Patents
Literature
5152 results about "Clinical treatment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
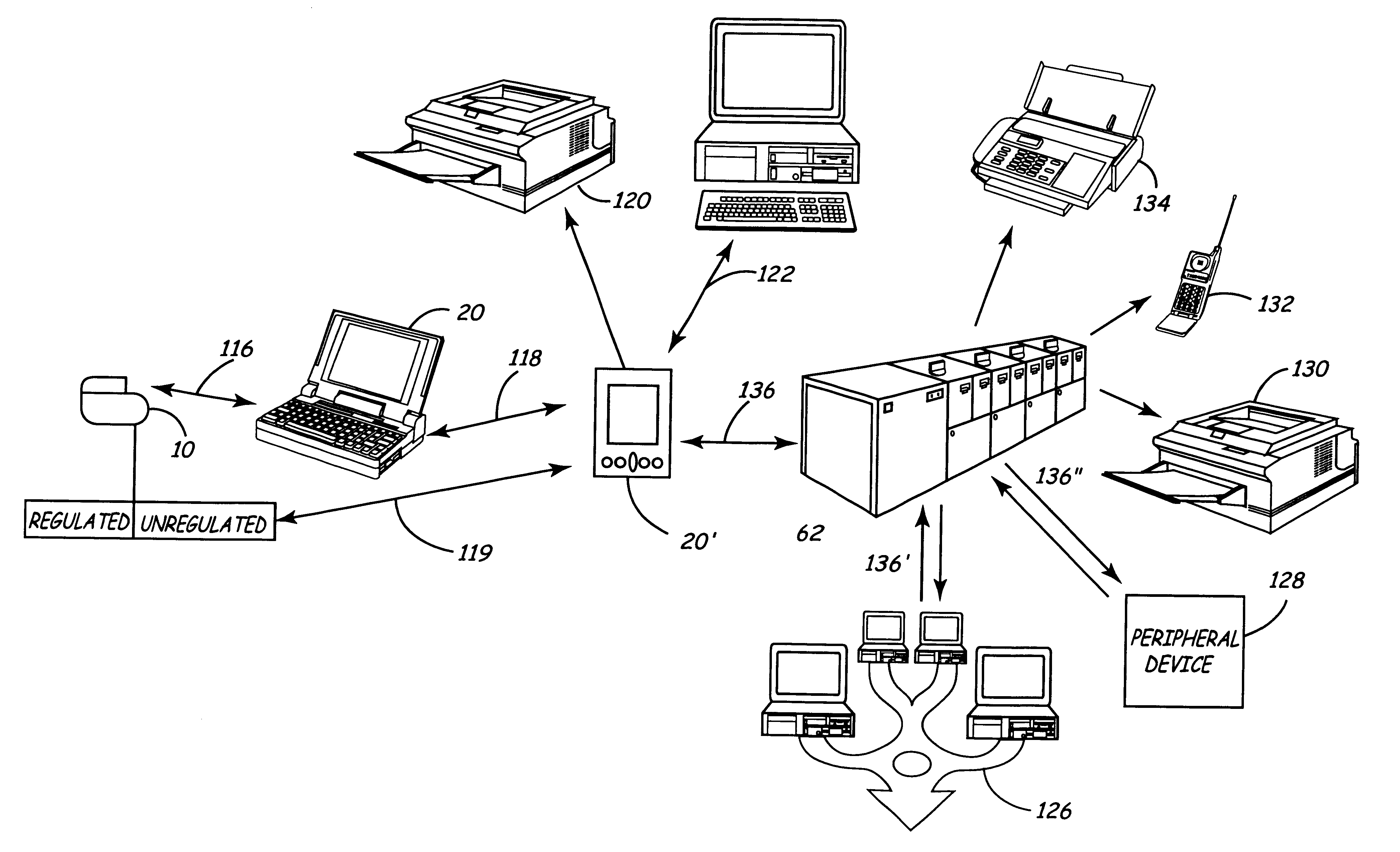
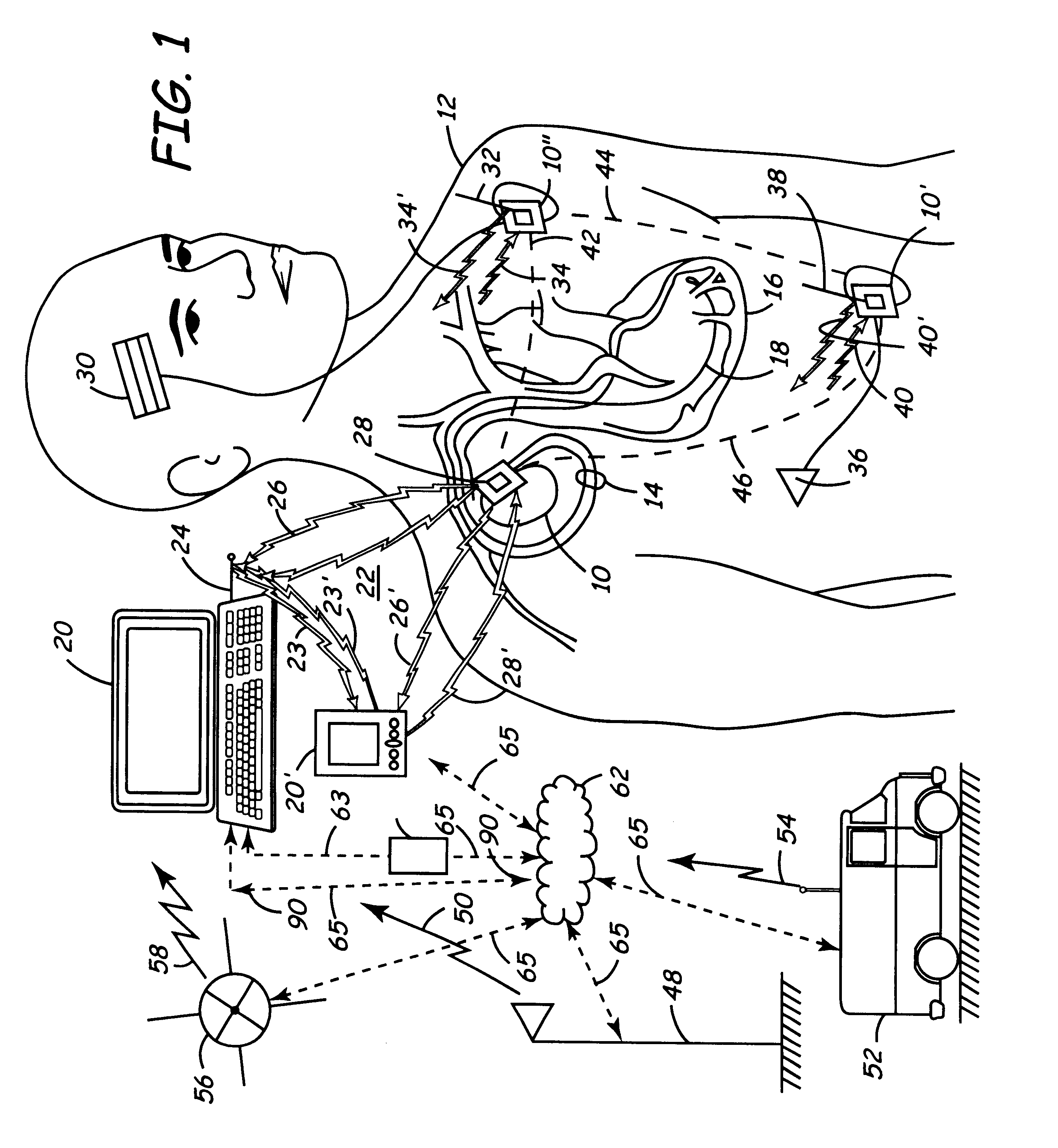
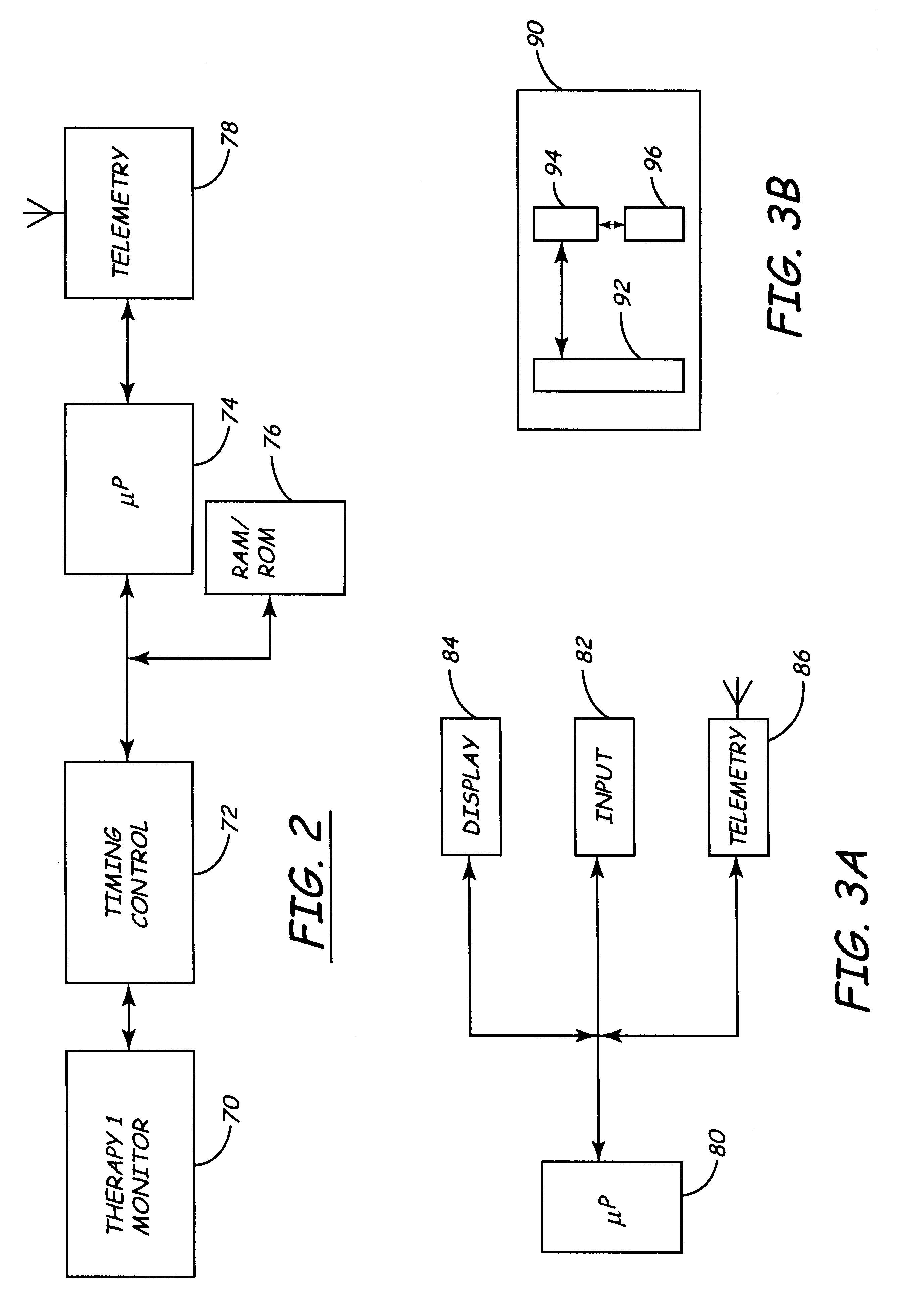
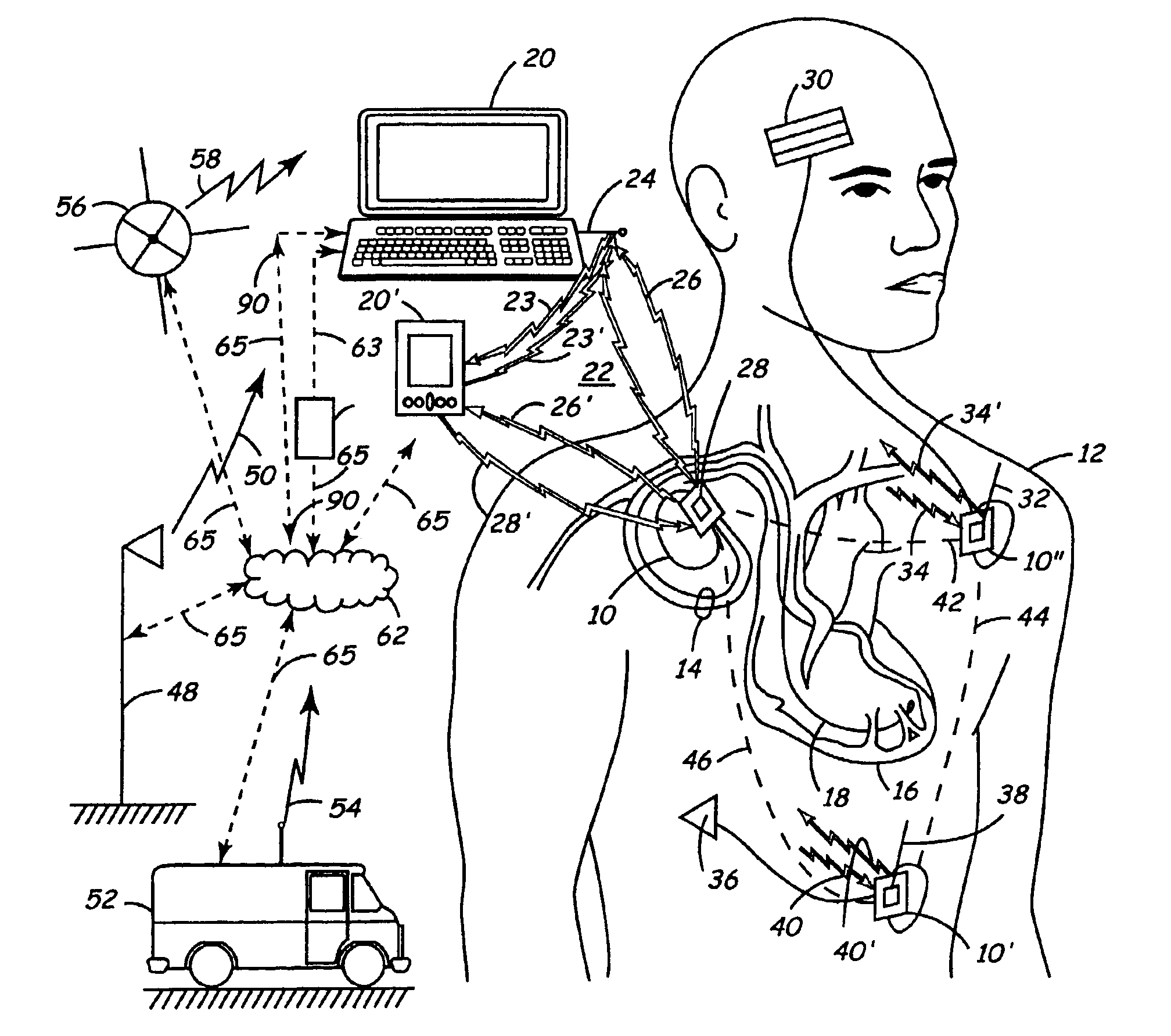
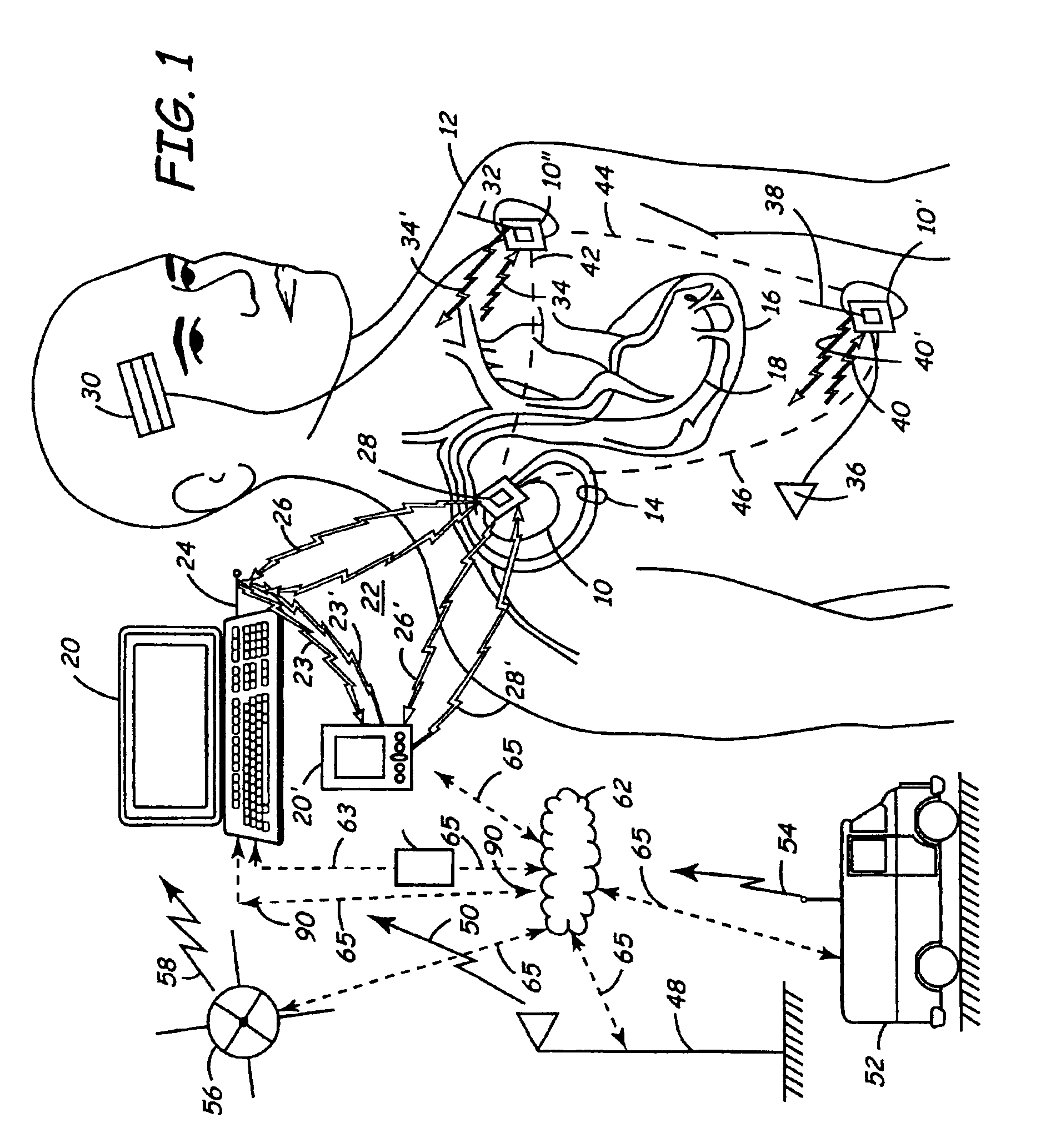
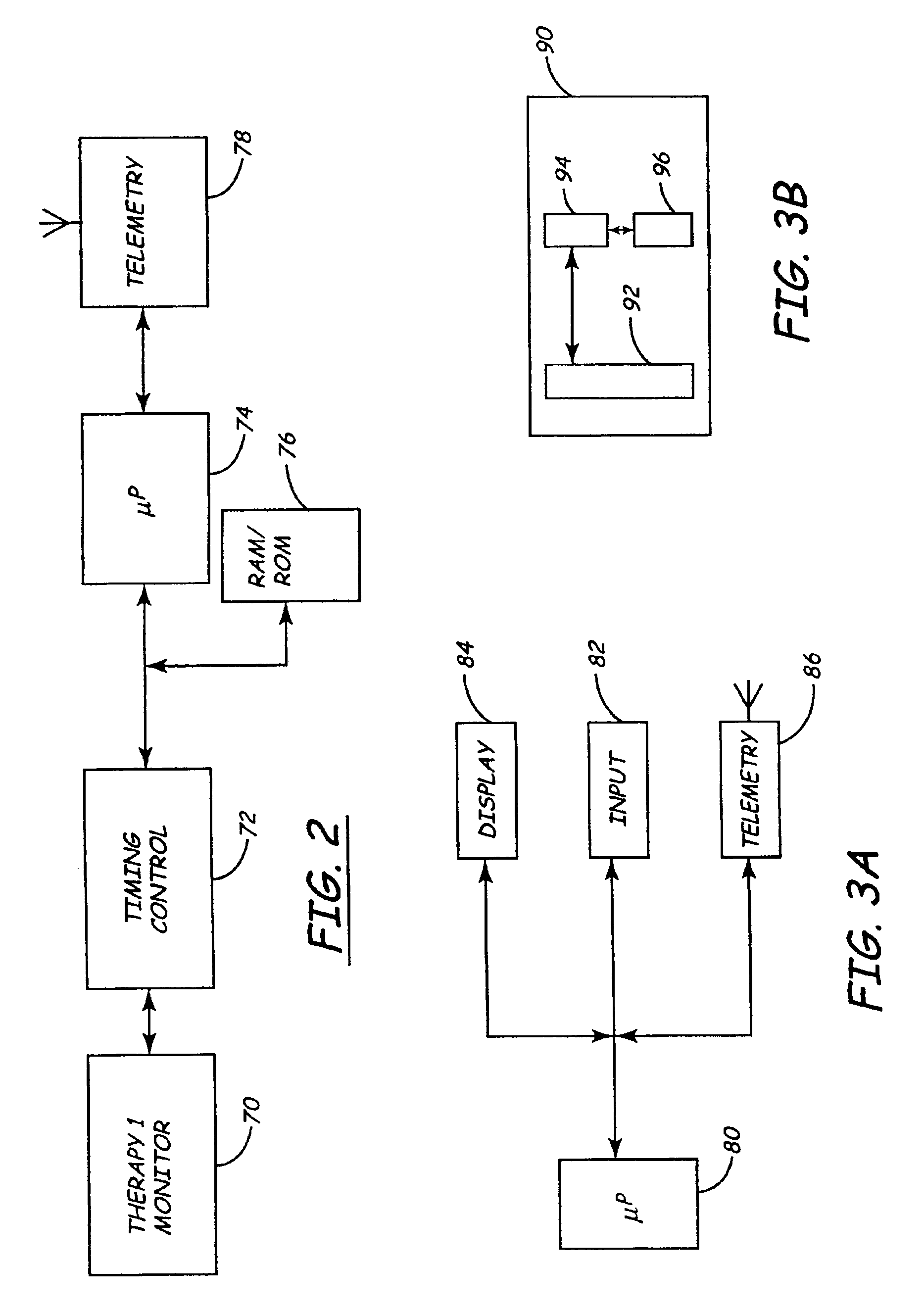
Apparatus and method for remote therapy and diagnosis in medical devices via interface systems
InactiveUS6418346B1Improve mobilityGood transferabilityElectrotherapyLocal control/monitoringStore and forwardMedical equipment
A system for transferring data into and out of medical devices wherein a personal data manager (PDM) is used in a web-based network is disclosed. The PDM co-operates with a programmer to enhance remote monitoring of implanted medical devices on a chronic basis to deliver clinical therapy in real time. The PDM is handheldable and mobile and expands the reach of the programmer by storing and forwarding data from the programmer to web-based network constituting a medical environment. The PDM is also implemented to store and forward information to PCs and similar peripheral equipment. In a specialized application, the PDM is configured to exchange data with the unregulated operational / functional segments of the IMD.
Owner:MEDTRONIC INC
Apparatus and method for remote therapy and diagnosis in medical devices via interface systems
InactiveUS7058453B2Readily configurable to transfer dataIncrease flexibilityElectrotherapyLocal control/monitoringMedical equipmentStore and forward
A system for transferring data into and out of medical devices wherein a personal data manager (PDM) is used in a web-based network is disclosed. The PDM co-operates with a programmer to enhance remote monitoring of implanted medical devices on a chronic basis to deliver clinical therapy in real time. The PDM is handheldable and mobile and expands the reach of the programmer by storing and forwarding data from the programmer to web-based network constituting a medical environment. The PDM is also implemented to store and forward information to PCs and similar peripheral equipment. In a specialized application, the PDM is configured to exchange data with the unregulated operational / functional segments of the IMD.
Owner:MEDTRONIC INC
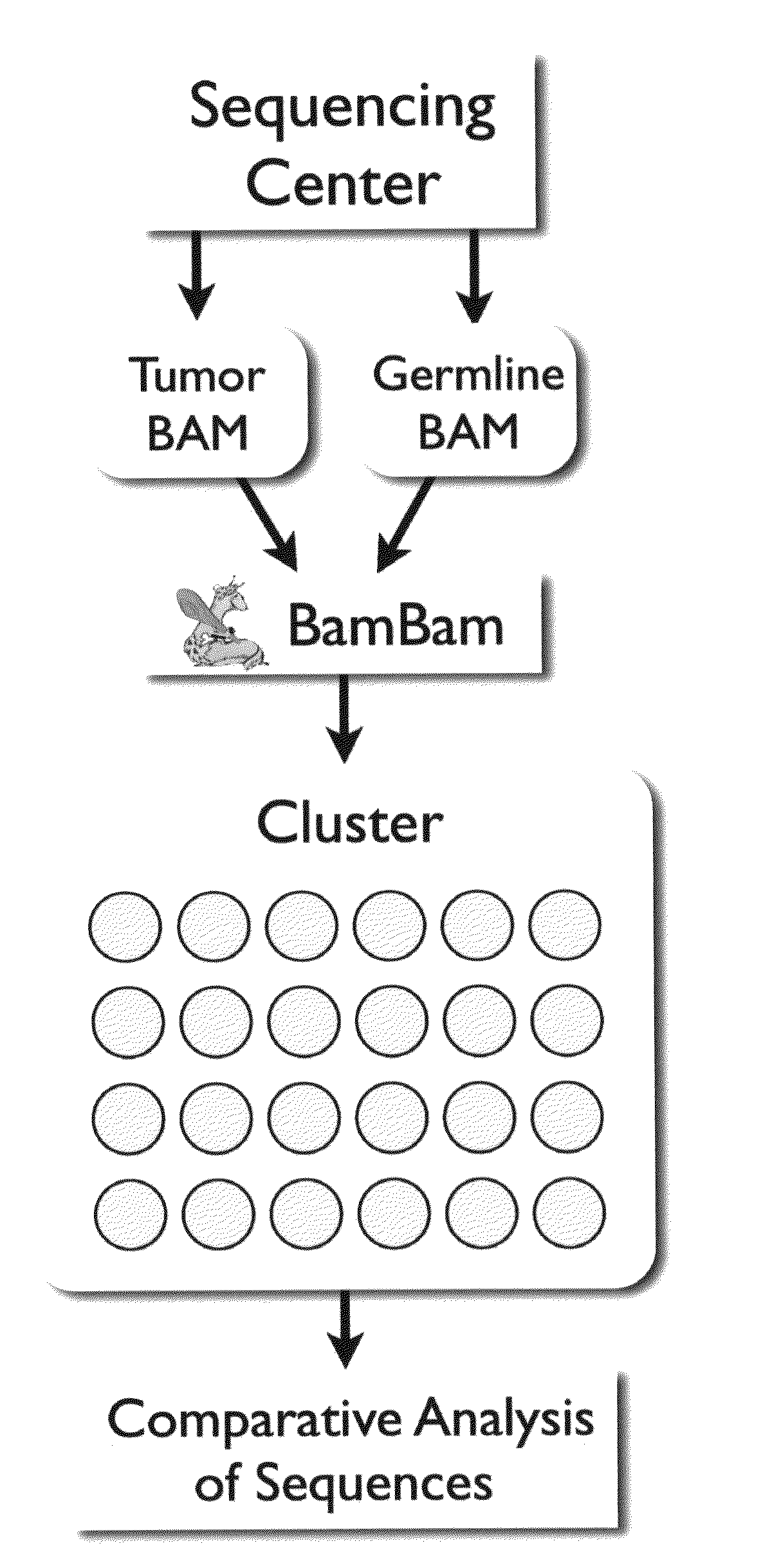
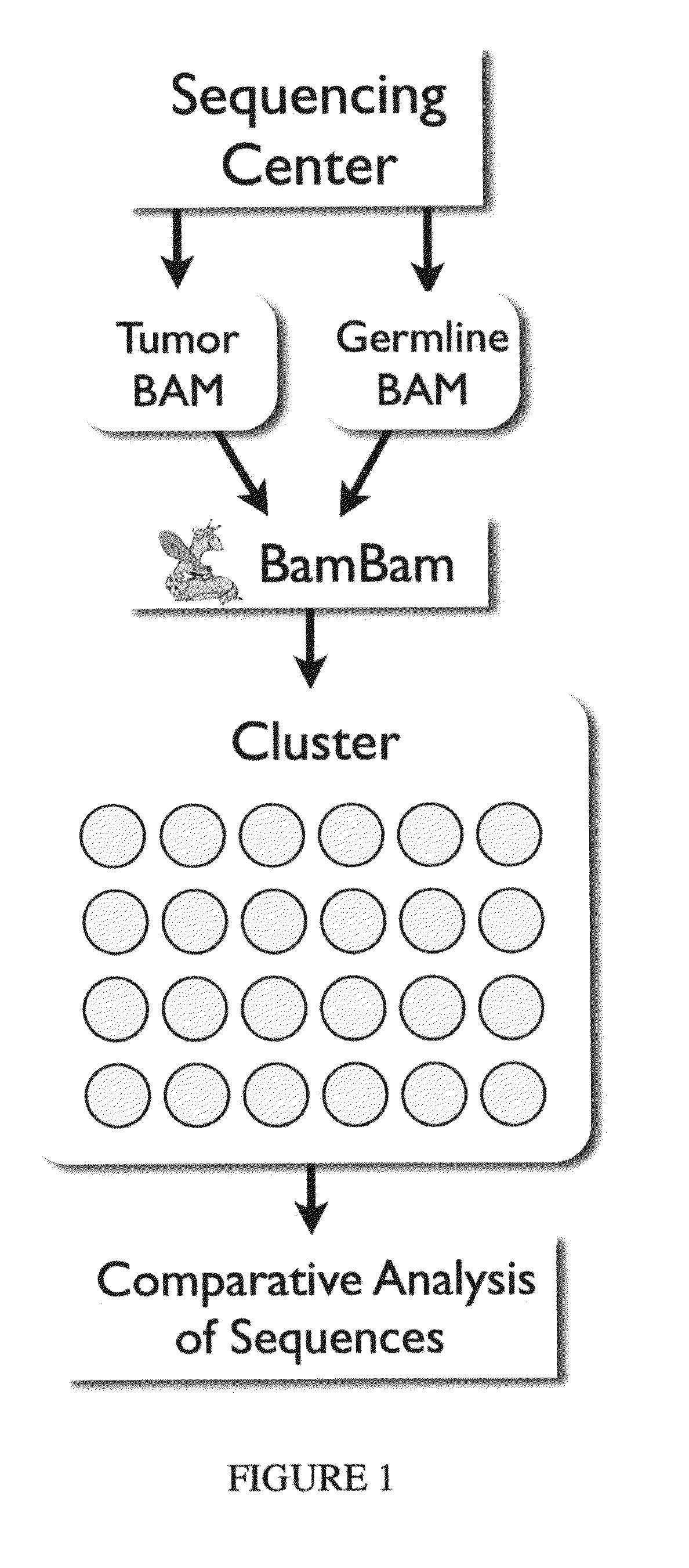
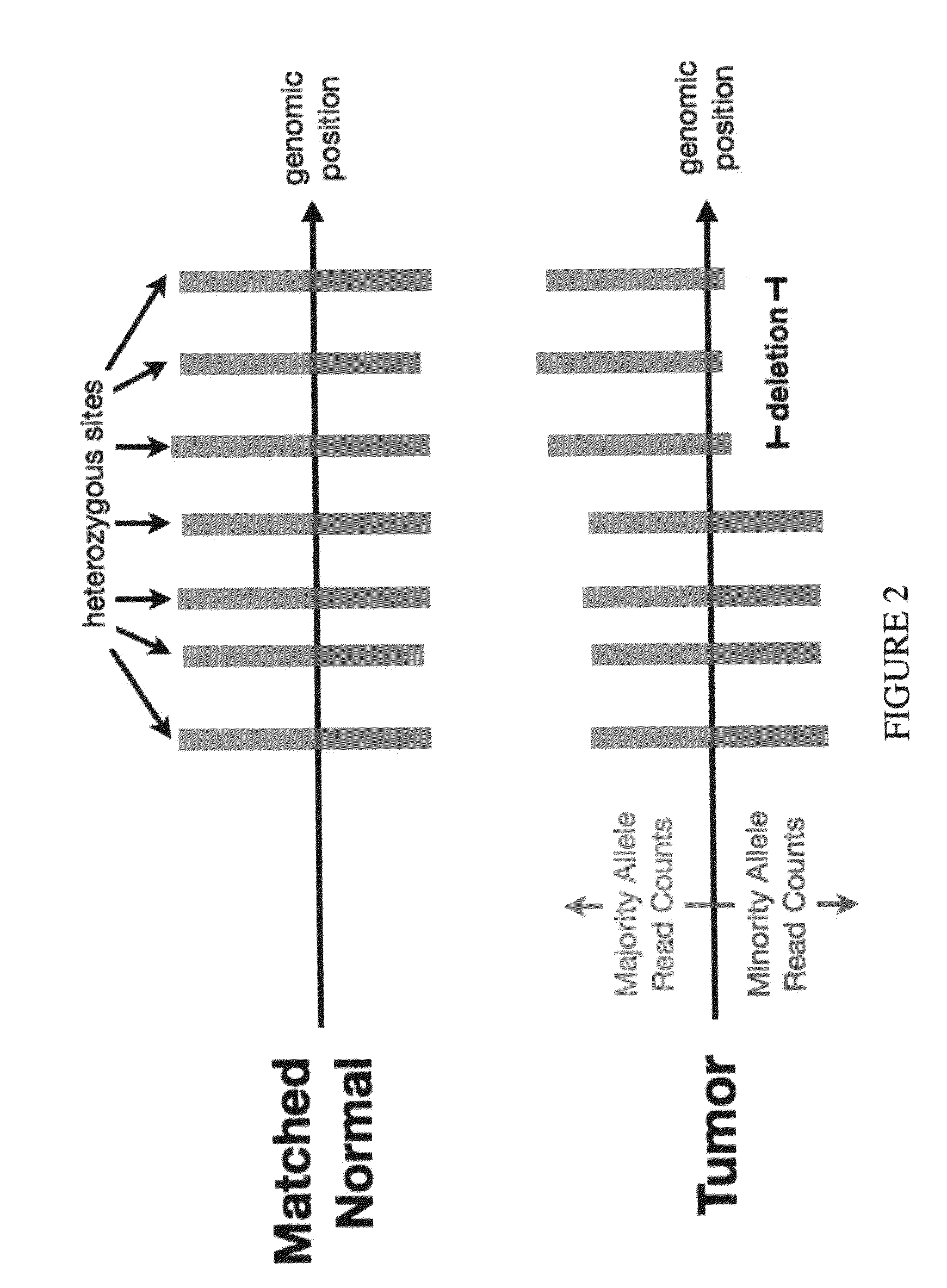
Bambam: parallel comparative analysis of high-throughput sequencing data
The present invention relates to methods for evaluating and / or predicting the outcome of a clinical condition, such as cancer, metastasis, AIDS, autism, Alzheimer's, and / or Parkinson's disorder. The methods can also be used to monitor and track changes in a patient's DNA and / or RNA during and following a clinical treatment regime. The methods may also be used to evaluate protein and / or metabolite levels that correlate with such clinical conditions. The methods are also of use to ascertain the probability outcome for a patient's particular prognosis.
Owner:RGT UNIV OF CALIFORNIA
Methods of treating chronic inflammatory diseases using carbonyl trapping agents
InactiveUS6444221B1Improved therapeutic propertyImprove propertiesBiocidePeptide/protein ingredientsEtiologyBenzoic acid
Owner:SECANT PHARMA
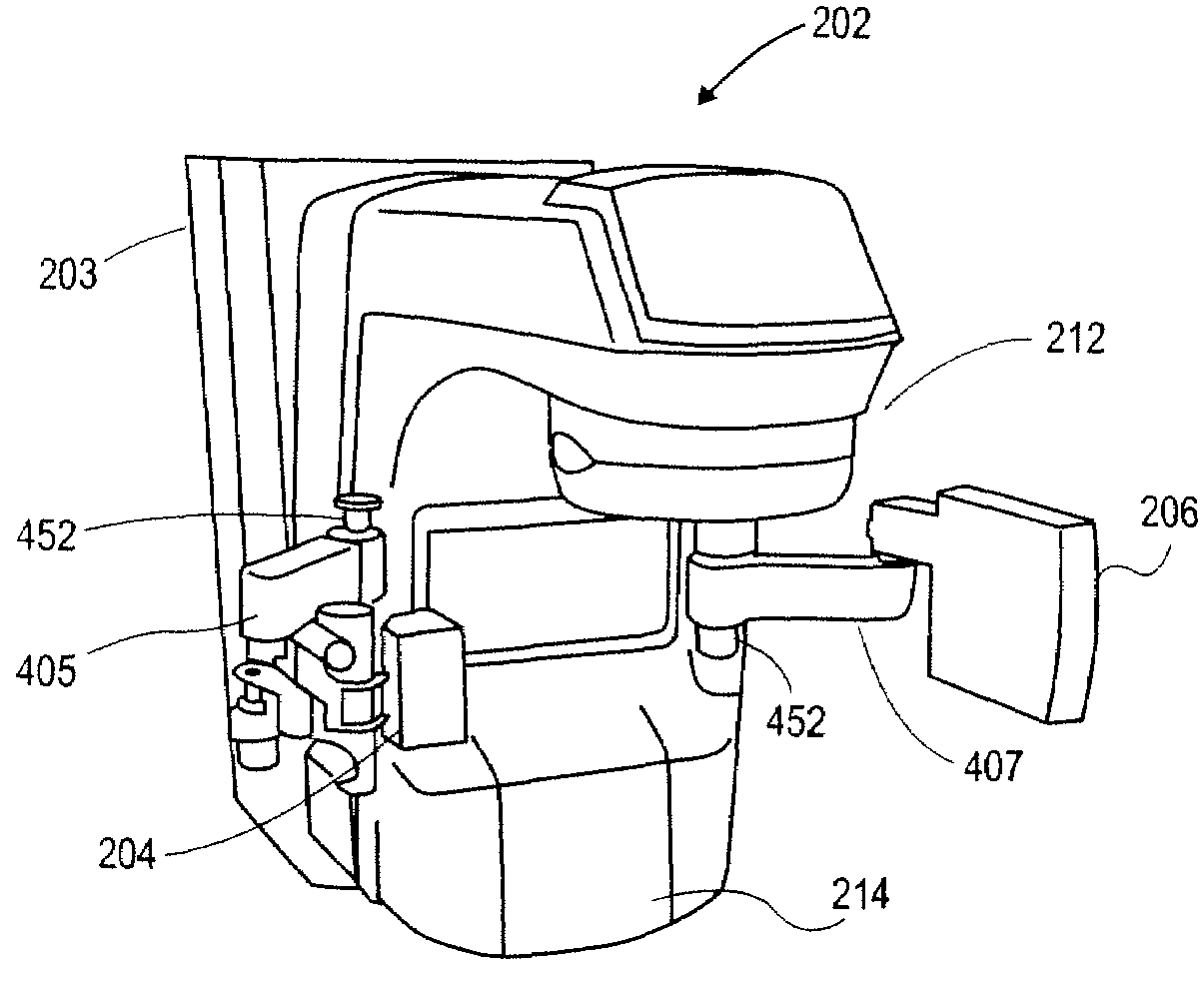
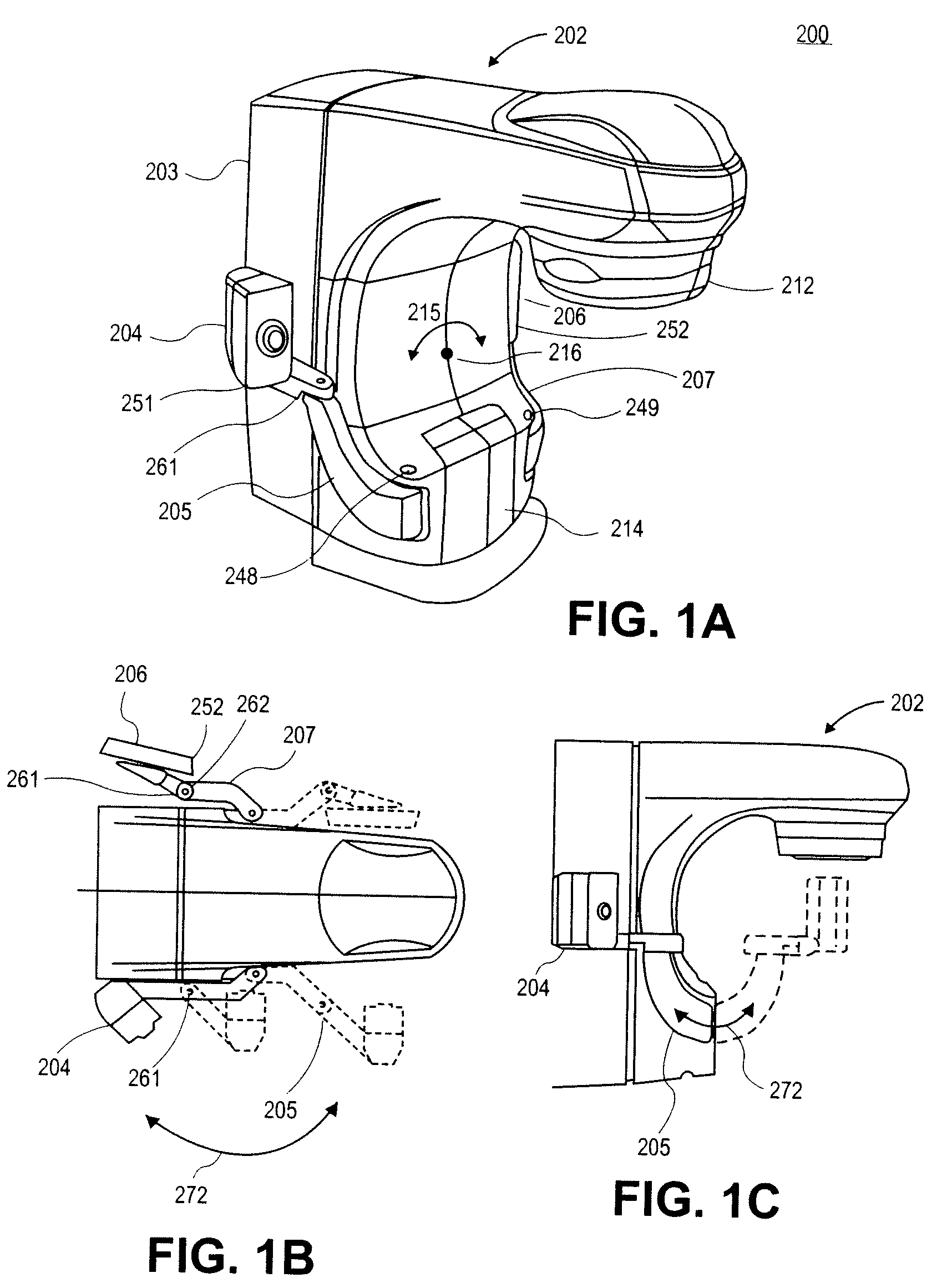
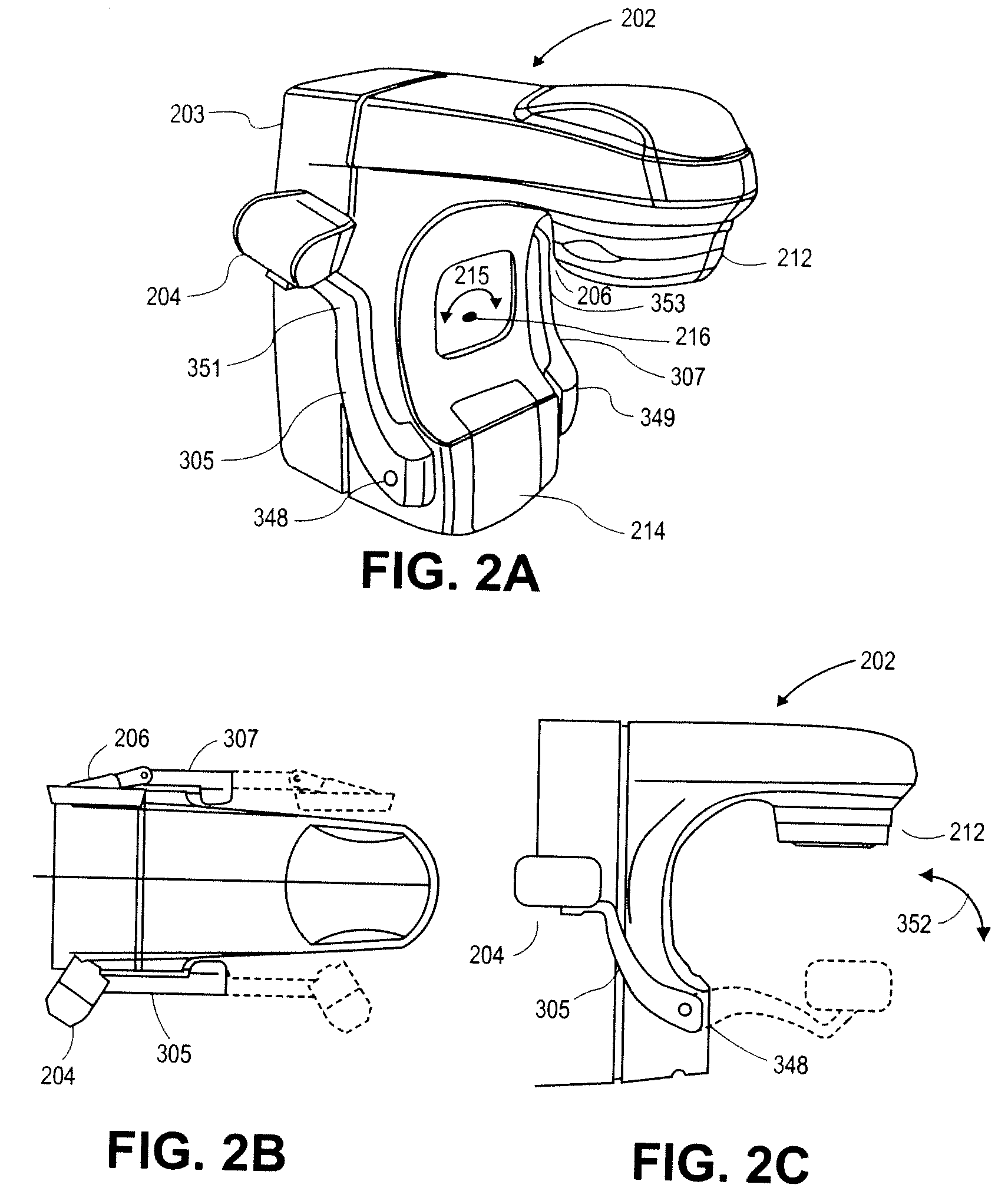
Imaging device for radiation treatment applications
ActiveUS7657304B2Material analysis using wave/particle radiationRadiation/particle handlingRobotic armEngineering
A radiotherapy clinical treatment machine is described having a rotatable gantry and an imaging device with articulating robotic arms to provide variable positioning and clearance for radiation treatment applications. According to one aspect of the invention, a first and a second robotic arms are pivotally coupled to the rotatable gantry, allowing the robotic arms to maneuver independently from the rotatable gantry.
Owner:VARIAN MEDICAL SYSTEMS
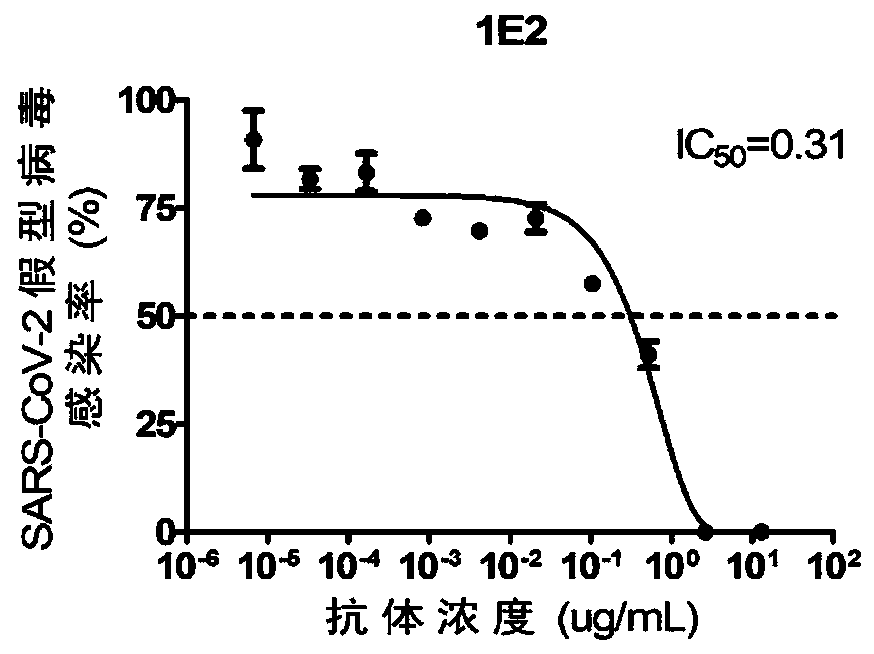
Single-domain antibodies for novel coronavirus and application of single-domain antibodies
ActiveCN111303279AHigh affinityAnd high activityImmunoglobulins against virusesAntiviralsAntigenReceptor
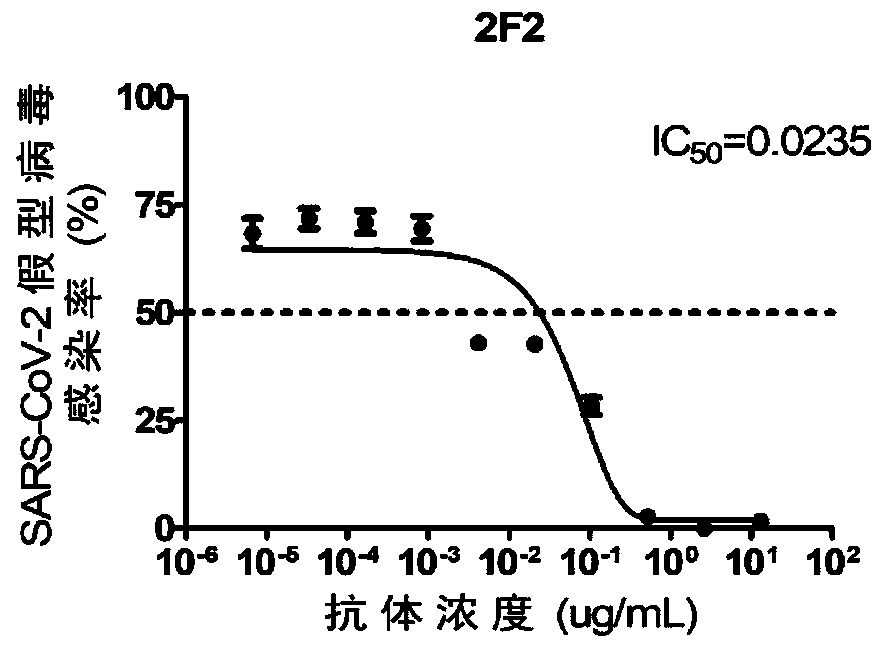
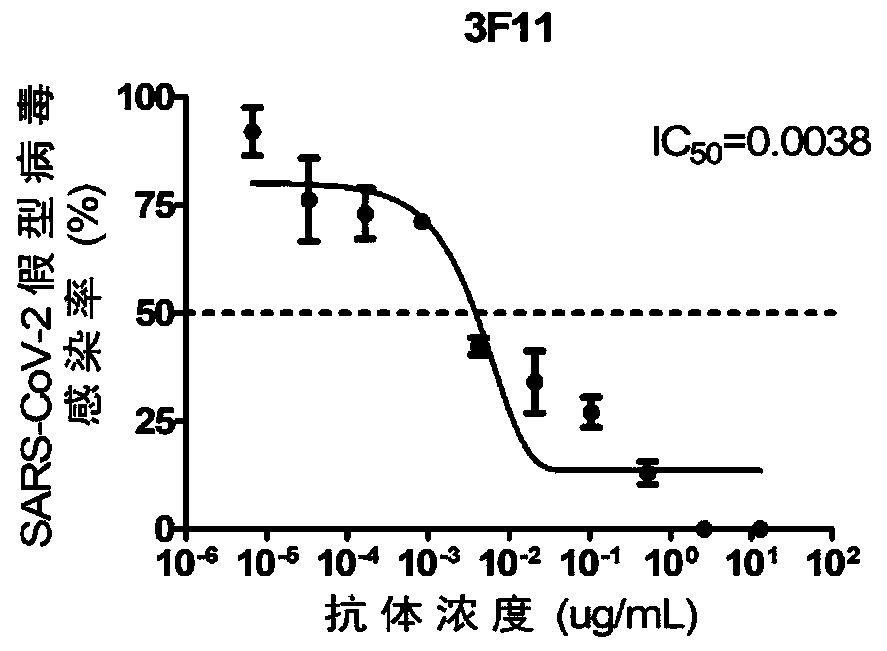
The invention discloses humanized single-domain antibodies for a novel coronavirus SARS-CoV-2 and an application of the humanized single-domain antibodies. The invention protects the single-domain antibodies described in any one of SEQ ID No.1 to SEQ ID No.5. Experimental results show that the single-domain antibodies provided by the invention have good affinity with receptor binding domain (RBD)antigens and have high neutralization activity on SARS-CoV-2 pseudovirus. The humanized single-domain antibodies have important scientific significance and application prospect for prevention and clinical treatment of the new coronavirus SARS-CoV-2 and for development of diagnostic reagents of the new coronavirus SARS-CoV-2.
Owner:BEIJING KAWIN TECH SHARE HLDG
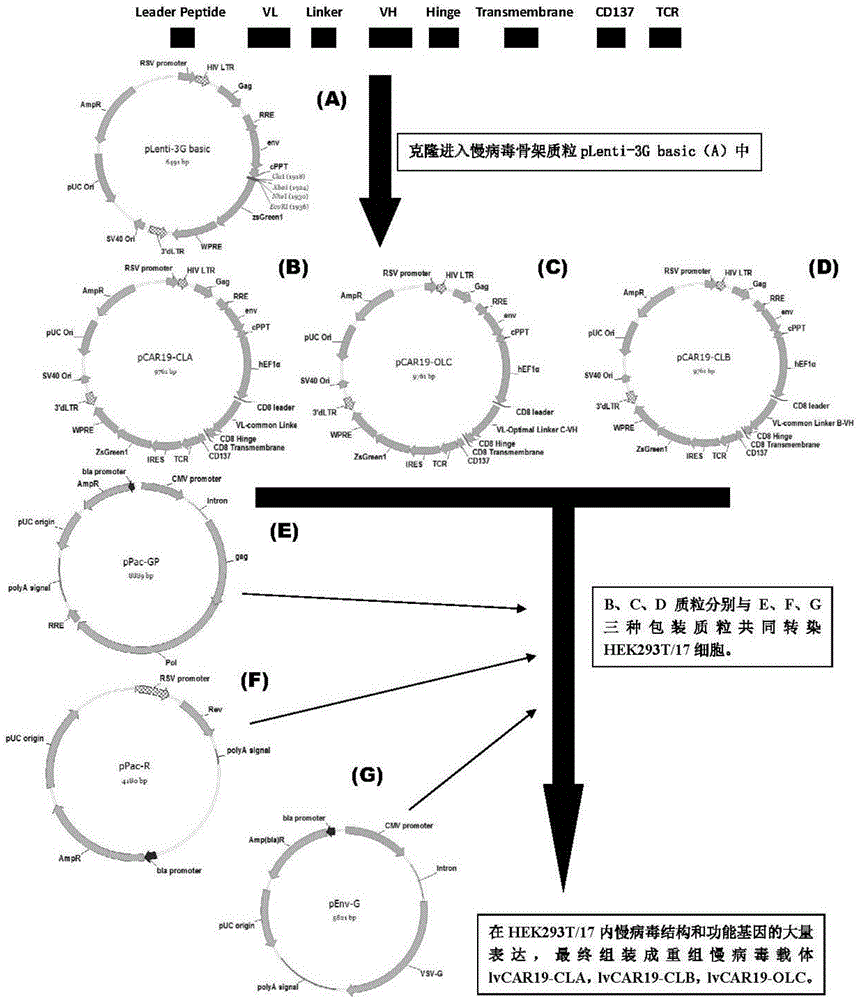
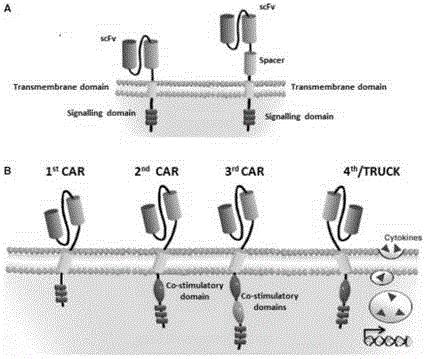
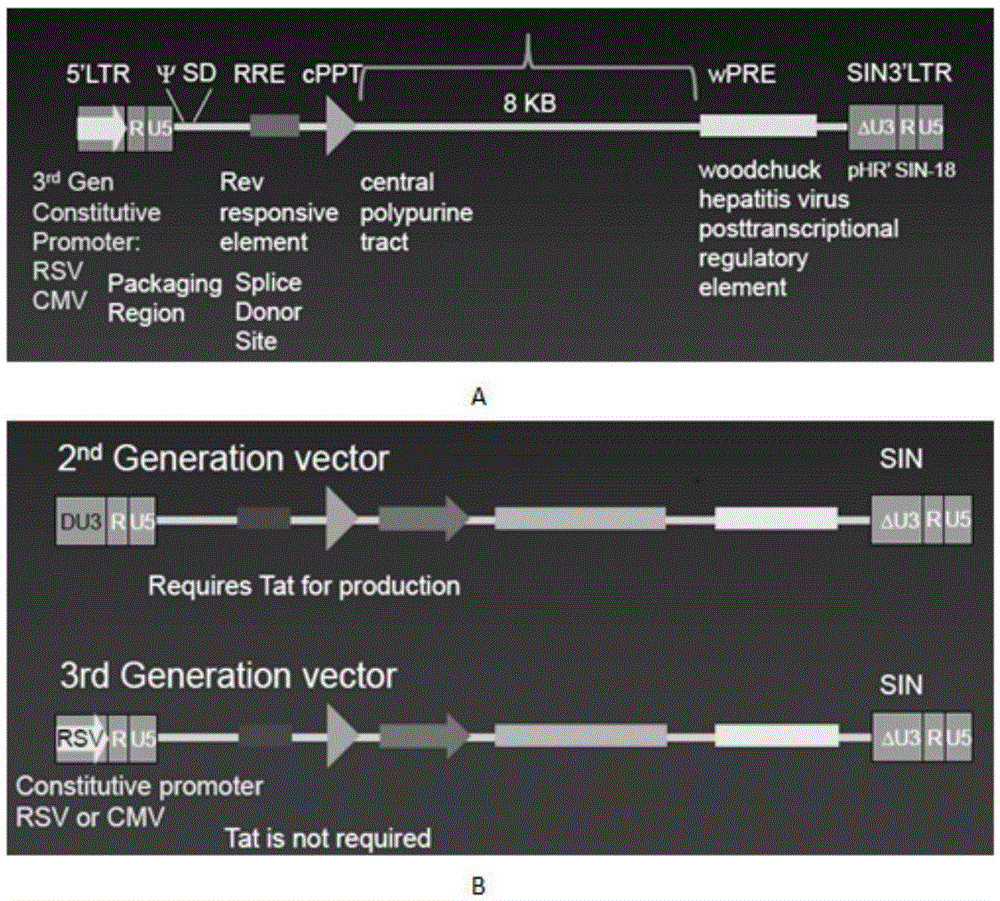
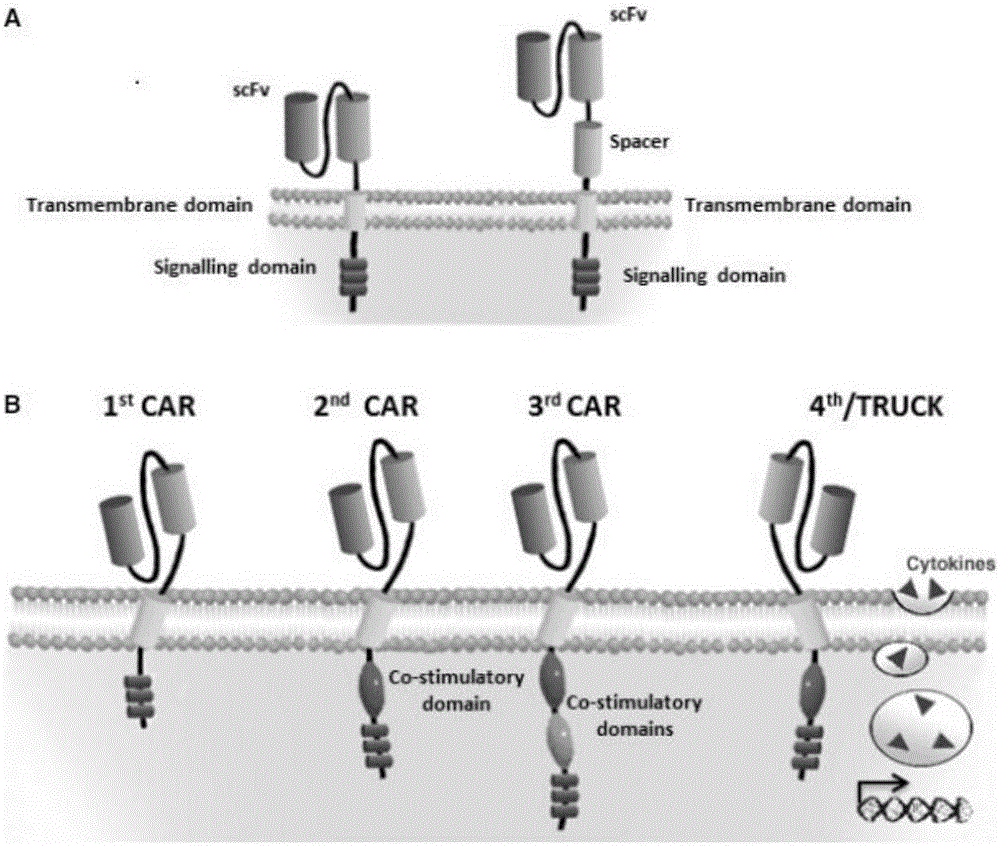
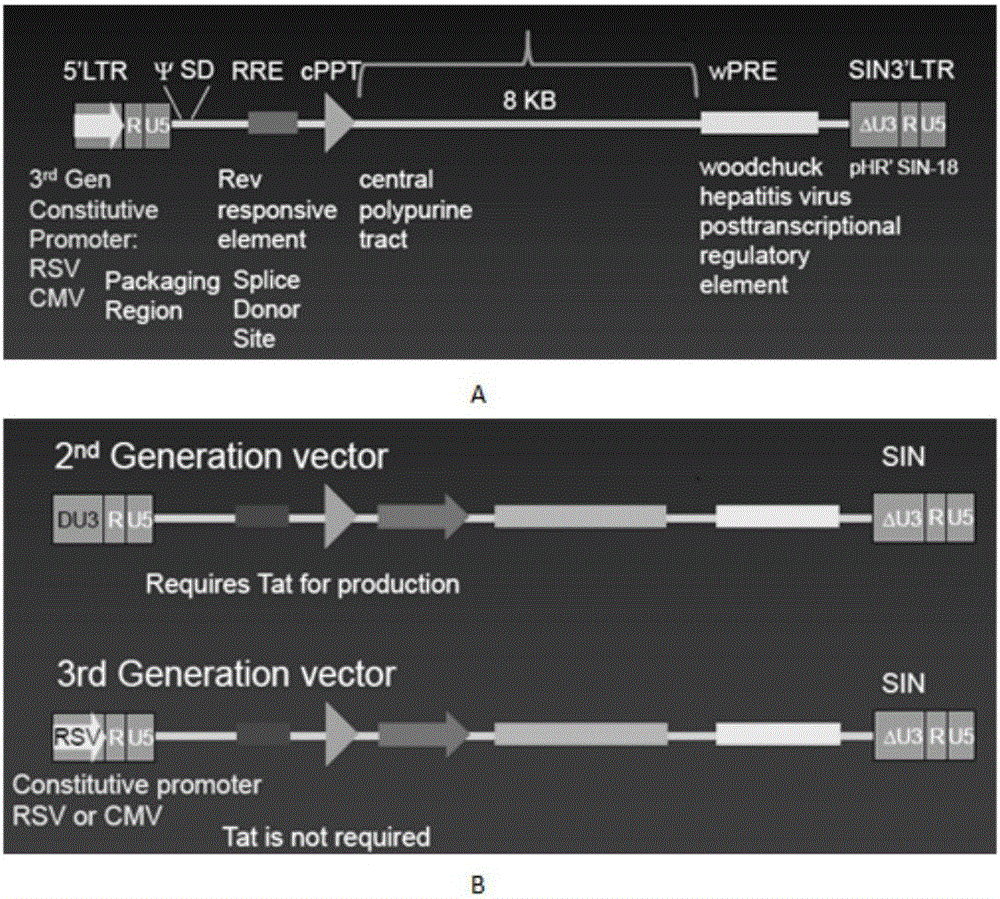
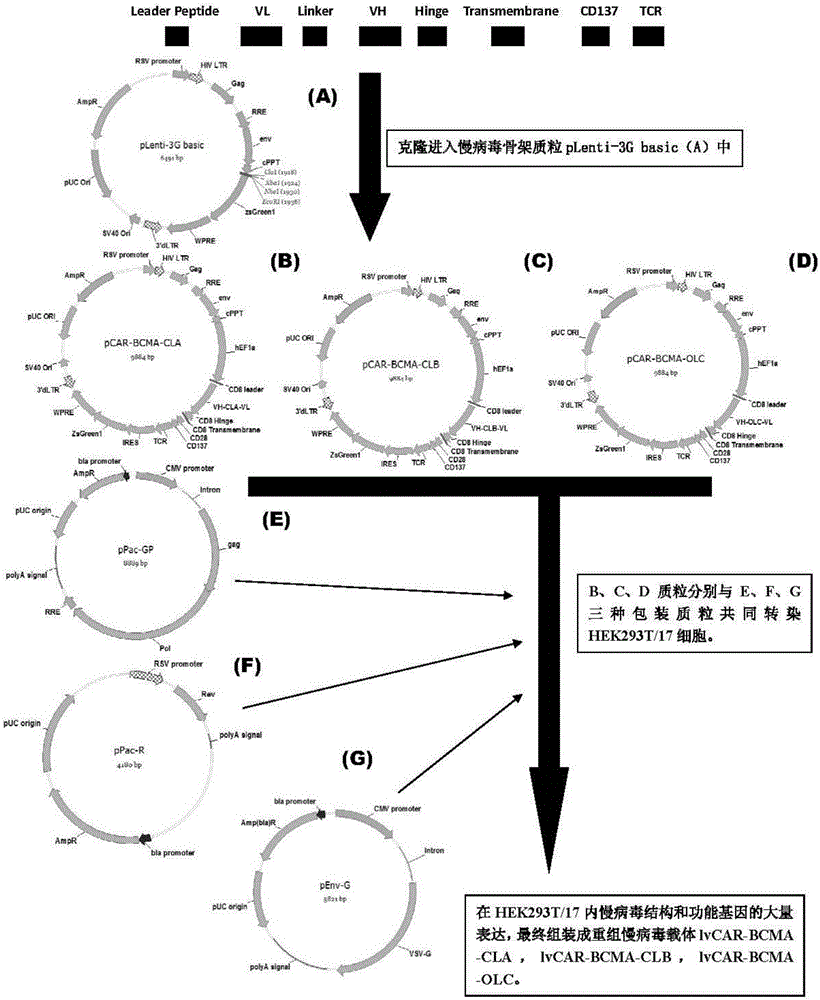
CAR-T transgene vector based on replication defective recombinant lentivirus and construction method and application of CAR-T transgene vector
ActiveCN105602992ASignificant effectPromote secretionGenetic material ingredientsFermentationEucaryotic cellAmpicillin
The invention discloses a CAR-T transgene vector based on replication defective recombinant lentivirus. The CAR-T transgene vector comprises an original nuclear replicon pUCOri sequence, a resistance gene AmpR sequence containing ampicillin, a virus replicon SV40 Ori sequence, a lentivirus packaging cis element, ZsGreen1 green fluorescent protein, an IRES ribosome binding sequence, a human EF1 alpha promoter , a chimeric antigen receptor of second-generation CAR or third-generation CAR and a regulating element, wherein the original nuclear replicon pUCOri sequence is used for plasmid replication; the resistance gene AmpR sequence is used for massively proliferating target strains; the virus replicon SV40 Ori sequence is used for enhancing replication in eukaryocyte; the lentivirus packaging cis element is used for lentivirus packaging; the ZsGreen1 green fluorescent protein is used for expressing green fluorescent for eukaryocyte; the IRES ribosome binding sequence is used for jointly transcribing and expressing protein; the human EF1 alpha promoter is used for conducting eukaryotic transcription on antigen receptor genes; the chimeric antigen receptor is used for forming the second-generation CAR or the third-generation CAR integrating recognition, transfer and start; the regulating element is used for enhancing expression efficiency of transgenes and used after eWPRE-enhanced type woodchuck hepatitis b virus is transcribed. Besides, the invention further discloses a construction method and application of the vector. By means of the CAR-T transgene vector and the construction method and application of the vector, secretion of cell factors and an in vitro killing effect of CAR-T cells can be remarkably improved, and the clinical treatment effect is remarkable.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
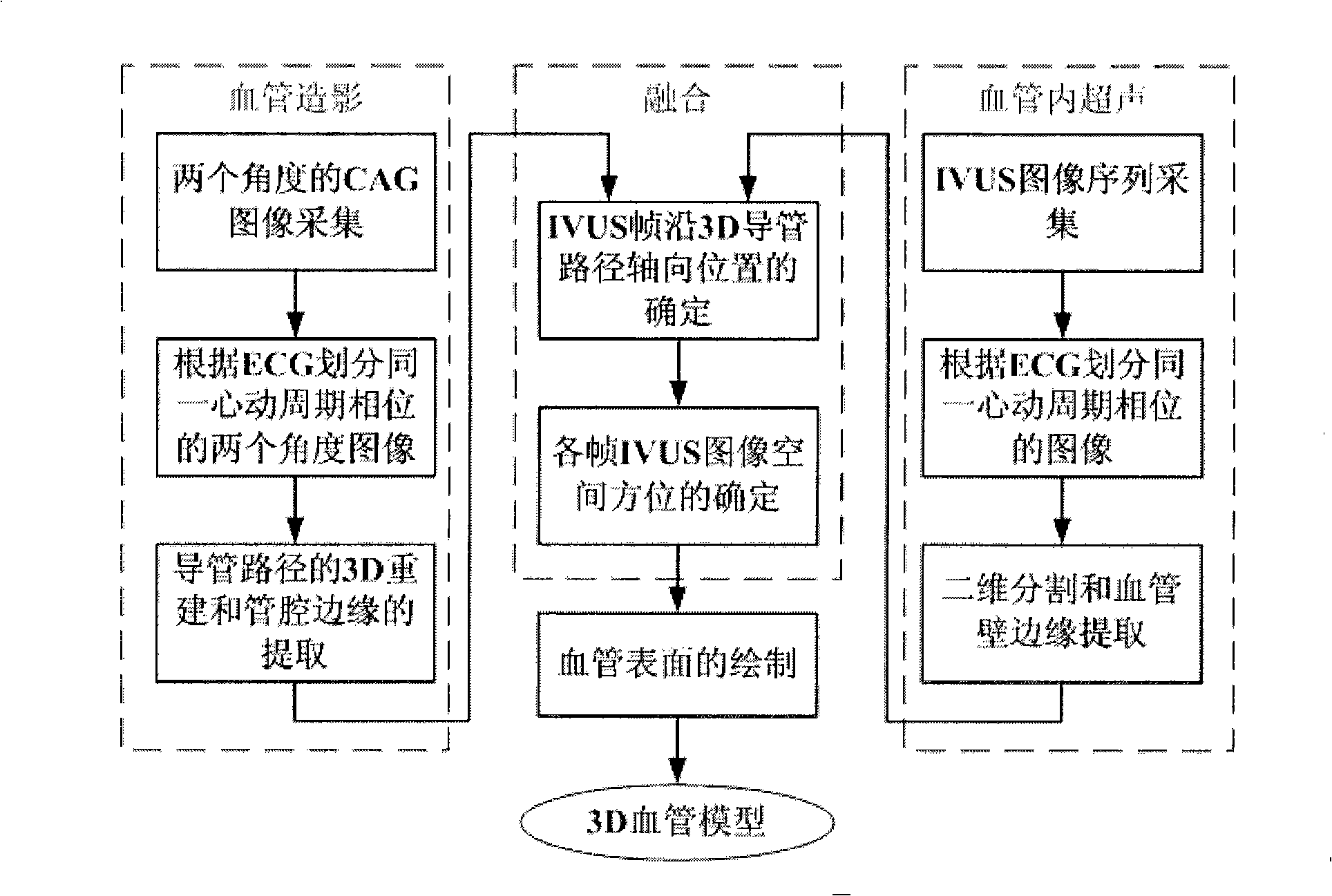
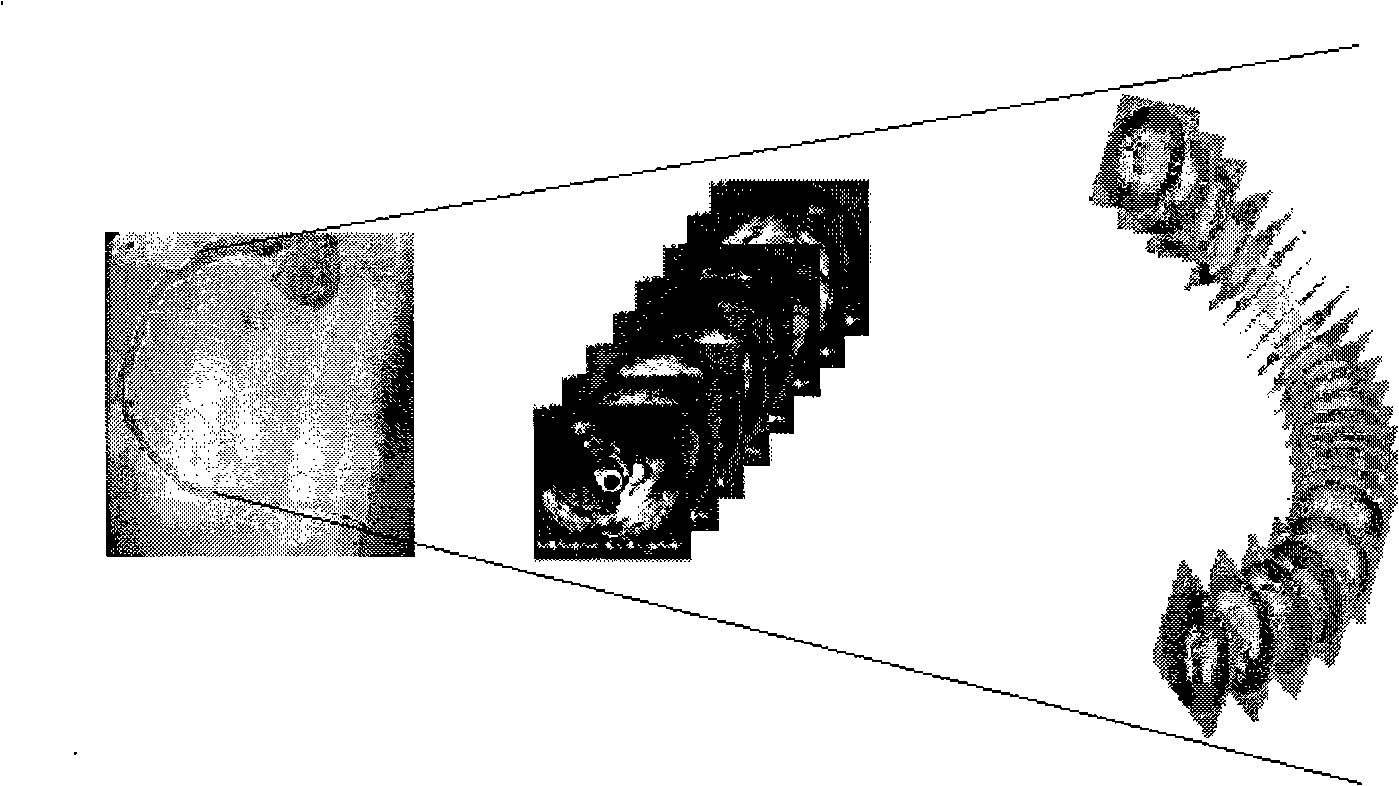
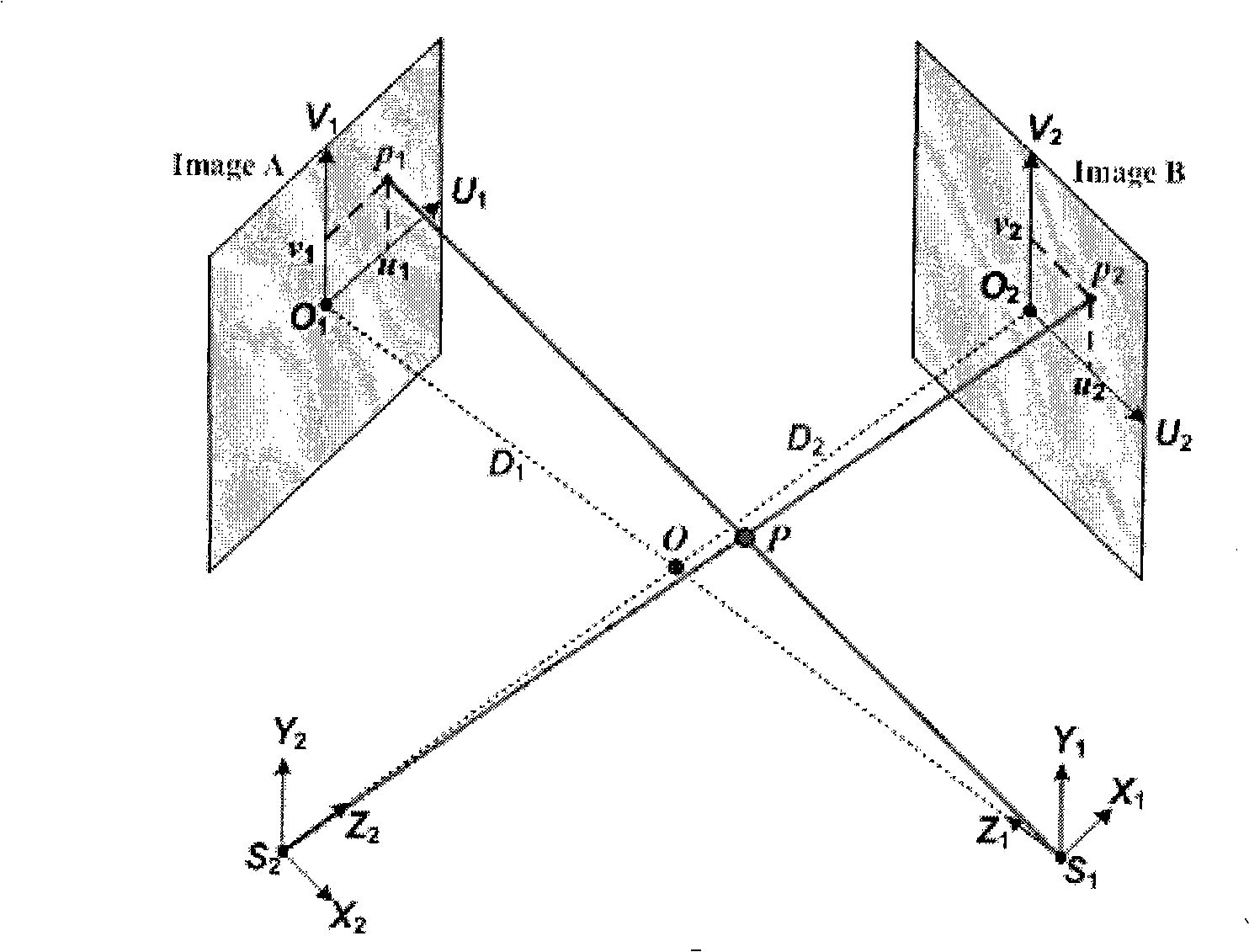
Rebuilding method of blood vessel three-dimensional model
InactiveCN101283929AReflect real bendReflection distortioUltrasonic/sonic/infrasonic diagnosticsImage analysisAnatomical structuresSonification
A method for reconstructing 3D blood vessels belongs to the field of medical detection technology for solving the accuracy problem in blood vessel reconstruction. The technical scheme is that the method can accurately reconstruct the anatomic structure of a blood vessel by combining cross section information of the blood vessel acquired by an IVUS image sequence and ultrasonic duct spatial geometrical information obtained by 3D reconstruction based on X-ray angiographic images. Compared with the reconstruction result obtained by using two single images, the method can completely and accurately reflect the actual shapes of blood vessels and possibly-existing plaques, thus providing more reliable basis for clinical treatment of coronary heart diseases and other angiopathies.
Owner:NORTH CHINA ELECTRIC POWER UNIV (BAODING)
Ultrasonic treatment of breast cancer
InactiveUS20050038339A1Ultrasonic/sonic/infrasonic diagnosticsUltrasound therapyEarly breast cancerWilms' tumor
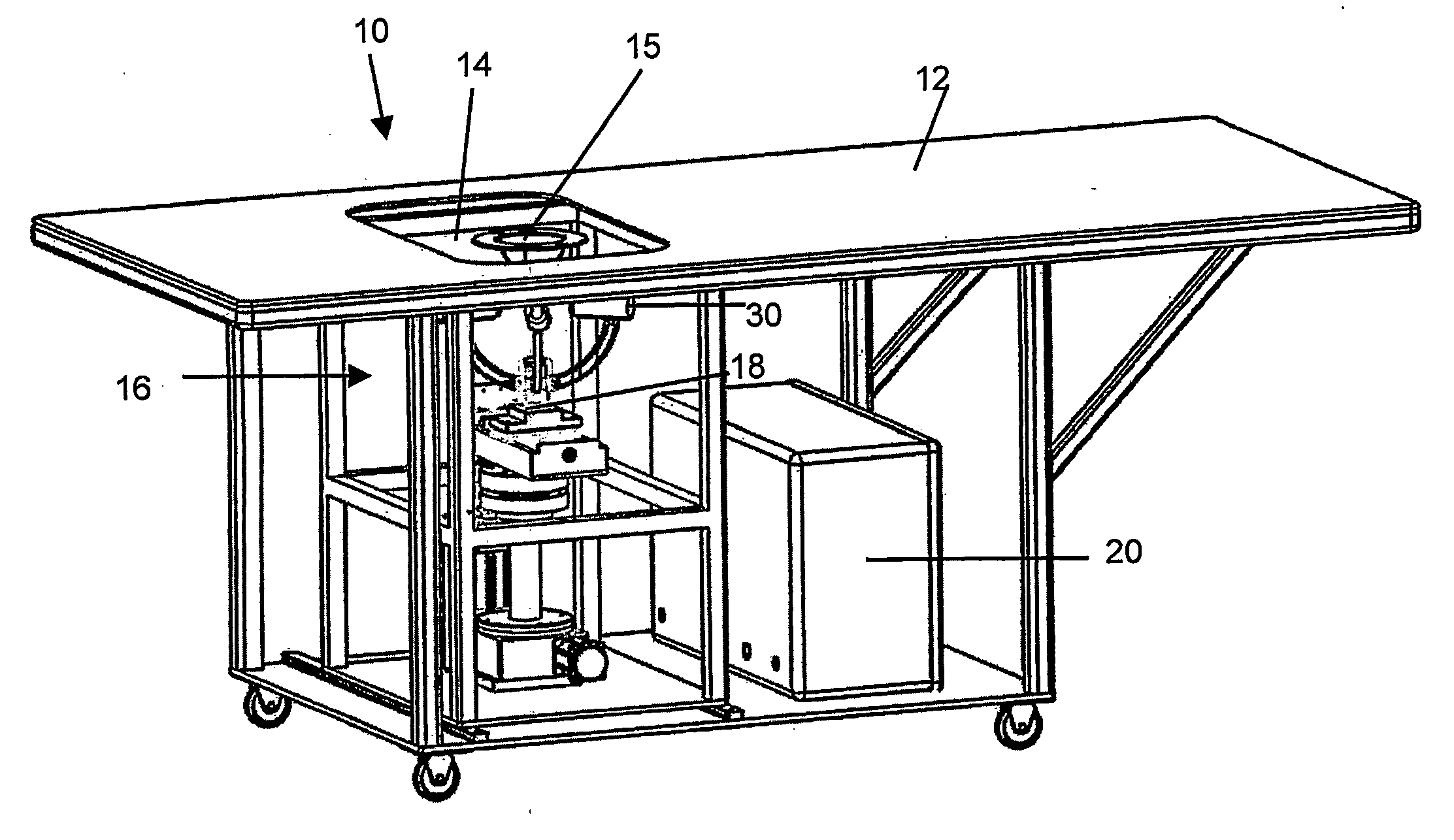
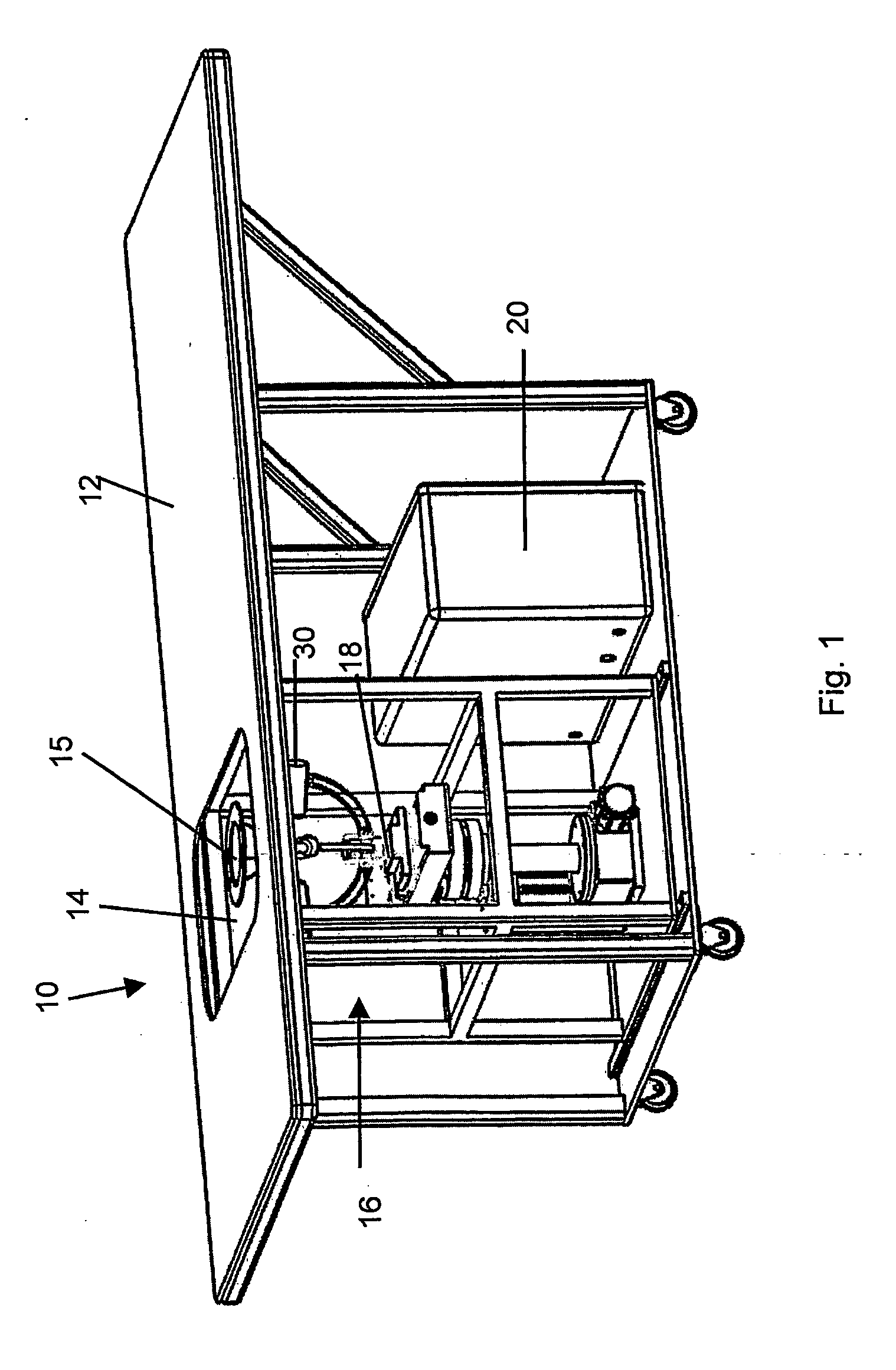
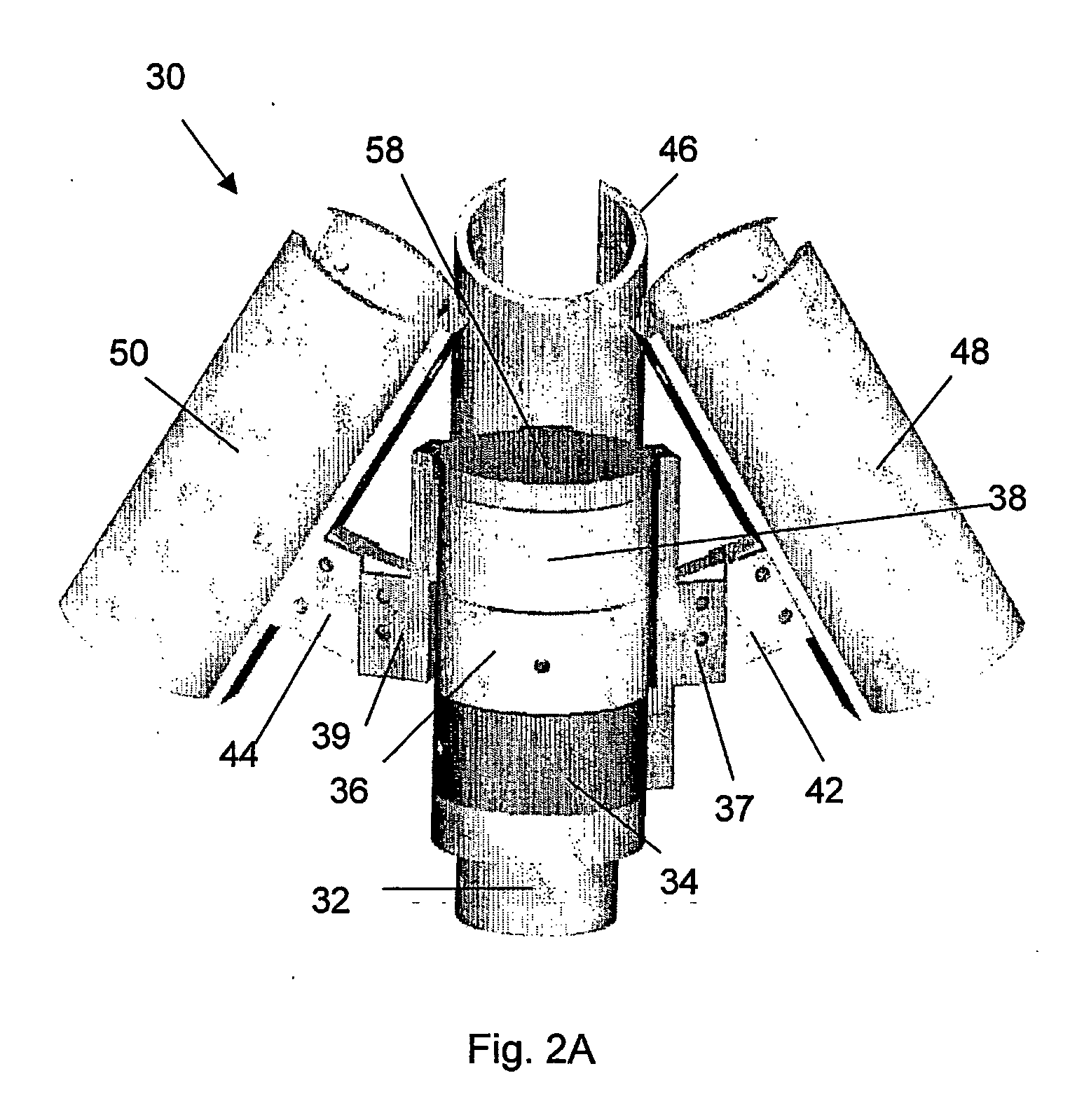
A method of treatment, clinical treatment assembly, robotic manipulator and controlling arrangements for the treatment of cancers are described. The invention has particular application in the treatment of breast cancer. A robotic manipulator (18) carries a jig assembly (30). The jig assembly (30) includes an array of treatment probes (52, 54, 56) and a single identification / diagnostic probe (58). The probes can be moved by the robotic manipulator (18) in three directions (x, y, θ). A subject breast tissue is received in a tank (16) through an operating window (14), and the robotic manipulator (18) is to firstly determine the site of a tumour in the breast tissue. Once the tumour has been located by use of the identification / diagnostic probe (58), the treatment probes (52, 54, 56) are used to ablate the tumour by the superposition of ultrasonic waves at a focal region. A series of such lesions may be performed in sequence to traverse the full extent of the tumour.
Owner:NANYANG TECH UNIV
Medical composition containing riluzole
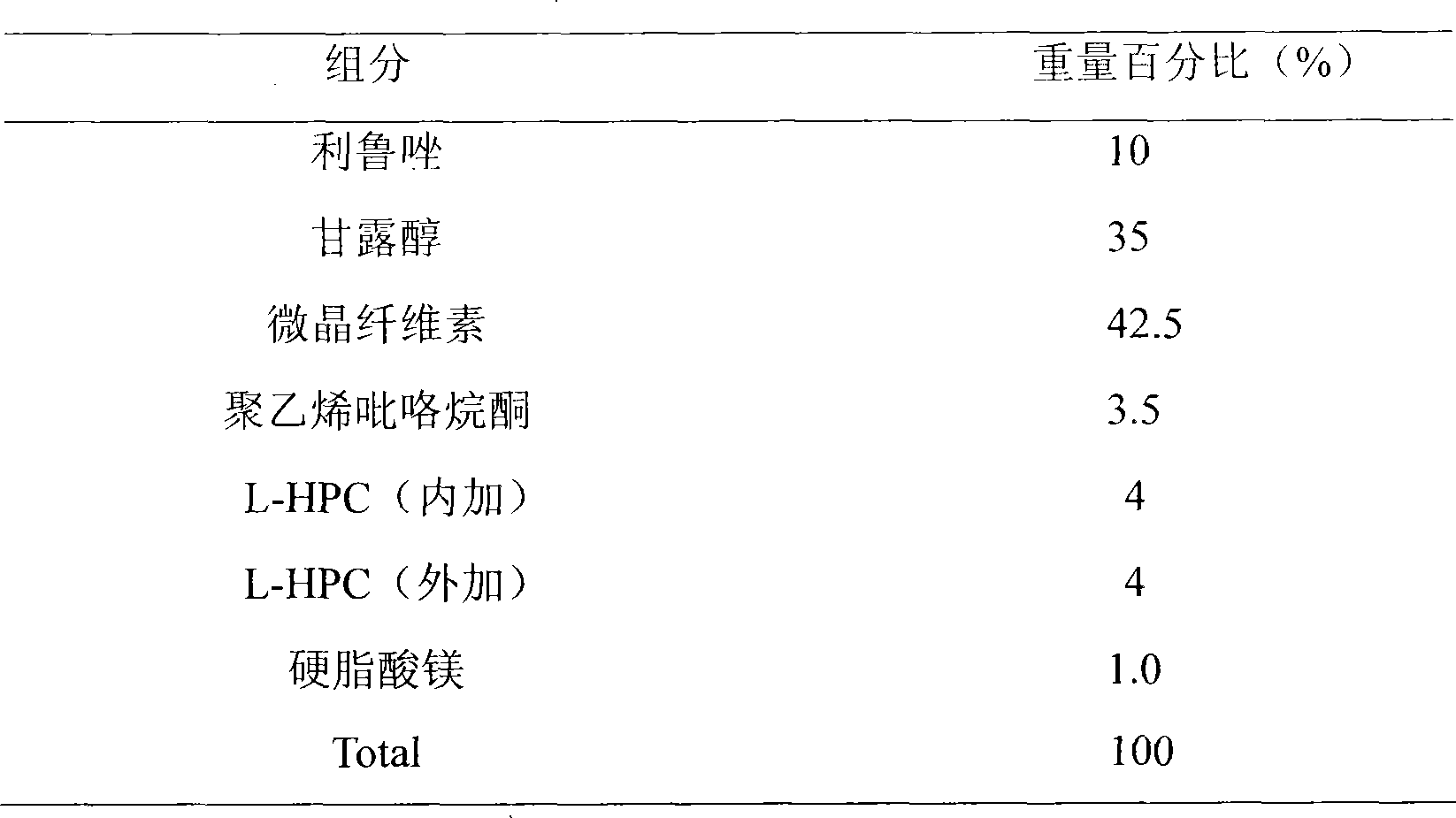
InactiveCN101390854AHigh dissolution rateEasy to takeOrganic active ingredientsNervous disorderChemical compositionBULK ACTIVE INGREDIENT
The invention discloses a stable medicinal combination which contains active ingredient riluzole and pharmaceutical auxiliary material. The medicine combination is mainly used for the clinical treatment of amyotrophic lateral sclerosis.
Owner:AVENTIS PHARMA HAINAN
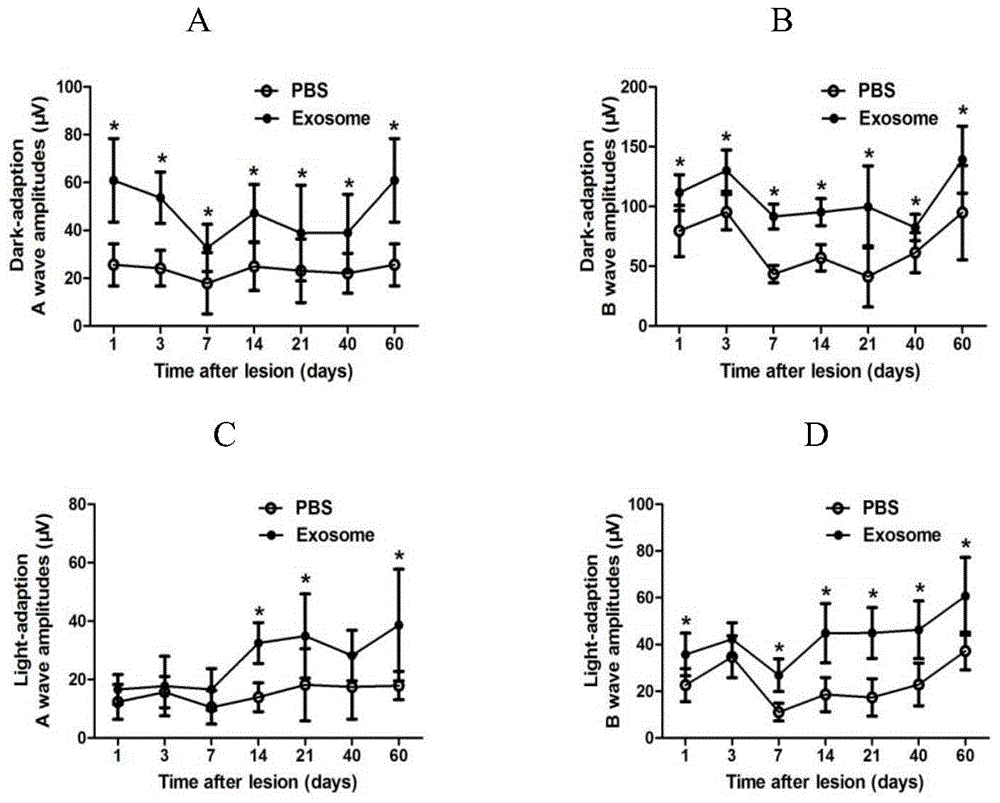
Stem cell exosome patch and preparation method and application thereof
InactiveCN104894062AAvoid the possibility of tumor formationStrong targetingImmobilised enzymesNervous system cellsBiological propertyAvailable drugs
The invention discloses a stem cell exosome patch and its preparation method and application and belongs to the field of tissue engineering. The stem cell exosome patch comprises the following steps: stem cell is cultured and stem cell exosome is collected; a patch scaffold is prepared; and the exosome obtained is attached to the patch scaffold to prepare the stem cell exosome patch. the stem cell exosome patch obtained has natural and easily available drug sources; action site accuracy of the exosome patch can be guaranteed; effective acting time of the patch after implantation can be prolonged; and potential safety hazard can be avoided to a great extent. Through the method, the stem cell exosome patch with good biological characteristics can be obtained. The invention is a new breakthrough of establishing a biological patch in the field of tissue engineering. Meanwhile, the product also provides a feasible scheme for clinical treatment of diseases. The principle of the invention is scientific and reliable, and the technology is simple and flexible.
Owner:JINAN UNIVERSITY
Double membrane tissue patching material and preparation method thereof
The invention discloses a double-layer membranous tissue repair material and a preparation method thereof, wherein, a cell-free membranous biological derivative material is used as a surface layer, and a fibroblast is compounded in the interior of a biological support material to form a substrate, and then the surface layer and the substrate are combined in a chimeric way to form the double-layer membranous tissue repair material; a compact surface layer structure can effectively reduce the loss of water, electrolytes and protein from surface of wound, avoid the invading and the reproduction of bacteria to the impaired surface of wound as well as prevent the infection of the surface of wound, thus being beneficial to epitheliosis and epithelial growth; the substrate can directly repair the surface of wound, promote the ingrowth of cells around the surface of wound and the angiogenesis, induce the differentiation from stem cells to skin cells and quicken wound healing; compared with the existing products, the tissue repair material has the advantages of being capable of promoting the regeneration of skin, improving the elasticity, the flexibility and the mechanical abrasion resistance of skin after the surface of wound is healed, reducing hyperplasia of scar tissues, controlling the contracture, having excellent biocompatibility, increasing the success rate of transplant and improving the quality of healing; the invention has wide material resources and simple production method; the double-layer membranous tissue repair material prepared is applicable to the clinical treatment of skin defect caused by inflammation, ulcer, thermal burns, iatrogenicity and the like.
Owner:SHAANXI RUISHENG BIOTECH
Systems and methods for disease knowledge modeling
InactiveUS20130268290A1Medical simulationData processing applicationsTreatment choicesTreatment options
Systems and methods are described herein for disease knowledge modeling and clinical treatment decision support, and the prioritization of possible treatment options based on tumor or other disease biomarkers. Disease or indication information, including identification of biomolecular entities associated with the indication may be culled through text data mining to create a knowledge model of the indication. In some embodiments, the knowledge model may comprise a network of associations between molecular entities, including such drug targets and biomakers, genes, pathways. The model may be combined with patient-specific variant information and historical treatment records to identify and prioritize treatment decisions and allow for the prediction of disease drivers and provide treatment options tailored to a patient's genetic data.
Owner:MOLECULAR HEALTH
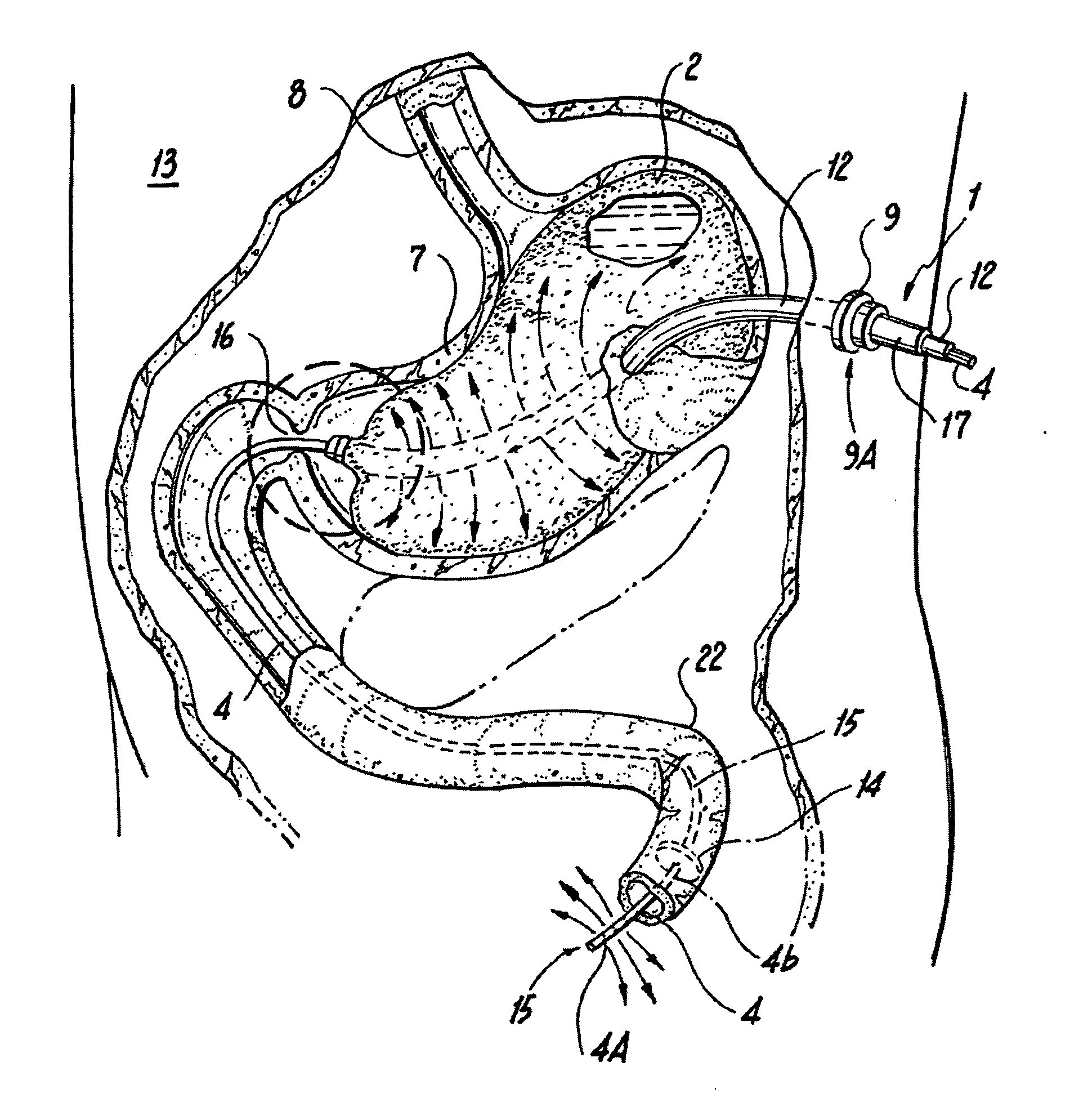
Endoscopically placed gastric balloon (EPGB) device and method for treating obesity involving the same
An endoscopically placed gastric balloon (EPGB) includes a gastric balloon. The EPGB is insertable in a patient and anchored in position between the lock and a portal. A port section enables the gastric balloon to be filled with mass containing a liquid medium during treatment, resulting in a patient receiving a true satiated neurogastric full feeling eliminating cravings and hunger feelings. The EPGB device allows for the balloon to be easily deflated or reduced and for a gastric suction and lavage to be accomplished without patient discomfort, while the device is in place and easily concealed from others during use. A method of using the EPGB enables a clinical treatment of a morbidly obese patient with greatly reduced health risks and neuro-psychiatric complications.
Owner:BASU PATRICK
Novel Imino Sugar Derivatives Demonstrate Potent Antiviral Activity and Reducted Toxicity
InactiveUS20110189771A1Good effectImprove performanceOrganic chemistryTissue cultureBovine Viral Diarrhea VirusesSide chain
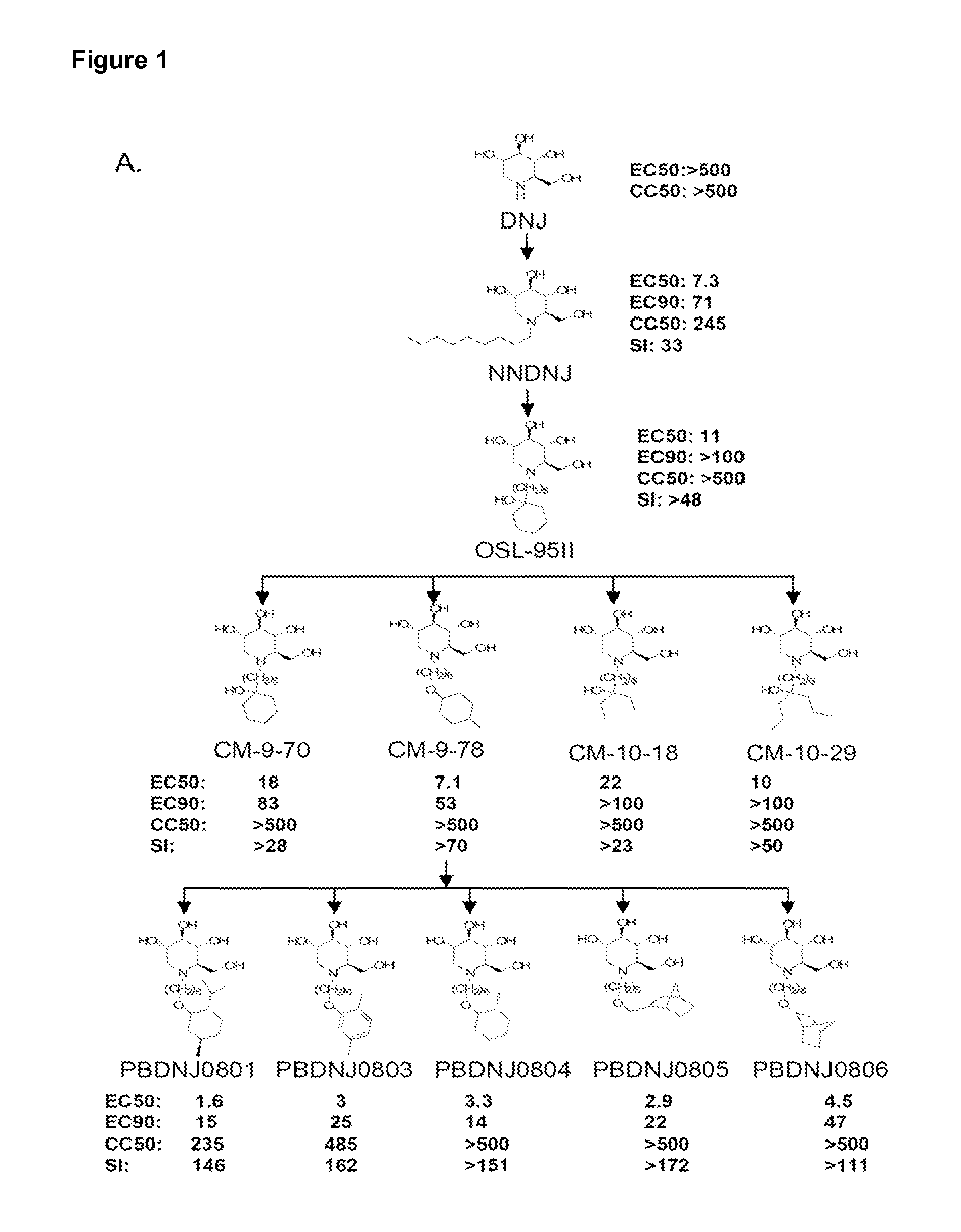
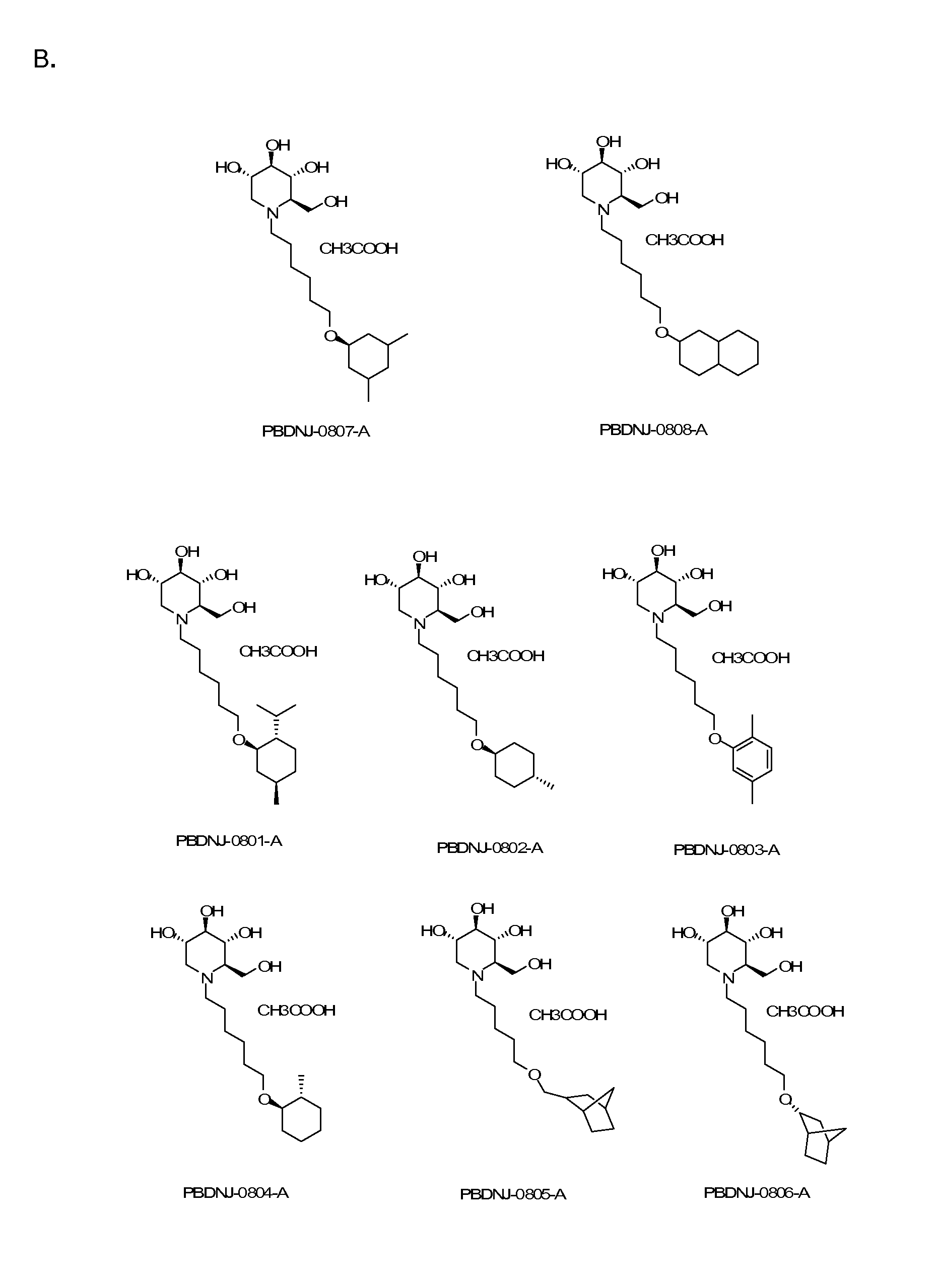
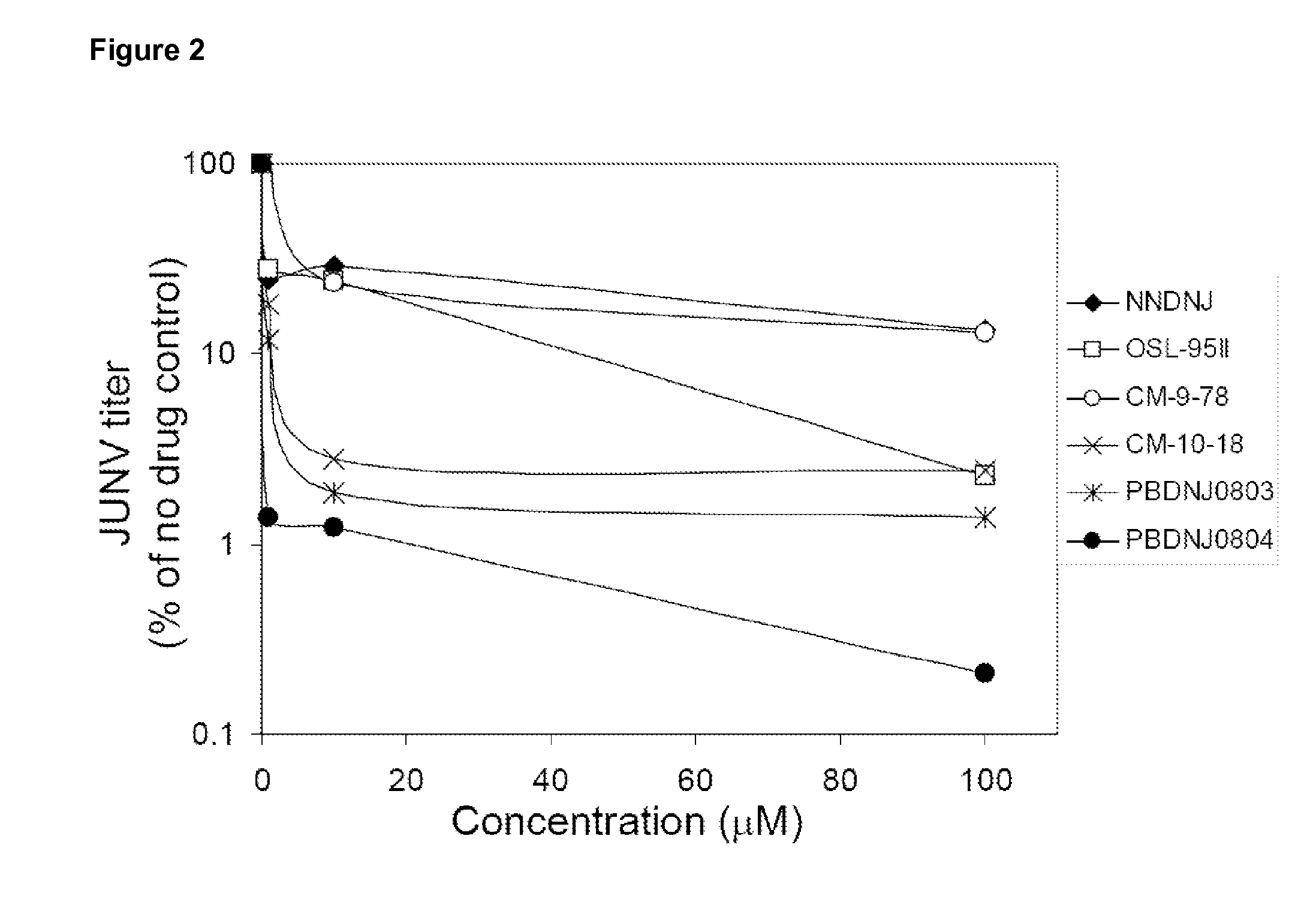
Imino sugars, such as deoxynojirimycin (DNJ), are glucose analogues that selectively inhibit cellular α-glucosidase I and II (enzymes that process N-linked glycans in glycoprotein) and exhibit broad spectrum antiviral activities against many enveloped viruses. Previously we have reported a novel DNJ derivative, OSL-95II, with antiviral activity and reduced cytotoxicity. In order to develop imino sugars with more potent antiviral activity as well as improved toxicity profile, OSL-95II was modified by diversifying the nitrogen linked alkylated side chain. The antiviral activities were initially tested in bovine viral diarrhea virus (BVDV) infected MDBK cells, yielding several imino sugar derivatives with novel structure and superior antiviral activity and toxicity profile. Furthermore, these new compounds were shown to be active against Dengue virus (DV) and West Nile virus (WNV) infection in BHK cells where potent anti-DV activity having submicromolar EC50 values and SI of greater than 900. These compounds represent a new generation of iminio sugars and their analogues, having application in the clinical treatment of infection of DV and other members of flaviviridae.
Owner:INST FOR HEPATITS & VIRUS RES +1
Method for preparing human umbilical cord mesenchymal stem cells
The invention relates to a method for preparing human umbilical cord mesenchymal stem cells, which comprises the steps of collection, transportation, handover, separation, freezing, recovering, primary culture and subculture of umbilical cords. The method has simple process, ensures low production cost, good freezing effect, little cell damage, low toxic side effect and no harm to human body and reduces the residual of the freezing protection liquid in the recovered cells of tissue blocks; and the prepared mesenchymal stem cells can be applied to the clinical treatment of various diseases.
Owner:江苏省北科生物科技有限公司 +1
Preparation method of allogenic mesenchymal stem cells by CRISPR (clustered regularly interspaced short palindromic repeats) technique editing and IGF (insulin-like growth factor) optimization and application of allogenic mesenchymal stem cells in treating myocardial infarction
ActiveCN105985985AImprove anti-apoptotic abilityPromote homingUnknown materialsFermentationAntigenInflammatory factors
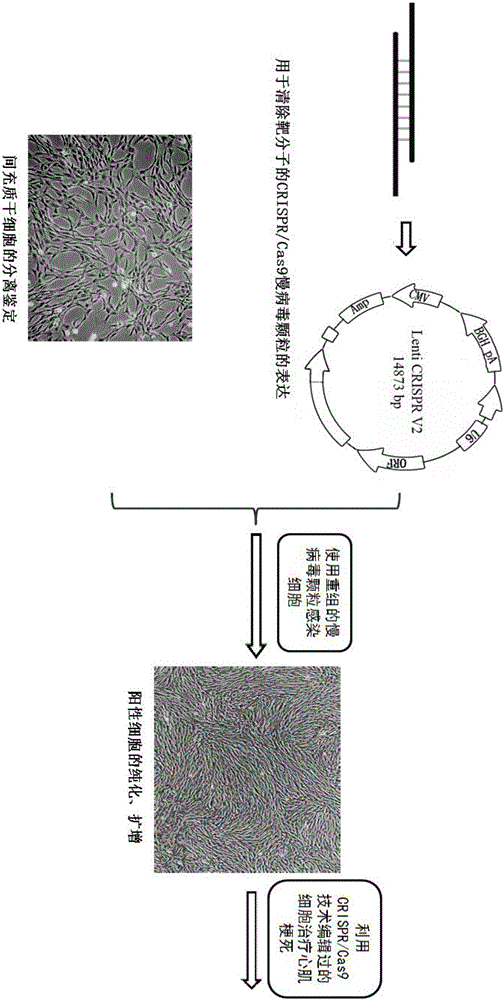
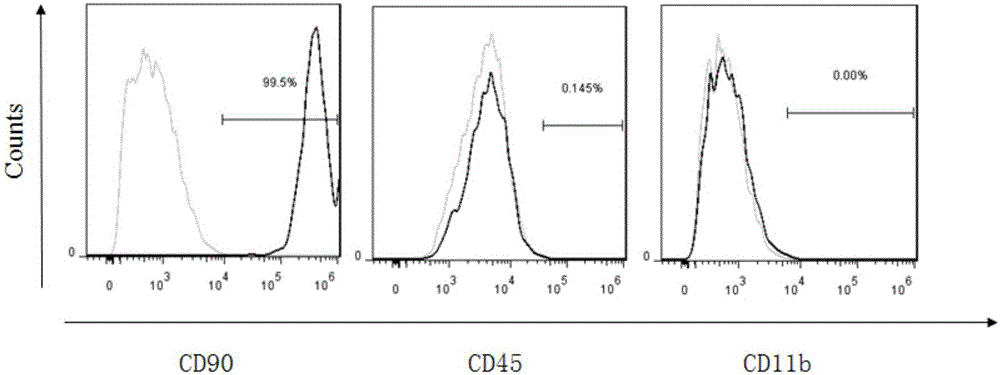
The invention belongs to the field of allogenic mesenchymal stem cells, and particularly relates to a preparation method of allogenic mesenchymal stem cells by CRISPR (clustered regularly interspaced short palindromic repeats) technique editing and IGF (insulin-like growth factor) optimization and application of the allogenic mesenchymal stem cells in treating myocardial infarction. The preparation method comprises the following steps: carrying out separation by density gradient centrifugation to obtain allogenic single karyocytes, and carrying out adherent culture to obtain mesenchymal stem cells; designing a mesenchymal stem cell surface antigen B2M-gRNA and an inflammatory factor TNF-alpha-gRNA; establishing recombinant slow virus particles, and transfecting the mesenchymal stem cells; optimizing the mesenchymal stem cells by using IGF-1; and preparing drugs for treating myocardial infarctions by using the modified and optimized mesenchymal stem cells. The CRISPR / Cas9 technique is utilized to remove the antigens capable of causing immunological rejection and the inflammatory factors capable of causing inflammatory reaction on the mesenchymal stem cell surface, and the IGF-1 is utilized to enhance the apoptosis resistance of the mesenchymal stem cells and promote the homing of the mesenchymal stem cells, thereby providing a new technical scheme for preparing drugs for treating cardiovascular diseases in clinic. The prepared allogenic mesenchymal stem cells can not cause immunological rejection after cell transplantation.
Owner:SUZHOU UNIV
Traditional Chinese medicine composition for treating insomnia and preparation method thereof
InactiveCN101757568AImprove self-coordinationEffective treatmentHeavy metal active ingredientsNervous disorderAsparagus cochinchinensisClinical efficacy
The invention discloses a new traditional Chinese medicine composition for treating insomnia and a preparation method thereof. The traditional Chinese medicine composition mainly comprises the following raw Chinese medicinal herbal materials: cinnabar, nacre mother of pearl, cortex albiziae, semen boitae, tuber fleeceflower stem, polygala root, amber, fossil fragments, spina date seed, glossy ganoderma, shell of abalone, oyster, gastrodia elata, root of straight ladybell, ophiopogon japonicus, lucid asparagus, fruit of Chinese wolfberry, fushen, motherwort, Chinese angelica, radix paeoniae alba, cortex lycii radicis, agilawood, root of three-nerved spicebush, rhizoma cyperi and the like. The traditional Chinese medicine composition can be prepared into any one common oral preparation according to a conventional traditional Chinese medicine preparation method. The invention can remarkably improve symptoms of insomnia, difficulty falling asleep, easy awakening, difficulty falling asleep after awakening, alternation of falling asleep and awakening, difficulty falling asleep all through the night, doldrums, slow response, fatigue, even vexation and the like, and has the advantages of accurate clinical treatment effect, remarkable treatment effect, low cost, basically no toxic or side effects, and the like.
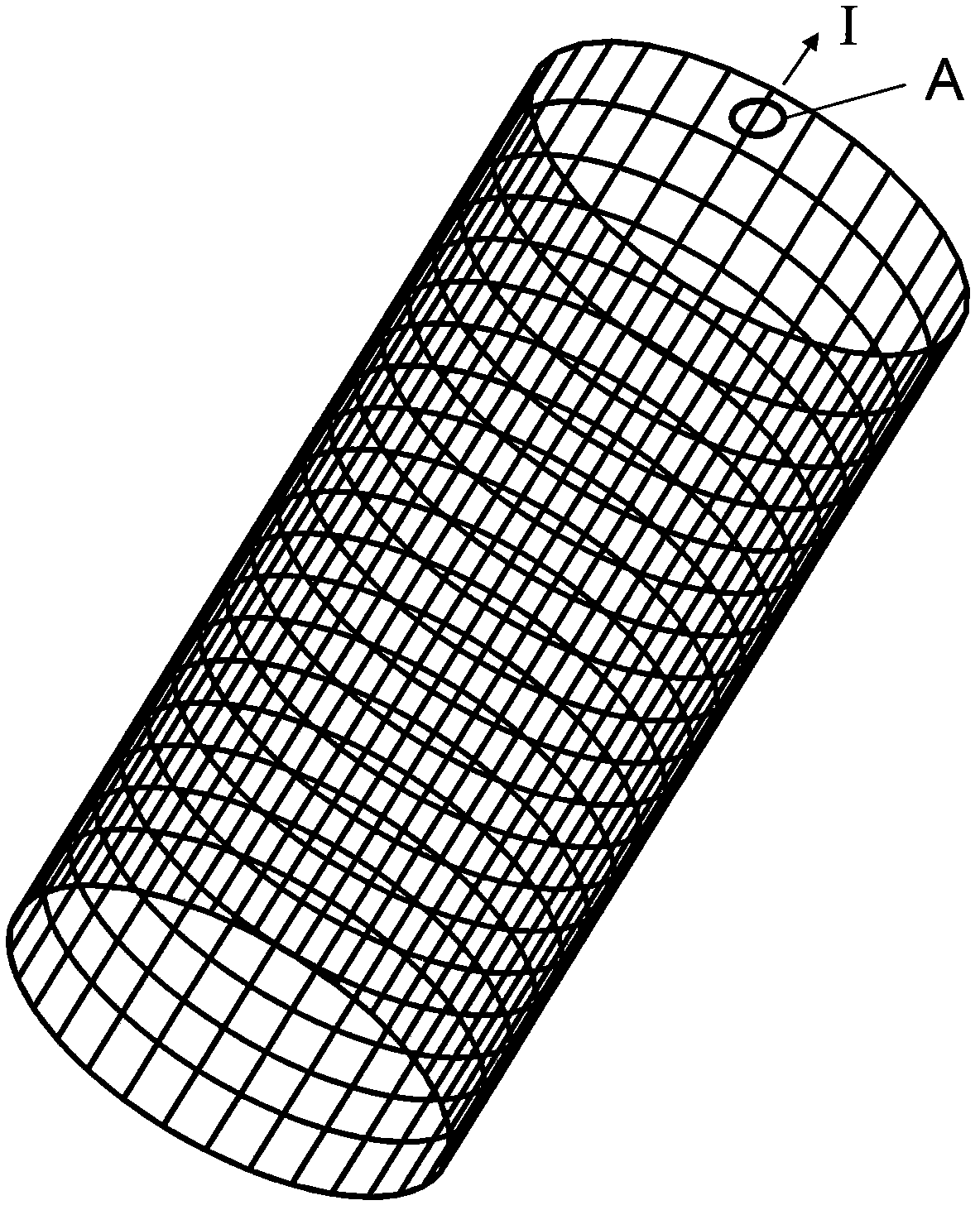
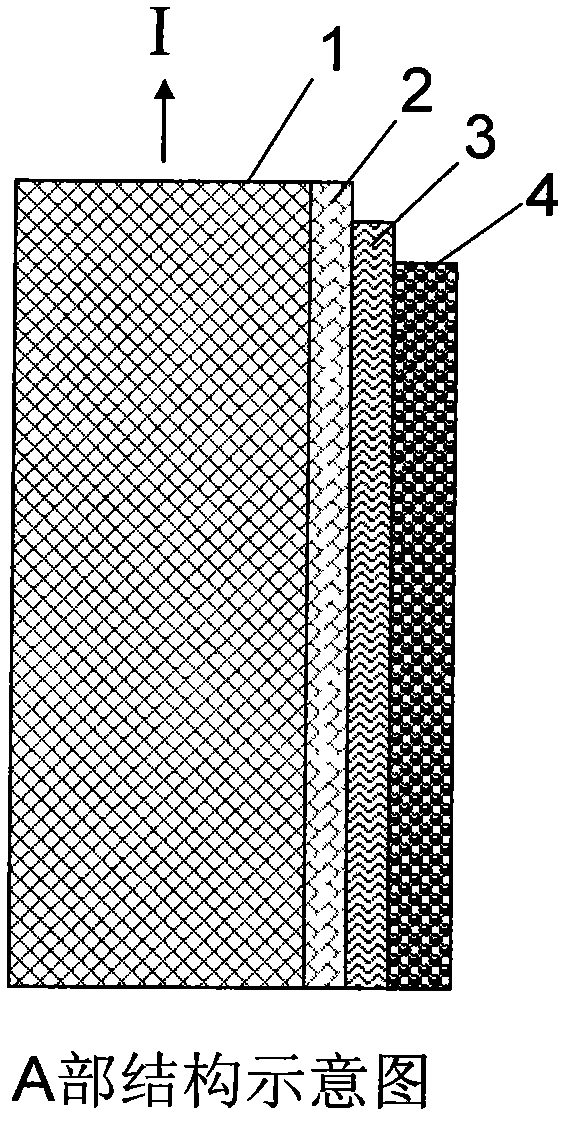
Biodegradable stent composite material and preparation method thereof
ActiveCN102008751AGood mechanical propertiesPromote degradationSuture equipmentsSurgical adhesivesBiocompatibility TestingAlloy
The invention relates to a biodegradable stent composite material and a preparation method thereof. The degradable material has a multi-gradient composite structure, and comprises a matrix made of a medical metal or an alloy thereof, a chemical coupling layer attached to the surface of the matrix, a high polymer transition layer attached to the surface of the chemical coupling layer and a degradable high polymer functional layer fixed on the surface of the high polymer transition layer. The stent composite material has high mechanical property, degradation property and biocompatibility, also can absorb and carry needed therapeutic medicaments, and meets the requirement on clinical treatment. The degradable composite material can be used as stent materials of esophagi, biliary ducts, intestinal tracts, urethrae, tracheae and other non-vessel lumens and blood vessels, and also can be used for manufacturing artificial bones, bone nails, bone connectors, bone sutures, anchors for suture, intervertebral discs, hemostatic clamps, hemostatic forceps, hemostatic plates, hemostatic screws, tissue adhesives, sealants and other medical devices and products.
Owner:清北高科(北京)科技有限公司
Focusing ultrasonic therapeutical system
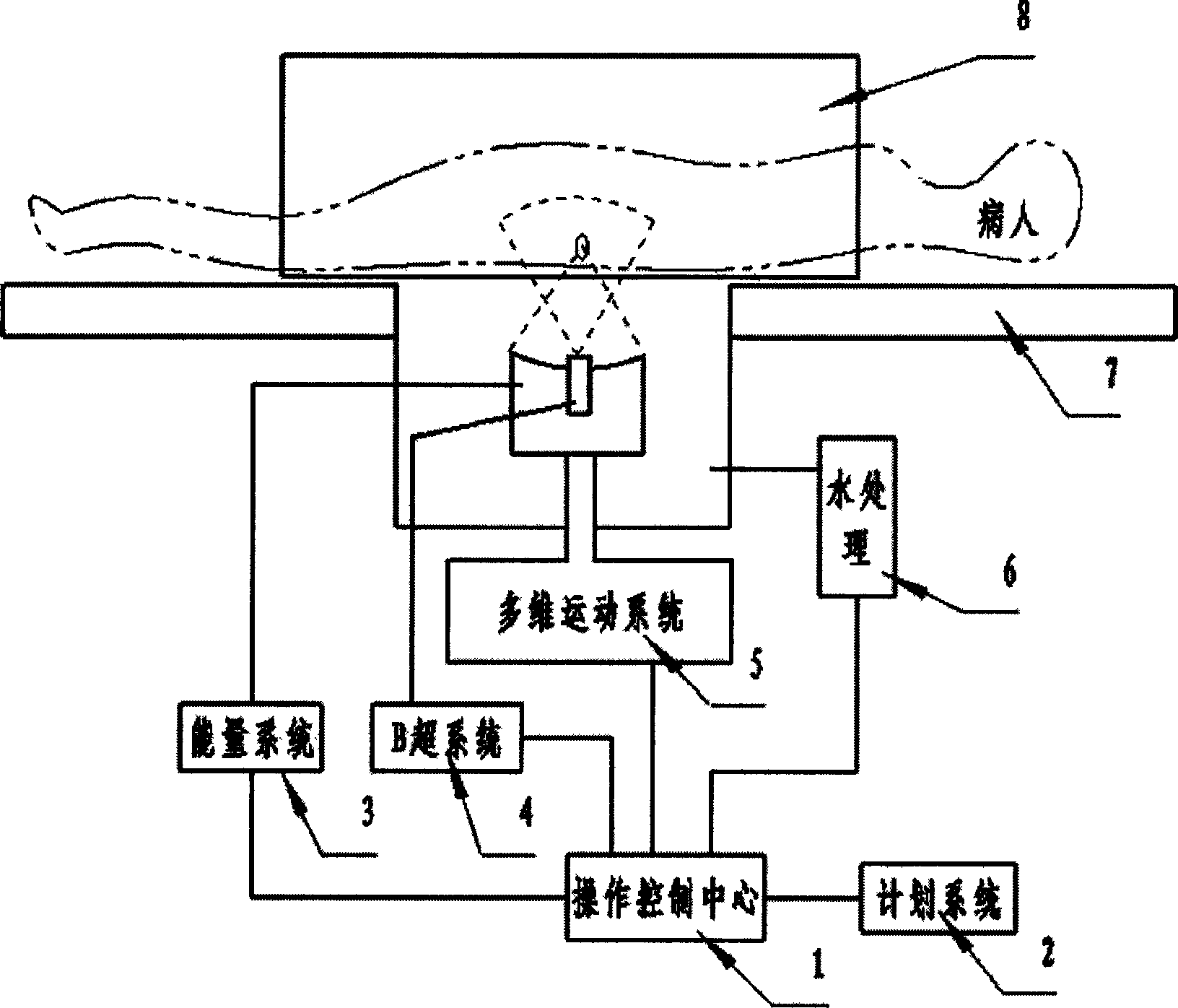
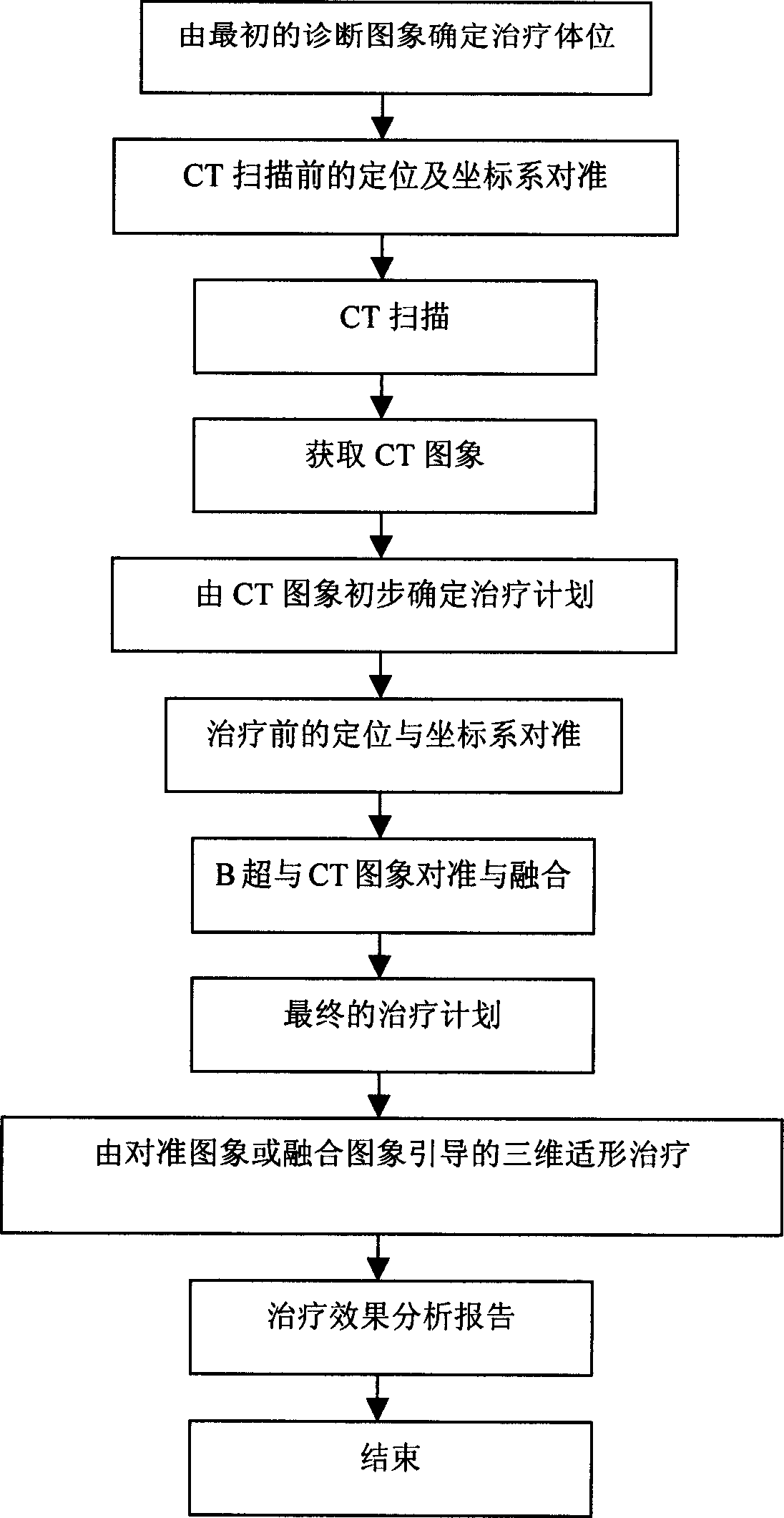
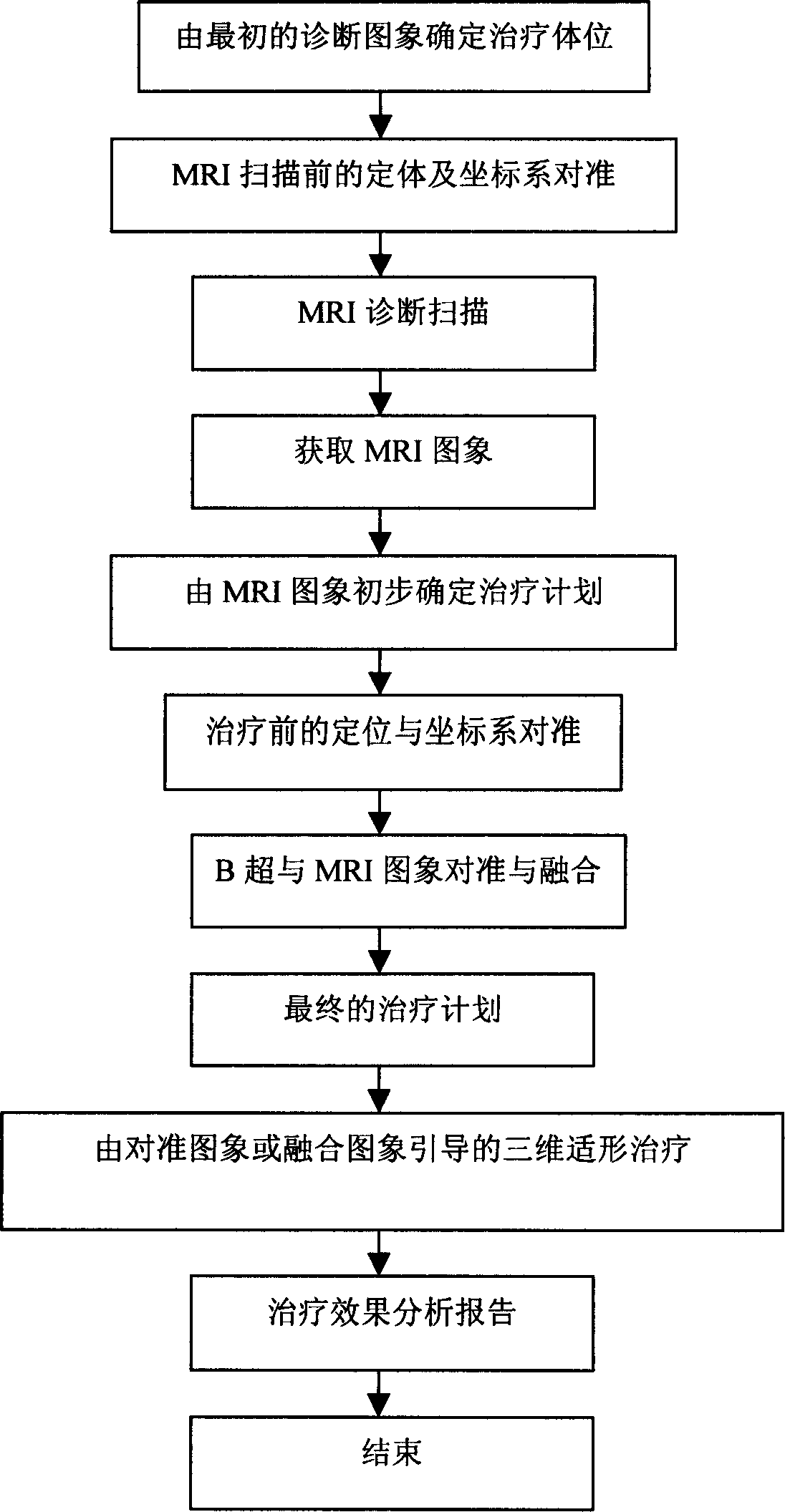
ActiveCN1814323ATreatment guidanceConvenient guidanceUltrasound therapyDiagnosticsHigh intensityBiomedical engineering
Owner:CHONGQING HAIFU (HIFU) TECHNOLOGY CO LTD
Targeted hollow gold nanostructures and methods of use
InactiveUS20090263485A1Easy to use detectionPowder deliverySynthetic resin layered productsSpherical shapedMolecular switch
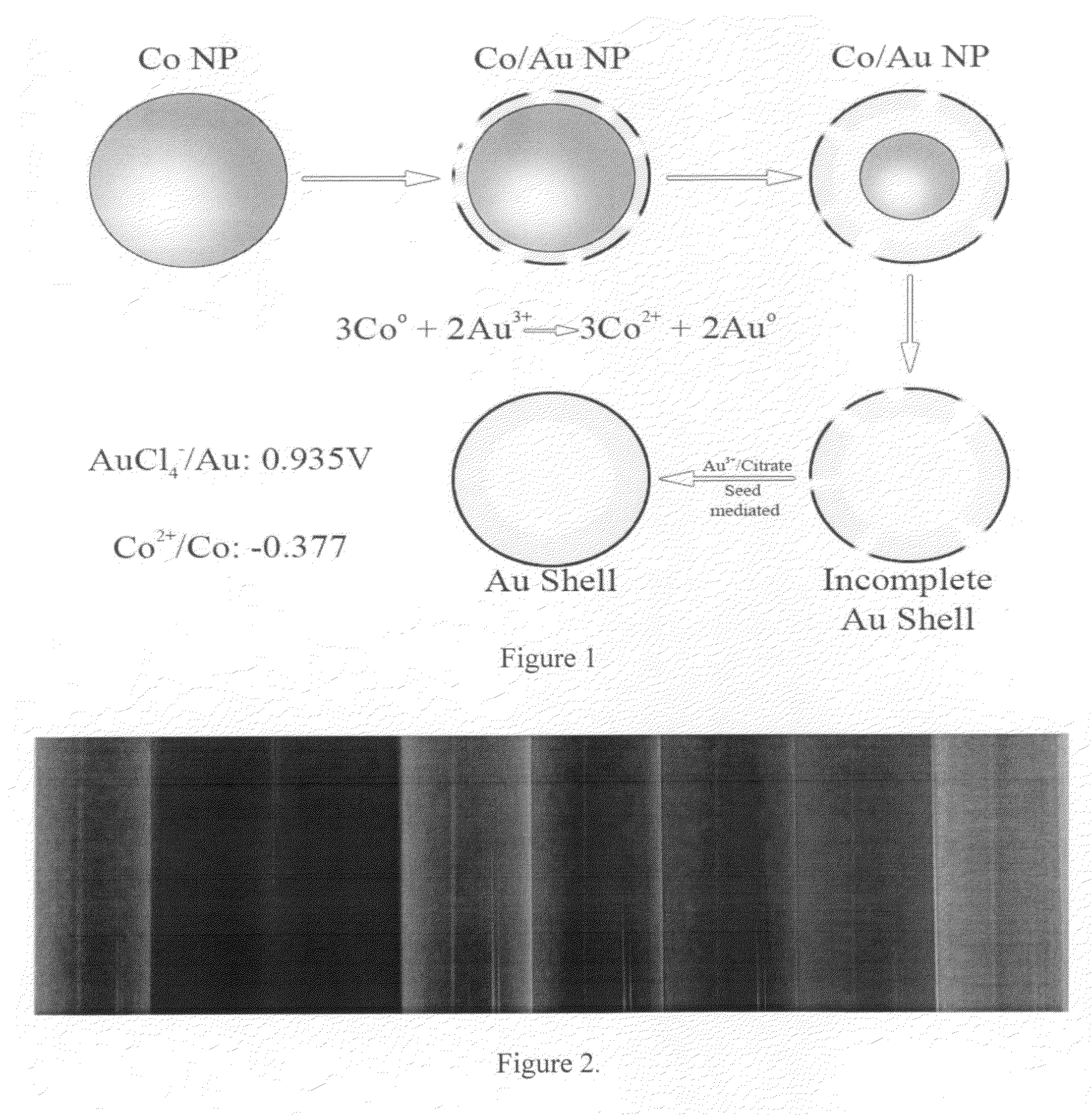
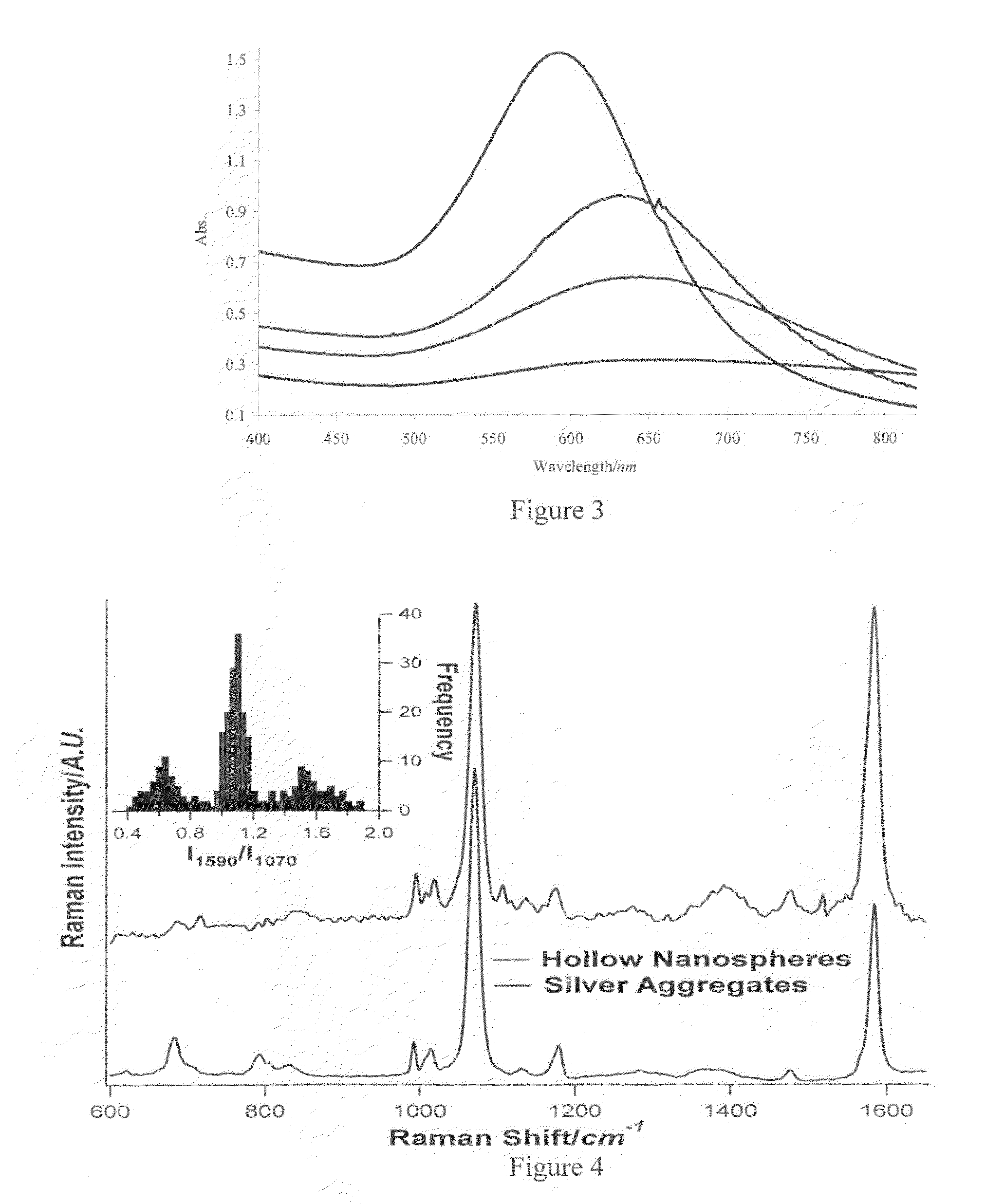
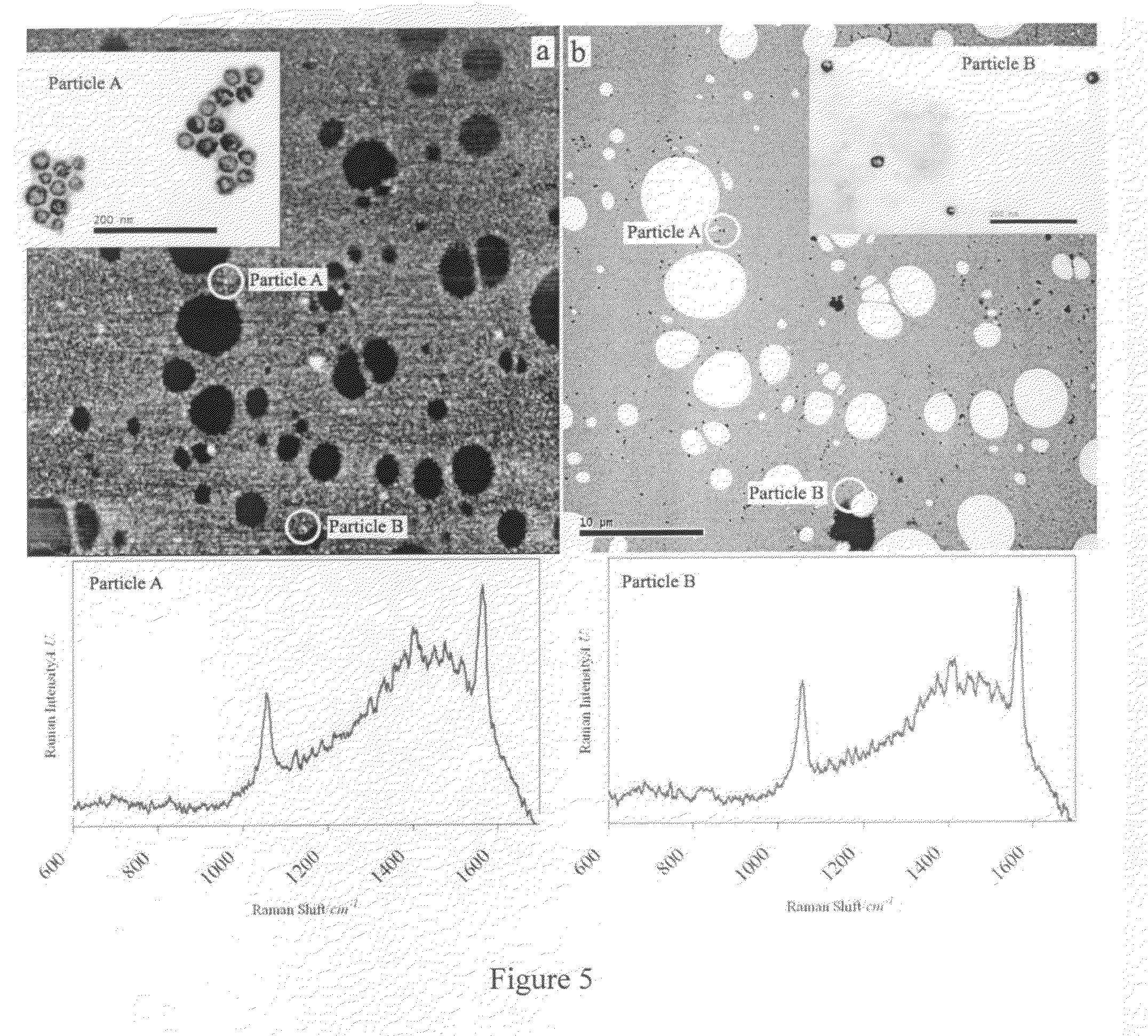
Provided are novel nanostructures comprising hollow nanospheres and nanotubes for use as chemical sensors and molecular specific photothermal coupling agents. The nanostructures can be used in laser-induced phototherapy for treatment of cancer and other disorders. The nanostructures can also be used as a sensor that detects molecules. The nanostructures are of particular use in the fields of clinical diagnosis, clinical therapy, clinical treatment, and clinical evaluation of various diseases and disorders, manufacture of compositions for use in the treatment of various diseases and disorders, for use in molecular biology, structural biology, cell biology, molecular switches, molecular circuits, and molecular computational devices, and the manufacture thereof. The hollow gold nanospheres have a unique combination of spherical shape, small size, and strong, tunable, and narrow surface plasmon resonance absorption covering the entire visible to near IR region.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Applications of exosome from mesenchymal stem cells
ActiveCN105267240AReduce retinal damageReduce inflammationSenses disorderUnknown materialsDiseaseUveitis
The invention relates to applications of exosome from mesenchymal stem cells. The concrete applications are applications of exosome from mesenchymal stem cells in preparation of medicines treating ophthalmic diseases. The ophthalmic diseases comprise uveitis, retinal detachment, retinal degeneration, retinal damage caused by various reasons and inflammation. Experiments prove that exosome has nerve nourishing and immunosuppression functions, can relief retina damage caused by various reasons and can reduce inflammatory reactions, therefore retina functions are improved, and a new approach is provided for clinic treatment of ophthalmic diseases.
Owner:天津医科大学眼科医院
Selective androgen receptor modulators
The present invention relates to androgen receptor targeting agents (ARTA) which demonstrate androgenic and anabolic activity, which are nonsteroidal ligands for the androgen receptor. The selective androgen receptor modulators (SARM) are useful for a) male contraception; b) treatment of a variety of hormone-related conditions, for example conditions associated with Androgen Decline in Aging Male (ADAM), such as fatigue, depression, decreased libido, sexual dysfunction, erectile dysfunction, hypogonadism, osteoporosis, hair loss, anemia, obesity, sarcopenia, osteopenia, osteoporosis, benign prostate hyperplasia, alterations in mood and cognition and prostate cancer; c) treatment of conditions associated with Androgen Decline in Female (ADIF), such as sexual dysfunction, decreased sexual libido, hypogonadism, sarcopenia, osteopenia, osteoporosis, alterations in cognition and mood, depression, anemia, hair loss, obesity, endometriosis, breast cancer, uterine cancer and ovarian cancer; d) treatment and / or prevention of chronic muscular wasting; e) decreasing the incidence of, halting or causing a regression of prostate cancer; f) oral androgen replacement and / or other clinical therapeutic and / or diagnostic areas.
Owner:UNIV OF TENNESSEE RES FOUND
Method and apparatus for performing qualitative and quantitative analysis of burn extent and severity using spatially structured illumination
Frequent monitoring of early-stage burns is necessary for deciding optimal treatment and management. Superficial-partial thickness and deep-partial thickness burns, while visually similar, differ dramatically in terms of clinical treatment and are known to progress in severity over time. The disclosed method uses spatial frequency domain imaging (SFDI) far noninvasively mapping quantitative changes in chromophore and optical properties that may be an indicative of burn wound severity. A controlled protocol of graded burn severity is developed and applied to 17 rats. SFDI data is acquired at multiple near-infrared wavelengths over a course of 3 h. Burn severity is verified using hematoxylin and eosin histology. Changes in water concentration (edema), deoxygenated hemoglobin concentration, and optical scattering (tissue denaturation) are statistically significant measures, which are used to differentiate superficial partial-thickness burns from deep-partial thickness burns.
Owner:RGT UNIV OF CALIFORNIA
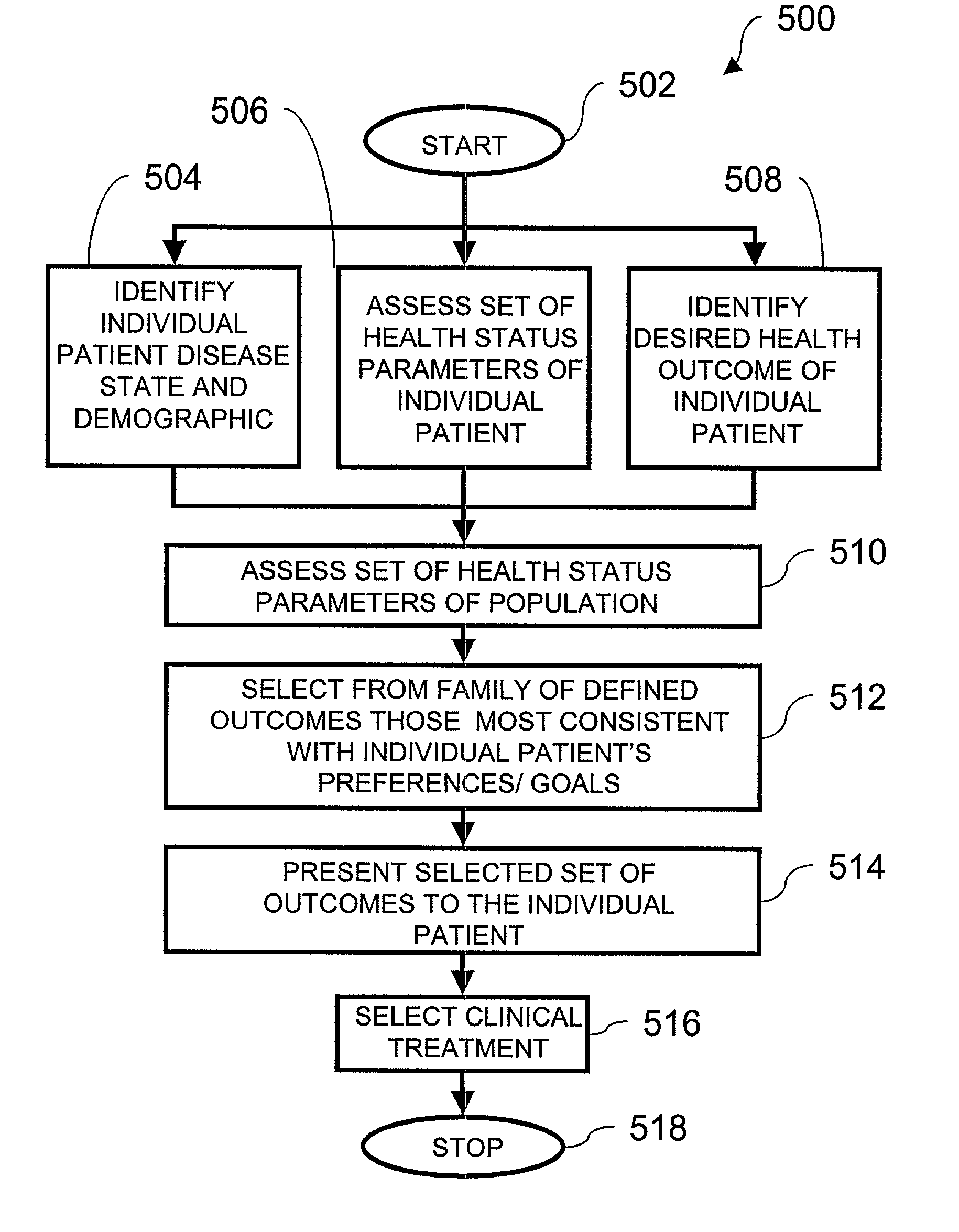
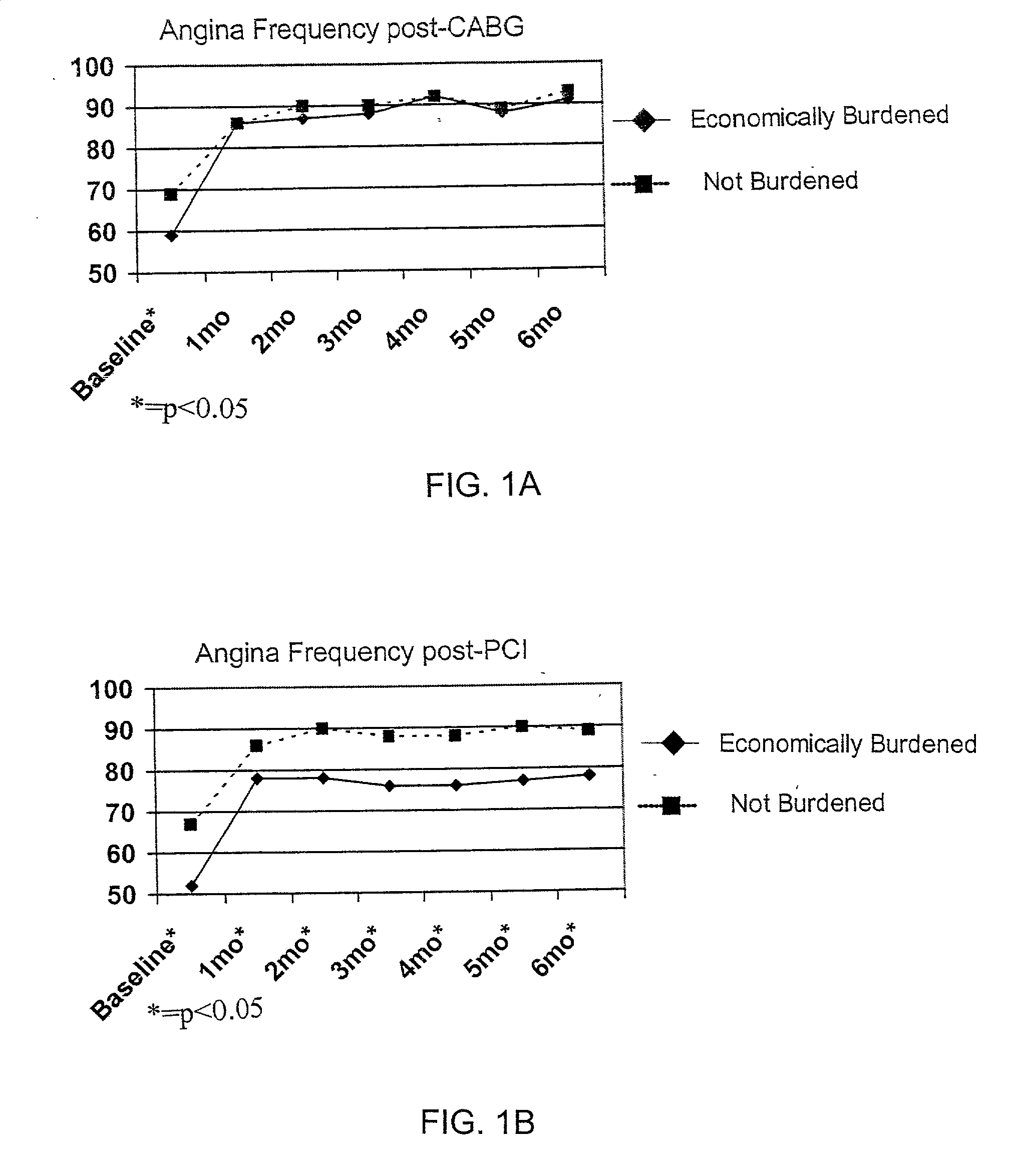
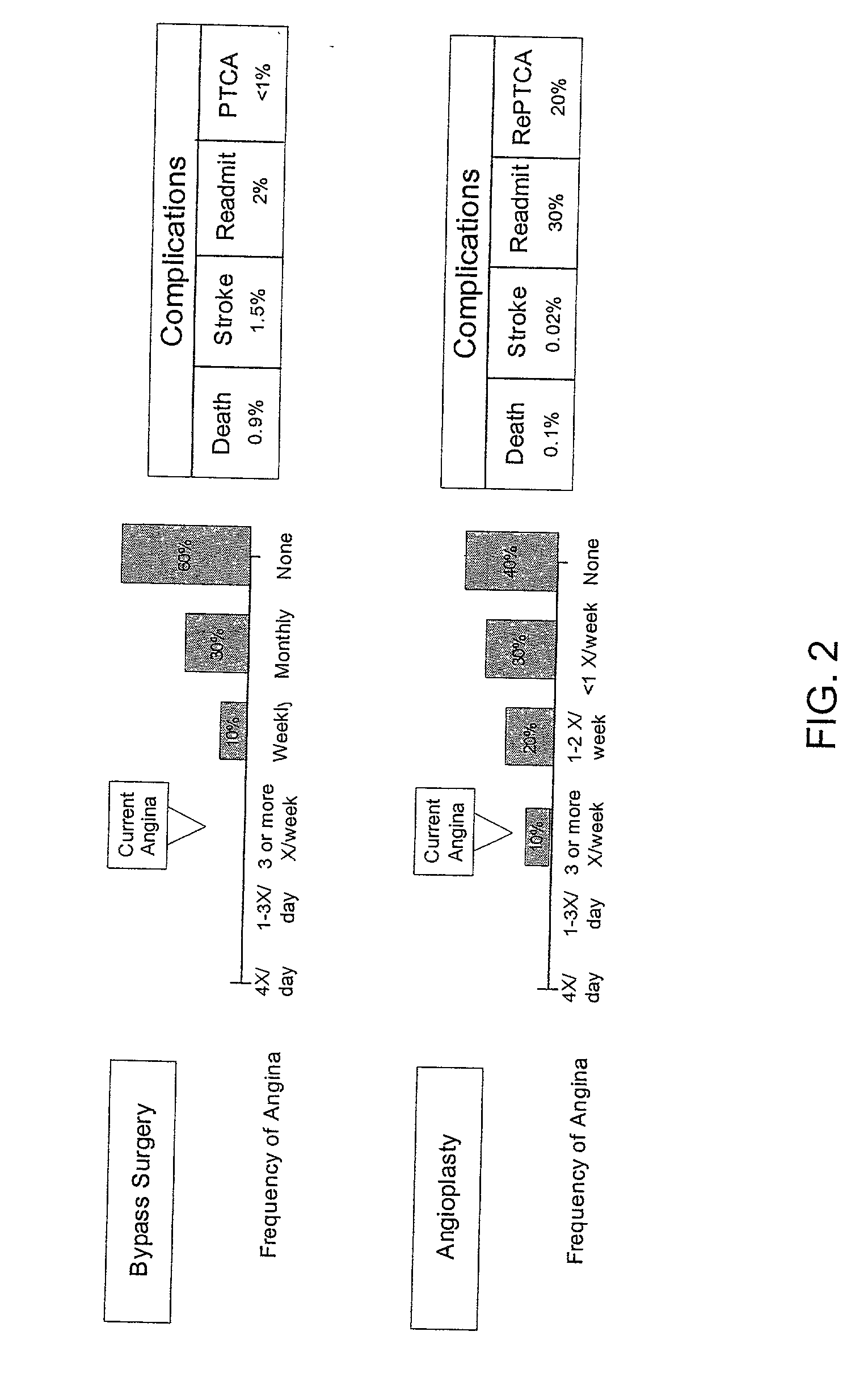
Method for selecting a clinical treatment plan tailored to patient defined health goals
ActiveUS20030229513A1TherapiesHealth-index calculationHer DiseaseCoronary Revascularization Procedure
The invention discloses a method by which the health care professional or patient may draw upon historical medical data concerning patients similarly situated in medical condition, to assist him / her in deciding on a clinical intervention procedure to select. This method is specifically tailored to the patient, as data is provided and evaluated from only similarly situated patients, and provides an expectation of potential outcome of the patient should one or the other of the options be selected. The invention further provides a database that may be used in order to provide this comparison based evaluation method. A computer based software system is further disclosed that implements the method. The invention more speiocifically provides a method by which a post-coronary event patient may make an informed decision of which post-coronary revascularization procedure to undergo in the future management of his disease. This method employs the patient's health status date (symptoms, function and quality of life), and provides projections of the patient's expected survival, risk, and 1-year health status outcome from the selection of revascularization procedure, such as Coronary Artery Bypass Grafting (CABG) or Percutaneous Coronary Intervention (PCI).
Owner:TERUMO MEDICAL CORP
Reversible heat gelling aquatic pharmaceutical composition of a Chinese medicine and compound prescription thereof
Disclosed is a reversible heat gelling aquatic pharmaceutical composition of a Chinese medicine and compound prescription, wherein the composition comprises traditional Chinese medicines and their compound recipe with various actions, methyl hydroxypropylcellulose of certain density, multiple valency salts of acid and pH regulator. The composition can realize gelatinization within a short period of time.
Owner:凌沛学
Traditional Chinese medicine composition for treating cerebral thrombosis and cerebral infarction and preparation method thereof
InactiveCN101757569AImprove self-coordinationEffective treatmentHeavy metal active ingredientsAnthropod material medical ingredientsMedicinal herbsTherapeutic effect
The invention discloses a new traditional Chinese medicine composition for treating cerebral thrombosis and cerebral infarction and a preparation method thereof. The traditional Chinese medicine composition mainly comprises the following Chinese medicinal herbs such as dutchmanspipe root, fruit of immature citron, dried orange peel, pangolin scales, ground beeltle, ligusticum wallichii, cassia twig, angelica sinensis, radix paeoniae alba, root of red-rooted salvia, agilawood, rhizoma cyperi, rhizoma corydalis, radix curcumae, pseudo-ginseng, common burreed rhizome, curcuma zedoary, red flower, peach kernel, rhizoma gastrodiae, uncaria rhynchophylla, white muscardine silkworm, scorpio, styrax asiatic styrax and rhizoma acori graminei. The traditional Chinese medicine composition can be prepared into any one common oral preparation according to a conventional traditional Chinese medicine preparation method. The invention can remarkably improve the symptoms of aphasia from apoplexy, facial paralysis, numb hands and feet, blindness, dizziness and faint, hypertension, blood viscosity increase, insufficiency of cerebral blood supply and the like, and has accurate clinical treatment effect, remarkable treatment effect and rapid effect. The traditional Chinese medicine composition has the advantages of low cost, basically no toxic or side effects and the like.
Owner:TAIYI HEPU BEIJING RES INST OF TCM
Chinese medicinal composition for treating antiaditis and preparation method thereof
InactiveCN101816757AImprove self-coordinationFunction increaseUnknown materialsRespiratory disorderPatriniaSide effect
The invention discloses a novel Chinese medicinal composition for treating antiaditis and a preparation method thereof. The Chinese medicinal composition mainly comprises the following raw material medicaments: blackberrykiky rhizome, immature bitter orange, Chinese eaglewood, szechuan lovage rhizome, tangerine peel, costustoot, radish seed, szechwan chinaberry fruit, longstamen onion bulb, honey suckle flower, philippine violet herb, mongolian dandelion herb, weeping forsythiae capsule, figwort root, fresh rehmannia, lightyellow sophora root, baical skullcap root, chrysanthemum, bupleurum, kudzuvine root, mint, great burdock achene, gypsum, anemarrhena asphodeloides bunge, common lophatherum herb, mongolian snakegourd root, gentian, heartleaf houttuynia herb, dahurian patrinia herb and the like. The Chinese medicinal composition can be prepared into any one common orally-taken preparation by a conventional Chinese medicinal preparation method. The Chinese medicinal composition can remarkably improve the symptoms: antiaditis, antiadoncus, inflamed and purulent pharynx, ardor and pain, bitter mouth and dry pharynx, dry congh, cough and the like, and has extract clinical treatment effect, remarkable treatment effect and quick response. The Chinese medicinal composition also has the advantages of low cost, basically no toxic or side effect and the like.
Owner:TAIYI HEPU BEIJING RES INST OF TCM
Anti-BCMA chimeric antigen receptor, encoding gene, recombinant expression vector and establishing method and application of anti-BCMA chimeric antigen receptor, encoding gene and recombinant expression vector
ActiveCN105777911AImprove in vitro killing effectGood clinical effectPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsSequence signalSingle-Chain Antibodies
The invention discloses an anti-BCMA chimeric antigen receptor, an encoding gene, a recombinant expression vector and an establishing method and application of the anti-BCMA chimeric antigen receptor, the encoding gene and the recombinant expression vector. The receptor comprises a CD8 leader chimeric receptor signal peptide, a BCMA single-chain antibody heavy chain VH, an Optimal Linker C, a BCMA single-chain antibody light chain VL, a CD8 Hinge chimeric receptor hinge, a CD8 Transmembrane chimeric receptor transmembrane domain, a CD137 chimeric receptor co-stimulatory factor and a TCR chimeric receptor T cell activating domain which are sequentially connected in series. In addition, the invention further discloses the encoding gene and the recombinant expression vector of the anti-BCMA chimeric antigen receptor and the establishing method and application of the encoding gene and the recombinant expression vector. The secretion of cell factors and the cytotoxicity in vitro of CAR-T cells can be remarkably improved, and the clinical treatment effect is outstanding.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Method for controlling quality of ginkgo leaves and extract thereof
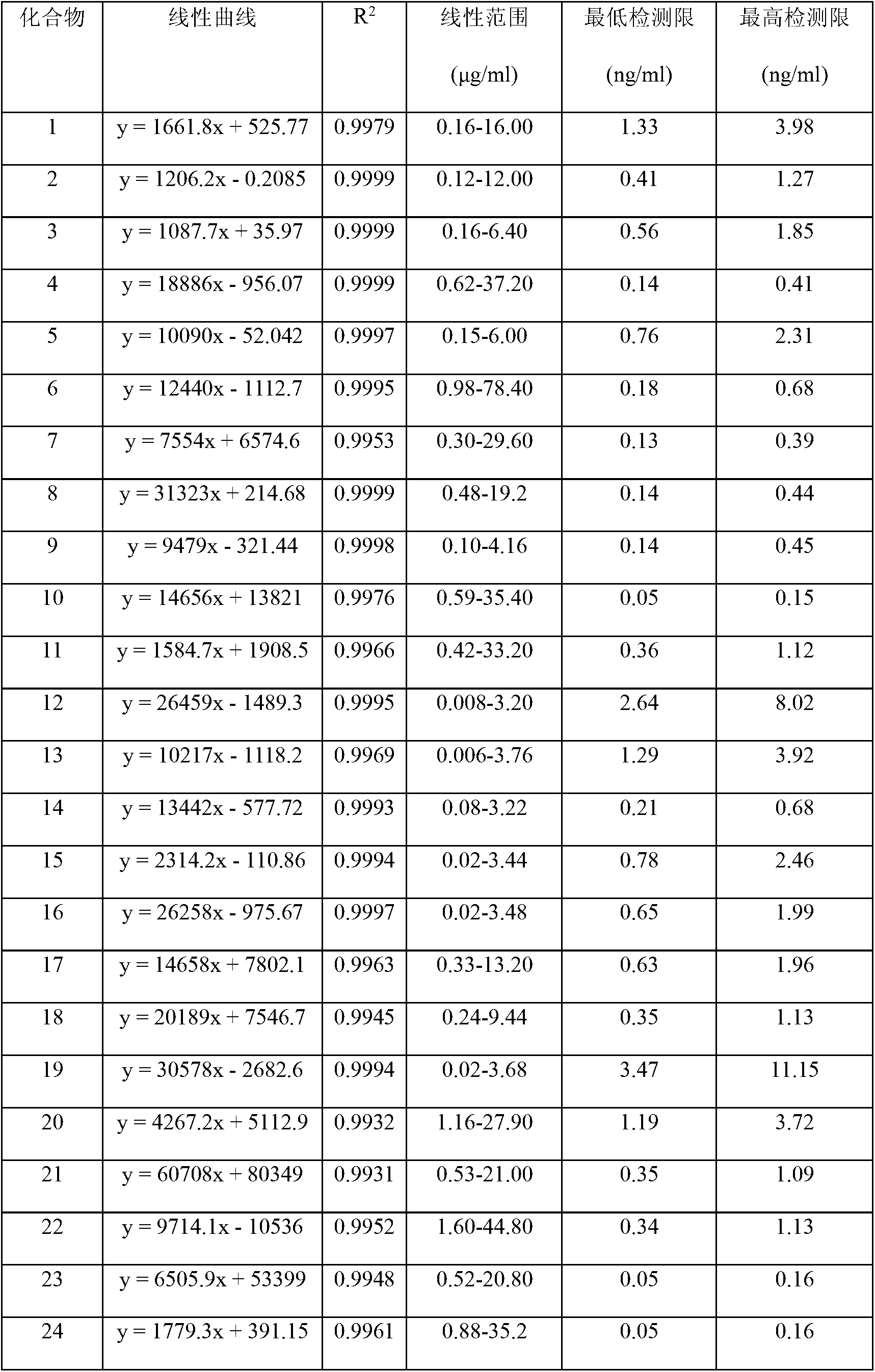
InactiveCN103175912AEasy to separateEasy CalibrationComponent separationCurative effectControl quality
The invention discloses a method for controlling the quality of ginkgo leaves and an extract thereof. After the screening of a great quantity of experiments, the method adopts ultra-high performance liquid chromatography for detection; and the method can be used for detecting three types of components including flavone, terpene lactones and phenolic acid, twenty-four active compounds in total, in the ginkgo leaves and the extract of the ginkgo leaves. The results of the experiments prove that the method is high in detection sensitivity and good in stability, and can be used for objectively, comprehensively and accurately evaluating the quality of ginkgo leaf medicines, the extract of the ginkgo leaves and ginkgo leaf preparations, thus having important significance on controlling the quality and guaranteeing the clinical treatment effect.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com