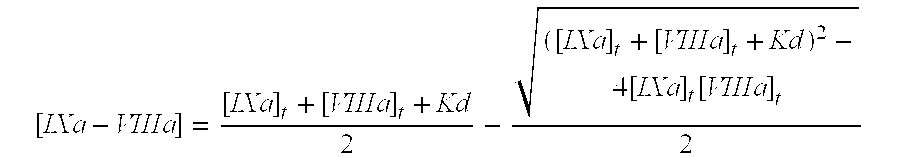Patents
Literature
1688 results about "Factor ii" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Factor II deficiency is a very rare blood clotting disorder. It results in excessive or prolonged bleeding after an injury or surgery. Factor II, also known as prothrombin, is a protein made in your liver. It plays an essential role in blood clot formation. It is one of about 13 clotting factors involved in the proper formation of blood clots.
Combined use of anti-cytokine antibodies or antagonists and anti-CD20 for treatment of B cell lymphoma
InactiveUS20020012665A1Avoiding and decreasing and resistanceOrganic active ingredientsIn-vivo radioactive preparationsFactor iiBiological activation
The present invention discloses combined therapies for treating hematologic malignancies, including B cell lymphomas and leukemias or solid non-hematologic tumors, comprising administration of anti-cytokine antibodies or antagonists to inhibit the activity of cytokines which play a role in perpetuating the activation of B cells. The administration of such antibodies and antagonists, particularly anti-IL10 antibodies and antagonists, is particularly useful for avoiding or decreasing the resistance of hematologic malignant cells or solid tumor cells to chemotherapeutic agents and anti-CD20 or anti-CD22 antibodies. The invention also provides combination therapies for solid tumors having B cell involvement comprising the administration of an anti-cytokine antibody and a B cell depleting antibody such as RITUXAN(R).
Owner:BIOGEN INC
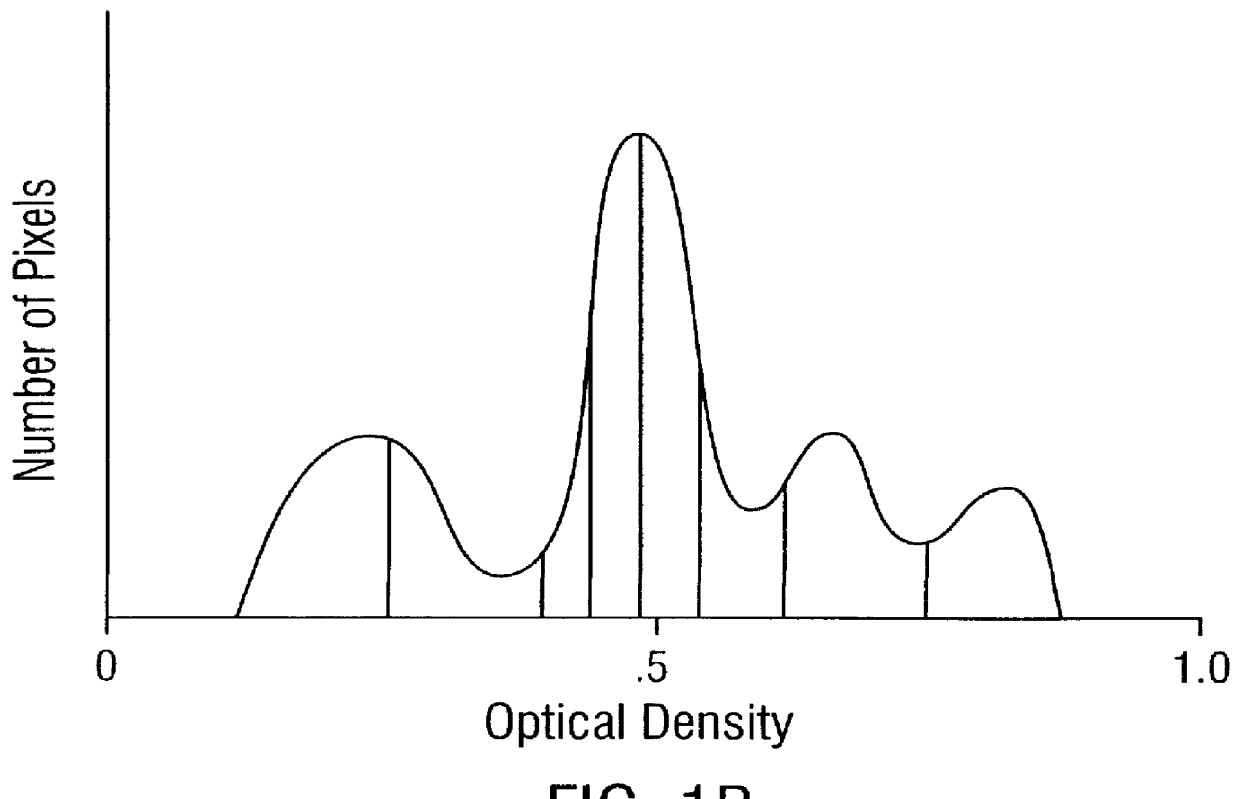
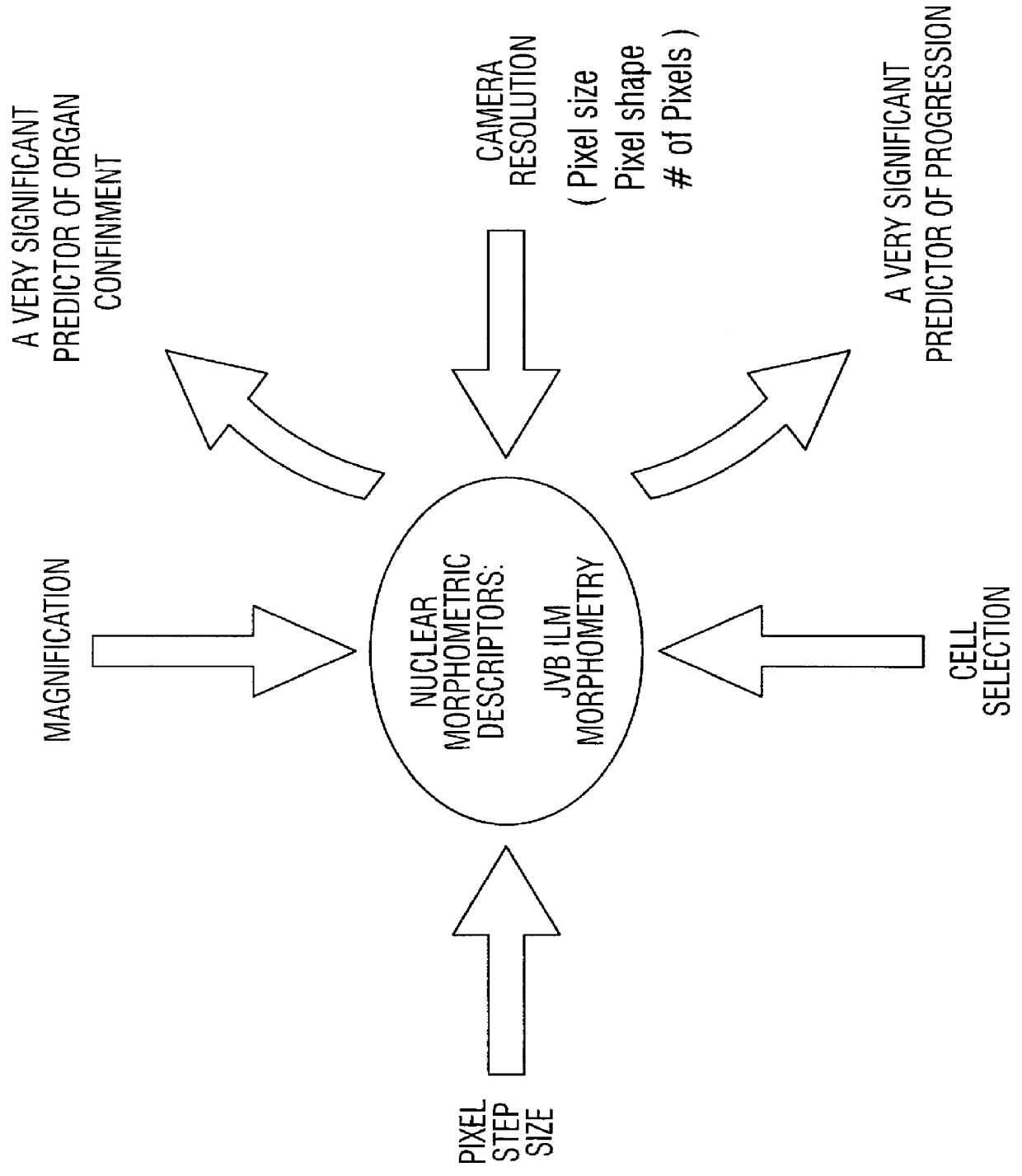
Prediction of prostate cancer progression by analysis of selected predictive parameters
InactiveUS6025128AImprove performanceMicrobiological testing/measurementBiological testingStatistical analysisFactor ii
A method for screening individuals at risk for prostate cancer progression is disclosed. The method is useful for evaluating cells from patients at risk for recurrence of prostate cancer following surgery for prostate cancer. Specifically, the method uses specific Markovian nuclear texture factors, alone or in combination with other biomarkers, to determine whether the cancer will progress or lose organ confinement. In addition, methods of predicting the development of fatal metastatic disease by statistical analysis of selected biomarkers is also disclosed. The invention also contemplates a method that uses a neural network to analyze and interpret cell morphology data. Utilizing Markovian factors and other biomarkers as parameters, the network is first trained with a sets of cell data from known progressors and known non-progressors. The trained network is then used to predict prostate cancer progression in patient samples.
Owner:CYTODIAGNOSTICS +3
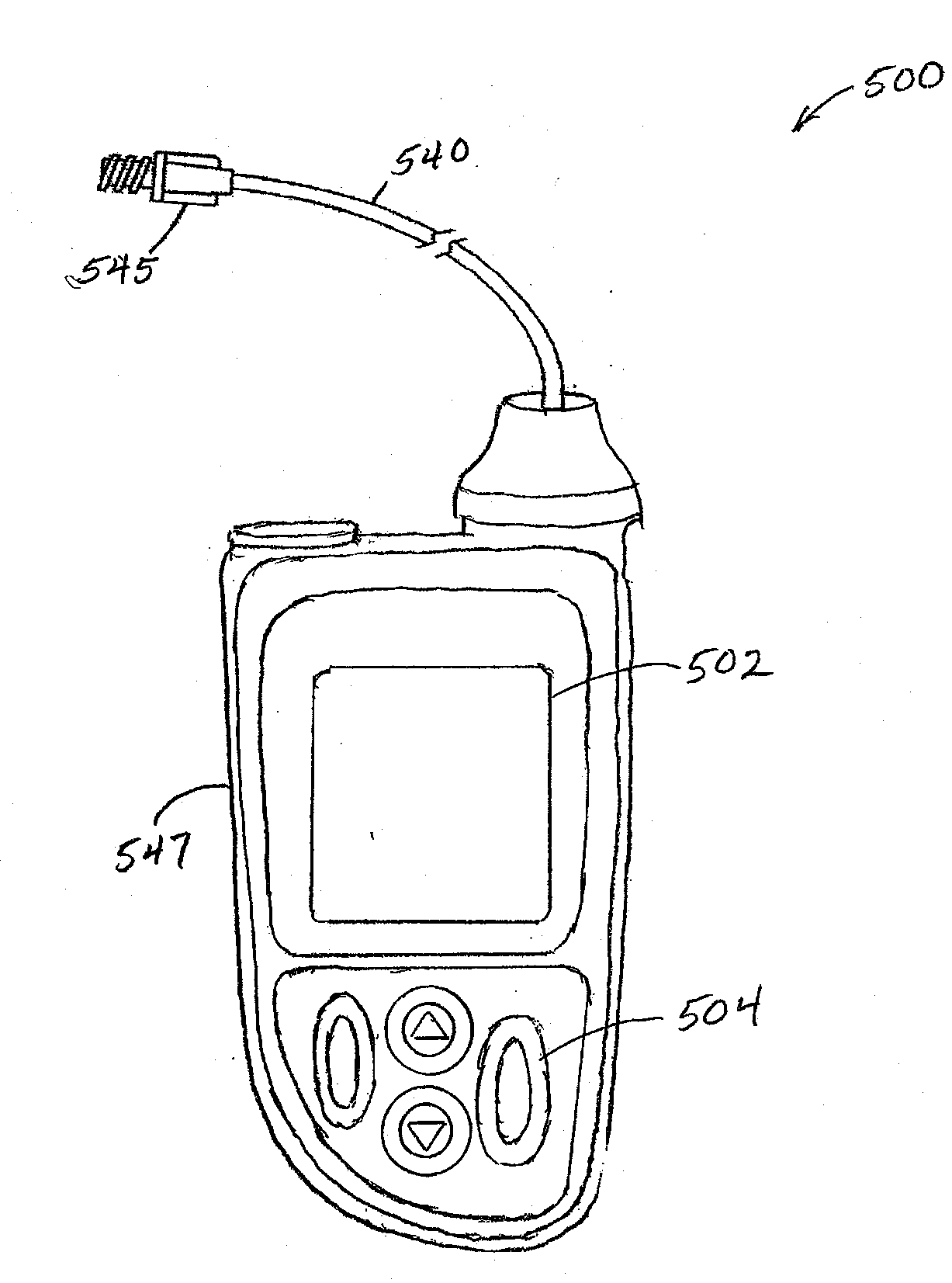
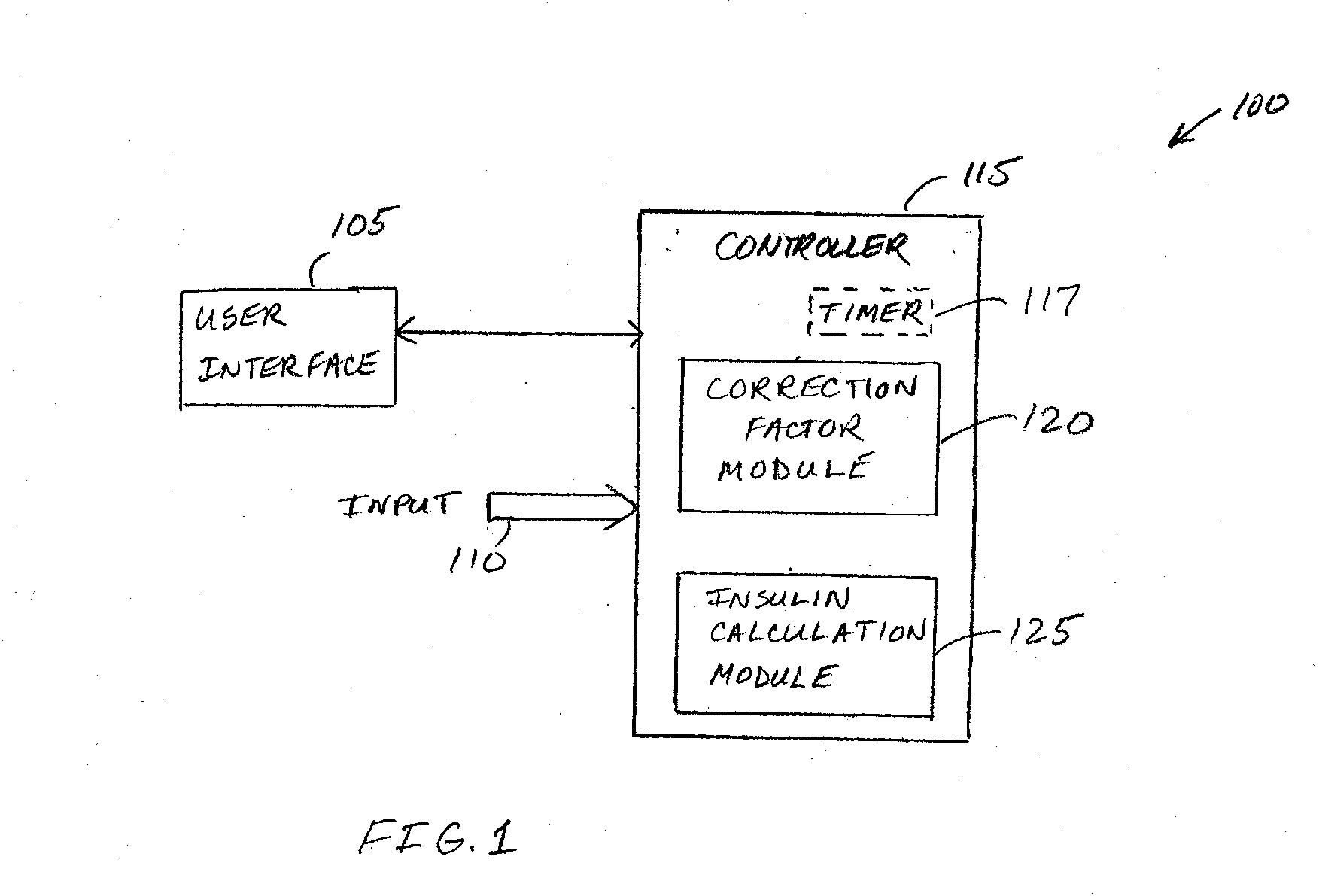
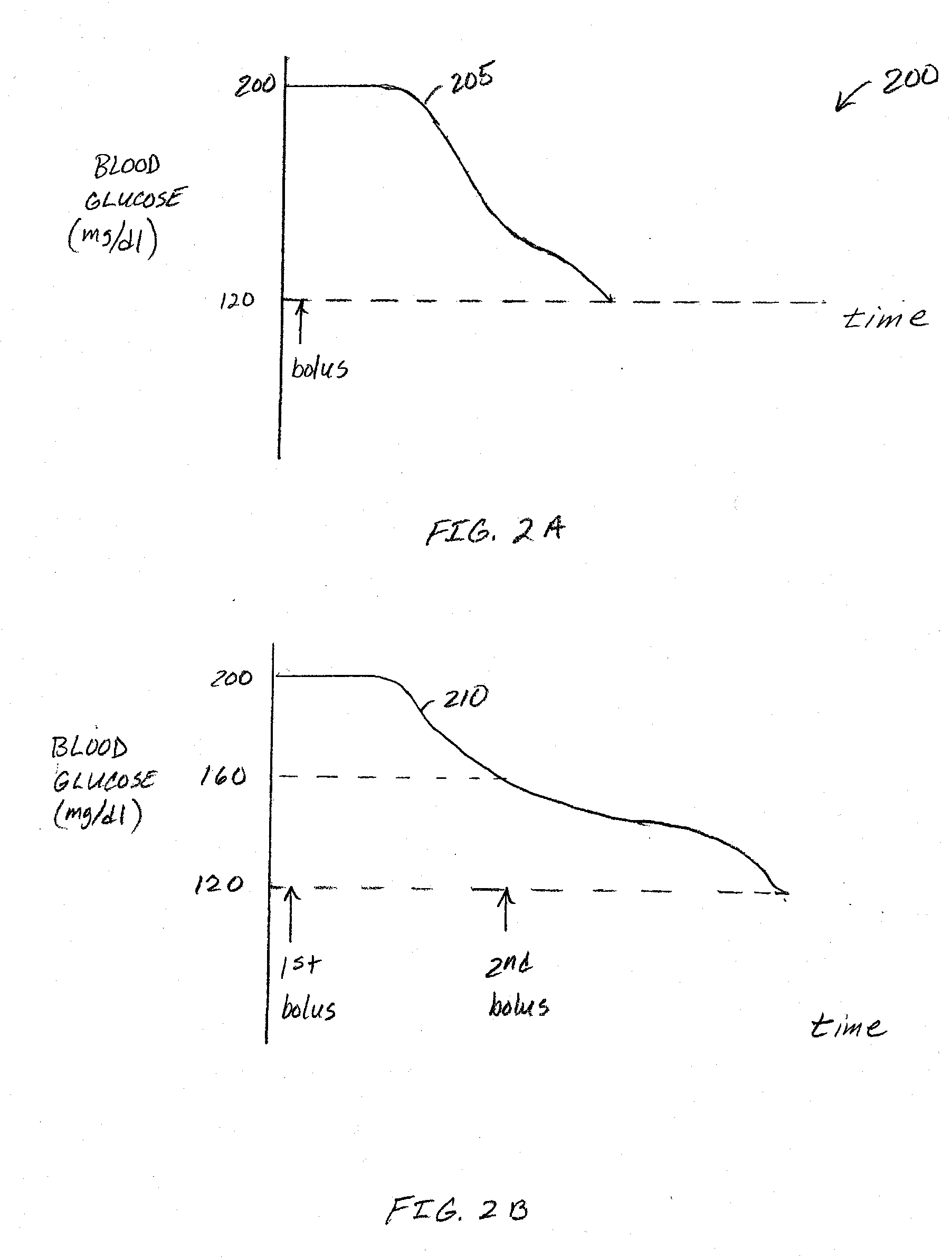
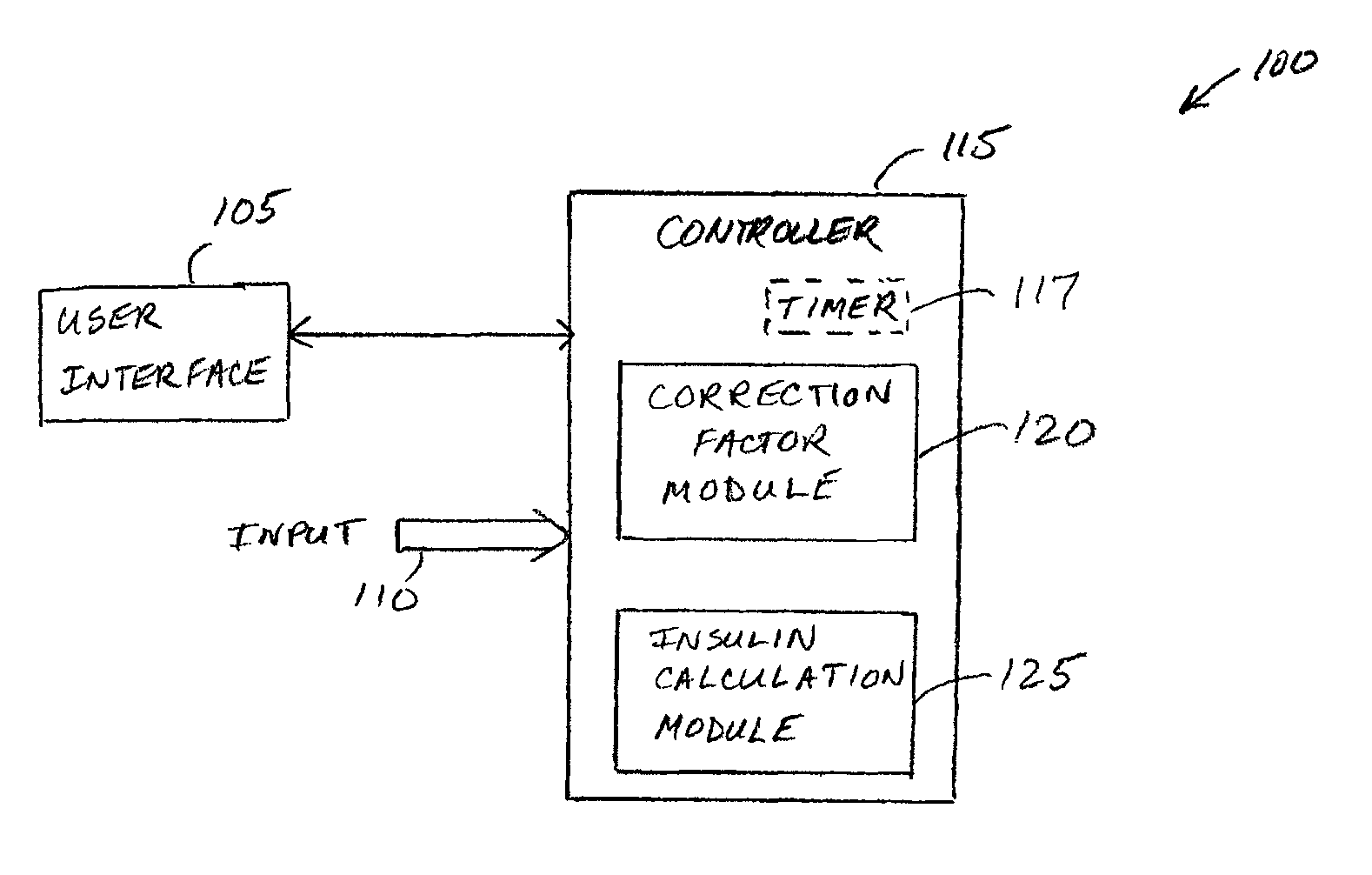
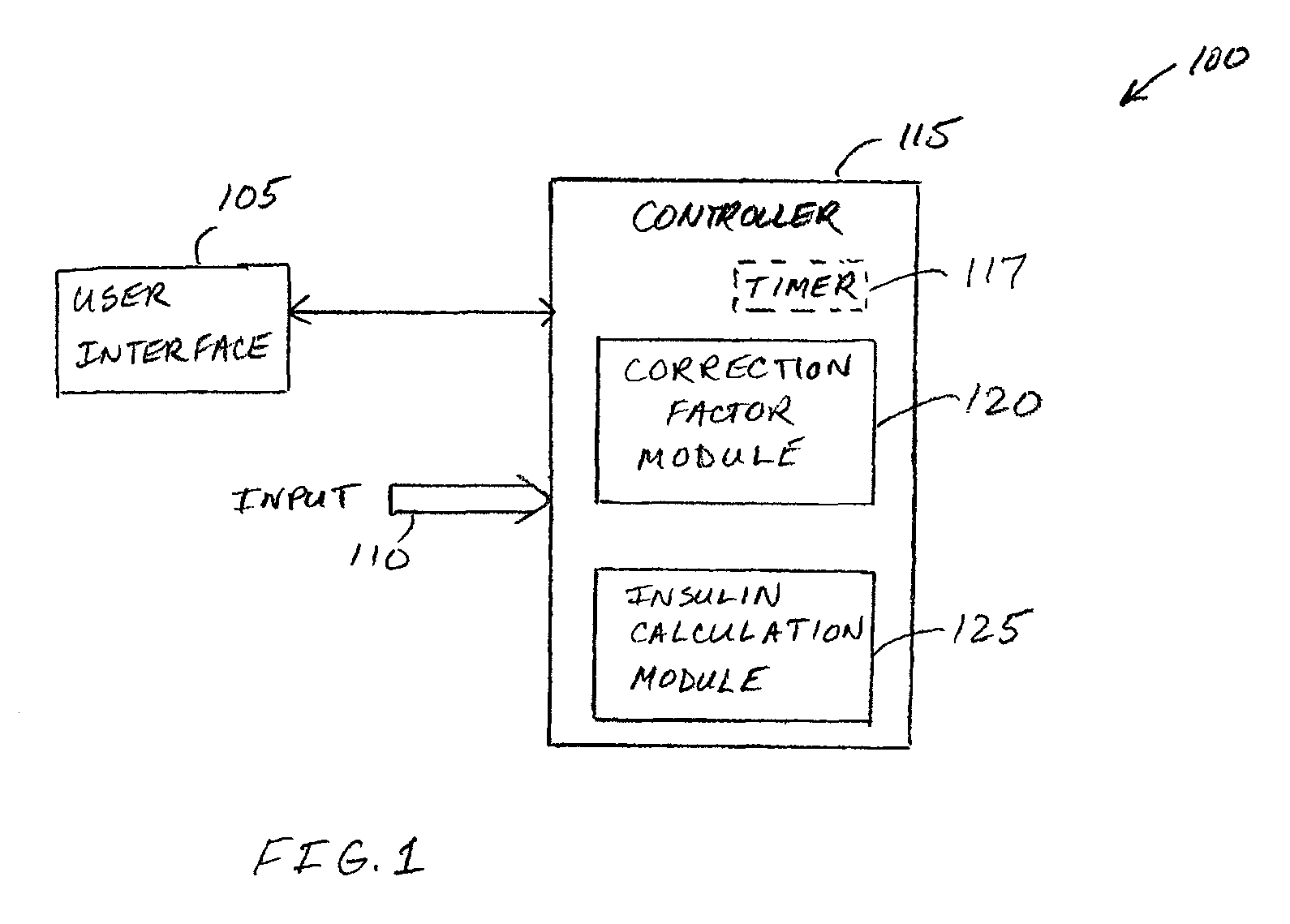
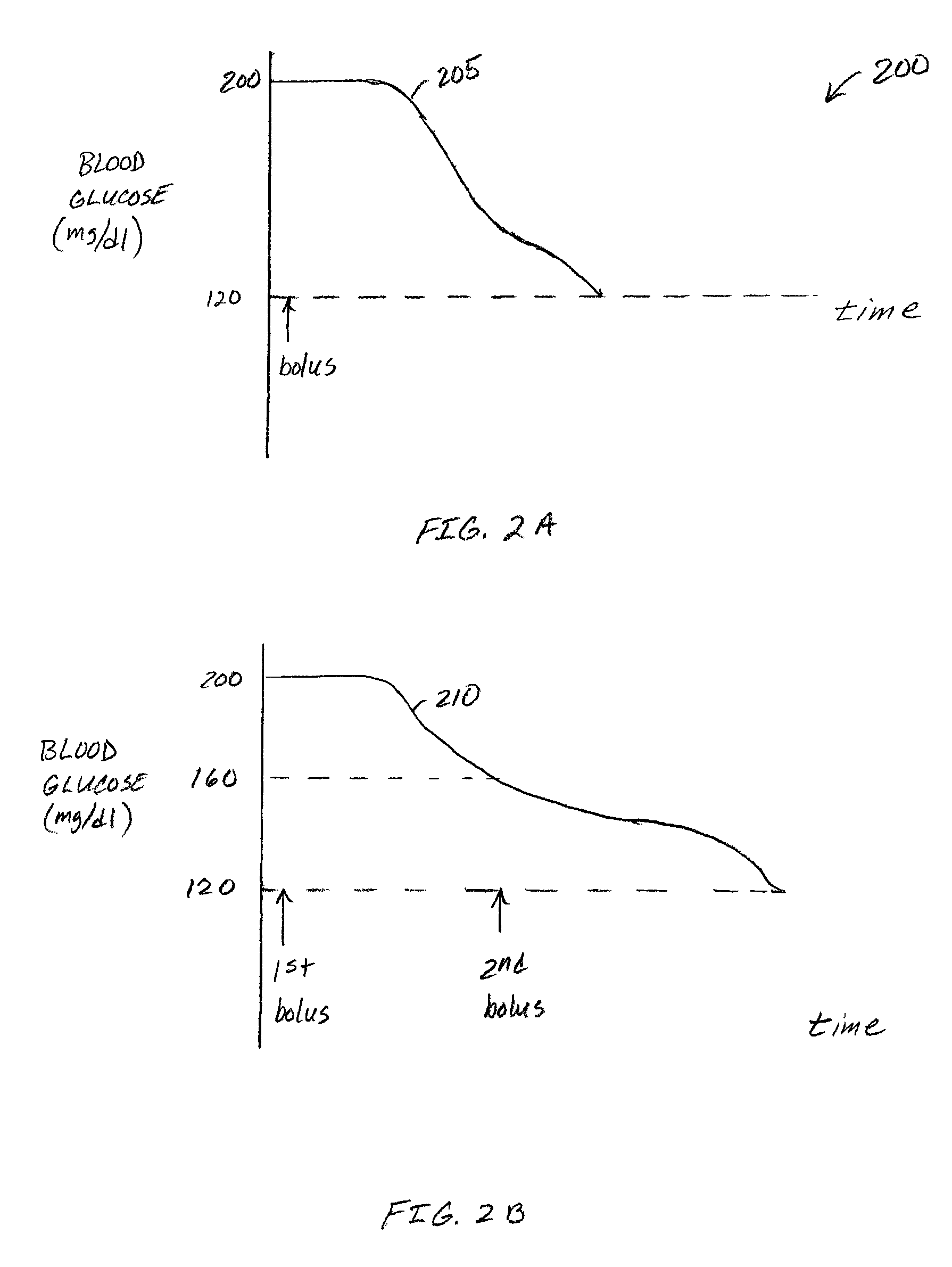
Correction factor testing using frequent blood glucose input
An apparatus comprising a user interface configured to generate an electrical signal to begin determination of an effective correction factor when prompted by a user, an input configured to receive sampled blood glucose data of a patient that is obtained during a specified time duration, including a time duration after delivery of an initial insulin correction bolus, and a controller in electrical communication with the input and the user interface. The controller includes a correction factor module configured for determining an effective correction factor according to an amount of insulin in the initial insulin correction bolus and a decrease in the blood glucose level determined using the sampled blood glucose data. Other devices and methods are disclosed.
Owner:TANDEM DIABETES CARE INC
Formulations for factor IX
InactiveUS6372716B1Pharmaceutical delivery mechanismSaccharide peptide ingredientsFactor iiFactor IX
Owner:GENETICS INST INC
Protein complexes having Factor VIII:C activity and production thereof
InactiveUS6060447AImprove stabilityHigh yieldPeptide/protein ingredientsMammal material medical ingredientsFactor iiADAMTS Proteins
Recombinant protein complexes having human Factor VIII:C activity are expressed in a eukaryotic host cell by transforming the host cell with first and second expression cassettes encoding a first polypeptide substantially homologous to human Factor VIII:C A domain and a second polypeptide substantially homologous to human Factor VIII:C C domain, respectively. In the present invention, the first polypeptide may be extended having at its C-terminal a human Factor VIII:C B domain N-terminal peptide, a polypeptide spacer of 3-40 amino acids, and a human Factor VIII:C B domain C-terminal peptide. Expression of the second polypeptide is improved by employing an .alpha..sub.1 -antitrypsin signal sequence.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Correction factor testing using frequent blood glucose input
An apparatus comprising a user interface configured to generate an electrical signal to begin determination of an effective correction factor when prompted by a user, an input configured to receive sampled blood glucose data of a patient that is obtained during a specified time duration, including a time duration after delivery of an initial insulin correction bolus, and a controller in electrical communication with the input and the user interface. The controller includes a correction factor module configured for determining an effective correction factor according to an amount of insulin in the initial insulin correction bolus and a decrease in the blood glucose level determined using the sampled blood glucose data. Other devices and methods are disclosed.
Owner:TANDEM DIABETES CARE INC
Preparation of recombinant factor VIII in a protein free medium
InactiveUS6171825B1Eliminate and at least greatly reduce riskImprove productivityFactor VIICulture processFactor iiManganese
Recombinant Factor VIII can be produced in relatively large quantities on a continuous basis from mammalian cells in the absence of any animal-derived proteins such as albumin by culturing the cells in a protein free medium supplemented with polyol copolymers, preferably in the presence of trace metals such as copper. In very preferred embodiments, the medium includes a polyglycol known as Pluronic F-68, copper sulfate, ferrous sulfate / EDTA complex, and salts of trace metals such as manganese, molybdenum, silicon, lithium and chromium. With an alternative medium which included trace copper ions alone (without polyol copolymers) we were also able to enhance the productivity of Factor VIII in recombinant cells such as BHK cells that are genetically engineered to express Factor VIII.
Owner:BAYER HEALTHCARE LLC +1
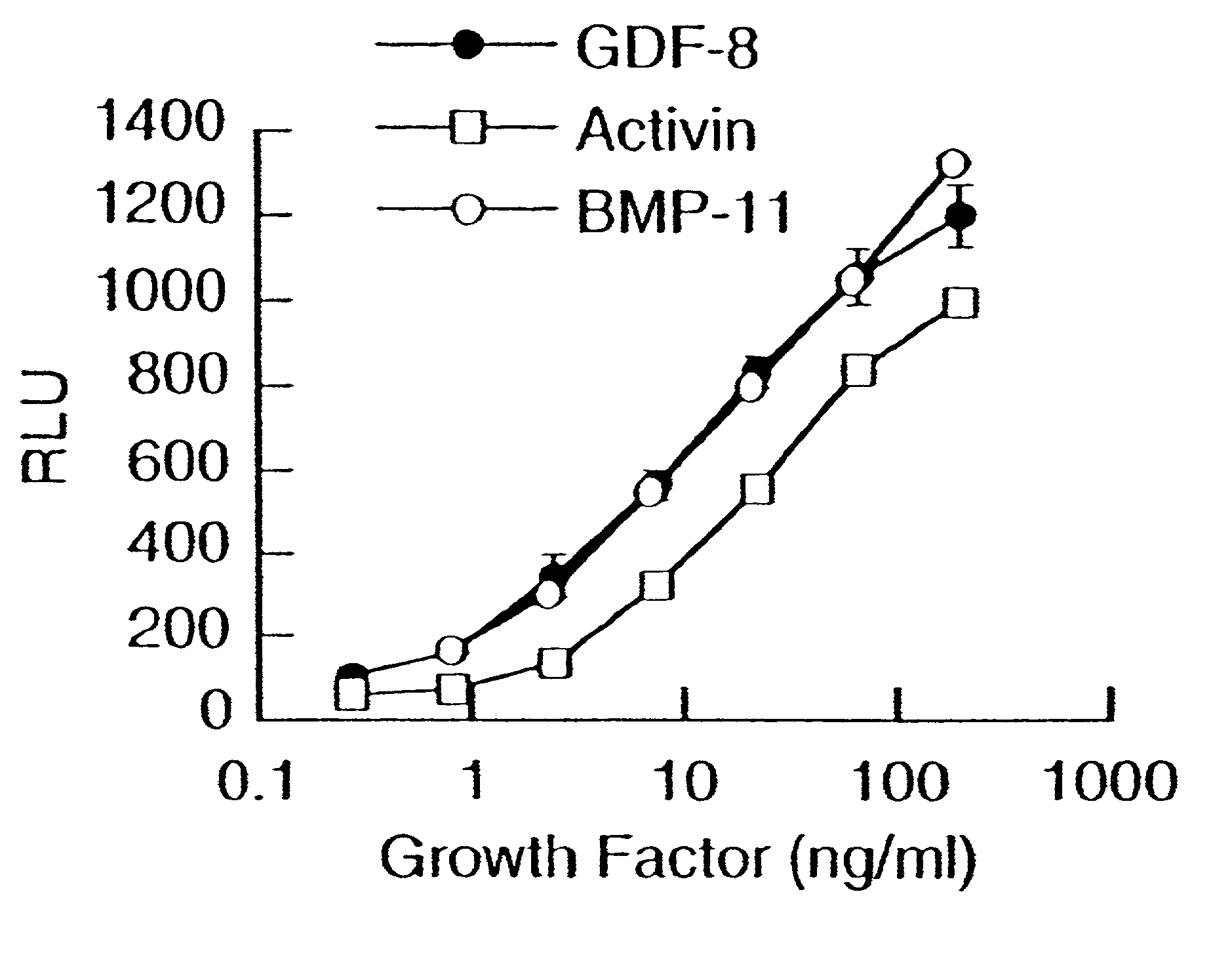
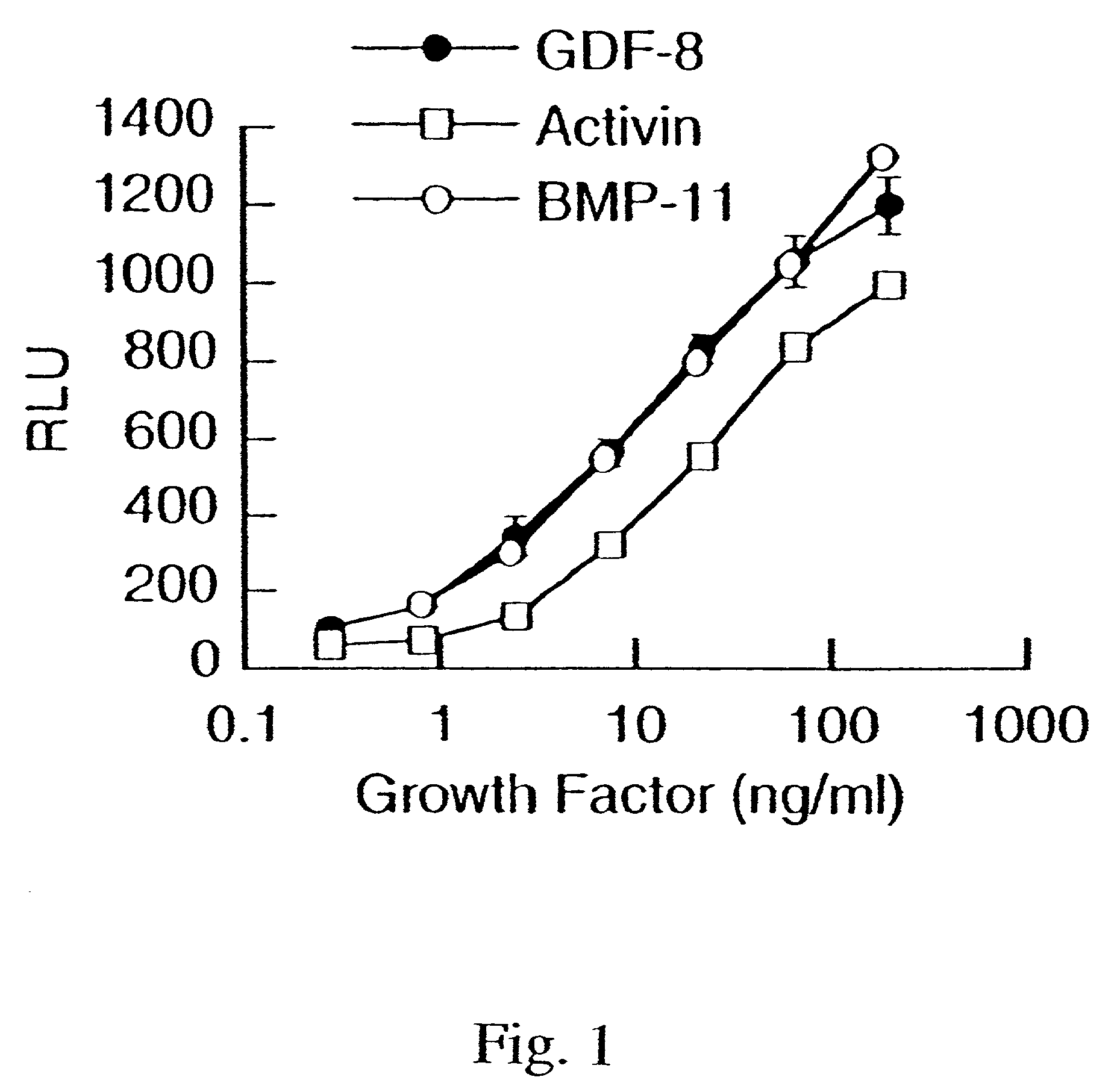
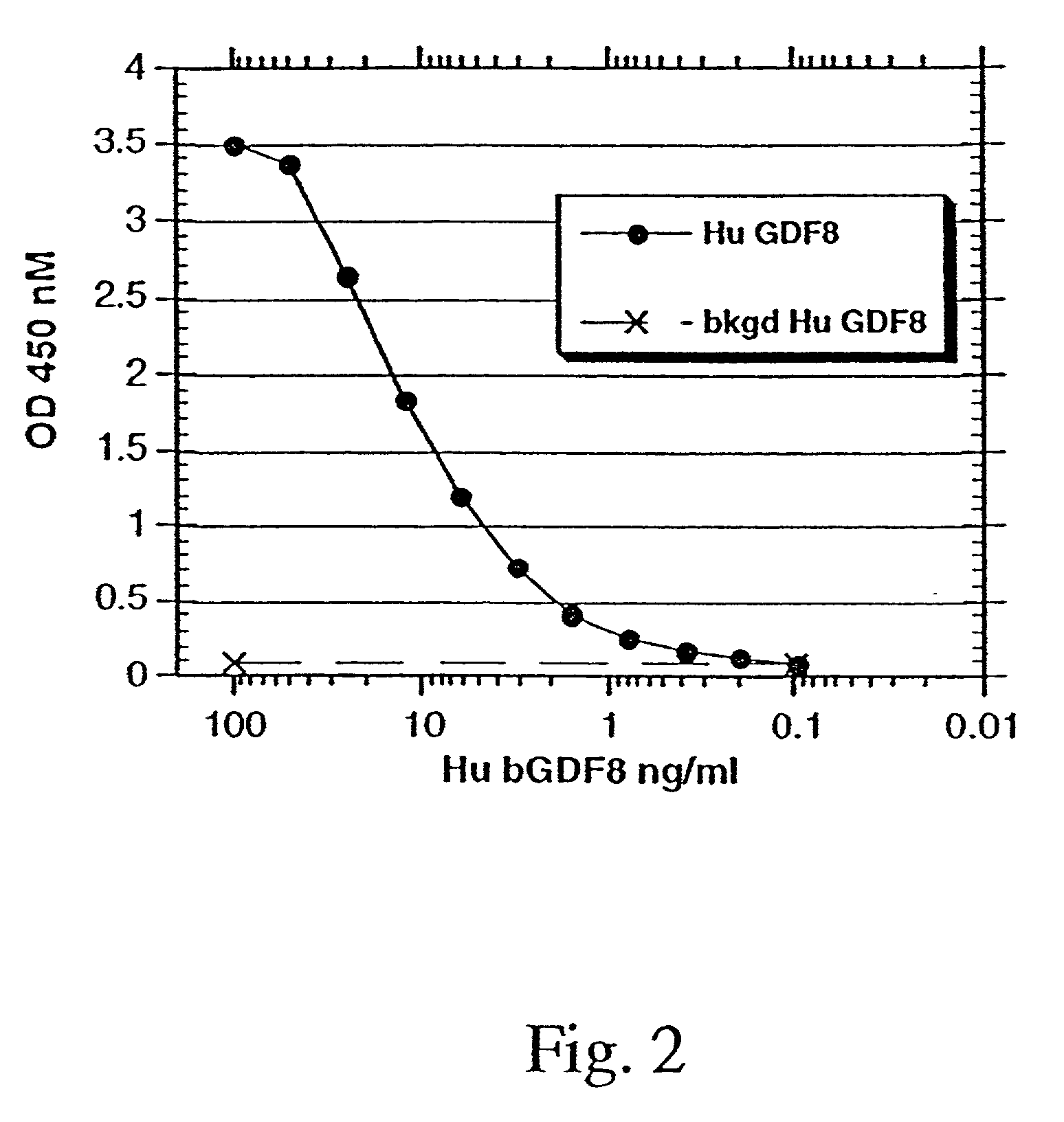
Modified and stabilized GDF propeptides and uses thereof
InactiveUS7202210B2Prevent practical therapeuticPrevent prophylactic utilityFungiBacteriaAcute hyperglycaemiaMuscle tissue
Modified and stabilized propeptides of Growth Differentiation Factor proteins, such as GDF-8 and Bone Morphogenetic Protein-11, are disclosed. Also disclosed are methods for making and using the modified propeptides to prevent or treat human or animal disorders in which an increase in muscle tissue would be therapeutically beneficial. Such disorders include muscle or neuromuscular disorders (such as amyotrophic lateral sclerosis, muscular dystrophy, muscle atrophy, congestive obstructive pulmonary disease, muscle wasting syndrome, sarcopenia, or cachexia), metabolic diseases or disorders (such as such as type 2 diabetes, noninsulin-dependent diabetes mellitus, hyperglycemia, or obesity), adipose tissue disorders (such as obesity), and bone degenerative diseases (such as osteoporosis).
Owner:WYETH LLC
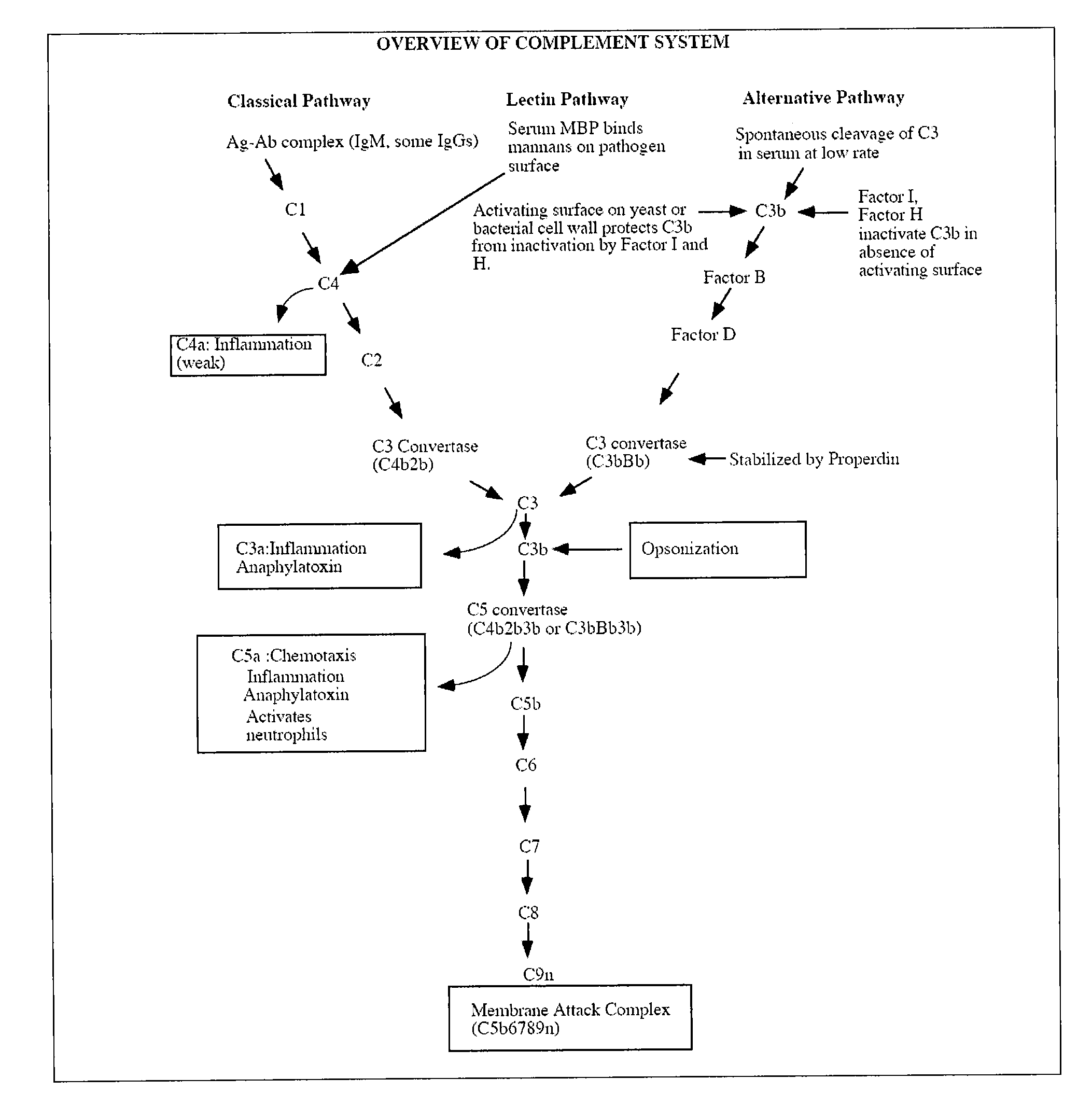
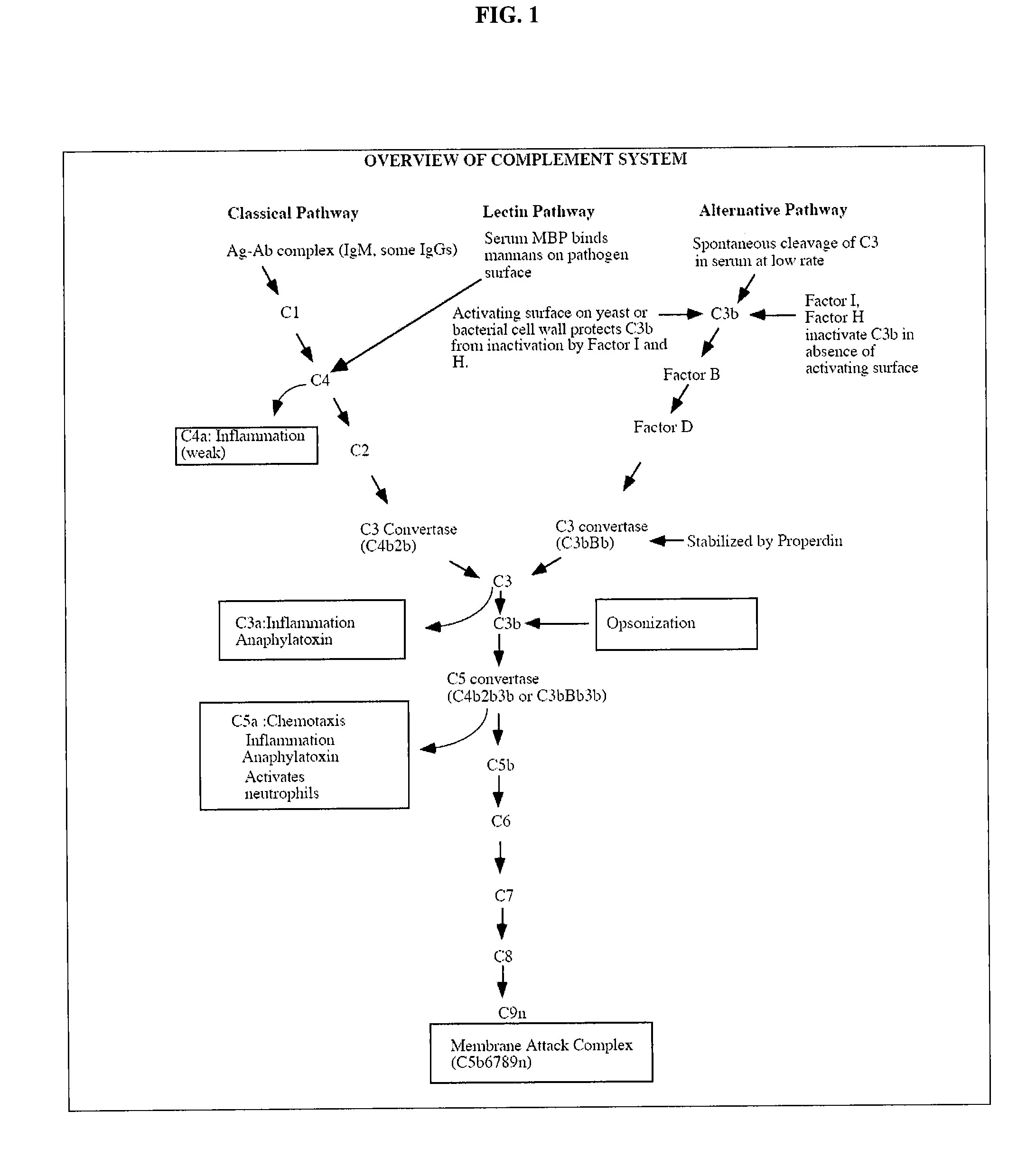
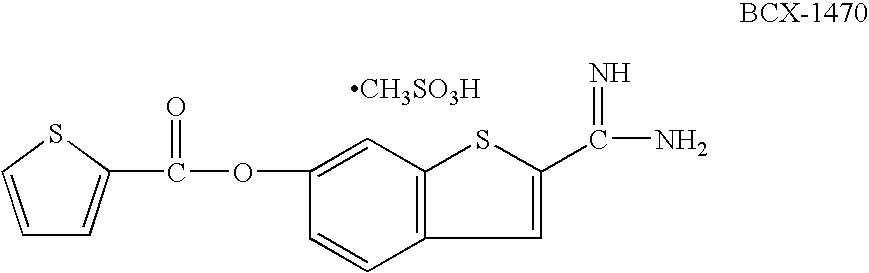
Compounds useful in the complement, coagulat and kallikrein pathways and method for their preparation
The present invention is concerned with new compounds, and particularly those having a fused bicyclic ring substituted with an amidine moiety. These compounds are each potent inhibitors of Factor D of the alternate pathway of complement, C1s of the classical pathway of complement, Factors Xa, XIIa, VIIa and thrombin of the coagulation pathway, plasmin in the fibrinolytic pathway, and kallikrein and high molecular weight kininogen in the inflammatory pathways. These proteases, which have serine in their active site, are called serine proteases and they are pivotal to most of the processes of inflammation and coagulation. In fact, these various systems are interactive with one another and it is difficult to activate one pathway without it influencing the others.
Owner:BIOCRYST PHARM INC
Modulation of hypoxia-inducible factor 1 alpha expression
InactiveUS7144999B2Modulate expressionSugar derivativesPeptide/protein ingredientsDiseaseHypoxia-Inducible Factor 1-Alpha
Compounds, compositions and methods are provided for modulating the expression of hypoxia-inducible factor 1 alpha. The compositions comprise oligonucleotides, targeted to nucleic acid encoding hypoxia-inducible factor 1 alpha. Methods of using these compounds for modulation of hypoxia-inducible factor 1 alpha expression and for diagnosis and treatment of disease associated with expression of hypoxia-inducible factor 1 alpha are provided.
Owner:IONIS PHARMA INC
Media and method for treating pathological syndrome
InactiveUS7572441B2Energy modified materialsImmunoglobulins against cytokines/lymphokines/interferonsNatural antibodyUltra low dose
A medicament based on antibodies contains an activated form of monoclonal, polyclonal, or natural antibodies to interferon in low or ultra-low doses prepared by multiple consecutive dilutions and exposure to external factors, preferably in accordance with homeopathic technology. In order to obtain antibodies, human or heterologous interferon alpha, beta, or gamma, including recombinant interferon, is used; a mixture of various, mostly centimal, homeopathic dilutions being employed. A method of treating a pathologic syndrome, whose formation is affected by interferon, consists in the use of activated forms of antibodies to interferon alpha, beta, or gamma in low or ultra-low doses obtained by multiple consecutive dilutions and exposure to external factors.
Owner:EPSHTEIN OLEG I
RNA interference mediated inhibition of vascular endothelial growth factor and vascular endothelial growth factor receptor gene expression using short interfering nucleic acid (siNA)
InactiveUS20050171039A1Improves various propertyImprove the immunityBiocideSugar derivativesDiseaseFactor ii
The present invention concerns methods and reagents useful in modulating vascular endothelial growth factor (VEGF, VEGF-A, VEGF-B, VEGF-C, VEGF-D) and / or vascular endothelial growth factor receptor (e.g., VEGFR1, VEGFR2 and / or VEGFr3) gene expression in a variety of applications, including use in therapeutic, diagnostic, target validation, and genomic discovery applications. Specifically, the invention relates to small nucleic acid molecules, such as short interfering nucleic acid (siNA), short interfering RNA (siRNA), double-stranded RNA (dsRNA), micro-RNA (miRNA), and short hairpin RNA (shRNA) molecules capable of mediating RNA interference (RNAi) against VEGF and / or VEGFr gene expression and / or activity. The small nucleic acid molecules are useful in the diagnosis and treatment of cancer, proliferative diseases, and any other disease or condition that responds to modulation of VEGF and / or VEGFr expression or activity.
Owner:SIRNA THERAPEUTICS INC
METHODS OF ADMINISTERING ANTI-TNFalpha ANTIBODIES
InactiveUS20120177596A1Reduce in quantityPatient compliance is goodSenses disorderNervous disorderHuman tumorFactor ii
Methods of treating disorders in which TFNα activity is detrimental via biweekly, subcutaneous administration of human antibodies, preferably recombinant human antibodies, that specifically bind to human tumor necrosis factor α (hTNFα) are disclosed. The antibody may be administered with or without methotrexate. These antibodies have high affinity for hTNFα (e.g., Kd=10−8 M or less), a slow off rate for hTNFα dissociation (e.g., Koff=10−3 sec−1 or less) and neutralize hTNFα activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. Kits containing a pharmaceutical composition and instructions for dosing, and preloaded syringes containing pharmaceutical compositions are also encompassed by the invention.
Owner:ABBVIE BIOTECHNOLOGY LTD
Treatment of age-related macular degeneration using inhibitors of complement factor d
InactiveUS20080269318A1Avoid developmentInhibit the loss of visual acuityBiocideSenses disorderComplement Factor H GeneFactor ii
The present invention provides methods for identifying a patient at risk for developing AMD by identifying the presence of the Y402H polymorphism or other at risk variants in the complement factor H gene. The present invention further provides methods for treating persons having AMD or at risk for developing AMD as a result of having the Y402H polymorphism or other at risk variants in the complement factor H gene.
Owner:ALCON RES LTD
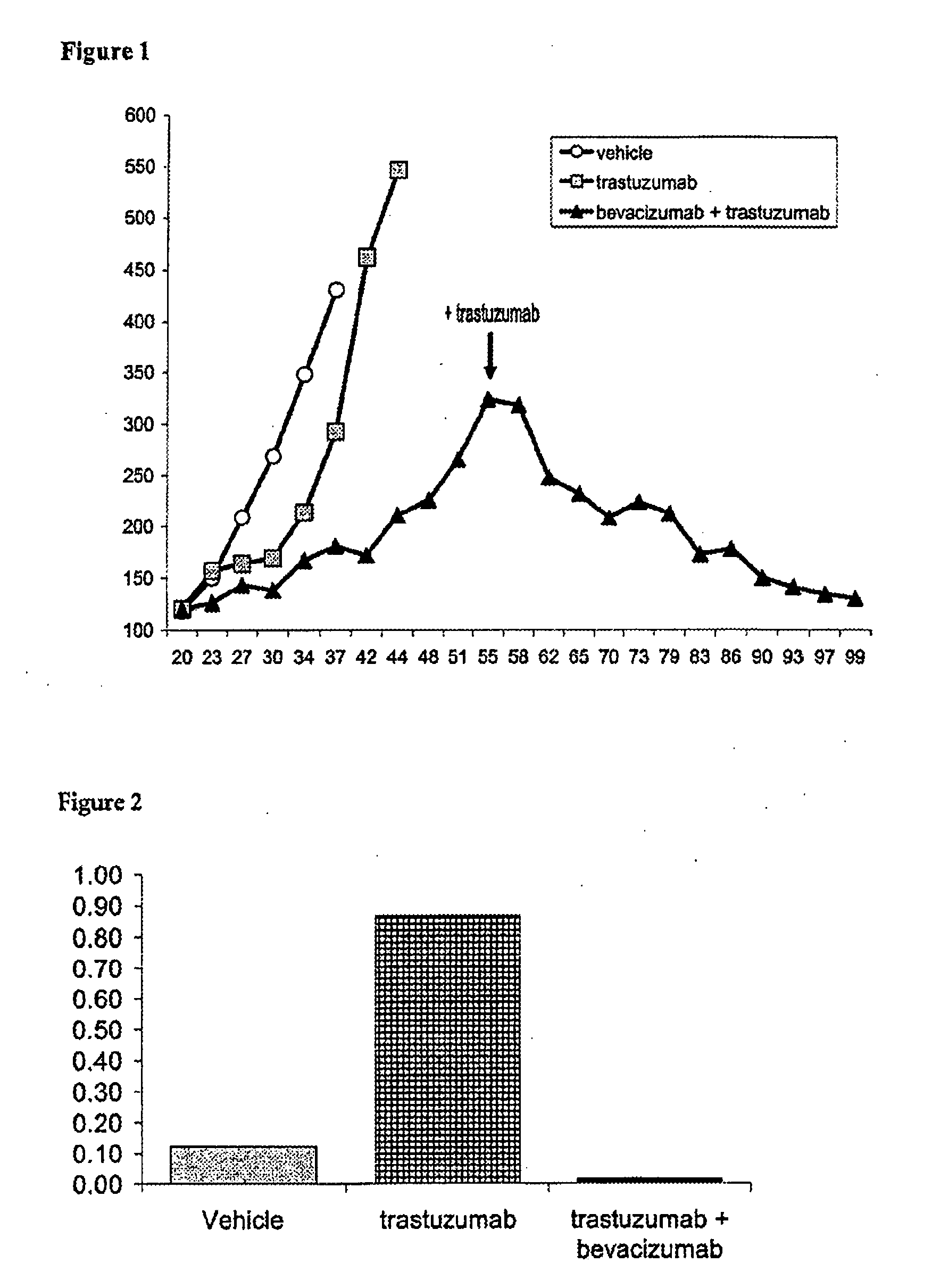
Tumor therapy with an antibody for vascular endothelial growth factor and an antibody for human epithelial growth factor receptor type 2
InactiveUS20070224203A1Prolong survival timeProlong progression-free survivalImmunoglobulins against growth factorsAntibody ingredientsDiseaseTumor therapy
The present invention provides a method of treating a breast cancer disease in a patient who has failed prior treatment with an anti-VEGF antibody, comprising administering to the patient a therapeutically effective amount of an anti-HER2 antibody while continuing said anti-VEGF antibody therapy. The invention also provides corresponding pharmaceutical kits and pharmaceutical compositions.
Owner:F HOFFMANN LA ROCHE & CO AG
Positive AMPA receptor modulation to enhance brain neurotrophic factor expression
InactiveUS6030968AImprove the level ofHigh expressionHeterocyclic compound active ingredientsFactor iiCiliary neurotrophic factor
Methods for increasing the level of neurotrophic factors and neurotrophic factor receptors in the brain of a mammal afflicted with a pathology which produces neurodegeneration without significant loss of memory or learning comprising administering to a mammal an effective amount of an allosteric upmodulator of alpha -amino-3-hydroxy-5-methyl-isoxazole-4-proprionic acid (AMPA) receptors.
Owner:RGT UNIV OF CALIFORNIA
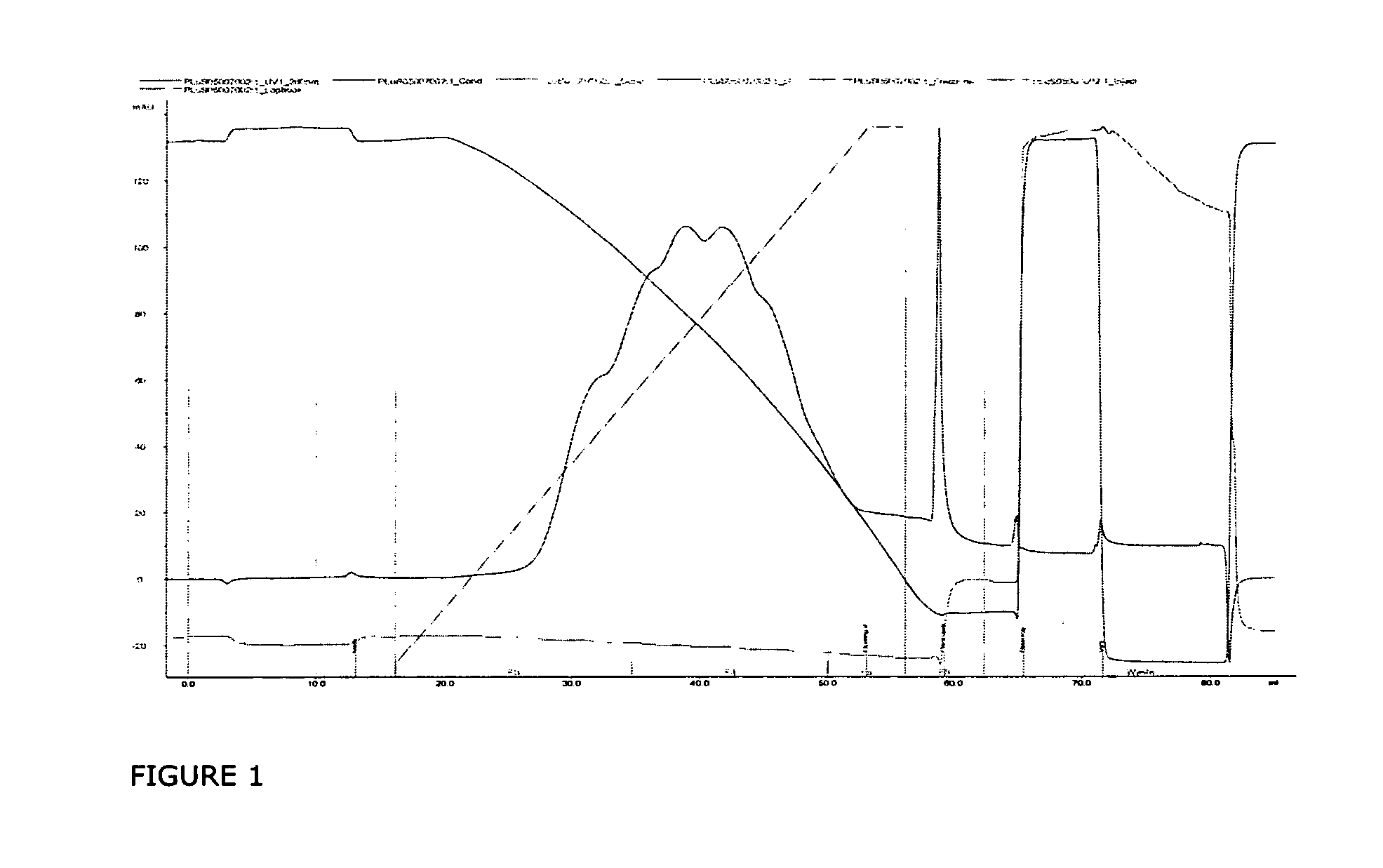
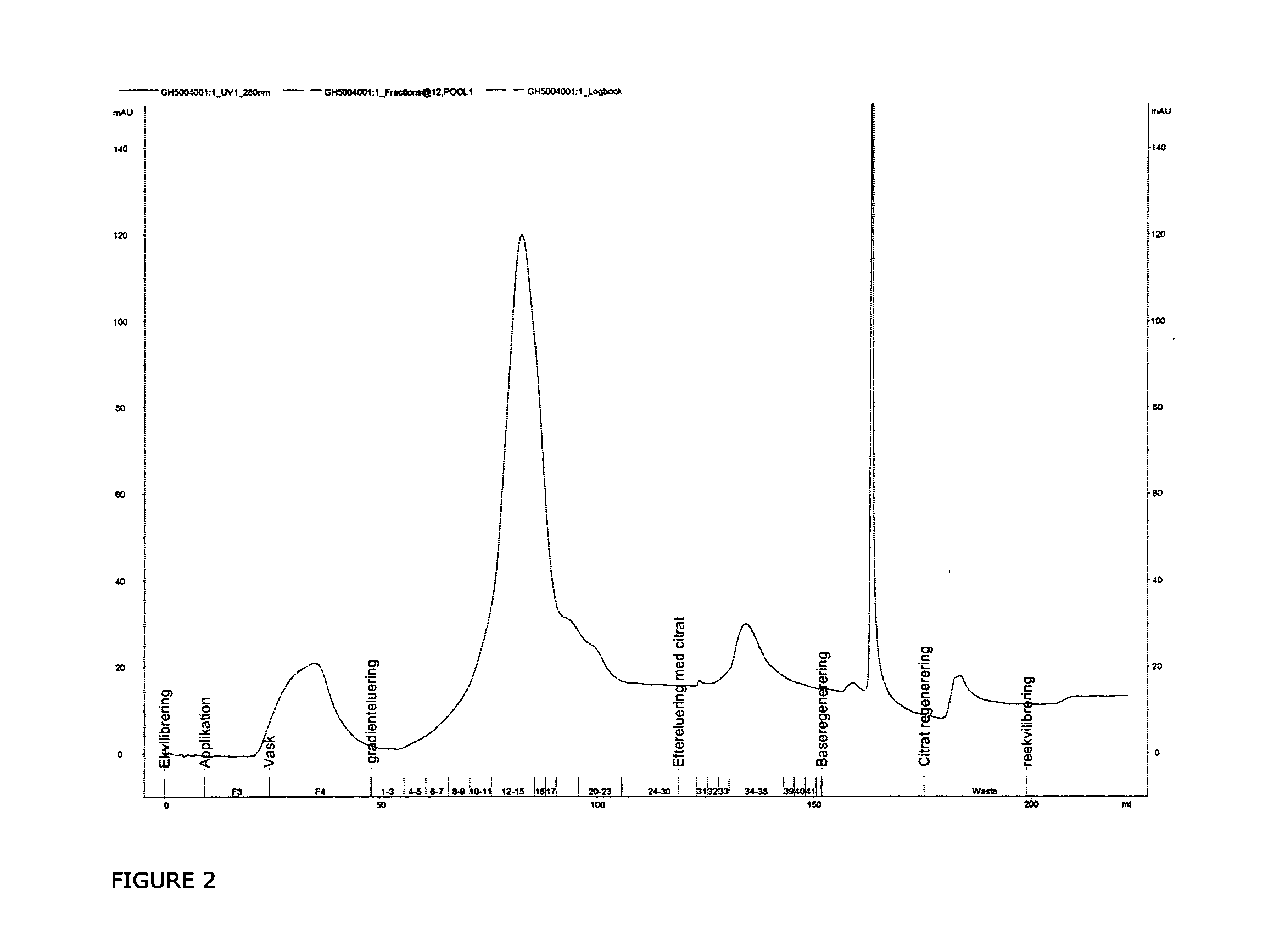
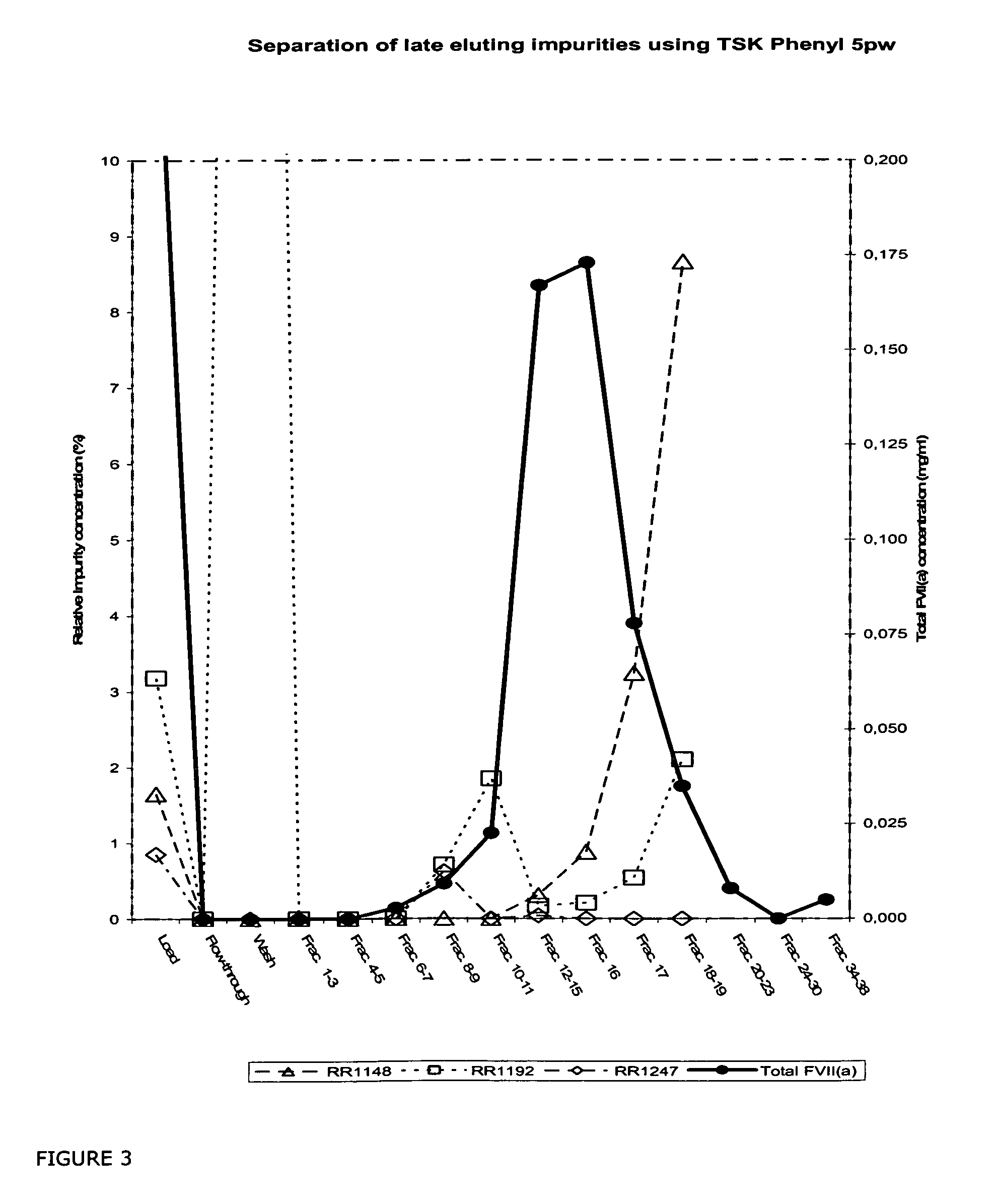
Hydrophobic interaction chromatography purification of factor VII polypeptides
InactiveUS20070037966A1Reduce the presence of impuritiesReduce contentMammal material medical ingredientsPeptide preparation methodsFactor iiRelated impurities
The invention described herein provides new methods of preparing purified Factor VII polypeptide drug substances in large quantities (industrial scale levels) that are associated with reduced content of product-related impurities (e.g., late eluting peaks) and / or that exhibit a relatively uniform glycosylation pattern.
Owner:NOVO NORDISK AS
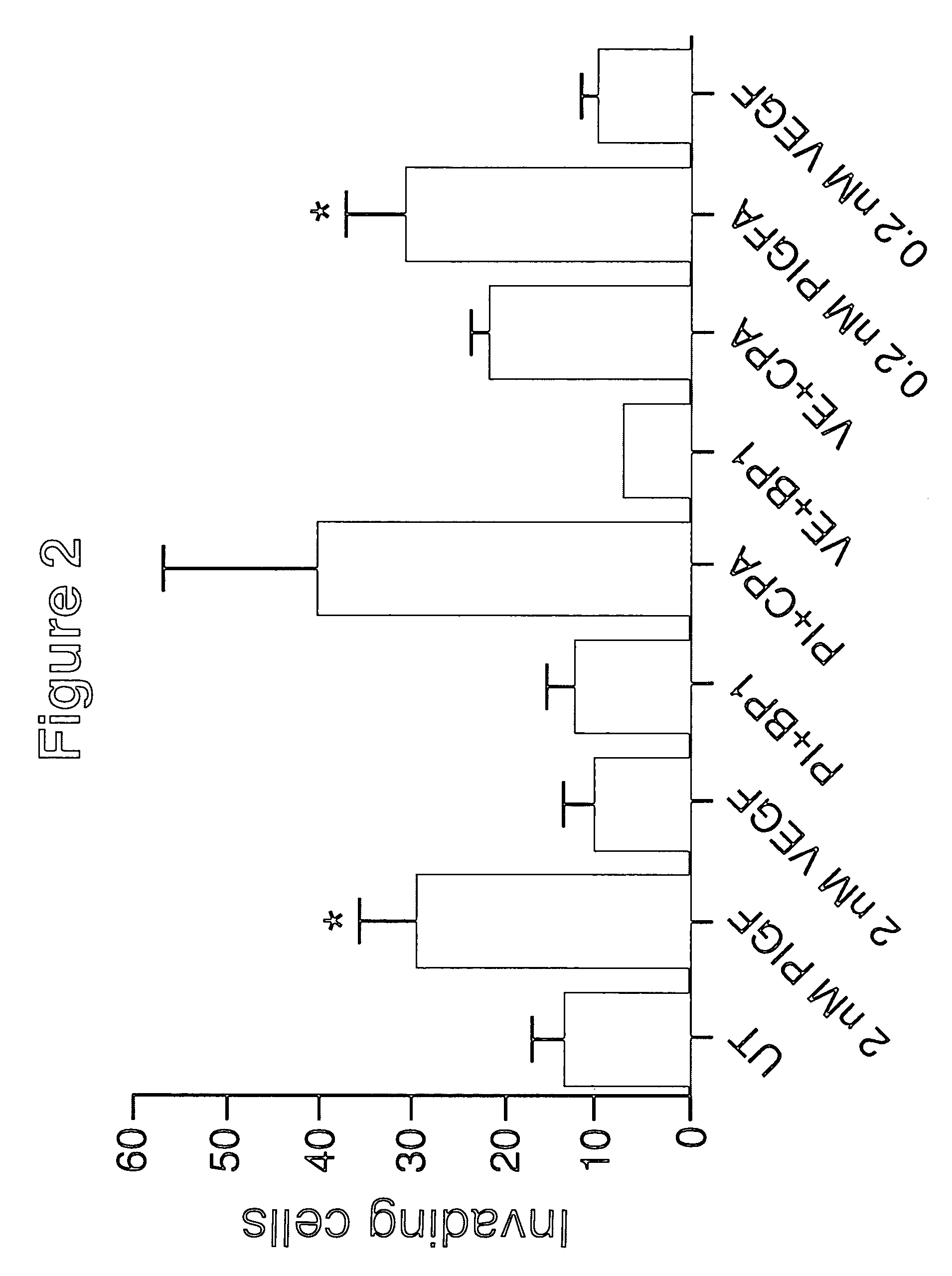
Inhibition of placenta growth factor (PLGF) mediated metastasis and/or angiogenesis
InactiveUS7642239B2Inhibit or eliminate tumor metastasisPrevent tumors from metastasizingSenses disorderPeptide/protein ingredientsLymphatic SpreadImmunotherapeutic agent
Owner:CENT FOR MOLECULAR BIOLOGY & MEDICINE +1
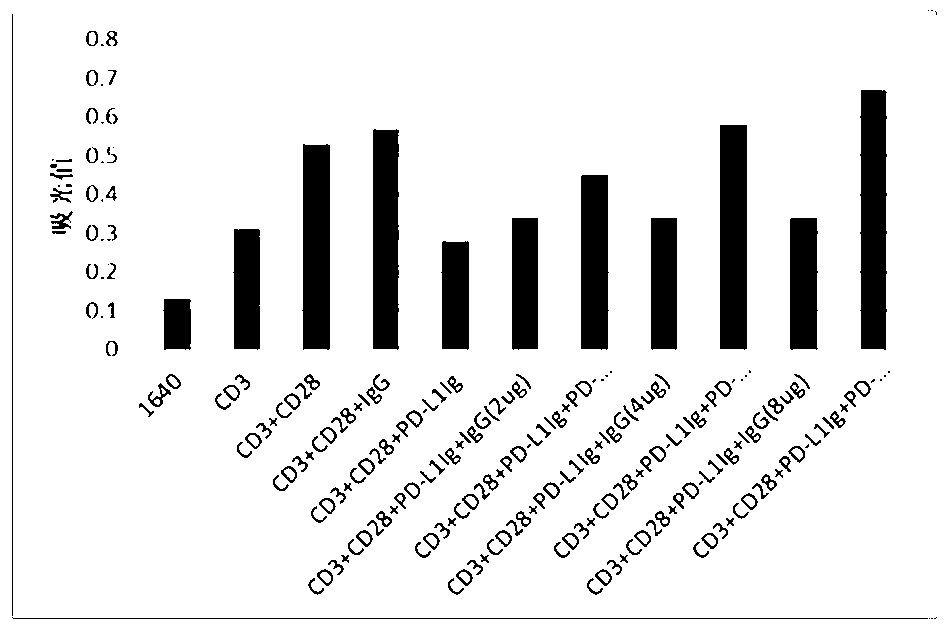
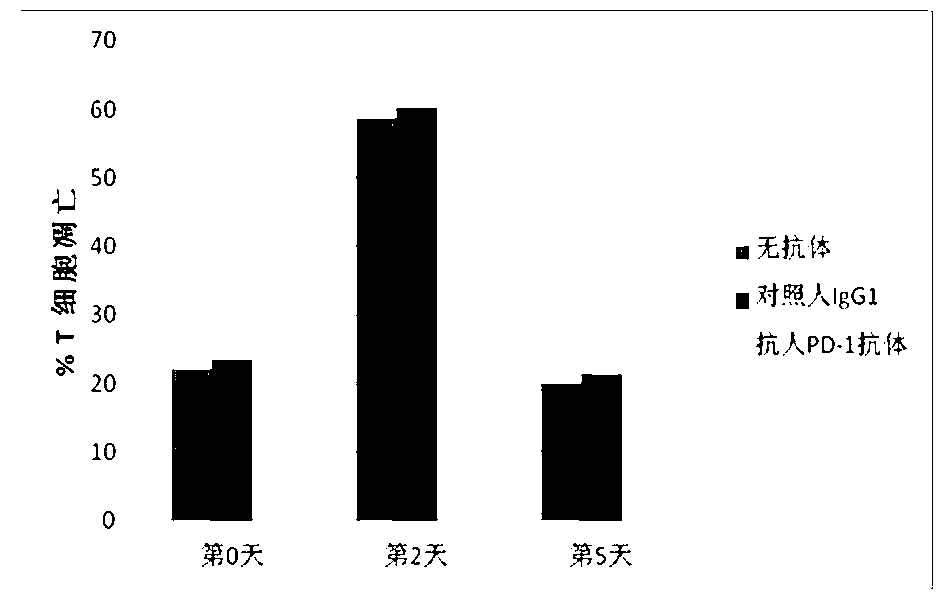
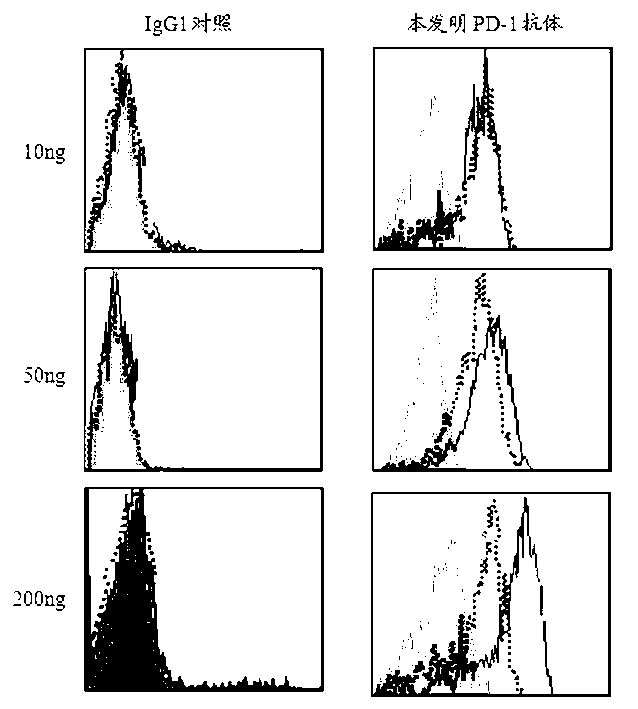
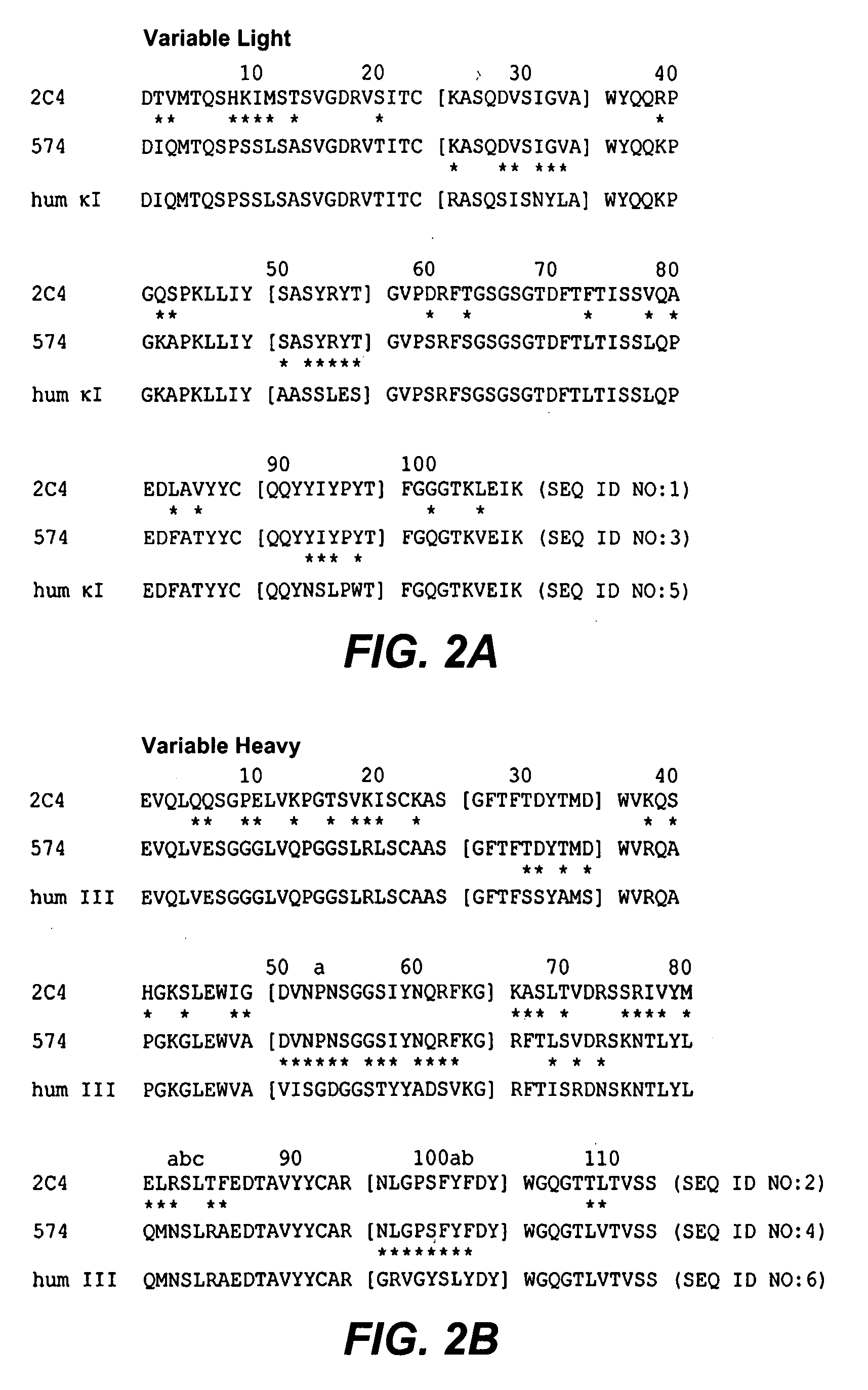
Full-humanized anti-PD-1 monoclonal antibody and preparation method and application thereof
InactiveCN103242448AHigh affinityImproving immunogenicityAntiviralsImmunoglobulins against cell receptors/antigens/surface-determinantsAutoimmune diseaseFactor ii
The invention discloses a full-humanized anti-PD-1 monoclonal antibody having a heavy-chain amino acid sequence shown in a sequence 7 in a sequence table and a light-chain amino acid sequence shown in a sequence 8 in the sequence table. The full-humanized anti-PD-1 monoclonal antibody has the advantages that the antibody has high appetency and low immunogenicity on PD-1, is efficiently expressed in animal cells, and can be used for industrial production. An experiment proves that the full-humanized anti-PD-1 monoclonal antibody specifically blocks a PD-1 / PD-L inhibiting signal, so that a disabled effector cell in an organism restores a biological function; activation proliferation of a tumor and a virus specificity CD8+T cell and secretion of a cell factor are facilitated; the killability of lymphocyte on tumor antigen, an exotic invasive virus and the like is enhanced; the immunity of the organism is improved; and the tumor cell and the virus are timely cleared. Therefore, the antibody disclosed by the invention has a wide application prospect on treatment of tumors, infectious diseases and autoimmune diseases.
Owner:ZHENGZHOU UNIV
Combination therapy with c-met and her antagonists
InactiveUS20090226455A1Significant anti-tumor activityFacilitates heterodimerizationAntibody ingredientsImmunoglobulinsC-MetFactor ii
The present invention relates generally to the fields of molecular biology and growth factor regulation. More specifically, the invention relates to combination therapies for the treatment of pathological conditions, such as cancer.
Owner:GENENTECH INC
Use of complement inhibitors to treat ocular diseases
Owner:GENENTECH INC
Conjugates comprising human IL-18 and substitution mutants thereof
Human interleukin-18 (IL-18) polypeptides and substitution mutants thereof were conjugated to water-soluble polymers at specific sites on the human IL-18 protein. These conjugated human IL-18 and substitution mutants thereof retain biological activity. These conjugated cytokines demonstrate enhanced and unexpected biological properties when compared to the corresponding unconjugated cytokines.
Owner:GLAXO SMITHKLINE LLC
Aneurysm stent with growth factor
The present invention relates to an aneurysm stent having a base and connector. The base has a vessel facing side and an aneurysm facing side, and is shaped to cover an aneurysm sufficiently. The connector is coupled to the aneurysm facing side of the base such that when deployed the connector is adapted to extend partially into the aneurysm to anchor the base about the aneurysm and alter flow into the aneurysm. Further, the stent can be used as a delivery mechanism to deliver growth factor.
Owner:THRAMANN JEFREY
Adoptive immunotherapy using macrophages sensitized with heat shock protein-epitope complexes
InactiveUS6156302AEnhancing host 's immunocompetenceHigh activityBiocideOrganic active ingredientsDiseaseInterleukin 6
The present invention relates to methods and compositions for enhancing immunological responses and for the prevention and treatment of infectious diseases or primary and metastatic neoplastic diseases based on the administration of macrophages and / or other antigen presenting cells (APC) sensitized with heat shock proteins non-covalently bound to peptide complexes and / or antigenic components. APC are incubated in the presence of hsp-peptide complexes and / or antigenic components in vitro. The sensitized cells are reinfused into the patient with or without treatment with cytokines including but not limited to interferon- alpha , interferon- alpha , interleukin-2, interleukin-4, interleukin-6 and tumor neurosis factor.
Owner:FORDHAM UNIVERSITY
Crystal of hypoxia inducible factor 1 alpha prolyl hydroxylase
The crystal structure of ligand-bound EGLN1 catalytic domain of prolyl hydroxylase is disclosed. These coordinates are useful in computer aided drug design for identifying compounds that regulate EGLN1 prolyl hydroxylase and thereby regulate HIF-regulated disorders.
Owner:AKEBIA THERAPEUTICS
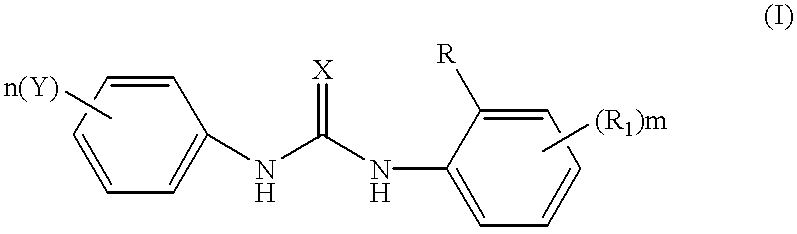
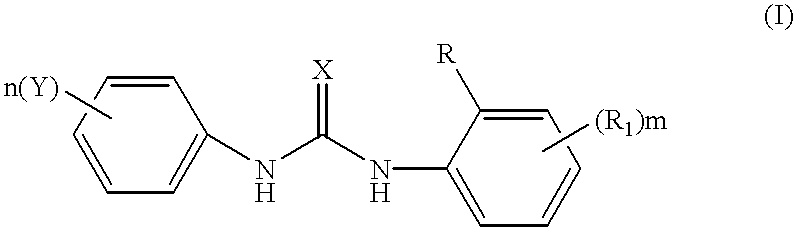
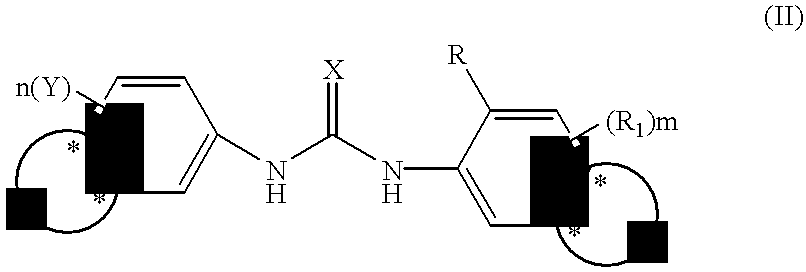
Phenyl urea antagonists of the IL-8 receptor
This invention relates to novel compounds of Formula (I), and a novel use of these compounds in treating chemokine mediated diseases, wherein the chemokine binds to an IL-8 a or b receptor. Compounds of Formula (1) are represented by the structure:wherein interalia, X is oxygen or sulfur;R is any functional moiety having an ionizable hydrogen and a pKa of 10 or less;R1 is independently selected from hydrogen; halogen; nitro; cyano; C1-C10 alkyl; halosubstituted C1-10 alkyl; C2-10 alkenyl; C1-10 alkoxy; halosubstituted C1-10 alkoxy; azide; (CR8R8)qS(O)tR4; hydroxy; hydroxy substituted C1-4 alkyl; aryl; aryl C1-4 alkyl; aryl C2-10 alkenyl; aryloxy; aryl C1-4 alkyloxy; heteroaryl; heteroarylalkyl; heteroaryl C2-10 alkenyl; heteroaryl C1-4 alkyloxy; heterocyclic, heterocyclic C1-4 alkyl; heterocyclic C1-4 alkyloxy; heterocyclic C2-10 alkenyl;q is 0 or an integer having a value of 1 to 10; n is an integer having a value of 1 to 3;m is an integer having a value of 1 to 3;n is an integer having a value of 1 to 3;Y is hydrogen; halogen; nitro; cyano; halosubstituted C1-10 alkyl; C1-10 alkyl; C2-10 alkenyl; C1-10 alkoxy; halosubstituted C1-1- alkoxy; azide; (CR8R8)qS(O)tR4, (CR8R8)qOR4; hydroxy; hydroxy substituted C1-4 alkyl; aryl; aryl C1-4 alkyl; aryloxy; aryC1-4 alkyloxy; aryl C2-10 alkenyl; heteroaryl; heteroarylalkyl; heteroaryl C1-4 alkyloxy; heteroaryl C2-10 alkenyl; heterocyclic, heterocyclic C1-4 alkyl; heterocyclic C2-10 alkenyl;or a pharmaceutically acceptable salt thereof.
Owner:GLAXO SMITHKLINE LLC
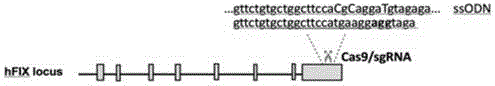
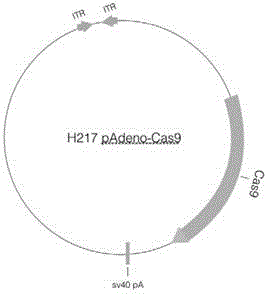
Site specific repairing carrier system and method of blood coagulation factor genetic mutation
The invention discloses a method for carrying out in-situ repairing on blood coagulation factor F8 / F9. The method comprises the following steps: in a target genome sequence, selecting the mutation sites of blood coagulation factor as the gene sites for in-situ repairing; designing the binding sites of nuclease of sgRNA sequence of a CRISPR / Cas system; designing a homologous recombinant repairing donor sequence for in-situ repairing; delivering nuclease protein and / or sgRNA and the nucleotide sequence of the homologous recombinant repairing donor to the gene sites of in-situ repairing by a delivering carrier; generating damages to the genome DNA by the nuclease on the gene in-situ repairing sites; and inserting the homologous recombinant repairing donor sequence into the gene in-situ repairing sites so as to repair the gene or supplement the expression of gene. The in-situ repairing of mutation sites of blood coagulation factor can be applied to the clinic, and the method has the advantages of precise induction, safer and controllable process, and definite target.
Owner:EAST CHINA NORMAL UNIV
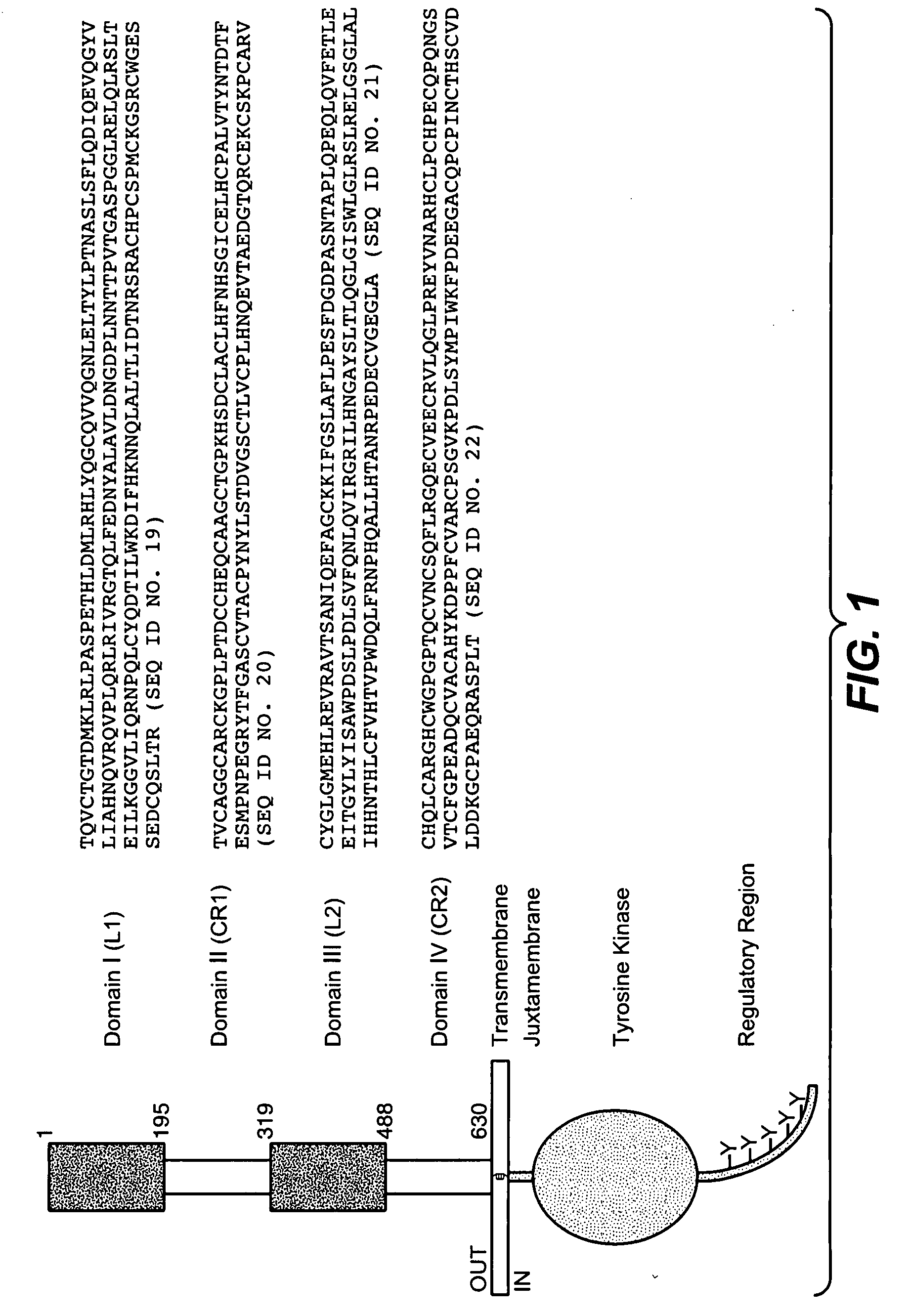
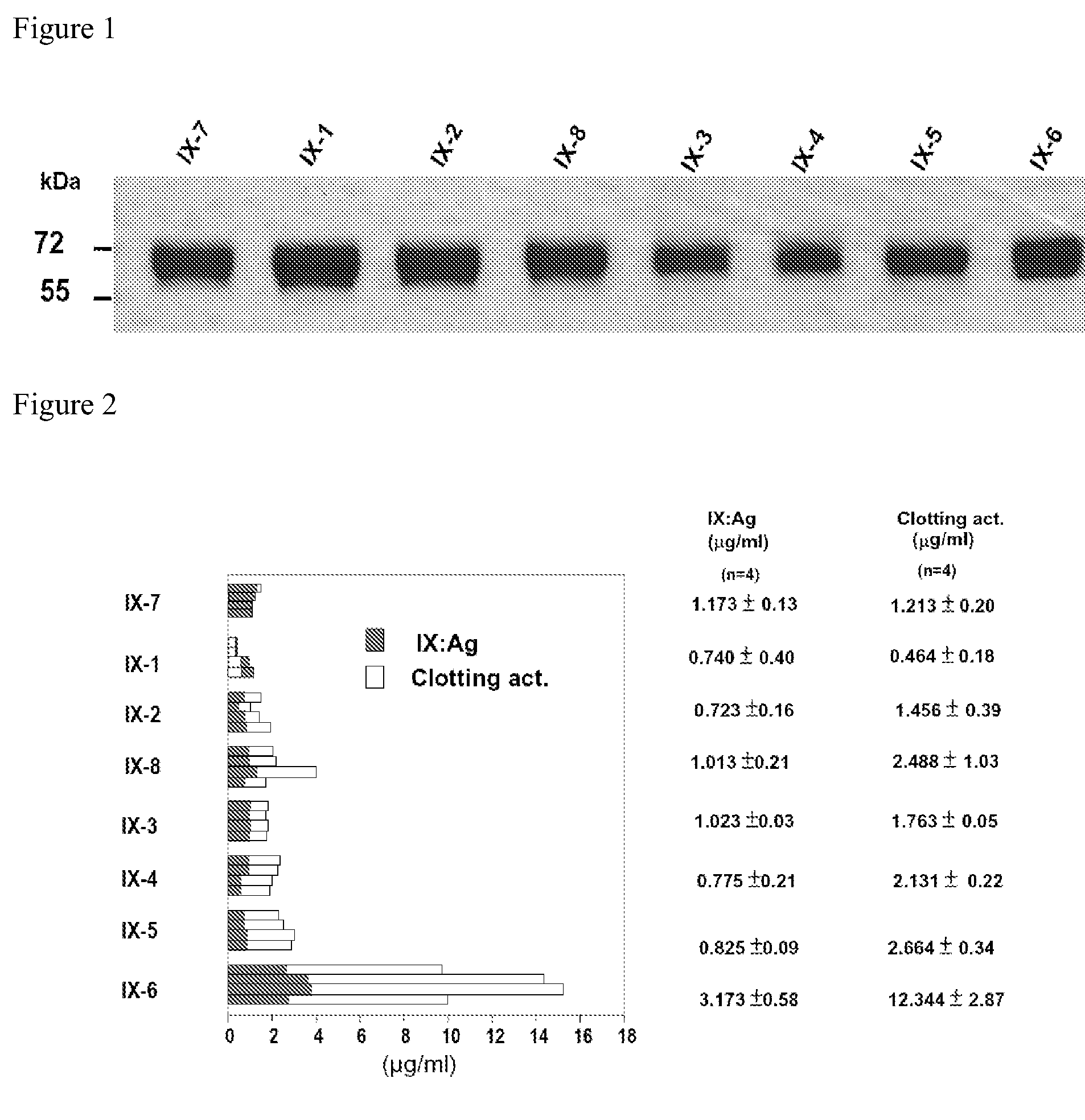
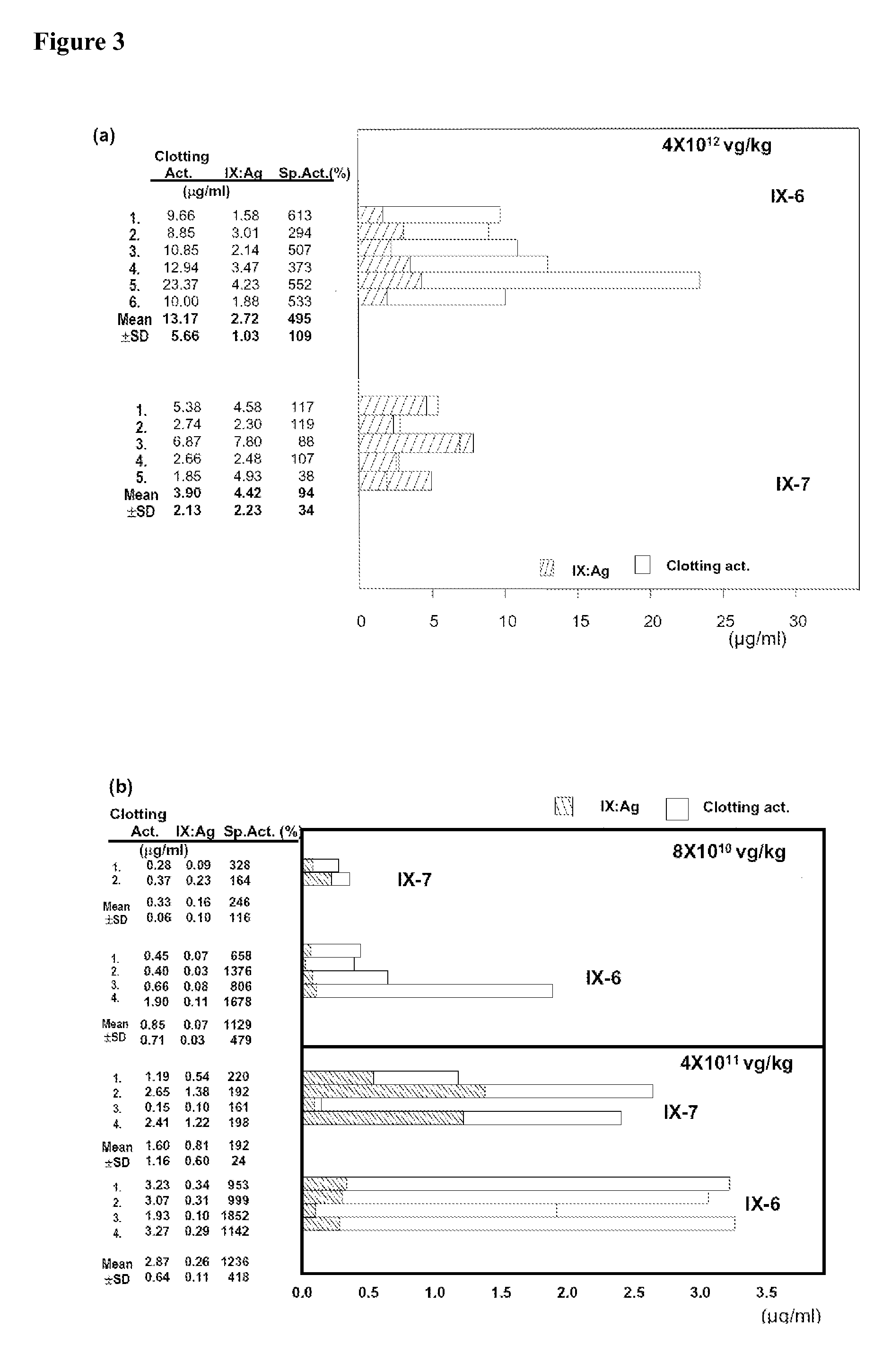
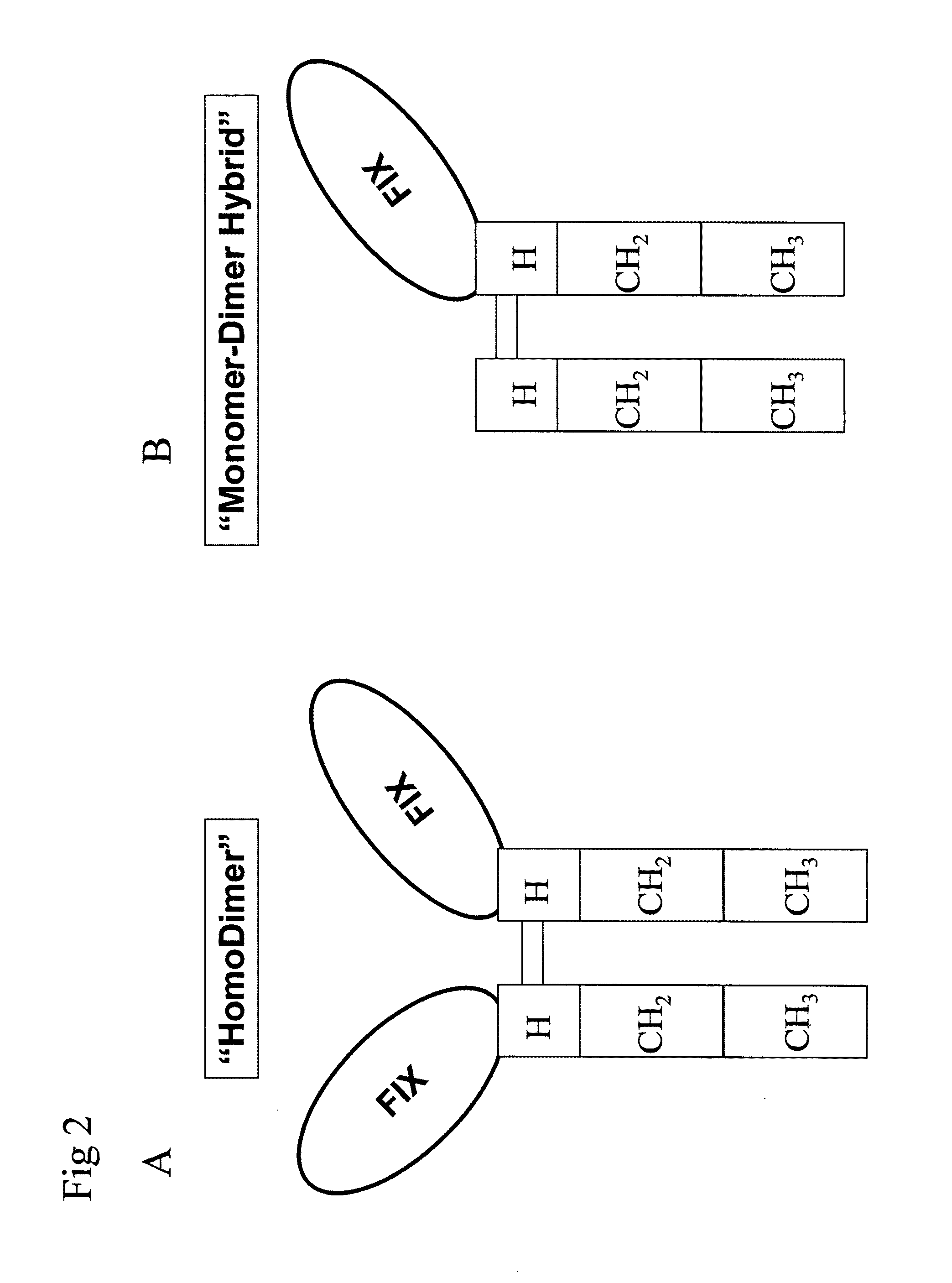
Recombinant human factor ix and use thereof
ActiveUS20080167219A1Easy to adaptPeptide/protein ingredientsGenetic material ingredientsWild typeFactor ii
The present invention aims at converting factor IX into a molecule with enhanced activity which provides an alternative for replacement therapy and gene therapy for hemophilia B. Using recombinant techniques, factor IX with replacement at positions 86, 277, and 338 exhibits better clotting activity than recombinant wild type factor IX.
Owner:LIN SHU WHA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com