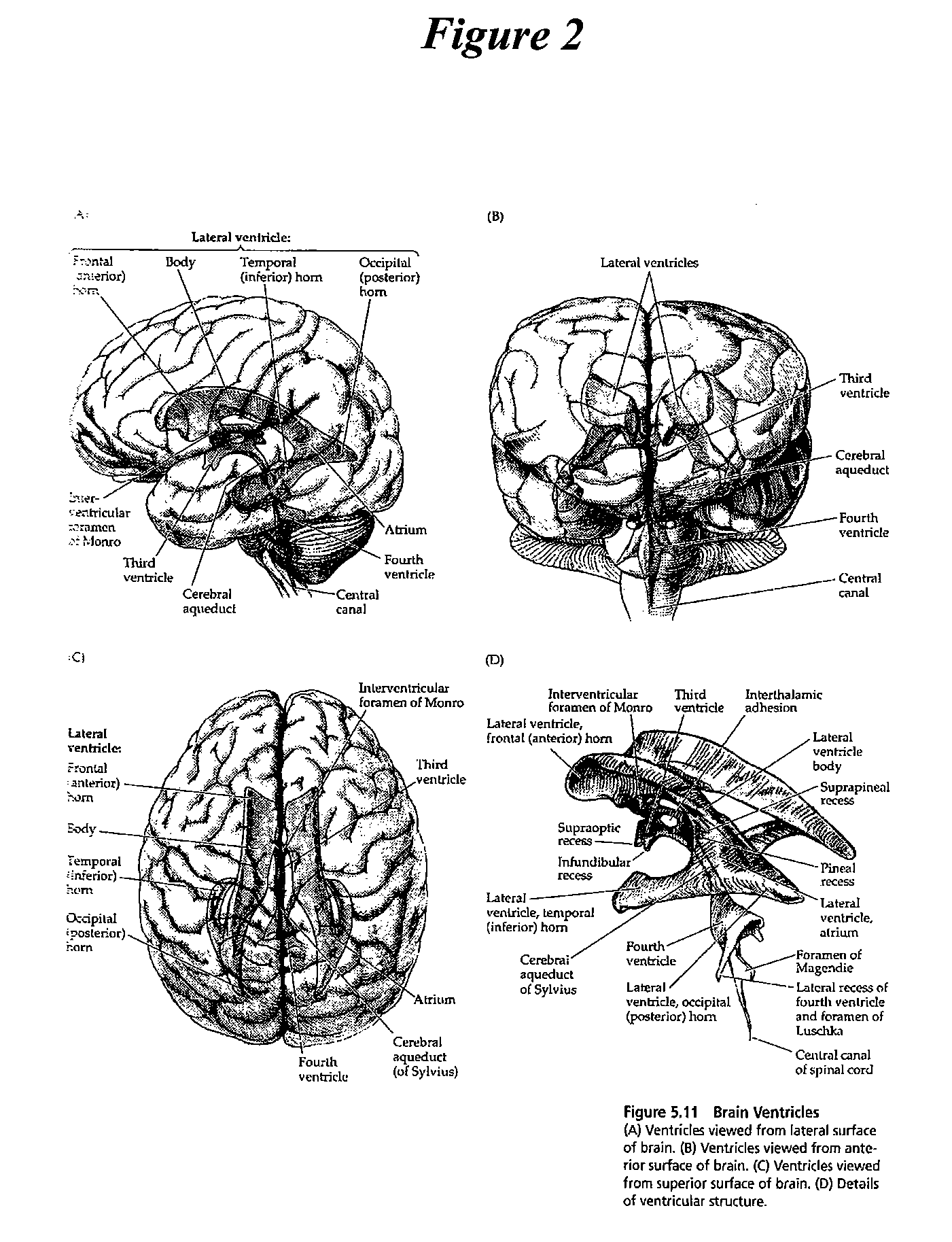
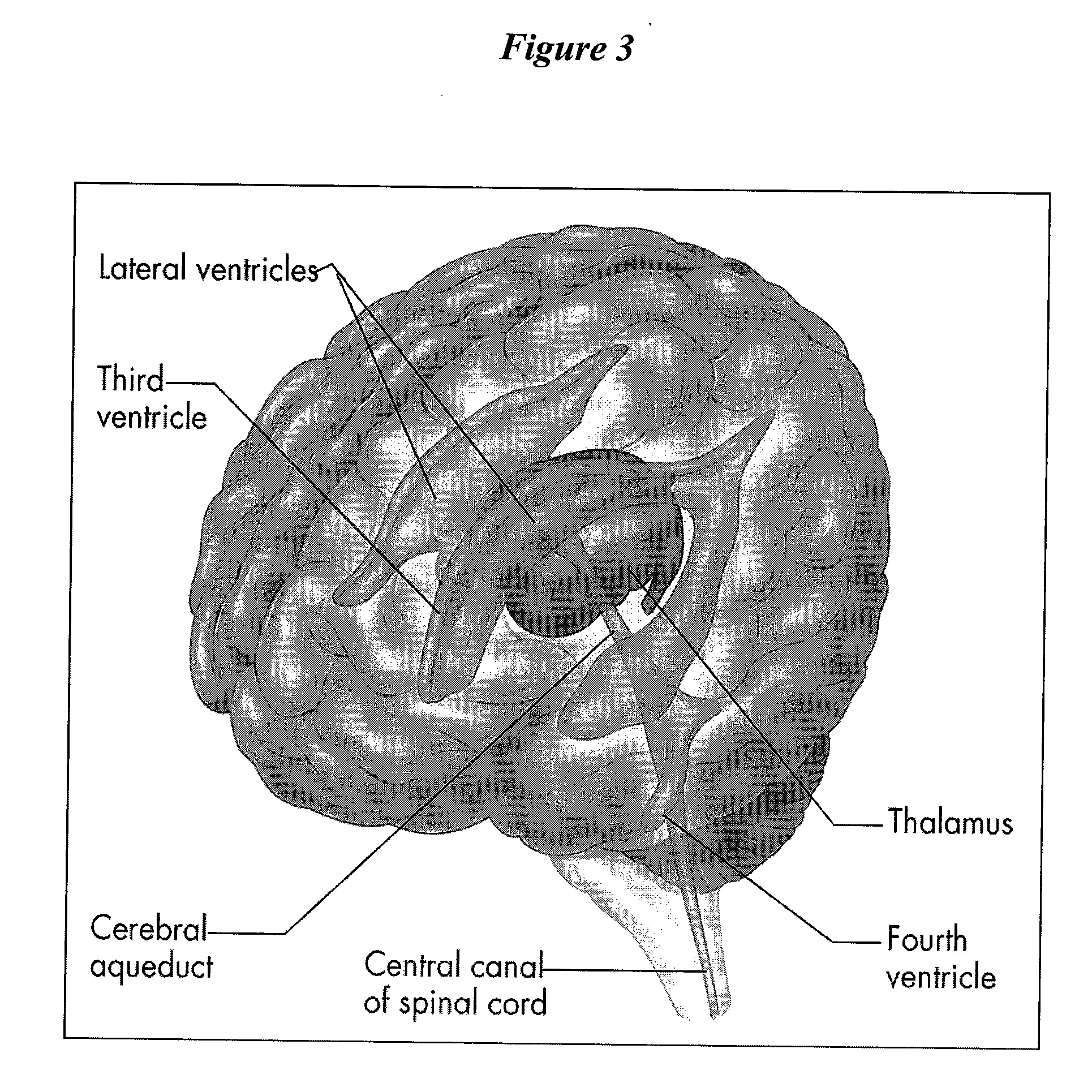
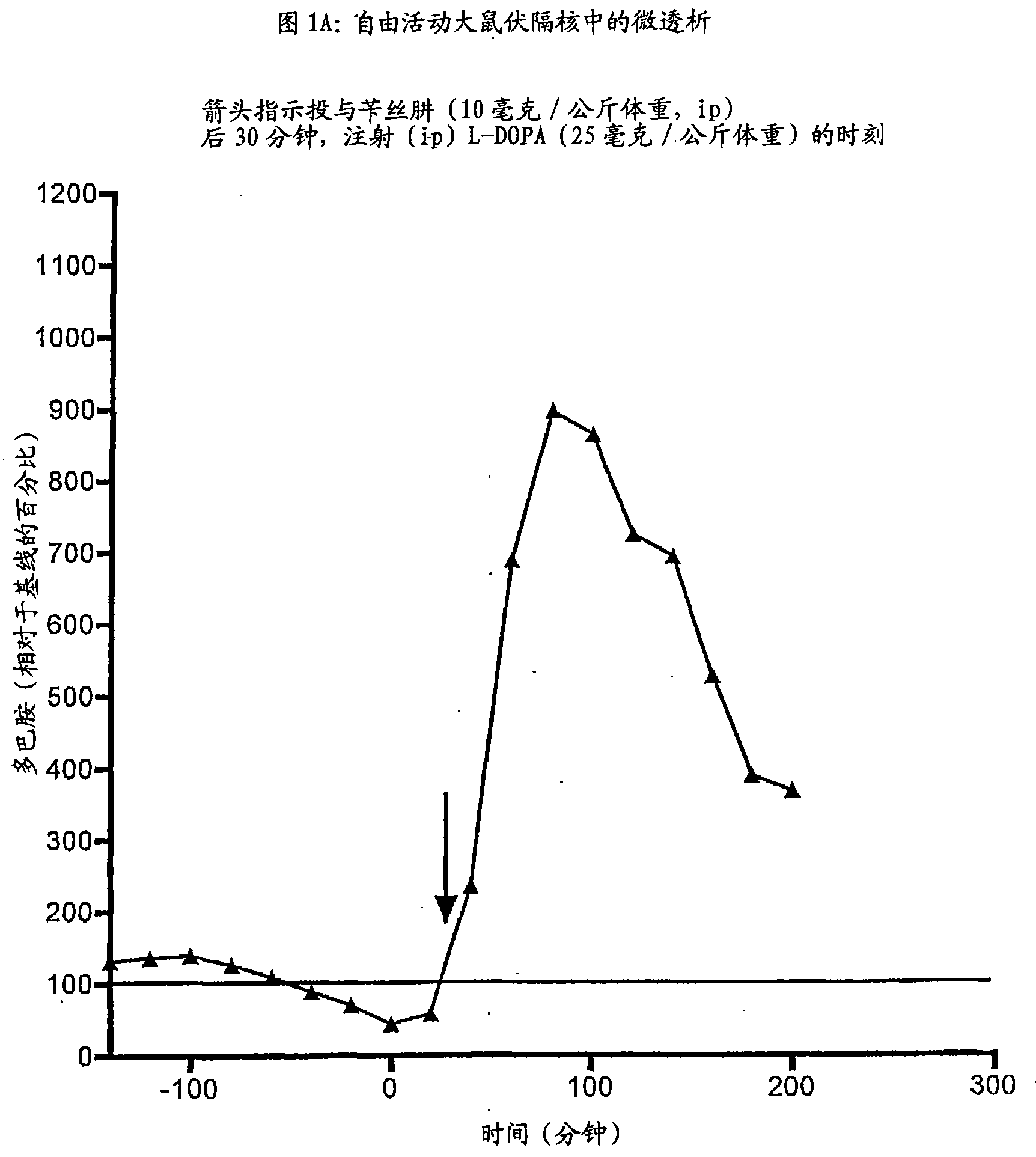
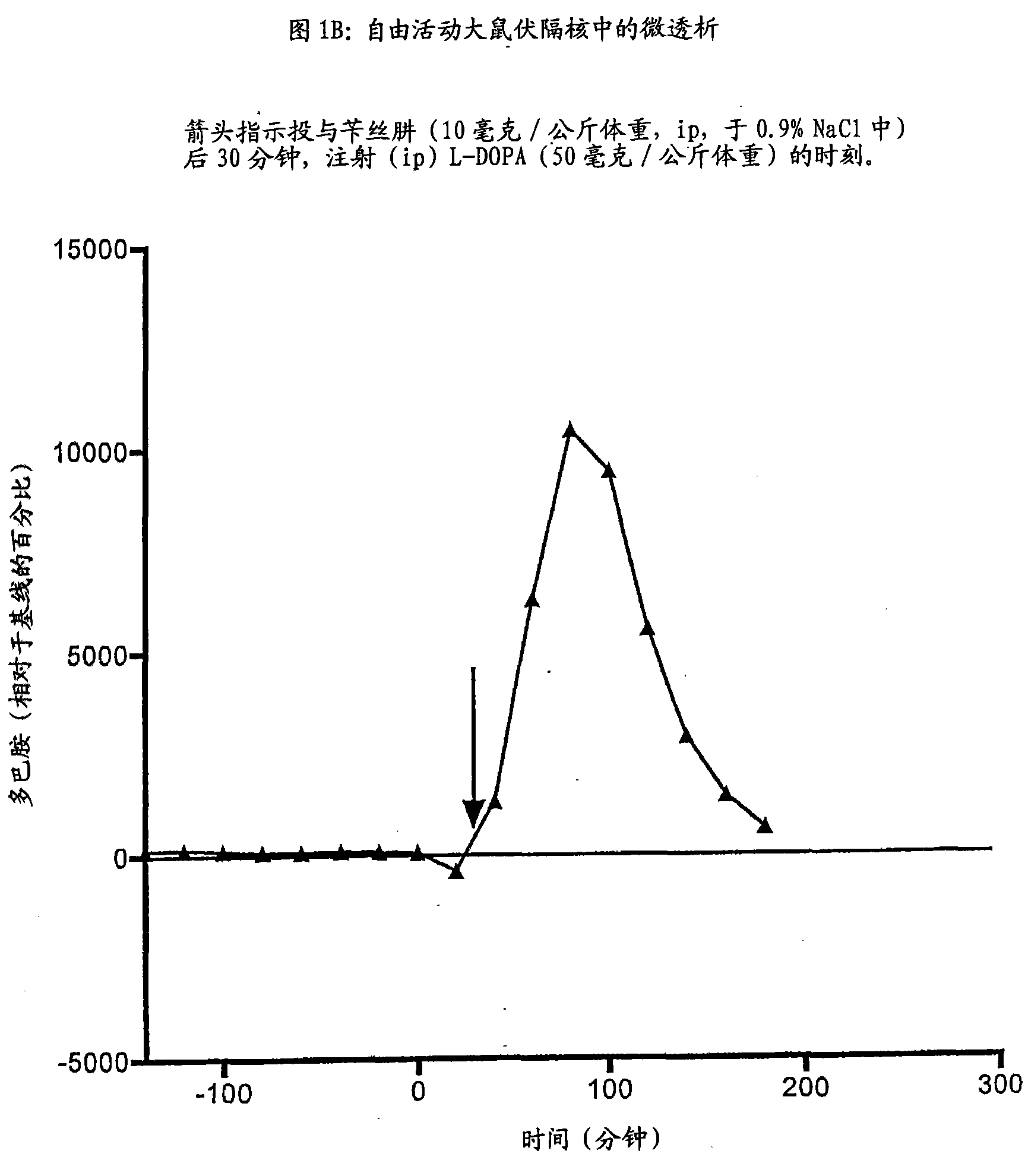
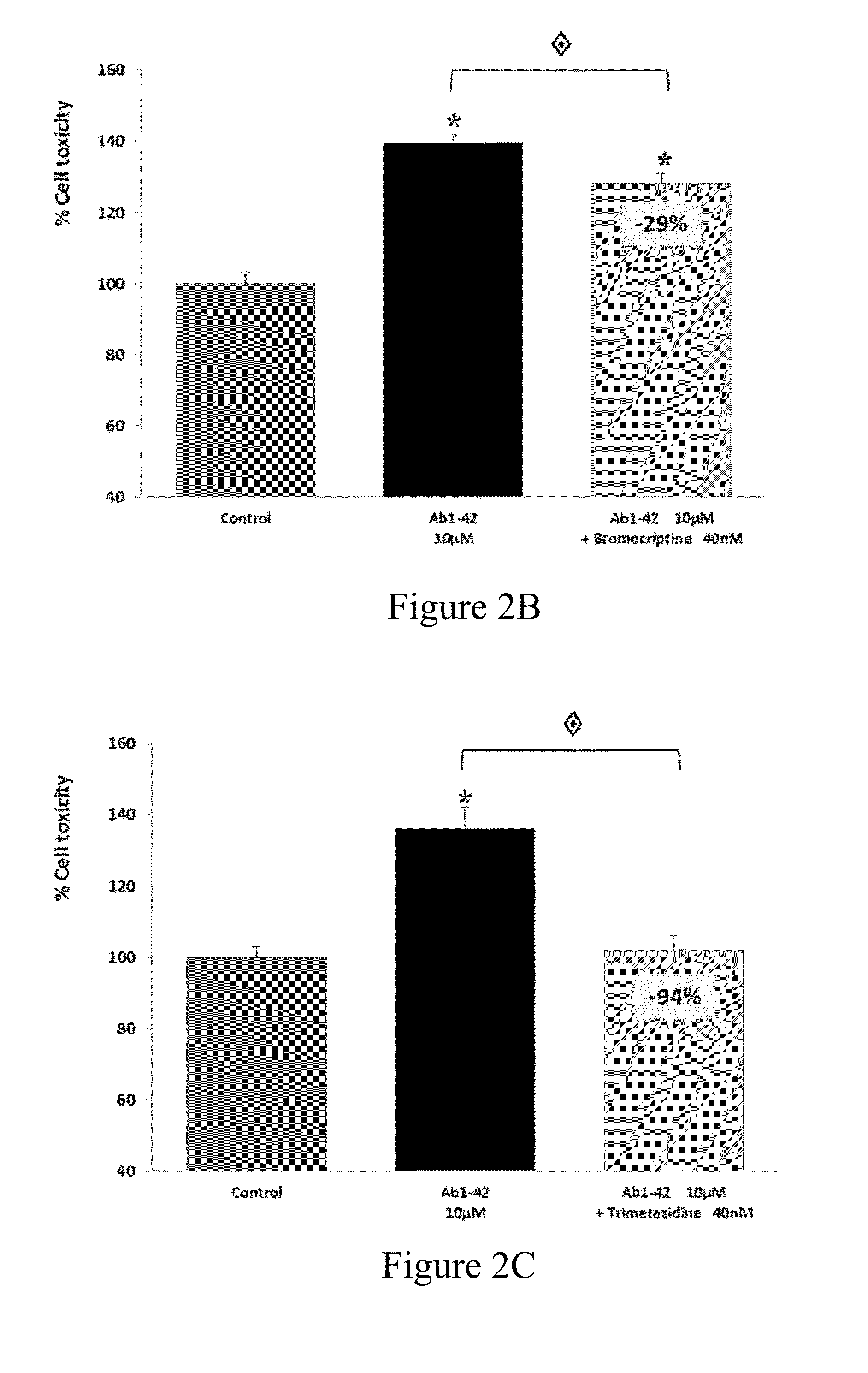
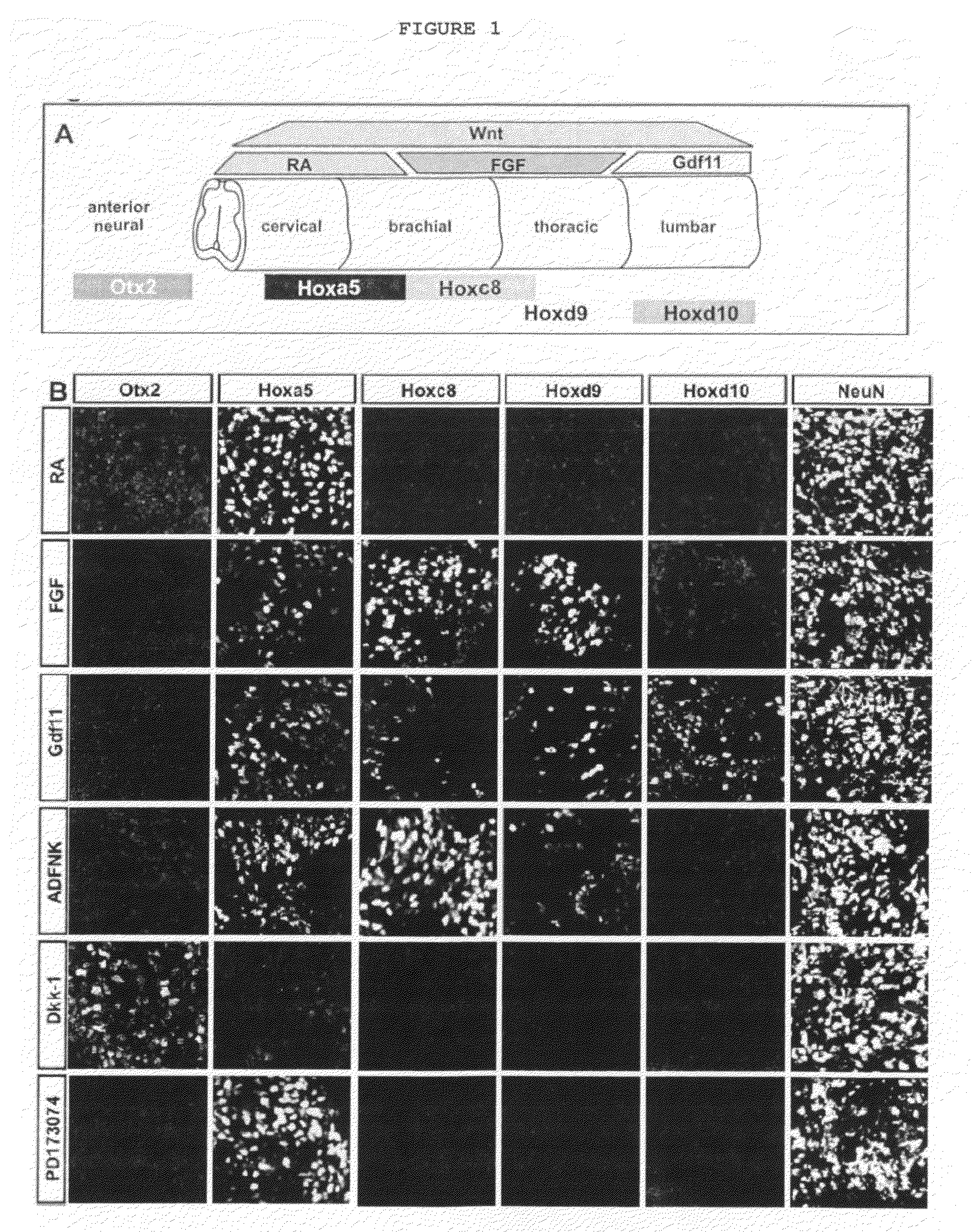
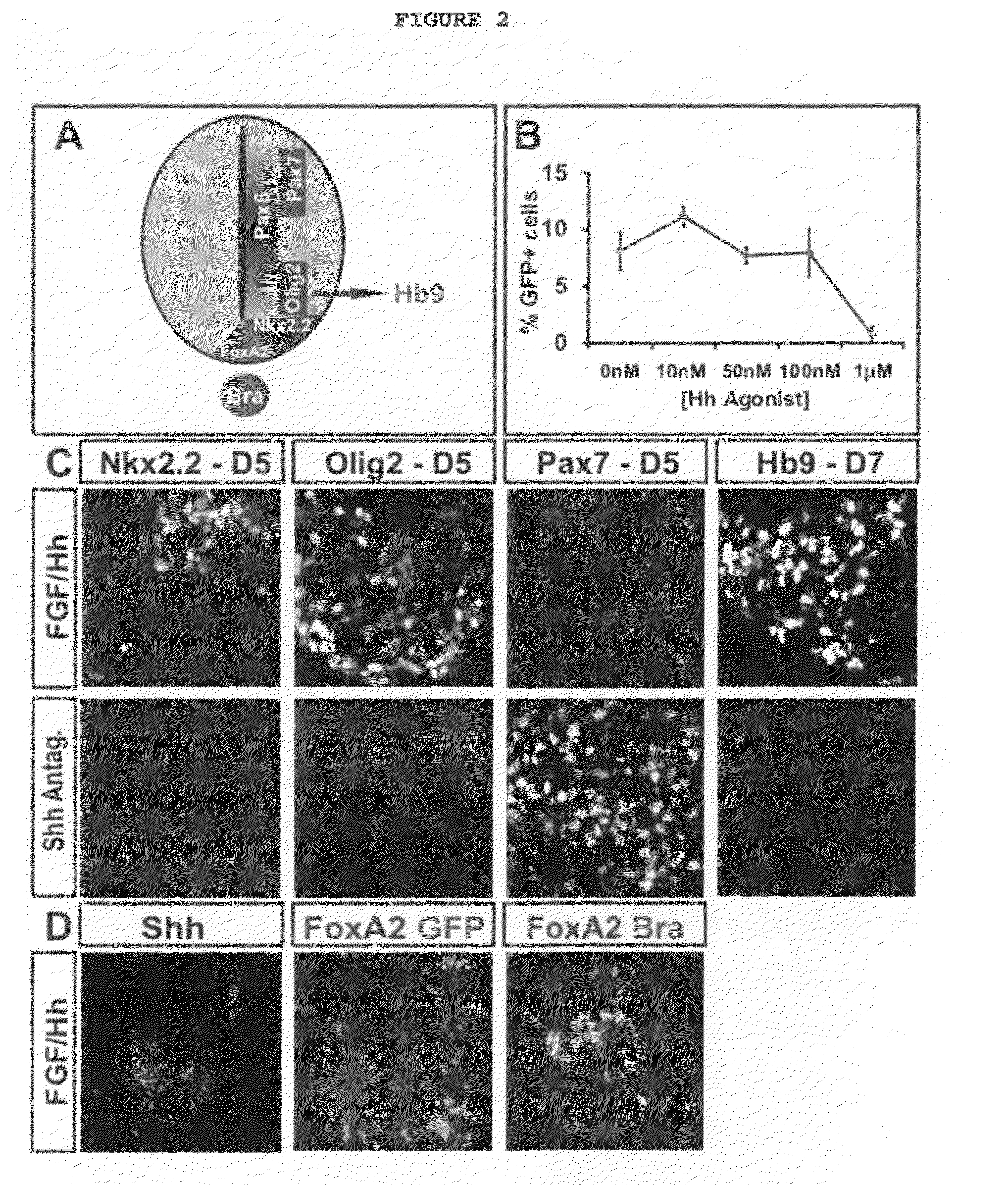
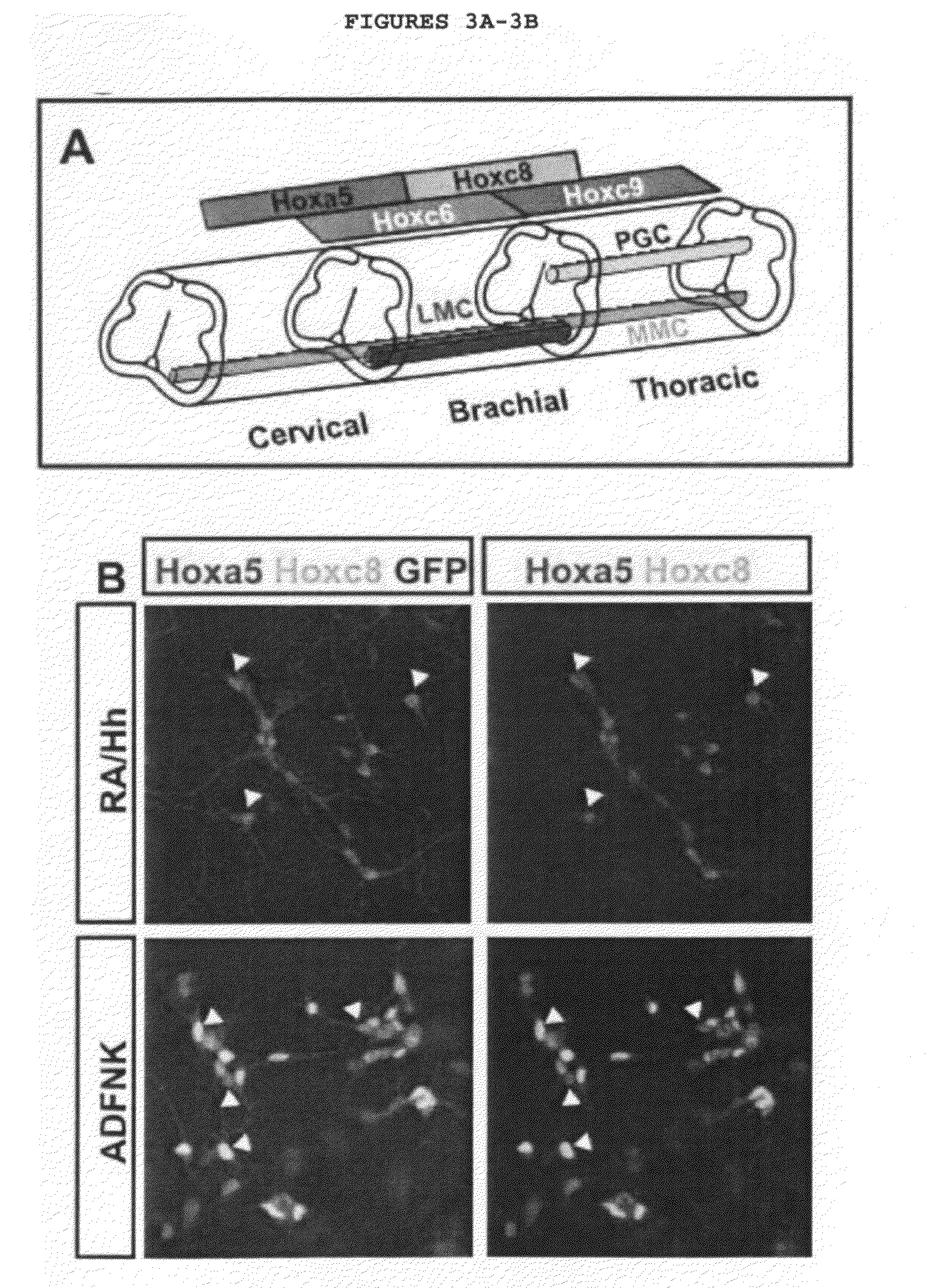
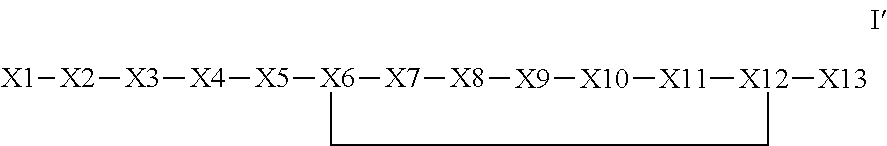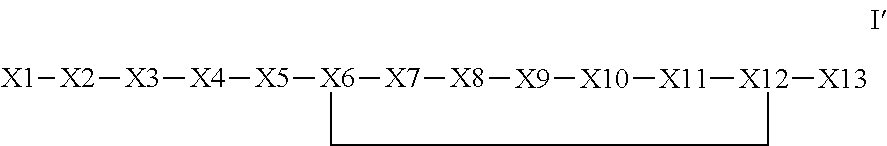Patents
Literature
775 results about "Amyotrophic lateral sclerosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A progressive neurological disorder which results in weakened muscles and deformity.
Methods and compositions using immunomodulatory compounds for the treatment and management of central nervous system disorders or diseases
InactiveUS20050143344A1Extension of timeBiocideNervous disorderMedicineAmyotrophic lateral sclerosis
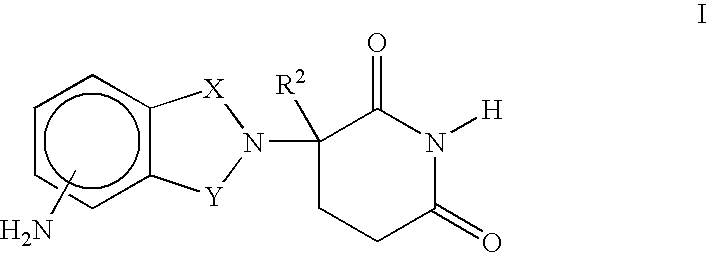
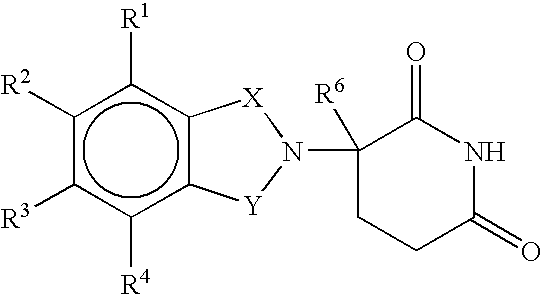
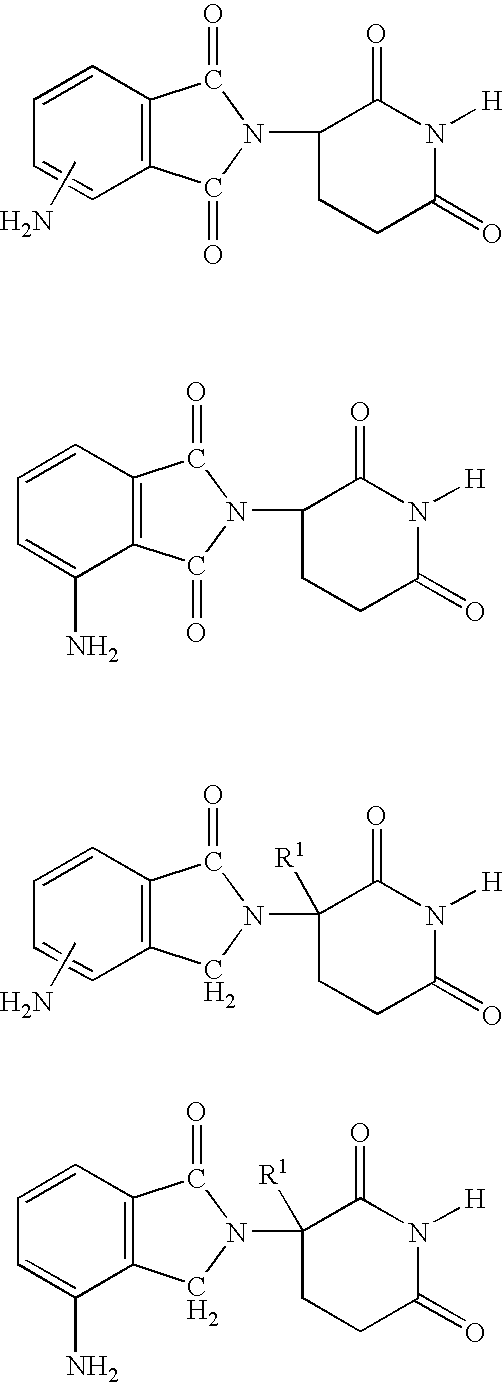
Methods of treating, preventing and / or managing central nervous system disorders, such as Amyotrophic Lateral Sclerosis (ALS or Lou Gehrig's Disease) and related syndromes are disclosed. Specific methods encompass the administration of an immunomodulatory compound of the invention, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active ingredient. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
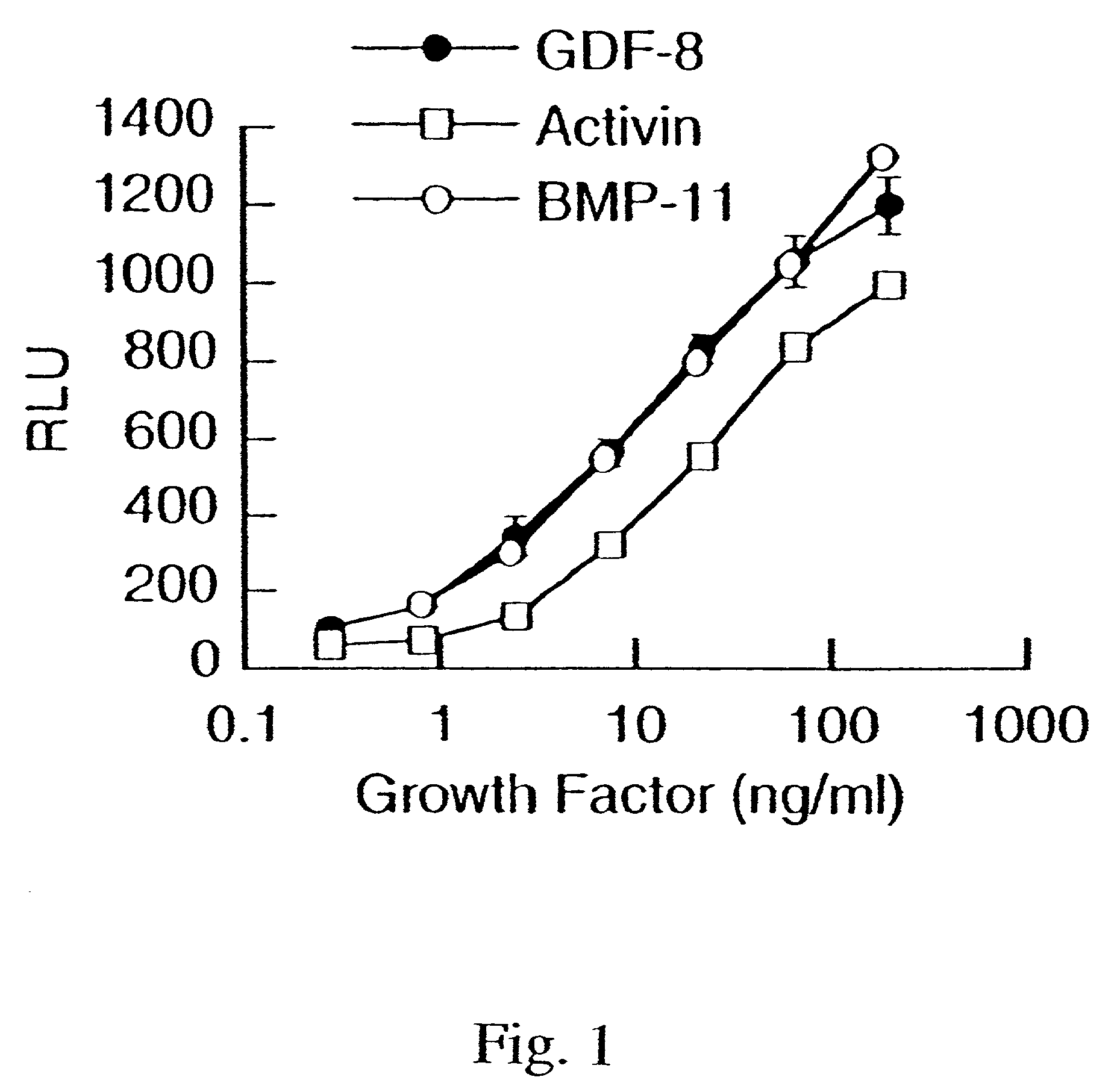
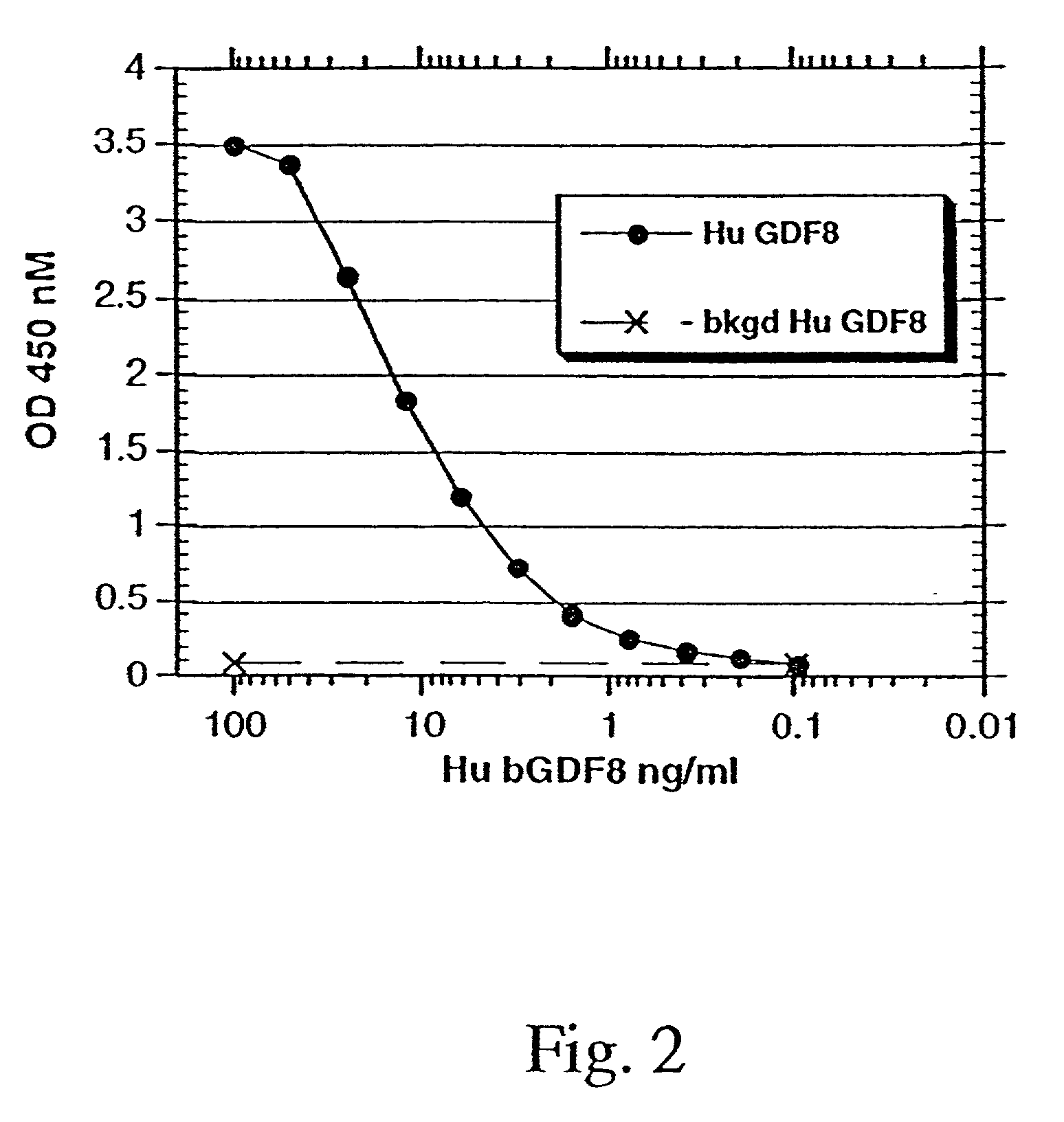
Modified and stabilized GDF propeptides and uses thereof
InactiveUS7202210B2Prevent practical therapeuticPrevent prophylactic utilityFungiBacteriaAcute hyperglycaemiaMuscle tissue
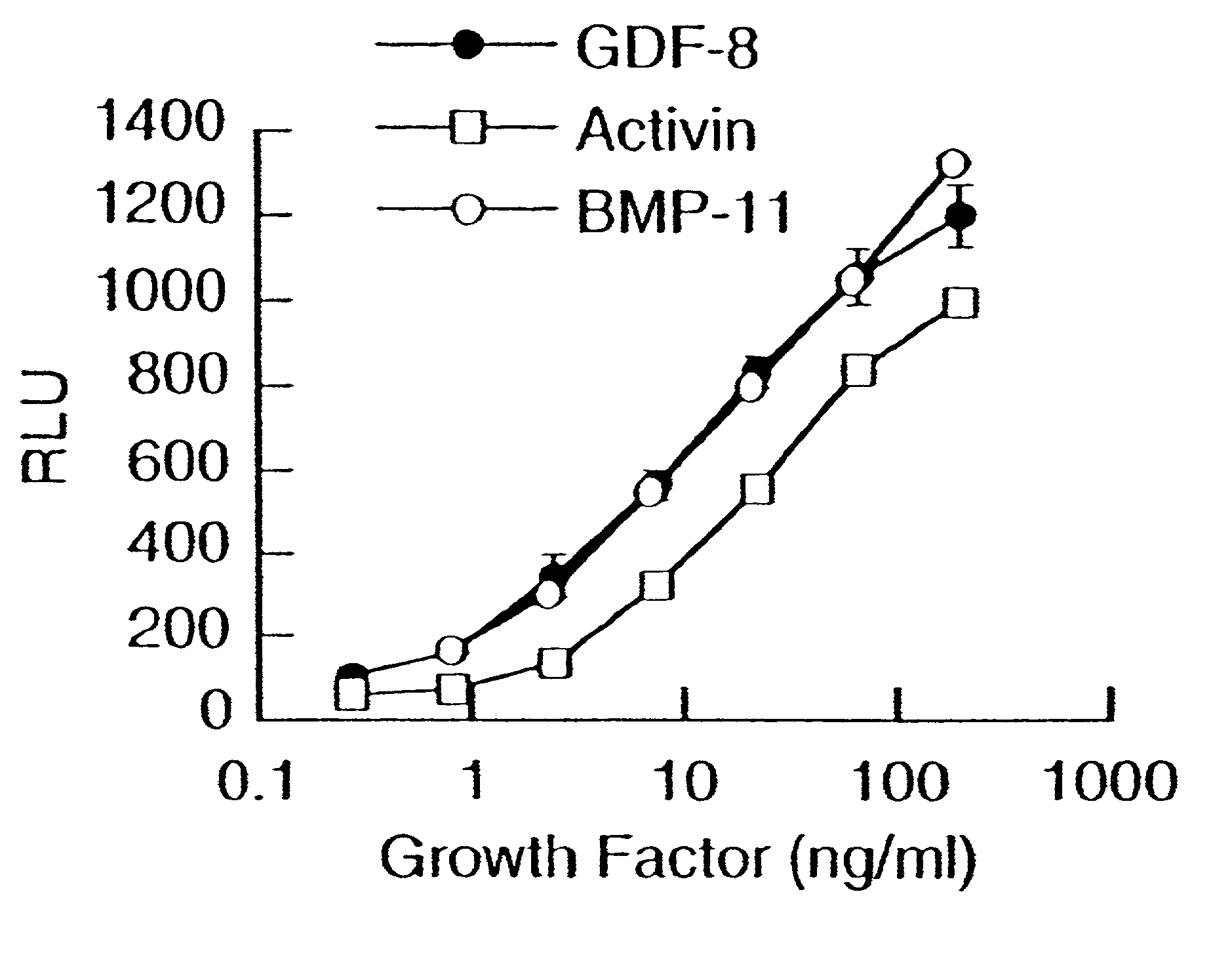
Modified and stabilized propeptides of Growth Differentiation Factor proteins, such as GDF-8 and Bone Morphogenetic Protein-11, are disclosed. Also disclosed are methods for making and using the modified propeptides to prevent or treat human or animal disorders in which an increase in muscle tissue would be therapeutically beneficial. Such disorders include muscle or neuromuscular disorders (such as amyotrophic lateral sclerosis, muscular dystrophy, muscle atrophy, congestive obstructive pulmonary disease, muscle wasting syndrome, sarcopenia, or cachexia), metabolic diseases or disorders (such as such as type 2 diabetes, noninsulin-dependent diabetes mellitus, hyperglycemia, or obesity), adipose tissue disorders (such as obesity), and bone degenerative diseases (such as osteoporosis).
Owner:WYETH LLC
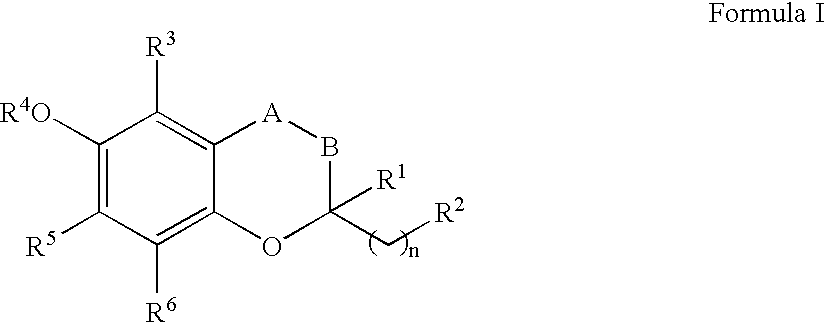
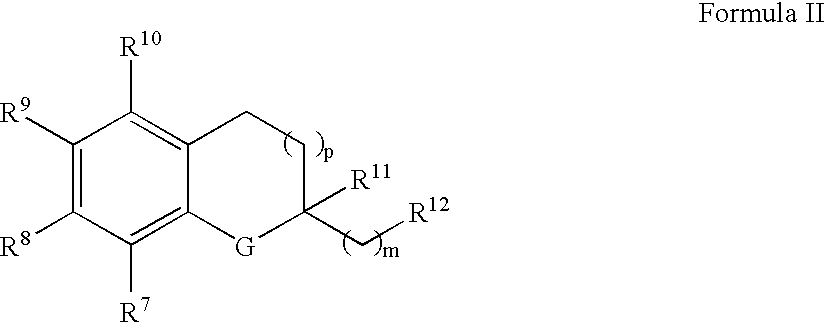
Treatment of mitochondrial diseases
InactiveUS20050065099A1Limit prevent damageBiocideSenses disorderHuntingtons choreaCerebellar ataxia
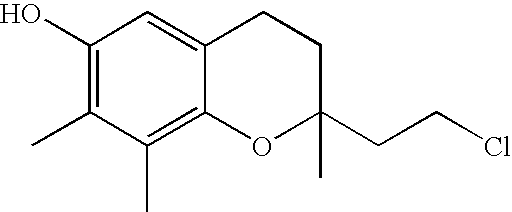
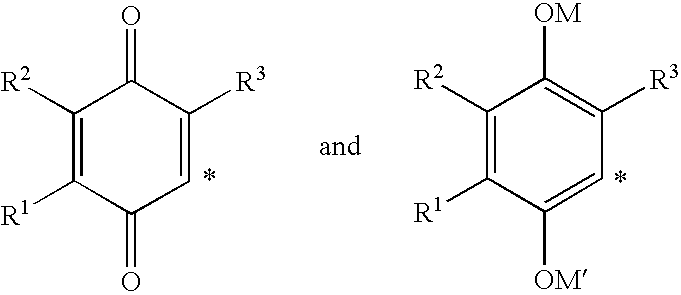
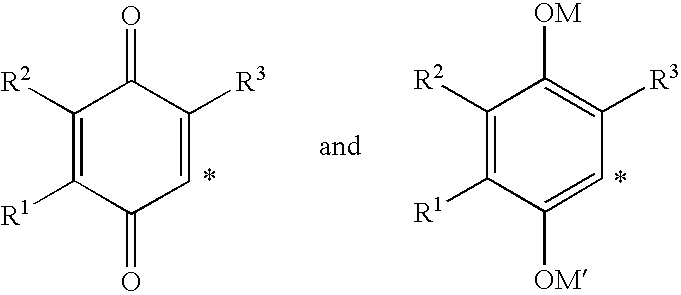
The invention relates the method of treatment or amelioration of mitochondrial disorders such as Alzheimer's disease, Parkinson's disease, Friedreich's ataxia (FRDA), cerebellar ataxias, Leber's hereditary optic neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), Myoclonic Epilepsy with Ragged Red Fibers (MERFF), amyotrophic lateral sclerosis (ALS), motor neuron diseases, Huntington's disease, macular degeneration, and epilepsy, with chroman derivatives of Formula I or Formula II as described herein.
Owner:EDISON PHARMA
Pro-neurogenic compounds
This technology relates generally to compounds and methods for stimulating neurogenesis (e.g., post-natal neurogenesis, including post-natal hippocampal and hypothalamic neurogenesis) and / or protecting neuronal cell from cell death. Various compounds are disclosed herein. In vivo activity tests suggest that these compounds may have therapeutic benefits in neuropsychiatric and / or neurodegenerative diseases such as schizophrenia, major depression, bipolar disorder, normal aging, epilepsy, traumatic brain injury, post-traumatic stress disorder, Parkinson's disease, Alzheimer's disease, Down syndrome, spinocerebellar ataxia, amyotrophic lateral sclerosis, Huntington's disease, stroke, radiation therapy, chronic stress, abuse of a neuro-active drug, retinal degeneration, spinal cord injury, peripheral nerve injury, physiological weight loss associated with various conditions, as well as cognitive decline associated with normal aging, chemotherapy, and the like.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Cytokine antagonists for neurological and neuropsychiatric disorders
Methods for treating neurological or neuropsychiatric diseases or disorders in humans by administering to the human a therapeutically effective dose of specific biologics are presented. The biologics of consideration include antagonists of tumor necrosis factor or of interleukin-1. The administration of these biologics is performed by specific methods, most, but not all of which fall into the category of anatomically localized administration designed for perispinal use. Anatomically localized administration involving perispinal use includes, but is not limited to the subcutaneous, intramuscular, interspinous, epidural, peridural, parenteral or intrathecal routes. Additonally, intranasal administration is discussed as a method to provide therapeutic benefit. The clinical conditions of consideration include, but are not limited to the following: diseases of the brain, including neurodegenerative diseases such as Alzheimer's Disease and Parkinson's Disease; migraine headache; spinal radiculopathy associated with intervertebral disc herniation, post-herpetic neuralgia, reflex sympathethic dystrophy, neuropathic pain, vertebral disc disease, low back pain, amyotrophic lateral sclerosis, chronic fatigue syndrome; and neuropsychiatric diseases, including bipolar affective disorder, anorexia nervosa, nicotine withdrawal, narcotic addiction, alcohol withdrawl, postpartum depression, and schizoaffective illness.
Owner:TACT IP
Glyceride esters for the treatment of diseases associated with reduced neuronal metabolism of glucose
Provided are alternative sources of ketone bodies for reducing or eliminating symptoms of Parkinson's disease, amyotrophic lateral sclerosis (ALS, also called Lou Gehrig's disease), Alzheimer's disease, Huntington's disease, epilepsy and other diseases or disorders characterized by impaired glucose metabolism. The alternative sources of ketone bodies include mono-, di- and triglyceride esters of acetoacetate and mixtures thereof, and / or mono-, di- and triglyceride esters of 3-hydroxybutyrate and mixtures thereof. These glyceride esters can be administered orally as a dietary supplement or in a nutritional composition.
Owner:NEUROENERGY VENTURES INC
Carbocyclic and heterocyclic substituted semicarbazones and thiosemicarbazones and the use thereof
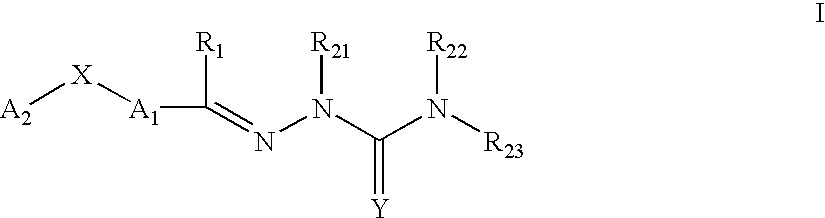
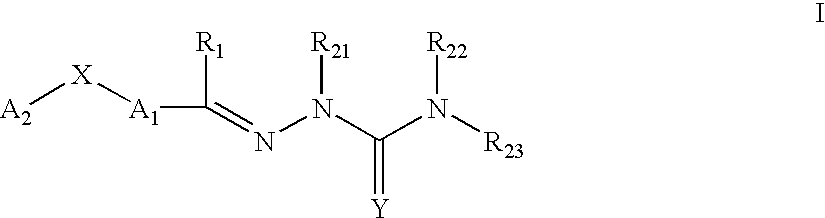
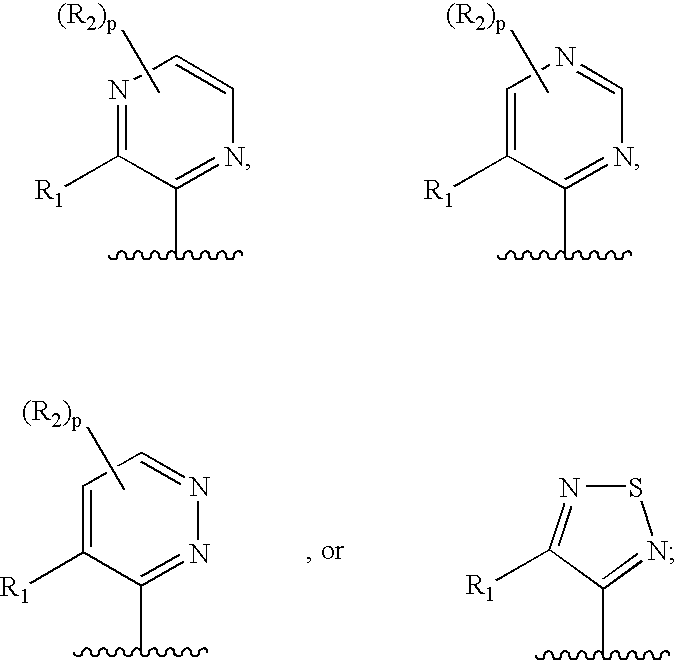
This invention is related to carbocyclic and heterocyclic substituted semicarbazones and thiosemicarbazones represented by Formula I: ##STR1## or a pharmaceutically acceptable salt or prodrug thereof, wherein: Y is oxygen or sulfur; R.sub.1, R.sub.21, R.sub.22 and R.sub.23 are independently hydrogen, alkyl, cycloalkyl, alkenyl, alkynyl, haloalkyl, aryl, aminoalkyl, hydroxyalkyl, alkoxyalkyl or carboxyalkyl; or R.sub.22 and R.sub.23, together with the N, form a heterocycle; A.sub.1 and A.sub.2 are independently aryl, heteroaryl, saturated or partially unsaturated carbocycle or saturated or partially unsaturated heterocycle, any of which is optionally substituted; X is one or O, S, NR.sub.24, CR.sub.25 R.sub.26, C(O), NR.sub.24 C(O), C(O)NR.sub.24, SO, SO.sub.2 or a covalent bond; where R.sub.24, R.sub.25 and R.sub.26 are independently hydrogen, alkyl, cycloalkyl, alkenyl, alkynyl, haloalkyl, aryl, aminoalkyl, hydroxyalkyl, alkoxyalkyl or carboxyalkyl. The invention also is directed to the use of carbocycle and heterocycle substituted semicarbazones and thiosemicarbazones for the treatment of neuronal damage following global and focal ischemia, for the treatment or prevention of neurodegenerative conditions such as amyotrophic lateral sclerosis (ALS), for the treatment and prevention of otoneurotoxicity and eye diseases involving glutamate toxicity and for the treatment, prevention or amelioration of pain, as anticonvulsants, and as antimanic depressants, as local anesthetics, as antiarrhythmics and for the treatment or prevention of diabetic neuropathy and urinary incontinence.
Owner:COCENSYS
Synthetic apelin mimetics for the treatment of heart failure
ActiveUS8673848B2Extended half-lifeIncrease constraintsNervous disorderSkeletal disorderCardiac fibrosisVentricular tachycardia
The invention provides a synthetic polypeptide of Formula I′:or an amide, an ester or a salt thereof, wherein X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X11, X12 and X13 are defined herein. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
Therapeutic agents useful for treating pain
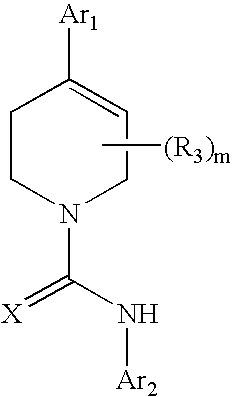
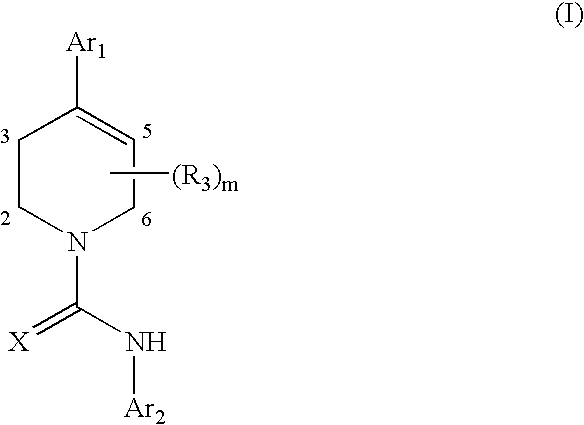
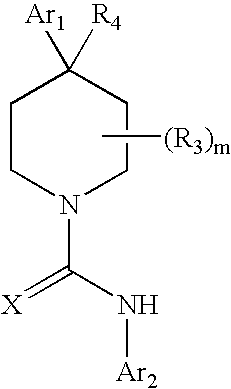
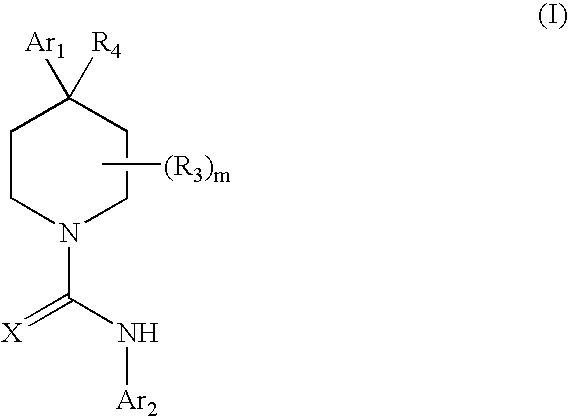
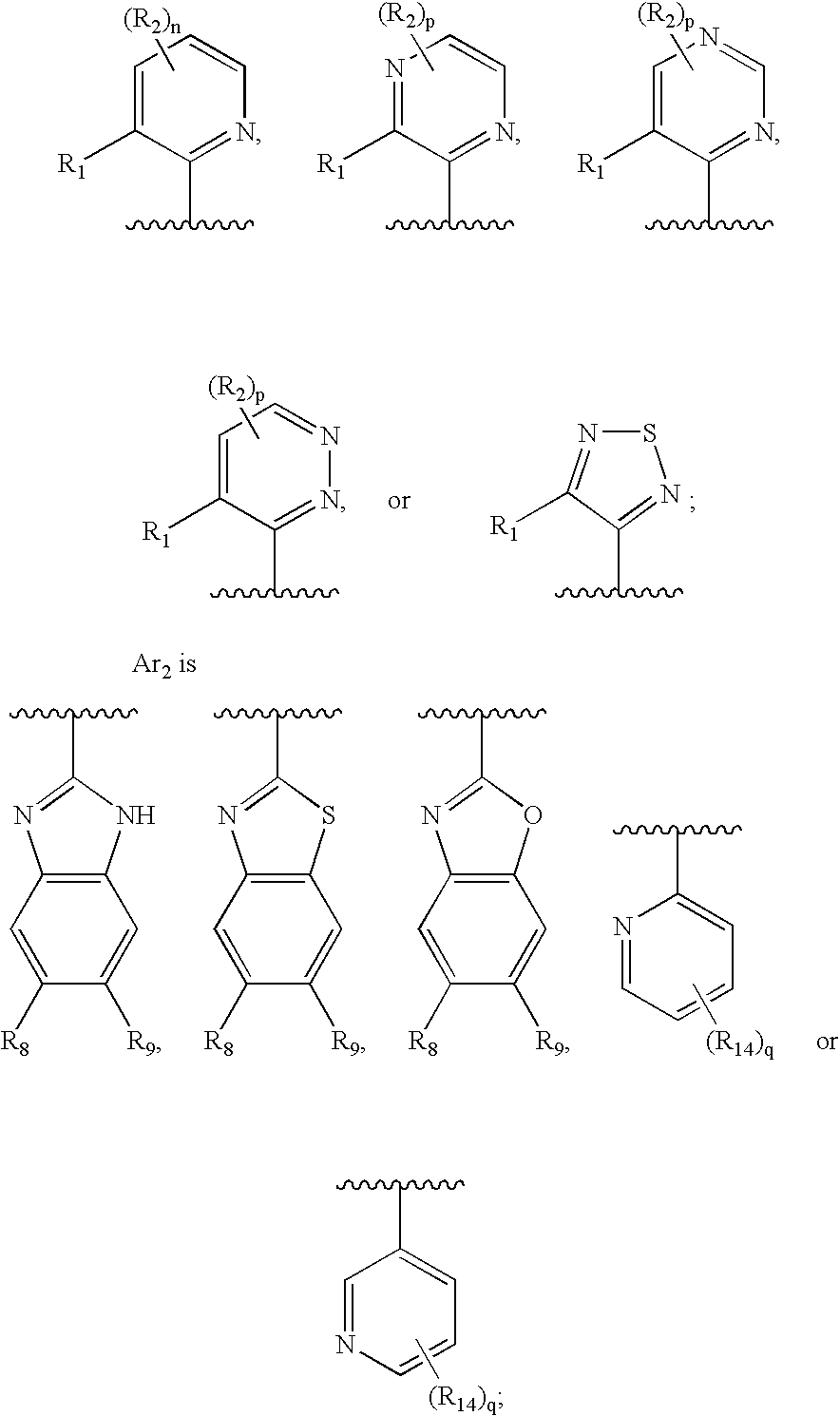
A compound of formula: where Ar1, Ar2, X, R3, and m are as disclosed herein or a pharmaceutically acceptable salt thereof (a “Tetrahydropiperidyl Compound”); compositions comprising an effective amount of a Tetrahydropiperidyl Compound; and methods for treating or preventing pain, UI, an ulcer, IBD, IBS, an addictive disorder, Parkinson's disease, parkinsonism, anxiety, epilepsy, stroke, a seizure, a pruritic condition, psychosis, a cognitive disorder, a memory deficit, restricted brain function, Huntington's chorea, amyotrophic lateral sclerosis, dementia, retinopathy, a muscle spasm, a migraine, vomiting, dyskinesia, or depression in an animal comprising administering to an animal in need thereof an effective amount of a Tetrahydropiperidyl Compound are disclosed herein.
Owner:PURDUE PHARMA LP
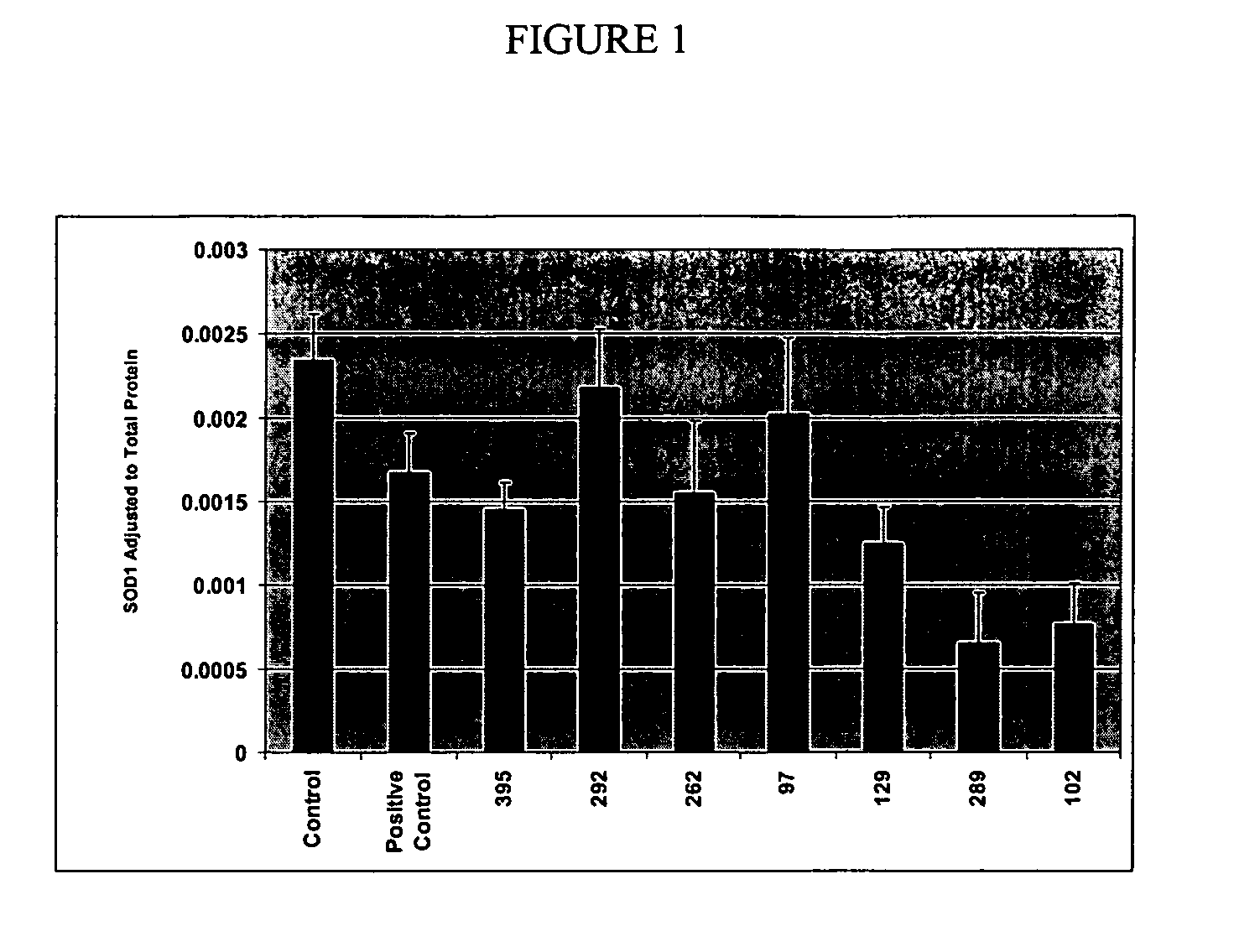
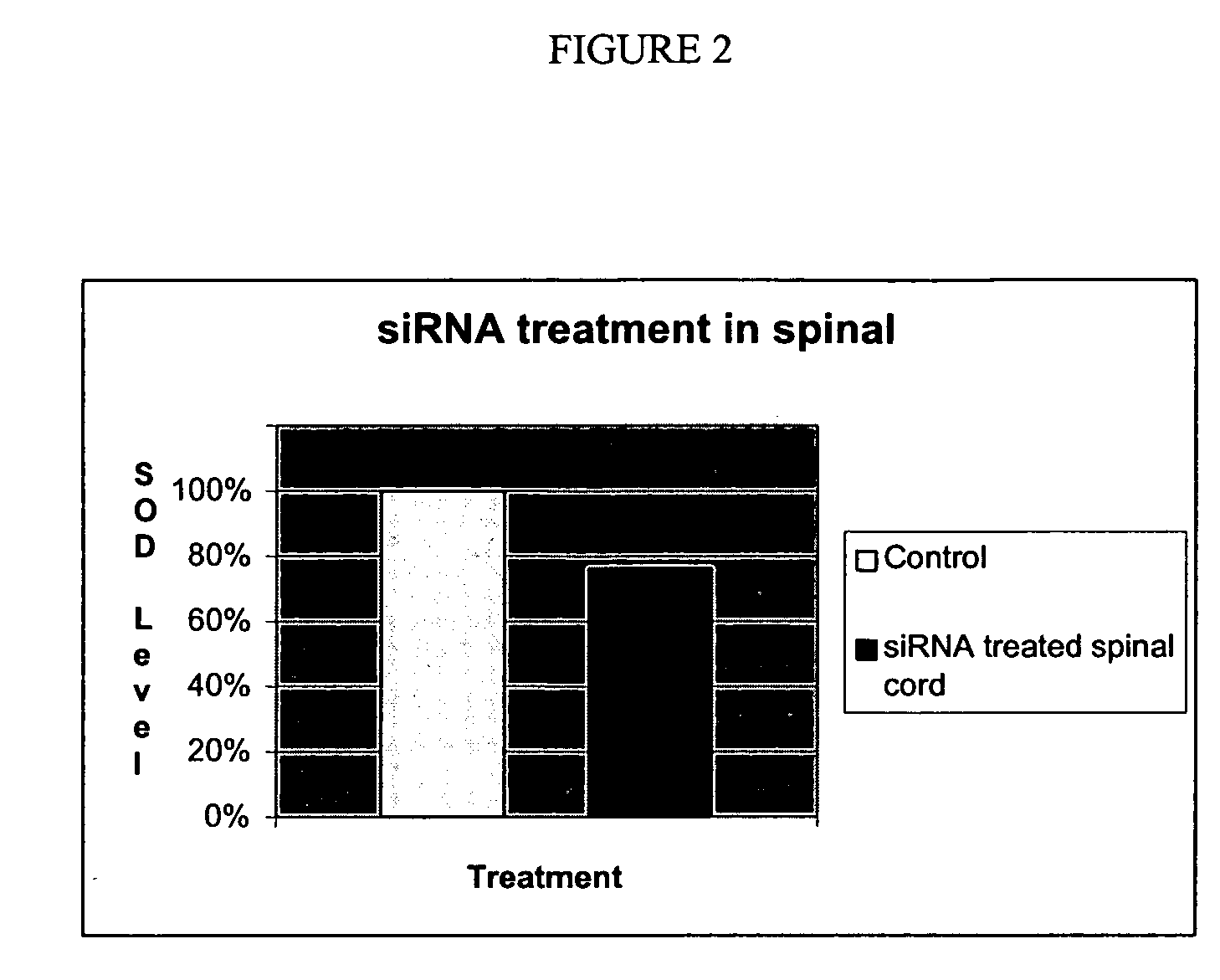
Small interference RNA (siRNA) molecules for modulating superoxide dismutase (SOD)
InactiveUS20060229268A1Good potencyIncrease contactNervous disorderGenetic material ingredientsProtein targetAmyotrophic lateral sclerosis
The invention pertains to using double stranded ribonucleic acid molecules such as small interfering RNA (siRNA) molecules to target an SOD gene to interfere with gene expression and SOD protein production. Method are disclosed for inhibiting expression of a target protein in a subject with a neurological disorder by introducing a small interference ribonucleic acid (siRNA) molecule into the subject with the neurological disorder, such as amyotrophic lateral sclerosis (ALS).
Owner:ALSGEN
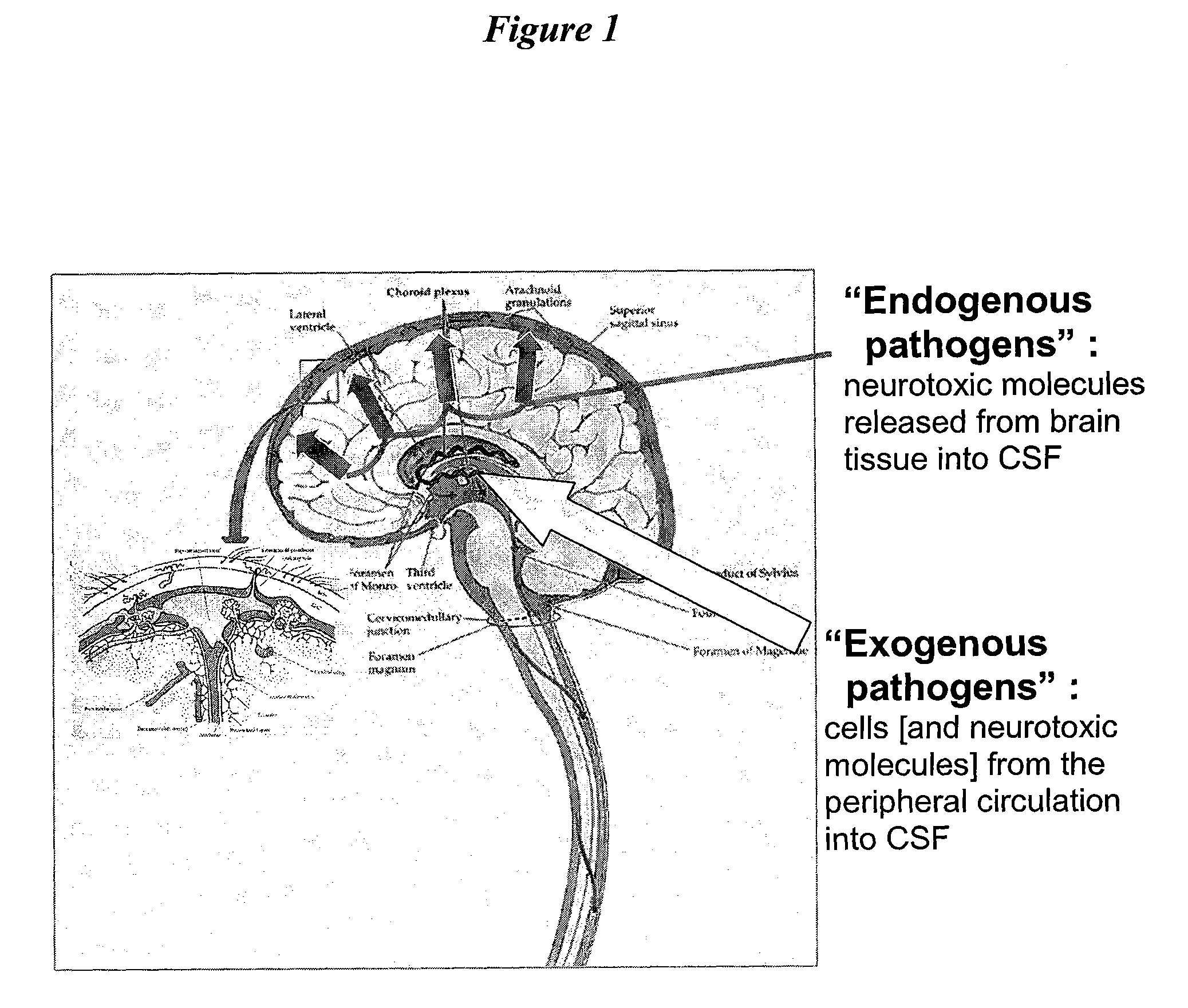
Cerebrospinal Fluid Purification System
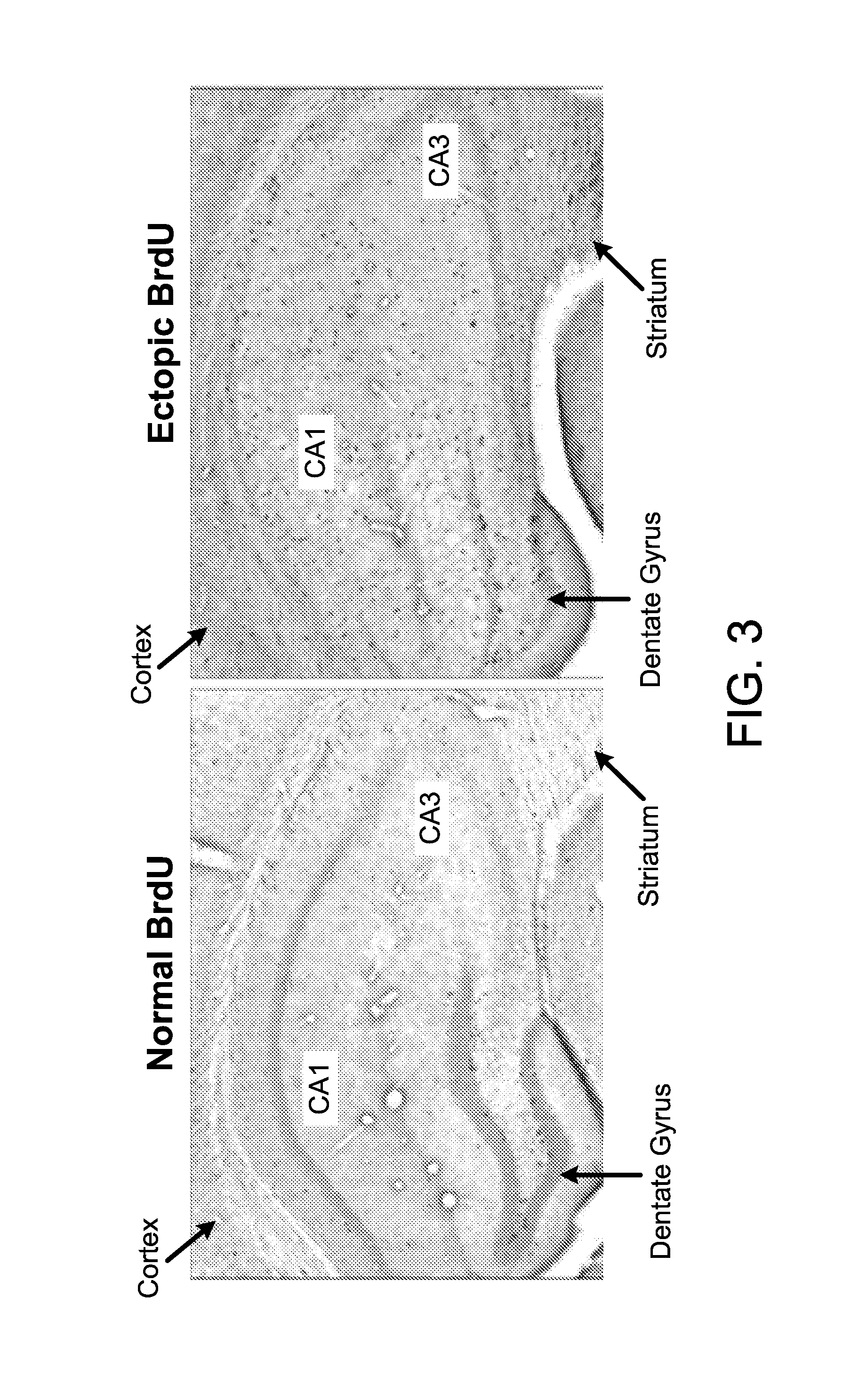
The present invention provides methods and systems for conditioning cerebrospinal fluid (CSF). The methods provide for efficiently removing target compounds from CSF. The systems provide for a multilumen flow path and exchange of a majority volume portion of CSF in the CSF space. The removal and / or delivery of specific compounds can be tailored to the pathology of the specific disease. The removal is targeted and specific, for example, through the use of specific size-exclusion thresholds, antibodies against specific toxins, and other chromatographic techniques, as well as delivery and / or removal of targeted therapeutic agents. The invention finds use as a diagnostic, therapeutic and drug delivery platform for a variety of diseases affecting the CNS by accessing the CSF space. Exemplified disease conditions treatable by the present CSF processing systems and methods include, but are not limited to: Cerebral Vasospasm, Guillain Bane Syndrome, illustrating multi-lumen lumbar approach Alzheimer's, Parkinson's, Huntington's, Multiple Sclerosis, Amyotrophic Lateral Sclerosis, Spinal Cord Injury, Traumatic Brain Injury, Stroke, Cancer affecting the brain or spinal cord, Prion disease, Encephalitis from various causes, Meningitis from various causes, diseases secondary to enzymatic or metabolic imbalances, Biological Warfare, etc. For the first time, the present invention offers patients a disease-modifying, disruptive technology treatment platform that addresses the known disease pathogenesis of a number of neurologic conditions to which there are presently limited and ineffective treatment options.
Owner:NEUROFLUIDICS
Derivate von dihydroxyphenylalanin
The invention relates to dihydroxyphenylalanine derivatives, the production thereof, and pharmaceutical compositions containing said dihydroxyphenylalanine derivatives. The invention further relates to the use of said dihydroxyphenylalanine derivatives and pharmaceutical compositions for the treatment and prevention of movement disorders, neurodegenerative diseases, Alzheimer, Parkinson's disease, hemiatrophy hemiparkinsonism, Parkinson's syndrome, Lewy bodies disease, frontotemporal dementia, Lytico-Bodig disease (Parkinsonism-dementia-amyotrophic lateral sclerosis, striatonigral degeneration, Shy-Drager syndrome, sporadic olivopontocerebellar degeneration, progressive pallidal atrophy, progressive supranuclear palsy, Hallervorden-Spatz disease, Huntington's disease, X chromosome-linked dystonia (Morbus Lubag), mitochondrial cytopathy with striatal necrosis, neuroacanthocytosis, restless leg syndrome, Wilson's disease.
Owner:ELLNEUROXX LTD
Antagonist antibodies against GDF-8 and uses in treatment of ALS and other GDF-8-associated disorders
InactiveUS20070087000A1Reduce wasteEffective therapyNervous disorderImmunoglobulins against cytokines/lymphokines/interferonsAntibody fragmentsNucleotide
The disclosure provides novel molecules related to growth and differentiation factor-8 (GDF-8), in particular mouse and humanized antibodies, and antibody fragments, including those that inhibit GDF-8 activity and signaling in vitro and / or in vivo. The disclosure also provides methods for diagnosing, treating, ameliorating, preventing, prognosing, or monitoring degenerative orders of muscle, bone, and insulin metabolism, etc., in particular amyotrophic lateral sclerosis (ALS). In addition, the disclosure provides pharmaceutical compositions for the treatment of such disorders by using the antibodies, polypeptides, polynucleotides, and vectors of the invention.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Therapeutic agents useful for treating pain
Piperidine Compounds; compositions comprising a Piperidine Compound; and methods for treating or preventing pain, UI, an ulcer, IBD, IBS, an addictive disorder, Parkinson's disease, parkinsonism, anxiety, epilepsy, stroke, a seizure, a pruritic condition, psychosis, a cognitive disorder, a memory deficit, restricted brain function, Huntington's chorea, amyotrophic lateral sclerosis, dementia, retinopathy, a muscle spasm, a migraine, vomiting, dyskinesia, or depression in an animal comprising administering to an animal in need thereof an effective amount of a Piperidine Compound are disclosed. In one embodiment, the Piperidine Compound has the formula: and pharmaceutically acceptable salts thereof, wherein Ar1, Ar2, X, R3, R4, and m are as disclosed herein.
Owner:PURDUE PHARMA LP
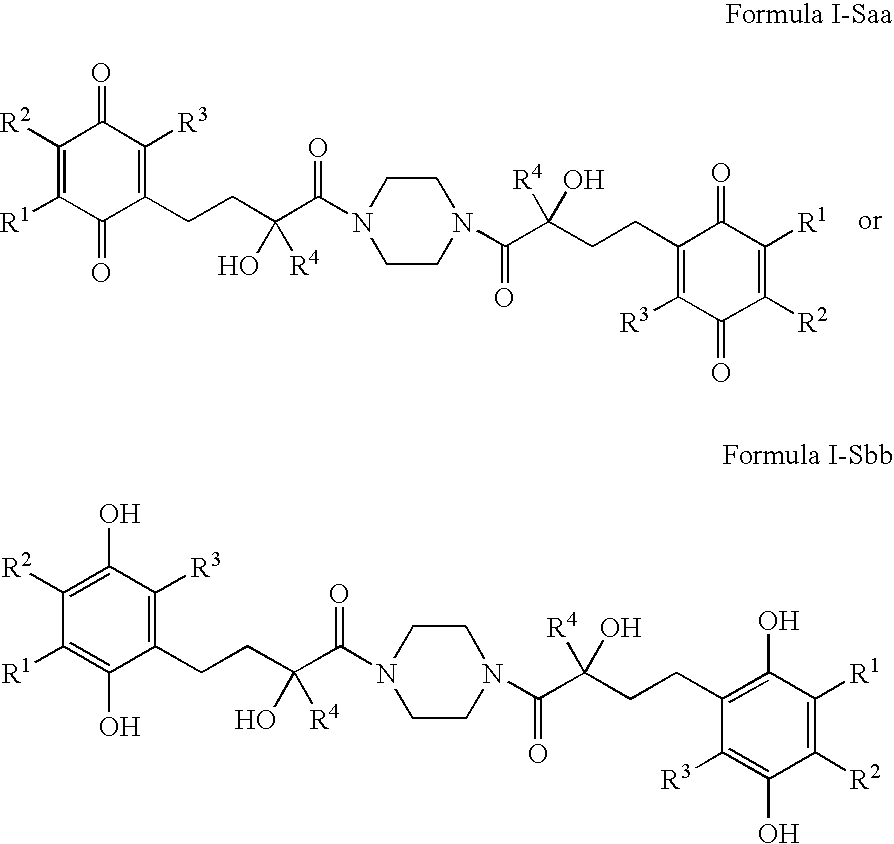
4-(p-QUINONYL)-2-HYDROXYBUTANAMIDE DERIVATIVES FOR TREATMENT OF MITOCHONDRIAL DISEASES
ActiveUS20090118257A1Good for healthRaise level of ATPBiocideSenses disorderKearn sayre syndromeHuntingtons chorea
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, and stroke (MELAS), Kearns-Sayre Syndrome (KSS), are disclosed, as well as compounds useful in the methods of the invention, such as 4-(p-quinolyl)-2-hydroxybutanamide derivatives. Methods and compounds useful in treating other disorders such as amyotrophic lateral sclerosis (ALS), Huntington's disease, Parkinson's disease, and pervasive developmental disorders such as autism are also disclosed. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed. Methods of modulating, normalizing, or enhancing energy biomarkers, as well as compounds useful for such methods, are also disclosed.
Owner:PTC THERAPEUTICS INC
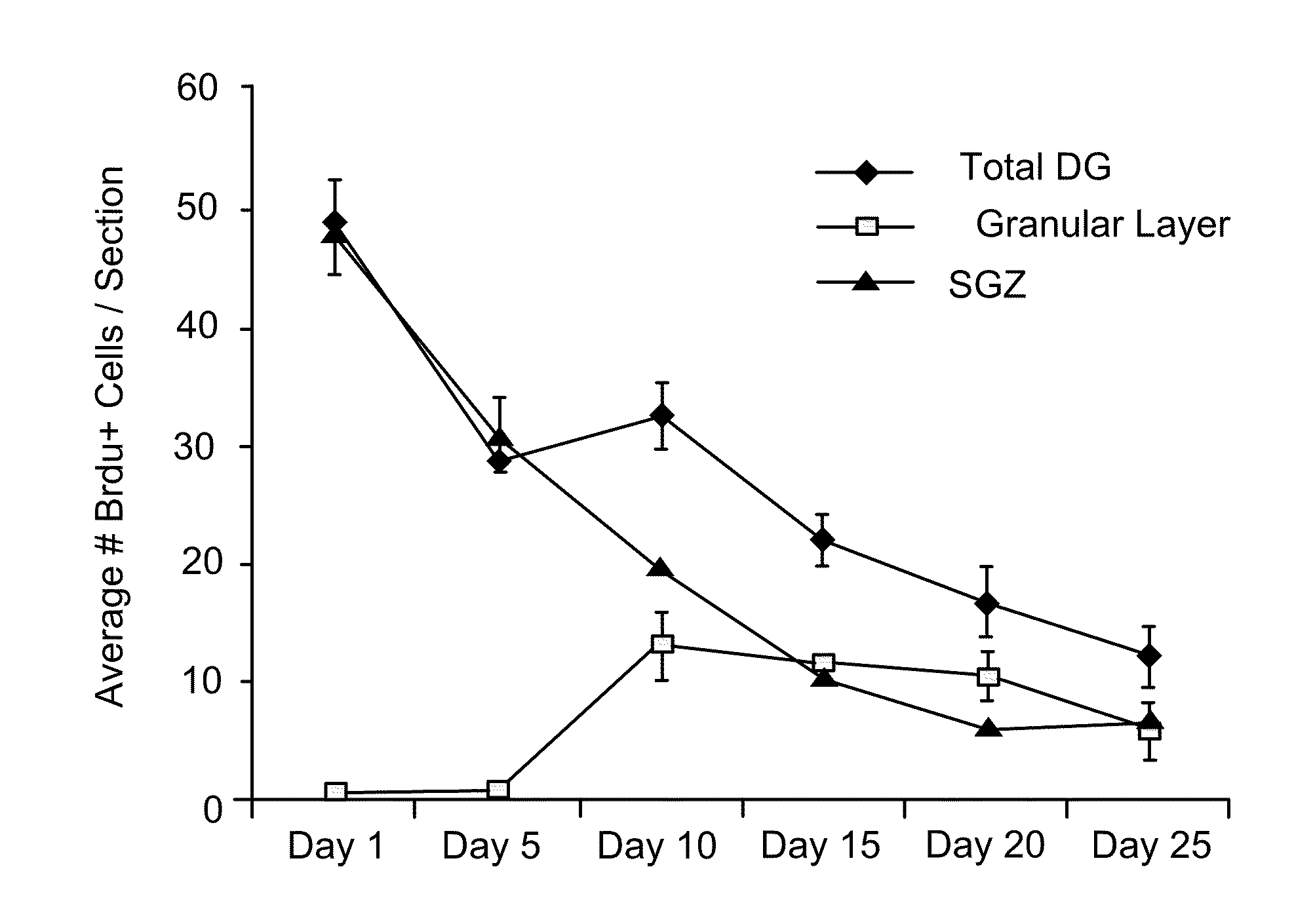
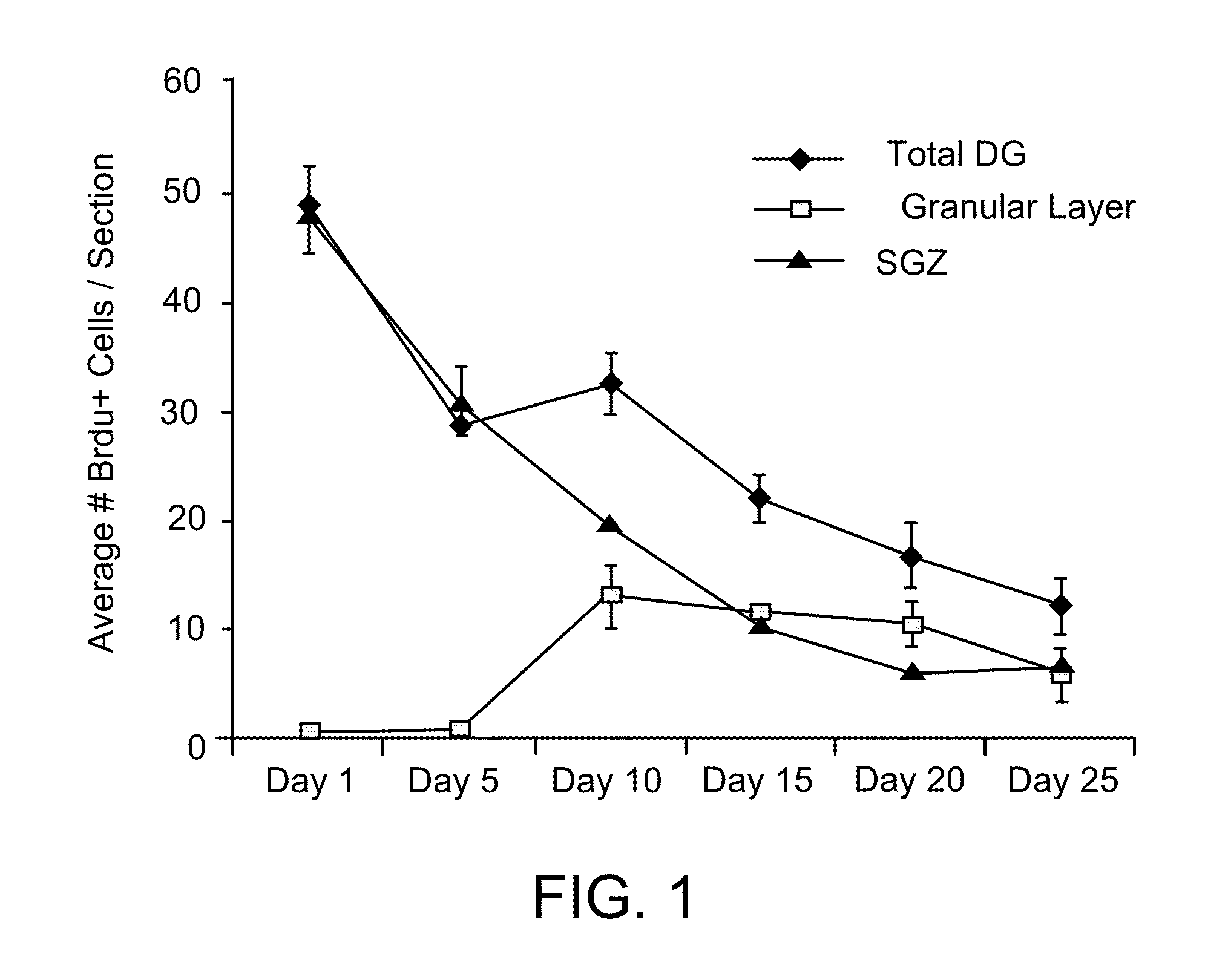
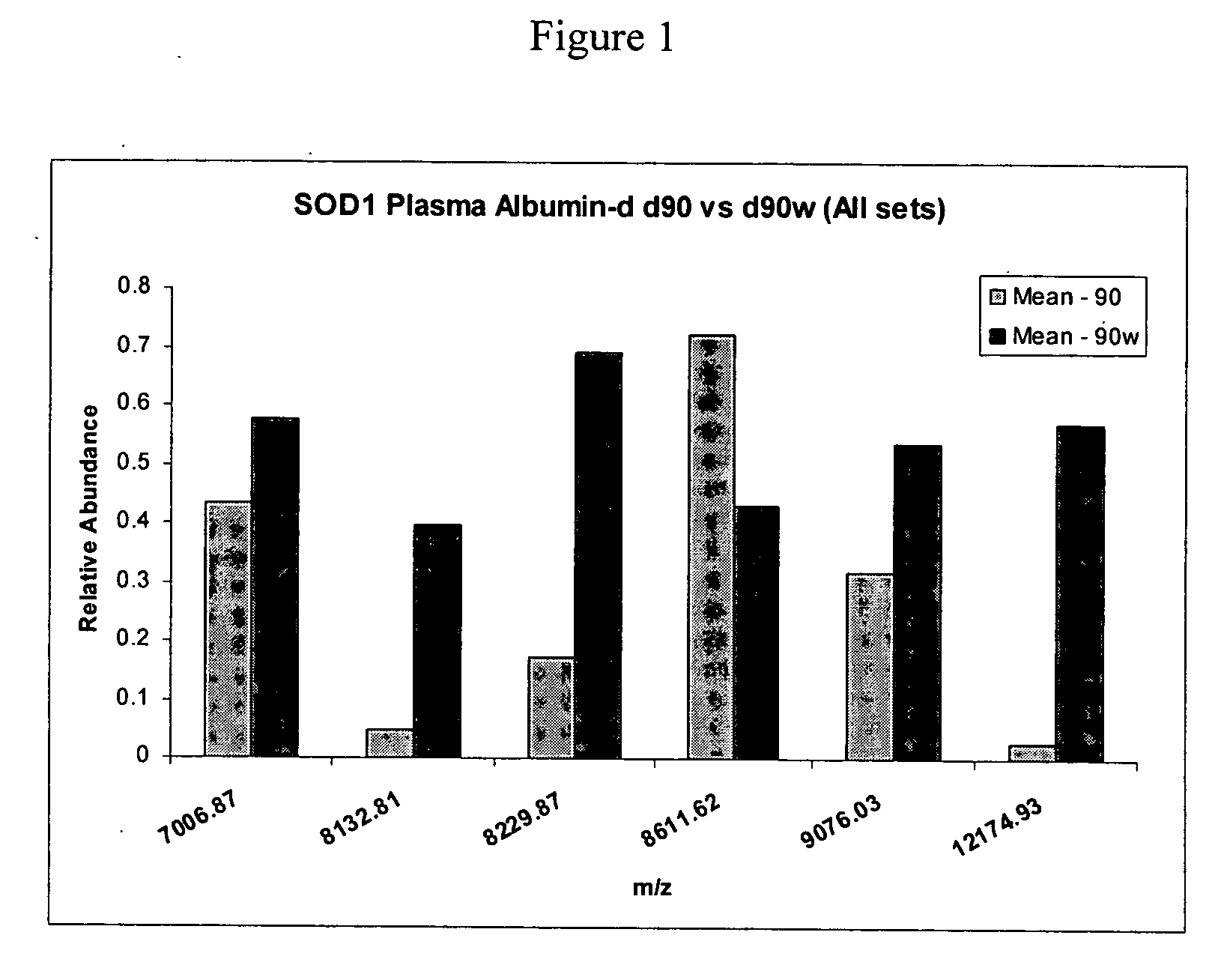
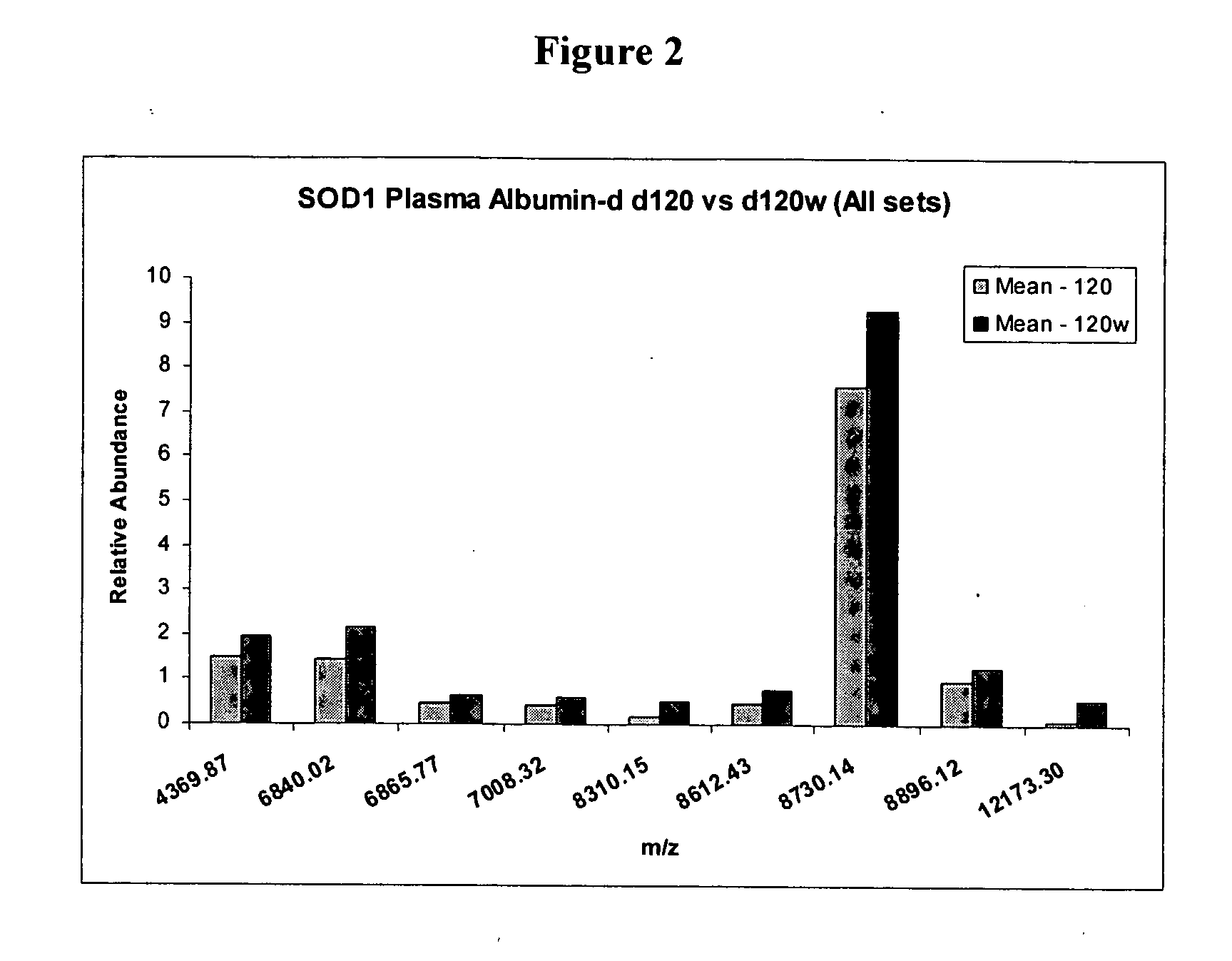
Biomarkers for amyotrophic lateral sclerosis
ActiveUS20050148026A1Microbiological testing/measurementMuscular disorderAmyotrophic lateral sclerosisPharmaceutical drug
The invention provides a method for diagnosing amyotrophic lateral sclerosis (ALS) in a subject, a method for assessing the effectiveness of a drug in treating ALS, and a method for determining the site of onset of ALS in a subject. Each method comprises (a) obtaining a sample from the subject, (b) analyzing the proteins in the sample by mass spectroscopy, and (c) determining a mass spectral profile for the sample. In some embodiments, the method comprises comparing the mass spectral profile of the sample to the mass spectral profile of a positive or a negative standard.
Owner:UNIVERSITY OF PITTSBURGH
Als treatment
ActiveUS20130158451A1Chiropractic devicesVibration massagePhysical medicine and rehabilitationNasal Cavity Epithelium
A method for treating amyotrophic lateral sclerosis (ALS) in a human subject is provided. A first vibration stimulation member is introduced into a posterior part of a first nasal cavity of the human subject. By means of the first vibration stimulation member, vibrations are imparted to the posterior part of the first nasal cavity at frequency in a range of from 60 to 70 Hz. A second vibration simulation member is arranged between the trapezius muscle and the sternocleidomastoid muscle on a first side of the neck of the human subject; and by means of said second vibration stimulation member, vibrations are imparted to the first side of the neck at a frequency in a range of from 30 to 50 Hz.
Owner:CHORDATE MEDICAL AB
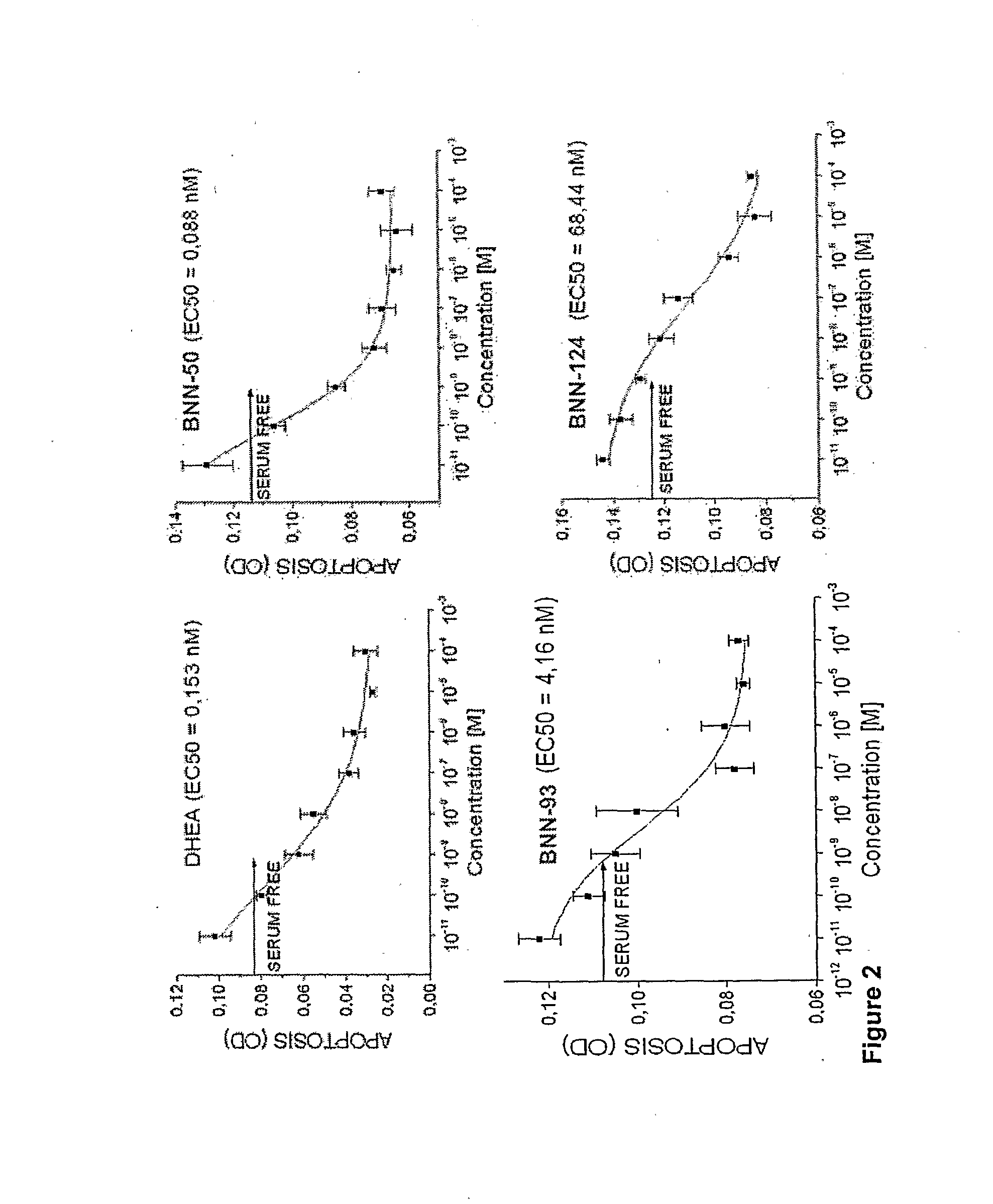
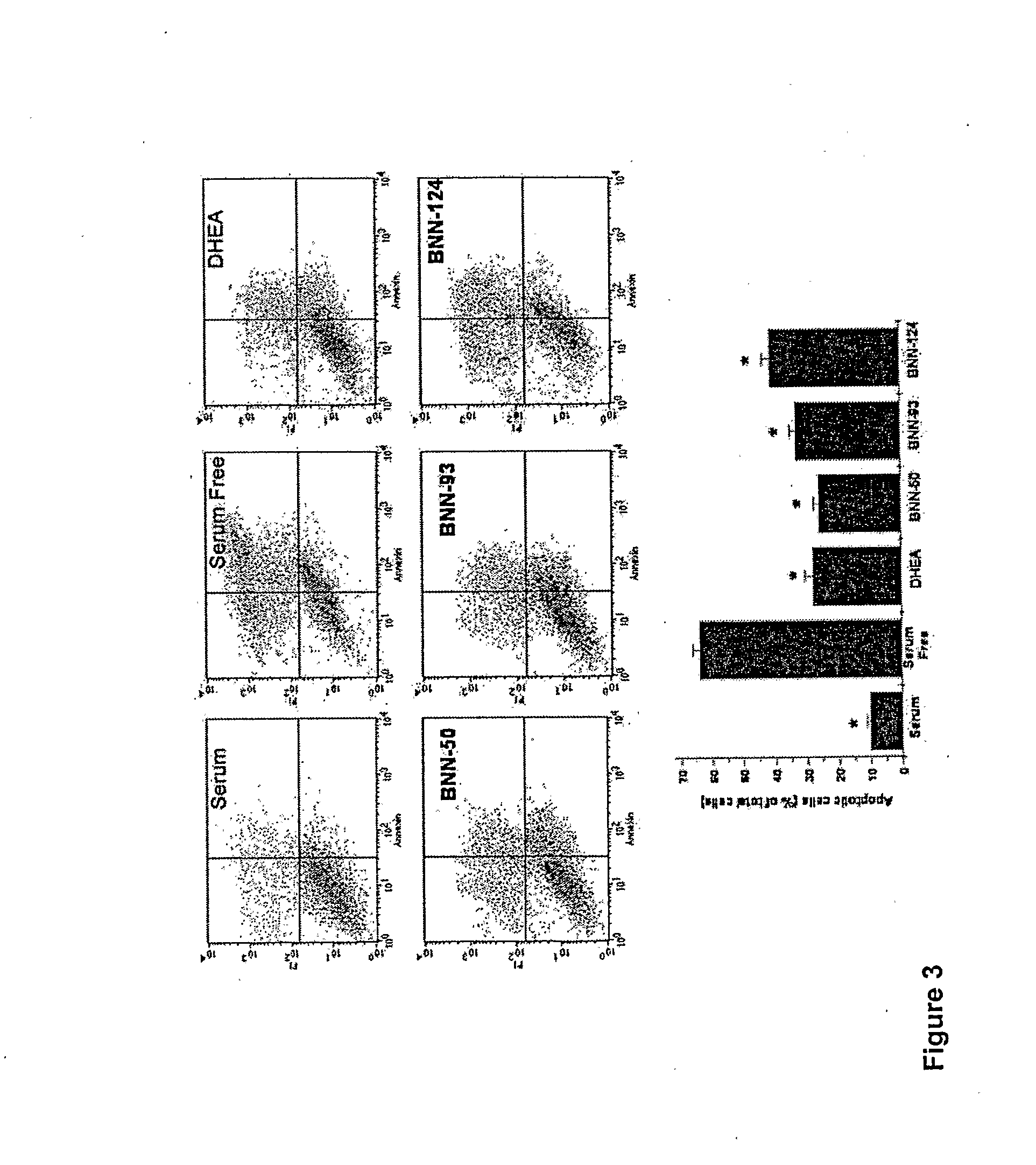
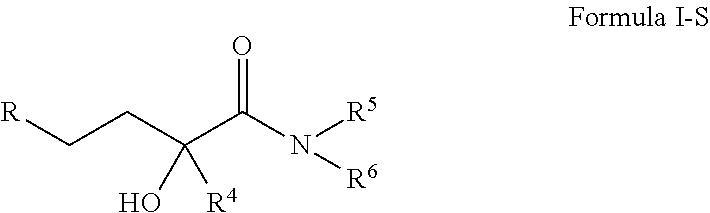
Neurosteroid compounds
The present invention relates to novel neurosteroid derivatives with anti-apoptotic, neuroprotective and neurogenic properties that act on the nervous system as well as methods for making the same and their applications in the treatment and / or prevention or amelioration of neurodegenerative diseases related to neuronal apoptosis or neuronal injury, or conditions related to or resulting from apoptosis, including but not limited to Alzheimer's disease, Parkinson's disease, Huntington's disease, multiple sclerosis and amyotrophic lateral sclerosis (ALS), retinal degeneration and detachment, peripheral neuropathy caused by genetic abnormalities, diabetes, polio, herpes, AIDS and chemotherapy, brain trauma, or ischemia and stroke. The active compounds are represented by Formula (I): wherein R1, R2, R3, R4, R5, R6, R7, A, B, X, Y and Z are defined in the description of the invention. The present invention also includes compositions which comprise one or more of the compounds of Formula (I).
Owner:BIONATURE E A LTD
4-(p-quinonyl)-2-hydroxybutanamide derivatives for treatment of mitochondrial diseases
ActiveUS7968746B2Reduce severityReduce in quantitySenses disorderNervous disorderHuntingtons choreaKearn sayre syndrome
Owner:PTC THERAPEUTICS INC
Neuroprotective small organic molecules, compositions and uses related thereto
InactiveUS20060014807A1Improve survival rateConducive to survivalBiocideOrganic chemistryMedicineAmyotrophic lateral sclerosis
The present application is directed to therapeutic compounds, compositions, and methods for culturing neuronal cells and for preventing and the treatment of neurodegenerative diseases, such as Parkinson's disease and amyotrophic lateral sclerosis (ALS).
Owner:LIN LEU FEN HOU
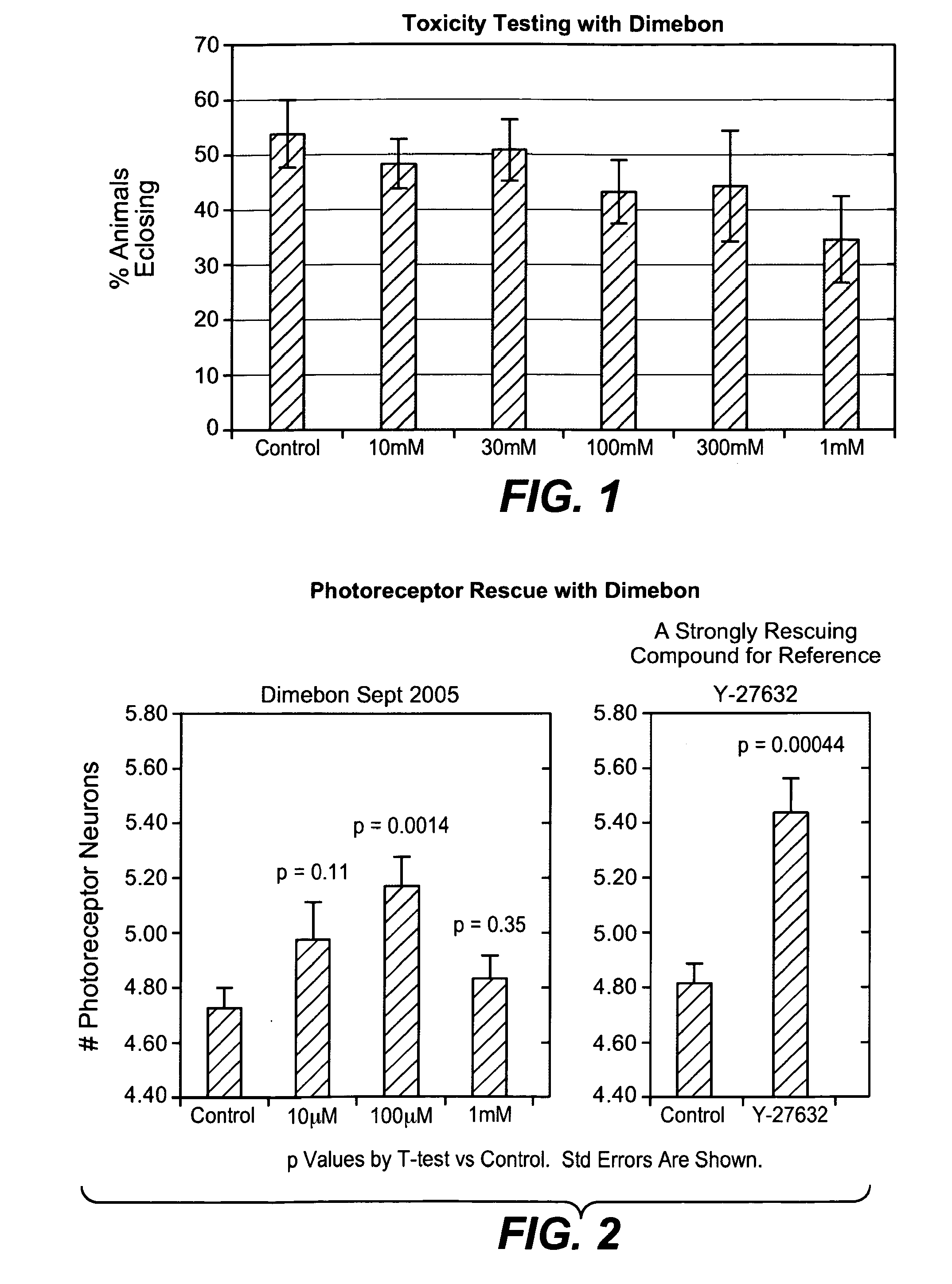
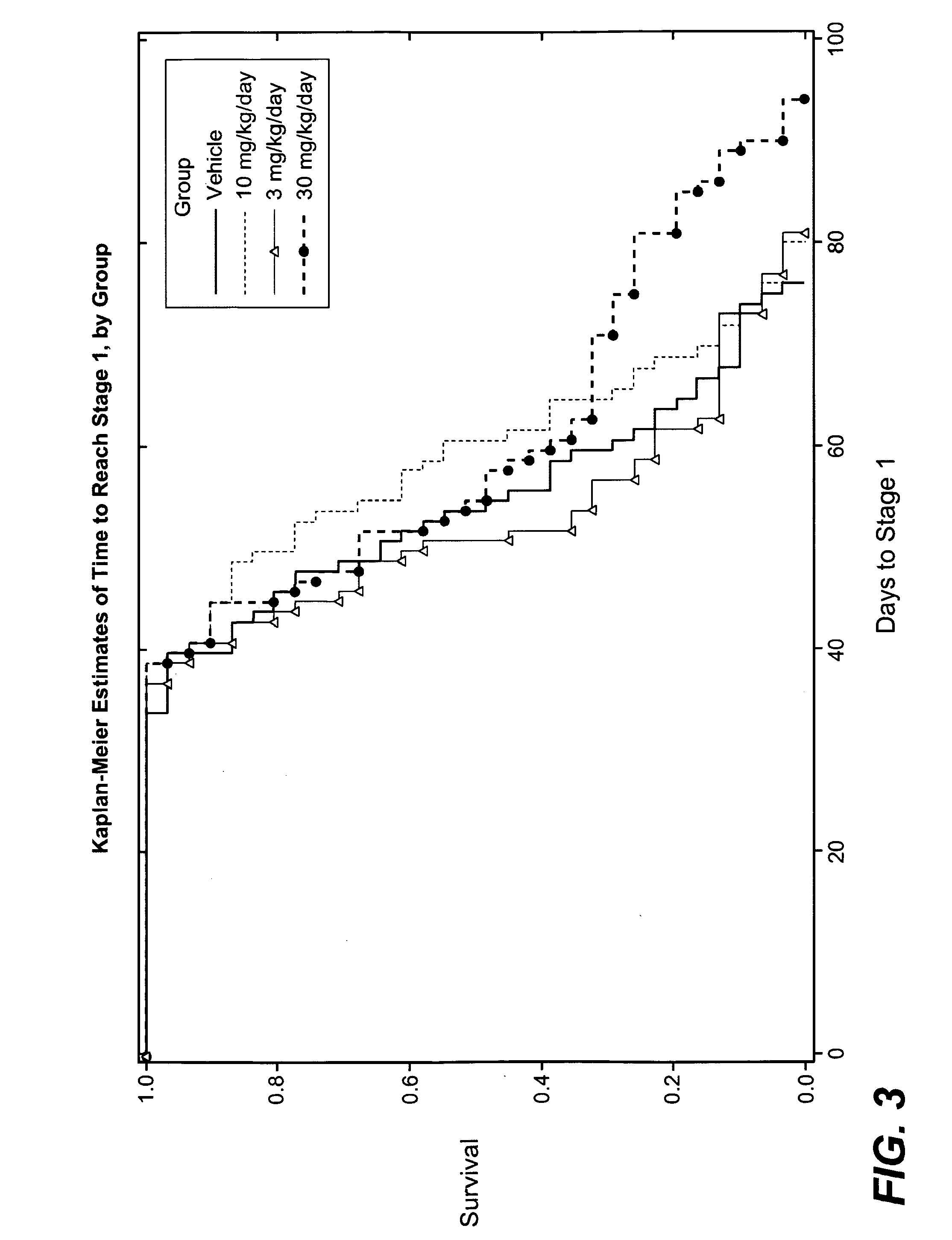
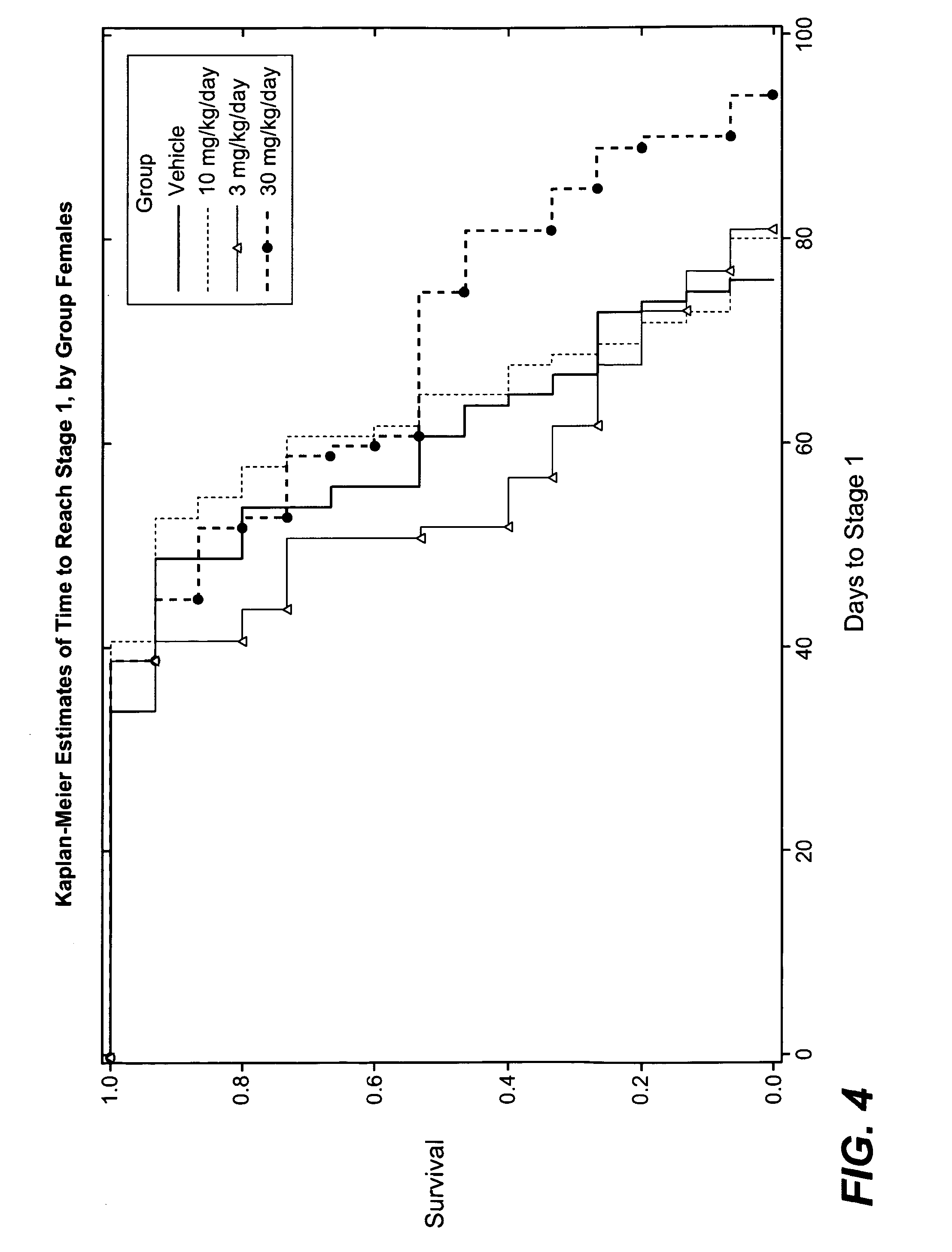
Hydrogenated pyrido (4,3-b) indoles for treating amyotrophic lateral sclerosis (ALS)
InactiveUS20100099700A1Quality improvementProlong survival timeBiocideNervous disorderAmyotrophic lateral sclerosisMedicine
Owner:MEDIVATION TECH INC
Protein biomarkers and therapeutic targets in an animal model for amyotrophic lateral sclerosis
The invention provides a method for determining the onset and / or progression of ALS in an animal. The method comprises (a) obtaining a sample from the animal, (b) analyzing the proteins in the sample by mass spectroscopy, and (c) determining a mass spectral profile for the sample. The invention also provides isolated protein biomarkers of ALS.
Owner:UNIVERSITY OF PITTSBURGH
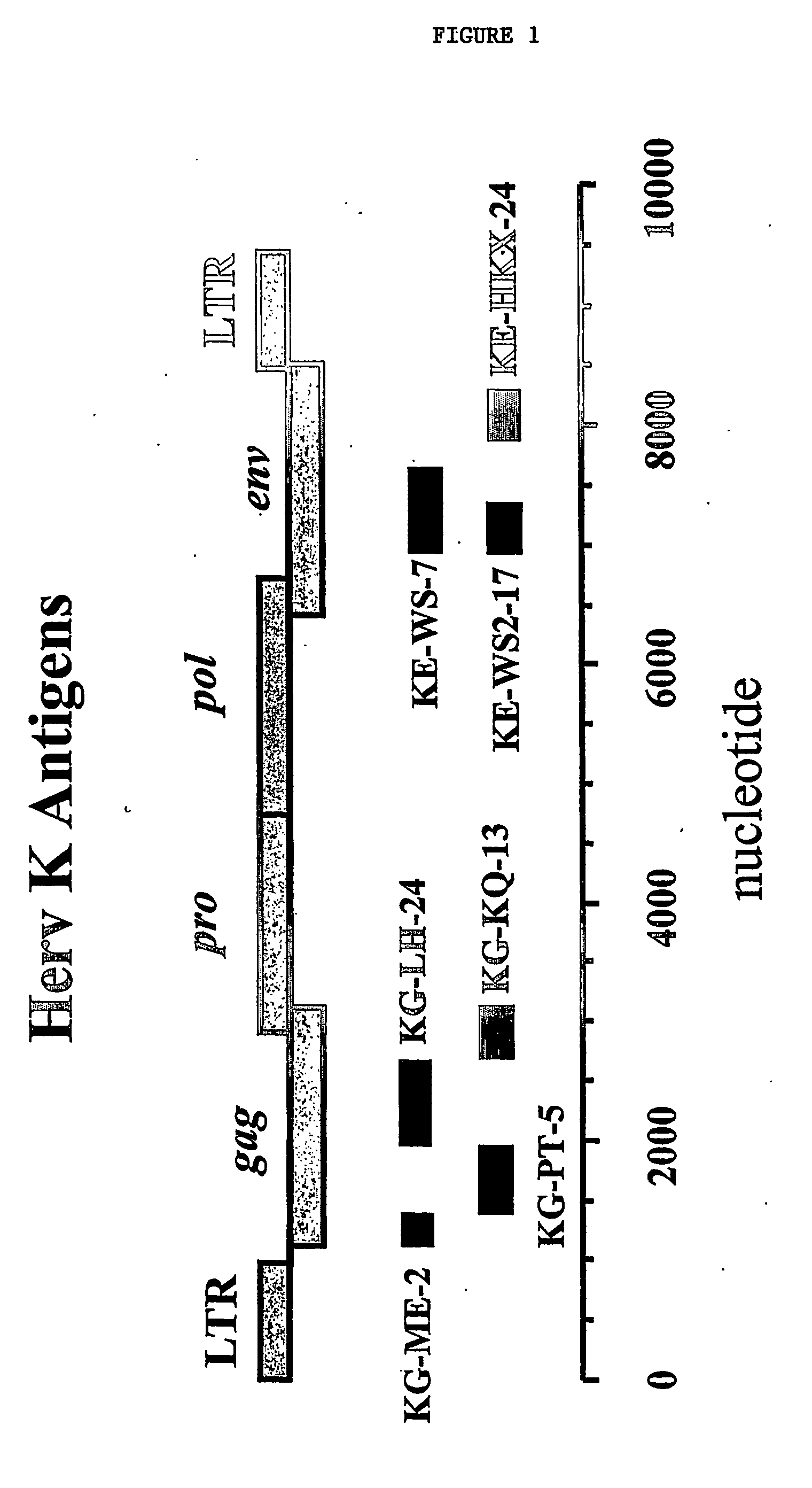
Monitoring and treatment of amyotrophic lateral sclerosis
InactiveUS20060160087A1Reduce expressionMicrobiological testing/measurementDisease diagnosisInfected cellAmyotrophic lateral sclerosis
The invention provides methods of monitoring amyotrophic lateral sclerosis (ALS) disease development or progression and monitoring an ALS therapy in an individual by determining the presence or absence of Herv-K / HML-2 expression in a biological sample from the individual. The invention is also directed to methods for aiding diagnosis of ALS by determining expression of Herv-K / HML-2 in a biological sample from the individual. The invention is also directed to methods of reducing Herv-K / HML-2 expression in infected cells and individuals. The invention includes reagents for use in these methods.
Owner:PATHOLOGICA
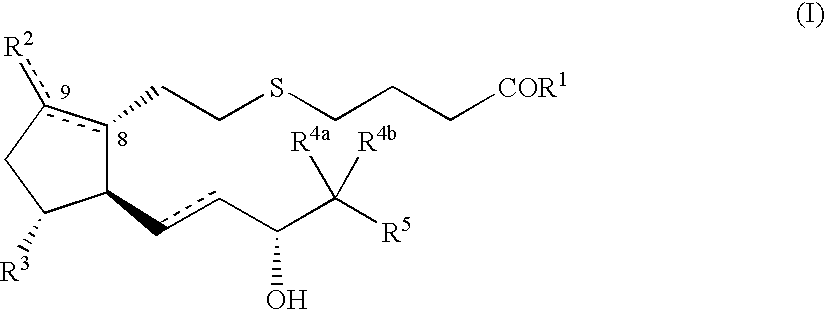
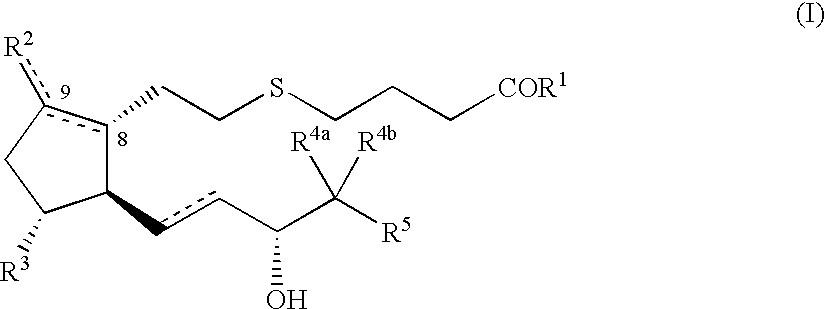
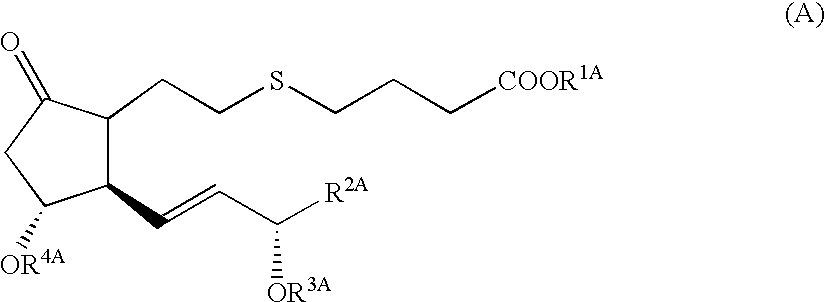
5-thia-omega-substituted phenyl-prostaglandin E derivatives, process for producing the same and drugs containing the same as the active ingredient
The present invention relates to 5-thia-omega-substituted phenylprostaglandin E derivatives of the formula (I)(wherein, all the symbols are as defined in the specification), process for producing them and pharmaceutical compositions comprising them as active ingredient.The compounds of the formula (I) can bind to PGE2 receptors (especially, subtype EP4) strongly, so they are expected to be useful for prevention and / or treatment of immunological diseases (autoimmune diseases such as amyotrophic lateral sclerosis (ALS), multiple sclerosis, Sjoegren's syndrome, chronic rheumarthrosis and systemic lupus erythematosus etc., and rejection after organ transplantation etc.), asthma, abnormal bone formation, neuronal cell death, lung failure, liver damage, acute hepatitis, nephritis, renal insufficiency, hypertension, myocardiac ischemia, systemic inflammatory response syndrome, ambustion pain, sepsis, hemophagous syndrome, macrophage activation syndrome, Still's disease, Kawasaki disease, burn, systemic granulomatosis, ulcerative colitis, Crohn's disease, hypercytokinemia at dialysis, multiple organ failure, and shock etc. Further, it is thought that EP4 subtype receptor relates to sleeping disorder and blood platelet aggregation, so the compounds of the present invention are expected to be useful for the prevention and / or treatment of such diseases.
Owner:ONO PHARMA CO LTD
Methods for treating Parkinson's disease using pro-neurogenic compounds
This technology relates generally to compounds and methods for stimulating neurogenesis (e.g., post-natal neurogenesis, including post-natal hippocampal and hypothalamic neurogenesis) and / or protecting neuronal cell from cell death. Various compounds are disclosed herein. In vivo activity tests suggest that these compounds may have therapeutic benefits in neuropsychiatric and / or neurodegenerative diseases such as schizophrenia, major depression, bipolar disorder, normal aging, epilepsy, traumatic brain injury, post-traumatic stress disorder, Parkinson's disease, Alzheimer's disease, Down syndrome, spinocerebellar ataxia, amyotrophic lateral sclerosis, Huntington's disease, stroke, radiation therapy, chronic stress, abuse of a neuro-active drug, retinal degeneration, spinal cord injury, peripheral nerve injury, physiological weight loss associated with various conditions, as well as cognitive decline associated with normal aging, chemotherapy, and the like.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
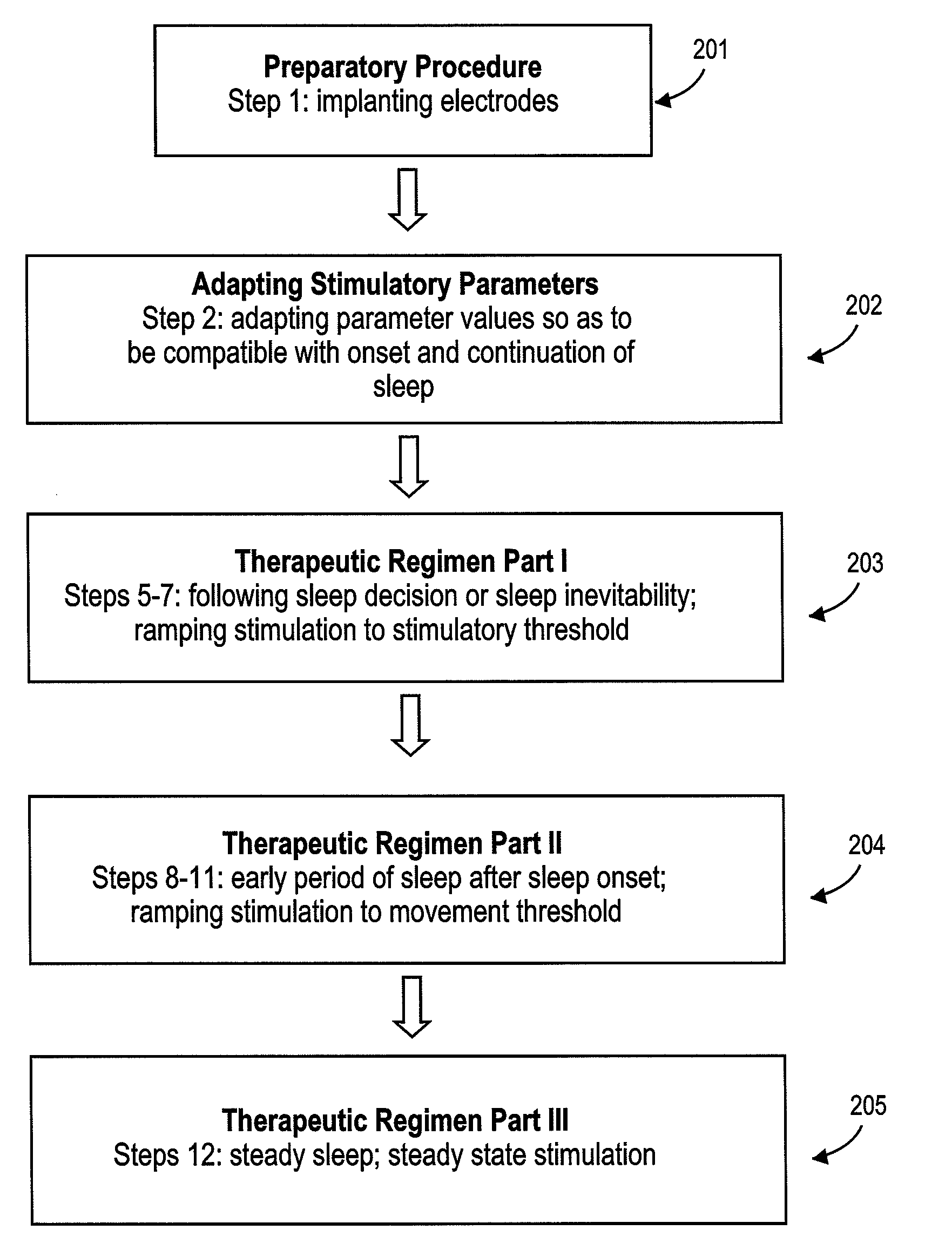
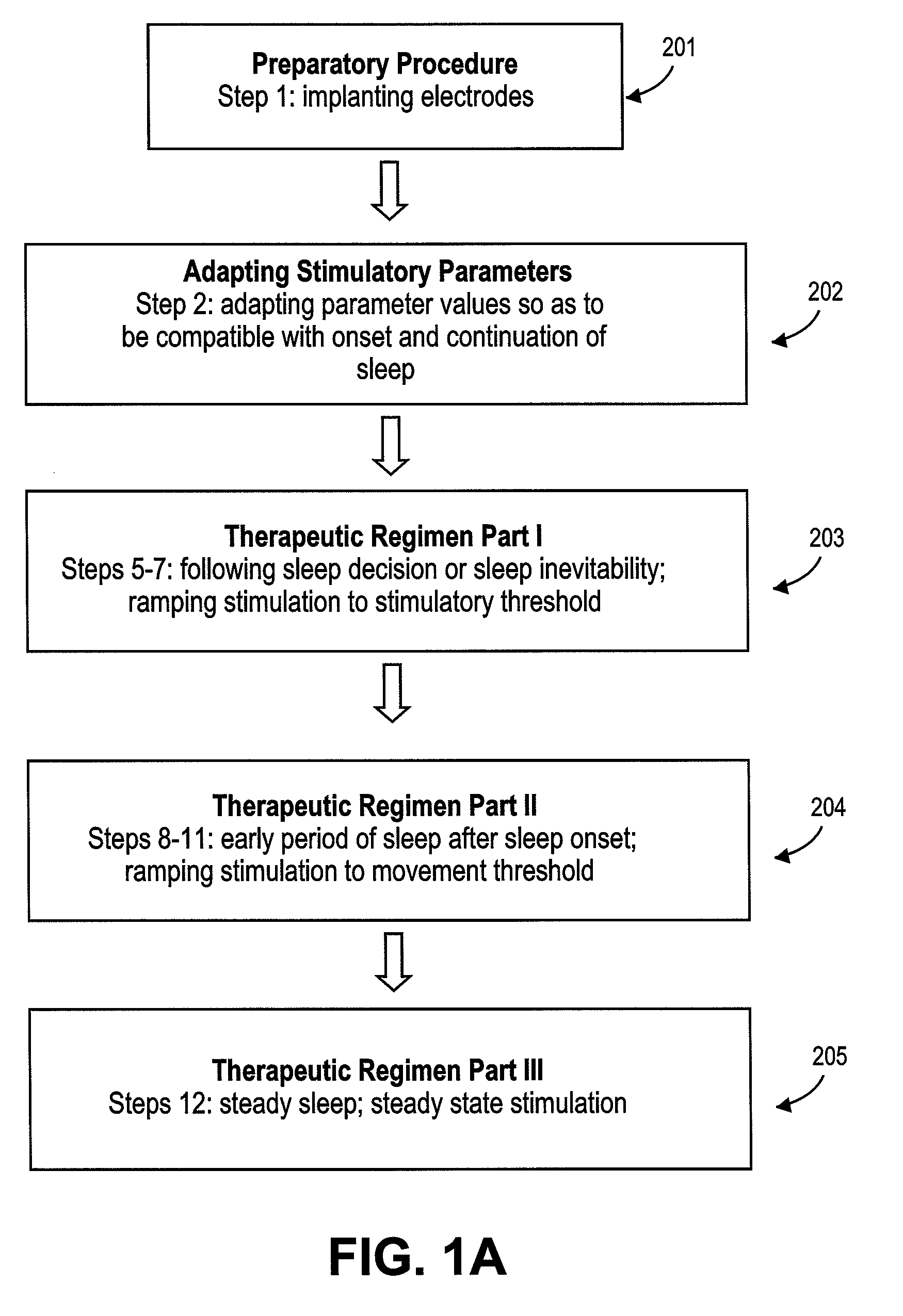
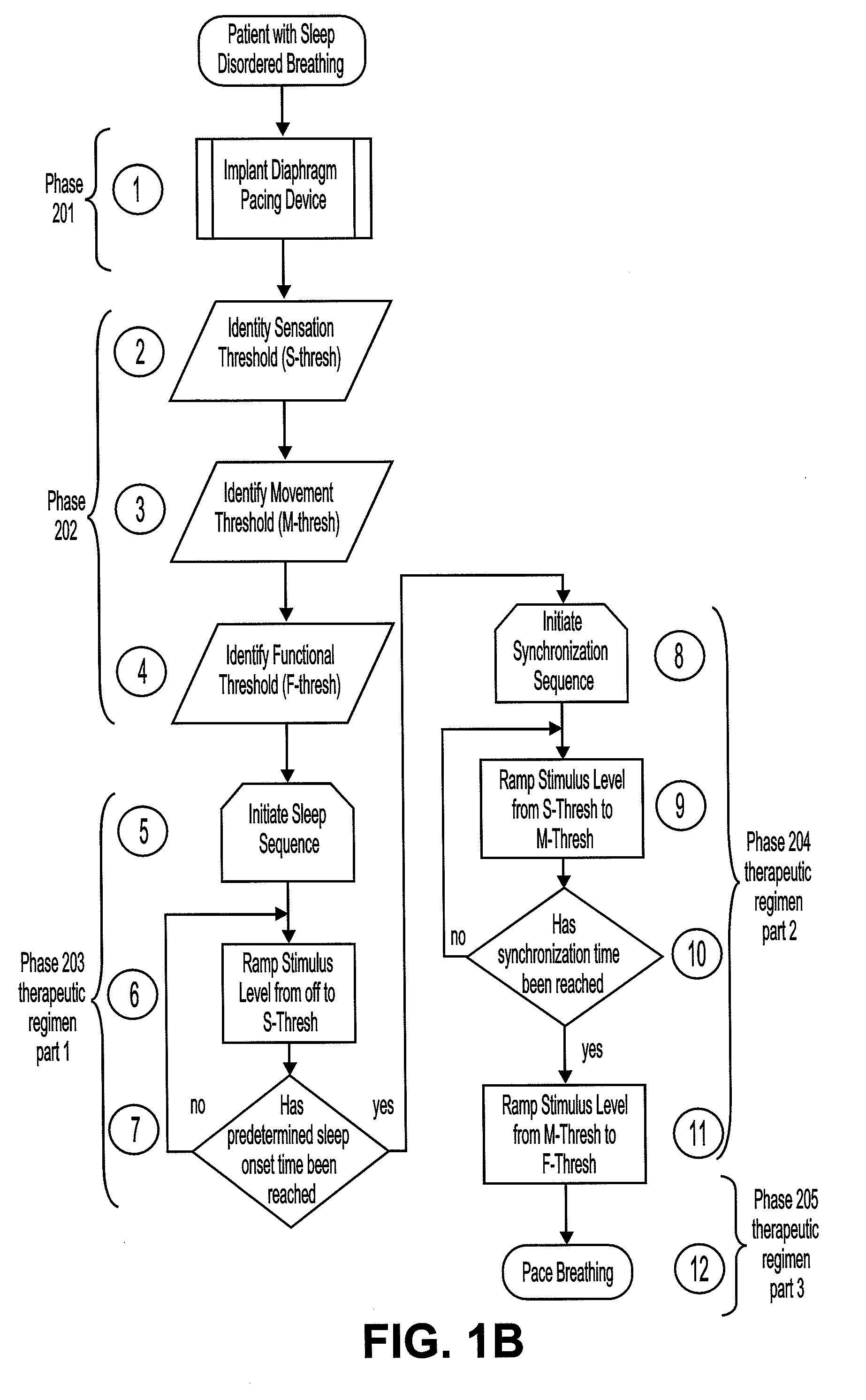
Method of improving sleep disordered breathing
ActiveUS8478412B2ElectrotherapyDiagnostic recording/measuringSleep disordered breathingSommeil paradoxal
A diaphragm pacing stimulatory method and a system to implement the method are provided to improve respiratory function and the quality of sleep in patients whose sleep is compromised by poor respiration. The diaphragm pacing method includes adaptations that make it particularly compatible with the onset of sleep and sustaining sleep. Embodiments of the method are operated independently of breathing effort the patient may make during sleep. Patients for whom the invention is appropriate include those with a neuromuscular disease, such as amyotrophic lateral sclerosis (ALS). System elements include an external electrical stimulator coupled to one or more implanted electrodes that stimulate diaphragm contraction. The system and method provide for a pacing of the diaphragm, improved breathing, and improved sleep. Features of improved sleep include longer sleep time, an increased amount of REM sleep, and fewer episodes of wakefulness and restlessness.
Owner:SYNAPSE BIOMEDICAL INC
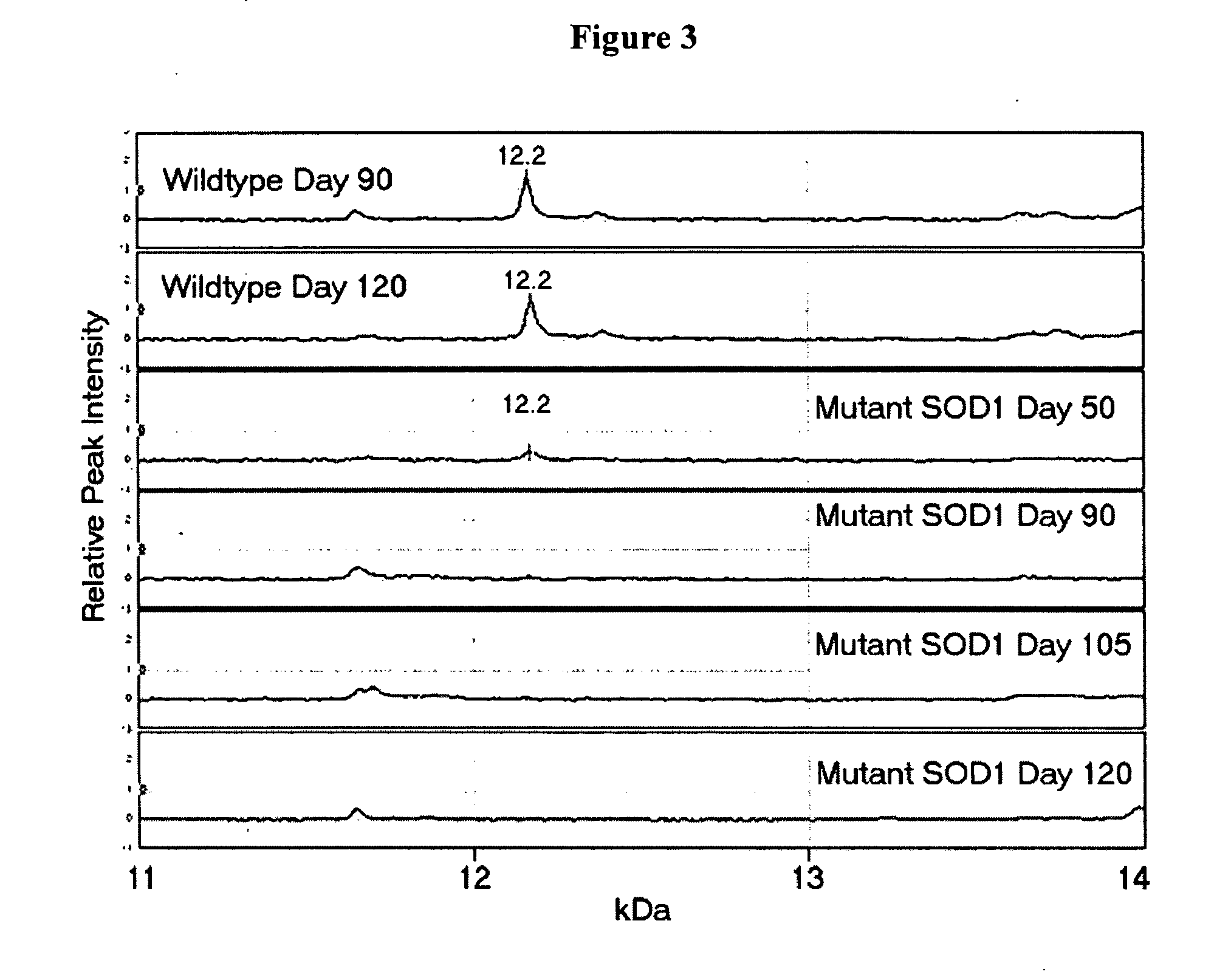
Mice having a mutant SOD-1-encoding transgene
Disclosed is the family of genes responsible for the neurodegenerative diseases, particularly Amyotrophic Lateral Sclerosis. Methods and compounds for the diagnosis, prevention, and therapy of the disease are also disclosed.
Owner:THE GENERAL HOSPITAL CORP +2
Combination of cell necrosis inhibitor and lithium for treating neuronal death or neurological dysfunction
InactiveUS20070049565A1Salicyclic acid active ingredientsBiocideDiabetic retinopathyHuntingtons chorea
The present invention relates to a combination of cell necrosis inhibitor and lithium, process for the preparation of the combination, pharmaceutical formulation containing the combination and use of the combination by either concomitant or sequential administration for improvement of treatment of neuronal death or neurological dysfunction. The combination of the present invention shows a synergic effect and thus is useful for treating neurological diseases, such as amyotrophic lateral sclerosis (ALS, Lou Gehrig's disease), Alzheimer's disease, Parkinson's disease, Huntington's disease, stroke, traumatic brain injury or spinal cord injury; and for treating ocular diseases such as glaucoma, diabetic retinopathy or macular degeneration.
Owner:NEUROTECH PHARMA
Compositions for treating neurological disorders
InactiveUS20140038927A1Significant comprehensive benefitsLow dosBiocideNervous disorderHuntingtons choreaAlcoholisms
The present invention relates to compositions and methods for the treatment of neurological disorders related to glutamate excitotoxicity and Amyloid β toxicity. More specifically, the present invention relates to novel combinatorial therapies of Multiple Sclerosis, Alzheimer's disease, Alzheimer's disease related disorders, Amyotrophic Lateral Sclerosis, Parkinson's disease, Huntington's disease, neuropathic pain, alcoholic neuropathy, alcoholism or alcohol withdrawal, or spinal cord injury.
Owner:PHARNEXT
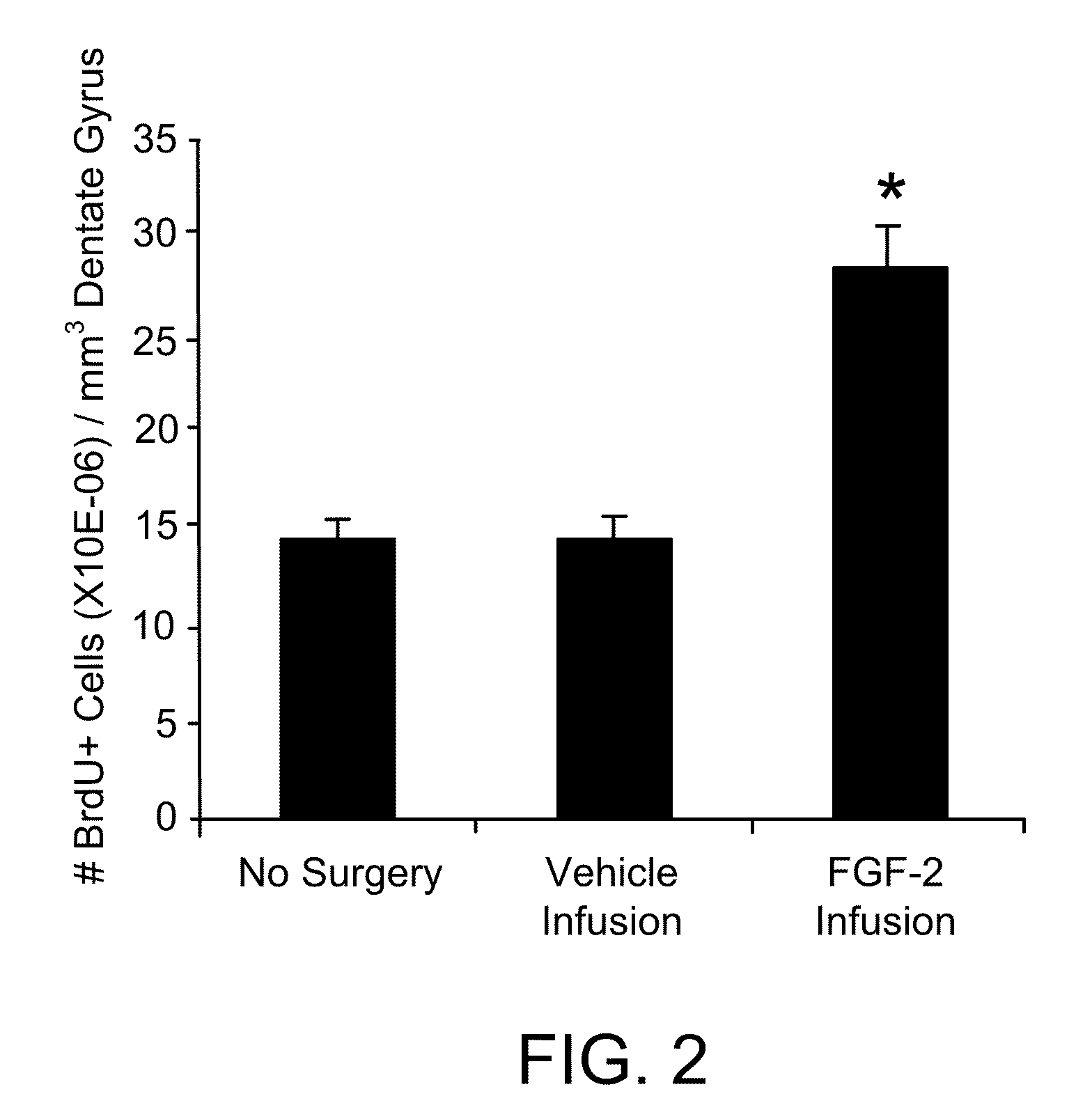
Generation of brachial, thoracic and lumbar spinal motor neurons from embryonic stem cells in the absence of all-trans retinoic acid supplement
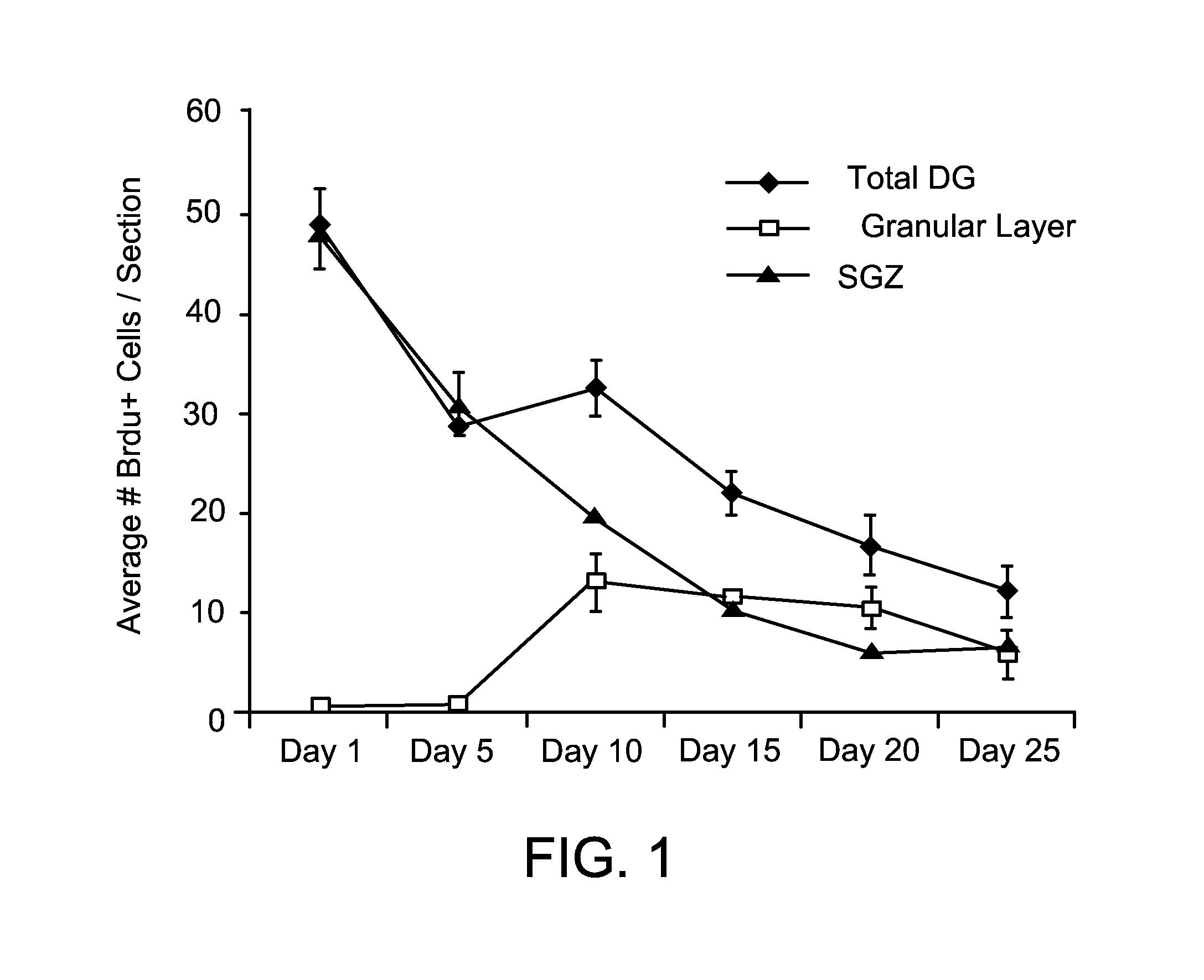
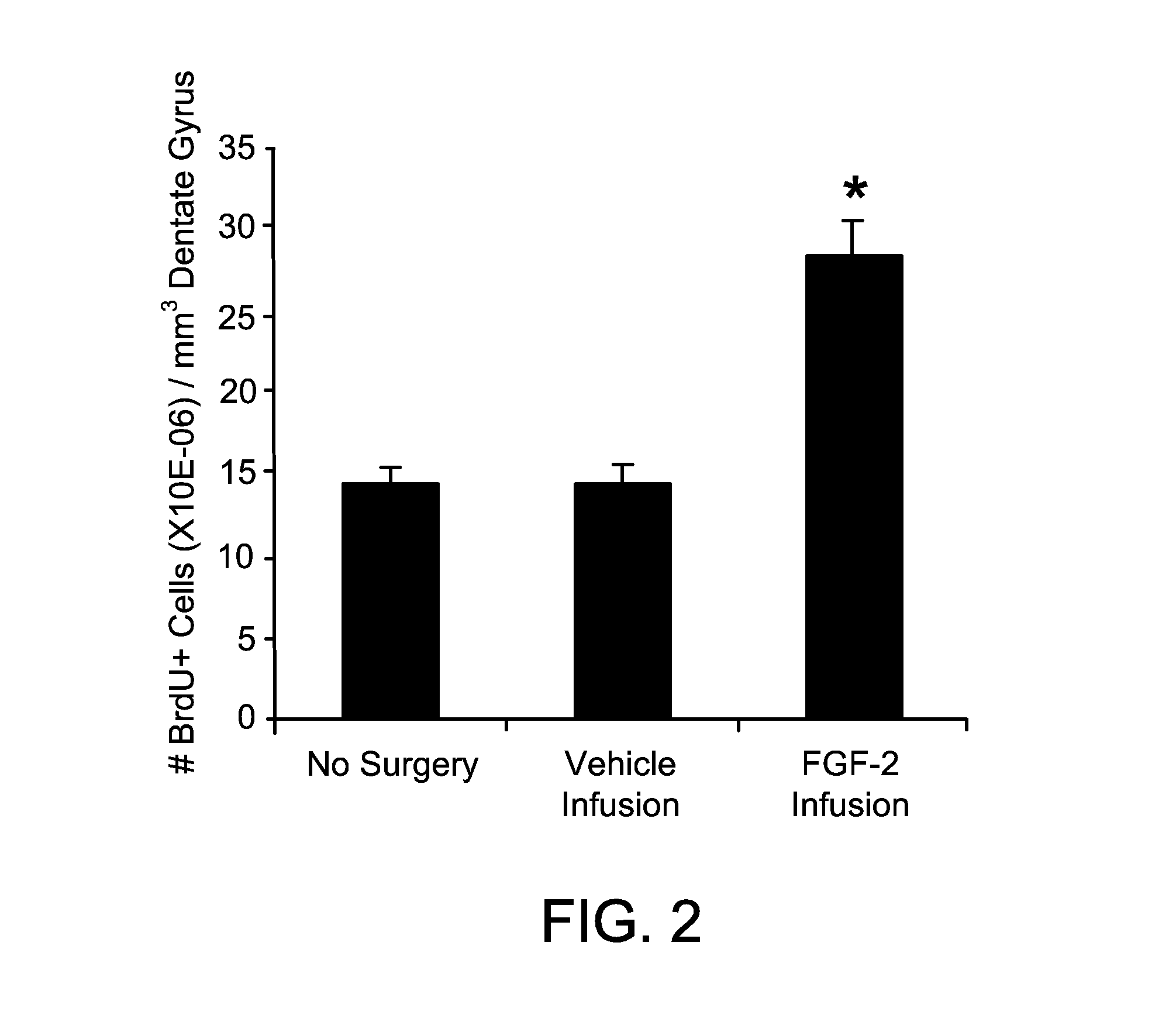
InactiveUS20100196332A1Increase the number ofAvoid signalingBiocidePeptide/protein ingredientsFOXP1Retinoid
Disclosed are methods for generating a neuron expressing Hoxc8 transcription factor or a caudal motor neuron comprising culturing an embryonic stem cell in a composition which is essentially free of retinoids and comprises an isotonic salt solution, so as to generate the neuron which expresses Hoxc8 transcription factor or the caudal motor neuron. Disclosed are also methods for generating a caudal brachial motor neuron, a thoracic motor neuron, or a lumbar motor neuron from an embryonic stem cell in a composition essentially free of retinoids and comprising ADFNK medium, an amount of FGF-2, or Gdf11 respectively. Disclosed are also methods of transplanting a motor neuron into a subject comprising generating the motor neuron and transplanting the motor neuron into the subject. Disclosed is also a population of motor neuron cells enriched for motor neuron cells expressing Foxp1 and expressing a gene associated with Spinal Muscular Atrophy (SMA) or Amyotrophic Lateral Sclerosis (ALS).
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com