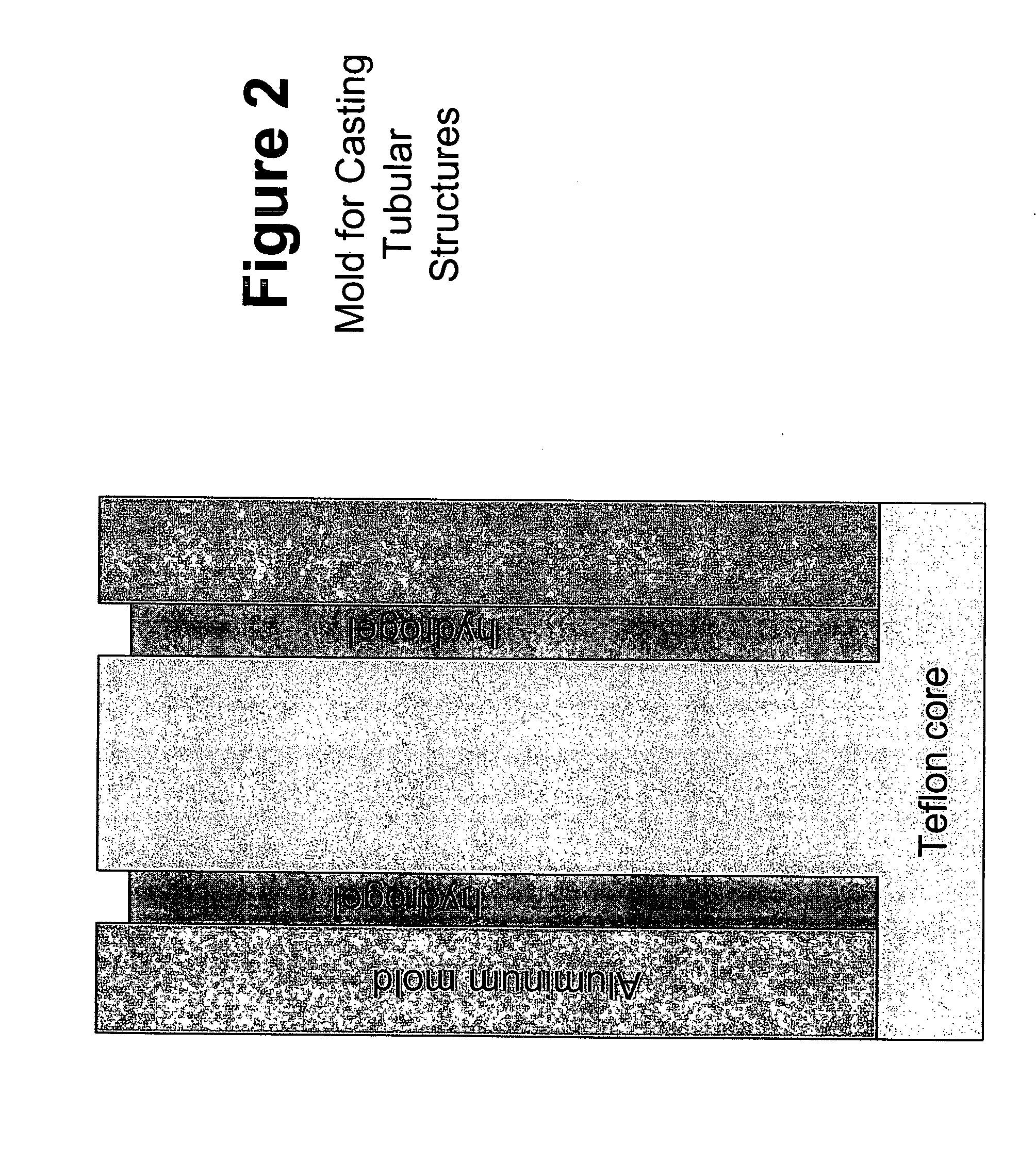
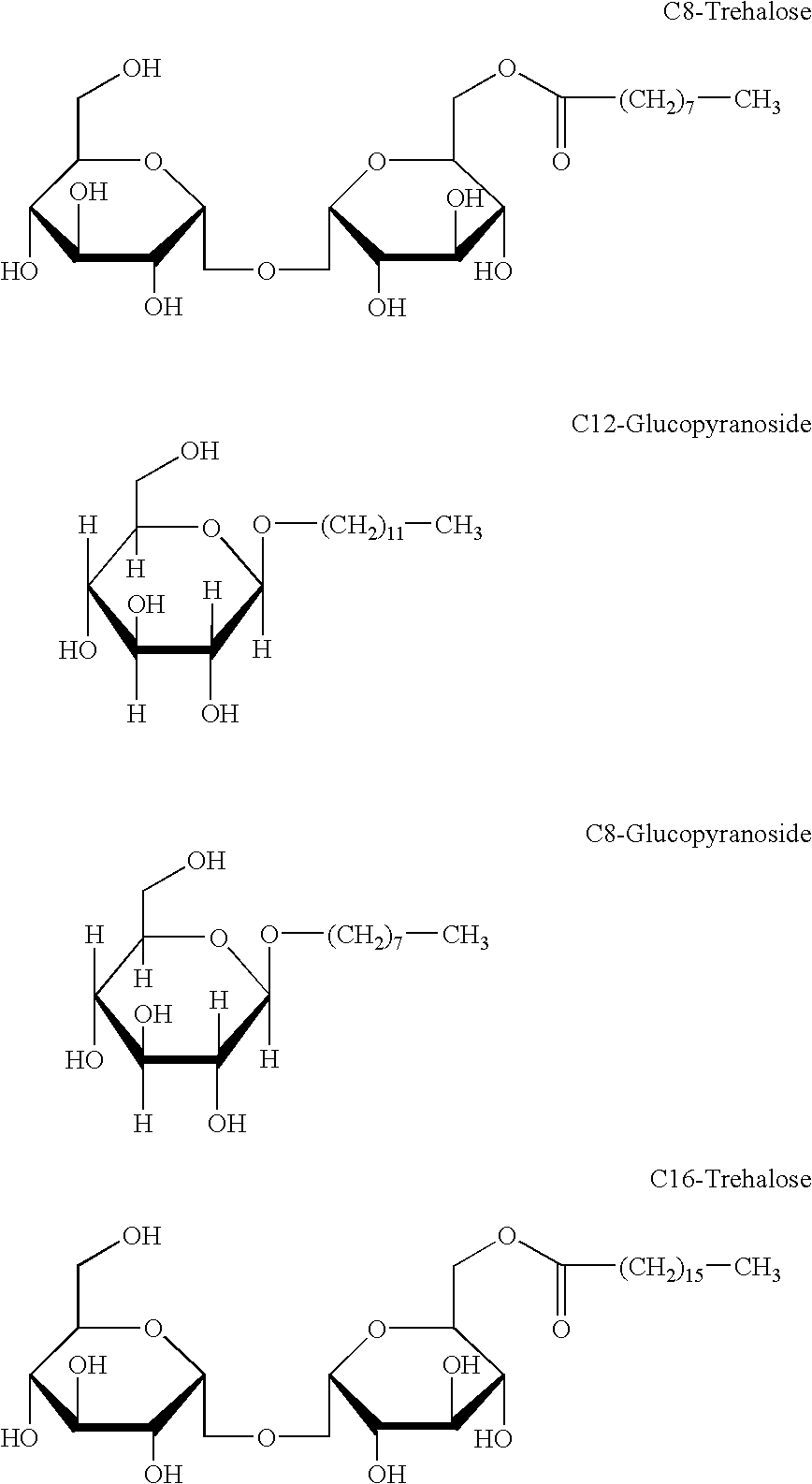
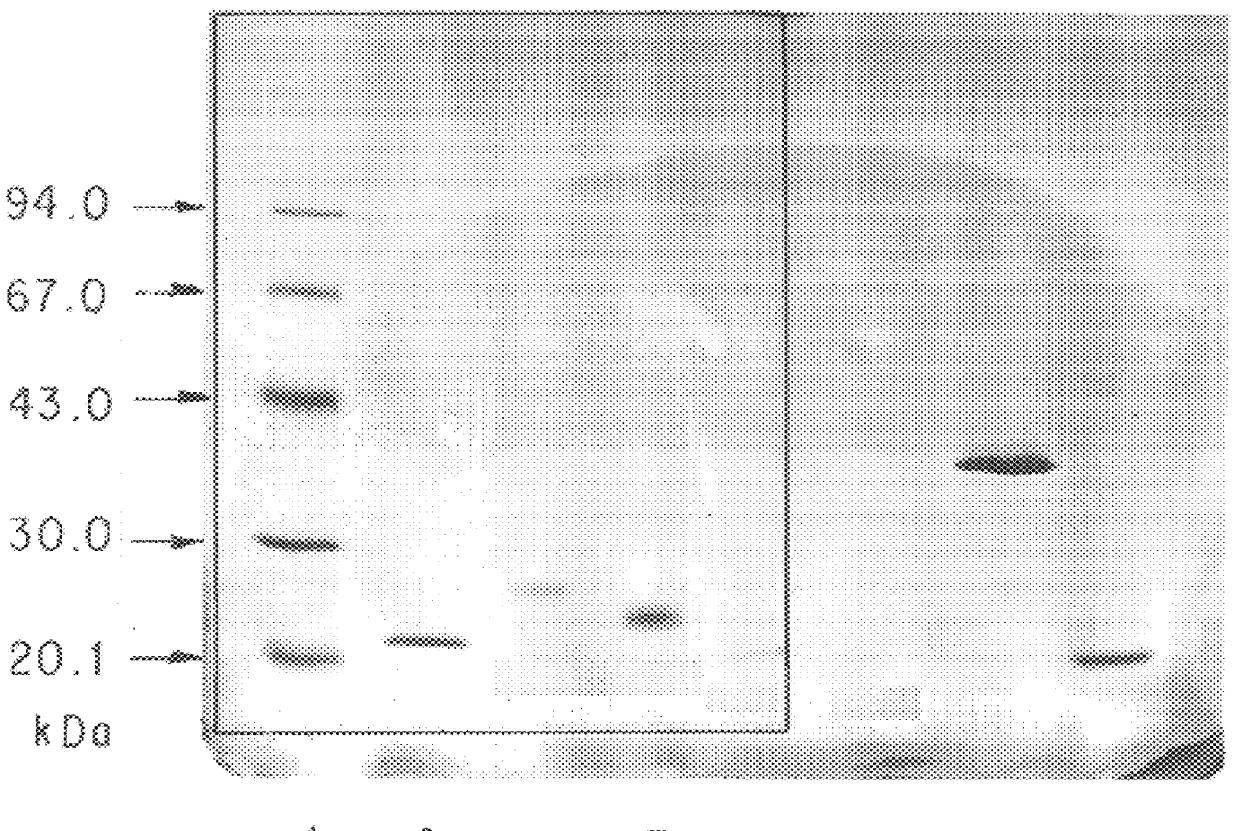
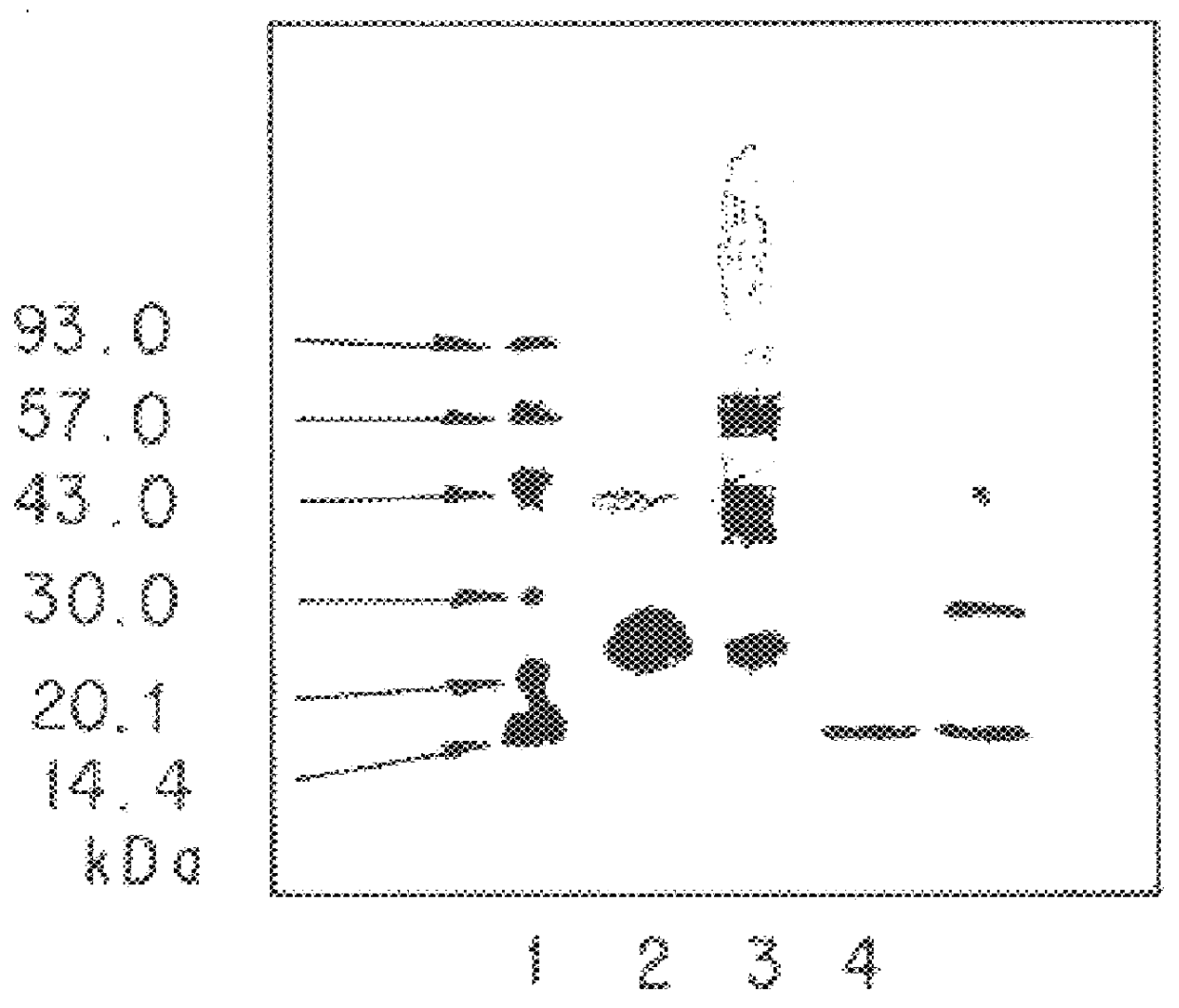
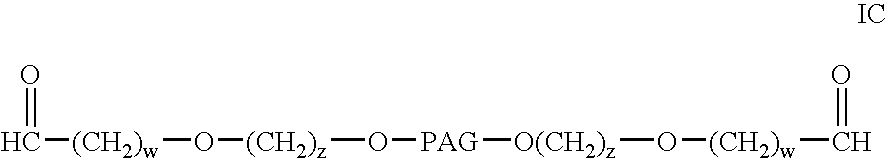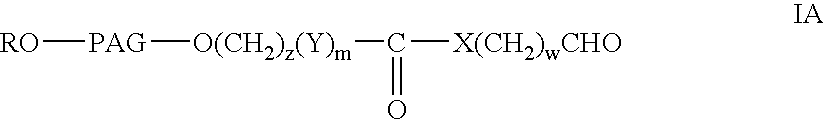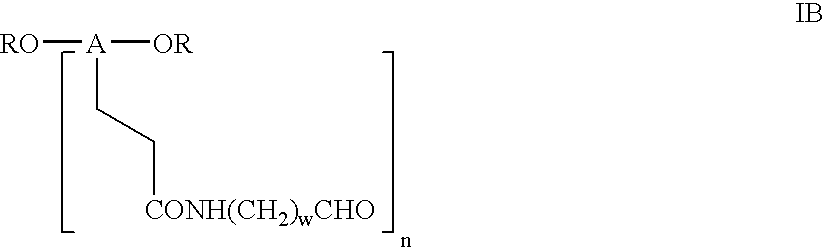Patents
Literature
977 results about "Active protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Active Protein. Proteins are large biomolecules (or macromolecules), consisting of one or more long chains of amino acid residues. Most proteins have unique 3-dimensional structures. The shape into which a protein naturally folds is known as its native conformation. If a protein can be modified and folded into the correct 3-D conformation.
Biologically active proteins having increased In Vivo and/or In Vitro stability
Owner:AMUNIX OPERATING INC
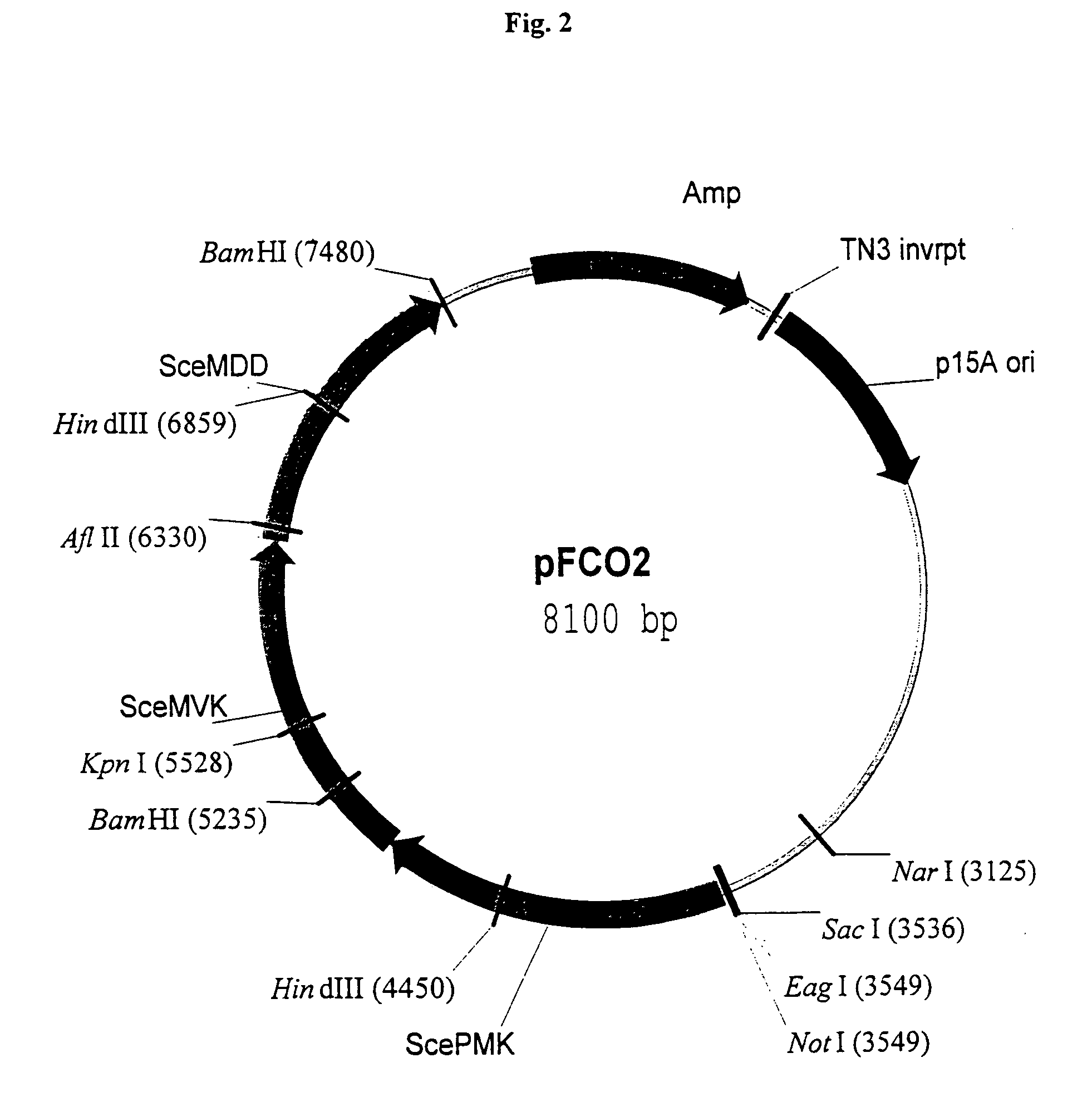
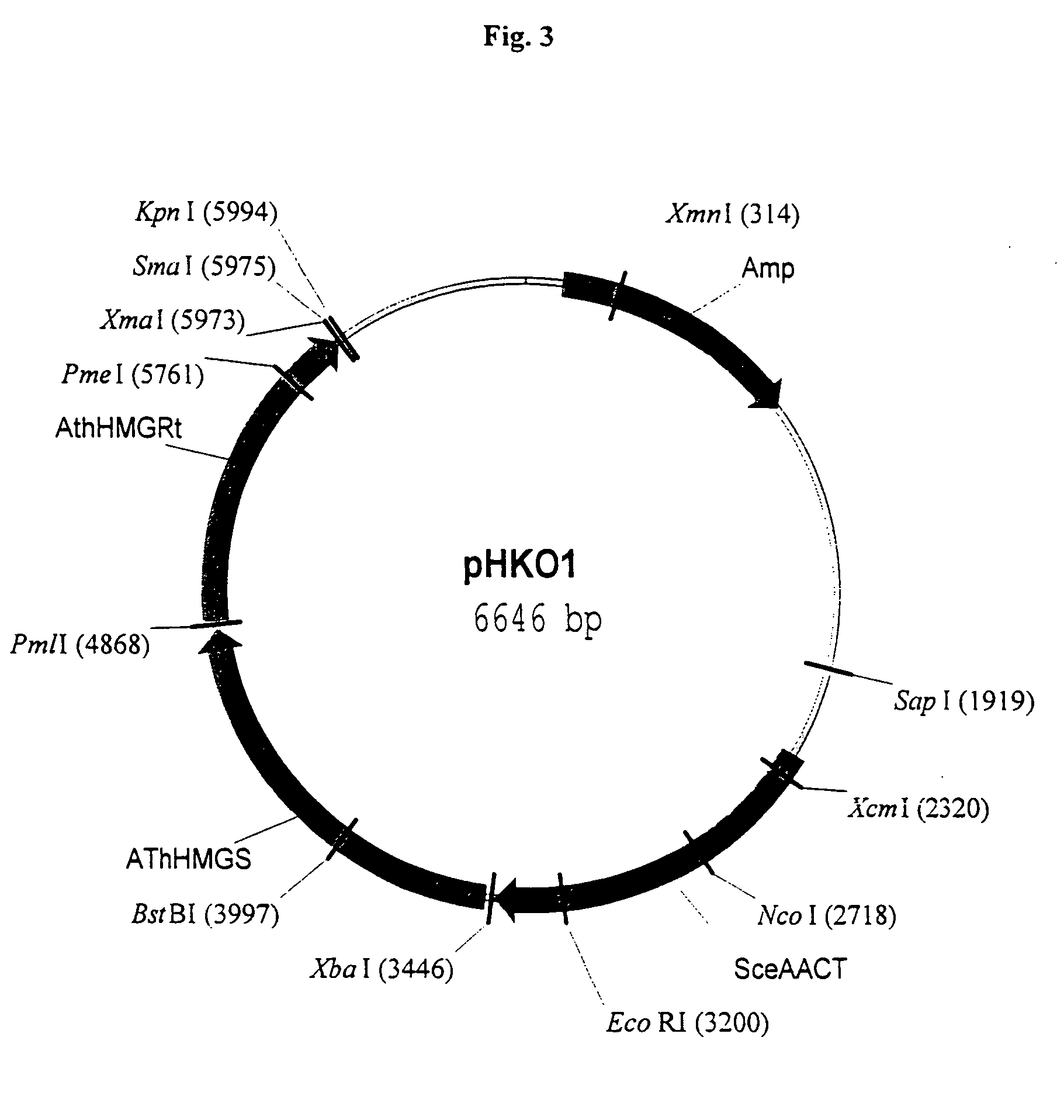
Materials and methods for increasing isoprenoid production in cells
InactiveUS7129392B2Other foreign material introduction processesIsomerasesPhytoene synthesisOpen reading frame
Owner:KUEHNLE AGROSYST COMPANY +1
Biocompatible polymers and Methods of use
InactiveUS20100254900A1Alter propertyUltrasonic/sonic/infrasonic diagnosticsPeptide/protein ingredientsPorosityNatural source
Compositions and methods for manufacturing polymers are disclosed. Compositions include novel plastics, including films and shaped forms comprising polymer matrices that are biologically compatible and biodegradable. Such plastics may comprise polymers derived from natural sources. Further, such plastics are useful in biological systems for wound repair, implants, stents, drug encapsulation and delivery, and other applications. The disclosed methods comprise mild manufacturing processes such that various additives, such as biologically active proteins, sugars, lipids, and the like may be incorporated into the polymer matrix without subsequent loss of bioactivity during processing. Additionally, methods of manufacture for controlling mechanical properties, such as elasticity, pliancy, and the porosity of such plastics are disclosed.
Owner:CARNEGIE MELLON UNIV
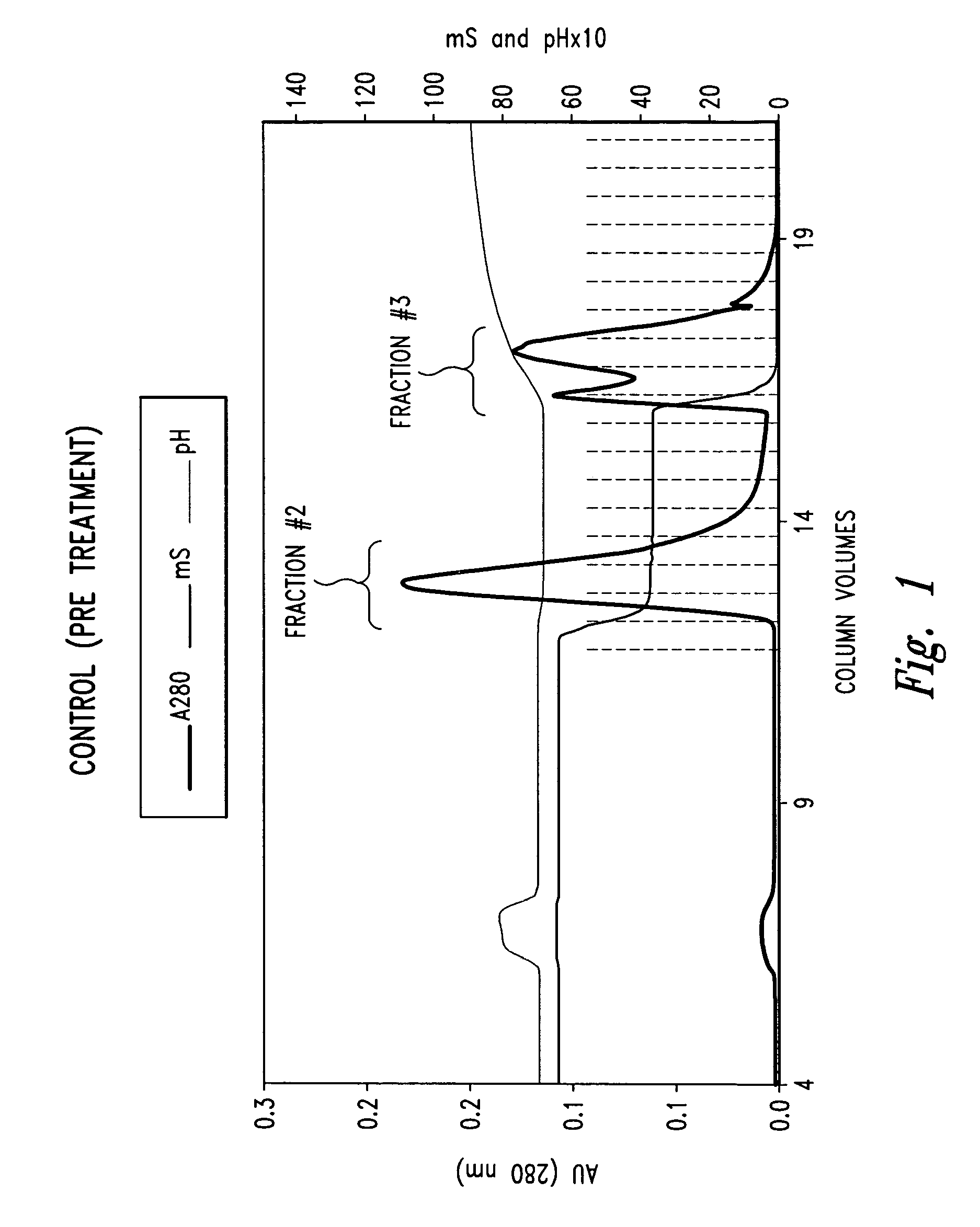
Method of purifying protein
InactiveUS20060142549A1Efficient removalSerum immunoglobulinsColony-stimulating factorActive proteinDNA Contamination
Problems to be Solved: The present invention provides a simpler and less expensive method for purifying physiologically active proteins, especially antibodies, which can ensure removal of impurities such as DNA contaminants and viruses, and which can minimize a loss of physiologically active proteins. Means for Solving the Problems: A method for removing impurities in a physiologically active protein-containing sample, which comprises the following steps: 1) allowing the physiologically active protein-containing sample to be converted into an aqueous solution of low conductivity at a pH below the isoelectric point of the physiologically active protein; and 2) removing the resulting particles.
Owner:CHUGAI PHARMA CO LTD
Method for stabilizing biomolecules in liquid formulations
InactiveUS20020110524A1Improve stabilityOrganic active ingredientsFactor VIIActive proteinPharmaceutical medicine
The invention is directed to a stable formulation of a biologically active protein useful for aerosol delivery to the respiratory tract of a patient in need of treatment comprising: (a) a carrier liquid comprising from about 10% to from about 100% V / V water and from about 0% to from about 90% V / V of an organic liquid; (b) a biologically effective amount of a protein suspended or dissolved in a carrier liquid; and (c) a stabilizing effective amount of a derivatized carbohydrate stabilizing agent suspended or dissolved in said carrier liquid. The stable formulations of the invention may optionally contain about 0.1% to about 5.0% W / V of a pharmaceutically acceptable excipient.
Owner:BATTELLE MEMORIAL INST
Modification of peptide and protein
PCT No. PCT / JP95 / 01994 Sec. 371 Date Sep. 8, 1997 Sec. 102(e) Date Sep. 8, 1997 PCT Filed Sep. 29, 1995 PCT Pub. No. WO96 / 10089 PCT Pub. Date Apr. 4, 1996A process for modifying a physiologically active peptide or a physiologically active protein which comprises reacting a physiologically active peptide or a physiologically active protein having at least a glutamine residue with a substance having an amino donor in the presence of a transglutaminase originating in a microorganism to thereby form an acid amide bond at the gamma -position acid amide group of the glutamine residue with the amino group of the amino donor; and the product of modification obtained thereby.
Owner:AJINOMOTO CO INC
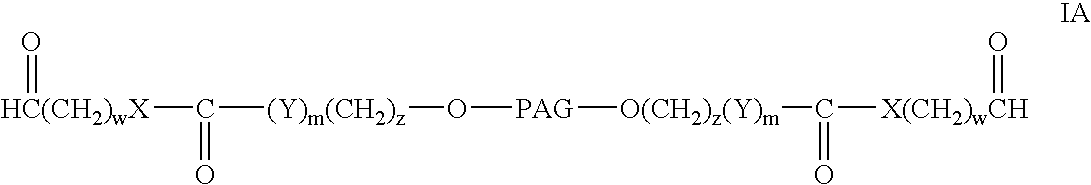
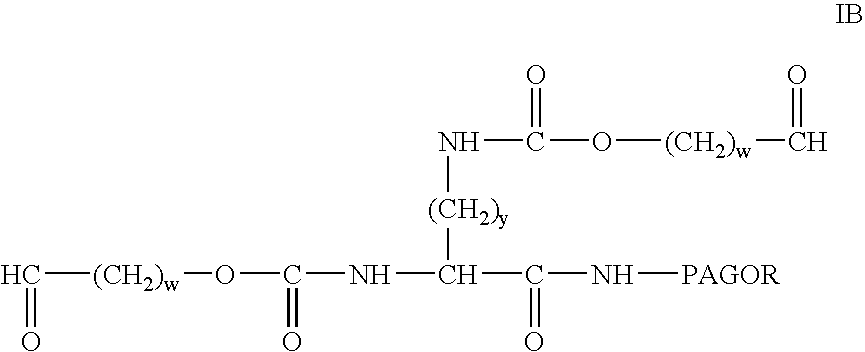
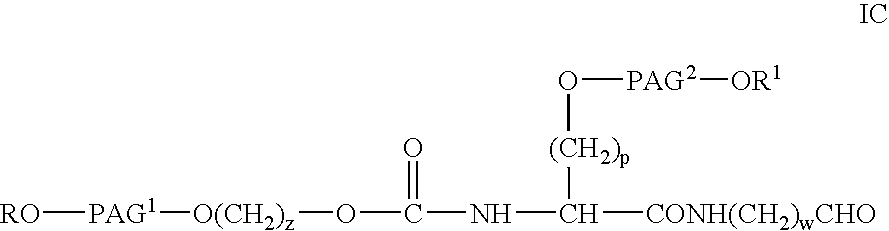
Bifunctional polyethylene glycol derivatives
InactiveUS20040115165A1Linkage stabilityDifficult to purifyOrganic chemistryPharmaceutical non-active ingredientsProtein insertionActive protein
The present invention provides novel heterobifunctional and monobifunctional polyethylene glycol derivatives for the pegylation of therapeutically active proteins. The heterobifunctional PEGs which bear two different functional groups as well as the monobifunctional PEGs which contain two similar functional groups, may be used for cross-linking purposes. The cross-linking may be intramolecular between two areas within the same molecule or intermolecular between two separate molecules. The pegylated protein conjugates that are produced, retain a substantial portion of their therapeutic activity and are less immunogenic than the protein from which the conjugate is derived. New syntheses for preparing such bifunctional derivatives are described.
Owner:SUN BIO INC
Method for purification of alpha-1 proteinase inhibitor
InactiveUS6093804AHigh yield isolationAchieve separationPeptide/protein ingredientsMammal material medical ingredientsPurification methodsEnzyme inhibitor
The methods of the present invention provide a simple means for separating active and inactive Alpha Proteinase Inhibitor (API). The methods further provide means for purifying API at high yield (>70%), and at levels of purity (>90%) and activity (>90%) not heretofore available. Moreover, the methods of the present invention are simple (i.e., two chromatographic steps) and efficient; and are thus especially suitable to large scale purification processes. These methods will contribute substantially to alleviating the unmet demand for API for therapeutic purposes.
Owner:KAMADA
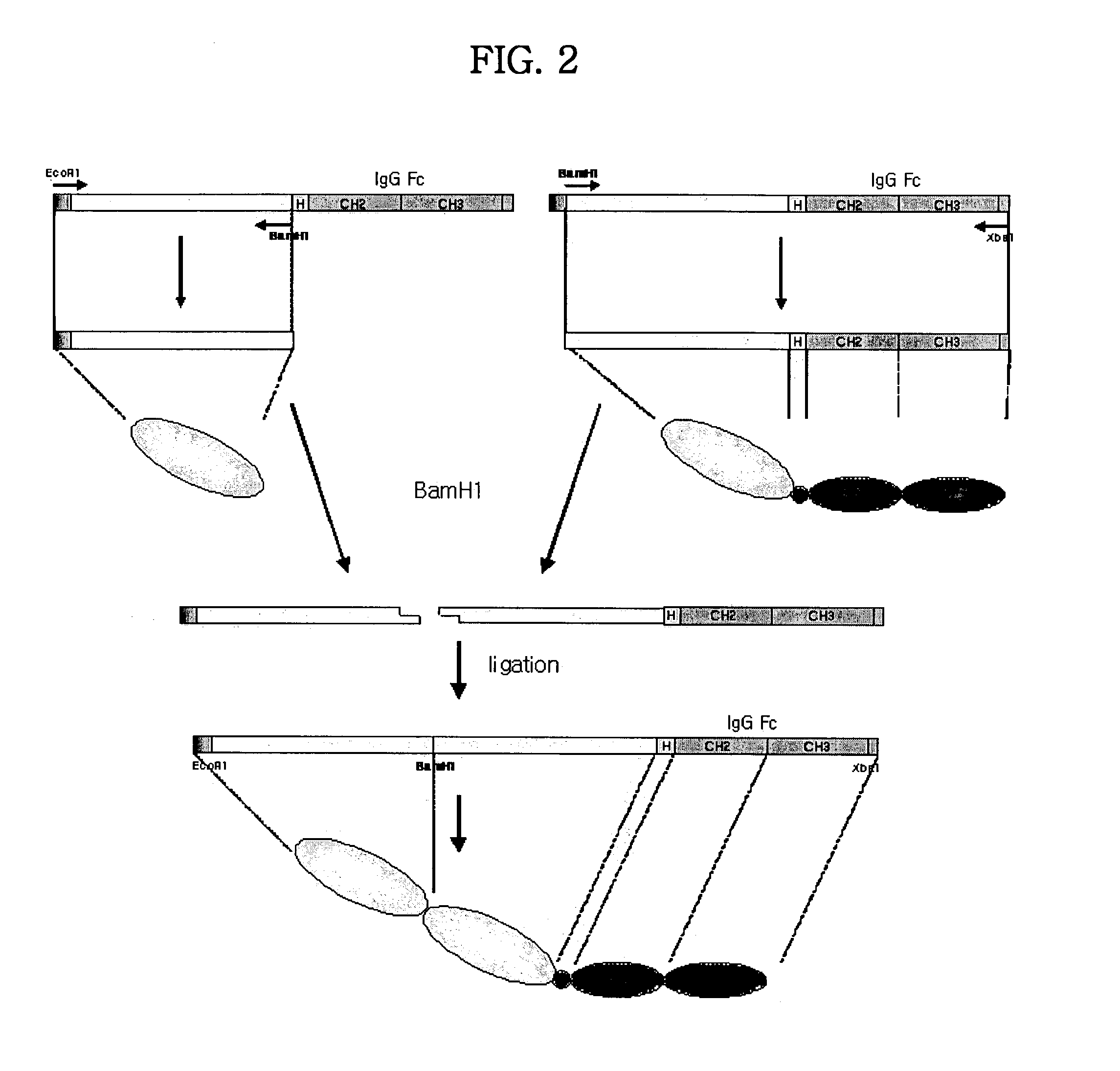
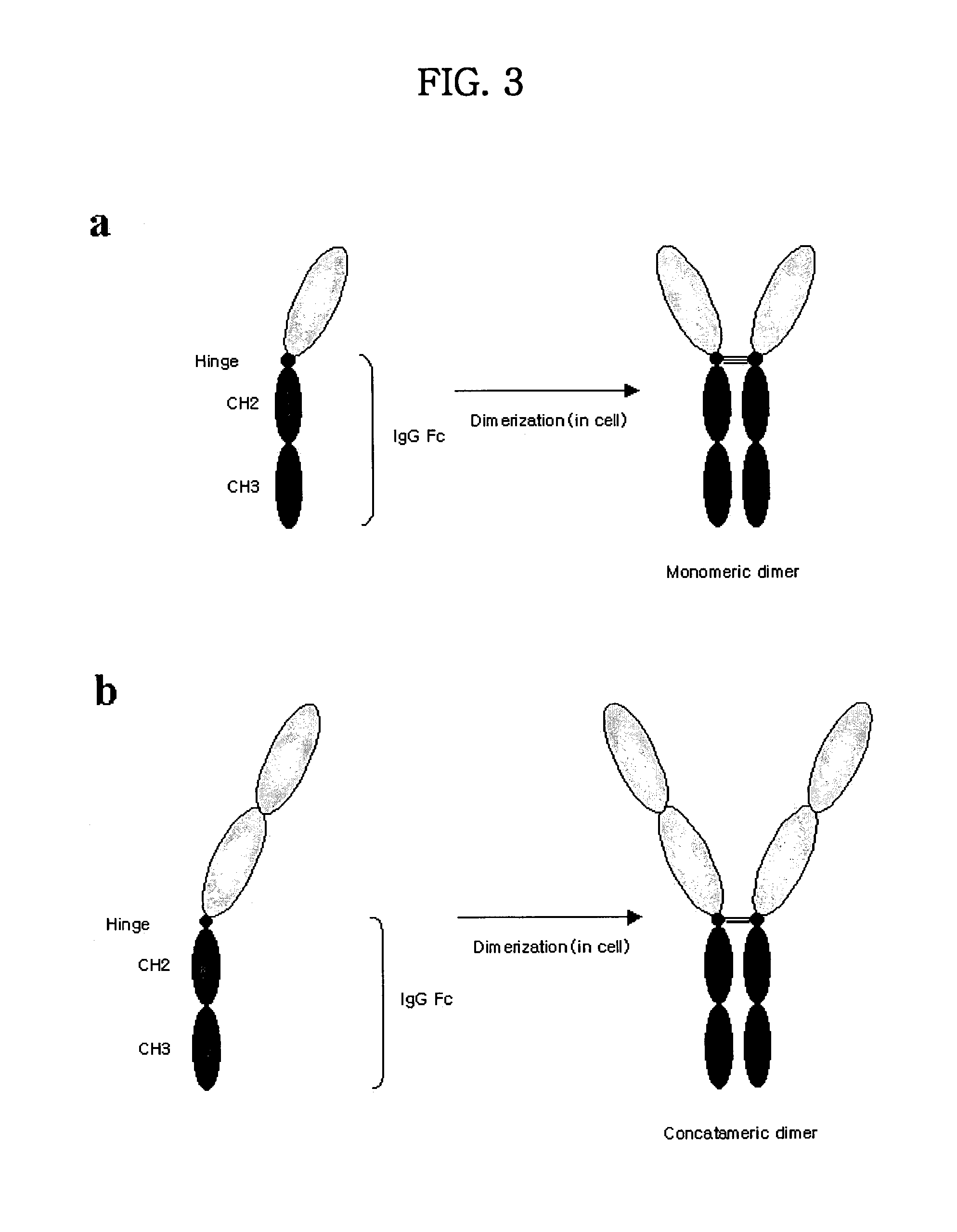
Concatameric immunoadhesion
ActiveUS20030195338A1Improve efficacyImprove stabilityAntipyreticAntibody mimetics/scaffoldsDNA constructActive protein
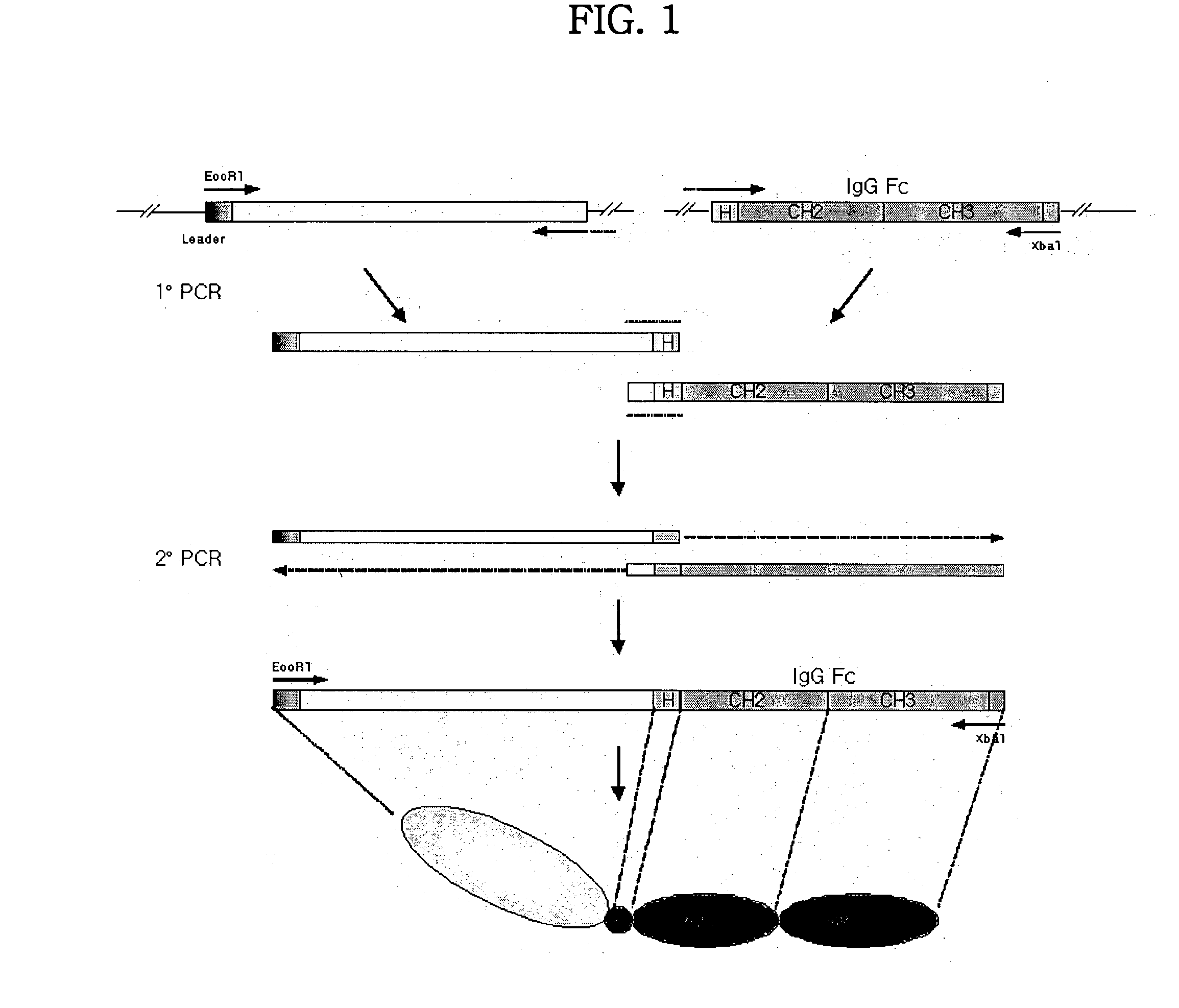
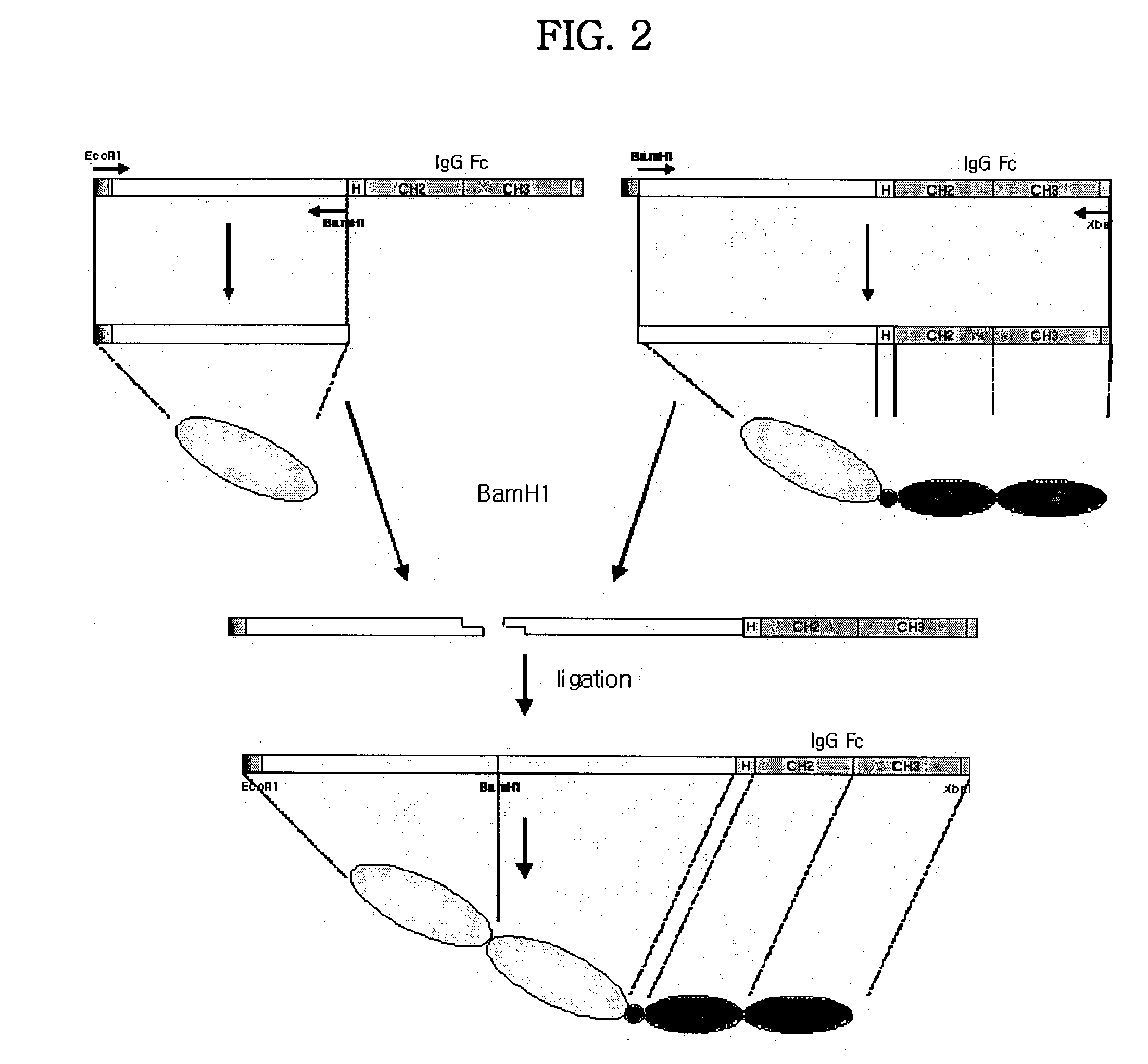
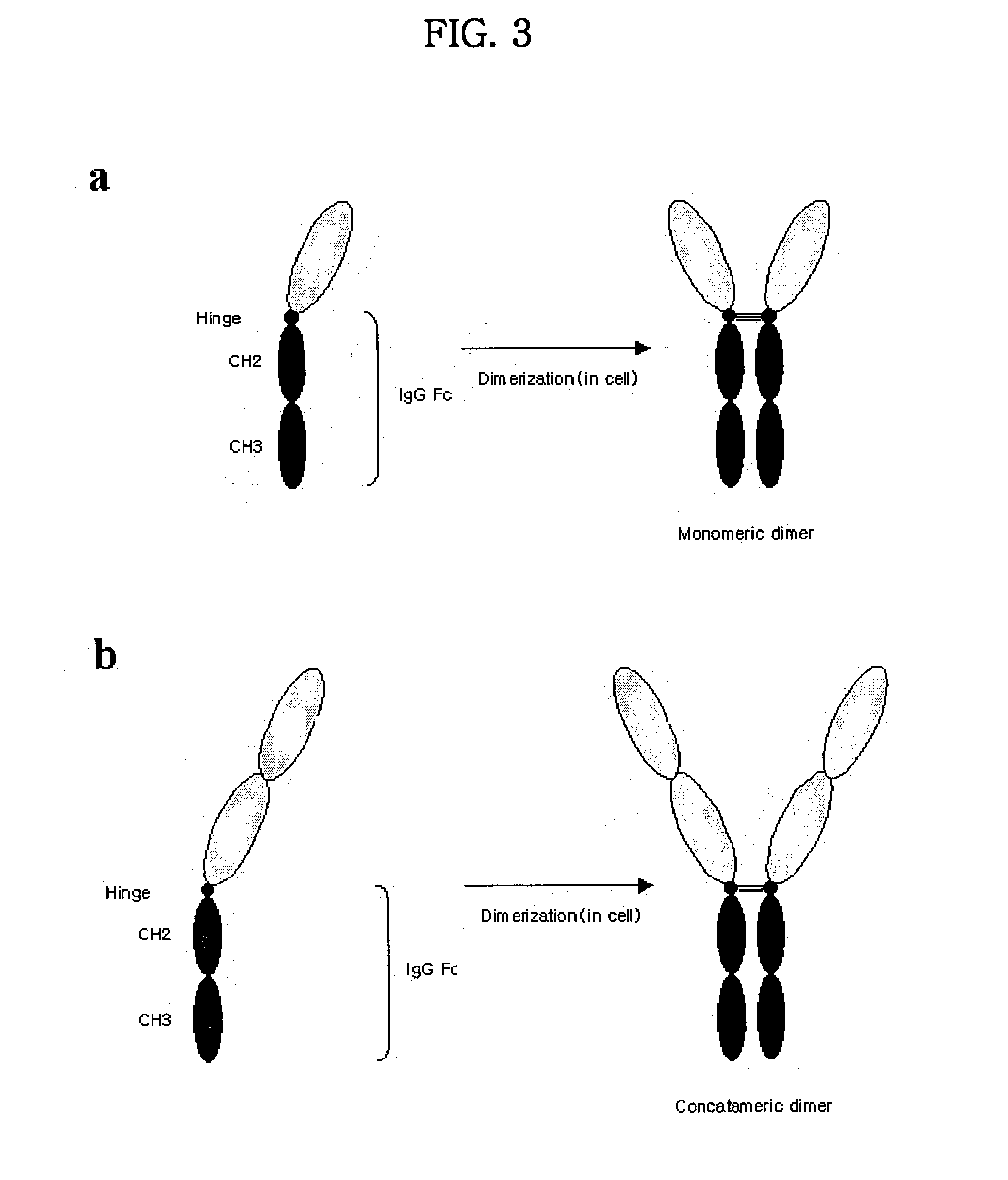
Disclosed are concatameric proteins comprising two soluble domains, in which the C-terminus of a soluble domain of a biologically active protein is linked to the N-terminus of an identical soluble domain or a distinct soluble domain of a biologically active protein. Also, the present invention discloses dimeric proteins formed by formation of intermolecular disulfide bonds at the hinge region of two monomeric proteins formed by linkage of a concatamer of two identical soluble extracellular regions of proteins involving immune response to an Fc fragment of an immunoglobulin molecule, their glycosylated proteins, DNA constructs encoding the monomeric proteins, recombinant expression plasmids containing the DNA construct, host cells transformed or transfected with the recombinant expression plasmids, and a method of preparing the dimeric proteins by culturing the host cells. Further, the present invention discloses pharmaceutical or diagnostic compositions comprising the dimeric protein or its glycosylated form.
Owner:MEDEXGEN
Method for preparing blend oil and cold-pressing and extracting peony seed oil
InactiveCN101653174AReduce lossesImprove nutrition and health valueEdible oils/fatsAlpha-TocopherolFiltration
The invention relates to a method for preparing blend oil and cold-pressing and extracting peony seed oil. The method comprises the following steps: collecting and dehulling seeds, cold-pressing and secondary-filtering, producing and filling the blend oil. The invention has a simple oil-extracting process and reduces the loss of active components in oil-extracting process. Cold pressing temperature is low so that phospholipid, pigment, and the like hardly enter oil, and the cold pressing oil has lighter color and lower phospholipid content and can achieve a new international four class oil standard only by mechanical filtration instead of any chemical refining. Cold pressing rap oil has high nutritive and healthcare values because thermosensitive alpha-tocopherol and phytosterols are fullyremained, active protein and other active components in peony seeds are remained so as to creates advantages for further developing new purposes of the seeds; no refining sewage is discharged in thecold pressing production process, therefore, the pollution controlling cost is lowered and the environmental protection is favorable.
Owner:兰州牡丹园艺开发公司
Method of purifying protein
InactiveUS7332289B2Efficient removalReduce conductivityMicrobiological testing/measurementPeptide preparation methodsActive proteinAqueous solution
A method for removing contaminant DNA in a sample containing a physiologically active protein, which comprises the following steps:1) converting the sample containing a physiologically active protein into a neutral aqueous solution of low conductivity; and2) removing the resulting particles.
Owner:CHUGAI PHARMA CO LTD
Cytokine concentration system
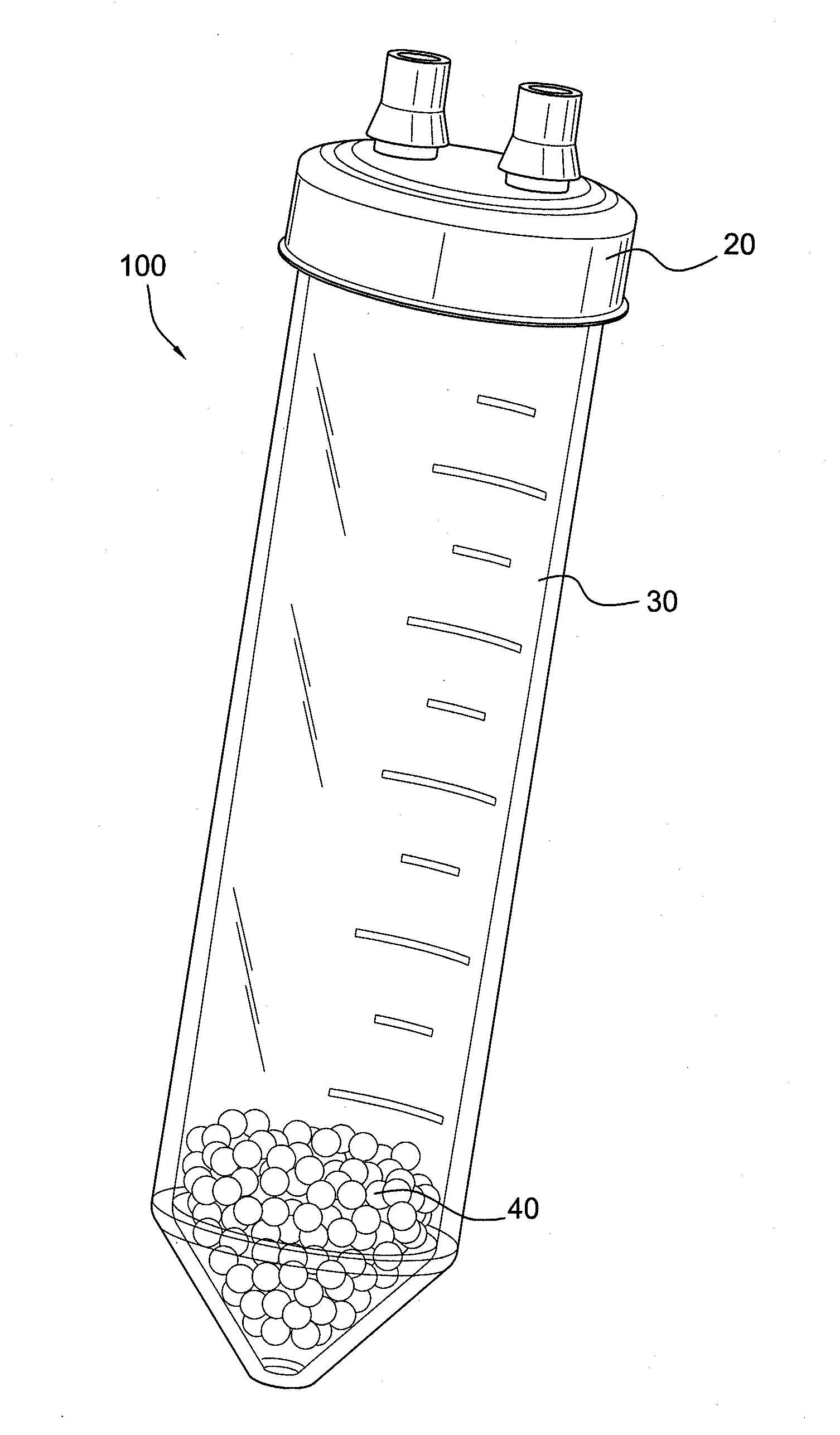
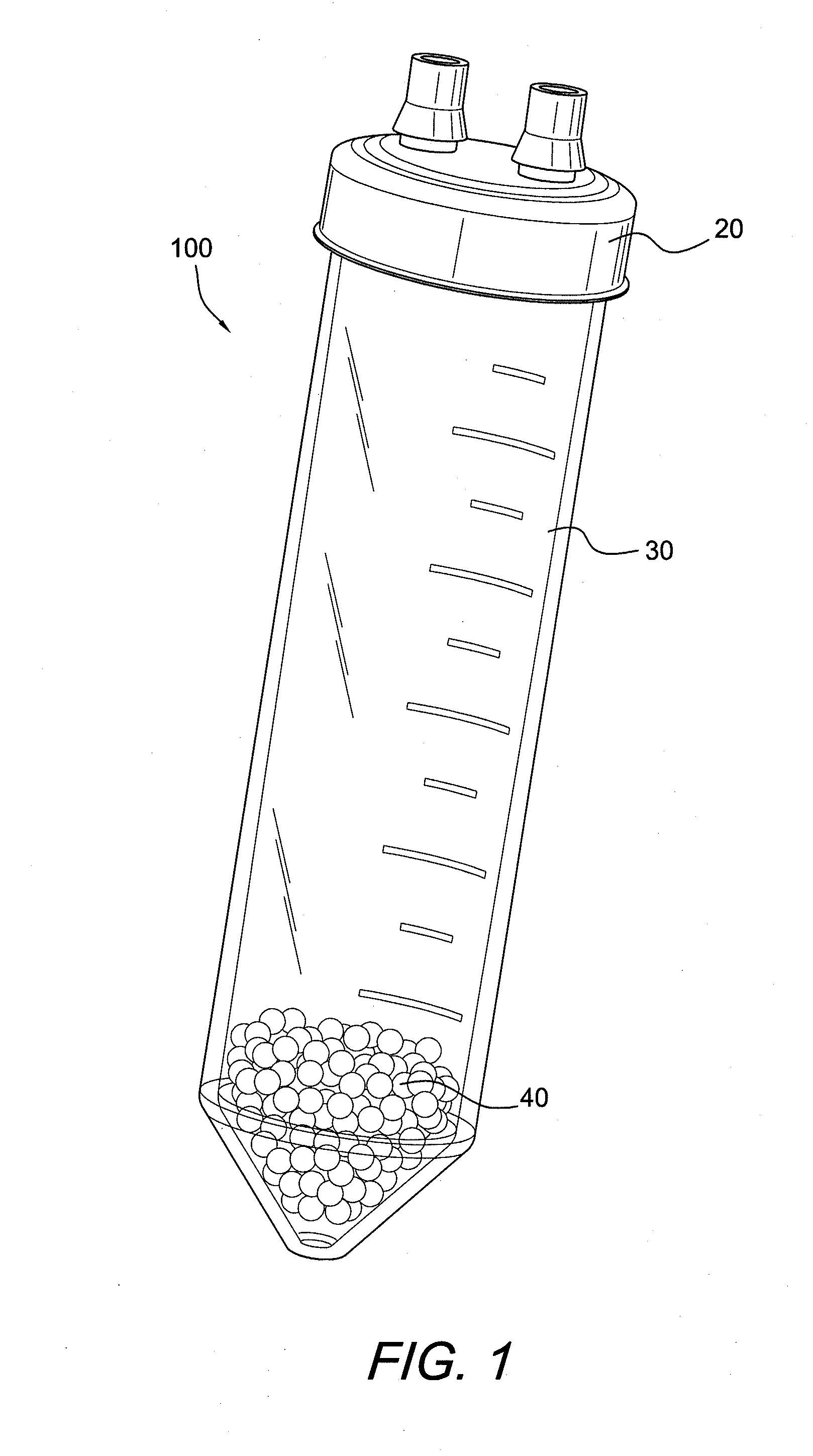
ActiveUS20100125236A1Avoid crackingOther blood circulation devicesDiagnosticsCentrifugationActive protein
Apparatus and methods for producing interleukin-1 receptor antagonist and / or other prophylatically or therapeutically effective protein. Blood is obtained from a patient with a conventional syringe and then introduced into dual luer lock centrifuge tube. The dual luer lock centrifuge tube is provided with beads that are coated with a silanized coating. The container is then incubated and centrifuged. Subsequent to the incubation and the centrifugation, the serum containing autologous therapeutically active protein, such as IL-1Ra, in the container is withdrawn through the luer lock of the container, and injected back into the patient.
Owner:ARTHREX
Tetravalent etanercept
ActiveUS7229962B2Improve efficacyImprove stabilityAntibody mimetics/scaffoldsAntipyreticDNA constructEtanercept
Disclosed are concatameric proteins comprising two soluble domains, in which the C-terminus of a soluble domain of a biologically active protein is linked to the N-terminus of an identical soluble domain or a distinct soluble domain of a biologically active protein. Also, the present invention discloses dimeric proteins formed by formation of intermolecular disulfide bonds at the hinge region of two monomeric proteins formed by linkage of a concatamer of two identical soluble extracellular regions of proteins involving immune response to an Fc fragment of an immunoglobulin molecule, their glycosylated proteins, DNA constructs encoding the monomeric proteins, recombinant expression plasmids containing the DNA construct, host cells transformed or transfected with the recombinant expression plasmids, and a method of preparing the dimeric proteins by culturing the host cells. Further, the present invention discloses pharmaceutical or diagnostic compositions comprising the dimeric protein or its glycosylated form.
Owner:MEDEXGEN
Bifunctional polyethylene glycol derivatives
InactiveUS7217845B2Organic chemistryPharmaceutical non-active ingredientsActive proteinImmunogenicity
The present invention provides novel heterobifunctional and monobifunctional polyethylene glycol derivatives for the pegylation of therapeutically active proteins. The heterobifunctional PEGs which bear two different functional groups as well as the monobifunctional PEGs which contain two similar functional groups, may be used for cross-linking purposes. The cross-linking may be intramolecular between two areas within the same molecule or intermolecular between two separate molecules. The pegylated protein conjugates that are produced, retain a substantial portion of their therapeutic activity and are less immunogenic than the protein from which the conjugate is derived. New syntheses for preparing such bifunctional derivatives are described.
Owner:SUN BIO INC
Expression of biologically active proteins in a bacterial cell-free synthesis system using bacterial cells transformed to exhibit elevated levels of chaperone expression
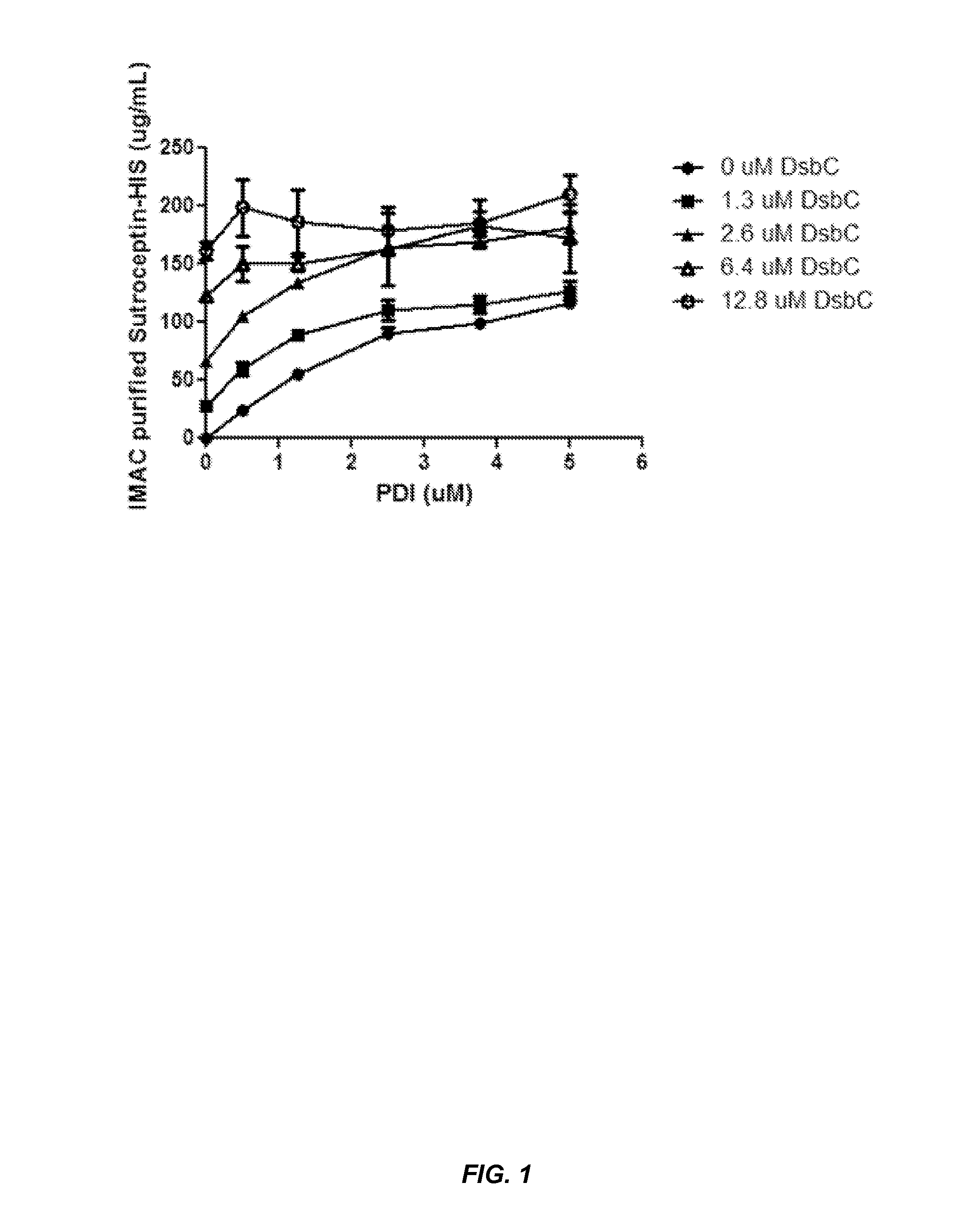
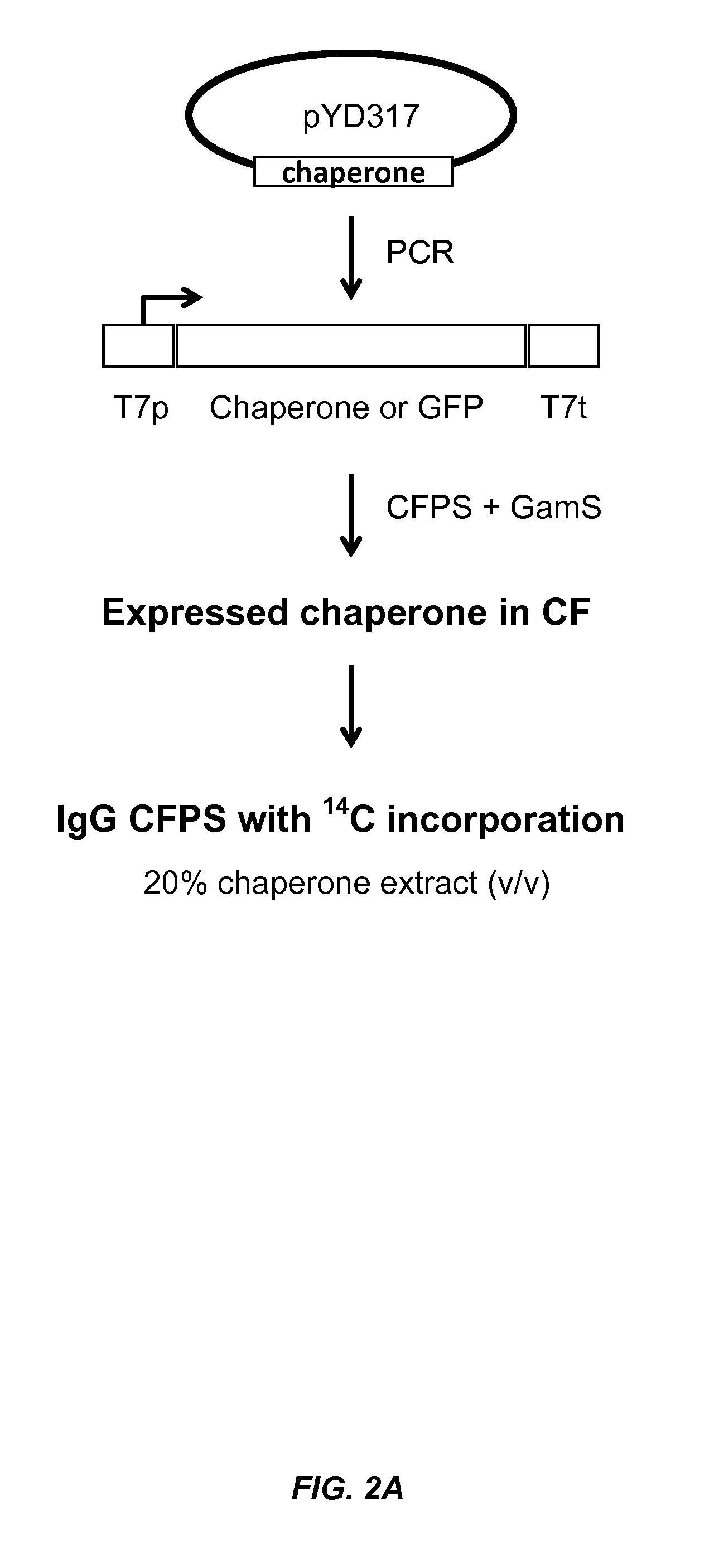
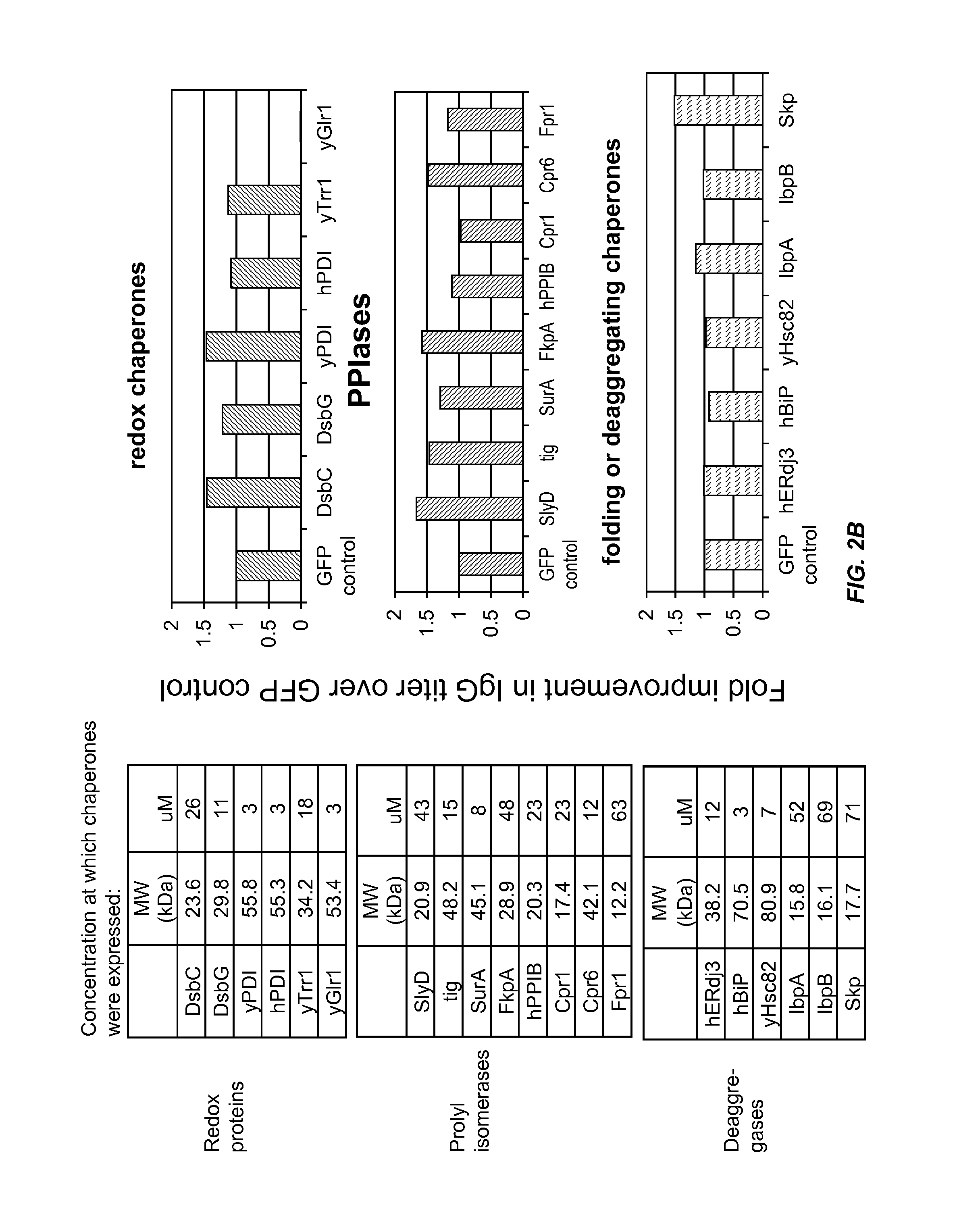
The present disclosure describes methods and systems for improving the expression of a properly folded, biologically active protein of interest in a cell free synthesis system. The methods and systems use a bacterial cell free extract having an active oxidative phosphorylation system, and include an exogenous protein chaperone. The exogenous protein chaperone can be expressed by the bacteria used to prepare the cell free extract. The exogenous protein chaperone can be a protein disulfide isomerase and / or a peptidyl-prolyl cis-trans isomerase. The inventors discovered that the combination of a protein disulfide isomerase and a peptidyl-prolyl cis-trans isomerase produces a synergistic increase in the amount of properly folded, biologically active protein of interest.
Owner:SUTRO BIOPHARMA
Surface expression of biologically active proteins in bacteria
Methods and compositions for targeting heterologous polypeptides to bacterial cell walls are provided.
Owner:OSEL
Stable, aerosolizable suspensions of proteins in ethanol
Stable suspensions of a biologically active protein are disclosed that are suited for aerosol delivery to the lungs of a patient in need of treatment, which comprise particles of biologically active protein suspended in ethanol. In a preferred embodiment, the invention describes a stable suspension of insulin useful for aerosol delivery to the lungs of a patient in need of treatment comprising particles of a pharmaceutically effective amount of insulin suspended in ethanol. A method of delivering a therapeutically effective amount of a protein to the respiratory tract of a patient is described which comprises producing an aerosol of a stable liquid suspension of a protein using an electrohydrodynamic spraying means wherein the liquid suspension comprises particles of the protein suspended in ethanol. The stable ethanol suspensions of the invention may optionally contain up to about 20% (V / V) of a pharmaceutically acceptable formulation additive such as glycerol, propylene glycol and polyethylene glycol as well as minor amounts (from about 0.05% to about 5.0% W / V) of a pharmaceutically acceptable excipient.
Owner:VENTAIRA PHARMA
Transgenic algae for delivering antigens to an animal
Delivery systems and methods are provided for delivering a biologically active protein to a host animal. The systems and methods provided include obtaining an algal cell transformed by an expression vector, the expression vector comprising a nucleotide sequence coding for the biologically active protein, operably linked to a promoter. In one illustrated embodiment, the biologically active protein is an antigenic epitope and upon administration to the animal the algal cell induces an immune response in the host animal.
Owner:PHYCOTRANSGENICS +1
Targeted delivery via biodegradable polymers
InactiveUS6911216B1Reduce deliveryEasy transferPowder deliveryPeptide/protein ingredientsActive proteinMicroparticle
Delivery of bioactive molecules such as nucleic acid molecules encoding a protein can be significantly enhanced by immobilization of the bioactive molecule in a polymeric material adjacent to the cells where delivery is desired, where the bioactive molecule is encapsulated in a vehicle such as liposomes which facilitates transfer of the bioactive molecules into the targeted tissue. Targeting of the bioactive molecules can also be achieved by selection of an encapsulating medium of an appropriate size whereby the medium serves to deliver the molecules to a particular target. For example, encapsulation of nucleic acid molecules or biologically active proteins within biodegradable, biocompatible polymeric microparticles which are appropriate sized to infiltrate, but remain trapped within, the capillary beds and alveoli of the lungs can be used for targeted delivery to these regions of the body following administration to a patient by infusion or injection.
Owner:GENZYME CORP
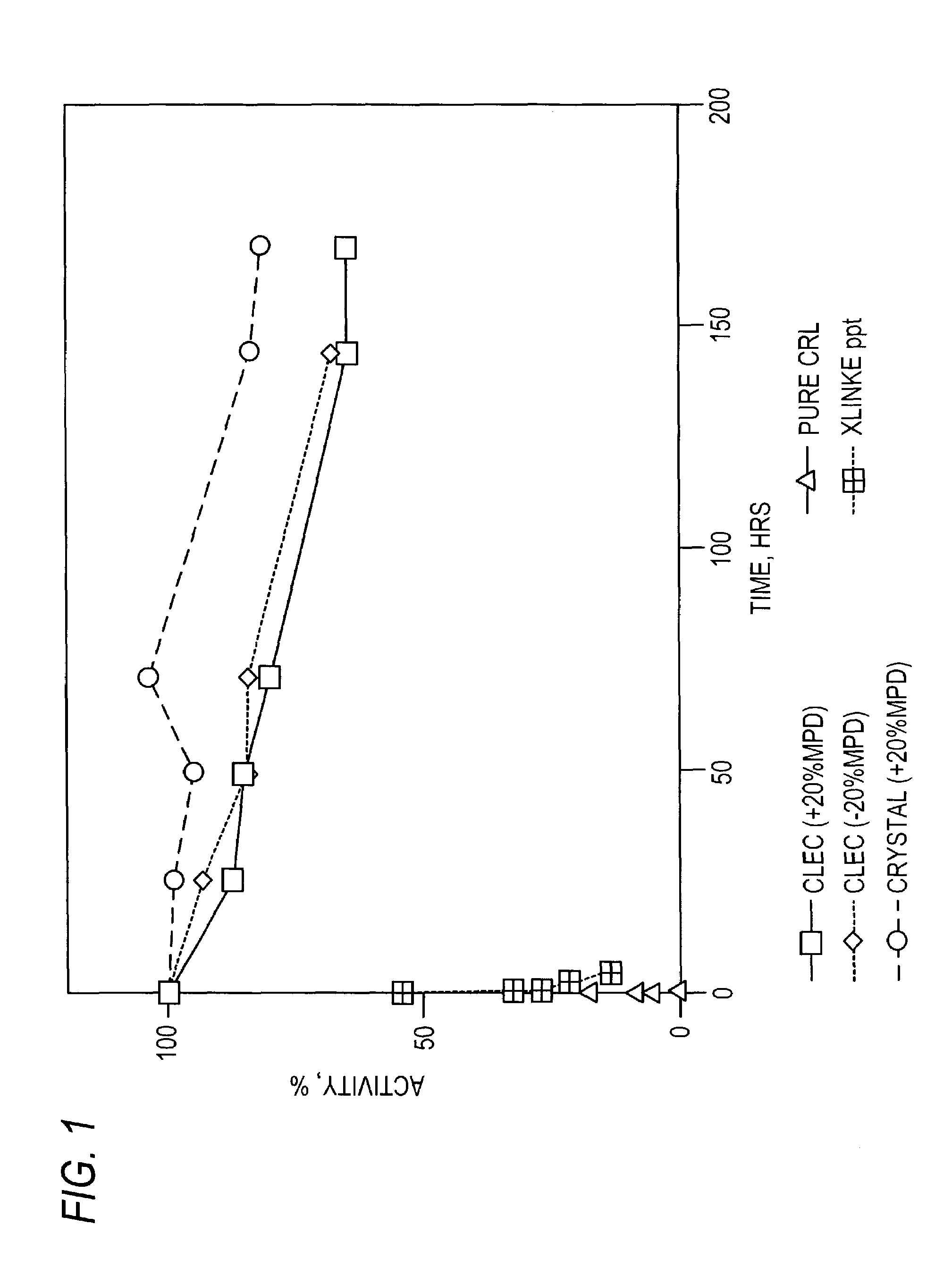
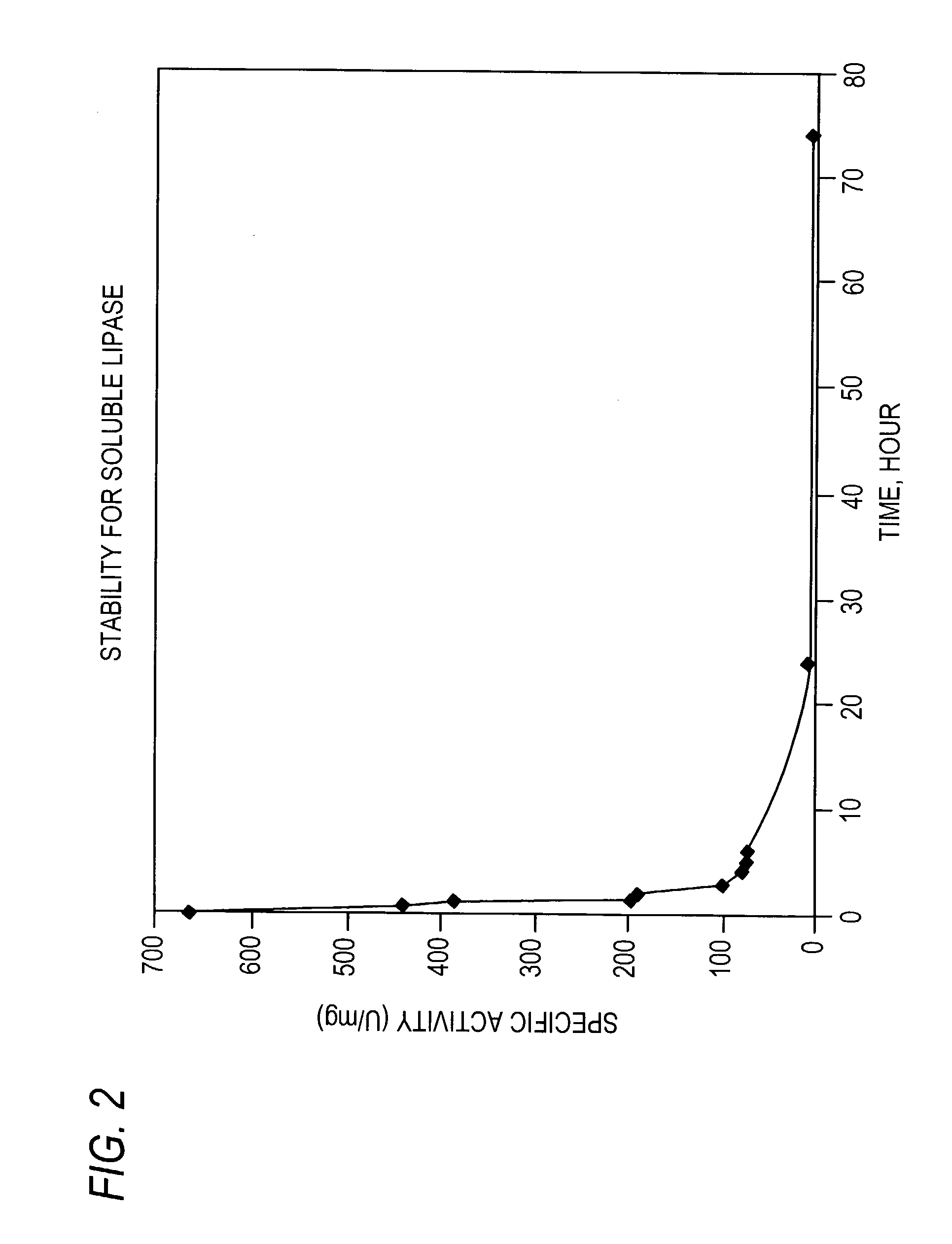
Stabilized protein crystals, formulations comprising them and methods of making them
InactiveUS7351798B2Further stabilizationComposition is stableImmobilised enzymesPowder deliveryPersonal careAdditive ingredient
This invention relates to methods for the stabilization, storage and delivery of biologically active macromolecules, such as proteins, peptides and nucleic acids. In particular, this invention relates to protein or nucleic acid crystals, formulations and compositions comprising them. Methods are provided for the crystallization of proteins and nucleic acids and for the preparation of stabilized protein or nucleic acid crystals for use in dry or slurry formulations. The present invention is further directed to encapsulating proteins, glycoproteins, enzymes, antibodies, hormones and peptide crystals or crystal formulations into compositions for biological delivery to humans and animals. According to this invention, protein crystals or crystal formulations are encapsulated within a matrix comprising a polymeric carrier to form a composition. The formulations and compositions enhance preservation of the native biologically active tertiary structure of the proteins and create a reservoir which can slowly release active protein where and when it is needed. Methods are provided preparing stabilized formulations using pharmaceutical ingredients or excipients and optionally encapsulating them in a polymeric carrier to produce compositions and using such protein crystal formulations and compositions for biomedical applications, including delivery of therapeutic proteins and vaccines. Additional uses for the protein crystal formulations and compositions of this invention involve protein delivery in human food, agricultural feeds, veterinary compositions, diagnostics, cosmetics and personal care compositions.
Owner:AJINOMOTO ALTHEA INC
Monofunctional polyethylene glycol aldehydes
The present invention provides novel monofunctional polyethylene glycol aldehydes for the pegylation of therapeutically active proteins. The pegylated protein conjugates that are produced, retain a substantial portion of their therapeutic activity and are less immunogenic than the protein from which the conjugate is derived. New syntheses for preparing such aldehydes are described.
Owner:SUN BIO INC
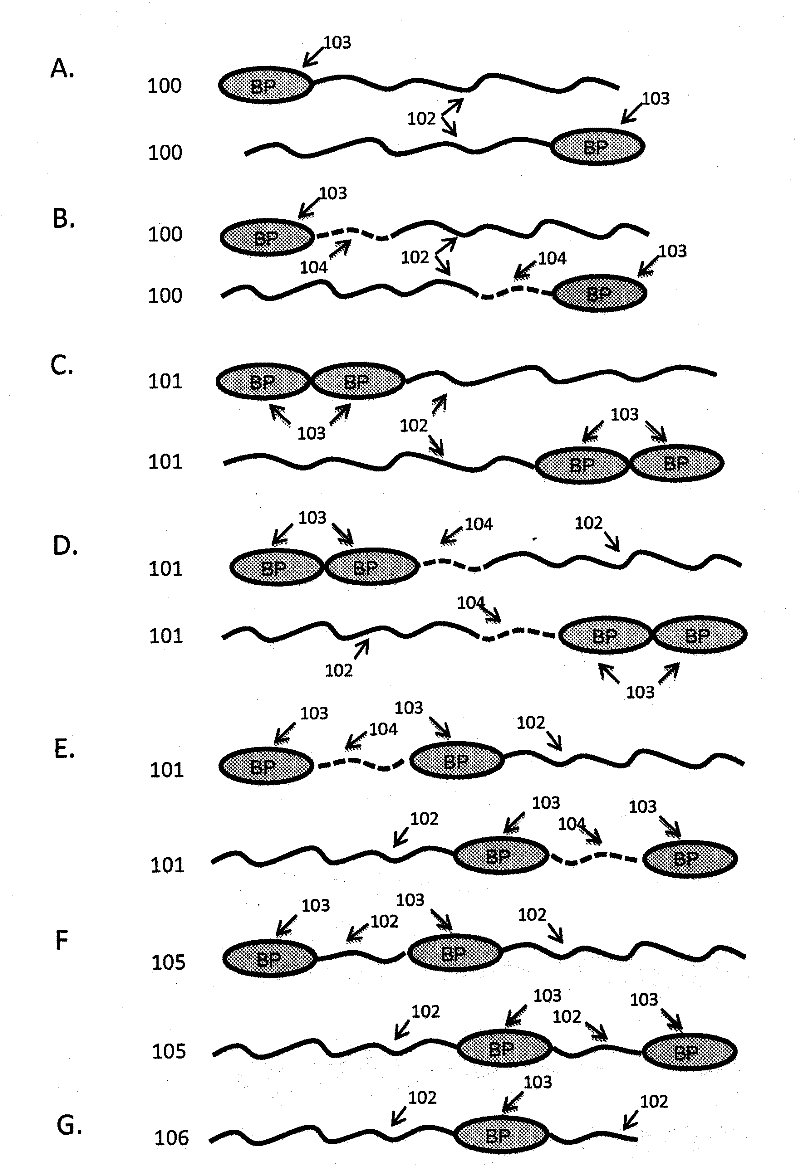
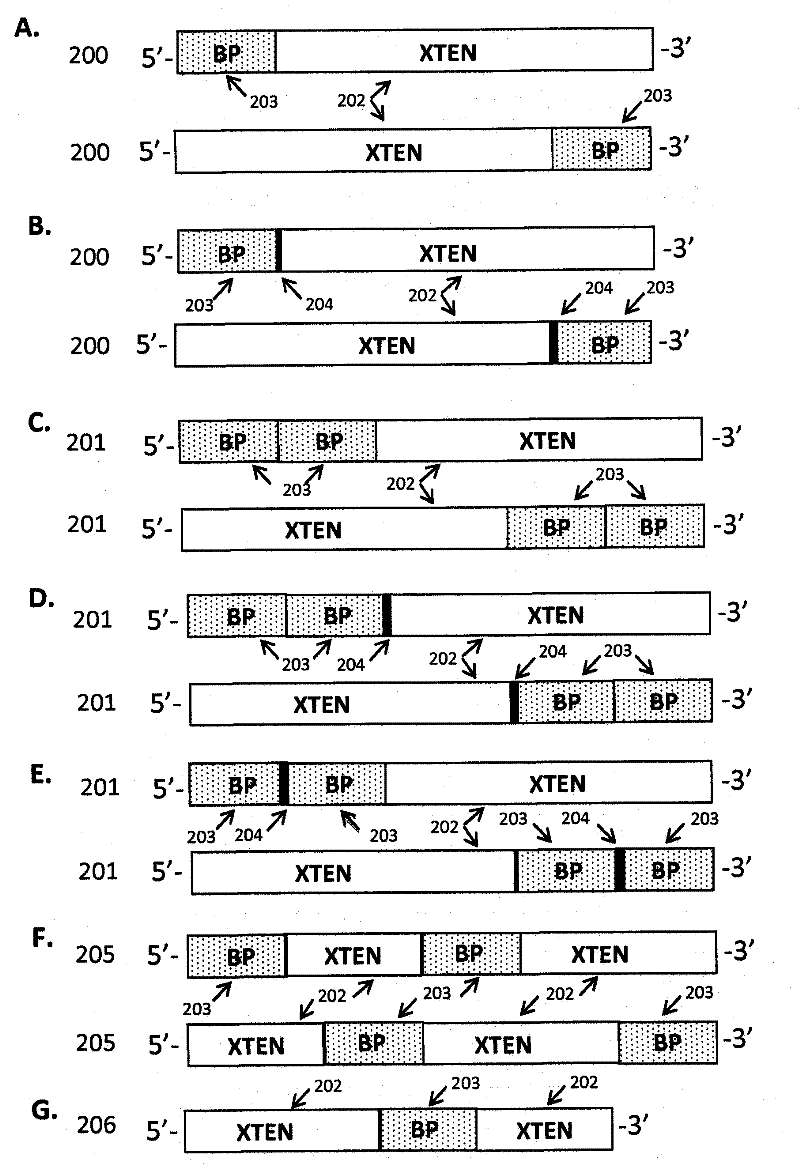
Extended recombinant polypeptides and compositions comprising same
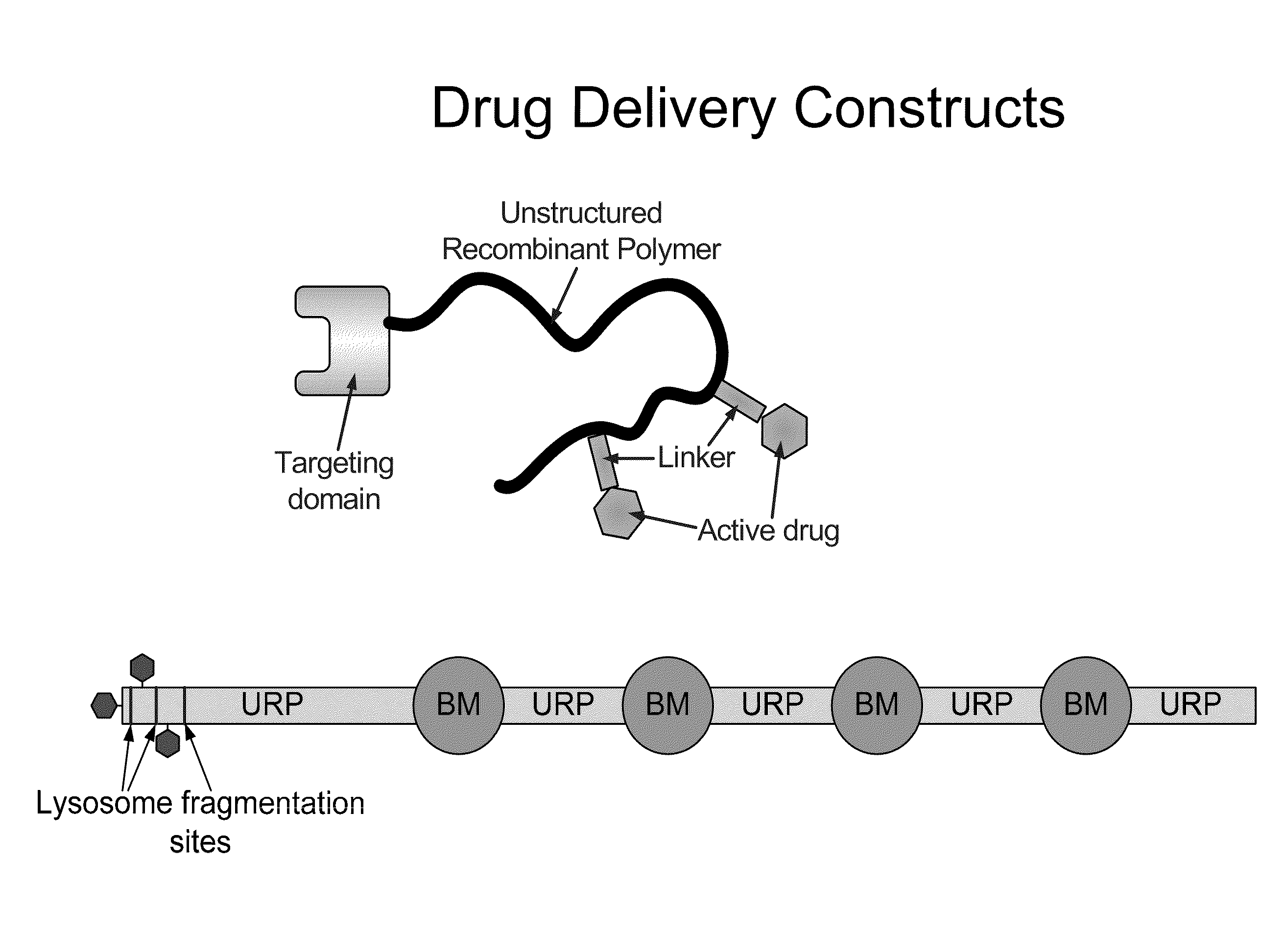
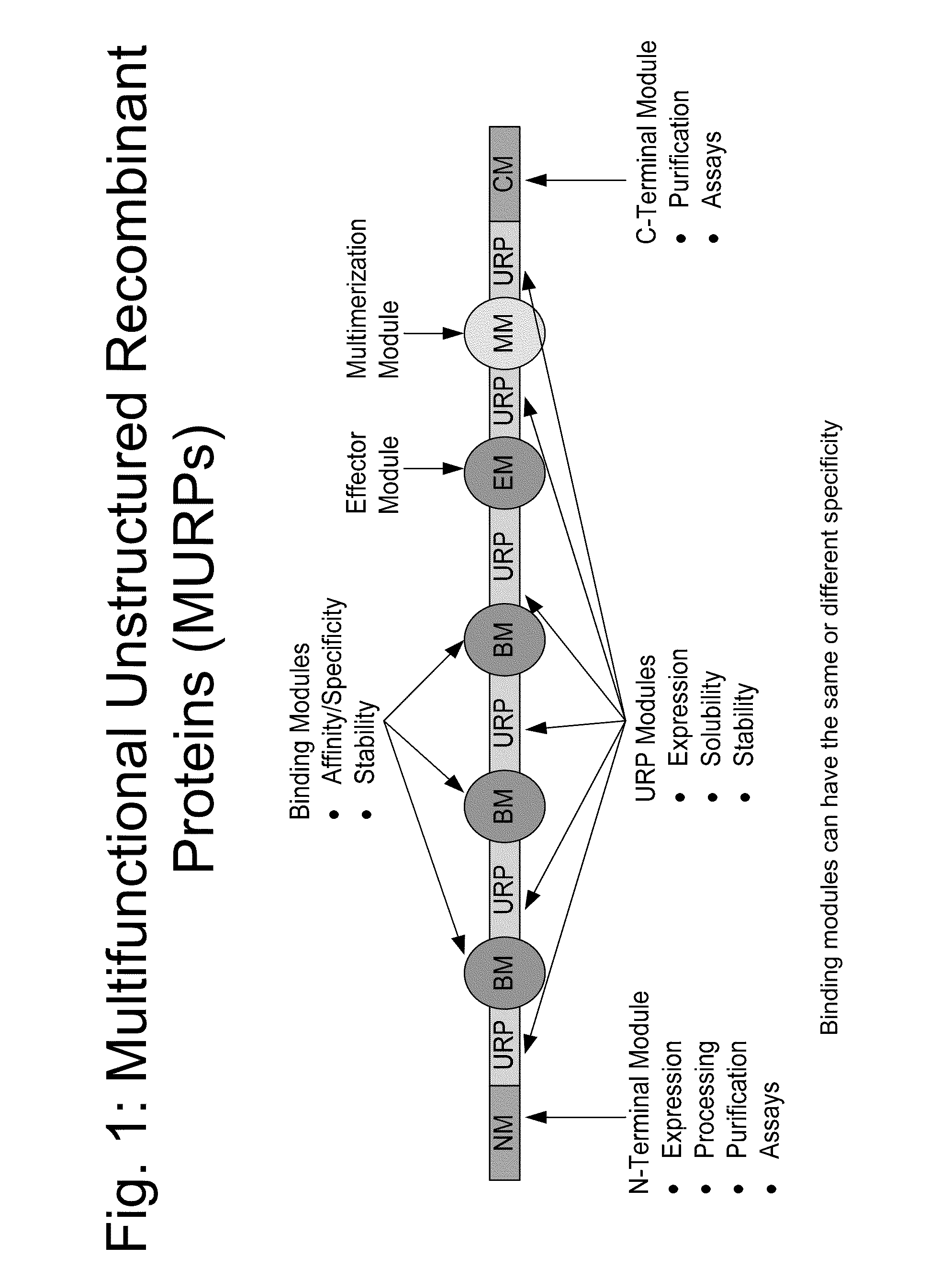
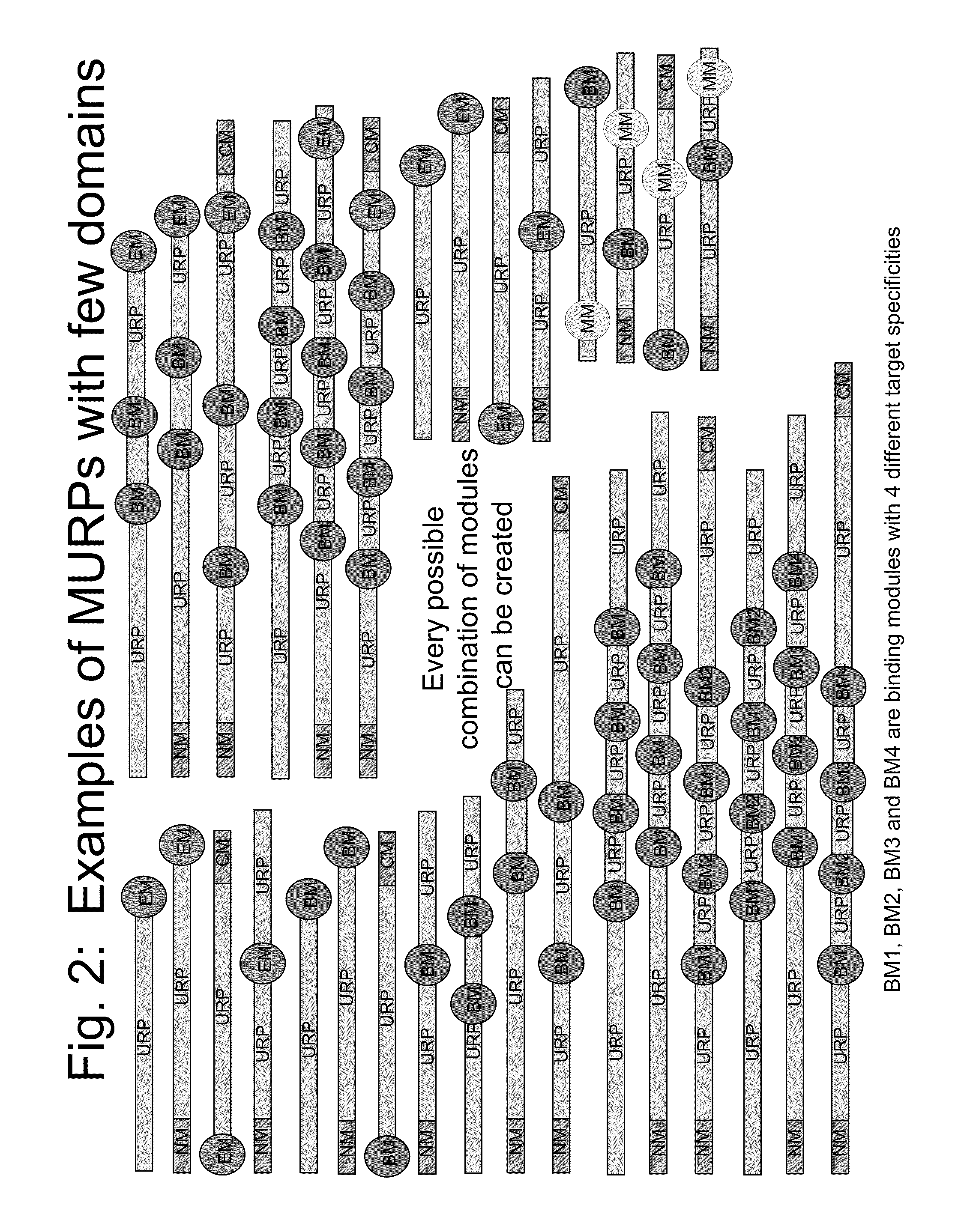
The present invention relates to compositions comprising biologically active proteins linked to extended recombinant polypeptide (XTEN), isolated nucleic acids encoding the compositions and vectors and host cells containing the same, and methods of using such compositions in treatment of glucose- related diseases, metabolic diseases, coagulation disorders, and growth hormone-related disorders and conditions.
Owner:阿穆尼克斯制药公司
Bacillussubtilis for decomposing aflatoxin and active protein secreted by same
ActiveCN102031234AImprove efficiencyStrong specificityAntibacterial agentsBacteriaDecompositionActive protein
Owner:CHINA AGRI UNIV
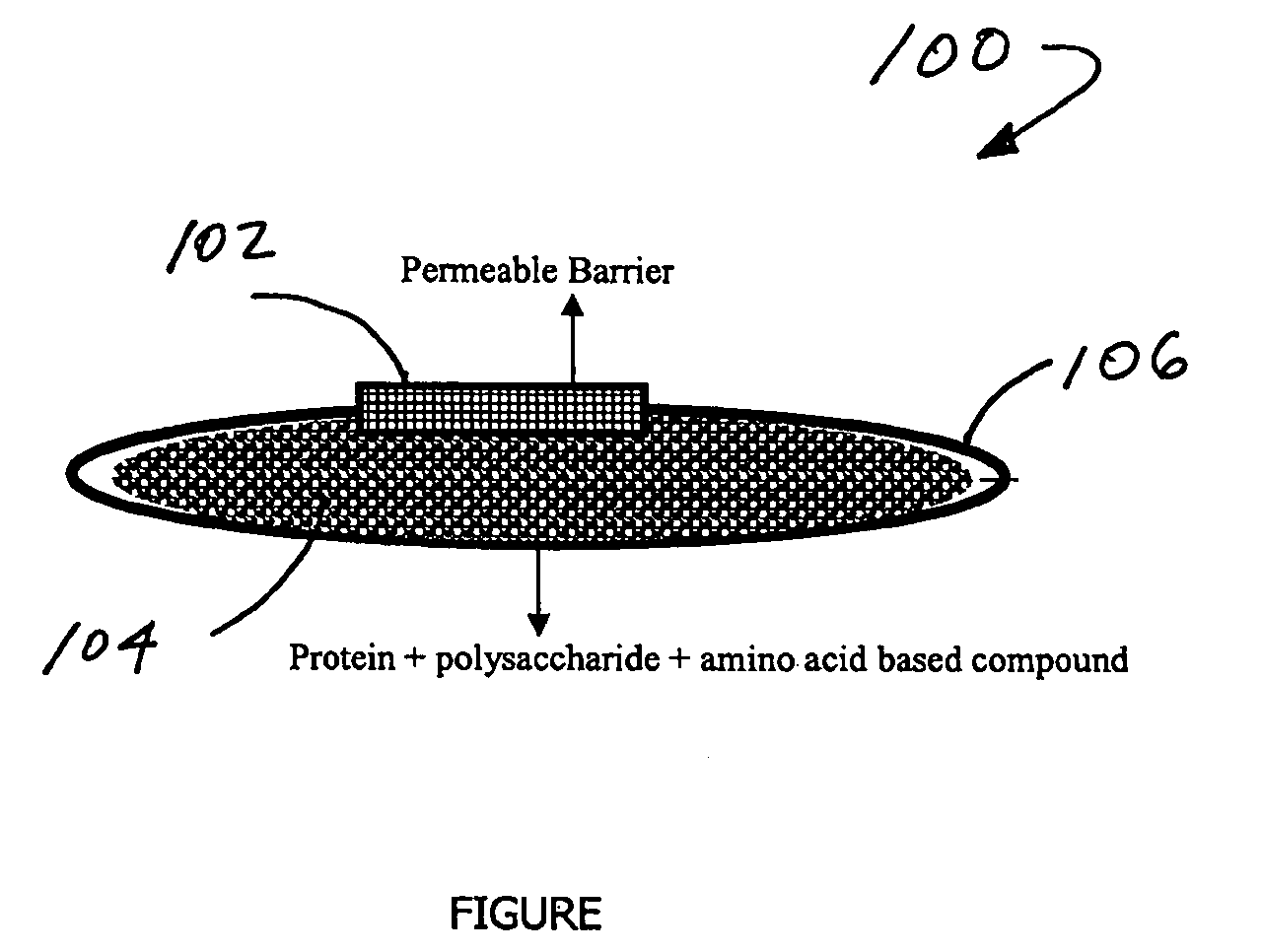
Stabilization of biologically active proteins with mixtures of polysaccharides and amino acid based compounds
InactiveUS20060024346A1Uniform pore sizeReduce concentrationOrganic active ingredientsBiocideActive proteinCompound (substance)
The invention provides heat stable aqueous solutions or gels comprising a biologically active protein and a stabilizing effective amount of a mixture of a polysaccharide and an amino acid based compound. The invention also discloses stabilized solutions or gels suitable for use in an implantable drug delivery device at body temperature, and a device containing the stabilized solution or gels.
Owner:BATTELLE MEMORIAL INST
Increased recovery of active proteins
InactiveUS7157557B2Raise the ratioFold preciselySugar derivativesPeptide/protein ingredientsSufficient timeActive protein
The invention provides methods of increasing yields of desired conformation of proteins. In particular embodiments, the invention includes contacting preparations of a recombinant protein with a reduction / oxidation coupling reagent for a time sufficient to increase the relative proportion of a desired configurational isomer.
Owner:IMMUNEX CORP
Long chain recombinant human bone morphogenesis protein-2 and its preparation method and uses
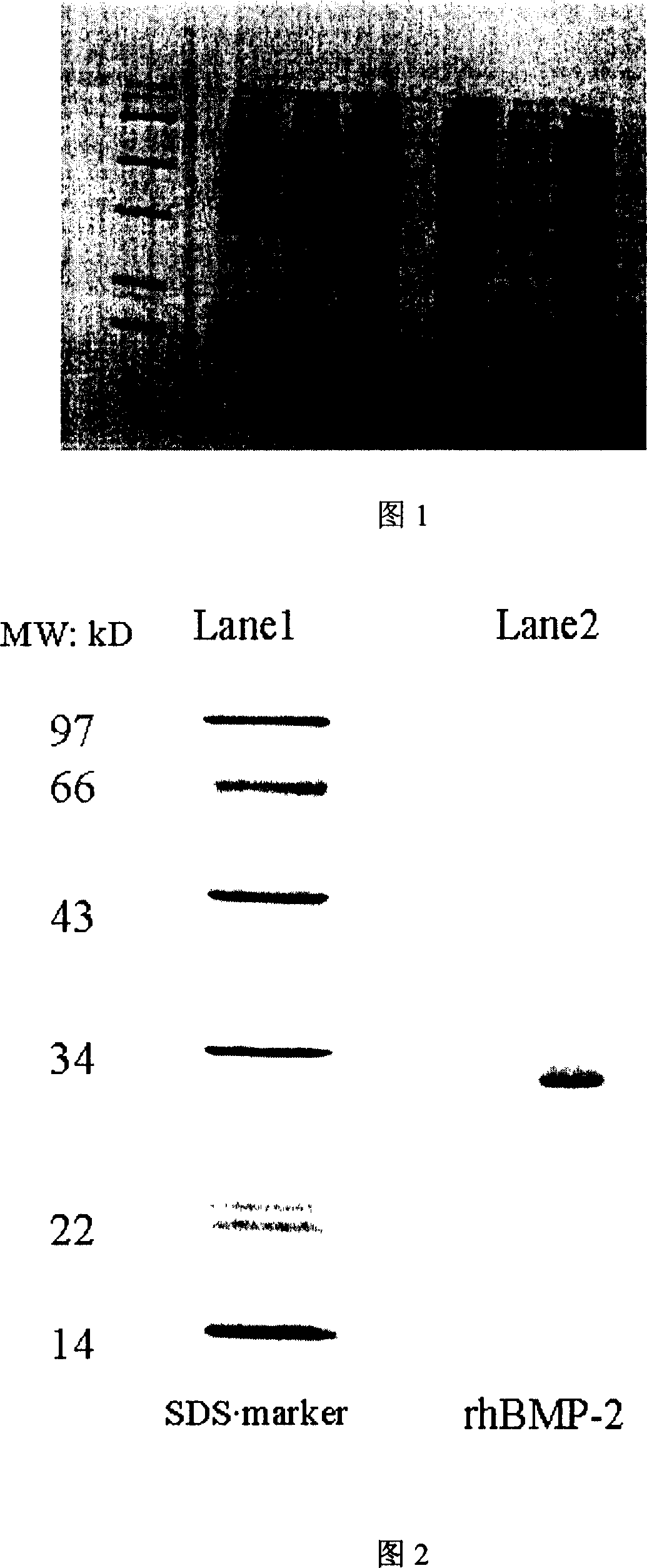
ActiveCN1951964AIncreased affinity binding sitesEasy to useBacteriaBone-inducing factorEscherichia coliNucleotide
The invention discloses a long-chain recombination human bone pattern generating protein-2 and preparing method and application, which is characterized by the following: utilizing gene project technique to grow the protein in the expressing system of escherichia coli and bacillus subtilis; adopting human bone sarcoma cell mRNA to do reverse transcription to obtain the cDNA as form; augmenting nucleotide sequence of entire 114 amino acids naturally from carboxyl end; adding a segment of nucleotide in front of the first codon of primer 5' end; increasing a segment of polypeptide at N end corresponding to amino acid sequence; obtaining long-chain rhBMP-2 gene with molecular weight at 30KD and purity over 95%.
Owner:SHANGHAI REBONE BIOMATERIALS
Vesicular phospholipid gels comprising proteinaceous substances
The present invention relates to a pharmaceutical composition for sustained release of a pharmaceutically active compound, the composition comprising a vesicular phospholipid gel. More particularly, the invention relates to a pharmaceutical composition comprising at least one proteinaceous substance as the pharmaceutically active compound in encapsulated form, the at least one proteinaceous substance being a biologically active protein, peptide or polypeptide. Furthermore, the present invention relates to a method for the production of said pharmaceutical composition comprising dual asymmetric centrifugation and to the use of said pharmaceutical composition for immunotherapy and / or for stimulating selective tissue regeneration in the treatment of surgical defects in the course of surgical interventions.
Owner:WINTER GERHARD DR
Method for preparing dephenolization cottonseed protein and raffinose
ActiveCN101785527AHigh nutritional valueSimple processSugar productsFood processingFiltrationActive protein
The invention relates to a method for deep processing cottonseeds, in particular to a method for preparing dephenolization cottonseed protein and raffinose. Gross cottonseeds are firstly delinted by a delinting machine to obtain naked seeds; after being husked by a husking machine, the naked seeds are subjected to kernel husk separation by an angle sieve to obtain cottonseed kernels and cottonseed husks; the cottonseed kernels are subjected to softening conditioning, compacting and drying; the dried blanks are sent to an extractor by a scraper blade and a closed auger and then are sprayed in stages by the No.6 solvent oil to obtain mixed oil; the mixed oil is subjected to sedimentation and centrifugal separation and purification, then is processed by a first evaporator and a second evaporator and is processed by a stripping tower to remove the solvent to obtain crude oil; the crude oil is refined to obtain edible oil; wet cotton dregs are put in a dephenolizing extractor and are subjected to liquid separation and filtration and purification to recover the solvent and also extract the crude product of the raffinose; and after being extruded, the wet cotton dregs are subjected to drying, desolventizing and conditioning and then are sent to a grinding packing department. The process technology has the advantages of high oil yielding rate, complete dephenolization, high content of active protein, simple process and low production cost.
Owner:邯郸晨光植物蛋白有限公司
Protein purification method
InactiveUS20080255342A1Efficient removalReduce conductivityImmunoglobulins against cell receptors/antigens/surface-determinantsPeptide preparation methodsActive proteinAqueous solution
A method for removing contaminant DNA in a sample containing a physiologically active protein, which comprises the following steps:1) converting the sample containing a physiologically active protein into a neutral aqueous solution of low conductivity; and2) removing the resulting particles.
Owner:CHUGAI PHARMA CO LTD
Method for stabilizing protein solution preparation
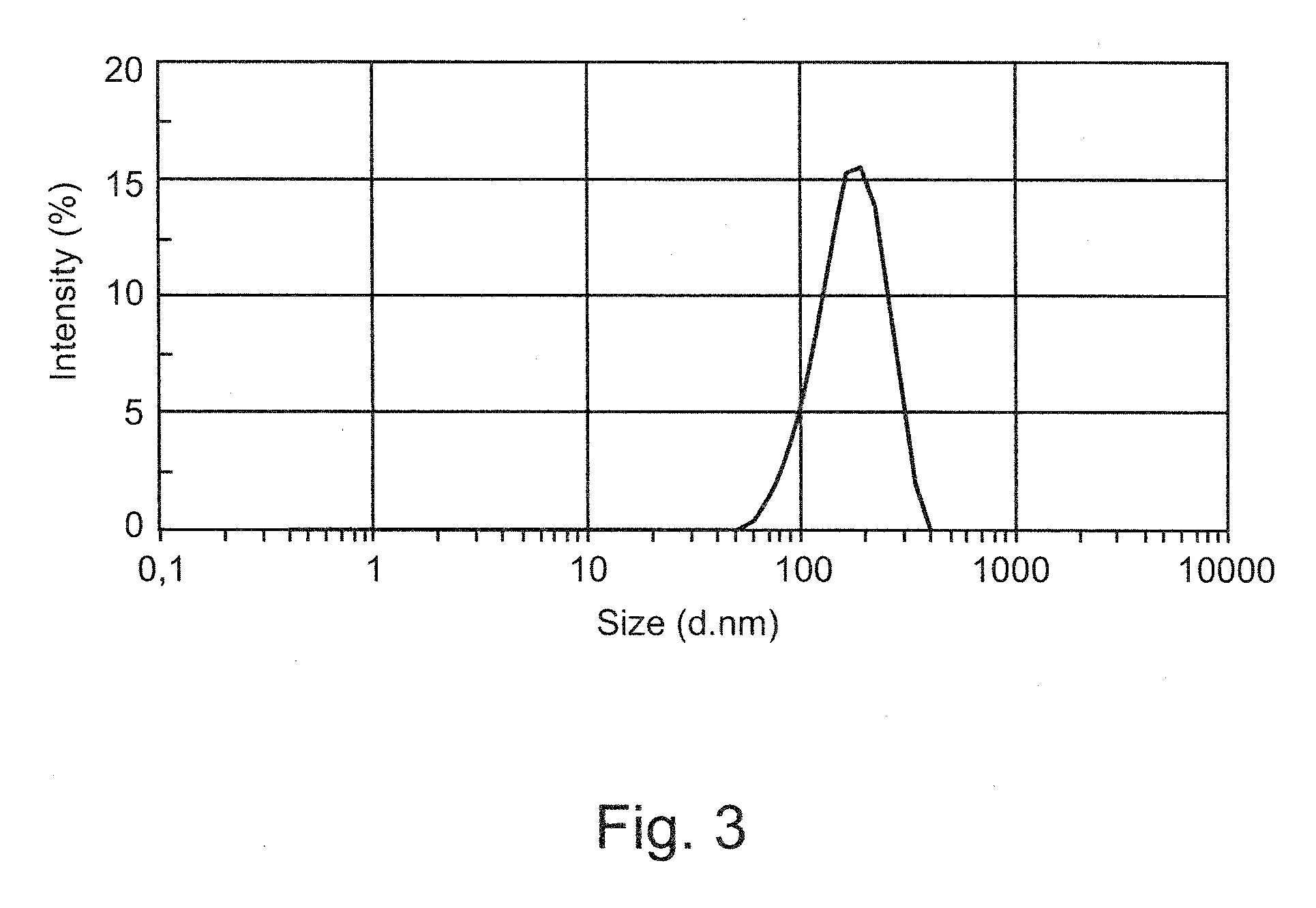
InactiveUS20060058511A1Suppress formationPeptide/protein ingredientsColony-stimulating factorProtein solutionActive protein
Problems to be Solved: The present invention provides a method for improving the stability of protein solution formulations by suppressing the formation of associated matter from physiologically active proteins (e.g., antibodies, enzymes, hormones, cytokines) in a solution form. Means for Solving the Problems: A method for stabilizing a protein solution formulation, which comprises storing the protein solution formulation under magnetic field lines, as well as a storage container for holding a protein solution formulation, which is equipped with a magnetic field generator.
Owner:CHUGAI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com