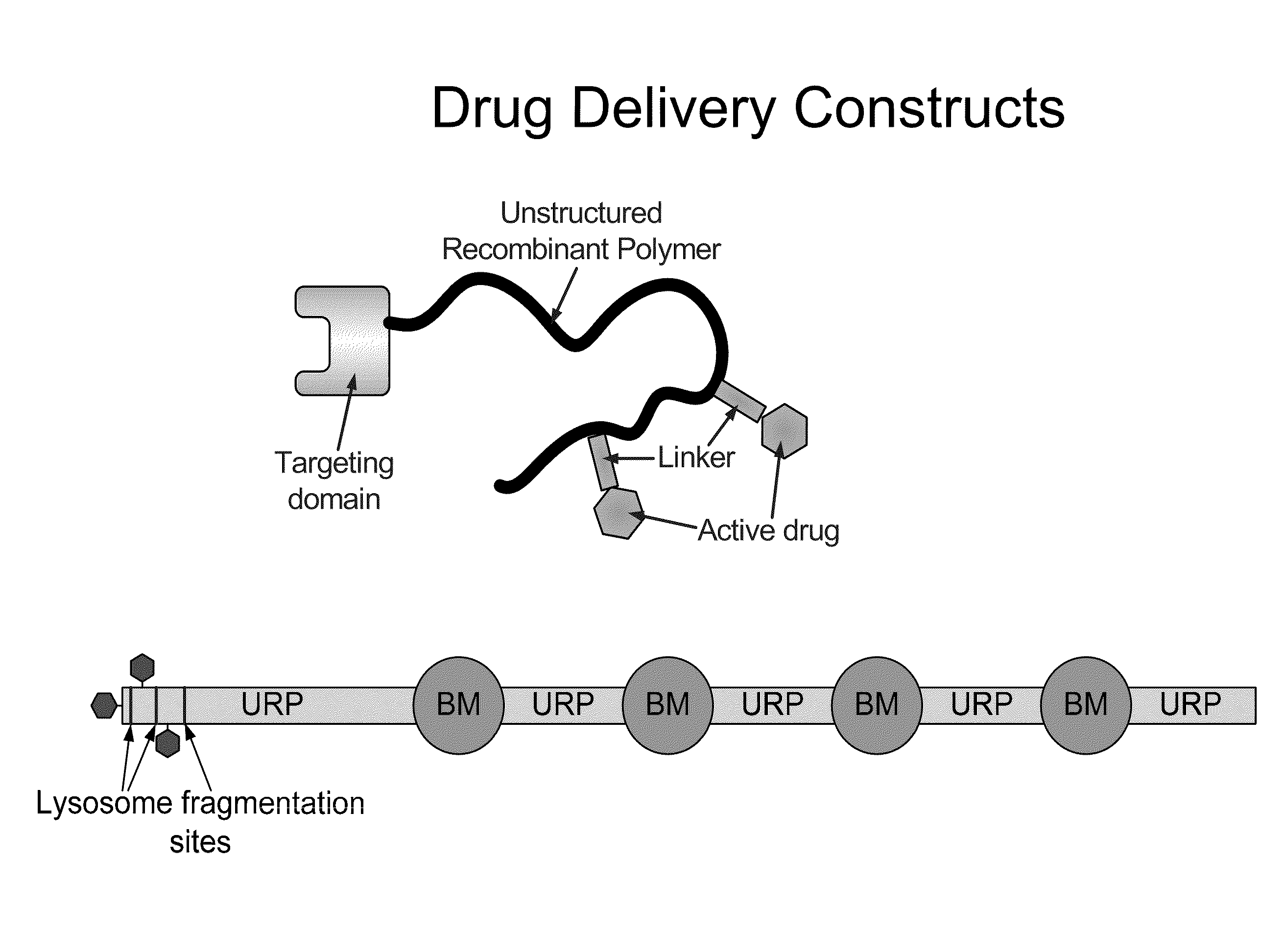
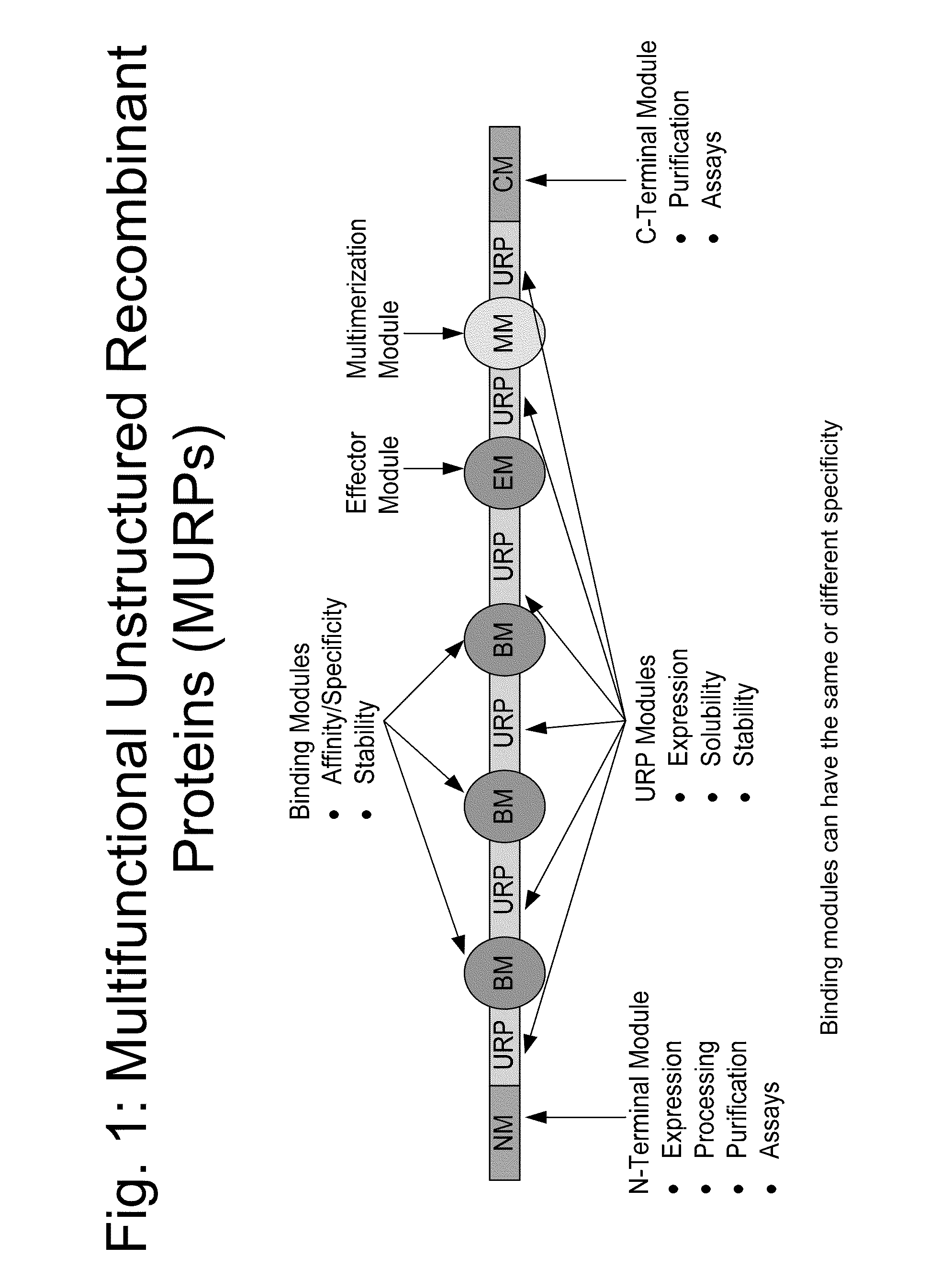
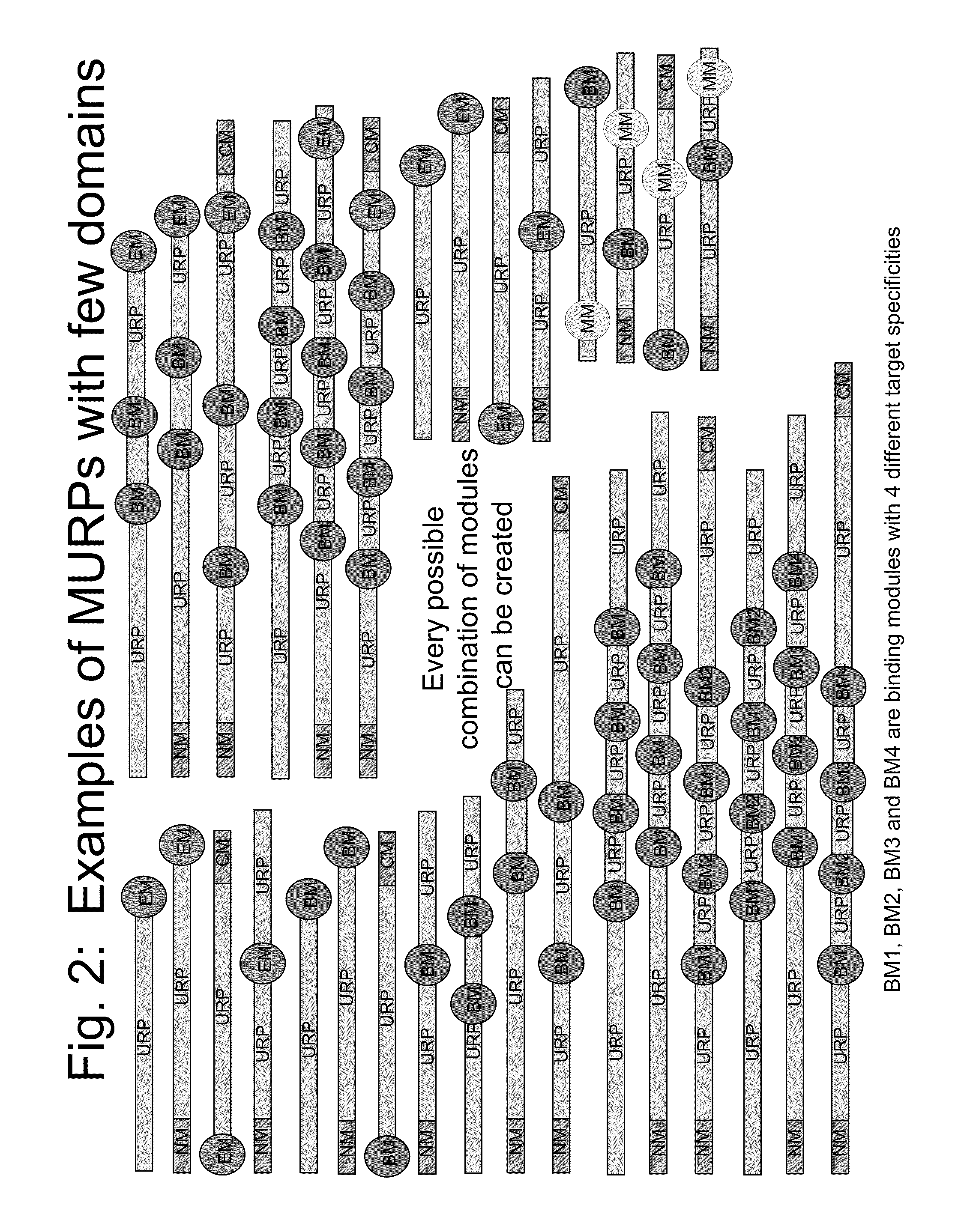
Biologically active proteins having increased In Vivo and/or In Vitro stability
a technology of biological active proteins and stability, which is applied in the direction of peptide/protein ingredients, depsipeptides, dna preparations, etc., can solve the problems of reduced or no therapeutic activity, significant product loss, and difficult manufacturing and characterization of mixtures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Example
Design of a Glycine-Serine Oligomer Based on Human Sequences
[0264]The human genome data base was searched for sequences that are rich in glycine. Three sequences were identified as suitable donor sequences as shown in Table X.
TABLE XDonor sequences for GRS design A.AminoAccessionSequencesacidProteinNP_009060GGGSGGGSGSGGGG486-499zinc finger proteinQ9Y2X9GSGSGGGGSGG19-31zinc finger proteinCAG38801SGGGGSGGGSGSG 7-19MAP2K4
[0265]Based on the sequences in Table X we designed a glycine rich sequence that contains multiple repeats of the peptide A with sequence GGGSGSGGGGS. Peptide A can be oligomerized to form structures with the formula (GGGSGSGGGGS)n where n is between 2 and 40. FIG. 5 shows that all possible 9mer subsequences in oligomers of peptide A are contained in at least one of the proteins listed in table 3. Thus oligomers of peptide A do not contain human T cell epitopes. Inspection of FIG. 5 reveals that GRS based on oligomers of peptide A can begin and end at any of the...
example
Serum Binding Activity of MURPs
[0344]One can coat MURPs of interest into microtiter plates and control proteins in other wells of the plate. Subsequently, one can add serum samples of interest to the wells for 1 hour. Subsequently, the wells can be washed with a plate washer. Bound serum proteins can be detected by adding antibodies against serum proteins that have been conjugated with enzymes like horse radish peroxidase or alkaline phosphatase for detection. Another way to detec serum binding to MURPs to add the MURP of interest to serum for about 1 hour to allow binding. Subsequently, one can immunoprecipitate the MURP using an antibody against an epitope in the MURP sequence. The precipitated samples can be analyzed by PAGE and optionally by Western to detect any proteins that co-precipitated with the MURP. One can identify the serum proteins that show co-precipitation by mass spectrometry.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| optical density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com