Patents
Literature
237 results about "Related impurities" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
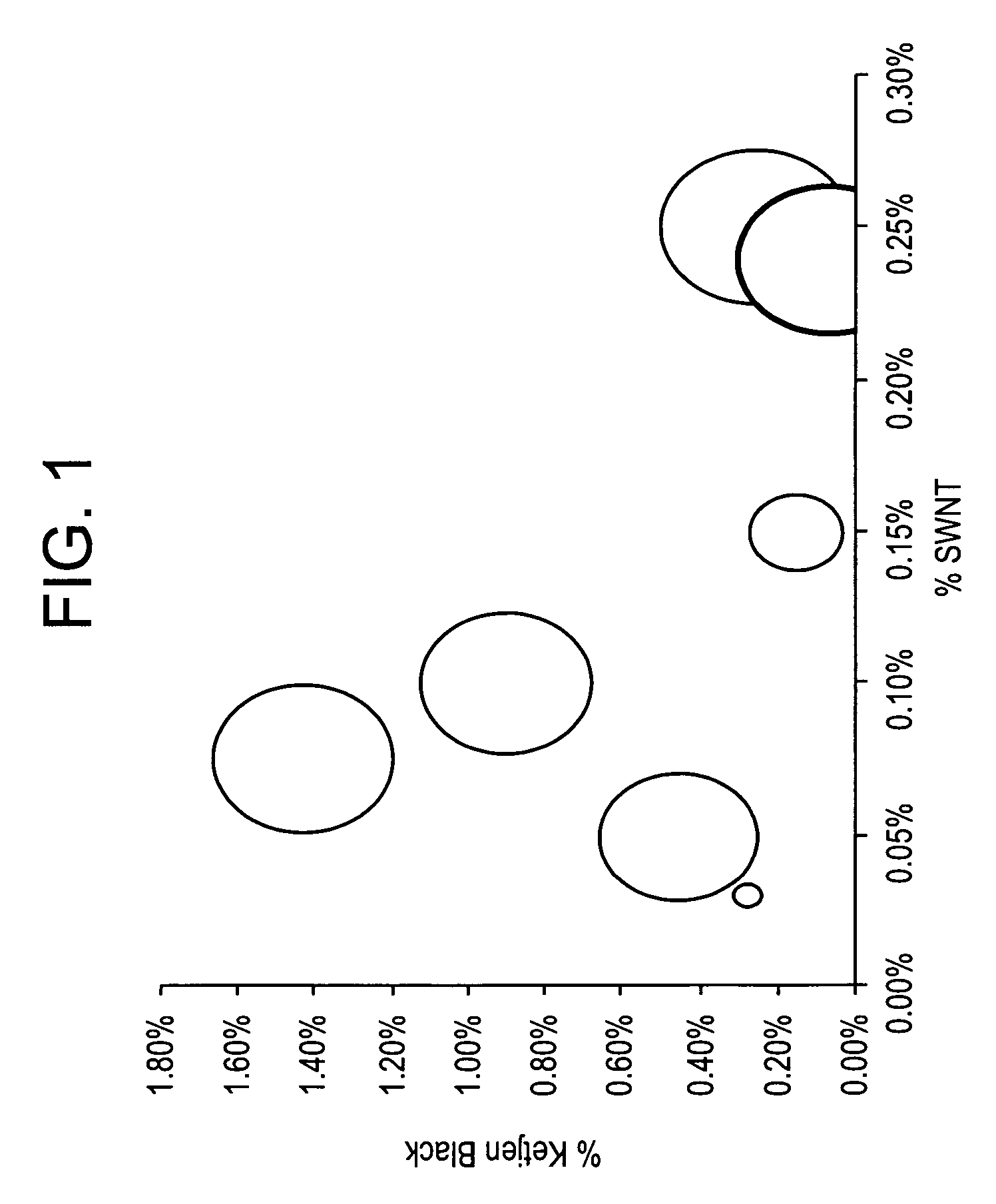
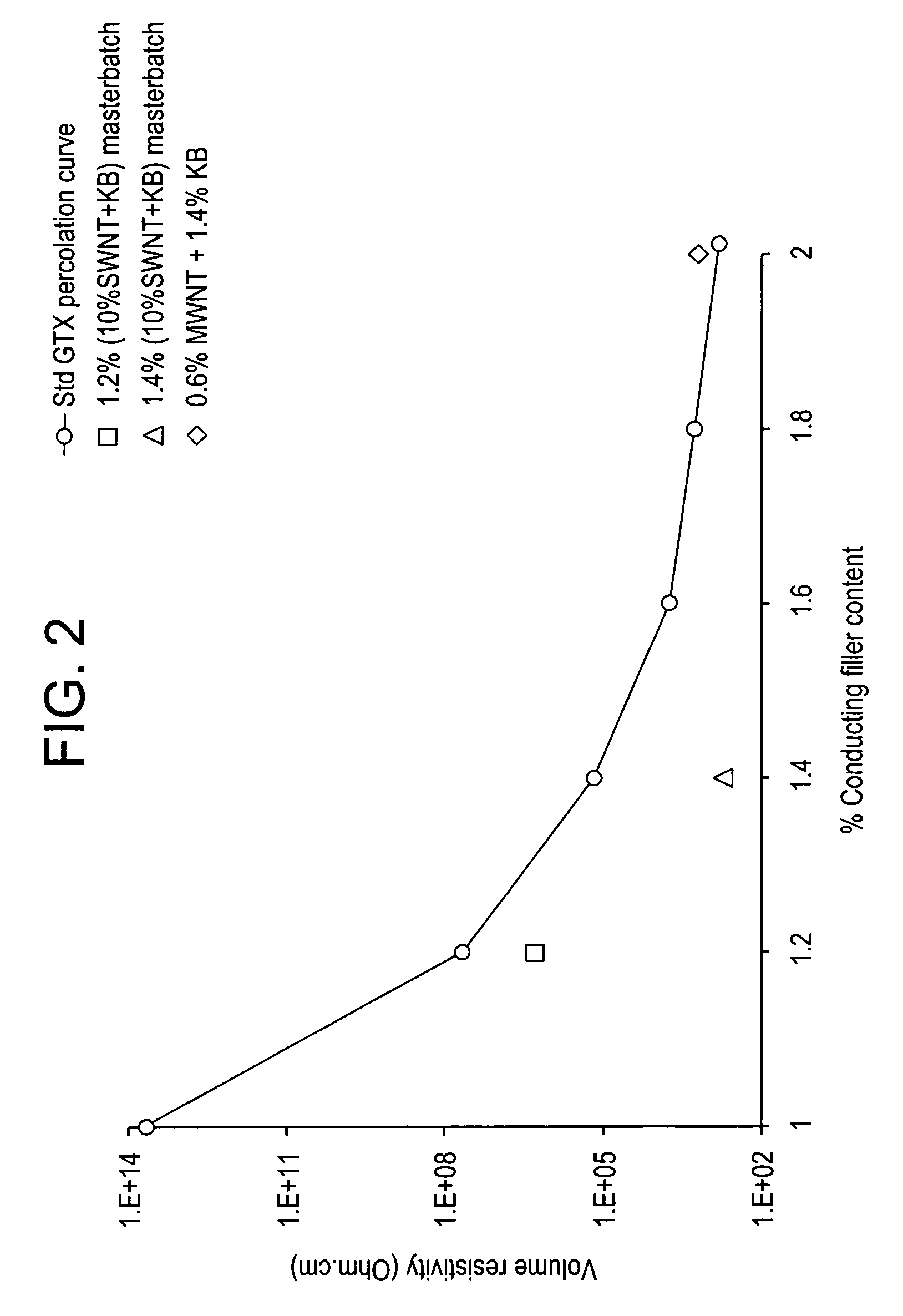
Conductive thermoplastic compositions, methods of manufacture and articles derived from such compositions
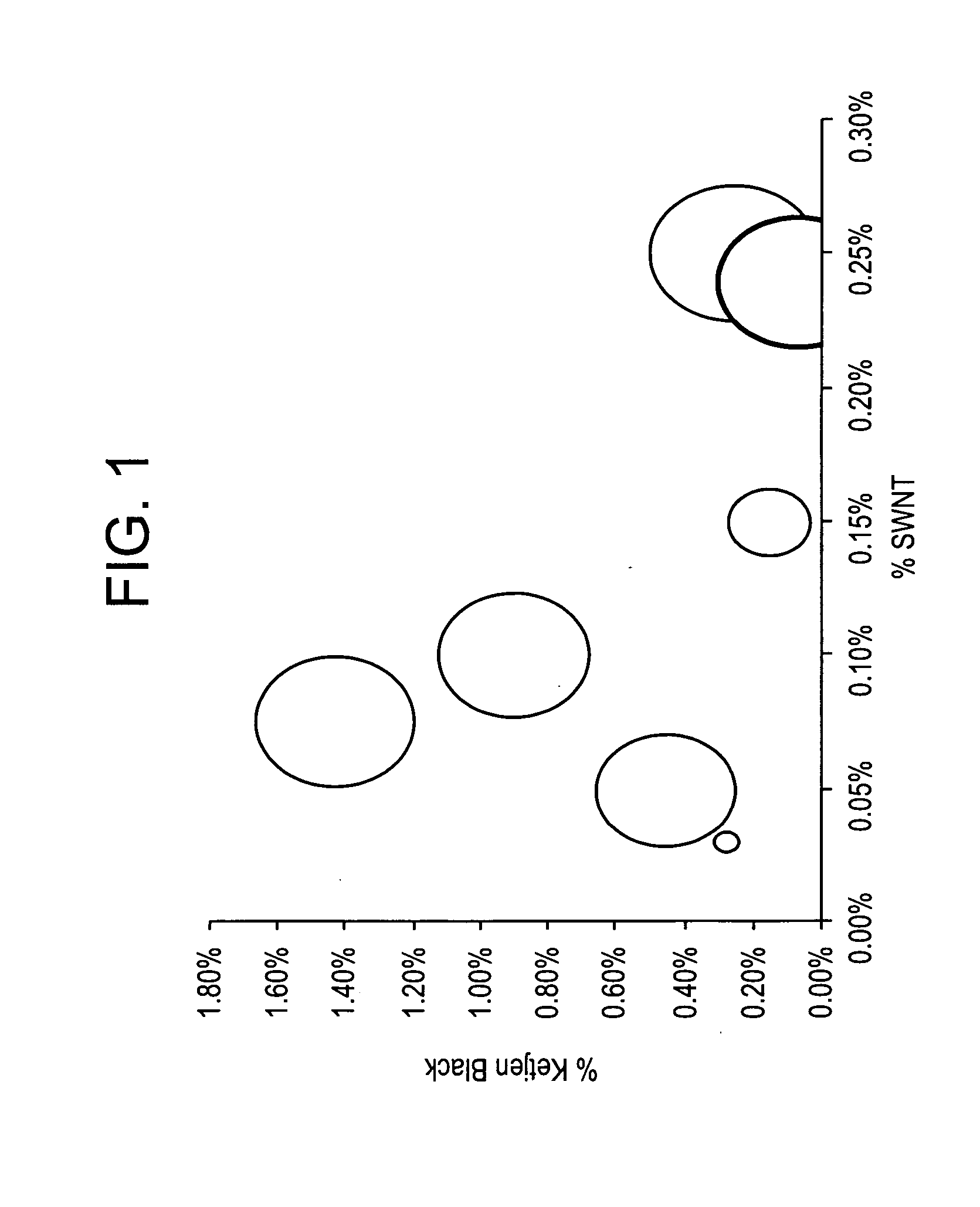
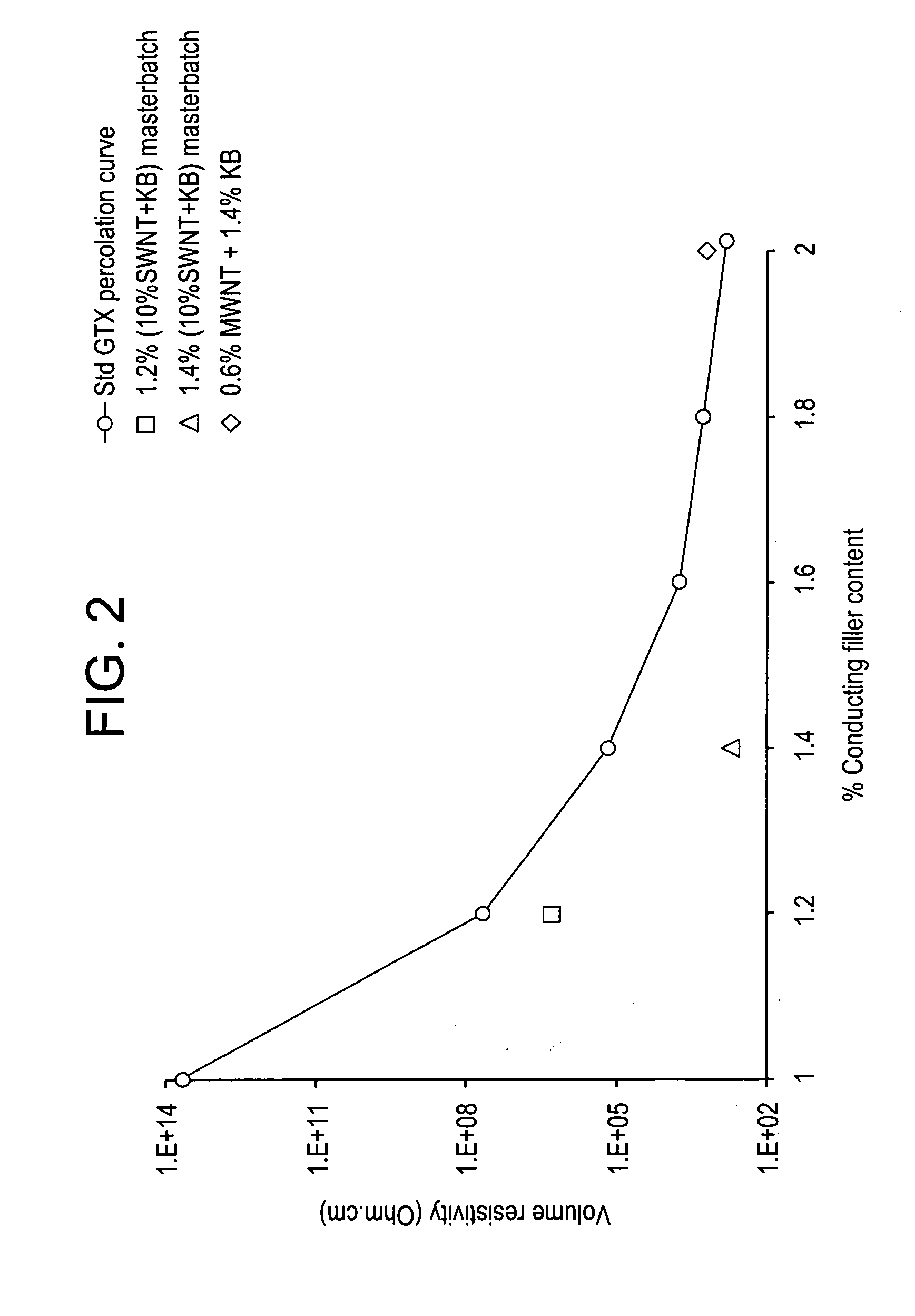
Disclosed herein is an electrically conductive precursor composition comprising an organic polymer precursor; a single wall nanotube composition, wherein the single wall nanotube composition contains at least 0.1 wt % of production related impurities; and an optional nanosized conductive filler. A conductive composition comprises an organic polymer; a single wall nanotube composition, wherein the single wall nanotube composition contains at least 0.1 wt % of production related impurities; and a nanosized conductive filler.
Owner:SABIC INNOVATIVE PLASTICS IP BV
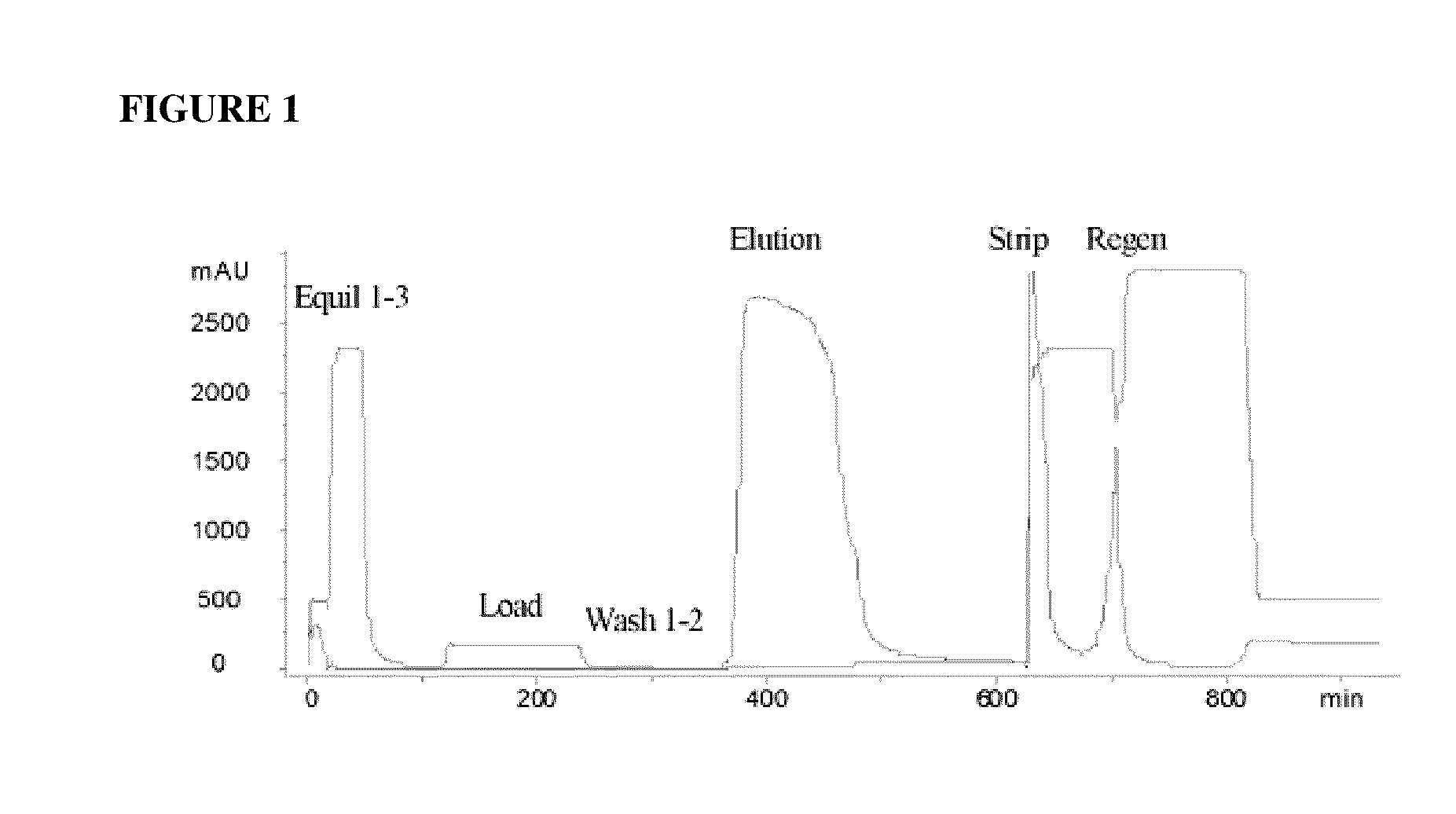
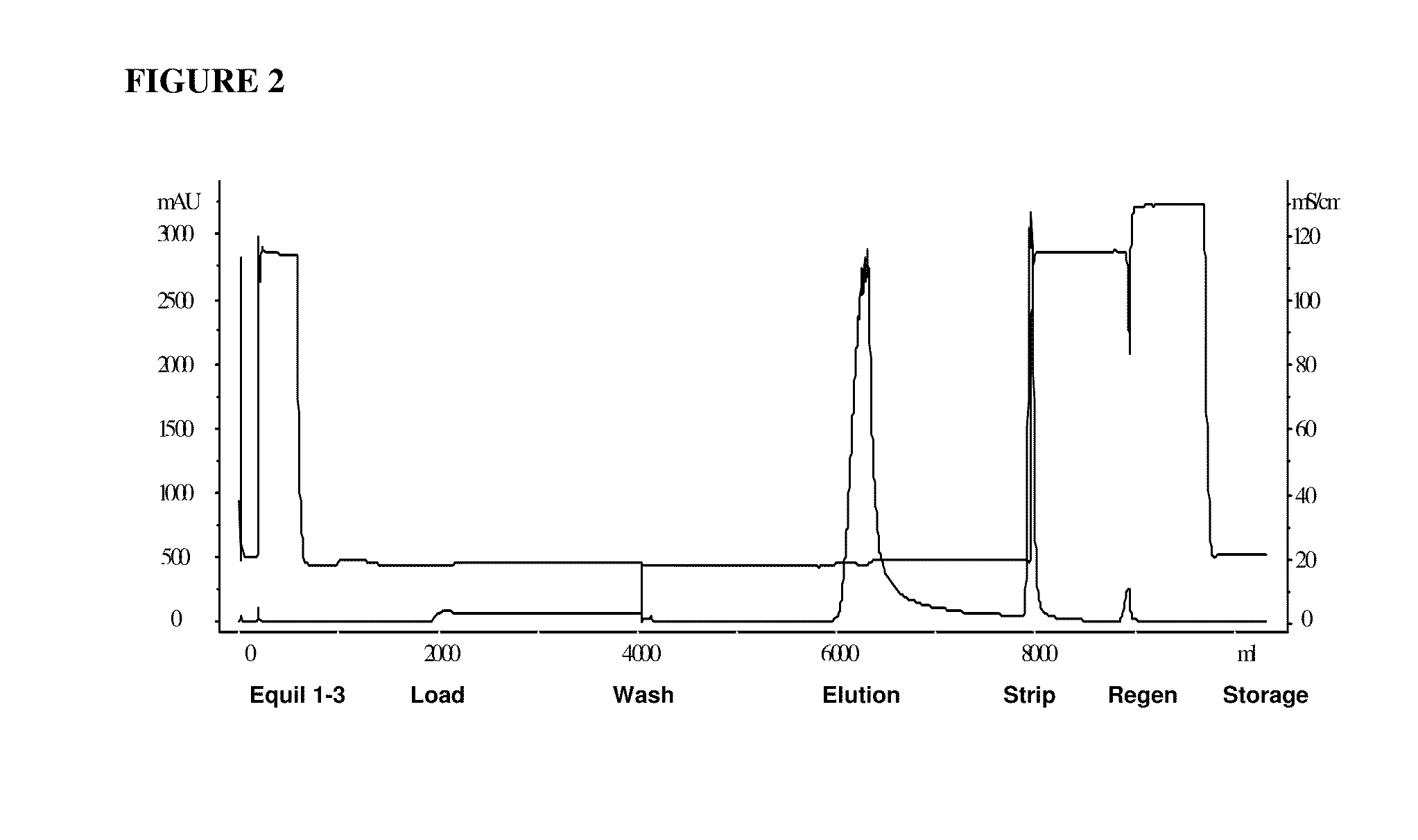
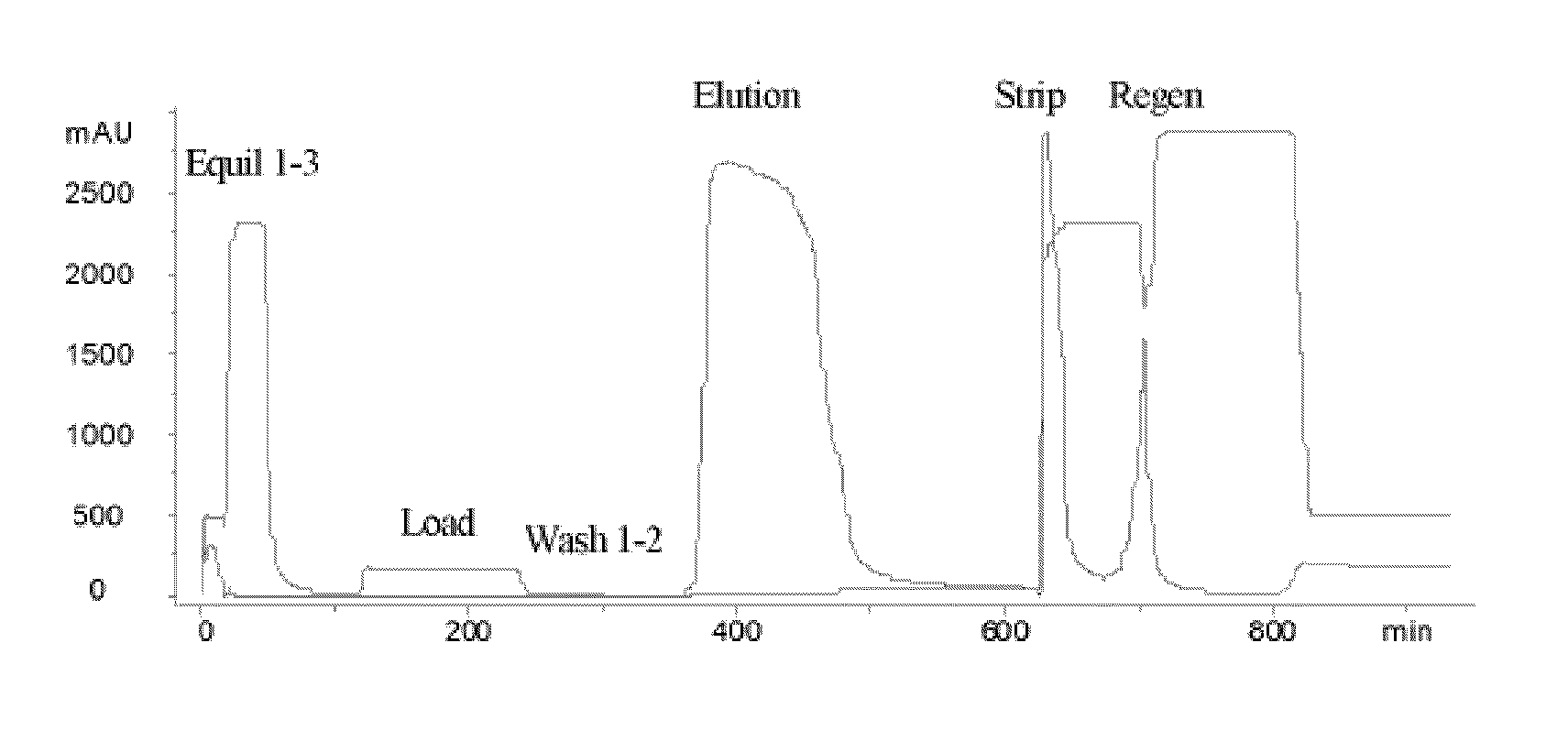
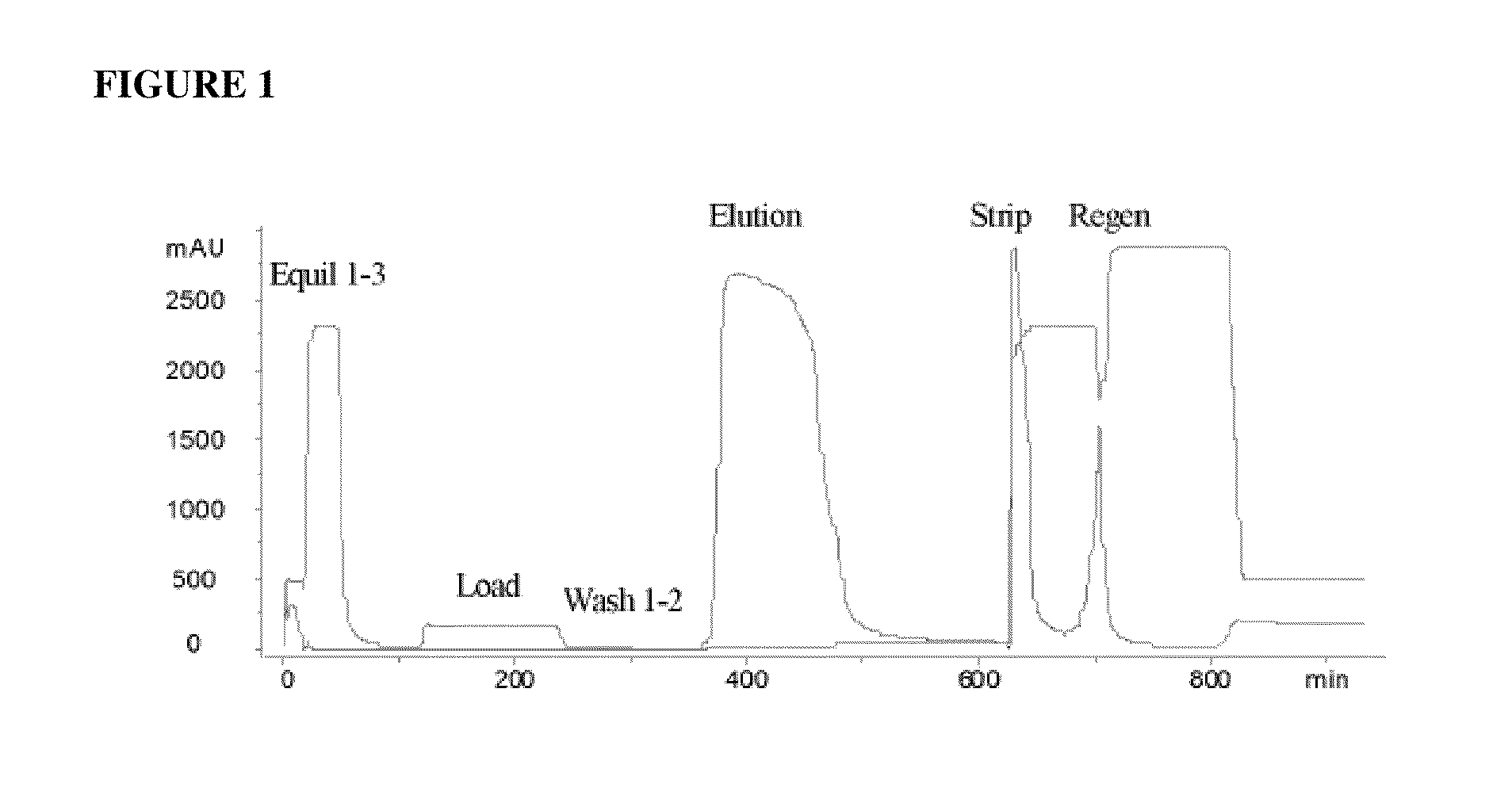
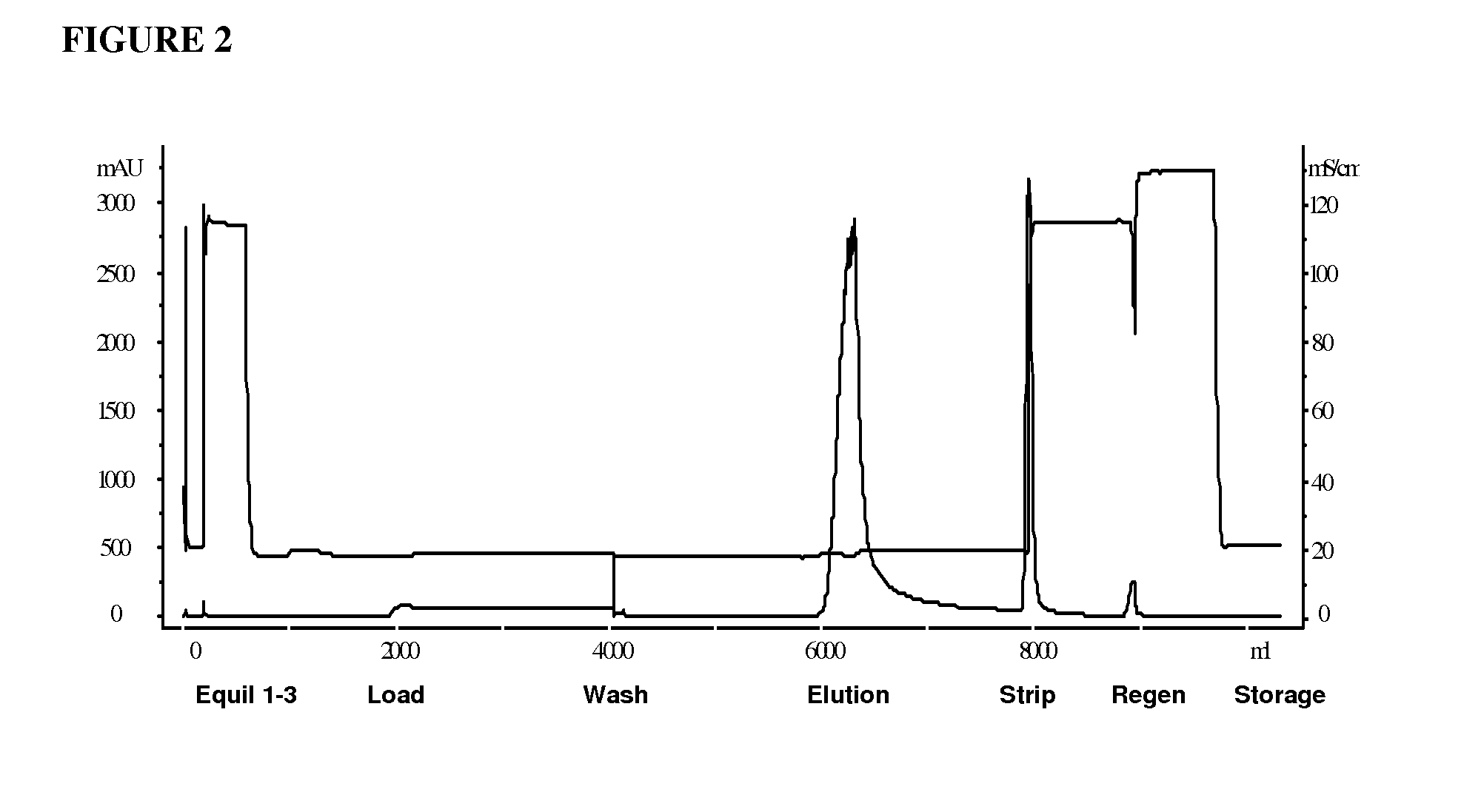
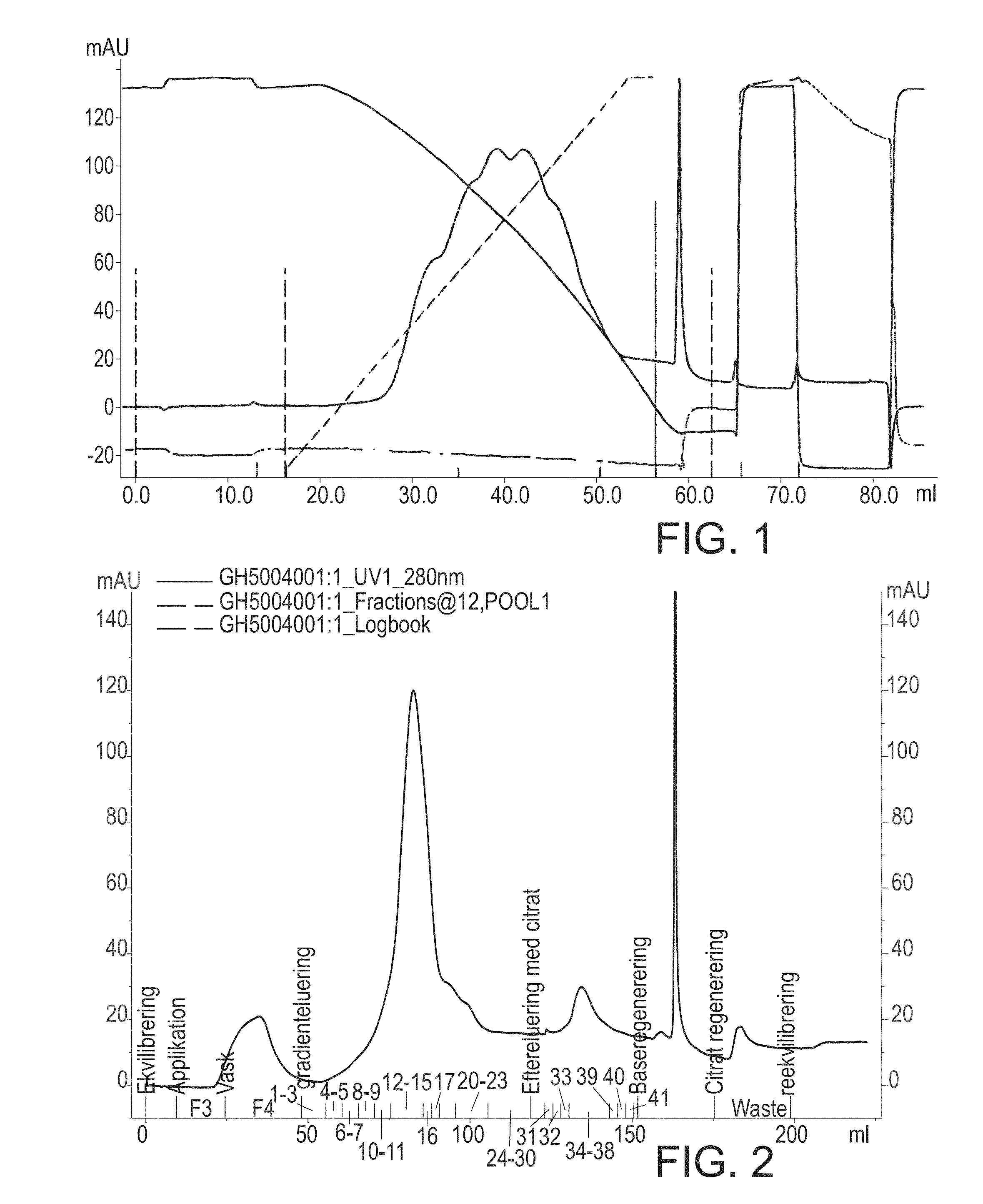
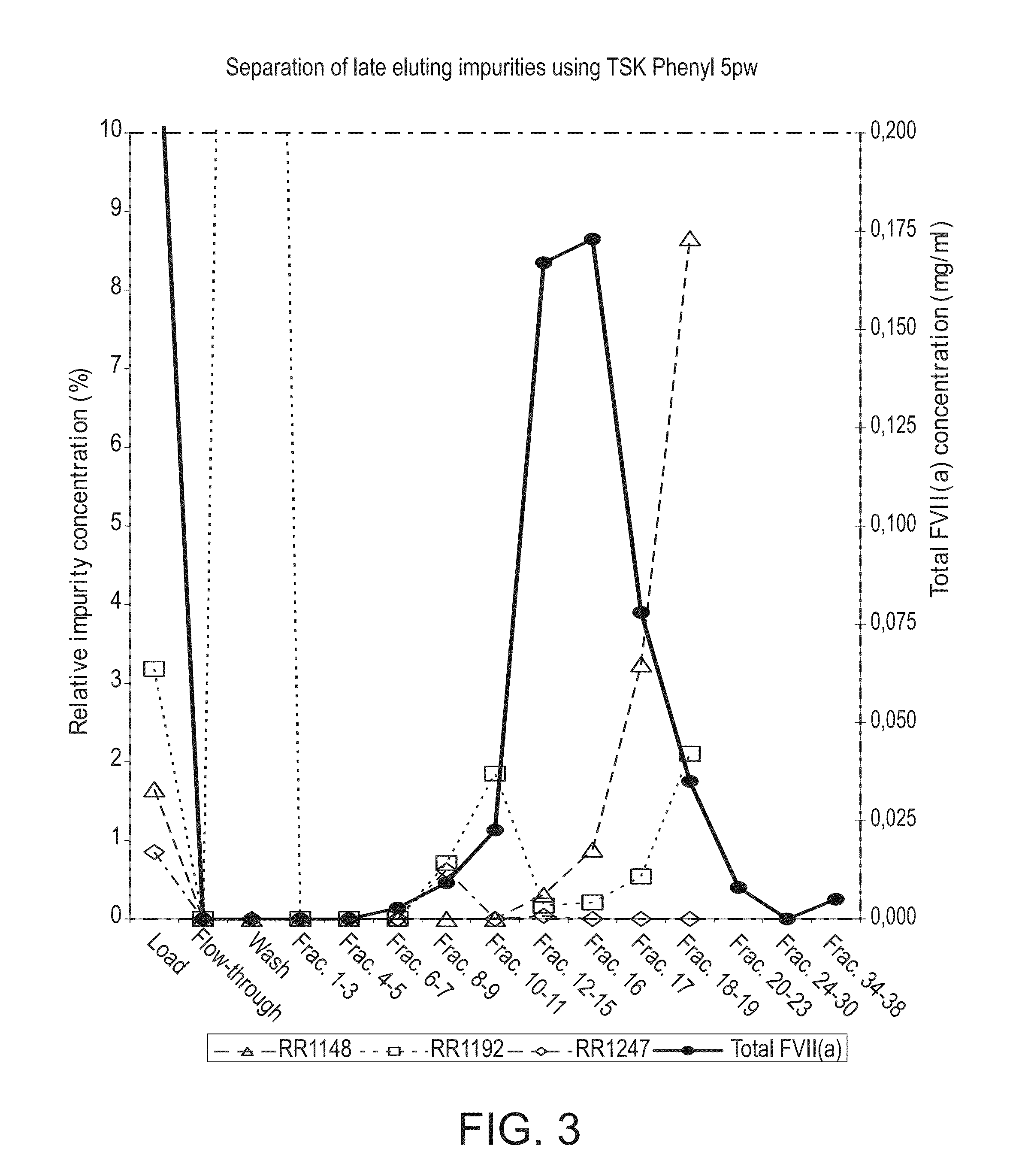
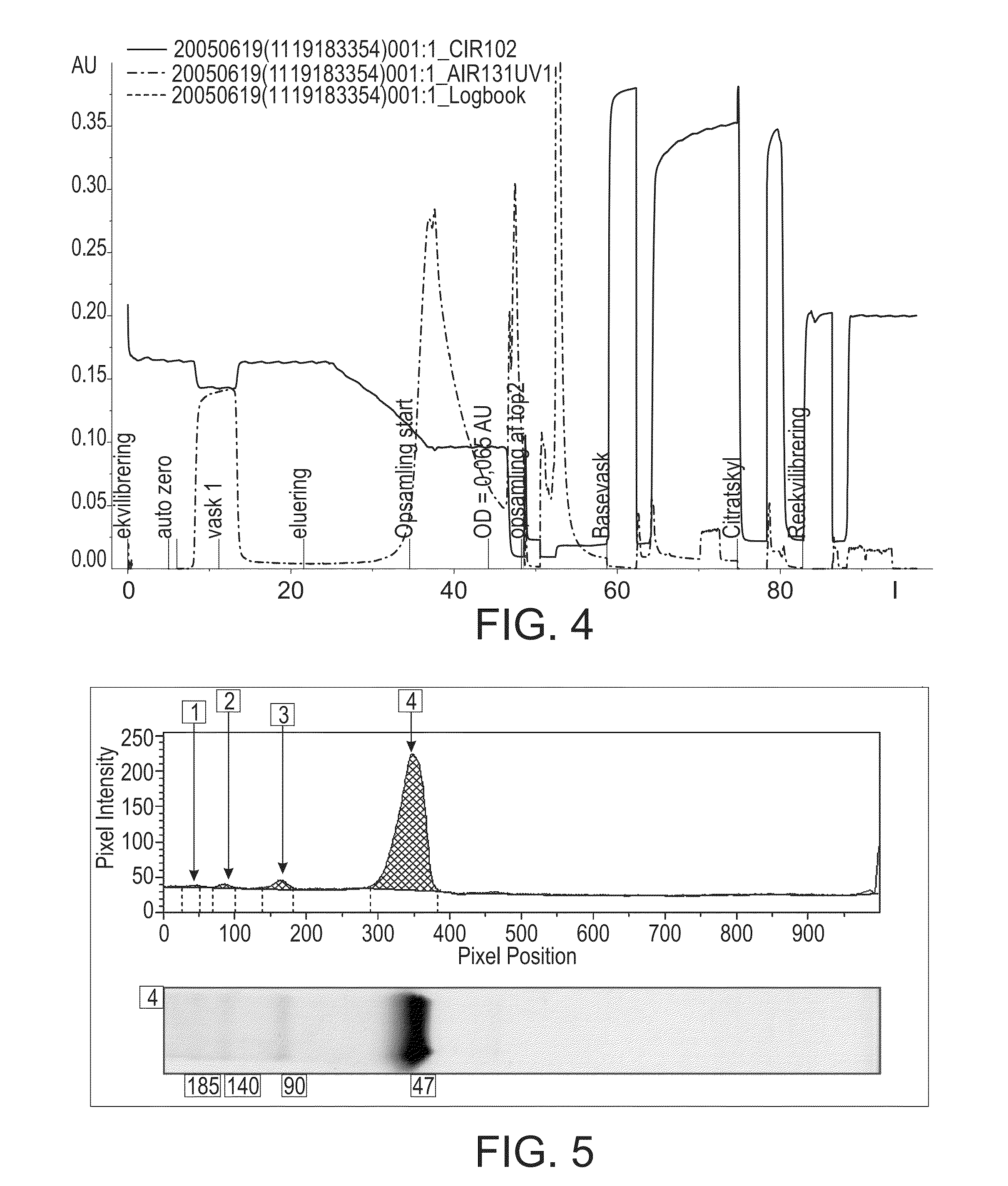
Hydrophobic interaction chromatography purification of factor VII polypeptides
InactiveUS20070037966A1Reduce the presence of impuritiesReduce contentMammal material medical ingredientsPeptide preparation methodsFactor iiRelated impurities
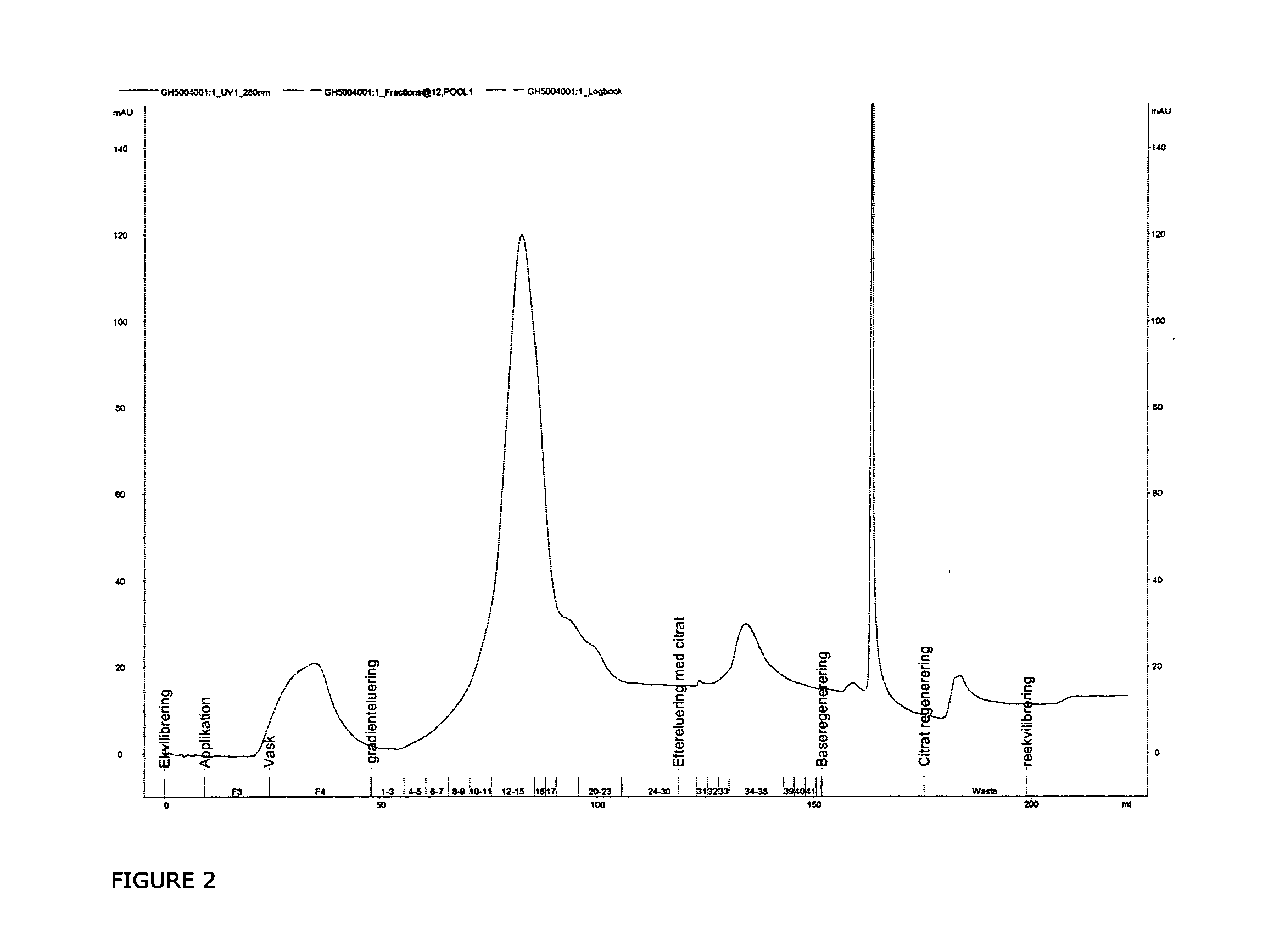
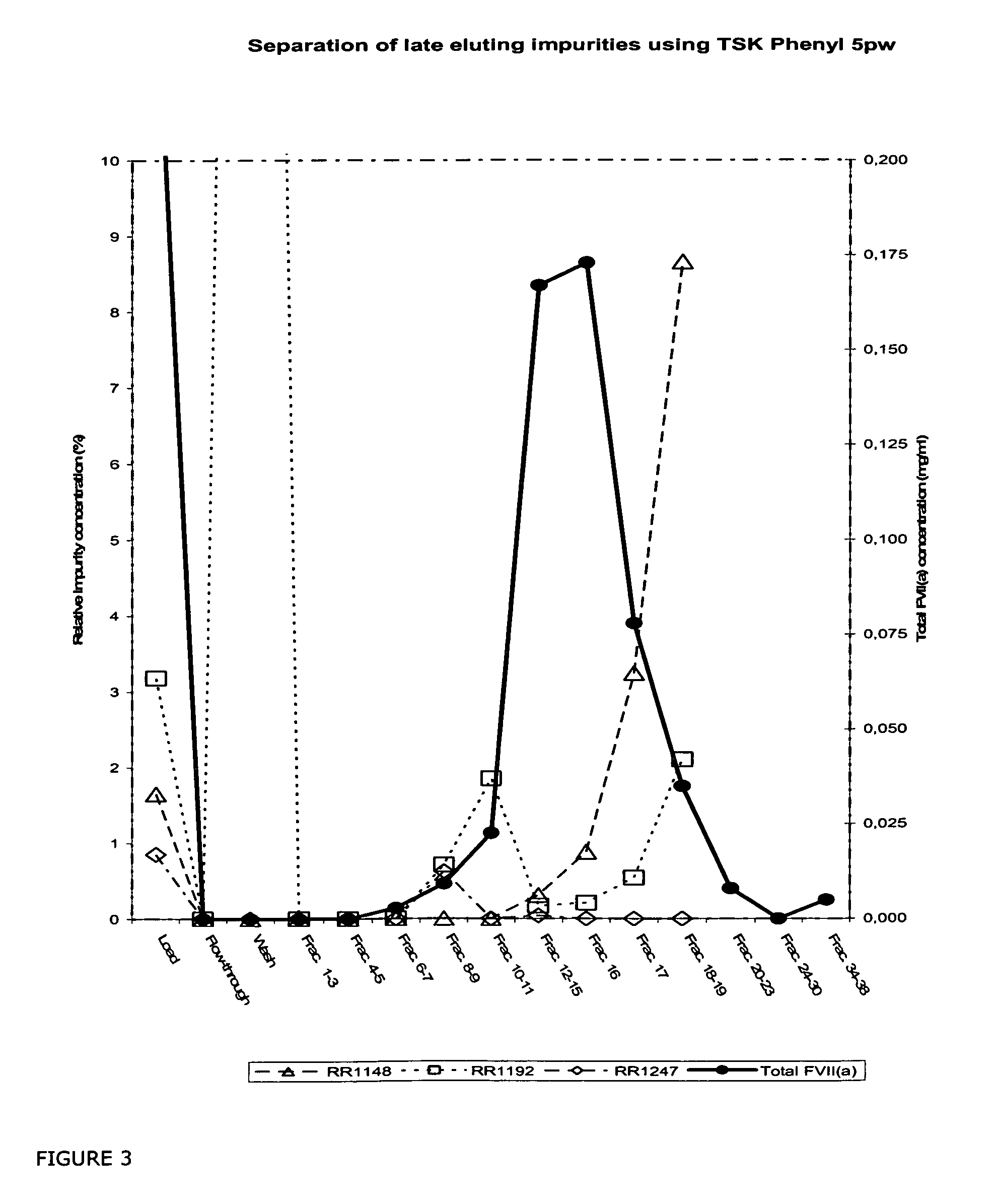
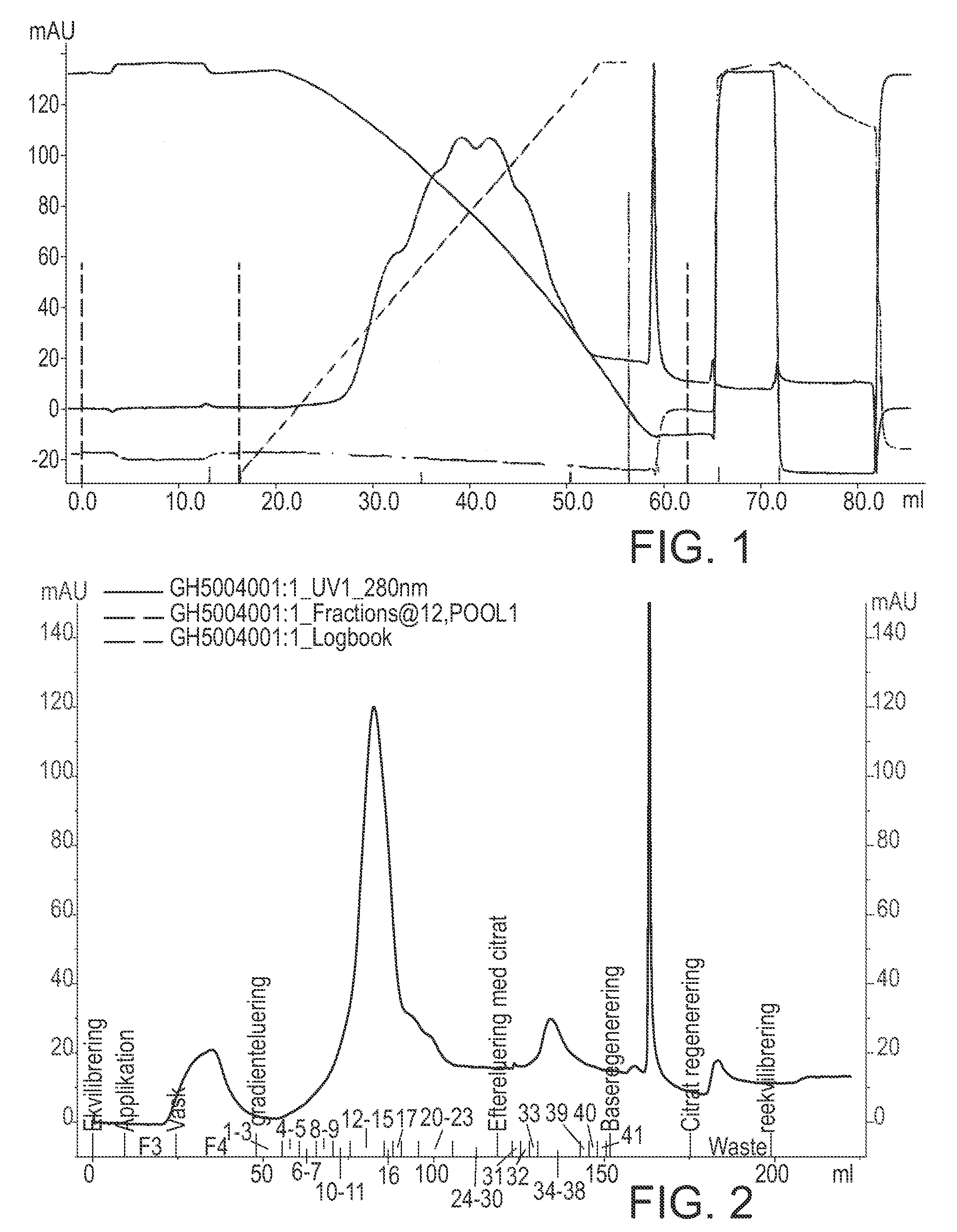
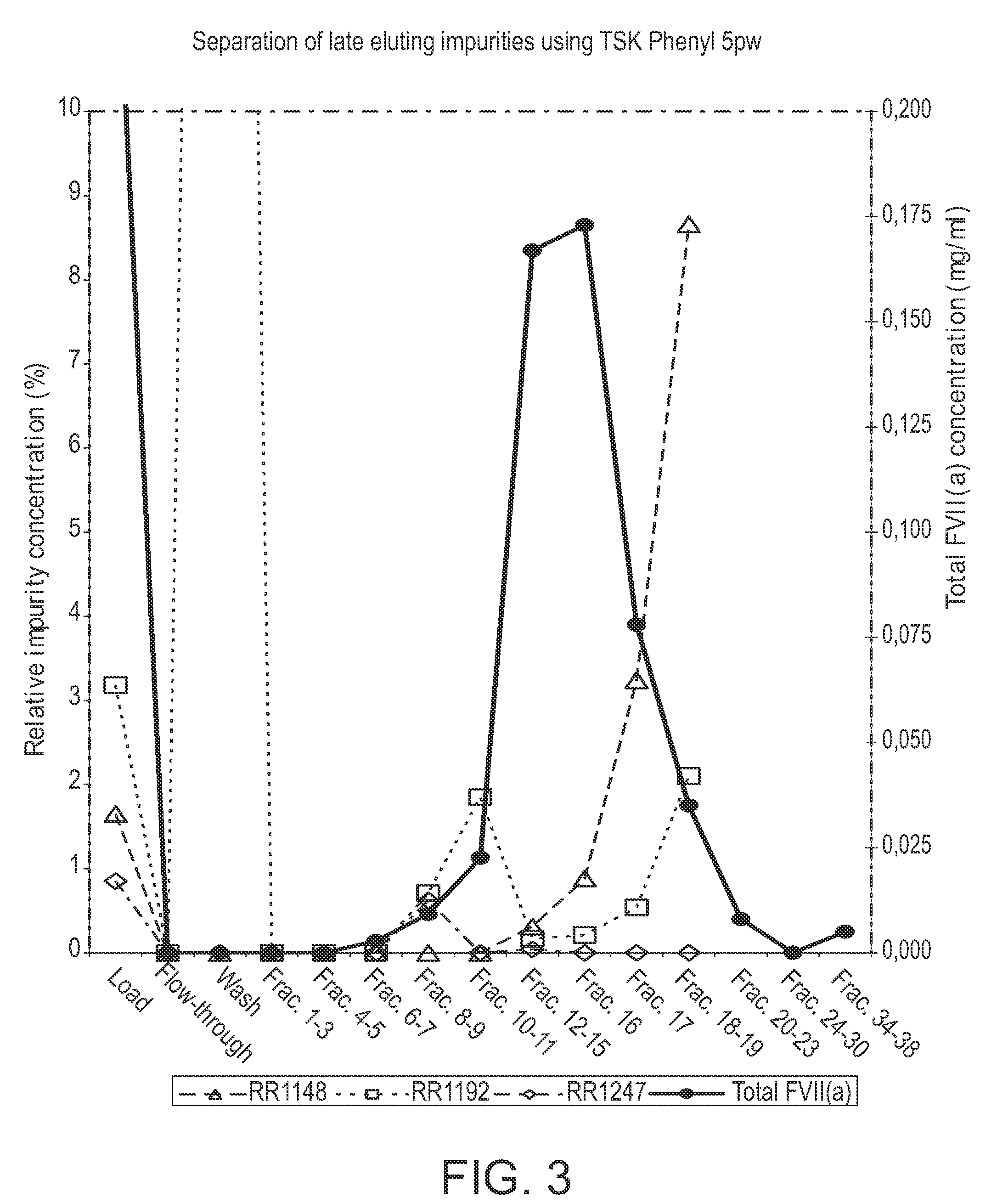
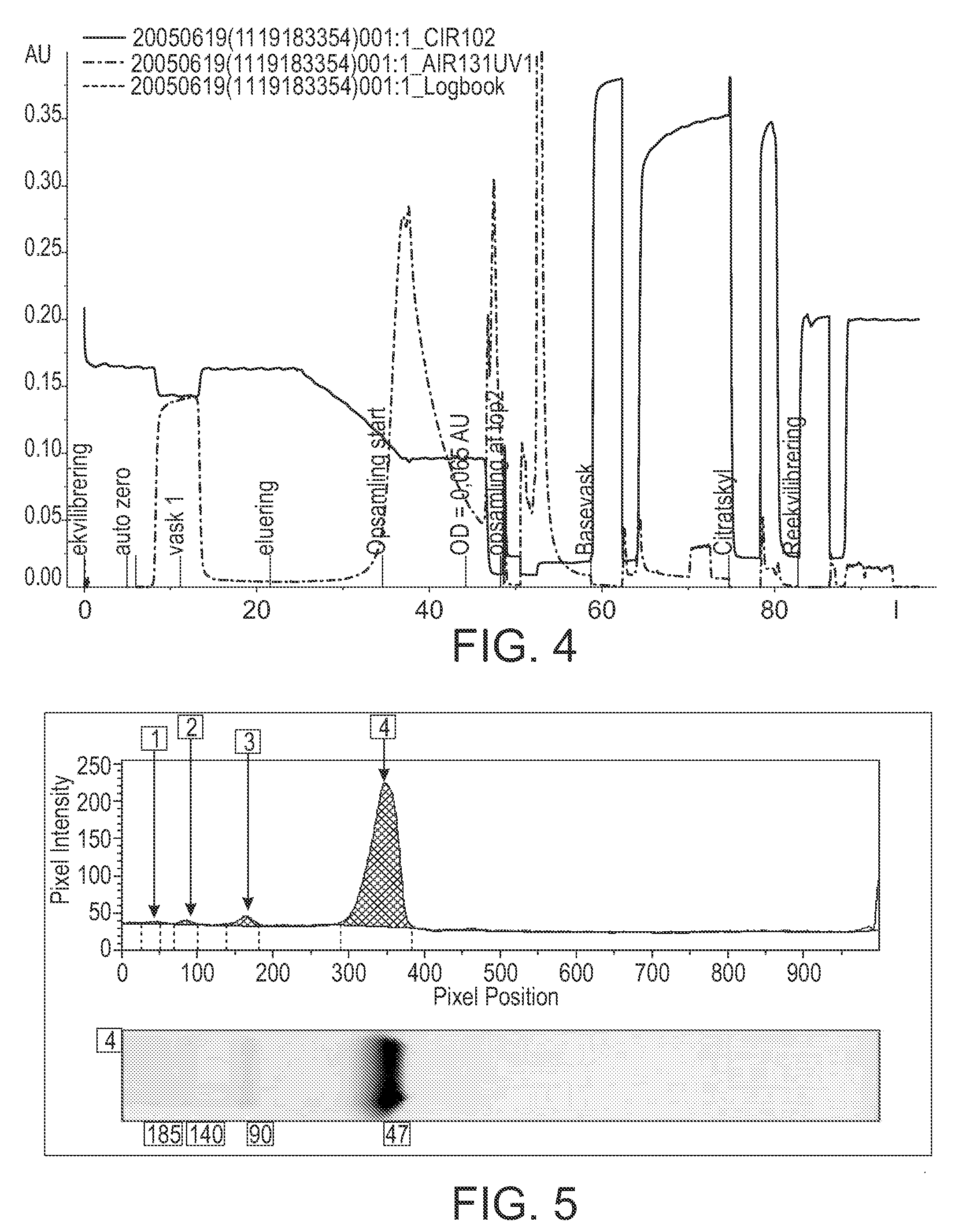
The invention described herein provides new methods of preparing purified Factor VII polypeptide drug substances in large quantities (industrial scale levels) that are associated with reduced content of product-related impurities (e.g., late eluting peaks) and / or that exhibit a relatively uniform glycosylation pattern.
Owner:NOVO NORDISK AS
Electrically conductive compositions and method of manufacture thereof
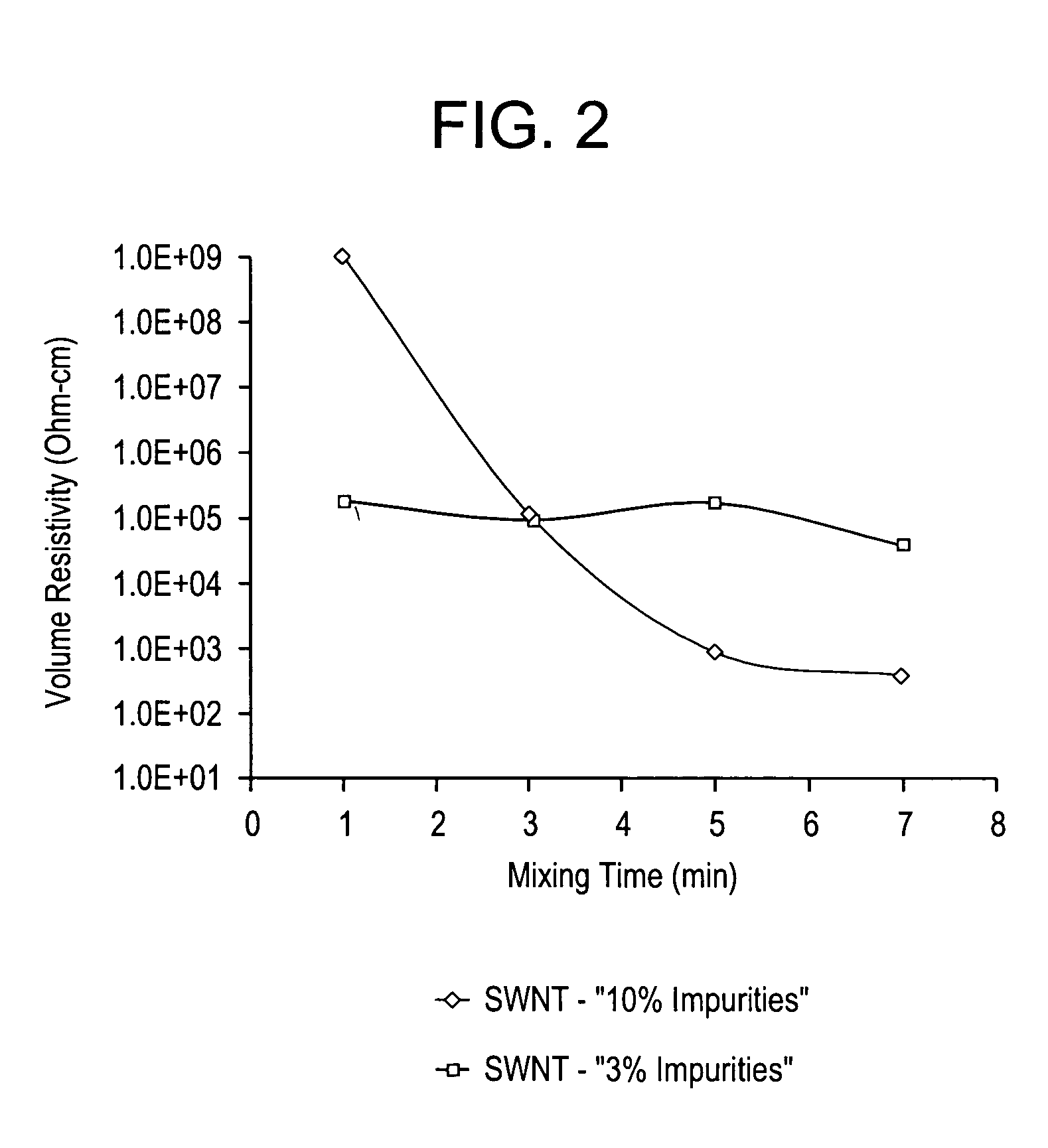
Disclosed herein is an electrically conductive composition comprising an organic polymer; and a carbon nanotube composition, wherein the carbon nanotube composition comprises carbon nanotubes that can rope and have greater than or equal to about 0.1 wt % production related impurities, based on the total weight of the carbon nanotube composition, and wherein the composition has a bulk volume resistivity less than or equal to about 1012 ohm-cm, and a notched Izod impact strength of greater than or equal to about 5 kilojoules / square meter.
Owner:SABIC INNOVATIVE PLASTICS IP BV
Purification of acidic proteins using ceramic hydroxyapatite chromatography
ActiveUS8058407B2Antibody mimetics/scaffoldsSolid sorbent liquid separationRelated impuritiesChemistry
The present invention provides a method of removing product-related inactive or partially active species, high molecular weight aggregates, as well as other process-related impurities from preparations of acidic proteins by using ceramic hydroxyapatite chromatography.
Owner:WYETH LLC
Conductive thermoplastic compositions, methods of manufacture and articles derived from such compositions
InactiveUS7309727B2Material nanotechnologyNon-metal conductorsSingle-Walled NanotubeRelated impurities
Disclosed herein is an electrically conductive precursor composition comprising an organic polymer precursor; a single wall nanotube composition, wherein the single wall nanotube composition contains at least 0.1 wt % of production related impurities; and an optional nanosized conductive filler. A conductive composition comprises an organic polymer; a single wall nanotube composition, wherein the single wall nanotube composition contains at least 0.1 wt % of production related impurities; and a nanosized conductive filler.
Owner:SABIC INNOVATIVE PLASTICS IP BV
Purification of acidic proteins using ceramic hydroxyapatite chromatography
The present invention provides a method of removing product-related inactive or partially active species, high molecular weight aggregates, as well as other process-related impurities from preparations of acidic proteins by using ceramic hydroxyapatite chromatography.
Owner:WYETH LLC
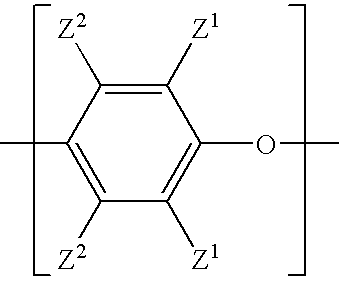
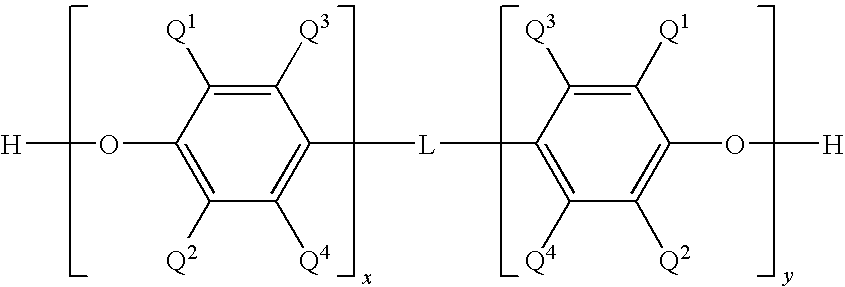
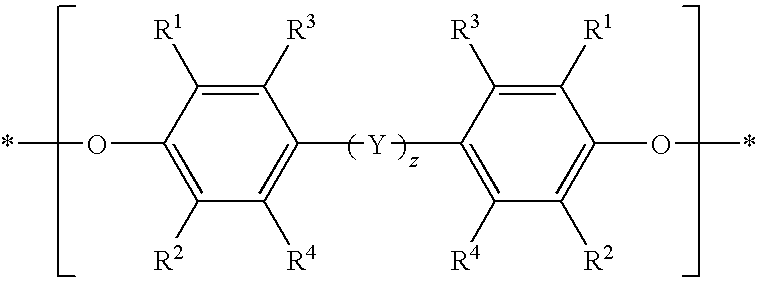
Poly(arylene ether) preparation method
Capped poly(arylene ether)s are prepared by a method that includes reacting a poly(arylene ether) with a capping agent to form a capping reaction mixture, washing the capping reaction mixture with a concentrated basic aqueous solution, and isolating the capped poly(arylene ether) by a total isolation method. The washing method is effective for removal of capping-related impurities, and surprisingly does not result in decomposition of the capped poly(arylene ether).
Owner:SHPP GLOBAL TECH BV
Protective layer for protecting a component against corrosion and oxidation at high temperatures, and component
InactiveUS20070065675A1Improve high temperature stabilityImprove long-term stabilityBiocideOrganic chemistryRheniumRare-earth element
Known protective layers with a high Al and / or Cr content and additionally strengthened by Re form brittle phases which become more brittle during use under the influence of carbon. The protective layer according to the invention has the composition 0.5 to 2% rhenium, 24 to 26% cobalt, 15 to 21% chromium, 9 to 11.5% aluminum, 0.05 to 0.7% yttrium and / or at least one equivalent metal selected from the group consisting of scandium and the rare earth elements, 0 to 1% ruthenium, remainder cobalt and / or nickel and manufacturing-related impurities, and reveals scarcely any embrittlement caused by Cr / Re precipitates.
Owner:SIEMENS ENERGY GLOBAL GMBH & CO KG
A method of purifying a peptide
InactiveCN101981048AHigh yieldCation exchanger materialsPeptide/protein ingredientsCyclic peptideCombinatorial chemistry
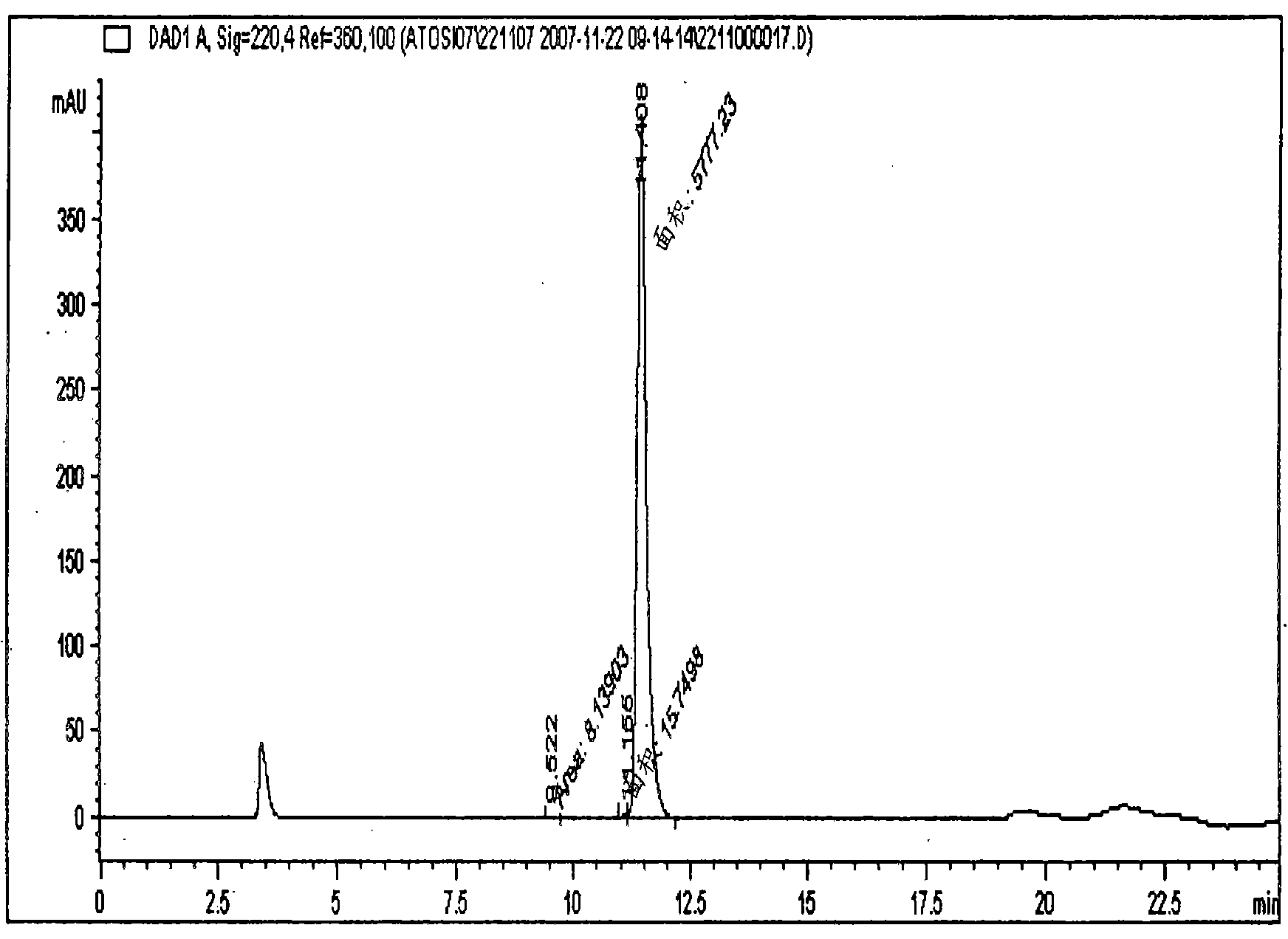
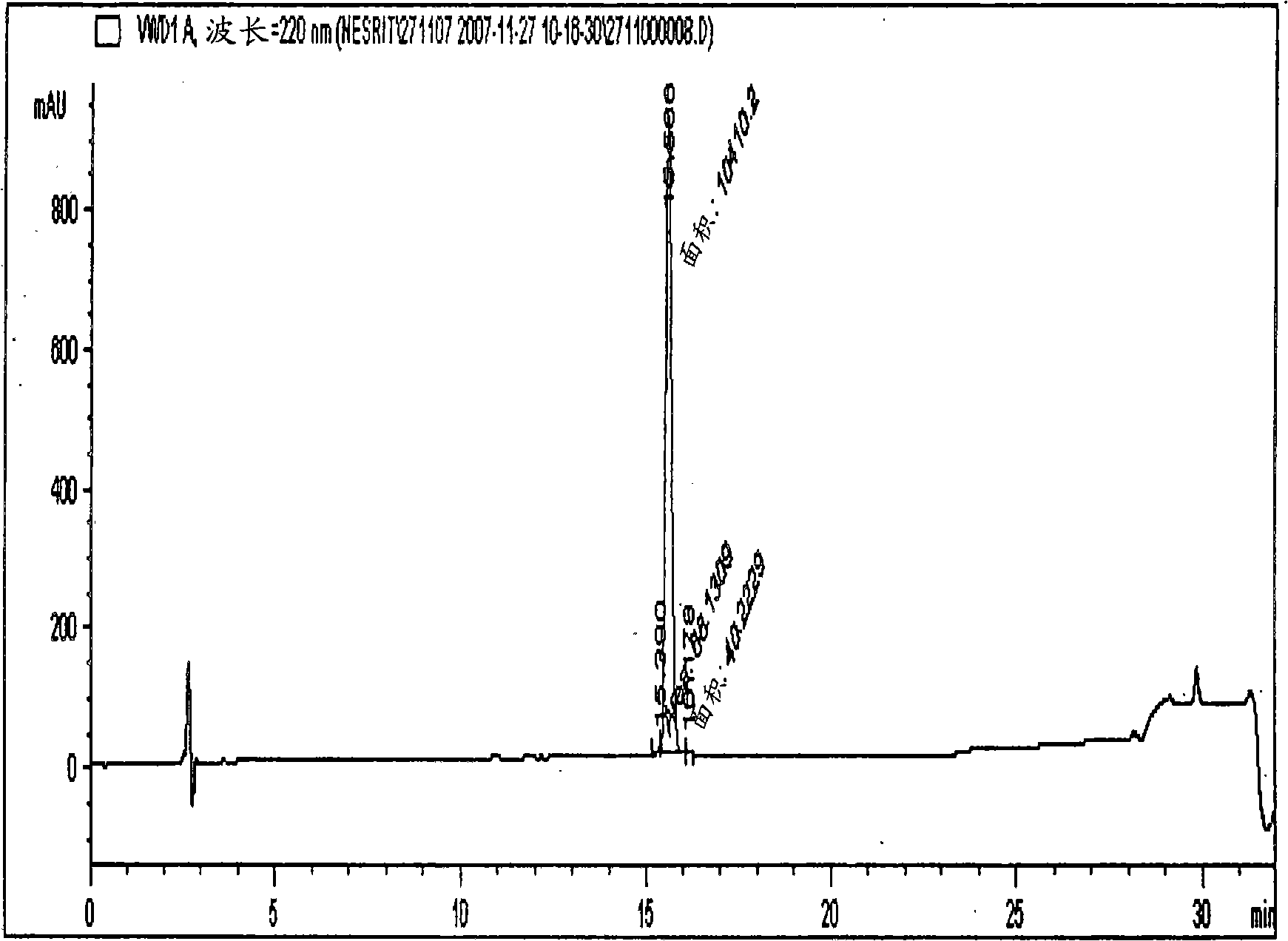
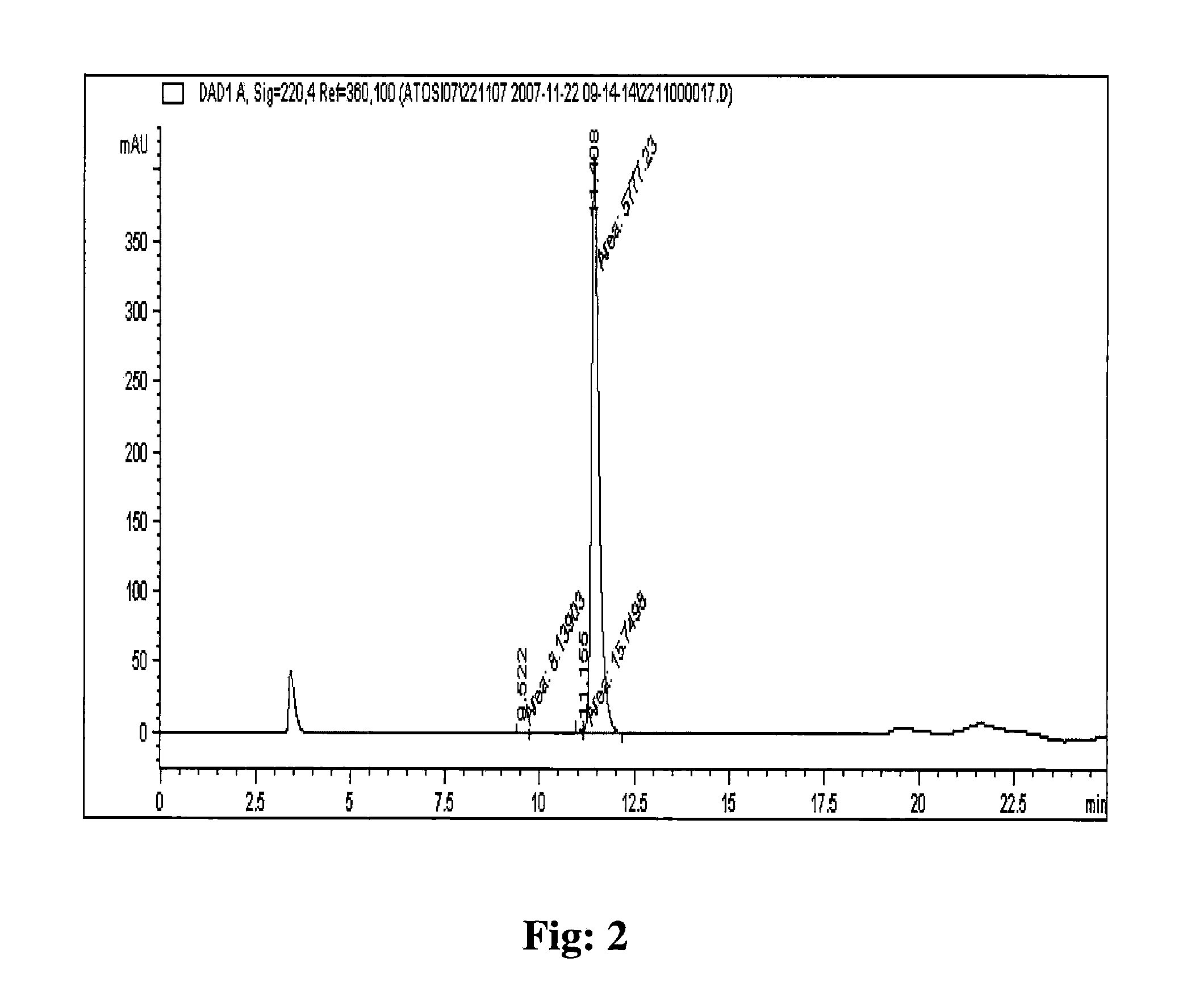
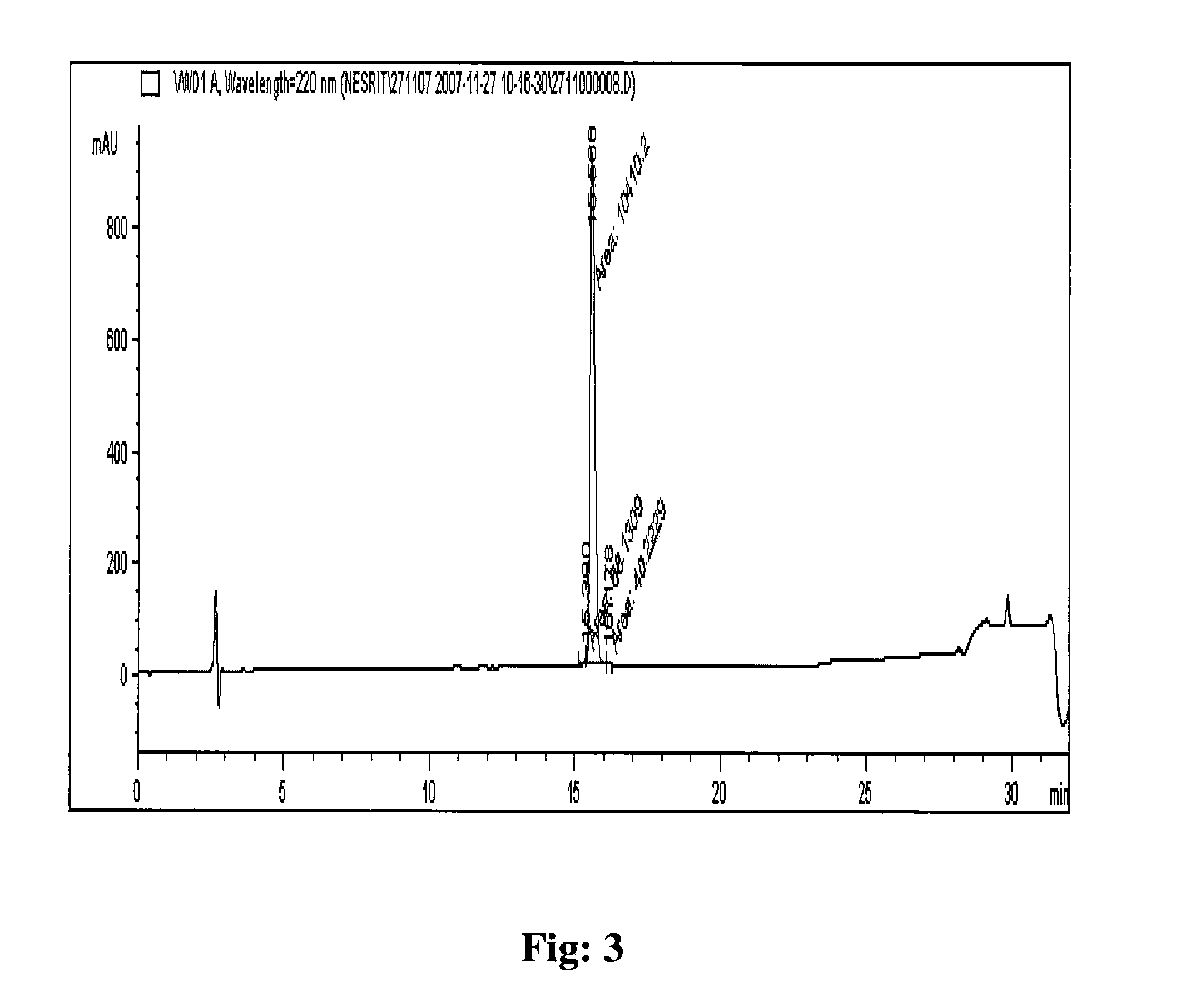
The invention relates, interalia, to the field of purification of peptides, notably cyclic or non-cyclic peptides their analogs or derivatives thereof. More particularly, the invention relates to a simplified and optimized purification process of cyclic peptides from a composition comprising the said peptide and at least one related impurity by chromatographic procedures enabling high yields, selectivity and purity of the desired end product. The improved process is particularly useful for the preparation of eptifibatide, exenatide, atosiban, nesiritide and their respective derivatives and analogs. The polypeptides are prepared in high purity of at least about 96 %, and preferably at least about 99 %.
Owner:BIOCON LTD
Iron-nickel alloy
Owner:OUTOKUMPU VDM GMBH
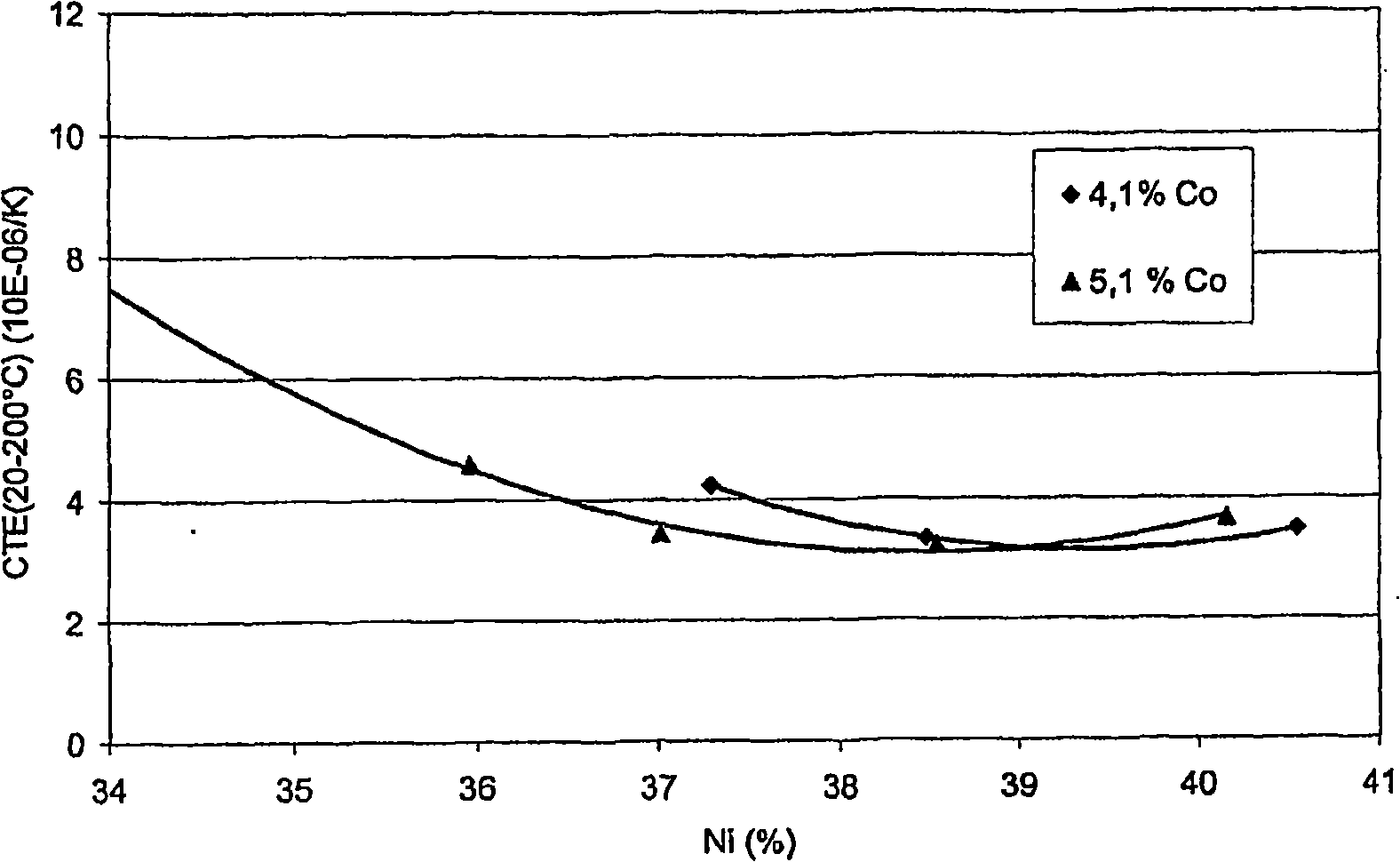
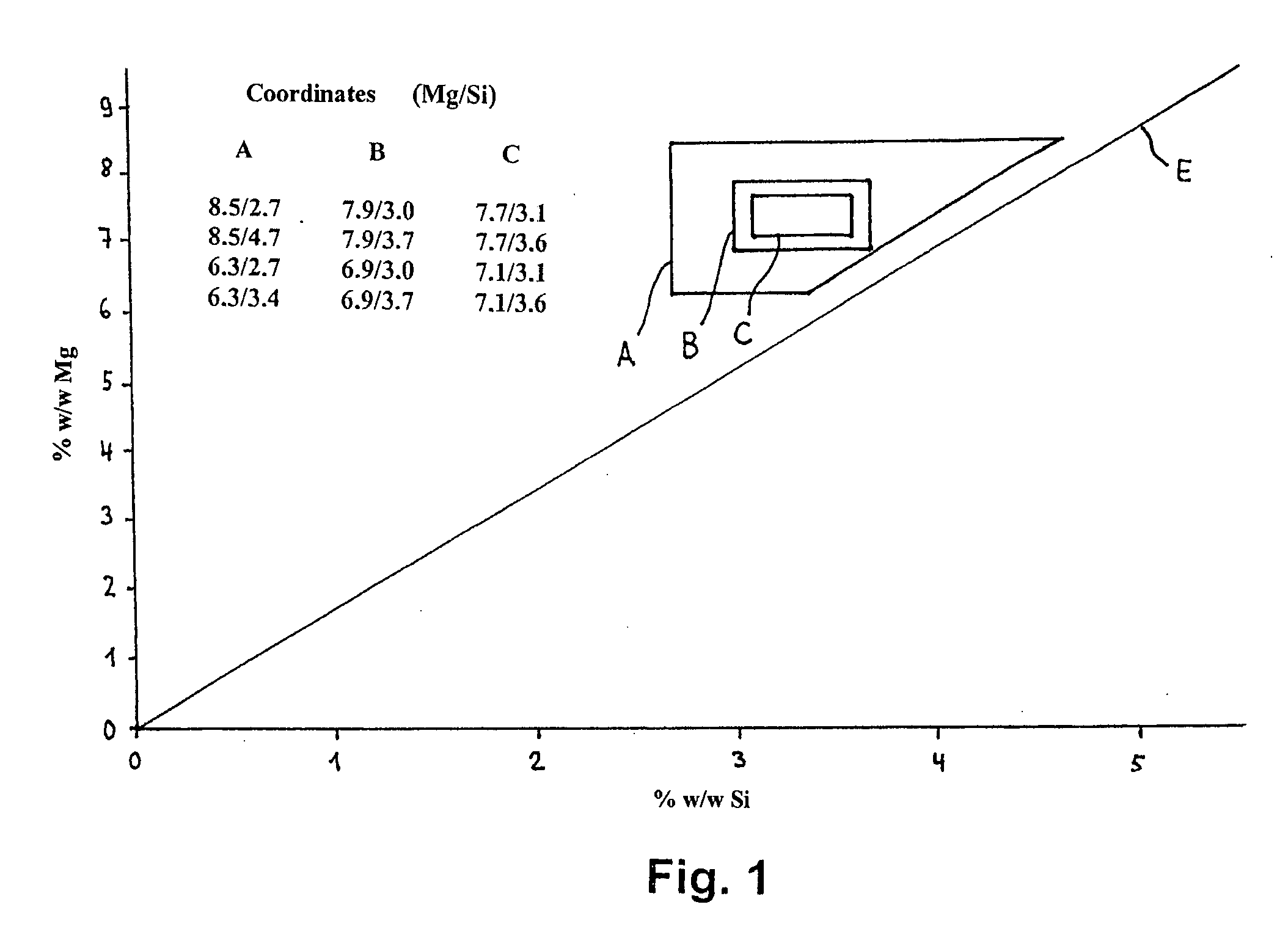
High temperature aluminium alloy
In an aluminium alloy of type AlMgSi with good creep strength at elevated temperatures for the production of castings subject to high thermal and mechanical stresses the contents of the alloying elements magnesium and silicon in % w / w in a Cartesian coordinate system are limited by a polygon A with the coordinates [Mg; Si] [8.5; 2.7] [8.5; 4.7] [6.3; 2.7] [6.3; 3.4] and that the alloy also contains0.1 to 1% w / w manganesemax. 1% w / w ironmax. 3% w / w coppermax. 2% w / w nickelmax. 0.5% w / w chromiummax. 0.6% w / w cobaltmax. 0.2% w / w zincmax. 0.2% w / w titaniummax. 0.5% w / w zirconiummax. 0.008% w / w berylliummax. 0.5% w / w vanadiumas well as aluminium remainder rest with further elements and manufacturing-related impurities of individually max. 0.05% w / w and max. 0.2% w / w in total.The alloy is suitable in particular for the production of cylinder crankcases by the pressure die casting method.
Owner:АЛЮМИНИУМ РАЙНФЕЛЬДЕН ГМБХ
Method for Producing a Wear-Resistant Aluminum Alloy,An Aluminum Alloy Obtained According to the Method, and Ues Thereof
The invention relates to a method for producing a wear-resistant aluminum alloy, to an aluminum alloy produced according to the method, and to the use thereof. The method comprises the steps of: (i) providing an aluminum alloy having the composition Fe: 3-10; X: 3-10; Y: 0-1.5; Z: 0-10; wherein X represents an element or combination of elements (a) V and Si; (b) Cr and Ti; (c) Ce; or (d) Mn; each time with the proviso that the proportion of the individual elements in the combinations of elements (a) and (b) is at least 0.5 wt %; Y represents one or more grain-refining elements selected from the group of B, Ce, Sr, Sc, Mg, Nb, Mn and Zr, unless already present as X; Z represents one or more additives increasing the heat resistance, selected from the group of ceramic fibers, particles and platelets, the figures referring to % by weight in the alloy, and Al and production-related impurities representing the remaining proportion in the alloy to make 100 wt %, with the proviso that the proportion of Al in the alloy is at least 80 wt %; (ii) melting the aluminum alloy, dissolving and homogenizing the alloy elements at temperatures of from 650° C. to 1,000° C.; and (iii) casting the melt into a casting mold at a casting temperature ranging from the melting temperature of the alloy up to a temperature 150° C. above the melting temperature.
Owner:BAM BUNDESANSTALT FUER MATERIALFORSCHUNG UND
Higher-strength, cold-formable steel and steel sheet product consisting of such a steel
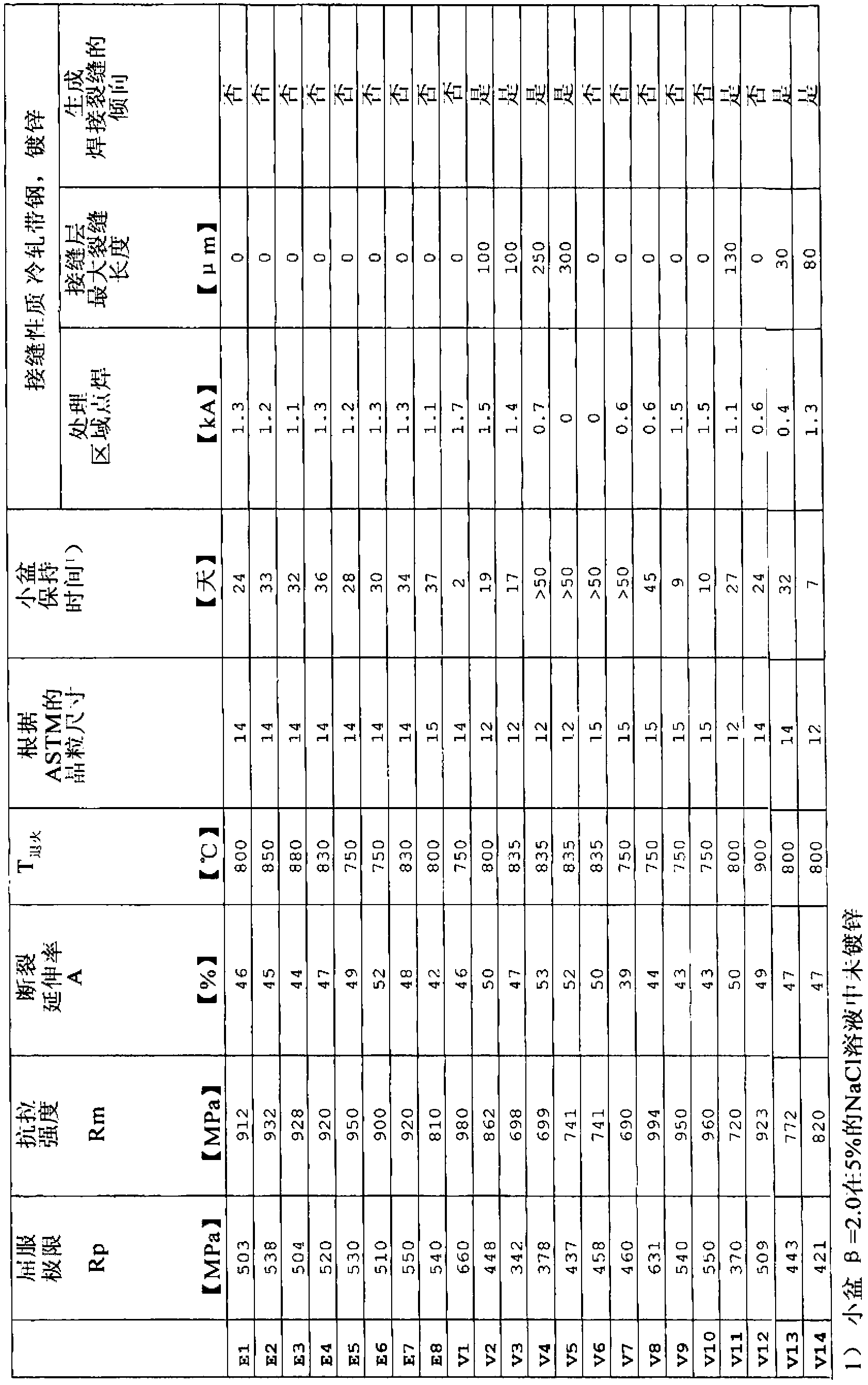
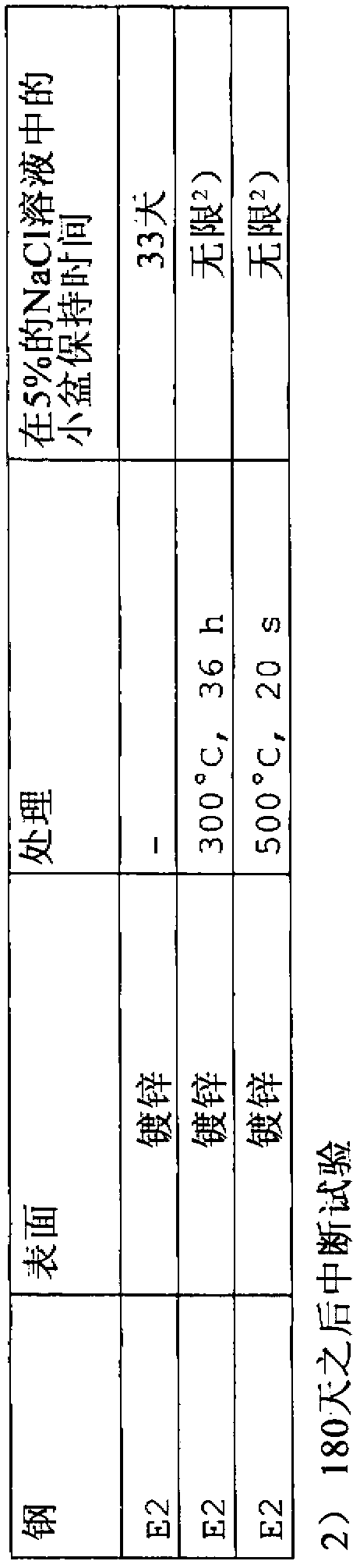
The invention relates to a higher-strength, cold-formable steel and to a steel sheet product produced from such a steel, in which an optimal combination of weldability and a low tendency toward delayed cracking as well as good strength and hot- and cold-formability are assured. In order to achieve this, a steel according to the invention contains (in % by weight) C: 0,1-1,0%, Mn: 10-25%, Si: up to 0.5%, Al: 0.3-2%, Cr: 1.5-3.5%, S: <0.03%, P: <0.08%, N: <0.1%, Mo: <2%, B: <0.01%, Ni: <8%, Cu: <5%, Ca: up to 0.015%, at least one element from the "V, Nb" group provided that: Nb: 0.01-0.5%, V: 0.01-0.5% and optionally Ti: 0.01-0.5%, and remainder being iron and unavoidable manufacturing-related impurities.
Owner:THYSSENKRUPP STEEL EURO AG
Method for synthesizing clindamycin phosphate
InactiveCN101830946AImprove conversion rateIncrease contentSugar derivativesSugar derivatives preparationSolubilityHydrolysis
The invention discloses a method for synthesizing clindamycin phosphate, which comprises the following steps of: performing ketal protection reaction on clindamycin hydrochloride alcoholate at the temperature of between 2.0 below zero and 2.0 DEG C under the action of acetone and phosphorus oxychloride to form propylidene clindamycin; and performing esterification, hydrolysis, adsorption, washing, deabsorption, concentration, coarse crystallization, decoloration, refining and drying to obtain the finished product of clindamycin phosphate. Because a new catalyst 4-dimethylaminopyridine participates in the esterification in the rection system, the phosphorylating reaction is performed completely, and the conversion rate of raw materials is improved. Meanwhile, due to the secondary crystallization method, the problems of poor color grade and poor powder solubility are solved, and the operating conditions are mild and simple. By adopting triethylamine to replace partial pyridine, the esterification is pushed forwards; the reaction period is shortened; and importantly, related impurities in the finished product and the production cost are reduced, and the content is improved.
Owner:南阳普康药业有限公司 +1
Hydrophobic interaction chromatography purification of factor VII polypeptides
ActiveUS9023992B2Reduce contentReduce, or virtually eliminate, the presence of late elution peaks in the drug substanceHydrolasesPeptide/protein ingredientsFactor VIIRelated impurities
The invention described herein provides new methods of preparing purified Factor VII polypeptide drug substances in large quantities (industrial scale levels) that are associated with reduced content of product-related impurities (e.g., late eluting peaks) and / or that exhibit a relatively uniform glycosylation pattern.
Owner:NOVO NORDISK HEALTH CARE AG
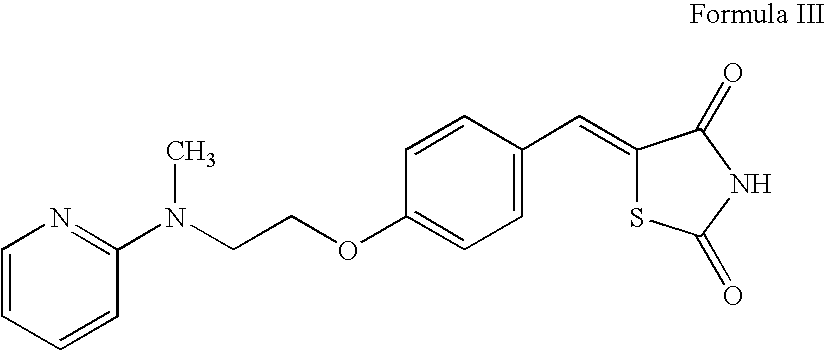
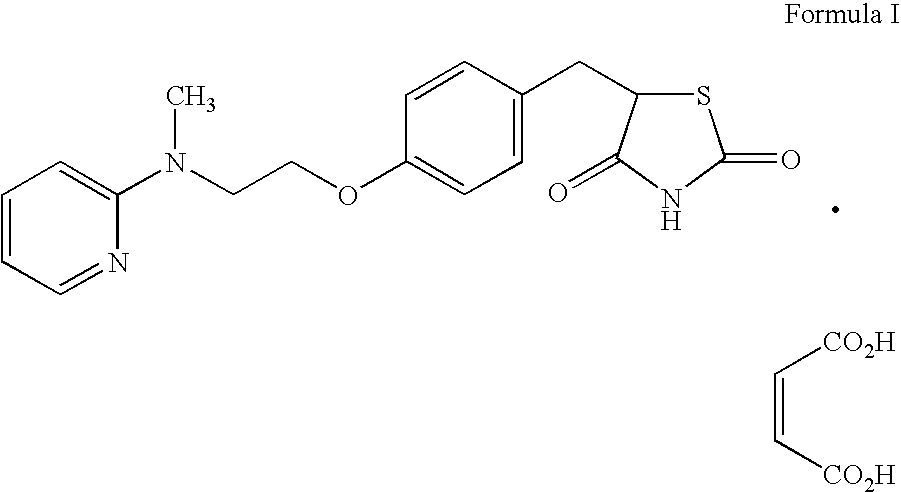
Preparation of rosiglitazone and its salts
The present invention relates to rosiglitazone and its pharmaceutically acceptable salts free of at least one of the process related impurities, in particular the dehydro and the succinic acid impurities of rosiglitazone, wherein said impurities are present in an amount of about 05 mg to not more than about 15 mg, and processes for their preparation.
Owner:DR REDDYS LAB LTD +1
Poly(arylene ether) preparation method
Capped poly(arylene ether)s are prepared by a method that includes reacting a poly(arylene ether) with a capping agent to form a capping reaction mixture, washing the capping reaction mixture with a concentrated basic aqueous solution, and isolating the capped poly(arylene ether) by a total isolation method. The washing method is effective for removal of capping-related impurities, and surprisingly does not result in decomposition of the capped poly(arylene ether).
Owner:SHPP GLOBAL TECH BV
Hydrophobic interaction chromatography purification of factor vii polypeptides
ActiveUS20110064719A1Reduce contentReduce, or virtually eliminate, the presence of late elution peaks in the drug substanceHydrolasesPeptide/protein ingredientsFactor iiFactor VII
The invention described herein provides new methods of preparing purified Factor VII polypeptide drug substances in large quantities (industrial scale levels) that are associated with reduced content of product-related impurities (e.g., late eluting peaks) and / or that exhibit a relatively uniform glycosylation pattern.
Owner:NOVO NORDISK AS
Analytic method of omeprazole related substance
The invention provides an analytic method of an omeprazole related substance. The analytic method is characterized in that high performance liquid chromatography is adopted, an organic solvent and salt mixed phase is used for dissolving a sample, acetonitrile and phosphate with the pH of 7.6 are used as mobile phases, and the gradient elution is carried out on octyl bonds and a silica gel chromatographic column. The analytic method can be used for simply and effectively solving the defects of incapability of separating the rated substance and solvent peaks, little detection amount of impurities and the like and efficiently separating and identifying related impurities of the omeprazole.
Owner:CSPC OUYI PHARM CO LTD
Steel, and processing method for the production of higher-strength fracture-splittable machine components
The invention relates to a steel and a processing method for higher-strength fracture-splittable machine components that are composed of at least two fracture-splittable parts. Said steel and method are characterized in that the chemical composition of the steel (expressed in percent by weight) is as follows: C is between 0.40% and 0.60%; Si is between 0.20% and 1.00%; Mn is between 0.540% and 1.50%; Cr is between 0% and 1.00%; Ni is between 0% and 0.50%; Mo is between 0% and 0.20%; Nb is between 0% and 0.050%; V is between 0% and 0.30%; Al is between 0% and 0.05%; N is between 0.005% and 0.020%; the rest is composed of iron and smelting-related impurities and residual matter.
Owner:GEORGSMARIENHUTTE
Method of Purifying a Peptide
InactiveUS20100317827A1High yieldPeptide/protein ingredientsSolid sorbent liquid separationCyclic peptidePurification methods
The invention relates, interalia, to the field of purification of peptides, notably cyclic or non-cyclic peptides their analogs or derivatives thereof. More particularly, the invention relates to a simplified and optimized purification process of cyclic peptides from a composition comprising the said peptide and at least one related impurity by chromatographic procedures enabling high yields, selectivity and purity of the desired end product. The improved process is particularly useful for the preparation of eptifibatide, exenatide, atosiban, nesiritide and their respective derivatives and analogs. The polypeptides are prepared in high purity of at least about 96%, and preferably at least about 99%.
Owner:BIOCON LTD
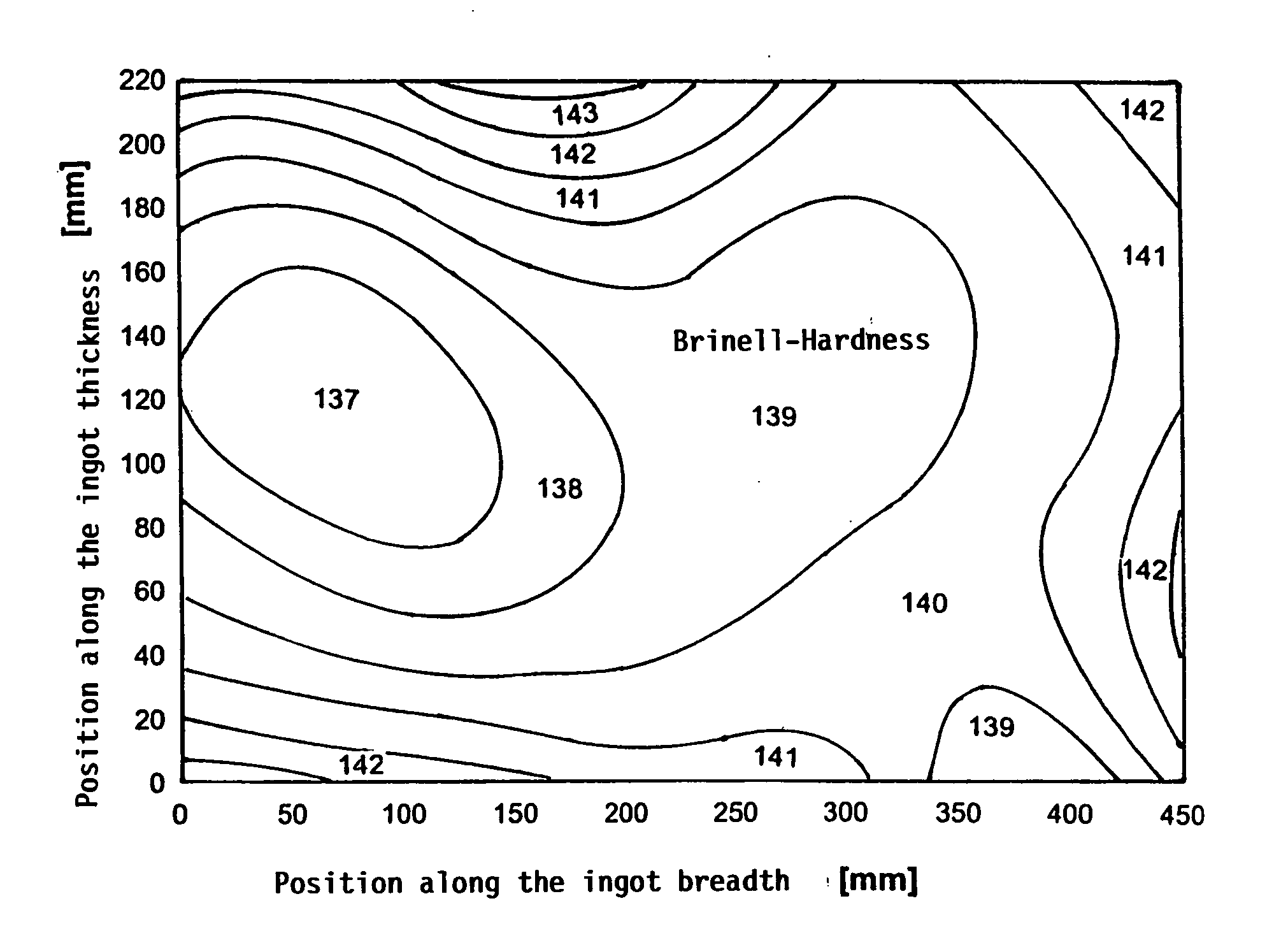
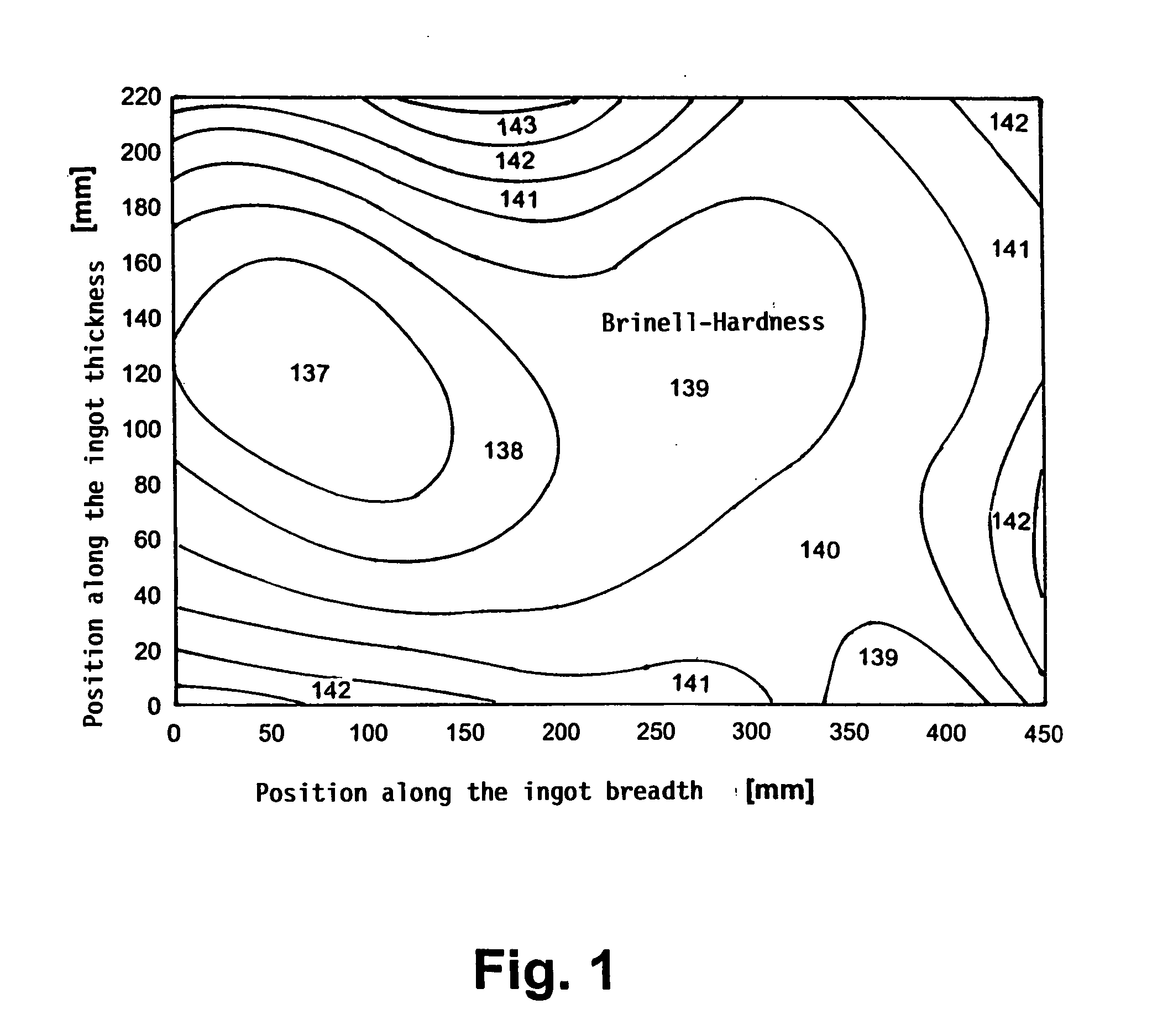
Aluminium alloy with increased resistance and low quench sensitivity
An aluminium alloy having high mechanical strength and low quench sensitivity comprising 4.6 to 5.2 wt. % Zn, 2.6 to 3.0 wt. % Mg, 0.1 to 0.2 wt. % Cu, 0.05 to 0.2 wt. % Zr, max. 0.05 wt. % Mn, max. 0.05 wt. % Cr, max. 0.15 wt. % Fe, max. 0.15 wt. % Si, max. 0.10 wt. % Ti and aluminium as the remainder along with production related impurities, individually max. 0.05 wt. %, in total max. 0.15 wt. %. A process for producing plates having a thickness of more than 300 mm for manufacturing moulds for injection-moulding plastics is made up of the following steps: continuous casting the alloy into ingots having a thickness greater than 300 mm, heating the ingots to a temperature of 470 to 490° C. with a max. heating rate of 20° C. / h between 170 and 410° C., homogenising the ingots for 10 to 14 h at a temperature of 470 to 490° C., cooling the ingots in still air to an intermediate temperature of 400-410° C., cooling the ingots by means of forced air cooling from the intermediate temperature of 400-410° C. to a temperature of less than 100° C., cooling the ingots to room temperature, artificially age-hardening the ingots at elevated temperature. The artificially age-hardened ingots can be employed for manu-facturing moulds for injection-moulding plastics.
Owner:ALCAN TECH & MANAGEMENT LTD
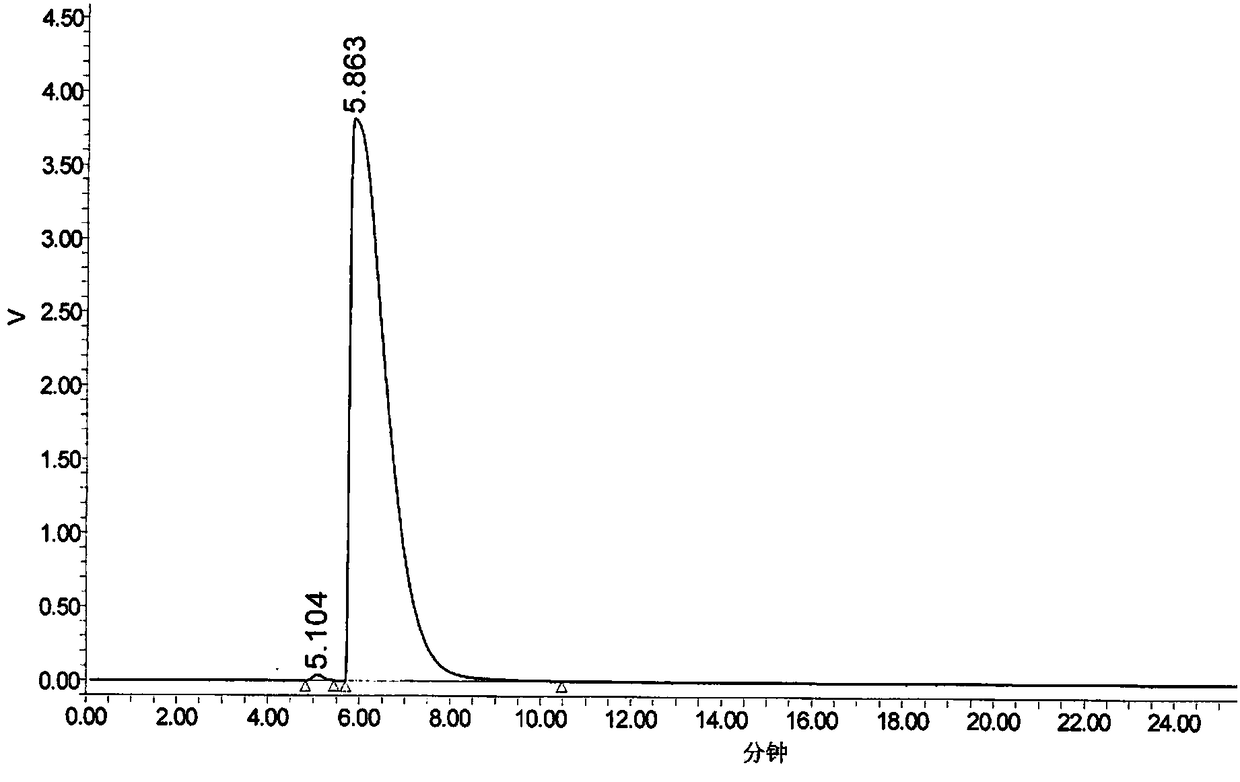
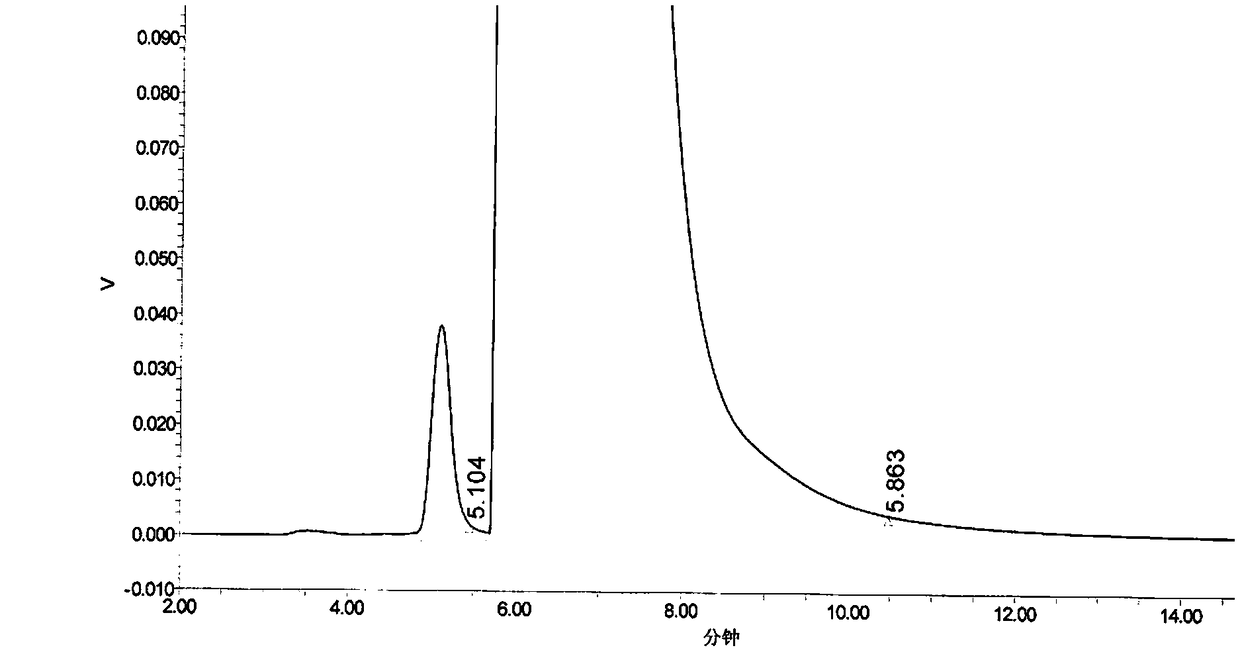
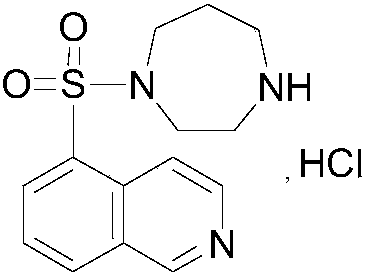
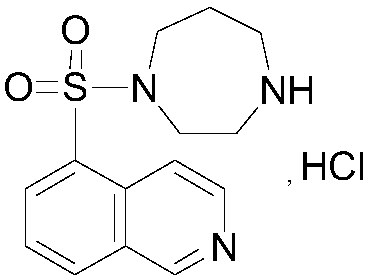
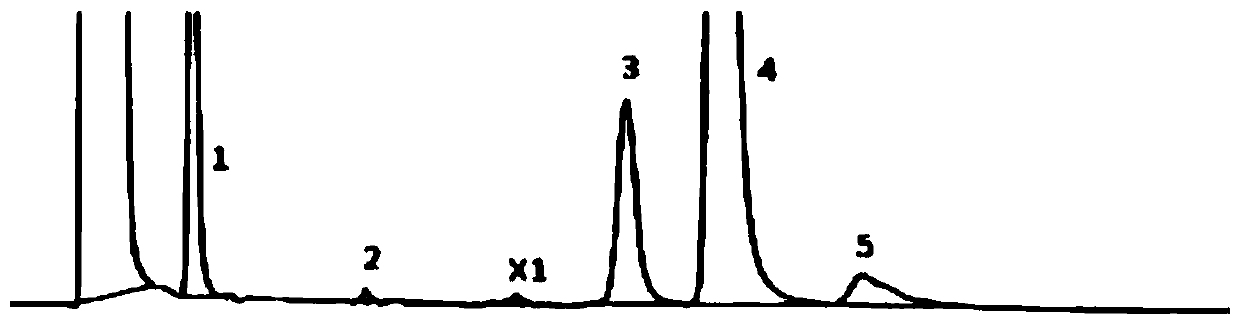
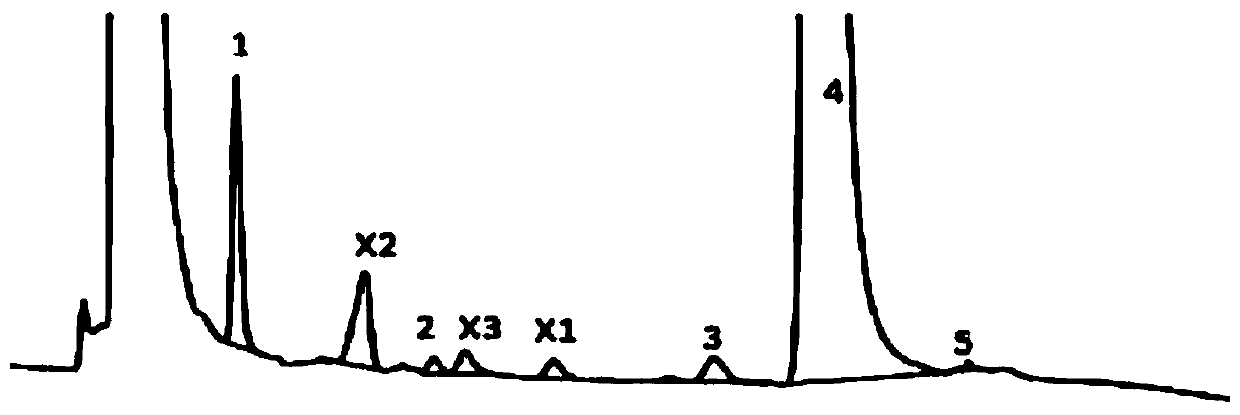
Method for determination of content of related impurity (5-methyl isoquinolinesulfonic acid) of fasudil hydrochloride
The invention provides a method for determination of the content of a related impurity (5-methyl isoquinolinesulfonic acid) of fasudil hydrochloride. The method is characterized in that a reversed-phase high-performance liquid chromatographic peak area normalization method is adopted for quantitative analysis; for the chromatographic conditions, the chromatographic column is an ACCHROM XAmide column; the flow speed is 0.9-1.1ml / min; the detection wavelength is 238nm; the column temperature is 28-32 DEG C; the sample injection amount is 20mu l; a mobile phase A is 0.05mol / l ammonium dihydrogenphosphate aqueous solution; a mobile phase B is methanol; A:B is equal to (91-94): (6-9) (V:V). The method provided by the invention is convenient to control the product quality in the processes of production and quality control, and has the advantages of low cost, simpleness, easy implementation, high accuracy and precision, good stability and reproducibility and high sensitivity.
Owner:SHANDONG XINHUA PHARMA CO LTD
HPLC detection method for fasudil hydrochloride related substances
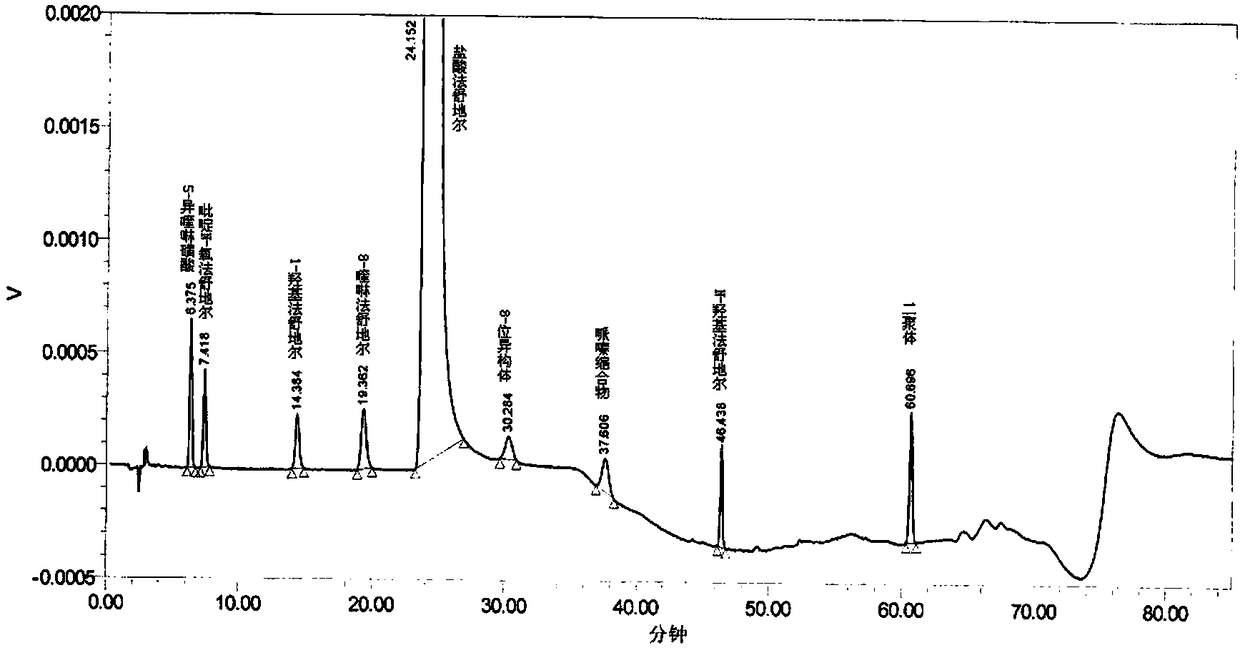
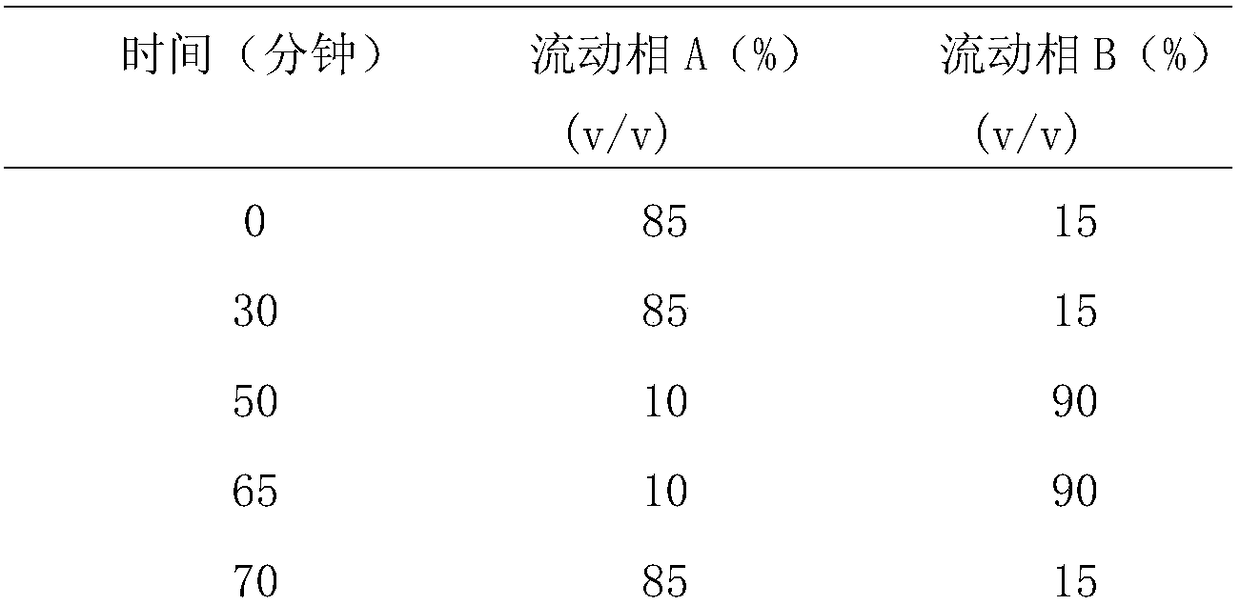
The invention provides a content determination method for fasudil hydrochloride related impurities, namely 5-isoquinoline sulfonic acid, pyridine N-oxyfasudil, 1-hydroxyfasudil, 8-quinoline fasudil, an 8-isomer, a piperazine condensation compound, N-hydroxyfasudil and a dimer. Quantitative analysis is performed by a reversed-phase high performance liquid chromatography peak area normalization method, and a chromatographic column with an octadecyl bonded phase as a stationary phase is used; the detection wavelength is 275nm; a mobile phase A is a phosphate buffer (pH of 6.95-7.05)-methanol (85:15), a mobile phase B is a phosphate buffer (pH of 6.95-7.05)-methanol (40:60), and gradient elution is performed. By the content determination method, product quality control during production and quality control is facilitated; the content determination method has the advantages of low cost, simplicity, easiness in implementation, high accuracy and precision and good stability and reproducibility.
Owner:SHANDONG XINHUA PHARMA CO LTD
Method of obtaining a purified, biologically active heterologous protein
ActiveUS8802816B2Polypeptide with localisation/targeting motifPeptide/protein ingredientsHeterologousOrganism
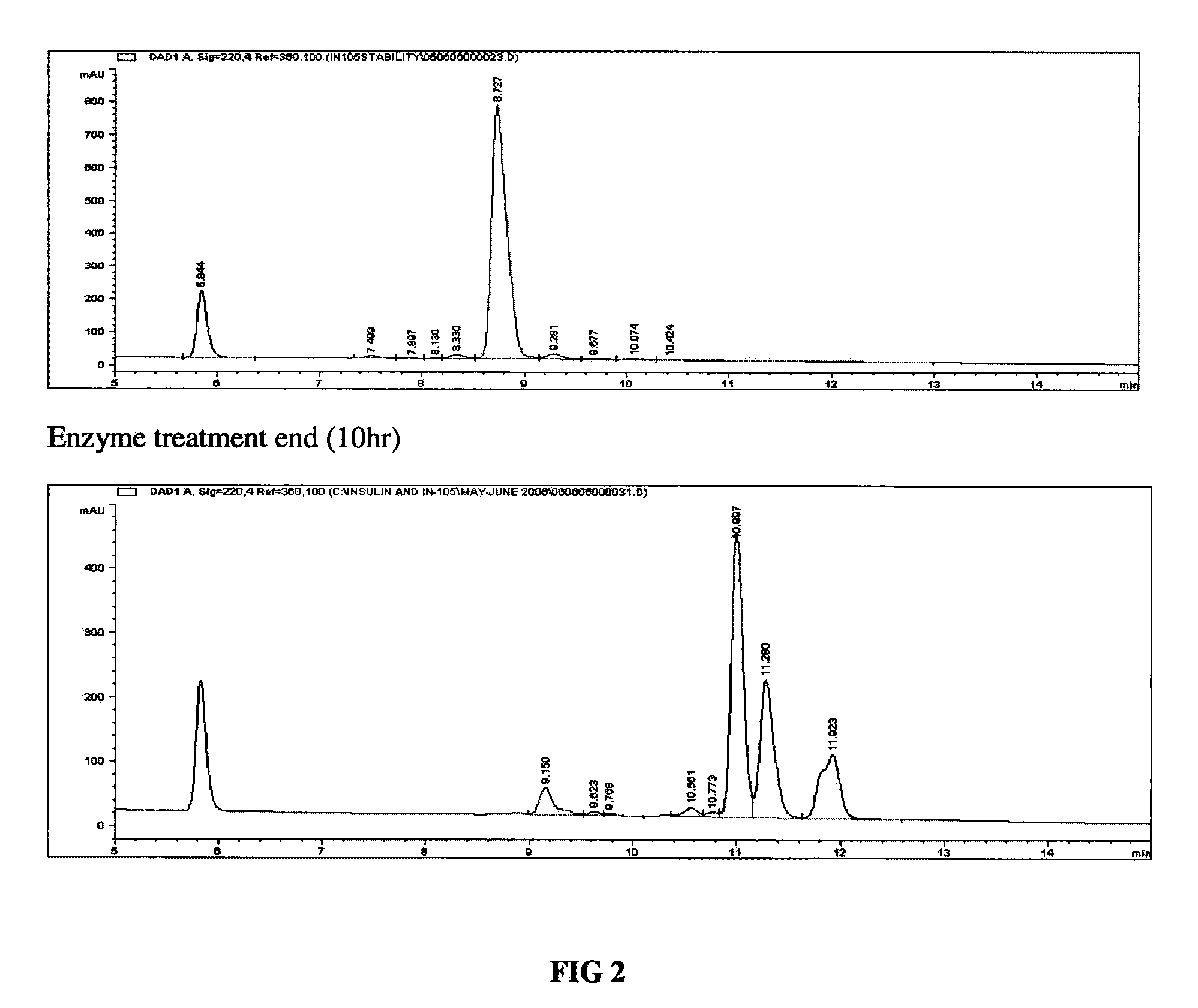
The invention relates to methods of separation and / or purification of impurities yielding a purified heterologous protein product devoid of related impurities or with substantially minimal quantities of such glycosylated impurities. More specifically, the invention relates to the identification of glycosylated forms of insulin analogues such as glargine impurities characterized post expression in yeast based systems such as Pichia pastoris. The invention also relates to methods used to clone gene encoding the protein insulin glargine; inserting the related gene in a suitable yeast host; producing culture of the recombinant strain, stimulating expression of the heterologous polypeptide, its secretion and purification post fermentation and related enzymatic conversions.
Owner:BIOCON LTD
Medicinal composition containing sitagliptin or pharmaceutically acceptable salt thereof and preparation method thereof and application
ActiveCN109157522AQuality improvementIncrease contentOrganic active ingredientsMetabolism disorderSitagliptinHydrogen phosphate
The invention relates to a medicinal composition. The medicinal composition comprises sitagliptin, pharmaceutically acceptable salt thereof and / or hydrate of the salt, and anhydrous calcium hydrogen phosphate. The medicinal composition is further used for preparing a solid preparation, and is especially prepared into a tablet by a direct tabletting method. Through selection of the medicinal composition, especially selection of the specific anhydrous calcium hydrogen phosphate as a raw material, the quality control way of the medicinal composition using the sitagliptin, the pharmaceutically acceptable salt thereof and / or the hydrate of the salt as an active component can be significantly improved, and even production of related impurities in the medicinal composition can be significantly improved so as to improve the stability of the medicinal composition and relevant dosage forms.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
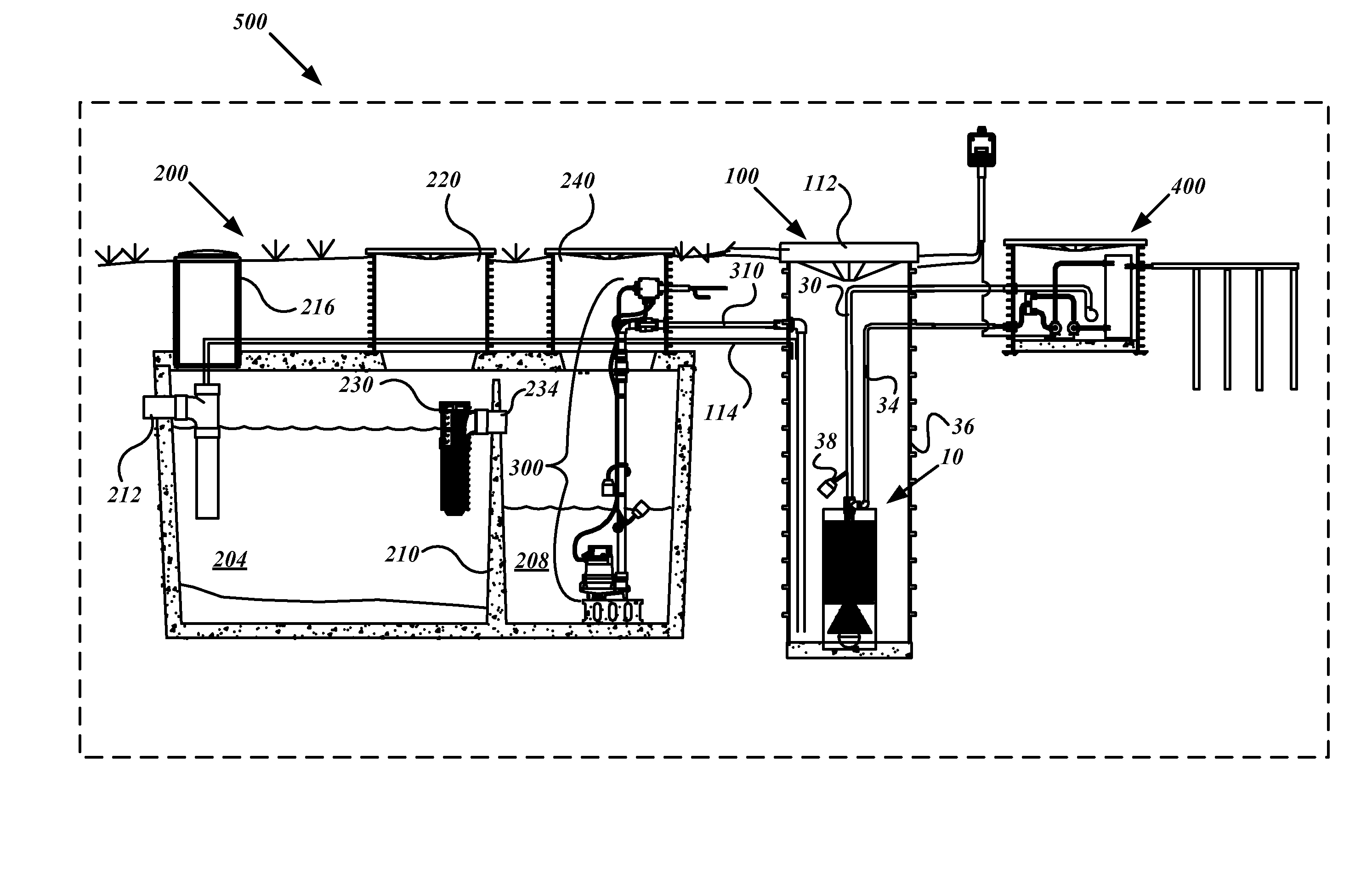
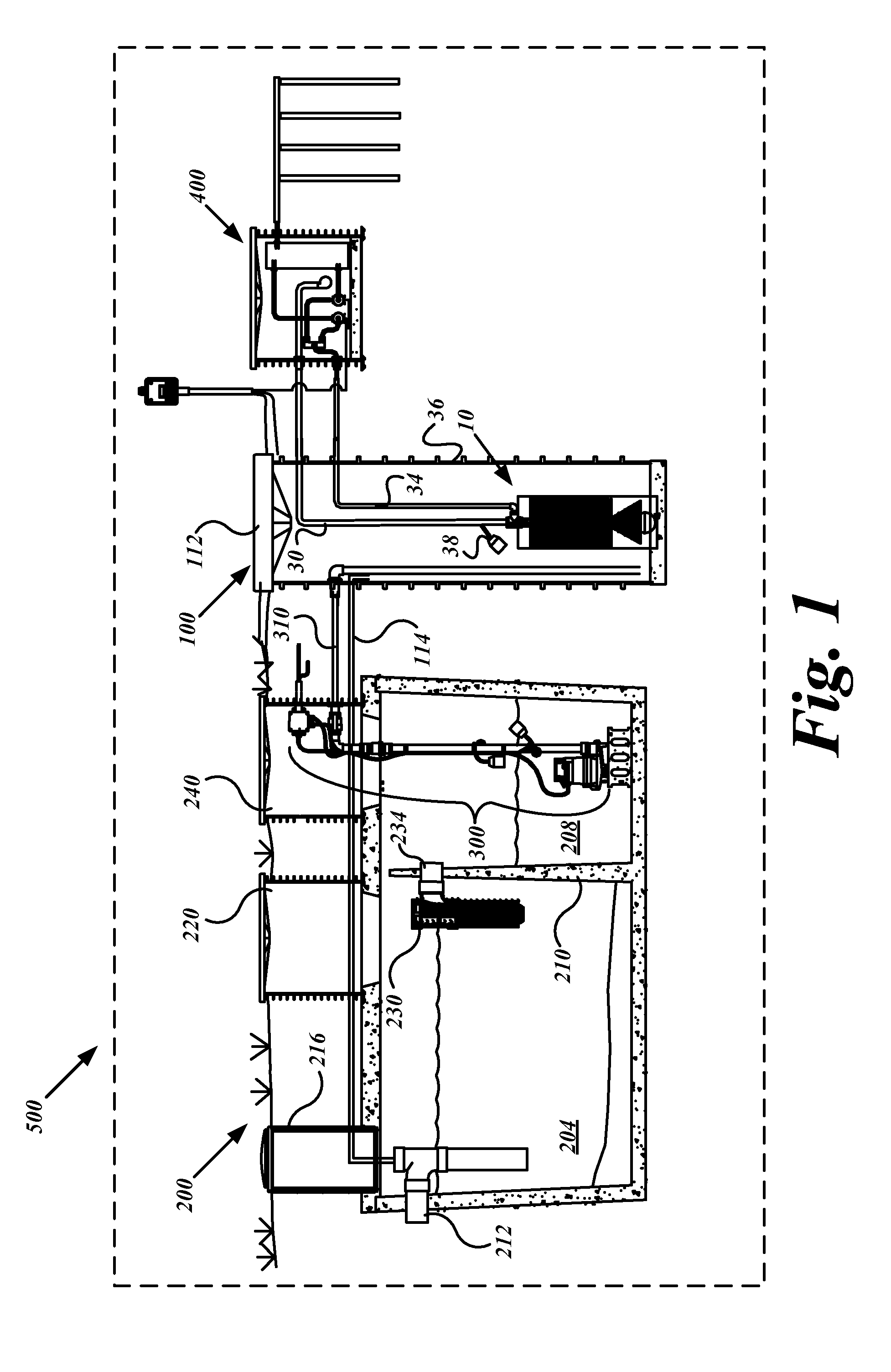
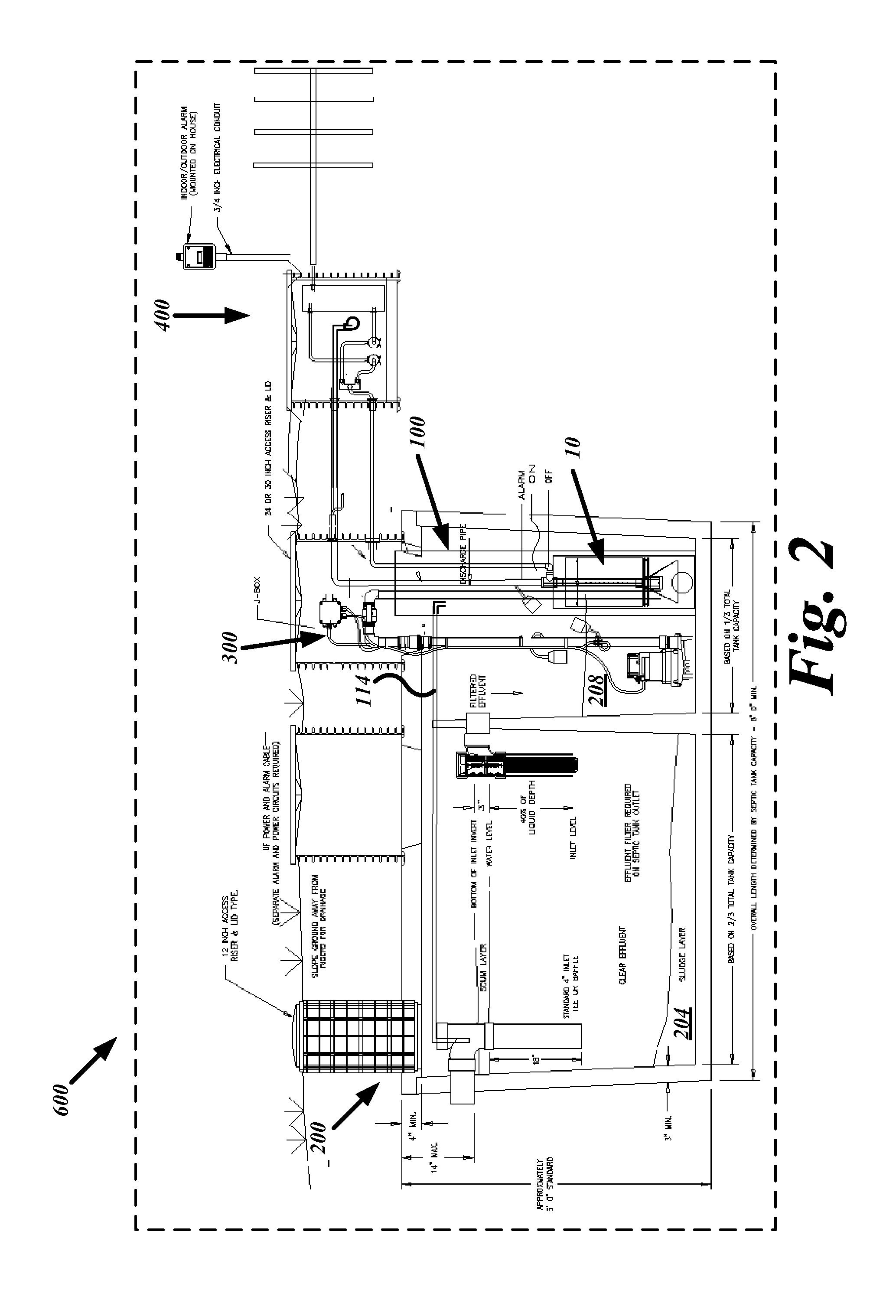
System, device and method for on-site wastewater processing
InactiveUS20090223891A1Sufficiently clean waterSmall sizeUltrafiltrationLiquid displacementFiltrationWastewater
A modular wastewater clarification device that may be positioned external to a septic tank or, alternatively, installed internally within a septic tank chamber to produce sufficiently clean water for lawn and agricultural uses. The modular clarification device includes a filter having a smaller size than the pre-filter bridging between the primary and secondary chambers of a septic tank. In one embodiment, the modular filtration unit resides outside the two-chambered septic tank and receives pre-filtered septic tank effluent fluids stored in the secondary chamber that has accumulated pre-filtered effluent. In another embodiment, the modular wastewater clarification device resides inside the secondary chamber and filters the accumulated pre-filtered effluent. The modular filtration device, having a substantially smaller pore size range than the inter-chamber pre-filter, releases clean water having substantially lowered bacterial and waster related impurities sufficient to meet water release standards suitable for lawn, garden, and agricultural uses.
Owner:GAUTHIER RAY
High-performance liquid chromatography separation and determination method for related impurities in fosfomycin trometamol raw material drug and preparations thereof and application thereof
The invention discloses a high-performance liquid chromatography separation and determination method for impurities in a fosfomycin trometamol raw material drug and preparations thereof and an application thereof, aiming at separating the impurities. According to the method, the aminopropyl silane-bonded silica gel is used as a chromatographic column of a filler, a differential detector is used for detection; and a phosphate buffer solution and methanol-acetonitrile are mixed as a mobile phase; the method includes the following steps of: 1, preparation of a system suitability solution: wettinga raw material drug or a preparation with water, heating, and dissolving and diluting the raw material drug or the preparation by the mobile phase to obtain a solution A; dissolving and diluting theraw material drug or the preparation by using the solution A to obtain the system suitability solution; 2, preparation of a test solution: taking the raw material drug or the preparation, and adding the mobile phase for dissolving and dilution to obtain the set content of fosfomycin trometamol as the test solution; 3, preparation of a reference solution: diluting the test solution by using the mobile phase to obtain a solution containing fosfomycin trometamol equivalent to a mass concentration of 0.3-0.5% of the test solution as the reference solution; and 4, determination method: separately injecting the above three solutions into a liquid chromatograph, and calculating the content of each impurity in the raw material drug by using a principal component self-control method.
Owner:SHANXI C&Y PHARMACEUTICAL GROUP CO LTD
Protective layer for protecting a component against corrosion and oxidation at high temperatures, and component
InactiveUS8025984B2Improve high temperature stabilityImprove long-term stabilityPropellersBlade accessoriesRare-earth elementRhenium
Known protective layers with a high Al and / or Cr content and additionally strengthened by Re form brittle phases which become more brittle during use under the influence of carbon. The protective layer according to the invention has the composition 0.5 to 2% rhenium, 24 to 26% cobalt, 15 to 21% chromium, 9 to 11.5% aluminum, 0.05 to 0.7% yttrium and / or at least one equivalent metal selected from the group consisting of scandium and the rare earth elements, 0 to 1% ruthenium, remainder cobalt and / or nickel and manufacturing-related impurities, and reveals scarcely any embrittlement caused by Cr / Re precipitates.
Owner:SIEMENS ENERGY GLOBAL GMBH & CO KG
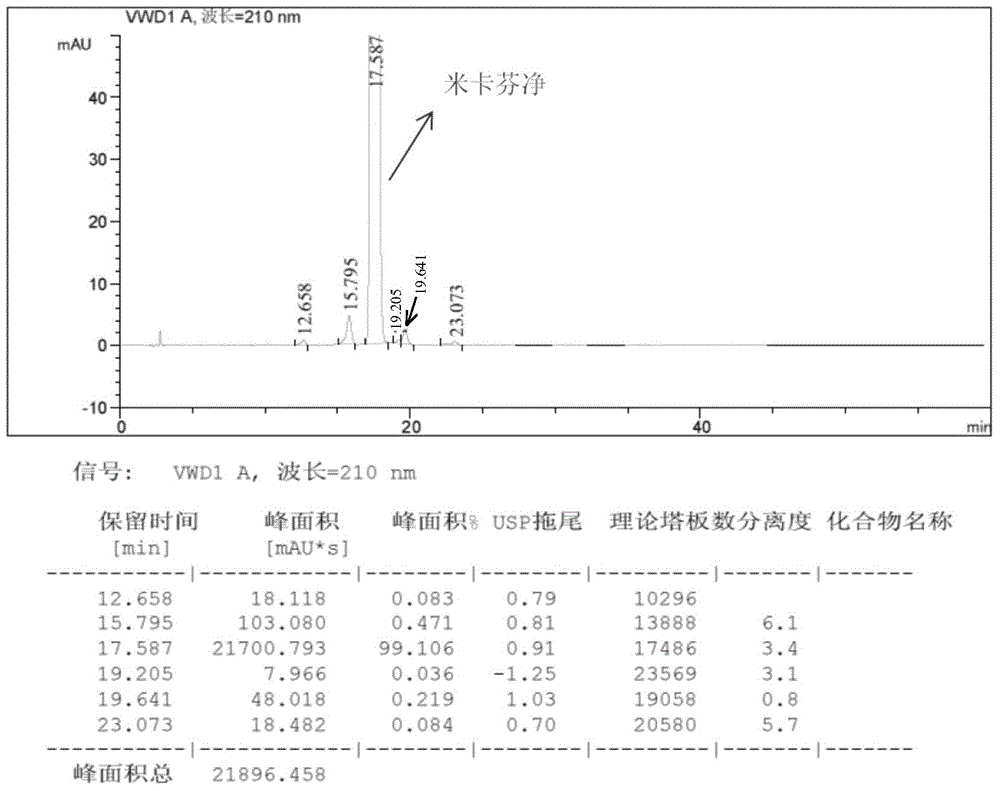
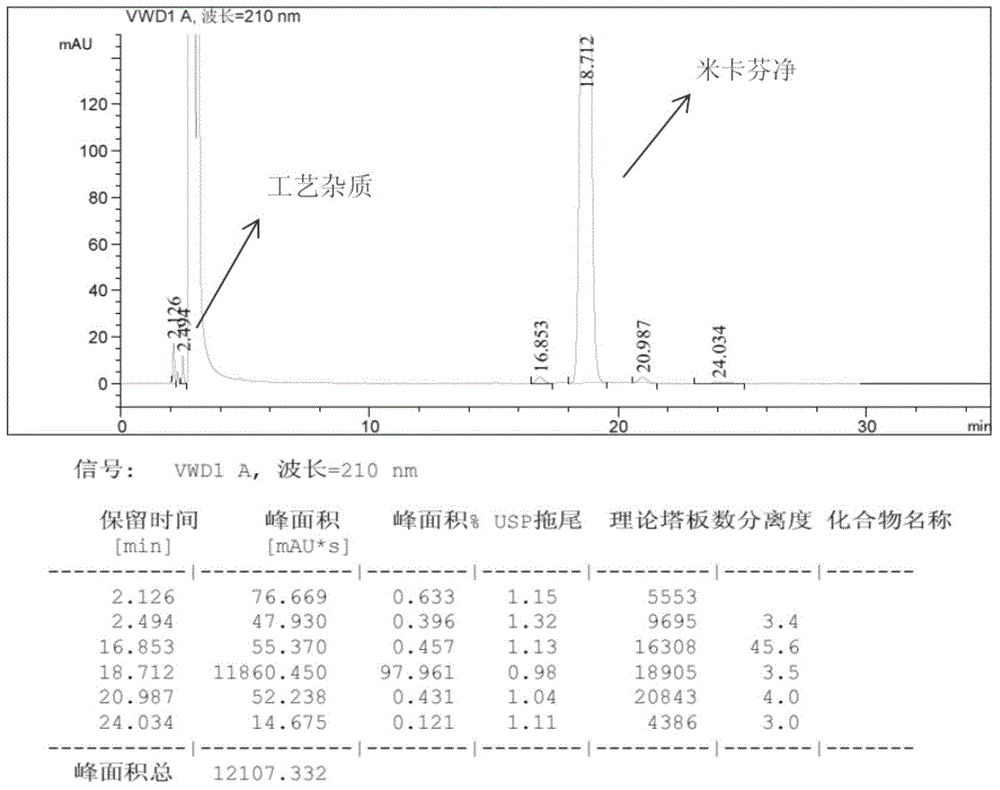
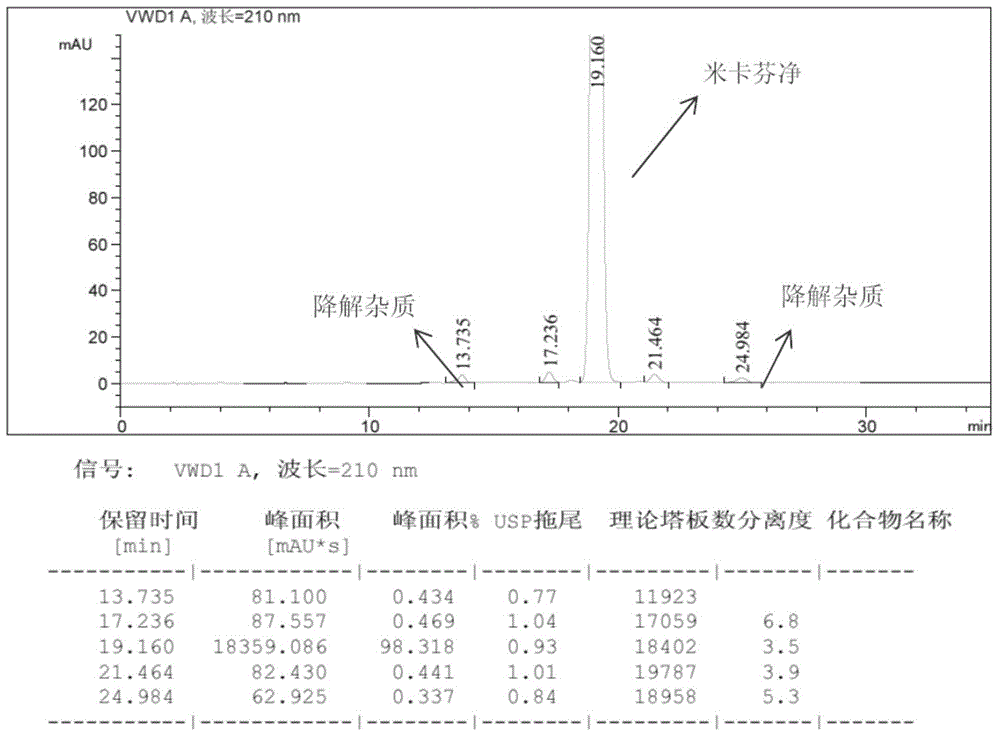
Purification salt conversion method of micafungin
ActiveCN105254721AHigh purityMeet the needs of industrial productionPeptide preparation methodsIon exchangeQuality control
The present invention relates to a purification salt conversion method of micafungin. According to the present invention, the method is mainly characterized in that a buffer solution of a corresponding salt and a micafungin N, N-diisopropylethylamine salt are subjected to ion exchange on a reverse phase preparative chromatographic column so as to complete salt conversion, remove the related impurities, and achieve the purification effect; and the process has characteristics of stable and controllable process, easy and convenient operation, easy industrial production achieving, and easy product quality control.
Owner:JIANGSU HANSOH PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com