buffer helps to maintain a constant pH. The osmolarity and ion concentrations of the solutions match those of the human body (isotonic).
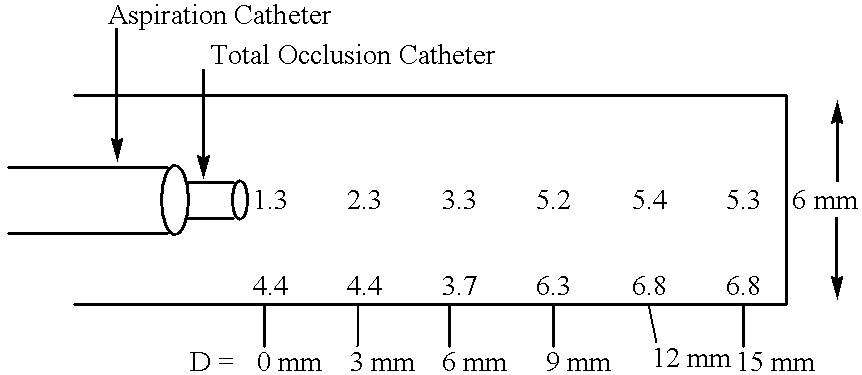
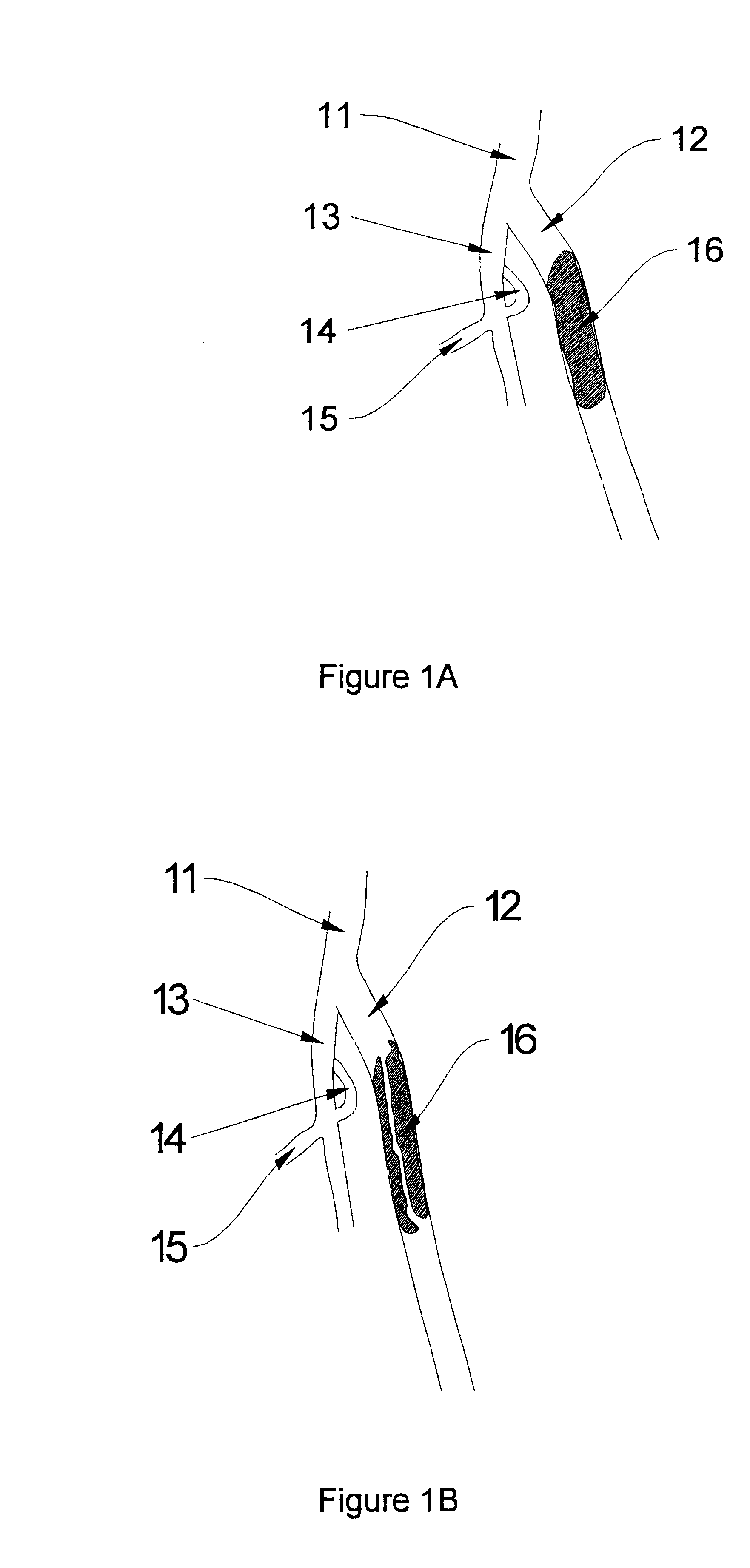
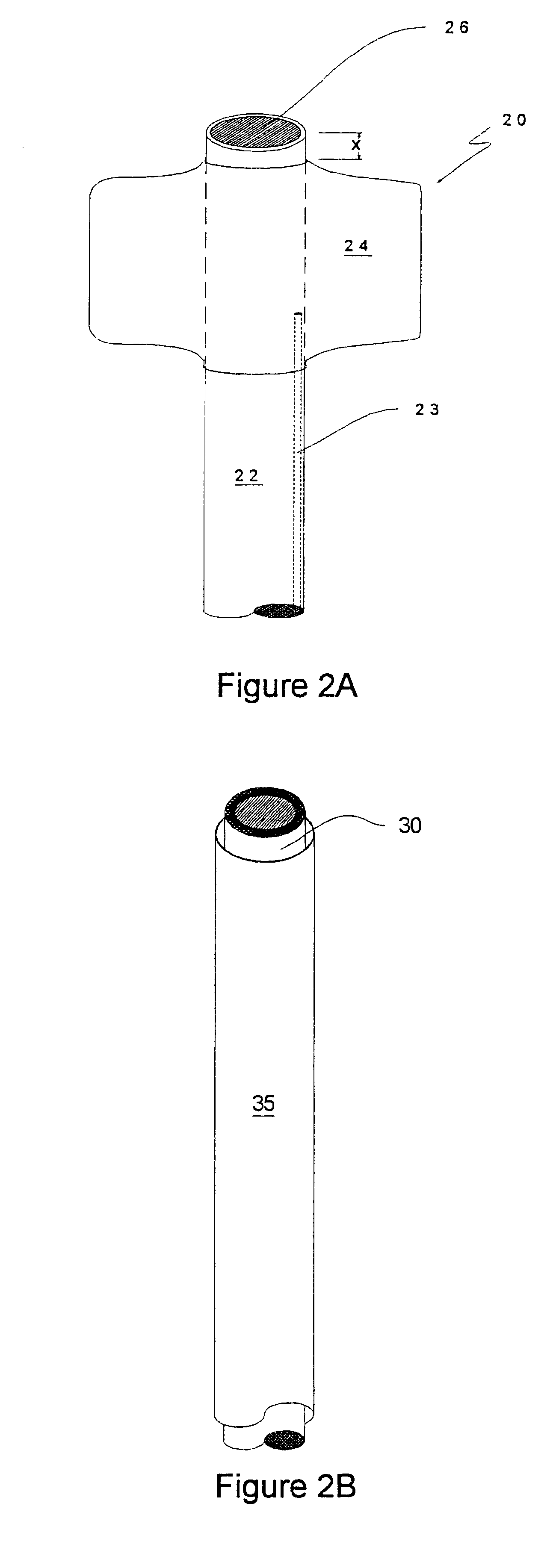
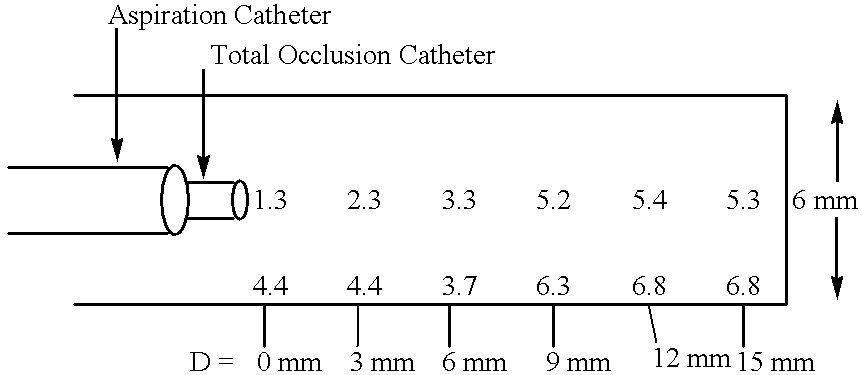
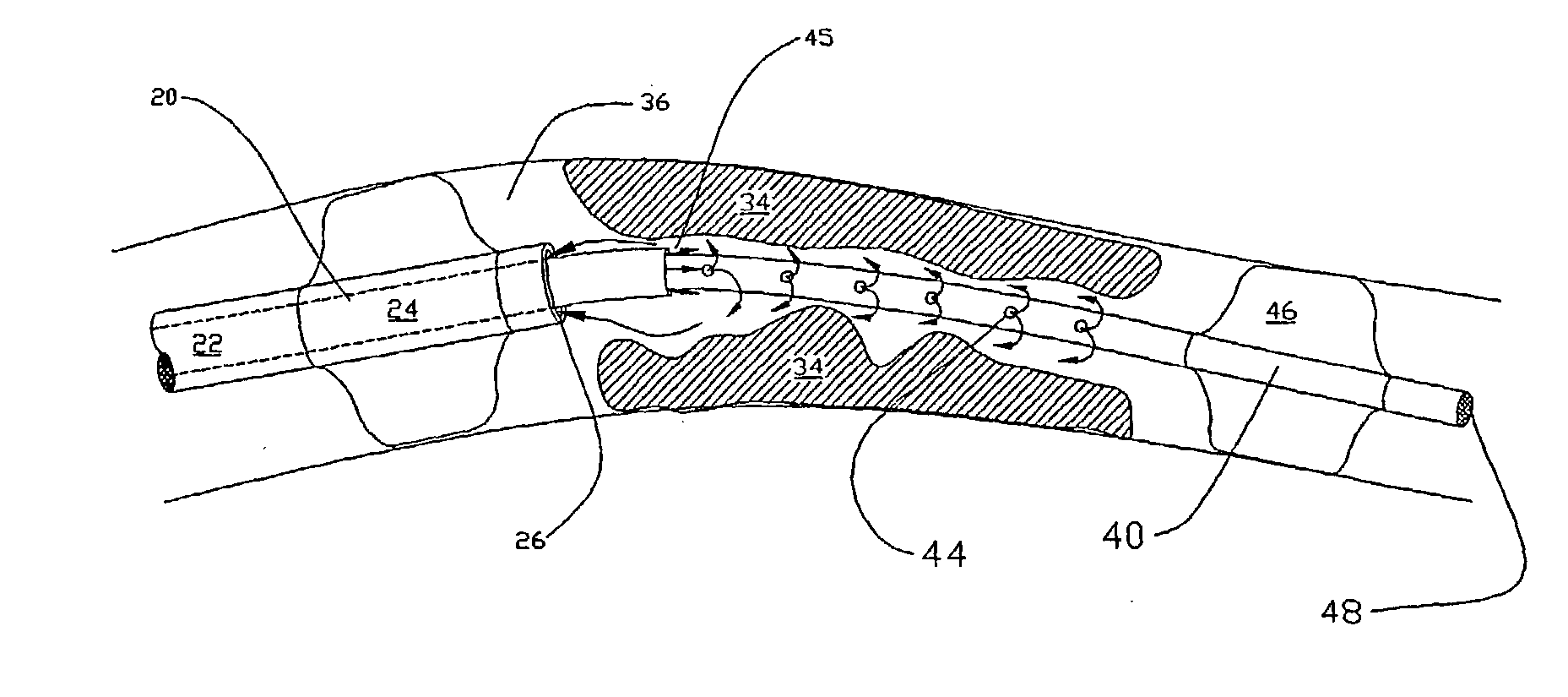
Methods for enhancing fluid flow through an obstructed vascular site, and systems and kits for use in practicing the same
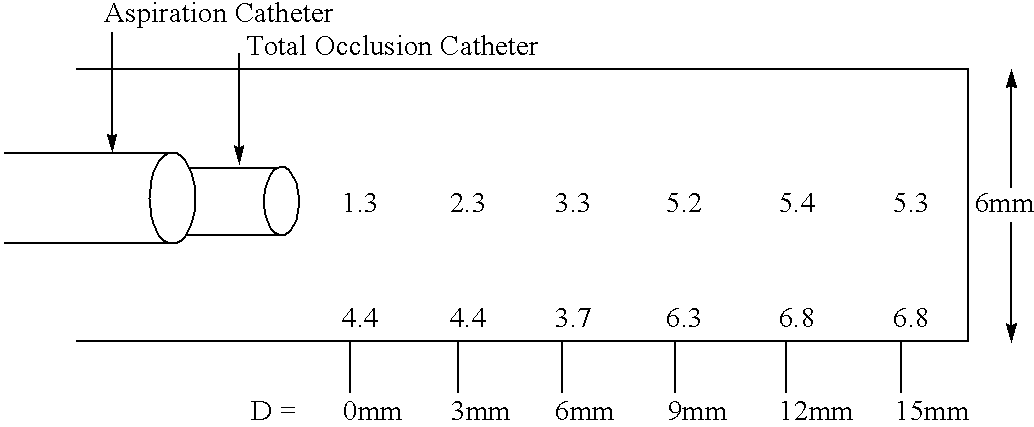
Methods of enhancing fluid flow through a vascular site occupied by a vascular occlusion, as well as systems and kits for use in practicing the same, are provided. In practicing the subject methods, the vascular site is flushed simultaneously with a first dissolution fluid (e.g., an organic matter dissolution fluid and / or an inorganic matter dissolution fluid), and a second dissolution fluid attenuating fluid, where flushing is carried out in a manner such that only a surface of the vascular occlusion is contacted with the non-attenuated dissolution fluid. Examples of dissolution fluid / dissolution fluid attenuating fluid pairs include: (1) oxidizing agent fluid and fluid comprising oxidizable neutralizing agent; (2) surfactant fluid and phosphate buffered saline; (3) acidic solution and phosphate buffered saline; etc. Flushing is carried out in this manner for a period of time sufficient for fluid flow through the vascular site to be enhanced, e.g. increased or established. The subject methods, systems and kits for practicing the same find use in the treatment of a variety of different vascular diseases characterized by the presence of vascular occlusions, including both partial and total occlusions.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Methods for enhancing fluid flow through an obstructed vascular site, and systems and kits for use in practicing the same
Methods of enhancing fluid flow through a vascular site occupied by a vascular occlusion, as well as systems and kits for use in practicing the same, are provided. In practicing the subject methods, the vascular site is flushed simultaneously with a first dissolution fluid (e.g., an organic matter dissolution fluid and / or an inorganic matter dissolution fluid), and a second dissolution fluid attenuating fluid, where flushing is carried out in a manner such that only a surface of the vascular occlusion is contacted with the non-attenuated dissolution fluid. Examples of dissolution fluid / dissolution fluid attenuating fluid pairs include: (1) oxidizing agent fluid and fluid comprising oxidizable neutralizing agent; (2) surfactant fluid and phosphate buffered saline; (3) acidic solution and phosphate buffered saline; etc. Flushing is carried out in this manner for a period of time sufficient for fluid flow through the vascular site to be enhanced, e.g. increased or established. The subject methods, systems and kits for practicing the same find use in the treatment of a variety of different vascular diseases characterized by the presence of vascular occlusions, including both partial and total occlusions.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
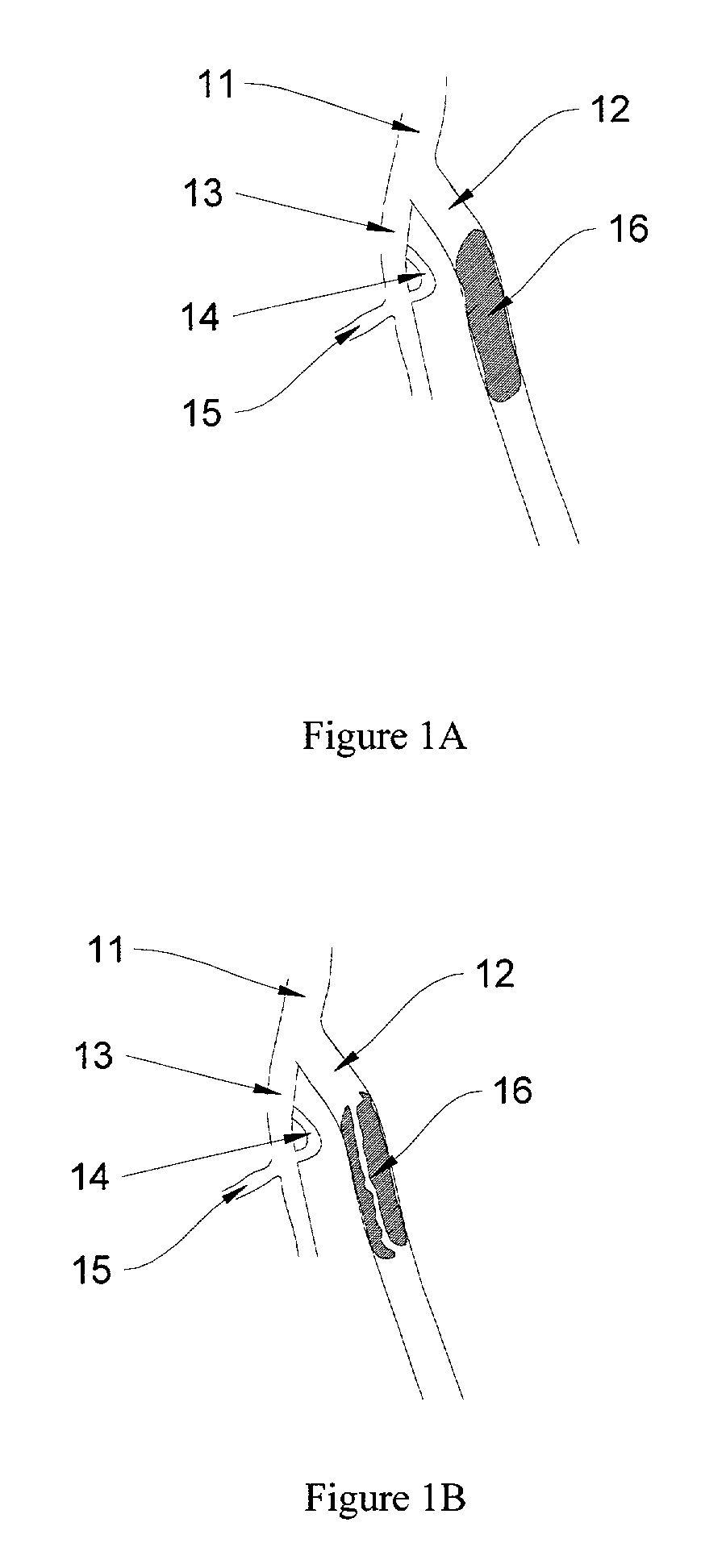
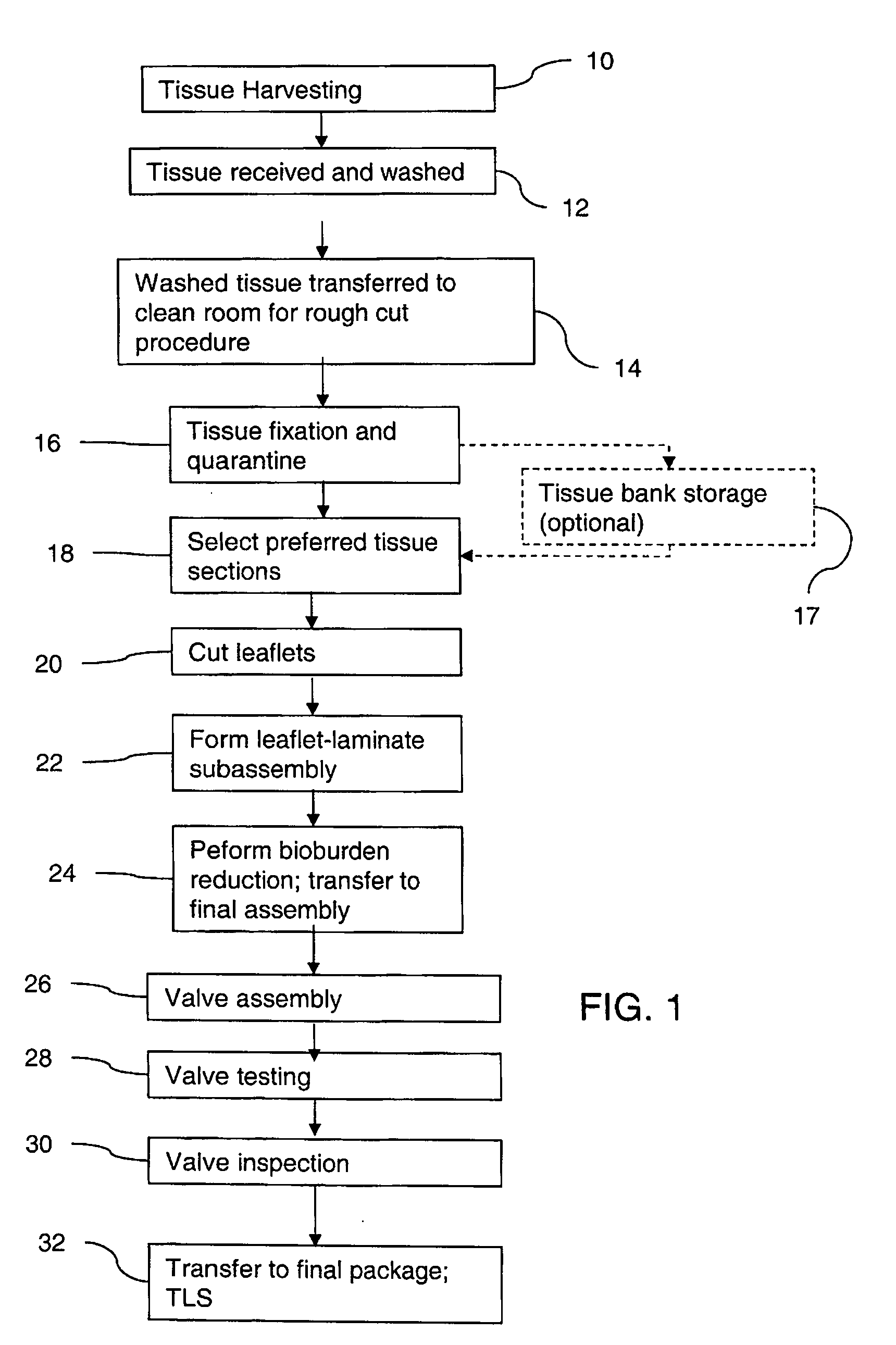
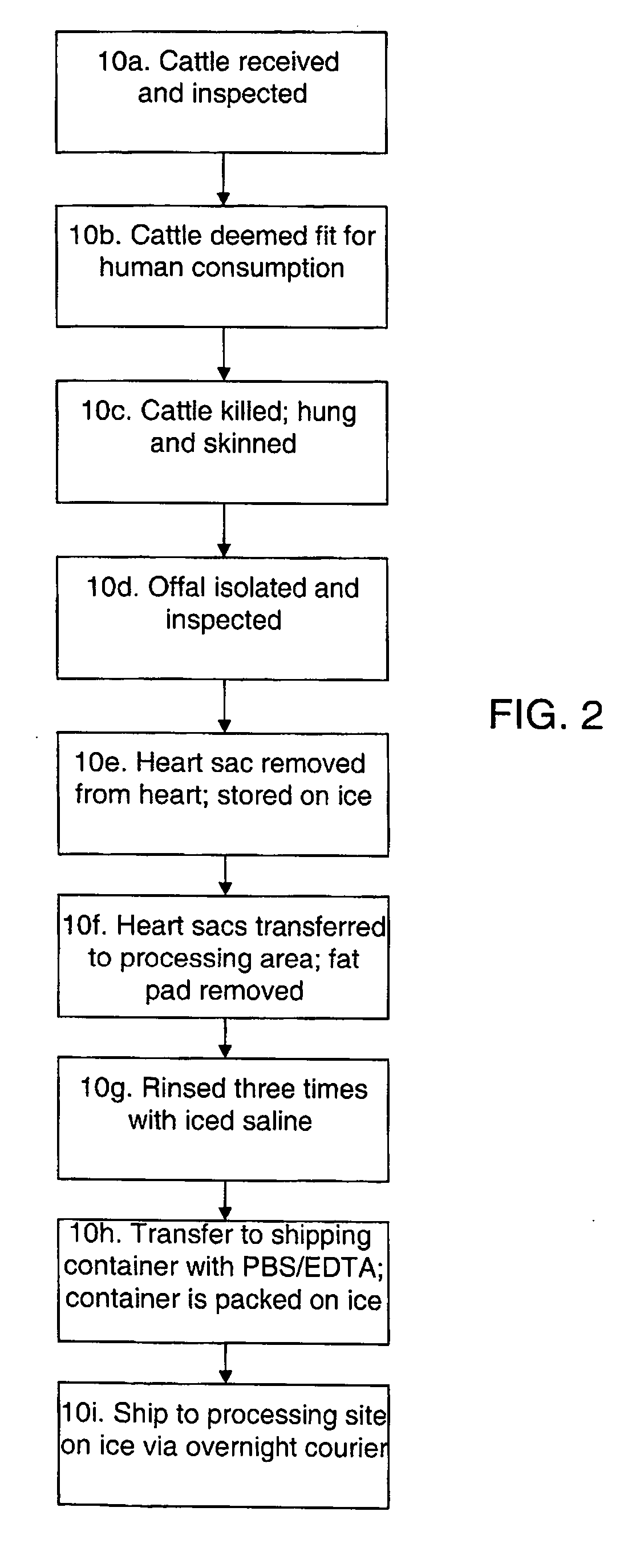
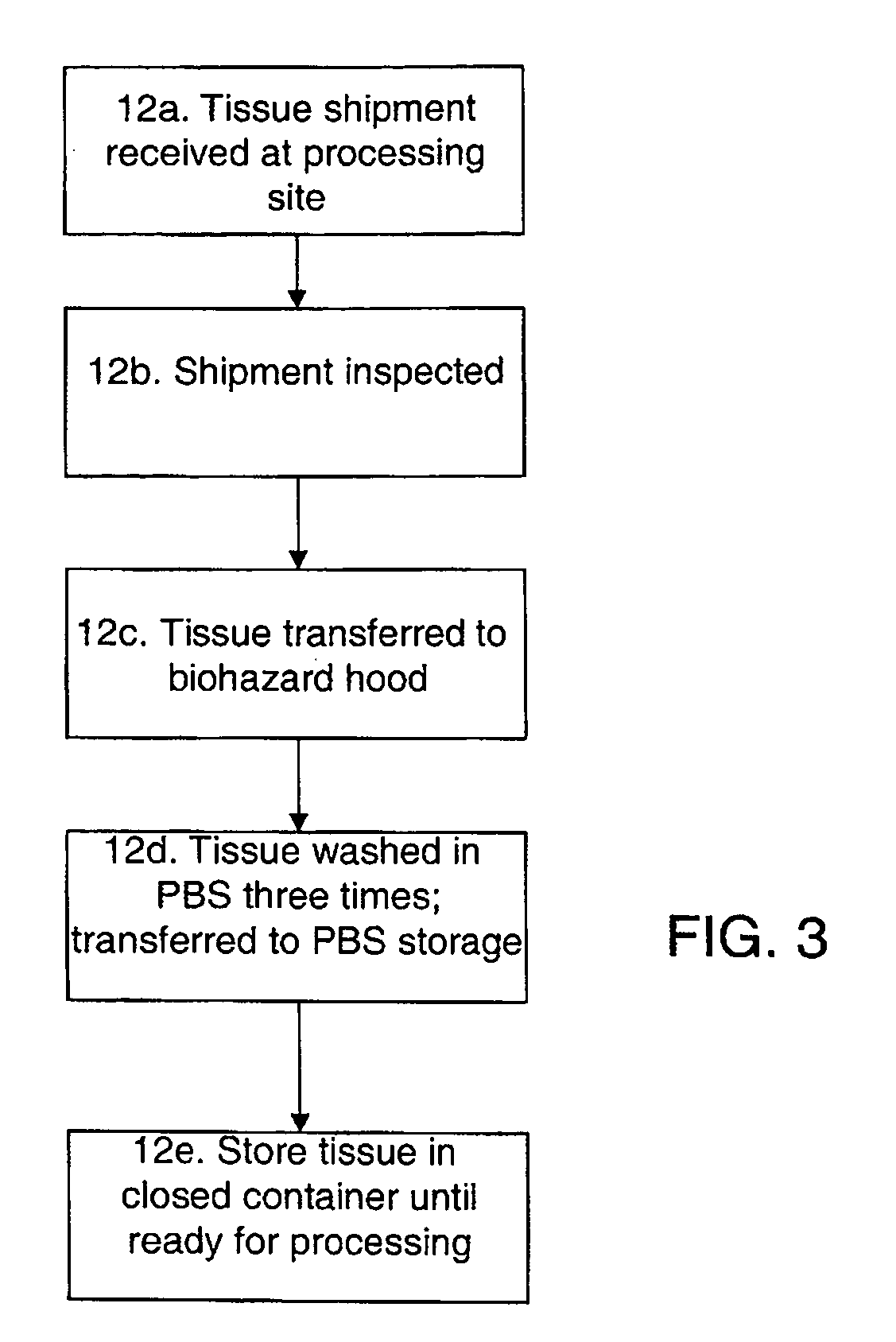
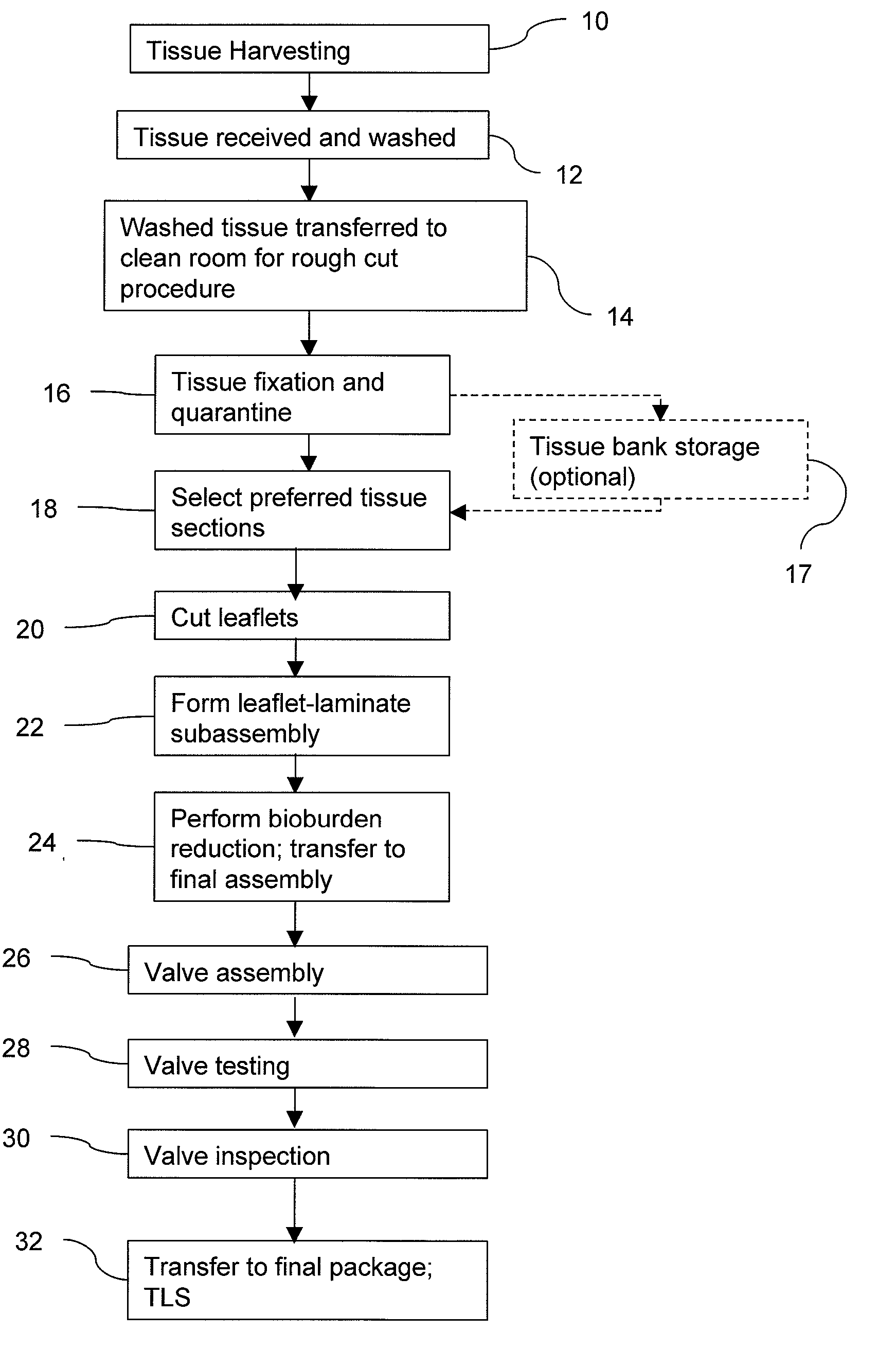
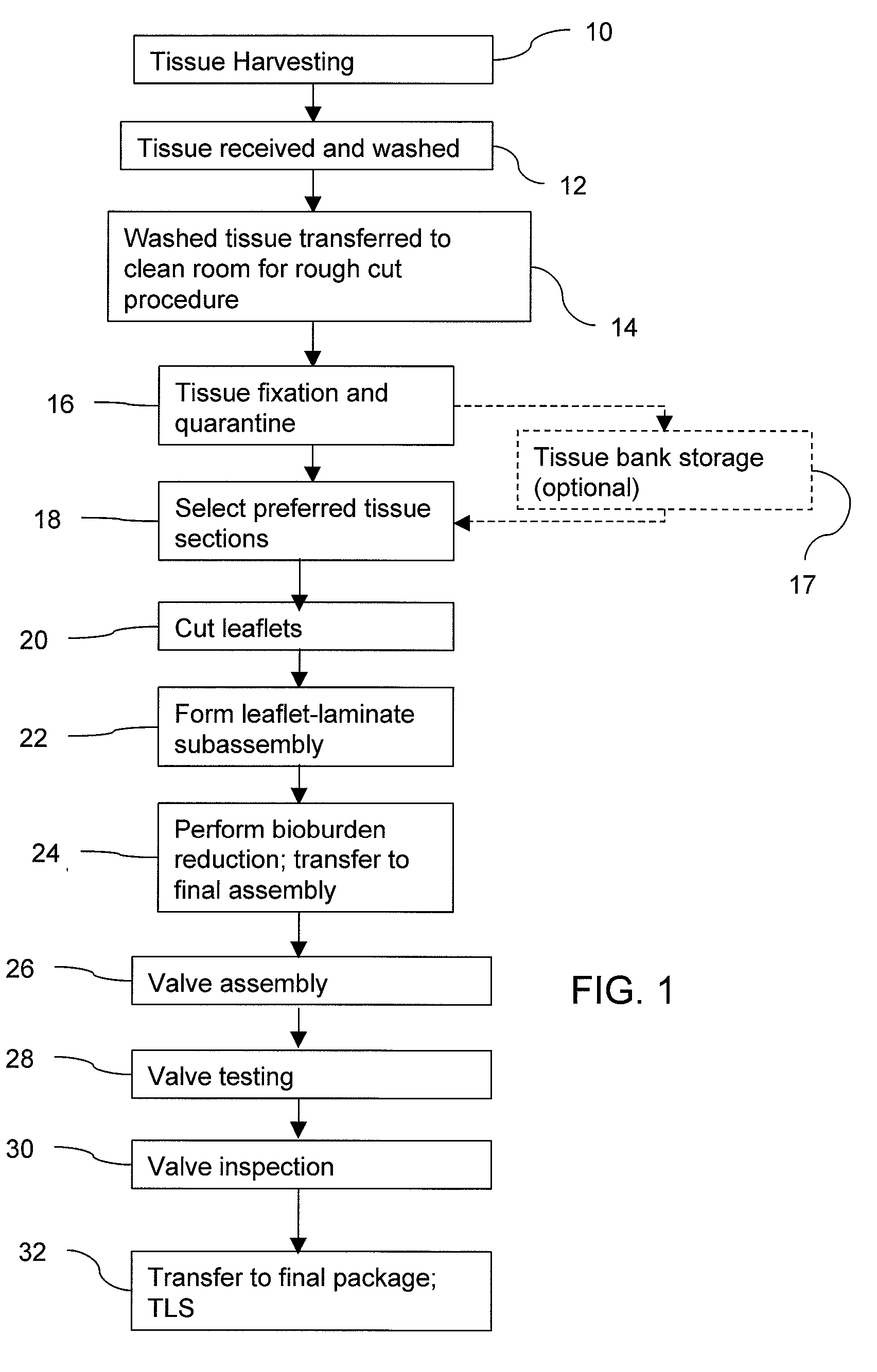
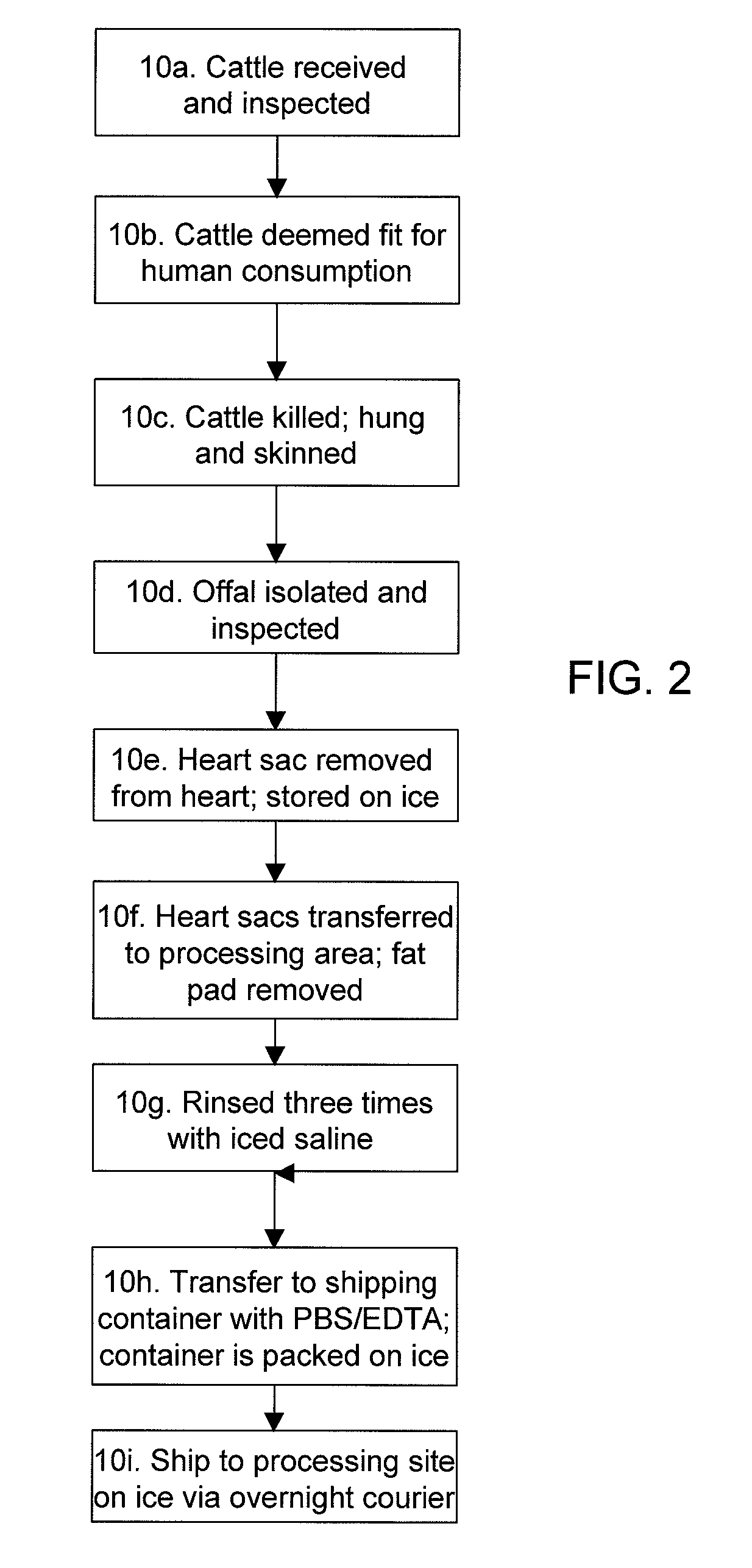
Methods for processing biological tissue
ActiveUS20060154230A1Remove background levelAvoid degradationDead animal preservationPhosphateCalcification
A method for processing biological tissue used in biological prostheses includes providing a tissue procurement solution formed from a phosphate buffered saline (PBS) solution and a chelating agent. The tissue is transferred from the tissue procurement solution and undergoes chemical fixation. The fixed tissue is then immersed in a series of fresh bioburden reduction process (BRP) solutions to extract phospholipids. The tissue procurement solution reduces the bioburden on the stored tissue and preserves tissue architecture by minimizing tissue swelling. The tissue procurement solution further reduces calcium from the incoming water and / or tissue, and inhibits enzymes that digest the collagen matrix. The serial immersion of the tissue in the fresh bioburden solutions ensures optimal extraction of phospholipids thereby mitigating subsequent calcification of the tissue.
Owner:MEDTRONIC INC
Methods for processing biological tissue
ActiveUS20060207031A1Remove background levelAvoid degradationSuture equipmentsDead animal preservationCalcificationPhospholipid
A method for processing biological tissue used in biological prostheses includes providing a tissue procurement solution formed from a phosphate buffered saline (PBS) solution and a chelating agent. The tissue is transferred from the tissue procurement solution and undergoes chemical fixation. The fixed tissue is then immersed in a series of fresh bioburden reduction process (BRP) solutions to extract phospholipids. The tissue procurement solution reduces the bioburden on the stored tissue and preserves tissue architecture by minimizing tissue swelling. The tissue procurement solution further reduces calcium from the incoming water and / or tissue, and inhibits enzymes that digest the collagen matrix. The serial immersion of the tissue in the fresh bioburden solutions ensures optimal extraction of phospholipids thereby mitigating subsequent calcification of the tissue.
Owner:MEDTRONIC INC
Vascular embolization material
InactiveUS20060069168A1Easy to observeImprove abilitiesSurgical adhesivesPharmaceutical non-active ingredientsSwelling ratioWater insoluble
This invention provides an embolization material used for blocking a blood vessel in vivo for stopping the blood flow. The most suitable embolization material has a water swelling ratio of 30% or more, is degradable in a phosphate buffered saline, is formed as virtually spherical particles, and is preferably composed of a water insoluble poly(ethylene glycol) copolymer, wherein when the film formed from said polymer is saturated with water, it has an elastic modulus in tension of 1500 MPa or less. The embolization material of this invention can reliably block a blood vessel at an intended site without causing cohesion or clogging in a catheter or in the blood vessel at other than the intended site. Thereafter, the blocked site concerned can be liberated from the embolized state by degradation, and the degraded components can be metabolized or excreted outside the body.
Owner:TORAY IND INC
Methods for enhancing fluid flow through an obstructed vascular site, and systems and kits for use in practicing the same
Methods of enhancing fluid flow through a vascular site occupied by a vascular occlusion, as well as systems and kits for use in practicing the same, are provided. In practicing the subject methods, the vascular site is flushed simultaneously with a first dissolution fluid (e.g., an organic matter dissolution fluid and / or an inorganic matter dissolution fluid), and a second dissolution fluid attenuating fluid, where flushing is carried out in a manner such that only a surface of the vascular occlusion is contacted with the non-attenuated dissolution fluid. Examples of dissolution fluid / dissolution fluid attenuating fluid pairs include: (1) oxidizing agent fluid and fluid comprising oxidizable neutralizing agent; (2) surfactant fluid and phosphate buffered saline; (3) acidic solution and phosphate buffered saline; etc. Flushing is carried out in this manner for a period of time sufficient for fluid flow through the vascular site to be enhanced, e.g. increased or established. The subject methods, systems and kits for practicing the same find use in the treatment of a variety of different vascular diseases characterized by the presence of vascular occlusions, including both partial and total occlusions.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Methods and compositions for treating gastric disorders
Improved efficacy in treatment of gastric disorders with botulinum toxin is obtained using liposomal encapsulated botulinum formulations for topical administration of the botulinum toxin. The liposomes are typically administered in a physiologically acceptable carrier such as saline or phosphate buffered saline by application onto the surface of the tissue within into the gastrointestinal (GI) tract in need of treatment. Preferably, the formulation is applied via a roller, sponge or nozzle that is attached to an endoscope.
Owner:LIPELLA PHARMA
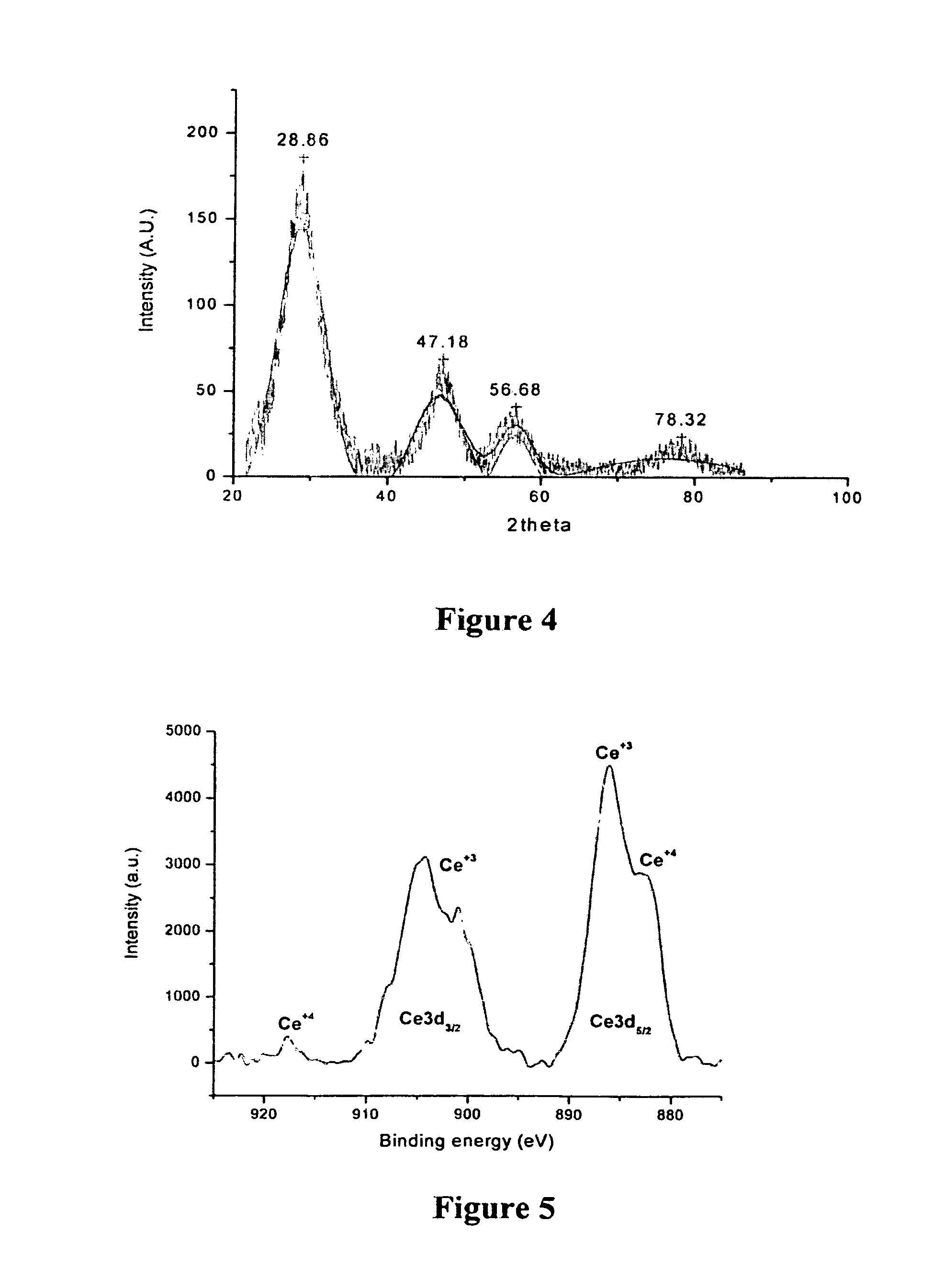
Synthesis of polymer coated ceria nanoparticles for biomedical applications
ActiveUS8333993B1Eliminate needImprove solubilityPowder deliveryInorganic active ingredientsSynthesis methodsPhosphate
Procedures and methods for synthesizing biodegradable polymer coated nanoceria result in stable nanoparticle preparations in aqueous systems and physiological relevant colloidal solutions, such as phosphate buffer saline. The coated nanoceria preparations increase the nanoparticle concentration in aqueous or colloidal solutions as most needed for antioxidant, free-radical scavenger, and autocatalytic biomedical applications, including, biological, pharmacological and potential clinical use. To meet this need, a facile synthetic procedure for preparation of a biodegradable polymer-coated nanoceria is disclosed; the preferred biodegradable polymer is dextran. The synthesis method occurs under ambient conditions in an aqueous phase without the use of surfactants and results in a monodispersed preparation that is dextran-coated as determined by dynamic light scattering (DLS). Preliminary characterization of polymer coated nanoceria by XPS, TEM, XRD, and the like shows that these nanoparticles have the necessary physical properties for the desired biological potency, such as Ce+4 / Ce+3 mixed valence state.
Owner:UNIV OF CENT FLORIDA RES FOUND INC
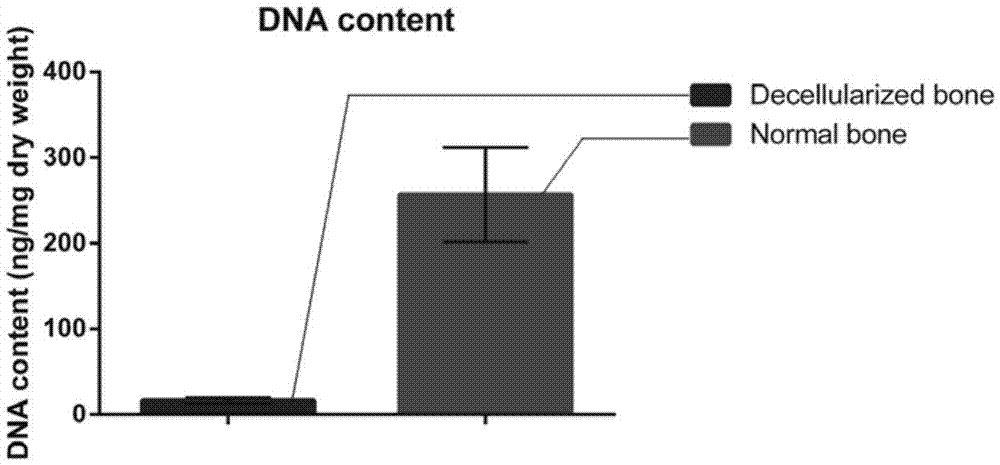
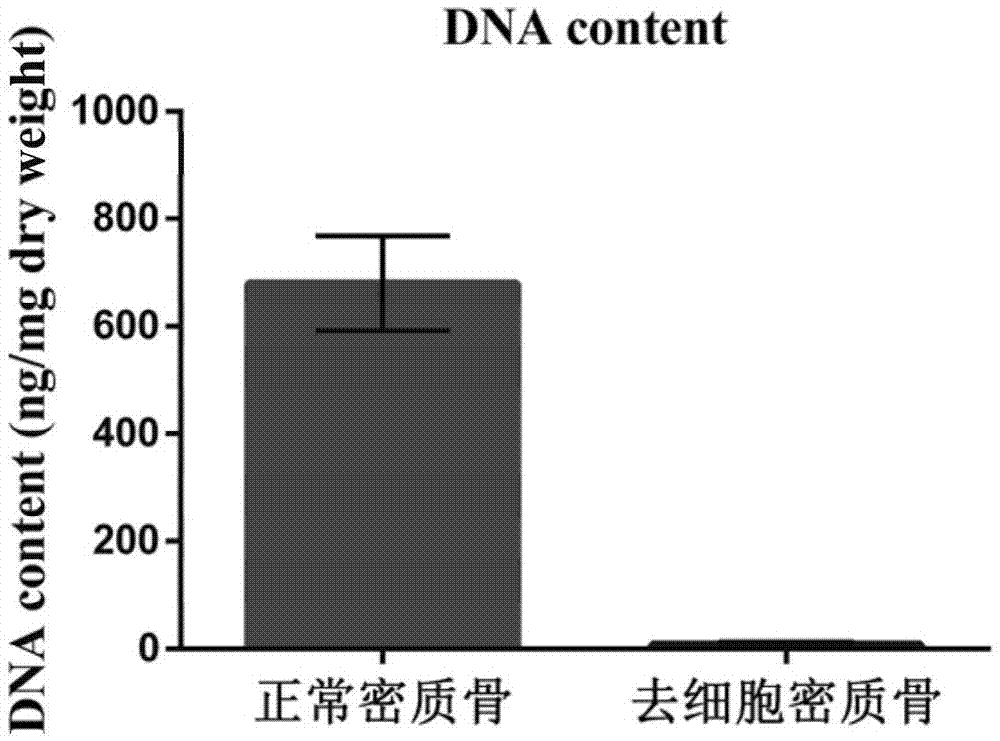
Natural-tissue-derived decellularized and decalcified bone material and preparation method thereof
InactiveCN105435307AImprove biological activityImprove manufacturing speedProsthesisCell-Extracellular MatrixBiocompatibility Testing
The invention discloses a preparation method of a natural-tissue-derived decellularized and decalcified bone material. According to the method, any bone tissue of a mammal is treated with a protease inhibitor-containing normal saline buffer, an organic solvent, Tirton X-containing PBS (phosphate buffer saline), SDS (sodium dodecyl sulfonate)-containing PBS, pancreatin-containing PBS, deoxyribonuclease-containing PBS, an EDTA (ethylene diamine tetraacetic acid) isotonic solution and ultrasonic waves, and the decellularized and decalcified bone material is obtained. Cancellous bones and cortical bones can be decellularized completely and simultaneously, the conditions are mild, damage to an ECM (extracellular matrix) is avoided, rapidness and stability are realized, and the obtained decellularized and decalcified bone material has the advantages of good biocompatibility, high plasticity, high biomechanical strength and the like and can be used for clinically repairing bone regeneration and repair disorder such as bone defects, nonunion and the like caused by various causes.
Owner:GUANGXI MEDICAL UNIVERSITY
In-vitro culture method of NK (natural killer) cells
InactiveCN103627672AEnhance killing activityPromote growthBlood/immune system cellsNatural Killer Cell Inhibitory ReceptorsBottle
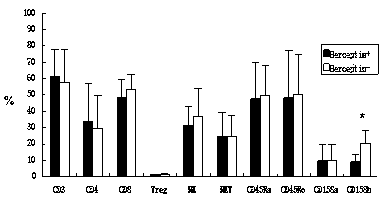
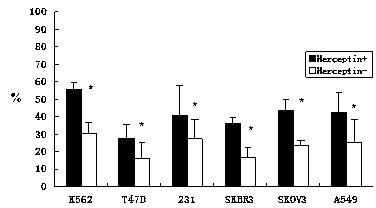
The invention discloses an in-vitro culture method of NK (natural killer) cells and belongs to culture of human cells. The in-vitro culture method disclosed by the invention comprises the following steps: merging herceptin diluted by PBS (phosphate buffered saline) and human immunoglobulin diluted by the PBS, then uniformly and fully spreading at the bottom of a culture bottle and standing overnight; additionally taking peripheral blood, performing density gradient centrifugation, sucking a single nuclear cell, adding into a serum-free culture medium, and adjusting the concentration of the cells to 1.0*10<6> / ml-3.0*10<6> / ml; and then adding cell factors IL-2 and IL-15, adding into the culture bottle coated by the herceptin and culturing in an incubator. Therefore, on the basis of ensuring the amplification multiple of various cell subgroups, the growth and the proliferation of the NK cells are promoted, the killing activity of lymphocytes is enhanced, the serum-free culture medium can replace a serum-containing complete culture medium, the number of obtained culture products is equivalent to the activity of the cells, the in-vitro large-scale culture of the NK cells is realized, the in-vitro culture method is used for clinical biological treatment of the NK cells, and the safety in clinical application can be increased by using the in-vitro culture method.
Owner:TIANJIN MEDICAL UNIV CANCER HOSPITAL
Primary separation and culture method of human amniotic mesenchymal stem cells
ActiveCN104450611AA large amountHigh puritySkeletal/connective tissue cellsMesenchymal stem cellPhosphate buffered saline
The invention relates to a primary separation and culture method of human amniotic mesenchymal stem cells. The primary separation and culture method of the human amniotic mesenchymal stem cells comprises the following steps of: providing a human amniotic tissue, washing with PBS (phosphate buffer saline), shearing into pieces, adding 0.1-0.3% (by mass) trypsinase to digest, adding I-type collagenase and basal high-glucose DMEM until the final concentration of the I-type collagenase is 0.1-0.2%, digesting, separating the digested human amniotic tissue centrifugally to obtain a precipitate, resuspending the precipitate with PBS, filtering, separating centrifugally, and removing the supernatant to obtain the human amniotic mesenchymal stem cells. Compared with the prior art, the primary separation and culture method provided by the invention has the advantages that because a small amount of trypsinase is used to pretreat and loosen the human amniotic tissue, and further the I-type collagenase with good tissue specificity is used to treat the human amniotic tissue, the digesting time can be shortened greatly, the primary separation steps can be simplified, the human amniotic mesenchymal stem cells can be obtained at a high yield, and the viability of the obtained human amniotic mesenchymal stem cells can be improved greatly compared with that of the human amniotic mesenchymal stem cells in the prior art.
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Micelle based on non-linear polyethylene glycol-polylactic acid block copolymer and preparation method thereof
ActiveCN101812227AGood biocompatibilityAvoid adsorptionPharmaceutical non-active ingredientsEnd-groupPolyethylene glycol
The invention relates to a micelle based on non-linear polyethylene glycol-polylactic acid block copolymer and a preparation method thereof. The preparation method comprises the following steps: using a single-end hydroxyl or a double-end-group modified polyethylene glycol to induce lactide for ring-opening polymerization, thereby obtaining a non-linear spindle polyethylene glycol-polylactic acid block copolymer, and then obtaining a micron-sized spindle micelle with high stability through self-assembly in aqueous solution. The particle sizes can be controlled through adjusting the molecular weights of polyethylene glycol and the polylactic acid, the critical micelle concentration is as high as 3.72*10-4g / L, and the drug loading rate and the drug encapsulation rate of the micelle are respectively up to 39.9 percent and 70.8 percent by mass percent, superior to a spherical micelle formed through assembly of a linear polyethylene glycol-polylactic acid block copolymer with an identical molecular weight. Simultaneously, the micelle coated with oil-soluble drugs demonstrates controllable release properties to drug molecules in phosphate buffer saline with the pH value of 7.4. The micelle can be used for slow release and targeted delivery of drugs, enhancement of drug effects and the like.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Method of Separating Collagen From the Various Animal Tissues for Producing Collagen Solution and Product Using the Same
ActiveUS20080118947A1Improve customer satisfactionQuality improvementConnective tissue peptidesPeptide/protein ingredientsPhosphateFractionation
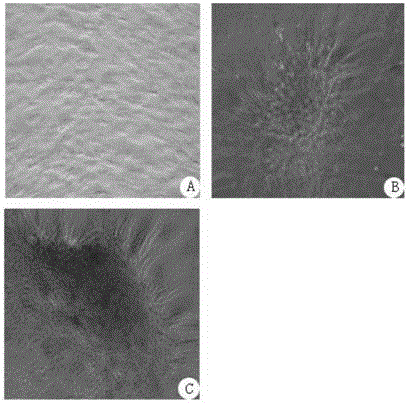
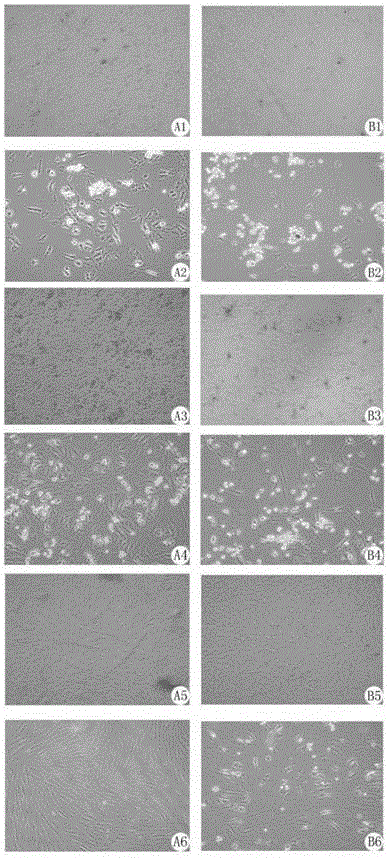
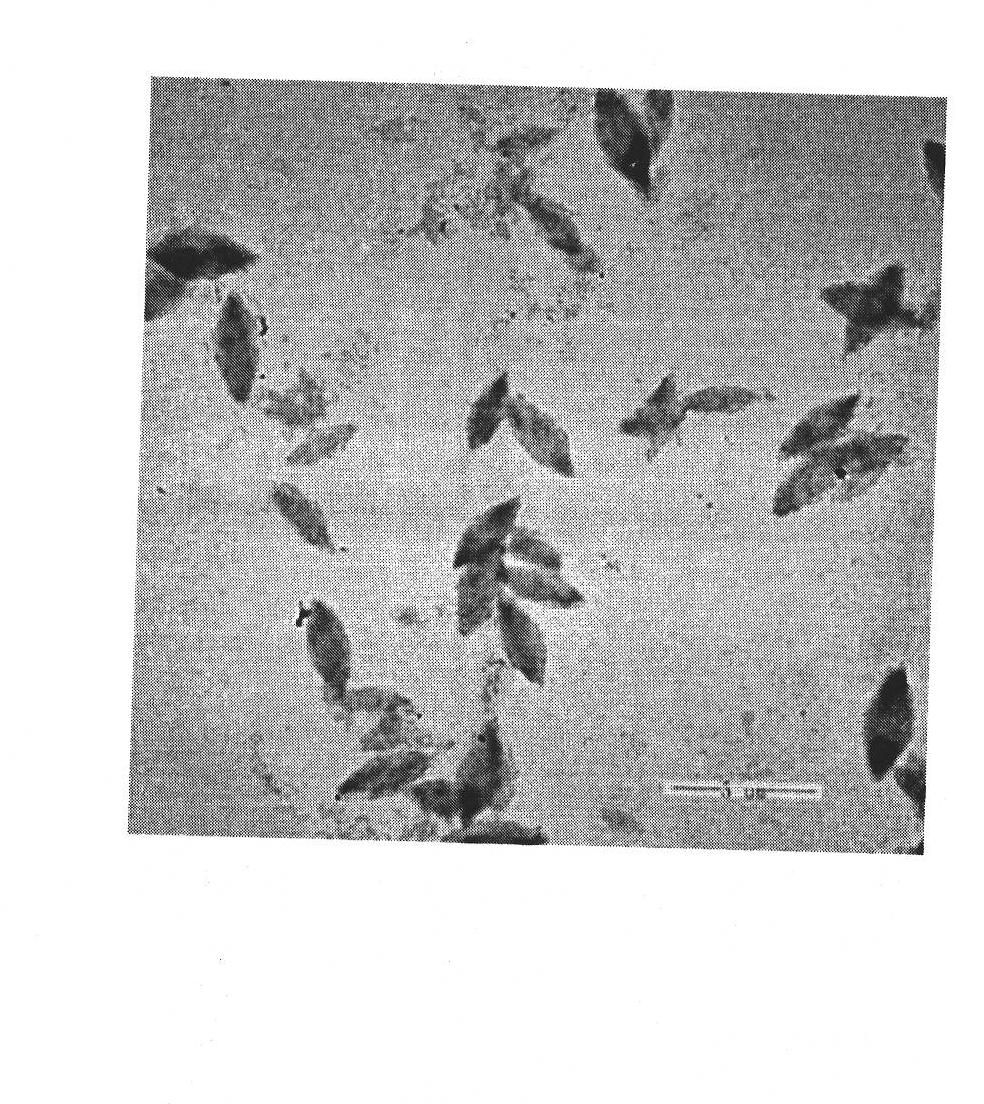
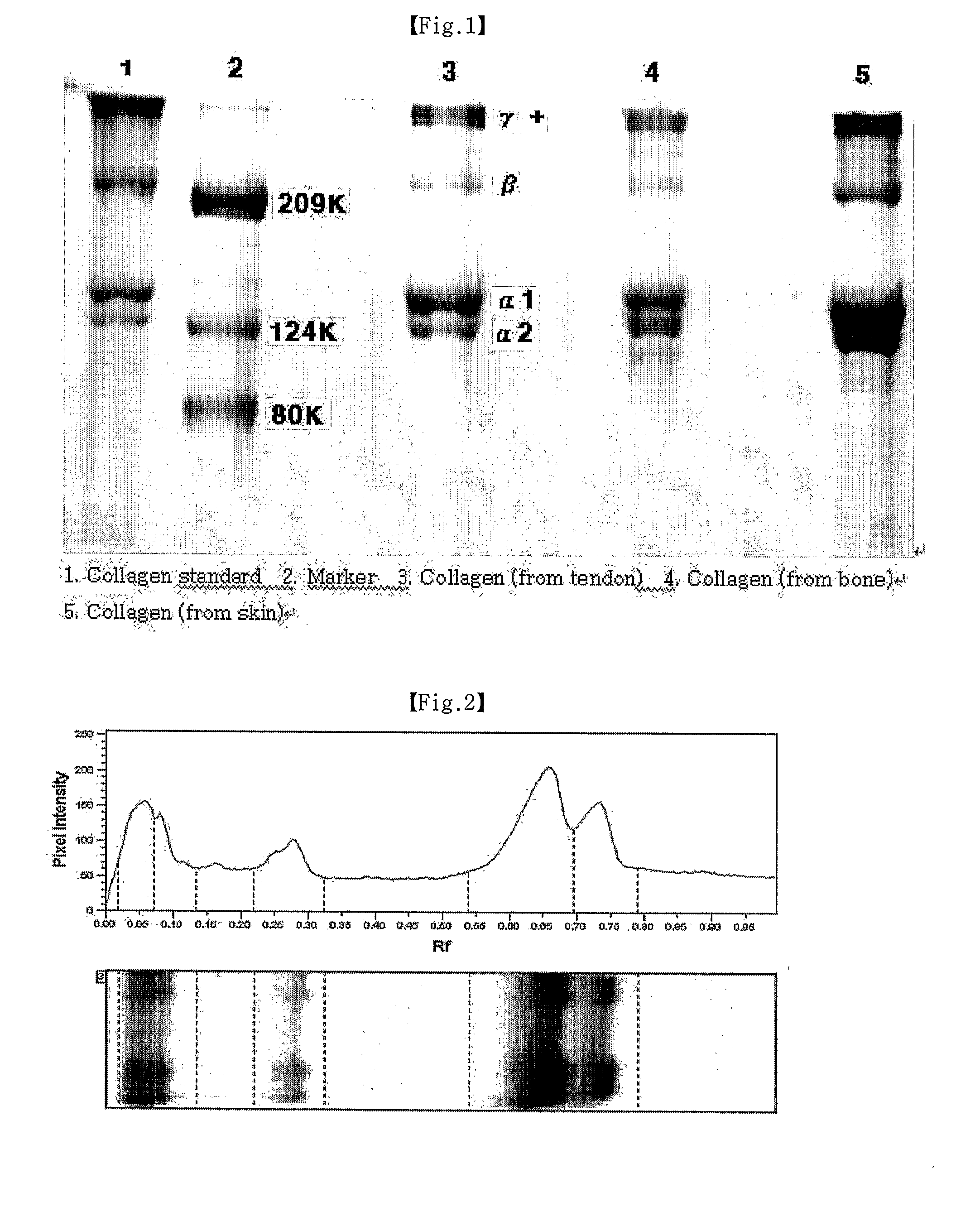
A method for separation the collagen from the various animal tissues is disclosed for preparing collagen solution and product using the same. The porcine tissues are processed to have proper form and size for acid-treatment. The acid-treatment is repeated with pepsin to separate type I or II collagens. The separated collagen is salt-treated for fractionation and ethanol-treated for obtaining 5˜10% of collagen from the initial tissue weight. The prepared tissues are processed for separating collagen through the collagen separating process. The separated collagen is processed for preparing product. The method for preparing product is comprised: treating a collagen solution having a predetermined concentration under a neutral condition at a low temperature, followed by overnight treatment at a temperature of 30 to 35° C.; concentrating collagen by centrifugation; and dissolving the thus-concentrated collagen in refrigerated weakly-acidic solvent or phosphate buffered saline (PBS), thereby preparing collagen having a concentration of 1 to 5 mg / mL.
Owner:CELLONTECH
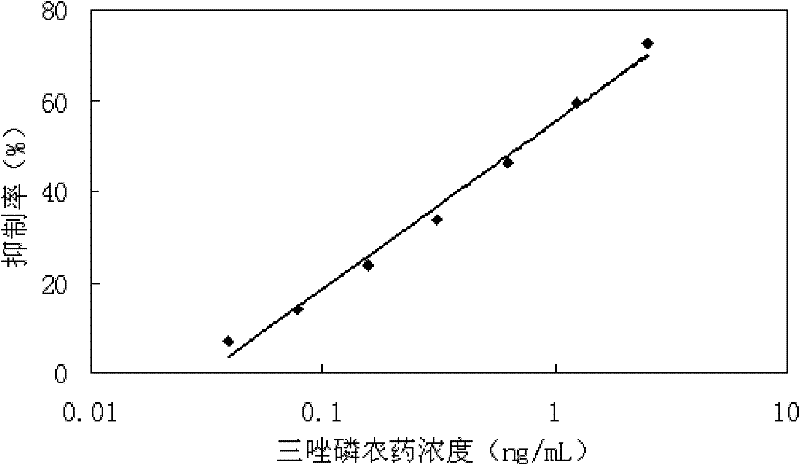
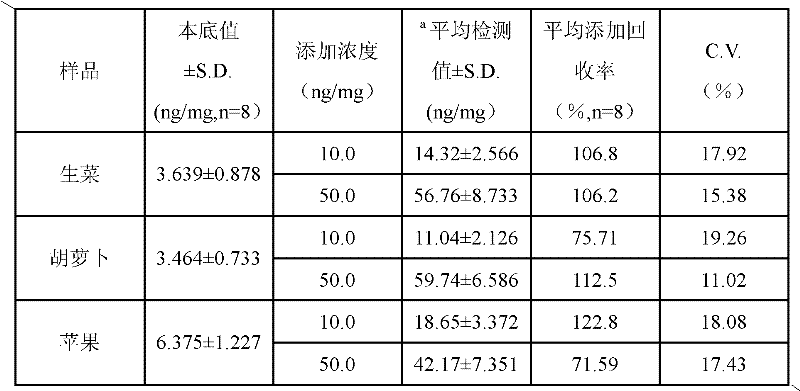
Determination kit for triazophos chemoluminescence immunoassay and preparation method and using method thereof
ActiveCN102175865AHigh sensitivityReduce distractionsMaterial analysisMonoclonal antibodyHorse radish peroxidase
The invention provides a preparation method of a determination kit for triazophos chemoluminescence immunoassay and relates to determination kits for triazophos chemoluminescence immunoassay and a preparation method and a using method thereof. The invention solves the technical problem of high minimum detection limit in the conventional direct competitive enzyme-linked immunosorbent assay method. The determination kit for triazophos chemoluminescence immunoassay consists of a triazophos standard substance, a carrier coating a triazophos monoclonal antibody, horse radish peroxidase-labeled triazophos hapten, a chemiluminescent sensitizing solution and phosphate buffer saline (PBS). The kit is prepared by putting the prepared triazophos standard substance, the carrier coating the triazophos monoclonal antibody, the chemiluminescent sensitizing solution and the PBS buffer solution into a box to obtain the determination kit. When the kit is used, the triazophosstandard substance and samples are added into carrier reactive pores respectively and washed, and the chemiluminescent sensitizing solution is added, then the luminescent intensity is determined and specification curve is drawn, and finally the concentration of triazophos in the sample is determined. The minimum detection limit of triazophos is 0.06ng / mL, so the invention can be used in the field of foods.
Owner:INST OF QUALITY STANDARD & TESTING TECH FOR AGRO PROD OF CAAS
Method for extracting high-molecular-weight genome from animal feces
InactiveCN102586234AHigh molecular weightSimple methodDNA preparationSodium acetateMolecular identification
The invention relates to a method for extracting high-molecular-weight genome from animal feces. The method comprises the steps of sample treatment, bacterial cell lysis and extraction of DNA (Deoxyribonucleic Acid). Specifically, the method comprises the steps of: dissolving an animal feces sample with aseptic PBS (Phosphate Buffered Saline) buffer, carrying out low-speed centrifuging and filtering to remove food residues in the feces, collecting bacterial cells, performing wall breaking on the bacteria by adopting lysozyme, decolorizing peptidase, protease and SDS (Sodium Dodecyl Sulfate), extracting with a mixture of phenol, chloroform and isoamyl alcohol as well as a mixture of chloroform and isoamyl alcohol, precipitating with isopropanol and sodium acetate, washing and drying, dissolving, and digesting RNA (Ribonucleic Acid) with RNAse (Ribonuclease). According to the invention, the method is simple in operation; and the DNA molecular weight of the extracted genome achieves more than 40kb, so that the researches on microorganism molecule ecological diversity, molecular identification, target gene amplification, macro genome library construction and the like are met.
Owner:YUNNAN NORMAL UNIV
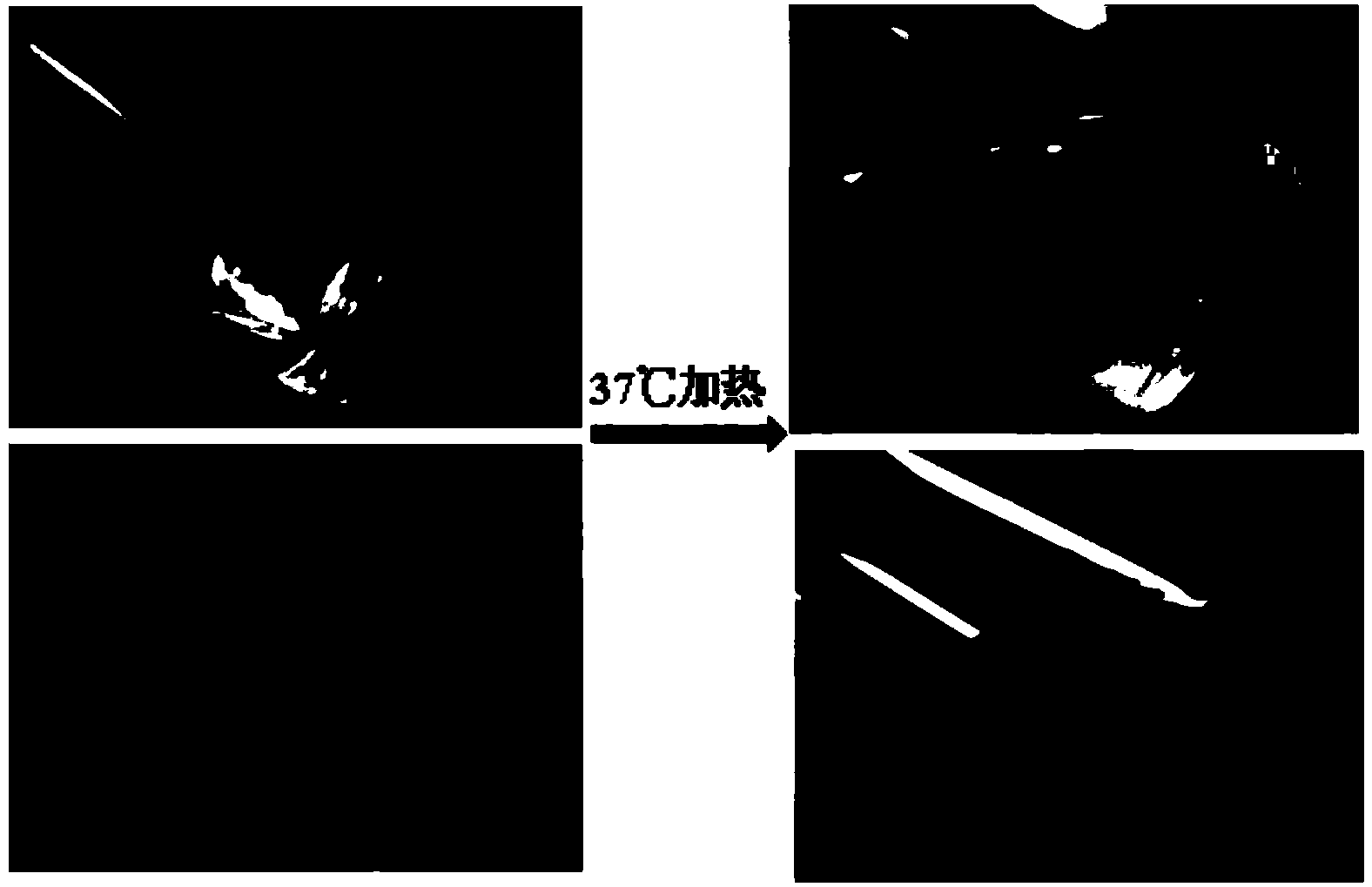
Angiogenesis-facilitating temperature-sensitive hydrogel powder and temperature-sensitive hydrogel prepared from same
The invention discloses a preparation method of angiogenesis-facilitating temperature-sensitive hydrogel. The preparation method comprises the following steps: under an ice-bath condition, adding the temperature-sensitive hydrogel powder provided by the invention and used as a raw material into a precooled PBS (phosphate buffer saline) solution, distilled water or DMEM (dulbecco's modified eagle medium), wherein the ratio of the temperature-sensitive hydrogel powder to the precooled PBS solution, distilled water or DMEM is 30mg:1ml, uniformly stirring, and placing the mixture in an environment with the temperature of 37 DEG C, so as to obtain the angiogenesis-facilitating temperature-sensitive hydrogel. The invention further discloses novel temperature-sensitive hydrogel powder and a preparation method thereof. The temperature-sensitive hydrogel powder can be stored for a long time at room temperature, the sterilization method for the temperature-sensitive hydrogel powder is simple, and the temperature-sensitive hydrogel powder can be conveniently produced in a large-scale manner and put into a market; the temperature-sensitive hydrogel prepared from the temperature-sensitive hydrogel powder has good temperature sensitiveness and biocompatibility, can be degraded in a body, has an angiogenesis-facilitating effect, and has a good application prospect in regenerative medicine and clinical treatment.
Owner:杭州倍荣生物科技有限公司
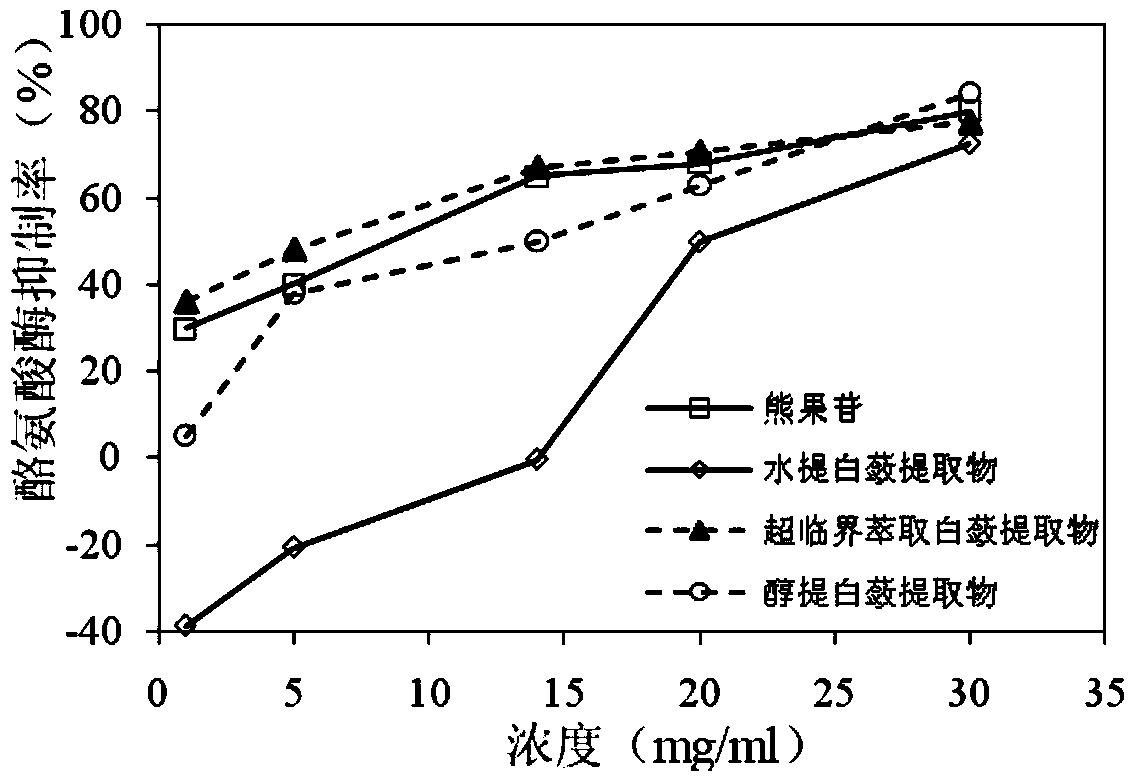
Method for detecting whitening efficacy of melothria heterophylla root extracts
InactiveCN103439280AReduce dosageEasy to operateColor/spectral properties measurementsWater bathsArbutin
The invention discloses a method for detecting whitening efficacy of melothria heterophylla root extracts and relates to natural melothria heterophylla root extracts. The method comprises the following steps: (1) preparing a tyrosinase solution, an L-tyrosine solution and a PBS (phosphate buffer saline) solution as base solutions, then preparing four to-be-detected solutions, namely an arbutin solution, a supercritical CO2 extracted melothria heterophylla root extract solution, a water extracted melothria heterophylla root extract solution and an alcohol extracted melothria heterophylla root extract solution, quantitatively adding each base solution and one of the four to-be-detected solutions in four test tubes and shaking the solutions to be uniform; (2) heating the four test tubes in a water bath kettle, respectively determining the absorbance of each solution in each test tube under a wave length of 475nm and calculating the inhibition ratio of the to-be-detected solution with the concentration to tyrosinase; and (3) repeating the steps (1) and (2), determining the inhibition ratio of the other to-be-detected solutions to tyrosinase, determining the inhibition ratio of the other to-be-detected solutions with different concentrations to tyrosinase, and then according to the comparison of determination results, obtaining the whitening efficacy of each melothria heterophylla root extract and arbutin under the same concentration as well as the whitening efficacy of each melothria heterophylla root extract or arbutin under different concentrations.
Owner:XIAMEN UNIV
Methods and compositions for treating rhinitis
InactiveUS20120263781A1Bacterial antigen ingredientsPeptide/protein ingredientsNasal passagesPhosphate buffered saline
Improved efficacy in treatment of rhinitis with botulinum toxin is obtained using liposomal encapsulated botulinum formulations for administration of the botulinum toxin. The liposomes are typically administered in a physiologically acceptable carrier such as saline or phosphate buffered saline by instillation into the nasal passages.
Owner:LIPELLA PHARMA
Isolated nanocapsule populations and surfactant-stabilized microcapsules and nanocapsules for diagnostic imaging and drug delivery and methods for their production
InactiveUS20080279783A1Reduce deliveryUltrasonic/sonic/infrasonic diagnosticsEchographic/ultrasound-imaging preparationsMicrobubblesPhosphate
A method for producing surfactant-stabilized microcapsules or nanocapsules including the steps of (a) preparing a suspension comprising a non-ionic sorbitan detergent and a salt in phosphate buffered saline; (b) adding to the suspension a nonionic polyoxyethylenesorbitan detergent to produce a solution; (c) heating while stirring the solution of step (b) to 55±5° C. and maintaining the temperature of the solution at 55±5° C. for several minutes; (d) allowing the solution to cool to room temperature; (e) autoclaving the solution; (f) creating surfactant-stabilized microbubbles and nanobubbles in the solution; and (g) collecting surfactant-stabilized nanocapsules and microcapsules formed from the microbubbles and nanobubbles.
Owner:DREXEL UNIV
Porous body comprising apatite/collagen composite fibers and its production method
InactiveUS20090149634A1Good biocompatibilityConnective tissue peptidesPeptide/protein ingredientsFiberApatite
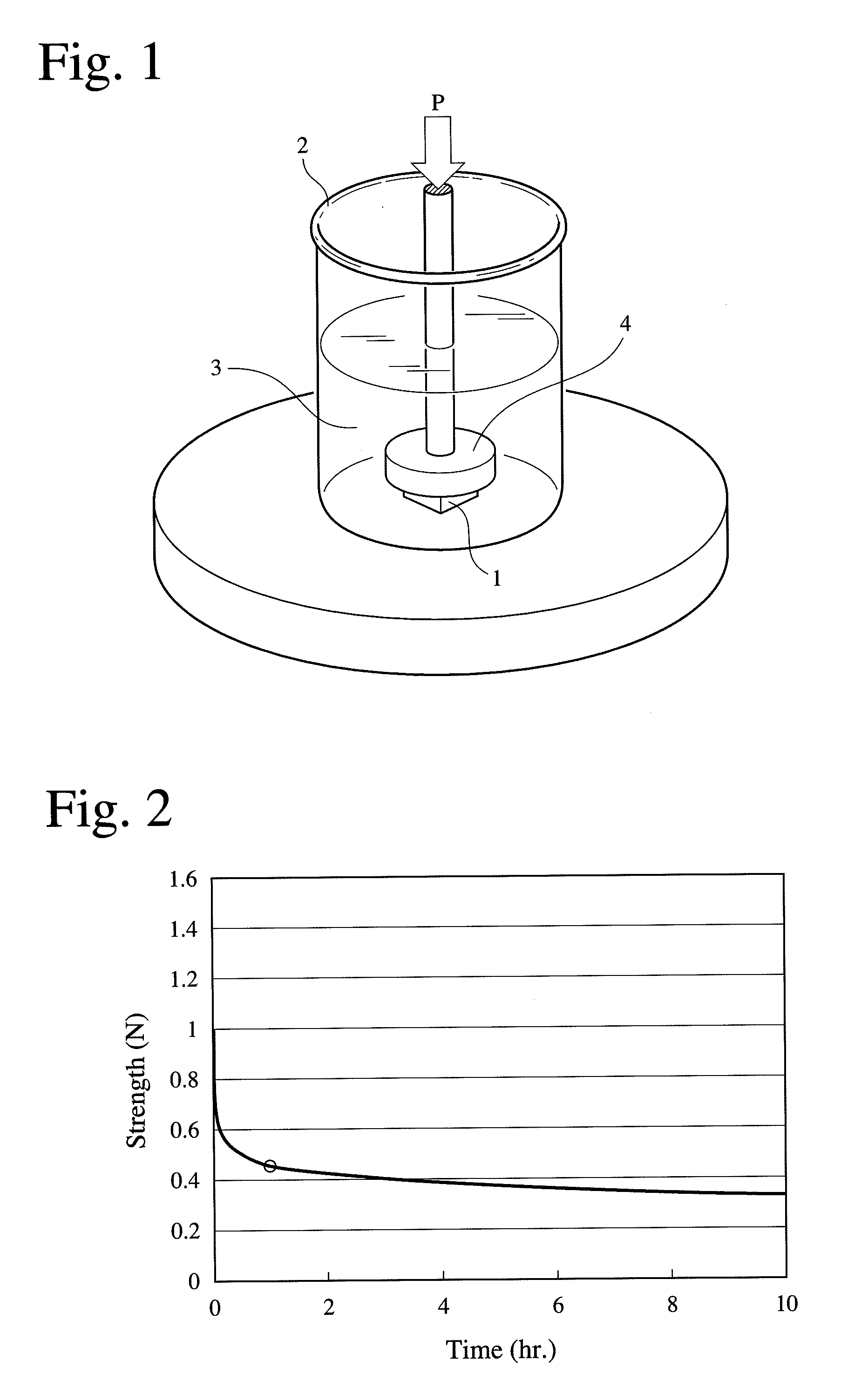
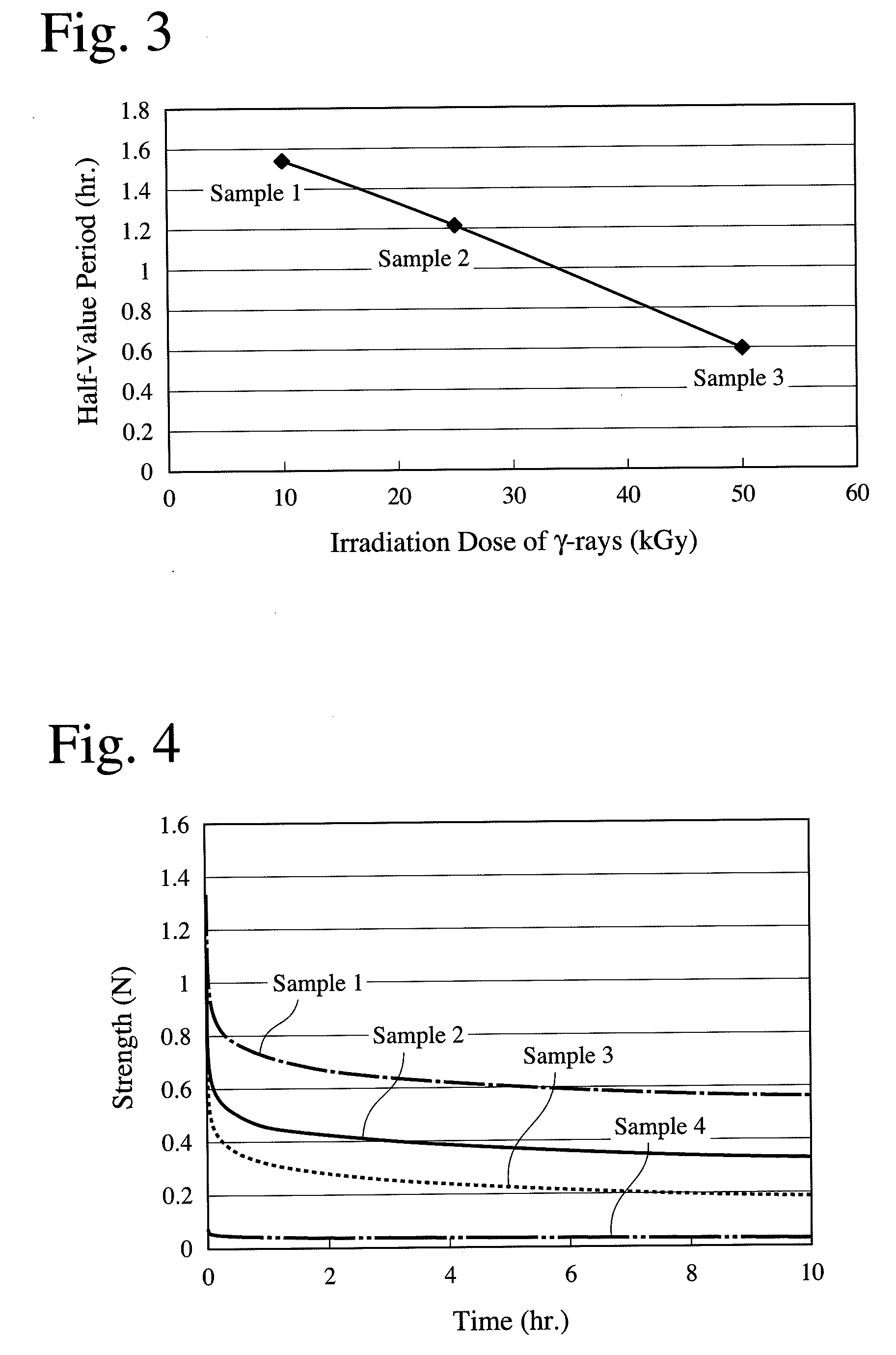
A porous body comprising apatite / collagen composite fibers, which has a half-value period of strength of 0.8-1.6 hours, the half-value period of strength being the time until the strength of the porous body of 10 mm×10 mm×4 mm comprising apatite / collagen composite fibers is reduced to half, after the porous body degassed by pressure reduction to 3 kPa (absolute pressure) for 10 minutes in a phosphate buffer saline is given a 20-% strain at a speed of 10 mm / minute.
Owner:HOYA CORP +1
Method for separating MVs (microvesicles) and MV exosomes from tumor cell supernatant
ActiveCN106289927ARetain structureReserve ingredientsPreparing sample for investigationMicrosphereCentrifugation
The invention discloses a method for separating MVs (microvesicles) and MV exosomes from a tumor cell supernatant. The method comprises the following steps: after adherent culture of tumor cells, a culture solution is taken and subjected to centrifugation, the supernatant is mixed with a coupling compound, the mixture is incubated at 4 DEG C under the 100-300-rpm horizontal vibration condition for 3-16 h, a liquid is removed, an incubated coupling compound is obtained and cleaned with PBS (phosphate buffered saline), and coupling microspheres with MVs adsorbed are obtained. The total MVs extracted with the method retain the structures and components of all the MVs, and the yield is high. Secondary sorting can be performed, specific MVs can be analyzed, and the extraction efficiency and the research value are increased. The total extraction time requires only at least 4 hours to finish purification of the MVs and requires only at least 5 hours to finish purification of the exosomes. The extraction purity is higher, and experiments show that the extracted MVs account for 90% or higher of the total extracted proteins while organic precipitate accounts for lower than 20%.
Owner:ZHEJIANG PROVINCIAL HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Preparation method of living cell based complex three-dimensional microchannel porous support
A preparation method of a living cell based complex three-dimensional microchannel porous support. The method comprises steps of: first, preparing two parts of hydrogel collosol with different hydrogel monomer concentrations; then preparing two mixed liquors containing the hydrogel collosol and cell culture solution; putting the two mixed liquors into two sets of different injectors of a cell printer; then printing the mixed liquors on a petri dish surface until formation of a required three-dimensional cell-laden hydrogel support; cultivating the cell-laden hydrogel support in an incubator; then soaking the cell-laden hydrogel support in lauryl sodium sulfate to complete split and kill the cells; flushing with Dulbecco's phosphate-buffered saline and deionized water successively; finally obtaining the three-dimensional porous hydrogel support with complex microchannels. According to the invention, living cells are employed as a porogenic agent; size and density of the holes are controlled through controlling of the cell concentration and culture conditions; distribution of the holes is controlled through controlling of a printing platform; and a complex and controllable microchannel structure is formed in the hydrogel support.
Owner:XI AN JIAOTONG UNIV
Test method for induced differentiating human embryo stem cells to retina pigment epithelial cells
The invention provides a test method for induced differentiating human embryo stem cells to retina pigment epithelial cells, which comprises the following steps that: A) 0.25% pancreatic enzyme and 0.1mg / mL collagenase iv are mixed into phosphate buffered saline (PBS) solution containing 1mM calcium chloride (CaCl2) and 20% KSR, the solution is used for digesting embryo stem (ES) clones, so the ES becomes an ES cell group with 5 to 10 cells; B) the ES clone is incubated in a culture dish which is wrapped by gelatin in a short time so as to remove the feeder layer cells; and C) the ES cell group is cultured in a continuous differential culture medium with the concentration of 6.7*102 cell groups per liters in a non-adhesive bacteria culture dish. The test method has the advantages such as simple and convenient method, good repeatability, high successful rate, good omnisexuality, rich and economical source and the like.
Owner:薛志刚 +1
Multipurpose Lens Care Solution with Benefits to Corneal Epithelial Barrier Function
A multipurpose lens care solution comprising 0.005 wt. % to 1 wt. % of an anionic biopolymer, and an antimicrobial agent selected from 0.5 ppm to 2 ppm of poly(hexamethylene biguanide), 0.5 ppm to 2 ppm polyquaternium-1, or 1 ppm to 4 ppm alexidine. The lens care solution exhibits a ZO-1 immunostaining of HCEpiC similar to phosphate buffered saline for after thirty minutes of contact time with the solution. The lens care solution will also have a transepithelial electrical resistance (TEER) of HCEpiC within a 25% difference or less than phosphate buffered saline in Ohm / cm2 after one hour of contact time with a 3:1 dilution (solution:DMEM), or the ECIS electrode arrays exhibit a 25% difference or less than phosphate buffered saline in Ohm after one hour of contact time with a 1:1 dilution (solution:DMEM).
Owner:BAUSCH & LOMB INC
Bastard halibut embryonic-period primordial germ cell tracking and positioning method
InactiveCN104878102AEasy to operateSlow down the speed of color developmentMicrobiological testing/measurementYolkPlant Germ Cells
The invention relates to a positioning and marking method for embryonic-period primordial germ cells (PGCs), in particular to a bastard halibut embryonic-period primordial germ cell tracking and positioning method. The bastard halibut embryonic-period primordial germ cell tracking and positioning method includes the steps of fixing collected various periods of embryo samples of the bastard halibuts by a 4% PFA solution; using a PBS (phosphate buffer saline) solution with 50% of deionized formamide to preserve the embryo samples at the temperature of -20 DEG C, and subjecting the fixed and preserved embryo samples to oolemma removing, gradient methanol dewatering and rewatering; after rewatering, washing the various periods of embryo samples with PBS buffering liquid without RNA ( ribonucleic acid) enzyme, pre-hybridizing at the temperature of 62-65 DEG C for 2-4 hours; after hybridization, adding a hybridization solution with bastard halibut RNA probes into the various periods of embryo samples subjected to pre-hybridization for hybridizing overnight at the temperature of 62-65 DEG C; after hybridization, subjecting the various periods of embryo samples to washing, antibody incubation and rewashing, keeping away from light, and developing colors to achieve marking for tracking and positioning of the embryonic-period primordial germ cells of the bastard halibuts. The bastard halibut embryonic-period primordial germ cell tracking and positioning method has the advantages that the problems that yolks and oolemma of the samples hybridized in situ conventionally are difficult to strip and a background color is too deep after color developing detection are solved, and operation steps are simplified.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Method of disposing kitchen waste and recovering electric power with air cathode microbial fuel cell
ActiveCN103165931AImprove economic efficiencyThe power generation effect is stable and goodBiochemical fuel cellsHigh concentrationChemical oxygen demand
The invention provides a method of disposing kitchen waste and recovering electric power with an air cathode microbial fuel cell, and relates to the method of using the microbial fuel cell to dispose the kitchen waste and recover electric power. The method is used for solving the problems that the existing disposing method of the kitchen waste is low in energy utilization rate, and triggers a secondary pollution easily. The method includes that : firstly, carbon powder is evenly mixed with a polytetrafluoroethylene (PTFE), and the mixture is daubed on a carbon cloth which is heated in a muffle furnace; secondly, high-concentration PTFE is coated on the cloth to heat again; thirdly, then platinum-carbon catalyst is daubed, and a platinized carbon cloth cathode is acquired; fourthly, a carbon brush anode is in preparation; fifthly, the carbon brush anode, a resistance and the air cathode are in connection through a wire, and a cell reactor is assembled; sixth, leachate of food is pretreated; seventh, phosphate buffered saline (PBS) buffer solution is in preparation, and the leachate is diluted; eighth, the leachate is diluted to be neutral; ninth, reactor water is injected into a reactor to dispose the kitchen waste and recover the electric power. The efficiency of disposing the kitchen waste is good, and chemical oxygen demand (COD) removal rate reaches to 86.4%. The method is mainly used for the disposing and utilization of the kitchen waste.
Owner:HARBIN INST OF TECH
Polyethylene glycol hydrogel for orthotopic injection and preparation method thereof
InactiveCN102898661AGood biocompatibilityPromote degradationPharmaceutical non-active ingredientsPolymer scienceThiomalic acid
The invention discloses a method for preparing polyethylene glycol hydrogel for orthotopic injection. The method comprises the following steps of: preparing a multi-mercapto linear polyethylene glycol polyether ester by polycondensation with polyethylene glycol and thiomalic acid as raw materials and rare earth trifluoromethanesulfonate as a catalyst; preparing a double-bond linear polyethylene glycol polyether ester by polycondensation with polyethylene glycol and maleic anhydride as starting raw materials and rare earth trifluoromethanesulfonate as a catalyst; and dissolving multi-mercapto linear polyethylene glycol polyether ester and multiple double-bond linear polyethylene glycol polyether esters in a phosphate buffered saline (PBS) buffer solution respectively, quickly mixing the two solutions uniformly and standing the mixed solution to obtain the polyethylene glycol hydrogel for orthotopic injection. The method is simple; conditions are easy to control, so that the method is suitable for industrial production. The invention also discloses the polyethylene glycol hydrogel for orthotopic injection, which is degradable.
Owner:ZHEJIANG UNIV
Preparation of flaky cross-linked sodium hyaluronate hydrogel
The invention provides a cross-linked sodium hyaluronate hydrogel material, which is good in tissue compatibility, high in strength, good in breathability and high in exudates absorbing capacity, and has certain transparency. According to the cross-linked sodium hyaluronate hydrogel material, with a sparse fiber mesh structure as a framework, the cross-linked sodium hyaluronate gel is combined with the sparse fiber mesh structure, so as to form hydrogel with a similar armored concrete structure; the hydrogel is formed by crosslinking the sodium hyaluronate and combining with phosphate buffer saline; and the phosphate buffer saline contains a bacteriostatic agent. According to the hydrogel with the structure, the characteristics of the sodium hyaluronate hydrogel are kept; and the strength and the toughness of the sodium hyaluronate hydrogel are increased.
Owner:BEIJING MENGBORUN BIOTECH
A kind of subcutaneous soft tissue filling localized fibrin compound that can be used for facial beauty and its preparation method
The invention relates to a facial fibrous protein composite filled and positioned in subcutaneous soft tissues, and a preparation method thereof. The composite comprises the following raw materials by weight: 10.0 to 95.0g of five-time autogenous concentrated plasma, 0.001 to 0.10g of calcium chloride, 1.0 to 15.0g of sodium sulfite, 0.005 to 0.5g of recombinant human vascular endothelial growth factor freeze-dried powder, 0.005 to 0.5g of recombinant human basic fibroblast growth factor freeze-fried powder, 0.1 to 10.0g of amino-silanized ferroferric oxide magnetic particle, and 5.0 to 90.0g of 0.1mol / L phosphate buffered saline injection. The autogenous concentrated plasma can form a stable composite fibrous protein scaffold in several hours under the double actions of calcium chloride and sodium sulfite to meet the requirement of plastic surgery; meanwhile, the composite can achieve the accurate positioning and filling effect under the action of an external magnetic field; moreover, the composite does not have irritability, and is high in safety and easy to prepare, has a good filling effect, promotes the proliferation of surrounding cells in which the composite is filled, restores tissues of the depression part and achieves the strengthening, filling and restoration effects.
Owner:董萍 +1
Tissue preserving fluid for treating HIV (human immunodeficiency virus) infection-accompanied colorectal cancer and decompensated cirrhosis complicated small liver carcinoma
The invention discloses a tissue preserving fluid. The tissue preserving fluid is composed of, by concentration, 1.0-2.5%w / v of poloamer, 1.0-2.5%w / v of polyvinylpyrrolidone, 0.2-1.0%w / v of glucose, 15-40%w / v of compound amino acid injection, 5-30%w / v of propylene glycol, 10-40%w / v of PBS (phosphate buffer saline) vitamin E, 0.1-1.5%w / v of vitamin C and 0.1-1.0%w / v of aqueous solvent for injection. Due to the fact of containing no DMSO (dimethylsulfoxide) or protein, the tissue preserving fluid can avoid toxicity produced due to DMSO reverse infusion as well as safety worries of serum and proteins on clinical application, thereby being high in safety.
Owner:南京三生生物技术股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com