Purification of acidic proteins using ceramic hydroxyapatite chromatography
a technology of hydroxyapatite and acidic proteins, which is applied in the direction of antibody medical ingredients, pharmaceutical active ingredients, separation processes, etc., can solve the problems of adversely affecting product safety, reducing product yield, and reducing product efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
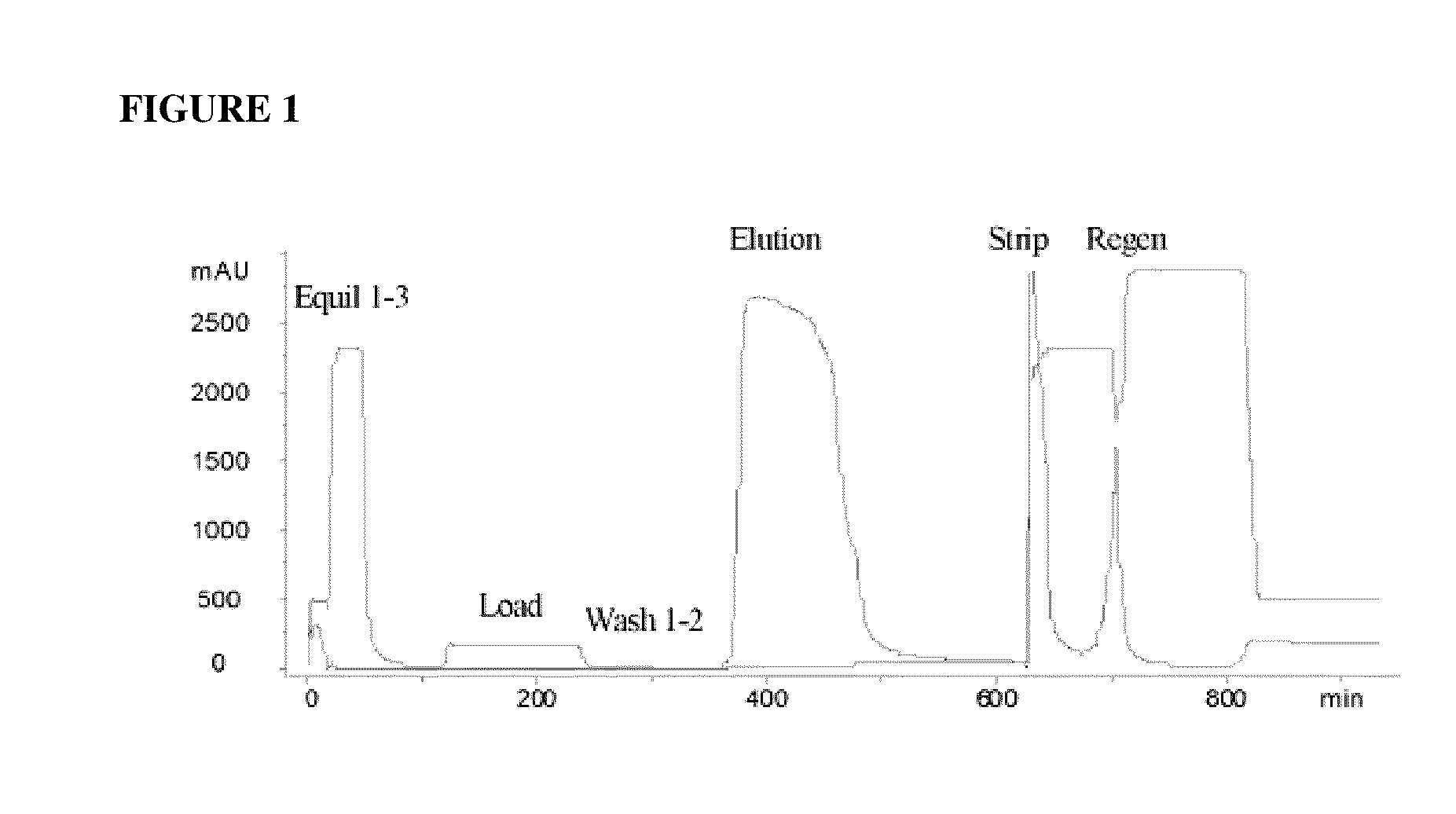
[0062]An acidic immunoglobulin fusion protein, ActRIIB-Fc, was expressed in CHO cells and purified from the harvest media by rProtein A chromatography (GE-Healthcare, Piscataway, N.J.). The protein elution from rProtein A chromatography, which contained ActRIIB-Fc protein, also contained a significant level of inactive and partially active species and HMWA (see “Load” row in Table 2). In order to remove inactive and partially active ActRIIB-Fc species and HMWA, the elution from the Protein A column was subjected to cHA chromatography.
[0063]The cHA column (BioRad Laboratories, Hercules, Calif.) was first equilibrated with Equilibration Buffer 1, containing 0.3 M sodium phosphate, 1.0 M NaCl, pH 6.8, followed by Equilibration Buffer 2, containing 10 mM HEPES, pH 7.2 (Table 1). Equilibration Buffer 1 is a buffer with high concentration of phosphate and the Equilibration Buffer 2 is used to wash out the phosphate. The column was subsequently equilibrated with calcium chloride solution a...
example 2
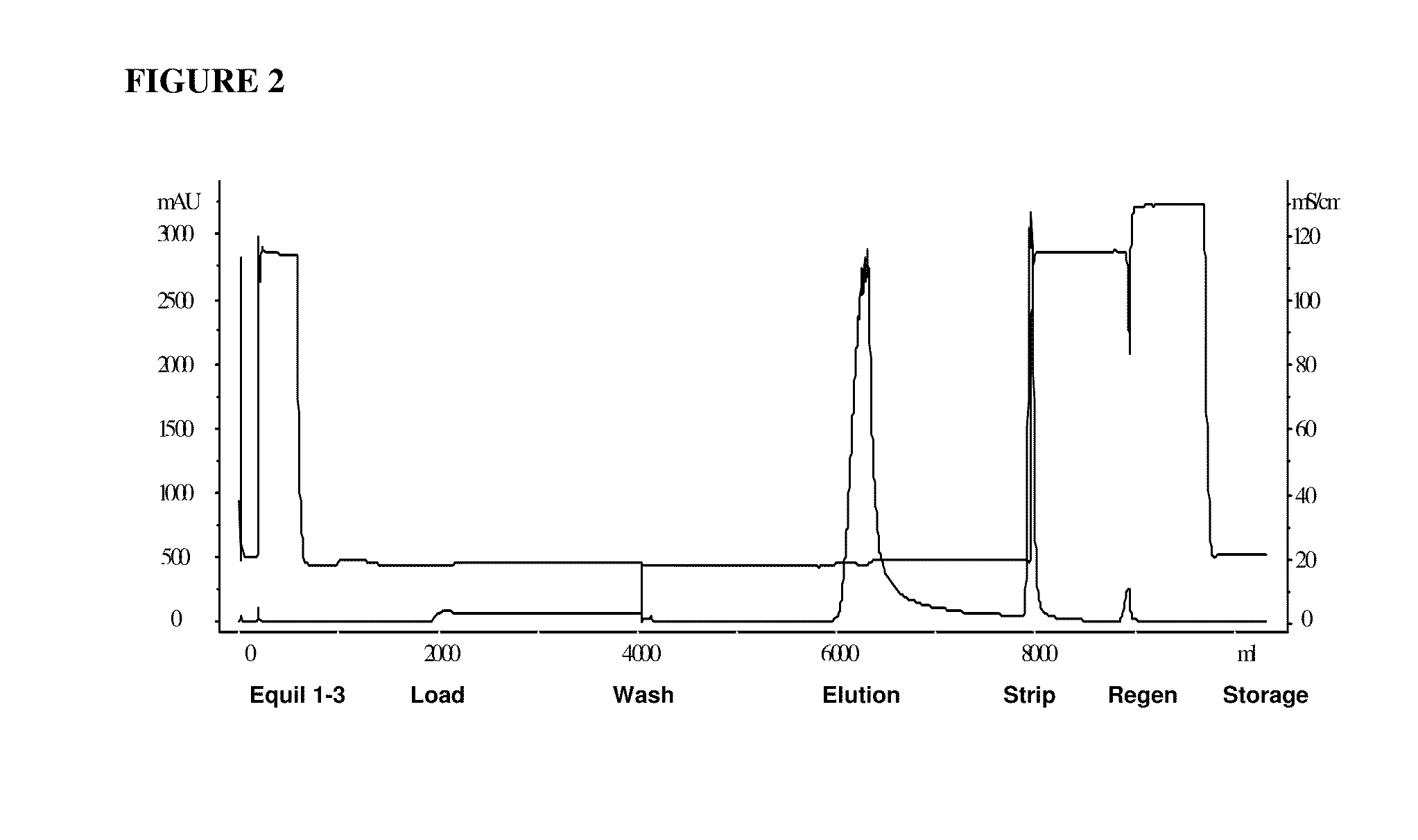
[0069]cHA chromatography was found to be superior for HMWA removal during purification of another acidic Ig fusion protein comprising IL21 fused to the Fc domain of Ig (sIL21r-Fc). See Ettinger et al., Ann Rheum Dis 2008; 67 (Suppl III):iii83-iii86 (for a description of the sIL21r-Fc fusion protein). One goal for this process step was to remove HMWA in order to improve the subsequent chromatographic step's capacity for HMWA and HCP. The column was equilibrated with a calcium chloride solution at neutral pH and low ionic strength. The rProtein A eluate pool was not spiked with calcium chloride for this process due to observed product instability upon exposure to calcium chloride, but rather, the rProtein A eluate pool was loaded directly onto the cHA column at 20-30 g / L resin load challenge. Under these buffer conditions, the product was bound to the cHA resin, with some species of HMWA flowing through the column. The column was then washed with a neutral pH and low ionic strength bu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com